CN112587455A - Plant composition and herbal whitening and freckle removing sleep mask gel - Google Patents
Plant composition and herbal whitening and freckle removing sleep mask gel Download PDFInfo
- Publication number
- CN112587455A CN112587455A CN202011538204.2A CN202011538204A CN112587455A CN 112587455 A CN112587455 A CN 112587455A CN 202011538204 A CN202011538204 A CN 202011538204A CN 112587455 A CN112587455 A CN 112587455A
- Authority
- CN
- China
- Prior art keywords
- extract
- root extract
- percent
- root
- whitening
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000002087 whitening effect Effects 0.000 title claims abstract description 33
- 208000003351 Melanosis Diseases 0.000 title claims abstract description 29
- 239000000203 mixture Substances 0.000 title claims abstract description 18
- 239000000284 extract Substances 0.000 claims abstract description 131
- 239000001947 glycyrrhiza glabra rhizome/root Substances 0.000 claims abstract description 24
- 241001313857 Bletilla striata Species 0.000 claims abstract description 19
- 244000223014 Syzygium aromaticum Species 0.000 claims abstract description 18
- 235000016639 Syzygium aromaticum Nutrition 0.000 claims abstract description 18
- 241000196324 Embryophyta Species 0.000 claims abstract description 17
- 244000197580 Poria cocos Species 0.000 claims abstract description 17
- 235000008599 Poria cocos Nutrition 0.000 claims abstract description 17
- 240000004534 Scutellaria baicalensis Species 0.000 claims abstract description 16
- 235000017089 Scutellaria baicalensis Nutrition 0.000 claims abstract description 16
- 206010014970 Ephelides Diseases 0.000 claims abstract description 12
- 244000303040 Glycyrrhiza glabra Species 0.000 claims abstract description 10
- 235000006200 Glycyrrhiza glabra Nutrition 0.000 claims abstract description 10
- 235000008434 ginseng Nutrition 0.000 claims abstract description 9
- 244000146462 Centella asiatica Species 0.000 claims abstract description 7
- 235000004032 Centella asiatica Nutrition 0.000 claims abstract description 7
- 235000011135 Salvia miltiorrhiza Nutrition 0.000 claims abstract description 7
- 241000304195 Salvia miltiorrhiza Species 0.000 claims abstract description 7
- 241001080798 Polygala tenuifolia Species 0.000 claims abstract description 6
- 244000042430 Rhodiola rosea Species 0.000 claims abstract description 6
- 235000003713 Rhodiola rosea Nutrition 0.000 claims abstract description 6
- 235000005035 Panax pseudoginseng ssp. pseudoginseng Nutrition 0.000 claims abstract description 5
- 235000003140 Panax quinquefolius Nutrition 0.000 claims abstract description 5
- LPLVUJXQOOQHMX-QWBHMCJMSA-N glycyrrhizinic acid Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@H](O[C@@H]1O[C@@H]1C([C@H]2[C@]([C@@H]3[C@@]([C@@]4(CC[C@@]5(C)CC[C@@](C)(C[C@H]5C4=CC3=O)C(O)=O)C)(C)CC2)(C)CC1)(C)C)C(O)=O)[C@@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O LPLVUJXQOOQHMX-QWBHMCJMSA-N 0.000 claims abstract description 4
- 235000011477 liquorice Nutrition 0.000 claims abstract description 4
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 claims description 27
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 21
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 20
- POJWUDADGALRAB-UHFFFAOYSA-N allantoin Chemical compound NC(=O)NC1NC(=O)NC1=O POJWUDADGALRAB-UHFFFAOYSA-N 0.000 claims description 18
- 229920001577 copolymer Polymers 0.000 claims description 18
- 238000003756 stirring Methods 0.000 claims description 18
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 claims description 14
- 229940107131 ginseng root Drugs 0.000 claims description 13
- 229940059958 centella asiatica extract Drugs 0.000 claims description 12
- 229940024332 polygala tenuifolia root extract Drugs 0.000 claims description 12
- 239000002994 raw material Substances 0.000 claims description 12
- 229940084801 salvia miltiorrhiza root extract Drugs 0.000 claims description 12
- -1 polydimethylsiloxane Polymers 0.000 claims description 11
- MXOAEAUPQDYUQM-QMMMGPOBSA-N (S)-chlorphenesin Chemical compound OC[C@H](O)COC1=CC=C(Cl)C=C1 MXOAEAUPQDYUQM-QMMMGPOBSA-N 0.000 claims description 9
- OMIGHNLMNHATMP-UHFFFAOYSA-N 2-hydroxyethyl prop-2-enoate Chemical compound OCCOC(=O)C=C OMIGHNLMNHATMP-UHFFFAOYSA-N 0.000 claims description 9
- QCDWFXQBSFUVSP-UHFFFAOYSA-N 2-phenoxyethanol Chemical compound OCCOC1=CC=CC=C1 QCDWFXQBSFUVSP-UHFFFAOYSA-N 0.000 claims description 9
- HIQIXEFWDLTDED-UHFFFAOYSA-N 4-hydroxy-1-piperidin-4-ylpyrrolidin-2-one Chemical compound O=C1CC(O)CN1C1CCNCC1 HIQIXEFWDLTDED-UHFFFAOYSA-N 0.000 claims description 9
- POJWUDADGALRAB-PVQJCKRUSA-N Allantoin Natural products NC(=O)N[C@@H]1NC(=O)NC1=O POJWUDADGALRAB-PVQJCKRUSA-N 0.000 claims description 9
- BIVBRWYINDPWKA-VLQRKCJKSA-L Glycyrrhizinate dipotassium Chemical compound [K+].[K+].O([C@@H]1[C@@H](O)[C@H](O)[C@H](O[C@@H]1O[C@H]1CC[C@]2(C)[C@H]3C(=O)C=C4[C@@H]5C[C@](C)(CC[C@@]5(CC[C@@]4(C)[C@]3(C)CC[C@H]2C1(C)C)C)C(O)=O)C([O-])=O)[C@@H]1O[C@H](C([O-])=O)[C@@H](O)[C@H](O)[C@H]1O BIVBRWYINDPWKA-VLQRKCJKSA-L 0.000 claims description 9
- 229920002385 Sodium hyaluronate Polymers 0.000 claims description 9
- 229960000458 allantoin Drugs 0.000 claims description 9
- 229960003993 chlorphenesin Drugs 0.000 claims description 9
- 239000004205 dimethyl polysiloxane Substances 0.000 claims description 9
- 229940101029 dipotassium glycyrrhizinate Drugs 0.000 claims description 9
- 235000011187 glycerol Nutrition 0.000 claims description 9
- WCVRQHFDJLLWFE-UHFFFAOYSA-N pentane-1,2-diol Chemical compound CCCC(O)CO WCVRQHFDJLLWFE-UHFFFAOYSA-N 0.000 claims description 9
- 229960005323 phenoxyethanol Drugs 0.000 claims description 9
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 claims description 9
- 229940010747 sodium hyaluronate Drugs 0.000 claims description 9
- YWIVKILSMZOHHF-QJZPQSOGSA-N sodium;(2s,3s,4s,5r,6r)-6-[(2s,3r,4r,5s,6r)-3-acetamido-2-[(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2- Chemical compound [Na+].CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 YWIVKILSMZOHHF-QJZPQSOGSA-N 0.000 claims description 9
- 240000004371 Panax ginseng Species 0.000 claims description 8
- BQMNFPBUAQPINY-UHFFFAOYSA-N azane;2-methyl-2-(prop-2-enoylamino)propane-1-sulfonic acid Chemical compound [NH4+].[O-]S(=O)(=O)CC(C)(C)NC(=O)C=C BQMNFPBUAQPINY-UHFFFAOYSA-N 0.000 claims description 8
- 229940082941 sedum roseum root extract Drugs 0.000 claims description 8
- NCZPCONIKBICGS-UHFFFAOYSA-N 3-(2-ethylhexoxy)propane-1,2-diol Chemical compound CCCCC(CC)COCC(O)CO NCZPCONIKBICGS-UHFFFAOYSA-N 0.000 claims description 6
- DFPAKSUCGFBDDF-UHFFFAOYSA-N Nicotinamide Chemical compound NC(=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-UHFFFAOYSA-N 0.000 claims description 6
- 241000207929 Scutellaria Species 0.000 claims description 6
- DFPAKSUCGFBDDF-ZQBYOMGUSA-N [14c]-nicotinamide Chemical compound N[14C](=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-ZQBYOMGUSA-N 0.000 claims description 6
- 238000001816 cooling Methods 0.000 claims description 6
- 229940100524 ethylhexylglycerin Drugs 0.000 claims description 6
- 238000010438 heat treatment Methods 0.000 claims description 6
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 claims description 5
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 claims description 5
- 229960002216 methylparaben Drugs 0.000 claims description 5
- 235000002789 Panax ginseng Nutrition 0.000 claims description 4
- 238000002360 preparation method Methods 0.000 claims description 4
- ANZUDYZHSVGBRF-UHFFFAOYSA-N 3-ethylnonane-1,2,3-triol Chemical compound CCCCCCC(O)(CC)C(O)CO ANZUDYZHSVGBRF-UHFFFAOYSA-N 0.000 claims description 3
- 241000202807 Glycyrrhiza Species 0.000 claims description 3
- 235000001188 Peltandra virginica Nutrition 0.000 claims description 3
- 230000001804 emulsifying effect Effects 0.000 claims description 3
- LTINPJMVDKPJJI-UHFFFAOYSA-N iodinated glycerol Chemical compound CC(I)C1OCC(CO)O1 LTINPJMVDKPJJI-UHFFFAOYSA-N 0.000 claims description 3
- 229960003966 nicotinamide Drugs 0.000 claims description 3
- 235000005152 nicotinamide Nutrition 0.000 claims description 3
- 239000011570 nicotinamide Substances 0.000 claims description 3
- 241000049624 Alisma plantago-aquatica subsp. orientale Species 0.000 claims description 2
- 241000282461 Canis lupus Species 0.000 claims 1
- 230000001815 facial effect Effects 0.000 abstract description 6
- 230000036564 melanin content Effects 0.000 abstract description 5
- 239000002085 irritant Substances 0.000 abstract description 3
- 231100000021 irritant Toxicity 0.000 abstract description 3
- 235000017443 Hedysarum boreale Nutrition 0.000 abstract 1
- 235000007858 Hedysarum occidentale Nutrition 0.000 abstract 1
- 244000131316 Panax pseudoginseng Species 0.000 abstract 1
- 239000002537 cosmetic Substances 0.000 description 17
- 238000012360 testing method Methods 0.000 description 15
- 239000000499 gel Substances 0.000 description 14
- 241001465754 Metazoa Species 0.000 description 12
- 230000000694 effects Effects 0.000 description 10
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 6
- 238000010171 animal model Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 206010015946 Eye irritation Diseases 0.000 description 5
- 231100000013 eye irritation Toxicity 0.000 description 5
- 102000003425 Tyrosinase Human genes 0.000 description 4
- 108060008724 Tyrosinase Proteins 0.000 description 4
- 241000700198 Cavia Species 0.000 description 3
- 241000283973 Oryctolagus cuniculus Species 0.000 description 3
- 230000001154 acute effect Effects 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 239000013642 negative control Substances 0.000 description 3
- 239000013641 positive control Substances 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 238000010998 test method Methods 0.000 description 3
- 230000037303 wrinkles Effects 0.000 description 3
- 229920000298 Cellophane Polymers 0.000 description 2
- 201000004624 Dermatitis Diseases 0.000 description 2
- VYZAHLCBVHPDDF-UHFFFAOYSA-N Dinitrochlorobenzene Chemical compound [O-][N+](=O)C1=CC=C(Cl)C([N+]([O-])=O)=C1 VYZAHLCBVHPDDF-UHFFFAOYSA-N 0.000 description 2
- 241001619461 Poria <basidiomycete fungus> Species 0.000 description 2
- AIGAZQPHXLWMOJ-UHFFFAOYSA-N Tanshinone I Chemical compound C1=CC2=C(C)C=CC=C2C(C(=O)C2=O)=C1C1=C2C(C)=CO1 AIGAZQPHXLWMOJ-UHFFFAOYSA-N 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 238000009395 breeding Methods 0.000 description 2
- 230000001488 breeding effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- 210000000744 eyelid Anatomy 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 2
- 229910052753 mercury Inorganic materials 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 238000010181 skin prick test Methods 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 229940126680 traditional chinese medicines Drugs 0.000 description 2
- 230000002936 tranquilizing effect Effects 0.000 description 2
- 206010067484 Adverse reaction Diseases 0.000 description 1
- 241000213006 Angelica dahurica Species 0.000 description 1
- 241000132012 Atractylodes Species 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 241000167550 Centella Species 0.000 description 1
- 206010008570 Chloasma Diseases 0.000 description 1
- 206010051559 Corneal defect Diseases 0.000 description 1
- 206010012442 Dermatitis contact Diseases 0.000 description 1
- 241000628997 Flos Species 0.000 description 1
- 240000008917 Glycyrrhiza uralensis Species 0.000 description 1
- 235000000554 Glycyrrhiza uralensis Nutrition 0.000 description 1
- 241000283977 Oryctolagus Species 0.000 description 1
- 208000012641 Pigmentation disease Diseases 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- 206010040914 Skin reaction Diseases 0.000 description 1
- 229930183118 Tanshinone Natural products 0.000 description 1
- GAMYVSCDDLXAQW-AOIWZFSPSA-N Thermopsosid Natural products O(C)c1c(O)ccc(C=2Oc3c(c(O)cc(O[C@H]4[C@H](O)[C@@H](O)[C@H](O)[C@H](CO)O4)c3)C(=O)C=2)c1 GAMYVSCDDLXAQW-AOIWZFSPSA-N 0.000 description 1
- 230000037374 absorbed through the skin Effects 0.000 description 1
- 239000002390 adhesive tape Substances 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 230000003712 anti-aging effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000003796 beauty Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 231100000739 chronic poisoning Toxicity 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 208000010247 contact dermatitis Diseases 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- NJDNXYGOVLYJHP-UHFFFAOYSA-L disodium;2-(3-oxido-6-oxoxanthen-9-yl)benzoate Chemical compound [Na+].[Na+].[O-]C(=O)C1=CC=CC=C1C1=C2C=CC(=O)C=C2OC2=CC([O-])=CC=C21 NJDNXYGOVLYJHP-UHFFFAOYSA-L 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 229930003944 flavone Natural products 0.000 description 1
- 150000002212 flavone derivatives Chemical class 0.000 description 1
- 235000011949 flavones Nutrition 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 229940020947 fluorescein sodium Drugs 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 241000411851 herbal medicine Species 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 239000008274 jelly Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000008099 melanin synthesis Effects 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 238000000034 method Methods 0.000 description 1
- 230000003020 moisturizing effect Effects 0.000 description 1
- 231100000286 mucous membrane, eye irritation or corrosion testing Toxicity 0.000 description 1
- YLGYACDQVQQZSW-UHFFFAOYSA-N n,n-dimethylprop-2-enamide Chemical compound CN(C)C(=O)C=C YLGYACDQVQQZSW-UHFFFAOYSA-N 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 239000000419 plant extract Substances 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 238000012827 research and development Methods 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 230000036559 skin health Effects 0.000 description 1
- 230000035483 skin reaction Effects 0.000 description 1
- 231100000430 skin reaction Toxicity 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 229940104261 taurate Drugs 0.000 description 1
- XOAAWQZATWQOTB-UHFFFAOYSA-N taurine Chemical compound NCCS(O)(=O)=O XOAAWQZATWQOTB-UHFFFAOYSA-N 0.000 description 1
- VHBFFQKBGNRLFZ-UHFFFAOYSA-N vitamin p Natural products O1C2=CC=CC=C2C(=O)C=C1C1=CC=CC=C1 VHBFFQKBGNRLFZ-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9789—Magnoliopsida [dicotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9728—Fungi, e.g. yeasts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9794—Liliopsida [monocotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/02—Preparations for care of the skin for chemically bleaching or whitening the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/59—Mixtures
- A61K2800/592—Mixtures of compounds complementing their respective functions
- A61K2800/5922—At least two compounds being classified in the same subclass of A61K8/18
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/74—Biological properties of particular ingredients
- A61K2800/78—Enzyme modulators, e.g. Enzyme agonists
- A61K2800/782—Enzyme inhibitors; Enzyme antagonists
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Botany (AREA)
- Biotechnology (AREA)
- Dermatology (AREA)
- Neurosurgery (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Anesthesiology (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Cosmetics (AREA)
Abstract
The invention relates to a herbal plant composition for whitening skin and removing freckles, which consists of glabrous licorice root, salvia miltiorrhiza, clove, liquorice, rhodiola rosea, scutellaria baicalensis, centella asiatica, polygala tenuifolia, rhizoma alismatis, ginseng, bletilla striata and poria cocos. Also relates to herbal whitening and freckle removing sleep mask gel prepared from the extract of the plant composition. The facial mask gel prepared from plant essence is mild and non-irritant to eyes and skin, and can be used for a long time. Further, within 6 weeks, the skin melanin content can be obviously reduced, and the skin color can be improved to a certain extent.
Description
Technical Field
The invention belongs to the technical field of Chinese herbal medicine cosmetics, relates to a herbal plant composition for whitening and removing freckles, and further relates to herbal facial mask gel for whitening and removing freckles.
Background
With the improvement of living standard of people, the skin health awareness and the appearance aesthetic level of people are improved. Functional skin care products are becoming more and more popular with oriental women. Among skin care products with different effects of whitening, removing freckles, resisting wrinkles, moisturizing and the like, Asian women mostly like whitening products, the whiter the skin is, and the better the whitening skin care products are, the skin care products are hot to sell every spring and summer. Therefore, many cosmetic companies have conducted a great deal of research on changing skin pigmentation and removing wrinkles, including development of products for whitening skin, removing freckles and removing wrinkles with high efficiency. Meanwhile, the development and application of natural ingredients are more and more important in the cosmetic industry, and the importance of publicity and promotion of manufacturers to consumers is also important.
The whitening cosmetics are cosmetics added with mercury and other harmful substances from old times, and have very quick and good effect. In particular, mercury binds to tyrosinase, and seriously affects the activity of the tyrosinase, thereby having the effect of inhibiting melanin production. Although the skin of a human body does not show excessive adverse reactions after being used, the skin of the human body can be absorbed through the skin, enter the body and accumulate to cause potential hazards of chronic poisoning, and influence the health of the body, so the method is strictly prohibited. The use safety of the cosmetics is ensured, and the important significance is achieved for ensuring the health of people.
Natural active ingredients separated from traditional Chinese medicines are receiving increasing attention for beauty cosmetics for treating skin diseases with increased pigment, such as chloasma and freckles. The existing research finds that the traditional Chinese medicines such as angelica dahurica, rhizoma typhonii, bighead atractylodes rhizome and the like have the effects of reducing the activity of tyrosinase or reducing the synthesis of melanoma cells. The traditional Chinese medicine components are high in safety, mild in skin effect and remarkable in effect, and are applied to various cosmetics such as whitening cosmetics and anti-aging cosmetics, functional cosmetics prepared from the traditional Chinese medicine components serving as raw materials have high research and development values and market prospects, and the application field and scale are required to be developed and strengthened day by day.
Disclosure of Invention
In view of the above, the invention aims to provide a herbal plant composition for whitening and removing freckles and herbal facial mask gel for whitening and removing freckles, which contains the herbal composition.
In order to achieve the purpose, the invention provides the following technical scheme:
1. a herbal plant composition for whitening skin and removing freckles comprises the following components: glycyrrhiza glabra, Salvia miltiorrhiza, clove, liquorice, rhodiola rosea, Scutellaria baicalensis, centella asiatica, polygala tenuifolia, rhizoma alismatis, ginseng, bletilla striata and poria cocos.
Further, the plant composition is an extract of each component, which is respectively an extract of glycyrrhiza glabra root, an extract of salvia miltiorrhiza root, an extract of clove leaf, an extract of glycyrrhiza glabra root, an extract of rhodiola rosea root, an extract of scutellaria baicalensis root, an extract of centella asiatica, an extract of polygala tenuifolia root, an extract of rhizoma alismatis tuber, an extract of ginseng root, an extract of bletilla striata root and an extract of poria cocos.
Further, the plant composition comprises, by mass, 0.08 to 0.12 part of glycyrrhiza glabra root extract, 0.0008 to 0.0012 part of salvia miltiorrhiza root extract, 0.0001 to 0.00015 part of clove leaf extract, 0.00024 to 0.00036 part of glycyrrhiza glabra root extract, 0.00016 to 0.00024 part of rhodiola rosea root extract, 0.0006 to 0.001 part of scutellaria baicalensis root extract, 0.0001 to 0.00015 part of centella asiatica extract, 0.0001 to 0.00015 part of polygala tenuifolia root extract, 0.0001 to 0.00015 part of rhizoma alismatis tuber extract, 0.0004 to 0.0006 part of ginseng root extract, 0.0008 to 0.0012 part of bletilla striata root extract and 0.0008 to 0.0012 part of poria cocos extract.
2. The herbal whitening and freckle removing sleep mask gel comprises the following raw materials in percentage by mass:
6 to 10 percent of propylene glycol, 3 to 6 percent of glycerin, 2 to 6 percent of isopropyl myristate, 1.5 to 3 percent of polydimethylsiloxane, 0.2 to 0.4 percent of dimethiconol, 1.5 to 2.5 percent of nicotinamide, 0.8 to 1.5 percent of 1, 2-pentanediol, 0.5 to 0.8 percent of hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, 0.5 to 0.8 percent of ammonium acryloyldimethyltaurate/VP copolymer, 0.36 to 0.7 percent of phenoxyethanol, 0.04 to 0.06 percent of ethylhexylglycerin, 0.1 to 0.3 percent of allantoin, 0.1 to 0.2 percent of methyl hydroxybenzoate, 0.08 to 0.12 percent of dipotassium glycyrrhizinate, 0.04 to 0.06 percent of chlorphenesin, 0.04 to 0.06 percent of sodium hyaluronate, 0.08 to 0.12 percent of glycyrrhiza glabra root extract, 0.0008 to 0.0012 percent of salvia miltiorrhiza root extract, 0.0001 to 0.15 percent of clove leaf extract, 0.001 to 24 percent of glycyrrhiza root extract, 0.00024 percent of scutellaria root extract, 0.0006 to 0.0006 percent of scutellaria root extract, 0.0001-0.00015% of centella asiatica extract, 0.0001-0.00015% of polygala tenuifolia root extract, 0.0001-0.00015% of rhizoma alismatis tuber extract, 0.0004-0.0006% of ginseng root extract, 0.0008-0.0012% of bletilla striata root extract, 0.0008-0.0012% of poria cocos extract, 0.00568-0.00852% of essence and the balance of water.
Further, the raw materials comprise the following components in percentage by mass:
propylene glycol 8%, glycerin 4%, isopropyl myristate 3%, polydimethylsiloxane 1.72%, dimethiconol 0.28%, nicotinamide 2%, 1, 2-pentanediol 1%, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer 0.6%, ammonium acryloyldimethyl taurate/VP copolymer 0.6%, phenoxyethanol 0.45%, ethylhexylglycerin 0.05%, allantoin 0.2%, methylparaben 0.15%, dipotassium glycyrrhizinate 0.1%, chlorphenesin 0.05%, sodium hyaluronate 0.05%, Glycyrrhiza glabra root extract 0.1%, Salvia miltiorrhiza root extract 0.001%, clove leaf extract 0.00012%, Glycyrrhiza glabra root extract 0.0003%, rhodiola rosea root extract 0.0002%, Scutellaria baicalensis root extract 0.0008%, centella asiatica extract 0.00012%, Polygala tenuifolia root extract 0.00012%, Alismatis rhizoma extract 0.00012%, Panax ginseng root extract 0.0005%, bletilla striata root extract 0.001%, and mixtures thereof, 0.001% of tuckahoe extract, 0.00568-0.00852% of essence and the balance of water.
3. The preparation method of the herbal whitening and freckle removing sleep mask gel specifically comprises the following steps:
a. adding water, propylene glycol, glycerol, ammonium acryloyldimethyl taurate/VP copolymer, allantoin, methylparaben, chlorphenesin and sodium hyaluronate into a water phase pot, heating to 80-85 ℃, and uniformly stirring to obtain water phase A solution;
b. adding isopropyl myristate, polydimethylsiloxane, dimethiconol, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer into an oil phase pot, heating to 80-85 ℃, and uniformly stirring to obtain an oil phase B solution;
c. adding the water phase A solution into an emulsifying pot, adding the oil phase B solution, keeping the temperature at about 80 ℃, homogenizing and stirring uniformly;
d. cooling to 45-50 ℃, adding nicotinamide, 1, 2-pentanediol, dipotassium glycyrrhizinate, glycyrrhiza glabra root extract, salvia miltiorrhiza root extract, clove leaf extract, glycyrrhiza glabra root extract, rhodiola rosea root extract, scutellaria baicalensis root extract, centella asiatica extract, polygala tenuifolia root extract, rhizoma alismatis tuber extract, ginseng root extract, bletilla striata root extract and poria cocos extract, and uniformly stirring;
e. cooling to 30-40 ℃, adding phenoxyethanol, ethylhexyl glycerol and essence, and uniformly stirring.
Further, the stirring speed is 60-100 r/min.
The invention has the beneficial effects that: the invention mainly utilizes plant effective ingredients to prepare the facial mask gel with whitening and freckle removing effects, and the facial mask gel is prepared from extracts of glycyrrhiza glabra, salvia miltiorrhiza, clove, liquorice, rhodiola rosea, scutellaria baicalensis, centella asiatica, polygala tenuifolia, rhizoma alismatis, ginseng, bletilla striata and poria cocos. The Glycyrrhiza glabra L.contains flavone, tanshinone in Salvia miltiorrhiza Bunge is a natural antioxidant component, so that oxidation reaction is reduced, oxidized lipid and free radical are eliminated, and melanin generation is reduced. The effective components of centella, bletilla striata, scutellaria baicalensis and the like can inhibit the activity of tyrosinase and block the generation of melanin, and the whitening effect can be achieved by multiple angles. Rhizoma Alismatis has tranquilizing and tranquilizing effects, and can be added into skin care product for relieving skin allergy. The jelly prepared from various plant active ingredients and suitable humectant and emollient proportions is mild and non-irritant to eyes and skin, and can be used for a long time. And experiments prove that the skin melanin content can be obviously reduced within 0-6 weeks, and the skin chromaticity and the like can be improved to a certain extent.
Detailed Description
The following describes in detail preferred embodiments of the present invention. The experimental procedures, in which specific conditions are not specified in the examples, are generally carried out under conventional conditions or under conditions recommended by the manufacturers.
The herbal whitening and freckle removing sleep mask gel provided by the invention is mainly prepared from various plant extracts and comprises the following components:
radix Glycytthizae (Glycyrrhiza Glabra) root extract, radix Salviae Miltiorrhizae (Salvia Miltiorrhiza) root extract, flos Caryophylli (Eugenia CARYOPHYLLUS) leaf extract, radix Glycyrrhizae (Glycyrrhiza URALENSIS) root extract, radix Rhodiolae (RHODIOLA ROSEA) root extract, radix Scutellariae (Scutellaria BAICALENSIS) root extract, herba Centellae (CENTELLA ASIATICA) extract, radix Polygalae (Polygala TENUIFOLIA) root extract, rhizoma Alismatis (Alisma ORIENTALE) tuber extract, radix Ginseng (Panax GINSENG) root extract, rhizoma Bletillae (BLETILLA STRIATA) root extract, and Poria (Poria coccos) extract. The extract of centella asiatica can be the extract of whole plant.
The herbal whitening and freckle removing sleep mask gel comprises the following raw materials in percentage by mass:
6 to 10 percent of propylene glycol, 3 to 6 percent of glycerin, 2 to 6 percent of isopropyl myristate, 1.5 to 3 percent of polydimethylsiloxane, 0.2 to 0.4 percent of dimethiconol, 1.5 to 2.5 percent of nicotinamide, 0.8 to 1.5 percent of 1, 2-pentanediol, 0.5 to 0.8 percent of hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, 0.5 to 0.8 percent of ammonium acryloyldimethyltaurate/VP copolymer, 0.36 to 0.7 percent of phenoxyethanol, 0.04 to 0.06 percent of ethylhexylglycerin, 0.1 to 0.3 percent of allantoin, 0.1 to 0.2 percent of methyl hydroxybenzoate, 0.08 to 0.12 percent of dipotassium glycyrrhizinate, 0.04 to 0.06 percent of chlorphenesin, 0.04 to 0.06 percent of sodium hyaluronate, 0.08 to 0.12 percent of glycyrrhiza glabra root extract, 0.0008 to 0.0012 percent of salvia miltiorrhiza root extract, 0.0001 to 0.15 percent of clove leaf extract, 0.001 to 24 percent of glycyrrhiza root extract, 0.00024 percent of scutellaria root extract, 0.0006 to 0.0006 percent of scutellaria root extract, 0.0001-0.00015% of centella asiatica extract, 0.0001-0.00015% of polygala tenuifolia root extract, 0.0001-0.00015% of rhizoma alismatis tuber extract, 0.0004-0.0006% of ginseng root extract, 0.0008-0.0012% of bletilla striata root extract, 0.0008-0.0012% of poria cocos extract, 0.00568-0.00852% of essence and the balance of water. Preferably, the water is deionized water.
Example 1
The herbal whitening and freckle removing sleep mask gel comprises the following raw materials in percentage by mass:
propylene glycol 8%, glycerin 4%, isopropyl myristate 3%, polydimethylsiloxane 1.72%, dimethiconol 0.28%, nicotinamide 2%, 1, 2-pentanediol 1%, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer 0.6%, ammonium acryloyldimethyl taurate/VP copolymer 0.6%, phenoxyethanol 0.45%, ethylhexylglycerin 0.05%, allantoin 0.2%, methylparaben 0.15%, dipotassium glycyrrhizinate 0.1%, chlorphenesin 0.05%, sodium hyaluronate 0.05%, Glycyrrhiza glabra root extract 0.1%, Salvia miltiorrhiza root extract 0.001%, clove leaf extract 0.00012%, Glycyrrhiza glabra root extract 0.0003%, rhodiola rosea root extract 0.0002%, Scutellaria baicalensis root extract 0.0008%, centella asiatica extract 0.00012%, Polygala tenuifolia root extract 0.00012%, Alismatis rhizoma extract 0.00012%, Panax ginseng root extract 0.0005%, bletilla striata root extract 0.001%, and mixtures thereof, 0.001% of tuckahoe extract, 0.0071% of essence and 77.63762% of water.
The preparation method comprises the following steps:
1) adding raw materials of water, propylene glycol, glycerol, acryloyl dimethyl ammonium taurate/VP copolymer, allantoin, methyl hydroxybenzoate, chlorphenesin and sodium hyaluronate into a water phase pot, heating to 80-85 ℃, and uniformly stirring to obtain water phase A solution;
2) adding raw materials of isopropyl myristate, polydimethylsiloxane, dimethiconol and hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer into an oil phase pot, heating to 80-85 ℃, and uniformly stirring to obtain oil phase B liquid;
3) adding the water phase A solution into an emulsifying pot, adding the oil phase B solution, keeping the temperature at about 80 ℃, homogenizing and stirring uniformly;
4) cooling to 45-50 ℃, adding raw materials of nicotinamide, 1, 2-pentanediol, dipotassium glycyrrhizinate, glycyrrhiza glabra root extract, salvia miltiorrhiza root extract, clove leaf extract, glycyrrhiza glabra root extract, rhodiola rosea root extract, scutellaria baicalensis root extract, centella asiatica extract, polygala tenuifolia root extract, rhizoma alismatis tuber extract, ginseng root extract, bletilla striata root extract and poria cocos extract, and uniformly stirring;
5) cooling to 30-40 ℃, adding the raw materials of phenoxyethanol, ethylhexyl glycerol and essence, and uniformly stirring;
6) inspecting quality, packaging after qualified.
Preferably, the stirring speed is 60-100 r/min.
Examples 2 to 5
The raw materials of examples 2 to 5 were the same as in example 1, and the components were as shown in the following table 1 in mass percent (%):
table 1:
the preparation method is the same as that of the example 1, and finally, the cream yellow jelly-shaped finished product is prepared.
Example 6
Acute eye irritation tests were performed on the samples prepared in examples 1 to 5 as received in accordance with technical Specification for cosmetic safety (2015 edition).
1. Experimental animals and breeding environment:
selecting 10 common-grade New Zealand rabbits; selecting female animal with no pregnancy and no farrowing; weight: 2.42 kg-2.68 kg.
At the temperature: 20 ℃ to 25 ℃, relative humidity: feeding the seeds 50-60%.
2. The test method comprises the following steps:
the experimental animals were kept in a single cage, the animals were acclimated in the experimental animal room for at least 3 days before the experiment, both eyes of the experimental animals were examined 24 hours before the experiment was started (including examination using 2% fluorescein sodium solution), and animals with eye irritation symptoms, corneal defects and conjunctival lesions could not be used for the experiment.
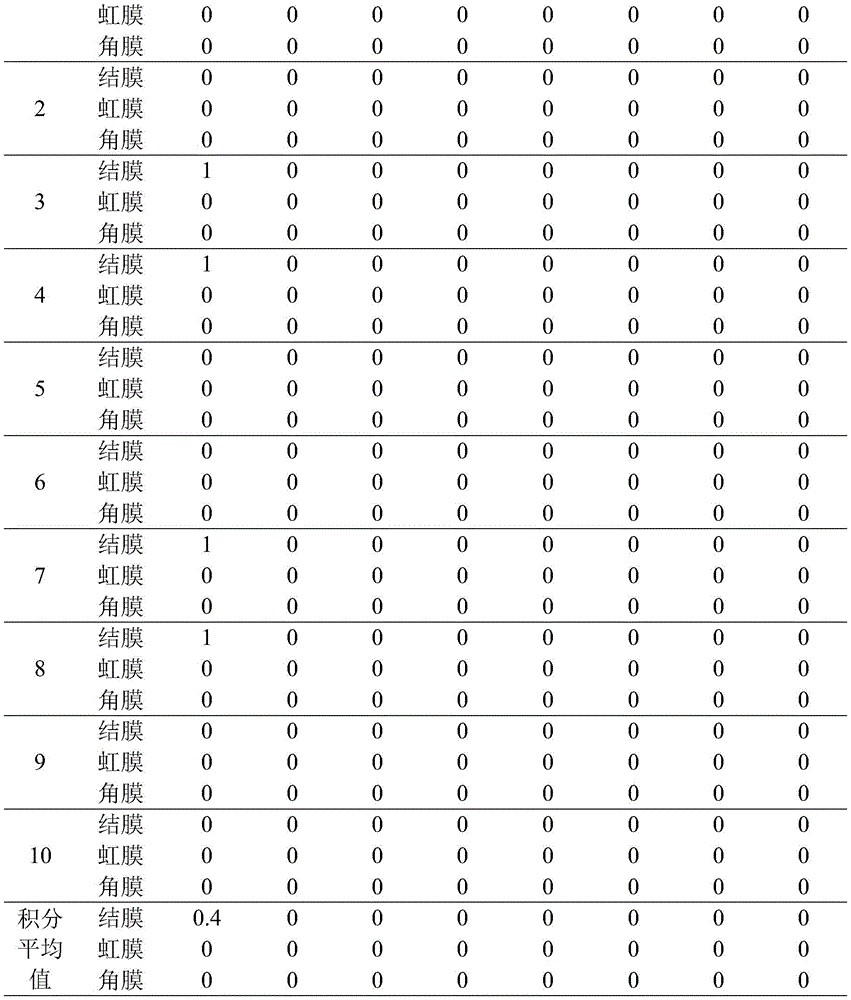
Gently pull the lower eyelid of one side of the rabbit's eye open, and add 0.1ml of the sample to the conjunctival sac to allow the upper and lower eyelids to close passively for 1s to prevent sample loss, while the other side of the eye is left untreated as a control. Eyes were not washed within 24h after the application of the sample, and the eyes of the animals were examined at 1h, 24h, 48h, 72h, and 4d and 7d after the application of the sample. If the stimulatory response does not occur for 72 hours, the test can be terminated, e.g., the stimulatory response does not recover within 7 days, and the observation time is extended to determine the reversibility or irreversibility of the lesion, typically not to exceed 21 days. After the end of the 24h observation and recording, the eyes of all animals were further examined with a 2% sodium fluorescein solution. The eye irritation/corrosion test was scored according to chapter six 5 of the technical Specification for cosmetic safety (2015 edition) Table 1, and the eye irritation intensity of the samples was determined according to Table 3. The following table 2 is the test result of the acute eye irritation of the sample to the experimental rabbit, and fully shows that the acute eye irritation of the sample to the experimental rabbit is not irritant under the condition of no flushing.
Table 2:
example 7
The samples of examples 1 to 5 were subjected to the skin allergy test-topical closed skin test (BT) according to the technical specifications for cosmetic safety (2015 edition).
1. Experimental animals and breeding environment:
common guinea pigs of the Netherlands, the test group and the positive control group each comprise 20 animals, the negative control group comprises 10 animals, and the female animals are selected from non-pregnant animals and non-farrowing animals; weight: 241.4g-276.8 g. At the temperature: 20 ℃ to 25 ℃, relative humidity: feeding the seeds 50-60%.
2. The test method comprises the following steps:
the animal is adapted to at least 3 days in the environment of the experimental animal room before the experiment, and the experimentAbout 24 hours before the onset, the left side of the back of the guinea pig was dehaired to a range of about 6cm2。
And (3) induction contact: 0.2ml of the sample was applied to the skin of the test group animals, which had been depilated at 2cmx2cm, covered with two layers of gauze and a layer of cellophane, and fixed in a sealing manner with a non-irritating adhesive tape for 6 h. The 7 th and 14 th steps were repeated in the same manner, and the negative control group was not subjected to induction contact. The positive control group was also smeared with 0.4% 2,4 dinitrochlorobenzene.
And (3) exciting contact: 14d after the last induction, a 0.2m1 sample was applied to the right 2cm x2cm depilated area (24 h before contact) on the back of the test and negative control guinea pigs, which was then covered with two layers of gauze and a layer of cellophane and fixed with non-stimulating tape for 6 h. The positive control group was also smeared with 0.2% 2,4 dinitrochlorobenzene.
Skin reactions were observed at 24h and 48h after the end of challenge exposure, scored according to chapter 6 skin allergy test table 1 of technical Specification for cosmetic safety (2015 edition), and the sensitization intensity of the samples was determined according to table 3. Table 3 below is the results of the skin allergy test of the samples to the experimental guinea pigs, which is a sufficient indication that the samples are not allergic to the skin.
Table 3:
the herbal whitening and freckle removing sleep mask gel provided by the invention has no stimulation to skin and can be used for a long time.
Example 8
Skin whiteness (L value): the higher the value of L, the higher the skin brightness; the lower the value of L, the more black the skin is. Skin dark yellowness (ITA ° value): the ITA ° value is a value that characterizes skin brightness. The greater the ITA value, the brighter the skin, whereas the darker the skin. Skin melanin content (MI value): the MI value characterizes the melanin index, the lower the test result, the lower the melanin content at the spot site.
The total number of volunteers was 50, and 25 women and men aged 20-40 years old, respectively. The exclusion conditions of the volunteers meet the inclusion and exclusion standards of the diagnosis standard and the treatment principle of the cosmetic contact dermatitis.
The test method comprises the following steps: the test part is the back of the hand, after the testee cleans the hand every day, the back of any one of the left hand and the right hand is uniformly smeared with the herbal whitening and freckle removing sleep mask gel, the skin is cleaned after being kept for 20 minutes, the service cycle is 6 weeks, and the test is carried out once a week. During the test period, the test area was not filled with any other cosmetic. Meanwhile, the other hand is coated with certain commercially available whitening facial mask gel as a control.
Testing environment requirements: under the constant temperature and humidity environment, the temperature is 25 +/-1 ℃, the relative humidity is 45% +/-5, and the volunteers are adapted to the environment for 20 minutes after cleaning hands during testing.
The test results are shown in table 4 below:
the herbal whitening and freckle removing sleep mask gel disclosed by the invention has no stimulation to the skin of a human, and has a certain improvement effect on the aspects of skin melanin content, skin chromaticity and the like within 0-6 weeks.
Finally, it is noted that the above-mentioned preferred embodiments illustrate rather than limit the invention, and that, although the invention has been described in detail with reference to the above-mentioned preferred embodiments, it will be understood by those skilled in the art that various changes in form and detail may be made therein without departing from the scope of the invention as defined by the appended claims.
Claims (7)
1. The herbal plant composition for whitening and removing freckles is characterized by comprising the following components: glycyrrhiza glabra, Salvia miltiorrhiza, clove, liquorice, rhodiola rosea, Scutellaria baicalensis, centella asiatica, polygala tenuifolia, rhizoma alismatis, ginseng, bletilla striata and poria cocos.
2. The herbal plant composition for whitening and removing freckles as claimed in claim 1, wherein the plant composition is an extract of each component, and the extract is glycyrrhiza glabra root extract, salvia miltiorrhiza root extract, clove leaf extract, glycyrrhiza glabra root extract, rhodiola rosea root extract, scutellaria baicalensis root extract, centella asiatica extract, polygala tenuifolia root extract, alisma orientale tuber extract, ginseng root extract, bletilla striata root extract, poria cocos wolf extract.
3. The herbal plant composition for whitening and removing freckles according to claim 2, wherein the herbal plant composition comprises, by mass, 0.08-0.12 part of glycyrrhiza glabra root extract, 0.0008-0.0012 part of salvia miltiorrhiza root extract, 0.0001-0.00015 part of clove leaf extract, 0.00024-0.00036 part of glycyrrhiza glabra root extract, 0.00016-0.00024 part of salidrosis radix extract, 0.0006-0.001 part of scutellaria baicalensis root extract, 0.0001-0.00015 part of centella asiatica extract, 0.0001-0.00015 part of polygala tenuifolia root extract, 0.0001-0.00015 part of rhizoma alismatis tuber extract, 0.0004-0.0006 part of ginseng root extract, 0.0008-0.0012 part of bletilla striata root extract and 0.0008-0.0012 part of poria cocos extract.
4. The herbal whitening and freckle removing sleep mask gel is characterized by comprising the following raw materials in percentage by mass:
6 to 10 percent of propylene glycol, 3 to 6 percent of glycerin, 2 to 6 percent of isopropyl myristate, 1.5 to 3 percent of polydimethylsiloxane, 0.2 to 0.4 percent of dimethiconol, 1.5 to 2.5 percent of nicotinamide, 0.8 to 1.5 percent of 1, 2-pentanediol, 0.5 to 0.8 percent of hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, 0.5 to 0.8 percent of ammonium acryloyldimethyltaurate/VP copolymer, 0.36 to 0.7 percent of phenoxyethanol, 0.04 to 0.06 percent of ethylhexylglycerin, 0.1 to 0.3 percent of allantoin, 0.1 to 0.2 percent of methyl hydroxybenzoate, 0.08 to 0.12 percent of dipotassium glycyrrhizinate, 0.04 to 0.06 percent of chlorphenesin, 0.04 to 0.06 percent of sodium hyaluronate, 0.08 to 0.12 percent of glycyrrhiza glabra root extract, 0.0008 to 0.0012 percent of salvia miltiorrhiza root extract, 0.0001 to 0.15 percent of clove leaf extract, 0.001 to 24 percent of glycyrrhiza root extract, 0.00024 percent of scutellaria root extract, 0.0006 to 0.0006 percent of scutellaria root extract, 0.0001-0.00015% of centella asiatica extract, 0.0001-0.00015% of polygala tenuifolia root extract, 0.0001-0.00015% of rhizoma alismatis tuber extract, 0.0004-0.0006% of ginseng root extract, 0.0008-0.0012% of bletilla striata root extract, 0.0008-0.0012% of poria cocos extract, 0.00568-0.00852% of essence and the balance of water.
5. The herbal whitening and freckle-removing sleep mask gel according to claim 4 is characterized by comprising the following raw materials in percentage by mass:
propylene glycol 8%, glycerin 4%, isopropyl myristate 3%, polydimethylsiloxane 1.72%, dimethiconol 0.28%, nicotinamide 2%, 1, 2-pentanediol 1%, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer 0.6%, ammonium acryloyldimethyl taurate/VP copolymer 0.6%, phenoxyethanol 0.45%, ethylhexylglycerin 0.05%, allantoin 0.2%, methylparaben 0.15%, dipotassium glycyrrhizinate 0.1%, chlorphenesin 0.05%, sodium hyaluronate 0.05%, Glycyrrhiza glabra root extract 0.1%, Salvia miltiorrhiza root extract 0.001%, clove leaf extract 0.00012%, Glycyrrhiza glabra root extract 0.0003%, rhodiola rosea root extract 0.0002%, Scutellaria baicalensis root extract 0.0008%, centella asiatica extract 0.00012%, Polygala tenuifolia root extract 0.00012%, Alismatis rhizoma extract 0.00012%, Panax ginseng root extract 0.0005%, bletilla striata root extract 0.001%, and mixtures thereof, 0.001% of tuckahoe extract, 0.00568-0.00852% of essence and the balance of water.
6. The herbal whitening and freckle-removing sleep mask gel according to claim 4 or 5, which is characterized in that the preparation method comprises the following specific steps:
a. adding water, propylene glycol, glycerol, ammonium acryloyldimethyl taurate/VP copolymer, allantoin, methylparaben, chlorphenesin and sodium hyaluronate into a water phase pot, heating to 80-85 ℃, and uniformly stirring to obtain water phase A solution;
b. adding isopropyl myristate, polydimethylsiloxane, dimethiconol, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer into an oil phase pot, heating to 80-85 ℃, and uniformly stirring to obtain an oil phase B solution;
c. adding the water phase A solution into an emulsifying pot, adding the oil phase B solution, keeping the temperature at about 80 ℃, homogenizing and stirring uniformly;
d. cooling to 45-50 ℃, adding nicotinamide, 1, 2-pentanediol, dipotassium glycyrrhizinate, glycyrrhiza glabra root extract, salvia miltiorrhiza root extract, clove leaf extract, glycyrrhiza glabra root extract, rhodiola rosea root extract, scutellaria baicalensis root extract, centella asiatica extract, polygala tenuifolia root extract, rhizoma alismatis tuber extract, ginseng root extract, bletilla striata root extract and poria cocos extract, and uniformly stirring;
e. cooling to 30-40 ℃, adding phenoxyethanol, ethylhexyl glycerol and essence, and uniformly stirring.
7. The herbal whitening and freckle removing sleep mask gel according to claim 6, wherein the stirring speed is 60-100 r/min.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011538204.2A CN112587455A (en) | 2020-12-23 | 2020-12-23 | Plant composition and herbal whitening and freckle removing sleep mask gel |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202011538204.2A CN112587455A (en) | 2020-12-23 | 2020-12-23 | Plant composition and herbal whitening and freckle removing sleep mask gel |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN112587455A true CN112587455A (en) | 2021-04-02 |
Family
ID=75200717
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202011538204.2A Pending CN112587455A (en) | 2020-12-23 | 2020-12-23 | Plant composition and herbal whitening and freckle removing sleep mask gel |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN112587455A (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113171314A (en) * | 2021-04-24 | 2021-07-27 | 广州市德创生物科技有限公司 | Whitening composition and preparation method and application thereof |
| CN115040441A (en) * | 2022-06-20 | 2022-09-13 | 广州雅纯化妆品制造有限公司 | Preparation method of composition containing dendrobium officinale fermentation product and essence |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107684536A (en) * | 2017-10-23 | 2018-02-13 | 宇妥藏药股份有限公司 | A kind of whitening moisturizing face masque and preparation method thereof |
| CN109512759A (en) * | 2018-12-05 | 2019-03-26 | 中山市美太保健制品有限公司 | A kind of facial mask containing plant herbal ingredients |
| CN110960455A (en) * | 2019-12-05 | 2020-04-07 | 广州科缇生物科技有限公司 | Essence composition with whitening effect and preparation method and application thereof |
-
2020
- 2020-12-23 CN CN202011538204.2A patent/CN112587455A/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107684536A (en) * | 2017-10-23 | 2018-02-13 | 宇妥藏药股份有限公司 | A kind of whitening moisturizing face masque and preparation method thereof |
| CN109512759A (en) * | 2018-12-05 | 2019-03-26 | 中山市美太保健制品有限公司 | A kind of facial mask containing plant herbal ingredients |
| CN110960455A (en) * | 2019-12-05 | 2020-04-07 | 广州科缇生物科技有限公司 | Essence composition with whitening effect and preparation method and application thereof |
Non-Patent Citations (1)
| Title |
|---|
| 王建新: "《化妆品植物原料大全》", 30 June 2012 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113171314A (en) * | 2021-04-24 | 2021-07-27 | 广州市德创生物科技有限公司 | Whitening composition and preparation method and application thereof |
| CN115040441A (en) * | 2022-06-20 | 2022-09-13 | 广州雅纯化妆品制造有限公司 | Preparation method of composition containing dendrobium officinale fermentation product and essence |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109288764B (en) | Hydrolat spray and preparation method thereof | |
| CN110585048B (en) | Composition with anti-saccharification and anti-oxidation functions and application thereof | |
| US20130101689A1 (en) | Composition containing paper mulberry extracts | |
| CN101849899A (en) | Chinese medicinal compound extract and cosmetics comprising same as active ingredient | |
| CN109662928A (en) | A kind of skin care compositions and its application and preparation method with sun-proof reparation effect | |
| CN112245363B (en) | Salvia miltiorrhiza whitening and freckle removing mask | |
| CN110090191B (en) | After-sun repair composition and cosmetic thereof | |
| CN110384653A (en) | It is a kind of to have effects that the multi-faceted polypeptide Essence for dispelling yellow and its preparation process | |
| CN105125466A (en) | Freckle removing night cream and preparing method thereof | |
| CN109044941B (en) | Skin firming, nourishing and moisturizing composition and preparation method and application thereof | |
| CN112587455A (en) | Plant composition and herbal whitening and freckle removing sleep mask gel | |
| CN113288854A (en) | Traditional Chinese medicine formula anti-aging whitening moisturizing mask and preparation method thereof | |
| CN111184824A (en) | Freckle-removing traditional Chinese medicine liquid and preparation method thereof | |
| CN110101648B (en) | Beauty-care concealer air cushion BB cream | |
| CN109568230A (en) | A kind of Chinese medicine cosmetic of the anti-aging of moisturizing whitening | |
| CN112370404B (en) | Moisturizing and whitening composition, and preparation method and application thereof | |
| CN115778870A (en) | Anti-wrinkle anti-aging composition and preparation method and application thereof | |
| CN116725935B (en) | Essence composition with whitening, anti-allergy and anti-wrinkle functions, and preparation method and application thereof | |
| CN109984986A (en) | A kind of pure Chinese medicine facial mask and preparation method thereof | |
| CN110478285B (en) | Composition for resisting scalp aging | |
| Gosavi et al. | Formulation and evaluation of polyherbal lotus oil | |
| CN115944570A (en) | Composition, application thereof and cosmetic | |
| CN114569520A (en) | Composition for plumping and moistening lips and application thereof | |
| CN113940910A (en) | Gingko extract toning lotion | |
| CN117582390A (en) | Composition for fading neck lines and head lines and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20210402 |