CN116199688A - Cyclic compounds and their use - Google Patents
Cyclic compounds and their use Download PDFInfo
- Publication number
- CN116199688A CN116199688A CN202211441252.9A CN202211441252A CN116199688A CN 116199688 A CN116199688 A CN 116199688A CN 202211441252 A CN202211441252 A CN 202211441252A CN 116199688 A CN116199688 A CN 116199688A
- Authority
- CN
- China
- Prior art keywords
- substituted
- unsubstituted
- pharmaceutically acceptable
- stereoisomer
- acceptable salt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 150000001923 cyclic compounds Chemical class 0.000 title claims abstract description 47
- 150000003839 salts Chemical class 0.000 claims abstract description 68
- 229940002612 prodrug Drugs 0.000 claims abstract description 36
- 239000000651 prodrug Substances 0.000 claims abstract description 36
- 101000596894 Homo sapiens High affinity nerve growth factor receptor Proteins 0.000 claims abstract description 29
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 25
- 239000003814 drug Substances 0.000 claims abstract description 14
- 101000850794 Homo sapiens Tropomyosin alpha-3 chain Proteins 0.000 claims abstract description 13
- 201000010099 disease Diseases 0.000 claims abstract description 11
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 11
- 201000011510 cancer Diseases 0.000 claims abstract description 9
- 206010006187 Breast cancer Diseases 0.000 claims abstract description 6
- 208000026310 Breast neoplasm Diseases 0.000 claims abstract description 6
- 208000024770 Thyroid neoplasm Diseases 0.000 claims abstract description 6
- 230000001404 mediated effect Effects 0.000 claims abstract description 6
- 201000002510 thyroid cancer Diseases 0.000 claims abstract description 6
- 206010009944 Colon cancer Diseases 0.000 claims abstract description 5
- 206010029260 Neuroblastoma Diseases 0.000 claims abstract description 5
- 206010060862 Prostate cancer Diseases 0.000 claims abstract description 5
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims abstract description 5
- 208000029742 colonic neoplasm Diseases 0.000 claims abstract description 5
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 claims abstract description 5
- 201000001441 melanoma Diseases 0.000 claims abstract description 5
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims abstract description 5
- 230000003248 secreting effect Effects 0.000 claims abstract description 5
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims abstract description 5
- -1 fused Chemical group 0.000 claims description 92
- 238000002360 preparation method Methods 0.000 claims description 71
- 125000000217 alkyl group Chemical group 0.000 claims description 45
- 229910052736 halogen Inorganic materials 0.000 claims description 38
- 150000002367 halogens Chemical class 0.000 claims description 38
- 238000000034 method Methods 0.000 claims description 26
- 238000011282 treatment Methods 0.000 claims description 22
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 19
- 125000000623 heterocyclic group Chemical group 0.000 claims description 16
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 15
- 229910052757 nitrogen Inorganic materials 0.000 claims description 14
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 11
- 239000003112 inhibitor Substances 0.000 claims description 11
- 125000003545 alkoxy group Chemical group 0.000 claims description 10
- 125000004433 nitrogen atom Chemical group N* 0.000 claims description 10
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 10
- 229910052799 carbon Inorganic materials 0.000 claims description 9
- 125000001313 C5-C10 heteroaryl group Chemical group 0.000 claims description 8
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 8
- 125000003118 aryl group Chemical group 0.000 claims description 8
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 8
- 125000002947 alkylene group Chemical group 0.000 claims description 7
- 125000006413 ring segment Chemical group 0.000 claims description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical group N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 6
- 125000004432 carbon atom Chemical group C* 0.000 claims description 6
- 125000005842 heteroatom Chemical group 0.000 claims description 6
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 6
- 125000004205 trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 claims description 6
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 6
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 claims description 4
- 125000006001 difluoroethyl group Chemical group 0.000 claims description 4
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 claims description 4
- 238000002601 radiography Methods 0.000 claims description 4
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 4
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 3
- 239000004480 active ingredient Substances 0.000 claims description 3
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 3
- 239000011737 fluorine Substances 0.000 claims description 3
- 229910052731 fluorine Inorganic materials 0.000 claims description 3
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 3
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 claims description 3
- 125000002950 monocyclic group Chemical group 0.000 claims description 3
- 125000002757 morpholinyl group Chemical group 0.000 claims description 3
- 239000008194 pharmaceutical composition Substances 0.000 claims description 3
- 125000004193 piperazinyl group Chemical group 0.000 claims description 3
- 125000003386 piperidinyl group Chemical group 0.000 claims description 3
- 125000000719 pyrrolidinyl group Chemical group 0.000 claims description 3
- 125000003003 spiro group Chemical group 0.000 claims description 3
- 125000006569 (C5-C6) heterocyclic group Chemical group 0.000 claims description 2
- 229910052770 Uranium Inorganic materials 0.000 claims description 2
- 238000011321 prophylaxis Methods 0.000 claims description 2
- 229910052721 tungsten Inorganic materials 0.000 claims description 2
- 125000001183 hydrocarbyl group Chemical group 0.000 claims 2
- 239000002671 adjuvant Substances 0.000 claims 1
- 239000002243 precursor Substances 0.000 claims 1
- 230000002265 prevention Effects 0.000 claims 1
- 150000001875 compounds Chemical class 0.000 abstract description 60
- 102100035108 High affinity nerve growth factor receptor Human genes 0.000 abstract description 26
- 210000004027 cell Anatomy 0.000 abstract description 16
- 230000000694 effects Effects 0.000 abstract description 13
- 229940079593 drug Drugs 0.000 abstract description 12
- 230000035755 proliferation Effects 0.000 abstract description 10
- 230000005764 inhibitory process Effects 0.000 abstract description 9
- 229940043355 kinase inhibitor Drugs 0.000 abstract description 5
- 206010059866 Drug resistance Diseases 0.000 abstract description 4
- 241000282414 Homo sapiens Species 0.000 abstract description 4
- 238000013508 migration Methods 0.000 abstract description 4
- 230000005012 migration Effects 0.000 abstract description 4
- 210000004881 tumor cell Anatomy 0.000 abstract description 4
- 229940121657 clinical drug Drugs 0.000 abstract description 3
- 230000009545 invasion Effects 0.000 abstract description 3
- 239000003909 protein kinase inhibitor Substances 0.000 abstract description 3
- 241000124008 Mammalia Species 0.000 abstract description 2
- 230000002062 proliferating effect Effects 0.000 abstract description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 abstract 1
- 230000008961 swelling Effects 0.000 abstract 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 abstract 1
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 72
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 24
- 238000006243 chemical reaction Methods 0.000 description 23
- 238000004440 column chromatography Methods 0.000 description 22
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 21
- 239000007787 solid Substances 0.000 description 21
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 19
- 238000001035 drying Methods 0.000 description 18
- 239000000243 solution Substances 0.000 description 18
- 238000001308 synthesis method Methods 0.000 description 18
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 16
- 230000015572 biosynthetic process Effects 0.000 description 16
- 238000003786 synthesis reaction Methods 0.000 description 16
- 239000002904 solvent Substances 0.000 description 15
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 14
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 12
- KHNNSMFTHAZLKZ-UHFFFAOYSA-N imidazo[1,2-b]pyridazine-6-carboxylic acid Chemical compound N1=C(C(=O)O)C=CC2=NC=CN21 KHNNSMFTHAZLKZ-UHFFFAOYSA-N 0.000 description 10
- 230000002401 inhibitory effect Effects 0.000 description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- 239000012044 organic layer Substances 0.000 description 9
- 239000011734 sodium Substances 0.000 description 9
- 229940125782 compound 2 Drugs 0.000 description 8
- 239000012043 crude product Substances 0.000 description 8
- 108090000623 proteins and genes Proteins 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 7
- 229910052786 argon Inorganic materials 0.000 description 6
- 229940126214 compound 3 Drugs 0.000 description 6
- 229940125898 compound 5 Drugs 0.000 description 6
- BRZYSWJRSDMWLG-CAXSIQPQSA-N geneticin Chemical compound O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](C(C)O)O2)N)[C@@H](N)C[C@H]1N BRZYSWJRSDMWLG-CAXSIQPQSA-N 0.000 description 6
- 125000001072 heteroaryl group Chemical group 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 125000001424 substituent group Chemical group 0.000 description 6
- 150000001721 carbon Chemical group 0.000 description 5
- 230000004663 cell proliferation Effects 0.000 description 5
- GVNVAWHJIKLAGL-UHFFFAOYSA-N 2-(cyclohexen-1-yl)cyclohexan-1-one Chemical compound O=C1CCCCC1C1=CCCCC1 GVNVAWHJIKLAGL-UHFFFAOYSA-N 0.000 description 4
- 101150065749 Churc1 gene Proteins 0.000 description 4
- 229910021595 Copper(I) iodide Inorganic materials 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 108091000080 Phosphotransferase Proteins 0.000 description 4
- 102100038239 Protein Churchill Human genes 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- 238000010609 cell counting kit-8 assay Methods 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 239000000460 chlorine Substances 0.000 description 4
- 229940125904 compound 1 Drugs 0.000 description 4
- 229940125773 compound 10 Drugs 0.000 description 4
- LSXDOTMGLUJQCM-UHFFFAOYSA-M copper(i) iodide Chemical compound I[Cu] LSXDOTMGLUJQCM-UHFFFAOYSA-M 0.000 description 4
- YNHIGQDRGKUECZ-UHFFFAOYSA-N dichloropalladium;triphenylphosphanium Chemical compound Cl[Pd]Cl.C1=CC=CC=C1[PH+](C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1[PH+](C=1C=CC=CC=1)C1=CC=CC=C1 YNHIGQDRGKUECZ-UHFFFAOYSA-N 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 238000000605 extraction Methods 0.000 description 4
- 230000004927 fusion Effects 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 4
- 150000002430 hydrocarbons Chemical group 0.000 description 4
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 231100000252 nontoxic Toxicity 0.000 description 4
- 230000003000 nontoxic effect Effects 0.000 description 4
- 102000020233 phosphotransferase Human genes 0.000 description 4
- 238000012216 screening Methods 0.000 description 4
- 238000012163 sequencing technique Methods 0.000 description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N tetrahydrofuran Substances C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 4
- 238000001262 western blot Methods 0.000 description 4
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 3
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 102100029166 NT-3 growth factor receptor Human genes 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 125000001931 aliphatic group Chemical group 0.000 description 3
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 3
- 238000010276 construction Methods 0.000 description 3
- 239000000706 filtrate Substances 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 3
- 150000007529 inorganic bases Chemical class 0.000 description 3
- OKKJLVBELUTLKV-VMNATFBRSA-N methanol-d1 Chemical compound [2H]OC OKKJLVBELUTLKV-VMNATFBRSA-N 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 230000026731 phosphorylation Effects 0.000 description 3
- 238000006366 phosphorylation reaction Methods 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- OEBIHOVSAMBXIB-SJKOYZFVSA-N selitrectinib Chemical compound C[C@@H]1CCC2=NC=C(F)C=C2[C@H]2CCCN2C2=NC3=C(C=NN3C=C2)C(=O)N1 OEBIHOVSAMBXIB-SJKOYZFVSA-N 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 108010064892 trkC Receptor Proteins 0.000 description 3
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- OOWSDKUFKGVADH-UHFFFAOYSA-N 1-diphenylphosphoryloxy-2,3,4,5,6-pentafluorobenzene Chemical compound FC1=C(F)C(F)=C(F)C(F)=C1OP(=O)(C=1C=CC=CC=1)C1=CC=CC=C1 OOWSDKUFKGVADH-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 239000007821 HATU Substances 0.000 description 2
- 102000000646 Interleukin-3 Human genes 0.000 description 2
- 108010002386 Interleukin-3 Proteins 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- 150000001204 N-oxides Chemical class 0.000 description 2
- 101150117329 NTRK3 gene Proteins 0.000 description 2
- 108010025020 Nerve Growth Factor Proteins 0.000 description 2
- 102000007072 Nerve Growth Factors Human genes 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 108091005682 Receptor kinases Proteins 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 102000005937 Tropomyosin Human genes 0.000 description 2
- 108010030743 Tropomyosin Proteins 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 230000003698 anagen phase Effects 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- 150000007514 bases Chemical class 0.000 description 2
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 2
- 229940125797 compound 12 Drugs 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000001085 cytostatic effect Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000003113 dilution method Methods 0.000 description 2
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 2
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 230000003463 hyperproliferative effect Effects 0.000 description 2
- 239000005457 ice water Substances 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 229940076264 interleukin-3 Drugs 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 229910001629 magnesium chloride Inorganic materials 0.000 description 2
- MGZILBNRSLWODC-UHFFFAOYSA-N methyl 3-iodo-2-methyl-5-nitrobenzoate Chemical compound COC(=O)C1=CC([N+]([O-])=O)=CC(I)=C1C MGZILBNRSLWODC-UHFFFAOYSA-N 0.000 description 2
- 239000012046 mixed solvent Substances 0.000 description 2
- 150000007530 organic bases Chemical class 0.000 description 2
- YWWARDMVSMPOLR-UHFFFAOYSA-M oxolane;tetrabutylazanium;fluoride Chemical compound [F-].C1CCOC1.CCCC[N+](CCCC)(CCCC)CCCC YWWARDMVSMPOLR-UHFFFAOYSA-M 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 2
- 239000013612 plasmid Substances 0.000 description 2
- 239000013600 plasmid vector Substances 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- NROKBHXJSPEDAR-UHFFFAOYSA-M potassium fluoride Chemical compound [F-].[K+] NROKBHXJSPEDAR-UHFFFAOYSA-M 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- FIKPXCOQUIZNHB-WDEREUQCSA-N repotrectinib Chemical compound C[C@H]1CNC(=O)C2=C3N=C(N[C@H](C)C4=C(O1)C=CC(F)=C4)C=CN3N=C2 FIKPXCOQUIZNHB-WDEREUQCSA-N 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 238000000967 suction filtration Methods 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- YAPQBXQYLJRXSA-UHFFFAOYSA-N theobromine Chemical compound CN1C(=O)NC(=O)C2=C1N=CN2C YAPQBXQYLJRXSA-UHFFFAOYSA-N 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- CWMFRHBXRUITQE-UHFFFAOYSA-N trimethylsilylacetylene Chemical group C[Si](C)(C)C#C CWMFRHBXRUITQE-UHFFFAOYSA-N 0.000 description 2
- 125000005273 2-acetoxybenzoic acid group Chemical group 0.000 description 1
- MSWZFWKMSRAUBD-IVMDWMLBSA-N 2-amino-2-deoxy-D-glucopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-IVMDWMLBSA-N 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- 229940013085 2-diethylaminoethanol Drugs 0.000 description 1
- QKUHRLBOOQGPNW-UHFFFAOYSA-N 3-iodo-2-methyl-5-nitrobenzoic acid Chemical compound CC1=C(I)C=C([N+]([O-])=O)C=C1C(O)=O QKUHRLBOOQGPNW-UHFFFAOYSA-N 0.000 description 1
- ZZLCFHIKESPLTH-UHFFFAOYSA-N 4-Methylbiphenyl Chemical compound C1=CC(C)=CC=C1C1=CC=CC=C1 ZZLCFHIKESPLTH-UHFFFAOYSA-N 0.000 description 1
- HVCNXQOWACZAFN-UHFFFAOYSA-N 4-ethylmorpholine Chemical compound CCN1CCOCC1 HVCNXQOWACZAFN-UHFFFAOYSA-N 0.000 description 1
- JLPQXFFMVVPIRW-UHFFFAOYSA-N 7-bromoheptanoic acid Chemical compound OC(=O)CCCCCCBr JLPQXFFMVVPIRW-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 102100033793 ALK tyrosine kinase receptor Human genes 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 102100035080 BDNF/NT-3 growth factors receptor Human genes 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 101100127166 Escherichia coli (strain K12) kefB gene Proteins 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- CWYNVVGOOAEACU-UHFFFAOYSA-N Fe2+ Chemical compound [Fe+2] CWYNVVGOOAEACU-UHFFFAOYSA-N 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- 101000779641 Homo sapiens ALK tyrosine kinase receptor Proteins 0.000 description 1
- 101000596896 Homo sapiens BDNF/NT-3 growth factors receptor Proteins 0.000 description 1
- 101000686031 Homo sapiens Proto-oncogene tyrosine-protein kinase ROS Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 102000043136 MAP kinase family Human genes 0.000 description 1
- 108091054455 MAP kinase family Proteins 0.000 description 1
- 108010006519 Molecular Chaperones Proteins 0.000 description 1
- 102000005431 Molecular Chaperones Human genes 0.000 description 1
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 101150111783 NTRK1 gene Proteins 0.000 description 1
- 102000038030 PI3Ks Human genes 0.000 description 1
- 108091007960 PI3Ks Proteins 0.000 description 1
- 102100023347 Proto-oncogene tyrosine-protein kinase ROS Human genes 0.000 description 1
- 241000508269 Psidium Species 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 125000002015 acyclic group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-N ammonia Natural products N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 229960003121 arginine Drugs 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid group Chemical group C(C1=CC=CC=C1)(=O)O WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 1
- 229960003237 betaine Drugs 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 229960001948 caffeine Drugs 0.000 description 1
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 125000005047 dihydroimidazolyl group Chemical group N1(CNC=C1)* 0.000 description 1
- 125000005048 dihydroisoxazolyl group Chemical group O1N(CC=C1)* 0.000 description 1
- 125000005049 dihydrooxadiazolyl group Chemical group O1N(NC=C1)* 0.000 description 1
- 125000005050 dihydrooxazolyl group Chemical group O1C(NC=C1)* 0.000 description 1
- 125000005051 dihydropyrazinyl group Chemical group N1(CC=NC=C1)* 0.000 description 1
- 125000005052 dihydropyrazolyl group Chemical group N1(NCC=C1)* 0.000 description 1
- 125000004655 dihydropyridinyl group Chemical group N1(CC=CC=C1)* 0.000 description 1
- 125000005053 dihydropyrimidinyl group Chemical group N1(CN=CC=C1)* 0.000 description 1
- 125000005054 dihydropyrrolyl group Chemical group [H]C1=C([H])C([H])([H])C([H])([H])N1* 0.000 description 1
- 125000005056 dihydrothiazolyl group Chemical group S1C(NC=C1)* 0.000 description 1
- 125000005057 dihydrothienyl group Chemical group S1C(CC=C1)* 0.000 description 1
- 125000005058 dihydrotriazolyl group Chemical group N1(NNC=C1)* 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000002359 drug metabolite Substances 0.000 description 1
- 229950000521 entrectinib Drugs 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- DBLXOVFQHHSKRC-UHFFFAOYSA-N ethanesulfonic acid;2-piperazin-1-ylethanol Chemical compound CCS(O)(=O)=O.OCCN1CCNCC1 DBLXOVFQHHSKRC-UHFFFAOYSA-N 0.000 description 1
- 238000003810 ethyl acetate extraction Methods 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 239000012065 filter cake Substances 0.000 description 1
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 1
- 229960002442 glucosamine Drugs 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- DQOCFCZRZOAIBN-WZHZPDAFSA-L hydroxycobalamin Chemical compound O.[Co+2].[N-]([C@@H]1[C@H](CC(N)=O)[C@@]2(C)CCC(=O)NC[C@@H](C)OP([O-])(=O)O[C@H]3[C@H]([C@H](O[C@@H]3CO)N3C4=CC(C)=C(C)C=C4N=C3)O)\C2=C(C)/C([C@H](C\2(C)C)CCC(N)=O)=N/C/2=C\C([C@H]([C@@]/2(CC(N)=O)C)CCC(N)=O)=N\C\2=C(C)/C2=N[C@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(N)=O DQOCFCZRZOAIBN-WZHZPDAFSA-L 0.000 description 1
- UWYVPFMHMJIBHE-OWOJBTEDSA-N hydroxymaleic acid group Chemical group O/C(/C(=O)O)=C/C(=O)O UWYVPFMHMJIBHE-OWOJBTEDSA-N 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 238000003674 kinase activity assay Methods 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- YNESATAKKCNGOF-UHFFFAOYSA-N lithium bis(trimethylsilyl)amide Chemical compound [Li+].C[Si](C)(C)[N-][Si](C)(C)C YNESATAKKCNGOF-UHFFFAOYSA-N 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- LGRLWUINFJPLSH-UHFFFAOYSA-N methanide Chemical compound [CH3-] LGRLWUINFJPLSH-UHFFFAOYSA-N 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- ACTNHJDHMQSOGL-UHFFFAOYSA-N n',n'-dibenzylethane-1,2-diamine Chemical compound C=1C=CC=CC=1CN(CCN)CC1=CC=CC=C1 ACTNHJDHMQSOGL-UHFFFAOYSA-N 0.000 description 1
- HAYYBYPASCDWEQ-UHFFFAOYSA-N n-[5-[(3,5-difluorophenyl)methyl]-1h-indazol-3-yl]-4-(4-methylpiperazin-1-yl)-2-(oxan-4-ylamino)benzamide Chemical compound C1CN(C)CCN1C(C=C1NC2CCOCC2)=CC=C1C(=O)NC(C1=C2)=NNC1=CC=C2CC1=CC(F)=CC(F)=C1 HAYYBYPASCDWEQ-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000003566 oxetanyl group Chemical group 0.000 description 1
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 1
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 125000003367 polycyclic group Chemical group 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 239000011698 potassium fluoride Substances 0.000 description 1
- 235000003270 potassium fluoride Nutrition 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- VVWRJUBEIPHGQF-MDZDMXLPSA-N propan-2-yl (ne)-n-propan-2-yloxycarbonyliminocarbamate Chemical compound CC(C)OC(=O)\N=N\C(=O)OC(C)C VVWRJUBEIPHGQF-MDZDMXLPSA-N 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- RUUOPSRRIKJHNH-UHFFFAOYSA-N pyridazine-3-carboxylic acid Chemical compound OC(=O)C1=CC=CN=N1 RUUOPSRRIKJHNH-UHFFFAOYSA-N 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 238000010517 secondary reaction Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid Substances OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- UYDIIUHJHUZDME-UHFFFAOYSA-N tert-butyl 5-bromopentanoate Chemical compound CC(C)(C)OC(=O)CCCCBr UYDIIUHJHUZDME-UHFFFAOYSA-N 0.000 description 1
- BDLPJHZUTLGFON-UHFFFAOYSA-N tert-butyl n-(6-hydroxyhexyl)carbamate Chemical compound CC(C)(C)OC(=O)NCCCCCCO BDLPJHZUTLGFON-UHFFFAOYSA-N 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 125000003554 tetrahydropyrrolyl group Chemical group 0.000 description 1
- 125000005958 tetrahydrothienyl group Chemical group 0.000 description 1
- 229960004559 theobromine Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- QIWRFOJWQSSRJZ-UHFFFAOYSA-N tributyl(ethenyl)stannane Chemical compound CCCC[Sn](CCCC)(CCCC)C=C QIWRFOJWQSSRJZ-UHFFFAOYSA-N 0.000 description 1
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- COIOYMYWGDAQPM-UHFFFAOYSA-N tris(2-methylphenyl)phosphane Chemical compound CC1=CC=CC=C1P(C=1C(=CC=CC=1)C)C1=CC=CC=C1C COIOYMYWGDAQPM-UHFFFAOYSA-N 0.000 description 1
- 229960000281 trometamol Drugs 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/22—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed systems contains four or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/12—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains three hetero rings
- C07D498/18—Bridged systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/22—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains four or more hetero rings
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The invention provides a cyclic compound with a structure shown in a formula (I) or pharmaceutically acceptable salt thereof or stereoisomer thereof or prodrug molecule thereof, and application thereof. The compound and the pharmaceutically acceptable salt thereof related by the invention can be used as protein kinase inhibitor, have strong inhibition activity on TRKs kinase, have strong inhibition activity on the proliferation of wild type and drug-resistant cells of Ba/F3-TRKs stable strain, can inhibit the proliferation, migration and invasion of various tumor cells, can especially overcome the drug resistance of the existing clinical drugs, and can be used for preparing drugs for preventing or treating diseases (such as swelling) mediated by TRK tyrosine kinaseTumor) is used for treating human and other mammal tumors and other transitional proliferative diseases, such as non-small cell lung cancer, breast cancer, colon cancer, prostatic cancer, thyroid cancer, malignant melanoma, neuroblastoma, breast-like secretory cancer and the like.
Description
Technical Field
The invention relates to the technical field of chemical medicines, in particular to a cyclic compound and application thereof.
Background
Cancer has become one of the important diseases threatening human health. In recent years, the incidence and mortality of cancer have seen a continuing trend. Traditional malignant tumor treatment means (operation treatment, radiation treatment and chemotherapy) have certain limitations and toxic and side effects. Improving survival rate and life quality of tumor patients becomes the primary problem to be solved in tumor treatment. Targeted antitumor drugs have played an increasingly important role in clinical treatment of tumors in recent 20 years, however, the problem of drug resistance is also increasing.
Tropomyosin receptor kinase (Tropomyosin receptor kinase, TRK) comprises three highly homologous subtypes TrkA, trkB and TrkC, encoded by NTRK1, NTRK2 and NTRK3 genes, respectively. The extracellular domain of Trk kinase acts by binding to different Neurotrophins (NTs). When the ligand is combined with the corresponding Trk protein, trk kinase is dimerized and phosphorylated in sequence, and downstream signal channels (mainly PI3K/AKT channel, ras/Raf/MAPK channel and PLC gamma/PKC channel) are activated to regulate and control a series of biological functions such as proliferation, differentiation, survival, migration and the like of cells. It was found that TRK promotes proliferation and survival of tumor cells mainly by gene fusion with its chaperone proteins. TRKs gene fusion is found in various tumors such as thyroid cancer, lung cancer, breast cancer and glioma, and the fusion forms comprise various types such as LMNA-NTRK1, BCR-NTRK2, ETV6-NTRK3 and the like. Two "unlimited cancer species" TRK kinase inhibitors, larrotinib and emtrictinib (Entrectinib), were marketed sequentially, demonstrating that TRK kinase is an effective "pan-cancer" target.
Larrottinib (larotentib) is a selective TRKA/B/C inhibitor developed by LOXO Oncology company. Entrictinib (entretinib) is a multi-target kinase inhibitor and has ALK, ROS1, TRKs and other kinase inhibitory activities. Larotigotine and emtrictinib are marketed in 2018 and 2019 for the treatment of TRK fusion positive tumors, respectively, belonging to the first generation of TRK inhibitors, but with clinical use, clinical resistance problems are rapidly generated. Clinical studies have successively found mutations such as G595R, G667C, F589L, G667S of NTRK1 and G623R, G696A of NTRK3, which lead to a great decrease in the clinical therapeutic effect of the drug. TRK second generation inhibitors LOXO-195, TPX-0005, etc. are under clinical study, which are mainly resistant to mutations such as the front of TRK solvents, e.g., G595R of NTRK1 and G623R of NTRK3, but not to xDFG mutation sites, and no inhibitors for these mutations are currently marketed.
Disclosure of Invention
Based on the above, the invention provides a novel cyclic compound or pharmaceutically acceptable salt or stereoisomer thereof, which can be used as a protein kinase inhibitor, can effectively inhibit the activity of TRK protein kinase and inhibit proliferation, migration and invasion of various tumor cells, and can especially overcome the drug resistance of the existing clinical drugs.
The method specifically comprises the following technical scheme:
a cyclic compound having a structure represented by formula (I) or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof:
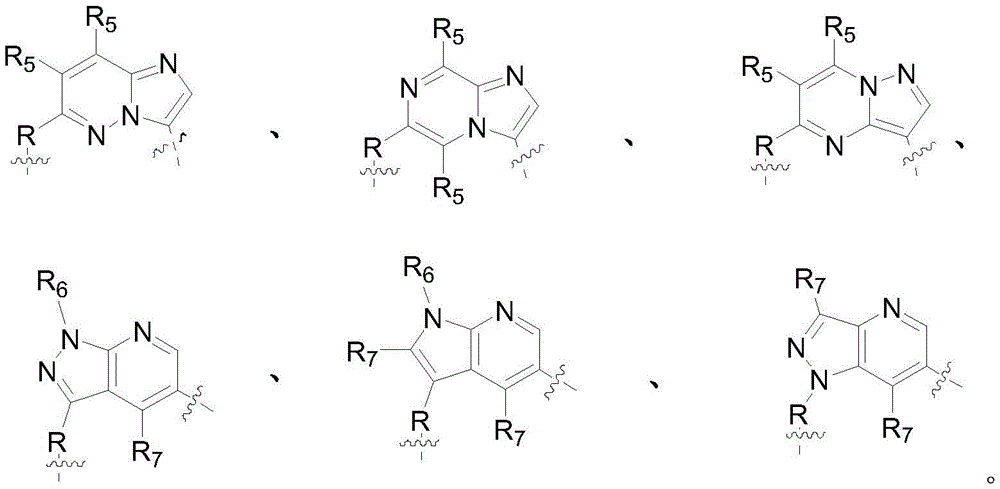
E. z, M, Q are each independently selected from: CR (computed radiography) 5 Or N;
t is selected from: n, CR 7 Or NR 6 The method comprises the steps of carrying out a first treatment on the surface of the V is selected from: n, C or CR 7 The method comprises the steps of carrying out a first treatment on the surface of the U, W are each independently selected from: CR (computed radiography) 7 Or N;
R 5 、R 6 、R 7 each independently selected from: H. halogen, C 1 ~C 6 Alkyl, substituted or unsubstituted C 3 ~C 7 Cycloalkyl;
r is selected from: substituted or unsubstituted phenyl, -C (=o) -, -C (=o) NR 8 R 9 or-NR 8 R 9 The method comprises the steps of carrying out a first treatment on the surface of the Wherein R is 8 Selected from: H. c (C) 1 ~C 20 Alkyl, - (CH) 2 ) m NR 10 R 11 、-(CH 2 ) n CR 10 R 11 R 12 ;R 9 Selected from: c (C) 1 ~C 20 Alkylene, or R 8 、R 9 To which they are connectedTogether form a substituted or unsubstituted heterocyclic group;
R 10 、R 11 、R 12 Each independently selected from: H. c (C) 1 ~C 20 Alkyl, or R 10 、R 11 Together with the nitrogen or carbon atom to which they are attached, form a substituted or unsubstituted monocyclic, fused, spiro or bridged ring containing 0 to 3 heteroatoms;
m and n are each independently selected from: an integer of 0 to 10;
x, Y are each independently selected from: -O-, -N (R) 13 )-、-S-、-S(=O)-、-S(O) 2 -、-C(=O)-、-NR 13 (C=O)-;R 13 Selected from: H. c (C) 1 ~C 6 An alkyl group;
l is selected from: substituted or unsubstituted C 2 ~C 12 Alkylene, substituted or unsubstituted C 2 ~C 12 Unsaturated chain hydrocarbon groups;
X 1 and X 2 Each independently selected from: -N (R) 13 ) Or none;
R 1 selected from: H. halogen, C 1 ~C 20 Alkyl, C 1 ~C 20 Alkoxy or halogen substituted C 1 ~C 20 An alkyl group;
g is selected from: r' substituted or unsubstituted C 6 ~C 10 Aryl, R' substituted or unsubstituted 5-10 membered heteroaryl;
each R' is independently selected from: H. halogen, substituted or unsubstituted C 1 ~C 20 Alkyl, substituted or unsubstituted C 1 ~C 20 Alkoxy, nitro, substituted or unsubstituted C 3 ~C 12 Cycloalkyl, substituted or unsubstituted C 6 ~C 10 Aryl, substituted or unsubstituted 3-12 membered heterocyclyl, substituted or unsubstituted 5-10 membered heteroaryl.
In some embodiments, G is selected from: r 'is a substituted or unsubstituted phenyl group, R' is a substituted or unsubstituted 5-6 membered heteroaryl group containing 1-3N ring atoms.
In some of these embodiments, the cyclic compound has a structure represented by the following formula (II) or formula (III):
Wherein R is 2 Selected from: H. halogen, C 1 ~C 20 Alkyl, C 1 ~C 20 Alkoxy, halogen substituted C 1 ~C 20 Alkyl, C 3 ~C 12 Cycloalkyl;
R 3 selected from: H. halogen, substituted or unsubstituted C 1 ~C 20 Alkyl, substituted or unsubstituted 5-6 membered heterocyclic group containing 1-3N ring atoms;
R 4 selected from: H. halogen, nitro, substituted or unsubstituted C 1 ~C 20 Alkyl, substituted or unsubstituted C 1 ~C 20 Alkoxy, substituted or unsubstituted C 3 ~C 12 Cycloalkyl, substituted or unsubstituted 3-12 membered heterocyclyl, substituted or unsubstituted 5-10 membered heteroaryl;
R’ 4 selected from: H. substituted or unsubstituted C 1 ~C 20 Alkyl, substituted or unsubstituted C 3 ~C 12 Cycloalkyl, substituted or unsubstituted C 6 ~C 10 Aryl, substituted or unsubstituted 3-12 membered heterocyclyl, substituted or unsubstituted 5-10 membered heteroaryl.
In some of these embodiments, R 4 Selected from: H. halogen, C 1 ~C 4 Alkyl, nitro, halogen substituted C 1 ~C 4 Alkyl, C 1 ~C 4 Alkoxy, halogen substituted C 1 ~C 4 Alkoxy, - (CH) 2 ) p NR 15 R 16 By 1 or more R 17 Substituted or unsubstituted 5-6 membered heterocyclyl, substituted with 1-3R 17 Substituted or unsubstituted 5-6 membered heteroaryl; p is selected from: 1. 2 or 3;
R 15 、R 16 together with the nitrogen atom to which they are attached form R 17 A substituted or unsubstituted 5-12 membered heterocyclyl;
each R is 17 Each independently selected from: H. c (C) 1 -C 6 Alkyl, C 3 ~C 8 Cycloalkyl, 5-8 membered heterocyclyl, dimethylamino, methanesulfonyl.
In some of these embodiments, R 4 Selected from: H. halogen, nitro, methyl, ethyl, propyl, methoxy, ethoxy, propoxy, trifluoromethyl, trifluoroethyl,
Each R is 17 Each independently selected from: H. methyl, ethyl, propyl, dimethylamino, cyclohexyl, methylsulfonyl.
In some of these embodiments, R 2 Selected from: H. halogen, C 1 ~C 6 Alkyl, C 1 ~C 6 Alkoxy, halogen substituted C 1 ~C 6 Alkyl, C 3 ~C 8 Cycloalkyl groups.
In some of these embodiments, R 2 Selected from: H. fluorine, methyl, ethyl, propyl, isopropyl, tert-butyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, cyclopropyl.
In some of these embodiments, R 3 Selected from: H. halogen, C 1 ~C 6 Alkyl, halogen substituted C 1 ~C 6 Alkyl, - (CH) 2 ) p NR 15 R 16 The method comprises the steps of carrying out a first treatment on the surface of the p is selected from: 1. 2 or 3;
R 15 、R 16 together with the nitrogen atom to which they are attached form R 17 A substituted or unsubstituted 5-8 membered heterocyclic group;
wherein R is 17 Selected from: H. c (C) 1 -C 6 An alkyl group.
In some of these embodiments, R 3 Selected from: H. halogen, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl,Wherein R is 17 Selected from: H. methyl, ethyl, propyl.
In some of these embodiments, R' 4 Selected from: H. c (C) 1 ~C 6 Alkyl, C 3 ~C 8 Cycloalkyl, R 17 A substituted or unsubstituted phenyl group; wherein R is 17 Selected from: H. c (C) 1 -C 6 An alkyl group.
In some embodiments, a is selected from:
in some embodiments, a is selected from:
in some embodiments, R is selected from: -C (=o) -, -C (=o) NR 8 R 9 or-NR 8 R 9 The method comprises the steps of carrying out a first treatment on the surface of the Wherein R is 8 Selected from: H. c (C) 1 ~C 6 Alkyl, - (CH) 2 ) m NR 10 R 11 ;R 9 Selected from: c (C) 1 ~C 6 Alkylene, or R 8 、R 9 Together with the nitrogen atom to which they are attached form one or more R 14 A substituted or unsubstituted 3-8 membered heterocyclic group;
R 10 、R 11 each independently selected from: H. c (C) 1 ~C 6 An alkyl group;
m is selected from: an integer of 1 to 5;
R 14 selected from: H. c (C) 1 ~C 6 An alkyl group.
In some embodiments, R is selected from: -C (=o) -, -C (=o) NR 8 R 9 or-NR 8 R 9 The method comprises the steps of carrying out a first treatment on the surface of the Wherein R is 8 Selected from: H. c (C) 1 ~C 3 An alkyl group; r is R 9 Selected from: c (C) 1 ~C 3 Alkylene, or R 8 、R 9 Together with the nitrogen atom to which they are attached form one or more R 14 Substituted or unsubstituted morpholinyl, pyrrolidinyl, piperidinyl or piperazinyl; r is R 14 Selected from: H. c (C) 1 ~C 3 An alkyl group.
In some embodiments, R is selected from: -C (=o) -,x is selected from: 0. 1, 2 and 3; y is selected from: an integer between 0 and 8; r is R 8 Selected from: H. c (C) 1 ~C 6 An alkyl group.
In some embodiments, a is selected from:
In some of these embodiments, X, Y are each independently selected from: -O-, -N (R) 13 )-、-NR 13 (C=O)-;R 13 Selected from: H. c (C) 1 ~C 3 An alkyl group.
In some embodiments, X is-O-; y is selected from: -N (R) 13 )-、-NR 13 (C=O)-;R 13 Selected from: H. methyl group.
In some embodiments, L is selected from: c (C) 2 ~C 8 Alkylene, C 2 ~C 8 Unsaturated chain hydrocarbon groups.
In some of these embodiments, a and Y together form the structure:
l is selected from:q is 3; x is-O-; r is R 4 Selected from:R 17 Selected from: H. methyl, ethyl, propyl.
In some of these embodiments, R 1 Selected from: H. halogen, C 1 ~C 3 An alkyl group.
In some of these embodiments, X 1 Is NH or not, X 2 Is NH.
The invention also provides application of the cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or prodrug molecule thereof.
The specific technical scheme is as follows:
the use of a cyclic compound as described above, or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a prodrug molecule thereof, in the preparation of a TRK inhibitor.
Use of a cyclic compound as described above or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof in the preparation of a medicament for the prophylaxis and/or treatment of a disease mediated by TRK kinase.
In some of these embodiments, the disease mediated by TRK kinase is a tumor.
In some of these embodiments, the tumor is: non-small cell lung cancer, breast cancer, colon cancer, prostate cancer, thyroid cancer, malignant melanoma, neuroblastoma, and breast-like secretory cancer.
The invention also provides a pharmaceutical composition for preventing and/or treating tumors.
The specific technical scheme is as follows:
a pharmaceutical composition for preventing and/or treating tumors is prepared from an active ingredient and pharmaceutically acceptable auxiliary materials, wherein the active ingredient comprises the cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or prodrug molecules thereof.
The cyclic compound provided by the invention can be used as a protein kinase inhibitor, has strong inhibition activity on TRKs kinase, has strong inhibition activity on the proliferation of wild type and drug-resistant cells of Ba/F3-TRKs stable strains, can inhibit the proliferation, migration and invasion of various tumor cells, and can especially overcome the drug resistance of the existing clinical drugs. The cyclic compound provided by the invention can be used for preparing medicines for preventing or treating diseases (such as tumors) mediated by TRK tyrosine kinase, and can be used for treating transitional proliferative diseases such as tumors of human beings and other mammals, such as non-small cell lung cancer, breast cancer, colon cancer, prostatic cancer, thyroid cancer, malignant melanoma, neuroblastoma, breast-like secretory cancer and the like.
Detailed Description
The experimental methods of the present invention, in which specific conditions are not specified in the following examples, are generally conducted under conventional conditions or under conditions recommended by the manufacturer. The various chemicals commonly used in the examples are commercially available.
Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. The terminology used in the description of the invention herein is for the purpose of describing particular embodiments only and is not intended to be limiting of the invention.
The terms "comprising" and "having" and any variations thereof, are intended to cover a non-exclusive inclusion. For example, a process, method, apparatus, article, or device that comprises a list of steps is not limited to the elements or modules listed but may alternatively include additional steps not listed or inherent to such process, method, article, or device.
In the present invention, the term "plurality" means two or more. "and/or", describes an association relationship of an association object, and indicates that there may be three relationships, for example, a and/or B, and may indicate: a exists alone, A and B exist together, and B exists alone. The character "/" generally indicates that the context-dependent object is an "or" relationship.
In the compounds of the invention, when any variable (e.g., R 14 、R 17 Etc.) occur more than once in any component, the definition of each occurrence is independent of the definition of each other occurrence. Also, combinations of substituents and variables are permissible provided that such combinations stabilize the compounds. The lines drawn from the substituents into the ring system indicate that the bond referred to may be attached to any substitutable ring atom. If the ring system is polycyclic, it means that such bonds are only attached to any suitable carbon atom adjacent to the ring. It is to be understood that substituents and substitution patterns of the compounds of this invention may be selected by one of ordinary skill in the art to provide compounds that are chemically stable and that may be readily synthesized from readily available starting materials by techniques in the art and methods set forth below. If the substituent itself is substituted with more than one group, it is understood that these groups may be on the same carbon atom or on different carbon atoms, as long as the structure is stabilized.
The term "alkyl" as used herein is meant to include both branched and straight chain saturated aliphatic hydrocarbon groups having a specified number of carbon atoms. For example, "C 1 -C 6 Alkyl "medium" C 1 -C 6 The definition of "includes groups having 1, 2, 3, 4, 5 or 6 carbon atoms arranged in a straight or branched chain. For example, "C 1 -C 6 The alkyl group includes, in particular, methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, isobutyl, pentyl, hexyl.
The term "alkylene" refers to groups having one less hydrogen on an "alkyl" basis, e.g., -CH 2 -、-CH 2 CH 2 -、-CH 2 CH 2 CH 2 -、-CH 2 CH 2 CH 2 CH 2 -and the like.
The term "cycloalkyl" refers to a monocyclic saturated aliphatic hydrocarbon group having a specified number of carbon atoms. For example "C 3 ~C 7 Cycloalkyl "includes cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and the like.
The term "unsaturated chain hydrocarbon group" refers to branched and straight chain unsaturated aliphatic hydrocarbon groups having a specific number of carbon atoms, i.e., acyclic chain hydrocarbon groups, and having 1 or more carbon-carbon double bonds in the carbon chain, or having carbon-carbon triple bonds, such as: -ch=ch-, -CH 2 CH=CH-、-CH 2 CH=CHCH 2 -、-CH=CHCH 2 CH 2 -、-(CH 2 ) 2 (CH=CH)(CH 2 ) 2 CH 2 -,-CH 2 CH=CHCH 2 CH=CHCH 2 -and the like.
The term "alkoxy" refers to a group having the structure of an-O-alkyl group, such as-OCH 3 、-OCH 2 CH 3 、-OCH 2 CH 2 CH 3 、-O-CH 2 CH(CH 3 ) 2 、-OCH 2 CH 2 CH 2 CH 3 、-O-CH(CH 3 ) 2 Etc.
The term "heterocycloalkyl" or "heterocyclyl" is a saturated or partially unsaturated cyclic substituent such as a single, fused, spiro, or bridged ring, wherein one or more ring atoms are selected from heteroatoms of N, O or S (O) m (where m is an integer from 0 to 2) and the remaining ring atoms are carbon, for example: morpholinyl, piperidinyl, tetrahydropyrrolyl, oxetanyl, piperazinyl, pyrrolidinyl, dihydroimidazolyl, dihydroisoxazolyl, dihydroisothiazolyl, dihydrooxadiazolyl, dihydrooxazolyl, dihydropyrazinyl, dihydropyrazolyl, dihydropyridinyl, dihydropyrimidinyl, dihydropyrrolyl, dihydrotetrazolyl, dihydrothiadiazolyl, dihydrothiazolyl, dihydrothienyl, dihydrotriazolyl, dihydroazetidinyl, tetrahydrofuranyl, tetrahydrothienyl, and the like, and the N-oxides thereof, and the attachment of the heterocyclic substituents may be effected through a carbon atom or through a heteroatom.
The term "heteroaryl" as used herein refers to an aromatic ring containing 1 or more heteroatoms selected from O, N or S, heteroaryl groups within the scope of the present invention include, but are not limited to: quinolinyl, pyrazolyl, pyrrolyl, thienyl, furyl, pyridyl, pyrimidinyl, pyrazinyl, triazolyl, imidazolyl, oxazolyl, isoxazolyl, pyridazinyl, benzofuranyl, benzothienyl, benzoxazolyl, indolyl, and the like; "heteroaryl" is also understood to include any N-oxide derivative of a heteroaryl group containing nitrogen. The attachment of the heteroaryl group may be through a carbon atom or through a heteroatom.
The term "substituted" as used herein refers to the replacement of a hydrogen group in a particular structure with a group of the specified substituent.
As understood by those skilled in the art, "halo" or "halogen" as used herein means chlorine, fluorine, bromine and iodine.
Unless otherwise defined, alkyl, cycloalkyl, aryl, heteroaryl, and heterocycloalkyl substituents may be unsubstituted or substituted. For example, C 1 -C 6 The alkyl group may be substituted with one, two or three substituents selected from OH, halogen, alkoxy, dialkylamino or heterocyclyl groups such as morpholino, piperidinyl and the like.
The present invention includes the free forms of the compounds of formulas (I) - (III), as well as pharmaceutically acceptable salts and stereoisomers thereof. The term "free form" refers to an amine compound in a non-salt form. Included are pharmaceutically acceptable salts including not only the exemplary salts of the specific compounds described herein, but also the typical pharmaceutically acceptable salts of all compounds of formulas (I) - (III) in free form. The free form of the particular salt of the compound may be isolated using techniques known in the art. For example, the free form can be regenerated by treating the salt with a suitable dilute aqueous base solution, such as dilute aqueous NaOH, dilute aqueous potassium carbonate, dilute aqueous ammonia, and dilute aqueous sodium bicarbonate. The free forms differ somewhat from their respective salt forms in certain physical properties, such as solubility in polar solvents, but for the purposes of this invention such acid and base salts are otherwise pharmaceutically comparable to their respective free forms.
Pharmaceutically acceptable salts of the present invention can be synthesized from the compounds of the present invention containing a basic moiety or an acidic moiety by conventional chemical methods. Typically, salts of basic compounds are prepared by ion exchange chromatography or by reacting the free base with a stoichiometric or excess of an inorganic or organic acid in the form of the desired salt in a suitable solvent or combination of solvents. Similarly, salts of acidic compounds are formed by reaction with suitable inorganic or organic bases.
Thus, pharmaceutically acceptable salts of the compounds of the invention include the conventional non-toxic salts of the compounds of the invention formed by the reaction of a basic compound of the invention with an inorganic or organic acid. For example, conventional nontoxic salts include salts derived from inorganic acids such as hydrochloric, hydrobromic, sulfuric, sulfamic, phosphoric, nitric and the like, and also salts prepared from organic acids such as acetic, propionic, succinic, glycolic, stearic, lactic, malic, tartaric, citric, ascorbic, pamoic, maleic, hydroxymaleic, phenylacetic, glutamic, benzoic, salicylic, sulfanilic, 2-acetoxy-benzoic, fumaric, toluenesulfonic, methanesulfonic, ethanedisulfonic, oxalic, isethionic, trifluoroacetic and the like.
If the compounds of the present invention are acidic, suitable "pharmaceutically acceptable salts" refer to salts prepared with pharmaceutically acceptable non-toxic bases including inorganic and organic bases. Salts derived from inorganic bases include aluminum, ammonium, calcium, copper, ferric, ferrous, lithium, magnesium, manganic, manganous, potassium, sodium, zinc, and the like. Ammonium, calcium, magnesium, potassium and sodium salts are particularly preferred. Salts derived from pharmaceutically acceptable organic non-toxic bases including salts of primary, secondary and tertiary amines, substituted amines including naturally occurring substituted amines, cyclic amines and basic ion exchange resins such as arginine, betaine, caffeine, choline, N' -dibenzylethylenediamine, diethylamine, 2-diethylaminoethanol, 2-dimethylaminoethanol, aminoethanol, ethanolamine, ethylenediamine, N-ethylmorpholine, N-ethylpiperidine, glucamine, glucosamine, histidine, hydroxycobalamin, isopropylamine, lysine, methylglucamine, morpholine, piperazine, piperidine, guava, polyamine resins, procaine, purines, theobromine, triethylamine, trimethylamine, tripropylamine, tromethamine and the like.
Berg et al, "pharmaceutical salts," j.pharm.sci.'1977:66:1-19 describe in more detail the preparation of pharmaceutically acceptable salts as described above and other typical pharmaceutically acceptable salts.
Since under physiological conditions the deprotonated acidic moiety, e.g. the completion group, in the compound may be anionic, and this charge may then be balanced out by the protonated or alkylated basic moiety, e.g. the tetravalent nitrogen atom, which is internally cationic, it should be noted that the compounds of the present invention are potentially internal salts or zwitterions.
In some embodiments, the invention provides a method of treating hyperproliferative diseases or conditions, such as human or other mammalian tumors, using compounds having structures of formulas (I) - (III) and pharmaceutically acceptable salts thereof.
In some of these embodiments, the compounds of the invention and pharmaceutically acceptable salts thereof are useful in the treatment or control of hyperproliferative diseases such as non-small cell lung cancer, breast cancer, colon cancer, prostate cancer, thyroid cancer, malignant melanoma, neuroblastoma, and breast-like secretory cancers.
Drug metabolites and prodrugs
Metabolites of the compounds and pharmaceutically acceptable salts thereof of the present invention, as well as prodrugs that can be converted in vivo to structures of the compounds and pharmaceutically acceptable salts thereof of the present invention are also encompassed by the claims of the present invention.
The invention is further illustrated by the following examples, which are not intended to limit the scope of the invention.
Example 1: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5 6 -methyl-7-oxo-6, 14-diazo-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -piperidin-5 (1, 3) -benzocyclotetradec-3-yn-5 5 Preparation of formamide (named WZQ-6C)
Step 1: preparation of 3-iodo-2-methyl-5-nitrobenzoic acid (Compound 2)
Compound 1 (4.0 g,20.49 mmol) was dissolved in 50mL of concentrated sulfuric acid using a 200mL round bottom flask, heated to 60℃and NIS (4.96 g,28.69 mmol) was added to the mixture in 3 portions over 30 minutes, after 2-3 hours the reaction solution was slowly added to ice water, an off-white precipitate was precipitated, filtered off with suction, the filter cake was rinsed 2-3 times with ice water and cyclohexane and column chromatography gave 4.0g of a white solid (yield: 60.79%).
1 H NMR(400MHz,DMSO-d 6 )δ8.71(d,J=2.5Hz,1H),8.45(d,J=2.5Hz,1H),2.69(s,3H).LC-MS(ESI)m/z 306.0[M-H] -
Step 2: preparation of N- (3-chloro-5- (trifluoromethyl) phenyl) -3-iodo-2-methyl-5-nitrobenzamide (Compound 4)
Compound 2 (6.0 g,19.69 mmol) was dissolved in 40mL of DMF solvent, HATU (7.4 g,19.69 mmol) and DIPEA (5.9 mL,35.8 mmol) were added and reacted for 0.5 h, compound 3 (3.5 g,17.90 mmol) was added and stirred overnight at ambient temperature, then extracted 2-3 times with ethyl acetate and water, the organic layer was quenched with anhydrous Na 2 SO 4 After drying, spin-drying and column chromatography gave 2.3g (yield: 26.52%) of a white solid.
1 H NMR(400MHz,DMSO-d 6 )δ11.08(s,1H),8.73(d,J=2.4Hz,1H),8.43(d,J=2.4Hz,1H),8.08(d,J=2.0Hz,1H),8.05(d,J=2.0Hz,1H),7.64(s,1H),2.55(s,3H).LC-MS(ESI)m/z482.3[M-H] - .
Step 3: preparation of N- (3-chloro-5- (trifluoromethyl) phenyl) -2-methyl-5-nitro-3- ((trimethylsilyl) ethynyl) benzamide (Compound 5)
Into a 100mL two-necked flask, compound 4 (3.2 g,6.60 mmol), cuprous iodide (125.7 mg,0.66 mmol), bis (triphenylphosphine) palladium dichloride (231.7 mg,0.33 mmol), anhydrous DMF solvent 30mL, N-diisopropylethylamine (3.3 mL,19.81 mmol), displacement argon, and closure of the reaction system were added, and then trimethylsilylacetylene (2.8 mL,19.81 mmol) was injected by syringe and stirred at 60℃for 6 hours. The reaction solution was filtered with celite and the solvent was dried to give a black mixture, which was directly used for the next reaction.
Step 4: preparation of N- (3-chloro-5- (trifluoromethyl) phenyl) -3-ethynyl-2-methyl-5-nitrobenzamide (Compound 6)
The crude product of the previous step is dissolved in methanol, and about 4mL of 1mol/L tetrabutylammonium fluoride-tetrahydrofuran solution is added and stirred at normal temperature for 2 hours. Spin-drying the reaction system, column chromatography gave 1.1g (total yield in two steps: 43.53%) of a white solid.
1 H NMR(400MHz,DMSO-d 6 )δ11.08(s,1H),8.43(d,J=2.5Hz,1H),8.38(d,J=2.5Hz,1H),8.09(d,J=2.0Hz,1H),8.07(d,J=2.0Hz,1H),7.64(s,1H),4.83(s,1H),2.58(s,3H).LC-MS(ESI)m/z 381.0[M-H] - .
Step 5: preparation of tert-butyl (S) - (1- (3-iodoimidazo [1,2-b ] pyridazin-6-yl) piperidin-3-yl) carbamate (Compound 9)
Compound 7 (1.0 g,3.58 mmol) was dissolved in 20mL of dimethyl sulfoxide (DMSO), compound 8 (3.58 g,17.89 mmol) was added, and potassium fluoride (2.49 g,42.94 mmol) was heated and stirred at 120℃for 3 hours. The reaction solution was extracted 3 times with ethyl acetate and water, and the organic layer was formed betweenAdding anhydrous Na 2 SO 4 After drying, spin-drying and column chromatography gave 1.1g (yield: 69.3%) of yellow solid.
1 H NMR(400MHz,DMSO-d 6 )δ7.79(d,J=9.9Hz,1H),7.58(s,1H),7.12(d,J=9.9Hz,1H),6.96(d,J=7.4Hz,1H),4.03–3.90(m,2H),3.43(s,1H),3.08(t,J=11.3Hz,1H),2.97(dd,J=12.9,9.1Hz,1H),1.88–1.78(m,2H),1.59–1.45(m,2H),1.39(s,9H).LC-MS(ESI)m/z 444.1[M+H] + .
Step 6: preparation of tert-butyl- (S) - (1- (3- ((3- ((3-chloro-5- (trifluoromethyl) phenyl) carbamoyl) -2-methyl-5-nitrophenyl) ethynyl) imidazo [1,2-b ] pyridazin-6-yl) piperidin-3-yl) carbamate (Compound 10)
Compound 6 (500 mg,1.31 mmol) and compound 9 (579.13 mg,1.31 mmol) were dissolved in 15mL anhydrous N, N-Dimethylformamide (DMF), and then cuprous iodide (24.8 mg,0.13 mmol), bis (triphenylphosphine) palladium dichloride (45.8 mg,0.065 mmol), DIPEA (0.65 mL,3.93 mmol) were added, and the reaction system was closed. Stir with heating at 80 ℃ and react overnight. After filtration through celite, the filtrate was dried by spin-drying, and column chromatography gave 550mg (yield: 60.31%) of a yellow solid.
1 H NMR(400MHz,DMSO-d 6 )δ11.14(s,1H),8.44(d,J=2.5Hz,1H),8.42(d,J=2.6Hz,1H),8.12(s,2H),7.97(s,1H),7.94(d,J=9.9Hz,1H),7.65(s,1H),7.30(d,J=10.0Hz,1H),6.97(d,J=7.7Hz,1H),4.15(d,J=11.4Hz,1H),4.00(d,J=13.0Hz,1H),3.45-3.38(m,1H),3.02(t,J=11.8Hz,1H),2.88–2.80(m,1H),2.73(s,3H),1.90–1.75(m,2H),1.59-1.42(m,2H),1.26(s,9H).LC-MS(ESI)m/z 697.9[M+H] + .
Step 7: preparation of tert-butyl- (S) - (1- (3- ((5-amino-3- ((3-chloro-5- (trifluoromethyl) phenyl) carbamoyl) -2-methylphenyl) ethynyl) imidazo [1,2-b ] pyridazin-6-yl) piperidin-3-yl) carbamate (Compound 11)
Compound 10 (1.0 g,1.43 mmol) was dissolved in 20mL of a 5:1 ethanol/water mixture and NH was added 4 Cl solid (383 mg,7.16 mmol) and iron powder (399 mg,7.16 mmol) were heated to 70℃and reacted for 4 hours, after filtration with celite, the filtrate was dried by spin-drying, and 600mg (yield: 62.69%) of an off-white solid was obtained by column chromatography.
1 H NMR(400MHz,DMSO-d 6 )δ10.85(s,1H),8.16(s,1H),8.14(s,1H),7.89(d,J=10.0Hz,1H),7.86(s,1H),7.56(s,1H),7.24(d,J=10.0Hz,1H),6.95(d,J=7.7Hz,1H),6.88(d,J=2.4Hz,1H),6.73(d,J=2.4Hz,1H),5.36(s,2H),4.13(d,J=12.7Hz,1H),3.98(d,J=12.9Hz,1H),3.44-3.38(m,1H),2.97(t,J=11.9Hz,1H),2.86–2.76(m,1H),2.44(s,3H),1.89–1.73(m,2H),1.54(q,J=11.8Hz,1H),1.49–1.39(m,1H),1.26(s,9H).LC-MS(ESI)m/z 668.3[M+H] + .
Step 8: preparation of tert-butyl- (S) - (1- (3- ((5- (7-bromoheptylamino) -3- ((3-chloro-5- (trifluoromethyl) phenyl) carbamoyl) -2-methylphenyl) ethynyl) imidazo [1,2-b ] pyridazin-6-yl) piperidin-3-yl) carbamate (Compound 12)
7-Bromoheptanoic acid (184.29 mg,0.881 mmol) was dissolved in DMF (10 mL), HATU (335 mg,0.881 mmol) and DIPEA (0.23 mL,1.36 mmol) were added and stirred for 0.5 hours, then compound 11 (457 mg,0.678 mmol) was added and reacted at room temperature for 4 to 5 hours, the reaction solution was extracted 2 to 3 times with ethyl acetate and water, and the organic layer was then dried over anhydrous Na 2 SO 4 After drying, spin-drying, column chromatography gave 219mg (yield: 37.59%) of an off-white solid.
1 H NMR(400MHz,DMSO-d 6 )δ10.98(s,1H),10.14(s,1H),8.13(s,2H),8.03(s,1H),7.94(s,1H),7.92(d,J=9.8Hz,1H),7.66(s,1H),7.60(s,1H),7.27(d,J=10.0Hz,1H),6.97(d,J=7.6Hz,1H),4.15(d,J=10.7Hz,1H),4.00(d,J=12.9Hz,1H),3.53(t,J=6.6Hz,2H),2.99(t,J=12.0Hz,1H),2.85–2.77(m,1H),2.55(s,3H),2.34(t,J=7.3Hz,2H),1.84-1.77(m,4H),1.64-1.55(m,3H),1.45-1.38(m,3H),1.37-1.29(m,3H),1.25(s,9H).LC-MS(ESI)m/z 858.3[M+H] + .
Step 9: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5 6 -methyl-7-oxo-6, 14-diazo-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -piperidin-5 (1, 3) -benzocyclotetradec-3-yn-5 5 Preparation of formamide (Compound WZQ-6C)
Compound 12 (200 mg,0.23 mmol) was dissolved in 10mL of dichloromethane, 4mL of trifluoroacetic acid was added and stirred at room temperature for 3-4 hours, and the solvent was dried with ethyl acetate and saturated NaHCO 3 After 3 times of extraction of the solution, the organic layer was added with anhydrous Na 2 SO 4 And drying and spin-drying to obtain a crude product of the yellow-white solid. The crude product was directly dissolved in 10mL of DMF and K was added 2 CO 3 (42 mg,0.3 mmol) and then heated to 80℃for 5 hours, and column chromatography gave 41mg of a white solid (total yield: 25.97%) after pumping off the solvent with an oil pump.
1 H NMR(600MHz,DMSO-d 6 )δ10.96(s,1H),10.17(s,1H),8.13(s,1H),8.11(s,1H),7.92(t,J=5.0Hz,3H),7.61(s,1H),7.53(d,J=2.2Hz,1H),7.33(d,J=10.1Hz,1H),4.62(d,J=8.5Hz,1H),4.03(d,J=12.4Hz,1H),2.88(t,J=11.2Hz,1H),2.79–2.55(m,4H),2.49(s,3H),2.37–2.30(m,2H),2.03–1.97(m,1H),1.81-1.79(m,1H),1.72-1.69(m,2H),1.61–1.52(m,1H),1.47-1.43(m,2H),1.38-1.33(m,5H).HRMS(ESI)for C 35 H 35 ClF 3 N 7 O 2 [M+H] + :calcd,678.2566,found.678.2552.
Example 2: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5-methyl-7-oxo-6, 13-diazonium-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -piperidin-5 (1, 3) -benzocyclotridec-3-yn-5 5 Preparation of formamide (named WZQ-5C)
The synthesis method is described in example 1.
1 H NMR(400MHz,DMSO-d 6 )δ10.95(s,1H),10.11(s,1H),8.12(d,J=2.3Hz,1H),8.11(s,1H),8.00(d,J=2.2Hz,1H),7.90(d,J=10.1Hz,2H),7.61(s,1H),7.39(d,J=2.2Hz,1H),7.33(d,J=10.1Hz,1H),4.58(d,J=12.6Hz,1H),4.06(d,J=12.6Hz,1H),2.82(t,J=12.0Hz,1H),2.76–2.66(m,1H),2.61(q,J=8.2,5.9Hz,2H),2.50(s,3H),2.32(d,J=7.0Hz,2H),2.10(d,J=12.2Hz,1H),1.86–1.67(m,2H),1.65–1.52(m,2H),1.42(dd,J=23.2,6.4Hz,4H),1.24(s,2H),1.20-1.05(m,1H).HRMS(ESI)for C 34 H 33 ClF 3 N 7 O 2 [M+H] + :calcd,664.2409,found.664.2398.
Example 3: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5 6 -methyl-7-oxo-6, 15-diazo-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -piperidin-5 (1, 3) -benzocyclopentane-3-yn-5 5 Preparation of formamide (named WZQ-7C)
The synthesis method is described in example 1.
1 H NMR(400MHz,DMSO-d 6 )δ10.95(s,1H),10.12(s,1H),8.12(dd,J=6.0,4.1Hz,2H),7.92(d,J=2.3Hz,1H),7.91–7.87(m,2H),7.71(d,J=2.2Hz,1H),7.61(s,1H),7.29(d,J=10.1Hz,1H),4.62(d,J=10.6Hz,1H),4.02(d,J=13.1Hz,1H),3.01–2.90(m,1H),2.69–2.54(m,4H),2.48(s,3H),2.38-2.26(m,2H),1.98(d,J=12.0Hz,1H),1.76(d,J=13.2Hz,1H),1.69(s,2H),1.49(q,J=12.7Hz,2H),1.37–1.27(m,8H).HRMS(ESI)for C 36 H 37 ClF 3 N 7 O 2 [M+H] + :calcd,692.2722,found,692.2710.
Example 4: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5 6 -methyl-11-oxo-6-oxo-12-aza-2 (6, 3) -imidazo [1,2-b]Pyridazine-1%1, 3) -piperidin-5 (1, 3) -benzocyclododecane-3-yn-5 5 Preparation of formamide (named WZQ 2-144)
Step 1: preparation of 3-iodo-2-methyl-5-nitrobenzoic acid methyl ester (Compound 2)
Compound 1 (1.0 g,3.26 mmol) was first dissolved in DMF and K was added 2 CO 3 (900 mg,6.51 mmol) and methyl iodide (0.24 mL,3.91 mmol) were reacted at room temperature for 4 hours, then ethyl acetate and water were used for extraction 3 times, and then the organic layer was dried by spin-drying, and column chromatography was performed to obtain 860mg of an off-white solid (yield: 82.24%).
1 H NMR(400 MHz,DMSO-d 6 )δ8.74(d,J=2.4 Hz,1H),8.47(d,J=2.4 Hz,1H),3.90(s,3H),2.66(s,3H).LC-MS(ESI)m/z 319.9[M-H] - .
Step 2: preparation of 3-iodo-2-methyl-5-nitrobenzoic acid methyl ester (Compound 3)
Compound 2 (750 mg,2.34 mmol) was dissolved in 15mL of ethanol, water 4:1, iron powder (652.2 mg,11.68 mmol) and NH were added to the mixed solvent 4 Cl (624.7 mg,11.68 mmol) was reacted at 70℃for 2 hours and then filtered with celite, followed by column chromatography to give 620mg (yield: 91.18%) of an off-white solid.
1 H NMR(400 MHz,DMSO-d 6 )δ7.29(d,J=2.4 Hz,1H),6.94(d,J=2.4 Hz,1H),5.34(s,2H),3.79(s,3H),2.35(s,3H).LC-MS(ESI)m/z 292.0[M+H] + .
Step 3: preparation of 5-hydroxy-3-iodo-2-methylbenzoic acid methyl ester (Compound 4)
At 50mL of H 2 SO 4 (2.0%) Compound 3 (4.2 g,14.43 mmol) was added to the solution, and after heating to 80℃to dissolve the whole, the reaction solution was moved to 0℃to remove NaNO 2 (1.49 g,21.64 mmol) was dissolved in 10mL of water and then slowly added dropwise to the reaction mixture, followed by reaction in an ice bath for 2 hours. The reaction solution was then added dropwise to boiling 20mL of H 2 SO 4 (2.0%) in solution, reacted at 80℃for 1 hour, extracted with ethyl acetate 2-3 times, and the organic layer was dried by spin-drying, and column chromatography gave 2.7g (yield: 64.07%) of a yellow solid.
1 H NMR(400 MHz,DMSO-d 6 )δ9.92(s,1H),7.46(d,J=2.6 Hz,1H),7.12(d,J=2.7 Hz,1H),3.81(s,3H),2.42(s,3H).LC-MS(ESI)m/z 293.0[M+H] + .
Step 4: preparation of 5- ((5- (tert-butoxy) -5-oxopentyl) oxy) -3-iodo-2-methylbenzoic acid methyl ester (Compound 5)
Compound 4 (1.02 g,3.49 mmol) was dissolved in 15mL of DMF and K was added 2 CO 3 (965 mg,6.98 mmol) and t-butyl 5-bromopentanoate (993.7 mg,4.19 mmol) were then stirred at room temperature overnight, and after DMF was pumped down with an oil pump, column chromatography gave 1.06g (yield: 67.71%) of a yellow oil.
1 H NMR(400MHz,CDCl 3 )δ7.55(d,J=2.8Hz,1H),7.30(d,J=2.8Hz,1H),3.96(t,J=5.8Hz,2H),3.91(s,3H),2.59(s,3H),2.30(t,J=6.8Hz,2H),1.82-1.75(m,4H),1.47(s,9H).LC-MS(ESI)m/z 449.1[M+H] + .
Step 5: preparation of methyl 5- ((5- (tert-butoxy) -5-oxo-oxy) -2-methyl-3- ((trimethylsilyl) ethynyl) benzoate (Compound 6)
Into a 50mL two-necked flask, compound 5 (1.06 g,2.36 mmol), cuprous iodide (45.03 mg,0.236 mmol), bis (triphenylphosphine) palladium dichloride (118.2 mg,0.118 mmol), anhydrous acetonitrile solvent 20mL, N-diisopropylethylamine (1.17 mL,7.09 mmol), replaced with argon, and the reaction was closed, and then trimethylsilylacetylene (1.0 mL,7.09 mmol) was injected by syringe and stirred at 60℃for 4 hours. The reaction solution was filtered with celite and the solvent was dried to give a black mixture, which was directly used for the next reaction.
Step 6: preparation of methyl 5- ((5- (tert-butoxy) -5-oxopentyloxy) -3-ethynyl-2-methylbenzoate (Compound 7)
The crude product of the previous step is dissolved in methanol, and about 3mL of 1mol/L tetrabutylammonium fluoride-tetrahydrofuran solution is added and stirred at normal temperature for 2 hours. Spin-drying the reaction system, column chromatography gave 687.6mg (total yield over two steps: 84%) of a yellow oil.
1 H NMR(400MHz,CDCl 3 )δ7.38(d,J=2.8Hz,1H),7.16(d,J=2.8Hz,1H),3.97(t,J=5.8Hz,2H),3.89(s,3H),3.31(s,1H),2.62(s,3H),2.30(t,J=6.9Hz,2H),1.82-1.74(m,4H),1.46(s,9H).LC-MS(ESI)m/z 347.3[M+H] + .
Step 7: preparation of methyl (S) -5- ((5- (tert-butoxy) -5-oxopentyl) oxy) -3- ((6- (3- ((tert-butoxycarbonyl) amino) piperidin-1-yl) imidazo [1,2-b ] pyridazin-3-yl) ethynyl) -2-methylbenzoate (Compound 9)
Compound 7 (430 mg,1.24 mmol) and compound 8 (660.28 mg,1.49 mmol) were dissolved in 15mL anhydrous N, N-Dimethylformamide (DMF), and then cuprous iodide (23.64 mg,0.12 mmol), bis (triphenylphosphine) palladium dichloride (43.56 mg,0.062 mmol), DIPEA (0.61 mL,3.72 mmol) were added, argon was replaced, and the reaction system was closed. Stir with heating at 80 ℃ and react overnight. After filtration through celite, the filtrate was dried by spin-drying, and column chromatography gave 300mg (yield: 36.52%) of a yellow oil.
1 H NMR(400MHz,DMSO-d 6 )δ7.90(d,J=9.9Hz,1H),7.86(s,1H),7.30(d,J=2.8Hz,1H),7.27(d,J=2.8Hz,1H),7.24(d,J=10.0Hz,1H),6.96(d,J=7.4Hz,1H),4.10(d,J=8.9Hz,1H),4.03(t,J=6.2Hz,2H),3.97(d,J=13.2Hz,1H),3.85(s,3H),3.44-3.41(m,1H),3.07(t,J=11.3Hz,1H),2.91(dd,J=12.9,9.4Hz,1H),2.66(s,3H),2.27(t,J=7.1Hz,2H),1.89–1.75(m,2H),1.75–1.61(m,4H),1.59–1.44(m,2H),1.40(s,9H),1.34(s,9H).LC-MS(ESI)m/z 661.8[M+H] + .
Step 8: methyl (1) 3 S)-5 6 -methyl-11-oxo-6-oxo-12-aza-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -piperidin-5 (1, 3) -benzocyclododecane-3-yn-5 5 Preparation of carboxylic acid esters (Compound 10)
Compound 9 (100 mg,0.15 mmol) was dissolved in 10mL of 1, 4-dioxane solvent, 2mL of 1, 4-dioxane solution of hydrochloric acid was added, stirring was performed at room temperature for 2 hours, a tan solid was obtained after spinning-dry the solvent, the obtained crude product was dissolved in 15mL of dichloromethane solvent, 2mL of DMF was added, DIPEA (0.12 mL,0.75 mmol) and FDPP (69.67 mg,0.18 mmol) were added, stirring was performed at room temperature overnight, extraction was performed 3 times with DCM and water, the organic layer was spun-dry, and column chromatography gave 54mg (yield: 73.30%) of a yellow-white solid.
1 H NMR(400MHz,CDCl 3 )δ7.77(s,1H),7.68(d,J=9.9Hz,1H),7.35(d,J=2.8Hz,1H),7.24(d,J=2.6Hz,1H),6.84(d,J=9.9Hz,1H),5.37(d,J=7.9Hz,1H),4.77(dd,J=12.7,4.4Hz,1H),4.09(s,1H),3.97(t,J=7.6Hz,2H),3.86(s,3H),2.87-2.80(m,1H),2.65(s,3H),2.55(dd,J=12.5,10.7Hz,1H),2.45-2.39(m,1H),2.13-2.06(m,2H),2.04-1.95(m,1H),1.93-1.75(m,4H),1.75–1.61(m,2H).LC-MS(ESI)m/z 488.3[M+H] + .
Step 9: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5 6 -methyl-11-oxo-6-oxo-12-aza-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -piperidin-5 (1, 3) -benzocyclododecane-3-yn-5 5 Preparation of formamide (Compounds WZQ 2-144)
Compound 10 (20 mg,0.041 mmol) and compound 11 (9.6 mg,0.049 mmol) were dissolved in 8mL of anhydrous THF under argon, and after stirring under ice bath for 5 minutes, lithium bis (trimethylsilyl) amide (25. Mu.L, 0.12 mmol) was added to the mixed system by syringe, reacted for 3 hours, and then reacted with NH 4 Cl solution and ethyl acetate extraction 1-2 times, column chromatography gave 13mg (yield: 48.67%) of a white solid.
1 H NMR(400MHz,DMSO-d 6 )δ10.88(s,1H),8.12(s,2H),7.93(d,J=3.5Hz,1H),7.90(d,J=2.2Hz,2H),7.60(s,1H),7.33(d,J=10.1Hz,1H),7.14(d,J=2.7Hz,1H),7.10(d,J=2.7Hz,1H),4.74–4.64(m,1H),4.14–4.04(m,3H),3.76(dd,J=11.8,7.1Hz,1H),2.88–2.81(m,1H),2.46(s,3H),2.20(d,J=5.8Hz,2H),2.00(q,J=7.4Hz,1H),1.90(d,J=11.8Hz,1H),1.83-1.77(m,4H),1.66–1.54(m,2H),1.49–1.40(m,1H).HRMS(ESI)for C 33 H 30 ClF 3 N 6 O 3 [M+H] + :calcd,651.2093,found.651.2064.
Example 5: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5 6 -methyl-7, 12-dioxo-6, 13-diaza-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -piperidin-5 (1, 3) -benzocyclotridec-3-yn-5 5 Preparation of formamide (named WZQ 2-148)
The synthesis method is described in example 1.
1 H NMR(400MHz,MeOD)δ8.09(d,J=2.1Hz,1H),8.03(s,1H),7.86(d,J=2.3Hz,1H),7.80–7.75(m,2H),7.57(d,J=2.2Hz,1H),7.48(d,J=1.9Hz,1H),7.28(d,J=10.0Hz,1H),4.75(dd,J=12.7,4.6Hz,1H),4.08(d,J=12.7Hz,1H),3.97-3.89(m,2H),2.93–2.86(m,1H),2.61(s,3H),2.52–2.45(m,2H),2.43-2.36(m,1H),2.28–2.20(m,1H),2.16(d,J=12.6Hz,1H),1.92(t,J=12.4Hz,2H),1.86–1.70(m,5H),1.51-1.41(m,2H).HRMS(ESI)for C 34 H 31 ClF 3 N 7 O 3 [M+H] + :calcd,678.2202,found.678.2182.
Example 6: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5 6 -methyl-11-oxo-6-oxo-12-aza-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -pyrrolidin-5 (1, 3) -benzocyclododecane-3-yne-5 5 Preparation of formamide (named WZQ 2-152)
The synthesis method is described in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.88(s,1H),8.33(d,J=6.8Hz,1H),8.12(s,2H),7.92(d,J=9.8Hz,1H),7.82(s,1H),7.59(s,1H),7.11(d,J=2.8Hz,1H),7.09(d,J=2.7Hz,1H),6.97(d,J=9.8Hz,1H),4.37–4.29(m,1H),4.15(dd,J=11.0,7.7Hz,1H),4.05-3.92(m,2H),3.72(t,J=9.1Hz,1H),3.52-3.45(m,1H),3.05(dd,J=11.1,8.7Hz,1H),2.46(s,3H),2.31–2.22(m,2H),2.20–2.13(m,1H),2.13–2.04(m,1H),1.91-1.78(m,3H),1.65(s,1H).HRMS(ESI)for C 32 H 28 ClF 3 N 6 O 3 [M+H] + :calcd,637.1936,found.637.1943.
Example 7: preparation of N- (3-chloro-5- (trifluoromethyl) phenyl) -46,14-dimethyl-10-oxo-5-oxo-11, 14-diazo-1 (3, 6) -imidazo [1,2-b ] pyridazin-4 (1, 3) -benzocyclotetradec-2-yne-45-carboxamide (designated WZQ 2-154)
The synthesis method is described in example 4.
1 H NMR(400MHz,CDCl 3 )δ9.15(s,1H),8.13(s,1H),7.93(s,1H),7.66(s,1H),7.57(d,J=9.8Hz,1H),7.40(d,J=1.9Hz,1H),7.09(d,J=2.7Hz,1H),6.86(d,J=2.7Hz,1H),6.64(d,J=9.9Hz,1H),6.15(t,J=5.6Hz,1H),3.98(t,J=6.1Hz,2H),3.73–3.66(m,2H),3.52-3.46(m,2H),3.08(s,3H),2.52(s,3H),2.20(t,J=7.0Hz,2H),1.81-1.78(m,4H).HRMS(ESI)for C 31 H 28 ClF 3 N 6 O 3 [M+H] + :calcd,625.1936,found.625.1917.
Example 8:4 6 14-dimethyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) -10-oxo-5-oxo-11, 14-diazo-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -phencymene-2-yne-4 5 Preparation of formamide (named WZQ 3-1)
The synthesis method is described in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.86(s,1H),8.19(d,J=1.5Hz,1H),8.18(s,1H),8.11(s,1H),7.97(s,1H),7.93(d,J=10.0Hz,1H),7.89(s,1H),7.74(s,1H),7.47(t,J=1.3Hz,1H),7.29(d,J=2.7Hz,1H),7.13(d,J=10.0Hz,1H),7.10(d,J=2.7Hz,1H),4.14(t,J=7.4Hz,2H),3.76–3.69(m,2H),3.43-3.37(m,2H),3.14(s,3H),2.49(s,3H),2.22-2.20(m,2H),2.19–2.18(m,3H),1.83–1.70(m,4H).HRMS(ESI)for C 35 H 33 F 3 N 8 O 3 [M+H] + :calcd,671.2700,found.671.2692.
Example 9: (1 3 S) -N- (3-chloro-5- (trifluoromethyl) phenyl) -5 6 -methyl-12-oxo-6-oxo-13-aza-2 (6, 3) -imidazo [1,2-b]Pyridazin-1 (1, 3) -piperidin-5 (1, 3) -benzocyclotridec-3-yn-5 5 Preparation of formamide (named WZQ 3-3)
The synthesis method is described in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.89(s,1H),8.12(d,J=2.3Hz,2H),7.91(dd,J=9.0,6.1Hz,3H),7.60(d,J=1.8Hz,1H),7.34(d,J=10.1Hz,1H),7.18(d,J=2.7Hz,1H),7.09(d,J=2.6Hz,1H),4.59(dd,J=12.6,4.5Hz,1H),4.17(t,J=5.7Hz,2H),4.10(d,J=12.9Hz,1H),3.81–3.70(m,1H),2.88–2.79(m,1H),2.46(s,3H),2.29-2.21(m,1H),2.07-2.0(m,1H),1.92(d,J=12.0Hz,1H),1.83(d,J=13.3Hz,1H),1.77-1.69(m,3H),1.63–1.51(m,2H),1.51–1.32(m,4H).HRMS(ESI)for C 34 H 32 ClF 3 N 6 O 3 [M+H] + :calcd,665.2249,found.665.2253.
Example 10:4 6 14-dimethyl-N- (3- ((4-methylpiperazin-1-yl) methyl) -5- (trifluoromethyl) phenyl) -10-oxo-5-oxo-11, 14-diazo-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradec-2-yn-4 5 Preparation of formamide (named WZQ 3-9)
The synthesis method is described in example 4.
1 H NMR(400MHz,MeOD)δ8.08(s,1H),7.89(s,1H),7.77–7.70(m,2H),7.46(s,1H),7.25(d,J=2.7Hz,1H),7.05(d,J=8.2Hz,1H),7.03(s,1H),4.13(t,J=7.0Hz,2H),3.88–3.80(m,2H),3.63(s,2H),3.60–3.52(m,2H),3.18(s,3H),2.56(d,J=16.1Hz,11H),2.34(s,5H),1.91-1.85(m,4H).HRMS(ESI)for C 37 H 41 F 3 N 8 O 3 [M+H] + :calcd,703.3326,found.703.3330.
Example 11:4 6 13-dimethyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) -9-oxo-5-oxo-10, 13-diazo-1 (3, 6) -imidazo [1,2-b ]Pyridazin-4 (1, 3) -phencyclitridec-2-yne-4 5 Preparation of formamide (named WZQ 3-19)
The synthesis method is described in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.86(s,1H),8.19(s,1H),8.18(s,1H),8.11(s,1H),8.07(t,J=5.3Hz,1H),7.93(d,J=9.9Hz,1H),7.87(s,1H),7.74(s,1H),7.47(s,1H),7.45(d,J=2.7Hz,1H),7.12(d,J=10.0Hz,1H),7.09(d,J=2.7Hz,1H),4.19(t,J=7.5Hz,2H),3.80–3.72(m,2H),3.42–3.37(m,2H),3.14(s,3H),2.49(s,3H),2.23-2.20(m,2H),2.18(s,3H),1.95(q,J=9.2,8.4Hz,2H).HRMS(ESI)for C 34 H 31 F 3 N 8 O 3 [M+H] + :calcd,657.2544,found.657.2528.
Example 12:4 6 13-dimethyl-N- (3- ((4-methylpiperazin-1-yl) methyl) -5- (trifluoromethyl) phenyl) -9-oxo-5-oxo-10, 13-diazo-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotridec-2-yn-4 5 Preparation of formamide (named WZQ 3-22)
The synthesis method is described in example 4.
1 HNMR(400MHz,MeOD)δ8.02(s,1H),7.97(s,1H),7.75(d,J=10.0Hz,1H),7.71(s,1H),7.47(s,1H),7.35(d,J=2.8Hz,1H),7.08(d,J=10.0Hz,1H),7.04(d,J=2.7Hz,1H),4.19(t,J=7.1Hz,2H),3.91–3.83(m,2H),3.69(s,2H),3.60–3.52(m,2H),3.19(s,3H),2.97(s,4H),2.69(s,4H),2.64(s,3H),2.54(s,3H),2.38–2.33(m,2H),2.12-2.07(m,2H).HRMS(ESI)for C 36 H 39 F 3 N 8 O 3 [M+H] + :calcd,689.3170,found.689.3143.
Example 13:4 6 11-dimethyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) -10-oxo-5-oxo-11, 14-diazo-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -phencymene-2-yne-4 5 Preparation of formamide (named WZQ 3-48)
The synthesis method is described in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.86(s,1H),8.21–8.16(m,2H),8.11(s,1H),7.79(d,J=10.3Hz,2H),7.75(s,1H),7.47(s,1H),7.23(d,J=2.7Hz,1H),7.13(s,1H),7.09(d,J=2.6Hz,1H),6.76(d,J=9.8Hz,1H),4.01(t,J=7.6Hz,2H),3.62-3.57(m,1H),3.52-3.48(m,3H),3.08(s,3H),2.48(s,3H),2.44(t,J=5.1Hz,2H),2.19(s,3H),1.86-1.73(m,4H).HRMS(ESI)for C 35 H 33 F 3 N 8 O 3 [M+H] + :calcd,671.2700,found.671.2698.
Example 14:4 6 -methyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5-trifluoromethylphenyl) -10-oxo-5-oxa-11, 14-diaza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradecane-2-yne-4 5 Preparation of formamide (named WZQ 3-99)
The synthesis method is described in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.87(s,1H),8.20(s,1H),8.18(s,1H),8.10(s,1H),8.08(t,J=5.2Hz,1H),7.79(m,2H),7.74(s,1H),7.47(s,1H),7.26(t,J=5.3Hz,1H),7.19(d,J=2.7Hz,1H),7.09(d,J=2.6Hz,1H),6.74(d,J=9.7Hz,1H),4.06(t,J=7.5Hz,2H),3.47–3.37(m,4H),2.48(s,3H),2.20(m,2H),2.18(s,3H),1.86–1.71(m,4H).HRMS(ESI)for C 34 H 31 F 3 N 8 O 3 [M+H] + :calcd,657.2544,found.657.2561.
Example 15:4 6 -methyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5-trifluoromethylphenyl) -11-oxo-5-oxa-10, 14-diaza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradecane-2-yne-4 5 Preparation of formamide (named WZQ 3-115)
The synthesis method is described in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.87(s,1H),8.20(s,1H),8.18(s,1H),8.11(s,1H),8.05(t,J=5.7Hz,1H),7.81(s,1H),7.78(d,J=9.7Hz,1H),7.74(s,1H),7.47(s,1H),7.34(t,J=5.7Hz,1H),7.18(d,J=2.7Hz,1H),7.09(d,J=2.6Hz,1H),6.73(d,J=9.7Hz,1H),4.19–4.09(m,2H),3.59-3.53(m,2H),3.18-3.14(m,2H),2.63–2.57(m,2H),2.49(s,3H),2.18(s,3H),1.85-1.77(m,2H),1.65-1.56(m,2H).HRMS(ESI)for C 34 H 31 F 3 N 8 O 3 [M+H] + :calcd,657.2544,found,657.2552.
Example 16:4 6 10-dimethyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) -11-oxo-5-oxa-10, 14-diaza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradode-2-ynyl-4 5 Preparation of formamide (named WZQ 3-151)
The synthesis method is described in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.86(s,1H),8.20(s,1H),8.17(s,1H),8.10(s,1H),7.80(s,1H),7.77(d,J=9.5Hz,1H),7.74(s,1H),7.47(s,1H),7.35(t,J=5.8Hz,1H),7.08(s,1H),7.05(s,1H),6.74(d,J=9.7Hz,1H),4.12(t,J=7.5Hz,2H),3.54(q,J=7.3Hz,2H),3.44–3.39(m,2H),2.93(s,3H),2.82-2.75(m,2H),2.47(s,3H),2.18(s,3H),1.78-1.64(m,4H).HRMS(ESI)for C 35 H 33 F 3 N 8 O 3 [M+H] + :calcd,671.2700,found,671.2738.
Example 17:4 6 -methyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) -13-oxo-5-oxo-12-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotridec-2-ynes-4 5 Preparation of formamide (named WZQ 3-87)
Step 1: preparation of methyl 5- ((6- ((t-butoxycarbonyl) amino) hexyl) oxy) -3-iodo-2-methylbenzoate (Compound 2)
Compound 1 (3.0 g,10.27 mmol), tert-butyl (6-hydroxyhexyl) carbamate (2.68 g,12.33 mmol) and PPh 3 (4.04 g,15.40 mmol) was added to a 100mL two-necked flask, and after evacuation under argon, 30mL dry THF was added, and DIAD (2.7 mL,15.4 mmol) was added over 20 minutes after the flask was placed under ice-bath, and after 0.5 hours was allowed to react at room temperature for 6 hours, the solvent was dried, and column chromatography gave 4.0g (yield: 79.25%) of a yellow oil.
1 H NMR(400MHz,CDCl 3 )δ7.54(d,J=2.8Hz,1H),7.29(d,J=2.6Hz,1H),4.61(s,1H),3.92(t,J=8.3Hz,2H),3.90(s,3H),3.13(q,J=6.8Hz,2H),2.58(s,3H),1.79-1.72(m,2H),1.56–1.48(m,4H),1.46(s,9H),1.41-1.35(m,2H).LC-MS(ESI)m/z 490.1[M-H] - .
Step 2: preparation of methyl 5- ((6- ((t-Butoxycarbonyl) amino) hexyl) oxy) -2-methyl-3- ((trimethylsilyl) ethynyl) benzoate (Compound 3)
The synthetic procedure of step 5 of example 4 was referenced to give compound 3, crude product was used directly in the next step.
Step 3: preparation of methyl 5- ((6- ((t-butoxycarbonyl) amino) hexyl) oxy) -3-ethynyl-2-methylbenzoate (Compound 4)
Compound 4 was obtained by the synthesis method according to step 6 of example 4.
1 H NMR(400MHz,CDCl 3 )δ7.32(d,J=2.9Hz,1H),7.10(d,J=2.9Hz,1H),4.71(s,1H),3.88(t,J=6.4Hz,2H),3.84(s,3H),3.29(s,1H),3.07(q,J=6.7Hz,2H),2.57(s,3H),1.74-1.68(m,2H),1.49-1.43(m,4H),1.40(s,9H),1.36–1.30(m,2H)。LC-MS(ESI)m/z 388.3[M-H] - .
Step 4: preparation of tert-butyl (6- (3-ethynyl-4-methyl-5- ((3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) carbamoyl) phenoxy) hexyl) carbamate (Compound 5)
Compound 5 was obtained by the synthesis method according to step 9 in example 4.
1 H NMR(400MHz,CDCl 3 )δ8.73(s,1H),8.24(s,1H),7.78(s,2H),7.35(s,1H),7.09(t,J=3.3Hz,2H),7.02(d,J=2.7Hz,1H),4.54(s,1H),3.96(t,J=6.3Hz,2H),3.32(s,1H),3.08(q,J=6.7Hz,2H),2.51(s,3H),2.28(s,3H),1.78-1.71(m,2H),1.51-1.42(m,4H),1.39(s,11H).LC-MS(ESI)m/z 597.3[M-H] - .
Step 5: preparation of methyl 3-iodoimidazo [1,2-b ] pyridazine-6-carboxylate (Compound 7)
Compound 6 (970 mg,5.47 mmol) was dissolved in 20mL of DMF, NIS (2.46 g,10.95 mmol) was added, after heating to 90℃and reaction for 2 hours, extracted 2-3 times with ethyl acetate and water, the organic layer was dried by spin-drying, and column chromatography gave 500mg (yield: 30.13%) of yellow solid.
1 H NMR(400MHz,DMSO-d 6 )δ8.27(d,J=9.5Hz,1H),8.10(s,1H),7.76(d,J=9.5Hz,1H),3.99(s,3H).LC-MS(ESI)m/z 304.0[M+H] + .
Step 6: preparation of methyl 3- ((5- ((tert-butoxycarbonyl) amino) hexyl) oxy) -2-methyl-3- ((3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) carbamoyl) phenyl) ethynyl) imidazo [1,2-b ] pyridazine-6-carboxylate (Compound 8)
Compound 8 was obtained by the synthesis method according to step 7 in example 4.
1 H NMR(400MHz,DMSO-d 6 )δ10.90(s,1H),8.40(d,J=9.7Hz,2H),8.22(s,1H),8.19(s,1H),8.11(s,1H),7.86(d,J=9.5Hz,1H),7.76(s,1H),7.50(s,1H),7.29(d,J=2.6Hz,1H),7.21(d,J=2.7Hz,1H),6.78(t,J=5.0Hz,1H),4.07(t,J=6.6Hz,2H),3.96(s,3H),2.91(q,J=6.6Hz,2H),2.60(s,3H),2.19(s,3H),1.76-1.70(m,2H),1.45-1.29(m,6H),1.36(s,9H).LC-MS(ESI)m/z774.7[M+H] + .
Step 7: preparation of 3- ((5- ((6- ((tert-butoxycarbonyl) amino) hexyl) oxy) -2-methyl-3- ((3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) carbamoyl) phenyl) ethynyl) imidazo [1,2-b ] pyridazine-6-carboxylic acid (Compound 9)
Compound 8 (150 mg,0.19 mmol) was dissolved in 12mL of THF/H 2 To a mixed solvent of o=5:1, liOH (23.2 mg,0.97 mmol) was added, and after stirring overnight at room temperature, THF was dried by spin-drying, pH was adjusted to acidity with 2N HCl solution, yellow solid precipitated, and suction filtration gave crude 108mg (yield about 73.33%) which was used directly in the next step.
Step 8:4 6 -methyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) -13-oxo-5-oxo-12-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotridec-2-yn-4 5 Preparation of formamide (Compounds WZQ 3-87)
Compound 9 (108 mg,0.14 mmol) was dissolved in 10mL of DCM, 2mL of TFA was added to react at room temperature for 2 hours, the solvent was dried to give crude product, after dissolution in 30mL of DMF/DCM mixture, DIPEA (0.12 mL,0.71 mmol) and FDPP (81.99 mg,0.21 mmol) were added to react overnight, after which the mixture was reacted with DCM and H 2 O extraction was performed 2 times, and column chromatography gave 28mg (total yield in two steps: 30.7%) of a yellow solid.
1 H NMR(400MHz,DMSO-d 6 )δ10.90(s,1H),8.91(t,J=5.6Hz,1H),8.36(t,J=4.7Hz,2H),8.20(s,1H),8.17(d,J=2.2Hz,1H),8.12(s,1H),7.77(d,J=9.4Hz,1H),7.75(s,1H),7.53(d,J=2.8Hz,1H),7.47(s,1H),7.14(d,J=2.7Hz,1H),4.29(t,J=8.1Hz,2H),3.45-3.41(m,2H),2.54(s,3H),2.19(s,3H),1.79-1.72(m,2H),1.66-1.58(m,4H),1.48(q,J=7.2Hz,2H).HRMS(ESI)for C 34 H 30 F 3 N 7 O 3 [M+H] + :calcd,642.2435,found,642.2462.
Example 18:4 6 -methyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5- (trifluoromethyl) phenyl) -12-oxo-5-oxo-11-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclododecane-2-yn-4 5 Preparation of formamide (named WZQ 3-95)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.88(s,1H),8.66(t,J=5.6Hz,1H),8.38(d,J=9.4Hz,1H),8.32(s,1H),8.20(s,2H),8.11(s,1H),7.77(d,J=9.4Hz,2H),7.48(s,1H),7.37(d,J=2.7Hz,1H),7.17(d,J=2.6Hz,1H),4.21(t,J=7.2Hz,2H),3.39(q,J=5.7Hz,2H),2.52(s,3H),2.19(s,3H),1.84–1.77(m,2H),1.71-1.66(m,2H),1.61–1.53(m,2H).HRMS(ESI)for C 33 H 28 F 3 N 7 O 3 [M+H] + :calcd,628.2278,found.628.2292.
Example 19:4 6 -methyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5-trifluoromethylphenyl) -14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradec-2-ynyl-4 5 Preparation of formamide (named WZQ 3-109)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.89(s,1H),8.88(t,J=5.6Hz,1H),8.37-8.35(m,2H),8.20(s,1H),8.19(s,1H),8.12(s,1H),7.80(d,J=9.4Hz,1H),7.75(s,1H),7.57(d,J=2.7Hz,1H),7.48(s,1H),7.16(d,J=2.6Hz,1H),4.24(t,J=7.5Hz,2H),3.40(q,J=6.0Hz,2H),2.54(s,3H),2.19(s,3H),1.80-1.73(m,2H),1.66-1.60(m,2H),1.52-1.48(m,2H),1.48-1.43(m,4H).HRMS(ESI)for C 35 H 32 F 3 N 7 O 3 [M+H] + :calcd,656.2591,found,656.2602.
Example 20:4 6 -methyl-N- (3- (4-methyl-1H-imidazol-1-yl) -5-trifluoromethylphenyl) -15-oxo-5-oxa-14-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclopentadode-2-ynyl-4 5 Preparation of formamide (named WZQ 3-143)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.91(s,1H),8.86(s,1H),8.39(s,1H),8.35(s,2H),8.22(s,1H),8.12(s,1H),7.81-7.78(m,2H),7.61(s,1H),7.56(s,1H),7.20(s,1H),4.16(t,J=6.8Hz,2H),3.40(q,J=6.6Hz,2H),2.54(s,3H),2.21(s,3H),1.83-1.77(m,2H),1.66-1.60(m,2H),1.49-1.42(m,8H).HRMS(ESI)for C36H34F3N7O3[M+H] + :calcd,670.2748,found,670.2780.
Example 21:4 6 methyl-N-)(3- (4-methyl-1H-imidazol-1-yl) -5-trifluoromethylphenyl) -11, 14-dioxo-5-oxa-10, 13-diaza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradode-2-yne-4 5 Preparation of formamide (named WZQ 3-152)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.90(s,1H),8.97(d,J=5.5Hz,1H),8.40(d,J=9.4Hz,1H),8.38(s,1H),8.26(t,J=6.0Hz,1H),8.20(s,2H),8.12(s,1H),7.86(d,J=9.4Hz,1H),7.75(s,1H),7.59(s,1H),7.48(s,1H),7.16(s,1H),4.23(t,J=7.6Hz,2H),4.02(d,J=5.2Hz,2H),3.23-3.19(m,2H),2.54(s,3H),2.18(s,3H),1.87-1.80(m,2H),1.67-1.62(m,2H).HRMS(ESI)for C 34 H 29 F 3 N 8 O 4 [M+H] + :calcd,671.2337,found,671.2353.
Example 22:4 6 -methyl-N- (4-methylpiperazin-1-yl) methyl) -3- (trifluoromethyl) phenyl) -14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradode-2-yne-4 5 Preparation of formamide (named WZQ 4-5)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.71(s,1H),8.87(t,J=5.5Hz,1H),8.36(d,J=8.3Hz,1H),8.35(s,1H),8.21(s,1H),7.97(d,J=8.6Hz,1H),7.80(d,J=9.4Hz,1H),7.71(d,J=8.5Hz,1H),7.54(s,1H),7.10(s,1H),4.23(t,J=7.6Hz,2H),3.63(s,2H),3.44-3.40(m,2H),2.86(s,4H),2.54(s,4H),2.48(s,3H),1.79-1.73(m,2H),1.66-1.60(m,2H),1.54-1.42(m,6H).HRMS(ESI)for C 37 H 40 F 3 N 7 O 3 [M+H] + :calcd,688.3217,found,688.3250.
Example 23: n- (3-chloro-5-trifluoromethyl) -4 6 -methyl-14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradecane-2-yne-4 5 Preparation of formamide (named WZQ 4-12)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.89(s,1H),8.87(t,J=5.5Hz,1H),8.36-8.34(m,2H),8.13(s,2H),7.80(d,J=9.4Hz,1H),7.59(s,1H),7.55(s,1H),7.15(s,1H),4.23(t,J=7.5Hz,2H),3.39(q,J=6.1Hz,2H),2.51(s,3H),1.83-1.72(m,2H),1.65-1.60(m,2H),1.51-1.45(m,6H).HRMS(ESI)for C 31 H 27 ClF 3 N 5 O 3 [M+H] + :calcd,610.1827,found,610.1847.
Example 24:4 6 -methyl-N- (3- (4-methylpiperazin-1-yl) methyl) -5- (trifluoromethyl) phenyl) -14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetraundec-2-yne-4 5 Preparation of formamide (named WZQ 4-13)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.74(s,1H),8.87(t,J=5.5Hz,1H),8.38-8.34(m,2H),8.14(s,1H),7.95(s,1H),7.80(d,J=9.5Hz,1H),7.54(d,J=2.7Hz,1H),7.39(s,1H),7.12(d,J=2.6Hz,1H),4.23(t,J=7.4Hz,2H),3.59(s,2H),3.40(q,J=5.5Hz,2H),2.67(s,8H),2.39(s,3H),1.80-1.73(m,2H),1.67-1.60(m,2H),1.54-1.42(m,6H).HRMS(ESI)for C 37 H 40 F 3 N 7 O 3 [M+H] + :calcd,688.3217,found,688.3220.
Example 25:4 6 -methyl-N- (3- (4-morpholinopiperidin-1-yl) -5- (tri)Fluoromethyl) phenyl) -14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradecane-2-yne-4 5 Preparation of formamide (named WZQ 4-21)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.54(s,1H),8.88(t,J=6.0Hz,1H),8.38-8.35(m,2H),7.80(d,J=9.4Hz,1H),7.62(s,1H),7.60(s,1H),7.55(s,1H),7.09(s,1H),7.03(s,1H),4.23(t,J=7.6Hz,2H),4.04-3.92(m,4H),3.66(t,J=12.4Hz,2H),3.50(d,J=12.5Hz,2H),3.39(q,J=6.8Hz,2H),3.19-3.05(m,2H),2.85(t,J=12.8Hz,2H),2.49(s,3H),2.46(t,J=10.5Hz,1H),2.16(d,J=10.2Hz,2H),1.79-1.74(m,2H),1.68-1.63(m,4H),1.51-1.45(m,6H).HRMS(ESI)for[M+H] + :calcd,744.3480,found,744.3484.
Example 26: n- (3-tert-butyl) -1- (4-chlorophenyl) -1H-pyrazol-5-yl) -4 6 -methyl-14-oxo-5-oxa-13-aza-1 (7, 2) -pyrrolo [1,2-b]Pyridin-4 (1, 3) -benzocyclotetraene-2-yn-4 5 Preparation of formamide (named WZQ 4-24)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.42(s,1H),8.85(t,J=5.6Hz,1H),8.36(t,J=4.7Hz,2H),7.79(d,J=9.4Hz,1H),7.58(q,J=2.5Hz,4H),7.52(d,J=2.7Hz,1H),7.01(d,J=2.6Hz,1H),6.47(s,1H),4.21(t,J=7.5Hz,2H),3.39(q,J=6.0Hz,2H),2.38(s,3H),1.79-1.71(m,2H),1.65-1.58(m,2H),1.50-1.44(m,6H),1.32(s,9H).HRMS(ESI)for C 37 H 38 ClN 7 O 3 [M+H] + :calcd,664.2797,found,664.2808.
Example 27:4 6 -methyl-N- (3- (4-methyl-1, 4-diazepin-1-yl) methyl)Phenyl) -5- (trifluoromethyl) phenyl) -14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradecan-2-ynyl-4 5 Preparation of formamide (named WZQ 4-28)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.72(s,1H),8.87(t,J=5.6Hz,1H),8.37-8.35(m,2H),8.15(s,1H),7.93(s,1H),7.80(d,J=9.4Hz,1H),7.54(d,J=2.7Hz,1H),7.39(s,1H),7.12(d,J=2.7Hz,1H),4.23(t,J=7.4Hz,2H),3.69(s,2H),3.42-3.39(m,2H),2.68-2.62(m,4H),2.59-2.55(m,4H),2.25(s,3H),1.78-1.70(m,4H),1.63(s,2H),1.52-1.45(m,6H).HRMS(ESI)for C 38 H 42 F 3 N 7 O 3 [M+Na] + :calcd,724.3193,found,724.3211.
Example 28:4 6 -methyl-N- (3-morpholinomethyl) -5-trifluoromethylphenyl) -14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradec-2-yne-4 5 Preparation of formamide (named WZQ 4-45)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.72(s,1H),8.86(s,1H),8.35(s,2H),8.16(s,1H),7.94(s,1H),7.79(d,J=9.4Hz,1H),7.53(s,1H),7.39(s,1H),7.13(s,1H),4.23(t,J=8.3Hz,2H),3.59(s,4H),3.55(s,2H),3.39(q,J=6.6Hz,2H),2.52(s,3H),2.39(s,4H),1.80-1.74(m,2H),1.65-1.60(m,2H),1.50-1.45(m,6H).HRMS(ESI)for C36H37F3N6O4[M+H] + :calcd,675.2901,found,675.2927.
Example 29: n- (3-isopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) -4 6 -methyl-14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2 ]b]Pyridazin-4 (1, 3) -benzocyclotetradec-2-ynyl-4 5 Preparation of formamide (named WZQ 4-48)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.34(s,1H),8.86(t,J=5.6Hz,1H),8.36(d,J=9.1Hz,1H),8.35(s,1H),7.80(d,J=9.4Hz,1H),7.60(s,1H),7.52(s,1H),7.51(s,1H),7.05(d,J=2.6Hz,1H),6.93(s,1H),4.22(t,J=7.4Hz,2H),3.49(s,2H),3.41(t,J=6.2Hz,2H),2.89-2.83(m,1H),2.49(s,8H),1.80-1.73(m,2H),1.66-1.60(m,2H),1.55-1.39(m,6H),1.22(s,3H),1.20(s,3H).HRMS(ESI)for C 39 H 47 N 7 O 3 [M+Na] + :calcd,684.3633,found,684.3662.
Example 30: n- (3-cyclopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) -4 6 -methyl-14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b ]Pyridazin-4 (1, 3) -benzocyclotetradecane-2-yne-4 5 Preparation of formamide (named WZQ 4-69)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.29(s,1H),8.86(t,J=5.6Hz,1H),8.36(d,J=9.4Hz,1H),8.35(s,1H),7.80(d,J=9.4Hz,1H),7.52(s,1H),7.51(s,1H),7.34(d,J=1.8Hz,1H),7.05(d,J=2.6Hz,1H),6.78(s,1H),4.22(t,J=7.4Hz,2H),3.42(s,2H),3.40(q,J=6.1Hz,2H),2.50(s,3H),2.48(s,4H),2.32(s,4H),1.93-1.86(m,1H),1.80-1.73(m,2H),1.66-1.60(m,2H),1.53-1.43(m,6H),0.98-0.93(m,2H),0.65-0.60(m,2H).HRMS(ESI)for C 39 H 45 N 7 O 3 [M+H] + :calcd,660.3657,found,660.3654.
Example 31: n- (3-tert-butyl) -5- (4-methylpiperazin-1-yl) methylphenyl)-4 6 -methyl-14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradec-2-yne-4 5 Preparation of formamide (named WZQ 4-85)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.31(s,1H),8.86(t,J=5.6Hz,1H),8.36(d,J=9.4Hz,1H),8.35(s,1H),7.79(d,J=9.4Hz,1H),7.65(s,1H),7.61(s,1H),7.51(d,J=2.7Hz,1H),7.07-7.05(m,2H),4.22(t,J=7.5Hz,2H),3.44(s,2H),3.40(q,J=7.1Hz,2H),2.50(s,3H),2.38(s,8H),2.18(s,3H),1.79-1.73(m,2H),1.66-1.60(m,2H),1.52-1.45(m,6H),1.28(s,9H).HRMS(ESI)for C 40 H 49 N 7 O 3 [M+Na] + :calcd,698.3789,found,698.3820.
Example 32: preparation of N- (3-cyclopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) -14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b ] pyridazin-4 (1, 3) -benzocyclotetradec-2-ynyl-44-carboxamide (designated WZQ-107)
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.10(s,1H),8.94(t,J=5.5Hz,1H),8.37(d,J=9.7Hz,1H),8.36(s,1H),7.82(d,J=9.4Hz,1H),7.70(d,J=7.9Hz,1H),7.59(s,1H),7.53(s,1H),7.30(s,1H),7.29(d,J=8.6Hz,1H),6.78(s,1H),4.36(t,J=7.8Hz,2H),3.48(s,3H),3.42(q,J=6.7Hz,2H),2.82(s,4H),2.52(s,4H),2.35(s,3H),1.94-1.88(m,1H),1.87-1.81(m,2H),1.65-1.61(m,2H),1.55-1.41(m,6H),0.99–0.93(m,2H),0.67–0.61(m,2H).HRMS(ESI)for C 38 H 43 N 7 O 3 [M+H] + :calcd,646.3500,found,646.3508.
Example 33:n- (3-isopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) -4 6 -methyl-14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzotetracyclotetradecane-4 5 Preparation of formamide (named WZQ 4-81)
The steps are as follows: compound 1 (30 mg,0.045 mmol) was dissolved in 10mL of methanol, palladium on carbon (10 mg) was added and reacted overnight under hydrogen, after which palladium on carbon was removed by suction filtration through celite, column chromatography gave 5mg (yield: 16.57%) of an off-white solid.
1 H NMR(400MHz,CDCl 3 )δ8.08(d,J=9.4Hz,1H),7.89(d,J=9.4Hz,1H),7.83(s,1H),7.54(s,1H),7.50(s,1H),7.47(t,J=5.3Hz,1H),7.41(s,1H),7.01(s,2H),6.92(d,J=2.6Hz,1H),4.07(t,J=6.1Hz,2H),3.57(q,J=6.4Hz,2H),3.54(s,2H),3.37-3.30(m,2H),3.09-3.03(m,2H),2.98-2.91(m,1H),2.51(s,8H),2.42(s,3H),2.32(s,3H),1.81-1.76(m,6H),1.56-1.49(m,4H),1.30(s,3H),1.29(s,3H).HRMS(ESI)for C 39 H 51 N 7 O 3 [M+Na] + :calcd,688.3946,found,688.3947.
Example 34: (E) -N- (3-isopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) -4 6 -methyl-14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridin-4 (1, 3) -benzocyclotetradec-2-en-4 5 Preparation of formamide (named WZQ 4-92)
Step 1: preparation of tert-butyl (7- (3-iodo-5- (3-isopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) carbamoyl) -4-methylphenoxy) heptyl) carbamate (Compound 2)
Compound 2 was obtained by the synthesis method according to step 9 in example 4.
1 H NMR(400MHz,CDCl 3 )δ7.73(s,1H),7.47(d,J=2.5Hz,1H),7.45(s,1H),7.39(s,1H),6.98(s,2H),4.58(s,1H),3.93(t,J=6.4Hz,2H),3.50(s,2H),3.10(q,J=6.7Hz,2H),2.95-1.88(m,1H),2.54-2.43(m,11H),2.30(s,3H),1.79-1.72(m,2H),1.52-1.43(m,13H),1.39-1.33(m,4H),1.27(s,3H),1.25(s,3H).LC-MS(ESI)m/z 721.2[M+H] + .
Step 2: preparation of methyl 3-vinylimidazo [1,2-b ] pyridazine-6-carboxylate (Compound 4)
Compound 3 (100 mg,0.33 mmol) and tributylvinyltin (418 mg,1.32 mmol) were dissolved in 10mL of toluene, and then tetrakis triphenylphosphine palladium (38.11 mg,0.03 mmol) was added and the reaction system was reacted overnight at 90℃under argon. Spin-drying the reaction system, column chromatography gave 54mg (yield: 80.54%) of a yellow solid.
1 H NMR(400MHz,CDCl 3 )δ8.06(d,J=9.4Hz,1H),8.03(s,1H),7.77(d,J=9.5Hz,1H),7.13(dd,J=17.9,11.7Hz,1H),6.34(dd,J=17.9,1.2Hz,1H),5.56(dd,J=11.7,1.3Hz,1H),4.07(s,3H).LC-MS(ESI)m/z 204.1[M+H] + .
Step 3: (E) Preparation of methyl-3- (5- (7-t-butoxycarbonylamino) heptyl) -3- (3-isopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) carbamoyl) -2-methylstyrene-yiimidazo [1,2-b ] pyridazine-6-carboxylate (Compound 5)
Compound 2 (200 mg,0.28 mmol) and Compound 4 (56.4 mg,0.28 mmol) were dissolved in 15mL of ultra-dry DMF, then palladium acetate (4.9 mg,0.02 mmol), tris (o-methylphenyl) phosphorus (13.5 mg,0.04 mmol) and N, N-diisopropylethylamine (0.18 mL,1.11 mmol) were added, after 12 hours at 90℃the extracts were performed 2-3 times with ethyl acetate and water and the organic layers were combined and column chromatography gave 107mg (yield: 48.44%) of a yellowish brown oil.
1 H NMR(400MHz,CDCl 3 )δ8.25(d,J=16.4Hz,1H),8.09(s,1H),8.08(d,J=9.0Hz,1H),7.83(s,1H),7.80(d,J=9.4Hz,1H),7.51(s,1H),7.49(s,1H),7.32(d,J=16.3Hz,1H),7.28(s,1H),6.99(s,1H),6.96(d,J=2.7Hz,1H),4.56(s,1H),4.07(s,3H),4.03(t,J=6.4Hz,2H),3.57(s,2H),3.13(q,J=6.0Hz,2H),2.98-2.93(m,1H),2.64(s,8H),2.53(s,3H),2.40(s,3H),1.86-1.79(m,2H),1.54-1.47(m,4H),1.44(s,9H),1.41-1.36(m,4H),1.30(s,3H),1.28(s,3H).
Step 4: (E) Preparation of (E) -3- (5- (7-tert-Butoxycarbonylamino) heptyl) oxy) -3- (3-isopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) carbamoyl) -2-methylstyrene yl imidazo [1,2-b ] pyridazine-6-carboxylic acid (Compound 6)
The procedure of step 7 of example 17 was followed to give compound 6, crude product was used directly in the next step.
Step 5: (E) -N- (3-isopropyl-5- (4-methylpiperazin-1-yl) methyl) phenyl) -4 6 -methyl-14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridin-4 (1, 3) -benzocyclotetradec-2-en-4 5 Preparation of formamide (Compounds WZQ 4-92)
Compounds WZQ to 92 were obtained by the synthetic method of step 8 in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ10.26(s,1H),8.97(t,J=5.4Hz,1H),8.56(s,1H),8.28(d,J=9.4Hz,1H),7.87(d,J=16.6Hz,1H),7.73(d,J=16.5Hz,1H),7.67(d,J=9.4Hz,1H),7.56(s,1H),7.54(s,2H),6.90(s,1H),6.89(s,1H),4.24(t,J=7.6Hz,2H),3.43(s,2H),3.40(q,J=6.6Hz,2H),2.89-2.82(m,1H),2.43-2.39(m,11H),2.24(s,3H),1.87-1.80(m,2H),1.66-1.60(m,2H),1.55-1.43(m,6H),1.21(s,3H),1.19(s,3H).HRMS(ESI)for C 39 H 49 N 7 O 3 [M+H] + :calcd,664.3970,found,664.3983.
Example 35:1- (4-chloro-3-trifluoromethylphenyl) -3- (-4) 6 -methyl-14-oxo-5-oxa-13-aza-1 (3, 6) -imidazo [1,2-b]Pyridazin-4 (1, 3) -benzocyclotetradec-2-ynyl-4 5 Preparation of urea (named WZQ-52).
The synthesis is described in example 17.
1 H NMR(400MHz,DMSO-d 6 )δ9.56(s,1H),8.81(t,J=5.6Hz,1H),8.35(d,J=9.4Hz,1H),8.33(s,1H),8.19(s,1H),8.10(s,1H),7.77(d,J=9.4Hz,1H),7.64(s,2H),7.52(d,J=2.4Hz,1H),7.15(d,J=2.4Hz,1H),4.15(t,J=7.3Hz,2H),3.39(q,J=5.3Hz,2H),2.42(s,3H),1.77-1.71(m,2H),1.65-1.58(m,2H),1.48-1.44(m,6H).
Example 36 IC of Compounds against TRKs kinase 50 Testing
Kinase activity assay: application of Z' -LYTE TM Techniques (detection by fluorescence, enzyme-coupled format, based on differential susceptibility of phosphorylated and non-phosphorylated polypeptides to proteolytic cleavage), employing Fluorescence Resonance Energy Transfer (FRET) principles, using Z' -LYTE TM The inhibitory activity of the FRET peptide substrate, the secondary reaction test compound on TRKs (TRKA, TRKC, TRKA-G667C, TRKA-G595R) kinase (American Life technologies Co., PV3144, PV3616, PV 3617).
Enzymatic reaction: into 384-well plates, 5. Mu.L of enzyme-substrate system [50mM 4-hydroxyethylpiperazine ethanesulfonic acid (HEPES) pH 7.5,0.01% BRIJ-35,10mM magnesium chloride (MgCl) was added 2 ) 1mM ethylene glycol bis (2-aminoethylether) tetraacetic acid (EGTA), 2. Mu. MTyr 01 peptide substrate]Transferring into 5nL of compound to be detected (concentration gradient) by using an echo520 ultra-trace liquid pipetting system, oscillating at room temperature for 10-20min, and using echo520 ultra-trace liquidThe pipetting system was transferred to 200nL,12.5nL and 25nLATP (final concentrations 400uM,25uM and 50uM respectively), and centrifuged after shaking and mixing, and reacted at 30℃for 1.5 hours in the absence of light.
Detection reaction: 2.5. Mu.L of reaction solution (Development Solution) (1:128 dilution) was added to each well and incubated at 37℃for 1h in the absence of light, followed by 5. Mu.L of Stop Reagent.
Reading a plate: the multi-label microplate detector (Perkin Elmer EnVision Multimode Plate Reader) detects fluorescent signals (excitation light wavelength 400nm, emission light wavelength 460nm, 535 nm).
And (3) calculating: the inhibition rate of each well was calculated from the total active well and the control signal well, and the data analysis method was as follows:
Phosphorylation ratio = 1- { (emission ratio xf100% -c100%)/[ c0% -c100% + emission ratio× (f100% -f0%) ] } ×100;
inhibition = 100× (1-compound phosphorylation ratio/negative control phosphorylation ratio).
IC 50 The values were calculated using medical mapping software (GraphPad prism 5.0).
The kinase activity test results are shown in table 1.
Results of the compound kinase Activity test (IC) 50 :nM)
IC 50 :*<10nM;10≦**<100nM;100≦***<1000nM;****≧1μM。
From the data in Table 1, it can be seen that the novel cyclic compounds of the present invention have a strong inhibitory activity against TRKs kinase.
Example 37: cell proliferation inhibition activity study based on Ba/F3-TRKs stable strain
BaF3 cells (pre-mouse B cells) used in the experiment were purchased from Japanese cell banks, and the BaF3-CD74-NTRK1, baF3-ETV6-NTRK3, CD74-NTRK1-G667C, CD-NTRK 1-G595R monoclonal stable strains were all constructed in the laboratory and were completely correct by experiments such as positive drug activity, protein expression and gene sequencing.
The brief steps for the construction of the stable strain are as follows: constructing pCDNA3.1 (+) plasmid vectors carrying genes such as CD74-NTRK1, ETV6-NTRK3, CD74-NTRK1-G667C, CD-NTRK 1-G595R and the like; usingCell LineThe Kit V Kit electrotransfers the plasmid into Ba/F3 cells; after 48 hours of electrotransformation, adding geneticin (G418) with a final concentration of 1000 mug/ml for screening for two weeks, and removing interleukin 3 (IL 3) for further screening to obtain a polyclonal stable strain; then selecting monoclonal by limiting dilution method; further, identifying the stable strain by using positive drugs, western Blot (WB) and gene sequencing; identification of the exact monoclonal can be used in studies of the cytostatic activity of the inhibitor.
Cell proliferation inhibition activity study: inoculating 8000-12000 cells in logarithmic growth phase into 96-well plate, adding inhibitor (0-10 μm) at different concentrations the next day, and culturing for 72 hr; then 10 mu L Cell Counting Kit-8 cell counting reagent (CCK-8 reagent) is added to each well, and incubation is continued for 1-3 hours; the absorbance at 450nm and 650nm was then measured with a super microplate reader. Half maximal Inhibitory Concentration (IC) was calculated using medical mapping software (GraphPad Prism 8.0.0) 50 ). The test results are shown in Table 2.
Results of test for inhibitory Activity of the compounds of Table 2 on cell proliferation (IC 50 :nM)
IC 50 :*<10nM;10≦**<100nM;100≦***<1000nM;****≧1μM。
As can be seen from the data in Table 2, the novel cyclic compounds of the present invention have a strong inhibitory activity on the proliferation of cells of the Ba/F3-TRKs-stable strain.
Example 38: drug-resistant cell proliferation inhibition activity research based on Ba/F3-TRKs stable strain
BaF3 cells (pre-mouse B cells) used in this experiment were purchased from Japanese cell bank, baF3-CD74-NTRK1-G667S, baF3-CD74-NTRK1-G667A, baF3-CD74-NTRK1-F589L, baF3-CD74-NTRK1-V573M, baF3-ETV6-NTRK3-G696C, baF-ETV 6-NTRK3-G696A, baF3-ETV6-NTRK3-G696S, baF3-ETV6-NTRK3-G623R, baF3-ETV6-NTRK3-G623E, baF-ETV 6-NTRK3-F617L, baF3-ETV 601M monoclonal stable strain was constructed from this laboratory and was completely correct by positive pharmacological activity, protein expression, gene sequencing and the like.
The brief steps for the construction of the stable strain are as follows: construction of a pCDNA3.1 (+) plasmid vector carrying the BaF3-CD74-NTRK1-G667S, baF3-CD74-NTRK1-G667A, baF3-CD74-NTRK1-F589L, baF3-CD74-NTRK1-V573M, baF3-ETV6-NTRK3-G696C, baF3-ETV6-NTRK3-G696A, baF3-ETV6-NTRK3-G696S, baF3-ETV6-NTRK3-G623R, baF3-ETV6-NTRK3-G623E, baF3-ETV6-NTRK3-F617L, baF-ETV 6-NTRK3-V601M isogene; usingCell LineThe Kit V Kit electrotransfers the plasmid into Ba/F3 cells; after 48 hours of electrotransformation, adding geneticin (G418) with a final concentration of 1000 mug/ml for screening for two weeks, and removing interleukin 3 (IL 3) for further screening to obtain a polyclonal stable strain; then selecting monoclonal by limiting dilution method; further, identifying the stable strain by using positive drugs, western Blot (WB) and gene sequencing; identification of the exact monoclonal can be used in studies of the cytostatic activity of the inhibitor.
Cell proliferationInhibition of proliferation activity study: inoculating 8000-12000 cells in logarithmic growth phase into 96-well plate, adding inhibitor (0-10 μm) at different concentrations the next day, and culturing for 72 hr; then 10 mu L Cell Counting Kit-8 cell counting reagent (CCK-8) is added to each well and incubation is continued for 1-3 hours; the absorbance at 450nm and 650nm was then measured with a super microplate reader. Half maximal Inhibitory Concentration (IC) was calculated using medical mapping software (GraphPad Prism 8.0.0) 50 )。
The test results are shown in Table 3.
Table 3 results of test for inhibitory Activity of Compounds WZQ4-69 against drug-resistant cell proliferation (IC 50 :nM)
| Numbering of compounds | WZQ4-69 | LOXO-195 | TPX-0005 |
| BaF3-CD74-NTRK1-G667S | ** | ** | * |
| BaF3-CD74-NTRK1-G667A | * | * | * |
| BaF3-CD74-NTRK1-F589L | * | ** | * |
| BaF3-CD74-NTRK1-V573M | * | ** | ** |
| BaF3-ETV6-NTRK3-G696C | * | ** | ** |
| BaF3-ETV6-NTRK3-G696A | * | * | * |
| BaF3-ETV6-NTRK3-G696S | * | ** | * |
| BaF3-ETV6-NTRK3-G623E | * | * | * |
| BaF3-ETV6-NTRK3-G623R | * | * | * |
| BaF3-ETV6-NTRK3-F617L | * | ** | * |
| BaF3-ETV6-NTRK3-V601M | ** | ** | ** |
IC 50 :*<10nM;10≦**<100nM;100≦***<1000nM;****≧1μM。
From the data in Table 3, it can be seen that the novel cyclic compounds WZQ-69 of the invention have strong inhibitory activity on the proliferation of various drug-resistant cells of the Ba/F3-TRKs stable strain, and the inhibitory effect is obviously better than that of the second-generation TRK inhibitor LOXO-195.
The technical features of the above-described embodiments may be arbitrarily combined, and all possible combinations of the technical features in the following embodiments are not described for brevity of description, however, as long as there is no contradiction between the combinations of the technical features, they should be considered as the scope of the description.
The foregoing examples illustrate only a few embodiments of the invention and are described in detail herein without thereby limiting the scope of the invention. It should be noted that it will be apparent to those skilled in the art that several variations and modifications can be made without departing from the spirit of the invention, which are all within the scope of the invention. Accordingly, the scope of protection of the present invention is to be determined by the appended claims.
Claims (31)
1. A cyclic compound having a structure represented by formula (I) or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof:
E. z, M, Q are each independently selected from: CR (computed radiography) 5 Or N;
t is selected from: n, CR 7 Or NR 6 The method comprises the steps of carrying out a first treatment on the surface of the V is selected from: n, C or CR 7 The method comprises the steps of carrying out a first treatment on the surface of the U, W are each independently selected from: CR (computed radiography) 7 Or N;
R 5 、R 6 、R 7 each independently selected from: H. halogen, C 1 ~C 6 Alkyl, substituted or unsubstituted C 3 ~C 7 Cycloalkyl;
r is selected from: substituted or unsubstituted phenyl, -C (=o) -, -C (=o) NR 8 R 9 or-NR 8 R 9 The method comprises the steps of carrying out a first treatment on the surface of the Wherein R is 8 Selected from: H. c (C) 1 ~C 20 Alkyl, - (CH) 2 ) m NR 10 R 11 、-(CH 2 ) n CR 10 R 11 R 12 ;R 9 Selected from: c (C) 1 ~C 20 Alkylene, or R 8 、R 9 Together with the nitrogen atom to which they are attached form a substituted or unsubstituted heterocyclic group;
R 10 、R 11 、R 12 each independently selected from: H. c (C) 1 ~C 20 Alkyl, or R 10 、R 11 Together with the nitrogen or carbon atom to which they are attached, form a substituted or unsubstituted monocyclic, fused, spiro or bridged ring containing 0 to 3 heteroatoms;
m and n are each independently selected from: an integer of 0 to 10;
x, Y are each independently selected from: -O-, -N (R) 13 )-、-S-、-S(=O)-、-S(O) 2 -、-C(=O)-、-NR 13 (C=O)-;R 13 Selected from: H. c (C) 1 ~C 6 An alkyl group;
l is selected from: substituted or unsubstituted C 2 ~C 12 Alkylene, substituted or unsubstituted C 2 ~C 12 Unsaturated chain hydrocarbon groups;
X 1 and X 2 Each independently selected from: -N (R) 13 ) Or none;
R 1 selected from: H. halogen, C 1 ~C 20 Alkyl, C 1 ~C 20 Alkoxy or halogen substituted C 1 ~C 20 An alkyl group;
g is selected from: r' substituted or unsubstituted C 6 ~C 10 Aryl, R' substituted or unsubstituted 5-10 membered heteroaryl;
each R' is independently selected from: H. halogen, substituted or unsubstituted C 1 ~C 20 Alkyl, substituted or unsubstituted C 1 ~C 20 Alkoxy, nitro, substituted or unsubstituted C 3 ~C 12 Cycloalkyl, substituted or unsubstituted C 6 ~C 10 Aryl, substituted or unsubstituted 3-12 membered heterocyclyl, substituted or unsubstituted 5-10 membered heteroaryl.
2. The cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or prodrug molecule thereof according to claim 1, wherein G is selected from the group consisting of: r 'is a substituted or unsubstituted phenyl group, R' is a substituted or unsubstituted 5-6 membered heteroaryl group containing 1-3N ring atoms.
3. The cyclic compound or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a prodrug molecule thereof according to claim 2, wherein the cyclic compound has a structure represented by the following formula (II) or formula (III):
wherein R is 2 Selected from: H. halogen, C 1 ~C 20 Alkyl, C 1 ~C 20 Alkoxy, halogen substituted C 1 ~C 20 Alkyl, C 3 ~C 12 Cycloalkyl;
R 3 selected from: H. halogen, substituted or unsubstituted C 1 ~C 20 Alkyl, substituted or unsubstituted 5-6 membered heterocyclic group containing 1-3N ring atoms;
R 4 selected from: H. halogen, nitro, substituted or unsubstituted C 1 ~C 20 Alkyl, substituted or unsubstituted C 1 ~C 20 Alkoxy, substituted or unsubstituted C 3 ~C 12 Cycloalkyl, substituted or unsubstituted 3-12 membered heterocyclyl, substituted or unsubstituted 5-10 membered heteroaryl;
R’ 4 selected from: H. substituted or unsubstituted C 1 ~C 20 Alkyl, substituted or unsubstituted C 3 ~C 12 Cycloalkyl, substituted or unsubstituted C 6 ~C 10 Aryl, substituted or unsubstituted 3-12 membered heterocyclyl, substituted or unsubstituted 5-10 membered heteroaryl.
4. A cyclic compound or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof according to claim 3, wherein R 4 Selected from: H. halogen, C 1 ~C 4 Alkyl, nitro, halogen substituted C 1 ~C 4 Alkyl, C 1 ~C 4 Alkoxy, halogen substituted C 1 ~C 4 Alkoxy, - (CH) 2 ) p NR 15 R 16 By 1 or more R 17 Substituted or unsubstituted 5-6 membered heterocyclyl, substituted with 1-3R 17 Substituted or unsubstituted 5-6 membered heteroaryl; p is selected from: 1. 2 or 3;
R 15 、R 16 together with the nitrogen atom to which they are attached form R 17 A substituted or unsubstituted 5-12 membered heterocyclyl;
Each R is 17 Each independently selected from: H. c (C) 1 -C 6 Alkyl, C 3 ~C 8 Cycloalkyl, 5-8 membered heterocyclyl, dimethylamineA methyl sulfonyl group.
5. The cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or prodrug molecule thereof according to claim 4, wherein R 4 Selected from: H. halogen, nitro, methyl, ethyl, propyl, methoxy, ethoxy, propoxy, trifluoromethyl, trifluoroethyl,
Each R is 17 Each independently selected from: H. methyl, ethyl, propyl, dimethylamino, cyclohexyl, methylsulfonyl.
6. A cyclic compound or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof according to claim 3, wherein R 2 Selected from: H. halogen, C 1 ~C 6 Alkyl, C 1 ~C 6 Alkoxy, halogen substituted C 1 ~C 6 Alkyl, C 3 ~C 8 Cycloalkyl groups.
7. The cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or prodrug molecule thereof according to claim 6, wherein R 2 Selected from: H. fluorine, methyl, ethyl, propyl, isopropyl, tert-butyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, cyclopropyl.
8. A cyclic compound or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof according to claim 3, wherein R 3 Selected from: H. halogen, C 1 ~C 6 Alkyl, halogen substituted C 1 ~C 6 Alkyl, - (CH) 2 ) p NR 15 R 16 The method comprises the steps of carrying out a first treatment on the surface of the p is selected from: 1. 2 or 3;
R 15 、R 16 together with the nitrogen atom to which they are attached form R 17 A substituted or unsubstituted 5-8 membered heterocyclic group;
wherein R is 17 Selected from: H. c (C) 1 -C 6 An alkyl group.
10. A cyclic compound or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof according to claim 3, wherein R' 4 Selected from: H. c (C) 1 ~C 6 Alkyl, C 3 ~C 8 Cycloalkyl, R 17 A substituted or unsubstituted phenyl group; wherein R is 17 Selected from: H. c (C) 1 -C 6 An alkyl group.
13. The cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or a prodrug molecule thereof according to any one of claims 1 to 10, wherein R is selected from: -C (=o) -, -C (=o) NR 8 R 9 or-NR 8 R 9 The method comprises the steps of carrying out a first treatment on the surface of the Wherein R is 8 Selected from: H. c (C) 1 ~C 6 Alkyl, - (CH) 2 ) m NR 10 R 11 ;R 9 Selected from: c (C) 1 ~C 6 Alkylene, or R 8 、R 9 Together with the nitrogen atom to which they are attached form one or more R 14 A substituted or unsubstituted 3-8 membered heterocyclic group;
R 10 、R 11 each independently selected from: H. c (C) 1 ~C 6 An alkyl group;
m is selected from: an integer of 1 to 5;
R 14 selected from: H. c (C) 1 ~C 6 An alkyl group.
14. The cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or prodrug molecule thereof according to claim 13, wherein R is selected from: -C (=o) -, -C (=o) NR 8 R 9 or-NR 8 R 9 The method comprises the steps of carrying out a first treatment on the surface of the Wherein R is 8 Selected from: H. c (C) 1 ~C 3 An alkyl group; r is R 9 Selected from: c (C) 1 ~C 3 Alkylene, or R 8 、R 9 Together with the nitrogen atom to which they are attached form one or more R 14 Substituted or unsubstituted morpholinyl, pyrrolidinyl, piperidinyl or piperazinyl; r is R 14 Selected from: H. c (C) 1 ~C 3 An alkyl group.
15. The cyclic compound according to any one of claims 1-10 or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a precursor thereofA pharmaceutical molecule, characterized in that R is selected from: -C (=o) -, x is selected from: 0. 1, 2 and 3; y is selected from: an integer between 0 and 8; r is R 8 Selected from: H. c (C) 1 ~C 6 An alkyl group.
17. the cyclic compound or pharmaceutically acceptable salt thereof, or stereoisomer thereof, or prodrug molecule thereof according to any one of claims 1 to 10, wherein X, Y is each independently selected from the group consisting of: -O-, -N (R) 13 )-、-NR 13 (C=O)-;R 13 Selected from: H. c (C) 1 ~C 3 An alkyl group.
18. The cyclic compound or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof according to claim 17, wherein X is-O-; y is selected from: -N (R) 13 )-、-NR 13 (C=O)-;R 13 Selected from: H. methyl group.
19. The cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or a prodrug molecule thereof according to any one of claims 1 to 10, wherein L is selected from the group consisting of: c (C) 2 ~C 8 Alkylene, C 2 ~C 8 Unsaturated chain hydrocarbon groups.
21. The cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or a prodrug molecule thereof according to any one of claims 3 to 10, wherein a and Y together form the structure:
23. The cyclic compound or pharmaceutically acceptable salt thereof or stereoisomer thereof or prodrug molecule thereof according to any one of claims 1 to 10, wherein R 1 Selected from: H. halogen, C 1 ~C 3 An alkyl group.
24. The cyclic compound according to any one of claims 1-10 or a pharmaceutically acceptable salt thereof or a stereoisomer thereofAn isomer or prodrug molecule thereof, characterized by X 1 Is NH or not, X 2 Is NH.
27. use of a cyclic compound as defined in any one of claims 1 to 26, or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a prodrug molecule thereof, for the preparation of a TRK inhibitor.
28. Use of a cyclic compound as defined in any one of claims 1 to 26 or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof for the preparation of a medicament for the prophylaxis and/or treatment of a disease mediated by TRK kinase.
29. The use according to claim 28, wherein the disease mediated by TRK kinase is a tumour.
30. The use of claim 29, wherein the tumor is: non-small cell lung cancer, breast cancer, colon cancer, prostate cancer, thyroid cancer, malignant melanoma, neuroblastoma, and breast-like secretory cancer.
31. A pharmaceutical composition for the prevention and/or treatment of tumors, characterized in that it is prepared from an active ingredient comprising a cyclic compound according to any one of claims 1 to 26 or a pharmaceutically acceptable salt thereof or a stereoisomer thereof or a prodrug molecule thereof, and a pharmaceutically acceptable adjuvant.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111395245 | 2021-11-23 | ||
| CN2021113952455 | 2021-11-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116199688A true CN116199688A (en) | 2023-06-02 |
Family
ID=86510208
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211441252.9A Pending CN116199688A (en) | 2021-11-23 | 2022-11-17 | Cyclic compounds and their use |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116199688A (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015108490A2 (en) * | 2014-01-17 | 2015-07-23 | Agency For Science, Technology And Research | Heteroaryl alkyne derivatives and uses thereof |
| CN104995172A (en) * | 2013-02-19 | 2015-10-21 | 小野药品工业株式会社 | Trk-INHIBITING COMPOUND |
-
2022
- 2022-11-17 CN CN202211441252.9A patent/CN116199688A/en active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104995172A (en) * | 2013-02-19 | 2015-10-21 | 小野药品工业株式会社 | Trk-INHIBITING COMPOUND |
| WO2015108490A2 (en) * | 2014-01-17 | 2015-07-23 | Agency For Science, Technology And Research | Heteroaryl alkyne derivatives and uses thereof |
Non-Patent Citations (3)
| Title |
|---|
| ZUQIN WANG ET AL.: ""Discovery of the First Highly Selective and Broadly Effective Macrocycle-Based Type II TRK Inhibitors that Overcome Clinically Acquired Resistance"", 《J. MED. CHEM.》, vol. 65, 15 April 2022 (2022-04-15), pages 6325 - 6337, XP093099038, DOI: 10.1021/acs.jmedchem.2c00308 * |
| ZUQIN WANG ET AL.: ""Structure-Based Optimization of the Third Generation Type II Macrocycle TRK Inhibitors with Improved Activity against Solvent-Front, xDFG, and Gatekeeper Mutations‘", 《J. MED. CHEM.》, vol. 66, 7 September 2023 (2023-09-07), pages 12950 - 12965 * |
| 王杰: "新一代TRK抑制剂JND4135体内外克服突变耐药的作用及机制研究", 《暨南大学硕士学位论文》, 20 June 2021 (2021-06-20), pages 1 - 128 * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN111153901B (en) | Nitrogen-containing fused heterocyclic SHP2 inhibitor compound, preparation method and application | |
| US8772311B2 (en) | Harmine derivatives, intermediates used in their preparations, preparation processes and use thereof | |
| EP3473626B1 (en) | Pyrrolopyrimidine crystal for preparing jak inhibitor | |
| EP4324833A1 (en) | Alkynylphenylbenzamide compound and use thereof | |
| TWI321566B (en) | Pyrido[2,3-d]pyrimidine derivatives, preparation thereof, therapeutic use thereof | |
| EP3533787B1 (en) | Pyridone compound as c-met inhibitor | |
| CN101973989B (en) | Thiazole amide compound and medicinal application thereof for treating malignancy | |
| WO2018177444A2 (en) | Azaphenalene-3-one derivative, preparation method therefor and application thereof | |
| WO2020221236A1 (en) | Trk kinase inhibitor and use thereof | |
| CN116199688A (en) | Cyclic compounds and their use | |
| CN111704613B (en) | Imidazole derivatives and their use as TRPV4 inhibitors | |
| CN116354988A (en) | Tetracyclic compound and medical application thereof | |
| CN115368355A (en) | Crystalline form of pyrazolo heteroaryl derivative | |
| CA3219001A1 (en) | Pharmaceutically acceptable salt of pyrazoloheteroaryl derivative and crystal form thereof | |
| CN115433207A (en) | Macrocyclic heterocyclic compound as EGFR inhibitor and application thereof | |
| CN115745975B (en) | JAK kinase domain and pseudokinase domain co-inhibition prodrug, preparation method and medical application | |
| WO2024056091A1 (en) | Pyridonopyrimidine derivative as rsk inhibitor and use thereof | |
| CN114989176B (en) | Imidazopyridazine derivative and application thereof | |
| CN115028648B (en) | Tri-fused ring compound and pharmaceutical composition and application thereof | |
| CN117510504A (en) | Protein kinase degradation agent, medicine and application | |
| WO2023011446A1 (en) | Novel sulfonamide menin-mll interaction inhibitor, preparation method therefor, and medical use thereof | |
| CN116891437A (en) | Amino acid derivative, pharmaceutical composition, preparation method and application thereof | |
| WO2019233461A1 (en) | Tropomyosin receptor kinase inhibitor, preparation method therefor and use thereof | |
| CN118108710A (en) | Indole iodized salt-pyridine bishemicyanine compound and preparation method and application thereof | |
| JP2024510520A (en) | Double ring miscellaneous ring FGFR4 inhibitor, pharmaceutical compositions and preparations containing double ring miscellaneous ring FGFR4 inhibitor, and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |