CN113039194A - Peptides with immunomodulatory properties - Google Patents
Peptides with immunomodulatory properties Download PDFInfo
- Publication number
- CN113039194A CN113039194A CN201880098522.6A CN201880098522A CN113039194A CN 113039194 A CN113039194 A CN 113039194A CN 201880098522 A CN201880098522 A CN 201880098522A CN 113039194 A CN113039194 A CN 113039194A
- Authority
- CN
- China
- Prior art keywords
- peptide
- immunomodulatory
- seq
- sequence
- amino acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/06—Linear peptides containing only normal peptide links having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/337—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having four-membered rings, e.g. taxol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/08—Peptides having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/14—Peptides containing saccharide radicals; Derivatives thereof, e.g. bleomycin, phleomycin, muramylpeptides or vancomycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55516—Proteins; Peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- General Chemical & Material Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Molecular Biology (AREA)
- Rheumatology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Pain & Pain Management (AREA)
- Genetics & Genomics (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
The present invention provides novel peptides having immunomodulatory activity in vitro and in vivo. The peptides may include a specific striapathic region composed of alternating hydrophilic and hydrophobic modules that may adopt an amphiphilic conformation under physiological conditions. The present invention provides peptides that can specifically bind to key functional regions on one or more signaling proteins, particularly proinflammatory cytokines, macrophage inhibitory proteins, and histone regulatory proteins. The invention includes peptides that are sufficiently stable in the circulation for intravenous administration. The present invention also provides pharmaceutical compositions comprising the subject peptides. The subject peptides are useful in methods for modulating macrophage activity. In some cases, the peptide is a CD206 binding agent. The invention also provides methods of treating chronic inflammation-related disorders in a subject with the peptides and compositions of the invention.
Description
Introduction to
Acute inflammation is the initial response of a tissue to a noxious stimulus. It involves a relatively complex, highly controlled process that begins when cells present in the damaged tissue (including macrophages, dendritic cells, histiocytes, kupffer cells, and mast cells) sense and activate damage-associated molecules. Upon activation, these cells release inflammatory mediators, such as vasodilators. Vasodilators increase blood flow and vascular permeability near the site of injury. This in turn results in increased movement of plasma and leukocytes (including neutrophils and macrophages) from the blood into the damaged tissue. Since inflammatory mediators are usually rapidly degraded, acute inflammation requires constant stimulation to maintain. Thus, acute inflammation can be resolved as long as the noxious stimulus is removed.
Depending on the specific agent and the genetic makeup of the animal to which it is exposed, a variety of agents (including but not limited to bacteria, viruses, physical injury, chemical injury, cancer, chemotherapy, and radiation therapy) can cause long-term, excessive inflammation. Such inflammation, known as chronic inflammation, is considered to be a contributing factor to many of the widespread debilitating diseases, including heart disease, cancer, respiratory diseases, stroke, neurological diseases (e.g., alzheimer's disease), diabetes and renal disease. Chronic inflammation results in the destruction of normal tissue, and instead occurs collagen-rich connective tissue. Collagen-rich connective tissue (also known as scar tissue) has reduced tissue function compared to normal tissue. Long-term, persistent formation of scar tissue can cause fibrosis. Fibrosis is a common symptom of diseases affecting the lung, skin, liver, heart and bone marrow and is a key factor in diseases such as idiopathic pulmonary fibrosis, scleroderma, keloids, cirrhosis, myocardial fibrosis, diabetic nephropathy, myelodysplastic syndrome, and other conditions.
Studies on chronic inflammation and fibrosis indicate that regardless of the activator and the affected tissue, signaling proteins in the shared network tend to work together to establish a pro-inflammatory state. This network of signaling proteins includes many different cytokines, cytokine receptors, transcription factors, and micrornas, including TGF β, TGF β RII, and miRNA19 b. Therefore, therapeutic agents that reduce inflammation without deleterious side effects are of great interest.
Disclosure of Invention
The present invention provides novel peptides having immunomodulatory activity in vitro and in vivo. The peptides may include a specific striapathic region composed of alternating hydrophilic and hydrophobic modules that may adopt an amphiphilic conformation under physiological conditions. The peptides may specifically bind to a key functional region on one or more signaling proteins, in particular pro-inflammatory cytokines, macrophage inhibitory proteins and/or histone regulatory proteins. The invention includes peptides that are sufficiently stable in the in vivo circulation following administration to a subject. The present invention also provides pharmaceutical compositions comprising the subject peptides.
The subject peptides are useful in methods for modulating macrophage activity. In some cases, the peptide is a CD206 binding agent. The invention also provides methods of treating chronic inflammation-related disorders in a subject with the peptides and compositions of the invention.
These and other features and advantages of the compositions and methods of the present invention will be set forth or will become more fully apparent from the following description and appended claims. For example, suitable immunomodulatory polypeptides can be identified by employing the formulae and sequences described herein. Additionally, the features and advantages of the described compositions and methods may be learned by the practice of the methods or will be obvious from the description.
Drawings
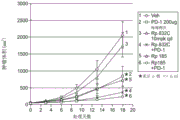
Figure 1 presents a graph of the results of bleomycin-induced reduction of pulmonary fibrosis in a mouse pulmonary fibrosis model. The fibrosis measurement is the Ashcroft score after trichrome staining. The collagen score is a quantitative measure after hydroxyproline staining. For more details, see the experimental section below.
Figure 2 shows that an exemplary peptide of interest acts synergistically with a PD-1 checkpoint inhibitor to reduce tumor volume in a mouse tumor suppression model. For more details, see the experimental section below.
Figure 3 shows that the exemplary peptides RP832C and RP837 reduced the viability of macrophages in samples from human scleroderma patients. Macrophage samples were evaluated after 96 hours incubation with various concentrations of peptide.
Figures 4A-4B show the selective effect of exemplary peptides RP832C and RP837 on macrophage samples from scleroderma patients with a high arginase to IFNg (interferon- γ) ratio (figure 4B) versus samples from healthy controls with a low arginase to IFNg ratio (figure 4A).
Detailed description of the preferred embodiments
The following description provides specific details in order to provide a thorough understanding of the present invention. That is, well-known structures, materials, processes, techniques, and operations have not been shown or described in detail to avoid obscuring aspects of the described immunomodulatory peptides and related methods of treating a subject. In addition, it will be understood by those skilled in the art that the described immunomodulatory peptides and related methods of treating a subject can be practiced and used without these specific details. Indeed, the described immunomodulatory peptides and methods can be applied in practice by modifying the indicated peptides, compositions, kits and methods, and can be used in conjunction with other methods, treatment regimens, devices and techniques that are routinely used.
Immunomodulatory polypeptides
As mentioned above, the present invention provides immunomodulatory peptides, particularly peptides having immunosuppressive properties; and methods of administering such immunomodulatory peptides to a subject, particularly a subject having a medical condition associated with persistent or chronic inflammation or likely to develop such a medical condition. The terms "immune-modulating" and "immunomodulation" are used interchangeably herein. In some cases, an immunomodulatory peptide described herein can be referred to as an anti-inflammatory peptide, and vice versa. In certain instances, the immunomodulatory peptide (e.g., as described herein) is an anti-inflammatory peptide, e.g., the peptide has at least one anti-inflammatory property.
Certain aspects of the peptides applicable to, or suitable for use in conjunction with, the present invention are described in Jaynes et al, WO2016/061133, the contents of which are incorporated herein by reference in their entirety.
The terms "peptide" and "polypeptide" are used synonymously herein to refer to a polymer made up of amino acid residues. The term "amino acid residue" as used herein refers to any naturally occurring amino acid, non-naturally occurring amino acid, or amino acid mimetic (e.g., peptoid monomer). The amino acid residues may be in the L-or D-form.
The invention includes immunomodulatory peptides having a striapathic region comprising at least 25% of the length of the polypeptide and at least one immunomodulatory property. The term "striapathic region" refers to a region or portion of a peptide sequence that is composed of alternating hydrophobic and hydrophilic modules. A "hydrophobic moiety" is a peptide sequence consisting of one to five (e.g., 1 to 3 or 1 to 2) hydrophobic amino acid residues (e.g., 1, 2, 3, 4 or 5 hydrophobic amino acid residues). A "hydrophilic moiety" is a peptide sequence consisting of one to five (e.g., 1 to 3 or 1 to 2) hydrophilic amino acid residues (e.g., 1, 2, 3, 4 or 5 hydrophilic amino acid residues).
Thus, the striapathic region can be represented by the formula (X)1-5J1-5)nOr (J)1-5X1-5)nWherein each X represents a hydrophilic amino acid residue, each J represents a hydrophobic amino acid residue, and each n is an integer between 1 and 10, such as 2 to 10. 2 to 8, 3 to 8, 4 to 8, or 5 to 10. Aspects of the invention include immunomodulatory peptides having a striapathic region with a specific degree of cationic charge. The immunomodulatory peptides of the invention can include a striapathic region having a cationic surface. In certain embodiments, the striapathic region has a cationic charge (i.e., charge)>0, e.g., +1, +2, +3, +4, +5, +6, or greater). In certain embodiments, the immunomodulatory peptide comprises a tail region (e.g., a hydrophobic tail sequence). In certain embodiments, the immunomodulatory peptide comprises two or more striapathic regions. In such embodiments, the two amphipathic regions of the peptide are in the form of a dimer, wherein the two amphipathic regions may have the same or different amino acid sequences (i.e., be homodimers or heterodimers). In certain embodiments, the two (or more) striapathic regions are connected by a linker or linking region. The linker may be a continuous (or linear) amino acid sequence or a non-amino acid moiety, as desired.
Hydrophobic amino acid residues are characterized by side chain groups that have primarily non-polar chemical or physical properties, such as in the environment in which the peptide is used (e.g., under physiological conditions). Such hydrophobic amino acid residues may or may not be naturally occurring. Hydrophobic amino acid residues can be mimetics of naturally occurring amino acids that are characterized by side chain groups that are predominantly non-polar in chemical or physical properties. In contrast, hydrophilic amino acid residues are characterized by side chain groups that exhibit predominantly polar (e.g., charged or neutral hydrophilic) properties, such as in the environment in which the peptide is used (e.g., physiological conditions). Such hydrophilic amino acid residues may or may not be naturally occurring. Hydrophilic amino acid residues can be mimetics of naturally occurring amino acids that are characterized by side chain groups that exhibit predominantly hydrophilic (e.g., charged or neutral polarity). Examples of hydrophilic and hydrophobic amino acid residues are shown in table 1 below. Suitable non-naturally occurring amino acid residues and amino acid mimetics are well known in the art. See, for example, Liang et al, (2013), "characterization indices for natural and unnatural amino acids that are suitable for peptidomimetics," PLoS ONE 8(7): e 67844.
Although most amino acid residues may be considered hydrophobic or hydrophilic, a few amino acid residues may appear hydrophobic or hydrophilic according to their background. For example, glycine, proline, serine, and/or cysteine may sometimes serve as hydrophilic amino acid residues due to their relatively weak non-polar character. In contrast, histidine and arginine may sometimes act as hydrophobic amino acid residues due to their larger, slightly hydrophobic side chains.
Table 1: hydrophobic and hydrophilic amino acid residues
| Hydrophilic residue (X) | Hydrophobic residue (J) |
| Arginine | Tryptophan |
| Histidine | Phenylalanine |
| Lysine | Tyrosine |
| Aspartic acid | Isoleucine |
| Glutamic acid | Leucine |
| Asparagine | Valine |
| Glutamine | Methionine |
| Pyrrolysine | Cysteine |
| Ornithine | Threonine |
| Serine | |
| Alanine | |
| Proline | |
| Glycine | |
| Selenocysteine | |
| N-formylmethionine | |
| Norleucine | |
| Norvaline |
The term "anti-inflammatory property" as used herein refers to any property of a polypeptide that can be evaluated in silico, in vitro and/or in vivo, which reduces or inhibits or is expected to reduce or inhibit pro-inflammatory signals mediated by a protein target and/or reduce or inhibit inflammation in a subject. The term "immunomodulatory property" as used herein refers to any property of a polypeptide that can be evaluated in silico, in vitro and/or in vivo, that modulates or is expected to modulate the expression or secretion of one or more cytokines involved in autoimmunity and/or in an immune response to a pathogenic agent, or modulates one or more components of a cytokine signaling pathway.
Selected immunomodulatory peptides of interest
The exemplary immunomodulatory peptide sequences described herein are merely examples and are not the only immunomodulatory polypeptides provided herein. Indeed, fragments and variants of the sequences of the disclosed peptides are also within the scope of the invention.
The present invention provides immunomodulatory polypeptides, sometimes referred to as "RP peptides", that satisfy the requirements of one or more of the following structural formulae. The present invention also provides immunomodulatory polypeptides having minimal homology to any of the exemplary RP peptides disclosed herein, or variants or fragments thereof. Thus, the peptides or polypeptides of the invention are immunomodulatory peptides satisfying one of the following formulae or having minimal homology to any of the exemplary RP peptides disclosed herein.
A "fragment" of the invention includes at least 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, or 23 consecutive amino acid residues of a peptide disclosed herein (or at most one less number of amino acid residues than in a target peptide) and retains at least one immunomodulatory property of the target peptide. Accordingly, fragments of the invention include peptides lacking one, two, three, four or more amino acids at the N-terminus and/or the C-terminus relative to the parent immunomodulatory peptides disclosed herein.
A "variant" of the invention is a polypeptide that is substantially similar to a polypeptide disclosed herein and retains at least one immunomodulatory property of the subject polypeptide. Variants may include a deletion (i.e., truncation) of one or more amino acid residues at the N-terminus or the C-terminus of a subject polypeptide disclosed herein; deletion and/or addition of one or more amino acid residues at one or more internal sites of a subject polypeptide disclosed herein; and/or substitution of one or more amino acid residues (e.g., one, two, three, or even more) at one or more positions of a subject polypeptide disclosed herein. For a subject polypeptide of 12 amino acid residues in length or less, a variant polypeptide can include three or fewer (e.g., three, two, one, or none) amino acid residues deleted at the N-terminus and/or the C-terminus, whether or not they are located internally.
Thus, the present invention further provides immunomodulatory polypeptides that are at least 50% (e.g., at least 50% sequence identity) identical to any one of the immunomodulatory polypeptides disclosed in a table disclosed herein (e.g., table 3) (e.g., at least 60%, 70%, 80%, 85%, 90%, 95%, or more) and still retain at least one immunomodulatory property. Sequence identity is determined based on a comparison of two peptide sequences or fragments thereof of the same or similar length.
Thus, in certain embodiments, the invention provides polypeptides that include an amino acid sequence that differs from any of the polypeptides disclosed herein by 1 to 10 amino acids (e.g., 10 or fewer, 9 or fewer, 8 or fewer, 7 or fewer, 6 or fewer, 5 or fewer, 4 or fewer, 3 or fewer, 2 or fewer, or 1 amino acid difference) and still retain at least one immunomodulatory property. As used herein, "amino acid differences" include: amino acid substitutions, amino acid insertions, terminal amino acid additions, amino acid deletions, terminal amino acid truncations, or any combination thereof. The differences between the striapathic region of the homologous immunomodulatory polypeptide and the striapathic region of any one of the immunomodulatory polypeptides shown in table 3 can include deletions, additions, and/or substitutions of amino acid residues as discussed herein. A substituted amino acid residue may be unrelated to the amino acid residue being substituted (e.g., unrelated in hydrophobicity/hydrophilicity, size, charge, polarity, etc.), or the substituted amino acid residue may constitute a similar, conservative, or highly conservative amino acid substitution. Definitions of "similar", "conservative" and "highly conservative" amino acid substitutions as used herein are shown in table 2 below. Determining whether an amino acid residue substitution is similar, conserved, or highly conserved is based entirely on the side chain of the amino acid residue, which may be modified to increase peptide stability, rather than the peptide backbone, as shown below.
Table 2: classification of amino acid substitutions
Specific interesting immunomodulatory peptides and fragments and variants thereof for use in the subject pharmaceutical compositions and methods are described in more detail below. In some cases, the subject immunomodulatory peptides have macrophage modulating activity.
The "length" of a polypeptide is the number of amino acid residues making up the polypeptide joined end-to-end, excluding any non-peptide linkers and/or modifications that the polypeptide may contain. In some embodiments, the peptide is 5 to 30 amino acid residues (e.g., 5 to 25, 10 to 20 or 5 to 18, 5 to 12 or 5 to 10, or 6 to 30, 6 to 25, 6 to 20, 6 to 18, 6 to 12, 6 to 10 or 7 to 12 or 7 to 10 amino acid residues) in length, and the peptide comprises a striapathic region consisting of alternating hydrophilic and hydrophobic modules that adopts an amphiphilic conformation under physiological conditions (e.g., as described herein). In some embodiments, the peptide is 5 to 12 amino acid residues (e.g., 6, 7, 8, 9, or 10 amino acid residues) in length and comprises a striapathic region consisting of alternating hydrophilic and hydrophobic modules that adopts an amphiphilic conformation under physiological conditions. In certain instances, the striapathic region of the peptide is 5 to 18 amino acid residues in length (e.g., 6 to 18, 6 to 14, 6 to 12, 7 to 12, or 5, 6, 7, 8, 9, 10, 11, or 12 amino acids in length), wherein the peptide is optionally further modified (e.g., as described herein). The striapathic region may comprise: 2 or more (e.g., 3 or more or 4 or more) hydrophobic modules; and one or more (e.g., 2 or more, 3 or more, or 4 or more) hydrophilic modules (e.g., each comprising at least one cationic residue). In some embodiments, the target immunomodulatory peptide (e.g., as described herein) is a CD206 binding peptide. In some cases, the striapathic region of the peptide is 6 to 12 amino acid residues in length, e.g., 7 to 12. In some cases, the striapathic region of the peptide is 6 to 10 amino acid residues in length.
The hydrophobic moiety may consist of any suitable residue. In some cases, the hydrophobic moiety comprises an amino acid residue selected from the group consisting of phenylalanine, tryptophan, alanine, valine, and glycine. The striapathic region can comprise a total of 1, 2, or more cationic amino acid residues, e.g., 3 or more, 4 or more, 5 or more, 6 or more, or even more. The immunomodulatory peptide can comprise 2, 3, or more hydrophilic moieties consisting of any suitable residue. In some cases, the hydrophilic moiety comprises an amino acid residue selected from the group consisting of lysine, arginine, histidine, aspartic acid, glutamic acid, asparagine, and glutamine.
In the formulae described herein, j (N) is used to refer to a particular hydrophobic moiety, where N represents a position in the linear formula. Similarly, x (N) is used to refer to a particular hydrophilic module, where N represents a position in the linear form.
In the formula described herein, J(nx)For specific hydrophobic amino acid residues, wherein n representsThe module in which x represents its position. Similarly, X(nx)Is used to refer to a particular hydrophilic amino acid residue, where n represents the moiety in which the residue is located and x represents its position within the moiety.
In certain instances of the immunomodulatory peptide, the striapathic region comprises hydrophobic and hydrophilic modules having the formula:
[J1] - [ X1] - [ J2] (formula 1).
In some embodiments of the immunomodulatory peptide, the striapathic region comprises the following formula of hydrophilic and hydrophobic modules:
[J1] - [ X1] - [ J2] - [ X2] (formula 2)
In some embodiments of the immunomodulatory peptide, the striapathic region comprises the following formula of hydrophilic and hydrophobic modules:
[ X1] - [ J1] - [ X2] - [ J2] (formula 3).
In some embodiments of the immunomodulatory peptide, the striapathic region comprises the following formula of hydrophobic and hydrophilic modules:
[J1] - [ X1] - [ J2] - [ X2] - [ J3] (formula 4).
In certain embodiments, the striapathic region comprises three or more hydrophilic modules and three or more hydrophobic modules, and comprises one of the following formulae:
[J1] - [ X1] - [ J2] - [ X2] - [ J3] - [ X3] (formula 5)
[J1] - [ X1] - [ J2] - [ X2] - [ J3] - [ X3] - [ J4] (formula 6).
In certain embodiments, the striapathic region comprises three or more hydrophilic modules and three or more hydrophobic modules, and comprises one of the following formulae:
[ X1] - [ J1] - [ X2] - [ J2] - [ X3] - [ J3] (formula 7).
In some cases of formula 1, the striapathic region has a sequence defined by one of the following formulae:
[J1aJ1b]-[X1aX1b]-[J2aJ2b](formula 1A)(ii) a And
[J2bJ2a]-[X1bX1a]-[J1bJ1a](formula 1B);
wherein:
J1a、J1b、J2aand J2bEach independently selected from hydrophobic amino acid residues (e.g., phenylalanine, tryptophan, and valine); and
X1aand X1bEach independently selected from hydrophilic amino acid residues (e.g., lysine or arginine).
In some cases of formula 1A, the peptide comprises the sequence FWKRFV (RP837N) (seq id no: 5) or a fragment or variant thereof (e.g., comprising a substituted variant).
In some embodiments of formula 2, the striapathic region has a sequence defined by the formula:
[J1aJ1b]-[X1aX1b]-[J2a]-[X2a](formula 2A);
wherein:
J1a、J1band J2aEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, tryptophan, or valine); and
X1a、X1band X2aEach independently selected from hydrophilic amino acid residues (e.g., lysine or arginine).
In some cases of formula 2A, the peptide includes the sequence fvrkr (RP837C)1) (sequence No.: 6) or a fragment or variant thereof (e.g., a variant comprising a substitution).
In some embodiments of formula 3, the striapathic region has a sequence defined by the formula:
[X1aX1b]-[J1aJ1bJ1cJ1d]-[X2aX2b]-[J2aJ2b](formula 3A);
wherein:
J1a、J1b、J1c、J1d、J2aand J2bEach independently selected from a hydrophobic amino acid residue (e.g., leucine, serine, alanine, or phenylalanine); and
X1a、X1b、X2aand X2bEach independently selected from a hydrophilic amino acid residue (e.g., glutamic acid, aspartic acid, lysine, asparagine, or arginine).
In some embodiments of formula 3A, the striapathic region has a sequence defined by the formula:
EX1bLSAFX2aNJ2aJ2b(SEQ ID NO: 25);
wherein:
J2aand J2bEach independently selected from alanine and phenylalanine; and
X1band X2aEach independently selected from lysine and arginine.
In some cases of formula 3A, the peptide includes sequence EKLSAFRNFF (RP843) (seq id no: 9) or a fragment or variant thereof (e.g., a variant including one or two substitutions).
In certain instances of formula 4, the striapathic region has a sequence defined by one of the following formulae:
[J1aJ1b]-[X1aX1b]-[J2aJ2b]-[X2aX2b]-[J3aJ3b](formula 4A); and
[J3aJ3b]-[X2aX2b]-[J2bJ2a]-[X1bX1a]-[J1bJ1a](formula 4B);
wherein:
J1a、J1b、J2a、J2b、J3aand J3bEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, tyrosine, isoleucine, or leucine); and
X1a、X1b、X2aand X2bEach independently selected from hydrophilic amino acid residues (e.g., lysine or arginine). In some embodiments of formulas 4A-4B, the striapathic region has a sequence defined by the formula:
LJ1bKKIIKKJ3al (SEQ ID NO: 26)
Wherein J1bAnd J3aIndependently phenylalanine, tyrosine or leucine (e.g., tyrosine or leucine).
In some cases of formulas 4A-4B, the peptide includes sequence LYKKIIKKLL (RP846) (SEQ ID NO: 12) or a fragment or variant thereof (e.g., a variant including one or two substitutions).
In some embodiments of formula 4, the striapathic region has a sequence defined by one of the following formulae:
[J1aJ1bJ1c]-[X1a]-[J2aJ2b]-[X2aX2b]-[J3aJ3b](formula 4C);
wherein:
J1a、J1b、J1c、J2a、J2b、J3aand J3bEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, tyrosine, or proline); and
X1a、X2aand X2bEach independently selected from a hydrophilic amino acid residue (e.g., aspartic acid, lysine, or arginine).
In some embodiments of formula 4C, the striapathic region has a sequence defined by the formula:
FYPDJ2aJ2bX2aX2bJ3aJ3b(SEQ ID NO: 27)
Wherein J2a、J2b、J3aAnd J3bEach independently phenylalanine or tyrosine (e.g., phenylalanine)
X2aAnd X2bEach independently lysine or arginine
In some cases of formula 4C, the peptide includes sequence FYPDFFKKFF (RP844) (seq id no: 10) or a fragment or variant thereof (e.g., a variant including one or two substitutions).
In some embodiments of formula 4, the striapathic region has a sequence defined by one of the following formulae:
[J1aJ1b]-[X1aX1b]-[J2a]-[X2aX2bX2c]-[J3aJ3b](formula 4D);
wherein:
J1a、J1b、J2a、J3aand J3bEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, serine, glycine, or isoleucine); and
X1a、X1b、X2a、X2band X2cEach independently selected from a hydrophilic amino acid residue (e.g., glutamic acid, aspartic acid, lysine, or arginine).
In some embodiments of formula 4D, the striapathic region has a sequence defined by the formula:
J1aJ1bX1aX1bSKEKIG (Serial number: 28)
Wherein:
J1aand J1bEach independently is phenylalanine or tyrosine (e.g., phenylalanine); and
X1aand X1bEach independently lysine or arginine.
In some cases of formula 4D, the peptide includes sequence FFRKSKEKIG (RP853) (seq id no: 18) or a fragment or variant thereof (e.g., a variant including one or two substitutions).
In some cases, the striapathic region has a sequence defined by the formula:
[J1aJ1b]-[X1aX1b]-[J2aJ2b]-[X2aX2b]-[J3a](formula 4E)
Wherein:
J1a、J1b、J2a、J2band J3aEach independently selected from phenylalanine, alanine and isoleucine; and X1a、X1b、X2a、X2bAnd X2cEach independently selected from ornithine, lysine and arginine.
In some cases, the striapathic region has a sequence defined by the formula:
[J1aJ1b]-[X1aX1b]-[J2aJ2b]-[X2aX2b]-[J3a]-[X3a](formula 5A)
Wherein:
J1a、J1b、J2a、J2band J3aEach independently selected from phenylalanine, tryptophan, alanine and valine;
and
X1a、X1b、X2a、X2band X3aEach independently selected from ornithine, lysine and arginine.
In some embodiments of formula 5A, the striapathic region has a sequence defined by the formula:
J1aJ1bOOJ2aJ2bOOJ3ao (Serial number: 29)
Wherein J1a、J1b、J2a、J2bAnd J3aEach independently selected from phenylalanine and alanine (e.g., each of the J1, J2, and J3 modules includes phenylalanine and alanine).
In some embodiments of formula 5A, the striapathic region has a sequence defined by the formula:
FAX1aX1bFAX2aX2bJ3aFX3a(SEQ ID NO: 30)
Wherein X1a、X1b、X2a、X2bAnd X3aEach independently selected from ornithine,Lysine and arginine.
In some cases of formula 5A, the peptide comprises the sequence FAOOFAOOFO (RP850) (seq id no: 19) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
In some embodiments of formula 5A, the striapathic region has a sequence defined by the formula:
FWKX1bFVX2aKWX3a(SEQ ID NO: 31)
Wherein X1b、X2aAnd X3aEach independently lysine or arginine.
In some cases of formula 5A, the peptide comprises sequence FWKRFVRKWR (RP837) (seq id no: 4) or FWKKFVKKWK (RP841) (seq id no: 7) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
In some cases, the immunomodulatory peptide of formula 5A is not FFRKFAKRFK (RP183) (SEQ ID NO: 21) or FFKKFFKKFK (RP185) (SEQ ID NO: 22).
In some cases, the striapathic region has a sequence defined by the formula:
[J1aJ1b]-[X1aX1b]-[J2aJ2b]-[X2aX2b]-[J3a]-[X3a](formula 5A)
Wherein:
J1a、J1b、J2a、J2band J3aEach independently selected from hydrophobic amino acid residues (e.g., phenylalanine, tryptophan, alanine, valine, and glycine); and
X1a、X1b、X2a、X2band X3aEach independently selected from a hydrophilic amino acid residue (e.g., lysine, ornithine, arginine, histidine, aspartic acid, glutamic acid, asparagine, or glutamine).
In some cases of formula 5A, J1a、J1b、J2a、J2bAnd J3aEach independently selected from phenylalanine, tryptophanAlanine and glycine; x1a、X1b、X2a、X2bAnd X3aEach independently selected from lysine and arginine. In some cases of formula 5A, J1a、J1b、J2a、J2bAnd J3aEach independently selected from phenylalanine, tryptophan, alanine and valine; x1a、X1b、X2a、X2bAnd X3aEach independently selected from ornithine, lysine and arginine (e.g., Lys or Arg). In some cases of formula 5A, J1a、J1b、J2a、J2bAnd J3aEach independently selected from phenylalanine and alanine; x1a、X1b、X2a、X2bAnd X3aEach independently selected from lysine and arginine. In some cases of formula 5A, J1a、J1b、J2a、J2bAnd J3aEach is phenylalanine; x1a、X1b、X2a、X2bAnd X3aEach independently selected from lysine and arginine. In some cases of formula 5A, J1a、J1b、J2a、J2bAnd J3aEach is tryptophan, and X1a、X1b、X2a、X2bAnd X3aEach independently selected from histidine, lysine and arginine. In some cases of formula 5A, J1a、J2aAnd J3aEach independently selected from phenylalanine and tryptophan, J1bSelected from tryptophan and alanine, J2bSelected from valine, tryptophan and alanine, and X1a、X1b、X2a、X2bAnd X3aEach independently selected from ornithine, lysine, arginine or histidine.
In some embodiments of formula 5A, the striapathic region has a sequence defined by the formula:
WWX1aHWWHX2bWX3a(SEQ ID NO: 32)
Wherein X1a、X2bAnd X3aEach independently of the other being histidineAcid, lysine or arginine.
In some cases of formula 5B, the peptide comprises sequence WWHHWWHHWH (RP847) (seq id no: 13), WWRHWWHRWR (RP848) (seq id no: 14), or WWKHWWHKWK (RP849) (seq id no: 15) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
In some embodiments of formula 5, the striapathic region has a sequence defined by the formula:
[J1aJ1b]-[X1aX1b]-[J2aJ2bJ2c]-[X2b]-[J3a]-[X3a](formula 5B);
wherein:
J1a、J1b、J2a、J2band J3aEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, alanine, threonine, or leucine); and
X1a、X1b、X2a、X2band X3aEach independently selected from a hydrophilic amino acid residue (e.g., histidine, aspartic acid, lysine, or arginine).
In some embodiments of formula 5B, the striapathic region has a sequence defined by the formula:
J1aJ1b X1aHJ2aJ2bTHLD (sequence number: 33)
Wherein:
J1a、J1b、J2aand J2bEach independently selected from phenylalanine and alanine; and
X1aindependently selected from lysine and arginine.
In some cases of formula 5C, the peptide includes sequence FFRHFATHLD (RP845) (seq id no: 11) or a fragment or variant thereof (e.g., a variant including one or two substitutions).
In some embodiments of formula 5, the striapathic region has a sequence defined by one of the following formulae:
[J1a]-[X1a]-[J2aJ2bJ2c]-[X2a]-[J3aJ3b]-[X3aX3b](formula 5C);
wherein:
J1a、J2a、J2b、J2c、J3aand J3bEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, tyrosine, leucine, glycine, or isoleucine); and
X1a、X2a、X3aand X3bEach independently selected from hydrophilic amino acid residues (e.g., glutamine, lysine, or histidine).
In some embodiments of formula 5C, the striapathic region has a sequence defined by the formula:
J1aQJ2aLGX2aIIHH (Serial number: 34)
Wherein:
J1aand J2aEach independently selected from phenylalanine, tyrosine and leucine; and
X2alysine and arginine.
In some cases of formula 5C, the peptide comprises sequence FQFLGKIIHH (RP852) (seq id no: 17) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
In some embodiments of formula 6, the striapathic region has a sequence defined by the formula:
[J1a]-[X1aX1b]-[J2aJ2b]-[X2a]-[J3a]-[X3aX3b]-[J4aJ4b](formula 6A);
wherein:
J1a、J2a、J2b、J3a、J4aand J4bEach independently selected from hydrophobic amino acid residues (e.g., phenylalanine, tryptophan, alanine, isoleucine, valine, and glycine); and
X1a、X1b、X2a、X3aand X3bEach independently selected from a hydrophilic amino acid residue (lysine, arginine, histidine, aspartic acid, glutamic acid, asparagine, or glutamine).
In some embodiments of formula 6A, the striapathic region has a sequence defined by the formula:
GX1aX1bGJ2bX2aGX3aX3bGJ4b(SEQ ID NO: 35)
Wherein:
J2band J4bEach independently selected from phenylalanine, tryptophan, alanine, isoleucine and valine; and
X1a、X1b、X2a、X3aand X3bEach independently selected from lysine, arginine, histidine, aspartic acid, glutamic acid, asparagine and glutamine.
In some embodiments of formula 6A, the striapathic region has a sequence defined by the formula:
GDX1bGIX2aGHX3bGF (SEQ ID NO: 36)
Wherein X1b、X2aAnd X3bEach independently selected from lysine and arginine.
In some cases of formula 6A, the peptide comprises sequence GDRGIKGHRGF (RP842) (seq id no: 8) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
In some embodiments of formula 7, the striapathic region has a sequence defined by one of the following formulae:
[X1aX1b]-[J1a]-[X2a]-[J2a]-[X3a]-[J3aJ3bJ3c](formula 7A);
wherein:
J1a、J2a、J3a、J3band J3cEach independently selected from hydrophobicA sex amino acid residue (e.g., isoleucine, valine, leucine, serine, or alanine); and
X1a、X1b、X2aand X3aEach independently selected from hydrophilic amino acid residues (e.g., lysine or arginine). In some embodiments of formula 7A, the striapathic region has a sequence defined by the formula:
X1aX1bIX2aVX3aLSA (Serial number: 37)
Wherein X1a、X1b、X2aAnd X3aEach independently selected from lysine and arginine.
In some cases of formula 7A, the peptide comprises sequence KKIRVRLSA (RP851) (seq id no: 16) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
Multimeric peptides
The invention includes multimers (e.g., dimers) of two or more immunomodulatory peptides (e.g., as described herein) linked by branched or linear linkers. Aspects of the invention include dimers of any one of the subject immunomodulatory polypeptides. The dimer may be a homodimer or a heterodimer. Any two immunomodulatory polypeptides may be linked by a linker. Any suitable linker may be used. Linkers that may be employed include, but are not limited to, covalent bonds, peptide linkers (e.g., glycine-containing linkers or Gly and Ser-containing linkers), C1-C12 linkers having terminal amino and/or carboxylic acid groups, or polymeric linkers (e.g., PEG or modified PEG). The dimer may include a linker that links the C-terminus of the first polypeptide to the N-terminus of the second polypeptide. In some cases, the two polypeptides may be linked through the C-terminus. In some cases, the two polypeptides may be linked through the N-terminus.
The invention further encompasses any two immunomodulatory polypeptides that have been linked together. The linkage may be formed by a peptide linker, such as a Gly-Gly-Gly (GGG), Gly-Gly-Gly-Arg (GGGR; SEQ ID NO: 40), Gly-Pro-Gly (GPG), or Gly-Pro-Gly-Arg (GPGR; SEQ ID NO: 41) sequence, which links the C-terminus of a first immunomodulatory polypeptide to the N-terminus of a second immunomodulatory polypeptide. Alternatively, the linkage may be a peptoid linker (e.g., a poly-N-substituted version of any of the foregoing peptide linkers), a polymer containing g-amino acids (e.g., corresponding to any of the foregoing peptide linkers), or a non-peptide, chemical linker. The linked immunomodulatory polypeptide can be any of the polypeptides disclosed herein (e.g., the polypeptides in table 3), and can include the same polypeptides linked to form a homodimer or different polypeptides linked to form a heterodimer. The technique of linking peptides via peptide and non-peptide linkers is well known in the art, and the polypeptide combinations of the invention are intended to encompass all such linkages.
Any two peptides containing a striapathic region can be linked (e.g., as described herein). The two regions of the dimeric peptide may be homodimeric or heterodimeric with respect to each other. By "homodimeric" is meant that the two peptide regions of the dimeric peptide have the same N-terminal to C-terminal sequence or their reverse C-terminal to N-terminal sequences. The subject immunomodulatory polypeptides described herein can be linked in any suitable configuration to form a multimer. In some cases, the multimer includes 3 or more immunomodulatory polypeptides (e.g., as described herein), wherein the polypeptides can be arranged in a linear or branched format. Linear multimers of immunomodulatory polypeptides may include linked peptides in a head-to-tail arrangement linked by covalent bonds or optional linkers (e.g., peptide linkers). In some cases, linear multimers can be referred to as oligomers, e.g., polypeptide chains that include sequence segments of an immunomodulatory polypeptide (e.g., as described herein). Alternatively, the immunomodulatory polypeptides of a linear multimer can be linked in a head-to-head (e.g., N-terminal to N-terminal) and/or tail-to-tail (e.g., C-terminal to C-terminal) configuration. In branched multimers, the immunomodulatory polypeptides may be linked by any suitable branched linker, e.g., a group comprising three functional groups for linking amino acid residues (e.g., lysine amino acids). In some cases, the multimer is a dimer.
In some cases, the immunomodulatory peptide dimer has the formula:
Z1-T-Z2
wherein:
t is a linker, e.g., a peptide linker;
Z1is a first polypeptide or region having 3-10 (e.g., 4-10, 5-10, or 3-6, or 3, 4, 5, or 6) amino acid residues consisting of a mixture of hydrophilic and hydrophobic amino acid residues (e.g., as described herein); and
Z2is a second polypeptide or region having 3-10 (e.g., 4-10, 5-10, or 3-6, or 3, 4, 5, or 6) amino acid residues consisting of a mixture of hydrophilic and hydrophobic amino acid residues (e.g., as described herein).
In some cases of the dimer, the hydrophilic moiety consists of an amino acid residue selected from lysine and arginine; and the hydrophobic moiety consists of an amino acid residue selected from the group consisting of phenylalanine and tryptophan. In some cases, the first and second polypeptides (Z)1And Z2) Comprising four amino acid residues. In some cases, Z1And Z2Each comprising four amino acid residues, two of which are hydrophilic residues (e.g., as described herein) and the remaining two of which are hydrophobic residues (e.g., as described herein).
In certain embodiments, the dimer has one of the following formulas:
[ X1] - [ J1] -T- [ J1] - [ X1] (formula 8);
[J1] - [ X1] -T- [ X1] - [ J1] (formula 9);
[ X1] - [ J1] -T- [ J2] - [ X2] (formula 10);
[J1] - [ X1] -T- [ X2] - [ J2] (formula 11);
wherein T is a linker (e.g., a peptide linker).
In some cases of formulae 8 and 9, the dimer has a sequence defined by one of the following formulae:
[X1aX1b]-[J1aJ1b]-T-[J1bJ1a]-[X1bX1a](formula 8A); or
[J1aJ1b]-[X1aX1b]-T-[X1bX1a]-[J1bJ1a](formula 9A);
wherein:
t is a peptide linker (e.g., a polyglycine linker);
J1aand J1bEach independently selected from a hydrophobic amino acid residue (e.g., tryptophan or phenylalanine); and
X1aand X1bEach independently selected from a hydrophilic amino acid residue (e.g., asparagine or arginine). In certain instances of formulas 8A and 9A, T is a peptide linker consisting of one, two, or three glycine residues.
In some embodiments of formula 9A, the dimer has a sequence defined by the formula:
FW-[X1aX1b]-T-[X1bX1a]WF (SEQ ID NO: 38)
Wherein X1aAnd X1bEach independently selected from lysine and arginine.
In some embodiments of formula 9A, the dimer has a sequence defined by the formula:
[J1aJ1b]-KR-T-RK-[J1bJ1a](SEQ ID NO: 39)
Wherein J1aAnd J1bEach independently selected from tryptophan and phenylalanine.
In some cases of formula 7A, the peptide comprises sequence FWKRGGRKWF (RP837A) (seq id no: 4) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
In some cases of formulas 10 and 11, the dimer has a sequence defined by one of the following formulas:
[J1aJ1b]-[X1aX1b]-T-[X2aX2b]-[J2aJ2b](formula (II)10A)
[X1aX1b]-[J1aJ1b]-T-[J2aJ2b]-[X2aX2b](formula 11A)
Wherein:
J1a、J1b、J2aand J2bEach independently selected from a hydrophobic amino acid residue (e.g., tryptophan or phenylalanine); and
X1a、X1b、X2aand X2bEach independently selected from a hydrophilic amino acid residue (e.g., asparagine or arginine). In certain instances of formulas 10A and 11A, T is a peptide linker consisting of one, two, or three glycine residues.
In some cases, the first and second polypeptides (Z) of the dimer1And Z2) One of the following formulas comprising hydrophilic and hydrophobic modules: [ X1]-[J1]-[X2]-[J2](formula 3); or [ J1]-[X1]-[J2]-[X2](formula 2).
In certain embodiments, the dimer has one of the following formulas:
[ X1] - [ J1] - [ X2] - [ J2] -T- [ J2] - [ X2] - [ J1] - [ X1] (formula 12);
[J1] - [ X1] - [ J2] - [ X2] -T- [ X2] - [ J2] - [ X1] - [ J1] (formula 13);
[ X1] - [ J1] - [ X2] - [ J2] -T- [ J3] - [ X3] - [ J4] - [ X4] (formula 14);
[J1] - [ X1] - [ J2] - [ X2] -T- [ X3] - [ J3] - [ X4] - [ J4] (formula 15);
wherein T is a peptide linker.
In some cases of formulae 12 and 13, the dimer has one of the following formulae:
[X1a]-[J1a]-[X2a]-[J2a]-T-[J2a]-[X2a]-[J1a]-[X1a](formula 12A); and
[J1a]-[X1a]-[J2a]-[X2a]-T-[X2a]-[J2a]-[X1a]-[J1a](formula 13A);
wherein:
t is a peptide linker (e.g., a polyglycine linker);
J1aand J2aEach independently selected from phenylalanine and tryptophan; and
X1aand X2aEach independently selected from lysine and arginine.
In some cases of formula 12A, the peptide comprises sequence RWKFGGFKWR (RP832C) (seq id no: 1) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
In some cases of formula 13A, the peptide comprises sequence FKWRGGRWKF (RP837C) (seq id no: 3) or a fragment or variant thereof (e.g., a variant comprising one or two substitutions).
In certain embodiments, the immunomodulatory peptide comprises a tail region.
In some cases of formulae 14 and 15, the dimer has one of the following formulae:
[X1a]-[J1a]-[X2a]-[J2a]-T-[J3a]-[X3a]-[J4a]-[X4a](formula 14A); and
[J1a]-[X1a]-[J2a]-[X2a]-T-[X3a]-[J3a]-[X4a]-[J4a](formula 15A);
wherein:
t is a peptide linker (e.g., a polyglycine linker);
J1a、J2a、J3aand J4aEach independently selected from phenylalanine and tryptophan; and
X1a、X2a、X3aand X4aEach independently selected from lysine and arginine.
Immunomodulatory peptides of interest include, but are not limited to, any of the polypeptides set forth in table 3, fragments thereof (e.g., as described herein), or variants thereof (e.g., as described herein).
Table 3: peptide of interest
In certain embodiments, the subject immunomodulatory polypeptide comprises a sequence selected from the group consisting of:
a) a sequence selected from the peptide sequences shown in table 3;
b) a sequence having at least 75% sequence identity (e.g., at least 80%, 85%, 90%, or 95% sequence identity) to a sequence defined in a); and
c) a sequence having one or two amino acid substitutions relative to the sequence defined in a), wherein the one or two amino acid substitutions are of amino acids according to table 2 (e.g., similar amino acid substitutions, conservative amino acid substitutions, or highly conservative amino acid substitutions).
In some cases, the sequence described in a) is RP 832C. In some cases, the sequence described in a) is RP 837. In some cases, the sequence described in a) is RP 837C. In some cases, the sequence described in a) is RP 837A. In some cases, the sequence described in a) is RP 837N. In some cases, the sequence described in a) is RP837C1. In some cases, the sequence described in a) is RP 841. In some cases, the sequence described in a) is RP 842. In some cases, the sequence described in a) is RP 843. In some cases, the sequence described in a) is RP 844. In some cases, the sequence described in a) is RP 845. In some cases, the sequence described in a) is RP 846. In some cases, the sequence described in a) is RP 847. In some cases, the sequence described in a) is RP 848. In some cases, the sequence described in a) is RP 849. In some cases, the sequence described in a) is RP 850. In some cases, the sequence described in a) is RP 851. In some cases, the sequence described in a) is RP 852. In some cases, the sequence described in a) is RP 853.
In some cases, the sequence described in b) has a sequence with at least 80% sequence identity to the sequence defined in a). In some cases, the sequence described in b) has a sequence with at least 85% sequence identity to the sequence defined in a). In some cases, the sequence described in b) has a sequence with at least 90% sequence identity to the sequence defined in a). In some cases, the sequence described in b) has a sequence with at least 95% sequence identity to the sequence defined in a).
In certain embodiments, the sequence described in c) has one or two amino acid substitutions relative to the sequence defined in a), wherein the one or two amino acid substitutions are similar amino acid substitutions according to table 2. In certain embodiments, the sequence described in c) has one or two amino acid substitutions relative to the sequence defined in a), wherein the one or two amino acid substitutions are conservative amino acid substitutions according to table 2. In certain embodiments, the sequence described in c) has one or two amino acid substitutions relative to the sequence defined in a), wherein the one or two amino acid substitutions are highly conservative amino acid substitutions according to table 2. Any of the variants of the immunomodulatory peptides described herein can be applied to the parent peptides shown in table 3.
Excluded Polypeptides
The compositions of the present invention optionally do not include polypeptides described in U.S. patent application nos. 2012/0270770 and 2003/0109452 and 6,559,281, the contents of which are incorporated herein by reference in their entirety. Thus, one or more polypeptides described in such publications and/or the use of such polypeptides may be excluded from the scope of the compositions and/or methods disclosed herein. In addition, any of the polypeptides disclosed by Jaynes et al in tables 3-9 of WO2016/061133 (the contents of which are incorporated herein by reference) may optionally be excluded from the compositions disclosed herein and/or methods of use of such compounds. In some cases, any of the polypeptides disclosed in table 4 herein may optionally be excluded from the compositions disclosed herein and/or methods of use of such compounds.
In certain instances, an immunomodulatory peptide of the formula described herein is not a polypeptide as set forth in table 4.
Table 4:
| RP numbering | Sequence number: | sequence of |
| 182 | 20 | KFRKAFKRFF |
| 183 | 21 | FFRKFAKRFK |
| 185 | 22 | FFKKFFKKFK |
| 186 | 23 | KFKKFFKKFF |
| 233 | 24 | KFKKAFKKAF |
Modified polypeptides
Embodiments of the invention include modifications to any of the immunomodulatory polypeptides of the invention by chemical or genetic means. Examples of such modifications include the structure of polypeptides having the non-natural amino acids in the L or D form and/or a partial or complete sequence of natural amino acids. For example, any of the peptides disclosed herein and any variants thereof can be produced in the full D form. Furthermore, the polypeptides of the invention may be modified to contain a carbohydrate or lipid moiety, such as a sugar or fatty acid, covalently linked to the side chain or the N-or C-terminus of the amino acid. In addition, the polypeptides of the invention may be modified to enhance solubility and/or half-life upon administration. For example, polyethylene glycol (PEG) and related polymers have been used to enhance the solubility and half-life of protein therapeutics in the blood. Thus, the polypeptide of the present invention may be modified by a PEG polymer or the like. The polypeptides of the invention may also be modified to contain sulfur, phosphorus, halogens, metals, etc., and amino acid mimetics may be used to produce the polypeptides of the invention (e.g., having a structure based on a structural algorithm or a structure similar to any of the immunomodulatory polypeptides disclosed herein). In certain embodiments, the polypeptides of the invention, including amino acid mimetics, have enhanced properties, such as resistance to degradation. For example, a polypeptide of the invention can include one or more (e.g., all) peptoid monomers.
The immunomodulatory polypeptide can be linked to another molecule via a degradable linkage (e.g., a disulfide bond). The disulfide bond may be mediated through the sulfhydryl group of a cysteine residue found in the immunomodulatory polypeptide as well as the sulfhydryl group in the other molecule. The cysteine residue may, for example, be located at the C-terminus or N-terminus of the immunomodulatory polypeptide. Using this type of disulfide linkage, the polypeptides of the invention can be suitably linked to various types of useful molecules. For example, the linkage can be achieved using another immunomodulatory polypeptide (which optionally includes a C-terminal or N-terminal cysteine residue), a fluorescent tag (e.g., Dylight 350), a chemotherapeutic agent (e.g., a paclitaxel derivative formed by adding a sulfhydryl group to an appropriate site on the paclitaxel ring structure followed by oxidation using a cysteine-containing peptide of the invention), and the like.
The linked immunomodulatory polypeptide (e.g., a homodimer or heterodimer) can bind to a target molecule (e.g., a target protein, such as a pro-inflammatory signaling protein), wherein the binding energy is greater than the binding energy of the monomeric polypeptide alone. Thus, for example, the binding energy of the linked immunomodulatory polypeptide to an NF-kB class II protein (e.g., RelB) can be at least-700 kcal/mol, and in some embodiments at least-750, -800, -900, -1000, -1100, -1200, -1250, -1300, -1350, -1400, -1425, -1450, -1475, -1500, -1525, -1550, -1575, -1600kcal/mol or greater. The binding energy can be measured, for example, in silico, in vitro, or in vivo using methods well known in the art (e.g., using ClusProTMAlgorithm) to determine.
In some cases, if the modified peptide is covalently linked to a molecule of interest, the resulting compound may be referred to as a peptide conjugate. Any suitable molecule of interest may be linked to the target immunomodulatory peptide. The molecule of interest may be peptidic or non-peptidic, and may be naturally occurring or synthetic. Molecules of interest suitable for use in conjunction with a target immunomodulatory peptide include, but are not limited to, protein domains, polypeptides, peptide tags, specific binding moieties (e.g., antibodies or antibody fragments), polymeric moieties (e.g., polyethylene glycol (PEG)), carbohydrates, dextrans or polyacrylates, linkers, moieties that confer a desired drug-like property (e.g., half-life extending moieties), tags, and solid supports. In some cases, the molecule of interest may confer enhanced and/or modified properties and functions to the resulting modified peptide, including but not limited to increased aqueous solubility, ease of chemical synthesis, cost, bioconjugate sites, stability, pI, aggregation, non-specific binding to a second target protein, and/or reduced specific binding, e.g., as described herein.
In some embodiments of any of the peptide sequences described herein, the peptide sequence may be extended to include one or more additional residues, e.g., two or more, three or more, four or more, five or more, six or more, or even more additional residues, at the N-terminus and/or C-terminus of the sequence. Any suitable residue may be included at the N-terminus and/or C-terminus of the peptide to provide a desired property or group, such as increased solubility through a water-soluble group, a linkage for dimerization or multimerization, a linkage for attachment of a tag or specific binding moiety.
In some cases, the subject modified peptides have the corresponding formula:
B-L-M
wherein B is an immunomodulatory peptide (e.g., as described herein); l is an optional linking group; m is a molecule of interest, wherein L is attached to B at any suitable position (e.g., N-terminus, C-terminus, or via the side chain of a residue not involved in target binding).
The modified peptide may include one or more molecules of interest. In some cases, the molecule of interest is covalently linked through the a-amino group of the N-terminal residue, or covalently linked to the a-carboxylic acid group of the C-terminal residue.
The molecule of interest may comprise a polypeptide or protein domain. Polypeptide and protein domains of interest include, but are not limited to: a gD tag, a c-Myc epitope, a FLAG tag, a His tag, a fluorescent protein (e.g., GFP), a β -galactosidase protein, GST, albumin, an immunoglobulin, an antibody, an Fc domain or similar antibody-like fragment, a leucine zipper motif, a coiled coil domain, a hydrophobic region, a hydrophilic region, a polypeptide comprising a free thiol group that forms an intermolecular disulfide bond between two or more multimerization domains, a "bulge-into-cavity" domain, a β -lactoglobulin, or a fragment thereof.
The molecule of interest may include a half-life extending moiety. The term "half-life extending moiety" refers to a pharmaceutically acceptable moiety, domain, or "carrier" covalently linked or conjugated to a target compound, which half-life extending moiety can prevent or reduce proteolytic degradation or other chemical modification of the target compound in vivo that reduces activity, as compared to a non-conjugated form of the target compound; increased half-life or other pharmacokinetic properties (e.g., absorption); the toxicity is reduced; the solubility is improved; increasing the biological activity and/or target selectivity of the subject compound relative to the target of interest; improving manufacturability and/or reducing immunogenicity of the subject compound.
In certain embodiments, the half-life extending moiety is a polypeptide that binds to a serum protein (e.g., an immunoglobulin (e.g., IgG) or serum albumin (e.g., Human Serum Albumin (HSA). polyethylene glycol is an example of a useful half-life extending moiety exemplary half-life extending moieties include polyalkylene glycol moieties (e.g., PEG), serum albumin or fragments thereof, transferrin receptor or transferrin binding portion thereof, moieties comprising binding sites for polypeptides suitable for increasing half-life in vivo, ethylene glycol copolymers, propylene glycol copolymers, carboxymethylcellulose, polyvinylpyrrolidone, poly-1, 3-dioxolane, poly-1, 3, 6-trioxane, ethylene/maleic anhydride copolymers, polyamino acids (e.g., polylysine), dextran n-vinylpyrrolidone, polyethylene glycol, polyethylene, Poly-n-vinylpyrrolidone, propylene glycol homopolymers, propylene oxide polymers, ethylene oxide polymers, polyoxyethylated polyols, polyvinyl alcohol, linear or branched glycosylated chains, polysialic acid, polyacetals, lipids, long chain fatty acids, long chain hydrophobic aliphatic groups, immunoglobulin Fc domains (see, e.g., U.S. patent No. 6,660,843), albumin (e.g., human serum albumin; see, e.g., U.S. patent No. 6,926,898 and U.S. 2005/0054051; U.S. patent No. 6,887,470), transthyretin (TTR; see, e.g., US 2003/0195154; 2003/0191056), or thyroxine-binding globulin (TBG).
In certain embodiments, the half-life extending moiety is a lipid. In certain embodiments, the half-life extending moiety is a fatty acid. Any suitable lipid and fatty acid may be used in the subject modified compounds. See, e.g., Chae et al, "fatty acid conjugated Exendin-4 analogs suitable for type 2 antidiabetic drugs", J.ControlRelease, 5.21/2010; 144(1):10-6.
In certain embodiments, the immunomodulatory peptide is modified to include a specific binding moiety. The specific binding member is a member complementary theretoTwo parts are specifically combined. In some cases, the specific binding member is at least 10-7Affinity of M (e.g., by a K of 100nM or less, e.g., 30nM or less, 10nM or less, 3nM or less, 1nM or less, 300pM or less, or 100pM or lessDAssay) binds to the complementary second moiety. Complementary binding moiety pairs of specific binding moieties include, but are not limited to, ligands or agonists/promoters and receptors, antibodies and antigens, complementary polynucleotides, complementary protein homo-or heterodimers, aptamers and small molecules, and polyhistidine tags and nickel. The specific binding pair may include analogs, derivatives and fragments of the original specific binding member. For example, antibodies to protein antigens may also recognize peptide fragments, chemically synthesized proteins, labeled proteins, derivatized proteins, and the like, as long as the epitope is present. Protein domains of interest for use as specific binding members include, but are not limited to, Fc domains or similar antibody-like fragments, leucine zipper motifs, coiled-coil domains, hydrophobic regions, hydrophilic regions, polypeptides comprising a free thiol group that forms an intermolecular disulfide bond between two or more multimerization domains or "bulge-into-cavity" domains (see, e.g., WO 94/10308; U.S. Pat. No. 5,731,168, Lovejoy et al (1993), science 259: 1288-1293; Harbury et al (1993), science 262: 262-05; Harbury et al (1994), Nature 371: 80-83; Hakansson et al (1999), structure 7: 255-64).
In certain embodiments, the peptide is an attached specific binding moiety that specifically binds to a target protein. The linked specific binding moiety may be an antibody, an antibody fragment, a receptor agonist or an aptamer. The linked specific binding member can specifically bind to any suitable target protein (e.g., a target protein that is expected to be targeted for binding to a target therapeutic method). Target proteins of interest include, but are not limited to, PDGF (e.g., PDGF-B), VEGF-B, VEGF-C, VEGF-D, EGF, EGFR, Her2, PD-1, PD-L1, OX-40, and LAG 3. In certain embodiments, the linked specific binding moiety is a receptor agonist or ligand, e.g., a protein ligand associated with an inflammatory pathway, such as interleukin 13(IL-13) or an activated molecule belonging to a member of the toll-like receptor (TLR) family, e.g., TLR 3. In certain instances, the linked specific binding moiety (e.g., a protein, antibody, or antibody fragment) can be further linked to an additional active agent (e.g., a chemotherapeutic agent, e.g., as described herein).
An immunomodulatory polypeptide (e.g., as described herein) can be conjugated with an additional active agent to provide a conjugate of the immunomodulatory polypeptide. After the target peptide is generated and/or prepared and selected according to the teachings herein, the target peptide can be linked, fused, conjugated (e.g., covalent or non-covalent), or otherwise associated with a pharmaceutically active or diagnostic moiety or a biocompatible modifier. The term "peptide conjugate" refers to any biologically active or detectable molecule or drug associated with the disclosed immunomodulatory peptide compounds, regardless of the method of association employed. In this regard, it is understood that such conjugates can comprise, in addition to the disclosed immunomodulatory peptides, polypeptides, proteins, prodrugs (which are metabolized in vivo to an active agent), polymers, nucleic acid molecules, small molecules, binding agents, mimetics, synthetic drugs, inorganic molecules, organic molecules, and radioisotopes. Furthermore, as described above, selected conjugates can be associated or linked covalently or non-covalently to a target peptide and exhibit various stoichiometric molar ratios based at least in part on the method used to effect conjugation.
In some cases, the molecule of interest is a second active agent, such as an active agent or drug used in conjunction with a target of interest in a targeted therapeutic approach. In certain instances, the molecule of interest is a small molecule, a chemotherapeutic agent, an antibody fragment, a bispecific antibody, an aptamer, or an L-protein. In some embodiments, the peptide is modified to include moieties (e.g., proteins, nucleic acids, small organic molecules, etc.) that can be used as a drug. Exemplary pharmaceutical proteins include, for example, cytokines, antibodies, chemokines, growth factors, interleukins, cell surface proteins, extracellular domains, cell surface receptors, cytotoxins, and the like. Exemplary small molecule drugs include small molecule toxins or therapeutic agents. Any suitable therapeutic or diagnostic agent (e.g., as described herein) can be conjugated to the immunomodulatory peptide. A variety of therapeutic agents are described in the section entitled "combination therapy," including, but not limited to, anti-cancer agents, antiproliferative agents, cytotoxic agents, and chemotherapeutic agents, any of which may be suitable for use with the subject peptide conjugates.
In certain embodiments, the modified peptide can be conjugated to a bispecific antibody, e.g., an engineered bispecific monoclonal antibody that can bind to two different types of antigens of interest simultaneously.
In certain embodiments, the modified peptide may comprise a cell penetrating peptide (e.g., tat). The cell penetrating peptide may facilitate uptake of the molecule into a cell. Any suitable marker polypeptide and its respective antibody may be used. Examples include a polyhistidine (poly-his) or polyhistidine-glycine (poly-his-gly) tag; flu HA-tagged polypeptide and its antibody 12CA5[ Field et al, molecular and cellular biology, 8:2159-2165(1988) ]; the C-myc marker and its 8F9, 3C7, 6E10, G4, B7 and 9E10 antibodies [ Evan et al, molecular and cellular biology, 5:3610-3616(1985) ]; and the herpes simplex virus glycoprotein D (gD) marker and its antibody [ Paborsky et al, protein engineering, 3(6):547-553(1990) ]. Other marker polypeptides include Flag-peptide [ Hopp et al, Biotechnology, 6:1204-1210(1988) ]; KT3 epitope peptide [ Martin et al, science 255: 192-; tubulin epitope peptides [ Skinner et al, J. Biochem., 266:15163-15166(1991) ]; and T7 gene 10 protein peptide marker [ Lutz-Freyermeth et al, Proc. Natl. Acad. Sci. USA 87:6393-6397(1990) ].
It will be appreciated by those skilled in the art that many different responses may be used to link or associate a therapeutic or diagnostic moiety and/or linker with a target immunomodulatory peptide. In certain embodiments, this may be achieved by reaction of amino acid residues (e.g., as described herein) of the peptide, including the amino acid terminus, the C-terminal carboxylic acid, the amine group of lysine, the free carboxylic acid groups of glutamic and aspartic acids, the thiol group of cysteine, and various portions of aromatic amino acids. One method of covalent attachment is a carbodiimide reaction, which attaches the carboxyl (or amino) group of the compound to the amino (or carboxyl) group of the target peptide. In addition, bifunctional agents (e.g., dialdehydes or imidates) have been used to link the amino group of a target peptide to an amino group of an antibody molecule. Also useful means for attaching drugs to immunomodulatory peptides are maleimide-thiol conjugation chemistry, click chemistry, e.g., between azide and alkyne groups, and the like. A further means for attaching drugs to peptides is the schiff base reaction. The method may involve periodate oxidation of a drug containing an ethylene glycol or hydroxyl group, thereby forming an aldehyde, which is then reacted with the binding agent. Attachment is achieved by the formation of schiff bases with the amino groups of the binding agent. Isothiocyanates and azlactones can also be used as coupling agents to covalently bind a drug to a binding agent.
It is to be understood that a variety of linker variants or types of linkers can be used to associate the disclosed immunomodulatory peptides with pharmaceutically active or diagnostic moieties or biocompatibility modifiers. In some embodiments, the linker is cleavable under intracellular conditions, and cleavage of the linker releases the drug unit in the antibody in the intracellular environment. In certain embodiments, the linker unit is non-cleavable. Bivalent linker reagents useful for linking two or more functional or biologically active moieties (e.g., peptides, nucleic acids, drugs, toxins, antibodies, haptens and reporter groups) are known and methods for obtaining conjugates thereof are described (Hermanson, G.T. (1996), bioconjugation techniques; academic Press: New York: p.234-242).
Composition comprising a metal oxide and a metal oxide
The compositions of the present invention include immunomodulatory polypeptides that meet the requirements of one of the structural formulae described herein. For example, the immunomodulatory polypeptide can have a striapathic region having a sequence corresponding to any one of the formulae disclosed herein. Typically, the immunomodulatory polypeptides included in the compositions of the invention are synthetic polypeptides (e.g., made by chemical synthesis and/or recombinantly produced).
The compositions of the invention may comprise a single immunomodulatory polypeptide or a combination thereof. The compositions may be substantially free of proteins and other polypeptides that do not meet the requirements of the structural algorithms disclosed herein. The term "substantially free of proteins and other polypeptides" as used herein means that less than 5% of the protein content in the composition is made up of proteins and other polypeptides that are not immunomodulatory polypeptides of the invention. Compositions that are substantially free of non-immunomodulatory polypeptides of the invention may have less than 4%, 3%, 2%, 1%, 0.5%, 0.1%, 0.05%, 0.01% or less of proteins or other polypeptides that do not meet the requirements of the structural algorithms disclosed herein. Thus, the composition may be substantially free of blood proteins, such as serum albumin, globulin, fibrinogen, and clotting factors. Alternatively, the composition may be substantially free of globulin, fibrinogen and clotting factors, but may include purified or recombinantly produced serum albumin.
In certain embodiments, the compositions of the invention comprise immunomodulatory polypeptides that do not naturally occur in humans or other mammals or animals. However, the compositions of the invention may include immunomodulatory polypeptides that occur naturally in humans or other mammals or animals, provided that the compositions are substantially free of biomolecules (e.g., non-immunomodulatory polypeptides, nucleic acids, lipids, carbohydrates, and metabolites) associated with or co-purified with the immunomodulatory polypeptides in vivo. The term "substantially free of biomolecules" as used herein means that less than 5% of the dry weight of the composition is comprised of biomolecules that are not immunomodulatory polypeptides. A composition that is substantially free of such biomolecules may have less than 4%, 3%, 2%, 1%, 0.5%, 0.1%, 0.05%, 0.01% or less of biomolecules that are not immunomodulatory polypeptides. Thus, for example, the composition can be substantially free of biomolecules enriched in blood, such as proteins, fatty acids, cholesterol, non-protein clotting factors, metabolites, and the like, as discussed above. Additionally, the composition may be substantially free of cells, including red blood cells, white blood cells, and platelets, as well as cellular debris.
The compositions of the invention can include at least 1mg (e.g., at least 5, 10, 20, 30, 40, 50, 75, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, 1000mg or more) of an immunomodulatory polypeptide. Thus, for example, the composition may include an amount equal to about 1mg to about 1000mg (e.g., about 5mg to about 900mg, about 5mg to about 800mg, about 5mg to about 700mg, about 5mg to about 600mg, about 10mg to about 500mg, about 10mg to about 400mg, about 10mg to about 300mg, about 10mg to about 250mg, about 10mg to about 200mg, about 10mg to about 150mg, about 10mg to about 100mg, about 50mg to about 500mg, about 50mg to about 400mg, about 50mg to about 300mg, about 50mg to about 250mg, about 50mg to about 200mg, about 50mg to about 150mg, about 50mg to about 100mg, about 75mg to about 500mg, about 75mg to about 400mg, about 75mg to about 300mg, about 75mg to about 250mg, about 75mg to about 200mg, about 75mg to about 150mg, about 75mg to about 100mg, about 100mg to about 100mg, about 100mg, About 100mg to about 200mg or any other range encompassing two of the foregoing endpoints).
The compositions of the invention can include solutions containing at least 1mg/ml (e.g., at least 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, 100mg/ml or more) of an immunomodulatory polypeptide. Thus, for example, the compositions can comprise a polypeptide having an immunomodulatory polypeptide concentration of about 1mg/ml to about 1000mg/ml (e.g., about 5mg/ml to about 900mg/ml, about 5mg/ml to about 800mg/ml, about 5mg/ml to about 700mg/ml, about 5mg/ml to about 600mg/ml, about 5mg/ml to about 500mg/ml, about 10mg/ml to about 400mg/ml, about 10mg/ml to about 300mg/ml, about 10mg/ml to about 250mg/ml, about 10mg/ml to about 200mg/ml, about 10mg/ml to about 150mg/ml, about 10mg/ml to about 100mg/ml, about 50mg/ml to about 500mg/ml, about 50mg/ml to about 400mg/ml, a, About 50mg/ml to about 300mg/ml, about 50mg/ml to about 250mg/ml, about 50mg/ml to about 200mg/ml, about 50mg/ml to about 150mg/ml, about 50mg/ml to about 100mg/ml, about 75mg/ml to about 500mg/ml, about 75mg/ml to about 400mg/ml, about 75mg/ml to about 300mg/ml, about 75mg/ml to about 250mg/ml, about 75mg/ml to about 200mg/ml, about 75mg/ml to about 150mg/ml, about 75mg/ml to about 100mg/ml, about 100mg/ml to about 500mg/ml, about 100mg/ml to about 400mg/ml, about 100mg/ml to about 300mg/ml, about 100mg/ml to about 250mg/ml, about 100mg/ml to about 200mg/ml, About 100mg/ml to about 150mg/ml or any other range containing two of the foregoing endpoints).
The compositions of the present invention include pharmaceutical compositions. Such pharmaceutical compositions may comprise one or more immunomodulatory polypeptides and a pharmaceutically acceptable carrier. The pharmaceutical composition may further comprise a protein and/or chemotherapeutic agent other than an immunomodulatory polypeptide of the invention. The other protein may be a therapeutic agent, such as a therapeutic antibody. The therapeutic protein or antibody may have immunomodulatory properties or other properties that are enhanced by or enhance the immunomodulatory polypeptide of the invention. Alternatively, the other protein may be a carrier protein, such as serum albumin (e.g., HSA). The serum albumin (e.g., HAS, BSA, etc.) may be purified or recombinantly produced. By mixing the immunomodulatory polypeptide in the pharmaceutical composition with serum albumin, the immunomodulatory polypeptide can be effectively "loaded" onto the serum albumin, thereby allowing a greater amount of immunomodulatory polypeptide to be successfully delivered to the site of inflammation. The chemotherapeutic agent may be, for example, an anticancer chemotherapeutic agent. Such chemotherapeutic agents include, but are not limited to, gemcitabine, docetaxel, bleomycin, erlotinib, gefitinib, lapatinib, imatinib, dasatinib, nilotinib, bosutinib, crizotinib, ceritinib, tremetinib, bevacizumab, sunitinib, sorafenib, trastuzumab, enreatrituzumab, rituximab, ipilimumab, rapamycin, temsirolimus, everolimus, methotrexate, doxorubicin, idelberry, Folfirinox, cisplatin, carboplatin, 5-fluorouracil, tegafur, paclitaxel, prednisone, levothyroxine, and pemetrexed.
In some cases of a subject pharmaceutical composition, the composition includes an immunomodulatory polypeptide that is a CD206 binding peptide (e.g., as described herein) and a chemotherapeutic agent. In some embodiments, the immunomodulatory polypeptide used in the combination composition is a peptide shown in table 3. In certain instances, the immunomodulatory peptides (e.g., the peptides shown in table 3) are combined with a chemotherapeutic agent. In certain instances of the pharmaceutical composition, the chemotherapeutic agent is gemcitabine. In some cases of the pharmaceutical composition, the chemotherapeutic agent is docetaxel. In some cases of the pharmaceutical composition, the chemotherapeutic agent is spruce.
In some cases of a subject pharmaceutical composition, the composition comprises an immunomodulatory polypeptide that is a CD206 binding peptide (e.g., as described herein) and is conjugated to a second additional agent (e.g., as described herein). In some cases, the other agent is a chemotherapeutic agent. In some embodiments, the immunomodulatory polypeptide used in a subject peptide conjugate is a peptide shown in table 3. In some cases, the immunomodulatory peptides (e.g., the peptides shown in table 3) are conjugated to chemotherapeutic agents. In certain instances of the subject peptide conjugates, the chemotherapeutic agent is gemcitabine. In some cases of the subject peptide conjugates, the chemotherapeutic agent is docetaxel. In some cases of the subject peptide conjugates, the chemotherapeutic agent is spruce. In some cases of the subject peptide conjugates, the chemotherapeutic agent is paclitaxel.
In some cases, a subject pharmaceutical composition for treating cancer (e.g., ovarian cancer) includes an immunomodulatory polypeptide used in combination with a vaccination therapy (e.g., a Dendritic Cell (DC) vaccination agent that promotes Th1/Th17 immunization). In some cases of the pharmaceutical composition, the immunomodulatory polypeptide is an adjuvant used in combination with a Th 17-inducing vaccination agent.
The pharmaceutical composition of the present invention may be formulated to be suitable for oral administration, parenteral administration, inhalation administration, topical administration, mucosal administration, and the like. In some embodiments, the administering is by a route selected from the group consisting of oral, intravenous, intraperitoneal, inhalation, intranasal, intraprostatic, and intratumoral. The present invention is not limited by the route of administration. Compositions formulated for oral administration may, for example, include an enteric coating to ensure that the peptides contained therein reach the intestine and outside the intestine. Enteric formulations (e.g. gastro-insoluble capsules for oral administration, suppositories for rectal or vaginal administration) also form part of the invention. Compositions formulated for topical administration may, for example, be suspended in a gel or cream, applied to microneedles, or impregnated into bandages or topical patches to prolong the duration of action of the peptides contained therein. Any inhalable formulation that can be provided in nebulized form, including the subject peptide for delivery to a patient via the intrapulmonary route, can be used in conjunction with the present invention. In some cases, the subject compositions are administered by intratumoral injection, such as into injectable epidermal, subcutaneous, and/or lymph node tumors.
In some embodiments, the compositions are administered mucosally (e.g., using standard techniques; see, e.g., Remington: pharmaceutical sciences and practices, Mack Publishing Company, Isston, Pa., 19 th edition, 1995 (e.g., for mucosal delivery techniques, including intranasal, pulmonary, vaginal, and rectal techniques), as well as European publication No. 517,565 and Illum et al, J.Controlated Release, 1994, 29: 133. 141 (e.g., for intranasal administration techniques). in some cases, the compositions of the invention may be administered transdermally or transdermally using standard techniques.
Also provided are liposomal pharmaceutical compositions comprising the subject immunomodulatory peptides. Any suitable nanocarriers and liposomes can be used to prepare liposomal formulations of the subject peptides, such as the nanocarriers and liposomes described in the following publications: arias, "liposomes for drug delivery: patent review ", review of experts in treatment, 23, 2013, stage 11, pages 1399-; and Torchilin, "multifunctional nanocarriers," advanced drug delivery reviews, vol 58, No. 14, p 2006, 12, 1, p 1532-1555.
Also provided are nanoparticle formulations or compositions comprising the subject immunomodulatory peptides. Nanoparticle formulations or compositions can increase the water solubility of a peptide of interest and can enable protected, sustained, and targeted delivery of the peptide in therapeutic applications (e.g., as described herein). In some cases, the formulation is a polymer-based nanoparticle formulation. The nanoparticle formulation of interest comprises albumin nanoparticles, for example a nanoparticle formulation comprising human serum albumin. In some cases, desolvation techniques may be used to prepare albumin nanoparticles. The particle size, peptide drug release mechanism, encapsulation efficiency and peptide drug polymer interaction may be determined and selected using any suitable in vitro method. Cell culture studies, in vivo pharmacokinetic profiles (e.g. in rats) can be used for biological characterization of the desired formulation.
In some cases, the nanoparticle formulation composition including the target immunomodulatory peptide consists of iron oxide nanoparticles (iopps). The IONPs may be used in a variety of biomedical applications. In some cases, the iopp preparation may exhibit high uptake in macrophages and/or target cancer cells. The IONPs having the desired cytotoxicity, in vivo distribution and/or clearance may be selected for use in combination with the target immunomodulatory peptide. Well characterized varieties of IONPs having different sizes and coatings may be used in the subject compositions and formulations. In some cases, Polyethyleneimine (PEI) coated iopps or pegylated iopps are used. The IONP can enhance cytotoxicity of the subject preparation through a variety of mechanisms (e.g., ROS production and apoptosis).
Also provided are kits comprising an immunomodulatory polypeptide that is a CD206 binding peptide (e.g., as described herein) and another agent (e.g., a chemotherapeutic or immunotherapeutic agent) for treating cancer. The kit can include a dose of an immunomodulatory peptide in an amount effective to inhibit proliferation of cancer cells in a subject. The kit can also include a dose of another agent, such as a chemotherapeutic agent or an immunotherapeutic agent (e.g., as described herein), in an amount effective to inhibit cancer cell proliferation in the subject. In some cases, the kit includes an insert carrying instructions for administration of the immunomodulatory peptide and/or the another agent (e.g., a chemotherapeutic agent or an immunotherapeutic agent). In some cases, the kit of instructions for the combination therapy may suggest (i) reducing the dose of the immunomodulatory peptide when used in combination with a chemotherapeutic agent; (ii) when used in combination with the immunomodulatory peptide, reducing the dose of another agent (e.g., a chemotherapeutic or immunotherapeutic agent); and/or (iii) a different dosing regimen than that normally recommended for one or both agents.
Method
The present invention provides methods of modulating macrophage activity using immunomodulatory peptides (e.g., as described herein). In some cases of the method, the modulated macrophage activity is macrophage polarization. The method may comprise contacting the macrophage with a CD206 binding agent (a peptide belonging to the invention) to modulate the activity of the macrophage. In some cases, modulating activity refers to inhibiting macrophage activity. The subject methods may reduce the viability of the macrophages, where viability may be determined using any suitable method.
In certain embodiments, an immunomodulatory polypeptide of the invention can bind to human CD206 with an affinity of at least-650 kcal/mol, and in certain embodiments at least-700, -750, -800, -850, -900, -925, -950, -975, -1000, -1025, -1050kcal/mol or higher. The requisite binding affinity may correspond to a binding affinity that may be detected in vitro or in vivo. Alternatively, the requisite binding affinity may correspond to a binding affinity that may be measured, for example, using ClusProTMThe binding affinity detected by the algorithm in silico.
The macrophage targeted using the subject methods can be a M2 macrophage or a Tumor Associated Macrophage (TAM). The macrophages targeted may be in vitro or in vivo.
In certain embodiments, the peptides of the invention bind to two or more targets (e.g., pro-inflammatory targets). In some embodiments, the variant polypeptide binds to three, four, five, or more pro-inflammatory targets. For example, as described below, a variant polypeptide can bind to any combination of the targets disclosed herein (e.g., NF-kB class II protein and Human Serum Albumin (HSA)). Such binding can be based on computer, in vitro or in vivo data.
Exemplary RP peptides of interest can interact with a variety of inflammation-associated signaling molecules, including NF-kB class II subunit RelB, TGF β, Notch1, Wnt8R, TRAIL, IL6R, IL10R, EGFR, and CDK6, as well as other membrane-associated signaling molecules, including CD206, CD47, and SIRP- α, the translation modifying proteins transglutaminase 2(TGM2), and the histone modifying enzymes Histone Methyltransferase (HMT). In some cases, the target peptide is a CD206 binding peptide. After folding these protein targets into their normal 3-dimensional conformation, amphipathic gaps are typically created that have high affinity for the immunomodulatory peptides described herein.
For more details on target signaling molecules that specifically bind to target immunomodulatory peptides, see the following publications: jaynes et al, WO2016/061133, the contents of which publication are incorporated herein in their entirety.
Immunomodulatory polypeptides used in the subject methods can be determined based on their ability to bind to a mannose binding site on CD206 and/or interfere with or block the binding of SIRP-mannose to CD 206. For example, the immunomodulatory polypeptide can bind to at least one amino acid residue in CD206 selected from the group consisting of Glu-725, Tyr-729, Glu-733, Asn-747, and Asp-748 or an equivalent amino acid residue in a CD206 protein of another species. Alternatively, the immunomodulatory polypeptide can bind to at least one amino acid residue in human CD206 selected from the group consisting of Phe-708, Thr-709, Trp-710, Pro-714, Glu-719, Asn-720, Trp-721, Ala-722, Glu-725, Tyr-729, Glu-733, Asn-747, Asp-748, Ser-1691, Cys-1693, Phe-1694, and Phe-1703, or equivalent amino acid residues in CD206 proteins of other species. In certain embodiments, the immunomodulatory polypeptide can bind to at least one amino acid residue in CD206 selected from the group consisting of Phe-708, Trp-710, Trp-721, Glu-725, Tyr-729, Glu-733, or an equivalent amino acid residue in a CD206 protein of another species.
In certain instances, the immunomodulatory polypeptide binds to the Fibronectin (FBN) domain of CD206 and/or interferes with or blocks collagen binding to CD 206. In some cases, the immunomodulatory polypeptide can specifically bind to the Fibronectin (FBN) domain of CD 206. In some cases, a subject immunomodulatory polypeptide binds to the type C Carbohydrate Recognition Domain (CRD) of CD206 to modulate (e.g., activate) the activity of CD 206. In some cases, a subject immunomodulatory polypeptide binds to the type C Carbohydrate Recognition Domain (CRD) of CD206 to modulate (e.g., interfere with, block, or inhibit) the activity of CD 206. In certain instances, the CRD domain to which the subject immunomodulatory polypeptide specifically binds to modulate the activity of CD206 is a CRD 4 or 5 domain.
In certain embodiments, the immunomodulatory polypeptide binds to two or more targets (e.g., pro-inflammatory targets). In some embodiments, the immunomodulatory polypeptide binds to three, four, five, or more proinflammatory targets. For example, an immunomodulatory polypeptide can bind to any combination of targets disclosed herein. Such binding can be based on computer, in vitro or in vivo data. Thus, an immunomodulatory polypeptide can bind to two or more NF-kB class II subunits (e.g., RelB and at least one other NF-kB class II subunit, such as RelA, cRel, NF-kB1, or NF-kB 2). Alternatively (or in addition), the immunomodulatory polypeptide can bind to an NF-kB class II subunit (e.g., RelB) and at least one other signaling molecule (e.g., at least one signaling molecule selected from the group consisting of TGF β, Notch1, Wnt8R, TRAIL, IL6R, IL10R, EGFR, CDK6, CD206, CD47, SIRP- α, HMT, and TGM 2). For example, an immunomodulatory polypeptide can bind to an NF-kB class II subunit (e.g., RelB) and at least one signaling molecule selected from the group consisting of TGF β, Notch1, Wnt8R, TRAIL, IL6R, IL10R, EGFR, and CDK 6. Alternatively, the immunomodulatory polypeptide can bind to a NF-kB class II subunit (e.g., RelB) and at least one signaling molecule selected from the group consisting of CD206, CD47, SIRP-a, and TGM 2. In other alternatives, the immunomodulatory polypeptide can bind to a NF-kB class II subunit (e.g., RelB) and HMT. In other alternatives, the immunomodulatory polypeptide can bind to at least one signaling molecule selected from the group consisting of TGF β, Notch1, Wnt8R, TRAIL, IL6R, IL10R, EGFR, and CDK6, and at least one signaling molecule selected from the group consisting of CD206, CD47, SIRP- α, and TGM 2. In other alternatives, the immunomodulatory polypeptide can bind to at least one signaling molecule selected from the group consisting of TGF β, Notch1, Wnt8R, TRAIL, IL6R, IL10R, EGFR, and CDK6, and can also bind to HMT. In still other embodiments, the immunomodulatory polypeptide can be combined with: NF-kB class II subunit (e.g., RelB); at least one signaling molecule selected from the group consisting of TGF β, Notch1, Wnt8R, TRAIL, IL6R, IL10R, EGFR, and CDK 6; at least one signaling molecule selected from the group consisting of CD206, CD47, SIRP-a, and TGM 2; and HMT. In certain embodiments, the immunomodulatory polypeptide binds to two or more proinflammatory targets, and also binds to serum albumin (e.g., human serum albumin).
Immunomodulatory polypeptides of the invention provide a powerful tool for reducing inflammation and/or treating disorders associated with excessive inflammation (whether acute or chronic). The term "treating" and similar words as used herein shall mean stabilizing a medical condition, reducing the symptoms of the medical condition, preventing the occurrence of the medical condition, or curing the medical condition.
Accordingly, the present invention provides methods of reducing/decreasing the expression level and/or activity of at least one (e.g., 2, 3, 4, 5 or more) pro-inflammatory cytokine at a site of inflammation in a subject. The methods comprise administering to the subject an immunomodulatory polypeptide (or, e.g., a pharmaceutical composition comprising an immunomodulatory polypeptide) of the invention. The proinflammatory cytokine can be selected from the group consisting of NF-kB, TNF α, IL-1, IL-6, IL-8, IL-12, IL-17, IL-23, MCP-1, MMP-1, and MMP-9. The reduction/decrease may be a reduction/decrease in the expression or activity of the cytokine of at least 10% (e.g., 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50% or greater).
The present invention also provides methods of inhibiting an increase in the level of expression and/or activity of at least one (e.g., 2, 3, 4, 5, or more) proinflammatory cytokine at a potential site of inflammation in a subject. The methods comprise administering to the subject an immunomodulatory polypeptide (or, e.g., a pharmaceutical composition comprising an immunomodulatory polypeptide) of the invention. The proinflammatory cytokine can be selected from the group consisting of NF-kB, TNF α, IL-1, IL-6, IL-8, IL-12, IL-17, IL-23, MCP-1, MMP-1, and MMP-9. The methods can inhibit cytokine expression and/or activity by limiting such increase to no more than 20% (e.g., 15%, 12.5%, 10%, 7.5%, 5%, 4%, 3%, 2%, 1% or less).
It is to be understood that, in some cases, modulating the level and/or activity of proinflammatory cytokines at a site of inflammation in a subject can effect downstream modulation of the activity of immune cells of interest (e.g., effector T cells, regulatory T cells (tregs), natural killer cells (NK cells), B cells, etc.), and can effect modulation of a target immune or inflammatory response.
The invention also provides a method of treating or preventing a chronic inflammation-related disorder. The chronic inflammation-related disorder can be irritable bowel syndrome, ulcerative colitis, crohn's disease, idiopathic pulmonary fibrosis, asthma, keratitis, arthritis, osteoarthritis, rheumatoid arthritis, an autoimmune disease, feline or human immunodeficiency virus (FIV or HIV) infection, cancer, age-related inflammation and/or stem cell dysfunction (e.g., age-related increases in Nlrp3 expression, age-related increases in SOCS3 in muscle stem cells, etc.), graft-versus-host disease (GVHD), keloids, scleroderma, obesity, diabetes, diabetic wounds, other chronic wounds, atherosclerosis, multiple sclerosis, parkinson's disease, alzheimer's disease, macular degeneration, gout, gastric ulcers, gastritis, mucositis, toxoplasmosis, and chronic viral or microbial infections (such as chronic bacterial or protozoal infections, for example). The methods comprise administering an immunomodulatory polypeptide (or, e.g., a pharmaceutical composition comprising an immunomodulatory polypeptide) of the invention to a subject suffering from, or at risk of developing, the disorder.
The invention also provides methods of treating or preventing fibrosis. The fibrosis may be, for example, pulmonary fibrosis, skin fibrosis, liver fibrosis, kidney fibrosis or fibrosis caused by ionizing radiation. The methods comprise administering an immunomodulatory polypeptide (or, e.g., a pharmaceutical composition comprising an immunomodulatory polypeptide) of the invention to a subject suffering from or at risk of developing fibrosis.
The invention also provides methods of treating cancer. The cancer may be colon cancer, breast cancer, leukemia, lymphoma, ovarian cancer, prostate cancer, liver cancer, lung cancer, testicular cancer, cervical cancer, bladder cancer, endometrial cancer, kidney cancer, melanoma, thyroid cancer, or brain or eye cancer. The methods comprise administering an immunomodulatory polypeptide of the invention (or, e.g., a pharmaceutical composition comprising an immunomodulatory polypeptide) to a subject afflicted with cancer. The disclosed subject matter also provides methods for treating a solid tumor cancer in a subject. In some embodiments, the method comprises administering to the subject a therapeutically effective amount of a compound disclosed herein.
For any of the foregoing methods, the subject can be an animal, such as a farm animal (e.g., horse, cow, pig, goat, sheep, rabbit, chicken, turkey, duck, etc.), a pet (e.g., dog, cat, rabbit, hamster, gerbil, bird, fish, etc.), a laboratory animal (e.g., mouse, rat, monkey, orangutan, owl, fish, etc.), a zoo animal (e.g., gorilla, orangutan, chimpanzee, monkey, elephant, camel, zebra, boar, lion, tiger, giraffe, bear, bird, etc.), a wild animal (e.g., deer, wolf, lion, bird, etc.), or a human.
In combination with any of the foregoing methods, the immunomodulatory polypeptide can be administered at a dose and frequency depending on the animal type, animal size, and condition to be treated. Typically, the immunomodulatory polypeptide is administered in an amount of about 1mg to about 1000mg (e.g., about 5mg to about 900mg, about 5mg to about 800mg, about 5mg to about 700mg, about 5mg to about 600mg, about 10mg to about 500mg, about 10mg to about 400mg, about 10mg to about 300mg, about 10mg to about 250mg, about 10mg to about 200mg, about 10mg to about 150mg, about 10mg to about 100mg, about 50mg to about 500mg, about 50mg to about 400mg, about 50mg to about 300mg, about 50mg to about 250mg, about 50mg to about 200mg, about 50mg to about 150mg, about 50mg to about 100mg, about 75mg to about 500mg, about 75mg to about 400mg, about 75mg to about 300mg, about 75mg to about 250mg, about 75mg to about 200mg, about 75mg to about 150mg, about 75mg to about 100mg, about 100mg to about 500mg, about 100mg to about 400mg, about 75mg to about 300mg, about 75mg to about 250mg, about 100mg, about 300 Once (every other day or once a week). The daily dose may be administered once daily, or divided into smaller doses to be taken at multiple time points daily. For humans (and other mammals of similar size), a dose of 5mg/kg may be administered every other day. The immunomodulatory polypeptide may be administered at intervals (e.g., 2-3 weeks of polypeptide administration, waiting 2-3 weeks, and then repeating the cycle) for a fixed period of time (e.g., 2-3 weeks), or until the level of proinflammatory cytokines has decreased or stabilized, the chronic inflammatory condition or fibrosis has been reduced, or the cancer has been alleviated.
The immunomodulatory polypeptides (or pharmaceutical compositions comprising such polypeptides), when administered in conjunction with any of the foregoing methods, can be administered intravenously, intraperitoneally, parenterally, in situ, subcutaneously, topically, by inhalation, nasally, orally, sublingually, intraocularly, via an implantable depot, using a nanoparticle-based delivery system, microneedle patch, microsphere, bead, osmotic or mechanical pump, and/or other mechanical device.
In combination with any of the foregoing methods, the immunomodulatory polypeptide (e.g., as described herein) (or a pharmaceutical composition comprising such polypeptide) can be administered in combination with another agent intended for reducing or preventing inflammation, treating or preventing chronic inflammation or fibrosis, or treating cancer. In each case, the immunomodulatory polypeptide can be administered before, during, or after administration of the other drug. For the treatment of cancer, the immunomodulatory polypeptide can be administered in combination with another therapeutic agent (e.g., a chemotherapeutic agent or an immunotherapeutic agent) selected from the group consisting of: taxanes, nucleoside analogs, steroids, anthracyclines, thyroid hormone replacement drugs, thymidylate targeting drugs, chimeric antigen receptor/T cell therapies, chimeric antigen receptor/NK cell therapies, apoptosis regulating gene inhibitors (e.g., B cell CLL/lymphoma 2(BCL-2) BCL-2-type 1(BCL-XL) inhibitors), CARP-1/CCAR1 (cell division cycle and apoptosis regulating gene 1) inhibitors, colony stimulating factor 1 receptor (CSF1R) inhibitors, CD47 inhibitors, cancer vaccines (e.g., dendritic cell vaccines that induce Th 17), and other cell therapies. Specific chemotherapeutic agents include, for example, gemcitabine, docetaxel, bleomycin, erlotinib, gefitinib, lapatinib, imatinib, dasatinib, nilotinib, bosutinib, crizotinib, ceritinib, tremetinib, bevacizumab, sunitinib, sorafenib, trastuzumab, emrituximab, rituximab, ipilimus, rapamycin, temsirolimus, everolimus, methotrexate, doxorubicin, idelberry, Folfirinox, cisplatin, carboplatin, 5-fluorouracil, tixol, paclitaxel, prednisone, levothyroxine, pemetrexed, naltexox, ABT-199.
In some embodiments, the immunomodulatory polypeptide used in combination therapy is a peptide having macrophage modulating activity (e.g., as described herein). In certain instances, the immunomodulatory polypeptide is a CD206 binding peptide (e.g., as described herein). In some embodiments, the immunomodulatory polypeptide used in combination therapy is a peptide shown in table 3. In certain instances, the immunomodulatory peptides (e.g., the peptides shown in table 3) can be administered in combination with a chemotherapeutic agent to treat cancer. In certain instances, the chemotherapeutic agent is gemcitabine. In some cases, the chemotherapeutic agent is docetaxel. In some cases, the chemotherapeutic agent is spruce.
For the treatment of cancer (e.g., melanoma, non-small cell lung cancer, or lymphoma, such as hodgkin's lymphoma), the immunomodulatory polypeptide may be administered in combination with an immunotherapeutic agent. An immunotherapeutic agent is any suitable agent used to treat a disease by inducing, enhancing or suppressing an immune response. In some cases, the immunotherapeutic agent is an immune checkpoint inhibitor. Any suitable checkpoint inhibitor may be used in combination with the subject peptides, including but not limited to cytotoxic T lymphocyte-associated antigen 4(CTLA-4) inhibitors, programmed death protein 1(PD-1) inhibitors, and PD-L1 inhibitors. Exemplary checkpoint inhibitors of interest include, but are not limited to, ipilimumab, pembrolizumab, and nivolumab. In certain embodiments, the immunomodulatory polypeptide may be administered in combination with a colony stimulating factor 1 receptor (CSF1R) inhibitor for the treatment of cancer and/or inflammatory diseases. Contemplated CSF1R inhibitors include, but are not limited to, imirituzumab.
Any suitable cancer vaccine therapies and agents can be used in conjunction with the subject immunomodulatory polypeptide compositions and methods. For the treatment of cancer (e.g., ovarian cancer), the immunomodulatory polypeptide can be administered in combination with a vaccination therapy (e.g., a Dendritic Cell (DC) vaccination agent that promotes Th1/Th17 immunization). Th17 cell infiltration was associated with a significant prolongation of overall survival in ovarian cancer patients. In some cases, the immunomodulatory polypeptide is used as an adjuvant treatment regimen with Th 17-inducing vaccination.
Also of interest are agents belonging to CARP-1/CCAR1 (cell division cycle and apoptosis regulatory gene 1) inhibitors (including but not limited to those described in the following publications: Rishi et al, J. biomedical nanotechnology, Vol. 11, No. 9, 9/2015, 9/pp. 1608-1627(20)) and CD47 inhibitors (including but not limited to anti-CD 47 antibody agents, such as Hu5F 9-G4).
In some cases, the combination provides an enhanced effect relative to the use of either component alone; in some cases, the combination provides a superadditive or synergistic effect relative to the combined or additive effect of the components. Various combinations of the subject polypeptides and chemotherapeutic agents may be employed, or used sequentially or simultaneously. For multiple administrations, for example, the two agents can be administered directly in alternation, or two or more administrations of one agent can be alternated with a single administration of the other agent. The simultaneous administration of the two agents may also be alternated or otherwise dispersed with the administration of each agent. In some cases, the time between administrations can be about 1-6 hours, about 6-12 hours, about 12-24 hours, about 1-2 days, about 1-2 weeks, or longer after treatment initiation.
In some embodiments, the method is a method of reducing proliferation of a cancer cell, wherein the method comprises contacting the cell with an effective amount of a subject immunomodulatory polypeptide (e.g., as described herein). The methods can be performed in conjunction with administration of a chemotherapeutic agent (e.g., as described herein). The cancer cell can be an in vitro cancer cell or an in vivo cancer cell. In certain instances, the method comprises contacting the cell with an immunomodulatory peptide (e.g., a peptide shown in table 3), and contacting the cell with a chemotherapeutic agent. Any suitable cancer cell can be targeted. In certain instances, the chemotherapeutic agent is gemcitabine. In some cases, the chemotherapeutic agent is docetaxel. In some cases, the chemotherapeutic agent is spruce.
Alternatively, for methods of treating cancer, the immunomodulatory polypeptides (or pharmaceutical compositions comprising such polypeptides) may be administered in combination with radiation therapy. Furthermore, the immunomodulatory polypeptide can be administered before or after administration of radiation therapy.
Any of the foregoing methods of the invention further comprises the step of assessing the efficacy of the treatment method. Since the immunomodulatory polypeptides of the invention have demonstrable abilities to reduce tissue inflammation and inhibit production of inflammatory mediators (e.g., IL-1, IL-6, IL-12, and TNF α) in tissue and serum (data not shown), the efficacy of the treatment methods can be assessed by measuring the levels of such cytokines (e.g., in serum) to determine whether the levels have responded appropriately to the treatment. Depending on the cytokine level, the dose of immunomodulatory polypeptide can be adjusted up or down as desired.
Definition of
It is to be understood that the invention is not limited to the particular embodiments described herein, as variations in actual practice are possible. It is also to be understood that the terminology used in the patent is for the purpose of describing particular embodiments only, and is not intended to limit the inventive concept, the scope of which will be limited only by the appended claims.
Where a range of values is provided, it is understood that each intervening value, to the extent that there is no such difference, to the upper and lower limits of that range, and any other stated or intervening value in that range, is encompassed within the invention. Unless the context clearly dictates otherwise, each intermediate value should be as low as one tenth of the unit of the lower limit. The upper and lower limits of these smaller ranges may independently be included in the smaller ranges, and are also encompassed within the invention, subject to any specifically excluded limit in the stated range. Where the stated range includes one or both of the limits, ranges excluding either or both of those included limits are also included in the invention.
Certain ranges set forth herein precede the value by the term "about". The term "about" is used herein for the purpose of providing literal support for the precise number following the term, as well as numbers that are near or similar to the number following the term. In determining whether a number is near or approximate to a specifically recited number, a near or approximate non-recited number may be a number substantially equal to the specifically recited number in the context of its occurrence.
Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. Representative exemplary methods and materials are described herein, although methods and materials similar or equivalent to those described herein can also be used in the practice or testing of the present invention.
All publications and patents cited in this specification are herein incorporated by reference as if each individual publication or patent were specifically and individually indicated to be incorporated by reference and were set forth for the purpose of disclosing and describing the methods and/or materials associated with the cited publications. The citation of any publication is for its disclosure prior to the filing date and should not be construed as an admission that the present invention is not entitled to antedate such publication by virtue of prior invention. Further, the dates of publication provided may be different from the actual publication dates which may need to be independently confirmed.
It is noted that, as used herein and in the appended claims, the singular forms "a," "an," and "the" include plural referents unless the context clearly dictates otherwise. It should also be noted that claims may be drafted to exclude any optional element. Accordingly, this statement is intended to serve as antecedent basis for use of such exclusive terminology as "solely," "only," and the like in connection with the recitation of claim elements, or use of a "negative" limitation.
As will be apparent to those skilled in the art upon reading this disclosure, each of the individual embodiments described and illustrated in this patent has layered components and features that can be readily separated or combined with the features of any of the other several embodiments without departing from the scope and spirit of the present invention. Any recited method may be implemented in the order of events recited or in any other order that is logically possible.
Examples of the invention
Methods and materials of interest for the preparation and evaluation of target immunomodulatory peptides include those disclosed in the experimental section of WO2016/061133 by Jaynes et al, the contents of which are incorporated herein in their entirety.
The following examples are put forth so as to provide those of ordinary skill in the art with a complete disclosure and description of methods of making and using the present invention, and are not intended to limit the scope of what the inventors regard as their invention nor are they intended to represent that the experiments below are all or the only experiments performed.
Example 1: tumor growth inhibition
The polypeptides of the invention were also tested for their effect on tumor growth in a mouse non-metastatic breast cancer model. MCF-7 human non-metastatic breast cancer cells were incubated in normal growth medium at 37 ℃ under 5% CO 2. Cells were harvested under conditions where fusion reached 80% to 90%. Athymic nude mice (J: NU) with impaired immune function were divided into 2 groups (9 animals per group). All mice were injected with-4.5X 106MCF-7 cells, which have been stained with VIVO Tracker 680 and suspended in 200. mu.l PBS/Matrigel mixture. Make itCells were injected subcutaneously into the dorsal side of the treated animals using a 22 gauge needle equipped with a 500 μ l syringe.
Animals were assigned to receive vehicle and peptide treatment. Animals receiving peptide treatment are treated with the target polypeptide. Freshly prepared peptides were dissolved in sterile physiological saline at a concentration of 100 μ M and animals of the peptide group were treated therewith. Animals receiving vehicle treatment are injected with saline buffer only. All therapeutic drugs were injected into the tumor mass using an 271/2 gauge needle fitted with a 1ml syringe twice a week for 5 weeks. Animal body weight and tumor volume were measured 3 times per week and fluorescently labeled, followed by VIVO Tracker 680 and IVIS imaging.
Figure 2 shows that peptides RP832C and RP185 can reduce tumor volume in a mouse tumor suppression model. The data show that the polypeptides of the invention can inhibit tumor growth in vivo.
Example 2: administration of peptides in combination with chemotherapy
Given that inflammation plays a significant role in tumor development and metastasis, and that M2 macrophage activity is known to be associated with tumor development, it is expected that administration of a peptide of the invention (e.g., a selected peptide shown in table 3) may have a positive impact on cancer treatment outcome.
To validate this theory, mice in the immunocompromised ("nude") group were injected under the left upper nipple at-5X 106Personal triple negative breast cancer cells (MDA-MB-231). After this administration, one group received vehicle only; both groups received the chemotherapeutic gemcitabine, at a dose of q4d of 40mg/kg body weight. One group also received a daily dose of 5mg/kg body weight of the test peptide; and the fourth group received only the peptide at a daily dose of 5mg/kg body weight. Starting on study day 32, the concentration of RP-182 was increased to 20mg/kg body weight in the gemcitabine + RP-182 group. Tumor volume was assessed at various time points after initial cell administration. After 50 days, the mice were sacrificed.
In the second experiment, xenografts of C42B prostate cancer cells were introduced into four groups of mice and tumors were allowed to grow to about 100m prior to treatment3. One group onlyTreating with a solvent; the second group was treated with docetaxel at a dose of 2.5mg/kg body weight once a week; the third group was treated with the test peptide at a dose of 10mg/kg body weight, once daily subcutaneously; the fourth group was treated with docetaxel (dose 2.5mg/kg, once a week) and the test peptide (dose 10mg/kg, once a day). Assessing tumor volume at various time points after initial cell administration; after 27 days, the mice were sacrificed. .
It is expected that the peptides of the invention (e.g., selected peptides shown in table 3) will produce a synergistic effect when administered with chemotherapeutic agents, including gemcitabine and docetaxel, as well as checkpoint inhibitor therapy and other immunotherapies. In particular, the peptides of the invention are particularly useful when used in conjunction with recently developed CAR-T (chimeric antigen receptor/T cell) therapies. Such therapies produce a very high systemic load of dead cell material while destroying tumor cells, thereby over-stimulating the immune system and producing a "cytokine storm" that may be fatal to the patient.
Example 3: study of selected peptides
Table 5: binding of selected peptides shown in tables 3-4 to CD206 can be calculated using the ClusPro algorithm:
example 4: selective effect of RP peptides on macrophage activity in scleroderma patients.
Peripheral blood-derived macrophages from healthy volunteers and scleroderma patients were cultured in MCS-F for 7 days, followed by qPCR for arginase 1(M2 marker) or IFNg (M1 marker), followed by treatment with RP peptide (dose range 0-100 uM). After 48 hours, the medium was changed and the cells were reprocessed with the peptide. After 96 hours, cell viability was determined by PrestoBlue assay. (FIG. 4A) macrophages from healthy controls (with low arginase: IFNg ratio: 3.6) were resistant to the effect of RP-peptides of interest (RP182, RP185, RP832C, RP837) on survival. (FIG. 4B): in scleroderma (SSc) patients (with homoarginase: IFNg ratio: 8.8), the RP peptides of interest (RP182, RP185, RP832C, RP837) resulted in a substantial reduction in survival at 96 hours, even at 0.01 uM.
Example 5: rescue of bleomycin-induced pulmonary fibrosis
Intratracheal Instillation (IT) of bleomycin was used as a pulmonary fibrosis model. The rescue of bleomycin-induced pulmonary fibrosis in mice by the subject peptide was investigated. Experimental parameters: four groups (n-8) of C57BL6 male mice were studied over 6-8 weeks. 2.5U/kg bleomycin the IT administration was carried out as a single bolus. After 72 hours, 1mg/kg of the peptide of interest (RP182, RP185, RP832, RP837) was administered by IT, once every two days. In this bleomycin challenge experiment, the fibrosis measurement is the Ashcroft score after trichrome staining. The collagen score is a quantitative measure after hydroxyproline staining. Fig. 1 shows a corresponding graph of these results.
The target peptide results in reduced fibrosis and collagen deposition. Sections of lung tissue were stained with hematoxylin and eosin (H/E) and Massachusetts trichrome. Unlike the alveoli of the treated and untreated lung groups, the alveoli of the vehicle group appeared to be surrounded by fibrotic tissue and collagen deposition increased. Weight change: there was no significant change in body weight of the RP peptide-treated group compared to the body weight of the vehicle-treated group. Analysis of Ashcroft score: lung specimens from the vehicle group showed a deformed lung structure with increased fibrosis and collagen deposition and therefore scored approximately 6, whereas lung specimens from the peptide treated group showed a well organized lung structure and therefore a lower score.
Fibrosis and collagen deposition level assessment: fibrosis and collagen deposition were significantly reduced in the treated group compared to the vehicle group as determined by ImageJ software. Lung weight change: the vehicle group had a higher lung weight compared to the peptide-treated group, as indicated by fibrosis and reduced collagen content in the peptide-treated group. IHC staining of TGF β 1 and α SMA: peptide-treated lung tissue showed a significant reduction in markers associated with fibrosis of TGF β 1 and α SMA.
These results indicate that the exemplified peptides provide a reduction in bleomycin-induced pulmonary fibrosis in a mouse pulmonary fibrosis model.
Example 6: the RP peptide of interest acts synergistically with PD-1 checkpoint inhibitors in CT26 xenografts.
FIG. 2 shows the results of studies on the effect of the peptide of interest (with and without anti-PD 1 antibody) on tumor volume in a mouse tumor suppression model. The peptides RP832C and RP185 were administered at a dose of 10mg/kg once per day. The anti-PD 1 antibody was administered intraperitoneally at a dose of 200ug, twice weekly. The results provided in the examples demonstrate the efficacy of the peptides of the invention.
While the claims are appended hereto, the following clauses are provided to illustrate aspects of the invention:
[J1aJ1b]-[X1aX1b]-[J2aJ2b]-[X2aX2b]-[J3a]-[X3a](formula 5A);
wherein: j. the design is a square1a、J1b、J2a、J2bAnd J3aEach independently selected fromHydrophobic amino acid residues (e.g., phenylalanine, tryptophan, alanine, valine, and glycine); x1a、X1b、X2a、X2bAnd X3aEach independently selected from a hydrophilic amino acid residue (e.g., lysine, arginine, histidine, aspartic acid, glutamic acid, asparagine, or glutamine).
Wherein X1a、X1b、X2a、X2bAnd X3aEach independently selected from ornithine, lysine and arginine.
Clause 7. The immunomodulatory peptide of clauses 2-4, having a sequence defined by the formula: FWKX1bFVX2aKWX3a(SEQ ID NO: 31)
Wherein X1b、X2aAnd X3aEach independently lysine or arginine.
Clause 9. The immunomodulatory peptide of clause 8, wherein: j. the design is a square1a、J1b、J2a、J2bAnd J3aEach is tryptophan, and X1a、X1b、X2a、X2bAnd X3aEach independently selected from histidine, lysine and arginine.
Wherein X1a、X2bAnd X3aEach independently histidine, lysine or arginine.
Clause 11. The immunomodulatory peptide of clause 10, having a sequence selected from WWHHWWHHWH (SEQ ID NO: 13), WWRHWWHRWR (SEQ ID NO: 14), and WWKHWWHKW (SEQ ID NO: 15) (RP 847-849).
[J1a]-[X1aX1b]-[J2aJ2b]-[X2a]-[J3a]-[X3aX3b]-[J4aJ4b](formula 6A);
wherein J1a、J2a、J2b、J3a、J4aAnd J4bEach independently selected from hydrophobic amino acid residues (e.g., phenylalanine, tryptophan, alanine, isoleucine, valine, and glycine); and
X1a、X1b、X2a、X3aand X3bEach independently selected from a hydrophilic amino acid residue (lysine, arginine, histidine, aspartic acid, glutamic acid, asparagine, or glutamine).
Clause 13. The immunomodulatory peptide of clause 12, having the sequence GDRGIKGHRGF (SEQ ID NO: 8) (RP 842).
wherein: j. the design is a square1a、J1b、J2aAnd J2bEach independently selected from hydrophobic amino acid residues (e.g., phenylalanine, tryptophan, and valine); x1aAnd X1bEach independently selected from hydrophilic amino acid residues (e.g., lysine or arginine).
Clause 15. The immunomodulatory peptide of clause 14, having the sequence FWKRFV (seq id no: 5) (RP 837N).
[J1aJ1b]-[X1aX1b]-[J2a]-[X2a](formula 2A)
Wherein: j. the design is a square1a、J1b、J2aAnd J2bEach independently selected from hydrophobic amino acid residues (e.g., phenylalanine, tryptophan, and valine); x1a、X1bAnd X2aEach independently selected from hydrophilic amino acid residues (e.g., lysine or arginine).
Clause 17. The immunomodulatory peptide of clause 16, having the sequence
FVRKWR (Serial number: 6) (RP837C1)。
[J1aJ1b]-[X1aX1b]-[J2aJ2b]-[X2aX2b]-[J3aJ3b](formula 4A); and [ J3aJ3b]-[X2aX2b]-[J2bJ2a]-[X1bX1a]-[J1bJ1a](formula 4B); wherein J1a、J1b、J2a、J2b、J3aAnd J3bEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, tyrosine, or leucine); x1a、X1b、X2aAnd X2bEach independently selected from hydrophilic amino acid residues (e.g., lysine or arginine).
Clause 19. The immunomodulatory peptide of clause 18, having the sequence LYKKIIKKLL (SEQ ID NO: 12) (RP 846).
[J1aJ1bJ1c]-[X1a]-[J2aJ2b]-[X2aX2b]-[J3aJ3b](formula 4C);
wherein J1a、J1b、J1c、J2a、J2b、J3aAnd J3bEach independently selected from hydrophobic amino acid residues (e.g., phenylalanine, tyrosine, and proline); x1a、X2aAnd X2bEach independently selected from a hydrophilic amino acid residue (e.g., aspartic acid, lysine, or arginine).
Clause 21. An immunomodulatory peptide according to clause 20 having the sequence FYPDFFKKFF (SEQ ID NO: 10) (RP 844).
Clause 22. The immunomodulatory peptide of clause 1, wherein the striapathic region has the corresponding formula shown in formula 4, and has a sequence defined by the formula: [ J ]1aJ1b]-[X1aX1b]-[J2a]-[X2aX2bX2c]-[J3aJ3b](formula 4D);wherein J1a、J1b、J2a、J3aAnd J3bEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, serine, glycine, or isoleucine); x1a、X1b、X2a、X2bAnd X2cEach independently selected from a hydrophilic amino acid residue (e.g., glutamic acid, aspartic acid, lysine, or arginine).
Clause 23. An immunomodulatory peptide according to clause 22 having the sequence FFRKSKEKIG (seq id no: 18) (RP 853).
Clause 24. The immunomodulatory peptide of clause 1, wherein the striapathic region has the corresponding formula shown in formula 4, and has a sequence defined by the formula: [ J ]1aJ1b]-[X1aX1b]-[J2a J2b]-[X2aX2b]-[J3a](formula 4E); wherein J1a、J1b、J2a、J2bAnd J3aEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, alanine, or isoleucine); x1a、X1b、X2a、X2bAnd X2cEach independently selected from hydrophilic amino acid residues (e.g., ornithine, lysine or arginine).
Clause 25. The immunomodulatory peptide of clause 1, wherein the striapathic region has the corresponding formula shown in formula 5, and has a sequence defined by the formula: [ J ]1a]-[X1a]-[J2aJ2bJ2c]-[X2a]-[J3aJ3b]-[X3aX3b](formula 5C); wherein J1a、J2a、J2b、J2c、J3aAnd J3bEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, leucine, glycine, or isoleucine); x1a、X2a、X3aAnd X3bEach independently selected from hydrophilic amino acid residues (e.g., glutamine, lysine, or histidine).
Clause 26. An immunomodulatory peptide according to clause 25 having the sequence FQFLGKIIHH (seq id no: 17) (RP 852).
Clause 27. The immunomodulatory peptide of clause 1, wherein the striapathic region has the corresponding formula shown in formula 7, and has a sequence defined by the formula: [ X ]1aX1b]-[J1a]-[X2a]-[J2a]-[X3a]-[J3aJ3bJ3c](formula 7A); wherein J1a、J2a、J3a、J3bAnd J3cEach independently selected from a hydrophobic amino acid residue (e.g., isoleucine, valine, leucine, serine, or alanine); x1a、X1b、X2aAnd X3aEach independently selected from hydrophilic amino acid residues (e.g., lysine or arginine).
Clause 28. An immunomodulatory peptide according to clause 27 having the sequence KKIRVRLSA (seq id no: 16) (RP 851).
Clause 29. The immunomodulatory peptide of clause 1, wherein the striapathic region has the corresponding formula shown in formula 5, and has a sequence defined by the formula: [ J ]1aJ1b]-[X1aX1b]-[J2aJ2bJ2c]-[X2b]-[J3a]-[X3a](formula 5B);
wherein J1a、J1b、J2a、J2bAnd J3aEach independently selected from a hydrophobic amino acid residue (e.g., phenylalanine, alanine, threonine, or leucine); x1a、X1b、X2a、X2bAnd X3aEach independently selected from a hydrophilic amino acid residue (e.g., histidine, aspartic acid, lysine, or arginine).
Clause 30. An immunomodulatory peptide according to clause 29, having the sequence FFRHFATHLD (seq id no: 11) (RP 845).
Clause 31. The immunomodulatory peptide of clause 40, wherein the striapathic region has the corresponding formula shown in formula 3 and has a sequence defined by the formula: [ X ]1aX1b]-[J1aJ1bJ1cJ1d]-[X2aX2b]-[J2aJ2b](formula 3A); wherein: j. the design is a square1a、J1b、J1c、J1d、J2aAnd J2bEach independently selected from a hydrophobic amino acid residue (e.g., leucine, serine, alanine, or phenylalanine); x1a、X1b、X2aAnd X2bEach independently selected from a hydrophilic amino acid residue (e.g., glutamic acid, aspartic acid, lysine, asparagine, or arginine).
Clause 32. The immunomodulatory peptide of clause 31, having the sequence EKLSAFRNFF (seq id no: 9) (RP 843).
Clause 33. The immunomodulatory peptide of clause 1, wherein the striapathic region comprises a dimer of first and second polypeptides linked by a peptide linker linking the C-terminus of the first polypeptide with the N-terminus of the second polypeptide.
Clause 34. The immunomodulatory peptide of clause 33, wherein:
the hydrophilic moiety consists of an amino acid residue selected from the group consisting of lysine, arginine, and ornithine; and
the hydrophobic moiety consists of an amino acid residue selected from the group consisting of phenylalanine and tryptophan.
Clause 35. The immunomodulatory peptide of clause 33 or 34, wherein the first and second polypeptides comprise one of the following formulae: [ X1] - [ J1] - [ X2] - [ J2] (formula 3); or [ J1] - [ X1] - [ J2] - [ X2] (formula 2).
Clause 36. The immunomodulatory peptide of any of clauses 33-35, wherein the dimer has one of the following formulae: [ X1] - [ J1] - [ X2] - [ J2] -T- [ J2] - [ X2] - [ J1] - [ X1 ]; or
[J1] - [ X1] - [ J2] - [ X2] -T- [ X2] - [ J2] - [ X1] - [ J1 ]; wherein T is the peptide linker.
Clause 37. The immunomodulatory peptide of clause 36, wherein the dimer has one of the following formulae: [ X ]1a]-[J1a]-[X2a]-[J2a]-T-[J2a]-[X2a]-[J1a]-[X1a](formula 12A); or [ J1a]-[X1a]-[J2a]-[X2a]-T-[X2a]-[J2a]-[X1a]-[J1a](formula 13A); wherein T is the peptide linker (e.g., a polyglycine linker).
Clause 38. The immunomodulatory peptide of clause 37, having a sequence selected from RWKFGGFKWR (seq id no: 1) (RP832C) and FKWRGGRWKF (seq id no: 3) (RP 837C).
Clause 39. The immunomodulatory peptide of clause 33 or 34, wherein the dimer has one of the following formulae: [ X ]1aX1b]-[J1aJ1b]-T-[J1bJ1a]-[X1bX1a](formula 8A); or [ J1aJ1b]-[X1aX1b]-T-[X1bX1a]-[J1bJ1a](formula 9A); wherein: t is the peptide linker (e.g., a polyglycine linker); j. the design is a square1aAnd J1bEach independently selected from hydrophobic amino acid residues (e.g., tryptophan and phenylalanine); x1aAnd X1bEach independently selected from a hydrophilic amino acid residue (e.g., asparagine or arginine).
Clause 40. The immunomodulatory peptide of clause 49, having the sequence FWKRGGRKWF (seq id no: 4) (peptide 837A).
Clause 41. The immunomodulatory peptide of any of clauses 1-40, comprising:
a) a sequence selected from the peptide sequences shown in table 3;
b) a sequence having at least 75% sequence identity (e.g., at least 80%, 85%, 90%, or 95% sequence identity) to a sequence defined in a); or
c) A sequence having one or two amino acid substitutions relative to the sequence defined in a), wherein the one or two amino acid substitutions are of amino acids according to table 2 (e.g., similar amino acid substitutions, conservative amino acid substitutions, or highly conservative amino acid substitutions).
Clause 42. The immunoregulatory peptide of clauses 1-41 consisting of a sequence selected from any one of the sequences set forth in Table 3 (SEQ ID NO: 1-19).
Clause 43. An immunomodulatory peptide of 6 to 30 amino acid residues in length, comprising:
a) selected from the group consisting of SEQ ID NO: (1-19) (e.g., RP832C, 837A, 837C, 837N, 841-842, 843-850, and 853); or
b) A sequence having one or two amino acid substitutions relative to the sequence defined in a), wherein the one or two amino acid substitutions are of amino acids according to table 2 (e.g., similar amino acid substitutions, conservative amino acid substitutions, or highly conservative amino acid substitutions).
Clause 44. The immunomodulatory peptide of clause 43, wherein one or two amino acid substitutions defined in b) consists of a cationic amino acid substitution in the sequence achieved with another cationic amino acid residue (e.g., K to O, O to K, K to R, etc.).
Clause 45. The immunomodulatory peptide of clause 43, comprising the peptide sequence selected from: RWKFGGFKWR (RP832C) (SEQ ID NO: 1), FKWRGGRWKF (RP837C) (SEQ ID NO: 3) and FWKRGGRKWF (RP837A) (SEQ ID NO: 4).
Clause 46. The immunomodulatory peptide of clause 43, comprising a peptide selected from FWKRFV (RP837N) (SEQ ID NO: 5) and FVRKR (RP837C)1) (sequence No.: 6) the peptide sequence of (1).
Clause 47. The immunomodulatory peptide of clause 43, comprising a peptide sequence selected from FAOOFAOOFO (RP850) (SEQ ID NO: 19), FWKRFVRKWR (RP837) (SEQ ID NO: 4) and FWKKFVKKWK (RP841) (SEQ ID NO: 7).
Clause 48. The immunomodulatory peptide of clause 43, comprising a peptide sequence selected from WWHHWWHHWH (SEQ ID NO: 13), WWRHWWHRWR (SEQ ID NO: 14), and WWKHWWHKWK (SEQ ID NO: 15) (RP 847-849).
Clause 49. The immunomodulatory peptide of clause 43, comprising the peptide sequence GDRGIKGHRGF (RP842) (SEQ ID NO: 8).
Clause 50. The immunomodulatory peptide of clause 43, comprising the peptide sequence LYKKIIKKLL (RP846) (SEQ ID NO: 12).
Clause 51. The immunomodulatory peptide of clause 43, comprising the peptide sequence FYPDFFKKFF (RP844) (SEQ ID NO: 10).
Clause 52. The immunomodulatory peptide of clause 43, comprising the peptide sequence FFRKSKEKIG (RP853) (SEQ ID NO: 18).
Clause 53. An immunomodulatory peptide according to clause 43, comprising the peptide sequence FFRHFATHLD (RP845) (SEQ ID NO: 11).
Clause 54. The immunomodulatory peptide of clause 43, comprising the peptide sequence EKLSAFRNFF (RP843) (SEQ ID NO: 9).
Clause 55. An immunomodulatory peptide (e.g., 12 amino acid residues or less in length) comprising a sequence selected from: RWKFGGFKWR (RP832C) (SEQ ID NO: 1), FKWRGGRWKF (RP837C) (SEQ ID NO: 3) and FWKRGGRKWF (RP837A) (SEQ ID NO: 4).
Clause 56. The immunomodulatory peptide of clause 55, consisting of the sequence: RWKFGGFKWR (RP832C) (SEQ ID NO: 1).
Clause 57. The immunomodulatory peptide of clause 55, consisting of the sequence: FKWRGGRWKF (RP837C) (SEQ ID NO: 3).
Clause 58. The immunomodulatory peptide of clause 55, consisting of the sequence: FWKRGGRKWF (RP837A) (SEQ ID NO: 4).
Clause 59. A pharmaceutical composition comprising the immunomodulatory peptide of any of clauses 1-58 and a pharmaceutically acceptable carrier.
Clause 60. The pharmaceutical composition of clause 59, wherein the composition is formulated for oral, parenteral, inhalation, or topical administration.
Clause 61. The pharmaceutical composition of clauses 59 or 60, wherein the composition is formulated for intravenous or subcutaneous administration.
Clause 62. The pharmaceutical composition of clauses 59 or 60, wherein the composition is formulated for oral administration and further comprises an enteric coating.
Clause 63. The pharmaceutical composition of clauses 59 or 60, wherein the composition is formulated for topical administration in a form selected from the group consisting of: gel suspensions, creams, microneedles, impregnated bandages or topical patches.
Clause 64. A method of modulating macrophage activity, the method comprising: contacting a macrophage with a CD206 binding agent to modulate the activity of the macrophage.
Clause 65. The method of clause 64, wherein the CD206 binding agent binds to a mannose binding site to modulate the binding of signal-regulatory protein (SIRP) -mannose to CD 206.
Clause 66. The method of any one of clauses 64-65, wherein the CD206 binding agent binds to CD206 and has a binding energy of at least-650 kcal/mol.
Clause 67. The method of any one of clauses 64-66, wherein the CD 206-binding agent is in direct contact with at least one amino acid residue of CD206 selected from the group consisting of Phe-708, Thr-709, Trp-710, Pro-714, Glu-719, Asn-720, Trp-721, Ala-722, Glu-725, Tyr-729, Glu-733, Asn-747, Asp-748, Ser-1691, Cys-1693, Phe-1694, and Phe-1703.
Clause 68. The method of any one of clauses 64-67, wherein the macrophage activity that is modulated is macrophage polarization.
Clause 69. The method of any of clauses 64-68, wherein the viability of the macrophages is reduced.
Clause 70. The method of any one of clauses 64-69, wherein the macrophage is a M2 macrophage or a tumor-associated macrophage (TAM).
Clause 71. The method of any one of clauses 64-70, wherein the CD206 binding agent inhibits macrophage activity.
Clause 72. The method of any one of clauses 64-71, wherein the CD206 binding agent is an immunomodulatory peptide.
Clause 73. The method of any of clauses 64-71, wherein the macrophage is in vitro.
Clause 74. The method of any of clauses 64-71, wherein the macrophage is in vivo.
Clause 75. The method of any one of clauses 64-74, wherein the CD206 binding agent is the immunomodulatory peptide of any one of clauses 1-58.
Clause 76. A method of treating a chronic inflammation-related disorder in a subject, the method comprising: administering to the subject an effective amount of a CD206 binding agent (e.g., an immunomodulatory peptide according to any of clauses 1-58) to treat a chronic inflammation-related disorder suffered by the subject.
Clause 77. The method of clause 76, wherein the chronic inflammation-related disorder is selected from the group consisting of: scleroderma or multiple sclerosis, irritable bowel syndrome, ulcerative colitis, crohn's disease, idiopathic pulmonary fibrosis, scleroderma, asthma, keratitis, arthritis, osteoarthritis, rheumatoid arthritis, autoimmune diseases, feline or human immunodeficiency virus (FIV or HIV) infection, cancer, age-related inflammation and/or stem cell dysfunction, Graft Versus Host Disease (GVHD), keloids, obesity, diabetes, diabetic wounds, other chronic wounds, atherosclerosis, parkinson's disease, alzheimer's disease, macular degeneration, gout, gastric ulcers, gastritis, mucositis, toxoplasmosis and chronic viral or microbial infections.
Clause 78. The method of any one of clauses 76-77, wherein the CD206 binding agent is the immunomodulatory peptide of any one of clauses 1-58.
Clause 79. The method of clause 77, wherein the disorder is cancer.
Clause 80. The method of clause 79, further comprising administering to the subject an effective amount of another agent.
Clause 81. The method of clause 80, wherein the other agent is a chemotherapeutic agent.
Clause 82. The method of clause 80, wherein the chemotherapeutic agent is selected from gemcitabine, docetaxel, bleomycin, erlotinib, gefitinib, lapatinib, imatinib, dasatinib, nilotinib, bosutinib, crizotinib, ceritinib, tremetinib, bevacizumab, sunitinib, sorafenib, trastuzumab, enrmetuzumab, rituximab, ipilimumab, rapamycin, temsirolimus, everolimus, methotrexate, doxorubicin, idelberry, Folfirinox, cisplatin, carboplatin, 5-fluorouracil, tixogil, paclitaxel, prednisone, levothyroxine, and pemetrexed.
Clause 83. The method of clause 81, wherein the chemotherapeutic agent is spruce.
Clause 84. The method of clause 81, wherein the chemotherapeutic agent is gemcitabine or docetaxel.
Clause 85. The method of clause 80, wherein the other agent is an immunotherapeutic agent.
Clause 86. The method of clause 85, wherein the immunotherapeutic agent is an immune checkpoint inhibitor.
Clause 87. The method of clause 86, wherein the immune checkpoint inhibitor is selected from the group consisting of a cytotoxic T lymphocyte-associated antigen 4(CTLA-4) inhibitor, a programmed death protein 1(PD-1) inhibitor, and a PD-L1 inhibitor.
Clause 88. The method of clause 87, wherein the immune checkpoint inhibitor is selected from the group consisting of ipilimumab, pembrolizumab, and nivolumab.
Clause 89. The method of clause 76, wherein the chronic inflammation-related disorder is fibrosis.
Clause 90. The method of clause 76, wherein the chronic inflammation-related disorder is scleroderma.
Clause 91. The method of any one of clauses 76-90, wherein the CD206 binding agent is the immunomodulatory peptide of any one of clauses 1-58.
Clause 92. The method of clause 91, wherein the CD206 binding agent consists of an immunomodulatory peptide set forth in table 3.
Claims (23)
1. An immunomodulatory peptide, comprising:
a) selected from the group consisting of SEQ ID NO: (1-19) the peptide sequence of (1-19); or
b) A sequence having one or two amino acid substitutions relative to the sequence defined in a), wherein the one or two amino acid substitutions are of amino acids according to table 2.
2. The immunomodulatory peptide of claim 1, wherein the one or two amino acid substitutions defined in b) consist of highly conserved cationic amino acid substitutions of the sequence.
3. The immunomodulatory peptide of claim 1, comprising the peptide sequence selected from: RWKFGGFKWR (RP832C) (SEQ ID NO: 1), FKWRGGRWKF (RP837C) (SEQ ID NO: 3) and FWKRGGRKWF (RP837A) (SEQ ID NO: 4).
4. The immunomodulatory peptide of claim 3, comprising a sequence selected from FWKRFV (RP837N) (SEQ ID NO: 5) and FVRKWR (RP837C)1) (sequence No.: 16) the peptide sequence of (1).
5. The immunomodulatory peptide of claim 1, comprising the peptide sequence selected from FAOOFAOOFO (RP850) (SEQ ID NO: 19), FWKRFVRKWR (RP837) (SEQ ID NO: 2), and FWKKFVKKWK (RP841) (SEQ ID NO: 7).
6. The immunomodulatory peptide of claim 1, comprising a peptide sequence selected from WWHHWWHHWH (SEQ ID NO: 13), WWRHWWHRWR (SEQ ID NO: 14), and WWKHWWHKWK (SEQ ID NO: 15) (RP 847-849).
7. The immunomodulatory peptide of claim 1, comprising the peptide sequence GDRGIKGHRGF (RP842) (SEQ ID NO: 8).
8. The immunomodulatory peptide of claim 1, comprising the peptide sequence LYKKIIKKLL (RP846) (SEQ ID NO: 12).
9. The immunomodulatory peptide of claim 1, comprising the peptide sequence FYPDFFKKFF (RP844) (SEQ ID NO: 10).
10. The immunomodulatory peptide of claim 1, comprising the peptide sequence FFRKSKEKIG (RP853) (SEQ ID NO: 18).
11. The immunomodulatory peptide of claim 1, comprising the peptide sequence FFRHFATHLD (RP845) (SEQ ID NO: 11).
12. The immunomodulatory peptide of claim 1, comprising the peptide sequence EKLSAFRNFF (RP843) (SEQ ID NO: 9).
13. A pharmaceutical composition comprising the immunomodulatory peptide of claim 1 and a pharmaceutically acceptable carrier.
14. A peptide of 12 amino acid residues or less in length comprising a sequence selected from: RWKFGGFKWR (RP832C) (SEQ ID NO: 1), FKWRGGRWKF (RP837C) (SEQ ID NO: 3) and FWKRGGRKWF (RP837A) (SEQ ID NO: 4).
15. The peptide of claim 14, comprising the sequence: RWKFGGFKWR (RP832C) (SEQ ID NO: 1).
16. The peptide of claim 14, comprising the sequence: FKWRGGRWKF (RP837C) (SEQ ID NO: 3).
17. The peptide of claim 14, comprising the sequence: FWKRGGRKWF (RP837A) (SEQ ID NO: 4).
18. A method of treating a chronic inflammation-related disorder in a subject, the method comprising:
administering to the subject an effective amount of the peptide of claim 1 to treat a chronic inflammation-related disorder suffered by the subject.
19. The method of claim 18, wherein the chronic inflammation-related disorder is selected from the group consisting of: scleroderma or multiple sclerosis, irritable bowel syndrome, ulcerative colitis, crohn's disease, idiopathic pulmonary fibrosis, scleroderma, asthma, keratitis, arthritis, osteoarthritis, rheumatoid arthritis, autoimmune diseases, feline or human immunodeficiency virus (FIV or HIV) infection, cancer, age-related inflammation and/or stem cell dysfunction, Graft Versus Host Disease (GVHD), keloids, obesity, diabetes, diabetic wounds, other chronic wounds, atherosclerosis, parkinson's disease, alzheimer's disease, macular degeneration, gout, gastric ulcers, gastritis, mucositis, toxoplasmosis and chronic viral or microbial infections.
20. The method of claim 19, wherein the disorder is cancer.
21. The method of claim 20, further comprising administering to the subject an effective amount of a chemotherapeutic or immunotherapeutic agent.
22. The method of claim 18, wherein the chronic inflammation-related disorder is fibrosis.
23. The method of claim 18, wherein the chronic inflammation-related disorder is scleroderma.
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/US2018/048632 WO2020046297A2 (en) | 2018-08-29 | 2018-08-29 | Peptides having immunomodulatory properties |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN113039194A true CN113039194A (en) | 2021-06-25 |
Family
ID=69645844
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201880098522.6A Pending CN113039194A (en) | 2018-08-29 | 2018-08-29 | Peptides with immunomodulatory properties |
Country Status (8)
| Country | Link |
|---|---|
| EP (1) | EP3844297A4 (en) |
| JP (2) | JP2022511280A (en) |
| KR (1) | KR20210052473A (en) |
| CN (1) | CN113039194A (en) |
| AU (1) | AU2018438604A1 (en) |
| CA (1) | CA3110618A1 (en) |
| WO (1) | WO2020046297A2 (en) |
| ZA (1) | ZA202101327B (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20190224275A1 (en) | 2017-05-12 | 2019-07-25 | Aurinia Pharmaceuticals Inc. | Protocol for treatment of lupus nephritis |
| WO2022034523A1 (en) * | 2020-08-13 | 2022-02-17 | Immunitybio, Inc. | Phagocytosis-inducing compounds and methods of use |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020099008A1 (en) * | 1999-04-26 | 2002-07-25 | Daniel R. Twardzik | Method for stimulating hematopoiesis using tgf-alpha |
| US20120270770A1 (en) * | 2010-08-03 | 2012-10-25 | Jesse Michael Jaynes | Anti-angiogenic peptides and their uses |
| US20160054304A1 (en) * | 2013-03-14 | 2016-02-25 | Galapagos Nv | Molecular targets and compounds, and methods to identify the same, useful in the treatment of fibrotic diseases |
| WO2016061133A1 (en) * | 2014-10-14 | 2016-04-21 | Riptide Bioscience, Inc. | Peptides having anti-inflammatory properties |
| CN107614004A (en) * | 2015-03-23 | 2018-01-19 | 激流生物科学有限公司 | Antimicrobial peptide and its application method |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1741790A1 (en) * | 2005-03-24 | 2007-01-10 | DIGILAB BioVisioN GmbH | Methods to identify and characterize prolyl oligopeptidases by using substrates and products thereof |
-
2018
- 2018-08-29 JP JP2021510646A patent/JP2022511280A/en active Pending
- 2018-08-29 CA CA3110618A patent/CA3110618A1/en active Pending
- 2018-08-29 CN CN201880098522.6A patent/CN113039194A/en active Pending
- 2018-08-29 WO PCT/US2018/048632 patent/WO2020046297A2/en unknown
- 2018-08-29 KR KR1020217008293A patent/KR20210052473A/en not_active Application Discontinuation
- 2018-08-29 EP EP18931536.9A patent/EP3844297A4/en active Pending
- 2018-08-29 AU AU2018438604A patent/AU2018438604A1/en active Pending
-
2021
- 2021-02-26 ZA ZA2021/01327A patent/ZA202101327B/en unknown
-
2023
- 2023-07-28 JP JP2023123680A patent/JP2023145635A/en active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020099008A1 (en) * | 1999-04-26 | 2002-07-25 | Daniel R. Twardzik | Method for stimulating hematopoiesis using tgf-alpha |
| US20120270770A1 (en) * | 2010-08-03 | 2012-10-25 | Jesse Michael Jaynes | Anti-angiogenic peptides and their uses |
| US20160054304A1 (en) * | 2013-03-14 | 2016-02-25 | Galapagos Nv | Molecular targets and compounds, and methods to identify the same, useful in the treatment of fibrotic diseases |
| WO2016061133A1 (en) * | 2014-10-14 | 2016-04-21 | Riptide Bioscience, Inc. | Peptides having anti-inflammatory properties |
| CN107614004A (en) * | 2015-03-23 | 2018-01-19 | 激流生物科学有限公司 | Antimicrobial peptide and its application method |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2022511280A (en) | 2022-01-31 |
| WO2020046297A2 (en) | 2020-03-05 |
| ZA202101327B (en) | 2022-09-28 |
| CA3110618A1 (en) | 2020-03-05 |
| EP3844297A2 (en) | 2021-07-07 |
| KR20210052473A (en) | 2021-05-10 |
| AU2018438604A1 (en) | 2021-03-18 |
| JP2023145635A (en) | 2023-10-11 |
| EP3844297A4 (en) | 2022-08-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| ES2363162T3 (en) | COMPOSITIONS AND PROCEDURES FOR THE TREATMENT OF FIBROTIC DISORDERS. | |
| AU2018273958B2 (en) | PD-1 and CTLA-4 dual inhibitor peptides | |
| CN107847583A (en) | PEGylated Interleukin 10 for treating cancer | |
| US9254309B2 (en) | Use of multivalent synthetic ligands of surface nucleolin for treating cancer or inflammation | |
| JP2023145635A (en) | Peptides having immunomodulatory properties | |
| US8906355B2 (en) | Synthetic stereoisomer peptides in the retro-inverso and inverso configuration, and with cyclic and linear structure, their polymer conjugates, their encapsulation in polymer particles, and uses thereof | |
| CN118873680A (en) | Antibody-urease conjugates for therapeutic purposes | |
| US8715986B2 (en) | Stereoisomer peptides, ligand-targeted multi- stereoisomer peptide polymer conjugates, and uses thereof | |
| US20210401927A1 (en) | Peptides Having Immunomodulatory Properties | |
| CA2945812A1 (en) | Trail receptor agonists for treatment of fibrotic diseases | |
| US11352404B2 (en) | Phosphatidylserine targeting fusion molecules and methods for their use | |
| JP2020501513A (en) | Therapeutic multi-target constructs and uses thereof | |
| US20150209335A1 (en) | Methods and small molecule therapeutics comprising fused elps | |
| US20220143130A1 (en) | Peptides Having Immunomodulatory Properties | |
| US11338040B2 (en) | Immunomodulatory compounds | |
| JP2022512541A (en) | Combination therapy including PD-1 chimeric protein | |
| JP2010509241A (en) | Self-aggregating nanoparticles composed of transmembrane peptides and their use for specific intratumoral delivery of anticancer agents | |
| CA3075670A1 (en) | Pharmaceutical constructs with enhanced binding affinity with albumin | |
| WO2015016608A1 (en) | Composition for carrying nucleic acid, comprising antibody and nucleic acid-binding peptide | |
| JP4566406B2 (en) | Retro-inverted peptides targeting GIT transport receptors and related methods | |
| WO2023097111A2 (en) | Methods and compositions for treating calcinosis associated conditions | |
| WO2019204402A1 (en) | Compositions and methods for treatment of cancer | |
| JP2003534387A (en) | Tumor activating prodrug compounds and methods for their production and use | |
| US20220112243A1 (en) | Immunomodulatory Peptides | |
| Liu et al. | Peptide Therapeutics: Oncology |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |