CN108529642B - Preparation method of Cu-SSZ-13 molecular sieve - Google Patents
Preparation method of Cu-SSZ-13 molecular sieve Download PDFInfo
- Publication number
- CN108529642B CN108529642B CN201810506476.0A CN201810506476A CN108529642B CN 108529642 B CN108529642 B CN 108529642B CN 201810506476 A CN201810506476 A CN 201810506476A CN 108529642 B CN108529642 B CN 108529642B
- Authority
- CN
- China
- Prior art keywords
- molecular sieve
- ssz
- hours
- product
- crystallized
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B39/00—Compounds having molecular sieve and base-exchange properties, e.g. crystalline zeolites; Their preparation; After-treatment, e.g. ion-exchange or dealumination
- C01B39/02—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof; Direct preparation thereof; Preparation thereof starting from a reaction mixture containing a crystalline zeolite of another type, or from preformed reactants; After-treatment thereof
- C01B39/06—Preparation of isomorphous zeolites characterised by measures to replace the aluminium or silicon atoms in the lattice framework by atoms of other elements, i.e. by direct or secondary synthesis
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B39/00—Compounds having molecular sieve and base-exchange properties, e.g. crystalline zeolites; Their preparation; After-treatment, e.g. ion-exchange or dealumination
- C01B39/02—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof; Direct preparation thereof; Preparation thereof starting from a reaction mixture containing a crystalline zeolite of another type, or from preformed reactants; After-treatment thereof
- C01B39/04—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof; Direct preparation thereof; Preparation thereof starting from a reaction mixture containing a crystalline zeolite of another type, or from preformed reactants; After-treatment thereof using at least one organic template directing agent, e.g. an ionic quaternary ammonium compound or an aminated compound
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/70—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data
- C01P2002/72—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data by d-values or two theta-values, e.g. as X-ray diagram
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
- C01P2004/03—Particle morphology depicted by an image obtained by SEM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/61—Micrometer sized, i.e. from 1-100 micrometer
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/12—Surface area
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Inorganic Chemistry (AREA)
- Catalysts (AREA)
- Silicates, Zeolites, And Molecular Sieves (AREA)
Abstract
The invention provides a preparation method of a Cu-SSZ-13 molecular sieve, which comprises the following steps: (1) separating aluminum source, sodium hydroxide, Cu-TEPA complex and template agentMixing the water, adding a silicon source after uniformly stirring, and continuously stirring until uniformly mixing to obtain a mixture; (2) crystallizing the mixture at 120-170 ℃ for 48-96 hours to obtain a crystallized product; (3) drying the crystallized product at 80-120 ℃ for 8-12 hours to obtain a dried product; (4) and roasting the dried product at 500-600 ℃ for 6-10 hours to obtain the Cu-SSZ-13 molecular sieve. The molecular sieve has wide application prospect in the fields of petrochemical industry, MTO reaction, tail gas purification, fine chemical industry and the like, and can be used as a catalyst for removing NO in automobile tail gasx。
Description
Technical Field
The invention relates to the technical field of modified catalysts, in particular to a preparation method of a Cu-SSZ-13 molecular sieve.
Background
Over the past decades, Nitrogen Oxides (NO)x) A number of environmental problems have been created, such as: acid rain and photochemical smog in cities, nitrogen oxides in motor vehicle exhaust, and the like. In the United states, European Union and Japan, a series of regulations for strictly controlling the emission of nitrogen oxide-containing exhaust gas have been issued to discharge NO in exhaust gasxThe content of (b) is required to be controlled at a lower level. Therefore, the development of newer and more effective exhaust gas purification technologies becomes a hot spot and urgent research.
In addition, in recent years, due to the soaring price of petroleum, a technology for converting methanol into low carbon olefins (MTO) has attracted attention, and research on the technology has focused mainly on the screening and preparation of catalysts. The smaller the pore size of the molecular sieve, the better the catalytic selectivity.
Due to the unique physicochemical properties of microporous molecular sieves, microporous molecular sieves have shown wide application prospects in more and more fields, and have become the focus of research in recent years. Since the successful synthesis of artificial zeolite in 1948, new zeolite is continuously emerging, and has been widely applied to the fields of petrochemical industry, metallurgy, metal processing, mechanical manufacturing, pesticides, environmental protection and the like, and becomes an important material which cannot be replaced in industry and agriculture. In the 80S of the 20 th century, a new molecular sieve SSZ-13 was synthesized by the united states chemist Zones S I by a hydrothermal process. The zeolite is a Chabazite (CHA) having a structure made of AlO4And SiO4The tetrahedrons are connected end to end through oxygen atoms and are orderly arranged into an ellipsoidal crystal structure with an eight-membered ring structure, and the size of a pore channel is only 0.3 nm. The SSZ-13 belongs to small-pore zeolite and is divided according to the size of zeolite pore channels, and the specific surface area can reach up to 700m2(ii) in terms of/g. SSZ-13 has good thermal stability due to its large specific surface area and structural characteristics of eight-membered ring, and can be used as adsorbent or catalyst carrier, such asAir purificant, automobile exhaust catalyst, etc. Meanwhile, SSZ-13 also has cation exchange property and acidity adjustability, so that the catalyst has good catalytic performance on various reaction processes, including catalytic cracking and hydrocracking of hydrocarbon compounds, olefin and aromatic hydrocarbon structural reaction and the like. For example, SSZ-13 has good catalytic effect in the catalytic reaction of MTO due to the characteristics of small pore channel size, high specific surface area and the like, can obtain low-carbon olefin with high yield, and has extremely wide development prospect.
Further, Cu-SSZ-13 molecular sieves have received much attention for their high catalytic activity and high hydrothermal stability. However, the current Cu-SSZ-13 molecular sieve has relatively few literature at home and abroad for the following reasons. Firstly, the template agent for preparing the Cu-SSZ-13 molecular sieve is difficult to buy at home and is expensive; secondly, the reaction period for preparing the Cu-SSZ-13 molecular sieve is longer, so that the cost for preparing the molecular sieve Cu-SSZ-13 is increased. In view of the above, it is necessary to select an optimal preparation process, reduce the preparation cost, and prepare the Cu-SSZ-13 molecular sieve catalyst in a one-step method, so that the Cu-SSZ-13 molecular sieve catalyst is more favorable for industrial production.
In view of this, the invention is particularly proposed.
Disclosure of Invention
The invention aims to provide a preparation method of a Cu-SSZ-13 molecular sieve.
In order to achieve the purpose, the technical scheme of the invention is as follows:
the invention relates to a preparation method of a Cu-SSZ-13 molecular sieve, which comprises the following steps:
(1) mixing an aluminum source, sodium hydroxide, a Cu-TEPA complex, a template agent and deionized water, adding a silicon source after uniformly stirring, and continuously stirring until uniformly mixing to obtain a mixture;
(2) crystallizing the mixture at 120-170 ℃ for 48-96 hours to obtain a crystallized product;
(3) drying the crystallized product at 80-120 ℃ for 8-12 hours to obtain a dried product;
(4) and roasting the dried product at 500-600 ℃ for 6-10 hours to obtain the Cu-SSZ-13 molecular sieve.
Preferably, the aluminum source is selected from at least one of sodium metaaluminate, alumina hydrate, and pseudoboehmite.
Preferably, the silicon source is selected from at least one of fumed silica, ethyl orthosilicate, silica sol and silica white.
Preferably, the molar ratio of the aluminum source, the silicon source, the sodium hydroxide, the template agent and the Cu-TEPA complex compound is (0.02-0.09): 1: (0.02-0.26): (0.01-0.35): (0.01-0.08): (10-43).
Preferably, the template agent is selected from at least one of N, N-trimethyladamantane ammonium hydroxide and choline chloride.
Preferably, the template agent is a composite template agent consisting of N, N, N-trimethyl adamantane ammonium hydroxide and choline chloride, and the molar ratio of the two is (1-5): 1.
preferably, cetyl trimethyl ammonium bromide is also added as an organic amine promoter in step (1).
Preferably, the molar ratio of the organic amine accelerant to the template agent is (0.1-0.5): 1.
preferably, step (2) comprises a secondary crystallization process:
(i) crystallizing the mixture at 120-130 ℃ for 10-15 hours to obtain a crystallized intermediate product;
(ii) crystallizing the crystallized intermediate product at 150-170 ℃ for 30-50 hours to obtain a crystallized product.
Preferably, step (3) is performed by washing the crystallized product for a plurality of times until the solution becomes neutral before drying.
The invention has the beneficial effects that:
the silicon-aluminum ratio of the Cu-SSZ-13 molecular sieve prepared by the invention is adjustable within the range of 10-40, and the composite template agent is used, so that the dosage of N, N, N-trimethyl adamantane ammonium hydroxide as a single template agent is greatly reduced, and the production cost is reduced. The molecular sieve has high crystallinity, good hydrothermal stability and specific surface area up to 850m2The average grain diameter is within 5-15 mu m, and the yield of crystallized products can reach more than 95 percent. The molecular sieve has wide application in the fields of petrochemical industry, MTO reaction, tail gas purification, fine chemical industry and the likeThe application prospect is as follows: can be used as a catalyst for removing NO in automobile exhaustxCan be used as a catalyst in the reaction process of preparing olefin (MTO) from methanol and can also be used as an adsorbent for CO in methane gas2Separation of (4).
Drawings
FIG. 1 is an XRD spectrum of the Cu-SSZ-13 molecular sieve prepared in example 1-1.
FIGS. 2a and 2b are SEM images of the Cu-SSZ-13 molecular sieve prepared in example 1-1.
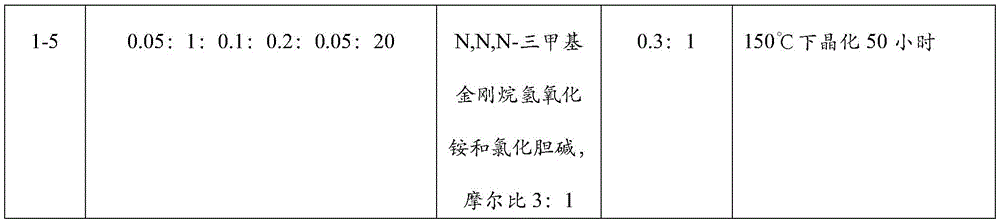
FIGS. 3a and 3b are SEM images of Cu-SSZ-13 molecular sieves prepared in examples 1-5.
FIG. 4 is a BET plot of the Cu-SSZ-13 molecular sieve prepared in example 1-1.
Detailed Description
In order to make the objects, technical solutions and advantages of the present invention more apparent, the technical solutions of the present invention will be described in detail below. It is to be understood that the described embodiments are merely exemplary of the invention, and not restrictive of the full scope of the invention. All other embodiments, which can be derived by a person skilled in the art from the examples given herein without any inventive step, are within the scope of the present invention.
The invention relates to a preparation method of a Cu-SSZ-13 molecular sieve, which comprises the following steps:
(1) mixing an aluminum source, sodium hydroxide, a Cu-TEPA complex, a template agent and deionized water, adding a silicon source after uniformly stirring, and continuously stirring until uniformly mixing to obtain a mixture;
(2) crystallizing the mixture at 120-170 ℃ for 48-96 hours to obtain a crystallized product;
(3) drying the crystallized product at 80-120 ℃ for 8-12 hours to obtain a dried product;
(4) and roasting the dried product at 500-600 ℃ for 6-10 hours to obtain the Cu-SSZ-13 molecular sieve.
Wherein the Cu-TEPA complex is prepared by reacting copper sulfate with tetraethylenepentamine.
In one embodiment of the invention, the aluminum source is selected from at least one of sodium metaaluminate, alumina siccative, and pseudoboehmite.
In one embodiment of the present invention, the silicon source is selected from at least one of fumed silicon, ethyl orthosilicate, silica sol, and silica white.
In one embodiment of the invention, the molar ratio of the aluminum source, the silicon source, the sodium hydroxide, the template and the Cu-TEPA complex is (0.02-0.09) to 1: (0.02-0.26): (0.01-0.35): (0.01-0.08): (10-43).
In one embodiment of the invention, the template agent is selected from at least one of N, N, N-trimethyladamantane ammonium hydroxide and choline chloride.
Further, the template agent is preferably a composite template agent consisting of N, N, N-trimethyl adamantane ammonium hydroxide and choline chloride, and the molar ratio of the two is (1-5): 1. the use of the composite template agent can reduce the dosage of N, N, N-trimethyl adamantane ammonium hydroxide and reduce the synthesis cost of the molecular sieve on the premise of ensuring the quality of the molecular sieve.
In one embodiment of the present invention, cetyltrimethylammonium bromide is also added as an organic amine promoter in step (1). The function of the gel is to improve the solubility of the organic amine template in the gel, so that a uniform Cu-SSZ-13 molecular sieve synthesis initial gel mixture is prepared.
In one embodiment of the invention, the molar ratio of the organic amine promoter to the template agent is (0.1-0.5): 1.
in one embodiment of the present invention, the step (2) includes a secondary crystallization process:
(i) crystallizing the mixture at 120-130 ℃ for 10-15 hours to obtain a crystallized intermediate product;
(ii) crystallizing the crystallized intermediate product at 150-170 ℃ for 30-50 hours to obtain a crystallized product.
In one embodiment of the present invention, step (3) is performed by washing the crystallized product several times until the solution becomes neutral before drying.
In one embodiment of the invention, the Cu-SSZ-13 molecular sieve is prepared by the following method:
(1) mixing an aluminum source, sodium hydroxide, a Cu-TEPA complex, a composite template agent, an organic amine promoter and deionized water according to the proportion, adding a silicon source after uniformly stirring, and continuously stirring until uniformly mixing to obtain a mixture;
(2) putting the mixture into a stainless steel synthesis kettle lined with polytetrafluoroethylene, sealing and carrying out secondary crystallization, namely crystallizing at 120-130 ℃ for 10-15 hours, and then crystallizing at 150-170 ℃ for 30-50 hours to obtain a crystallized product;
(3) washing the crystallized product for multiple times until the solution is neutral, and drying the crystallized product at the temperature of 80-120 ℃ for 8-12 hours to obtain a dried product;
(4) and roasting the dried product at 500-600 ℃ for 6-10 hours to obtain the Cu-SSZ-13 molecular sieve.
Example 1
(1) Mixing sodium metaaluminate, sodium hydroxide, a Cu-TEPA complex, a template agent, an organic amine promoter and deionized water, stirring uniformly, adding silica sol, and continuously stirring until the mixture is uniformly mixed to obtain a mixture. The molar ratio of the materials is shown in table 1;
(2) crystallizing the mixture at 120-170 ℃ for 48-96 hours to obtain a crystallized product, wherein crystallization parameters are shown in table 1;
(3) drying the crystallized product at 100 ℃ for 10 hours to obtain a dried product;
(4) and roasting the dried product at 550 ℃ for 8 hours to obtain the Cu-SSZ-13 molecular sieve.
TABLE 1
The Cu-SSZ-13 molecular sieve prepared in example 1-1 was subjected to X-ray diffraction measurement, and its XRD spectrum is shown in FIG. 1. It can be seen that the spectral line is flat, there is no impurity peak, and the crystallinity is good.
The Cu-SSZ-13 molecular sieves prepared in examples 1-1 and 1-5 were subjected to Scanning Electron Microscope (SEM) test, and the SEM images thereof are shown in FIGS. 2a and 2b (corresponding to examples 1-1) and FIGS. 3a and 3b (corresponding to examples 1-5). It can be seen that through the secondary crystallization process, the particles on the surface of the molecular sieve are obviously reduced, and the crystal grains obtain a more regular shape.
The Cu-SSZ-13 molecular sieve prepared in example 1-1 was subjected to specific surface area measurement and testing by nitrogen adsorption-desorption (BET) method, and its BET spectrum is shown in FIG. 4. The adsorbability is stable, and the specific surface area can reach 850m2/g。
The Cu-SSZ-13 molecular sieve prepared in the examples 1-1 to 1-5 is subjected to diesel vehicle tail gas purification at the space velocity of 400000h-1At a reaction temperature of 250 ℃ and NOxThe conversion results are shown in Table 2.
TABLE 2
| Examples | NOxConversion rate/% |
| 1-1 | 98 |
| 1-2 | 95 |
| 1-3 | 90 |
| 1-4 | 91 |
| 1-5 | 94 |
As can be seen from Table 2, NO in examples 1-1 to 1-5xThe conversion rates are all above 90%, wherein example 1-1 can reach 98%, and the catalyst has excellent catalytic activity. When a single template agent is used, an organic amine promoter is not used, or one-step crystallization is carried out, NO thereofxThe conversion rate was decreased to various degrees.
The above description is only for the specific embodiments of the present invention, but the scope of the present invention is not limited thereto, and any person skilled in the art can easily conceive of the changes or substitutions within the technical scope of the present invention, and all the changes or substitutions should be covered within the scope of the present invention. Therefore, the protection scope of the present invention shall be subject to the protection scope of the appended claims.
Claims (5)
1. A preparation method of a Cu-SSZ-13 molecular sieve is characterized by comprising the following steps:
(1) mixing an aluminum source, sodium hydroxide, a Cu-TEPA complex, a template agent and deionized water, adding a silicon source after uniformly stirring, and continuously stirring until uniformly mixing to obtain a mixture; the template agent is a composite template agent consisting of N, N, N-trimethyl adamantane ammonium hydroxide and choline chloride, and the molar ratio of the N, N, N-trimethyl adamantane ammonium hydroxide to the choline chloride is (1-5): 1; cetyl trimethyl ammonium bromide is also added in the step (1) as an organic amine promoter;
(2) and (3) secondary crystallization process:
(i) crystallizing the mixture at 120-130 ℃ for 10-15 hours to obtain a crystallized intermediate product;
(ii) crystallizing the crystallized intermediate product at 150-170 ℃ for 30-50 hours to obtain a crystallized product;
(3) drying the crystallized product at 80-120 ℃ for 8-12 hours to obtain a dried product;
(4) and roasting the dried product at 500-600 ℃ for 6-10 hours to obtain the Cu-SSZ-13 molecular sieve.
2. The process of claim 1 wherein the aluminum source is selected from at least one of sodium metaaluminate, alumina xerogel and pseudoboehmite.
3. The method of claim 1, wherein the silicon source is selected from at least one of fumed silicon, ethyl orthosilicate, and silica sol.
4. The method according to claim 1, wherein the molar ratio of the organic amine promoter to the templating agent is (0.1-0.5): 1.
5. the method as claimed in claim 1, wherein the step (3) is carried out by washing the crystallized product several times until the solution becomes neutral before the drying is carried out.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201810506476.0A CN108529642B (en) | 2018-05-24 | 2018-05-24 | Preparation method of Cu-SSZ-13 molecular sieve |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201810506476.0A CN108529642B (en) | 2018-05-24 | 2018-05-24 | Preparation method of Cu-SSZ-13 molecular sieve |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN108529642A CN108529642A (en) | 2018-09-14 |
| CN108529642B true CN108529642B (en) | 2021-07-20 |
Family
ID=63472587
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201810506476.0A Active CN108529642B (en) | 2018-05-24 | 2018-05-24 | Preparation method of Cu-SSZ-13 molecular sieve |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN108529642B (en) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109319806A (en) * | 2018-10-08 | 2019-02-12 | 中海油天津化工研究设计院有限公司 | A kind of method of the integral formula SSZ-13 molecular sieve of mixed templates dry glue |
| CN109364989B (en) * | 2018-11-20 | 2020-09-11 | 中国科学院生态环境研究中心 | Modified Cu-SSZ-13 catalyst and preparation method and application thereof |
| CN109399661A (en) * | 2018-12-02 | 2019-03-01 | 天津大沽化工股份有限公司 | A kind of preparation method of Fe-SSZ-24 molecular sieve |
| CN110201711B (en) * | 2019-07-11 | 2021-09-28 | 中国华能集团有限公司 | Catalyst for synthesizing low-carbon mixed alcohol by carbon dioxide hydrogenation and preparation method thereof |
| CN111298831B (en) * | 2019-11-25 | 2022-10-25 | 上海绿强新材料有限公司 | Preparation method of SSZ-13 molecular sieve for MTO catalytic reaction |
| CN111943224B (en) * | 2020-08-18 | 2022-11-11 | 桂林理工大学 | Preparation method of Cu-SSZ-13 molecular sieve catalyst, obtained product and application |
| CN115140745B (en) * | 2021-03-30 | 2023-11-10 | 中国石油化工股份有限公司 | Metal modified hierarchical pore ZSM-5 molecular sieve and preparation method thereof |
| CN112939020B (en) * | 2021-04-09 | 2023-09-29 | 南京诚志清洁能源有限公司 | Stepped crystallization preparation method and application of Cu-SSZ-13 molecular sieve catalyst |
| CN114655963B (en) * | 2022-03-31 | 2022-12-20 | 山东泓泰恒瑞新材料有限公司 | Preparation method of SSZ-13 molecular sieve composite material |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103232044B (en) * | 2013-04-25 | 2015-04-29 | 上海卓悦化工科技有限公司 | Synthesis method of nanoscale MCM-49 (Multi Chip Module) molecular sieve |
| CN105645426B (en) * | 2014-11-18 | 2017-09-01 | 中触媒有限公司 | A kind of synthetic method of the molecular sieves of SSZ 13 |
| CN105692647B (en) * | 2016-03-17 | 2018-03-06 | 山东齐鲁华信高科有限公司 | A kind of preparation method of the molecular sieves of SSZ 13 |
| CN106238092A (en) * | 2016-07-13 | 2016-12-21 | 无锡威孚环保催化剂有限公司 | The method of one-step synthesis method Cu SSZ 13 molecular sieve catalyst |
-
2018
- 2018-05-24 CN CN201810506476.0A patent/CN108529642B/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN108529642A (en) | 2018-09-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN108529642B (en) | Preparation method of Cu-SSZ-13 molecular sieve | |
| CN108217680B (en) | Method for synthesizing mordenite MOR molecular sieve, product and application thereof | |
| KR20130133245A (en) | Chabazite type zeolite and process for production thereof, copper-carrying low-silica zeolite, nox reductive elimination catalyst including said zeolite, and method for reductive elimination of nox employing said catalyst | |
| CN109201109B (en) | Catalyst for preparing olefin from methanol and preparation method thereof | |
| CN112794338B (en) | ZSM-5 molecular sieve and preparation method and application thereof | |
| CN111072043B (en) | Hydrogen mordenite, preparation method and application thereof | |
| CN110615444A (en) | Mordenite molecular sieve, and preparation method and application thereof | |
| WO2016145619A1 (en) | Method for preparation of, and application of, mordenite having mesopores and micropores | |
| CN103030156B (en) | Preparation method of binderless ZSM-5 molecular sieve | |
| CN113247918A (en) | Preparation method for synthesizing SSZ-13 molecular sieve by crystal transformation of A-type molecular sieve | |
| KR102517895B1 (en) | Manufacturing method of zeolite SSZ-98 | |
| CN111056561B (en) | Small-grain SSZ-13 molecular sieve containing hierarchical pores and synthesis method thereof | |
| CN112537778A (en) | Preparation method and application of mordenite with high silica-alumina ratio | |
| CN108946756B (en) | Hierarchical pore EUO structure molecular sieve and synthesis method thereof | |
| CN108033462B (en) | Hierarchical porous LTL molecular sieve and synthesis method and application thereof | |
| CN107777699B (en) | ZSM-11/SSZ-13 composite structure molecular sieve and synthetic method thereof | |
| CN115196650B (en) | Metal modified mesoporous ZSM-5 molecular sieve and preparation method thereof | |
| CN108996517B (en) | Hierarchical-pore wide-silica-alumina-ratio EU-1 molecular sieve and preparation method thereof | |
| CN111298831B (en) | Preparation method of SSZ-13 molecular sieve for MTO catalytic reaction | |
| CN110614121B (en) | ZSM-35 molecular sieve, preparation method and application thereof | |
| CN114425397A (en) | Non-noble metal catalyst and preparation method thereof and method for preparing propylene by propane dehydrogenation | |
| JPH0859566A (en) | Production of methylamines | |
| CN111410207B (en) | Normal-pressure synthesis method of SAPO-11 molecular sieve | |
| CN106799256B (en) | alkane isomerization catalyst and preparation method thereof | |
| CN113860323B (en) | Synthesis method of molecular sieve |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |