CN113173857A - Cannabidiol derivative and preparation method and application thereof - Google Patents
Cannabidiol derivative and preparation method and application thereof Download PDFInfo
- Publication number
- CN113173857A CN113173857A CN202110248495.XA CN202110248495A CN113173857A CN 113173857 A CN113173857 A CN 113173857A CN 202110248495 A CN202110248495 A CN 202110248495A CN 113173857 A CN113173857 A CN 113173857A
- Authority
- CN
- China
- Prior art keywords
- compound
- cannabidiol
- derivative
- nmr
- magnetic resonance
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- QHMBSVQNZZTUGM-ZWKOTPCHSA-N cannabidiol Chemical class OC1=CC(CCCCC)=CC(O)=C1[C@H]1[C@H](C(C)=C)CCC(C)=C1 QHMBSVQNZZTUGM-ZWKOTPCHSA-N 0.000 title claims abstract description 45
- 238000002360 preparation method Methods 0.000 title abstract description 7
- QHMBSVQNZZTUGM-UHFFFAOYSA-N Trans-Cannabidiol Natural products OC1=CC(CCCCC)=CC(O)=C1C1C(C(C)=C)CCC(C)=C1 QHMBSVQNZZTUGM-UHFFFAOYSA-N 0.000 claims description 19
- 229950011318 cannabidiol Drugs 0.000 claims description 18
- ZTGXAWYVTLUPDT-UHFFFAOYSA-N cannabidiol Natural products OC1=CC(CCCCC)=CC(O)=C1C1C(C(C)=C)CC=C(C)C1 ZTGXAWYVTLUPDT-UHFFFAOYSA-N 0.000 claims description 18
- PCXRACLQFPRCBB-ZWKOTPCHSA-N dihydrocannabidiol Natural products OC1=CC(CCCCC)=CC(O)=C1[C@H]1[C@H](C(C)C)CCC(C)=C1 PCXRACLQFPRCBB-ZWKOTPCHSA-N 0.000 claims description 17
- 239000003814 drug Substances 0.000 claims description 11
- 238000004519 manufacturing process Methods 0.000 claims description 8
- 238000000034 method Methods 0.000 claims description 6
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 5
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid group Chemical group C(C1=CC=CC=C1)(=O)O WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims description 4
- 230000000259 anti-tumor effect Effects 0.000 claims description 3
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N phenol group Chemical group C1(=CC=CC=C1)O ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 claims description 3
- 239000005711 Benzoic acid Substances 0.000 claims description 2
- 235000010233 benzoic acid Nutrition 0.000 claims description 2
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 27
- 150000001875 compounds Chemical class 0.000 description 23
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- 238000001228 spectrum Methods 0.000 description 12
- 210000004027 cell Anatomy 0.000 description 11
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 10
- 238000005481 NMR spectroscopy Methods 0.000 description 10
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 10
- 238000005160 1H NMR spectroscopy Methods 0.000 description 8
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 8
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 8
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 8
- 229940125898 compound 5 Drugs 0.000 description 8
- 229940079593 drug Drugs 0.000 description 8
- 239000011541 reaction mixture Substances 0.000 description 8
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 8
- 239000007832 Na2SO4 Substances 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 238000001035 drying Methods 0.000 description 7
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 7
- 229910052938 sodium sulfate Inorganic materials 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 6
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 229940125904 compound 1 Drugs 0.000 description 6
- 229940125782 compound 2 Drugs 0.000 description 6
- 229940126214 compound 3 Drugs 0.000 description 6
- 229910052739 hydrogen Inorganic materials 0.000 description 6
- 239000001257 hydrogen Substances 0.000 description 6
- 239000002994 raw material Substances 0.000 description 6
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 5
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 5
- 229910052799 carbon Inorganic materials 0.000 description 5
- 239000000706 filtrate Substances 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- OKKJLVBELUTLKV-VMNATFBRSA-N methanol-d1 Chemical compound [2H]OC OKKJLVBELUTLKV-VMNATFBRSA-N 0.000 description 5
- -1 methoxy, methyl Chemical group 0.000 description 5
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 5
- 239000012044 organic layer Substances 0.000 description 5
- 230000004224 protection Effects 0.000 description 5
- 238000010898 silica gel chromatography Methods 0.000 description 5
- 238000003756 stirring Methods 0.000 description 5
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 4
- 206010006187 Breast cancer Diseases 0.000 description 4
- 208000026310 Breast neoplasm Diseases 0.000 description 4
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 4
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 4
- 201000011510 cancer Diseases 0.000 description 4
- 238000012258 culturing Methods 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 229920006395 saturated elastomer Polymers 0.000 description 4
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- HBENZIXOGRCSQN-VQWWACLZSA-N (1S,2S,6R,14R,15R,16R)-5-(cyclopropylmethyl)-16-[(2S)-2-hydroxy-3,3-dimethylpentan-2-yl]-15-methoxy-13-oxa-5-azahexacyclo[13.2.2.12,8.01,6.02,14.012,20]icosa-8(20),9,11-trien-11-ol Chemical compound N1([C@@H]2CC=3C4=C(C(=CC=3)O)O[C@H]3[C@@]5(OC)CC[C@@]2([C@@]43CC1)C[C@@H]5[C@](C)(O)C(C)(C)CC)CC1CC1 HBENZIXOGRCSQN-VQWWACLZSA-N 0.000 description 3
- VIMMECPCYZXUCI-MIMFYIINSA-N (4s,6r)-6-[(1e)-4,4-bis(4-fluorophenyl)-3-(1-methyltetrazol-5-yl)buta-1,3-dienyl]-4-hydroxyoxan-2-one Chemical compound CN1N=NN=C1C(\C=C\[C@@H]1OC(=O)C[C@@H](O)C1)=C(C=1C=CC(F)=CC=1)C1=CC=C(F)C=C1 VIMMECPCYZXUCI-MIMFYIINSA-N 0.000 description 3
- IOOMXAQUNPWDLL-UHFFFAOYSA-N 2-[6-(diethylamino)-3-(diethyliminiumyl)-3h-xanthen-9-yl]-5-sulfobenzene-1-sulfonate Chemical compound C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=C(S(O)(=O)=O)C=C1S([O-])(=O)=O IOOMXAQUNPWDLL-UHFFFAOYSA-N 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 239000001963 growth medium Substances 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- SHAHPWSYJFYMRX-GDLCADMTSA-N (2S)-2-(4-{[(1R,2S)-2-hydroxycyclopentyl]methyl}phenyl)propanoic acid Chemical compound C1=CC([C@@H](C(O)=O)C)=CC=C1C[C@@H]1[C@@H](O)CCC1 SHAHPWSYJFYMRX-GDLCADMTSA-N 0.000 description 2
- PHDIJLFSKNMCMI-ITGJKDDRSA-N (3R,4S,5R,6R)-6-(hydroxymethyl)-4-(8-quinolin-6-yloxyoctoxy)oxane-2,3,5-triol Chemical compound OC[C@@H]1[C@H]([C@@H]([C@H](C(O1)O)O)OCCCCCCCCOC=1C=C2C=CC=NC2=CC=1)O PHDIJLFSKNMCMI-ITGJKDDRSA-N 0.000 description 2
- IGVKWAAPMVVTFX-BUHFOSPRSA-N (e)-octadec-5-en-7,9-diynoic acid Chemical compound CCCCCCCCC#CC#C\C=C\CCCC(O)=O IGVKWAAPMVVTFX-BUHFOSPRSA-N 0.000 description 2
- JNPGUXGVLNJQSQ-BGGMYYEUSA-M (e,3r,5s)-7-[4-(4-fluorophenyl)-1,2-di(propan-2-yl)pyrrol-3-yl]-3,5-dihydroxyhept-6-enoate Chemical compound CC(C)N1C(C(C)C)=C(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)C(C=2C=CC(F)=CC=2)=C1 JNPGUXGVLNJQSQ-BGGMYYEUSA-M 0.000 description 2
- HIHOEGPXVVKJPP-JTQLQIEISA-N 5-fluoro-2-[[(1s)-1-(5-fluoropyridin-2-yl)ethyl]amino]-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyridine-3-carbonitrile Chemical compound N([C@@H](C)C=1N=CC(F)=CC=1)C(C(=CC=1F)C#N)=NC=1NC=1C=C(C)NN=1 HIHOEGPXVVKJPP-JTQLQIEISA-N 0.000 description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 2
- 208000002454 Nasopharyngeal Carcinoma Diseases 0.000 description 2
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- KCXMKQUNVWSEMD-UHFFFAOYSA-N benzyl chloride Chemical compound ClCC1=CC=CC=C1 KCXMKQUNVWSEMD-UHFFFAOYSA-N 0.000 description 2
- 239000012230 colorless oil Substances 0.000 description 2
- 239000012043 crude product Substances 0.000 description 2
- 238000010520 demethylation reaction Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 201000005202 lung cancer Diseases 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- GVOISEJVFFIGQE-YCZSINBZSA-N n-[(1r,2s,5r)-5-[methyl(propan-2-yl)amino]-2-[(3s)-2-oxo-3-[[6-(trifluoromethyl)quinazolin-4-yl]amino]pyrrolidin-1-yl]cyclohexyl]acetamide Chemical compound CC(=O)N[C@@H]1C[C@H](N(C)C(C)C)CC[C@@H]1N1C(=O)[C@@H](NC=2C3=CC(=CC=C3N=CN=2)C(F)(F)F)CC1 GVOISEJVFFIGQE-YCZSINBZSA-N 0.000 description 2
- 201000011216 nasopharynx carcinoma Diseases 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- RWWYLEGWBNMMLJ-YSOARWBDSA-N remdesivir Chemical compound NC1=NC=NN2C1=CC=C2[C@]1([C@@H]([C@@H]([C@H](O1)CO[P@](=O)(OC1=CC=CC=C1)N[C@H](C(=O)OCC(CC)CC)C)O)O)C#N RWWYLEGWBNMMLJ-YSOARWBDSA-N 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- FANCTJAFZSYTIS-IQUVVAJASA-N (1r,3s,5z)-5-[(2e)-2-[(1r,3as,7ar)-7a-methyl-1-[(2r)-4-(phenylsulfonimidoyl)butan-2-yl]-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol Chemical compound C([C@@H](C)[C@@H]1[C@]2(CCCC(/[C@@H]2CC1)=C\C=C\1C([C@@H](O)C[C@H](O)C/1)=C)C)CS(=N)(=O)C1=CC=CC=C1 FANCTJAFZSYTIS-IQUVVAJASA-N 0.000 description 1
- VUDZSIYXZUYWSC-DBRKOABJSA-N (4r)-1-[(2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-1,3-diazinan-2-one Chemical compound FC1(F)[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)N[C@H](O)CC1 VUDZSIYXZUYWSC-DBRKOABJSA-N 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- MZSAMHOCTRNOIZ-UHFFFAOYSA-N 3-[4-(aminomethyl)-6-(trifluoromethyl)pyridin-2-yl]oxy-N-phenylaniline Chemical compound NCC1=CC(=NC(=C1)C(F)(F)F)OC=1C=C(NC2=CC=CC=C2)C=CC=1 MZSAMHOCTRNOIZ-UHFFFAOYSA-N 0.000 description 1
- APKFDSVGJQXUKY-KKGHZKTASA-N Amphotericin-B Natural products O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1C=CC=CC=CC=CC=CC=CC=C[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-KKGHZKTASA-N 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 244000025254 Cannabis sativa Species 0.000 description 1
- 235000008697 Cannabis sativa Nutrition 0.000 description 1
- BUDQDWGNQVEFAC-UHFFFAOYSA-N Dihydropyran Chemical compound C1COC=CC1 BUDQDWGNQVEFAC-UHFFFAOYSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 1
- 101100272976 Panax ginseng CYP716A53v2 gene Proteins 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 229940125907 SJ995973 Drugs 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- YLEIFZAVNWDOBM-ZTNXSLBXSA-N ac1l9hc7 Chemical compound C([C@H]12)C[C@@H](C([C@@H](O)CC3)(C)C)[C@@]43C[C@@]14CC[C@@]1(C)[C@@]2(C)C[C@@H]2O[C@]3(O)[C@H](O)C(C)(C)O[C@@H]3[C@@H](C)[C@H]12 YLEIFZAVNWDOBM-ZTNXSLBXSA-N 0.000 description 1
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 1
- 229960003942 amphotericin b Drugs 0.000 description 1
- 230000036592 analgesia Effects 0.000 description 1
- 230000000561 anti-psychotic effect Effects 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 229940073608 benzyl chloride Drugs 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229930003827 cannabinoid Natural products 0.000 description 1
- 239000003557 cannabinoid Substances 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 206010015037 epilepsy Diseases 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 229910052736 halogen Chemical group 0.000 description 1
- 150000002367 halogens Chemical group 0.000 description 1
- 150000002431 hydrogen Chemical group 0.000 description 1
- 230000005918 in vitro anti-tumor Effects 0.000 description 1
- 239000013067 intermediate product Substances 0.000 description 1
- 230000003340 mental effect Effects 0.000 description 1
- COTNUBDHGSIOTA-UHFFFAOYSA-N meoh methanol Chemical compound OC.OC COTNUBDHGSIOTA-UHFFFAOYSA-N 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 239000011259 mixed solution Substances 0.000 description 1
- XZMHJYWMCRQSSI-UHFFFAOYSA-N n-[5-[2-(3-acetylanilino)-1,3-thiazol-4-yl]-4-methyl-1,3-thiazol-2-yl]benzamide Chemical compound CC(=O)C1=CC=CC(NC=2SC=C(N=2)C2=C(N=C(NC(=O)C=3C=CC=CC=3)S2)C)=C1 XZMHJYWMCRQSSI-UHFFFAOYSA-N 0.000 description 1
- 230000004112 neuroprotection Effects 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- ZDYVRSLAEXCVBX-UHFFFAOYSA-N pyridinium p-toluenesulfonate Chemical compound C1=CC=[NH+]C=C1.CC1=CC=C(S([O-])(=O)=O)C=C1 ZDYVRSLAEXCVBX-UHFFFAOYSA-N 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- UIIMBOGNXHQVGW-UHFFFAOYSA-M sodium bicarbonate Substances [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000007447 staining method Methods 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C69/00—Esters of carboxylic acids; Esters of carbonic or haloformic acids
- C07C69/76—Esters of carboxylic acids having a carboxyl group bound to a carbon atom of a six-membered aromatic ring
- C07C69/84—Esters of carboxylic acids having a carboxyl group bound to a carbon atom of a six-membered aromatic ring of monocyclic hydroxy carboxylic acids, the hydroxy groups and the carboxyl groups of which are bound to carbon atoms of a six-membered aromatic ring
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C69/00—Esters of carboxylic acids; Esters of carbonic or haloformic acids
- C07C69/62—Halogen-containing esters
- C07C69/65—Halogen-containing esters of unsaturated acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C69/00—Esters of carboxylic acids; Esters of carbonic or haloformic acids
- C07C69/76—Esters of carboxylic acids having a carboxyl group bound to a carbon atom of a six-membered aromatic ring
- C07C69/84—Esters of carboxylic acids having a carboxyl group bound to a carbon atom of a six-membered aromatic ring of monocyclic hydroxy carboxylic acids, the hydroxy groups and the carboxyl groups of which are bound to carbon atoms of a six-membered aromatic ring
- C07C69/92—Esters of carboxylic acids having a carboxyl group bound to a carbon atom of a six-membered aromatic ring of monocyclic hydroxy carboxylic acids, the hydroxy groups and the carboxyl groups of which are bound to carbon atoms of a six-membered aromatic ring with etherified hydroxyl groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/12—Systems containing only non-condensed rings with a six-membered ring
- C07C2601/16—Systems containing only non-condensed rings with a six-membered ring the ring being unsaturated
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
The invention discloses a cannabidiol derivative and a preparation method and application thereof, belonging to the technical field of pharmaceutical chemistry.
Description
Technical Field
The invention belongs to the technical field of medicinal chemistry, and particularly relates to a cannabidiol derivative, a preparation method thereof and application thereof in tumor inhibition.
Background
The incidence of cancer and the mortality rate are rapidly increasing globally, and cancer is the leading cause of human death in the 21 st century and is one of the most important obstacles for extending the life expectancy of people. Cannabidiol ((-) -cannabidiol, CBD) is one of the major components of the unique cannabinoid component in Cannabis sativa L plants. The research shows that the cannabidiol has no mental activity, but has various pharmacological effects, such as neuroprotection, epilepsy resistance, anxiety resistance, antipsychotic, analgesia, anti-inflammation, asthma treatment and the like.
Disclosure of Invention
The invention mainly provides cannabidiol derivatives with novel structures, a preparation method thereof and application thereof in tumor inhibition.
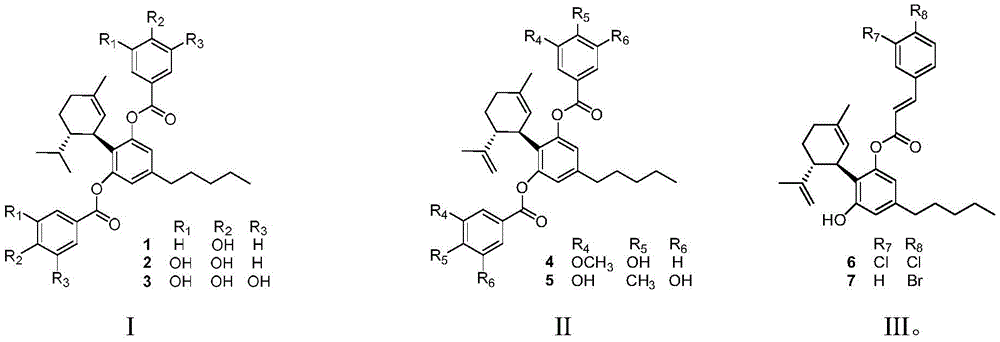
The structure of the cannabidiol derivative is shown as I, II and III:
wherein R in I, II and III1,R2,R3,R4,R5,R6,R7And R8Can be a substituent such as hydrogen, hydroxyl, methoxy, methyl or halogen.
The cannabidiol derivatives I, II and III are obtained by using cannabidiol as a basic skeleton and esterifying phenolic hydroxyl groups of the cannabidiol derivatives with benzoic acid substituted by different groups, and the obtained cannabidiol derivatives are new compounds.
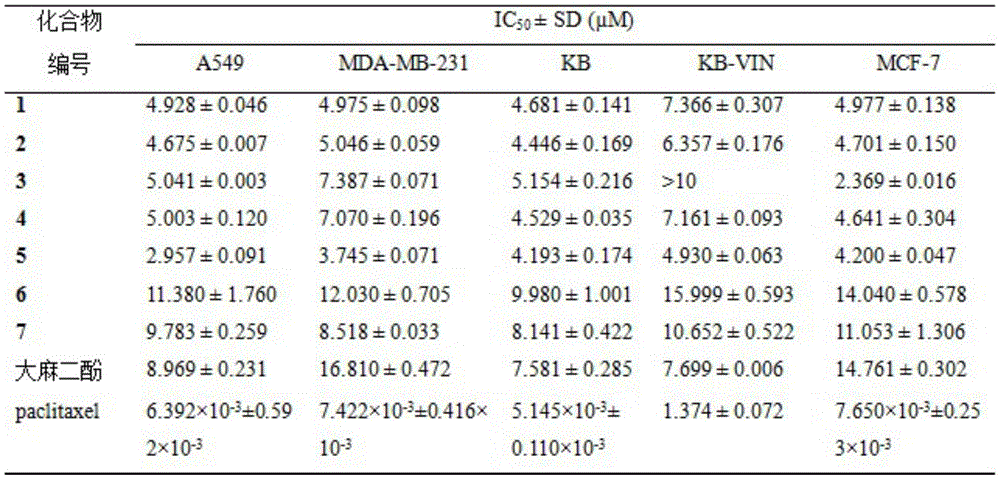
The in vitro anti-tumor activity proves that the cannabidiol derivative has obvious inhibitory activity on lung cancer cell strains (A549), human breast cancer cell strains (MDA-MB-231), nasopharyngeal carcinoma and drug-resistant strains (KB, KB-VIN) thereof and human breast cancer cell strains (MCF-7), wherein the activity of the compound 5 is most obvious, and IC (integrated Circuit) is shown in the specification50The value is between 2.96 and 4.93. mu.M.
The invention has the advantages and positive effects that:
(1) the cannabidiol derivative provided by the invention takes cannabidiol as a parent nucleus and carries out the treatment on phenolic hydroxyl group of the cannabidiol derivativeReasonably modifying to construct a cannabidiol derivative with novel structure, and obtaining the product with chemical structure1H NMR,13C NMR and MS confirmation.
(2) The cannabidiol derivative compound 1-5 provided by the invention has stronger inhibiting effect on 5 cancer cell lines (A549, MDA-MB-231, KB, KB-VIN and MCF-7) than cannabidiol, and is expected to be developed into a new anti-tumor drug.
Drawings
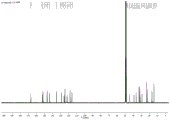
FIG. 1 shows CD of Compound 1 of the present invention3Nuclear magnetic resonance hydrogen spectrum in OD;
FIG. 2 shows CD of Compound 1 of the present invention3Nuclear magnetic resonance carbon spectrum in OD;
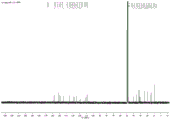
FIG. 3 shows CD of Compound 2 of the present invention3Nuclear magnetic resonance hydrogen spectrum in OD;
FIG. 4 shows CD of Compound 2 of the present invention3Nuclear magnetic resonance carbon spectrum in OD;
FIG. 5 shows CD of Compound 3 of the present invention3Nuclear magnetic resonance hydrogen spectrum in OD;
FIG. 6 shows CD of Compound 3 of the present invention3Nuclear magnetic resonance carbon spectrum in OD;
FIG. 7 shows CD of Compound 4 of the present invention3Nuclear magnetic resonance hydrogen spectrum in OD;
FIG. 8 shows CD of Compound 4 of the present invention3Nuclear magnetic resonance carbon spectrum in OD;
FIG. 9 shows CD of Compound 5 of the present invention3Nuclear magnetic resonance hydrogen spectrum in OD;
FIG. 10 shows CD of Compound 5 of the present invention3Nuclear magnetic resonance carbon spectrum in OD.
Detailed Description
The present invention will be described in further detail below with reference to specific embodiments and drawings, and the protected contents of the present invention include, but are not limited to, the following embodiments. All other embodiments, which can be derived by a person skilled in the art from the embodiments given herein without making any inventive effort, are within the scope of the present invention. The procedures, conditions, reagents, experimental methods, etc. in the following examples are all common general knowledge in the art, except for those specifically mentioned below. The reagents used in the invention are all commercially available chemically pure or analytically pure products.
The cannabidiol derivatives have the structures shown as I, II and III:
the synthesis of the cannabidiol derivative comprises the following 3 routes:
route 1:
taking 1a and 2a as raw materials, performing methylation reaction, benzyl protection and demethylation reaction on the raw materials, esterifying the raw materials with cannabidiol, removing benzyl protection to obtain a compound 1 and a compound 2, taking 3a as a raw material, performing benzyl protection and demethylation reaction on the raw materials, esterifying the raw materials with cannabidiol, and removing benzyl protection to obtain a compound 3.
Route 2:
the compound 4a and 5a are respectively reacted with 3, 4-Dihydropyran (DHP) to protect the hydroxyl group, then respectively reacted with cannabidiol to obtain intermediate products 4c and 5c, and finally deprotected by 2-Tetrahydropyran (THP) to obtain compound 4 and compound 5.
Route 3:
cannabidiol reacts with 6a and 7a under the action of CMPI and DMAP to give compound 6 and compound 7, respectively.
Example 1
A process for preparing cannabidiol derivative compound 1, comprising the steps of:
step 3. Compound 1c (14.88mmol) is added to methanol (MeOH, 20mL), dioxane (40mL) and H2To a mixed solution of O (15mL), NaOH (0.50mol) was added, the reaction was stirred overnight, the reaction mixture was refluxed at 120 ℃ overnight, the solvent was removed by concentration under reduced pressure, the residue was extracted with DCM, the organic layer was washed with saturated brine, and anhydrous Na2SO4Drying, filtering and concentrating in vacuo to give compound 1d (2.69 g);
step 4, adding the compound 1d (0.96mmol) and cannabidiol (0.48mmol) into DCM (15mL) solution at 0 ℃ under the protection of nitrogen, stirring, adding CMPI (0.96mmol) and DMAP (0.96mmol) into the reaction solution, stirring the reaction mixture for reaction for 1 hour, filtering the reaction precipitate, and sequentially using saturated NaHCO for the filtrate3Washing with saturated brine, and purifying with Na2SO4Drying, vacuum concentrating, and purifying by silica gel column chromatography (n-hexane: acetone, volume ratio 95:5) to obtain compound 1e (110.4 mg);
The nuclear magnetic resonance spectrum of the compound 1 is shown in figures 1 and 2, and the data are as follows:1H NMR(400MHz,CD3OD)δH 7.99(d,J=8.7Hz,4H),6.88(d,J=8.8Hz,4H),6.83(s,2H),5.11(s,1H),3.45(d,J=10.5Hz,1H),2.63-2.59(m,2H),1.97-1.91(m,1H),1.71-1.59(overlap,5H),1.55-1.50(m,1H),1.36-1.35(m,4H),1.23(s,3H),1.17-1.06(m,1H),0.90(t,J=6.7Hz,3H),0.82(d,J=6.8Hz,3H),0.65(d,J=6.8Hz,3H).13C NMR(100MHz,CH3OD):δC 166.7,166.7,164.3,164.3,151.7,151.7,143.5,134.7,133.6,133.6,133.6,133.6,128.7,128.7,125.5,125.5,121.2,121.2,116.4,116.4,116.4,116.4,44.4,39.1,36.1,32.6,31.8,31.4,29.1,23.5,23.5,23.4,22.0,16.8,14.4.ESI-MS:m/z 555.80[M-H]-。
the formula of the compound obtained in this example was derived as follows:
example 2
A process for preparing cannabidiol derivative compound 2, comprising the steps of:
by substituting compound 1a in example 1 with compound 2a and keeping the other reagents unchanged, 34.2mg of a colorless oil, compound 2, was prepared according to the method of example 1, with a final yield of 96.89%.
The nuclear magnetic resonance spectrum of the compound 2 is shown in figures 3 and 4, and the data are as follows:1H NMR(400MHz,CD3OD)δH 7.57-7.55(overlap,4H),6.87(d,J=8.8Hz,2H),6.83(s,2H),5.09(s,1H),3.46(d,J=10.6Hz,1H),2.64-2.60(m,2H),2.01-1.95(m,1H),1.70-1.63(overlap,5H),1.57-1.50(m,1H),1.39-1.34(m,4H),1.24(s,3H),1.19-1.09(m,1H),0.91(t,J=6.8Hz,3H),0.85(d,J=6.8Hz,3H),0.67(d,J=6.8Hz,3H).13C NMR(100MHz,CH3OD):δC 166.9,166.9,152.4,152.4,151.8,151.8,146.4,146.4,143.5,134.9,128.8,128.8,125.4,125.4,124.5,124.5,121.7,121.7,118.0,118.0,115.9,115.9,44.3,39.0,36.1,32.6,31.8,31.3,29.1,23.5,23.5,23.5,22.0,16.8,14.4.ESI-MS:m/z 587.75[M-H]-。
the formula of the compound obtained in this example was derived as follows:
example 3
A process for preparing cannabidiol derivative compound 3, comprising the steps of:
the preparation was carried out by following the steps 2 to 5 in example 1 except for replacing the compound 1b in example 1 with the compound 3a and leaving the other reagents unchanged, to obtain 98.1mg of a pale purple powder, i.e., the compound 3, in 93.07% yield as the final step.
The nuclear magnetic resonance spectrum of the compound 3 is shown in fig. 5 and 6, and the data is as follows:1H NMR(400MHz,CD3OD)δH 7.19(s,4H),6.81(s,2H),5.07(s,1H),3.47(d,J=10.5Hz,1H),2.65-2.61(m,2H),2.04-1.99(m,1H),1.83-1.71(m,2H),1.68-1.64(m,3H),1.58-1.49(s,1H),1.40-1.34(m,4H),1.24(s,3H),1.21-1.10(m,1H),0.92(t,J=6.6Hz,3H),0.86(d,J=6.8Hz,3H),0.68(d,J=6.8Hz,3H).13C NMR(100MHz,CH3OD):δC 167.1,167.1,151.8,151.8,146.6,146.6,146.6,146.6,143.4,140.7,140.7,135.1,128.9,128.9,125.2,125.2,120.4,120.4,110.8,110.8,110.8,110.8,44.3,39.0,36.2,32.6,31.8,31.3,29.2,23.6,23.6,23.5,22.0,16.9,14.4.ESI-MS:m/z 619.65[M-H]-。
the formula of the compound obtained in this example was derived as follows:
example 4
A process for preparing cannabidiol derivative compound 4 comprising the steps of:
step 3. adding the compound 4c (0.36mmol) and p-toluenesulfonic acid (PTSA, 0.01mmol) into 25mL of methanol at room temperature, stirring for reaction for 4 hours, concentrating under reduced pressure, and then adding NaHCO with the mass fraction of 5%3Neutralizing the solution, extracting with ethyl acetate, washing the organic layer with water and saturated brine, and passing through anhydrous Na2SO4Drying and distillation under the reduced pressure gave compound 4(204.0mg, yield: 92.25%) as a pale brown solid.
The nuclear magnetic resonance spectrum of the compound 4 is shown in fig. 7 and 8, and the data are as follows:1H NMR(400MHz,CD3OD)δH 7.70(d,J=8.3Hz,2H),7.66(s,2H),6.92(d,J=8.3Hz,2H),6.84(s,2H),5.14(s,1H),4.58(s,1H),4.53(s,1H),3.92(s,6H),3.53(d,J=10.4,1H),2.85-2.79(m,1H),2.65-2.61(m,2H),1.73-1.62(m,6H),1.59(s,3H),1.38-1.34(m,4H),1.22(s,3H),0.92(t,J=6.9Hz,3H).13C NMR(100MHz,CH3OD):δC 166.6,166.6,153.6,153.6,151.5,151.5,149.1,148.9,148.9,143.5,134.5,128.4,128.4,125.9,125.9,125.0,125.0,121.7,121.7,116.1,116.1,114.1,114.1,111.7,56.5,47.3,40.0,36.1,32.5,31.8,31.1,30.0,23.6,23.5,20.2,14.4,14.4.ESI-MS:m/z 613.70[M-H]-。
the formula of the compound obtained in this example was derived as follows:
example 5
A process for preparing cannabidiol derivative compound 5, comprising the steps of:
the compound 4a in example 4 was replaced with the compound 5a, and the other reagents were not changed, according to the procedures of steps 1 to 3 in example 4, to give 7.1mg of a colorless oil, i.e., compound 5, in a final step at a yield of 28.87%.
The nuclear magnetic resonance spectrum of the compound 5 is shown in fig. 9 and 10, and the data is as follows:1H NMR(400MHz,CD3OD)δH 7.12(s,4H),6.81(s,2H),5.08(s,1H),4.54(s,1H),4.50(s,1H),3.54(dd,J=10.4,1.5Hz,1H),2.91-2.84(m,1H),2.64-2.60(m,2H),2.13(s,6H),1.72-1.56(m,6H),1.55(s,3H),1.37-1.33(m,4H),1.18(s,3H),0.91(t,J=6.9Hz,3H).13C NMR(100MHz,CH3OD):δC 166.9,166.9,157.6,157.6,157.6,157.6,151.5,151.5,149.1,143.4,135.0,128.4,128.4,128.2,124.7,119.2,119.2,119.2,119.2,111.8,109.0,109.0,109.0,109.0,47.2,40.0,36.1,32.5,31.8,30.9,29.9,23.5,23.5,20.0,14.4,9.0,9.0.ESI-MS:m/z 613.50[M-H]-。
the formula of the compound obtained in this example was derived as follows:
example 6
A process for preparing cannabidiol derivative compound 6, comprising the steps of:
adding compound 6a (0.38mmol) and cannabidiol (0.19mmol) to a solution of DCM (5mL) at 0 deg.C under nitrogen, stirring, adding CMPI (0.38mmol) and DMAP (0.38mmol) to the reaction mixture, stirring for 1 hr, filtering off the reaction precipitate, sequentially adding saturated NaHCO to the filtrate3Washing with saturated brine and Na2SO4Drying, concentrating, and purifying with silica gel column chromatography (n-hexane: acetone, volume ratio, 95:5) to obtain 10.6mg of white oil, i.e., compound 6, with a yield of 10.89%.
Data for the nmr spectrum of compound 6 are as follows:1H NMR(400MHz,CDCl3)δH 7.71(d,J=16.0Hz,1H),7.64(s,1H),7.50(d,J=8.3Hz,1H),7.39(d,J=8.3Hz,1H),6.58(s,1H),6.54(d,J=16.0Hz,1H),6.47(s,1H),5.56(br s,1H),4.63(s,1H),4.45(s,1H),3.54(br s,1H),2.54-2.50(overlap,3H),2.23-2.17(m,1H),2.08-2.03(m,1H),1.81-1.70(overlap,4H),1.61(s,3H),1.59(s,3H),1.33-1.29(m,4H),0.88(t,J=6.9Hz,3H).ESI-MS:m/z 511.25[M-H]-。
the formula of the compound obtained in this example was derived as follows:
example 7
A process for preparing cannabidiol derivative compound 7 comprising the steps of:
by substituting compound 6a in example 6 with compound 7a and leaving the other reagents unchanged, the procedure of example 6 was followed to give 20.0mg of a white oil, i.e., compound 7, in 20.16% yield.
Data for the nmr spectrum of compound 7 are as follows:1H NMR(400MHz,CDCl3)δH 7.76(d,J=16.0Hz,1H),7.56(d,J=8.4Hz,2H),7.42(d,J=8.4Hz,2H),6.58(s,1H),6.55(d,J=16.0Hz,1H),6.47(s,1H),5.56(br s,1H),4.62(s,1H),4.45(s,1H),3.55(br s,1H),2.53-2.49(overlap,3H),2.22-2.19(m,1H),2.08-2.03(m,1H),1.83-1.67(overlap,4H),1.61(s,3H),1.59(s,3H),1.33-1.28(m,4H),0.88(t,J=6.9Hz,3H).ESI-MS:m/z 523.20[M+H]+。
the formula of the compound obtained in this example was derived as follows:
example 8
A Sulforhodamine B protein staining method (Sulforhodamine B, SRB) is adopted to determine the drug concentration (IC) of cannabidiol derivatives (half maximum inhibition) when the inhibition rate of the cannabidiol derivatives on lung cancer cell lines (A549), human breast cancer cell lines (MDA-MB-231), nasopharyngeal carcinoma and drug-resistant strains (KB, KB-VIN) thereof and human breast cancer cell lines (MCF-7) reaches 50 percent50) Paclitaxel is a positive drug.
The method comprises the following specific steps:
(1) RPMI 1640 medium was supplemented with 25mM HEPES (4- (2-hydroxyethyl) -1-piperazineethanesulfonic acid), 2mM l-glutamine, 10% heat-inactivated fetal bovine serum, 100IU penicillin, 100. mu.g/mL streptomycin and 0.25. mu.g/mL amphotericin B;
(2) and (3) culturing the cells: different tumor cell strains were cultured in the above culture medium at 37 deg.C and 5% CO2Culturing in an incubator;
(3) preparing the medicine: seven compounds prepared in examples 1-7 were prepared as 10mM stock solutions in DMSO;
(4) plate preparation: adjusting the cell suspension concentration to 8000-2Culturing in an incubator;
(5) adding medicine: adding the medicines in the step (3) into a 96-well culture plate respectively until the final concentrations are respectively 100 mu M, 10 mu M, 1 mu M and 0.1 mu M, setting 3 flat wells for each concentration gradient, and culturing for 72 h; the experiment was divided into drug group, control group (only culture medium and cells) and blank group (only culture medium);
(6) and (3) detection: cells were fixed in 10% by mass trichloroacetic acid and then stained with 0.04% sulforhodamine B, the protein-bound dye was solubilized with 10mM Tris base, and the absorbance (OD) was measured at 515nm using a ELx800 microplate reader with Gen5 software.
Cell viability (%) - (experimental OD-blank OD)/(control OD-blank OD). times.100%
According to the standard curve of the concentration of the drug and the cell growth inhibition rate, the IC of the drug is obtained50。
The inhibitory effect of cannabidiol derivatives on tumor cells is shown in table 1.
TABLE 1 test results of antitumor Activity
The results show that the compounds 1-5 have better inhibiting effect on 5 cancer cell lines (A549, MDA-MB-231, KB, KB-VIN and MCF-7) than cannabidiol, especially the compound 5 has the most remarkable activity and IC50Values between 2.96-4.93 μ M, compounds 6 and 7 are comparable to the antitumor activity of cannabidiol.
Claims (3)
2. the method of producing cannabidiol derivatives as claimed in claim 1, wherein cannabidiol is used as a nucleus, and the phenolic hydroxyl group of cannabidiol is esterified with benzoic acid substituted with different groups to obtain cannabidiol derivatives.
3. Use of the cannabidiol derivative as claimed in claim 1 in the manufacture of an anti-tumour medicament.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110248495.XA CN113173857B (en) | 2021-03-09 | 2021-03-09 | Cannabidiol derivative and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110248495.XA CN113173857B (en) | 2021-03-09 | 2021-03-09 | Cannabidiol derivative and preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113173857A true CN113173857A (en) | 2021-07-27 |
| CN113173857B CN113173857B (en) | 2023-05-23 |
Family
ID=76922863
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110248495.XA Active CN113173857B (en) | 2021-03-09 | 2021-03-09 | Cannabidiol derivative and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113173857B (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114292249A (en) * | 2022-03-07 | 2022-04-08 | 中国农业科学院农产品加工研究所 | Cannabidiol-2-piperazinoate and application thereof |
| CN114349695A (en) * | 2022-03-07 | 2022-04-15 | 中国农业科学院农产品加工研究所 | Cannabidiol-2-nicotinate and application thereof |
| WO2024134682A1 (en) * | 2022-12-21 | 2024-06-27 | Council Of Scientific And Industrial Research An Indian Registered Body Incorporated Under The Regn. Of Soc. Act (Act Xxi Of 1860) | (n-alkyldihydrol-2h-oxazinyl)-cannabidiol as anti-proliferative agent and process for preparation thereof |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1543647A (en) * | 1966-11-04 | 1968-10-25 | Process for preparing resorcinol derivatives substituted in position 2 | |
| US3562312A (en) * | 1966-11-04 | 1971-02-09 | Albert Eschenmoser | Manufacture of 2-substituted resorcinol derivatives |
| US6017919A (en) * | 1996-02-06 | 2000-01-25 | Japan Tobacco Inc. | Compounds and pharmaceutical use thereof |
| CN101198324A (en) * | 2005-06-16 | 2008-06-11 | 欧洲凯尔特公司 | Cannabinoid active pharmaceutical ingredient for improved dosage forms |
| US20100298579A1 (en) * | 2009-04-29 | 2010-11-25 | Thc Pharm Gmbh | Process for preparing synthetic cannabinoids |
| US20190316144A1 (en) * | 2017-07-11 | 2019-10-17 | Trait Biosciences Inc. | In vivo generation of water-soluble cannabinoids in plant cell suspension cultures |
-
2021
- 2021-03-09 CN CN202110248495.XA patent/CN113173857B/en active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1543647A (en) * | 1966-11-04 | 1968-10-25 | Process for preparing resorcinol derivatives substituted in position 2 | |
| US3562312A (en) * | 1966-11-04 | 1971-02-09 | Albert Eschenmoser | Manufacture of 2-substituted resorcinol derivatives |
| US6017919A (en) * | 1996-02-06 | 2000-01-25 | Japan Tobacco Inc. | Compounds and pharmaceutical use thereof |
| CN101198324A (en) * | 2005-06-16 | 2008-06-11 | 欧洲凯尔特公司 | Cannabinoid active pharmaceutical ingredient for improved dosage forms |
| US20100298579A1 (en) * | 2009-04-29 | 2010-11-25 | Thc Pharm Gmbh | Process for preparing synthetic cannabinoids |
| US20190316144A1 (en) * | 2017-07-11 | 2019-10-17 | Trait Biosciences Inc. | In vivo generation of water-soluble cannabinoids in plant cell suspension cultures |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114292249A (en) * | 2022-03-07 | 2022-04-08 | 中国农业科学院农产品加工研究所 | Cannabidiol-2-piperazinoate and application thereof |
| CN114349695A (en) * | 2022-03-07 | 2022-04-15 | 中国农业科学院农产品加工研究所 | Cannabidiol-2-nicotinate and application thereof |
| CN114349695B (en) * | 2022-03-07 | 2022-05-20 | 中国农业科学院农产品加工研究所 | Cannabidiol-2-nicotinate and application thereof |
| CN114292249B (en) * | 2022-03-07 | 2022-07-19 | 中国农业科学院农产品加工研究所 | Cannabidiol-2-piperazinoate and application thereof |
| WO2024134682A1 (en) * | 2022-12-21 | 2024-06-27 | Council Of Scientific And Industrial Research An Indian Registered Body Incorporated Under The Regn. Of Soc. Act (Act Xxi Of 1860) | (n-alkyldihydrol-2h-oxazinyl)-cannabidiol as anti-proliferative agent and process for preparation thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113173857B (en) | 2023-05-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN113173857B (en) | Cannabidiol derivative and preparation method and application thereof | |
| CN113683557B (en) | Application of cyclopentadienyl iridium/rhodium dimer | |
| EP2840088B1 (en) | Method for preparing 5,6,4'-trihydroxyflavone-7-0-d-glucuronic acid | |
| CN105541773B (en) | A kind of preparation method of 3,4- dihydros -4- aryl-coumarin class compounds | |
| CN111825615B (en) | Oxidized isoaporphine alkaloid derivative and application thereof | |
| CN113880872A (en) | Preparation of camptothecin boric acid compound and application of camptothecin boric acid compound in anti-tumor aspect | |
| CN109251196B (en) | Aminobenzo [ d ] aza-quinazoline compound and preparation method and application thereof | |
| CN108014113B (en) | Application of butyrylamidodimethoxybenzo [ d ] aza-based quinazoline compound in preparation of drugs for treating cervical cancer | |
| CN115073406B (en) | Eucalyptus type sesquiterpene lactone TBA derivative and application thereof | |
| CN113880855A (en) | Preparation of 9-fluoro camptothecin derivative and application of 9-fluoro camptothecin derivative in anti-tumor aspect | |
| CN108276384B (en) | acetaminobenzo [ d ] azepinyl quinazoline compound and preparation and application thereof | |
| CN107382944B (en) | Coumarin gossypol derivatives with anti-tumor activity and synthesis method thereof | |
| CN108329300B (en) | Nitrobenzo [ d ] aza-quinazoline compound and preparation method and application thereof | |
| CN108324718B (en) | Application of cyclohexyl methoxy formyl amino chloro benzo aza group quinazoline compound in leukemia treatment drug | |
| CN1310920C (en) | Prepn of racemized and optically active poon essence A and its analog | |
| CN111018885B (en) | 1, 2-dioxycyclohexene [3,4-f ] nitrogen oxo cyclononane derivative and synthetic method and application thereof | |
| CN112110902B (en) | 1-deoxynojirimycin-kaempferol compound, intermediate, preparation method and application | |
| CN115181112B (en) | Synthesis and anti-tumor application of 6-bromo-cycloicaritin chromane 3, 4-diketone derivative | |
| CN109776411B (en) | Nitrogen mustard carbostyril derivative and preparation method and application thereof | |
| CN108245520B (en) | Application of acetamido quinazoline compound in preparation of drugs for treating lung cancer | |
| CN108245519B (en) | Application of butyrylaminoquinazoline compound in preparation of drugs for treating leukemia | |
| CN103044423B (en) | Substituted pyridine-2-ketone compounds and preparation method thereof | |
| CN110194740B (en) | 4-tert-butyloxycarbonylpiperazine-1, 8-naphthalimide derivative and synthetic method and application thereof | |
| CN108409654B (en) | Tetrahydroisoquinoline-2-radical aryloxy phenoxyalkyl ketone compound with antineoplastic activity and pharmaceutical application thereof | |
| CN110105316B (en) | Resveratrol-phthalide hybrid compound and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |