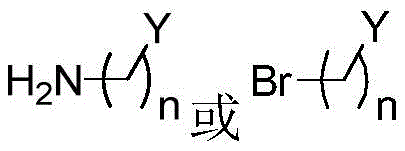CN115572282A - Pyrazole amide compound containing aromatic heterocyclic structure, and preparation method and application thereof - Google Patents
Pyrazole amide compound containing aromatic heterocyclic structure, and preparation method and application thereof Download PDFInfo
- Publication number
- CN115572282A CN115572282A CN202110757318.4A CN202110757318A CN115572282A CN 115572282 A CN115572282 A CN 115572282A CN 202110757318 A CN202110757318 A CN 202110757318A CN 115572282 A CN115572282 A CN 115572282A
- Authority
- CN
- China
- Prior art keywords
- group
- substituted
- radical
- unsubstituted
- substitution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- -1 Pyrazole amide compound Chemical class 0.000 title claims abstract description 180
- 238000002360 preparation method Methods 0.000 title claims abstract description 38
- 125000006615 aromatic heterocyclic group Chemical group 0.000 title claims abstract description 14
- 150000001875 compounds Chemical class 0.000 claims abstract description 60
- 125000001424 substituent group Chemical group 0.000 claims abstract description 29
- 241000238631 Hexapoda Species 0.000 claims abstract description 8
- 238000006467 substitution reaction Methods 0.000 claims description 68
- 229910052736 halogen Inorganic materials 0.000 claims description 61
- 150000002367 halogens Chemical class 0.000 claims description 61
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 45
- 125000000171 (C1-C6) haloalkyl group Chemical group 0.000 claims description 32
- 125000000217 alkyl group Chemical group 0.000 claims description 32
- 239000000203 mixture Substances 0.000 claims description 28
- 125000004423 acyloxy group Chemical group 0.000 claims description 25
- 125000003368 amide group Chemical group 0.000 claims description 24
- 125000003601 C2-C6 alkynyl group Chemical group 0.000 claims description 23
- 125000002057 carboxymethyl group Chemical group [H]OC(=O)C([H])([H])[*] 0.000 claims description 23
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims description 23
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 23
- 125000001072 heteroaryl group Chemical group 0.000 claims description 21
- 239000001257 hydrogen Substances 0.000 claims description 21
- 229910052739 hydrogen Inorganic materials 0.000 claims description 21
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 19
- 125000005842 heteroatom Chemical group 0.000 claims description 17
- 150000003839 salts Chemical class 0.000 claims description 17
- 125000000623 heterocyclic group Chemical group 0.000 claims description 16
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 claims description 15
- 125000000304 alkynyl group Chemical group 0.000 claims description 15
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 15
- 238000000034 method Methods 0.000 claims description 15
- 125000000392 cycloalkenyl group Chemical group 0.000 claims description 14
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 claims description 13
- 125000003545 alkoxy group Chemical group 0.000 claims description 11
- 125000001624 naphthyl group Chemical group 0.000 claims description 11
- 125000003342 alkenyl group Chemical group 0.000 claims description 10
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 9
- 230000003287 optical effect Effects 0.000 claims description 9
- 241000607479 Yersinia pestis Species 0.000 claims description 8
- 125000000262 haloalkenyl group Chemical group 0.000 claims description 8
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 8
- 229910052760 oxygen Inorganic materials 0.000 claims description 8
- 125000004076 pyridyl group Chemical group 0.000 claims description 8
- 150000001408 amides Chemical class 0.000 claims description 7
- 229910052717 sulfur Inorganic materials 0.000 claims description 7
- 125000002252 acyl group Chemical group 0.000 claims description 6
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 6
- 125000004122 cyclic group Chemical group 0.000 claims description 6
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 claims description 6
- 125000005843 halogen group Chemical group 0.000 claims description 5
- 239000002917 insecticide Substances 0.000 claims description 5
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 claims description 5
- 230000000361 pesticidal effect Effects 0.000 claims description 4
- 229920006395 saturated elastomer Polymers 0.000 claims description 4
- 239000000642 acaricide Substances 0.000 claims description 3
- LBQAJLBSGOBDQF-UHFFFAOYSA-N nitro azanylidynemethanesulfonate Chemical compound [O-][N+](=O)OS(=O)(=O)C#N LBQAJLBSGOBDQF-UHFFFAOYSA-N 0.000 claims description 3
- 206010061217 Infestation Diseases 0.000 claims description 2
- 230000000895 acaricidal effect Effects 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 125000001188 haloalkyl group Chemical group 0.000 claims description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N naphthalene-acid Natural products C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 claims description 2
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 2
- 239000002689 soil Substances 0.000 claims description 2
- 125000006555 (C3-C5) cycloalkyl group Chemical group 0.000 claims 1
- 241000238876 Acari Species 0.000 abstract description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 112
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 54
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 48
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 48
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 42
- 238000006243 chemical reaction Methods 0.000 description 40
- 238000003786 synthesis reaction Methods 0.000 description 34
- 230000015572 biosynthetic process Effects 0.000 description 33
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 30
- 239000002244 precipitate Substances 0.000 description 25
- 239000002904 solvent Substances 0.000 description 25
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 24
- 229910052757 nitrogen Inorganic materials 0.000 description 24
- 239000007787 solid Substances 0.000 description 23
- 238000013100 final test Methods 0.000 description 22
- 238000010438 heat treatment Methods 0.000 description 20
- 238000010992 reflux Methods 0.000 description 20
- 239000012265 solid product Substances 0.000 description 20
- 239000007858 starting material Substances 0.000 description 19
- 238000001914 filtration Methods 0.000 description 17
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 16
- 238000004440 column chromatography Methods 0.000 description 16
- 239000003480 eluent Substances 0.000 description 16
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 14
- RDHPKYGYEGBMSE-UHFFFAOYSA-N bromoethane Chemical compound CCBr RDHPKYGYEGBMSE-UHFFFAOYSA-N 0.000 description 12
- 238000001704 evaporation Methods 0.000 description 11
- 239000000706 filtrate Substances 0.000 description 11
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 10
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 10
- FORBXGROTPOMEH-UHFFFAOYSA-N 5-bromo-2-(3-chloropyridin-2-yl)pyrazole-3-carboxylic acid Chemical compound OC(=O)C1=CC(Br)=NN1C1=NC=CC=C1Cl FORBXGROTPOMEH-UHFFFAOYSA-N 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- MCTWTZJPVLRJOU-UHFFFAOYSA-N 1-methyl-1H-imidazole Chemical compound CN1C=CN=C1 MCTWTZJPVLRJOU-UHFFFAOYSA-N 0.000 description 8
- 235000019270 ammonium chloride Nutrition 0.000 description 8
- 125000004432 carbon atom Chemical group C* 0.000 description 8
- 239000002994 raw material Substances 0.000 description 8
- 238000002390 rotary evaporation Methods 0.000 description 8
- 238000005406 washing Methods 0.000 description 8
- QMNWYGTWTXOQTP-UHFFFAOYSA-N 1h-triazin-6-one Chemical compound O=C1C=CN=NN1 QMNWYGTWTXOQTP-UHFFFAOYSA-N 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 7
- 239000012043 crude product Substances 0.000 description 7
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 6
- 238000001035 drying Methods 0.000 description 6
- 230000008020 evaporation Effects 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- 239000011541 reaction mixture Substances 0.000 description 6
- 230000035484 reaction time Effects 0.000 description 6
- 238000000926 separation method Methods 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 6
- 241001477931 Mythimna unipuncta Species 0.000 description 5
- 244000061458 Solanum melongena Species 0.000 description 5
- 235000002597 Solanum melongena Nutrition 0.000 description 5
- 125000003118 aryl group Chemical group 0.000 description 5
- 229940126214 compound 3 Drugs 0.000 description 5
- 239000008187 granular material Substances 0.000 description 5
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 4
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 4
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 4
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 4
- 240000008042 Zea mays Species 0.000 description 4
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 4
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 235000005822 corn Nutrition 0.000 description 4
- 239000012065 filter cake Substances 0.000 description 4
- 239000005457 ice water Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- 239000003595 mist Substances 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- 239000004094 surface-active agent Substances 0.000 description 4
- PYOKUURKVVELLB-UHFFFAOYSA-N trimethyl orthoformate Chemical compound COC(OC)OC PYOKUURKVVELLB-UHFFFAOYSA-N 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- ANYWGXDASKQYAD-UHFFFAOYSA-N 5-nitroisoindole-1,3-dione Chemical compound [O-][N+](=O)C1=CC=C2C(=O)NC(=O)C2=C1 ANYWGXDASKQYAD-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 241000500437 Plutella xylostella Species 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- 229940125898 compound 5 Drugs 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 150000002170 ethers Chemical class 0.000 description 3
- 239000006260 foam Substances 0.000 description 3
- DDRPCXLAQZKBJP-UHFFFAOYSA-N furfurylamine Chemical compound NCC1=CC=CO1 DDRPCXLAQZKBJP-UHFFFAOYSA-N 0.000 description 3
- 125000002541 furyl group Chemical group 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 239000012442 inert solvent Substances 0.000 description 3
- 230000000749 insecticidal effect Effects 0.000 description 3
- 229960001669 kinetin Drugs 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 125000003226 pyrazolyl group Chemical group 0.000 description 3
- 125000000168 pyrrolyl group Chemical group 0.000 description 3
- 239000000376 reactant Substances 0.000 description 3
- 238000001953 recrystallisation Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000000391 smoking effect Effects 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 3
- 125000001544 thienyl group Chemical group 0.000 description 3
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 2
- VYELVDIXBRKOMJ-UHFFFAOYSA-N 1,1-dioxo-4h-1$l^{6},2,4-benzothiadiazin-7-amine Chemical compound N1=CNS(=O)(=O)C2=CC(N)=CC=C21 VYELVDIXBRKOMJ-UHFFFAOYSA-N 0.000 description 2
- GUMZPHOQHLZJOY-UHFFFAOYSA-N 1,3-oxazine-2,4-dione Chemical compound O=C1C=COC(=O)N1 GUMZPHOQHLZJOY-UHFFFAOYSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- SGUVLZREKBPKCE-UHFFFAOYSA-N 1,5-diazabicyclo[4.3.0]-non-5-ene Chemical compound C1CCN=C2CCCN21 SGUVLZREKBPKCE-UHFFFAOYSA-N 0.000 description 2
- JTLAIKFGRHDNQM-UHFFFAOYSA-N 1-bromo-2-fluoroethane Chemical compound FCCBr JTLAIKFGRHDNQM-UHFFFAOYSA-N 0.000 description 2
- FFBUYJAHSNPQDH-UHFFFAOYSA-N 2-ethyl-5-nitroisoindole-1,3-dione Chemical compound C1=C([N+]([O-])=O)C=C2C(=O)N(CC)C(=O)C2=C1 FFBUYJAHSNPQDH-UHFFFAOYSA-N 0.000 description 2
- LEPAHWUHXOWZCL-UHFFFAOYSA-N 5-amino-2-ethylisoindole-1,3-dione Chemical compound C1=C(N)C=C2C(=O)N(CC)C(=O)C2=C1 LEPAHWUHXOWZCL-UHFFFAOYSA-N 0.000 description 2
- MOBNCKURXDGQCB-UHFFFAOYSA-N 6-nitro-1h-quinazolin-4-one Chemical compound N1C=NC(=O)C2=CC([N+](=O)[O-])=CC=C21 MOBNCKURXDGQCB-UHFFFAOYSA-N 0.000 description 2
- IBAFKJJPXIXQEO-UHFFFAOYSA-N 7-nitro-2H-1,2,4-benzothiadiazine Chemical compound [N+](=O)([O-])C1=CC2=C(NC=NS2)C=C1 IBAFKJJPXIXQEO-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- ATRRKUHOCOJYRX-UHFFFAOYSA-N Ammonium bicarbonate Chemical compound [NH4+].OC([O-])=O ATRRKUHOCOJYRX-UHFFFAOYSA-N 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- CXXRMDFANWFMGM-UHFFFAOYSA-N CCN(C(C(C1=C2)=CC=C2NC(C2=CC(Br)=NN2C2=NC=CC=C2Cl)=O)=O)C1=O Chemical compound CCN(C(C(C1=C2)=CC=C2NC(C2=CC(Br)=NN2C2=NC=CC=C2Cl)=O)=O)C1=O CXXRMDFANWFMGM-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 239000004606 Fillers/Extenders Substances 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- 239000005909 Kieselgur Substances 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 241000500441 Plutellidae Species 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 150000001335 aliphatic alkanes Chemical class 0.000 description 2
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 2
- 239000001099 ammonium carbonate Substances 0.000 description 2
- 235000012501 ammonium carbonate Nutrition 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical compound BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 2
- 229910000024 caesium carbonate Inorganic materials 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- 229940125904 compound 1 Drugs 0.000 description 2
- 229940125782 compound 2 Drugs 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 239000012973 diazabicyclooctane Substances 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 239000000975 dye Substances 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 239000000417 fungicide Substances 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 235000010755 mineral Nutrition 0.000 description 2
- 239000005645 nematicide Substances 0.000 description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 description 2
- 239000012074 organic phase Substances 0.000 description 2
- 125000004043 oxo group Chemical group O=* 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000000575 pesticide Substances 0.000 description 2
- 235000021317 phosphate Nutrition 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 239000011736 potassium bicarbonate Substances 0.000 description 2
- 229910000028 potassium bicarbonate Inorganic materials 0.000 description 2
- 235000015497 potassium bicarbonate Nutrition 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- 235000011181 potassium carbonates Nutrition 0.000 description 2
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 125000000714 pyrimidinyl group Chemical group 0.000 description 2
- 125000006413 ring segment Chemical group 0.000 description 2
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 235000012239 silicon dioxide Nutrition 0.000 description 2
- 238000002791 soaking Methods 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 235000017550 sodium carbonate Nutrition 0.000 description 2
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 2
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 238000000967 suction filtration Methods 0.000 description 2
- 235000001508 sulfur Nutrition 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- 239000008399 tap water Substances 0.000 description 2
- 235000020679 tap water Nutrition 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 description 1
- FKTXDTWDCPTPHK-UHFFFAOYSA-N 1,1,1,2,3,3,3-heptafluoropropane Chemical group FC(F)(F)[C](F)C(F)(F)F FKTXDTWDCPTPHK-UHFFFAOYSA-N 0.000 description 1
- NDQXKKFRNOPRDW-UHFFFAOYSA-N 1,1,1-triethoxyethane Chemical compound CCOC(C)(OCC)OCC NDQXKKFRNOPRDW-UHFFFAOYSA-N 0.000 description 1
- FWLWTILKTABGKQ-UHFFFAOYSA-N 1-(bromomethyl)-3-methylbenzene Chemical compound CC1=CC=CC(CBr)=C1 FWLWTILKTABGKQ-UHFFFAOYSA-N 0.000 description 1
- GIGRWGTZFONRKA-UHFFFAOYSA-N 1-(bromomethyl)-4-methoxybenzene Chemical compound COC1=CC=C(CBr)C=C1 GIGRWGTZFONRKA-UHFFFAOYSA-N 0.000 description 1
- WZRKSPFYXUXINF-UHFFFAOYSA-N 1-(bromomethyl)-4-methylbenzene Chemical compound CC1=CC=C(CBr)C=C1 WZRKSPFYXUXINF-UHFFFAOYSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- SKATTZSUUSDLRS-UHFFFAOYSA-N 2-amino-5-nitrobenzenesulfonamide Chemical compound NC1=CC=C([N+]([O-])=O)C=C1S(N)(=O)=O SKATTZSUUSDLRS-UHFFFAOYSA-N 0.000 description 1
- VSBVFLVLPDTKPJ-UHFFFAOYSA-N 2-amino-6-nitrobenzamide Chemical compound NC(=O)C1=C(N)C=CC=C1[N+]([O-])=O VSBVFLVLPDTKPJ-UHFFFAOYSA-N 0.000 description 1
- GGKYLHNARFFORH-UHFFFAOYSA-N 2-amino-6-nitrobenzoic acid Chemical compound NC1=CC=CC([N+]([O-])=O)=C1C(O)=O GGKYLHNARFFORH-UHFFFAOYSA-N 0.000 description 1
- NAMYKGVDVNBCFQ-UHFFFAOYSA-N 2-bromopropane Chemical compound CC(C)Br NAMYKGVDVNBCFQ-UHFFFAOYSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- ZAJALNCZCSSGJC-UHFFFAOYSA-N 2-chloro-5-nitrobenzenesulfonamide Chemical compound NS(=O)(=O)C1=CC([N+]([O-])=O)=CC=C1Cl ZAJALNCZCSSGJC-UHFFFAOYSA-N 0.000 description 1
- AKCRQHGQIJBRMN-UHFFFAOYSA-N 2-chloroaniline Chemical compound NC1=CC=CC=C1Cl AKCRQHGQIJBRMN-UHFFFAOYSA-N 0.000 description 1
- OKDGRDCXVWSXDC-UHFFFAOYSA-N 2-chloropyridine Chemical compound ClC1=CC=CC=N1 OKDGRDCXVWSXDC-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- CVKOOKPNCVYHNY-UHFFFAOYSA-N 3-(bromomethyl)benzonitrile Chemical compound BrCC1=CC=CC(C#N)=C1 CVKOOKPNCVYHNY-UHFFFAOYSA-N 0.000 description 1
- 125000006284 3-fluorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(F)=C1[H])C([H])([H])* 0.000 description 1
- 125000004180 3-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C(F)=C1[H] 0.000 description 1
- HUDPLKWXRLNSPC-UHFFFAOYSA-N 4-aminophthalhydrazide Chemical compound O=C1NNC(=O)C=2C1=CC(N)=CC=2 HUDPLKWXRLNSPC-UHFFFAOYSA-N 0.000 description 1
- MMVIDXVHQANYAE-UHFFFAOYSA-N 5-nitro-2-benzofuran-1,3-dione Chemical compound [O-][N+](=O)C1=CC=C2C(=O)OC(=O)C2=C1 MMVIDXVHQANYAE-UHFFFAOYSA-N 0.000 description 1
- IWUDYSJCRIVVNK-UHFFFAOYSA-N 5-nitroisoindol-1-one Chemical compound [O-][N+](=O)C1=CC=C2C(=O)N=CC2=C1 IWUDYSJCRIVVNK-UHFFFAOYSA-N 0.000 description 1
- TWJZVXRMXVNSIE-UHFFFAOYSA-N 6-nitro-1h-quinazoline-2,4-dione Chemical compound N1C(=O)NC(=O)C2=CC([N+](=O)[O-])=CC=C21 TWJZVXRMXVNSIE-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- 230000002407 ATP formation Effects 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 229910014265 BrCl Inorganic materials 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- RNZSKDXNBTVALM-UHFFFAOYSA-N CC1=CC=C(CN(C(C(C2=C3)=CC=C3NC(C3=CC(Br)=NN3C3=NC=CC=C3Cl)=O)=O)C2=O)C=C1 Chemical compound CC1=CC=C(CN(C(C(C2=C3)=CC=C3NC(C3=CC(Br)=NN3C3=NC=CC=C3Cl)=O)=O)C2=O)C=C1 RNZSKDXNBTVALM-UHFFFAOYSA-N 0.000 description 1
- 229910021532 Calcite Inorganic materials 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 239000005886 Chlorantraniliprole Substances 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 244000060011 Cocos nucifera Species 0.000 description 1
- 235000013162 Cocos nucifera Nutrition 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 241000790917 Dioxys <bee> Species 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 241000255777 Lepidoptera Species 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- FVOOVCNBSCELQS-UHFFFAOYSA-N O=C(C1=CC(Br)=NN1C1=NC=CC=C1Cl)NC(C=C1C(N2CCF)=O)=CC=C1C2=O Chemical compound O=C(C1=CC(Br)=NN1C1=NC=CC=C1Cl)NC(C=C1C(N2CCF)=O)=CC=C1C2=O FVOOVCNBSCELQS-UHFFFAOYSA-N 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 239000004113 Sepiolite Substances 0.000 description 1
- 229930182692 Strobilurin Natural products 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000003905 agrochemical Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000004450 alkenylene group Chemical group 0.000 description 1
- 125000002877 alkyl aryl group Chemical group 0.000 description 1
- 150000008055 alkyl aryl sulfonates Chemical class 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- 150000008051 alkyl sulfates Chemical class 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 125000004419 alkynylene group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000001409 amidines Chemical class 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-N ammonia Natural products N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 229960000892 attapulgite Drugs 0.000 description 1
- 239000000987 azo dye Substances 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 150000001558 benzoic acid derivatives Chemical class 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- VEMKTZHHVJILDY-UXHICEINSA-N bioresmethrin Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OCC1=COC(CC=2C=CC=CC=2)=C1 VEMKTZHHVJILDY-UXHICEINSA-N 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- CODNYICXDISAEA-UHFFFAOYSA-N bromine monochloride Chemical compound BrCl CODNYICXDISAEA-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- PSOVNZZNOMJUBI-UHFFFAOYSA-N chlorantraniliprole Chemical compound CNC(=O)C1=CC(Cl)=CC(C)=C1NC(=O)C1=CC(Br)=NN1C1=NC=CC=C1Cl PSOVNZZNOMJUBI-UHFFFAOYSA-N 0.000 description 1
- 150000008280 chlorinated hydrocarbons Chemical class 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 229910052570 clay Inorganic materials 0.000 description 1
- 239000008199 coating composition Substances 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 229910000428 cobalt oxide Inorganic materials 0.000 description 1
- IVMYJDGYRUAWML-UHFFFAOYSA-N cobalt(ii) oxide Chemical compound [Co]=O IVMYJDGYRUAWML-UHFFFAOYSA-N 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 229910000365 copper sulfate Inorganic materials 0.000 description 1
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 1
- 239000006184 cosolvent Substances 0.000 description 1
- 125000006165 cyclic alkyl group Chemical group 0.000 description 1
- 125000005724 cycloalkenylene group Chemical group 0.000 description 1
- 125000002993 cycloalkylene group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001162 cycloheptenyl group Chemical group C1(=CCCCCC1)* 0.000 description 1
- 125000000596 cyclohexenyl group Chemical group C1(=CCCCC1)* 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000002433 cyclopentenyl group Chemical group C1(=CCCC1)* 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000010459 dolomite Substances 0.000 description 1
- 229910000514 dolomite Inorganic materials 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 239000003630 growth substance Substances 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 150000008282 halocarbons Chemical class 0.000 description 1
- 125000005347 halocycloalkyl group Chemical group 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 239000004009 herbicide Substances 0.000 description 1
- 125000001183 hydrocarbyl group Chemical group 0.000 description 1
- 150000003840 hydrochlorides Chemical class 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- DCYOBGZUOMKFPA-UHFFFAOYSA-N iron(2+);iron(3+);octadecacyanide Chemical compound [Fe+2].[Fe+2].[Fe+2].[Fe+3].[Fe+3].[Fe+3].[Fe+3].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-] DCYOBGZUOMKFPA-UHFFFAOYSA-N 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical class [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000004579 marble Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 125000004957 naphthylene group Chemical group 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 229910052754 neon Inorganic materials 0.000 description 1
- GKAOGPIIYCISHV-UHFFFAOYSA-N neon atom Chemical compound [Ne] GKAOGPIIYCISHV-UHFFFAOYSA-N 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N nitrogen dioxide Inorganic materials O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 238000013386 optimize process Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 229910052625 palygorskite Inorganic materials 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 125000006340 pentafluoro ethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 125000000843 phenylene group Chemical group C1(=C(C=CC=C1)*)* 0.000 description 1
- 239000001007 phthalocyanine dye Substances 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000002568 propynyl group Chemical group [*]C#CC([H])([H])[H] 0.000 description 1
- 229960003351 prussian blue Drugs 0.000 description 1
- 239000013225 prussian blue Substances 0.000 description 1
- 239000008262 pumice Substances 0.000 description 1
- 125000004309 pyranyl group Chemical group O1C(C=CC=C1)* 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 150000003217 pyrazoles Chemical class 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- TXQWFIVRZNOPCK-UHFFFAOYSA-N pyridin-4-ylmethanamine Chemical compound NCC1=CC=NC=C1 TXQWFIVRZNOPCK-UHFFFAOYSA-N 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 125000001567 quinoxalinyl group Chemical class N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000035806 respiratory chain Effects 0.000 description 1
- 229910052624 sepiolite Inorganic materials 0.000 description 1
- 235000019355 sepiolite Nutrition 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 235000010288 sodium nitrite Nutrition 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- ZRRGOUHITGRLBA-UHFFFAOYSA-N stattic Chemical compound [O-][N+](=O)C1=CC=C2C=CS(=O)(=O)C2=C1 ZRRGOUHITGRLBA-UHFFFAOYSA-N 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012956 testing procedure Methods 0.000 description 1
- 238000009210 therapy by ultrasound Methods 0.000 description 1
- 150000004897 thiazines Chemical class 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000000464 thioxo group Chemical group S=* 0.000 description 1
- 150000003852 triazoles Chemical class 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- UCPYLLCMEDAXFR-UHFFFAOYSA-N triphosgene Chemical compound ClC(Cl)(Cl)OC(=O)OC(Cl)(Cl)Cl UCPYLLCMEDAXFR-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052845 zircon Inorganic materials 0.000 description 1
- GFQYVLUOOAAOGM-UHFFFAOYSA-N zirconium(iv) silicate Chemical compound [Zr+4].[O-][Si]([O-])([O-])[O-] GFQYVLUOOAAOGM-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/58—1,2-Diazines; Hydrogenated 1,2-diazines
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/64—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with three nitrogen atoms as the only ring hetero atoms
- A01N43/707—1,2,3- or 1,2,4-triazines; Hydrogenated 1,2,3- or 1,2,4-triazines
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/80—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,2
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/88—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms six-membered rings with three ring hetero atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Dentistry (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Plant Pathology (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Pest Control & Pesticides (AREA)
- Agronomy & Crop Science (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
The invention relates to a pyrazole amide compound containing an aromatic heterocyclic structure, and a preparation method and application thereof. Specifically, the invention discloses a structure shown in formula (I), wherein the definition of each group and substituent is described in the specification; the invention also discloses a preparation method of the compound and application of the compound in the aspects of killing insects and mites.
Description
Technical Field
The invention relates to the technical field of agricultural chemicals and preparation thereof, in particular to a pyrazole amide compound containing an aromatic heterocyclic structure and a preparation method and application thereof.
Background
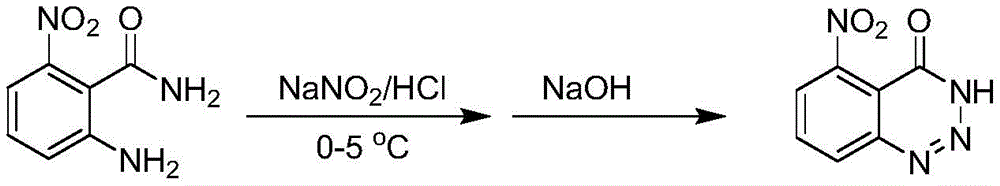
The 5-amidopyrazole insecticide is a medicament with high efficiency, wide insecticidal spectrum, wide application range and excellent effect, and has the functions of killing insects, inhibiting food, inhibiting spawning and sterilizing. The compounds act on a respiratory chain complex I to inhibit the transmission of electrons, the electron transmission is blocked, an electrochemical (proton) gradient cannot be established, ATP synthesis cannot be driven, ATP supply is insufficient, and insects, mites, bacteria and fungi die.
Disclosure of Invention
The invention aims to provide a pyrazole amide compound containing an aromatic heterocyclic structure shown in a formula I, a preparation method thereof and application thereof in the aspect of disinsection.
The invention provides a pyrazole amide compound containing an aromatic heterocyclic structure, an optical isomer, a cis-trans isomer or an agriculturally and pharmaceutically acceptable salt thereof, which is characterized in that the compound has a structure shown in a formula I,
wherein,
R 1 selected from the group consisting of substituted or unsubstituted:C 1-6 alkyl radical, C 3-6 Cycloalkyl, C 2-6 Alkenyl radical, C 2-6 Alkynyl, C 1-8 Alkoxy, hydrogen, phenyl, naphthalenyl, 5-6 membered heteroaryl, amino, hydroxyl, amido, acyloxy, carboxymethyl, nitro, cyano, sulfonic, halogen, formyl, acyl, carboxyl; by substituted is meant substituted with one or more (e.g., 2,3,4, or 5) substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amido, acyloxy, C 3-6 Cycloalkyl radical, C 3-6 Halogenocycloalkyl, C 2-6 Alkenyl radical, C 2-6 Haloalkenyl, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 A cycloalkenyl group;
R 2 selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl radical, C 3-6 Cycloalkyl radical, C 2-6 Alkenyl radical, C 2-6 Alkynyl, C 1-6 Alkoxy, phenyl, hydrogen, amino, hydroxyl, amide, acyloxy, carboxymethyl, nitro, cyano, sulfonic, halogen, formyl, acyl, carboxyl; the substitution refers to substitution by one or more halogens;
a is selected from substituted or unsubstituted phenyl, substituted or unsubstituted naphthalene cyclyl, substituted or unsubstituted 4-8 membered heteroaryl, substituted or unsubstituted C 8-14 A heteroaromatic bicyclic or tricyclic ring system; by substituted is meant substituted with one or more (e.g., 2,3,4, or 5) substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amido, acyloxy, C 3-6 Cycloalkyl radical, C 3-6 Halogenocycloalkyl, C 2-6 Alkenyl radical, C 2-6 Halogenated alkenyl group, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, phenyl, naphthyl;
b is an unsaturated or saturated 5-6 membered nitrogen containing heterocyclic group, wherein said heterocyclic group contains at least one N heteroatom and 0-2 heteroatoms selected from N, O or S;
n is selected from 0 or 1;
y is selected from the group consisting of: hydrogen, C 1-6 Alkoxy radical, C 1-8 Alkyl radical, C 1-6 Haloalkyl, C 3-6 Cycloalkyl radical, C 3-6 Halogenocycloalkyl, C 2-6 Alkenyl radical, C 2-6 Halogenated alkenyl group, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, substituted or unsubstituted phenyl, substituted or unsubstituted naphthalenylcyclyl, substituted or unsubstituted 4-8 membered heteroaryl, substituted or unsubstituted C 8-14 A heteroaromatic bicyclic or tricyclic ring system, or a 3-7 membered heterocyclyl; by substituted is meant substituted with one or more (e.g., 2,3,4, or 5) substituents selected from the group consisting of: c 1-6 Alkyl radical, C 2-6 Alkenyl, halogen, C 1-6 Haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy, C 3-6 Cycloalkyl radical, C 3-6 Halogenocycloalkyl, C 2-6 Haloalkenyl, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, phenyl, naphthyl.
In another preferred embodiment, R 1 Selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl radical, C 2-6 Alkenyl radical, C 1-6 Alkoxy, phenyl, 5-6 membered heteroaryl, hydrogen, amino, amido, halogen, formyl, acyl, carboxy; said substitution means substitution with one or more (e.g. 2,3,4 or 5) substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amide, acyloxy;
R 2 selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl radical, C 3-5 Cycloalkyl radical, C 2-5 Alkenyl radical, C 2-6 Alkynyl, C 1-6 Alkoxy, halogen; the substitution refers to substitution by one or more halogens;
a is selected from substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution by one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amido, acyloxy, C 3-6 Cycloalkyl radical, C 3-6 Halocycloalkyl radical, C 2-6 Alkenyl radical, C 2-6 Haloalkenyl, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, phenyl, naphthyl;
b is an unsaturated 5-6 membered nitrogen containing heterocyclyl wherein said heterocyclyl contains at least one N heteroatom and 0-2 heteroatoms selected from N, O or S;
y is selected from the group consisting of: hydrogen, C 1-6 Alkoxy radical, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 3-6 Cycloalkyl, C 2-6 Alkenyl radical, C 2-6 Alkynyl, substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: c 1-6 Alkyl radical, C 2-6 Alkenyl, halogen, C 1-6 Haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy, C 3-6 Cycloalkyl, C 3-6 Halogenocycloalkyl, C 2-6 Haloalkenyl, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, phenyl, naphthyl.
In another preferred embodiment, B is a 5-or 6-membered nitrogen-containing heterocyclic group containing 1-3N heteroatoms and 0-2S heteroatoms in the ring.
In another preferred embodiment, B is a compound containing 1-3 oxo (= O), thioxo (= S), or dioxy (e.g. having two oxo groups on S, forming S (O) 2 ) A substituted nitrogen-containing heterocyclic group.
In another preferred embodiment, B is a 5-6 membered nitrogen containing heterocyclyl, wherein said heterocyclyl contains 1,2 or 3N heteroatoms and 0-2 heteroatoms selected from N, O or S, and the number of ring heteroatoms in said heterocyclyl is 3 or less (i.e., 1,2, 3).
In another preferred embodiment, said 3-7 membered heterocyclyl contains 1-3 heteroatoms selected from N, O and S.
In another preferred embodiment, B is through the ring N atom with- (CH) 2 ) n Y being linked, i.e. forming
In another preferred embodiment, B is selected from the group consisting of:
in another preferred embodiment, R 1 Selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl, phenyl, 5-6 membered heteroaryl, hydrogen, amino, amido, halo; the substitution means substitution with one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl;
R 2 selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl radical, C 1-6 Alkoxy radical, C 2-5 Alkenyl, halogen; the substitution refers to substitution by one or more halogens;
a is selected from substituted or unsubstituted phenyl; the substitution means substitution with one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, cyano, C 1-6 Haloalkyl, amide, acyloxy;
b is selected from the following group:
y is selected from the group consisting of: hydrogen, C 1-6 Alkoxy radical, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 2-6 Alkenyl, substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: c 1-6 Alkyl radical, C 2-6 Alkenyl, halogen, C 1-6 Haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy.
In another preferred embodiment, R 1 Selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl, phenyl, 5-6 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl;
R 2 selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl, halogen; said substitution refers to substitution by one or more halogens;
y is selected from the group consisting of: hydrogen, C 1-6 Alkyl radical, C 1-6 Haloalkyl, substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: c 1-6 Alkyl radical, C 2-6 Alkenyl, halogen, C 1-6 Haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy.
In another preferred embodiment, R 1 Selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl, phenyl, pyridyl; said substitution refers to substitution by one or more halogens;
R 2 selected from halogen and C 1-5 A haloalkyl group.
In another preferred embodiment, R 1 Selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl radical, C 3-6 Cycloalkyl radical, C 2-5 Alkenyl radical, C 2-5 Alkynyl, C 1-4 An alkoxy group; the substitution means substitution with one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amido, acyloxy.
In another preferred embodiment, R 1 Selected from the group consisting of substituted or unsubstituted: phenyl, 5-6 membered heteroaryl, hydrogen, amino, nitro, cyano, sulfonic acid, halogen(ii) a The substitution refers to substitution by one or more halogens.
In another preferred embodiment, R is 1 Is a substituted phenyl ring or a substituted 5-6 membered heteroaryl.
In another preferred embodiment, R is 1 Is a halogenated benzene ring or a halogenated 5-6 membered heteroaryl.
In another preferred embodiment, the 5-6 membered heteroaryl is a 6 membered heteroaryl containing 1-2N heteroatoms, preferably pyridinyl.
In another preferred embodiment, the 5-6 membered heteroaryl is pyridyl, preferably with the N atom of the ring of the pyridyl in the ortho position.
In another preferred embodiment, C is 1-6 The alkyl group is a methyl group.
In another preferred embodiment, the 5-6 membered heteroaryl is substituted with a halogen, such as: 2-chloropyridine.
In another preferred embodiment, R 2 Selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl radical, C 3-6 Cycloalkyl radical, C 2-6 Alkenyl, phenyl, hydrogen, amino, hydroxyl, amido, nitro, cyano, halogen; the substitution refers to substitution by one or more halogens.
In another preferred embodiment, C is 1-6 Alkyl is halo-substituted, such as difluoromethyl.
In another preferred embodiment, the halogen is Br.
In another preferred embodiment, R 2 Selected from Br or difluoromethyl.
In another preferred embodiment, a is selected from the group consisting of substituted or unsubstituted: phenyl, pyridyl, pyrimidinyl, pyrazolyl, furanyl, thienyl, pyrrolyl; the substitution is selected from one or moreSubstituent group substitution: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl radical, C 3-6 Cycloalkyl, C 2-6 Alkenyl radical, C 2-6 Alkynyl, halo C 1-6 Alkyl, amino, nitro.
In another preferred embodiment, Y is selected from the group consisting of: hydrogen, C1-6 alkoxy, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution refers to substitution by one or more substituents selected from the group consisting of: c1-6 alkyl, C2-6 alkenyl, halogen, C1-6 haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy.
In another preferred embodiment, Y is selected from the group consisting of: hydrogen, C1-6 alkyl, C1-6 haloalkyl, substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: c1-6 alkyl, halogen, C1-6 haloalkyl, cyano, amino, nitro, carboxyl.
In another preferred embodiment, Y is a substituted or unsubstituted group selected from phenyl, pyridyl, pyrazolyl, furyl, thienyl, pyrrolyl; the substitution means substitution with one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl radical, C 3-6 Cycloalkyl, C 2-6 Alkenyl radical, C 2-6 Alkynyl, halo C 1-6 Alkyl, amino, nitro.
In another preferred embodiment, Y is selected from the group consisting of: CH (CH) 3 、CH 2 CH 3 、CH 2 CF 3 、CH 2 CH 2 CH 3 、CH 2 CH 2 CF 3 、CH(CH 3 ) 2 、CH 2 CH 2 CH 2 CH 3 、CH 2 CH(CH 3 ) 2 、CH 2 CH 2 CH 2 CH 2 CH 3 、CH 2 CH(CH 3 )CH 2 CH 3 、CH 2 C(CH 3 ) 3 。
In another preferred embodiment, B is selected from the group consisting of:
in another preferred embodiment, ring A, ring B, R1, R2 and Y are each independently the corresponding groups in compounds 1-423.
In another preferred embodiment, said compound of formula I is selected from compounds 1 to 423 in table 1.
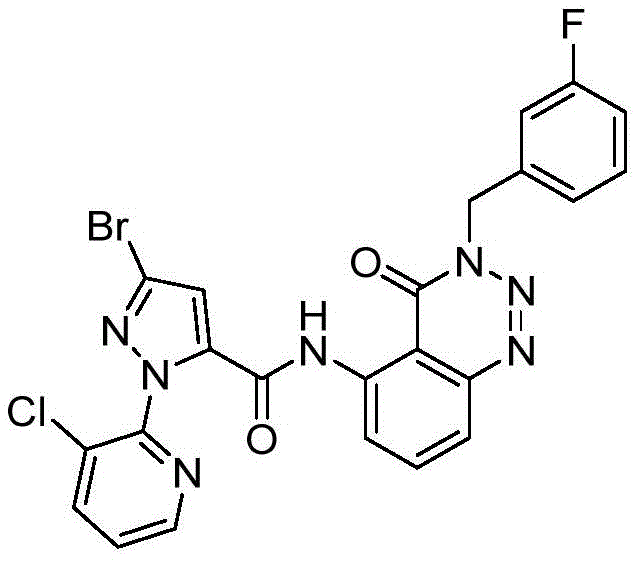
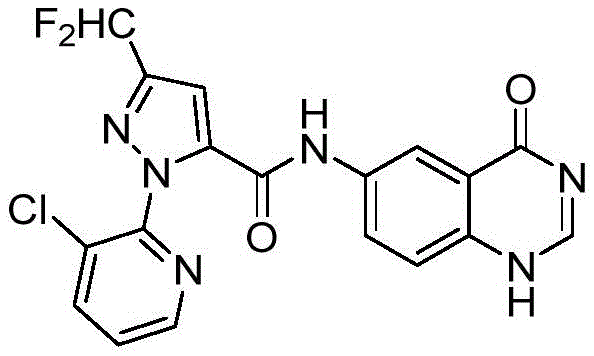
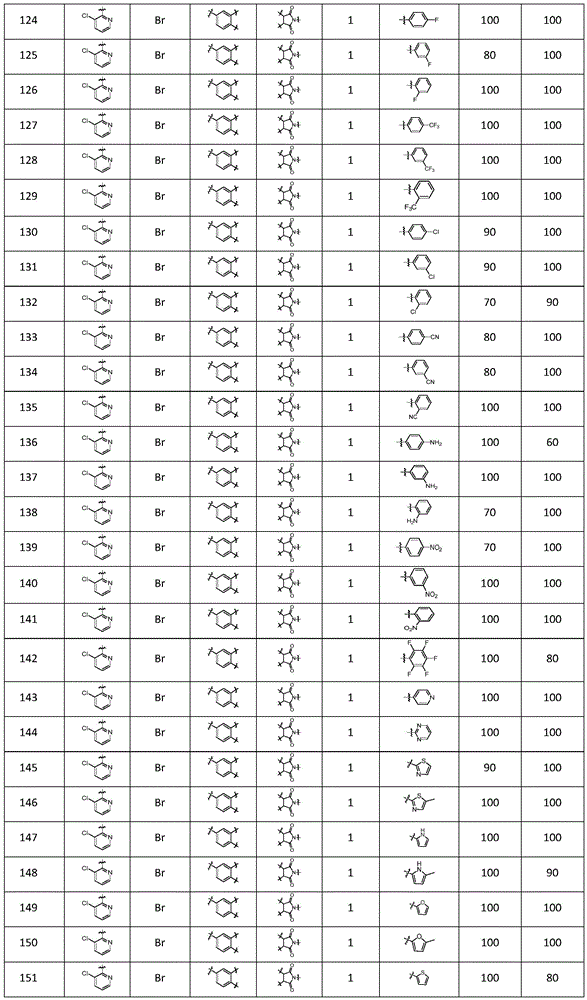
In another preferred embodiment, the compound is selected from the following numbered compounds in table 1: 1.2, 3,4, 5,13, 14,15, 16, 17, 18, 19, 20, 21, 22, 23, 38, 39, 45, 49, 52, 61, 65, 66, 68, 70, 71, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 296, 297, 298, 299, 300, 301, 302, 303, 304, 305, 306, 309, 310, 311, 312, 313, 314, 325, 326, 327, 328, 329, 330, 331, 332, 333, 334, 340, 341, 342, 343, 363, 364, 365, 366, 367, 368, 369, 370, 371, 372, 373, 375, 376, 377, 378, 379, 380, 381, 382, 383, 384, 385, 386, 387, 388, 389, 390, 391, 392, 393, 394, 395, 396.
In a second aspect of the present invention, there is provided a pesticidal composition comprising:
1) 0.001 to 99.99% by weight of a compound of claim 1, an optical isomer, a cis-trans isomer, or an agriculturally pharmaceutically acceptable salt thereof, or a combination thereof; and
2) An agriculturally pharmaceutically acceptable carrier and/or excipient.
In a third aspect of the present invention, there is provided a use of the compound of claim 1, an optical isomer, a cis-trans isomer, or an agriculturally pharmaceutically acceptable salt thereof for controlling lepidopteran pests; or for preparing insecticides and/or acaricides for controlling lepidopteran pests.
In another preferred example, the pests are armyworm and diamondback moth.
In a fourth aspect of the invention, there is provided a method of combating and/or killing pests which comprises applying to a plant suffering from a pest infestation, the soil surrounding or to the environment a compound according to claims 1 to 7 or an agriculturally acceptable salt thereof or a pesticidal composition according to claim 8.
In another preferred embodiment, the aromatic heterocyclic structure-containing pyrazole amide compound or the agriculturally and pharmaceutically acceptable salt thereof or the pesticide composition is applied at a concentration of 0.05 to 200ppm; preferably, 0.1 to 100ppm; more preferably, it is 0.5 to 50ppm.
It is to be understood that within the scope of the present invention, the above-described features of the present invention and those specifically described below (e.g., in the examples) may be combined with each other to form new or preferred embodiments. Not to be reiterated herein, but to the extent of space.
Detailed Description
The inventor of the invention has long and intensive research and discovers and synthesizes a series of pyrazole amide compounds containing aromatic heterocyclic structures, which have novel structures and remarkable insecticidal effects. On this basis, the inventors have completed the present invention.
Term(s) for
In the present invention, unless otherwise specified, the terms used have the ordinary meaning known to those skilled in the art.
The term "C 1-6 Alkyl "means a straight or branched chain alkyl group having 1 to 6 carbon atoms, such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl orLike groups.
The term "C 2-6 The alkenyl group "means a straight-chain or branched alkenyl group having 2 to 6 carbon atoms, such as vinyl, allyl, 1-propenyl, isopropenyl, 1-butenyl, 2-butenyl or the like.
The term "C 2-6 Alkynyl "means a straight or branched chain alkynyl group having 2 to 6 carbon atoms, such as ethynyl, propynyl or the like.
The term "C 3-6 Cycloalkyl "refers to a cyclic alkyl group having 3 to 6 carbon atoms, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, or the like.
The term "C 5-7 Cycloalkenyl "means a cyclic alkenyl group having 5 to 7 carbon atoms with one or more double bonds, such as cyclopentenyl, cyclohexenyl, cycloheptenyl, 1, 3-cyclohexadienyl, 1, 4-cyclohexadienyl or the like.
As used herein, the term "C 1-8 Alkoxy "means a straight or branched chain alkoxy group having 1 to 8 carbon atoms, such as methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy, tert-butoxy or the like.
The term "halogen" refers to fluorine, chlorine, bromine, or iodine. The term "halogenated" refers to a group substituted with one or more of the above halogen atoms, which may be the same or different, such as trifluoromethyl, pentafluoroethyl, heptafluoroisopropyl, or the like.
The term "heterocycle" means that at least one of the atoms forming the skeleton of the heterocycle is not carbon, and is nitrogen, oxygen or sulfur. Typically, the heterocyclic ring contains no more than 4 nitrogens, no more than 2 oxygens, and/or no more than 2 sulfurs. Unless otherwise indicated, the heterocyclic ring may be a saturated, partially unsaturated, or fully unsaturated ring.
The term "alkyl" refers to a group of an alkane molecule lacking one hydrogen atom; the term "alkylene" refers to a group of an alkane molecule lacking two hydrogen atoms. Similarly, "alkenylene", "alkynylene", "cycloalkylene", "cycloalkenylene", "phenylene", "naphthylene", "heterocyclylene" or "heteroarylene bicyclic or tricyclic ring system" are defined analogously.
The term "aryl" denotes a hydrocarbyl moiety comprising one or more aromatic rings. For example, the term "C 6-10 Aryl "refers to an aromatic ring group having 6 to 10 carbon atoms, such as phenyl, naphthyl, and the like, which does not contain heteroatoms in the ring.
The term "heteroaryl" denotes a heteroaromatic system containing 1 to 4 heteroatoms including nitrogen, oxygen and S (O) r (where r is an integer 0,1, 2), e.g., 4-8 membered heteroaryl refers to a heteroaromatic system containing 4-8 ring atoms, 4-10 membered heteroaryl refers to a heteroaromatic system containing 4-10 ring atoms, including but not limited to pyrrolyl, furanyl, thienyl, pyrazolyl, thiazolyl, imidazolyl, oxazolyl, isoxazolyl, pyridyl, pyranyl, pyridazinyl, pyrimidinyl, pyrazinyl, benzimidazolyl, triazolyl, and the like.
Unless specifically stated to be "substituted or unsubstituted", the groups of the present invention may be substituted with a substituent selected from the group consisting of: halogen, acyloxy, cyano, amino, nitro, carboxyl, amido, carboxymethyl, C 1-6 Alkyl radical, C 1-6 Alkoxy radical, C 1-6 Haloalkyl, C 2-6 Alkenyl radical, C 2-6 Halogenated alkenyl group, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, hydroxy, C 3-6 Cycloalkyl radical, C 3-6 Halocycloalkyl, hydroxy C 1-4 Alkyl radical, C 5-7 Cycloalkenyl, phenyl, naphthyl, and the like.
The inert solvent refers to various solvents which do not react with the raw materials, including various straight, branched or cyclic alcohols, ethers or ketones, alkyl halides, 1, 4-dioxane, acetonitrile, tetrahydrofuran, N, N-Dimethylformamide (DMF), dimethyl sulfoxide (DMSO), etc.
The term "agriculturally pharmaceutically acceptable salt" means that the anion of the salt is known and acceptable in forming a pharmaceutically acceptable salt of the nematicide. Preferably, the salt is water soluble. Suitably, the acid addition salts formed by the compounds of formula (I) include salts formed with inorganic acids, such as hydrochlorides, phosphates, sulphates, nitrates; and salts formed with organic acids such as acetates, benzoates, and the like.
The term "optical isomer" means that the chiral carbon atom involved in the compound of the present invention may be in the R configuration, or may be in the S configuration, or a combination thereof. The compounds of the invention may contain one or more asymmetric centers and thus occur as racemates, racemic mixtures, single enantiomers, diastereomeric compounds and individual diastereomers. Asymmetric centers that may be present depend on the nature of the various substituents on the molecule. Each such asymmetric center will independently produce two optical isomers and all possible optical isomers and diastereomeric mixtures and pure or partially pure compounds are included within the scope of the invention. The present invention includes all isomeric forms of the compounds.
Process for the preparation of the compounds of the invention
The compound represented by the general formula of the present invention can be produced by the following method, however, the conditions of the method, such as reactants, solvent, base, amount of the compound used, reaction temperature, time required for the reaction, etc., are not limited to the following explanation. The compounds of the present invention may also be conveniently prepared by optionally combining various synthetic methods described in the present specification or known in the art, and such combinations may be readily carried out by those skilled in the art to which the present invention pertains. Reagents may be purchased commercially if feasible.
Typical embodiments of the compounds of the present invention may be synthesized using the following general reaction schemes. It is clear from the description given herein that the general scheme can be modified by substituting other materials with similar structures to obtain correspondingly different products. Synthetic methods can be used as needed to provide large scale production. The starting materials can be obtained commercially or synthesized using published methods. The examples given herein, through simple testing procedures, the characteristics of the final product often make the characteristics of the necessary starting materials apparent.
Synthesis reaction parameters the compounds of the present invention may be prepared from readily available starting materials using, for example, the following general methods and procedures. It will be appreciated that where typical or optimized process conditions (i.e., reaction temperatures, times, molar ratios of reactants, solvents, catalysts, pressures, etc.) are given, other process conditions may also be used, unless otherwise indicated. Optimal reaction conditions may vary with the particular reactants or solvents used, but such conditions may be determined by one skilled in the art by routine optimization procedures.
The starting materials for the following reactions are generally known compounds or can be prepared by known procedures or obvious modifications thereof. For example, many of the starting materials are available from commercial suppliers, others may be prepared by procedures described in the text of standard references or obvious modifications.
In the production process of the present invention, each reaction is usually carried out in an inert solvent at a reaction temperature of-20 to 120 ℃ (preferably 50 to 100 ℃ or 120 to 180 ℃). The reaction time is usually 2 to 24 hours, preferably 4 to 18 hours, and the reaction time can be appropriately extended according to the reaction requirement, and the specific reaction time is determined according to the degree of reaction.
Typically, bases used in the reactions of the present invention include (but are not limited to): triethylamine, diisopropylethylamine, diethylamine, piperidine, piperazine, morpholine, N-methylmorpholine, triethylenediamine (DABCO), 1, 8-diazabicyclo [5.4.0] undec-7-ene (DBU), 1, 5-diazabicyclo [4.3.0] non-5-ene (DBN), potassium carbonate, potassium bicarbonate, sodium carbonate, sodium bicarbonate, cesium carbonate, sodium hydroxide, potassium hydroxide, sodium methoxide, sodium ethoxide, or a combination thereof.
The invention provides a preparation method of the pyrazole amide compound containing the aromatic heterocyclic structure, which comprises the following steps:
heating and refluxing the compound Z1 and the compound Z2 under an alkaline condition to react to obtain a compound 3; reducing nitro on the compound 3 to obtain a compound 4; heating and refluxing the compound 4 and the compound 5 for reaction to obtain a final compound, namely the pyrazole amide compound containing the aromatic heterocyclic structure.
Wherein, the compound 1 has the following structural formula:
the structural formula of the compound 2 is as follows:
the structural formula of the compound 3 is as follows:
the structural formula of the compound 4 is as follows:
the structural formula of the compound 5 is as follows:
the structural formula of the final compound is as follows:
A. b, n and Y are defined as the same as the above.
In another preferred embodiment, the preparation method of the invention comprises the following steps:
the method comprises the following steps: compound 1 was added to an eggplant-shaped bottle, and DMF was added to the eggplant-shaped bottle to dissolve it. Sequentially adding the compound 2 and triethylamine, heating to 90 ℃, and carrying out reflux reaction for 5 hours. The mixture was then poured into ice water and a precipitate precipitated out. The resulting precipitate was separated by filtration, washed with excess water and dried at 70-75 deg.C to give a crude product which was purified by ethanol recrystallization to give compound 3.
Step two: adding compound 3, iron powder, ammonium chloride, ethanol and water (E) into eggplant-shaped bottle t OH:H 2 O = 2.5. Heated to 75 ℃ and refluxed for 0.5h. Filtering with diatomite to obtain filtrate. And (4) removing ethanol and water by rotary evaporation to obtain a solid. Separating and purifying by column chromatography (eluent: 5-10% methanol/dichloromethane), and evaporating the solvent to obtain a compound 4.
Step three: adding compound 4, compound 5, N-methylimidazole and N, N, N ', N' -tetramethyl chlorourea hexafluorophosphate into an eggplant-shaped bottle, and dissolving the mixture in acetonitrile. Heating to 60 ℃, refluxing for 6h, and detecting the completion of the reaction by TLC. The mixture was poured into water and a precipitate precipitated out. The precipitate was filtered and washed with excess water to give a crude solid. Separating and purifying by column chromatography (eluent: 15% -25% methanol/dichloromethane), evaporating the solvent to dryness to obtain the final product, namely the pyrazole amide compound containing aromatic heterocyclic structure,
in the production process of the present invention, each reaction is usually carried out in an inert solvent at a reaction temperature of-20 to 120 ℃ (preferably 50 to 100 ℃ or 120 to 180 ℃). The reaction time is usually 2 to 24 hours, preferably 4 to 18 hours, and the reaction time can be appropriately extended according to the reaction requirement, and the specific reaction time is determined according to the degree of the reaction.
The inert gas includes at least one of nitrogen, helium, neon, or argon.
Bases used in the reaction include (but are not limited to): triethylamine, diisopropylethylamine, diethylamine, piperidine, piperazine, morpholine, N-methylmorpholine, triethylenediamine (DABCO), 1, 8-diazabicyclo [5.4.0] undec-7-ene (DBU), 1, 5-diazabicyclo [4.3.0] non-5-ene (DBN), potassium carbonate, potassium bicarbonate, sodium carbonate, sodium bicarbonate, cesium carbonate, sodium hydroxide, potassium hydroxide, sodium methoxide, sodium ethoxide, or a combination thereof.
Composition comprising a metal oxide and a metal oxide
The active substances according to the invention can be prepared in a customary manner to give pesticide compositions. These active compounds can be formulated in the customary formulations, for example as solutions, emulsions, suspensions, powders, foams, pastes, granules, aerosols, natural and synthetic materials impregnated with active substance, microcapsules in polymers, coating compositions for seeds, and formulations for use with combustion devices, for example smoking cartridges, smoking pots and smoking trays, and ULV Cold mist (Cold mist) and hot mist (Warm mist) formulations.
These formulations can be produced in a known manner, for example by mixing the active compounds with extenders, that is liquid or liquefied gas or solid diluents or carriers, and optionally with surfactants, that is emulsifiers and/or dispersants and/or foam formers. Organic solvents may also be used as adjuvants, for example when water is used as extender.
When liquid solvents are used as diluents or carriers, it is basically suitable, for example: aromatic hydrocarbons such as xylene, toluene or alkylnaphthalene; chlorinated aromatic or chlorinated aliphatic hydrocarbons, such as chlorobenzene, vinyl chloride or dichloromethane; aliphatic hydrocarbons, such as cyclohexane or paraffins, for example mineral oil fractions; alcohols, such as ethanol or ethylene glycol and their ethers and lipids; ketones such as acetone, methyl ethyl ketone, methyl isobutyl ketone or cyclohexanone; or less commonly used polar solvents such as dimethylformamide and dimethylsulfoxide, and water.
Liquid gas diluents or carriers refer to liquids that will become gases at normal temperature and pressure, such as aerosol propellants, such as halogenated hydrocarbons, as well as butane, propane, nitrogen, and carbon dioxide.
The solid carrier may be a finely divided natural mineral such as kaolin, clay, talc, quartz, attapulgite, montmorillonite or diatomaceous earth; and ground synthetic minerals such as highly dispersed silicic acid, alumina and silicates. Solid carriers for granules are crushed and classified natural zircon, such as calcite, marble, pumice, sepiolite and dolomite, as well as synthetic granules of inorganic and organic coarse powders, and granules of organic materials, such as sawdust, coconut shells, corn cobs and tobacco stalks, and the like.
Nonionic and anionic emulsifying trains may be used as emulsifiers and/or foam formers. Such as polyoxyethylene-fatty acid esters, polyoxyethylene-fatty alcohol ethers, such as alkylaryl polyethylene glycol ethers, alkyl sulfonates, alkyl sulfates, aryl sulfonates and albumin hydrolysates. The dispersant comprises lignin sulfite waste liquor and methyl cellulose.
Binders such as carboxymethylcellulose and natural and synthetic polymers in the form of powders, granules or emulsions, for example gum arabic, polyvinyl alcohol and polyvinyl acetate, can be used in the formulations.
Colorants such as inorganic dyes, e.g., iron oxide, cobalt oxide, and prussian blue; organic dyes such as azo dyes or metal phthalocyanine dyes; and with trace nutrients such as salts of iron, manganese, boron, copper, cobalt, aluminum, and zinc, and the like.
The active compounds according to the invention can be present in their commercial preparations in a mixture with other active compounds, such as insecticides, fungicides, herbicides, growth regulators, etc., or in the use forms prepared from these preparations. Insecticides include, for example, phosphates, carbamates, pyrethroids, chlorinated hydrocarbons, substances produced by microorganisms, and the like, fungicides include strobilurins, amides, triazoles, and the like, and miticides include quinoxalines, amidines, organosulfurs, organotins, thiazines, and the like.
Furthermore, the active compounds according to the invention can also be present in their commercial preparations in a mixture with synergists, i.e. compounds which increase the activity of the compounds, which are not necessarily added, since the active compounds themselves are active.
These formulations generally contain from 0.001 to 99.99% by weight, preferably from 0.01 to 99.9% by weight, more preferably from 0.05 to 90% by weight, of the active compound according to the invention, based on the total weight of the nematicide composition. The concentration of the active compound in the commercial preparations or dosage forms used can vary within wide limits. The concentration of active compound in the dosage form to be used can be from 0.0000001 to 100% (g/v), preferably between 0.0001 and 1% (g/v).
Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. In addition, any methods and materials similar or equivalent to those described herein can be used in the methods of the present invention. The preferred embodiments and materials described herein are intended to be exemplary only.
Compared with the prior art, the invention has the following main advantages:
the compound has excellent insecticidal activity and can be used for controlling lepidoptera pests, particularly armyworms and diamondback moths.
The invention will be further illustrated with reference to the following specific examples. It should be understood that these examples are for illustrative purposes only and are not intended to limit the scope of the present invention. Experimental procedures without specific conditions noted in the following examples, generally according to conventional conditions, or according to conditions recommended by the manufacturer. Unless otherwise indicated, percentages and parts are by weight.
Examples
Example 1 preparation of N- (2-ethyl-1, 3-dioxo-5-isoindolyl) -3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxamide
Step 1: synthesis of 2-ethyl-5-nitroisoindoline-1, 3-dione
In a 100mL eggplant type bottle, 5-nitrophthalimide (10.00 mmol) was added, 40mL of N, N-dimethylformamide was added to dissolve, and bromoethane (20.00 mmol) and triethylamine (TEA, 30.00 mmol) were added to the eggplant type bottle in this order. The reaction mixture was then poured into ice water and the precipitate precipitated out after heating to 90 ℃ and refluxing for 5h. Filtering and separating to obtain precipitate, washing the precipitate with excessive water, drying at 70-75 ℃, and recrystallizing and purifying the obtained crude product with ethanol to obtain 2.00g of white solid compound with the yield of 91%.
Step 2: synthesis of 2-ethyl-5-aminoisoindoline-1, 3-dione
2-Ethyl-5-nitroisoindoline-1, 3-dione (8 mmol) obtained in step 1, iron powder (40 mmol) and ammonium chloride (24 mmol) were added to a 100mL eggplant-shaped flask, and 30mL ethanol and 12mL water were added to dissolve. Heating to 75 deg.C, reflux reacting for 0.5h, filtering with diatomite, and collecting filtrate. Ethanol and water were removed by rotary evaporation to give a solid, which was purified by column chromatography (eluent: 10% methanol/dichloromethane), and the solvent was evaporated to dryness to give 1.22g of a yellow solid product in 80.5% yield.
And step 3: synthesis of N- (2-ethyl-1, 3-dioxo-5-isoindolyl) -3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxamide
In a 100mL round-bottom flask, 2-ethyl-5-aminoisoindoline-1, 3-dione (5 mmol) obtained in step 2, 3-bromo-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid (10 mmol), N-methylimidazole (35 mmol), and N, N, N ', N' -tetramethylchlorourea hexafluorophosphate (12 mmol) were added and dissolved in 40mL acetonitrile. Heating to 60 ℃, refluxing for 6h, and detecting the completion of the reaction by TLC. The reaction mixture was poured into water and a precipitate precipitated out. Filtering and separating to obtain precipitate, and washing the precipitate with excessive water to obtain solid crude product. Separation and purification by column chromatography (eluent: 20% methanol/dichloromethane) and evaporation of the solvent afforded the product as a white solid, 1.21g, 51% yield.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.05(s,1H),8.55-8.54(m,1H),8.27-8.25(m,1H),8.12(d,J=1.7Hz,1H),7.98-7.96(m,1H),7.84(d,J=8.2Hz,1H),7.69-7.66(m,1H),7.51(s,1H),3.65-3.52(m,2H),1.14(t,J=7.1Hz,3H). 13 c NMR (101MHz, DMSO). Delta.167.25, 167.20,155.97,148.09,147.28,143.24,139.62,139.02,133.06,127.64,126.86,126.83,126.62,124.42,124.11,113.75,111.42,32.37,13.60 HRMS (ESI-TOF) molecular formula C 19 H 13 BrClN 5 O 3 :
[M-H] - Calculated relative molecular mass:471.9818, found 471.9809.
Example 2: preparation of N- (2- (2-fluoroethyl) -1, 3-dioxo-5-isoindolyl) -3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 1 was used, except that:
the raw material bromoethane in the step 1 adopts 1-bromo-2-fluoroethane.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.07(s,1H),8.56-8.54(m,1H),8.27-8.24(m,1H),8.14(d,J=0.9Hz,1H),8.00-7.98(m,1H),7.87(d,J=8.2Hz,1H),7.69-7.65(m,1H),7.53(s,1H),4.42(m,2H),3,83(t,2H). 13 C NMR(101MHz,DMSO)δ167.23,166.85,155.87,148.69,147.18,143.44,139.52,139.12,133.03,127.94,126.76,126.63,126.42,124.72,124.01,113.55,111.42,60.26,44.01. 19 f NMR (376 MHz, DMSO) delta-60.02 HRMS (ESI-TOF) with a molecular formula of C 19 H 12 BrClFN 5 O:[M-H] - Calculated relative molecular mass: 489.9723, found 489.9725.
Example 3: preparation of N- (2- (4-methylbenzyl) -1, 3-dioxo-5-isoindolyl) -3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 1 was used, except that:
example 1 the starting material, ethyl bromide in step 1, was 4-methylbenzyl bromide.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.07(s,1H),8.56-8.54(m,1H),8.27-8.24(m,1H),8.14(d,J=0.9Hz,1H),8.00-7.98(m,1H),7.87(d,J=8.2Hz,1H),7.69-7.66(m,1H),7.51(s,1H),7.18(d,J=8.0Hz,2H),7.12(d,J=7.9Hz,2H),4.69(s,2H),2.25(s,3H). 13 C NMR(101MHz,DMSO)δ167.22,167.13155.99,148.09,147.27,143.44,139.61,139.01,136.58,133.65,132.89,129.07,127.63,127.38,126.85,126.81,126.41,124.62,124.38,113.95,111.45,40.64,20.60 HRMS (ESI-TOF) molecular formula C 25 H 17 BrClN 5 O 3 :[M-H] - Calculated relative molecular mass: 548.0131, found 548.0130.
Example 4: preparation of N- (2-methylfuranyl-1, 3-dioxo-5-isoindolyl) -3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxamide
Step 1
5-Nitrophthalic anhydride (10.00 mmol) was added to a 100mL eggplant type bottle, and dissolved in 8mL of glacial acetic acid. Then 2-furanmethanamine (12.00 mmol) is dropped into an eggplant-shaped bottle, heated to 110 ℃, and refluxed for 18h. The reaction mixture was poured into ice water to precipitate. The precipitate is isolated by filtration, washed with excess water and dried at 70-75 ℃. The obtained crude product was purified by recrystallization from ethanol to obtain 2.20g of a white solid compound with a yield of 81%.
Step 2, synthesis of N- (2-methylfuryl) -5-aminoisoindoline-1, 3-dione
The N-2- (furyl-2-methyl) -5-nitroisoindoline-1, 3-dione (5 mmol) obtained in step 1, iron powder (25 mmol) and ammonium chloride (15 mmol) were added to a 100mL eggplant-type bottle, and 20mL ethanol and 8mL water were added to dissolve. Heating to 75 deg.C, reflux reacting for 0.5h, filtering with diatomite, and collecting filtrate. Ethanol and water were removed by rotary evaporation to give a crude solid product which was purified by column chromatography (eluent: 10% methanol/dichloromethane) and the solvent was evaporated to dryness to give 1.03g of a yellow solid product in 85% yield.
Step 3 Synthesis of N- (2-methylfuranyl-1, 3-dioxo-5-isoindolyl) -3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxamide
In a 100mL round-bottom flask, N-2- (furyl-2-methyl) -5-aminoisoindoline-1, 3-dione (4 mmol) obtained in step 2, 3-bromo-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid (8 mmol), N-methylimidazole (28 mmol), and N, N, N ', N' -tetramethylchlorourea hexafluorophosphate (9.6 mmol) were charged, and dissolved in 20mL of acetonitrile. Heating to 60 ℃, refluxing for 6h, and detecting the completion of the reaction by TLC. The reaction mixture was poured into water and a precipitate precipitated out. Filtering and separating to obtain precipitate, and washing the precipitate with excessive water to obtain a solid crude product. Separation and purification by column chromatography (eluent: 22% methanol/dichloromethane) and evaporation of the solvent afforded the product as a white solid, 1.07g, 51% yield.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.09(s,1H),8.56-8.54(m,1H),8.27-8.25(m,1H),8.15(d,J=1.7Hz,1H),8.01-7.99(m,1H),7.88(d,J=8.2Hz,1H),7.70-7.66(m,1H),7.56(d,J=1.0Hz,1H),7.52(s,1H),6.40-6.39(m,1H),6.36(d,J=3.1Hz,1H),4.74(s,2H). 13 c NMR (101MHz, DMSO). Delta.166.75, 166.65,155.99,149.32,148.06,147.30,143.48,142.64,139.64,138.97,132.79,127.63,126.89,126.84,126.29,124.66,124.48,113.93,111.47,110.61,108.06,34.12 HRMS (ESI-TOF) molecular formula C 22 H 13 BrClN 5 O 4 :[M-H] - Calculated relative molecular mass: 523.9767, found 523.9770.
Example 5: preparation of N- (4-methylpyridyl-1, 3-dioxo-5-isoindolyl) -3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 4 was used, except that:
example 4 the starting material, 2-furanmethanamine, in step 1, was 4-pyridinemethanamine.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.10(s,1H),8.56-8.54(m,1H),8.28-8.25(m,1H),8.16(d,J=1.6Hz,1H),8.01-7.99(m,1H),7.88(d,J=8.2Hz,1H),7.70-7.67(m,1H),7.52(s,1H),7.44-7.42(m,2H),7.09-7.05(m,2H),4.91(s,2H). 13 c NMR (101MHz, DMSO). Delta.166.75, 166.65,156.00,148.06,147.30,143.52,139.63,138.97,138.58,132.76,127.62,127.01,126.92,126.88,126.84,126.24,126.09,124.69,124.53,113.97,111.48,109.25,35.60 HRMS (ESI-TOF) molecular formula C 23 H 14 BrClN 6 O 3 :[M-H] - Calculated relative molecular mass: 534.9927, found 534.9928.
Example 6: preparation of N- (2-chlorophenyl-1, 3-dioxo-5-isoindolyl) -3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 4 was used, except that:
example 4 in step 1, 2-chloroaniline was used as the starting material, 2-furanmethanamine.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.08(s,1H),8.58-8.54(m,1H),8.26-8.25(m,1H),8.18(d,J=1.6Hz,1H),8.01-7.97(m,1H),7.88(d,J=8.2Hz,1H),7.70-7.67(m,1H),7.52(s,1H),7.48-7.45(m,1H),7.44-7.42(m,1H),7.09-7.05(m,1H),6.97-6.95(m,1H). 13 c NMR (101MHz, DMSO). Delta.166.72, 166.65,156.00,148.06,147.30,143.52,139.63,138.97,138.58,132.76,127.62,127.01,126.92,126.88,126.84,126.24,126.09,124.69,124.53,113.97,111.48,110.98,110.52 HRMS (ESI-TOF) molecular formula C 23 H 12 BrCl 2 N 5 O 3 :[M-H] - Calculated relative molecular mass: 553.9428, found 553.9426.
Example 7: preparation of N- (2-benzyl-1, 3-dioxo-5-isoindolyl) -3-bromo-1- (2-chlorophenyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 1 was used, except that:
example 1 the starting material, ethyl bromide in step 1, was benzyl bromide; in the step 3, 3-bromo-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid is used as the raw material, and 3-bromo-1- (3-chloro-2-phenyl) -1H-pyrazole-5-carboxylic acid is used as the raw material.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ10.99(s,1H),8.16(d,J=0.8Hz,1H),8.02(d,J=1.2Hz,1H),7.85(d,J=8.2Hz,1H),7.65-7.61(m,2H),7.58-7.52(m,2H),7.48(s,1H),7.40(m,1H),7.36-7.33(m,2H),7.15-7.11(t,J=8.8Hz,2H),4.72(s,2H). 13 c NMR (101MHz, DMSO). Delta.165.89, 164.57,162.80,161.62,143.34,139.08,134.57,132.20,131.83,130.81,130.62,130.54,129.40,128.51,128.40,127.72, 127.13.87, 126.05,123.91,119.90,115.28,113.25,109.35,44.52 HRMS (ESI-TOF) of formula C 25 H 16 BrClN 4 O 3 :[M-H] - Calculated relative molecular mass: 533.0022, found 533.0023.
Example 8: preparation of N- (2- (3-cyanobenzyl) -1, 3-dioxo-5-isoindolyl) -3-difluoromethyl-1-methyl-1H-pyrazole-5-carboxamide
A synthesis similar to that of example 1 was used, except that:
example 1 the starting material, ethyl bromide in step 1, was 3-cyanobenzyl bromide; in the step 3, 3-difluoromethyl-1-methyl-1H-pyrazole-5-carboxylic acid is adopted as the raw material 3-bromo-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ10.58(s,1H),8.55(s,1H),8.30(d,J=1.6Hz,1H),8.07-8.04(m,1H),7.89(d,J=8.2Hz,1H),7.40-7.31(m,1H),7.19-7.14(m,2H),6.78(s,1H),6.49(s,1H),4.74(s,2H),3.99(s,3H). 19 FNMR(376MHz,DMSO)δ-113.48. 13 c NMR (101MHz, DMSO). Delta.168.66, 167.53,166.72,165.43,162.81,161.67,155.82,143.32,139.76,137.12,132.25,131.80,130.62,128.57,126.05,123.90,119.92,115.20,109.65,99.97,44.52,38.55 HRMS (ESI-TOF) formula C 22 H 15 F 2 N 5 O 3 :[M-H] - Calculated relative molecular mass: 434.1070, found 434.1071.
Example 9: preparation of N- (3-methylphenyl-1, 3-dioxo-5-isoindolyl) -3-difluoromethyl-1- (5-bromo-2-pyridinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 1 was used, except that:
example 1 in step 1, ethyl bromide was used as the starting material, 3-methylbenzyl bromide was used.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.10(s,1H),8.56-8.54(m,1H),8.28-8.25(m,1H),8.16(d,J=1.6Hz,1H),8.01-7.99(m,1H),7.88(d,J=8.2Hz,1H),7.70-7.67(m,1H),7.52(s,1H),7.44-7.42(m,1H),7.09-7.05(m,1H),6.97-6.95(m,1H),6.88(s,1H),6.38(s,1H),2.99(s,3H). 19 F NMR(376MHz,DMSO)δ-110.29. 13 c NMR (101MHz, DMSO) delta 166.75,166.65,156.00,148.06,147.30,143.52,139.63,138.97,138.58,136.74,134.82,132.76,127.62,127.01,126.92,126.88,126.84,126.24,126.09,124.69,124.53, 118.66.97, 111.48,35.60 HRMS (ESI-TOF) has the formula C 25 H 16 BrF 2 N 5 O 3 :[M-H] - Calculated relative molecular mass: 551.0410, found 551.0408.
Example 10: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (4-oxo-3, 4-dihydro-5-benzo [ d ] [1,2,3] triazinyl) -1H-pyrazole-5-carboxamide
Step 1 Synthesis of 5-Nitro-2H-benzo [ d ] [1,3] oxazine-2, 4 (1H) -dione
2-amino-6-nitrobenzoic acid (40 mmol) was added to tetrahydrofuran (120 mL), stirred, and cooled to-10 ℃. Triphosgene (40 mmol) was dissolved in tetrahydrofuran (20 mL), the temperature was controlled between-10 ℃ and-5 ℃, and the solution was added dropwise slowly over 1 hour. Slowly warm up and reflux. After the reaction is finished, the solvent is evaporated under reduced pressure, anhydrous ether (150 mL) is added into the residue, the mixture is fully stirred and filtered, and a filter cake is washed by the anhydrous ether and dried to obtain a solid product 7,32g, wherein the yield is more than 88%.
Step 2
5-Nitro-2H-benzo [ d ] [1,3] oxazine-2, 4 (1H) -dione (35 mmol) and ammonium carbonate (70 mmol) were added to 1, 4-dioxane (100 mL) and stirred to give a suspension. The reaction was heated to 60 ℃ and the progress of the reaction was followed by TLC. After the reaction, the reaction mixture was cooled to room temperature, and the solvent was evaporated under reduced pressure. To the residue was added water (150 mL), extracted with ethyl acetate (60 mL. Times.3), the organic phases combined, washed with saturated aqueous sodium bicarbonate solution, dried over anhydrous sodium sulfate, filtered, and the filtrate concentrated to give 5.38g of a solid product with a yield of 85% or more.
Step 3 Synthesis of 5-nitrobenzo [ d ] [1,2,3] triazin-4 (3H) -one
Dissolving 2-amino-6-nitrobenzamide (30 mmol) in 0.5M hydrochloric acid (240 mL), stirring under the condition of ice salt bath (controlling the temperature between 0 and 5 ℃), slowly dropwise adding an aqueous solution containing sodium nitrite (60 mmol), after dropwise adding for about 40 minutes, naturally heating to room temperature and continuing to react for 2 to 3 hours. Adjusting pH to 7-8 with sodium hydroxide water solution, stirring for 15 min, vacuum filtering, washing filter cake with water, and drying to obtain solid product 4.90g with yield over 85%.
Step 4 Synthesis of 5-aminobenzo [ d ] [1,2,3] triazin-4 (3H) -one
To a 100mL eggplant-type bottle were added 5-nitrobenzo [ d ] [1,2,3] triazin-4 (3H) -one (10 mmol), iron powder (50 mmol), ammonium chloride (30 mmol), 20mL of ethanol and 8mL of water were added to dissolve. Heating to 75 deg.C, reflux reacting for 0.5h, filtering with diatomite, and collecting filtrate. Removing ethanol and water from the filtrate by rotary evaporation to obtain solid, separating and purifying by column chromatography (eluent: 8% methanol/dichloromethane), and evaporating the solvent to obtain yellow solid product 1.22g with yield over 75%.
Step 5 Synthesis of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (4-oxo-3, 4-dihydro-5-benzo [ d ] [1,2,3] triazinyl) -1H-pyrazole-5-carboxamide
A100 mL round-bottomed flask was charged with 5-aminobenzo [ d ] [1,2,3] triazin-4 (3H) -one (7 mmol), 3-bromo-1- (3-chloro-2-pyridinyl) -1H-pyrazole-5-carboxylic acid (14 mmol), N-methylimidazole (49 mmol), N, N, N ', N' -tetramethylchlorourea hexafluorophosphate (16.8 mmol), and dissolved in 40mL acetonitrile. The reaction was heated to 60 ℃ and refluxed for 6h, and the completion of the reaction was monitored by TLC. The mixture was poured into water and a precipitate precipitated out. Filtering, washing with excessive water to obtain solid, separating and purifying by column chromatography (eluent: 20% methanol/dichloromethane), and evaporating solvent to obtain final white solid product 1.60g with yield over 51%.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ15.32(s,1H),12.58(s,1H),8.58(d,J=1.4Hz,1H),8.56(d,J=3.6Hz,1H),8.28(d,J=8.1Hz,1H),8.01-7.97(m,1H),7.89(d,J=8.1Hz,1H),7.73-7.69(m,1H),7.27(s,1H). 13 CNMR (101MHz, DMSO) delta 155.97,155.49,148.14,147.29,141.22,140.80,139.61,138.96,129.15,127.69,127.00,126.87,126.85,121.06,112.90,111.45 HRMS (ESI-TOF) with molecular formula C 16 H 8 BrClN 7 O 2 :[M-H] - Calculated relative molecular mass: 443.9617, found 443.9621.
Example 11: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (3-ethyl-4-oxo-3, 4-dihydro-5-benzo [ d ] [1,2,3] triazinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 10 was used, except that:
example 10 step 4 ethyl followed by reduction was performed as in step 1 of example 1.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.18(s,1H),8.58(d,J=1.4Hz,1H),8.56(d,J=3.6Hz,1H),8.27(d,J=8.1,1.4Hz,1H),8.25-8.22(m,1H),8.18(d,J=8.9Hz,1H),7.71-7.67(m,1H),7.56(s,1H),4.39(q,J=7.2Hz,2H),1.38(t,J=7.2Hz,3H). 13 c NMR (101MHz, DMSO). Delta.156.00, 154.41,148.12,147.30,141.50,140.41,139.63,138.95,129.23,127.67,126.88,126.87,120.16,113.06,111.49,44.44,13.91 HRMS (ESI-TOF) molecular formula C 18 H 13 BrClN 7 O 2 :[M-H] - Calculated relative molecular mass: 471.9930, found 471.9925.
Example 12: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (3- (3-fluorophenyl) -4-oxo-3, 4-dihydro-5-benzo [ d ] [1,2,3] triazinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 10 was used, except that:
example 10 step 4 was followed by 3-fluorobenzyl and reduction as in step 1 of example 1.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ12.49(s,1H),8.61(d,J=8.1Hz,1H),8.55(d,J=4.4Hz,1H),8.27(d,J=7.8Hz,1H),8.04-8.00(m,1H),7.94(d,J=8.0Hz,1H),7.71-7.67(m,1H),7.40(d,J=8.5Hz,2H),7.30(s,1H),6.93(d,J=8.6Hz,2H),4.78(s,2H). 19 F NMR(376MHz,DMSO)δ-90.88. 13 CNMR (101MHz, DMSO) delta 158.97,157.14,155.08,147.89,147.37,144.28,139.65,139.20,137.61,136.63,129.58,127.83,127.80,127.40,127.02,123.02,121.49,118.78,115.27,114.03,110.18,107.42,55.09 HRMS (ESI-TOF) molecular formula C 23 H 14 BrClFN 7 O 2 :[M-H] - Calculated relative molecular mass: 553.0070, found 553.0068.
Example 13: preparation of 3-bromo-1- (3-chloropyridin-2-yl) -N- (1, 1-dioxide-4H-7-benzo [ e ] [1,2,4] thiadiazinyl) -1H-pyrazole-5-carboxamide 5-aminobenzo [ d ] [1,2,3] triazin-4 (3H) -one
Step 1
2-chloro-5-nitrobenzenesulfonamide (40 mmol), ammonium carbonate (80 mmol) and copper sulfate (12 mmol) were added to aqueous ammonia (100 mL) and heated in a high pressure flask at 120 ℃ for 4 hours. And (4) detecting the completion of the reaction by TLC, cooling the reaction solution to normal temperature, and pouring the reaction solution into water for precipitation. And (4) carrying out suction filtration, washing a filter cake with water, and drying to obtain a solid product 7.38g with the yield of more than 85%.
Step 2 Synthesis of 7-Nitro-4H-benzo [ e ] [1,2,4] thiadiazine 1, 1-dioxide
2-amino-5-nitrobenzenesulfonamide (30 mmol) was added to trimethyl orthoformate (100 mL) and stirred to give a suspension. The reaction was heated to 140 ℃ and the progress of the reaction was followed by TLC. After the reaction is finished, cooling to room temperature, and pouring into water for precipitation. And (4) carrying out suction filtration, washing a filter cake with water, and drying to obtain a solid product of 4.77g with the yield of more than 70%.
Step 3 Synthesis of 7-amino-4H-benzo [ e ] [1,2,4] thiadiazine 1, 1-dioxide
7-Nitro-4H-benzo [ e ] [1,2,4] thiadiazine 1, 1-dioxide (10 mmol), iron powder (50 mmol), ammonium chloride (30 mmol) were added to a 100mL eggplant-type bottle, and 20mL of ethanol and 8mL of water were added to dissolve them. Heating to 75 ℃, and carrying out reflux reaction for 0.5h. Filtering with diatomite, collecting filtrate, removing ethanol and water by rotary evaporation to obtain solid, separating and purifying by column chromatography (eluent: 10% methanol/dichloromethane), and evaporating solvent to obtain yellow solid product 1.48g with yield over 75%.
Step 4 Synthesis of 3-bromo-1- (3-chloropyridin-2-yl) -N- (1, 1-dioxido-4H-7-benzo [ e ] [1,2,4] thiadiazinyl) -1H-pyrazole-5-carboxamide 5-aminobenzo [ d ] [1,2,3] triazin-4 (3H) -one
A100 mL round-bottomed flask was charged with 7-amino-4H-benzo [ e ] [1,2,4] thiadiazine 1, 1-dioxide (7 mmol), 3-bromo-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid (14 mmol), N-methylimidazole (49 mmol), N, N, N ', N' -tetramethylchlorourea hexafluorophosphate (16.8 mmol), and dissolved in 40mL of acetonitrile. Heating to 60 ℃, refluxing for 6h, and detecting the completion of the reaction by TLC. The mixture was poured into water and a precipitate precipitated out. Filtering, washing precipitate with excessive water, and drying to obtain solid. The final white solid product (1.72 g) was obtained by column chromatography for separation and purification (eluent: 15% methanol/dichloromethane) and evaporation of the solvent to dryness, with a yield of over 51%.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ12.34(s,1H),10.87(s,1H),8.54(dd,J=4.7,1.4Hz,1H),8.25(dd,J=8.1,1.4Hz,1H),8.15(d,J=2.3Hz,1H),7.96(s,1H),7.86(dd,J=9.0,2.3Hz,1H),7.67(dd,J=8.1,4.7Hz,1H),7.48(s,1H),7.34(d,J=9.0Hz,1H). 13 c NMR (101MHz, DMSO). Delta.160.66, 155.90,148.27,146.69,140.02,136.85,136.52,133.71,132.79,125.71,125.42,123.42,119.91,118.69,115.01,114.38 HRMS (ESI-TOF) molecular formula C 16 H 10 BrClN 6 O 3 S:[M-H] - Calculated relative molecular mass: 478.9334, found 478.9328.
Example 14: preparation of 3-bromo-1- (3-chloropyridin-2-yl) -N- (3-methyl-1, 1-dioxide-4H-7-benzo [ e ] [1,2,4] thiadiazinyl) -1H-pyrazole-5-carboxamide 5-aminobenzo [ d ] [1,2,3] triazin-4 (3H) -one
A synthesis similar to that of example 13 was used, except that:
example 13 trimethyl orthoformate as solvent in step 2 triethyl orthoacetate was used.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ10.85(s,1H),8.56-8.48(m,1H),8.29-8.21(m,1H),8.10(d,J=1.9Hz,1H),7.88-7.83(m,1H),7.70-7.65(m,1H),7.47(s,1H),7.30(d,J=9.0Hz,1H),7.48(s,1H),2.87(s,3H). 13 c NMR (101MHz, DMSO). Delta.160.60, 158.01,155.89,148.21,140.19,137.79,136.82,135.13,132.82,125.63,125.48,124.15,121.87,119.18,116.82,114.43,19.25 HRMS (ESI-TOF) molecular formula C 17 H 12 BrClN 6 O 3 S:[M-H] - Calculated relative molecular mass: 492.9491, found 492.9489.
Example 15: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (1-ethyl-4-oxo-1, 4-dihydro-6-quinazolinyl) -1H-pyrazole-5-carboxamide
Step 1
6-Nitro-4 (1H) -quinazolinone (20.00 mmol) was added to a 100mL eggplant-shaped flask, dissolved by adding 40mL acetonitrile, and bromoethane (40.00 mmol) and triethylamine (TEA, 60.00 mmol) were added to the eggplant-shaped flask in this order. Heating to 80 ℃, and refluxing for 5h. After the reaction, the reaction mixture was cooled to room temperature, and the solvent was evaporated under reduced pressure. To the residue was added water, extracted with dichloromethane three times, and the organic phases were combined. Drying with anhydrous sodium sulfate, filtering, and concentrating the filtrate to obtain solid product 3.72g with yield over 85%.
Step 2
1-Ethyl-6-amino-4 (1H) -quinazolinone (10 mmol), iron powder (50 mmol), ammonium chloride (30 mmol) were added to a 100mL eggplant-shaped bottle, 20mL ethanol and 8mL water were added to dissolve. Heating to 75 deg.C, reflux reacting for 0.5h, filtering with diatomite, and collecting filtrate. Ethanol and water were removed by rotary evaporation to obtain a solid, which was separated and purified by column chromatography (eluent: 10% methanol/dichloromethane), and the solvent was evaporated to dryness to obtain 1.42g of a yellow solid product with a yield of 75% or more.
Step 3 Synthesis of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (1-ethyl-4-oxo-1, 4-dihydro-6-quinazolinyl) -1H-pyrazole-5-carboxamide
In a 100mL round bottom flask, 1-ethyl-6-amino-4 (1H) -quinazolinone (7 mmol), 3-bromo-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid (14 mmol), N-methylimidazole (49 mmol), N' -tetramethylchlorourea hexafluorophosphate (16.8 mmol) was added and dissolved in 40mL acetonitrile. Heating to 60 ℃, refluxing for 6h, and detecting the completion of the reaction by TLC. The mixture was poured into water, and the precipitate was precipitated and filtered. The precipitate was washed with excess water and dried to give a solid. The crude product was purified by column chromatography (eluent: 16% methanol/dichloromethane), and the solvent was evaporated to dryness to give 1.69g of the final white solid product with a yield of over 51%.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ10.88(s,1H),8.58-8.54(m,1H),8.49(d,J=2.4Hz,1H),8.36(s,1H),8.29-8.23(m,1H),8.05-8.01(m,1H),7.72-7.68(m,1H),7.67(d,J=3.3Hz,1H),7.52(s,1H),4.00(q,J=7.1Hz,2H),1.28(t,J=7.1Hz,3H). 13 c NMR (101MHz, DMSO). Delta.163.28, 161.62,160.57,155.85,148.27,140.18,139.39,137.87,136.85,132.75,128.77,125.71,125.42,123.91,117.87,114.46,114.42,45.38,12.39 HRMS (ESI-TOF) molecular formula C 19 H 13 BrClN 6 O 2 :[M-H] - Calculated relative molecular mass: 470.9977, found 470.9980.
Example 16: preparation of 3-difluoromethyl-1- (3-chloro-2-pyridinyl) -N- (4-oxo-1, 4-dihydro-6-quinazolinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 15 was used, except that:
example 15 direct nitro reduction of starting material 6-nitro-4 (1H) -quinazolinone in step 1; in the step 3, 3-difluoromethyl-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid is used as the raw material 3-bromo-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ12.24(s,1H),10.86(s,1H),8.58-8.54(m,1H),8.46(d,J=2.2Hz,1H),8.28-8.24(m,1H),8.04(s,1H),8.03-7.98(m,1H),7.71-7.67(m,1H),7.66(d,J=3.6Hz,1H),7.52(s,1H),6.45(s,1H). 19 F NMR(376MHz,DMSO)δ-115.52. 13 c NMR (101MHz, DMSO). Delta.163.22, 161.60,155.91,150.35,148.27,140.14,137.25,136.91,135.56,132.79,125.70,125.48,122.94,122.91,116.89,116.17,114.45,113.82 HRMS (ESI-TOF) molecular formula C 18 H 11 ClF 2 N 6 O 2 :[M-H] - Calculated relative molecular mass: 416.0606, found 416.0603.
Example 17: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (1-benzyl-4-oxo-1, 4-dihydro-6-quinazolinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 15 was used, except that:
example 15 ethyl bromide, starting material in step 1, benzyl bromide was used.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ10.85(s,1H),8.56-8.52(m,1H),8.42(d,J=2.2Hz,1H),8.26-8.21(m,1H),8.02(s,1H),8.01-7.97(m,1H),7.40-7.35(m,5H)7.70-7.65(m,1H),7.62(d,J=3.6Hz,1H),7.50(s,1H),5.23(s,2H). 13 c NMR (101MHz, DMSO). Delta.163.22, 161.60,155.91,150.35,148.27,140.14,137.25,136.91,135.56,132.79,125.70,125.48,122.94,122.91,116.89,116.17,114.45 HRMS (ESI-TOF) molecular formula C 24 H 16 BrClN 6 O 2 :[M-H] - Calculated relative molecular mass: 533.0134, found 533.0141.
Example 18: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (2-isopropyl-1, 3-tetraoxide-2H-benzo [ d ] [1,3,2] dithiazolyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 1 was used, except that:
example 1 the starting material bromoethane in step 1, 2-bromopropane was used, starting material 5-nitrophthalimide, and 6-nitro-1, 1-dioxide-benzo [ d ] isothiazole was used.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ10.89(s,1H),8.56-8.54(m,1H),8.27-8.25(m,1H),8.09(d,J=1.3Hz,1H),7.98-7.96(m,1H),7.81(d,J=8.2Hz,1H),7.70-7.67(m,1H),7.51(s,1H),4.41-4.33(m,1H),1.38(d,J=6.9Hz,6H). 13 c NMR (101MHz, DMSO). Delta.163.86, 161.60,155.90,148.22,140.75,140.13,138.74,136.88,133.35,132.81,130.37,125.70,125.41,124.12,120.89,114.46,53.92,20.41 HRMS (ESI-TOF) molecular formula C 19 H 15 BrClN 5 O 4 S:[M-H] - Calculated relative molecular mass: 522.9722, found 522.9720.
Example 19: preparation of 3-difluoromethyl-1-methyl-N- (2- (4-methoxybenzyl) -1-oxo-5-isoindolyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 1 was used, except that:
example 1 starting material bromoethane in step 1, 4-methoxybenzyl bromide, starting material 5-nitrophthalimide, 5-nitro-1-isoindolone; in the step 3, 3-difluoromethyl-1-methyl-1H-pyrazole-5-carboxylic acid is adopted as the raw material 3-bromo-1- (3-chloro-2-pyridyl) -1H-pyrazole-5-carboxylic acid.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.02(s,1H),8.58-8.52(m,1H),8.29-8.22(m,1H),8.12(d,J=0.9Hz,1H),7.52(s,1H),7.19(d,J=8.0Hz,2H),7.11(d,J=7.9Hz,2H),6,52(s,1H),4.72(s,2H),4.22(s,2H)3.96(s,3H).3.81(s,3H). 19 F NMR(376MHz,DMSO)δ-112.38. 13 c NMR (101MHz, DMSO). Delta.161.69, 159.52,155.87,148.27,141.49,140.15,137.97,136.88,136.75,132.99,132.76,130.04,128.95,128.54,125.72,122.56,114.48,114.09,55.67,50.45,40.98,21.17.HRMS (ESI-TOF) molecular formula C 22 H 20 F 2 N 4 O 3 :[M-H] - Calculated relative molecular mass: 426.1509, found 426.1511.
Example 20: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (2-ethyl-1, 1-dioxo-3-oxo-3, 4-dihydro-2H-benzo [ e ] [1,2,4] thiadiazinyl) -1H-pyrazole-5-carboxamide
Step 1 Synthesis of 2-ethyl-7-nitro-2H-benzo [ e ] [1,2,4] thiadiazine-3 (4H) -1, 1-dioxide
7-Nitro-2H-benzo [ e ] [1,2,4] thiadiazine-3 (4H) -1, 1-dioxide (20.00 mmol) was added to a 100mL eggplant type bottle, 50mL of N, N-dimethylformamide was added thereto to dissolve, and bromoethane (20.00 mmol) and triethylamine (TEA, 60.00 mmol) were added to the eggplant type bottle in this order. Heating to 90 ℃, and refluxing for 5h. The mixture was then poured into ice water and a precipitate precipitated out. The precipitate was filtered, washed with excess water and dried at 70-75 ℃. The obtained crude solid product was purified by recrystallization from ethanol to obtain 4,36g as a white solid with a yield of 80.5%.
Step 2 Synthesis of 2-ethyl-7-amino-2H-benzo [ e ] [1,2,4] thiadiazine-3 (4H) -1, 1-dioxide
2-Ethyl-7-nitro-2H-benzo [ e ] [1,2,4] thiadiazine-3 (4H) -1, 1-dioxide (10 mmol), iron powder (50 mmol), ammonium chloride (30 mmol) were added to a 100mL eggplant-shaped bottle, 30mL of ethanol and 12mL of water were added to dissolve. Heating to 75 ℃, and carrying out reflux reaction for 0.5h. Filtering with diatomite to obtain filtrate. And (4) removing ethanol and water by rotary evaporation to obtain a solid. Separation and purification by column chromatography (eluent: 10% methanol/dichloromethane) and evaporation of the solvent gave 1.81g of a yellow solid product in 75% or more yield.
And step 3: synthesis of 3-bromo-1- (3-chloro-2-pyridyl) -N- (2-ethyl-1, 1-dioxo-3-oxo-3, 4-dihydro-2H-benzo [ e ] [1,2,4] thiadiazinyl) -1H-pyrazole-5-carboxamide
A100 mL round-bottomed flask was charged with 2-ethyl-7-amino-2H-benzo [ e ] [1,2,4] thiadiazine-3 (4H) -1, 1-dioxide (5 mmol), 3-bromo-1- (2-chloropyridyl) -1H-pyrazole-5-carboxylic acid (10 mmol), N-methylimidazole (35 mmol), N, N, N ', N' -tetramethylchlorourea hexafluorophosphate (12 mmol), and dissolved in 40mL of acetonitrile. Heating to 60 ℃, refluxing for 6h, and detecting the completion of the reaction by TLC. The mixture was poured into water and a precipitate precipitated out. The precipitate was filtered and washed with excess water to give a solid. The crude product was purified by column chromatography (eluent: 15% methanol/dichloromethane), and the solvent was evaporated to dryness to give 1.34g of the final white solid product with a yield of over 51%.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ12.02(s,1H),10.85(s,1H),8.61-8.55(m,1H),8.48(d,J=2.4Hz,1H),8.35(s,1H),8.27-8.21(m,1H),8.05-7.98(m,1H),7.69-7.62(m,1H),7.51(s,1H),4.00(q,J=7.1Hz,2H),1.28(t,J=7.1Hz,3H). 13 C NMR(101MHz,DMSO)δ161.60,155.98,149.11,148.32,140.18,136.85,136.53,132.79,131.57,126.32,125.70,125.46,123.77,121.16,114.71,114.42,38.45,13.62.HRMS (ESI-TOF) molecular formula is C 18 H 14 BrClN 6 O 4 S:[M-H] - Calculated relative molecular mass: 522.9596, found 522.9591.
Example 21: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (2-fluoroethyl-2, 4-dioxo-1, 2,3, 4-tetrahydroquinazolinyl) -1H-pyrazole-5-carboxamide
A synthesis similar to that of example 20 was used, except that:
example 20 starting material 7-nitro-2H-benzo [ e ] [1,2,4] thiadiazine-3 (4H) -1, 1-dioxide in step 1, using 6-nitroquinazoline-2, 4 (1h, 3h) -dione; 1-bromo-2-fluoroethane is used as the raw material bromoethane.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.96(s,1H),10.79(s,1H),8.62-8.53(m,1H),8.50(d,J=2.4Hz,1H),8.37(s,1H),8.26-8.19(m,1H),8.07-7.99(m,1H),7.71-7.64(m,1H),7.53(s,1H),4.52-4.50(m,2H),3.35(t,J=7.1Hz,2H). 19 F NMR(376MHz,DMSO)δ-90.82. 13 c NMR (101MHz, DMSO). Delta.162.72, 161.60,155.95,150.57,148.31,140.19,138.44,137.02,136.88,132.81,127.34,125.71,125.42,124.05,117.65,114.47,110.24,35.25,14.57 HRMS (ESI-TOF) molecular formula C 19 H 13 BrClFN 6 O 3 :[M-H] - Calculated relative molecular mass: 505.9911, found 505.9910.
Example 22: preparation of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (1, 4-dioxo-1, 2,3, 4-tetrahydrophthalazinyl) -1H-pyrazole-5-carboxamide
Step 1: synthesis of 7-amino-2, 3-dihydrophthalazine-1, 4-dione
2-Ethyl-7-nitro-2, 3-dihydrophthalazine-1, 4-dione (10 mmol), iron powder (50 mmol), ammonium chloride (30 mmol) were added to a 100mL eggplant-shaped bottle, and 30mL ethanol and 12mL water were added to dissolve. Heated to 75 ℃ and refluxed for 0.5h. Filtering with diatomaceous earth, collecting filtrate, and removing ethanol and water by rotary evaporation to obtain solid. Separation and purification by column chromatography (eluent: 10% methanol/dichloromethane) and evaporation of the solvent gave 1.33g of a yellow solid product in 75% or more yield.
Step 2: synthesis of 3-bromo-1- (3-chloro-2-pyridinyl) -N- (1, 4-dioxo-1, 2,3, 4-tetrahydrophthalazinyl) -1H-pyrazole-5-carboxamide
In a 100mL round-bottomed flask was added 2-ethyl-7-amino-2, 3-dihydrophthalazine-1, 4-dione (5 mmol), 3-bromo-1- (2-chloropyridyl) -1H-pyrazole-5-carboxylic acid (10 mmol), N-methylimidazole (35 mmol), N, N, N ', N' -tetramethylchlorourea hexafluorophosphate (12 mmol), and dissolved in 40mL of acetonitrile. Heating to 60 ℃, refluxing for 6h, and detecting the completion of the reaction by TLC. The mixture was poured into water and a precipitate precipitated out. The precipitate was filtered and washed with excess water to give a solid. The final white solid product (1.17 g) was obtained by column chromatography for separation and purification (eluent: 15% methanol/dichloromethane) and evaporation of the solvent to dryness, with a yield of over 51%.
The final test results were as follows: 1 H NMR(400MHz,DMSO)δ11.55-11.46(m,2H),11.03(s,1H),8.56-8.54(m,1H),8.39(s,1H),8.27-8.25(m,1H),8.09-8.03(m,1H),7.70-7.67(m,2H),7.56(s,1H). 13 c NMR (101MHz, DMSO). Delta.160.82, 156.91,155.95,154.02,148.31,141.80,140.19,138.63,136.90,132.82,129.93,127.25,125.96,125.77,125.46,118.01,114.42 HRMS (ESI-TOF) molecular formula C 17 H 10 BrClN 6 O 3 :[M-H] - Calculated relative molecular mass: 459.9692, found 459.9694.
The other compounds in Table 1 were prepared in a similar manner to examples 1-22, using different starting materials, pyrazole compounds.
In order to further prove the biological activity of the pyrazole amide compound containing the aromatic heterocyclic structure, the inventor designs the following test method.
The same test method is adopted for diamondback moth and armyworm, and the leaf soaking method is adopted. Firstly, dissolving the surfactant with the concentration of less than 10% in tap water, and performing microwave ultrasonic treatment to promote the uniform dissolution of the surfactant. Dimethyl sulfoxide (DMSO) with concentration not higher than 5% is used as cosolvent, tap water with dissolved surfactant is added to prepare different preparations with different concentrations. Soaking corn leaf seedlings in different agents with different concentrations for treatment, airing, placing the corn leaf seedlings in culture dishes, then respectively inoculating 10 larvae of diamondback moths or armyworms with the age of 2-3 in each culture dish, setting three groups of parallel experiments, adding blank control and chlorantraniliprole as positive control, observing every 24 hours, supplementing fresh leaves to the corn leaf seedlings when the leaves are eaten by the insects, recording death and survival numbers after 72 hours, and calculating the death rate.
Mortality (%) = (number of control live insects-number of treated live insects)/number of control live insects × 100%.
The structures of the pyrazole amide compounds containing aromatic heterocyclic structures and the measurement results of the biological activities of the pyrazole amide compounds on armyworm larvae and diamondback moth larvae are shown in the table 1.
TABLE 1
All documents referred to herein are incorporated by reference into this application as if each were individually incorporated by reference. Furthermore, it should be understood that various changes and modifications of the present invention can be made by those skilled in the art after reading the above teachings of the present invention, and these equivalents also fall within the scope of the present invention as defined by the appended claims.
Claims (10)
1. A pyrazole amide compound containing an aromatic heterocyclic structure, an optical isomer, a cis-trans isomer or an agriculturally and pharmaceutically acceptable salt thereof is characterized in that the compound has a structure shown in a formula I,
wherein,
R 1 selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl radical, C 3-6 Cycloalkyl radical, C 2-6 Alkenyl radical, C 2-6 Alkynyl, C 1-8 Alkoxy, hydrogen, phenyl, naphthalenylcyclyl, 5-6 membered heteroaryl, amino, hydroxyl, amido, acyloxy, carboxymethyl, nitro, cyano, sulfonic acid, halogen, formyl, acyl, carboxyl; by substituted is meant substituted with one or more (e.g., 2,3,4, or 5) substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amido, acyloxy, C 3-6 Cycloalkyl radical, C 3-6 Halogenocycloalkyl, C 2-6 Alkenyl radical, C 2-6 Haloalkenyl, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 A cycloalkenyl group;
R 2 selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl radical, C 3-6 Cycloalkyl, C 2-6 Alkenyl radical, C 2-6 Alkynyl radical、C 1-6 Alkoxy, phenyl, hydrogen, amino, hydroxyl, amide, acyloxy, carboxymethyl, nitro, cyano, sulfonic, halogen, formyl, acyl, carboxyl; the substitution refers to substitution by one or more halogens;
a is selected from substituted or unsubstituted phenyl, substituted or unsubstituted naphthalene cyclyl, substituted or unsubstituted 4-8 membered heteroaryl, substituted or unsubstituted C 8-14 A heteroaromatic bicyclic or tricyclic ring system; by substituted is meant substituted with one or more (e.g., 2,3,4, or 5) substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amido, acyloxy, C 3-6 Cycloalkyl radical, C 3-6 Halogenocycloalkyl, C 2-6 Alkenyl radical, C 2-6 Halogenated alkenyl group, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, phenyl, naphthyl;
b is an unsaturated or saturated 5-6 membered nitrogen containing heterocyclic group, wherein said heterocyclic group contains at least one N heteroatom and 0-2 heteroatoms selected from N, O or S;
n is selected from 0 or 1;
y is selected from the group consisting of: hydrogen, C 1-6 Alkoxy radical, C 1-8 Alkyl radical, C 1-6 Haloalkyl, C 3-6 Cycloalkyl radical, C 3-6 Halocycloalkyl radical, C 2-6 Alkenyl radical, C 2-6 Halogenated alkenyl group, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, substituted or unsubstituted phenyl, substituted or unsubstituted naphthalenylcyclyl, substituted or unsubstituted 4-8 membered heteroaryl, substituted or unsubstituted C 8-14 A heteroaromatic bicyclic or tricyclic ring system, or a 3-7 membered heterocyclic group; by substituted is meant substituted with one or more (e.g., 2,3,4, or 5) substituents selected from the group consisting of: c 1-6 Alkyl radical, C 2-6 Alkenyl, halogen, C 1-6 Haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy, C 3-6 Cycloalkyl, C 3-6 Halogenocycloalkyl, C 2-6 Haloalkenyl, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl group,C 5-7 Cycloalkenyl, phenyl, naphthyl.
2. The compound of claim 1, wherein R is 1 Selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl radical, C 2-6 Alkenyl radical, C 1-6 Alkoxy, phenyl, 5-6 membered heteroaryl, hydrogen, amino, amido, halo, formyl, acyl, carboxy; said substitution means substitution by one or more (e.g. 2,3,4 or 5) substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amide, acyloxy;
R 2 selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl radical, C 3-5 Cycloalkyl, C 2-5 Alkenyl radical, C 2-6 Alkynyl, C 1-6 Alkoxy, halogen; said substitution refers to substitution by one or more halogens;
a is selected from substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution by one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, carboxymethyl, cyano, C 1-6 Haloalkyl, amido, acyloxy, C 3-6 Cycloalkyl radical, C 3-6 Halogenocycloalkyl, C 2-6 Alkenyl radical, C 2-6 Haloalkenyl, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, phenyl, naphthyl;
b is an unsaturated 5-6 membered nitrogen containing heterocyclyl wherein said heterocyclyl contains at least one N heteroatom and 0-2 heteroatoms selected from N, O or S;
y is selected from the group consisting of: hydrogen, C 1-6 Alkoxy radical, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 3-6 Cycloalkyl, C 2-6 Alkenyl radical, C 2-6 Alkynyl, substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: c 1-6 Alkyl radical、C 2-6 Alkenyl, halogen, C 1-6 Haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy, C 3-6 Cycloalkyl radical, C 3-6 Halogenocycloalkyl, C 2-6 Haloalkenyl, C 2-6 Alkynyl, C 2-6 Halogenated alkynyl, C 5-7 Cycloalkenyl, phenyl, naphthyl.
3. The compound of claim 2, wherein R is 1 Selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl, phenyl, 5-6 membered heteroaryl, hydrogen, amino, amido, halogen; the substitution means substitution with one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl;
R 2 selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl radical, C 1-6 Alkoxy radical, C 2-5 Alkenyl, halogen; said substitution refers to substitution by one or more halogens;
a is selected from substituted or unsubstituted phenyl; the substitution refers to substitution by one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl, cyano, C 1-6 Haloalkyl, amide, acyloxy;
b is selected from the following group:
y is selected from the group consisting of: hydrogen, C 1-6 Alkoxy radical, C 1-6 Alkyl radical, C 1-6 Haloalkyl, C 2-6 Alkenyl, substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: c 1-6 Alkyl radical, C 2-6 Alkenyl, halogen, C 1-6 Haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy.
4. A compound of claim 3 wherein R is 1 Selected from the group consisting of substituted or unsubstituted: c 1-6 Alkyl, phenyl, 5-6 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: halogen, hydroxy, C 1-6 Alkoxy radical, C 1-6 Alkyl, amino, nitro, carboxyl;
R 2 selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl, halogen; the substitution refers to substitution by one or more halogens;
y is selected from the group consisting of: hydrogen, C 1-6 Alkyl radical, C 1-6 Haloalkyl, substituted or unsubstituted phenyl, substituted or unsubstituted 4-8 membered heteroaryl; the substitution means substitution with one or more substituents selected from the group consisting of: c 1-6 Alkyl radical, C 2-6 Alkenyl, halogen, C 1-6 Haloalkyl, cyano, amino, nitro, carboxyl, carboxymethyl, amido, acyloxy.
5. A compound of claim 4, wherein R is 1 Selected from the group consisting of substituted or unsubstituted: c 1-5 Alkyl, phenyl, pyridyl; the substitution refers to substitution by one or more halogens;
R 2 selected from halogen and C 1-5 A haloalkyl group.
6. The compound of claim 1, wherein R is 1 Selected from the group consisting of substituted or unsubstituted: phenyl, 5-6 membered heteroaryl, hydrogen, amino, nitro, cyano, sulfonic acid, halogen; the substitution refers to substitution by one or more halogens.
8. a pesticidal composition, comprising:
1) 0.001 to 99.99% by weight of a compound of claim 1, an optical isomer, a cis-trans isomer, or an agriculturally pharmaceutically acceptable salt thereof, or a combination thereof; and
2) An agriculturally pharmaceutically acceptable carrier and/or excipient.
9. Use of a compound of claim 1, an optical isomer, a cis-trans isomer, or an agriculturally pharmaceutically acceptable salt thereof, for controlling lepidopteran pests; or for the preparation of insecticides and/or acaricides for controlling lepidopteran pests.
10. A method of combating and/or killing insects, which comprises applying a compound according to claims 1 to 7 or an agriculturally acceptable salt thereof or a pesticidal composition according to claim 8 to a plant suffering from a condition susceptible to insect infestation, the soil surrounding the same or to the environment.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110757318.4A CN115572282B (en) | 2021-07-05 | 2021-07-05 | Pyrazole amide compound containing aromatic heterocyclic structure, and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110757318.4A CN115572282B (en) | 2021-07-05 | 2021-07-05 | Pyrazole amide compound containing aromatic heterocyclic structure, and preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN115572282A true CN115572282A (en) | 2023-01-06 |
| CN115572282B CN115572282B (en) | 2024-07-09 |
Family
ID=84579470
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110757318.4A Active CN115572282B (en) | 2021-07-05 | 2021-07-05 | Pyrazole amide compound containing aromatic heterocyclic structure, and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115572282B (en) |
Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004106324A1 (en) * | 2003-05-27 | 2004-12-09 | E.I. Dupont De Nemours And Company | Azolecarboxamide herbicides |
| WO2008036540A2 (en) * | 2006-09-20 | 2008-03-27 | Boehringer Ingelheim International Gmbh | Rho kinase inhibitors |
| CN101426779A (en) * | 2006-02-16 | 2009-05-06 | 先正达参股股份有限公司 | Pesticide containing bicyclic bisamide structure |
| CN101743237A (en) * | 2007-07-16 | 2010-06-16 | 先正达参股股份有限公司 | The condensed anthranilamide derivatives as insectisides |
| CN101827837A (en) * | 2007-08-22 | 2010-09-08 | 先正达参股股份有限公司 | The condensed anthranilamide derivatives as insectisides |
| WO2010114726A1 (en) * | 2009-03-31 | 2010-10-07 | Merck Sharp & Dohme Corp. | Aminobenzotriazole derivatives |
| US20110124654A1 (en) * | 2008-06-11 | 2011-05-26 | Huifen Chen | Diazacarbazoles and methods of use |
| CN102791714A (en) * | 2010-03-26 | 2012-11-21 | 霍夫曼-拉罗奇有限公司 | N-(imidazopyrimidin-7-yl)-heteroarylamide derivatives and their use as PDE10A inhibitors |
| WO2013059587A1 (en) * | 2011-10-20 | 2013-04-25 | Sirtris Pharmaceuticals, Inc. | Substituted bicyclic aza-heterocycles and analogues as sirtuin modulators |
| CN103467380A (en) * | 2013-09-29 | 2013-12-25 | 南开大学 | Substituted phenyl pyrazole amide derivative and preparation method and application thereof |
| CN103755700A (en) * | 2013-12-26 | 2014-04-30 | 青岛科技大学 | Novel pyrazol amides compound and application thereof |
| CN110234650A (en) * | 2017-02-01 | 2019-09-13 | 菲尼克斯药品股份公司 | Aryl hydrocarbon receptor (AhR) adjusts immunomodulator compounds |
| CN110248940A (en) * | 2017-02-01 | 2019-09-17 | 菲尼克斯药品股份公司 | Aryl hydrocarbon receptor (AhR) adjusts immunomodulator compounds |
| CN110325532A (en) * | 2017-02-21 | 2019-10-11 | 菲尼克斯药品股份公司 | Aryl hydrocarbon receptor (AhR) adjusts immunomodulator compounds |
| WO2020021024A1 (en) * | 2018-07-26 | 2020-01-30 | Phenex Pharmaceuticals Ag | Substituted bicyclic compounds as modulators of the aryl hydrocarbon receptor (ahr) |
| CN111163766A (en) * | 2017-08-17 | 2020-05-15 | 医肯纳肿瘤学公司 | AHR inhibitors and uses thereof |
| CN111662269A (en) * | 2019-03-07 | 2020-09-15 | 湖南化工研究院有限公司 | 1-pyridyl pyrazole amide compound and preparation method and application thereof |
| CN111670186A (en) * | 2017-12-20 | 2020-09-15 | Pi工业有限公司 | Pyrazolopyridine-diamides, their use as pesticides and processes for their preparation |
| WO2021000770A1 (en) * | 2019-07-02 | 2021-01-07 | 凯复制药有限公司 | Heterocyclic compound capable of enhancing immune activity, preparation method therefor and application in medicine |
-
2021
- 2021-07-05 CN CN202110757318.4A patent/CN115572282B/en active Active
Patent Citations (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004106324A1 (en) * | 2003-05-27 | 2004-12-09 | E.I. Dupont De Nemours And Company | Azolecarboxamide herbicides |
| CN101426779A (en) * | 2006-02-16 | 2009-05-06 | 先正达参股股份有限公司 | Pesticide containing bicyclic bisamide structure |
| WO2008036540A2 (en) * | 2006-09-20 | 2008-03-27 | Boehringer Ingelheim International Gmbh | Rho kinase inhibitors |
| CN101743237A (en) * | 2007-07-16 | 2010-06-16 | 先正达参股股份有限公司 | The condensed anthranilamide derivatives as insectisides |
| CN101827837A (en) * | 2007-08-22 | 2010-09-08 | 先正达参股股份有限公司 | The condensed anthranilamide derivatives as insectisides |
| US20110124654A1 (en) * | 2008-06-11 | 2011-05-26 | Huifen Chen | Diazacarbazoles and methods of use |
| CN102119162A (en) * | 2008-06-11 | 2011-07-06 | 健泰科生物技术公司 | Diazacarbazoles and methods of use |
| WO2010114726A1 (en) * | 2009-03-31 | 2010-10-07 | Merck Sharp & Dohme Corp. | Aminobenzotriazole derivatives |
| CN102791714A (en) * | 2010-03-26 | 2012-11-21 | 霍夫曼-拉罗奇有限公司 | N-(imidazopyrimidin-7-yl)-heteroarylamide derivatives and their use as PDE10A inhibitors |
| WO2013059587A1 (en) * | 2011-10-20 | 2013-04-25 | Sirtris Pharmaceuticals, Inc. | Substituted bicyclic aza-heterocycles and analogues as sirtuin modulators |
| CN103467380A (en) * | 2013-09-29 | 2013-12-25 | 南开大学 | Substituted phenyl pyrazole amide derivative and preparation method and application thereof |
| CN103755700A (en) * | 2013-12-26 | 2014-04-30 | 青岛科技大学 | Novel pyrazol amides compound and application thereof |
| CN110234650A (en) * | 2017-02-01 | 2019-09-13 | 菲尼克斯药品股份公司 | Aryl hydrocarbon receptor (AhR) adjusts immunomodulator compounds |
| CN110248940A (en) * | 2017-02-01 | 2019-09-17 | 菲尼克斯药品股份公司 | Aryl hydrocarbon receptor (AhR) adjusts immunomodulator compounds |
| CN110325532A (en) * | 2017-02-21 | 2019-10-11 | 菲尼克斯药品股份公司 | Aryl hydrocarbon receptor (AhR) adjusts immunomodulator compounds |
| CN111163766A (en) * | 2017-08-17 | 2020-05-15 | 医肯纳肿瘤学公司 | AHR inhibitors and uses thereof |
| CN111670186A (en) * | 2017-12-20 | 2020-09-15 | Pi工业有限公司 | Pyrazolopyridine-diamides, their use as pesticides and processes for their preparation |
| WO2020021024A1 (en) * | 2018-07-26 | 2020-01-30 | Phenex Pharmaceuticals Ag | Substituted bicyclic compounds as modulators of the aryl hydrocarbon receptor (ahr) |
| CN111662269A (en) * | 2019-03-07 | 2020-09-15 | 湖南化工研究院有限公司 | 1-pyridyl pyrazole amide compound and preparation method and application thereof |
| WO2021000770A1 (en) * | 2019-07-02 | 2021-01-07 | 凯复制药有限公司 | Heterocyclic compound capable of enhancing immune activity, preparation method therefor and application in medicine |
Non-Patent Citations (6)
Also Published As
| Publication number | Publication date |
|---|---|
| CN115572282B (en) | 2024-07-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6905521B2 (en) | New pyridadinone herbicide | |
| WO2020177778A1 (en) | 1-pyridylpyrazole amide compound, the preparation method and application thereof | |
| UA120656C2 (en) | New alkynyl-substituted 3-phenylpyrrolidine-2,4-diones and use thereof as herbicides | |
| EP1072598B9 (en) | Pyrazolinone derivatives | |
| CN111087345B (en) | Azobenzene heterocyclic amide derivative, and preparation method and application thereof | |
| CN110963973A (en) | Triketone compound containing quinazolinedione fragment, preparation method and application thereof, and herbicide | |
| CN113045561B (en) | Diarylamine derivatives as fungicides | |
| CN102464592A (en) | Acyl benzylamine compound and application thereof | |
| WO2019020981A1 (en) | Pyrazole, isothiazole and isoxazole derivatives useful as agricultural fungicides | |
| CN106543139B (en) | Triazolone compound and application thereof | |
| CN110551148B (en) | Compound containing silicon acyl acetonitrile, preparation method and application thereof | |
| CN115572282B (en) | Pyrazole amide compound containing aromatic heterocyclic structure, and preparation method and application thereof | |
| CN113549053B (en) | Pyrazoloquine (azolyl) ether compound and application thereof | |
| WO2024174317A1 (en) | N-phenylimine-containing derivative, preparation method therefor and use thereof | |
| CN111072568B (en) | Phenyl pyrazole compound containing azo structure, and preparation method and application thereof | |
| EP4003976B1 (en) | Picolinamide derivatives useful as agricultural fungicides | |
| CN112390727B (en) | Oxime carboxylate compound and application thereof | |
| JP6430518B2 (en) | Pesticide | |
| CN110964037B (en) | Pyrimidine-fused ring-containing compound and preparation method and application thereof | |
| CN110396083B (en) | Pyridazinonyl-contained butenolide compound and application thereof | |
| CN115197131A (en) | Azo 2-amino benzyl nicotinate derivative and preparation method and application thereof | |
| CN113512002B (en) | Pyrazole amide compound containing azo structure, and preparation and application thereof | |
| WO2020063982A1 (en) | Quinazolinedione fragment-containing compound, preparation method therefor, use thereof, and herbicide | |
| WO1995025730A1 (en) | Nicotinic acid derivative and herbicide | |
| CN105777640B (en) | Pyrazole cyclohexanediol ether compound and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |