CN113087806B - Novel CAR-T cells targeting multiple tumors, and preparation and methods thereof - Google Patents
Novel CAR-T cells targeting multiple tumors, and preparation and methods thereof Download PDFInfo
- Publication number
- CN113087806B CN113087806B CN202011635074.4A CN202011635074A CN113087806B CN 113087806 B CN113087806 B CN 113087806B CN 202011635074 A CN202011635074 A CN 202011635074A CN 113087806 B CN113087806 B CN 113087806B
- Authority
- CN
- China
- Prior art keywords
- car
- cells
- cell
- gly
- leu
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
- A61K39/001129—Molecules with a "CD" designation not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70517—CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70578—NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/515—Animal cells
- A61K2039/5156—Animal cells expressing foreign proteins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/02—Fusion polypeptide containing a localisation/targetting motif containing a signal sequence
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/70—Fusion polypeptide containing domain for protein-protein interaction
- C07K2319/74—Fusion polypeptide containing domain for protein-protein interaction containing a fusion for binding to a cell surface receptor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/15011—Lentivirus, not HIV, e.g. FIV, SIV
- C12N2740/15041—Use of virus, viral particle or viral elements as a vector
- C12N2740/15043—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/10—Plasmid DNA
- C12N2800/106—Plasmid DNA for vertebrates
- C12N2800/107—Plasmid DNA for vertebrates for mammalian
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Zoology (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Cell Biology (AREA)
- Biophysics (AREA)
- Biomedical Technology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Virology (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Oncology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Hematology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
The present invention relates to novel CAR-T cells that target multiple tumors and methods of making and using the same, in particular, the invention provides a chimeric antigen receptor CAR comprising an antigen binding domain that specifically binds to the CD155 antigen. The CD 155-specifically targeted engineered immune cell can specifically and selectively kill various tumors and has a remarkable killing effect.
Description
Technical Field
The present invention relates to the field of immunotherapy, and in particular, to novel CAR-T cells that target multiple tumors, and their preparation and methods.
Background
Adoptive cell therapy of Chimeric Antigen Receptor (CAR) modified T cells (CAR-T) is currently one of the most promising immunotherapeutic approaches, especially against refractory leukemias and lymphomas, with enormous success. Although about 90% of cancer cases worldwide are solid tumors, the antigenic heterogeneity of solid tumors, immunosuppressive microenvironments, and the dilemma that complex vasculature influences CAR-T infiltration make CAR-T cell therapy difficult to work effectively, therefore, the technology starts from two points of development and overcoming of immunosuppressive tumor environment and tumor antigen heterogeneity, discovers a novel antigen target highly expressed in a plurality of advanced solid tumors, meanwhile, the target can be used as an immune check point to inhibit the functions of NK and T cells, and CAR-T is designed aiming at the target, so that various solid tumors can be directly killed, the immune inhibition effect of the target on the NK and T cells can be reduced, the application value of the CAR-T in the solid tumors is effectively improved, and the two pain points of antigen target heterogeneity and tumor immune inhibition microenvironment in the solid tumors are solved.
There is therefore an urgent need in the art to develop a chimeric antigen receptor T cell that has a significant killing effect against a variety of tumors while also fighting the immunosuppressive microenvironment.
Disclosure of Invention
The invention aims to provide a chimeric antigen receptor T cell which has a remarkable killing effect on various tumors and can also resist an immunosuppressive microenvironment.
In a first aspect, the invention provides a chimeric antigen receptor CAR comprising an antigen binding domain that specifically binds to the CD155 antigen.
In another preferred embodiment, the antigen binding domain is an antibody, extracellular domain or antigen binding fragment.
In another preferred embodiment, the antigen binding fragment is a Fab or scFv or a single domain antibody sdFv.
In another preferred embodiment, the antigen binding domain comprises the extracellular segment of TIGIT.
In another preferred embodiment, the antigen binding domain comprises an antibody single chain variable region sequence targeting CD 155.
In another preferred embodiment, the CAR has the structure shown in formula I:
L-Z1-Z2-TM-C-CD3ζ (I)
in the formula (I), the compound is shown in the specification,
each "-" is independently a linker peptide or a peptide bond;
l is an optional signal peptide sequence;
z1 is an antigen binding domain comprising the extracellular segment of TIGIT or an antibody single chain variable region sequence targeting CD 155; and
z2 is a null or hinge region;
TM is a transmembrane domain;
c is a costimulatory signal molecule;
CD3 ζ is the cytoplasmic signaling sequence derived from CD3 ζ.
In another preferred embodiment, the amino acid sequence of the extracellular domain protein of TIGIT is selected from the group consisting of:
(a) 1 amino acid sequence of the protein as shown in SEQ ID NO;
(b) 1, a protein which is formed by substituting, deleting or adding one or more (such as 1-10) amino acid residues in the amino acid sequence of SEQ ID NO, has the functions of the protein (a) and is derived from the protein (a); or
(c) And (b) a protein derived from (a) and having more than 90% (preferably more than or equal to 95%) homology with the protein sequence defined by (a) and having the protein function of (a).
In another preferred embodiment, the nucleotide sequence encoding extracellular domain protein of TIGIT is selected from the group consisting of:
(a) the polynucleotide with the nucleotide sequence shown in SEQ ID NO. 2; (b) polynucleotide having homology of more than or equal to 70% (preferably more than or equal to 80%, > 90%, > 95% or more than or equal to 98%) and having activity of targeting or binding to CD155 with the sequence shown in SEQ ID NO. 2;
(c) the polynucleotide shown in SEQ ID NO. 2 is truncated by 1-60 (preferably 1-30, more preferably 1-6) nucleotides at the 5 'end and/or 3' end, and has the activity of targeting or binding to CD 155.
In another preferable example, the extracellular segment of TIGIT is human.
In another preferred embodiment, the CD 155-targeting antibody single chain variable region sequence has the structure shown in formula A1 or A2:
V L1 -V H1 (A1) (ii) a Or
V H1 -V L1 (A2);
Wherein, V L1 Is the light chain variable region of an anti-CD 155 antibody; v H1 Is the heavy chain variable region of an anti-CD 155 antibody; "-" is a linker peptide (or flexible linker) or peptide bond.
In another preferred embodiment, V is L1 And V H1 Connected by a flexible joint.
In another preferred embodiment, the flexible linker is 1 to 5 (preferably 2 to 4) consecutive sequences of SEQ ID NO:20 (GGGGS).
In another preferred embodiment, V L1 The amino acid sequence of (1) is shown as position 152-289 of SEQ ID NO 10, and V H1 The amino acid sequence of (1) is shown as positions 22-136 of SEQ ID NO. 10.
In another preferred embodiment, V L1 The amino acid sequence of (1) is shown in SEQ ID NO. 12.
In another preferred embodiment, V H1 The amino acid sequence of (1) is shown in SEQ ID NO. 14.
In another preferred embodiment, the amino acid sequence of the CD 155-targeting antibody single chain variable region sequence is shown in SEQ ID NO. 19.
In another preferred embodiment, the CD 155-targeting antibody single chain variable region sequence is a murine, human, chimeric of human and murine, or fully humanized single chain antibody variable region fragment.
In another preferred embodiment, the chimeric antigen receptor CAR has the structure shown in formula II or II':
L-V L1 -V H1 -Z2-TM-C-CD3 ζ (II); or
L-V H1 -V L1 -Z2-TM-C-CD3ζ(II’)
Wherein each element is as described above.
In another preferred embodiment, L is a signal peptide of a protein selected from the group consisting of: TIGIT, CD8, CD8 α, CD28, GM-CSF, CD4, CD137, FcR γ, FcR β, CD3 ζ, CD3 γ, CD3 δ, CD3 ∈, CD5, CD22, CD20, CD79a, CD79b, CD278(ICOS), FcERI, CD66d, DAP10, DAP12, or a combination thereof.
In another preferred embodiment, L is a signal peptide of a protein selected from the group consisting of: TIGIT, CD8, CD8 α, CD28, GM-CSF, CD4, CD137, or a combination thereof.
In another preferred example, the signal peptide of L is an extracellular signal peptide of TIGIT, and the amino acid sequence is MRWCLLLIWAQGLRQAPLASG (SEQ ID NO: 3).
In another preferred embodiment, the signal peptide of L is a CD8 alpha signal peptide, and the amino acid sequence is MALPVTALLLPLALLLHAARP (SEQ ID NO: 18).
In another preferred embodiment, said Z2 is the hinge region of a protein selected from the group consisting of: CD8, Ig (immunoglobulin) hinge, or a combination thereof.
In another preferred embodiment, the Z2 is a CD8hinge region.
In another preferred embodiment, the amino acid sequence of Z2 is: TTTPAPRPPTPAPTIASQPLSLRPEACRPAAGGAVHTRGLDFACD (SEQ ID NO: 8).
In another preferred embodiment, the TM is a transmembrane region of a protein selected from the group consisting of: an alpha chain of a T cell receptor, a beta chain of a T cell receptor, a zeta chain of a T cell receptor, CD28, CD3 epsilon, CD45, CD4, CD5, CD8, CD9, CD16, CD22, CD33, CD37, CD64, CD80, CD86, CD134, CD137, CD154, CD8 alpha, ICOS, CD19, CD45, or a combination thereof.
In another preferred embodiment, the TM is a transmembrane region of a protein selected from the group consisting of: CD8, CD28, CD33, CD37, CD8 a, CD5, CD16, ICOS, CD9, CD22, CD134, CD137, CD154, CD19, CD45, CD4, CD3 epsilon, or a combination thereof.
In another preferred embodiment, the TM is a transmembrane region of a protein selected from the group consisting of: CD8 α.
In another preferred embodiment, the amino acid sequence of TM is: IYIWAPLAGTCGVLLLSLVITLYC (SEQ ID NO: 9).
In another preferred embodiment, C is a costimulatory signal molecule for a protein selected from the group consisting of: CD, CD zeta, CD gamma, CD delta, CD epsilon, CD79, CD66, CD134, CD137, ICOS, CD154, 4-1BB, OX, CD, LIGHT, NKG2, B-H, OX, activated NK cell receptor, BTLA, Toll ligand receptor, CD, CDS, ICAM-L LFA-1(CD 11/CD), B-H, CDS, ICAM-1, ICOS (CD278), GITR, BAFFR, HVEM (LIGHT), KIRDS, SLAMF, NKp (KLRF), NKp, CD alpha, CD beta, IL2 gamma, IL7 alpha, ITGA, VLA, CD49, ITGA, VLA-6, GAITCD 49, GAITD 11, GAMMA 11, GAMGB, GAMMA-11, GAMGK, GAITGB, GAITGA, CD49, GAITGA, CD 11-L11, GAITGB, GAITGA, GAITGB, GAITCD 49, GAITGA-11, GAITGB, GAITCD 11, GAITGB, CD11, GAITGB, NKE-L-1, NKCD 11, NKG-1, NKCD 11, GAITGB, NKG-1, GAITGB, NKG-1, NKCD 11, NKG-1, NKG 244, NKG, CD11, NKG, CD11, NKG, CD11, NKG, CD11, NKG, CD11, CD278, NKG, CD11, NKG, NK, 2B4) CD84, CD96 (tactile), CEACAM1, CRTAM, Ly9(CD229), CD160(BY55), PSGL1, CD100(SEMA4D), CD69, SLAMF6(NTBA, Ly108), SLAM (SLAMF1, CD150, IPO-3), BLAME (SLAMF8), SELPLG (CD162), LTBR, LAT, GADS, SLP-76, PAG/Cbp, CD19a, DAP10, DAP12, ligands for CD83, MHC class I molecules, TNF receptor proteins, immunoglobulin-like proteins, cytokine receptors, integrins, signaling lymphocyte activation molecules, or combinations thereof.
In another preferred embodiment, C is a costimulatory signal molecule for a protein selected from the group consisting of: CD27, CD3 ζ, CD3 γ, CD3 δ, CD3 ε, CD5, CD22, CD79a, CD79B, CD66d, CD2, CD4, CD5, CD28, CD30, CD40, CD134, CD137, ICOS, CD154, 4-1BB, OX40, CD7, LIGHT, NKG2C, B7-H3, or a combination thereof.
In another preferred embodiment, C is a co-stimulatory signaling molecule from 4-1 BB.
In another preferred embodiment, the amino acid sequence of the co-stimulatory signaling molecule of C is KRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCEL (SEQ ID No.: 4).
In another preferred example, the amino acid sequence of CD3 ζ is RVKFSRSADAPAYKQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR (SEQ ID No.: 5).
In another preferred embodiment, the amino acid sequence of the CAR is as set forth in SEQ ID No. 6 or 10.
In a second aspect, the invention provides a nucleic acid molecule encoding a Chimeric Antigen Receptor (CAR) according to the first aspect of the invention.
In another preferred embodiment, the nucleic acid molecule is selected from the group consisting of:
(a) a polynucleotide encoding a polypeptide as set forth in SEQ ID NO. 6 or 10;
(b) a polynucleotide having a sequence shown in SEQ ID NO. 7 or 11;
(c) a polynucleotide having a nucleotide sequence having a homology of 75% or more (preferably 80% or more, more preferably 90% or more, still more preferably 95% or more, still more preferably 98% or more, still more preferably 99% or more) to the sequence of (b);
(d) a polynucleotide in which 1 to 60 (preferably 1 to 30, more preferably 1 to 10) nucleotides are truncated or added to the 5 'end and/or the 3' end of the polynucleotide shown in (b);
(e) a polynucleotide complementary to any one of the polynucleotides of (a) - (d).
In another preferred embodiment, the nucleotide sequence of the nucleic acid molecule is shown in SEQ ID NO 7 or 11.
In another preferred embodiment, the nucleic acid molecule is a polynucleotide.
In a third aspect, the invention provides a vector comprising a nucleic acid molecule according to the second aspect of the invention.
In another preferred embodiment, the carrier is selected from the group consisting of: a plasmid, a lentiviral vector, an adenoviral vector, a retroviral vector, or a combination thereof.
In another preferred embodiment, the vector is a lentiviral vector.
In a fourth aspect, the invention provides a host cell comprising a vector or chromosome of the third aspect of the invention into which has been integrated an exogenous nucleic acid molecule of the second aspect of the invention or which expresses a CAR of the first aspect of the invention.
In another preferred embodiment, the cell is an isolated cell, and/or the cell is a genetically engineered cell.
In another preferred embodiment, the cell is a mammalian cell.
In another preferred embodiment, the cell is a T cell or NK cell.
In another preferred embodiment, the host cell is an engineered immune cell.
In another preferred embodiment, the engineered immune cells comprise T cells or NK cells, preferably (i) chimeric antigen receptor T cells (CAR-T cells); (ii) chimeric antigen receptor NK cells (CAR-NK cells); or (iii) exogenous T Cell Receptor (TCR) T cells (TCR-T cells).
In another preferred embodiment, the immune cells are autologous.
In another preferred embodiment, the immune cells are non-autologous.
In another preferred embodiment, the immune cells target the CD155 antigen.
In a fifth aspect, the invention provides a method of making an engineered immune cell expressing a CAR according to the first aspect of the invention, comprising the steps of: transferring the nucleic acid molecule of the second aspect of the invention or the vector of the third aspect of the invention into a T cell or NK cell, thereby obtaining the engineered immune cell.
In another preferred embodiment, the introducing includes introducing simultaneously, sequentially or sequentially.
In another preferred embodiment, the cell is a CAR-T cell or CAR-NK cell.
In another preferred embodiment, the method further comprises the step of performing function and effectiveness detection on the obtained engineered immune cells.
In a sixth aspect, the present invention provides a pharmaceutical composition comprising a chimeric antigen receptor according to the first aspect of the present invention, a nucleic acid molecule according to the second aspect of the present invention, a vector according to the third aspect of the present invention, or a host cell according to the fourth aspect of the present invention, and a pharmaceutically acceptable carrier, diluent or excipient.
In another preferred embodiment, the pharmaceutical composition is a liquid formulation.
In another preferred embodiment, the dosage form of the pharmaceutical composition is an injection.
In another preferred embodiment, the concentration of the cells in the pharmaceutical composition is 1 × 10 5 -1×10 8 One cell/ml, preferably 1X 10 6 -1×10 7 One cell/ml, more preferably 1X 10 6 -5×10 6 Individual cells/ml.
In another preferred embodiment, the pharmaceutical composition further comprises other drugs for treating cancer or tumor (such as emerging antibody drugs, other CAR-T drugs, or chemotherapeutic drugs).
In a seventh aspect, the present invention provides a use of the chimeric antigen receptor according to the first aspect, the nucleic acid molecule according to the second aspect, the vector according to the third aspect, the host cell according to the fourth aspect, or the pharmaceutical composition according to the sixth aspect, for preparing a medicament or an agent for selectively killing tumor.
In another preferred embodiment, the tumor comprises a tumor that highly expresses CD 155.
In another preferred embodiment, the tumor includes a solid tumor and a hematologic tumor.
In another preferred embodiment, the solid tumor is selected from the group consisting of: liver cancer, head and neck cancer, melanoma, non-hodgkin's lymphoma, bladder cancer, glioblastoma, cervical cancer, lung cancer, chondrosarcoma, thyroid cancer, renal cancer, mesothelioma, osteosarcoma, cholangiocarcinoma, ovarian cancer, gastric cancer, bladder cancer, prostate cancer, meningioma, pancreatic cancer, multiple squamous cell tumor, esophageal cancer, lung small cell carcinoma, colorectal cancer, breast cancer, medulloblastoma, breast cancer, or a combination thereof.
In another preferred embodiment, the hematological tumor is selected from the group consisting of: acute Myeloid Leukemia (AML), Multiple Myeloma (MM), Chronic Lymphocytic Leukemia (CLL), Acute Lymphoblastic Leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), or a combination thereof.
In an eighth aspect, the invention provides a kit for selectively killing a tumor, the kit comprising a container, and a chimeric antigen receptor of the first aspect of the invention, a nucleic acid molecule of the second aspect of the invention, a vector of the third aspect of the invention, or a host cell of the fourth aspect of the invention, in the container.
In another preferred embodiment, the kit further comprises a label or instructions for use.
The ninth aspect of the present invention provides a method for selectively killing tumors, comprising:
administering to a subject in need thereof a safe and effective amount of a host cell according to the fourth aspect of the invention, or a pharmaceutical composition according to the sixth aspect of the invention.
In another preferred embodiment, the subject comprises a human or non-human mammal.
In another preferred embodiment, the non-human mammal includes a rodent (e.g., mouse, rat, rabbit), primate (e.g., monkey).
In another preferred embodiment, the method is non-therapeutic and non-diagnostic.
In a tenth aspect, the present invention provides a method for treating a disease, comprising administering to a subject in need thereof a safe and effective amount of the pharmaceutical composition according to the sixth aspect of the present invention.
In another preferred embodiment, the method further comprises administering to a subject in need of treatment an additional agent for treating cancer or tumor.
In another preferred embodiment, the other drug comprises a CAR-T drug.
In another preferred embodiment, the disease is cancer or a tumor.
In another preferred embodiment, the tumor comprises a tumor that highly expresses CD 155.
In another preferred embodiment, the tumor includes a solid tumor and a hematologic tumor.
In another preferred embodiment, the solid tumor is selected from the group consisting of: liver cancer, head and neck cancer, melanoma, non-hodgkin's lymphoma, bladder cancer, glioblastoma, cervical cancer, lung cancer, chondrosarcoma, thyroid cancer, renal cancer, mesothelioma, osteosarcoma, cholangiocarcinoma, ovarian cancer, gastric cancer, bladder cancer, prostate cancer, meningioma, pancreatic cancer, multiple squamous cell tumor, esophageal cancer, lung small cell carcinoma, colorectal cancer, breast cancer, medulloblastoma, breast cancer, or a combination thereof.
In another preferred embodiment, the hematological tumor is selected from the group consisting of: acute Myeloid Leukemia (AML), Multiple Myeloma (MM), Chronic Lymphocytic Leukemia (CLL), Acute Lymphoblastic Leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), or a combination thereof.
It is to be understood that within the scope of the present invention, new or preferred solutions may be constructed between the above-described features of the present invention and the features specifically described below (e.g., in the examples). Not to be repeated herein, depending on the space.
Drawings
FIG. 1 is a graph of the expression level of CD155 in various solid tumor cell lines in the CCLE database.
FIG. 2 shows the data of the tissue chip for CD155 expression. FIG. 2-A is the expression of CD155 in tumor tissue. FIG. 2-B shows the expression of CD155 in tissues adjacent to cancer, and FIG. 2-C shows the expression of CD155 in normal tissues.
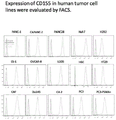
FIG. 3 shows the flow-type detection of CD155 expression in tumor cell lines
FIG. 4 is a schematic structural diagram of an expression cassette in the examples.
FIG. 5 is a graph showing the results of viral titer for pELPS-TIGIT-CD8H-BBz and pELPS-TIGIT-BBz.
Figure 6 is a flow chart of the expression level of CAR of TIGIT-CAR-T cells.
FIG. 7 is a graph of the cytotoxicity results of TIGIT-CAR-T on different tumor cells.
Figure 8 is a flow chart of the expression levels of CAR of PVRbbz-CAR-T cells.
FIG. 9 is a graph showing the results of flow-through validation of CD155 knockdown in PC3 cells.
Figure 10 is a graph of killing of CD155 knock-out PC3 cells by PVRbbz-CAR-T cells.
FIG. 11 is a graph of the killing of various tumor cells by PVRbbz-CAR-T cells.
Figure 12 is a graph of experimental tracking results of animal models of PVRbbz-CAR-T treated bone metastasis.
FIG. 13 is a graph showing the results of HE staining of the major organs of experimental animals treated with PVRbbz-CAR-T.
FIG. 14 is a graph showing the results of an experiment on the immune function of CD 155-inhibited T cells, FIG. 14A is a graph showing the flow chart of the cytokine secretion of CD 155-inhibited T cells, and FIG. 14B is a graph showing the results of the CAR-T killing function of CD 155-inhibited.
Detailed Description
The inventor of the invention has extensively and deeply studied, and through a large amount of screening of targets, the invention unexpectedly discovers an engineered immune cell specifically targeting CD155 for the first time, which can specifically and selectively kill a plurality of tumors and has a remarkable killing effect. On this basis, the present inventors have completed the present invention.
The present invention is representatively illustrated in detail for the engineered immune cells of the present invention, taking CAR-T cells as an example. The engineered immune cells of the invention are not limited to the CAR-T cells described above and below, and the engineered immune cells of the invention have the same or similar technical features and benefits as the CAR-T cells described above and below. Specifically, when the immune cells express the chimeric antigen receptor CAR, the NK cells are identical to T cells (or T cells can replace NK cells); when the immune cell is a T cell, the TCR is identical to the CAR (or the CAR can be replaced with a TCR).
CD155
CD155, also known as PVR, was first discovered to mediate viral entry into cells as a receptor for poliovirus. CD155 is highly expressed in many cancer cells and overexpression of CD155 may induce tumor immune escape.
The present study found that CD155 overexpression promotes tumor cell invasion and migration, and is associated with tumor progression and poor prognosis. As an immunomodulatory molecule, CD155 can bind to the co-stimulatory molecule CD226 and the co-inhibitory molecules TIGIT and CD96, and exert their respective effects of activating NK cells and inhibiting T cells and NK cells, and has a dual function in tumor immunity, while tumor-infiltrating immune cells express reduced CD226 and increased TIGIT. Thus, high expression of CD155 in tumor cells can be used as a key point for tumor therapy.
The research of the invention finds that CD226, TIGIT and CD96 are three receptors known at present as CD155, and TIGIT has the highest affinity to CD155, so based on the research result of the invention, the invention uses TIGIT as the extracellular end part of CAR structure to be more efficient than CD226 and can neutralize the inhibition effect of CD155 when designing CAR-T, thereby achieving two purposes. Meanwhile, CD155 is hardly expressed in normal tissues, but highly expressed in many malignant tumors such as liver cancer, head and neck cancer, melanoma, non-hodgkin's lymphoma, and the like. Is very relevant to disease prognosis. Thus a CAR-T targeting CD155 can be designed or can function in a variety of solid tumors while TIGIT serves as an immune checkpoint and, using its extracellular end designed CAR-T, can bind to CD155, thereby activating CAR intracellular signaling to exert a killing target cell effect while relieving CD155 from its immunosuppressive effect on T cells.
TIGIT extracellular domain protein
The invention unexpectedly discovers that the special TIGIT extracellular domain protein can be specifically combined with CD 155.
TIGIT (also known as WUCAM, Vstm3, VSIG9) is a member of the poliovirus receptor (PVR/nectin family, a member of the immunoglobulin superfamily. its expression is strictly restricted to lymphocytes, and the highest expression is effector cells and regulatory CD4 + T cell, follicular helper CD4 + T cell, effector CD8 + T cells and natural killer cells (NK). TIGIT may suppress immune cells in various aspects of the cancer immune cycle. First, TIGIT can inhibit NK cell effector function, thereby preventing primary tumor cell death andrelease of cancer cell antigens. Second, TIGIT on T cells can inhibit dendritic cell costimulatory capacity, resulting in decreased cancer antigen presentation and increased anti-inflammatory cytokines such as IL-10. TIGIT can also induce PVR signaling on other cells, such as tumor cells. Third, TIGIT + Tregs or PVR stimulated bone marrow cells can inhibit CD8 + T cell effector function or to CD4 + T cells are polarized. Fourth, TIGIT may inhibit CD8 directly or through Tregs + T cells and prevent the elimination of cancer cells. Blocking the TIGIT-CD155 pathway can promote to reverse the mediated inhibition effect and promote the killing effect of the immune system on the tumor. The invention constructs a chimeric antigen receptor T cell taking a TIGIT extracellular segment as a target, and the TIGIT extracellular segment is connected with a CD8 transmembrane region, a costimulatory factor 4-1BB and an intracellular signal activation sequence CD3zeta to construct a structure of a second-generation CAR, which is used for treating a solid tumor highly expressing a CD155 protein.
In a preferred embodiment of the invention, the amino acid sequence of the TIGIT extracellular domain protein of the invention is shown in SEQ ID No. 1.
MMTGTIETTGNISAEKGGSIILQCHLSSTTAQVTQVNWEQQDQLLAICNADLGWHISPSFKDRVAPGPGLGLTLQSLTVNDTGEYFCIYHTYPDGTYTGRIFLEVLESSVAEHGARFQIP(SEQ ID NO.:1)
The nucleotide sequence for encoding the TIGIT extracellular segment protein is shown as SEQ ID No. 2.
ATGATGACAGGCACAATAGAAACAACGGGGAACATTTCTGCAGAGAAAGGTGGCTCTATCATCTTACAATGTCACCTCTCCTCCACCACGGCACAAGTGACCCAGGTCAACTGGGAGCAGCAGGACCAGCTTCTGGCCATTTGTAATGCTGACTTGGGGTGGCACATCTCCCCATCCTTCAAGGATCGAGTGGCCCCAGGTCCCGGCCTGGGCCTCACCCTCCAGTCGCTGACCGTGAACGATACAGGGGAGTACTTCTGCATCTATCACACCTACCCTGATGGGACGTACACTGGGAGAATCTTCCTGGAGGTCCTAGAAAGCTCAGTGGCTGAGCACGGTGCCAGGTTCCAGATTCCA(SEQ ID NO.:2)
Chimeric Antigen Receptors (CAR)
Chimeric immune antigen receptors (CARs) consist of an extracellular antigen recognition region, usually a scFv (single-chain variable fragment), a transmembrane region, and an intracellular costimulatory signal region. The design of CARs goes through the following process: the first generation CARs had only one intracellular signaling component, CD3 ζ or Fc γ RI molecule, and due to the single intracellular activation domain, it caused only transient T cell proliferation and less cytokine secretion, and did not provide long-term T cell proliferation signaling and sustained in vivo anti-tumor effects, and thus did not achieve good clinical efficacy. The second generation CARs introduce a costimulatory molecule such as CD28, 4-1BB, OX40 and ICOS on the basis of the original structure, and compared with the first generation CARs, the function of the second generation CARs is greatly improved, and the persistence of CAR-T cells and the killing capability of the CAR-T cells on tumor cells are further enhanced. On the basis of the second generation CARs, a plurality of novel immune co-stimulatory molecules such as CD27 and CD134 are connected in series, and the development is three-generation and four-generation CARs.
The extracellular domain of CARs recognizes a specific antigen and subsequently transduces this signal through the intracellular domain, causing activated proliferation, cytolytic toxicity and cytokine secretion of the cell, thereby clearing the target cell. Autologous cells from the patient (or a heterologous donor) are first isolated, activated and genetically engineered to produce immune cells for CAR production, and then injected into the same patient. In this way, the probability of graft versus host disease is very low and antigens are recognized by immune cells in a non-MHC restricted manner.
CAR-immune cell therapy has achieved very high clinical response rates in the treatment of hematological malignancies, which rates were previously unattainable by any therapeutic approach, and have triggered a hot surge of clinical research in the world.
Specifically, the Chimeric Antigen Receptors (CARs) of the invention include an extracellular domain, a transmembrane domain, and an intracellular domain. The extracellular domain includes a target-specific binding element (also referred to as an antigen-binding domain). The intracellular domain includes a costimulatory signaling region and/or a zeta chain moiety. The costimulatory signaling region refers to a portion of the intracellular domain that includes the costimulatory molecule. Costimulatory molecules are cell surface molecules required for efficient response of lymphocytes to antigens, rather than antigen receptors or their ligands.
A linker may be incorporated between the extracellular domain and the transmembrane domain of the CAR, or between the cytoplasmic domain and the transmembrane domain of the CAR. As used herein, the term "linker" generally refers to any oligopeptide or polypeptide that functions to connect a transmembrane domain to an ectodomain or a cytoplasmic domain of a polypeptide chain. The linker may comprise 0-300 amino acids, preferably 2 to 100 amino acids and most preferably 3 to 50 amino acids.
The CARs of the invention, when expressed in T cells, are capable of antigen recognition based on antigen binding specificity. When it binds its associated antigen, it affects the tumor cells, causing the tumor cells to not grow, to be driven to death, or to otherwise be affected, and causing the patient's tumor burden to shrink or be eliminated. The antigen binding domain is preferably fused to an intracellular domain from one or more of the costimulatory molecules and/or the zeta chain. Preferably, the antigen binding domain is fused to the intracellular domain of the 4-1BB signaling domain and/or the CD3zeta signaling domain combination.
As used herein, "antigen binding domain" and "single chain antibody fragment" each refer to an Fab fragment, Fab 'fragment, F (ab') 2 fragment, or single Fv fragment having antigen binding activity. Fv antibodies contain the variable regions of the antibody heavy chain, the variable regions of the light chain, but no constant regions, and have the smallest antibody fragment of the entire antigen binding site. Generally, Fv antibodies also comprise a polypeptide linker between the VH and VL domains and are capable of forming the structures required for antigen binding. The antigen binding domain is typically a scFv (single-chain variable fragment). The size of the scFv is typically 1/6 for a whole antibody. Single chain antibodies are preferably a sequence of amino acids encoded by a single nucleotide chain. In a preferred embodiment of the present invention, the scFv comprises an antibody, preferably a single chain antibody, that specifically recognizes the tumor highly expressed antigen CD 155.
In a preferred embodiment, the antigen binding portion of the CAR of the invention targets the CD155 antigen. In a preferred embodiment, the antigen binding portion of the CAR of the invention is a TIGIT extracellular domain protein targeting CD 155.
In a preferred embodiment, the TIGIT extracellular segment protein contains variant forms, and the variant has homology of more than or equal to 80%, more than or equal to 85%, more than or equal to 90%, more than or equal to 95%, more than or equal to 98% or more than or equal to 99% with the TIGIT extracellular segment protein sequence table of the wild type.
In the present invention, the TIGIT extracellular domain protein of the present invention also includes conservative variants thereof, which means that at most 10, preferably at most 8, more preferably at most 5, and most preferably at most 3 amino acids are replaced by amino acids with similar or similar properties to form a polypeptide compared with the amino acid sequence of the TIGIT extracellular domain protein of the present invention.
In the present invention, the number of amino acids to be added, deleted, modified and/or substituted is preferably not more than 40%, more preferably not more than 35%, more preferably 1 to 33%, more preferably 5 to 30%, more preferably 10 to 25%, more preferably 15 to 20% of the total number of amino acids in the original amino acid sequence.
In the present invention, the number of the amino acids to be added, deleted, modified and/or substituted is usually 1, 2, 3, 4 or 5, preferably 1 to 3, more preferably 1 to 2, and most preferably 1.
For the hinge region and transmembrane region (transmembrane domain), the CAR can be designed to include a transmembrane domain fused to the extracellular domain of the CAR. In one embodiment, a transmembrane domain that is naturally associated with one of the domains in the CAR is used. In some examples, the transmembrane domains may be selected, or modified by amino acid substitutions, to avoid binding such domains to the transmembrane domains of the same or different surface membrane proteins, thereby minimizing interaction with other members of the receptor complex.
The extracellular domain of the CAR of the invention includes an extracellular domain protein of TIGIT, preferably an extracellular domain protein of TIGIT having a specific sequence.
In the present invention, the intracellular domain in the CAR of the invention includes the transmembrane region of CD8, the costimulatory factor of 4-1BB, the signaling domain of CD3 zeta.
In a preferred embodiment of the invention, the amino acid sequence of the CAR (containing the antigen binding domain of the extracellular segment of TIGIT) is as set forth in SEQ ID No. 6.
MRWCLLLIWAQGLRQAPLASGMMTGTIETTGNISAEKGGSIILQCHLSSTTAQVTQVNWEQQDQLLAICNADLGWHISPSFKDRVAPGPGLGLTLQSLTVNDTGEYFCIYHTYPDGTYTGRIFLEVLESSVAEHGARFQIPTTTPAPRPPTPAPTIASQPLSLRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLSLVITLYCKRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFSRSADAPAYKQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR(SEQ ID NO.:6)
In a preferred embodiment of the invention, the nucleotide sequence of the CAR (containing the antigen binding domain of the extracellular segment of TIGIT) is as shown in SEQ ID No. 7.
ATGCGCTGGTGTCTCCTCCTGATCTGGGCCCAGGGGCTGAGGCAGGCTCCCCTCGCCTCAGGAATGATGACAGGCACAATAGAAACAACGGGGAACATTTCTGCAGAGAAAGGTGGCTCTATCATCTTACAATGTCACCTCTCCTCCACCACGGCACAAGTGACCCAGGTCAACTGGGAGCAGCAGGACCAGCTTCTGGCCATTTGTAATGCTGACTTGGGGTGGCACATCTCCCCATCCTTCAAGGATCGAGTGGCCCCAGGTCCCGGCCTGGGCCTCACCCTCCAGTCGCTGACCGTGAACGATACAGGGGAGTACTTCTGCATCTATCACACCTACCCTGATGGGACGTACACTGGGAGAATCTTCCTGGAGGTCCTAGAAAGCTCAGTGGCTGAGCACGGTGCCAGGTTCCAGATTCCAaccacgacgccagcgccgcgaccaccaacaccggcgcccaccatcgcgtcgcagcccctgtccctgcgcccagaggcgtgccggccagcggcggggggcgcagtgcacacgagggggctggacttcgcctgtgatatctacatctgggcgcccttggccgggacttgtggggtccttctcctgtcactggttatcaccctttactgcaaacggggcagaaagaaactcctgtatatattcaaacaaccatttatgagaccagtacaaactactcaagaggaagatggctgtagctgccgatttccagaagaagaagaaggaggatgtgaactgagagtgaagttcagcaggagcgcagacgcccccgcgtacaagcagggccagaaccagctctataacgagctcaatctaggacgaagagaggagtacgatgttttggacaagagacgtggccgggaccctgagatggggggaaagccgagaaggaagaaccctcaggaaggcctgtacaatgaactgcagaaagataagatggcggaggcctacagtgagattgggatgaaaggcgagcgccggaggggcaaggggcacgatggcctttaccagggtctcagtacagccaccaaggacacctacgacgcccttcacatgcaggccctgccccctcgc(SEQ ID NO.:7)
Wherein, the 1 st to 21 st positions in SEQ ID NO. 6 are signal peptides; extracellular segment of TIGIT at 22-141 bits; 142 th and 186 th are hinge regions; the 187-210 th transmembrane region (e.g., the transmembrane region of CD 8); 211 st-252 th site is a co-stimulation element (e.g., 4-1 BB); the 253 rd and 364 th bits are CD3 ζ.
The extracellular antigen recognition region of the CAR of the invention may also be an antigen-specific scFv.
When the extracellular antigen-recognition region of the CAR is a single chain antibody fragment, the signal peptide is the CD8 a signal peptide.
The signal peptide of the CAR is a nucleotide sequence of a CD8 alpha signal peptide (CD8 alpha Leader) shown in SEQ ID NO. 17:
atggccttaccagtgaccgccttgctcctgccgctggccttgctgctccacgccgccaggccg;
the amino acid sequence of the CD8 alpha signal peptide (CD8 alpha Leader) of the CAR is shown as SEQ ID NO. 18: MALPVTALLLPLALLLHAARP, respectively;
in a preferred embodiment of the invention, the amino acid sequence of the CAR (comprising an antigen binding domain which is an scFv specific for an antigen of the invention) is as shown in SEQ ID No. 10:
MALPVTALLLPLALLLHAARPEVQLQQSGAELVRPGTSVKLSCKALGYTFTDHEMHWVKQTPVHGLEWIGTIHPGSGVTAYNQKFKGKATLTADKSSSTAYMELSTLTSEDSAVYYCTPLWLRRDWGQGTTLTVSTGGGGSGGGGSGGGGSALDIQMTQTPKFMSTSVGDRVSVTCKASQNVATNVVWFQQKSGQSPKALIYSASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCQQYNNYPLTFGAGTKLELKAAAGAPVPYPDPLEPRGAASAWSHPQFEKTTTPAPRPPTPAPTIASQPLSLRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLSLVITLYCKRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFSRSADAPAYKQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR。
in a preferred embodiment of the invention, the nucleotide sequence of the CAR (comprising an antigen binding domain that is an scFv specific for an antigen of the invention) is as shown in SEQ ID No. 11:
atggccttaccagtgaccgccttgctcctgccgctggccttgctgctccacgccgccaggccgGAGGTTCAGCTGCAGCAGTCTGGGGCTGAGCTGGTGAGGCCTGGGACTTCAGTGAAGCTGTCCTGCAAGGCTTTGGGCTACACATTTACTGACCATGAAATGCACTGGGTGAAACAGACACCTGTGCATGGCCTGGAATGGATTGGAACTATTCATCCAGGAAGTGGTGTTACTGCCTACAATCAGAAGTTCAAGGGCAAGGCCACACTGACTGCAGACAAATCCTCCAGCACAGCCTACATGGAGCTCAGCACCCTGACATCTGAAGACTCTGCTGTCTATTACTGTACACCACTTTGGTTACGACGGGACTGGGGCCAAGGCACCACTCTCACAGTGTCGACAGGTGGAGGCGGTTCAGGCGGAGGTGGCTCTGGCGGTGGCGGAAGTGCACTCGATATCCAGATGACACAGACTCCAAAATTCATGTCCACATCAGTAGGAGACAGGGTCAGCGTCACCTGCAAGGCCAGTCAGAATGTGGCTACTAATGTAGTCTGGTTTCAACAGAAATCAGGGCAATCTCCTAAAGCACTGATTTACTCGGCATCCTACCGGTACAGTGGAGTCCCTGATCGCTTCACAGGCAGTGGATCTGGGACAGATTTCACTCTCACCATCAGCAATGTGCAGTCTGAAGACTTGGCAGAGTATTTCTGTCAGCAATATAACAACTATCCTCTCACGTTCGGTGCTGGGACCAAGCTGGAGCTGAAAGCGGCCGCAGGTGCGCCGGTGCCGTATCCAGATCCGCTGGAACCGCGTGGGGCCGCAAGCGCTTGGAGCCACCCGCAGTTCGAAAAAaccacgacgccagcgccgcgaccaccaacaccggcgcccaccatcgcgtcgcagcccctgtccctgcgcccagaggcgtgccggccagcggcggggggcgcagtgcacacgagggggctggacttcgcctgtgatatctacatctgggcgcccttggccgggacttgtggggtccttctcctgtcactggttatcaccctttactgcaaacggggcagaaagaaactcctgtatatattcaaacaaccatttatgagaccagtacaaactactcaagaggaagatggctgtagctgccgatttccagaagaagaagaaggaggatgtgaactgagagtgaagttcagcaggagcgcagacgcccccgcgtacaagcagggccagaaccagctctataacgagctcaatctaggacgaagagaggagtacgatgttttggacaagagacgtggccgggaccctgagatggggggaaagccgagaaggaagaaccctcaggaaggcctgtacaatgaactgcagaaagataagatggcggaggcctacagtgagattgggatgaaaggcgagcgccggaggggcaaggggcacgatggcctttaccagggtctcagtacagccaccaaggacacctacgacgcccttcacatgcaggccctgccccctcgc。
in a preferred embodiment of the invention, V of said CAR L1 The amino acid sequence of (1) is shown in SEQ ID NO. 12:
ALDIQMTQTPKFMSTSVGDRVSVTCKASQNVATNVVWFQQKSGQSPKALIYSASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCQQYNNYPLTFGAGTKLELKAAAGAPVPYPDPLEPRGAASAWSHPQFEK。
in a preferred embodiment of the invention, V of said CAR L1 The nucleotide sequence of (1) is shown as SEQ ID NO. 13:
GCACTCGATATCCAGATGACACAGACTCCAAAATTCATGTCCACATCAGTAGGAGACAGGGTCAGCGTCACCTGCAAGGCCAGTCAGAATGTGGCTACTAATGTAGTCTGGTTTCAACAGAAATCAGGGCAATCTCCTAAAGCACTGATTTACTCGGCATCCTACCGGTACAGTGGAGTCCCTGATCGCTTCACAGGCAGTGGATCTGGGACAGATTTCACTCTCACCATCAGCAATGTGCAGTCTGAAGACTTGGCAGAGTATTTCTGTCAGCAATATAACAACTATCCTCTCACGTTCGGTGCTGGGACCAAGCTGGAGCTGAAAGCGGCCGCAGGTGCGCCGGTGCCGTATCCAGATCCGCTGGAACCGCGTGGGGCCGCAAGCGCTTGGAGCCACCCGCAGTTCGAAAAA。
in a preferred embodiment of the invention, V of said CAR H1 The amino acid sequence is shown as SEQ ID No. 14:
EVQLQQSGAELVRPGTSVKLSCKALGYTFTDHEMHWVKQTPVHGLEWIGTIHPGSGVTAYNQKFKGKATLTADKSSSTAYMELSTLTSEDSAVYYCTPLWLRRDWGQGTTLTVST。
in a preferred embodiment of the invention, V of said CAR H1 The nucleotide sequence of (1) is shown in SEQ ID NO. 15:
GAGGTTCAGCTGCAGCAGTCTGGGGCTGAGCTGGTGAGGCCTGGGACTTCAGTGAAGCTGTCCTGCAAGGCTTTGGGCTACACATTTACTGACCATGAAATGCACTGGGTGAAACAGACACCTGTGCATGGCCTGGAATGGATTGGAACTATTCATCCAGGAAGTGGTGTTACTGCCTACAATCAGAAGTTCAAGGGCAAGGCCACACTGACTGCAGACAAATCCTCCAGCACAGCCTACATGGAGCTCAGCACCCTGACATCTGAAGACTCTGCTGTCTATTACTGTACACCACTTTGGTTACGACGGGACTGGGGCCAAGGCACCACTCTCACAGTGTCGACA。
in a preferred embodiment of the invention, V of said CAR L1 And V H1 The connecting amino acid sequence is shown as SEQ ID NO. 16:
GGGGSGGGGSGGGGS。
wherein, the 1 st to 21 st positions in SEQ ID No. 10 are signal peptides; the antibody single-chain variable region sequence targeting CD155 at the 22 th position to the 289 th position; 290 th-334 th site is a hinge region; positions 335 and 358 are transmembrane regions (such as the transmembrane region of CD 8); 359-400 as a co-stimulation element (e.g., 4-1 BB); the 401 st and 512 th bits are CD3 ζ.
The amino acid sequence (VH-VL) of the CD 155-targeting antibody single chain variable region sequence is shown in SEQ ID NO. 19:
EVQLQQSGAELVRPGTSVKLSCKALGYTFTDHEMHWVKQTPVHGLEWIGTIHPGSGVTAYNQKFKGKATLTADKSSSTAYMELSTLTSEDSAVYYCTPLWLRRDWGQGTTLTVSTGGGGSGGGGSGGGGSALDIQMTQTPKFMSTSVGDRVSVTCKASQNVATNVVWFQQKSGQSPKALIYSASYRYSGVPDRFTGSGSGTDFTLTISNVQSEDLAEYFCQQYNNYPLTFGAGTKLELKAAAGAPVPYPDPLEPRGAASAWSHPQFEK(SEQ ID NO.19)
chimeric antigen receptor T cells (CAR-T cells)
As used herein, the terms "CAR-T cell", "CAR-T cell of the invention" all refer to a CAR-T cell according to the sixth aspect of the invention, which can target the CD155 protein, for the treatment of tumors with high expression of CD155, particularly solid tumors.
CAR-T cells have the following advantages over other T cell-based therapies: (1) the action process of the CAR-T cell is not limited by MHC; (2) given that many tumor cells express the same tumor antigen, CAR gene construction for a certain tumor antigen can be widely utilized once it is completed; (3) the CAR can utilize tumor protein antigens and glycolipid non-protein antigens, so that the target range of the tumor antigens is expanded; (4) the use of patient autologous cells reduces the risk of rejection; (5) the CAR-T cells have the function of immunological memory and can survive in vivo for a long time.
In the present invention, the CAR of the invention comprises (i) an extracellular domain comprising the extracellular domain of TIGIT; (ii) optionally a hinge region; (iii) a transmembrane domain; (iv) a co-stimulatory factor; and (v) the signaling domain of CD3 ζ.
Chimeric antigen receptor NK cells (CAR-NK cells)
As used herein, the terms "CAR-NK cell", "CAR-NK cell of the invention" all refer to a CAR-NK cell according to the first aspect of the invention. The CAR-NK cells of the invention can target the CD155 protein for the treatment of tumors with high expression of CD155, in particular solid tumors.
Natural Killer (NK) cells are a major class of immune effector cells that protect the body from viral infection and tumor cell invasion through non-antigen specific pathways. By engineering (genetically modifying) NK cells it is possible to obtain new functions, including the ability to specifically recognize tumor antigens and having an enhanced anti-tumor cytotoxic effect.
CAR-NK cells also have advantages compared to autologous CAR-T cells, such as: (1) directly kills tumor cells by releasing perforin and granzyme, but has no killing effect on normal cells of an organism; (2) they release very small amounts of cytokines and thus reduce the risk of cytokine storm; (3) is easy to be amplified in vitro and can be developed into ready-made products. Otherwise, similar to CAR-T cell therapy.
Exogenous T cell antigen receptor
As used herein, a foreign T cell antigen receptor (TCR) is a TCR that is exogenously transferred into a T cell by means of genetic engineering, using lentivirus or retrovirus as a vector, by cloning the α chain and β chain of the TCR from a tumor-reactive T cell by gene transfer technique.
The exogenous TCR modified T cell can specifically recognize and kill tumor cells, and affinity of the T cell and tumor can be improved and anti-tumor effect can be improved by optimizing affinity of TCR and tumor specific antigen.
Carrier
Nucleic acid sequences encoding the desired molecule can be obtained using recombinant methods known in the art, such as, for example, by screening libraries from cells expressing the gene, by obtaining the gene from vectors known to include the gene, or by direct isolation from cells and tissues containing the gene using standard techniques. Alternatively, the gene of interest may be produced synthetically.
The present invention also provides a vector into which the expression cassette of the present invention is inserted. Vectors derived from retroviruses such as lentiviruses are suitable tools for achieving long-term gene transfer, as they allow long-term, stable integration of the transgene and its propagation in daughter cells. Lentiviral vectors have advantages over vectors derived from oncogenic retroviruses such as murine leukemia virus, in that they can transduce non-proliferating cells such as hepatocytes. They also have the advantage of low immunogenicity.
In brief summary, an expression cassette or nucleic acid sequence of the invention is typically operably linked to a promoter and incorporated into an expression vector. The vector is suitable for replication and integration into eukaryotic cells. Typical cloning vectors contain transcriptional and translational terminators, initial sequences, and promoters that may be used to regulate the expression of the desired nucleic acid sequence.
The expression constructs of the invention may also be used for nucleic acid immunization and gene therapy using standard gene delivery protocols. Methods of gene delivery are known in the art. See, for example, U.S. Pat. nos. 5,399,346, 5,580,859, 5,589,466, which are incorporated herein by reference in their entirety. In another embodiment, the invention provides a gene therapy vector.
The nucleic acid can be cloned into many types of vectors. For example, the nucleic acid can be cloned into vectors including, but not limited to, plasmids, phagemids, phage derivatives, animal viruses, and cosmids. Specific vectors of interest include expression vectors, replication vectors, probe generation vectors, and sequencing vectors.
Further, the expression vector may be provided to the cell in the form of a viral vector. Viral vector technology is well known in the art and is described, for example, in Sambrook et al (2001, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, New York) and other virology and Molecular biology manuals. Viruses that may be used as vectors include, but are not limited to, retroviruses, adenoviruses, adeno-associated viruses, herpes viruses, and lentiviruses. Typically, suitable vectors comprise an origin of replication functional in at least one organism, a promoter sequence, a convenient restriction enzyme site, and one or more selectable markers (e.g., WO 01/96584; WO 01/29058; and U.S. Pat. No. 6,326,193).
Many virus-based systems have been developed for gene transfer into mammalian cells. For example, retroviruses provide a convenient platform for gene delivery systems. The selected gene can be inserted into a vector and packaged into a retroviral particle using techniques known in the art. The recombinant virus can then be isolated and delivered to the subject cells in vivo or ex vivo. Many retroviral systems are known in the art. In some embodiments, an adenoviral vector is used. Many adenoviral vectors are known in the art. In one embodiment, a lentiviral vector is used.
Additional promoter elements, such as enhancers, may regulate the frequency of transcription initiation. Typically, these are located in the 30-110bp region upstream of the start site, although many promoters have recently been shown to also contain functional elements downstream of the start site. The spacing between promoter elements is often flexible so that promoter function is maintained when the elements are inverted or moved relative to one another. In the thymidine kinase (tk) promoter, the spacing between promoter elements can be increased by 50bp apart, before activity begins to decrease. Depending on the promoter, it appears that the individual elements may function cooperatively or independently to initiate transcription.
An example of a suitable promoter is the immediate early Cytomegalovirus (CMV) promoter sequence. The promoter sequence is a strong constitutive promoter sequence capable of driving high level expression of any polynucleotide sequence operably linked thereto. Another example of a suitable promoter is elongation growth factor-1 α (EF-1 α). However, other constitutive promoter sequences may also be used, including, but not limited to, the simian virus 40(SV40) early promoter, the mouse mammary cancer virus (MMTV), the Human Immunodeficiency Virus (HIV) Long Terminal Repeat (LTR) promoter, the MoMuLV promoter, the avian leukemia virus promoter, the Epstein-Barr (Epstein-Barr) virus immediate early promoter, the rous sarcoma virus promoter, and human gene promoters such as, but not limited to, the actin promoter, myosin promoter, heme promoter, and creatine kinase promoter. Further, the present invention should not be limited to the use of constitutive promoters. Inducible promoters are also contemplated as part of the invention. The use of an inducible promoter provides a molecular switch that is capable of turning on expression of a polynucleotide sequence operably linked to the inducible promoter when such expression is desired, or turning off expression when expression is not desired. Examples of inducible promoters include, but are not limited to, the metallothionein promoter, the glucocorticoid promoter, the progesterone promoter, and the tetracycline promoter.
To assess the expression of the CAR polypeptide or portion thereof, the expression vector introduced into the cells can also comprise either or both of a selectable marker gene or a reporter gene to facilitate identification and selection of expressing cells from a population of cells sought to be transfected or infected by the viral vector. In other aspects, the selectable marker may be carried on a single piece of DNA and used in a co-transfection procedure. Both the selectable marker and the reporter gene may be flanked by appropriate regulatory sequences to enable expression in a host cell. Useful selectable markers include, for example, antibiotic resistance genes, such as neo and the like.
Reporter genes are used to identify potentially transfected cells and to evaluate the functionality of regulatory sequences. Typically, the reporter gene is the following: which is not present in or expressed by the recipient organism or tissue and which encodes a polypeptide whose expression is clearly indicated by some readily detectable property, such as enzymatic activity. After the DNA has been introduced into the recipient cell, the expression of the reporter gene is assayed at an appropriate time. Suitable reporter genes may include genes encoding luciferase, beta-galactosidase, chloramphenicol acetyltransferase, secreted alkaline phosphatase, or green fluorescent protein (e.g., Ui-Tei et al, 2000FEBS Letters479: 79-82). Suitable expression systems are well known and can be prepared using known techniques or obtained commercially. Generally, the construct with the minimum of 5 flanking regions showing the highest level of reporter gene expression was identified as the promoter. Such promoter regions can be linked to reporter genes and used to evaluate the ability of an agent to modulate promoter-driven transcription.
Methods for introducing and expressing genes into cells are known in the art. In the context of expression vectors, the vector may be readily introduced into a host cell by any method known in the art, e.g., mammalian, bacterial, yeast or insect cells. For example, the expression vector may be transferred into the host cell by physical, chemical or biological means.
Physical methods for introducing polynucleotides into host cells include calcium phosphate precipitation, lipofection, particle bombardment, microinjection, electroporation, and the like. Methods for producing cells comprising vectors and/or exogenous nucleic acids are well known in the art. See, e.g., Sambrook et al (2001, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, New York). A preferred method for introducing the polynucleotide into a host cell is calcium phosphate transfection.
Biological methods for introducing a polynucleotide of interest into a host cell include the use of DNA and RNA vectors. Viral vectors, particularly retroviral vectors, have become the most widely used method for inserting genes into mammalian, e.g., human, cells. Other viral vectors may be derived from lentiviruses, poxviruses, herpes simplex virus I, adenoviruses, adeno-associated viruses, and the like. See, for example, U.S. patent nos. 5,350,674 and 5,585,362.
Chemical means of introducing polynucleotides into host cells include colloidal dispersion systems such as macromolecular complexes, nanocapsules, microspheres, beads; and lipid-based systems including oil-in-water emulsions, micelles, mixed micelles, and liposomes. Exemplary colloidal systems for use as delivery vehicles in vitro and in vivo are liposomes (e.g., artificial membrane vesicles).
In the case of non-viral delivery systems, an exemplary delivery vehicle is a liposome. Lipid formulations are contemplated for use to introduce nucleic acids into host cells (ex vivo or in vivo). In another aspect, the nucleic acid can be associated with a lipid. The nucleic acid associated with the lipid may be encapsulated in the aqueous interior of the liposome, dispersed within the lipid bilayer of the liposome, attached to the liposome via a linker molecule associated with both the liposome and the oligonucleotide, entrapped in the liposome, complexed with the liposome, dispersed in a solution comprising the lipid, mixed with the lipid, associated with the lipid, contained as a suspension in the lipid, contained in or complexed with a micelle, or otherwise associated with the lipid. The lipid, lipid/DNA or lipid/expression vector associated with the composition is not limited to any particular structure in solution. For example, they may be present in bilayer structures, either as micelles or with a "collapsed" structure. They may also simply be dispersed in a solution, possibly forming aggregates that are not uniform in size or shape. Lipids are fatty substances, which may be naturally occurring or synthetic lipids. For example, lipids include fatty droplets that occur naturally in the cytoplasm as well as such compounds that contain long-chain aliphatic hydrocarbons and their derivatives such as fatty acids, alcohols, amines, amino alcohols, and aldehydes.
In a preferred embodiment of the invention, the vector is a lentiviral vector.
Preparation
The invention provides a CAR according to the first aspect of the invention, a nucleic acid molecule according to the second aspect of the invention, a vector according to the third aspect of the invention, or a vector according to the fourth aspect of the inventionThe host cell, and a pharmaceutically acceptable carrier, diluent or excipient. In one embodiment, the formulation is a liquid formulation. Preferably, the formulation is an injection. Preferably, the CAR-T cells are present in the formulation at a concentration of 1X 10 5 -1×10 8 One cell/ml, preferably 1X 10 6 -1×10 7 One cell/ml, more preferably 1X 10 6 -5×10 6 Individual cells/ml. In one embodiment, the formulation may include a buffer such as neutral buffered saline, sulfate buffered saline, and the like; carbohydrates such as glucose, mannose, sucrose or dextran, mannitol; a protein; polypeptides or amino acids such as glycine; an antioxidant; chelating agents such as EDTA or glutathione; adjuvants (e.g., aluminum hydroxide); and a preservative. The formulations of the present invention are preferably formulated for intravenous administration.
Therapeutic applications
The invention includes therapeutic applications of cells (e.g., T cells) transduced with Lentiviral Vectors (LV) encoding expression cassettes of the invention. The transduced T cells can target a marker CD155 protein of tumor cells, and the T cells are synergistically activated to cause cellular immune response, so that the killing efficiency of the T cells on solid tumors is remarkably improved.
Accordingly, the present invention also provides a method of stimulating a T cell-mediated immune response to a target cell population or tissue of a mammal comprising the steps of: administering to the mammal the CAR-T cells of the invention.
In one embodiment, the invention includes a class of cell therapy in which autologous T cells (or allogeneic donors) from a patient are isolated, activated, genetically engineered to produce CAR-T cells, and subsequently injected into the same patient. In this way, the probability of graft versus host disease is very low and antigens are recognized by T cells in a non-MHC restricted manner. Furthermore, one CAR-T can treat all cancers expressing this antigen. Unlike antibody therapy, CAR-T cells are able to replicate in vivo, resulting in long-term persistence that can lead to sustained tumor control.
In one embodiment, the CAR-T cells of the invention can undergo robust in vivo T cell expansion and can persist for an extended amount of time. Additionally, the CAR-mediated immune response can be part of an adoptive immunotherapy step, wherein the CAR-modified T cell induces an immune response specific to the antigen binding domain in the CAR. For example, CAR-T cells of CD155 elicit a specific immune response against cells expressing CD 155.
Although the data disclosed herein specifically disclose lentiviral vectors comprising the extracellular segment, hinge and transmembrane regions, and the 4-1BB and CD3zeta signaling domains of TIGIT against CD155, the invention should be construed to include any number of variations to each of the construct components.
Treatable cancers include tumors that are not vascularized or have not substantially vascularized, as well as vascularized tumors. The cancer may comprise a non-solid tumor (such as a hematological tumor, e.g., leukemia and lymphoma) or may comprise a solid tumor. The types of cancer treated with the CARs of the invention include, but are not limited to, carcinomas, blastomas and sarcomas, and certain leukemias or lymphoid malignancies, benign and malignant tumors, such as sarcomas, carcinomas and melanomas. Adult tumors/cancers and pediatric tumors/cancers are also included.
Hematologic cancers are cancers of the blood or bone marrow. Examples of hematologic (or hematological) cancers include leukemias, including acute leukemias (such as acute lymphocytic leukemia, acute myelogenous leukemia and myeloblastic, promyelocytic, granulo-monocytic, and erythroleukemia), chronic leukemias (such as chronic myelogenous (granulocytic) leukemia, chronic myelogenous leukemia, and chronic lymphocytic leukemia), polycythemia vera, lymphomas, hodgkin's disease, non-hodgkin's lymphoma (indolent and higher order forms), multiple myeloma, waldenstrom's macroglobulinemia, heavy chain disease, myelodysplastic syndrome, hairy cell leukemia, and myelodysplasia.
A solid tumor is an abnormal mass of tissue that generally does not contain cysts or fluid areas. Solid tumors can be benign or malignant. Different types of solid tumors are named for the cell types that form them (such as sarcomas, carcinomas, and lymphomas). Examples of solid tumors such as sarcomas and carcinomas include fibrosarcoma, myxosarcoma, liposarcoma mesothelioma, lymphoid malignancies, pancreatic cancer, ovarian cancer.
The CAR-modified T cells of the invention may also be used as a type of vaccine for ex vivo immunization and/or in vivo therapy of mammals. Preferably, the mammal is a human.
For ex vivo immunization, at least one of the following occurs in vitro prior to administration of the cells into a mammal: i) expanding the cell, ii) introducing a nucleic acid encoding the CAR into the cell, and/or iii) cryopreserving the cell.
Ex vivo procedures are well known in the art and are discussed more fully below. Briefly, cells are isolated from a mammal (preferably a human) and genetically modified (i.e., transduced or transfected in vitro) with a vector expressing a CAR disclosed herein. The CAR-modified cells can be administered to a mammalian recipient to provide a therapeutic benefit. The mammalian recipient can be a human, and the CAR-modified cells can be autologous with respect to the recipient. Alternatively, the cells may be allogeneic, syngeneic (syngeneic), or xenogeneic with respect to the recipient.
In addition to using cell-based vaccines for ex vivo immunization, the present invention also provides compositions and methods for in vivo immunization to elicit an immune response against an antigen in a patient.
The invention provides a method of treating a tumor comprising administering to a subject in need thereof a therapeutically effective amount of a CAR-modified T cell of the invention.
The CAR-modified T cells of the invention can be administered alone or as a pharmaceutical composition in combination with diluents and/or with other components or other cytokines or cell populations. Briefly, a pharmaceutical composition of the invention may comprise a target cell population as described herein, in combination with one or more pharmaceutically or physiologically acceptable carriers, diluents, or excipients. Such compositions may include buffers such as neutral buffered saline, sulfate buffered saline, and the like; carbohydrates such as glucose, mannose, sucrose or dextran, mannitol; a protein; polypeptides or amino acids such as glycine; an antioxidant; chelating agents such as EDTA or glutathione; adjuvants (e.g., aluminum hydroxide); and a preservative. The compositions of the present invention are preferably formulated for intravenous administration.
The pharmaceutical compositions of the present invention may be administered in a manner suitable for the disease to be treated (or prevented). The number and frequency of administration will be determined by such factors as the condition of the patient, and the type and severity of the patient's disease-although the appropriate dosage may be determined by clinical trials.
When referring to an "immunologically effective amount", "an anti-tumor effective amount", "a tumor-inhibiting effective amount", or a "therapeutic amount", the precise amount of the composition of the invention to be administered can be determined by a physician, taking into account the age, weight, tumor size, extent of infection or metastasis, and individual differences in the condition of the patient (subject). It can be generally pointed out that: pharmaceutical compositions comprising T cells described herein can be in the range of 10 4 To 10 9 Dosage of individual cells/kg body weight, preferably 10 5 To 10 6 Doses of individual cells per kg body weight (including all integer values within those ranges) were administered. The T cell composition may also be administered multiple times at these doses. Cells can be administered by using infusion techniques well known in immunotherapy (see, e.g., Rosenberg et al, New Eng.J.of Med.319:1676, 1988). Optimal dosages and treatment regimens for a particular patient can be readily determined by those skilled in the medical arts by monitoring the patient for signs of disease and adjusting the treatment accordingly.
Administration of the subject composition may be carried out in any convenient manner, including by spraying, injection, swallowing, infusion, implantation or transplantation. The compositions described herein can be administered to a patient subcutaneously, intradermally, intratumorally, intranodal, intraspinally, intramuscularly, by intravenous (i.v.) injection, or intraperitoneally. In one embodiment, the T cell composition of the invention is administered to a patient by intradermal or subcutaneous injection. In another embodiment, the T cell composition of the invention is preferably administered by i.v. injection. The composition of T cells can be injected directly into the tumor, lymph node or site of infection.
In certain embodiments of the invention, cells activated and expanded using the methods described herein or other methods known in the art for expanding T cells to therapeutic levels are administered to a patient in conjunction with (e.g., prior to, concurrently with, or subsequent to) any number of relevant treatment modalities, including but not limited to treatment with: such as antiviral therapy, cidofovir and interleukin-2, cytarabine (also known as ARA-C) or natalizumab therapy for MS patients or efavirenz therapy for psoriasis patients or other therapy for PML patients. In further embodiments, the T cells of the invention may be used in combination with: chemotherapy, radiation, immunosuppressive agents such as cyclosporine, azathioprine, methotrexate, mycophenolate mofetil, and FK506, antibodies, or other immunotherapeutic agents. In a further embodiment, the cell composition of the invention is administered to the patient in conjunction with (e.g., prior to, concurrently with, or subsequent to) bone marrow transplantation with a chemotherapeutic agent such as fludarabine, external beam radiation therapy (XRT), cyclophosphamide. For example, in one embodiment, the subject may undergo standard treatment with high dose chemotherapy followed by peripheral blood stem cell transplantation. In some embodiments, after transplantation, the subject receives an injection of the expanded immune cells of the invention. In an additional embodiment, the expanded cells are administered pre-or post-surgery.
The dosage of the above treatments administered to a patient will vary with the precise nature of the condition being treated and the recipient of the treatment. The proportion of doses administered to a human can be effected in accordance with accepted practice in the art. Typically, 1X 10 may be used per treatment or per course of treatment 6 1 to 10 10 The modified T cells of the invention are administered to a patient, for example, by intravenous infusion.
The main advantages of the invention include:
(1) the engineered immune cells of the invention can specifically target CD155, thereby selectively killing tumors from which CD155 is specifically and highly expressed.
(2) The invention discovers for the first time that the T cell TIGIT CAR-T modified by the chimeric antigen receptor has obvious and specific killing effect on tumor cells with high expression of CD155, and the prepared TIGIT CAR-T cell can be used for treating various tumor patients due to the high expression of CD155 in various tumor cells.
(3) The invention discovers for the first time that the engineered immune cells of the invention can greatly reduce the off-target effect of CAR-T therapy and can treat a variety of tumors.
(4) The invention discovers for the first time that T cells modified by a chimeric antigen receptor containing an extracellular segment of TIGIT or a CD 155-targeted antibody single-chain variable region sequence have very excellent killing effect on tumors with high expression of CD155, have no killing effect on normal cells and have very good specificity.
(5) The extracellular segment of TIGIT used in the invention is humanized, and the problem of immunogenicity of a murine single-chain antibody as a target is solved.
(6) The TIGIT CAR-T disclosed by the invention not only has a killing effect on various tumors, but also can neutralize and relieve the inhibiting effect on T cell, NK, DC and other immune cell functions mediated by TIGIT.
The invention will be further illustrated with reference to the following specific examples. It should be understood that these examples are for illustrative purposes only and are not intended to limit the scope of the present invention. Experimental procedures without specific conditions noted in the following examples, generally following conventional conditions, such as Sambrook et al, molecular cloning: the conditions described in the Laboratory Manual (New York: Cold Spring Harbor Laboratory Press,1989), or according to the manufacturer's recommendations. Unless otherwise indicated, percentages and parts are percentages and parts by weight.
Unless otherwise specified, materials and reagents used in examples of the present invention are commercially available products.
CD155 is highly expressed in a variety of tumors.
Example 1
The expression of CD155 in tumor cell lines (https:// ports. branched. org/capsule/pagegene ═ PVR) was retrieved using the CCLE database, and as a result, as shown in FIG. 1, CD155 was highly expressed in various solid tumors, even in hematological tumors such as T-cell _ lymphoma, lymphoma _ Hodgkin, and the like.
Example 2
As a result of immunohistochemical staining of various tumor tissues and tissues adjacent to the tumor and normal tissues using the tissue chip, as shown in FIG. 2, CD155 was highly expressed in tumors such as liver cancer, prostate cancer, ovarian cancer, stomach cancer and pancreatic cancer (FIG. 2-A), was also expressed in tissues adjacent to the tumor (FIG. 2-B), and was less expressed or not expressed in normal tissues (FIG. 2-C).
Example 3
Further, the expression of CD155 in different tumor cell lines was stained by flow cytometry, well-grown tumor cells were collected and trypsinized, and then antibody staining was performed on the cells, and as a result, as shown in fig. 3, CD155 was highly expressed in cells such as a liver cancer cell line huh7, a prostate cancer cell line PC3, an ovarian cancer cell line ES-1, a stomach cancer cell line HGC, and a pancreatic cancer cell line PANC 1.
The CAR extracellular antigen recognition region is the TIGIT extracellular domain protein as an example.
Example 4
The plasmid vector of the expression cassette was constructed, the structure and positional relationship of each element on the expression cassette are referred to fig. 2, and the original backbone of the plasmid vector was an empty vector (lentivirus transfer vector obtained from gay biosciences, inc. of shanghai). Three CAR sequences were designed according to CAR structural sequence. The device comprises the following components:
(1)pELPS-TIGIT-CD8H-BBz:TIGIT ECD+CD8hinge+CD8αTM+4-1BB+CD3ζ(SEQ ID NO.6);
(2) to assess the effect of the presence or absence of a hinge region on anti-tumor activity, a hinge region-free CD8hinge CAR sequence corresponding to pels-TIGIT-CD 8H-BBz was constructed:
pELPS-TIGIT-BBz:TIGIT ECD+CD8αTM+4-1BB+CD3ζ
(3)pELPS-PVR-CD8H-BBz:anti-CD155 scfv+CD8hinge+CD8αTM+4-1BB+CD3ζ(SEQ ID NO.10);
the front end of the probe has a CD8 alpha signal peptide.
After the plasmid was designed, the map was sent to a plasmid company and synthesized and purchased from the company. The resulting lentiviral plasmid vectors were designated pELPS-TIGIT-CD8H-BBz and pELPS-TIGIT-BBz, pELPS-PVR-CD8H-BBz plasmids, as shown in FIG. 4.
Hereinafter, plasmids pELPS-TIGIT-CD8H-BBz are abbreviated as CD8H-CD8TM, pELPS-TIGIT-BBz are abbreviated as CD8TM and are collectively referred to as TIGIT-CAR, obtained CAR-T is referred to as TIGIT-CAR-T, pELPS-PVR-CD8H-BBz is abbreviated as PVRbbz, and obtained CAR-T is referred to as PVRbbz-CAR-T.
Example 5
Construction of lentiviruses containing chimeric antigen receptors expressing the above
The method comprises the following steps:
the two target plasmids in example 1, the lentiviral packaging plasmid psPAX2 and the lentiviral enveloping plasmid pMD2.G are transfected into 293T cells by a transfection reagent PEI, and culture supernatant is obtained and then subjected to ultrafiltration and super-separation concentration to obtain the lentivirus expressing the target element.
Example 6
Construction of chimeric antigen receptor expressing T cells
T cells were isolated from human blood using the magnetic bead method, activated with CD3/CD28 complex, infected with packaged lentivirus, and expression levels were measured by 48h flow cytometry. The specific process is as follows:
(1) a lymphocyte separation medium was first added to a 350mL centrifuge tube, and then blood was added to the upper layer of the lymphocyte separation medium (the volume ratio of the lymphocyte separation medium to the blood was 2:1) while keeping the interface clear, and the mixture was centrifuged at 800g for 25 minutes (the centrifuge was set to 1 at the rising rate and 0 at the falling rate).
(2) After the centrifugation is finished, the eukaryotic cell layer is carefully sucked, added with PBS and gently blown and uniformly mixed, and centrifuged for 10 minutes at 500 g.
(3) After resuspending the cells with PBS, the cells were counted. After counting, after centrifugation at 300g for 10 minutes, the supernatant was discarded and buffer in x-vivo medium containing 10% serum was added (10 × 1) 7 Adding 70 mu L of each cell
(4) According to 1 x 10 7 After adding 20. mu.L of CD4/CD8 magnetic beads to each cell, the cells were washed with cell buffer at 4 ℃ and resuspended.
(5) The column was placed in a magnetic field, rinsed with buffer, and the cell suspension was added, after the liquid had run off, the column was rinsed twice with buffer.
(6) The column was removed from the magnetic field, and after adding buffer, the cells were quickly pushed into the centrifuge tube with a plunger and centrifuged at 300g for 10 minutes.
(7) After completion of centrifugation, the supernatant was discarded, and cells were resuspended in an x-vivo complete medium (containing 10% serum and containing IL-7, IL-15, and IL-21 at a ratio of 1: 1000) and counted.
(8) Adjusting cell density to 1 x 10 6 Cells were activated by adding a complex of CD3 and CD28 at a ratio of 1: 100/mL.
(9) After 48h of activation, the cells can be counted for subsequent lentiviral infection. According to the titer of the prepared virus, adding lentivirus into T cells, centrifuging at 1800rpm at 32 ℃ for 1h, placing the T cells in an incubator for culture, changing the culture solution after 12h, and detecting the expression of CAR by 48h flow cytometry.
Example 7
20 ten thousand 293t cells are paved in a 24-well plate, different numbers of viruses are added after the cells are attached to the wall, and the titer of the viruses is calculated by detecting the expression of TIGIT in the cells after 48 hours. As a result, as shown in FIG. 5, a high positive rate was obtained with a virus amount of 1. mu.L, indicating a high virus titer.
Example 8
Detecting a CAR expression level of a TIGIT-CAR-T cell; infected CAR-T cells and uninfected T cells in the same batch are taken as a control, the cells are incubated with a Flow antibody of TIGIT for 30 minutes, washed twice and detected by a Flow cytometer Fortessa, and the expression result is obtained by Flow Jo analysis. The results are shown in FIG. 6. Figure 6 results show that CAR expression levels of T cells after infection with both viruses were 58.3% and 60.8%, respectively.
Example 9
The killing effect of different cancer cells (such as human prostate cancer cell line PC3, pancreatic cancer cell line Panc-1 and ovarian cancer cell line OVCAR-83 cancer cell lines) is tested.
Adding target cells 2X 10 in a low adsorption well plate 4 Number/well, in an effective target ratio (number of effector cells: number of target cells) 4: 1. 2: 1. 1: 1. 0.5: 1. 0: 1 corresponding number of CAR-T cells were added while different gradient target cell wells were made (0, 1, 2, 4, 8, 10 ten thousand). Co-incubationAfter (co-culture: CC) for 12-48h, because the target cells can express luciferase, after the substrate is added, the light absorption value and the number of the cells are in a linear relationship, and the killing effect of two CAR-T cells on the target cells can be embodied through reading.
From the results of fig. 7, it is seen that the unmodified T cell has no killing function on the target cell, but the killing effect of the two CAR-T cells is obvious, and the killing effect gradually increases with the increase of the effective-to-target ratio, which indicates that when the CAR extracellular antigen recognition region targeting CD155 is TIGIT extracellular segment protein, the TIGIT-CAR-T cell has a good anti-tumor activity.
A single-chain antibody fragment in which the CAR extracellular antigen recognition region is antigen-specific is exemplified.
Example 10
Construction of chimeric antigen receptor (PVRbbz) expressing T cells
After lentivirus is packaged and T cells obtained by collection and separation by the method, the T cells are infected by MOI (maximum organization of identity) 10 according to the calculation of virus titer, centrifuged at 1800rpm at 32 ℃ for 2h, cultured in an incubator, changed after 12h, and the expression of CAR is detected by 48h flow cytometry.
The results are shown in FIG. 8, and the positive rate can reach 61.5%.
Example 11
Validation of the PVRbbz CAR-T specific killing of CD 155-positive cells
CD155 knock-out of PC3 cells using crispr cas9, the results of which are shown in FIG. 9, by adding 2X 10 target cells to low adsorption well plates 4 Number/well, in an effective target ratio (number of effector cells: number of target cells) of 0.625: 1 corresponding amount of CAR-T cells were added and after co-incubation (co-culture: CC)12, the killing effect was examined, and the results are shown in FIG. 10, in which PVRbbz had no killing effect on PC3 cells of CD155KO, showing that PVRbbzCAR-T can specifically kill CD155 positive tumor cells, but no effect on CD155 negative cells.
Example 12
The PVRbbz CAR-T is used for detecting the killing effect of different cancer cells (human prostate cancer cell line PC3, pancreatic cancer cell line Panc-1, ovarian cancer cell line ES-1, osteosarcoma cell line U205, liver cancer cell line Huh7 and blood tumor cell line mm1s 6).
Example 13
Rapid regression of PVRbbz CAR-T tumors in bone metastasis models
The bone metastasis osteosarcoma model constructed by injecting human osteosarcoma cell line 143B into tibia and imaging the tumor change by IVIS in vivo imaging system using 5 × 106 effector cells/mouse was followed, and as a result, as shown in fig. 12, PVRbbz CAR-T exhibited a significant antitumor effect and no significant toxicity was found in the main organs in vivo after the mice were sacrificed (as shown in fig. 13).
Example 14
CD155 inhibits immune function of T cells
After a CD155 knockout PC3 cell (PC3-CD155KO) or a CD155 knockout PC3 cell (PC3-WT) and a T cell are incubated for 24h at a ratio of 1:1, the expression of IFN gamma, TNF alpha and granzyme B (GraB) of the T cell (CD4 and CD8) is detected by flow, and the result is shown in FIG. 14A, and the capability of the T cell to express a functional cytokine is obviously reduced after the incubation of the T cell and a CD155 positive tumor cell. For example, in CD8, the ratio of expression of IFN γ in T cells incubated with PC3-CD155KO cells was 48.4%, the ratio of expression of TNF α was 70%, and the ratio of expression of IFN γ in T cells incubated with PC3-WT cells was 58.2%, the ratio of expression of IFN γ in T cells incubated with PC3-WT cells was 33.4%, the ratio of expression of TNF α was 36.3%, and the ratio of expression of granzyme B was 43.9%.
CD155 knock-out PC3-PSMA + Cell (PC3+ CD155KO) or PC3-PSMA without knockout of CD155 + Cell (PC3+ WT) and PSMA-CAR-T-targeting PSMACells with low (target antigen multi) effective target ratio E: T (effector cells: target cells): the killing efficiency was calculated after incubation for 12h at 1:1, 0.5:1, 0.25:1, 0.125: 1. As a result, as shown in FIG. 14B, the expression of CD155 significantly reduced the killing effect of PSMA-CAR-T on target cells.
In conclusion, the present invention finds that CD155 is highly expressed in solid tumor and hematological tumor such as liver cancer, prostate cancer, ovarian cancer, gastric cancer, pancreatic cancer, etc., and uniform antigen expression is very important for CAR T cell-based therapy, because tumor target heterogeneity is a limiting factor in the treatment of malignant tumor using these powerful and specific therapies, the CD 155-targeted CAR T cells of the present invention can exhibit strong activity on various tumor cells (including solid tumor, liquid tumor). And can also play an obvious anti-tumor effect in vivo under the non-toxic effect. Cellular immunotherapy targeting CD155 may alternatively exert anti-tumor effects in a variety of tumors.
All documents referred to herein are incorporated by reference into this application as if each were individually incorporated by reference. Furthermore, it should be understood that various changes or modifications of the present invention can be made by those skilled in the art after reading the above teachings of the present invention, and these equivalents also fall within the scope of the appended claims of the present application.
Sequence listing
<110> university of east China
Shanghai Bangyao Biological Technology Co.,Ltd.
<120> novel CAR-T cells targeting multiple tumors and preparation and methods thereof
<130> P2020-2785
<150> CN 201911415453.X
<151> 2019-12-31
<160> 20
<170> SIPOSequenceListing 1.0
<210> 1
<211> 120
<212> PRT
<213> Intelligent (Homo sapiens)
<400> 1
Met Met Thr Gly Thr Ile Glu Thr Thr Gly Asn Ile Ser Ala Glu Lys
1 5 10 15
Gly Gly Ser Ile Ile Leu Gln Cys His Leu Ser Ser Thr Thr Ala Gln
20 25 30
Val Thr Gln Val Asn Trp Glu Gln Gln Asp Gln Leu Leu Ala Ile Cys
35 40 45
Asn Ala Asp Leu Gly Trp His Ile Ser Pro Ser Phe Lys Asp Arg Val
50 55 60
Ala Pro Gly Pro Gly Leu Gly Leu Thr Leu Gln Ser Leu Thr Val Asn
65 70 75 80
Asp Thr Gly Glu Tyr Phe Cys Ile Tyr His Thr Tyr Pro Asp Gly Thr
85 90 95
Tyr Thr Gly Arg Ile Phe Leu Glu Val Leu Glu Ser Ser Val Ala Glu
100 105 110
His Gly Ala Arg Phe Gln Ile Pro
115 120
<210> 2
<211> 360
<212> DNA
<213> Intelligent (Homo sapiens)
<400> 2
atgatgacag gcacaataga aacaacgggg aacatttctg cagagaaagg tggctctatc 60
atcttacaat gtcacctctc ctccaccacg gcacaagtga cccaggtcaa ctgggagcag 120
caggaccagc ttctggccat ttgtaatgct gacttggggt ggcacatctc cccatccttc 180
aaggatcgag tggccccagg tcccggcctg ggcctcaccc tccagtcgct gaccgtgaac 240
gatacagggg agtacttctg catctatcac acctaccctg atgggacgta cactgggaga 300
atcttcctgg aggtcctaga aagctcagtg gctgagcacg gtgccaggtt ccagattcca 360
<210> 3
<211> 21
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 3
Met Arg Trp Cys Leu Leu Leu Ile Trp Ala Gln Gly Leu Arg Gln Ala
1 5 10 15
Pro Leu Ala Ser Gly
20
<210> 4
<211> 42
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 4
Lys Arg Gly Arg Lys Lys Leu Leu Tyr Ile Phe Lys Gln Pro Phe Met
1 5 10 15
Arg Pro Val Gln Thr Thr Gln Glu Glu Asp Gly Cys Ser Cys Arg Phe
20 25 30
Pro Glu Glu Glu Glu Gly Gly Cys Glu Leu
35 40
<210> 5
<211> 112
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 5
Arg Val Lys Phe Ser Arg Ser Ala Asp Ala Pro Ala Tyr Lys Gln Gly
1 5 10 15
Gln Asn Gln Leu Tyr Asn Glu Leu Asn Leu Gly Arg Arg Glu Glu Tyr
20 25 30
Asp Val Leu Asp Lys Arg Arg Gly Arg Asp Pro Glu Met Gly Gly Lys
35 40 45
Pro Arg Arg Lys Asn Pro Gln Glu Gly Leu Tyr Asn Glu Leu Gln Lys
50 55 60
Asp Lys Met Ala Glu Ala Tyr Ser Glu Ile Gly Met Lys Gly Glu Arg
65 70 75 80
Arg Arg Gly Lys Gly His Asp Gly Leu Tyr Gln Gly Leu Ser Thr Ala
85 90 95
Thr Lys Asp Thr Tyr Asp Ala Leu His Met Gln Ala Leu Pro Pro Arg
100 105 110
<210> 6
<211> 364
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 6
Met Arg Trp Cys Leu Leu Leu Ile Trp Ala Gln Gly Leu Arg Gln Ala
1 5 10 15
Pro Leu Ala Ser Gly Met Met Thr Gly Thr Ile Glu Thr Thr Gly Asn
20 25 30
Ile Ser Ala Glu Lys Gly Gly Ser Ile Ile Leu Gln Cys His Leu Ser
35 40 45
Ser Thr Thr Ala Gln Val Thr Gln Val Asn Trp Glu Gln Gln Asp Gln
50 55 60
Leu Leu Ala Ile Cys Asn Ala Asp Leu Gly Trp His Ile Ser Pro Ser
65 70 75 80
Phe Lys Asp Arg Val Ala Pro Gly Pro Gly Leu Gly Leu Thr Leu Gln
85 90 95
Ser Leu Thr Val Asn Asp Thr Gly Glu Tyr Phe Cys Ile Tyr His Thr
100 105 110
Tyr Pro Asp Gly Thr Tyr Thr Gly Arg Ile Phe Leu Glu Val Leu Glu
115 120 125
Ser Ser Val Ala Glu His Gly Ala Arg Phe Gln Ile Pro Thr Thr Thr
130 135 140
Pro Ala Pro Arg Pro Pro Thr Pro Ala Pro Thr Ile Ala Ser Gln Pro
145 150 155 160
Leu Ser Leu Arg Pro Glu Ala Cys Arg Pro Ala Ala Gly Gly Ala Val
165 170 175
His Thr Arg Gly Leu Asp Phe Ala Cys Asp Ile Tyr Ile Trp Ala Pro
180 185 190
Leu Ala Gly Thr Cys Gly Val Leu Leu Leu Ser Leu Val Ile Thr Leu
195 200 205
Tyr Cys Lys Arg Gly Arg Lys Lys Leu Leu Tyr Ile Phe Lys Gln Pro
210 215 220
Phe Met Arg Pro Val Gln Thr Thr Gln Glu Glu Asp Gly Cys Ser Cys
225 230 235 240
Arg Phe Pro Glu Glu Glu Glu Gly Gly Cys Glu Leu Arg Val Lys Phe
245 250 255
Ser Arg Ser Ala Asp Ala Pro Ala Tyr Lys Gln Gly Gln Asn Gln Leu
260 265 270
Tyr Asn Glu Leu Asn Leu Gly Arg Arg Glu Glu Tyr Asp Val Leu Asp
275 280 285
Lys Arg Arg Gly Arg Asp Pro Glu Met Gly Gly Lys Pro Arg Arg Lys
290 295 300
Asn Pro Gln Glu Gly Leu Tyr Asn Glu Leu Gln Lys Asp Lys Met Ala
305 310 315 320
Glu Ala Tyr Ser Glu Ile Gly Met Lys Gly Glu Arg Arg Arg Gly Lys
325 330 335
Gly His Asp Gly Leu Tyr Gln Gly Leu Ser Thr Ala Thr Lys Asp Thr
340 345 350
Tyr Asp Ala Leu His Met Gln Ala Leu Pro Pro Arg
355 360
<210> 7
<211> 1092
<212> DNA
<213> Artificial sequence (artificial sequence)
<400> 7
atgcgctggt gtctcctcct gatctgggcc caggggctga ggcaggctcc cctcgcctca 60
ggaatgatga caggcacaat agaaacaacg gggaacattt ctgcagagaa aggtggctct 120
atcatcttac aatgtcacct ctcctccacc acggcacaag tgacccaggt caactgggag 180
cagcaggacc agcttctggc catttgtaat gctgacttgg ggtggcacat ctccccatcc 240
ttcaaggatc gagtggcccc aggtcccggc ctgggcctca ccctccagtc gctgaccgtg 300
aacgatacag gggagtactt ctgcatctat cacacctacc ctgatgggac gtacactggg 360
agaatcttcc tggaggtcct agaaagctca gtggctgagc acggtgccag gttccagatt 420
ccaaccacga cgccagcgcc gcgaccacca acaccggcgc ccaccatcgc gtcgcagccc 480
ctgtccctgc gcccagaggc gtgccggcca gcggcggggg gcgcagtgca cacgaggggg 540
ctggacttcg cctgtgatat ctacatctgg gcgcccttgg ccgggacttg tggggtcctt 600
ctcctgtcac tggttatcac cctttactgc aaacggggca gaaagaaact cctgtatata 660
ttcaaacaac catttatgag accagtacaa actactcaag aggaagatgg ctgtagctgc 720
cgatttccag aagaagaaga aggaggatgt gaactgagag tgaagttcag caggagcgca 780
gacgcccccg cgtacaagca gggccagaac cagctctata acgagctcaa tctaggacga 840
agagaggagt acgatgtttt ggacaagaga cgtggccggg accctgagat ggggggaaag 900
ccgagaagga agaaccctca ggaaggcctg tacaatgaac tgcagaaaga taagatggcg 960
gaggcctaca gtgagattgg gatgaaaggc gagcgccgga ggggcaaggg gcacgatggc 1020
ctttaccagg gtctcagtac agccaccaag gacacctacg acgcccttca catgcaggcc 1080
ctgccccctc gc 1092
<210> 8
<211> 45
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 8
Thr Thr Thr Pro Ala Pro Arg Pro Pro Thr Pro Ala Pro Thr Ile Ala
1 5 10 15
Ser Gln Pro Leu Ser Leu Arg Pro Glu Ala Cys Arg Pro Ala Ala Gly
20 25 30
Gly Ala Val His Thr Arg Gly Leu Asp Phe Ala Cys Asp
35 40 45
<210> 9
<211> 24
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 9
Ile Tyr Ile Trp Ala Pro Leu Ala Gly Thr Cys Gly Val Leu Leu Leu
1 5 10 15
Ser Leu Val Ile Thr Leu Tyr Cys
20
<210> 10
<211> 512
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 10
Met Ala Leu Pro Val Thr Ala Leu Leu Leu Pro Leu Ala Leu Leu Leu
1 5 10 15
His Ala Ala Arg Pro Glu Val Gln Leu Gln Gln Ser Gly Ala Glu Leu
20 25 30
Val Arg Pro Gly Thr Ser Val Lys Leu Ser Cys Lys Ala Leu Gly Tyr
35 40 45
Thr Phe Thr Asp His Glu Met His Trp Val Lys Gln Thr Pro Val His
50 55 60
Gly Leu Glu Trp Ile Gly Thr Ile His Pro Gly Ser Gly Val Thr Ala
65 70 75 80
Tyr Asn Gln Lys Phe Lys Gly Lys Ala Thr Leu Thr Ala Asp Lys Ser
85 90 95
Ser Ser Thr Ala Tyr Met Glu Leu Ser Thr Leu Thr Ser Glu Asp Ser
100 105 110
Ala Val Tyr Tyr Cys Thr Pro Leu Trp Leu Arg Arg Asp Trp Gly Gln
115 120 125
Gly Thr Thr Leu Thr Val Ser Thr Gly Gly Gly Gly Ser Gly Gly Gly
130 135 140
Gly Ser Gly Gly Gly Gly Ser Ala Leu Asp Ile Gln Met Thr Gln Thr
145 150 155 160
Pro Lys Phe Met Ser Thr Ser Val Gly Asp Arg Val Ser Val Thr Cys
165 170 175
Lys Ala Ser Gln Asn Val Ala Thr Asn Val Val Trp Phe Gln Gln Lys
180 185 190
Ser Gly Gln Ser Pro Lys Ala Leu Ile Tyr Ser Ala Ser Tyr Arg Tyr
195 200 205
Ser Gly Val Pro Asp Arg Phe Thr Gly Ser Gly Ser Gly Thr Asp Phe
210 215 220
Thr Leu Thr Ile Ser Asn Val Gln Ser Glu Asp Leu Ala Glu Tyr Phe
225 230 235 240
Cys Gln Gln Tyr Asn Asn Tyr Pro Leu Thr Phe Gly Ala Gly Thr Lys
245 250 255
Leu Glu Leu Lys Ala Ala Ala Gly Ala Pro Val Pro Tyr Pro Asp Pro
260 265 270
Leu Glu Pro Arg Gly Ala Ala Ser Ala Trp Ser His Pro Gln Phe Glu
275 280 285
Lys Thr Thr Thr Pro Ala Pro Arg Pro Pro Thr Pro Ala Pro Thr Ile
290 295 300
Ala Ser Gln Pro Leu Ser Leu Arg Pro Glu Ala Cys Arg Pro Ala Ala
305 310 315 320
Gly Gly Ala Val His Thr Arg Gly Leu Asp Phe Ala Cys Asp Ile Tyr
325 330 335
Ile Trp Ala Pro Leu Ala Gly Thr Cys Gly Val Leu Leu Leu Ser Leu
340 345 350
Val Ile Thr Leu Tyr Cys Lys Arg Gly Arg Lys Lys Leu Leu Tyr Ile
355 360 365
Phe Lys Gln Pro Phe Met Arg Pro Val Gln Thr Thr Gln Glu Glu Asp
370 375 380
Gly Cys Ser Cys Arg Phe Pro Glu Glu Glu Glu Gly Gly Cys Glu Leu
385 390 395 400
Arg Val Lys Phe Ser Arg Ser Ala Asp Ala Pro Ala Tyr Lys Gln Gly
405 410 415
Gln Asn Gln Leu Tyr Asn Glu Leu Asn Leu Gly Arg Arg Glu Glu Tyr
420 425 430
Asp Val Leu Asp Lys Arg Arg Gly Arg Asp Pro Glu Met Gly Gly Lys
435 440 445
Pro Arg Arg Lys Asn Pro Gln Glu Gly Leu Tyr Asn Glu Leu Gln Lys
450 455 460
Asp Lys Met Ala Glu Ala Tyr Ser Glu Ile Gly Met Lys Gly Glu Arg
465 470 475 480
Arg Arg Gly Lys Gly His Asp Gly Leu Tyr Gln Gly Leu Ser Thr Ala
485 490 495
Thr Lys Asp Thr Tyr Asp Ala Leu His Met Gln Ala Leu Pro Pro Arg
500 505 510
<210> 11
<211> 1536
<212> DNA
<213> Artificial sequence (artificial sequence)
<400> 11
atggccttac cagtgaccgc cttgctcctg ccgctggcct tgctgctcca cgccgccagg 60
ccggaggttc agctgcagca gtctggggct gagctggtga ggcctgggac ttcagtgaag 120
ctgtcctgca aggctttggg ctacacattt actgaccatg aaatgcactg ggtgaaacag 180
acacctgtgc atggcctgga atggattgga actattcatc caggaagtgg tgttactgcc 240
tacaatcaga agttcaaggg caaggccaca ctgactgcag acaaatcctc cagcacagcc 300
tacatggagc tcagcaccct gacatctgaa gactctgctg tctattactg tacaccactt 360
tggttacgac gggactgggg ccaaggcacc actctcacag tgtcgacagg tggaggcggt 420
tcaggcggag gtggctctgg cggtggcgga agtgcactcg atatccagat gacacagact 480
ccaaaattca tgtccacatc agtaggagac agggtcagcg tcacctgcaa ggccagtcag 540
aatgtggcta ctaatgtagt ctggtttcaa cagaaatcag ggcaatctcc taaagcactg 600
atttactcgg catcctaccg gtacagtgga gtccctgatc gcttcacagg cagtggatct 660
gggacagatt tcactctcac catcagcaat gtgcagtctg aagacttggc agagtatttc 720
tgtcagcaat ataacaacta tcctctcacg ttcggtgctg ggaccaagct ggagctgaaa 780
gcggccgcag gtgcgccggt gccgtatcca gatccgctgg aaccgcgtgg ggccgcaagc 840
gcttggagcc acccgcagtt cgaaaaaacc acgacgccag cgccgcgacc accaacaccg 900
gcgcccacca tcgcgtcgca gcccctgtcc ctgcgcccag aggcgtgccg gccagcggcg 960
gggggcgcag tgcacacgag ggggctggac ttcgcctgtg atatctacat ctgggcgccc 1020
ttggccggga cttgtggggt ccttctcctg tcactggtta tcacccttta ctgcaaacgg 1080
ggcagaaaga aactcctgta tatattcaaa caaccattta tgagaccagt acaaactact 1140
caagaggaag atggctgtag ctgccgattt ccagaagaag aagaaggagg atgtgaactg 1200
agagtgaagt tcagcaggag cgcagacgcc cccgcgtaca agcagggcca gaaccagctc 1260
tataacgagc tcaatctagg acgaagagag gagtacgatg ttttggacaa gagacgtggc 1320
cgggaccctg agatgggggg aaagccgaga aggaagaacc ctcaggaagg cctgtacaat 1380
gaactgcaga aagataagat ggcggaggcc tacagtgaga ttgggatgaa aggcgagcgc 1440
cggaggggca aggggcacga tggcctttac cagggtctca gtacagccac caaggacacc 1500
tacgacgccc ttcacatgca ggccctgccc cctcgc 1536
<210> 12
<211> 138
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 12
Ala Leu Asp Ile Gln Met Thr Gln Thr Pro Lys Phe Met Ser Thr Ser
1 5 10 15
Val Gly Asp Arg Val Ser Val Thr Cys Lys Ala Ser Gln Asn Val Ala
20 25 30
Thr Asn Val Val Trp Phe Gln Gln Lys Ser Gly Gln Ser Pro Lys Ala
35 40 45
Leu Ile Tyr Ser Ala Ser Tyr Arg Tyr Ser Gly Val Pro Asp Arg Phe
50 55 60
Thr Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser Asn Val
65 70 75 80
Gln Ser Glu Asp Leu Ala Glu Tyr Phe Cys Gln Gln Tyr Asn Asn Tyr
85 90 95
Pro Leu Thr Phe Gly Ala Gly Thr Lys Leu Glu Leu Lys Ala Ala Ala
100 105 110
Gly Ala Pro Val Pro Tyr Pro Asp Pro Leu Glu Pro Arg Gly Ala Ala
115 120 125
Ser Ala Trp Ser His Pro Gln Phe Glu Lys
130 135
<210> 13
<211> 414
<212> DNA
<213> Artificial sequence (artificial sequence)
<400> 13
gcactcgata tccagatgac acagactcca aaattcatgt ccacatcagt aggagacagg 60
gtcagcgtca cctgcaaggc cagtcagaat gtggctacta atgtagtctg gtttcaacag 120
aaatcagggc aatctcctaa agcactgatt tactcggcat cctaccggta cagtggagtc 180
cctgatcgct tcacaggcag tggatctggg acagatttca ctctcaccat cagcaatgtg 240
cagtctgaag acttggcaga gtatttctgt cagcaatata acaactatcc tctcacgttc 300
ggtgctggga ccaagctgga gctgaaagcg gccgcaggtg cgccggtgcc gtatccagat 360
ccgctggaac cgcgtggggc cgcaagcgct tggagccacc cgcagttcga aaaa 414
<210> 14
<211> 115
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 14
Glu Val Gln Leu Gln Gln Ser Gly Ala Glu Leu Val Arg Pro Gly Thr
1 5 10 15
Ser Val Lys Leu Ser Cys Lys Ala Leu Gly Tyr Thr Phe Thr Asp His
20 25 30
Glu Met His Trp Val Lys Gln Thr Pro Val His Gly Leu Glu Trp Ile
35 40 45
Gly Thr Ile His Pro Gly Ser Gly Val Thr Ala Tyr Asn Gln Lys Phe
50 55 60
Lys Gly Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser Thr Ala Tyr
65 70 75 80
Met Glu Leu Ser Thr Leu Thr Ser Glu Asp Ser Ala Val Tyr Tyr Cys
85 90 95
Thr Pro Leu Trp Leu Arg Arg Asp Trp Gly Gln Gly Thr Thr Leu Thr
100 105 110
Val Ser Thr
115
<210> 15
<211> 345
<212> DNA
<213> Artificial sequence (artificial sequence)
<400> 15
gaggttcagc tgcagcagtc tggggctgag ctggtgaggc ctgggacttc agtgaagctg 60
tcctgcaagg ctttgggcta cacatttact gaccatgaaa tgcactgggt gaaacagaca 120
cctgtgcatg gcctggaatg gattggaact attcatccag gaagtggtgt tactgcctac 180
aatcagaagt tcaagggcaa ggccacactg actgcagaca aatcctccag cacagcctac 240
atggagctca gcaccctgac atctgaagac tctgctgtct attactgtac accactttgg 300
ttacgacggg actggggcca aggcaccact ctcacagtgt cgaca 345
<210> 16
<211> 15
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 16
Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser
1 5 10 15
<210> 17
<211> 63
<212> DNA
<213> Artificial sequence (artificial sequence)
<400> 17
atggccttac cagtgaccgc cttgctcctg ccgctggcct tgctgctcca cgccgccagg 60
ccg 63
<210> 18
<211> 21
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 18
Met Ala Leu Pro Val Thr Ala Leu Leu Leu Pro Leu Ala Leu Leu Leu
1 5 10 15
His Ala Ala Arg Pro
20
<210> 19
<211> 268
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 19
Glu Val Gln Leu Gln Gln Ser Gly Ala Glu Leu Val Arg Pro Gly Thr
1 5 10 15
Ser Val Lys Leu Ser Cys Lys Ala Leu Gly Tyr Thr Phe Thr Asp His
20 25 30
Glu Met His Trp Val Lys Gln Thr Pro Val His Gly Leu Glu Trp Ile
35 40 45
Gly Thr Ile His Pro Gly Ser Gly Val Thr Ala Tyr Asn Gln Lys Phe
50 55 60
Lys Gly Lys Ala Thr Leu Thr Ala Asp Lys Ser Ser Ser Thr Ala Tyr
65 70 75 80
Met Glu Leu Ser Thr Leu Thr Ser Glu Asp Ser Ala Val Tyr Tyr Cys
85 90 95
Thr Pro Leu Trp Leu Arg Arg Asp Trp Gly Gln Gly Thr Thr Leu Thr
100 105 110
Val Ser Thr Gly Gly Gly Gly Ser Gly Gly Gly Gly Ser Gly Gly Gly
115 120 125
Gly Ser Ala Leu Asp Ile Gln Met Thr Gln Thr Pro Lys Phe Met Ser
130 135 140
Thr Ser Val Gly Asp Arg Val Ser Val Thr Cys Lys Ala Ser Gln Asn
145 150 155 160
Val Ala Thr Asn Val Val Trp Phe Gln Gln Lys Ser Gly Gln Ser Pro
165 170 175
Lys Ala Leu Ile Tyr Ser Ala Ser Tyr Arg Tyr Ser Gly Val Pro Asp
180 185 190
Arg Phe Thr Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu Thr Ile Ser
195 200 205
Asn Val Gln Ser Glu Asp Leu Ala Glu Tyr Phe Cys Gln Gln Tyr Asn
210 215 220
Asn Tyr Pro Leu Thr Phe Gly Ala Gly Thr Lys Leu Glu Leu Lys Ala
225 230 235 240
Ala Ala Gly Ala Pro Val Pro Tyr Pro Asp Pro Leu Glu Pro Arg Gly
245 250 255
Ala Ala Ser Ala Trp Ser His Pro Gln Phe Glu Lys
260 265
<210> 20
<211> 5
<212> PRT
<213> Artificial sequence (artificial sequence)
<400> 20
Gly Gly Gly Gly Ser
1 5
Claims (13)
1. A chimeric antigen receptor CAR, comprising an antigen binding domain that specifically binds to CD155 antigen, wherein the CAR has the structure of formula I:
L-Z1-Z2-TM-C-CD3ζ (I)
in the formula (I), the compound is shown in the specification,
each "-" is independently a linker peptide or a peptide bond;
l is an optional signal peptide sequence;
z1 is an antigen binding domain comprising the extracellular segment of TIGIT or an antibody single chain variable region sequence targeting CD 155; and
z2 is a null or hinge region;
TM is a transmembrane domain;
c is a costimulatory signal molecule;
CD3 ζ is the cytoplasmic signaling sequence derived from CD3 ζ;
wherein the amino acid sequence of the extracellular segment protein of TIGIT is shown in SEQ ID NO 1, and V in the variable region sequence of the CD 155-targeted antibody single chain L1 The amino acid sequence of (1) is shown as position 152-289 of SEQ ID NO 10, and V H1 The amino acid sequence of (1) is shown as positions 22-136 of SEQ ID NO. 10.
2. The chimeric receptor CAR of claim 1, wherein said CD 155-targeting antibody single chain variable region sequence has the structure of formula a1 or a 2:
V L1 -V H1 (A1) (ii) a Or
V H1 -V L1 (A2);
Wherein, V L1 Is the light chain variable region of an anti-CD 155 antibody; v H1 Is the heavy chain variable region of an anti-CD 155 antibody; "-" is a linker peptide or flexible linker or peptide bond.
3. The chimeric antigen receptor CAR of claim 1, wherein the amino acid sequence of the CD 155-targeting antibody single chain variable region sequence is set forth in SEQ ID No. 19.
4. The chimeric antigen receptor CAR of claim 1, wherein said chimeric antigen receptor CAR has the structure of formula II or II':
L-V L1 -V H1 -Z2-TM-C-CD3 ζ (II); or
L-V H1 -V L1 -Z2-TM-C-CD3ζ(II’)
Wherein each element is as described above.
5. The chimeric antigen receptor CAR of claim 1, wherein the amino acid sequence of the CAR is as set forth in SEQ ID No. 6 or 10.
6. A nucleic acid molecule encoding the Chimeric Antigen Receptor (CAR) of claim 1.
7. A vector comprising the nucleic acid molecule of claim 6.
8. A host cell comprising the vector or chromosome of claim 7 having integrated therein the exogenous nucleic acid molecule of claim 6 or expressing the CAR of claim 1.
9. A method of making an engineered immune cell expressing the CAR of claim 1, comprising the steps of: transforming the nucleic acid molecule of claim 6 or the vector of claim 7 into a T cell or NK cell, thereby obtaining the engineered immune cell.
10. A pharmaceutical composition comprising the chimeric antigen receptor of claim 1, the nucleic acid molecule of claim 6, the vector of claim 7, or the host cell of claim 8, and a pharmaceutically acceptable carrier, diluent, or excipient.
11. Use of the chimeric antigen receptor of claim 1, the nucleic acid molecule of claim 6, the vector of claim 7, the host cell of claim 8, or the pharmaceutical composition of claim 10 for the preparation of a medicament or formulation for selective tumor killing.
12. The use of claim 11, wherein said tumor comprises a tumor that is highly expressing CD 155.
13. A kit for selective killing of tumors, comprising a container, and within the container the chimeric antigen receptor of claim 1, the nucleic acid molecule of claim 6, the vector of claim 7, or the host cell of claim 8.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911415453X | 2019-12-31 | ||
| CN201911415453 | 2019-12-31 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN113087806A CN113087806A (en) | 2021-07-09 |
| CN113087806B true CN113087806B (en) | 2022-09-06 |
Family
ID=76663746
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202011635074.4A Active CN113087806B (en) | 2019-12-31 | 2020-12-31 | Novel CAR-T cells targeting multiple tumors, and preparation and methods thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN113087806B (en) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114213536B (en) * | 2021-12-24 | 2022-07-12 | 北京市神经外科研究所 | CD133 antibodies, chimeric antigen receptors, and uses thereof |
| CN114957483A (en) * | 2022-04-13 | 2022-08-30 | 郑州大学第三附属医院(河南省妇幼保健院) | CD 155-targeted CAR (CAR-based protein) structural design and application thereof |
| CN114874335B (en) * | 2022-05-06 | 2024-04-19 | 北京大学深圳研究生院 | Three-target-point regulatable CAR immune cell composition and application thereof |
| CN114836428B (en) * | 2022-06-08 | 2024-03-26 | 华东师范大学 | TIGIT gene interference chimeric antigen receptor NK cell and application thereof |
| CN115838439B (en) * | 2022-12-06 | 2023-10-31 | 上海恩凯细胞技术有限公司 | Preparation method and application of chimeric transition receptor gene modified NK cells |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108368176A (en) * | 2015-10-01 | 2018-08-03 | 波滕扎治疗公司 | Anti- TIGIT antigen-binding proteins and its application method |
| CN110035768A (en) * | 2016-07-26 | 2019-07-19 | 泰莎治疗私人有限公司 | Chimeric antigen receptor |
| CN110352245A (en) * | 2016-10-20 | 2019-10-18 | 高山免疫科学股份有限公司 | Variant immune modulator and engineering cell therapy can be secreted |
| CN110511912A (en) * | 2018-08-30 | 2019-11-29 | 上海斯丹赛生物技术有限公司 | The function point analysis of immunocyte |
| EP3632461A1 (en) * | 2018-10-05 | 2020-04-08 | St. Anna Kinderkrebsforschung | A group of chimeric antigen receptors (cars) |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2997217A1 (en) * | 2015-09-14 | 2017-03-23 | Alpine Immune Sciences, Inc. | Tunable variant immunoglobulin superfamily domains and engineered cell therapy |
| US11471488B2 (en) * | 2016-07-28 | 2022-10-18 | Alpine Immune Sciences, Inc. | CD155 variant immunomodulatory proteins and uses thereof |
| SG11202004158QA (en) * | 2017-12-28 | 2020-06-29 | Nanjing Legend Biotech Co Ltd | Single-domain antibodies and variants thereof against tigit |
| US11701385B2 (en) * | 2018-08-31 | 2023-07-18 | Innovative Cellular Therapeutics Holdings, Ltd. | Modulation of cell function for immunotherapy |
| CN112142854B (en) * | 2020-09-18 | 2021-06-15 | 南京凯地生物科技有限公司 | Immune regulation specific chimeric antigen receptor cell and preparation method and application thereof |
-
2020
- 2020-12-31 CN CN202011635074.4A patent/CN113087806B/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108368176A (en) * | 2015-10-01 | 2018-08-03 | 波滕扎治疗公司 | Anti- TIGIT antigen-binding proteins and its application method |
| CN110035768A (en) * | 2016-07-26 | 2019-07-19 | 泰莎治疗私人有限公司 | Chimeric antigen receptor |
| CN110352245A (en) * | 2016-10-20 | 2019-10-18 | 高山免疫科学股份有限公司 | Variant immune modulator and engineering cell therapy can be secreted |
| CN110511912A (en) * | 2018-08-30 | 2019-11-29 | 上海斯丹赛生物技术有限公司 | The function point analysis of immunocyte |
| EP3632461A1 (en) * | 2018-10-05 | 2020-04-08 | St. Anna Kinderkrebsforschung | A group of chimeric antigen receptors (cars) |
Non-Patent Citations (2)
| Title |
|---|
| A TIGIT-based chimeric co-stimulatory switch receptor improves T-cell anti-tumor function;Shiran Hoogi等;《Journal for ImmunoTherapy of Cancer》;20190909;第7卷(第1期);1-13 * |
| CD155, an onco-immunologic molecule in human tumors;Gao J等;《Cancer Sci.》;20171231;第108卷(第10期);1934-1938 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113087806A (en) | 2021-07-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN114957475B (en) | anti-B7-H3 monoclonal antibodies and their use in cell therapy | |
| CN110760007B (en) | CD7-CAR-T cell and preparation and application thereof | |
| JP7148611B2 (en) | CHIMERIC ANTIGEN RECEPTOR TARGETING BCMA AND METHOD FOR PRODUCING AND USE THEREOF | |
| CN113087806B (en) | Novel CAR-T cells targeting multiple tumors, and preparation and methods thereof | |
| CN111051502B (en) | Preparation technology of universal chimeric antigen receptor T cell | |
| CN113784732B (en) | Engineered immune cells targeting BCMA and uses thereof | |
| KR102520550B1 (en) | Combined chimeric antigen receptors targeting CD19 and CD20 and their applications | |
| CN112778427B (en) | Bispecific CS1-BCMA CAR-T cells and uses thereof | |
| CN110157738B (en) | Engineered immune cells targeting CD19 and CD22 and application thereof | |
| CN113784733A (en) | BCMA-targeted engineered immune cells and uses thereof | |
| CN113087805A (en) | Preparation and application of chimeric antigen receptor T cell of co-expression immune regulatory molecule | |
| US20210395362A1 (en) | Car-t cells with humanized cd19 scfv with mutation in cdr 1 region | |
| CN116478929B (en) | Bispecific CAR-T cells targeting BCMA and CD19 | |
| CN114763388A (en) | B7-H3-targeted CAR-T cells and application thereof in treatment of acute myeloid leukemia | |
| CN109897114B (en) | CD 47-targeted engineered immune cells with suicide gene switch | |
| WO2023161846A1 (en) | Gpc3-targeting chimeric antigen receptor t cell and use thereof | |
| CN110577932A (en) | Chimeric antigen receptor T cell derived from umbilical cord blood | |
| US20230144447A1 (en) | Cd22-targeted chimeric antigen receptor, preparation method therefor and application thereof | |
| CN116462770B (en) | Humanized antibody of CD19, CAR-T cell expressing bispecific chimeric antigen receptor and application thereof | |
| CN116444669B (en) | Humanized antibodies targeting BCMA CAR-T cells | |
| CN116496397B (en) | Humanized antibodies targeting CD19CAR-T cells | |
| CN114685684A (en) | MUC1-Tn chimeric antigen receptor modified V gamma 9V delta 2T cell and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |