CN113145112B - 一种用于二硝基甲苯选择性加氢的Pd-Pt/C催化剂的制备方法 - Google Patents
一种用于二硝基甲苯选择性加氢的Pd-Pt/C催化剂的制备方法 Download PDFInfo
- Publication number
- CN113145112B CN113145112B CN202110477286.2A CN202110477286A CN113145112B CN 113145112 B CN113145112 B CN 113145112B CN 202110477286 A CN202110477286 A CN 202110477286A CN 113145112 B CN113145112 B CN 113145112B
- Authority
- CN
- China
- Prior art keywords
- catalyst
- minutes
- stirring
- preparation
- carbon black
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000003054 catalyst Substances 0.000 title claims abstract description 88
- JRTYPQGPARWINR-UHFFFAOYSA-N palladium platinum Chemical compound [Pd].[Pt] JRTYPQGPARWINR-UHFFFAOYSA-N 0.000 title claims abstract description 37
- 238000002360 preparation method Methods 0.000 title claims abstract description 17
- DYSXLQBUUOPLBB-UHFFFAOYSA-N 2,3-dinitrotoluene Chemical compound CC1=CC=CC([N+]([O-])=O)=C1[N+]([O-])=O DYSXLQBUUOPLBB-UHFFFAOYSA-N 0.000 title claims abstract description 13
- 238000005984 hydrogenation reaction Methods 0.000 title claims abstract description 7
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims abstract description 40
- 238000006243 chemical reaction Methods 0.000 claims abstract description 20
- 239000006229 carbon black Substances 0.000 claims abstract description 19
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 claims description 40
- 238000003756 stirring Methods 0.000 claims description 29
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 18
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 15
- 238000000034 method Methods 0.000 claims description 13
- PIBWKRNGBLPSSY-UHFFFAOYSA-L palladium(II) chloride Chemical compound Cl[Pd]Cl PIBWKRNGBLPSSY-UHFFFAOYSA-L 0.000 claims description 13
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 12
- 239000008367 deionised water Substances 0.000 claims description 12
- 229910021641 deionized water Inorganic materials 0.000 claims description 12
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 10
- 229910052763 palladium Inorganic materials 0.000 claims description 10
- 229910052700 potassium Inorganic materials 0.000 claims description 10
- 239000011591 potassium Substances 0.000 claims description 10
- 238000001035 drying Methods 0.000 claims description 8
- 238000001914 filtration Methods 0.000 claims description 8
- 229910052697 platinum Inorganic materials 0.000 claims description 8
- 125000004429 atom Chemical group 0.000 claims description 7
- 238000000227 grinding Methods 0.000 claims description 7
- 238000001291 vacuum drying Methods 0.000 claims description 7
- 239000011780 sodium chloride Substances 0.000 claims description 6
- 229910052739 hydrogen Inorganic materials 0.000 claims description 5
- 239000001257 hydrogen Substances 0.000 claims description 5
- 238000010438 heat treatment Methods 0.000 claims description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 4
- 238000001132 ultrasonic dispersion Methods 0.000 claims description 4
- 238000001816 cooling Methods 0.000 claims description 3
- 239000010453 quartz Substances 0.000 claims description 3
- 238000004321 preservation Methods 0.000 claims description 2
- 238000009210 therapy by ultrasound Methods 0.000 claims description 2
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 claims 1
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims 1
- VOZKAJLKRJDJLL-UHFFFAOYSA-N 2,4-diaminotoluene Chemical compound CC1=CC=C(N)C=C1N VOZKAJLKRJDJLL-UHFFFAOYSA-N 0.000 abstract description 14
- 238000009903 catalytic hydrogenation reaction Methods 0.000 abstract description 12
- 239000000956 alloy Substances 0.000 abstract description 9
- 229910045601 alloy Inorganic materials 0.000 abstract description 9
- 229910021126 PdPt Inorganic materials 0.000 abstract description 7
- 230000003197 catalytic effect Effects 0.000 abstract description 3
- 238000006073 displacement reaction Methods 0.000 abstract description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 10
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 238000009826 distribution Methods 0.000 description 5
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 5
- 235000017557 sodium bicarbonate Nutrition 0.000 description 5
- 238000003917 TEM image Methods 0.000 description 4
- 238000011068 loading method Methods 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- 239000011148 porous material Substances 0.000 description 4
- 239000002994 raw material Substances 0.000 description 4
- 238000001308 synthesis method Methods 0.000 description 4
- DVKJHBMWWAPEIU-UHFFFAOYSA-N toluene 2,4-diisocyanate Chemical compound CC1=CC=C(N=C=O)C=C1N=C=O DVKJHBMWWAPEIU-UHFFFAOYSA-N 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000000151 deposition Methods 0.000 description 2
- WQOXQRCZOLPYPM-UHFFFAOYSA-N dimethyl disulfide Chemical compound CSSC WQOXQRCZOLPYPM-UHFFFAOYSA-N 0.000 description 2
- -1 dinitrotoluene iron Chemical compound 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 239000000806 elastomer Substances 0.000 description 2
- 239000006260 foam Substances 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 239000007791 liquid phase Substances 0.000 description 2
- 238000013507 mapping Methods 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 230000035484 reaction time Effects 0.000 description 2
- RMBFBMJGBANMMK-UHFFFAOYSA-N 2,4-dinitrotoluene Chemical compound CC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O RMBFBMJGBANMMK-UHFFFAOYSA-N 0.000 description 1
- AOFIWCXMXPVSAZ-UHFFFAOYSA-N 4-methyl-2,6-bis(methylsulfanyl)benzene-1,3-diamine Chemical compound CSC1=CC(C)=C(N)C(SC)=C1N AOFIWCXMXPVSAZ-UHFFFAOYSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 229920002334 Spandex Polymers 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 239000004566 building material Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000003889 chemical engineering Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 208000012839 conversion disease Diseases 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000003822 epoxy resin Substances 0.000 description 1
- 239000006261 foam material Substances 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 239000001995 intermetallic alloy Substances 0.000 description 1
- 239000002649 leather substitute Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000002923 metal particle Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000002086 nanomaterial Substances 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- 230000033116 oxidation-reduction process Effects 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- NXJCBFBQEVOTOW-UHFFFAOYSA-L palladium(2+);dihydroxide Chemical compound O[Pd]O NXJCBFBQEVOTOW-UHFFFAOYSA-L 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- DYFXGORUJGZJCA-UHFFFAOYSA-N phenylmethanediamine Chemical compound NC(N)C1=CC=CC=C1 DYFXGORUJGZJCA-UHFFFAOYSA-N 0.000 description 1
- NFOHLBHARAZXFQ-UHFFFAOYSA-L platinum(2+);dihydroxide Chemical compound O[Pt]O NFOHLBHARAZXFQ-UHFFFAOYSA-L 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920003225 polyurethane elastomer Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000004759 spandex Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 239000004753 textile Substances 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/40—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals of the platinum group metals
- B01J23/44—Palladium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/20—Catalysts, in general, characterised by their form or physical properties characterised by their non-solid state
- B01J35/23—Catalysts, in general, characterised by their form or physical properties characterised by their non-solid state in a colloidal state
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/391—Physical properties of the active metal ingredient
- B01J35/393—Metal or metal oxide crystallite size
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/396—Distribution of the active metal ingredient
- B01J35/399—Distribution of the active metal ingredient homogeneously throughout the support particle
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/0201—Impregnation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/03—Precipitation; Co-precipitation
- B01J37/031—Precipitation
- B01J37/035—Precipitation on carriers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/16—Reducing
- B01J37/18—Reducing with gases containing free hydrogen
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/34—Irradiation by, or application of, electric, magnetic or wave energy, e.g. ultrasonic waves ; Ionic sputtering; Flame or plasma spraying; Particle radiation
- B01J37/348—Electrochemical processes, e.g. electrochemical deposition or anodisation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C209/00—Preparation of compounds containing amino groups bound to a carbon skeleton
- C07C209/30—Preparation of compounds containing amino groups bound to a carbon skeleton by reduction of nitrogen-to-oxygen or nitrogen-to-nitrogen bonds
- C07C209/32—Preparation of compounds containing amino groups bound to a carbon skeleton by reduction of nitrogen-to-oxygen or nitrogen-to-nitrogen bonds by reduction of nitro groups
- C07C209/36—Preparation of compounds containing amino groups bound to a carbon skeleton by reduction of nitrogen-to-oxygen or nitrogen-to-nitrogen bonds by reduction of nitro groups by reduction of nitro groups bound to carbon atoms of six-membered aromatic rings in presence of hydrogen-containing gases and a catalyst
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Electrochemistry (AREA)
- Physics & Mathematics (AREA)
- Health & Medical Sciences (AREA)
- Plasma & Fusion (AREA)
- Toxicology (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Catalysts (AREA)
Abstract
本发明公开了一种用于二硝基甲苯选择性加氢的Pd‑Pt/C催化剂的制备方法,属于催化加氢技术领域。其是以炭黑为载体,先制备出Pt/C催化剂,然后利用电化学置换反应,采用Pd2+置换出Pt/C催化剂中的部分Pt原子,从而制得所述Pd‑Pt/C催化剂。本发明利用Pd2+/Pd和PtCl2‑/Pt之间的氧化还原电势差,用Pd2+置换出Pt/C催化剂上部分的Pt原子,从而在炭黑载体表面形成明确结构的PdPt合金,所得具有PdPt合金结构的双金属催化剂中Pd用量少,但在二硝基甲苯选择性加氢制备甲苯二胺反应中仍具有良好的催化性能,因而具有较好应用前景。
Description
技术领域
本发明属于催化加氢技术领域,具体涉及一种Pd-Pt双金属催化剂及其制备方法并用于二硝基甲苯的催化加氢生成甲苯二胺。
背景技术
甲苯二胺(TDA)又名二氨基甲苯,在工业上有广泛的应用,如TDA与二甲基二硫催化合成的二甲硫基甲苯二胺可用作环氧树脂的液体固化剂、聚氨醋防水涂料的固化剂、聚氨酷弹性体材料预聚体的交联剂等;TDA中加入氟化聚酞胺弹性体可使其具有优异的防锈涂饰性能。此外,TDA最主要的用途是作为生产甲苯二异氰酸酯(TDI)的原料,而TDI是生产聚氨醋的重要原料之一,由它制成的泡沫材料、弹性体、氨纶、合成革、粘合剂、涂料等产品目前已广泛应用于石油、化工、轻工、纺织、建材、机械、电子、汽车印刷及造纸等领域中。随着TDI在聚酯工业中的地位日益突出,绿色高效的制备甲苯二胺成为近年国内外研究的热点。
甲苯二胺合成工艺主要有:二硝基甲苯铁粉还原法、二硝基甲苯硫化碱还原法、二硝基甲苯电解还原法、二硝基甲苯催化加氢法等。采用催化加氢的方法可以大幅度降低产品成本,提高产品质量,增加收率,缩短反应时间和减少三废污染,并且可以制备用其他还原方法不能得到的化合物,因此受到人们的普遍重视。催化加氢分为气相催化加氢和液相催化加氢。气相催化加氢由于受原料沸点限制,应用范围较窄;相比之下,液相催化加氢以其环境友好,产品质量稳定,工艺先进而受到人们重视。
催化加氢常用的催化剂有Pd、Pt、Ni。为了提高催化剂的活性、稳定性和选择性,以及进一步降低贵金属组分的用量等,往往会在催化剂中添加其他金属组分,形成了多金属或多金属催化剂。这类催化剂中两种或多种金属粒子在分布上的彼此穿插和相互配合,往往表现出更高的催化活性、选择性和更长的使用寿命。
发明内容
本发明的目的在于提供一种具有PdPt合金结构、Pd用量少、催化剂活性高的用于二硝基甲苯制备甲苯二胺的Pd-Pt/C催化剂的制备方法。
为实现上述目的,本发明采用如下技术方案:
一种用于二硝基甲苯选择性加氢的Pd-Pt/C催化剂,其是以炭黑为载体,先制备出Pt/C催化剂,然后利用电化学置换反应,采用Pd2+置换出Pt/C催化剂中的部分Pt原子,从而制得具有PdPt合金结构的Pd-Pt/C催化剂。该Pd-Pt/C催化剂中Pd-Pt含量为4~6wt%,其中,Pd与Pt的摩尔比为1:5~10。
所述Pd-Pt/C催化剂通过原位合成方法制成,其制备方法包括如下步骤:
(1)Pt/C催化剂的制备:将氯亚铂酸钾于去离子水中溶解完全,得氯亚铂酸钾溶液,然后加入盐酸调节溶液pH<7,再加入载体炭黑,超声分散均匀后加入碳酸氢钠,并升温到90℃,保温反应30分钟后过滤,所得沉淀分散于去离子水中后,放到反应釜中用氢气进行还原,再经40℃~60℃干燥36~48小时,研磨,得到Pt/C催化剂;
(2)Pd-Pt/C催化剂的制备:将氯化钠于去离子水中溶解完全后,加入氯化钯,并加入盐酸调节溶液pH<7,待氯化钯充分溶解后,再加入制备好的Pt/C催化剂,超声分散均匀后搅拌6~8小时,然后经过滤,40℃~60℃干燥36~48小时,研磨,得到所述Pd-Pt/C催化剂。
进一步地,步骤(1)中所述氯亚铂酸钾溶液的浓度为0.1~0.3g/L;所用炭黑与氯亚铂酸钾的质量比为10~8:1;所用碳酸氢钠与氯亚铂酸钾的质量比为10~20:1。
进一步地,步骤(1)中所述还原是于压力1~3MPa、温度100~200℃的条件下反应30分钟。
进一步地,步骤(2)中所用氯化钠和氯化钯的摩尔比为2:1,所用氯化钯和氯亚铂酸钾的摩尔比为1:1~3。
本发明所得Pd-Pt/C催化剂可用于二硝基甲苯选择性加氢制备甲苯二胺。
本发明利用Pd2+/Pd(0.915V)的氧化还原电势比PtCl2-/Pt(0.758V)高的特性,利用Pd2+部分取代己经制备得到的负载在炭黑表面的Pt原子,并使Pd原子扩散到Pt纳米粒子内,最终形成PdPt纳米合金。
本发明所制备的催化剂与传统的Pd/C催化剂比较,具有以下优点:(1)炭黑载体表面具有明确的PdPt合金结构。(2)催化剂中Pd用量少,可降低成本。(3)催化剂加氢活性高且容易分离,反应转化率可达99%,收率达38%。
附图说明
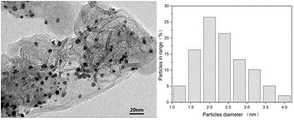
图1为实施例1所得Pd-Pt/C催化剂的透射电镜图和孔径分布图。
图2为实施例1所得Pd-Pt/C催化剂的mapping图。
图3为实施例1所得Pd/C催化剂和Pd-Pt/C催化剂的XRD对比图。
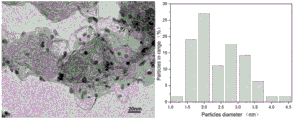
图4为对比例所得Pd-Pt/C催化剂的透射电镜图和孔径分布图。
具体实施方式
为了使本发明所述的内容更加便于理解,下面结合具体实施方式对本发明所述的技术方案做进一步的说明,但是本发明不仅限于此。
实施例1
(1)Pt/C催化剂的制备:将110mg(0.265mmol)氯亚铂酸钾加入圆底烧瓶中,加入500mL去离子水,搅拌溶解10分钟后,加入1mL盐酸使溶液pH<7,以防止铂沉积过快,再继续搅拌15分钟,然后加入1g 100%压缩炭黑,搅拌15分钟保证炭黑充分湿润并分散,然后超声10分钟。继续搅拌10分钟后慢慢加入2g碳酸氢钠(防止在加入过程中形成泡沫)。搅拌10分钟后将溶液升温至90±1℃,保温反应30分钟后冷却至60℃,此时铂以氢氧化物的形式沉积在炭黑上,将催化剂过滤后放到装有50mL水的石英杯中,然后在反应釜中用氢气还原30分钟(还原时的压力为1MPa、温度为110℃),再放到真空干燥箱中,在60℃下干燥36小时,然后将催化剂研磨后保存在样品瓶中。
(2)Pd-Pt/C催化剂的制备:将28mg(0.48mmol)氯化钠加入圆底烧瓶中,加入500mL去离子水溶解,然后加入42mg(0.24mmol)氯化钯,搅拌10分钟后加入1mL盐酸使氯化钯完全溶解并使溶液pH<7,以防止钯沉积过快,再继续搅拌15分钟后加入制备好的Pt/C催化剂,超声分散均匀后搅拌6小时,过滤后放到真空干燥箱中,60℃下干燥36小时,得到的Pd-Pt/C催化剂(Pd:Pt摩尔比1:1)研磨保存好。
经ICP表征表明,所得催化剂中Pd和Pt原子的质量分别为0.32%和4.97%。
图1为本实施例所得Pd-Pt/C催化剂的透射电镜图和孔径分布图。如图1所示,所得Pd-Pt/C催化剂的平均粒径为2.36nm。
图2为本实施例所得Pd-Pt/C催化剂的mapping图。图中清楚地展示了所制备的Pd-Pt/C催化剂中Pd、Pt和C元素的分布情况。可以看出,Pd和Pt元素均匀分散在炭黑的表面上,Pd2+通过置换出Pt原子和剩余的Pt形成PdPt合金颗粒。
图3为本实施例所得Pd/C催化剂和Pd-Pt/C催化剂的XRD对比图。XRD是表征纳米材料物相的最基础的技术方法。对于金属间合金,两种金属的特征衍射峰消失,一种新型的不同于两种金属的特征衍射峰的布拉格衍射峰被观察到。图中Pd/C在40.1°、46.6°、68.1°和82.1°的衍射峰,相对应与面心立方Pd(JCPDS NO.46-1043)的(111)、(200)、(220)和(311)。Pd-Pt/C的XRD图与Pd/C相似,但由于Pt的加入衍射峰的位置稍稍向左移动,这表明合成的合金是面心立方相。
实施例2
(1)Pt/C催化剂的制备:将110mg(0.265mmol)氯亚铂酸钾加入烧杯中,加入500mL去离子水,搅拌溶解10分钟后,加入1mL盐酸使溶液pH<7,以防止铂沉积过快,再继续搅拌15分钟,然后加入1g 100%压缩炭黑,搅拌15分钟保证炭黑充分湿润并分散,然后超声10分钟。继续搅拌10分钟后慢慢加入1.8g碳酸氢钠(防止在加入过程中形成泡沫)。搅拌10分钟后将溶液升温至90±1℃,保温反应30分钟后冷却至60℃,此时铂以氢氧化物的形式沉积在炭黑上,将催化剂过滤后放到装有50mL水的石英杯中,然后在反应釜中用氢气还原30分钟(还原时的压力为1MPa、温度为110℃),再放到真空干燥箱中,在60℃下干燥36小时,然后将催化剂研磨后保存在样品瓶中。
(2)Pd-Pt/C催化剂的制备:将2.2mg(0.038mmol)氯化钠加入圆底烧瓶中,加入500mL去离子水溶解,然后加入3.3mg(0.019mmol)氯化钯,搅拌10分钟后加入1mL盐酸使氯化钯完全溶解并使溶液pH<7,以防止钯沉积过快,再继续搅拌15分钟后加入制备好的Pt/C催化剂,超声分散均匀后搅拌6小时,过滤后放到真空干燥箱中,60℃下干燥36小时,得到的Pd-Pt/C催化剂研磨保存好。所得催化剂中Pd和Pt原子的质量分别为0.2wt%和4.8wt%。
对比例
共还原合成法是制备合金最为普通和简单的方法,本实施例使用共还原法制备Pd-Pt/C催化剂作为对比,其具体步骤如下:
将4.6mg(0.08mmol)氯化钠加入圆底烧瓶中,加入500mL去离子水溶解,然后加入8mg(0.04mmol)氯化钯,搅拌10分钟后加入1mL盐酸使氯化钯完全溶解并使溶液pH<7,再继续搅拌15分钟使其生成氯化钯钠。向该溶液中加入100mg(0.24mmol)氯亚铂酸钾,搅拌10分钟使其完全溶解,然后加入1g 100%压缩炭黑,搅拌15分钟保证炭黑充分湿润并分散,然后超声10分钟。继续搅拌10分钟后慢慢加入2g碳酸氢钠,搅拌10分钟后将溶液升温至90±1℃,保温反应30分钟,然后冷却至60℃。此时把钯和铂以氢氧化物的形式沉积在炭黑上,将催化剂过滤、烘干。烘干后的催化剂加入100mL石英杯中并加50mL去离子水稀释并搅拌形成混合物,然后在反应釜内(t=30min,T=110℃,转速为600rpm)利用氢气将氢氧化钯和氢氧化铂同时还原成金属,再放到真空干燥箱中,在60℃下干燥36小时,所得催化剂研磨后保存在样品瓶中。 所得Pd-Pt/C催化剂中Pd含量为0.5wt%,Pt含量为4.5wt%。
图4为本对比例所得Pd-Pt/C催化剂的透射电镜图和孔径分布图。如图4所示,所得Pd-Pt/C催化剂的平均粒径为2.45nm。
2,4-二硝基甲苯选择性加氢试验
在高温高压反应釜中,以50mL无水乙醇为溶剂,10g/L二硝基甲苯为反应原料,加入10mg催化剂进行二硝基甲苯催化加氢合成甲苯二胺的反应,反应压力为1MPa、温度为100℃、转速为600rpm,反应3min。反应后产物用气相色谱检测分析组成,结果见表1。
表1 不同催化剂催化性能对比
由实施例1可以看出,Pd使用过量时,所得Pd-Pt/C催化剂中Pd的负载量也只能达到0.3wt%左右,从中可以得出在Pd-Pt/C催化剂中Pd的最大负载量。且通过与实施例2对比可知,0.3wt%为Pd-Pt/C催化剂中Pd的最优负载量。而由与Pt/C催化剂的对比可见,Pd的少量负载即可实现催化性能的明显提高。同时,与共还原合成法制备的Pd-Pt/C催化剂相比,本发明原位合成法制备的催化剂具有更好的转化率和收率。
以上所述仅为本发明的较佳实施例,凡依本发明申请专利范围所做的均等变化与修饰,皆应属本发明的涵盖范围。
Claims (1)
1.一种用于二硝基甲苯选择性加氢的Pd-Pt/C催化剂的制备方法,其特征在于:步骤如下:
(1)Pt/C催化剂的制备:将0.265mmol氯亚铂酸钾加入圆底烧瓶中,加入500mL去离子水,搅拌溶解10分钟后,加入1mL盐酸使溶液pH<7,以防止铂沉积过快,再继续搅拌15分钟,然后加入1g 100%压缩炭黑,搅拌15分钟保证炭黑充分湿润并分散,然后超声10分钟,继续搅拌10分钟后慢慢加入2g碳酸氢钠,搅拌10分钟后将溶液升温至90±1℃,保温反应30分钟后冷却至60℃,过滤后放到装有50mL水的石英杯中,然后在反应釜中用氢气还原30分钟,再放到真空干燥箱中,在60℃下干燥36小时,然后将催化剂研磨后保存在样品瓶中;还原时的压力为1MPa、温度为110℃;
(2)Pd-Pt/C催化剂的制备:将0.48mmol氯化钠加入圆底烧瓶中,加入500mL去离子水溶解,然后加入0.24mmol氯化钯,搅拌10分钟后加入1mL盐酸使氯化钯完全溶解并使溶液pH<7,以防止钯沉积过快,再继续搅拌15分钟后加入制备好的Pt/C催化剂,超声分散均匀后搅拌6小时,过滤后放到真空干燥箱中,60℃下干燥36小时,得到Pd-Pt/C催化剂,其中Pd和Pt原子的质量分别为0.32%和4.97%。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110477286.2A CN113145112B (zh) | 2021-04-30 | 2021-04-30 | 一种用于二硝基甲苯选择性加氢的Pd-Pt/C催化剂的制备方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202110477286.2A CN113145112B (zh) | 2021-04-30 | 2021-04-30 | 一种用于二硝基甲苯选择性加氢的Pd-Pt/C催化剂的制备方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113145112A CN113145112A (zh) | 2021-07-23 |
| CN113145112B true CN113145112B (zh) | 2023-03-24 |
Family
ID=76872660
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202110477286.2A Active CN113145112B (zh) | 2021-04-30 | 2021-04-30 | 一种用于二硝基甲苯选择性加氢的Pd-Pt/C催化剂的制备方法 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113145112B (zh) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN115501886B (zh) * | 2022-03-31 | 2023-10-24 | 西南民族大学 | 一种用于低温硝基苯加氢合成苯胺催化剂的制备方法 |
| CN116440898A (zh) * | 2023-05-08 | 2023-07-18 | 中国科学院金属研究所 | 用于二硝基甲苯加氢反应的原子级分散Pd-Pt催化剂及其制备方法和应用 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB799871A (en) * | 1955-05-10 | 1958-08-13 | Du Pont | Hydrogenation catalysts and process |
| EP1192989A1 (de) * | 2000-09-28 | 2002-04-03 | Degussa AG | Katalysator zur Hydrierung aromatischer Nitroverbindungen |
| EP1441850A1 (en) * | 2001-11-08 | 2004-08-04 | Degussa AG | Supported catalyst for hydrogenation of nitroaromatics |
| CN101612566A (zh) * | 2009-07-14 | 2009-12-30 | 复旦大学 | 一种低铂炭载纳米Pd-Pt合金催化剂、制备方法及其应用 |
| CN103480370A (zh) * | 2012-06-15 | 2014-01-01 | 中国石油化工股份有限公司 | 一种催化加氢用碳载钯铂金属催化剂的制备方法 |
| CN108686652B (zh) * | 2018-05-09 | 2019-10-18 | 南通龙翔新材料科技股份有限公司 | 一种1,8-二硝基萘加氢催化剂及其制备方法 |
-
2021
- 2021-04-30 CN CN202110477286.2A patent/CN113145112B/zh active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN113145112A (zh) | 2021-07-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110302769A (zh) | 一种催化剂载体、负载型催化剂及其制备方法和用途 | |
| CN113145112B (zh) | 一种用于二硝基甲苯选择性加氢的Pd-Pt/C催化剂的制备方法 | |
| CN112387295B (zh) | 一种氮掺杂碳负载钌单原子催化剂及其制备方法和应用 | |
| CN106914255B (zh) | 一种非合金金属复合物及其制备方法和应用 | |
| CN112808288A (zh) | 一种氮磷或氮磷硫共掺杂碳负载金属单原子的催化剂及其微波辅助制备方法 | |
| CN109876866B (zh) | 一种用于芳香醛合成芳香胺的催化剂及其制备方法 | |
| CN110743544A (zh) | 一种苯乙酮选择加氢制备α-苯乙醇用钯炭催化剂及其制备方法与应用 | |
| CN112675865B (zh) | 一种高活性、高稳定性担载镍催化剂及其制备方法和应用 | |
| CN107684921B (zh) | 一种用于tmbq转化为tmhq的催化剂及其制备方法 | |
| CN104888853B (zh) | 一种石墨烯负载PVP稳定纳米Ru催化剂、制备方法及其用途 | |
| CN112774681B (zh) | 非晶态合金催化剂及其制备方法和应用 | |
| CN114588940B (zh) | 一种用于酚类化合物加氢的镍基催化剂及其制备方法和应用 | |
| CN110339844B (zh) | Fe纳米棒与Pt@Fe纳米棒催化剂及合成和应用 | |
| CN111135848B (zh) | 木质基碳催化剂、其制备方法及苯酚加氢制备环己酮的方法 | |
| CN110538651B (zh) | 一种铂炭催化剂及其制备方法 | |
| Liao et al. | In situ growing PtCo bimetallic catalyst on plant tannin-grafted collagen fiber for catalytic hydrogenation of cinnamaldehyde with desirable performance | |
| CN114733530B (zh) | 一种有机液体储氢载体的加氢催化剂及其制备方法和应用 | |
| CN102600891A (zh) | 一种非酸介质中硝基苯选择加氢制对氨基苯酚的催化剂 | |
| CN114931946A (zh) | 一种Pt/C复合催化剂及其制备方法和应用 | |
| CN112371170B (zh) | 一种异质结纳米复合催化剂及其制备方法和应用 | |
| CN115888716A (zh) | 金属有机框架-离子液体复合催化剂及制备方法和用途 | |
| CN109894131B (zh) | 一种对苯二甲酸二甲酯(dmt)加氢催化剂及其制备方法 | |
| JP2000117104A (ja) | 炭素含有担体材料上の貴金属からなる触媒の製造方法 | |
| CN113292519A (zh) | 磁性金钴复合物催化剂及其制备方法和应用 | |
| CN107570157B (zh) | 一种制备对氨基酚的有序介孔炭催化剂的制备方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |