CN107174586B - Pharmaceutical composition with arundoin derivative as active ingredient and application thereof - Google Patents
Pharmaceutical composition with arundoin derivative as active ingredient and application thereof Download PDFInfo
- Publication number
- CN107174586B CN107174586B CN201710092931.2A CN201710092931A CN107174586B CN 107174586 B CN107174586 B CN 107174586B CN 201710092931 A CN201710092931 A CN 201710092931A CN 107174586 B CN107174586 B CN 107174586B
- Authority
- CN
- China
- Prior art keywords
- arundoin
- derivatives
- melatonin
- pharmaceutical composition
- application
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- MRNPHCMRIQYRFU-KXUMSINMSA-N Arundoin Chemical class C([C@]1(C)[C@@H](C(C)C)CC[C@H]1[C@]1(C)CC=C23)C[C@]1(C)[C@H]3CC[C@@H]1[C@]2(C)CC[C@H](OC)C1(C)C MRNPHCMRIQYRFU-KXUMSINMSA-N 0.000 title claims abstract description 55
- 239000008194 pharmaceutical composition Substances 0.000 title claims abstract description 20
- 239000004480 active ingredient Substances 0.000 title abstract description 7
- DRLFMBDRBRZALE-UHFFFAOYSA-N melatonin Chemical compound COC1=CC=C2NC=C(CCNC(C)=O)C2=C1 DRLFMBDRBRZALE-UHFFFAOYSA-N 0.000 claims abstract description 27
- YJPIGAIKUZMOQA-UHFFFAOYSA-N Melatonin Natural products COC1=CC=C2N(C(C)=O)C=C(CCN)C2=C1 YJPIGAIKUZMOQA-UHFFFAOYSA-N 0.000 claims abstract description 26
- 229960003987 melatonin Drugs 0.000 claims abstract description 23
- 102000001419 Melatonin receptor Human genes 0.000 claims abstract description 18
- 108050009605 Melatonin receptor Proteins 0.000 claims abstract description 18
- 239000003814 drug Substances 0.000 claims abstract description 16
- 208000015114 central nervous system disease Diseases 0.000 claims abstract description 7
- 239000000952 serotonin receptor agonist Substances 0.000 claims abstract description 3
- 238000002360 preparation method Methods 0.000 claims description 21
- 108091032151 5-hydroxytryptamine receptor family Proteins 0.000 claims description 14
- 102000014630 G protein-coupled serotonin receptor activity proteins Human genes 0.000 claims description 8
- 102000040125 5-hydroxytryptamine receptor family Human genes 0.000 claims description 6
- 239000000018 receptor agonist Substances 0.000 claims description 6
- 229940044601 receptor agonist Drugs 0.000 claims description 6
- 229940122081 5 Hydroxytryptamine receptor agonist Drugs 0.000 claims description 2
- 229940121723 Melatonin receptor agonist Drugs 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims 2
- 229940079593 drug Drugs 0.000 abstract description 10
- 102000005962 receptors Human genes 0.000 abstract description 9
- 108020003175 receptors Proteins 0.000 abstract description 9
- 229940076279 serotonin Drugs 0.000 abstract 1
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 14
- 239000000546 pharmaceutical excipient Substances 0.000 description 14
- 230000000694 effects Effects 0.000 description 10
- 239000000203 mixture Substances 0.000 description 10
- 150000001875 compounds Chemical class 0.000 description 9
- 238000009472 formulation Methods 0.000 description 8
- 230000001270 agonistic effect Effects 0.000 description 7
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- NWUMSRKLBRWRAS-UHFFFAOYSA-N Arundoin Natural products COC1CCC2(C)C(CCC3C2=CCC4(C)C5CC(C)(C)CCC5(C)CCC34C)C1(C)C NWUMSRKLBRWRAS-UHFFFAOYSA-N 0.000 description 6
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- YJYPHIXNFHFHND-UHFFFAOYSA-N agomelatine Chemical compound C1=CC=C(CCNC(C)=O)C2=CC(OC)=CC=C21 YJYPHIXNFHFHND-UHFFFAOYSA-N 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 238000000034 method Methods 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 229960002629 agomelatine Drugs 0.000 description 5
- 125000004005 formimidoyl group Chemical group [H]\N=C(/[H])* 0.000 description 5
- 238000005160 1H NMR spectroscopy Methods 0.000 description 4
- 241001494508 Arundo donax Species 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 4
- 230000000144 pharmacologic effect Effects 0.000 description 4
- 208000020016 psychiatric disease Diseases 0.000 description 4
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- VFSMDMJLLJMJQD-UHFFFAOYSA-N N1C(=CC=C1)NCC1=CNC2=CC=CC=C12 Chemical compound N1C(=CC=C1)NCC1=CNC2=CC=CC=C12 VFSMDMJLLJMJQD-UHFFFAOYSA-N 0.000 description 3
- 239000003513 alkali Substances 0.000 description 3
- 239000012043 crude product Substances 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- RKHCTAKUYDTFHE-QMMMGPOBSA-N ly-156,735 Chemical compound C1=C(Cl)C(OC)=CC2=C1NC=C2[C@@H](C)CNC(C)=O RKHCTAKUYDTFHE-QMMMGPOBSA-N 0.000 description 3
- 239000013641 positive control Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- FQUYSHZXSKYCSY-UHFFFAOYSA-N 1,4-diazepane Chemical compound C1CNCCNC1 FQUYSHZXSKYCSY-UHFFFAOYSA-N 0.000 description 2
- FXHRAKUEZPSMLJ-UHFFFAOYSA-N 1-methyl-1,4-diazepane Chemical compound CN1CCCNCC1 FXHRAKUEZPSMLJ-UHFFFAOYSA-N 0.000 description 2
- WHKWMTXTYKVFLK-UHFFFAOYSA-N 1-propan-2-ylpiperazine Chemical compound CC(C)N1CCNCC1 WHKWMTXTYKVFLK-UHFFFAOYSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 241000709675 Coxsackievirus B3 Species 0.000 description 2
- WVVXBPKOIZGVNS-UHFFFAOYSA-N N-[2-[2-(phenylmethyl)-1H-indol-3-yl]ethyl]acetamide Chemical compound N1C2=CC=CC=C2C(CCNC(=O)C)=C1CC1=CC=CC=C1 WVVXBPKOIZGVNS-UHFFFAOYSA-N 0.000 description 2
- 102000004960 NAD(P)H dehydrogenase (quinone) Human genes 0.000 description 2
- 108020000284 NAD(P)H dehydrogenase (quinone) Proteins 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- 235000014676 Phragmites communis Nutrition 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 2
- 239000012091 fetal bovine serum Substances 0.000 description 2
- 210000001985 kidney epithelial cell Anatomy 0.000 description 2
- 210000004560 pineal gland Anatomy 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 239000000741 silica gel Substances 0.000 description 2
- 229910002027 silica gel Inorganic materials 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 229960000660 tasimelteon Drugs 0.000 description 2
- PTOIAAWZLUQTIO-GXFFZTMASA-N tasimelteon Chemical compound CCC(=O)NC[C@@H]1C[C@H]1C1=CC=CC2=C1CCO2 PTOIAAWZLUQTIO-GXFFZTMASA-N 0.000 description 2
- CZDYPVPMEAXLPK-UHFFFAOYSA-N tetramethylsilane Chemical compound C[Si](C)(C)C CZDYPVPMEAXLPK-UHFFFAOYSA-N 0.000 description 2
- CYHOMWAPJJPNMW-JIGDXULJSA-N tropine Chemical class C1[C@@H](O)C[C@H]2CC[C@@H]1N2C CYHOMWAPJJPNMW-JIGDXULJSA-N 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- YIMPWIJIKJBRDM-UHFFFAOYSA-N 1-indol-1-yl-n,n-dimethylmethanamine Chemical compound C1=CC=C2N(CN(C)C)C=CC2=C1 YIMPWIJIKJBRDM-UHFFFAOYSA-N 0.000 description 1
- 206010001497 Agitation Diseases 0.000 description 1
- 241001494510 Arundo Species 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 244000025254 Cannabis sativa Species 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 231100000491 EC50 Toxicity 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 239000012981 Hank's balanced salt solution Substances 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 229940123784 Melatonin receptor antagonist Drugs 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 102000006833 Multifunctional Enzymes Human genes 0.000 description 1
- 108010047290 Multifunctional Enzymes Proteins 0.000 description 1
- YBKLLOANIOYZAN-UHFFFAOYSA-N N-(1H-indol-3-ylmethyl)piperazin-1-amine Chemical compound N1(CCNCC1)NCC1=CNC2=CC=CC=C12 YBKLLOANIOYZAN-UHFFFAOYSA-N 0.000 description 1
- RCYLUNPFECYGDW-UHFFFAOYSA-N N-(4-phenyl-1,2,3,4-tetrahydronaphthalen-2-yl)propanamide Chemical compound C12=CC=CC=C2CC(NC(=O)CC)CC1C1=CC=CC=C1 RCYLUNPFECYGDW-UHFFFAOYSA-N 0.000 description 1
- YLXDSYKOBKBWJQ-LBPRGKRZSA-N N-[2-[(8S)-2,6,7,8-tetrahydro-1H-cyclopenta[e]benzofuran-8-yl]ethyl]propanamide Chemical compound C1=C2OCCC2=C2[C@H](CCNC(=O)CC)CCC2=C1 YLXDSYKOBKBWJQ-LBPRGKRZSA-N 0.000 description 1
- 241000209504 Poaceae Species 0.000 description 1
- 241000270942 Rana pipiens Species 0.000 description 1
- 206010047139 Vasoconstriction Diseases 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- REAYFGLASQTHKB-UHFFFAOYSA-N [2-[3-(1H-pyrazol-4-yl)phenoxy]-6-(trifluoromethyl)pyridin-4-yl]methanamine Chemical compound N1N=CC(=C1)C=1C=C(OC2=NC(=CC(=C2)CN)C(F)(F)F)C=CC=1 REAYFGLASQTHKB-UHFFFAOYSA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000008484 agonism Effects 0.000 description 1
- 230000003064 anti-oxidating effect Effects 0.000 description 1
- GAIINJYDKIFVHG-XZOQPEGZSA-N arundinine Natural products O(c1cc2c(CCN(C)C)c[nH]c2cc1)[C@]12[C@H](N(C)CC1)Nc1c2cccc1 GAIINJYDKIFVHG-XZOQPEGZSA-N 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- DUEPRVBVGDRKAG-UHFFFAOYSA-N carbofuran Chemical compound CNC(=O)OC1=CC=CC2=C1OC(C)(C)C2 DUEPRVBVGDRKAG-UHFFFAOYSA-N 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- PBAYDYUZOSNJGU-UHFFFAOYSA-N chelidonic acid Natural products OC(=O)C1=CC(=O)C=C(C(O)=O)O1 PBAYDYUZOSNJGU-UHFFFAOYSA-N 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 230000027288 circadian rhythm Effects 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940053999 hypnotics and sedatives melatonin receptor agonists Drugs 0.000 description 1
- 230000002267 hypothalamic effect Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 108010082117 matrigel Proteins 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 230000003020 moisturizing effect Effects 0.000 description 1
- RCGABEQHDDFFRR-UHFFFAOYSA-N n-ethyl-n-(1h-indol-3-ylmethyl)ethanamine Chemical compound C1=CC=C2C(CN(CC)CC)=CNC2=C1 RCGABEQHDDFFRR-UHFFFAOYSA-N 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 230000000955 neuroendocrine Effects 0.000 description 1
- 239000002858 neurotransmitter agent Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 239000008227 sterile water for injection Substances 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 238000004809 thin layer chromatography Methods 0.000 description 1
- 230000025033 vasoconstriction Effects 0.000 description 1
- 230000029812 viral genome replication Effects 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 230000002087 whitening effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/551—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having two nitrogen atoms, e.g. dilazep
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/404—Indoles, e.g. pindolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/404—Indoles, e.g. pindolol
- A61K31/4045—Indole-alkylamines; Amides thereof, e.g. serotonin, melatonin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4523—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems
- A61K31/454—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. pimozide, domperidone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Indole Compounds (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
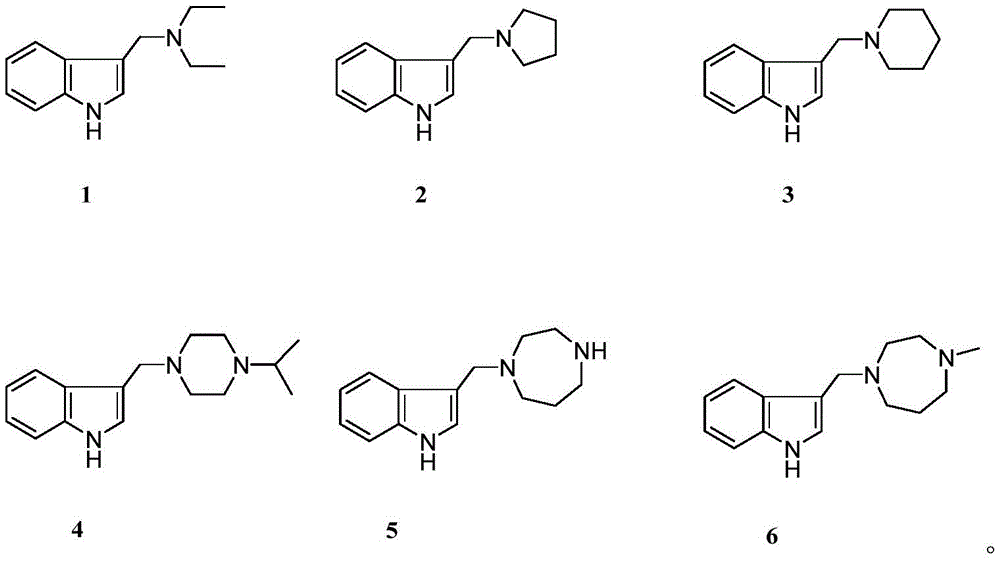
The invention provides a pharmaceutical composition taking arundoin derivatives (1-6) as active ingredients, a derivative and a pharmaceutical composition thereof which are used as melatonin and serotonin receptor agonists, and application thereof in preparing medicines for treating or improving central nervous system diseases related to melatonin and 5-serotonin receptors.
Description
Technical Field
The invention belongs to the technical field of medicines, and particularly relates to a pharmaceutical composition taking a graminine derivative shown in structural formulas 1-6 as an active ingredient, a preparation method thereof, a melatonin and 5-hydroxytryptamine receptor agonist serving as the derivative and the pharmaceutical composition thereof, and application of the derivative and the pharmaceutical composition thereof in preparation of medicines for treating or improving central nervous system diseases related to melatonin and serotonin receptors.
Background
Melatonin (Melatonin) receptor is a neuroendocrine hormone secreted by the pineal gland, controlled by the supranuclear hypothalamic cross, and regulates circadian rhythms, which are manifested by a peak at nightDaytime decreases to a trough value. McCord and Allan found in 1917 that the bovine pineal bodies can regulate the changes of Rana pipiens tadpole skin in the environment, and determined that the structure of melatonin is N-acetyl-5-methoxytryptamine. Melatonin is classified as MT according to melatonin function and pharmacological profile1,MT2And MT3Three subtypes, these receptors are considered as potential pharmaceutically active targets. Wherein MT3Receptor affinity sites are predominantly located in human homeosomal hamster Quinone Reductase (QR) enzyme with detoxifying properties2) The above. Melatonin receptor subtype MT found in mammals1And MT2Currently, common melatonin receptor agonists are Tasimelteon (VEC-162), Ramelteon (TAK-375), β -methyl-6-chloromelatonin (LY-156735, TIK-301), Agomelatine (Agomelatine)[11]Etc.; common melatonin receptor antagonists: n-acetyl-2-benzyltryptamine (Luzindole), 4-Phenyl-2-propionoaminotetralin (4-P-PDOT), and the like.
With the intensive research on natural products, more and more natural small molecules are attracting the interest of medicinal chemists. Natural small molecule arundoin (N, N-dimethylaminomethylindole), originally isolated from Arundo Donax (Arundo Donax L.) of the Arundo genus of asian grass (Gramineae) by Orekhov and Norkina, has a chemical structure similar to that of melatonin receptors and a broad range of biological activities: including vasoconstriction, antioxidation, neurotransmitter 5-HT2AReceptor antagonists, etc. The arundoin can inhibit CVB3 host Cell Pathological Effect (CPE), and can be used as an anti-CVB 3 virus drug [ CN 105748474 ] by inhibiting early CVB3 virus replication in host cells]. Giantreed alkali or pharmaceutically acceptable salt as active ingredient has good skin whitening, elasticity, anti-wrinkling or skin moisturizing effects on human body [ KR 2016020240]。
At present, no report is found on the use of the arundoin derivatives (1-6) provided by the invention as melatonin and 5-hydroxytryptamine receptor agonists and medicaments for resisting mental diseases as active ingredients, and no report is found on the use of the derivatives or the pharmaceutical compositions thereof as melatonin and 5-hydroxytryptamine receptor agonists in the preparation or treatment of anti-mental disease medicaments.
Disclosure of Invention
The invention aims to provide a novel pharmaceutical composition taking the Giantreed alkali derivatives (1-6) with medicinal value as active ingredients, a preparation method thereof, application of the derivatives and the pharmaceutical composition thereof as melatonin and 5-hydroxytryptamine receptor agonists and application of the derivatives and the pharmaceutical composition thereof in preparing medicines for treating or improving central nervous system diseases related to melatonin and 5-hydroxytryptamine receptors.
In order to achieve the above purpose of the present invention, the present invention provides the following technical solutions:
the pharmaceutical composition contains the arundoin derivatives 1-6 shown in the following structural formula and a pharmaceutically acceptable carrier or excipient.
The invention also provides a method for preparing the arundoin derivatives 1-6 in the pharmaceutical composition, wherein the arundoin is respectively reacted with ethylamine, pyrrole, piperidine, N-isopropylpiperazine, N-methyl homopiperazine and homopiperazine in anhydrous toluene, the reaction is performed at the temperature of 110 ℃ under reflux until the reaction is finished, the solvent is recovered under reduced pressure to prepare a crude product, and the crude product is subjected to silica gel column chromatography purification (diethylamine/methanol/chloroform, v/v/v, 2/4/94-3/5/92) to prepare target compounds 1-6.
The invention also provides application of the pharmaceutical composition in preparing melatonin and 5-hydroxytryptamine receptor agonists, and application of the pharmaceutical composition in preparing medicines for treating or improving central nervous system diseases related to melatonin and 5-hydroxytryptamine receptors.
The invention also provides application of the arundoin derivatives 1-6 shown in the following structural formula in preparing medicines for treating or improving central nervous system diseases related to melatonin and 5-hydroxytryptamine receptors, application of the arundoin derivatives 1-6 in preparing anti-mental disease medicines, and application of the arundoin derivatives 1-6 in preparing melatonin and 5-hydroxytryptamine receptor agonists.
Melatonin and 5-hydroxytryptamine receptor agonistic activity are used as guidance to discover that the arundoin derivatives 1-6 have the effect of resisting mental diseases. The invention obtains 6 derivatives through the structural modification of the arundoin, and the derivatives have better agonistic activity, wherein the activity of the compounds 2 and 6 is better, and the derivatives have better activity on a melatonin receptor MT1EC of (1)50Values of 0.51 and 0.50 mm respectively; for 5-HT1AEC of (1)50The values were 0.28 and 0.23 mm respectively.
When the compound of the present invention is used as a medicament, it may be used as it is or in the form of a pharmaceutical composition. The pharmaceutical composition contains 0.1-99%, preferably 0.5-90%, of the compound of the present invention, the remainder being pharmaceutically acceptable, non-toxic and inert pharmaceutically acceptable carriers or excipients for humans and animals.
The pharmaceutically acceptable carrier or excipient is one or more of solid, semi-solid and liquid diluents, fillers and pharmaceutical adjuvants. The pharmaceutical composition of the present invention is used in the form of a dose per unit body weight. The medicine of the present invention may be administrated through injection and oral taking.
Description of the drawings:
FIG. 1 is a schematic structural diagram of the arundoin derivatives (1-6) of the present invention.
Detailed Description
In order to better understand the substance of the present invention, the preparation method and pharmacological effect results of the arundoin derivatives (1-6) of the present invention are illustrated by the examples of the present invention, but the present invention is not limited thereto.
In the following examples, high resolution electrospray ionization mass spectrometry (HRESIMS) was performed on an LCMS-IT-TOF mass spectrometer (Shimadzu, Kyoto, Japan)Fixed, nuclear magnetic resonance spectrum (1H and13c NMR from Bruker AM 400(1H/13C, 400MHz/100MHz) nuclear magnetic resonance apparatus (Bruker, Bremerhaven, Germany) using TMS (tetramethylsilane) as internal standard. Column chromatography silica gel (200-300 mesh) and thin-layer chromatography silica gel GF254 are all produced by Qingdao Meigaoji Co. The reagents were purchased from Alfa Aesar, carbofuran and Acros.
Compound preparation example 1:
dissolving arundoin (2mmol) and ethylamine, pyrrole, piperidine, N-isopropylpiperazine, N-methyl homopiperazine and homopiperazine (2-10 mmol) in 10mL of anhydrous toluene respectively, refluxing at 110 ℃ until the reaction is finished, recovering the solvent under reduced pressure to obtain a crude product, and purifying by silica gel column chromatography (diethylamine/methanol/chloroform, v/v/v, 2/4/94-3/5/92) to obtain a target compound (1-6):
compounds 1-6 structure determination data:
n, N-Diethyl-3-indolylmethylamine (N, N-Diethyl-3-indolylmethylamine, 1) was a white powder, and the yield was 97%.1H NMR (400MHz,CDCl3)H:8.93(s,1H,NH),7.78‐7.00(m,5H,H‐2,4,5,6,7),3.87(s,2H,‐CH2N),2.69‐2.65(m,4H,
N-Pyrrolyl-3-indolylmethylamine (2) was obtained in the form of a white powder with a yield of 96%.1H NMR(400MHz, CDCl3)H:9.27(s,1H,NH),7.75‐7.00(m,5H,H‐2,4,5,6,7),3.91(s,2H,CH2N),2.68(m,4H,H‐2',5'),Piperazinyl-3-indolylmethylamine, 4) as a white powder in a yield of 93%.1H NMR(400MHz,CDCl3)H:9.57(s,1H,NH), 7.73‐6.94(m,5H,H‐2,4,5,6,7),3.78(s,2H,CH2N),2.69‐2.60(m,9H,H‐1”,2',3',5',6'),1.05(d,6H,Me).13C NMR(100MHz,CDCl3):136.2(s,C‐8),128.3(s,C‐9),124.4(d,C‐2),121.7(d,C‐6),119.3(d,C‐5),119.3 (d,C‐4),111.3(s,C‐3),111.2(d,C‐7),54.5(d,C‐1”),53.1(t,CH2N),53.1(t,C‐3',5'),48.7(t,C‐2',6'),18.7(q,7'),2.17(s,1H,NH),1.82‐1.76(m,2H,H‐3'),.13C NMR(100MHz,CDCl3):136.2 (s,C‐8),128.1(s,C‐9),123.8(d,C‐2),121.7(d,C‐6),119.3(d,C‐5),119.2(d,3-indolymethyalamine, 6) yellow amorphous powder, yield 80%.1H NMR(400MHz,CDCl3)H:10.05(s,1H,NH),7.80‐7.02(m,5H,H‐2,4,5,6,7),3.89(s,2H,CH2N),2.89‐2.85(m,4H,H‐2',4'), 2.75‐2.69(m,4H,H‐6',7'),2.40(s,3H,NMe),1.89‐1.85(m,2H,H‐3').13C NMR(100MHz,CDCl3):136.4(s, C‐8),128.1(s,C‐9),124.3(d,C‐2),121.6(d,C‐6),119.4(d,C‐5),119.1(d,C‐4),112.6(s,C‐3),111.3(d,C‐7),57.5(t,C‐6'),56.8(t,C‐7'),54.3(t,C‐4'),54.0(t,C‐2'),53.6(t,CH2N),46.9(q,NMe),27.0(t,C‐3').ESIMS: m/z 244[M+H]+,HRESIMS:C15H21N3[M+H]+Found 244.1742, calculated 244.1808.
The pharmacological effects of the arundoin derivatives of the present invention are illustrated below by the melatonin and 5-hydroxytryptamine receptor agonistic activity pharmacological effects test:
test example 1:
the arundoin derivatives (1-6) prepared in the preparation examples of the compounds of the invention are applied to human renal epithelial cells MT1-HEK 293 and 5-HT1A-measurement of the agonist activity on the receptor in a model of HEK293 cells.
1 agonist Activity test section
1.1 materials and instruments
MT for Activity screening1And 5-HT1AThe cell lines correspond to human kidney epithelial cells MT respectively1-HEK 293 and 5-HT1AHEK293, cell culture Medium containing 10% fetal bovine serum (Dulbecco's Modified Eagle Medium, DMEM), 10% FBS, HBSS from GIBCO; melatonin (CAS: 73-31-4) was purchased from Damas-beta, Inc. (Basel, Switzerland); the positive control drugs agomelatine (CAS: 138112-76-2) and 5-hydroxytryptamine (CAS: 50-67-9) were purchased from Saen chemical technology (Shanghai) Inc. and Yunnan Zehao commercial Inc., respectively; the leave-in Calcium 8 Kit (Wash Free Fluo-8 Calcium Asaay Kit, HD 03-0010, HDB Biosciences Co. Ltd, Shanghai, China). CO2 constant temperature incubator Thermo Forma 3310 (USA); inverted biomicroscope model XD-101 (nanjing); flexstation 3 desktop multifunctional microplate reader (Molecular Devices, Calif., USA).
1.2 Experimental procedures
Preparing to obtain tropine esters of organic acids with different skeletons, and applying the tropine esters to human kidney epithelial cells MT1-HEK 293 and 5-HT1AMT of the synthesized derivatives on a model of HEK293 cells with agomelatine and 5-hydroxytryptamine as positive controls1And 5-HT1AAnd (3) measuring the receptor agonistic activity. MT (multiple terminal)1And 5-HT1ACompound pair MT for receptor agonistic activity1And 5-HT1AReceptor activation rate and half maximal effective concentration Expression (EC)50), EC50The concentration at which the drug produces 50% of the maximal effect on the corresponding symptom. EC was performed using the basic Biometrics and mapping integration software GraphPad Prism 5.0 developed by GraphPadSoftware Inc50References to computational, specific test methods[17‐19]Cells were plated at a density of 4 × 104/well in Matrigel-coated 96-well black-walled transfixion plates in CO using Dulbecco's modified Eagle Medium2Culturing in a constant temperature incubator at 37 ℃ with the concentration of 5% for 24 h. The supernatant was aspirated, the original medium was discarded, and 100. mu.l/well of freshly prepared dye solution was added, and the mixture was protected from light at 37 ℃ for 60 min. Preparing a sample to be tested: preparing samples to be tested with different concentrations, and placing the samples to be tested into the other transparent bottom plate. The two plates are simultaneously placed in a FlexStation 3 desktop multifunctional microplate reader. Experimental data from Flex Station 3Reading by multi-functional enzyme-linked immunosorbent assay (EC)50Values were calculated by Graph Pad Prism 5 software, 100% agonism Δ a/Δ c × 100 (a: test sample; c: positive control).
2. As a result: the concentration is 1.00mM, and the agonistic activity of the arundoin and the derivatives (1-6) thereof is shown in Table (1):
TABLE 1 Giantrodia alkali derivatives vs. MT1And 5-HT1AAgonistic activity of receptorsa
Note:aall tested compounds were at a concentration of about 1Mm, agomelatine at a test concentration of 1.11 μ M, and 5-hydroxytryptamine at a test concentration of 6.67 μ M.The agonistic activity is expressed as the average of three determinations(n=3).
TABLE 2 half Effective Concentration (EC) of derivatives 2 and 650,mM)
Note: the test concentration range of the compound 1-6 is 0.02-1.52 mu M;average of three determinations, EC50Is expressed as(n=3)。
3. And (4) conclusion: the experimental results show that determined Arundinine derivative pairs MT1And 5-HT1AThe receptor has stronger excitability activity and has potential regulation or treatment effects related to melatonin and the receptor.
Formulation example 1:
the obtained arundoin derivatives 1-6 prepared by the method of preparation example 1 are dissolved by a small amount of DMSO respectively or mixed, and then are added with water for injection by a conventional method, and then are subjected to fine filtration, encapsulation and sterilization to prepare injection.
Formulation example 2:
the preparation method of the preparation example 1 is that the arundoin derivatives 1-6 are prepared firstly, and are dissolved by a small amount of DMSO respectively or mixed, then the obtained solution is dissolved in sterile water for injection, stirred to be dissolved, filtered by a sterile suction filter funnel, then sterile fine filtered, subpackaged in ampoules, frozen and dried at low temperature, and then sterile melt-sealed to obtain the powder injection.
Formulation example 3:
the method of preparation example 1 is firstly used for preparing the arundoin derivatives 1-6, and the arundoin derivatives and the excipient are added into the mixture respectively or mixed according to the weight ratio of the arundoin derivatives to the excipient of 9:1 to prepare powder.
Formulation example 4:
the preparation method of the preparation example 1 is firstly used for preparing and obtaining the arundoin derivatives 1-6, and the arundoin derivatives are respectively or mixed, added with excipient according to the weight ratio of the arundoin derivatives to the excipient of 5:1, granulated and tabletted.
Formulation example 5:
the arundoin derivatives 1-6 obtained by the method of preparation example 1 were prepared first, and prepared into oral liquid by a conventional oral liquid preparation method, separately or mixed.
Formulation example 6:
the arundoin derivatives 1-6 obtained by the method of preparation example 1 are prepared, respectively or mixed, and the excipient is added according to the weight ratio of the arundoin derivatives to the excipient of 5:1 to prepare capsules.
Formulation example 7:
the preparation method of the preparation example 1 is firstly used for preparing the arundoin derivatives 1-6, and the arundoin derivatives and the excipients are respectively or mixed, added with the excipients according to the weight ratio of 3:1 and prepared into capsules.
Formulation example 8:
the arundoin derivatives 1-6 obtained by the method of preparation example 1 are prepared, respectively or mixed, and the excipient is added according to the weight ratio of the arundoin derivatives to the excipient of 5:1 to prepare granules.
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201710092931.2A CN107174586B (en) | 2017-02-21 | 2017-02-21 | Pharmaceutical composition with arundoin derivative as active ingredient and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201710092931.2A CN107174586B (en) | 2017-02-21 | 2017-02-21 | Pharmaceutical composition with arundoin derivative as active ingredient and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107174586A CN107174586A (en) | 2017-09-19 |
| CN107174586B true CN107174586B (en) | 2020-08-11 |
Family
ID=59830435
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201710092931.2A Active CN107174586B (en) | 2017-02-21 | 2017-02-21 | Pharmaceutical composition with arundoin derivative as active ingredient and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN107174586B (en) |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1720225A (en) * | 2002-11-28 | 2006-01-11 | 苏文生命科学有限公司 | N-arylsulfonyl-3-substituted indoles having serotonin receptor affinity, process for their preparation and pharmaceutical composition containing them |
-
2017
- 2017-02-21 CN CN201710092931.2A patent/CN107174586B/en active Active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1720225A (en) * | 2002-11-28 | 2006-01-11 | 苏文生命科学有限公司 | N-arylsulfonyl-3-substituted indoles having serotonin receptor affinity, process for their preparation and pharmaceutical composition containing them |
Non-Patent Citations (3)
| Title |
|---|
| Indole;美国化学会;《Registry》;20090914;1-3 * |
| Oxytocic activity of basic (aminomethyl) derivatives of phenols and related compounds;COHEN A.等;《Brit.J.Pharmacol.》;19571231;第12卷;194-208 * |
| Structure-Activity Relationships at 5-HT1A Receptors: Binding Profiles and Intrinsic Activity;DAVID L. NELSON等;《Pharmacology Biochemistry & Behavio》;19911231;第40卷;1041-1051 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107174586A (en) | 2017-09-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101406727B1 (en) | Agomelatine hydrochloride hydrate and preparation thereof | |
| CN109563060B (en) | IDO1 inhibitor and preparation method and application thereof | |
| JP2021503443A (en) | Antagonist of muscarinic acetylcholine receptor M4 | |
| CN107235992B (en) | Indolone spiral shell thiophane class compound and its salt, preparation method and application | |
| KR20130136544A (en) | New crystal form vii of agomelatine, preparation method and use thereof and pharmaceutical composition containing same | |
| CN113387957B (en) | Spirocyclic indolone-pyrrolidine carbonate compound and composition, preparation method and application thereof | |
| CN104086551B (en) | Compound and its production and use | |
| JP5986635B2 (en) | 5,6,7,8-Tetrahydro-6- [N, N-bis [(2-thienyl) ethyl]] amino-1-naphthol, process for its preparation and use thereof | |
| CN109809971B (en) | Poly-benzyl derivative, pharmaceutical composition thereof, preparation method and application thereof | |
| CN110372557B (en) | Cyclohexanamines D3/D2Partial receptor agonists | |
| CN107174586B (en) | Pharmaceutical composition with arundoin derivative as active ingredient and application thereof | |
| KR20190034609A (en) | Crystals of cyclic amine derivatives and uses thereof | |
| CN108143741B (en) | Application of magnolol glucoside in preparation of medicine for treating central nervous system diseases | |
| CN104379557B (en) | The preparation method of agomelatine crystal form I | |
| CN107235991B (en) | The raceme and its salt, preparation method and application of indolone spiral shell tetrahydro thio-pyrylium class compound | |
| CN107365265B (en) | apocynin water-soluble prodrug, preparation method, pharmaceutical composition and application thereof | |
| KR101406736B1 (en) | Agomelatine hydrobromide hydrate and preparation thereof | |
| JP2021509897A (en) | Heterocyclic compounds and their use as CSF-1R inhibitors | |
| CN108997121A (en) | Application of the magnolia bark phenol derivative in preparation treatment central nervous system disease drug | |
| CN109942537B (en) | ALDH2 agonist, preparation method and application thereof | |
| CN106905300B (en) | Giantreed alkali derivant and its pharmaceutical composition and its application in pharmacy | |
| CN114364658A (en) | Preparation method and composition of levo-praziquantel and chiral intermediate thereof | |
| RU2603770C2 (en) | Substituted pyrazine pyrimidinones as trpa1 channel blockers, pharmaceutical composition, methods of production and use thereof | |
| CN108586341B (en) | Amide compounds and medicinal salts thereof, and preparation method and medicinal application thereof | |
| CN110066253B (en) | 1,2, 5-oxadiazole derivative, preparation method thereof and application thereof in medicines |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |