Structure directing agent, preparation method and application thereof
Technical Field
The invention belongs to the technical field of molecular sieve preparation, and particularly relates to a structure directing agent, a preparation method and application thereof.
Background
The zeolite molecular sieve has unique pore channel shape selectivity, unique solid acid property and excellent ion exchange property, so that the zeolite molecular sieve has wide application range and has great commercial application value in the aspects of adsorption, separation, catalysis and the like. Wherein the microporous molecular sieve, such as SSZ-13(CHA), SSZ-39(AEI), LZ-214(RHO) and SSZ-16(AFX) isThe sub-sieve being treated to contain NOxNH of diesel vehicle exhaust3The selective catalytic reduction (NH3-SCR) technology shows excellent activity and catalytic stability.
The AFX type molecular sieve is a small-pore molecular sieve with a unique 8-membered ring pore and cage structure, has commercial value, and plays an important role in the light olefin (MTO) reaction produced from methanol and the NH3-SCR denitration reaction. The AFX topology has a hexagonal crystal structure that can be seen as being built up from 6-membered rings along the c-axis in an AABBCCBB fashion, with a framework density of 15.1T/1000A3Identical to the CHA structure, with channels consisting of micropores with 8-membered rings (3.73A) containing one elongated oval aft cage (5.5X 13.5A) and one smaller gme cage (3.3X 7.4A). A typical AFX previously synthesized by structure directing agents is SSZ-16, with a lower Si/Al ratio (<6) AFX structured molecular sieves were first found in SAPO-56 synthesis, while aluminosilicate structured SSZ-16 was first synthesized from Zones, and the structure directing agent used was 1,1- (butane-1, 4-diyl) bis [ 4-aza-1-ane]Dibromide (DABCO). Since then, several other structure directing agents were discovered, such as gemini quaternary ammonium salts (N, N-bistiethylpentanedidiammonium), which generally produce AFX with a maximum Si/Al ratio close to 5, and only silicon-rich (Si/Al = 16.8) AFX molecular sieves were obtained by using the bulky and rigid, expensive structure directing agent 1, 3-bis (1-adamantyl) imidazolium salt. In the AFX synthesis process, the catalyst can be synthesized by common inorganic silicon sources and aluminum sources or by FAU molecular sieve crystal transformation. It is reported that the prepared AFX zeolite SiO2/Al2O3The molar ratio is generally in the range of 8 to 15. Lobo et al report that zeolite AFX (SSZ-16) is only in limited SiO2/Al2O3Synthesized in a molar ratio range. In order to improve the Si/Al ratio of the AFX molecular sieve, an expensive structure directing agent is generally needed to be adopted for synthesis, the preparation process is long in flow and low in yield, the FAU molecular sieve with high silicon-aluminum ratio is needed to be added to be used as an aluminum source for crystal transformation synthesis, the commercial synthesis cost is improved, the synthesis cost can be saved by designing a cheap novel structure directing agent, the AFX molecular sieve with high silicon-aluminum ratio can be obtained, and the hydrothermal stability of the AFX molecular sieve is improvedSex; in addition, the hydrothermal one-step synthesis of AFX molecular sieve and the adoption of cheap raw materials are also an advantage of the patent.
The AFX molecular sieve is taken as a catalyst at present and mainly faces the following problems: (1) because the structure-directing agent has weak stabilization effect on the inorganic framework structure, the change range of the Si/Al composition in the synthesis is narrow, and the Si/Al ratio is difficult to improve; (2) the structure directing agent used in the synthesis has high cost, high price and complex synthesis, and is not suitable for industrial amplification, and (3) the synthesis needs long crystallization time. Therefore, it is necessary to develop a new low-cost structure-directing agent for synthesizing AFX molecular sieve to improve Si/Al ratio and reduce synthesis cost.
Disclosure of Invention
In view of the above, the present invention aims to provide a structure directing agent, a preparation method and an application thereof, so as to improve the Si/Al ratio of the AFX molecular sieve and reduce the synthesis cost.
In order to achieve the purpose, the technical scheme of the invention is realized as follows:
the structure directing agent is Gemini quaternary ammonium salt or Gemini quaternary ammonium base, and the molecular formula of the structure directing agent is (CH)3CH2)3N+RN+(CH3CH2)3 2X-Wherein R is benzene or alkylbenzene, X-Is halogen or hydroxyl.
A method for preparing a structure directing agent as described above, comprising the steps of: adding a raw material A into a solvent, stirring until the raw material A is completely dissolved, adding tertiary amine, heating and refluxing for reaction for 24 hours, washing with diethyl ether, recrystallizing, filtering, and drying at 70 ℃ to obtain the required structure directing agent, wherein the raw material A is one of alpha, alpha-dibromo-o-xylene, alpha-dibromo-m-xylene or alpha, alpha-dibromo-p-xylene.
Preferably, the reflux reaction temperature is 60 ℃.
Preferably, the drying time is 8 h.
A preparation method of an AFX molecular sieve comprises the following steps:
(1) stirring silicon source, alkali source and secondary deionized water at room temperature, wherein OH-The mol ratio of Si is 0.5-1.2;
(2) adding a structure directing agent, and uniformly stirring;
(3) adding an aluminum source, and uniformly stirring to obtain a mixed solution;
(4) transferring the mixed solution to a hydrothermal kettle for hydrothermal crystallization to obtain a reaction solution;
(5) and centrifuging, washing, drying and roasting the solid crystals in the reaction liquid to obtain the required AFX molecular sieve.
Preferably, the molar ratio of Si/Al in the mixed liquid is 5 to 50.
Preferably, the silicon source is one or more of sodium silicate, silica sol, fumed silica, white carbon black and ethyl orthosilicate.
Preferably, the aluminum source is one or more of H-FAU, aluminum nitrate, aluminum sulfate, aluminum chloride, aluminum hydroxide, sodium metaaluminate, pseudo-boehmite and aluminum isopropoxide.
Preferably, the hydrothermal synthesis conditions in the step (4) comprise static hydrothermal synthesis and dynamic hydrothermal synthesis, the temperature of the hydrothermal kettle is 130-170 ℃, and the crystallization time is 5-336 h.
Preferably, the silicon source is sodium silicate, the aluminum source is H-FAU, and SiO is contained in the mixed solution2:Al2O3:Na2O structural directing agent H2The molar ratio of O is 5-50:0.5:13.05-16.95:6-12: 1000-2100.
Compared with the prior art, the structure directing agent, the preparation method and the application thereof have the following advantages:
the preparation method of the AFX molecular sieve adopts the novel structure directing agent which is low in price and easy to synthesize, and the AFX molecular sieve with high Si/Al ratio can be directly synthesized by a one-step hydrothermal method.
Drawings
The accompanying drawings, which are incorporated in and constitute a part of this specification, illustrate an embodiment of the invention and, together with the description, serve to explain the invention and not to limit the invention. In the drawings:
FIG. 1 shows an embodiment of the present inventionOSDA1 and OSDA31H、13C, nuclear magnetic spectrum schematic diagram;
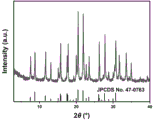
FIG. 2 is an XRD spectrum of the AFX molecular sieve synthesized in example 1 of the present invention;
FIG. 3 is an XRD spectrum of the AFX molecular sieve synthesized in example 2 of the present invention;
FIG. 4 is an XRD spectrum of the AFX molecular sieve synthesized in example 3 of the present invention;
FIG. 5 is an XRD spectrum of the AFX molecular sieve synthesized in example 4 of the present invention.
Detailed Description
Unless defined otherwise, technical terms used in the following examples have the same meanings as commonly understood by one of ordinary skill in the art to which the present invention belongs. The test reagents used in the following examples, unless otherwise specified, are all conventional biochemical reagents; the experimental methods are conventional methods unless otherwise specified.
The present invention will be described in detail with reference to the following examples and accompanying drawings.
The synthesis method of the structure directing agent p-diethylbenzene hexa-ethyl gemini quaternary ammonium salt comprises the following steps: adding dibromoparaxylene (0.0367 mol) serving as a raw material into 80 mL absolute ethyl alcohol serving as a solvent, stirring until the dibromoparaxylene is completely dissolved, slowly dripping triethylamine (0.0734 mol) into the mixed solution, refluxing and heating to 60 ℃, reacting for 24 hours, washing with diethyl ether, recrystallizing, filtering, and drying at 70 ℃ overnight to obtain a white solid structure directing agent OSDA1, wherein the yield is 91%. Subsequent treatment of Br with ion exchange resin-Exchange to OH-And (3) evaporating part of the solution to obtain a 40% solution which is p-diethylbenzene hexaethyl gemini quaternary ammonium hydroxide and is marked as a structure directing agent OSDA 2.
The synthesis method of the structure directing agent m-diethylbenzene hexa-ethyl gemini quaternary ammonium salt comprises the following steps: adding dibromo-m-xylene (0.0367 mol) serving as a raw material into 80 mL of absolute ethanol solvent, stirring until the dibromo-m-xylene is completely dissolved, slowly dripping triethylamine (0.0734 mol) into the solution, carrying out reflux reaction at 60 ℃ for 24 hours, washing with diethyl ether, recrystallizing, filtering, and drying at 70 ℃ for 8 hours to obtain a white solid, wherein the yield is 90%. And then Br-is exchanged into OH-through ion exchange resin, and partial solution is evaporated in a rotary mode to obtain 40% solution of m-diethylbenzene hexaethyl Gemini quaternary ammonium hydroxide which is marked as a structure directing agent OSDA 3.
In addition, similar structure directing agents also include: p-xylene hexaethyl gemini quaternary ammonium cations, p-diethylbenzene hexaethyl gemini quaternary ammonium cations, p-dipropylbenzene hexaethyl gemini quaternary ammonium cations, m-xylene hexaethyl gemini quaternary ammonium cations, m-diethylbenzene hexaethyl gemini quaternary ammonium cations and m-dipropylbenzene hexaethyl gemini quaternary ammonium cations. In addition, the ethyl group may be substituted with methyl, propyl, butyl, etc., to form a novel structure directing agent.
OSDA1 and OSDA31H、13The C nuclear magnetic spectrum schematic diagram is shown in FIG. 1, and the structural formula of the AFX molecular sieve prepared in each example is shown in Table 1.
Table 1 examples 1-4 structural directing agents
Example 1
(1) Weighing 0.4 g of fumed silica, 0.278 g of sodium hydroxide and 7.21 g of deionized water, and uniformly stirring at room temperature;
(2) adding 0.311 g of structure directing agent OSDA1 into the solution obtained in the step (1), and uniformly stirring again;
(3) adding 0.083 g of aluminum nitrate into the solution obtained in the step (2), and stirring to form a uniform solution;
(4) the resulting solution was transferred to a 100 mL hydrothermal kettle. At 150oC, dynamic hydrothermal crystallization for 96 hours;
(5) and (4) centrifuging, washing, drying and roasting the solid obtained in the step (4) to obtain the pure-phase AFX molecular sieve, wherein an XRD spectrogram is shown in figure 2.
Example 2
(1) Weighing 0.737g of white carbon black, 0.344 g of sodium hydroxide and 5.4 g of deionized water, and uniformly stirring at room temperature;
(2) adding 0.459 g of OSDA2 into the solution obtained in the step (1), and uniformly stirring again;
(3) adding 0.215 g H-FAU (Si/Al =2.73) serving as an aluminum source into the solution obtained in the step (2), and stirring to form a uniform solution;
(4) the resulting solution was transferred to a 100 mL hydrothermal kettle. At 140oC, dynamic hydrothermal crystallization for 48 hours;
(5) and (4) centrifuging, washing, drying and roasting the solid obtained in the step (4) to obtain the pure-phase AFX molecular sieve, wherein an XRD spectrogram is shown in figure 3.
Example 3
(1) 3.192 g of sodium Silicate (SiO) are weighed out2: 23.1 wt%,Na25.96 wt% of O), 0.098 g of sodium hydroxide and 3.4 g of deionized water, and stirring uniformly at room temperature;
(2) adding 0.459 g of OSDA2 into the solution obtained in the step (1), and uniformly stirring again;
(3) adding 0.215 g H-FAU (Si/Al =2.73) serving as an aluminum source into the solution obtained in the step (2), and stirring to form a uniform solution;
(4) the resulting solution was transferred to a 100 mL hydrothermal kettle. At 140oC, dynamic hydrothermal crystallization for 48 hours;
(5) and (4) centrifuging, washing, drying and roasting the solid obtained in the step (4) to obtain the pure-phase AFX molecular sieve, wherein an XRD spectrogram is shown in figure 4.
Example 4
(1) 3.192 g of sodium Silicate (SiO) are weighed out2: 23.1 wt%,Na25.96 percent by weight of O), 0.1088 g of sodium hydroxide and 2.5 g of deionized water are stirred uniformly at room temperature;
(2) adding 0.699 g of OSDA3 into the solution obtained in the step (1), and uniformly stirring again;
(3) adding 0.215 g H-FAU (Si/Al =2.73) serving as an aluminum source into the solution obtained in the step (2), and stirring to form a uniform solution;
(4) the resulting solution was transferred to a 100 mL hydrothermal kettle. At 150oC, dynamic hydrothermal crystallization for 96 hours;
(5) and (4) centrifuging, washing, drying and roasting the solid obtained in the step (4) to obtain the pure-phase AFX molecular sieve, wherein an XRD spectrogram is shown in figure 5.
The above description is only for the purpose of illustrating the preferred embodiments of the present invention and is not to be construed as limiting the invention, and any modifications, equivalents, improvements and the like that fall within the spirit and principle of the present invention are intended to be included therein.