CN113230240B - 1,3-二苯基丙-2-烯-1-酮衍生物及其应用 - Google Patents
1,3-二苯基丙-2-烯-1-酮衍生物及其应用 Download PDFInfo
- Publication number
- CN113230240B CN113230240B CN202110317148.8A CN202110317148A CN113230240B CN 113230240 B CN113230240 B CN 113230240B CN 202110317148 A CN202110317148 A CN 202110317148A CN 113230240 B CN113230240 B CN 113230240B
- Authority
- CN
- China
- Prior art keywords
- compound
- dmso
- group
- preparation
- yield
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- DQFBYFPFKXHELB-VAWYXSNFSA-N trans-chalcone Chemical class C=1C=CC=CC=1C(=O)\C=C\C1=CC=CC=C1 DQFBYFPFKXHELB-VAWYXSNFSA-N 0.000 title claims abstract description 20
- 238000002360 preparation method Methods 0.000 claims abstract description 49
- 108091008099 NLRP3 inflammasome Proteins 0.000 claims abstract description 30
- 150000001875 compounds Chemical class 0.000 claims abstract description 24
- 201000010099 disease Diseases 0.000 claims abstract description 14
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 14
- 206010034674 peritonitis Diseases 0.000 claims abstract description 13
- 206010009887 colitis Diseases 0.000 claims abstract description 8
- 239000004480 active ingredient Substances 0.000 claims abstract description 7
- 150000003839 salts Chemical class 0.000 claims abstract description 6
- 108010001946 Pyrin Domain-Containing 3 Protein NLR Family Proteins 0.000 claims description 4
- 102000000874 Pyrin Domain-Containing 3 Protein NLR Family Human genes 0.000 claims description 4
- 239000003112 inhibitor Substances 0.000 claims description 3
- 206010061218 Inflammation Diseases 0.000 claims description 2
- 239000003814 drug Substances 0.000 claims description 2
- 230000004054 inflammatory process Effects 0.000 claims description 2
- 230000002265 prevention Effects 0.000 claims 1
- 230000004913 activation Effects 0.000 abstract description 12
- 229940126585 therapeutic drug Drugs 0.000 abstract description 2
- 229940127107 NLRP3 inflammasome inhibitor Drugs 0.000 abstract 1
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 48
- 239000007787 solid Substances 0.000 description 46
- 239000000243 solution Substances 0.000 description 32
- 238000000034 method Methods 0.000 description 31
- -1 C 1 -C 6 Alkylsulfinyl radical Chemical class 0.000 description 24
- HUWSZNZAROKDRZ-RRLWZMAJSA-N (3r,4r)-3-azaniumyl-5-[[(2s,3r)-1-[(2s)-2,3-dicarboxypyrrolidin-1-yl]-3-methyl-1-oxopentan-2-yl]amino]-5-oxo-4-sulfanylpentane-1-sulfonate Chemical compound OS(=O)(=O)CC[C@@H](N)[C@@H](S)C(=O)N[C@@H]([C@H](C)CC)C(=O)N1CCC(C(O)=O)[C@H]1C(O)=O HUWSZNZAROKDRZ-RRLWZMAJSA-N 0.000 description 22
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 21
- 229910052739 hydrogen Inorganic materials 0.000 description 20
- 239000001257 hydrogen Substances 0.000 description 20
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 18
- 210000004027 cell Anatomy 0.000 description 18
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 18
- 239000000741 silica gel Substances 0.000 description 18
- 229910002027 silica gel Inorganic materials 0.000 description 18
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 17
- 239000012044 organic layer Substances 0.000 description 16
- 238000003756 stirring Methods 0.000 description 16
- 239000002158 endotoxin Substances 0.000 description 14
- 229920006008 lipopolysaccharide Polymers 0.000 description 14
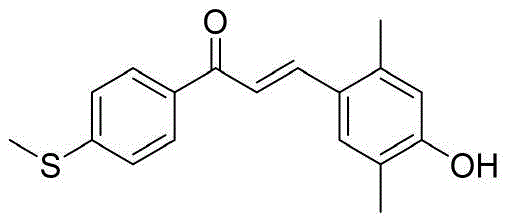
- PHRSDLZWVTXEOY-RUDMXATFSA-N (e)-3-(4-hydroxy-3,5-dimethylphenyl)-1-(4-methylsulfanylphenyl)prop-2-en-1-one Chemical compound C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC(C)=C(O)C(C)=C1 PHRSDLZWVTXEOY-RUDMXATFSA-N 0.000 description 13
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 12
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- 238000006243 chemical reaction Methods 0.000 description 12
- 102000000589 Interleukin-1 Human genes 0.000 description 11
- 108010002352 Interleukin-1 Proteins 0.000 description 11
- 102000003777 Interleukin-1 beta Human genes 0.000 description 11
- 108090000193 Interleukin-1 beta Proteins 0.000 description 11
- 229910052736 halogen Inorganic materials 0.000 description 10
- 150000002367 halogens Chemical class 0.000 description 10
- 241000699670 Mus sp. Species 0.000 description 9
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 9
- 125000000217 alkyl group Chemical group 0.000 description 9
- 150000007942 carboxylates Chemical class 0.000 description 9
- 229920003045 dextran sodium sulfate Polymers 0.000 description 9
- 229910000027 potassium carbonate Inorganic materials 0.000 description 9
- 239000006228 supernatant Substances 0.000 description 9
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 8
- 230000002401 inhibitory effect Effects 0.000 description 8
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 7
- 108090000426 Caspase-1 Proteins 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 6
- 210000001072 colon Anatomy 0.000 description 6
- 238000003304 gavage Methods 0.000 description 6
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical group NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- 239000000460 chlorine Substances 0.000 description 5
- 230000037213 diet Effects 0.000 description 5
- 235000005911 diet Nutrition 0.000 description 5
- 239000012153 distilled water Substances 0.000 description 5
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 5
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 5
- 125000001424 substituent group Chemical group 0.000 description 5
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 4
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- 125000003545 alkoxy group Chemical group 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 239000011737 fluorine Substances 0.000 description 4
- 229910052731 fluorine Inorganic materials 0.000 description 4
- 125000000623 heterocyclic group Chemical group 0.000 description 4
- 239000007928 intraperitoneal injection Substances 0.000 description 4
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 4
- NFHFRUOZVGFOOS-UHFFFAOYSA-N palladium;triphenylphosphane Chemical compound [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 4
- 125000000565 sulfonamide group Chemical group 0.000 description 4
- 125000006559 (C1-C3) alkylamino group Chemical class 0.000 description 3
- 125000004890 (C1-C6) alkylamino group Chemical group 0.000 description 3
- 125000006700 (C1-C6) alkylthio group Chemical group 0.000 description 3
- GPRYKVSEZCQIHD-UHFFFAOYSA-N 1-(4-aminophenyl)ethanone Chemical compound CC(=O)C1=CC=C(N)C=C1 GPRYKVSEZCQIHD-UHFFFAOYSA-N 0.000 description 3
- JECUZQLBQKNEMW-UHFFFAOYSA-N 1-(4-methylsulfanylphenyl)ethanone Chemical compound CSC1=CC=C(C(C)=O)C=C1 JECUZQLBQKNEMW-UHFFFAOYSA-N 0.000 description 3
- 125000003903 2-propenyl group Chemical class [H]C([*])([H])C([H])=C([H])[H] 0.000 description 3
- UGNJKENOQWQDTN-VGOFMYFVSA-N COC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)NC(=O)NC1=CC=C(C=C1)C)C Chemical compound COC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)NC(=O)NC1=CC=C(C=C1)C)C UGNJKENOQWQDTN-VGOFMYFVSA-N 0.000 description 3
- YXDKOMLVUSTTHB-IZZDOVSWSA-N COC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)NC(=O)NCCC)C Chemical compound COC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)NC(=O)NCCC)C YXDKOMLVUSTTHB-IZZDOVSWSA-N 0.000 description 3
- 102100035904 Caspase-1 Human genes 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- 238000008157 ELISA kit Methods 0.000 description 3
- TVULOZPIZRTGFX-YIXHJXPBSA-N FC1=CC=C(C=C1)NC(=O)NC1=CC=C(C=C1)C(\C=C\C1=CC(=C(C(=C1)C)OC)C)=O Chemical compound FC1=CC=C(C=C1)NC(=O)NC1=CC=C(C=C1)C(\C=C\C1=CC(=C(C(=C1)C)OC)C)=O TVULOZPIZRTGFX-YIXHJXPBSA-N 0.000 description 3
- 101000979572 Homo sapiens NLR family CARD domain-containing protein 4 Proteins 0.000 description 3
- 102100023435 NLR family CARD domain-containing protein 4 Human genes 0.000 description 3
- SYCIWORSGGMZAP-KRXBUXKQSA-N O1CCC2=C1C=CC(=C2)/C=C/C(=O)C1=CC=C(C=C1)SC Chemical compound O1CCC2=C1C=CC(=C2)/C=C/C(=O)C1=CC=C(C=C1)SC SYCIWORSGGMZAP-KRXBUXKQSA-N 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- KHBQMWCZKVMBLN-IDEBNGHGSA-N benzenesulfonamide Chemical group NS(=O)(=O)[13C]1=[13CH][13CH]=[13CH][13CH]=[13CH]1 KHBQMWCZKVMBLN-IDEBNGHGSA-N 0.000 description 3
- 210000004979 bone marrow derived macrophage Anatomy 0.000 description 3
- 150000001721 carbon Chemical group 0.000 description 3
- 125000004432 carbon atom Chemical group C* 0.000 description 3
- 238000001514 detection method Methods 0.000 description 3
- DANUORFCFTYTSZ-UHFFFAOYSA-N epinigericin Natural products O1C2(C(CC(C)(O2)C2OC(C)(CC2)C2C(CC(O2)C2C(CC(C)C(O)(CO)O2)C)C)C)C(C)C(OC)CC1CC1CCC(C)C(C(C)C(O)=O)O1 DANUORFCFTYTSZ-UHFFFAOYSA-N 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- DANUORFCFTYTSZ-BIBFWWMMSA-N nigericin Chemical compound C([C@@H]1C[C@H]([C@H]([C@]2([C@@H](C[C@](C)(O2)C2O[C@@](C)(CC2)C2[C@H](CC(O2)[C@@H]2[C@H](C[C@@H](C)[C@](O)(CO)O2)C)C)C)O1)C)OC)[C@H]1CC[C@H](C)C([C@@H](C)C(O)=O)O1 DANUORFCFTYTSZ-BIBFWWMMSA-N 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 125000003367 polycyclic group Chemical group 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 125000006413 ring segment Chemical group 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 125000000335 thiazolyl group Chemical group 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- GLGNXYJARSMNGJ-VKTIVEEGSA-N (1s,2s,3r,4r)-3-[[5-chloro-2-[(1-ethyl-6-methoxy-2-oxo-4,5-dihydro-3h-1-benzazepin-7-yl)amino]pyrimidin-4-yl]amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide Chemical compound CCN1C(=O)CCCC2=C(OC)C(NC=3N=C(C(=CN=3)Cl)N[C@H]3[C@H]([C@@]4([H])C[C@@]3(C=C4)[H])C(N)=O)=CC=C21 GLGNXYJARSMNGJ-VKTIVEEGSA-N 0.000 description 2
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 2
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 2
- WYECURVXVYPVAT-UHFFFAOYSA-N 1-(4-bromophenyl)ethanone Chemical compound CC(=O)C1=CC=C(Br)C=C1 WYECURVXVYPVAT-UHFFFAOYSA-N 0.000 description 2
- UNILWMWFPHPYOR-KXEYIPSPSA-M 1-[6-[2-[3-[3-[3-[2-[2-[3-[[2-[2-[[(2r)-1-[[2-[[(2r)-1-[3-[2-[2-[3-[[2-(2-amino-2-oxoethoxy)acetyl]amino]propoxy]ethoxy]ethoxy]propylamino]-3-hydroxy-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-[(2r)-2,3-di(hexadecanoyloxy)propyl]sulfanyl-1-oxopropan-2-yl Chemical compound O=C1C(SCCC(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](CSC[C@@H](COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)C(=O)NCC(=O)N[C@H](CO)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(N)=O)CC(=O)N1CCNC(=O)CCCCCN\1C2=CC=C(S([O-])(=O)=O)C=C2CC/1=C/C=C/C=C/C1=[N+](CC)C2=CC=C(S([O-])(=O)=O)C=C2C1 UNILWMWFPHPYOR-KXEYIPSPSA-M 0.000 description 2
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 2
- GOUHYARYYWKXHS-UHFFFAOYSA-N 4-formylbenzoic acid Chemical compound OC(=O)C1=CC=C(C=O)C=C1 GOUHYARYYWKXHS-UHFFFAOYSA-N 0.000 description 2
- UYGBSRJODQHNLQ-UHFFFAOYSA-N 4-hydroxy-3,5-dimethylbenzaldehyde Chemical compound CC1=CC(C=O)=CC(C)=C1O UYGBSRJODQHNLQ-UHFFFAOYSA-N 0.000 description 2
- 125000002373 5 membered heterocyclic group Chemical group 0.000 description 2
- 108091008098 AIM2 inflammasome Proteins 0.000 description 2
- 102100029647 Apoptosis-associated speck-like protein containing a CARD Human genes 0.000 description 2
- 238000011740 C57BL/6 mouse Methods 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- 108010034143 Inflammasomes Proteins 0.000 description 2
- 102000003810 Interleukin-18 Human genes 0.000 description 2
- 108090000171 Interleukin-18 Proteins 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 108010087999 Steryl-Sulfatase Proteins 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 125000002619 bicyclic group Chemical group 0.000 description 2
- 238000004440 column chromatography Methods 0.000 description 2
- 229940125773 compound 10 Drugs 0.000 description 2
- 229940125797 compound 12 Drugs 0.000 description 2
- 229940126543 compound 14 Drugs 0.000 description 2
- 229940125758 compound 15 Drugs 0.000 description 2
- 229940125782 compound 2 Drugs 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000002550 fecal effect Effects 0.000 description 2
- 239000012065 filter cake Substances 0.000 description 2
- 125000001153 fluoro group Chemical group F* 0.000 description 2
- 125000001207 fluorophenyl group Chemical group 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 2
- 125000002816 methylsulfanyl group Chemical group [H]C([H])([H])S[*] 0.000 description 2
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 2
- 239000003330 peritoneal dialysis fluid Substances 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- ASGMFNBUXDJWJJ-JLCFBVMHSA-N (1R,3R)-3-[[3-bromo-1-[4-(5-methyl-1,3,4-thiadiazol-2-yl)phenyl]pyrazolo[3,4-d]pyrimidin-6-yl]amino]-N,1-dimethylcyclopentane-1-carboxamide Chemical compound BrC1=NN(C2=NC(=NC=C21)N[C@H]1C[C@@](CC1)(C(=O)NC)C)C1=CC=C(C=C1)C=1SC(=NN=1)C ASGMFNBUXDJWJJ-JLCFBVMHSA-N 0.000 description 1
- UAOUIVVJBYDFKD-XKCDOFEDSA-N (1R,9R,10S,11R,12R,15S,18S,21R)-10,11,21-trihydroxy-8,8-dimethyl-14-methylidene-4-(prop-2-enylamino)-20-oxa-5-thia-3-azahexacyclo[9.7.2.112,15.01,9.02,6.012,18]henicosa-2(6),3-dien-13-one Chemical compound C([C@@H]1[C@@H](O)[C@@]23C(C1=C)=O)C[C@H]2[C@]12C(N=C(NCC=C)S4)=C4CC(C)(C)[C@H]1[C@H](O)[C@]3(O)OC2 UAOUIVVJBYDFKD-XKCDOFEDSA-N 0.000 description 1
- AOSZTAHDEDLTLQ-AZKQZHLXSA-N (1S,2S,4R,8S,9S,11S,12R,13S,19S)-6-[(3-chlorophenyl)methyl]-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-9,13-dimethyl-6-azapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one Chemical compound C([C@@H]1C[C@H]2[C@H]3[C@]([C@]4(C=CC(=O)C=C4[C@@H](F)C3)C)(F)[C@@H](O)C[C@@]2([C@@]1(C1)C(=O)CO)C)N1CC1=CC=CC(Cl)=C1 AOSZTAHDEDLTLQ-AZKQZHLXSA-N 0.000 description 1
- ABJSOROVZZKJGI-OCYUSGCXSA-N (1r,2r,4r)-2-(4-bromophenyl)-n-[(4-chlorophenyl)-(2-fluoropyridin-4-yl)methyl]-4-morpholin-4-ylcyclohexane-1-carboxamide Chemical compound C1=NC(F)=CC(C(NC(=O)[C@H]2[C@@H](C[C@@H](CC2)N2CCOCC2)C=2C=CC(Br)=CC=2)C=2C=CC(Cl)=CC=2)=C1 ABJSOROVZZKJGI-OCYUSGCXSA-N 0.000 description 1
- IUSARDYWEPUTPN-OZBXUNDUSA-N (2r)-n-[(2s,3r)-4-[[(4s)-6-(2,2-dimethylpropyl)spiro[3,4-dihydropyrano[2,3-b]pyridine-2,1'-cyclobutane]-4-yl]amino]-3-hydroxy-1-[3-(1,3-thiazol-2-yl)phenyl]butan-2-yl]-2-methoxypropanamide Chemical compound C([C@H](NC(=O)[C@@H](C)OC)[C@H](O)CN[C@@H]1C2=CC(CC(C)(C)C)=CN=C2OC2(CCC2)C1)C(C=1)=CC=CC=1C1=NC=CS1 IUSARDYWEPUTPN-OZBXUNDUSA-N 0.000 description 1
- WWTBZEKOSBFBEM-SPWPXUSOSA-N (2s)-2-[[2-benzyl-3-[hydroxy-[(1r)-2-phenyl-1-(phenylmethoxycarbonylamino)ethyl]phosphoryl]propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)O)C(=O)C(CP(O)(=O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1C=CC=CC=1)CC1=CC=CC=C1 WWTBZEKOSBFBEM-SPWPXUSOSA-N 0.000 description 1
- STBLNCCBQMHSRC-BATDWUPUSA-N (2s)-n-[(3s,4s)-5-acetyl-7-cyano-4-methyl-1-[(2-methylnaphthalen-1-yl)methyl]-2-oxo-3,4-dihydro-1,5-benzodiazepin-3-yl]-2-(methylamino)propanamide Chemical compound O=C1[C@@H](NC(=O)[C@H](C)NC)[C@H](C)N(C(C)=O)C2=CC(C#N)=CC=C2N1CC1=C(C)C=CC2=CC=CC=C12 STBLNCCBQMHSRC-BATDWUPUSA-N 0.000 description 1
- LOGFVTREOLYCPF-KXNHARMFSA-N (2s,3r)-2-[[(2r)-1-[(2s)-2,6-diaminohexanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxybutanoic acid Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-KXNHARMFSA-N 0.000 description 1
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 1
- IWZSHWBGHQBIML-ZGGLMWTQSA-N (3S,8S,10R,13S,14S,17S)-17-isoquinolin-7-yl-N,N,10,13-tetramethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-amine Chemical compound CN(C)[C@H]1CC[C@]2(C)C3CC[C@@]4(C)[C@@H](CC[C@@H]4c4ccc5ccncc5c4)[C@@H]3CC=C2C1 IWZSHWBGHQBIML-ZGGLMWTQSA-N 0.000 description 1
- NRIYPIBRPGAWDD-UHFFFAOYSA-N (5-methylthiophen-2-yl)boronic acid Chemical compound CC1=CC=C(B(O)O)S1 NRIYPIBRPGAWDD-UHFFFAOYSA-N 0.000 description 1
- 125000004739 (C1-C6) alkylsulfonyl group Chemical group 0.000 description 1
- 125000006569 (C5-C6) heterocyclic group Chemical group 0.000 description 1
- IEOIMIMGAKYMNQ-KRXBUXKQSA-N (E)-3-(3-chloro-4-hydroxyphenyl)-1-(4-methylsulfanylphenyl)prop-2-en-1-one Chemical compound C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC=C(O)C(Cl)=C1 IEOIMIMGAKYMNQ-KRXBUXKQSA-N 0.000 description 1
- OIPGYOCQGCKRQC-RUDMXATFSA-N (E)-3-(4-hydroxy-3,5-dimethoxyphenyl)-1-(4-methylsulfanylphenyl)prop-2-en-1-one Chemical compound COC1=C(O)C(OC)=CC(\C=C\C(=O)C=2C=CC(SC)=CC=2)=C1 OIPGYOCQGCKRQC-RUDMXATFSA-N 0.000 description 1
- WYANTWYCXMAWEF-BJMVGYQFSA-N (E)-3-(4-hydroxy-3,5-dimethylphenyl)-1-(4-thiophen-2-ylphenyl)prop-2-en-1-one Chemical compound OC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)C=1SC=CC=1)C WYANTWYCXMAWEF-BJMVGYQFSA-N 0.000 description 1
- HXESECVVROEVID-BJMVGYQFSA-N (E)-3-(4-hydroxy-3,5-dimethylphenyl)-1-[4-(5-methylthiophen-2-yl)phenyl]prop-2-en-1-one Chemical compound OC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)C=1SC(=CC=1)C)C HXESECVVROEVID-BJMVGYQFSA-N 0.000 description 1
- WZEUJSSCFIUAIH-YCRREMRBSA-N (E)-3-(4-hydroxy-3-methoxyphenyl)-1-(4-methylsulfanylphenyl)prop-2-en-1-one Chemical compound OC1=C(C=C(C=C1)/C=C/C(=O)C1=CC=C(C=C1)SC)OC WZEUJSSCFIUAIH-YCRREMRBSA-N 0.000 description 1
- KQAWGRKSGAMKJO-BJMVGYQFSA-N (E)-3-[3,5-dimethyl-4-(3-morpholin-4-ylpropoxy)phenyl]-1-(4-methylsulfanylphenyl)prop-2-en-1-one Chemical compound CC=1C=C(C=C(C=1OCCCN1CCOCC1)C)/C=C/C(=O)C1=CC=C(C=C1)SC KQAWGRKSGAMKJO-BJMVGYQFSA-N 0.000 description 1
- IDNZQWAOIWOIKU-FPYGCLRLSA-N (e)-1-(4-chlorophenyl)-3-(4-hydroxy-3,5-dimethylphenyl)prop-2-en-1-one Chemical compound CC1=C(O)C(C)=CC(\C=C\C(=O)C=2C=CC(Cl)=CC=2)=C1 IDNZQWAOIWOIKU-FPYGCLRLSA-N 0.000 description 1
- RGSIGQQHMBKCKZ-FPYGCLRLSA-N (e)-1-(4-fluorophenyl)-3-(4-hydroxy-3,5-dimethylphenyl)prop-2-en-1-one Chemical compound CC1=C(O)C(C)=CC(\C=C\C(=O)C=2C=CC(F)=CC=2)=C1 RGSIGQQHMBKCKZ-FPYGCLRLSA-N 0.000 description 1
- PGWKBUGWWIWWSE-IZZDOVSWSA-N (e)-3-(3,5-diethyl-4-hydroxyphenyl)-1-(4-methylsulfanylphenyl)prop-2-en-1-one Chemical compound CCC1=C(O)C(CC)=CC(\C=C\C(=O)C=2C=CC(SC)=CC=2)=C1 PGWKBUGWWIWWSE-IZZDOVSWSA-N 0.000 description 1
- JPNPMEFSDBMGGP-KRXBUXKQSA-N (e)-3-(3-fluoro-4-hydroxyphenyl)-1-(4-methylsulfanylphenyl)prop-2-en-1-one Chemical compound C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC=C(O)C(F)=C1 JPNPMEFSDBMGGP-KRXBUXKQSA-N 0.000 description 1
- PVJWWTLLOMENRE-FPYGCLRLSA-N (e)-3-(4-hydroxy-3,5-dimethylphenyl)-1-(4-iodophenyl)prop-2-en-1-one Chemical compound CC1=C(O)C(C)=CC(\C=C\C(=O)C=2C=CC(I)=CC=2)=C1 PVJWWTLLOMENRE-FPYGCLRLSA-N 0.000 description 1
- INTNLMHRANSODS-RUDMXATFSA-N (e)-3-(4-hydroxy-3,5-dimethylphenyl)-1-(4-methylsulfonylphenyl)prop-2-en-1-one Chemical compound CC1=C(O)C(C)=CC(\C=C\C(=O)C=2C=CC(=CC=2)S(C)(=O)=O)=C1 INTNLMHRANSODS-RUDMXATFSA-N 0.000 description 1
- VZCHCIWHJLPPNF-UHFFFAOYSA-N 1-(4-thiophen-2-ylphenyl)ethanone Chemical compound C1=CC(C(=O)C)=CC=C1C1=CC=CS1 VZCHCIWHJLPPNF-UHFFFAOYSA-N 0.000 description 1
- KQZLRWGGWXJPOS-NLFPWZOASA-N 1-[(1R)-1-(2,4-dichlorophenyl)ethyl]-6-[(4S,5R)-4-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]-5-methylcyclohexen-1-yl]pyrazolo[3,4-b]pyrazine-3-carbonitrile Chemical compound ClC1=C(C=CC(=C1)Cl)[C@@H](C)N1N=C(C=2C1=NC(=CN=2)C1=CC[C@@H]([C@@H](C1)C)N1[C@@H](CCC1)CO)C#N KQZLRWGGWXJPOS-NLFPWZOASA-N 0.000 description 1
- WZZBNLYBHUDSHF-DHLKQENFSA-N 1-[(3s,4s)-4-[8-(2-chloro-4-pyrimidin-2-yloxyphenyl)-7-fluoro-2-methylimidazo[4,5-c]quinolin-1-yl]-3-fluoropiperidin-1-yl]-2-hydroxyethanone Chemical compound CC1=NC2=CN=C3C=C(F)C(C=4C(=CC(OC=5N=CC=CN=5)=CC=4)Cl)=CC3=C2N1[C@H]1CCN(C(=O)CO)C[C@@H]1F WZZBNLYBHUDSHF-DHLKQENFSA-N 0.000 description 1
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 1
- FQPFIOXJCQCZNM-UHFFFAOYSA-N 1-[4-(5-methylthiophen-2-yl)phenyl]ethanone Chemical compound C1=CC(C(=O)C)=CC=C1C1=CC=C(C)S1 FQPFIOXJCQCZNM-UHFFFAOYSA-N 0.000 description 1
- OQURWGJAWSLGQG-UHFFFAOYSA-N 1-isocyanatopropane Chemical compound CCCN=C=O OQURWGJAWSLGQG-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- ZVNSDZSAOHBAJU-UHFFFAOYSA-N 2-(sulfamoylamino)propane Chemical compound CC(C)NS(N)(=O)=O ZVNSDZSAOHBAJU-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- HPYMCGXNFIZOGE-RUDMXATFSA-N 2-[2,6-dimethyl-4-[(e)-3-(4-methylsulfanylphenyl)-3-oxoprop-1-enyl]phenoxy]acetic acid Chemical compound C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC(C)=C(OCC(O)=O)C(C)=C1 HPYMCGXNFIZOGE-RUDMXATFSA-N 0.000 description 1
- YSUIQYOGTINQIN-UZFYAQMZSA-N 2-amino-9-[(1S,6R,8R,9S,10R,15R,17R,18R)-8-(6-aminopurin-9-yl)-9,18-difluoro-3,12-dihydroxy-3,12-bis(sulfanylidene)-2,4,7,11,13,16-hexaoxa-3lambda5,12lambda5-diphosphatricyclo[13.2.1.06,10]octadecan-17-yl]-1H-purin-6-one Chemical compound NC1=NC2=C(N=CN2[C@@H]2O[C@@H]3COP(S)(=O)O[C@@H]4[C@@H](COP(S)(=O)O[C@@H]2[C@@H]3F)O[C@H]([C@H]4F)N2C=NC3=C2N=CN=C3N)C(=O)N1 YSUIQYOGTINQIN-UZFYAQMZSA-N 0.000 description 1
- TVTJUIAKQFIXCE-HUKYDQBMSA-N 2-amino-9-[(2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-ynyl-1H-purine-6,8-dione Chemical compound NC=1NC(C=2N(C(N(C=2N=1)[C@@H]1O[C@@H]([C@H]([C@H]1O)F)CO)=O)CC#C)=O TVTJUIAKQFIXCE-HUKYDQBMSA-N 0.000 description 1
- NPRYCHLHHVWLQZ-TURQNECASA-N 2-amino-9-[(2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-ynylpurin-8-one Chemical compound NC1=NC=C2N(C(N(C2=N1)[C@@H]1O[C@@H]([C@H]([C@H]1O)F)CO)=O)CC#C NPRYCHLHHVWLQZ-TURQNECASA-N 0.000 description 1
- LDLCZOVUSADOIV-UHFFFAOYSA-N 2-bromoethanol Chemical compound OCCBr LDLCZOVUSADOIV-UHFFFAOYSA-N 0.000 description 1
- GSLTVFIVJMCNBH-UHFFFAOYSA-N 2-isocyanatopropane Chemical compound CC(C)N=C=O GSLTVFIVJMCNBH-UHFFFAOYSA-N 0.000 description 1
- QBWKPGNFQQJGFY-QLFBSQMISA-N 3-[(1r)-1-[(2r,6s)-2,6-dimethylmorpholin-4-yl]ethyl]-n-[6-methyl-3-(1h-pyrazol-4-yl)imidazo[1,2-a]pyrazin-8-yl]-1,2-thiazol-5-amine Chemical compound N1([C@H](C)C2=NSC(NC=3C4=NC=C(N4C=C(C)N=3)C3=CNN=C3)=C2)C[C@H](C)O[C@H](C)C1 QBWKPGNFQQJGFY-QLFBSQMISA-N 0.000 description 1
- PIAZYBLGBSMNLX-UHFFFAOYSA-N 4-(3-chloropropyl)morpholine Chemical compound ClCCCN1CCOCC1 PIAZYBLGBSMNLX-UHFFFAOYSA-N 0.000 description 1
- RYJBGMLJVKGXIT-NYYWCZLTSA-N 4-[(E)-3-(4-methylsulfanylphenyl)-3-oxoprop-1-enyl]benzoic acid Chemical compound C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC=C(C(O)=O)C=C1 RYJBGMLJVKGXIT-NYYWCZLTSA-N 0.000 description 1
- BIANZVZZKGIQKG-UHFFFAOYSA-N 4-[4-(5-hydroxypentoxy)phenyl]benzonitrile Chemical compound C1=CC(OCCCCCO)=CC=C1C1=CC=C(C#N)C=C1 BIANZVZZKGIQKG-UHFFFAOYSA-N 0.000 description 1
- BFXHJFKKRGVUMU-UHFFFAOYSA-N 4-fluorobenzenesulfonyl chloride Chemical compound FC1=CC=C(S(Cl)(=O)=O)C=C1 BFXHJFKKRGVUMU-UHFFFAOYSA-N 0.000 description 1
- SNIGEINPLSDHBQ-UHFFFAOYSA-N 4-methoxy-3,5-dimethylbenzaldehyde Chemical compound COC1=C(C)C=C(C=O)C=C1C SNIGEINPLSDHBQ-UHFFFAOYSA-N 0.000 description 1
- 108060000255 AIM2 Proteins 0.000 description 1
- HGINCPLSRVDWNT-UHFFFAOYSA-N Acrolein Chemical compound C=CC=O HGINCPLSRVDWNT-UHFFFAOYSA-N 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- XUUJMZJAQBXROZ-IZZDOVSWSA-N C(C)(C)NC(=O)NC1=CC=C(C=C1)C(\C=C\C1=CC(=C(C(=C1)C)OC)C)=O Chemical compound C(C)(C)NC(=O)NC1=CC=C(C=C1)C(\C=C\C1=CC(=C(C(=C1)C)OC)C)=O XUUJMZJAQBXROZ-IZZDOVSWSA-N 0.000 description 1
- APJKNHDUMFSYLM-KRXBUXKQSA-N C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC=C(O)C(O)=C1 Chemical compound C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC=C(O)C(O)=C1 APJKNHDUMFSYLM-KRXBUXKQSA-N 0.000 description 1
- FWLUCRFKYIFJER-NYYWCZLTSA-N C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC=C(O)C=C1 Chemical compound C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC=C(O)C=C1 FWLUCRFKYIFJER-NYYWCZLTSA-N 0.000 description 1
- NUTNWDSYPXIWQM-IZZDOVSWSA-N CC1=C(OC(C(=O)O)CC)C(=CC(=C1)\C=C\C(=O)C1=CC=C(C=C1)SC)C Chemical compound CC1=C(OC(C(=O)O)CC)C(=CC(=C1)\C=C\C(=O)C1=CC=C(C=C1)SC)C NUTNWDSYPXIWQM-IZZDOVSWSA-N 0.000 description 1
- RLLAVYLLEFQNHS-KPKJPENVSA-N CC1=C(OCCCCC(=O)O)C(=CC(=C1)\C=C\C(=O)C1=CC=C(C=C1)SC)C Chemical compound CC1=C(OCCCCC(=O)O)C(=CC(=C1)\C=C\C(=O)C1=CC=C(C=C1)SC)C RLLAVYLLEFQNHS-KPKJPENVSA-N 0.000 description 1
- YYZRCAGJCUZKIA-IZZDOVSWSA-N CN(C1=CC=C(C=C1)C(\C=C\C1=CC(=C(C(=C1)C)OC)C)=O)C Chemical compound CN(C1=CC=C(C=C1)C(\C=C\C1=CC(=C(C(=C1)C)OC)C)=O)C YYZRCAGJCUZKIA-IZZDOVSWSA-N 0.000 description 1
- ZMZLMWUNDPFSNY-BJMVGYQFSA-N COC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)SC)C Chemical compound COC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)SC)C ZMZLMWUNDPFSNY-BJMVGYQFSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 229940126657 Compound 17 Drugs 0.000 description 1
- 229940126639 Compound 33 Drugs 0.000 description 1
- 229940127007 Compound 39 Drugs 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- BSGGYUXXMRKKFV-WEVVVXLNSA-N FC1=C(C=CC(=C1)O)/C=C/C(=O)C1=CC=C(C=C1)SC Chemical compound FC1=C(C=CC(=C1)O)/C=C/C(=O)C1=CC=C(C=C1)SC BSGGYUXXMRKKFV-WEVVVXLNSA-N 0.000 description 1
- 108010040721 Flagellin Proteins 0.000 description 1
- 208000012671 Gastrointestinal haemorrhages Diseases 0.000 description 1
- 201000005569 Gout Diseases 0.000 description 1
- 101710088172 HTH-type transcriptional regulator RipA Proteins 0.000 description 1
- 229940122390 Inflammasome inhibitor Drugs 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 102100024064 Interferon-inducible protein AIM2 Human genes 0.000 description 1
- RDQOIMCGWUUIRX-BJMVGYQFSA-N N-[4-[(E)-3-(4-hydroxy-3,5-dimethylphenyl)prop-2-enoyl]phenyl]prop-2-enamide Chemical compound CC1=CC(/C=C/C(C(C=C2)=CC=C2NC(C=C)=O)=O)=CC(C)=C1O RDQOIMCGWUUIRX-BJMVGYQFSA-N 0.000 description 1
- 150000001204 N-oxides Chemical class 0.000 description 1
- OPFJDXRVMFKJJO-ZHHKINOHSA-N N-{[3-(2-benzamido-4-methyl-1,3-thiazol-5-yl)-pyrazol-5-yl]carbonyl}-G-dR-G-dD-dD-dD-NH2 Chemical compound S1C(C=2NN=C(C=2)C(=O)NCC(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(N)=O)=C(C)N=C1NC(=O)C1=CC=CC=C1 OPFJDXRVMFKJJO-ZHHKINOHSA-N 0.000 description 1
- UKAWSRZDFWKBOI-RUDMXATFSA-N NC1=CC=C(C=C1)C(\C=C\C1=CC(=C(C(=C1)C)OC)C)=O Chemical compound NC1=CC=C(C=C1)C(\C=C\C1=CC(=C(C(=C1)C)OC)C)=O UKAWSRZDFWKBOI-RUDMXATFSA-N 0.000 description 1
- 102000012064 NLR Proteins Human genes 0.000 description 1
- 108091005686 NOD-like receptors Proteins 0.000 description 1
- GGIXQMMATOKHLO-ONNFQVAWSA-N OC1=C(C=C(C=C1)/C=C/C(=O)C1=CC=C(C=C1)SC)C Chemical compound OC1=C(C=C(C=C1)/C=C/C(=O)C1=CC=C(C=C1)SC)C GGIXQMMATOKHLO-ONNFQVAWSA-N 0.000 description 1
- RMLGVKYCWTYOQM-RUDMXATFSA-N OC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)S(=O)C)C Chemical compound OC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)S(=O)C)C RMLGVKYCWTYOQM-RUDMXATFSA-N 0.000 description 1
- DETNTGFGHWCWQT-CMDGGOBGSA-N OC1=CC(=C(C(=C1)C)/C=C/C(=O)C1=CC=C(C=C1)SC)C Chemical compound OC1=CC(=C(C(=C1)C)/C=C/C(=O)C1=CC=C(C=C1)SC)C DETNTGFGHWCWQT-CMDGGOBGSA-N 0.000 description 1
- JMFIRFSBSIPPJK-RMKNXTFCSA-N OC1=CC(=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)SC)C Chemical compound OC1=CC(=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)SC)C JMFIRFSBSIPPJK-RMKNXTFCSA-N 0.000 description 1
- XMLTZANZJAQSEC-RUDMXATFSA-N OCCOC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)SC)C Chemical compound OCCOC1=C(C=C(C=C1C)/C=C/C(=O)C1=CC=C(C=C1)SC)C XMLTZANZJAQSEC-RUDMXATFSA-N 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- PNUZDKCDAWUEGK-CYZMBNFOSA-N Sitafloxacin Chemical compound C([C@H]1N)N(C=2C(=C3C(C(C(C(O)=O)=CN3[C@H]3[C@H](C3)F)=O)=CC=2F)Cl)CC11CC1 PNUZDKCDAWUEGK-CYZMBNFOSA-N 0.000 description 1
- LJOOWESTVASNOG-UFJKPHDISA-N [(1s,3r,4ar,7s,8s,8as)-3-hydroxy-8-[2-[(4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-yl] (2s)-2-methylbutanoate Chemical compound C([C@H]1[C@@H](C)C=C[C@H]2C[C@@H](O)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)CC1C[C@@H](O)CC(=O)O1 LJOOWESTVASNOG-UFJKPHDISA-N 0.000 description 1
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 1
- SMNRFWMNPDABKZ-WVALLCKVSA-N [[(2R,3S,4R,5S)-5-(2,6-dioxo-3H-pyridin-3-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [[[(2R,3S,4S,5R,6R)-4-fluoro-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl] hydrogen phosphate Chemical compound OC[C@H]1O[C@H](OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)C2C=CC(=O)NC2=O)[C@H](O)[C@@H](F)[C@@H]1O SMNRFWMNPDABKZ-WVALLCKVSA-N 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- HFBMWMNUJJDEQZ-UHFFFAOYSA-N acryloyl chloride Chemical compound ClC(=O)C=C HFBMWMNUJJDEQZ-UHFFFAOYSA-N 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- 125000004397 aminosulfonyl group Chemical group NS(=O)(=O)* 0.000 description 1
- 210000003567 ascitic fluid Anatomy 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- XRWSZZJLZRKHHD-WVWIJVSJSA-N asunaprevir Chemical compound O=C([C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)OC1=NC=C(C2=CC=C(Cl)C=C21)OC)N[C@]1(C(=O)NS(=O)(=O)C2CC2)C[C@H]1C=C XRWSZZJLZRKHHD-WVWIJVSJSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 150000008331 benzenesulfonamides Chemical class 0.000 description 1
- KGNDCEVUMONOKF-UGPLYTSKSA-N benzyl n-[(2r)-1-[(2s,4r)-2-[[(2s)-6-amino-1-(1,3-benzoxazol-2-yl)-1,1-dihydroxyhexan-2-yl]carbamoyl]-4-[(4-methylphenyl)methoxy]pyrrolidin-1-yl]-1-oxo-4-phenylbutan-2-yl]carbamate Chemical compound C1=CC(C)=CC=C1CO[C@H]1CN(C(=O)[C@@H](CCC=2C=CC=CC=2)NC(=O)OCC=2C=CC=CC=2)[C@H](C(=O)N[C@@H](CCCCN)C(O)(O)C=2OC3=CC=CC=C3N=2)C1 KGNDCEVUMONOKF-UGPLYTSKSA-N 0.000 description 1
- 125000000319 biphenyl-4-yl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1C1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- XTEOJPUYZWEXFI-UHFFFAOYSA-N butyl n-[3-[4-(imidazol-1-ylmethyl)phenyl]-5-(2-methylpropyl)thiophen-2-yl]sulfonylcarbamate Chemical compound S1C(CC(C)C)=CC(C=2C=CC(CN3C=NC=C3)=CC=2)=C1S(=O)(=O)NC(=O)OCCCC XTEOJPUYZWEXFI-UHFFFAOYSA-N 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 229940126142 compound 16 Drugs 0.000 description 1
- 229940125810 compound 20 Drugs 0.000 description 1
- 229940126086 compound 21 Drugs 0.000 description 1
- 229940126208 compound 22 Drugs 0.000 description 1
- 229940125833 compound 23 Drugs 0.000 description 1
- 229940125961 compound 24 Drugs 0.000 description 1
- 229940125846 compound 25 Drugs 0.000 description 1
- 229940125851 compound 27 Drugs 0.000 description 1
- 229940127204 compound 29 Drugs 0.000 description 1
- 229940126214 compound 3 Drugs 0.000 description 1
- 229940125877 compound 31 Drugs 0.000 description 1
- 229940125878 compound 36 Drugs 0.000 description 1
- 229940125807 compound 37 Drugs 0.000 description 1
- 229940127573 compound 38 Drugs 0.000 description 1
- 229940125898 compound 5 Drugs 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 125000005047 dihydroimidazolyl group Chemical group N1(CNC=C1)* 0.000 description 1
- 125000005048 dihydroisoxazolyl group Chemical group O1N(CC=C1)* 0.000 description 1
- 125000005049 dihydrooxadiazolyl group Chemical group O1N(NC=C1)* 0.000 description 1
- 125000005050 dihydrooxazolyl group Chemical group O1C(NC=C1)* 0.000 description 1
- 125000005051 dihydropyrazinyl group Chemical group N1(CC=NC=C1)* 0.000 description 1
- 125000005052 dihydropyrazolyl group Chemical group N1(NCC=C1)* 0.000 description 1
- 125000004655 dihydropyridinyl group Chemical group N1(CC=CC=C1)* 0.000 description 1
- 125000005053 dihydropyrimidinyl group Chemical group N1(CN=CC=C1)* 0.000 description 1
- 125000005054 dihydropyrrolyl group Chemical group [H]C1=C([H])C([H])([H])C([H])([H])N1* 0.000 description 1
- 125000005056 dihydrothiazolyl group Chemical group S1C(NC=C1)* 0.000 description 1
- 125000005057 dihydrothienyl group Chemical group S1C(CC=C1)* 0.000 description 1
- 125000005058 dihydrotriazolyl group Chemical group N1(NNC=C1)* 0.000 description 1
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- AFRWBGJRWRHQOV-UHFFFAOYSA-N ethyl 5-bromopentanoate Chemical compound CCOC(=O)CCCCBr AFRWBGJRWRHQOV-UHFFFAOYSA-N 0.000 description 1
- PQJJJMRNHATNKG-UHFFFAOYSA-N ethyl bromoacetate Chemical compound CCOC(=O)CBr PQJJJMRNHATNKG-UHFFFAOYSA-N 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- AFLFKFHDSCQHOL-IZZDOVSWSA-N gft505 Chemical compound C1=CC(SC)=CC=C1C(=O)\C=C\C1=CC(C)=C(OC(C)(C)C(O)=O)C(C)=C1 AFLFKFHDSCQHOL-IZZDOVSWSA-N 0.000 description 1
- JAXFJECJQZDFJS-XHEPKHHKSA-N gtpl8555 Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@H](B1O[C@@]2(C)[C@H]3C[C@H](C3(C)C)C[C@H]2O1)CCC1=CC=C(F)C=C1 JAXFJECJQZDFJS-XHEPKHHKSA-N 0.000 description 1
- 208000035861 hematochezia Diseases 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- FUKUFMFMCZIRNT-UHFFFAOYSA-N hydron;methanol;chloride Chemical compound Cl.OC FUKUFMFMCZIRNT-UHFFFAOYSA-N 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 125000002346 iodo group Chemical group I* 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- CFTUQSLVERGMHL-UHFFFAOYSA-N methyl 2-(bromomethyl)prop-2-enoate Chemical compound COC(=O)C(=C)CBr CFTUQSLVERGMHL-UHFFFAOYSA-N 0.000 description 1
- UFQQDNMQADCHGH-UHFFFAOYSA-N methyl 2-bromobutanoate Chemical compound CCC(Br)C(=O)OC UFQQDNMQADCHGH-UHFFFAOYSA-N 0.000 description 1
- UCFFGYASXIPWPD-UHFFFAOYSA-N methyl hypochlorite Chemical compound COCl UCFFGYASXIPWPD-UHFFFAOYSA-N 0.000 description 1
- 125000006216 methylsulfinyl group Chemical group [H]C([H])([H])S(*)=O 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 125000002950 monocyclic group Chemical group 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- GWKWCUBXWGJMGK-UHFFFAOYSA-N n-(4-acetylphenyl)-4-fluorobenzenesulfonamide Chemical compound C1=CC(C(=O)C)=CC=C1NS(=O)(=O)C1=CC=C(F)C=C1 GWKWCUBXWGJMGK-UHFFFAOYSA-N 0.000 description 1
- DZFGSPKLORYTIN-UHFFFAOYSA-N n-(4-acetylphenyl)prop-2-enamide Chemical compound CC(=O)C1=CC=C(NC(=O)C=C)C=C1 DZFGSPKLORYTIN-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- AGRDPCWQGGNEQL-UHFFFAOYSA-N n-propan-2-ylsulfamoyl chloride Chemical compound CC(C)NS(Cl)(=O)=O AGRDPCWQGGNEQL-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- PIDFDZJZLOTZTM-KHVQSSSXSA-N ombitasvir Chemical compound COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NC1=CC=C([C@H]2N([C@@H](CC2)C=2C=CC(NC(=O)[C@H]3N(CCC3)C(=O)[C@@H](NC(=O)OC)C(C)C)=CC=2)C=2C=CC(=CC=2)C(C)(C)C)C=C1 PIDFDZJZLOTZTM-KHVQSSSXSA-N 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 238000010814 radioimmunoprecipitation assay Methods 0.000 description 1
- 125000003003 spiro group Chemical group 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- IGVNJALYNQVQIT-UHFFFAOYSA-N tert-butyl 2-bromo-2-methylpropanoate Chemical compound CC(C)(C)OC(=O)C(C)(C)Br IGVNJALYNQVQIT-UHFFFAOYSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 description 1
- 125000003554 tetrahydropyrrolyl group Chemical group 0.000 description 1
- 125000005958 tetrahydrothienyl group Chemical group 0.000 description 1
- ARYHTUPFQTUBBG-UHFFFAOYSA-N thiophen-2-ylboronic acid Chemical compound OB(O)C1=CC=CS1 ARYHTUPFQTUBBG-UHFFFAOYSA-N 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/12—Ketones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/136—Amines having aromatic rings, e.g. ketamine, nortriptyline having the amino group directly attached to the aromatic ring, e.g. benzeneamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/165—Amides, e.g. hydroxamic acids having aromatic rings, e.g. colchicine, atenolol, progabide
- A61K31/167—Amides, e.g. hydroxamic acids having aromatic rings, e.g. colchicine, atenolol, progabide having the nitrogen of a carboxamide group directly attached to the aromatic ring, e.g. lidocaine, paracetamol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/17—Amides, e.g. hydroxamic acids having the group >N—C(O)—N< or >N—C(S)—N<, e.g. urea, thiourea, carmustine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/18—Sulfonamides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/192—Carboxylic acids, e.g. valproic acid having aromatic groups, e.g. sulindac, 2-aryl-propionic acids, ethacrynic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/21—Esters, e.g. nitroglycerine, selenocyanates
- A61K31/27—Esters, e.g. nitroglycerine, selenocyanates of carbamic or thiocarbamic acids, meprobamate, carbachol, neostigmine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/34—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide
- A61K31/343—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having five-membered rings with one oxygen as the only ring hetero atom, e.g. isosorbide condensed with a carbocyclic ring, e.g. coumaran, bufuralol, befunolol, clobenfurol, amiodarone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/38—Heterocyclic compounds having sulfur as a ring hetero atom
- A61K31/381—Heterocyclic compounds having sulfur as a ring hetero atom having five-membered rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/06—Anti-spasmodics, e.g. drugs for colics, esophagic dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
Landscapes
- Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Pain & Pain Management (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Emergency Medicine (AREA)
- Rheumatology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
技术领域
本发明涉及药物化学领域,具体涉及一类1,3-二苯基丙-2-烯-1-酮衍生物及其应用。
背景技术
NLRP3炎症小体是一种NOD样受体,由炎症小体传感器分子(NLRP3蛋白),接头蛋白ASC,以及效应分子caspase-1前体蛋白(pro-caspase-1)三部分组成,是存在于胞浆中的一种多蛋白复合物。NLRP3炎症小体激活后,Pro-caspase-1自剪切为有活性的caspase-1,进一步将pro-IL-1β和Pro-IL-18切割为有活性的白介素-1β(IL-1β)和白介素-18(IL-18),最终导致炎症反应和细胞焦亡。大量证据表明很多人类疾病,如阿尔茨海默症,痛风,多发性硬化,II型糖尿病,炎症性肠病等,与NLRP3炎症小体有着密切关联。到目前为止,已经有多种NLRP3炎症小体抑制剂被发现,但并没有一个可用于临床。因此,发现新的NLRP3炎症小体抑制剂对治疗NLRP3相关疾病具有重要意义。
发明内容
基于此,本发明发现了一类1,3-二苯基丙-2-烯-1-酮衍生物,这类1,3-二苯基丙-2-烯-1-酮衍生物能够选择性抑制NLRP3炎症小体的激活,从而可以治疗或者改善与NLRP3炎症小体相关的疾病,例如:急性腹膜炎和结肠炎。
具体技术方案如下:
式(I)所示的1,3-二苯基丙-2-烯-1-酮衍生物或其药学上可接受的盐作为活性成分在制备NLRP3炎症小体抑制剂中的应用;
其中,
Q选自:-COOH、-OR4;
R1选自:C1-C6烷基、C1-C6烷氧基、C1-C6烷硫基、氨基、C1-C6烷基胺基、苯基、R7取代的苯基、卤素、C1-C6烷基亚磺酰基、C1-C6烷基磺酰基、噻唑基、R7取代的噻唑基、R7取代的苯磺酰胺基、C1-C6烷基氨基磺酰胺基、C1-C6烷基磺酰胺基、丙烯酰胺基、C1-C6烷基酰胺基、或-NHCONH-R8;
R2、R3、R5、R6、R7各自分别独立地选自:氢、羟基、C1-C6烷基、C1-C6烷氧基、C1-C6烷硫基、卤素、羧基;
R4选自:氢、C1-C6烷基、羧基取代的C1-C6烷基、羟基取代的C1-C6烷基、5-6元杂环基取代的C1-C6烷基、-CONH-R9、羧基取代的烯丙基,或者R4与R3连接形成5-6元杂环结构;
R8选自:氢、C1-C6烷基、R7取代的苯基;
R9选自:氢、C1-C6烷基。
在其中一些实施例中,R1选自:C1-C3烷硫基、氨基、C1-C3烷基胺基、卤素、C1-C3烷基亚磺酰基、C1-C3烷基磺酰基、卤素取代的苯磺酰胺基、C1-C3烷基氨基磺酰胺基、C1-C3烷基磺酰胺基、丙烯酰胺基、C1-C3烷基酰胺基、或-NHCONH-R8。
在其中一些实施例中,R1选自:甲硫基、氨基、二甲胺基、苯基、氟、氯、碘、溴、甲基亚磺酰基、甲磺酰基、噻唑基、甲基取代的噻唑基、乙基磺酰胺基、对氟苯磺酰胺基、异丙基氨基磺酰胺基、丙烯酰胺基、-NHCONH-R8,其中,R8选自:异丙基、丙基、氟苯基、甲苯基。
在其中一些实施例中,R2、R3、R5、R6、R7各自分别独立地选自:氢、羟基、C1-C3烷基、C1-C3烷氧基、卤素。
在其中一些实施例中,R2、R3、R5、R6、R7各自分别独立地选自:氢、羟基、甲基、甲氧基、乙基、卤素。
在其中一些实施例中,R2选自:氢、卤素、C1-C3烷基。
在其中一些实施例中,R2选自:氢、氟、甲基。
在其中一些实施例中,R3选自:氢、卤素、C1-C3烷基、C1-C3烷氧基、羟基。
在其中一些实施例中,R3选自:氢、甲基、甲氧基、氯、氟,乙基、羟基。
在其中一些实施例中,R5选自:氢、C1-C3烷基、C1-C3烷氧基。
在其中一些实施例中,R5选自:氢、甲基、乙基、甲氧基。
在其中一些实施例中,R6选自:氢、C1-C3烷基。
在其中一些实施例中,R6选自:氢、甲基。
在其中一些实施例中,R7选自:氢、卤素、C1-C3烷基。
在其中一些实施例中,R7选自:氢、氟、甲基。
在其中一些实施例中,R4选自:氢、C1-C3烷基、羧基取代的C1-C6烷基、羟基取代的C1-C3烷基、6元杂环基取代的C1-C3烷基、-CONH-R9、羧基取代的烯丙基,或者R4与R3连接形成5元杂环结构;其中,R9选自:氢、C1-C3烷基。
在其中一些实施例中,R4选自:氢、甲基、或以下结构:
在其中一些实施例中,Q选自:-OH、COOH、羟基取代的乙氧基,并且,R2和R6不同时为C1-C6烷基,R3和R5不同时为C1-C6烷氧基。
在其中一些实施例中,所述1,3-二苯基丙-2-烯-1-酮衍生物具有式(II)所示结构:
其中,Q选自:-OH、COOH、羟基取代的乙氧基,并且,R2和R6不同时为C1-C6烷基,R3和R5不同时为C1-C6烷氧基。
在其中一些实施例中,所述1,3-二苯基丙-2-烯-1-酮衍生物具有式(III)所示结构:
R1选自:C1-C6烷基、C1-C6烷氧基、C1-C6烷硫基、氨基、C1-C6烷基胺基、卤素、C1-C6烷基亚磺酰基、C1-C6烷基磺酰基、R7取代的苯磺酰胺基、C1-C6烷基氨基磺酰胺基、C1-C6烷基磺酰胺基、丙烯酰胺基、C1-C6烷基酰胺基、或-NHCONH-R8。
在其中一些实施例中,R1选自:C1-C3烷硫基、氨基、C1-C3烷基胺基、卤素、C1-C3烷基亚磺酰基、C1-C3烷基磺酰基、卤素取代的苯磺酰胺基、C1-C3烷基氨基磺酰胺基、C1-C3烷基磺酰胺基、丙烯酰胺基、C1-C3烷基酰胺基、或-NHCONH-R8。
在其中一些实施例中,R1选自:C1-C3烷基亚磺酰基、卤素取代的苯磺酰胺基、C1-C3烷基氨基磺酰胺基、丙烯酰胺基。
在其中一些实施例中,R1选自:甲硫基、氨基、二甲胺基、氟、氯、甲基亚磺酰基、甲磺酰基、乙基磺酰胺基、对氟苯磺酰胺基、异丙基氨基磺酰胺基、丙烯酰胺基、-NHCONH-R8,其中,R8选自:异丙基、氟苯基。
在其中一些实施例中,所述1,3-二苯基丙-2-烯-1-酮衍生物具有式(IV)所示结构:
其中,R4选自:C1-C3烷基、羟基取代的C1-C3烷基、6元杂环基取代的C1-C3烷基、-CONH-R9、羧基取代的烯丙基、羧基取代的C4-C6烷基;其中,R9选自:氢、C1-C3烷基。
在其中一些实施例中,所述1,3-二苯基丙-2-烯-1-酮衍生物具有式(V)所示结构:
上述的1,3-二苯基丙-2-烯-1-酮衍生物或其药学上可接受的盐作为活性成分在制备预防和/或治疗与NLRP3炎症小体相关的疾病的药物中的应用。
在其中一些实施例中,所述与NLRP3炎症小体相关的疾病为腹膜炎和结肠炎。
在其中一些实施例中,所述腹膜炎为急性腹膜炎。
本发明还提供了一种防治NLRP3炎症小体相关的疾病的药物组合物。
具体技术方案如下:
一种防治NLRP3炎症小体相关的疾病的药物组合物,由活性成分和药学上可接受的辅料制备而成,所述活性成分包括有上述的1,3-二苯基丙-2-烯-1-酮衍生物或其药学上可接受的盐。
与现有技术相比,本发明具有以下有益效果:
本发明发现了一类1,3-二苯基丙-2-烯-1-酮衍生物,该类化合物能够选择性抑制NLRP3炎症小体的激活,从而可以治疗或者改善与NLRP3炎症小体相关的疾病,例如:急性腹膜炎和结肠炎,从而可用于制备与NLRP3炎症小体相关疾病的治疗药物。
附图说明
图1为化合物40在体外特异性抑制NLRP3炎性体的活化并抑制细胞焦亡的结果图。
图2为化合物40抑制LPS诱导的急性腹膜炎的结果图。
图3为化合物40改善硫酸葡聚糖钠(DSS)诱导的结肠炎的结果图。
具体实施方式
本发明所述化合物中,当任何变量(例如R7等)在任何组分中出现超过一次,则其每次出现的定义独立于其它每次出现的定义。同样,允许取代基及变量的组合,只要这种组合使化合物稳定。自取代基划入环系统的线表示所指的键可连接到任何能取代的环原子上。如果环系统为多环,其意味着这种键仅连接到邻近环的任何适当的碳原子上。要理解本领域普通技术人员可选择本发明化合物的取代基及取代型式而提供化学上稳定的并可通过本领域技术和下列提出的方法自可容易获得的原料容易的合成的化合物。如果取代基自身被超过一个基团取代,应理解这些基团可在相同碳原子上或不同碳原子上,只要使结构稳定。
本文所用术语“烷基”意指包括具有特定碳原子数目的支链的和直链的饱和脂肪烃基。例如,“C1-C6烷基”中“C1-C6”的定义包括以直链或支链排列的具有1、2、3、4、5或6个碳原子的基团。例如,“C1-C6烷基”具体包括甲基、乙基、正丙基、异丙基、正丁基、叔丁基、异丁基、戊基、己基。
本文所用术语“烷氧基”指具有-O-烷基结构的基团,如-OCH3、-OCH2CH3、-OCH2CH2CH3、-O-CH2CH(CH3)2、-OCH2CH2CH2CH3、-O-CH(CH3)2等。
本文所用术语“烷硫基”指具有-S-烷基结构的基团,如-SCH3、-SCH2CH3、-SCH2CH2CH3、-S-CH2CH(CH3)2、-SCH2CH2CH2CH3、-S-CH(CH3)2等。
本文所用术语“烷基胺基”指氨基中的一个或两个氢原子被烷基取代后的基团,如二甲胺基、二乙胺基、甲胺基、乙胺基等。
本文所用术语“杂环基”指饱和或部分不饱和的单环、双环或多环环状取代基,其中一个或多个环原子选自N、O或S(O)m(其中m是0-2的整数)的杂原子,其余环原子为碳,双环或多环包括螺环、稠环和桥环。例如:吗啉基、哌啶基、四氢吡咯基、吡咯烷基、二氢咪唑基、二氢异噁唑基、二氢异噻唑基、二氢噁二唑基、二氢噁唑基、二氢吡嗪基、二氢吡唑基、二氢吡啶基、二氢嘧啶基、二氢吡咯基、二氢四唑基、二氢噻二唑基、二氢噻唑基、二氢噻吩基、二氢三唑基、二氢氮杂环丁烷基、四氢呋喃基、四氢噻吩基等,及其N-氧化物。杂环取代基的连接可通过碳原子或通过杂原子实现。
正如本领域技术人员所理解的,本文中所用“卤素”(“halo”)或“卤”意指氯、氟、溴和碘。
除非另行定义,文中所使用的所有专业与科学用语与本领域技术人员所熟悉的意义相同。此外,任何与所记载内容相似或均等的方法及材料皆可应用于本发明方法中。文中所述的较佳实施方法与材料仅作示范之用。
以下实施例中的原料可以从商业途径获得,或者通过本领域已知的方法制备,或根据本文所述方法制备。
本发明化合物的合成路线如下:
其中,中间体1h-1l的合成路线如下:
实施例1:(E)-3-(4-甲氧基-3,5-二甲基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物1)的制备
将4-甲巯基苯乙酮(200mg,1.20mmol)和3,5-二甲基-4-甲氧基苯甲醛(197mg,1.20mmol)溶于5mL甲醇中,然后加入氢氧化钠(481mg,12.03mmol),室温下搅拌24小时,析出固体并用甲醇重结晶得到白色固体270mg,产率77%。1H NMR(400MHz,CDCl3)δ7.97–7.93(m,2H),7.72(d,J=15.6Hz,1H),7.42(d,J=15.6Hz,1H),7.33–7.27(m,2H),3.75(s,3H),2.53(s,3H),2.32(s,6H);13C NMR(100MHz,CDCl3)δ189.4,159.4,145.6,144.6,134.8,131.7,130.6,129.4,129.1,125.2,120.7,59.9,16.4,15.0;HRMS(ESI)calcd forC19H20O2S(M+H)+313.1257,found 313.1278.
实施例2:(E)-1-(4-氨基苯基)-3-(4-甲氧基-3,5-二甲基苯基)丙-2-烯-1-酮(化合物2)的制备
参照实施例1的方法,得到黄色固体,产率63%。1HNMR(400MHz,DMSO-d6)δ7.97–7.87(m,2H),7.75(d,J=15.4Hz,1H),7.57–7.42(m,3H),6.69–6.56(m,2H),6.15(s,2H),3.68(s,3H),2.25(s,3H);13C NMR(100MHz,DMSO-d6)δ185.8,158.3,153.8,141.3,131.1,130.9,130.6,129.2,125.5,121.1,112.7,59.4,15.9;HRMS(ESI)calcd for C18H19NO2(M+H)+282.1489,282.1489.
实施例3:(E)-1-(4-(二甲基氨基)苯基)-3-(4-甲氧基-3,5-二甲基苯基)丙-2-烯-1-酮(化合物3)的制备
参照实施例1的方法,得到黄色固体,产率75%。1HNMR(400MHz,DMSO-d6)δ8.14–8.00(m,2H),7.78(d,J=15.5Hz,1H),7.59–7.51(m,3H),6.80–6.70(m,2H),3.68(s,3H),3.04(s,6H),2.26(s,6H);13C NMR(100MHz,DMSO-d6)δ186.1,158.4,153.3,141.5,130.9,130.7,130.6,129.2,125.3,121.1,110.8,59.4,39.6,15.8;HRMS(ESI)calcd for C20H23NO2(M+H)+310.1802,found 310.1804.
实施例4:(E)-3-(2,3-二氢苯并呋喃-5-基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物4)的制备
参照实施例1的方法,得到黄色固体,产率74%。1HNMR(400MHz,DMSO-d6)δ8.12–8.05(m,2H),7.85(d,J=1.7Hz,1H),7.77(d,J=15.4Hz,1H),7.69(d,J=15.4Hz,1H),7.61(dd,J=8.3,1.9Hz,1H),7.42–7.36(m,2H),6.84(d,J=8.3Hz,1H),4.61(t,J=8.7Hz,2H),3.23(t,J=8.7Hz,2H),2.55(s,3H);13C NMR(100MHz,DMSO-d6)δ187.7,162.2,145.2,144.2,134.1,130.7,128.9,128.6,127.5,125.4,124.9,118.6,109.3,71.8,28.6,14.0;HRMS(ESI)calcd for C18H16O2S(M+H)+297.0944,found297.0967.
实施例5:(E)-1-异丙基-3-(4-(3-(4-甲氧基-3,5-二甲基苯基)丙烯酰基)苯基)脲(化合物5)的制备
将化合物2(50mg,0.18mmol)和异氰酸异丙酯(18mg,0.21mmol)溶解于1.5mL甲苯中。反应液加热到70℃搅拌过夜后,将形成的沉淀物过滤并用甲苯洗涤,干燥,得到黄色固体48mg,产率74%。1H NMR(400MHz,DMSO-d6)δ8.78(s,1H),8.10–8.03(m,2H),7.80(d,J=15.5Hz,1H),7.63–7.52(m,5H),6.19(d,J=7.5Hz,1H),3.84–3.74(m,1H),3.69(s,3H),2.26(s,6H),1.11(d,J=6.5Hz,6H);13C NMR(100MHz,DMSO-d6)δ187.1,158.7,154.0,145.3,142.7,131.0,130.4,130.3,130.1,129.5,120.8,116.6,59.4,41.1,22.9,15.9;HRMS(ESI)calcd for C22H26N2O3(M+H)+367.2016,found 367.2013.
实施例6:(E)-1-(4-(3-(4-甲氧基-3,5-二甲基苯基)丙烯酰基)苯基)-3-丙基脲(化合物6)的制备
参照实施例5的方法,得到黄色固体,产率74%。1HNMR(400MHz,DMSO-d6)δ8.91(s,1H),8.11–8.00(m,2H),7.80(d,J=15.5Hz,1H),7.69–7.49(m,5H),6.33(t,J=5.7Hz,1H),3.69(s,3H),3.11–3.02(m,2H),2.26(s,6H),1.54–1.39(m,2H),0.88(t,J=7.4Hz,3H);13CNMR(100MHz,DMSO-d6)δ187.1,158.7,154.8,145.4,142.7,131.0,130.4,130.4,129.5,120.8,116.6,59.4,40.9,22.9,15.9,11.4;HRMS(ESI)calcd for C22H26N2O3(M+H)+367.2016,found367.2019.
实施例7:(E)-1-(4-氟苯基)-3-(4-(3-(4-甲氧基-3,5-二甲基苯基)丙烯酰基)苯基)脲(化合物7)的制备
参照实施例5的方法,得到黄色固体,产率95%。1HNMR(400MHz,DMSO-d6)δ9.14(s,1H),8.85(s,1H),8.18–8.04(m,2H),7.82(d,J=15.5Hz,1H),7.67–7.55(m,5H),7.53–7.44(m,2H),7.21–7.08(m,2H),3.69(s,3H),2.26(s,6H);13C NMR(100MHz,DMSO-d6)δ187.2,158.7,157.6(d,J=237.0Hz),152.3,144.4,142.9,135.6(d,J=2.6Hz),131.2,130.9,130.3,130.0,129.5,120.3(d,J=7.9Hz),120.3,117.3,115.4(d,J=22.3Hz)59.4,15.8;HRMS(ESI)calcd for C25H23FN2O3(M+H)+419.1765,found419.1777.
实施例8:(E)-1-(4-(3-(4-甲氧基-3,5-二甲基苯基)丙烯酰基)苯基)-3-(对甲苯基)脲(化合物8)的制备
参照实施例5的方法,得到黄色固体,产率90%。1HNMR(400MHz,DMSO-d6)δ9.10(s,1H),8.70(s,1H),8.18–8.01(m,2H),7.82(d,J=15.4Hz,1H),7.67–7.50(m,5H),7.41–7.28(m,2H),7.16–7.02(m,2H),3.69(s,3H),2.27(s,6H),2.25(s,3H);13C NMR(100MHz,DMSO-d6)δ187.2,158.7,152.2,144.5,142.9,136.7,131.1,131.1,130.9,130.3,130.0,129.5,129.2,120.7,118.6,117.2,59.4,20.4,15.8;HRMS(ESI)calcd for C26H26N2O3(M+H)+415.2016,found415.2021.
实施例9:(E)-3-(4-羟基-3,5-二甲基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物9)的制备
将4-甲巯基苯乙酮(2.33g,14.00mmol)和3,5-二甲基-4-羟基苯甲醛(2.10g,14.00mmol)加入到20mL氯化氢甲醇溶液(4mol/L)中,在室温下搅拌24h,将所形成的沉淀物过滤并干燥得到黄色固体3.20g,产率77%。1H NMR(400MHz,DMSO-d6)δ8.94(s,1H),8.14–8.02(m,2H),7.71(d,J=15.4Hz,1H),7.60(d,J=15.4Hz,1H),7.49(s,2H),7.43–7.34(m,2H),2.55(s,3H),2.20(s,6H);13C NMR(100MHz,DMSO-d6)δ187.8,156.3,145.2,144.6,134.2,129.7,129.0,125.8,125.0,124.7,118.2,16.7,14.0;HRMS(ESI)calcd forC18H18O2S(M+H)+299.1100,found 299.1106.
实施例10:(E)-2-(2,6-二甲基-4-(3-(4-(甲硫基)苯基)-3-氧代丙-1-烯-1-基)苯氧基)乙酸(化合物10)的制备
将化合物9(200mg,0.67mmol)和溴乙酸乙酯(224mg,1.34mmol)溶解于2mL无水DMF溶液中,加入碳酸钾(463mg,3.35mmol),90℃搅拌6h,然后将该反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到中间体羧酸酯,为黄色油状物,183mg,产率71%。将上述羧酸酯(300mg,0.78mmol)溶解于THF/H2O(8mL/8mL)中,加入氢氧化锂(112mg,4.68mmol),并在50℃下搅拌过夜,然后用盐酸(1mol/L)将溶液调节至pH=2,并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到化合物10,为黄色固体,180mg,产率65%。1HNMR(400MHz,DMSO-d6)δ8.14–8.03(m,2H),7.80(d,J=15.5Hz,1H),7.61(d,J=15.5Hz,1H),7.56(s,2H),7.43–7.29(m,2H),4.43(s,2H),2.55(s,3H),2.27(s,6H);13C NMR(100MHz,DMSO-d6)δ187.9,170.2,157.4,145.5,143.5,133.9,131.1,130.5,129.7,129.1,125.0,120.8,68.8,16.1,14.0;HRMS(ESI)calcd for C20H20O4S(M+H)+357.1155,found357.1159.
实施例11:(E)-3-(4-(2-羟基乙氧基)-3,5-二甲基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物11)的制备
将化合物9(100mg,0.34mmol)和2-溴乙醇(125mg,1.00mmol)溶解于2mL无水DMF溶液中,加入碳酸钾(232mg,1.68mmol),90℃搅拌6h,然后将该反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到黄色固体65mg,产率57%。1HNMR(400MHz,DMSO-d6)δ8.12–8.06(m,2H),7.80(d,J=15.5Hz,1H),7.62(d,J=15.5Hz,1H),7.56(s,2H),7.42–7.37(m,2H),4.91(t,J=5.5Hz,1H),3.84–3.79(m,2H),3.74–3.68(m,2H),2.56(s,3H),2.28(s,6H);13C NMR(100MHz,DMSO-d6)δ187.8,157.9,145.4,143.6,133.9,131.2,130.0,129.7,129.0,125.0,120.5,74.0,60.5,16.0,14.0;HRMS(ESI)calcd for C20H22O3S(M+H)+343.1362,found 343.1364.
实施例12:(E)-2-(2,6-二甲基-4-(3-(4-(甲硫基)苯基)-3-氧代丙-1-烯-1-基)苯氧基)丁酸(化合物12)的制备
将化合物9(200mg,0.67mmol)和2-溴丁酸甲酯(363mg,2.01mmol)溶解于2mL无水DMF溶液中,加入碳酸钾(463mg,3.35mmol),90℃搅拌6h,然后将该反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到中间体羧酸酯,为黄色油状物,210mg,产率79%。将上述羧酸酯(200mg,0.50mmol)溶解于THF/H2O(5mL/5mL)中,加入氢氧化锂(72mg,3.00mmol),并在50℃下搅拌过夜,然后用盐酸(1mol/L)将溶液调节至pH=2,并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到化合物12,为黄色固体,120mg,产率62%。1HNMR(400MHz,DMSO-d6)δ8.14–8.04(m,2H),7.80(d,J=15.6Hz,1H),7.61(d,J=15.6Hz,1H),7.55(s,2H),7.44–7.35(m,2H),4.44(t,J=5.9Hz,1H),2.55(s,3H),2.28(s,6H),1.97–1.81(m,2H),0.98(t,J=7.4Hz,3H);13C NMR(100MHz,DMSO-d6)δ187.8,172.1,157.0,145.5,143.5,133.9,130.8,129.9,129.9,129.1,125.0,120.6,81.2,25.7,16.9,14.0,9.0;HRMS(ESI)calcd for C22H24O4S(M+H)+385.1468,found 385.1473.
实施例13:(E)-2-(2,6-二甲基-4-(3-(4-(甲硫基)苯基)-3-氧代丙-1-烯-1-基)苯氧基)-2-甲基丙酸(化合物13)的制备
将化合物9(150mg,0.50mmol)和2-溴-2-甲基丙酸叔丁酯(336mg,1.51mmol)溶解于2mL无水DMF溶液中,加入碳酸钾(347mg,2.51mmol),90℃搅拌6h,然后将该反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到中间体羧酸酯,为黄色油状物,180mg,产率82%。将上述羧酸酯(180mg,0.41mmol)溶解于二氯甲烷中,加入三氟乙酸(466mg,4.08mmol),并在常温下搅拌过夜,浓缩并通过硅胶柱纯化,得到化合物13,为黄色固体,110mg,产率70%。1HNMR(400MHz,DMSO-d6)δ8.21–7.99(m,2H),7.80(d,J=15.6Hz,1H),7.61(d,J=15.6Hz,1H),7.55(s,2H),7.42–7.36(m,2H),2.56(s,3H),2.22(s,6H),1.39(s,6H);13C NMR(101MHz,DMSO-d6)δ187.78,174.96,154.94,145.40,143.32,133.87,133.14,130.19,129.33,129.00,124.93,120.84,80.69,24.99,17.53,13.94;HRMS(ESI)calcd for C22H24O4S(M+H)+385.1468,found 385.1468.
实施例14:(E)-2-((2,6-二甲基-4-(3-(4-(甲硫基)苯基)-3-氧代丙-1-烯-1-基)苯氧基)甲基)丙烯酸(化合物14)的制备
将化合物9(200mg,0.67mmol)和2-(溴甲基)丙烯酸甲酯(239mg,1.34mmol)溶解于2mL无水DMF溶液中,加入碳酸钾(463mg,3.35mmol),90℃搅拌6h,然后将该反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到中间体羧酸酯,为黄色油状物,185mg,产率70%。将上述羧酸酯(185mg,0.47mmol)溶解于THF/H2O(5mL/5mL)中,加入氢氧化锂(67mg,2.80mmol),并在50℃下搅拌过夜,然后用盐酸(1mol/L)将溶液调节至pH=2,并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到化合物14,为黄色固体,55mg,产率31%。1HNMR(400MHz,CDCl3)δ7.98–7.94(m,2H),7.73(d,J=15.6Hz,1H),7.42(d,J=15.6Hz,1H),7.35–7.28(m,4H),6.60–6.56(m,1H),6.29–6.25(m,1H),4.56–4.52(m,2H),2.54(s,3H),2.31(s,6H);13C NMR(100MHz,DMSO-d6)δ187.9,166.8,157.5,145.5,143.6,137.6,133.9,131.3,130.4,129.7,129.1,126.7,125.0,120.8,70.2,16.1,14.0;HRMS(ESI)calcd for C22H22O4S(M+H)+383.1312,found 383.1315.
实施例15:(E)-5-(2,6-二甲基-4-(3-(4-(甲硫基)苯基)-3-氧代丙-1-烯-1-基)苯氧基)戊酸(化合物15)的制备
将化合物9(200mg,0.67mmol)和5-溴戊酸乙酯(420mg,2.01mmol)溶解于2mL无水DMF溶液中,加入碳酸钾(463mg,3.35mmol),90℃搅拌6h,然后将该反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到中间体羧酸酯,为黄色油状物,240mg,产率84%。将上述羧酸酯(180mg,0.42mmol)溶解于THF/H2O(4mL/4mL)中,加入氢氧化锂(50mg,2.10mmol),并在50℃下搅拌过夜,然后用盐酸(1mol/L)将溶液调节至pH=2,并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到化合物15,为黄色固体,121mg,产率72%。1HNMR(400MHz,DMSO-d6)δ8.12–8.06(m,2H),7.80(d,J=15.5Hz,1H),7.62(d,J=15.5Hz,1H),7.57(s,2H),7.43–7.36(m,2H),3.77(t,J=5.8Hz,2H),2.56(s,3H),2.31(t,J=6.9Hz,2H),2.25(s,6H),1.77–1.70(m,4H);13C NMR(100MHz,DMSO-d6)δ187.8,174.5,157.9,145.4,143.6,133.9,131.1,130.1,129.6,129.0,124.9,120.5,71.5,33.5,29.4,21.3,16.0,14.0;HRMS(ESI)calcd for C23H26O4S(M+H)+399.1625,found 399.1629.
实施例16:(E)-2,6-二甲基-4-(3-(4-(甲硫基)苯基)-3-氧代丙-1-烯-1-基)苯基丙基氨基甲酸酯(化合物16)的制备
将化合物9(50mg,0.17mmol)和三乙胺(3mg,0.03mmol)溶解在无水CH2Cl2中,加入异氰酸丙酯,并在室温下搅拌5h,然后将反应液浓缩并通过硅胶柱纯化得到白色固体46mg,产率72%。1HNMR(400MHz,CDCl3)δ7.99–7.90(m,2H),7.72(d,J=15.6Hz,1H),7.42(d,J=15.6Hz,1H),7.36–7.27(m,4H),5.21(t,J=6.1Hz,1H),3.29–3.17(m,2H),2.53(s,3H),2.22(s,6H),1.66–1.53(m,2H),0.97(t,J=7.4Hz,3H);13C NMR(100MHz,CDCl3)δ189.3,153.8,150.1,145.6,144.3,134.6,132.3,132.0,129.1,128.8,125.2,121.3,43.1,23.3,16.4,14.9,11.3;HRMS(ESI)calcd for C22H25NO3S(M+H)+384.1628,found 384.1631.
实施例17:(E)-3-(3,5-二甲基-4-(3-吗啉代丙氧基)苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物17)的制备
将化合物9(100mg,0.34mmol)和4-(3-氯丙基)吗啉(110mg,0.67mmol)溶解于2mL无水DMF溶液中,加入碳酸钾(232mg,1.68mmol),90℃搅拌6h,然后将该反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到白色固体101mg,产率71%。1HNMR(400MHz,CDCl3)δ8.00–7.86(m,2H),7.71(d,J=15.6Hz,1H),7.41(d,J=15.6Hz,1H),7.34–7.27(m,4H),3.84(t,J=6.3Hz,2H),3.73(t,J=4.7Hz,4H),2.59(t,J=7.3Hz,2H),2.53(s,3H),2.48(t,J=4.7Hz,4H),2.30(s,6H),2.05–1.95(m,2H);13C NMR(100MHz,CDCl3)δ189.3,158.3,145.5,144.6,134.8,131.8,130.5,129.3,129.0,125.2,120.6,70.3,67.1,55.5,53.8,27.5,16.5,15.0;HRMS(ESI)calcd for C25H31NO3S(M+H)+426.2097,found426.2101.
实施例18:(E)-4-(3-(4-(甲硫基)苯基)-3-氧代丙-1-烯-1-基)苯甲酸(化合物18)的制备
将4-甲巯基苯乙酮(366mg,2.20mmol)和4-甲酰基苯甲酸(300mg,2.00mmol)溶解在10mL甲醇中,加入4mL40%的氢氧化钠溶液,室温下搅拌24h,随后,将溶液调节至pH=2,然后过滤沉淀,用甲醇洗涤并干燥,得到黄色固体415mg,产率70%。1H NMR(400MHz,DMSO-d6)δ8.14–8.10(m,2H),8.03(d,J=15.6Hz,1H),8.00–7.97(m,4H),7.76(d,J=15.6Hz,1H),7.47–7.35(m,2H),2.56(s,3H);13C NMR(100MHz,DMSO-d6)δ187.9,167.2,145.9,142.4,138.4,133.6,129.7,129.2,128.8,125.0,123.8,14.0;HRMS(ESI)calcd forC17H14O3S(M+H)+299.0736,found 299.0740.
实施例19:(E)-3-(4-羟苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物19)的制备
参照实施例9的方法,得到黄色固体,产率60%。1HNMR(400MHz,DMSO-d6)δ10.11(s,1H),8.10–8.03(m,2H),7.76–7.64(m,4H),7.43–7.32(m,2H),6.88–6.80(m,2H),2.55(s,3H);13C NMR(100MHz,DMSO-d6)δ187.8,160.1,145.1,144.2,134.1,131.0,128.9,125.9,124.9,118.3,115.9,14.0;HRMS(ESI)calcd for C16H14O2S(M+H)+271.0787,found271.0812.
实施例20:(E)-3-(4-羟基-3-甲基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物20)的制备
参照实施例9的方法,得到黄色固体,产率63%。1HNMR(400MHz,DMSO-d6)δ10.00(s,1H),8.12–7.99(m,2H),7.73–7.66(m,2H),7.63(d,J=15.4Hz,1H),7.51(dd,J=8.3,2.2Hz,1H),7.41–7.35(m,2H),6.85(d,J=8.3Hz,1H),2.55(s,3H),2.17(s,3H);13C NMR(100MHz,DMSO-d6)δ187.7,158.4,145.0,144.4,134.2,131.3,128.9,125.7,124.9,124.7,118.0,114.9,15.9,14.0;HRMS(ESI)calcd for C17H16O2S(M+H)+285.0944,found285.0968.
实施例21:(E)-3-(4-羟基-3-甲氧基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物21)的制备
参照实施例9的方法,得到黄色固体,产率51%。1HNMR(400MHz,DMSO-d6)δ9.68(s,1H),8.11–8.02(m,2H),7.75(d,J=15.4Hz,1H),7.67(d,J=15.4Hz,1H),7.51(d,J=2.0Hz,1H),7.43–7.35(m,2H),7.28(dd,J=8.3,1.9Hz,1H),6.84(d,J=8.1Hz,1H),3.87(s,3H),2.55(s,3H);13C NMR(100MHz,DMSO-d6)δ187.8,149.7,148.0,145.1,144.6,134.2,128.9,126.3,124.9,124.1,118.5,115.6,111.7,55.8,14.0;HRMS(ESI)calcd forC17H16O3S(M+H)+301.0893,found 301.0911.
实施例22:(E)-3-(3-氟-4-羟基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物22)的制备
参照实施例9的方法,得到黄色固体,产率30%。1HNMR(400MHz,DMSO-d6)δ10.51(s,1H),8.12–8.03(m,2H),7.85(dd,J=12.6,2.1Hz,1H),7.79(d,J=15.5Hz,1H),7.64(d,J=15.5Hz,1H),7.52–7.47(m,1H),7.41–7.36(m,2H),7.06–6.88(m,1H),2.55(s,3H);13C NMR(100MHz,DMSO-d6)δ187.7,151.2(d,J=241.6Hz),147.5(d,J=12.5Hz),145.3,143.0(d,J=2.5Hz),133.9,129.0,126.9(d,J=2.6Hz),126.7(d,J=6.6Hz),124.9,120.0,117.8(d,J=3.3Hz),115.8(d,J=18.6Hz),14.0;HRMS(ESI)calcd for C16H13FO2S(M+H)+289.0693,found289.0712.
实施例23:(E)-3-(2-氟-4-羟基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物23)的制备
参照实施例9的方法,得到黄色固体,产率18%。1HNMR(400MHz,DMSO-d6)δ10.58(s,1H),8.09–8.01(m,2H),7.94(t,J=8.8Hz,1H),7.81–7.70(m,2H),7.43–7.35(m,2H),6.72(dd,J=8.6,2.3Hz,1H),6.66(dd,J=12.6,2.3Hz,1H),2.55(s,3H);13C NMR(100MHz,DMSO-d6)δ187.7,162.2(d,J=250.0Hz),,161.7(d,J=12.6Hz),145.4,135.5(d,J=3.7Hz),133.9,130.3(d,J=4.5Hz),128.9,125.0,120.1(d,J=4.3Hz),113.4(d,J=11.5Hz),112.64(d,J=2.3Hz),102.9(d,J=24.1Hz),14.0;HRMS(ESI)calcd for C16H13FO2S(M+H)+289.0693,found 289.0708.
实施例24:(E)-3-(3-氯-4-羟基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物24)的制备
参照实施例9的方法,得到黄色固体,产率23%。1HNMR(400MHz,DMSO-d6)δ10.81(s,1H),8.14–8.06(m,2H),8.02(d,J=2.1Hz,1H),7.81(d,J=15.5Hz,1H),7.67–7.60(m,2H),7.41–7.36(m,2H),7.02(d,J=8.4Hz,1H),2.55(s,1H);13C NMR(100MHz,DMSO-d6)δ187.7,155.3,145.3,142.7,133.9,130.1,129.7,129.0,127.2,124.9,120.5,119.8,116.7,14.0;HRMS(ESI)calcd for C16H13ClO2S(M+H)+305.0398,found 305.0411.
实施例25:(E)-3-(4-羟基-2,5-二甲基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物25)的制备
参照实施例9的方法,得到黄色固体,产率71%。1HNMR(400MHz,DMSO-d6)δ9.87(s,1H),8.17–8.01(m,2H),7.92(d,J=15.3Hz,1H),7.81(s,1H),7.64(d,J=15.3Hz,1H),7.45–7.28(m,2H),6.67(s,1H),2.55(s,3H),2.33(s,3H),2.15(s,3H);13C NMR(100MHz,DMSO-d6)δ187.7,158.1,145.0,141.0,137.8,134.2,129.5,128.9,124.9,123.9,122.4,118.4,116.6,19.0,15.5,14.0;HRMS(ESI)calcd for C18H18O2S(M+H)+299.1100,found299.1114.
实施例26:(E)-3-(4-羟基-2,6-二甲基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物26)的制备
参照实施例9的方法,得到黄色固体,产率48%。1HNMR(400MHz,DMSO-d6)1HNMR(400MHz,DMSO-d6)δ9.73(s,1H),δ8.02–7.95(m,2H),7.85(d,J=15.8Hz,1H),7.40–7.35(m,2H),7.28(d,J=15.8Hz,1H),6.56(s,2H),2.53(s,3H),2.34(s,6H);13C NMR(100MHz,DMSO-d6)δ188.1,158.0,145.4,141.6,139.8,134.1,129.0,125.1,124.6,124.4,115.8,21.6,14.0;HRMS(ESI)calcd for C18H18O2S(M+H)+299.1100,found 299.1113.
实施例27:(E)-3-(4-羟基-3,5-二甲氧基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物27)的制备
参照实施例9的方法,得到黄色固体,产率45%。1HNMR(400MHz,DMSO-d6)δ9.04(s,1H),8.13–8.06(m,2H),7.78(d,J=15.4Hz,1H),7.67(d,J=15.4Hz,1H),7.43–7.36(m,2H),7.20(s,2H),3.85(s,6H),2.56(s,3H);13C NMR(100MHz,DMSO-d6)δ187.8,148.1,145.1,145.0,138.7,134.1,129.0,125.1,124.9,118.9,106.9,56.2,14.0;HRMS(ESI)calcd for C18H18O4S(M+H)+331.0999,found 331.1000.
实施例28:(E)-3-(3,5-二乙基-4-羟苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物28)的制备
参照实施例9的方法,得到黄色固体,产率69%。1HNMR(400MHz,DMSO-d6)δ8.81(s,1H),8.19–7.99(m,2H),7.72(d,J=15.4Hz,1H),7.64(d,J=15.4Hz,1H),7.48(s,2H),7.42–7.34(m,2H),2.63(q,J=7.5Hz,4H),2.55(s,3H),1.17(t,J=7.5Hz,6H);13C NMR(100MHz,DMSO-d6)δ187.7,155.2,145.0,144.8,134.2,130.9,128.9,128.1,126.1,124.9,118.1,23.0,14.4,14.0;HRMS(ESI)calcd for C20H22O2S(M+H)+327.1413,found 327.1429.
实施例29:(E)-3-(3,4-二羟基苯基)-1-(4-(甲硫基)苯基)丙-2-烯-1-酮(化合物29)的制备
参照实施例9的方法,得到黄色固体,产率46%。1HNMR(400MHz,DMSO-d6)δ9.61(s,1H),9.25(s,1H),8.14–7.93(m,2H),7.66–7.53(m,2H),7.43–7.34(m,2H),7.26(d,J=2.1Hz,1H),7.18(dd,J=8.2,2.1Hz,1H),6.81(d,J=8.2Hz,1H),2.55(s,3H);13C NMR(100MHz,DMSO-d6)δ187.8,148.7,145.6,145.1,144.7,134.2,128.9,126.4,125.0,122.2,118.3,115.8,115.6,14.0;HRMS(ESI)calcd for C16H14O3S(M+H)+287.0736,found287.0740.
实施例30:(E)-1-(4-氟苯基)-3-(4-羟基-3,5-二甲基苯基)丙-2-烯-1-酮(化合物30)的制备
参照实施例9的方法,得到黄色固体,产率63%。1HNMR(400MHz,DMSO-d6)δ8.94(s,1H),8.29–8.09(m,2H),7.71(d,J=15.4Hz,1H),7.62(d,J=15.4Hz,1H),7.48(s,2H),7.41–7.31(m,2H);13C NMR(100MHz,DMSO-d6)δ187.4,164.9(d,J=251.3Hz),156.4,145.1,134.7(d,J=2.9Hz),131.3(d,J=9.2Hz),129.7,125.7,124.7,118.0,115.7(d,J=21.7Hz),16.6;HRMS(ESI)calcd for C17H15FO2(M+H)+271.1129,found 271.1131.
实施例31:(E)-1-(4-氯苯基)-3-(4-羟基-3,5-二甲基苯基)丙-2-烯-1-酮(化合物31)的制备
参照实施例9的方法,得到黄色固体,产率67%。1HNMR(400MHz,DMSO-d6)δ8.18–8.10(m,2H),7.70(d,J=15.4Hz,1H),7.65–7.58(m,3H),7.49(s,2H),2.21(s,6H);13C NMR(100MHz,DMSO-d6)δ187.7,156.5,145.4,137.7,136.7,130.3,129.8,128.8,125.6,124.7,117.9,16.5;HRMS(ESI)calcd for C17H15ClO2(M+H)+287.0833,found 287.0832.
实施例32:(E)-3-(4-羟基-3,5-二甲基苯基)-1-(4-碘苯基)丙-2-烯-1-酮(化合物32)的制备
参照实施例9的方法,得到黄色固体,产率44%。1HNMR(400MHz,DMSO-d6)δ7.96–7.92(m,2H),7.91–7.86(m,2H),7.67(d,J=15.5Hz,1H),7.61(d,J=15.5Hz,3H),7.49(s,2H),2.20(s,6H);13C NMR(100MHz,DMSO-d6)δ188.3,156.5,145.4,137.7,137.3,130.2,129.8,125.7,124.7,117.9,101.4,16.6;HRMS(ESI)calcd for C17H15IO2(M+H)+379.0189,found379.0197.
实施例33:(E)-3-(4-羟基-3,5-二甲基苯基)-1-(4-(甲基亚磺酰基)苯基)丙-2-烯-1-酮(化合物33)的制备
在0℃搅拌条件下,将化合物9(500mg,1.68mmol)溶解在15mL无水CH2Cl2溶液中,然后将3-氯过氧苯甲酸(289mg,1.68mmol)缓慢加入到上述溶液中并在室温下继续搅拌5h,然后将溶液过滤,滤饼通过色谱柱纯化得到黄色固体354mg,产率67%。1HNMR(400MHz,DMSO-d6)δ8.99(s,1H),8.31–8.25(m,2H),7.87–7.82(m,2H),7.73(d,J=15.5Hz,1H),7.65(d,J=15.5Hz,1H),7.50(s,2H),2.81(s,3H),2.21(s,6H);13C NMR(100MHz,DMSO-d6)δ188.4,156.6,151.0,145.7,139.8,129.9,129.1,125.6,124.7,123.9,118.2,43.1,16.6;HRMS(ESI)calcd for C18H18O3S(M+H)+315.1049,found 315.1051.
实施例34:(E)-3-(4-羟基-3,5-二甲基苯基)-1-(4-(甲基磺酰基)苯基)丙-2-烯-1-酮(化合物34)的制备
在搅拌条件下,将化合物9(500mg,1.68mmol)溶解在15mL无水CH2Cl2溶液中,然后将3-氯过苯甲酸(754mg,4.37mmol)缓慢加入到上述溶液中丙在室温搅拌5h,然后将溶液过滤,滤饼通过色谱柱纯化得到黄色固体338mg,产率61%。1H NMR(400MHz,DMSO-d6)δ8.38–8.27(m,2H),8.15–8.02(m,2H),7.72(d,J=15.6Hz,1H),7.66(d,J=15.6Hz,1H),7.52(s,2H),3.31(s,3H),2.20(s,6H);13C NMR(100MHz,DMSO-d6)δ188.4,156.9,146.4,143.9,142.0,130.1,129.3,127.5,125.5,124.8,118.1,43.4,16.7;HRMS(ESI)calcd forC18H18O4S(M+H)+331.0999,found 331.0996.
实施例35:(E)-1-([[1,1'-联苯]-4-基)-3-(4-羟基-3,5-二甲基苯基)丙-2-烯-1-酮(化合物35)的制备
参照实施例9的方法,得到黄色固体,产率47%。1HNMR(400MHz,DMSO-d6)δ8.27–8.18(m,2H),7.87–7.81(m,2H),7.80–7.71(m,3H),7.65(d,J=15.5Hz,1H),7.55–7.47(m,4H),7.46–7.39(m,1H),2.21(s,6H);13C NMR(100MHz,DMSO-d6)δ188.5,156.5,145.0,144.3,139.1,136.9,129.9,129.3,128.5,127.1,127.0,125.8,124.8,118.4,16.7;HRMS(ESI)calcd for C23H20O2(M+H)+329.1536,found 329.1537.
实施例36:
中间体1-(4-(噻吩-2-基)苯基)乙-1-酮(中间体1h)的制备
N2保护下,将4-溴苯乙酮(200mg,1.01mmol),噻吩2-基硼酸(386mg,3.02mmol),碳酸钾(277mg,2.01mmol),和四(三苯基膦)钯(116mg,0.10mmol)溶解于5mLDME溶液中,并在90℃条件下搅拌过夜,然后将反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到白色固体145mg,产率71%。1H NMR(400MHz,CDCl3)δ7.99–7.95(m,2H),7.73–7.67(m,2H),7.44(dd,J=3.6,1.1Hz,1H),7.37(dd,J=5.1,1.1Hz,1H),7.12(dd,J=5.1,3.6Hz,1H),2.62(s,3H).
(E)-3-(4-羟基-3,5-二甲基苯基)-1-(4-(噻吩-2-基)苯基)丙-2-烯-1-酮(化合物36)的制备的制备
参照实施例9的方法,得到黄色固体,产率38%。1HNMR(400MHz,DMSO-d6)δ8.96(s,1H),8.24–8.12(m,2H),7.88–7.79(m,2H),7.78–7.70(m,2H),7.69–7.60(m,2H),7.51(s,2H),7.20(dd,J=5.1,3.7Hz,1H),2.22(s,6H);13C NMR(100MHz,DMSO-d6)δ187.9,156.4,144.9,142.2,137.7,136.7,129.8,129.4,128.9,127.5,125.7,125.5,125.4,124.7,118.2,16.6;HRMS(ESI)calcd for C21H18O2S(M+H)+335.1100,found 35.1103.
实施例37:
中间体1-(4-(5-甲基噻吩-2-基)苯基)乙-1-酮(中间体1i)的制备
N2保护下,将4-溴苯乙酮(200mg,1.01mmol),(5-甲基噻吩-2-基)硼酸(285mg,2.01mmol),碳酸钾(277mg,2.01mmol),和四(三苯基膦)钯(116mg,0.10mmol)溶解于5mLDME溶液中,并在90℃条件下搅拌过夜,然后将反应液用水稀释并用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到白色固体148mg,产率68%。1HNMR(400MHz,CDCl3)δ7.98–7.90(m,2H),7.69–7.57(m,2H),7.24(d,J=3.6Hz,1H),6.77(m,1H),2.60(s,3H),2.53(d,J=1.1Hz,3H).
(E)-3-(4-羟基-3,5-二甲基苯基)-1-(4-(5-甲基噻吩-2-基)苯基)丙-2-烯-1-酮(化合物37)的制备
参照实施例9的方法,得到黄色固体,产率34%。1HNMR(400MHz,DMSO-d6)δ8.95(s,1H),8.18–8.08(m,2H),7.80–7.68(m,3H),7.62(d,J=15.4Hz,1H),7.55–7.46(m,3H),6.94–6.81(m,1H),2.50–2.46(m,3H),2.21(s,6H);13C NMR(100MHz,DMSO-d6)δ187.8,156.3,144.7,141.2,139.7,138.0,136.3,129.7,129.4,127.4,125.8,125.5,124.8,124.7,118.2,16.6,15.2;HRMS(ESI)calcd for C22H20O2S(M+H)+349.1257,found349.1261.
实施例38:
中间体N-(4-乙酰基苯基)-4-氟苯磺酰胺(中间体1j)的制备
0℃搅拌条件下,将对氨基苯乙酮(100mg,0.74mmol)溶解于1.5mL无水THF溶液中,加入吡啶(175mg,2.22mmol),再缓慢加入对氟苯磺酰氯(172mg,0.89mmol),并在室温下继续搅拌5h,然后将溶液用水稀释,用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到白色固体205mg,产率77%。1HNMR(400MHz,CDCl3)δ7.90–7.83(m,4H),7.39(s,1H),7.19–7.11(m,4H),2.54(s,3H)。
(E)-4-氟-N-(4-(3-(4-羟基-3,5-二甲基苯基)丙烯酰基)苯基)苯磺酰胺(化合物38)的制备
参照实施例9的方法,得到黄色固体,产率49%。1HNMR(400MHz,DMSO-d6)δ8.93(s,1H),8.07–7.99(m,2H),7.95–7.86(m,2H),7.62(d,J=15.4Hz,1H),7.55(d,J=15.4Hz,1H),7.49–7.37(m,4H),7.32–7.20(m,2H),2.19(s,6H);13C NMR(100MHz,DMSO-d6)δ187.4,164.6(d,J=252.3Hz),156.3,144.5,141.8,135.7(d,J=2.9Hz),133.4,130.0,129.9(d,J=9.7Hz),129.6,125.8,124.7,118.4,118.1,116.8(d,J=23.0Hz),16.6;HRMS(ESI)calcdfor C23H20FNO4S(M+H)+426.1170,found426.1175.
实施例39:
中间体N-(4-乙酰基苯基)异丙基氨基磺酰胺(中间体1k)的制备
0℃搅拌条件下,将对氨基苯乙酮(50mg,0.37mmol)溶解于2mL无水CH2Cl2溶液中,加入三乙胺(74mg,0.74mmol),再缓慢加入异丙基氨基磺酰氯(70mg,0.44mmol),并在室温下继续搅拌5h,然后将溶液用水稀释,用乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到白色固体54mg,产率57%。1HNMR(400MHz,CDCl3)δ7.98–7.86(m,2H),7.21–7.13(m,2H),7.07(s,1H),4.56(d,J=7.7Hz,1H),3.64–3.50(m,1H),2.57(s,3H),1.13(d,J=6.5Hz,6H).
(E)-3-(4-羟基-3,5-二甲基苯基)-1-(4-{[((丙-2-基)氨磺酰基]氨基}苯基)丙-2-烯-1-酮(化合物39)的制备
参照实施例9的方法,得到黄色固体,产率37%。1HNMR(400MHz,DMSO-d6)δ10.22(s,1H),8.90(s,1H),8.13–8.01(m,2H),7.77–7.64(m,2H),7.57(d,J=15.4Hz,1H),7.47(s,2H),7.29–7.17(m,2H),3.34–3.29(m,1H),2.20(s,6H),0.99(d,J=6.5Hz,6H);13C NMR(100MHz,DMSO-d6)δ187.4,156.2,144.1,143.6,131.5,129.9,129.6,125.9,124.7,118.3,116.5,45.2,23.1,16.6;HRMS(ESI)calcd for C20H24N2O4S(M+H)+389.1560,found389.1532.
实施例40:
中间体N-(4-乙酰基苯基)丙烯酰胺(中间体1l)的制备
0℃搅拌条件下,将对氨基苯乙酮(405mg,3.00mmol)溶解于7mL无水THF溶液中,加入三乙胺(413mg,4.20mmol),再缓慢加入丙烯酰氯(299mg,3.30mmol),并在室温下继续搅拌5h,然后将溶液过滤,将滤液用水和乙酸乙酯萃取,合并有机层,浓缩并通过硅胶柱纯化,得到白色固体428mg,产率75%。1HNMR(400MHz,CDCl3)δ7.99–7.89(m,3H),7.74–7.67(m,2H),6.47(dd,J=16.9,1.2Hz,1H),6.30(dd,J=16.9,10.2Hz,1H),5.81(dd,J=10.2,1.2Hz,1H),2.58(s,3H).
(E)-N-(4-(3-(4-羟基-3,5-二甲基苯基)丙烯酰基)苯基)丙烯酰胺(化合物40)的制备
参照实施例9的方法,得到黄色固体,产率66%。1HNMR(400MHz,DMSO-d6)δ10.51(s,1H),8.94(s,1H),8.17–8.11(m,2H),7.87–7.81(m,2H),7.71(d,J=15.4Hz,1H),7.60(d,J=15.4Hz,1H),7.48(s,2H),6.48(dd,J=17.0,10.0Hz,1H),6.32(dd,J=17.0,2.0Hz,1H),5.82(dd,J=10.0,2.0Hz,1H),2.20(s,6H);13C NMR(100MHz,DMSO-d6)δ187.5,163.7,156.3,144.4,143.2,133.1,131.6,129.8,129.7,128.0,125.9,124.8,118.9,118.3,16.6;HRMS(ESI)calcd for C20H19NO2(M+H)+322.1438,found 322.1437.
实施例41:1,3-二苯基丙-2-烯-1-酮衍生物对NLRP3炎症小体抑制作用的体外研究
将J774A.1细胞分到96孔板上,每个孔5×105个细胞,种板过夜,弃上清,每孔加入100μL含细菌脂多糖(LPS)(1μg/ml)的含10%血清的DMEM培养基,然后加入不同浓度的(1μM、2μM、5μM、10μM、20μM、40μM)1,3-二苯基丙-2-烯-1-酮衍生物处理1小时,再加入尼日利亚菌素(Nigericin,10μM)处理1小时,之后收集细胞上清液,采用Mouse IL-1βELISA试剂盒测定IL-1β含量,计算出本发明化合物对NLRP3炎症小体的抑制作用,结果见表1。
表1 1,3-二苯基丙-2-烯-1-酮衍生物对J774A.1细胞中NLRP3炎症小体活化介导的IL-1β释放的抑制活性(IC50,μM)
实施例42:化合物40在体外特异性抑制NLRP3炎性体的活化并抑制细胞焦亡
1、NLRP3炎症小体激活及IL-1β检测:将J774A.1细胞或小鼠骨髓来源的巨噬细胞(BMDMs)分到96孔板上,每个孔5×105个细胞,种板过夜,弃上清,每孔加入100μL含细菌脂多糖(LPS)(1μg/ml)的含10%血清的DMEM培养基,然后加入不同浓度的(1μM、2μM、5μM)化合物40处理1h,再加入尼日利亚菌素(Nigericin,10μM)处理1h,之后收集细胞上清液,采用Mouse IL-1βELISA试剂盒测定IL-1β含量。
2、细胞焦亡检测:如前所述处理J774A.1细胞,然后使用LDH检测试剂盒评估细胞上清液中LDH的释放。
3、蛋白质免疫印迹分析:将步骤1处理后的J774A.1细胞样品在带有蛋白酶抑制剂的RIPA裂解缓冲液中于4℃裂解30min。裂解液或上清液中的蛋白质用12%SDS-聚丙烯酰胺凝胶分离,转移到PVDF膜上,并与抗鼠IL-1β抗体,抗ASC抗体,抗casepase-1抗体,抗NLRP3抗体,抗β-actin抗体进行蛋白免疫印迹分析。
4、NLRC4和AIM2炎症小体激活及IL-1β检测:对于NLRC4或AIM2炎性体激活,用1μg/mLLPS刺激J774A.1细胞5h,然后加入不同浓度的(1μM、2μM、5μM)化合物40处理1h,然后用细菌鞭毛蛋白(FLA-STUltrapure)(2.5μg/mL)感染细胞4h,或用poly(dA:dT)(0.25μg/mL)转染细胞4h,之后收集细胞上清液,采用Mouse IL-1βELISA试剂盒测定IL-1β含量。
结果如图1所示,从图1的结果来看,在NLRP3炎症小体激活的J774A.1和BMDMs细胞模型中,化合物40可以浓度依赖性地抑制IL-1β的分泌(图1中的A图和B图)。蛋白质免疫印迹实验显示化合物40抑制caspase-1p20成熟和IL-1β分泌的作用呈剂量依赖性,但不影响细胞裂解物中的pro-IL-1β,pro-caspase-1,NLRP3或ASC(图1中的C图)。化合物40还可以抑制LPS/尼日利亚霉素诱导的细胞焦亡(图1中的D图和E图)。与此同时,化合物40对AIM2或NLRC4炎性小体的激活没有抑制作用(图1中的F图)。以上结果表明,化合物40可以特异性抑制NLRP3炎性小体依赖性的caspase-1激活和IL-1β分泌。
实施例43:化合物40抑制LPS诱导的急性腹膜炎
1、6-8周雌性C57BL/6小鼠分成4组,每组6只,具体分组处理如下:
第一组:连续三天灌胃空白媒介物;
第二组:连续三天灌胃空白媒介物,第三天腹腔内注射LPS(20mg/kg);
第三组:连续三天灌胃化合物40(25mg/kg/天,第三天腹腔内注射LPS(20mg/kg);
第四组:连续三天灌胃化合物40(100mg/kg/天),第三天腹腔内注射LPS(20mg/kg);
2、LPS腹腔注射6h后,收集血液,同时对每组小鼠腹腔灌注1mlPBS,取腹腔灌洗液,对血液和腹腔灌洗液离心。
3、将步骤2所得血液上清和腹腔灌洗液上清用ELISA的方法进行IL-1β含量测定,结果见图2。
从图2的结果看,注射LPS后,小鼠血液中和腹腔液中的IL-1β含量明显上升,而灌胃化合物40可以剂量依赖性降低IL-1β水平,表明化合物40可以有效抑制LPS诱导的急性腹膜炎。
实施例44:化合物40改善硫酸葡聚糖钠(DSS)诱导的结肠炎
1、6-8周雌性C57BL/6小鼠分成4组,每组5只,具体分组处理如下:
第一组:第1-10天在饮食中给予蒸馏水,同时每天灌胃媒介物;
第二组:第1-3天饮食中给予蒸馏水,第4-10天开始每天在饮食中给予2%DSS的蒸馏水,同时每天灌胃媒介物;
第三组:连续10天灌胃化合物40(25mg/kg/天),第四天开始每天在饮食中给予2%DSS的蒸馏水;
第四组:连续10天灌胃化合物40(100mg/kg/天),第四天开始每天在饮食中给予2%DSS的蒸馏水;
2、从给药后开始每天监测每组小鼠的便血程度和体重,并在第11天取小鼠结肠进行长度测量及对结肠中的IL-1β含量测定。
结果如图3所示,从图3的结果来看,在饮食中给予2%DSS后,小鼠的便血严重程度增加,而且小鼠结肠长度缩短,结肠中IL-1β显著升高,灌胃化合物40可以剂量依赖性改善小鼠便血,改善结肠长度缩短,降低结肠中IL-1β水平。
活性测试结果表明,本发明的1,3-二苯基丙-2-烯-1-酮类化合物对受试细胞J774A.1细胞显示出抑制NLRP3炎症小体的活性。同时代表性化合物40可以选择性抑制NLRP3炎症小体的激活,同时可以改善LPS诱导的急性腹膜炎和DSS诱导的结肠炎,可见,本发明的1,3-二苯基丙-2-烯-1-酮类化合物具有用于治疗NLRP3炎症小体相关疾病的用途。
以上所述实施例的各技术特征可以进行任意的组合,为使描述简洁,未对以下实施例中的各个技术特征所有可能的组合都进行描述,然而,只要这些技术特征的组合不存在矛盾,都应当认为是本说明书记载的范围。
以上所述实施例仅表达了本发明的几种实施方式,其描述较为具体和详细,但并不能因此而理解为对本发明专利范围的限制。应当指出的是,对于本领域的普通技术人员来说,在不脱离本发明构思的前提下,还可以做出若干变形和改进,这些都属于本发明的保护范围。因此,本发明专利的保护范围应以所附权利要求为准。
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110317148.8A CN113230240B (zh) | 2021-03-22 | 2021-03-22 | 1,3-二苯基丙-2-烯-1-酮衍生物及其应用 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110317148.8A CN113230240B (zh) | 2021-03-22 | 2021-03-22 | 1,3-二苯基丙-2-烯-1-酮衍生物及其应用 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113230240A CN113230240A (zh) | 2021-08-10 |
| CN113230240B true CN113230240B (zh) | 2022-12-27 |
Family
ID=77130715
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110317148.8A Active CN113230240B (zh) | 2021-03-22 | 2021-03-22 | 1,3-二苯基丙-2-烯-1-酮衍生物及其应用 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113230240B (zh) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116554050A (zh) * | 2022-01-28 | 2023-08-08 | 四川大学 | 酰胺苯酚类衍生物及其用途 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019094777A1 (en) * | 2017-11-13 | 2019-05-16 | Gilead Sciences, Inc. | Compositions and methods for identifying and treating liver diseases and monitoring treatment outcomes |
| CN112424207A (zh) * | 2018-07-25 | 2021-02-26 | 诺华股份有限公司 | Nlrp3炎性小体抑制剂 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2841900B1 (fr) * | 2002-07-08 | 2007-03-02 | Genfit S A | Nouveaux derives de 1,3-diphenylprop-2-en-1-one substitues, preparation et utilisations |
| US20040181075A1 (en) * | 2002-12-19 | 2004-09-16 | Weingarten M. David | Process of making chalcone derivatives |
| FR2857361B1 (fr) * | 2003-07-08 | 2005-09-09 | Genfit S A | PREPARATION DE DERIVES DE 1,3-DIPHENYPROP-2-¼n-1-one |
| CN111848461A (zh) * | 2019-04-29 | 2020-10-30 | 苏州大学 | Nlrp3炎症小体抑制剂及其制备方法和应用 |
| CA3144430A1 (en) * | 2019-07-16 | 2021-01-21 | Shijun Zhang | Compounds as nlrp3 inflammasome inhibitors and compositions and uses thereof |
-
2021
- 2021-03-22 CN CN202110317148.8A patent/CN113230240B/zh active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019094777A1 (en) * | 2017-11-13 | 2019-05-16 | Gilead Sciences, Inc. | Compositions and methods for identifying and treating liver diseases and monitoring treatment outcomes |
| CN112424207A (zh) * | 2018-07-25 | 2021-02-26 | 诺华股份有限公司 | Nlrp3炎性小体抑制剂 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113230240A (zh) | 2021-08-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN105358550A (zh) | 新型组蛋白脱乙酰基酶抑制剂 | |
| JP4880678B2 (ja) | 肝臓のカルニチンパルミトイルトランスフェラーゼ(l−cpt1)阻害剤として有用なスルホンアミド誘導体 | |
| CN110101700A (zh) | 作为丝氨酸蛋白酶抑制剂的多取代芳族化合物的制药用途 | |
| JP5898230B2 (ja) | S−ニトロソグルタチオンレダクターゼ阻害薬としての新規な置換二環芳香族化合物 | |
| AU2006212761A1 (en) | Combination therapy | |
| PT1258484E (pt) | Novos compostos de isoxazol e de tiazol e sua utilização enquanto fármacos | |
| JP2003507372A (ja) | 糖尿病を処置するための安息香酸誘導体 | |
| JP2012153726A (ja) | 新規3−ヒドロキシ−5−アリールイソチアゾール誘導体 | |
| JP7344959B2 (ja) | マトリックスメタロプロテイナーゼ(mmp)阻害剤及びその使用方法 | |
| KR100710958B1 (ko) | 데하이드로 아미노산 | |
| TW202325702A (zh) | 新穎之酸分泌抑制劑及其用途 | |
| JP2004511456A (ja) | 糖尿病用薬剤 | |
| JP2021519312A (ja) | カルパインモジュレーター及びその治療的使用 | |
| EP1783116B1 (en) | 2-phenylpyridine derivative | |
| JP2021529193A (ja) | カルパインインヒビターを用いて肝線維症を処置する方法 | |
| CN113230240B (zh) | 1,3-二苯基丙-2-烯-1-酮衍生物及其应用 | |
| HU193348B (en) | Process for producing piroxicam salts of antiflogistic activity | |
| WO2020108661A1 (zh) | 作为cdk-hdac双通路抑制剂的杂环化合物 | |
| KR20210097100A (ko) | 2-(1-아실옥시-n-펜틸)벤조산 및 염기성 아미노산 또는 아미노구아니딘이 형성하는 염, 이의 제조 방법 및 용도 | |
| HUT69690A (en) | Pharmaceutical compositions containing 6-chloro-5-fluoro-3-(2-thenoyl)-2-oxindole-1-carboxamide having analgesic and antiinflammatory effect | |
| AU2005202553A1 (en) | Nitrosated and nitrosylated proton pump inhibitors, compositions and methods of use | |
| KR20040027927A (ko) | 심장 및 대사 장애의 치료를 위해 주요 환이 치환된갑상선 수용체 길항근 | |
| JP2002053557A (ja) | アポリポ蛋白a−i産生促進薬 | |
| WO2017198179A1 (zh) | 新型噻唑类衍生物在治疗炎性肠病中的应用 | |
| HU199785B (en) | Process for producing pyrrolidine-2-(1,3-dicarbonyl) derivatives and pharmaceutical compositions comprising same as active ingredient |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |