CN107841010B - Preparation method of easy-to-process reinforced styrene butadiene rubber - Google Patents
Preparation method of easy-to-process reinforced styrene butadiene rubber Download PDFInfo
- Publication number
- CN107841010B CN107841010B CN201610833898.XA CN201610833898A CN107841010B CN 107841010 B CN107841010 B CN 107841010B CN 201610833898 A CN201610833898 A CN 201610833898A CN 107841010 B CN107841010 B CN 107841010B
- Authority
- CN
- China
- Prior art keywords
- rubber
- parts
- softener
- reinforcing agent
- solvent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 229920003048 styrene butadiene rubber Polymers 0.000 title claims abstract description 50
- 238000000034 method Methods 0.000 title claims abstract description 28
- 238000002360 preparation method Methods 0.000 title claims description 15
- 239000012744 reinforcing agent Substances 0.000 claims abstract description 70
- 239000005060 rubber Substances 0.000 claims abstract description 67
- 239000002904 solvent Substances 0.000 claims abstract description 63
- 229920001971 elastomer Polymers 0.000 claims abstract description 58
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 47
- 239000000839 emulsion Substances 0.000 claims abstract description 43
- 239000004816 latex Substances 0.000 claims abstract description 41
- 229920000126 latex Polymers 0.000 claims abstract description 41
- 239000003054 catalyst Substances 0.000 claims abstract description 37
- 239000003995 emulsifying agent Substances 0.000 claims abstract description 36
- 238000002156 mixing Methods 0.000 claims abstract description 30
- 230000003014 reinforcing effect Effects 0.000 claims abstract description 24
- MTAZNLWOLGHBHU-UHFFFAOYSA-N butadiene-styrene rubber Chemical compound C=CC=C.C=CC1=CC=CC=C1 MTAZNLWOLGHBHU-UHFFFAOYSA-N 0.000 claims abstract description 22
- 238000001035 drying Methods 0.000 claims abstract description 17
- 238000005406 washing Methods 0.000 claims abstract description 17
- 239000003292 glue Substances 0.000 claims abstract description 15
- 150000001336 alkenes Chemical class 0.000 claims abstract description 12
- 238000000354 decomposition reaction Methods 0.000 claims abstract description 11
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 claims abstract description 11
- 239000002174 Styrene-butadiene Substances 0.000 claims abstract description 6
- 239000011115 styrene butadiene Substances 0.000 claims abstract description 6
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 claims description 48
- -1 thioamide compound Chemical class 0.000 claims description 44
- 238000003756 stirring Methods 0.000 claims description 31
- 238000006243 chemical reaction Methods 0.000 claims description 28
- 238000005865 alkene metathesis reaction Methods 0.000 claims description 27
- 239000000701 coagulant Substances 0.000 claims description 20
- 238000009833 condensation Methods 0.000 claims description 16
- 230000005494 condensation Effects 0.000 claims description 16
- XUDBVJCTLZTSDC-UHFFFAOYSA-N 2-ethenylbenzoic acid Chemical compound OC(=O)C1=CC=CC=C1C=C XUDBVJCTLZTSDC-UHFFFAOYSA-N 0.000 claims description 13
- 125000004432 carbon atom Chemical group C* 0.000 claims description 13
- 239000003795 chemical substances by application Substances 0.000 claims description 13
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 11
- YNQLUTRBYVCPMQ-UHFFFAOYSA-N Ethylbenzene Chemical compound CCC1=CC=CC=C1 YNQLUTRBYVCPMQ-UHFFFAOYSA-N 0.000 claims description 10
- IRTACFOVZDBFEX-UHFFFAOYSA-N ethenyl-diethoxy-ethylsilane Chemical compound CCO[Si](CC)(C=C)OCC IRTACFOVZDBFEX-UHFFFAOYSA-N 0.000 claims description 9
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 claims description 7
- 230000008569 process Effects 0.000 claims description 7
- KBIWOJBFYNSQKW-UHFFFAOYSA-N 3-ethenylphthalic acid Chemical compound OC(=O)C1=CC=CC(C=C)=C1C(O)=O KBIWOJBFYNSQKW-UHFFFAOYSA-N 0.000 claims description 6
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 claims description 6
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 claims description 6
- 238000004519 manufacturing process Methods 0.000 claims description 6
- 125000001424 substituent group Chemical group 0.000 claims description 6
- 125000003545 alkoxy group Chemical group 0.000 claims description 5
- FWDBOZPQNFPOLF-UHFFFAOYSA-N ethenyl(triethoxy)silane Chemical compound CCO[Si](OCC)(OCC)C=C FWDBOZPQNFPOLF-UHFFFAOYSA-N 0.000 claims description 5
- NKSJNEHGWDZZQF-UHFFFAOYSA-N ethenyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)C=C NKSJNEHGWDZZQF-UHFFFAOYSA-N 0.000 claims description 4
- HZVOZRGWRWCICA-UHFFFAOYSA-N methanediyl Chemical compound [CH2] HZVOZRGWRWCICA-UHFFFAOYSA-N 0.000 claims description 4
- 239000000203 mixture Substances 0.000 claims description 4
- 229920006395 saturated elastomer Polymers 0.000 claims description 4
- 239000000344 soap Substances 0.000 claims description 4
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 claims description 3
- 229910000329 aluminium sulfate Inorganic materials 0.000 claims description 3
- 239000001110 calcium chloride Substances 0.000 claims description 3
- 229910001628 calcium chloride Inorganic materials 0.000 claims description 3
- 230000015271 coagulation Effects 0.000 claims description 3
- 238000005345 coagulation Methods 0.000 claims description 3
- RNCZBYQGHGAXTP-UHFFFAOYSA-N ethenyl-hexyl-dipentoxysilane Chemical compound C(=C)[Si](CCCCCC)(OCCCCC)OCCCCC RNCZBYQGHGAXTP-UHFFFAOYSA-N 0.000 claims description 3
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 claims description 3
- 229910001629 magnesium chloride Inorganic materials 0.000 claims description 3
- 229910052943 magnesium sulfate Inorganic materials 0.000 claims description 3
- 229910052751 metal Inorganic materials 0.000 claims description 3
- 239000002184 metal Substances 0.000 claims description 3
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 3
- 150000002832 nitroso derivatives Chemical group 0.000 claims description 3
- 150000007524 organic acids Chemical class 0.000 claims description 3
- 229910000077 silane Inorganic materials 0.000 claims description 3
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 claims description 3
- MMILTFOIUWTZFM-UHFFFAOYSA-N 2-ethenoxybenzoic acid Chemical compound OC(=O)C1=CC=CC=C1OC=C MMILTFOIUWTZFM-UHFFFAOYSA-N 0.000 claims description 2
- PGGMLBNYLZDCBN-UHFFFAOYSA-N 7-cyclohexyl-2-ethenyl-3-sulfanylideneisoindol-1-one Chemical compound C(=C)N1C(C=2C(C1=O)=C(C=CC=2)C1CCCCC1)=S PGGMLBNYLZDCBN-UHFFFAOYSA-N 0.000 claims description 2
- OLTTVMKWLZAVBQ-UHFFFAOYSA-N 7-cyclohexyl-2-prop-1-enyl-3-sulfanylideneisoindol-1-one Chemical compound C(=CC)N1C(C=2C(C1=O)=C(C=CC=2)C1CCCCC1)=S OLTTVMKWLZAVBQ-UHFFFAOYSA-N 0.000 claims description 2
- FSOOWVYTLGDBFZ-UHFFFAOYSA-N C(=C)(C)[SiH2]CC(OCCCC)OCCCC Chemical compound C(=C)(C)[SiH2]CC(OCCCC)OCCCC FSOOWVYTLGDBFZ-UHFFFAOYSA-N 0.000 claims description 2
- 229910021592 Copper(II) chloride Inorganic materials 0.000 claims description 2
- 229910021578 Iron(III) chloride Inorganic materials 0.000 claims description 2
- GLKXEWVWWWLALC-UHFFFAOYSA-N N(=O)N(C1=CC2=CC=CC=C2C=C1)C=CC1=CC=CC=C1 Chemical compound N(=O)N(C1=CC2=CC=CC=C2C=C1)C=CC1=CC=CC=C1 GLKXEWVWWWLALC-UHFFFAOYSA-N 0.000 claims description 2
- 239000012327 Ruthenium complex Substances 0.000 claims description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N Salicylic acid Natural products OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 claims description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 claims description 2
- BBZZUDQCNMQSSE-UHFFFAOYSA-L [Ru](Cl)Cl.C(C)(C)OC1=C(C=C2C(CCCC2)P(C2CCCCC2)C2CCCCC2)C=CC=C1 Chemical compound [Ru](Cl)Cl.C(C)(C)OC1=C(C=C2C(CCCC2)P(C2CCCCC2)C2CCCCC2)C=CC=C1 BBZZUDQCNMQSSE-UHFFFAOYSA-L 0.000 claims description 2
- RSWGJHLUYNHPMX-ONCXSQPRSA-N abietic acid Chemical compound C([C@@H]12)CC(C(C)C)=CC1=CC[C@@H]1[C@]2(C)CCC[C@@]1(C)C(O)=O RSWGJHLUYNHPMX-ONCXSQPRSA-N 0.000 claims description 2
- 125000005336 allyloxy group Chemical group 0.000 claims description 2
- DIZPMCHEQGEION-UHFFFAOYSA-H aluminium sulfate (anhydrous) Chemical compound [Al+3].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O DIZPMCHEQGEION-UHFFFAOYSA-H 0.000 claims description 2
- 150000001412 amines Chemical class 0.000 claims description 2
- 125000003118 aryl group Chemical group 0.000 claims description 2
- 125000004104 aryloxy group Chemical group 0.000 claims description 2
- 125000000051 benzyloxy group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])O* 0.000 claims description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 2
- RBTARNINKXHZNM-UHFFFAOYSA-K iron trichloride Chemical compound Cl[Fe](Cl)Cl RBTARNINKXHZNM-UHFFFAOYSA-K 0.000 claims description 2
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 claims description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 2
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 claims description 2
- 229920000642 polymer Polymers 0.000 claims description 2
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 claims description 2
- 229960004889 salicylic acid Drugs 0.000 claims description 2
- 150000004671 saturated fatty acids Chemical group 0.000 claims description 2
- 125000004469 siloxy group Chemical group [SiH3]O* 0.000 claims description 2
- 150000004670 unsaturated fatty acids Chemical class 0.000 claims description 2
- 235000021122 unsaturated fatty acids Nutrition 0.000 claims description 2
- OCKPCBLVNKHBMX-UHFFFAOYSA-N butylbenzene Chemical compound CCCCC1=CC=CC=C1 OCKPCBLVNKHBMX-UHFFFAOYSA-N 0.000 claims 4
- 125000000753 cycloalkyl group Chemical group 0.000 claims 1
- 239000004902 Softening Agent Substances 0.000 abstract description 10
- 241001441571 Hiodontidae Species 0.000 abstract description 5
- 230000009471 action Effects 0.000 abstract description 4
- 238000012545 processing Methods 0.000 abstract description 3
- 230000001112 coagulating effect Effects 0.000 abstract 1
- 239000000047 product Substances 0.000 description 29
- 238000012360 testing method Methods 0.000 description 15
- 235000014113 dietary fatty acids Nutrition 0.000 description 10
- 239000000194 fatty acid Substances 0.000 description 10
- 229930195729 fatty acid Natural products 0.000 description 10
- 229910052700 potassium Inorganic materials 0.000 description 9
- 239000011591 potassium Substances 0.000 description 9
- 239000010692 aromatic oil Substances 0.000 description 7
- 230000000052 comparative effect Effects 0.000 description 7
- 239000003921 oil Substances 0.000 description 5
- 229910052708 sodium Inorganic materials 0.000 description 5
- 239000011734 sodium Substances 0.000 description 5
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 239000010426 asphalt Substances 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 239000006229 carbon black Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 239000002994 raw material Substances 0.000 description 3
- ITCAUAYQCALGGV-XTICBAGASA-M sodium;(1r,4ar,4br,10ar)-1,4a-dimethyl-7-propan-2-yl-2,3,4,4b,5,6,10,10a-octahydrophenanthrene-1-carboxylate Chemical compound [Na+].C([C@@H]12)CC(C(C)C)=CC1=CC[C@@H]1[C@]2(C)CCC[C@@]1(C)C([O-])=O ITCAUAYQCALGGV-XTICBAGASA-M 0.000 description 3
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- MBGQQKKTDDNCSG-UHFFFAOYSA-N ethenyl-diethoxy-methylsilane Chemical compound CCO[Si](C)(C=C)OCC MBGQQKKTDDNCSG-UHFFFAOYSA-N 0.000 description 2
- 239000007791 liquid phase Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 238000010057 rubber processing Methods 0.000 description 2
- 229910052707 ruthenium Inorganic materials 0.000 description 2
- 238000005728 strengthening Methods 0.000 description 2
- MMQSDCNBALQLIU-UHFFFAOYSA-N C(=C)CCCCCO[SiH](CCCCCC)CCCCCC Chemical compound C(=C)CCCCCO[SiH](CCCCCC)CCCCCC MMQSDCNBALQLIU-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 239000005662 Paraffin oil Substances 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000012752 auxiliary agent Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- ORTQZVOHEJQUHG-UHFFFAOYSA-L copper(II) chloride Chemical compound Cl[Cu]Cl ORTQZVOHEJQUHG-UHFFFAOYSA-L 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- MQBPUUJYMURCOU-UHFFFAOYSA-N ethenyl-dihexyl-pentoxysilane Chemical compound C(=C)[Si](CCCCCC)(CCCCCC)OCCCCC MQBPUUJYMURCOU-UHFFFAOYSA-N 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000000295 fuel oil Substances 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 125000006606 n-butoxy group Chemical group 0.000 description 1
- 125000001298 n-hexoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 125000006609 n-nonyloxy group Chemical group 0.000 description 1
- 125000003935 n-pentoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 150000001282 organosilanes Chemical class 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 238000012805 post-processing Methods 0.000 description 1
- NVJCKICOBXMJIJ-UHFFFAOYSA-M potassium;1,4a-dimethyl-7-propan-2-yl-2,3,4,4b,5,6,10,10a-octahydrophenanthrene-1-carboxylate Chemical compound [K+].C12CCC(C(C)C)=CC2=CCC2C1(C)CCCC2(C)C([O-])=O NVJCKICOBXMJIJ-UHFFFAOYSA-M 0.000 description 1
- 125000002572 propoxy group Chemical group [*]OC([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229940099259 vaseline Drugs 0.000 description 1
- 238000004073 vulcanization Methods 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L9/00—Compositions of homopolymers or copolymers of conjugated diene hydrocarbons
- C08L9/06—Copolymers with styrene
- C08L9/08—Latex
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08C—TREATMENT OR CHEMICAL MODIFICATION OF RUBBERS
- C08C19/00—Chemical modification of rubber
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08C—TREATMENT OR CHEMICAL MODIFICATION OF RUBBERS
- C08C19/00—Chemical modification of rubber
- C08C19/08—Depolymerisation
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08C—TREATMENT OR CHEMICAL MODIFICATION OF RUBBERS
- C08C19/00—Chemical modification of rubber
- C08C19/25—Incorporating silicon atoms into the molecule
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08C—TREATMENT OR CHEMICAL MODIFICATION OF RUBBERS
- C08C19/00—Chemical modification of rubber
- C08C2019/09—Metathese
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Processes Of Treating Macromolecular Substances (AREA)
Abstract
The invention provides a method for easily processing reinforced butadiene styrene rubber, which comprises the steps of firstly dissolving butadiene styrene rubber in a solvent to prepare a solution, adding an olefin double decomposition catalyst and an anti-scorching rubber reinforcing agent, and removing the reacted glue solution through the solvent to obtain a reinforcing softener; dispersing the softening agent into water under the action of an emulsifier to form emulsion, mixing the emulsion with styrene butadiene latex, and then coagulating, washing and drying to obtain the easily processed reinforced styrene butadiene rubber product. The softener product can reduce Mooney 0.2-2.5 units/part of base rubber, improve strength by more than 0.15 MPa/part and delay scorching time by 1-15 s/part.
Description
Technical Field
The invention relates to a preparation method of styrene butadiene rubber, in particular to a preparation method of reinforced styrene butadiene rubber which is easy to process.
Background
The Mooney viscosity of the emulsion polymerized styrene-butadiene rubber raw rubber is generally 40-130ML1+4 100℃Mooney requirements of the mixes are generally below 90ML1+4 100℃(ii) a The raw rubber has lower strength, generally only 5.5 +/-0.5 MPa, and the strength is improved mainly by reinforcing agent in the post-processing process, generally reaching 21.5-24.5 MPa. The reinforcing agent mainly comprises inorganic powder such as carbon black, white carbon black, nano calcium carbonate and montmorillonite, the self-agglomeration of the inorganic powder is serious, and high energy is required for mixing the inorganic powder with rubber, and the inorganic powder is generally repeatedly kneaded and mixed for a long time on an open mill or an internal mixer. In the open milling or banburying process, a certain amount of softener is generally required to be added. Generally, the low molecular weight substances can provide a certain flexibility to the rubber compound, and can increase the plasticity, fluidity and adhesiveness of the rubber compound so as to facilitate the technological operations such as compression, molding and the like. And the dispersion of the powdery compounding agent is facilitated, the mixing temperature is reduced, and the processability of the rubber is improved.
The softener most used in rubber processing is a petroleum softener, and CN201410018005.7 provides a wet-skid resistant rubber softener and a preparation process, wherein the softener comprises the following raw materials in parts by weight: naphthenic oil: 70-90 parts of alkene mixed resin: 10-30 parts of aluminum trichloride: 0.1 to 1.0 portion. The anti-wet-skid rubber softener has the advantages of various performances of environment-friendly aromatic oil and environment-friendly naphthenic oil, and has good anti-wet-skid performance, lower rolling resistance and good wear resistance. CN201110345185.6 discloses a rubber softener and a preparation method thereof, which comprises the following raw materials in parts by weight: 10-30% of asphalt and 90-70% of naphthenic oil; the asphalt modified rubber softener is nontoxic and lower than European Union safety standards, fills up the blank of domestic production, and greatly saves the cost of domestic tire production enterprises. The softeners reported in the patent are actually physical plasticizers, mainly comprising aromatic oil, paraffin oil, naphthenic oil, heavy oil, paraffin, vaseline, asphalt, petroleum resin and the like, have only filling and softening effects on rubber, have no reinforcing effect, and are easy to separate out during the use of the product.
The Mooney scorch time of the styrene butadiene rubber can be used for measuring the scorch difficulty of the rubber material, and the longer the scorch time is, the less prone to the occurrence of the phenomenon of early vulcanization in the processing process. Scorch time can generally be measured by Mooney viscometer, the time required for the Mooney torque to reach 10%, denoted by T10, and the T10 of styrene butadiene rubber under standard test formulation is 2-3 min.
Disclosure of Invention
The invention aims to provide a preparation method of easily processed reinforced styrene butadiene rubber, which comprises the following specific steps:
1) firstly, preparing a reinforcing softener, dissolving styrene-butadiene rubber in a solvent to prepare a solution with the concentration of 1-50% w, preferably 5-15% w, adding 0.1-1.0 part, preferably 0.1-0.3 part of olefin double decomposition catalyst and 1-50 parts, preferably 10-30 parts of scorch-proof rubber reinforcing agent into the solution based on 100 parts by mass of rubber, isolating air and water at the temperature of 15-50 ℃, preferably 20-30 ℃, reacting for 0.5-5 hours, preferably 1-2 hours, and removing glue solution after the reaction by using the solvent to obtain a reinforcing softener product with the Mn of 3000-; the scorch-proof rubber reinforcing agent is prepared by olefin double decomposition reaction of a scorch-proof agent and a reinforcing agent, wherein the scorch-proof agent is a nitroso compound containing an ethylenically unsaturated substituent, an organic acid or a thioamide compound; the reinforcing agent is an organic silane compound containing an ethylenically unsaturated substituent.
2) Adding the prepared reinforced softener product, an emulsifier and water, mixing, and stirring to prepare a softener emulsion;
3) adding styrene-butadiene latex and softener emulsion into a condensation kettle, stirring, and mixing according to a dry basis ratio of 100: (1-50) mixing to prepare mixed latex, wherein the preferable ratio is 100: (10-30), adding a coagulant for coagulation at the reaction temperature of 10-80 ℃, and washing, centrifugally dewatering and drying to obtain the easily-processed reinforced styrene-butadiene rubber.
The specific preparation method of the scorch-proof rubber reinforcing agent comprises the following steps: the scorch retarder and the reinforcing agent are mixed in a ratio of 1: (0.1-5.0), preparing a solution with the mass concentration of 1% -30% by using a solvent, adding an olefin double decomposition catalyst accounting for 0.01-0.1% of the total mass of the anti-scorching agent and the reinforcing agent into the solution, isolating air and water, reacting at normal temperature for 0.5-8 h, and then removing the solvent by using a vacuum solvent removal device to prepare the anti-scorching rubber reinforcing agent.
The scorch retarder is a nitroso compound containing an ethylenically unsaturated substituent, or an organic acid or a thioimide, and can be N-nitrosodi (vinyl benzene) amine, N-nitroso-styryl-beta-naphthylamine, N-nitroso-2-propenyl-2, 4-dimethyl-1, 2-dihydroquinoline polymer, vinyl benzoic acid, vinyl salicylic acid, butenyl salicylic acid, vinyl phthalic acid, N-vinyl cyclohexyl thiophthalimide, or N-propenyl cyclohexyl thiophthalimide;
the solvent is preferably a solvent which does not contain alkene in the structure and can dissolve styrene butadiene rubber; selected from hexane, cyclohexane, toluene, ethylbenzene, etc.
The reinforcing agent is an organic silane compound containing an ethylenically unsaturated substituent, and the structural formula of the reinforcing agent is as follows:
wherein:
R4-contains 2 to 18 carbonsThe unsaturated olefinic aliphatic segment of (2) may be a vinyl group, a propenyl group, an isopropenyl group, an isopentenyl group, a 3-pentenyl group, a 6-octenyl group, a trimethylvinyl group, a 1-heptenyl group, a methacryloxypropyl group, a methacryloxy group, preferably a vinyl group;
R1、R3an alkoxy group having 1 to 12 carbon atoms, an aryloxy group having 6 to 12 carbon atoms, and a siloxy group having 3 to 12 carbon atoms, and may be a methoxy group, an ethoxy group, a propoxy group, an isopropoxy group, a n-butoxy group, a n-hexyloxy group, a n-pentyloxy group, a n-heptyloxy group, an isooctyloxy group, a n-nonyloxy group, a phenoxy group, a chlorophenoxy group, an allyloxy group, a benzyloxy group, or a trimethylsiloxy group, preferably a methoxy group or an ethoxy group; r1And R3The purpose of the alkoxy group is to hydrolyze the alkoxy group by the action of heat and acid during the processing of styrene-butadiene rubber, thereby forming an-O-Si-space network structure in the rubber system and reinforcing the matrix rubber; if the organosilane has only one alkoxy group, it will form dimer after hydrolysis, and will not form-O-Si-space network structure, and will not play a role in reinforcement.
R2May be and R1The same group can also be a saturated chain segment containing 1-20 carbon atoms, a naphthenic group containing 3-8 carbon atoms or an aromatic group containing 6-8 carbon atoms; methyl, ethyl, methoxy, ethoxy are preferred.
The reinforcing agent of the present invention may be vinyltriethoxysilane, vinyldiethoxy-ethylsilane, vinyltrimethoxysilane, vinyldipentyloxy-hexylsilane, isopropenyldi-n-butoxyethylsilane, etc.
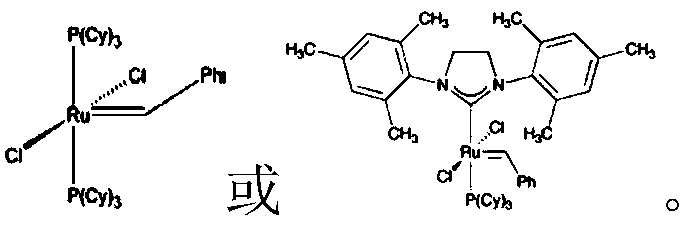
The olefin metathesis catalyst of the invention is a ruthenium carbene complex catalyst, which can be (o-isopropoxy benzylidene) (tricyclohexylphosphine) ruthenium (II) dichlorideDichloro [ o-isopropoxybenzylidene ] group][1, 3-bis (2,4, 6-trimethylphenyl) -2-imidazolinylidene]Ruthenium complexEtc. of Grubbs series catalysts having similar functions or other metal carbene catalysts having olefin metathesis function, preferably
The invention does not specially limit the types and the dosage of the emulsifier and the coagulant, and can adopt the commonly used auxiliary agents and dosage in the field. The emulsifier can be saturated or unsaturated fatty acid soap, rosin acid soap and other anionic emulsifiers; the dosage of the reinforcing type softening agent can be 0.1-20 parts, preferably 0.5-10 parts, added into 100 parts of reinforcing type softening agent products.
The addition amount of water in the preparation process of the softener emulsion is not particularly required, as long as the materials can form an emulsion state, and the addition amount of water is preferably 50-500 parts of water added into 100 parts of reinforced softener products.
The temperature of the process for preparing the softener emulsion is not particularly limited in the present invention as long as the emulsion can be formed under stirring, and for example, the temperature may be preferably 10 to 80 ℃.
The coagulant of the invention can be CaCl2、MgSO4、MgCl2、Al2(SO4)3、FeCl3、CuCl2、Ca(HSO4)2And soluble metal salts and mixtures thereof. The amount of the coagulant is preferably 1 to 25 parts by mass, preferably 5 to 15 parts by mass, based on 100 parts by mass of the styrene-butadiene latex.
The term "part" as used herein means a part by mass.
The invention is based on the technical principle that:
wherein n > m
The chemical reaction equation (1) takes vinyl benzoic acid as a scorch retarder and vinyl triethoxysilane as a reinforcing agent to carry out olefin double decomposition reaction to prepare the scorch retarder rubber reinforcing agent; the formula (2) is that the molecular weight of the styrene-butadiene rubber is reduced under the action of an olefin metathesis catalyst, and simultaneously reacts with the scorch-proof rubber reinforcing agent generated by the formula (1) to introduce a reinforcing group and a scorch-proof group on a side group.
The softener is a double decomposition reaction product of styrene butadiene rubber and a scorch-proof rubber reinforcing agent, has a similar composition with styrene butadiene rubber, and has a softening effect on rubber due to low molecular weight. Usually, the rubber processing formula adopts the operating oil as a softening agent, and small molecular aids such as a reinforcing agent, an anti-scorching agent and the like are added, and the aids are easily adsorbed to the surface by carbon black to reduce the action effect. This scheme will prevent burnt agent, reinforcer and softener chemical bonding, can effectively strengthen to prevent the scorching of rubber.
The easy-to-process reinforced butadiene styrene rubber prepared by the invention is tested to reduce Mooney 0.2-2.5 units/part of base rubber, improve the strength by more than 0.15 MPa/part and delay the scorching time by 1-15 s/part.
Detailed Description
The raw material auxiliaries required for the specific implementation of the invention are as follows:
(1) styrene butadiene rubber SBR1500, SBR1712, technical grade, langzhou petrochemical division;
(2) vinyltriethoxysilane, vinylethyldiethoxysilane, vinyltrimethoxysilane, reagent grade, Shanghai Virgillosiloxane GmbH;
(3) olefin metathesis catalyst:ruthenium carbene catalyst, 500 mg/bottle, purity more than or equal to 98% w, Bailingwei science and technology Limited;
(4) hexane, cyclohexane, toluene, ethylbenzene, technical grade, langzhou petrochemical division;
(5) sodium aliphatate, sodium abietate, potassium aliphatate, potassium abietate, technical grade, Lanzhou petrochemical division;
(6)CaCl2、MgSO4、MgCl2、Al2(SO4)3industrial grade, langzhou petrochemical division;
(7) 2-vinylbenzoic acid, 3-vinylphthalic acid, reagent grade, gansu gacheng auxiliaries;
mixing the softener emulsion and the styrene-butadiene latex liquid phase, and testing the Mooney viscosity, the scorching time and the mechanical property of the rubber; the Mooney viscosity and the scorch time of the rubber are tested according to GB/T1232.1-2000, the mechanical property of the rubber is tested according to GB/T528-.
Example 1:
mixing 2-vinylbenzoic acid serving as an anti-scorching agent and vinyltriethoxysilane serving as a reinforcing agent in a ratio of 1.0: 5.0 mol ratio, preparing a solution with 30 percent of mass concentration by using a hexane solvent, and adding 0.1 percent of olefin metathesis catalyst accounting for the total mass of the scorch retarder and the reinforcing agent into the solutionIsolating air and water, reacting at normal temperature for 8h, and removing the solvent by using a vacuum solvent removal device to obtain the scorch-proof rubber reinforcing agent 1-1.
Styrene butadiene rubber SBR1500 was dissolved in a hexane solvent to prepare a 1.5% w solution, and 0.13 part by mass of an olefin metathesis catalyst was added to 100 parts by mass of the rubberAnd 1.9 parts of scorch-proof rubber reinforcing agent 1-1, isolating air and water at 15.5 ℃, reacting for 0.53 hour, and removing the reacted glue solution through a solvent to obtain a reinforcing type softener product with Mn of 88000;
adding 100 parts by mass of the prepared reinforced softener product, 0.15 part by mass of emulsifier sodium fatty acid and 55 parts by mass of desalted water into an emulsifier, controlling the temperature to be 75 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 10 are mixed to prepare mixed latex, 20 parts by mass of coagulant CaCl is added at the reaction temperature of 78 DEG C2Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 48 from 52, the tensile strength is improved to 26.8MPa from 25.3MPa, and the scorch time is improved to 189s from 125 s.
Comparative example 1:
compared with the embodiment 1, the other conditions are the same, except that the scorch-proof reinforcing agent is not added, specifically comprising the following steps: styrene butadiene rubber SBR1500 was dissolved in a hexane solvent to prepare a 1.5% w solution, and 0.13 part by mass of an olefin metathesis catalyst was added to 100 parts by mass of the rubberAir and water are isolated at 15.5 ℃, the reaction is carried out for 0.53 hour, and glue solution after the reaction is removed through a solvent, so as to obtain a reinforced softener product with Mn of 98000;
adding 100 parts of the prepared reinforced softener product, 0.15 part of emulsifier sodium fatty acid and 55 parts of desalted water into an emulsifier, controlling the temperature to be 75 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 10 are mixed to prepare mixed latex, 20 parts by mass of coagulant CaCl is added at the reaction temperature of 78 DEG C2Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 50 from 52, the tensile strength is 25.2MPa, the improvement is not realized, and the scorching time is as short as 90 s.
Example 2:
the scorch retarder 3-vinylphthalic acid and the reinforcing agent vinyldiethoxymethylsilane were mixed at a ratio of 1.0: 0.1 mol ratio, preparing a solution with the mass concentration of 1% by using a hexane solvent, adding an olefin double decomposition catalyst accounting for 0.01% of the total substance of the scorch retarder and the reinforcing agent into the solution, isolating air and water, reacting at normal temperature for 0.5h, and then removing the solvent by using a vacuum desolventizing device to prepare the scorch retarder 2-1.
Styrene butadiene rubber SBR1500 is dissolved in toluene solvent to prepare 10 percent w solution, and 0.98 part of olefin metathesis catalyst is added according to 100 parts of rubber by massAnd 48 parts of scorch-proof rubber reinforcing agent 2-1, isolating air and water at 48.9 ℃, reacting for 4.5 hours, and removing the reacted glue solution through a solvent to obtain a reinforcing type softener product with Mn of 3200;
adding 100 parts of the prepared reinforced softener product, 19 parts of emulsifier sodium fatty acid and 490 parts of desalted water into an emulsifier, controlling the temperature to be 50 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 50 are mixed to prepare mixed latex, 20 parts by mass of coagulant MgSO is added at the reaction temperature of 55 DEG C4Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 32 from 52, the tensile strength is improved to 32.9MPa from 25.3MPa, and the scorch time is improved to 143s from 125 s.
Comparative example 2:
compared with example 2, the other conditions are the same, except that no olefin metathesis catalyst is added, specifically: the scorch retarder 3-vinylphthalic acid and the reinforcing agent vinyldiethoxymethylsilane were mixed at a ratio of 1.0: mixing the components in a molar ratio of 0.1, preparing a solution with the mass concentration of 1% by using a hexane solvent, isolating air and water, reacting at normal temperature for 0.5h, and removing the solvent by using a vacuum solvent removal device to prepare the scorch-proof rubber reinforcing agent 2-2. Styrene butadiene rubber SBR1500 is dissolved in toluene solvent to prepare 10% w solution, based on 100 parts by mass of rubber, 48 parts of scorch-proof rubber reinforcing agent is added 2-2, air and water are isolated at 48.9 ℃, the reaction is carried out for 4.5 hours, and glue solution after the reaction is removed through the solvent, so as to obtain a reinforcing type softener product with Mn of 170000;
adding 100 parts of the prepared reinforced softener product, 19 parts of emulsifier sodium fatty acid and 490 parts of desalted water into an emulsifier, controlling the temperature to be 50 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass ratio of a dry base of 100: 50 are mixed to prepare mixed latex20 parts by mass of a coagulant MgSO was added at a reaction temperature of 55 ℃4Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
The test shows that the Mooney viscosity is 52 without reduction, the tensile strength is improved from 25.3MPa to 26.5MPa, and the scorch time is improved from 125s to 129 s.
Example 3:
the scorch retarder 3-vinylphthalic acid and the reinforcing agent vinyltrimethoxysilane were mixed in a ratio of 1.0: 4.0 mol ratio, preparing 10% solution by mass with hexane solvent, adding 0.09% olefin metathesis catalyst of total amount of scorch retarder and strengthening agent into the solutionIsolating air and water, reacting at normal temperature for 4.5h, and removing the solvent by using a vacuum desolventizing device to prepare the scorch-proof rubber reinforcing agent 3-1.
Styrene butadiene rubber SBR1712 is dissolved in cyclohexane solvent to prepare 25 percent w solution, and 0.4 part of olefin metathesis catalyst is added according to 100 parts of rubber by massAnd 25 parts of scorch-proof rubber reinforcing agent 3-1, isolating air and water at 25 ℃, reacting for 2.5 hours, and removing the reacted glue solution through a solvent to obtain a reinforcing type softening agent product with Mn of 58000;
adding 100 parts of the prepared reinforced softener product, 10 parts of emulsifier sodium abietate and 250 parts of desalted water into an emulsifier, controlling the temperature to be 40 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 25 to prepare a mixed latex, and adding 20 parts by mass of a coagulant MgSO into the mixed latex at a reaction temperature of 55 DEG C4Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 90 from 120, the tensile strength is improved to 27.5MPa from 26.3MPa, and the scorch time is improved to 212s from 125 s.
Comparative example 3:
compared with the example 3, the other conditions are the same, except that the aromatic oil is adopted as the softening agent, specifically: adding 100 parts of aromatic oil softener, 10 parts of sodium abietate emulsifier and 250 parts of desalted water into an emulsifier, controlling the temperature to be 40 ℃, and stirring to prepare softener emulsion;
adding latex and softener emulsion into a condensation kettle, wherein the mass portion ratio of the latex to the softener emulsion on a dry basis is 100: 25 to prepare a mixed latex, and adding 20 parts by mass of a coagulant MgSO into the mixed latex at a reaction temperature of 55 DEG C4Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
The test shows that the Mooney viscosity is reduced to 80 from 120, the tensile strength is reduced to 23.5MPa from 26.3MPa, and the scorch time is 130 s.
Example 4:
2-vinylbenzoic acid serving as an anti-scorching agent and vinyl dipentyloxy-hexylsilane serving as a reinforcing agent are mixed in a ratio of 1.0: 3.0 mol ratio, preparing a solution with 15 percent of mass concentration by using a hexane solvent, and adding 0.029 percent of olefin metathesis catalyst accounting for the total mass of the scorch retarder and the reinforcing agent into the solutionIsolating air and water, reacting at normal temperature for 4.8h, and removing the solvent by using a vacuum desolventizing device to obtain the scorch-proof rubber reinforcing agent 4-1.
Styrene butadiene rubber SBR1712 is dissolved in ethylbenzene solvent to prepare 45 percent w solution, and 0.5 part of olefin metathesis catalyst is added according to 100 parts by mass of rubberAnd 1.1 parts of scorch-proof rubber reinforcing agent 4-1, isolating air and water at 15 ℃, reacting for 4.9 hours, and removing the reacted glue solution through a solvent to obtain a reinforcing type softener product with Mn of 40000;
adding 100 parts of the prepared reinforced softener product, 18 parts of emulsifier potassium rosinate and 450 parts of desalted water into an emulsifier, controlling the temperature to be 60 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 40 are mixed to prepare mixed latex, and 15 parts by mass of coagulant Al is added at the reaction temperature of 30 DEG C2(SO4)3Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 55 from 120, the tensile strength is improved to 27.9MPa from 26.3MPa, and the scorch time is improved to 194s from 125 s.
Comparative example 4:
the same conditions as in example 4, except that the reinforcing agent vinylpentyloxy-dihexylsilane, which contains only one siloxane, were used, specifically: 2-vinyl benzoic acid serving as an anti-scorching agent and vinyl pentoxy-dihexyl silane serving as a reinforcing agent are mixed in a ratio of 1.0: 3.0 mol ratio, preparing a 15 mass percent solution by using a hexane solvent, adding an olefin double decomposition catalyst accounting for 0.029 percent of the total substance of the scorch retarder and the reinforcing agent into the solution, isolating air and water, reacting at normal temperature for 4.8 hours, and then removing the solvent by using a vacuum desolventizing device to prepare the scorch retarder 4-2.
Styrene butadiene rubber SBR1712 is dissolved in ethylbenzene solvent to prepare 45 percent w solution, and 0.5 part of olefin metathesis catalyst is added according to 100 parts by mass of rubberAnd 1.1 parts of scorch-proof rubber reinforcing agent 4-2, isolating air and water at 15 ℃, reacting for 4.9 hours, and removing the reacted glue solution through a solvent to obtain a reinforcing type softening agent product with Mn of 58000;
adding 100 parts of the prepared reinforced softener product, 18 parts of emulsifier potassium rosinate and 450 parts of desalted water into an emulsifier, controlling the temperature to be 60 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 40 are mixed to prepare mixed latex, and 15 parts by mass of coagulant Al is added at the reaction temperature of 30 DEG C2(SO4)3Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 78 from 120, the tensile strength is improved to 26.4MPa from 26.3MPa, no obvious change is caused, and the scorch time is improved to 196s from 125 s.
Example 5:
2-vinylbenzoic acid serving as a scorch retarder and vinyl ethyl diethoxysilane serving as a reinforcing agent are mixed in a proportion of 1.0: 3.7 mol ratio, preparing a solution with 19 mass percent by using a hexane solvent, and adding 0.05 percent of olefin metathesis catalyst accounting for the total mass of the scorch retarder and the reinforcing agent into the solutionIsolating air and water, reacting for 7h at normal temperature, and removing the solvent by using a vacuum solvent removal device to obtain the scorch-proof rubber reinforcing agent 5-1.
Styrene butadiene rubber SBR1500 was dissolved in a hexane solvent to prepare a 37.5% w solution, and 0.18 part by mass of an olefin metathesis catalyst was added to 100 parts by mass of the rubberAnd 12 parts of scorch-proof rubber reinforcing agent 5-1, isolating air and water at 35 ℃, reacting for 4 hours, and removing glue solution after reaction through a solvent to obtain a reinforcing type softener product with Mn of 91000;
adding 100 parts of the prepared reinforced softener product, 0.5 part of emulsifier potassium fatty acid and 400 parts of desalted water into an emulsifier, controlling the temperature to be 15 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 6.8 mixing to prepare mixed latex, and adding 1.5 parts by mass of coagulant Al at the reaction temperature of 12 DEG C2(SO4)3Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 45 from 52, the tensile strength is improved to 28.4MPa from 25.3MPa, and the scorch time is improved to 174s from 125 s.
Comparative example 5:
compared with the embodiment 5, the other conditions are the same, except that the reinforcing agent and the scorch retarder are added in the liquid-phase mixing procedure, and the concrete steps are as follows: styrene butadiene rubber SBR1500 was dissolved in a hexane solvent to prepare a 37.5% w solution, and 0.18 part by mass of an olefin metathesis catalyst was added to 100 parts by mass of the rubberIsolating air and water at 35 ℃, reacting for 4 hours, and removing glue solution after reaction through a solvent to obtain a reinforced softener product with Mn of 101000;
adding 100 parts of the prepared reinforced softener product, 12 parts of rubber reinforcing agent vinyl ethyl diethoxysilane, 12 parts of scorch retarder 2-vinyl benzoic acid, 0.5 part of emulsifier fatty acid potassium and 400 parts of desalted water into an emulsifier, controlling the temperature to be 15 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 6.8 mixing to prepare mixed latex, and adding 1.5 parts by mass of coagulant Al at the reaction temperature of 12 DEG C2(SO4)3Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 50 from 52, the tensile strength is improved to 25.8MPa from 25.3MPa, the strength is slightly improved, and the scorching time is improved to 134s from 125 s.
Example 6:
2-vinylbenzoic acid serving as a scorch retarder and vinyl ethyl diethoxysilane serving as a reinforcing agent are mixed in a proportion of 1.0: 4.5 mol ratio, preparing a solution with 25 percent mass concentration by using a hexane solvent, and adding 0.06 percent of olefin metathesis catalyst accounting for the total mass of the scorch retarder and the reinforcing agent into the solutionIsolating air and water, reacting at normal temperature for 4h, and removing the solvent by using a vacuum solvent removal device to obtain the scorch-proof rubber reinforcing agent 6-1.
Styrene butadiene rubber SBR1500 was dissolved in cyclohexane solvent to prepare a 20% w solution, and 0.9 part by mass of olefin metathesis catalyst was added to 100 parts by mass of the rubberAnd 38 parts of scorch-proof rubber reinforcing agent 6-1, isolating air and water at 28 ℃, reacting for 3.2 hours, and removing glue solution after reaction through a solvent to obtain a reinforcing type softening agent product with Mn of 6000;
adding 100 parts of the prepared reinforced softener product, 0.2 part of emulsifier potassium fatty acid and 443 parts of desalted water into an emulsifier, controlling the temperature to be 78 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 1.3 mixing to prepare mixed latex, and adding 5.5 parts by mass of coagulant Al at the reaction temperature of 35 DEG C2(SO4)3Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 49.5 from 52, the tensile strength is improved to 25.9MPa from 25.3MPa, and the scorch time is improved to 184s from 125 s.
Comparative example 6:
compared with the example 6, the other conditions are the same, except that the aromatic oil is adopted as the softening agent, and the strengthening agent and the scorch retarder are added, specifically: adding 100 parts of aromatic oil softener, 0.2 part of emulsifier potassium fatty acid, 443 parts of desalted water and 38 parts of rubber reinforcing agent vinyl ethyl diethoxysilane and 1 part of scorch retarder 2-vinyl benzoic acid in mass relative to the aromatic oil into an emulsifier, controlling the temperature to be 78 ℃, and stirring to prepare softener emulsion;
adding latex and softener emulsion into a condensation kettle, wherein the mass portion ratio of the latex to the softener emulsion on a dry basis is 100: 1.3, mixing to prepare mixed latex, adding 5.5 parts by mass of coagulant at the reaction temperature of 35 ℃ for coagulation, washing, centrifugally dewatering and drying to prepare the high-strength styrene-butadiene rubber which is easy to process.
The Mooney viscosity is reduced from 52 to 49, the tensile strength is not improved, and the scorch time is improved from 125s to 274 s.
Example 7:
2-vinylbenzoic acid serving as a scorch retarder and vinyl ethyl diethoxysilane serving as a reinforcing agent are mixed in a proportion of 1.0: 4.5 mol ratio, preparing a solution with a hexane solvent to a mass concentration of 25%, and adding an olefin metathesis catalyst in an amount of 0.06% based on the total amount of the scorch retarder and the reinforcing agent to the solutionIsolating air and water, reacting for 4h at normal temperature, and removing the solvent by using a vacuum solvent removal device to obtain the scorch-proof rubber reinforcing agent 7-1.
Styrene butadiene rubber SBR1500 was dissolved in cyclohexane solvent to prepare a 18% w solution, and 0.95 part by mass of olefin metathesis catalyst was added to 100 parts by mass of the rubberAnd 43 parts of scorch-proof rubber reinforcing agent 7-1, isolating air and water at 48 ℃, reacting for 3.5 hours, and removing the reacted glue solution through a solvent to obtain a reinforcing type softener product with Mn of 5500;
adding 100 parts of the prepared reinforced softener product, 0.29 part of emulsifier potassium fatty acid and 98 parts of desalted water into an emulsifier, controlling the temperature to be 63 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 33 are mixed to prepare a mixed latex, 21 parts by mass of a coagulant Ca (HSO) is added at a reaction temperature of 55 DEG C4)2Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced from 52 to 39, the tensile strength is improved from 25.3MPa to 32.9MPa, and the scorch time is improved from 125s to 194 s.
Comparative example 7:
compared with example 7, the other conditions are the same, except that the softening agent is added with the reinforcing agent after the double decomposition catalyst is added in the preparation processReacting for a period of time, and adding a scorch retarder for reacting for a period of time, wherein the reaction comprises the following steps: styrene butadiene rubber SBR1500 was dissolved in cyclohexane solvent to prepare a 18% w solution, and 0.95 part by mass of olefin metathesis catalyst was added to 100 parts by mass of the rubberIsolating air and water at 48 ℃ for reacting for 2h, adding 43 parts of rubber reinforcing agent vinyl ethyl diethoxysilane, isolating air and water at 48 ℃, reacting for 1.5 h, adding 10 parts of scorch retarder 2-vinylbenzoic acid, isolating air and water at 48 ℃, reacting for 2h, and removing the reacted glue solution through a solvent to obtain a reinforcing softener product with Mn of 7500;
adding 100 parts of the prepared reinforced softener product, 0.29 part of emulsifier potassium fatty acid and 98 parts of desalted water into an emulsifier, controlling the temperature to be 63 ℃, and stirring to prepare a softener emulsion;
adding latex and softener emulsion into a condensation kettle, stirring, and mixing according to the mass portion ratio of a dry base of 100: 33 are mixed to prepare a mixed latex, 21 parts by mass of a coagulant Ca (HSO) is added at a reaction temperature of 55 DEG C4)2Coagulating, washing, centrifugal dewatering and drying to obtain the easily processed reinforced butadiene styrene rubber.
Tests show that the Mooney viscosity is reduced to 49 from 52, the tensile strength is improved to 27.9MPa from 25.3MPa, and the scorch time is improved to 144s from 125 s.
Claims (17)
1. A preparation method of easily processed reinforced styrene butadiene rubber comprises the following specific steps:
1) firstly, preparing a reinforcing softener, dissolving styrene-butadiene rubber in a solvent to prepare a solution with the concentration of 1-50 w%, adding 0.1-1.0 part of olefin metathesis catalyst and 1-50 parts of scorch-proof rubber reinforcing agent into the solution based on 100 parts by mass of rubber, isolating air and water at 15-50 ℃, reacting for 0.5-5 hours, and removing glue solution after the reaction through the solvent to obtain a reinforcing softener product; the scorch-proof rubber reinforcing agent is prepared by olefin double decomposition reaction of a scorch-proof agent and a reinforcing agent, wherein the scorch-proof agent is a nitroso compound containing an ethylenically unsaturated substituent, an organic acid or a thioamide compound; the reinforcing agent is an organic silane compound containing an ethylenically unsaturated substituent; the preparation method of the scorch-proof rubber reinforcing agent comprises the following steps: mixing a scorch retarder and a reinforcing agent in a molar ratio of 1: 0.1-5.0, preparing a solution with a mass concentration of 1-30% by using a solvent, adding an olefin double decomposition catalyst accounting for 0.01-0.1% of the total mass of the scorch retarder and the reinforcing agent into the solution, isolating air and water, reacting at normal temperature for 0.5-8 h, and removing the solvent by using a vacuum solvent removal device to prepare the scorch retarder;
2) mixing the prepared reinforced softener product with an emulsifier and water, and stirring to prepare a softener emulsion;
3) adding styrene-butadiene latex and softener emulsion into a condensation kettle, stirring, and mixing according to a dry basis ratio of 100: (1-50) mixing to prepare mixed latex, adding a coagulant at the reaction temperature of 10-80 ℃ for coagulation, washing, centrifugally dewatering and drying to obtain the easily processed reinforced styrene butadiene rubber.
2. The method of claim 1, wherein the styrene-butadiene rubber is dissolved in a solvent to prepare a solution with a concentration of 5 w% to 15 w%.
3. The method of preparing easily processed reinforced styrene-butadiene rubber according to claim 1, wherein 0.1 to 0.3 part by mass of olefin metathesis catalyst is added to 100 parts by mass of the rubber in the preparation of the reinforcing softener.
4. The method of claim 1, wherein 10 to 30 parts by mass of scorch retarder is added to 100 parts by mass of the rubber during the preparation of the reinforcing softener.
5. The method of claim 1, wherein the solvent is a solvent capable of dissolving styrene butadiene rubber without containing an olefin in the structure.
6. The process for producing easy-to-process reinforced styrene-butadiene rubber according to claim 1, wherein the solvent is selected from the group consisting of hexane, cyclohexane, toluene and ethylbenzene.
7. A process for preparing easy-to-process reinforced styrene-butadiene rubber as claimed in claim 1, wherein said scorch retarder is selected from the group consisting of N-nitrosodi (vinylbenzene) amine, N-nitroso-styryl- β -naphthylamine, N-nitroso-2-propenyl-2, 4-dimethyl-1, 2-dihydroquinoline polymer, vinylbenzoic acid, vinyl salicylic acid, butenyl salicylic acid, vinyl phthalic acid, N-vinylcyclohexyl thiophthalimide and N-propenyl cyclohexylthiophthalimide.
8. The method of preparing easy-to-process reinforced styrene-butadiene rubber according to claim 1, wherein the reinforcing agent has a structural formula of:wherein: r4Is an unsaturated olefin aliphatic chain segment containing 2-18 carbon atoms; r1、R3Is an alkoxy group having 1 to 12 carbon atoms, an aryloxy group having 6 to 12 carbon atoms or a siloxy group having 3 to 12 carbon atoms; r2Is a reaction with R1The same group, or a saturated chain segment containing 1-20 carbon atoms, a cycloalkyl containing 3-8 carbon atoms, or an aryl containing 6-8 carbon atoms.
9. The method of claim 8, wherein R is the structural formula of the reinforcing agent4Selected from the group consisting of ethenyl, propenyl, isopropenyl, isopentenyl, 3-pentenyl, 6-octenyl, trimethylethenyl, 1-heptenyl, methacryloxypropyl, and methacryloxy.
10. The easily processable reinforced styrene-butadiene rubber as claimed in claim 8The preparation method is characterized in that R in the structural formula of the reinforcing agent1、R3Selected from methoxy, ethoxy, propoxy, isopropoxy, n-butoxy, n-hexyloxy, n-pentyloxy, n-heptyloxy, isooctyloxy, n-nonyloxy, phenoxy, chlorophenoxy, allyloxy, benzyloxy or trimethylsilyloxy.
11. The method of claim 8, wherein R is the structural formula of the reinforcing agent2Selected from methyl, ethyl, methoxy or ethoxy.
12. The process for producing a processable reinforcing styrene-butadiene rubber as claimed in claim 1 or 8, wherein the reinforcing agent is selected from the group consisting of vinyltriethoxysilane, vinyldiethoxy-ethylsilane, vinyltrimethoxysilane, vinyldipentyloxy-hexylsilane and isopropenyldi-n-butoxyethylsilane.
13. The method of preparing easy-to-process reinforced styrene-butadiene rubber according to claim 1, wherein the olefin metathesis catalyst is a metal carbene catalyst having an olefin metathesis function.
14. The process for preparing easy-to-process reinforced styrene-butadiene rubber according to claim 1, wherein the olefin metathesis catalyst is selected from the group consisting of (o-isopropoxybenzylidene) (tricyclohexylphosphine) ruthenium (II) dichlorideDichloro [ o-isopropoxybenzylidene ] group][1, 3-bis (2,4, 6-trimethylphenyl) -2-imidazolinylidene]Ruthenium complex
15. The method of claim 1, wherein the emulsifier is selected from saturated or unsaturated fatty acid soap or rosin acid soap, and the amount of the emulsifier is 0.1-20 parts by weight based on 100 parts by weight of the reinforcing softener product.
16. The process for preparing easy-to-process reinforced styrene-butadiene rubber according to claim 1, wherein said coagulant is selected from the group consisting of CaCl2、MgSO4、MgCl2、Al2(SO4)3、FeCl3、CuCl2Or Ca (HSO)4)2The dosage of the one or the mixture is 1 to 25 parts of butylbenzene latex added by 100 parts of butylbenzene latex.
17. The method according to claim 1, wherein the dry-basis ratio of the styrene-butadiene latex to the softener emulsion in step 3) is 100: 10 to 30.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201610833898.XA CN107841010B (en) | 2016-09-20 | 2016-09-20 | Preparation method of easy-to-process reinforced styrene butadiene rubber |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201610833898.XA CN107841010B (en) | 2016-09-20 | 2016-09-20 | Preparation method of easy-to-process reinforced styrene butadiene rubber |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107841010A CN107841010A (en) | 2018-03-27 |
| CN107841010B true CN107841010B (en) | 2020-09-04 |
Family
ID=61656651
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201610833898.XA Active CN107841010B (en) | 2016-09-20 | 2016-09-20 | Preparation method of easy-to-process reinforced styrene butadiene rubber |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN107841010B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022181545A1 (en) * | 2021-02-24 | 2022-09-01 | 株式会社カネカ | Manufacturing method for polymer comprising hydrolyzable silyl group, and polymer, curable composition, and cured product |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102603928A (en) * | 2011-01-11 | 2012-07-25 | 赞南科技(上海)有限公司 | Preparation method of hydrogenated nitrile rubber and degradation and hydrogenation method of butadiene type rubber |
| CN103204959A (en) * | 2012-01-16 | 2013-07-17 | 中国石油天然气股份有限公司 | Preparation method of oil-extended powder rubber |
| CN105732690A (en) * | 2014-12-11 | 2016-07-06 | 中国石油天然气股份有限公司 | Rubber reinforcing agent, preparation method thereof and preparation method of styrene butadiene rubber |
-
2016
- 2016-09-20 CN CN201610833898.XA patent/CN107841010B/en active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102603928A (en) * | 2011-01-11 | 2012-07-25 | 赞南科技(上海)有限公司 | Preparation method of hydrogenated nitrile rubber and degradation and hydrogenation method of butadiene type rubber |
| CN103204959A (en) * | 2012-01-16 | 2013-07-17 | 中国石油天然气股份有限公司 | Preparation method of oil-extended powder rubber |
| CN105732690A (en) * | 2014-12-11 | 2016-07-06 | 中国石油天然气股份有限公司 | Rubber reinforcing agent, preparation method thereof and preparation method of styrene butadiene rubber |
Non-Patent Citations (1)
| Title |
|---|
| 用复分解催化剂促进橡胶化学反应;粷谷信三;《橡胶参考资料》;19881126(第11期);第31-37页 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107841010A (en) | 2018-03-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN100406509C (en) | Rubber composition for a tire and tire using the same | |
| GB2026499A (en) | Process for the preparation of a pulverulent filled rubber | |
| EP2917251B1 (en) | Uses of biobased styryene | |
| US3922240A (en) | Process for the production of pourable, pulverulent rubber-filler mixtures | |
| JP2017160412A (en) | Functionalized elastomer containing boron group | |
| CN105732690A (en) | Rubber reinforcing agent, preparation method thereof and preparation method of styrene butadiene rubber | |
| JP2008285626A (en) | Rubber composition for tire and pneumatic tire using the same | |
| CN104650400B (en) | Cyclopentadiene-modified carbon nanotube/rubber composite material and preparation method thereof | |
| CN107841010B (en) | Preparation method of easy-to-process reinforced styrene butadiene rubber | |
| CN106566034B (en) | The method of the wet-process modified white carbon black of boric acid ester interface activating agent | |
| JPH01249812A (en) | Production of diene-based polymer rubber and rubber composition therefrom | |
| CN107841008B (en) | Easily-processed reinforced styrene butadiene rubber and preparation method thereof | |
| US9133281B2 (en) | Preparation and use of functionalized elastomers in rubber compositions containing silica filler and tire components thereof | |
| JP7019818B2 (en) | A functionalized polymer, a process for preparing the functionalized polymer, and a rubber composition containing the functionalized polymer. | |
| CN101319063A (en) | Rubber composition for a tire and tire using the same | |
| CN107841007B (en) | Preparation method of easily processed reinforced nitrile rubber | |
| US20030158325A1 (en) | Preparation and use of composite of rubber and carbon black aggregates and articles of manufacture, including tires, having a component comprised thereof | |
| CN107840903B (en) | Preparation method of reinforced softener for styrene butadiene rubber | |
| EP3628692B1 (en) | Silica reinforced rubber composition containing a multifunctional group functionalized elastomer and tire with tread | |
| CN107841005B (en) | Easily-processed reinforced nitrile rubber and preparation method thereof | |
| EP1191072B1 (en) | Electropolymerization modified carbon black and articles including tires having at least one component containing such modified carbon black | |
| CN107840902B (en) | Preparation method of reinforcing plasticizer for nitrile rubber | |
| JP6263526B2 (en) | Process for the preparation of functionalized and branched elastomeric copolymers | |
| CN1296991A (en) | Rubber mixture contg. hydroxy and/or carboxy rubber and hydrophobic silicon oxide or silicates filler | |
| JPH01284503A (en) | Production of modified diene polymer rubber and composition comprising the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |