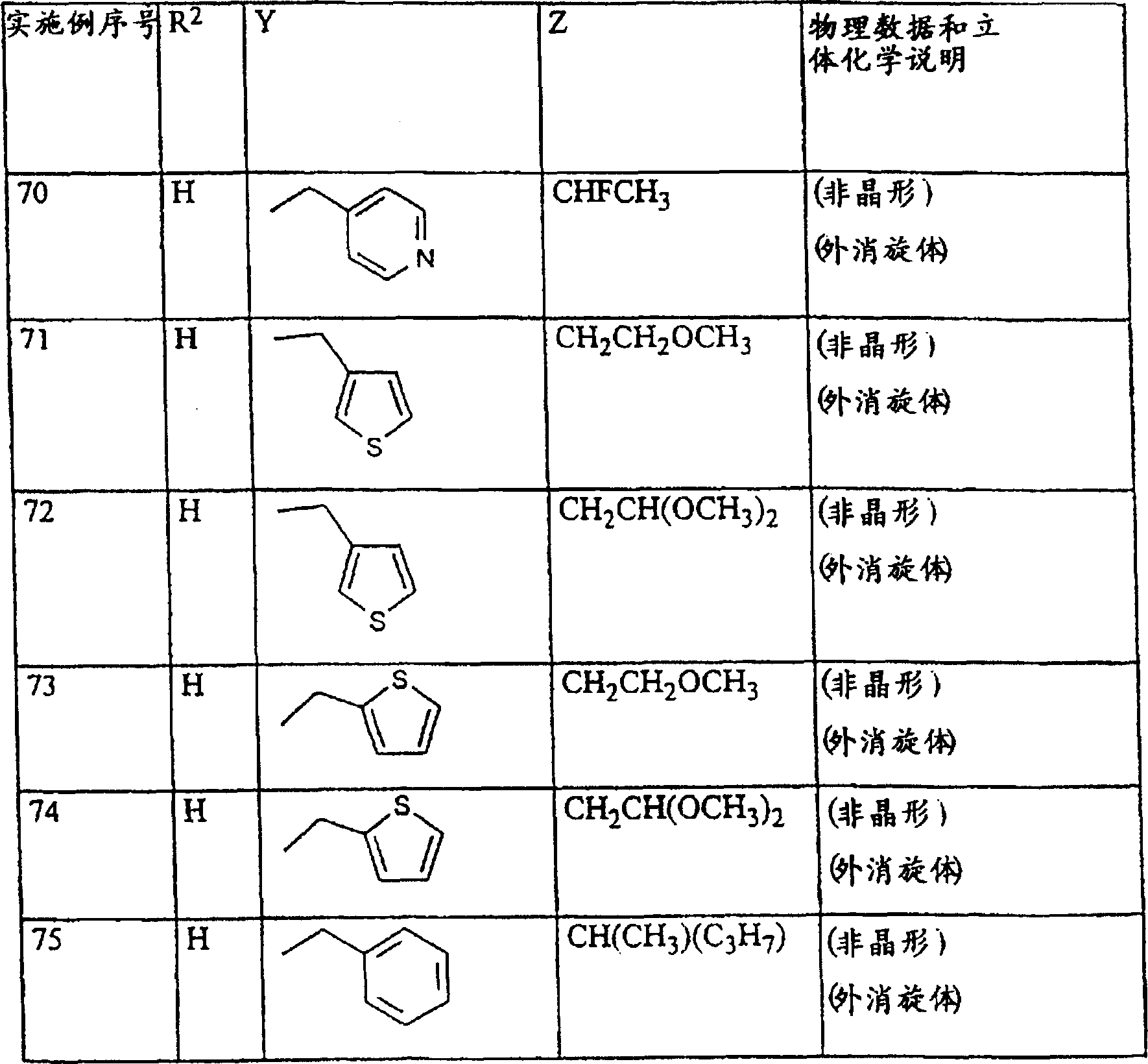
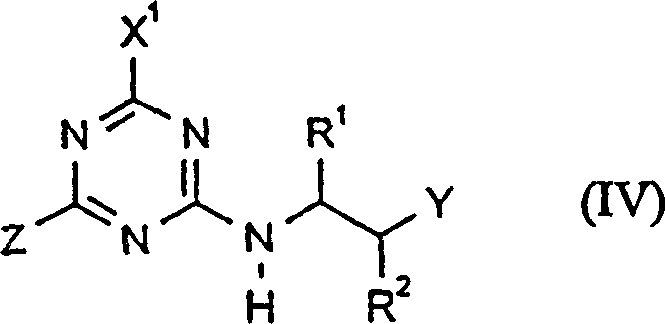
CN1113060C - 作为除草剂的取代的2-氨基-4-烷基氨基-1,3,5-三嗪 - Google Patents
作为除草剂的取代的2-氨基-4-烷基氨基-1,3,5-三嗪Info
- Publication number
- CN1113060C CN1113060C CN97180460A CN97180460A CN1113060C CN 1113060 C CN1113060 C CN 1113060C CN 97180460 A CN97180460 A CN 97180460A CN 97180460 A CN97180460 A CN 97180460A CN 1113060 C CN1113060 C CN 1113060C
- Authority
- CN
- China
- Prior art keywords
- methyl
- propylcarbamic
- phenyl
- phenyls
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 230000002363 herbicidal effect Effects 0.000 title claims description 10
- 239000004009 herbicide Substances 0.000 title abstract description 9
- 150000001875 compounds Chemical class 0.000 claims abstract description 106
- 238000000034 method Methods 0.000 claims abstract description 29
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims abstract description 15
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 10
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 10
- 239000001257 hydrogen Substances 0.000 claims abstract description 10
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 10
- 125000003545 alkoxy group Chemical group 0.000 claims abstract description 9
- -1 methoxyl group Chemical group 0.000 claims description 75
- 241000196324 Embryophyta Species 0.000 claims description 27
- 238000006243 chemical reaction Methods 0.000 claims description 25
- 125000001424 substituent group Chemical group 0.000 claims description 16
- 229910052801 chlorine Inorganic materials 0.000 claims description 13
- 229910052731 fluorine Inorganic materials 0.000 claims description 12
- 239000003085 diluting agent Substances 0.000 claims description 10
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 claims description 8
- 229940123208 Biguanide Drugs 0.000 claims description 8
- 239000003795 chemical substances by application Substances 0.000 claims description 8
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 7
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims description 7
- 150000004283 biguanides Chemical class 0.000 claims description 6
- JUJWROOIHBZHMG-UHFFFAOYSA-N pyridine Substances C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 6
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 claims description 5
- 150000003918 triazines Chemical class 0.000 claims description 5
- 229910021529 ammonia Inorganic materials 0.000 claims description 4
- 150000003973 alkyl amines Chemical class 0.000 claims description 3
- 238000007796 conventional method Methods 0.000 claims description 3
- 150000003839 salts Chemical class 0.000 claims description 3
- 125000001544 thienyl group Chemical group 0.000 claims description 3
- QQOWHRYOXYEMTL-UHFFFAOYSA-N triazin-4-amine Chemical class N=C1C=CN=NN1 QQOWHRYOXYEMTL-UHFFFAOYSA-N 0.000 claims description 3
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 claims description 2
- 239000004094 surface-active agent Substances 0.000 claims description 2
- 125000001309 chloro group Chemical group Cl* 0.000 claims 3
- 125000001153 fluoro group Chemical group F* 0.000 claims 3
- 125000001246 bromo group Chemical group Br* 0.000 claims 1
- 230000002508 compound effect Effects 0.000 claims 1
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 abstract description 8
- 125000004923 naphthylmethyl group Chemical group C1(=CC=CC2=CC=CC=C12)C* 0.000 abstract description 7
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 abstract description 6
- 150000002367 halogens Chemical class 0.000 abstract description 6
- 125000004414 alkyl thio group Chemical group 0.000 abstract description 5
- 125000004453 alkoxycarbonyl group Chemical group 0.000 abstract description 4
- 125000004448 alkyl carbonyl group Chemical group 0.000 abstract description 4
- 125000004644 alkyl sulfinyl group Chemical group 0.000 abstract description 4
- 125000004390 alkyl sulfonyl group Chemical group 0.000 abstract description 4
- 125000003342 alkenyl group Chemical group 0.000 abstract description 3
- 150000002431 hydrogen Chemical class 0.000 abstract description 3
- 125000000547 substituted alkyl group Chemical group 0.000 abstract description 2
- 125000006193 alkinyl group Chemical group 0.000 abstract 1
- 239000013067 intermediate product Substances 0.000 abstract 1
- 125000000068 chlorophenyl group Chemical group 0.000 description 55
- 239000000203 mixture Substances 0.000 description 45
- 125000004799 bromophenyl group Chemical group 0.000 description 33
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 30
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 28
- 239000004178 amaranth Substances 0.000 description 28
- 230000004048 modification Effects 0.000 description 19
- 238000012986 modification Methods 0.000 description 19
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 19
- 239000007858 starting material Substances 0.000 description 18
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 15
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 15
- 241000209140 Triticum Species 0.000 description 15
- 235000021307 Triticum Nutrition 0.000 description 15
- 239000000706 filtrate Substances 0.000 description 15
- 240000001592 Amaranthus caudatus Species 0.000 description 14
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 14
- 239000012074 organic phase Substances 0.000 description 14
- 229910052938 sodium sulfate Inorganic materials 0.000 description 14
- 235000011152 sodium sulphate Nutrition 0.000 description 14
- 239000002904 solvent Substances 0.000 description 14
- 235000009328 Amaranthus caudatus Nutrition 0.000 description 13
- 241000192043 Echinochloa Species 0.000 description 13
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 13
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 12
- 240000006122 Chenopodium album Species 0.000 description 12
- 235000009344 Chenopodium album Nutrition 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- 125000004201 2,4-dichlorophenyl group Chemical group [H]C1=C([H])C(*)=C(Cl)C([H])=C1Cl 0.000 description 11
- 125000004215 2,4-difluorophenyl group Chemical group [H]C1=C([H])C(*)=C(F)C([H])=C1F 0.000 description 11
- HIXDQWDOVZUNNA-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-hydroxy-7-methoxychromen-4-one Chemical compound C=1C(OC)=CC(O)=C(C(C=2)=O)C=1OC=2C1=CC=C(OC)C(OC)=C1 HIXDQWDOVZUNNA-UHFFFAOYSA-N 0.000 description 11
- 125000004198 2-fluorophenyl group Chemical group [H]C1=C([H])C(F)=C(*)C([H])=C1[H] 0.000 description 11
- 125000004189 3,4-dichlorophenyl group Chemical group [H]C1=C([H])C(Cl)=C(Cl)C([H])=C1* 0.000 description 11
- 125000004211 3,5-difluorophenyl group Chemical group [H]C1=C(F)C([H])=C(*)C([H])=C1F 0.000 description 11
- 125000004180 3-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C(F)=C1[H] 0.000 description 11
- 125000001255 4-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1F 0.000 description 11
- 125000004199 4-trifluoromethylphenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C(F)(F)F 0.000 description 11
- 244000025254 Cannabis sativa Species 0.000 description 11
- 239000002585 base Substances 0.000 description 11
- 229910052799 carbon Inorganic materials 0.000 description 11
- 125000004432 carbon atom Chemical group C* 0.000 description 11
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 11
- 125000000040 m-tolyl group Chemical group [H]C1=C([H])C(*)=C([H])C(=C1[H])C([H])([H])[H] 0.000 description 11
- 125000003261 o-tolyl group Chemical group [H]C1=C([H])C(*)=C(C([H])=C1[H])C([H])([H])[H] 0.000 description 11
- 125000000636 p-nitrophenyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)[N+]([O-])=O 0.000 description 11
- 125000001037 p-tolyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1*)C([H])([H])[H] 0.000 description 11
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 10
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 10
- 235000012735 amaranth Nutrition 0.000 description 10
- 239000000460 chlorine Substances 0.000 description 10
- 238000003756 stirring Methods 0.000 description 10
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 9
- 241000209219 Hordeum Species 0.000 description 9
- 241001106042 Mandragora Species 0.000 description 9
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- 235000010086 Setaria viridis var. viridis Nutrition 0.000 description 9
- 235000002597 Solanum melongena Nutrition 0.000 description 9
- 244000061458 Solanum melongena Species 0.000 description 9
- 125000004093 cyano group Chemical group *C#N 0.000 description 9
- 239000011737 fluorine Substances 0.000 description 9
- 244000304962 green bristle grass Species 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 8
- 244000017020 Ipomoea batatas Species 0.000 description 8
- 235000002678 Ipomoea batatas Nutrition 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 239000003921 oil Substances 0.000 description 8
- 235000019198 oils Nutrition 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 229920002554 vinyl polymer Polymers 0.000 description 8
- 241000219198 Brassica Species 0.000 description 7
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 7
- 235000007340 Hordeum vulgare Nutrition 0.000 description 7
- 244000292697 Polygonum aviculare Species 0.000 description 7
- 235000006386 Polygonum aviculare Nutrition 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 7
- 239000003995 emulsifying agent Substances 0.000 description 7
- 235000019441 ethanol Nutrition 0.000 description 7
- 235000011167 hydrochloric acid Nutrition 0.000 description 7
- 239000000376 reactant Substances 0.000 description 7
- 235000003351 Brassica cretica Nutrition 0.000 description 6
- 235000003343 Brassica rupestris Nutrition 0.000 description 6
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 241000209149 Zea Species 0.000 description 6
- QKSKPIVNLNLAAV-UHFFFAOYSA-N bis(2-chloroethyl) sulfide Chemical compound ClCCSCCCl QKSKPIVNLNLAAV-UHFFFAOYSA-N 0.000 description 6
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 6
- 229910052794 bromium Inorganic materials 0.000 description 6
- 239000001273 butane Substances 0.000 description 6
- 235000010460 mustard Nutrition 0.000 description 6
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 6
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 240000006995 Abutilon theophrasti Species 0.000 description 5
- 240000002791 Brassica napus Species 0.000 description 5
- 244000042664 Matricaria chamomilla Species 0.000 description 5
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 5
- 125000003784 fluoroethyl group Chemical group [H]C([H])(F)C([H])([H])* 0.000 description 5
- 238000010992 reflux Methods 0.000 description 5
- 235000011121 sodium hydroxide Nutrition 0.000 description 5
- KAESVJOAVNADME-UHFFFAOYSA-N 1H-pyrrole Natural products C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 4
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 4
- 235000005781 Avena Nutrition 0.000 description 4
- 244000075850 Avena orientalis Species 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 241000219146 Gossypium Species 0.000 description 4
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 4
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 4
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 4
- 241000209117 Panicum Species 0.000 description 4
- 235000006443 Panicum miliaceum subsp. miliaceum Nutrition 0.000 description 4
- 235000009037 Panicum miliaceum subsp. ruderale Nutrition 0.000 description 4
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 4
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 4
- 235000002634 Solanum Nutrition 0.000 description 4
- 241000207763 Solanum Species 0.000 description 4
- 241000209072 Sorghum Species 0.000 description 4
- 235000011684 Sorghum saccharatum Nutrition 0.000 description 4
- 241001251949 Xanthium sibiricum Species 0.000 description 4
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 4
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 4
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 235000005822 corn Nutrition 0.000 description 4
- QGBSISYHAICWAH-UHFFFAOYSA-N dicyandiamide Chemical class NC(N)=NC#N QGBSISYHAICWAH-UHFFFAOYSA-N 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 229940043265 methyl isobutyl ketone Drugs 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000002689 soil Substances 0.000 description 4
- 239000010902 straw Substances 0.000 description 4
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 235000004977 Brassica sinapistrum Nutrition 0.000 description 3
- 229920000742 Cotton Polymers 0.000 description 3
- 241000234653 Cyperus Species 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 241000801118 Lepidium Species 0.000 description 3
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 3
- 235000004429 Matricaria chamomilla var recutita Nutrition 0.000 description 3
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 125000002877 alkyl aryl group Chemical group 0.000 description 3
- 239000008346 aqueous phase Substances 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 125000002603 chloroethyl group Chemical group [H]C([*])([H])C([H])([H])Cl 0.000 description 3
- 150000002012 dioxanes Chemical class 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 125000005816 fluoropropyl group Chemical group [H]C([H])(F)C([H])([H])C([H])([H])* 0.000 description 3
- 238000001640 fractional crystallisation Methods 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 125000005843 halogen group Chemical group 0.000 description 3
- 229910052500 inorganic mineral Inorganic materials 0.000 description 3
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 3
- 150000002576 ketones Chemical class 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 3
- GRVDJDISBSALJP-UHFFFAOYSA-N methyloxidanyl Chemical group [O]C GRVDJDISBSALJP-UHFFFAOYSA-N 0.000 description 3
- 125000006216 methylsulfinyl group Chemical group [H]C([H])([H])S(*)=O 0.000 description 3
- 235000010755 mineral Nutrition 0.000 description 3
- 239000011707 mineral Substances 0.000 description 3
- 239000002480 mineral oil Substances 0.000 description 3
- 235000010446 mineral oil Nutrition 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 229920000151 polyglycol Polymers 0.000 description 3
- 239000010695 polyglycol Substances 0.000 description 3
- 229910000027 potassium carbonate Inorganic materials 0.000 description 3
- 125000005344 pyridylmethyl group Chemical group [H]C1=C([H])C([H])=C([H])C(=N1)C([H])([H])* 0.000 description 3
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 3
- 235000002639 sodium chloride Nutrition 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 125000005301 thienylmethyl group Chemical group [H]C1=C([H])C([H])=C(S1)C([H])([H])* 0.000 description 3
- HWWYDZCSSYKIAD-UHFFFAOYSA-N 3,5-dimethylpyridine Chemical compound CC1=CN=CC(C)=C1 HWWYDZCSSYKIAD-UHFFFAOYSA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 2
- 241000219144 Abutilon Species 0.000 description 2
- 241000209136 Agropyron Species 0.000 description 2
- 241000234282 Allium Species 0.000 description 2
- 241000219318 Amaranthus Species 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- 244000099147 Ananas comosus Species 0.000 description 2
- 241000404028 Anthemis Species 0.000 description 2
- 244000105624 Arachis hypogaea Species 0.000 description 2
- 235000005340 Asparagus officinalis Nutrition 0.000 description 2
- 241000611157 Brachiaria Species 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Chemical compound CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 241000219312 Chenopodium Species 0.000 description 2
- 235000000509 Chenopodium ambrosioides Nutrition 0.000 description 2
- 244000098897 Chenopodium botrys Species 0.000 description 2
- 235000005490 Chenopodium botrys Nutrition 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 241000132536 Cirsium Species 0.000 description 2
- GHSWVJODQBRTJM-UHFFFAOYSA-N ClC(C1=CC=CC=C1)OO Chemical compound ClC(C1=CC=CC=C1)OO GHSWVJODQBRTJM-UHFFFAOYSA-N 0.000 description 2
- 235000010071 Cucumis prophetarum Nutrition 0.000 description 2
- 244000024469 Cucumis prophetarum Species 0.000 description 2
- 241000208296 Datura Species 0.000 description 2
- 241000208175 Daucus Species 0.000 description 2
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 2
- 241000202829 Eleocharis Species 0.000 description 2
- 239000005977 Ethylene Substances 0.000 description 2
- 241000234642 Festuca Species 0.000 description 2
- 241001290564 Fimbristylis Species 0.000 description 2
- 241000816457 Galeopsis Species 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 235000009438 Gossypium Nutrition 0.000 description 2
- 235000021506 Ipomoea Nutrition 0.000 description 2
- 241000207783 Ipomoea Species 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- 241000208822 Lactuca Species 0.000 description 2
- 241000208204 Linum Species 0.000 description 2
- 241000209082 Lolium Species 0.000 description 2
- 241000227653 Lycopersicon Species 0.000 description 2
- 235000017945 Matricaria Nutrition 0.000 description 2
- 235000007232 Matricaria chamomilla Nutrition 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 239000005583 Metribuzin Substances 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 241000208125 Nicotiana Species 0.000 description 2
- 241000209094 Oryza Species 0.000 description 2
- 235000011096 Papaver Nutrition 0.000 description 2
- 240000001090 Papaver somniferum Species 0.000 description 2
- 241001268782 Paspalum dilatatum Species 0.000 description 2
- 241000219833 Phaseolus Species 0.000 description 2
- 241000219843 Pisum Species 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- 241000205407 Polygonum Species 0.000 description 2
- 241000219295 Portulaca Species 0.000 description 2
- 241000218206 Ranunculus Species 0.000 description 2
- 241000209056 Secale Species 0.000 description 2
- 241000780602 Senecio Species 0.000 description 2
- 244000275012 Sesbania cannabina Species 0.000 description 2
- 235000005775 Setaria Nutrition 0.000 description 2
- 241000232088 Setaria <nematode> Species 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 241000488874 Sonchus Species 0.000 description 2
- 244000273618 Sphenoclea zeylanica Species 0.000 description 2
- 235000017967 Sphenoclea zeylanica Nutrition 0.000 description 2
- 240000006694 Stellaria media Species 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 2
- 241000219793 Trifolium Species 0.000 description 2
- 241000219422 Urtica Species 0.000 description 2
- 241000219873 Vicia Species 0.000 description 2
- 241000405217 Viola <butterfly> Species 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 241001506766 Xanthium Species 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 244000193174 agave Species 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 150000001345 alkine derivatives Chemical class 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 238000004176 ammonification Methods 0.000 description 2
- 235000011114 ammonium hydroxide Nutrition 0.000 description 2
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 2
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 2
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 2
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 2
- 229940073608 benzyl chloride Drugs 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- BTANRVKWQNVYAZ-UHFFFAOYSA-N butan-2-ol Chemical compound CCC(C)O BTANRVKWQNVYAZ-UHFFFAOYSA-N 0.000 description 2
- WRMFBHHNOHZECA-UHFFFAOYSA-N butan-2-olate Chemical compound CCC(C)[O-] WRMFBHHNOHZECA-UHFFFAOYSA-N 0.000 description 2
- MZZBPDKVEFVLFF-UHFFFAOYSA-N cyanazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)(C)C#N)=N1 MZZBPDKVEFVLFF-UHFFFAOYSA-N 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- DIOQZVSQGTUSAI-UHFFFAOYSA-N decane Chemical compound CCCCCCCCCC DIOQZVSQGTUSAI-UHFFFAOYSA-N 0.000 description 2
- 150000004816 dichlorobenzenes Chemical class 0.000 description 2
- 125000004598 dihydrobenzofuryl group Chemical group O1C(CC2=C1C=CC=C2)* 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 230000008029 eradication Effects 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 2
- OSUHJPCHFDQAIT-UHFFFAOYSA-N ethyl 2-{4-[(6-chloroquinoxalin-2-yl)oxy]phenoxy}propanoate Chemical group C1=CC(OC(C)C(=O)OCC)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 OSUHJPCHFDQAIT-UHFFFAOYSA-N 0.000 description 2
- 125000006125 ethylsulfonyl group Chemical group 0.000 description 2
- 230000004907 flux Effects 0.000 description 2
- 239000004088 foaming agent Substances 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 125000002541 furyl group Chemical group 0.000 description 2
- 125000004836 hexamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 2
- 150000002430 hydrocarbons Chemical group 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- ZXEKIIBDNHEJCQ-UHFFFAOYSA-N isobutanol Chemical compound CC(C)CO ZXEKIIBDNHEJCQ-UHFFFAOYSA-N 0.000 description 2
- 125000005956 isoquinolyl group Chemical group 0.000 description 2
- FOXFZRUHNHCZPX-UHFFFAOYSA-N metribuzin Chemical compound CSC1=NN=C(C(C)(C)C)C(=O)N1N FOXFZRUHNHCZPX-UHFFFAOYSA-N 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 125000001715 oxadiazolyl group Chemical group 0.000 description 2
- 125000002971 oxazolyl group Chemical group 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 2
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 2
- 125000003226 pyrazolyl group Chemical group 0.000 description 2
- 125000005493 quinolyl group Chemical group 0.000 description 2
- BACHBFVBHLGWSL-JTQLQIEISA-N rac-diclofop methyl Natural products C1=CC(O[C@@H](C)C(=O)OC)=CC=C1OC1=CC=C(Cl)C=C1Cl BACHBFVBHLGWSL-JTQLQIEISA-N 0.000 description 2
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 2
- 238000004162 soil erosion Methods 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- XLNZEKHULJKQBA-UHFFFAOYSA-N terbufos Chemical compound CCOP(=S)(OCC)SCSC(C)(C)C XLNZEKHULJKQBA-UHFFFAOYSA-N 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 2
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 2
- 229960000278 theophylline Drugs 0.000 description 2
- 125000001113 thiadiazolyl group Chemical group 0.000 description 2
- 125000000335 thiazolyl group Chemical group 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 2
- 238000005292 vacuum distillation Methods 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- IPPAUTOBDWNELX-UHFFFAOYSA-N (2-ethoxy-2-oxoethyl) 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate Chemical group C1=C([N+]([O-])=O)C(C(=O)OCC(=O)OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 IPPAUTOBDWNELX-UHFFFAOYSA-N 0.000 description 1
- ROBSGBGTWRRYSK-SNVBAGLBSA-N (2r)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propanoic acid Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=CC=C(C#N)C=C1F ROBSGBGTWRRYSK-SNVBAGLBSA-N 0.000 description 1
- NYHLMHAKWBUZDY-QMMMGPOBSA-N (2s)-2-[2-chloro-5-[2-chloro-4-(trifluoromethyl)phenoxy]benzoyl]oxypropanoic acid Chemical compound C1=C(Cl)C(C(=O)O[C@@H](C)C(O)=O)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 NYHLMHAKWBUZDY-QMMMGPOBSA-N 0.000 description 1
- 239000001605 (5-methyl-2-propan-2-ylcyclohexyl) acetate Substances 0.000 description 1
- 125000004765 (C1-C4) haloalkyl group Chemical group 0.000 description 1
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 1
- 125000006002 1,1-difluoroethyl group Chemical group 0.000 description 1
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 1
- 125000004066 1-hydroxyethyl group Chemical group [H]OC([H])([*])C([H])([H])[H] 0.000 description 1
- BXKKQFGRMSOANI-UHFFFAOYSA-N 1-methoxy-3-[4-[(2-methoxy-2,4,4-trimethyl-3h-chromen-7-yl)oxy]phenyl]-1-methylurea Chemical compound C1=CC(NC(=O)N(C)OC)=CC=C1OC1=CC=C2C(C)(C)CC(C)(OC)OC2=C1 BXKKQFGRMSOANI-UHFFFAOYSA-N 0.000 description 1
- PAMIQIKDUOTOBW-UHFFFAOYSA-N 1-methylpiperidine Chemical class CN1CCCCC1 PAMIQIKDUOTOBW-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- 125000004793 2,2,2-trifluoroethoxy group Chemical group FC(CO*)(F)F 0.000 description 1
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 1
- SBASXUCJHJRPEV-UHFFFAOYSA-N 2-(2-methoxyethoxy)ethanol Chemical compound COCCOCCO SBASXUCJHJRPEV-UHFFFAOYSA-N 0.000 description 1
- NUPJIGQFXCQJBK-UHFFFAOYSA-N 2-(4-isopropyl-4-methyl-5-oxo-4,5-dihydro-1H-imidazol-2-yl)-5-(methoxymethyl)nicotinic acid Chemical compound OC(=O)C1=CC(COC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 NUPJIGQFXCQJBK-UHFFFAOYSA-N 0.000 description 1
- GOCUAJYOYBLQRH-UHFFFAOYSA-N 2-(4-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=NC=C(C(F)(F)F)C=C1Cl GOCUAJYOYBLQRH-UHFFFAOYSA-N 0.000 description 1
- YUVKUEAFAVKILW-UHFFFAOYSA-N 2-(4-{[5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoic acid Chemical compound C1=CC(OC(C)C(O)=O)=CC=C1OC1=CC=C(C(F)(F)F)C=N1 YUVKUEAFAVKILW-UHFFFAOYSA-N 0.000 description 1
- XNWFRZJHXBZDAG-UHFFFAOYSA-N 2-METHOXYETHANOL Chemical compound COCCO XNWFRZJHXBZDAG-UHFFFAOYSA-N 0.000 description 1
- GQQIAHNFBAFBCS-UHFFFAOYSA-N 2-[2-chloro-5-(1,3-dioxo-4,5,6,7-tetrahydroisoindol-2-yl)-4-fluorophenoxy]acetic acid Chemical compound C1=C(Cl)C(OCC(=O)O)=CC(N2C(C3=C(CCCC3)C2=O)=O)=C1F GQQIAHNFBAFBCS-UHFFFAOYSA-N 0.000 description 1
- ONNQFZOZHDEENE-UHFFFAOYSA-N 2-[5-(but-3-yn-2-yloxy)-4-chloro-2-fluorophenyl]-4,5,6,7-tetrahydro-1H-isoindole-1,3(2H)-dione Chemical compound C1=C(Cl)C(OC(C)C#C)=CC(N2C(C3=C(CCCC3)C2=O)=O)=C1F ONNQFZOZHDEENE-UHFFFAOYSA-N 0.000 description 1
- ZBMRKNMTMPPMMK-UHFFFAOYSA-N 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid;azane Chemical compound [NH4+].CP(O)(=O)CCC(N)C([O-])=O ZBMRKNMTMPPMMK-UHFFFAOYSA-N 0.000 description 1
- CFWRDBDJAOHXSH-SECBINFHSA-N 2-azaniumylethyl [(2r)-2,3-diacetyloxypropyl] phosphate Chemical compound CC(=O)OC[C@@H](OC(C)=O)COP(O)(=O)OCCN CFWRDBDJAOHXSH-SECBINFHSA-N 0.000 description 1
- VOHIQFRJLJPDFS-UHFFFAOYSA-N 2-chloro-4-methylbenzene-1,3-dicarboxylic acid Chemical class ClC1=C(C(=O)O)C(=CC=C1C(=O)O)C VOHIQFRJLJPDFS-UHFFFAOYSA-N 0.000 description 1
- QEGVVEOAVNHRAA-UHFFFAOYSA-N 2-chloro-6-(4,6-dimethoxypyrimidin-2-yl)sulfanylbenzoic acid Chemical compound COC1=CC(OC)=NC(SC=2C(=C(Cl)C=CC=2)C(O)=O)=N1 QEGVVEOAVNHRAA-UHFFFAOYSA-N 0.000 description 1
- JLYFCTQDENRSOL-UHFFFAOYSA-N 2-chloro-N-(2,4-dimethylthiophen-3-yl)-N-(1-methoxypropan-2-yl)acetamide Chemical compound COCC(C)N(C(=O)CCl)C=1C(C)=CSC=1C JLYFCTQDENRSOL-UHFFFAOYSA-N 0.000 description 1
- IRCMYGHHKLLGHV-UHFFFAOYSA-N 2-ethoxy-3,3-dimethyl-2,3-dihydro-1-benzofuran-5-yl methanesulfonate Chemical compound C1=C(OS(C)(=O)=O)C=C2C(C)(C)C(OCC)OC2=C1 IRCMYGHHKLLGHV-UHFFFAOYSA-N 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- UPMXNNIRAGDFEH-UHFFFAOYSA-N 3,5-dibromo-4-hydroxybenzonitrile Chemical compound OC1=C(Br)C=C(C#N)C=C1Br UPMXNNIRAGDFEH-UHFFFAOYSA-N 0.000 description 1
- XMTQQYYKAHVGBJ-UHFFFAOYSA-N 3-(3,4-DICHLOROPHENYL)-1,1-DIMETHYLUREA Chemical compound CN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XMTQQYYKAHVGBJ-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- CSDQQAQKBAQLLE-UHFFFAOYSA-N 4-(4-chlorophenyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Chemical compound C1=CC(Cl)=CC=C1C1C(C=CS2)=C2CCN1 CSDQQAQKBAQLLE-UHFFFAOYSA-N 0.000 description 1
- NYRMIJKDBAQCHC-UHFFFAOYSA-N 5-(methylamino)-2-phenyl-4-[3-(trifluoromethyl)phenyl]furan-3(2H)-one Chemical compound O1C(NC)=C(C=2C=C(C=CC=2)C(F)(F)F)C(=O)C1C1=CC=CC=C1 NYRMIJKDBAQCHC-UHFFFAOYSA-N 0.000 description 1
- NTSLROIKFLNUIJ-UHFFFAOYSA-N 5-Ethyl-2-methylpyridine Chemical compound CCC1=CC=C(C)N=C1 NTSLROIKFLNUIJ-UHFFFAOYSA-N 0.000 description 1
- QQOGZMUZAZWLJH-UHFFFAOYSA-N 5-[2-chloro-6-fluoro-4-(trifluoromethyl)phenoxy]-n-ethylsulfonyl-2-nitrobenzamide Chemical compound C1=C([N+]([O-])=O)C(C(=O)NS(=O)(=O)CC)=CC(OC=2C(=CC(=CC=2F)C(F)(F)F)Cl)=C1 QQOGZMUZAZWLJH-UHFFFAOYSA-N 0.000 description 1
- PVSGXWMWNRGTKE-UHFFFAOYSA-N 5-methyl-2-[4-methyl-5-oxo-4-(propan-2-yl)-4,5-dihydro-1H-imidazol-2-yl]pyridine-3-carboxylic acid Chemical compound N1C(=O)C(C(C)C)(C)N=C1C1=NC=C(C)C=C1C(O)=O PVSGXWMWNRGTKE-UHFFFAOYSA-N 0.000 description 1
- HZKBYBNLTLVSPX-UHFFFAOYSA-N 6-[(6,6-dimethyl-5,7-dihydropyrrolo[2,1-c][1,2,4]thiadiazol-3-ylidene)amino]-7-fluoro-4-prop-2-ynyl-1,4-benzoxazin-3-one Chemical compound C#CCN1C(=O)COC(C=C2F)=C1C=C2N=C1SN=C2CC(C)(C)CN21 HZKBYBNLTLVSPX-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- VTNQPKFIQCLBDU-UHFFFAOYSA-N Acetochlor Chemical compound CCOCN(C(=O)CCl)C1=C(C)C=CC=C1CC VTNQPKFIQCLBDU-UHFFFAOYSA-N 0.000 description 1
- 239000002890 Aclonifen Substances 0.000 description 1
- 241000743339 Agrostis Species 0.000 description 1
- XKJMBINCVNINCA-UHFFFAOYSA-N Alfalone Chemical compound CON(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XKJMBINCVNINCA-UHFFFAOYSA-N 0.000 description 1
- RGCKGOZRHPZPFP-UHFFFAOYSA-N Alizarin Natural products C1=CC=C2C(=O)C3=C(O)C(O)=CC=C3C(=O)C2=C1 RGCKGOZRHPZPFP-UHFFFAOYSA-N 0.000 description 1
- OSDWBNJEKMUWAV-UHFFFAOYSA-N Allyl chloride Chemical compound ClCC=C OSDWBNJEKMUWAV-UHFFFAOYSA-N 0.000 description 1
- 241000743985 Alopecurus Species 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 239000003666 Amidosulfuron Substances 0.000 description 1
- CTTHWASMBLQOFR-UHFFFAOYSA-N Amidosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)N(C)S(C)(=O)=O)=N1 CTTHWASMBLQOFR-UHFFFAOYSA-N 0.000 description 1
- 235000007119 Ananas comosus Nutrition 0.000 description 1
- 241001666377 Apera Species 0.000 description 1
- 235000003911 Arachis Nutrition 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- 235000007320 Avena fatua Nutrition 0.000 description 1
- 241001647031 Avena sterilis Species 0.000 description 1
- 235000004535 Avena sterilis Nutrition 0.000 description 1
- 239000005469 Azimsulfuron Substances 0.000 description 1
- QGQSRQPXXMTJCM-UHFFFAOYSA-N Benfuresate Chemical compound CCS(=O)(=O)OC1=CC=C2OCC(C)(C)C2=C1 QGQSRQPXXMTJCM-UHFFFAOYSA-N 0.000 description 1
- 239000005476 Bentazone Substances 0.000 description 1
- JDWQITFHZOBBFE-UHFFFAOYSA-N Benzofenap Chemical compound C=1C=C(Cl)C(C)=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OCC(=O)C1=CC=C(C)C=C1 JDWQITFHZOBBFE-UHFFFAOYSA-N 0.000 description 1
- DTCJYIIKPVRVDD-UHFFFAOYSA-N Benzthiazuron Chemical compound C1=CC=C2SC(NC(=O)NC)=NC2=C1 DTCJYIIKPVRVDD-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- 239000005484 Bifenox Substances 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 101000742062 Bos taurus Protein phosphatase 1G Proteins 0.000 description 1
- 241000339490 Brachyachne Species 0.000 description 1
- 235000011331 Brassica Nutrition 0.000 description 1
- XTFNPKDYCLFGPV-OMCISZLKSA-N Bromofenoxim Chemical compound C1=C(Br)C(O)=C(Br)C=C1\C=N\OC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O XTFNPKDYCLFGPV-OMCISZLKSA-N 0.000 description 1
- 241000209200 Bromus Species 0.000 description 1
- 239000007848 Bronsted acid Substances 0.000 description 1
- 229910021532 Calcite Inorganic materials 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000217446 Calystegia sepium Species 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 241000320316 Carduus Species 0.000 description 1
- 244000036828 Carduus nutans Species 0.000 description 1
- 241000132570 Centaurea Species 0.000 description 1
- 240000004385 Centaurea cyanus Species 0.000 description 1
- 235000005940 Centaurea cyanus Nutrition 0.000 description 1
- DXXVCXKMSWHGTF-UHFFFAOYSA-N Chlomethoxyfen Chemical compound C1=C([N+]([O-])=O)C(OC)=CC(OC=2C(=CC(Cl)=CC=2)Cl)=C1 DXXVCXKMSWHGTF-UHFFFAOYSA-N 0.000 description 1
- 239000005496 Chlorsulfuron Substances 0.000 description 1
- 235000005979 Citrus limon Nutrition 0.000 description 1
- 244000131522 Citrus pyriformis Species 0.000 description 1
- LPXYXBZAXFTFKH-UHFFFAOYSA-N ClC=1C(=C(C(=NC1)C(=O)O)C(=O)O)Cl Chemical compound ClC=1C(=C(C(=NC1)C(=O)O)C(=O)O)Cl LPXYXBZAXFTFKH-UHFFFAOYSA-N 0.000 description 1
- 239000005497 Clethodim Substances 0.000 description 1
- 239000005498 Clodinafop Substances 0.000 description 1
- 239000005499 Clomazone Substances 0.000 description 1
- 239000005500 Clopyralid Substances 0.000 description 1
- 235000013162 Cocos nucifera Nutrition 0.000 description 1
- 244000060011 Cocos nucifera Species 0.000 description 1
- 241000207892 Convolvulus Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 241000219122 Cucurbita Species 0.000 description 1
- 235000019093 Cucurbita foetidissima Nutrition 0.000 description 1
- 244000149213 Cucurbita foetidissima Species 0.000 description 1
- VYNOULHXXDFBLU-UHFFFAOYSA-N Cumyluron Chemical compound C=1C=CC=CC=1C(C)(C)NC(=O)NCC1=CC=CC=C1Cl VYNOULHXXDFBLU-UHFFFAOYSA-N 0.000 description 1
- OFSLKOLYLQSJPB-UHFFFAOYSA-N Cyclosulfamuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)NC=2C(=CC=CC=2)C(=O)C2CC2)=N1 OFSLKOLYLQSJPB-UHFFFAOYSA-N 0.000 description 1
- 239000005501 Cycloxydim Substances 0.000 description 1
- XHXUANMFYXWVNG-UHFFFAOYSA-N D-menthyl acetate Natural products CC(C)C1CCC(C)CC1OC(C)=O XHXUANMFYXWVNG-UHFFFAOYSA-N 0.000 description 1
- 241000320605 Dactyloctenium Species 0.000 description 1
- NNYRZQHKCHEXSD-UHFFFAOYSA-N Daimuron Chemical compound C1=CC(C)=CC=C1NC(=O)NC(C)(C)C1=CC=CC=C1 NNYRZQHKCHEXSD-UHFFFAOYSA-N 0.000 description 1
- 239000005503 Desmedipham Substances 0.000 description 1
- SPANOECCGNXGNR-UITAMQMPSA-N Diallat Chemical compound CC(C)N(C(C)C)C(=O)SC\C(Cl)=C\Cl SPANOECCGNXGNR-UITAMQMPSA-N 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- LBGPXIPGGRQBJW-UHFFFAOYSA-N Difenzoquat Chemical compound C[N+]=1N(C)C(C=2C=CC=CC=2)=CC=1C1=CC=CC=C1 LBGPXIPGGRQBJW-UHFFFAOYSA-N 0.000 description 1
- 239000005507 Diflufenican Substances 0.000 description 1
- 235000017896 Digitaria Nutrition 0.000 description 1
- 241001303487 Digitaria <clam> Species 0.000 description 1
- ZAFNJMIOTHYJRJ-UHFFFAOYSA-N Diisopropyl ether Chemical compound CC(C)OC(C)C ZAFNJMIOTHYJRJ-UHFFFAOYSA-N 0.000 description 1
- 239000005508 Dimethachlor Substances 0.000 description 1
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- QAHFOPIILNICLA-UHFFFAOYSA-N Diphenamid Chemical compound C=1C=CC=CC=1C(C(=O)N(C)C)C1=CC=CC=C1 QAHFOPIILNICLA-UHFFFAOYSA-N 0.000 description 1
- 239000005630 Diquat Substances 0.000 description 1
- YUBJPYNSGLJZPQ-UHFFFAOYSA-N Dithiopyr Chemical compound CSC(=O)C1=C(C(F)F)N=C(C(F)(F)F)C(C(=O)SC)=C1CC(C)C YUBJPYNSGLJZPQ-UHFFFAOYSA-N 0.000 description 1
- 239000005510 Diuron Substances 0.000 description 1
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Divinylene sulfide Natural products C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 1
- 241001057636 Dracaena deremensis Species 0.000 description 1
- GUVLYNGULCJVDO-UHFFFAOYSA-N EPTC Chemical compound CCCN(CCC)C(=O)SCC GUVLYNGULCJVDO-UHFFFAOYSA-N 0.000 description 1
- 240000003133 Elaeis guineensis Species 0.000 description 1
- 235000001950 Elaeis guineensis Nutrition 0.000 description 1
- 235000007351 Eleusine Nutrition 0.000 description 1
- 241000209215 Eleusine Species 0.000 description 1
- 235000006369 Emex spinosa Nutrition 0.000 description 1
- 244000294661 Emex spinosa Species 0.000 description 1
- 241001517310 Eria Species 0.000 description 1
- BXEHUCNTIZGSOJ-UHFFFAOYSA-N Esprocarb Chemical compound CC(C)C(C)N(CC)C(=O)SCC1=CC=CC=C1 BXEHUCNTIZGSOJ-UHFFFAOYSA-N 0.000 description 1
- PTFJIKYUEPWBMS-UHFFFAOYSA-N Ethalfluralin Chemical compound CC(=C)CN(CC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O PTFJIKYUEPWBMS-UHFFFAOYSA-N 0.000 description 1
- 239000005512 Ethofumesate Substances 0.000 description 1
- ICWUMLXQKFTJMH-UHFFFAOYSA-N Etobenzanid Chemical compound C1=CC(OCOCC)=CC=C1C(=O)NC1=CC=CC(Cl)=C1Cl ICWUMLXQKFTJMH-UHFFFAOYSA-N 0.000 description 1
- RXCPQSJAVKGONC-UHFFFAOYSA-N Flumetsulam Chemical compound N1=C2N=C(C)C=CN2N=C1S(=O)(=O)NC1=C(F)C=CC=C1F RXCPQSJAVKGONC-UHFFFAOYSA-N 0.000 description 1
- 239000005533 Fluometuron Substances 0.000 description 1
- VEVZCONIUDBCDC-UHFFFAOYSA-N Flurprimidol Chemical compound C=1N=CN=CC=1C(O)(C(C)C)C1=CC=C(OC(F)(F)F)C=C1 VEVZCONIUDBCDC-UHFFFAOYSA-N 0.000 description 1
- 239000005559 Flurtamone Substances 0.000 description 1
- 241000748465 Galinsoga Species 0.000 description 1
- 235000018914 Galinsoga parviflora Nutrition 0.000 description 1
- 244000214240 Galinsoga parviflora Species 0.000 description 1
- 241001101998 Galium Species 0.000 description 1
- 235000014820 Galium aparine Nutrition 0.000 description 1
- 240000005702 Galium aparine Species 0.000 description 1
- 239000005562 Glyphosate Substances 0.000 description 1
- 241001138408 Glyptostrobus pensilis Species 0.000 description 1
- CAWXEEYDBZRFPE-UHFFFAOYSA-N Hexazinone Chemical compound O=C1N(C)C(N(C)C)=NC(=O)N1C1CCCCC1 CAWXEEYDBZRFPE-UHFFFAOYSA-N 0.000 description 1
- 239000005566 Imazamox Substances 0.000 description 1
- XVOKUMIPKHGGTN-UHFFFAOYSA-N Imazethapyr Chemical compound OC(=O)C1=CC(CC)=CN=C1C1=NC(C)(C(C)C)C(=O)N1 XVOKUMIPKHGGTN-UHFFFAOYSA-N 0.000 description 1
- 239000005567 Imazosulfuron Substances 0.000 description 1
- NAGRVUXEKKZNHT-UHFFFAOYSA-N Imazosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N3C=CC=CC3=NC=2Cl)=N1 NAGRVUXEKKZNHT-UHFFFAOYSA-N 0.000 description 1
- 241001327265 Ischaemum Species 0.000 description 1
- NEKOXWSIMFDGMA-UHFFFAOYSA-N Isopropalin Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(C(C)C)C=C1[N+]([O-])=O NEKOXWSIMFDGMA-UHFFFAOYSA-N 0.000 description 1
- 239000005570 Isoxaben Substances 0.000 description 1
- 239000005571 Isoxaflutole Substances 0.000 description 1
- 241000520028 Lamium Species 0.000 description 1
- 239000005572 Lenacil Substances 0.000 description 1
- 241000209510 Liliopsida Species 0.000 description 1
- 241000064140 Lindernia Species 0.000 description 1
- 239000005573 Linuron Substances 0.000 description 1
- 235000002262 Lycopersicon Nutrition 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- SUSRORUBZHMPCO-UHFFFAOYSA-N MC-4379 Chemical compound C1=C([N+]([O-])=O)C(C(=O)OC)=CC(OC=2C(=CC(Cl)=CC=2)Cl)=C1 SUSRORUBZHMPCO-UHFFFAOYSA-N 0.000 description 1
- 239000005574 MCPA Substances 0.000 description 1
- XHXUANMFYXWVNG-ADEWGFFLSA-N Menthyl acetate Natural products CC(C)[C@@H]1CC[C@@H](C)C[C@H]1OC(C)=O XHXUANMFYXWVNG-ADEWGFFLSA-N 0.000 description 1
- 239000005579 Metamitron Substances 0.000 description 1
- RRVIAQKBTUQODI-UHFFFAOYSA-N Methabenzthiazuron Chemical compound C1=CC=C2SC(N(C)C(=O)NC)=NC2=C1 RRVIAQKBTUQODI-UHFFFAOYSA-N 0.000 description 1
- 239000005581 Metobromuron Substances 0.000 description 1
- WLFDQEVORAMCIM-UHFFFAOYSA-N Metobromuron Chemical compound CON(C)C(=O)NC1=CC=C(Br)C=C1 WLFDQEVORAMCIM-UHFFFAOYSA-N 0.000 description 1
- 239000005582 Metosulam Substances 0.000 description 1
- VGHPMIFEKOFHHQ-UHFFFAOYSA-N Metosulam Chemical compound N1=C2N=C(OC)C=C(OC)N2N=C1S(=O)(=O)NC1=C(Cl)C=CC(C)=C1Cl VGHPMIFEKOFHHQ-UHFFFAOYSA-N 0.000 description 1
- 239000005584 Metsulfuron-methyl Substances 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- 235000003990 Monochoria hastata Nutrition 0.000 description 1
- 240000000178 Monochoria vaginalis Species 0.000 description 1
- LKJPSUCKSLORMF-UHFFFAOYSA-N Monolinuron Chemical compound CON(C)C(=O)NC1=CC=C(Cl)C=C1 LKJPSUCKSLORMF-UHFFFAOYSA-N 0.000 description 1
- 240000005561 Musa balbisiana Species 0.000 description 1
- 235000018290 Musa x paradisiaca Nutrition 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- WXZVAROIGSFCFJ-UHFFFAOYSA-N N,N-diethyl-2-(naphthalen-1-yloxy)propanamide Chemical compound C1=CC=C2C(OC(C)C(=O)N(CC)CC)=CC=CC2=C1 WXZVAROIGSFCFJ-UHFFFAOYSA-N 0.000 description 1
- CMEWLCATCRTSGF-UHFFFAOYSA-N N,N-dimethyl-4-nitrosoaniline Chemical compound CN(C)C1=CC=C(N=O)C=C1 CMEWLCATCRTSGF-UHFFFAOYSA-N 0.000 description 1
- LVKTWOXHRYGDMM-UHFFFAOYSA-N Naproanilide Chemical compound C=1C=C2C=CC=CC2=CC=1OC(C)C(=O)NC1=CC=CC=C1 LVKTWOXHRYGDMM-UHFFFAOYSA-N 0.000 description 1
- 239000005585 Napropamide Substances 0.000 description 1
- CCGPUGMWYLICGL-UHFFFAOYSA-N Neburon Chemical compound CCCCN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 CCGPUGMWYLICGL-UHFFFAOYSA-N 0.000 description 1
- 239000005586 Nicosulfuron Substances 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- 239000005587 Oryzalin Substances 0.000 description 1
- 239000005590 Oxyfluorfen Substances 0.000 description 1
- OQMBBFQZGJFLBU-UHFFFAOYSA-N Oxyfluorfen Chemical compound C1=C([N+]([O-])=O)C(OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 OQMBBFQZGJFLBU-UHFFFAOYSA-N 0.000 description 1
- 239000005591 Pendimethalin Substances 0.000 description 1
- 241000745991 Phalaris Species 0.000 description 1
- 239000005594 Phenmedipham Substances 0.000 description 1
- 241000746981 Phleum Species 0.000 description 1
- UNLYSVIDNRIVFJ-UHFFFAOYSA-N Piperophos Chemical compound CCCOP(=S)(OCCC)SCC(=O)N1CCCCC1C UNLYSVIDNRIVFJ-UHFFFAOYSA-N 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 239000005600 Propaquizafop Substances 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 239000005603 Prosulfocarb Substances 0.000 description 1
- 239000005604 Prosulfuron Substances 0.000 description 1
- LTUNNEGNEKBSEH-UHFFFAOYSA-N Prosulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)CCC(F)(F)F)=N1 LTUNNEGNEKBSEH-UHFFFAOYSA-N 0.000 description 1
- 108010009736 Protein Hydrolysates Proteins 0.000 description 1
- VXMNDQDDWDDKOQ-UHFFFAOYSA-N Pyrazosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N(N=CC=2C(O)=O)C)=N1 VXMNDQDDWDDKOQ-UHFFFAOYSA-N 0.000 description 1
- 239000005606 Pyridate Substances 0.000 description 1
- JTZCTMAVMHRNTR-UHFFFAOYSA-N Pyridate Chemical compound CCCCCCCCSC(=O)OC1=CC(Cl)=NN=C1C1=CC=CC=C1 JTZCTMAVMHRNTR-UHFFFAOYSA-N 0.000 description 1
- 239000005608 Quinmerac Substances 0.000 description 1
- 239000005616 Rimsulfuron Substances 0.000 description 1
- 241000490453 Rorippa Species 0.000 description 1
- 241000341978 Rotala Species 0.000 description 1
- 241000209051 Saccharum Species 0.000 description 1
- 240000000111 Saccharum officinarum Species 0.000 description 1
- 235000007201 Saccharum officinarum Nutrition 0.000 description 1
- 240000009132 Sagittaria sagittifolia Species 0.000 description 1
- 241000202758 Scirpus Species 0.000 description 1
- 239000004113 Sepiolite Substances 0.000 description 1
- CSPPKDPQLUUTND-NBVRZTHBSA-N Sethoxydim Chemical compound CCO\N=C(/CCC)C1=C(O)CC(CC(C)SCC)CC1=O CSPPKDPQLUUTND-NBVRZTHBSA-N 0.000 description 1
- 241000220261 Sinapis Species 0.000 description 1
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 239000005618 Sulcotrione Substances 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 239000005864 Sulphur Substances 0.000 description 1
- STSCVKRWJPWALQ-UHFFFAOYSA-N TRIFLUOROACETIC ACID ETHYL ESTER Chemical class CCOC(=O)C(F)(F)F STSCVKRWJPWALQ-UHFFFAOYSA-N 0.000 description 1
- 241000245665 Taraxacum Species 0.000 description 1
- 240000001949 Taraxacum officinale Species 0.000 description 1
- 235000005187 Taraxacum officinale ssp. officinale Nutrition 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- KDWQYMVPYJGPHS-UHFFFAOYSA-N Thenylchlor Chemical compound C1=CSC(CN(C(=O)CCl)C=2C(=CC=CC=2C)C)=C1OC KDWQYMVPYJGPHS-UHFFFAOYSA-N 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- YIJZJEYQBAAWRJ-UHFFFAOYSA-N Thiazopyr Chemical compound N1=C(C(F)F)C(C(=O)OC)=C(CC(C)C)C(C=2SCCN=2)=C1C(F)(F)F YIJZJEYQBAAWRJ-UHFFFAOYSA-N 0.000 description 1
- QHTQREMOGMZHJV-UHFFFAOYSA-N Thiobencarb Chemical compound CCN(CC)C(=O)SCC1=CC=C(Cl)C=C1 QHTQREMOGMZHJV-UHFFFAOYSA-N 0.000 description 1
- PHSUVQBHRAWOQD-UHFFFAOYSA-N Tiocarbazil Chemical compound CCC(C)N(C(C)CC)C(=O)SCC1=CC=CC=C1 PHSUVQBHRAWOQD-UHFFFAOYSA-N 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- 239000005624 Tralkoxydim Substances 0.000 description 1
- WHKUVVPPKQRRBV-UHFFFAOYSA-N Trasan Chemical compound CC1=CC(Cl)=CC=C1OCC(O)=O WHKUVVPPKQRRBV-UHFFFAOYSA-N 0.000 description 1
- 239000005625 Tri-allate Substances 0.000 description 1
- MWBPRDONLNQCFV-UHFFFAOYSA-N Tri-allate Chemical compound CC(C)N(C(C)C)C(=O)SCC(Cl)=C(Cl)Cl MWBPRDONLNQCFV-UHFFFAOYSA-N 0.000 description 1
- 239000005627 Triclopyr Substances 0.000 description 1
- IBZHOAONZVJLOB-UHFFFAOYSA-N Tridiphane Chemical compound ClC1=CC(Cl)=CC(C2(CC(Cl)(Cl)Cl)OC2)=C1 IBZHOAONZVJLOB-UHFFFAOYSA-N 0.000 description 1
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 1
- 239000005628 Triflusulfuron Substances 0.000 description 1
- 241001573053 Vandellia Species 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- JFBZPFYRPYOZCQ-UHFFFAOYSA-N [Li].[Al] Chemical compound [Li].[Al] JFBZPFYRPYOZCQ-UHFFFAOYSA-N 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000001785 acacia senegal l. willd gum Substances 0.000 description 1
- 230000000895 acaricidal effect Effects 0.000 description 1
- 239000000642 acaricide Substances 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- NUFNQYOELLVIPL-UHFFFAOYSA-N acifluorfen Chemical compound C1=C([N+]([O-])=O)C(C(=O)O)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 NUFNQYOELLVIPL-UHFFFAOYSA-N 0.000 description 1
- DDBMQDADIHOWIC-UHFFFAOYSA-N aclonifen Chemical compound C1=C([N+]([O-])=O)C(N)=C(Cl)C(OC=2C=CC=CC=2)=C1 DDBMQDADIHOWIC-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- XCSGPAVHZFQHGE-UHFFFAOYSA-N alachlor Chemical compound CCC1=CC=CC(CC)=C1N(COC)C(=O)CCl XCSGPAVHZFQHGE-UHFFFAOYSA-N 0.000 description 1
- 125000002723 alicyclic group Chemical group 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- HFVAFDPGUJEFBQ-UHFFFAOYSA-M alizarin red S Chemical compound [Na+].O=C1C2=CC=CC=C2C(=O)C2=C1C=C(S([O-])(=O)=O)C(O)=C2O HFVAFDPGUJEFBQ-UHFFFAOYSA-M 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 150000004703 alkoxides Chemical class 0.000 description 1
- 150000008051 alkyl sulfates Chemical class 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- RQVYBGPQFYCBGX-UHFFFAOYSA-N ametryn Chemical compound CCNC1=NC(NC(C)C)=NC(SC)=N1 RQVYBGPQFYCBGX-UHFFFAOYSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- MXWJVTOOROXGIU-UHFFFAOYSA-N atrazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)C)=N1 MXWJVTOOROXGIU-UHFFFAOYSA-N 0.000 description 1
- VJBCNMFKFZIXHC-UHFFFAOYSA-N azanium;2-(4-methyl-5-oxo-4-propan-2-yl-1h-imidazol-2-yl)quinoline-3-carboxylate Chemical compound N.N1C(=O)C(C(C)C)(C)N=C1C1=NC2=CC=CC=C2C=C1C(O)=O VJBCNMFKFZIXHC-UHFFFAOYSA-N 0.000 description 1
- MAHPNPYYQAIOJN-UHFFFAOYSA-N azimsulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2N(N=CC=2C2=NN(C)N=N2)C)=N1 MAHPNPYYQAIOJN-UHFFFAOYSA-N 0.000 description 1
- 239000000987 azo dye Substances 0.000 description 1
- 150000003851 azoles Chemical class 0.000 description 1
- ZOMSMJKLGFBRBS-UHFFFAOYSA-N bentazone Chemical compound C1=CC=C2NS(=O)(=O)N(C(C)C)C(=O)C2=C1 ZOMSMJKLGFBRBS-UHFFFAOYSA-N 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- CNBGNNVCVSKAQZ-UHFFFAOYSA-N benzidamine Natural products C12=CC=CC=C2C(OCCCN(C)C)=NN1CC1=CC=CC=C1 CNBGNNVCVSKAQZ-UHFFFAOYSA-N 0.000 description 1
- GINJFDRNADDBIN-FXQIFTODSA-N bilanafos Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCP(C)(O)=O GINJFDRNADDBIN-FXQIFTODSA-N 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- WZDDLAZXUYIVMU-UHFFFAOYSA-N bromobutide Chemical compound CC(C)(C)C(Br)C(=O)NC(C)(C)C1=CC=CC=C1 WZDDLAZXUYIVMU-UHFFFAOYSA-N 0.000 description 1
- HKPHPIREJKHECO-UHFFFAOYSA-N butachlor Chemical compound CCCCOCN(C(=O)CCl)C1=C(CC)C=CC=C1CC HKPHPIREJKHECO-UHFFFAOYSA-N 0.000 description 1
- 125000000480 butynyl group Chemical group [*]C#CC([H])([H])C([H])([H])[H] 0.000 description 1
- KVNRLNFWIYMESJ-UHFFFAOYSA-N butyronitrile Chemical compound CCCC#N KVNRLNFWIYMESJ-UHFFFAOYSA-N 0.000 description 1
- HFEJHAAIJZXXRE-UHFFFAOYSA-N cafenstrole Chemical compound CCN(CC)C(=O)N1C=NC(S(=O)(=O)C=2C(=CC(C)=CC=2C)C)=N1 HFEJHAAIJZXXRE-UHFFFAOYSA-N 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- VSGNNIFQASZAOI-UHFFFAOYSA-L calcium acetate Chemical compound [Ca+2].CC([O-])=O.CC([O-])=O VSGNNIFQASZAOI-UHFFFAOYSA-L 0.000 description 1
- 239000001639 calcium acetate Substances 0.000 description 1
- 235000011092 calcium acetate Nutrition 0.000 description 1
- 229960005147 calcium acetate Drugs 0.000 description 1
- NKWPZUCBCARRDP-UHFFFAOYSA-L calcium bicarbonate Chemical compound [Ca+2].OC([O-])=O.OC([O-])=O NKWPZUCBCARRDP-UHFFFAOYSA-L 0.000 description 1
- 229910000020 calcium bicarbonate Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- NQIZDFMZAXUZCZ-UHFFFAOYSA-N carbifene Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(OCC)C(=O)N(C)CCN(C)CCC1=CC=CC=C1 NQIZDFMZAXUZCZ-UHFFFAOYSA-N 0.000 description 1
- 229950003365 carbifene Drugs 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- GGWHBJGBERXSLL-NBVRZTHBSA-N chembl113137 Chemical compound C1C(=O)C(C(=N/OCC)/CCC)=C(O)CC1C1CSCCC1 GGWHBJGBERXSLL-NBVRZTHBSA-N 0.000 description 1
- MXZACTZQSGYANA-UHFFFAOYSA-N chembl545463 Chemical compound Cl.C1=CC(OC)=CC=C1C(N=C1)=CN2C1=NC(C)=C2O MXZACTZQSGYANA-UHFFFAOYSA-N 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- WYKYKTKDBLFHCY-UHFFFAOYSA-N chloridazon Chemical compound O=C1C(Cl)=C(N)C=NN1C1=CC=CC=C1 WYKYKTKDBLFHCY-UHFFFAOYSA-N 0.000 description 1
- RIUXZHMCCFLRBI-UHFFFAOYSA-N chlorimuron Chemical compound COC1=CC(Cl)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(O)=O)=N1 RIUXZHMCCFLRBI-UHFFFAOYSA-N 0.000 description 1
- 150000008422 chlorobenzenes Chemical class 0.000 description 1
- 125000004775 chlorodifluoromethyl group Chemical group FC(F)(Cl)* 0.000 description 1
- 125000004773 chlorofluoromethyl group Chemical group [H]C(F)(Cl)* 0.000 description 1
- 125000004218 chloromethyl group Chemical group [H]C([H])(Cl)* 0.000 description 1
- VJYIFXVZLXQVHO-UHFFFAOYSA-N chlorsulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)Cl)=N1 VJYIFXVZLXQVHO-UHFFFAOYSA-N 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- SILSDTWXNBZOGF-JWGBMQLESA-N clethodim Chemical compound CCSC(C)CC1CC(O)=C(C(CC)=NOC\C=C\Cl)C(=O)C1 SILSDTWXNBZOGF-JWGBMQLESA-N 0.000 description 1
- YUIKUTLBPMDDNQ-MRVPVSSYSA-N clodinafop Chemical compound C1=CC(O[C@H](C)C(O)=O)=CC=C1OC1=NC=C(Cl)C=C1F YUIKUTLBPMDDNQ-MRVPVSSYSA-N 0.000 description 1
- KIEDNEWSYUYDSN-UHFFFAOYSA-N clomazone Chemical compound O=C1C(C)(C)CON1CC1=CC=CC=C1Cl KIEDNEWSYUYDSN-UHFFFAOYSA-N 0.000 description 1
- HUBANNPOLNYSAD-UHFFFAOYSA-N clopyralid Chemical compound OC(=O)C1=NC(Cl)=CC=C1Cl HUBANNPOLNYSAD-UHFFFAOYSA-N 0.000 description 1
- YIANBKOBVRMNPR-UHFFFAOYSA-N cloransulam Chemical compound N=1N2C(OCC)=NC(F)=CC2=NC=1S(=O)(=O)NC1=C(Cl)C=CC=C1C(O)=O YIANBKOBVRMNPR-UHFFFAOYSA-N 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 239000006184 cosolvent Substances 0.000 description 1
- MGNCLNQXLYJVJD-UHFFFAOYSA-N cyanuric chloride Chemical compound ClC1=NC(Cl)=NC(Cl)=N1 MGNCLNQXLYJVJD-UHFFFAOYSA-N 0.000 description 1
- 125000001047 cyclobutenyl group Chemical group C1(=CCC1)* 0.000 description 1
- 239000002837 defoliant Substances 0.000 description 1
- WZJZMXBKUWKXTQ-UHFFFAOYSA-N desmedipham Chemical compound CCOC(=O)NC1=CC=CC(OC(=O)NC=2C=CC=CC=2)=C1 WZJZMXBKUWKXTQ-UHFFFAOYSA-N 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 239000012973 diazabicyclooctane Substances 0.000 description 1
- IWEDIXLBFLAXBO-UHFFFAOYSA-N dicamba Chemical compound COC1=C(Cl)C=CC(Cl)=C1C(O)=O IWEDIXLBFLAXBO-UHFFFAOYSA-N 0.000 description 1
- 125000004772 dichloromethyl group Chemical group [H]C(Cl)(Cl)* 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- WYEHFWKAOXOVJD-UHFFFAOYSA-N diflufenican Chemical compound FC1=CC(F)=CC=C1NC(=O)C1=CC=CN=C1OC1=CC=CC(C(F)(F)F)=C1 WYEHFWKAOXOVJD-UHFFFAOYSA-N 0.000 description 1
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- BWUPSGJXXPATLU-UHFFFAOYSA-N dimepiperate Chemical compound C=1C=CC=CC=1C(C)(C)SC(=O)N1CCCCC1 BWUPSGJXXPATLU-UHFFFAOYSA-N 0.000 description 1
- SCCDDNKJYDZXMM-UHFFFAOYSA-N dimethachlor Chemical compound COCCN(C(=O)CCl)C1=C(C)C=CC=C1C SCCDDNKJYDZXMM-UHFFFAOYSA-N 0.000 description 1
- BTVWZWFKMIUSGS-UHFFFAOYSA-N dimethylethyleneglycol Natural products CC(C)(O)CO BTVWZWFKMIUSGS-UHFFFAOYSA-N 0.000 description 1
- 239000010459 dolomite Substances 0.000 description 1
- 229910000514 dolomite Inorganic materials 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- IRLGCAJYYKDTCG-UHFFFAOYSA-N ethametsulfuron Chemical compound CCOC1=NC(NC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)C(O)=O)=N1 IRLGCAJYYKDTCG-UHFFFAOYSA-N 0.000 description 1
- QMTNOLKHSWIQBE-FGTMMUONSA-N exo-(+)-cinmethylin Chemical compound O([C@H]1[C@]2(C)CC[C@@](O2)(C1)C(C)C)CC1=CC=CC=C1C QMTNOLKHSWIQBE-FGTMMUONSA-N 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- IANUJLZYFUDJIH-UHFFFAOYSA-N flufenacet Chemical compound C=1C=C(F)C=CC=1N(C(C)C)C(=O)COC1=NN=C(C(F)(F)F)S1 IANUJLZYFUDJIH-UHFFFAOYSA-N 0.000 description 1
- FOUWCSDKDDHKQP-UHFFFAOYSA-N flumioxazin Chemical compound FC1=CC=2OCC(=O)N(CC#C)C=2C=C1N(C1=O)C(=O)C2=C1CCCC2 FOUWCSDKDDHKQP-UHFFFAOYSA-N 0.000 description 1
- RZILCCPWPBTYDO-UHFFFAOYSA-N fluometuron Chemical compound CN(C)C(=O)NC1=CC=CC(C(F)(F)F)=C1 RZILCCPWPBTYDO-UHFFFAOYSA-N 0.000 description 1
- 125000003983 fluorenyl group Chemical class C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 description 1
- 125000005817 fluorobutyl group Chemical group [H]C([H])(F)C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004216 fluoromethyl group Chemical group [H]C([H])(F)* 0.000 description 1
- OQZCSNDVOWYALR-UHFFFAOYSA-N flurochloridone Chemical compound FC(F)(F)C1=CC=CC(N2C(C(Cl)C(CCl)C2)=O)=C1 OQZCSNDVOWYALR-UHFFFAOYSA-N 0.000 description 1
- BGZZWXTVIYUUEY-UHFFFAOYSA-N fomesafen Chemical compound C1=C([N+]([O-])=O)C(C(=O)NS(=O)(=O)C)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 BGZZWXTVIYUUEY-UHFFFAOYSA-N 0.000 description 1
- ZHNUHDYFZUAESO-UHFFFAOYSA-N formamide Substances NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 1
- 150000003948 formamides Chemical class 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000000855 fungicidal effect Effects 0.000 description 1
- 239000000417 fungicide Substances 0.000 description 1
- 239000003502 gasoline Substances 0.000 description 1
- XDDAORKBJWWYJS-UHFFFAOYSA-N glyphosate Chemical compound OC(=O)CNCP(O)(O)=O XDDAORKBJWWYJS-UHFFFAOYSA-N 0.000 description 1
- 229940097068 glyphosate Drugs 0.000 description 1
- ZEKANFGSDXODPD-UHFFFAOYSA-N glyphosate-isopropylammonium Chemical compound CC(C)N.OC(=O)CNCP(O)(O)=O ZEKANFGSDXODPD-UHFFFAOYSA-N 0.000 description 1
- 230000026030 halogenation Effects 0.000 description 1
- 238000005658 halogenation reaction Methods 0.000 description 1
- 244000144980 herd Species 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- GNOIPBMMFNIUFM-UHFFFAOYSA-N hexamethylphosphoric triamide Chemical compound CN(C)P(=O)(N(C)C)N(C)C GNOIPBMMFNIUFM-UHFFFAOYSA-N 0.000 description 1
- 239000010903 husk Substances 0.000 description 1
- 150000004678 hydrides Chemical class 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000003840 hydrochlorides Chemical class 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- 239000005457 ice water Substances 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 239000001023 inorganic pigment Substances 0.000 description 1
- 239000002917 insecticide Substances 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- NRXQIUSYPAHGNM-UHFFFAOYSA-N ioxynil Chemical compound OC1=C(I)C=C(C#N)C=C1I NRXQIUSYPAHGNM-UHFFFAOYSA-N 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- DCYOBGZUOMKFPA-UHFFFAOYSA-N iron(2+);iron(3+);octadecacyanide Chemical compound [Fe+2].[Fe+2].[Fe+2].[Fe+3].[Fe+3].[Fe+3].[Fe+3].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-] DCYOBGZUOMKFPA-UHFFFAOYSA-N 0.000 description 1
- 229960004592 isopropanol Drugs 0.000 description 1
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 1
- PUIYMUZLKQOUOZ-UHFFFAOYSA-N isoproturon Chemical compound CC(C)C1=CC=C(NC(=O)N(C)C)C=C1 PUIYMUZLKQOUOZ-UHFFFAOYSA-N 0.000 description 1
- PMHURSZHKKJGBM-UHFFFAOYSA-N isoxaben Chemical compound O1N=C(C(C)(CC)CC)C=C1NC(=O)C1=C(OC)C=CC=C1OC PMHURSZHKKJGBM-UHFFFAOYSA-N 0.000 description 1
- OYIKARCXOQLFHF-UHFFFAOYSA-N isoxaflutole Chemical compound CS(=O)(=O)C1=CC(C(F)(F)F)=CC=C1C(=O)C1=C(C2CC2)ON=C1 OYIKARCXOQLFHF-UHFFFAOYSA-N 0.000 description 1
- 229940088649 isoxaflutole Drugs 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- CONWAEURSVPLRM-UHFFFAOYSA-N lactofen Chemical compound C1=C([N+]([O-])=O)C(C(=O)OC(C)C(=O)OCC)=CC(OC=2C(=CC(=CC=2)C(F)(F)F)Cl)=C1 CONWAEURSVPLRM-UHFFFAOYSA-N 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- ZTMKADLOSYKWCA-UHFFFAOYSA-N lenacil Chemical compound O=C1NC=2CCCC=2C(=O)N1C1CCCCC1 ZTMKADLOSYKWCA-UHFFFAOYSA-N 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- 239000012280 lithium aluminium hydride Substances 0.000 description 1
- AFRJJFRNGGLMDW-UHFFFAOYSA-N lithium amide Chemical compound [Li+].[NH2-] AFRJJFRNGGLMDW-UHFFFAOYSA-N 0.000 description 1
- SIAPCJWMELPYOE-UHFFFAOYSA-N lithium hydride Chemical compound [LiH] SIAPCJWMELPYOE-UHFFFAOYSA-N 0.000 description 1
- 229910000103 lithium hydride Inorganic materials 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000004579 marble Substances 0.000 description 1
- XIGAUIHYSDTJHW-UHFFFAOYSA-N mefenacet Chemical compound N=1C2=CC=CC=C2SC=1OCC(=O)N(C)C1=CC=CC=C1 XIGAUIHYSDTJHW-UHFFFAOYSA-N 0.000 description 1
- VHCNQEUWZYOAEV-UHFFFAOYSA-N metamitron Chemical compound O=C1N(N)C(C)=NN=C1C1=CC=CC=C1 VHCNQEUWZYOAEV-UHFFFAOYSA-N 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 125000001160 methoxycarbonyl group Chemical group [H]C([H])([H])OC(*)=O 0.000 description 1
- BACHBFVBHLGWSL-UHFFFAOYSA-N methyl 2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate Chemical group C1=CC(OC(C)C(=O)OC)=CC=C1OC1=CC=C(Cl)C=C1Cl BACHBFVBHLGWSL-UHFFFAOYSA-N 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 125000004092 methylthiomethyl group Chemical group [H]C([H])([H])SC([H])([H])* 0.000 description 1
- DSRNRYQBBJQVCW-UHFFFAOYSA-N metoxuron Chemical compound COC1=CC=C(NC(=O)N(C)C)C=C1Cl DSRNRYQBBJQVCW-UHFFFAOYSA-N 0.000 description 1
- RSMUVYRMZCOLBH-UHFFFAOYSA-N metsulfuron methyl Chemical group COC(=O)C1=CC=CC=C1S(=O)(=O)NC(=O)NC1=NC(C)=NC(OC)=N1 RSMUVYRMZCOLBH-UHFFFAOYSA-N 0.000 description 1
- 239000011785 micronutrient Substances 0.000 description 1
- 235000013369 micronutrients Nutrition 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- DEDOPGXGGQYYMW-UHFFFAOYSA-N molinate Chemical compound CCSC(=O)N1CCCCCC1 DEDOPGXGGQYYMW-UHFFFAOYSA-N 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- QTGVGIVRLSGTJJ-UHFFFAOYSA-N n-(acetamidomethyl)-2-chloro-n-(2,6-diethylphenyl)acetamide Chemical compound CCC1=CC=CC(CC)=C1N(CNC(C)=O)C(=O)CCl QTGVGIVRLSGTJJ-UHFFFAOYSA-N 0.000 description 1
- XRKQMIFKHDXFNQ-UHFFFAOYSA-N n-cyclohexyl-n-ethylcyclohexanamine Chemical compound C1CCCCC1N(CC)C1CCCCC1 XRKQMIFKHDXFNQ-UHFFFAOYSA-N 0.000 description 1
- JIKUXBYRTXDNIY-UHFFFAOYSA-N n-methyl-n-phenylformamide Chemical class O=CN(C)C1=CC=CC=C1 JIKUXBYRTXDNIY-UHFFFAOYSA-N 0.000 description 1
- 229940042880 natural phospholipid Drugs 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 239000005645 nematicide Substances 0.000 description 1
- RTCOGUMHFFWOJV-UHFFFAOYSA-N nicosulfuron Chemical compound COC1=CC(OC)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CN=2)C(=O)N(C)C)=N1 RTCOGUMHFFWOJV-UHFFFAOYSA-N 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- NVGOPFQZYCNLDU-UHFFFAOYSA-N norflurazon Chemical compound O=C1C(Cl)=C(NC)C=NN1C1=CC=CC(C(F)(F)F)=C1 NVGOPFQZYCNLDU-UHFFFAOYSA-N 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 235000014571 nuts Nutrition 0.000 description 1
- LLLFASISUZUJEQ-UHFFFAOYSA-N orbencarb Chemical compound CCN(CC)C(=O)SCC1=CC=CC=C1Cl LLLFASISUZUJEQ-UHFFFAOYSA-N 0.000 description 1
- 239000002420 orchard Substances 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 239000005416 organic matter Substances 0.000 description 1
- UNAHYJYOSSSJHH-UHFFFAOYSA-N oryzalin Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(S(N)(=O)=O)C=C1[N+]([O-])=O UNAHYJYOSSSJHH-UHFFFAOYSA-N 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- FIKAKWIAUPDISJ-UHFFFAOYSA-L paraquat dichloride Chemical compound [Cl-].[Cl-].C1=C[N+](C)=CC=C1C1=CC=[N+](C)C=C1 FIKAKWIAUPDISJ-UHFFFAOYSA-L 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- CHIFOSRWCNZCFN-UHFFFAOYSA-N pendimethalin Chemical compound CCC(CC)NC1=C([N+]([O-])=O)C=C(C)C(C)=C1[N+]([O-])=O CHIFOSRWCNZCFN-UHFFFAOYSA-N 0.000 description 1
- QWENMOXLTHDKDL-UHFFFAOYSA-M pentoxymethanedithioate Chemical compound CCCCCOC([S-])=S QWENMOXLTHDKDL-UHFFFAOYSA-M 0.000 description 1
- 125000005004 perfluoroethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 1
- 125000005005 perfluorohexyl group Chemical group FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)* 0.000 description 1
- 125000005008 perfluoropentyl group Chemical group FC(C(C(C(C(F)(F)F)(F)F)(F)F)(F)F)(F)* 0.000 description 1
- 125000005009 perfluoropropyl group Chemical group FC(C(C(F)(F)F)(F)F)(F)* 0.000 description 1
- IDOWTHOLJBTAFI-UHFFFAOYSA-N phenmedipham Chemical compound COC(=O)NC1=CC=CC(OC(=O)NC=2C=C(C)C=CC=2)=C1 IDOWTHOLJBTAFI-UHFFFAOYSA-N 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 239000001007 phthalocyanine dye Substances 0.000 description 1
- 230000008654 plant damage Effects 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 238000012805 post-processing Methods 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 235000011056 potassium acetate Nutrition 0.000 description 1
- RPDAUEIUDPHABB-UHFFFAOYSA-N potassium ethoxide Chemical compound [K+].CC[O-] RPDAUEIUDPHABB-UHFFFAOYSA-N 0.000 description 1
- BDAWXSQJJCIFIK-UHFFFAOYSA-N potassium methoxide Chemical compound [K+].[O-]C BDAWXSQJJCIFIK-UHFFFAOYSA-N 0.000 description 1
- AAEVYOVXGOFMJO-UHFFFAOYSA-N prometryn Chemical compound CSC1=NC(NC(C)C)=NC(NC(C)C)=N1 AAEVYOVXGOFMJO-UHFFFAOYSA-N 0.000 description 1
- MFOUDYKPLGXPGO-UHFFFAOYSA-N propachlor Chemical compound ClCC(=O)N(C(C)C)C1=CC=CC=C1 MFOUDYKPLGXPGO-UHFFFAOYSA-N 0.000 description 1
- OYJMHAFVOZPIOY-UHFFFAOYSA-N propan-2-yl 2-chloro-5-[3-methyl-2,6-dioxo-4-(trifluoromethyl)pyrimidin-1-yl]benzoate Chemical compound C1=C(Cl)C(C(=O)OC(C)C)=CC(N2C(N(C)C(=CC2=O)C(F)(F)F)=O)=C1 OYJMHAFVOZPIOY-UHFFFAOYSA-N 0.000 description 1
- LFULEKSKNZEWOE-UHFFFAOYSA-N propanil Chemical compound CCC(=O)NC1=CC=C(Cl)C(Cl)=C1 LFULEKSKNZEWOE-UHFFFAOYSA-N 0.000 description 1
- FROBCXTULYFHEJ-OAHLLOKOSA-N propaquizafop Chemical compound C1=CC(O[C@H](C)C(=O)OCCON=C(C)C)=CC=C1OC1=CN=C(C=C(Cl)C=C2)C2=N1 FROBCXTULYFHEJ-OAHLLOKOSA-N 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- FVSKHRXBFJPNKK-UHFFFAOYSA-N propionitrile Chemical compound CCC#N FVSKHRXBFJPNKK-UHFFFAOYSA-N 0.000 description 1
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- NQLVQOSNDJXLKG-UHFFFAOYSA-N prosulfocarb Chemical compound CCCN(CCC)C(=O)SCC1=CC=CC=C1 NQLVQOSNDJXLKG-UHFFFAOYSA-N 0.000 description 1
- 239000003531 protein hydrolysate Substances 0.000 description 1
- 229960003351 prussian blue Drugs 0.000 description 1
- 239000013225 prussian blue Substances 0.000 description 1
- ASRAWSBMDXVNLX-UHFFFAOYSA-N pyrazolynate Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OS(=O)(=O)C1=CC=C(C)C=C1 ASRAWSBMDXVNLX-UHFFFAOYSA-N 0.000 description 1
- FKERUJTUOYLBKB-UHFFFAOYSA-N pyrazoxyfen Chemical compound C=1C=C(Cl)C=C(Cl)C=1C(=O)C=1C(C)=NN(C)C=1OCC(=O)C1=CC=CC=C1 FKERUJTUOYLBKB-UHFFFAOYSA-N 0.000 description 1
- VTRWMTJQBQJKQH-UHFFFAOYSA-N pyributicarb Chemical compound COC1=CC=CC(N(C)C(=S)OC=2C=C(C=CC=2)C(C)(C)C)=N1 VTRWMTJQBQJKQH-UHFFFAOYSA-N 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- ALZOLUNSQWINIR-UHFFFAOYSA-N quinmerac Chemical compound OC(=O)C1=C(Cl)C=CC2=CC(C)=CN=C21 ALZOLUNSQWINIR-UHFFFAOYSA-N 0.000 description 1
- MEFOUWRMVYJCQC-UHFFFAOYSA-N rimsulfuron Chemical compound CCS(=O)(=O)C1=CC=CN=C1S(=O)(=O)NC(=O)NC1=NC(OC)=CC(OC)=N1 MEFOUWRMVYJCQC-UHFFFAOYSA-N 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229910052624 sepiolite Inorganic materials 0.000 description 1
- 235000019355 sepiolite Nutrition 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- ODCWYMIRDDJXKW-UHFFFAOYSA-N simazine Chemical compound CCNC1=NC(Cl)=NC(NCC)=N1 ODCWYMIRDDJXKW-UHFFFAOYSA-N 0.000 description 1
- MGLWZSOBALDPEK-UHFFFAOYSA-N simetryn Chemical compound CCNC1=NC(NCC)=NC(SC)=N1 MGLWZSOBALDPEK-UHFFFAOYSA-N 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- ODZPKZBBUMBTMG-UHFFFAOYSA-N sodium amide Chemical compound [NH2-].[Na+] ODZPKZBBUMBTMG-UHFFFAOYSA-N 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229960002668 sodium chloride Drugs 0.000 description 1
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 1
- 239000012312 sodium hydride Substances 0.000 description 1
- 229910000104 sodium hydride Inorganic materials 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- SZXAJDQTPWYNBN-NWBUNABESA-M sodium;4-methoxycarbonyl-5,5-dimethyl-3-oxo-2-[(e)-n-prop-2-enoxy-c-propylcarbonimidoyl]cyclohexen-1-olate Chemical compound [Na+].C=CCO\N=C(/CCC)C1=C([O-])CC(C)(C)C(C(=O)OC)C1=O SZXAJDQTPWYNBN-NWBUNABESA-M 0.000 description 1
- 238000009331 sowing Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- PQTBTIFWAXVEPB-UHFFFAOYSA-N sulcotrione Chemical compound ClC1=CC(S(=O)(=O)C)=CC=C1C(=O)C1C(=O)CCCC1=O PQTBTIFWAXVEPB-UHFFFAOYSA-N 0.000 description 1
- PXQLVRUNWNTZOS-UHFFFAOYSA-N sulfanyl Chemical compound [SH] PXQLVRUNWNTZOS-UHFFFAOYSA-N 0.000 description 1
- OORLZFUTLGXMEF-UHFFFAOYSA-N sulfentrazone Chemical compound O=C1N(C(F)F)C(C)=NN1C1=CC(NS(C)(=O)=O)=C(Cl)C=C1Cl OORLZFUTLGXMEF-UHFFFAOYSA-N 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- RJKCKKDSSSRYCB-UHFFFAOYSA-N tebutam Chemical compound CC(C)(C)C(=O)N(C(C)C)CC1=CC=CC=C1 RJKCKKDSSSRYCB-UHFFFAOYSA-N 0.000 description 1
- IROINLKCQGIITA-UHFFFAOYSA-N terbutryn Chemical compound CCNC1=NC(NC(C)(C)C)=NC(SC)=N1 IROINLKCQGIITA-UHFFFAOYSA-N 0.000 description 1
- 229930192474 thiophene Natural products 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- DQFPEYARZIQXRM-LTGZKZEYSA-N tralkoxydim Chemical compound C1C(=O)C(C(/CC)=N/OCC)=C(O)CC1C1=C(C)C=C(C)C=C1C DQFPEYARZIQXRM-LTGZKZEYSA-N 0.000 description 1
- XOPFESVZMSQIKC-UHFFFAOYSA-N triasulfuron Chemical compound COC1=NC(C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2)OCCCl)=N1 XOPFESVZMSQIKC-UHFFFAOYSA-N 0.000 description 1
- YMXOXAPKZDWXLY-QWRGUYRKSA-N tribenuron methyl Chemical group COC(=O)[C@H]1CCCC[C@@H]1S(=O)(=O)NC(=O)N(C)C1=NC(C)=NC(OC)=N1 YMXOXAPKZDWXLY-QWRGUYRKSA-N 0.000 description 1
- IMFACGCPASFAPR-UHFFFAOYSA-N tributylamine Chemical compound CCCCN(CCCC)CCCC IMFACGCPASFAPR-UHFFFAOYSA-N 0.000 description 1
- REEQLXCGVXDJSQ-UHFFFAOYSA-N trichlopyr Chemical compound OC(=O)COC1=NC(Cl)=C(Cl)C=C1Cl REEQLXCGVXDJSQ-UHFFFAOYSA-N 0.000 description 1
- 125000003866 trichloromethyl group Chemical group ClC(Cl)(Cl)* 0.000 description 1
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 1
- 125000005034 trifluormethylthio group Chemical group FC(S*)(F)F 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- ZSDSQXJSNMTJDA-UHFFFAOYSA-N trifluralin Chemical compound CCCN(CCC)C1=C([N+]([O-])=O)C=C(C(F)(F)F)C=C1[N+]([O-])=O ZSDSQXJSNMTJDA-UHFFFAOYSA-N 0.000 description 1
- AKTQJCBOGPBERP-UHFFFAOYSA-N triflusulfuron Chemical compound FC(F)(F)COC1=NC(N(C)C)=NC(NC(=O)NS(=O)(=O)C=2C(=CC=CC=2C)C(O)=O)=N1 AKTQJCBOGPBERP-UHFFFAOYSA-N 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 238000009333 weeding Methods 0.000 description 1
- 239000004563 wettable powder Substances 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D251/00—Heterocyclic compounds containing 1,3,5-triazine rings
- C07D251/02—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/64—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with three nitrogen atoms as the only ring hetero atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/64—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with three nitrogen atoms as the only ring hetero atoms
- A01N43/66—1,3,5-Triazines, not hydrogenated and not substituted at the ring nitrogen atoms
- A01N43/68—1,3,5-Triazines, not hydrogenated and not substituted at the ring nitrogen atoms with two or three nitrogen atoms directly attached to ring carbon atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/64—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with three nitrogen atoms as the only ring hetero atoms
- A01N43/66—1,3,5-Triazines, not hydrogenated and not substituted at the ring nitrogen atoms
- A01N43/68—1,3,5-Triazines, not hydrogenated and not substituted at the ring nitrogen atoms with two or three nitrogen atoms directly attached to ring carbon atoms
- A01N43/70—Diamino—1,3,5—triazines with only one oxygen, sulfur or halogen atom or only one cyano, thiocyano (—SCN), cyanato (—OCN) or azido (—N3) group directly attached to a ring carbon atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C279/00—Derivatives of guanidine, i.e. compounds containing the group, the singly-bound nitrogen atoms not being part of nitro or nitroso groups
- C07C279/20—Derivatives of guanidine, i.e. compounds containing the group, the singly-bound nitrogen atoms not being part of nitro or nitroso groups containing any of the groups, X being a hetero atom, Y being any atom, e.g. acylguanidines
- C07C279/24—Y being a hetero atom
- C07C279/26—X and Y being nitrogen atoms, i.e. biguanides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D251/00—Heterocyclic compounds containing 1,3,5-triazine rings
- C07D251/02—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings
- C07D251/12—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members
- C07D251/14—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with hydrogen or carbon atoms directly attached to at least one ring carbon atom
- C07D251/16—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with hydrogen or carbon atoms directly attached to at least one ring carbon atom to only one ring carbon atom
- C07D251/18—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with hydrogen or carbon atoms directly attached to at least one ring carbon atom to only one ring carbon atom with nitrogen atoms directly attached to the two other ring carbon atoms, e.g. guanamines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D251/00—Heterocyclic compounds containing 1,3,5-triazine rings
- C07D251/02—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings
- C07D251/12—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members
- C07D251/26—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with only hetero atoms directly attached to ring carbon atoms
- C07D251/40—Nitrogen atoms
- C07D251/48—Two nitrogen atoms
- C07D251/50—Two nitrogen atoms with a halogen atom attached to the third ring carbon atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D251/00—Heterocyclic compounds containing 1,3,5-triazine rings
- C07D251/02—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings
- C07D251/12—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members
- C07D251/26—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with only hetero atoms directly attached to ring carbon atoms
- C07D251/40—Nitrogen atoms
- C07D251/48—Two nitrogen atoms
- C07D251/52—Two nitrogen atoms with an oxygen or sulfur atom attached to the third ring carbon atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Agronomy & Crop Science (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
本发明涉及式(I)新的取代的2-氨基-4-烷基氨基-1,3,5-三嗪,其中R1代表任选被取代的甲基,R2代表氢或烷基,Y代表各种情况下任选被取代的苄基,萘基甲基,杂环基甲基或杂环基氧基,和Z代表氢,代表卤素,或者代表各种情况下任选被取代的烷基,烷氧基,烷基羰基,烷氧羰基,烷硫基,烷基亚磺酰基,烷基磺酰基,链烯基或炔烃基。本发明还涉及这些化合物的制备方法和新的中间体,并且涉及这些化合物作为除草剂的用途。
Description
技术领域
本发明涉及新的取代的2-氨基-4-烷基氨基-1,3,5-三嗪,其多种制备方法和新的中间体,以及其作为除草剂的用途。
背景技术
从(专利)文献中已知多种取代的2,4-二氨基-三嗪(参见US3816419,US3932167,EP191496,EP273328,EP411153/WO90/09378,WO97/00254,WO97/08156)。但是,迄今为止这些化合物没有获得任何特殊的重要性。
发明内容
因此,本发明提供通式(I)的新的取代的2-氨基-4-烷基氨基-1,3,5-三嗪:其中R1代表任选被取代的甲基,R2代表氢或烷基,Y代表各种情况下任选被取代的苄基,萘基甲基,杂环基甲基或杂环基氧基,和Z代表氢,代表卤素,或者代表各种情况下任选被取代的烷基,烷氧基,烷基羰基,烷氧羰基,烷硫基,烷基亚磺酰基,烷基磺酰基,链烯基或炔烃基。
当进行下面的反应时,得到通式(I)的新的2-氨基-4-烷基氨基-1,3,5-三嗪:(a)如果适当在反应助剂存在下和如果适当在稀释剂存在下,通式(II)的取代的双胍--和/或通式(II)的化合物的酸加成物--与通式(III)的的烷氧羰基化合物反应,其中R1,R2和Y各自如上定义,
Z-CO-OR’ (III)其中Z如上定义,和R’代表烷基,或者(b)如果适当在反应助剂存在下和如果适当在稀释剂存在下,通式(IV)的取代的三嗪与氨反应,其中R1,R2,Y和Z各自如上定义,和X1代表卤素或烷氧基,(c)如果适当在反应助剂存在下和如果适当在稀释剂存在下,通式(V)的取代的氨基三嗪与通式(VI)的取代的烷基胺反应,其中Z如上定义,和X2代表卤素或烷氧基,其中R1,R2和Y各自如上定义,并且,如果适当,通过常规方法对由(a),(b)或(c)描述的方法获得的通式(I)的化合物在上述取代基定义范围内进行进一步转化。
通式(I)的新的取代的2-氨基-4-烷基氨基-1,3,5-三嗪具有强的和选择性的除草活性。
本发明通式(I)的化合物含有至少一个不对称取代的碳原子,因此可以以不同的对映体(R-和S-构型)或者非对映异构体形式存在。本发明涉及通式(I)的化合物的不同的可能的各个对映体或立体异构体形式,和这些异构体化合物的混合物。
在定义中,烃链,例如烷基—也包括与杂原子组合的情况,例如在烷氧基或烷硫基中--在各种情况下是直链或支链的。
卤素一般代表氟,氯,溴或碘,优选代表氟,氯或溴,特别代表氟或氯。
本发明优选提供下列式(I)的化合物,其中R1代表任选被卤素-,氰基-,羧基-,氨基甲酰基-,硫代氨基甲酰基-或C1-C4-烷氧基-取代的甲基,R2代表氢或者具有1-3个碳原子的烷基,Y代表各种情况下任选被取代的苄基,萘基甲基,杂环基甲基或杂环基氧基,其中可能的杂环基优选选自下组:呋喃基,苯并呋喃基,二氢苯并呋喃基,四氢呋喃基,噻吩基,苯并噻吩基,噻唑基,苯并噻唑基,噁唑基,苯并噁唑基,噻二唑基,噁二唑基,吡唑基,吡咯基,吲哚基,吡啶基,喹啉基,异喹啉基和嘧啶基,其中可能的取代基在各种情况下优选选自下组:羟基,氰基,硝基,卤素,各种情况下任选被羟基-,氰基-或卤素-取代的各种情况下具有1-6个碳原子的烷基或烷氧基,各种情况下任选被卤素-取代的各种情况下烷基中具有1-6个碳原子的烷基羰基,烷氧羰基,烷硫基,烷基亚磺酰基或烷基磺酰基,各种情况下任选被羟基-,氰基-,硝基-,卤素-,C1-C4-烷基-,C1-C4-卤代烷基-,C1-C4-烷氧基-或C1-C4-卤代烷氧基-取代的苯基或苯氧基,还有各种情况下任选被卤素-取代的亚甲基二氧基或亚乙基二氧基,和Z代表氢,代表卤素,代表各种情况下任选被羟基-,氰基-,硝基-,卤素-,C1-C4-烷氧基,-C1-C4-烷基-羰基-,C1-C4-烷氧基-羰基-,C1-C4-烷硫基-,C1-C4-烷基亚磺酰基-或C1-C4-烷基磺酰基-取代的各种情况下烷基中具有1-6个碳原子的烷基,烷氧基,烷基羰基,烷氧羰基,烷硫基,烷基亚磺酰基或烷基磺酰基,或者代表任选被卤素-取代的各种情况下具有2-6个碳原子的链烯基或炔烃基。
在上面作为优选(优选地)定义的式(I)的化合物中,特别强调的是下组:(A)式(I)的化合物,其中R1,R2和Z各自如上定义且Y代表各种情况下任选被取代的苄基或萘基甲基,其中可能的取代基是如上定义的;(B)式(I)的化合物,其中R1,R2和Z各自如上定义且Y代表各种情况下任选被取代的杂环基甲基或杂环基氧基,其中可能的杂环基和可能的取代基是如上定义的。
本发明特别涉及下列式(I)的化合物,其中R1代表任选被氟-和/或氯-取代的甲基,R2代表氢,甲基或乙基,Y代表各种情况下任选被取代的苄基,萘基甲基,杂环基甲基或杂环基氧基,其中可能的杂环基优选选自下组:呋喃基,苯并呋喃基,二氢苯并呋喃基,四氢呋喃基,噻吩基,苯并噻吩基,噻唑基,苯并噻唑基,噁唑基,苯并噁唑基,噻二唑基,噁二唑基,吡唑基,吡咯基,吲哚基,吡啶基,喹啉基,异喹啉基和嘧啶基,其中可能的取代基在各种情况下优选选自下组:羟基,氰基,硝基,氟,氯,溴,各种情况下任选被羟基-,氰基-,氟-或氯-取代的甲基,乙基,正-或异-丙基,正-、异-、仲-或叔-丁基,甲氧基,乙氧基,正-或异-丙氧基,正-、异-、仲-或叔-丁氧基,各种情况下任选被氟-或氯-取代的乙酰基,丙酰基,正-或异-丁酰基,甲氧羰基,乙氧羰基,正-或异-丙氧羰基,甲硫基,乙硫基,正-或异-丙硫基,甲基亚磺酰基,乙基亚磺酰基,正-或异-丙基亚磺酰基,甲基磺酰基,乙基磺酰基,正-或异-丙基磺酰基,各种情况下任选被羟基-,氰基-,硝基-,氟-,氯-,溴-,甲基-,乙基-,正-或异-丙基-,正-、异-、仲-或叔-丁基-,三氟甲基-,甲氧基-,乙氧基-,正-或异-丙氧基-,正-、异-、仲-或叔-丁氧基-,二氟甲氧基-或三氟甲氧基-取代的苯基或苯氧基,还有各种情况下任选被氟-或氯-取代的亚甲基二氧基或亚乙基二氧基,和Z代表氢,代表氟,氯,溴,代表各种情况下任选被羟基-,氰基-,硝基-,氟-,氯-,甲氧基-,乙氧基-,正-或并-丙氧基-,正-、异-、仲-或叔-丁氧基-,甲硫基-,乙硫基-,正-或异-丙硫基-,甲基亚磺酰基-,乙基亚磺酰基-,正-或异-丙基亚磺酰基-,甲基磺酰基-,乙基磺酰基-,正-或异-丙基磺酰基-取代的甲基,乙基,正-或异-丙基,正-、异-、仲-或叔-丁基,甲氧基,乙氧基,正-或异-丙氧基,正-、异-、仲-或叔-丁氧基,甲硫基,乙硫基,正-或异-丙硫基,甲基亚磺酰基,乙基亚磺酰基,正-或异-丙基亚磺酰基,甲基磺酰基,乙基磺酰基,正-或异-丙基磺酰基,或者代表各种情况下任选被氟-,氯-或溴-取代的乙烯基,丙烯基,丁烯基,乙炔基,丙炔基或丁炔基。在上面作为特别优选定义的式(I)的化合物中,特别强调的是下组:(AA)式(I)的化合物,其中R1,R2和Z各自如上定义且Y代表各种情况下任选被取代的苄基或萘基甲基,可能的取代基是如上定义的,前提是与R1连接的碳原子的取代基是R构型;(BB)式(I)的化合物,其中R1,R2和Z各自如上定义且Y代表各种情况下任选被取代的苄基或萘基甲基,可能的取代基是如上定义的,前提是与R1连接的碳原子的取代基是S构型;(CC)式(I)的化合物,其中R1,R2和Z各自如上定义且Y代表各种情况下任选被取代的呋喃基甲基,噻吩基甲基,吡啶基甲基或嘧啶基甲基,可能的取代基是如上定义的,前提是这些化合物以外消旋混合物存在;(DD)式(I)的化合物,其中R1,R2和Z各自如上定义且Y代表各种情况下任选被取代的呋喃基甲基,噻吩基甲基,吡啶基甲基或嘧啶基甲基,可能的取代基是如上定义的,前提是与R1连接的碳原子的取代基是R构型;(EE)式(I)的化合物,其中R1,R2和Z各自如上定义且Y代表各种情况下任选被取代的呋喃基甲基,噻吩基甲基,吡啶基甲基或嘧啶基甲基,可能的取代基是如上定义的,前提是与R1连接的碳原子的取代基是S构型。
上述一般或优选的基团的定义适用于式(I)的终产物和相应地适用于制备的各种情况下需要的起始物或中间体。这些基团的定义可以根据需要相互组合,即也包括上述优选的范围之间的组合。
本发明的式(I)化合物的实施例列在下面的组中。这里的通式在各种情况下代表R对映体,S对映体和外消旋体。第1组这里Z具有例如下面给出的定义;氢,甲基,乙基,正-或异-丙基,正-、异-、仲或叔-丁基,氟代甲基,二氟甲基,三氟甲基,氯代甲基,二氯甲基,氯代氟代甲基,氯代溴代甲基,氯代二氟甲基,氟代二氯甲基,溴代二氟甲基,三氯甲基,1-氟-乙基,2-氟-乙基,1-氯-乙基,2-氯-乙基,1-氯-1-氟-乙基,1-氟-丙基,2-氟-丙基,3-氟-丙基,1-氟-1-甲基-乙基,2-氟-1-甲基-乙基,1-氯-1-甲基-乙基,1-氟-1-甲基-丙基,1-氯-1-乙基-丙基,1-氟-1-乙基-丙基,1-氯-1-乙基-丙基,1-氟-2-甲基-丙基,1-氯-2-甲基-丙基,1-氯-丙基,2-氯-丙基,3-氯-丙基,1-氯-1-甲基-乙基,2-氯-1-甲基-乙基,1,1-二氟-乙基,1,2-二氟-乙基,1,1-二氯-乙基,2,2,2-三氟-乙基,1,2,2,2-四氟-乙基,全氟乙基,1,1-二氟-丙基,1,1-二氯-丙基,全氟丙基,1-氟-丁基,1-氯-丁基,全氟戊基,全氟己基,1-羟基-乙基,乙酰基,1,1-双-乙酰基-甲基,1-乙酰基-1-甲氧羰基-甲基,1-乙酰基-1-乙氧羰基-甲基,甲氧基甲基,1,1-二甲氧基-甲基,1-甲氧基-乙基,2-甲氧基-乙基,1,1-二甲氧基-乙基,乙氧基甲基,1-乙氧基乙基,2-乙氧基-乙基,2-甲氧基-1-甲基-乙基,2-甲氧基-1-乙基-乙基,2-乙氧基-1-甲基-乙基,2-乙氧基-1-乙基-乙基,甲硫基甲基,乙基硫基甲基,1-甲硫基-乙基,2-甲硫基-乙基,1-乙硫基-乙基,2-乙硫基乙基,甲基亚磺酰基甲基,乙基亚磺酰基甲基,甲基磺酰基甲基,乙基磺酰基甲基,甲氧基,乙氧基,正-或异-丙氧基,甲硫基,乙硫基,正-或异-丙硫基,甲基亚磺酰基,乙基亚磺酰基,甲基磺酰基,乙基磺酰基,氟代甲氧基,二氟甲氧基,三氟甲氧基,氟代乙氧基,二氟乙氧基,三氟乙氧基,二氟甲硫基,三氟甲硫基,乙烯基,1-氯-乙烯基,2-氯-乙烯基,1-氟-乙烯基,2-氟-乙烯基,1-溴-乙烯基,2-溴-乙烯基,1,2-二氯-乙烯基,1,2-二溴-乙烯基,1,2-二氟-乙烯基,2,2-二氯-乙烯基,2,2-二氟-乙烯基,2,2-二溴-乙烯基,1-氯-2-氟-乙烯基,2-溴-2-氯-乙烯基,三氯乙烯基,烯丙基,2-氯-烯丙基,3-氯-烯丙基,3,3-二氯-烯丙基,1-丙烯基,异丙烯基,1-氯-2-丙烯基,1-氟-2-丙烯基,1-溴-2-丙烯基,1,2-二氯-1-丙烯基,1,2-二溴-1-丙烯基,1,2-二氟-1-丙烯基,1,1-二氯-2-丙烯基,1,1-二溴-2-丙烯基,1,1-二氟-2-丙烯基,1,1,3,3,3-五氟-2-丙烯基,2-丁烯-1-基,2-丁烯-2-基,3-氯-2-丁烯基,3-溴-2-丁烯基,3,3,3-三氟-2-丁烯基,乙炔基,2-氯-乙炔基,2-溴-乙炔基,1-丙炔基,2-丙炔基,3,3,3-三氟-1-丙炔基。第2组 这里Z具有例如上面第1组中给出的定义。第3组 这里Z具有例如上面第1组中给出的定义。第4组 这里Z具有例如上面第1组中给出的定义。第5组 这里Z具有例如上面第1组中给出的定义。第6组 这里Z具有例如上面第1组中给出的定义。第7组 这里Z具有例如上面第1组中给出的定义。第8组 这里Z具有例如上面第1组中给出的定义。第9组 这里Z具有例如上面第1组中给出的定义。第10组 这里Z具有例如上面第1组中给出的定义。第11组 这里Z具有例如上面第1组中给出的定义。第12组 这里Z具有例如上面第1组中给出的定义。第13组 这里Z具有例如上面第1组中给出的定义。第14组 这里Z具有例如上面第1组中给出的定义。第15组 这里Z具有例如上面第1组中给出的定义。第16组 这里Z具有例如上面第1组中给出的定义。第17组 这里Z具有例如上面第1组中给出的定义。第18组 这里Z具有例如上面第1组中给出的定义。第19组 这里Z具有例如上面第1组中给出的定义。第20组 这里Z具有例如上面第1组中给出的定义。第21组 这里Z具有例如上面第1组中给出的定义。第22组 这里Z具有例如上面第1组中给出的定义。第23组 这里Z具有例如上面第1组中给出的定义。第24组 这里Z具有例如上面第1组中给出的定义。第25组 这里Z具有例如上面第1组中给出的定义。第26组 这里Z具有例如上面第1组中给出的定义。第27组 这里Z具有例如上面第1组中给出的定义。第28组 这里Z具有例如上面第1组中给出的定义。第29组 这里Z具有例如上面第1组中给出的定义。第30组 这里Z具有例如上面第1组中给出的定义。第31组 这里Z具有例如上面第1组中给出的定义。第32组 这里Z具有例如上面第1组中给出的定义。第33组 这里Z具有例如上面第1组中给出的定义。第34组 这里Z具有例如上面第1组中给出的定义。第35组 这里Z具有例如上面第1组中给出的定义。第36组 这里Z具有例如上面第1组中给出的定义。第37组 这里Z具有例如上面第1组中给出的定义。第38组 这里Z具有例如上面第1组中给出的定义。第39组 这里Z具有例如上面第1组中给出的定义。第40组 这里Z具有例如上面第1组中给出的定义。第41组 这里Z具有例如上面第1组中给出的定义。第42组 这里Z具有例如上面第1组中给出的定义。第43组 这里Z具有例如上面第1组中给出的定义。第44组 这里Z具有例如上面第1组中给出的定义。第45组 这里Z具有例如上面第1组中给出的定义。第46组 这里Z具有例如上面第1组中给出的定义。第47组 这里Z具有例如上面第1组中给出的定义。第48组 这里Z具有例如上面第1组中给出的定义。第49组 这里Z具有例如上面第1组中给出的定义。第50组 这里Z具有例如上面第1组中给出的定义。第51组 这里Z具有例如上面第1组中给出的定义。第52组 这里Z具有例如上面第1组中给出的定义。第53组 这里Z具有例如上面第1组中给出的定义。第54组 这里Z具有例如上面第1组中给出的定义。第55组 这里Z具有例如上面第1组中给出的定义。第56组 这里Z具有例如上面第1组中给出的定义。第57组 这里Z具有例如上面第1组中给出的定义。第58组 这里Z具有例如上面第1组中给出的定义。第59组 这里Z具有例如上面第1组中给出的定义。第60组 这里Z具有例如上面第1组中给出的定义。第61组 这里Z具有例如上面第1组中给出的定义。第62组 这里Z具有例如上面第1组中给出的定义。第63组 这里Z具有例如上面第1组中给出的定义。第64组 这里Z具有例如上面第1组中给出的定义。第65组 这里Z具有例如上面第1组中给出的定义。第66组 这里Z具有例如上面第1组中给出的定义。第67组 这里Z具有例如上面第1组中给出的定义。第68组 这里Z具有例如上面第1组中给出的定义。第69组 这里Z具有例如上面第1组中给出的定义。第70组 这里Z具有例如上面第1组中给出的定义。第71组 这里Z具有例如上面第1组中给出的定义。第72组 这里Z具有例如上面第1组中给出的定义。第73组 这里Z具有例如上面第1组中给出的定义。第74组 这里Z具有例如上面第1组中给出的定义。第75组 这里Z具有例如上面第1组中给出的定义。第76组 这里Z具有例如上面第1组中给出的定义。
使用例如1-(1-甲基-3-苯基-丙基)-双胍和三氟乙酸甲酯为起始物,则本发明方法(a)的反应过程可以通过下面的反应式来详细说明:
式(II)提供了在为了制备式(I)的化合物的本发明方法(a)中用作起始物的取代的双胍的一般定义。在式(II)中,R1,R2和Y各自优选地或特别地具有上文已经指出的、与描述本发明式(I)的化合物相关的、作为优选的或作为特别优选的R1,R2和Y的那些定义。
式(II)的取代的双胍的例子可以提到的是:1-(1-甲基-3-苯基-丙基)-,1-(1,2-二甲基-3-苯基-丙基)-,1-(1-甲基-3-(2-氟-苯基)-丙基)-,1-(1-甲基-3-(3-氟-苯基)-丙基)-,1-(1-甲基-3-(4-氟-苯基)-丙基)-,1-(1-甲基-3-(2-氯-苯基)-丙基)-,1-(1-甲基-3-(3-氯-苯基)-丙基)-,1-(1-甲基-3-(4-氯-苯基)-丙基)-,1-(1-甲基-3-(2-溴-苯基)-丙基)-,1-(1-甲基-3-(3-溴-苯基)-丙基)-,1-(1-甲基-3-(4-溴-苯基)-丙基)-,1-(1-甲基-3-(2-硝基-苯基)-丙基)-,1-(1-甲基-3-(3-硝基-苯基)-丙基)-,1-(1-甲基-3-(4-硝基-苯基)-丙基)-,1-(1-甲基-3-(2-甲基-苯基)-丙基)-,1-(1-甲基-3-(3-甲基-苯基)-丙基)-,1-(1-甲基-3-(4-甲基-苯基)-丙基)-,1-(1-甲基-3-(2-三氟甲基-苯基)-丙基)-,1-(1-甲基-3-(3-三氟甲基-苯基)-丙基)-,1-(1-甲基-3-(4-三氟甲基-苯基)-丙基)-,1-(1-甲基-3-(2-甲氧基-苯基)-丙基)-,1-(1-甲基-3-(3-甲氧基-苯基)-丙基)-,1-(1-甲基-3-(4-甲氧基-苯基)-丙基)-,1-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基)-,1-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基)-,1-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基)-,1-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基)-,1-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基)-,1-(1-甲基-3-(2-甲氧羰基-苯基)-丙基)-,1-(1-甲基-3-(2-乙氧羰基-苯基)-丙基)-,1-(1-甲基-3-(4-甲氧羰基-苯基)-丙基)-,1-(1-甲基-3-(4-乙氧羰基-苯基)-丙基)-,1-(1-甲基-3-(2-甲硫基-苯基)-丙基)-,1-(1-甲基-3-(4-甲硫基-苯基)-丙基)-,1-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基)-,1-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基)-,1-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基)-,1-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基)-,1-(1-甲基-3-(3,4-二氯-苯基)-丙基)-,1-(1-甲基-3-(2,4-二氯-苯基)-丙基)-,1-(1-甲基-3-(2,5-二氯-苯基)-丙基)-,1-(1-甲基-3-(2,6-二氯-苯基)-丙基)-,1-(1-甲基-3-(2,6-二氟-苯基)-丙基)-,1-(1-甲基-3-(2,5-二氟-苯基)-丙基)-,1-(1-甲基-3-(2,4-二氟-苯基)-丙基)-,1-(1-甲基-3-(3,4-二氟-苯基)-丙基)-,1-(1-甲基-3-(3,5-二氟-苯基)-丙基)-,1-(1-甲基-3-(2-氟-4-氯-苯基)-丙基)-,1-(1-甲基-3-(4-氟-2-氯-苯基)-丙基)-,1-(1-甲基-3-(2,4-二甲基-苯基)-丙基)-,1-(1-甲基-3-(3,4-二甲基-苯基)-丙基)-,1-(1-甲基-3-(3,5-二甲基-苯基)-丙基)-,1-(1-甲基-3-(2,5-二甲基-苯基)-丙基)-,1-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基)-,1-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基)-,1-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基)-,1-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基)-,1-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基)-,1-(1-甲基-3-噻吩-2-基-丙基)-,1-(1-甲基-3-噻吩-3-基-丙基)-,1-(1-甲基-3-吡啶-2-基-丙基)-,1-(1-甲基-3-吡啶-3-基-丙基)-和1-(1-甲基-3-吡啶-4-基-丙基)-双胍。
式(II)的化合物的合适的酸加成物是与质子酸的加成产物,例如与盐酸,氢溴酸,硫酸,甲磺酸,苯磺酸和对甲苯磺酸的加成产物。
通式(II)的起始物迄今为止还没有在文献中公开;其作为新的物质也是本中请主题的一部分。
如果适当在反应助剂例如盐酸存在下,和如果适当在稀释剂例如正癸烷或1,2-二氯-苯存在下,在100℃和200℃之间的温度下,当通式(VI)的取代的烷基氨基化合物--和/或通式(V)的化合物的酸加成物,例如盐酸盐--与式(VII)的氰基胍(“二氰基二酰胺”)反应时,得到通式(II)的新的取代的双胍,其中R1,R2和Y各自如上定义,(参见EP492615,制备实施例)。
需要用作本发明目的的母体的通式(VI)的取代的烷基氨基化合物是已知的和/或可以通过本身已知的方法制备(参见DE3426919;DE4000610;DE4332738;EP320898;EP443606;四面体:Asymmetry 5(1994),817-820;四面体通讯29(1988),223-224;上述引文36(1995),3917-1920;制备实施例)。
式(III)提供了在制备式(I)的化合物的本发明方法(a)中也用作起始物的烷氧羰基化合物的一般定义。在式(III)中,Z优选地或特别地具有上文已经指出的、与描述本发明式(I)化合物相关的、作为优选的或作为特别优选的Z的定义;R’优选代表具有1-4个碳原子的烷基,特别代表甲基或乙基。
式(III)的起始物是用于合成的已知的化合物。
式(IV)提供了在制备式(I)化合物的本发明方法(b)中要用作起始物的取代的三嗪的一般定义。在式(IV)中,R1,R2,Y和Z各自优选地或特别地具有上文已经指出的、与描述本发明式(I)化合物相关的、作为优选的或作为特别优选的R1,R2,Y和Z的定义;X1优选代表氟,氯,溴,甲氧基或乙氧基,并且特别代表氯。可以提到的式(IV)的取代的三嗪的例子是:2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4,6-二氯-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-甲基-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-三氟甲基-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-(1-氟-乙基)-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-(1-氟-1-甲基-乙基)-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-甲氧基-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-(2,2,2-三氟乙氧基)-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-甲硫基-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-甲基亚磺酰基-1,3,5-三嗪;2-(1-甲基-3-苯基-丙基氨基)-,2-(1,2-二甲基-3-苯基-丙基氨基)-,2-(1-甲基-3-(2-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(3-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(3-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(4-溴-苯基)-丙基氨基)-,2-(1-甲基-3-(2-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-硝基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基-苯基)丙基氨基)-,2-(1-甲基-3-(3-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(3-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-二氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-三氟甲氧基-苯基)-丙基氨基)-,1-(1-甲基-3-(3-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-三氟甲氧基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-乙氧羰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基亚磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-甲基磺酰基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,6-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二氟-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-氯-苯基)-丙基氨基)-,2-(1-甲基-3-(2,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,4-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(3,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2,5-二甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氯-6-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(4-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-4-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(2-氟-5-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-(5-氟-2-甲基-苯基)-丙基氨基)-,2-(1-甲基-3-噻吩-2-基-丙基氨基)-,2-(1-甲基-3-噻吩-3-基-丙基氨基)-,2-(1-甲基-3-吡啶-2-基-丙基氨基)-,2-(1-甲基-3-吡啶-3-基-丙基氨基)-和2-(1-甲基-3-吡啶-4-基-丙基氨基)--4-氯-6-甲磺酰基-1,3,5-三嗪。
通式(IV)的起始物迄今为止还没有在文献中公开过;其作为新的物质也是本申请主题的一部分。
如果适当在酸受体例如乙基二异丙基胺存在下,和如果适当在稀释剂例如四氢呋喃或二噁烷存在下,在-50℃和+50℃之间的温度下,当通式(VIII)的三嗪与通式(VI)的取代的烷基氨基化合物反应时,得到通式(IV)的新的取代的三嗪,其中X1和Z各自如上定义,和X3代表卤素,其中R1,R2和Y各自如上定义(参见制备实施例)。
式(V)提供了在制备式(I)的化合物的本发明方法(c)中用作起始物的取代的氨基三嗪的一般定义。在式(V)中,Z优选地或特别地具有上文已经指出的、与描述本发明式(I)化合物相关的,作为优选的或作为特别优选的Z的定义;X2优选代表氟,氯,溴,甲氧基或乙氧基,并且特别代表氯或甲氧基。
通式(V)的起始物是已知的和/或可以通过本身已知的方法制备(参见WO95/11237)。
式(VI)提供了在本发明方法(c)中也要用作起始物的取代的烷基胺的一般定义。在式(VI)中,R1,R2和Y各自优选地或特别地具有上文已经指出的、与描述本发明式(I)化合物相关的、作为优选的或作为特别优选的R1,R2和Y的定义。
通式(VI)的起始物是已知的和/或可以通过本身已知的方法制备(DE3426919;DE4000610;DE4332738;EP320898;EP443606;四面体:Asymmetry 5(1994),817-820;四面体通讯29(1988),223-224;上述引文36(1995),3917-1920;制备实施例)。
如果适当,本发明制备式(I)的化合物的方法使用反应助剂进行。方法(a),(b)和(c)的合适的反应助剂一般是常规的无机或有机碱或酸受体。这些优选包括碱土金属或碱金属乙酸盐,氨化物,碳酸盐,碳酸氢盐,氢化物,氢氧化物或烷氧化物,例如乙酸钠,乙酸钾或乙酸钙,氨化锂,氨化钠,氨化钾或氨化钙,碳酸钠,碳酸钾或碳酸钙,碳酸氢钠,碳酸氢钾或碳酸氢钙,氢化锂,氢化钠,氢化钾或氢化钙,氢氧化锂,氢氧化钠,氢氧化钾或氢氧化钙,甲醇钠,乙醇钠,正-或异-丙醇钠,正-、异-、仲-或叔丁醇钠,甲醇钾,乙醇钾,正-或异-丙醇钾,正-、异-、仲-或叔丁醇钾,还有碱性有机氮化合物,例如三甲胺,三乙胺,三丙胺,三丁胺,乙基-二异丙基胺,N,N-二甲基-环己基胺,二环己基胺,乙基-二环己基胺,N,N-二甲基苯胺,N,N-二甲基苄基胺,吡啶,2-甲基-、3-甲基-、4-乙基-、2,4-二甲基-、2,6-二甲基-、3,4-二甲基-和3,5-二甲基-吡啶,5-乙基-2-甲基吡啶,4-二甲基氨基吡啶,N-甲基哌啶,1,4-二-氮杂双环[2.2.2]-辛烷(DABCO),1,5-二氮杂双环[4.3.0]-壬-5-烯(DBN)或1,8-二氮杂双环[5.4.0]-十一-7-烯(DBU)。
进行本发明方法(a),(b)和(c)的合适的稀释剂是所有惰性有机溶剂。这些特别包括脂肪族、脂环族或芳香族的、任意卤化的烃,例如汽油,苯,甲苯,二甲苯,氯苯,二氯苯,石油醚,己烷,环己烷,二氯甲烷,氯仿,四氯化碳;醚类,例如乙醚,二异丙基醚,二噁烷,四氢呋喃或乙二醇二甲基醚或乙二醇二乙基醚;酮类,例如丙酮,丁酮,或甲基异丁基酮;腈类,例如乙腈,丙腈或丁腈;酰胺类,例如N,N-二甲基甲酰胺,N,N-二甲基乙酰胺,N-甲基-N-甲酰苯胺,N-甲基-吡咯烷酮或六甲基磷酰三胺;酯类,例如乙酸甲酯,乙酸乙酯,乙酸正-或异-丙酯,乙酸正-、异-、仲-或叔-丁酯;亚砜类,例如二甲亚砜;醇类,例如甲醇,乙醇,正-或异-丙醇,正-、异-、仲-或叔-丁醇,乙二醇单甲基醚,乙二醇单乙基醚,二甘醇单甲基醚,二甘醇单乙基醚,其与水的混合物,或纯水。
进行本发明方法(a),(b)和(c)时,反应温度可以在较宽的范围内变化。一般情况下,反应在0℃和300℃之间的温度下进行,优选在10℃和250℃之间的温度下进行。
本发明方法(a),(b)和(c)一般在大气压下进行。但是也可以在高压或低压下进行本发明方法-一般在0.1至10巴之间。
在实施本发明方法时,一般以大约等摩尔量使用起始物。但是,也可以以相对大的过量使用其中之一反应组分。反应一般在合适的稀释剂中在反应助剂存在下进行,并且反应混合物一般在需要的温度下搅拌数小时。通过常规方法进行后处理(参见制备实施例)。
本发明活性化合物可以用作脱叶剂,干燥剂,杀稻草剂,特别是作为杀杂草剂。从广义上说,所谓杂草被理解是在其所不期望的地点生长的所有植物。本发明物质是作为灭生除草剂还是选择性除草剂主要取决于使用的量。
与例如下面的植物相关,可以使用本发明活性化合物:双子叶杂草种类:芥属(Sinapis),独行菜属(Lepidium),猪殃殃属(Galium),繁缕属(Stellaria),母菊属(Matricaria),春黄菊属(Anthemis),辣子草(Galinsoga),藜属(Chenopodium),荨麻属(Urtica),千里光属(Senecio),苋属(Amaranthus),马齿苋属(Portulaca),苍耳属(Xanthium),旋花属(Convolvulus),甘薯属(Ipomoea),蓼属(Polygonum),田菁(Sesbania),豚草属(Ambrosia),蓟属(Cirsium),飞廉属(Carduus),苦苣菜属(Sonchus),茄属(Solanum),焊菜属(Rorippa),水松叶属(Rotala),母草属(Lindernia),野芒麻属(Lamium),婆婆纳属(Veronica),苘麻属(Abutilon),刺果(Emex),曼陀罗属(Datura),堇菜属(Viola),鼬瓣花属(Galeopsis),罂粟属(Papaver),矢车菊属(Centaurea),车轴草属(Trifolium),毛茛属(Ranunculus)和蒲公英属(Taraxacum)。双子叶作物种类:棉属(Gossypium),大豆属(Glycine),Beta,胡萝卜属(Daucus),菜豆属(Phaseolus),豌豆属(Pisum),茄属(Solanum),亚麻属(Linum),甘薯属(Ipomoea),巢菜属(Vicia),烟草属(Nicotiana),番茄属(Lycopersicon),花生(Arachis),芥属(Brassica),莴苣属(Lactuca),香瓜属(Cucumis),和臭瓜(Cucurbita)。单子叶杂草种类:稗属(Echinochloa),狗尾草属(Setaria),黍属(Panicum),马唐属(Digitaria),梯牧属(Phleum),早熟禾属(Poa),羊茅属(Festuca),蟋蟀菜属(Eleusine),臂形草属(Brachiaria),黑麦草属(Lolium),雀麦属(Bromus),燕麦属(Avena),莎草属(Cyperus),蜀黍属(Sorghum),冰草属(Agropyron),Cynodon,雨久花属(Monchoria),飘拂草属(Fimbristylis),慈姑(Sagittaria),荸荠属(Eleocharis),镳草(Scirpus),雀稗草(Paspalum),Ischaemum,尖瓣花属(Sphenoclea),龙爪茅属(Dactyloctenium),剪股颍属(Agrostis),看麦娘属(Alopecurus),Apera和Phalaris。单子叶作物种类:稻属(Oryza),玉米属(Zea),小麦属(Triticum),大麦属(Hordeum),燕麦属(Avena),黑麦属(Secale),蜀黍属(Sorghum),黍属(Panicum),甘蔗(Saccharum),菠萝(Ananas),天门冬属(Asparagus)和葱属(Allium)。
但是,本发明活性化合物的用途不受这些种类的限制,而是以相同的方式扩展到其它植物。
根据浓度,本发明化合物适于例如在工业场所和铁道,有或没有树木的路或广场上全部控制杂草。这些化合物同样被用来在多年生种植植物场所,草坪,运动场和牧场控制杂草,所述多年生种植植物场所例如树林,装饰性种植的树,果园,葡萄园,柠檬园,坚果园,香蕉种植园,咖啡种植园,茶种植园,橡胶种植园,油棕种植园,可可种植园,软果种植植物和蛇麻草田,以及用来在年生种植植物场所选择性控制杂草。
本发明式(I)的化合物特别适用于在芽前和芽后在单子叶和双子叶农作物中选择性控制单子叶和双子叶杂草。
活性化合物可以配制成常规制剂,例如溶液、乳剂、可湿性粉末、悬浮剂、粉剂、细粉剂、糊剂、可溶性粉末、颗粒剂、混悬乳油、浸有活性化合物的天然和合成材料,以及包裹在聚合物中的细微胶囊。
这些型剂是用已知的方式生产的,例如,通过将活性化合物与扩充剂即液体溶剂和/或固体载体混合,并任选使用表面活性剂,即乳化剂和/或分散剂和/或起泡剂。
如果使用的扩充剂是水,则也可以用例如有机溶剂作助溶剂。合适的液体溶剂主要有:芳族化合物,如二甲苯,甲苯或烷基萘,氯代芳族化合物和氯代脂肪烃,如氯代苯类、氯乙烯类或二氯甲烷,脂肪烃,如环己烷或石蜡,例如矿物油馏份,矿物油和植物油,醇类,如丁醇或乙二醇以及其醚和酯,酮类,如丙酮、甲乙酮、甲基异丁基酮或环己酮,强极性溶剂,如二甲基甲酰胺和二甲亚砜,以及水。
合适的固体载体是:例如铵盐,磨碎的天然矿物质如高岭土、粘土、滑石、白垩、石英、硅镁土、蒙脱石或硅藻土,和磨碎的合成矿物质,如高分散二氧化硅、矾土和硅酸盐;用于颗粒剂的适合的固体载体有:例如压碎和破碎的天然矿物质如方解石、大理石、浮石、海泡石和白云石,以及有机和无机粉的合成颗粒,和如下有机物的颗粒:例如锯木屑、椰壳、玉米穗轴和烟茎;适合的乳化剂和/或起泡剂有:例如非离子和阴离子乳化剂,如聚氧乙烯脂肪酸酯、聚氧乙烯脂肪醇醚,例如,烷基芳基聚乙二醇醚,烷基磺酸盐,烷基硫酸盐,芳基磺酸盐以及蛋白水解产物;适合的分散剂有:例如,木素亚硫酸废液和甲基纤维素。
制剂中可以使用粘合剂如羧甲基纤维素和粉状、颗粒或乳胶形式的天然和合成聚合物,如阿拉伯胶、聚乙烯醇和聚乙酸乙烯酯,以及天然磷脂,如脑磷脂和卵磷脂,和合成磷脂。其它可能的添加剂是矿物油和植物油。
也可以使用染料,如无机颜料,例如氧化铁、氧化钛和普鲁士蓝,和有机染料,如茜素染料、偶氮染料和金属酞菁染料,和微量营养素如铁、锰、硼、铜、钴、钼和锌的盐。
一般情况下,制剂含有0.1-95%重量的活性化合物,优选0.5-90%。
对于控制杂草,本发明活性化合物本身或其制剂形式也可以作为与已知除草剂的混合物使用,可以是最终制剂或容器混合物。
适用于所述混合物的可能的成分是已知的除草剂,例如乙草胺,三氟羧草醚(-钠盐),苯草醚,甲草胺,禾草灭(钠盐),莠灭净,amidochlor,amidosulfuron,磺草灵,莠去津,azimsulfuron,除草灵,呋草黄,苄嘧草隆(-甲酯),灭草松,吡草酮,新燕灵(-乙酯),双丙氨酰膦,甲羧除草醚,溴丁酰草胺,溴酚肟,溴苯腈,丁草胺,丁草特,cafenstrole,卡草胺,甲氧除草醚,灭草平,杀草敏,氯嘧黄隆(-乙酯),草枯醚,氯黄隆,氯麦隆,环庚草醚,醚草隆,烯草酮,clodinafop(-propargyl),异噁草酮,二氯吡啶酸,clopyrasulfuron,cloransulam(-methyl),cumyluron,氰草津,灭草特,cyclosulfamuron,噻草酮,cyhalofop(-buty1),2,4-滴,2,4-滴丁酸,2,4-滴丙酸,甜菜安,燕麦敌,麦草畏,禾草灵(-甲酯),双苯唑快,吡氟草胺,丁噁隆,哌草丹,二甲草胺,二甲丙乙净,dimethenamid,氨基乙氟灵,双苯酰草胺,敌草快,氟硫草定,敌草隆,杀草隆,EPTC,禾草畏,乙丁烯氟灵,ethametsulfuron(-methyl),乙呋草黄,ethoxyfen,etobenzanid,噁唑禾草灵,麦草伏(-异丙酯),麦草伏(-异丙酯-L),麦草伏(-甲酯),啶嘧黄隆,吡氟禾草灵(-丁酯),flumetsulam,flumiclorac(-penty1),flumioxazin,flumipropyn,伏草隆,氟咯草酮,乙羧氟草醚(-乙酯),胺草唑,flupropacil,芴丁酸,氟定酮,氟草烟,调嘧醇,呋草酮,氟黄胺草醚,草铵膦(-铵盐),草甘膦(-异丙基铵盐),halosafen,吡氟氯禾灵(-乙氧基乙酯),环嗪酮,咪草酯(-甲酯),imazamethapyr,imazamox,灭草烟,灭草喹,咪草烟,imazosulfuron,碘苯腈,异丙乐灵,异丙隆,isoxaben,isoxaflutole,噁草醚,乳氟禾草灵,环草定,利谷隆,MCPA,MCPP,苯噻草胺,苯嗪草酮,吡草安,甲基苯噻隆,metobenzuron,秀谷隆,丙草安,metosulam,甲氧隆,嗪草酮,赛克津,甲黄隆(-甲酯),草达灭,绿谷隆,萘丙胺,萘氧丙草胺,草不隆,烟嘧黄隆,哒草伏,坪草丹,安磺灵,噁草酮,乙氧氟草醚,百草枯,二甲戊乐灵,甜菜宁,哌草磷,丙草安,氟嘧黄隆(-甲酯),扑草净,毒草安,敌稗,喔草酯,戊炔草胺,苄草丹,prosulfuron,吡唑特,吡嘧黄隆(-乙酯),苄草唑,稗草畏,哒草特,pyrithiobac(-sodium),二氯喹啉酸,喹草酸,喹禾灵(-乙酯),喹禾灵(-四氢糠基酯),rimsulfuron,稀禾定,西玛津,西草净,sulcotrione,sulfentrazone,嘧黄隆(-甲酯),草甘膦,牧草胺,特丁噻草隆,特丁津,特丁净,thenylchlor,thiafluamide,thiazopyr,thidiazimin,噻黄隆(-甲酯),杀草丹,仲草丹,肟草酮,野燕畏,醚苯黄隆,苯黄隆(-甲酯),绿草定,灭草环,氟乐灵和triflusulfuron。
也可以是与其它已知活性化合物的混合物,所述其它活性化合物是例如杀真菌剂,杀昆虫剂,杀螨剂,杀线虫剂,鸟驱避剂,植物营养剂和改善土壤结构的试剂。
这些活性化合物可以使用其本身,以其制剂形式使用,或者以通过进一步稀释而从中制备的使用形式使用,例如即用型溶液,混悬剂,乳剂,粉剂,糊剂和颗粒剂。它们可以以常规方式使用,例如通过浇灌,喷雾,弥雾或播撒。
本发明活性化合物可以在植物芽前或芽后施用。其也可以在播种前掺合到土壤中。
使用的活性化合物的量可以在一个宽的范围内变化。这主要取决于期望的效果的性质。一般情况下,使用的量在每公顷土壤面积使用1g-10kg活性化合物,优选每公顷土壤面积使用5g-5kg活性化合物。
具体实施方式
根据下面的实施例可以说明本发明活性化合物的制备和应用。制备实施例 实施例1 (方法(a))
在20℃至30℃下,搅拌下,向31.5g(0.10mol)(R/S)-1-(1-甲基-3-(4-甲硫基-苯基)-丙基)-双胍盐酸盐(外消旋),15.5g(0.10mol)三氟乙酸乙酯和150ml甲醇的混合物中滴加饱和的6.0g(0.11mol)甲醇钠的甲醇溶液,然后反应混合物在大约20℃下搅拌大约20小时。然后混合物用二氯甲烷稀释至其大约3倍体积,用水洗涤后用1N氢氧化钠水溶液洗涤,硫酸钠干燥并过滤。用水泵真空小心地从滤液中蒸馏出溶剂。
得到12.1g(理论量的34%)(R/S)-2-氨基-4-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-6-三氟甲基-1,3,5-三嗪(外消旋体),为非晶形残余物。实施例2 (方法(b))
在20℃至30℃下,搅拌下,向5.4g(18.2mmol)(R/S)-2,4-二氯-6-(1-甲基-3-苯基-丙基氨基)-1,3,5-三嗪(外消旋体)和35ml四氢呋喃的混合物中滴加5.7ml 25%强度的氨水溶液,然后反应混合物在大约20℃下搅拌大约4小时。用水泵真空浓缩混合物,然后残余物溶解于乙酸乙酯/饱和的氯化钠水溶液中,分离有机相,并且用乙酸乙酯萃取水相;合并有机相,用硫酸钠干燥并过滤。用水泵真空浓缩滤液,且残余物通过用乙酸乙酯/己烷溶解进行结晶。然后通过吸滤分离结晶产物。
在20℃至30℃下,搅拌下向6.0g(16.8mmol)(R/S)-2-氨基-4-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-6-三氟甲基-1,3,5-三嗪(外消旋体)和100ml二氯甲烷的混合物中加入4.6g(大约17mmol)3-氯-过苯甲酸(技术级),将反应混合物在大约20℃下搅拌1小时。然后向混合物中加入水至大约其原来体积的二倍,并且用氨水使其呈碱性。然后分离有机相,硫酸钠干燥并过滤。用水泵真空浓缩滤液,且残余物溶解于石油英/乙醇。然后通过吸滤分离结晶产物。
在20℃至30℃下,搅拌下向9.0g(25mmol)(R/S)-2-氨基-4-(1-甲基-3-(4-甲硫基-苯基)-丙基氨基)-6-三氟甲基-1,3,5-三嗪(外消旋体)和150ml二氯甲烷的混合物中加入14g(大约55mmol)3-氯-过苯甲酸(技术级),将反应混合物在大约20℃下搅拌2小时。然后向混合物中加入水至大约其原来体积的二倍,并且用氨水使其呈碱性。然后分离有机相,硫酸钠干燥并过滤。用水泵真空小心从滤液中蒸馏出溶剂。
1.0g(3.6mmol)(R/S)-2-氨基-4-氯-6-(1-甲基-3-苯基-丙基氨基)-1,3,5-三嗪(外消旋体),0.84g(7.5mmol)叔丁醇钾,10ml 2,2,2-三氟-乙醇和5ml二噁烷的混合物在大约70℃下搅拌4小时,并接着用水泵真空浓缩。残余物溶解于乙酸乙酯/水,分离有机相并再萃取水相。用硫酸钠干燥合并的有机相并过滤。用水泵真空小心从滤液中蒸馏出溶剂。
得到0.98g(理论量的80%)(R/S)-2-氨基-4-(2,2,2-三氟-乙氧基)-6-(1-甲基-3-苯基-丙基氨基)-1,3,5-三嗪(外消旋体),为非晶形残余物。
通过制备实施例1-5的方法,并且根据本发明制备方法的一般说明,可以制备例如下面表1中列出的式(I)的化合物。 表1:式(I)化合物的实施例,其中R1=CH3--在表1中列出的化合物中,R1总是代表甲基,所以表中对于各个实施例不再提及。表1(续)表1(续)表1(续)表1(续)表1(续)表1(续)表1(续) 式(II)的起始物: 实施例(II-1)
在大约140℃(内部温度,水浴温度大约175℃),将23.2g(0.10mol)(R/S)-1-甲基-3-(4-甲硫基-苯基)-丙基胺盐酸盐(外消旋体),8.5g(0.10mol)二氰基二酰胺(氰基胍)和100ml二氯苯的混合物搅拌3小时。然后用油泵真空小心蒸馏出溶剂。
得到非晶形残余物(R/S)-1-(1-甲基-3-(4-甲硫基-苯基)-丙基)-双胍盐酸盐(外消旋体),其不用进一步纯化即可用于方法(a)的反应中。
即使没有溶剂,即在熔融状态下,在相同的温度下也可以进行该反应。
通过实施例(II-1)的方法,也能制备例如下面表2中列出的式(II)的化合物或其盐酸盐。 表2:式(II)化合物的实施例,其中R1=CH3--在所有的例子中,这些都是相应的盐酸盐! 式(IV)的起始物 实施例(IV-1)
搅拌下,向冷却到-40℃至-50℃的20.2g(0.11mol)氰尿酰氯(2,4,6-三氯-1,3,5-三嗪)和80ml四氢呋喃的混合物中加入溶解于20ml四氢呋喃中的16.34g(0.11mol)(R/S)-1-甲基-3-苯基-丙基胺和14.2g(0.11mol)乙基二异丙基胺的溶液。反应混合物在上述温度下搅拌30分钟后在室温(大约20℃)下又搅拌30分钟。浓缩混合物后残余物用乙醚/饱和的氯化铵水溶液振荡,分离有机相,反复萃取水相,合并的有机相用硫酸钠干燥并过滤。滤液用水泵真空浓缩,残余物溶解于石油醚/甲基叔丁基醚,吸滤分离结晶产物。
得到27.5g(理论量的84%)(R/S)-2,4-二氯-6-(1-甲基-3-苯基-丙基氨基)-1,3,5-三嗪(外消旋体),熔点79℃。式(V)的起始物 实施例(V-1)
将8.0g(0.08mol)戊烷-2,4-二酮,14.5g(0.08mol)4-甲硫基-苄基氯,12g碳酸钾和100ml乙醇的混合物在回流下加热15小时。冷却后,用大约等量体积的水和二氯甲烷将混合物稀释到大约其原来体积的三倍,剧烈摇荡后分离有机相,硫酸钠干燥并过滤。用水泵真空浓缩滤液并且残余物用油泵真空蒸馏残余物。
室温(大约20℃)下,向7.2g(0.04mo)1-(4-甲硫基-苯基)-丁烷-3-酮,4.0g羟基胺-盐酸盐(0.058mol)和50ml乙醇的混合物中加入2.0g氢氧化钠,并且混合物在20℃至35℃下搅拌2小时。用大约等量体积的水和二氯甲烷将混合物稀释到大约其原来体积的三倍,剧烈摇荡后分离有机相,硫酸钠干燥并过滤。用水泵真空小心从滤液中蒸馏出溶剂。
得到4.0g(理论量的48%)1-(4-甲硫基-苯基)-丁烷-3-酮肟,为熔点71℃的固体残余物。步骤3
搅拌下,向25g四氢铝锂(铝锂氢化物,氢化铝锂)和300ml四氢呋喃的混合物中滴加在200ml四氢呋喃中的63g(0.3mol)1-(4-甲硫基-苯基)-丁烷-3-酮肟的溶液,内部的温度从开始的大约20℃升至大约60℃,然后反应混合物回流下搅拌5小时。在20℃至40℃下滴加100ml水。然后分离有机相,硫酸钠干燥并过滤。用水泵真空浓缩滤液,并用油泵真空蒸馏残余物。
步骤1
将8.0g(0.08mol)戊烷-2,4-二酮,14.5g(0.08mol)4-甲硫基-苄基氯,12g碳酸钾和100ml乙醇的混合物在回流下加热15小时。冷却后,用大约等量体积的水和二氯甲烷将混合物稀释到大约其原来体积的三倍,剧烈摇荡后分离有机相,硫酸钠干燥并过滤。用水泵真空浓缩滤液并且残余物用油泵真空蒸馏残余物。
经大约60分钟,搅拌下向加热到140℃的83g(0.46mol)1-(4-甲硫基-苯基)-丁烷-3-酮,300ml甲酰胺和10ml甲酸的混合物中滴加70ml甲酸。混合物在140℃至150℃下搅拌大约3小时。冷却后,用大约等量体积的水和二氯甲烷将混合物稀释到大约其原来体积的三倍,并剧烈摇荡。然后分离有机相,硫酸钠干燥并过滤。用水泵真空小心从滤液中蒸馏出溶剂。
得到94g(理论量的92%)(R/S)-N-(1-甲基-3-(4-甲硫基-苯基)-丙基)-甲酰胺(外消旋体),为非晶形残余物,其不用进一步纯化即可用于下一步骤。步骤3
67g(0.3mol)(R/S)-N-(1-甲基-3-(4-甲硫基-苯基)-丙基)-甲酰胺(外消旋体)和250ml浓盐酸的混合物在回流下加热大约150分钟。用冰-水冷却后,用2N氢氧化钠水溶液使混合物呈碱性,与二氯甲烷振荡,分离有机相,硫酸钠干燥并过滤。用水泵真空浓缩滤液,并用油泵真空蒸馏残余物。
得到40g(理论量的68%)(R/S)-1-甲基-3-(4-甲硫基-苯基)-丙基胺(外消旋体),熔点105℃(0.8巴)。实施例(V-3)
搅拌下,向20g(0.1mol)(R/S)-1-甲基-3-(4-甲硫基-苯基)-丙基胺(外消旋体)和20ml水的混合物中滴加11ml 33%强度盐酸,并且混合物在室温下再搅拌大约1小时。然后用水泵真空小心蒸馏出溶剂,定量得到(R/S)-1-甲基-3-(4-甲硫基-苯基)-丙基胺盐酸盐(外消旋体),为固体残余物。实施例(V-4) 对映体的分离:
首先向11.9g(0.08mol)(R/S)-1-甲基-3-苯基-丙基胺中加入34ml甲基叔丁基醚和8.32g(0.08mol)甲氧基乙酸甲酯,并且与0.42gNovozym-435混合。混合物在室温(大约20℃)下搅拌2小时后过滤,并用25ml甲基叔丁基醚洗涤。每次用30ml 10%强度的盐酸水溶液将滤液洗涤三次,然后用硫酸钠干燥并过滤。用水泵真空小心从滤液中蒸馏出溶剂。
得到7.4g(96.25%ee,理论量的84%)(R)-N-甲氧基乙酰基-1-甲基-3-苯基-丙基胺,其然后与50ml 18%强度的盐酸在回流下加热4小时。后处理后得到5.0g(96%ee,理论量的98%)(R)-1-甲基-3-苯基-丙基胺。
合并根据上述说明得到的三份盐酸水溶液,并且在冰冷却下用10%强度的氢氧化钠水溶液碱化。然后每次用50ml二氯甲烷萃取混合物三次。合并的有机萃取液用硫酸钠干燥并过滤。用水泵真空小心从滤液中蒸馏出溶剂。
得到4.1g(71.3%ee,理论量的69%)(S)-1-甲基-3-苯基-丙基胺,为固体残留物。应用实施例 实施例A芽前试验溶剂: 5份重量丙酮乳化剂: 1份重量烷基芳基聚乙二醇醚
为了制备活性化合物合适的制剂,将1份重量活性化合物与所述量溶剂混合,加入所述量乳化剂,用水将乳油稀释到需要的浓度。
在正常土壤中播种试验植物的种子。24小时后,用活性化合物制剂浇灌土壤。每单位面积水的量保持不变是有利的。制剂中活性化合物的浓度并不关键,只有每单位面积活性化合物的施用比例是重要的。
三星期后,以与未处理对照物生长相比较的损害百分率目测评分对植物损害的程度。数据说明:
0%=没有效果(象未处理对照物一样)
100%=完全破坏
在该项试验中,例如制备实施例5,7,9,11,12,15,52,53,59,60,61,62和63的化合物显示出非常强的除杂草活性,并且农作物例如玉米,小麦,大麦,油菜子和棉花对它们中的一些有好的耐受性(参见表A)。在下面的表中,“ai”指“活性成分”。表A:芽前试验/温室活性化合物的制备 施用比例 玉米 苋草 猪殃殃实施例序号 (ai的克数/公顷) 1000 10 100 100表A(续)活性化合物的制备 施用比例 茴麻 苋草 猪殃殃 芥草 苍耳实施例序号 (ai的克数/公顷) 1000 80 100 80 80 90表A(续)活性化合物的制备 施用比例 大麦 棉花 看麦娘 马唐 稗 苋草 曼陀罗草 母菊 堇菜实施例序号 (ai的克数/公顷) 125 0 0 95 90 95 100 - 100 100 250 15 0 100 100 95 100 100 100 100表A(续)活性化合物的制备 施用比例 大麦 油菜籽 马唐 稗 苋草 藜 母菊 堇菜实施例序号 (ai的克数/公顷) 500 0 0 100 90 100 100 - 100表A(续)活性化合物的制备 施用比例 大麦 油菜籽 马唐 稗 苋草 藜 母菊 堇菜实施例序号 (ai的克数/公顷) 125 0 0 100 90 100 100 100 100表A(续)活性化合物的制备 施用比例 小麦 马唐 稗 苋草 曼陀罗草 茄实施例序号 (ai的克数/公顷) 500 0 100 100 100 100 100 250 0 100 100 100 100 100表A(续)活性化合物的制备 施用比例 小麦 马唐 稗 苋草 曼陀罗草 茄实施例序号 (ai的克数/公顷) 500 0 100 100 100 95 100表A(续)活性化合物的制备 施用比例 看麦娘 苘麻 苋草实施例序号 (ai的克数/公顷) 1000 95 100 90表A(续)活性化合物的制备 施用比例 小麦 棉花 马唐 稗 苋草 曼陀罗草 茄实施例序号 (ai的克数/公顷) 250 0 0 90 100 80 100 100表A(续)活性化合物的制备 施用比例 大麦 马唐 稗 苋草 曼陀罗草 茄实施例序号 (ai的克数/公顷) 500 10 100 100 100 100 100表A(续)活性化合物的制备 施用比例 看麦娘 狗尾草 苘麻 苋草 猪殃殃实施例序号 (ai的克数/公顷) 1000 90 100 90 100 100实施例B芽后试验溶剂: 5份重量丙酮乳化剂: 1份重量烷基芳基聚乙二醇醚
为了制备活性化合物合适的制剂,将1份重量活性化合物与所述量溶剂混合,加入所述量乳化剂,用水将乳油稀释到需要的浓度。
用活性化合物制剂对高5-15cm的试验植物喷雾,使对每单位面积施用特定量的期望的活性化合物。选择喷雾液的浓度,使以1000L水/公顷施用特定量的期望的活性化合物。
三星期后,以与未处理对照物生长相比较的伤害百分率目测评分对植物的伤害程度。数据说明:
0%=没有效果(象未处理对照物一样)
100%=完全破坏
在该项试验中,例如制备实施例2,6,7,8,9,10,11,12,13,14,15,16,18,52,53,60,61,62,63和67的化合物显示出非常强的除杂草活性,并且农作物例如玉米,小麦,大麦和油菜子对它们中的一些有好的耐受性(参见表B)。表B:芽后试验/温室活性化合物的制备 施用比例 苋草 芥草实施例序号 (ai的克数/公顷) 1000 100 100表B(续)活性化合物的制备 施用比例 苋草 芥草实施例序号 (ai的克数/公顷) 1000 100 95表B(续)活性化合物的制备 施用比例 小麦 油菜籽 苋草 藜 甘薯 蓼 茄实施例序号 (ai的克数/公顷) 125 0 0 100 100 100 100 100表B(续)活性化合物的制备 施用比例 小麦 稗 狗尾草 苋草 藜 甘薯 蓼 茄实施例序号 (ai的克数/公顷) 250 10 80 90 95 95 95 95 95表B(续)活性化合物的制备 施用比例 莎草 苋草 猪殃殃 芥草实施例序号 (ai的克数/公顷) 1000 90 100 80 100表B(续)活性化合物的制备 施用比例 玉米 苋草 曼陀罗草 甘薯 蓼 婆婆纳实施例序号 (ai的克数/公顷) 250 10 95 100 100 100 100表B(续)活性化合物的制备 施用比例 小麦 苋草 藜 曼陀罗草 甘薯 蓼 茄实施例序号 (ai的克数/公顷) 125 30 95 100 100 95 100 100表B(续)活性化合物的制备 施用比例 小麦 苋草 藜 曼陀罗草 甘薯 蓼 茄实施例序号 (ai的克数/公顷) 250 0 100 100 100 95 100 100表B(续)活性化合物的制备 施用比例 小麦 苋草 藜 曼陀罗草 甘薯 蓼 茄实施例序号 (ai的克数/公顷) 250 0 95 95 100 100 100 100表B(续)活性化合物的制备 施用比例 狗尾草 苘麻 苋草 猪殃殃 芥草 苍耳实施例序号 (ai的克数/公顷) 1000 100 100 100 100 100 100表B(续)活性化合物的制备 施用比例 狗尾草 苘麻 苋草 猪殃殃 芥草 苍耳实施例序号 (ai的克数/公顷) 1000 100 100 100 100 100 100表B(续)活性化合物的制备 施用比例 大麦 稗 狗尾草 苋草 藜 堇菜实施例序号 (ai的克数/公顷) 500 10 100 95 100 100 100表B(续)活性化合物的制备 施用比例 稗 狗尾草 苋草 藜 堇菜实施例序号 (ai的克数/公顷) 500 100 90 100 100 100 500 100 100 100 100 100表B(续)活性化合物的制备 施用比例 马唐 狗尾草 苋草 藜 堇菜实施例序号 (ai的克数/公顷) 500 90 100 100 100 100 500 70 100 100 100 100表B(续)活性化合物的制备 施用比例 马唐 狗尾草 苋草 藜 堇菜实施例序号 (ai的克数/公顷) 1000 95 100 100 100 100表B(续)活性化合物的制备 施用比例 稗 苋草 藜 堇菜实施例序号 (ai的克数/公顷) 500 100 100 100 100 500 100 100 100 100表B(续)活性化合物的制备 施用比例 野燕麦 狗尾草 苘麻 苋草 芥草实施例序号 (ai的克数/公顷) 1000 100 90 100 100 100表B(续)活性化合物的制备 施用比例 狗尾草 苘麻 苋草 苍耳实施例序号 (ai的克数/公顷) 1000 100 100 100 100
Claims (7)
2.制备权利要求1的式(I)的化合物的方法,特征在于
其中R1,R2和Y各自如权利要求1中的定义,
Z-CO-OR’ (III)
其中
Z如权利要求1中的定义,和
R’代表烷基,
或者
其中
R1,R2,Y和Z各自如上定义,和
X1代表卤素或烷氧基,
其中
Z如上定义,和
X2代表卤素或烷氧基,
其中
R1,R2和Y各自如上定义,
并且,如果适当,通过常规方法对由(a),(b)或(c)描述的方法获得的通式(I)的化合物在上述取代基定义范围内进行进一步转化。
3.除草组合物,特征在于,其含有至少一种权利要求1的式(I)的化合物。
4.权利要求1的式(I)的化合物的用途,该用途为防治不需要的植物。
5.防治杂草的方法,特征在于,使权利要求1的式(I)的化合物作用于杂草或其生长地域。
6.制备除草组合物的方法,特征在于,将权利要求1的式(I)的化合物与扩充剂和/或表面活性剂混合。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19641693.0 | 1996-10-10 | ||
| DE19641693A DE19641693A1 (de) | 1996-10-10 | 1996-10-10 | Substituierte 2-Amino-4-alkylamino-1,3,5-triazine |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1239957A CN1239957A (zh) | 1999-12-29 |
| CN1113060C true CN1113060C (zh) | 2003-07-02 |
Family
ID=7808311
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN97180460A Expired - Fee Related CN1113060C (zh) | 1996-10-10 | 1997-09-29 | 作为除草剂的取代的2-氨基-4-烷基氨基-1,3,5-三嗪 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US6284710B1 (zh) |
| EP (1) | EP0934285A1 (zh) |
| JP (1) | JP2001501940A (zh) |
| KR (1) | KR20000048679A (zh) |
| CN (1) | CN1113060C (zh) |
| AU (1) | AU730012B2 (zh) |
| BR (1) | BR9712519A (zh) |
| CA (1) | CA2267933A1 (zh) |
| DE (1) | DE19641693A1 (zh) |
| WO (1) | WO1998015539A1 (zh) |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19641694A1 (de) | 1996-10-10 | 1998-04-16 | Bayer Ag | Substituierte 2,4-Diamino-1,3,5-triazine |
| DE19744711A1 (de) * | 1997-10-10 | 1999-04-15 | Bayer Ag | Substituierte 2,4-Diamino-1,3,5-triazine |
| DE19810394A1 (de) * | 1998-03-11 | 1999-09-16 | Bayer Ag | Substituierte Aminotriazine mit mindestens zwei asymmetrisch substituierten Kohlenstoffatomen |
| DE19816055A1 (de) * | 1998-04-09 | 1999-10-14 | Bayer Ag | Thienylalkylamino-1,3,5-triazine |
| DE19825379A1 (de) * | 1998-06-06 | 1999-12-09 | Bayer Ag | Verwendung von substituierten 2,4-Diamino-1,3,5-triazinen zur Bekämpfung tierischer Schädlinge |
| DE19830902A1 (de) | 1998-07-10 | 2000-01-13 | Hoechst Schering Agrevo Gmbh | Verfahren zur Herstellung von 2-Amino-4-chlor-1,3,5-triazinen |
| DE19842894A1 (de) * | 1998-09-18 | 2000-03-23 | Hoechst Schering Agrevo Gmbh | Synergistische Wirkstoffkombinationen zur Bekämpfung von Schadpflanzen in Nutzpflanzenkulturen |
| DE19921883A1 (de) | 1999-05-12 | 2000-11-16 | Bayer Ag | Substituierte Thienocycloalk(en)ylamino-1,3,5-triazine |
| DE19933937A1 (de) * | 1999-07-20 | 2001-01-25 | Bayer Ag | Optisch aktive Thienylalkylamino-1,3,5-triazine |
| US7112587B2 (en) * | 2001-09-21 | 2006-09-26 | Reddy Us Therapeutics, Inc. | Methods and compositions of novel triazine compounds |
| US7169785B2 (en) * | 2001-09-21 | 2007-01-30 | Reddy Us Therapeutics, Inc. | Methods and compositions of novel triazine compounds |
| US7163943B2 (en) * | 2001-09-21 | 2007-01-16 | Reddy Us Therapeutics, Inc. | Methods and compositions of novel triazine compounds |
| US7132423B2 (en) * | 2001-09-21 | 2006-11-07 | Reddy Us Therapeutics, Inc. | Methods and compositions of novel triazine compounds |
| US7173032B2 (en) * | 2001-09-21 | 2007-02-06 | Reddy Us Therapeutics, Inc. | Methods and compositions of novel triazine compounds |
| US7335770B2 (en) * | 2004-03-24 | 2008-02-26 | Reddy U5 Therapeutics, Inc. | Triazine compounds and their analogs, compositions, and methods |
| DE102004034571A1 (de) * | 2004-07-17 | 2006-02-23 | Bayer Cropscience Gmbh | Herbizide Mittel |
| EP1836894A1 (de) | 2006-03-25 | 2007-09-26 | Bayer CropScience GmbH | Neue Sulfonamid-haltige feste Formulungen |
| EP1790227A1 (de) | 2005-11-25 | 2007-05-30 | Bayer CropScience AG | Wässrige Suspensionskonzentrate aus 2,4-Diamino-s-triazinherbiziden |
| EP1854355A1 (de) * | 2006-03-15 | 2007-11-14 | Bayer CropScience AG | Wässrige Suspensionskonzentrate |
| DE102007013362A1 (de) | 2007-03-16 | 2008-09-18 | Bayer Cropscience Ag | Penetrationsförderer für herbizide Wirkstoffe |
| EP1844654A1 (de) | 2006-03-29 | 2007-10-17 | Bayer CropScience GmbH | Penetrationsförderer für agrochemische Wirkstoffe |
| EP1958509A1 (de) * | 2007-02-19 | 2008-08-20 | Bayer CropScience AG | Herbizid-Kombination |
| WO2011076731A1 (de) | 2009-12-23 | 2011-06-30 | Bayer Cropscience Ag | Flüssige formulierung von 2-iodo-n-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoyl] benzolsulfonamid |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0273328A2 (en) * | 1986-12-27 | 1988-07-06 | Idemitsu Kosan Company Limited | Triazine derivatives and herbicides containing said derivatives |
| EP0411153A1 (en) * | 1989-02-20 | 1991-02-06 | Idemitsu Kosan Company Limited | Triazine derivative and herbicide containing the same as active ingredient |
| WO1997008156A1 (de) * | 1995-08-24 | 1997-03-06 | Hoechst Schering Agrevo Gmbh | 2,4-diamino-1,3,5-triazine, verfahren zu deren herstellung und deren verwendung als herbizide und pflanzenwachstumsregulatoren |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3816419A (en) | 1972-10-27 | 1974-06-11 | American Cyanamid Co | Substituted s-triazines |
| US3932167A (en) | 1972-10-27 | 1976-01-13 | American Cyanamid Company | Substituted s-triazines as herbicidal agents |
| DE3426919A1 (de) | 1984-07-21 | 1986-01-23 | Bayer Ag, 5090 Leverkusen | Optisch aktive 3,4-methylendioxyphenylbutanderivate verfahren zu ihrer herstellung und ihre verwendung |
| JPS61189277A (ja) | 1985-02-15 | 1986-08-22 | Idemitsu Kosan Co Ltd | トリアジン誘導体,その製造方法およびそれを有効成分とする除草剤 |
| HUT56050A (en) | 1987-12-18 | 1991-07-29 | Schering Corp | Process for resolving 2-amino-4phenyl-butane |
| DE4000610A1 (de) | 1990-01-11 | 1991-07-18 | Knoll Ag | Verfahren zur herstellung von optisch aktiven 3-amino-1-arylbutanen und 3-benzylamino-1-arylbutanen |
| JP2818958B2 (ja) | 1990-02-23 | 1998-10-30 | 塩野義製薬株式会社 | 4―(4―アルコキシフェニル)―2―ブチルアミン誘導体およびその製造法 |
| US5286905A (en) | 1990-12-28 | 1994-02-15 | Idemitsu Kosan Company Limited | Process for producing biguanide derivative |
| DE4332738A1 (de) | 1993-09-25 | 1995-03-30 | Basf Ag | Racematspaltung primärer und sekundärer Amine durch Enzym-katalysierte Acylierung |
| DE4335497A1 (de) | 1993-10-19 | 1995-04-20 | Basf Ag | Verfahren zur Herstellung unsymmetrisch substituierter Triazine |
| DE19522137A1 (de) | 1995-06-19 | 1997-01-02 | Hoechst Schering Agrevo Gmbh | 2-Amino-1,3,5-triazine, Verfahren zu deren Herstellung und deren Verwendung als Herbizide und Pflanzenwachstumsregulatoren |
-
1996
- 1996-10-10 DE DE19641693A patent/DE19641693A1/de not_active Withdrawn
-
1997
- 1997-09-29 JP JP10517140A patent/JP2001501940A/ja active Pending
- 1997-09-29 EP EP97943911A patent/EP0934285A1/de not_active Withdrawn
- 1997-09-29 US US09/284,052 patent/US6284710B1/en not_active Expired - Fee Related
- 1997-09-29 CN CN97180460A patent/CN1113060C/zh not_active Expired - Fee Related
- 1997-09-29 CA CA002267933A patent/CA2267933A1/en not_active Abandoned
- 1997-09-29 AU AU45578/97A patent/AU730012B2/en not_active Ceased
- 1997-09-29 BR BR9712519-9A patent/BR9712519A/pt not_active IP Right Cessation
- 1997-09-29 WO PCT/EP1997/005320 patent/WO1998015539A1/de not_active Application Discontinuation
- 1997-09-29 KR KR1019990702627A patent/KR20000048679A/ko not_active Application Discontinuation
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0273328A2 (en) * | 1986-12-27 | 1988-07-06 | Idemitsu Kosan Company Limited | Triazine derivatives and herbicides containing said derivatives |
| EP0411153A1 (en) * | 1989-02-20 | 1991-02-06 | Idemitsu Kosan Company Limited | Triazine derivative and herbicide containing the same as active ingredient |
| WO1997008156A1 (de) * | 1995-08-24 | 1997-03-06 | Hoechst Schering Agrevo Gmbh | 2,4-diamino-1,3,5-triazine, verfahren zu deren herstellung und deren verwendung als herbizide und pflanzenwachstumsregulatoren |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20000048679A (ko) | 2000-07-25 |
| BR9712519A (pt) | 1999-10-19 |
| CN1239957A (zh) | 1999-12-29 |
| CA2267933A1 (en) | 1998-04-16 |
| DE19641693A1 (de) | 1998-04-16 |
| AU4557897A (en) | 1998-05-05 |
| US6284710B1 (en) | 2001-09-04 |
| JP2001501940A (ja) | 2001-02-13 |
| WO1998015539A1 (de) | 1998-04-16 |
| EP0934285A1 (de) | 1999-08-11 |
| AU730012B2 (en) | 2001-02-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1113060C (zh) | 作为除草剂的取代的2-氨基-4-烷基氨基-1,3,5-三嗪 | |
| CN1233242A (zh) | 作为除草剂的取代的2,4-二氨基-1,3,5-三嗪 | |
| CN1119337C (zh) | 作为除草剂的取代的2,4-二氨基-1,3,5-三嗪 | |
| CN1090623C (zh) | 具有除草性质的3-芳基-1,2,4-三唑衍生物 | |
| CN1239956A (zh) | 作为除草剂的取代的2-氨基-4-烷基氨基-1,3,5-三嗪 | |
| KR20010024389A (ko) | 치환된 2,4-디아미노-1,3,5-트리아진 및 제초제로서 그의용도 | |
| CN1293060C (zh) | 用作除草剂的取代的芳基磺酰基(硫代)脲类 | |
| CN1196725A (zh) | 取代的氨基尿嘧啶 | |
| US6399541B1 (en) | Substituted 2,4-diamino-1,3,5-triazines as herbicides | |
| CN1057765C (zh) | 制备4-氰基苯基亚氨基杂环化合物的中间体 | |
| CN1121392C (zh) | 取代嘧啶(硫)酮 | |
| CN1113063C (zh) | 杂环基尿嘧啶 | |
| CN1227545A (zh) | 3-氰基芳基-吡唑及其作为除草剂的应用 | |
| CN1227548A (zh) | 取代的对三氟甲基苯基尿嘧啶 | |
| CN1130360C (zh) | 取代的噻吩并环烷(烯)基氨基-1,3,5-三嗪类化合物 | |
| CN1070855C (zh) | 4-硫代氨基甲酰基-1-(3-吡唑基)-吡唑化合物及其作除草剂的用途 | |
| CN1370160A (zh) | 取代的杂环基-2h-苯并吡喃类化合物 | |
| RU2252937C2 (ru) | Производные 1,3,5-триазина, гербицидная композиция на их основе и промежуточные соединения | |
| CN1101390C (zh) | 杂环基尿嘧啶 | |
| CN1103764C (zh) | 取代的苯基尿嘧啶 | |
| CN1227546A (zh) | 具有除草活性的取代的1-(3-吡唑基)-吡唑和用于其制备的中间体 | |
| CN1140514C (zh) | 取代的亚氨基烷氧基苯基尿嘧啶及其制法和作为除草剂的应用 | |
| JP4911820B2 (ja) | 置換された1,3,5−トリアジン類 | |
| CN1216533A (zh) | 3-氰基芳基-吡唑及其作为除草剂的用途 | |
| CN1361772A (zh) | 取代的杂芳基氧基乙酰苯胺类化合物及其作为除草剂的应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| C19 | Lapse of patent right due to non-payment of the annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee | ||
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: WD Ref document number: 1024483 Country of ref document: HK |