CN108362535B - Method for extracting extracellular vesicles from body fluid based on aqueous two-phase system - Google Patents
Method for extracting extracellular vesicles from body fluid based on aqueous two-phase system Download PDFInfo
- Publication number
- CN108362535B CN108362535B CN201810107478.2A CN201810107478A CN108362535B CN 108362535 B CN108362535 B CN 108362535B CN 201810107478 A CN201810107478 A CN 201810107478A CN 108362535 B CN108362535 B CN 108362535B
- Authority
- CN
- China
- Prior art keywords
- extracellular vesicles
- body fluid
- polyethylene glycol
- phase
- solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N1/00—Sampling; Preparing specimens for investigation
- G01N1/28—Preparing specimens for investigation including physical details of (bio-)chemical methods covered elsewhere, e.g. G01N33/50, C12Q
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/62—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light
- G01N21/63—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light optically excited
- G01N21/64—Fluorescence; Phosphorescence
- G01N21/6402—Atomic fluorescence; Laser induced fluorescence
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/62—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light
- G01N21/63—Systems in which the material investigated is excited whereby it emits light or causes a change in wavelength of the incident light optically excited
- G01N21/64—Fluorescence; Phosphorescence
- G01N21/6486—Measuring fluorescence of biological material, e.g. DNA, RNA, cells
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Optics & Photonics (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Medicinal Preparation (AREA)
Abstract
The invention relates to a method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system, which comprises the steps of adding polyethylene glycol and glucan into a body fluid sample, adjusting the pH value of a system to be 6.4-8.5 by adding a buffer solution, fully mixing, and oscillating to form a white emulsion; then centrifuging, and dividing the solution into an upper layer and a lower layer; and separating to obtain the extracellular vesicles. The invention obviously improves the efficiency of extracting the ATPS extracellular vesicles and the purity of the product by optimizing the pH value of a polyethylene glycol/glucan system. The method for extracting the extracellular vesicles by using the ATPS method has the greatest advantages of being fast and convenient to operate and capable of recovering about 60% of the extracellular vesicles in body fluid within 15-20 minutes.
Description
Technical Field
The invention relates to the technical field of biology, in particular to a method for extracting extracellular vesicles from body fluid based on a two-aqueous-phase system.
Background
Most organisms, including complex eukaryotes, gram-negative and positive bacteria, mycobacteria and fungi, secrete extracellular vesicles (excell μ Lar vesicles). The types of the cell mainly comprise Exosome (with the particle size of 50-100nm), Microvesicle (with the particle size of 20-1000nm), Apoptotic bodies (with the particle size of 1000-5000nm) and the like1,2. In humans, the endogenous extracellular vesicles have been shown to play a role in blood coagulation, intercellular signaling and waste management3. Meanwhile, the extracellular vesicles contain various biomolecules such as nucleic acids, proteins and lipids, and thus have great potential in diagnosis and treatment of diseases4。
The existing methods for extracting and separating extracellular vesicles from body fluid mainly comprise an ultracentrifugation method, a membrane affinity capture method, an immunocapture method, an ultrafiltration membrane method, a polymer sedimentation method and the like. The ultracentrifugation method is based on the difference of the size and density of the extracellular vesicles and other components in body fluid, and the extracellular vesicles are separated by the ultracentrifuge, and the ultracentrifugation method needs expensive equipment, is complex to operate and has low recovery rate5-7. The membrane affinity capture method and the immunocapture method require the use of a solid phase surface modified with a membrane affinity molecule or an antibody specific to an exosomeNoodle, resulting in higher extraction cost and unsuitability for systematic enlargement8-11. The ultrafiltration membrane method is a screening method based on the particle size of extracellular vesicles, can realize rapid separation, but cannot realize concentration of the extracellular vesicles, and the extracellular vesicles may be captured by an ultrafiltration membrane with micron or nanometer pore size to influence the final extraction efficiency12,13. The polymer sedimentation method is to utilize polymers such as polyethylene glycol to reduce the solubility of extracellular vesicles by changing the microenvironment in body fluid, so as to settle the extracellular vesicles, and the method does not need large-scale equipment, but because the sedimentation process of the vesicles needs to be incubated for a period of time (0.5 hours-overnight), the time required for completing one operation is still long14,15。
An Aqueous Two-Phase System (ATPS) is a liquid-liquid separation technique, and has wide application prospect in separation and purification of biomolecules such as proteins, viruses and nucleic acids16. The method has high separation efficiency, and more importantly, the operation time can be greatly reduced. At present, the work of separating the extracellular vesicles by adopting an ATPS method is reported internationally, two mutually insoluble aqueous two-phase systems formed by two different high-molecular polymers (polyethylene glycol and dextran) under certain concentration are mainly used for redistributing the extracellular vesicles between two phases, and then a polymer solution is formed into an upper layer and a lower layer by matching low-speed centrifugation, wherein the extracellular vesicles are enriched in the dextran positioned at the lower layer17,18。
However, in addition to polymer concentration, parameters that affect the redistribution efficiency of ATPS to target particles or biomacromolecules include pH and temperature16. Especially, for example, blood plasma is used, wherein the contents of the most important proteins and their isoelectric points are: 55% of albumin and 4.7-5.3 of pI; 35% of globulin and 7.5% of pI; 4% of fibrinogen and 55-5.8% of pI. And the isoelectric point of the extracellular vesicles is not less than 819. It has been reported that biomolecules tend to enter the polyethylene glycol (PEG) phase during redistribution when the pH is above the isoelectric point of the biomolecule20And vice versa.
Disclosure of Invention
The invention aims to provide a method for extracting extracellular vesicles from body fluid based on a two-aqueous-phase system, which can remarkably improve the extraction efficiency of ATPS extracellular vesicles and the purity of products by optimizing the pH value of a polyethylene glycol/dextran system.
The research of the invention finds that when the pH value of the ATPS aqueous solution is between a certain value of the impurity protein and the extracellular vesicles, the extraction efficiency and the purity of the extracellular vesicles can be further improved.
The principle of the invention is briefly described as follows:
according to the improved model, the redistribution coefficient K (upper solute concentration/lower solute concentration) of proteins in aqueous two-phase systems (ATPS) conforms to the following expression:
K=Khphob·Kel·Ksize·Ksol·Kaff
wherein Khphob,Kel,Ksize,KsolAnd K andaffrespectively represents the contribution values of hydrophilicity and hydrophobicity, electrostatic acting force, particle diameter, solubility and affinity to the total distribution coefficient21. Altering the pH can affect the electrical and surface properties of the solute, with the net charge of the protein being 0 when the pH of the ATPS is equal to the isoelectric point pI of the protein, and the protein being positively or negatively charged when the pH is below or above the isoelectric point pI, respectively. There is a literature showing a higher pH value (>pI) will make the protein of interest more prone to be enriched in the PEG phase. The principle schematic of the method of the invention is shown in figure 1.
The technical scheme of the invention is as follows:
a method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system comprises adding polyethylene glycol (PEG) and Dextran (DEX) into a body fluid sample, adjusting pH value of the system to 6.4-8.5 by adding buffer solution, mixing completely, and shaking to form white emulsion (ATPS); then centrifuging (typically 1500 Xg for 10 min) to separate the solution into an upper (polyethylene glycol phase) and a lower (dextran) layer; because of the difference in partition coefficients between the two phases, extracellular vesicles will be concentrated primarily in the lower dextran phase and at the interface of the two phases; and separating to obtain the extracellular vesicles.
Further, polyethylene glycol (PEG) having a molecular weight of 35,000 and Dextran (DEX) having a molecular weight of 450,000 and 650,000 were used.
Further, the pH value of the system is adjusted to be 6.30-7.20 or 7.60-9.40 by adding a buffer solution. For example, the pH value of the system can be adjusted to be 6.30, 6.32, 6.81, 7.18, 7.20, 7.60, 7.64, 8.07, 9.36 and 9.40; preferably 6.81 + -0.3 or 8.07 + -0.4.
Further, the buffer solution is a sodium carbonate-sodium bicarbonate buffer solution, or a disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solution; preferably, the buffer solution has a pH of 6.3 to 8.5.
Because the pH value of human plasma is about 7.35-7.45, the pH value of the system can be adjusted to a required range (for example, about 6.81) by adopting the buffer solution (buffer) with a lower pH value (for example, pH value of 6.3-6.6); the volume is then removed or the dilution factor is determined again with a buffer solution (e.g. around 6.81) of the same pH as the system. Studies have shown that the pH of the above system or the buffer solution is acceptable within a suitable range (e.g. 6.81 ± 0.4 or 8.07 ± 0.4). So >2 sample volumes of the buffer solution (e.g., 6.81 + -0.4 or 8.07 + -0.4) can be used to directly dilute the sample.
Further, the mass fraction of polyethylene glycol in the above system is 3 to 7%, preferably 4 to 6%, more preferably 5%.
Further, the mass fraction of glucan in the above system is 0.5 to 2.5%, preferably 1 to 2%, more preferably 1.5%.
Further, the mass concentration ratio of polyethylene glycol to glucan in the system is (2-6): 1; e.g., 2:1, 3:1, 4:1.5, 4:1, 5:1.5, 5:1, 6: 1; preferably (4-5): 1.5.
Furthermore, the mass fractions of the polyethylene glycol and the glucan in the system are respectively 6% and 1%; 5% and 1%; 5% and 1.5%; 4% and 1%; 4% and 1.5%; 4% and 2%; more preferably 5% and 1.5%.
Further, preparing mother liquor with a certain mass concentration from polyethylene glycol and glucan, and adding the mother liquor into the system as required. For example, the mass concentration of the mother solution of polyethylene glycol and dextran is 30-60% and 5-25%, respectively. Preferably, the polyethylene glycol and dextran are prepared as a mother solution having a concentration of about 10 times the desired concentration in the system, for example, if the polyethylene glycol concentration in the system is required to be 5% by mass, the polyethylene glycol is prepared as a mother solution having a concentration of 50% by mass. This is advantageous for practical operation.
Further, the body fluid sample includes plasma, serum, urine, spinal fluid, ascites, etc. of human or animal.
Further, the body fluid sample is diluted 1-4 times with Phosphate Buffered Saline (PBS) and then the extracellular vesicles in the body fluid sample are extracted according to the method.
The starting materials used in the present invention are commercially available or may be prepared by methods conventional in the art.
On the basis of the common knowledge in the field, the above preferred conditions can be combined with each other to obtain the preferred embodiments of the invention.
Specifically, the method for extracting extracellular vesicles from body fluid based on the aqueous two-phase system comprises the following steps:
1) adding a polyethylene glycol solution and a glucan solution into a body fluid sample, and adjusting the pH value of a system to be 6.81 +/-0.3 or 8.07 +/-0.4 by adding the buffer solution;
2) mixing thoroughly, and shaking vigorously to obtain white emulsion (ATPS);
3) after centrifugation at 1500 Xg for 10 min at room temperature, the upper polyethylene glycol phase is discarded, and the liquid near the interface and the bottom dextran phase are taken out, or a proper amount of Phosphate Buffer Solution (PBS) is further added, so that the enriched extracellular vesicles are obtained.
The Phosphate Buffered Saline (PBS) may be formulated according to methods conventional in the art, preferably at a pH of 7.4.
By respectively experimental determination of plasma protein without extracellular vesicles and extracellular vesicles extracted from human plasma, the distribution coefficients of the protein and the extracellular vesicles are significantly different under the conditions of different pH values. When the pH was 6.81 and 8.07, the redistribution rate of the extracellular vesicles was significantly higher than the other conditions.
At the same time, 1/4 diluted plasma to the original concentration reduced the recovery of impurity proteins in the product by 10-15% without significant change in extracellular vesicle recovery. Extracellular vesicle recovery was higher than both existing commercial kits (exoQuick by SBI and Exoeasy by Qiagen).
The method for extracting the extracellular vesicles by using the ATPS method has the greatest advantages of being fast and convenient to operate and capable of recovering about 60% of the extracellular vesicles in body fluid within 15-20 minutes.
Drawings
FIG. 1 is a schematic diagram of the method of the present invention.
FIG. 2 is an ATPS phase separation curve of Experimental example 1.
FIG. 3 shows the recovery efficiency of extracellular vesicles in Experimental example 2.
FIG. 4 shows the recovery rates of impurity proteins in Experimental example 3.
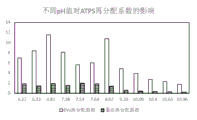
FIG. 5 shows the effect of pH on extracellular vesicle extraction in Experimental example 4.
FIG. 6 shows a comparison of the effects of SBI ExoQuick and Qiagen ExoEasy in Experimental example 5.
FIG. 7 shows the results of Western blot and TEM characterization of extracellular vesicles isolated by ATPS.
Detailed Description
The following examples are intended to illustrate the invention but are not intended to limit the scope of the invention. The examples do not show specific techniques or conditions, according to the techniques or conditions described in the literature in the field, or according to the product specifications. The reagents or instruments used are conventional products available from regular distributors, not indicated by the manufacturer.
Experimental example 1ATPS phase separation Curve
First, reagent preparation
The solutions were diluted in PBS using the polymers polyethylene glycol (PEG) molecular weight 35,000, Dextran (DEX) molecular weight 450,000 and 650,000 to concentrations 35% wt and 15% wt, respectively. 35% wt PEG was mixed with PBS to make a total volume of 1mL, and PEG concentrations were 2%, 4%, 6%, 8% and 10% in this order. PBS buffer pH 7.4.
Second, determination of ATPS phase transition curve
Then, a 15% wt DEX solution was added dropwise thereto until the mixed solution became turbid, and the volume of the DEX solution used was recorded and converted into the final PEG and DEX concentrations, resulting in a phase diagram shown in FIG. 2 (the abscissa indicates the DEX concentration, the ordinate indicates the PEG concentration, and the ATPS phasegraph indicates the ATPS phase diagram). The results show that when the PEG and DEX concentrations lie above this curve, the solution is able to form two immiscible phases, with the upper PEG phase and the lower DEX phase.
Experimental example 2ATPS Polymer concentration optimization
First, reagent preparation
The polymer polyethylene glycol (PEG) molecular weight of 35,000 and Dextran (DEX) molecular weight of 450,000-650,000 were used. PEG 35,000 solutions with concentrations of 60%, 50% and 40% and DEX 450,000-650,000 solutions with concentrations of 10%, 15% and 20% were prepared, respectively. Cell membrane green fluorescent probe DIO. PBS buffer pH 7.4.
Secondly, preparing green fluorescence labeled extracellular vesicles
After thawing the commercial human plasma 1300g were centrifuged for 15 minutes to remove cell debris. The supernatant was then transferred to a fresh centrifuge tube, centrifuged at 3000g for 15 minutes, mixed with PBS at a volume ratio of 1:9, and centrifuged at 13000g for 30 minutes at 4 ℃. The supernatant was dispensed into 20mL ultracentrifuge tubes (2 mL plasma per tube), centrifuged at 100,000g for 1 hour, the supernatant discarded, the pellet at the bottom of the 20mL ultracentrifuge tubes was resuspended in 1mL PBS, centrifugation at 100,000g was continued for 1 hour, and the extracellular vesicles pelleted at the bottom of the tubes were resuspended in 100. mu.L (corresponding to 2mL plasma) of PBS containing 5% DIO dye and incubated at room temperature for 2 hours. Dialyzed overnight to remove the remaining DIO dye.
Thirdly, preparing human plasma without extracellular vesicles
After thawing commercial human plasma, centrifugation was carried out at 160,000g for 18 hours, and the supernatant was aliquoted and frozen for future use.
Fourth, ATPS concentration optimization
1mL of green fluorescently labeled extracellular vesicles was added to 9mL of non-vesicular human plasma to constitute Standard A. mu.L of the standard A solution was mixed with 100. mu.L of 60% PEG and 100. mu.L of 10% DEX to prepare 6% + 1% working solution (6%, 1% representing the concentration of PEG and DEX, respectively). Similarly, a working fluid of 5% + 1%, 5% + 1.5%, 4% + 1%, 4% + 1.5%, 4% + 2% can be prepared. Because these six conditions are all located above the PEG/DEX phase transition curve, two immiscible phases can be formed.
The prepared ATPS working solution is vigorously shaken on a shaker and then centrifuged at 1500g for 10 minutes, and obvious layering can be observed. The supernatant was discarded, and the liquid at the bottom and near the interface was removed and made up to 800. mu.L by adding PBS. Taking 200 mu L of the extracellular vesicles, performing fluorescence quantification by using a multifunctional microplate reader, and comparing the fluorescence quantification with a standard substance A to obtain the recovery efficiency of the extracellular vesicles under the condition. The results are shown in FIG. 3. Although 6% + 1% is slightly higher than 5% + 1.5%, the final concentration of 5% + 1.5% was selected as the ATPS working solution concentration, considering that the viscosity of the mother solution required for the preparation of 6% PEG is too high.
Experimental example 3 Effect of plasma dilution factor on extracellular vesicle extraction
First, reagent preparation
The polymer polyethylene glycol (PEG) molecular weight of 35,000 and Dextran (DEX) molecular weight of 450,000-650,000 were used. PEG 35,000 solution with the concentration of 50% and DEX solution with the concentration of 15% are respectively prepared. PBS buffer pH 7.4.
Secondly, preparing green fluorescence labeled extracellular vesicles
Same as Experimental example 2
Thirdly, preparing human plasma without extracellular vesicles
Same as Experimental example 2
Fourth, optimization of plasma dilution factor
1mL of green fluorescently labeled extracellular vesicles was added to 9mL of non-vesicular human plasma to constitute Standard A. The standard A and PBS were mixed and diluted at 1:1 (standard B), 1:2 (standard C) and 1:3 (standard D), respectively, then the standards A, B, C and D were mixed with 100. mu.L of 50% PEG and 100. mu.L of 15% DEX, respectively, and shaken, and then centrifuged at 1500g for 10 minutes, the supernatant was discarded, and the liquid near the bottom and interface was taken out and added with PBS to make the volume of 800. mu.L (A ', B', C ', D'). And comparing the fluorescence intensity differences of A '/A, B'/B, C '/C and D'/D by using a multifunctional microplate reader to obtain the extraction efficiency of the extracellular vesicles. The impurity protein recovery rates of A '/A, B'/B, C '/C and D'/D can be obtained by BCA detection. The results are shown in fig. 4(EVs yield indicates the recovery rate of extracellular vesicles, and Protein yield indicates the recovery rate of total Protein (total Protein includes not only the Protein contained in extracellular vesicles but also plasma-derived foreign Protein in non-vesicular human plasma).
Experimental example 4 Effect of pH on extracellular vesicle extraction
First, reagent preparation
The polymer polyethylene glycol (PEG) molecular weight of 35,000 and Dextran (DEX) molecular weight of 450,000-650,000 were used. PEG 35,000 solution with the concentration of 50% and DEX solution with the concentration of 15% are respectively prepared. Sodium carbonate-sodium bicarbonate buffer solutions with different pH values and disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solutions are prepared. PBS buffer pH 7.4.
Secondly, preparing green fluorescence labeled extracellular vesicles
Same as Experimental example 2
Thirdly, preparing human plasma without extracellular vesicles
Same as Experimental example 2
Fourth, influence of pH value on extraction of extracellular vesicles
Mixing the fluorescence-labeled extracellular vesicles with buffers with different pH values, namely 1:3, mixing the mixture with 50% of PEG and 15% of DEX according to the volume ratio of 8:1:1, shaking the mixture, centrifuging the mixture at 1500g, and comparing the concentration difference between the lower DEX phase and the upper PEG phase by using fluorescence (K ═ Con)DEX/ConPEG)。
Mixing vesicle-free human plasma with buffers with different pH values 1:3, mixing with 50% PEG and 15% DEX according to the volume ratio of 8:1:1, shaking, centrifuging at 1500g, and comparing the concentration difference of the lower DEX phase and the upper PEG phase by using BCA method (K ═ Con)DEX/ConPEG). The results are shown in FIG. 5.
It can be seen that the conditions of pH6.81 and pH8.07 have significant advantages over other conditions.
Experimental example 5 comparison of Effect with SBI ExoQuick and Qiagen ExoEasy
First, reagent preparation
The polymer polyethylene glycol (PEG) molecular weight of 35,000 and Dextran (DEX) molecular weight of 450,000-650,000 were used. PEG 35,000 solution with the concentration of 50% and DEX solution with the concentration of 15% are respectively prepared. Sodium carbonate-sodium bicarbonate buffer solutions with different pH values of 8.07 were prepared. PBS buffer pH 7.4. SBI ExoQuick kit, and ExoEasy kit from Qiagen.
Secondly, preparing green fluorescence labeled extracellular vesicles
Same as Experimental example 2
Thirdly, preparing human plasma without extracellular vesicles
Same as Experimental example 2
Fourth, comparison with other commercial kits
1mL of green fluorescently labeled extracellular vesicles was added to 9mL of non-vesicular human plasma to constitute Standard A. 250 mu LA was mixed with pH8.07 sodium carbonate-sodium bicarbonate buffer at 1:3, then mixed with 50% PEG and 15% DEX at 8:1:1 ratio, shaken and centrifuged at 1500 g. 250 μ LA was taken and processed according to the SBI standard extraction protocol. 250 μ LA were taken and processed according to the standard extraction protocol of Qiagen. The results are shown in FIG. 6. The vesicle extraction efficiency of the ATPS method and ExoQuick was determined by comparing the fluorescence intensity of the product and the standard a, and the vesicle extraction efficiency of ExoEasy was determined by comparing the fluorescence intensity of the overflow and the standard a.
FIG. 7 shows Western blot results (FIG. 7A) and transmission mirror results (FIG. 7B) of extracellular vesicles isolated by ATPS. Among them, TSG 101 and CD63 are exosome-specific proteins (one of extracellular vesicles that is difficult to separate), and a positive result indicates that ATPS can be separated into extracellular vesicles. Calnexin is a negative control protein.
Example 1
Preparation of reagents:
the polymer polyethylene glycol (PEG) molecular weight of 35,000 and Dextran (DEX) molecular weight of 450,000-650,000 were used. PEG 35,000 solution with the concentration of 50% and DEX solution with the concentration of 15% are respectively prepared. Preparing sodium carbonate-sodium bicarbonate buffer solutions with different pH values and disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solutions; PBS buffer solution with pH 7.4 was prepared. The supernatant was retained after centrifugation at 3000Xg for 15 minutes in human or animal plasma (cell and cell debris removed).
A method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system comprises the following steps:
1) adding a polyethylene glycol solution and a glucan solution into the plasma, and adjusting the pH value of the system to be 6.81 by adding the buffer solution; the mass fractions of polyethylene glycol and dextran in the system are 5% and 1.5%, respectively;
2) mixing thoroughly, and shaking vigorously to obtain white emulsion (ATPS);
3) after centrifugation at 1500 Xg for 10 min at room temperature, the upper polyethylene glycol phase is discarded, and the liquid near the interface and the bottom dextran phase are taken out, or a proper amount of Phosphate Buffer Solution (PBS) is further added, so that the enriched extracellular vesicles are obtained.
Example 2
A method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system, which differs from example 1 only in that: the pH value of the system is adjusted to 8.07 by adding the buffer solution.
Example 3
A method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system, which differs from example 1 only in that: the body fluid sample is serum.
Example 4
A method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system, which differs from example 2 only in that: the body fluid sample is serum.
Example 5
Preparation of reagents:
the polymer polyethylene glycol (PEG) molecular weight of 35,000 and Dextran (DEX) molecular weight of 450,000-650,000 were used. PEG 35,000 solution with the concentration of 50% and DEX solution with the concentration of 15% are respectively prepared. Preparing 10% NaOH solution; PBS buffer solution with pH 7.4 was prepared. Human or animal urine is collected, centrifuged at 2000Xg for 20 minutes and the supernatant is retained (cells are removed from the urine).
A method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system comprises the following steps:
1) adding NaOH solution into the plasma dropwise until the pH value of urine is 6.81;
2) adding polyethylene glycol and dextran into the system to make the mass fractions of the polyethylene glycol and the dextran reach 5% and 1.5% respectively;
3) mixing thoroughly, and shaking vigorously to obtain white emulsion (ATPS);
4) after centrifugation at 1500 Xg for 10 min at room temperature, the upper polyethylene glycol phase is discarded, and the liquid near the interface and the bottom dextran phase are taken out, or a proper amount of Phosphate Buffer Solution (PBS) is further added, so that the enriched extracellular vesicles are obtained.
Example 6
A method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system, which differs from example 1 only in that: the pH of the system was adjusted to 8.07 by the addition of NaOH.
Example 7
Preparation of reagents:
the polymer polyethylene glycol (PEG) molecular weight of 35,000 and Dextran (DEX) molecular weight of 450,000-650,000 were used. PEG 35,000 solution with the concentration of 50% and DEX solution with the concentration of 15% are respectively prepared. 10% NaOH solution is prepared. Ascites fluid from a human or animal is collected, centrifuged at 300Xg for 20 minutes and the supernatant is transferred to a new centrifuge tube and centrifuged at 800Xg for 30 minutes and then filtered through a 0.45 μm filter.
A method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system comprises the following steps:
1) adding NaOH solution into the ascites drop by drop until the pH value is 8.07;
2) adding polyethylene glycol and dextran into the system to make the mass fractions of the polyethylene glycol and the dextran reach 5% and 1.5% respectively;
3) mixing thoroughly, and shaking vigorously to obtain white emulsion (ATPS);
4) after centrifugation at 1500 Xg for 10 min at room temperature, the upper polyethylene glycol phase is discarded, and the liquid near the interface and the bottom dextran phase are taken out, or a proper amount of Phosphate Buffer Solution (PBS) is further added, so that the enriched extracellular vesicles are obtained.
[ REFERENCE ] to
(1)S,E.L.A.;Mager,I.;Breakefield,X.O.;Wood,M.J.ExtracellμLar vesicles:biology and emerging therapeutic opportunities.Nature reviews.Drug discovery 2013,12,347-357.
(2)Raposo,G.;Stoorvogel,W.ExtracellμLar vesicles:exosomes,microvesicles,and friends. The Journal of cell biology 2013,200,373-383.
(3)van der Pol,E.;Boing,A.N.;Harrison,P.;Sturk,A.;Nieuwland,R.Classification, functions,and clinical relevance of extracellμLar vesicles.Pharmacological reviews 2012,64, 676-705.
(4)Thery,C.Cancer:Diagnosis by extracellμLar vesicles.Nature 2015,523,161-162.
(5)Momen-Heravi,F.;Balaj,L.;Alian,S.;Trachtenberg,A.J.;Hochberg,F.H.;Skog,J.;Kuo, W.P.Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Frontiers in physiology 2012,3,162.
(6)Livshits,M.A.;Khomyakova,E.;Evtushenko,E.G.;Lazarev,V.N.;KμLemin,N.A.; Semina,S.E.;Generozov,E.V.;Govorun,V.M.Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol.Scientific reports 2015,5,17319.
(7)Lamparski,H.G.;Metha-Damani,A.;Yao,J.Y.;Patel,S.;Hsu,D.H.;Ruegg,C.;Le Pecq,J. B.Production and characterization of clinical grade exosomes derived from dendritic cells.Journal of immunological methods 2002,270,211-226.
(8)Balaj,L.;Atai,N.A.;Chen,W.;Mu,D.;Tannous,B.A.;Breakefield,X.O.;Skog,J.; Maguire,C.A.Heparin affinity purification of extracellμLar vesicles.Scientific reports 2015,5, 10266.
(9)Nakai,W.;Yoshida,T.;Diez,D.;Miyatake,Y.;Nishibu,T.;Imawaka,N.;Naruse,K.; Sadamura,Y.;Hanayama,R.A novel affinity-based method for the isolation of highly purified extracellμLar vesicles.Scientific reports 2016,6,33935.
(10)Taylor,D.D.;Gercel-Taylor,C.MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer.Gynecologic oncology 2008,110,13-21.
(11)Mathivanan,S.;Lim,J.W.;Tauro,B.J.;Ji,H.;Moritz,R.L.;Simpson,R.J.Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature.MolecμLar&cellμLar proteomics:MCP 2010, 9,197-208.
(12)Momen-Heravi,F.;Balaj,L.;Alian,S.;Mantel,P.Y.;Halleck,A.E.;Trachtenberg,A.J.; Soria,C.E.;Oquin,S.;Bonebreak,C.M.;Saracoglu,E.;Skog,J.;Kuo,W.P.Current methods for the isolation of extracellμLar vesicles.Biological chemistry 2013,394,1253-1262.
(13)Liang,L.G.;Kong,M.Q.;Zhou,S.;Sheng,Y.F.;Wang,P.;Yu,T.;Inci,F.;Kuo,W.P.;Li, L.J.;Demirci,U.;Wang,S.An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellμLar vesicles for detection of bladder cancer. Scientific reports 2017,7,46224.
(14)Taylor,D.D.;Zacharias,W.;Gercel-Taylor,C.Exosome isolation for proteomic analyses and RNA profiling.Methods in molecμLar biology 2011,728,235-246.
(15)Yamada,T.;Inoshima,Y.;Matsuda,T.;Ishiguro,N.Comparison of methods for isolating exosomes from bovine milk.The Journal of veterinary medical science 2012,74,1523-1525.
(16)Iqbal,M.;Tao,Y.;Xie,S.;Zhu,Y.;Chen,D.;Wang,X.;Huang,L.;Peng,D.;Sattar,A.; Shabbir,M.A.;Hussain,H.I.;Ahmed,S.;Yuan,Z.Aqueous two-phase system(ATPS):an overview and advances in its applications.Biological procedures online 2016,18,18.
(17)Kim,J.;Shin,H.;Kim,J.;Kim,J.;Park,J.Isolation of High-Purity ExtracellμLar Vesicles by Extracting Proteins Using Aqueous Two-Phase System.PloS one 2015,10,e0129760.
(18)Shin,H.;Han,C.;Labuz,J.M.;Kim,J.;Kim,J.;Cho,S.;Gho,Y.S.;Takayama,S.;Park,J. High-yield isolation of extracellμLar vesicles using aqueous two-phase system.Scientific reports 2015,5,13103.
(19)Graner,M.W.;Alzate,O.;Dechkovskaia,A.M.;Keene,J.D.;Sampson,J.H.;Mitchell,D. A.;Bigner,D.D.Proteomic and immunologic analyses of brain tumor exosomes.FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2009,23, 1541-1557.
(20)Andrews,B.A.;Schmidt,A.S.;Asenjo,J.A.Correlation for the partition behavior of proteins in aqueous two-phase systems:effect of surface hydrophobicity and charge.Biotechnology and bioengineering 2005,90,380-390.
(21)KμLa,M.R.Trends and future prospects of aqueous two-phase extraction.Bioseparation 1990,1,181-189.
Although the invention has been described in detail hereinabove with respect to specific embodiments thereof, it will be apparent to those skilled in the art that modifications and improvements can be made thereto based on the invention. Accordingly, such modifications and improvements are intended to be within the scope of the invention as claimed.
Claims (3)
1. A method for extracting extracellular vesicles from body fluid based on an aqueous two-phase system is characterized by comprising the steps of adding polyethylene glycol and glucan into a body fluid sample, adjusting the pH value of the system to be 6.81 +/-0.3 or 8.07 +/-0.4 by adding a buffer solution, fully mixing and then oscillating to form a white emulsion; centrifuging, separating the solution into an upper layer and a lower layer, removing the upper polyethylene glycol phase, taking out the liquid near the interface and the bottom glucan phase, and further adding a proper amount of phosphate buffer solution; separating to obtain extracellular vesicles;
wherein the buffer solution is a sodium carbonate-sodium bicarbonate buffer solution with pH of 6.3-8.5;
the mass fractions of polyethylene glycol and glucan in the system are respectively 6% and 1%, or 5% and 1.5%;
the molecular weight of the polyethylene glycol is 35,000, and the molecular weight of the glucan is 450,000-650,000;
the body fluid sample is human or animal plasma;
and diluting the body fluid sample by 1-4 times by using phosphate buffer solution, and extracting extracellular vesicles in the body fluid sample.
2. The method of claim 1, wherein the pH of the system is adjusted to 6.81 or 8.07.
3. The method of claim 1, comprising the steps of:
1) adding a polyethylene glycol solution and a glucan solution into a body fluid sample, and adjusting the pH value of a system to be 6.81 or 8.07 by adding the buffer solution;
2) mixing thoroughly, and shaking vigorously to obtain white emulsion;
3) centrifuging at 1500 Xg for 10 min at room temperature, removing the upper polyethylene glycol phase, and taking out the liquid near the interface and the bottom dextran phase, or obtaining the enriched extracellular vesicles.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201810107478.2A CN108362535B (en) | 2018-02-02 | 2018-02-02 | Method for extracting extracellular vesicles from body fluid based on aqueous two-phase system |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201810107478.2A CN108362535B (en) | 2018-02-02 | 2018-02-02 | Method for extracting extracellular vesicles from body fluid based on aqueous two-phase system |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN108362535A CN108362535A (en) | 2018-08-03 |
| CN108362535B true CN108362535B (en) | 2021-01-15 |
Family
ID=63004292
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201810107478.2A Active CN108362535B (en) | 2018-02-02 | 2018-02-02 | Method for extracting extracellular vesicles from body fluid based on aqueous two-phase system |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN108362535B (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102174177B1 (en) * | 2018-11-19 | 2020-11-05 | 포항공과대학교 산학협력단 | Aqueous two-phase system nano filter and separation method threrefor |
| CN109929795B (en) * | 2019-03-22 | 2022-08-19 | 南昌大学第二附属医院 | Improved extraction method of urine small extracellular vesicle |
| KR102294264B1 (en) * | 2019-08-07 | 2021-08-26 | 포항공과대학교 산학협력단 | Separation method of bio-nano particles by aqueous two-phase composition |
| CN115873786A (en) * | 2022-12-29 | 2023-03-31 | 陕西慧康生物科技有限责任公司 | Large-scale extraction method of exosomes in fresh milk |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1048561A (en) * | 1989-07-01 | 1991-01-16 | 中国科学院微生物研究所 | A kind of method of aqueous two-phase system enzyme purification |
| KR101745455B1 (en) * | 2015-03-24 | 2017-06-09 | 포항공과대학교 산학협력단 | Multistage purification method of extracellular vesicles by aqueous two-phase system |
| KR101761680B1 (en) * | 2015-03-31 | 2017-08-23 | 포항공과대학교 산학협력단 | Isolation Method of Extracellular Vesicles by Aqueous Two-phase System |
| CN105061772B (en) * | 2015-08-05 | 2017-06-16 | 西南交通大学 | Polyethylene glycol/glucan double-aqueous phase system emulsion stabilizer and preparation method thereof |
-
2018
- 2018-02-02 CN CN201810107478.2A patent/CN108362535B/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN108362535A (en) | 2018-08-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN108362535B (en) | Method for extracting extracellular vesicles from body fluid based on aqueous two-phase system | |
| Konoshenko et al. | Isolation of extracellular vesicles: general methodologies and latest trends | |
| Liangsupree et al. | Modern isolation and separation techniques for extracellular vesicles | |
| CN106124282B (en) | A kind of method of lamination centrifugal filtration separation and Extraction excretion body | |
| Martins et al. | A review on comparative studies addressing exosome isolation methods from body fluids | |
| KR101761680B1 (en) | Isolation Method of Extracellular Vesicles by Aqueous Two-phase System | |
| Kang et al. | Methods to isolate extracellular vesicles for diagnosis | |
| US9101925B2 (en) | Centrifuge and separation vessel therefore | |
| US20210079377A1 (en) | Method of Isolating Exosomes Using Encapsulation And Aqueous Micellar System | |
| US10669535B2 (en) | Methods for the isolation of extracellular vesicles and other bioparticles from urine and other biofluids | |
| CN111321108A (en) | High-purity exosome density gradient centrifugation method | |
| Omrani et al. | Global trend in exosome isolation and application: an update concept in management of diseases | |
| EP2352533A1 (en) | System and method for separating cells from body fluids | |
| CN103443275A (en) | Method for recovering sperm nucleic acid from a forensic sample | |
| US20070272612A1 (en) | Method for the fractionation and separation of particles by step-wise gradient density extraction | |
| US20090265184A1 (en) | Method for the fractionation and separation of particles by step-wise gradient density extraction | |
| CN108546671A (en) | A kind of separation method of cells in biological samples microcapsule bubble | |
| CN117330481B (en) | Flow detection method for exosomes and application thereof | |
| Shami-shah et al. | Advances in extracellular vesicle isolation methods: a path towards cell-type specific EV isolation | |
| CN112852725A (en) | Preparation method and application for extracting and purifying stem cell exosome by using protein cross-linked nano affinity microspheres | |
| CN114574437A (en) | Plasma exosome extraction reagent, enrichment method, extraction kit and application thereof | |
| CN116116385A (en) | Extraction of exosomes in blood and proteomic analysis method thereof | |
| RU2824663C1 (en) | Exosome recovery kit | |
| Maggio et al. | Current methods for the isolation of urinary extracellular vesicles | |
| Kralj-Iglič et al. | Morphology and Formation Mechanisms of Cellular Vesicles Harvested from Blood |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| TR01 | Transfer of patent right | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20221215 Address after: 102,609 108, Building 1, China Resources Life Science Park, Baocan South Street, Daxing District, Beijing Patentee after: Beijing Enkang Pharmaceutical Co.,Ltd. Address before: 102206 room 106, building 1, hospital 8, shengshengyuan Road, Zhongguancun Life Science Park, Changping District, Beijing Patentee before: BEIJING ECHO BIOTECH Co.,Ltd. |