CN112759546B - 3-(二甲氨基甲基)哌啶-4-醇衍生物及其制备方法和药物用途 - Google Patents
3-(二甲氨基甲基)哌啶-4-醇衍生物及其制备方法和药物用途 Download PDFInfo
- Publication number
- CN112759546B CN112759546B CN201911076330.8A CN201911076330A CN112759546B CN 112759546 B CN112759546 B CN 112759546B CN 201911076330 A CN201911076330 A CN 201911076330A CN 112759546 B CN112759546 B CN 112759546B
- Authority
- CN
- China
- Prior art keywords
- methyl
- piperidin
- dimethylamino
- methoxyphenyl
- sulfonyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000002360 preparation method Methods 0.000 title abstract description 11
- CAJTZENXFRGKHX-UHFFFAOYSA-N 3-[(dimethylamino)methyl]piperidin-4-ol Chemical class CN(C)CC1CNCCC1O CAJTZENXFRGKHX-UHFFFAOYSA-N 0.000 title 1
- 150000001875 compounds Chemical class 0.000 claims abstract description 34
- 150000003839 salts Chemical class 0.000 claims abstract description 15
- -1 1- (1- (benzylsulfonyl) -4- (difluoromethoxy) -4- (3-methoxyphenyl) piperidin-3-yl) -N, N-dimethylaminomethyl amine Chemical class 0.000 claims description 41
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 20
- 208000002193 Pain Diseases 0.000 claims description 17
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 15
- 230000036407 pain Effects 0.000 claims description 14
- DXASQZJWWGZNSF-UHFFFAOYSA-N n,n-dimethylmethanamine;sulfur trioxide Chemical group CN(C)C.O=S(=O)=O DXASQZJWWGZNSF-UHFFFAOYSA-N 0.000 claims description 12
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 11
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 10
- 229910052736 halogen Inorganic materials 0.000 claims description 9
- 150000002367 halogens Chemical group 0.000 claims description 9
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 claims description 8
- 108090000137 Opioid Receptors Proteins 0.000 claims description 7
- 102000003840 Opioid Receptors Human genes 0.000 claims description 7
- 229910052739 hydrogen Inorganic materials 0.000 claims description 7
- 239000001257 hydrogen Substances 0.000 claims description 7
- 239000008194 pharmaceutical composition Substances 0.000 claims description 7
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 6
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 claims description 5
- 239000003937 drug carrier Substances 0.000 claims description 5
- NVDIESCTJSWZJX-UHFFFAOYSA-N C1(=CC=CC=C1)CS(=O)(=O)N1CC(C(CC1)(C1=CC=CC(OC)=C1)O)CN(C)C Chemical compound C1(=CC=CC=C1)CS(=O)(=O)N1CC(C(CC1)(C1=CC=CC(OC)=C1)O)CN(C)C NVDIESCTJSWZJX-UHFFFAOYSA-N 0.000 claims description 4
- ZNZNEYZRPFKPRA-UHFFFAOYSA-N CN(C)CC(CN(CC1)S(CC2=CC=CC=C2)(=O)=O)C1(C1=CC(OC(F)(F)F)=CC=C1)O Chemical compound CN(C)CC(CN(CC1)S(CC2=CC=CC=C2)(=O)=O)C1(C1=CC(OC(F)(F)F)=CC=C1)O ZNZNEYZRPFKPRA-UHFFFAOYSA-N 0.000 claims description 4
- 239000003814 drug Substances 0.000 claims description 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 4
- IAVKGLZUNCTOFD-UHFFFAOYSA-N C(S(=O)(=O)N1CCC(C(CN(C)C)C1)(O)C1=CC(OC)=CC=C1)C Chemical compound C(S(=O)(=O)N1CCC(C(CN(C)C)C1)(O)C1=CC(OC)=CC=C1)C IAVKGLZUNCTOFD-UHFFFAOYSA-N 0.000 claims description 3
- OMFMJGMBGHNRJR-UHFFFAOYSA-N C(S(=O)(=O)N1CCC(O)(C2=CC(OC)=CC=C2)C(CN(C)C)C1)CC Chemical compound C(S(=O)(=O)N1CCC(O)(C2=CC(OC)=CC=C2)C(CN(C)C)C1)CC OMFMJGMBGHNRJR-UHFFFAOYSA-N 0.000 claims description 3
- XIOIASUBOHCNQX-UHFFFAOYSA-N C1(=CC=C(C)C=C1)CS(=O)(=O)N1CC(C(C2=CC(OC)=CC=C2)(O)CC1)CN(C)C Chemical compound C1(=CC=C(C)C=C1)CS(=O)(=O)N1CC(C(C2=CC(OC)=CC=C2)(O)CC1)CN(C)C XIOIASUBOHCNQX-UHFFFAOYSA-N 0.000 claims description 3
- ICIVKTGPZFLOGE-UHFFFAOYSA-N C1(=CC=C(C=C1)F)CS(=O)(=O)N1CC(C(CC1)(O)C1=CC(OC)=CC=C1)CN(C)C Chemical compound C1(=CC=C(C=C1)F)CS(=O)(=O)N1CC(C(CC1)(O)C1=CC(OC)=CC=C1)CN(C)C ICIVKTGPZFLOGE-UHFFFAOYSA-N 0.000 claims description 3
- XCGOMDJATHJHQB-UHFFFAOYSA-N C1(=CC=CC(=C1)Br)CS(=O)(=O)N1CC(C(CC1)(O)C1=CC(OC)=CC=C1)CN(C)C Chemical compound C1(=CC=CC(=C1)Br)CS(=O)(=O)N1CC(C(CC1)(O)C1=CC(OC)=CC=C1)CN(C)C XCGOMDJATHJHQB-UHFFFAOYSA-N 0.000 claims description 3
- HHTVZBJSJWCUDU-UHFFFAOYSA-N C1(=CC=CC(=C1)Cl)CS(=O)(=O)N1CCC(C2=CC=CC(OC)=C2)(O)C(CN(C)C)C1 Chemical compound C1(=CC=CC(=C1)Cl)CS(=O)(=O)N1CCC(C2=CC=CC(OC)=C2)(O)C(CN(C)C)C1 HHTVZBJSJWCUDU-UHFFFAOYSA-N 0.000 claims description 3
- ARDVLGSZPVCWNT-UHFFFAOYSA-N C1(F)=C(CS(=O)(=O)N2CC(C(O)(C3=CC=CC(OC)=C3)CC2)CN(C)C)C=CC=C1 Chemical compound C1(F)=C(CS(=O)(=O)N2CC(C(O)(C3=CC=CC(OC)=C3)CC2)CN(C)C)C=CC=C1 ARDVLGSZPVCWNT-UHFFFAOYSA-N 0.000 claims description 3
- ZGEQUKPGCACQFV-UHFFFAOYSA-N C1=C(CS(=O)(=O)N2CCC(C3=CC(OC)=CC=C3)(C(CN(C)C)C2)O)C=C(C(F)(F)F)C=C1 Chemical compound C1=C(CS(=O)(=O)N2CCC(C3=CC(OC)=CC=C3)(C(CN(C)C)C2)O)C=C(C(F)(F)F)C=C1 ZGEQUKPGCACQFV-UHFFFAOYSA-N 0.000 claims description 3
- QRTPKXHPTHMFST-UHFFFAOYSA-N C1=C(CS(=O)(=O)N2CCC(O)(C3=CC(OC)=CC=C3)C(CN(C)C)C2)C=C(N(=O)=O)C=C1 Chemical compound C1=C(CS(=O)(=O)N2CCC(O)(C3=CC(OC)=CC=C3)C(CN(C)C)C2)C=C(N(=O)=O)C=C1 QRTPKXHPTHMFST-UHFFFAOYSA-N 0.000 claims description 3
- KPDQCCADXLLPCA-UHFFFAOYSA-N C1=C(CS(=O)(=O)N2CCC(O)(C3=CC(OC)=CC=C3)C(CN(C)C)C2)C=CC(C(F)(F)F)=C1 Chemical compound C1=C(CS(=O)(=O)N2CCC(O)(C3=CC(OC)=CC=C3)C(CN(C)C)C2)C=CC(C(F)(F)F)=C1 KPDQCCADXLLPCA-UHFFFAOYSA-N 0.000 claims description 3
- USTMGQYBTQSFGW-UHFFFAOYSA-N C1=CC=CC=C1S(=O)(=O)N1CCC(O)(C2=CC=CC(OC)=C2)C(CN(C)C)C1 Chemical compound C1=CC=CC=C1S(=O)(=O)N1CCC(O)(C2=CC=CC(OC)=C2)C(CN(C)C)C1 USTMGQYBTQSFGW-UHFFFAOYSA-N 0.000 claims description 3
- QARHEKHLAUFVPF-UHFFFAOYSA-N CCCCS(N(CC1)CC(CN(C)C)C1(C1=CC(OC)=CC=C1)O)(=O)=O Chemical compound CCCCS(N(CC1)CC(CN(C)C)C1(C1=CC(OC)=CC=C1)O)(=O)=O QARHEKHLAUFVPF-UHFFFAOYSA-N 0.000 claims description 3
- ZOECFKNQYIMPLV-UHFFFAOYSA-N CN(C)CC(CN(CC1)S(CC(C=C2)=CC=C2Cl)(=O)=O)C1(C1=CC(OC)=CC=C1)O Chemical compound CN(C)CC(CN(CC1)S(CC(C=C2)=CC=C2Cl)(=O)=O)C1(C1=CC(OC)=CC=C1)O ZOECFKNQYIMPLV-UHFFFAOYSA-N 0.000 claims description 3
- NUESOWFMPJQFPJ-UHFFFAOYSA-N CS(=O)(=O)N1CCC(C(CN(C)C)C1)(O)C1=CC(OC)=CC=C1 Chemical compound CS(=O)(=O)N1CCC(C(CN(C)C)C1)(O)C1=CC(OC)=CC=C1 NUESOWFMPJQFPJ-UHFFFAOYSA-N 0.000 claims description 3
- 206010012335 Dependence Diseases 0.000 claims description 3
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 claims description 3
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 3
- OBWBAIVWLKBMQS-UHFFFAOYSA-N C(S(=O)(=O)N1CCC(C2=CC(OC)=CC=C2)(O)C(CN(C)C)C1)CC1=CC=CC=C1 Chemical compound C(S(=O)(=O)N1CCC(C2=CC(OC)=CC=C2)(O)C(CN(C)C)C1)CC1=CC=CC=C1 OBWBAIVWLKBMQS-UHFFFAOYSA-N 0.000 claims description 2
- JUKOQNIFESKMAX-HMKHSRINSA-N CC(C)([C@@H](CC1)C2)[C@@]1(CS(N(CC1)CC(CN(C)C)C1(C1=CC(OC)=CC=C1)O)(=O)=O)C2=O Chemical compound CC(C)([C@@H](CC1)C2)[C@@]1(CS(N(CC1)CC(CN(C)C)C1(C1=CC(OC)=CC=C1)O)(=O)=O)C2=O JUKOQNIFESKMAX-HMKHSRINSA-N 0.000 claims description 2
- JUKOQNIFESKMAX-YGNMWTRNSA-N CC(C)([C@H](CC1)C2)[C@]1(CS(N(CC1)CC(CN(C)C)C1(C1=CC(OC)=CC=C1)O)(=O)=O)C2=O Chemical compound CC(C)([C@H](CC1)C2)[C@]1(CS(N(CC1)CC(CN(C)C)C1(C1=CC(OC)=CC=C1)O)(=O)=O)C2=O JUKOQNIFESKMAX-YGNMWTRNSA-N 0.000 claims description 2
- 208000000094 Chronic Pain Diseases 0.000 claims description 2
- 208000004296 neuralgia Diseases 0.000 claims description 2
- 208000021722 neuropathic pain Diseases 0.000 claims description 2
- 150000002431 hydrogen Chemical class 0.000 claims 3
- 206010058019 Cancer Pain Diseases 0.000 claims 1
- 208000003251 Pruritus Diseases 0.000 claims 1
- 208000002551 irritable bowel syndrome Diseases 0.000 claims 1
- 238000004519 manufacturing process Methods 0.000 claims 1
- 238000001356 surgical procedure Methods 0.000 claims 1
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 48
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 31
- 239000007788 liquid Substances 0.000 description 16
- 239000000243 solution Substances 0.000 description 16
- 238000003756 stirring Methods 0.000 description 12
- 125000001424 substituent group Chemical group 0.000 description 12
- 239000000203 mixture Substances 0.000 description 11
- 239000007787 solid Substances 0.000 description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 11
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 10
- 125000003118 aryl group Chemical group 0.000 description 10
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 10
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 9
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 9
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- 230000000202 analgesic effect Effects 0.000 description 9
- 230000027455 binding Effects 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 125000000753 cycloalkyl group Chemical group 0.000 description 9
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 8
- TVYLLZQTGLZFBW-ZBFHGGJFSA-N (R,R)-tramadol Chemical compound COC1=CC=CC([C@]2(O)[C@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-ZBFHGGJFSA-N 0.000 description 7
- 229960004380 tramadol Drugs 0.000 description 7
- TVYLLZQTGLZFBW-GOEBONIOSA-N tramadol Natural products COC1=CC=CC([C@@]2(O)[C@@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-GOEBONIOSA-N 0.000 description 7
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 5
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 5
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical class [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 5
- 125000003545 alkoxy group Chemical group 0.000 description 5
- 125000004432 carbon atom Chemical group C* 0.000 description 5
- 239000012065 filter cake Substances 0.000 description 5
- 125000001072 heteroaryl group Chemical group 0.000 description 5
- 239000005457 ice water Substances 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 239000012074 organic phase Substances 0.000 description 5
- 238000000967 suction filtration Methods 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 125000004390 alkyl sulfonyl group Chemical group 0.000 description 4
- 239000008365 aqueous carrier Substances 0.000 description 4
- 239000008346 aqueous phase Substances 0.000 description 4
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 4
- 125000000392 cycloalkenyl group Chemical group 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 239000000706 filtrate Substances 0.000 description 4
- 229940090044 injection Drugs 0.000 description 4
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 238000000926 separation method Methods 0.000 description 4
- 239000012453 solvate Substances 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- 208000000114 Pain Threshold Diseases 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 125000003342 alkenyl group Chemical group 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 210000004027 cell Anatomy 0.000 description 3
- 238000004440 column chromatography Methods 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 125000003709 fluoroalkyl group Chemical group 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 239000008103 glucose Substances 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 3
- 230000001404 mediated effect Effects 0.000 description 3
- 238000000034 method Methods 0.000 description 3
- 230000037040 pain threshold Effects 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 229940032147 starch Drugs 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- YBBRCQOCSYXUOC-UHFFFAOYSA-N sulfuryl dichloride Chemical compound ClS(Cl)(=O)=O YBBRCQOCSYXUOC-UHFFFAOYSA-N 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- PLDWAJLZAAHOGG-UHFFFAOYSA-N 1-bromo-3-methoxybenzene Chemical compound COC1=CC=CC(Br)=C1 PLDWAJLZAAHOGG-UHFFFAOYSA-N 0.000 description 2
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 2
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical group [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 2
- 206010067484 Adverse reaction Diseases 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- RQFPIMOOHRJCNG-UHFFFAOYSA-N C(S(=O)(=O)N1CCC(O)(C2=CC(OC)=CC=C2)C(CN(C)C)C1)(C)C Chemical compound C(S(=O)(=O)N1CCC(O)(C2=CC(OC)=CC=C2)C(CN(C)C)C1)(C)C RQFPIMOOHRJCNG-UHFFFAOYSA-N 0.000 description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- MVNWQTVWQCWDEW-UHFFFAOYSA-N FC(OC(OC(F)(F)F)OF)F Chemical compound FC(OC(OC(F)(F)F)OF)F MVNWQTVWQCWDEW-UHFFFAOYSA-N 0.000 description 2
- ULMHMJAEGZPQRY-UHFFFAOYSA-N N-(tert-butoxycarbonyl)piperidin-2-one Chemical compound CC(C)(C)OC(=O)N1CCCCC1=O ULMHMJAEGZPQRY-UHFFFAOYSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 208000004756 Respiratory Insufficiency Diseases 0.000 description 2
- 206010038678 Respiratory depression Diseases 0.000 description 2
- 239000008156 Ringer's lactate solution Substances 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- 206010048010 Withdrawal syndrome Diseases 0.000 description 2
- 230000006838 adverse reaction Effects 0.000 description 2
- 229940035676 analgesics Drugs 0.000 description 2
- 239000000730 antalgic agent Substances 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Chemical compound BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- DCFKHNIGBAHNSS-UHFFFAOYSA-N chloro(triethyl)silane Chemical compound CC[Si](Cl)(CC)CC DCFKHNIGBAHNSS-UHFFFAOYSA-N 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 125000004093 cyano group Chemical group *C#N 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 229960002428 fentanyl Drugs 0.000 description 2
- IVLVTNPOHDFFCJ-UHFFFAOYSA-N fentanyl citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 IVLVTNPOHDFFCJ-UHFFFAOYSA-N 0.000 description 2
- 125000001153 fluoro group Chemical group F* 0.000 description 2
- 238000007429 general method Methods 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 125000001786 isothiazolyl group Chemical group 0.000 description 2
- 239000007951 isotonicity adjuster Substances 0.000 description 2
- 238000002386 leaching Methods 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- RLSSMJSEOOYNOY-UHFFFAOYSA-N m-cresol Chemical compound CC1=CC=CC(O)=C1 RLSSMJSEOOYNOY-UHFFFAOYSA-N 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 102000006240 membrane receptors Human genes 0.000 description 2
- 108020004084 membrane receptors Proteins 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 125000002950 monocyclic group Chemical group 0.000 description 2
- 229960005181 morphine Drugs 0.000 description 2
- 102000051367 mu Opioid Receptors Human genes 0.000 description 2
- 229940005483 opioid analgesics Drugs 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 125000000547 substituted alkyl group Chemical group 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 108020001612 μ-opioid receptors Proteins 0.000 description 2
- SFLSHLFXELFNJZ-QMMMGPOBSA-N (-)-norepinephrine Chemical compound NC[C@H](O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-QMMMGPOBSA-N 0.000 description 1
- PPKXEPBICJTCRU-XMZRARIVSA-N (R,R)-tramadol hydrochloride Chemical compound Cl.COC1=CC=CC([C@]2(O)[C@H](CCCC2)CN(C)C)=C1 PPKXEPBICJTCRU-XMZRARIVSA-N 0.000 description 1
- 125000001781 1,3,4-oxadiazolyl group Chemical group 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- WORJRXHJTUTINR-UHFFFAOYSA-N 1,4-dioxane;hydron;chloride Chemical compound Cl.C1COCCO1 WORJRXHJTUTINR-UHFFFAOYSA-N 0.000 description 1
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 1
- 125000006020 2-methyl-1-propenyl group Chemical group 0.000 description 1
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 1
- 125000006163 5-membered heteroaryl group Chemical group 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- CLUYGXJGAYELSK-UHFFFAOYSA-N CN(C)CC1CNCCC1O.C1CCNCC1 Chemical class CN(C)CC1CNCCC1O.C1CCNCC1 CLUYGXJGAYELSK-UHFFFAOYSA-N 0.000 description 1
- ZFQHRYXNCMKMCB-UHFFFAOYSA-N CNCNC(C(CN(CC1)S(CC2=CC=CC=C2)(=O)=O)C1(C1=CC(OC)=CC=C1)OC(F)F)NC Chemical compound CNCNC(C(CN(CC1)S(CC2=CC=CC=C2)(=O)=O)C1(C1=CC(OC)=CC=C1)OC(F)F)NC ZFQHRYXNCMKMCB-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- 206010010774 Constipation Diseases 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 208000018522 Gastrointestinal disease Diseases 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 239000007818 Grignard reagent Substances 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 208000008454 Hyperhidrosis Diseases 0.000 description 1
- 208000013016 Hypoglycemia Diseases 0.000 description 1
- 206010021036 Hyponatraemia Diseases 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 238000006683 Mannich reaction Methods 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-M Methanesulfonate Chemical compound CS([O-])(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-M 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 229920000715 Mucilage Polymers 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-L Phosphate ion(2-) Chemical compound OP([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-L 0.000 description 1
- 206010062519 Poor quality sleep Diseases 0.000 description 1
- 101100244562 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) oprD gene Proteins 0.000 description 1
- 208000032140 Sleepiness Diseases 0.000 description 1
- 208000026137 Soft tissue injury Diseases 0.000 description 1
- 206010041349 Somnolence Diseases 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 206010044565 Tremor Diseases 0.000 description 1
- AJKNYEHIJAMGHX-UHFFFAOYSA-M [Br-].COC1=CC=CC([Mg+])=C1 Chemical compound [Br-].COC1=CC=CC([Mg+])=C1 AJKNYEHIJAMGHX-UHFFFAOYSA-M 0.000 description 1
- BMWRQNIRQHXSLA-UHFFFAOYSA-M [Br-].FC(F)(F)OC1=CC=CC([Mg+])=C1 Chemical compound [Br-].FC(F)(F)OC1=CC=CC([Mg+])=C1 BMWRQNIRQHXSLA-UHFFFAOYSA-M 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 1
- 239000012346 acetyl chloride Substances 0.000 description 1
- 208000005298 acute pain Diseases 0.000 description 1
- 125000002252 acyl group Chemical class 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 230000036592 analgesia Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 125000006615 aromatic heterocyclic group Chemical group 0.000 description 1
- 125000005251 aryl acyl group Chemical group 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000004603 benzisoxazolyl group Chemical group O1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000002047 benzodioxolyl group Chemical group O1OC(C2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M bisulphate group Chemical group S([O-])(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- BPKIGYQJPYCAOW-FFJTTWKXSA-I calcium;potassium;disodium;(2s)-2-hydroxypropanoate;dichloride;dihydroxide;hydrate Chemical compound O.[OH-].[OH-].[Na+].[Na+].[Cl-].[Cl-].[K+].[Ca+2].C[C@H](O)C([O-])=O BPKIGYQJPYCAOW-FFJTTWKXSA-I 0.000 description 1
- BMLSTPRTEKLIPM-UHFFFAOYSA-I calcium;potassium;disodium;hydrogen carbonate;dichloride;dihydroxide;hydrate Chemical compound O.[OH-].[OH-].[Na+].[Na+].[Cl-].[Cl-].[K+].[Ca+2].OC([O-])=O BMLSTPRTEKLIPM-UHFFFAOYSA-I 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 229960001681 croscarmellose sodium Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 108700023159 delta Opioid Receptors Proteins 0.000 description 1
- 102000048124 delta Opioid Receptors Human genes 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 description 1
- 125000006222 dimethylaminomethyl group Chemical group [H]C([H])([H])N(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 208000002173 dizziness Diseases 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 125000004612 furopyridinyl group Chemical group O1C(=CC2=C1C=CC=N2)* 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 229940093181 glucose injection Drugs 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 150000004795 grignard reagents Chemical class 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 125000001183 hydrocarbyl group Chemical group 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 230000002218 hypoglycaemic effect Effects 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 125000004857 imidazopyridinyl group Chemical group N1C(=NC2=C1C=CC=N2)* 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 125000005990 isobenzothienyl group Chemical group 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 102000048260 kappa Opioid Receptors Human genes 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229960001375 lactose Drugs 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 229960001855 mannitol Drugs 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 239000002756 mu opiate receptor agonist Substances 0.000 description 1
- 229940126487 mu opioid receptor agonist Drugs 0.000 description 1
- VGIVLIHKENZQHQ-UHFFFAOYSA-N n,n,n',n'-tetramethylmethanediamine Chemical compound CN(C)CN(C)C VGIVLIHKENZQHQ-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000002560 nitrile group Chemical group 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 239000000346 nonvolatile oil Substances 0.000 description 1
- 229960002748 norepinephrine Drugs 0.000 description 1
- SFLSHLFXELFNJZ-UHFFFAOYSA-N norepinephrine Natural products NCC(O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-UHFFFAOYSA-N 0.000 description 1
- 238000005935 nucleophilic addition reaction Methods 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 239000000014 opioid analgesic Substances 0.000 description 1
- 239000008203 oral pharmaceutical composition Substances 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 125000004043 oxo group Chemical group O=* 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 125000003367 polycyclic group Chemical group 0.000 description 1
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 208000037821 progressive disease Diseases 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- 125000002098 pyridazinyl group Chemical group 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 238000002390 rotary evaporation Methods 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 239000008354 sodium chloride injection Substances 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000011146 sterile filtration Methods 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 229910021653 sulphate ion Inorganic materials 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000035900 sweating Effects 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical class CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 108020001588 κ-opioid receptors Proteins 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/92—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with a hetero atom directly attached to the ring nitrogen atom
- C07D211/96—Sulfur atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/04—Antipruritics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Pain & Pain Management (AREA)
- Psychiatry (AREA)
- Dermatology (AREA)
- Rheumatology (AREA)
- Addiction (AREA)
- Hydrogenated Pyridines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
本发明提供式(FWBH)化合物或其药学上可接受的盐,及其制备方法和药物用途,
Description
技术领域
本发明属于制药领域,涉及具有通式(FWBH)的3-(二甲氨基甲基)哌啶-4-醇哌啶衍生物或其盐类与制备方法,并涉及所述化合物在治疗阿片受体介导的疾病中的用途。
背景技术
疼痛是多种疾病进程中所出现的常见症状,是困扰患者的主要问题之一,已被列为继体温、脉搏、呼吸、血压之后的第五大生命体征。目前,阿片类镇痛药物在疼痛治疗中有着不可替代的作用,如吗啡、芬太尼等。但长期使用会出现耐药性,成瘾性,戒断反应,呼吸抑制等不良反应。曲马多是Grünenthal公司1977年开发的一种人工合成的阿片类中枢系统镇痛剂,商品名为tramal。它是相对较弱的μ阿片受体激动剂(对μ阿片受体的Ki=2400nM,EC50>1000nM),并且可以抑制5-羟色胺和去甲肾上腺素的再摄取。它主要经肝脏代谢,且几乎完全经肾脏排泄。曲马多作为一种不典型的阿片类药物,不同于其他传统的阿片类药物,有其独特的药理学特点,不仅有较强的镇痛效果,而且不良反应少,现已经被广泛应用于疼痛的治疗。然而,临床上的应用显示,曲马多的镇痛效果略低于吗啡、芬太尼等镇痛药。此外,曲马多还存在呼吸抑制、成瘾性、恶心、腹泻、头痛、头晕、嗜睡和便秘等副作用。长期服药还可以出现出汗、焦虑、睡眠不良、疼痛和身体颤抖等戒断反应。另外,有研究表明,曲马多的使用与需要住院治疗的低钠血症和低血糖风险增加有关。因此,有必要开发镇痛作用更强、副作用更低的镇痛药。
发明内容
本发明提供了式(FWBH)所示的化合物,或其药学上可接受的盐:
其中,
R1选自氢、C1-6烷基、氟代烷基、环烷基、链状烯基、环烯基、取代或未取代芳基C1-6烷基;
R2选自C1-6烷基,环烷基,取代或未取代桥环烷基,取代或未取代芳基,取代或未取代杂芳基,其取代基可选自芳基、卤素、C1-6烷基、氰基、烷氧基、氨基、硝基、烷磺酰基、酯基、三氟甲基、三氟甲氧基、二氟甲氧基、甲氧基、氟、硝基、酚羟基;
n=0,1,2,或3;
R3选自氢、C1-6烷基、氟代烷基、环烷基、链状烯基、环烯基、芳基C1-6烷基。优选实施方式中,R1选自甲基、三氟甲基。
优选实施方式中,R2选自甲基、乙基、异丙基、烯丙基、取代或未取代苯基、取代或未取代桥环烷基。其中取代苯基中的取代基优选为单取代,取代基优选自卤素、甲基、三氟甲基、硝基,取代位点可为邻位、间位、对位。取代桥环烷基选自以下基团:
优选实施方式中,R3选自氢、氟代烷基。更优选实施方式中,R3选自氢、氟代甲基。
优选实施方式中,式(FWBH)化合物选自:
1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-((3-氯苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((4-甲基苄基)磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(苯乙基磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((3-(三氟甲基)苄基)磺酰基)哌啶-4-醇;
1-((4-氯苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-1-((4-氟苄基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-((3-溴苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-1-((2-氟苄基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-(丁基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((3-硝基苄基)磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(苯基磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((4-(三氟甲基)苄基)磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-1-(乙基磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(丙基磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-1-(异丙基磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-(烯丙基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
(1R,4S)-1-(((3-((二甲氨基)甲基)-4-羟基-4-(3-甲氧基苯基)哌啶-1-基)磺酰基)甲基)-7,7-二甲双环[2.2.1]庚-2-酮;
3-((二甲氨基)甲基)-1-((((1R,4S)-2-羟基-7,7-二甲基双环[2.2.1]庚-1-基)甲基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(甲磺酰基)哌啶-4-醇;
(1S,4R)-1-(((3-((二甲氨基)甲基)-4-羟基-4-(3-甲氧基苯基)哌啶-1-基)磺酰基)甲基)-7,7-二甲基双环[2.2.1]庚-2-酮;
3-((二甲氨基)甲基)-1-((((1S,4R)-2-羟基-7,7-二甲基双环[2.2.1]庚-1-基)甲基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-(三氟甲氧基)苯基)哌啶-4-醇;
1-(1-(苄基磺酰基)-4-(二氟甲氧基)-4-(3-甲氧基苯基)哌啶-3-基)-N,N-二甲氨基甲胺。
本发明还提供了式(FWBH)化合物药学上可接受的盐类,可是与无机酸或有机酸形成的盐,如盐酸盐、氢溴酸盐、氢碘酸盐、硫酸盐或硫酸氢盐、磷酸盐或磷酸氢盐、乙酸盐、苯甲酸盐、酒石酸盐、琥珀酸盐、马来酸盐、富马酸盐、乳酸盐、柠檬酸盐、葡糖酸盐、甲磺酸盐、苯磺酸盐或对甲苯磺酸盐,优选盐酸盐。
本发明还提供了式(FWBH)化合物药学上可接受的溶剂化物或水合物。
本发明还提供了一种药物组合物,其包括:式(FWBH)化合物或其药学上可接受的盐、溶剂化物或水合物;以及药学上可接受的载体。
上述化合物制备的药物可用于治疗或改善与阿片受体有关的疾病。所述疾病可选自但不限于疼痛、胃肠道疾病和抑郁症。例如,疼痛可选自但不限于中枢介导的疼痛、外周介导的疼痛、与结构或软组织损伤有关的疼痛、与炎症有关的疼痛、与进行性疾病有关的疼痛、神经病疼痛、急性疼痛和慢性疼痛。
这种方法可以通过给予对象有效治疗量的式(FWBH)化合物或其药学上可接受的盐、溶剂化物或其水合物来实现。
本发明还提供了合成通式(FWBH)化合物的方法,包括Mannich反应、格式试剂亲核加成、脱保护基Boc、上保护基TES、与磺酰氯缩合、脱保护基、成盐等步骤。
反应路线中的取代基和基团的定义如上所述。
具体实施方式
如本申请全文,包括权利要求书中所使用,除非另外特别表明,否则以下术语具有下文定义的含义如本文中所使用。
术语“C1-C6烷基”指含有1至6个碳原子的饱和的支链或直链烷基,诸如(但不限于)甲基、乙基、正丙基、异丙基、正丁基、仲丁基、异丁基、叔丁基、正戊基、异戊基、新戊基及正己基。
术语“C1-6单取代或多取代烷基”指如上文所定义的C1-C6烷基中的一个或多个氢原子被选自下列的取代基代替:OH、卤素、烷氧基、二烷基氨基或杂环基,例如吗啉基、哌啶基等。
术语“C1-6单取代或多取代烷基酰基”指上文所定义的“C1-6单取代或多取代烷基”通过羰基与连接于母体分子部分。
术语“环烷基”是指碳原子的环状饱和单价单环或双环碳氢基团,例如环丙基、环丁基、环戊基、环己基,或类似基团。所述环烷基可任选地被一个、两个或三个取代基所取代,所述取代基选自卤原子、羟基、芳基。
术语“链状烯基”指具有至少一个碳-碳双键的脂族烃基,包括具有至少一个碳-碳双键的直链或支链基团。其例如具有2至6个碳原子。代表性实例包括(但不限于)乙烯基、1-丙烯基、2-丙烯基(烯丙基)、异丙烯基、2-甲基-1-丙烯基、1-丁烯基、2-丁烯基等。当本发明的化合物含有C2-C6链状烯基时,所述化合物可以纯E(entgegen)形式、纯Z(zusammen)形式或其任何混合物形式存在。
术语“环烯基”是指通过在环烷基移除额外的氢原子形成双键基团而得到相应的环烯基。
术语“桥环烷基”指任意两个碳环共用两不直接相连的碳原子的环烃基。
术语“取代或未取代桥环烷基”是指桥环烷基上的0个或至少一个氢原子被选自下列的取代基代替:羟基、氧代、卤素、C1-6烷基、腈基、烷氧基、氨基、硝基、烷磺酰基、酯基、三氟甲基、三氟甲氧基、二氟甲氧基、甲氧基、氟、硝基。
术语“芳基”指含有6至10个碳原子且具有共轭π电子系统的所有碳单环或稠环多环芳族基团,诸如苯基或萘基。
术语“取代或未取代芳基”是指芳基上的0至3个氢原子被选自下列的取代基代替:芳基、卤素、C1-6烷基、腈基、烷氧基、氨基、硝基、烷磺酰基、酯基、三氟甲基、三氟甲氧基、二氟甲氧基、甲氧基、氟、硝基、酚羟基。
术语“卤代”或“卤素”指氯、氟、溴或碘原子。
术语“取代或未取代芳基酰基”指上文所定义的“取代或未取代芳基”通过羰基与连接于母体分子部分。
术语“取代或未取代芳基烷基”是指如上文所定义的C1-C6烷基中的一个或多个氢原子被如上文所定义的“取代或未取代芳基”所取代。
术语“杂芳基”指单环或稠环多环芳族杂环基团,其中至少一个环中的一个或多个杂原子环成员(成环原子)各自独立地选自氧(O)、硫(S)及氮(N)。杂芳基的实例包括(但不限于)6元环取代基,诸如吡啶基、吡嗪基、嘧啶基及哒嗪基;5元杂芳基,诸如三唑基、咪唑基、呋喃基、异噁唑基、异噻唑基、1,2,3-、1,2,4、1,2,5-或1,3,4-噁二唑基、噁唑基、噻吩基、噻唑基、异噻唑基及吡唑基;6/5元稠环取代基,诸如吲哚基、吲唑基、苯并呋喃基、苯并咪唑基、苯并噻吩基、苯并噁二唑基、苯并噻唑基、异苯并噻吩基、苯并噻吩基、苯并异噁唑基、苯并噁唑基、苯并间二氧杂环戊烯基、呋喃并吡啶基、嘌呤基、咪唑并吡啶基、咪唑并嘧啶基、吡咯并吡啶基、吡唑并吡啶基、吡唑并嘧啶基、噻吩并吡啶基、三唑并嘧啶基、三唑并吡啶基(例如5,6,7,8-四氢[1,2,4]三唑并[1,5-a]吡啶-2-基)及邻氨基苯甲酰基;及6/6元稠环取代基,诸如喹啉基、异喹啉基、噌啉基、喹唑啉基、氧代色烷基及1,4-苯并噁嗪基。
术语“取代或未取代杂芳基”是指杂芳基上的0至3个氢原子被选自下列的取代基代替:芳基、卤素、C1-6烷基、氰基、烷氧基、氨基、硝基、烷磺酰基、酯基、三氟甲基、三氟甲氧基、二氟甲氧基、甲氧基、氟、硝基、酚羟基。
除非特别说明,本发明中,所有出现的化合物均意在包括所有可能的异构体,例如互变异构体、对映异构体、非对映异构体、及其混合物形式。
“有效治疗量”指在某种程度上减轻所治疗病症中一种或多种症状的化合物的给药量。
术语“药学上可接受的载体”表示能用于制备药物组合物的载体,它们一般是安全的、无毒性的,不是生物上或其他方面不期待的,且包括能被动物和人类药学上接受的载体。在说明书和权利要求书中使用的“药学上可接受的载体”包括一种或一种以上的这类载体。
本发明所述的药物组合物可以是液体、半液体或固体形式,按照适合于所用的给药途径的方式配制。本发明所述的药物组合物可以按照下列给药方式给药:口服、肠胃外、腹膜内、静脉内、透皮、舌下、肌内、直肠、口腔、鼻内、脂质体等方式。
口服药物组合物可以是固体、凝胶或液体。固体制剂的实例包括但不限于片剂、胶囊剂、颗粒剂和散装粉剂。这些制剂可以选择地含有粘合剂、稀释剂、崩解剂、润滑剂、助流剂、甜味剂和矫味剂等。粘合剂的实例包括但不限于微晶纤维素、葡萄糖溶液、阿拉伯胶浆、明胶溶液、蔗糖和淀粉糊;润滑剂的实例包括但不限于滑石、淀粉、硬脂酸镁、硬脂酸钙、硬脂酸;稀释剂的实例包括但不限于乳糖、蔗糖、淀粉、甘露糖醇、磷酸二钙;助流剂的实例包括但不限于二氧化硅;崩解剂的实例包括但不限于交联羧甲基纤维素钠、淀粉羟乙酸钠、藻酸、玉米淀粉、马铃薯淀粉、甲基纤维素、琼脂和羧甲基纤维素。
以肠胃外给予本发明药物组合物,一般以注射为主,包括皮下、肌内或静脉内注射。注射剂可以被制成任何常规形式,如液体溶液或悬液、适合于在注射之前溶解或悬浮在液体中的固体形式或者乳剂。可用于本发明注射剂的药学上可接受的载体的实例包括但不限于水性载体、非水性载体、抗微生物剂、等渗剂、缓冲剂、抗氧剂、悬浮与分散剂、乳化剂、螯合剂和其它药学上可接受的物质。水性载体的实例包括氯化钠注射液、林格式注射液、等渗葡萄糖注射液、无菌水注射液、葡萄糖与乳酸化林格氏注射液;非水性载体的实例包括植物来源的固定油、棉籽油、玉米油、芝麻油和花生油;抗微生物剂的实例包括间甲酚、苄醇、氯丁醇、苯扎氯铵等;等渗剂的实例包括氯化钠和葡萄糖;缓冲剂包括磷酸盐和柠檬酸盐。
本发明药物组合物还可以制备成无菌的冻干粉针剂,将化合物溶于磷酸钠缓冲溶液,其中含有葡萄糖或其他适合的赋形剂,随后在本领域技术人员已知的标准条件下将溶液无菌过滤,继之以冷冻干燥,得到所需的制剂。
术语“式(FWBH)”或“式(FWBH)化合物”可称作“本发明的化合物”。这样的术语还被定义为包括本发明的化合物的所有形式,包括水合物、溶剂合物、异构体、结晶及非结晶形式、同晶型体、多晶型物及其代谢物。
本发明的化合物一般按照IUPAC或CAS命名体系命名。可以使用本领域技术人员公知的缩写(例如,“Ph”表示苯基、“Me”表示甲基、“Et”表示乙基、“h”表示小时,“r.t.”表示室温)。
本发明通过以下实施例阐述,这些实施仅用于说明,不限制本发明的范围。式FWBH化合物可如下面的通用合成路线和实施例描述而制备。
实施例1
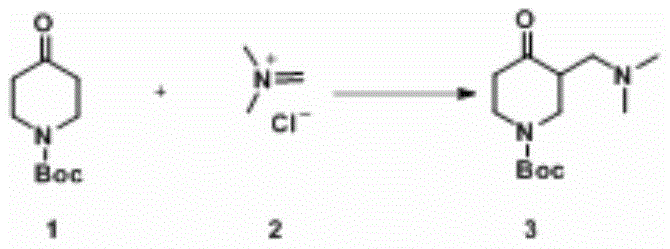
中间体2的制备
伴有氮气气球、温度计和恒压滴液漏斗的1L三口瓶加N,N,N',N'-四甲基甲烷二胺(60g,587.2mmol,1eq.)、甲基叔丁基醚(500ml),冷却至0℃,30℃下滴加乙酰氯(46.1g,587.2mmol,1eq.,约20min)。滴加完毕,搅拌30分钟,抽滤,滤饼加乙腈(100ml)和MTBE(25ml)搅拌10min,抽滤,滤饼减压蒸干(55℃),得46g类白色固体(极易吸潮),收率83.7%。
实施例2
中间体3的制备:
伴有温度计、氮气球的1L单口瓶加Boc-哌啶酮(35g,175.66mmol,1eq.)、乙腈(350ml),搅拌溶解,加中间体2(19.72g,210.8mmol,1.2eq.)。内温30~35℃反应24h,24h后TLC显示基本反应完全。将乙腈减压蒸除,加DCM(300ml),再加饱和碳酸氢钠(250ml),混合、静置、分层、分液。水相DCM(200+100ml)萃取。有机相合并,水洗(50ml),无水硫酸镁干燥,抽滤,滤液减压蒸除,得43.3g红棕色粘稠液体,收率93.5%。
实施例3
中间体5的制备:
伴有氮气球、恒压滴液漏斗、冷凝管和温度计的1L四口瓶加镁屑(11g,451.68mmol,3eq.)、THF(300ml)、3粒碘,少量间溴苯甲醚(84.5g,451.68mmol,3eq.)的THF(70ml)溶液,升温回流引发。待黄色褪去,停止加热。缓慢滴加间溴苯甲醚的THF溶液(约1h),直至滴加完全。滴加完毕,搅拌自然降至室温。30min后,25℃下滴加中间体3(37.76g,1eq.)的THF(100ml)溶液。滴加完毕,撤冰水浴搅拌,室温过夜(18h)。将反应液倒入氯化铵的水溶液中(200ml)和冰(约100g)的混合液中,搅拌5min,将THF减压蒸除(30℃)。加乙酸乙酯(300ml),搅拌、静置、分层、分液。水相乙酸乙酯萃取(300ml)。有机相合并,水洗(100ml),无水硫酸镁干燥,抽滤,滤液减压蒸除,得76.5g黄色液体,柱层析纯化,得21.4g浅黄色粘稠状液体,收率38.9%(两步,以boc-哌啶酮计)。1H NMR(400MHz,CD3OD),δ7.26(t,J=8Hz,1H),7.04~7.05(m,1H),6.98(d,J=8Hz,1H),6.81(dd,J=8Hz,J=4Hz,1H),4.20~4.25(m,1H),3.96~4.00(m,1H),3.79(s,3H),3.35(s,1H),3.03~3.22(m,2H),2.31~2.37(m,1H),2.07~2.11(m,1H),2.04(s,6H),1.94~2.01(m,1H),1.78~1.81(m,1H),1.58~1.62(m,1H),1.50(s,9H).。
实施例4
中间体6的制备:
250ml单口瓶加Boc-氨基醇(9.5g,26.06mmol,1eq.))、甲醇(76ml),搅拌,滴加HCl/1,4-二氧六环溶液(16.3ml,65.15mmol,2.5eq.),内温升至约36℃。搅拌2小时后,TLC显示有大量原料。升温至50℃搅拌,2小时后TLC显示基本反应完全。加MTBE(150ml)搅拌,逐渐析出固体,搅拌过夜(24h)。抽滤,滤饼MTBE(20ml)淋洗,旋蒸减压旋干,得8.63g类白色固体,收率98.3%。1H NMR(400MHz,CD3OD),δ7.38(t,J=8Hz,1H),7.15~7.16(m,1H),7.12(d,J=8Hz,1H),6.92(dd,J1=8Hz,J2=4Hz,1H),3.83(s,3H),3.75(dd,J1=12Hz,J2=4Hz,1H),3.41~3.43(m,3H),3.09~3.15(m,1H),2.85~2.92(m,1H),2.75(s,3H),2.75~2.78(m,1H),2.57(s,3H),2.54~2.62(m,1H),1.89~1.94(m,1H).。
实施例5
中间体7的制备:
伴有氮气气球、恒压滴液漏斗和温度计的250ml三口瓶加氨基醇盐酸盐(10g,33.22mmol,1eq.)、咪唑(20.35g,298.98mmol,9eq.)、DCM(100ml),搅拌溶解。冷却,10℃下滴加三乙基氯硅烷(35.06g,232.6mmol,7eq.)。滴加完毕,搅拌5min,撤冰水浴搅拌过夜(26h)。TLC(DCM:MeOH=10:1和4:1)显示反应完全。冰水浴下,将反应液缓慢倒入饱和碳酸氢钠水溶液中(100ml),搅拌10分钟,加二氯甲烷(25ml)萃取。水相二氯甲烷萃取。有机相合并,水洗(20ml),无水硫酸镁干燥,抽滤,滤液减压蒸除,得36.72g浅黄色液体,三氧化二铝柱层析纯化,得10.2g浅黄色液体,收率81%。LC-MS:ESI+[M+H]+379.1。
实施例6
中间体7与磺酰氯缩合通用方法:
100ml单口瓶加中间体7(1eq.)、DCM(10ml)、TEA(2eq.),冰水浴下搅拌,加磺酰氯(1eq.)。30℃下搅拌一定时间。加水(30ml),DCM,混合、静置、分层、分液。水相DCM(20ml)萃取。有机相合并,水洗。减压蒸除,得浅黄色液体。未经进一步纯化,直接用于下一步。
实施例7
脱TES通用方法:
50ml单口瓶加原料(1eq.),THF,搅拌溶解,加TBAF(1.5eq.)。室温搅拌,LC-MS监测直至反应完全。将THF减压蒸除,加碳酸氢钠溶液(30ml),DCM萃取。有机相合并,水洗,无水硫酸镁干燥,抽滤,滤液减压蒸除,得液体。柱层析纯化得目标产物。
实施例8
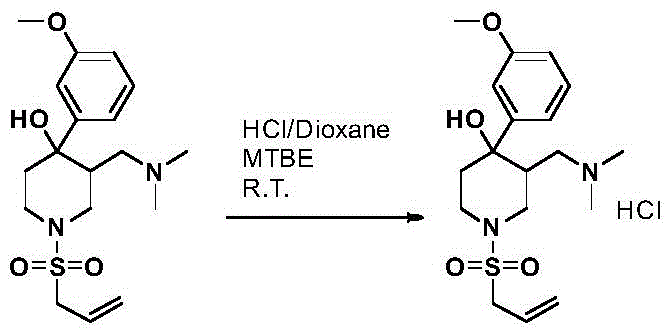
成盐通用方法:
50ml单口瓶加原料(1eq.),加二氯甲烷,搅拌溶解,加甲基叔丁基醚.。加HCl的1,4-二氧六环溶液(1.2eq.),析出固体,搅拌一定时间,抽滤,滤饼甲基叔丁基醚淋洗,油泵抽干,得目标产物。
实施例9
1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH6)
由1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:95.8%.1H NMR(400MHz,CDOD),δ7.50(dd,J1=8Hz,J2=4Hz,1H),7.36~7.44(m,3H),7.33(t,J=8Hz,1H),7.07(s,1H),70.03(d,J=8Hz,1H),6.85~6.87(m,1H),4.45(s,2H),3.79~3.83(m,4H),3.52~3.57(m,1H),3.12~3.20(m,2H),2.95~3.01(m,1H),2.60~2.69(m,1H),2.60(s,6H),2.29~2.35(m,1H),2.13~2.21(m,1H),1.65~1.70(m,1H).LC-MS-ESI+:[M+H]+419.3.。
实施例10
1-((3-氯苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH7)
由1-((3-氯苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:92.1%.1H NMR(400MHz,CDOD),δ7.56~7.57(m,1H),7.41~7.47(m,3H),7.34(t,J=8Hz,1H),7.10~7.11(m,1H),7.05(d,J=12Hz,1H),6.88(dd,J1=8Hz,J2=4Hz,H),4.49(t,2H),3.89(dd,J1=12Hz,J2=4Hz,1H),3.82(s,3H),3.56~3.61(m,1H),3.18~3.25(m,2H),2.99~3.05(m,1H),2.71(s,6H),2.69~2.73(m,1H),2.53(s,3H),2.34~2.41(m,1H),2.15~2.23(m,1H),1.68~1.73(m,1H).LC-MS-ESI+:[M+H]+453.2.。
实施例11
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((4-甲基苄基)磺酰基)哌啶-4-醇盐酸盐(FWBH8)
由3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((4-甲基苄基)磺酰基)哌啶-4-醇成盐得到,收率:96.8%.1H NMR(400MHz,CD3OD),δ7.31~7.38(m,3H),7.23~7.26(m,2H),7.02~7.07(m,2H),6.87(dd,J=8Hz,J=4Hz,1H),4.40(s,2H),3.81(s,3H),3.75~3.82(m,1H),3.51~3.56(m,1H),3.12~3.21(m,3H),2.95~3.01(m,1H),2.66~2.70(m,1H),2.60(s,6H),2.35(s,3H),2.28~2.37(m,1H),2.13~2.21(m,1H),1.65~1.70(m,1H).LC-MS-ESI+:[M+H]+433.3.。
实施例12
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(苯乙基磺酰基)哌啶-4-醇盐酸盐(FWBH9)
由3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(苯乙基磺酰基)哌啶-4-醇成盐得到,收率:87.2%.1H NMR(400MHz,CD3OD),δ7.23~7.37(m,6H),7.11(s,1H),7.07(d,J=8Hz,1H),6.88(dd,J1=8Hz,J2=4Hz,H),3.86~3.91(m,1H),3.82(s,3H),3.70(d,J=12Hz,1H),3.39~3.43(m,2H),3.10~3.27(m,4H),3.01~3.06(m,1H),2.69~2.74(m,1H),2.65(s,6H),2.41~2.46(m,1H),2.21~2.29(m,1H),1.76(dd,J1=12Hz,J2=4Hz,H).LC-MS-ESI+:[M+H]+433.3.。
实施例13
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((3-(三氟甲基)苄基)磺酰基)哌啶-4-醇盐酸盐(FWBH10)
由3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((3-(三氟甲基)苄基)磺酰基)哌啶-4-醇成盐得到,收率:90.6%.1H NMR(400MHz,CD3OD),δ7.70~7.81(m,3H),7.83(t,J=8Hz,1H),7.33(t,J=8Hz,1H),7.04~7.10(m,2H),6.87(d,J=8Hz,1H),4.57(s,2H),3.82~3.87(m,1H),3.81(s,3H),3.58~3.61(m,1H),3.18~3.25(m,2H),2.96~3.04(m,1H),2.69~2.73(m,1H),2.62(s,6H),2.39(m,1H),2.18~2.26(m,1H),1.70~1.74(m,1H).LC-MS-ESI+:[M+H]+487.2.。
实施例14
1-((4-氯苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH11)
由1-((4-氯苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:68.4%.1H NMR(400MHz,CD3OD),δ7.41~7.50(m,4H),7.30~7.37(m,1H),7.04~7.09(m,2H),6.85~6.88(m,1H),4.44~4.46(m,2H),3.80~3.84(m,4H),3.59(m,1H),3.14~3.24(m,3H),2.96~3.03(m,1H),2.69~2.71(m,4H),2.54(s,3H),2.35(s,1H),2.16~2.23(m,1H),1.68~1.72(m,1H).LC-MS-ESI+:[M+H]+453.2.。
实施例15
3-((二甲氨基)甲基)-1-((4-氟苄基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH12)
由3-((二甲氨基)甲基)-1-((4-氟苄基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:98.33%.1H NMR(400MHz,CD3OD),δ7.50~7.54(m,2H),7.31~7.35(m,1H),7.14~7.19(m,2H),7.09(s,1H),7.05(d,J=8Hz,1H),6.87(d,J=8Hz,1H),4.45(s,2H),3.82~3.84(m,1H),3.81(s,3H),3.54~3.59(m,1H),3.15~3.21(m,1H),2.97~3.03(m,1H),2.68~2.72(m,1H),2.62(s,6H),2.34~2.40(m,1H),2.16~2.24(m,1H),1.67~1.72(m,1H).LC-MS-ESI+:[M+H]+437.2.。
实施例16
1-((3-溴苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH13)
由1-((3-溴苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:85%.1H NMR(400MHz,CD3OD),δ7.57~7.58(m,1H),7.43(d,J=8Hz,1H),7.35(d,J=8Hz,1H),7.18~7.24(m,2H),6.90~6.98(m,2H),6.72~6.76(m,1H),4.33(s,2H),3.68~3.75(m,1H),3.67(s,3H),3.45(dd,J1=16Hz,J2=8Hz,1H),3.03~3.11(m,2H),2.84~2.90(m,1H),2.54~2.59(m,1H),2.48(s,6H),2.19~2.24(m,1H),2.01~2.09(m,1H),1.55~1.59(m,1H).LC-MS-ESI+:[M+H]+497.2.。
实施例17
3-((二甲氨基)甲基)-1-((2-氟苄基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH14)
由3-((二甲氨基)甲基)-1-((2-氟苄基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:94%.1H NMR(400MHz,CD3OD),δ7.54~7.60(m,1H),7.41~7.49(m,1H),7.32~7.38(m,1H),7.19~7.29(m,2H),7.04~7.11(m,2H),6.86~6.91(m,1H),4.52(s,2H),3.82~3.87(m,1H),3.81(s,3H),3.54~3.57(m,1H),3.20~3.27(m,2H),2.99~3.06(m,1H),2.67~2.73(m,1H),2.62(s,6H),2.36~2.41(m,1H),2.15~2.25(m,1H),1.61~1.74(m,1H).LC-MS-ESI+:[M+H]+437.2.。
实施例18
1-(丁基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH15)
由1-(丁基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇盐成盐得到,收率:88.6%.1H NMR(400MHz,CD3OD),δ7.32~7.39(m,1H),7.08~7.15(m,2H),6.86~6.92(m,1H),3.87~3.91(m,1H),3.81(s,3H),3.67~3.72(m,1H),3.20~3.27(m,1H),3.03~3.16(m,3H),2.70~2.77(m,1H),2.65(s,6H),2.45~2.50(m,1H),2.25~2.33(m,1H),1.76~1.84(m,3H),1.46~1.56(m,2H),0.91~1.04(m,3H).LC-MS-ESI+:[M+H]+385.2.。
实施例19
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((3-硝基苄基)磺酰基)哌啶-4-醇盐酸盐(FWBH22)
由3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((3-硝基苄基)磺酰基)哌啶-4-醇成盐得到收率:98.4%.1H NMR(400MHz,CD3OD),δ8.28~8.34(m,2H),7.74~7.80(m,2H),7.32~7.40(m,1H),7.05~7.13(m,2H),6.86~6.92(m,1H),4.62~4.66(m,2H),3.81~3.88(m,4H),3.60~3.69(m,2H),3.18~3.27(m,3H),2.98~3.08(m,1H),2.64~2.74(m,7H),2.40~2.48(m,1H),2.19~2.28(m,1H),1.70~1.77(m,1H).LC-MS-ESI+:[M+H]+464.2.。
实施例20
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(苯基磺酰基)哌啶-4-醇盐酸盐(FWBH16)
由3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(苯基磺酰基)哌啶-4-醇成盐得到,收率:92.3%.1H NMR(400MHz,CD3OD),δ7.89(d,J=8Hz,1H),7.72~7.76(m,1H),7.65~7.69(m,2H),7.32(t,J=8Hz,1H),7.02~7.06(m,2H),6.84~6.87(m,1H),3.98(dd,J1=12Hz,J2=4Hz,1H),3.79(s,3H),3.71~3.73(m,1H),2.95~3.01(m,1H),2.53~2.77(m,10H),2.29~2.36(m,1H),1.73~1.77(m,1H).LC-MS-ESI+:[M+H]+405.2.。
实施例21
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((4-(三氟甲基)苄基)磺酰基)哌啶-4-醇盐酸盐(FWBH17)
由3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((4-(三氟甲基)苄基)磺酰基)哌啶-4-醇成盐得到,收率:98.5%.1H NMR(400MHz,CD3OD),δ7.69~7.75(m,4H),7.31~7.36(m,1H),7.04~7.10(m,2H),6.86~6.89(m,1H),4.56(s,2H),3.82~3.88(m,1H),3.81(s,3H),3.58~3.62(m,1H),3.22(t,J=8Hz,2H),2.98~3.05(m,1H),2.69~2.74(m,1H),2.63(s,6H),2.35~2.41(m,1H),2.17~2.25(m,1H),1.69~1.74(m,1H).LC-MS-ESI+:[M+H]+487.2.。
实施例22
3-((二甲氨基)甲基)-1-(乙基磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH18)
由3-((二甲氨基)甲基)-1-(乙基磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:98.7%.1H NMR(400MHz,CD3OD),δ7.34(t,J=8Hz,1H),7.13(s,1H),7.09(d,J=8Hz,1H),6.88(dd,J1=8Hz,J2=4Hz,1H),3.91(dd,J1=12Hz,J2=8Hz,1H),3.82(s,3H),3.70(dd,J1=12Hz,J2=4Hz,1H),3.22~3.36(m,2H),3.13~3.18(m,2H),3.03~3.09(m,1H),2.74(s,3H),2.70~2.73(m,1H),2.58(s,3H),2.45~2.51(m,1H),2.25~2.33(m,1H),1.75~1.80(m,1H),1.37(t,J=8Hz,3H).LC-MS-ESI+:[M+H]+357.3.。
实施例23
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(丙基磺酰基)哌啶-4-醇盐酸盐(FWBH19)
由3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(丙基磺酰基)哌啶-4-醇成盐得到,收率:80%.1H NMR(400MHz,CD3OD),δ7.32~7.36(m,1H),7.08~7.13(m,2H),6.88(dd,J1=8Hz,J2=4Hz,1H),3.87~3.91(m,1H),3.81(s,3H),3.66~3.71(m,1H),3.19~3.33(m,2H),3.03~3.12(m,3H),2.61~2.75(m,7H),2.45~2.51(m,1H),2.25~2.33(m,1H),1.82~1.89(m,2H),1.76~1.81(m,1H),1.08~1.12(m,3H).LC-MS-ESI+:[M+H]+371.2.。
实施例24
3-((二甲氨基)甲基)-1-(异丙基磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH20)
由3-((二甲氨基)甲基)-1-(异丙基磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:85.5%.1H NMR(400MHz,CD3OD),δ7.34(t,J=8Hz,1H),7.04~7.11(m,2H),6.88(dd,J1=8Hz,J2=4Hz,1H),3.89~3.93(m,1H),3.81(s,3H),3.70~3.75(m,1H),3.34~3.44(m,3H),3.03~3.09(m,1H),2.61~2.73(m,7H),2.41~2.45(m,1H),2.22~2.30(m,1H),1.71~1.76(m,1H),1.37(d,J=4Hz,6H),.LC-MS-ESI+:[M+H]+371.3.。
实施例25
1-(烯丙基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH21)
由1-(烯丙基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:89%.1H NMR(400MHz,CD3OD),δ7.32~7.36(m,1H),7.07~7.12(m,2H),6.88(dd,J1=8Hz,J2=4Hz,1H),5.91~6.02(m,1H),5.45~5.53(m,2H),3.94(d,J=8Hz,3H),3.82(s,3H),3.70~3.75(m,1H),3.25~3.39(m,3H),3.02~3.08(m,1H),2.74(s,3H),2.57(s,3H),2.42~2.47(m,1H),2.23~2.31(m,1H),1.77(dd,J1=16Hz,J2=4Hz,1H).LC-MS-ESI+:[M+H]+369.2.。
实施例26
(1R,4S)-1-(((3-((二甲氨基)甲基)-4-羟基-4-(3-甲氧基苯基)哌啶-1-基)磺酰基)甲基)-7,7-二甲双环[2.2.1]庚-2-酮盐酸盐(FWBH24)
由(1R,4S)-1-(((3-((二甲氨基)甲基)-4-羟基-4-(3-甲氧基苯基)哌啶-1-基)磺酰基)甲基)-7,7-二甲双环[2.2.1]庚-2-酮成盐得到,收率:84.4%.1H NMR(400MHz,CD3OD),δ7.32~7.37(m,1H),7.07~7.13(m,2H),6.88(dd,J1=8Hz,J2=4Hz,H),3.92~3.95(m,1H),3.82(s,3H),3.73~3.77(m,1H),3.46(t,J=16Hz,1H),3.20~3.35(m,2H),2.96~3.12(m,2H),2.39~2.75(m,10H),2.24~2.35(m,1H),2.06~2.14(m,2H),1.98(dd,J1=20Hz,J2=4Hz,1H),1.80(dd,J1=12Hz,J2=4Hz,1H),1.62~1.70(m,1H),1.45~1.53(m,1H),1.15(d,J=4Hz,3H),0.93(d,J=4Hz,3H).LC-MS-ESI+:[M+H]+479.3.。
实施例27
3-((二甲氨基)甲基)-1-((((1R,4S)-2-羟基-7,7-二甲基双环[2.2.1]庚-1-基)甲基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH26)
由3-((二甲氨基)甲基)-1-((((1R,4S)-2-羟基-7,7-二甲基双环[2.2.1]庚-1-基)甲基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:84.2%.1H NMR(400MHz,CD3OD),δ7.32~7.39(m,1H),7.09~7.17(m,2H),6.87~6.93(m,1H),4.03~4.07(m,1H),3.92~3.97(m,1H),3.72~3.86(m,4H),3.46~3.52(m,1H),3.18~3.35(m,2H),3.05~3.10(m,1H),2.05~2.77(m,8H),2.28~2.36(m,1H),1.73~1.86(m,5H),1.43~1.48(m,1H),1.08~1.23(m,5H),0.99~1.00(m,1H),0.90~0.95(m,3H).LC-MS-ESI+:[M+H]+481.3.。
实施例28
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(甲磺酰基)哌啶-4-醇盐酸盐(FWBH23)
由3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(甲磺酰基)哌啶-4-醇成盐得到,收率:94%.1H NMR(400MHz,CD3OD),δ7.34(t,J=8Hz,1H),7.09~7.14(m,2H),6.86~6.89(m,1H),3.88(dd,J1=8Hz,J2=4Hz,1H),3.82(s,3H),3.65~3.70(m,1H),3.11~3.25(m,2H),3.03~3.09(m,1H),2.95(s,3H),2.72~2.78(m,1H),2.65(s,6H),2.49~2.55(m,1H),2.29~2.37(m,1H),1.79~1.84(m,1H).LC-MS-ESI+:[M+H]+343.2.。
实施例29
(1S,4R)-1-(((3-((二甲氨基)甲基)-4-羟基-4-(3-甲氧基苯基)哌啶-1-基)磺酰基)甲基)-7,7-二甲基双环[2.2.1]庚-2-酮盐酸盐(FWBH25)
由(1S,4R)-1-(((3-((二甲氨基)甲基)-4-羟基-4-(3-甲氧基苯基)哌啶-1-基)磺酰基)甲基)-7,7-二甲基双环[2.2.1]庚-2-酮成盐得到,收率:76.3%.1H NMR(400MHz,CD3OD),δ7.34(t,J=8Hz,1H),7.07~7.13(m,2H),7.86~7.89(m,1H),3.95(d,J=12Hz,1H),3.82(s,3H),3.74(t,J=8Hz,1H),3.42~3.50(m,1H),3.20~3.35(m,2H),3.06~3.12(m,1H),2.97~3.03(m,1H),2.68~2.74(m,7H),2.39~2.57(m,3H),2.23~2.35(m,1H),2.06~2.15(m,2H),1.98(dd,J1=20Hz,J2=4Hz,1H),1.80(d,J=16Hz,1H),1.62~1.70(m,1H),1.45~1.53(m,1H),1.14(d,J=8Hz,3H),0.93(d,J=8Hz,3H).LC-MS-ESI+:[M+H]+479.3.。
实施例30
3-((二甲氨基)甲基)-1-((((1S,4R)-2-羟基-7,7-二甲基双环[2.2.1]庚-1-基)甲基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇盐酸盐(FWBH27)
由3-((二甲氨基)甲基)-1-((((1S,4R)-2-羟基-7,7-二甲基双环[2.2.1]庚-1-基)甲基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇成盐得到,收率:88.6%.1H NMR(400MHz,CD3OD),δ7.32~7.38(m,1H),7.09~7.14(m,2H),6.89(dd,J1=8Hz,J2=4Hz,1H),4.02~4.07(m,1H),3.93~3.99(m,1H),3.82(s,3H),3.72~3.78(m,1H),3.46~3.52(m,1H),3.18~3.30(m,2H),3.05~3.11(m,1H),2.87~2.93(m,1H),2.50~2.75(m,8H),2.28~2.36(m,1H),1.73~1.86(m,5H),1.43~1.148(m,1H),0.91~1.20(m,8H).LC-MS-ESI+:[M+H]+481.3.。
实施例31
1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-(三氟甲氧基)苯基)哌啶-4-醇盐酸盐(FWBH29)
将实施例3中的3-(甲氧基)苯基溴化镁替换为3-(三氟甲氧基)苯基溴化镁,经与制备1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇相同的路线,可制得1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-(三氟甲氧基)苯基)哌啶-4-醇。由1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-(三氟甲氧基)苯基)哌啶-4-醇成盐得到,收率:91.4%.1H NMR(400MHz,CD3OD),δ7.49~7.55(m,4H),7.47(s,1H),7.39~7.44(m,3H),7.25(d,J=8Hz,1H),4.46(s,2H),3.82(dd,J1=12Hz,J2=4Hz,1H),3.54~3.59(m,1H),3.12~3.21(m,2H),3.00~3.07(m,1H),2.58~2.63(m,7H),2.35~2.41(m,1H),2.13~2.22(m,1H),1.68~1.73(m,1H).LC-MS-ESI+:[M+H]+473.2.。
实施例32
1-(1-(苄基磺酰基)-4-(二氟甲氧基)-4-(3-甲氧基苯基)哌啶-3-基)-N,N-二甲氨基甲胺
25ml单口瓶加原料(100mg,0.24mmol,1eq.)、二氯甲烷(0.5ml)、水(0.5ml)、KOAc(188.43mmol,1.92mmol,8eq.),加TMSCF2Br(195mg,0.96mmol,4eq.)。室温搅拌析出固体,补加二氯甲烷(0.5ml)、水(0.5ml)。LC-MS监测直至反应完全。抽滤,滤饼水洗,烘干,得约115mg类白色固体。甲醇和乙酸乙酯(0.4ml:0.6ml)结晶得90mg类白色固体,收率80.4%。
1-(1-(苄基磺酰基)-4-(二氟甲氧基)-4-(3-甲氧基苯基)哌啶-3-基)-N,N-二甲氨基甲胺盐酸盐(FWBH30)
由1-(1-(苄基磺酰基)-4-(二氟甲氧基)-4-(3-甲氧基苯基)哌啶-3-基)-N,N-二甲氨基甲胺成盐得到,收率84%。1H NMR(400MHz,CD3OD),δ7.485~7.501(m,2H),7.339~7.421(m,4H),7.107~7.148(m,2H),6.890~6.914(m,2H),6.838(s,J=58.4Hz,1H),4.456(s,2H),3.823(s,3H),3.765(d,J=13.2Hz,2H),3.564(d,J=12.8Hz,1H),3.117~3.362(m,2H),2.977(s,3H),2.637(s,3H),2.482~2.504(m,1H),2.326~2.395(m,1H),1.715(d,J=14.8Hz,1H).LC-MS-ESI+:
[M+H]+469.2.。
实施例33
膜受体的制备
分别表达有μ阿片受体、δ阿片受体、κ阿片受体的CHO细胞种于10cm2培养皿中培养(F-12培养基+10%新生牛血清)数天,细胞长满皿底后吸去培养液;加PBS/EDTA溶液(0.1MNaCl,0.01M NaH2PO4,0.04%EDTA)3ml消化3-5min,用吸管吹打,使细胞完全脱落,收集细胞于40ml离心管,5000rpm离心5min,去上清液;加入冰冷的匀浆液(50mM HEPES PH 7.4,3mMMgCl,1mM EGTA)于离心管,将溶液和沉淀转移到匀浆器中匀浆;然后将匀浆液转移到离心管中,18000rpm离心15min,共离心2次;得到的沉淀加入适量的50mM Tris-HCl,pH 7.4的缓冲液匀浆并分装于离心管,-70℃冰箱保存待用。
竞争结合试验
总结合管加相当于20-30μg的表达的膜受体蛋白和[3H]标记的配体(1-2nM),相对应的非特异性结合管另加1μM的相应配体,样品管加不同浓度的各种筛选的阿片配体类药物,终体积为100μl,30℃孵育30min,置冰水中终止反应。在Millipore样品收集器上经GF/C(whatman)玻璃纤维滤纸负压抽滤。用4ml 50mM Tris-HCl(pH 7.4)冲洗三次,滤纸烘干后,置于0.5ml Eppendorf管,加0.5ml亲脂闪烁液,PERKIN ELMER PRI-CARB 2910液体闪烁计数仪测定放射性强度,计算抑制率,实验重复三次以上,每组三复管。
抑制率(或称结合率)=(总结合率dpm-样品管dpm)/(总结合管dpm-非特异性结合管dpm)×100%
用Graphpad Prism 5.0软件计算IC50。按下式计算Ki值,Ki=IC50/(1+[L]/Kd),[L]为所加标记配体的浓度,Kd为标记配体的平衡解离参数
表1为代表性化合物对阿片受体的亲和常数Ki值,采用三次独立的测量平均值±标准偏差表示。
表1化合物1μM浓度下阿片受体结合率或Ki
a.在0.1μM下抑制率或结合率。
表1的“结合率(%)或Ki(nM)”一栏中,用百分比表示的数值是指结合率,以nM为单位的数值是指Ki。
从表1可以看出,本发明化合物均表现出比曲马多更强或相当的阿片受体亲和力。
实施例34
体内热板法镇痛试验
将体重20g左右雌性小鼠放在预热至55℃热板仪上,以小鼠舔后足反应的潜伏期为痛阈指标。实验前筛选动物,将反应潜伏期小于5s或大于30s的动物剔除。为防止足部烫伤,最长观察时间设为60s。基础痛阈为2次测量值的平均值,两次测量之间相隔5min。各组小鼠痛阈值分别在腹腔给药后15分钟、30分钟、60分钟和120分钟进行测定。镇痛有效百分率(%MPE)根据以下公式计算:镇痛有效百分率(%MPE)。根据镇痛有效百分率用Graphpadprism 5.0软件计算ED50值。
表2 5mg/kg剂量下化合物热板最大镇痛有效百分比或ED50值
表2的“%MPE或ED50”一栏中,用百分比表示的数值是指%MPE,以mg/kg为单位的数值是指ED50。
从表2可以看出,本发明化合物均表现出比曲马多更强的体内镇痛作用。
Claims (9)
2.根据权利要求1所述的化合物,或其药学上可接受的盐,其特征在于,R1选自甲基、三氟甲基。
4.根据权利要求1或2所述的化合物,或其药学上可接受的盐,其特征在于,R3选自氢、氟代甲基。
5.根据权利要求1或2所述的化合物,或其药学上可接受的盐,其特征在于,式(FWBH)化合物选自:
1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-((3-氯苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((4-甲基苄基)磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(苯乙基磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((3-(三氟甲基)苄基)磺酰基)哌啶-4-醇;
1-((4-氯苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-1-((4-氟苄基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-((3-溴苄基)磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-1-((2-氟苄基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-(丁基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((3-硝基苄基)磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(苯基磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-((4-(三氟甲基)苄基)磺酰基)哌啶-4-醇;
3-((二甲氨基)甲基)-1-(乙基磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(丙基磺酰基)哌啶-4-醇;
1-(烯丙基磺酰基)-3-((二甲氨基)甲基)-4-(3-甲氧基苯基)哌啶-4-醇;
(1R,4S)-1-(((3-((二甲氨基)甲基)-4-羟基-4-(3-甲氧基苯基)哌啶-1-基)磺酰基)甲基)-7,7-二甲双环[2.2.1]庚-2-酮;
3-((二甲氨基)甲基)-1-((((1R,4S)-2-羟基-7,7-二甲基双环[2.2.1]庚-1-基)甲基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
3-((二甲氨基)甲基)-4-(3-甲氧基苯基)-1-(甲磺酰基)哌啶-4-醇;
(1S,4R)-1-(((3-((二甲氨基)甲基)-4-羟基-4-(3-甲氧基苯基)哌啶-1-基)磺酰基)甲基)-7,7-二甲基双环[2.2.1]庚-2-酮;
3-((二甲氨基)甲基)-1-((((1S,4R)-2-羟基-7,7-二甲基双环[2.2.1]庚-1-基)甲基)磺酰基)-4-(3-甲氧基苯基)哌啶-4-醇;
1-(苄基磺酰基)-3-((二甲氨基)甲基)-4-(3-(三氟甲氧基)苯基)哌啶-4-醇;
1-(1-(苄基磺酰基)-4-(二氟甲氧基)-4-(3-甲氧基苯基)哌啶-3-基)-N,N-二甲氨基甲胺。
6.一种药物组合物,其特征在于,包括:权利要求1至5中任一项所述的化合物或其药学上可接受的盐;以及药学上可接受的载体。
7.权利要求1-5中任一项所述的化合物或者其药学上可接受的盐类在制备治疗与阿片受体相关适应症的药物中的用途。
8.如权利要求7所述的用途,其特征在于,所述与阿片受体相关适应症为疼痛、肠易激综合征、瘙痒、成瘾、抑郁症。
9.如权利要求8所述的用途,其特征在于,所述的疼痛包括治疗或缓解手术期间的疼痛、慢性疼痛、神经性疼痛、癌性疼痛。
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911076330.8A CN112759546B (zh) | 2019-11-06 | 2019-11-06 | 3-(二甲氨基甲基)哌啶-4-醇衍生物及其制备方法和药物用途 |
| US17/755,780 US20230027752A1 (en) | 2019-11-06 | 2020-11-02 | Opioid receptor agonist, preparation method therefor and pharmaceutical use thereof |
| PCT/CN2020/125799 WO2021088758A1 (zh) | 2019-11-06 | 2020-11-02 | 一类阿片受体激动剂及其制备方法和药物用途 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911076330.8A CN112759546B (zh) | 2019-11-06 | 2019-11-06 | 3-(二甲氨基甲基)哌啶-4-醇衍生物及其制备方法和药物用途 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN112759546A CN112759546A (zh) | 2021-05-07 |
| CN112759546B true CN112759546B (zh) | 2022-08-26 |
Family
ID=75692750
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201911076330.8A Active CN112759546B (zh) | 2019-11-06 | 2019-11-06 | 3-(二甲氨基甲基)哌啶-4-醇衍生物及其制备方法和药物用途 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112759546B (zh) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117447388B (zh) * | 2022-12-15 | 2024-05-10 | 南昌双天使生物科技开发有限公司 | 氘代的4-苯基哌啶-4-醇类化合物的制备及其用途 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1157815A (zh) * | 1995-12-20 | 1997-08-27 | 格吕伦塔尔有限公司 | 用作药物活性成分的1-苯基-2-二甲氨基甲基-环己-1-醇化合物 |
| CN1547570A (zh) * | 2000-09-29 | 2004-11-17 | 取代的c-环己基甲胺衍生物 | |
| CN105646332A (zh) * | 2014-11-09 | 2016-06-08 | 复旦大学 | 氨基甲基哌啶类衍生物及其制备方法和药物用途 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10153346A1 (de) * | 2001-10-29 | 2004-04-22 | Grünenthal GmbH | Substituierte Indole |
| DE10153347A1 (de) * | 2001-10-29 | 2003-05-08 | Gruenenthal Gmbh | Substituierte 1H-Chinolin-2-on-Verbindungen |
| DE10153345A1 (de) * | 2001-10-29 | 2003-05-08 | Gruenenthal Gmbh | Substituierte 1H-Chinoxalin-2-on-Verbindungen und substituierte 4-Aryl- und 4-Heteroarylcyclohexan-Verbindungen |
| DE10153348A1 (de) * | 2001-10-29 | 2003-05-08 | Gruenenthal Gmbh | Substituierte Benzo(b)azepin-2-on-Verbindungen |
-
2019
- 2019-11-06 CN CN201911076330.8A patent/CN112759546B/zh active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1157815A (zh) * | 1995-12-20 | 1997-08-27 | 格吕伦塔尔有限公司 | 用作药物活性成分的1-苯基-2-二甲氨基甲基-环己-1-醇化合物 |
| CN1547570A (zh) * | 2000-09-29 | 2004-11-17 | 取代的c-环己基甲胺衍生物 | |
| CN105646332A (zh) * | 2014-11-09 | 2016-06-08 | 复旦大学 | 氨基甲基哌啶类衍生物及其制备方法和药物用途 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN112759546A (zh) | 2021-05-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI403511B (zh) | 脯胺酸衍生物之鹽、或其溶媒合物,以及其製造方法 | |
| CA2401711C (en) | Cyclic amide derivatives | |
| AU2011208139B2 (en) | Piperazine compound having a PGDS inhibitory effect | |
| TW201311674A (zh) | 吲唑-及吡咯并吡啶-衍生物及其醫藥用途 | |
| CN107530349B (zh) | 作为a2b拮抗剂的黄嘌呤取代的炔基氨基甲酸酯/反式氨基甲酸酯 | |
| JP6498672B2 (ja) | 疼痛に対して多重モードの活性を有するピペリジン化合物 | |
| TWI444376B (zh) | 脯胺醯胺吡啶化合物、其藥學組成物及醫藥用途 | |
| TW200526573A (en) | Organic compounds | |
| JP6585718B2 (ja) | 抗ヒスタミン剤としての新規ベンゾイミダゾール誘導体 | |
| EP1603914B1 (en) | Compounds having activity at 5ht2c receptor and uses thereof | |
| CN112759545B (zh) | 3-(二甲氨基甲基)哌啶-4-醇类衍生物及其制备方法和药物用途 | |
| CN112759587B (zh) | 3-(二甲氨基甲基)哌啶-4-醇类衍生物及其制备方法和药物用途 | |
| JP2011518213A (ja) | 1,3−ジヒドロ−2H−ピロロ[3,2−b]ピリジン−2−オン誘導体、この調製、およびこの治療用途 | |
| CN112759544B (zh) | 3-(二甲氨基甲基)哌啶-4-醇衍生物制备方法和药物用途 | |
| CN112759546B (zh) | 3-(二甲氨基甲基)哌啶-4-醇衍生物及其制备方法和药物用途 | |
| JP2007530648A (ja) | Cns障害の処置のための、5−ht6受容体アンタゴニストとしての3−((ヘテロ)アリールスルフォニル)−8−((アミノアルキル)オキシ)キノリン | |
| CZ290442B6 (cs) | (R)-5-Brom-N-(1-ethyl-4-methylhexahydro-1H-1,4-diazepin-6-yl)-2-methoxy-6-methylamino-3-pyridinkarboxamid, způsob jeho přípravy a farmaceutický prostředek, který jej obsahuje | |
| CN112759538B (zh) | 3-(二甲氨基甲基)环己-4-醇衍生物及其制备方法和药物用途 | |
| WO2004056788A1 (en) | Benzoxazocines and their use as monoamine-reuptake inhibitors | |
| CA2317515A1 (en) | Oxazole derivatives as serotonin-1a receptor agonists | |
| US20240182443A1 (en) | Polymorphic forms of compound and preparation method therefor and application thereof | |
| CN117447385A (zh) | 氘代的3-氨甲基-4-苯基哌啶-4-醇类化合物的制备及其用途 | |
| CN115246817B (zh) | 化合物A苯甲酸盐的晶型β及其制备方法和含有该晶型的药物组合物 | |
| WO2019015640A1 (zh) | 一种氮杂环酰胺衍生物的盐、其晶型及其制备方法和用途 | |
| US10435408B2 (en) | Azepine derivatives as 5-HT7 receptor modulators |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |