CN107001342B - 新化合物 - Google Patents
新化合物 Download PDFInfo
- Publication number
- CN107001342B CN107001342B CN201580039289.0A CN201580039289A CN107001342B CN 107001342 B CN107001342 B CN 107001342B CN 201580039289 A CN201580039289 A CN 201580039289A CN 107001342 B CN107001342 B CN 107001342B
- Authority
- CN
- China
- Prior art keywords
- methyl
- chloro
- phenyl
- piperazin
- carbonyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 150000001875 compounds Chemical class 0.000 title claims description 265
- 238000011282 treatment Methods 0.000 claims abstract description 22
- 150000003839 salts Chemical class 0.000 claims description 62
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 41
- 125000001424 substituent group Chemical group 0.000 claims description 31
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 22
- 239000008194 pharmaceutical composition Substances 0.000 claims description 16
- 125000000217 alkyl group Chemical group 0.000 claims description 15
- 125000004076 pyridyl group Chemical group 0.000 claims description 15
- 229910052736 halogen Inorganic materials 0.000 claims description 14
- 150000002367 halogens Chemical class 0.000 claims description 14
- 125000003718 tetrahydrofuranyl group Chemical group 0.000 claims description 14
- 239000003814 drug Substances 0.000 claims description 10
- 201000006417 multiple sclerosis Diseases 0.000 claims description 10
- 125000001412 tetrahydropyranyl group Chemical group 0.000 claims description 10
- 206010002556 Ankylosing Spondylitis Diseases 0.000 claims description 8
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 8
- 229910052731 fluorine Inorganic materials 0.000 claims description 7
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 6
- XVGTUBJIMYRFHI-NPMXOYFQSA-N 5-chloro-N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)Cl)CN2C[C@@H](N(CC2)C(=O)[C@@H]2OCCC2)C)C)C1)C XVGTUBJIMYRFHI-NPMXOYFQSA-N 0.000 claims description 5
- XVGTUBJIMYRFHI-WNSKOXEYSA-N 5-chloro-N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)Cl)CN2C[C@@H](N(CC2)C(=O)[C@H]2OCCC2)C)C)C1)C XVGTUBJIMYRFHI-WNSKOXEYSA-N 0.000 claims description 5
- HAEULXIXALXHKV-SFHVURJKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C HAEULXIXALXHKV-SFHVURJKSA-N 0.000 claims description 5
- 238000004519 manufacturing process Methods 0.000 claims description 5
- VUWZPRWSIVNGKG-UHFFFAOYSA-N fluoromethane Chemical compound F[CH2] VUWZPRWSIVNGKG-UHFFFAOYSA-N 0.000 claims description 4
- 125000003838 furazanyl group Chemical group 0.000 claims description 4
- 125000002541 furyl group Chemical group 0.000 claims description 4
- 125000002883 imidazolyl group Chemical group 0.000 claims description 4
- 125000001786 isothiazolyl group Chemical group 0.000 claims description 4
- 125000000842 isoxazolyl group Chemical group 0.000 claims description 4
- 125000001715 oxadiazolyl group Chemical group 0.000 claims description 4
- 125000002971 oxazolyl group Chemical group 0.000 claims description 4
- 125000003373 pyrazinyl group Chemical group 0.000 claims description 4
- 125000003226 pyrazolyl group Chemical group 0.000 claims description 4
- 125000002098 pyridazinyl group Chemical group 0.000 claims description 4
- 125000000714 pyrimidinyl group Chemical group 0.000 claims description 4
- 125000000168 pyrrolyl group Chemical group 0.000 claims description 4
- 125000005247 tetrazinyl group Chemical group N1=NN=NC(=C1)* 0.000 claims description 4
- 125000003831 tetrazolyl group Chemical group 0.000 claims description 4
- 125000001113 thiadiazolyl group Chemical group 0.000 claims description 4
- 125000000335 thiazolyl group Chemical group 0.000 claims description 4
- 125000001544 thienyl group Chemical group 0.000 claims description 4
- 125000004306 triazinyl group Chemical group 0.000 claims description 4
- 125000001425 triazolyl group Chemical group 0.000 claims description 4
- USPQTGUCNDTTBM-YJBOKZPZSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3S)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1COCC1)C USPQTGUCNDTTBM-YJBOKZPZSA-N 0.000 claims description 3
- 238000002560 therapeutic procedure Methods 0.000 claims description 3
- 239000003937 drug carrier Substances 0.000 claims description 2
- 108091008680 RAR-related orphan receptors Proteins 0.000 abstract description 69
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 21
- 201000010099 disease Diseases 0.000 abstract description 13
- 230000001404 mediated effect Effects 0.000 abstract description 12
- 102100023421 Nuclear receptor ROR-gamma Human genes 0.000 abstract description 3
- 108091008773 RAR-related orphan receptors γ Proteins 0.000 abstract description 2
- 239000000203 mixture Substances 0.000 description 179
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 152
- 239000000243 solution Substances 0.000 description 112
- 239000007787 solid Substances 0.000 description 83
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 73
- 229910001868 water Inorganic materials 0.000 description 72
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 69
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 69
- 238000005160 1H NMR spectroscopy Methods 0.000 description 46
- -1 homopiperidinyl Chemical group 0.000 description 46
- 238000000034 method Methods 0.000 description 43
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 40
- 229910052938 sodium sulfate Inorganic materials 0.000 description 40
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-dimethylformamide Substances CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 36
- 239000007832 Na2SO4 Substances 0.000 description 36
- 239000007821 HATU Substances 0.000 description 34
- 239000012044 organic layer Substances 0.000 description 33
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 28
- 238000002953 preparative HPLC Methods 0.000 description 25
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 24
- 210000004027 cell Anatomy 0.000 description 24
- 239000002904 solvent Substances 0.000 description 24
- 125000004429 atom Chemical group 0.000 description 22
- 239000011541 reaction mixture Substances 0.000 description 22
- 238000012360 testing method Methods 0.000 description 22
- 239000012267 brine Substances 0.000 description 20
- 229940079322 interferon Drugs 0.000 description 20
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 20
- 238000006243 chemical reaction Methods 0.000 description 19
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 18
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 18
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 18
- 238000004440 column chromatography Methods 0.000 description 17
- 238000001035 drying Methods 0.000 description 17
- 229920006395 saturated elastomer Polymers 0.000 description 17
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 16
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 16
- 239000002253 acid Substances 0.000 description 16
- WSFSSNUMVMOOMR-BJUDXGSMSA-N methanone Chemical compound O=[11CH2] WSFSSNUMVMOOMR-BJUDXGSMSA-N 0.000 description 16
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 15
- 239000000706 filtrate Substances 0.000 description 15
- 238000001914 filtration Methods 0.000 description 15
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 14
- 230000001064 anti-interferon Effects 0.000 description 14
- 108090000623 proteins and genes Proteins 0.000 description 14
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 13
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 13
- 239000002552 dosage form Substances 0.000 description 13
- 239000012074 organic phase Substances 0.000 description 13
- KDLHZDBZIXYQEI-UHFFFAOYSA-N palladium Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 13
- 235000018102 proteins Nutrition 0.000 description 13
- 102000004169 proteins and genes Human genes 0.000 description 13
- WYURNTSHIVDZCO-UHFFFAOYSA-N tetrahydrofuran Substances C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 13
- 238000004293 19F NMR spectroscopy Methods 0.000 description 12
- 208000023275 Autoimmune disease Diseases 0.000 description 12
- 239000000460 chlorine Substances 0.000 description 12
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 12
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 12
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 12
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 12
- 241000282414 Homo sapiens Species 0.000 description 11
- VFRSADQPWYCXDG-LEUCUCNGSA-N ethyl (2s,5s)-5-methylpyrrolidine-2-carboxylate;2,2,2-trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.CCOC(=O)[C@@H]1CC[C@H](C)N1 VFRSADQPWYCXDG-LEUCUCNGSA-N 0.000 description 11
- 239000010410 layer Substances 0.000 description 11
- 239000012071 phase Substances 0.000 description 11
- 238000002360 preparation method Methods 0.000 description 11
- 210000000068 Th17 cell Anatomy 0.000 description 10
- 239000002585 base Substances 0.000 description 10
- 208000027866 inflammatory disease Diseases 0.000 description 10
- UIIMBOGNXHQVGW-UHFFFAOYSA-M sodium bicarbonate Substances [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 10
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 10
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 10
- 238000003556 assay Methods 0.000 description 9
- 239000006184 cosolvent Substances 0.000 description 9
- 201000002491 encephalomyelitis Diseases 0.000 description 9
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 9
- 239000007858 starting material Substances 0.000 description 9
- 238000003756 stirring Methods 0.000 description 9
- 108020004414 DNA Proteins 0.000 description 8
- OGXXMKMAKDFHHV-AWEZNQCLSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](NCC1)C OGXXMKMAKDFHHV-AWEZNQCLSA-N 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 108010004469 allophycocyanin Proteins 0.000 description 8
- 208000035475 disorder Diseases 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 125000001072 heteroaryl group Chemical group 0.000 description 8
- 125000005842 heteroatom Chemical group 0.000 description 8
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 8
- 238000001727 in vivo Methods 0.000 description 8
- BVWJUKIAFKMHFI-LBPRGKRZSA-N tert-butyl (2s)-4-[(3-amino-5-chloro-2-methylphenyl)methyl]-2-methylpiperazine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)[C@@H](C)CN1CC1=CC(Cl)=CC(N)=C1C BVWJUKIAFKMHFI-LBPRGKRZSA-N 0.000 description 8
- UJJLJRQIPMGXEZ-BYPYZUCNSA-N (2s)-oxolane-2-carboxylic acid Chemical compound OC(=O)[C@@H]1CCCO1 UJJLJRQIPMGXEZ-BYPYZUCNSA-N 0.000 description 7
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 7
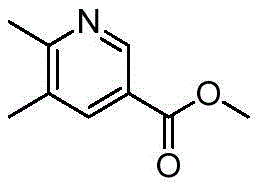
- AIDXHOVOUCHIPI-UHFFFAOYSA-N 5-chloro-6-methylpyridine-3-carboxylic acid Chemical compound CC1=NC=C(C(O)=O)C=C1Cl AIDXHOVOUCHIPI-UHFFFAOYSA-N 0.000 description 7
- 102000014150 Interferons Human genes 0.000 description 7
- 108010050904 Interferons Proteins 0.000 description 7
- 108060001084 Luciferase Proteins 0.000 description 7
- 239000005089 Luciferase Substances 0.000 description 7
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 7
- 239000012043 crude product Substances 0.000 description 7
- 125000000753 cycloalkyl group Chemical group 0.000 description 7
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- CDBYLPFSWZWCQE-UHFFFAOYSA-L sodium carbonate Substances [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 7
- 229910000029 sodium carbonate Inorganic materials 0.000 description 7
- UJJLJRQIPMGXEZ-SCSAIBSYSA-N tetrahydrofuran-2-carboxylic acid Chemical compound OC(=O)[C@H]1CCCO1 UJJLJRQIPMGXEZ-SCSAIBSYSA-N 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 6
- LVEYOSJUKRVCCF-UHFFFAOYSA-N 1,3-bis(diphenylphosphino)propane Chemical compound C=1C=CC=CC=1P(C=1C=CC=CC=1)CCCP(C=1C=CC=CC=1)C1=CC=CC=C1 LVEYOSJUKRVCCF-UHFFFAOYSA-N 0.000 description 6
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 6
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 6
- LKTUUSBGSVWDAL-UHFFFAOYSA-N 5-fluoro-6-methylpyridine-3-carboxylic acid Chemical compound CC1=NC=C(C(O)=O)C=C1F LKTUUSBGSVWDAL-UHFFFAOYSA-N 0.000 description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 6
- 229910052693 Europium Inorganic materials 0.000 description 6
- 241000699670 Mus sp. Species 0.000 description 6
- JHLBCRRBLWDJLP-GFCCVEGCSA-N N-[5-chloro-2-methyl-3-[[(3R)-3-methylpiperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@H](NCC1)C JHLBCRRBLWDJLP-GFCCVEGCSA-N 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- 150000007513 acids Chemical class 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 238000004296 chiral HPLC Methods 0.000 description 6
- 235000019439 ethyl acetate Nutrition 0.000 description 6
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 6
- 239000012458 free base Substances 0.000 description 6
- 125000000524 functional group Chemical group 0.000 description 6
- 230000002757 inflammatory effect Effects 0.000 description 6
- 239000003112 inhibitor Substances 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 239000003446 ligand Substances 0.000 description 6
- GSUBXIVOZXWGKF-UHFFFAOYSA-N oxolane-3-carbaldehyde Chemical compound O=CC1CCOC1 GSUBXIVOZXWGKF-UHFFFAOYSA-N 0.000 description 6
- 239000002953 phosphate buffered saline Substances 0.000 description 6
- 229910000027 potassium carbonate Inorganic materials 0.000 description 6
- 108090000765 processed proteins & peptides Proteins 0.000 description 6
- 125000006239 protecting group Chemical group 0.000 description 6
- KSLZRIXXMKXDEO-UHFFFAOYSA-N 5-chloro-2-methyl-3-nitrobenzoic acid Chemical compound CC1=C(C(O)=O)C=C(Cl)C=C1[N+]([O-])=O KSLZRIXXMKXDEO-UHFFFAOYSA-N 0.000 description 5
- 102000004127 Cytokines Human genes 0.000 description 5
- 108090000695 Cytokines Proteins 0.000 description 5
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 5
- PIMQDMQZFHZZPF-ZDUSSCGKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-5-cyano-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)C#N)C)=O)C)CN1C[C@@H](NCC1)C PIMQDMQZFHZZPF-ZDUSSCGKSA-N 0.000 description 5
- JHLBCRRBLWDJLP-LBPRGKRZSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@@H](NCC1)C JHLBCRRBLWDJLP-LBPRGKRZSA-N 0.000 description 5
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 5
- 108010090804 Streptavidin Proteins 0.000 description 5
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 5
- 150000001412 amines Chemical class 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 5
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 5
- 239000006071 cream Substances 0.000 description 5
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 230000002452 interceptive effect Effects 0.000 description 5
- 239000000543 intermediate Substances 0.000 description 5
- 108020001756 ligand binding domains Proteins 0.000 description 5
- 239000002609 medium Substances 0.000 description 5
- 239000013612 plasmid Substances 0.000 description 5
- 239000012453 solvate Substances 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- KNKSPMDGDIIUTP-AWEZNQCLSA-N 3-cyano-n-[5-fluoro-2-methyl-3-[[(3s)-3-methylpiperazin-1-yl]methyl]phenyl]benzamide Chemical compound C1CN[C@@H](C)CN1CC1=CC(F)=CC(NC(=O)C=2C=C(C=CC=2)C#N)=C1C KNKSPMDGDIIUTP-AWEZNQCLSA-N 0.000 description 4
- OTLMJDZYTWGGGP-UHFFFAOYSA-N 5,6-dimethylpyridine-3-carboxylic acid Chemical compound CC1=CC(C(O)=O)=CN=C1C OTLMJDZYTWGGGP-UHFFFAOYSA-N 0.000 description 4
- DLEUXLJMIZVCEQ-LBPRGKRZSA-N C[C@H]1CN(Cc2cc(Cl)cc(c2C)[N+]([O-])=O)CCN1C(=O)OC(C)(C)C Chemical compound C[C@H]1CN(Cc2cc(Cl)cc(c2C)[N+]([O-])=O)CCN1C(=O)OC(C)(C)C DLEUXLJMIZVCEQ-LBPRGKRZSA-N 0.000 description 4
- ZCOXUBZJISFEJR-LBPRGKRZSA-N C[C@H]1CN(Cc2cc(F)cc(c2C)[N+]([O-])=O)CCN1C(=O)OC(C)(C)C Chemical compound C[C@H]1CN(Cc2cc(F)cc(c2C)[N+]([O-])=O)CCN1C(=O)OC(C)(C)C ZCOXUBZJISFEJR-LBPRGKRZSA-N 0.000 description 4
- 108091029433 Conserved non-coding sequence Proteins 0.000 description 4
- 201000004624 Dermatitis Diseases 0.000 description 4
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 4
- 101100396718 Homo sapiens IL17A gene Proteins 0.000 description 4
- CDPBCEPCZBNKOU-MAUKXSAKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methyl-1-oxidopyridin-1-ium-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(=O)C=1C=C(C(=[N+](C1)[O-])C)F)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCC1)C CDPBCEPCZBNKOU-MAUKXSAKSA-N 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- 201000004681 Psoriasis Diseases 0.000 description 4
- 210000001744 T-lymphocyte Anatomy 0.000 description 4
- 230000002378 acidificating effect Effects 0.000 description 4
- 239000012190 activator Substances 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- 230000001363 autoimmune Effects 0.000 description 4
- 230000006287 biotinylation Effects 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- 239000012634 fragment Substances 0.000 description 4
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 4
- CSNNHWWHGAXBCP-UHFFFAOYSA-L magnesium sulphate Substances [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 4
- HWZINXYBRIQTEJ-UHFFFAOYSA-N methyl 5,6-dichloropyridine-3-carboxylate Chemical compound COC(=O)C1=CN=C(Cl)C(Cl)=C1 HWZINXYBRIQTEJ-UHFFFAOYSA-N 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 102000006255 nuclear receptors Human genes 0.000 description 4
- 108020004017 nuclear receptors Proteins 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 229940002612 prodrug Drugs 0.000 description 4
- 239000000651 prodrug Substances 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 4
- 206010039073 rheumatoid arthritis Diseases 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 235000011152 sodium sulphate Nutrition 0.000 description 4
- 239000011550 stock solution Substances 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- 238000011200 topical administration Methods 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- QAEDZJGFFMLHHQ-UHFFFAOYSA-N trifluoroacetic anhydride Chemical compound FC(F)(F)C(=O)OC(=O)C(F)(F)F QAEDZJGFFMLHHQ-UHFFFAOYSA-N 0.000 description 4
- KIFWPSWOZXXLMM-VIFPVBQESA-N (3S)-1-[(5-chloro-2-methyl-3-nitrophenyl)methyl]-3-methylpiperazine Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](NCC2)C)C1)C)[N+](=O)[O-] KIFWPSWOZXXLMM-VIFPVBQESA-N 0.000 description 3
- IHPWWVPOLYUYMG-UHFFFAOYSA-N (5-chloro-2-methyl-3-nitrophenyl)methanol Chemical compound CC1=C(CO)C=C(Cl)C=C1[N+]([O-])=O IHPWWVPOLYUYMG-UHFFFAOYSA-N 0.000 description 3
- OVEMFXRHESVQEF-UHFFFAOYSA-N (5-fluoro-2-methyl-3-nitrophenyl)methanol Chemical compound CC1=C(CO)C=C(F)C=C1[N+]([O-])=O OVEMFXRHESVQEF-UHFFFAOYSA-N 0.000 description 3
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 3
- YPCLLVYLWXIIBW-UHFFFAOYSA-N 5-bromo-3-methylpyridine-2-carbonitrile Chemical compound CC1=CC(Br)=CN=C1C#N YPCLLVYLWXIIBW-UHFFFAOYSA-N 0.000 description 3
- UORDMUKGFFTCOC-UHFFFAOYSA-N 5-chloro-1-(chloromethyl)-2-methyl-3-nitrobenzene Chemical compound ClC=1C=C(CCl)C(=C(C1)[N+](=O)[O-])C UORDMUKGFFTCOC-UHFFFAOYSA-N 0.000 description 3
- DIHBEWMXYDGNED-UHFFFAOYSA-N 5-cyano-6-methylpyridine-3-carboxylic acid Chemical compound CC1=NC=C(C(O)=O)C=C1C#N DIHBEWMXYDGNED-UHFFFAOYSA-N 0.000 description 3
- RUFNWXXFCIKZBX-UHFFFAOYSA-N 5-fluoro-2-methyl-3-nitrobenzaldehyde Chemical compound CC1=C(C=O)C=C(F)C=C1[N+]([O-])=O RUFNWXXFCIKZBX-UHFFFAOYSA-N 0.000 description 3
- CMZCBPLTJBYJHZ-UHFFFAOYSA-N 5-fluoro-2-methyl-3-nitrobenzoic acid Chemical compound CC1=C(C(O)=O)C=C(F)C=C1[N+]([O-])=O CMZCBPLTJBYJHZ-UHFFFAOYSA-N 0.000 description 3
- LYFRBMMDMZOHML-UHFFFAOYSA-N 5-fluoro-6-methyl-1-oxidopyridin-1-ium-3-carboxylic acid Chemical compound C(=O)(O)C=1C=C(C(=[N+](C1)[O-])C)F LYFRBMMDMZOHML-UHFFFAOYSA-N 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 3
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- OUDDGKWCGPFKKR-KRWDZBQOSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-6-cyanopyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C OUDDGKWCGPFKKR-KRWDZBQOSA-N 0.000 description 3
- XOWQTTHBANMMIS-ZDUSSCGKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-3-cyano-4-fluorobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=C(C=C1)F)C#N)=O)C)CN1C[C@@H](NCC1)C XOWQTTHBANMMIS-ZDUSSCGKSA-N 0.000 description 3
- GSSYBZHVCOORFS-ZDUSSCGKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-3-cyano-5-fluorobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC(=C1)F)C#N)=O)C)CN1C[C@@H](NCC1)C GSSYBZHVCOORFS-ZDUSSCGKSA-N 0.000 description 3
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 3
- 108020005497 Nuclear hormone receptor Proteins 0.000 description 3
- 206010033799 Paralysis Diseases 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- QAXOVBGJNQZZHN-ZDUSSCGKSA-N [(2S)-4-[(3-amino-5-chloro-2-methylphenyl)methyl]-2-methylpiperazin-1-yl]-(oxan-4-yl)methanone Chemical compound NC=1C(=C(CN2C[C@@H](N(CC2)C(=O)C2CCOCC2)C)C=C(C1)Cl)C QAXOVBGJNQZZHN-ZDUSSCGKSA-N 0.000 description 3
- FJQHVFWGPLIBBL-ZDUSSCGKSA-N [(2S)-4-[(5-chloro-2-methyl-3-nitrophenyl)methyl]-2-methylpiperazin-1-yl]-(oxan-4-yl)methanone Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)C2CCOCC2)C)C1)C)[N+](=O)[O-] FJQHVFWGPLIBBL-ZDUSSCGKSA-N 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- 239000000556 agonist Substances 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000008346 aqueous phase Substances 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 229960002685 biotin Drugs 0.000 description 3
- 235000020958 biotin Nutrition 0.000 description 3
- 239000011616 biotin Substances 0.000 description 3
- 238000007413 biotinylation Methods 0.000 description 3
- 125000004432 carbon atom Chemical group C* 0.000 description 3
- 230000024245 cell differentiation Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000007884 disintegrant Substances 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- VDAPYXHAXWXNDK-UHFFFAOYSA-N ethyl 2-chloro-5-cyano-6-methylpyridine-3-carboxylate Chemical compound CCOC(=O)C1=CC(C#N)=C(C)N=C1Cl VDAPYXHAXWXNDK-UHFFFAOYSA-N 0.000 description 3
- IOFDGVXYARBFOQ-UHFFFAOYSA-N ethyl 5-cyano-6-methyl-2-oxo-1h-pyridine-3-carboxylate Chemical compound CCOC(=O)C1=CC(C#N)=C(C)NC1=O IOFDGVXYARBFOQ-UHFFFAOYSA-N 0.000 description 3
- QUAMCESTXLYSGN-UHFFFAOYSA-N ethyl 5-cyano-6-methylpyridine-3-carboxylate Chemical compound CCOC(=O)C1=CN=C(C)C(C#N)=C1 QUAMCESTXLYSGN-UHFFFAOYSA-N 0.000 description 3
- MIRZZLBANFMLGV-UHFFFAOYSA-N ethyl 5-fluoro-6-methyl-1-oxidopyridin-1-ium-3-carboxylate Chemical compound C(C)OC(=O)C=1C=C(C(=[N+](C1)[O-])C)F MIRZZLBANFMLGV-UHFFFAOYSA-N 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 239000000499 gel Substances 0.000 description 3
- 150000004677 hydrates Chemical class 0.000 description 3
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 3
- 229960002751 imiquimod Drugs 0.000 description 3
- DOUYETYNHWVLEO-UHFFFAOYSA-N imiquimod Chemical compound C1=CC=CC2=C3N(CC(C)C)C=NC3=C(N)N=C21 DOUYETYNHWVLEO-UHFFFAOYSA-N 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 238000002955 isolation Methods 0.000 description 3
- 210000003141 lower extremity Anatomy 0.000 description 3
- 239000000314 lubricant Substances 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- WADLLLSMEPLCNO-UHFFFAOYSA-N methyl 2,6-dichloro-5-fluoropyridine-3-carboxylate Chemical compound COC(=O)C1=CC(F)=C(Cl)N=C1Cl WADLLLSMEPLCNO-UHFFFAOYSA-N 0.000 description 3
- FQYVDJYSYAEPIP-UHFFFAOYSA-N methyl 5,6-dimethylpyridine-3-carboxylate Chemical compound COC(=O)C1=CN=C(C)C(C)=C1 FQYVDJYSYAEPIP-UHFFFAOYSA-N 0.000 description 3
- ASEMVDGUOASAMH-UHFFFAOYSA-N methyl 6-cyano-5-methylpyridine-3-carboxylate Chemical compound COC(=O)C1=CN=C(C#N)C(C)=C1 ASEMVDGUOASAMH-UHFFFAOYSA-N 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 229910017604 nitric acid Inorganic materials 0.000 description 3
- 150000002828 nitro derivatives Chemical class 0.000 description 3
- 239000012299 nitrogen atmosphere Substances 0.000 description 3
- 239000008184 oral solid dosage form Substances 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- BOTREHHXSQGWTR-UHFFFAOYSA-N oxolane-3-carboxylic acid Chemical compound OC(=O)C1CCOC1 BOTREHHXSQGWTR-UHFFFAOYSA-N 0.000 description 3
- 238000007911 parenteral administration Methods 0.000 description 3
- 230000008506 pathogenesis Effects 0.000 description 3
- 239000003208 petroleum Substances 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 229940044601 receptor agonist Drugs 0.000 description 3
- 239000000018 receptor agonist Substances 0.000 description 3
- 239000000741 silica gel Substances 0.000 description 3
- 229910002027 silica gel Inorganic materials 0.000 description 3
- 125000003003 spiro group Chemical group 0.000 description 3
- PHCVDOATVJUEJN-NSHDSACASA-N tert-butyl (2S)-4-(5-chloro-2-methyl-3-nitrobenzoyl)-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(C(=O)N2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)[N+](=O)[O-] PHCVDOATVJUEJN-NSHDSACASA-N 0.000 description 3
- AETMTTJRWLMBMN-INIZCTEOSA-N tert-butyl (2S)-4-[(5-chloro-2-methyl-3-nitrophenyl)methyl]-2-ethylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)CC)C1)C)[N+](=O)[O-] AETMTTJRWLMBMN-INIZCTEOSA-N 0.000 description 3
- BDUUSJKAJXRTAD-INIZCTEOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(3-cyano-5-fluorobenzoyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CC(=CC(=C1)F)C#N)=O BDUUSJKAJXRTAD-INIZCTEOSA-N 0.000 description 3
- JGRKCQXTMATMQC-KRWDZBQOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(3-cyanobenzoyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CC(=CC=C1)C#N)=O JGRKCQXTMATMQC-KRWDZBQOSA-N 0.000 description 3
- GTZJJLLSVVOGKR-HNNXBMFYSA-N tert-butyl (2S)-4-[[5-chloro-3-[(5-fluoro-6-methyl-1-oxidopyridin-1-ium-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound C(C)(C)(C)OC(=O)N1[C@H](CN(CC1)CC=1C(=C(C=C(C1)Cl)NC(=O)C=1C=C(C(=[N+](C1)[O-])C)F)C)C GTZJJLLSVVOGKR-HNNXBMFYSA-N 0.000 description 3
- BADYGPCOTZMVLR-HNNXBMFYSA-N tert-butyl (2S)-4-[[5-chloro-3-[(5-fluoro-6-methylpyridine-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C(=C1)F)C)=O BADYGPCOTZMVLR-HNNXBMFYSA-N 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- LEIMLDGFXIOXMT-UHFFFAOYSA-N trimethylsilyl cyanide Chemical compound C[Si](C)(C)C#N LEIMLDGFXIOXMT-UHFFFAOYSA-N 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- 239000003643 water by type Substances 0.000 description 3
- BOTREHHXSQGWTR-SCSAIBSYSA-N (3r)-oxolane-3-carboxylic acid Chemical compound OC(=O)[C@@H]1CCOC1 BOTREHHXSQGWTR-SCSAIBSYSA-N 0.000 description 2
- BOTREHHXSQGWTR-BYPYZUCNSA-N (3s)-oxolane-3-carboxylic acid Chemical compound OC(=O)[C@H]1CCOC1 BOTREHHXSQGWTR-BYPYZUCNSA-N 0.000 description 2
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Substances CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 2
- XQISPVJWRLHHLT-UHFFFAOYSA-N 1-hydroxycyclohexa-2,4-diene-1-carboxylic acid Chemical compound OC(=O)C1(O)CC=CC=C1 XQISPVJWRLHHLT-UHFFFAOYSA-N 0.000 description 2
- HOZKVIZTKBAMIA-UHFFFAOYSA-N 2-chloro-5-fluoro-6-methylpyridine-3-carboxylic acid Chemical compound Cc1nc(Cl)c(cc1F)C(O)=O HOZKVIZTKBAMIA-UHFFFAOYSA-N 0.000 description 2
- GWSXYSZAGOCSNP-UHFFFAOYSA-N 3-cyano-4-methylbenzoic acid Chemical compound CC1=CC=C(C(O)=O)C=C1C#N GWSXYSZAGOCSNP-UHFFFAOYSA-N 0.000 description 2
- UEJFQUFYBURWAU-NPMXOYFQSA-N 5-chloro-N-[5-fluoro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)F)CN2C[C@@H](N(CC2)C(=O)[C@@H]2OCCC2)C)C)C1)C UEJFQUFYBURWAU-NPMXOYFQSA-N 0.000 description 2
- UEJFQUFYBURWAU-WNSKOXEYSA-N 5-chloro-N-[5-fluoro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)F)CN2C[C@@H](N(CC2)C(=O)[C@H]2OCCC2)C)C)C1)C UEJFQUFYBURWAU-WNSKOXEYSA-N 0.000 description 2
- GBIVKBUFSLXYOJ-YJBOKZPZSA-N 5-chloro-N-[5-fluoro-2-methyl-3-[[(3S)-3-methyl-4-[(3S)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)F)CN2C[C@@H](N(CC2)C(=O)[C@@H]2COCC2)C)C)C1)C GBIVKBUFSLXYOJ-YJBOKZPZSA-N 0.000 description 2
- ULOJHSLPFZBIRU-UHFFFAOYSA-N 5-fluoro-1-oxidopyridin-1-ium-3-carboxylic acid Chemical compound OC(=O)C1=CC(F)=C[N+]([O-])=C1 ULOJHSLPFZBIRU-UHFFFAOYSA-N 0.000 description 2
- AYOUQXBXIGKZHH-NPMXOYFQSA-N 5-fluoro-N-[5-fluoro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound FC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)F)CN2C[C@@H](N(CC2)C(=O)[C@@H]2OCCC2)C)C)C1)C AYOUQXBXIGKZHH-NPMXOYFQSA-N 0.000 description 2
- BVUMVUIXTLQBIE-UHFFFAOYSA-N 5-methoxy-6-methylpyridine-3-carboxylic acid Chemical compound COC1=CC(C(O)=O)=CN=C1C BVUMVUIXTLQBIE-UHFFFAOYSA-N 0.000 description 2
- FAMBJGGXXNEVAR-UHFFFAOYSA-N 6-cyano-5-methylpyridine-3-carboxylic acid Chemical compound C(#N)C1=NC=C(C(=O)O)C=C1C FAMBJGGXXNEVAR-UHFFFAOYSA-N 0.000 description 2
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- 102000001381 Arachidonate 5-Lipoxygenase Human genes 0.000 description 2
- 108010093579 Arachidonate 5-lipoxygenase Proteins 0.000 description 2
- 208000032116 Autoimmune Experimental Encephalomyelitis Diseases 0.000 description 2
- 239000005711 Benzoic acid Substances 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- PGNWIJGYNRQABE-LBPRGKRZSA-N C[C@H]1CN(Cc2cc(F)cc(N)c2C)CCN1C(=O)OC(C)(C)C Chemical compound C[C@H]1CN(Cc2cc(F)cc(N)c2C)CCN1C(=O)OC(C)(C)C PGNWIJGYNRQABE-LBPRGKRZSA-N 0.000 description 2
- LBUYALIKCFHUHP-KRWDZBQOSA-N C[C@H]1CN(Cc2cc(F)cc(NC(=O)c3cccc(c3)C#N)c2C)CCN1C(=O)OC(C)(C)C Chemical compound C[C@H]1CN(Cc2cc(F)cc(NC(=O)c3cccc(c3)C#N)c2C)CCN1C(=O)OC(C)(C)C LBUYALIKCFHUHP-KRWDZBQOSA-N 0.000 description 2
- GBIVKBUFSLXYOJ-MAUKXSAKSA-N ClC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)F)CN2C[C@@H](N(CC2)C(=O)[C@H]2COCC2)C)C)C1)C Chemical compound ClC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)F)CN2C[C@@H](N(CC2)C(=O)[C@H]2COCC2)C)C)C1)C GBIVKBUFSLXYOJ-MAUKXSAKSA-N 0.000 description 2
- SELLTOADNZULHM-HNNXBMFYSA-N ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=CC(=C1)F)=O Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=CC(=C1)F)=O SELLTOADNZULHM-HNNXBMFYSA-N 0.000 description 2
- 229920002261 Corn starch Polymers 0.000 description 2
- 208000011231 Crohn disease Diseases 0.000 description 2
- 238000001712 DNA sequencing Methods 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 208000009386 Experimental Arthritis Diseases 0.000 description 2
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 2
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 101000998146 Homo sapiens Interleukin-17A Proteins 0.000 description 2
- 101000686034 Homo sapiens Nuclear receptor ROR-gamma Proteins 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 102000013691 Interleukin-17 Human genes 0.000 description 2
- 108050003558 Interleukin-17 Proteins 0.000 description 2
- 102100033461 Interleukin-17A Human genes 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 2
- 229920000881 Modified starch Polymers 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- KOFBXSAUSTXLQO-IQMFZBJNSA-N N-[5-chloro-2-methyl-3-[[(3R)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@H](N(CC1)C(=O)[C@@H]1OCCC1)C KOFBXSAUSTXLQO-IQMFZBJNSA-N 0.000 description 2
- KOFBXSAUSTXLQO-CMJOXMDJSA-N N-[5-chloro-2-methyl-3-[[(3R)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@H](N(CC1)C(=O)[C@H]1OCCC1)C KOFBXSAUSTXLQO-CMJOXMDJSA-N 0.000 description 2
- USPQTGUCNDTTBM-CRAIPNDOSA-N N-[5-chloro-2-methyl-3-[[(3R)-3-methyl-4-[(3R)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@H](N(CC1)C(=O)[C@H]1COCC1)C USPQTGUCNDTTBM-CRAIPNDOSA-N 0.000 description 2
- USPQTGUCNDTTBM-QAPCUYQASA-N N-[5-chloro-2-methyl-3-[[(3R)-3-methyl-4-[(3S)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@H](N(CC1)C(=O)[C@@H]1COCC1)C USPQTGUCNDTTBM-QAPCUYQASA-N 0.000 description 2
- BUZCHOLWUAWZJH-KRWDZBQOSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-2-cyanopyridine-4-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=NC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C BUZCHOLWUAWZJH-KRWDZBQOSA-N 0.000 description 2
- CKOBGOQZYXZFPE-IBGZPJMESA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-3-cyano-4-methylbenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=C(C=C1)C)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C CKOBGOQZYXZFPE-IBGZPJMESA-N 0.000 description 2
- QWYWHOHMGDKYNH-KRWDZBQOSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-5-cyano-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)C#N)C)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C QWYWHOHMGDKYNH-KRWDZBQOSA-N 0.000 description 2
- XFBDQIZUKQMZQP-INIZCTEOSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C XFBDQIZUKQMZQP-INIZCTEOSA-N 0.000 description 2
- BCDWRSHKPZPGFU-SFHVURJKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-6-cyano-5-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)C)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C BCDWRSHKPZPGFU-SFHVURJKSA-N 0.000 description 2
- LZNYIDFBJLOFNS-SFHVURJKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-6-ethylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)CC)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C LZNYIDFBJLOFNS-SFHVURJKSA-N 0.000 description 2
- FYJPHDOPMMSQIC-KRWDZBQOSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-6-methoxypyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)OC)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C FYJPHDOPMMSQIC-KRWDZBQOSA-N 0.000 description 2
- HTJOCHRWFJGXRW-SFHVURJKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-(oxane-4-carbonyl)piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)C)=O)C)CN1C[C@@H](N(CC1)C(=O)C1CCOCC1)C HTJOCHRWFJGXRW-SFHVURJKSA-N 0.000 description 2
- XCLJMHRWMWSOFW-UPCLLVRISA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyano-4-fluorobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=C(C=C1)F)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCC1)C XCLJMHRWMWSOFW-UPCLLVRISA-N 0.000 description 2
- IIOWMUIJVUSKCS-UPCLLVRISA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyano-5-fluorobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC(=C1)F)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCC1)C IIOWMUIJVUSKCS-UPCLLVRISA-N 0.000 description 2
- ZPOVMLBJLQEZEQ-BXKMTCNYSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCC1)C ZPOVMLBJLQEZEQ-BXKMTCNYSA-N 0.000 description 2
- YPPRSYZJDCKDNP-BXKMTCNYSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-fluoro-5-methylbenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC(=C1)C)F)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCC1)C YPPRSYZJDCKDNP-BXKMTCNYSA-N 0.000 description 2
- APOGNFGPRUXEAF-QMHKHESXSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-fluorobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)F)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCC1)C APOGNFGPRUXEAF-QMHKHESXSA-N 0.000 description 2
- IFIXQYGYOFKUQS-BXKMTCNYSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5,6-dimethylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C=1)NC(C1=CN=C(C(=C1)C)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCC1)C IFIXQYGYOFKUQS-BXKMTCNYSA-N 0.000 description 2
- NNUBTGPLWUTDED-UPCLLVRISA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5-cyano-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)C#N)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCC1)C NNUBTGPLWUTDED-UPCLLVRISA-N 0.000 description 2
- KOFBXSAUSTXLQO-NPMXOYFQSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCC1)C KOFBXSAUSTXLQO-NPMXOYFQSA-N 0.000 description 2
- XCLJMHRWMWSOFW-FYSMJZIKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyano-4-fluorobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=C(C=C1)F)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCC1)C XCLJMHRWMWSOFW-FYSMJZIKSA-N 0.000 description 2
- IIOWMUIJVUSKCS-FYSMJZIKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyano-5-fluorobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC(=C1)F)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCC1)C IIOWMUIJVUSKCS-FYSMJZIKSA-N 0.000 description 2
- ZPOVMLBJLQEZEQ-XDHUDOTRSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCC1)C ZPOVMLBJLQEZEQ-XDHUDOTRSA-N 0.000 description 2
- YPPRSYZJDCKDNP-XDHUDOTRSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-fluoro-5-methylbenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC(=C1)C)F)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCC1)C YPPRSYZJDCKDNP-XDHUDOTRSA-N 0.000 description 2
- IFIXQYGYOFKUQS-XDHUDOTRSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5,6-dimethylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C=1)NC(C1=CN=C(C(=C1)C)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCC1)C IFIXQYGYOFKUQS-XDHUDOTRSA-N 0.000 description 2
- NNUBTGPLWUTDED-FYSMJZIKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5-cyano-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)C#N)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCC1)C NNUBTGPLWUTDED-FYSMJZIKSA-N 0.000 description 2
- KOFBXSAUSTXLQO-WNSKOXEYSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCC1)C KOFBXSAUSTXLQO-WNSKOXEYSA-N 0.000 description 2
- ZQSHVXZBTNVEIK-LAUBAEHRSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCC1)C ZQSHVXZBTNVEIK-LAUBAEHRSA-N 0.000 description 2
- USPQTGUCNDTTBM-MAUKXSAKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoro-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCC1)C USPQTGUCNDTTBM-MAUKXSAKSA-N 0.000 description 2
- ZQSHVXZBTNVEIK-UWJYYQICSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3S)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1COCC1)C ZQSHVXZBTNVEIK-UWJYYQICSA-N 0.000 description 2
- RRTYFLLTXVCGFV-AWEZNQCLSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-5,6-dimethylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C=1)NC(C1=CN=C(C(=C1)C)C)=O)C)CN1C[C@@H](NCC1)C RRTYFLLTXVCGFV-AWEZNQCLSA-N 0.000 description 2
- XKIXFMDIIHVXRN-LBPRGKRZSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-5-fluoro-6-methyl-1-oxidopyridin-1-ium-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(=O)C=1C=C(C(=[N+](C1)[O-])C)F)C)CN1C[C@@H](NCC1)C XKIXFMDIIHVXRN-LBPRGKRZSA-N 0.000 description 2
- TVRITUKKXLUTHG-ZDUSSCGKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-5-methoxy-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)OC)C)=O)C)CN1C[C@@H](NCC1)C TVRITUKKXLUTHG-ZDUSSCGKSA-N 0.000 description 2
- BLRWAHSTAWELAI-AWEZNQCLSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-6-ethylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)CC)=O)C)CN1C[C@@H](NCC1)C BLRWAHSTAWELAI-AWEZNQCLSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 206010033892 Paraplegia Diseases 0.000 description 2
- 108010081690 Pertussis Toxin Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- LOUPRKONTZGTKE-WZBLMQSHSA-N Quinine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-WZBLMQSHSA-N 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- 108091023040 Transcription factor Proteins 0.000 description 2
- 102000040945 Transcription factor Human genes 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 235000010233 benzoic acid Nutrition 0.000 description 2
- 230000027455 binding Effects 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- UWTDFICHZKXYAC-UHFFFAOYSA-N boron;oxolane Chemical compound [B].C1CCOC1 UWTDFICHZKXYAC-UHFFFAOYSA-N 0.000 description 2
- 229940098773 bovine serum albumin Drugs 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 230000003081 coactivator Effects 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- DOBRDRYODQBAMW-UHFFFAOYSA-N copper(i) cyanide Chemical compound [Cu+].N#[C-] DOBRDRYODQBAMW-UHFFFAOYSA-N 0.000 description 2
- 239000008120 corn starch Substances 0.000 description 2
- 229940111134 coxibs Drugs 0.000 description 2
- 125000000392 cycloalkenyl group Chemical group 0.000 description 2
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical group C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 208000012997 experimental autoimmune encephalomyelitis Diseases 0.000 description 2
- 239000013613 expression plasmid Substances 0.000 description 2
- BRZYSWJRSDMWLG-CAXSIQPQSA-N geneticin Chemical compound O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](C(C)O)O2)N)[C@@H](N)C[C@H]1N BRZYSWJRSDMWLG-CAXSIQPQSA-N 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 125000001183 hydrocarbyl group Chemical group 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000010212 intracellular staining Methods 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 238000003670 luciferase enzyme activity assay Methods 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- KTMKRRPZPWUYKK-UHFFFAOYSA-N methylboronic acid Chemical compound CB(O)O KTMKRRPZPWUYKK-UHFFFAOYSA-N 0.000 description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 description 2
- 239000008108 microcrystalline cellulose Substances 0.000 description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 2
- 125000002950 monocyclic group Chemical group 0.000 description 2
- 125000006578 monocyclic heterocycloalkyl group Chemical group 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 150000007530 organic bases Chemical class 0.000 description 2
- 230000002018 overexpression Effects 0.000 description 2
- AVPKHOTUOHDTLW-UHFFFAOYSA-N oxane-4-carboxylic acid Chemical compound OC(=O)C1CCOCC1 AVPKHOTUOHDTLW-UHFFFAOYSA-N 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Chemical group 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- 229960001639 penicillamine Drugs 0.000 description 2
- 235000021317 phosphate Nutrition 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- 229920001592 potato starch Polymers 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- LEHBURLTIWGHEM-UHFFFAOYSA-N pyridinium chlorochromate Chemical compound [O-][Cr](Cl)(=O)=O.C1=CC=[NH+]C=C1 LEHBURLTIWGHEM-UHFFFAOYSA-N 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 229930195734 saturated hydrocarbon Natural products 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 229910052717 sulfur Chemical group 0.000 description 2
- 239000011593 sulfur Chemical group 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 238000007910 systemic administration Methods 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 229940095064 tartrate Drugs 0.000 description 2
- PHCVDOATVJUEJN-LLVKDONJSA-N tert-butyl (2R)-4-(5-chloro-2-methyl-3-nitrobenzoyl)-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(C(=O)N2C[C@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)[N+](=O)[O-] PHCVDOATVJUEJN-LLVKDONJSA-N 0.000 description 2
- BADYGPCOTZMVLR-OAHLLOKOSA-N tert-butyl (2R)-4-[[5-chloro-3-[(5-fluoro-6-methylpyridine-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C(=C1)F)C)=O BADYGPCOTZMVLR-OAHLLOKOSA-N 0.000 description 2
- VGMJZBJKBOMXBC-INIZCTEOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(5-cyano-6-methylpyridine-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C(=C1)C#N)C)=O VGMJZBJKBOMXBC-INIZCTEOSA-N 0.000 description 2
- FKGQWAOAGVXPCF-HNNXBMFYSA-N tert-butyl (2S)-4-[[5-chloro-3-[(5-fluoro-1-oxidopyridin-1-ium-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound C(C)(C)(C)OC(=O)N1[C@H](CN(CC1)CC=1C(=C(C=C(C1)Cl)NC(=O)C=1C=[N+](C=C(C1)F)[O-])C)C FKGQWAOAGVXPCF-HNNXBMFYSA-N 0.000 description 2
- KSOHGBRGKURNEH-HNNXBMFYSA-N tert-butyl (2S)-4-[[5-chloro-3-[(6-cyano-5-fluoropyridine-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C(=C1)F)C#N)=O KSOHGBRGKURNEH-HNNXBMFYSA-N 0.000 description 2
- QBHKDKLXWNYLFE-KRWDZBQOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(6-ethylpyridine-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C=C1)CC)=O QBHKDKLXWNYLFE-KRWDZBQOSA-N 0.000 description 2
- DATRVIMZZZVHMP-QMMMGPOBSA-N tert-butyl (2s)-2-methylpiperazine-1-carboxylate Chemical compound C[C@H]1CNCCN1C(=O)OC(C)(C)C DATRVIMZZZVHMP-QMMMGPOBSA-N 0.000 description 2
- UOIGNCCAYVCSKO-KRWDZBQOSA-N tert-butyl (2s)-4-[[5-chloro-2-methyl-3-[(6-methylpyridine-3-carbonyl)amino]phenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)[C@@H](C)CN1CC1=CC(Cl)=CC(NC(=O)C=2C=NC(C)=CC=2)=C1C UOIGNCCAYVCSKO-KRWDZBQOSA-N 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- FWPIDFUJEMBDLS-UHFFFAOYSA-L tin(II) chloride dihydrate Chemical compound O.O.Cl[Sn]Cl FWPIDFUJEMBDLS-UHFFFAOYSA-L 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 239000012049 topical pharmaceutical composition Substances 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- 239000013598 vector Substances 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- UVGHPGOONBRLCX-NJSLBKSFSA-N (2,5-dioxopyrrolidin-1-yl) 6-[5-[(3as,4s,6ar)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]hexanoate Chemical compound C([C@H]1[C@H]2NC(=O)N[C@H]2CS1)CCCC(=O)NCCCCCC(=O)ON1C(=O)CCC1=O UVGHPGOONBRLCX-NJSLBKSFSA-N 0.000 description 1
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- LJRDOKAZOAKLDU-UDXJMMFXSA-N (2s,3s,4r,5r,6r)-5-amino-2-(aminomethyl)-6-[(2r,3s,4r,5s)-5-[(1r,2r,3s,5r,6s)-3,5-diamino-2-[(2s,3r,4r,5s,6r)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-hydroxycyclohexyl]oxy-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]oxyoxane-3,4-diol;sulfuric ac Chemical compound OS(O)(=O)=O.N[C@@H]1[C@@H](O)[C@H](O)[C@H](CN)O[C@@H]1O[C@H]1[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](N)C[C@@H](N)[C@@H]2O)O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)N)O[C@@H]1CO LJRDOKAZOAKLDU-UDXJMMFXSA-N 0.000 description 1
- CKUHLVROMDZYID-ZDUSSCGKSA-N (3S)-1-[(5-chloro-2-methyl-3-nitrophenyl)methyl]-3-ethylpiperazine Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](NCC2)CC)C1)C)[N+](=O)[O-] CKUHLVROMDZYID-ZDUSSCGKSA-N 0.000 description 1
- OWDJJQVSMNGUPK-VIFPVBQESA-N (3s)-1-[(5-fluoro-2-methyl-3-nitrophenyl)methyl]-3-methylpiperazine Chemical compound C1CN[C@@H](C)CN1CC1=CC(F)=CC([N+]([O-])=O)=C1C OWDJJQVSMNGUPK-VIFPVBQESA-N 0.000 description 1
- POCJOGNVFHPZNS-ZJUUUORDSA-N (6S,7R)-2-azaspiro[5.5]undecan-7-ol Chemical compound O[C@@H]1CCCC[C@]11CNCCC1 POCJOGNVFHPZNS-ZJUUUORDSA-N 0.000 description 1
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 1
- DELJOESCKJGFML-DUXPYHPUSA-N (e)-3-aminobut-2-enenitrile Chemical compound C\C(N)=C/C#N DELJOESCKJGFML-DUXPYHPUSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- AUYBSFAHQLKXSW-UHFFFAOYSA-N 1,2-dichloroethane;3-(ethyliminomethylideneamino)-n,n-dimethylpropan-1-amine;hydrochloride Chemical compound Cl.ClCCCl.CCN=C=NCCCN(C)C AUYBSFAHQLKXSW-UHFFFAOYSA-N 0.000 description 1
- IGERFAHWSHDDHX-UHFFFAOYSA-N 1,3-dioxanyl Chemical group [CH]1OCCCO1 IGERFAHWSHDDHX-UHFFFAOYSA-N 0.000 description 1
- JPRPJUMQRZTTED-UHFFFAOYSA-N 1,3-dioxolanyl Chemical group [CH]1OCCO1 JPRPJUMQRZTTED-UHFFFAOYSA-N 0.000 description 1
- ILWJAOPQHOZXAN-UHFFFAOYSA-N 1,3-dithianyl Chemical group [CH]1SCCCS1 ILWJAOPQHOZXAN-UHFFFAOYSA-N 0.000 description 1
- KFHQOZXAFUKFNB-UHFFFAOYSA-N 1,3-oxathiolanyl Chemical group [CH]1OCCS1 KFHQOZXAFUKFNB-UHFFFAOYSA-N 0.000 description 1
- MUQYPLLTMWVNSU-UHFFFAOYSA-N 1,6-dimethylcyclohexa-2,4-diene-1-carboxylic acid Chemical compound CC1C=CC=CC1(C)C(O)=O MUQYPLLTMWVNSU-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- LIMXEVCFAUTBCK-UHFFFAOYSA-N 2,5-dibromo-3-methylpyridine Chemical compound CC1=CC(Br)=CN=C1Br LIMXEVCFAUTBCK-UHFFFAOYSA-N 0.000 description 1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical compound CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 1
- MSWZFWKMSRAUBD-IVMDWMLBSA-N 2-amino-2-deoxy-D-glucopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-IVMDWMLBSA-N 0.000 description 1
- WMQAQMYIZDJCDJ-UHFFFAOYSA-N 2-methyloxolane-2-carboxylic acid Chemical compound OC(=O)C1(C)CCCO1 WMQAQMYIZDJCDJ-UHFFFAOYSA-N 0.000 description 1
- BSKHPKMHTQYZBB-UHFFFAOYSA-N 2-methylpyridine Chemical compound CC1=CC=CC=N1 BSKHPKMHTQYZBB-UHFFFAOYSA-N 0.000 description 1
- LGCYVLDNGBSOOW-UHFFFAOYSA-N 2H-benzotriazol-4-ol 1-hydroxybenzotriazole Chemical compound OC1=CC=CC2=C1N=NN2.C1=CC=C2N(O)N=NC2=C1 LGCYVLDNGBSOOW-UHFFFAOYSA-N 0.000 description 1
- UUKWKUSGGZNXGA-UHFFFAOYSA-N 3,5-dinitrobenzamide Chemical compound NC(=O)C1=CC([N+]([O-])=O)=CC([N+]([O-])=O)=C1 UUKWKUSGGZNXGA-UHFFFAOYSA-N 0.000 description 1
- DVLFYONBTKHTER-UHFFFAOYSA-N 3-(N-morpholino)propanesulfonic acid Chemical compound OS(=O)(=O)CCCN1CCOCC1 DVLFYONBTKHTER-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- JMHGATOBRPWPBZ-UHFFFAOYSA-N 3-cyano-4-fluorobenzoic acid Chemical compound OC(=O)C1=CC=C(F)C(C#N)=C1 JMHGATOBRPWPBZ-UHFFFAOYSA-N 0.000 description 1
- HADZSOZVTCEMNP-UHFFFAOYSA-N 3-cyano-5-fluorobenzoic acid Chemical compound OC(=O)C1=CC(F)=CC(C#N)=C1 HADZSOZVTCEMNP-UHFFFAOYSA-N 0.000 description 1
- GYLKKXHEIIFTJH-UHFFFAOYSA-N 3-cyanobenzoic acid Chemical compound OC(=O)C1=CC=CC(C#N)=C1 GYLKKXHEIIFTJH-UHFFFAOYSA-N 0.000 description 1
- XPUFVYIUPYNLPD-UHFFFAOYSA-N 3-fluoro-5-methylbenzoic acid Chemical compound CC1=CC(F)=CC(C(O)=O)=C1 XPUFVYIUPYNLPD-UHFFFAOYSA-N 0.000 description 1
- SYVNVEGIRVXRQH-UHFFFAOYSA-N 3-fluorobenzoyl chloride Chemical compound FC1=CC=CC(C(Cl)=O)=C1 SYVNVEGIRVXRQH-UHFFFAOYSA-N 0.000 description 1
- LDDHMKANNXWUAK-UHFFFAOYSA-N 3-iodo-4-methylbenzoic acid Chemical compound CC1=CC=C(C(O)=O)C=C1I LDDHMKANNXWUAK-UHFFFAOYSA-N 0.000 description 1
- IYTJRMRETHPZAC-UHFFFAOYSA-N 4,4-dibenzylpiperidine Chemical compound C1CNCCC1(CC=1C=CC=CC=1)CC1=CC=CC=C1 IYTJRMRETHPZAC-UHFFFAOYSA-N 0.000 description 1
- RNRLTTNKVLFZJS-UHFFFAOYSA-N 5,6-dichloropyridine-3-carboxylic acid Chemical compound OC(=O)C1=CN=C(Cl)C(Cl)=C1 RNRLTTNKVLFZJS-UHFFFAOYSA-N 0.000 description 1
- 102000004023 5-Lipoxygenase-Activating Proteins Human genes 0.000 description 1
- 108090000411 5-Lipoxygenase-Activating Proteins Proteins 0.000 description 1
- CSAPESWNZDOAFU-UHFFFAOYSA-N 5-chloro-2-methylbenzoic acid Chemical compound CC1=CC=C(Cl)C=C1C(O)=O CSAPESWNZDOAFU-UHFFFAOYSA-N 0.000 description 1
- IGCUHEPJCBRXFP-UHFFFAOYSA-N 5-chloro-6-methylpyridine-3-carbonyl chloride Chemical compound ClC=1C(=NC=C(C(=O)Cl)C1)C IGCUHEPJCBRXFP-UHFFFAOYSA-N 0.000 description 1
- AUDVURYRGMHMMA-ZDUSSCGKSA-N 5-chloro-N-[5-cyano-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C(=NC=C(C(=O)NC2=C(C(=CC(=C2)C#N)CN2C[C@@H](NCC2)C)C)C1)C AUDVURYRGMHMMA-ZDUSSCGKSA-N 0.000 description 1
- JVBLXLBINTYFPR-UHFFFAOYSA-N 5-fluoro-2-methylbenzoic acid Chemical compound CC1=CC=C(F)C=C1C(O)=O JVBLXLBINTYFPR-UHFFFAOYSA-N 0.000 description 1
- BXZSBDDOYIWMGC-UHFFFAOYSA-N 5-fluoropyridine-3-carboxylic acid Chemical compound OC(=O)C1=CN=CC(F)=C1 BXZSBDDOYIWMGC-UHFFFAOYSA-N 0.000 description 1
- WMHSQCDPPJRWIL-UHFFFAOYSA-N 6-cyanopyridine-3-carboxylic acid Chemical compound OC(=O)C1=CC=C(C#N)N=C1 WMHSQCDPPJRWIL-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 208000023328 Basedow disease Diseases 0.000 description 1
- KHBQMWCZKVMBLN-UHFFFAOYSA-N Benzenesulfonamide Chemical compound NS(=O)(=O)C1=CC=CC=C1 KHBQMWCZKVMBLN-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 238000011740 C57BL/6 mouse Methods 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 1
- LUKZNWIVRBCLON-GXOBDPJESA-N Ciclesonide Chemical compound C1([C@H]2O[C@@]3([C@H](O2)C[C@@H]2[C@@]3(C[C@H](O)[C@@H]3[C@@]4(C)C=CC(=O)C=C4CC[C@H]32)C)C(=O)COC(=O)C(C)C)CCCCC1 LUKZNWIVRBCLON-GXOBDPJESA-N 0.000 description 1
- 235000001258 Cinchona calisaya Nutrition 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- KGEBYJITDCJHHF-YCRPNKLZSA-N ClC=1C=C(C(=C(C1)NC(=O)C1=CN=C(S1)C)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCCC1)C Chemical compound ClC=1C=C(C(=C(C1)NC(=O)C1=CN=C(S1)C)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1OCCCC1)C KGEBYJITDCJHHF-YCRPNKLZSA-N 0.000 description 1
- WUYDYOXJISCXTH-QYBDOPJKSA-N ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@]1(OCCC1)C)C Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@]1(OCCC1)C)C WUYDYOXJISCXTH-QYBDOPJKSA-N 0.000 description 1
- LESNYGWZJLVNMZ-INIZCTEOSA-N ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C=C1)C#N)=O Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C=C1)C#N)=O LESNYGWZJLVNMZ-INIZCTEOSA-N 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- 102000010918 Cysteinyl leukotriene receptors Human genes 0.000 description 1
- 108050001116 Cysteinyl leukotriene receptors Proteins 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- VVNCNSJFMMFHPL-VKHMYHEASA-N D-penicillamine Chemical compound CC(C)(S)[C@@H](N)C(O)=O VVNCNSJFMMFHPL-VKHMYHEASA-N 0.000 description 1
- 230000004568 DNA-binding Effects 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- 239000001692 EU approved anti-caking agent Substances 0.000 description 1
- YQYJSBFKSSDGFO-UHFFFAOYSA-N Epihygromycin Natural products OC1C(O)C(C(=O)C)OC1OC(C(=C1)O)=CC=C1C=C(C)C(=O)NC1C(O)C(O)C2OCOC2C1O YQYJSBFKSSDGFO-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 208000015023 Graves' disease Diseases 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 208000035895 Guillain-Barré syndrome Diseases 0.000 description 1
- 229910004373 HOAc Inorganic materials 0.000 description 1
- 101100005713 Homo sapiens CD4 gene Proteins 0.000 description 1
- 101000853012 Homo sapiens Interleukin-23 receptor Proteins 0.000 description 1
- 101001103036 Homo sapiens Nuclear receptor ROR-alpha Proteins 0.000 description 1
- 101001103034 Homo sapiens Nuclear receptor ROR-beta Proteins 0.000 description 1
- 101000669402 Homo sapiens Toll-like receptor 7 Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 102000001617 Interferon Receptors Human genes 0.000 description 1
- 108010054267 Interferon Receptors Proteins 0.000 description 1
- 102000003777 Interleukin-1 beta Human genes 0.000 description 1
- 108090000193 Interleukin-1 beta Proteins 0.000 description 1
- 102100036672 Interleukin-23 receptor Human genes 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- UETNIIAIRMUTSM-UHFFFAOYSA-N Jacareubin Natural products CC1(C)OC2=CC3Oc4c(O)c(O)ccc4C(=O)C3C(=C2C=C1)O UETNIIAIRMUTSM-UHFFFAOYSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 206010049567 Miller Fisher syndrome Diseases 0.000 description 1
- HZQDCMWJEBCWBR-UUOKFMHZSA-N Mizoribine Chemical compound OC1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 HZQDCMWJEBCWBR-UUOKFMHZSA-N 0.000 description 1
- 101001034845 Mus musculus Interferon-induced transmembrane protein 3 Proteins 0.000 description 1
- 241001049988 Mycobacterium tuberculosis H37Ra Species 0.000 description 1
- BKFYAHBQWWFDIE-XRHLQHRESA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2R)-2-methyloxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@]1(OCCC1)C)C BKFYAHBQWWFDIE-XRHLQHRESA-N 0.000 description 1
- BKFYAHBQWWFDIE-MYUZEXMDSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-2-methyloxolane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@]1(OCCC1)C)C BKFYAHBQWWFDIE-MYUZEXMDSA-N 0.000 description 1
- WDYQRTDNBRPCDT-BVZFJXPGSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCCC1)C WDYQRTDNBRPCDT-BVZFJXPGSA-N 0.000 description 1
- ROMZHFBAFYHUIP-HJPURHCSSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoropyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=CC(=C1)F)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCCC1)C ROMZHFBAFYHUIP-HJPURHCSSA-N 0.000 description 1
- HCSQSMBXWPTDSV-UUOWRZLLSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(2S)-oxane-2-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1OCCCC1)C HCSQSMBXWPTDSV-UUOWRZLLSA-N 0.000 description 1
- BZMGEEQFOCYTDO-PGRDOPGGSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-3-cyanobenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=CC=C1)C#N)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCCC1)C BZMGEEQFOCYTDO-PGRDOPGGSA-N 0.000 description 1
- FJDVPWUDRDOLJU-FUHWJXTLSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-fluoropyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=CC(=C1)F)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCCC1)C FJDVPWUDRDOLJU-FUHWJXTLSA-N 0.000 description 1
- OIYAOGRZLZSSDU-GHTZIAJQSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCCC1)C OIYAOGRZLZSSDU-GHTZIAJQSA-N 0.000 description 1
- DTFAEOMDBUHZPC-FXAWDEMLSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5,6-dimethylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C=1)NC(C1=CN=C(C(=C1)C)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCC1)C DTFAEOMDBUHZPC-FXAWDEMLSA-N 0.000 description 1
- LZIQBFJBWNWROB-QFBILLFUSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-cyano-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)C#N)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCC1)C LZIQBFJBWNWROB-QFBILLFUSA-N 0.000 description 1
- AUWLQLJVTMZBQI-QFBILLFUSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3R)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-methoxy-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)OC)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@H]1COCC1)C AUWLQLJVTMZBQI-QFBILLFUSA-N 0.000 description 1
- UHQDEBZYNJHRPN-YJBOKZPZSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3S)-oxane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-2-methyl-1,3-thiazole-5-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(=O)C1=CN=C(S1)C)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1COCCC1)C UHQDEBZYNJHRPN-YJBOKZPZSA-N 0.000 description 1
- DTFAEOMDBUHZPC-PXNSSMCTSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3S)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5,6-dimethylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C=1)NC(C1=CN=C(C(=C1)C)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1COCC1)C DTFAEOMDBUHZPC-PXNSSMCTSA-N 0.000 description 1
- LZIQBFJBWNWROB-LPHOPBHVSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3S)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-cyano-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)C#N)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1COCC1)C LZIQBFJBWNWROB-LPHOPBHVSA-N 0.000 description 1
- AUWLQLJVTMZBQI-LPHOPBHVSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3S)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-5-methoxy-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)OC)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1COCC1)C AUWLQLJVTMZBQI-LPHOPBHVSA-N 0.000 description 1
- CNBGPAAWDKRCMA-PXNSSMCTSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methyl-4-[(3S)-oxolane-3-carbonyl]piperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)C)=O)C)CN1C[C@@H](N(CC1)C(=O)[C@@H]1COCC1)C CNBGPAAWDKRCMA-PXNSSMCTSA-N 0.000 description 1
- AEIJXNUKJNYXTP-ZDUSSCGKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-2-cyanopyridine-4-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=NC=C1)C#N)=O)C)CN1C[C@@H](NCC1)C AEIJXNUKJNYXTP-ZDUSSCGKSA-N 0.000 description 1
- OZSCORFOBMVEBO-NSHDSACASA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-2-methyl-1,3-thiazole-5-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(=O)C1=CN=C(S1)C)C)CN1C[C@@H](NCC1)C OZSCORFOBMVEBO-NSHDSACASA-N 0.000 description 1
- XBCZESXVGNHWAE-HNNXBMFYSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-3-cyano-4-methylbenzamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CC(=C(C=C1)C)C#N)=O)C)CN1C[C@@H](NCC1)C XBCZESXVGNHWAE-HNNXBMFYSA-N 0.000 description 1
- LWPYQCXNCLGZJN-LBPRGKRZSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-5-fluoropyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=CC(=C1)F)=O)C)CN1C[C@@H](NCC1)C LWPYQCXNCLGZJN-LBPRGKRZSA-N 0.000 description 1
- UEDKLXOEMNTNNL-LBPRGKRZSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-6-cyano-5-fluoropyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)F)C#N)=O)C)CN1C[C@@H](NCC1)C UEDKLXOEMNTNNL-LBPRGKRZSA-N 0.000 description 1
- QCZVRRAJBVHBNZ-AWEZNQCLSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-6-cyano-5-methylpyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C(=C1)C)C#N)=O)C)CN1C[C@@H](NCC1)C QCZVRRAJBVHBNZ-AWEZNQCLSA-N 0.000 description 1
- RQJBHWDUEBUTSH-ZDUSSCGKSA-N N-[5-chloro-2-methyl-3-[[(3S)-3-methylpiperazin-1-yl]methyl]phenyl]-6-cyanopyridine-3-carboxamide Chemical compound ClC=1C=C(C(=C(C1)NC(C1=CN=C(C=C1)C#N)=O)C)CN1C[C@@H](NCC1)C RQJBHWDUEBUTSH-ZDUSSCGKSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 150000001204 N-oxides Chemical class 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- BSPUVYFGURDFHE-UHFFFAOYSA-N Nitramine Natural products CC1C(O)CCC2CCCNC12 BSPUVYFGURDFHE-UHFFFAOYSA-N 0.000 description 1
- 102100039614 Nuclear receptor ROR-alpha Human genes 0.000 description 1
- 102100039617 Nuclear receptor ROR-beta Human genes 0.000 description 1
- 208000003435 Optic Neuritis Diseases 0.000 description 1
- 102000016978 Orphan receptors Human genes 0.000 description 1
- 108070000031 Orphan receptors Proteins 0.000 description 1
- 206010033885 Paraparesis Diseases 0.000 description 1
- BELBBZDIHDAJOR-UHFFFAOYSA-N Phenolsulfonephthalein Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)C2=CC=CC=C2S(=O)(=O)O1 BELBBZDIHDAJOR-UHFFFAOYSA-N 0.000 description 1
- 102100038277 Prostaglandin G/H synthase 1 Human genes 0.000 description 1
- 108050003243 Prostaglandin G/H synthase 1 Proteins 0.000 description 1
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 1
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 102000042839 ROR family Human genes 0.000 description 1
- 108091082331 ROR family Proteins 0.000 description 1
- 101150042659 RORC gene Proteins 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 244000082988 Secale cereale Species 0.000 description 1
- 208000021386 Sjogren Syndrome Diseases 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 239000012505 Superdex™ Substances 0.000 description 1
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 241000610741 Tetraneuris Species 0.000 description 1
- 229910021626 Tin(II) chloride Inorganic materials 0.000 description 1
- 102000002689 Toll-like receptor Human genes 0.000 description 1
- 108020000411 Toll-like receptor Proteins 0.000 description 1
- 102100039390 Toll-like receptor 7 Human genes 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 206010046851 Uveitis Diseases 0.000 description 1
- JVVXZOOGOGPDRZ-SLFFLAALSA-N [(1R,4aS,10aR)-1,4a-dimethyl-7-propan-2-yl-2,3,4,9,10,10a-hexahydrophenanthren-1-yl]methanamine Chemical compound NC[C@]1(C)CCC[C@]2(C)C3=CC=C(C(C)C)C=C3CC[C@H]21 JVVXZOOGOGPDRZ-SLFFLAALSA-N 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- VJHCJDRQFCCTHL-UHFFFAOYSA-N acetic acid 2,3,4,5,6-pentahydroxyhexanal Chemical compound CC(O)=O.OCC(O)C(O)C(O)C(O)C=O VJHCJDRQFCCTHL-UHFFFAOYSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 239000012042 active reagent Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- IAJILQKETJEXLJ-RSJOWCBRSA-N aldehydo-D-galacturonic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-RSJOWCBRSA-N 0.000 description 1
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 229940061720 alpha hydroxy acid Drugs 0.000 description 1
- 150000001280 alpha hydroxy acids Chemical class 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- VZTDIZULWFCMLS-UHFFFAOYSA-N ammonium formate Chemical compound [NH4+].[O-]C=O VZTDIZULWFCMLS-UHFFFAOYSA-N 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- HOPRXXXSABQWAV-UHFFFAOYSA-N anhydrous collidine Natural products CC1=CC=NC(C)=C1C HOPRXXXSABQWAV-UHFFFAOYSA-N 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000011914 asymmetric synthesis Methods 0.000 description 1
- AUJRCFUBUPVWSZ-XTZHGVARSA-M auranofin Chemical compound CCP(CC)(CC)=[Au]S[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O AUJRCFUBUPVWSZ-XTZHGVARSA-M 0.000 description 1
- 229960005207 auranofin Drugs 0.000 description 1
- 230000005784 autoimmunity Effects 0.000 description 1
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 1
- 229960002170 azathioprine Drugs 0.000 description 1
- 125000002785 azepinyl group Chemical group 0.000 description 1
- 125000002393 azetidinyl group Chemical group 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 229960003270 belimumab Drugs 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 125000006580 bicyclic heterocycloalkyl group Chemical group 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- MOYGZHXDRJNJEP-UHFFFAOYSA-N buclizine Chemical compound C1=CC(C(C)(C)C)=CC=C1CN1CCN(C(C=2C=CC=CC=2)C=2C=CC(Cl)=CC=2)CC1 MOYGZHXDRJNJEP-UHFFFAOYSA-N 0.000 description 1
- 229960001705 buclizine Drugs 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- XAAHAAMILDNBPS-UHFFFAOYSA-L calcium hydrogenphosphate dihydrate Chemical compound O.O.[Ca+2].OP([O-])([O-])=O XAAHAAMILDNBPS-UHFFFAOYSA-L 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000007894 caplet Substances 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 150000005829 chemical entities Chemical class 0.000 description 1
- 239000007810 chemical reaction solvent Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 229960003728 ciclesonide Drugs 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- LOUPRKONTZGTKE-UHFFFAOYSA-N cinchonine Natural products C1C(C(C2)C=C)CCN2C1C(O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-UHFFFAOYSA-N 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 125000000259 cinnolinyl group Chemical group N1=NC(=CC2=CC=CC=C12)* 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- UTBIMNXEDGNJFE-UHFFFAOYSA-N collidine Natural products CC1=CC=C(C)C(C)=N1 UTBIMNXEDGNJFE-UHFFFAOYSA-N 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000013066 combination product Substances 0.000 description 1
- 229940127555 combination product Drugs 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 235000008504 concentrate Nutrition 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229960005168 croscarmellose Drugs 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 239000001767 crosslinked sodium carboxy methyl cellulose Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 239000003260 cyclooxygenase 1 inhibitor Substances 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- DEZRYPDIMOWBDS-UHFFFAOYSA-N dcm dichloromethane Chemical compound ClCCl.ClCCl DEZRYPDIMOWBDS-UHFFFAOYSA-N 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-M decanoate Chemical compound CCCCCCCCCC([O-])=O GHVNFZFCNZKVNT-UHFFFAOYSA-M 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 125000004431 deuterium atom Chemical group 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- 229940095079 dicalcium phosphate anhydrous Drugs 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- LTMHNWPUDSTBKD-UHFFFAOYSA-N diethyl 2-(ethoxymethylidene)propanedioate Chemical compound CCOC=C(C(=O)OCC)C(=O)OCC LTMHNWPUDSTBKD-UHFFFAOYSA-N 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 125000004852 dihydrofuranyl group Chemical group O1C(CC=C1)* 0.000 description 1
- 125000005043 dihydropyranyl group Chemical group O1C(CCC=C1)* 0.000 description 1
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 description 1
- UXGNZZKBCMGWAZ-UHFFFAOYSA-N dimethylformamide dmf Chemical compound CN(C)C=O.CN(C)C=O UXGNZZKBCMGWAZ-UHFFFAOYSA-N 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 1
- UZZWBUYVTBPQIV-UHFFFAOYSA-N dme dimethoxyethane Chemical compound COCCOC.COCCOC UZZWBUYVTBPQIV-UHFFFAOYSA-N 0.000 description 1
- CETRZFQIITUQQL-UHFFFAOYSA-N dmso dimethylsulfoxide Chemical compound CS(C)=O.CS(C)=O CETRZFQIITUQQL-UHFFFAOYSA-N 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 239000006167 equilibration buffer Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- WSJUEQKKDPVZKU-UHFFFAOYSA-N ethyl 5-fluoro-6-methylpyridine-3-carboxylate Chemical compound FC=1C(=NC=C(C(=O)OCC)C1)C WSJUEQKKDPVZKU-UHFFFAOYSA-N 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 238000003818 flash chromatography Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 210000003194 forelimb Anatomy 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 238000001030 gas--liquid chromatography Methods 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 230000014509 gene expression Effects 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 229960002442 glucosamine Drugs 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 229960004275 glycolic acid Drugs 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 239000003979 granulating agent Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 102000047214 human RORC Human genes 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- XXSMGPRMXLTPCZ-UHFFFAOYSA-N hydroxychloroquine Chemical compound ClC1=CC=C2C(NC(C)CCCN(CCO)CC)=CC=NC2=C1 XXSMGPRMXLTPCZ-UHFFFAOYSA-N 0.000 description 1
- 229960004171 hydroxychloroquine Drugs 0.000 description 1
- 239000005457 ice water Substances 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000002649 immunization Methods 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 230000002584 immunomodulator Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000003406 indolizinyl group Chemical group C=1(C=CN2C=CC=CC12)* 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 210000004969 inflammatory cell Anatomy 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- POCJOGNVFHPZNS-UHFFFAOYSA-N isonitramine Natural products OC1CCCCC11CNCCC1 POCJOGNVFHPZNS-UHFFFAOYSA-N 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 125000005956 isoquinolyl group Chemical group 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- VHOGYURTWQBHIL-UHFFFAOYSA-N leflunomide Chemical compound O1N=CC(C(=O)NC=2C=CC(=CC=2)C(F)(F)F)=C1C VHOGYURTWQBHIL-UHFFFAOYSA-N 0.000 description 1
- 229960000681 leflunomide Drugs 0.000 description 1
- 150000002617 leukotrienes Chemical class 0.000 description 1
- 238000012417 linear regression Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- AARWZYFWYNJHOQ-UHFFFAOYSA-N methyl 2-chloro-5-fluoro-6-methylpyridine-3-carboxylate Chemical compound COC(=O)C1=CC(F)=C(C)N=C1Cl AARWZYFWYNJHOQ-UHFFFAOYSA-N 0.000 description 1
- SFHNAZRNPCBTEJ-UHFFFAOYSA-N methyl 5-chloro-6-methylpyridine-3-carboxylate Chemical compound ClC=1C(=NC=C(C(=O)OC)C1)C SFHNAZRNPCBTEJ-UHFFFAOYSA-N 0.000 description 1
- ZFIHBTMALUQXAL-UHFFFAOYSA-N methyl 5-fluoro-6-methylpyridine-3-carboxylate Chemical compound COC(=O)C1=CN=C(C)C(F)=C1 ZFIHBTMALUQXAL-UHFFFAOYSA-N 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- 239000011325 microbead Substances 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 229950000844 mizoribine Drugs 0.000 description 1
- 102000035118 modified proteins Human genes 0.000 description 1
- 108091005573 modified proteins Proteins 0.000 description 1
- 125000002757 morpholinyl group Chemical group 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- ACTNHJDHMQSOGL-UHFFFAOYSA-N n',n'-dibenzylethane-1,2-diamine Chemical compound C=1C=CC=CC=1CN(CCN)CC1=CC=CC=C1 ACTNHJDHMQSOGL-UHFFFAOYSA-N 0.000 description 1
- ITOZGXQFBZMJPY-AWEZNQCLSA-N n-[5-chloro-2-methyl-3-[[(3s)-3-methylpiperazin-1-yl]methyl]phenyl]-6-methylpyridine-3-carboxamide Chemical compound C1CN[C@@H](C)CN1CC1=CC(Cl)=CC(NC(=O)C=2C=NC(C)=CC=2)=C1C ITOZGXQFBZMJPY-AWEZNQCLSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- WOOWBQQQJXZGIE-UHFFFAOYSA-N n-ethyl-n-propan-2-ylpropan-2-amine Chemical compound CCN(C(C)C)C(C)C.CCN(C(C)C)C(C)C WOOWBQQQJXZGIE-UHFFFAOYSA-N 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004593 naphthyridinyl group Chemical group N1=C(C=CC2=CC=CN=C12)* 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 208000008795 neuromyelitis optica Diseases 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- VWBWQOUWDOULQN-UHFFFAOYSA-N nmp n-methylpyrrolidone Chemical compound CN1CCCC1=O.CN1CCCC1=O VWBWQOUWDOULQN-UHFFFAOYSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-M octanoate Chemical compound CCCCCCCC([O-])=O WWZKQHOCKIZLMA-UHFFFAOYSA-M 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 239000006186 oral dosage form Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 229940116315 oxalic acid Drugs 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 125000000160 oxazolidinyl group Chemical group 0.000 description 1
- 125000003566 oxetanyl group Chemical group 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 229960003531 phenolsulfonphthalein Drugs 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 125000003386 piperidinyl group Chemical group 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229920002704 polyhistidine Polymers 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 159000000001 potassium salts Chemical class 0.000 description 1
- 229940069328 povidone Drugs 0.000 description 1
- 238000000634 powder X-ray diffraction Methods 0.000 description 1
- 238000004262 preparative liquid chromatography Methods 0.000 description 1
- 238000012746 preparative thin layer chromatography Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- UORVCLMRJXCDCP-UHFFFAOYSA-M propynoate Chemical compound [O-]C(=O)C#C UORVCLMRJXCDCP-UHFFFAOYSA-M 0.000 description 1
- 230000006920 protein precipitation Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 125000001042 pteridinyl group Chemical group N1=C(N=CC2=NC=CN=C12)* 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000004309 pyranyl group Chemical group O1C(C=CC=C1)* 0.000 description 1
- 125000003072 pyrazolidinyl group Chemical group 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- 229960000948 quinine Drugs 0.000 description 1
- ZHNFLHYOFXQIOW-LPYZJUEESA-N quinine sulfate dihydrate Chemical compound [H+].[H+].O.O.[O-]S([O-])(=O)=O.C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OC)C=C21.C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OC)C=C21 ZHNFLHYOFXQIOW-LPYZJUEESA-N 0.000 description 1
- 125000005493 quinolyl group Chemical group 0.000 description 1
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 125000006413 ring segment Chemical group 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 229940083542 sodium Drugs 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 239000012321 sodium triacetoxyborohydride Substances 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 235000011150 stannous chloride Nutrition 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 239000003270 steroid hormone Substances 0.000 description 1
- 108020003113 steroid hormone receptors Proteins 0.000 description 1
- 102000005969 steroid hormone receptors Human genes 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- TYFQFVWCELRYAO-UHFFFAOYSA-L suberate(2-) Chemical compound [O-]C(=O)CCCCCCC([O-])=O TYFQFVWCELRYAO-UHFFFAOYSA-L 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- NCEXYHBECQHGNR-QZQOTICOSA-N sulfasalazine Chemical compound C1=C(O)C(C(=O)O)=CC(\N=N\C=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-QZQOTICOSA-N 0.000 description 1
- 229960001940 sulfasalazine Drugs 0.000 description 1
- NCEXYHBECQHGNR-UHFFFAOYSA-N sulfasalazine Natural products C1=C(O)C(C(=O)O)=CC(N=NC=2C=CC(=CC=2)S(=O)(=O)NC=2N=CC=CC=2)=C1 NCEXYHBECQHGNR-UHFFFAOYSA-N 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- GFYHSKONPJXCDE-UHFFFAOYSA-N sym-collidine Natural products CC1=CN=C(C)C(C)=C1 GFYHSKONPJXCDE-UHFFFAOYSA-N 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 229960001967 tacrolimus Drugs 0.000 description 1
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 239000006068 taste-masking agent Substances 0.000 description 1
- BVWJUKIAFKMHFI-GFCCVEGCSA-N tert-butyl (2R)-4-[(3-amino-5-chloro-2-methylphenyl)methyl]-2-methylpiperazine-1-carboxylate Chemical compound NC=1C(=C(CN2C[C@H](N(CC2)C(=O)OC(C)(C)C)C)C=C(C1)Cl)C BVWJUKIAFKMHFI-GFCCVEGCSA-N 0.000 description 1
- DLEUXLJMIZVCEQ-GFCCVEGCSA-N tert-butyl (2R)-4-[(5-chloro-2-methyl-3-nitrophenyl)methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)[N+](=O)[O-] DLEUXLJMIZVCEQ-GFCCVEGCSA-N 0.000 description 1
- BCXQPOAZVWSVCS-AWEZNQCLSA-N tert-butyl (2S)-4-[[5-chloro-2-methyl-3-[(2-methyl-1,3-thiazole-5-carbonyl)amino]phenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(=O)C1=CN=C(S1)C BCXQPOAZVWSVCS-AWEZNQCLSA-N 0.000 description 1
- YNVGEBJDBFVWGT-INIZCTEOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(2-cyanopyridine-4-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CC(=NC=C1)C#N)=O YNVGEBJDBFVWGT-INIZCTEOSA-N 0.000 description 1
- VGJWCUNFNDNAJW-INIZCTEOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(3-cyano-4-fluorobenzoyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CC(=C(C=C1)F)C#N)=O VGJWCUNFNDNAJW-INIZCTEOSA-N 0.000 description 1
- XYYSNJWZHBGPFX-SFHVURJKSA-N tert-butyl (2S)-4-[[5-chloro-3-[(3-cyano-4-methylbenzoyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CC(=C(C=C1)C)C#N)=O XYYSNJWZHBGPFX-SFHVURJKSA-N 0.000 description 1
- CYZJKRXNEFRQIA-KRWDZBQOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(5,6-dimethylpyridine-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C=1)C)NC(C1=CN=C(C(=C1)C)C)=O CYZJKRXNEFRQIA-KRWDZBQOSA-N 0.000 description 1
- IQUPZMAETDNGJI-INIZCTEOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(5-methoxy-6-methylpyridine-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C(=C1)OC)C)=O IQUPZMAETDNGJI-INIZCTEOSA-N 0.000 description 1
- BIQGCYLBPWSFGJ-KRWDZBQOSA-N tert-butyl (2S)-4-[[5-chloro-3-[(6-cyano-5-methylpyridine-3-carbonyl)amino]-2-methylphenyl]methyl]-2-methylpiperazine-1-carboxylate Chemical compound ClC=1C=C(C(=C(CN2C[C@@H](N(CC2)C(=O)OC(C)(C)C)C)C1)C)NC(C1=CN=C(C(=C1)C)C#N)=O BIQGCYLBPWSFGJ-KRWDZBQOSA-N 0.000 description 1
- DATRVIMZZZVHMP-MRVPVSSYSA-N tert-butyl (2r)-2-methylpiperazine-1-carboxylate Chemical compound C[C@@H]1CNCCN1C(=O)OC(C)(C)C DATRVIMZZZVHMP-MRVPVSSYSA-N 0.000 description 1
- CTCGRXDGXGUOTE-VIFPVBQESA-N tert-butyl (2s)-2-ethylpiperazine-1-carboxylate Chemical compound CC[C@H]1CNCCN1C(=O)OC(C)(C)C CTCGRXDGXGUOTE-VIFPVBQESA-N 0.000 description 1
- GCRNGPNGNJSZCC-UHFFFAOYSA-N tert-butyl 3-amino-3a,4,6,6a-tetrahydro-1h-pyrrolo[3,4-c]pyrazole-5-carboxylate Chemical compound N1N=C(N)C2CN(C(=O)OC(C)(C)C)CC21 GCRNGPNGNJSZCC-UHFFFAOYSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- WHRNULOCNSKMGB-UHFFFAOYSA-N tetrahydrofuran thf Chemical compound C1CCOC1.C1CCOC1 WHRNULOCNSKMGB-UHFFFAOYSA-N 0.000 description 1
- 125000005958 tetrahydrothienyl group Chemical group 0.000 description 1
- WROMPOXWARCANT-UHFFFAOYSA-N tfa trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.OC(=O)C(F)(F)F WROMPOXWARCANT-UHFFFAOYSA-N 0.000 description 1
- 125000001984 thiazolidinyl group Chemical group 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 125000004568 thiomorpholinyl group Chemical group 0.000 description 1
- 210000001541 thymus gland Anatomy 0.000 description 1
- GZNAASVAJNXPPW-UHFFFAOYSA-M tin(4+) chloride dihydrate Chemical compound O.O.[Cl-].[Sn+4] GZNAASVAJNXPPW-UHFFFAOYSA-M 0.000 description 1
- AXZWODMDQAVCJE-UHFFFAOYSA-L tin(II) chloride (anhydrous) Chemical compound [Cl-].[Cl-].[Sn+2] AXZWODMDQAVCJE-UHFFFAOYSA-L 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000759 toxicological effect Toxicity 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- WLPUWLXVBWGYMZ-UHFFFAOYSA-N tricyclohexylphosphine Chemical compound C1CCCCC1P(C1CCCCC1)C1CCCCC1 WLPUWLXVBWGYMZ-UHFFFAOYSA-N 0.000 description 1
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 229940046728 tumor necrosis factor alpha inhibitor Drugs 0.000 description 1
- 239000002452 tumor necrosis factor alpha inhibitor Substances 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/50—Pyridazines; Hydrogenated pyridazines
- A61K31/501—Pyridazines; Hydrogenated pyridazines not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/506—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D241/00—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings
- C07D241/02—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings
- C07D241/04—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/06—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Dermatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
本发明涉及新的类视黄醇相关孤儿受体γ(RORγ)调节剂以及它们在治疗由RORγ介导的疾病中的用途。
Description
本发明涉及新的类视黄醇相关孤儿受体γ(RORγ)调节剂以及它们在治疗由RORγ介导的疾病中的用途。
背景技术
类视黄醇相关孤儿受体(Retinoid-related orphan receptors,ROR)是转录因子,其属于类固醇激素核受体超家族(Jetten&Joo(2006)Adv.Dev.Biol.16:313-355)。所述ROR家族由三个成员组成,ROR alpha(RORα)、ROR beta(RORβ)和ROR gamma(RORγ),其各自由单独的基因(分别是RORA、RORB和RORC)编码。ROR包含由大多数核受体所共享的四个主要结构域:N-末端A/B结构域、DNA-结合结构域、铰链结构域和配体结合结构域。各ROR基因产生数种亚型,它们的区别仅在于其N-末端A/B结构域。RORγ的两种亚型已被鉴定:RORγ1和RORγt(也称为RORγ2)。RORγ是用于描述RORγ1和/或RORγt的术语。
虽然RORγ1表达于多种组织中,包括胸腺、肌肉、肾和肝,但RORγt仅唯一地表达于免疫系统的细胞中。RORγt已经被确定为Th17细胞分化的关键调节剂。Th17细胞是产生IL-17和其他促炎细胞因子的T辅助细胞的亚类。已经显示,Th17细胞在多种小鼠自身免疫疾病模型中(包括实验性自身免疫性脑脊髓炎(EAE)和胶原诱发性关节炎(CIA))具有关键作用。此外,已经显示,Th17细胞或其产物与多种人类炎症和自身免疫疾病(包括多发性硬化、类风湿性关节炎、银屑病、强直性脊柱炎、克罗恩病和哮喘)的病理学有关(Jetten(2009)Nucl.Recept.Signal.7:e003;Manel等人(2008)Nat.Immunol.9:641-649;Miossec&Kolls(2012)Nat.Rev.Drug.Discov.10:763-776)。慢性自身免疫疾病(包括多发性硬化和类风湿性关节炎)的发病机制起因于对自身抗原耐受性的破坏和自体侵袭性效应T细胞浸润靶组织的发展。研究已显示,Th17细胞是组织特异性自身免疫中炎症过程的重要驱动因子之一(Steinman(2008)J.Exp.Med.205:1517-1522;Leung等人(2010)Cell.Mol.Immunol.7:182-189)。有证据表明,Th17细胞在疾病过程中被激活并负责募集其他炎症细胞类型,特别是嗜中性粒细胞,以介导靶组织中的病理(Korn等人(2009)Annu.Rev.Immunol.27:485-517)。
RORγt在Th17细胞的致病响应中起着决定性作用(Ivanov等人(2006)Cell 126:1121-1133)。RORγt缺陷的小鼠显示了非常少的Th17细胞。此外,RORγt缺陷导致EAE的改善。RORγt在自身免疫性或炎性疾病的发病中的作用的进一步证据可见于以下参考文献:Jetten&Joo(2006)Adv.Dev.Biol.16:313-355;Meier等人(2007)Immunity 26:643-654;Aloisi&Pujol-Borrell(2006)Nat.Rev.Immunol.6:205-217;Jager等人(2009)J.Immunol.183:7169-7177;Serafini等人(2004)Brain Pathol.14:164-174;Magliozzi等人(2007)Brain 130:1089-1104;Barnes(2008)Nat.Rev.Immunol.8:183-192;Miossec&Kolls(2012)Nat.Rev.Drug.Discov.10:763-776。
考虑到RORγ在疾病的发病学中所起的作用,期望的是制备能够调节RORγ活性的化合物,所述化合物可用于治疗RORγ介导的疾病。
发明内容
本发明涉及新RORγ调节剂和它们在治疗由RORγ介导的疾病中的用途。特别地,本发明涉及式I化合物及其药学上可接受的盐
其中R1-R7如下文所定义。
在另一方面,本发明提供了式I化合物在治疗由RORγ介导的疾病中的用途。所述疾病的实例包括自身免疫性或炎性疾病,例如多发性硬化、类风湿性关节炎、银屑病和强直性脊柱炎。还在另一方面,本发明涉及治疗所述疾病的方法。
发明详述
术语和定义
“烷基”是指具有特定成员原子数的一价饱和烃链。例如,C1-C6烷基是指具有1-6个成员原子的烷基。烷基可任选被一个或多个本申请所定义的取代基取代。烷基可为直链或支链的。代表性的支链烷基具有一个、两个或三个支链。烷基的实例包括甲基、乙基、丙基(正丙基和异丙基)、丁基(正丁基、异丁基和叔丁基)、戊基(正戊基、异戊基和新戊基)和己基。
“环烷基”是指具有特定成员原子数的饱和烃环。环烷基是单环系统或是稠合或桥连的双环系统。例如,C3-C7环烷基是指具有3-7个成员原子的环烷基。环烷基可任选被一个或多个本申请所定义的取代基取代。环烷基的实例包括环丙基、环丁基、环戊基和环己基。
“对映体过量”或“ee”是一种对映体相对于另一种过量,以百分比表示。因此,当两种对映体以相等的量存在于外消旋混合物中时,所述对映体过量为零(0%ee)。但是,如果一种对映体富集使得其构成该产物的95%,那么该对映体过量将为90%ee(富集的对映体的量,95%,减去另一对映体的量,5%)。
“对映体纯”是指其对映体过量为99%ee或更大的产物。
“半衰期”是指一半量的物质在体外或体内转化成另一化学上不同的种类所需要的时间。
“卤代”是指卤素基团氟、氯、溴和碘。
“杂芳基”是指在环中含有1-4个作为成员原子的杂原子的芳香环。含有一个以上杂原子的杂芳基可含有不同的杂原子。杂芳基可任选被一个或多个本申请所定义的取代基取代。杂芳基是单环系统,或是稠合或桥连的双环系统。单环杂芳基环具有5-7个成员原子。双环杂芳基环具有7-11个成员原子。双环杂芳基环包括其中苯基与单环杂环烷基环连接形成稠合、螺环或桥连的双环系统的那些环以及其中单环杂芳基环与单环环烷基、环烯基、杂环烷基或杂芳基环连接形成稠合、螺环或桥连的双环系统的那些环。杂芳基的实例包括吡咯基、吡唑基、咪唑基、噁唑基、异噁唑基、噁二唑基、噻唑基、异噻唑基、噻二唑基、呋喃基、呋咱基、噻吩基、三唑基、吡啶基、嘧啶基、哒嗪基、吡嗪基、三嗪基、四嗪基、四唑基、吲哚基、异吲哚基、吲嗪基、吲唑基、嘌呤基、喹啉基、异喹啉基、喹喔啉基、喹唑啉基、蝶啶基、噌啉基、苯并咪唑基、呋喃并吡啶基和二氮杂萘基。如本文所用“5-6元单环杂芳基”代表含有芳香一价单环基团的基团或部分,所述基团或部分含有5或6个环原子,包括至少1个碳原子和1-4个独立地选自氮、氧和硫的杂原子。经选择的5元单环杂芳基包含1个氮、氧或硫环杂原子和任选地含有1、2或3个额外的氮环原子。经选择的6-元单环杂芳基含有1、2或3个氮环杂原子。本发明有用的5-6元单环杂芳基的说明性的实例包括,但不限于吡咯基、吡唑基、咪唑基、噁唑基、异噁唑基、噁二唑基、噻唑基、异噻唑基、噻二唑基、呋喃基、呋咱基、噻吩基、三唑基、吡啶基、嘧啶基、哒嗪基、吡嗪基、三嗪基、四嗪基和四唑基。
“杂原子”是指氮、硫或氧原子。
“杂环烷基”是指在环中含有1-4个作为成员原子的杂原子的饱和环。但是,杂环烷基环不是芳香性的。含有一个以上杂原子的杂环烷基可含有不同的杂原子。杂环烷基可任选被本申请所定义的一个或多个取代基取代。杂环烷基是单环系统或是稠合、螺环或桥连的双环系统。单环杂环烷基环具有4-7个成员原子。双环杂环烷基环具有7-11个成员原子。杂环烷基的实例包括吡咯烷基、四氢呋喃基、二氢呋喃基、吡喃基、四氢吡喃基、二氢吡喃基、四氢噻吩基、吡唑烷基、噁唑烷基、噻唑烷基、哌啶基、高哌啶基、哌嗪基、吗啉基、硫吗啉基、氮杂环庚烯基、1,3-二氧杂环戊烷基、1,3-二氧杂环己烷基、1,3-氧硫杂环戊烷基、1,3-二噻烷基、氮杂环丁烷基、氧杂环丁烷基、氮杂双环[3.2.1]辛基和氧杂双环[2.2.1]庚基。
“成员原子”是指形成链或环的一个或多个原子。当在链中或在环内存在一个以上的成员原子时,各成员原子与该链或环中的相邻成员原子共价结合。构成链或环上取代基的原子不是该链或环的成员原子。
“任选取代的”是指基团,例如烷基、烯基、炔基、芳基、环烷基、环烯基、杂环烷基或杂芳基,可为未取代的,或所述基团可被一个或多个所定义的取代基取代。
“RORγ”是指由RORC基因编码的所有亚型,其包括RORγ1和RORγt。
“RORγ调节剂”是指直接或间接抑制RORγ的活性的化合物。RORγ调节剂包括RORγ的拮抗剂和反激动剂。
“药学上可接受的”是指在合理的医学判断范围内,适用于与人和动物的组织接触而没有过度的毒性、刺激性或其他问题或并发症,且具有合理的利益/风险比的那些化合物、材料、组合物和剂型。
提及基团“取代的”时,表示与在基团中的成员原子相连的一个或多个氢原子被选自所定义的取代基的取代基替换。应当理解的是术语“取代的”包括隐含规定,即所述取代符合所取代的原子和取代基的允许的化合价,以及所述取代产生稳定的化合物(即,不会自发地通过诸如重排、环化或消除进行转化,且足以稳固以便经得起从反应混合物中分离的化合物)。当陈述一个基团可含有一个或多个取代基时,所述基团中的一个或多个(适当时)成员原子可被取代。此外,基团中的单个的成员原子可取代有超过一个取代基,只要这样的取代符合所述原子的允许的化合价。
化合物
本发明提供了式I化合物或其药学上可接受的盐。
其中:
R1为:
-5-6元单环杂芳基,其任选被以下取代:i)C1-C5烷基,其任选被CF3或CN取代,ii)CH2F;或iii)1-2个独立地选自以下的取代基:卤素、甲基、甲氧基和CN;其中所述5-6元单环杂芳基选自:吡咯基、吡唑基、咪唑基、噁唑基、异噁唑基、噁二唑基、噻唑基、异噻唑基、噻二唑基、呋喃基、呋咱基、噻吩基、三唑基、吡啶基、嘧啶基、哒嗪基、吡嗪基、三嗪基、四嗪基和四唑基,或其N-氧化物;或
-苯基,其被1-2个独立地选自以下的取代基取代:CN、卤素和甲基;
R2为C1-C3烷基;
R3为卤素;
R4为H;
R5为C1-C3烷基;
R6为H或甲基;和
R7为四氢呋喃基或四氢吡喃基,其中所述四氢呋喃基或四氢吡喃基任选被甲基取代。
在一个实施方案中,本发明涉及式I化合物,其中:
R1为-5-6元单环杂芳基,其任选被以下取代:i)C1-C5烷基,其任选被CF3或CN取代,ii)CH2F;或iii)1-2个独立地选自以下的取代基:卤素、甲基、甲氧基和CN;或
-苯基,其被1-2个独立地选自以下的取代基取代:CN、卤素和甲基;
R2为C1-C3烷基;
R3为卤素;
R4为H;
R5为C1-C3烷基;
R6为H或甲基;和
R7为四氢呋喃基或四氢吡喃基,其中所述四氢呋喃基或四氢吡喃基任选被甲基取代。
在一个实施方案中,本发明涉及式I化合物,其中R1为:
-噻唑基或吡啶基,其任选被以下取代:i)C1-C5烷基,其任选被CF3或CN取代,ii)CH2F;或iii)1-2个独立地选自以下的取代基:卤素、甲基、甲氧基和CN;或
-苯基,其被1-2个独立地选自以下的取代基取代:CN、卤素和甲基。
在一个实施方案中,本发明涉及式I化合物,其中R1为被1或2个选自CN和卤素的取代基取代的苯基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为被CN取代的苯基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为被CN和F取代的苯基。
在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为6元单环杂芳基,其被2个独立地选自以下的取代基取代:甲基、卤素、CN和甲氧基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为被2个独立地选自以下的取代基取代的吡啶基:甲基、卤素、CN和甲氧基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为被2个独立地选自以下的取代基取代的吡啶基:甲基、F和CN。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为被甲基和F取代的吡啶基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为被甲基和Cl取代的吡啶基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为被甲基和CN取代的吡啶基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R1为被CN和F取代的吡啶基。
在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R2为甲基。
在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R3为Cl。
在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R5为甲基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R5为乙基。
在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R6为H。
在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R7为四氢呋喃基,其任选被甲基取代。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R7为四氢呋喃基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R7为四氢吡喃基。在一个实施方案中,本发明还涉及任意上述实施方案的化合物,其中R7为甲基四氢呋喃基。
在一个实施方案中,本发明涉及式(I)化合物,其中R1为被以下取代的吡啶基:i)甲基和CN或ii)甲基和Cl,R2为甲基,R3为Cl,R4为H,R5为甲基,R6为H和R7为四氢呋喃基。
在一个实施方案中,本发明涉及式(I)化合物,其中R1为被甲基和F取代的吡啶基,R2为甲基,R3为Cl,R4为H,R5为甲基,R6为H和R7为四氢呋喃基。
在另一实施方案中,本发明涉及式(I)化合物,其中R1为被CN取代的苯基,R2为甲基,R3为Cl,R4为H,R5为甲基,R6为H和R7为四氢呋喃基或四氢吡喃基。
在另一实施方案中,本发明涉及式(I)化合物,其中R1为被CN和F取代的苯基,R2为甲基,R3为Cl,R4为H,R5为甲基,R6为H和R7为四氢呋喃基。
在一个实施方案中,所述式I化合物选自:
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
3-氰基-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺;
3-氰基-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺;
(S)-3-氰基-N-(5-氟-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺;
3-氰基-N-(5-氟-2-甲基-3-(((3S)-3-甲基-4-(四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺;
3-氰基-N-(5-氟-2-甲基-3-(((3S)-3-甲基-4-(四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺;
N-(5-氯-2-甲基-3-(((3S)-3-甲基-4-(四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((3S)-3-甲基-4-(四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-乙基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-乙基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-甲基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-甲基苯甲酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-乙基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-2-氰基异烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-2-氰基异烟酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-氟烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-氟烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-2-甲基四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-2-甲基四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-2-甲基四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-2-甲基四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟苯甲酰胺;
3-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟-4-甲基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟-4-甲基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3,5-二氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3,5-二氟苯甲酰胺;
3-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟-5-甲基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟-5-甲基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺;
N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺;
N-(5-氯-3-(((S)-3-乙基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)-2-甲基苯基)-5-氟-6-甲基烟酰胺;
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)烟酰胺;
5-氟-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺;
5-氯-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
5-氯-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-氰基烟酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-2-氰基异烟酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-甲基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-(氟甲基)烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-(氟甲基)烟酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-甲氧基烟酰胺;
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
5-氯-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
5-氯-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-3-(((S)-3-乙基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)-2-甲基苯基)-6-甲基烟酰胺;
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-甲氧基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-甲氧基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;和
5-((5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)氨基甲酰基)-3-氟-2-甲基吡啶1-氧化物;
或其药学上可接受的盐。
在另一实施方案中,所述式I化合物选自:
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;和
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;
或其药学上可接受的盐。
在另一实施方案中,所述式I化合物选自:
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-甲氧基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-甲氧基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;和
5-((5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)氨基甲酰基)-3-氟-2-甲基吡啶1-氧化物;
或其药学上可接受的盐。
式I化合物可含有一个或多个不对称中心(也称为手性中心),因此可以单独的对映异构体、非对映异构体或其它立体异构体的形式或以其混合物的形式存在。手性中心诸如手性碳原子也可存在于取代基诸如烷基中。当存在于式I或本申请所说明的任何化学结构中的手性中心的立体化学未指明时,该结构意在包含所有单独的立体异构体及其所有的混合物。因此,含有一个或多个手性中心的式I化合物可以以外消旋混合物、对映异构体富集混合物或对映异构体纯的单独立体异构体存在。
含有一个或多个不对称中心的根据式I的化合物的单独立体异构体可以通过本领域技术人员已知的方法拆分。例如,可如下进行所述拆分:(1)通过形成非对映异构体盐、复合物或其它衍生物;(2)通过与立体异构体特异性试剂的选择性反应,例如通过酶促氧化或还原;或(3)通过在手性环境中的气-液色谱或液相色谱,所述手性环境例如在手性载体(例如连接有手性配体的硅胶)或在手性溶剂存在下。本领域技术人员将会理解,当将所需立体异构体通过上述分离方法之一转化成另一化学实体时,需要其它步骤来释放所需形式。或者,特异性立体异构体可通过使用光学活性试剂、基质、催化剂或溶剂的不对称合成法来合成,或通过不对称转化将一种对映异构体转化成另一种异构体。
式I的化合物也可含有双键或其它几何不对称中心。当存在于式I或本申请所说明的任何化学结构中的几何不对称中心的立体化学未指明时,所述结构旨在涵盖反式(E)几何异构体、顺式(Z)几何异构体,以及其所有的混合物。同样,所有互变异构形式也包括在式I中,不论这样的互变异构体以平衡形式还是主要以一种形式存在。
在一些实施方案中,式I化合物可以以游离碱或游离酸存在。
在某些实施方案中,式I的化合物可含有酸性官能团。在某些其他实施方案中,式I的化合物可含有碱性官能团。由此,本领域技术人员将理解,可制备式I化合物的药学上可接受的盐。实际上,在本发明的某些实施方案中,式I化合物的药学上可接受的盐相对于各自的游离碱或游离酸可能是优选的,因为这样的盐可赋予所述分子更大的稳定性或溶解性,由此促进配制为剂型。因此,本发明还涉及式I的化合物的药学上可接受的盐的用途。
本申请使用的术语"药学上可接受的盐"是指这样的盐,其保留主题化合物所需的生物活性并表现出最小的非期望的毒理学效应。这些药学上可接受的盐可以在化合物的最终分离和纯化过程中原位制备,或通过分别使呈其游离酸或游离碱形式的纯化的化合物分别与合适的碱或酸反应来制备。合适的药学上可接受的盐包括Berge、Bighley和Monkhouse,J.Pharm.Sci.(1977)66,第1-19页中描述的那些。
含有碱性胺或其他碱性官能团的所公开化合物的盐可通过本领域已知的任意合适方法进行制备,包括将该游离碱用无机酸或有机酸进行处理,所述无机酸例如盐酸、氢溴酸、硫酸、硝酸、磷酸等,所述有机酸例如乙酸、三氟乙酸、马来酸、琥珀酸、扁桃酸、富马酸、丙二酸、丙酮酸、草酸、羟乙酸、水杨酸、吡喃糖苷酸(例如葡糖醛酸或半乳糖醛酸)、α-羟基酸(例如柠檬酸或酒石酸)、氨基酸(例如天冬氨酸或谷氨酸)、芳香酸(例如苯甲酸或肉桂酸)、磺酸(例如对甲苯磺酸、甲磺酸、乙磺酸等)。药学上可接受的盐的实例包括硫酸盐、焦硫酸盐、硫酸氢盐、亚硫酸盐、亚硫酸氢盐、磷酸盐、氯化物、溴化物、碘化物、乙酸盐、丙酸盐、癸酸盐、辛酸盐、丙烯酸盐、甲酸盐、异丁酸盐、己酸盐、庚酸盐、丙炔酸盐、草酸盐、丙二酸盐、琥珀酸盐、辛二酸盐、癸二酸盐、富马酸盐、马来酸盐,丁炔-1,4-二酸盐、己炔-1,6-二酸盐、苯甲酸盐、氯苯甲酸盐、甲基苯甲酸盐、二硝基苯甲酸盐、羟基苯甲酸盐、甲氧基苯甲酸盐、酞酸盐、苯基乙酸盐、苯基丙酸盐、苯基丁酸盐、柠檬酸盐、乳酸盐、γ-羟基丁酸盐、乙醇酸盐、酒石酸盐、扁桃酸盐和磺酸盐,例如二甲苯磺酸盐、甲磺酸盐、丙磺酸盐、萘-1-磺酸盐和萘-2-磺酸盐。
含有酸性官能团的所公开化合物的盐可通过与合适的碱反应进行制备。这种药学上可接受的盐可用提供药学上可接受的阳离子的碱制备,其包括碱金属盐(特别是钠盐和钾盐)、碱土金属盐(特别是钙盐和镁盐)、铝盐和铵盐,以及由生理学可接受的有机碱制成的盐,所述有机碱例如三甲胺、三乙胺、吗啉、吡啶、哌啶、甲基吡啶、二环己胺、N,N’-二苄基乙二胺、2-羟基乙胺、双-(2-羟基乙基)胺、三-(2-羟基乙基)胺、普鲁卡因、二苄基哌啶、去氢枞胺、N,N’-双去氢枞胺、葡萄糖胺、N-甲基葡萄糖胺、可力丁、胆碱、奎宁、喹啉和碱性氨基酸(例如赖氨酸和精氨酸)。
不是药学上可接受的其他盐可用于制备本发明化合物并且这些应被认为形成本发明的其他方面。这些盐,例如三氟乙酸盐,尽管其本身不是药学上可接受的,但可用于制备在获得本发明化合物和它们的药学上可接受的盐中用作中间体的盐。
如果将含有碱性胺或其他碱性官能团的本发明化合物作为盐分离,则该化合物相应的游离碱形式可通过本领域已知的任意合适的方法进行制备,包括用无机或有机碱处理该盐,适当的无机或有机碱具有比该化合物游离碱形式更高的pKa。同样地,如果含有酸性官能团的本发明化合物作为盐分离,那么该化合物的相应的游离酸形式可通过本领域已知的任意合适的方法进行制备,包括将该盐用无机或有机酸处理,适当的无机或有机酸具有比该化合物游离酸形式更低的pKa。
本申请所用术语“本发明化合物”是指式I化合物(作为游离碱或游离酸)及其药学上可接受的盐。术语“本发明化合物”在本申请中也出现并且是指式I化合物(作为游离碱或游离酸)和它的药学上可接受的盐。
本发明还包括各种氘化形式的式(I)化合物。与碳原子连接的各个可用的氢原子可独立地被氘原子替换。本领域技术人员将知晓如何合成氘化形式的式(I)化合物。在制备氘代形式的式(I)化合物时可使用市售的氘代起始物质,或它们可使用常规技术采用氘代试剂(例如氘代氢化锂铝)合成。
本发明化合物可以固体或液体形式存在。在固态时,本发明化合物可以晶体或非晶体形式或它们的混合物存在。对于呈晶体形式的本发明化合物,本领域技术人员将理解,可形成药学上可接受的溶剂合物,其中在结晶时溶剂分子被掺入晶格中。溶剂合物可包含非水溶剂例如乙醇、异丙醇、DMSO、乙酸、乙醇胺和乙酸乙酯,或者它们可包含水作为溶剂(该溶剂被掺入到晶格中)。其中水为溶剂(该溶剂被并入晶格中)的溶剂合物通常称为“水合物”。水合物包括化学计量的水合物以及含有可变量水的组合物(compositions)。本发明包括所有该类溶剂合物。
本领域技术人员还将理解,可以晶体形式(包括其各种溶剂合物)存在的本发明的某些化合物也可显示多晶型(即出现不同晶体结构的能力)。这些不同晶型,通常称为“多晶型物”。本发明包括所有这样的多晶型物。多晶型物有相同的化学组成,但是不同的堆积(packing)、几何排列和其它可描述的晶体固态的性质。因此,多晶型物可具有不同的物理性质,例如形状、密度、硬度、可变形性、稳定性和溶解性质。多晶型物通常具有不同的熔点、IR光谱和X-射线粉末衍射图,其可用于鉴定。本领域技术人员将会理解,可制备不同多晶型物,例如,通过改变或调整在制备所述化合物中所用的条件或试剂。例如,温度、压力或溶剂的变化可产生多晶型物。此外,一种多晶型物在某些条件下可自发转化成另一种多晶型物。
式I化合物及其药学上可接受的盐可单独使用或与其他治疗剂组合使用。因此本发明的组合疗法包括给药至少一种式I化合物或其药学上可接受的盐并使用至少一种其他治疗活性剂。式I化合物或其药学上可接受的盐以及所述其他治疗活性剂可在单一药物组合物中一起给药或分开给药,并且当分开给药时,可同时进行或以任意顺序连续进行。
在另一方面,提供了组合产品,其包含式I化合物或其药学上可接受的盐,以及一种或多种其他治疗活性剂和任选的药学上可接受的载体或赋形剂。
合适的其他治疗剂包括,但不限于,(1)TNF-α抑制剂;(2)非选择性COX-1/COX-2抑制剂;(3)COX-2抑制剂;(4)用于治疗炎性和自身免疫性疾病的其他药剂,包括糖皮质激素、甲氨蝶呤、来氟米特、柳氮磺胺吡啶、硫唑嘌呤、环孢菌素、他克莫司、青霉胺、布西拉明、阿克他利、咪唑立宾、氯苯扎利、环索奈德、羟氯喹、d-青霉胺、金硫代苹果酸盐、金诺芬或胃肠外或口服金、环磷酰胺、Lymphostat-B、BAFF/APRIL抑制剂,例如belimumab和CTLA-4-Ig或其模拟物;(5)白三烯生物合成抑制剂,5-脂氧合酶(5-LO)抑制剂或5-脂氧合酶活化蛋白(FLAP)拮抗剂;(6)LTD4受体拮抗剂;(7)PDE4抑制剂;(8)抗组胺H1受体拮抗剂;(9)a1-和a2-肾上腺素受体激动剂;(10)抗胆碱能药物;(11)β-肾上腺素受体激动剂;(12)胰岛素样生长因子I型(IGF-1)模拟物;(13)糖皮质激素;(14)激酶抑制剂,例如Janus激酶(JAK1和/或JAK2和/或JAK3和/或TYK2)、p38MAPK和IKK2的抑制剂;(15)B细胞靶生物剂(B-celltargeting biologies),例如利妥昔单抗;(16)选择性共刺激调节剂,例如阿巴他塞;(17)白介素抑制剂,例如IL-1抑制剂阿那白滞素、IL-6抑制剂托珠单抗(tocilizumab)或sirukumab、IL-12/IL-23抑制剂ustekinumab、IL-23抑制剂guselkumab和抗-IL17抗体;(18)抗-GM-CSF抗体;(19)检查点阻断(checkpoint blockade)和其他免疫疗法,例如抗-PD-1/抗-PD-L1抗体,包括pembrolizumab和nivolumab和抗-CTLA4抗体,包括易普利姆玛(ipilimumab);(20)BET抑制剂,例如GSK525762;和(21)其他肿瘤药物,例如氟尿嘧啶、贝伐单抗、盐酸依立替康、卡培他滨、西妥昔单抗、雷莫芦单抗(ramucirumab)、奥沙利铂、亚叶酸钙、帕尼单抗(panitumumab)、瑞戈非尼(regorafenib)、阿柏西普(ziv-aflibercept)、曲妥珠单抗、甲磺酸伊马替尼、苹果酸舒尼替尼、甲苯磺酸索拉非尼、紫杉醇、依维莫司、盐酸厄洛替尼、盐酸吉西他滨、丝裂霉素C、达拉非尼(dabrafenib)、曲美替尼(trametinib)、拉帕替尼(lapatinib)、奥法木单抗(ofatumumab)、托泊替康(topotecan)、盐酸多柔比星和依鲁替尼(ibrutinib)。
化合物的制备
式I的化合物可使用常规有机合成制备。合适的合成途径描述在下文的以下一般反应方案中。
本领域技术人员将理解,若本申请描述的取代基与本申请所述的合成方法不相容,则可用对反应条件稳定的合适的保护基对所述取代基进行保护。可在反应顺序中的合适点脱除保护基,从而提供期望的中间体或目标化合物。合适的保护基和使用所述合适的保护基对不同的取代基进行保护和脱保护的方法对本领域技术人员而言是熟知的;其实例可参见T.Greene and P.Wuts,Protecting Groups in Chemical Synthesis(第3版),JohnWiley&Sons,NY(1999)。在一些情形中,可具体选择在使用的反应条件下具有反应性的取代基。在这些情况中,所述反应条件将所选的取代基转化为另一种取代基,所述另一种取代基要么是在中间体化合物中有用的,要么是靶标化合物中期望的取代基。
方案1
[示例性条件:a)BH3·THF,THF,0℃-RT;b)PCC,CH2Cl2;c)NaBH(OAc)3,HOAc,DCM,3;d)Pd,H2,乙醇,RT;e)R1CO2H,HOBt,EDC,DMF;f)TFA,DCM;g)R7CO2H,HOBt,EDC,DMF]。
方案1代表制备式I化合物的一般反应方案,其中R1-R7如上文所定义。所述起始材料或试剂是市售可得的或是使用本领域技术人员已知的方法由市售可得的起始材料制备的。
苯甲酸1可由BH3·THF还原,以提供苯甲醇2。醇2可被PCC氧化成相应的醛,然后用3还原胺化,以提供硝基化合物4。在H2的存在下,用Pd还原硝基化合物4得到胺,将其与各种酸反应得到酰胺5。5的Boc保护通过用TFA处理脱除并将所得胺与各种酸反应,以提供最终的式I化合物。
方案2
[示例性条件:a)TFA,DCM,RT;b)HATU,DIPEA,DMF;c)SnCl2.2H2O,乙醇,RT;d)R1CO2H,HATU,DIPEA,DMF]。
方案2代表制备式I化合物的另一反应方案,其中R1-R7如上文所定义。所述起始材料或试剂是市售可得的或是使用本领域技术人员已知的方法由市售可得的起始材料制备的。
通过TFA脱除硝基化合物1上的Boc保护,以提供硝基胺2,然后可将其与各种酸反应,得到硝基酰胺3。通过氯化锡(II)脱水物(dehydrate)将所述硝基还原成胺,得到关键中间体4,然后将其与各种酸缩合,得到最终式I化合物。
实施例
缩写
ACN 乙腈
DCE 1,2-二氯乙烷
DCM 二氯甲烷
DIPEA N,N-二异丙基乙胺
DMAP N,N-二甲基吡啶-4-胺
DME 1,2-二甲氧基乙烷
DMF N,N-二甲基甲酰胺
DMSO 二甲基亚砜
DPPP 1,3-双(二苯基膦)丙烷
EA 乙酸乙酯
EDC N-(3-二甲基氨基丙基)-N′-乙基碳二亚胺盐酸盐
ESI 电喷雾离子化
HATU O-(7-氮杂苯并三唑-1-基)-N,N,N′,N′-四甲基脲六氟磷酸盐
HOBt 羟基苯并三唑
HPLC 高效液相色谱
LCMS 液相色谱质谱
MDAP 质谱导向的自动化制备液相色谱
MS 质谱
NMP N-甲基-2-吡咯烷酮
PE 石油醚
PCC 氯铬酸吡啶
PG 保护基
RT 室温
sat. 饱和
SM 起始材料
TEA 三乙胺
TFA 三氟乙酸
TFAA 三氟乙酸酐
THF 四氢呋喃
TMSCN 三甲基甲硅烷基氰化物
色谱
除非另外提及,所有色谱使用硅胶柱进行。
LCMS条件:
1)酸性条件:
移动相:含0.05%TFA的水/乙腈
柱:Agilent SB-C18 4.6x30mm 1.8m;
检测:MS和光电二极管阵列检测器(PDA)
2)碱性条件:
移动相:10mM NH4HCO3水溶液/乙腈
柱:Waters XBridge C18 4.6x50mm 3.5m;
检测:MS和光电二极管阵列检测器(PDA)
MDAP条件:
1)酸性条件:
仪器:Waters质量导向的自动纯化系统
柱:Waters Sunfire Prep C18柱(5um,19x50mm)
移动相:含0.05%TFA的水/乙腈
2)碱性条件:
仪器:质量导向的自动纯化系统
柱:Xbridge Prep C18柱(5um,19x50mm)
移动相:含0.05%氨的水/乙腈
在以下步骤中,各起始材料后通常提及了中间体。这仅是为了帮助本领域化学人员。所述起始材料可能不一定是从所提及的批次制备的。
描述1
5,6-二氯烟酸甲酯(D1)
将5,6-二氯烟酸(5g)和亚硫酰氯(3.10g)在甲醇(20mL)中的混合物在25℃搅拌过夜。加入冷水(100mL)并将所得混合物用饱和NaHCO3溶液中和。将该水层用DCM(2×100mL)萃取被将合并的有机层用Na2SO4干燥。过滤后,将该滤液真空浓缩,得到标题化合物(5g),其为白色固体。MS(ESI):C7H5Cl2NO2理论值205;实测值206[M+H]+。
描述2
5,6-二甲基烟酸甲酯(D2)
将K2CO3(1.342g)、三环己基膦(0.272g)、Pd2(dba)3(0.444g)、甲基硼酸(0.291g)和5,6-二氯烟酸甲酯(D1,1g)在1,4-二噁烷(20mL)中的混合物加热至110℃过夜。加入冷水(30mL)并将该水层用DCM(2×100mL)萃取。将合并的有机层用Na2SO4干燥,过滤并真空浓缩。将所得残余物用柱色谱纯化(用EA:PE=0%-50%洗脱),得到标题化合物(1g),其为黄色油。MS(ESI):C9H11NO2理论值165;实测值166[M+H]+。
描述3
5,6-二甲基烟酸(D3)
将氢氧化钠(121mg)和5,6-二甲基烟酸甲酯(D2,500mg)在甲醇(10mL)和水(10mL)中的混合物搅拌2小时。加入冷水(50mL)并将所得混合物的pH用HCl溶液(7M)调节至5。将该水层用DCM(2×100mL)萃取。将合并的有机层用Na2SO4干燥,过滤并真空浓缩,得到标题化合物(400mg),其为白色固体。MS(ESI):C8H9NO2理论值151;实测值152[M+H]+。
描述4
5-氯-6-甲基烟酸甲酯(D4)
将5,6-二氯烟酸甲酯(D1,2g)、甲基硼酸(0.581g)、K2CO3(2.68g)和Pd(PPh3)4(0.561g)在1,4-二噁烷(100mL)中的混合物在75℃搅拌过夜。将所得混合物过滤并将该滤液真空浓缩,得到粗产物,将其用柱色谱进一步纯化(用EA:PE=50%-100%洗脱),得到标题化合物(420mg),其为黄色固体。MS(ESI):C8H8ClNO2理论值185;实测值186[M+H]+。
描述5
5-氯-6-甲基烟酸(D5)
将5-氯-6-甲基烟酸甲酯(D4,450mg)、氢氧化钠(485mg)在甲醇(20mL)和水(5mL)中的混合物在室温搅拌1小时。使用HCl溶液(4M)以调节pH值至4。将该溶液浓缩并用EA(20mL)萃取。将该有机相用水(2×10mL)洗涤,用Na2SO4干燥并真空浓缩,得到标题化合物(400mg),其为白色固体。MS(ESI):C7H6ClNO2理论值171;实测值172[M+H]+。
描述6
5-溴-3-甲基-2-氰基吡啶(D6)
向2,5-二溴-3-甲基吡啶(5g)在DMF(20mL)中的溶液中加入氰化亚铜(1.785g)。将该混合物在120℃搅拌过夜,然后冷却至室温。将该混合物分配至EA(50mL)和水(50mL)之间。将该有机层用盐水(50mL)洗涤,用Na2SO4干燥并真空浓缩。将所得残余物用柱色谱纯化(用EA:PE=20%洗脱),得到标题化合物(600mg),其为白色固体。MS(ESI):C7H5BrN2理论值195;实测值196[M+H]+。
描述7
6-氰基-5-甲基烟酸甲酯(D7)
将5-溴-3-甲基-2-氰基吡啶(D6,700mg)、Pd(OAc)2(160mg)、DPPP(394mg)和TEA(1.486mL)在甲醇(12mL)和DMF(3mL)中的混合物在CO气氛下(10atm)加热至120℃,持续12小时。冷却至室温后,将该混合物真空浓缩。将该残余物用柱色谱纯化(用EA:PE=20%洗脱),得到标题化合物(300mg),其为粘性油状物。MS(ESI):C9H8N2O2理论值176;实测值177[M+H]+。
描述8
6-氰基-5-甲基烟酸(D8)
将6-氰基-5-甲基烟酸甲酯(D7,250mg)和LiOH(68.0mg)在THF(15mL)和水(5mL)中的混合物在室温搅拌过夜。将该混合物分配至水(10mL)和EA(16mL)之间。将该水相用HCl溶液(1M)酸化以调节pH至约6,然后用EA(20mL)萃取。将所得有机相用Na2SO4干燥,过滤并真空浓缩,得到标题化合物(160mg),其为淡色固体。MS(ESI):C8H6N2O2理论值162;实测值163[M+H]+。
描述9
3-羧基-5-氟吡啶1-氧化物(D9)
向5-氟烟酸(2g)在(CH3CO)2O(5mL)和乙酸(5mL)中的溶液中加入过氧化氢水溶液(30%,4.82g)。将该混合物在110℃搅拌2小时,然后冷却至室温。加入水(50mL)。将该混合物用EA(3×50mL)萃取。将合并的有机层用饱和NaHCO3溶液(50mL)、水(50mL)和盐水(50mL)洗涤。将该溶液用MgSO4干燥并真空蒸发,得到标题化合物(2g),其为白色固体。MS(ESI):C6H4FNO3理论值157;实测值158[M+H]+。
描述10
5-氰基-2-羟基-6-甲基烟酸乙酯(D10)
在圆底烧瓶中,将2-(乙氧基亚甲基)丙二酸二乙酯(21.6g)和(E)-3-氨基丁-2-烯腈(8.20g)的混合物在150℃搅拌2小时并静置过夜。将该混合物过滤。将沉淀物用冰冷甲醇洗涤,得到标题化合物(5g),其为黄色固体。MS(ESI):C10H10N2O3理论值206;实测值207[M+H]+。
描述11
2-氯-5-氰基-6-甲基烟酸乙酯(D11)
在圆底烧瓶中,将5-氰基-2-羟基-6-甲基烟酸乙酯(D10,3mg)和三氯氧磷(22.3mg)的混合物在90℃搅拌5小时并静置过夜。将该溶液真空浓缩。将该残余物倒至冰上。将所得混合物过滤,得到标题化合物(3g),其为黄色固体。MS(ESI):C10H9ClN2O2理论值224;实测值225[M+H]+。
描述12
5-氰基-6-甲基烟酸乙酯(D12)
向2-氯-5-氰基-6-甲基烟酸乙酯(D11,1.5g)、甲醇(50mL)和钯(10%在碳上,0.071g)的混合物中加入甲酸铵(6.32g)。将该混合物在室温搅拌3小时,然后过滤。将该溶液真空浓缩。将该残余物用柱色谱纯化(用PE:EA=20%洗脱),得到标题化合物(1g),其为白色固体。MS(ESI):C10H10N2O2理论值190;实测值191[M+H]+。
描述13
5-氰基-6-甲基烟酸(D13)
向5-氰基-6-甲基烟酸乙酯(D12,1g)、甲醇(15mL)和水(30mL)的混合物中加入氢氧化钠(2.103g)。将该混合物在室温搅拌30min。将该溶液的pH用盐酸调节至4。将该混合物用EA(2×100mL)洗涤。将合并的有机层真空浓缩,得到标题化合物(800mg),其为白色固体。1H NMR(400MHz,MeOD-d4):9.20(s,1H),8.62(s,1H),2.83(s,3H)。MS(ESI):C8H6N2O2理论值162;实测值163[M+H]+。
描述14
2,6-二氯-5-氟烟酸甲酯(D14)
在室温,向2,6-二氯-5-氟烟酸(5g)和一滴DMF在DCM(20mL)中的混合物中滴加草酰氯(5mL)。将该混合物在室温搅拌1小时,然后浓缩。将所得酰氯再次溶于DCM(10mL)中,然后滴加至DCM(20mL)和MeOH(20mL)的混合物中。将所得混合物在室温再搅拌1小时,然后浓缩,得到标题化合物(6g),其为油状物。MS(ESI):C7H4Cl2FNO2理论值223;实测值224[M+H]+。
描述15
2-氯-5-氟-6-甲基烟酸甲酯(D15)
将2,6-二氯-5-氟烟酸甲酯(D14,6g)、2,4,6-三甲基-1,3,5,2,4,6-三氧杂三硼杂己环(trioxatriborinane)(3.36g)、K2CO3(9.99g)和Pd(Ph3P)4(1.548g)在1,4-二噁烷(50mL)中的混合物加热至110℃,持续20小时。将该混合物过滤并将该滤液浓缩。将该残余物用柱色谱纯化(用EA:PE=1:10洗脱),得到标题化合物(3.5g),其为油状物。MS(ESI):C8H7ClFNO2理论值203;实测值204[M+H]+。
描述16
5-氟-6-甲基烟酸甲酯(D16)
将2-氯-5-氟-6-甲基烟酸甲酯(D15,4.2g)、Pd/C(0.5g)和乙酸钠(6.77g)在EA(50mL)中的混合物在室温氢气氛下(1atm)搅拌过夜。将该混合物过滤并将该滤液浓缩。将该残余物用柱色谱纯化(用EA:PE=1:10洗脱),得到标题化合物(3.5g),其为白色固体。MS(ESI):C8H8FNO2理论值169;实测值170[M+H]+。
描述17
5-氟-6-甲基烟酸(D17)
向5-氟-6-甲基烟酸甲酯(D16,2.3g)在THF(10mL)和甲醇(10mL)中的溶液中加入NaOH(0.707g)在水(5mL)中的溶液。将该混合物在室温搅拌1小时,然后真空浓缩。向该残余物中加入水(5mL)。将该混合物的pH调节至3。收集固体并真空干燥,得到标题化合物(800mg),其为白色固体。1HNMR(400MHz,DMSO-d6):8.83(s,1H),8.00(dd,J=1.2Hz,9.6Hz,1H),2.57(s,3H)。MS(ESI):C7H6FNO2理论值155;实测值156[M+H]+。
描述18
3-氰基-4-甲基苯甲酸(D18)
将3-碘-4-甲基苯甲酸(3.0g)和氰化亚铜(I)(1.333g)在DMF(12mL)中的混合物在100℃搅拌20小时。将该混合物倒至冰水中并用EA萃取。将该有机相浓缩。将该残余物用PE:EA(5:1)的混合溶剂洗涤,得到标题化合物(800mg),其为绿色固体。MS(ESI):C9H7NO2理论值161;实测值160[M-H]-。
描述19
5-氟-2-甲基-3-硝基苯甲酸(D19)
将5-氟-2-甲基苯甲酸(20g)分批加至冰冷的浓硫酸(98%,80mL)中。将该混合物在0℃搅拌直至所有固体溶解。将硝酸(65%,6mL)和H2SO4(98%,12mL)的混合物分批加入,然后逐渐温热至室温。将所得混合物在室温搅拌6小时,然后倒至冰上(500mL)。收集固体并用水(100mL)洗涤。将该固体再次溶于EA(200mL)中并用盐水洗涤。将该有机层用无水Na2SO4干燥并真空浓缩,得到标题化合物(11g),其为褐色固体。MS(ESI):C8H6FNO4理论值199;实测值198[M-H]-。
描述20
5-氯-2-甲基-3-硝基苯甲酸(D20)
在0℃,向5-氯-2-甲基苯甲酸(50g)在浓H2SO4(300mL)中的溶液中分批加入硝酸(65%,1.92g)和浓硫酸(50mL)的混合物。将该混合物搅拌6小时,然后倒至冰上(1kg)。将所得混合物用水(100mL)稀释。过滤后,收集固体和再次溶于EA(300mL)。将该溶液用盐水洗涤,用Na2SO4干燥,过滤并真空浓缩。将该残余物用EA和PE(2:1,50mL)洗涤两次,得到标题化合物(39g),其为黄色固体。MS(ESI):C8H6ClNO4理论值215;实测值216[M+H]+。
描述21
(5-氟-2-甲基-3-硝基苯基)甲醇(D21)
将5-氟-2-甲基-3-硝基苯甲酸(D19,11g)和BH3.THF(1M的THF溶液,72mL)的混合物加热至80℃,持续2小时。将MeOH(20mL)缓慢加至该混合物中以淬灭该反应。将所得溶液真空浓缩。将该残余物溶于DCM(50mL)并用饱和NaHCO3溶液(2×50mL)和盐水(2×50mL)洗涤。将该有机相用Na2SO4干燥,过滤并浓缩,得到标题化合物(9g),其为黄色固体。MS(ESI):C8H8FNO3理论值185;未获得质量实测值。
描述22
(5-氯-2-甲基-3-硝基苯基)甲醇(D22)
在0℃,向5-氯-2-甲基-3-硝基苯甲酸(D20,10.7g)在THF(60mL)中的混合物中分批加入BH3.THF(1M的THF溶液,99mL)。将该混合物逐渐温热至室温并搅拌5小时。将MeOH(50mL)缓慢加至混合物。将该混合物真空浓缩,得到标题化合物(8.5g)。1H NMR(400MHz,CDCl3):7.67(s,1H),7.65(s,1H),4.73(d,2H),2.33(s,3H)。
描述23
5-氯-1-(氯甲基)-2-甲基-3-硝基苯(D23)
将(5-氯-2-甲基-3-硝基苯基)甲醇(D22,7g)溶于亚硫酰氯(24.78g)。在80℃搅拌过夜后,将该混合物浓缩,得到标题化合物(7g),其为黄色固体。MS(ESI):C8H7Cl2NO2理论值219;未获得质量实测值。
描述24
5-氟-2-甲基-3-硝基苯甲醛(D24)
向(5-氟-2-甲基-3-硝基苯基)甲醇(D21,9g)在DCM(100mL)中的混合物中分批加入PCC(14g)。将该混合物在室温搅拌过夜。将该溶剂真空除去,得到粗产物,将其用柱色谱纯化(用EA:PE=5%洗脱),得到标题化合物(5g),其为淡黄色固体。MS(ESI):C8H6FNO3理论值185;未获得质量实测值。
描述25
(S)-4-(5-氯-2-甲基-3-硝基苯甲酰基)-2-甲基哌嗪-1-甲酸叔丁酯(D25)
在0℃,向5-氯-2-甲基-3-硝基苯甲酸(D20,32.3g)、(S)-2-甲基哌嗪-1-甲酸叔丁酯(25g)和DIPEA(43.6mL)在DMF(100mL)中的溶液中加入HATU(57.0g)。将该混合物在室温搅拌过夜,然后倒至水中。将所得混合物过滤。将该固体溶于EA并用盐水洗涤三次。将该溶液用Na2SO4干燥并真空浓缩,得到标题化合物(47g),其为亮橙色固体。MS(ESI):C18H24ClN3O5理论值397;实测值342[M-tBu+H+H]+。
描述26
(S)-4-(5-氟-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D26)
向5-氟-2-甲基-3-硝基苯甲醛(D24,10g)和(S)-2-甲基哌嗪-1-甲酸叔丁酯(12.03g)在DCM(120mL)中的溶液中加入数滴乙酸(3.28g)。将该混合物在室温搅拌1小时。将三乙酰氧基硼氢化钠(23.15g)加至冰浴中。将该混合物在室温搅拌过夜并用饱和NaHCO3溶液淬灭。将该有机层用无水Na2SO4干燥,过滤并真空浓缩,得到标题化合物(22.17g),其为糖浆。MS(ESI):C18H26FN3O4理论值367;实测值368[M+H]+。
描述27
(S)-4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D27)
在0℃,将BH3.THF(1.0M的THF溶液,151mL)滴加至(S)-4-(5-氯-2-甲基-3-硝基苯甲酰基)-2-甲基哌嗪-1-甲酸叔丁酯(D25,30g)在THF(200mL)中的溶液中,历时10min。将该反应混合物加热至75℃并搅拌1小时。将所得混合物浓缩,得到标题化合物(28g),其为黄色油。MS(ESI):C18H26ClN3O4理论值383;实测值384[M+H]+。
描述28
(S)-4-(5-氯-2-甲基-3-硝基苄基)-2-乙基哌嗪-1-甲酸叔丁酯(D28)
在60℃,向5-氯-1-(氯甲基)-2-甲基-3-硝基苯(D23,1.232g)在DMF(20mL)中的溶液中加入(S)-2-乙基哌嗪-1-甲酸叔丁酯(1g)和K2CO3(1.935g)。搅拌过夜后,将该混合物倒至冰/水中并然后用DCM(3×100mL)萃取。将合并的有机层用Na2SO4干燥,过滤并浓缩,得到黄色油,将其用柱色谱纯化(用EA:PE=5%洗脱),得到标题化合物(1.3g),其为黄色固体。MS(ESI):C19H28ClN3O4理论值397;实测值398[M+H]+。
描述29
(S)-4-(3-氨基-5-氟-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D29)
在氢气下,向(S)-4-(5-氟-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D26,5g)在乙醇(65mL)中的溶液中加入钯(0.145g)。将该混合物在室温搅拌24小时,然后过滤。将该滤液真空蒸发,得到标题化合物(4.5g)。MS(ESI):C18H28FN3O2理论值337;实测值338[M+H]+。
描述30
(S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D30)
向在50℃氮气氛下搅拌的(S)-4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D27,30g)和镍(4.59g)在甲醇(200mL)中的溶液中加入肼(80%,12.26mL)。将该反应混合物在50℃搅拌1小时。将催化剂过滤并将该滤液浓缩。将该残余物真空干燥,得到标题化合物(27g),其为亮黄色油状物。MS(ESI):C18H28ClN3O2理论值353;实测值354[M+H]+。
描述31
(S)-1-(5-氟-2-甲基-3-硝基苄基)-3-甲基哌嗪,二盐酸盐(D31)
向(S)-4-(5-氟-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D26,4g)在DCM(15mL)中的溶液中加入HCl/MeOH(27.2mL)。将该混合物脱气并在室温氮气氛下搅拌12小时。将该混合物浓缩,得到标题化合物(3.1g)。MS(ESI):C13H18FN3O2理论值267;实测值268[M+H]+。
描述32
(S)-1-(5-氯-2-甲基-3-硝基苄基)-3-甲基哌嗪(D32)
向(S)-4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D27,1.5138g)在DCM(15mL)中的溶液中滴加TFA(3.04mL)。将所得混合物在室温搅拌过夜。将该溶剂真空除去。将该残余物用DCM(10mL)稀释并用饱和Na2CO3溶液碱化至pH=9。加入NaOH溶液(2M)以调节pH至11。将该水相分离并用DCM(2×15mL)萃取。将合并的有机层用Na2SO4干燥,过滤并浓缩,得到标题化合物(1.17g),其为淡黄色油状物。MS(ESI):C13H18ClN3O2理论值283;实测值284[M+H]+。
描述33
(S)-1-(5-氯-2-甲基-3-硝基苄基)-3-乙基哌嗪,二盐酸盐(D33)
向(S)-4-(5-氯-2-甲基-3-硝基苄基)-2-乙基哌嗪-1-甲酸叔丁酯(D28,1.3g)在甲醇(30mL)中的溶液中加入HCl(1.191g)的MeOH溶液。将该混合物在室温搅拌18小时,然后真空浓缩,得到标题化合物(900mg),其为白色固体。MS(ESI):C14H20ClN3O2理论值297;实测值298[M+H]+。
描述34
(S)-4-(5-氯-3-(3-氰基苯甲酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D34)
向3-氰基苯甲酸(3.06g)在DCM(30mL)中的混悬液中加入两滴DMF。滴加草酰氯(1.948mL)。将所得混合物在室温搅拌过夜。将该溶剂真空除去。将该残余物再次溶于乙腈(10mL)。将该溶液滴加至(S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D30,6.7g)和碳酸钾(7.85g)在乙腈(50mL)中的混合物中。将该混合物搅拌过夜,然后用水(10mL)淬灭。将所得混合物过滤并将所述固体用DCM(20mL)洗涤。将该滤液浓缩至干,然后溶于DCM(50mL)中。将该DCM溶液用饱和Na2CO3溶液洗涤,用Na2SO4干燥和过滤。将该残余物用柱色谱纯化(用DCM:MeOH=1:0-99:1洗脱),得到标题化合物(5.99g),其为灰白色泡沫状固体。MS(ESI):C26H31ClN4O3理论值482;实测值483[M+H]+。
描述35-36
描述35-36使用所述与描述34类似的步骤制备。
D35:(S)-4-(5-氯-3-(6-乙基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯
D36:(S)-4-(5-氯-2-甲基-3-(6-甲基烟酰氨基)苄基)-2-甲基哌嗪-1-甲酸叔丁酯
描述37
(S)-4-(5-氯-3-(3-氰基-4-氟苯甲酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯,三氟乙酸盐(D37)
向(S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D30,220.5mg)和3-氰基-4-氟苯甲酸(114.5mg)在DCM(10mL)中的溶液中加入DMAP(7.4mg)和EDC(250.8mg)。将该混合物搅拌过夜。将该混合物用DCM(10mL)稀释,然后用水(5mL)洗涤。将该有机层分离并浓缩至干。将该残余物用反相色谱纯化(用ACN/水洗脱(含有0.05%TFA),ACN%=10%-95%,50mL/min),得到标题化合物(315.3mg),其为淡黄色固体。MS(ESI):C26H30ClFN4O3理论值500;实测值501[M+H]+。
描述38-39
描述38-39使用与就描述所述37类似的步骤制备。
D38:(S)-4-(5-氯-3-(3-氰基-4-甲基苯甲酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯,三氟乙酸盐
D39:(S)-4-(3-(3-氰基苯甲酰氨基)-5-氟-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯,三氟乙酸盐
描述40
(S)-4-(5-氯-3-(5-氟-6-甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D40)
将(S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D30,913mg)、5-氟-6-甲基烟酸(D17,400mg)、HATU(980mg)和DIPEA(0.450mL)在DCM(100mL)中的溶液在室温搅拌18小时。将该混合物真空浓缩,得到标题化合物(1.2g),其为红色油状物。MS(ESI):C25H32ClFN4O3理论值490;实测值491[M+H]+。
描述41
描述41使用与描述40所述类似的步骤制备,其中具体的反应碱或溶剂列于下表。
D41:(S)-4-(5-氯-3-(2-氰基异烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯,三氟乙酸盐
描述42
(S)-4-(5-氯-3-(5-氰基-6-甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯,三氟乙酸盐(D42)
向(S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D30,264.5mg)和5-氰基-6-甲基烟酸(D13,158mg)在无水DMF(5mL)中的溶液中加入HATU(510.3mg)和DIPEA(0.261mL),将该反应混合物搅拌过周末。用DCM(10mL)稀释,用水(5mL x 2)洗涤两次,将该有机层浓缩至干,将该粗产物用反相色谱纯化(Biotage,SNAP Cartridge,KP-C18-HS 120g柱,ACN/水(含0.05%TFA),ACN%=10%-90%,50mL/min),得到标题化合物(350.8mg),其为灰白色固体。1H NMR(400MHz,CDCl3)δppm 1.33(d,3H),1.44(s,9H),2.27(s,3H),2.51-2.67(m,1H),2.77-2.84(m,1H),2.88(s,3H),3.14(d,1H),3.28(br s,1H),3.41(d,1H),3.94-4.17(m,3H),4.46(brs,1H),7.24(s,1H),7.63(s,1H),8.53(s,1H),8.89(br s,1H),9.25(s,1H)。19F NMR(376MHz,CDCl3)δppm-75.5。MS(ESI):C26H32ClN5O3理论值497;实测值498[M+H]+。
描述43-45
描述43-45使用与描述40所述类似的步骤制备,其中具体的反应碱或溶剂列于下表。
D43:(S)-4-(5-氯-3-(6-氰基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯,三氟乙酸盐
D44:(S)-3-((3-((4-(叔丁氧羰基)-3-甲基哌嗪-1-基)甲基)-5-氯-2-甲基苯基)氨基甲酰基)-5-氟吡啶1-氧化物
D45:(S)-4-(5-氯-3-(5-氟烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯
描述46
(S)-4-(5-氯-3-(3-氰基-5-氟苯甲酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D46)
向3-氰基-5-氟苯甲酸(200mg)在DCM(20mL)中的溶液中加入亚硫酰氯(288mg)。将该混合物在40℃搅拌8小时,然后减压浓缩至干。将该残余物再溶于DCM(20mL)中。在0℃,将所得溶液缓慢加至(S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D30,429mg)和DIPEA(470mg)在DCM(20mL)中的混合物中。将该反应混合物温热至室温并搅拌1小时。然后将所得混合物用水(15mL)分配。将该有机相用Na2SO4干燥,过滤并真空浓缩,得到标题化合物(320mg),其为黄色固体。MS(ESI):C26H30ClFN4O3理论值500;实测值501[M+H]+。
描述47-48
描述47-48使用与描述46所述类似的步骤制备。
D47:(S)-4-(5-氯-2-甲基-3-(2-甲基噻唑-5-甲酰氨基)苄基)-2-甲基哌嗪-1-甲酸叔丁酯
D48:(S)-4-(5-氯-3-(6-氰基-5-甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯
描述49
(S)-4-(5-氯-3-(6-氰基-5-氟烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D49)
向(S)-3-((3-((4-(叔丁氧羰基)-3-甲基哌嗪-1-基)甲基)-5-氯-2-甲基苯基)氨基甲酰基)-5-氟吡啶1-氧化物(D44,500mg)在CH3CN(20mL)中的溶液中加入TMSCN(0.204mL)和TEA(0.212mL)。将该混合物在80℃搅拌2小时。冷却至室温后,加入水。将该混合物用EA(3×50mL)萃取。将合并的有机层用饱和NaHCO3溶液、水和盐水洗涤。将所得溶液用MgSO4干燥和过滤。将该滤液真空浓缩。将该残余物用柱色谱纯化(用EA:PE=20%洗脱),得到标题化合物(250mg),其为白色固体。MS(ESI):C25H29ClFN5O3理论值501;实测值502[M+H]+。
描述50
(S)-3-氰基-N-(5-氟-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)苯甲酰胺(D50)
在室温,搅拌下向(S)-4-(3-(3-氰基苯甲酰氨基)-5-氟-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯,三氟乙酸盐(D39,4.2g)在DCM(60mL)中的溶液中加入TFA(20.81mL)。将所得混合物加热回流过夜。将该混合物冷却至室温并用饱和Na2CO3溶液小心地淬灭。将该pH调节至大约10。将该水相分离并用THF/EA萃取五次。将合并的有机相真空浓缩至大约100mL的体积。然后将该溶液用Na2SO4干燥,过滤并浓缩,得到标题化合物(2.8g)。MS(ESI):C21H23FN4O理论值366;实测值367[M+H]+。
描述51
(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺,二盐酸盐(D51)
将(S)-4-(5-氯-2-甲基-3-(6-甲基烟酰氨基)苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D36,1.0g)在DCM(6mL)中的混合物加至HCl溶液(4M的二噁烷溶液,1.057mL)。将该混合物在室温搅拌2小时,然后减压浓缩,得到标题化合物(1.07g),其为亮黄色固体。MS(ESI):C20H25ClN4O理论值372;实测值373[M+H]+。
描述52
(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(D52)
在室温,向(S)-4-(5-氯-3-(3-氰基苯甲酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D34,5.99g)在DCM(20mL)中的溶液中滴加TFA(9.55mL)。将该混合物在40℃加热2小时。将该溶剂真空除去。将该残余物用饱和Na2CO3溶液中和至pH=10,然后用DCM(2×50mL)萃取。将合并的有机层用Na2SO4干燥,过滤并浓缩,得到标题化合物(4.89g),其为淡黄色固体。MS(ESI):C21H23ClN4O理论值:382;实测值383[M+H]+。
描述53-54
描述53和54使用与描述52所述类似的步骤制备。
D53:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺
D54:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基-4-甲基苯甲酰胺
描述55
(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(D55)
向(S)-4-(5-氯-3-(5-氟-6-甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D40,900mg)在DCM(10mL)中的溶液中滴加TFA(1.130mL),将该反应混合物在室温搅拌过周末。LCMS显示反应完成。用饱和Na2CO3溶液中和至pH=10,萃取,将该水层用DCM萃取两次(10mL x 2)。将合并的有机层用Na2SO4干燥。过滤,将该滤液浓缩至干,得到标题化合物(686.9mg),其为黄色固体(95%纯度)。1H NMR(400MHz,CDCl3)δppm 1.01(d,3H),1.71(t,1H),2.04(td,1H),2.27(s,3H),2.62(d,3H),2.70(d,2H),2.76-2.84(m,1H),2.87(d,1H),2.95(d,1H),3.33-3.50(m,2H),7.18(s,1H),7.72(brs,1H),7.81(brs,1H),7.87(d,1H),8.77(s,1H)。19F NMR(376MHz,CDCl3)δppm-122.7。MS(ESI):C20H24ClFN4O理论值390;实测值391[M+H]+。
描述56
描述56使用与描述52所述类似的步骤制备。
D56:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-2-氰基异烟酰胺
描述57
(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺(D57)
在室温,向(S)-4-(5-氯-3-(5-氰基-6-甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D42,3.08g)在DCM(12mL)中的溶液中滴加TFA(4.29mL)。将该反应混合物搅拌过夜,LCMS显示反应完成。用DCM(20mL)稀释,用饱和Na2CO3水溶液中和至pH=10,然后加入2M NaOH水溶液直至pH=11。萃取,将该水层用DCM(10mL)再次萃取。将合并的有机层用Na2SO4干燥。过滤,将该滤液浓缩至干,得到标题化合物(2.6g),其为黄色固体(92%的纯度,基于LCMS)。MS(ESI):C21H24ClN5O理论值397;实测值398[M+H]+。
描述58
描述58使用与描述52所述类似的步骤制备。
D58:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-6-氰基烟酰胺
描述59
(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-6-乙基烟酰胺(D59)
将(S)-4-(5-氯-3-(6-乙基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D35,1.6g)和HCl水溶液(4M,3.29mL)在DCM(10mL)中的溶液搅拌1小时。加入冷水(30mL)。将所得混合物用饱和NaHCO3溶液中和。将该水层用DCM(2×100mL)萃取。将合并的有机层用Na2SO4干燥,过滤并真空浓缩,得到标题化合物(1.0g),其为亮黄色固体。MS(ESI):C21H27ClN4O理论值386;未获得质量实测值。
描述60
(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺,二盐酸盐(D60)
向(S)-4-(5-氯-3-(3-氰基-5-氟苯甲酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D46,320mg)在甲醇(20mL)中的溶液中加入HCl(46.6mg)。在60℃搅拌5小时后,将该混合物浓缩,得到标题化合物(250mg),其为白色固体。MS(ESI):C21H22ClFN4O理论值400;实测值401[M+H]+。
描述61-64
描述61-64使用与描述60所述类似的步骤制备。
D61:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺,二盐酸盐
D62:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-6-氰基-5-氟烟酰胺,二盐酸盐
D63:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-6-氰基-5-甲基烟酰胺,二盐酸盐
D64:(S)-5-氯-N-(5-氰基-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺,二盐酸盐
描述65
(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟烟酰胺,二盐酸盐(D65)
将(S)-4-(5-氯-3-(5-氟烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D45,300mg)和HCl(2293mg)在1,4-二噁烷(6mL)中的混合物搅拌2小时。将该混合物浓缩,得到标题化合物(210mg)。MS(ESI):C19H22ClFN4O理论值376;实测值377[M+H]+。
描述66
(S)-(4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)(四氢-2H-吡喃-4-基)甲酮(D66)
向四氢-2H-吡喃-4-甲酸(484mg)在DCM(20mL)中的溶液中加入DMAP(46.0mg)和EDC(1307mg)。搅拌20min后,加入(S)-1-(5-氯-2-甲基-3-硝基苄基)-3-甲基哌嗪(D32,960mg)在DCM(5mL)中的溶液。将所得混合物搅拌过夜并用水(2×15mL)洗涤。将该有机层用Na2SO4干燥,过滤和真空浓缩,得到标题化合物(1.43g)。MS(ESI):C19H26ClN3O4,理论值395;实测值396[M+H]+。
描述67
((S)-4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮(D67)
向(R)-四氢呋喃-2-甲酸(364mg),DIPEA(1215mg)和HATU(1783mg)在DMF(15mL)中的混合物中加入(S)-1-(5-氯-2-甲基-3-硝基苄基)-3-甲基哌嗪(D32,890mg)。将该混合物在25℃搅拌6小时。将所得混合物用水(70mL)稀释并用EA(3×40mL)萃取。将合并的有机层用水(3×50mL)和盐水(2×50mL)洗涤,用硫酸钠干燥并浓缩。将该残余物用柱色谱纯化(用PE:EA=3:1洗脱),得到标题化合物(1.2g),其为黄色油。MS(ESI):C18H24ClN3O4理论值381;实测值382[M+H]+。
描述68-73
描述68-73使用与描述67所述类似的步骤制备。
D68:((S)-4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)((S)-四氢呋喃-2-基)甲酮
D69:((S)-4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)(四氢呋喃-3-基)甲酮
D70:((S)-4-(5-氟-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮
D71:((S)-4-(5-氟-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)((S)-四氢呋喃-2-基)甲酮
D72:((S)-4-(5-氯-2-甲基-3-硝基苄基)-2-乙基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮
D73:((S)-4-(5-氟-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)(四氢呋喃-3-基)甲酮
描述74
((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)((S)-四氢呋喃-2-基)甲酮(D74)
向((S)-4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)((S)-四氢呋喃-2-基)甲酮(D68,1.2g)、铁(0.877g)在甲醇(15mL)中的混合物中加入氯化铵(0.841g)在水(3mL)中的溶液。将该混合物在70℃搅拌1小时,然后过滤。将该滤液浓缩并将该残余物溶于EA和水中。将该有机层用盐水洗涤,用硫酸钠干燥并浓缩,得到标题化合物(800mg),其为黄色固体。MS(ESI):C18H26ClN3O2理论值351;实测值352[M+H]+。
描述75-80
描述75-80使用与描述74所述类似的步骤制备。
D75:((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮
D76:((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)(四氢呋喃-3-基)甲酮
D77:((S)-4-(3-氨基-5-氟-2-甲基苄基)-2-甲基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮
D78:((S)-4-(3-氨基-5-氟-2-甲基苄基)-2-甲基哌嗪-1-基)((S)-四氢呋喃-2-基)甲酮
D79:((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-乙基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮
D80:((S)-4-(3-氨基-5-氟-2-甲基苄基)-2-甲基哌嗪-1-基)(四氢呋喃-3-基)甲酮
描述81
(S)-(4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)(四氢-2H-吡喃-4-基)甲酮(D81)
向(S)-(4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-基)(四氢-2H-吡喃-4-基)甲酮(D66,1.3g)在乙醇(20mL)中的溶液中加入氯化锡(II)二水合物(3.70g)。将该混合物在室温搅拌过夜,然后浓缩。将该残余物混悬于DCM(30mL)中。加入NaOH水溶液(2M)直至形成澄清溶液。将该有机相分离。将该水层用DCM(20mL)萃取。将合并的有机层用Na2SO4干燥,过滤并浓缩,得到标题化合物(1.09g)。MS(ESI):C19H28ClN3O2理论值365;实测值366[M+H]+。
描述82
5-甲氧基-6-甲基烟酸(D82)
将5-氟-6-甲基烟酸(D17,3.5g)和甲醇钠(12.2g)在DMF(50mL)中的混合物加热至140℃,持续16小时。冷却至室温后,将该混合物过滤和收集固体。在0℃,将该固体溶于水(10mL)中并将该pH值使用HCl溶液(2M)调节至3~4。将沉淀物通过过滤收集,用水洗涤并干燥,得到标题化合物(2.45g),其为白色固体。MS(ESI):C8H9NO3理论值167;实测值168[M+H]+。
描述83
(R)-4-(5-氯-2-甲基-3-硝基苯甲酰基)-2-甲基哌嗪-1-甲酸叔丁酯(D83)
将5-氯-2-甲基-3-硝基苯甲酸(D20,5g)在亚硫酰氯(20ml)中的混悬液搅拌并加热回流3小时。将该混合物真空浓缩并将该残余物加至在氮气下用冰水浴冷却的(R)-2-甲基哌嗪-1-甲酸叔丁酯(4.18g)和TEA(7.04g)在DCM(50mL)中的溶液中。将该反应在室温搅拌过夜。将该反应混合物用饱和NaHCO3溶液(50mL)和盐水(2×50mL)洗涤,然后用Na2SO4干燥,过滤后,将该溶剂真空除去,得到标题化合物(8g),其为白色固体。
描述84
描述84使用与描述27所述类似的步骤制备。
D84:(R)-4-(5-氯-2-甲基-3-硝基苄基)-2-甲基哌嗪-1-甲酸叔丁酯
描述85
描述85使用与描述74所述类似的步骤制备。
D85:(R)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯
描述86-87
描述86-87使用与描述40所述类似的步骤制备,具体反应溶剂列于表中。
D86:(S)-4-(5-氯-3-(5,6-二甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯
D87:(R)-4-(5-氯-3-(5-氟-6-甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯
描述88
描述88使用与描述34所述类似的步骤制备。
D88:(S)-4-(5-氯-3-(5-甲氧基-6-甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯
描述89-91
描述89-91使用与描述65所述类似的步骤制备。
D89:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺,二盐酸盐
D90:(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-甲氧基-6-甲基烟酰胺,二盐酸盐
D91:(R)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺,二盐酸盐
描述92
(R)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺,2三氟乙酸盐(D92)
在室温,向(R)-4-(5-氯-3-(5-氟-6-甲基烟酰氨基)-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D87,814mg)在DCM(8mL)中的溶液中滴加三氟乙酸(1.3ml)。将该反应混合物搅拌过夜。溶剂真空除去,将该残余物用EtOAc(20mL)溶解,用饱和Na2CO3溶液中和至pH=10。萃取,将该水层用EtOAc萃取三次(3×10mL)。将合并的有机层用Na2SO4干燥。过滤,将该滤液浓缩至干,得到标题化合物(950mg,19F NMR显示其为TFA盐)。1H NMR(400MHz,MeOD-d4):8.87(s,1H),8.07(d,J=9.8Hz,1H),7.37(s,1H),7.34(s,1H),3.69(s,2H),3.41-3.36(m,2H),3.16(t,J=12.0Hz,1H),3.03(t,J=12.8Hz,2H),2.60(s,3H),2.47(t,J=12.3Hz,1H),2.32-2.22(m,4H),1.31(d,J=6.6Hz,3H)。19F NMR(376MHz,MeOD-d4):-77.3,-125.3。MS(ESI):C20H24ClFN4O理论值390;实测值391[M+H]+。
描述93
5-(乙氧基羰基)-3-氟-2-甲基吡啶1-氧化物(D93)
在25℃,向5-氟-6-甲基烟酸乙酯(使用与D16所述类似的步骤制备,200mg)在DCM(5mL)中的溶液中加入3-氯苯并过氧酸(226mg),过夜。将该反应混合物浓缩并将该残余物用快速硅胶色谱(PE/EA=1/1)纯化,以提供标题化合物(200mg),其为白色固体。MS(ESI):C9H10FNO3理论值199;实测值200[M+H]+。
描述94
5-羧基-3-氟-2-甲基吡啶1-氧化物(D94)
在25℃,向5-(乙氧基羰基)-3-氟-2-甲基吡啶1-氧化物(D93,200mg)在THF(2mL)中的溶液中加入水(2.000mL)和氢氧化锂(72.1mg),添加完成后,将该反应混合物在25℃搅拌1h。将有机溶剂真空除去,将水层用氯化氢(1M水溶液)酸化至pH~3并用乙酸乙酯(30mL)萃取,将该有机相用无水硫酸钠干燥并浓缩,得到标题化合物(170mg),其为白色固体。MS(ESI):C7H6FNO3理论值171;实测值172[M+H]+。
描述95
(S)-5-((3-((4-(叔丁氧羰基)-3-甲基哌嗪-1-基)甲基)-5-氯-2-甲基苯基)氨基甲酰基)-3-氟-2-甲基吡啶1-氧化物(D95)
在25℃,向5-羧基-3-氟-2-甲基吡啶1-氧化物(D94,170mg)在DMF(5mL)中的溶液中加入HATU(378mg)、DIPEA(0.174mL)和(S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-甲酸叔丁酯(D30,352mg),将该反应混合物在25℃搅拌过夜。将该溶剂减压蒸发并将该残余物溶于二氯甲烷中。将该有机层用水、盐水洗涤并用硫酸钠干燥。将该溶剂浓缩并将该残余物用硅胶柱色谱纯化(石油醚/乙酸乙酯=2/1),得到标题化合物(300mg),其为黄色固体。MS(ESI):C25H32ClFN4O4理论值506;实测值507[M+H]+。
描述96
(S)-5-((5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)氨基甲酰基)-3-氟-2-甲基吡啶1-氧化物,二盐酸盐(D96)
向(S)-5-((3-((4-(叔丁氧羰基)-3-甲基哌嗪-1-基)甲基)-5-氯-2-甲基苯基)氨基甲酰基)-3-氟-2-甲基吡啶1-氧化物(D95,300mg)在1,4-二噁烷(10mL)中的溶液中加入氯化氢(108mg),然后将该反应混合物在25℃搅拌1h,然后过滤,得到标题化合物(280mg),其为黄色固体。MS(ESI):C20H24ClFN4O2理论值406;实测值407[M+H]+。
实施例1
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(E1)
向四氢-2H-吡喃-4-甲酸(1.896g)在DCM(20mL)中的溶液中加入3滴DMF,然后在氮气氛下滴加草酰氯(1.403mL)在DCM(5mL)中的溶液。将该反应混合物在室温搅拌2小时。将该溶剂真空除去。将该残余物溶于乙腈(10mL)中。在0℃,将所得混合物滴加至(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(D52,4.65g)和碳酸钾(5.04g)在乙腈(30mL)中的混合物中。加入后,将该反应混合物在室温搅拌过夜。过滤后,将该滤液浓缩至干。将该残余物溶于DCM(40mL)并用饱和Na2CO3溶液洗涤。将该有机层用Na2SO4干燥,过滤并浓缩。将该残余物用柱色谱纯化(用DCM/MeOH洗脱,MeOH%=0.5~2%),得到该粗产物(5.2g),其为白色固体,将其用柱色谱,然后用反相色谱再次纯化。收集纯的级分并冷冻干燥,得到标题化合物的TFA盐,将其用DCM(30mL)溶解,用NaOH溶液(0.6g在40mL水中)和水(20mL)洗涤。将该有机层浓缩至干。将该残余物溶于乙腈/水中并再次冷冻干燥,得到标题化合物(1.9g),其为白色固体。1HNMR(400MHz,MeOD-d4):8.32(s,1H),8.25(d,J=7.6Hz,1H),7.94(d,J=7.6Hz,1H),7.71(t,J=7.8Hz,1H),7.36(brs,1H),7.29(brs,1H),4.74-4.59(m,0.5H),4.38-4.19(m,1H),4.00-3.88(m,2H),3.88-3.75(m,0.5H),3.61-3.34(m,4.5H),3.02-2.63(m,3.5H),2.30(s,3H),2.24-1.93(m,2H),1.91-1.49(m,4H),1.42-1.14(m,3H)。MS(ESI):C27H31ClN4O3理论值494;实测值495[M+H]+。
实施例2
3-氰基-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺,三氟乙酸盐(E2)
向(S)-3-氰基-N-(5-氟-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)苯甲酰胺(D50,109.7mg)和(R)-四氢呋喃-2-甲酸(40.9mg)在DCM(5mL)中的溶液中加入EDC(116.4mg)和HOBt(60.7mg)。将该反应混合物在室温搅拌过夜。将该混合物用DCM(10mL)稀释并用水(10mL)洗涤。将该有机层浓缩至干并将该残余物用MDAP(酸性条件)纯化得到标题化合物(65.9mg),其为白色固体。1H NMR(400MHz,MeOD-d4):8.34(s,1H),8.28(d,J=7.8Hz,1H),7.99(d,J=7.6Hz,1H),7.75(t,J=7.8Hz,1H),7.40-7.32(m,2H),4.77-4.55(m,2H),4.47(brs,2H),4.26(d,J=13.7Hz,0.5H),3.98-3.79(m,2H),3.66-3.39(m,2.5H),3.29-2.97(m,3H),2.34(s,3H),2.25-2.05(m,2H),2.00-1.90(m,2H),1.54-1.24(m,3H)。19F NMR(376MHz,MeOD-d4):-76.6,-116.0。MS(ESI):C26H29FN4O3理论值464;实测值465[M+H]+。
实施例3
3-氰基-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺,三氟乙酸盐(E3)
实施例3使用与实施例2所述类似的步骤进行制备。1H NMR(400MHz,MeOD-d4):8.34(s,1H),8.28(d,J=8.1Hz,1H),7.99(d,J=7.8Hz,1H),7.75(t,J=7.8Hz,1H),7.40-7.31(m,2H),4.82-4.75(m,0.5H),4.70(t,J=6.7Hz,1H),4.61(brs,0.5H),4.52-4.39(m,2H),4.32-4.15(m,0.5H),3.95-3.80(m,2H),3.61(brs,0.5H),3.55-3.40(m,2H),3.28-3.00(m,3H),2.33(s,3H),2.23-2.07(m,2H),2.02-1.89(m,2H),1.54-1.25(m,3H)。19F NMR(376MHz,MeOD-d4):-76.6,-116.1。MS(ESI):C26H29FN4O3理论值464;实测值465[M+H]+。
实施例4
(S)-3-氰基-N-(5-氟-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺(E4)
在室温,向(S)-3-氰基-N-(5-氟-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)苯甲酰胺(D50,240mg)在DMF(5mL)中的混合物中加入HATU(374mg)。将该混合物在25℃搅拌10min,然后加入DIPEA(169mg)。将该混合物在25℃搅拌过夜。冷却后,将该混合物用EA萃取。将该有机相用Na2SO4干燥,通过薄硅藻土垫过滤并浓缩,得到棕色油,将其用柱色谱纯化(用PE:EA=10:1洗脱),得到无色油,将其用反相色谱纯化(C18,移动相0.01%NH4HCO3:H2O,CH3CN,10~95%,9.5min,30mL/min),得到淡黄色固体(250mg),将其用制备型HPLC纯化(Gilson GX-281,移动相:0.01%NH4HCO3:H2O,CH3CN,50~95%,9.0min,30mL/min)得到标题化合物(110mg),其为白色固体。1H NMR(400MHz,CDCl3):8.20(s,1H),8.16(d,J=7.8Hz,1H),7.94-7.82(m,2H),7.68(t,J=7.8Hz,1H),7.62(d,J=8.5Hz,1H),6.95(d,J=6.5Hz,1H),4.76(brs,0.5H),4.38(d,J=13.3Hz,0.5H),4.11-3.96(m,2.5H),3.63(d,J=12.0Hz,0.5H),3.53-3.33(m,4.5H),2.98-2.87(m,0.5H),2.84-2.75(m,1H),2.75-2.62(m,2H),2.31(s,3H),2.25-2.15(m,1H),2.09-1.79(m,3H),1.70-1.52(m,2H),1.42-1.18(m,3H)。MS(ESI):C27H31FN4O3理论值478;实测值479[M+H]+。
实施例5-21
实施例5-21使用与实施例4所述类似的步骤制备,其中具体的反应碱或溶剂列于下表。
E5:3-氰基-N-(5-氟-2-甲基-3-(((3S)-3-甲基-4-(四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺
E6:3-氰基-N-(5-氟-2-甲基-3-(((3S)-3-甲基-4-(四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺
E7:N-(5-氯-2-甲基-3-(((3S)-3-甲基-4-(四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺
E8:N-(5-氯-2-甲基-3-(((3S)-3-甲基-4-(四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺
E9:(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-乙基烟酰胺
E10:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-乙基烟酰胺
E11:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-甲基苯甲酰胺
E12:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-甲基苯甲酰胺
E13:(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺
E14:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-乙基烟酰胺
E15:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-2-氰基异烟酰胺
E16:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-2-氰基异烟酰胺
E17:(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-甲基烟酰胺
E18:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-甲基烟酰胺
E19:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-甲基烟酰胺
E20:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-氟烟酰胺
E21:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-氰基-5-氟烟酰胺
实施例22
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺(E22)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺,二盐酸盐(D60,150mg)在DCM(20mL)中的溶液中加入(R)-四氢呋喃-2-甲酸(43.4mg)、HATU(142mg)和DIPEA(0.065mL)。搅拌2小时后,将该混合物减压浓缩。将该残余物用制备型HPLC纯化(Gilson GX-281,移动相:0.01%NH4HCO3/H2O,CH3CN,50~95%,9.0min,30mL/min)得到标题化合物(100mg),其为白色固体。1H NMR(400MHz,CDCl3):8.16(brs,0.5H),8.04-8.02(m,1.5H),7.94(t,J=8.2Hz 1H),7.69(s,1H),7.58(d,J=6.8Hz,1H),7.19(d,J=7.3Hz,1H),4.68-4.61(m,1H),4.58-4.55(m,0.5H),4.24-4.13(m,1H),3.98-3.93(m,1H),3.89-3.81(m,1.5H),3.48-3.26(m,2.5H),2.88-2.72(m,1H),2.69-2.63(m,1H),2.57(d,J=11.3Hz,0.5H),2.30(s,3H),2.24-1.87(m,6H),1.36-1.17(m,3H)。MS(ESI):C26H28ClFN4O3理论值498;实测值499[M+H]+。
实施例23
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺(E23)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺,二盐酸盐(D60,100mg)在DCM(20mL)中的溶液中加入(S)-四氢呋喃-2-甲酸(29.0mg)、HATU(114mg)和DIPEA(0.131mL)。搅拌2小时后,将该混合物减压浓缩。将该残余物用制备型HPLC纯化(Gilson GX-281,移动相:0.01%NH4HCO3/H2O,CH3CN,50~95%,9.0min,30mL/min)得到标题化合物(54mg),其为白色固体。1H NMR(400MHz,CDCl3):7.99(brs,1H),7.91(brs,1.5H),7.82-7.72(m,1.5H),7.58(d,J=7.3Hz,1H),7.20(d,J=2.0Hz,1H),4.73-4.65(m,0.5H),4.57(brs,1H),4.40-4.25(m,1H),3.97-3.91(m,1H),3.88-3.83(m,1H),3.78-3.70(m,0.5H),3.48-3.36(m,2.5H),2.94-2.85(m,0.5H),2.80-2.61(m,2H),2.35-2.15(m,5H),2.10-1.86(m,4H),1.34-1.22(m,3H)。MS(ESI):C26H28ClFN4O3理论值498;实测值499[M+H]+。
实施例24
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(E24)
在室温,向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(D52,200mg)在DMF(5mL)中的混合物中加入HATU(298mg)。将该混合物在25℃搅拌10min,然后加入DIPEA(135mg)。将该混合物在25℃搅拌过夜。将该混合物分配至水和EA之间。将该有机相用Na2SO4干燥,通过薄硅藻土垫过滤并浓缩,得到棕色油,将其用制备型HPLC纯化(GilsonGX-281,移动相:0.01%NH4HCO3/H2O,CH3CN,50~95%,9.0min,30mL/min)得到标题化合物(50mg),其为白色固体。1H NMR(400MHz,DMSO-d6):10.19(s,1H),8.40(s,1H),8.26(d,J=7.8Hz,1H),8.08(d,J=7.8Hz,1H),7.76(t,J=7.8Hz,1H),7.39(d,J=1.5Hz,1H),7.29-7.27(m,1H),4.67-4.59(m,1H),4.54-4.45(m,0.5H),4.24-4.11(m,1H),3.83-3.70(m,2.5H),3.49-3.40(m,2H),3.23-3.12(m,0.5H),2.85-2.72(m,1.5H),2.64(d,J=11.3Hz,1H),2.22(s,3H),2.18-1.75(m,6H),1.28-1.11(m,3H)。MS(ESI):C26H29ClN4O3理论值480;实测值481[M+H]+。
实施例25
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(E25)
在室温,向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(D52,200mg)在DMF(5mL)中的混合物中加入HATU(298mg)。将该混合物在25℃搅拌10min,然后加入DIPEA(135mg)。将该混合物在25℃搅拌过夜。将所得混合物分配至水和EA之间。将该有机相用Na2SO4干燥,通过薄硅藻土垫过滤并浓缩,得到棕色油,将其用制备型HPLC纯化(GilsonGX-281,移动相:0.01%NH4HCO3/H2O,CH3CN,50~95%,9.0min,30mL/min),得到标题化合物(60mg),其为白色固体。1H NMR(400MHz,DMSO-d6):10.19(s,1H),8.41(s,1H),8.26(d,J=7.8Hz,1H),8.08(d,J=7.8Hz,1H),7.76(t,J=7.8Hz,1H),7.39(d,J=2.0Hz,1H),7.28(brs,1H),4.56(brs,0.5H),4.22-4.19(m,1H),3.96-3.92(m,0.5H),3.85-3.60(m,4H),3.50-3.43(m,2H),3.34-3.27(m,1H),3.22-3.16(m,0.5H),2.84-2.74(m,1.5H),2.65(d,J=11.3Hz,1H),2.22(s,3H),2.16-1.90(m,4H),1.27-1.11(m,3H)。MS(ESI):C26H29ClN4O3理论值480;实测值481[M+H]+。
实施例26
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(E26)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(D52,600mg)在DMF(50mL)中的溶液中加入(S)-四氢呋喃-2-甲酸(110mg)、HATU(1192mg)和DIPEA(0.547mL)。将该混合物在室温搅拌2小时。加入水。将该溶液用EA(3×50mL)萃取。将该滤液用饱和NaHCO3溶液、水和盐水洗涤。将所得混合物用MgSO4干燥。过滤后,将该残余物用制备型HPLC纯化,得到标题化合物(50mg),其为白色固体。1H NMR(400MHz,CDCl3):8.22(s,1H),8.17(d,J=7.5Hz,1H),8.06-7.96(m,1H),7.86(d,J=7.8Hz,1H),7.75(brs,1H),7.66(t,J=7.9Hz,1H),7.18(d,J=2.0Hz,1H),4.67(brs,0.5H),4.57(t,J=6.1Hz,1H),4.36(brs,0.5H),4.31-4.22(m,0.5H),3.95-3.90(m,1H),3.87-3.82(m,1H),3.77-3.68(m,0.5H),3.47-3.39(m,2.5H),2.95-2.84(m,0.5H),2.81-2.59(m,2H),2.35-2.15(m,5H),2.10-1.85(m,4H),1.33-1.21(m,3H)。MS(ESI):C26H29ClN4O3理论值480;实测值481[M+H]+。
实施例27
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(E27)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(D52,150mg)在DMF(50mL)中的溶液中加入(S)-四氢呋喃-3-甲酸(54.6mg)、HATU(149mg)和DIPEA(0.137mL)。将该混合物在室温搅拌2小时。加入水。将该溶液用EA(3×50mL)萃取。将该滤液用饱和NaHCO3溶液、水和盐水洗涤。将所得溶液用MgSO4干燥。过滤后,将该残余物用手性制备型HPLC纯化,得到标题化合物(20mg),其为白色固体。1H NMR(400MHz,CDCl3):8.19(s,1H),8.15(d,J=7.8Hz,1H),7.89(d,J=7.8Hz,1H),7.82(brs,1H),7.76(d,J=7.5Hz,1H),7.68(t,J=7.8Hz,1H),7.18(d,J=2.0Hz,1H),4.77(brs,0.5H),4.39(d,J=14.1Hz,0.5H),4.07-3.79(m,4.5H),3.64(d,J=13.6Hz,0.5H),3.52-3.32(m,2.5H),3.27-3.14(m,1H),2.98-2.66(m,2.5H),2.32(s,3H),2.26-2.00(m,4H),1.36-1.22(m,3H)。MS(ESI):C26H29ClN4O3理论值480;实测值481[M+H]+。
实施例28
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺(E28)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺(D53,96.5mg)和(R)-四氢呋喃-2-甲酸(36.5mg)在无水DMF(5mL)中的溶液中加入HATU(182.3mg)和DIPEA(0.126mL)。将该反应混合物搅拌过夜,然后用MDAP(碱性条件)直接纯化,得到标题化合物(65.9mg),其为白色固体。1H NMR(400MHz,MeOD-d4):8.39(dd,J=5.9Hz,2.0Hz,1H),8.34-8.30(m,1H),7.54(t,J=8.9Hz,1H),7.34(s,1H),7.30(brs,1H),4.75-4.67(m,1H),4.66-4.55(m,1.5H),4.27(d,J=13.4Hz,0.5H),4.22-4.14(m,0.5H),4.00-3.90(m,1H),3.89-3.78(m,1.5H),3.55-3.45(m,2H),3.39-3.33(m,0.5H),3.05-2.96(m,0.5H),2.89-2.78(m,1H),2.77-2.69(m,1H),2.30(s,3H),2.27-2.07(m,3H),2.02-1.87(m,3H),1.38-1.23(m,3H)。19F NMR(376MHz,MeOD-d4):-105.1。MS(ESI):C26H28ClFN4O3理论值498;实测值499[M+H]+。
实施例29
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺(E29)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺(D53,99.8mg)和(S)-四氢呋喃-2-甲酸(39.6mg)在无水DMF(5mL)中的溶液中加入HATU(189.3mg)和DIPEA(0.130mL)。将该混合物搅拌过夜,然后用MDAP(碱性条件)直接纯化,得到标题化合物(68.9mg),其为白色固体。1H NMR(400MHz,MeOD-d4):8.39(dd,J=6.1Hz,2.0Hz,1H),8.36-8.29(m,1H),7.54(t,J=8.8Hz,1H),7.35(d,J=2.0Hz,1H),7.31(d,J=2.0Hz,1H),4.75-4.53(m,2.5H),4.39-4.24(m,1H),3.97-3.89(m,1H),3.88-3.80(m,1H),3.76(d,J=13.7Hz,0.5H),3.57-3.45(m,2H),3.45-3.36(m,0.5H),3.07-2.93(m,0.5H),2.84(d,J=10.8Hz,1H),2.72(d,J=11.5Hz,1H),2.30(s,3H),2.27-1.87(m,6H),1.42-1.21(m,3H)。19F NMR(376MHz,MeOD-d4):-106.7。MS(ESI):C26H28ClFN4O3理论值498;实测值499[M+H]+。
实施例30
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(E30)
在0℃,向(R)-四氢呋喃-2-甲酸(44.6mg),(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(D55,150mg)和DIPEA(0.201mL)在DCM(10mL)中的溶液中加入HATU(175mg)。将该混合物在室温搅拌过夜,然后用NaHCO3水溶液和盐水洗涤三次。将所得溶液用Na2SO4干燥,过滤并浓缩。将该残余物用制备型HPLC纯化,得到标题化合物(34mg),其为白色固体。1H NMR(400MHz,DMSO-d6):10.19(s,1H),8.89(s,1H),8.12(d,J=10.0Hz,1H),7.39(s,1H),7.29(d,J=4.8Hz,1H),4.68-4.58(m,1H),4.55-4.47(m,0.5H),4.24-4.12(m,1H),3.85-3.69(m,2.5H),3.51-3.41(m,2H),3.22-3.13(m,0.5H),2.86-2.69(m,1.5H),2.64(d,J=11.0Hz,1H),2.54(d,J=2.8Hz,3H),2.22(s,3H),2.18-1.73(m,6H),1.29-1.12(m,3H)。19F NMR(376MHz,DMSO-d6):-124.7。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。
实施例31
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(E31)
在0℃,向(S)-四氢呋喃-2-甲酸(44.6mg),(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(D55,150mg)和DIPEA(0.201mL)在DCM(10mL)中的溶液中加入HATU(175mg)。将该混合物在室温搅拌过夜,然后用NaHCO3水溶液和盐水洗涤三次。将所得溶液用Na2SO4干燥,过滤并浓缩。将该残余物用制备型HPLC纯化,得到标题化合物(31mg),其为白色固体。1H NMR(400MHz,DMSO-d6):10.19(s,1H),8.89(s,1H),8.12(d,J=10.0Hz,1H),7.39(d,J=2.0Hz,1H),7.28(d,J=1.8Hz,1H),4.59(t,J=6.5Hz,1H),4.56-4.46(m,0.5H),4.37-4.26(m,0.5H),4.16(d,J=12.0Hz,0.5H),3.85-3.67(m,2.5H),3.52-3.38(m,2H),3.28-3.14(m,0.5H),2.89-2.70(m,1.5H),2.64(d,J=10.8Hz,1H),2.54(d,J=2.8Hz,3H),2.30-2.14(m,4H),2.05-1.73(m,5H),1.32-1.06(m,3H)。19F NMR(376MHz,DMSO-d6):-124.7。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。
实施例32
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺(E32)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺(D57,95.0mg)和(R)-四氢呋喃-2-甲酸(40.5mg)在无水DMF(5mL)中的溶液中加入HATU(182.8mg)和DIPEA(0.13mL)。将该混合物在室温搅拌过夜,然后用MDAP直接纯化(碱性条件,ACN/H2O(含0.05%NH3H2O),ACN%=30-70%),得到标题化合物(78.5mg),其为白色固体。1H NMR(400MHz,MeOD-d4):9.21(s,1H),8.64(s,1H),7.44(brs,2H),4.79-4.62(m,1.5H),4.60-4.11(m,2H),3.99-3.79(m,2.5H),3.76-3.35(m,2.5H),3.24-2.96(m,1.5H),2.94-2.67(m,4H),2.33(s,3H),2.27-1.78(m,5H),1.52-1.13(m,3H)。MS(ESI):C26H30ClN5O3理论值495;实测值496[M+H]+。
实施例33
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺(E33)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺(D57,95.6mg)和(S)-四氢呋喃-2-甲酸(40.0mg)在无水DMF(5mL)中的溶液中加入HATU(176.3mg)和DIPEA(0.13mL)。将该混合物在室温搅拌过夜,然后用MDAP直接纯化(碱性条件,ACN/H2O(含0.05%NH3H2O),ACN%=30-70%),得到标题化合物(75.9mg),其为白色固体。1H NMR(400MHz,MeOD-d4):9.21(s,1H),8.64(s,1H),7.43(brs,2H),4.76-4.60(m,1.5H),4.58-4.11(m,2H),3.96-3.77(m,2.5H),3.75-3.36(m,2.5H),3.21-2.96(m,1.5H),2.93-2.60(m,4H),2.33(s,3H),2.25-1.83(m,5H),1.51-1.13(m,3H)。MS(ESI):C26H30ClN5O3理论值495;实测值496[M+H]+。
实施例34&35
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-2-甲基四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-2-甲基四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(E34&E35)
在室温,向2-甲基四氢呋喃-2-甲酸(52.1mg)和(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺(D52,153mg)在DMF(6mL)中的混合物中加入HATU(228mg)。搅拌10min后,加入DIPEA(0.105mL)。将该混合物在50℃搅拌18小时。将所得混合物用水(10mL)稀释,然后用EA(30mL)萃取。将该有机相用Na2SO4干燥,过滤并浓缩,得到棕色油。将该残余物用制备型HPLC纯化(Gilson GX-281,移动相:0.01%NH4HCO3/H2O,CH3CN,50~95%,9.0min,30mL/min),得到粗产物(150mg),其为白色固体,将其用制备型手性HPLC纯化(柱:AD-H 4.6×250mm,5um;共溶剂:MeOH(0.1%DEA);柱温39.9℃;CO2流速:2.25mL/min;共溶剂流速:0.75mL/min;共溶剂:25%),得到标题化合物(50mg和65mg),其为白色固体。异构体1:1H NMR(400MHz,CDCl3):8.21(brs,1H),8.16(d,J=8.0Hz,1H),7.87(d,J=7.8Hz,1H),7.82-7.75(m,1H),7.67(t,J=7.8Hz,1H),7.22-7.15(m,1H),5.18-5.06(m,0.5H),4.82-4.68(m,0.5H),4.49(d,J=13.8Hz,0.5H),4.29-4.18(m,0.5H),4.08-3.87(m,1H),3.87-3.64(m,1H),3.46-3.30(m,2.5H),3.00-2.67(m,2.5H),2.61(d,J=11.3Hz,1H),2.31(s,3H),2.27-2.13(m,1H),2.07-1.77(m,3H),1.68-1.53(m,2H),1.50-1.41(m,3H),1.38-1.15(m,3H)。MS(ESI):C27H31ClN4O3理论值494;实测值495[M+H]+。异构体2:1H NMR(400MHz,CDCl3):8.20(brs,1H),8.16(d,J=7.8Hz,1H),7.87(d,J=7.8Hz,1H),7.83-7.76(m,1H),7.67(t,J=7.8Hz,1H),7.18(s,1H),4.90-4.80(m,0.5H),4.72(d,J=12.5Hz,0.5H),4.67-4.57(m,0.5H),4.38-4.29(m,0.5H),3.99-3.91(m,1H),3.85-3.72(m,1H),3.48-3.32(m,2H),3.27-3.15(m,0.5H),2.96-2.81(m,1.5H),2.80-2.59(m,2H),2.32(s,3H),2.23-1.78(m,4H),1.70-1.55(m,2H),1.51-1.38(m,3H),1.37-1.17(m,3H)。MS(ESI):C27H31ClN4O3理论值494;实测值495[M+H]+。
实施例36-55
实施例36-55使用与实施例34&35所述类似的步骤制备,其中具体的反应碱或溶剂列于下表。
E36&E37:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺
E38&E39:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-2-甲基四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-2-甲基四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺
E40&E41:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺
E42&E43:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺
E44&E45:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟烟酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟烟酰胺
E46&E47:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟烟酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟烟酰胺
E48&E49:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺
E50&E51:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-2-甲基噻唑-5-甲酰胺
E52&E53:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺
E54&E55:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢-2H-吡喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺
实施例56&57
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(E56)
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(E57)
将HATU(438mg)、DIPEA(0.402mL)、(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(D55,300mg)和四氢呋喃-3-甲酸(89mg)在DCM(150mL)中的混合物在室温搅拌过夜。将该混合物真空浓缩并用制备型HPLC纯化,得到粗产物(260mg),将其用手性HPLC分离,得到标题化合物(E56(55mg)和E57(50mg)),其为白色固体。E56:手性SFC:柱:OJ-H,4.6×250mm,5um;共溶剂:MeOH(含0.1%DEA);柱温:36.7℃;CO2流速:2.4mL/min;共溶剂流速:0.6mL/min;共溶剂:20%;tR=7.40min。1H NMR(400MHz,MeOD-d4):8.88(s,1H),8.08(d,J=9.6Hz,1H),7.37(d,J=2.0Hz,1H),7.32(s,1H),4.68(brs,0.5H),4.34-4.31(m,0.5H),4.25(brs,0.5H),4.04-3.99(m,0.5H),3.96-3.77(m,4H),3.56-3.36(m,3.5H),3.05-2.97(m,0.5H),2.88-2.83(m,1H),2.80-2.75(m,1H),2.61(brs,3H),2.32(s,3H),2.24-2.00(m,4H),1.32-1.28(m,3H)。19F NMR(376MHz,MeOD-d4):-125.4。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。E57:手性SFC:柱:OJ-H,4.6×250mm,5um;共溶剂:MeOH(含0.1%DEA);柱温:40℃;CO2流速:2.4mL/min;共溶剂流速:0.6mL/min;共溶剂:20%;tR=8.51min。1H NMR(400MHz,MeOD-d4):8.88(s,1H),8.08(d,J=9.6Hz,1H),7.37(d,J=2.0Hz,1H),7.32(s,1H),4.67(brs,0.5H),4.34-4.31(m,1H),3.98-3.78(m,4.5H),3.53-3.48(m,2H),3.43-3.41(m,1.5H),2.99-2.85(m,0.5H),2.88-2.85(m,1H),2.80-2.75(m,1H),2.61(brs,3H),2.32(s,3H),2.24-2.00(m,4H),1.32-1.28(m,3H)。19FNMR(376MHz,MeOD-d4):-125.4。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。
实施例58
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟苯甲酰胺(E58)
向((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮(D75,100mg)、DMAP(38.2mg)在DCM(10mL)中的混合物中缓慢加入3-氟苯甲酰氯(45.1mg)。将该混合物在20℃搅拌16小时。除去溶剂。将该残余物用制备型HPLC纯化,得到标题化合物(63mg),其为白色固体。1HNMR(400MHz,MeOD-d4):7.83-7.81(d,J=8.4Hz,1H),7.74-7.71(d,J=12.0Hz,1H),7.60-7.55(m,1H),7.40-7.32(m,3H),4.74-4.70(m,1H),4.66-4.64(m,0.5H),4.31-4.28(brs,0.5H),4.25-4.15(m,0.5H),4.00-3.82(m,2.5H),3.56-3.48(m,2H),3.39-3.33(m,0.5H),3.06-2.99(m,0.5H),2.89-2.78(m,1H),2.77-2.74(m,1H),2.32(s,3H),2.27-1.94(m,6H),1.40-1.25(m,3H)。MS(ESI):C25H29ClFN3O3理论值473;实测值474[M+H]+。
实施例59-65
实施例59-65使用与实施例58所述类似的步骤制备。
E59:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟苯甲酰胺
E60:3-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺
E61:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟-4-甲基苯甲酰胺
E62:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟-4-甲基苯甲酰胺
E63:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3,5-二氟苯甲酰胺
E64:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3,5-二氟苯甲酰胺
E65:3-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)苯甲酰胺
实施例66
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟-5-甲基苯甲酰胺(E66)
将3-氟-5-甲基苯甲酸(65.7mg)在亚硫酰氯(338mg)中的混合物在80℃搅拌1小时。然后将该混合物真空浓缩。将该残余物加至((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮(D75,100mg)、DMAP(38.2mg)在DCM(8mL)中的混合物中。将该混合物在20℃搅拌16小时,然后浓缩。将该残余物用制备型HPLC和手性HPLC纯化,得到标题化合物(5mg),其为白色固体。1H NMR(400MHz,MeOD-d4):7.66(s,1H),7.50(d,J=9.6Hz,1H),7.35-7.31(m,2H),7.21(d,J=9.0Hz,1H),4.76-4.63(m,1.5H),4.40-4.30(m,1H),3.98-3.70(m,2.5H),3.54-3.46(m,2H),3.37-3.31(m,0.5H),3.04-2.97(m,0.5H),2.95-2.73(m,2H),2.46(s,3H),2.29(s,3H),2.25-1.90(m,6H),1.41-1.17(m,3H)。MS(ESI):C26H31ClFN3O3理论值487;实测值488[M+H]+。
实施例67
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氟-5-甲基苯甲酰胺(E67)
实施例67使用与实施例66所述类似的步骤制备。1H NMR(400MHz,MeOD-d4):7.65(s,1H),7.50(d,J=9.6Hz,1H),7.32(d,J=9.6Hz,2H),7.21(d,J=9.0Hz,1H),4.76-4.60(m,1.5H),4.39-4.27(m,1H),3.98-3.70(m,2.5H),3.54-3.46(m,2H),3.37-3.31(m,0.5H),3.04-2.96(m,0.5H),2.87-2.62(m,2H),2.46(s,3H),2.29(s,3H),2.25-1.88(m,6H),1.37-1.13(m,3H)。MS(ESI):C26H31ClFN3O3理论值487;实测值488[M+H]+。
实施例68
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺(E68)
在氮气氛下,向5,6-二甲基烟酸(D3,55mg)和1滴DMF在DCM(5mL)中的溶液中滴加草酰氯(0.080mL)。将该混合物在室温搅拌1小时,然后减压浓缩。将该残余物溶于DCM(5mL)中,向其中加入((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮(D75,128mg)和DIPEA(0.191mL)。将所得混合物在室温搅拌3小时,然后真空浓缩。将该粗产物用制备型TLC纯化(用PE:EA=1:3洗脱),得到油状物。将该油状物用制备型HPLC纯化,得到标题化合物(20mg),其为白色固体。1H NMR(400MHz,MeOD-d4):8.84(s,1H),8.14(s,1H),7.36(s,1H),7.32(d,J=1.6Hz,1H),4.75-4.70(m,1H),4.69-4.62(m,0.5H),4.33-4.26(d,0.5H),4.22-4.18(m,0.5H),3.98-3.94(m,1H),3.89-3.82(m,1.5H),3.56-3.49(m,2H),3.36-3.34(m,0.5H),3.03-2.98(m,0.5H),2.90-2.84(m,1H),2.77-2.74(m,1H),2.61(s,3H),2.44(s,3H),2.32(s,3H),2.29-1.94(m,6H),1.40-1.25(m,3H)。MS(ESI):C26H33ClN4O3理论值484;实测值485[M+H]+。
实施例69-72
实施例69-72使用实施例68所述的类似步骤制备,具体的反应碱列于表中。
E69:N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺
E70:N-(5-氯-3-(((S)-3-乙基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)-2-甲基苯基)-5-氟-6-甲基烟酰胺
E71:5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)烟酰胺,三氟乙酸盐
E72:5-氟-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺
实施例73&74
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺&N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺(E73&E74)
将5,6-二甲基烟酸(D3,0.129g)在草酰氯(3.07mL)中的混合物在室温搅拌1小时。将该混合物真空浓缩。将该残余物加至((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)(四氢呋喃-3-基)甲酮(D76,200mg)和DIPEA(0.147g)在DCM(20mL)中的混合物中。将该混合物在20℃搅拌16小时,然后浓缩。将所得混合物用制备型HPLC和手性HPLC纯化,得到标题化合物(10mg和8mg),其为白色固体。异构体1:1H NMR(400MHz,MeOD-d4):8.82(d,J=2.0Hz,1H),8.12(d,J=1.2Hz,1H),7.35(d,J=2.4Hz,1H),7.30(d,J=2.0Hz,1H),4.67(brs,0.5H),4.33-4.24(m,1H),4.04-4.00(0.5H),3.95-3.76(m,4H),3.55-3.34(m,3.5H),3.03-2.96(m,0.5H),2.86(d,J=11.6Hz,1H),2.77-2.72(m,1H),2.59(s,3H),2.42(s,3H),2.31(s,3H),2.24-1.98(m,4H),1.38-1.23(m,3H)。MS(ESI):C26H33ClN4O3理论值484;实测值485[M+H]+。异构体2:1H NMR(400MHz,MeOD-d4):8.82(d,J=1.6Hz,1H),8.12(d,J=1.2Hz,1H),7.35(d,J=2.0Hz,1H),7.31(s,1H),4.66(brs,0.5H),4.34-4.30(m,1H),3.97-3.75(m,4.5H),3.56-3.37(m,3.5H),3.03-2.95(m,0.5H),2.86(d,J=11.6Hz,1H),2.78-2.72(m,1H),2.59(s,3H),2.42(s,3H),2.31(s,3H),2.26-1.98(m,4H),1.36-1.24(m,3H)。MS(ESI):C26H33ClN4O3理论值484;实测值485[M+H]+。
实施例75&76
5-氯-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺&5-氯-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺(E75&E76)
向5-氯-6-甲基烟酸(D5,400mg)在DCM(2mL)中的溶液中小心地加入草酰氯(0.612mL)。将该混合物搅拌0.5小时,然后浓缩,得到5-氯-6-甲基烟酰氯(500mg)。将一部分该残余物(113mg)加至((S)-4-(3-氨基-5-氟-2-甲基苄基)-2-甲基哌嗪-1-基)(四氢呋喃-3-基)甲酮(D80,200mg)和DMAP(219mg)在DCM(3mL)中的溶液中。将该混合物搅拌2小时,然后过滤。浓缩后,加入水。将所得混合物用EA萃取。将该有机相干燥,浓缩并用制备型HPLC纯化,得到标题化合物(6mg和12mg)。异构体1:1H NMR(400MHz,MeOD-d4):8.94(d,J=1.2Hz,1H),8.36(d,J=1.6Hz,1H),7.14-7.08(m,2H),4.67(m,0.5H),4.33-4.24(m,1H),4.04-4.00(m,0.5H),3.95-3.76(m,4H),3.56-3.35(m,3.5H),3.04-2.97(m,0.5H),2.87(d,J=11.2Hz,1H),2.78-2.73(m,1H),2.70(s,3H),2.29(s,3H),2.25-1.98(m,4H),1.39-1.24(m,3H)。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。异构体2:1H NMR(400MHz,MeOD-d4):8.94(d,J=1.2Hz,1H),8.36(d,J=1.2Hz,1H),7.14-7.08(m,2H),4.66(m,0.5H),4.33-4.31(m,1H),3.97-3.75(m,4.5H),3.56-3.37(m,3.5H),3.03-2.95(m,0.5H),2.87(d,J=11.6Hz,1H),2.79-2.73(m,1H),2.70(s,3H),2.29(s,3H),2.27-1.98(m,4H),1.37-1.24(m,3H)。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。
实施例77
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-氰基烟酰胺(E77)
向(S)-(4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)(四氢-2H-吡喃-4-基)甲酮(D81,112.7mg)和6-氰基烟酸(51.7mg)在无水DMF(5mL)中的溶液中加入HATU(178.7mg)和DIPEA(0.161mL)。将该混合物在室温搅拌过夜并用MDAP纯化(碱性条件,ACN/H2O(含0.05%NH3H2O),ACN%=30%-80%),得到标题化合物(40.7mg),其为灰白色固体。1HNMR(400MHz,MeOD-d4):9.23(s,1H),8.50(dd,J=8.1Hz,1.7Hz,1H),8.04(d,J=8.1Hz,1H),7.39(d,J=1.7Hz,1H),7.32(d,J=1.7Hz,1H),4.72-4.54(m,1H),4.37-4.22(m,1H),4.01-3.90(m,2H),3.84(d,J=13.0Hz,0.5H),3.58-3.44(m,4.5H),3.44-3.36(m,0.5H),3.03-2.82(m,2.5H),2.80-2.68(m,1H),2.32(s,3H),2.28-1.95(m,2H),1.93-1.50(m,4H),1.42-1.17(m,3H)。MS(ESI):C26H30ClN5O3理论值495;实测值496[M+H]+。
实施例78-87
实施例78-87使用实施例77所述的类似步骤制备。
E78:(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-2-氰基异烟酰胺
E79:(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺,三氟乙酸盐
E80:(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-甲基苯甲酰胺,三氟乙酸盐
E81:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-(氟甲基)烟酰胺
E82:N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-(氟甲基)烟酰胺
E83:(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-6-甲氧基烟酰胺
E84:(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺
E85:5-氯-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺
E86:5-氯-N-(5-氟-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺
E87:N-(5-氯-3-(((S)-3-乙基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)-2-甲基苯基)-6-甲基烟酰胺
实施例88
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺(E88)
向((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)((S)-四氢呋喃-2-基)甲酮(D74,100mg)、5-氯-6-甲基烟酸(D5,48.8mg)、HATU(162mg)在DCM(15mL)中的溶液中加入DIPEA(0.099mL)。将该混合物在室温搅拌过夜。加入冷水(30mL)并将该水层用DCM(2×30mL)萃取。将合并的有机层用Na2SO4干燥,过滤并真空浓缩。将该残余物用柱色谱(用PE:EA=50%-100%洗脱)和制备型HPLC纯化,得到标题化合物(26mg),其为白色固体。1H NMR(400MHz,MeOD-d4):8.94(s,1H),8.36(s,1H),7.36(d,J=1.8Hz,1H),7.31(d,J=1.8Hz,1H),4.74-4.59(m,1.5H),4.37-4.25(m,1H),3.97-3.88(m,1H),3.88-3.80(m,1H),3.80-3.73(m,0.5H),3.55-3.46(m,2H),3.45-3.35(m,0.5H),3.06-2.95(m,0.5H),2.88-2.80(m,1H),2.76-2.72(m,1H),2.70(s,3H),2.31(s,3H),2.27-1.68(m,6H),1.41-1.22(m,3H)。MS(ESI):C25H30Cl2N4O3理论值504;实测值505[M+H]+。
实施例89
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺(E89)
向((S)-4-(3-氨基-5-氯-2-甲基苄基)-2-甲基哌嗪-1-基)((R)-四氢呋喃-2-基)甲酮(D75,100mg)、5-氯-6-甲基烟酸(D5,48.8mg)和HATU(162mg)在DCM(10mL)中的混合物中加入DIPEA(0.099mL)。将该混合物在室温搅拌过夜。加入冷水(30mL)并将该水层用DCM(2×30mL)萃取。将合并的有机层用Na2SO4干燥,过滤并真空浓缩。将该残余物用柱色谱(用EA:PE=0%-50%洗脱)和制备型HPLC纯化,得到标题化合物(10mg),其为白色固体。1HNMR(400MHz,MeOD-d4):8.94(d,J=1.6Hz,1H),8.36(d,J=1.6Hz,1H),7.36(s,1H),7.32(d,J=4.2Hz,1H),4.90-4.69(m,1H),4.64-4.62(m,0.5H),4.33-4.20(m,1H),3.97-3.94(m,1H),3.88-3.81(m,1.5H),3.53-4.99(m,2H),3.34-3.33(m,0.5H),3.04-2.99(m,0.5H),2.88-2.81(m,1H),2.75-2.71(m,4H),2.31(s,3H),2.26-1.92(m,6H),1.38-1.25(m,3H)。MS(ESI):C25H30Cl2N4O3理论值504;实测值505[M+H]+。
实施例90
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺(E90)
将(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5,6-二甲基烟酰胺,二盐酸盐(D89,100mg)、(S)-四氢呋喃-2-甲酸(30.0mg)、DIPEA(66.8mg)和HATU(147mg)在DCM(2mL)中的混合物搅拌2小时。将该混合物浓缩并用制备型HPLC纯化,得到标题化合物(10mg)。1H NMR(400MHz,MeOD-d4):8.82(s,1H),8.13(s,1H),7.35(d,J=2.3Hz,1H),7.30(d,J=2.0Hz,1H),4.73-4.63(m,1.5H),4.35-4.28(m,1H),3.96-3.75(m,2.5H),3.54-3.47(m,2H),3.43-3.37(m,0.5H),3.04-2.98(m,0.5H),2.86-2.83(m,1H),2.74-2.71(m,1H),2.59(s,3H),2.42(s,3H),2.31(s,3H),2.26-1.89(m,6H),1.38-1.25(m,3H)。MS(ESI):C26H33ClN4O3理论值484;实测值485[M+H]+。
实施例91&92
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺和N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺(E91&E92)
向(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺(D57,238mg)在DMF(3mL)中的溶液中加入四氢呋喃-3-甲酸(83mg)、HATU(273mg)和TEA(182mg),将所得混合物在室温搅拌过夜。将该混合物分配至EA和水之间,将该有机层用盐水洗涤,用无水Na2SO4干燥。浓缩后,将所述混合物先用制备型HPLC纯化,用手性HPLC进一步纯化,得到标题化合物(56mg和54mg),其为黄色固体。异构体1:1H NMR(400MHz,DMSO-d6):10.27(s,1H),9.21(d,J=1.8Hz,1H),8.73(d,J=1.8Hz,1H),7.41(d,J=1.8Hz,1H),7.29(s,1H),4.56(brs,0.5H),4.22-4.19(m,1H),3.96-3.59(m,5H),3.50-3.44(m,2H),3.30-3.26(m,0.5H),3.22-3.16(m,0.5H),2.84-2.73(m,4.5H),2.66-2.63(m,1H),2.23(s,3H),2.16-1.86(m,4H),1.26-1.11(m,3H)。MS(ESI):C26H30ClN5O3理论值495;实测值496[M+H]+。异构体2:1H NMR(400MHz,DMSO-d6):10.28(s,1H),9.21(d,J=1.6Hz,1H),8.73(d,J=1.6Hz,1H),7.41(d,J=1.8Hz,1H),7.29(s,1H),4.55(brs,0.5H),4.25-4.19(m,1H),3.96-3.64(m,5H),3.50-3.43(m,2H),3.29-3.25(m,0.5H),3.24-3.18(m,0.5H),2.84-2.74(m,4.5H),2.66-2.63(m,1H),2.23(s,3H),2.18-1.85(m,4H),1.25-1.12(m,3H)。MS(ESI):C26H30ClN5O3理论值495;实测值496[M+H]+。
实施例93&94
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-甲氧基-6-甲基烟酰胺和N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-甲氧基-6-甲基烟酰胺(E93&E94)
向四氢呋喃-3-甲酸(17.29mg)在DCM(20mL)中的混合物中加入(S)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-甲氧基-6-甲基烟酰胺,二盐酸盐(D90,60mg)、HATU(67.9mg)、TEA(45.2mg)并将该反应在室温搅拌过夜。将该混合物分配至EA和水之间,将该有机层用盐水洗涤,用无水Na2SO4干燥。浓缩后,将该混合物先用制备型HPLC纯化,然后用手性HPLC进一步纯化,得到标题化合物(5mg和17mg),其为黄色固体。异构体1:1HNMR(400MHz,CDCl3)8.51(s,1H),7.87(s,1H),7.79(s,1H),7.66(s,1H),7.14(s,1H),4.77(brs,0.5H),4.42-4.39(m,0.5H),4.05-3.81(m,7H),3.65-3.62(m,0.5H),3.51-3.32(m,2.5H),3.20(brs,1H),2.99-2.93(m,0.5H),2.83-2.78(m,1H),2.70-2.67(m,1H),2.54(s,3H),2.31-2.14(m,4.5H),2.05-2.03(m,2H),1.35-1.23(m,4H)。MS(ESI):C26H33ClN4O4理论值500;实测值501[M+H]+。异构体2:1H NMR(400MHz,CDCl3)8.52(d,J=1.8Hz,1H),7.86(s,1H),7.82(s,1H),7.67(d,J=2.0Hz,1H),7.15(brs,1H),4.76(brs,0.5H),4.43-4.40(m,0.5H),4.12-3.84(m,7H),3.62-3.58(m,0.5H),3.51-3.35(m,2.5H),3.24-3.15(m,1H),2.98-2.94(m,0.5H),2.83-2.79(m,1H),2.70-2.67(m,1H),2.54(s,3H),2.31(s,3H),2.24-1.98(m,3.5H),1.37-1.23(m,4H)。MS(ESI):C26H33ClN4O4理论值500;实测值501[M+H]+。
实施例95
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(E95)
将(R)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺,二盐酸盐(D91,100mg)、(R)-四氢呋喃-2-甲酸(25.04mg)、HATU(123mg)和DIPEA(0.188mL)在DMF(5mL)中的混合物在室温搅拌2小时。将该混合物用水(50mL)稀释并用EA(3×50mL)萃取。将合并的有机层用盐水洗涤并用Na2SO4干燥。浓缩后,将该残余物用制备型HPLC纯化,得到标题化合物(60mg)。1H NMR(400MHz,MeOD-d4):8.87(s,1H),8.06(dd,J=9.8,1.8Hz,1H),7.36(d,J=2.3Hz,1H),7.30(d,J=2.3Hz,1H),4.71-4.65(m,1.5H),4.32-4.28(m,1H),3.95-3.75(m,2.5H),3.54-3.47(m,2H),3.42-3.36(m,0.5H),3.03-2.98(m,0.5H),2.85-2.83(m,1H),2.73-2.71(m,1H),2.60(d,J=3.0Hz,3H),2.30-1.89(m,9H),1.37-1.24(m,3H)。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。
实施例96
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(E96)
将(R)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺,二盐酸盐(D91,100mg)、(S)-四氢呋喃-2-甲酸(25.04mg)、HATU(123mg)和DIPEA(0.188mL)在DMF(5mL)中的混合物在室温搅拌2小时。将该混合物用水(50mL)稀释并用EA(3×50mL)萃取。将合并的有机层用盐水洗涤并用Na2SO4干燥。浓缩后,将该残余物用制备型HPLC纯化,得到标题化合物(60mg)。1H NMR(400MHz,MeOD-d4):8.87(s,1H),8.06(dd,J=9.6,1.2Hz,1H),7.36(brs,1H),7.30(brs,1H),4.72-4.62(m,1.5H),4.29-4.26(m,0.5H),4.18(brs,0.5H),3.98-3.91(m,1H),3.87-3.80(m,1.5H),3.54-3.46(m,2H),3.37-3.33(m,0.5H),3.04-2.98(m,0.5H),2.87-2.81(m,1H),2.74-2.72(m,1H),2.60(d,J=3.2Hz,3H),2.30-1.91(m,9H),1.38-1.23(m,3H)。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。
实施例97
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(E97)
向(R)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺,2三氟乙酸盐(D92,415mg)在DMF(4mL)中的溶液中加入(S)-四氢呋喃-3-甲酸(137.4mg,97%ee)在DMF(1mL)中的溶液,然后加入HATU(741.3mg)和DIPEA(0.55ml)。将该反应混合物搅拌过夜。将该混合物用制备型HPLC纯化并用手性SFC进一步纯化,得到标题化合物(20mg)。1H NMR(400MHz,MeOD-d4):8.87(s,1H),8.07(dd,J=9.8,1.7Hz,1H),7.36(d,J=2.2Hz,1H),7.31(d,J=2.0Hz,1H),4.67(brs,0.5H),4.32(d,J=13.7Hz,0.5H),4.24(brs,0.5H),4.06-3.75(m,4.5H),3.57-3.46(m,2H),3.46-3.34(m,1.5H),3.05-2.95(m,0.5H),2.86(d,J=11.2Hz,1H),2.79-2.70(m,1H),2.60(d,J=2.9Hz,3H),2.31(s,3H),2.26-1.97(m,4H),1.40-1.19(m,3H)。19F NMR(376MHz,MeOD-d4)-126.9。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。
实施例98
N-(5-氯-2-甲基-3-(((R)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺(E98)
向(R)-N-(5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺,2三氟乙酸盐(D92,415mg)在DMF(4mL)中的溶液中加入(R)-四氢呋喃-3-甲酸(136.7mg,90%ee)在DMF(1mL)中的溶液,然后加入HATU(741.3mg)和DIPEA(0.55ml)。将该反应混合物搅拌过夜。将该混合物用制备型HPLC纯化并用手性SFC进一步纯化,得到标题化合物(65mg)。1H NMR(400MHz,MeOD-d4)8.87(s,1H),8.07(dd,J=9.9,1.6Hz,1H),7.36(d,J=2.2Hz,1H),7.31(d,J=1.7Hz,1H),4.66(brs,0.5H),4.37-4.24(m,1H),3.99-3.72(m,4.5H),3.58-3.46(m,2H),3.45-3.35(m,1.5H),3.05-2.95(m,0.5H),2.86(d,J=11.5Hz,1H),2.79-2.70(m,1H),2.60(d,J=2.9Hz,3H),2.31(s,3H),2.28-1.96(m,4H),1.38-1.20(m,3H)。19F NMR(376MHz,MeOD-d4)-125.4。MS(ESI):C25H30ClFN4O3理论值488;实测值489[M+H]+。
实施例99
5-((5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)氨基甲酰基)-3-氟-2-甲基吡啶1-氧化物(E99)
在25℃,向(S)-5-((5-氯-2-甲基-3-((3-甲基哌嗪-1-基)甲基)苯基)氨基甲酰基)-3-氟-2-甲基吡啶1-氧化物,二盐酸盐(D96,280mg)在DMF(10mL)中的溶液中加入HATU(333mg)、DIPEA(0.612mL)和(R)-四氢呋喃-3-甲酸(102mg),将该反应混合物在25℃搅拌过夜。将溶剂减压蒸发并将该残余物溶于二氯甲烷中。将该有机层用水、盐水洗涤并用硫酸钠干燥。将溶剂浓缩并将该残余物用硅胶柱色谱纯化(石油醚/乙酸乙酯=1/2),得到标题化合物(140mg),其为白色固体。MS(ESI):C25H30ClFN4O4理论值505;实测值505[M+H]+。
生物学数据
如上所述,所述式I化合物是RORγ调节剂且用于治疗由RORγ介导的疾病。式I化合物的生物活性可使用用于确定作为RORγ调节剂的候选化合物的活性的任意合适测试以及组织和体内模型进行确定。
荧光能量转移(FRET)测试
该测试是在384-孔板上(Greiner 784076,Longwood,FL),在由50mM NaF、50mM 3-(N-吗啉代)丙磺酸,pH 7.5、50μM 3-[(3-胆酰胺丙基)二甲基氨基]-丙磺酸盐、0.1mg/mL牛血清白蛋白和10mM二硫基苏糖醇组成的测试缓冲液中进行的。总体积为10μL/孔。通过将适当量的生物素化SRC和铕标记的链霉亲和素(PerkinElmer Life and AnalyticalSciences,Waltham,MA)加至测试缓冲液中,其最终浓度分别是27和3.3nM,来制备所述铕标记的SRC1溶液。别藻蓝蛋白(APC)-标记的-LBD溶液通过加入适当量的生物素化RORγ-LBD和APC-标记的链霉亲和素(CR130-100;PerkinElmer Life and Analytical Sciences)至各自最终浓度为33nM来制备。在室温培养15min后,加入20倍过量的生物素以阻断剩余游离的链霉亲和素。然后将等体积的铕标记的SRC-和APC-标记的RORγ-LBD与0.2μM替代激动剂N-(2-氯-6-氟苄基)-N-((2’-甲氧基-[1,1’-联苯]-4-基)甲基)苯磺酰胺(Zhang,W.,等人,Mol.Pharmacol.2012,82,583-590)混合并以10μL体积/孔分配于384孔测试板中。所述384孔测试板在预先分配至各孔中的DMSO中具有100nL的测试化合物。将板在室温培养1h,然后在配置用于铕-APC标记的LANCE模式下的ViewLux(PerkinElmer Life and AnalyticalSciences)上读取。收集数据,然后通过Activitybase分析。
双重荧光共振能量转移(FRET)测试
该测试是基于这样的知识,即核受体以配体依赖性方式与辅因子(转录因子)相互作用。RORγ是典型的核受体,即其在配体结合域(LBD)中具有与辅激活因子相互作用的AF2结构域。相互作用的位点被绘图为辅激活因子SRC1(2)序列的LXXLL基序。含有LXXLL基序的短肽序列模拟全长辅激活因子的行为。
该测试测量辅激活因子肽与纯化的细菌表达的RORγ配体结合域(RORγ-LBD)的配体介导的相互作用,来间接评估配体结合。在不存在配体的情况下,RORγ具有与辅激活因子SRC1(2)的相互作用的基础水平,从而有可能发现抑制或增强RORγ/SRC1(2)相互作用的配体。
材料
产生RORγ-LBD细菌表达质粒
人RORγ配体结合域(RORγ-LBD)在大肠杆菌菌株BL21(DE3)中表达为氨基末端多组氨酸标记的融合蛋白。将编码该重组蛋白的DNA亚克隆至修饰的pET21a表达载体(Novagen)中。将修饰的多组氨酸标记(MKKHHHHHHLVPRGS)在框(frame)中融合至人RORγ序列的残基263-518。
蛋白质纯化
将约50g大肠杆菌细胞团粒再悬浮于300mL裂解缓冲液(30mM咪唑pH 7.0和150mMNaCl)中。通过超声处理裂解细胞,然后通过在4℃以20,000g离心30分钟来除去细胞碎片。澄清的上清液过滤通过0.45uM乙酸纤维素膜滤器。将澄清的裂解液装载至柱(XK-26)上,所述柱用ProBond镍螯合树脂(Invitrogen)填充,用30mM咪唑pH 7.0和150mM NaCl预平衡。用平衡缓冲液洗涤至基线吸收后,将柱用30-500mM咪唑pH 7.0的梯度展开。将含有RORγ-LBD蛋白的柱级份汇集并浓缩至体积5ml。将浓缩的蛋白质装载至Superdex 200柱上,所述柱用20mM Tris-Cl pH 7.2和200mM NaCl预平衡。将含有期望的RORγ-LBD蛋白的级份汇集在一起。
蛋白质生物素化
如下对纯化的RORγ-LBD进行缓冲液更换:相对PBS[100mM磷酸钠,pH 8和150mMNaCl]彻底透析[至少20体积的(>8000x)的3次更换]。RORγ-LBD在PBS中的浓度为约30uM。将5倍克分子浓度过量的NHS-LC-Biotin(Pierce)加入最小体积的PBS中。将该溶液在周围室温孵育60分钟,并不时轻轻混合。修饰的RORγ-LBD相对2次缓冲液更换-含有5mMDTT、2mMEDTA和2%蔗糖的TBS pH 8.0–进行透析,每次至少20倍体积。将修饰的蛋白质分配成等份,在干冰上冷冻,并在-80℃贮存。对生物素化的RORγ-LBD进行质谱分析以揭示经生物素化试剂修饰的程度。一般而言,约95%的所述蛋白质具有至少一个生物素化位点,以及生物素化的总体程度遵循范围为1-5个的多个位点的正态分布。使用类似方法产生对应于辅激活因子甾体激素受体共活化物SRC1(2)的氨基酸676至700(CPSSHSSLTERHKILHRLLQEGSPS)的生物素化的肽。
测试
制备铕标记的SRC1(2)肽:生物素化的SRC1(2)溶液如下制备:从100uM储备溶液中加入适量的生物素化的SRC1(2)至含有10mM新鲜添加的DTT(来自固体)的缓冲液,得到40nM的最终浓度。然后将适量的铕标记的链霉亲和素加到于管中的生物素化的SRC1(2)溶液中,得到10nM的最终浓度。将管轻轻倒置并在室温孵育15分钟。加入来自10mM储备溶液的过量20倍的生物素,然后将管轻轻倒置并在室温孵育10分钟。
制备APC标记的RORγ-LBD:生物素化的RORγ-LBD溶液如下制备:从储备溶液中加入适量的生物素化的RORγ-LBD至含有10mM新鲜添加的DTT(来自固体)的缓冲液,得到40nM的最终浓度。然后将适量的APC标记的链霉亲和素加到于管中的生物素化的RORγ-LBD溶液中,得到20nM的最终浓度。将管轻轻倒置并在室温孵育15分钟。加入来自10mM储备溶液的过量20倍的生物素,然后将管轻轻倒置并在室温孵育10分钟。
将等体积的上述铕标记的SRC1(2)肽和APC标记的RORγ-LBD轻轻混合在一起,得到20nM RORγ-LBD、10nM APC-链霉亲和素、20nM SRC1(2)和5nM铕-链霉亲和素。将反应混合物孵育5分钟。使用Thermo Combi Multidrop 384stacker装置,将25ul反应混合物/孔加至384孔测试板,所述板每孔含有于100%DMSO中的1ul测试化合物。将板孵育1小时,然后在ViewLux上以EU/APC的Lance模式读取。
Jurkat细胞荧光素酶测试
已知RORγ结合至IL17启动子中的CNS(保守的非编码序列)增强子元件。在该测试中,RORγ活性使用荧光素酶报道分子构建物间接评价,所述荧光素酶报道分子构建物含有具有RORγ特异性CNS增强子元件的人IL17启动子。化合物对RORγ活性的抑制将导致用所述报道分子构建物转染的Jurkat细胞的荧光素酶活性的降低。
材料
Jurkat细胞系
对于荧光素酶报道分子质粒,从人基因组DNA对含有RORγ特异性CNS增强子元件的3Kb人IL17启动子进行PCR扩增,然后克隆到测序为XhoI-HindIII(1.1Kb)和KpnI-XhoI(1.9Kb)片段的pGL4-Luc2/hygro报道分子质粒中。对于1.1Kb片段,使用PCR从293T细胞的基因组DNA扩增人IL17近侧启动子区,使用的引物如下:正向引物,5'-CTCGAGTAGAGCAGGACAGGGAGGAA-3'(XhoI位点加了下划线)和反向引物,5'-AAGCTTGGATGGATGAGTTTGTGCCT-3'(HindIII位点加了下划线)。切割所述1.1kb DNA带,纯化,并插入到pMD19-T Simple载体(Takara)中。DNA序列测定确认后,所述1.1kb DNA用XhoI和HindIII消化并插入到pGL4.31[luc2P/GAL4UAS/Hygro](Promega)的XhoI/HindIII位点,产生所述pIL17-1kb-luc报道分子构建物。对于所述1.9Kb片段,使用PCR从基因组DNA扩增人IL17启动子区,使用的引物如下:正向引物,5'-GGTACCTGCCCTGCTCTATCCTGAGT-3'(KpnI位点加了下划线)和反向引物,5'-CTCGAGTGGTGAGTGCTGAGAGATGG-3'(XhoI位点加了下划线)。切割所得1.9kb DNA带,凝胶纯化,并克隆到pMD19-T Simple载体(Takara)中。DNA序列测定分析揭示,存在三个点突变,但是没有一个影响RORγ结合。通过用KpnI和XhoI双重消化释放1.9kb DNA片段并插入到pIL17-1kb-luc中,产生荧光素酶报道分子质粒“pIL17-3kb-CNS-luc.”。为了表达RORγt,将与所公开的序列NM_001001523一致的人RORγt的全长cDNA在KpnI-NotI克隆位点克隆到pcDNA3.1中,产生RORγt过表达质粒“CDNA3.1DhRORγ49-8”。
将荧光素酶报道分子质粒和RORγt过表达质粒转染到Jurkat细胞系中,然后鉴定稳定的克隆。将稳定的克隆在于RPMI(1640)中的10%透析的FBS中培养,所述RPMI(1640)含有800ug/ml遗传霉素和400ug/ml潮霉素。
测试
将化合物以三个浓度(10mM、400uM和16uM)溶于DMSO中,然后分别以40nl、12.5nl、5nl分配到384孔测试板中。用纯的DMSO调节体积,得到最终均一的体积40nl。对上述Jurkat细胞进行计数并离心。弃去生长培养基,然后将细胞用测试培养基(不含酚红的RPMI)以1E-6/ml再悬浮。将细胞加到测试板中的每种化合物中。细胞为未处理的或用CD3微珠子(MiltenyiBiotec)以1ul珠子/500,000个细胞处理。将细胞培养过夜并进行荧光素酶测试(Promega)。经ViewLux(使用荧光素酶Greiner 384设置)收集数据。
Th17细胞分化测试
ELISA
使用CD4+T细胞分离II试剂盒根据制造商(Miltenyi Biotec)指示纯化小鼠CD4+细胞。96孔板用抗mCD3抗体预涂覆。未涂覆的孔作为对照。将CD4+细胞再悬浮于RPMI 1640完全培养基中,然后加到所述96孔板中。然后将细胞因子混合物(Cytokine cocktail)和化合物加到孔中。用于所述测试的抗体和细胞因子(所有都来自R&D系统)选自以下:抗mCD3;抗mCD28;抗mIFNγ;抗mIL4;mIL-6;mIL-23;mIL-1β;hTGF-β1。将培养基在37℃孵育3天,然后收集上清液进行ELISA。根据制造商(R&D系统)指示进行IL-17ELISAs。结果使用Prism软件分析,用非线性回归确定pIC50。
细胞内染色
将上述的Th17分化培养基维持5天,然后根据制造商(BD Biosciences)指示经IL-17和IFN-γ细胞内染色分析细胞。
测试数据
如果进行多次测定,那么下述数据表示多次测试结果的平均pIC50值。应理解,根据进行该测试人员所用的具体条件和步骤,下示数据可具有合理的变化。
所有示例性化合物均在上述FRET测试中进行测定,除了实施例97-99。发现所有测试化合物均具有5-8的pIC50。例如,发现实施例57和91的化合物分别具有约6.9和6.6的pIC50值。
所有示例性化合物均在上述双重FRET测试中进行测定,除了实施例14、18、45、46、53、66、74、75、81、82、93、97和98。发现所有测试化合物均具有5-8的pIC50。例如,发现实施例57和91的化合物分别具有大约6.7和6.1的pIC50值。
所有示例性化合物均在上述Jurkat细胞荧光素酶测试中进行测定,除了实施例2-6、10、15、16、20、21、28、29、34、35、38、39、44-55、63、64、68-72、75-79、81-84、87、89和95-99。发现所有测试化合物均具有5-9的pIC50。例如,发现实施例57和91的化合物分别具有大约7.6和7.9的pIC50值。
所有示例性化合物均在上述Th17细胞分化测试中进行测定,除了实施例20、21、38、39、48-51、54、55、61-64、66、67、77、79、81、82和95-99。发现所有测试化合物均具有6-9的pIC50。例如,发现实施例57和91的化合物分别具有大约7.09和7.76的pIC50值。
EAE研究
实验性自身免疫性脑脊髓炎(EAE)是多发性硬化的动物模型。在EAE研究中测量了测试化合物改善EAE的能力。将C57BL/6(B6)品系的野生型小鼠维持在无病原体的条件下。EAE如下诱导:静脉注射100ng百日咳毒素(List Biological Laboratories),然后在第0天用由在PBS中的MOG35-55肽(300μg/小鼠)和等体积的完全弗氏佐剂(Difco Laboratories)组成的乳液进行皮下免疫,所述完全弗氏佐剂含有5mg/ml热灭活的结核分枝杆菌H37Ra,接着如前所述在第2天进行另一静脉注射100ng的百日咳毒素(Wang等人(2006)J.Clin.Invest.116:2434-2441)。为了治疗EAE,每种化合物或媒介物PBS从第0天开始以选自3、10、30和100mg/kg的不同剂量口服给药,一天两次。使用EAE评分系统对小鼠的每日疾病严重度进行评分(Wang等人(2006)J.Clin.Invest.116:2434-2441):0,没有明显的疾病迹象;1,柔弱的尾部(limp tail)或后肢无力但两者不同时存在;2,柔弱的尾部和下肢轻瘫(无力,一个或两个后肢不完全麻痹);3,截瘫(两个后肢完全麻痹);4,截瘫伴前肢无力或麻痹;5,濒死状态或死亡。临床评分数据可表示为平均值±S.E.M。
体外经皮研究
体外经皮研究旨在预测针对银屑病的局部制剂的化合物获得的经皮渗透水平。该测试与化合物的内在效能一起用于预测化合物与靶标接合的成功的可能性。经皮渗透与内在效能的比例越高,局部皮肤浓度与内在效能的比例就越高,因此在局部制剂中化合物与靶标接合的可能性就越大。
化合物在经pH=6的改性的水性乳膏中制备。
水性乳膏组成
该研究可以用来自三种皮肤供体的经植皮的(dermatomed)人腹部皮肤进行,使用2cm2Franz扩散池(diffusion cell)。接收流体由在0.1%w/v叠氮化钠/磷酸盐缓冲生理盐水中的牛血清白蛋白(4%w/v)组成,然后可在37℃加热,为的是在皮肤表面达到32℃。可将乳膏制剂施用在供体侧,以10mg剂量,即,5mg/cm2。可在以下时间点取样:t=0、3、6、9和24h。然后可如下测试接受者样品:使用基于用乙腈使蛋白质沉淀的方法,接着进行LC/MS/MS分析。可使用历时24小时每cm2渗透至接受者隔室的个体的API(以多种组成)确定经皮流量(表示为ng/cm2/小时)。
咪喹莫特-诱导的皮肤炎症
咪喹莫特是一种免疫调节剂,其有效地活化特异性Toll样受体(例如,TLR7)并诱导需要免疫系统IL23R/RORγ/IL17轴的皮肤的刺激/炎症(van der Fits等人,(2009)JImmunol;182:5836-5845;Gray等人,(2013)Nature Immunol;Jun;14(6):584-92)。所述咪喹莫特-诱导的皮肤炎症模型可用于评估RORγ抑制剂降低小鼠中Th17-驱动的炎症的能力。对于耳厚度用计数工程师的测径器(Mitutoyo PK-0505)测量的仅耳皮肤炎症模型而言,8-12周龄的雌性野生型C57BL/6NTac小鼠可获自Taconic(Hudson,NY),并被给予每日局部剂量的10mg市售咪喹莫特乳膏(5%)(Aldara;Medicis),所述乳膏在大约11:00h分布于双耳,连续至多4天。或者,在大约11:00h将72mg的Aldara分布于小鼠的双耳和剃毛/脱毛的背部皮肤上连续3天,以检测RORγ-依赖性基因表达(使用Qiazol从双耳分离RNA,然后用RNeasy方案(Qiagen,Germantown,MD)净化;Taqman探针/引物针对B2M(Mm00437762_m1)、IL-17A(Mm00439619_m1)、IL-17F(Mm00521423_m1)或IL-22(Mm00444241_m1)(ThermoFisher Scientific,Inc.,Waltham,MA)设置,并离体刺激(抗-CD3(2ug/ml,克隆eBio500A2,eBioscience,San Diego,CA)、抗-CD28(1ug/ml,克隆37.51,BD Bioscience,San Jose,CA)、重组小鼠IL-1β(20ng/ml,R&D Systems,Minneapolis,MN)和重组小鼠IL-23(20ng/ml,R&D Systems,Minneapolis,MN)全血中(Meso Scale Discovery,Rockville,MD)IL-17A蛋白的表达。对于治疗这些模型中的皮肤炎症,将各化合物或媒介物(甲基纤维素的水溶液,1%w/v,Sigma Aldrich,St.Louis,MO)在大约08:00h和16:00h,通过口服强饲法以选自1、3、10和30mg/kg的不同剂量每日给药。
人外周血CD4+T细胞培养和细胞因子分析
人生物样品是购自AllCells,LLC和/或Stemcell Technologies,Inc的冷冻保存的人CD4+T细胞。所述CD4+T细胞通过以下被分化成Th17亚型:在Th17偏移混合物(skewingcocktail)(包含IL-1β(10ng/mL)、IL-6(30ng/mL)、TGFβ(0.5ng/mL)、IL-21(10ng/mL)、IL-23(10ng/mL)、抗-IFNγ(10μg/mL)和抗-IL-4(10μg/mL))的存在下,在用抗-CD3抗体(2μg/mL)涂布的组织培养板中的Iscove改良的Dulbecco培养基(IMDM)中培养5天,所述培养基含有10%HI-FBS、55μM 2-巯基乙醇和可溶性抗-CD28(3μg/mL)。为了检测化合物对Th17极化的影响,将在补充有所有Th17极化混合成分(上文)的IMDM中的新鲜解冻的CD4+细胞以低细胞密度(20,000细胞/孔)直接接种到已含有连续稀释的化合物的抗-CD3涂布的圆底96孔板上。将细胞在37℃不受干扰地培养5天。培养后,分别立即通过MSD电化学发光细胞因子测定(Mesoscale Discovery)和ELISA(Quantikine assay,R&D Systems)分析上清液中的分泌的IL-17A和IL-22蛋白。化合物处理以一式三份进行。
使用方法
式I化合物是RORγ调节剂且可用于治疗由RORγ介导的疾病,特别是自身免疫性或炎性疾病。本发明炎性或自身免疫性疾病的实例包括多发性硬化、类风湿性关节炎、银屑病、强直性脊柱炎、克罗恩病、炎性肠病、斯耶格伦综合征、视神经炎、慢性阻塞性肺病、哮喘、Ⅰ型糖尿病、视神经脊髓炎、重症肌无力、葡萄膜炎、格林-巴利综合症、银屑病性关节炎、格雷夫斯病和变态反应。因此,在另一方面,本发明涉及治疗由RORγ介导的自身免疫性和炎性疾病的方法。
在另一方面,本发明还提供了式(I)化合物,或其药学上可接受的盐,其用于治疗。
在另一方面,本发明还提供了式(I)化合物,或其药学上可接受的盐,其用于治疗由RORγ介导的炎性和自身免疫性疾病。
在另一方面,本发明提供了式(I)化合物,或其药学上可接受的盐,其用于治疗多发性硬化。
在另一方面,本发明提供了式(I)化合物,或其药学上可接受的盐,其用于治疗强直性脊柱炎。
在另一方面,本发明涉及治疗由RORγ介导的炎性或自身免疫性疾病的方法,其包括向有此需要的人给药治疗有效量的式(I)化合物,或其药学上可接受的盐。
还在其他方面,本发明涉及治疗多发性硬化的方法,其包括向有此需要的人给药治疗有效量的式(I)化合物,或其药学上可接受的盐。
还在其他方面,本发明涉及治疗强直性脊柱炎的方法,其包括向有此需要的人给药治疗有效量的式(I)化合物,或其药学上可接受的盐。
在另一方面,本发明涉及式(I)化合物或其药学上可接受的盐在制备用于治疗由RORγ介导的炎性或自身免疫性疾病的药物中的用途。
还在其他方面,本发明涉及式(I)化合物或其药学上可接受的盐在制备用于治疗多发性硬化的药物中的用途。
还在其他方面,本发明涉及式(I)化合物或其药学上可接受的盐在制备用于治疗强直性脊柱炎的药物中的用途。
本申请使用的"治疗"涉及病症时是指:(1)改善或预防所述病症的一种或多种生物学表现,(2)干扰(a)导致所述病症或对所述病症负责的生物学级联中的一个或多个点或(b)所述病症的一种或多种生物学表现,(3)减轻与所述病症相关的一种或多种症状或影响,或(4)减慢所述病症的进展或所述病症的一种或多种生物学表现。
如上所述,病症的"治疗"包括所述病症的预防。本领域技术人员将理解,"预防"不是一个绝对的术语。在医学上,"预防"被理解为是指对药物的预防性给药以基本上减小病症或其生物学表现的可能性或严重度,或延迟所述病症或其生物学表现的发作。
本发明化合物可以以任一合适的给药途径给药,包括全身给药和局部给药。全身给药包括口服给药、肠胃外给药、透皮给药、直肠给药和吸入给药。肠胃外给药表示除肠内、透皮或吸入以外的给药途径,通常为注射或输注。肠胃外给药包括静脉、肌内和皮下注射或输注。吸入表示给药至患者肺部,无论是通过嘴或是鼻通道吸入。局部给药包括施用至皮肤以及眼内、耳、阴道内和鼻内给药。
在给定时间内,本发明化合物可给药一次或根据给药方案以不同的时间间隔给药多个剂量。例如,剂量可以每天给药一、二、三或四次。剂量可以给药直到实现所需治疗效果,或无限期给药以维持所需治疗效果。本发明化合物的合适给药方案取决于化合物的药代动力学性质,例如吸收、分布和半衰期,其可由本领域技术人员确定。此外,本发明化合物的合适的给药方案(包括该给药方案的持续时间)取决于待治疗的病症、待治疗的病症的严重程度、待治疗的个体的年龄和身体状况、待治疗的个体的医学史、并行治疗的性质、所需治疗效果和本领域技术人员知识和专业内的类似因素。所述技术人员还将理解,合适的给药方案可根据个体对于给药方案的反应或由于个体需要随时间变化来进行调整。
典型的日剂量可取决于具体的所选的给药途径而变化。用于口服给药的典型的日剂量为0.1mg至1000mg。用于局部给药的典型的日剂量为约0.001%至约10%w/w(重量百分数),且优选约0.01%至约1%w/w。
此外,本发明化合物可作为前药给药。本申请使用的本发明化合物的“前药”是化合物的功能性衍生物,其一旦给药至个体,最终在体内释放本发明化合物。给药作为前药的本发明化合物可使得本领域技术人员能够进行以下的一种或多种:(a)调节化合物在体内的作用开始;(b)调节化合物在体内的作用持续时间;(c)调节化合物在体内的运输或分布;(d)调节化合物在体内的溶解度;以及(e)克服化合物遭受的副作用或其他困难。用于制备前药的典型的功能性衍生物包括在体内经化学或酶法断裂的化合物的修饰。这种修饰,包括制备磷酸酯、酰胺、酯、硫酯、碳酸酯和氨基甲酸酯,是本领域技术人员所熟知的。
组合物
在给药至个体前,通常(但不一定)将本发明化合物配制成药物组合物。因此,在另一方面,本发明涉及药物组合物,其包含本发明化合物和一种或多种药学上可接受的赋形剂。
本发明的药物组合物可被制备和包装成散装形式,其中可以提取安全且有效量的本发明化合物,然后给予个体,例如用粉末或浆状物的形式。或者,本发明的药物组合物可被制备和包装成单位剂型,其中每个物理离散单位含有安全且有效量的本发明化合物。当制备单位剂型时,本发明的药物组合物通常含有0.1mg到1000mg。
本发明的药物组合物通常含有一种本发明化合物。然而,在某些实施方案中,本发明的药物组合物含有超过一种本发明化合物。例如,在某些实施方案中,本发明的药物组合物含有两种本发明化合物。此外,本发明的药物组合物可任选还包含一种或多种另外的药学上可接受的化合物。
本申请使用的“药学上可接受的赋形剂”表示在给药形式中涉及的或与药物组合物相容的药学上可接受的物质、组合物或媒介物。当混合时,各赋形剂必须与药物组合物的其它成分相容,使得对个体给药时将基本上降低本发明化合物功效的相互作用以及将导致药物组合物成为药学上不可接受的相互作用得以避免。此外,各赋形剂的纯度当然必须足够高,使其为药学上可接受的。
本发明化合物和药学上可接受的一种或多种赋形剂通常被配制成通过所需给药途径适于给药至个体的剂型。例如,剂型包括那些(1)适于口服给药的剂型,例如片剂、胶囊剂、小胶囊剂、丸剂、锭剂、粉末、浆状物、酏剂、悬浮液、溶液、乳剂、囊袋剂和扁囊剂;(2)适于肠胃外给药的剂型,例如无菌溶液、悬浮液和用于重构(reconstitution)的粉末;(3)适于透皮给药的剂型,例如透皮贴剂;(4)适于直肠给药的剂型,例如栓剂;(5)适于吸入的剂型,例如干粉、气雾剂、悬浮液和溶液;和(6)适于局部给药的剂型,例如乳膏、软膏、洗剂、溶液、糊剂、喷雾剂、泡沫剂和凝胶剂。
合适的药学上可接受的赋形剂将根据所选具体的剂型而变化。此外,可根据在组合物中所起的具体功能来选择合适的药学上可接受的赋形剂。例如,可根据促进制备均一剂型的能力来选择某些药学上可接受的赋形剂。可根据促进制备稳定的剂型的能力来选择某些药学上可接受的赋形剂。可根据在给药至个体后促进本发明化合物从身体的一个器官或部分携带或运输至身体的另一器官或部分的能力来选择某些药学上可接受的赋形剂。某些药学上可接受的赋形剂可根据其提高患者顺应性的能力进行选择。
合适的药学上可接受的赋形剂包括下列赋形剂类型:稀释剂、填充剂、粘合剂、崩解剂、润滑剂、助流剂、制粒剂、包衣剂、润湿剂、溶剂、共溶剂、助悬剂、乳化剂、增甜剂、调味剂、掩味剂、着色剂、防结块剂、保湿剂、螯合剂、增塑剂、增粘剂、抗氧化剂、防腐剂、稳定剂、表面活性剂和缓冲剂。本领域技术人员将理解,某些药学上可接受的赋形剂可以以多于一种功能和以替代性功能来使用,取决于所述赋形剂在制剂中存在多少和在制剂中存在何种其它成分。
具有本领域的知识和技术的技术人员能够选择出以适当量用于本发明的合适的药学上可接受的赋形剂。此外,有许多本领域技术人员可用的资源,这些资源描述了药学上可接受的赋形剂且其可用于选择合适的药学上可接受的赋形剂。实例包括Remington's Pharmaceutical Sciences(Mack Publishing Company),The Handbook of Pharmaceutical Additives(Gower Publishing Limited),和The Handbook of Pharmaceutical Excipients(the American Pharmaceutical Association and thePharmaceutical Press)。
本发明的药物组合物使用本领域技术人员已知的技术和方法制备。通常用于本领域的一些方法描述在Remington's Pharmaceutical Sciences(Mack PublishingCompany)中。
一方面,本发明涉及固体口服剂型,例如片剂或胶囊剂,其包含安全且有效量的本发明化合物以及稀释剂或填充剂。合适的稀释剂和填充剂包括乳糖、蔗糖、葡萄糖、甘露醇、山梨醇、淀粉(例如玉米淀粉、马铃薯淀粉和预胶化淀粉)、纤维素及其衍生物(例如微晶纤维素)、硫酸钙和磷酸氢钙。口服固体剂型还可包含粘合剂。合适的粘合剂包括淀粉(例如玉米淀粉、马铃薯淀粉和预胶化淀粉)、明胶、阿拉伯胶、海藻酸钠、海藻酸、西黄蓍胶、瓜尔胶、聚维酮和纤维素及其衍生物(例如微晶纤维素)。口服固体剂型还可包含崩解剂。合适的崩解剂包括交聚维酮、淀粉羟乙酸钠、交联羧甲基纤维素、海藻酸和羧甲基纤维素钠。口服固体剂型还可包含润滑剂。合适的润滑剂包括硬脂酸、硬脂酸镁、硬脂酸钙和滑石。
Claims (20)
1.式I化合物或其药学上可接受的盐:
其中:
R1为:
-5-6元单环杂芳基,其任选被以下取代:i)任选被CF3或CN取代的C1-C5烷基;ii)CH2F;或iii)1-2个独立地选自以下的取代基:卤素、甲基、甲氧基和CN;其中所述5-6元单环杂芳基选自:吡咯基、吡唑基、咪唑基、噁唑基、异噁唑基、噁二唑基、噻唑基、异噻唑基、噻二唑基、呋喃基、呋咱基、噻吩基、三唑基、吡啶基、嘧啶基、哒嗪基、吡嗪基、三嗪基、四嗪基和四唑基;或
-苯基,其被1-2个独立地选自以下的取代基取代:CN、卤素和甲基;
R2为C1-C3烷基;
R3为卤素;
R4为H;
R5为C1-C3烷基;
R6为H或甲基;和
R7为四氢呋喃基或四氢吡喃基,其中所述四氢呋喃基或四氢吡喃基任选被甲基取代。
2.权利要求1的化合物或盐,其中R1为被CN取代的苯基。
3.权利要求1的化合物或盐,其中R1为被CN和F取代的苯基。
4.权利要求1的化合物或盐,其中R1为被以下取代的吡啶基:i)甲基和F;ii)甲基和Cl;iii)甲基和CN;或iv)CN和F。
5.权利要求1的化合物或盐,其中R1为被以下取代的吡啶基:i)甲基和F。
6.权利要求1-5中任一项的化合物或盐,其中R2为甲基。
7.权利要求1-5中任一项的化合物或盐,其中R3为Cl。
8.权利要求1-5中任一项的化合物或盐,其中R5为甲基。
9.权利要求1-5中任一项的化合物或盐,其中R6为H。
10.权利要求1-5中任一项的化合物或盐,其中R7为四氢呋喃基。
11.权利要求1-5中任一项的化合物或盐,其中R7为四氢吡喃基。
12.权利要求1的化合物或其药学上可接受的盐,所述化合物选自:
(S)-N-(5-氯-2-甲基-3-((3-甲基-4-(四氢-2H-吡喃-4-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-5-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-3-氰基苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-3-氰基-4-氟苯甲酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-5-氰基-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺;
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺;和
5-氯-N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((R)-四氢呋喃-2-羰基)哌嗪-1-基)甲基)苯基)-6-甲基烟酰胺。
13.权利要求1的化合物或其药学上可接受的盐,所述化合物为N-(5-氯-2-甲基-3-(((S)-3-甲基-4-((S)-四氢呋喃-3-羰基)哌嗪-1-基)甲基)苯基)-5-氟-6-甲基烟酰胺。
14.药物组合物,其包含权利要求1-13中任一项的式I化合物或其药学上可接受的盐和药学上可接受的赋形剂。
15.药物组合物,其包含权利要求1-13中任一项的式I化合物或其药学上可接受的盐和药学上可接受的载体。
16.权利要求1-5、12或13中任一项的化合物或其药学上可接受的盐,其用于治疗。
17.权利要求1-5、12或13中任一项的化合物或其药学上可接受的盐,其用于治疗多发性硬化。
18.权利要求1-5、12或13中任一项的化合物或其药学上可接受的盐,其用于治疗强直性脊柱炎。
19.权利要求1-13中任一项的化合物或其药学上可接受的盐在制备用于治疗多发性硬化的药物中的用途。
20.权利要求1-13中任一项的化合物或其药学上可接受的盐在制备用于治疗强直性脊柱炎的药物中的用途。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNPCT/CN2014/078701 | 2014-05-28 | ||
| CN2014078701 | 2014-05-28 | ||
| PCT/CN2015/079755 WO2015180614A1 (en) | 2014-05-28 | 2015-05-26 | Novel compounds |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107001342A CN107001342A (zh) | 2017-08-01 |
| CN107001342B true CN107001342B (zh) | 2020-02-07 |
Family
ID=54698095
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201580039289.0A Active CN107001342B (zh) | 2014-05-28 | 2015-05-26 | 新化合物 |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US9868724B2 (zh) |
| EP (1) | EP3148987B1 (zh) |
| JP (1) | JP6522665B2 (zh) |
| KR (1) | KR20170008852A (zh) |
| CN (1) | CN107001342B (zh) |
| AU (1) | AU2015267911B2 (zh) |
| CA (1) | CA2953637C (zh) |
| CL (1) | CL2016003023A1 (zh) |
| CR (1) | CR20160549A (zh) |
| DO (1) | DOP2016000311A (zh) |
| EA (1) | EA032272B1 (zh) |
| ES (1) | ES2716966T3 (zh) |
| IL (1) | IL248983A0 (zh) |
| MX (1) | MX2016015631A (zh) |
| PE (1) | PE20170137A1 (zh) |
| PH (1) | PH12016502331A1 (zh) |
| SG (1) | SG11201609722UA (zh) |
| WO (1) | WO2015180614A1 (zh) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PL2928885T3 (pl) | 2012-12-06 | 2017-09-29 | Glaxo Group Limited | Modulatory związanego z retinoidem receptora sierocego gamma (ror-gramma) do stosowania w leczeniu chorób autoimmunizacyjnych i zapalnych |
| JP6759110B2 (ja) | 2014-05-28 | 2020-09-23 | グラクソスミスクライン、インテレクチュアル、プロパティー、ディベロップメント、リミテッドGlaxosmithkline Intellectual Property Development Limited | 新規な化合物 |
| EP3148985A4 (en) | 2014-05-28 | 2018-01-17 | GlaxoSmithKline Intellectual Property Development Limited | Novel compounds |
| SG11201609722UA (en) | 2014-05-28 | 2016-12-29 | Glaxosmithkline Ip Dev Ltd | Novel compounds |
| CN109476653B (zh) * | 2016-07-13 | 2022-04-22 | 利奥制药有限公司 | 类视黄醇相关孤儿受体γ的杂芳族调节剂 |
| CN109134476B (zh) * | 2017-06-15 | 2021-03-19 | 复旦大学 | 桥环哌嗪类衍生物或其盐及其制备方法和用途 |
Family Cites Families (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7790726B2 (en) | 2005-08-16 | 2010-09-07 | Chemocentryx, Inc. | Monocyclic and bicyclic compounds and methods of use |
| US20070208000A1 (en) | 2005-12-15 | 2007-09-06 | Morgan Bradley P | Certain chemical entities, compositions and methods |
| US7825120B2 (en) | 2005-12-15 | 2010-11-02 | Cytokinetics, Inc. | Certain substituted ((piperazin-1-ylmethyl)benzyl)ureas |
| US7989455B2 (en) | 2005-12-19 | 2011-08-02 | Cytokinetics, Inc. | Compounds, compositions and methods |
| WO2012028100A1 (en) | 2010-09-01 | 2012-03-08 | Glaxo Group Limited | Novel compounds |
| WO2012027965A1 (en) | 2010-09-01 | 2012-03-08 | Glaxo Group Limited | Novel compounds |
| WO2012100734A1 (en) | 2011-01-24 | 2012-08-02 | Glaxo Group Limited | Compounds useful as retinoid-related orphan receptor gamma modulators |
| WO2012100732A1 (en) | 2011-01-24 | 2012-08-02 | Glaxo Group Limited | Retinoid-related orphan receptor gamma modulators, composition containing them and uses thereof |
| EP2511263A1 (en) | 2011-04-14 | 2012-10-17 | Phenex Pharmaceuticals AG | Pyrrolo sulfonamide compounds for modulation of orphan nuclear receptor RAR-related orphan receptor-gamma (RORgamma, NR1F3) activity and for the treatment of chronic inflammatory and autoimmune diseases |
| WO2012145254A2 (en) | 2011-04-16 | 2012-10-26 | Board Of Regents, The University Of Texas System | Methods of using inhibitors of rorϒt to treat disease |
| AR086156A1 (es) | 2011-04-28 | 2013-11-20 | Japan Tobacco Inc | Compuesto de amida y su uso medicinal |
| US9586928B2 (en) * | 2011-05-16 | 2017-03-07 | The Scripps Research Institute | Modulators of the nuclear hormone receptor ROR |
| SG11201400456RA (en) * | 2011-09-09 | 2014-04-28 | Univ New York | Amido compounds as ror?tmodulators and uses thereof |
| GB201116641D0 (en) | 2011-09-27 | 2011-11-09 | Glaxo Group Ltd | Novel compounds |
| WO2013100027A1 (ja) * | 2011-12-28 | 2013-07-04 | 武田薬品工業株式会社 | 複素環化合物 |
| PE20142400A1 (es) | 2012-04-27 | 2015-02-04 | Glaxo Group Ltd | Compuestos novedosos |
| GB201207406D0 (en) | 2012-04-27 | 2012-06-13 | Glaxo Group Ltd | Novel compounds |
| JO3215B1 (ar) * | 2012-08-09 | 2018-03-08 | Phenex Pharmaceuticals Ag | حلقات غير متجانسة بها 5 ذرات تحتوي على النيتروجين بها استبدال بكربوكساميد أو سلفوناميد كمعدلات لمستقبل نووي غير محمي RORy |
| EA026415B1 (ru) * | 2012-10-16 | 2017-04-28 | Янссен Фармацевтика Нв | Связанные с метиленом хинолиниловые модуляторы ror-гамма-t |
| PL2928885T3 (pl) | 2012-12-06 | 2017-09-29 | Glaxo Group Limited | Modulatory związanego z retinoidem receptora sierocego gamma (ror-gramma) do stosowania w leczeniu chorób autoimmunizacyjnych i zapalnych |
| WO2013171729A2 (en) | 2013-01-08 | 2013-11-21 | Glenmark Pharmaceuticals S.A. | Aryl and heteroaryl amide compounds as rorgamat modulator |
| WO2015061515A1 (en) | 2013-10-25 | 2015-04-30 | Glaxosmithkline Llc | Novel compounds |
| US10286000B2 (en) | 2013-10-25 | 2019-05-14 | St. Jude Children's Research Hospital, Inc. | Retinoid X receptor-gamma agonists and retinoid X receptor-alpha antagonists for treatment of cancer |
| SG11201609722UA (en) | 2014-05-28 | 2016-12-29 | Glaxosmithkline Ip Dev Ltd | Novel compounds |
| EP3148985A4 (en) | 2014-05-28 | 2018-01-17 | GlaxoSmithKline Intellectual Property Development Limited | Novel compounds |
| JP6759110B2 (ja) | 2014-05-28 | 2020-09-23 | グラクソスミスクライン、インテレクチュアル、プロパティー、ディベロップメント、リミテッドGlaxosmithkline Intellectual Property Development Limited | 新規な化合物 |
-
2015
- 2015-05-26 SG SG11201609722UA patent/SG11201609722UA/en unknown
- 2015-05-26 CA CA2953637A patent/CA2953637C/en active Active
- 2015-05-26 EP EP15800053.9A patent/EP3148987B1/en active Active
- 2015-05-26 MX MX2016015631A patent/MX2016015631A/es unknown
- 2015-05-26 PE PE2016002268A patent/PE20170137A1/es not_active Application Discontinuation
- 2015-05-26 ES ES15800053T patent/ES2716966T3/es active Active
- 2015-05-26 AU AU2015267911A patent/AU2015267911B2/en not_active Ceased
- 2015-05-26 CN CN201580039289.0A patent/CN107001342B/zh active Active
- 2015-05-26 JP JP2016569773A patent/JP6522665B2/ja active Active
- 2015-05-26 KR KR1020167036018A patent/KR20170008852A/ko unknown
- 2015-05-26 EA EA201692395A patent/EA032272B1/ru not_active IP Right Cessation
- 2015-05-26 US US15/314,107 patent/US9868724B2/en active Active
- 2015-05-26 WO PCT/CN2015/079755 patent/WO2015180614A1/en active Application Filing
- 2015-05-26 CR CR20160549A patent/CR20160549A/es unknown
-
2016
- 2016-11-15 IL IL248983A patent/IL248983A0/en unknown
- 2016-11-23 PH PH12016502331A patent/PH12016502331A1/en unknown
- 2016-11-24 CL CL2016003023A patent/CL2016003023A1/es unknown
- 2016-11-28 DO DO2016000311A patent/DOP2016000311A/es unknown
Also Published As
| Publication number | Publication date |
|---|---|
| EP3148987A4 (en) | 2017-11-15 |
| CL2016003023A1 (es) | 2017-04-21 |
| CN107001342A (zh) | 2017-08-01 |
| US9868724B2 (en) | 2018-01-16 |
| JP6522665B2 (ja) | 2019-05-29 |
| EA201692395A1 (ru) | 2017-03-31 |
| WO2015180614A1 (en) | 2015-12-03 |
| JP2017519738A (ja) | 2017-07-20 |
| EP3148987A1 (en) | 2017-04-05 |
| AU2015267911B2 (en) | 2017-10-26 |
| PE20170137A1 (es) | 2017-03-05 |
| AU2015267911A1 (en) | 2016-12-01 |
| MX2016015631A (es) | 2017-04-25 |
| SG11201609722UA (en) | 2016-12-29 |
| US20170101399A1 (en) | 2017-04-13 |
| CR20160549A (es) | 2017-04-28 |
| IL248983A0 (en) | 2017-01-31 |
| KR20170008852A (ko) | 2017-01-24 |
| DOP2016000311A (es) | 2017-02-28 |
| PH12016502331A1 (en) | 2017-02-06 |
| ES2716966T3 (es) | 2019-06-18 |
| CA2953637A1 (en) | 2015-12-03 |
| CA2953637C (en) | 2022-11-29 |
| EP3148987B1 (en) | 2019-01-02 |
| EA032272B1 (ru) | 2019-05-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107001342B (zh) | 新化合物 | |
| CN110248939B (zh) | 作为rho-激酶抑制剂的酪氨酸酰胺衍生物 | |
| EP3468960B1 (en) | Chemical compounds as atf4 pathway inhibitors | |
| US10005731B2 (en) | Modulators of the retinoid-related orphan receptor gamma (ROR-gamma) for use in the treatment of autoimmune and inflammatory diseases | |
| CN106536489B (zh) | 类视黄醇相关孤儿受体γ调节剂及其用途 | |
| US9902715B2 (en) | Compounds | |
| CN111050765A (zh) | 螺环化合物及其制造和使用方法 | |
| TW201714884A (zh) | 新穎化合物 | |
| BR112016027749B1 (pt) | Composto modulador do receptor órfão relacionado à retinóide gama (rory), composição farmacêutica compreendendo dito composto e uso do mesmo para o tratamento de esclerose múltipla e espondilite anquilosante |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |