CN112656805A - Application of substance for inhibiting YTHDF1 activity in preparation of product for preventing or treating gastric cancer - Google Patents
Application of substance for inhibiting YTHDF1 activity in preparation of product for preventing or treating gastric cancer Download PDFInfo
- Publication number
- CN112656805A CN112656805A CN201910981163.5A CN201910981163A CN112656805A CN 112656805 A CN112656805 A CN 112656805A CN 201910981163 A CN201910981163 A CN 201910981163A CN 112656805 A CN112656805 A CN 112656805A
- Authority
- CN
- China
- Prior art keywords
- ythdf1
- gastric cancer
- inhibiting
- substance
- gene
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
The invention discloses application of a substance for inhibiting YTHDF1 activity in preparation of a product for preventing or treating gastric cancer. The invention firstly discloses the application of a substance inhibiting the expression of YTHDF1 in a cell or a substance inhibiting the activity of YTHDF1 in the cell in preparing a product for preventing and/or treating gastric cancer. The invention further discloses a product for preventing and/or treating gastric cancer. The substance (such as shRNA) interfering with YTHDF1 gene expression by taking YTHDF1 as a target point can effectively inhibit the proliferation, migration and/or invasion of gastric cancer cells and the growth of gastric cancer tumors, and can be used for treating gastric cancer or/and preventing gastric cancer. And gastric cancer tissues can be distinguished by detecting the expression quantity of the YTHDF1 gene, the pathological stage of pTNM of a gastric cancer patient is evaluated, whether the gastric cancer patient has nerve invasion or vein invasion is identified, and the diagnosis of gastric cancer can be assisted.
Description
Technical Field
The invention relates to the field of biological medicines, in particular to application of a substance for inhibiting YTHDF1 activity in preparation of a product for preventing or treating gastric cancer.
Background
Stomach cancer (GC) is located in the front among the worldwide onset and death of cancer, a complex disease that is commonly regulated by environmental factors and individual genetic factors. Despite the steady decline in the incidence of gastric cancer in recent years, gastric cancer still results in over 723,000 deaths per year. The main causes of high mortality from gastric cancer are that early symptoms of the disease are not noticeable to the patient, late clinical manifestations are intricate and complex, and have potential biological and genetic heterogeneity at the molecular level, show significant individual differences, and have a very poor prognosis. Especially for patients in late stage, the optimal surgical treatment opportunity is lost, and chemotherapy is still an indispensable treatment means, so that the molecular mechanism of gastric cancer occurrence and development is deeply understood, and the molecular marker of gastric cancer occurrence is found to find the optimal way of drug treatment, which becomes an irreparable task of gastric cancer treatment.
YTHDF1(YTH domain N6-methyl adenine (m6A) RNA binding protein 1, Ensembl: ENSG00000149658 MIM: 616529), which can selectively bind with N6-methyl adenine modified RNA molecule and regulate the fate of RNA. At present, no report about YTHDF1 is related to gastric cancer.
Disclosure of Invention
The technical problem to be solved by the invention is how to inhibit the proliferation, migration and/or invasion of gastric cancer cells and the growth of gastric cancer tumors, and how to identify or assist in identifying tumor tissues, identifying or assist in evaluating the stage of pTNM pathology and whether neuro-invasion or venous invasion occurs in gastric cancer patients.
In order to solve the problems, the invention firstly provides the application of a substance for inhibiting the expression of YTHDF1 in a cell or a substance for inhibiting the activity of YTHDF1 in the cell.
The above application is any one of the following:
A1. the application of a substance for inhibiting the expression of YTHDF1 gene in cells in preparing a product for preventing and/or treating gastric cancer;
A2. the application of a substance for inhibiting YTHDF1 activity in cells in preparing a product for preventing and/or treating gastric cancer;
A3. the application of the substance for inhibiting the expression of YTHDF1 gene in cells in preparing products for inhibiting the proliferation of gastric cancer cells;
A4. the application of substance for inhibiting YTHDF1 activity in cells in preparing products for inhibiting gastric cancer cell proliferation;
A5. the application of substance for inhibiting YTHDF1 gene expression in cells in preparing products for inhibiting gastric cancer cell migration;
A6. the application of substance for inhibiting YTHDF1 activity in cells in preparing products for inhibiting gastric cancer cell migration;
A7. the application of substance for inhibiting YTHDF1 gene expression in cells in preparing products for inhibiting gastric cancer cell migration;
A8. the application of a substance for inhibiting YTHDF1 activity in cells in preparing products for inhibiting the invasion of liver and stomach cancer cells.
In the application, the product can be a medicine, a vaccine, a health product and/or a food.
In the application, the substance for inhibiting the expression of YTHDF1 gene in the cell and the substance for inhibiting the activity of YTHDF1 gene in the cell can be any one of the following biological materials 1) to 6):
1) generating shRNA of siRNA or a chemical modifier of the shRNA; the siRNA is small interfering RNA interfering the expression of YTHDF1 gene;
2) an expression vector for expressing 1) the shRNA;
3) a recombinant microorganism expressing 1) the shRNA;
4) siRNA generated by 1) the shRNA or a chemical modification of the siRNA;
5) an expression vector expressing 4) the siRNA;
6) a recombinant microorganism expressing 5) said siRNA.
In the application, the chemical modifier of the shRNA is a substance obtained by chemically modifying the shRNA. The chemical modification may include one or a combination of several selected from ribose modification, base modification, and phosphate backbone modification.
The chemical modifier of the siRNA is a substance obtained by chemically modifying the siRNA. The chemical modification may include one or a combination of several selected from ribose modification, base modification, and phosphate backbone modification.
In the application, the target sequences of the siRNA are shown as a sequence 1 and a sequence 2 respectively.
In the application, the target sequence of the siRNA is shown in sequence 1, the stem sequence corresponding to the shRNA can be 1-20 th nucleotides of sequence 5 and 33-52 th nucleotides of sequence 5, and the loop sequence is 21-32 th nucleotides of sequence 5; specifically, the sequence of the shRNA is shown as a sequence 5. The target sequence of the siRNA is shown in a sequence 2, the stem sequence corresponding to the shRNA can be the 1 st to 20 th nucleotides of a sequence 6 and the 33 th to 52 th nucleotides of the sequence 6, and the loop sequence is the 21 st to 32 th nucleotides of the sequence 6; specifically, the sequence of the shRNA is shown as a sequence 6.
In the above application, the recombinant microorganism may be a recombinant lentivirus expressing the shRNA or siRNA.
In the above application, the cell may be a mammalian cell, such as a human, specifically a human gastric cancer cell line, and the human gastric cancer cell line used in the present invention is a human gastric cancer cell line MGC-803 or a human gastric cancer cell line HGC-27.
In the above applications, the active ingredients of the product for preventing and/or treating gastric cancer, the product for inhibiting gastric cancer cell proliferation, the product for inhibiting gastric cancer cell migration and the product for inhibiting gastric cancer cell can be substances for inhibiting the expression of YTHDF1 gene in cells, the active ingredients of the product can also contain other ingredients, and the other active ingredients of the product can be determined by those skilled in the art according to the effect of resisting gastric cancer cells.
The product for preventing and/or treating gastric cancer, the product for inhibiting gastric cancer cell proliferation and the product for inhibiting gastric cancer cell migration and the product for inhibiting gastric cancer cells can also contain a pharmaceutically acceptable carrier. As used herein, a "pharmaceutically acceptable carrier" should be compatible with the RNA molecule of the agent of the invention. The pharmaceutically acceptable carrier refers to an in vivo transfection reagent, such as Polyethyleneimine (PEI), linear polyethyleneimine (jetPEI), liposome, transferrin, folic acid, nanoemulsion, nanoparticle and the like. Other examples of substances which may serve as pharmaceutically acceptable carriers or components thereof are lyoprotectants sugars, such as lactose, glucose and sucrose; starches, such as corn starch and potato starch; powdered gum tragacanth; malt; gelatin; talc; solid lubricants, such as stearic acid and magnesium stearate; calcium sulfate; vegetable oils such as peanut oil, cottonseed oil, sesame oil, olive oil, corn oil and cocoa butter; polyols, such as glycerol, mannitol; alginic acid; emulsifiers, such as Tween; phospholipids, such as lecithin, soya lecithin, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylinositol, phosphatidylserine, stearamide; cholesterol; macromolecular polymers such as polyethyleneimine, chitosan, hyaluronic acid; wetting agents, such as sodium lauryl sulfate; a colorant; a flavoring agent; tabletting agents, stabilizers; an antioxidant; a preservative; pyrogen-free water; isotonic saline solution; and phosphate buffer and the like; physiological saline, glycerol and phosphate buffered saline.
The product for preventing and/or treating gastric cancer, the product for inhibiting gastric cancer cell proliferation, the product for inhibiting gastric cancer cell migration and the product for inhibiting gastric cancer cells also belong to the protection scope of the invention.
The invention further provides application of the substance for detecting the expression quantity or relative expression quantity of YTHDF1 gene.
The above application is any one of the following:
B1) the application of the substance for detecting the expression quantity or relative expression quantity of YTHDF1 gene in preparing products for identifying or assisting in identifying tumor tissues;
B2) the application of the substance for detecting the expression quantity or relative expression quantity of the YTHDF1 gene in preparing products for evaluating or assisting in evaluating the pathological stage of pTNM of a gastric cancer patient;
B3) the application of the substance for detecting the expression quantity or relative expression quantity of YTHDF1 gene in preparing products for identifying or assisting in identifying whether gastric cancer patients have nerve invasion or vein invasion.
In the application, the product can be a medicine, a vaccine, a health product and/or a food.
In the application, the substance for detecting the expression quantity or the relative expression quantity of the YTHDF1 gene utilizes a system for detecting the expression quantity or the relative expression quantity of the YTHDF1 gene by quantitative PCR;
the system for detecting the expression quantity or relative expression quantity of the YTHDF1 gene by quantitative PCR comprises primers, probes, a kit and/or other reagents and/or instruments required for quantitative PCR.
Specifically, the system for detecting the expression level or relative expression level of the YTHDF1 gene by quantitative PCR may be:
1) primers for amplifying the YTHDF1 gene;
2) comprises the reagents required for quantitative PCR of 1);
3) a kit comprising 1) or 2);
4) the apparatus required for quantitative PCR.
Wherein, the primer for amplifying YTHDF1 gene can be single-stranded DNA molecule shown in sequence 4. The primers for amplifying YTHDF1 gene and other reagents required for quantitative PCR reaction can be independently packaged.
In the invention, the relative expression level of the YTHDF1 gene is the expression level of YTHDF1 gene relative to GAPDH.
In the above application, the substance for detecting the expression level or relative expression level of the YTHDF1 gene further comprises a data processing device, and the data processing device is used for converting the expression level or relative expression level of the YTHDF1 gene from the subject to be detected into a diagnosis result of the subject to be detected.
Specifically, the data processing device comprises a data input module, a data comparison module and a conclusion output module; the data input module is used for inputting the expression quantity or relative expression quantity of the YTHDF1 gene of the object to be detected, the data comparison module is used for comparing the expression quantity or relative expression quantity of the YTHDF1 gene of the object to be detected, and the conclusion output module is used for outputting the diagnosis result of the object to be detected.
When the tumor tissue is identified or assisted to be identified, detecting the expression amount or relative expression amount of YTHDF1 gene from the tissue to be identified: the higher the expression or relative expression of YTHDF1 gene of the tissue to be identified is, the higher the risk of tumor tissue of the tissue to be identified is or is selected as a candidate; the lower the expression or relative expression of the YTHDF1 gene of the tissue to be identified, the lower the risk that the tissue to be identified is or is candidate for being a tumor tissue.
Aiming at the assessment or the auxiliary assessment of the pathological stage of pTNM of the gastric cancer patient, detecting the expression quantity or the relative expression quantity of YTHDF1 gene of the gastric cancer patient to be detected: the higher the expression or relative expression of YTHDF1 gene of the patient to be detected, the higher the possibility of the gastric cancer patient in stage III and/or IV of pTNM pathological stage is; the lower the expression level or relative expression level of YTHDF1 gene of the patient to be tested is, the higher the possibility that the gastric cancer patient is in stage I and/or stage II of pTNM pathological stage is.
Aiming at identifying or assisting in identifying whether the gastric cancer patient has nerve invasion or vein invasion, detecting the expression or relative expression of YTHDF1 gene of the gastric cancer patient to be detected: the higher the expression or relative expression of the YTHDF1 gene of the gastric cancer patient to be detected is, the higher the risk of nerve invasion and/or vein invasion of the gastric cancer patient to be detected is; the lower the expression or relative expression of the YTHDF1 gene of the gastric cancer patient to be detected is, the smaller the risk of nerve invasion and/or vein invasion of the gastric cancer patient to be detected is.
Experiments show that substances (such as shRNA) interfering with YTHDF1 gene expression by taking YTHDF1 as a target spot can effectively inhibit proliferation, migration and/or invasion of gastric cancer cells and growth of gastric cancer tumors, and can be used for treating gastric cancer or/and preventing gastric cancer. And gastric cancer tissues can be distinguished by detecting the expression quantity of the YTHDF1 gene, the pathological stage of pTNM of a gastric cancer patient is evaluated, whether the gastric cancer patient has nerve invasion or vein invasion is identified, and the diagnosis of gastric cancer can be assisted.
Drawings
FIG. 1 shows m in gastric cancer according to the cBioPortal database6Mutation rate at the first 10 th position of the a-related gene (n ═ 630). The distribution of the different mutation types of the YTHDF1 gene is shown in the right panel.
Fig. 2 shows the correlation between DNA methylation levels of the YTHDF1 promoter and their expression from RNA sequencing results (n 338).
Fig. 3 is a database of 24 cancer types according to TCGA, which shows the change in relative expression of YTHDF1 in tumor tissues compared to normal tissues.
Fig. 4 is a violin-like plot of YTHDF1 in normal, good, and malignant stomach tissues (n: 32 cases normal, n: 164 cases benign, n: 188 cases malignant;. p < 0.001, one-way ANOVA analysis using Newman-Keuls post-hoc tests.
Com, overall survival was calculated for patients with high expression of YTHDF1 (n-215) and low expression of YTHDF1 (n-58) in kmplot.com.
Fig. 6 shows relative expression of YTHDF1 (n 113) in gastric cancer tissue and its adjacent normal tissue in 113 pairs.
Fig. 7 is an immunohistochemical image of YTHDF1 in 2 gastric cancer tissues and adjacent paracarcinoma tissues. Scale, 50 μm.
Fig. 8 shows the relationship between the expression of YTHDF1 and nerve invasion, pTNM pathological stage, and venous invasion (n 113) in gastric cancer patients.
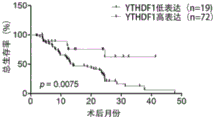
FIG. 9 shows that Kaplan-Meier survival analysis shows that YTHDF1 expression (low expression, n 19, high expression, n 72) of gastric cancer patients is correlated with overall survival (log-rank with Mantel-Cox test). Data are expressed as mean ± standard deviation.
FIG. 10 shows the Western blot analysis of knockdown of shRNAs targeting YTHDF1 in MGC-803 and HGC-27 cells.
FIG. 11 is an experimental analysis of the proliferation of knockdown YTHDF1 gene in MGC-803 and HGC-27 cells. Wherein n is 3; p < 0.05, p < 0.01, one-way ANOVA analysis using Newman-Keuls post hoc tests; scale, 100 μm.
FIG. 12 is experimental analysis of the migration and invasion of knockdown YTHDF1 gene in MGC-803 and HGC-27 cells. Wherein n is 3; p < 0.01, p < 0.001, one-way ANOVA analysis was performed using Newman-Keuls post hoc tests.
Fig. 13 is a tumor experiment (day 28) (n-8) of YTHDF1 inoculated mice after MGC-803 cell knockdown.
FIG. 14 shows the subcutaneous tumor volume of nude mice knockdown YTHDF1 in MGC-803 cells, as well as a control group; wherein n is 8; p < 0.001, one-way ANOVA analysis was performed with Newman-Keuls post hoc test.
FIG. 15 shows the subcutaneous tumor weight knockdown of YTHDF1 in MGC-803 cells, as well as control nude mice. Wherein n is 8; p < 0.001, one-way ANOVA analysis was performed with Newman-Keuls post hoc test.
FIG. 16 is a statistical result of Ki-67 and Caspase-3 positive staining in immunohistochemistry for xenograft tumors. Wherein n is 8; p < 0.001, one-way ANOVA analysis using Newman-Keuls post hoc tests; scale, 20 μm.
Fig. 17 shows the results of dissection and metastatic nodule statistics for the mouse lung metastasis model. Wherein n is 4; p < 0.001, one-way ANOVA analysis was performed with Newman-Keuls post hoc test. Arrows represent metastatic nodules.
Fig. 18 is a histological result of the lung metastasis model. Scale, 200 μm.
Fig. 19 shows MGC-803 injected into abdominal cavity metastasis model after knockdown of YTHDF1 and statistics of xenograft tumor counts. Wherein n is 4; p < 0.001, one-way ANOVA analysis was performed with Newman-Keuls post hoc test. Arrows represent tumors.
Detailed Description
The following examples are given to facilitate a better understanding of the invention, but do not limit the invention. The experimental procedures used in the following examples are all conventional procedures unless otherwise specified. Materials, reagents and the like used in the following examples are commercially available unless otherwise specified.
The experimental procedures used in the following examples are as follows:
first, protein immunoblotting experiment (western blot)
1. Extraction of total protein of cell or tissue and determination of protein concentration
(1) And (3) total protein extraction: extracting total protein of cell, directly centrifuging to collect cell, washing with PBS twice, centrifuging to precipitate, removing supernatant, adding appropriate amount of protein lysis buffer containing protease inhibitor, reacting on ice for 30min, centrifuging at 4 deg.C and 10000rpm × 15min, and collecting supernatant. For extracting total protein of tissue, shearing tissue with scissors, adding appropriate amount of protein lysis buffer containing protease inhibitor, homogenizing with homogenizer until tissue is completely pulverized, reacting on ice for 30min, centrifuging at 4 deg.C and 10000rpm × 15min, and collecting supernatant. If the tissue is not completely disrupted, the above steps are repeated. The preparation of the protein lysate and the whole cracking process are carried out on ice;
(2) protein concentration determination: the concentration of the protein sample was determined using the BCA protein concentration assay kit from wiglas bio. Firstly, drawing a standard curve according to the concentration and the light absorption value of the standard substance, calculating a functional relation, and then calibrating the concentration of the sample to be detected according to the light absorption value of the sample to be detected.
2. Preparation of electrophoretic protein samples
Adding 5 × loading buffer and ddH according to the concentration and volume ratio of each protein sample2And O is mixed uniformly, and the samples loaded in the same batch are preferably kept consistent in final concentration. Boiling in boiling water for 10min, centrifuging at 4 deg.C, and packaging. The split protein samples can be frozen at-80 ℃ for later use.
3. SDS-PAGE electrophoresis
Firstly, preparing separating gel and concentrating gel with proper concentration, and standing at room temperature until the gel is completely solidified. And (3) sampling 10-40 mu g of total protein sample. 80V constant voltage electrophoresis, when the sample enters the separation gel, the voltage is adjusted to 120V. The electrophoresis was terminated when bromophenol blue was 0.5cm from the bottom, and the gel was carefully removed.
4. Protein transfer membrane
(1) Preparing electrotransfer buffer solution and pre-cooling in advance.
(2) The PVDF membrane is soaked in methanol for 30s, and then is put into an electric transfer buffer solution together with the sponge and filter paper used for transferring the membrane for soaking for 10 min.
(3) Placing the film transfer material in the following sequence: the device comprises an electrotransformation instrument plastic bracket, a sponge, two layers of filter paper, gel, a PVDF film, two layers of filter paper, a sponge and an electrotransformation instrument plastic bracket; during placement, air bubbles are prevented from being reserved between the gel and the PVDF film, and the plastic bracket is clamped after placement is finished.
(4) Gel is placed into an electric rotating tank in the direction of a negative electrode according to the direction of a positive electrode of the PVDF membrane, the electric rotating tank is placed in an ice bath environment, and the membrane is rotated for 1h at a constant current of 200 mA.
(5) After the electrotransfer is finished, taking out the PVDF membrane, and marking the sample loading sequence.
5. Hybridization and development
(1) The electroporated PVDF membrane was washed 5 min/times X3 times in TBST (20mM (pH 7.6-8.0), 100mM NaCl, 0.1% Tween-20 solution.
(2) Placing in a sealing solution containing 5% skimmed milk powder, sealing at room temperature for 1 hr.
(3) The primary antibody was diluted with blocking solution to the appropriate concentration and the PVDF membrane was incubated overnight at 4 ℃.
(4) The membrane was washed 3 times with 1 × TBST for 10min each time.
(5) And (3) diluting the horseradish peroxidase-labeled secondary antibody to a proper concentration by using a confining liquid, and incubating the PVDF membrane for 1-2 h at room temperature.
(6) The membrane was washed 3 times with 1 × TBST for 10min each time.
(7) Developing by an ECL method: developing with X-ray film in dark room, absorbing excessive water on the film, and placing the protein surface on the preservative film. And (3) uniformly dripping ECL reaction liquid on the surface of the PVDF membrane, carrying out light-shielding reaction for 1min, carefully wrapping the membrane, and developing by using an X-ray film in a dark room.
Second, Real-time PCR
1. Trizol method for extracting total RNA in cells and tissues
1) MGC-803 cells are taken as adherent cells, pancreatin digestion is not needed, and Trizol can be directly used for blowing, digesting and cracking after the culture medium is removed; the amount of Trizol is generally determined by the area of the culture dish, and is generally 1mL/10cm2。
Adding 1mL of Trizol to each 50-100 mg of the gastric cancer tissue/paracancer normal tissue to ensure that the sample volume does not exceed 10% of the volume of the Trizol as much as possible, and fully homogenizing for 1-2 min;
2) transferring the cell suspension added with Trizol into a 1.5mL EP tube, standing the mixture for 5min at a warm state to fully crack the cells, and storing the mixture in a refrigerator at the temperature of-80 ℃ for a long time;
3) adding 0.2mL of chloroform into 1mL of Trizol, oscillating for 10-15 s violently, and standing for 2-3 min at room temperature;
4) centrifuging at 12,000rpm at 4 deg.C for 15min, transferring the upper water phase into a new 1.5mL LEP tube, adding 0.5mL isopropanol per 1mL LTrizol, mixing, and standing at room temperature for 5-10 min;
5) centrifugation was carried out at 12,000rpm for 10min at 4 ℃ at which time RNA was visible at the bottom of the EP tube, the supernatant was discarded, cells were washed with 1mL of 75% ethanol per 1mL of trizol, and the pellet was suspended by gentle shaking. At this point, the mixture can be kept at-80 ℃ overnight;
6) centrifuging at 4 ℃ and 7,500rpm for 5min, sucking the supernatant as dry as possible, drying at room temperature for 5-10 min, and not completely drying to avoid affecting RNA dissolution;
7) dissolving RNA in appropriate volume of DEPC H2O, TE buffer or 0.5% SDS, and stored in a refrigerator at-80 ℃ for further use.
2. Detection of RNA quality
The quality of the RNA sample is measured by OD280, OD260 and OD230 values, and the existence of other organic solvents and protein pollution in the RNA sample is judged by OD260/OD280 and OD260/OD230 generally. Good RNA quality is OD260/OD280 ═ 1.8-2.1; OD260/OD230 > 1.8.
3. Reverse transcription
(1) M-MLV reverse transcription of first strand cDNA: the following ingredients were added to sterile centrifuge tubes without RNase:
(2) mixing the above solutions, placing in 65 deg.C water bath, incubating for 5min, immediately cooling on ice, and adding the following components:
(3) mixing the above solutions, centrifuging, placing in 37 deg.C water bath for 5min, and adding 1.0 μ LM-MLV (200U/. mu.L).
(4) After the solution is mixed evenly, the mixture is reacted for 60min in 37 ℃ water bath, and then the mixture is moved to 70 ℃ water bath for 15min to stop the reaction, and the mixture is frozen and stored at minus 20 ℃ for standby, thus obtaining cDNA.
(5) GAPDH was amplified by PCR and the quality of the reverse transcribed cDNA was determined.
2.2.2.3PCR detection of YTHDF1 levels
(1) GAPDH is used as a relative quantitative internal reference, each sample is subjected to 3 times of repetition, and when the samples are fully mixed, bubbles are prevented from being generated, and fluorescence interference is avoided. The PCR reaction system is as follows:
YTHDF1 primer:
YTHDF1-F:TCAGGCTGGAGAATAACGA
YTHDF 1-R:GGTTGTGTGCTTGTAGGAACT
GAPDH primer:
GAPDH-F:TCAACGACCACTTTGTCAAGCTCAGCT
GAPDH-R:GGTGGTCCAGGGGTCTTACT
(2) selecting sample type, serial number and sample repetition serial number on a PCR instrument, setting a primer/probe, and setting PCR reaction conditions as follows:
(3) click "Stat", the PCR instrument starts to run automatically and the fluorescence of the product is collected.
(4) Setting a threshold value of 2-ΔΔCTThe relative expression level of YTHDF1 gene was calculated.
III, H & E staining
(1) Material taking and fixing: 10% formalin is prepared as a fixing solution, and after gastric cancer tissues are taken out, the gastric cancer tissues are quickly put into the formalin for fixation. Prevent cell autolysis and degradation, quickly denature protein, and maintain the original morphological structure of tissue.
(2) And (3) dehydrating: gradient alcohol (50%, 75%, 85%, 95%, absolute ethanol) dehydrates tissues and prevents tissue deformation. Xylene replaces the dehydrating agent and the transparent tissue blocks in the tissue, so that light can penetrate through the tissue, and the tissue blocks play a role of a bridge for next-step wax dipping.
(3) Wax dipping: and putting the transparent tissue block into a 60 ℃ constant temperature wax box for wax dipping to replace the transparent agent in the tissue so as to prepare for embedding.
(4) Embedding: pouring melted paraffin liquid into the embedding frame, clamping the tissue block with toothless forceps, placing in the embedding frame, cooling to solidify at room temperature, removing the embedding frame, and taking out the paraffin block.
(5) Slicing: the embedded wax block is trimmed into a regular quadrangular frustum by a blade and clamped in a wax block clamp, so that the cut surface of the wax block is parallel to a slicing blade, and the wax block is sliced by a Leica slicer to be 4-7 microns thick. Flattening the wax sheet, attaching the wax sheet to a glass slide coated with an adhesive, and drying the glass slide in a 45 ℃ incubator.
(6) Dewaxing: baking the slices at 60 deg.C for 20min, xylene I (20min, 60 deg.C), xylene II (2 min), gradient alcohol (100%, 95%, 80%, 70%) for 2min, and washing with distilled water for 5 min.
(7) Dyeing: hematoxylin 5min, distilled water washing for 3s, 1% hydrochloric acid alcohol for 10s, distilled water slightly washing, saturated lithium carbonate for 2min, 70% ethanol for 2min, 80% ethanol for 2min, 100% ethanol for 2min, and 0.5% eosin for 3 min.
(8) And (3) dehydrating: absolute ethyl alcohol for 2min, xylene I for 2min, and xylene II for 2 min.
(9) Drying the slices by blowing, and sealing the slices by neutral resin. In this step the slices must be thoroughly dehydrated and transparent before they can be capped with neutral gum. If the dehydration is not complete, the film will be white and foggy after being sealed, which is not easy to observe, and the film is easy to fade.
Fourth, Immunohistochemistry (IHC)
The steps of material taking, fixing, dehydrating, wax dipping, embedding and slicing are the same as the steps (1) - (5) of H & E staining.
(6) Antigen retrieval: heating 0.01M sodium citrate buffer solution in an open port of the pressure cooker, after boiling, putting the slice rack with the slices into the pressure cooker, pressing the cooker cover, and continuing heating. And (3) starting timing when the steam valve is aerated, closing the heating source after 2min, opening the pot cover after the pressure is reduced, continuously soaking the slices in the citric acid buffer solution, and naturally cooling to room temperature. (because the antigen in the tissue is fixed by formaldehyde or paraformaldehyde, the antigen epitope is lost by the cross-linking between proteins and the blocking action of aldehyde group, and the antigenic determinant in the tissue cell is exposed again by antigen repair, thus improving the possibility of antigen-antibody recognition)
(7) Deionized water 3min × 3 times, PBS 3min × 3 times.
(8)3%H2O2Incubate 15min at room temperature, deionized water 3min × 3 times, PBS 3min × 3 times.
(9) Sealing goat serum at room temperature for 1 h.
(10) Serum was decanted, washed free, primary antibody diluted with PBS was added dropwise, and incubated overnight in a wet box at 4 ℃.
(11) Taking out the plate the next day, re-warming at 37 deg.C for 60min (to prevent the plate from falling off due to temperature change and to prevent non-specific staining), deionized water for 3min × 3 times, PBS for 3min × 3 times.
(12) Adding reaction enhancing solution dropwise, and incubating at room temperature for 20 min. Deionized water 3min × 3 times, PBS 3min × 3 times.
(13) The enzyme-labeled anti-mouse/rabbit IgG polymer was added dropwise, incubated at room temperature for 30min, PBS 3min X3 times.
(14) DAB color development: and preparing the stable DAB buffer solution, the stable DAB substrate and the stable DAB chromogen into a DAB color developing solution according to a proportion, and placing the DAB color developing solution on ice for later use. The PBS on the slice is wiped off, DAB developing solution is dripped, and when yellow particles are generated under the observation of a microscope, the slice is washed by deionized water to stop the reaction, and the PBS is used for 3min multiplied by 3 times.
(15) Counterdyeing: wiping off PBS, dropping hematoxylin to stain for 10-30 s, and washing with distilled water to terminate the reaction. 5s of hydrochloric acid alcohol, washing with distilled water, treating with saturated lithium carbonate, returning blue for 2min, and washing with distilled water.
(16) Gradient alcohol (70%, 80%, 90%, 95%, 100%) is dehydrated, 2 min/gradient, xylene I, II are each 2min, after blowing dry by a blower, neutral resin is sealed and examined under a microscope.
Example 1 high expression of YTHDF1 in gastric cancer
YTHDF1 is highly expressed in gastric cancer in the cBioPortal database and the TCGA database
The invention analyzes the cBioPortal (cBio Cancer Genomics Portal) database of 630 cases of primary gastric adenocarcinoma and researches the RNA methylation modification (m) in the gastric adenocarcinoma development6A) The genetic changes of the related genes resulted in the results shown in fig. 1, and the mutation rate of YTH family readers (except YTHDF2) was higher in the database than those of writers (YTHDF1, WTAP, METTL14) and erasers (ALKBH5, FTO), and YHTDF1 in the YTH family readers was the highest, reaching about 7%. Analyzing the mutation type of YTHDF1, it is found that 65% of the mutations are gene amplification, and the gene amplification can cause the over-expression of the gene product, which indicates that YTHDF1 has high expression in gastric cancer.
The DNA methylation levels of YTHDF1 initiator in 338 gastric Cancer cases in the tcga (the Cancer Genome atlas) database were further analyzed, and it was found that the DNA methylation levels of YTHDF1 initiator in 338 gastric Cancer cases were lower (β value < 0.1) (fig. 2), indicating that YTHDF1 is in an active transcriptional state in gastric Cancer. Subsequent analysis of the TCGA database comprising 24 different cancer types revealed that YTHDF1 was significantly higher expressed in gastric cancer patients than normal tissue, and that upregulation of YTHDF1 was observed in most tumors, suggesting that YTHDF1 has a general oncogenic role in tumor development (fig. 3). The biological information analysis shows that the high expression of YTHDF1 is obviously related to the progression of gastric cancer (figure 4) and the lower overall survival rate (figure 5), the expression level of YTHDF1 is obviously increased compared with normal tissues in the initial period and the progressive period, and the data deviation value of the progressive period is more compact (figure 5).
Second, YTHDF1 is highly expressed in gastric cancer patients
In order to further clarify the correlation between YTHDF1 and gastric cancer occurrence and verify the accuracy of TCGA data, a total of 113 new gastric cancer patients who are from Shanxi tumor hospital, China civil liberation military 301 hospital and China medical science institute tumor hospital and have tumor removed through surgical treatment are collected, the gastric cancer tissues and tissues beside the cancer of the same patient are respectively sampled, and the clinical data of the patients are statistically analyzed. The expression of YTHDF1 in gastric cancer tissues and paracarcinoma tissues in gastric cancer cases is verified 113 by the three-time PCR method, and the result is shown in FIG. 6, compared with the paracarcinoma tissues, the mRNA level of YTHDF1 is remarkably increased in the gastric cancer tissues (p is 0.0005), which indicates that the expression of YTHDF1 is positively correlated with the onset of gastric cancer patients. The results of the five, Immunohistochemistry (IHC) analysis are shown in fig. 7, and the up-regulation of YTHDF1 protein level in gastric cancer tissues (2 gastric cancer patients) was consistent with RNA level.
The abnormal high expression of YTHDF1 in gastric cancer tissues and the correlation of poor clinical pathological characteristics, such as nerve invasion, pTNM pathological stages (III, IV, vs I and II) and vascular invasion, are analyzed, and the results are shown in FIG. 8, the YTHDF1 shows a high expression trend in the III and IV stages of the pTNM pathological stages as a whole, and shows a low expression trend in the I and II stages of the pTNM pathological stages, which indicates that the high expression YTHDF1 aggravates the pathological process of gastric cancer. The 113 cases were classified into invasive group (positive) and non-invasive group (negative) depending on whether or not invasion occurred, and the results are shown in fig. 8, and YTHDF1 showed a significant tendency to be up-regulated in both neuroinvasive and vascular invasive groups compared to the non-invasive group, indicating that YTHDF1 promoted metastasis of gastric cancer cells. The results show that the expression of YTHDF1 is positively correlated with nerve invasion, pTNM pathological stage (stage III, IV vs I, II) and vascular invasion.
Kaplan-Meier survival analysis was performed, and the results are shown in FIG. 9, wherein the overall survival rate of 4 years is poor in patients with YTHDF1 high-expression gastric cancer (Log-rank (Mantel-Cox) p ═ 0.0075).
The above analysis all showed that YTHDF1 has carcinogenic effect in gastric cancer.
Example 2YTHDF1 knockdown inhibits progression and metastasis of gastric cancer
First, construct shYTHDF1-1, shYTHDF1-2 and shControl
1. According to the general principle of shRNA design, siRNA targets are designed. The sequences of two siRNA targets of YTHDF1 gene are 5'-GATACAGTTCATGACAATGA-3' (SEQ ID NO: 1) and 5'-GAAACGTCCAGCCTAATTCT-3' (SEQ ID NO: 2), respectively.
Primers used to prepare the coding genes for the shRNA that produced the two sirnas were YTHDF 1-1F: 5'-CCGGGATACAGTTCATGACAATGACTTCCTGTCAGATCATTGTCATGAACTGTATCTTTTTG-3'
YTHDF1-1R:5′-AATTCAAAAAGATACAGTTCATGACAATGATCTGACAGGAAGTCATTGTCATGAACTGTATC-3′
YTHDF1-2F:5′-CCGGGAAACGTCCAGCCTAATTCTCTTCCTGTCAGAAGAATTAGGCTGGACGTTTCTTTTTG-3′
YTHDF1-2R:5′-AATTCAAAAAGAAACGTCCAGCCTAATTCTTCTGACAGGAAGAGAATTAGGCTGGACGTTTC-3′
2. Primer annealing to form double-stranded fragments with cohesive ends
Primers YTHDF1-1F and YTHDF1-1R anneal to form a double-stranded fragment with sticky ends, which is named shYTHDF 1-1:
primers YTHDF1-2F and YTHDF1-2R anneal to form a double-stranded fragment with sticky ends, which is named shYTHDF 1-2:
wherein, the annealing system is as follows:
the annealing procedure is as follows: at 95 ℃ for 3 min; reducing the temperature by 1 ℃ every 60s, and slowly annealing until the temperature reaches 25 ℃; storing at 3.4 deg.C.
3. Construction of interference vectors
The vector pLKO.1-puro (Addgene, cat. 10878) was double digested with EcoR I and Age I to obtain a vector fragment, which was recovered and purified. And (3) connecting YTHDF1-1 and YTHDF1-2 with the vector fragment respectively to obtain YTHDF1-1 and YTHDF1-2 connecting products. Respectively transforming the YTHDF1-1 and YTHDF1-2 connecting products into competent escherichia coli JM109, after bacteria grow out, selecting a single bacterial colony in 5ml of LB culture medium containing corresponding antibiotics, and carrying out shake culture at 37 ℃ for 8-12 h; detecting the picked clones by PCR; observing the amplification result by agarose gel electrophoresis; and selecting PCR amplification positive clone and sequencing to further identify the correctness of the nucleic acid sequence of the coding region of the cloned gene, checking to determine whether the reading frame is correct, sequencing the correct plasmid, carrying out large-scale extraction on the plasmid to obtain the plasmid, and carrying out subsequent experiments.
The recombinant expression vector obtained by replacing the fragment (small fragment) between the Age I and EcoR I recognition sites of the vector pLKO.1-puro with the DNA molecule of sequence 3 (5'-GATACAGTTCATGACAATGACTTCCTGTCAGATCATTGTCATGAACTGTATCTTTTT-3') as a result of sequencing and keeping the other sequences of the vector pLKO.1-puro unchanged was named shYTHDF1-1 (interference vector). The DNA molecule shown in the sequence 3 codes shRNA taking YTHDF1 gene as a target spot, the shRNA is named as YTHDF1-1-shRNA, and the sequence of YTHDF1-1-shRNA is sequence 5 (5'-GAUACAGUUCAUGACAAUGACUUCCUGUCAGAUCAUUGUCAUGAACUGU AUCUUUUU-3'). The stem sequence of YTHDF1-1-shRNA is the 1 st to 20 th nucleotides of the sequence 5 and the 33 th to 52 th nucleotides of the sequence 5, and the loop sequence of YTHDF1-1-shRNA is the 21 st to 32 th nucleotides of the sequence 5. YTHDF1-1-shRNA generates siRNA interfering YTHDF1 gene, the siRNA is named as YTHDF1-1-siRNA, and the target sequence of YTHDF1-1-siRNA is 5'-GATACAGTTCATGACAATGA-3' (sequence 1).
The recombinant expression vector obtained by replacing the fragment (small fragment) between the Age I and EcoR I recognition sites of the vector pLKO.1-puro with the DNA molecule of sequence 4 (5'-GAAACGTCCAGCCTAATTCTCTTCCTGTCAGAAGAATTAGGCTGGACGTTTCTTTTT-3') as a result of sequencing and keeping the other sequences of the vector pLKO.1-puro unchanged was named shYTHDF1-2 (interference vector). The DNA molecule shown in the sequence 4 encodes shRNA taking YTHDF1 gene as a target spot, the shRNA is named as YTHDF1-2-shRNA, and the sequence of YTHDF1-2-shRNA is sequence 6 (5'-GAAACGUCCAGCCUAAUUCUCUUCCUGUCAGAAGAAUUAGGCUGGACGU UUCUUUUU-3'). The stem sequence of YTHDF1-2-shRNA is the 1 st to 20 th nucleotides of the sequence 6 and the 33 th to 52 th nucleotides of the sequence 6, and the loop sequence of YTHDF1-2-shRNA is the 21 st to 32 th nucleotides of the sequence 6. YTHDF1-2-shRNA generates siRNA interfering YTHDF1 gene, the siRNA is named as YTHDF1-2-siRNA, and the target sequence of YTHDF1-2-siRNA is 5'-GAAACGTCCAGCCTAATTCT-3' (sequence 2).
Transfection experiment of gastric cancer cell line
The day before transfection, the gastric cancer cell lines (i.e., MGC-803 and HGC-27) were passaged at appropriate densities to place the human gastric cancer cell lines in log phase growth at the time of transfection. Washing the cells once with serum-free medium, then washing with 1-2 × 106cells/well density cells were resuspended in complete medium without antibiotics, 1600 μ l/well, and transferred to 6-well plates for culture (adherent cells need to be plated one day earlier, cell density is 50% -70%, transfection is performed within 24h, and cells are in logarithmic phase). Adding 3 μ g shYTHDF1-1, 3 μ g shYTHDF1-2 and 3 μ g shControl (unmodified vector pLKO.1-puro, Addgene, Inc., Cat. 10878) into 200 μ l serum-free culture medium, mixing to obtain serum-free solution containing shYTHDF1-1, shYTHDF1-2 and shControl, and standing at room temperature for 5 min; and adding three parts of 3 mul of liposome lipofectamine2000 into 200 mul of serum-free culture medium respectively to obtain serum-free solution containing liposome lipofectamine2000, standing for 5min at room temperature, mixing the serum-free solutions containing shYTHDF1-1, shYTHDF1-2 and shControl in the three serum-free solutions containing liposome lipofectamine2000 respectively to obtain a three-part mixture, and standing for 20min at room temperature. The mixture was added directly to a 6-well plate of a human gastric cancer cell line (i.e., MG)C-803 and HGC-27), and mixing the mixture by slight shaking, culturing the mixture in an incubator at 37 ℃ for 4 to 6 hours, then replacing the culture medium with a culture medium containing serum and antibiotics, and continuing culturing the mixture for 24 to 48 hours. Changes in RNA levels were detected 24h after transfection and changes in protein were detected 48h after transfection.
The expression levels of shYTHDF1-1, shYTHDF1-2 and shControl in gastric cancer cell lines (namely MGC-803 and HGC-27) are respectively detected by using a 'one-and-protein immunoblotting experiment (western blot)' method, and the result shows that shYTHDF1-1, shYTHDF1-2 and shControl can be stably expressed in MGC-803 and HGC-27 (figure 10). Therefore, the gastric cancer cell lines MGC-803 and HGC-27 were selected as transfected cell lines to study the effect in gastric cancer after knockdown of YTHDF 1.
Through the method for transfecting the gastric cancer cell line, shYTHDF1-1, shYTHDF1-2 and shControl are used for transfecting gastric cancer cell lines MGC-803 and HGC-27 respectively, so that gastric cancer cell lines MGC-803 and HGC-27 of a shYTHDF1-1 group, a shYTHDF1-2 group and a shControl group are obtained after transfection respectively, and the following experiments are carried out, wherein the specific steps are as follows:
1. cell proliferation assay
The gastric cancer cell lines MGC-803 and HGC-27 after 8 hours of transfection were trypsinized and the cells were harvested by centrifugation at 800rpm × 5min, resuspended in fresh complete medium and expressed at 1 × 103Cells/well were plated in a 96-well plate, and 3 replicates were set, and after cells were attached (about 6 hours), 10. mu.l of CCK8 reagent was added per well, incubated at 37 ℃ for 2 hours, and then the absorbance at a wavelength of 450nm was measured using a microplate reader as 0 hour cell activity, and then cell activity was measured every 24 hours in the same manner.
The results showed that YTHDF1 was knocked down (indicated by "shYTHDF 1-1" and "shYTHDF 1-2" in the figure) to inhibit gastric cancer cell proliferation (FIG. 11) compared to the control group (indicated by "shControl" in the figure).
2. Cell invasion assay
(1) The Wegener Matrigel is taken out from the temperature of minus 80 ℃, and then is subjected to sol-gel overnight at the temperature of 4 ℃, chamber, gun head, 1.5ml of EP tube and 24-hole plate which are put at the temperature of minus 20 ℃ for precooling overnight, and the diluted Matrigel is subjected to precooling overnight at the temperature of 4 ℃ by using serum-free culture medium, so that the Matrigel is prevented from being solidified due to too high temperature in the processes of diluting the gel and absorbing the gel.
(2) An ice-on-ice operation step: the Matrigel was diluted to a final concentration of 1mg/ml using a pre-cooled serum-free medium, mixed well and placed on ice for use to prevent Matrigel from freezing.
(3) The pre-cooled chamber and 24-well plate are taken out, and the chamber is put into the 24-well plate. And taking the precooled gun head, vertically adding 100 mu l of diluted Matrigel below the center of the chamber, and taking a notice that the gun head does not touch the membrane at the bottom of the chamber to prevent the gun head from puncturing or scratching the bottom mould. Incubating for 3h in an incubator at 37 ℃ to completely solidify the matrigel.
(4) And taking out the solidified chamber, slightly washing the serum-free culture medium once, sucking the culture medium by attaching a gun head to the side wall of the chamber, and putting the culture medium into a 24-well plate for later use.
(5) The gastric cancer cell lines MGC-803 and HGC-27 after 8h of transfection were trypsinized, and after collection, washed 2 times with serum-free medium (to remove the influence of residual serum on cell surface on cell growth), and the cell pellet was resuspended in the serum-free medium. After counting, the number of cells was adjusted to 2X 105Perml, 150. mu.l/chamber of cell suspension was added to the upper chamber of the chamber, and 800. mu.l of 20% FCS-containing medium was added to the lower chamber at 37 ℃ and 5% CO2And culturing for 24 h.
(6) The chamber was removed, the medium carefully discarded from the upper chamber, placed in a new 24-well plate, 1ml of methanol added to the lower chamber and fixed at room temperature for 30 min.
(7) The methanol was discarded and 600. mu.l of crystal violet stain was added to each well in the lower chamber and stained for 30min at room temperature.
(8)ddH2And (3) slightly soaking the chamber for a plurality of times, washing out crystal violet, taking out the chamber, slightly wiping off cells on the surfaces of matrigel and membrane by using a cotton swab, and reversely buckling and drying the chamber.
(9) Carefully remove the chamber bottom membrane with forceps, place on a glass slide for fixation, and perform microscopic examination of the cells after sealing with neutral resin. And counting cells in 10 visual fields of each group, and counting the results.
The results showed that knocking down YTHDF1 (shown as "shYTHDF 1-1" and "shYTHDF 1-2" in the figure) significantly inhibited the invasion ability of gastric cancer cells, compared to the control group (shown as "shControl" in the figure) (FIG. 12).
3. Cell migration assay
Pancreatin digestion of gastric cancer cell lines MGC-803 and HGC-27 after 24h transfection, centrifuging at 900rpm × 5min to collect cells, washing with serum-free medium three times, suspending the cells with serum-free medium and counting, adjusting the concentration to 2 × 105And/ml. 400. mu.l of the cell suspension was added to the upper chamber of the transwell chamber, and 600. mu.l of the cell complete medium containing 20% serum was added to the lower chamber, and incubated at 37 ℃ for 24 h. The chamber was removed, the medium was discarded, washed twice with PBS, the non-invasive cells on the upper indoor surface were wiped off with a cotton swab, the chamber was inverted and a drop of serum was added to the back, and air dried naturally at room temperature. Adding 1ml of methanol or 4% formaldehyde for fixation for ten minutes, removing the fixation solution, and naturally drying at room temperature. Adding 1ml of 0.1% crystal violet staining solution, staining for 10min at room temperature, slightly rinsing in tap water to a proper shade, reversing the chamber, and naturally air-drying at room temperature. Carefully peeling the basement membrane of the chamber by using a surgical blade, fixing the basement membrane on a glass slide, and performing microscopic examination on cells and photographing and counting after the cells are sealed by neutral resin.
The results showed that knocking down YTHDF1 (shown as "shYTHDF 1-1" and "shYTHDF 1-2" in the figure) significantly inhibited the migration ability of gastric cancer cells, compared to the control group (shown as "shControl" in the figure) (FIG. 12).
Example 3 Effect of YTHDF1 on subcutaneous neoplasia in nude mice
Through a nude mouse subcutaneous tumor forming experiment, the regulation and control effect of YTHDF1 on the generation and development of gastric cancer and the potential treatment value of YTHDF1 are researched in an animal body. And (3) testing the subcutaneous tumor formation of the nude mice after knocking down YTHDF 1.
1. Detection of tumor size
MGC-803 cells were transfected with shYTHDF1-1, shYTHDF1-2, and shControl, respectively, to obtain a population of 5X 10 cells, as described in example 2 for the transfection experiment of gastric cancer cell lines6The gastric cancer cells MGC-803 with the same amount in the shYTHDF1-1 group, shYTHDF1-2 group and shControl group in the logarithmic growth phase are suspended in 0.1ml of Phosphate Buffered Saline (PBS) to obtain suspensions of the gastric cancer cells MGC-803 in the shYTHDF1-1 group, shYTHDF1-2 group and shControl group.
Purchasing 5-6 weeks old female nude mice, randomly dividing into three groups, and adapting to 2E to E in animal roomsAfter 3 days, each group of female nude mice was injected subcutaneously on the posterior side with the suspension of the gastric cancer cell MGC-803 (3X 10)6Cell/cell), the size of the growth of the tumor mass was observed in real time.
Statistics of tumor size revealed that, in MGC-803, YTHDF1 (indicated as "shYTHDF 1-1" and "shYTHDF 1-2" in the figure) was knocked down, the tumor size was significantly smaller than that in the control group (indicated as "shControl" in the figure) (FIG. 13)
Statistics on the tumor volume showed that the tumor volume was significantly lower in MGC-803 mice knocked-down with YTHDF1 (indicated as "shYTHDF 1-1" and "shYTHDF 1-2") (FIG. 14).
Statistics on tumor mass weight showed that tumor mass weight was significantly lower in MGC-803-knocked-down YTHDF1 (indicated as "shYTHDF 1-1" and "shYTHDF 1-2") nude mice than in control group (indicated as "shControl" in the figure) (FIG. 15).
And secondly, after tumor masses are obtained, taking a part of the tumor masses to perform H & E staining and immunohistochemical experiments.
Tumor mass tissues are stained by a 'three-step and H & E staining' method, and the proliferation antigen Ki-67 and the apoptosis protein Caspase3 are qualitatively and quantitatively determined respectively. Compared with the control group (shown as "shControl"), the nude mice with knockdown YTHDF1 (shown as "shYTHDF 1-1" and "shYTHDF 1-2") showed tumor tissues with low Ki-67 expression and high Caspase3 expression (FIG. 16).
Immunohistochemistry of Ki-67 and Caspase3 by "four, Immunohistochemistry (IHC)" was quantified and the results showed: compared with the control group (shown as "shControl" in the figure), the nude mice with YTHDF1 (shown as "shYTHDF 1-1" and "shYTHDF 1-2" in the figure) knocked down in MGC-803 show tumor tissues with low Ki-67 expression and high Caspase3 expression (FIG. 16).
Further, the cancer promotion function of YTHDF1 is shown.
Example 4 in vivo validation of the Effect of knockdown of YTHDF1 on gastric cancer cell metastasis
And (3) detecting an in-vivo experiment of the mice, namely a nude mice subcutaneous tumor forming experiment to test whether the proliferation of the gastric cancer cells can be inhibited after knocking down YTHDF 1.
Firstly, establishing a gastric cancer mouse model, which comprises the following specific steps:
MGC-803 cells were transfected with shYTHDF1-1, shYTHDF1-2, and shControl, respectively, to obtain a population of 5X 10 cells, as described in example 2 for the transfection experiment of gastric cancer cell lines6The gastric cancer cells MGC-803 with the same amount in the shYTHDF1-1 group, shYTHDF1-2 group and shControl group in the logarithmic growth phase are suspended in 0.1m1 Phosphate Buffered Saline (PBS) to obtain suspensions of the gastric cancer cells MGC-803 in the shYTHDF1-1 group, shYTHDF1-2 group and shControl group.
5-6 weeks old female NOD/SCID mice are purchased and randomly divided into 3 groups, after the animal house is adapted for 2-3 days, the mice are subjected to sublethal dose X-ray irradiation (250cGy), suspensions of gastric cancer cells MGC-803 of the shYTHDF1-1 group, the shYTHDF1-2 group and the shControl group are respectively injected through tail veins on the irradiation day, and after about thirty days of culture, dissected mice show obvious tumor metastasis symptoms (cachexia), thereby proving that the gastric cancer mouse model is successfully constructed. The number of lung nodules in nude mice was counted.
The results showed that knock-down of YTHDF1 (designated as "shYTHDF 1-1" and "shYTHDF 1-2" in the figure) in MGC-803 greatly reduced the formation of lung nodules compared to the control group (designated as "shControl" in the figure) (FIGS. 17 and 18).
Secondly, a nude mouse abdominal cavity transfer model comprises the following specific steps:
MGC-803 cells were transfected with shYTHDF1-1, shYTHDF1-2, and shControl, respectively, to obtain a population of 5X 10 cells, as described in example 2 for the transfection experiment of gastric cancer cell lines6The gastric cancer cells MGC-803 with the same amount in the shYTHDF1-1 group, shYTHDF1-2 group and shControl group in the logarithmic growth phase are suspended in 0.1m1 Phosphate Buffered Saline (PBS) to obtain suspensions of the gastric cancer cells MGC-803 in the shYTHDF1-1 group, shYTHDF1-2 group and shControl group.
Female NOD/SCID mice 5-6 weeks old are purchased and randomly divided into three groups, after the animals are adapted to the animal rooms for 2-3 days, suspensions of gastric cancer cells MGC-803 of the shYTHDF1-1 group, the shYTHDF1-2 group and the shControl group are respectively injected into the abdominal cavity of a nude mouse, the mice are killed after 4 weeks, the abdominal cavity is opened, and the number of intestinal nodules is counted.
The results showed that knock-down of YTHDF1 (designated as "shYTHDF 1-1" and "shYTHDF 1-2" in the figure) in MGC-803 significantly reduced the formation of intestinal nodules compared to the control group (designated as "shControl" in the figure) (FIG. 19).
Taken together, the results indicate that YTHDF1 acts as an oncogene in gastric cancer to regulate tumor proliferation and metastasis.
The present invention has been described in detail above. It will be apparent to those skilled in the art that the invention can be practiced in a wide range of equivalent parameters, concentrations, and conditions without departing from the spirit and scope of the invention and without undue experimentation. While the invention has been described with reference to specific embodiments, it will be appreciated that the invention can be further modified. In general, this application is intended to cover any variations, uses, or adaptations of the invention following, in general, the principles of the invention and including such departures from the present disclosure as come within known or customary practice within the art to which the invention pertains. The use of some of the essential features is possible within the scope of the claims attached below.
SEQUENCE LISTING
<110> institute of basic medicine of Chinese academy of medical sciences
Application of substance for inhibiting YTHDF1 activity in preparation of product for preventing or treating gastric cancer
<130> GNCFY191528
<160> 6
<170> PatentIn version 3.5
<210> 1
<211> 20
<212> DNA
<213> Artificial Sequence (Artificial Sequence)
<400> 1
<210> 2
<211> 20
<212> DNA
<213> Artificial Sequence (Artificial Sequence)
<400> 2
<210> 3
<211> 57
<212> DNA
<213> Artificial Sequence (Artificial Sequence)
<400> 3
gatacagttc atgacaatga cttcctgtca gatcattgtc atgaactgta tcttttt 57
<210> 4
<211> 57
<212> DNA
<213> Artificial Sequence (Artificial Sequence)
<400> 4
gaaacgtcca gcctaattct cttcctgtca gaagaattag gctggacgtt tcttttt 57
<210> 5
<211> 57
<212> RNA
<213> Artificial Sequence (Artificial Sequence)
<400> 5
gauacaguuc augacaauga cuuccuguca gaucauuguc augaacugua ucuuuuu 57
<210> 6
<211> 57
<212> RNA
<213> Artificial Sequence (Artificial Sequence)
<400> 6
gaaacgucca gccuaauucu cuuccuguca gaagaauuag gcuggacguu ucuuuuu 57
Claims (10)
1. The application of the substance for inhibiting the YTHDFl gene expression in cells in preparing products for preventing and/or treating gastric cancer.
2. Use of a substance that inhibits the expression of the YTHDF1 gene in a cell as described in any one of:
A1) the application in preparing products for inhibiting gastric cancer cell proliferation;
A2) the application in preparing products for inhibiting the migration of gastric cancer cells;
A3) the application in preparing products for inhibiting the invasion of liver and stomach cancer cells.
3. The application of a substance for inhibiting YTHDF1 activity in cells in preparing a product for preventing and/or treating gastric cancer.
4. Use of a substance that inhibits the activity of YTHDF1 in a cell as described in any one of:
B1) the application in preparing products for inhibiting gastric cancer cell proliferation;
B2) the application in preparing products for inhibiting the migration of gastric cancer cells;
B3) the application in preparing products for inhibiting the invasion of liver and stomach cancer cells.
5. Use according to any one of claims 1 to 4, characterized in that: the substance inhibiting YTHDF1 gene expression in the cell and the substance inhibiting YTHDF1 activity in the cell are all any one of the following biological materials 1) -6):
1) generating shRNA of siRNA or a chemical modifier of the shRNA; the siRNA is small interfering RNA interfering the expression of YTHDF1 gene;
2) an expression vector for expressing the shRNA of 1);
3) a recombinant microorganism expressing the shRNA of 1);
4) siRNA generated by 1) the shRNA or a chemical modification of the siRNA;
5) an expression vector expressing 4) the siRNA;
6) a recombinant microorganism expressing 5) said siRNA.
6. A product for preventing and/or treating gastric cancer, or a product for inhibiting proliferation and/or migration and/or invasion of gastric cancer cells, wherein the product comprises any one of the following biomaterials 1) to 6):
1) generating shRNA of siRNA or a chemical modifier of the shRNA; the siRNA is small interfering RNA interfering YTHDF l gene expression;
2) an expression vector for expressing the shRNA of 1);
3) a recombinant microorganism expressing the shRNA of 1);
4) siRNA generated by 1) the shRNA or a chemical modification of the siRNA;
5) an expression vector expressing 4) the siRNA;
6) a recombinant microorganism expressing 5) said siRNA.
7. The application of the substance for detecting the expression quantity or relative expression quantity of YTHDF1 gene in preparing products for identifying or assisting in identifying tumor tissues.
8. The application of the substance for detecting the expression quantity or relative expression quantity of the YTHDF1 gene in preparing products for evaluating or assisting in evaluating the pathological stage of pTNM of a gastric cancer patient.
9. The application of the substance for detecting the expression quantity or relative expression quantity of YTHDF1 gene in preparing products for identifying or assisting in identifying whether gastric cancer patients have nerve invasion or vein invasion.
10. Use according to any one of claims 7 to 9, characterized in that: the substance for detecting the expression quantity or relative expression quantity of the YTHDF1 gene is a system for detecting the expression quantity or relative expression quantity of the YTHDF1 gene by utilizing quantitative PCR.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910981163.5A CN112656805A (en) | 2019-10-15 | 2019-10-15 | Application of substance for inhibiting YTHDF1 activity in preparation of product for preventing or treating gastric cancer |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910981163.5A CN112656805A (en) | 2019-10-15 | 2019-10-15 | Application of substance for inhibiting YTHDF1 activity in preparation of product for preventing or treating gastric cancer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN112656805A true CN112656805A (en) | 2021-04-16 |
Family
ID=75400662
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201910981163.5A Pending CN112656805A (en) | 2019-10-15 | 2019-10-15 | Application of substance for inhibiting YTHDF1 activity in preparation of product for preventing or treating gastric cancer |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112656805A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114395629A (en) * | 2022-01-24 | 2022-04-26 | 上海交通大学医学院附属第九人民医院 | YTHDF1 inhibitor and detection reagent and application thereof |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109337980A (en) * | 2018-11-23 | 2019-02-15 | 中国科学院昆明动物研究所 | The purposes of people's YTHDF1 gene |
-
2019
- 2019-10-15 CN CN201910981163.5A patent/CN112656805A/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109337980A (en) * | 2018-11-23 | 2019-02-15 | 中国科学院昆明动物研究所 | The purposes of people's YTHDF1 gene |
Non-Patent Citations (2)
| Title |
|---|
| CHENG ZHANG等: "Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K‐Akt signaling in gastric cancer", 《CANCER MEDICINE》, vol. 8, no. 10, 26 June 2019 (2019-06-26), pages 4766 - 4781 * |
| 张杰: "m6A甲基化识别蛋白YTHDF1、YTHDF2对胃癌细胞的功能探究", 《中国优秀博硕士学位论文全文数据库(硕士) 医药卫生科技辑》, no. 01, 15 January 2019 (2019-01-15), pages 072 - 1359 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114395629A (en) * | 2022-01-24 | 2022-04-26 | 上海交通大学医学院附属第九人民医院 | YTHDF1 inhibitor and detection reagent and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN106222170B (en) | Circular rna circ-CCNY and application thereof | |
| CN108486060B (en) | Exosome for treating tumors and preparation method and application thereof | |
| Wang et al. | RhoC is essential for angiogenesis induced by hepatocellular carcinoma cells via regulation of endothelial cell organization | |
| CN111378753A (en) | Application of human PNO1 gene in lung cancer and related product | |
| CN108203732A (en) | Applications of the TRIM24 in diagnosis of glioma | |
| CN108660212B (en) | Application of WDR1 gene in preparation of non-small cell lung cancer treatment and detection products | |
| CN112656805A (en) | Application of substance for inhibiting YTHDF1 activity in preparation of product for preventing or treating gastric cancer | |
| CN109939222B (en) | Medical application of CREG protein for promoting skeletal muscle regeneration | |
| CN109364249B (en) | Application of MANF-targeted substance in preparation of product for treating intrahepatic bile duct cancer | |
| CN108465108B (en) | Specific gene target for preventing or treating brain glioma | |
| CN114225036B (en) | Application of ATXN1 protein as target in preparation of medicine or kit for treating, preventing or diagnosing medulloblastoma | |
| CN114807364A (en) | Application of YRNA fragment hY4F as molecular marker in preparation of lung cancer diagnostic reagent and anti-lung cancer drug | |
| CN106834288A (en) | A kind of long non-coding RNA and its application in diagnosis/treatment stomach cancer | |
| CN109097358A (en) | A kind of lncRNA is preventing or is treating the application in hypertension | |
| CN110742899A (en) | Application of miR-140 in preparation of medicine for inhibiting breast cancer proliferation and migration | |
| CN114921548B (en) | Application of ZNF526 in preparation of liver cancer diagnosis and/or prognosis and treatment preparation, diagnosis, prognosis and treatment preparation | |
| CN116814700B (en) | Application of ACSM5-P425T in construction of drug detection model for treating Xuanwei lung cancer | |
| Zhang et al. | The role of corneal endothelium in macular corneal dystrophy development and recurrence | |
| CN116875694A (en) | Oral squamous carcinoma biomarker and application thereof | |
| CN112725449B (en) | Application of siRNA inhibitor of circ0058792 in preparation of medicine for treating renal clear cell carcinoma | |
| US20240093194A1 (en) | Mir-7-5p mimic for inhibiting migration and invasion of breast cancer, screening method and application thereof | |
| CN110656174B (en) | Specific marker closely related to occurrence and development of primary hepatocellular carcinoma and application thereof | |
| CN118853813A (en) | Test method for researching promotion of pancreatic cancer malignant progress by FLRT3 | |
| CN115094134A (en) | Application of PCSK9 in macrophage M2 type polarization and related diseases thereof | |
| CN117482103A (en) | Application of miR-3919, polynucleotide derivative or agonist thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20210416 |
|
| RJ01 | Rejection of invention patent application after publication |