CN114605380A - Hyperbranched macromolecular photoinitiator and preparation method and application thereof - Google Patents
Hyperbranched macromolecular photoinitiator and preparation method and application thereof Download PDFInfo
- Publication number
- CN114605380A CN114605380A CN202210382254.9A CN202210382254A CN114605380A CN 114605380 A CN114605380 A CN 114605380A CN 202210382254 A CN202210382254 A CN 202210382254A CN 114605380 A CN114605380 A CN 114605380A
- Authority
- CN
- China
- Prior art keywords
- formula
- hyperbranched
- reaction
- compound
- molar ratio
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000002360 preparation method Methods 0.000 title claims description 15
- 150000001875 compounds Chemical class 0.000 claims description 23
- 238000006243 chemical reaction Methods 0.000 claims description 17
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 17
- 229920002521 macromolecule Polymers 0.000 claims description 11
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical group CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 claims description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 10
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 claims description 10
- 239000003054 catalyst Substances 0.000 claims description 9
- NBOMNTLFRHMDEZ-UHFFFAOYSA-N thiosalicylic acid Chemical compound OC(=O)C1=CC=CC=C1S NBOMNTLFRHMDEZ-UHFFFAOYSA-N 0.000 claims description 9
- 229940103494 thiosalicylic acid Drugs 0.000 claims description 9
- 238000009835 boiling Methods 0.000 claims description 8
- 238000005886 esterification reaction Methods 0.000 claims description 8
- 238000000034 method Methods 0.000 claims description 7
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid Substances OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 6
- 238000000576 coating method Methods 0.000 claims description 6
- 239000012024 dehydrating agents Substances 0.000 claims description 6
- 239000001257 hydrogen Substances 0.000 claims description 6
- 229910052739 hydrogen Inorganic materials 0.000 claims description 6
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims description 6
- 239000002904 solvent Substances 0.000 claims description 6
- 125000006527 (C1-C5) alkyl group Chemical group 0.000 claims description 5
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical group C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 claims description 5
- 239000011248 coating agent Substances 0.000 claims description 5
- 229910052757 nitrogen Inorganic materials 0.000 claims description 5
- 239000000376 reactant Substances 0.000 claims description 5
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims description 5
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 claims description 4
- LGRFSURHDFAFJT-UHFFFAOYSA-N Phthalic anhydride Natural products C1=CC=C2C(=O)OC(=O)C2=C1 LGRFSURHDFAFJT-UHFFFAOYSA-N 0.000 claims description 4
- UHOVQNZJYSORNB-UHFFFAOYSA-N monobenzene Natural products C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 claims description 4
- -1 phthalic anhydride compound Chemical class 0.000 claims description 4
- 239000005548 dental material Substances 0.000 claims description 3
- 229910052736 halogen Inorganic materials 0.000 claims description 3
- 150000002367 halogens Chemical class 0.000 claims description 3
- 229920002120 photoresistant polymer Polymers 0.000 claims description 3
- 125000006833 (C1-C5) alkylene group Chemical group 0.000 claims description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 2
- 239000000463 material Substances 0.000 claims description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 2
- 230000035484 reaction time Effects 0.000 claims description 2
- 125000001424 substituent group Chemical group 0.000 claims description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims 1
- 239000005977 Ethylene Substances 0.000 claims 1
- 239000000976 ink Substances 0.000 claims 1
- 238000004519 manufacturing process Methods 0.000 claims 1
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 claims 1
- 230000008901 benefit Effects 0.000 abstract description 7
- 238000009826 distribution Methods 0.000 abstract description 6
- 238000007306 functionalization reaction Methods 0.000 abstract 1
- 239000000047 product Substances 0.000 description 37
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 27
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 24
- 238000003756 stirring Methods 0.000 description 12
- 238000005160 1H NMR spectroscopy Methods 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 6
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 239000011259 mixed solution Substances 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- 238000012546 transfer Methods 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 4
- 238000001723 curing Methods 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 4
- 230000037230 mobility Effects 0.000 description 4
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 230000008034 disappearance Effects 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000002390 rotary evaporation Methods 0.000 description 3
- 238000000967 suction filtration Methods 0.000 description 3
- 238000003848 UV Light-Curing Methods 0.000 description 2
- JHIWVOJDXOSYLW-UHFFFAOYSA-N butyl 2,2-difluorocyclopropane-1-carboxylate Chemical compound CCCCOC(=O)C1CC1(F)F JHIWVOJDXOSYLW-UHFFFAOYSA-N 0.000 description 2
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- BJMLLSSSTGHJJE-UHFFFAOYSA-N (4-methylbenzoyl) 4-methylbenzoate Chemical compound C1=CC(C)=CC=C1C(=O)OC(=O)C1=CC=C(C)C=C1 BJMLLSSSTGHJJE-UHFFFAOYSA-N 0.000 description 1
- BTJPUDCSZVCXFQ-UHFFFAOYSA-N 2,4-diethylthioxanthen-9-one Chemical compound C1=CC=C2C(=O)C3=CC(CC)=CC(CC)=C3SC2=C1 BTJPUDCSZVCXFQ-UHFFFAOYSA-N 0.000 description 1
- KOVQQOMROOKART-UHFFFAOYSA-N 2-[2-hydroxyethyl(methyl)amino]propan-1-ol Chemical compound OCC(C)N(C)CCO KOVQQOMROOKART-UHFFFAOYSA-N 0.000 description 1
- 239000004925 Acrylic resin Substances 0.000 description 1
- 229920000178 Acrylic resin Polymers 0.000 description 1
- HVVWZTWDBSEWIH-UHFFFAOYSA-N [2-(hydroxymethyl)-3-prop-2-enoyloxy-2-(prop-2-enoyloxymethyl)propyl] prop-2-enoate Chemical compound C=CC(=O)OCC(CO)(COC(=O)C=C)COC(=O)C=C HVVWZTWDBSEWIH-UHFFFAOYSA-N 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 238000009776 industrial production Methods 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D335/00—Heterocyclic compounds containing six-membered rings having one sulfur atom as the only ring hetero atom
- C07D335/04—Heterocyclic compounds containing six-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D335/10—Dibenzothiopyrans; Hydrogenated dibenzothiopyrans
- C07D335/12—Thioxanthenes
- C07D335/14—Thioxanthenes with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached in position 9
- C07D335/16—Oxygen atoms, e.g. thioxanthones
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2/00—Processes of polymerisation
- C08F2/46—Polymerisation initiated by wave energy or particle radiation
- C08F2/48—Polymerisation initiated by wave energy or particle radiation by ultraviolet or visible light
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D4/00—Coating compositions, e.g. paints, varnishes or lacquers, based on organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond ; Coating compositions, based on monomers of macromolecular compounds of groups C09D183/00 - C09D183/16
- C09D4/06—Organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond in combination with a macromolecular compound other than an unsaturated polymer of groups C09D159/00 - C09D187/00
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
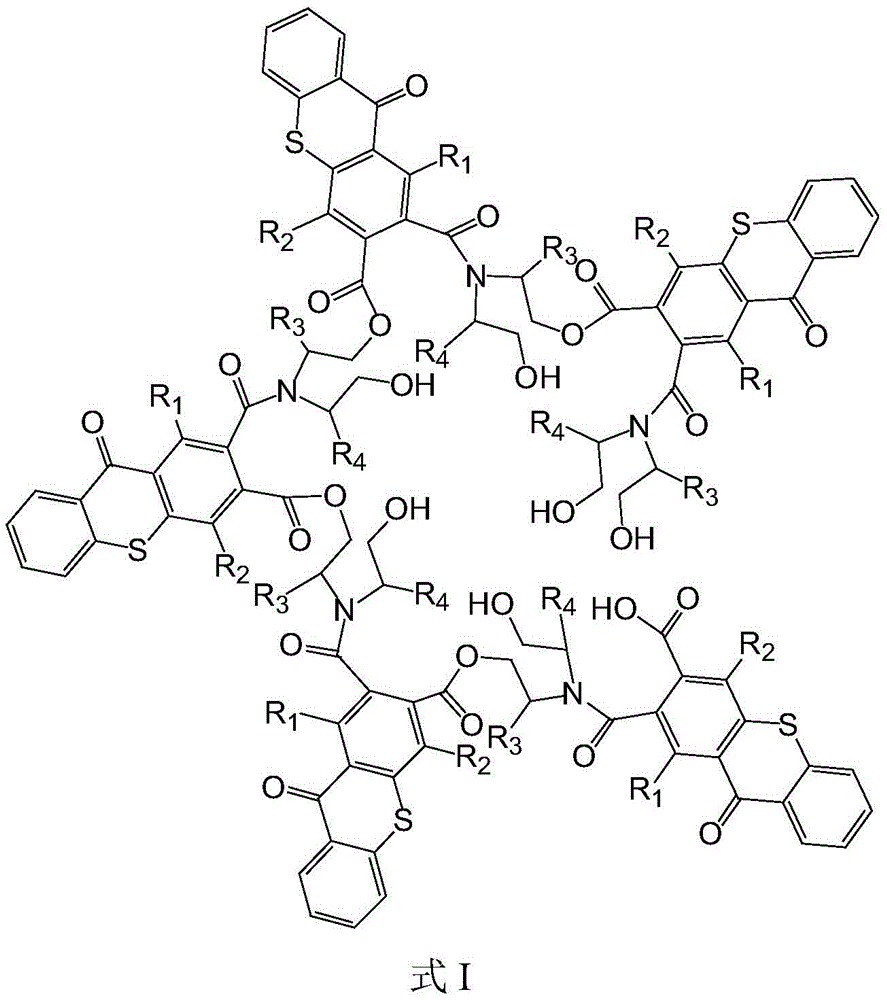
The hyperbranched macromolecular photoinitiator has a structure shown in a formula I, has more molecular branched chains and wider relative molecular mass distribution, and therefore has better solubility and lower viscosity, and has a large number of end groups around molecules, so that the hyperbranched macromolecular photoinitiator also has the advantage of multiple functionalization.
Description
Technical Field
The invention belongs to the technical field of photopolymerization, and relates to a hyperbranched macromolecular photoinitiator and a preparation method and application thereof.
Background
The ultraviolet curing technology has the outstanding advantages of high coating curing speed, environmental friendliness and the like. The photoinitiator plays an important role in the light curing technology and has an important influence on the ultraviolet light curing technology. The macromolecular photoinitiator can solve the problems of migration and volatilization of the micromolecular photoinitiator, but the solubility of macromolecules is poor due to the increase of the relative molecular mass of the macromolecules. Polymerizable macrophotoinitiators have recently been attracting attention from developers, but have also had problems such as poor monomer solubility. The hyperbranched macromolecular photoinitiator has more molecular branched chains and wider distribution of relative molecular mass, thereby having better solubility and lower viscosity, and having a plurality of end groups around the molecules, thereby having the advantage of multifunction. And the energy transfer between the photoinitiator unit and the coinitiator unit is intramolecular transfer, the transfer speed is high, and the initiation efficiency is high. The invention can further solve the problems of pollution and compatibility and greatly broadens the field of UV light curing.
Disclosure of Invention
The hyperbranched macromolecular photoinitiator has a structure shown in a formula I, and has the advantages of good solubility and low viscosity due to the existence of cavities in molecules, an ellipsoidal three-dimensional structure and relatively wide molecular mass distribution, and multiple functions due to the existence of a large number of end groups around the molecules.
In order to achieve the purpose, the invention adopts the following technical scheme:
in one aspect, the present invention provides a hyperbranched macrophotoinitiator having a structure represented by formula I:
wherein R is1Is absent, or is selected from substituted or unsubstituted C1-C5 alkylene, R2、R3And R4Independently selected from hydrogen, substituted or unsubstituted C1-C5 alkyl, when said group has a substituent selected from halogen or C1-C5 alkyl.
The hyperbranched macromolecular photoinitiator has the advantages of more molecular branched chains and wider distribution of relative molecular mass, thereby having better solubility and lower viscosity, and having a plurality of end groups around the molecules, thereby having multiple functions.
In the invention R1、R2、R3And R4Independently selected from hydrogen, substituted or unsubstituted C1-C5 alkyl, representing R1、R2、R3And R4Independently selected from hydrogen, substituted or unsubstituted C1, C2, C3, C4 or C5 alkyl; preferably, R1、R2、R3And R4Independently selected from hydrogen, methyl or ethyl.
As a preferred technical scheme of the invention, the hyperbranched macromolecular photoinitiator is selected from any one of compounds a to c:
the hyperbranched macromolecular photoinitiator has the advantages of multiple molecular branches, wide distribution of relative molecular mass, good solubility and low viscosity, and multiple functions because a large number of end groups exist around the molecules.
In another aspect, the present invention provides a method for preparing the hyperbranched macromolecular photoinitiator described above, the method comprising the steps of:
(1) reacting thiosalicylic acid with a phthalic anhydride compound shown in a formula II to obtain a compound shown in a formula III, wherein the reaction formula is as follows:
(2) reacting the compound shown in the formula III with an alcamines compound to obtain a compound shown in the formula IV, wherein the reaction formula is as follows:
(3) and (3) carrying out hyperbranched macromolecule esterification reaction on the compound shown in the formula IV to obtain the hyperbranched macromolecule photoinitiator shown in the formula I, wherein the reaction formula is as follows:
preferably, the molar ratio of the thiosalicylic acid to the benzene anhydride compound represented by the formula II in the step (1) is 1: 3-9, such as 1:3, 1:3.3, 1:3.5, 1:3.8, 1:4, 1:4.5, 1:4.8, 1:5, 1:5.5, 1:5.8, 1:6, 1:6.5, 1:6.8, 1:7, 1:7.5, 1:7.8, 1:8, 1:8.5 or 1: 9.
Preferably, the reaction of step (1) is carried out in concentrated sulfuric acid.
Preferably, the molar ratio of the concentrated sulfuric acid to the thiosalicylic acid is 5 to 10:1, such as 5:1, 5.3:1, 5.5:1, 5.8:1, 6:1, 6.4:1, 6.8:1, 7:1, 7.3:1, 7.5:1, 7.8:1, 9:1, 9.2:1, 9.5:1, 9.8:1, or 10: 1.
Preferably, the reaction in step (1) is performed by reacting at room temperature for 1-5 h (e.g. 1h, 1.5h, 2h, 2.5h, 3h, 3.5h, 4h, 4.5h or 5h), then heating to 50-80 ℃ (e.g. 50 ℃, 53 ℃, 55 ℃, 58 ℃, 60 ℃, 62 ℃, 65 ℃, 68 ℃, 70 ℃, 75 ℃, 78 ℃ or 80 ℃) for 3-10 h (e.g. 3h, 3.5h, 3.8h, 4h, 4.3h, 4.5h, 5h, 5.5h, 6h, 6.5h, 7h, 7.5h, 8h, 8.5h, 9h, 9.5h or 10h), and finally pouring the reactant into boiling water for 10-30 min (e.g. 10min, 13min, 15min, 18min, 20min, 23min, 25min, 28min or 30 min).
Preferably, the molar ratio of the compound of formula III to the alkanolamine compound in step (2) is 1: 2-5, such as 1:2, 1:2.5, 1:2.8, 1:3, 1:3.5, 1:3.8, 1:4, 1:4.5, 1:4.8 or 1:5.
Preferably, the reaction of step (2) is carried out under nitrogen protection.
Preferably, the solvent for the reaction in step (2) is any one or a combination of at least two of tetrahydrofuran, acetone or butanone.
Preferably, the temperature of the reaction in step (2) is 25 to 50 ℃, such as 25 ℃, 28 ℃,30 ℃,35 ℃, 38 ℃, 40 ℃, 45 ℃, 48 ℃ or 50 ℃.
Preferably, the reaction time in step (2) is 2-5 h, such as 2h, 2.5h, 2.8h, 3h, 3.5h, 4h, 4.5h or 5 h.
Preferably, the hyperbranched esterification reaction of step (3) is carried out in the presence of a catalyst and a dehydrating agent.
Preferably, the catalyst is 4-dimethylaminopyridine.
Preferably, the dehydrating agent is dicyclohexylcarbodiimide.
Preferably, the molar ratio of the catalyst to the compound of formula IV is 1 (1-10), such as 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9 or 1: 10.
Preferably, the molar ratio of the dehydrating agent to the compound of formula IV is 1 (1-10), such as 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, or 1: 10.
Preferably, the temperature of the hyperbranched macromolecule esterification reaction in the step (3) is 50-80 ℃, such as 50 ℃, 53 ℃, 55 ℃, 58 ℃, 60 ℃, 62 ℃, 65 ℃, 68 ℃, 70 ℃, 75 ℃, 78 ℃ or 80 ℃.
Preferably, the time of the esterification reaction of the hyperbranched macromolecule in the step (3) is 5-10 h, such as 5h, 5.5h, 6h, 6.5h, 7h, 7.5h, 8h, 8.5h, 9h, 9.5h or 10 h.
The preparation method is simple and efficient, is simple to operate, has low cost, does not relate to toxic substances such as halogen and high-boiling-point solvents, is suitable for industrial production, and can be used in important technical fields such as coatings, printing ink, dental materials and photoresist.
In another aspect, the present invention provides the use of a hyperbranched macrophotoinitiator as described above in the preparation of a coating, an ink, a dental material or a photoresist material.
Compared with the prior art, the invention has the following beneficial effects:
the hyperbranched macromolecular photoinitiator has more molecular branched chains and wider relative molecular mass distribution, thereby having better solubility and lower viscosity, and having a plurality of end groups around the molecules, thereby having the advantage of multifunction. And the energy transfer between the photoinitiator unit and the coinitiator unit is intramolecular transfer, the transfer speed is high, the initiation efficiency is high, the mobility is low, and the mobility is as low as below 0.07 mg/kg. The invention can further solve the problems of pollution and compatibility and greatly broadens the field of UV light curing.
Detailed Description
The technical solution of the present invention is further explained by the following embodiments. It should be understood by those skilled in the art that the examples are only for the understanding of the present invention and should not be construed as the specific limitations of the present invention.
Example 1
In this example, a process for the preparation of compound a is provided:
the preparation method comprises the following steps:
(1) slowly adding 0.01mol of thiosalicylic acid into 0.09mol of concentrated sulfuric acid, fully stirring, then adding 0.05mol of phthalic anhydride in batches, mechanically stirring, reacting at room temperature for 1h, heating to 80 ℃ for reacting for 3h, pouring the reactant into boiling water, boiling for 15min, carrying out suction filtration, and recrystallizing by using a mixed solution of ethanol and water (the volume ratio of ethanol to water is 4: 1) to obtain a product shown in the formula III. The occurrence of the product anhydride carbonyl peak is observed by infrared, and the nuclear magnetic peaks are 1H NMR (300MHz, DMSO) and delta 8.4-7.2 (m,7H), which indicates that the product is successfully synthesized.
(2) Adding 0.01mol of the product of the formula III into a DMF flask, fully stirring, adding 0.07mol of diethanolamine, mechanically stirring, reacting for 2 hours at room temperature under the protection of nitrogen, filtering, and recrystallizing with a mixed solution of ethanol and water (the volume ratio of ethanol to water is 4: 1) to obtain the product of the formula IV. The appearance of the carboxyl peak of the product is observed by infrared; nuclear magnetic peaks 1H NMR (300MHz, DMSO). delta.13.4 (s,1H), 8.2-7.4 (m,7H), 4.9(d,2H), 3.7(m,4H), 3.59(m,4H), indicating successful synthesis of the product.
(3) Adding 0.036mol of the product into 50ml of tetrahydrofuran for dissolving, reacting for 5 hours at 72 ℃ by using 0.002mol of 4-dimethylaminopyridine catalyst and 0.002mol of dicyclohexylcarbodiimide as dehydrating agents, and removing the solvent by rotary evaporation to obtain the target product. The disappearance of the carboxyl peak and the appearance of the ester peak of the product are observed by infrared; nuclear magnetic peak1H NMR (300MHz, DMSO), delta 13.29(s,1H), 8.1-7.2 (m,35H), 4.81-4.72 (m,14H), 3.7-3.4 (m, 32H). By Agilent Technologies GPC measurement MS (m/z): 1865(M +1)+The successful synthesis of the product is demonstrated.
Example 2
In this example, a process for the preparation of compound b is provided:
the preparation method comprises the following steps:
(1) slowly adding 0.01mol of thiosalicylic acid into 0.09mol of concentrated sulfuric acid, fully stirring, then adding 0.05mol of p-methyl benzoic anhydride in batches, mechanically stirring, reacting at room temperature for 1h, heating to 80 ℃ for reacting for 3h, pouring the reactant into boiling water, boiling for 15min, carrying out suction filtration, and recrystallizing by using a mixed solution of ethanol and water (the volume ratio of ethanol to water is 4: 1) to obtain a product shown in the formula III. The occurrence of the product anhydride carbonyl peak is observed by infrared, and the nuclear magnetic peaks are 1H NMR (300MHz, DMSO), delta 8.0-6.9 (m,7H) and 2.3-2.5 (m,6H), which indicates that the product is successfully synthesized. Indicating the successful synthesis of the product.
(2) Adding 0.01mol of the product of the formula III into a DMF flask, fully stirring, adding 0.07mol of diethanolamine, mechanically stirring, reacting for 2 hours at room temperature under the protection of nitrogen, filtering, and recrystallizing with a mixed solution of ethanol and water (the volume ratio of ethanol to water is 4: 1) to obtain the product of the formula IV. The appearance of the carboxyl peak of the product is observed by infrared, and the nuclear magnetic peaks 1H NMR (300MHz, DMSO) delta 13.6(s,1H), 7.9-6.8 (m,7H), 4.9(d,2H), 3.62-3.71 (m,8H), 2.3-2.55 (m,6H) indicate that the product is successfully synthesized.
(3) Adding 0.036mol of the product into 50ml of tetrahydrofuran for dissolving, reacting for 5 hours at 72 ℃ by using 0.002mol of 4-dimethylaminopyridine catalyst and 0.002mol of dicyclohexylcarbodiimide as dehydrating agents, and removing the solvent by rotary evaporation to obtain the target product. The disappearance of the carboxyl peak and the appearance of the ester peak of the product are observed by infrared; nuclear magnetic peak1H NMR (300MHz, DMSO), delta 13.76(s,1H), 8.1-7.2 (m,35H), 4.85-4.71 (m,14H), 3.71-3.4 (m,32H), 2.35(m,30H), MS (m/z) using Agilent Technologies GPC: 2005(M +1)+The successful synthesis of the product is demonstrated.
Example 3
In this example, a process for the preparation of compound c is provided:
the preparation method comprises the following steps:
(1) slowly adding 0.01mol of thiosalicylic acid into 0.09mol of concentrated sulfuric acid, fully stirring, then adding 0.05mol of phthalic anhydride in batches, mechanically stirring, reacting at room temperature for 1h, heating to 80 ℃ for reacting for 3h, pouring the reactant into boiling water, boiling for 15min, carrying out suction filtration, and recrystallizing by using a mixed solution of ethanol and water (the volume ratio of ethanol to water is 4: 1) to obtain a product shown in the formula III. The occurrence of the product anhydride carbonyl peak is observed by infrared, and the nuclear magnetic peaks are 1H NMR (300MHz, DMSO) and delta 8.6-7.2 (m,7H), which indicates that the product is successfully synthesized. Indicating the successful synthesis of the product.
(2) Adding 0.01mol of the product of the formula III into a DMF flask, fully stirring, adding 0.07mol of dimethyl-diethanolamine, mechanically stirring, reacting at room temperature for 2h under the protection of nitrogen, filtering, and recrystallizing with a mixed solution of ethanol and water (the volume ratio of ethanol to water is 4: 1) to obtain the product of the formula IV. The appearance of the carboxyl peak of the product is observed by infrared, and the nuclear magnetic peaks 1H NMR (300MHz, DMSO) delta 13.4(s,1H), 8.2-7.4 (m,7H), 4.9(d,2H), 3.7(d,2H), 3.59(m,4H), 3.7(m,4H) indicate that the product is successfully synthesized.
(3) Adding 0.036mol of the product into 50ml of tetrahydrofuran for dissolving, reacting for 5 hours at 72 ℃ by using 0.002mol of 4-dimethylaminopyridine catalyst and 0.002mol of dicyclohexylcarbodiimide as dehydrating agents, and removing the solvent by rotary evaporation to obtain the target product. The disappearance of the carboxyl peak and the appearance of the ester peak of the product are observed by infrared; nuclear magnetic peak1H NMR (300MHz, DMSO), delta 13.77(s,1H), 8.1-7.21 (m,35H), 4.82-4.72 (m,14H), 3.7-3.45 (m,32H), 1.26(m,30H), MS (m/z) using Agilent Technologies GPC: 2004(M +1)+The successful synthesis of the product is demonstrated.
Example 4
In a yellow light laboratory, the final products prepared in examples 1 to 3 and commercially available DETX were used as initiators, and the photocureable coating was prepared with acrylic resin, pentaerythritol triacrylate and a fluorine-modified leveling agent. Coating on a 7cm × 7cm blank glass plate on a rotary spin coater at 500rpm, pre-baking at 100 deg.C for 2min, exposing at 50mJ, exposing at 250 deg.C for 30min, and making into cured film. The pencil hardness of the cured film is tested by reference to GB/T6739-2006, the adhesive force of the cured film is tested by reference to GB/T9286-1998, and the test data are shown in Table 1. The solidified membrane is dissolved in acetonitrile solution, filtrate is obtained through extraction and purification, and the mobility is tested by adopting gas chromatography-mass spectrometry (GC-MS), and the test results are shown in Table 1.
TABLE 1
As can be seen from Table 1, the photoinitiators of the invention have low mobilities, as low as below 0.07 mg/kg.
The applicant states that the present invention is illustrated by the above examples to the hyperbranched macrophotoinitiators of the invention, the preparation and the use thereof, but the present invention is not limited to the above examples, i.e. it does not mean that the present invention has to be implemented by relying on the above examples. It should be understood by those skilled in the art that any modification of the present invention, equivalent substitutions of the raw materials of the product of the present invention, addition of auxiliary components, selection of specific modes, etc., are within the scope and disclosure of the present invention.
Claims (10)
1. A hyperbranched macromolecular photoinitiator, which is characterized by having a structure shown in formula I:
wherein R is1Is absent, or is selected from substituted or unsubstituted C1-C5 alkylene, R2、R3、R4Independently selected from hydrogen, substituted or unsubstituted C1-C5 alkyl, when said group has a substituent selected from halogen or C1-C5 alkyl.
2. The hyperbranched macrophotoinitiator according to claim 1, wherein R is R1Absent, or methylene or ethylene.
3. Hyperbranched macrophotoinitiator according to claim 1 or 2, wherein R is R2、R3、R4Independently selected from hydrogen, methyl or ethyl.
5. the method of preparing a hyperbranched macrophotoinitiator according to any one of claims 1 to 4, comprising the steps of:
(1) reacting thiosalicylic acid with a phthalic anhydride compound shown in a formula II to obtain a compound shown in a formula III, wherein the reaction formula is as follows:
(2) reacting the compound shown in the formula III with an alcamines compound to obtain a compound shown in the formula IV, wherein the reaction formula is as follows:
(3) and (3) carrying out hyperbranched macromolecule esterification reaction on the compound shown in the formula IV to obtain the hyperbranched macromolecule photoinitiator shown in the formula I, wherein the reaction formula is as follows:
wherein R is1、R2、R3And R4The definition of (a) is the same as the definition in the structure shown in formula I.
6. The preparation method according to claim 5, wherein the molar ratio of the thiosalicylic acid in the step (1) to the benzene anhydride compound shown in the formula II is 1: 4-10.
7. The production method according to claim 5 or 6, wherein the reaction in step (1) is carried out in concentrated sulfuric acid;
preferably, the molar ratio of the concentrated sulfuric acid to the thiosalicylic acid is 6-10: 1;
preferably, the reaction in the step (1) is firstly carried out at room temperature for 1-5 h, then the temperature is raised to 50-80 ℃ for reaction for 3-10 h, and finally the reactant is poured into boiling water and boiled for 10-30 min.
8. The preparation method according to any one of claims 5 to 7, wherein the molar ratio of the compound represented by the formula III to the alkanolamine compound in the step (2) is 1: 2-5.
9. The method according to any one of claims 5 to 8, wherein the reaction of step (2) is carried out under a nitrogen blanket;
preferably, the solvent for the reaction in step (2) is any one or a combination of at least two of tetrahydrofuran, acetone or butanone;
preferably, the reaction temperature in the step (2) is 25-50 ℃;
preferably, the reaction time in the step (2) is 2-5 h;
preferably, the esterification reaction of the hyperbranched macromolecules in the step (3) is carried out in the presence of a catalyst and a dehydrating agent;
preferably, the catalyst is 4-dimethylaminopyridine;
preferably, the dehydrating agent is dicyclohexylcarbodiimide;
preferably, the molar ratio of the catalyst to the compound of formula IV is 1 (1-10);
preferably, the molar ratio of the dehydrating agent to the compound of formula IV is 1 (1-10);
preferably, the temperature of the esterification reaction of the hyperbranched macromolecules in the step (3) is 50-80 ℃;
preferably, the time of the esterification reaction of the hyperbranched macromolecules in the step (3) is 5-10 h.
10. Use of a hyperbranched macrophotoinitiator according to any one of claims 1 to 4 in the preparation of a coating, ink, dental material or photoresist material.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210382254.9A CN114605380A (en) | 2022-04-12 | 2022-04-12 | Hyperbranched macromolecular photoinitiator and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210382254.9A CN114605380A (en) | 2022-04-12 | 2022-04-12 | Hyperbranched macromolecular photoinitiator and preparation method and application thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN114605380A true CN114605380A (en) | 2022-06-10 |
Family
ID=81868902
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210382254.9A Pending CN114605380A (en) | 2022-04-12 | 2022-04-12 | Hyperbranched macromolecular photoinitiator and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114605380A (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016192610A1 (en) * | 2015-06-03 | 2016-12-08 | 江苏和成新材料有限公司 | Acyloxime ester compound used for uv curing material, synthesis method of same, and application of same |
| CN111072794A (en) * | 2019-12-31 | 2020-04-28 | 阜阳欣奕华材料科技有限公司 | Polymerizable photoinitiator and preparation method and application thereof |
| CN111087494A (en) * | 2019-12-31 | 2020-05-01 | 阜阳欣奕华材料科技有限公司 | Polymerizable photoinitiator and preparation method and application thereof |
-
2022
- 2022-04-12 CN CN202210382254.9A patent/CN114605380A/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016192610A1 (en) * | 2015-06-03 | 2016-12-08 | 江苏和成新材料有限公司 | Acyloxime ester compound used for uv curing material, synthesis method of same, and application of same |
| CN111072794A (en) * | 2019-12-31 | 2020-04-28 | 阜阳欣奕华材料科技有限公司 | Polymerizable photoinitiator and preparation method and application thereof |
| CN111087494A (en) * | 2019-12-31 | 2020-05-01 | 阜阳欣奕华材料科技有限公司 | Polymerizable photoinitiator and preparation method and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN108586639B (en) | Photopolymerisable thioxanthone photoinitiator containing coinitiator amine and preparation method thereof | |
| CN103130919B (en) | Carbazole ketone oxime ester high-photosensibility photoinitiator | |
| CN102212150B (en) | Polymerizable thioxanthone photoinitiator containing auxiliary initiator amine and preparation method thereof | |
| EP1051665A1 (en) | Photopolymerization compositions including maleimides and processes for using the same | |
| JP2009191179A (en) | Photopolymerization initiator, polymerizable composition, and method for producing polymer | |
| CN112105672B (en) | Photoreactive composition, reaction product, and method for producing reaction product | |
| JP2010106278A (en) | Derivatized polyhydroxystyrene having novolac type structure | |
| CN109384670A (en) | A kind of water-soluble light trigger and preparation method thereof | |
| KR100406016B1 (en) | Method for preparing thioxant and its derivative | |
| Chen et al. | Novel multifunctional polymeric photoinitiators and photo‐coinitiators derived from hyperbranched polyglycerol | |
| WO2019101142A1 (en) | Dibutylfluorene derivative and application thereof as photoinitiator | |
| CN111087494B (en) | Polymerizable photoinitiator and preparation method and application thereof | |
| CN113508106A (en) | Photoinitiator composition containing acylcarbazole derivative and carbazolyl oxime ester and application of photoinitiator composition in photocuring composition | |
| CN114605380A (en) | Hyperbranched macromolecular photoinitiator and preparation method and application thereof | |
| CN111072794B (en) | Polymerizable photoinitiator and preparation method and application thereof | |
| CN110950977B (en) | Acylphosphine oxide photoinitiator and synthesis method thereof | |
| CN106279470A (en) | Amphipathy macromolecule hydrogen-capture-type light initiator based on hyperbranched polyetheramine and preparation method thereof | |
| CN114369179A (en) | Photoinitiator composition, photocuring composition and product containing photoinitiator composition | |
| CN109134710A (en) | A kind of arylsulfonium salts oxime ester lightlike initiating agent and its synthesis and application | |
| CN114181110B (en) | Bisphenol fluorene oxime ester photoinitiator and preparation method and application thereof | |
| CN102120783A (en) | Aliphatic tertiary amine-containing thioxanthone photoinitiator and preparation method thereof | |
| CN112409510A (en) | Anthracene nucleus column aromatic hydrocarbon initiator and preparation method thereof | |
| CN107765510B (en) | 9-phenylacridine macromolecular photosensitizer and preparation method and application thereof | |
| CN114316092A (en) | Photoinitiator, preparation method and application thereof | |
| CN116589618B (en) | Single-component macromolecular photoinitiator and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20220610 |
|
| RJ01 | Rejection of invention patent application after publication |