CN111153991A - Human SARS-CoV-2 monoclonal antibody and its preparation method and use - Google Patents
Human SARS-CoV-2 monoclonal antibody and its preparation method and use Download PDFInfo
- Publication number
- CN111153991A CN111153991A CN202010120349.4A CN202010120349A CN111153991A CN 111153991 A CN111153991 A CN 111153991A CN 202010120349 A CN202010120349 A CN 202010120349A CN 111153991 A CN111153991 A CN 111153991A
- Authority
- CN
- China
- Prior art keywords
- cov
- sars
- monoclonal antibody
- human
- virus
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 241000282414 Homo sapiens Species 0.000 title claims abstract description 63
- 241001678559 COVID-19 virus Species 0.000 title claims abstract description 54
- 238000002360 preparation method Methods 0.000 title claims abstract description 18
- 238000000034 method Methods 0.000 claims abstract description 37
- 201000003176 Severe Acute Respiratory Syndrome Diseases 0.000 claims abstract description 31
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 claims abstract description 29
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 claims abstract description 29
- 210000004027 cell Anatomy 0.000 claims abstract description 28
- 238000001514 detection method Methods 0.000 claims abstract description 25
- 101001024647 Severe acute respiratory syndrome coronavirus Nucleoprotein Proteins 0.000 claims abstract description 24
- 238000012216 screening Methods 0.000 claims abstract description 12
- 101001024637 Severe acute respiratory syndrome coronavirus 2 Nucleoprotein Proteins 0.000 claims abstract description 9
- 208000025370 Middle East respiratory syndrome Diseases 0.000 claims abstract description 8
- 108090001074 Nucleocapsid Proteins Proteins 0.000 claims abstract description 8
- 206010003445 Ascites Diseases 0.000 claims abstract description 7
- 239000003814 drug Substances 0.000 claims abstract description 7
- 230000004927 fusion Effects 0.000 claims abstract description 6
- 230000002163 immunogen Effects 0.000 claims abstract description 6
- 238000011725 BALB/c mouse Methods 0.000 claims abstract description 5
- 230000002401 inhibitory effect Effects 0.000 claims abstract description 5
- 230000009385 viral infection Effects 0.000 claims abstract description 5
- 230000002265 prevention Effects 0.000 claims abstract description 4
- 210000004989 spleen cell Anatomy 0.000 claims abstract description 3
- 125000003275 alpha amino acid group Chemical group 0.000 claims abstract 2
- 108090000623 proteins and genes Proteins 0.000 claims description 21
- 102000004169 proteins and genes Human genes 0.000 claims description 15
- 238000002965 ELISA Methods 0.000 claims description 14
- 241000315672 SARS coronavirus Species 0.000 claims description 12
- 241000699666 Mus <mouse, genus> Species 0.000 claims description 11
- 238000000746 purification Methods 0.000 claims description 11
- 241000699670 Mus sp. Species 0.000 claims description 4
- 239000013604 expression vector Substances 0.000 claims description 4
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 claims description 4
- 241000588724 Escherichia coli Species 0.000 claims description 3
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 3
- 239000013613 expression plasmid Substances 0.000 claims description 3
- 201000000050 myeloid neoplasm Diseases 0.000 claims description 3
- 239000002773 nucleotide Substances 0.000 claims description 3
- 125000003729 nucleotide group Chemical group 0.000 claims description 3
- 238000001261 affinity purification Methods 0.000 claims description 2
- 201000010099 disease Diseases 0.000 claims description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 2
- 230000036046 immunoreaction Effects 0.000 claims description 2
- 239000000825 pharmaceutical preparation Substances 0.000 claims 1
- 241000700605 Viruses Species 0.000 abstract description 16
- 210000004408 hybridoma Anatomy 0.000 abstract description 11
- 238000003745 diagnosis Methods 0.000 abstract description 8
- 230000003053 immunization Effects 0.000 abstract description 5
- 230000028327 secretion Effects 0.000 abstract description 5
- 210000004072 lung Anatomy 0.000 abstract description 3
- 238000011160 research Methods 0.000 abstract description 3
- 210000001185 bone marrow Anatomy 0.000 abstract 1
- 210000004881 tumor cell Anatomy 0.000 abstract 1
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 12
- 239000006228 supernatant Substances 0.000 description 12
- 241000711573 Coronaviridae Species 0.000 description 10
- 108091006197 SARS-CoV-2 Nucleocapsid Protein Proteins 0.000 description 9
- 102000036639 antigens Human genes 0.000 description 9
- 108091007433 antigens Proteins 0.000 description 9
- 239000000427 antigen Substances 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 7
- UQLDLKMNUJERMK-UHFFFAOYSA-L di(octadecanoyloxy)lead Chemical compound [Pb+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O UQLDLKMNUJERMK-UHFFFAOYSA-L 0.000 description 7
- 230000014509 gene expression Effects 0.000 description 7
- 238000005406 washing Methods 0.000 description 7
- 101710141454 Nucleoprotein Proteins 0.000 description 6
- 239000003480 eluent Substances 0.000 description 6
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 6
- 238000004153 renaturation Methods 0.000 description 6
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 6
- 239000000872 buffer Substances 0.000 description 5
- 238000000338 in vitro Methods 0.000 description 5
- 150000007523 nucleic acids Chemical group 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 238000010828 elution Methods 0.000 description 4
- 238000007710 freezing Methods 0.000 description 4
- 230000008014 freezing Effects 0.000 description 4
- 108020004707 nucleic acids Proteins 0.000 description 4
- 102000039446 nucleic acids Human genes 0.000 description 4
- 230000009465 prokaryotic expression Effects 0.000 description 4
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 239000007853 buffer solution Substances 0.000 description 3
- 238000005138 cryopreservation Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 108010078144 glutaminyl-glycine Proteins 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 229930027917 kanamycin Natural products 0.000 description 3
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 3
- 229960000318 kanamycin Drugs 0.000 description 3
- 229930182823 kanamycin A Natural products 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 230000001717 pathogenic effect Effects 0.000 description 3
- 238000007789 sealing Methods 0.000 description 3
- 230000003612 virological effect Effects 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 2
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 2
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 241000127282 Middle East respiratory syndrome-related coronavirus Species 0.000 description 2
- 206010035664 Pneumonia Diseases 0.000 description 2
- 206010036790 Productive cough Diseases 0.000 description 2
- 239000012980 RPMI-1640 medium Substances 0.000 description 2
- 230000003187 abdominal effect Effects 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 150000001413 amino acids Chemical group 0.000 description 2
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical class N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 2
- 108010008355 arginyl-glutamine Proteins 0.000 description 2
- 239000012148 binding buffer Substances 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 108010036413 histidylglycine Proteins 0.000 description 2
- 238000002649 immunization Methods 0.000 description 2
- 239000007928 intraperitoneal injection Substances 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 108010054155 lysyllysine Proteins 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 230000008520 organization Effects 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 239000012474 protein marker Substances 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- JQWHASGSAFIOCM-UHFFFAOYSA-M sodium periodate Chemical compound [Na+].[O-]I(=O)(=O)=O JQWHASGSAFIOCM-UHFFFAOYSA-M 0.000 description 2
- 210000003802 sputum Anatomy 0.000 description 2
- 208000024794 sputum Diseases 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 1
- 206010000060 Abdominal distension Diseases 0.000 description 1
- UCIYCBSJBQGDGM-LPEHRKFASA-N Ala-Arg-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)O)N UCIYCBSJBQGDGM-LPEHRKFASA-N 0.000 description 1
- BUDNAJYVCUHLSV-ZLUOBGJFSA-N Ala-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O BUDNAJYVCUHLSV-ZLUOBGJFSA-N 0.000 description 1
- FUSPCLTUKXQREV-ACZMJKKPSA-N Ala-Glu-Ala Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O FUSPCLTUKXQREV-ACZMJKKPSA-N 0.000 description 1
- RZZMZYZXNJRPOJ-BJDJZHNGSA-N Ala-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](C)N RZZMZYZXNJRPOJ-BJDJZHNGSA-N 0.000 description 1
- JAQNUEWEJWBVAY-WBAXXEDZSA-N Ala-Phe-Phe Chemical compound C([C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 JAQNUEWEJWBVAY-WBAXXEDZSA-N 0.000 description 1
- MAZZQZWCCYJQGZ-GUBZILKMSA-N Ala-Pro-Arg Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O MAZZQZWCCYJQGZ-GUBZILKMSA-N 0.000 description 1
- NCQMBSJGJMYKCK-ZLUOBGJFSA-N Ala-Ser-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O NCQMBSJGJMYKCK-ZLUOBGJFSA-N 0.000 description 1
- LSMDIAAALJJLRO-XQXXSGGOSA-N Ala-Thr-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O LSMDIAAALJJLRO-XQXXSGGOSA-N 0.000 description 1
- IETUUAHKCHOQHP-KZVJFYERSA-N Ala-Thr-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)N)[C@@H](C)O)C(O)=O IETUUAHKCHOQHP-KZVJFYERSA-N 0.000 description 1
- OTOXOKCIIQLMFH-KZVJFYERSA-N Arg-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N OTOXOKCIIQLMFH-KZVJFYERSA-N 0.000 description 1
- NONSEUUPKITYQT-BQBZGAKWSA-N Arg-Asn-Gly Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)NCC(=O)O)N)CN=C(N)N NONSEUUPKITYQT-BQBZGAKWSA-N 0.000 description 1
- IIABBYGHLYWVOS-FXQIFTODSA-N Arg-Asn-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O IIABBYGHLYWVOS-FXQIFTODSA-N 0.000 description 1
- OBFTYSPXDRROQO-SRVKXCTJSA-N Arg-Gln-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCN=C(N)N OBFTYSPXDRROQO-SRVKXCTJSA-N 0.000 description 1
- YNSGXDWWPCGGQS-YUMQZZPRSA-N Arg-Gly-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O YNSGXDWWPCGGQS-YUMQZZPRSA-N 0.000 description 1
- LXMKTIZAGIBQRX-HRCADAONSA-N Arg-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O LXMKTIZAGIBQRX-HRCADAONSA-N 0.000 description 1
- DNBMCNQKNOKOSD-DCAQKATOSA-N Arg-Pro-Gln Chemical compound NC(N)=NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O DNBMCNQKNOKOSD-DCAQKATOSA-N 0.000 description 1
- FRBAHXABMQXSJQ-FXQIFTODSA-N Arg-Ser-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O FRBAHXABMQXSJQ-FXQIFTODSA-N 0.000 description 1
- ASQKVGRCKOFKIU-KZVJFYERSA-N Arg-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O ASQKVGRCKOFKIU-KZVJFYERSA-N 0.000 description 1
- ISVACHFCVRKIDG-SRVKXCTJSA-N Arg-Val-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O ISVACHFCVRKIDG-SRVKXCTJSA-N 0.000 description 1
- ZZXMOQIUIJJOKZ-ZLUOBGJFSA-N Asn-Asn-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(N)=O ZZXMOQIUIJJOKZ-ZLUOBGJFSA-N 0.000 description 1
- GJFYPBDMUGGLFR-NKWVEPMBSA-N Asn-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CC(=O)N)N)C(=O)O GJFYPBDMUGGLFR-NKWVEPMBSA-N 0.000 description 1
- JTXVXGXTRXMOFJ-FXQIFTODSA-N Asn-Pro-Asn Chemical compound NC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(O)=O JTXVXGXTRXMOFJ-FXQIFTODSA-N 0.000 description 1
- HPBNLFLSSQDFQW-WHFBIAKZSA-N Asn-Ser-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O HPBNLFLSSQDFQW-WHFBIAKZSA-N 0.000 description 1
- ZNYKKCADEQAZKA-FXQIFTODSA-N Asn-Ser-Met Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(O)=O ZNYKKCADEQAZKA-FXQIFTODSA-N 0.000 description 1
- WLVLIYYBPPONRJ-GCJQMDKQSA-N Asn-Thr-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O WLVLIYYBPPONRJ-GCJQMDKQSA-N 0.000 description 1
- WUQXMTITJLFXAU-JIOCBJNQSA-N Asn-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)N)N)O WUQXMTITJLFXAU-JIOCBJNQSA-N 0.000 description 1
- BLQBMRNMBAYREH-UWJYBYFXSA-N Asp-Ala-Tyr Chemical compound N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O BLQBMRNMBAYREH-UWJYBYFXSA-N 0.000 description 1
- UQBGYPFHWFZMCD-ZLUOBGJFSA-N Asp-Asn-Asn Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O UQBGYPFHWFZMCD-ZLUOBGJFSA-N 0.000 description 1
- RDRMWJBLOSRRAW-BYULHYEWSA-N Asp-Asn-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O RDRMWJBLOSRRAW-BYULHYEWSA-N 0.000 description 1
- BKXPJCBEHWFSTF-ACZMJKKPSA-N Asp-Gln-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O BKXPJCBEHWFSTF-ACZMJKKPSA-N 0.000 description 1
- VAWNQIGQPUOPQW-ACZMJKKPSA-N Asp-Glu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O VAWNQIGQPUOPQW-ACZMJKKPSA-N 0.000 description 1
- TVIZQBFURPLQDV-DJFWLOJKSA-N Asp-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CC(=O)O)N TVIZQBFURPLQDV-DJFWLOJKSA-N 0.000 description 1
- NVFSJIXJZCDICF-SRVKXCTJSA-N Asp-Lys-Lys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)O)N NVFSJIXJZCDICF-SRVKXCTJSA-N 0.000 description 1
- YIDFBWRHIYOYAA-LKXGYXEUSA-N Asp-Ser-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YIDFBWRHIYOYAA-LKXGYXEUSA-N 0.000 description 1
- BJDHEININLSZOT-KKUMJFAQSA-N Asp-Tyr-Lys Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(O)=O BJDHEININLSZOT-KKUMJFAQSA-N 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 208000025721 COVID-19 Diseases 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 238000012270 DNA recombination Methods 0.000 description 1
- 238000009007 Diagnostic Kit Methods 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 241001198387 Escherichia coli BL21(DE3) Species 0.000 description 1
- NUMFTVCBONFQIQ-DRZSPHRISA-N Gln-Ala-Phe Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O NUMFTVCBONFQIQ-DRZSPHRISA-N 0.000 description 1
- WOACHWLUOFZLGJ-GUBZILKMSA-N Gln-Arg-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O WOACHWLUOFZLGJ-GUBZILKMSA-N 0.000 description 1
- JESJDAAGXULQOP-CIUDSAMLSA-N Gln-Arg-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)CN=C(N)N JESJDAAGXULQOP-CIUDSAMLSA-N 0.000 description 1
- ZPDVKYLJTOFQJV-WDSKDSINSA-N Gln-Asn-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O ZPDVKYLJTOFQJV-WDSKDSINSA-N 0.000 description 1
- PKVWNYGXMNWJSI-CIUDSAMLSA-N Gln-Gln-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKVWNYGXMNWJSI-CIUDSAMLSA-N 0.000 description 1
- YRWWJCDWLVXTHN-LAEOZQHASA-N Gln-Ile-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CCC(=O)N)N YRWWJCDWLVXTHN-LAEOZQHASA-N 0.000 description 1
- LGIKBBLQVSWUGK-DCAQKATOSA-N Gln-Leu-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LGIKBBLQVSWUGK-DCAQKATOSA-N 0.000 description 1
- ZBKUIQNCRIYVGH-SDDRHHMPSA-N Gln-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N ZBKUIQNCRIYVGH-SDDRHHMPSA-N 0.000 description 1
- JUUNNOLZGVYCJT-JYJNAYRXSA-N Gln-Phe-Lys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)N)N JUUNNOLZGVYCJT-JYJNAYRXSA-N 0.000 description 1
- XQDGOJPVMSWZSO-SRVKXCTJSA-N Gln-Pro-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(=O)N)N XQDGOJPVMSWZSO-SRVKXCTJSA-N 0.000 description 1
- OKARHJKJTKFQBM-ACZMJKKPSA-N Gln-Ser-Asn Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)N)C(=O)O)N OKARHJKJTKFQBM-ACZMJKKPSA-N 0.000 description 1
- YRHZWVKUFWCEPW-GLLZPBPUSA-N Gln-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O YRHZWVKUFWCEPW-GLLZPBPUSA-N 0.000 description 1
- MXOODARRORARSU-ACZMJKKPSA-N Glu-Ala-Ser Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)O)N MXOODARRORARSU-ACZMJKKPSA-N 0.000 description 1
- CUXJIASLBRJOFV-LAEOZQHASA-N Glu-Gly-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O CUXJIASLBRJOFV-LAEOZQHASA-N 0.000 description 1
- WGYHAAXZWPEBDQ-IFFSRLJSSA-N Glu-Val-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGYHAAXZWPEBDQ-IFFSRLJSSA-N 0.000 description 1
- BRFJMRSRMOMIMU-WHFBIAKZSA-N Gly-Ala-Asn Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O BRFJMRSRMOMIMU-WHFBIAKZSA-N 0.000 description 1
- VSVZIEVNUYDAFR-YUMQZZPRSA-N Gly-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN VSVZIEVNUYDAFR-YUMQZZPRSA-N 0.000 description 1
- UPOJUWHGMDJUQZ-IUCAKERBSA-N Gly-Arg-Arg Chemical compound NC(=N)NCCC[C@H](NC(=O)CN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O UPOJUWHGMDJUQZ-IUCAKERBSA-N 0.000 description 1
- JVACNFOPSUPDTK-QWRGUYRKSA-N Gly-Asn-Phe Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 JVACNFOPSUPDTK-QWRGUYRKSA-N 0.000 description 1
- NPSWCZIRBAYNSB-JHEQGTHGSA-N Gly-Gln-Thr Chemical compound [H]NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O NPSWCZIRBAYNSB-JHEQGTHGSA-N 0.000 description 1
- UFPXDFOYHVEIPI-BYPYZUCNSA-N Gly-Gly-Asp Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O UFPXDFOYHVEIPI-BYPYZUCNSA-N 0.000 description 1
- UUYBFNKHOCJCHT-VHSXEESVSA-N Gly-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN UUYBFNKHOCJCHT-VHSXEESVSA-N 0.000 description 1
- GMTXWRIDLGTVFC-IUCAKERBSA-N Gly-Lys-Glu Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O GMTXWRIDLGTVFC-IUCAKERBSA-N 0.000 description 1
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 1
- PCPOYRCAHPJXII-UWVGGRQHSA-N Gly-Lys-Met Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(O)=O PCPOYRCAHPJXII-UWVGGRQHSA-N 0.000 description 1
- OMOZPGCHVWOXHN-BQBZGAKWSA-N Gly-Met-Ser Chemical compound CSCC[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)CN OMOZPGCHVWOXHN-BQBZGAKWSA-N 0.000 description 1
- JJGBXTYGTKWGAT-YUMQZZPRSA-N Gly-Pro-Glu Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O JJGBXTYGTKWGAT-YUMQZZPRSA-N 0.000 description 1
- GLACUWHUYFBSPJ-FJXKBIBVSA-N Gly-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CN GLACUWHUYFBSPJ-FJXKBIBVSA-N 0.000 description 1
- CSMYMGFCEJWALV-WDSKDSINSA-N Gly-Ser-Gln Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(N)=O CSMYMGFCEJWALV-WDSKDSINSA-N 0.000 description 1
- YXTFLTJYLIAZQG-FJXKBIBVSA-N Gly-Thr-Arg Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N YXTFLTJYLIAZQG-FJXKBIBVSA-N 0.000 description 1
- YGHSQRJSHKYUJY-SCZZXKLOSA-N Gly-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN YGHSQRJSHKYUJY-SCZZXKLOSA-N 0.000 description 1
- PBJOQLUVSGXRSW-YTQUADARSA-N His-Trp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CNC3=CC=CC=C32)NC(=O)[C@H](CC4=CN=CN4)N)C(=O)O PBJOQLUVSGXRSW-YTQUADARSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101001123943 Human cytomegalovirus (strain AD169) Large structural phosphoprotein Proteins 0.000 description 1
- YKRYHWJRQUSTKG-KBIXCLLPSA-N Ile-Ala-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N YKRYHWJRQUSTKG-KBIXCLLPSA-N 0.000 description 1
- NCSIQAFSIPHVAN-IUKAMOBKSA-N Ile-Asn-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N NCSIQAFSIPHVAN-IUKAMOBKSA-N 0.000 description 1
- LWWILHPVAKKLQS-QXEWZRGKSA-N Ile-Gly-Met Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CCSC)C(=O)O)N LWWILHPVAKKLQS-QXEWZRGKSA-N 0.000 description 1
- 241000581650 Ivesia Species 0.000 description 1
- RFUBXQQFJFGJFV-GUBZILKMSA-N Leu-Asn-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O RFUBXQQFJFGJFV-GUBZILKMSA-N 0.000 description 1
- YKNBJXOJTURHCU-DCAQKATOSA-N Leu-Asp-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N YKNBJXOJTURHCU-DCAQKATOSA-N 0.000 description 1
- DLFAACQHIRSQGG-CIUDSAMLSA-N Leu-Asp-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O DLFAACQHIRSQGG-CIUDSAMLSA-N 0.000 description 1
- WQWSMEOYXJTFRU-GUBZILKMSA-N Leu-Glu-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O WQWSMEOYXJTFRU-GUBZILKMSA-N 0.000 description 1
- AVEGDIAXTDVBJS-XUXIUFHCSA-N Leu-Ile-Arg Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AVEGDIAXTDVBJS-XUXIUFHCSA-N 0.000 description 1
- IAJFFZORSWOZPQ-SRVKXCTJSA-N Leu-Leu-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O IAJFFZORSWOZPQ-SRVKXCTJSA-N 0.000 description 1
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 1
- UCXQIIIFOOGYEM-ULQDDVLXSA-N Leu-Pro-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 UCXQIIIFOOGYEM-ULQDDVLXSA-N 0.000 description 1
- YEIYAQQKADPIBJ-GARJFASQSA-N Lys-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCCCN)N)C(=O)O YEIYAQQKADPIBJ-GARJFASQSA-N 0.000 description 1
- WTZUSCUIVPVCRH-SRVKXCTJSA-N Lys-Gln-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N WTZUSCUIVPVCRH-SRVKXCTJSA-N 0.000 description 1
- DCRWPTBMWMGADO-AVGNSLFASA-N Lys-Glu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O DCRWPTBMWMGADO-AVGNSLFASA-N 0.000 description 1
- PBLLTSKBTAHDNA-KBPBESRZSA-N Lys-Gly-Phe Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PBLLTSKBTAHDNA-KBPBESRZSA-N 0.000 description 1
- WOEDRPCHKPSFDT-MXAVVETBSA-N Lys-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCCCN)N WOEDRPCHKPSFDT-MXAVVETBSA-N 0.000 description 1
- PYFNONMJYNJENN-AVGNSLFASA-N Lys-Lys-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N PYFNONMJYNJENN-AVGNSLFASA-N 0.000 description 1
- YXPJCVNIDDKGOE-MELADBBJSA-N Lys-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)N)C(=O)O YXPJCVNIDDKGOE-MELADBBJSA-N 0.000 description 1
- PLDJDCJLRCYPJB-VOAKCMCISA-N Lys-Lys-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PLDJDCJLRCYPJB-VOAKCMCISA-N 0.000 description 1
- HKXSZKJMDBHOTG-CIUDSAMLSA-N Lys-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCCN HKXSZKJMDBHOTG-CIUDSAMLSA-N 0.000 description 1
- YCJCEMKOZOYBEF-OEAJRASXSA-N Lys-Thr-Phe Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O YCJCEMKOZOYBEF-OEAJRASXSA-N 0.000 description 1
- GILLQRYAWOMHED-DCAQKATOSA-N Lys-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN GILLQRYAWOMHED-DCAQKATOSA-N 0.000 description 1
- TUSOIZOVPJCMFC-FXQIFTODSA-N Met-Asp-Asp Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O TUSOIZOVPJCMFC-FXQIFTODSA-N 0.000 description 1
- RDLSEGZJMYGFNS-FXQIFTODSA-N Met-Ser-Asp Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O RDLSEGZJMYGFNS-FXQIFTODSA-N 0.000 description 1
- 101900332836 Middle East respiratory syndrome-related coronavirus Nucleoprotein Proteins 0.000 description 1
- 108010079364 N-glycylalanine Proteins 0.000 description 1
- 241001292005 Nidovirales Species 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- QMMRHASQEVCJGR-UBHSHLNASA-N Phe-Ala-Pro Chemical compound C([C@H](N)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=CC=C1 QMMRHASQEVCJGR-UBHSHLNASA-N 0.000 description 1
- WEDZFLRYSIDIRX-IHRRRGAJSA-N Phe-Ser-Arg Chemical compound NC(=N)NCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=CC=C1 WEDZFLRYSIDIRX-IHRRRGAJSA-N 0.000 description 1
- 229920002535 Polyethylene Glycol 1500 Polymers 0.000 description 1
- DZZCICYRSZASNF-FXQIFTODSA-N Pro-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 DZZCICYRSZASNF-FXQIFTODSA-N 0.000 description 1
- ILMLVTGTUJPQFP-FXQIFTODSA-N Pro-Asp-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O ILMLVTGTUJPQFP-FXQIFTODSA-N 0.000 description 1
- DWGFLKQSGRUQTI-IHRRRGAJSA-N Pro-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1 DWGFLKQSGRUQTI-IHRRRGAJSA-N 0.000 description 1
- RCYUBVHMVUHEBM-RCWTZXSCSA-N Pro-Pro-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O RCYUBVHMVUHEBM-RCWTZXSCSA-N 0.000 description 1
- BGWKULMLUIUPKY-BQBZGAKWSA-N Pro-Ser-Gly Chemical compound OC(=O)CNC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 BGWKULMLUIUPKY-BQBZGAKWSA-N 0.000 description 1
- CXGLFEOYCJFKPR-RCWTZXSCSA-N Pro-Thr-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O CXGLFEOYCJFKPR-RCWTZXSCSA-N 0.000 description 1
- 101000933967 Pseudomonas phage KPP25 Major capsid protein Proteins 0.000 description 1
- 208000037847 SARS-CoV-2-infection Diseases 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- YQHZVYJAGWMHES-ZLUOBGJFSA-N Ser-Ala-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YQHZVYJAGWMHES-ZLUOBGJFSA-N 0.000 description 1
- HQTKVSCNCDLXSX-BQBZGAKWSA-N Ser-Arg-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O HQTKVSCNCDLXSX-BQBZGAKWSA-N 0.000 description 1
- QVOGDCQNGLBNCR-FXQIFTODSA-N Ser-Arg-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O QVOGDCQNGLBNCR-FXQIFTODSA-N 0.000 description 1
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 1
- NUEHQDHDLDXCRU-GUBZILKMSA-N Ser-Pro-Arg Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O NUEHQDHDLDXCRU-GUBZILKMSA-N 0.000 description 1
- KQNDIKOYWZTZIX-FXQIFTODSA-N Ser-Ser-Arg Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KQNDIKOYWZTZIX-FXQIFTODSA-N 0.000 description 1
- VAIWUNAAPZZGRI-IHPCNDPISA-N Ser-Trp-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)NC(=O)[C@H](CO)N VAIWUNAAPZZGRI-IHPCNDPISA-N 0.000 description 1
- BSNZTJXVDOINSR-JXUBOQSCSA-N Thr-Ala-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O BSNZTJXVDOINSR-JXUBOQSCSA-N 0.000 description 1
- OYTNZCBFDXGQGE-XQXXSGGOSA-N Thr-Gln-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](C)C(=O)O)N)O OYTNZCBFDXGQGE-XQXXSGGOSA-N 0.000 description 1
- GUZGCDIZVGODML-NKIYYHGXSA-N Thr-Gln-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N)O GUZGCDIZVGODML-NKIYYHGXSA-N 0.000 description 1
- MSIYNSBKKVMGFO-BHNWBGBOSA-N Thr-Gly-Pro Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N1CCC[C@@H]1C(=O)O)N)O MSIYNSBKKVMGFO-BHNWBGBOSA-N 0.000 description 1
- MEJHFIOYJHTWMK-VOAKCMCISA-N Thr-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)[C@@H](C)O MEJHFIOYJHTWMK-VOAKCMCISA-N 0.000 description 1
- NCXVJIQMWSGRHY-KXNHARMFSA-N Thr-Leu-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N)O NCXVJIQMWSGRHY-KXNHARMFSA-N 0.000 description 1
- CJXURNZYNHCYFD-WDCWCFNPSA-N Thr-Lys-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O CJXURNZYNHCYFD-WDCWCFNPSA-N 0.000 description 1
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 1
- MXDOAJQRJBMGMO-FJXKBIBVSA-N Thr-Pro-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O MXDOAJQRJBMGMO-FJXKBIBVSA-N 0.000 description 1
- NLWDSYKZUPRMBJ-IEGACIPQSA-N Thr-Trp-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(C)C)C(=O)O)N)O NLWDSYKZUPRMBJ-IEGACIPQSA-N 0.000 description 1
- ABCLYRRGTZNIFU-BWAGICSOSA-N Thr-Tyr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N)O ABCLYRRGTZNIFU-BWAGICSOSA-N 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- UIRVSEPRMWDVEW-RNXOBYDBSA-N Trp-Tyr-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CNC4=CC=CC=C43)N UIRVSEPRMWDVEW-RNXOBYDBSA-N 0.000 description 1
- IELISNUVHBKYBX-XDTLVQLUSA-N Tyr-Ala-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 IELISNUVHBKYBX-XDTLVQLUSA-N 0.000 description 1
- JRXKIVGWMMIIOF-YDHLFZDLSA-N Tyr-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CC=C(C=C1)O)N JRXKIVGWMMIIOF-YDHLFZDLSA-N 0.000 description 1
- DWAMXBFJNZIHMC-KBPBESRZSA-N Tyr-Leu-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O DWAMXBFJNZIHMC-KBPBESRZSA-N 0.000 description 1
- GPLTZEMVOCZVAV-UFYCRDLUSA-N Tyr-Tyr-Arg Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)C1=CC=C(O)C=C1 GPLTZEMVOCZVAV-UFYCRDLUSA-N 0.000 description 1
- GVNLOVJNNDZUHS-RHYQMDGZSA-N Val-Thr-Lys Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O GVNLOVJNNDZUHS-RHYQMDGZSA-N 0.000 description 1
- KJFBXCFOPAKPTM-BZSNNMDCSA-N Val-Trp-Val Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O)=CNC2=C1 KJFBXCFOPAKPTM-BZSNNMDCSA-N 0.000 description 1
- 108010003533 Viral Envelope Proteins Proteins 0.000 description 1
- 108010067390 Viral Proteins Proteins 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 108010044940 alanylglutamine Proteins 0.000 description 1
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 1
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 1
- 235000011130 ammonium sulphate Nutrition 0.000 description 1
- 238000011091 antibody purification Methods 0.000 description 1
- 108010068380 arginylarginine Proteins 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000007910 cell fusion Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 238000003115 checkerboard titration Methods 0.000 description 1
- 238000003759 clinical diagnosis Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 238000003113 dilution method Methods 0.000 description 1
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 238000013399 early diagnosis Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 238000011067 equilibration Methods 0.000 description 1
- 235000019441 ethanol Nutrition 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 238000012268 genome sequencing Methods 0.000 description 1
- 239000007973 glycine-HCl buffer Substances 0.000 description 1
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 1
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 1
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 1
- 108010072405 glycyl-aspartyl-glycine Proteins 0.000 description 1
- 108010079413 glycyl-prolyl-glutamic acid Proteins 0.000 description 1
- 108010089804 glycyl-threonine Proteins 0.000 description 1
- 108010010147 glycylglutamine Proteins 0.000 description 1
- 108010087823 glycyltyrosine Proteins 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000003771 laboratory diagnosis Methods 0.000 description 1
- 230000031700 light absorption Effects 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 108010003700 lysyl aspartic acid Proteins 0.000 description 1
- 108010056582 methionylglutamic acid Proteins 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 1
- 108010089198 phenylalanyl-prolyl-arginine Proteins 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 239000003761 preservation solution Substances 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 108010070643 prolylglutamic acid Proteins 0.000 description 1
- 108010015796 prolylisoleucine Proteins 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 238000010079 rubber tapping Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 238000009589 serological test Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 108010026333 seryl-proline Proteins 0.000 description 1
- 108010007375 seryl-seryl-seryl-arginine Proteins 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 239000012089 stop solution Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 108010051110 tyrosyl-lysine Proteins 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/70—Vectors or expression systems specially adapted for E. coli
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56983—Viruses
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/577—Immunoassay; Biospecific binding assay; Materials therefor involving monoclonal antibodies binding reaction mechanisms characterised by the use of monoclonal antibodies; monoclonal antibodies per se are classified with their corresponding antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/20011—Coronaviridae
- C12N2770/20022—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Organic Chemistry (AREA)
- Virology (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Hematology (AREA)
- Biotechnology (AREA)
- Urology & Nephrology (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Pathology (AREA)
- Zoology (AREA)
- Analytical Chemistry (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Engineering & Computer Science (AREA)
- General Physics & Mathematics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Plant Pathology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Gastroenterology & Hepatology (AREA)
Abstract
The invention discloses a human SARS-CoV-2 monoclonal antibody, its preparation method is: using SARS-CoV Nucleocapsid recombinant protein as immunogen, immunizing BALB/c mouse, making fusion and subcloning of mouse spleen cell and mouse bone marrow tumour cell, then making lots of repeated screening and domestication of cell strain by using commercial products SARS-CoV-2Nucleocapsid and MERS Nucleocapsid, finally successfully obtaining high affinity and high specificity anti SARS-CoV-2N monoclonal antibody hybridoma cell strain, finally preparing and purifying ascites so as to obtain monoclonal antibody; the amino acid sequence of the SARS-CoV Nucleocapsid recombinant protein is shown in SEQ ID NO. 1. The invention also discloses the application of the monoclonal antibody in preparing SARS-CoV-2 virus detection products and in preparing SARS-CoV-2 virus inhibiting medicine. The monoclonal antibody of the invention can detect SARS-CoV-2 in human throat swab/lung secretion and other samples by a double antibody sandwich method, can be applied to a SARS-CoV-2 detection kit, and is suitable for diagnosis, prevention and control of SARS-CoV-2 virus infection, scientific research of virus and the like.
Description
Technical Field
The invention relates to a human SARS-CoV-2 monoclonal antibody and its preparation method and application, belonging to the technical field of monoclonal antibody preparation.
Background
On day 9/1 of 2020, scientists observed under an electron microscope that the pathogen responsible for this pneumonia presented an enveloped typical coronavirus morphology with a coronal appearance. Meanwhile, the sequencing result of pathogenic genome shows that the nucleic acid sequence of the coronavirus is not completely consistent with the 6 coronavirus (such as SARS, MERS and the like) discovered before. Thus, the World Health Organization (WHO) named the new virus at 12.1/2020: 2019Novel Coronavirus (2019Novel Coronavir, 2019-nCoV), day 11 month 2, the International Committee for Classification of viruses (ICTV) named SARS-CoV-2. The virus has long incubation period and strong infectivity, and as long as 24 days 2, 18 and 18 in 2020, 74185 cases (severe cases 11977) have been diagnosed, 14376 cases are cured cumulatively, 2004 cases are died cumulatively, and 5248 cases are suspected. Close contacts 574418 were cumulatively tracked, close contacts 135881 who were still under medical observation (information from the national health Care official website). There have been 25 diagnosed cases in countries and regions worldwide (world health organization 2/10/2020), which have constituted the emergent public health event of international concern. At present, specific targeted drug treatment is not available, so that the prevention of healthy people, the diagnosis and isolation of suspected and confirmed cases and treatment work are very important.
Coronaviruses belong to the order of the nested viruses (Nidovirales), the family of Coronaviridae (Coronaviridae), and the genus coronaviruses (Coronavirus), and are the largest viruses of the RNA viruses known to humans at present, and have a length of 27 to 32 kb. Coronaviruses have at least 4 major structural proteins, including spike (S), membrane (M), envelope (E) and nucleocapsid (N) proteins, which are essential for binding of the virus to cellular receptors and are essential for the complete structure of the virus. Wherein the nucleocapsid protein (N) is a basic phosphoprotein, the central region of which is combined with the viral genome RNA to form a coiled nucleocapsid helix, which is the core structure of the viral genetic material coated therein and is one of the viral proteins with the highest expression in the infected cells.
Current laboratory diagnosis of viral infections generally involves 3 methods: molecular diagnostics based on PCR technology; immunological-based serological tests; pathogenic biological diagnosis based on in vitro virus culture. In vitro virus culture is generally not a routine diagnostic method because it is highly demanding both on assay conditions and on the skill of the operator. With the continuous innovation of gene sequencing technology in recent years, scientists complete the genome sequencing of viruses at the initial stage of the new coronary pneumonia epidemic situation, and then a plurality of nucleic acid detection kits are developed and put into use. At present, laboratory detection of suspected cases of the new coronavirus is multipurpose nucleic acid detection (PCR), but most of nucleic acid detection samples are upper respiratory tract samples (throat swab and sputum are main samples), and the collection process has great exposure risk to medical workers. And the experimental process is long, about 4-5 h, and pollution (namely false positive or false negative results) is easy to occur. And due to frequent virus variation, primer design and the like, the nucleic acid detection method also has the problems of high false negative, low coincidence rate of detection results of different manufacturers and the like in the practical application process. The diagnosis method for detecting the virus antigen can greatly reduce the risk, and because serum is generally adopted as a detection sample, the sample is convenient to collect and low in toxic content, meanwhile, a complex processing procedure of the sample in a laboratory is omitted, a detection result can be obtained in 15-20 min, the operation is simple, and the current huge clinical diagnosis and treatment pressure can be greatly relieved while medical care personnel are protected.
The key to the development of SARS-CoV-2 virus antigen detection kit is to find out the antibody with high specificity and high affinity. Compared with polyclonal antibodies, the monoclonal antibody has the advantage of high specificity, so that the detection specificity and the anti-interference capability of the constructed kit can be greatly improved. In addition, because the protein on the virus envelope is easy to be mutated, the N protein is relatively stable, is the main antigen part of the virus, and has stronger antigenicity than other proteins. And at present, no commercial diagnostic kit taking SARS-CoV-2N protein monoclonal antibody as a core raw material is available.
Disclosure of Invention
Aiming at the prior art, the invention provides a human SARS-CoV-2 monoclonal antibody, a preparation method and application thereof. The invention utilizes DNA recombination technology to synthesize SARS-CoV Nucleocapsid full-length gene, constructs into prokaryotic expression vector pET28a, and obtains a large amount of SARS-CoVNucleocapsid protein in vitro through optimized fermentation, purification and renaturation processes; the monoclonal antibody of anti-human SARS-CoVNucleucoapsid is successfully prepared by immunizing BALB/c female mice and adopting a hybridoma technology; then, a large amount of repeated screening and cell strain domestication are carried out on the commercialized products SARS-CoV-2Nucleocapsid and MERSNuclocapsid, and finally, the anti-SARS-CoV-2N monoclonal antibody with high affinity and high specificity is successfully obtained; on the basis of the above-mentioned diagnosis method, a quick and quick diagnosis method for SARS-CoV-2 and its diagnosis reagent are developed, so that it provides a quick and accurate method for clinically diagnosing SARS-CoV-2 infection, at the same time it also provides strong support for scientific research of new virus.
The invention is realized by the following technical scheme:
a human SARS-CoV-2 monoclonal antibody, using SARS-CoV nucleoapsid recombinant protein as immunogen to immunize mouse to obtain monoclonal antibody; the amino acid sequence of the SARS-CoV Nucleocapsid recombinant protein is shown as SEQID NO. 1.
The SARS-CoV Nucleocapsid recombinant protein is prepared by the following method: human SARS-CoV nucleoapsid gene (nucleotide sequence shown in SEQ ID NO.2) is inserted into expression vector pET28a to construct pET28a-SARS-CoV-N fusion expression plasmid, and then converted into colibacillus, and then induced by IPTG, purified and renatured to obtain SARS-CoV nucleoapsid recombinant protein.
Amino acid sequence of SARS-CoV nucleoapsid recombinant protein (SEQ ID NO. 1):
MSDNGPQSNQRSAPRITFGGPTDSTDNNQNGGRNGARPKQRRPQGLPNNTASWFTALTQHGKEELRFPRGQGVPINTNSGPDDQIGYYRRATRRVRGGDGKMKELSPRWYFYYLGTGPEASLPYGANKEGIVWVATEGALNTPKDHIGTRNPNNNAATVLQLPQGTTLPKGFYAEGSRGGSQASSRSSSRSRGNSRNSTPGSSRGNSPARMASGGGETALALLLLDRLNQLESKVSGKGQQQQGQTVTKKSAAEASKKPRQKRTATKQYNVTQAFGRRGPEQTQGNFGDQDLIRQGTDYKHWPQIAQFAPSASAFFGMSRIGMEVTPSGTWLTYHGAIKLDDKDPQFKDNVILLNKHIDAYKTFPPTEPKKDKKKKTDEAQPLPQRQKKQPTVTLLPAADMDDFSRQLQNSMSGASADSTQA
nucleotide sequence of human SARS-CoV nucleoapsid gene (SEQ ID NO. 2):
atgtctgataatggaccccaatcaaaccaacgtagtgccccccgcattacatttggtggacccacagattcaactgacaataaccagaatggaggacgcaatggggcaaggccaaaacagcgccgaccccaaggtttacccaataatactgcgtcttggttcacagctctcactcagcatggcaaggaggaacttagattccctcgaggccagggcgttccaatcaacaccaatagtggtccagatgaccaaattggctactaccgaagagctacccgacgagttcgtggtggtgacggcaaaatgaaagagctcagccccagatggtacttctattacctaggaactggcccagaagcttcacttccctacggcgctaacaaagaaggcatcgtatgggttgcaactgagggagccttgaatacacccaaagaccacattggcacccgcaatcctaataacaatgctgccaccgtgctacaacttcctcaaggaacaacattgccaaaaggcttctacgcagagggaagcagaggcggcagtcaagcctcttctcgctcctcatcacgtagtcgcggtaattcaagaaattcaactcctggcagcagtaggggaaattctcctgctcgaatggctagcggaggtggtgaaactgccctcgcgctattgctgctagacagattgaaccagcttgagagcaaagtttctggtaaaggccaacaacaacaaggccaaactgtcactaagaaatctgctgctgaggcatctaaaaagcctcgccaaaaacgtactgccacaaaacagtacaacgtcactcaagcatttgggagacgtggtccagaacaaacccaaggaaatttcggggaccaagacctaatcagacaaggaactgattacaaacattggccgcaaattgcacaatttgctccaagtgcctctgcattctttggaatgtcacgcattggcatggaagtcacaccttcgggaacatggctgacttatcatggagccattaaattggatgacaaagatccacaattcaaagacaacgtcatactgctgaacaagcacattgacgcatacaaaacattcccaccaacagagcctaaaaaggacaaaaagaaaaagactgatgaagctcagcctttgccgcagagacaaaagaagcagcccactgtgactcttcttcctgcggctgacatggatgatttctccagacaacttcaaaattccatgagtggagcttctgctgattcaactcaggcataa
the preparation method of the human SARS-CoV-2 monoclonal antibody comprises the following steps: using SARS-CoV Nucleocapsid recombinant protein as immunogen to immunize BALB/c mouse, then making fusion and subcloning of mouse spleen cell and mouse myeloma cell, and making specific screening and domestication by using SARS-CoV-2Nucleocapsid and MERS Nucleocapsid to finally obtain monoclonal cell strain with good specificity and high potency, then making ascites preparation and purification so as to obtain a large quantity of monoclonal antibody resisting human SARS-CoV Nucleocapsid.
Preferably, female mice 8-12 weeks old are selected for said BALB/c mice.
Preferably, the screening method is an ELISA method (enzyme-linked immunosorbent assay).
Preferably, the purification method is Protein a affinity purification.
The human SARS-CoV-2 monoclonal antibody is applied in preparing SARS-CoV-2 virus detecting product.
The human SARS-CoV-2 monoclonal antibody is applied in preparing medicine for inhibiting SARS-CoV-2 virus.
The human SARS-CoV-2 monoclonal antibody is applied in preparing medicine preparation for preventing and treating SARS-CoV-2 virus infection caused diseases.
A method for detecting the level of SARS-CoV-2 virus for non-diagnostic purposes: contacting the sample to be detected with the human SARS-CoV-2 monoclonal antibody, and detecting the immunoreaction of the sample and the human SARS-CoV-2 monoclonal antibody. The sample to be detected is selected from sputum, oral/nasopharyngeal secretions, pulmonary secretions and the like.
A reagent kit for detecting SARS-CoV-2 virus, which comprises the above-mentioned human SARS-CoV-2 monoclonal antibody.
A medicine for inhibiting SARS-CoV-2 virus contains the above-mentioned human SARS-CoV-2 monoclonal antibody.
The invention provides an expression and purification method of human SARS-CoV Nucleocapsid in vitro recombinant protein, which solves the problems of difficult acquisition of natural human SARS-CoV Nucleocapsid protein, and in vitro expression yield, purity and stability. Moreover, the SARS-CoV Nucleocapsid recombinant protein is used to immunize animal, so that it can prepare wider anti-SARS-CoVNucleocapsid antibody, and through the specific screening and domestication of SARS-CoV-2Nucleocapsid and MERS Nucleocapsid, it can prepare mouse anti-human SARS-CoV-2N monoclonal antibody with high affinity and good specificity.
The monoclonal antibody of the invention can detect SARS-CoV-2 in human throat swab/lung secretion and other samples by a double antibody sandwich method, can be applied to a SARS-CoV-2 detection kit, and is suitable for diagnosis, prevention and control of SARS-CoV-2 virus infection, scientific research of virus and the like.
The invention obtains the hybridoma cell strain N6A8 and hybridoma cell strain N7A7 which can generate the anti-human SARS-CoV-2N monoclonal antibody, and the anti-human SARS-CoV-2N monoclonal antibody N6A8 and N7A7 by screening, the antibody can detect the SARS-CoV-2N concentration in the human body fluid by a double antibody sandwich method, and has important application prospect. The invention establishes the ELISA method for detecting the human SARS-CoV-2N protein, realizes high sensitivity (pg level) and specificity of detection, can quickly and accurately determine the content of the human SARS-CoV-2N protein in samples such as throat swab/lung secretion and the like, and can provide reliable clinical reference value for early diagnosis and early treatment of SARS-CoV-2 infected patients.
The various terms and phrases used herein have the ordinary meaning as is well known to those skilled in the art.
Drawings
FIG. 1: the recombinant plasmid pET28a-SARS-CoV-N small sample induces expression of SDS-PAGE identification result, wherein, M: the pre-stained protein Marker, lanes 1-3 are respectively pET28a-SARS-CoV-N bacterial liquid post-ultrasonic precipitation after induction, pET28a-SARS-CoV-N bacterial liquid ultrasonic supernatant after induction, and pET28a-SARS-CoV-N bacterial liquid before induction.
FIG. 2: the result of SDS-PAGE identification after the purification and renaturation of human SARS-CoV-N recombinant protein, wherein, M: pre-staining a protein Marker; lane 1: after purification and renaturation, human SARS-CoV-N recombinant protein is obtained.
FIG. 3: the WB identification result of the monoclonal antibody (N7A7) against human SARS-CoV-2N protein.
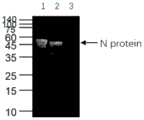
FIG. 4: the WB identification result of the anti-human SARS-CoV-2N protein monoclonal antibody (N6A8), wherein 1, recombinant human SARS-CoV-2N protein (E.coli); 2. recombinant human SARS-CoV N protein (e.coli); 3. recombinant human MERS-CoV N protein (Baculovirus-instect Cells).
FIG. 5: anti-human SARS-CoV-2N protein monoclonal antibody and recombinant prokaryotic expression SARS-CoV-2-N, SARS-CoV-N and MERS-CoV-N protein antigen ELISA detection result.
Detailed Description
The present invention will be further described with reference to the following examples. However, the scope of the present invention is not limited to the following examples. It will be understood by those skilled in the art that various changes and modifications may be made to the invention without departing from the spirit and scope of the invention.
The instruments, reagents, materials and the like used in the following examples are conventional instruments, reagents, materials and the like in the prior art and are commercially available in a normal manner unless otherwise specified. Unless otherwise specified, the experimental methods, detection methods, and the like described in the following examples are conventional experimental methods, detection methods, and the like in the prior art.
EXAMPLE 1 preparation of human SARS-CoV Nucleocapsid recombinant protein
(1) Construction of human SARS-CoV nucleoapsid prokaryotic expression vector
Human SARS-CoV nucleoapsid gene sequence SEQ ID NO.2 was synthesized artificially, inserted into NdeI and XhoI double-digested prokaryotic expression plasmid pET2a, and transformed into competent Escherichia coli BL21(DE 3). Screening positive clone on 0.1g/L kanamycin resistant plate, inducing with 1mM IPTG at 16 deg.C, 25 deg.C, 30 deg.C, 37 deg.C, preferably 25 deg.C, and shaking culturing at 200rpm for 14h to induce expression of human SARS-CoV nucleoapsid recombinant protein. The results of the final SDS-PAGE identification are shown in FIG. 1.
(2) Expression of pET28a-SARS-CoV-N fusion protein
The correctly identified pET28a-SARS-CoV-N strain was transferred to LB medium containing 100ug/ml kanamycin for overnight culture, transferred to fresh LB medium containing 100ug/ml kanamycin at a ratio of 1:100 the next day, and cultured with shaking at 37 ℃ and 200rpm until OD600 became 0.4-0.6, IPTG (final concentration of 1mmol/L) was added, and the temperature was adjusted to 25 ℃ to induce expression for 14 hours. Centrifuging at 4 deg.C and 10000r/min for 10min, and collecting precipitate.
(3) purification of pET28a-SARS-CoV-N fusion protein
A. The cell pellet was resuspended in lysis Buffer A (20mM Tris-HCl (pH8.0), 1mM EDTA, 0.1% Triton X-100), sonicated at 600W, centrifuged at 10000rpm for 30min, and the supernatant was collected.
B. And (3) purifying the thallus cracking supernatant through Ni column affinity chromatography: the invention adopts an AKTA Pure25 purifier to purify a protein sample.
Column assembling: 5ml of Ni Sepharose excel (GE) affinity packing were transferred to a XK 16/20Colum (GE) column, and the nickel column was equilibrated with 5 column volumes of binding buffer I (20mM Tris-HCl (pH 8.0)).
Loading: filtering a protein sample by a 0.45 mu m filter membrane to prepare a sample, wherein the flow rate is 2 ml/min; finally, 5-10 column volumes of buffer III were used for equilibration.
And (3) elution: elution was carried out with eluent I (20mM Tris-HCl (pH8.0), 20mM imidazole), eluent II (20mM Tris-HCl (pH8.0), 100mM imidazole), eluent III (20mM Tris-HCl (pH8.0), 250mM imidazole) and eluent IV (20mM Tris-HCl (pH8.0), 500mM imidazole), respectively, and different elution peaks were collected.
Balancing and storing: the nickel column was equilibrated with 5 column volumes of binding buffer I, purified water, 20% ethanol, respectively, and stored at 4 ℃.
(4) Protein dialysis renaturation: dialyzing the collected target peak into a proper buffer solution for storage, wherein the renaturation buffer solution is as follows: 10mm PBS (pH7.4), finally obtaining the soluble human SARS-CoV-2N recombinant protein.
(5) SDS-PAGE identification
SDS-PAGE was used to identify the samples after the purification and renaturation of the recombinant human SARS-CoV-N protein, the results are shown in FIG. 2.
EXAMPLE 2 preparation of monoclonal antibody against human SARS-CoV-2N
(1) Animal immunization
Using purified human SARS-CoV-N recombinant protein as immunogen, immunizing BALB/c female mouse of 8-12 weeks age by conventional method, emulsifying with Freund's complete adjuvant and antigen for the first time, and performing subcutaneous and abdominal multipoint immunization at dosage of 100 μ g/mouse; boosting the immunity once every two weeks for three times, wherein the adjuvant is Freund incomplete adjuvant, and the dosage is 100 mu g/time; detection of mouse tail blood titer by indirect ELISA method>1:104Three days before fusion, 100 mug of human SARS-CoV-2N antigen is boosted by intraperitoneal injection.
(2) Cell fusion
Mixing immune splenocytes with myeloma cells (SP2/0) at a ratio of 5:1, centrifuging at 1000rpm for 10min, and discarding supernatant; resuspending the cells in RPMI-1640 medium, centrifuging at 1000rpm for 10min, discarding the supernatant, sucking out the residual liquid with a dropper, tapping the bottom of the dropper to loosen and homogenize the precipitated cells, preheating in a 37 ℃ water bath, adding 1ml of preheated PEG1500(Roche) within 60s, then slowly dripping the RPMI-1640 medium to 20ml, centrifuging at 1000rpm for 10min, and discarding the supernatant. Cells were resuspended in HAT-containing medium and plated in 96-well cell culture plates and placed at 37 ℃ in 5% CO2Culturing in an incubator.
(3) Cell selection and monoclonalization
The indirect ELISA method is adopted to screen the positive culture hole specifically combined with the human SARS-CoV-N recombinant protein, and the specific process is as follows:
① preparation of antigen ELISA plate
Diluting human SARS-CoV-2N recombinant protein to 2 microgram/ml with 50mM pH9.6 carbonate buffer solution, adding to 96-well enzyme label plate, 100 microliter/well, coating overnight at 4 deg.C; after washing 3-5 times with PBST (10mM PBS (pH7.4), 0.05% Tween-80), spin-drying, blocking with blocking solution (10mM PBS (pH7.4), 5% BSA) at 37 ℃ for 2h for use.
② cell supernatant ELISA detection
Respectively taking 100 mu l of cell supernatant of 96-well cells to be screened, adding the cell supernatant into a prepared antigen ELISA plate of 96-well, incubating for 1h at 37 ℃, and washing the plate for 3-5 times by PBST. Further, 100. mu.l of goat anti-mouse-HRP diluted 1:5000 was added to each well, incubated at 37 ℃ for 45min, and the plate was washed 3-5 times with PBST. Finally adding substrate H2O2100. mu.l/well of TMB ((3,3 ', 5, 5' -tetramethylbenzidine)), developed in the dark for 10min, stopped by adding 50 ul/well of 2M sulfuric acid, and measured for absorbance of 450nM in a microplate reader.
③ cell monoclonality
And (3) selecting the wells with positive ELISA detection, and performing cell monoclonality by adopting a limiting dilution method to ensure that each culture well contains at most one hybridoma cell. When the cells grow to the culture holes 1/3-1/2, the cells are screened by an indirect ELISA method again, and finally, the single cell strain can be ensured to stably secrete the antibody of the anti-human SARS-CoV N recombinant protein.
(3) Hybridoma cell rescreening and monoclonalization
The method is the same as the operation method of the cell screening and monoclonality of the (3), the recombinant human SARS-CoV-2Nucleocapsid (bs-41408P, purchased from Beijing Boaosen biotechnology Co., Ltd.) and the recombinant human MERS-CoVNucleocsid (40068-V08B, purchased from Beijing Yiwangshan) are respectively coated on an enzyme label plate, the supernatant of the hybridoma is detected by adopting an indirect ELISA method, the hybridoma cell strain which has no cross with the MERS-CoV Nucleocapsid and has stronger reaction with the SARS-CoV-2Nucleocapsid is selected, and the hybridoma cell strain which has specific reaction with the human SARS-CoV-2Nucleocapsid and high affinity and secretes the anti-SARS-CoV-2 Nucleocapsid monoclonal antibody is finally obtained by multi-round screening.
(4) Cell cryopreservation
Cell cryopreservation solution: 90% fetal bovine serum + 10% DMSO, the hybridoma cells were centrifuged and resuspended in a pre-chilled cell cryopreservation solution at a cell density of 107cells/ml, 1 ml/count, subpackaging into freezing tubes, placing the freezing tubes into a programmed cooling box, placing the freezing tubes into a refrigerator at minus 80 ℃, and placing the freezing tubes into liquid nitrogen for long-term storage the next day.
(5) Preparation of monoclonal antibodies
In the invention, the monoclonal antibody of the anti-human SARS-CoV-2N recombinant protein is obtained by preparing a mouse ascites method, and the specific steps are as follows:
firstly, BALB/c male sensitization (intraperitoneal injection of liquid paraffin, 0.5 ml/mouse) of 8-12 weeks old is performed, hybridoma cells obtained in the mode of 2 weeks later are inoculated in an abdominal cavity (at least one time of serum-free culture and washing), and the inoculation amount is 106And (4) repeatedly extracting abdominal ascites and 4000rmp after the abdominal distension of the mice is obvious, centrifuging for 30min, collecting supernatant, and subpackaging at-20 ℃ for later use.
EXAMPLE 3 monoclonal antibody purification of recombinant protein against human SARS-CoV-2N
(1) Purification of monoclonal antibodies
The monoclonal antibody ascites obtained in example 2 was purified by Protein a affinity chromatography, comprising the following steps:
the method comprises the following basic steps: equilibration-loading-equilibration-elution-equilibration-preservation.
Balance liquid: 20mM PB (pH7.2) buffer;
eluent: 0.1M Glycine-HCl buffer pH 3.0;
neutralization buffer: 1M, pH9.0, Tris-HCl buffer;
preservation solution: 20% absolute ethyl alcohol.
Collecting 3-5ml ascites, precipitating with 50% saturated ammonium sulfate for more than 2 hr, centrifuging for 20min at 10000rmp, and removing supernatant; dissolving the precipitate with balance solution, filtering with 0.45 μm filter membrane, and preparing for sample loading; the Protein A affinity column is balanced by 5 times of column volume of balance liquid in advance, then the Protein precipitated by ammonium sulfate is loaded at the flow rate of 2ml/min, and finally the balance liquid with 5 times of column volume is used for balancing; and (3) replacing the eluent for elution, collecting the target protein, and then performing antibody: neutralizing the buffer at a volume ratio of 20:1 to obtain a purified monoclonal antibody against human SARS-CoV-2N recombinant protein.
Example 4: antibody pairing detection
(1) Antibody Horse Radish Peroxidase (HRP) labeling
HRP labeling was performed by sodium periodate oxidation. The labeled antibody is subjected to titer detection by an indirect ELISA method, and is stored at-20 ℃ for later use.
(2) Antibody pair screening
Antibody matching is carried out by adopting a chessboard titration method, and human SARS-CoV-2N recombinant proteins with the concentrations of 0.3ng/ml, 0.6ng/ml, 1.25ng/ml, 2.5ng/ml, 5ng/ml, 10ng/ml and 20ng/ml are respectively selected to screen the optimal matching antibody. Coating 24 enzyme-labeled antibodies with an empirical concentration of 200 ng/well, standing overnight at 4 ℃, washing for 3-5 times by using PBST, adding 300 mu l/well of a sealing solution, sealing for 2h at 37 ℃, and washing for 3-5 times by using PBST; adding a fixed value of human SARS-CoV-2N recombinant protein, incubating for 1h at 37 ℃, and washing for 3-5 times by PBST; respectively adding 13 HRP labeled antibodies diluted by 1:500 times, incubating for 45min at 37 ℃, and washing for 3-5 times by PBST; adding substrate TMB, developing in dark at 37 deg.C for 10min, adding stop solution 2M sulfuric acid 50 μ l, and measuring light absorption value of 450nM in microplate reader. The results are shown in Table 1, and the best match results were determined.
TABLE 1 optimal paired antibody detection results screened by checkerboard titration
Example 5 detection of specific reactivity of anti-N protein antibodies with viral antigens
To screen for antibodies specifically reactive with SARS virus. Therefore, the N protein of the recombinant expressed SARS-CoV-2 and MERS are selected to carry out SDS-PAGE electrophoresis, then the transformation and the sealing are carried out, Western Blot is respectively carried out with the N protein monoclonal antibody of the SARS-CoV-2, and finally the specificity of the N protein monoclonal antibody of the SARS-CoV-2 is determined; at the same time, the recombinant expressed SARS-CoV N protein and the recombinant expressed SARS-CoV-2N protein are respectively coated, and the two monoclonal antibodies of N6A8 and N7A7 described in example 4 are used to react with them, and the result shows that the recombinant expressed SARS-CoVN protein and the recombinant expressed SARS-CoV-2N protein have the same structure and activity, and do not cross with MERS-CoV N protein, and the details are shown in FIGS. 3-5.
The above examples are provided to those of ordinary skill in the art to fully disclose and describe how to make and use the claimed embodiments, and are not intended to limit the scope of the disclosure herein. Modifications apparent to those skilled in the art are intended to be within the scope of the appended claims.
Sequence listing
<110> Beijing Boaosen Biotechnology Ltd
<120> human SARS-CoV-2 monoclonal antibody, its preparation method and application
<141>2020-02-26
<160>2
<170>SIPOSequenceListing 1.0
<210>1
<211>422
<212>PRT
<213>Artificial Sequence
<400>1
Met Ser Asp Asn Gly Pro Gln Ser Asn Gln Arg Ser Ala Pro Arg Ile
1 5 10 15
Thr Phe Gly Gly Pro Thr Asp Ser Thr Asp Asn Asn Gln Asn Gly Gly
20 25 30
Arg Asn Gly Ala Arg Pro Lys Gln Arg Arg Pro Gln Gly Leu Pro Asn
35 40 45
Asn Thr Ala Ser Trp Phe Thr Ala Leu Thr Gln His Gly Lys Glu Glu
50 55 60
Leu Arg Phe Pro Arg Gly Gln Gly Val Pro Ile Asn Thr Asn Ser Gly
65 70 75 80
Pro Asp Asp Gln Ile Gly Tyr Tyr Arg Arg Ala Thr Arg Arg Val Arg
85 90 95
Gly Gly Asp Gly Lys Met Lys Glu Leu Ser Pro Arg Trp Tyr Phe Tyr
100 105 110
Tyr Leu Gly Thr Gly Pro Glu Ala Ser Leu Pro Tyr Gly Ala Asn Lys
115 120 125
Glu Gly Ile Val Trp Val Ala Thr Glu Gly Ala Leu Asn Thr Pro Lys
130 135 140
Asp His Ile Gly Thr Arg Asn Pro Asn Asn Asn Ala Ala Thr Val Leu
145 150 155 160
Gln Leu Pro Gln Gly Thr Thr Leu Pro Lys Gly Phe Tyr Ala Glu Gly
165 170 175
Ser Arg Gly Gly Ser Gln Ala Ser Ser Arg Ser Ser Ser Arg Ser Arg
180 185 190
Gly Asn Ser Arg Asn Ser Thr Pro Gly Ser Ser Arg Gly Asn Ser Pro
195 200 205
Ala Arg Met Ala Ser Gly Gly Gly Glu Thr Ala Leu Ala Leu Leu Leu
210 215 220
Leu Asp Arg Leu Asn Gln Leu Glu Ser Lys Val Ser Gly Lys Gly Gln
225 230 235 240
Gln Gln Gln Gly Gln Thr Val Thr Lys Lys Ser Ala Ala Glu Ala Ser
245 250 255
Lys Lys Pro Arg Gln Lys Arg Thr Ala Thr Lys Gln Tyr Asn Val Thr
260 265 270
Gln Ala Phe Gly Arg Arg Gly Pro Glu Gln Thr Gln Gly Asn Phe Gly
275 280 285
Asp Gln Asp Leu Ile Arg Gln Gly Thr Asp Tyr Lys His Trp Pro Gln
290 295 300
Ile Ala Gln Phe Ala Pro Ser Ala Ser Ala Phe Phe Gly Met Ser Arg
305 310 315 320
Ile Gly Met Glu Val Thr Pro Ser Gly Thr Trp Leu Thr Tyr His Gly
325 330 335
Ala Ile Lys Leu Asp Asp Lys Asp Pro Gln Phe Lys Asp Asn Val Ile
340 345 350
Leu Leu Asn Lys His Ile Asp Ala Tyr Lys Thr Phe Pro Pro Thr Glu
355 360 365
Pro Lys Lys Asp Lys Lys Lys Lys Thr Asp Glu Ala Gln Pro Leu Pro
370 375 380
Gln Arg Gln Lys Lys Gln Pro Thr Val Thr Leu Leu Pro Ala Ala Asp
385 390 395 400
Met Asp Asp Phe Ser Arg Gln Leu Gln Asn Ser Met Ser Gly Ala Ser
405 410 415
Ala Asp Ser Thr Gln Ala
420
<210>2
<211>1269
<212>DNA
<213>Homo sapiens
<400>2
atgtctgata atggacccca atcaaaccaa cgtagtgccc cccgcattac atttggtgga 60
cccacagatt caactgacaa taaccagaat ggaggacgca atggggcaag gccaaaacag 120
cgccgacccc aaggtttacc caataatact gcgtcttggt tcacagctct cactcagcat 180
ggcaaggagg aacttagatt ccctcgaggc cagggcgttc caatcaacac caatagtggt 240
ccagatgacc aaattggcta ctaccgaaga gctacccgac gagttcgtgg tggtgacggc 300
aaaatgaaag agctcagccc cagatggtac ttctattacc taggaactgg cccagaagct 360
tcacttccct acggcgctaa caaagaaggc atcgtatggg ttgcaactga gggagccttg 420
aatacaccca aagaccacat tggcacccgc aatcctaata acaatgctgc caccgtgcta 480
caacttcctc aaggaacaac attgccaaaa ggcttctacg cagagggaag cagaggcggc 540
agtcaagcct cttctcgctc ctcatcacgt agtcgcggta attcaagaaa ttcaactcct 600
ggcagcagta ggggaaattc tcctgctcga atggctagcg gaggtggtga aactgccctc 660
gcgctattgc tgctagacag attgaaccag cttgagagca aagtttctgg taaaggccaa 720
caacaacaag gccaaactgt cactaagaaa tctgctgctg aggcatctaa aaagcctcgc 780
caaaaacgta ctgccacaaa acagtacaac gtcactcaag catttgggag acgtggtcca 840
gaacaaaccc aaggaaattt cggggaccaa gacctaatca gacaaggaac tgattacaaa 900
cattggccgc aaattgcaca atttgctcca agtgcctctg cattctttgg aatgtcacgc 960
attggcatgg aagtcacacc ttcgggaaca tggctgactt atcatggagc cattaaattg 1020
gatgacaaag atccacaatt caaagacaac gtcatactgc tgaacaagca cattgacgca 1080
tacaaaacat tcccaccaac agagcctaaa aaggacaaaa agaaaaagac tgatgaagct 1140
cagcctttgc cgcagagaca aaagaagcag cccactgtga ctcttcttcc tgcggctgac 1200
atggatgatt tctccagaca acttcaaaat tccatgagtg gagcttctgc tgattcaact 1260
caggcataa 1269
Claims (10)
1. A human SARS-CoV-2 monoclonal antibody, which is characterized in that: using SARS-CoV nucleoapsid recombinant protein as immunogen to immunize mouse to obtain monoclonal antibody; the amino acid sequence of the SARS-CoV Nucleocapsid recombinant protein is shown in SEQ ID NO. 1.
2. The human SARS-CoV-2 monoclonal antibody of claim 1, wherein: the SARS-CoVNucleucoapsid recombinant protein is prepared by the following method: human SARS-CoV nucleoapsid gene whose nucleotide sequence is shown in SEQ ID No.2 is inserted into expression vector pET28a to construct pET28a-SARS-CoV-N fusion expression plasmid, and then the Escherichia coli is transformed, and then the induced, purified and renatured by IPTG to obtain SARS-CoV nucleoapsid recombinant protein.
3. The method for preparing human SARS-CoV-2 monoclonal antibody according to claim 1 or 2, wherein: using SARS-CoV Nucleocapsid recombinant protein as immunogen to immunize BALB/c mouse, then making fusion and subcloning of mouse spleen cell and mouse myeloma cell, and making specific screening and domestication by using SARS-CoV-2Nucleocapsid and MERS Nucleocapsid to finally obtain monoclonal cell strain with good specificity and high potency, then making ascites preparation and purification so as to obtain a large quantity of monoclonal antibody resisting human SARS-CoV Nucleocapsid.
4. The method for preparing human SARS-CoV-2 monoclonal antibody according to claim 3, wherein: selecting female mice 8-12 weeks old from the BALB/c mice;
or/and: the screening method is an ELISA method;
or/and: the purification method is Protein A affinity purification.
5. Use of the human SARS-CoV-2 monoclonal antibody of claim 1 or 2 in the preparation of SARS-CoV-2 virus detection products.
6. Use of the human SARS-CoV-2 monoclonal antibody of claim 1 or 2 for the preparation of a medicament for inhibiting SARS-CoV-2 virus.
7. Use of the human SARS-CoV-2 monoclonal antibody of claim 1 or 2 for the preparation of a pharmaceutical preparation for the prevention or treatment of a disease caused by SARS-CoV-2 virus infection.
8. A method for detecting the level of SARS-CoV-2 virus for non-diagnostic purposes, comprising: contacting the sample to be detected with the human SARS-CoV-2 monoclonal antibody as described in claim 1 or 2, and detecting the immunoreaction of the sample with the human SARS-CoV-2 monoclonal antibody.
9. A kit for detecting SARS-CoV-2 virus comprising the human SARS-CoV-2V monoclonal antibody of claim 1 or 2.
10. A medicament for inhibiting SARS-CoV-2 virus comprising the human SARS-CoV-2 monoclonal antibody of claim 1 or 2.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010120349.4A CN111153991A (en) | 2020-02-26 | 2020-02-26 | Human SARS-CoV-2 monoclonal antibody and its preparation method and use |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010120349.4A CN111153991A (en) | 2020-02-26 | 2020-02-26 | Human SARS-CoV-2 monoclonal antibody and its preparation method and use |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111153991A true CN111153991A (en) | 2020-05-15 |
Family
ID=70566544
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010120349.4A Pending CN111153991A (en) | 2020-02-26 | 2020-02-26 | Human SARS-CoV-2 monoclonal antibody and its preparation method and use |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111153991A (en) |
Cited By (75)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111458520A (en) * | 2020-04-10 | 2020-07-28 | 中国人民解放军军事科学院军事医学研究院 | Novel coronavirus Alpha L ISA antigen rapid detection kit |
| CN111499699A (en) * | 2020-05-31 | 2020-08-07 | 深圳晶蛋生物医药科技有限公司 | Novel coronavirus COVID-19-N protein expression and purification method |
| CN111518204A (en) * | 2020-05-27 | 2020-08-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies against novel coronaviruses for immunodetection |
| CN111518202A (en) * | 2020-05-27 | 2020-08-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Novel coronavirus antibody and ELISA detection kit for same |
| CN111518203A (en) * | 2020-05-27 | 2020-08-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Kit for detecting novel coronavirus |
| CN111560070A (en) * | 2020-05-27 | 2020-08-21 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibody aiming at novel coronavirus NP protein and detection application thereof |
| CN111647077A (en) * | 2020-06-02 | 2020-09-11 | 深圳市因诺赛生物科技有限公司 | Novel coronavirus (SARS-COV-2) spike protein binding molecule and application thereof |
| CN111647079A (en) * | 2020-07-03 | 2020-09-11 | 北部湾大学 | Neutralizing antibody for resisting novel coronavirus N protein |
| CN111733141A (en) * | 2020-06-19 | 2020-10-02 | 清华大学深圳国际研究生院 | Hybridoma cell capable of secreting monoclonal antibody against novel coronavirus N protein, monoclonal antibody and application |
| CN111732655A (en) * | 2020-07-01 | 2020-10-02 | 中国人民解放军军事科学院军事医学研究院 | RBD-targeted high-neutralization-activity anti-SARS-CoV-2 fully-humanized monoclonal antibody and application thereof |
| CN111748033A (en) * | 2020-05-27 | 2020-10-09 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Separation antibody combined with novel coronavirus NP protein and detection kit containing same |
| CN111793129A (en) * | 2020-07-28 | 2020-10-20 | 上海市公共卫生临床中心 | Antibody or antigen binding fragment thereof specifically binding to coronavirus |
| CN111875700A (en) * | 2020-07-28 | 2020-11-03 | 武汉华美生物工程有限公司 | Single-chain antibody of anti SARS-COV-2 virus N protein and its use |
| CN111875701A (en) * | 2020-08-14 | 2020-11-03 | 江苏中慧元通生物科技有限公司 | Single-chain antibody of SARS-CoV-2 virus and its use |
| CN111909261A (en) * | 2020-08-19 | 2020-11-10 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111909263A (en) * | 2020-08-19 | 2020-11-10 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111909260A (en) * | 2020-08-19 | 2020-11-10 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111909262A (en) * | 2020-08-19 | 2020-11-10 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925441A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925440A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925444A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925442A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925443A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111978378A (en) * | 2020-08-10 | 2020-11-24 | 武汉大学 | SARS-CoV-2 antigen polypeptide and its application |
| CN112010963A (en) * | 2020-07-20 | 2020-12-01 | 江苏集萃医学免疫技术研究所有限公司 | SARS-COV-2 antibody and use thereof |
| CN112079920A (en) * | 2020-09-18 | 2020-12-15 | 北京华大蛋白质研发中心有限公司 | Monoclonal antibody for detecting SARS-CoV-2 virus N protein and its application |
| CN112111007A (en) * | 2020-09-22 | 2020-12-22 | 通用生物系统(安徽)有限公司 | Preparation method of novel coronavirus nucleocapsid protein monoclonal antibody |
| CN112175071A (en) * | 2020-09-22 | 2021-01-05 | 通用生物系统(安徽)有限公司 | Preparation method of novel coronavirus spike protein monoclonal antibody |
| CN112210004A (en) * | 2020-07-20 | 2021-01-12 | 江苏集萃医学免疫技术研究所有限公司 | Coronavirus COVID-19 detection antibody and application thereof |
| CN112225797A (en) * | 2020-09-24 | 2021-01-15 | 杭州医学院 | Monoclonal antibody for resisting SARS-CoV-2 nucleocapsid protein and application thereof |
| CN112251414A (en) * | 2020-10-12 | 2021-01-22 | 中国科学院苏州纳米技术与纳米仿生研究所 | Hybridoma cell strain, preparation method and application thereof |
| CN112341540A (en) * | 2020-11-11 | 2021-02-09 | 英科博雅基因科技(天津)有限公司 | Polyclonal antibodies against the receptor binding domain of the S1 protein for the treatment of COVID-19 infection |
| CN112390879A (en) * | 2021-01-21 | 2021-02-23 | 上海科技大学 | Antibody targeting SARS-CoV-2 and its preparation method and use |
| CN112521494A (en) * | 2020-06-19 | 2021-03-19 | 武汉生物制品研究所有限责任公司 | Monoclonal antibody 2B11 for resisting SARS-CoV-2 |
| CN112666350A (en) * | 2021-03-18 | 2021-04-16 | 北京百普赛斯生物科技股份有限公司 | Test paper and kit for detecting novel coronavirus |
| CN112679605A (en) * | 2021-03-15 | 2021-04-20 | 安源医药科技(上海)有限公司 | Antibodies or antigen binding fragments thereof against novel coronavirus nucleocapsid proteins and uses thereof |
| CN112684168A (en) * | 2020-09-11 | 2021-04-20 | 必欧瀚生物技术(合肥)有限公司 | N antigen detection kit for SARS-CoV-2 virus and its preparation method |
| CN112898415A (en) * | 2020-05-27 | 2021-06-04 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibody for detecting novel coronavirus and detection kit |
| CN112898416A (en) * | 2020-05-27 | 2021-06-04 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Binding protein of novel coronavirus NP protein and application thereof |
| CN112940087A (en) * | 2021-03-17 | 2021-06-11 | 郑州大学 | Common epitope peptide of SARS-CoV and SARS-CoV-2 and its application |
| CN112979790A (en) * | 2020-05-27 | 2021-06-18 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies and use in detecting novel coronaviruses |
| CN112979793A (en) * | 2020-05-27 | 2021-06-18 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies for detecting novel coronaviruses |
| CN112979791A (en) * | 2020-05-27 | 2021-06-18 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies against novel coronaviruses |
| CN112979792A (en) * | 2020-05-27 | 2021-06-18 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies against novel coronaviruses, encoding nucleic acids, vectors, host cells, derivatives and uses thereof |
| CN113150130A (en) * | 2021-01-31 | 2021-07-23 | 中南大学湘雅医院 | Novel coronavirus monoclonal antibody and application thereof |
| CN113150133A (en) * | 2021-03-15 | 2021-07-23 | 安源医药科技(上海)有限公司 | Monoclonal antibodies or antigen-binding fragments thereof against SARS-CoV-2 |
| CN113234146A (en) * | 2021-05-07 | 2021-08-10 | 南京川博生物技术有限公司 | Preparation method of coronavirus spike protein monoclonal antibody |
| CN113461810A (en) * | 2021-05-17 | 2021-10-01 | 深圳市福田区格物智康病原研究所 | Fully human monoclonal antibody for resisting novel coronavirus spike protein and application thereof |
| CN113461813A (en) * | 2021-09-03 | 2021-10-01 | 上海良润生物医药科技有限公司 | Paired antibody for detecting new coronavirus and application thereof |
| CN113493508A (en) * | 2021-06-15 | 2021-10-12 | 北京华大蛋白质研发中心有限公司 | Double-antibody sandwich ELISA kit for detecting new coronavirus N protein |
| CN113603769A (en) * | 2021-07-20 | 2021-11-05 | 贵州省人民医院 | Hybridoma cell strain capable of stably secreting anti-novel coronavirus nucleocapsid protein monoclonal antibody and establishment method and application thereof |
| CN113683692A (en) * | 2021-08-26 | 2021-11-23 | 深圳市亚辉龙生物科技股份有限公司 | SARS-CoV-2N protein antibody and its application |
| WO2021232541A1 (en) * | 2020-05-18 | 2021-11-25 | 博奥赛斯(天津)生物科技有限公司 | Igm/igg colloidal gold detection kit for novel coronavirus |
| WO2021233433A1 (en) * | 2020-05-22 | 2021-11-25 | 南京金斯瑞生物科技有限公司 | Anti-sars-cov-2 spike protein monoclonal antibody |
| WO2021237534A1 (en) * | 2020-05-27 | 2021-12-02 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibody against novel coronavirus, antibody combination, preparation method, and detection kit containing same |
| CN113735969A (en) * | 2021-09-20 | 2021-12-03 | 中国人民解放军军事科学院军事医学研究院 | Fully human anti-new coronavirus broad-spectrum high-neutralization-activity monoclonal antibody and application thereof |
| CN113740538A (en) * | 2020-05-27 | 2021-12-03 | 复旦大学 | Method and product for detecting SARS-CoV-2 novel coronavirus IgM/IgG antibody |
| CN113817051A (en) * | 2020-06-19 | 2021-12-21 | 武汉生物制品研究所有限责任公司 | Monoclonal antibody 1B6 for resisting SARS-CoV-2 |
| WO2021255755A1 (en) * | 2020-06-15 | 2021-12-23 | Vangala Rajanikanth | Lateral flow assay device for detection of analytes and method of detection thereof |
| WO2021254403A1 (en) * | 2020-06-16 | 2021-12-23 | Hifibio (Hangzhou) Co., Ltd. | Methods and compositions related to neutralizing antibodies against human coronavirus |
| CN113831409A (en) * | 2020-06-08 | 2021-12-24 | 中国科学院深圳先进技术研究院 | anti-SAR-COV-2 antibody or antigen binding fragment thereof and application thereof |
| WO2021258625A1 (en) * | 2020-06-22 | 2021-12-30 | 中国科学院深圳先进技术研究院 | Anti-sar-cov-2 (covid-19) fully humanized monoclonal antibody, and preparation method therefor and application thereof |
| CN113945714A (en) * | 2020-07-16 | 2022-01-18 | 南京蓬勃生物科技有限公司 | Method for detecting neutralizing capacity of novel coronavirus neutralizing antibody drugs |
| EP3945319A1 (en) * | 2020-07-29 | 2022-02-02 | Sysmex Corporation | Method for measuring viral antigen in sample, antibody set, and reagent kit |
| WO2022029494A1 (en) * | 2020-08-04 | 2022-02-10 | Abbott Rapid Diagnostics International Unlimited Company | Assays for detecting sars-cov-2 |
| WO2022027703A1 (en) * | 2020-08-06 | 2022-02-10 | 中国人民解放军陆军军医大学 | Monoclonal antibody of n antigen of sars-cov-2, detection method therefor and use thereof |
| CN114231497A (en) * | 2022-02-24 | 2022-03-25 | 广州伯尼兹生物科技有限公司 | Monoclonal antibody hybridoma cell line for expressing novel coronavirus S1 protein and neutralizing active antibody |
| WO2022068844A1 (en) * | 2020-09-30 | 2022-04-07 | Vazyme Biotech Co., Ltd. | Neutralizing antibody against sars-cov-2 |
| WO2022031360A3 (en) * | 2020-06-04 | 2022-04-14 | Massachusetts Institute Of Technology | Systems and methods for analyte detection using electromagnetically induced resonance |
| KR20220052645A (en) * | 2020-10-21 | 2022-04-28 | 재단법인 바이오나노헬스가드연구단 | A severe acute respiratory syndrome coronavirus specific antigen tagged with streptavidin binding peptide and use thereof |
| CN114751980A (en) * | 2022-04-22 | 2022-07-15 | 厦门博昂生物技术有限公司 | Monoclonal antibody blocking agent for detecting neocorona antigen |
| WO2022181550A1 (en) * | 2021-02-25 | 2022-09-01 | 花王株式会社 | Anti-sars-cov-2 antibody |
| WO2022216223A1 (en) * | 2020-04-09 | 2022-10-13 | Intra-Immusg Private Limited (Sg) | Vaccine and/or antibody for viral infection |
| WO2023199955A1 (en) * | 2022-04-12 | 2023-10-19 | 花王株式会社 | Anti-sars-cov-2 antibody |
| US11820811B2 (en) | 2020-09-22 | 2023-11-21 | Shihezi University | Nano-antibody and its application based on SARS-CoV-2 S protein |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1673231A (en) * | 2004-07-15 | 2005-09-28 | 中国科学院上海生命科学研究院 | Monoclonal antibody of SARS coronavirus N protein and its application |
| CN1820021A (en) * | 2003-05-09 | 2006-08-16 | 亚特斯公司 | Peptides and mixtures thereof for use in the detection of severe acute respiratory syndrome-associated coronavirus (SARS) |
| CN101283093A (en) * | 2005-10-11 | 2008-10-08 | 希森美康株式会社 | Method for determination of SARS virus nucleocapsid protein, reagent kit for the determination, test device, monoclonal antibody directed against SARS virus nucleocapsid protein, and hybridoma capable |
-
2020
- 2020-02-26 CN CN202010120349.4A patent/CN111153991A/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1820021A (en) * | 2003-05-09 | 2006-08-16 | 亚特斯公司 | Peptides and mixtures thereof for use in the detection of severe acute respiratory syndrome-associated coronavirus (SARS) |
| CN1673231A (en) * | 2004-07-15 | 2005-09-28 | 中国科学院上海生命科学研究院 | Monoclonal antibody of SARS coronavirus N protein and its application |
| CN101283093A (en) * | 2005-10-11 | 2008-10-08 | 希森美康株式会社 | Method for determination of SARS virus nucleocapsid protein, reagent kit for the determination, test device, monoclonal antibody directed against SARS virus nucleocapsid protein, and hybridoma capable |
Non-Patent Citations (1)
| Title |
|---|
| AHMED SF, ET AL.: "Preliminary Identification of Potential Vaccine Targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies.", 《VIRUSES》 * |
Cited By (111)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022216223A1 (en) * | 2020-04-09 | 2022-10-13 | Intra-Immusg Private Limited (Sg) | Vaccine and/or antibody for viral infection |
| CN111458520B (en) * | 2020-04-10 | 2023-09-15 | 中国人民解放军军事科学院军事医学研究院 | Novel coronavirus alpha LISA antigen rapid detection kit |
| CN111458520A (en) * | 2020-04-10 | 2020-07-28 | 中国人民解放军军事科学院军事医学研究院 | Novel coronavirus Alpha L ISA antigen rapid detection kit |
| WO2021232541A1 (en) * | 2020-05-18 | 2021-11-25 | 博奥赛斯(天津)生物科技有限公司 | Igm/igg colloidal gold detection kit for novel coronavirus |
| WO2021233433A1 (en) * | 2020-05-22 | 2021-11-25 | 南京金斯瑞生物科技有限公司 | Anti-sars-cov-2 spike protein monoclonal antibody |
| CN111518202B (en) * | 2020-05-27 | 2021-10-19 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Novel coronavirus antibody and ELISA detection kit for same |
| CN112898416B (en) * | 2020-05-27 | 2022-02-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Binding protein of novel coronavirus NP protein and application thereof |
| CN112898415B (en) * | 2020-05-27 | 2021-12-10 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibody for detecting novel coronavirus and detection kit |
| CN112979791B (en) * | 2020-05-27 | 2021-12-10 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies against novel coronaviruses |
| CN113740538A (en) * | 2020-05-27 | 2021-12-03 | 复旦大学 | Method and product for detecting SARS-CoV-2 novel coronavirus IgM/IgG antibody |
| CN111748033A (en) * | 2020-05-27 | 2020-10-09 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Separation antibody combined with novel coronavirus NP protein and detection kit containing same |
| WO2021237534A1 (en) * | 2020-05-27 | 2021-12-02 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibody against novel coronavirus, antibody combination, preparation method, and detection kit containing same |
| CN111560070A (en) * | 2020-05-27 | 2020-08-21 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibody aiming at novel coronavirus NP protein and detection application thereof |
| CN111518203A (en) * | 2020-05-27 | 2020-08-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Kit for detecting novel coronavirus |
| CN111518204A (en) * | 2020-05-27 | 2020-08-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies against novel coronaviruses for immunodetection |
| CN111560070B (en) * | 2020-05-27 | 2021-10-01 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibody aiming at novel coronavirus NP protein and detection application thereof |
| CN111518202A (en) * | 2020-05-27 | 2020-08-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Novel coronavirus antibody and ELISA detection kit for same |
| CN111518204B (en) * | 2020-05-27 | 2021-09-14 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies against novel coronaviruses for immunodetection |
| CN112979793B (en) * | 2020-05-27 | 2022-02-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies for detecting novel coronaviruses |
| CN112979790B (en) * | 2020-05-27 | 2022-02-11 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies and use in detecting novel coronaviruses |
| CN112979792A (en) * | 2020-05-27 | 2021-06-18 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies against novel coronaviruses, encoding nucleic acids, vectors, host cells, derivatives and uses thereof |
| CN112979791A (en) * | 2020-05-27 | 2021-06-18 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies against novel coronaviruses |
| CN112979793A (en) * | 2020-05-27 | 2021-06-18 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies for detecting novel coronaviruses |
| CN112979790A (en) * | 2020-05-27 | 2021-06-18 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibodies and use in detecting novel coronaviruses |
| CN112898416A (en) * | 2020-05-27 | 2021-06-04 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Binding protein of novel coronavirus NP protein and application thereof |
| CN112898415A (en) * | 2020-05-27 | 2021-06-04 | 江苏省疾病预防控制中心(江苏省公共卫生研究院) | Antibody for detecting novel coronavirus and detection kit |
| CN111499699A (en) * | 2020-05-31 | 2020-08-07 | 深圳晶蛋生物医药科技有限公司 | Novel coronavirus COVID-19-N protein expression and purification method |
| CN111647077A (en) * | 2020-06-02 | 2020-09-11 | 深圳市因诺赛生物科技有限公司 | Novel coronavirus (SARS-COV-2) spike protein binding molecule and application thereof |
| CN111647077B (en) * | 2020-06-02 | 2021-02-09 | 深圳市因诺赛生物科技有限公司 | Novel coronavirus (SARS-COV-2) spike protein binding molecule and application thereof |
| WO2022031360A3 (en) * | 2020-06-04 | 2022-04-14 | Massachusetts Institute Of Technology | Systems and methods for analyte detection using electromagnetically induced resonance |
| CN113831409A (en) * | 2020-06-08 | 2021-12-24 | 中国科学院深圳先进技术研究院 | anti-SAR-COV-2 antibody or antigen binding fragment thereof and application thereof |
| CN113831409B (en) * | 2020-06-08 | 2023-04-25 | 中国科学院深圳先进技术研究院 | anti-SAR-COV-2 antibody or antigen binding fragment thereof and application thereof |
| WO2021255755A1 (en) * | 2020-06-15 | 2021-12-23 | Vangala Rajanikanth | Lateral flow assay device for detection of analytes and method of detection thereof |
| WO2021254403A1 (en) * | 2020-06-16 | 2021-12-23 | Hifibio (Hangzhou) Co., Ltd. | Methods and compositions related to neutralizing antibodies against human coronavirus |
| CN113817051A (en) * | 2020-06-19 | 2021-12-21 | 武汉生物制品研究所有限责任公司 | Monoclonal antibody 1B6 for resisting SARS-CoV-2 |
| CN112521494A (en) * | 2020-06-19 | 2021-03-19 | 武汉生物制品研究所有限责任公司 | Monoclonal antibody 2B11 for resisting SARS-CoV-2 |
| CN113817051B (en) * | 2020-06-19 | 2023-08-29 | 武汉生物制品研究所有限责任公司 | Monoclonal antibody 1B6 against SARS-CoV-2 |
| CN111733141B (en) * | 2020-06-19 | 2022-02-18 | 清华大学深圳国际研究生院 | Hybridoma cell capable of secreting monoclonal antibody against novel coronavirus N protein, monoclonal antibody and application |
| CN111733141A (en) * | 2020-06-19 | 2020-10-02 | 清华大学深圳国际研究生院 | Hybridoma cell capable of secreting monoclonal antibody against novel coronavirus N protein, monoclonal antibody and application |
| WO2021258625A1 (en) * | 2020-06-22 | 2021-12-30 | 中国科学院深圳先进技术研究院 | Anti-sar-cov-2 (covid-19) fully humanized monoclonal antibody, and preparation method therefor and application thereof |
| CN113896788B (en) * | 2020-06-22 | 2023-05-23 | 中国科学院深圳先进技术研究院 | anti-SAR-COV-2 (COVID-19) fully human monoclonal antibody and preparation method and application thereof |
| CN113896788A (en) * | 2020-06-22 | 2022-01-07 | 中国科学院深圳先进技术研究院 | anti-SAR-COV-2 (COVID-19) fully human monoclonal antibody and preparation method and application thereof |
| CN111732655A (en) * | 2020-07-01 | 2020-10-02 | 中国人民解放军军事科学院军事医学研究院 | RBD-targeted high-neutralization-activity anti-SARS-CoV-2 fully-humanized monoclonal antibody and application thereof |
| CN111732655B (en) * | 2020-07-01 | 2021-10-22 | 中国人民解放军军事科学院军事医学研究院 | RBD-targeted high-neutralization-activity anti-SARS-CoV-2 fully-humanized monoclonal antibody and application thereof |
| CN111647079A (en) * | 2020-07-03 | 2020-09-11 | 北部湾大学 | Neutralizing antibody for resisting novel coronavirus N protein |
| CN113945714B (en) * | 2020-07-16 | 2023-01-31 | 南京蓬勃生物科技有限公司 | Method for detecting neutralizing capacity of novel coronavirus neutralizing antibody drugs |
| CN113945714A (en) * | 2020-07-16 | 2022-01-18 | 南京蓬勃生物科技有限公司 | Method for detecting neutralizing capacity of novel coronavirus neutralizing antibody drugs |
| CN112010963A (en) * | 2020-07-20 | 2020-12-01 | 江苏集萃医学免疫技术研究所有限公司 | SARS-COV-2 antibody and use thereof |
| CN112210004A (en) * | 2020-07-20 | 2021-01-12 | 江苏集萃医学免疫技术研究所有限公司 | Coronavirus COVID-19 detection antibody and application thereof |
| CN112010963B (en) * | 2020-07-20 | 2022-05-20 | 江苏集萃医学免疫技术研究所有限公司 | SARS-COV-2 antibody and use thereof |
| CN111875700B (en) * | 2020-07-28 | 2022-03-25 | 武汉华美生物工程有限公司 | Single-chain antibody of anti SARS-COV-2 virus N protein and its use |
| CN111793129B (en) * | 2020-07-28 | 2021-09-24 | 上海市公共卫生临床中心 | Antibody or antigen binding fragment thereof specifically binding to coronavirus |
| CN111793129A (en) * | 2020-07-28 | 2020-10-20 | 上海市公共卫生临床中心 | Antibody or antigen binding fragment thereof specifically binding to coronavirus |
| CN111875700A (en) * | 2020-07-28 | 2020-11-03 | 武汉华美生物工程有限公司 | Single-chain antibody of anti SARS-COV-2 virus N protein and its use |
| EP3945319A1 (en) * | 2020-07-29 | 2022-02-02 | Sysmex Corporation | Method for measuring viral antigen in sample, antibody set, and reagent kit |
| WO2022029494A1 (en) * | 2020-08-04 | 2022-02-10 | Abbott Rapid Diagnostics International Unlimited Company | Assays for detecting sars-cov-2 |
| CN114058593A (en) * | 2020-08-06 | 2022-02-18 | 中国人民解放军陆军军医大学 | Monoclonal antibody of N antigen of SARS-CoV-2, detection method and use thereof |
| WO2022027703A1 (en) * | 2020-08-06 | 2022-02-10 | 中国人民解放军陆军军医大学 | Monoclonal antibody of n antigen of sars-cov-2, detection method therefor and use thereof |
| CN111978378A (en) * | 2020-08-10 | 2020-11-24 | 武汉大学 | SARS-CoV-2 antigen polypeptide and its application |
| CN111875701A (en) * | 2020-08-14 | 2020-11-03 | 江苏中慧元通生物科技有限公司 | Single-chain antibody of SARS-CoV-2 virus and its use |
| CN111925443A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925444A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111909261A (en) * | 2020-08-19 | 2020-11-10 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111909263A (en) * | 2020-08-19 | 2020-11-10 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925442A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| WO2022037616A1 (en) * | 2020-08-19 | 2022-02-24 | 重庆医科大学 | Novel coronavirus rbd specific monoclonal antibody cqts004 and use thereof |
| CN111909260A (en) * | 2020-08-19 | 2020-11-10 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111909262A (en) * | 2020-08-19 | 2020-11-10 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925441A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN111925440A (en) * | 2020-08-19 | 2020-11-13 | 重庆医科大学 | New coronavirus RBD specific monoclonal antibody and application |
| CN112684168A (en) * | 2020-09-11 | 2021-04-20 | 必欧瀚生物技术(合肥)有限公司 | N antigen detection kit for SARS-CoV-2 virus and its preparation method |
| CN112079920A (en) * | 2020-09-18 | 2020-12-15 | 北京华大蛋白质研发中心有限公司 | Monoclonal antibody for detecting SARS-CoV-2 virus N protein and its application |
| CN112111007A (en) * | 2020-09-22 | 2020-12-22 | 通用生物系统(安徽)有限公司 | Preparation method of novel coronavirus nucleocapsid protein monoclonal antibody |
| US11820811B2 (en) | 2020-09-22 | 2023-11-21 | Shihezi University | Nano-antibody and its application based on SARS-CoV-2 S protein |
| CN112175071A (en) * | 2020-09-22 | 2021-01-05 | 通用生物系统(安徽)有限公司 | Preparation method of novel coronavirus spike protein monoclonal antibody |
| CN112225797A (en) * | 2020-09-24 | 2021-01-15 | 杭州医学院 | Monoclonal antibody for resisting SARS-CoV-2 nucleocapsid protein and application thereof |
| CN112225797B (en) * | 2020-09-24 | 2022-01-25 | 杭州医学院 | Monoclonal antibody for resisting SARS-CoV-2 nucleocapsid protein and application thereof |
| WO2022068844A1 (en) * | 2020-09-30 | 2022-04-07 | Vazyme Biotech Co., Ltd. | Neutralizing antibody against sars-cov-2 |
| CN112251414A (en) * | 2020-10-12 | 2021-01-22 | 中国科学院苏州纳米技术与纳米仿生研究所 | Hybridoma cell strain, preparation method and application thereof |
| KR102437114B1 (en) | 2020-10-21 | 2022-08-26 | 재단법인 바이오나노헬스가드연구단 | A severe acute respiratory syndrome coronavirus specific antigen tagged with streptavidin binding peptide and use thereof |
| KR20220052645A (en) * | 2020-10-21 | 2022-04-28 | 재단법인 바이오나노헬스가드연구단 | A severe acute respiratory syndrome coronavirus specific antigen tagged with streptavidin binding peptide and use thereof |
| CN112341540A (en) * | 2020-11-11 | 2021-02-09 | 英科博雅基因科技(天津)有限公司 | Polyclonal antibodies against the receptor binding domain of the S1 protein for the treatment of COVID-19 infection |
| CN112341540B (en) * | 2020-11-11 | 2022-09-06 | 英科博雅基因科技(天津)有限公司 | Polyclonal antibodies against the receptor binding domain of the S1 protein for the treatment of COVID-19 infection |
| CN112390879B (en) * | 2021-01-21 | 2021-04-02 | 上海科技大学 | Antibody targeting SARS-CoV-2 and its preparation method and use |
| CN112390879A (en) * | 2021-01-21 | 2021-02-23 | 上海科技大学 | Antibody targeting SARS-CoV-2 and its preparation method and use |
| CN113150130A (en) * | 2021-01-31 | 2021-07-23 | 中南大学湘雅医院 | Novel coronavirus monoclonal antibody and application thereof |
| WO2022181550A1 (en) * | 2021-02-25 | 2022-09-01 | 花王株式会社 | Anti-sars-cov-2 antibody |
| CN113150133A (en) * | 2021-03-15 | 2021-07-23 | 安源医药科技(上海)有限公司 | Monoclonal antibodies or antigen-binding fragments thereof against SARS-CoV-2 |
| CN112679605A (en) * | 2021-03-15 | 2021-04-20 | 安源医药科技(上海)有限公司 | Antibodies or antigen binding fragments thereof against novel coronavirus nucleocapsid proteins and uses thereof |
| CN112679605B (en) * | 2021-03-15 | 2021-07-09 | 安源医药科技(上海)有限公司 | Antibodies or antigen binding fragments thereof against novel coronavirus nucleocapsid proteins and uses thereof |
| WO2022193980A1 (en) * | 2021-03-15 | 2022-09-22 | 安源医药科技(上海)有限公司 | Antibody or antigen-binding fragment thereof for novel coronavirus nucleocapsid protein, and application thereof |
| CN112940087A (en) * | 2021-03-17 | 2021-06-11 | 郑州大学 | Common epitope peptide of SARS-CoV and SARS-CoV-2 and its application |
| CN112666350B (en) * | 2021-03-18 | 2021-06-22 | 北京百普赛斯生物科技股份有限公司 | Test paper and kit for detecting novel coronavirus |
| CN112666350A (en) * | 2021-03-18 | 2021-04-16 | 北京百普赛斯生物科技股份有限公司 | Test paper and kit for detecting novel coronavirus |
| CN113234146A (en) * | 2021-05-07 | 2021-08-10 | 南京川博生物技术有限公司 | Preparation method of coronavirus spike protein monoclonal antibody |
| CN113461810A (en) * | 2021-05-17 | 2021-10-01 | 深圳市福田区格物智康病原研究所 | Fully human monoclonal antibody for resisting novel coronavirus spike protein and application thereof |
| CN113461810B (en) * | 2021-05-17 | 2021-12-28 | 深圳市福田区格物智康病原研究所 | Fully human monoclonal antibody for resisting novel coronavirus spike protein and application thereof |
| CN113493508A (en) * | 2021-06-15 | 2021-10-12 | 北京华大蛋白质研发中心有限公司 | Double-antibody sandwich ELISA kit for detecting new coronavirus N protein |
| CN113603769A (en) * | 2021-07-20 | 2021-11-05 | 贵州省人民医院 | Hybridoma cell strain capable of stably secreting anti-novel coronavirus nucleocapsid protein monoclonal antibody and establishment method and application thereof |
| CN113683692B (en) * | 2021-08-26 | 2023-03-07 | 深圳市亚辉龙生物科技股份有限公司 | SARS-CoV-2N protein antibody and its application |
| WO2023025278A1 (en) * | 2021-08-26 | 2023-03-02 | 深圳市亚辉龙生物科技股份有限公司 | Sars-cov-2n protein antibody and application thereof |
| CN113683692A (en) * | 2021-08-26 | 2021-11-23 | 深圳市亚辉龙生物科技股份有限公司 | SARS-CoV-2N protein antibody and its application |
| CN113461813A (en) * | 2021-09-03 | 2021-10-01 | 上海良润生物医药科技有限公司 | Paired antibody for detecting new coronavirus and application thereof |
| CN113461813B (en) * | 2021-09-03 | 2021-12-14 | 上海良润生物医药科技有限公司 | Paired antibody for detecting new coronavirus and application thereof |
| CN113735969B (en) * | 2021-09-20 | 2023-05-12 | 中国人民解放军军事科学院军事医学研究院 | Fully human anti-new coronavirus broad-spectrum high-neutralization activity monoclonal antibody and application thereof |
| CN113735969A (en) * | 2021-09-20 | 2021-12-03 | 中国人民解放军军事科学院军事医学研究院 | Fully human anti-new coronavirus broad-spectrum high-neutralization-activity monoclonal antibody and application thereof |
| CN114231497A (en) * | 2022-02-24 | 2022-03-25 | 广州伯尼兹生物科技有限公司 | Monoclonal antibody hybridoma cell line for expressing novel coronavirus S1 protein and neutralizing active antibody |
| CN114231497B (en) * | 2022-02-24 | 2022-05-20 | 广州伯尼兹生物科技有限公司 | Monoclonal antibody hybridoma cell line expressing SARS-CoV-2S 1 protein and neutralizing active antibody |
| WO2023199955A1 (en) * | 2022-04-12 | 2023-10-19 | 花王株式会社 | Anti-sars-cov-2 antibody |
| CN114751980B (en) * | 2022-04-22 | 2022-10-28 | 厦门博昂生物技术有限公司 | Monoclonal antibody blocking agent for detecting neocorona antigen |
| CN114751980A (en) * | 2022-04-22 | 2022-07-15 | 厦门博昂生物技术有限公司 | Monoclonal antibody blocking agent for detecting neocorona antigen |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN111153991A (en) | Human SARS-CoV-2 monoclonal antibody and its preparation method and use | |
| CN111269313B (en) | Monoclonal antibody for detecting novel coronavirus and application of monoclonal antibody in preparation of kit | |
| CN112094326B (en) | New coronavirus antigen and application thereof | |
| CN113527476A (en) | Novel nano antibody for resisting H5 subtype avian influenza virus and application thereof | |
| CN114957454A (en) | Nano antibody and fusion protein for resisting CSFV E2 protein, and preparation method and application thereof | |
| CN113087792A (en) | Canine distemper virus nano antibody and application thereof | |
| CN113150138B (en) | KPC-2 monoclonal antibody, and preparation method and application thereof | |
| CN114276445A (en) | Rotavirus recombinant protein specific antibody, plasmid vector and method | |
| CN110527668B (en) | Toxoplasma gondii-resistant coryneform protein 4 (ROP 4) monoclonal antibody, and preparation method and application thereof | |
| CN102690351A (en) | Preparation method of plasmodium vivax aldolase protein monoclonal antibody | |
| CN110702913A (en) | Monoclonal antibody composition for quantitatively detecting coxiella burnetii I strain | |
| CN110894236B (en) | Anti-aspergillus galactomannan monoclonal antibody and application thereof | |
| CN113150139B (en) | PBP2a monoclonal antibody and preparation method and application thereof | |
| CN107098980A (en) | A kind of fusion protein for detecting Detecting Rubella Virus Antibodies In Human Sera and preparation method thereof | |
| CN112679607B (en) | Preparation method of troponin I E13 single-chain antibody | |
| CN110540966B (en) | Human haemophilus influenzae surface protein monoclonal antibody and antigen capture ELISA kit | |
| CN110894235A (en) | Rabbit-derived monoclonal antibody for resisting cryptococcus neoformans tunica polysaccharide and application thereof | |
| CN117534750B (en) | Antibody for resisting novel coronavirus nucleocapsid protein or antigen binding fragment thereof and application thereof | |
| CN114751963B (en) | Protein for detecting foot-and-mouth disease virus antibody and application thereof | |
| CN116444653B (en) | Preparation and application of blocking African swine fever virus monoclonal antibody hybridoma cell strain | |
| CN116769040B (en) | Mouse anti-GDH hybridoma cell strain, monoclonal antibody and application | |
| CN118562012B (en) | Mouse anti-candida parapsilosis mannan hybridoma cell strain and monoclonal antibody | |
| CN114685619B (en) | Antigen protein, monoclonal antibody or polyclonal antibody and application thereof | |
| CN117363582B (en) | Hybridoma cell strain secreting anti-peste des petits ruminants virus F protein monoclonal antibody, monoclonal antibody thereof and application | |
| CN107286240B (en) | anti-CTRP 4 monoclonal antibody and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |