CN107978779B - Self-repairing anion exchange membrane for fuel cell and preparation method thereof - Google Patents
Self-repairing anion exchange membrane for fuel cell and preparation method thereof Download PDFInfo
- Publication number
- CN107978779B CN107978779B CN201711152612.2A CN201711152612A CN107978779B CN 107978779 B CN107978779 B CN 107978779B CN 201711152612 A CN201711152612 A CN 201711152612A CN 107978779 B CN107978779 B CN 107978779B
- Authority
- CN
- China
- Prior art keywords
- fuel cell
- exchange membrane
- anion exchange
- repairing
- self
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1065—Polymeric electrolyte materials characterised by the form, e.g. perforated or wave-shaped
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F212/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring
- C08F212/02—Monomers containing only one unsaturated aliphatic radical
- C08F212/04—Monomers containing only one unsaturated aliphatic radical containing one ring
- C08F212/14—Monomers containing only one unsaturated aliphatic radical containing one ring substituted by heteroatoms or groups containing heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/42—Nitriles
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/42—Nitriles
- C08F220/44—Acrylonitrile
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F230/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and containing phosphorus, selenium, tellurium or a metal
- C08F230/04—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and containing phosphorus, selenium, tellurium or a metal containing a metal
- C08F230/08—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and containing phosphorus, selenium, tellurium or a metal containing a metal containing silicon
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1067—Polymeric electrolyte materials characterised by their physical properties, e.g. porosity, ionic conductivity or thickness
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/1016—Fuel cells with solid electrolytes characterised by the electrolyte material
- H01M8/1018—Polymeric electrolyte materials
- H01M8/1069—Polymeric electrolyte materials characterised by the manufacturing processes
- H01M8/1072—Polymeric electrolyte materials characterised by the manufacturing processes by chemical reactions, e.g. insitu polymerisation or insitu crosslinking
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Polymers & Plastics (AREA)
- Manufacturing & Machinery (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Sustainable Development (AREA)
- Electrochemistry (AREA)
- Sustainable Energy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Fuel Cell (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Conductive Materials (AREA)
Abstract
The invention discloses a self-repairing anion exchange membrane for a fuel cell and a preparation method thereof, and the method comprises the steps of mixing trimethyl [1- (trifluoromethyl) vinyl ] silane, a polymerization monomer, 4-vinylbenzyl chloride, tetra-allylammonium chloride and an emulsifier, placing the mixture in a microwave reaction kettle in the atmosphere of nitrogen or inert gas, reacting for 15-25 minutes at 60-90 ℃, and carrying out polymerization reaction to obtain a polymer membrane; then the polymer membrane is subjected to ion exchange and other steps. The self-repairing anion exchange membrane for the fuel cell has the advantages of simple and easy preparation method, more excellent mechanical property, alkali resistance and chemical stability and higher conductivity, and can be automatically repaired and reused after being degraded in the using process, so that the service life is prolonged, and the waste problem is reduced.
Description
Technical Field
The invention belongs to the technical field of high polymer materials, relates to a fuel cell component, and particularly relates to a self-repairing anion exchange membrane for a fuel cell and a preparation method thereof.
Background
The anion exchange membrane fuel cell is a clean energy device, can directly convert chemical energy into electric energy used by electric appliances, has the advantages of high energy conversion rate, high power density, quick start, no pollution and the like, and has attracted extensive attention of researchers in the industry.
The anion exchange membrane is one of the key components of the anion exchange membrane fuel cell, plays a role in blocking fuel and transferring anions in the fuel cell, and the performance of the anion exchange membrane directly influences the working performance and the service life of the fuel cell. Anion exchange membranes for anion exchange membrane fuel cells are required to have good mechanical properties, thermal stability, chemical stability and alkali resistance, and also to have high ionic conductivity.
The traditional anion exchange membrane has the problems of low thermal and chemical stability, easy degradation of quaternary ammonium groups in the polymer at high temperature or under the alkaline condition, reduced ion exchange capacity, shortened service life of the whole membrane and waste.
Therefore, an anion exchange membrane with excellent mechanical property, alkali resistance and chemical stability and high conductivity is needed in the industry.
Disclosure of Invention
In order to overcome the defects in the prior art, the invention provides a self-repairing anion exchange membrane for a fuel cell and a preparation method thereof, the preparation method is simple and easy to implement, the requirement on equipment is not high, raw materials are easy to obtain, and the price is low.
In order to achieve the purpose, the invention adopts the technical scheme that the preparation method of the self-repairing anion exchange membrane for the fuel cell comprises the following steps:
1) preparation of polymer film: mixing trimethyl [1- (trifluoromethyl) vinyl ] silane, a polymerization monomer, 4-vinylbenzyl chloride, tetraallylammonium chloride and an emulsifier, placing the mixture in a microwave reaction kettle in the atmosphere of nitrogen or inert gas, and reacting for 15-25 minutes at 60-90 ℃ to perform polymerization reaction to obtain a polymer film;
2) ion exchange: soaking the polymer film prepared in the step 1) in a potassium hydroxide solution with the mass fraction of 5-10% at the temperature of 60-70 ℃ for 65-75 hours, taking out, soaking in deionized water for 12-24 hours, taking out, and drying in a vacuum drying oven at the temperature of 60-70 ℃ for 12-18 hours;
wherein, the mass ratio of trimethyl [1- (trifluoromethyl) vinyl ] silane, the polymerization monomer, 4-vinylbenzyl chloride, tetraallylammonium chloride and the emulsifier in the step 1) is (2-3): (2-3): (1-2): (1-2): (0.1-0.3);
the polymerization type monomer is selected from one or more of acrylonitrile, styrene, α -methyl styrene, methacrylonitrile, sulfonated styrene, octafluorostyrene, methyl methacrylate, ethyl acrylate or methyl sulfonated styrene;
the emulsifier is one or more selected from sodium dodecyl benzene sulfonate, polyoxypropylene polyethylene glycerol ether and nonylphenol polyoxyethylene ether;
the inert gas is selected from one or more of neon, helium and argon;
a self-repairing anion exchange membrane for a fuel cell is prepared by adopting the preparation method of the self-repairing anion exchange membrane for the fuel cell;
an anion exchange membrane fuel cell adopts the self-repairing anion exchange membrane for the fuel cell as a polymer electrolyte membrane.
Adopt the produced beneficial effect of above-mentioned technical scheme to lie in:
1) the preparation method of the self-repairing anion exchange membrane for the fuel cell provided by the invention is simple and easy to implement, has low requirements on equipment, easily available raw materials and low price.
2) The self-repairing anion exchange membrane for the fuel cell, provided by the invention, contains fluorine and silicon elements in a molecular structure, has excellent performances of a fluorine-silicon material, and has better mechanical properties, chemical stability, thermal stability and alkali resistance.
3) According to the self-repairing anion exchange membrane for the fuel cell, provided by the invention, the molecular structure contains chlorine groups, and when quaternary amino groups degrade and break bonds under an alkaline condition, the chlorine groups can spontaneously react with degraded product amino groups to play a role in recovering performance, so that the service life of the membrane is prolonged, and waste is reduced.
4) According to the self-repairing anion exchange membrane for the fuel cell, the raw material of the tetra-allylammonium chloride simultaneously plays a role of a cross-linking agent and an ion exchange group in a molecular structure to form a three-dimensional network structure, so that the polymer membrane is excellent in mechanical property and good in chemical and thermodynamic stability, an ion transfer channel is formed, ion transfer is facilitated, and the ionic conductivity is improved.
Detailed Description
In order to make the technical solutions of the present invention better understood and make the above features, objects, and advantages of the present invention more comprehensible, the present invention is further described with reference to the following examples. The examples are intended to illustrate the invention only and are not intended to limit the scope of the invention.
The raw material used in the following examples of the present invention was obtained from Shanghai spring Xin import & export trade company, Inc.
Example 1
A preparation method of a self-repairing anion exchange membrane for a fuel cell comprises the following steps:
1) preparation of polymer film: mixing 2g of trimethyl [1- (trifluoromethyl) vinyl ] silane, 2g of acrylonitrile, 1g of 4-vinylbenzyl chloride, 1g of tetra-allylammonium chloride and 0.1g of sodium dodecyl benzene sulfonate, placing the mixture in a microwave reaction kettle in a nitrogen atmosphere, and reacting for 15 minutes at 60 ℃ to perform polymerization reaction to obtain a polymer membrane;
2) ion exchange: soaking the polymer film prepared in the step 1) in a potassium hydroxide solution with the mass fraction of 5% at 60 ℃ for 65 hours, taking out the polymer film, soaking the polymer film in deionized water for 12 hours, taking out the polymer film, and drying the polymer film in a vacuum drying oven at 60 ℃ for 12 hours;
a self-repairing anion exchange membrane for a fuel cell is prepared by adopting the preparation method of the self-repairing anion exchange membrane for the fuel cell;
an anion exchange membrane fuel cell adopts the self-repairing anion exchange membrane for the fuel cell as a polymer electrolyte membrane.
Example 2
A preparation method of a self-repairing anion exchange membrane for a fuel cell comprises the following steps:
1) preparation of polymer film: mixing 2.3g of trimethyl [1- (trifluoromethyl) vinyl ] silane, 2.5g of octafluorostyrene, 1.2g of 4-vinylbenzyl chloride, 1.6g of tetra allyl ammonium chloride and 0.2g of polyoxypropylene polyethylene glycerol ether, placing the mixture in a microwave reaction kettle in an argon atmosphere, and reacting at 70 ℃ for 20 minutes to perform polymerization reaction to obtain a polymer film;
2) ion exchange: soaking the polymer film prepared in the step 1) in a potassium hydroxide solution with the mass fraction of 7% at 64 ℃ for 69 hours, taking out, soaking in deionized water for 15 hours, taking out, and drying in a vacuum drying oven at 65 ℃ for 14 hours;
a self-repairing anion exchange membrane for a fuel cell is prepared by adopting the preparation method of the self-repairing anion exchange membrane for the fuel cell;
an anion exchange membrane fuel cell adopts the self-repairing anion exchange membrane for the fuel cell as a polymer electrolyte membrane.
Example 3
A preparation method of a self-repairing anion exchange membrane for a fuel cell comprises the following steps:
1) preparing a polymer film, namely mixing 2.8g of trimethyl [1- (trifluoromethyl) vinyl ] silane, 2g of α -methyl styrene, 1.8g of 4-vinyl benzyl chloride, 2g of tetra allyl ammonium chloride and 0.25g of nonylphenol polyoxyethylene ether, placing the mixture in a microwave reaction kettle in a neon atmosphere, and reacting for 22 minutes at 80 ℃ to perform polymerization reaction to obtain the polymer film;
2) ion exchange: soaking the polymer film prepared in the step 1) in a potassium hydroxide solution with the mass fraction of 9% at 68 ℃ for 72 hours, taking out the polymer film, soaking the polymer film in deionized water for 22 hours, taking out the polymer film, and drying the polymer film in a vacuum drying oven at 68 ℃ for 16 hours;
a self-repairing anion exchange membrane for a fuel cell is prepared by adopting the preparation method of the self-repairing anion exchange membrane for the fuel cell;
an anion exchange membrane fuel cell adopts the self-repairing anion exchange membrane for the fuel cell as a polymer electrolyte membrane.
Example 4
A preparation method of a self-repairing anion exchange membrane for a fuel cell comprises the following steps:
1) preparation of polymer film: mixing 3g of trimethyl [1- (trifluoromethyl) vinyl ] silane, 3g of methacrylonitrile, 1g of 4-vinylbenzyl chloride, 2g of tetra allyl ammonium chloride and 0.3g of sodium dodecyl benzene sulfonate, placing the mixture in a microwave reaction kettle in a nitrogen atmosphere, and reacting at 90 ℃ for 25 minutes to perform polymerization reaction to obtain a polymer film;
2) ion exchange: soaking the polymer film prepared in the step 1) in a potassium hydroxide solution with the mass fraction of 10% at 70 ℃ for 75 hours, taking out the polymer film, soaking the polymer film in deionized water for 24 hours, taking out the polymer film, and drying the polymer film in a vacuum drying oven at 70 ℃ for 18 hours;
a self-repairing anion exchange membrane for a fuel cell is prepared by adopting the preparation method of the self-repairing anion exchange membrane for the fuel cell;
an anion exchange membrane fuel cell adopts the self-repairing anion exchange membrane for the fuel cell as a polymer electrolyte membrane.
Comparative example
Commercially available conventional homogeneous anion exchange membranes are available from Beijing Runfan technology development, Inc.
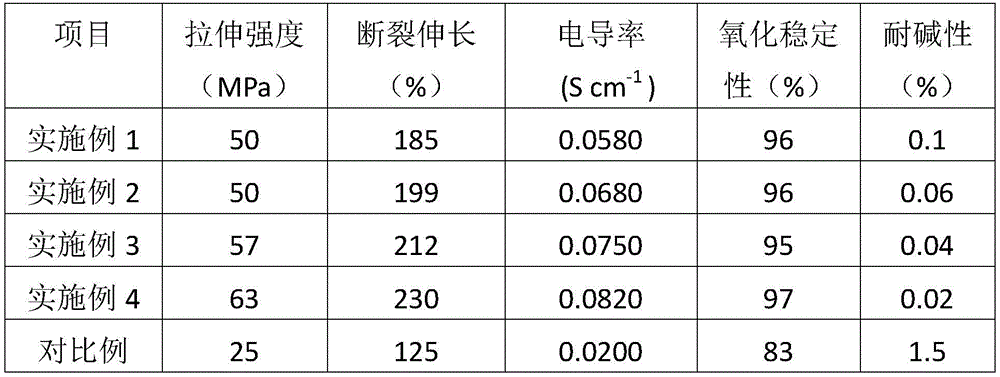
The samples obtained in the above examples 1 to 4 and comparative example were subjected to the relevant performance tests, the test results are shown in table 1, the test methods are as follows,
(1) and (3) testing tensile strength: testing according to GB/T1040-2006 Plastic tensile Property test method;
(2) conductivity: the impedance of the prepared anion-exchange membrane is measured on an electrochemical workstation (Zahner IM6 EX) by adopting a two-electrode alternating-current impedance method, and the testing frequency is 1 Hz-1 MHz. The conductivity test was performed in a vessel filled with deionized water in order to ensure that the relative humidity of the membrane was 100% and the temperature was controlled at 30 ℃. Before the test at this temperature point, the sample was kept at this temperature for 30min, and the conductivity was calculated according to the following formula:
wherein σ is the conductivity (S cm)-1) L is the distance (cm) between the two electrodes, R is the AC impedance of the sample being measured, and S is the cross-sectional area of the membrane.
(3) Oxidation stability: prepared vaginaOxidation stability of the ion exchange membranes was achieved by soaking the membranes in Fenton's reagent (containing 4ppm Fe) at 70 deg.C2+3% hydrogen peroxide solution) for 20 hours, and the weight retention of the film was weighed and calculated. The calculation formula is as follows: retention rate (weight of membrane after soaking-weight of membrane before soaking)/weight of membrane before soaking × 100%.
(4) Alkali resistance: the alkali resistance of the membrane was measured by immersing the membrane in a 1mol/L KOH aqueous solution at 80 ℃ for 60 days and calculating the rate of change in conductivity before and after immersion. The calculation formula is as follows: change rate (conductivity before soaking-conductivity after soaking)/conductivity before soaking × 100%.
As can be seen from Table 1, the self-repairing anion exchange membrane for the fuel cell disclosed by the invention has better mechanical property, oxidation resistance and alkali resistance, and the conductivity is higher than that of the traditional anion exchange membrane, so that the self-repairing anion exchange membrane for the fuel cell meets the use requirement of the anion exchange membrane fuel cell.
TABLE 1 Properties of samples of examples and comparative examples
The foregoing is merely a preferred embodiment of the invention and is not intended to limit the invention in any manner; as will be readily apparent to those skilled in the art from the disclosure herein, the present invention may be practiced without these specific details; however, those skilled in the art should appreciate that they can readily use the disclosed conception and specific embodiments as a basis for designing or modifying other structures for carrying out the same purposes of the present invention; meanwhile, any changes, modifications, and evolutions of the equivalent changes of the above embodiments according to the actual techniques of the present invention are still within the protection scope of the technical solution of the present invention.
Claims (7)
1. A preparation method of a self-repairing anion exchange membrane for a fuel cell is characterized by comprising the following steps:
1) preparation of polymer film: mixing trimethyl [1- (trifluoromethyl) vinyl ] silane, a polymerization monomer, 4-vinylbenzyl chloride, tetraallylammonium chloride and an emulsifier, placing the mixture in a microwave reaction kettle in the atmosphere of nitrogen or inert gas, and reacting for 15-25 minutes at 60-90 ℃ to perform polymerization reaction to obtain a polymer film;
2) ion exchange: soaking the polymer film prepared in the step 1) in a potassium hydroxide solution with the mass fraction of 5-10% at the temperature of 60-70 ℃ for 65-75 hours, taking out, soaking in deionized water for 12-24 hours, taking out, and drying in a vacuum drying oven at the temperature of 60-70 ℃ for 12-18 hours;
wherein the polymerized monomer is selected from one or more of acrylonitrile, styrene, α -methyl styrene, methacrylonitrile, sulfonated styrene, octafluorostyrene, methyl methacrylate, ethyl acrylate or methyl sulfonated styrene.
2. The method for preparing the self-repairing anion-exchange membrane for the fuel cell according to claim 1, wherein the mass ratio of trimethyl [1- (trifluoromethyl) vinyl ] silane, the polymerized monomer, 4-vinylbenzyl chloride, tetraallylammonium chloride and the emulsifier in the step 1) is (2-3): (2-3): (1-2): (1-2): (0.1-0.3).
3. The method for preparing the self-repairing anion exchange membrane for the fuel cell as claimed in claim 1, wherein the emulsifier is one or more selected from sodium dodecyl benzene sulfonate, polyoxypropylene polyethylene glycerol ether and nonylphenol polyoxyethylene ether.
4. The method as claimed in claim 1, wherein the inert gas is selected from one or more of neon, helium and argon.
5. A self-repairing anion exchange membrane for a fuel cell is characterized by being prepared by the preparation method of the self-repairing anion exchange membrane for the fuel cell as claimed in any one of claims 1 to 4.
6. An application method of the self-repairing anion exchange membrane for the fuel cell, which is characterized in that the self-repairing anion exchange membrane for the fuel cell of claim 5 is adopted as a polymer electrolyte membrane of the cell.
7. An anion exchange membrane fuel cell, characterized in that the self-repairing anion exchange membrane for a fuel cell according to claim 5 is used as a polymer electrolyte membrane.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201711152612.2A CN107978779B (en) | 2017-11-19 | 2017-11-19 | Self-repairing anion exchange membrane for fuel cell and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201711152612.2A CN107978779B (en) | 2017-11-19 | 2017-11-19 | Self-repairing anion exchange membrane for fuel cell and preparation method thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107978779A CN107978779A (en) | 2018-05-01 |
| CN107978779B true CN107978779B (en) | 2020-03-27 |
Family
ID=62010497
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201711152612.2A Active CN107978779B (en) | 2017-11-19 | 2017-11-19 | Self-repairing anion exchange membrane for fuel cell and preparation method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN107978779B (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109265616A (en) * | 2018-08-01 | 2019-01-25 | 湖南辰砾新材料有限公司 | A kind of insulating foam and preparation method thereof |
| JP2021082576A (en) * | 2019-11-20 | 2021-05-27 | ロベルト・ボッシュ・ゲゼルシャフト・ミト・ベシュレンクテル・ハフツングRobert Bosch Gmbh | Separator, fuel battery, and manufacturing method of separator |
| CN114976477B (en) * | 2022-06-02 | 2023-09-15 | 界首市天鸿新材料股份有限公司 | Tape casting forming process of high-performance lithium battery diaphragm |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006021193A (en) * | 2004-06-07 | 2006-01-26 | Dainichiseika Color & Chem Mfg Co Ltd | Ion exchange composite membrane and electrodialyser |
| CN101024690A (en) * | 2006-02-20 | 2007-08-29 | 三星Sdi株式会社 | Multiblock copolymer, method of preparing the same, polymer electrolyte membrane prepared from the multiblock copolymer, method of preparing the polymer electrolyte membrane, and fuel cell employing t |
| CN102725316A (en) * | 2009-09-17 | 2012-10-10 | 联合利华有限公司 | Use of branched addition copolymers in films and membranes |
| CN104703697A (en) * | 2012-10-04 | 2015-06-10 | 伊沃夸水处理技术有限责任公司 | High-performance anion exchange membranes and methods of making same |
| CN104927079A (en) * | 2015-07-10 | 2015-09-23 | 常州大学 | Preparation method of alkaline anion exchange membrane |
| CN106345324A (en) * | 2016-08-31 | 2017-01-25 | 山东天维膜技术有限公司 | Method for preparing hybridized ion exchange membrane |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060088749A1 (en) * | 2004-10-26 | 2006-04-27 | Gangadhar Panambur | Novel compositions of monomers, oligomers and polymers and methods for making the same |
| US20100133171A1 (en) * | 2009-03-27 | 2010-06-03 | Chunqing Liu | Polybenzoxazole Polymer-Based Mixed Matrix Membranes |
-
2017
- 2017-11-19 CN CN201711152612.2A patent/CN107978779B/en active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006021193A (en) * | 2004-06-07 | 2006-01-26 | Dainichiseika Color & Chem Mfg Co Ltd | Ion exchange composite membrane and electrodialyser |
| CN101024690A (en) * | 2006-02-20 | 2007-08-29 | 三星Sdi株式会社 | Multiblock copolymer, method of preparing the same, polymer electrolyte membrane prepared from the multiblock copolymer, method of preparing the polymer electrolyte membrane, and fuel cell employing t |
| CN102725316A (en) * | 2009-09-17 | 2012-10-10 | 联合利华有限公司 | Use of branched addition copolymers in films and membranes |
| CN104703697A (en) * | 2012-10-04 | 2015-06-10 | 伊沃夸水处理技术有限责任公司 | High-performance anion exchange membranes and methods of making same |
| CN104927079A (en) * | 2015-07-10 | 2015-09-23 | 常州大学 | Preparation method of alkaline anion exchange membrane |
| CN106345324A (en) * | 2016-08-31 | 2017-01-25 | 山东天维膜技术有限公司 | Method for preparing hybridized ion exchange membrane |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107978779A (en) | 2018-05-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110862516A (en) | Cardo structure-containing isatin aromatic hydrocarbon copolymer, and preparation method and application thereof | |
| CN107978779B (en) | Self-repairing anion exchange membrane for fuel cell and preparation method thereof | |
| CN112185712B (en) | Imidazole polyion liquid gel electrolyte and preparation method thereof | |
| KR101351280B1 (en) | Anion exchange membrane for redox flow battery and method for preparing the same | |
| CN108794784B (en) | Anion exchange membrane | |
| CN110797561B (en) | Proton exchange membrane based on carbon quantum dots and preparation method thereof | |
| CN110054792B (en) | SBS-based anion exchange membrane and preparation method thereof | |
| CN108219086B (en) | Anion exchange membrane based on fullerene and preparation method thereof | |
| Wang et al. | PVDF based ion exchange membrane prepared by radiation grafting of ethyl styrenesulfonate and sequent hydrolysis | |
| CN108091930A (en) | New single-ion polymer electrolyte and preparation method and application | |
| CN110071328B (en) | Cross-linked modified polyethyleneimine solid electrolyte and application thereof | |
| CN110247110B (en) | Preparation method of lithium ion solid electrolyte with room-temperature high ionic conductivity | |
| CN101488572B (en) | Ionic exchange film for fuel cell and preparation thereof | |
| CN102945975A (en) | Pyridine onium salt polymer anion exchange film and preparation method thereof | |
| CN108711632B (en) | Anion exchange membrane for fuel cell and preparation method thereof | |
| CN116613362A (en) | Composite amphoteric ion exchange membrane for vanadium battery and preparation method thereof | |
| Li et al. | Trimethyl-ammonium alkaline anion exchange membranes with the vinylbenzyl chloride/acrylonitrile main chain | |
| CN110828872B (en) | High-temperature proton exchange membrane for fuel cell and preparation method thereof | |
| CN112531189A (en) | Anion exchange membrane for fuel cell and preparation method thereof | |
| CN113078340B (en) | Polysulfone/polyvinyl alcohol composite anion exchange membrane and preparation method thereof | |
| CN107978769A (en) | A kind of vanadium cell is used based on pyrrolotriazine derivatives membrane and preparation method thereof | |
| CN107978778B (en) | High-temperature anhydrous proton exchange membrane and preparation method thereof | |
| CN106519282B (en) | A kind of Kynoar is grafted the preparation method of poly- (α-methylstyrene) copolymer sulfonic acid proton exchange film | |
| CN114695933A (en) | Semi-interpenetrating anion exchange membrane and preparation method and application thereof | |
| CN113121764A (en) | Branched polyarylether ion exchange membrane and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20201105 Address after: Taihu County, Anhui city of Anqing Province Jin Xi Zhen 246400 Patentee after: Taihu County market supervision and Inspection Institute (Taihu County functional membrane Testing Institute) Address before: 410217 Changsha, Changsha City, Hunan Wangcheng economic and Technological Development Zone gold Pioneer Park 4 Building C5 Patentee before: HUNAN CHENLI NEW MATERIAL Co.,Ltd. |
|
| TR01 | Transfer of patent right |