CN114057724A - 一种btk抑制剂 - Google Patents
一种btk抑制剂 Download PDFInfo
- Publication number
- CN114057724A CN114057724A CN202010743104.7A CN202010743104A CN114057724A CN 114057724 A CN114057724 A CN 114057724A CN 202010743104 A CN202010743104 A CN 202010743104A CN 114057724 A CN114057724 A CN 114057724A
- Authority
- CN
- China
- Prior art keywords
- compound
- preparation
- reaction
- tlc
- reduced pressure
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 229940124291 BTK inhibitor Drugs 0.000 title abstract description 17
- 150000001875 compounds Chemical class 0.000 claims abstract description 419
- 230000000694 effects Effects 0.000 claims abstract description 14
- 125000001424 substituent group Chemical group 0.000 claims abstract description 11
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 188
- 238000002360 preparation method Methods 0.000 claims description 140
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 claims description 117
- 230000002829 reductive effect Effects 0.000 claims description 101
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 claims description 99
- 238000004809 thin layer chromatography Methods 0.000 claims description 81
- 238000006243 chemical reaction Methods 0.000 claims description 79
- 238000001035 drying Methods 0.000 claims description 79
- 238000001914 filtration Methods 0.000 claims description 76
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 74
- 238000001704 evaporation Methods 0.000 claims description 67
- -1 C3-6Cycloalkyl radical Chemical class 0.000 claims description 51
- 238000010992 reflux Methods 0.000 claims description 46
- 238000003756 stirring Methods 0.000 claims description 46
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 claims description 45
- 238000010438 heat treatment Methods 0.000 claims description 44
- 238000001816 cooling Methods 0.000 claims description 42
- 238000002425 crystallisation Methods 0.000 claims description 42
- 230000008025 crystallization Effects 0.000 claims description 42
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 41
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Substances CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 claims description 39
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 claims description 38
- 238000002386 leaching Methods 0.000 claims description 38
- 239000012065 filter cake Substances 0.000 claims description 37
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 claims description 32
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 27
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 22
- 201000010099 disease Diseases 0.000 claims description 18
- 238000000034 method Methods 0.000 claims description 15
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 claims description 12
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 12
- 239000003795 chemical substances by application Substances 0.000 claims description 12
- 125000001072 heteroaryl group Chemical group 0.000 claims description 12
- 239000003960 organic solvent Substances 0.000 claims description 12
- 150000003839 salts Chemical class 0.000 claims description 12
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 claims description 11
- 125000003118 aryl group Chemical group 0.000 claims description 11
- 229940125782 compound 2 Drugs 0.000 claims description 11
- 229940126214 compound 3 Drugs 0.000 claims description 11
- 239000008194 pharmaceutical composition Substances 0.000 claims description 11
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 claims description 10
- 229940125898 compound 5 Drugs 0.000 claims description 10
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 claims description 9
- 125000000217 alkyl group Chemical group 0.000 claims description 8
- 229940125773 compound 10 Drugs 0.000 claims description 8
- 239000003085 diluting agent Substances 0.000 claims description 8
- 229910052736 halogen Inorganic materials 0.000 claims description 8
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 claims description 8
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 8
- 239000007787 solid Substances 0.000 claims description 8
- 239000012453 solvate Substances 0.000 claims description 7
- 238000011282 treatment Methods 0.000 claims description 7
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 claims description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 6
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 claims description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 claims description 6
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 claims description 6
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 claims description 6
- 150000002367 halogens Chemical class 0.000 claims description 6
- NWZSZGALRFJKBT-KNIFDHDWSA-N (2s)-2,6-diaminohexanoic acid;(2s)-2-hydroxybutanedioic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O.NCCCC[C@H](N)C(O)=O NWZSZGALRFJKBT-KNIFDHDWSA-N 0.000 claims description 5
- BNGPVKSKKYIJCR-UHFFFAOYSA-N 2-chloro-1,3-dimethylimidazolidine;hydrochloride Chemical compound [Cl-].CN1CC[NH+](C)C1Cl BNGPVKSKKYIJCR-UHFFFAOYSA-N 0.000 claims description 5
- ONELILMJNOWXSA-UHFFFAOYSA-N 3-bromo-4-fluorobenzoic acid Chemical compound OC(=O)C1=CC=C(F)C(Br)=C1 ONELILMJNOWXSA-UHFFFAOYSA-N 0.000 claims description 5
- 125000000592 heterocycloalkyl group Chemical group 0.000 claims description 5
- IKDUDTNKRLTJSI-UHFFFAOYSA-N hydrazine monohydrate Substances O.NN IKDUDTNKRLTJSI-UHFFFAOYSA-N 0.000 claims description 5
- 239000000651 prodrug Substances 0.000 claims description 5
- 229940002612 prodrug Drugs 0.000 claims description 5
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 claims description 5
- 239000002904 solvent Substances 0.000 claims description 5
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 claims description 4
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 claims description 4
- 229910002651 NO3 Inorganic materials 0.000 claims description 4
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 claims description 4
- 125000004104 aryloxy group Chemical group 0.000 claims description 4
- 239000002585 base Substances 0.000 claims description 4
- 229940001468 citrate Drugs 0.000 claims description 4
- 229940125904 compound 1 Drugs 0.000 claims description 4
- 125000003107 substituted aryl group Chemical group 0.000 claims description 4
- 238000000967 suction filtration Methods 0.000 claims description 4
- QAIPRVGONGVQAS-DUXPYHPUSA-N trans-caffeic acid Chemical compound OC(=O)\C=C\C1=CC=C(O)C(O)=C1 QAIPRVGONGVQAS-DUXPYHPUSA-N 0.000 claims description 4
- 125000006583 (C1-C3) haloalkyl group Chemical group 0.000 claims description 3
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 3
- 239000002671 adjuvant Substances 0.000 claims description 3
- 239000003513 alkali Substances 0.000 claims description 3
- 125000003545 alkoxy group Chemical group 0.000 claims description 3
- 239000003937 drug carrier Substances 0.000 claims description 3
- 230000008569 process Effects 0.000 claims description 3
- 238000003786 synthesis reaction Methods 0.000 claims description 3
- LSPHULWDVZXLIL-UHFFFAOYSA-N (+/-)-Camphoric acid Chemical compound CC1(C)C(C(O)=O)CCC1(C)C(O)=O LSPHULWDVZXLIL-UHFFFAOYSA-N 0.000 claims description 2
- 125000004890 (C1-C6) alkylamino group Chemical group 0.000 claims description 2
- ACEAELOMUCBPJP-UHFFFAOYSA-N (E)-3,4,5-trihydroxycinnamic acid Natural products OC(=O)C=CC1=CC(O)=C(O)C(O)=C1 ACEAELOMUCBPJP-UHFFFAOYSA-N 0.000 claims description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 claims description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 claims description 2
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 claims description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 claims description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 claims description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims description 2
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 claims description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 claims description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 claims description 2
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 claims description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 claims description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 claims description 2
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 claims description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 2
- IOVCWXUNBOPUCH-UHFFFAOYSA-M Nitrite anion Chemical compound [O-]N=O IOVCWXUNBOPUCH-UHFFFAOYSA-M 0.000 claims description 2
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 claims description 2
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 claims description 2
- 229910019142 PO4 Inorganic materials 0.000 claims description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 claims description 2
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 claims description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 claims description 2
- 229940072107 ascorbate Drugs 0.000 claims description 2
- 235000010323 ascorbic acid Nutrition 0.000 claims description 2
- 239000011668 ascorbic acid Substances 0.000 claims description 2
- 229940077388 benzenesulfonate Drugs 0.000 claims description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 claims description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims description 2
- 230000015572 biosynthetic process Effects 0.000 claims description 2
- 235000004883 caffeic acid Nutrition 0.000 claims description 2
- 229940074360 caffeic acid Drugs 0.000 claims description 2
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical compound C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 claims description 2
- QAIPRVGONGVQAS-UHFFFAOYSA-N cis-caffeic acid Natural products OC(=O)C=CC1=CC=C(O)C(O)=C1 QAIPRVGONGVQAS-UHFFFAOYSA-N 0.000 claims description 2
- 238000006482 condensation reaction Methods 0.000 claims description 2
- 230000018044 dehydration Effects 0.000 claims description 2
- 238000006297 dehydration reaction Methods 0.000 claims description 2
- 238000001514 detection method Methods 0.000 claims description 2
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 claims description 2
- 229940050411 fumarate Drugs 0.000 claims description 2
- 229930195712 glutamate Natural products 0.000 claims description 2
- 229940049906 glutamate Drugs 0.000 claims description 2
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 claims description 2
- 230000007062 hydrolysis Effects 0.000 claims description 2
- 238000006460 hydrolysis reaction Methods 0.000 claims description 2
- 229940001447 lactate Drugs 0.000 claims description 2
- 229940049920 malate Drugs 0.000 claims description 2
- BJEPYKJPYRNKOW-UHFFFAOYSA-N malic acid Chemical compound OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 claims description 2
- 150000007522 mineralic acids Chemical class 0.000 claims description 2
- 230000003287 optical effect Effects 0.000 claims description 2
- NIXKBAZVOQAHGC-UHFFFAOYSA-M phenylmethanesulfonate Chemical compound [O-]S(=O)(=O)CC1=CC=CC=C1 NIXKBAZVOQAHGC-UHFFFAOYSA-M 0.000 claims description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 2
- 239000010452 phosphate Substances 0.000 claims description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 claims description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 claims description 2
- 229960001860 salicylate Drugs 0.000 claims description 2
- 239000012312 sodium hydride Substances 0.000 claims description 2
- 229910000104 sodium hydride Inorganic materials 0.000 claims description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 claims description 2
- 229940095064 tartrate Drugs 0.000 claims description 2
- DUYAAUVXQSMXQP-UHFFFAOYSA-M thioacetate Chemical compound CC([S-])=O DUYAAUVXQSMXQP-UHFFFAOYSA-M 0.000 claims description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 claims description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 2
- 239000008096 xylene Substances 0.000 claims description 2
- 230000006806 disease prevention Effects 0.000 claims 1
- 238000004519 manufacturing process Methods 0.000 claims 1
- 150000007524 organic acids Chemical class 0.000 claims 1
- 235000005985 organic acids Nutrition 0.000 claims 1
- 206010028980 Neoplasm Diseases 0.000 abstract description 19
- 239000002177 L01XE27 - Ibrutinib Substances 0.000 abstract description 14
- 229960001507 ibrutinib Drugs 0.000 abstract description 14
- XYFPWWZEPKGCCK-GOSISDBHSA-N ibrutinib Chemical compound C1=2C(N)=NC=NC=2N([C@H]2CN(CCC2)C(=O)C=C)N=C1C(C=C1)=CC=C1OC1=CC=CC=C1 XYFPWWZEPKGCCK-GOSISDBHSA-N 0.000 abstract description 14
- 239000003814 drug Substances 0.000 abstract description 13
- 230000005764 inhibitory process Effects 0.000 abstract description 13
- 229940079593 drug Drugs 0.000 abstract description 10
- 238000000338 in vitro Methods 0.000 abstract description 9
- 201000011510 cancer Diseases 0.000 abstract description 7
- 102000004190 Enzymes Human genes 0.000 abstract description 6
- 108090000790 Enzymes Proteins 0.000 abstract description 6
- 108091000080 Phosphotransferase Proteins 0.000 abstract description 6
- 231100000135 cytotoxicity Toxicity 0.000 abstract description 6
- 230000003013 cytotoxicity Effects 0.000 abstract description 6
- 238000002474 experimental method Methods 0.000 abstract description 6
- 102000020233 phosphotransferase Human genes 0.000 abstract description 6
- 238000013461 design Methods 0.000 abstract description 3
- 125000000539 amino acid group Chemical group 0.000 abstract description 2
- 230000001093 anti-cancer Effects 0.000 abstract description 2
- 230000003993 interaction Effects 0.000 abstract description 2
- 238000005556 structure-activity relationship Methods 0.000 abstract description 2
- 239000000126 substance Substances 0.000 abstract 1
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 116
- 239000010410 layer Substances 0.000 description 34
- 239000008213 purified water Substances 0.000 description 33
- 239000012044 organic layer Substances 0.000 description 31
- 238000005406 washing Methods 0.000 description 31
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 30
- 238000005160 1H NMR spectroscopy Methods 0.000 description 30
- 210000004027 cell Anatomy 0.000 description 30
- 239000000203 mixture Substances 0.000 description 22
- 238000009472 formulation Methods 0.000 description 14
- 235000002639 sodium chloride Nutrition 0.000 description 14
- 239000000243 solution Substances 0.000 description 14
- 102100029823 Tyrosine-protein kinase BTK Human genes 0.000 description 11
- 239000004480 active ingredient Substances 0.000 description 10
- 150000003254 radicals Chemical class 0.000 description 10
- 206010006187 Breast cancer Diseases 0.000 description 8
- 208000026310 Breast neoplasm Diseases 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 7
- 206010025323 Lymphomas Diseases 0.000 description 7
- 235000019441 ethanol Nutrition 0.000 description 7
- 201000005202 lung cancer Diseases 0.000 description 7
- 208000020816 lung neoplasm Diseases 0.000 description 7
- 210000004881 tumor cell Anatomy 0.000 description 7
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 description 6
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- 125000005843 halogen group Chemical group 0.000 description 6
- 208000032839 leukemia Diseases 0.000 description 6
- 239000003826 tablet Substances 0.000 description 6
- 206010059866 Drug resistance Diseases 0.000 description 5
- 230000002159 abnormal effect Effects 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 210000003719 b-lymphocyte Anatomy 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 239000000314 lubricant Substances 0.000 description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 5
- 229920001223 polyethylene glycol Polymers 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 208000023275 Autoimmune disease Diseases 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- 208000005718 Stomach Neoplasms Diseases 0.000 description 4
- WDENQIQQYWYTPO-IBGZPJMESA-N acalabrutinib Chemical compound CC#CC(=O)N1CCC[C@H]1C1=NC(C=2C=CC(=CC=2)C(=O)NC=2N=CC=CC=2)=C2N1C=CN=C2N WDENQIQQYWYTPO-IBGZPJMESA-N 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 125000000753 cycloalkyl group Chemical group 0.000 description 4
- 206010017758 gastric cancer Diseases 0.000 description 4
- 201000007270 liver cancer Diseases 0.000 description 4
- 208000014018 liver neoplasm Diseases 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 201000011549 stomach cancer Diseases 0.000 description 4
- 239000000375 suspending agent Substances 0.000 description 4
- 238000013268 sustained release Methods 0.000 description 4
- 241000220479 Acacia Species 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 3
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- 230000000259 anti-tumor effect Effects 0.000 description 3
- 206010003246 arthritis Diseases 0.000 description 3
- 230000001363 autoimmune Effects 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 239000001768 carboxy methyl cellulose Substances 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 239000013078 crystal Substances 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 239000002270 dispersing agent Substances 0.000 description 3
- 239000000796 flavoring agent Substances 0.000 description 3
- 235000013355 food flavoring agent Nutrition 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 3
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 239000002502 liposome Substances 0.000 description 3
- 201000007924 marginal zone B-cell lymphoma Diseases 0.000 description 3
- 208000021937 marginal zone lymphoma Diseases 0.000 description 3
- 125000002950 monocyclic group Chemical group 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 230000019491 signal transduction Effects 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 239000012730 sustained-release form Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 108091008875 B cell receptors Proteins 0.000 description 2
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 2
- 102100026008 Breakpoint cluster region protein Human genes 0.000 description 2
- 208000011691 Burkitt lymphomas Diseases 0.000 description 2
- 206010009900 Colitis ulcerative Diseases 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 2
- HSHXDCVZWHOWCS-UHFFFAOYSA-N N'-hexadecylthiophene-2-carbohydrazide Chemical compound CCCCCCCCCCCCCCCCNNC(=O)c1cccs1 HSHXDCVZWHOWCS-UHFFFAOYSA-N 0.000 description 2
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- 201000006704 Ulcerative Colitis Diseases 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 125000003282 alkyl amino group Chemical group 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 201000003444 follicular lymphoma Diseases 0.000 description 2
- 235000003599 food sweetener Nutrition 0.000 description 2
- 210000001035 gastrointestinal tract Anatomy 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 description 2
- 125000005842 heteroatom Chemical group 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000001965 increasing effect Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 239000008108 microcrystalline cellulose Substances 0.000 description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 125000003367 polycyclic group Chemical group 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 235000010413 sodium alginate Nutrition 0.000 description 2
- 239000000661 sodium alginate Substances 0.000 description 2
- 229940005550 sodium alginate Drugs 0.000 description 2
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 2
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000003765 sweetening agent Substances 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 231100000820 toxicity test Toxicity 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- 230000004614 tumor growth Effects 0.000 description 2
- 239000002691 unilamellar liposome Substances 0.000 description 2
- RNOAOAWBMHREKO-QFIPXVFZSA-N (7S)-2-(4-phenoxyphenyl)-7-(1-prop-2-enoylpiperidin-4-yl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide Chemical compound C(C=C)(=O)N1CCC(CC1)[C@@H]1CCNC=2N1N=C(C=2C(=O)N)C1=CC=C(C=C1)OC1=CC=CC=C1 RNOAOAWBMHREKO-QFIPXVFZSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 1
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- CFWRDBDJAOHXSH-SECBINFHSA-N 2-azaniumylethyl [(2r)-2,3-diacetyloxypropyl] phosphate Chemical compound CC(=O)OC[C@@H](OC(C)=O)COP(O)(=O)OCCN CFWRDBDJAOHXSH-SECBINFHSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 108010029445 Agammaglobulinaemia Tyrosine Kinase Proteins 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 206010073478 Anaplastic large-cell lymphoma Diseases 0.000 description 1
- 230000024704 B cell apoptotic process Effects 0.000 description 1
- 208000036170 B-Cell Marginal Zone Lymphoma Diseases 0.000 description 1
- GUBGYTABKSRVRQ-DCSYEGIMSA-N Beta-Lactose Chemical compound OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-DCSYEGIMSA-N 0.000 description 1
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 description 1
- 208000033222 Biliary cirrhosis primary Diseases 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 102100031383 Fibulin-7 Human genes 0.000 description 1
- 206010051066 Gastrointestinal stromal tumour Diseases 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 1
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 1
- 208000024869 Goodpasture syndrome Diseases 0.000 description 1
- 208000001204 Hashimoto Disease Diseases 0.000 description 1
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 1
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 1
- 102100024233 High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A Human genes 0.000 description 1
- 101000846874 Homo sapiens Fibulin-7 Proteins 0.000 description 1
- 101001117267 Homo sapiens High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A Proteins 0.000 description 1
- 101000934996 Homo sapiens Tyrosine-protein kinase JAK3 Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 206010020651 Hyperkinesia Diseases 0.000 description 1
- 208000000269 Hyperkinesis Diseases 0.000 description 1
- 206010020850 Hyperthyroidism Diseases 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 208000032004 Large-Cell Anaplastic Lymphoma Diseases 0.000 description 1
- 201000003791 MALT lymphoma Diseases 0.000 description 1
- 231100000002 MTT assay Toxicity 0.000 description 1
- 238000000134 MTT assay Methods 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 206010027406 Mesothelioma Diseases 0.000 description 1
- 208000003250 Mixed connective tissue disease Diseases 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 1
- 229910003827 NRaRb Inorganic materials 0.000 description 1
- 206010029240 Neuritis Diseases 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 241000721454 Pemphigus Species 0.000 description 1
- 208000031845 Pernicious anaemia Diseases 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 208000007452 Plasmacytoma Diseases 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 206010036105 Polyneuropathy Diseases 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 208000012654 Primary biliary cholangitis Diseases 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- BCKXLBQYZLBQEK-KVVVOXFISA-M Sodium oleate Chemical compound [Na+].CCCCCCCC\C=C/CCCCCCCC([O-])=O BCKXLBQYZLBQEK-KVVVOXFISA-M 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 208000004732 Systemic Vasculitis Diseases 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 102100025387 Tyrosine-protein kinase JAK3 Human genes 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- 230000001594 aberrant effect Effects 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 229940124532 absorption promoter Drugs 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 1
- 238000010009 beating Methods 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- 235000012216 bentonite Nutrition 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 239000012964 benzotriazole Substances 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000004067 bulking agent Substances 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 235000011132 calcium sulphate Nutrition 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001717 carbocyclic compounds Chemical group 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 208000006990 cholangiocarcinoma Diseases 0.000 description 1
- 208000016644 chronic atrophic gastritis Diseases 0.000 description 1
- 229940121657 clinical drug Drugs 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 239000012050 conventional carrier Substances 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 229940097362 cyclodextrins Drugs 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 201000001981 dermatomyositis Diseases 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- FRKBLBQTSTUKOV-UHFFFAOYSA-N diphosphatidyl glycerol Natural products OP(O)(=O)OCC(OP(O)(O)=O)COP(O)(O)=O FRKBLBQTSTUKOV-UHFFFAOYSA-N 0.000 description 1
- ZGSPNIOCEDOHGS-UHFFFAOYSA-L disodium [3-[2,3-di(octadeca-9,12-dienoyloxy)propoxy-oxidophosphoryl]oxy-2-hydroxypropyl] 2,3-di(octadeca-9,12-dienoyloxy)propyl phosphate Chemical compound [Na+].[Na+].CCCCCC=CCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COP([O-])(=O)OCC(O)COP([O-])(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COC(=O)CCCCCCCC=CCC=CCCCCC ZGSPNIOCEDOHGS-UHFFFAOYSA-L 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000000235 effect on cancer Effects 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- UPBDXRPQPOWRKR-UHFFFAOYSA-N furan-2,5-dione;methoxyethene Chemical compound COC=C.O=C1OC(=O)C=C1 UPBDXRPQPOWRKR-UHFFFAOYSA-N 0.000 description 1
- 201000011243 gastrointestinal stromal tumor Diseases 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 230000008105 immune reaction Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 239000012442 inert solvent Substances 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 201000000564 macroglobulinemia Diseases 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- CUONGYYJJVDODC-UHFFFAOYSA-N malononitrile Chemical compound N#CCC#N CUONGYYJJVDODC-UHFFFAOYSA-N 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 210000003593 megakaryocyte Anatomy 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 239000007932 molded tablet Substances 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 210000000822 natural killer cell Anatomy 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 125000006574 non-aromatic ring group Chemical group 0.000 description 1
- 102000037979 non-receptor tyrosine kinases Human genes 0.000 description 1
- 108091008046 non-receptor tyrosine kinases Proteins 0.000 description 1
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- 230000009437 off-target effect Effects 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 239000008203 oral pharmaceutical composition Substances 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 1
- 150000003905 phosphatidylinositols Chemical class 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 208000019629 polyneuritis Diseases 0.000 description 1
- 229920003124 powdered cellulose Polymers 0.000 description 1
- 235000019814 powdered cellulose Nutrition 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 229940126586 small molecule drug Drugs 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- RYYKJJJTJZKILX-UHFFFAOYSA-M sodium octadecanoate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC([O-])=O RYYKJJJTJZKILX-UHFFFAOYSA-M 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000011476 stem cell transplantation Methods 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 125000001712 tetrahydronaphthyl group Chemical group C1(CCCC2=CC=CC=C12)* 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000004565 tumor cell growth Effects 0.000 description 1
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 1
- 208000035408 type 1 diabetes mellitus 1 Diseases 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 description 1
- 239000007762 w/o emulsion Substances 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000000230 xanthan gum Substances 0.000 description 1
- 235000010493 xanthan gum Nutrition 0.000 description 1
- 229920001285 xanthan gum Polymers 0.000 description 1
- 229940082509 xanthan gum Drugs 0.000 description 1
- 229930195724 β-lactose Natural products 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
- A61P5/16—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4 for decreasing, blocking or antagonising the activity of the thyroid hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/48—Drugs for disorders of the endocrine system of the pancreatic hormones
- A61P5/50—Drugs for disorders of the endocrine system of the pancreatic hormones for increasing or potentiating the activity of insulin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Diabetes (AREA)
- Immunology (AREA)
- Endocrinology (AREA)
- Hematology (AREA)
- Physical Education & Sports Medicine (AREA)
- Neurology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Pulmonology (AREA)
- Urology & Nephrology (AREA)
- Rheumatology (AREA)
- Oncology (AREA)
- Emergency Medicine (AREA)
- Obesity (AREA)
- Dermatology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Transplantation (AREA)
Abstract
本发明属于医药化工领域,具体涉及一种BTK抑制剂;本发明的目的在于对已上市药物依鲁替尼的结构特点分析,结合最新报道的构效关系,哌啶‑2,6‑二酮N2位取代基R1的不同对Btk酶活性影响不大,且R1朝向酶的一个亲水口袋,引入不同的亲水性基团可以调节化合物的理化性质。N9位取代基R2的空间取向朝向激酶的gatekeeper区域,并与特定的残基相互作用在Tec家族中,不同激酶的gatekeeper区域产生相互作用的氨基酸残基存在差异:期望设计合成更高细胞毒性化合物,发挥抗癌效果,并具备成药性潜质。细胞毒性实验发现本发明化合物对多种癌细胞具有较强的体外抑制作用。
Description
技术领域
本发明属于药物化学领域,具体为一种新型的3-氨基-2,7-二氢吡喃并[4,3-c]吡唑-4,6-二酮类BTK抑制剂以及这种抑制剂的制备方法和用途。
背景技术
肿瘤是一种严重威胁人类健康的常见病和多发病,是由于人体内细胞的不正常增生所导致。肺癌、消化道肿瘤和肝癌是男性最常见的肿瘤,占所有病例的70%以上(肺癌23%、胃癌15.2%、肝癌13.57%、食管癌10.46%、结直肠癌9.39%),而乳腺癌、肺癌、消化道肿瘤和肝癌是女性最常见的肿瘤,占所有病例的60%以上(乳腺癌16.97%、肺癌14.85%、结直肠癌9.68%、胃癌8.53%、肝癌6.17%)。随着中国人口老龄化的加剧,癌症的患病率势必增加。另外环境污染情况也增加了一些癌症的患病率,如淋巴癌。自身免疫性疾病是免疫系统对自身机体的成份发生免疫反应,造成损害而引发疾病。
BTK(brutontyrosinekinase)是一种胞浆蛋白,属于非受体络氨酸激酶Tec家族,其表达于多数的造血细胞中,如B细胞、肥大细胞、巨核细胞等,但在T细胞、NK细胞及浆细胞中不表达。BTK对B细胞受体信号通路起非常关键的作用,对B细胞的增殖、分化和凋亡有重要影响。
在恶性B细胞中,B细胞受体信号通路过度活跃,从而抑制B细胞的正常分化和凋亡,促进异常增殖。已知多种B细胞类型的恶性肿瘤中经常存在BCR通路的异常调节,如弥漫性大B细胞淋巴瘤(DLBCL)、套细胞淋巴瘤(MCL)、慢性淋巴细胞白血病(CLL)、滤泡性淋巴瘤(FL)、华氏巨球蛋白血症(WM)、边缘区淋巴瘤(MZL)、B淋巴母细胞性白血病(B-LBL)和伯基特淋巴瘤(BL)等。
自从BTK抑制剂在1993年第一次被发现之后,针对BTK的药物研究一直没有停止过。BTK抑制剂的适应症仍在不断拓展中,除抗肿瘤外,自身免疫型疾病的应用将是把BTK抑制剂推向另一个市场高度的关键所在。目前国内相继研发BTK的多种小分子抑制剂并且在体内及体外试验中均表现出良好的抗肿瘤作用,除了已上市的药物Ibrutinib和ACP-196,更多新的BTK抑制剂正在研究中,目前进展较快的除BGB-3111之外,CT1530、GS-4059已经进入临床Ⅱ期
目前,针对BTK靶点的肿瘤靶向小分子药物已经研发到了第2代,第一代BTK抑制剂为依鲁替尼(Ibrutinib)于2013年美国上市用于临床治疗复发性或难治性套细胞淋巴瘤(MCL),是靶向BTK的明星药物,Ibrutinib能够抑制恶性增殖的B细胞的生长和转移,同时Ibrutinib是第一个被FDA构批准上市的BTK抑制剂。Ibrutinib除了被用于治疗各种血液疾病,它还用于治疗自身免疫相关疾病包括干细胞移植、移植后的免疫抵抗和关节炎等。但是Ibrutinib在治疗中的响应率并非百分之百,而且疾病会复发,该药物存在着未知的原发性耐药和获得性耐药。随着药物的上市和临床使用,Ibrutinib开始在不同病人体内产生了不同机制的耐药性。获得性耐药迄今为止只有这两个靶点突变的发现,在BCR信号通路中,BTK与ibrutinib的结合位点481位发生了突变Ibrutinib剂量依赖性抑制关节炎模型中的疾病Ibrutinib在临床上的耐药问题越来越多,也出现了许多副作用。
第二代BTK抑制剂Acalabrutinib(ACP-196),ACP-196被称之为第二代BTK抑制剂,就是因为在抑制BTK激酶靶点的基础上还不对其他的激酶产生抑制作用。在2017年8月被FDA批准用于套细胞淋巴瘤(MCL)的治疗,目前也还有许多适应症正处于临床,例如:慢性淋巴白血病(CLL)正处于临床三期;一些实体瘤比如非小细胞肺癌,头颈癌等正处于临床二期,发展非常迅速。ACP-196对关节炎的治疗目前也处于临床二是因为Ibrutinib的脱靶效应导致的。因此第二代选择性更好的BTK抑制剂开始应运而期阶段,由于其的高选择性,副作用少,在治疗例如自身免疫疾病之类的慢性病具有良好的发展前景。
目前已知BTK抑制剂的选择性不理想,除了抑制BTK,还抑制其他多种激酶(如ETK,EGF,BLK,FGR,HCK,YES,BRK和JAK3等),从而产生较多的副作用;同时,BTK结合位点发生突变后往往会导致耐药性的产生。因此临床上需要更多的BTK抑制剂,用于治疗肿瘤等疾病,同时可以克服此类不良事件。
本发明设计合成一种新型的3-氨基-2,7-二氢吡喃并[4,3-c]吡唑-4,6-二酮类化合物,体外实验表明这种化合物对癌细胞具有较好的细胞毒性,其中化合物TM10对肺癌细胞的IC50达到0.86μM,是一种具有开发为新型抗癌药物的化合物;在制备方法上,本发明通过简单方法高效地合成了关键中间体及其目标化合物。
发明内容
本发明的目的在于对已上市药物依鲁替尼的结构特点分析,结合最新报道的构效关系,哌啶-2,6-二酮N2位取代基R1的不同对Btk酶活性影响不大,且R1朝向酶的一个亲水口袋,引入不同的亲水性基团(如:酸、酰胺、胺)可以调节化合物的理化性质。N9位取代基R2的空间取向朝向激酶的gatekeeper区域,并与特定的残基相互作用在Tec家族中,不同激酶的gatekeeper区域产生相互作用的氨基酸残基存在差异:
期望设计合成更高细胞毒性化合物,发挥抗癌效果,并具备成药性潜质。细胞毒性实验发现本发明化合物对多种癌细胞具有较强的体外抑制作用。
术语解释
以下列出了用于描述本申请的各种术语的定义。这些定义适用于整个说明书和权利要求书中使用的术语,除非在特定情况下单独地或作为较大组的一部分另有限制。
本发明中的术语“烷基”是指饱和的直链或支链烃基,在某些实施方案中,分别含有1至4个,C1-4烷基的实例包括但不限于甲基、乙基、丙基、异丙基、正丁基、叔丁基或类似基团。
本发明中的术语“芳基”是指单环或多环碳环系统,其具有一个或多个稠合或非稠合的芳环,包括但不限于苯基、萘基、四氢萘基等类似基团,并且其环碳上的氢原子还可以被一至多个取代基取代。
本发明中的术语“卤代”是指卤素原子取代碳原子上的氢原子所形成的基团,其中卤素原子包括但不限于F、Cl、Br、I。
本发明中的术语“烷氧基”是指-O-烷基,其中所述烷基包括但不限于C1-3烷基和C3-6环烷基,具体的实例包括但不限于甲氧基、乙氧基、丙氧基、异丙氧基、环丙氧基、环丁氧基、环戊氧基、环己氧基及其卤代形式的基团。
本发明中的术语“烷氨基”是指基团-N-烷基,其中所述烷基包括但不限于C1-3烷基和C3-6环烷基,具体的实例包括但不限于甲氨基、乙氨基、丙氨基、环丙氨基、环丁氧基等基团。本发明的术语“环烷基”是指单环或多环的饱和或部分不饱和碳环化合物的单价基团,C3-6环烷基的包括但不限于环丙基、环丁基、环戊基、环己基等,并且其环碳上的氢原子还可以被一至多个取代基取代。
本发明的术语“杂环烷基”指含有2至6个环碳原子和1至3个环杂原子的单环或多环非芳香族环体系,其中所述杂原子选自N、O、S。
本发明的术语“杂芳基”是指含有1至6个碳和至少一个杂原子的芳香环体系,其中所述杂原子选自N、S、O。
本发明的术语“烷氨基”是指基团-烷基-NRaRb,其中烷基包括但不限于C1-3烷基、C1-3卤代烷基、C1-3烷氧基和C3-6环烷基,Ra和Rb各自独立的选自H、C1-3烷基或C3-6环烷基。
本发明所述的“药学上可接受的盐”指本发明化合物的盐,有本发明发现的具有特定取代基的化合物与相对无毒的酸或碱制备。本发明中含有相对碱性的官能团,可通过在纯的溶液或者合适的惰性溶剂中用足量的酸与这类化合物的中性形式接触的方式获得酸加成盐,药学上可接受的酸加成盐包括无机盐和有机酸盐。所述无机酸盐包括但不限于:盐酸盐、硝酸盐、硼酸盐、氢氰酸盐、氢氟酸盐、氢溴酸盐、氢碘酸盐、亚硝酸盐、高卤酸盐、卤酸盐、亚卤酸盐、次卤酸盐、偏铝酸盐、硫酸盐、磷酸盐、硝酸盐;所述的有机酸盐包括但不限于:甲酸盐、乙酸盐、丙酸盐、丁酸盐、丙烯酸盐、乙二酸盐、丙二酸盐、丁二酸盐、苯甲酸盐、邻苯二甲酸羧酸盐、甲磺酸盐、乙磺酸盐、苯磺酸盐、苯甲磺酸盐、对甲苯亚磺酸盐、硫代乙酸盐、三氟乙酸盐、酒石酸盐、苹果酸盐、枸椽酸盐、抗坏血酸盐、水杨酸盐、咖啡酸富马酸盐、马来酸盐、乳酸盐、柠檬酸盐、谷氨酸盐、樟脑酸盐、樟脑磺酸盐等。
本发明所述的“溶剂合物”是指含有化学计量或非化学计量的溶剂加成形式,溶剂可选自水、乙醇、异丙醇、乙醚、丙酮等。
本发明所述的“前药”是指通过代谢的方式在体内可转化的化合物,以提供通过本申请化学式所描述的任何化合物,且各种形式的药物是本领域已知的。
本发明所述的“有效量”是指能够在所需的对象中达到所需的治疗效果,但不会造成过度的负面影响的剂量,具体的剂量通常可以由本领域的技术人员根据需要确定。
本发明所述的“治疗”是指减轻或缓和疾病及其并发症状的方法;所述的预防是指减少或消除疾病、病状或病症的症状或并发症的发作。
应该理解的是在上文中未解释的,但出现在本发明中的其它术语,应按照本领域专业技术人员的通常理解来定义。
本发明的具体技术方案如下:
本发明第一方面,提供一种式(I)所示吡唑-4,6-二酮类化合物或其药学上可接受的盐的水合物、溶剂合物、前药、立体异构体或互变异构体:
其中:
R独立的选自H、卤素、芳基、杂芳基、C1-4烷基、C1-6烷氧基、C3-6环烷基、C2-6杂环烷基、芳氧基、-NR2R3;或取代的芳基、取代的杂芳基、取代的C3-6环烷基、取代的C2-6杂环烷基、取代的芳氧基、取代的C1-6烷氧基中的一个或多个独立的基团,其中取代基基团选自卤素、芳基、杂芳基、C1-6烷氧基、C1-6烷氨基、C1-4烷基、取代的C2-6杂环烷基、卤素取代的C2-6杂环烷基、C2-6杂环烷基中的一个或多个取代基。
R优选为-NR2R3、芳基、杂芳基、C1-4烷基、C2-6杂环烷基、取代的芳基、取代的杂芳基。
R进一步优选为-NR2R3、芳基、杂芳基、取代的芳基。
R2独立的选自H或C1-4烷基;R2进一步优选为甲基。
R3独立的选自H、C1-4烷基、C3-6环烷基、C1-3卤代烷基、芳基、杂芳基、C1-6烷氧基、C1-6烷氨基、取代的C2-6杂环烷基、卤素取代的C2-6杂环烷基、C2-6杂环烷基中的一个或多个;R3进一步优选为甲基。
Z独立的选自C1-4烷基或芳基;Z优选为甲基、乙基、苯;Z进一步优选为甲基、苯。
本发明优选方案,所述的式(I)化合物选自下组:
本发明第二方面,提供一种式(I)所示吡唑-4,6-二酮类化合物的制备方法。本申请化合物可以通过使用本领域技术人员已知的多种方式制备得到。本申请可以使用本文描述的方法及有机化学合成方法或者本领域技术人员所理解的其变化来合成。优选地方法包括但不限于下述这些方法。
优选地,本发明式(I)所示吡唑-4,6-二酮类化合物的制备方法,包括以下步骤:
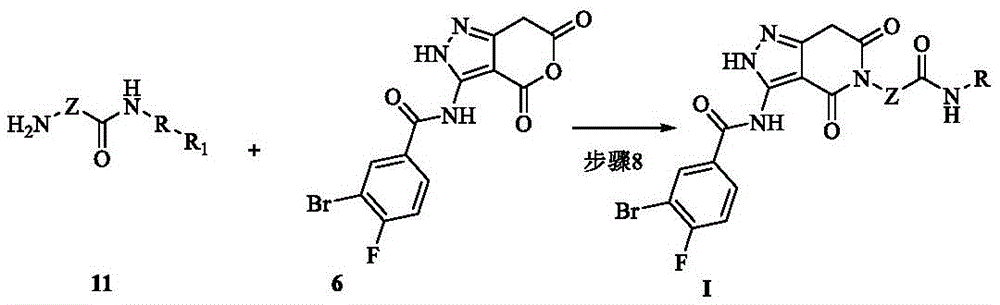
化合物6的制备:
化合物11的通用合成方法:
化合物I的制备:
其中,R和Z为本发明第一方面式(I)结构化合物所定义;
优选地,式(I)所示吡唑-4,6-二酮类化合物的制备方法,反应步骤包括:
步骤1:化合物1在乙醇钠溶液中发生缩合反应,得化合物2;
步骤2:化合物2与水合肼反应,得到化合物3;
步骤3:化合物3碱性条件下反应,得化合物4;
步骤4:化合物4控温回流脱水生成中间体化合物5;
步骤5:化合物5与3-溴4-氟苯甲酸、EDCI、HOBt在二氯甲烷中反应,得化合物6。
步骤6:化合物8和化合物9反应得到化合物10。
步骤7:化合物10水解得到化合物11。
步骤8:化合物6和化合物11在有机溶剂中反应,经过析晶,过滤干燥得目标化合物I。优选地,式(I)所示吡唑-4,6-二酮类化合物的制备方法,具体反应步骤包括:
步骤1:将乙醇钠加入乙醇溶剂中,搅拌溶解,加入化合物1,升温至回流,TLC检测,反应完毕,控温析晶,抽滤,滤饼用乙醇淋洗,干燥得粗品化合物2;粗品重结晶得最终化合物2。
优选地,所述的析晶温度为15~30℃。
步骤2:将化合物2加入到水中,搅拌溶解,加入水合肼,升温至回流,TLC检测,反应完毕,降温析出固体,过滤,干燥得化合物3。
优选地,所述的析晶温度为-10~5℃。
步骤3:化合物3加入到碱溶液中,搅拌溶解,加热至回流,TLC检测,反应完毕,降温加入盐酸调PH到3~4,析出固体,过滤,干燥得化合物4。
优选地,所述的碱为氢化钠,氢氧化钾,氢氧化钠,氢氧化锂,进一步优选为氢氧化钠。
步骤4:将化合物4溶于乙酸酐中,加入缩合剂,加热至回流,TLC检测,反应完毕,
降温析晶,抽滤,用甲基叔丁基醚淋洗滤饼,干燥得化合物5。
优选地,所述的缩合剂为二环己基碳二亚胺(DCC)、1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐[EDCI]、2-氯-1,3-二甲基氯化咪唑啉(DMC),进一步优选为二环己基碳二亚胺(DC C)。
步骤5:将3-溴4-氟苯甲酸溶于经过干燥的二氯甲烷,依次加入EDCI、HOBt和化合物5,室温下搅拌反应,TLC检测,反应完毕,过滤,浓缩,减压蒸干,经柱层分离得得化合物6。
步骤6:化合物8加入到干燥过的二氯甲烷中,依次加入化合物9和缩合剂,室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10。
优选地,所述的缩合剂选自二环己基碳二亚胺(DCC)、1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐[EDCI]、2-氯-1,3-二甲基氯化咪唑啉(DMC)、1-羰基苯并三唑(HOBt),进一步优选为1-羰基苯并三唑(HOBt)和1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐[EDCI]。
步骤7:将化合物10溶于有机溶剂中,滴加浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11。
优选地,所述的有机溶剂为乙酸乙酯,二氯甲烷,甲醇,乙醇,其中优选为乙酸乙酯。
步骤8:化合物6溶于有机溶剂,加入化合物11,加热至回流反应,TLC检测反应结束,降温析晶,有机溶剂淋洗滤饼,干燥,得化合物I。
优选地,所述的有机溶剂为甲苯、苯、二甲苯,其中优选为甲苯。
优选地,所述的析晶温度为-5~5℃,其中优选为0℃。
本发明第三方面,提供一种药物组合物,所述的药物组合物包括:治疗有效量的本发明所述式(I)化合物或其药学上可接受的盐、溶剂合物、前药、立体异构体或互变异构体中的一种或多种,以及任选的药学上可接受的载体、赋形剂、佐剂、辅料或稀释剂。
优选方案,所述的药物组合物可用于治疗与酪氨酸激酶表达异常或酪氨酸激酶活性较高相关的疾病。
优选方案,所述的与酪氨酸激酶表达异常或酪氨酸激酶活性较高相关的疾病包括但不限于细胞异常增值、形态变化、运动功能亢进、血管新生疾病、肿瘤生长、肿瘤转移疾病。
优选方案,一种BTK抑制剂,所述的抑制剂含有抑制有效量的如本发明所述的式(I)吡唑-4,6-二酮类化合物,或其药学上可接受的盐、互变异构体、光学异构体、药学上可接受的溶剂合物中的一种或多种,以及任选的药学上可接受的载体、赋形剂、佐剂、辅料或稀释剂。
本发明化合物可直接用于预防和治疗,或者优选地以药物组合物的形式。尽管活性成份能够单独给药,但优选为药物制剂或组合物的形式。因此,本发明提供了一种药物制剂,其包含本发明的化合物以及药学上可接受的稀释剂、赋形剂或载体(在这里统称为“载体”材料)。本发明的药物组合物可采取下文所述的药物制剂的形式。因此,本发明涉及一种药物组合物,其包含至少一种式(I)的化合物以及常规的赋形剂。
用于口服给药的示例性组合物包括:悬浮剂,其可包含例如微晶纤维素用于赋予体积,藻酸或藻酸钠作为助悬剂,甲基纤维素作为粘度增强剂,以及甜味剂或调味剂,例如本领域已知的那些;如速释片剂,其可包含例如微晶纤维素、磷酸二钙、淀粉、硬脂酸镁、硫酸钙、山梨糖醇、葡萄糖和/或乳糖和/或其他赋形剂、粘合剂、膨胀剂、崩解剂、稀释剂和润滑剂,例如本领域已知的那些。合适的粘合剂包括:淀粉、明胶、天然的糖如葡萄糖或β-乳糖、玉米甜味剂、天然和合成树胶如阿拉伯胶、黄芪胶或藻酸钠、羧甲基纤维素、聚乙二醇和蜡等。崩解剂包括但不限于:淀粉、甲基纤维素、琼脂、膨润土和黄原胶等。式(I)的化合物也可通过舌下和/或含服方式经口腔递送。模制片、压制片或冻干片是可使用的示例性形式。示例性的组合物包括将本发明化合物与快速溶解的稀释剂如甘露醇、乳糖、蔗糖和/或环糊精配制的那些。这些制剂中还可包括高分子量赋形剂如纤维素(微晶粉末纤维素)或聚乙二醇(PEG)。这些制剂也可包括有助于粘膜粘附的赋形剂,例如羟丙基纤维素(HPC),羟丙基甲基纤维素(HPMC),羧甲基纤维素钠(SCMC),马来酸酐共聚物(例如,Gantrez)和控制释放的试剂如聚丙烯酸类共聚物(例如Carbopo1934)。还可加入润滑剂,助流剂,调味剂,着色剂和稳定剂以便于制备和使用。这些剂型中使用的润滑剂包括:油酸钠,硬脂酸钠,硬脂酸镁,苯甲酸钠,乙酸钠和氯化钠等。对于液体形式的口服给药,口服药物组分可以与任何口服、非毒性的药学上可接受的惰性载体如乙醇、甘油和水等组合。
本发明的药物制剂包括适用于口服、胃肠外[包括皮下、皮内、肌内、静脉内(推注或输注)和关节内],吸入(包括可通过各种类型的计量加压气溶胶的方式产生的细颗粒粉剂或喷雾剂),喷雾器或吸入器,直肠、腹膜内和局部(包括皮肤、含服、舌下和眼内)给药的制剂,虽然最合适的途径可能取决于例如接受者的病症和状态。
适用于口服给药的本发明的制剂可以是各种含有预定量的活性成分的独立单位的形式,例如胶囊、扁胶囊、丸剂或片剂;粉末或颗粒;在水性液体或非水性液体的溶液或悬液,例如酏剂、酊剂、混悬剂或糖浆剂;或水包油乳液剂或油包水乳液剂。该活性成分也可制成大丸剂、药糖剂或糊剂。
片剂可以通过与任选的一种或多种辅助成份压缩或模塑来制作。压缩片剂的制作方法可以是在合适的机器中将任选与粘结剂、润滑剂、惰性稀释剂、润滑剂、表面活性剂或分散剂混合的诸如粉末或颗粒等自由流动形式的活性成份进行压缩。模塑片剂可通过在合适的机器中对用惰性液态稀释剂湿润的粉末状化合物的混合物进行模塑。片剂可任选地包衣或刻痕,并可配制成能够提供其中活性成分的缓释或控释。本发明化合物可以适用于速释或缓释的形式给予。速释或缓释可通过利用包含本发明化合物的合适的药物组合物来实现,或者尤其是在缓释的情况下,利用例如皮下植入物或渗透泵的装置来实现。本发明化合物也可以脂质体方式给予。优选的单位剂量制剂是包含有效剂量(如下文所述)或者其合适部分的活性成分的制剂。
应该理解的是,除上述特别提到的成分,本发明的制剂还可以包括与所研究制剂类型有关领域的其他常规药剂,例如,适宜于口服的制剂可以包括调味剂。
所述制剂可以作为单位剂型方便地提供,且可通过药学领域熟知的任何方法制造。所有方法均包括使活性成分与构成一种或多种附加成分的载体结合的步骤。通常,使活性成分与液体载体和/或细分固体载体均匀且密切地结合以制备制剂,然后,如果需要,将该产物成型,得到所需制剂。
本发明的化合物也可脂质体递送系统,例如小单室囊泡、大单室囊泡和多室囊泡的形式给予。脂质体可以由各种磷脂、1,2-二棕榈酰磷脂酰胆碱、磷脂酰乙醇胺(脑磷脂)、磷脂酰丝氨酸、磷脂酰肌醇、二磷脂酰甘油(心磷脂)或磷脂酰胆碱(卵磷脂)形成。
用于胃肠外给药的制剂包括水性和非水性无菌注射溶液,其可包含抗氧化剂、缓冲剂、抑菌剂以及使得制剂与指定接受者的血液等渗的溶质;以及水性和非水性无菌混悬液,其可包含助悬剂和增稠剂。可以用单位剂量或多剂量容器,例如密封的安瓿和药瓶提供该制剂,也可通过冷冻干燥(冻干)条件保存该组合物,临用前只需要加入无菌液体载体如盐水或注射用水即可使用。临时用的注射溶液和混悬液可以由前文描述的无菌粉末、颗粒和片剂进行制备。用于胃肠外给药的示例性组合物包括:可注射溶液剂或悬浮剂,其可包含例如合适的非毒性胃肠外可接受的稀释剂或溶剂,例如聚乙二醇、乙醇、1,3-丁二醇、水、林格溶液、氯化钠等渗溶液、或其他合适的分散剂或润湿剂以及助悬剂,包括合成的单或二甘油酯和脂肪酸,包括油酸和Cremaphor。
用于鼻腔、气溶胶或吸入给药的示例性组合物包括盐水中的溶液,其可包含例如苄基醇或其他合适的防腐剂,吸收促进剂以提高生物利用度,和/或其他增溶剂或分散剂如本领域已知的那些。
用于直肠给药的制剂可以用常规载体如可可油/合成甘油酯或聚乙二醇制成栓剂形式。这些载体在常温条件下通常为固体,但在直肠管腔内液化和/或溶解以释放药物。
口腔内局部给药例如含服或舌下给药的制剂,包括锭剂,其包含在调味基质如蔗糖和阿拉伯胶或西黄蓍胶中的活性成分,和软锭剂,其包含在明胶和甘油或蔗糖和阿拉伯胶中的基质中的活性成分。局部给药的示例性组合物包括局部载体如Plastibase(用聚乙烯胶化的矿物油)。
本发明第四方面,提供一种如式(I)的吡唑-4,6-二酮类化合物的用途,具体的可用于制备酪氨酸激酶抑制剂,用于体外非治疗性的抑制酪氨酸激酶的活性,用于体外非治疗性的抑制肿瘤细胞生长或其组合用途,其在治疗或预防其中BTK起作用的疾病中的用途。
优选方案,本发明所述的如式(I)的吡唑-4,6-二酮类化合物,其用于制备治疗或预防其中BTK起作用的疾病的药物。
优选方案,所述的BTK起作用的疾病包括癌症或自身免疫系统相关的疾病,具体的所述癌症包括但不限于非小癌肺细胞、淋巴瘤、乳腺癌、白血病和头颈部鳞癌细胞肉瘤、肺癌、卵巢癌、宫颈癌、结肠直肠癌、乳腺癌、胰腺癌、胶质瘤、胶质母细胞瘤、黑色素瘤、前列腺癌、浆细胞瘤、结外边缘区B细胞淋巴瘤、淋巴结边缘区B细胞淋巴瘤、非霍奇金淋巴瘤、胃癌、肺癌、肝细胞癌、胃癌、胃肠道间质瘤、甲状腺癌、胆管癌、子宫内膜癌、肾癌、间变性大细胞淋巴瘤、急性髓细胞白血病、多发性骨髓瘤、黑色素瘤或间皮瘤;所述自身免疫性相关的疾病包括但不限于慢性淋巴细胞性甲状炎、甲状腺功能亢进、胰岛素依赖型糖尿病、重症肌无力、溃疡性结肠炎、恶性贫血伴慢性萎缩性胃炎、肺出血肾炎综合征、原发性胆汁性肝硬化、多发性脑脊髓硬化症、急性特发性多神经炎、系统性红斑狼疮、类风湿关节炎、系统性血管炎、硬皮病、天胞疮、皮肌炎、混合型结缔组织病、自身免疫性溶血性贫血、甲状腺自身免疫病、溃疡性结肠炎。
优选方案,所述的治疗是指减轻或缓和疾病及其并发症状的方法;所述的预防是指减少或消除疾病、病状或病症的症状或并发症的发作。
与现有技术相比,本发明的主要优点包括:
(1)本发明提供了一种结构新颖的式(I)吡唑-4,6-二酮类化合物。
(2)本发明提供的式(I)吡唑-4,6-二酮类化合物,对于肿瘤细胞的生长显著的抑制作用。
(3)本发明提供的式(I)吡唑-4,6-二酮类化合物,起始物料便宜易得,合成路线较短,操作更为简单,适合工业化放大生产。
应该理解的是,在本发明的范围内,本发明的上述各技术特征和在下文中(包括实施例)具体描述的各技术特征之间都可以相互结合,从而构成新的或优选的技术方案。
具体实施方式
下面通过实施例进一步说明本发明的方法。应正确理解的是,本发明实施例的制备方法仅仅是用于说明本发明,而不是对本发明的限制,在本发明的构思前提下对本发明制备方法的简单改进都属于本发明要求保护的范围。
本文中所使用的缩写:
DCC:二环己基碳二亚胺
EDCI:1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐
DMC:2-氯-1,3-二甲基氯化咪唑啉
HOBt:1-羰基苯并三唑
TLC:薄层色谱
Boc基团:叔丁氧羰基
化合物6的制备:
化合物2的制备:将乙醇钠固体(4g,0.01mol)加至无水乙醇(40ml)中,室温搅拌溶解,倒入(13.20g,0.02mol)丙二腈,缓慢升温至回流,TLC检测,反应完毕,控温至20℃,搅拌析晶,析晶完毕,抽滤,乙醇淋洗,所得固体减压烘干后转移至三口瓶,倒入纯净水,室温搅拌至溶解,用5mol/L盐酸调pH到3~4,析出晶体,过滤,滤饼用纯净水重结晶,得白色针状晶体,减压干燥得化合物2,收率85.6%,纯度99.92%。
化合物3的制备:
将中间体2加入到500ml水中,搅拌溶解,加入水合肼50ml,缓慢升温至回流,TLC检测,反应完毕,降温至0℃析晶,过滤析出沉淀,减压干燥得化合物3,收率87.8%,纯度99.89%。
化合物4的制备:
将化合物3加入到0.2MNaOH(200ml)中,搅拌溶解,加热至回流反应,TLC检测,反应完毕,降温到0℃,反应液用5mol/L盐酸调PH到3~4,过滤,减压干燥得中间体4,收率92.5%,纯度99.90%。
化合物5的制备:
将化合物4(150g,0.90mol)溶于乙酸酐(500ml)、加入DCC(240.5g,1.17mol),加热至回流,TLC检测,反应完毕,降温至0℃析晶,抽滤,滤饼用甲基叔丁基醚淋洗,减压干燥得化合物5,收率91.7%,纯度99.88%。
化合物6的制备:
将3-溴4-氟苯甲酸(2.19g,0.1mol)加入到300ml二氯甲烷中,依次加入EDCI(1.92g,0.1mol),HOBt(1.35g,0.1mol)和中间体5(83.6g,0.08mol),室温下搅拌反应,TLC检测,反应完毕,过滤,浓缩,减压蒸干,经柱层分离得得化合物6,收率90.5%,纯度99.87%。
实施例1
化合物TM1的制备:
化合物10-1的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-1(8.90g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-1,收率91.8%,纯度99.78%。
化合物11-1的制备:
将化合物10-1(23.13g,0.1mol)溶于150ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11,收率90.3%,纯度99.85%。
化合物TM1的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-1(15.74g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM1,收率92.4%,纯度99.92%。
1HNMR(400MHz,DMSOd6):δ12.15(s,1H),9.76(s,1H),8.98(s,1H),7.82~7.74(m,3H),4.66(s,2H),4.32(s,2H),3.42(s,2H),2.76(s,6H);
13CNMR(100MHz,DMSOd6):173.4,168.5,164.6,159.8,155.5,150.2,141.1,133.4,133.5,128.9,118.7,112.2,98.7,78.1,54.8,45.5(2C),30.4.
MS(m/z):481.24[M+H]+;
实施例2
化合物TM2的制备:
化合物10-2的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-2(12.89g,0.13mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-2,收率91.5%,纯度99.77%。
化合物11-2的制备:
将化合物10-2(25.64g,0.1mol)溶于150ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-2,收率91.1%,纯度99.89%。
化合物TM2的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-2(18.75g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM2,收率92.1%,纯度99.90%。
1HNMR(400MHz,DMSO-d6)δ12.15(s,1H),9.76(s,1H),8.98(s,1H),7.82~7.74(m,3H),4.35(s,2H),3.92(s,2H),3.55~3.47(m,1H),1.74~1.69(m,2H),1.50~1.43(m,4H),1.23~1.11(m,4H);
13C-NMR(100MHz,DMSO-d6):168.4,157.6,155.5,150.2,141.1,133.4,132.6,129.5,120.9,118.7,112.2,98.7,54.8,50.1,35.6(2C),30.5,27.0,25.6,26.7(2C).
MS(m/z):506.12[M+H]+;
实施例3
化合物TM3的制备:
化合物10-3的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-3(11.18g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-3,收率92.0%,纯度99.82%。
化合物11-3的制备:
将化合物10-3(25.03g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-3,收率91.6%,纯度99.90%。
化合物TM3的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-3(18.02g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM3,收率92.1%,纯度99.90%。
1HNMR(400MHz,DMSO-d6)δ12.15(s,1H),9.76(s,1H),8.98(s,1H),7.82~7.74(m,3H),7.23~7.03(m,5H),4.65(s,2H),3.42(s,2H);
13C-NMR(100MHz,DMSO-d6):168.4,157.6,155.5,150.2,141.1,137.1,133.4,134.3,130.4,129.5,128.8(2C),127.5,121.8(2C),120.9,118.7,112.2,98.7,54.8,30.5.
MS(m/z):500.18[M+H]+;
实施例4
化合物TM4的制备:
化合物10-4的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-4(13.33g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-4,收率92.2%,纯度99.85%。
化合物11-4的制备:
将化合物10-4(26.83g,0.1mol)溶于150ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-4,收率91.8%,纯度99.91%。
化合物TM4的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-4(20.18g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM4,收率91.7%,纯度99.87%。
1HNMR(400MHz,DMSO-d6)δ12.15(s,1H),9.76(s,1H),8.98(s,1H),7.82~7.74(m,3H),7.23~7.03(m,4H),4.65(s,2H),3.42(s,2H);
13C-NMR(100MHz,DMSO-d6):168.4,162.9,157.6,155.5,150.2,141.1,137.1,133.4,134.3,130.4,129.5,128.8(2C),127.5,121.8(2C),118.7,112.2,98.7,54.8,30.5.
MS(m/z):518.31[M+H]+;
实施例5
化合物TM5的制备:
化合物10-5的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-5(11.29g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-5,收率91.9%,纯度99.81%。
化合物11-5的制备:
将化合物10-5(25.13g,0.1mol)溶于150ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-5,收率92.0%,纯度99.92%。
化合物TM5的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-5(18.14g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM5,收率91.9%,纯度99.89%。
1HNMR(400MHz,DMSO-d6)δ12.15(s,1H),9.76(s,1H),8.98(s,1H),7.82~7.74(m,3H),7.33~7.23(m,3H),7.11~7.04(m,1H),4.65(s,2H),3.42(s,2H);
13C-NMR(100MHz,DMSO-d6):168.4,162.9,157.6,155.5,150.2,141.1,137.1,133.4,134.3,130.4,129.5,128.8,127.5,124.8,121.8,118.7,112.2,98.7,48.8,30.5.
MS(m/z):501.16[M+H]+;
实施例6
化合物TM6的制备:
化合物10-6的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-6(16.46g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-6,收率92.2%,纯度99.84%。
化合物11-6的制备:
将化合物10-6(29.44g,0.1mol)溶于150ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-6,收率92.2%,纯度99.91%。
化合物TM6的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-6(23.71g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM6,收率92.4%,纯度99.91%。
1HNMR(400MHz,DMSO-d6)δ12.15(s,1H),9.76(s,1H),8.98(s,1H),7.82~7.74(m,3H),7.63(s,1H),7.23~7.33(m,2H),4.92(s,2H),3.57(s,2H),3.32(s,3H),2.15(s,3H);
13C-NMR(100MHz,DMSO-d6):168.4,167.9,157.6,155.5,150.2,141.1,135.1,133.4,132.5,131.0,129.5,128.1,125.4,123.3,122.8,120.9,118.7,112.2,98.7,56.4,54.8,27.0,18.2.
MS(m/z):544.22[M+H]+;
实施例7
化合物TM7的制备:
化合物10-7的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-7(15.98g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-7,收率92.0%,纯度99.82%。
化合物11-7的制备:
将化合物10-7(29.03g,0.1mol)溶于150ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-7,收率91.8%,纯度99.88%。
化合物TM7的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-7(22.82g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM7,收率92.0%,纯度99.87%。
1HNMR(400MHz,DMSO-d6)δ12.15(s,1H),10.56(s,1H),9.76(s,1H),8.98(s,1H),7.82~7.74(m,3H),7.23~7.13(m,4H),4.65(s,2H),3.72(s,2H);
13C-NMR(100MHz,DMSO-d6):168.4,157.6,155.5,150.2,141.1,136.2(2C),133.4,130.3,129.5,126.8,123.2(2C),122.5,120.9,118.7,115.2(2C),112.2,98.7,54.8,27.2.
MS(m/z):540.13[M+H]+;
实施例8
化合物TM8的制备:
化合物10-8的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-8(32.93g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-8,收率92.5%,纯度99.87%。
化合物11-8的制备:
将化合物10-8(43.16g,0.1mol)溶于150ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-8,收率92.4%,纯度99.90%。
化合物TM8的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-8(39.78g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM8,收率92.3%,纯度99.90%。
1HNMR(400MHz,DMSO-d6)δ12.15(s,1H),9.76(s,1H),8.98(s,1H),7.82~7.74(m,4H),7.23~7.33(m,3H),4.72(s,2H),3.49(s,2H),3.11(m,4H);2.74(m,1H);2.45(m,8H);2.36(s,3H);1.82(m,4H);
13C-NMR(100MHz,DMSO-d6):168.4,167.2,157.6,155.5,150.2,141.1,133.4,132.7,129.5,120.9,120.6(2C),118.7,118.4,118.3,117.7(2C),112.2,98.7,63.4(2C),56.7(2C),54.8,54.3(2C),53.3,45.3,29.4(2C),27.0.
MS(m/z):681.16[M+H]+;
实施例9
化合物TM9的制备:
化合物10-9的制备:
化合物8(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-9(16.46g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-9,收率92.1%,纯度99.85%。
化合物11-8的制备:
将化合物10-9(19.44g,0.1mol)溶于150ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-9,收率92.0%,纯度99.87%。
化合物TM9的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-9(23.31g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM9,收率92.0%,纯度99.89%。
MS(m/z):544.15[M+H]+;1H-NMR(400MHz,DMSO-d6):δ11.08(s,1H),10.24(s,1H),8.70(s,1H),7.55(s,1H),7.28~7.16(m,5H),4.83~4.64(m,2H),3.77(s,3H),3.46(s,2H),2.68~2.54(m,2H);2.18(s,3H);13C-NMR(400MHz,DMSO-d6):δ173.0,168.8,167.9,162.4,158.9,149.8,137.7,135.5,130.8,128.3,125.7,123.0,122.5,121.5,119.4,118.9,118.6,115.3,114.6,72.2,56.6,39.7,35.5,19.9.
实施例10
化合物TM10的制备:
化合物10-10的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-10(16.46g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-10,收率91.7%,纯度99.82%。
化合物11-10的制备:
将化合物10-10(35.64g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-10,收率92.0%,纯度99.87%。
化合物TM10的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-10(30.76g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM10,收率92.2%,纯度99.91%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),7.71~7.54(m,4H),7.45(s,1H),7.25~7.17(m,5H),3.71(s,3H),3.59(s,2H),2.15(s,3H);
13C-NMR(400MHz,DMSO-d6):δ172.8,168.8,167.7,162.8,158.6,149.7,142.4,137.5,135.7,131.2(2C),130.4,128.5,125.8,124.3,123.4,122.3,121.2,119.5,118.7,118.4,115.1,114.8,113.5(2C),72.4,56.8,19.7.
MS(m/z):606.20[M+H]+;
实施例11
化合物TM11的制备:
化合物10-11的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-11(8.90g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-11,收率92.0%,纯度99.85%。
化合物11-11的制备:
将化合物10-11(29.34g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-11,收率92.4%,纯度99.89%。
化合物TM11的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-11(23.19g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM11,收率92.0%,纯度99.88%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),7.71~7.54(m,3H),7.75(s,1H),7.25~7.17(m,3H),4.15(s,2H),3.59(s,2H),2.33(s,6H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,158.6,149.7,142.4,137.5,131.2,130.4,128.5(2C),125.8(2C),123.4,121.2,119.5,114.8,110.5,99.5,72.4,47.4(2C),31.2.
MS(m/z):543.13[M+H]+;
实施例12
化合物TM12的制备:
化合物10-12的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-12(8.90g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-12,收率92.0%,纯度99.85%。
化合物11-12的制备:
将化合物10-12(29.34g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-12,收率92.4%,纯度99.89%。
化合物TM12的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-12(23.19g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM12,收率92.0%,纯度99.88%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),7.71~7.54(m,3H),7.75(s,1H),7.25~7.17(m,3H),3.59(s,2H),3.49~3.41(m,1H),1.59~1.22(m,10H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,158.6,149.7,142.4,137.5,131.2,130.4,128.5(2C),125.8(2C),123.4,121.2,119.5,114.8,110.5,99.5,55.4,33.4(2C),31.2,26.8(2C),23.9.
MS(m/z):568.09[M+H]+;
实施例13
化合物TM13的制备:
化合物10-13的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-13(11.18g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-13,收率91.8%,纯度99.84%。
化合物11-13的制备:
将化合物10-13(31.24g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-13,收率92.6%,纯度99.91%。
化合物TM13的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-13(25.47g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM13,收率91.7%,纯度99.82%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),7.75(s,1H),7.71~7.54(m,5H),7.25~7.17(m,5H),7.15~7.07(m,1H),3.59(s,2H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,158.6,149.7,142.4,137.5,136.7,131.2,130.4,129.1(2C),128.5(2C),127.4(2C),125.8(2C),123.4,122.5,121.2,119.5,114.8,110.5,99.5,31.2.
MS(m/z):562.21[M+H]+;
实施例14
化合物TM14的制备:
化合物10-14的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-14(13.33g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-14,收率91.4%,纯度99.81%。
化合物11-14的制备:
将化合物10-14(33.04g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-14,收率92.0%,纯度99.87%。
化合物TM14的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-14(27.63g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM14,收率91.1%,纯度99.80%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),7.99~7.75(m,2H),7.71~7.54(m,4H),7.25~7.17(m,5H),3.59(s,2H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,161.5,158.6,149.7,142.4,137.5,136.7,133.4,130.4,128.5(2C),125.8(2C),127.7(2C),123.4,122.5,121.2,119.5,117.1(2C),110.5,99.5,31.2.
MS(m/z):580.15[M+H]+;
实施例15
化合物TM15的制备:
化合物10-15的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-15(11.29g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-15,收率92.0%,纯度99.87%。
化合物11-15的制备:
将化合物10-15(31.34g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-15,收率92.3%,纯度99.89%。
化合物TM15的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-15(25.59g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM15,收率91.9%,纯度99.88%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.03~7.92(m,3H),7.81~7.70(m,3H),7.25~7.17(m,5H),3.59(s,2H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,158.6,154.3,149.7,146.8,142.4,139.4,137.5,136.7,133.4,130.4,128.5(2C),125.8(2C),123.4,122.5,121.2,119.5,117.9,113.5,99.5,31.2.
MS(m/z):563.11[M+H]+;
实施例16
化合物TM16的制备:
化合物10-16的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-16(17.18g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-16,收率91.5%,纯度99.84%。
化合物11-16的制备:
将化合物10-16(36.24g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-16,收率91.8%,纯度99.82%。
化合物TM16的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-16(31.48g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM16,收率91.4%,纯度99.85%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.03~7.92(m,2H),7.85~7.71(m,4H),7.57~7.41(m,4H),7.25~7.17(m,4H),3.59(s,2H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,158.6,154.3,149.7,146.8,142.4,139.4,137.5,135.7,133.4,132.8,130.4,129.1,128.5(2C),126.7,125.8(2C),124.3,122.5,120.5,119.5,119.1,117.9,116.7,113.5,99.5,31.2.
MS(m/z):612.16[M+H]+;
实施例17
化合物TM17的制备:
化合物10-17的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-17(15.98g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-17,收率91.9%,纯度99.86%。
化合物11-17的制备:
将化合物10-17(33.52g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-17,收率92.1%,纯度99.89%。
化合物TM17的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-17(30.27g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM17,收率91.7%,纯度99.86%。
1H-NMR(400MHz,DMSO-d6):δ12.11(s,1H),11.03(s,1H),10.20(s,1H),8.74(s,1H),7.71~7.54(m,3H),7.75(s,1H),7.25~7.17(m,3H),7.11~7.04(m,4H),3.59(s,2H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,158.6,150.7,149.7,142.4,139.1,137.5,136.2,131.2,130.4,128.5(2C),125.8(2C),124.6(2C),123.4,121.2,119.5,117.0(2C),114.8,110.5,99.5,31.2;
MS(m/z):602.18[M+H]+;
实施例18
化合物TM18的制备:
化合物10-18的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-18(32.93g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-18,收率92.4%,纯度99.88%。
化合物11-18的制备:
将化合物10-18(49.37g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-18,收率92.3%,纯度99.92%。
化合物TM18的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-18(47.22g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM18,收率92.1%,纯度99.88%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),7.71~7.54(m,3H),7.75(s,1H),7.31~7.22(m,5H),7.11~6.94(m,2H),3.59(s,2H),3.34~7.21(m,4H),2.77~2.63(m,1H),2.36~2.18(m,11H),1.77~1.65(m,4H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,158.6,149.7,145.7,142.4,137.5,131.2,130.4,129.4,128.5(2C),125.8(2C),123.4,122.6(2C),121.2,119.5,115.2(2C),114.8,110.5,99.5,76.2,66.1(2C),61.5(2C),55.3(2C),47.6,31.2,29.3(2C).
MS(m/z):743.24[M+H]+;
实施例19
化合物TM19的制备:
化合物10-19的制备:
化合物8-1(23.73g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-19(17.79g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-19,收率91.7%,纯度99.80%。
化合物11-19的制备:
将化合物10-19(36.85g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-19,收率91.8%,纯度99.89%。
化合物TM19的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-19(32.20g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM19,收率91.6%,纯度99.84%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),7.84~7.75(m,2H),7.70~7.56(m,5H),7.35~7.20(m,5H),3.59(s,2H);
13C-NMR(400MHz,DMSO-d6):δ168.8,167.7,162.8,158.6,149.7,142.4,139.2,138.4,137.5,131.2,130.4,128.5(2C),127.3,125.8(2C),124.6,124.3,123.4,123.0,122.5,121.2,119.5,114.8,111.0,110.5,99.5,31.2.
MS(m/z):618.19[M+H]+;
实施例20
化合物TM20的制备:
化合物10-20的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-20(8.90g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-20,收率91.1%,纯度99.75%。
化合物11-20的制备:
将化合物10-20(24.53g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-20,收率91.2%,纯度99.83%。
化合物TM20的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-20(17.43g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM20,收率91.0%,纯度99.80%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.25~7.17(m,1H),4.88~4.81(m,2H),4.31(s,2H),3.59(s,2H),2.71~2.64(m,2H),2.26(s,6H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,149.7,145.6,142.4,133.1,132.3,128.2,118.5,111.0,99.6,79.2,47.2(2C),40.1,33.7,31.2.
MS(m/z):495.32[M+H]+;
实施例21
化合物TM21的制备:
化合物10-21的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-21(11.90g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-21,收率91.4%,纯度99.78%。
化合物11-21的制备:
将化合物10-21(27.04g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-21,收率91.9%,纯度99.88%。
化合物TM21的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-21(20.43g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM21,收率92.1%,纯度99.89%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.25~7.17(m,1H),4.31(s,2H),3.59(s,2H),3.51~3.42(m,1H),2.71~2.64(m,2H),1.74~1.69(m,2H),1.50~1.43(m,4H),1.23~1.11(m,4H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,149.7,145.6,142.4,133.1,132.3,128.2,118.5,111.0,99.6,50.5,40.1,35.6(2C),33.7,31.2,25.6,26.7(2C).
MS(m/z):520.11[M+H]+;
实施例22
化合物TM22的制备:
化合物10-22的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-22(11.18g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-22,收率91.6%,纯度99.79%。
化合物11-22的制备:
将化合物10-22(26.43g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-22,收率91.1%,纯度99.82%。
化合物TM22的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-22(19.71g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM22,收率91.5%,纯度99.82%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.25~7.17(m,6H),4.31(s,2H),3.59(s,2H),2.71~2.64(m,2H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,149.7,145.6,142.4,139.5,133.1,132.3,128.8(2C),128.2,127.1,121.8(2C),118.5,111.0,99.6,40.1,33.7,31.2.
MS(m/z):514.17[M+H]+;
实施例23
化合物TM23的制备:
化合物10-23的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-23(13.33g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-23,收率91.0%,纯度99.74%。
化合物11-23的制备:
将化合物10-23(28.23g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-23,收率91.4%,纯度99.83%。
化合物TM23的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-23(21.86g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM23,收率91.7%,纯度99.84%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.25~7.17(m,5H),4.31(s,2H),3.59(s,2H),2.71~2.64(m,2H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,162.3,159.2,149.7,145.6,142.4,133.1,132.3,131.5,128.2,121.8(2C),118.5,115.2(2C),111.0,99.6,40.1,33.7,31.2.
MS(m/z):532.24[M+H]+;
实施例24
化合物TM24的制备:
化合物10-24的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-24(11.29g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-24,收率91.6%,纯度99.77%。
化合物11-24的制备:
将化合物10-24(26.53g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-24,收率91.8%,纯度99.85%。
化合物TM24的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-24(19.82g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM24,收率91.8%,纯度99.87%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.33~7.19(m,4H),7.11~7.04(m,1H),4.31(s,2H),3.59(s,2H),2.71~2.64(m,2H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,151.3,149.7,147.3,145.6,142.4,139.2,133.1,132.3,128.2,124.5,118.5,115.6,111.0,99.6,40.1,33.7,31.2.
MS(m/z):515.19[M+H]+;
实施例25
化合物TM25的制备:
化合物10-25的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-25(16.46g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-25,收率91.0%,纯度99.71%。
化合物11-25的制备:
将化合物10-25(30.84g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-25,收率91.3%,纯度99.83%。
化合物TM25的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-25(24.99g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM25,收率91.0%,纯度99.81%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.82~7.74(m,3H),7.25~7.17(m,1H),4.31(s,2H),3.59(s,2H),3.32(s,3H),2.71~2.64(m,2H),2.15(s,3H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,157.4,149.7,145.6,142.4,137.3,133.1,132.3,130.3,128.2,122.1,118.5,114.2,111.0,103.9,99.6,55.6,40.1,33.7,31.2,15.9.
MS(m/z):558.07[M+H]+;
实施例26
化合物TM26的制备:
化合物10-26的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-26(15.98g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-26,收率91.9%,纯度99.82%。
化合物11-26的制备:
将化合物10-26(30.44g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-26,收率91.9%,纯度99.87%。
化合物TM26的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-26(24.51g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM26,收率92.2%,纯度99.90%。
1H-NMR(400MHz,DMSO-d6):δ12.15(s,1H),11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.25~7.17(m,5H),4.31(s,2H),3.59(s,2H),2.71~2.64(m,2H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,149.7,146.5,145.6,142.4,137.2,136.4,133.1,132.3,128.2,123.2(2C),118.5,115.2(2C),111.0,99.6,40.1,33.7,31.2.
MS(m/z):554.30[M+H]+;
实施例27
化合物TM27的制备:
化合物10-27的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-27(32.93g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-27,收率92.3%,纯度99.87%。
化合物11-27的制备:
将化合物10-27(46.17g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-27,收率92.1%,纯度99.89%。
化合物TM27的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-27(41.46g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM27,收率92.4%,纯度99.91%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.82~7.74(m,4H),7.25~7.17(m,1H),4.31(s,2H),3.59(s,2H),3.34~7.21(m,4H),2.77~2.63(m,3H),2.36~2.18(m,11H),1.77~1.65(m,4H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,149.7,146.6,145.6,142.4,133.1,132.3,128.2,127.3,126.1(2C),118.5,113.4(2C),111.0,99.6,63.4(2C),70.5,56.7(2C),54.3(2C),46.8,40.1,33.7,31.2,29.4(2C).
MS(m/z):695.27[M+H]+;
实施例28
化合物TM28的制备:
化合物10-28的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-28(18.02g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-28,收率91.5%,纯度99.82%。
化合物11-28的制备:
将化合物10-28(32.14g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-28,收率91.1%,纯度99.80%。
化合物TM28的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-28(26.55g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM28,收率91.8%,纯度99.87%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.18~7.96(m,4H),7.62~7.54(m,2H),7.25~7.17(m,1H),4.31(s,2H),3.59(s,2H),2.71~2.64(m,2H);
13C-NMR(400MHz,DMSO-d6):δ174.0,170.1,168.6,167.8,159.2,155.2,149.7,145.6,142.4,133.1,132.3,131.2,128.2,122.5,123.6,120.3,119.1,118.5,111.0,99.6,40.1,33.7,31.2.
MS(m/z):571.22[M+H]+;
实施例29
化合物TM29的制备:
化合物10-29的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-29(17.18g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-29,收率91.2%,纯度99.81%。
化合物11-29的制备:
将化合物10-29(31.44g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-29,收率91.4%,纯度99.83%。
化合物TM29的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-29(25.71g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM29,收率91.4%,纯度99.85%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.38~8.32(m,1H),8.11~7.98(m,4H),7.62~7.47(m,3H),7.25~7.18(m,2H),4.31(s,2H),3.59(s,2H),2.71~2.64(m,2H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,149.7,145.6,142.4,136.2,134.7,133.1,132.3,129.5,128.2,127.0,126.6,124.3,122.7,121.3,119.5,118.5,117.1,111.0,99.6,40.1,33.7,31.2.
MS(m/z):564.16[M+H]+;
实施例30
化合物TM30的制备:
化合物10-30的制备:
化合物8-2(18.92g,0.1mol)加入到干燥过的二氯甲烷中,依次加入化合物9-30(15.31g,0.12mol)和HOBt(13.51g,0.1mol)、EDCI(19.17g,0.1mol),室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10-30,收率91.6%,纯度99.83%。
化合物11-30的制备:
将化合物10-30(29.88g,0.1mol)溶于180ml乙酸乙酯中,滴加30mL浓度为3mol/L盐酸,脱去Boc基团,纯化水洗涤,有机层无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11-30,收率91.9%,纯度99.88%。
化合物TM30的制备:
将中间体6(18.41g,0.05mol)加入到100ml三口瓶中,倒入80ml甲苯,加入化合物11-30(23.84g,0.12mol),加热至回流反应,TLC检测反应结束,降温至0℃析晶,析晶结束,过滤,滤饼用适量甲苯淋洗,减压干燥得化合物TM30,收率92.0%,纯度99.89%。
1H-NMR(400MHz,DMSO-d6):δ11.03(s,1H),10.20(s,1H),8.74(s,1H),8.11~7.98(m,2H),7.53~7.40(m,4H),7.25~7.17(m,1H),4.31(s,2H),3.59(s,2H),2.71~2.64(m,2H);
13C-NMR(400MHz,DMSO-d6):δ170.1,168.6,167.8,159.2,149.7,145.6,142.4,136.5,134.2,133.1,132.3,130.1(2C),128.2,119.3(2C),118.5,111.0,99.6,40.1,33.7,31.2.
MS(m/z):548.27[M+H]+;
对癌细胞的体外细胞毒活性测试
为了研究本次实验中所合成的目标化合物抑制肿瘤细胞增殖的能力,我们测定了本发明化合物对淋巴瘤细胞株(Raji)、乳腺癌细胞(MCF7)、白血病细胞(K562)和头颈部鳞癌细胞(HN5)四种肿瘤细胞的体外细胞毒性。实验采用的检测方法是标准的MTT法。
实验方法具体为:
淋巴瘤细胞株、乳腺癌细胞、白血病细胞、头颈部鳞癌细胞(上海和序生物科技有限公司)四种肿瘤细胞经胰酶消化处理10min后弃掉液体,用5%血清的培养液吹打,调细胞浓度为300~400个/μL,依次加入药物,预留只含培养液的空白组,在培养箱中培养24h。弃掉上清液,将稀释好的不同浓度的目标化合物加入96孔板(上海卧宏生物科技有限公司),对照组只加细胞液,每组浓度均设置三个副孔,混匀后继续培养36h,观察不同时间段细胞形态变化,细胞与药物充分作用后,每孔加入现配的MTT溶液显色,继续培养6h。弃掉上层液体,100μLDMSO溶解形成紫色结晶,选定490nm,酶标仪(HBS-1096A酶标分析仪,南京德铁实验设备有限公司)测定吸光度。通过公式:细胞抑制率(%)=(1-A实验/A对照)×100%,求出IC50值(用细胞存活率对剂量对数作图法)。
化合物TM1~8对淋巴瘤细胞株(Raji)、乳腺癌细胞(MCF7)、白血病细胞(K562)和头颈部鳞癌细胞(HN5)四种肿瘤细胞增殖抑制的体外毒性试验结果见表1。
表1部分化合物对肿瘤细胞毒性实验(IC50,μM)a
注:a表中数值为3次实验的平均值;b药物作用时间36h
本发明提供的一类新型抗肿瘤活性化合物TM1~30对淋巴瘤细胞株(Raji)、乳腺癌细胞(MCF7)、白血病细胞(K562)和头颈部鳞癌细胞(HN5)四种肿瘤细胞生长抑制活性均较高,而化合物TM10对淋巴瘤细胞株(Raji)和白血病细胞(K562)的抑制率均达到nM级别;TM19对MCF7的抑制率达到nM级别;以及TM26对Raji和MCF7抑制率均达到nM级别;且三个化合物对四种肿瘤细胞的抑制率显著高于对照化合物依鲁替尼;化合物TM25对MCF7细胞的抑制活性与选择性优于其他三种细胞。
本发明化合物TM10还显示出较好的物理性质,水溶性达到0.115mg/ml;同时小鼠体内代谢良好,生物利用度达到96.8%。
Claims (13)
1.一种式I所示吡唑-4,6-二酮类化合物或其药学上可接受的盐的水合物、溶剂合物、前药、立体异构体或互变异构体:
其中,R独立的选自H、卤素、芳基、杂芳基、C1-4烷基、C1-6烷氧基、C3-6环烷基、C2-6杂环烷基、芳氧基、-NR2R3;或取代的芳基、取代的杂芳基、取代的C3-6环烷基、取代的C2-6杂环烷基、取代的芳氧基、取代的C1-6烷氧基中的一个或多个独立的基团,其中取代基基团选自卤素、芳基、杂芳基、C1-6烷氧基、C1-6烷氨基、C1-4烷基、取代的C2-6杂环烷基、卤素取代的C2-6杂环烷基、C2-6杂环烷基中的一个或多个取代基。R2独立的选自H或C1-4烷基;
R3独立的选自H、C1-4烷基、C3-6环烷基、C1-3卤代烷基、芳基、杂芳基、C1-6烷氧基、C1-6烷氨基、取代的C2-6杂环烷基、卤素取代的C2-6杂环烷基、C2-6杂环烷基中的一个或多个;
Z独立的选自C1-4烷基或芳基。
2.根据权利要求1所述的吡唑-4,6-二酮类化合物,其特征在于,所述药学上可接受的盐为无机酸的盐或有机酸的盐,无机酸盐包括但不限于:盐酸盐、硝酸盐、硼酸盐、氢氰酸盐、氢氟酸盐、氢溴酸盐、氢碘酸盐、亚硝酸盐、高卤酸盐、卤酸盐、亚卤酸盐、次卤酸盐、偏铝酸盐、硫酸盐、磷酸盐、硝酸盐;所述的有机酸盐包括但不限于:甲酸盐、乙酸盐、丙酸盐、丁酸盐、丙烯酸盐、乙二酸盐、丙二酸盐、丁二酸盐、苯甲酸盐、邻苯二甲酸羧酸盐、甲磺酸盐、乙磺酸盐、苯磺酸盐、苯甲磺酸盐、对甲苯亚磺酸盐、硫代乙酸盐、三氟乙酸盐、酒石酸盐、苹果酸盐、枸椽酸盐、抗坏血酸盐、水杨酸盐、咖啡酸富马酸盐、马来酸盐、乳酸盐、柠檬酸盐、谷氨酸盐、樟脑酸盐、樟脑磺酸盐等。
5.根据权利要求4所述的吡唑-4,6-二酮类化合物的制备方法,其特征在于,反应步骤包括:
步骤1:化合物1在乙醇钠溶液中发生缩合反应,得化合物2;
步骤2:化合物2与水合肼反应,得到化合物3;
步骤3:化合物3碱性条件下反应,得化合物4;
步骤4:化合物4控温回流脱水生成中间体化合物5;
步骤5:化合物5与3-溴4-氟苯甲酸、EDCI、HOBt在二氯甲烷中反应,得化合物6。
步骤6:化合物8和化合物9反应得到化合物10。
步骤7:化合物10水解得到化合物11。
步骤8:化合物6和化合物11在有机溶剂中反应,经过析晶,过滤干燥得目标化合物I。
6.根据权利要求4所述的吡唑-4,6-二酮类化合物的制备方法,其特征在于,反应具体步骤包括:
步骤1:将乙醇钠加入乙醇溶剂中,搅拌溶解,加入化合物1,升温至回流,TLC检测,反应完毕,降温析晶,抽滤,干燥得化合物2。
步骤2:将化合物2加入到水中,搅拌溶解,加入水合肼,升温至回流,TLC检测,反应完毕,降温析出固体,过滤,干燥得化合物3。
步骤3:化合物3加入到碱溶液中,搅拌溶解,加热至回流,TLC检测,反应完毕,降温加入盐酸调PH到3~4,析出固体,过滤,干燥得化合物4。
步骤4:将化合物4溶于乙酸酐溶液,加入缩合剂,加热至回流,TLC检测,反应完毕,降温析晶,抽滤,用甲基叔丁基醚淋洗滤饼,干燥得化合物5。
步骤5:将3-溴4-氟苯甲酸溶于经过干燥的二氯甲烷,依次加入EDCI、HOBt和化合物5,室温下搅拌反应,TLC检测,反应完毕,过滤,浓缩,减压蒸干,经柱层分离得得化合物6。
步骤6:化合物8加入到干燥过的二氯甲烷中,依次加入化合物9和缩合剂,室温搅拌,TLC检测,反应结束,减压蒸干,经柱层分离得化合物10。
步骤7:将化合物10溶于有机溶剂中,滴加盐酸,脱去Boc基团,萃取剂萃取,无水硫酸钠干燥,过滤,浓缩,减压蒸干,得化合物11。
步骤8:化合物6溶于有机溶剂,加入化合物11,加热至回流反应,TLC检测反应结束,降温析晶,有机溶剂淋洗滤饼,干燥,得化合物I。
7.根据权利要求6所述的吡唑-4,6-二酮类化合物的制备方法,其特征在于,步骤3所述的碱为氢化钠,氢氧化钾,氢氧化钠,氢氧化锂中的一种或两种。
8.根据权利要求6所述的吡唑-4,6-二酮类化合物的制备方法,其特征在于,步骤4所述的缩合剂为二环己基碳二亚胺、1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐、2-氯-1,3-二甲基氯化咪唑啉中的一种或两种。
9.根据权利要求6所述的吡唑-4,6-二酮类化合物的制备方法,其特征在于,步骤6所述的缩合剂为二环己基碳二亚胺、1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐、2-氯-1,3-二甲基氯化咪唑啉、1-羰基苯并三唑一种或两种。
10.根据权利要求6所述的吡唑-4,6-二酮类化合物的制备方法,其特征在于,步骤7所述的有机溶剂为乙酸乙酯,二氯甲烷,甲醇,乙醇一种。
11.根据权利要求6所述的吡唑-4,6-二酮类化合物的制备方法,其特征在于,步骤8所述的有机溶剂为甲苯、苯、二甲苯中的一种。
12.一种药物组合物,其特征在于,所述的药物组合物包括:治疗有效量的如本发明所述的式(I)化合物,或其药学上可接受的盐、互变异构体、光学异构体、药学上可接受的溶剂合物中的一种或多种,以及任选的药学上可接受的载体、赋形剂、佐剂、辅料或稀释剂。
13.一种如式(I)所示吡唑-4,6-二酮类化合物的用途,其特征在于,可用于治疗与酪氨酸激酶表达异常或酪氨酸激酶活性较高相关的疾病。,其在治疗或预防其中BTK起作用的疾病中的用途。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010743104.7A CN114057724A (zh) | 2020-07-29 | 2020-07-29 | 一种btk抑制剂 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010743104.7A CN114057724A (zh) | 2020-07-29 | 2020-07-29 | 一种btk抑制剂 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN114057724A true CN114057724A (zh) | 2022-02-18 |
Family
ID=80226730
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010743104.7A Pending CN114057724A (zh) | 2020-07-29 | 2020-07-29 | 一种btk抑制剂 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN114057724A (zh) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080076921A1 (en) * | 2006-09-22 | 2008-03-27 | Pharmacyclics, Inc. | Inhibitors of bruton's tyrosine kinase |
| CN108779078A (zh) * | 2015-10-12 | 2018-11-09 | 北京大学深圳研究生院 | 激酶的新型抑制剂和探针及其用途 |
-
2020
- 2020-07-29 CN CN202010743104.7A patent/CN114057724A/zh active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080076921A1 (en) * | 2006-09-22 | 2008-03-27 | Pharmacyclics, Inc. | Inhibitors of bruton's tyrosine kinase |
| CN108779078A (zh) * | 2015-10-12 | 2018-11-09 | 北京大学深圳研究生院 | 激酶的新型抑制剂和探针及其用途 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2024050645A (ja) | ヘテロアリールで置換されたピラゾール化合物及びその医薬用途 | |
| TWI822666B (zh) | Janus激酶抑制劑之結晶型 | |
| CN113677680A (zh) | Egfr抑制剂及其组合物和应用 | |
| CN106554347B (zh) | Egfr激酶抑制剂及其制备方法和应用 | |
| CN111484435B (zh) | 四氢吡咯烷类化合物或其药学上可接受的盐及其制备方法和应用 | |
| WO2019070167A1 (ru) | Ингибиторы рецептора эпидермального фактора роста | |
| EP4353724A1 (en) | Compound as cdk kinase inhibitor and use thereof | |
| CN114181161B (zh) | (2-((取代氧基)苯基)氨基)嘧啶-4-基)氨基苯甲酰衍生物及其制备方法与应用 | |
| US10280174B2 (en) | Salt of fused pyrimidine compound and crystal thereof | |
| JP2024123117A (ja) | ジヒドロクロメン誘導体 | |
| WO2021129841A1 (zh) | 用作ret激酶抑制剂的化合物及其应用 | |
| CN114057724A (zh) | 一种btk抑制剂 | |
| WO2019228330A1 (zh) | 取代的苯并[d]咪唑类化合物及其药物组合物 | |
| CN111233774B (zh) | 一种胺基嘧啶类化合物 | |
| CN112125914A (zh) | 5-取代的小檗胺衍生物,其制备方法和应用 | |
| TW202144358A (zh) | 用作ret激酶抑制劑的化合物及其應用 | |
| CN116940581A (zh) | 一类新型蛋白降解剂及其应用 | |
| CN114874189A (zh) | 取代的杂芳基衍生物及其组合物及用途 | |
| CN106279136B (zh) | 治疗中枢神经系统退行性疾病或脑肿瘤的化合物及其应用 | |
| EP4393930A1 (en) | Crystal forms, preparation method and application of aryl phosphine oxide compound | |
| US20240376138A1 (en) | Crystal Forms, Preparation Method And Application Of Aryl Phosphine Oxide Compound | |
| JP7287929B2 (ja) | ジヒドロクロメン誘導体を含有する医薬 | |
| CN115304502A (zh) | Foxm1抑制剂及其制备方法和应用 | |
| CN118619950A (zh) | 一类三环类化合物及其制备和应用 | |
| CN113968861A (zh) | 具有PI3Kδ/BTK双靶点活性的化合物及其制备方法和应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |