CN112661882B - Application of cyclohexene-1,2-dicarboxylic acid ester compound - Google Patents
Application of cyclohexene-1,2-dicarboxylic acid ester compound Download PDFInfo
- Publication number
- CN112661882B CN112661882B CN201910983439.3A CN201910983439A CN112661882B CN 112661882 B CN112661882 B CN 112661882B CN 201910983439 A CN201910983439 A CN 201910983439A CN 112661882 B CN112661882 B CN 112661882B
- Authority
- CN
- China
- Prior art keywords
- dimethyl ether
- butyl
- pentyl
- propyl
- methyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Landscapes
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Transition And Organic Metals Composition Catalysts For Addition Polymerization (AREA)
Abstract
The invention provides application of a cyclohexene-1,2-diformate compound shown as a formula (I) as an electron donor, in particular to an internal electron donor in a catalyst system for olefin polymerization. In addition, when the cyclohexene-1,2-dicarboxylic acid ester compound shown in the formula (I) and the diether compound are compounded to be used as an internal electron donor, the obtained polymer has wider molecular weight distribution.
Description
Technical Field
The invention belongs to the field of olefin polymerization catalysts, and particularly relates to an application of cyclohexene-1,2-dicarboxylate in a solid catalyst component for olefin polymerization.
Background
Solid titanium catalyst group with magnesium, titanium, halogen and internal electron donor as basic componentsSeparately, ziegler-Natta (Z-N) catalysts, well known in the art, may be used for CH 2 = CHR olefin polymerization polymer with higher yield and higher stereoregularity can be obtained especially in alpha-olefin polymerization having 3 or more carbon atoms. It is well known that internal electron donor compounds are one of the essential components in Ziegler-Natta catalyst components. Starting from the fourth generation of Z-N catalysts, the alternation of each generation of catalysts is marked by the use of a novel internal electron donor. In recent years, the development of fifth generation Ziegler-Natta, i.e., non-phthalate Z-N, catalysts has been a focus of the polypropylene industry and academia. The fifth generation catalyst uses a novel non-phthalate internal electron donor as a main sign. Specifically, the internal electron donor is 1,3-diether and succinate, which are invented by BASELL corporation, and 1,3-diol ester, which is developed by my hospital. Compared with the phthalate internal electron donor used by the traditional fourth-generation Z-N catalyst, the fifth-generation catalyst not only avoids the use of phthalate (plasticizer), but also endows the catalyst and resin with unique performances. Different internal electron donors can endow the catalyst with different properties, for example, 1,3-diether internal electron donors can enable the catalyst to have good hydrogen regulation sensitivity, but the molecular weight distribution of the polymer is narrow. In summary, the performance of the catalyst is continuously improved, and one of the key factors is the replacement of the internal electron donor during the preparation of the catalyst. It can be said that the internal electron donor plays a very important role in the development of polypropylene catalysts.
Disclosure of Invention
The invention solves the first technical problem by providing a novel internal electron donor which can be used in an olefin polymerization catalyst and widens the selection range of the internal electron donor.
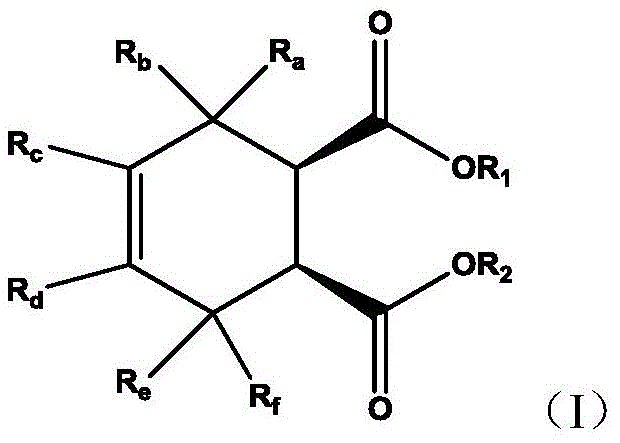
In a first aspect, the invention provides a use of a cyclohexene-1,2-dicarboxylate compound shown in formula (I) as an electron donor, especially an internal electron donor, in a catalyst system for olefin polymerization,
in the formula (I), R 1 And R 2 Are the same or different and are each independently selected from C 1 -C 20 Straight chain alkyl, C 3 -C 20 Branched alkyl or cycloalkyl, C 6 -C 20 Aryl radical, C 7 -C 20 Alkaryl and C 7 -C 20 Aralkyl, any of said alkyl, cycloalkyl, aryl, alkaryl and aralkyl groups being optionally substituted by one or more substituents selected from C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy, hydroxy, halogen, cyano, nitro, amino, mono-C 1 -C 10 Alkylamino and bis-C 1 -C 10 An alkylamino group; or R 1 And R 2 Can be connected into a ring in any way; r a 、R b 、R c 、R d 、R e And R f The same or different, each independently selected from hydrogen and C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy and halogen.
According to a preferred embodiment of the invention, in formula (I), R 1 And R 2 Identical or different, R 1 And R 2 Each independently selected from C 1 -C 10 Straight chain alkyl, C 3 -C 10 Branched alkyl or cycloalkyl, C 6 -C 10 And (4) an aryl group.
According to a preferred embodiment of the invention, in formula (I), R 1 And R 2 Is selected from C 1 -C 6 Straight-chain or branched alkyl, in particular selected from methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, n-pentyl, isopentyl, tert-pentyl, cyclopentyl and phenyl.
According to some embodiments of the invention, the cyclohexene-1,2-dicarboxylate compound is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylic acid methyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid ethyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-propyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isopropyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-butyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isobutyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-pentyl ester, and cis-4-cyclohexene-1,2-dicarboxylic acid isoamyl ester.
According to a preferred embodiment of the invention, said cyclohexene-1,2-dicarboxylate compound is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylic acid methyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid ethyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-propyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isopropyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-butyl ester and cis-4-cyclohexene-1,2-dicarboxylic acid isobutyl ester.
According to a preferred embodiment of the invention, said cyclohexene-1,2-dicarboxylate compound is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylate, cis-4-cyclohexene-1,2-n-propyl dicarboxylate, cis-4-cyclohexene-1,2-dicarboxylate, cis-4-cyclohexene-1,2-n-butyl dicarboxylate and cis-4-cyclohexene-1,2-dicarboxylate.
In a second aspect, the present invention provides a solid catalyst component for olefin polymerization, which comprises magnesium, titanium, halogen and an internal electron donor, wherein the internal electron donor comprises a first internal electron donor compound, the first internal electron donor compound is a cyclohexene-1,2-dicarboxylate compound shown in formula (I),
in the formula (I), R 1 And R 2 Are the same or different and are each independently selected from C 1 -C 20 Straight chain alkyl, C 3 -C 20 Branched alkyl or cycloalkyl, C 6 -C 20 Aryl radical, C 7 -C 20 Alkylaryl and C 7 -C 20 Aralkyl, any of said alkyl, cycloalkyl, aryl, alkaryl and aralkyl groups being optionally substituted by one or more substituents selected from C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy, hydroxy, halogen, cyano, nitro, amino, mono-C 1 -C 10 Alkylamino and bis-C 1 -C 10 An alkylamino group; or R 1 And R 2 Can be connected into a ring in any mode; r a 、R b 、R c 、R d 、R e And R f The same or different, each independently selected from hydrogen and C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy and halogen.
According to a preferred embodiment of the invention, in formula (I), R 1 And R 2 Identical or different, R 1 And R 2 Each independently selected from C 1 -C 10 Straight chain alkyl, C 3 -C 10 Branched alkyl or cycloalkyl, C 6 -C 10 And (4) an aryl group.
According to a preferred embodiment of the invention, in formula (I), R 1 And R 2 Is selected from C 1 -C 6 Straight-chain or branched alkyl, in particular selected from methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, n-pentyl, isopentyl, tert-pentyl, cyclopentyl and phenyl.
According to some embodiments of the invention, the cyclohexene-1,2-dicarboxylate compound is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylic acid methyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid ethyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-propyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isopropyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-butyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isobutyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-pentyl ester, and cis-4-cyclohexene-1,2-dicarboxylic acid isoamyl ester.
According to a preferred embodiment of the invention, said cyclohexene-1,2-dicarboxylate compound is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylic acid methyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid ethyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-propyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isopropyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-butyl ester and cis-4-cyclohexene-1,2-dicarboxylic acid isobutyl ester.
According to a preferred embodiment of the invention, said cyclohexene-1,2-dicarboxylate compound is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylate, cis-4-cyclohexene-1,2-n-propyl dicarboxylate, cis-4-cyclohexene-1,2-dicarboxylate, cis-4-cyclohexene-1,2-n-butyl dicarboxylate and cis-4-cyclohexene-1,2-dicarboxylate.
The inventor of the invention finds that when the Z-N catalyst prepared by compounding the cyclohexene-1,2-diformate compound and the diether compound shown in the formula (II) as the internal electron donor is used for olefin polymerization, the polymer has wider molecular weight distribution, so that the problem of narrow molecular weight distribution of the polymer when only the 1,3-diether internal electron donor is used is solved.
According to some preferred embodiments of the present invention, the internal electron donor further comprises a second internal electron donor compound, preferably the second internal electron donor compound is a diether compound represented by formula (II),
in the formula (II), R 3 And R 4 The same or different, each independently selected from hydrogen and C 1 -C 20 Straight chain alkyl, C 3 -C 20 Branched alkyl or cycloalkyl, C 6 -C 20 Aryl radical, C 7 -C 20 Alkylaryl and C 7 -C 20 Aralkyl, any of said alkyl, cycloalkyl, aryl, alkaryl and aralkyl groups being optionally substituted by one or more substituents selected from C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy, hydroxy, halogen, cyano, nitro, amino, mono-C 1 -C 10 Alkylamino and bis-C 1 -C 10 Alkylamino, the carbon atoms of the backbone optionally being substituted with heteroatoms; or R 3 And R 4 May be linked to form a ring in any manner, and may contain a double bond or a hetero atom in the resulting ring skeleton; r 5 、R 6 、R 9 And R 10 The same or different, each independently selected from hydrogen and C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy and halogen; r 7 And R 8 Are the same or different and are each independently selected from C 1 -C 10 An alkyl group.
According to some embodiments of the invention, in formula (II), R 3 And R 4 Is selected from C 1 -C 10 Straight chain alkyl, C 3 -C 10 Branched alkyl or cycloalkyl, C 6 -C 10 And (4) an aryl group.
According to a preferred embodiment of the invention, in formula (II), R 3 And R 4 Is selected from C 1 -C 6 Straight-chain or branched alkyl, in particular selected from methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, pentyl, isopentyl, tert-pentyl and phenyl.
According to a preferred embodiment of the invention, in formula (II), R 7 And R 8 Are the same or different and are each independently selected from C 1 -C 6 Alkyl, preferably selected from methyl, ethyl and isopropyl.
According to some embodiments of the invention, the dimethyl ether-based compound is selected from the group consisting of ' -dimethyl ether, ' -diethyl-dimethyl ether, ' -di-n-propyl-dimethyl ether, ' -diisopropyl-dimethyl ether, ' -di-n-butyl-dimethyl ether, ' -diisobutyl-dimethyl ether, ' -ditert-butyl-dimethyl ether, ' -di-n-pentyl-dimethyl ether, ' -diisoamyl-dimethyl ether, ' -di (1-methyl) butyl-dimethyl ether, ' -di (2-methyl) butyl-dimethyl ether, ' -di (1-ethyl) propyl-dimethyl ether, ' -ditert-pentyl-dimethyl ether, ' -di-n-hexyl-dimethyl ether, ' -diisohexyl-dimethyl ether, ' -di (1-methyl) pentyl-dimethyl ether, ' -di (2-methyl) pentyl-dimethyl ether, ' -di (3-methyl) pentyl-dimethyl ether, ' -di (1-ethyl) butyl-dimethyl ether, ' -di (2-ethyl) butyl-dimethyl ether, ' -ditert-hexyl-dimethyl ether, 2-methyl-2-ethyl-1,3-dimethyl ether, 2-methyl-2-n-propyl-1,3-dimethyl ether, 2-methyl-2-isopropyl-1,3-dimethyl ether, 2-methyl-2-n-butyl-1,3-dimethyl ether, 2-methyl-2-isobutyl-1,3-dimethyl ether, 2-methyl-2-n-pentyl-1,3-dimethyl ether, 2-methyl-2-isoamyl-1,3-dimethyl ether, 2-methyl-2-n-hexyl-1,3-dimethyl ether, 2-methyl-2-isohexyl-1,3-dimethyl ether, 2-ethyl-2-n-propyl-1,3-dimethyl ether 2-ethyl-2-isopropyl-1,3-dimethyl ether, 2-ethyl-2-n-butyl-1,3-dimethyl ether, 2-ethyl-2-isobutyl-1,3-dimethyl ether, 2-ethyl-2-n-pentyl-1,3-dimethyl ether, 2-ethyl-2-isoamyl-1,3-dimethyl ether, 2-ethyl-2-n-hexyl-1,3-dimethyl ether, 2-ethyl-2-isohexyl-1,3-dimethyl ether, 2-n-propyl-2-isopropyl-1,3-dimethyl ether, 2-n-propyl-2-n-butyl-1,3-dimethyl ether, 2-n-propyl-2-isobutyl-1,3-dimethyl ether, 2-n-propyl-2-n-pentyl-1,3-dimethyl ether, 2-n-propyl-2-isoamyl-1,3-dimethyl ether, 2-n-propyl-2-n-hexyl-1,3-dimethyl ether, 2-n-propyl-2-isohexyl-1,3-dimethyl ether, 2-isopropyl-2-n-butyl-3535-dimethyl ether, 2-isopropyl-2-isobutyl-1,3-dimethyl ether, 2-isopropyl-2-n-pentyl-1,3-dimethyl ether, 2-isopropyl-2-isoamyl-1,3-dimethyl ether, 2-isopropyl-2-n-hexyl-1,3-dimethyl ether 2-isopropyl-2-isohexyl-1,3-dimethyl ether, 2-n-butyl-2-isobutyl-1,3-dimethyl ether, 2-n-butyl-2-n-pentyl-1,3-dimethyl ether, 2-n-butyl-2-isopentyl-1,3-dimethyl ether, 2-n-butyl-2-n-hexyl-1,3-dimethyl ether, 2-n-butyl-2-isohexyl-1,3-dimethyl ether, 2-isobutyl-2-n-pentyl-1,3-dimethyl ether, 2-isobutyl-2-isopentyl-1,3-dimethyl ether, 2-isobutyl-2-n-hexyl-1,3-dimethyl ether, 2-isobutyl-2-isohexyl-1,3-dimethyl ether, 2-n-pentyl-2-isopentyl-1,3-dimethyl ether, 2-n-pentyl-2-n-hexyl-1,3-dimethyl ether, 2-n-pentyl-2-isohexyl-1,3-dimethyl ether, 2-isopentyl-2-n-hexyl-1,3-dimethyl ether, 2-isopentyl-2-isohexyl-1,3-dimethyl ether, and 2-n-hexyl-2-isohexyl-1,3-dimethyl ether.
According to a preferred embodiment of the invention, the dimethyl ethers are selected from the group consisting of ' -dimethyl ether, ' -diethyl-dimethyl ether, ' -di-n-propyl-dimethyl ether, ' -diisopropyl-dimethyl ether, ' -di-n-butyl-dimethyl ether, ' -diisobutyl-dimethyl ether, ' -di-tert-butyl-dimethyl ether, ' -di-n-pentyl-dimethyl ether, ' -diisoamyl-dimethyl ether, ' -di (1-ethyl) propyl-dimethyl ether, ' -ditert-pentyl-dimethyl ether, ' -di-n-hexyl-dimethyl ether, ' -diisohexyl-diethyl-dimethyl ether, ' -di (2-ethyl) butyl-dimethyl ether, ' -ditert-hexyl-dimethyl ether, 2-methyl-2-ethyl-dimethyl ether, 2-methyl-2-n-propyl-dimethyl ether, 2-methyl-2-isopropyl-dimethyl ether, 2-methyl-2-n-butyl-dimethyl ether, 2-methyl-2-isobutyl-dimethyl ether, 2-methyl-2-n-pentyl-dimethyl ether, 2-methyl-2-isoamyl-dimethyl ether, 2-methyl-2-isohexyl-1,3-dimethyl ether, 2-ethyl-2-n-propyl-1,3-dimethyl ether, 2-ethyl-2-isopropyl-1,3-dimethyl ether, 2-ethyl-2-n-butyl-1,3-dimethyl ether, 2-ethyl-2-isobutyl-1,3-dimethyl ether, 2-ethyl-2-n-pentyl-1,3-dimethyl ether, 2-ethyl-2-isoamyl-1,3-dimethyl ether, 2-ethyl-2-isohexyl-1,3-dimethyl ether, 2-n-propyl-2-isopropyl-1,3-dimethyl ether, 2-n-butyl-1,3-dimethyl ether 2-n-propyl-2-isobutyl-1,3-dimethyl ether, 2-n-propyl-2-n-pentyl-1,3-dimethyl ether, 2-n-propyl-2-isoamyl-1,3-dimethyl ether, 2-n-propyl-2-isohexyl-1,3-dimethyl ether, 2-isopropyl-2-n-butyl-1,3-dimethyl ether, 2-isopropyl-2-isobutyl-1,3-dimethyl ether, 2-isopropyl-2-n-pentyl-1,3-dimethyl ether, 2-isopropyl-2-isoamyl-1,3-dimethyl ether, 2-isopropyl-2-isohexyl-1,3-dimethyl ether, 2-n-butyl-2-isobutyl-1,3-dimethyl ether, 2-n-butyl-2-n-pentyl-1,3-dimethyl ether, 2-n-butyl-2-isoamyl-1,3-dimethyl ether, 2-n-butyl-2-isohexyl-1,3-dimethyl ether, 2-isobutyl-2-n-pentyl-1,3-dimethyl ether, 2-isobutyl-2-isoamyl-1,3-dimethyl ether, 2-isobutyl-2-isohexyl-1,3-dimethyl ether, 2-n-pentyl-2-isoamyl-1,3-dimethyl ether, 2-n-pentyl-2-isohexyl-1,3-dimethyl ether, 2-isopentyl-2-n-hexyl-1,3-dimethyl ether, 2-isoamyl-2-isohexyl-3282-and 2-n-hexyl-3434-dimethyl ether.
According to a preferred embodiment of the present invention, the dimethyl ether compound is selected from 2,2 '-dimethyl-1,3-dimethyl ether, 2,2' -diethyl-1,3-dimethyl ether, 2,2 '-di-n-propyl-1,3-dimethyl ether, 2,2' -diisopropyl-2,2-dimethyl ether, 2,2 '-di-n-butyl-2,2-dimethyl ether, 2,2' -diisobutyl-2,2-dimethyl ether, 2,2 '-di-n-pentyl-2,2-dimethyl ether, 2,2' -diisoamyl-6258-dimethyl ether, 2,2 '-di-hexyl-2,2-dimethyl ether, 6258 zxft 58' -diisohexyl-2,2-dimethyl ether, 6258 2-methyl-2-ethyl-2,2-dimethyl ether, 2-methyl-2-isopropyl-2,2-dimethyl ether, 2-methyl-2-isobutyl-2,2-dimethyl ether, 2-methyl-2-isoamyl-2,2-dimethyl ether, 2-ethyl-2-isopropyl-2,2-dimethyl ether, 2-ethyl-2-isobutyl-2,2-dimethyl ether, 2-ethyl-2-isoamyl-2,2-dimethyl ether, 2-ethyl-2-isohexyl-2,2-dimethyl ether, 2-n-propyl-2-isopropyl-2,2-dimethyl ether, 2-n-propyl-2-isobutyl-2,2-dimethyl ether, 2-n-propyl-2-isoamyl-2,2-dimethyl ether, 2-n-propyl-2-isohexyl-1,3-dimethyl ether, 2-isopropyl-2-n-butyl-1,3-dimethyl ether, 2-isopropyl-2-isobutyl-1,3-dimethyl ether, 2-isopropyl-2-n-pentyl-1,3-dimethyl ether, 2-isopropyl-2-isoamyl-1,3-dimethyl ether, 2-isopropyl-2-isohexyl-1,3-dimethyl ether, 2-n-butyl-2-isobutyl-1,3-dimethyl ether, 2-n-butyl-2-isoamyl-1,3-dimethyl ether, 2-n-butyl-2-isohexyl-1,3-dimethyl ether, 2-isobutyl-2-n-pentyl-1,3-dimethyl ether, 2-isobutyl-2-isoamyl-1,3-isobutyl-2-isohexyl-4924-isopentyl-4924-n-pentyl-4924-zxft 4924-dimethyl ether and at least one of the group of the above-n-propyl-2-isobutyl-2-isopentyl-3735-dimethyl ether.
According to some embodiments of the present invention, the molar ratio of the first internal electron donor compound and the second internal electron donor compound is from 0.1.
According to some embodiments of the present invention, the titanium atom is present in an amount of 1.0 to 8.0wt%, preferably 1.6 to 6.0wt%, based on the total amount of the catalyst components; the content of magnesium atoms is 10 to 70wt%, preferably 15 to 40wt%; the content of halogen atoms is 20 to 90wt%, preferably 30 to 85 wt%; the total internal electron donor compound content is 2-30wt%, preferably 3-20wt%.
The preparation method of the solid catalyst component can be that a magnesium compound, a titanium compound and an internal electron donor are contacted and reacted under certain conditions. The amounts of the titanium compound, the magnesium compound and the internal electron donor used for preparing the solid catalyst component are not particularly limited and may be those conventionally used in the art, respectively.
According to a preferred embodiment of the present invention, the magnesium compound may be at least one of a magnesium compound represented by formula (III), a hydrate of the magnesium compound represented by formula (III), and an alcohol adduct of the magnesium compound represented by formula (III),
MgR 11 R 12 (III)
in the formula (III), R 11 And R 12 Each selected from at least one of halogen, C1-5 linear or branched alkoxy and C1-5 linear or branched alkyl, preferably R 11 And R 12 Each selected from halogens, for example at least one selected from chlorine, bromine and iodine.
In the solid catalyst component of the present invention, the hydrate of the magnesium compound represented by the formula (III) is MgR 11 R 12 ·qH 2 O, wherein q is in the range of 0.1 to 6, preferably 2 to 3.5; the alcohol adduct is MgR 11 R 12 ·pR 0 OH, wherein R 0 Is a hydrocarbon group having 1 to 18 carbon atoms, preferably an alkyl group having 1 to 5 carbon atoms, more preferably at least one of a methyl group, an ethyl group, an n-propyl group and an isopropyl group; p is in the range of 0.1 to 6, preferably 2 to 3.5.
According to a preferred embodiment of the present invention, the magnesium compound is selected from at least one of dimethoxymagnesium, diethoxymagnesium, dipropoxymagnesium, diisopropoxymagnesium, dibutoxymagnesium, diisobutoxymagnesium, dipentyloxymagnesium, dihexomagnesium, di (2-methyl) hexyloxymagnesium, methoxymethylmagnesium chloride, methoxymethylmagnesium bromide, methoxymethylmagnesium iodide, ethoxymagnesium chloride, ethoxymagnesium bromide, ethoxymagnesium iodide, propoxymagnesium chloride, propoxymagnesium bromide, propoxymasium iodide, butoxymagnesium chloride, butoxymagnesium bromide, butoxymagnesium iodide, magnesium dichloride, magnesium dibromide, magnesium diiodide, an alcohol adduct of magnesium dichloride, an alcohol adduct of magnesium dibromide, and an alcohol adduct of magnesium diiodide. Most preferably, the magnesium compound is diethoxymagnesium or magnesium dichloride.
The solid catalyst component according to the invention in which the titanium compound is a compound of formula (IV),
TiX m (OR 13 ) 4-m (IV)
in formula (IV), X is a halogen, for example selected from chlorine, bromine and iodine; r is 13 Is a hydrocarbon group having 1 to 20 carbon atoms,preferably an alkyl group having 1 to 5 carbon atoms; m is an integer from 0 to 4, for example m may be 0, 1,2, 3 or 4.
According to a preferred embodiment of the present invention, the titanium compound is at least one selected from the group consisting of titanium tetrachloride, titanium tetrabromide, titanium tetraiodide, titanium tetrabutoxide, titanium tetraethoxide, titanium monochlorotriethoxyxide, titanium dichlorodiethoxide and titanium trichloromonoethoxyxide. Most preferably, the titanium compound is titanium tetrachloride.
In the present invention, the method of preparing the olefin polymerization solid catalyst component of the present invention by reacting a titanium compound, a magnesium compound and an internal electron donor may be performed by a method of preparing an olefin catalyst component, which is conventional in the art. The solid catalyst component of the present invention can be prepared, for example, by the following method.
Method one, with reference to the CN102453150B method, the catalyst component was prepared as follows. (1) Contacting a magnesium alkoxide or magnesium alkoxide halide compound with a titanium compound and an internal electron donor compound in the presence of an inert diluent; (2) Washing the solid obtained by the step (1) with an inert solvent to obtain a solid catalyst component.
The magnesium alkoxide compound used in the first process may be selected from one or a mixture of dimethoxy magnesium, diethoxy magnesium, dipropoxy magnesium, diisopropoxy magnesium, dibutoxy magnesium, diisobutyoxy magnesium, dipentyoxy magnesium, dihexyl magnesium, di (2-methyl) hexyl magnesium, preferably diethoxy magnesium or a mixture of diethoxy magnesium and other alkoxy magnesium. The alkoxy magnesium compound can be prepared by a method known in the art, such as preparing metal magnesium and fatty alcohol in the presence of a small amount of iodine.
The alkoxy magnesium halide compound used in the first process may be selected from at least one of methoxy magnesium chloride, ethoxy magnesium chloride, propoxy magnesium chloride and butoxy magnesium chloride, preferably ethoxy magnesium chloride. The alkoxy magnesium halide compound can be prepared by a method well known in the art, such as preparing magnesium ethoxy chloride by mixing grignard reagent butyl magnesium chloride with tetraethoxy titanium and tetraethoxy silicon.
In step (1) of method oneThe inert diluent is selected from C 6 -C 10 At least one of an alkane or an arene. Specific examples of the inert diluent may employ one or a mixture of hexane, heptane, octane, decane, benzene, toluene, xylene, and preferably toluene. The order of contacting is not particularly limited, and for example, the components may be contacted in the presence of an inert diluent, or the components may be diluted with an inert solvent and then contacted. The number of times of contact is not particularly limited, and may be once or more.
In step (2) of the first process, the inert solvent washing is selected from hydrocarbon compounds, for example, one selected from hexane, heptane, octane, decane, benzene, toluene, xylene or a mixture thereof, preferably hexane.
In step (2) of the first method, the method of washing is not particularly limited, and a method such as decantation or filtration is preferable. The amount of the inert solvent to be used, the washing time and the number of washing times are not particularly limited, and the amount of the inert solvent to be used is usually 1 to 1000 mol, preferably 10 to 500 mol, based on 1 mol of the magnesium compound, and the washing time is usually 1 to 24 hours, preferably 10 to 6 hours. In addition, from the viewpoint of washing uniformity and washing efficiency, it is preferable to carry out stirring during the washing operation. It is to be noted that the obtained solid catalyst component may be stored in a dry state or in an inert solvent.
The amount of each component used in the first process is 0.5 to 100 moles, preferably 1 to 50 moles, per mole of magnesium; the inert diluent is used in an amount of 0.5 to 100 moles, preferably 1 to 50 moles; the total amount of the internal electron donor compound is usually 0.005 to 10 moles, preferably 0.01 to 1 mole.
The contact temperature of the components in the first method is usually-40-200 ℃, and preferably-20-150 ℃; the contact time is usually 1 minute to 20 hours, preferably 5 minutes to 8 hours.
The second method, referring to the method of patent CN85100997, dissolves magnesium dihalide in a solvent system composed of organic epoxy compound, organic phosphorus compound and inert diluent to form a uniform solution, then mixes the uniform solution with titanium compound, and precipitates solid in the presence of precipitation assistant; then the solid is contacted with an internal electron donor to be carried on the solid to obtain the solid catalyst component.
The secondary precipitant used in the second method may be at least one of an organic acid anhydride, an organic acid, an ether and a ketone. Specific examples of the organic acid anhydride may be at least one of acetic anhydride, phthalic anhydride, succinic anhydride, maleic anhydride, and the like, specific examples of the organic acid may be at least one of acetic acid, propionic acid, butyric acid, acrylic acid, methacrylic acid, and the like, specific examples of the ether may be at least one of methyl ether, ethyl ether, propyl ether, butyl ether, and pentyl ether, and the ketone may be at least one of acetone, methyl ethyl ketone, and benzophenone.
The organic epoxy compound used in the second process may be at least one selected from the group consisting of ethylene oxide, propylene oxide, butylene oxide, butadiene double oxide, epichlorohydrin, methyl glycidyl ether, diglycidyl ether, and the like, and epichlorohydrin is preferable.
The organophosphorus compound used in the second process may be a hydrocarbyl or halohydrocarbyl ester of orthophosphoric acid or phosphorous acid, and specific examples of the organophosphorus compound include: trimethyl orthophosphate, triethyl orthophosphate, tributyl orthophosphate, triphenyl orthophosphate, trimethyl phosphite, triethyl phosphite, tributyl phosphite, benzyl phosphite, or the like, with tributyl orthophosphate being preferred.
The inert diluent used in the second method may employ at least one of hexane, heptane, octane, decane, benzene, toluene and xylene.
The amount of each component used in the second method is 0.2 to 10 moles, preferably 0.5 to 4 moles, of the organic epoxy compound per mole of the magnesium halide; 0.1 to 3 moles, preferably 0.3 to 1.5 moles of an organophosphorus compound; the titanium compound may be in the range of 0.5 to 20 moles, preferably 5 to 15 moles; the precipitation-assisting component may be 0.01 to 0.3 mol, preferably 0.02 to 0.08 mol; the total amount of the electron donor compound may be 0 to 10 moles, preferably 0.02 to 0.3 moles.
In a third method, referring to the method of CN1091748, a catalyst component is prepared by stirring and dispersing a magnesium chloride alcoholate melt at a high speed in a dispersion system of white oil and silicone oil to form an emulsion, and the emulsion is discharged into a cooling liquid to be rapidly cooled and shaped to form magnesium chloride alcoholate microspheres. The cooling liquid is inert hydrocarbon solvent with low boiling point, such as petroleum ether, pentane, hexane, heptane, etc. The obtained magnesium chloride alcoholate microspheres are washed and dried to form spherical carriers, and the molar ratio of alcohol to magnesium chloride is 2-3, preferably 2-2.5. The particle size of the carrier is 10 to 300 microns, preferably 30 to 150 microns.
Treating the spherical carrier with excessive titanium tetrachloride at low temperature, gradually heating, adding electron donor during the treatment, washing with inert solvent for several times, and drying to obtain solid powdered spherical catalyst. The molar ratio of titanium tetrachloride to magnesium chloride is 20 to 200, preferably 30 to 60; the initial treatment temperature is-30-0 ℃, preferably-25-20 ℃; the final treatment temperature is 80-136 deg.C, preferably 100-130 deg.C.
The spherical catalyst obtained has the following characteristics: 1.5-3.5% of titanium, 6.0-20.0% of ester, 52-60% of chlorine, 10-20% of magnesium and 1-6% of inert solvent.
The method four comprises the following steps: the catalyst component was prepared by the method disclosed in CN 1506384. Firstly, mixing a magnesium compound and an organic alcohol compound with an inert solvent according to a molar ratio of 2-5, heating to 120-150 ℃ to form a uniform solution, and selectively adding phthalic anhydride used as a precipitation aid, a silicon-containing compound or other auxiliary agents beneficial to obtaining good particles; then, according to the molar ratio of titanium/magnesium of 20-50, an alcohol compound and a titanium compound are contacted and reacted for 2-10h, the reaction temperature is-15 to-40 ℃, and the temperature is raised to 90-110 ℃ in the presence of a precipitation aid; adding an internal electron donor compound according to the molar ratio of magnesium/ester of 2-10, reacting for 1-3 hours at 100-130 ℃, and filtering to separate solid particles; then (optionally repeating for 2-3 times) contacting and reacting the solid particles with a titanium compound at a titanium/magnesium molar ratio of 20-50 at 100-130 ℃ for 1.5-3 hours, and filtering to separate out the solid particles; finally, washing the solid particles by using an inert solvent with the temperature of 50-80 ℃, and drying to obtain the catalyst component.
In any of the above four methods for preparing the solid catalyst component of the present invention, the first internal electron donor and the second internal electron donor may be used alone or in combination.
In any of the above four methods for preparing the olefin polymerization catalyst component of the present invention, the internal electron donor can also be added before or during the contacting of the magnesium compound and the titanium compound, for example, in the first method, the internal electron donor is added to the suspension of the alkoxy magnesium or alkoxy magnesium halide in the inert diluent, and then mixed with the titanium compound to prepare the olefin polymerization catalyst; in the second method, the internal electron donor is added into the magnesium halide solution before the magnesium halide solution contacts with the titanide.
In the preparation of the above-mentioned olefin polymerization catalyst component, the molar ratio of the sum of the amounts of the internal electron donor compounds represented by the formula (I) and the formula (II) as internal electron donors to magnesium atoms may be usually 0.01 to 3, preferably 0.02 to 0.3.
In a third aspect, the present invention provides a catalyst system for the polymerization of olefins comprising the reaction product of:
1) The solid catalyst component according to the second aspect,
2) An alkyl-aluminium compound, which is a mixture of,
3) Optionally, an external electron donor compound.
The amount of the aluminum alkyl compound used according to the catalyst system of the present invention may be those conventionally used in the art. Preferably, the alkyl aluminium compound is calculated as aluminium, the catalyst component is calculated as titanium, the molar ratio of the alkyl aluminium compound to the catalyst component is between 5 and 5000:1, preferably 20 to 1000:1, more preferably 50 to 500:1.
in the present invention, the aluminum alkyl compound may be any of various aluminum alkyl compounds commonly used in the field of olefin polymerization, which can be used as a cocatalyst of a Ziegler-Natta type catalyst. Preferably, the alkyl aluminum compound may be a compound represented by formula (V),
AlR' n 'X' 3-n ' (V),
in the formula (V), R' is selected from hydrogen and C 1 -C 20 Alkyl or C 6 -C 20 X 'is halogen and n' is an integer of 1 to 3. Preferably, specific examples of the alkyl aluminum compound may be at least one of trimethylaluminum, triethylaluminum, triisobutylaluminum, trioctylaluminum, diethylaluminum monohydrogen, diisobutylaluminum monohydrogen, diethylaluminum monochloride, diisobutylaluminum monochloride, ethylaluminum sesquichloride and ethylaluminum dichloride.
According to the catalyst system of the present invention, the kind and content of the external electron donor compound are not particularly limited. Preferably, the molar ratio of the alkylaluminum compound to the external electron donor compound, expressed as aluminum, is from 0.1 to 500, preferably from 1 to 300, more preferably from 3 to 100.
According to the catalyst system of the present invention, the external electron donor compound may be any of various external electron donor compounds commonly used in the field of olefin polymerization, which can be used as a co-catalyst of a ziegler-natta type catalyst. Preferably, the external electron donor compound may be an organosilicon compound represented by formula (VI),
R 1” m” R 2” n” Si(OR 3” ) 4-m”-n” (VI),
in the formula (VI), R 1” And R 2” Can be the same or different and are respectively selected from halogen, hydrogen atom and C 1 -C 20 Alkyl of (C) 3 -C 20 Cycloalkyl of, C 6 -C 20 Aryl and C 1 -C 20 One of the haloalkyl groups of (a); r 3” Is selected from C 1 -C 20 Alkyl of (C) 3 -C 20 Cycloalkyl of, C 6 -C 20 Aryl and C 1 -C 20 One of haloalkyl groups of (a); m 'and n' are each an integer of 0 to 3, and m "+ n"<4。
<xnotran> , , , , , , , , , , , , , , , , , , , () , , , , , , , , , , , , , , , , , , , , (2- ) , , </xnotran> At least one of diphenyldiethoxysilane, phenyltriethoxysilane, methyltrimethoxysilane, methyltriethoxysilane, ethyltrimethoxysilane, ethyltriethoxysilane, propyltrimethoxysilane, isopropyltrimethoxysilane, butyltrimethoxysilane, butyltriethoxysilane, isobutyltrimethoxysilane, tert-butyltrimethoxysilane, sec-butyltrimethoxysilane, pentyltrimethoxysilane, isopentyltrimethoxysilane, cyclopentyltrimethoxysilane, cyclohexyltrimethoxysilane, diphenyldimethoxysilane, diphenyldiethoxysilane, phenyltrimethoxysilane, phenyltriethoxysilane, n-propyltrimethoxysilane, vinyltrimethoxysilane, tetramethoxysilane, tetraethoxysilane, tetrabutoxysilane, 2-ethylpiperidinyl-2-tert-butyldimethoxysilane, (1,1,1-trifluoro-2-propyl) -2-ethylpiperidinyldimethoxysilane, and (1,1,1-trifluoro-2-propyl) -methyldimethoxysilane. More preferably, the external electron donor compound may be at least one of dicyclopentyldimethoxysilane, diisopropyldimethoxysilane, diisobutyldimethoxysilane, cyclohexylmethyldimethoxysilane, methyl-t-butyldimethoxysilane, and tetramethoxysilane.
In a fourth aspect, the present invention provides the use of a solid catalyst component according to the second aspect or a catalyst system according to the third aspect in the polymerization of olefins.
According to some embodiments of the present invention, the solid catalyst component and/or the catalyst system of the present invention may be used for homopolymerization of olefins, or for copolymerization of a plurality of olefins. At least one of the olefins is represented by the general formula CH 2 Olefin represented by = CHR where R is hydrogen or C 1 -C 6 Alkyl group of (1). Specific examples of the olefins may include: at least one of ethylene, propylene, 1-n-butene, 1-n-pentene, 1-n-hexene, 1-n-octene, and 4-methyl-1-pentene. Preferably, the olefin may be at least one of ethylene, propylene, 1-n-butene, 4-methyl-1-pentene, and 1-n-hexene. More preferably, the olefin is propylene.
In a fifth aspect, the present invention provides a process for the polymerisation of olefins, the process comprising: contacting one or more olefins, at least one of which is represented by the general formula CH, with the solid catalyst component provided according to the first aspect or/and the catalyst system provided according to the second aspect under olefin polymerization conditions 2 An olefin represented by = CHR, wherein R is hydrogen or C 1 -C 6 Alkyl group of (1).
The method for polymerizing the olefin can be used for homopolymerization of the olefin and copolymerization of a plurality of olefins. Specific examples of the olefins may include: at least one of ethylene, propylene, 1-n-butene, 1-n-pentene, 1-n-hexene, 1-n-octene, and 4-methyl-1-pentene. Preferably, the olefin may be at least one of ethylene, propylene, 1-n-butene, 4-methyl-1-pentene, and 1-n-hexene. More preferably, the olefin is propylene.
According to the present invention, the solid catalyst component, the alkylaluminum compound as cocatalyst and the external electron donor compound may be contacted prior to contacting the olefin monomer, referred to in the art as "precontacting" or "preconplexing"; it is also possible to add the three components separately to the olefin monomer and then carry out the polymerization, i.e.without "precontacting". According to the olefin polymerization process provided by the present invention, it is preferred that the components of the olefin polymerization catalyst system are subjected to a "pre-contact" process. The "precontacting" time is between 0.1 and 30 minutes, preferably between 1 and 10 minutes; the temperature of the "precontacting" is from-20 ℃ to 80 ℃, preferably from 10 ℃ to 50 ℃.
According to the invention, the catalyst system is polymerized to a certain extent in the presence of a small amount of olefin monomer to obtain a prepolymerized catalyst, and the prepolymerized catalyst is further contacted with the olefin monomer to react to obtain the olefin polymer. This technique, known in the industry as a "prepolymerization" process, contributes to, among other things, increasing the polymerization activity of the catalyst and increasing the bulk density of the polymer. According to the olefin polymerization method provided by the invention, a prepolymerization process can be adopted, a prepolymerization process can also be not adopted, and a prepolymerization process is preferably adopted. When the olefin monomer is propylene, the rate of the "prepolymerization" is 5-1000g PP/g Cat, preferably 10-500g PP/g Cat; the temperature of the "prepolymerization" is from-20 ℃ to 80 ℃ and preferably from 10 to 50 ℃.
According to the polymerization method for preparing polyolefin of the present invention, the polymerization conditions may be conventional in the art. The amount of catalyst used may be any of the various catalysts known in the art.
Detailed Description
The present invention will be described in detail below by way of examples. However, the present invention is not limited to the following examples.
In the following examples, the test methods involved are as follows:
1. yield of catalyst component (%) = (mass of obtained catalyst/mass of magnesium chloride used) × 100%;
2. titanium content in catalyst component: measuring with 721 spectrophotometer;
3. particle size distribution of the solids of the catalyst component: measuring by using a Malvern 2000 laser particle size analyzer according to a normal hexane dispersing agent laser diffraction method;
4. purity of internal electron donor compound: measuring by gas chromatography;
5. polymer Melt Index (MI): measured according to GB/T3682-2000;
6. propylene polymer Isotacticity Index (II): measuring by adopting a heptane extraction method; extracting 2g of dried polymer sample in an extractor with boiling heptane for 6 hours, and drying the residue to constant weight to obtain the ratio of the weight (g) of the polymer to 2 (g), namely the isotacticity;
7. polymer molecular weight distribution MWD (MWD = Mw/Mn): using PL-GPC220 and trichlorobenzene as a solvent, the measurement was carried out at 150 ℃ (standard: polystyrene, flow rate: 1.0mL/min, column: 3xPlgel 10um MlxED-B300x7.5nm).
8. And (3) activity calculation: catalyst activity = (mass of polyolefin produced)/(mass of catalyst solid component) g/g
9. And (3) measuring the bulk density: the polymer powder obtained by the preparation is freely dropped into a 100mL container from the height of 10cm in a funnel, the weight of the polymer powder in the container is weighed to be Mg, and the polymer bulk density is M/100g/cm 3 。
1. Synthesis of internal electron donor compound:
a compound A: cis-4-Cyclohexenyl-1,2 Ethyl dicarboxylate
50.0g of cis-4-cyclohexenyl-1,2-dicarboxylic acid and 2.5g of tetrabutylammonium bromide were dissolved in a dried mixed solvent of 500mL of N, N' -dimethylformamide and 100mL of acetone, and stirred until completely dissolved. 101.6g of potassium carbonate was added and stirred until no bubbles were released. 76.9g of bromoethane was added thereto, and the reaction was carried out at 20 ℃ for 1 hour. The temperature is raised to 35 ℃ for reaction for 4 hours, and the temperature is raised to 50 ℃ for reaction for 4 hours. The reaction was stopped, cooled, filtered to remove the solid, washed clean solvent, added with 300mL of water, extracted with ethyl acetate (180mL, 100mL, 80mL) three times, combined with the organic phase, dried over anhydrous magnesium sulfate, filtered, washed free of solvent, and distilled under reduced pressure to obtain 54.5g of cis-4-cyclohexenyl-1,2-dicarboxylic acid ethyl ester as a final product, with a yield of 82.1% and a purity of 99.2% (GC).
Compound B: cis-4-cyclohexenyl-1,2-n-propyl dicarboxylate
Using a synthetic method analogous to that for Compound A, conversion of bromoethane to n-bromopropane produced cis-4-cyclohexenyl-1,2-dicarboxylic acid n-propyl ester 60.3g, yield: 83.6% and 99.0% purity (GC).
Compound C: cis-4-cyclohexenyl-1,2 n-butyl dicarboxylate
Using a synthetic method analogous to that for Compound A, the conversion of bromoethane to n-bromobutane produced cis-4-cyclohexenyl-1,2-n-butyl dicarboxylate 54.3g, yield: 78.4% and 99.6% pure (GC).
Compound D: cis-4-cyclohexenyl-1,2-isobutyl dicarboxylate
Using a synthesis similar to compound A, conversion of bromoethane to bromoisobutane produced cis-4-cyclohexenyl-1,2-isobutyl dicarboxylate 61.3g, yield: 82.5% and 99.1% purity (GC).
2. Preparation of solid catalyst component
Example 1
(1) Preparation of alcohol compound solution
Sequentially adding 20g of anhydrous magnesium chloride, 80mL of toluene and 80mL of isooctanol into a reaction kettle repeatedly substituted by high-purity nitrogen, reacting for 3.0 hours under the conditions of stirring speed of 300rpm and temperature of 110 ℃, adding 3.0mL of tetrabutyl titanate, continuing to react for 1.5 hours, and adding 120mL of toluene to obtain a stable and uniform alcohol compound solution.
(2) Preparation of the catalyst component
75mL of the alcohol compound solution and 2.4g of 2,4-pentanediol benzoate are dropwise added into a reactor which is fully replaced by nitrogen and is filled with 60mL of titanium tetrachloride and 40mL of toluene, the mixture is fully contacted for 1.5 hours at minus 25 ℃ by stirring, then the temperature is raised to 110 ℃ by 3.5 hours, the temperature is kept constant for 1 hour, 108mL of toluene and 12mL of titanium tetrachloride are added, the mixture is stirred for 1 hour, cooled and pressure-filtered, 12mL of titanium tetrachloride and 108mL of toluene are added, the temperature is raised to 100 ℃, a mixture of 1.2g of the compound A and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (the mass ratio is 1:1) is added, and the temperature is kept constant for 1 hour. The temperature was raised to 110 ℃ and 96mL of toluene and 24mL of titanium tetrachloride were added and stirred for 1 hour, and the liquid was removed by pressure filtration and repeated twice. After stirring for 1 hour with 108mL of toluene and 12mL of titanium tetrachloride, the resulting solid was washed 4 times with 150mL of hexane after pressure filtration. And (3) carrying out filter pressing, transferring and drying to obtain the olefin polymerization catalyst component.
Example 2
The solid catalyst component was prepared by replacing the internal electron donor with a mixture of 1.2g of compound B and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (1:1 mass ratio) using the same preparation method as in example 1.
Example 3
The same preparation method as that used in example 1 was used to prepare a solid catalyst component by replacing the internal electron donor with a mixture of 1.2g of compound C and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (mass ratio 1:1).
Example 4
The same preparation method as that used in example 1 was used to prepare a solid catalyst component by replacing the internal electron donor with a mixture of 1.2g of compound D and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (mass ratio 1:1).
Example 5
(1) Preparation of alkoxy magnesium:
after a 1L reactor equipped with a stirrer, reflux condenser, thermometer and burette was sufficiently purged with nitrogen, 550mL of ethanol, 10mL of isopropanol and 0.68g of iodine were added to the reactor to dissolve the mixture. And (4) starting the stirrer, and then heating until the reflux temperature of the reaction system is reached. Then 32g of magnesium powder is added successively; the reaction was continued until no more hydrogen was discharged. Then washing, filtering and drying are carried out to obtain 147g of alkoxy magnesium carrier.
(2) Preparation of the catalyst component:
taking 10g of the prepared alkoxy magnesium carrier, 50mL of toluene, 3.0g of a mixture (the mass ratio is 1:1) of the compound A and 2-isopropyl-2-isoamyl-1,3-dimethyl ether to prepare a suspension; adding 40mL of toluene and 60mL of titanium tetrachloride into a 300mL reaction kettle repeatedly replaced by high-purity nitrogen, then adding the prepared suspension into the kettle, heating to 65 ℃, keeping the temperature for 0.5 hour, then continuously heating to 115 ℃, keeping the temperature for 1.5 hours, and then carrying out pressure filtration on the liquid to be clean. Adding 90mL of mixed solution of toluene and 60mL of titanium tetrachloride, heating to 110 ℃, stirring for 1 hour, performing filter pressing on the liquid, adding 120mL of mixed solution of toluene and 30mL of titanium tetrachloride, heating to 110 ℃, stirring for 1 hour, filtering the liquid, washing the obtained solid with 150mL of n-hexane for 3 times at 55 ℃, washing the solid with n-hexane once at room temperature, filtering the liquid, and drying to obtain the solid catalyst component.
Example 6
The solid catalyst component was prepared by replacing the internal electron donor with a mixture of 3.0g of compound B and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (1:1 mass ratio) using the same preparation method as in example 5.
Example 7
The same preparation method as that used in example 5 was used to prepare a solid catalyst component by replacing the internal electron donor with a mixture of 3.0g of compound C and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (mass ratio 1:1).
Example 8
The same preparation method as that used in example 5 was used to prepare a solid catalyst component by replacing the internal electron donor with a mixture of 3.0g of compound D and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (mass ratio 1:1).
Example 9
(1) Preparation of magnesium chloride solution:
in a reaction kettle repeatedly replaced by high-purity nitrogen, 20g of anhydrous magnesium chloride, 80mL of toluene, 32mL of epichlorohydrin and 36mL of tributyl phosphate are sequentially added at normal temperature, the temperature is raised to 50 ℃, and the mixture is reacted for 5 hours to be completely dissolved. A uniform magnesium chloride solution was formed, and 80mL of toluene was added dropwise, followed by stirring at 50 ℃ for 1 hour.
(2) Preparation of catalyst component:
mixing 60mL of titanium tetrachloride and 60mL of toluene, cooling to-28 ℃, dropwise adding 60mL of the magnesium chloride solution and 0.64g of 2, 4-pentanediol dibenzoate into the mixed solution for 1 hour, stirring after dropwise adding to ensure that the magnesium chloride solution and the 2, 4-pentanediol dibenzoate are fully contacted for 0.5 hour at-28 ℃, then heating to 85 ℃ for 4.5 hours, keeping the temperature for 1 hour, performing pressure filtration to remove liquid, adding 120mL of toluene, washing, and washing twice. 24mL of titanium tetrachloride and 96mL of toluene, and a mixture of 1.0g of Compound A and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (mass ratio 1:1) were added, and the mixture was stirred at 100 ℃ for 1 hour. 72mL of toluene and 48mL of titanium tetrachloride were added, stirred for 1 hour, and the liquid was removed by pressure filtration, and this was repeated three times. The solid obtained after removal of the liquid by pressure filtration was washed 4 times with 150mL of hexane. And (3) carrying out filter pressing, transferring and drying to obtain the olefin polymerization catalyst component.
Example 10
The same preparation method as in example 9 was used to replace the internal electron donor with a mixture of 1.0g of compound B and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (mass ratio 1:1) to prepare a solid catalyst component.
Example 11
The same preparation method as that used in example 9 was used to prepare a solid catalyst component by replacing the internal electron donor with a mixture of 1.0g of compound C and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (mass ratio 1:1).
Example 12
The same preparation method as in example 9 was used to prepare a solid catalyst component by replacing the internal electron donor with a mixture of 1.0g of compound D and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (mass ratio 1:1).
Example 13
The same preparation method as in example 1 was used to prepare a solid catalyst component by replacing the internal electron donor with a mixture of 1.2g of compound a and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (the mass ratios were 1:2, respectively).
Example 14
The same preparation method as in example 1 was used to prepare a solid catalyst component by replacing the internal electron donor with a mixture of 1.2g of compound a and 2-isopropyl-2-isoamyl-1,3-dimethyl ether (the mass ratios were 2:1, respectively).
Example 15
The catalyst solid component was prepared using the same preparation method as example 1, using only 1.2g of compound a as the internal electron donor.
Comparative example 1
The same preparation method as that used in example 1 was used, and only 1.2g of 2-isopropyl-2-isoamyl-1,3-dimethyl ether was used as an internal electron donor to prepare a solid catalyst component.
3. Polymerization of propylene
In a 5L autoclave, after sufficient replacement with vapor phase propylene, 5mL of a hexane solution of triethylaluminum (concentration of triethylaluminum: 0.5 mmol/mL), 1mL of a hexane solution of Cyclohexylmethyldimethoxysilane (CHMMS) (concentration of CHMMS: 0.10 mmol/mL), 10mL of anhydrous hexane, and 10mg of the solid catalyst component were added at room temperature. The autoclave was closed and a quantity of hydrogen and 1.2kg of liquid propylene were introduced. 4.5L of hydrogen is added, the polymerization temperature is 70 ℃, and the material is discharged after the polymerization time is 1 hour.
TABLE 1 Performance of the catalyst
As can be seen from the data in Table 1, the cyclohexene-1,2-dicarboxylate compound disclosed by the invention and the diether compound shown in the formula (II) are compounded to serve as internal electron donors, so that the internal electron donors maintain higher activity, excellent hydrogen regulation performance and higher stereotacticity in different Z-N catalyst systems, and the molecular weight distribution of the obtained polymer is obviously improved. Therefore, the polypropylene catalyst compounded by the two internal electron donors provided by the invention is very suitable for preparing the general polypropylene brand without plasticizer.
The preferred embodiments of the present invention have been described above in detail, but the present invention is not limited thereto. Within the scope of the technical idea of the invention, many simple modifications can be made to the technical solution of the invention, including combinations of various technical features in any other suitable way, and these simple modifications and combinations should also be regarded as the disclosure of the invention, and all fall within the scope of the invention.
Claims (22)
1. The cyclohexene-1,2-diformate compound shown in the formula (I) and the diether compound shown in the formula (II) are compounded to be used as an internal electron donor in a catalyst system for olefin polymerization,
in the formula (I), R 1 And R 2 Are the same or different and are each independently selected from C 1 -C 20 Straight chain alkyl, C 3 -C 20 Branched alkyl or cycloalkyl, C 6 -C 20 Aryl radical, C 7 -C 20 Alkaryl and C 7 -C 20 Aralkyl, any of said alkyl, cycloalkyl, aryl, alkaryl and aralkyl groups being optionally substituted by one or more substituents selected from C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy, hydroxy, halogen, cyano, nitro, amino, mono-C 1 -C 10 Alkylamino and bis-C 1 -C 10 An alkylamino group; or R 1 And R 2 Connected into a ring in any mode; r a 、R b 、R c 、R d 、R e And R f Same or different, each independently selected from hydrogen, C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy and halogen;
in the formula (II), R 3 And R 4 The same or different, each independently selected from hydrogen and C 1 -C 20 Straight chain alkyl, C 3 -C 20 Branched alkyl or cycloalkyl, C 6 -C 20 Aryl radical, C 7 -C 20 Alkylaryl and C 7 -C 20 Aralkyl, any of which may be optionally substituted with one or more substituents selected from C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy, hydroxy, halogen, cyano, nitro, amino, mono-C 1 -C 10 Alkylamino and bis-C 1 -C 10 Alkylamino, the carbon atoms of the backbone optionally being substituted with heteroatoms; or R 3 And R 4 Linked to form a ring in any way and containing, in the resulting ring skeleton, a double bond or a heteroatom; r 5 、R 6 、R 9 And R 10 The same or different, each independently selected from hydrogen and C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy and halogen; r 7 And R 8 Are the same or different and are each independently selected from C 1 -C 10 An alkyl group.
2. A solid catalyst component for olefin polymerization comprising magnesium, titanium, halogen and an internal electron donor, wherein the internal electron donor comprises a first internal electron donor compound and a second internal electron donor compound,
the first internal electron donor compound is a cyclohexene-1,2-dicarboxylate compound shown in a formula (I),
in the formula (I), R 1 And R 2 Are the same or different and are each independently selected from C 1 -C 20 Straight chain alkyl, C 3 -C 20 Branched alkyl or cycloalkyl, C 6 -C 20 Aryl radical, C 7 -C 20 Alkylaryl and C 7 -C 20 Aralkyl, any of said alkyl, cycloalkyl, aryl, alkaryl and aralkyl groups being optionally substituted by one or more substituents selected from C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy, hydroxy, halogen, cyano, nitro, amino, mono-C 1 -C 10 Alkylamino and bis-C 1 -C 10 An alkylamino group; or R 1 And R 2 Connected into a ring in any mode; r a 、R b 、R c 、R d 、R e And R f The same or different, each independently selected from hydrogen and C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy and halogen;
the second internal electron donor compound is a diether compound shown as a formula (II),
in the formula (II), R 3 And R 4 The same or different, each independently selected from hydrogen and C 1 -C 20 Straight chain alkyl, C 3 -C 20 Branched alkyl or cycloalkyl, C 6 -C 20 Aryl radical, C 7 -C 20 Alkylaryl and C 7 -C 20 Aralkyl, any of said alkyl, cycloalkyl, aryl, alkaryl and aralkyl groups being optionally substituted by one or more substituents selected from C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy, hydroxy, halogen, cyano, nitro, amino, mono-C 1 -C 10 Alkylamino and bis-C 1 -C 10 Alkylamino, the carbon atoms of the backbone optionally being substituted with heteroatoms; or R 3 And R 4 Linked to form a ring in any way and containing, in the resulting ring skeleton, a double bond or a heteroatom; r 5 、R 6 、R 9 And R 10 The same or different, each independently selected from hydrogen and C 1 -C 10 Alkyl radical, C 1 -C 10 Alkoxy and halogen; r 7 And R 8 Are the same or different and are each independently selected from C 1 -C 10 An alkyl group.
3. According to the rightThe solid catalyst component for olefin polymerization according to claim 2, wherein R in the formula (II) 7 And R 8 Are the same or different and are each independently selected from C 1 -C 6 An alkyl group.
4. The solid catalyst component for the polymerization of olefins according to claim 2 characterized in that in formula (II), R 7 And R 8 Identical or different, each independently selected from methyl, ethyl and isopropyl.
5. The solid catalyst component according to claim 2 in which in formula (I), R is 1 And R 2 Identical or different, R 1 And R 2 Each independently selected from C 1 -C 10 Straight chain alkyl, C 3 -C 10 Branched alkyl or cycloalkyl, C 6 -C 10 An aryl group; and/or
In the formula (II), R 3 And R 4 Is selected from C 1 -C 10 Straight chain alkyl, C 3 -C 10 Branched alkyl or cycloalkyl, C 6 -C 10 And (3) an aryl group.
6. The solid catalyst component according to claim 5 in which in formula (I), R is 1 And R 2 Identical or different, R 1 And R 2 Is selected from C 1 -C 6 Straight chain alkyl or C 3 -C 6 Branched alkyl or C 6 -C 10 An aryl group; and/or
In the formula (II), R 3 And R 4 Is selected from C 1 -C 6 Straight chain alkyl or C 3 -C 6 Branched alkyl or C 6 -C 10 And (4) an aryl group.
7. The solid catalyst component according to claim 5 in which in formula (I), R is 1 And R 2 The same or different, each is independently selected from methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, n-pentyl, isopentyl, tert-pentyl, and cycloPentyl and phenyl; and/or
In the formula (II), R 3 And R 4 Selected from the group consisting of methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, pentyl, isopentyl, tert-pentyl and phenyl.
8. The solid catalyst component according to any of claims 2 to 7 wherein the cyclohexene-1,2-dicarboxylate compound is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylic acid methyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid ethyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-propyl ester, cis-4-cyclohexene-1,2-dimethyloate, cis-4-cyclohexene-1,2-dicarboxylic acid n-butyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isobutyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-pentyl ester and cis-4-cyclohexene-1,2-dicarboxylic acid isoamyl ester.
9. The solid catalyst component according to claim 8 wherein the cyclohexene-1,2-dicarboxylate is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylic acid methyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid ethyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-propyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isopropyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-butyl ester and cis-4-cyclohexene-1,2-dicarboxylic acid isobutyl ester.
10. The solid catalyst component according to claim 9 wherein the cyclohexene-1,2-dicarboxylate compound is selected from at least one of cis-4-cyclohexene-1,2-dicarboxylic acid ethyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-propyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid isopropyl ester, cis-4-cyclohexene-1,2-dicarboxylic acid n-butyl ester, and cis-4-cyclohexene-1,2-dicarboxylic acid isobutyl ester.
11. The solid catalyst component according to claim 2, characterized in that the diether-based compound is selected from the group consisting of '-dimethyl ether,' -diethyl-dimethyl ether, '-di-n-propyl-dimethyl ether,' -diisopropyl-dimethyl ether, '-di-n-butyl-dimethyl ether,' -diisobutyl-dimethyl ether, '-di-tert-butyl-dimethyl ether,' -di-n-pentyl-dimethyl ether, '-diisoamyl-dimethyl ether,' -di (1-methyl) butyl-dimethyl ether, '-di (2-methyl) butyl-dimethyl ether,' -di (1-ethyl) propyl-dimethyl ether, '-di-tert-pentyl-dimethyl ether,' -di-n-hexyl-dimethyl ether, '-diisohexyl-diether,' -di (1-methyl) pentyl-dimethyl ether, '-di (2-methyl) pentyl-dimethyl ether,' -di (3-methyl) pentyl-dimethyl ether, '-di (1-ethyl) butyl-dimethyl ether,' -di (2-ethyl) butyl-dimethyl ether, 2,2' -ditert-hexyl-1,3-dimethyl ether, 2-methyl-2-ethyl-1,3-dimethyl ether, 2-methyl-2-n-propyl-1,3-dimethyl ether, 2-methyl-2-isopropyl-1,3-dimethyl ether, 2-methyl-2-n-butyl-1,3-dimethyl ether, 2-methyl-2-isobutyl-1,3-dimethyl ether, 2-methyl-2-n-pentyl-1,3-dimethyl ether, 2-methyl-2-isopentyl-1,3-dimethyl ether, 2-methyl-2-n-hexyl-1,3-dimethyl ether, 2-methyl-2-isohexyl-1,3-dimethyl ether 2-ethyl-2-n-propyl-1,3-dimethyl ether, 2-ethyl-2-isopropyl-1,3-dimethyl ether, 2-ethyl-2-n-butyl-1,3-dimethyl ether, 2-ethyl-2-isobutyl-1,3-dimethyl ether, 2-ethyl-2-n-pentyl-1,3-dimethyl ether, 2-ethyl-2-isoamyl-1,3-dimethyl ether, 2-ethyl-2-n-hexyl-1,3-dimethyl ether, 2-ethyl-2-isohexyl-3826-dimethyl ether, 2-n-propyl-2-isopropyl-1,3-dimethyl ether, 2-n-propyl-2-n-butyl-1,3-dimethyl ether, 2-n-propyl-2-isobutyl-1,3-dimethyl ether, 2-n-propyl-2-n-pentyl-1,3-dimethyl ether, 2-n-propyl-2-isoamyl-1,3-dimethyl ether, 2-n-propyl-2-n-hexyl-1,3-dimethyl ether, 2-n-propyl-2-isohexyl-1,3-dimethyl ether, 2-isopropyl-2-n-butyl-3535-dimethyl ether, 2-isopropyl-2-isobutyl-1,3-dimethyl ether, 2-isopropyl-2-n-pentyl-1,3-dimethyl ether, 2-isopropyl-2-isoamyl-1,3-dimethyl ether, 2-isopropyl-2-n-hexyl-1,3-dimethyl ether 2-isopropyl-2-isohexyl-1,3-dimethyl ether, 2-n-butyl-2-isobutyl-1,3-dimethyl ether, 2-n-butyl-2-n-pentyl-1,3-dimethyl ether, 2-n-butyl-2-isopentyl-1,3-dimethyl ether, 2-n-butyl-2-n-hexyl-1,3-dimethyl ether, 2-n-butyl-2-isohexyl-1,3-dimethyl ether, 2-isobutyl-2-n-pentyl-1,3-dimethyl ether, 2-isobutyl-2-isopentyl-1,3-dimethyl ether, 2-isobutyl-2-n-hexyl-1,3-dimethyl ether, 2-isobutyl-2-isohexyl-1,3-dimethyl ether, 2-n-pentyl-2-isopentyl-1,3-dimethyl ether, 2-n-pentyl-2-n-hexyl-1,3-dimethyl ether, 2-n-pentyl-2-isohexyl-1,3-dimethyl ether, 2-isopentyl-2-n-hexyl-1,3-dimethyl ether, 2-isopentyl-2-isohexyl-1,3-dimethyl ether, and 2-n-hexyl-2-isohexyl-1,3-dimethyl ether.
12. The solid catalyst component according to claim 11, characterized in that the diether-based compound is selected from the group consisting of '-dimethyl ether,' -diethyl-dimethyl ether, '-di-n-propyl-dimethyl ether,' -diisopropyl-dimethyl ether, '-di-n-butyl-dimethyl ether,' -diisobutyl-dimethyl ether, '-di-tert-butyl-dimethyl ether,' -di-n-pentyl-dimethyl ether, '-diisoamyl-dimethyl ether,' -di-iso-pentyl-dimethyl ether, '-di-tert-hexyl-dimethyl ether,' -2-methyl-2-ethyl-dimethyl ether, 2-methyl-2-n-propyl-dimethyl ether, 2-methyl-2-isopropyl-dimethyl ether, 2-methyl-2-n-butyl-dimethyl ether, 2-methyl-2-isobutyl-dimethyl ether, 2-methyl-2-n-pentyl-dimethyl ether, 2-methyl-2-isoamyl-1,3-dimethyl ether, 2-methyl-2-isohexyl-1,3-dimethyl ether, 2-ethyl-2-n-propyl-1,3-dimethyl ether, 2-ethyl-2-isopropyl-1,3-dimethyl ether, 2-ethyl-2-n-butyl-1,3-dimethyl ether, 2-ethyl-2-isobutyl-1,3-dimethyl ether, 2-ethyl-2-n-pentyl-1,3-dimethyl ether, 2-ethyl-2-isoamyl-1,3-dimethyl ether, 2-ethyl-2-isohexyl-1,3-dimethyl ether, 2-n-propyl-2-isopropyl-1,3-dimethyl ether 2-n-propyl-2-n-butyl-1,3-dimethyl ether, 2-n-propyl-2-isobutyl-1,3-dimethyl ether, 2-n-propyl-2-n-pentyl-1,3-dimethyl ether, 2-n-propyl-2-isopentyl-1,3-dimethyl ether, 2-n-propyl-2-isohexyl-1,3-dimethyl ether, 2-isopropyl-2-n-butyl-1,3-dimethyl ether, 2-isopropyl-2-isobutyl-1,3-dimethyl ether, 2-isopropyl-2-n-pentyl-1,3-dimethyl ether, 2-isopropyl-2-isopentyl-1,3-dimethyl ether, 2-isopropyl-2-isohexyl-1,3-dimethyl ether, 2-n-butyl-2-isobutyl-1,3-dimethyl ether, 2-n-butyl-2-n-pentyl-1,3-dimethyl ether, 2-n-butyl-2-isoamyl-1,3-dimethyl ether, 2-n-butyl-2-isohexyl-1,3-dimethyl ether, 2-isobutyl-2-n-pentyl-1,3-dimethyl ether, 2-isobutyl-2-isoamyl-1,3-dimethyl ether, 2-isobutyl-2-isohexyl-1,3-dimethyl ether, 2-n-pentyl-2-isopentyl-1,3-dimethyl ether, 2-n-pentyl-2-isohexyl-1,3-dimethyl ether, 2-isopentyl-2-n-hexyl-3638 z3638-dimethyl ether, 2-isopentyl-2-n-hexyl-4924-hexyl-4924-dimethyl ether and at least one of the group consisting of 2-isobutyl-2-isohexyl-5329-dimethyl ether.
13. The solid catalyst component according to claim 12, the diether compound is selected from 2,2 '-dimethyl-1,3-dimethyl ether, 2,2' -diethyl-1,3-dimethyl ether, 2,2 '-di-n-propyl-1,3-dimethyl ether, 2,2' -diisopropyl-2,2-dimethyl ether, 2,2 '-di-n-butyl-2,2-dimethyl ether, 2,2' -diisobutyl-2,2-dimethyl ether, 2,2 '-di-n-pentyl-2,2-dimethyl ether, 2,2' -diisoamyl-6258-dimethyl ether, 2,2 '-di-hexyl-2,2-dimethyl ether, 6258-zxft 58' -diisohexyl-2,2-dimethyl ether, 6258 zxft 2-methyl-2-ethyl-2,2-dimethyl ether, 2-methyl-2-isopropyl-2,2-dimethyl ether, 2-methyl-2-isobutyl-2,2-dimethyl ether, 2-methyl-2-isoamyl-2,2-dimethyl ether, 2-ethyl-2-isopropyl-2,2-dimethyl ether, 2-ethyl-2-isobutyl-2,2-dimethyl ether, 2-ethyl-2-isoamyl-2,2-dimethyl ether, 2-ethyl-2-isohexyl-2,2-dimethyl ether, 2-n-propyl-2-isopropyl-2,2-dimethyl ether, 2-n-propyl-2-isobutyl-2,2-dimethyl ether, 2-n-propyl-2-isopentyl-2,2-dimethyl ether, 2-n-propyl-2-isoamyl-2,2-dimethyl ether, 2-n-propyl-2-isohexyl-1,3-dimethyl ether, 2-isopropyl-2-n-butyl-1,3-dimethyl ether, 2-isopropyl-2-isobutyl-1,3-dimethyl ether, 2-isopropyl-2-n-pentyl-1,3-dimethyl ether, 2-isopropyl-2-isoamyl-1,3-dimethyl ether, 2-isopropyl-2-isohexyl-1,3-dimethyl ether, 2-n-butyl-2-isobutyl-1,3-dimethyl ether, 2-n-butyl-2-isoamyl-1,3-dimethyl ether, 2-n-butyl-2-isohexyl-1,3-dimethyl ether, 2-isobutyl-2-n-pentyl-1,3-dimethyl ether, 2-isobutyl-2-isoamyl-1,3-isobutyl-2-isohexyl-4924-isopentyl-4924-n-pentyl-4924-zxft 4924-dimethyl ether and at least one of the group of the above-n-propyl-2-isobutyl-2-isopentyl-3735-dimethyl ether.
14. The solid catalyst component according to any of claims 2 to 7, characterized in that the molar ratio of the first internal electron donor compound and the second internal electron donor compound is from 0.1.
15. The solid catalyst component according to claim 14, characterized in that the molar ratio of the first internal electron donor compound and the second internal electron donor compound is 0.2.
16. The solid catalyst component according to claim 15, characterized in that the molar ratio of the first internal electron donor compound and the second internal electron donor compound is between 0.3 and 1 and 0.3.
17. The solid catalyst component according to any one of claims 2 to 7, characterized in that the total content of the components in the catalyst component is 100wt% and the content of titanium atoms is 1.0 to 8.0wt%, based on the total amount of the catalyst component; the content of magnesium atoms is 10-70wt%; the content of halogen atoms is 20-90wt%; the total internal electron donor compound content is 2-30wt%.
18. The solid catalyst component according to claim 17, wherein the total content of each component in the catalyst component is 100wt% and the content of titanium atom is 1.6 to 6.0wt%, based on the total amount of the catalyst component; the content of magnesium atoms is 15-40wt%; the content of halogen atoms is 30-85%; the total internal electron donor compound content is 3-20wt%.
19. A catalyst system for olefin polymerization comprising the reaction product of:
1) The solid catalyst component of any of claims 2 to 18,
2) An alkyl-aluminium compound, which is a mixture of,
3) Optionally, an external electron donor compound.
20. Use of a solid catalyst component according to any one of claims 2-18 or a catalyst system according to claim 19 in the polymerization of olefins.
21. A process for the polymerization of olefins having the general formula CH, in the presence of the solid catalyst component according to any of claims 2 to 18 or the catalyst system according to claim 19 2 = CHR where R is hydrogen or C 1 -C 6 An alkyl group; the olefin polymerization is homopolymerization of a single olefin or copolymerization of a plurality of olefins.
22. The method of claim 21, wherein the olefin is selected from at least one of ethylene, propylene, 1-butene, 4-methyl-1-pentene, and 1-hexene.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910983439.3A CN112661882B (en) | 2019-10-16 | 2019-10-16 | Application of cyclohexene-1,2-dicarboxylic acid ester compound |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910983439.3A CN112661882B (en) | 2019-10-16 | 2019-10-16 | Application of cyclohexene-1,2-dicarboxylic acid ester compound |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN112661882A CN112661882A (en) | 2021-04-16 |
| CN112661882B true CN112661882B (en) | 2022-10-21 |
Family
ID=75400665
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201910983439.3A Active CN112661882B (en) | 2019-10-16 | 2019-10-16 | Application of cyclohexene-1,2-dicarboxylic acid ester compound |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112661882B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021064078A1 (en) * | 2019-10-04 | 2021-04-08 | Borealis Ag | Ziegler-natta catalysts for olefin polymerization |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003040918A (en) * | 2001-07-25 | 2003-02-13 | Toho Catalyst Co Ltd | Solid catalyst component and catalyst for polymerizing olefins |
| CN102212154A (en) * | 2011-04-19 | 2011-10-12 | 中国科学院化学研究所 | Catalyst solid component for olefin polymerization and preparation method thereof |
| CN105504110A (en) * | 2015-12-30 | 2016-04-20 | 神华集团有限责任公司 | Preparation method of catalyst solid component for olefin polymerization |
| CN107344976A (en) * | 2016-05-05 | 2017-11-14 | 中国石油化工股份有限公司 | Catalytic component, catalyst system and its application for olefinic polymerization |
-
2019
- 2019-10-16 CN CN201910983439.3A patent/CN112661882B/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003040918A (en) * | 2001-07-25 | 2003-02-13 | Toho Catalyst Co Ltd | Solid catalyst component and catalyst for polymerizing olefins |
| CN102212154A (en) * | 2011-04-19 | 2011-10-12 | 中国科学院化学研究所 | Catalyst solid component for olefin polymerization and preparation method thereof |
| CN105504110A (en) * | 2015-12-30 | 2016-04-20 | 神华集团有限责任公司 | Preparation method of catalyst solid component for olefin polymerization |
| CN107344976A (en) * | 2016-05-05 | 2017-11-14 | 中国石油化工股份有限公司 | Catalytic component, catalyst system and its application for olefinic polymerization |
Also Published As
| Publication number | Publication date |
|---|---|
| CN112661882A (en) | 2021-04-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5695093B2 (en) | Internal and external electron donor compounds for olefin polymerization catalysts II | |
| CN109111539B (en) | Catalyst component for olefin polymerization and catalyst thereof | |
| KR102381124B1 (en) | A solid catalyst component for polymerization of olefins, a method for preparing a solid catalyst component for polymerization of olefins, a catalyst for polymerization of olefins, a method for producing an olefin polymer, a method for producing a propylene-based copolymer, and a propylene-based copolymer | |
| JP2016510835A (en) | 1-mixed internal donor structure for olefin polymerization catalyst | |
| JP2013510941A (en) | Method for producing solid catalyst for propylene polymerization | |
| CN107344973B (en) | Catalyst component for olefin polymerization, catalyst system and application thereof | |
| CN107344978B (en) | Catalyst component for olefin polymerization, catalyst system and application thereof | |
| CN112661882B (en) | Application of cyclohexene-1,2-dicarboxylic acid ester compound | |
| CN112661883B (en) | Solid catalyst component for preparing polyolefin, catalyst system and application thereof | |
| CN107344976B (en) | Catalyst component for olefin polymerization, catalyst system and application thereof | |
| CN112661881B (en) | Olefin polymerization catalyst component, catalyst system and olefin polymerization method | |
| CN107344979B (en) | Catalyst component for olefin polymerization, catalyst system and application thereof | |
| CN109111536B (en) | Catalyst component for olefin polymerization and catalyst thereof | |
| CN109111535B (en) | Catalyst component for olefin polymerization and catalyst thereof | |
| CN111234062B (en) | Catalyst system for olefin polymerization and use thereof | |
| CN112300304B (en) | Catalyst system for olefin polymerization and prepolymerized catalyst composition | |
| CN107344980B (en) | Catalyst component for olefin polymerization, catalyst system and application thereof | |
| CN116023553B (en) | Catalyst component for olefin polymerization reaction, catalyst system and application | |
| TWI823951B (en) | Catalyst component for olefin polymerization, preparation method thereof and catalyst containing same | |
| CN109111538B (en) | Catalyst component for olefin polymerization and catalyst thereof | |
| CN106496366B (en) | A kind of olefin polymerization catalysis and its application | |
| US11840508B2 (en) | Catalyst system for olefin polymerization and use thereof | |
| CN112300302B (en) | Twelve-membered ring compound and application thereof | |
| CN111072815A (en) | Catalyst component and catalyst for olefin polymerization, application thereof and olefin polymerization method | |
| CN107344977B (en) | Catalyst component for olefin polymerization, catalyst system and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |