CN116271079A - anti-DLL 3 antibody, preparation method thereof, drug conjugate and application thereof - Google Patents
anti-DLL 3 antibody, preparation method thereof, drug conjugate and application thereof Download PDFInfo
- Publication number
- CN116271079A CN116271079A CN202210911943.4A CN202210911943A CN116271079A CN 116271079 A CN116271079 A CN 116271079A CN 202210911943 A CN202210911943 A CN 202210911943A CN 116271079 A CN116271079 A CN 116271079A
- Authority
- CN
- China
- Prior art keywords
- seq
- amino acid
- acid sequence
- antibody
- sequence shown
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000003814 drug Substances 0.000 title claims abstract description 54
- 229940079593 drug Drugs 0.000 title claims abstract description 43
- 238000002360 preparation method Methods 0.000 title claims abstract description 15
- 101000928513 Homo sapiens Delta-like protein 3 Proteins 0.000 claims abstract description 68
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 55
- 230000027455 binding Effects 0.000 claims abstract description 42
- 206010041067 Small cell lung cancer Diseases 0.000 claims abstract description 28
- 210000004027 cell Anatomy 0.000 claims description 88
- 229940049595 antibody-drug conjugate Drugs 0.000 claims description 61
- 239000000611 antibody drug conjugate Substances 0.000 claims description 60
- 102100036466 Delta-like protein 3 Human genes 0.000 claims description 57
- 229940127276 delta-like ligand 3 Drugs 0.000 claims description 49
- 238000000034 method Methods 0.000 claims description 42
- 238000011282 treatment Methods 0.000 claims description 32
- 239000008194 pharmaceutical composition Substances 0.000 claims description 24
- 208000000587 small cell lung carcinoma Diseases 0.000 claims description 24
- 125000000217 alkyl group Chemical group 0.000 claims description 16
- 201000011510 cancer Diseases 0.000 claims description 16
- 150000001875 compounds Chemical class 0.000 claims description 16
- 230000014509 gene expression Effects 0.000 claims description 16
- 229940127089 cytotoxic agent Drugs 0.000 claims description 15
- 239000002254 cytotoxic agent Substances 0.000 claims description 12
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 12
- 239000013604 expression vector Substances 0.000 claims description 12
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 claims description 10
- 239000000427 antigen Substances 0.000 claims description 10
- 102000036639 antigens Human genes 0.000 claims description 10
- 108091007433 antigens Proteins 0.000 claims description 10
- 101100226845 Strongylocentrotus purpuratus EGF2 gene Proteins 0.000 claims description 9
- COLNVLDHVKWLRT-QMMMGPOBSA-N phenylalanine group Chemical group N[C@@H](CC1=CC=CC=C1)C(=O)O COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 9
- 241001529936 Murinae Species 0.000 claims description 8
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 claims description 8
- 201000010099 disease Diseases 0.000 claims description 8
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 8
- 230000002159 abnormal effect Effects 0.000 claims description 7
- 239000007788 liquid Substances 0.000 claims description 7
- 125000006708 (C5-C14) heteroaryl group Chemical group 0.000 claims description 6
- 230000005856 abnormality Effects 0.000 claims description 6
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims description 6
- 238000012258 culturing Methods 0.000 claims description 6
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 claims description 6
- 125000005842 heteroatom Chemical group 0.000 claims description 6
- 201000011519 neuroendocrine tumor Diseases 0.000 claims description 6
- 229940124597 therapeutic agent Drugs 0.000 claims description 6
- CKLJMWTZIZZHCS-REOHCLBHSA-N aspartic acid group Chemical group N[C@@H](CC(=O)O)C(=O)O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 5
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 5
- -1 gaseous Substances 0.000 claims description 5
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 claims description 5
- 206010052399 Neuroendocrine tumour Diseases 0.000 claims description 4
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 claims description 4
- 210000003527 eukaryotic cell Anatomy 0.000 claims description 4
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 claims description 4
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 4
- 208000016065 neuroendocrine neoplasm Diseases 0.000 claims description 4
- 108020004707 nucleic acids Proteins 0.000 claims description 4
- 102000039446 nucleic acids Human genes 0.000 claims description 4
- 150000007523 nucleic acids Chemical class 0.000 claims description 4
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 claims description 4
- 238000003259 recombinant expression Methods 0.000 claims description 4
- 229910052717 sulfur Inorganic materials 0.000 claims description 4
- 230000001225 therapeutic effect Effects 0.000 claims description 4
- 241000588724 Escherichia coli Species 0.000 claims description 3
- 108010003723 Single-Domain Antibodies Proteins 0.000 claims description 3
- 239000002246 antineoplastic agent Substances 0.000 claims description 3
- 125000003118 aryl group Chemical group 0.000 claims description 3
- 210000004978 chinese hamster ovary cell Anatomy 0.000 claims description 3
- 238000012377 drug delivery Methods 0.000 claims description 3
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 claims description 3
- 229960003444 immunosuppressant agent Drugs 0.000 claims description 3
- 230000001861 immunosuppressant effect Effects 0.000 claims description 3
- 239000003018 immunosuppressive agent Substances 0.000 claims description 3
- 238000001802 infusion Methods 0.000 claims description 3
- 239000007924 injection Substances 0.000 claims description 3
- 238000002347 injection Methods 0.000 claims description 3
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 claims description 3
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 claims description 3
- 238000012544 monitoring process Methods 0.000 claims description 3
- 229910052760 oxygen Inorganic materials 0.000 claims description 3
- 230000005855 radiation Effects 0.000 claims description 3
- 239000008299 semisolid dosage form Substances 0.000 claims description 3
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 claims description 3
- 239000007909 solid dosage form Substances 0.000 claims description 3
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 claims description 3
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 claims description 3
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 claims description 3
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 2
- 230000006806 disease prevention Effects 0.000 claims description 2
- 210000005260 human cell Anatomy 0.000 claims description 2
- 210000002865 immune cell Anatomy 0.000 claims description 2
- 210000000822 natural killer cell Anatomy 0.000 claims description 2
- 210000001236 prokaryotic cell Anatomy 0.000 claims description 2
- 230000009465 prokaryotic expression Effects 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 132
- 239000002253 acid Substances 0.000 claims 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims 1
- 230000000694 effects Effects 0.000 abstract description 16
- 108090000623 proteins and genes Proteins 0.000 abstract description 14
- 102000053255 human DLL3 Human genes 0.000 abstract description 11
- 102000004169 proteins and genes Human genes 0.000 abstract description 10
- 230000005917 in vivo anti-tumor Effects 0.000 abstract description 9
- 230000001472 cytotoxic effect Effects 0.000 abstract description 7
- 230000004071 biological effect Effects 0.000 abstract description 3
- 231100000433 cytotoxic Toxicity 0.000 abstract description 3
- 230000000955 neuroendocrine Effects 0.000 abstract description 3
- 230000005918 in vitro anti-tumor Effects 0.000 abstract description 2
- 238000001727 in vivo Methods 0.000 abstract 1
- 150000001413 amino acids Chemical class 0.000 description 163
- 230000035772 mutation Effects 0.000 description 63
- 239000002953 phosphate buffered saline Substances 0.000 description 27
- 239000006228 supernatant Substances 0.000 description 26
- 241000699670 Mus sp. Species 0.000 description 24
- 241001465754 Metazoa Species 0.000 description 18
- 239000000562 conjugate Substances 0.000 description 18
- 238000002965 ELISA Methods 0.000 description 16
- 238000001514 detection method Methods 0.000 description 14
- 210000004408 hybridoma Anatomy 0.000 description 13
- 239000000243 solution Substances 0.000 description 12
- UQLDLKMNUJERMK-UHFFFAOYSA-L di(octadecanoyloxy)lead Chemical compound [Pb+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O UQLDLKMNUJERMK-UHFFFAOYSA-L 0.000 description 11
- 238000012360 testing method Methods 0.000 description 11
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 10
- 229920001213 Polysorbate 20 Polymers 0.000 description 10
- 235000001014 amino acid Nutrition 0.000 description 10
- 229940024606 amino acid Drugs 0.000 description 10
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 10
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 10
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 10
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 9
- 235000018102 proteins Nutrition 0.000 description 9
- 238000005406 washing Methods 0.000 description 9
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 8
- 241000699666 Mus <mouse, genus> Species 0.000 description 8
- 238000005859 coupling reaction Methods 0.000 description 8
- 238000007865 diluting Methods 0.000 description 8
- 238000010790 dilution Methods 0.000 description 8
- 239000012895 dilution Substances 0.000 description 8
- 239000013613 expression plasmid Substances 0.000 description 8
- 238000000338 in vitro Methods 0.000 description 8
- 239000002609 medium Substances 0.000 description 8
- 230000035945 sensitivity Effects 0.000 description 8
- 241000282693 Cercopithecidae Species 0.000 description 7
- 230000004083 survival effect Effects 0.000 description 7
- 230000008685 targeting Effects 0.000 description 7
- 239000013598 vector Substances 0.000 description 7
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 6
- 241000283707 Capra Species 0.000 description 6
- 238000002835 absorbance Methods 0.000 description 6
- 238000003556 assay Methods 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 6
- 239000012634 fragment Substances 0.000 description 6
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 6
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 6
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 6
- 238000006467 substitution reaction Methods 0.000 description 6
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 5
- 241000700159 Rattus Species 0.000 description 5
- 230000008878 coupling Effects 0.000 description 5
- 238000010168 coupling process Methods 0.000 description 5
- 238000012217 deletion Methods 0.000 description 5
- 230000037430 deletion Effects 0.000 description 5
- 238000010494 dissociation reaction Methods 0.000 description 5
- 230000005593 dissociations Effects 0.000 description 5
- 238000011156 evaluation Methods 0.000 description 5
- 239000007789 gas Substances 0.000 description 5
- 238000002649 immunization Methods 0.000 description 5
- 230000003053 immunization Effects 0.000 description 5
- 239000013612 plasmid Substances 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 238000012163 sequencing technique Methods 0.000 description 5
- 210000002966 serum Anatomy 0.000 description 5
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- HMUNWXXNJPVALC-UHFFFAOYSA-N 1-[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]piperazin-1-yl]-2-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)N1CCN(CC1)C(CN1CC2=C(CC1)NN=N2)=O HMUNWXXNJPVALC-UHFFFAOYSA-N 0.000 description 4
- 102100037642 Elongation factor G, mitochondrial Human genes 0.000 description 4
- 239000004471 Glycine Substances 0.000 description 4
- 101000880344 Homo sapiens Elongation factor G, mitochondrial Proteins 0.000 description 4
- 108060003951 Immunoglobulin Proteins 0.000 description 4
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 4
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 4
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 4
- 238000007792 addition Methods 0.000 description 4
- 235000004279 alanine Nutrition 0.000 description 4
- 230000000903 blocking effect Effects 0.000 description 4
- 230000037396 body weight Effects 0.000 description 4
- 229910002091 carbon monoxide Inorganic materials 0.000 description 4
- 230000009260 cross reactivity Effects 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 208000035475 disorder Diseases 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 102000018358 immunoglobulin Human genes 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 4
- 239000002244 precipitate Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 238000012216 screening Methods 0.000 description 4
- 230000001988 toxicity Effects 0.000 description 4
- 231100000419 toxicity Toxicity 0.000 description 4
- 230000009466 transformation Effects 0.000 description 4
- 210000004881 tumor cell Anatomy 0.000 description 4
- 239000004474 valine Substances 0.000 description 4
- 239000003981 vehicle Substances 0.000 description 4
- 102100033553 Delta-like protein 4 Human genes 0.000 description 3
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical class O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 101000872077 Homo sapiens Delta-like protein 4 Proteins 0.000 description 3
- 239000004697 Polyetherimide Substances 0.000 description 3
- 101100226846 Strongylocentrotus purpuratus EGF3 gene Proteins 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 238000010276 construction Methods 0.000 description 3
- 230000000875 corresponding effect Effects 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 231100000673 dose–response relationship Toxicity 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 238000000605 extraction Methods 0.000 description 3
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 3
- 229910052737 gold Inorganic materials 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
- 239000001963 growth medium Substances 0.000 description 3
- 229910052736 halogen Inorganic materials 0.000 description 3
- 150000002367 halogens Chemical class 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 239000012160 loading buffer Substances 0.000 description 3
- 235000013336 milk Nutrition 0.000 description 3
- 239000008267 milk Substances 0.000 description 3
- 210000004080 milk Anatomy 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 229920001601 polyetherimide Polymers 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 239000012898 sample dilution Substances 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 230000007480 spreading Effects 0.000 description 3
- 238000003892 spreading Methods 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 231100000331 toxic Toxicity 0.000 description 3
- 230000002588 toxic effect Effects 0.000 description 3
- 238000001890 transfection Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 230000004580 weight loss Effects 0.000 description 3
- GTBCXYYVWHFQRS-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-(pyridin-2-yldisulfanyl)pentanoate Chemical compound C=1C=CC=NC=1SSC(C)CCC(=O)ON1C(=O)CCC1=O GTBCXYYVWHFQRS-UHFFFAOYSA-N 0.000 description 2
- BQWBEDSJTMWJAE-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-[(2-iodoacetyl)amino]benzoate Chemical compound C1=CC(NC(=O)CI)=CC=C1C(=O)ON1C(=O)CCC1=O BQWBEDSJTMWJAE-UHFFFAOYSA-N 0.000 description 2
- 238000011729 BALB/c nude mouse Methods 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- 206010012735 Diarrhoea Diseases 0.000 description 2
- 101001130157 Dioclea sclerocarpa Lectin alpha chain Proteins 0.000 description 2
- 206010059866 Drug resistance Diseases 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 101100499372 Homo sapiens DLL1 gene Proteins 0.000 description 2
- GRRNUXAQVGOGFE-UHFFFAOYSA-N Hygromycin-B Natural products OC1C(NC)CC(N)C(O)C1OC1C2OC3(C(C(O)C(O)C(C(N)CO)O3)O)OC2C(O)C(CO)O1 GRRNUXAQVGOGFE-UHFFFAOYSA-N 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 2
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 2
- 101100499378 Mus musculus Dll3 gene Proteins 0.000 description 2
- 230000004988 N-glycosylation Effects 0.000 description 2
- 102000014736 Notch Human genes 0.000 description 2
- 108010070047 Notch Receptors Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 230000003698 anagen phase Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 238000011190 asparagine deamidation Methods 0.000 description 2
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical class C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 229960004562 carboplatin Drugs 0.000 description 2
- 230000022534 cell killing Effects 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 2
- 229960004316 cisplatin Drugs 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 230000006240 deamidation Effects 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 230000002121 endocytic effect Effects 0.000 description 2
- 230000012202 endocytosis Effects 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 239000012091 fetal bovine serum Substances 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 239000012737 fresh medium Substances 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 230000006801 homologous recombination Effects 0.000 description 2
- 238000002744 homologous recombination Methods 0.000 description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 2
- 238000004191 hydrophobic interaction chromatography Methods 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- GRRNUXAQVGOGFE-NZSRVPFOSA-N hygromycin B Chemical compound O[C@@H]1[C@@H](NC)C[C@@H](N)[C@H](O)[C@H]1O[C@H]1[C@H]2O[C@@]3([C@@H]([C@@H](O)[C@@H](O)[C@@H](C(N)CO)O3)O)O[C@H]2[C@@H](O)[C@@H](CO)O1 GRRNUXAQVGOGFE-NZSRVPFOSA-N 0.000 description 2
- 229940097277 hygromycin b Drugs 0.000 description 2
- 230000016784 immunoglobulin production Effects 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 239000007928 intraperitoneal injection Substances 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 2
- 230000002147 killing effect Effects 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 201000005202 lung cancer Diseases 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- 108010082117 matrigel Proteins 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 230000001124 posttranscriptional effect Effects 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000006916 protein interaction Effects 0.000 description 2
- 230000000306 recurrent effect Effects 0.000 description 2
- 238000011519 second-line treatment Methods 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 239000012085 test solution Substances 0.000 description 2
- 229940126585 therapeutic drug Drugs 0.000 description 2
- 125000003396 thiol group Chemical group [H]S* 0.000 description 2
- 229960000303 topotecan Drugs 0.000 description 2
- 229960002190 topotecan hydrochloride Drugs 0.000 description 2
- 230000004614 tumor growth Effects 0.000 description 2
- MFRNYXJJRJQHNW-DEMKXPNLSA-N (2s)-2-[[(2r,3r)-3-methoxy-3-[(2s)-1-[(3r,4s,5s)-3-methoxy-5-methyl-4-[methyl-[(2s)-3-methyl-2-[[(2s)-3-methyl-2-(methylamino)butanoyl]amino]butanoyl]amino]heptanoyl]pyrrolidin-2-yl]-2-methylpropanoyl]amino]-3-phenylpropanoic acid Chemical compound CN[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MFRNYXJJRJQHNW-DEMKXPNLSA-N 0.000 description 1
- LDXJRKWFNNFDSA-UHFFFAOYSA-N 2-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)-1-[4-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidin-5-yl]piperazin-1-yl]ethanone Chemical compound C1CN(CC2=NNN=C21)CC(=O)N3CCN(CC3)C4=CN=C(N=C4)NCC5=CC(=CC=C5)OC(F)(F)F LDXJRKWFNNFDSA-UHFFFAOYSA-N 0.000 description 1
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 description 1
- LTPSRQRIPCVMKQ-UHFFFAOYSA-N 2-amino-5-methylbenzenesulfonic acid Chemical compound CC1=CC=C(N)C(S(O)(=O)=O)=C1 LTPSRQRIPCVMKQ-UHFFFAOYSA-N 0.000 description 1
- OMNVYXHOSHNURL-WPRPVWTQSA-N Ala-Phe Chemical compound C[C@H](N)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 OMNVYXHOSHNURL-WPRPVWTQSA-N 0.000 description 1
- 210000003771 C cell Anatomy 0.000 description 1
- YSRHEJDCILVGQN-UHFFFAOYSA-N C(C)(=O)SCCC=C=O Chemical group C(C)(=O)SCCC=C=O YSRHEJDCILVGQN-UHFFFAOYSA-N 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 102100036462 Delta-like protein 1 Human genes 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 208000001976 Endocrine Gland Neoplasms Diseases 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 1
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 1
- 101000928537 Homo sapiens Delta-like protein 1 Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 238000012449 Kunming mouse Methods 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 229940122255 Microtubule inhibitor Drugs 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 102100027370 Parathymosin Human genes 0.000 description 1
- 240000005546 Piper methysticum Species 0.000 description 1
- 235000016787 Piper methysticum Nutrition 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 238000002123 RNA extraction Methods 0.000 description 1
- 101000702488 Rattus norvegicus High affinity cationic amino acid transporter 1 Proteins 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 101100187186 Solanum lycopersicum LE16 gene Proteins 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 206010042618 Surgical procedure repeated Diseases 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- IEDXPSOJFSVCKU-HOKPPMCLSA-N [4-[[(2S)-5-(carbamoylamino)-2-[[(2S)-2-[6-(2,5-dioxopyrrolidin-1-yl)hexanoylamino]-3-methylbutanoyl]amino]pentanoyl]amino]phenyl]methyl N-[(2S)-1-[[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]-N-methylcarbamate Chemical compound CC[C@H](C)[C@@H]([C@@H](CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCc1ccc(NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@@H](NC(=O)CCCCCN2C(=O)CCC2=O)C(C)C)cc1)C(C)C IEDXPSOJFSVCKU-HOKPPMCLSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 108010011559 alanylphenylalanine Proteins 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 108010004469 allophycocyanin Proteins 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000003302 anti-idiotype Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 239000007640 basal medium Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 238000003570 cell viability assay Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 230000037029 cross reaction Effects 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 238000011033 desalting Methods 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- OFDNQWIFNXBECV-VFSYNPLYSA-N dolastatin 10 Chemical class CC(C)[C@H](N(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C=1SC=CN=1)CC1=CC=CC=C1 OFDNQWIFNXBECV-VFSYNPLYSA-N 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 201000011523 endocrine gland cancer Diseases 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000011354 first-line chemotherapy Methods 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 239000005090 green fluorescent protein Substances 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000010005 growth-factor like effect Effects 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 238000012165 high-throughput sequencing Methods 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 229940127121 immunoconjugate Drugs 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000012405 in silico analysis Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000012516 mab select resin Substances 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- WKPWGQKGSOKKOO-RSFHAFMBSA-N maytansine Chemical class CO[C@@H]([C@@]1(O)C[C@](OC(=O)N1)([C@H]([C@@H]1O[C@@]1(C)[C@@H](OC(=O)[C@H](C)N(C)C(C)=O)CC(=O)N1C)C)[H])\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 WKPWGQKGSOKKOO-RSFHAFMBSA-N 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 231100000782 microtubule inhibitor Toxicity 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 239000003883 ointment base Substances 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 229950006765 rovalpituzumab tesirine Drugs 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 238000012807 shake-flask culturing Methods 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 238000011301 standard therapy Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 231100000041 toxicology testing Toxicity 0.000 description 1
- 239000012096 transfection reagent Substances 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 210000001835 viscera Anatomy 0.000 description 1
- 230000036642 wellbeing Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6849—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a receptor, a cell surface antigen or a cell surface determinant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/537—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines spiro-condensed or forming part of bridged ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7028—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages
- A61K31/7034—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin
- A61K31/704—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin attached to a condensed carbocyclic ring system, e.g. sennosides, thiocolchicosides, escin, daunorubicin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/65—Peptidic linkers, binders or spacers, e.g. peptidic enzyme-labile linkers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57423—Specifically defined cancers of lung
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57492—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving compounds localized on the membrane of tumor or cancer cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Hematology (AREA)
- Cell Biology (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Oncology (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- Hospice & Palliative Care (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Microbiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pulmonology (AREA)
- Peptides Or Proteins (AREA)
Abstract
The invention discloses an anti-DLL 3 antibody, a preparation method thereof, a drug conjugate and application thereof. The anti-DLL 3 antibody has good internalization activity, better binding activity with human DLL3 protein and stronger affinity at protein level; the DLL 3-targeted antibody coupled drug has good drug property, biological activity and in-vivo and in-vitro anti-tumor activity, and can realize the application of cytotoxic drugs in treating tumor patients with neuroendocrine characteristics including SCLC.
Description
The present application claims priority from chinese patent application CN2021108755368, whose application date is 2021, 7, 30, and chinese patent application CN2022102395912, whose application date is 2022, 3, 11. The present application refers to the entirety of the above-mentioned patent application.
Technical Field
The invention belongs to the field of biotechnology and medicine, and particularly relates to an anti-DLL 3 antibody, a preparation method thereof, a drug conjugate and application thereof.
Background
Small Cell Lung Cancer (SCLC) is the highest malignant type of lung cancer, and has the characteristics of rapid progress, early metastasis, easy recurrence and the like, and is about 15% -20% of new lung cancer, and the occurrence of the small cell lung cancer is closely related to long-term smoking. SCLC is largely divided into limited patients and extensive patients, with extensive patients being the major factor accounting for about 70% of SCLC. At present, treatment of SCLC is mainly chemotherapy, and first-line chemotherapy is mainly combination therapy based on platinum drugs, and most commonly etoposide+cisplatin or carboplatin (EP or CE regimen). Guidelines suggest that a combination of cisplatin/carboplatin treatment should be administered for 4-6 courses of treatment, either in the restricted or in the extensive phase, wherein the response rate for first-line treatment of SCLC in the restricted phase is 70% -90% and the response rate for SCLC in the extensive phase is 50% -60%. Current SCLC recurs or progresses within 1 year of first-line treatment with about 80% of patients with restricted periods and almost all patients with extensive periods, and it can be seen that the effect of second-line treatment is related to the final survival of SCLC patients. Topotecan single as standard therapy for second line treatment with a patient response rate of about 22% and a total patient survival of about 8 months; for drug-resistant recurrent patients, the response rate is only about 4%, the median survival is only 5 months, the clinical benefit is extremely limited, and the clinical benefit is greatly unsatisfied. In view of the above, SCLC still faces many challenges in terms of treatment due to the high recurrence rate and drug resistance rate, and limited selection of two-line therapeutic drugs, limited patient survival benefit, and there is a strong need for new therapeutic approaches to change existing clinical unmet. Several studies now suggest that Notch pathway inhibition is highly correlated with the development of SCLC, and that ligand DLL3 is considered one of the most potential targets for treatment of SCLC.
DLL3 is an atypical Notch pathway ligand identified by high-throughput sequencing, which is a single transmembrane protein consisting of 619 amino acids, the complete structure comprising 1 DSL domain, 1 intracellular domain and 6 epidermal growth factor-like domains (i.e. domains EGF1, EGF2, EGF3, EGF4, EGF5 and EGF 6), which are important targets for SCLC development and progression. Immunohistochemistry showed that this target was hardly expressed in normal tissues and not in other tumors, whereas it was expressed in large amounts specifically in neuroendocrine tumors, with high levels of DLL3 present on the tumor tissue and cancer cell surface of approximately 80% of SCLCs, and approximately 85% of recurrent SCLCs also expressed DLL3 proteins, a constitutive expression receptor for SCLCs. DLL3 is not expressed in normal tissues, but specifically expressed in neuroendocrine tumors such as SCLC, making it one of the targets for antibody or antibody drug conjugate (antibody drug conjugate, ADC) development.
There are a number of drugs currently being developed that target DLL3 targets, mainly bispecific antibodies, cytotherapeutic and ADC drugs (see for example CN104520324 a). The Rova-T is the first ADC drug targeting the DLL3 target point developed by ibovine and is also the first targeting therapeutic drug of SCLC in clinical research, the drug utilizes DLL3 expressed on the surface of tumor cells to identify the tumor cells and convey cytotoxic drug PBD into the tumor cells so as to achieve the effect of directionally killing the tumor cells, but in clinical phase 3 research, the drug is intolerable to patients due to too great toxic and side effect of the cytotoxic drug PBD, thereby resulting in insufficient effectiveness, and the development is stopped at present.
Disclosure of Invention
Aiming at the defects of the anti-DLL 3 antibody in the prior art and the current situation that the ADC drug targeting the DLL3 has not been successfully developed clinically, the invention provides a brand-new anti-DLL 3 antibody, the ADC targeting the DLL3, an intermediate, a preparation method and application thereof. Aiming at the defect of lack of the antibody drug conjugate for targeting DLL3 in the prior art, the antibody drug conjugate can realize the effect of treating tumor patients with neuroendocrine characteristics including SCLC. The invention mainly solves the technical problems through the following technical means.
The present invention provides an anti-DLL 3 antibody comprising a heavy chain variable region (VH) and a light chain variable region (VL); wherein,,
the VH comprises the following Complementarity Determining Regions (CDRs) or mutations thereof: VH CDR1 as shown in the amino acid sequence of SEQ ID NO 9, 19, 29, 39, 59, 69, 79, 89, 99 or 63, VH CDR2 as shown in the amino acid sequence of SEQ ID NO 10, 20, 30, 40, 60, 70, 80, 90, 100, 110 or 83, and/or VH CDR3 as shown in the amino acid sequence of SEQ ID NO 11, 21, 31, 41, 61, 71, 81, 91, 101, 111 or 93;
the VL comprises the following CDRs or mutations thereof: VL CDR1 as shown in the amino acid sequence of SEQ ID No. 12, 32, 42, 62, 72, 82, 92, 102, 112 or 103, VL CDR2 as shown in GAS, GAT, TTS, NAK, YTS, RAN, WAS, FTS or NAN, and/or VL CDR3 as shown in the amino acid sequence of SEQ ID No. 14, 24, 34, 44, 64, 74, 84, 94, 104, 114 or 113;
Wherein the mutation is a 3, 2 or 1 amino acid insertion, deletion or substitution in the amino acid sequence of the CDR.
In this application, an "amino acid mutation" in "an analogous" insertion, deletion or substitution of 3, 2 or 1 amino acids "refers to a mutation in which an amino acid is present in the sequence of the variant as compared to the original amino acid sequence, including an insertion, deletion or substitution of an amino acid that occurs on the basis of the original amino acid sequence. An exemplary interpretation is that mutations to a CDR may comprise 3, 2 or 1 amino acid mutations, and optionally the same or different numbers of amino acid residues may be selected between these CDRs for mutation, e.g., 1 amino acid mutation to CDR1, and no amino acid mutation to CDR2 and CDR 3.
In this application, the mutations may include mutations which are currently known to those skilled in the art, for example, during the production or use of the antibody, and may be performed on the antibody, for example, mutations at sites which may exist, particularly post-transcriptional modification of the CDR regions (post-translational modifications, PTMs), including aggregation, deamidation sensitivity (asparagine deamidation, site (NG, NS, NH, etc.), aspartic acid isomerism (DG, DP) sensitivity, N glycosylation (N- { P } S/T) sensitivity, oxidation sensitivity, and the like.
Preferably, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown in SEQ ID NOs 9, 10 and 11 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown in SEQ ID NO 19, 20 and 21 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown in SEQ ID NO. 29, 30 and 31 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown in SEQ ID NO 39, 40 and 41 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown as SEQ ID NO 59, 60 and 61 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown as SEQ ID NO 69, 70 and 71 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown as SEQ ID NO 79, 80 and 81 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown as SEQ ID NO 89, 90 and 91 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown as SEQ ID NO 99, 100 and 101 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown as SEQ ID NO 89, 110 and 111 respectively; or, the VH comprises the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 shown in SEQ ID NO. 63, 83 and 93 respectively.
Preferably, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 12, GAS and SEQ ID NO. 14, respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 12, GAS and SEQ ID NO. 24 respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 32, GAT and SEQ ID NO. 34 respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 42, TTS and SEQ ID NO. 44 respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 62, NAK and SEQ ID NO. 64, respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO 72, NAK and SEQ ID NO 74 respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 82, YTS and SEQ ID NO. 84 respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 92, RAN and SEQ ID NO. 94 respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 102, WAS and SEQ ID NO. 104 respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown as SEQ ID NO. 112, FTS and SEQ ID NO. 114 respectively; or, the VL comprises the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 shown in SEQ ID NO. 103, NAN and 113 respectively.
In a preferred embodiment, the amino acid sequences of the VH CDR1, VH CDR2 and VH CDR3 of the anti-DLL 3 antibody are shown in SEQ ID NOs 9, 10 and 11, respectively, and the amino acid sequences of the VL CDR1, VL CDR2 and VL CDR3 of the VL antibody are shown in SEQ ID NOs 12, GAS and 14, respectively. In a preferred embodiment, the amino acid sequences of the VH CDR1, VH CDR2 and VH CDR3 are shown in SEQ ID NO. 19, 20 and 21, respectively, and the amino acid sequences of the VL CDR1, VL CDR2 and VL CDR3 are shown in SEQ ID NO. 12, GAS and SEQ ID NO. 24, respectively. In a preferred embodiment, the amino acid sequences of the VH CDR1, VH CDR2 and VH CDR3 are shown in SEQ ID NO. 29, 30 and 31, respectively, and the amino acid sequences of the VL CDR1, VL CDR2 and VL CDR3 are shown in SEQ ID NO. 32, GAT and SEQ ID NO. 34, respectively. In a preferred embodiment, the amino acid sequences of the VH CDR1, VH CDR2 and VH CDR3 are shown in SEQ ID NO 39, 40 and 41, respectively, and the amino acid sequences of the VL CDR1, VL CDR2 and VL CDR3 are shown in SEQ ID NO 42, TTS and SEQ ID NO 44, respectively. In a preferred embodiment, the amino acid sequences of the VH comprising VH CDR1, VH CDR2 and VH CDR3 are shown in SEQ ID NO 59, 60 and 61, respectively, and the amino acid sequences of the VL comprising VL CDR1, VL CDR2 and VL CDR3 are shown in SEQ ID NO 62, NAK and SEQ ID NO 64, respectively. In a preferred embodiment, the amino acid sequences of the VH comprising VH CDR1, VH CDR2 and VH CDR3 are shown in SEQ ID NO:69, 70 and 71, respectively, and the amino acid sequences of the VL comprising VL CDR1, VL CDR2 and VL CDR3 are shown in SEQ ID NO:72, NAK and SEQ ID NO:74, respectively. In a preferred embodiment, the amino acid sequences of the VH CDR1, VH CDR2 and VH CDR3 are shown in SEQ ID NO. 79, 80 and 81, respectively, and the amino acid sequences of the VL CDR1, VL CDR2 and VL CDR3 are shown in SEQ ID NO. 82, YTS and SEQ ID NO. 84, respectively. In a preferred embodiment, the amino acid sequences of VH CDR1, VH CDR2 and VH CDR3 comprised by the VH are shown in SEQ ID NOs 89, 90 and 91, respectively, and the amino acid sequences of VL CDR1, VL CDR2 and VL CDR3 comprised by the VL are shown in SEQ ID NOs 92, RAN and 94, respectively. In a preferred embodiment, the amino acid sequences of the VH CDR1, VH CDR2 and VH CDR3 are shown as SEQ ID NO 99, 100 and 101, respectively, and the amino acid sequences of the VL CDR1, VL CDR2 and VL CDR3 are shown as SEQ ID NO 102, WAS and SEQ ID NO 104, respectively, in the anti-DLL 3 antibody. In a preferred embodiment, the amino acid sequences of the VH CDR1, VH CDR2 and VH CDR3 are shown in SEQ ID NO 89, 110 and 111, respectively, and the amino acid sequences of the VL CDR1, VL CDR2 and VL CDR3 are shown in SEQ ID NO 112, FTS and SEQ ID NO 114, respectively. In a preferred embodiment, the amino acid sequences of the VH CDR1, VH CDR2 and VH CDR3 of the anti-DLL 3 antibody are shown in SEQ ID nos. 63, 83 and 93, respectively, and the amino acid sequences of the VL CDR1, VL CDR2 and VL CDR3 of the VL antibody are shown in SEQ ID nos. 103, NAN and 113, respectively.
Preferably, in the anti-DLL 3 antibody, the heavy chain variable region (VH) further comprises a heavy chain variable region framework region (VH FWR), wherein the VH FWR is a heavy chain variable region framework region of a human or murine antibody. In a preferred embodiment of the invention, the VH may comprise an amino acid sequence as shown in SEQ ID NO 15, 25, 35, 45, 65, 75, 85, 95, 105, 13 or 22 or a mutation thereof.
Preferably, in the anti-DLL 3 antibody, the light chain variable region (VL) further comprises a light chain variable region framework region (VL FWR); wherein the VL FWR is a human or murine antibody light chain variable region framework region. In a preferred embodiment of the invention, the VL may comprise an amino acid sequence as shown in SEQ ID NO. 16, 26, 36, 46, 66, 76, 86, 96, 106, 33 or 23 or a mutation thereof.
In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 15 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 16 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 25 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 26 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 35 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 36 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 45 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 46 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 65 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 66 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 75 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 76 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 85 or a mutation thereof, and the VL comprises the amino acid sequence shown as SEQ ID NO. 86 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 95 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 96 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 105 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 106 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 13 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 33 or a mutation thereof. In a preferred embodiment, the VH comprises the amino acid sequence shown as SEQ ID NO. 22 or a mutation thereof and the VL comprises the amino acid sequence shown as SEQ ID NO. 23 or a mutation thereof.
The mutation described above is a deletion, substitution or addition of one or more amino acid residues in the amino acid sequence of the VH and/or VL, and the mutated amino acid sequence has at least 85% sequence identity to the amino acid sequence of the VH and/or VL and maintains or improves binding of the antibody to DLL 3; the at least 85% sequence identity is preferably at least 90% sequence identity, more preferably at least 95%, 96%, 97%, 98% sequence identity, most preferably at least 99% sequence identity.
In the present application, the amino acid sequences of the CDRs listed above are all shown according to the kabat definition rules (in the present application also shown according to the kabat definition rules). However, it is well known to those skilled in the art that CDRs of antibodies can be defined in the art by a variety of methods, such as Kabat definition rules based on sequence variability (see, kabat et al, protein sequences for immunology, fifth edition, national institutes of health, besseda, malyland (1991)) and Chothia definition rules based on structural loop region position (see, jmol Biol 273:927-48,1997). It will be appreciated by those skilled in the art that unless otherwise specified, the terms "CDR" and "complementarity determining region" of a given antibody or region thereof (e.g., variable region) are to be understood as encompassing complementarity determining regions defined in any of the above known schemes as described by the present invention. Although the scope of the claimed invention is based on the sequences shown by the kabat definition rules, amino acid sequences corresponding to the definition rules according to other CDRs should also fall within the scope of the claimed invention.
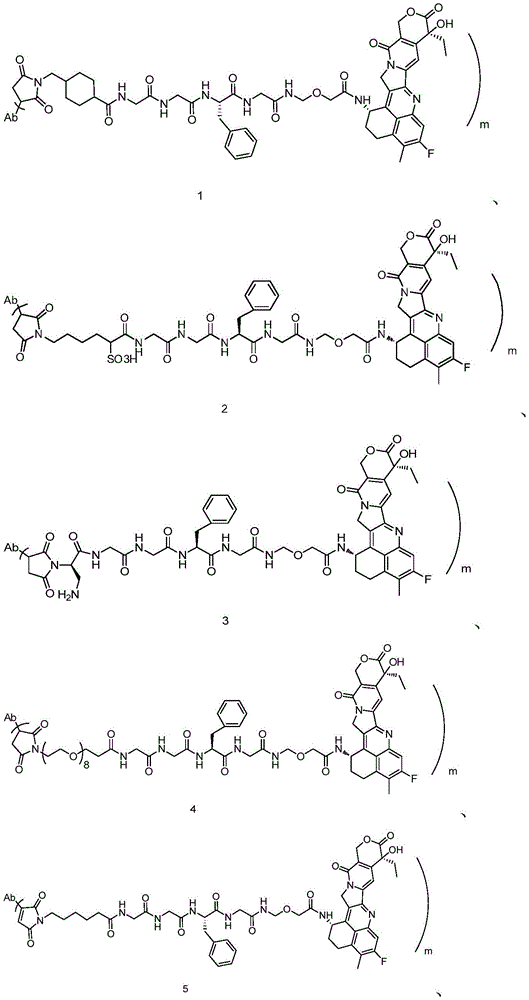
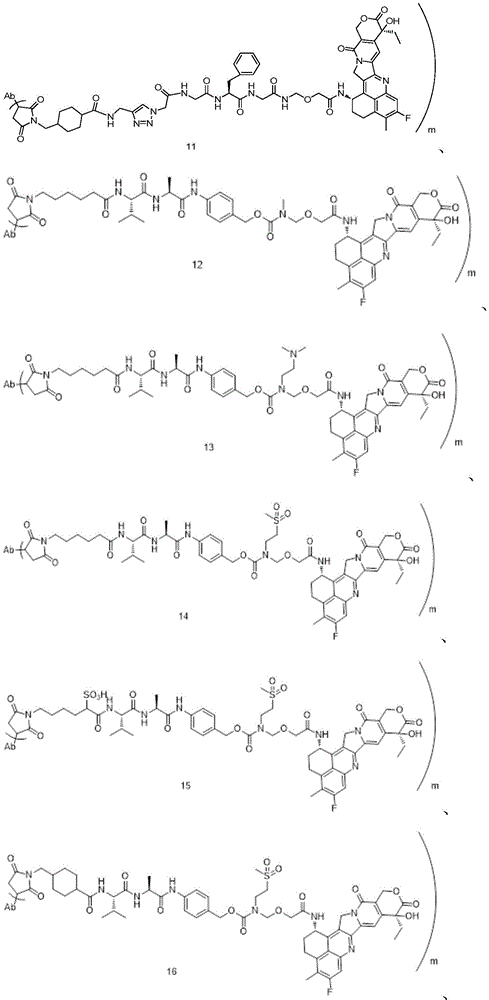
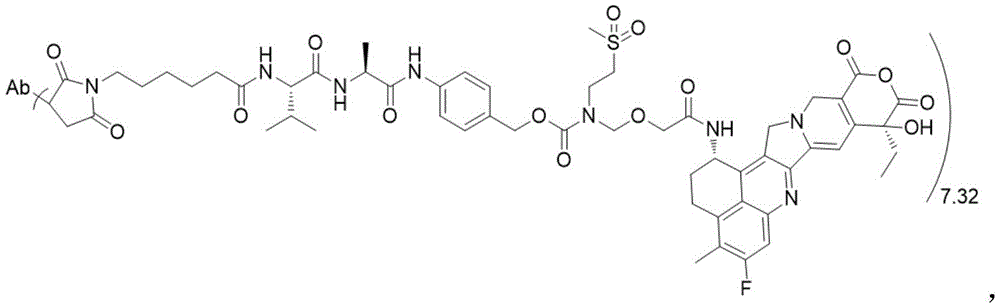
Preferably, the anti-DLL 3 antibody is a full length antibody, fab ', F (ab') 2, fv is preferably an scFv, a bispecific antibody, a multispecific antibody, a heavy chain antibody or a single domain antibody.
In a preferred embodiment, the anti-DLL 3 antibody is a full length antibody, which comprises a heavy chain and a light chain; the heavy chain constant region included in the heavy chain is preferably a human or mouse-derived antibody heavy chain constant region; light chain constant regions included in the light chain the antibody light chain constant regions are preferably human or mouse-derived antibody light chain constant regions. Preferably, the human light chain constant region is a human kappa or lambda light chain constant region; the human heavy chain constant region is human IgG1, igG2, igG3, or IgG4.
In a preferred embodiment, the heavy chain comprises an amino acid sequence as shown in SEQ ID NO. 17, 27, 37, 47, 67, 77, 87, 97, 107, 43 or 73 or a mutation thereof and/or the light chain comprises an amino acid sequence as shown in SEQ ID NO. 18, 28, 38, 48, 68, 78, 88, 98, 108, 53 or 109 or a mutation thereof.
In a preferred embodiment, the full length antibody, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 17 or a mutation thereof, and the light chain comprises the amino acid sequence shown as SEQ ID NO. 18 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 27 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 28 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 37 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 38 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 47 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 48 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 67 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 68 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 77 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 78 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 87 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 88 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 97 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 98 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 107 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 108 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 43 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 53 or a mutation thereof. In a preferred embodiment, the heavy chain comprises the amino acid sequence shown as SEQ ID NO. 73 or a mutation thereof and the light chain comprises the amino acid sequence shown as SEQ ID NO. 109 or a mutation thereof.
The mutation described above is a deletion, substitution or addition of one or more amino acid residues in the amino acid sequence of the heavy and/or light chain, and the mutated amino acid sequence has at least 85% sequence identity to the amino acid sequence of the heavy and/or light chain and maintains or improves the binding of the antibody to DLL 3; the at least 85% sequence identity is preferably at least 90% sequence identity; more preferably at least 95%, 96%, 97% or 98% sequence identity; most preferably at least 99% sequence identity.
In one aspect, the invention also provides an anti-DLL 3 antibody that competitively binds to DLL3 protein with an anti-DLL 3 antibody as described above.
In a further aspect the invention provides an isolated nucleic acid encoding an anti-DLL 3 antibody as described above.
In a further aspect the invention provides a recombinant expression vector comprising an isolated nucleic acid as described above. Preferably, the expression vector comprises a eukaryotic expression vector and/or a prokaryotic expression vector.
In a further aspect the invention provides a transformant comprising a recombinant expression vector as described above. Preferably, the host cells of the transformant are prokaryotic cells, preferably E.coli cells such as TG1, BL21 cells, and/or eukaryotic cells, preferably HEK293 cells or CHO cells.
In one aspect, the invention also provides an Antibody Drug Conjugate (ADC) having the structural formula Ab- (L) 3 -L 2 -L 1 -D) m ;
Wherein Ab is an anti-DLL 3 antibody;
m is 2-8;
L 1 the structure of which is shown as formula I, II, III or IV, the a end of which is connected with the cytotoxic drug, the e end of which is connected with the L 2 C end of (2) is connected;
(L) p wherein L is independently one or more of a phenylalanine residue, an alanine residue, a glycine residue, a glutamic acid residue, an aspartic acid residue, a cysteine residue, a glutamic acid residue, a histidine residue, an isoleucine residue, a leucine residue, a lysine residue, a methionine residue, a proline residue, a serine residue, a threonine residue, a tryptophan residue, a tyrosine residue, and a valine residue; p is 2-4;
R 1 is one or more of-NR 1-1 R 1-2 Substituted C 1 ~C 6 Alkyl, substituted by one or more R 1-3 S(O) 2 -substituted C 1 ~C 6 Alkyl, C 1 ~C 6 Alkyl, C 3 ~C 10 Cycloalkyl, C 6 ~C 14 Aryl or 5-14 membered heteroaryl; the hetero atom in the 5-14 membered heteroaryl is selected from one or more of N, O and S, and the number of the hetero atom is 1, 2, 3 or 4; said R is 1-1 、R 1-2 And R is 1-3 Each independently is C 1 ~C 6 An alkyl group;
Wherein n is independently 1 to 12, e.g., 8, 9, 10, 11 and 12, c-terminal is bonded to L by carbonyl 1 The f end is connected with the L 3 Is connected with the d end of the (C);
L 3 is thatWherein the b-terminal is connected with the Ab and the d-terminal is connected with the L 2 Is connected to the f terminal of (c).
In a certain preferred embodiment, the anti-DLL 3 antibody binds to one of the following antigen binding epitopes in the DLL3 protein: DSL domain, N-terminal, EGF2 domain and EGF3-EGF6 domain.
In a certain preferred embodiment, the anti-DLL 3 antibody is preferably an anti-DLL 3 antibody as described above.
In a certain preferred embodiment, the anti-DLL 3 antibody binds to an antigen binding epitope of the EGF2 domain in the DLL3 protein.
In a preferred embodiment, the anti-DLL 3 antibody comprises a VH having the amino acid sequence shown in SEQ ID NO. 15 and a VL having the amino acid sequence shown in SEQ ID NO. 16, or comprises a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26.
In a preferred embodiment, the anti-DLL 3 antibody is an anti-DLL 3 antibody having a light chain with an amino acid sequence shown in SEQ ID NO. 1 and a heavy chain with an amino acid sequence shown in SEQ ID NO. 2, or an anti-DLL 3 antibody having a light chain with an amino acid sequence shown in SEQ ID NO. 3 and a heavy chain with an amino acid sequence shown in SEQ ID NO. 4.
In a preferred embodiment, L is one or more of phenylalanine, alanine, glycine, isoleucine, leucine, proline and valine, preferably one or more of phenylalanine, alanine, glycine and valine.
In a preferred embodiment, L is a valine residue and/or an alanine residue, the plurality of L is two or three, and the p is 2. More preferably, said (L) p Is thatWherein the g-terminus is bound to the L via a carbonyl group 2 Is connected to the c terminal of (c).
In a preferred embodiment, said R 1 Is one or more of-NR 1-1 R 1-2 Substituted C 1 ~C 6 Alkyl, substituted by one or more R 1-3 S(O) 2 -substituted C 1 ~C 6 Alkyl, or C 1 ~C 6 Alkyl, said R 1-1 、R 1-2 And R is 1-3 Each independently is C 1 ~C 4 An alkyl group.
In a preferred embodiment, when R is 1 Is one or more of-NR 1-1 R 1-2 Substituted C 1 ~C 6 In the case of alkyl, said C 1 ~C 6 Alkyl is C 1 ~C 4 Alkyl, preferably methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl, preferably ethyl; the plurality is preferably two or three. Preferably, said R 1-1 And R is 1-2 Each independently is C 1 ~C 4 Alkyl is preferably methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl, more preferably methyl. More preferably, said-NR 1-1 R 1-2 is-N (CH) 3 ) 2 . Further preferably, when R is 1 Is covered by a-NR 1-1 R 1-2 Substituted C 1 ~C 6 In the case of alkyl, said radical is substituted by one-NR 1-1 R 1-2 Substituted C 1 ~C 6 Alkyl is
In a preferred embodiment, when R is 1 To be covered by one or more R 1-3 S(O) 2 -substituted C 1 ~C 6 In the case of alkyl, said C 1 ~C 6 Alkyl is C 1 ~C 4 Alkyl, preferably methylA group, ethyl, n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl group, more preferably ethyl; the plurality is preferably two or three. Preferably, said R 1-3 Is C 1 ~C 4 Alkyl is preferably methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl, more preferably methyl. More preferably, when said R 1 Is covered by one R 1-3 S(O) 2 -substituted C 1 ~C 6 In the case of alkyl, said radical is substituted by one R 1-3 S(O) 2 -substituted C 1 ~C 6 Alkyl is
In a preferred embodiment, when R is 1 Is C 1 ~C 6 In the case of alkyl, said C 1 ~C 6 Alkyl is C 1 ~C 4 Alkyl is more preferably methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl, and still more preferably methyl.
In a preferred embodiment, when L 1 When the structure of the formula I is shown in the specification, L 2 Preferably is The L is 3 Preferably +.>
In a preferred embodiment, when L 1 When the structure of (C) is shown as formula II, L 2 Preferably isThe L is 3 PreferablyIs that
In a preferred embodiment of the invention, when L 1 When the structure of (C) is shown as formula III, L 2 Preferably is The L is 3 Preferably +. >
In a preferred embodiment of the invention, when L 1 When the structure of (C) is shown as formula IV, the L 2 Preferably isThe L is 3 Preferably +.>
In a preferred embodiment, L 1 The structure of (a) is preferably as shown in formula I or III.
In a preferred embodiment, the compound of formula III is preferably
In a preferred embodiment, the formula III is further preferred
In a preferred embodiment, said L 3 Preferably linked to the thiol group of said antibody as a thioether bond. To be used forFor example, a->The form of linkage to the cysteine residues in the antibodies is
In a preferred embodiment, m is an integer from 2 to 8, e.g., 2, 3, 4, 5, 6, 7, 8, or a non-integer, e.g.: 7.68, 7.53, 4.43, 7.12, 6.92, 7.43, 7.23, 6.83, 7.32, 7.56, 7.54, 7.47, 5.82, 6.78, 2.28, 6.32, 7.45, 7.65, 7.64, 7.36, 7.75, 7.80, 7.77 or 7.76.
Preferably, the antibody drug conjugate is any one of the compounds shown as follows:
where Ab is the anti-DLL 3 antibody described above, m is from 2 to 8, preferably 7.68, 7.53, 4.43, 7.12, 6.92, 7.43, 7.23, 6.83, 7.32, 7.56, 7.54, 7.47, 5.82, 6.78, 2.28, 6.32, 7.45, 7.65, 7.64, 7.36, 7.75, 7.80, 7.77 or 7.76.
In a preferred embodiment, the antibody drug conjugate is any one of the compounds shown below:
wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown as SEQ ID NO. 25 and a VL having an amino acid sequence shown as SEQ ID NO. 26; preferably comprises a heavy chain having an amino acid sequence shown in SEQ ID NO. 27 and a light chain having an amino acid sequence shown in SEQ ID NO. 28.
In a preferred embodiment, the antibody drug conjugate is any one of the compounds shown below:
wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown as SEQ ID NO. 15 and a VL having an amino acid sequence shown as SEQ ID NO. 16; preferably comprises a heavy chain having an amino acid sequence shown in SEQ ID NO. 17 and a light chain having an amino acid sequence shown in SEQ ID NO. 18.
In a preferred embodiment, the antibody drug conjugate is a compound as shown below:
wherein Ab is an anti-DLL 3 antibody comprising a heavy chain having an amino acid sequence shown in SEQ ID NO. 2 and a light chain having an amino acid sequence shown in SEQ ID NO. 1.
In a preferred embodiment, the antibody drug conjugate is a compound as shown below:
wherein Ab is an anti-DLL 3 antibody comprising a heavy chain having an amino acid sequence shown in SEQ ID NO. 4 and a light chain having an amino acid sequence shown in SEQ ID NO. 3.
In a preferred embodiment, the antibody drug conjugate is a compound as shown below:
wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown as SEQ ID NO. 22 and a VL having an amino acid sequence shown as SEQ ID NO. 23; preferably comprises a heavy chain having an amino acid sequence shown in SEQ ID NO. 73 and a light chain having an amino acid sequence shown in SEQ ID NO. 109.
In a preferred embodiment, the antibody drug conjugate is a compound as shown below:
wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 45 and a VL having the amino acid sequence shown in SEQ ID NO. 46; preferably comprises a heavy chain having an amino acid sequence shown in SEQ ID NO. 47 and a light chain having an amino acid sequence shown in SEQ ID NO. 48.
In a further aspect the invention provides a chimeric antigen receptor comprising an anti-DLL 3 antibody as described above.
In a further aspect the invention provides a genetically modified cell comprising a chimeric antigen receptor as described above. Preferably, the genetically modified cell is a eukaryotic cell, preferably an isolated human cell; more preferably immune cells such as T cells, or NK cells.
In one aspect, the invention also provides a method for preparing an anti-DLL 3 antibody, the method comprising the steps of: the transformant as described above was cultured, and an anti-DLL 3 antibody was obtained from the culture.
In a further aspect the invention provides a pharmaceutical composition comprising an anti-DLL 3 antibody as described above, an antibody drug conjugate as described above, a chimeric antigen receptor as described above, and/or a genetically modified cell as described above. Preferably, the pharmaceutical composition is in liquid, gaseous, solid and semi-solid dosage forms, and/or the pharmaceutical composition may be administered orally, by injection, nasally, transdermally or mucosally. More preferably, the pharmaceutical composition further comprises a combination therapeutic agent comprising a chemotherapeutic agent, a radiation therapeutic agent, an immunosuppressant, and/or a cytotoxic drug.
In a further aspect the invention provides the use of an anti-DLL 3 antibody as described above, an antibody drug conjugate as described above, a chimeric antigen receptor as described above, a genetically modified cell as described above, and/or a pharmaceutical composition as described above for the preparation of a medicament, kit and/or administration device for the treatment and/or prevention of a disease associated with abnormal DLL3 expression; or an anti-DLL 3 antibody as described above, an antibody drug conjugate as described above, a chimeric antigen receptor as described above, a genetically modified cell as described above, and/or a pharmaceutical composition as described above for use in the treatment and/or prevention of a disease associated with abnormal DLL3 expression. The DLL3 expression abnormality related disease is preferably a tumor, the tumor is preferably a cancer, the cancer is preferably an endocrine tumor such as neuroendocrine tumor, prostate cancer, pancreatic cancer and colorectal cancer, and the like, and more preferably small cell lung cancer.
In a further aspect the invention provides a kit comprising an anti-DLL 3 antibody as described above, an antibody drug conjugate as described above, a chimeric antigen receptor as described above, a genetically modified cell as described above, and/or a pharmaceutical composition as described above; and optionally, instructions.
In one aspect, the invention provides a drug delivery device comprising: (1) An infusion module for administering a pharmaceutical composition as described above to a subject in need thereof, and (2) optionally a efficacy monitoring module.
In one aspect the invention also provides a method of detecting DLL3 comprising the step of detecting using an anti-DLL 3 antibody as described above. Preferably, the method is for non-diagnostic and/or therapeutic purposes.
In a further aspect the invention provides a method of diagnosing, preventing and/or treating a disease associated with abnormal DLL3 expression comprising administering to a subject in need thereof an anti-DLL 3 antibody as described above, an antibody drug conjugate as described above, a genetically modified cell as described above, and/or a pharmaceutical composition as described above. The DLL3 expression abnormality related disease is preferably a tumor, the tumor is preferably a cancer, the cancer is preferably a neuroendocrine tumor, and more preferably a small cell lung cancer.
The invention also provides a panel of antibodies (including molecules comprising or consisting of antibody fragments or variants), wherein the members of the panel correspond to one, two, three, four, five, or more different antibodies of the invention [ e.g., complete antibodies, fab, F (ab) ] 2 Fragments and scFv and the like]。
In one aspect, the invention also provides an antibody drug conjugate, which has a structural formula of Ab- (L) 5 -L 4 -L 3 -L 2 -L 1 -D) m ;
Wherein Ab is an anti-DLL 3 antibody; the anti-DLL 3 antibody binds to a DSL domain and/or the N-terminus in a DLL3 protein;
d is a cytotoxic drug;
m is 2-8;
L 1 the structure of which is shown as formula I, II, III or IV, the a end of which is connected with the cytotoxic drug, the e end of which is connected with the L 2 C end of (2) is connected;
(L) p wherein L is independently one or more of a phenylalanine residue, an alanine residue, a glycine residue, a glutamic acid residue, an aspartic acid residue, a cysteine residue, a glutamic acid residue, a histidine residue, an isoleucine residue, a leucine residue, a lysine residue, a methionine residue, a proline residue, a serine residue, a threonine residue, a tryptophan residue, a tyrosine residue, and a valine residue; p is 2-4;
R 1 is one or more of-NR 1-1 R 1-2 Substituted C 1 ~C 6 Alkyl, substituted by one or more R 1-3 S(O) 2 -substituted C 1 ~C 6 Alkyl, C 1 ~C 6 Alkyl, C 3 ~C 10 Cycloalkyl, C 6 ~C 14 Aryl or 5-14 membered heteroaryl;the hetero atom in the 5-14 membered heteroaryl is selected from one or more of N, O and S, and the number of the hetero atom is 1, 2, 3 or 4; said R is 1-1 、R 1-2 And R is 1-3 Each independently is C 1 ~C 6 An alkyl group;
L 2 is that Wherein n is independently 1 to 12, c-terminal is bound to L via a carbonyl group 1 The f end is connected with the L 3 Is connected with the d end of the (C);
L 3 is thatWherein the b-terminal is connected with the Ab and the d-terminal is connected with the L 2 Is connected with the f end of the (E);
the L is 4 Absent, or selected from cleavable linkers, non-cleavable linkers, hydrophilic linkers, pre-charged linkers and dicarboxylic acid-based linkers;
the L is 5 Absent, or a compound of formula V:
wherein X is 1 Selected from the group consisting of hydrogen, halogen, hydroxy, cyano, alkyl, alkoxy, and cycloalkyl;
X 2 selected from alkyl, cycloalkyl and heterocyclyl; m is 0-5; s is a sulfur atom.
In a preferred embodiment, D is a cytotoxic drug containing a hydroxyl, sulfhydryl or amino group, such as a microtubule inhibitor and/or a topoisomerase inhibitor.
In a preferred embodiment, L is one or more of phenylalanine, alanine, glycine, isoleucine, leucine, proline and valine, preferably one or more of phenylalanine, alanine, glycine and valine; more preferably, L is a valine residue and/or an alanine residue, the plurality of L is two or three, and p is 2.
In a preferred embodiment, said R 1 Is one or more of-NR 1-1 R 1-2 Substituted C 1 ~C 6 Alkyl, substituted by one or more R 1-3 S(O) 2 -substituted C 1 ~C 6 Alkyl, or C 1 ~C 6 Alkyl, said R 1-1 、R 1-2 And R is 1-3 Each independently is C 1 ~C 4 An alkyl group.
In a preferred embodiment, when L 1 When the structure of the formula I is shown in the specification, L 2 Is that
In a preferred embodiment, when L 1 When the structure of (C) is shown as formula IV, the L 2 Is that
In a certain preferred embodiment, said n is independently 8, 9, 10, 11 and 12.
In a preferred embodiment, m is an integer or non-integer from 2 to 8.
In a preferred embodiment, the L 4 Selected from the group consisting of N-succinimidyl 4- (2-pyridyldithio) pentanoate (SPP), N-succinimidyl (4-iodoacetyl) aminobenzoate (SIAB), N-succinimidyl 4- (maleimidomethyl) cyclohexanecarboxylate (SMCC), 6-Maleimidocaproyl (MC), maleimidopropionyl (MP), valine-citrulline (VC), alanine-phenylalanine (ala-phe), p-aminobenzyloxycarbonyl (PAB) and MC-VC-PAB.
In a preferred embodiment, the L 5 In (1), when X 1 Is a hydrogen atom, X 2 When the alkyl group and m are 1, the compound shown in the formula V is S- (3-carbonyl propyl) thioacetate.
In a preferred embodiment, D is one or more of a maytansine derivative, a dolastatin 10 derivative, an doxorubicin analog or a camptothecin analog.
Such as DM1, DM3 and DM4.
Such as MMAE and MMAF.
Such as doxorubicin and cambriubicin.
More preferably, D is a camptothecin analog, further preferably a compound having the structure shown as formula Va or Vb:
R 2 and R is 5 H, C each independently of the other 1 -C 6 Alkyl or halogen;
R 3 and R is 6 H, C each independently of the other 1 -C 6 Alkyl or halogen;
R 4 and R is 7 Each independently is C 1 -C 6 An alkyl group.
In a preferred embodiment, the said (L) p Is thatWherein the g-terminus is bound to the L via a carbonyl group 2 Is connected to the c terminal of (c).
In a preferred embodiment, the compound of formula III
In a preferred embodiment, the antibody drug conjugate is any one of the compounds shown below:
the anti-DLL 3 antibody is an anti-DLL 3 antibody with a light chain with an amino acid sequence shown as SEQ ID NO. 1 and a heavy chain with an amino acid sequence shown as SEQ ID NO. 2, or an anti-DLL 3 antibody with a light chain with an amino acid sequence shown as SEQ ID NO. 3 and a heavy chain with an amino acid sequence shown as SEQ ID NO. 4;
And m is 7.23 or 7.32.
Preferably, the antibody drug conjugate is any one of the compounds shown as follows:
wherein Ab is an anti-DLL 3 antibody comprising a heavy chain having an amino acid sequence shown in SEQ ID NO. 2 and a light chain or +.>
Wherein Ab is an anti-DLL 3 antibody comprising a heavy chain having an amino acid sequence shown in SEQ ID NO. 4 and a light chain having an amino acid sequence shown in SEQ ID NO. 3.
In a certain aspect, the invention also provides a preparation method of the antibody drug conjugate, which comprises the following steps: and (3) dropwise adding the excessive connecting group-drug conjugate dissolved in DMSO into a buffer solution containing the anti-DLL 3 antibody to obtain the antibody drug conjugate.
In a further aspect the invention provides a pharmaceutical composition comprising an antibody drug conjugate according to the invention.
Preferably, the pharmaceutical composition is in a liquid, gaseous, solid or semi-solid dosage form, and/or the pharmaceutical composition may be administered orally, by injection, nasally, transdermally or mucosally.
More preferably, the pharmaceutical composition further comprises a combination therapeutic agent comprising a chemotherapeutic agent, a radiation therapeutic agent, an immunosuppressant, and/or a cytotoxic drug.
In one aspect, the invention also provides the use of an antibody drug conjugate and/or a pharmaceutical composition according to the invention for the preparation of a medicament, kit and/or administration device for the treatment and/or prevention of diseases associated with abnormal DLL3 expression;
the DLL3 expression abnormality related disease is preferably a tumor, the tumor is preferably a cancer, the cancer is preferably a neuroendocrine tumor, and more preferably a small cell lung cancer.
In a further aspect the invention provides a kit comprising an antibody drug conjugate according to the invention and/or a pharmaceutical composition according to the invention; and optionally, instructions.
In one aspect, the invention provides a drug delivery device comprising: (1) An infusion module for administering an antibody drug conjugate according to the invention and/or a pharmaceutical composition according to the invention to a subject in need thereof, and (2) optionally a efficacy monitoring module.
In a further aspect the invention provides a method of detecting DLL3, which comprises the step of detecting using an antibody drug conjugate according to the invention. Preferably, the method of detecting DLL3 is for non-diagnostic and/or therapeutic purposes.
In a further aspect the invention provides a method of diagnosing, preventing and/or treating a disease associated with abnormal DLL3 expression comprising administering to a subject in need thereof an antibody drug conjugate according to the invention and/or a pharmaceutical composition according to the invention.
In the present invention, the conditions and operations of the coupling reaction may be those conventional in the art.
In the present invention, m represents the molar ratio of cytotoxic drug molecule to Ab (also known as DAR, i.e. drug antibody coupling ratio), m may be an integer or a fraction, preferably understood as: the average molar ratio of the drug molecules in the antibody drug conjugate obtained after coupling the monoclonal antibody molecules with the cytotoxic drugs can be generally determined by means of Hydrophobic chromatography (Hydrophobic-Interaction Chromatography, HIC), polyacrylamide-SDS gel electrophoresis (SDS-PAGE), liquid phase mass spectrometry (liquid chromatograph-mass spectrometer, LC-MS) and the like.
In the present invention, the term "C 1 ~C 6 Alkyl "alone or in combination denotes a saturated, straight-chain or branched alkyl radical having from 1 to 6, in particular from 1 to 4, carbon atoms, for example methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl," C 1 ~C 6 The alkyl group "is preferably methyl or ethyl.
Antibodies of the invention may be prepared using techniques well known in the art, such as hybridoma methods, recombinant DNA techniques, phage display techniques, synthetic techniques, or combinations thereof, or other techniques known in the art.
The term "selective" or "specific" as used herein refers to the fact that: the disclosed antagonists do not show significant binding to substances other than DLL3, except in those particular cases: wherein the supplemental antagonist imparts an additional specificity (e.g., a bispecific or bifunctional molecule wherein the molecule is designed to bind or perform both functions, at least one of which is specifically binding to DLL 3) to a moiety other than the DLL3 specific binding moiety.
An "antibody molecule" or "antibody" as described herein refers to an immunoglobulin molecule and an immunologically active portion of an immunoglobulin molecule, i.e., a molecule that contains an antigen binding site that immunospecifically binds an antigen. Thus, the term antibody encompasses not only intact antibody molecules, but also fragments of such antibodies as well as variants (including derivatives) of such antibodies and antibody fragments. The term antibody molecule as described herein includes, for example, but is not limited to, single chain Fv (scFv), fab fragments, fab 'fragments, F (ab') 2, disulfide-linked Fv (sdFv), fv, and whole or full length antibodies. The term "single chain Fv" or "scFv" refers to a polypeptide comprising the VL domain of an antibody linked to the VH domain of an antibody. Antibodies that immunospecifically bind DLL3 can cross-react with other antigens. Preferably, antibodies that immunospecifically bind DLL3 do not cross react with other antigens. Antibodies that immunospecifically bind DLL3 can be identified, for example, by immunoassays or other methods known to those skilled in the art. "intact antibody" or "full length antibody" refers to a protein comprising two heavy chains (H) and two light chains (L), said heavy and light chains being linked to each other by disulfide bonds, said protein comprising: (1) In the case of heavy chains, comprising a heavy chain variable region (abbreviated herein as "VH") and a heavy chain constant region comprising three domains CH1, CH2, CH 3; and (2) in the case of a light chain, a light chain variable region (abbreviated herein as "VL") and a light chain constant region comprising one domain CL. Antibodies of the invention include, but are not limited to, monoclonal, multispecific, human or chimeric antibodies, single-chain antibodies, fab fragments, F (ab') fragments, anti-idiotype (anti-Id) antibodies (including, for example, anti-Id antibodies of the invention), and epitope-binding fragments of any of the antibodies described above. The immunoglobulin molecules of the invention may be of any type (e.g., igG, igE, igM, igD, igA and IgY), class (e.g., igG1, igG2, igG3, igG4, igA1 and IgA 2) or subclass of immunoglobulin. The antibody of the present invention may be a monoclonal antibody, preferably a murine anti-human monoclonal antibody, or a polyclonal antibody. The antibodies of the invention may be super-humanized antibodies or diabodies.
In this application, the term "heavy chain antibody" refers to an antibody comprising only one heavy chain variable region (VHH) and two conventional CH2 and CH3 regions, also known as HCAbs.
"Single-domain antibody", also known as "nanobody", refers to a VHH structure cloned from a heavy chain antibody, which is the smallest unit known to bind an antigen of interest.
The amino acid sequence with the homology of 90%, 95%, 98% or more than 99% is obtained by inserting, deleting or replacing the amino acid sequence shown in the sequence table, wherein the replacement can be as follows: for example, the sequences are subjected to in silico analysis, and analysis and substitution of possible post-transcriptional modification (PTMS) sites, particularly CDR regions, including aggregation, deamidation sensitivity (asparagine deamidation, site (NG, NS, NH, etc.), aspartate isomerism (DG, DP) sensitivity, N glycosylation (N- { P } S/T) sensitivity, oxidation sensitivity, etc. of antibodies.
"KD" refers to the dissociation constant obtained from the ratio (or Kd/Ka, expressed in molar concentration (M)) of Kd (the rate of dissociation of a particular binding molecule-target protein interaction) to Ka (the rate of binding of a particular binding molecule-target protein interaction). KD values can be determined using methods well established in the art. A preferred method of determining the KD of a binding molecule is by using surface plasmon resonance, e.g., a biosensor system, such as the Biacore TM (GE Healthcare Life Sciences) system.
The term "treatment" or its equivalent, when used in reference to, for example, cancer, refers to a procedure or process used to reduce or eliminate the number of cancer cells in a patient or to alleviate symptoms of cancer. "treating" of cancer or another proliferative disorder does not necessarily mean that the cancer cells or other disorder will actually be eliminated, that the number of cells or disorders will actually be reduced or that the symptoms of the cancer or other disorder will actually be reduced. In general, methods of treating cancer are performed even with low likelihood of success, but are still considered to induce an overall beneficial course of action, given the patient's medical history and estimated survival expectancy.
The term "pharmaceutically acceptable carrier" refers to any formulation or carrier medium representative of a carrier capable of delivering an effective amount of the active agents of the present invention, which does not interfere with the biological activity of the active agents and which does not have toxic or side effects to the host or patient, including water, oils, vegetables and minerals, cream bases, lotion bases, ointment bases, and the like. Such matrices include suspending agents, viscosity enhancers, transdermal enhancers, and the like. Their formulations are well known to those skilled in the cosmetic or topical pharmaceutical arts.
The above preferred conditions can be arbitrarily combined on the basis of not deviating from the common knowledge in the art, and thus, each preferred embodiment of the present invention can be obtained.
The reagents and materials used in the present invention are commercially available.
The invention has the positive progress effects that: the anti-DLL 3 antibody has good internalization activity, better binding activity with human DLL3 protein and stronger affinity at protein level. The antibody-conjugated drug has good drug property, biological activity and in-vitro and in-vivo anti-tumor activity, and can realize the application of cytotoxic drugs in treating tumor patients with neuroendocrine characteristics including SCLC.
Drawings
FIG. 1 is a map of the pV81 vector.
FIG. 2 shows the internalization results of the antibodies of example 4.
Figure 3 shows the dose-response curve of the binding activity of chimeric antibodies to hlll 3.
Figure 4 shows the dose-response curves of the binding activity of chimeric antibodies to hDLL1 (left), hDLL4 (right).
Fig. 5 shows dose-response curves of binding activities of chimeric antibodies to mice (left side), monkey DLL3 (right side).
Figure 6 shows graphs of cytotoxic activity of different ADC candidates for 6 days of DLL3 target cell treatment.
FIG. 7 shows the in vivo anti-tumor status of ADC drugs in NCI-H82 model.
Detailed Description
Table a. Abbreviation description
| DMEM | Basal medium |
| HAT | Screening media |
| PEI | Polyether imide |
| MOI | Multiplicity of infection |
| GFP | Green fluorescent protein |
| SPF | Animals without specific pathogen |
| PBST | Tween-containing phosphate buffer salt solution |
| HRP | Horseradish peroxidase |
| TMB | 3,3', 5' -tetramethylbenzidine |
| PBS | Phosphate buffered saline solution |
| APC | Allophycocyanin |
The invention is further illustrated by means of the following examples, which are not intended to limit the scope of the invention. The experimental methods, in which specific conditions are not noted in the following examples, were selected according to conventional methods and conditions, or according to the commercial specifications.
Example 1 preparation of monoclonal antibodies FDA027 and FDA031 specifically targeting hDLL3
In the invention, monoclonal antibodies FDA027 and FDA031 with high affinity and specificity targeting hDLL3 are selected, wherein FDA027 has a light chain amino acid sequence shown as SEQ ID NO. 1 and a heavy chain amino acid sequence shown as SEQ ID NO. 2, and FDA031 has a light chain amino acid sequence shown as SEQ ID NO. 3 and a heavy chain amino acid sequence shown as SEQ ID NO. 4.
FDA027 light and heavy chain nucleotide sequences were obtained by total gene synthesis (Suzhou Jin Weizhi), double-digested single constructs of EcoR I (purchased from NEB, R3104S) and Hind III (purchased from NEB, R3101S) into the pV81 vector (see FIG. 1), transformation into Trans 1-T1 competent cells (purchased from full gold, CD 501) by ligation, sequencing confirmation, amplification-positive clones were picked up from them, plasmid medium extraction was performed to obtain antibody light chain eukaryotic expression plasmid FDA027-L/pV81 and antibody heavy chain eukaryotic expression plasmid FDA027-H/pV81, the mass ratio of light and heavy chain eukaryotic expression plasmid was 1.5:1, transformation into suspension-grown adapted CHO cells (purchased from ATCC) by electric shock, inoculation of the shock-adapted cells into 96 well plates at 2000-5000 cells/well plates, shaking-up to a maximum expression of 37% CO (37 ml) in shake flask (37% by shake flask) according to the kit (purchased from CisO, 62 HFEG) after 3 weeks culture 2 Shake culturing at 130r/min, spreading into 1000ml shake flask (culture volume 300 ml) after 3 days, 37deg.C, 5.0% CO 2 Shake culturing at 130r/min, feeding culture medium with 5-8% of initial culture volume every other day, culturing until 10-12 days, centrifuging the obtained solution at 9500r/min for 15min, removing cell precipitate, collecting supernatant, filtering with 0.22 μm filter membrane, purifying the treated sample with MabSelect affinity chromatography column (purchased from GE company), and finally obtaining high purity anti-DLL 3 antibody FDA027.FDA031 is prepared by the same procedure as FDA027.
Example 2 preparation of human hDLL3 overexpression vector and stably transformed cell line
Human DLL3 expression plasmid pCMV3-DLL3-T1 (purchased from Beijing Yiqiao, HG 20010-UT) was used to transform E.coli competent cells Trans1-T1 (purchased from full gold, CD 501) for amplification of the transfected expression plasmid. The transformation process was referred to the instructions for use of competent cells. The single clone was picked from the transformation plate and amplified in the medium overnight, centrifuged at 6000r/min for 20min, the bacterial liquid was collected for plasmid extraction, and plasmid DNA was extracted according to the procedure of the specification using a plasmid medium extraction kit (purchased from MN, product number: DP 117).
HEK293 cells (purchased from a cell bank of the department of Chinese sciences) with good growth state in logarithmic growth phase are selected, 10% fetal bovine serum is added to fresh culture medium DMEM to dilute the cells to 5×10 5 The cells/ml density, 2 ml/well, were inoculated into 6-well plates, and placed in an incubator (37 ℃ C., 5% CO) 2 ) Culturing. The following day, transfection reagent lipofectamine 3000 (purchased from Thermo, L3000-008) transfected plasmid pCMV3-DLL3-t1 to HEK293 cells, the procedure was performed according to the instructions of lipofectamine 3000 transfection kit. 48hr after transfection, cells were seeded at a 1-per-well seeding density in 96-well plates and cultured with fresh medium containing 200 μg/ml hygromycin B (available from Thermo, 10687010), and the fresh medium containing hygromycin B was changed every 3 to 4 days to obtain stably transfected monoclonal cells stably expressing hDLL 3. Flow cytometry detection screening hDLL3 expressing HEK293 cells, PBS dilution detection antibody goat anti-hDLL3-PE (purchased from R)&D, FAB 4315P) to 10. Mu.g/ml, 100. Mu.l to 5X 10 are added 5 The cells to be tested were incubated at 4℃for 1hr. Then, 1ml of PBS containing 2% fetal bovine serum was added to resuspend the cells, and the supernatant was discarded after centrifugation at 1500r/min for 5min, and the procedure was repeated twice. 1ml PBS was added to resuspend the cells, and the cells were tested with CytoFLEX (purchased from Beckman), and the positive clones with more than 85% positive expression rate of human DLL3 were successfully constructed with HEK293-DLL3 stable cell lines.
HEK293 overexpressing cell lines containing different hDLL3 domains, i.e., hDLL3 (the sequence of which is shown in SEQ ID NO: 5) overexpressing cell lines containing NO N-terminal, hDLL3 (the sequence of which is shown in SEQ ID NO: 6) overexpressing cell lines containing NO N-terminal, DSL and EGF1 domains, hDLL3 (the sequence of which is shown in SEQ ID NO: 7) overexpressing cell lines containing NO N-terminal, DSL and EGF1 and EGF2 domains, respectively, were stably transfected by the same method as described above.
SEQ ID NO:5
GKPIPNPLLGLDSTSGARCEPPAVGTACTRLCRPRSAPSRCGPGLRPCAPLEDECEAPLVCRAGCSPEHGFCEQPGECRCLEGWTGPLCTVPVSTSSCLSPRGPSSATTGCLVPGPGPCDGNPCANGGSCSETPRSFECTCPRGFYGLRCEVSGVTCADGPCFNGGLCVGGADPDSAYICHCPPGFQGSNCEKRVDRCSLQPCRNGGLCLDLGHALRCRCRAGFAGPRCEHDLDDCAGRACANGGTCVEGGGAHRCSCALGFGGRDCRERADPCAARPCAHGGRCYAHFSGLVCACAPGYMGARCEFPVHPDGASALPAAPPGLRPGDPQRYLSGGGGSGAGVIAVIVVVVIAIVAGIVVLVISRKKRMAKYEKAEIKEMGEMHRELNA
SEQ ID NO:6
GKPIPNPLLGLDSTSGAPLVCRAGCSPEHGFCEQPGECRCLEGWTGPLCTVPVSTSSCLSPRGPSSATTGCLVPGPGPCDGNPCANGGSCSETPRSFECTCPRGFYGLRCEVSGVTCADGPCFNGGLCVGGADPDSAYICHCPPGFQGSNCEKRVDRCSLQPCRNGGLCLDLGHALRCRCRAGFAGPRCEHDLDDCAGRACANGGTCVEGGGAHRCSCALGFGGRDCRERADPCAARPCAHGGRCYAHFSGLVCACAPGYMGARCEFPVHPDGASALPAAPPGLRPGDPQRYLSGGGGSGAGVIAVIVVVVIAIVAGIVVLVISRKKRMAKYEKAEIKEMGEMHRELNA
SEQ ID NO:7
GKPIPNPLLGLDSTSGGPGPCDGNPCANGGSCSETPRSFECTCPRGFYGLRCEVSGVTCADGPCFNGGLCVGGADPDSAYICHCPPGFQGSNCEKRVDRCSLQPCRNGGLCLDLGHALRCRCRAGFAGPRCEHDLDDCAGRACANGGTCVEGGGAHRCSCALGFGGRDCRERADPCAARPCAHGGRCYAHFSGLVCACAPGYMGARCEFPVHPDGASALPAAPPGLRPGDPQRYLSGGGGSGAGVIAVIVVVVIAIVAGIVVLVISRKKRMAKYEKAEIKEMGEMHRELNA
SEQ ID NO:8
GKPIPNPLLGLDSTSGSGVTCADGPCFNGGLCVGGADPDSAYICHCPPGFQGSNCEKRVDRCSLQPCRNGGLCLDLGHALRCRCRAGFAGPRCEHDLDDCAGRACANGGTCVEGGGAHRCSCALGFGGRDCRERADPCAARPCAHGGRCYAHFSGLVCACAPGYMGARCEFPVHPDGASALPAAPPGLRPGDPQRYLSGGGGSGAGVIAVIVVVVIAIVAGIVVLVISRKKRMAKYEKAEIKEMGEMHRELNA
Note that: underlined is the V5 tag, italics is the intracellular sequence
Example 3 preparation of hybridoma monoclonal antibodies and screening of antibodies
3-1 immunization and serum Titer detection
6 female healthy mice of SPF grade Balb/C6 weeks old (purchased from Shanghai Ji Hui laboratory animal feeding Co., ltd.) were selected, randomly divided into A, B two groups, and the DLL3 protein (purchased from ACRO, DL 3-H5255) was subjected to immunization recombination once at two weeks intervals, the first immunization was performed using Freund's complete adjuvant, and the subsequent immunization was performed using Freund's incomplete adjuvant. Mice were bled orbital at day 35, day 49 and their immune serum titers were detected by ELISA and flow cytometry (FACS) conventional methods.
ELISA detection method: the DLL3 protein was diluted to a concentration of 1. Mu.g/ml with PBS, 100. Mu.l/well was added to 96-well ELISA plate at 4℃overnight, the plate supernatant was discarded the next day, 200. Mu.l of PBS solution containing 2% milk was added to each well for blocking, blocking solution was discarded after 2hr and 200ul of PBST (1%Tween 20) was added to wash the plate wells 3 times, after which the serum to be examined was pre-diluted 100-fold as the initial concentration, 3-fold gradient diluted to 11 concentrations, 100ul of each well was added to the ELISA plate, plate supernatant was discarded after 1hr reaction at room temperature, 200ul of PBST (1%Tween 20) was added to wash 5 times, 100ul of HRP-labeled goat anti-mouse antibody (Jackson, 1:10000) was added, and after 1hr incubation at room temperature, the supernatant was discarded, 200ul of PBST (1%Tween 20) was added to wash 7 times. 100ul of TMB chromogenic solution was added to each well and developed for 10min, and after termination of the reaction by addition of 2M HCl, the microplate reader read the absorbance at 450nm (available from biotek, elx 808).
FACS detection method: a96 well V-shaped microplate (available from axygen, wipp 02280) was added 3X 10 per well 5 HEK293-DLL3 stably or endogenously expressed cells SHP-77 (purchased from ATCC), centrifuged at 1500r/min for 1min and the supernatant discarded. After diluting immune serum with PBS at 1:50, diluting 8 concentrations in 4-fold gradient, adding 50 mu L/well into a microplate, incubating on ice for 30min, adding 150 mu L of PBS per well to resuspend cells, centrifuging at 1500r/min for 1min, and repeating the operation for 2 times. APC-labeled goat anti-mouse IgG (Jackson, cat. No. 115-605-164, PBS 1:800 dilution) was added and incubated on ice for 30min at 50. Mu.L per well. Cells were resuspended in 150. Mu.L PBS per well, centrifuged at 1500r/min for 1min, and the procedure repeated 2 times for detection with CytoFLEX (available from Beckman). After detection, select the mice with highest titers and smooth serial two immune serum titers, and perform one by intraperitoneal injection of DLL3 protein 3 days before fusion Secondary impact immunization.
3-2 hybridoma fusion screening and cloning
Preparing cell suspension from spleen of Balb/C mouse with impact immunity, fusing spleen cells with SP2/0 cells (purchased from cell bank of Chinese sciences, TCM 42) by electrofusion method, and mixing hybridoma cells according to 2×10 4 Each cell was plated into 96-well plates and screened with HAT medium, and after 7 days the supernatant of the plates was assayed by ELISA, as described in example 3-1, and ELISA positive cells were assayed by FACS assay for DLL3 overexpressing 293 cell line and SHP-77 endogenous cell line, respectively. Cell-binding positive clones were subjected to limiting dilution and repeated 2 times, and the diluted clones were examined to confirm positive clones by ELISA and FACS methods of example 3-1. The positive cloned hybridomas detected after 2 limiting dilutions were amplified for antibody production purification and sequencing of antibody expression genes.
3-3 purification of hybridoma antibody production
To generate mg amounts of antibody for functional characterization, selected hybridoma cells were grown at 2.5X10 5 The density of/mL was inoculated into a cell culture flask containing 250mL of hybridoma serum-free medium (purchased from source culture organism, H630 KJ), shake flask culture was performed at 37℃for 130r/min, and cell supernatant was harvested by centrifugation after the cell viability was reduced to about 30%. The mouse monoclonal antibody was purified using Protein G medium and the antibody was replaced by dialysis into PBS ph7.2 buffer. Antibody concentration and purity were determined by measuring absorbance with a micro-spectrophotometer (available from Hangzhou o Cheng Yiqi, nano-300).
Example 4 analysis of endocytic Activity of antibodies
Endocytosis of the hybridoma antibodies obtained in example 3-3 was detected using SHP-77 cells by the FACS detection method described in example 3-1. The detection method is briefly described as follows, 6 parts of 5×10 are packaged 5 The SHP-77 cells were centrifuged at 1500r/min for 1min in 96-well V-shaped microplates and the supernatant was discarded. Saturated 1. Mu.g/ml of antibody to be detected was added, 200. Mu.L of antibody was added to each well, and incubated on ice for 30min. Then 150. Mu.L of PBS was added to each well, centrifuged at 1500r/min for 1min, the supernatant was discarded, and the procedure was repeated 4 times. Resuspension with 200 μl PBSTaking out 1 part, placing into 37 deg.C cell incubator, standing for 5hr, 3hr, 1hr, 0.5hr, and 15min respectively, placing the rest last cell on ice, standing for incubation time, centrifuging at 4deg.C for 1min at 1500r/min, discarding supernatant, adding APC labeled goat anti-mouse IgG (Jackson, cat No. 109-605-098, PBS 1:800 for dilution), adding 50 μl per well, incubating on ice for 30min, adding 150 μl PBS per well for resuspension cell, centrifuging at 1500r/min for 1min, discarding supernatant, and repeating the operation for 4 times. Finally, 100. Mu.L PBS was added to each well to resuspend the cells, and the cells were assayed by CytoFLEX (available from Beckman), and the assay fluorescence statistics are shown in Table 1 below, and FIG. 2 shows the internalization results of the antibodies obtained in 3-3 of example 3, which indicate that the antibodies obtained in 3-3 of example 3 have good internalization activity.
TABLE 1 flow cytometry detection of fluorescence values for antibody internalization
Example 5 acquisition of hybridoma antibody expression Gene sequences
TABLE 2 amino acid sequence numbering of the CDRs and variable regions of the resulting antibodies (encoded by kabat)
EXAMPLE 6 construction and preparation of chimeric antibodies and detection of binding Activity
The hybridoma clone heavy chain variable region sequence with better binding activity and endocytosis activity obtained in example 5 above was subjected to gene synthesis and cloned into pfuses-CHIg-hG 1 vector (purchased from invitrogen, trade name pFUSEss-hchg 1) containing the amino acid sequence of the heavy chain constant region of the human antibody IgG1 by means of homologous recombination to obtain chimeric antibody heavy chain expression vector. The cloned light chain variable region sequences were cloned into pFUSE2ss-CLIg-hk vectors (available from InvivoGen under the trade designation pFUSE2 ss-hclk) containing the amino acid sequence CL of the human antibody Kappa light chain constant region, respectively, by homologous recombination using the same method, to obtain chimeric antibody light chain expression vectors. The amino acid sequence numbers corresponding to the full length of the heavy and light chains of the chimeric antibody thus constructed are shown in Table 3-1. 293F cells of a well-grown logarithmic growth phase were collected, inoculated into 250mL cell culture flasks and cultured in 50mL medium, and PEI co-transfected with 25. Mu.g each of the light and heavy chain expression plasmids. Cell supernatants from day 7 post-transfection cultures were collected, centrifuged and filtered using a 0.45 μm filter, protein a medium purified for antibodies and the antibodies were replaced by dialysis into PBS ph7.2 buffer. The antibody concentration was determined by measuring absorbance with a micro-spectrophotometer (available from Hangzhou O Cheng Yiqi, nano-300). The binding activity of the obtained chimeric antibody to human DLL3 protein was detected by ELISA (results are shown in FIG. 3 and Table 3-2), and ELISA detection method is described in example 3-1. As can be seen from FIG. 3, the obtained chimeric antibodies all have better binding activity to human DLL3 protein.
TABLE 3-1 chimeric antibody light and heavy chain full-length amino acid sequence numbering
| Chimeric antibodies | Heavy chain full length | Full length of light chain |
| ch1A5 | SEQ ID NO:17 | SEQ ID NO:18 |
| ch33F11 | SEQ ID NO:27 | SEQ ID NO:28 |
| ch23G2 | SEQ ID NO:37 | SEQ ID NO:38 |
| ch32E8 | SEQ ID NO:47 | SEQ ID NO:48 |
| ch13C2 | SEQ ID NO:57 | SEQ ID NO:58 |
| ch19C7 | SEQ ID NO:67 | SEQ ID NO:68 |
| ch26D9 | SEQ ID NO:77 | SEQ ID NO:78 |
| ch10B1 | SEQ ID NO:87 | SEQ ID NO:88 |
| ch36H10 | SEQ ID NO:97 | SEQ ID NO:98 |
| ch4H7 | SEQ ID NO:107 | SEQ ID NO:108 |
| ch6A2 | SEQ ID NO:43 | SEQ ID NO:53 |
| ch15G2 | SEQ ID NO:73 | SEQ ID NO:109 |
TABLE 3-2 binding Activity of chimeric antibodies to human DLL3 protein
| Chimeric antibodies | EC50(ng/mL) |
| FDA027 | 10.84* |
| FDA031 | 23.35 |
| ch32E8 | 8.828 |
| ch1A5 | 9.213 |
| ch33F11 | 9.546 |
| ch23G2 | 9.406 |
| ch4H7 | 11.48 |
| ch19C7 | 8.658 |
| ch6A2 | 13.13 |
| ch36H10 | 15.88 |
| ch10B1 | 16.77 |
| ch26D9 | 11.78 |
| ch13C2 | 394.8 |
| ch15G2 | 8.526 |
Example 7 chimeric antibody Competition assay and binding epitope assay
The chimeric antibodies obtained in example 6 were labeled with Biotin, and the activity of competing the antibodies for binding was detected by ELISA. Briefly, the assay was performed by diluting the DLL3 protein to 1. Mu.g/ml in PBS, spreading the solution over a 384-well ELISA plate (from Corning, 3700) for 40 days, discarding the plate well supernatant the next day, blocking with 80. Mu.l of 3% milk (dissolved in PBS) for 2hr, washing with 80. Mu.l of PBST (1. Mu.m Tween 20) for 3 times, pre-diluting the unlabeled chimeric antibody to 100. Mu.g/ml, respectively, then diluting the solution by a 3-fold gradient for 11 concentration spots, adding 25. Mu.l of each well to the blocked ELISA plate, reacting at room temperature for 1hr, discarding the plate well supernatant, washing with 80. Mu.l of PBST (1. Mu.m Tween 20) for 5 times, adding 25. Mu.l of Biotin-labeled chimeric antibody, incubating at room temperature for 1hr, discarding the supernatant again, washing with 80. Mu.l of PBST (1. Mu.m Tween 20) for 5 times. Finally, HRP-labeled streptavidin secondary antibody 1:5000 Incubation (from gold, M00091) at room temperature for 1hr, discarding the supernatant and washing 7 times with 80 μl of PBST (1%o Tween 20). After the reaction was stopped by adding 25. Mu.l of TMB chromogenic solution and allowing to develop for 10min and adding 2M HCl, the absorbance at 450nm was read by an ELISA reader (available from biotek, elx 808).
To determine the region to which the antibody specifically binds, HEK293 overexpressing cell lines containing different hDLL3 domains (corresponding sequences: SEQ ID NO:5, SEQ ID NO:6, SEQ ID NO:7 and SEQ ID NO: 8) as described in example 2 were examined by FACS for the binding capacity of the chimeric antibody to these four cells to determine the binding region of the antibody. Briefly: will be 5X 10 5 The over-expressed cells of DSL, EGF1, EGF2 and EGF3 were centrifuged at 1500r/min for 1min in 96-well V-shaped microplates and the supernatant was discarded. Saturated 1. Mu.g/ml of antibody to be detected was added, 200. Mu.L per well and incubated on ice for 30min. Then 150. Mu.L PBS was added to each well, centrifuged at 1500r/min for 1min, the supernatant was discarded, and the plate was washed 4 times. APC-labeled goat anti-human IgG (Jackson, PBS 1:800 dilution) was added and incubated on ice for 30min at 50. Mu.L per well. After washing the plates 4 times with PBS, 100. Mu.L of PBS was added to each well to resuspend the cells, and the assay was performed with CytoFLEX (available from Beckman) and the results showed that: antibodies ch1A5, ch33F11, and ch32E8 bind to the EGF2 domain of DLL3, FDA031, ch13C2, and ch15G2 antibodies bind to the N-terminus of DLL3, FDA027 binds to the DSL domain of DLL3, and the remaining antibodies all bind to the EGF3 to EGF6 domains of DLL3, giving a binding epitope for ch15G2 as the N-terminal domain; the binding epitope of ch32E8, ch1A5 and ch33F11 is the EGF2 domain; the binding epitopes of ch36H10, ch6A2, ch19C7, ch26D9, ch23G2, ch10B1 and ch4H7 are EGF3-EGF6 domains.
Example 8 Cross-reactivity of antibodies to human DLL1, DLL4 proteins and murine and monkey DLL3 cells
The chimeric antibody obtained in example 6 was tested for its binding capacity to human DLL1 (purchased from Acro, DL1-H52H 8), DLL4 (purchased from Acro, DL 4-H5227) protein by ELISA, by ELISA test methods, respectively diluting DLL1 and DLL4 proteins with PBS to a final concentration of 1. Mu.g/ml, respectively spreading onto 384-well ELISA plates (purchased from corning, 3700) at 4℃overnight, discarding the plate well supernatant the next day, blocking 2hr with 80. Mu.l of 3% milk (dissolved in PBS), washing the plate well 3 times with 80. Mu.l of PBST (1. Mu.l of Tween 20), then diluting the chimeric antibody to be tested, FDA027 and FDA031 to 20. Mu.g/ml, 3-fold gradient dilution of 11 concentration spots, 25. Mu.l each well to the enzyme plate which has been blocked, room temperature reacting for 1hr, washing 5 mu.l of PBST (1. Mu.m) with 80. Mu.l of Tween20, washing 5 mu.l of PBST (1. Mu.m.m) each well with 7, and 7. Mu.l of anti-human Tween (7: 7). After addition of 25. Mu.l of TMB chromogenic solution for 10min and termination of the reaction with 2M HCl, the microplate reader reads the absorbance at 450nm (from biotek, elx 808) and the results (see FIG. 4) show that there is no significant cross-over between the 12 candidate antibodies and both hDLL1 and hDLL4 proteins, with the 36H10, 4H7 antibodies having a weaker cross-reaction with hDLL1 at high concentration (20. Mu.g/mL); the 6A2 antibody had some cross-reactivity with hDLL4 at high concentrations (20. Mu.g/mL).
To test the cross-reactivity of antibodies to murine and monkey DLL3, the present study used murine DLL3 expression plasmid pCMV3-mDLL3 (purchased from beijing yiqiao, MG 58052-UT) and monkey DLL3 expression plasmid pCMV3-rheDLL3 (purchased from beijing yiqiao, CG 90919-UT) to construct overexpressed cell lines. The construction of the overexpressing cells was as described in examples 2-3. The binding capacity of the chimeric antibody to these two cells was examined by flow cytometry, and the species cross-reactivity of the antibody was determined. The 5×10 of the construction 5 The Mouse DLL3 cell Mouse-DLL3 and the monkey DLL3 cell Rhesus-DLL3 were inoculated into 96-well V-type microplates (available from Axygen, wipp 02280), respectively, and centrifuged at 1500r/min for 1min, and the supernatants were discarded. The chimeric antibody, FDA027 and FDA031 were pre-diluted to 50. Mu.g/ml, diluted 4-fold in a gradient to 8 concentration points, and the antibodies to be detected were added at different concentrations, 200. Mu.L per well, and incubated on ice for 30min. Then 150. Mu.L PBS was added to each well, centrifuged at 1500r/min for 1min, the supernatant was discarded, and the plate was washed 4 times. APC-labeled goat anti-human IgG (Jackson, PBS 1:800 dilution) was added and incubated on ice for 30min at 50. Mu.L per well. After washing the plate 4 times with PBS, 100. Mu.L of PBS was added to each well to resuspend the cells, and the detection was performed with CytoFLEX (Beckman), and the obtained results (shown in FIG. 5) show that the remaining 9 antibodies were able to bind to mouse and monkey DLL3 except that ch32E8, ch13C2 and ch15G2 did not recognize monkey and mouse DLL 3.
Example 9 affinity detection of antibody binding to human DLL3 protein
To examine the affinity of antibodies for human DLL3 protein binding, the present study examined the binding kinetics of immobilized antibodies to free DLL3 using BLI, which was performed with reference to the instructions of the instrument (vendor: fortebio, apparatus model: octet 96 e), briefly, the AMC sensor was equilibrated for 60s with Loading Buffer/Sample dilution Buffer (1 XPBS, pH7.4, with 0.1%BSA and 0.02%Tween-20), yielding Baseline 1. And diluting the antibody to be detected to a concentration of 10ug/ml by using a Loading Buffer, combining the diluted antibody with the balanced sensor, and re-balancing the combined sensor by using the Loading Buffer again to obtain Baseline 2. The antibody-loaded sensor was then placed in human DLL3, his tag (suppliers: acrobiosystems, cat# DL3-H52H 4) diluted to 100 to 3.13nM with sample dilution for 90s to give an antibody-protein binding curve. The antigen-bound sensor was then again placed in Sample Dilution Buffer for dissociation 180s, resulting in a dissociation curve. The k-on, k-off values of antibody binding to protein were calculated by binding and dissociation curves, respectively, and KD values were calculated, and the results obtained (see Table 4) are shown. As a result, these chimeric antibodies were found to have strong affinity at the protein level.
TABLE 4 affinity of antibodies to human DLL3 protein binding
| Antibody name | KD(M) | kon(1/Ms) | Koff(1/s) |
| ch1A5 | 5.97E-11 | 1.24E+06 | 7.40E-05 |
| ch33F11 | 5.21E-11 | 1.21E+06 | 6.31E-05 |
| ch23G2 | 1.58E-11 | 1.76E+06 | 2.79E-05 |
| ch13C2 | 5.37E-09 | 2.23E+05 | 1.20E-03 |
| ch32E8 | 9.00E-11 | 1.49E+06 | 1.34E-04 |
| ch19C7 | 7.39E-10 | 1.30E+06 | 9.62E-04 |
| ch26D9 | 3.81E-11 | 1.73E+06 | 6.58E-05 |
| ch10B1 | 2.04E-10 | 1.37E+06 | 2.79E-04 |
| ch36H10 | 9.91E-11 | 1.69E+06 | 1.68E-04 |
| ch4H7 | 1.04E-10 | 1.07E+06 | 1.11E-04 |
| ch6A2 | 4.66E-10 | 1.47E+06 | 6.84E-04 |
| ch15G2 | 7.02E-11 | 1.72E+06 | 1.21E-04 |
Example 10 preparation of linker drug conjugates
The structures of the linker drug conjugates LE00 and LE01-LE24 used in the invention are shown in Table 5, wherein LE00 (GGFG-Dxd) is synthesized by the method reported in WO2015146132A, and LE01-LE24 is synthesized by the method reported in WO2020259258A 1.
TABLE 5 linker drug conjugate structures
Example 11 preparation of ADC by conjugation of antibody to linker drug conjugate
The different anti-DLL 3 antibodies obtained in examples 1 and 6 above were each replaced to 50mM PB/1.0mM EDTA buffer (pH 7.0) using a G25 desalting column, 12 equivalents TECP were added, and stirred at 37 ℃ for 2 hr to allow the inter-chain disulfide bond of the antibody to be completely opened, followed by adjusting the pH of the reduced antibody solution to 6.0 using phosphoric acid, and reducing the water bath temperature to 25 ℃ for the coupling reaction. The linker drug conjugate prepared according to example 10 was dissolved in DMSO, 12 equivalents of the linker-drug conjugate were extracted therefrom and added drop-wise to the reduced anti-DLL 3 antibody solution, the samples were prepared according to the highest DAR (i.e., excessive conjugation), the occurrence of precipitation at each coupling reaction was observed, DMSO was added to a final concentration of 10% (v/v), the reaction was stirred at 25 ℃ for 0.5 hr, and after the reaction was completed, the samples were filtered using a 0.22 μm membrane. Uncoupled small molecules were removed by purification using a tangential flow ultrafiltration system with buffer 50mM PB/1.0mM EDTA solution (pH 6.0), and after purification 6% sucrose was added and stored in a-20deg.C freezer. The absorbance values were measured at 280nm and 370nm by UV method, respectively, and DAR values were calculated, and the results are shown in Table 6. The results show that the antibodies of ch1A5, ch33F11, ch32E8, ch13C2, FDA027, FDA031, ch15G2 and ch4H7 can be coupled with LE14 normally, the antibodies of ch33F11, LE01-LE11, LE23 and LE24 can be coupled normally, the antibodies of ch1A5, LE00, LE12, LE13, LE15, LE16, LE17, LE18, LE19, LE20, LE21 and LE22 can be coupled normally, and DAR values, free Dxd content and the like of the coupled ADC meet the expected requirements. The ADC samples of the four antibodies of ch19C7, ch23G2, ch6A2 and ch26D9 have obvious flocculent precipitation phenomenon during centrifugal concentration when being coupled with LE14, so that the collection amount is small, which indicates that the coupling is not feasible, the DAR values of the ADC samples obtained by coupling the two antibodies of ch36H10 and ch10B1 are respectively 1.89 and 1.90, the impurity content of the two antibodies is very high, and particularly the small molecule residue of the ch36H10 is up to 49.35%, which indicates that the coupling is failed.
TABLE 6 evaluation of the drug resistance of ADC samples prepared by different antibody-conjugated LE14
Example 12 evaluation of in vitro cytotoxic Activity of ADC samples
HEK293 cells which are stably transfected by DLL3 and high in expression are selected as cell strains for detecting the in vitro activity of experiments, and the quantitative effect of different antibody drug conjugates on cell killing is observed. 2000 cells per well are inoculated in a 96-well cell culture plate and cultured for 20 to 24 hours; antibody drug conjugates prepared according to the method of example 11 were formulated into 9 concentration gradient test solutions of 80, 20, 5, 1.25, 0.3125, 0.0781, 0.0195, 0.00488 and 0.000488. Mu.g/ml, 100. Mu.l/well of diluted test solution was added to a cell-inoculated culture plate and placed at 37℃in 5% CO 2 Culturing in incubator for 144 hr, addingLuminescent Cell Viability Assay Reagent (50. Mu.l/well), shaking at 500rpm at room temperature for 10 minutes, mixing, data processing and IC50 calculation were performed with 100% viability of the whole cell group without drug after SpectraMaxL microplate reader reading (OD 570nm,2s interval reading), and the results are shown in FIG. 6 and Table 7./>
TABLE 7 in vitro killing of ADCs of candidate antibodies against DLL3/HEK293 cells IC 50 Statistical summary of values and maximum kill rates
| Sample name | IC 50 (μg/mL) | Maximum kill rate (%) |
| ch1A5-LE00 | 0.321 | 96.3 |
| ch33F11-LE01 | 0.432 | 95.4 |
| ch33F11-LE02 | 0.564 | 97.3 |
| ch33F11-LE03 | 0.375 | 95.2 |
| ch33F11-LE04 | 0.453 | 94.8 |
| ch33F11-LE05 | 0.532 | 96.2 |
| ch33F11-LE06 | 0.383 | 95.7 |
| ch33F11-LE07 | 0.432 | 95.2 |
| ch33F11-LE08 | 0.634 | 96.8 |
| ch33F11-LE09 | 0.563 | 94.3 |
| ch33F11-LE10 | 0.276 | 96.7 |
| ch33F11-LE11 | 0.364 | 93.1 |
| ch1A5-LE12 | 0.412 | 94.8 |
| ch1A5-LE13 | 0.337 | 96.3 |
| ch1A5-LE15 | 0.432 | 95.2 |
| ch1A5-LE16 | 0.386 | 96.5 |
| ch1A5-LE17 | 0.254 | 93.5 |
| ch1A5-LE18 | 0.227 | 95.6 |
| ch1A5-LE19 | 0.521 | 97.2 |
| ch1A5-LE20 | 0.632 | 95.4 |
| ch1A5-LE21 | 0.534 | 92.7 |
| ch1A5-LE22 | 0.432 | 97.1 |
| ch33F11-LE23 | 0.369 | 93.6 |
| ch33F11-LE24 | 0.463 | 96.5 |
| ch36H10-LE14 | 0.695 | 96.9 |
| ch32E8-LE14 | 0.225 | 96.8 |
| ch1A5-LE14 | 0.277 | 96.6 |
| ch19C7-LE14 | 8.69 | 88.4 |
| ch23G2-LE14 | 0.303 | 96.8 |
| ch33F11-LE14 | 0.377 | 98.3 |
| ch6A2-LE14 | 0.992 | 96.2 |
| ch10B1-LE14 | 0.46 | 97.6 |
| ch4H7-LE14 | 0.718 | 96.9 |
| ch13C2-LE14 | 2.72 | 97.9 |
| ch15G2-LE14 | 0.385 | 90.5 |
| ch26D9-LE14 | 0.563 | 95.6 |
| FDA031-LE14 | 0.389 | 95.2 |
| FDA027-LE14 | 0.087 1 | 95.1 2 |
1 And 2 : all are averages.
The experimental results in Table 7 show that besides relatively weak in-vitro cytotoxic activities of ch19C7-LE14 and ch13C2-LE14, the ADC disclosed by the invention has obvious in-vitro cell killing effect on DLL3 positive cells, relatively strongest in-vitro cytotoxic activity of FDA027-LE14 and optimal in-vitro activity IC50 of FDA027-LE 14. The IC90 value of each ADC disclosed by the invention is not greatly different from that of FDA027-LE 14.
EXAMPLE 13 in vivo anti-tumor Activity of ADC drugs in NCI-H82 model
Female Balb/c nude mice (available from Shanghai Ling Biotech Co., ltd.) 6-8 weeks old were selected for 5X 10 subcutaneous injection in 200ul Matrigel on the right back 6 Human small cell lung cancer cell (NCI-H82) for tumor growth to average volume of 140mm 3 When the mice were left and right, the mice were randomly divided into 15 groups according to tumor size and mouse weight, 8 animals in each of 1-13 groups were respectively a vehicle blank group, and each of the two dose groups of ch1A5-LE14, ch33F11-LE14, ch23G2LE14, ch32E8-LE14, FDA031-LE14 and FDA027-LE14 conjugates was respectively 2.5mg/kg and 5.0mg/kg, and were intravenously administered once a week for 3 times. The number of animals in each of 14-15 groups is 6, namely, the Lurbinectein and topotecan hydrochloride in the control group are respectively 0.18mg/kg and 1.8mg/kg, the Lurbinectein group is administrated once a week and intravenously, the total dose is 3 times, the topotecan hydrochloride group is administrated twice a week and continuously administrated on days 1 and 2, The administration was carried out 3 times. Body weight and tumor volume were measured three times a week and animal survival was observed during the course of the experiment. The results are shown in FIG. 7 and Table 8, and the 21 st balance average tumor volume of the vehicle blank (01 group) mice at the end of administration was 2773mm 3 FDA027-LE14 (2.5 mg/kg, group 12) treatment group had a 21 st balance average tumor volume of 486mm after termination of dosing 3 FDA027-LE14 (5 mg/kg, group 13) treatment group had a 21 st balance average tumor volume of 305mm after the end of dosing 3 . The 21 st balance average tumor volume of the ch1A5-LE14 treatment group (02 group) with 2.5mg/kg test agent was 607mm after the end of the administration 3 The 21 st balance average tumor volume of the ch1A5-LE14 treatment group (03 group) with 5mg/kg of test drug was 245mm after the end of the administration 3 . Test 2.5mg/kg ch33F11-LE14 treatment group (04 group) with a 21 st balance average tumor volume of 715mm after end of dosing 3 The 21 st balance average tumor volume of the ch33F11-LE14 treatment group (05 group) with 5mg/kg of test drug was 293mm after the end of the administration 3 . Test 2.5mg/kg ch23G2-LE14 treatment group (group 06) at 21 st balance average tumor volume of 1322mm after end of dosing 3 The 21 st balance average tumor volume of the ch23G2-LE14 treatment group (07 group) with 5mg/kg of test drug was 441mm after the end of the administration 3 . 2.5mg/kg of test agent ch32E8-LE14 treatment group (group 08) had a 21 st balance average tumor volume of 945mm after the end of dosing 3 The 21 st balance average tumor volume of the ch32E8-LE14 treatment group (09 group) with 5mg/kg of test drug was 433mm after the end of the administration 3 . FDA031-LE14 (2.5 mg/kg, group 10) treatment group had a balance 21 average tumor volume of 564mm after the end of dosing 3 FDA031-LE14 (5 mg/kg, group 11) treatment group had a 21 st balance average tumor volume of 245mm after the end of dosing 3 . A control Lurbinecedein (from Zhongkeyuan pharmaceutical Co., ltd., lot 000039-87-CE013BA-a 1) at 0.18mg/kg had a balance 21 with a tumor volume of 2268mm after the end of administration 3 1.8mg/kg control topotecan hydrochloride (available from Saen chemical technology (Shanghai) Co., lot R3URR 4E) had a 21 st balance average tumor volume of 1553mm after the end of dosing 3 . Experimental results show that the treatment groups (especially ch1A5-LE 14) have better in-vivo anti-tumor activity, and meanwhile, the control group hydrochloric acid topotecan is also usedThe experimental mice have no death condition and no weight loss condition, which indicates that each test drug has good safety.
TABLE 8 in vivo anti-tumor Condition of ADC drugs in NCI-H82 model
EXAMPLE 14 in vivo anti-tumor Activity of ADC drugs in NCI-H209 model
Female Balb/c nude mice 6-8 weeks old were selected for right back subcutaneous injection of 1X 10 in 200ul Matrigel 7 Human small cell lung cancer cell (NCI-H209) for tumor growth to average volume of 150mm 3 When the mice were left and right, the mice were randomly divided into 7 groups according to tumor size and mouse weight, 6 animals in each group were vehicle blank group, and three dose groups of ch1A5-LE14 and FDA027-LE14 conjugate were 1.5mg/kg, 2.5mg/kg and 5.0mg/kg, respectively, for single administration. Body weight and tumor volume were measured three times a week and animal survival was observed during the course of the experiment. The results are shown in Table 9 below, with the average tumor volume at the end of dosing of 2006mm in the vehicle blank (group 1) mice 3 1.5mg/kg FDA027-LE14 treatment group (group 5) had a 21 st balance average tumor volume of 0mm after the end of dosing 3 2.5mg/kg FDA027-LE14 treatment group (group 6) had a 21 st balance average tumor volume of 0mm after the end of dosing 3 5mg/kg FDA027-LE14 treatment group (group 7) had a balance 21 average tumor volume of 0mm after the end of dosing 3 .1.5mg/kg of ch1A5-LE14 treatment group (group 2) had a 21 st balance average tumor volume of 0mm after the end of the administration 3 2.5mg/kg of ch1A5-LE14 treatment group (group 3) had a 21 st balance average tumor volume of 0mm after the end of the administration 3 The 5mg/kg ch1A5-LE14 treatment group (group 4) had a 21 st balance average tumor volume of 0mm after the end of the dose 3 . The experimental results show that FDA027-LE14 and ch1A5-LE14 have better in vivo antitumor activity, and all experimental mice have no death condition and no weight loss condition, so that the test medicament has good safety.
TABLE 9 in vivo anti-tumor Activity of ADC drugs in NCI-H209 model
EXAMPLE 15 evaluation of toxicity of ADC drugs in mice
The mice of the KM strain are selected for toxicity evaluation, the mice are randomly divided into 4 groups according to the weight of the mice, 10 mice in each group are half of the mice, 600ul of ch1A5-LE14 conjugate is injected into the tail vein of each group, the doses are respectively 200mg/kg, 400mg/kg, 500mg/kg and 640mg/kg, toxic reaction conditions caused by the ch1A5-LE14 conjugate are observed within 14 days after single administration, the research result shows that only one animal in the 200mg/kg group is recovered and increased after the D3 day, other animals show an increasing trend in the whole experiment process, animals in the other groups are clinically observed to be abnormal except for the 200mg/kg group, the mice in the other groups are recovered normally and are not dying or dying soon after Mao Pengsong days after administration, and the results show that the conjugate has good tolerance in the mice and the maximum tolerance dose of 640mg/kg. In another study evaluating the toxicity of ch1A5-LE14 conjugates in KM mice, the intraperitoneal injection doses were 410mg/kg, 512mg/kg, 640mg/kg, 800mg/kg and 1000mg/kg, respectively, and the animals in each group were seen to be symptomatic of Mao Pengsong, bow-backed, reduced activity, crunched, diarrhea, soft stool and anal Zhou Wuhui, and 5 mice (10 mice per group) died in the 1000mg/kg group, and one mouse in the 800mg/kg group had a weight loss of more than 30% in three consecutive days over the 14 day observation period, and the animals were treated for well-being according to animal welfare, and the other groups did not see animal death, and the results further showed that the conjugates of the present invention had very good tolerability in mice.
EXAMPLE 16 evaluation of toxicity of ADC drugs in rats
The SD rat tail was selected for intravenous injection of the ch1A5-LE14 conjugate of the invention to evaluate the tolerability of ch1A5-LE14 in rats, and the mice were randomly divided into 3 groups of 8 male and female halves based on their body weight, one group was given 100mg/kg once a week, 3 times a week, one group was given 300mg/kg once, while a blank control group was established, the dosing volume was 10ul/g based on their body weight, and 12 days after completion of the last dosing. After the observation period of the last administration for 12 days is over, the animals do not die or die in the whole experiment process; all animals showed no abnormality after administration of the high dose 300mg/kg group by Mao Pengsong and 100mg/kg group; the weight of the high-dose 300mg/kg group is reduced, and the weight of all the surviving animals is recovered after D7 days; all animals were treated for general examination with ease after the end of the observation period, and no abnormalities were found in the viscera. The results of the toxicity studies in rats further indicate that the conjugates of the invention also have very well tolerated doses in rats.
While particular embodiments of the present invention have been described above, it will be appreciated by those skilled in the art that these are merely illustrative, and that many changes and modifications may be made to these embodiments without departing from the principles and spirit of the invention. Accordingly, the scope of the invention is defined by the appended claims.
Claims (19)
1. An anti-DLL 3 antibody comprising a heavy chain variable region (VH) and a light chain variable region (VL); wherein,,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO. 9, 10 and 11, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO. 12, GAS and SEQ ID NO. 14; or alternatively, the first and second heat exchangers may be,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO. 19, 20 and 21, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO. 12, GAS and SEQ ID NO. 24; or alternatively, the first and second heat exchangers may be,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO. 29, 30 and 31, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO. 32, GAT and SEQ ID NO. 34; or alternatively, the first and second heat exchangers may be,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO. 39, 40 and 41, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO. 42, TTS and SEQ ID NO. 44; or alternatively, the first and second heat exchangers may be,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO 59, 60 and 61, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO 62, NAK and SEQ ID NO 64; or alternatively, the first and second heat exchangers may be,
The amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO 69, 70 and 71, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO 72, NAK and SEQ ID NO 74; or alternatively, the first and second heat exchangers may be,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO. 79, 80 and 81, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO. 82, YTS and SEQ ID NO. 84; or alternatively, the first and second heat exchangers may be,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO. 89, 90 and 91, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO. 92, RAN and SEQ ID NO. 94; or alternatively, the first and second heat exchangers may be,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO 99, 100 and 101, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO 102, WAS and SEQ ID NO 104; or alternatively, the first and second heat exchangers may be,
the amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 contained in the VH are respectively shown as SEQ ID NO. 89, 110 and 111, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 contained in the VL are respectively shown as SEQ ID NO. 112, FTS and SEQ ID NO. 114; or alternatively, the first and second heat exchangers may be,
The amino acid sequences of the VH CDR1, the VH CDR2 and the VH CDR3 are shown as SEQ ID NO. 63, 83 and 93 respectively, and the amino acid sequences of the VL CDR1, the VL CDR2 and the VL CDR3 are shown as SEQ ID NO. 103, NAN and 113 respectively.
2. The anti-DLL 3 antibody of claim 1, wherein the heavy chain variable region (VH) further comprises a heavy chain variable region framework region (VH FWR), and/or the light chain variable region (VL) further comprises a light chain variable region framework region (VL FWR); wherein the VH FWR is a heavy chain variable region framework region of a human or murine antibody and the VL FWR is a light chain variable region framework region of a human or murine antibody;
preferably:
the VH comprises an amino acid sequence shown as SEQ ID NO. 15, 25, 35, 45, 65, 75, 85, 95, 105, 13 or 22; and/or the VL comprises an amino acid sequence as set forth in SEQ ID NO 16, 26, 36, 46, 66, 76, 86, 96, 106, 33 or 23;
more preferably:
the VH comprises an amino acid sequence shown as SEQ ID NO. 15, and the VL comprises an amino acid sequence shown as SEQ ID NO. 16; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 25, and the VL comprises an amino acid sequence shown as SEQ ID NO. 26; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 35, and the VL comprises an amino acid sequence shown as SEQ ID NO. 36; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 45, and the VL comprises an amino acid sequence shown as SEQ ID NO. 46; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 65, and the VL comprises an amino acid sequence shown as SEQ ID NO. 66; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 75, and the VL comprises an amino acid sequence shown as SEQ ID NO. 76; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 85, and the VL comprises an amino acid sequence shown as SEQ ID NO. 86; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 95, and the VL comprises an amino acid sequence shown as SEQ ID NO. 96; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 105, and the VL comprises an amino acid sequence shown as SEQ ID NO. 106; or (b)
The VH comprises an amino acid sequence shown as SEQ ID NO. 13, and the VL comprises an amino acid sequence shown as SEQ ID NO. 33; or (b)
The VH comprises the amino acid sequence shown as SEQ ID NO. 22, and the VL comprises the amino acid sequence shown as SEQ ID NO. 23.
3. The anti-DLL 3 antibody of claim 1 or 2, which is a full length antibody, fab ', F (ab') 2, fv preferably scFv, heavy chain antibody or single domain antibody, and/or which is a monoclonal antibody, a bispecific antibody or a multispecific antibody.
4. The anti-DLL 3 antibody of claim 3, which is a full length antibody comprising a heavy chain and a light chain; wherein the heavy chain comprises an amino acid sequence as set forth in SEQ ID NO. 17, 27, 37, 47, 67, 77, 87, 97, 107, 43 or 73 and/or the light chain comprises an amino acid sequence as set forth in SEQ ID NO. 18, 28, 38, 48, 68, 78, 88, 98, 108, 53 or 109;
preferably:
the heavy chain comprises an amino acid sequence shown as SEQ ID NO. 17, and the light chain comprises an amino acid sequence shown as SEQ ID NO. 18; or (b)
The heavy chain comprises the amino acid sequence shown as SEQ ID NO. 27, and the light chain comprises the amino acid sequence shown as SEQ ID NO. 28; or (b)
The heavy chain comprises an amino acid sequence as shown in SEQ ID NO. 37, and the light chain comprises an amino acid sequence as shown in SEQ ID NO. 38; or (b)
The heavy chain comprises an amino acid sequence as shown in SEQ ID NO. 47, and the light chain comprises an amino acid sequence as shown in SEQ ID NO. 48; or (b)
The heavy chain comprises an amino acid sequence as shown in SEQ ID NO. 67, and the light chain comprises an amino acid sequence as shown in SEQ ID NO. 68; or (b)
The heavy chain comprises an amino acid sequence as shown in SEQ ID NO. 77, and the light chain comprises an amino acid sequence as shown in SEQ ID NO. 78; or (b)
The heavy chain comprises the amino acid sequence shown as SEQ ID NO. 87, and the light chain comprises the amino acid sequence shown as SEQ ID NO. 88; or (b)
The heavy chain comprises an amino acid sequence as shown in SEQ ID NO. 97, and the light chain comprises an amino acid sequence as shown in SEQ ID NO. 98; or (b)
The heavy chain comprises an amino acid sequence as shown in SEQ ID NO. 107, and the light chain comprises an amino acid sequence as shown in SEQ ID NO. 108; or (b)
The heavy chain comprises an amino acid sequence shown as SEQ ID NO. 43, and the light chain comprises an amino acid sequence shown as SEQ ID NO. 53; or (b)
The heavy chain comprises the amino acid sequence shown as SEQ ID NO. 73 and the light chain comprises the amino acid sequence shown as SEQ ID NO. 109.
5. An isolated nucleic acid encoding the anti-DLL 3 antibody of any one of claims 1-4.
6. A recombinant expression vector comprising the isolated nucleic acid of claim 5;
Preferably, the expression vector comprises a eukaryotic expression vector and/or a prokaryotic expression vector.
7. A transformant comprising the recombinant expression vector of claim 6;
preferably, the host cells of the transformant are prokaryotic cells, preferably E.coli cells such as TG1, BL21 cells, and/or eukaryotic cells, preferably HEK293 cells or CHO cells.
8. An antibody drug conjugate has a structural general formula Ab- (L) 3 -L 2 -L 1 -D) m ;
Wherein Ab is an anti-DLL 3 antibody;
m is 2-8;
L 1 the structure of which is shown as formula I, II, III or IV, the a end of which is connected with the cytotoxic drug, the e end of which is connected with the L 2 C end of (2) is connected;
(L) p wherein L is independently one or more of a phenylalanine residue, an alanine residue, a glycine residue, a glutamic acid residue, an aspartic acid residue, a cysteine residue, a glutamic acid residue, a histidine residue, an isoleucine residue, a leucine residue, a lysine residue, a methionine residue, a proline residue, a serine residue, a threonine residue, a tryptophan residue, a tyrosine residue, and a valine residue; p is 2-4;
R 1 is one or more of-NR 1-1 R 1-2 Substituted C 1 ~C 6 Alkyl, substituted by one or more R 1-3 S(O) 2 -substituted C 1 ~C 6 Alkyl, C 1 ~C 6 Alkyl, C 3 ~C 10 Cycloalkyl, C 6 ~C 14 Aryl or 5-14 membered heteroaryl; the hetero atom in the 5-14 membered heteroaryl is selected from one or more of N, O and S, and the number of the hetero atom is 1, 2, 3 or 4; said R is 1-1 、R 1-2 And R is 1-3 Each independently is C 1 ~C 6 An alkyl group;
L 2 is that Wherein n is independently 1 to 12, c-terminal is bound to L via a carbonyl group 1 The f end is connected with the L 3 Is connected with the d end of the (C);
9. The antibody drug conjugate of claim 8,
the anti-DLL 3 antibody binds to one or more of the following antigen binding epitopes in a DLL3 protein: DSL domain, N-terminal, EGF2 domain and EGF3-EGF6 domain;
and/or, L is one or more of phenylalanine residue, alanine residue, glycine residue, isoleucine residue, leucine residue, proline residue and valine residue, preferably one or more of phenylalanine residue, alanine residue, glycine residue and valine residue; more preferably, L is a valine residue and/or an alanine residue, the plurality of L is two or three, and p is 2;
And/or, the R 1 Is one or more of-NR 1-1 R 1-2 Substituted C 1 ~C 6 Alkyl, substituted by one or more R 1-3 S(O) 2 -substituted C 1 ~C 6 Alkyl, or C 1 ~C 6 Alkyl, said R 1-1 、R 1-2 And R is 1-3 Each independently is C 1 ~C 4 An alkyl group;
and/or when L 1 When the structure of the formula I is shown in the specification, L 2 Preferably is
And/or, the n is independently 8, 9, 10, 11, and 12;
and/or m is an integer or non-integer of 2-8;
10. The antibody drug conjugate of claim 8 or 9,
the anti-DLL 3 antibody binds to an antigen binding epitope of an EGF2 domain in the DLL3 protein;
and/or, said (L) p Is thatWherein the g-terminus is bound to the L via a carbonyl group 2 C end of (2) is connected;
11. The antibody drug conjugate of claim 8, which is any one of the compounds shown below:
the Ab is an anti-DLL 3 antibody, wherein the anti-DLL 3 antibody is an anti-DLL 3 antibody according to any one of claims 1 to 4, an anti-DLL 3 antibody having a light chain with an amino acid sequence shown as SEQ ID NO. 1 and a heavy chain with an amino acid sequence shown as SEQ ID NO. 2, or an anti-DLL 3 antibody having a light chain with an amino acid sequence shown as SEQ ID NO. 3 and a heavy chain with an amino acid sequence shown as SEQ ID NO. 4;
M is 7.68, 7.53, 4.43, 7.12, 6.92, 7.43, 7.23, 6.83, 7.32, 7.56, 7.54, 7.47, 5.82, 6.78, 2.28, 6.32, 7.45, 7.65, 7.64, 7.36, 7.75, 7.80, 7.77 or 7.76;
preferably, the antibody-conjugated drug is any one of the compounds shown below:
wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26, < >>
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26, < > >
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising an amino acid sequence such asVH shown in SEQ ID NO. 25 and VL shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 25 and a VL having the amino acid sequence shown in SEQ ID NO. 26,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 15 and a VL having the amino acid sequence shown in SEQ ID NO. 16, < >>
Wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown in SEQ ID NO. 15 and a VL having an amino acid sequence shown in SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown in SEQ ID NO. 15 and a VL having an amino acid sequence shown in SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown in SEQ ID NO. 15 and a VL having an amino acid sequence shown in SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 15 and a VL having the amino acid sequence shown in SEQ ID NO. 16, < >>
Wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown in SEQ ID NO. 15 and a VL having an amino acid sequence shown in SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown in SEQ ID NO. 15 and a VL having an amino acid sequence shown in SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown in SEQ ID NO. 15 and a VL having an amino acid sequence shown in SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 15 and a VL having the amino acid sequence shown in SEQ ID NO. 16, < >>
Wherein Ab is an anti-DLL 3 antibody comprising an amino groupVH with acid sequence shown as SEQ ID NO. 15 and VL with amino acid sequence shown as SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown in SEQ ID NO. 15 and a VL having an amino acid sequence shown in SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having an amino acid sequence shown in SEQ ID NO. 15 and a VL having an amino acid sequence shown in SEQ ID NO. 16,
Wherein Ab is an anti-DLL 3 antibody comprising a heavy chain having an amino acid sequence shown in SEQ ID NO. 2 and a light chain having an amino acid sequence shown in SEQ ID NO. 1,
Wherein Ab is an anti-DLL 3 antibody comprising a heavy chain having an amino acid sequence shown in SEQ ID NO. 4 and a light chain having an amino acid sequence shown in SEQ ID NO. 3,
Wherein Ab is an anti-DLL 3 antibody comprising a VH having the amino acid sequence shown in SEQ ID NO. 22 and a VL having the amino acid sequence shown in SEQ ID NO. 23
12. A chimeric antigen receptor comprising the anti-DLL 3 antibody of any one of claims 1-4.
13. A genetically modified cell comprising an anti-DLL 3 antibody according to any one of claims 1 to 4; preferably, the genetically modified cell is a eukaryotic cell, preferably an isolated human cell; more preferably immune cells such as T cells, or NK cells.
14. A method of preparing an anti-DLL 3 antibody, the method comprising the steps of: culturing the transformant according to claim 7, and obtaining the anti-DLL 3 antibody from the culture.
15. A pharmaceutical composition comprising the anti-DLL 3 antibody of any one of claims 1-4, the antibody drug conjugate of any one of claims 8-11, the chimeric antigen receptor of claim 12, and/or the genetically modified cell of claim 13;
preferably, the pharmaceutical composition is in liquid, gaseous, solid and semi-solid dosage forms, and/or the pharmaceutical composition may be administered orally, by injection, nasally, transdermally or mucosally;
more preferably, the pharmaceutical composition further comprises a combination therapeutic agent comprising a chemotherapeutic agent, a radiation therapeutic agent, an immunosuppressant, and/or a cytotoxic drug.
16. Use of an anti-DLL 3 antibody according to any one of claims 1 to 4, an antibody drug conjugate according to any one of claims 8 to 11, a chimeric antigen receptor according to claim 12, a genetically modified cell according to claim 13 and/or a pharmaceutical composition according to claim 15 for the preparation of a medicament, kit and/or administration device for the treatment and/or prevention of diseases associated with abnormal DLL3 expression;
the DLL3 expression abnormality related disease is preferably a tumor, the tumor is preferably a cancer, and the cancer is preferably a neuroendocrine tumor, more preferably a small cell lung cancer.
17. A kit comprising an anti-DLL 3 antibody according to any one of claims 1-4, an antibody drug conjugate according to any one of claims 8-11, a chimeric antigen receptor according to claim 12, a genetically modified cell according to claim 13 and/or a pharmaceutical composition according to claim 15; and optionally, instructions.
18. A drug delivery device, comprising: (1) An infusion module for administering the pharmaceutical composition of claim 15 to a subject in need thereof, and (2) optionally a efficacy monitoring module.
19. A method of detecting DLL3 comprising the step of detecting using an anti-DLL 3 antibody according to any one of claims 1 to 4, said method being for non-diagnostic and/or therapeutic purposes.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2021108755368 | 2021-07-30 | ||
| CN202110875536 | 2021-07-30 | ||
| CN2022102395912 | 2022-03-11 | ||
| CN202210239591 | 2022-03-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116271079A true CN116271079A (en) | 2023-06-23 |
Family
ID=86792850
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210911943.4A Pending CN116271079A (en) | 2021-07-30 | 2022-07-29 | anti-DLL 3 antibody, preparation method thereof, drug conjugate and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116271079A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024179470A1 (en) * | 2023-02-27 | 2024-09-06 | 苏州盛迪亚生物医药有限公司 | Anti-dll3 antibody, and antibody-drug conjugate and pharmaceutical use thereof |
-
2022
- 2022-07-29 CN CN202210911943.4A patent/CN116271079A/en active Pending
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024179470A1 (en) * | 2023-02-27 | 2024-09-06 | 苏州盛迪亚生物医药有限公司 | Anti-dll3 antibody, and antibody-drug conjugate and pharmaceutical use thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230072897A1 (en) | Anti-b7-h4 antibody-drug conjugate and medicinal use thereof | |
| RU2766586C2 (en) | Pharmaceutical composition for treatment and/or prevention of malignant tumor | |
| AU2019272250B2 (en) | Anti-mesothelin antibody and antibody-drug conjugate thereof | |
| US20220098319A1 (en) | Cd73 antibody, preparation method therefor and application thereof | |
| ES2710916T3 (en) | Substitute binding proteins | |
| WO2023006084A1 (en) | Anti-dll3 antibody and preparation method therefor, drug conjugate and application thereof | |
| US20240269315A1 (en) | Drug conjugate and use thereof | |
| US20210214447A1 (en) | Axl-targeting antibody, antibody-drug conjugate, preparation method therefor, and use thereof | |
| JP2013502209A (en) | Targeted immunoconjugate | |
| CN111051348A (en) | PSMA-binding therapeutic molecules | |
| US20220356246A1 (en) | Anti-ROR1 antibodies and preparation method and uses thereof | |
| CN113366016B (en) | Monoclonal antibody for resisting human interleukin 5 (IL-5) and application thereof | |
| US20240024498A1 (en) | Pharmaceutical composition comprising antibody-drug conjugate, and use of pharmaceutical composition | |
| US10947316B2 (en) | Antibody-drug conjugates containing anti-Globo H antibodies and uses thereof | |
| US12065496B2 (en) | Antibody molecules and conjugates | |
| US20240252664A1 (en) | Product and process for employing gc7 (n1-guanyl-1,7-diaminoheptane) based antigen binding conjugates in cancer therapy | |
| TW201813671A (en) | Pharmaceutical use of anti-c-Met antibody-cytotoxic drug conjugate | |
| WO2021190564A1 (en) | Antibody-drug conjugate and medical use thereof | |
| CN117897407A (en) | anti-Nectin-4 antibody, drug conjugate, preparation method and application thereof | |
| CN113045659B (en) | anti-CD73 humanized antibodies | |
| CN116271079A (en) | anti-DLL 3 antibody, preparation method thereof, drug conjugate and application thereof | |
| JP2024514855A (en) | Binding molecules for DLL3 and their uses | |
| JP2023506805A (en) | Anti-CEA Antibody-Exatecan Analog Conjugate and Its Medical Use | |
| WO2023174213A1 (en) | Antibody-drug conjugate and use thereof | |
| WO2024227426A1 (en) | Antigen-binding protein for hror1 and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |