CN114561557B - Process for rapidly dissolving nickel beans - Google Patents
Process for rapidly dissolving nickel beans Download PDFInfo
- Publication number
- CN114561557B CN114561557B CN202210047082.XA CN202210047082A CN114561557B CN 114561557 B CN114561557 B CN 114561557B CN 202210047082 A CN202210047082 A CN 202210047082A CN 114561557 B CN114561557 B CN 114561557B
- Authority
- CN
- China
- Prior art keywords
- nickel
- beans
- sulfuric acid
- nickel beans
- hydrogen peroxide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B23/00—Obtaining nickel or cobalt
- C22B23/04—Obtaining nickel or cobalt by wet processes
- C22B23/0407—Leaching processes
- C22B23/0415—Leaching processes with acids or salt solutions except ammonium salts solutions
- C22B23/043—Sulfurated acids or salts thereof
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B3/00—Extraction of metal compounds from ores or concentrates by wet processes
- C22B3/04—Extraction of metal compounds from ores or concentrates by wet processes by leaching
- C22B3/06—Extraction of metal compounds from ores or concentrates by wet processes by leaching in inorganic acid solutions, e.g. with acids generated in situ; in inorganic salt solutions other than ammonium salt solutions
- C22B3/08—Sulfuric acid, other sulfurated acids or salts thereof
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B3/00—Extraction of metal compounds from ores or concentrates by wet processes
- C22B3/20—Treatment or purification of solutions, e.g. obtained by leaching
- C22B3/44—Treatment or purification of solutions, e.g. obtained by leaching by chemical processes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P10/00—Technologies related to metal processing
- Y02P10/20—Recycling
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Metallurgy (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Geology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- Geochemistry & Mineralogy (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Manufacture And Refinement Of Metals (AREA)
- Silicon Compounds (AREA)
Abstract
The invention discloses a method for rapidly dissolving nickel beansA process of adsorbing Fe by adding a coupling agent and aluminum silicate gel particles while dissolving nickel beans 3+ Preventing the complex formed by the nickel-containing compound, water and anions from adhering to the surface of the nickel beans to prevent the dissolution of the nickel beans; the process increases the dissolution rate of nickel beans, reduces the dosage of sulfuric acid, and reduces the content of sulfate ions in the subsequent nickel-containing products.
Description
Technical Field
The invention relates to the technical field of dissolution recovery of nickel, in particular to a process for rapidly dissolving nickel beans.
Background
With the increasing application of nickel in ternary polymer lithium batteries and chemical catalysts, the efficient recovery of nickel and the preparation of high-purity nickel products become key technologies in the current nickel recovery industry.
At present, the sources of recovered nickel mainly comprise electroplating sludge, recovered terpolymer lithium batteries and the like, but with the increase of the demand of nickel, the sources of recovered nickel mainly depend on purchased or imported nickel beans at present.
Nickel beans are high nickel-containing metal blocks, the single grain weight of the nickel beans is 70g-100g, the block shape is large, and the reaction is slow when the nickel beans are dissolved by concentrated sulfuric acid, so that the concentrated sulfuric acid in a dissolution system generally needs to be excessive so as to improve the dissolution rate of the nickel beans; however, this also results in more sulfuric acid entering the subsequent purification extraction process, which not only affects the extraction efficiency, but also additionally introduces sulfate ions, which affects the quality of the subsequent preparation of alkaline nickel carbonate or other nickel-containing products.
Disclosure of Invention
The invention discloses a process for rapidly dissolving nickel beans, which comprises the steps of adding coupling agent and aluminum silicate gel particles to adsorb Fe when dissolving nickel beans 3+ Preventing the complex formed by the nickel-containing compound, water and anions from adhering to the surface of the nickel beans to prevent the dissolution of the nickel beans; the process greatly improves the dissolution rate of nickel beans, can reduce the dosage of sulfuric acid, and reduces the content of sulfate ions in the subsequent nickel-containing products.
A process for rapidly dissolving nickel beans, which comprises the following steps:
(1) Adding nickel beans into a reaction tank, and thenAdding coupling agent of 0.5-0.8%o by mass of nickel bean, and then mixing according to a liquid-solid ratio of 2.8-3.0m 3 Adding sulfuric acid solution with equivalent concentration of 4.5N-4.6N per t, adding 10% -15% hydrogen peroxide 3 times after reacting for 0.5-0.6h, wherein the interval time is 0.5h each time, and the total amount of the added hydrogen peroxide is 5% -8% of the volume of the sulfuric acid solution;
(2) After the reaction is finished, removing solid impurities by a filter pressing mode, and obtaining the high nickel-containing solution.
The coupling agent is sodium pyrophosphate or sodium polymetaphosphate.
The process introduces phosphate ions and affects the quality of the subsequent nickel-containing products, so the invention develops another process for rapidly dissolving nickel beans, which comprises the following steps:
a process for rapidly dissolving nickel beans, which comprises the following steps:
(1) Dissolving sodium silicate into hot water to prepare 5% -6% sodium silicate aqueous solution, then dripping dilute sulfuric acid with the mass fraction of 0.5% -1% into the solution to be acidic, continuously stirring until the solution becomes thick, adding microsilica with the mass of 1-2 times of that of sodium silicate and a silane coupling agent with the mass of 3% -5% of that of sodium silicate, uniformly dispersing, and standing to form sol;
(2) Washing with dilute sulfuric acid of 0.5-1% in the same volume for 2-3 times, heating to make the sol lose water by 30-40%, adding 30% hydrogen peroxide to make the gel contain 10-12% hydrogen peroxide, cutting into blocks, and making into aluminum silicate gel particles;
(3) Adding nickel beans into a reaction tank, adding aluminum silicate gel particles with the mass fraction of 1-3%o of the nickel beans, and then adding the aluminum silicate gel particles according to the liquid-solid ratio of 2.9-3.0m 3 Adding sulfuric acid solution with equivalent concentration of 4.0N-4.2N; after 0.2 to 0.3 hour of reaction, adding 10 to 15 percent of hydrogen peroxide for 3 times, wherein the interval time of each time is 0.3 hour, and the total amount of the added hydrogen peroxide is 5 to 8 percent of the volume of the sulfuric acid solution;
(4) After the reaction is finished, removing solid impurities by a filter pressing mode, and obtaining the high nickel-containing solution.
Further, the modulus of the sodium silicate is 2-3.
Further, the grain diameter of the micro silicon powder is 8-10 mu m.
Further, the silane coupling agent is KH560 or KH570.
Further, the particle size of the aluminum silicate gel particles is 3-5mm.
The invention has the advantages that:
1. the invention adds coupling agent and aluminum silicate gel particles to adsorb Fe when dissolving nickel bean 3+ Preventing the complex formed by the nickel-containing compound, water and anions from adhering to the surface of the nickel beans to prevent the dissolution of the nickel beans; the process increases the dissolution rate of nickel beans, reduces the dosage of sulfuric acid, and reduces the content of sulfate ions in the subsequent nickel-containing products;
2. after a small amount of hydrogen peroxide is adsorbed by the aluminum silicate gel particles, dissolved Fe can be dissolved out 2+ Oxidation to Fe 3+ And is fixed in the form of ferric aluminum silicate, so that the introduction of phosphate ion impurities by using a coupling agent is avoided.
Detailed Description
Example 1
A process for rapidly dissolving nickel beans, which comprises the following steps:
(1) Adding nickel beans, then adding coupling agent sodium polymetaphosphate with the mass fraction of 0.6 per mill of the nickel beans into a reaction tank, and then adding sodium polymetaphosphate with the mass fraction of the nickel beans into the reaction tank according to the liquid-solid ratio of 2.9m 3 Adding sulfuric acid solution with equivalent concentration of 4.6N per t, adding 15% hydrogen peroxide 3 times after reacting for 0.5h, wherein the interval time is 0.5h each time, and the total amount of the added hydrogen peroxide is 7% of the volume of the sulfuric acid solution;
(2) After the reaction is finished, removing solid impurities by a filter pressing mode, and obtaining the high nickel-containing solution.
Example 2
A process for rapidly dissolving nickel beans, which comprises the following steps:
(1) Dissolving sodium silicate with the modulus of 2.3 in hot water to prepare a 6% sodium silicate aqueous solution, then dripping dilute sulfuric acid with the mass fraction of 1% into the solution to be acidic, continuously stirring until the solution becomes thick, adding microsilica with the particle size of 10 mu m, which is 2 times of the mass of the sodium silicate, and a silane coupling agent KH560 with the mass of 5% of the sodium silicate, uniformly dispersing, and standing to form sol;
(2) Washing with 1% dilute sulfuric acid with the same volume for 2 times, heating to dehydrate 40% of sol, adding 30% hydrogen peroxide to make gel contain 12% hydrogen peroxide, cutting into blocks, and making into aluminum silicate gel particles with particle size of 3 mm;
(3) Adding nickel beans into a reaction tank, adding aluminum silicate gel particles with the mass fraction of 3 per mill of the nickel beans, and then adding the aluminum silicate gel particles according to the liquid-solid ratio of 3.0m 3 Adding sulfuric acid solution with equivalent concentration of 4.0N; after 0.2h of reaction, adding 15% hydrogen peroxide for 3 times, wherein the interval time of each time is 0.3h, and the total amount of the added hydrogen peroxide is 5% of the volume of the sulfuric acid solution;
(4) After the reaction is finished, removing solid impurities by a filter pressing mode, and obtaining the high nickel-containing solution.
Example 3
A process for rapidly dissolving nickel beans, which comprises the following steps:
(1) Dissolving sodium silicate with the modulus of 2.9 in hot water to prepare 5% sodium silicate aqueous solution, then dripping dilute sulfuric acid with the mass fraction of 0.5% into the solution to be acidic, continuously stirring until the solution becomes thick, adding micro silicon powder with the particle size of 8 mu m, which is 1 time of the mass of the sodium silicate, and silane coupling agent KH570 with the mass of 3% of the sodium silicate, uniformly dispersing, and standing to form sol;
(2) Washing with 0.5% dilute sulfuric acid with the same volume for 3 times, heating to make the sol lose water by 30%, then adding 30% hydrogen peroxide to make the gel contain 10% hydrogen peroxide, cutting into blocks, and making into aluminum silicate gel particles with the particle size of 5 mm;
(3) Adding nickel beans into a reaction tank, then adding aluminum silicate gel particles with the mass fraction of 1 per mill of the nickel beans, and then according to the liquid-solid ratio of 2.9m 3 Adding sulfuric acid solution with equivalent concentration of 4.2N; after 0.3h of reaction, adding 10% hydrogen peroxide for 3 times, wherein the interval time of each time is 0.3h, and the total amount of the added hydrogen peroxide is 8% of the volume of the sulfuric acid solution;
(4) After the reaction is finished, removing solid impurities by a filter pressing mode, and obtaining the high nickel-containing solution.
Comparative example 1
A process for dissolving nickel beans, which is free of addition of sodium polymetaphosphate as a coupling agent, and the rest is the same as in example 1.
Comparative example 2
A process for dissolving nickel beans, which is free of micro silicon powder, is the same as in example 2.
Comparative example 3
A process for dissolving nickel beans, which is free of adding a silane coupling agent KH560, and the rest is the same as in example 2.
Comparative example 4
A process for dissolving nickel beans, which is not washed with dilute sulfuric acid in step (2), is the same as in example 2.
Comparative example 5
A process for dissolving nickel beans, wherein in the step (2) of the process, hydrogen peroxide is not used for replacing water in gel, and the rest is the same as in the example 2.
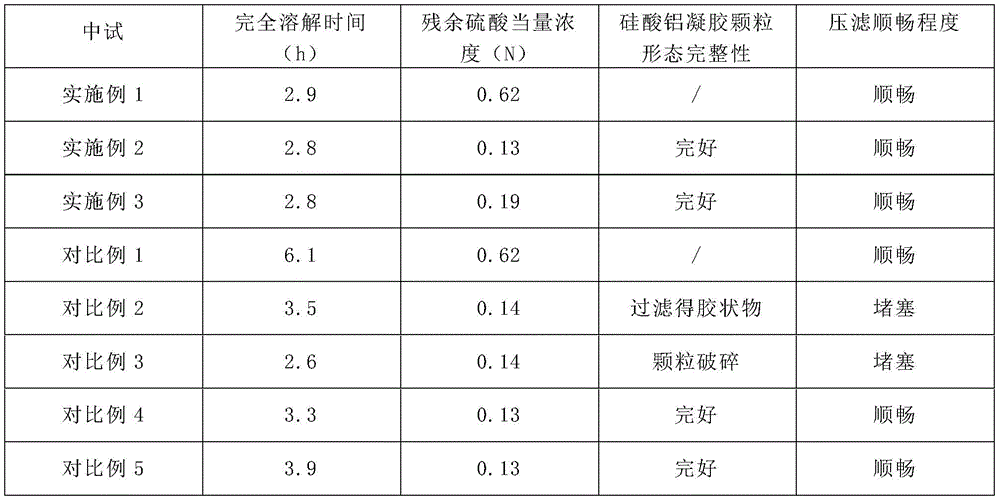
And (3) testing:
pilot tests were performed on the same batch of nickel beans according to the dissolution process of the above examples and comparative examples, using 100kg of nickel beans for each experiment, dissolving in the same reaction tank and controlling the same stirring speed, recording the time taken for the nickel beans to completely dissolve, and detecting the residual sulfuric acid equivalent concentration in the prepared high nickel-containing solution;
and meanwhile, after the dissolution is complete, the morphological integrity of the aluminum silicate gel particles and whether the filtration is smooth or not during the filter pressing are observed.
In the comparative example 2, the alumina silicate gel particles are difficult to maintain in a fixed form, and after the nickel beans are completely dissolved, flocculent insoluble matters are formed, and the filter is blocked during filtration, so that the filtration is difficult; in contrast, the aluminum silicate gel particles in comparative example 3 disintegrated into minute solid particles, which easily caused an increase in filtration resistance, and filtration was also difficult.
Finally: the foregoing description of the preferred embodiments of the invention is not intended to limit the invention to the precise form disclosed, and any such modifications, equivalents, and alternatives falling within the spirit and principles of the invention are intended to be included within the scope of the invention.
Claims (5)
1. A process for rapidly dissolving nickel beans is characterized in that: the process is specifically as follows:
(1) Dissolving sodium silicate into hot water to prepare 5% -6% sodium silicate aqueous solution, then dripping dilute sulfuric acid with the mass fraction of 0.5% -1% into the solution to be acidic, continuously stirring until the solution becomes thick, adding microsilica with the mass of 1-2 times of that of sodium silicate and a silane coupling agent with the mass of 3% -5% of that of sodium silicate, uniformly dispersing, and standing to form sol;
(2) Washing with dilute sulfuric acid of 0.5-1% in the same volume for 2-3 times, heating to make the sol lose water by 30-40%, adding 30% hydrogen peroxide to make the gel contain 10-12% hydrogen peroxide, cutting into blocks, and making into aluminum silicate gel particles;
(3) Adding nickel beans into a reaction tank, adding aluminum silicate gel particles with the mass fraction of 1-3%o of the nickel beans, and then adding the aluminum silicate gel particles according to the liquid-solid ratio of 2.9-3.0m 3 Adding sulfuric acid solution with equivalent concentration of 4.0N-4.2N; after 0.2 to 0.3 hour of reaction, adding 10 to 15 percent of hydrogen peroxide for 3 times, wherein the interval time of each time is 0.3 hour, and the total amount of the added hydrogen peroxide is 5 to 8 percent of the volume of the sulfuric acid solution;
(4) After the reaction is finished, removing solid impurities by a filter pressing mode, and obtaining the high nickel-containing solution.
2. The process for rapidly dissolving nickel beans according to claim 1, wherein: the modulus of the sodium silicate is 2-3.
3. The process for rapidly dissolving nickel beans according to claim 1, wherein: the grain diameter of the micro silicon powder is 8-10 mu m.
4. The process for rapidly dissolving nickel beans according to claim 1, wherein: the silane coupling agent is KH560 or KH570.
5. The process for rapidly dissolving nickel beans according to claim 1, wherein: the particle size of the aluminum silicate gel particles is 3-5mm.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210047082.XA CN114561557B (en) | 2022-01-15 | 2022-01-15 | Process for rapidly dissolving nickel beans |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210047082.XA CN114561557B (en) | 2022-01-15 | 2022-01-15 | Process for rapidly dissolving nickel beans |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN114561557A CN114561557A (en) | 2022-05-31 |
| CN114561557B true CN114561557B (en) | 2023-06-30 |
Family
ID=81712710
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210047082.XA Active CN114561557B (en) | 2022-01-15 | 2022-01-15 | Process for rapidly dissolving nickel beans |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114561557B (en) |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2088842B (en) * | 1980-12-05 | 1984-09-19 | Interox Chemicals Ltd | Separation of cobalt and nickel |
| EP0337047A1 (en) * | 1988-04-13 | 1989-10-18 | Rijksuniversiteit Gent Fakulteit Landbouwwetenschappen Leerstoel Voor Bodemfysika | Process for increasing the specific area and the activity of a sorbent material comprising aluminosilicates, and material obtained |
| KR100618165B1 (en) * | 2001-10-23 | 2006-08-31 | 아토테크더치랜드게엠베하 | Electrolytic method of and compositions for stripping electroless nickel |
| CN109292933B (en) * | 2018-11-12 | 2020-02-07 | 神美科技有限公司 | COD (chemical oxygen demand) remover with oxidation and flocculation combined function for sewage treatment |
| CN113149091A (en) * | 2021-05-26 | 2021-07-23 | 广东佳纳能源科技有限公司 | Battery-grade nickel salt and preparation method thereof |
| CN113562783B (en) * | 2021-07-30 | 2023-05-12 | 福建常青新能源科技有限公司 | Preparation method of nickel sulfate solution |
-
2022
- 2022-01-15 CN CN202210047082.XA patent/CN114561557B/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN114561557A (en) | 2022-05-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN113025822B (en) | Method for extracting nickel from nickel-containing iron powder and preparing iron phosphate and application | |
| JP2013095951A (en) | Method for recovering lithium | |
| KR20170061206A (en) | Collection method of precursor material using disposed lithum-ion battery | |
| CN110668483A (en) | Method for preparing aluminum fluoride by electrolyzing aluminum carbon slag | |
| US11695170B2 (en) | Battery-level Ni—Co—Mn mixed solution and preparation method for battery-level Mn solution | |
| CN112499686A (en) | Method for preparing aluminum-doped battery-grade manganese oxyhydroxide by using waste manganese liquid | |
| CN1227148A (en) | High purity high dispersiveness spherical super fine silver powder and its producing method | |
| CN113020615A (en) | Method for preparing high-purity rhodium powder by using rhodium trichloride | |
| CN110980819B (en) | Method for preparing basic nickel carbonate by using copper-nickel electroplating alloy waste | |
| CN113122725A (en) | Method for improving metal recovery rate and purity of waste lithium battery | |
| CN111137869A (en) | Preparation method of lithium iron phosphate | |
| CN114561557B (en) | Process for rapidly dissolving nickel beans | |
| CN108264100B (en) | Efficient synthesis method of rhodium nitrate solution | |
| CN112342383B (en) | Method for separating and recovering nickel, cobalt, manganese and lithium in ternary waste | |
| CN109930000B (en) | Method for purifying lepidolite leaching solution | |
| CN109809502B (en) | Method for producing nickel sulfate by using electrodeposited nickel anolyte | |
| CN113921932B (en) | Precursor solution, preparation method thereof, positive electrode material and lithium ion battery | |
| CN113611857B (en) | Method for preparing ternary cathode material by using manganese-containing cobalt-nickel waste residues | |
| CN113897490A (en) | Defluorination method and application of lithium ion battery anode material leaching solution | |
| CN111889697A (en) | Preparation method of high-purity gold | |
| CN116216749B (en) | Method for preparing battery grade lithium carbonate by using salt lake lithium carbonate | |
| CN113651352B (en) | Method for preparing low-calcium lanthanum cerium carbonate from high-calcium lanthanum cerium chloride solution | |
| CN115216629B (en) | Method for comprehensively recovering metal elements in tungsten-doped ternary precursor waste | |
| CN111057856B (en) | Method for leaching and recovering cobalt, nickel and molybdenum in catalyst | |
| CN110371939B (en) | Preparation method of diammonium phosphate based on phosphoric acid extraction spent acid |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |