CN111195266B - Probiotic composition with effect of relieving hyperuricemia and application thereof - Google Patents
Probiotic composition with effect of relieving hyperuricemia and application thereof Download PDFInfo
- Publication number
- CN111195266B CN111195266B CN201911330780.5A CN201911330780A CN111195266B CN 111195266 B CN111195266 B CN 111195266B CN 201911330780 A CN201911330780 A CN 201911330780A CN 111195266 B CN111195266 B CN 111195266B
- Authority
- CN
- China
- Prior art keywords
- parts
- freeze
- dried powder
- controlled
- probiotic composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000006041 probiotic Substances 0.000 title claims abstract description 82
- 235000018291 probiotics Nutrition 0.000 title claims abstract description 82
- 230000000529 probiotic effect Effects 0.000 title claims abstract description 59
- 230000000694 effects Effects 0.000 title claims abstract description 55
- 239000000203 mixture Substances 0.000 title claims abstract description 48
- 201000001431 Hyperuricemia Diseases 0.000 title claims abstract description 23
- 239000000843 powder Substances 0.000 claims abstract description 46
- DLRVVLDZNNYCBX-UHFFFAOYSA-N Polydextrose Polymers OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(O)O1 DLRVVLDZNNYCBX-UHFFFAOYSA-N 0.000 claims abstract description 38
- 238000000034 method Methods 0.000 claims abstract description 21
- 241000186606 Lactobacillus gasseri Species 0.000 claims abstract description 20
- 241001608472 Bifidobacterium longum Species 0.000 claims abstract description 19
- 239000004375 Dextrin Substances 0.000 claims abstract description 19
- 229920001353 Dextrin Polymers 0.000 claims abstract description 19
- 240000006024 Lactobacillus plantarum Species 0.000 claims abstract description 19
- 235000013965 Lactobacillus plantarum Nutrition 0.000 claims abstract description 19
- 241000186869 Lactobacillus salivarius Species 0.000 claims abstract description 19
- 229920001100 Polydextrose Polymers 0.000 claims abstract description 19
- 229940009291 bifidobacterium longum Drugs 0.000 claims abstract description 19
- 235000019425 dextrin Nutrition 0.000 claims abstract description 19
- FTSSQIKWUOOEGC-RULYVFMPSA-N fructooligosaccharide Chemical compound OC[C@H]1O[C@@](CO)(OC[C@@]2(OC[C@@]3(OC[C@@]4(OC[C@@]5(OC[C@@]6(OC[C@@]7(OC[C@@]8(OC[C@@]9(OC[C@@]%10(OC[C@@]%11(O[C@H]%12O[C@H](CO)[C@@H](O)[C@H](O)[C@H]%12O)O[C@H](CO)[C@@H](O)[C@@H]%11O)O[C@H](CO)[C@@H](O)[C@@H]%10O)O[C@H](CO)[C@@H](O)[C@@H]9O)O[C@H](CO)[C@@H](O)[C@@H]8O)O[C@H](CO)[C@@H](O)[C@@H]7O)O[C@H](CO)[C@@H](O)[C@@H]6O)O[C@H](CO)[C@@H](O)[C@@H]5O)O[C@H](CO)[C@@H](O)[C@@H]4O)O[C@H](CO)[C@@H](O)[C@@H]3O)O[C@H](CO)[C@@H](O)[C@@H]2O)[C@@H](O)[C@@H]1O FTSSQIKWUOOEGC-RULYVFMPSA-N 0.000 claims abstract description 19
- 229940107187 fructooligosaccharide Drugs 0.000 claims abstract description 19
- 229940072205 lactobacillus plantarum Drugs 0.000 claims abstract description 19
- 239000001259 polydextrose Substances 0.000 claims abstract description 19
- 235000013856 polydextrose Nutrition 0.000 claims abstract description 19
- 229940035035 polydextrose Drugs 0.000 claims abstract description 19
- 230000008569 process Effects 0.000 claims abstract description 14
- 244000005700 microbiome Species 0.000 claims abstract description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 27
- 239000000463 material Substances 0.000 claims description 22
- 238000005469 granulation Methods 0.000 claims description 16
- 230000003179 granulation Effects 0.000 claims description 16
- 239000007788 liquid Substances 0.000 claims description 16
- 238000002360 preparation method Methods 0.000 claims description 14
- 239000011812 mixed powder Substances 0.000 claims description 13
- 238000009835 boiling Methods 0.000 claims description 10
- 239000001963 growth medium Substances 0.000 claims description 10
- 239000007921 spray Substances 0.000 claims description 10
- 238000000855 fermentation Methods 0.000 claims description 9
- 230000004151 fermentation Effects 0.000 claims description 9
- 238000004321 preservation Methods 0.000 claims description 8
- 239000002002 slurry Substances 0.000 claims description 7
- 230000001580 bacterial effect Effects 0.000 claims description 6
- 239000003814 drug Substances 0.000 claims description 6
- 238000002156 mixing Methods 0.000 claims description 6
- 239000002131 composite material Substances 0.000 claims description 4
- 238000001035 drying Methods 0.000 claims description 4
- 238000012360 testing method Methods 0.000 claims description 4
- 241001052560 Thallis Species 0.000 claims description 3
- 230000003213 activating effect Effects 0.000 claims description 3
- 239000003995 emulsifying agent Substances 0.000 claims description 3
- 230000001939 inductive effect Effects 0.000 claims description 3
- 238000011081 inoculation Methods 0.000 claims description 3
- 238000009630 liquid culture Methods 0.000 claims description 3
- 230000001681 protective effect Effects 0.000 claims description 3
- 238000007873 sieving Methods 0.000 claims description 3
- 230000002906 microbiologic effect Effects 0.000 claims description 2
- 238000009629 microbiological culture Methods 0.000 claims 2
- 238000007710 freezing Methods 0.000 claims 1
- 230000008014 freezing Effects 0.000 claims 1
- 238000005507 spraying Methods 0.000 claims 1
- LEHOTFFKMJEONL-UHFFFAOYSA-N Uric Acid Chemical compound N1C(=O)NC(=O)C2=C1NC(=O)N2 LEHOTFFKMJEONL-UHFFFAOYSA-N 0.000 abstract description 25
- TVWHNULVHGKJHS-UHFFFAOYSA-N Uric acid Natural products N1C(=O)NC(=O)C2NC(=O)NC21 TVWHNULVHGKJHS-UHFFFAOYSA-N 0.000 abstract description 25
- 229940116269 uric acid Drugs 0.000 abstract description 25
- 238000003860 storage Methods 0.000 abstract description 7
- 230000001976 improved effect Effects 0.000 abstract description 6
- 208000024891 symptom Diseases 0.000 abstract description 4
- 230000000052 comparative effect Effects 0.000 description 23
- 239000008176 lyophilized powder Substances 0.000 description 16
- 239000002158 endotoxin Substances 0.000 description 14
- 229920006008 lipopolysaccharide Polymers 0.000 description 13
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 12
- 241000894006 Bacteria Species 0.000 description 11
- 238000001514 detection method Methods 0.000 description 9
- 206010061218 Inflammation Diseases 0.000 description 8
- 230000004054 inflammatory process Effects 0.000 description 8
- 241000700159 Rattus Species 0.000 description 7
- 210000004369 blood Anatomy 0.000 description 7
- 239000008280 blood Substances 0.000 description 7
- 230000000968 intestinal effect Effects 0.000 description 7
- 239000003973 paint Substances 0.000 description 7
- 210000001035 gastrointestinal tract Anatomy 0.000 description 6
- 235000014655 lactic acid Nutrition 0.000 description 6
- 239000004310 lactic acid Substances 0.000 description 6
- 238000011160 research Methods 0.000 description 6
- 230000037213 diet Effects 0.000 description 5
- 235000005911 diet Nutrition 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 230000002757 inflammatory effect Effects 0.000 description 5
- 210000002966 serum Anatomy 0.000 description 5
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 4
- NYHBQMYGNKIUIF-UUOKFMHZSA-N Guanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NYHBQMYGNKIUIF-UUOKFMHZSA-N 0.000 description 4
- 235000013361 beverage Nutrition 0.000 description 4
- 230000001276 controlling effect Effects 0.000 description 4
- 235000013305 food Nutrition 0.000 description 4
- 230000006872 improvement Effects 0.000 description 4
- 235000013406 prebiotics Nutrition 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 241000186000 Bifidobacterium Species 0.000 description 3
- 241000193468 Clostridium perfringens Species 0.000 description 3
- 241000194033 Enterococcus Species 0.000 description 3
- 108090001005 Interleukin-6 Proteins 0.000 description 3
- 241000186660 Lactobacillus Species 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- 239000001768 carboxy methyl cellulose Substances 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 230000002550 fecal effect Effects 0.000 description 3
- 210000004347 intestinal mucosa Anatomy 0.000 description 3
- 239000007928 intraperitoneal injection Substances 0.000 description 3
- 229940039696 lactobacillus Drugs 0.000 description 3
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- YDBHVMTTYXWHLI-UHFFFAOYSA-N 2,4,6-tribromo-3-hydroxybenzoic acid Chemical compound OC(=O)C1=C(Br)C=C(Br)C(O)=C1Br YDBHVMTTYXWHLI-UHFFFAOYSA-N 0.000 description 2
- RYYCJUAHISIHTL-UHFFFAOYSA-N 5-azaorotic acid Chemical compound OC(=O)C1=NC(=O)NC(=O)N1 RYYCJUAHISIHTL-UHFFFAOYSA-N 0.000 description 2
- MIKUYHXYGGJMLM-GIMIYPNGSA-N Crotonoside Natural products C1=NC2=C(N)NC(=O)N=C2N1[C@H]1O[C@@H](CO)[C@H](O)[C@@H]1O MIKUYHXYGGJMLM-GIMIYPNGSA-N 0.000 description 2
- NYHBQMYGNKIUIF-UHFFFAOYSA-N D-guanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(CO)C(O)C1O NYHBQMYGNKIUIF-UHFFFAOYSA-N 0.000 description 2
- 241000305071 Enterobacterales Species 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 210000000683 abdominal cavity Anatomy 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 229940029575 guanosine Drugs 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000003870 intestinal permeability Effects 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000000813 microbial effect Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 239000002777 nucleoside Substances 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 229950000193 oteracil Drugs 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 230000000770 proinflammatory effect Effects 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 238000013112 stability test Methods 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 238000009777 vacuum freeze-drying Methods 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- UDMBCSSLTHHNCD-UHFFFAOYSA-N Coenzym Q(11) Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(O)=O)C(O)C1O UDMBCSSLTHHNCD-UHFFFAOYSA-N 0.000 description 1
- 206010010774 Constipation Diseases 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 208000036649 Dysbacteriosis Diseases 0.000 description 1
- 208000027244 Dysbiosis Diseases 0.000 description 1
- 238000008157 ELISA kit Methods 0.000 description 1
- 241000588914 Enterobacter Species 0.000 description 1
- 201000005569 Gout Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 1
- 229930010555 Inosine Natural products 0.000 description 1
- 240000001929 Lactobacillus brevis Species 0.000 description 1
- 235000013957 Lactobacillus brevis Nutrition 0.000 description 1
- 244000199866 Lactobacillus casei Species 0.000 description 1
- 235000013958 Lactobacillus casei Nutrition 0.000 description 1
- 101001076687 Lactobacillus gasseri Inulosucrase Proteins 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 241000700157 Rattus norvegicus Species 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 108010093894 Xanthine oxidase Proteins 0.000 description 1
- 102100033220 Xanthine oxidase Human genes 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- UDMBCSSLTHHNCD-KQYNXXCUSA-N adenosine 5'-monophosphate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O UDMBCSSLTHHNCD-KQYNXXCUSA-N 0.000 description 1
- 229950006790 adenosine phosphate Drugs 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 229960003235 allopurinol sodium Drugs 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 206010013663 drug dependence Diseases 0.000 description 1
- 230000007140 dysbiosis Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 210000003608 fece Anatomy 0.000 description 1
- 238000003304 gavage Methods 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- RQFCJASXJCIDSX-UUOKFMHZSA-N guanosine 5'-monophosphate Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O RQFCJASXJCIDSX-UUOKFMHZSA-N 0.000 description 1
- 235000013928 guanylic acid Nutrition 0.000 description 1
- 239000004226 guanylic acid Substances 0.000 description 1
- 244000005709 gut microbiome Species 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 201000001421 hyperglycemia Diseases 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229960003786 inosine Drugs 0.000 description 1
- 210000005027 intestinal barrier Anatomy 0.000 description 1
- 230000004673 intestinal mucosal barrier function Effects 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 229940017800 lactobacillus casei Drugs 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 125000003835 nucleoside group Chemical group 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 238000011552 rat model Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- PTJRZVJXXNYNLN-UHFFFAOYSA-M sodium;2h-pyrazolo[3,4-d]pyrimidin-1-id-4-one Chemical compound [Na+].[O-]C1=NC=NC2=C1C=NN2 PTJRZVJXXNYNLN-UHFFFAOYSA-M 0.000 description 1
- 210000004872 soft tissue Anatomy 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 208000011117 substance-related disease Diseases 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 230000031068 symbiosis, encompassing mutualism through parasitism Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 230000003313 weakening effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
- A61K35/747—Lactobacilli, e.g. L. acidophilus or L. brevis
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Their preparation
- A23L2/385—Concentrates of non-alcoholic beverages
- A23L2/39—Dry compositions
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/125—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives containing carbohydrate syrups; containing sugars; containing sugar alcohols; containing starch hydrolysates
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/135—Bacteria or derivatives thereof, e.g. probiotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
- A61K35/745—Bifidobacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/06—Antigout agents, e.g. antihyperuricemic or uricosuric agents
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2400/00—Lactic or propionic acid bacteria
- A23V2400/11—Lactobacillus
- A23V2400/145—Gasseri
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2400/00—Lactic or propionic acid bacteria
- A23V2400/11—Lactobacillus
- A23V2400/169—Plantarum
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2400/00—Lactic or propionic acid bacteria
- A23V2400/11—Lactobacillus
- A23V2400/181—Salivarius
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2400/00—Lactic or propionic acid bacteria
- A23V2400/51—Bifidobacterium
- A23V2400/533—Longum
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Mycology (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Polymers & Plastics (AREA)
- Nutrition Science (AREA)
- Food Science & Technology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Biochemistry (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Physical Education & Sports Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
The invention relates to the field of microorganisms, in particular to a probiotic composition with a function of relieving hyperuricemia and application thereof; the probiotic composition comprises the following components in parts by weight: 30-50 parts of polydextrose, 10-20 parts of resistant dextrin, 23-33 parts of fructo-oligosaccharide, 4-8 parts of Lactobacillus gasseri LG08 freeze-dried powder, 2-6 parts of Bifidobacterium longum BL21 freeze-dried powder, 3-5 parts of Lactobacillus salivarius LS97 freeze-dried powder and 2-4 parts of Lactobacillus plantarum Lp90 freeze-dried powder; under the condition of equal intake of probiotics, the effect of reducing uric acid is better than that of single probiotics, the side effect caused by high uric acid symptom can be improved, and the stability of the probiotics in the storage process can be improved.
Description
Technical Field
The invention relates to the field of microorganisms, in particular to a probiotic composition with a function of relieving hyperuricemia and application thereof.
Background
With the improvement of living standard of people, the incidence of a plurality of chronic diseases is higher, such as hyperuricemia and the like. Hyperuricemia is a disease in which blood uric acid exceeds normal values, and uric acid is easily precipitated in joints, soft tissues, cartilage and kidneys in the form of sodium salt, causing organ and tissue lesions of the human body, resulting in gout and serious complications. According to statistics, the number of hyperuricemia patients is about 1.2 hundred million in China at present, and the traditional treatment methods comprise the problems of drug dependence, great side effect, difficult diet control and the like in the aspects of drugs, diet restriction and the like, so that the development of a new method for preventing and relieving the symptoms of hyperuricemia is not slow.
Hundreds of millions of bacteria inhabit the human intestinal tract, most of which live in symbiosis with the human body and form a unique micro-ecosystem. Researches find that intestinal micro-ecological imbalance is one of the key factors causing diseases, and the regulation of intestinal microbial flora and the improvement of the internal environment of the intestinal tract by using probiotics become research hotspots for preventing and treating related diseases, and a plurality of researches show that the probiotics have remarkable effects on relieving diarrhea, constipation, hypertension, hyperglycemia and the like. In the aspect of intervention of hyperuricemia by probiotics, dalian medical university breeds and obtains a Lactobacillus brevis DM9218 and recombinant protein (CN 106834162A) thereof, and the decomposition rates of inosine and guanosine are 99.31 percent and 99.64 percent respectively. Gold (screening of probiotic strains for reducing blood uric acid and exploration of mechanism of reducing blood uric acid, page 1757-1769 of 8 th stage in 2018 of microbiological report) and the like, further widens the range of degraded substrates, and breeds a lactobacillus casei ZM15 which can efficiently degrade nucleosides (adenosine and guanosine) and nucleotides (adenylic acid and guanylic acid), and can alleviate symptoms of hyperuricemia to a certain extent. The applicant also applies for lactobacillus gasseri LG08 with the effect of degrading uric acid and application thereof (Chinese application number CN201911191600. X), has excellent nucleoside and nucleotide degradation capability, can decompose most precursors synthesized by uric acid, reduce absorption of the precursors synthesized by uric acid by organisms, can directly and efficiently decompose uric acid in intestinal tracts, and further inhibit the generation of uric acid and relieve inflammation and the like by reducing the serum endotoxin level and inhibiting the xanthine oxidase activity.
However, it should be noted that, the above mentioned methods all use a single probiotic to intervene in hyperuricemia, and the species of the used probiotic is small, compared with the large number of flora in human intestinal tract, the effect of regulating intestinal flora by using a single probiotic is limited and the synergistic effect between multiple probiotics cannot be exerted. Furthermore, hyperuricemia is accompanied by various side effects such as dysbacteriosis and increased intestinal wall permeability, etc., and the above single strain protocol focuses on the effect of reducing uric acid concentration without intensive studies on the mitigating effects of other side effects. Finally, in the preparation process, the problems of stability and the like of probiotics in the storage process are not fully considered in the scheme, so that the problems of too fast loss of viable count, weakening of effect and the like are easily caused.
Disclosure of Invention
In order to solve the above technical problems, it is an object of the present invention to provide a probiotic composition having an effect of relieving hyperuricemia; under the condition of equal intake of probiotics, the effect of reducing uric acid is better than that of single probiotics, the side effect caused by high uric acid symptoms can be improved, and the stability of the probiotics in the storage process can be improved.
The invention provides a probiotic composition with a function of relieving hyperuricemia, which comprises the following components in parts by weight: 30-50 parts of polydextrose, 10-20 parts of resistant dextrin, 23-33 parts of fructo-oligosaccharide, 4-8 parts of lactobacillus gasseri LG08 freeze-dried powder, 2-6 parts of bifidobacterium longum BL21 freeze-dried powder, 3-5 parts of lactobacillus salivarius LS97 freeze-dried powder and 2-4 parts of lactobacillus plantarum Lp90 freeze-dried powder;
the Lactobacillus gasseri LG08 is preserved in the common microorganism center of the China Committee for culture Collection of microorganisms at 18.7.2018, the preservation number is CGMCC No.16131, the Lactobacillus gasseri is classified and named as Lactobacillus gasseri, and the preservation address is the microbial research institute of China academy of sciences No. 3 of West Lu No.1 of the sunward district of Beijing city;
the Bifidobacterium longum BL21 is preserved in the common microorganism center of China Committee for culture Collection of microorganisms (CGMCC) at 27.1.2015, the preservation number is CGMCC No.10452, the Bifidobacterium longum is named as Bifidobacterium longum by classification, and the preservation address is the institute of microbiology of China academy of sciences No. 3 of West Lu 1 of Beijing Korean district, beijing;
the Lactobacillus salivarius LS97 is stored in the common microorganism center of the China Committee for Culture Collection of Microorganisms (CCM) in 2018, 12 months and 10 days, the storage number is CGMCC No. 169922, the classification is named as Lactobacillus salivarius (Lactobacillus salivarius), and the storage address is the microorganism research institute of China academy of sciences No. 3 of West Lu 1 on the north Chen of the sunward area in Beijing;
the Lactobacillus plantarum Lp90 is already preserved in the ordinary microorganism center of China Committee for Culture Collection of Microorganisms (CCM) at 27.1.2015, the preservation number is CGMCC No.10453, the classification name is Lactobacillus plantarum (Lactobacillus plantarum), and the preservation address is the microorganism research institute of China academy of sciences No. 3 of the North Cheng Xilu No.1 of the sunward district in Beijing.
Specifically, the number of viable bacteria of the lyophilized powder of lactobacillus gasseri LG08, the lyophilized powder of bifidobacterium longum BL21, the lyophilized powder of lactobacillus salivarius LS97 and the lyophilized powder of lactobacillus plantarum Lp90 is 108-1010CFU/g, the water content is lower than 4%, and the water activity content is lower than 0.1.
Specifically, the polydextrose, the resistant dextrin and the fructo-oligosaccharide are treated by a boiling granulation process, the water content is lower than 3.5%, and the water activity is lower than 0.1.
In a second aspect, the invention provides the use of the probiotic composition in food, health care products and medicines.
In particular to application of the probiotic composition to a probiotic solid beverage.
The third aspect of the invention provides a preparation method of the probiotic solid beverage, which specifically comprises the following steps:
s1, preparing a composite probiotic composition: activating Lactobacillus gasseri LG08, bifidobacterium longum BL21, lactobacillus salivarius LS97 and Lactobacillus plantarum Lp90 in an MRS culture medium test tube respectively to obtain primary seed liquid, inoculating the primary seeds into an MRS liquid culture medium respectively under the culture conditions of 30-38 ℃, carrying out anaerobic culture for 16-24h to obtain secondary seed liquid, inoculating the secondary seed liquid into a fermentation culture medium respectively for high-density culture, wherein the inoculation amount is 0.5-5%, the culture conditions are 30-38 ℃, carrying out anaerobic culture for 12-18h, and centrifuging and collecting fermentation liquid respectively;
s2, respectively centrifuging the fermentation liquor obtained in the step S1, collecting thalli, adding a protective emulsifier, respectively carrying out vacuum freeze drying to obtain bacterial powder, wherein the water content is controlled to be below 4%, the water activity is controlled to be below 0.1, and then mixing the bacterial powder according to the weight part in the formula to obtain probiotic mixed powder A;
s3, auxiliary material granulation treatment: respectively sieving polydextrose, resistant dextrin and fructo-oligosaccharide with 80-100 mesh sieve, adding 3 powders into a boiling granulator according to the weight parts of the formula, taking out a part of the powder, adding water according to the proportion of 5-10% of the mass fraction to prepare liquid slurry, and controlling the temperature of the slurry at 60-70 ℃; controlling the water content of the material to be below 3.5% and the water activity to be lower than 0.1 by the granulation process, and taking the material out of the pot to obtain auxiliary mixed powder B;
and S4, mixing the probiotic mixed powder A and the auxiliary material mixed powder B to obtain the solid beverage.
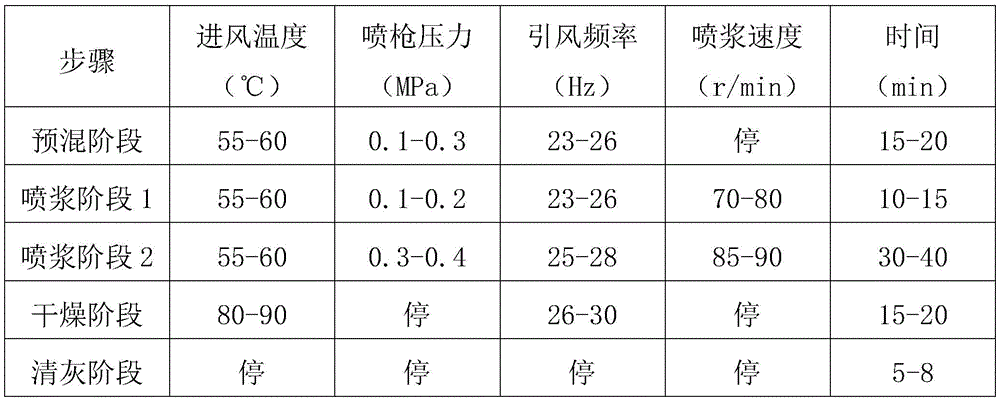
Furthermore, the air inlet temperature is controlled to be 55-60 ℃, the pressure of a spray gun is controlled to be 0.1-0.3MPa, the air inducing frequency is controlled to be 23-26Hz, and the time is 15-20min when no guniting is carried out in the premixing stage of the granulation process;
in the guniting stage 1, the air inlet temperature is controlled to be 55-60 ℃, the pressure of a spray gun is controlled to be 0.1-0.2MPa, the induced air frequency is controlled to be 23-26Hz, the guniting speed is 70-80r/min, and the time is 10-15min;
in the guniting stage 2, the air inlet temperature is controlled to be 55-60 ℃, the pressure of a spray gun is controlled to be 0.3-0.4MPa, the induced air frequency is 25-28Hz, the guniting speed is 85-90r/min, and the time is 30-40min;
in the drying stage, the air inlet temperature is controlled to be 80-90 ℃, the spray gun and the guniting are stopped, the air inducing frequency is 26-30Hz, and the time is 15-20min;
and in the ash removal stage, air inlet, a spray gun, induced air and guniting are controlled to be stopped for 5-8min.
The granulation process parameters are specifically shown in table 1.
TABLE 1 granulation Process of adjuvants
By means of the scheme, the invention at least has the following advantages:
(1) According to the invention, by adding multiple probiotics, the relieving effect on uric acid in blood is effectively enhanced compared with the effect of adding single probiotics under the condition of adding the same number of probiotics;
(2) The composition and the preparation thereof provided by the invention can effectively relieve the side effects brought by hyperuricemia, such as increasing the diversity of intestinal flora, enhancing the intestinal mucosa barrier, relieving inflammation and the like;
(3) According to the invention, the added auxiliary materials including the polydextrose, the fructo-oligosaccharide and the resistant dextrin are subjected to boiling granulation, so that the stability of the strain in the storage process is effectively improved by controlling the water content and water activity of the materials.
The foregoing description is only an overview of the technical solutions of the present invention, and in order to make the technical solutions of the present invention more clearly understood and to implement them in accordance with the contents of the description, the following detailed description is given with reference to the preferred embodiments of the present invention and the accompanying drawings.
Drawings
FIG. 1 is a graph of the effect of different intervention modes on blood uric acid in the present invention;
FIG. 2 is the effect of different intervention modes on LPS in the present invention;
FIG. 3 is a graph showing the effect of different intervention modes on IL-6/IL-10 in the present invention;
fig. 4 is a stability test of probiotic compositions of the present invention.
Detailed Description
The following detailed description of embodiments of the present invention is provided in connection with the accompanying drawings and examples. The following examples are intended to illustrate the invention, but are not intended to limit the scope of the invention.
Example 1
The embodiment provides a probiotic composition with an effect of relieving hyperuricemia, and the formula is as follows:
the paint comprises the following components in parts by weight: 30 parts of polydextrose, 20 parts of resistant dextrin, 33 parts of fructooligosaccharide, 4 parts of freeze-dried powder of Lactobacillus gasseri LG08, 6 parts of freeze-dried powder of Bifidobacterium longum BL21, 5 parts of freeze-dried powder of Lactobacillus salivarius LS97 and 2 parts of freeze-dried powder of Lactobacillus plantarum Lp 90.
And provides a preparation method thereof, which comprises the following steps:
s1, preparing a composite probiotic composition: activating Lactobacillus gasseri LG08, bifidobacterium longum BL21, lactobacillus salivarius LS97 freeze-dried powder and Lactobacillus plantarum Lp90 in an MRS culture medium test tube respectively to obtain primary seed liquid, inoculating the primary seeds into an MRS liquid culture medium respectively under the culture conditions of 30-38 ℃, carrying out anaerobic culture for 16-24h to obtain secondary seed liquid, inoculating the secondary seed liquid into a fermentation culture medium respectively for high-density culture, wherein the inoculation amount is 0.5-5%, the culture condition is 30-38 ℃, carrying out anaerobic culture for 12-18h, and centrifuging respectively to collect fermentation liquid;
s2, respectively centrifuging the fermentation liquor obtained in the step S1, collecting thalli, adding a protective emulsifier, respectively carrying out vacuum freeze drying to obtain bacterial powder, wherein the water content is controlled to be below 4%, the water activity is controlled to be below 0.1, and then mixing the bacterial powder according to the weight part in the formula to obtain probiotic mixed powder A;
s3, auxiliary material granulation treatment: respectively sieving polydextrose, resistant dextrin and fructo-oligosaccharide with 80-100 mesh sieve, adding 3 powders into a boiling granulator according to the weight parts of the formula, taking out 5-10% of the powder by weight parts, adding water to prepare liquid slurry, and controlling the temperature of the slurry at 60-70 ℃; the granulation process is shown in table 1, the water content of the material is controlled to be below 3.5%, the water activity is controlled to be lower than 0.1, and then the material is taken out of the pot, so that the auxiliary material mixed powder B is obtained;
and S4, mixing the probiotic mixed powder A and the auxiliary material mixed powder B to obtain the solid beverage.
Example 2
This example provides another probiotic composition with hyperuricemic effect formulated as follows:
the paint comprises the following components in parts by weight: 50 parts of polydextrose, 10 parts of resistant dextrin, 23 parts of fructooligosaccharide, 8 parts of freeze-dried powder of Lactobacillus gasseri LG08, 2 parts of freeze-dried powder of Bifidobacterium longum BL21, 3 parts of freeze-dried powder of Lactobacillus salivarius LS97 and 4 parts of freeze-dried powder of Lactobacillus plantarum Lp 90.
The preparation method is the same as in example 1.
Example 3
This example provides another probiotic composition with a hyperuricemic effect, formulated as follows:
the paint comprises the following components in parts by weight: 40 parts of polydextrose, 15 parts of resistant dextrin, 28 parts of fructooligosaccharide, 6 parts of freeze-dried powder of Lactobacillus gasseri LG08, 4 parts of freeze-dried powder of Bifidobacterium longum BL21, 4 parts of freeze-dried powder of Lactobacillus salivarius LS97 and 3 parts of freeze-dried powder of Lactobacillus plantarum Lp 90.
The preparation method is the same as in example 1.
Comparative example 1
The present comparative example provides a probiotic composition, formulated as follows:
the paint comprises the following components in parts by weight: 28 parts of polydextrose, 22 parts of resistant dextrin, 35 parts of fructo-oligosaccharide, 3 parts of lyophilized powder of Lactobacillus gasseri LG08, 1 part of lyophilized powder of Bifidobacterium longum BL21, 6 parts of lyophilized powder of Lactobacillus salivarius LS97 and 5 parts of lyophilized powder of Lactobacillus plantarum Lp 90.
The preparation method is the same as in example 1.
Comparative example 2
This comparative example provides another probiotic composition formulated as follows:
the paint comprises the following components in parts by weight: 55 parts of polydextrose, 8 parts of resistant dextrin, 18 parts of fructo-oligosaccharide, 9 parts of lyophilized powder of Lactobacillus gasseri LG08, 7 parts of lyophilized powder of Bifidobacterium longum BL21, 2 parts of lyophilized powder of Lactobacillus salivarius LS97 and 1 part of lyophilized powder of Lactobacillus plantarum Lp 90.
The preparation method is the same as that of example 1.
Comparative example 3
This comparative example provides another probiotic composition formulated as follows:
the paint comprises the following components in parts by weight: 40 parts of polydextrose, 15 parts of resistant dextrin, 28 parts of fructo-oligosaccharide and 17 parts of Lactobacillus gasseri LG08 freeze-dried powder.
The preparation method is the same as that of example 1.
Comparative example 4
This comparative example provides another probiotic composition formulated as follows:
the paint comprises the following components in parts by weight: 40 parts of polydextrose, 15 parts of resistant dextrin, 28 parts of fructo-oligosaccharide, 6 parts of lyophilized powder of Lactobacillus gasseri LG08, 4 parts of lyophilized powder of Bifidobacterium longum BL21, 4 parts of lyophilized powder of Lactobacillus salivarius LS97 and 3 parts of lyophilized powder of Lactobacillus plantarum Lp 90.
Wherein, the auxiliary materials of the polydextrose, the resistant dextrin and the fructo-oligosaccharide are not subjected to boiling granulation and are directly mixed with the probiotic freeze-dried powder; except for this, the preparation method was the same as in example 1.
Example 4
This example provides a method and results for testing the probiotic compositions mentioned in the foregoing examples 1-3 and comparative examples 1-4 (the formulation and process differences are shown in table 2), specifically as follows:
table 2 comparison table of each formulation difference
1. Experiment for reducing uric acid
The rat model with high uric acid is established by injecting potassium oxonate into abdominal cavity of rat and adding high purine diet, 90 male Wistar rats are adopted for experimental animals, and the experimental animals are raised at room temperature, fed by free diet, and are randomly divided into a normal control group, a hyperuricemia model group, an allopurines treatment group, an example 1 group, an example 2 group, an example 3 group, a comparative example 1 group, a comparative example 2 group and a comparative example 3 group (since the proportion of the comparative example 4 is consistent with that of the example 3, the difference is mainly whether the auxiliary material is granulated or not, the formula function comparison is not carried out on the comparative example 4), and 10 animals are each group. Then normal control group is fed with common mouse food, and 8 groups of rats are fed with high purine mouse food and injected with potassium oxonate-sodium carboxymethylcellulose suspension every 100g body weight per day by 250mg standard intraperitoneal injection. Starting on day 7, feeding high-purine mouse food and injecting potassium oxonate-sodium carboxymethylcellulose suspension into the abdominal cavity of the 6 groups, wherein the probiotic dry-preparation group comprises the following steps: gavage each rat 1 times daily the probiotic composition of examples 1-3 or comparative examples 1, 2, 3; allopurine group: each rat was given a standard intraperitoneal injection of allopurinol-sodium carboxymethyl cellulose suspension at 4.2mg per 100g body weight per day.
The intervention time lasts 21d, and the serum uric acid level, the fecal flora, the Lipopolysaccharide (LPS) and the inflammatory factor level are respectively detected before and after the intervention.
Wherein, the serum uric acid is detected by a TBHBA (2, 4, 6-tribromo-3-hydroxybenzoic acid) method.
Fecal flora detection is performed by continuously diluting a sample by 10 times, sampling 0.2mL, coating the sample on LBS, TPY, EMB, enterococcus agar plate and TSC culture medium, performing anaerobic culture at 37 ℃ for 48h, and performing plate counting detection on lactobacillus, bifidobacterium, enterobacter, enterococcus and clostridium perfringens respectively, wherein the results are shown in Table 3.
Lipopolysaccharide (LPS) detection was performed using a Lipopolysaccharide (LPS) detection kit (Shanghai Xinyu Biotechnology, inc.).
The inflammatory factor assay was performed using an enzyme linked immunosorbent assay kit (Abcam antibody coating, UK).
TABLE 3 detection of intestinal flora in rats (lg CFU/g feces)
Regarding the serum uric acid level detection, the results are shown in fig. 1, after 7 days of continuous intraperitoneal injection of potassium oxonate and supplementation of a high-purine diet, the blood uric acid content of rats in the model group, the allopurin drug intervention group and each probiotic intervention group is significantly increased, which indicates that the hyperuricemia model is successfully established. The intervention groups were then started with different formulations of probiotic composition and allopurin drug intervention for 21 days. From the results, examples 1 to 3 are superior to comparative examples 1 and 2. Therefore, when the addition amount of the probiotics is not in the range disclosed by the invention, the effect of the obtained probiotic composition on relieving uric acid is not obvious. In addition, compared with the auxiliary material formula (comparison example 3) consisting of single probiotics and prebiotics, the composite probiotic composition consisting of different probiotics and prebiotics can more effectively reduce the content of uric acid in serum under the same intake amount. Comparative examples 1 to 3 showed the best effect of reducing uric acid and the effect of example 3 was superior to that of the allopurin drug group.
The fecal flora change measurements are shown in Table 3. Bifidobacteria and lactic acid bacteria are generally considered to be beneficial microorganisms for the gut, helping to maintain the gut microbiota balance. On the contrary, enterobacteria, enterococci and clostridium perfringens are harmful bacteria and easily produce toxins, etc. The comparison result shows that the examples 1, 2 and 3 can effectively improve the content of lactic acid bacteria and bifidobacteria in the intestinal tract, and obviously reduce the content of harmful bacteria, namely enterobacteria, enterococcus and clostridium perfringens, and the effect is better than that of the comparative examples 1 and 2. Therefore, when the adding amount of the probiotics is not in the range disclosed by the invention, the obtained probiotic composition has no obvious effect of relieving the flora imbalance caused by hyperuricemia. In addition, the result of comparing the auxiliary material formula consisting of single probiotics and prebiotics (comparative example 3) shows that the adjusting effect of the probiotic composition on the flora is obviously better than that of the formula made of single probiotics. On the other hand, it was found after comparison of examples 1 to 3 that the formulation of example 3 has an optimum effect on the regulation of the intestinal flora.
Lipopolysaccharide and inflammatory factors are important factors influencing inflammation generation and can be used as one of important indexes reflecting in-vivo inflammation degree, hyperuricemia is often accompanied by side reaction of in-vivo inflammation aggravation, lipopolysaccharide can also be used as an important index reflecting intestinal permeability, and the increase of the content of lipopolysaccharide in blood indicates that the barrier effect of intestinal mucosa is reduced and the intestinal permeability is increased. On the other hand, lipopolysaccharide and proinflammatory factor IL-6 are important potential substances for generating inflammation, and the anti-inflammatory factor IL-10 can inhibit antigen presentation and inflammatory cytokine production. As shown in FIGS. 2 and 3, the results of measurement of LPS and inflammatory factors are shown in examples 1, 2 and 3, which are superior to those of comparative examples 1 and 2 in the effects of effectively reducing the contents of LPS and proinflammatory factor IL-6 and increasing the content of anti-inflammatory factor IL-10 in rats. It can be seen that when the addition amount of the probiotics is out of the range disclosed in the invention, the obtained probiotic composition has no obvious effect on relieving the side effect of hyperuricemia (inflammation improvement and intestinal mucosa barrier enhancement). In addition, the result of comparing the auxiliary material formula consisting of single probiotics and prebiotics (comparative example 3) shows that the regulation effect of the probiotic composition on the flora is obviously better than that of the formula made of single probiotics. On the other hand, it was found that the formulation of example 3 is optimal for the improvement of inflammation and enhancement of the intestinal mucosal barrier after comparative examples 1-3.
2. Stability test
The prepared probiotic composition is placed under the storage condition of 25 ℃ and 75% of relative humidity, the tracking monitoring period is set to be 2 years, the total number of lactobacillus of the product is detected in 0, 3, 6, 9, 12, 15, 18, 21 and 24 months respectively, the detection scheme adopts the lactobacillus detection method in the national standard GB 4789.35, and the detection result is shown in figure 4.
As can be seen from the results in fig. 4, the viable count of the lactic acid bacteria in the probiotic composition prepared by the boiling granulation treatment (example 3) is reduced slowly, and the survival rate of the lactic acid bacteria after 24 months can reach 66.7%, compared with the viable count of the lactic acid bacteria in the probiotic composition without the boiling granulation treatment (comparative example 4), which is reduced rapidly, and the survival rate of the lactic acid bacteria after 24 months is only 3.3%, indicating that the screening rate of the probiotic bacteria can be significantly reduced and the stability of the probiotic composition can be effectively improved by performing the boiling drying treatment on the auxiliary materials including polydextrose, resistant dextrin and fructo-oligosaccharide to control the water content and water activity.
The above description is only a preferred embodiment of the present invention and is not intended to limit the present invention, it should be noted that, for those skilled in the art, many modifications and variations can be made without departing from the technical principle of the present invention, and these modifications and variations should also be regarded as the protection scope of the present invention.
Claims (7)
1. A probiotic composition with a function of relieving hyperuricemia is characterized in that: the probiotic composition comprises the following components in parts by weight: 30-50 parts of polydextrose, 10-20 parts of resistant dextrin, 23-33 parts of fructo-oligosaccharide, 4-8 parts of lactobacillus gasseri LG08 freeze-dried powder, 2-6 parts of bifidobacterium longum BL21 freeze-dried powder, 3-5 parts of lactobacillus salivarius LS97 freeze-dried powder and 2-4 parts of lactobacillus plantarum Lp90 freeze-dried powder;
the Lactobacillus gasseri LG08 is preserved in China general microbiological culture Collection center (CGMCC) at 7, 18 and 2018 with the preservation number of CGMCC No. 16131;
the bifidobacterium longum BL21 is preserved in the common microorganism center of China Committee for culture Collection of microorganisms (CGMCC) No.10452 in 2015, 1 month and 27 days;
the Lactobacillus salivarius LS97 is preserved in the China general microbiological culture Collection center of the culture Collection of microorganisms in 2018, 12 months and 10 days, and the preservation number is CGMCC No. 1699;
the lactobacillus plantarum Lp90 is preserved in the general microbiological center of China Committee for culture Collection of microorganisms (CGMCC) No.10453 in 2015, 1 month and 27 days.
2. The probiotic composition with hyperuricemic effect according to claim 1, characterized in that: the viable count of the freeze-dried powder of the Lactobacillus gasseri LG08, the freeze-dried powder of the Bifidobacterium longum BL21, the freeze-dried powder of the Lactobacillus salivarius LS97 and the freeze-dried powder of the Lactobacillus plantarum Lp90 is 108-1010CFU/g, water content less than 4%, and water activity less than 0.1.
3. The probiotic composition with hyperuricemic effect according to claim 1, characterized in that: and the polydextrose, the resistant dextrin and the fructo-oligosaccharide are treated by a boiling granulation process, the water content is lower than 3.5 percent, and the water activity is lower than 0.1.
4. The probiotic composition with hyperuricemic effect according to claim 1, characterized in that: the probiotic composition comprises the following components in parts by weight: 40 parts of polydextrose, 15 parts of resistant dextrin, 28 parts of fructooligosaccharide, 6 parts of freeze-dried powder of Lactobacillus gasseri LG08, 4 parts of freeze-dried powder of Bifidobacterium longum BL21, 4 parts of freeze-dried powder of Lactobacillus salivarius LS97 and 3 parts of freeze-dried powder of Lactobacillus plantarum Lp 90.
5. Use of a probiotic composition having a hyperuricemic effect according to any one of claims 1 to 4 for the preparation of a medicament.
6. The method for preparing a probiotic composition with hyperuricemic effect according to any one of claims 1 to 4, wherein: the method specifically comprises the following steps:
s1, preparing a composite probiotic composition: activating Lactobacillus gasseri LG08, bifidobacterium longum BL21, lactobacillus salivarius LS97 and Lactobacillus plantarum Lp90 in an MRS culture medium test tube respectively to obtain primary seed liquid, inoculating the primary seed liquid into an MRS liquid culture medium respectively under the conditions of 30-38 ℃, carrying out anaerobic culture for 16-24h to obtain secondary seed liquid, inoculating the secondary seed liquid into a fermentation culture medium respectively for high-density culture with the inoculation amount of 0.5-5%, the culture conditions of 30-38 ℃, carrying out anaerobic culture for 12-18h, and centrifuging respectively to collect fermentation liquid;
s2, respectively centrifuging the fermentation liquor obtained in the step S1, collecting thalli, adding a protective emulsifier, respectively freezing and drying in vacuum to obtain bacterial powder, wherein the water content is controlled to be lower than 4%, and the water activity is controlled to be lower than 0.1, and then mixing the bacterial powder according to the parts by weight in the formula to obtain probiotic mixed powder A;
s3, auxiliary material granulation treatment: respectively sieving polydextrose, resistant dextrin and fructo-oligosaccharide with 80-100 mesh sieve, adding 3 powders into boiling granulator according to weight parts of the formula, taking out a part, adding water according to the proportion of 5-10% of the mass fraction to prepare liquid slurry, and controlling the temperature of the slurry at 60-70 ℃; controlling the water content of the materials to be lower than 3.5% and the water activity to be lower than 0.1 by the granulation process, taking the materials out of the pot, and obtaining auxiliary material mixed powder B;
and S4, mixing the probiotic mixed powder A and the auxiliary material mixed powder B to obtain the probiotic mixed powder.
7. The preparation method of probiotic composition with effect of relieving hyperuricemia according to claim 6, wherein the preparation method comprises the following steps: in the premixing stage of the granulation process, the air inlet temperature is controlled to be 55-60 ℃, the pressure of a spray gun is controlled to be 0.1-0.3MPa, the induced air frequency is controlled to be 23-26Hz, and the time is 15-20min without spraying slurry;
in the guniting stage 1, the air inlet temperature is controlled to be 55-60 ℃, the pressure of a spray gun is controlled to be 0.1-0.2MPa, the induced air frequency is controlled to be 23-26Hz, the guniting speed is 70-80r/min, and the time is 10-15min;
in the guniting stage 2, the air inlet temperature is controlled to be 55-60 ℃, the pressure of a spray gun is controlled to be 0.3-0.4MPa, the induced air frequency is controlled to be 25-28Hz, the guniting speed is 85-90r/min, and the time is 30-40min;
in the drying stage, the air inlet temperature is controlled to be 80-90 ℃, the spray gun and the guniting are stopped, the air inducing frequency is 26-30Hz, and the time is 15-20min;
and (3) controlling to stop air inlet, spray gun, induced air and guniting in the ash removal stage for 5-8min.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911330780.5A CN111195266B (en) | 2019-12-20 | 2019-12-20 | Probiotic composition with effect of relieving hyperuricemia and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201911330780.5A CN111195266B (en) | 2019-12-20 | 2019-12-20 | Probiotic composition with effect of relieving hyperuricemia and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN111195266A CN111195266A (en) | 2020-05-26 |
| CN111195266B true CN111195266B (en) | 2022-11-01 |
Family
ID=70741710
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201911330780.5A Active CN111195266B (en) | 2019-12-20 | 2019-12-20 | Probiotic composition with effect of relieving hyperuricemia and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111195266B (en) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110819569B (en) * | 2019-11-25 | 2022-12-06 | 微康益生菌(苏州)股份有限公司 | Lactobacillus salivarius LS97 and application thereof |
| CN111990647A (en) * | 2020-09-04 | 2020-11-27 | 内蒙古蒙牛乳业(集团)股份有限公司 | Dietary fiber composition, yoghourt and preparation method of yoghourt |
| CN112263669A (en) * | 2020-09-21 | 2021-01-26 | 康美华大基因技术有限公司 | Oligopeptide traditional Chinese medicine probiotic compound for relieving hyperuricemia |
| CN113181365B (en) * | 2021-03-12 | 2022-08-09 | 宁波倍益嘉生物科技有限公司 | Composition capable of reducing uric acid, dissolving uric acid crystals and tophus and application thereof |
| CN115252656B (en) * | 2021-07-19 | 2023-03-28 | 山东新时代药业有限公司 | Probiotic composition and application thereof |
| CN115039737B (en) * | 2021-08-09 | 2023-07-25 | 北京中医药大学 | Method for establishing uronate kidney deposition animal model |
| CN114917257A (en) * | 2022-05-19 | 2022-08-19 | 大禺(广州)健康研究有限公司 | Vaginal health probiotic composition, preparation method and application thereof |
| CN115518130A (en) * | 2022-09-16 | 2022-12-27 | 西双版纳傣密药业有限公司 | Traditional Chinese medicine formula for treating gout and preparation method thereof |
| JP7429076B1 (en) | 2023-04-04 | 2024-02-07 | ウィステリア製薬株式会社 | Powder containing lactic acid bacteria, disinfectant, oral care agent |
| CN116515694A (en) * | 2023-04-27 | 2023-08-01 | 广东南芯医疗科技有限公司 | Bifidobacterium longum NX-3 and application thereof in preparing uric acid lowering drugs |
| CN116585360B (en) * | 2023-05-24 | 2023-11-14 | 微康益生菌(苏州)股份有限公司 | Probiotic agent for improving chronic kidney disease and application thereof |
| CN118147021B (en) * | 2024-05-11 | 2024-07-09 | 微康益生菌(苏州)股份有限公司 | Composite probiotics for relieving gouty arthritis and application thereof |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008005834A (en) * | 2006-05-31 | 2008-01-17 | Meiji Milk Prod Co Ltd | Lactobacillus having blood uric acid level-reducing activity |
| CN101932697A (en) * | 2007-11-29 | 2010-12-29 | 明治乳业株式会社 | Lactic acid bacteria having action of lowering blood uric acid level |
| CN104839661A (en) * | 2015-04-10 | 2015-08-19 | 劲膳美生物科技股份有限公司 | High uric acid formula food |
| JP2017031102A (en) * | 2015-08-03 | 2017-02-09 | 雪印メグミルク株式会社 | Blood uric acid level reducing agent |
| CN108486007A (en) * | 2018-03-22 | 2018-09-04 | 嘉兴益诺康生物科技有限公司 | A kind of lactobacterium casei strains, probiotic composition and its application for reducing blood uric acid |
| CN110055199A (en) * | 2019-05-24 | 2019-07-26 | 吉林省命之元生物科技有限公司 | One UA149 plants of lactobacillus plantarum and its application |
| CN110313599A (en) * | 2018-03-30 | 2019-10-11 | 广州溯原生物科技有限公司 | A kind of anti-trioxypurine jelly and preparation method thereof |
-
2019
- 2019-12-20 CN CN201911330780.5A patent/CN111195266B/en active Active
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008005834A (en) * | 2006-05-31 | 2008-01-17 | Meiji Milk Prod Co Ltd | Lactobacillus having blood uric acid level-reducing activity |
| CN101932697A (en) * | 2007-11-29 | 2010-12-29 | 明治乳业株式会社 | Lactic acid bacteria having action of lowering blood uric acid level |
| CN104839661A (en) * | 2015-04-10 | 2015-08-19 | 劲膳美生物科技股份有限公司 | High uric acid formula food |
| JP2017031102A (en) * | 2015-08-03 | 2017-02-09 | 雪印メグミルク株式会社 | Blood uric acid level reducing agent |
| CN108486007A (en) * | 2018-03-22 | 2018-09-04 | 嘉兴益诺康生物科技有限公司 | A kind of lactobacterium casei strains, probiotic composition and its application for reducing blood uric acid |
| CN110313599A (en) * | 2018-03-30 | 2019-10-11 | 广州溯原生物科技有限公司 | A kind of anti-trioxypurine jelly and preparation method thereof |
| CN110055199A (en) * | 2019-05-24 | 2019-07-26 | 吉林省命之元生物科技有限公司 | One UA149 plants of lactobacillus plantarum and its application |
Non-Patent Citations (1)
| Title |
|---|
| 降血尿酸乳酸菌筛选及其对高尿酸血症模型大鼠作用研究;杨殿斌等;《中国微生态学杂志》;20130228;第25卷(第2期);第125-128页 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN111195266A (en) | 2020-05-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN111195266B (en) | Probiotic composition with effect of relieving hyperuricemia and application thereof | |
| CN109749957B (en) | Preparation and application of lactobacillus gasseri preparation with aquatic pathogenic bacteria antagonistic property | |
| CN114854643A (en) | Culture medium for promoting lactobacillus and bifidobacterium to co-proliferate and application thereof | |
| CN115094012B (en) | Preparation method and application of bacillus coagulans BC-HYC strain microbial inoculum | |
| CN108753650B (en) | Enterococcus faecium and composite microecological preparation prepared from enterococcus faecium | |
| US20190298784A1 (en) | Bifidobacterium breve cbt br3 strain for promotion of growth and nutraceutical composition for promotion of growth containing the same | |
| CN115039883B (en) | Use of lactobacillus plantarum TSP05 isolates to reduce purine content and uric acid levels | |
| CN116024130A (en) | Lactobacillus fermentum A21215 for reducing blood uric acid and application thereof | |
| CN116083325B (en) | Lactobacillus rhamnosus for improving helicobacter pylori related gastrointestinal diseases and application thereof | |
| CN111700157A (en) | Probiotic feed additive for improving immunity of aquatic animals | |
| CN105132321B (en) | A kind of VREF and the culture medium of high density solid state fermentation thereof and method | |
| CN113122467A (en) | Lactobacillus paracasei and composition thereof | |
| CN116083262A (en) | Lactobacillus plantarum strain with aquatic pathogenic bacteria antagonistic property and preparation and application of preparation thereof | |
| CN117264829A (en) | Lactobacillus plantarum for preventing and treating hypercholesterolemia, fermented product and application thereof | |
| CN115838675B (en) | Lactobacillus rhamnosus and composition and application thereof | |
| CN112980735A (en) | Clostridium butyricum, microbial inoculum, application of clostridium butyricum and microbial inoculum and preparation method of microbial inoculum | |
| CN114806975B (en) | Microecological preparation containing intestinal probiotics and preparation method and application thereof | |
| CN112940984B (en) | Compound lactobacillus preparation for resisting helicobacter pylori, reducing blood sugar, regulating intestines and stomach and increasing immunity and preparation method thereof | |
| CN118086155B (en) | Fermented lactobacillus mucilaginosus UN-P with weight-losing and lipid-lowering effects and application thereof | |
| CN114617202A (en) | Compound microecological preparation for improving disease resistance and production performance of ducks as well as preparation method and application of compound microecological preparation | |
| JP2019517810A (en) | Bacillus licheniformis NY1505 strain producing a large amount of α-glucosidase inhibitor | |
| CN116555074B (en) | Lactobacillus brevis JT1 and application thereof in preparation of hypoglycemic drugs | |
| CN118028148A (en) | Enterococcus faecalis MB2 and application thereof in preparation of anti-inflammatory and hypoglycemic foods and medicines | |
| CN107929329B (en) | Thrombolytic lipid-lowering probiotic composite bacteria traditional Chinese medicine granule and preparation method thereof | |
| KR20150145692A (en) | food include the microorganism products and method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| CB02 | Change of applicant information | ||
| CB02 | Change of applicant information |
Address after: 215000 No. 1033, long Qiao Road, Wujiang economic and Technological Development Zone, Suzhou, Jiangsu Applicant after: Weikang probiotics (Suzhou) Co.,Ltd. Address before: 215000 No. 1033, long Qiao Road, Wujiang economic and Technological Development Zone, Suzhou, Jiangsu Applicant before: JIANGSU WECARE BIOTECHNOLOGY Co.,Ltd. |
|
| GR01 | Patent grant | ||
| GR01 | Patent grant |