CN110408947B - Nickel-cobalt oxide electrode material of composite silver oxide and preparation method and application thereof - Google Patents
Nickel-cobalt oxide electrode material of composite silver oxide and preparation method and application thereof Download PDFInfo
- Publication number
- CN110408947B CN110408947B CN201910655151.3A CN201910655151A CN110408947B CN 110408947 B CN110408947 B CN 110408947B CN 201910655151 A CN201910655151 A CN 201910655151A CN 110408947 B CN110408947 B CN 110408947B
- Authority
- CN
- China
- Prior art keywords
- nickel
- electrode material
- cobalt
- silver
- oxide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000007772 electrode material Substances 0.000 title claims abstract description 54
- NDVLTYZPCACLMA-UHFFFAOYSA-N silver oxide Chemical compound [O-2].[Ag+].[Ag+] NDVLTYZPCACLMA-UHFFFAOYSA-N 0.000 title claims abstract description 54
- YTBWYQYUOZHUKJ-UHFFFAOYSA-N oxocobalt;oxonickel Chemical compound [Co]=O.[Ni]=O YTBWYQYUOZHUKJ-UHFFFAOYSA-N 0.000 title claims abstract description 37
- 239000002131 composite material Substances 0.000 title claims abstract description 31
- 229910001923 silver oxide Inorganic materials 0.000 title claims abstract description 27
- 238000002360 preparation method Methods 0.000 title abstract description 14
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims abstract description 102
- 229910052759 nickel Inorganic materials 0.000 claims abstract description 51
- 238000001027 hydrothermal synthesis Methods 0.000 claims abstract description 19
- 239000006260 foam Substances 0.000 claims abstract description 18
- BFDHFSHZJLFAMC-UHFFFAOYSA-L nickel(ii) hydroxide Chemical compound [OH-].[OH-].[Ni+2] BFDHFSHZJLFAMC-UHFFFAOYSA-L 0.000 claims abstract description 17
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims abstract description 15
- 239000001301 oxygen Substances 0.000 claims abstract description 15
- 229910052760 oxygen Inorganic materials 0.000 claims abstract description 15
- 239000002070 nanowire Substances 0.000 claims abstract description 14
- 239000000758 substrate Substances 0.000 claims abstract description 12
- 239000002243 precursor Substances 0.000 claims abstract description 10
- 239000012266 salt solution Substances 0.000 claims abstract description 8
- QXZUUHYBWMWJHK-UHFFFAOYSA-N [Co].[Ni] Chemical compound [Co].[Ni] QXZUUHYBWMWJHK-UHFFFAOYSA-N 0.000 claims abstract description 7
- 238000000137 annealing Methods 0.000 claims abstract description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 claims abstract description 7
- 239000002057 nanoflower Substances 0.000 claims abstract description 7
- 239000002105 nanoparticle Substances 0.000 claims abstract description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 19
- 238000000034 method Methods 0.000 claims description 16
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 claims description 16
- 239000003054 catalyst Substances 0.000 claims description 13
- 239000000243 solution Substances 0.000 claims description 11
- 239000008367 deionised water Substances 0.000 claims description 8
- 229910021641 deionized water Inorganic materials 0.000 claims description 8
- 150000002815 nickel Chemical class 0.000 claims description 8
- 229910001961 silver nitrate Inorganic materials 0.000 claims description 8
- 238000005406 washing Methods 0.000 claims description 8
- 238000001035 drying Methods 0.000 claims description 7
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 claims description 6
- 239000004202 carbamide Substances 0.000 claims description 6
- 150000001868 cobalt Chemical class 0.000 claims description 6
- 229910021586 Nickel(II) chloride Inorganic materials 0.000 claims description 4
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 claims description 4
- UFMZWBIQTDUYBN-UHFFFAOYSA-N cobalt dinitrate Chemical group [Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O UFMZWBIQTDUYBN-UHFFFAOYSA-N 0.000 claims description 4
- 229910001981 cobalt nitrate Inorganic materials 0.000 claims description 4
- QMMRZOWCJAIUJA-UHFFFAOYSA-L nickel dichloride Chemical compound Cl[Ni]Cl QMMRZOWCJAIUJA-UHFFFAOYSA-L 0.000 claims description 4
- 229910052709 silver Inorganic materials 0.000 claims description 4
- 239000004332 silver Substances 0.000 claims description 4
- GVPFVAHMJGGAJG-UHFFFAOYSA-L cobalt dichloride Chemical compound [Cl-].[Cl-].[Co+2] GVPFVAHMJGGAJG-UHFFFAOYSA-L 0.000 claims description 2
- 229940044175 cobalt sulfate Drugs 0.000 claims description 2
- 229910000361 cobalt sulfate Inorganic materials 0.000 claims description 2
- KTVIXTQDYHMGHF-UHFFFAOYSA-L cobalt(2+) sulfate Chemical compound [Co+2].[O-]S([O-])(=O)=O KTVIXTQDYHMGHF-UHFFFAOYSA-L 0.000 claims description 2
- 150000001875 compounds Chemical class 0.000 claims description 2
- 238000005868 electrolysis reaction Methods 0.000 claims description 2
- LGQLOGILCSXPEA-UHFFFAOYSA-L nickel sulfate Chemical compound [Ni+2].[O-]S([O-])(=O)=O LGQLOGILCSXPEA-UHFFFAOYSA-L 0.000 claims description 2
- 229910000363 nickel(II) sulfate Inorganic materials 0.000 claims description 2
- KBJMLQFLOWQJNF-UHFFFAOYSA-N nickel(ii) nitrate Chemical compound [Ni+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O KBJMLQFLOWQJNF-UHFFFAOYSA-N 0.000 claims description 2
- 239000002994 raw material Substances 0.000 claims description 2
- 230000003197 catalytic effect Effects 0.000 abstract description 10
- 239000000463 material Substances 0.000 abstract description 8
- 230000007797 corrosion Effects 0.000 abstract description 2
- 238000005260 corrosion Methods 0.000 abstract description 2
- 238000005265 energy consumption Methods 0.000 abstract description 2
- 230000007613 environmental effect Effects 0.000 abstract description 2
- 241000446313 Lamella Species 0.000 abstract 1
- 239000004094 surface-active agent Substances 0.000 abstract 1
- 230000000052 comparative effect Effects 0.000 description 14
- 238000006243 chemical reaction Methods 0.000 description 11
- 229910003266 NiCo Inorganic materials 0.000 description 10
- 229910000510 noble metal Inorganic materials 0.000 description 7
- 238000012360 testing method Methods 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 238000001000 micrograph Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 238000009210 therapy by ultrasound Methods 0.000 description 3
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000009776 industrial production Methods 0.000 description 2
- 238000004502 linear sweep voltammetry Methods 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 238000012876 topography Methods 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- MXEHMOBFYMIGAE-UHFFFAOYSA-L cobalt(2+);nickel;dihydroxide Chemical compound [OH-].[OH-].[Co+2].[Ni] MXEHMOBFYMIGAE-UHFFFAOYSA-L 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- ZOMNIUBKTOKEHS-UHFFFAOYSA-L dimercury dichloride Chemical class Cl[Hg][Hg]Cl ZOMNIUBKTOKEHS-UHFFFAOYSA-L 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000003912 environmental pollution Methods 0.000 description 1
- 235000019441 ethanol Nutrition 0.000 description 1
- 239000006261 foam material Substances 0.000 description 1
- HTXDPTMKBJXEOW-UHFFFAOYSA-N iridium(IV) oxide Inorganic materials O=[Ir]=O HTXDPTMKBJXEOW-UHFFFAOYSA-N 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000002086 nanomaterial Substances 0.000 description 1
- 239000002135 nanosheet Substances 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000027756 respiratory electron transport chain Effects 0.000 description 1
- 238000001878 scanning electron micrograph Methods 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- -1 therefore Substances 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/002—Mixed oxides other than spinels, e.g. perovskite
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/70—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper
- B01J23/89—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of the iron group metals or copper combined with noble metals
- B01J23/892—Nickel and noble metals
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/33—Electric or magnetic properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y40/00—Manufacture or treatment of nanostructures
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/02—Hydrogen or oxygen
- C25B1/04—Hydrogen or oxygen by electrolysis of water
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/055—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the substrate or carrier material
- C25B11/057—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the substrate or carrier material consisting of a single element or compound
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/073—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material
- C25B11/091—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material consisting of at least one catalytic element and at least one catalytic compound; consisting of two or more catalytic elements or catalytic compounds
- C25B11/093—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material consisting of at least one catalytic element and at least one catalytic compound; consisting of two or more catalytic elements or catalytic compounds at least one noble metal or noble metal oxide and at least one non-noble metal oxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2523/00—Constitutive chemical elements of heterogeneous catalysts
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Nanotechnology (AREA)
- Electrochemistry (AREA)
- Metallurgy (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Composite Materials (AREA)
- Inorganic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
- Battery Electrode And Active Subsutance (AREA)
Abstract
The invention discloses a nickel-cobalt oxide electrode material of composite silver oxide and a preparation method and application thereof. The electrode material takes foamed nickel as a substrate, layered nickel hydroxide grows on the surface of the substrate, silver oxide nano particles are attached to the layered nickel hydroxide, nanowires grow on the lamella, and nickel-cobalt oxide nanoflowers grow on the nanowires. The preparation method of the material comprises the following steps: firstly, preparing a precursor salt solution in the first step, adding pretreated nickel foam, and generating silver-doped layered nickel hydroxide by adopting a hydrothermal method; preparing a precursor salt solution in the second step, adding the foamed nickel obtained in the first step, and generating silver-doped nickel-cobalt double hydroxide by adopting a hydrothermal method; finally, annealing in the air to obtain the nickel-cobalt oxide of the composite silver oxide. The invention realizes the combination of the surface active substance and the foam nickel substrate, and has simple preparation process, time saving, low energy consumption, energy saving and environmental protection; has larger active area and excellent oxygen evolution catalytic activity, and resists corrosion under alkaline conditions.
Description
Technical Field
The invention relates to a nickel-cobalt oxide electrode material of composite silver oxide and a preparation method and application thereof, belonging to the technical field of electrolytic water catalytic oxygen evolution.
Background
Energy is the fundamental driving force for the development of human productivity as a cornerstone for various production activities in the human society. From ancient times to the present, fossil energy, which is a main energy source utilized by human, is consumed at a rate that becomes faster and faster as human develops, and particularly, since the fossil energy enters an industrial society, the existing storage is about to be depleted over centuries. The consumption of fossil energy also brings environmental pollution problems, and therefore, the development of sustainable and clean alternative energy is urgent. Due to combustion of hydrogenThe process energy releases huge energy and the product is water, therefore, hydrogen is considered as a sustainable clean energy source. From the aspect of environmental friendliness, hydrogen production by electrochemically catalyzing and decomposing water is one of ideal ways for preparing hydrogen. The electrolytic water comprises an anodic oxygen generation reaction (OER) and a cathodic Hydrogen Evolution Reaction (HER), wherein the OER reaction is a kinetic slow process of four electron transfer and tends to consume higher energy. At present, noble metal based catalysts (RuO)2/IrO2) Is a high-efficiency oxygen-producing catalyst, however, the noble metal-based catalysts are expensive and have limited reserves, which limits the large-scale industrial application of the noble metal-based catalysts. Therefore, in order to meet the requirement of developing sustainable energy, it is important to develop and apply an efficient and cheap oxygen evolution catalyst to replace an expensive noble metal catalyst.
In recent years, some non-noble metals have been used as oxygen evolution catalysts, but the catalytic stability and catalytic performance cannot meet the requirements of industrial production. Therefore, the development of a non-noble metal catalyst which is low in cost, easy to prepare and high in performance has important significance for promoting the industrial development of the oxygen evolution reaction. Researches find that the oxygen evolution reaction under the alkaline condition has the advantages of no pollution, convenient operation, mature technology, easy large-scale production and the like, and becomes one of the research hotspots. However, the catalyst for oxygen evolution reaction under alkaline conditions has problems of poor catalytic activity and stability, and further research is required.
Disclosure of Invention
Aiming at the defects in the prior art, the invention aims to provide a silver-doped nickel-cobalt oxide electrode material, a preparation method and application thereof.
In order to achieve the purpose, the technical scheme adopted by the invention is as follows: the nickel-cobalt oxide electrode material of the composite silver oxide is characterized in that foamed nickel is used as a substrate, layered nickel hydroxide grows on the surface of the foamed nickel, silver oxide nano particles are attached to the layered nickel hydroxide, nickel-cobalt oxide nano wires also grow on the layered nickel hydroxide, and nickel-cobalt oxide nano flowers grow on the nano wires.
The invention also provides a preparation method of the nickel-cobalt oxide electrode material of the composite silver oxide, which is characterized by comprising the following steps:
1) putting the foamed nickel into a silver nitrate solution, carrying out hydrothermal reaction, washing and drying a product to obtain a silver-doped layered nickel hydroxide electrode material;
2) preparing a precursor salt solution by taking nickel salt, cobalt salt, urea and deionized water as raw materials;
3) putting the silver-doped layered nickel hydroxide electrode material obtained in the step 1) into the precursor solution obtained in the step 2), performing hydrothermal reaction, and washing and drying a product to obtain a silver-doped nickel-cobalt double hydroxide electrode material;
4) annealing the silver-doped nickel-cobalt double hydroxide electrode material obtained in the step 3) in the air to obtain the silver oxide-compounded nickel-cobalt oxide electrode material.
According to the scheme, preferably, the concentration of the silver nitrate solution in the step 1) is 0.001-0.002 mol/L.
According to the scheme, preferably, the purity of the foamed nickel in the step 1) is more than 99.8 percent, and the areal density is 300-450g/cm2。
According to the scheme, preferably, the foamed nickel in the step 1) is pretreated for removing surface oil stains and oxide pollution layers, and is removed by an ultrasonic method after being soaked in an organic solvent and an acid solution. More preferably, the organic solvent is one or more of methanol, ethanol, tetrahydrofuran or chloroform, the acid is diluted hydrochloric acid, and the ultrasonic treatment time is 10-50 min.
According to the scheme, the temperature of the hydrothermal reaction in the step 1) is preferably 160 ℃, and the time is 6-8 hours.
According to the scheme, preferably, the nickel salt in the step 2) is nickel nitrate, nickel sulfate or nickel chloride; the cobalt salt is cobalt nitrate, cobalt sulfate or cobalt chloride. More preferably, the molar ratio of nickel salt to cobalt salt, urea is 1: 2: 3. more preferably, the dosage ratio of the nickel salt to the used deionized water is 0.05-0.1 mol: 1L of the compound.
According to the scheme, the temperature of the hydrothermal reaction in the step 3) is preferably 120 ℃, and the time is 6-8 hours.
According to the scheme, preferably, the annealing temperature in the step 4) is 250-350 ℃, and the heat preservation time is 2-3 h.
The invention also provides application of the nickel-cobalt oxide electrode material of the composite silver oxide, which is characterized in that the electrode material can be used as an electrode or a catalyst for preparing oxygen by electrolyzing water.
Compared with the prior art, the technical scheme of the invention has the following beneficial effects:
(1) according to the invention, the nickel foam is used as a substrate, and the hydrothermal method is adopted for preparation, so that the prepared electrode material has good corrosion resistance and controllable appearance; and the nickel-based metal has low price and stable catalytic performance under alkaline conditions.
(2) The preparation method of the nickel-cobalt oxide electrode material of the composite silver oxide, provided by the invention, has the advantages of simple process and low energy consumption in the preparation process, and is convenient for realizing industrial production.
(3) The nickel-cobalt oxide electrode material of the composite silver oxide prepared by the invention has unique appearance, is composed of zero-dimensional silver oxide nano particles, one-dimensional nano wires, two-dimensional nano sheets and three-dimensional nano flowers, can expose more active sites, and has larger active area.
(4) The nickel-cobalt oxide electrode material of the composite silver oxide prepared by the invention has higher catalytic activity. Compared with a pure nickel-cobalt oxide material, the electrode material provided by the invention has the current density of 10mA/cm2When the oxygen evolution performance is improved by 57 mv. Can be widely used as an alkaline electrolyzed water oxygen evolution electrode material and has wide application prospect.
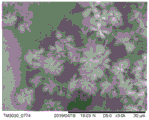
Drawings
FIG. 1 is a scanning image of the surface morphology of the NiCoO/AgO/foam nickel composite electrode material prepared in example 1 of the present invention.
FIG. 2 is a scanned surface topography of NiCo LDH/foamed nickel electrode material prepared in comparative example 1 of the present invention.
FIG. 3 is a scanned surface topography of a NiCoO/foam nickel composite electrode material prepared in comparative example 2 of the present invention.
FIG. 4 is a linear sweep voltammetry curve of pure nickel foam obtained by the test of the present invention, composite electrode materials prepared in comparative examples 1-2 and example 1.
Detailed Description
For a further understanding of the present invention, reference will now be made in detail to the following examples.
Example 1
A preparation method of NiCoO/AgO/foamed nickel composite electrode material comprises the following steps:
I. pretreatment of a foamed nickel substrate: selecting the cut 1X 3cm2The nickel foam (purity: 99.8%, areal density: 400 g/cm)2) Soaking in absolute ethyl alcohol, carrying out ultrasonic treatment for 15min, then using 2mol/L hydrochloric acid to carry out ultrasonic treatment for 15min, removing oil stains and oxide pollution layers on the surface, and finally washing the foam nickel substrate clean by deionized water.
II. Forming NiCo LDH/Ag (silver-doped nickel cobalt double hydroxide) on the pretreated foamed nickel through two-step hydrothermal reaction:
1) preparing a diluted silver nitrate solution: 0.5mL of silver nitrate solution with the concentration of 0.15mol/L is added into 39.5mL of deionized water to obtain diluted silver nitrate solution.
2) And (3) putting the foamed nickel substrate pretreated in the step (I) into the diluted silver nitrate solution, performing hydrothermal reaction for 6 hours in a high-pressure reaction kettle at 160 ℃, and washing and drying a product to obtain the foamed nickel material with the layered nickel hydroxide with the silver attached on the surface.
3) 2.5mmol of nickel chloride, 5mmol of cobalt nitrate and 7.5mmol of urea are dissolved in 30ml of deionized water to obtain a precursor salt solution.
4) And (3) putting the foamed nickel material with the surface growing with the layered nickel hydroxide attached with silver after hydrothermal reaction in the step 2) into the precursor salt solution, performing hydrothermal reaction in a high-pressure reaction kettle at 120 ℃ for 6h, and washing and drying the product after the reaction is finished to obtain the foamed nickel material with the surface growing with NiCo LDH/Ag.
III, annealing treatment: and (3) putting the foamed nickel material with the NiCo LDH/Ag growing on the surface obtained in the step (II) into a tube furnace, heating to 300 ℃ at the heating rate of 5 ℃/min in the air atmosphere, preserving the heat for 2h, and cooling to room temperature at the cooling rate of 5 ℃/min to obtain the NiCoO/AgO/foamed nickel composite electrode material.
Fig. 1 is a scanning electron microscope image of the surface morphology of the electrode material in which the layered nickel hydroxide with silver oxide attached thereto is grown on the foamed nickel prepared in this example, the nanowire is grown on the sheet layer, and the nickel-cobalt oxide nanoflower is grown on the nanowire.
Comparative example 1
Preparation of a NiCo LDH/foamed nickel electrode material, which comprises the following steps:
I. pretreatment of a foamed nickel substrate: the procedure is as in step I of example 1.
II. Hydrothermal reaction on pretreated nickel foam to form NiCo LDH:
2.5mmol of nickel chloride, 5mmol of cobalt nitrate and 7.5mmol of urea are dissolved in 30ml of deionized water to obtain a precursor salt solution. And (3) putting the foamed nickel substrate pretreated in the step (I) into the precursor salt solution, performing hydrothermal reaction for 6 hours at 120 ℃ in a high-pressure reaction kettle, and washing and drying a product after the reaction is finished to obtain the foamed nickel material with the linear NiCo LDH growing on the surface.
FIG. 2 is a scanning electron microscope image of the surface morphology of the NiCo LDH/foamed nickel composite nanomaterial prepared in this comparative example 1.
Comparative example 2
A preparation method of NiCoO/foamed nickel composite electrode material comprises the following steps:
I. pretreatment of a foamed nickel substrate: the procedure is as in comparative example 1, step I.
II. Hydrothermal reaction on pretreated nickel foam to form NiCo LDH: the procedure is as in step II of comparative example 1.
III, annealing to form NiCoO: and (3) putting the NiCo LDH/foamed nickel obtained by the hydrothermal method in the step (II) into a tube furnace, heating to 300 ℃ at the heating rate of 5 ℃/min in the air atmosphere, preserving the heat for 2h, and cooling to room temperature at the cooling rate of 5 ℃/min.
FIG. 3 is a scanning electron microscope image of the surface morphology of the NiCoO/foam nickel electrode material prepared in this comparative example 2.
And (4) analyzing results:
as is clear from the scanning electron micrographs of fig. 1 to 3, in comparative example 1, nanowires of nickel cobalt double hydroxide were uniformly grown on nickel foam; in comparative example 2, the nanowires were uniformly grown on the nickel foam, and nanoflowers of nickel-cobalt oxide were grown on the nanowires; in example 1, layered nickel hydroxide attached with silver oxide is grown on the nickel foam, nanowires are grown on the sheet layer, and nanoflowers of nickel-cobalt oxide are grown on the nanowires, so that the active area and catalytic activity of the material can be greatly increased.
And (3) effect testing:
the electrode materials prepared in the comparative examples 1-2 and the example 1 are used as electrolytic water electrodes for catalytic oxygen generation by electrolytic water, and pure nickel foam is used as a blank control.
The pure nickel foam and the composite electrode materials prepared respectively in the three examples are subjected to a linear sweep voltammetry curve test:
in the testing process, a three-electrode system is adopted, 1mol/L KOH is used as electrolyte, a carbon rod is used as a counter electrode, a saturated calomel electrode is used as a reference electrode, foamed nickel and composite electrode materials prepared in three embodiments are respectively used as working electrodes in sequence for testing, the foamed nickel and the composite electrode materials prepared in the three embodiments are guaranteed to have the same geometric area, and the testing result is shown in figure 4.
As can be seen from FIG. 4, when the current density was 10mA/cm2Compared with pure nickel foam, the performance of the silver-doped nickel-cobalt oxide electrode material is greatly improved. As can be seen from the above comparison, when the silver-doped nickel cobalt oxide electrode material prepared in example 1 of the present invention is used as a catalyst for water electrolysis, the silver-doped nickel cobalt oxide electrode material is compared with the nickel cobalt dihydroxide prepared in comparative example 1Compared with the nickel foam material of nickel cobalt oxide prepared in comparative example 2, the oxide has large active area and excellent catalytic performance, and is hopeful to replace noble metal catalyst in the field of oxygen production by electrolyzing water.
While the foregoing is directed to the preferred embodiment of the present invention, other and further embodiments of the invention may be devised without departing from the basic scope thereof, and the scope thereof is determined by the claims that follow.
Claims (10)
1. The nickel-cobalt oxide electrode material of the composite silver oxide is characterized in that foamed nickel is used as a substrate, layered nickel hydroxide grows on the surface of the foamed nickel, silver oxide nano particles are attached to the layered nickel hydroxide, nickel-cobalt oxide nano wires also grow on the layered nickel hydroxide, and nickel-cobalt oxide nano flowers grow on the nano wires.
2. The method for preparing the nickel cobalt oxide electrode material of composite silver oxide according to claim 1, comprising the steps of:
1) putting the foamed nickel into a silver nitrate solution, carrying out hydrothermal reaction, washing and drying a product to obtain a silver-doped layered nickel hydroxide electrode material;
2) preparing a precursor salt solution by taking nickel salt, cobalt salt, urea and deionized water as raw materials;
3) putting the silver-doped layered nickel hydroxide electrode material obtained in the step 1) into the precursor solution obtained in the step 2), performing hydrothermal reaction, and washing and drying a product to obtain a silver-doped nickel-cobalt double hydroxide electrode material;
4) annealing the silver-doped nickel-cobalt double hydroxide electrode material obtained in the step 3) in the air to obtain the silver oxide-compounded nickel-cobalt oxide electrode material.
3. The method for preparing the nickel cobalt oxide electrode material of composite silver oxide according to claim 2, wherein the concentration of the silver nitrate solution in the step 1) is 0.001-0.002 mol/L.
4. The method for preparing the nickel cobalt oxide electrode material of composite silver oxide as claimed in claim 2, wherein the purity of the nickel foam in step 1) is more than 99.8%, and the surface density is 300-450g/cm2。
5. The method for preparing the nickel cobalt oxide electrode material of composite silver oxide according to claim 2, wherein the temperature of the hydrothermal reaction in the step 1) is 160 ℃ and the time is 6-8 h.
6. The method for preparing the nickel cobalt oxide electrode material of composite silver oxide according to claim 2, wherein the nickel salt in step 2) is nickel nitrate, nickel sulfate or nickel chloride; the cobalt salt is cobalt nitrate, cobalt sulfate or cobalt chloride.
7. The method for preparing a nickel cobalt oxide electrode material of composite silver oxide according to claim 6, wherein the molar ratio of the nickel salt to the cobalt salt and urea is 1: 2: 3; the dosage ratio of the nickel salt to the used deionized water is 0.05-0.1 mol: 1L of the compound.
8. The method for preparing the nickel cobalt oxide electrode material of composite silver oxide according to claim 2, wherein the temperature of the hydrothermal reaction in the step 3) is 120 ℃ and the time is 6-8 h.
9. The method for preparing the nickel cobalt oxide electrode material of composite silver oxide according to claim 2, wherein the annealing temperature in the step 4) is 250-350 ℃, and the holding time is 2-3 h.
10. The use of the nickel cobalt oxide electrode material of silver oxide composite of claim 1, wherein the electrode material is used as an electrode or catalyst for the electrolysis of water to produce oxygen.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910655151.3A CN110408947B (en) | 2019-07-19 | 2019-07-19 | Nickel-cobalt oxide electrode material of composite silver oxide and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201910655151.3A CN110408947B (en) | 2019-07-19 | 2019-07-19 | Nickel-cobalt oxide electrode material of composite silver oxide and preparation method and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN110408947A CN110408947A (en) | 2019-11-05 |
| CN110408947B true CN110408947B (en) | 2021-12-03 |
Family
ID=68362088
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201910655151.3A Active CN110408947B (en) | 2019-07-19 | 2019-07-19 | Nickel-cobalt oxide electrode material of composite silver oxide and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN110408947B (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114976038B (en) * | 2022-07-28 | 2022-10-25 | 华中科技大学 | Silver-silver oxide heterogeneous nanoflower modified foamy copper and preparation method and application thereof |
| CN115505958A (en) * | 2022-09-30 | 2022-12-23 | 武汉工程大学 | Foam metal loaded bi-spinel type oxide CuCo 2 O 4 -Co 3 O 4 Preparation and application of derivatives thereof |
| CN116422333A (en) * | 2023-03-23 | 2023-07-14 | 华南协同创新研究院 | Three-dimensional foam-based photocatalytic material for efficiently removing composite pollutants in water as well as preparation method and application thereof |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS60159184A (en) * | 1984-01-27 | 1985-08-20 | Agency Of Ind Science & Technol | Anode for electrolyzing water |
| CN106807379A (en) * | 2015-11-27 | 2017-06-09 | 中国科学院大连化学物理研究所 | A kind of flower ball-shaped nickel cobalt oxide oxygen-separating catalyst and its preparation method and application |
| CN106807378A (en) * | 2015-11-27 | 2017-06-09 | 中国科学院大连化学物理研究所 | A kind of hexagon nickel cobalt oxide oxygen-separating catalyst and its preparation method and application |
| CN107146711A (en) * | 2017-04-10 | 2017-09-08 | 华南理工大学 | A kind of conductive substrates growth nano lamellar metal compound electrode material and its preparation and application |
| CN108448117A (en) * | 2018-03-07 | 2018-08-24 | 中国科学院上海高等研究院 | Ultra-thin nickel cobalt oxide nanometer sheet electrod-array rich in oxygen defect and preparation method |
| CN108754532A (en) * | 2018-05-29 | 2018-11-06 | 武汉工程大学 | A kind of iron of molybdenum doping/nickel layer shape array@nickel foam based combined electrode materials and the preparation method and application thereof |
-
2019
- 2019-07-19 CN CN201910655151.3A patent/CN110408947B/en active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS60159184A (en) * | 1984-01-27 | 1985-08-20 | Agency Of Ind Science & Technol | Anode for electrolyzing water |
| CN106807379A (en) * | 2015-11-27 | 2017-06-09 | 中国科学院大连化学物理研究所 | A kind of flower ball-shaped nickel cobalt oxide oxygen-separating catalyst and its preparation method and application |
| CN106807378A (en) * | 2015-11-27 | 2017-06-09 | 中国科学院大连化学物理研究所 | A kind of hexagon nickel cobalt oxide oxygen-separating catalyst and its preparation method and application |
| CN107146711A (en) * | 2017-04-10 | 2017-09-08 | 华南理工大学 | A kind of conductive substrates growth nano lamellar metal compound electrode material and its preparation and application |
| CN108448117A (en) * | 2018-03-07 | 2018-08-24 | 中国科学院上海高等研究院 | Ultra-thin nickel cobalt oxide nanometer sheet electrod-array rich in oxygen defect and preparation method |
| CN108754532A (en) * | 2018-05-29 | 2018-11-06 | 武汉工程大学 | A kind of iron of molybdenum doping/nickel layer shape array@nickel foam based combined electrode materials and the preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN110408947A (en) | 2019-11-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110201670B (en) | Ferronickel double-metal hydroxide/foamed nickel catalyst based on ferric trichloride/urea eutectic solvent, and preparation method and application thereof | |
| CN108796535B (en) | Copper-cobalt-molybdenum/nickel foam porous electrode material with trimetal, and preparation method and application thereof | |
| CN109201060B (en) | Preparation method of foamed nickel-nickel iron oxide composite oxygen evolution catalyst | |
| CN107376958B (en) | NiFeP difunctional transition metal phosphide catalyst and preparation and application thereof | |
| CN110331414B (en) | MOF (Metal organic framework) composite copper-based nanorod array @ foam copper-based composite electrode material as well as preparation method and application thereof | |
| CN108754532B (en) | Molybdenum-doped iron/nickel layered array @ foam nickel-based composite electrode material and preparation method and application thereof | |
| CN108439549B (en) | Preparation of array structure transition metal selenide electrode and application thereof in electrolytic water | |
| CN111636074B (en) | Preparation and application of copper electrode for electrochemical reduction of carbon dioxide | |
| CN109852992B (en) | Efficient electrocatalytic full-decomposition water nanosheet array electrode and preparation method and application thereof | |
| CN109433228B (en) | Angular Ni with hierarchical structure3S2/VS4Electrode material and preparation method thereof | |
| CN109621981B (en) | Metal oxide-sulfide composite oxygen evolution electrocatalyst and preparation method and application thereof | |
| CN110408947B (en) | Nickel-cobalt oxide electrode material of composite silver oxide and preparation method and application thereof | |
| CN109277110B (en) | Irregular spherical V-doped Ni3S2/NF oxygen evolution electric catalyst and preparation method thereof | |
| CN110306204B (en) | Silver-doped layered nickel hydroxide composite electrode material and preparation method and application thereof | |
| CN112080759B (en) | Preparation method of bismuth-doped bimetallic sulfide electrode for electrocatalytic oxidation of urea | |
| CN108611659B (en) | High-efficiency stable Co3O4Nanoribbon array chlorine evolution electrode | |
| CN110965076A (en) | Preparation method of electrolytic water electrode with double-function three-dimensional layered core-shell structure | |
| CN113957456A (en) | Nickel-based alkaline electrolytic water catalyst with co-doped combination heterostructure and preparation method thereof | |
| CN113862726A (en) | Preparation method and application of molybdenum-selenium double-element doped porous sheet layered nickel phosphide material | |
| CN114289043B (en) | Preparation method and application of self-supporting porous nano-plate cobalt-nickel phosphide catalyst | |
| CN111001414A (en) | Structure-controllable hollow nickel cobaltate nanowire/flaky manganese oxide core-shell array material and preparation method thereof | |
| CN114892206B (en) | Multi-metal nitride heterojunction nanorod array composite electrocatalyst and preparation method and application thereof | |
| CN113235125B (en) | Nickel-based NiCo 2 O 4 Electrocatalyst and its use in electrocatalytic oxidation of glycerol | |
| CN112921351B (en) | Preparation method and application of self-supporting catalytic electrode | |
| CN113249743B (en) | Catalyst for electrocatalytic oxidation of glycerol and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |