CN106977494B - Substituted pyrazole amide compounds and application thereof - Google Patents
Substituted pyrazole amide compounds and application thereof Download PDFInfo
- Publication number
- CN106977494B CN106977494B CN201610028679.4A CN201610028679A CN106977494B CN 106977494 B CN106977494 B CN 106977494B CN 201610028679 A CN201610028679 A CN 201610028679A CN 106977494 B CN106977494 B CN 106977494B
- Authority
- CN
- China
- Prior art keywords
- alkyl
- radical
- halo
- compounds
- substituted
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Dentistry (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Plant Pathology (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Pest Control & Pesticides (AREA)
- Agronomy & Crop Science (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
The invention discloses a substituted pyrazole amide compound, an N-oxide, a salt and a composition thereof, wherein the structure is shown as a general formula I, and the definition of each substituent group in the formula is shown in the specification. The substituted pyrazole amide compound has broad-spectrum insecticidal activity, and can obtain good control effect on aphids, chilo suppressalis, diamond back moths, beet armyworms and the like at very low dosage.
Description
Technical Field
The invention belongs to the field of pesticides, and particularly relates to a substituted pyrazole amide compound, an N-oxide, a salt and a composition, and application of the substituted pyrazole amide compound and the N-oxide, the salt and the composition as pesticides in agriculture or other fields.
Background
Anthranilic acid amides (ryanodine receptor inhibitors) are effective insecticides developed in recent years for controlling lepidopteran pests.
The following compounds with insecticidal activity are disclosed in patent WO 03015519:
the following compounds with insecticidal activity are disclosed in patent WO 2004067528:
all of the compounds disclosed in the above patents (applications) have similarities to the compounds of the present invention, but there are significant differences in structure.
Disclosure of Invention
The invention aims to provide a substituted pyrazole amide compound and an N-oxide or salt thereof, which can control various pests and diseases under a small dosage, and can be applied to control invertebrate pests in agricultural and non-agricultural environments.
The technical scheme of the invention is as follows:
the invention provides a substituted pyrazole amide compound, which is shown as a general formula I:
in the formula:
a and B are the same or different and are independently selected from O, S or NRa;Ra=H、CN、NO2、NH2、NHCH3、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6An alkynyl group;
x is selected from H, halogen, CN, NO2、NH2、CHO、CH=NOCH3、CH=NNHCH3、CH=NN(CH3)2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C2-C6Alkenyl radicalHalogen substituted C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6An alkynyl group;
z is selected from N or CRb;RbH, halogen or NO2;
R1Selected from halogen, CN, NO2、NH2、CHO、Si(CH3)3、P(CH3)2、P(O)(CH3)2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl, oxaC2-C8Cycloalkyl, aza C2-C8Cycloalkyl, thia C2-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkylsulfinyl group C1-C6Alkyl, halo C1-C6Alkylsulfinyl group C1-C6Alkyl radical, C1-C6Alkoxysulfinyl C1-C6Alkyl, halo C1-C6Alkoxysulfinyl C1-C6Alkyl radical, C1-C6Alkanesulfonyl group C1-C6Alkyl, halo C1-C6Alkanesulfonyl group C1-C6Alkyl radical, C1-C6Alkoxy sulfonyl C1-C6Alkyl, halo C1-C6Alkoxy sulfonyl C1-C6Alkyl, amino C1-C6Alkyl, amino-halo C1-C6Alkyl, hydroxy C1-C6Alkyl, hydroxy-halogeno-C1-C6Alkyl, mercapto C1-C6Alkyl, mercapto halogenated C1-C6Alkyl, cyano C1-C6Alkyl, cyano-halo C1-C6Alkyl radical, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical、C1-C6Alkoxy halogeno C1-C6Alkyl, halo C1-C6Alkoxy halogeno C1-C6Alkyl radical, C1-C6Alkylthio group C1-C6Alkyl, halo C1-C6Alkylthio group C1-C6Alkyl radical, C1-C6Alkylthio halogeno C1-C6Alkyl, halo C1-C6Alkylthio halogeno C1-C6Alkyl radical, C1-C6Alkylamino radical C1-C6Alkyl, halo C1-C6Alkylamino radical C1-C6Alkyl radical, C1-C6Alkylamino halogeno C1-C6Alkyl radical, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylthio carbonyl, halo C1-C6Alkylthio carbonyl group, C1-C6Alkylaminocarbonyl, halogeno C1-C6Alkylamino carbonyl, C1-C6Alkylamino thiocarbonyl, halogeno C1-C6Alkylamino thiocarbonyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkylamino carbonyl group C1-C6Alkyl, halo C1-C6Alkylamino carbonyl group C1-C6Alkyl radical, C1-C6Alkylthio carbonyl group C1-C6Alkyl, halo C1-C6Alkylthio carbonyl group C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkyl, substituted or unsubstituted aryl halo C1-C6Alkyl, substituted or unsubstituted aryloxy C1-C6Alkyl, substituted or unsubstitutedAryloxy halogeno C of1-C6Alkyl, substituted or unsubstituted arylamine group C1-C6Alkyl, substituted or unsubstituted arylamino halogeno C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkyl, substituted or unsubstituted heteroaryl halo C1-C6Alkyl, substituted or unsubstituted heteroaryloxy C1-C6Alkyl, substituted or unsubstituted heteroaryloxy-halogeno C1-C6Alkyl, substituted or unsubstituted heteroarylamino C1-C6Alkyl, substituted or unsubstituted heteroarylamine halo C1-C6Alkyl, substituted or unsubstituted arylcarbonyl C1-C6Alkyl, substituted or unsubstituted arylcarbonyl halogeno C1-C6Alkyl, substituted or unsubstituted aryloxycarbonyl C1-C6Alkyl, substituted or unsubstituted aryloxycarbonyl halo C1-C6Alkyl, substituted or unsubstituted arylaminocarbonyl C1-C6Alkyl, substituted or unsubstituted arylaminocarbonyl haloC1-C6Alkyl, substituted or unsubstituted heteroarylcarbonyl C1-C6Alkyl, substituted or unsubstituted heteroarylcarbonyl halide C1-C6Alkyl, substituted or unsubstituted heteroaryloxycarbonyl C1-C6Alkyl, substituted or unsubstituted heteroaryloxycarbonyl-halogeno C1-C6Alkyl, substituted or unsubstituted heteroarylaminocarbonyl C1-C6Alkyl, substituted or unsubstituted heteroarylaminocarbonyl halo C1-C6Alkyl, substituted or unsubstituted arylcarbonyl, substituted or unsubstituted aryloxycarbonyl, substituted or unsubstituted arylaminocarbonyl, substituted or unsubstituted arylaminothocarbonyl, substituted or unsubstituted heteroarylcarbonyl, substituted or unsubstituted heteroaryloxycarbonyl, substituted or unsubstituted heteroarylaminocarbonyl, substituted or unsubstituted arylC1-C6Alkoxy radical C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkoxy radical C1-C6An alkyl group;
R2,R3can be the same or different and is respectively selected from H, halogen, CN and NO2、NH2、CONH2、C1-C6Alkyl, halo C1-C6Alkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylamino, halogeno C1-C6Alkylamino radical, di (C)1-C6Alkyl) amino, C1-C6Alkyl (halo C)1-C6Alkyl) amino, C1-C6Alkylcarbonylamino, halo C1-C6Alkylcarbonylamino group, C1-C6Alkoxycarbonylamino, halo C1-C6Alkoxycarbonylamino group, C1-C6Alkylsulfinylamino, halogeno C1-C6Alkylsulfinylamino group, C1-C6Alkylsulfonylamino, halo C1-C6Alkylsulfonylamino group, C1-C6Alkylcarbonyl (C)1-C6Alkyl) amino, halo C1-C6Alkylcarbonyl (C)1-C6Alkyl) amino, C1-C6Alkoxycarbonyl (C)1-C6Alkyl) amino, halo C1-C6Alkoxycarbonyl (C)1-C6Alkyl) amino, C1-C6Alkylsulfinyl (C)1-C6Alkyl) amino, halo C1-C6Alkylsulfinyl (C)1-C6Alkyl) amino, C1-C6Alkylsulfonyl (C)1-C6Alkyl) amino, halo C1-C6Alkylsulfonyl (C)1-C6Alkyl) amino, C1-C6Alkylthio, halo C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylaminocarbonyl, halogeno C1-C6Alkylamino carbonyl, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkoxy, halo C1-C6Alkyl carbonyl radical C1-C6Alkoxy radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkoxy, halo C1-C6Alkoxycarbonyl radical C1-C6Alkoxy, unsubstituted or substituted amino C1-C6Alkyl, aryl, aryloxy, aryl C1-C6Alkyl, aryl C1-C6Alkoxy, heteroaryl C1-C6Alkyl, heteroaryl C1-C6Alkoxy groups: halogen, cyano, nitro, C1-C6Alkyl, halo C1-C6Alkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylthio or C1-C6An alkylcarbonyl group;
R4selected from H, halogen, CN, CONH2、CSNH2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C2-C6Alkenyloxy, halogeno C2-C6Alkenyloxy radical, C2-C6Alkynyloxy, halo C2-C6Alkynyloxy, cyano C1-C6Alkyl, cyano-halo C1-C6Alkyl, cyano C1-C6Alkoxy, cyano-halogeno C1-C6Alkoxy radical, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkylsulfinyloxy, halogeno C1-C6Alkylsulfinyloxy, C1-C6Alkylsulfinylamino, halogeno C1-C6Alkylsulfinylamino group, C1-C6Alkylsulfinyloxy C1-C6Alkyl, halo C1-C6Alkylsulfinyloxy C1-C6Alkyl radical, C1-C6Alkanesulfinylamino C1-C6Alkyl, halo C1-C6Alkanesulfinylamino C1-C6Alkyl radical, C1-C6Alkylsulfonyloxy, halo C1-C6Alkylsulfonyloxy, C1-C6Alkanesulfonylamino, halo C1-C6Alkylsulfonylamino group, C1-C6Alkylsulfonyloxy C1-C6Alkyl, halo C1-C6Alkylsulfonyloxy C1-C6Alkyl radical, C1-C6Alkanesulfonylamino C1-C6Alkyl, halo C1-C6Alkanesulfonylamino C1-C6Alkyl radical, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylaminocarbonyl, halogeno C1-C6Alkylamino carbonyl, C1-C6Alkoxycarbonyl radical C1-C6Alkoxy, halo C1-C6Alkoxycarbonyl radical C1-C6Alkoxy radical, C1-C6Alkylamino carbonyl group C1-C6Alkoxy, halo C1-C6Alkylamino carbonyl group C1-C6Alkoxy radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkylamino carbonyl group C1-C6Alkyl, halo C1-C6Alkylamino carbonyl group C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkoxy, halo C1-C6Alkyl carbonyl radical C1-C6Alkoxy, substituted or unsubstituted aryl C1-C6Alkyl, substituted or unsubstituted aryl halo C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkoxy, substituted or unsubstituted arylhalo C1-C6Alkoxy, substituted or unsubstituted heteroaryl C1-C6Alkyl, substituted or unsubstituted heteroaryl halo C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkoxy, substituted or unsubstituted heteroaryl halo C1-C6An alkoxy group;
R5,R6can be the same or different and is respectively selected from H, halogen, CN and NO2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl radicalHalogen substituted C2-C6Alkynyl, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylthio, halo C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylamino radical, C2-C6Dialkylamino radical, C3-C8Cycloalkylamino, C1-C6Alkylaminocarbonyl radical, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkoxy radical C1-C6Alkoxy, halo C1-C6Alkoxy radical C1-C6Alkoxy radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkoxy, halo C1-C6Alkoxycarbonyl radical C1-C6Alkoxy radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkoxy, halo C1-C6Alkyl carbonyl radical C1-C6An alkoxy group;
R7is selected from H; c1-C6Alkyl radical, C3-C8Cycloalkyl radical, C2-C6Alkenyl radical, C2-C6Alkynyl, each of which is optionally substituted with one or more of the following substituents: halogen, CN, NO2、OH、C1-C6Alkyl, halo C1-C6Alkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylthio, halo C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, phenyl, phenoxy, 5-membered heteroaromatic ring and 6-membered heteroaromatic ring; phenyl, phenoxy, 5-membered heteroaromatic ring, and 6-membered heteroaromatic ring are each optionally substituted with 1 to 3 independent substituents selected from the group consisting of: c1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, halogen, CN, NO2、C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylamino radical, C2-C6Dialkylamino radical, C3-C8Cycloalkylamino, C1-C6(alkyl) (cycloalkyl) amino, C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl group, C1-C6Alkylaminocarbonyl radical, C2-C8A dialkylaminocarbonyl group; c1-C6An alkoxy group; c1-C6An alkylamino group; c2-C6A dialkylamino group; c3-C8A cycloalkylamino group; c1-C6Alkylcarbonyl or C1-C6An alkoxycarbonyl group;
R8selected from H, OH, NH2、C(CH3)2CH2S(O)(NH)CH3、C(CH3)2CH2S(O)(NCN)CH3、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C3-C8Cycloalkyl radical C1-C6Alkyl, halo C3-C8Cycloalkyl radical C1-C6Alkyl radical, C3-C8Oxacycloalkyl radical, C3-C8Oxacycloalkyl radical C1-C6Alkyl, hydroxy C1-C6Alkyl radical, C1-C6Alkylamino radical C1-C6Alkyl, di (C)1-C6Alkyl) amino C1-C6Alkyl, substituted or unsubstituted pyridyl C1-C6Alkyl, substituted or unsubstituted thiazolyl C1-C6Alkyl, substituted or unsubstituted pyridyloxy C1-C6Alkyl, substituted or unsubstituted pyridylthio C1-C6Alkyl, substituted or unsubstituted pyridylamino C1-C6Alkyl, substituted or unsubstituted morphinyl, substituted or unsubstituted piperazinyl, morphinyl C1-C6Alkyl radical, C1-C6Alkylthio group C1-C6Alkyl radical, C1-C6Alkyl sulfoxide radical C1-C6Alkyl radical, C1-C6Alkyl sulfone radical C1-C6Alkyl radical, C1-C6Alkylcarbonyloxy, C1-C6Alkoxycarbonyloxy, C1-C6Alkylamino carbonyloxy radical, C1-C6Alkylsulfonyloxy, halo C1-C6Alkylsulfonyloxy, C1-C6Alkylsulfonamido, halo C1-C6Alkylsulfonamide group, substituted or unsubstituted arylcarbonyloxy group, substituted or unsubstituted aryloxycarbonyloxy group, substituted or unsubstituted arylaminocarbonyloxy group, substituted or unsubstituted heteroarylcarbonyloxy group, substituted or unsubstituted heteroaryloxycarbonyloxy group, substituted or unsubstituted heteroarylaminocarbonyloxy group, C1-C6Alkylamino thiocarbonyloxy, substituted or unsubstituted arylamino thiocarbonyloxy, substituted or unsubstituted aryl C1-C6Alkoxy, substituted or unsubstituted pyridyl C1-C6Alkoxy, substituted or unsubstituted thiazolyl C1-C6Alkoxy radical, C1-C6Alkylcarbonylamino group, C1-C6Alkoxycarbonylamino group, C1-C6Alkylamino carbonylamino group, C1-C6Alkylamino thiocarbonylamino, substituted or unsubstituted arylcarbonylamino, substituted or unsubstituted aryloxycarbonylamino, substituted or unsubstituted arylaminocarbonylamino, substituted or unsubstituted arylaminothiocarbonylamino, substituted or unsubstituted heteroarylcarbonylamino, substituted or unsubstituted heteroaryloxycarbonylamino, substituted or unsubstituted heteroarylaminocarbonylamino, substituted or unsubstituted heteroarylaminothiocarbonylamino, aminocarbonylamino, C1-C6Alkylamino radical, di (C)1-C6) Alkylamino, substituted or unsubstituted aryl C1-C6Alkylamino, substituted or unsubstituted pyridyl C1-C6Alkylamino, substituted or unsubstituted thiazolyl C1-C6Alkylamino, N (t-Bu) (COPh), N ═ CHN (CH)3)2、N=C(CH3)2、N=CHCF3、N=CHPh、N=CH(2-Py)、N=CH(3-Py)、N=CH(4-Py);
R9Selected from H, C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylaminocarbonyl, halogeno C1-C6Alkylamino carbonyl, C1-C6Alkylamino thiocarbonyl, halogeno C1-C6Alkylamino thiocarbonyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkylamino carbonyl group C1-C6Alkyl, halo C1-C6Alkylamino carbonyl group C1-C6Alkyl, substituted or unsubstituted arylcarbonyl, substituted or unsubstituted aryloxycarbonyl, substituted or unsubstituted heteroarylcarbonyl, substituted or unsubstituted heteroaryloxycarbonyl, substituted or unsubstituted arylaminocarbonyl, substituted or unsubstituted arylaminothiocarbonyl, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkoxysulfinyl, halo C1-C6Alkoxysulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkoxysulfonyl, halo C1-C6An alkoxysulfonyl group;
preferred compounds of the invention are: in the general formula I
A and B are the same or different and are independently selected from O, S or NRa;Ra=H、CN、NO2、NH2Or NHCH3;
X is selected from H, halogen, CN, NO2、NH2、CHO、CH=NOCH3、CH=NNHCH3Or CH (CH) NN (CH)3)2;
Z is selected from N or CRd;RdH, halogen or NO2;
R1Selected from halogen, CN, NO2、NH2、CHO、Si(CH3)3、P(CH3)2、P(O)(CH3)2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl, oxaC2-C8Cycloalkyl, aza C2-C8Cycloalkyl, thia C2-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkylsulfinyl group C1-C6Alkyl, halo C1-C6Alkylsulfinyl group C1-C6Alkyl radical, C1-C6Alkoxysulfinyl C1-C6Alkyl, halo C1-C6Alkoxysulfinyl C1-C6Alkyl radical, C1-C6Alkanesulfonyl group C1-C6Alkyl, halo C1-C6Alkanesulfonyl group C1-C6Alkyl radical, C1-C6Alkoxy sulfonyl C1-C6Alkyl, halo C1-C6Alkoxy sulfonyl C1-C6Alkyl, amino C1-C6Alkyl, amino-halo C1-C6Alkyl, hydroxy C1-C6Alkyl, hydroxy-halogeno-C1-C6Alkyl, mercapto C1-C6Alkyl, mercapto halogenated C1-C6Alkyl, cyano C1-C6Alkyl, cyano-halo C1-C6Alkyl radical, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkoxy halogeno C1-C6Alkyl, halo C1-C6Alkoxy halogeno C1-C6Alkyl radical, C1-C6Alkylthio group C1-C6Alkyl, halo C1-C6Alkylthio group C1-C6Alkyl radical, C1-C6Alkylthio halogeno C1-C6Alkyl, halo C1-C6Alkylthio halogeno C1-C6Alkyl radical, C1-C6Alkylamino radical C1-C6Alkyl, halo C1-C6Alkylamino radical C1-C6Alkyl radical, C1-C6Alkylamino halogeno C1-C6Alkyl radical, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylthio carbonyl, halo C1-C6Alkylthio carbonyl group, C1-C6Alkylaminocarbonyl, halogeno C1-C6Alkylamino carbonyl, C1-C6Alkylamino thiocarbonyl, halogeno C1-C6Alkylamino thiocarbonyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkylamino carbonyl group C1-C6Alkyl, halo C1-C6Alkylamino carbonyl group C1-C6Alkyl radical, C1-C6Alkylthio carbonyl group C1-C6Alkyl, halo C1-C6Alkylthio carbonyl group C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkyl, substituted or unsubstituted aryl halo C1-C6Alkyl, substituted or unsubstituted aryloxy C1-C6Alkyl, substituted or unsubstituted aryloxyRadical halo C1-C6Alkyl, substituted or unsubstituted arylamine group C1-C6Alkyl, substituted or unsubstituted arylamino halogeno C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkyl, substituted or unsubstituted heteroaryl halo C1-C6Alkyl, substituted or unsubstituted heteroaryloxy C1-C6Alkyl, substituted or unsubstituted heteroaryloxy-halogeno C1-C6Alkyl, substituted or unsubstituted heteroarylamino C1-C6Alkyl, substituted or unsubstituted heteroarylamine halo C1-C6Alkyl, substituted or unsubstituted arylcarbonyl C1-C6Alkyl, substituted or unsubstituted arylcarbonyl halogeno C1-C6Alkyl, substituted or unsubstituted aryloxycarbonyl C1-C6Alkyl, substituted or unsubstituted aryloxycarbonyl halo C1-C6Alkyl, substituted or unsubstituted arylaminocarbonyl C1-C6Alkyl, substituted or unsubstituted arylaminocarbonyl haloC1-C6Alkyl, substituted or unsubstituted heteroarylcarbonyl C1-C6Alkyl, substituted or unsubstituted heteroarylcarbonyl halide C1-C6Alkyl, substituted or unsubstituted heteroaryloxycarbonyl C1-C6Alkyl, substituted or unsubstituted heteroaryloxycarbonyl-halogeno C1-C6Alkyl, substituted or unsubstituted heteroarylaminocarbonyl C1-C6Alkyl, substituted or unsubstituted heteroarylaminocarbonyl halo C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkoxy radical C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkoxy radical C1-C6An alkyl group;
R2,R3can be the same or different and is respectively selected from H, halogen, CN and NO2、NH2、CONH2、C1-C6Alkyl, halo C1-C6Alkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylamino, halogeno C1-C6Alkylamino radical, di (C)1-C6Alkyl) amino, C1-C6Alkylcarbonylamino, halo C1-C6Alkylcarbonylamino group, C1-C6Alkoxycarbonylamino group, C1-C6Alkylsulfinylamino, halogeno C1-C6Alkylsulfinylamino group, C1-C6Alkylsulfonylamino, halo C1-C6Alkylsulfonylamino group, C1-C6Alkylcarbonyl (C)1-C6Alkyl) amino, halo C1-C6Alkylcarbonyl (C)1-C6Alkyl) amino, C1-C6Alkoxycarbonyl (C)1-C6Alkyl) amino, C1-C6Alkylsulfinyl (C)1-C6Alkyl) amino, halo C1-C6Alkylsulfinyl (C)1-C6Alkyl) amino, C1-C6Alkylsulfonyl (C)1-C6Alkyl) amino, halo C1-C6Alkylsulfonyl (C)1-C6Alkyl) amino, C1-C6Alkylthio, halo C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylaminocarbonyl, halogeno C1-C6Alkylamino carbonyl, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkoxy, halo C1-C6Alkyl carbonyl radical C1-C6Alkoxy radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkoxy, halo C1-C6Alkoxycarbonyl radical C1-C6An alkoxy group;
R4selected from H, halogen, CN, CONH2、CSNH2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C2-C6Alkenyloxy, halogeno C2-C6Alkenyloxy radical, C2-C6Alkynyloxy, halo C2-C6Alkynyloxy, cyano C1-C6Alkyl, cyano-halo C1-C6Alkyl, cyano C1-C6Alkoxy, cyano-halogeno C1-C6Alkoxy radical, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkylsulfinyloxy, halogeno C1-C6Alkylsulfinyloxy, C1-C6Alkylsulfinylamino, halogeno C1-C6Alkylsulfinylamino group, C1-C6Alkylsulfinyloxy C1-C6Alkyl, halo C1-C6Alkylsulfinyloxy C1-C6Alkyl radical, C1-C6Alkanesulfinylamino C1-C6Alkyl, halo C1-C6Alkanesulfinylamino C1-C6Alkyl radical, C1-C6Alkylsulfonyloxy, halo C1-C6Alkylsulfonyloxy, C1-C6Alkanesulfonylamino, halo C1-C6Alkylsulfonylamino group, C1-C6Alkylsulfonyloxy C1-C6Alkyl, halo C1-C6Alkylsulfonyloxy C1-C6Alkyl radical, C1-C6Alkanesulfonylamino C1-C6Alkyl, halo C1-C6Alkanesulfonylamino C1-C6Alkyl radical, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylaminocarbonyl, halogeno C1-C6Alkylamino carbonyl, C1-C6Alkoxycarbonyl radical C1-C6Alkoxy, halo C1-C6Alkoxycarbonyl radical C1-C6Alkoxy radical, C1-C6Alkylamino carbonyl group C1-C6Alkoxy, halo C1-C6Alkylamino carbonyl group C1-C6Alkoxy radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkylamino carbonyl group C1-C6Alkyl, halo C1-C6Alkylamino carbonyl group C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkoxy, halo C1-C6Alkyl carbonyl radical C1-C6Alkoxy, substituted or unsubstituted aryl C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkoxy, substituted or unsubstituted heteroaryl C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6An alkoxy group;
R5,R6can be the same or different and is respectively selected from H, halogen, CN and NO2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkylamino radical, C2-C6Dialkylamino radical, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkoxy radical C1-C6Alkoxy, halo C1-C6Alkoxy radical C1-C6Alkoxy radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkoxy, halo C1-C6Alkoxycarbonyl radical C1-C6Alkoxy radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkoxy, halo C1-C6Alkyl carbonyl radical C1-C6An alkoxy group;
R7is selected from H; c1-C6Alkyl radical, C3-C8Cycloalkyl radical, C2-C6Alkenyl radical, C2-C6Alkynyl, each of which is optionally substituted with one or more of the following substituents: halogen, CN, NO2、OH、C1-C6Alkyl, halo C1-C6Alkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylthio, halo C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, phenyl, phenoxy, 5-membered heteroaromatic ring and 6-membered heteroaromatic ring; phenyl, phenoxy, 5-membered heteroaromatic ring, and 6-membered heteroaromatic ring are each optionally substituted with 1 to 3 independent substituents selected from the group consisting of: c1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, halogen, CN, NO2、C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylamino radical, C2-C6Dialkylamino radical, C3-C8Cycloalkylamino, C1-C6(alkyl) (cycloalkyl) amino, C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl radical、C1-C6Alkylaminocarbonyl radical, C2-C8A dialkylaminocarbonyl group;
R8selected from H, OH, NH2、C(CH3)2CH2S(O)(NH)CH3、C(CH3)2CH2S(O)(NCN)CH3、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkoxy radical C1-C6Alkyl radical, C3-C8Cycloalkyl radical C1-C6Alkyl radical, C3-C8Oxacycloalkyl radical C1-C6Alkyl, halo C3-C8Cycloalkyl radical C1-C6Alkyl, hydroxy C1-C6Alkyl radical, C1-C6Alkylamino radical C1-C6Alkyl, di (C)1-C6Alkyl) amino C1-C6Alkyl, substituted or unsubstituted pyridyl C1-C6Alkyl, substituted or unsubstituted thiazolyl C1-C6Alkyl, substituted or unsubstituted pyridyloxy C1-C6Alkyl, substituted or unsubstituted pyridylthio C1-C6Alkyl, substituted or unsubstituted pyridylamino C1-C6Alkyl radical, C1-C6Alkylthio group C1-C6Alkyl radical, C1-C6Alkyl sulfoxide radical C1-C6Alkyl radical, C1-C6Alkyl sulfone radical C1-C6Alkyl radical, C1-C6Alkylcarbonyloxy, C1-C6Alkoxycarbonyloxy, C1-C6Alkylamino carbonyloxy radical, C1-C6Alkylsulfonyloxy, halo C1-C6Alkylsulfonyloxy, C1-C6Alkylsulfonamido, halo C1-C6Alkylsulfonamide group, substituted or unsubstituted arylcarbonyloxy group, substituted or unsubstituted aryloxycarbonyloxy group, substituted or unsubstituted heteroarylcarbonyloxy group, substituted or unsubstituted heteroaryloxycarbonyloxy group, C1-C6Alkylamino thiocarbonyloxy, substituted or unsubstituted arylamino thiocarbonyloxy, substituted or unsubstituted aryl C1-C6Alkoxy, substituted or unsubstituted pyridyl C1-C6Alkoxy, substituted or unsubstituted thiazolyl C1-C6Alkoxy radical, C1-C6Alkylcarbonylamino group, C1-C6Alkoxycarbonylamino group, C1-C6Alkylamino carbonylamino group, C1-C6Alkylamino thiocarbonylamino, substituted or unsubstituted arylcarbonylamino, substituted or unsubstituted aryloxycarbonylamino, substituted or unsubstituted arylaminocarbonylamino, substituted or unsubstituted arylaminothiocarbonylamino, aminocarbonylamino, C1-C6Alkylamino radical, di (C)1-C6) Alkylamino, substituted or unsubstituted aryl C1-C6Alkylamino, substituted or unsubstituted pyridyl C1-C6Alkylamino, substituted or unsubstituted thiazolyl C1-C6An alkylamino group;
R9selected from H, C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl group, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl, substituted or unsubstituted arylcarbonyl, substituted or unsubstituted aryloxycarbonyl, substituted or unsubstituted heteroarylcarbonyl, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6An alkylsulfonyl group;
again preferred compounds of the invention are: in the general formula I
A and B are the same or different and are independently selected from O, S or NRa;Ra=H、CN、NO2;
X is selected from H, halogen, CN, NO2、NH2Or CHO;
z is selected from N or CRd;RdH, halogen or NO2;
R1Selected from halogen, CN, NO2、NH2、CHO、Si(CH3)3、P(CH3)2、P(O)(CH3)2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl, oxaC2-C8Cycloalkyl, aza C2-C8Cycloalkyl, thia C2-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkylsulfinyl group C1-C6Alkyl, halo C1-C6Alkylsulfinyl group C1-C6Alkyl radical, C1-C6Alkoxysulfinyl C1-C6Alkyl, halo C1-C6Alkoxysulfinyl C1-C6Alkyl radical, C1-C6Alkanesulfonyl group C1-C6Alkyl, halo C1-C6Alkanesulfonyl group C1-C6Alkyl radical, C1-C6Alkoxysulfonyl groupC1-C6Alkyl, halo C1-C6Alkoxy sulfonyl C1-C6Alkyl, amino C1-C6Alkyl, amino-halo C1-C6Alkyl, hydroxy C1-C6Alkyl, hydroxy-halogeno-C1-C6Alkyl, mercapto C1-C6Alkyl, mercapto halogenated C1-C6Alkyl, cyano C1-C6Alkyl, cyano-halo C1-C6Alkyl radical, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkoxy halogeno C1-C6Alkyl, halo C1-C6Alkoxy halogeno C1-C6Alkyl radical, C1-C6Alkylthio group C1-C6Alkyl, halo C1-C6Alkylthio group C1-C6Alkyl radical, C1-C6Alkylthio halogeno C1-C6Alkyl, halo C1-C6Alkylthio halogeno C1-C6Alkyl radical, C1-C6Alkylamino radical C1-C6Alkyl, halo C1-C6Alkylamino radical C1-C6Alkyl radical, C1-C6Alkylamino halogeno C1-C6Alkyl radical, C1-C6Alkyl carbonyl radical C1-C6Alkyl, halo C1-C6Alkyl carbonyl radical C1-C6Alkyl radical, C1-C6Alkoxycarbonyl radical C1-C6Alkyl, halo C1-C6Alkoxycarbonyl radical C1-C6Alkyl radical, C1-C6Alkylamino carbonyl group C1-C6Alkyl, halo C1-C6Alkylamino carbonyl group C1-C6Alkyl radical, C1-C6Alkylthio carbonyl group C1-C6Alkyl, halo C1-C6Alkylthio carbonyl group C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkyl, substituted or notSubstituted aryl halo C1-C6Alkyl, substituted or unsubstituted aryloxy C1-C6Alkyl, substituted or unsubstituted aryloxy halogeno C1-C6Alkyl, substituted or unsubstituted arylamine group C1-C6Alkyl, substituted or unsubstituted arylamino halogeno C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkyl, substituted or unsubstituted heteroaryl halo C1-C6Alkyl, substituted or unsubstituted heteroaryloxy C1-C6Alkyl, substituted or unsubstituted heteroaryloxy-halogeno C1-C6Alkyl, substituted or unsubstituted heteroarylamino C1-C6Alkyl, substituted or unsubstituted heteroarylamine halo C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkoxy radical C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkoxy radical C1-C6An alkyl group;
R2,R3can be the same or different and is respectively selected from H, halogen, CN and NO2、NH2、CONH2、C1-C6Alkyl, halo C1-C6Alkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylamino, halogeno C1-C6Alkylamino radical, di (C)1-C6Alkyl) amino, C1-C6Alkylcarbonylamino, halo C1-C6Alkylcarbonylamino group, C1-C6Alkoxycarbonylamino group, C1-C6Alkylsulfinylamino, halogeno C1-C6Alkylsulfinylamino group, C1-C6Alkylsulfonylamino, halo C1-C6Alkylsulfonylamino, halo C1-C6Alkylcarbonyl (C)1-C6Alkyl) amino, halo C1-C6Alkylsulfinyl (C)1-C6Alkyl) amino, C1-C6Alkylsulfonyl (C)1-C6Alkyl) amino, halo C1-C6Alkylsulfonyl (C)1-C6Alkyl) amino, C1-C6Alkylthio, halo C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6An alkyl group;
R4selected from H, halogen, CN, C1-C6Alkyl, halo C1-C6Alkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C2-C6Alkenyloxy, halogeno C2-C6Alkenyloxy radical, C2-C6Alkynyloxy, halo C2-C6Alkynyloxy, cyano C1-C6Alkyl, cyano-halo C1-C6Alkyl, cyano C1-C6Alkoxy, cyano-halogeno C1-C6Alkoxy radical, C1-C6Alkylsulfinyloxy, halogeno C1-C6Alkylsulfinyloxy, C1-C6Alkylsulfinyloxy C1-C6Alkyl, halo C1-C6Alkylsulfinyloxy C1-C6Alkyl radical, C1-C6Alkylsulfonyloxy, halo C1-C6Alkylsulfonyloxy, C1-C6Alkylsulfonyloxy C1-C6Alkyl, halo C1-C6Alkylsulfonyloxy C1-C6Alkyl radical, C1-C6An alkylcarbonyl group,Halogen substituted C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl, substituted or unsubstituted heteroaryl C1-C6An alkyl group;
R5,R6can be the same or different and is respectively selected from H, halogen, CN and NO2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6An alkoxycarbonyl group;
R7is selected from H; c1-C6Alkyl radical, C3-C8Cycloalkyl radical, C2-C6Alkenyl radical, C2-C6Alkynyl, each of which is optionally substituted with one or more of the following substituents: halogen, CN, NO2、OH、C1-C6Alkyl, halo C1-C6Alkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylthio, halo C1-C6Alkylthio radical, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6Alkanesulfonyl group, C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, phenyl, phenoxy, 5-membered heteroaromatic ring and 6-membered heteroaromatic ring;
R8is selected fromH、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkylthio group C1-C6Alkyl radical, C3-C8Cycloalkyl radical C1-C6Alkyl, halo C3-C8Cycloalkyl radical C1-C6Alkyl, hydroxy C1-C6Alkyl, di (C)1-C6Alkyl) amino C1-C6Alkyl radical, C3-C8Oxacycloalkyl radical C1-C6Alkyl, substituted or unsubstituted pyridyl C1-C6Alkyl, substituted or unsubstituted thiazolyl C1-C6Alkyl radical, C1-C6Alkylthio group C1-C6Alkyl radical, C1-C6Alkyl sulfoxide radical C1-C6Alkyl radical, C1-C6Alkyl sulfone radical C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkoxy, substituted or unsubstituted pyridyl C1-C6Alkoxy, substituted or unsubstituted thiazolyl C1-C6An alkoxy group;
R9selected from H, C1-C6Alkyl, halo C1-C6Alkyl radical, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl, halo C1-C6Alkoxycarbonyl, substituted or unsubstituted arylcarbonyl, substituted or unsubstituted heteroarylcarbonyl, C1-C6Alkylsulfinyl, halogeno C1-C6Alkylsulfinyl radical, C1-C6Alkanesulfonyl, halo C1-C6An alkylsulfonyl group;
further preferred compounds of the invention are: in the general formula I
A and B are the same or different and are independently selected from O, S or NRa;RaH or CN;
x is selected from H, halogen or CN;
z is selected from N or CRd;RdH, halogen or NO2;
R1Selected from halogen, CN, NO2、NH2、CHO、Si(CH3)3、P(CH3)2、P(O)(CH3)2、C1-C6Alkyl, halo C1-C6Alkyl radical, C3-C8Cycloalkyl, halo C3-C8Cycloalkyl, oxaC2-C8Cycloalkyl, aza C2-C8Cycloalkyl, thia C2-C8Cycloalkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkylsulfinyl group C1-C6Alkyl, halo C1-C6Alkylsulfinyl group C1-C6Alkyl radical, C1-C6Alkoxysulfinyl C1-C6Alkyl, halo C1-C6Alkoxysulfinyl C1-C6Alkyl radical, C1-C6Alkanesulfonyl group C1-C6Alkyl, halo C1-C6Alkanesulfonyl group C1-C6Alkyl radical, C1-C6Alkoxy sulfonyl C1-C6Alkyl, halo C1-C6Alkoxy sulfonyl C1-C6Alkyl, amino C1-C6Alkyl, amino-halo C1-C6Alkyl, hydroxy C1-C6Alkyl, hydroxy-halogeno-C1-C6Alkyl, mercapto C1-C6Alkyl, mercapto halogenated C1-C6Alkyl, cyano C1-C6Alkyl, cyano-halo C1-C6Alkyl radical、C1-C6Alkoxy radical C1-C6Alkyl, halo C1-C6Alkoxy radical C1-C6Alkyl radical, C1-C6Alkoxy halogeno C1-C6Alkyl, halo C1-C6Alkoxy halogeno C1-C6Alkyl radical, C1-C6Alkylthio group C1-C6Alkyl, halo C1-C6Alkylthio group C1-C6Alkyl radical, C1-C6Alkylthio halogeno C1-C6Alkyl, halo C1-C6Alkylthio halogeno C1-C6Alkyl radical, C1-C6Alkylamino radical C1-C6Alkyl, halo C1-C6Alkylamino radical C1-C6Alkyl radical, C1-C6Alkylamino halogeno C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkyl, substituted or unsubstituted aryl halo C1-C6Alkyl, substituted or unsubstituted aryloxy C1-C6Alkyl, substituted or unsubstituted aryloxy halogeno C1-C6Alkyl, substituted or unsubstituted arylamine group C1-C6Alkyl, substituted or unsubstituted arylamino halogeno C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkyl, substituted or unsubstituted heteroaryl halo C1-C6Alkyl, substituted or unsubstituted heteroaryloxy C1-C6Alkyl, substituted or unsubstituted heteroaryloxy-halogeno C1-C6Alkyl, substituted or unsubstituted heteroarylamino C1-C6Alkyl, substituted or unsubstituted heteroarylamine halo C1-C6Alkyl, substituted or unsubstituted aryl C1-C6Alkoxy radical C1-C6Alkyl, substituted or unsubstituted heteroaryl C1-C6Alkoxy radical C1-C6An alkyl group;
R2,R3can be the same or different and is respectively selected from H, halogen, CN and NO2、NH2、CONH2、C1-C6Alkyl, halo C1-C6Alkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkoxy, halo C1-C6Alkoxy radical, C1-C6Alkylthio, halo C1-C6Alkylthio, di (C)1-C6Alkyl) amino, C1-C6Alkylcarbonylamino, halo C1-C6Alkylcarbonylamino group, C1-C6Alkoxycarbonylamino group, C1-C6Alkylsulfinylamino, halogeno C1-C6Alkylsulfinylamino group, C1-C6Alkylsulfonylamino, halo C1-C6An alkylsulfonylamino group;
R4selected from H, halogen, CN, C1-C6Alkyl, halo C1-C6Alkyl radical, C1-C6Alkoxy, halo C1-C6Alkoxy, cyano C1-C6Alkyl, cyano C1-C6Alkoxy, cyano-halogeno C1-C6Alkoxy radical, C1-C6Alkylsulfonyloxy, halo C1-C6Alkylsulfonyloxy, C1-C6Alkylsulfonyloxy C1-C6Alkyl, halo C1-C6Alkylsulfonyloxy C1-C6Alkyl radical, C1-C6Alkoxycarbonyl, substituted or unsubstituted heteroaryl C1-C6An alkyl group;
R5,R6can be the same or different and are respectively selected from H, halogen, CN, CF3Or NO2;
R7Is selected from H; c1-C6Alkyl radical, C3-C8Cycloalkyl radical, C2-C6Alkenyl radical, C2-C6An alkynyl group;
R8selected from H,C1-C6Alkyl, halo C1-C6Alkyl radical, C2-C6Alkenyl radical, C2-C6Alkynyl, C3-C8Cycloalkyl, C (CH)3)2CH2SCH3、CH2CH2CH2N(CH3)2、CH(CH3)CH2OH、
R9Selected from H, C1-C6Alkyl, halo C1-C6Alkyl radical, C1-C6Alkylcarbonyl, halo C1-C6Alkylcarbonyl group, C1-C6Alkoxycarbonyl or halo C1-C6An alkoxycarbonyl group;
still further preferred compounds of the invention are: in the general formula I
A and B can be the same or different and are respectively and independently selected from O, S or NH;
x is selected from H, F, Cl, Br, I and CN;
z is selected from N or CRd;Rd=H、F、Cl、Br;
R1Selected from F, Cl, Br, I, CN, NO2、NH2、CHO、Si(CH3)3、P(CH3)2、P(O)(CH3)2、CH3、CH2CH3、CH(CH3)2、CF3、CF2H、CF2CF2H、CH2CF3、CF2CF3A cyclopropyl group,CH=CH2、CH2-CH=CH2、CH2CH=CHCl、C≡CH、CH2-C≡CH、CH2C≡CI、CH2S(O)CH3、CH2S(O)CF3、CH2S(O)2CH3、CH2S(O)2CF3、CH2CN、CH2CH2CN、CH2OCH3、CH2CH2OCH3、CH2OCF3、CH2OCF2H、CH2OCF2CF2H、CH2OCH2CF3、CH2OCF2CF3、CH2SCH3、CH2CH2SCH3、CH2SCF3、CH2SCF2H、CH2SCF2CF2H、CH2SCH2CF3、CH2SCF2CF3、CH2NHCH3、CH2NHCF3、CH2NHCF2H、CH2NHCF2CF2H、CH2NHCH2CF3、CH2NHCF2CF3、CF2NHCH3、CH2Ph、CH2OPh、CH2NHPh、 CH2OCH2Ph、CH2OCH2Py、
R2,R3Can be the same or different and is respectively selected from H, F, Cl, Br, I, CN and C1-C6Alkyl, halo C1-C6Alkyl radical, C2-C6Alkenyl, halo C2-C6Alkenyl radical, C2-C6Alkynyl, halo C2-C6Alkynyl, C1-C6Alkylcarbonylamino, halo C1-C6Alkylcarbonylamino group, C1-C6Alkoxycarbonylamino, halo C1-C6Alkylsulfinylamino, halogeno C1-C6An alkylsulfonylamino group;
R4selected from H, F, Cl, Br, I, CN, C1-C3Alkoxy, halo C1-C3Alkoxy, cyano C1-C3Alkyl, cyano C1-C3Alkoxy, substituted or unsubstituted heteroaryl C1-C6An alkyl group;
R5,R6can be the same or different and is respectively selected from H, F, Cl, Br, I, CN, CF3、NO2;
R7Selected from H, C1-C6An alkyl group;
R8selected from H, CH3、CH2CH3、CH(CH3)2、C(CH3)3、CH2CH=CH2、CH2C≡CH、C(CH3)2C≡CH、C(CH3)2CH2SCH3、CH2CH2Cl、CH2CH2Br, cyclopropyl, CH2CH2CH2N(CH3)2、CH(CH3)CH2OH、
R9Is selected from H or C1-C6An alkyl group;
further preferred compounds of the invention are: in the general formula I
A and B can be the same or different and are respectively and independently selected from O or S;
x is selected from H, F, Cl or CN;
z is selected from N or CRd;RdH, F or Cl;
R1selected from F, Cl, Br, I, CN, NO2、NH2、CHO、Si(CH3)3、CH3、CF3、CF2H、CH2CF3、CF2CF3A cyclopropyl group,CH2C≡CI、CH2S(O)CH3、CH2S(O)CF3、CH2S(O)2CH3、CH2S(O)2CF3、CH2CN、CH2CH2CN、CH2OCH3、CH2OCF3、CH2OCF2CF2H、CH2OCH2CF3、CH2SCH3、CH2SCF3、CH2NHCH3、CH2NHCF3、CH2Ph、CH2OPh、CH2NHPh、 CH2OCH2Ph、
R2,R3Can be the same or different and is respectively selected from H, F, Cl, Br, CN, CH3、CH2CH3、CH2CH2CH3、CH(CH3)2、CH2F、CF2H、CF3、CH2Cl、CCl2H、CCl3、CFClH、NHCOCH3、NHCOCF3、NHC(O)OCH3、NHSOCF3、NHSO2CF3;
R4Selected from F, Cl, Br, CN, OCH3、OCH2CH3、OCF3、OCHF2、OCH2F、OCCl3、OCHCl2、OCH2Cl、OCHFCl、OCF2CF2H、OCH2CF3、CH2CN、OCH2CN、
R5,R6Can be the same or different and is respectively selected from H, F, Cl, Br, CF3、CN;
R7Selected from H, CH3、CH2CH3、CH2CH2CH3、CH(CH3)2、C(CH3)3;
R8Selected from H, CH3、CH2CH3、CH(CH3)2、C(CH3)3、CH2CH=CH2、CH2C≡CH、C(CH3)2C≡CH、C(CH3)2CH2SCH3、CH2CH2Cl、CH2CH2Br, cyclopropyl, CH2CH2CH2N(CH3)2、CH(CH3)CH2OH、
R9Is selected from H or CH3;
Still further preferred compounds of the invention are: in the general formula I
A and B can be the same or different and are respectively and independently selected from O or S;
x is selected from H, F, Cl or CN;
z is selected from N or CRd;RdH, F or Cl;
R1selected from F, Cl, Br, I, CN, NO2、NH2、CHO、Si(CH3)3、CH3、CF3、CF2H、CH2CF3、CF2CF3A cyclopropyl group,CH2S(O)CF3、CH2S(O)2CF3、CH2CN、CH2CH2CN、CH2OCH3、CH2OCF3、CH2OCF2CF2H、CH2OCH2CF3、CH2SCH3、CH2SCF3、CH2Ph、CH2OPh、 CH2OCH2Ph、
R2,R3Can be the same or different and is respectively selected from H, F, Cl, Br, CN, CH3、CH2CH3、CH(CH3)2、CH2F、CF2H、CF3、CFClH、NHCOCH3、NHCOCF3、NHC(O)OCH3、NHSOCF3、NHSO2CF3;
R5,R6Can be the same or different and is respectively selected from H, F, Cl, Br, CF3、CN;
R7Selected from H, CH3、CH2CH3、CH(CH3)2、C(CH3)3;
R8Selected from H, CH3、CH2CH3、CH(CH3)2、C(CH3)3、CH2CH=CH2、CH2C≡CH、C(CH3)2C≡CH、C(CH3)2CH2SCH3A cyclopropyl group,
R9Is selected from H or CH3;
In the definitions of the compounds of the general formula I given above, the terms used are generally defined as follows:
unsubstituted means that all substituents are hydrogen.
The number of substituents in the substituted amino group may be 1 to 2.
Halogen: refers to fluorine, chlorine, bromine or iodine.
Alkyl groups: straight-chain or branched alkyl groups, such as methyl, ethyl, propyl, isopropyl or tert-butyl.
Halogenated alkyl groups: straight-chain or branched alkyl groups, the hydrogen atoms on these alkyl groups may be partially or fully substituted by halogen atoms. For example, a haloalkyl group such as chloromethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl, or trifluoromethyl.
Alkoxy groups: straight or branched chain alkyl groups attached to the structure via oxygen atom linkages.
Haloalkoxy groups: straight-chain or branched alkoxy groups in which the hydrogen atoms may be partially or completely replaced by halogen atoms. For example, a haloalkoxy group such as chloromethoxy, dichloromethoxy, trichloromethoxy, fluoromethoxy, difluoromethoxy, trifluoromethoxy, chlorofluoromethoxy or trifluoroethoxy.
Alkenyl: straight-chain or branched and may have a double bond in any position, for example a vinyl or allyl group.
Alkynyl: straight or branched chain and may have a triple bond at any position, for example ethynyl or propargyl.
Aryl groups and the aryl moieties in aralkyl, arylalkenyl, aralkynyl, aryloxy and aryloxyalkyl groups include phenyl or naphthyl.
The heteroaryl group referred to in the present invention is a 5-or 6-membered ring containing 1 or more N, O, S heteroatoms. Such as pyridine, furan, pyrimidine, pyrazine, pyridazine, triazine, quinoline, or benzofuran.
The compounds of the present invention may exist as one or more stereoisomers. One skilled in the art will appreciate that when one stereoisomer is present in large concentrations relative to the other stereoisomers, or when it is separated from the other stereoisomers, it may be more active and/or it may exhibit beneficial effects. In addition, one skilled in the art would direct how to isolate, enrich, and/or selectively prepare the stereoisomers. Accordingly, the present invention includes compounds selected from formula I, N-oxides or salts thereof. The compounds of the present invention may exist as mixtures of stereoisomers, individual stereoisomers, or as optically active forms.
The compounds listed in tables 1-262 below are illustrative of the invention and are not intended to be limiting.
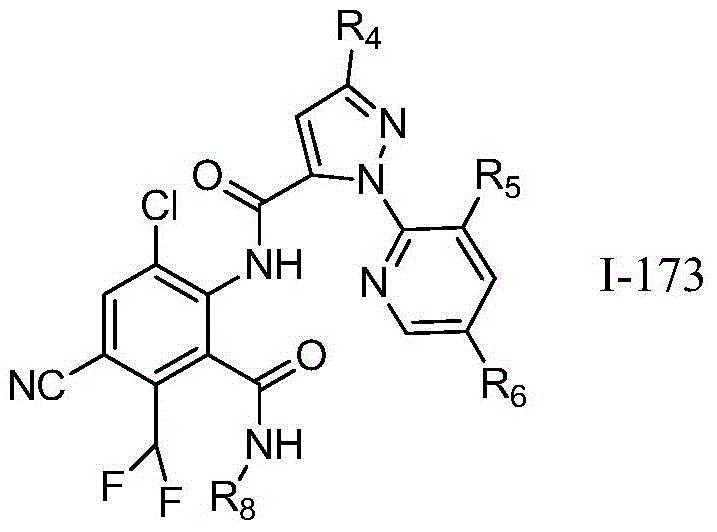
Table 1, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When F is equal to F, the following compounds are selected, and the serial numbers are 1-1 to 1-709
Table 2, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 2-1 to 2-709.
Table 3, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=F,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 3-1 to 3-709.
Table 4, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Br,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 4-1 to 4-709.
Table 5, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CF3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 5-1 to 5-709.
Table 6, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=H,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 6-1 to 6-709.
Table 7, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When F is equal to F, the compound with the same substituent as the compound in the table 1 is selected, and the number is 7-1 to 7-709
Table 8, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 8-1 to 8-709.
Table 9, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=F,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 9-1 to 9-709.
Table 10, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Br,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 10-1 to 10-709.
Table 11, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CF3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 11-1 to 11-709.
Table 12, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=H,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 12-1 to 12-709.
Table 13, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=Cl,R7=H,R9=H,R1When F is not satisfied, the same substituents as in Table 1 are selectedThe compound (1) is numbered from 13-1 to 13-709.
Table 14, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=CH3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 14-1 to 14-709.
Table 15, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=F,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 15-1 to 15-709.
Table 16, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=Br,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 16-1 to 16-709.
Table 17, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=CF3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 17-1 to 17-709.
Table 18, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=H,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 18-1 to 18-709.
Table 19, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=Cl,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 19-1 to 19-709.
Table 20, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=CH3,R7=H,R9=H,R1When F is equal to F, the compound with the same substituent as the compound in the table 1 is selected, and the number is 20-1 to 20-709.
Table 21, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=F,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 21-1 to 21-709.
Table 22, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=Br,R7=H,R9=H,R1When F is equal to F, compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 22-1 to 22-709.
Table 23, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=CF3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 23-1 to 23-709.
Table 24, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=H,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 24-1 to 24-709.
Table 25, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=Cl,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 25-1 to 25-709.
Table 26, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=CH3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 26-1 to 26-709.
Table 27, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=F,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 27-1 to 27-709.
Table 28, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=Br,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 28-1 to 28-709.
Table 29, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=CF3,R7=H,R9=H,R1When F is equal to F, the compounds with the same substituent groups as those in the table 1 are selected, and the numbers are 29-1 to 29-709.
Table 30, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=H,R7=H,R9=H,R1When F is equal to F, the compound with the same substituent as the compound in the table 1 is selected, and the number is 30-1 to 30-709.
Table 31, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When Cl is observed, the same substituents as those in Table 1 are selected, and the numbers are 31-1 to 31-709.
Table 32, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When Cl, the same substituents as those in Table 1 are selected, and the numbering is 32-1~32-709。
Table 33, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=F,R7=H,R9=H,R1When Cl is observed, compounds having the same substituents as those in Table 1 are selected and numbered 33-1 to 33-709.
Table 34, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Br,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 34-1 to 34-709.
Table 35, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CF3,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered from 35-1 to 35-709.
Table 36, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=H,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 36-1 to 36-709.
Table 37, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered from 37-1 to 37-709.
Table 38, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered 38-1 to 38-709.
Table 39, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=F,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers are 39-1 to 39-709.
Table 40, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Br,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered from 40-1 to 40-709.
Table 41, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CF3,R7=H,R9=H,R1When Cl is observed, the same substituents as those in Table 1 are selected, and the numbers are 41-1 to 41-709.
Table 42, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=H,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 42-1 to 42-709.
Table 43, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=Cl,R7=H,R9=H,R1When Cl is observed, compounds having the same substituents as those in Table 1 are selected and numbered from 43-1 to 43-709.
Table 44, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=CH3,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 44-1 to 44-709.
Table 45, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=F,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered from 45-1 to 45-709.
Table 46, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=Br,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 46-1 to 46-709.
Table 47, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=CF3,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers are 47-1 to 47-709.
Table 48, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=H,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered from 48-1 to 48-709.
Table 49, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=Cl,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are shown in numbers of 49-1 to 49-709.
Table 50, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=CH3,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 50-1 to 50-709.
Table 51, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=F,R7=H,R9=H,R1When Cl is not satisfied, compounds having the same substituents as those in Table 1 are selected and numbered 51-1 to 51-709。
Table 52, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=Br,R7=H,R9=H,R1When Cl is added, compounds having the same substituents as those in Table 1 are selected and numbered 52-1 to 52-709.
Table 53, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=CF3,R7=H,R9=H,R1When Cl is observed, compounds having the same substituents as those in Table 1 are selected and numbered from 53-1 to 53-709.
Table 54, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=H,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered 54-1 to 54-709.
Table 55, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=Cl,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered from 55-1 to 55-709.
Table 56, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=CH3,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered 56-1 to 56-709.
Table 57, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=F,R7=H,R9=H,R1When Cl is satisfied, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 57-1 to 57-709.
Table 58, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=Br,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered from 58-1 to 58-709.
Table 59, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=CF3,R7=H,R9=H,R1When Cl is added, compounds having the same substituents as those in Table 1 are selected and numbered 59-1 to 59-709.
Table 60, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=H,R7=H,R9=H,R1When Cl is satisfied, compounds having the same substituents as those in Table 1 are selected and numbered from 60-1 to 60-709.
Table 61, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When CN is not included, compounds having the same substituent as in Table 1 are selected and numbered from 61-1 to 61-709.
Table 62, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers of CN are 62-1 to 62-709.
Table 63, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=F,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituents as those in Table 1 are selected, and the numbers of the compounds are 63-1 to 63-709.
Table 64, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Br,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituent as in Table 1 are selected, and the numbers of the compounds are 64-1 to 64-709.
Table 65, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CF3,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the numbers are 65-1 to 65-709.
Table 66, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=H,R7=H,R9=H,R1When CN is not included, compounds having the same substituent as in Table 1 are selected and numbered from 66-1 to 66-709.
Table 67, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituent as in Table 1 are selected, and the numbers are 67-1 to 67-709.
Table 68, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 68-1 to 68-709.
Table 69, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=F,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers are 69-1 to 69-709.
Table 70, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Br,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the number is 70-1 to 70-709.
Table 71, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CF3,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituent as in Table 1 are selected, and the numbers of the compounds are 71-1 to 71-709.
Table 72, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=H,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 72-1 to 72-709.
Table 73, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=Cl,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituents as those in Table 1 are selected and numbered from 73-1 to 73-709.
Table 74, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=CH3,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 74-1 to 74-709.
Table 75, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=F,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the number is 75-1 to 75-709.
Table 76, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=Br,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 76-1 to 76-709.
Table 77, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=CF3,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituent as in Table 1 are selected, and the numbers of 77-1 to 77-709 are shown.
Table 78, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Br,R3=H,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the numbers are 78-1 to 78-709.
Table 79, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=Cl,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituent as in Table 1 are selected, and the numbers of 79-1 to 79-709 are shown.
TABLE 80, A=O,B=O,X=H,Z=N,R2=F,R3=CH3,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the number is 80-1 to 80-709.
Table 81, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=F,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 81-1 to 81-709.
Table 82, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=Br,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituent as in Table 1 are selected, and the numbers of the compounds are 82-1 to 82-709.
Table 83, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=CF3,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers are 83-1 to 83-709.
Table 84, a ═ O, B ═ O, X ═ H, Z ═ N, R2=F,R3=H,R7=H,R9=H,R1When CN is not substituted, the same substituents as those in Table 1 are selected, and the numbers of CN are 84-1 to 84-709.
Table 85, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=Cl,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the number is 85-1 to 85-709.
Table 86, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=CH3,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the numbers of CN are 86-1 to 86-709.
Table 87, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=F,R7=H,R9=H,R1When CN is not substituted, compounds having the same substituent as in Table 1 are selected, and the numbers of the compounds are 87-1 to 87-709.
Table 88, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=Br,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the numbers of the compounds are 88-1 to 88-709.
Table 89, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=CF3,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the number is 89-1 to 89-709.
Table 90, a ═ O, B ═ O, X ═ H, Z ═ N, R2=H,R3=H,R7=H,R9=H,R1When CN is not substituted, the same substituent as in Table 1 is selected, and the number is 90-1 to 90-709.
Table 91, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When Br is not included, compounds having the same substituents as those in Table 1 are selected and numbered from 91-1 to 91-709.
Table 92, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When Br is not included, compounds having the same substituents as those in Table 1 are selected and numbered from 92-1 to 92-709.
Table 93, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When Br is not greater than Br, compounds having the same substituents as those in Table 1 are selected and numbered from 93-1 to 93-709.
Table 94, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When Br is not included, compounds having the same substituents as those in Table 1 are selected and numbered 94-1 to 94-709.
Table 95, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When the substituent is the same as that in Table 1, the compound is selected as I, and the number is 95-1 to 95-709.
Table 96, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When the substituent is represented by I, compounds with the same substituent as in the table 1 are selected, and the numbers are 96-1 to 96-709.
Table 97, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When the substituent is the same as that in Table 1, compounds having the same number of 97-1 to 97-709 are selected.
Table 98, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When the substituent is I, the compound is the same as the substituent in the table 1, and the number is 98-1 to 98-709.
Table 99, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=NO2Then, compounds having the same substituents as those in Table 1 are selected, and the numbers are 99-1 to 99-709.
Table 100, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=NO2Then, the compounds with the same substituents as those in Table 1 are selected and numbered as 100-1 to 100-709.
Table 101, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=NO2Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 101-1 to 101-709.
Table 102, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=NO2Then, the compounds with the same substituents as those in Table 1 are selected and numbered 102-1 to 102-709.
Table 103, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2CN, the same substituents as those in Table 1 are selected, and the numbers of the compounds are 103-1 to 103-709.
Table 104, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2CN, the same substituents as those in Table 1 are selected, and the numbers of the compounds are 104-1 to 104-709.
Table 105, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2CN, the same substituents as those in Table 1 are selected, and the numbers of compounds are 105-1 to 105-709.
Table 106, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2CN, the same substituent as that in Table 1 is selected, and the numbers of the compounds are 106-1 to 106-709.
Table 107, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=Si(CH3)3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 107-1 to 107-709.
Table 108, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=Si(CH3)3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 108-1 to 108-709.
Table 109, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=Si(CH3)3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 109-1 to 109-709.
Table 110, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=Si(CH3)3Then, the compounds with the same substituents as those in Table 1 are selected and numbered as 110-1 to 110-709.
Table 111, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of which are 111-1 to 111-709.
Table 112, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH3Then, the compounds with the same substituents as those in Table 1 are selected and numbered 112-1 to 112-709.
Table 113, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH3Then, the compounds with the same substituents as those in Table 1 are selected and numbered as 113-1 to 113-709.
Table 114, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH3Then, the compounds with the same substituents as those in Table 1 are selected and numbered 114-1 to 114-709.
Table 115, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CF3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of which are 115-1 to 115-709.
Table 116, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CF3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers are 116-1 to 116-709.
Table 117, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CF3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers of 117-1 to 117-709 are shown.
Table 118, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CF3Then, the compounds with the same substituents as those in Table 1 are selected and numbered 118-1 to 118-709.
Table 119, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When the compound is cyclopropyl, the same substituent as in Table 1 is selected, and the compound is numbered 119-1 to 119-709.
Table 120, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When the compound is cyclopropyl, the same substituent as in Table 1 is selected, and the number is 120-1 to 120-709.
Table 121, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When the compound is cyclopropyl, compounds with the same substituents as those in Table 1 are selected, and the numbers are 121-1 to 121-709.
Table 122, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When the compound is cyclopropyl, compounds with the same substituents as those in Table 1 are selected, and the numbers are 122-1 to 122-709.
Table 123, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=Then, compounds with the same substituents as those in Table 1 are selected, and the numbers are 123-1 to 123-709.
Table 124, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 124-1 to 124-709.
Table 125, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 125-1 to 125-709.
Table 126, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers are 126-1 to 126-709.
Table 127, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2CF3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 127-1 to 127-709.
Table 128, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2CF3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 128-1 to 128-709.
Table 129, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2CF3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of which are 129-1 to 129-709.
Table 130, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2CF3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 130-1 to 130-709.
Table 131, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CF2CF3Then, the compounds with the same substituents as those in Table 1 are selected and numbered as 131-1 to 131-709.
Table 132, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CF2CF3Then, the compounds with the same substituents as those in Table 1 are selected and numbered 132-1 to 132-709.
Table 133, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CF2CF3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers are 133-1 to 133-709.
Table 134, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CF2CF3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of the compounds are 134-1 to 134-709.
Table 135, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2S(O)CH3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers of 135-1 to 135-709-
Table 136, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2S(O)CH3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of 136-1 to 136-709-
Table 137, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2S(O)CH3When compounds with the same substituents as those in Table 1 are selected, the numbers of which are 137-1 to 137-709-
Table 138, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2S(O)CH3Then, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 138-1 to 138-709-
Table 139, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2S(O)2CH3Then, the same substituent as that in Table 1 is selected, and the numbers of 139-1 to 139-709-
Table 140, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2S(O)2CH3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 140-1 to 140-
Table 141, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2S(O)2CH3When the compounds with the same substituents as those in Table 1 are selected, the numbers of 141-1 to 141-709-
Table 142, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2S(O)2CH3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 142-1 to 142-709-
Table 143, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2S(O)CF3When compounds having the same substituents as those in Table 1 are selected, the numbers of which are 143-1 to 143-709-
Table 144, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2S(O)CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 144-1 to 144-709-
Table 145, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2S(O)CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 145-1 to 145-709-
Table 146, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2S(O)CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 146-1 to 146-
Table 147, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2S(O)2CF3When the compound is selected from the same substituents as those in Table 1, the numbers are 147-1 to 147-709-
Table 148, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2S(O)2CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 148-1 to 148-
Table 149, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2S(O)2CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 149-1 to 149-709-
Table 150, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2S(O)2CF3When the compound is selected from the same substituents as those in Table 1, the numbers are 150-1 to 150-
Table 151, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2OCH3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 151-1 to 151-709-
Table 152, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2OCH3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers of which are 152-1 to 152-
Table 153, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2OCH3When compounds with the same substituents as those in Table 1 are selected, the numbers of 153-1 to 153-709
Table 154, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2OCH3When compounds with the same substituents as those in Table 1 are selected, the numbers of which are 154-1 to 154-
Table 155, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2OCH2CF3When compounds with the same substituents as those in Table 1 are selected, the numbers of which are 155-1 to 155-
Table 156, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2OCH2CF3Then, the same substituents as those in Table 1 are selected, and the numbers of the substituents are 156-1 to 156-709-
Table 157, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2OCH2CF3When compounds with the same substituents as those in Table 1 are selected, the numbers of which are 157-1 to 157-709-
Table 158, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2OCH2CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 158-1 to 158-709-
Table 159, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2SCH3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 159-1 to 159-709-
Table 160, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2SCH3When selected, the same substituents as in Table 1The material is numbered 160-1 to 160-
Table 161, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2SCH3When the compounds with the same substituents as those in Table 1 are selected, the numbers of which are 161-1 to 161-709-
Table 162, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2SCH3When the compounds with the same substituents as those in Table 1 are selected, the numbers of which are 162-1 to 162-709-
Table 163, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When the substituent is CHO, compounds having the same substituent as in Table 1 are selected, and the numbers are 163-1 to 163-709
Table 164, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When the substituent is CHO, compounds with the same substituent as in Table 1 are selected, and the numbers are 164-1 to 164-
TABLE 165,A=O,B=O,X=H,Z=N,R2=CN,R3=Cl,R7=H,R9=H,R1When the substituent is CHO, compounds having the same substituent as in Table 1 are selected, and the numbers are 165-1 to 165-
Table 166, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When the substituent is CHO, compounds with the same substituent as in Table 1 are selected, numbered 166-1 to 166-709-
Table 167, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=NH2Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of 167-1 to 167-
Table 168, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=NH2When the compound is selected from the same substituents as those in Table 1, the numbers of which are 168-1 to 168-709-
Table 169, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=NH2When the compounds with the same substituents as those in Table 1 are selected, the numbers thereof are 169-1 to 169-709-
Table 170, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=NH2When the compound is selected from the same substituents as those in Table 1, the numbers are 170-1 to 170-709-
Table 171, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CF2H is the same substituent as in Table 1, and is numbered 171-1 to 171-
Table 172, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CF2H, a compound having the same substituent as that in Table 1 is selected, numbered 172-1 to 172-709
Table 173, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CF2H is the same substituent as shown in Table 1, and the numbers of 173-1 to 173-
Table 174, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CF2H, a compound having the same substituent as that in Table 1 is selected, and the number is 174-1 to 174-709-
Table 175, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2OCF3When the compound is selected from the same substituents as those in Table 1, the numbers are 175-1 to 175-
Table 176, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2OCF3When the compound is the same substituent as that in Table 1, the numbers are 176-1 to 176-
Table 177, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2OCF3When, compounds with the same substituents as those in Table 1 are selected, the numbers of which are 177-1 to 177-709-
Table 178, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2OCF3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers of 178-1 to 178-709-
Table 179, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2CH2CN, the same substituents as those in Table 1 are selected, and the numbers are 179-1 to 179-709
Table 180, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2CH2CN, the same substituent as that in Table 1 is selected, numbered 180-1 to 180-709-
Table 181, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2CH2CN, the same substituents as those in Table 1 are selected, and the numbers of 181-1 to 181-709
Table 182, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2CH2CN, the same substituent as that in Table 1 is selected, and the numbers of CN are 182-1 to 182-709-
Table 183, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2CH2OCH3Then, compounds with the same substituents as those in Table 1 are selected and numbered 183-1 to 183-709
Table 184, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2CH2OCH3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers of 184-1 to 184-
Table 185, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2CH2OCH3When the compound is the same substituent as that in Table 1, the numbers of which are 185-1 to 185-709-
Table 186, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2CH2OCH3When the compound is selected from the same substituents as those in Table 1, the numbers are 186-1 to 186-709-
Table 187 where a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2OCF2CF3When the same substituents as those in Table 1 are selected, the compound is numbered 187-1~187-709
Table 188, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2OCF2CF3When, compounds with the same substituents as those in Table 1 are selected, numbered as 188-1-188-
Table 189, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2OCF2CF3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers are 189-1 to 189-709-
Table 190, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2OCF2CF3Then, the compounds with the same substituents as those in Table 1 are selected and numbered 190-1 to 190-709-
Table 191, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2OCF2CF2H, a compound having the same substituent as that in Table 1 is selected, and the number of the compound is 191-1 to 191-709-
Table 192, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2OCF2CF2H, a compound having the same substituent as that in Table 1 is selected, and the number is 192-1 to 192-
Table 193, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2OCF2CF2H is selected from the same substituents as those in Table 1, numbered 193-1-193-
Table 194, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2OCF2CF2H, a compound having the same substituent as that in Table 1 is selected and numbered 194-1 to 194-709
Table 195, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2OCF2H, a compound having the same substituent as that in Table 1 is selected, and the number of the compound is 195-1 to 195-709-
Table 196, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2OCF2H, a compound having the same substituent as that in Table 1, is selected and numbered 196-1 to 196-709
Table 197, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2OCF2H, a compound having the same substituent as that in Table 1 is selected, and the number is 197-1 to 197-709-
Table 198, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2OCF2H, a compound having the same substituent as that in Table 1, numbered 198-1 to 198-709
Table 199, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2CH2SCH3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers of 199-1 to 199-709-
Table 200, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2CH2SCH3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 200-1 to 200-
Table 201, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2CH2SCH3When the compounds with the same substituents as those in Table 1 are selected, the numbers of 201-1 to 201-709-
Table 202, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2CH2SCH3When the compounds with the same substituents as those in Table 1 are selected, the numbers of 202-1 to 202-
Table 203, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2SCF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 203-1 to 203-709-
Table 204, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2SCF3When the compounds with the same substituents as those in Table 1 are selected, the numbers of which are 204-1 to 204-
Table 205, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2SCF3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers are 205-1 to 205-709-
Table 206, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2SCF3Then, compounds with the same substituents as those in Table 1 are selected, and the numbers of 206-1 to 206-709-
Table 207, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2SCH2CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 207-1 to 207-709-
Table 208, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2SCH2CF3Then, the compounds with the same substituents as those in Table 1 are selected, and the numbers of which are 208-1 to 208-
Table 209, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2SCH2CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 209-1 to 209-709-
Table 210, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2SCH2CF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 210-1 to 210-
Table 211, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2SCF2CF3When compounds with the same substituents as those in Table 1 are selected, the numbers of which are 211-1 to 211-709-
Table 212, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2SCF2CF3When the compounds with the same substituents as those in Table 1 are selected, the numbers of which are 212-1 to 212-
Table 213, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2SCF2CF3When compounds having the same substituents as those in Table 1 are selected, the numbers of which are 213-1 to 213-709-
Table 214, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2SCF2CF3When the compounds with the same substituents as those in Table 1 are selected, the numbers of which are 214-1 to 214-709-
Table 215, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2SCF2H, a compound having the same substituent as that in Table 1, numbered 215-1 to 215-
Table 216, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2SCF2H is a compound having the same substituent as that in Table 1, numbered 216-1 to 216-709
Table 217, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2SCF2H, a compound having the same substituent as that in Table 1, numbered 217-1 to 217-709
Table 218, a ═ O, B ═ O,X=H,Z=N,R2=CN,R3=CH3,R7=H,R9=H,R1=CH2SCF2H, a compound having the same substituent as that in Table 1, numbered 218-1 to 218-709
Table 219, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2SCF2CF2H, a compound having the same substituent as that in Table 1 is selected, numbered 219-1 to 219-
Table 220, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2SCF2CF2H, a compound having the same substituent as that in Table 1 is selected, numbered 220-1 to 220-709-
Table 221, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2SCF2CF2H, compounds with the same substituents as those in Table 1 are selected, and the numbers are 221-1 to 221-709-
Table 222, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2SCF2CF2When H isSelecting compounds with the same substituents as those in Table 1, the numbers of which are 222-1 to 222-
Table 223, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2OCH2Selecting compounds with the same substituents as those in Table 1 at Ph, with numbers of 223-1-223-
Table 224, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2OCH2Selecting compounds with the same substituents as those in Table 1 at Ph, numbered as 224-1-224-709-
Table 225, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2OCH2Selecting compounds with the same substituents as those in Table 1 at Ph, with the numbers of 225-1-225-
Table 226, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2OCH2Selecting compounds with the same substituents as those in Table 1 in Ph, numbered 226-1-226-709-
Table 227, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=When compounds with the same substituents as those in Table 1 are selected, the numbers of 227-1 to 227-
Table 228, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=When the compound with the same substituent as that in Table 1 is selected, the number of which is 228-1 to 228-
Table 229, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=When the compound is selected from the same substituents as those in Table 1, the numbers of which are 229-1 to 229-709-
Table 230, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=When the compound is selected from the same substituents as those in Table 1, the numbers of which are 230-1 to 230-
Table 231, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=When compounds with the same substituents as those in Table 1 are selected, the numbers of 231-1 to 231-
Table 232, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=When the compound is selected from the same substituents as those in Table 1, the numbers are 232-1 to 232-
Table 233, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=When compounds with the same substituents as those in Table 1 are selected, the numbers of which are 233-1 to 233-709-
Table 234, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=Then, the compounds with the same substituents as those in Table 1 are selected and numbered 234-1 to 234-
Table 235, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1=CH2NHCF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 235-1 to 235-709-
Table 236, a ═ O, B ═ O, X ═ H, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1=CH2NHCF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 236-1 to 236-709-
Table 237 where a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1=CH2NHCF3When the compound is selected from the same substituents as those in Table 1, the numbers are 237-1 to 237-
Table 238, a ═ O, B ═ O, X ═ H, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1=CH2NHCF3When the compound is selected from the same substituents as those in Table 1, the numbers of which are 238-1 to 238-709-
Table 239, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When F is equal to F, the compound with the same substituent as that in Table 1 is selected, and the compound is numbered as 239-1-239-
Table 240, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When F is equal to F, the compound with the same substituent as that in Table 1 is selected, and the number is 240-1 to 240-
Table 241, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When F is equal to F, the compound with the same substituent as that in Table 1 is selected, and the compound is numbered 241-1-241-
Table 242, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When F is equal to F, the same substituent as in Table 1 is selected as compound No. 242-1-242-
Table 243, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When the substituent is Cl, a compound with the same substituent as that in Table 1 is selected, and the number is 243-1 to 243-
Table 244, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When the substituent is Cl, the compound is selected from the same substituents as those in Table 1, numbered 244-1 to 244-
Table 245, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When Cl, compounds with the same substituents as those in Table 1 are selected, numbered 245-1 to 245-
Table 246, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When Cl, compounds with the same substituents as those in Table 1 are selected, and the numbers are 246-1-246-
Table 247, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=Cl,R3=Cl,R7=H,R9=H,R1When CN is not substituted, compounds with the same substituents as those in Table 1 are selected, numbered 247-1 to 247-
Table 248, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=Cl,R3=CH3,R7=H,R9=H,R1When CN is not substituted, compounds with the same substituents as those in Table 1 are selected, and the numbers are 248-1 to 248-
Table 249, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=CN,R3=Cl,R7=H,R9=H,R1When CN is not substituted, compounds with the same substituents as those in Table 1 are selected, and the numbers are 249-1 to 249-709-
Table 250, a ═ O, B ═ O, X ═ Cl, Z ═ N, R2=CN,R3=CH3,R7=H,R9=H,R1When CN is not substituted, compounds with the same substituents as those in Table 1 are selected, numbered 250-1 to 250-
Table 251, a ═ O, B ═ O, X ═ H, R2=Cl,R3=Cl,R7=H,R9=H,R1When F is equal to F, the following compounds are selected, and the serial numbers are 251-1 to 251-980.
Table 252, a ═ O, B ═ O, X ═ H, R2=Cl,R3=CH3,R7=H,R9=H,R1When F, the same substituents as those in Table 251 are selected, and the numbers are as follows252-1 to 252-.
Table 253, a ═ O, B ═ O, X ═ H, R2=CN,R3=Cl,R7=H,R9=H,R1When F is equal to F, compounds with the same substituents as those in Table 251 are selected, and the numbers are 253-1 to 253-containing 980.
Table 254, a ═ O, B ═ O, X ═ H, R2=CN,R3=CH3,R7=H,R9=H,R1When F is equal to F, the same substituent as in Table 251 is selected, and the numbers are 254-1 to 254-980.
Table 255, a ═ O, B ═ O, X ═ H, R2=Cl,R3=Cl,R7=H,R9=H,R1When Cl, compounds having the same substituents as those in Table 251 are selected and numbered 255-1 to 255-980.
Table 256, a ═ O, B ═ O, X ═ H, R2=Cl,R3=CH3,R7=H,R9=H,R1When Cl, compounds having the same substituents as those in Table 251 are selected and numbered 256-1 to 256-980.
Table 257, a ═ O, B ═ O, X ═ H, R2=CN,R3=Cl,R7=H,R9=H,R1When Cl, compounds having the same substituents as those in Table 251 are selected and numbered 257-1 to 257-980.
Table 258, a ═ O, B ═ O, X ═ H, R2=CN,R3=CH3,R7=H,R9=H,R1When Cl, compounds with the same substituents as those in Table 251 are selected, and the numbers are 258-1 to 258-980.
Table 259, a ═ O, B ═ O, X ═ H, R2=Cl,R3=Cl,R7=H,R9=H,R1When CN is not shown, compounds having the same substituents as those in Table 251 are selected as compounds numbered 259-1 to 259-980.
Table 260, a ═ O, B ═ O, X ═ H, R2=Cl,R3=CH3,R7=H,R9=H,R1When CN is not shown, compounds having the same substituents as those in Table 251 are selected and numbered 260-1 to 260-980.
Table 261, a ═ O, B ═ O, X ═ H, R2=CN,R3=Cl,R7=H,R9=H,R1CN represents a compound having the same substituent as that in Table 251, numbered 261-1 to 261-.
Table 262, a ═ O, B ═ O, X ═ H, R2=CN,R3=CH3,R7=H,R9=H,R1When CN is not shown, compounds having the same substituents as those in Table 251 are selected and numbered 262-1 to 262-980.
The compounds of formula I of the present invention can be prepared according to the following method:
the method comprises the following steps:
the compound of the general formula I can be an oxazinone compound shown in the general formula II and R9Lg (Lg is a leaving group, such as halogen, alkyl or aryl sulfonate or alkyl sulfate) to prepare an intermediate II', and reacting with HNR7R8The preparation method comprises the following steps:
the second method comprises the following steps:
the compound of the general formula I can be an oxazinone compound and HNR shown in the general formula II7R8Reacting to obtain an intermediate I', and reacting with R9Lg (Lg is a leaving group, such as halogen, alkyl or aryl sulfonate or alkyl sulfate) by reaction:
the third method comprises the following steps:
the compound of the general formula I can be prepared from a compound shown in the general formula III and (Cl)3CO)2Reacting CO to obtain intermediate IV, and reacting with R9Lg (Lg is a leaving group, e.g. halogen, alkyl or aryl sulfonate or alkyl sulfate) to produce intermediate V, which is reacted with HNR7R8The intermediate VI is prepared by reaction, and finally the intermediate VI is reacted with pyrazole acyl chloride. Wherein the pyrazolylchloride can be prepared from the corresponding carboxylic acid by conventional methods, e.g. by reaction with thionyl chloride:
The definition of each group in the general formula is the same as that of the previous group.
The reaction is carried out in a solvent, suitably selected from, for example, acetonitrile, tetrahydrofuran, diethyl ether, dichloromethane, chloroform, ethyl acetate, dioxane, toluene and the like.
The reaction temperature is between room temperature and the boiling point temperature of the solvent, and is usually 20-100 ℃.
The reaction time is 30 minutes to 20 hours, usually 1 to 10 hours.
The raw material intermediates and sources involved in the preparation method are as follows:
oxazinones of formula II are described in general overview by Bioorganic and Medicinal Chemistry 2008,8, 2095-. The preparation method refers to WO03015519 and WO2005118552 methods.
Anthranilic acid compounds of formula III can be prepared from aromatic hydrocarbons in a two-step reaction, as follows: organic Synthesis, Coll.Vol.10.p.23 (2004); vol.79, p.196 (2002); adv.heterocyclic.chem.1975, 18, 1-58; journal of the Brazilian Chemical Society 2001,12(3),273- "324; angew. chem. int. Ed. Engl.1980,19, 222-.
The preparation method of the carboxylic acid compound shown as the general formula VII refers to WO2006062978 and WO 03015519.
The compounds of the general formula I show high insecticidal activity on adults, larvae and eggs of harmful insects in the fields of agriculture, civil use and animal technology. Part of the compounds show better bactericidal activity. The invention therefore also includes the use of the compounds of the general formula I as pesticides and/or fungicides in agriculture and in other fields.
In particular, the compounds of formula I are active against important species of the following families and purposes: lepidoptera (Spodoptera, Cyamopsis, codling moth, etc.). For example, the compound pesticide is very effective to European corn borers, sugarcane borers, apple skin loopers, apple budworms, gypsy moths and the like, has better activity to diamond back moths, and can obtain good effect at very low dosage. Meanwhile, part of the compounds of the invention show better bactericidal activity and can be used for preventing and treating rice blast, cucumber downy mildew and anthracnose.
At the same time, the compounds of the general formula I have low toxicity to many beneficial insects and acarids, mammals, fish, birds and no phytotoxicity.
Owing to their positive properties, the abovementioned compounds can be used advantageously for protecting crops, domestic and livestock animals of agricultural and horticultural importance, as well as the environment in which humans are often exposed, against harmful insects and fungi.
The amount of the compound used to achieve the desired effect will vary depending on factors such as the compound used, the crop to be protected, the type of pest, the extent of infection, the climatic conditions, the method of application, and the dosage form employed.
A dose of 10 g to 5 kg of compound per hectare provides adequate control.
A further object of the present invention is also a method for controlling insects and/or phytopathogenic fungi of crops of agricultural and horticultural importance and/or of livestock and breeding animals and/or of the environment frequented by humans by applying the compounds of the general formula I. In particular, the amount of compound used varies from 10 g to 5 kg per hectare.
For practical use in agriculture, it is often beneficial to use compositions containing one or more compounds of formula I.
Accordingly, a further object of the present invention is to devise insecticidal and/or fungicidal compositions containing one or more compounds of formula I as active ingredients.
The composition can be used in the forms of dry powder, wettable powder, missible oil, microemulsion, paste, granules, solution, suspending agent and the like: the type of composition chosen depends on the particular application.
The compositions are prepared in a known manner, for example by dissolving the active substance or the solvent medium and/or a solid diluent in the presence of an optional surfactant.
Useful solid diluents or carriers are, for example: silica, kaolin, bentonite, talc, diatomaceous earth, dolomite, calcium carbonate, magnesium oxide, chalk, clay, synthetic silicate attapulgite, sepiolite and the like.
Besides water, usable liquid diluents also include aromatic organic solvents (xylene or a mixture of alkylbenzenes, chlorobenzene, etc.), paraffins (petroleum fractions), alcohols (methanol, propanol, butanol, octanol, glycerol), esters (ethyl acetate, isobutyl acetate, etc.), ketones (cyclohexanone, acetone, acetophenone, isophorone, ethyl amyl ketone, etc.), amides (N, N-dimethylformamide, N-methylpyrrolidone, etc.).
Useful surfactants are the sodium, calcium, triethyl or triethanolamine salts of alkyl sulfonates, alkylaryl sulfonates, polyoxyethylene alkylphenols, polyoxyethylene esters of sorbitol, lignosulfonates, and the like.
The composition may further contain specific additives for specific purposes, for example, binders such as gum arabic, polyvinyl alcohol, polyvinyl pyrrolidone, etc.
The concentration of the active ingredient in the above composition may vary within wide limits depending on the active ingredient, the purpose of use, the environmental conditions and the type of formulation employed. In general, the concentration of the active ingredient is from 1% to 90%, preferably from 5% to 60%.
If desired, further active ingredients compatible with the compounds of the formula I can be added to the compositions, for example further acaricides/insecticides, fungicides, plant growth regulators, antibiotics, herbicides, fertilizers.
Several formulation methods are exemplified below:
preparation of a suspending agent: the content of active components in the common formula is 5-35%. Adding water as medium, raw medicine, dispersant, suspending agent and anti-freezing agent into a sand mill, grinding, and making into suspension.
Preparing the aqueous emulsion: the raw medicine, the solvent and the emulsifier are added together to be dissolved into a uniform oil phase. Mixing water and antifreeze agent to obtain uniform water phase. Under high-speed stirring, adding the water phase into the oil phase or adding the oil phase into the water phase to form the aqueous emulsion with good dispersibility. The content of the active component of the aqueous emulsion is generally 5 to 15 percent. To prepare a concentrated emulsion, the compounds of the invention can be dissolved in one or more solvents and emulsifiers added to enhance the dispersion of the compounds in water.
Preparing wettable powder: according to the formula requirement, the technical material, various surfactants, solid diluents and the like are fully mixed and crushed by an ultrafine crusher to obtain a wettable powder product with a preset content (for example, 10-40%). To prepare wettable powders suitable for spraying, the compounds of the invention can be mixed with finely divided solid powders such as clays, non-polar silicates, carbonates and wetting, binding and/or dispersing agents.
Preparing water dispersible granules: mixing and crushing the original medicine, the powdery solid diluent, the wetting and spreading agent, the adhesive and the like, adding water, kneading, adding the mixture into a granulating agent with a screen with a certain specification for granulation, and then drying and screening (according to the range of the screen). Or adding the original drug, the dispersing agent, the disintegrating agent, the wetting agent and the solid diluent into a sand mill, grinding by taking water as a medium to prepare a suspending agent, and then carrying out spray drying granulation to prepare a granular product with the content of 20-30 percent.
Detailed Description
The following specific examples are intended to further illustrate the invention, but the invention is by no means limited to these examples.
Synthetic examples
Example 1: preparation of Compound 1-1
To a 25 ml reaction flask, II-1(1.0mmol,0.491g) and 5 ml of N, N-dimethylformamide were added at room temperature, and 0.102 g of 25% aqueous ammonia was added with stirring to conduct reaction at room temperature. After TLC monitoring reaction, the reaction liquid is dropped into 30 ml water, solid is separated out, and 0.315g of product, namely compound 1-1, is obtained after suction filtration, water washing and drying, and the yield is 62%.
Example 2: preparation of Compounds 1-2
To a 25 ml reaction flask, II-1(1.0mmol,0.491g) and 5 ml of N, N-dimethylformamide were added at room temperature, and 0.116 g of a 40% aqueous methylamine solution was added with stirring to conduct a reaction at room temperature. After TLC monitoring reaction, the reaction liquid is dropped into 30 ml water, solid is separated out, and 0.334g of product, namely compound 1-2, is obtained after suction filtration, water washing and drying, and the yield is 64%.
Example 3: preparation of Compounds 1-10
To a 25 ml reaction flask, II-1(1.0mmol,0.491g) and 5 ml of N, N-dimethylformamide were added under stirring at room temperature3)2CHNH2(1.5mmol,0.089g), reaction at room temperature. After TLC monitoring reaction, the reaction liquid is dropped into 30 ml water, solid is separated out, and 0.336g of product, namely compound 1-10, is obtained after suction filtration, washing and drying, and the yield is 61%.
Other compounds of formula I may be prepared by the preparation methods provided by the present invention.
Melting point and mass spectrometry (ESI) data for a portion of the compounds of formula I are as follows:
compound 1-1: melting point 126-; mass Spectrometry ESI (M/z, M + H)+):505.7/507.7/509.7
Compounds 1-2: melting point 233-; mass Spectrometry ESI (M/z, M + H)+):519.7/521.7/523.7
Compounds 1-3: melting point 205-; mass Spectrometry ESI (M/z, M + H)+):533.7/535.7/537.7
Compounds 1-10: melting point 116-; mass Spectrometry ESI (M/z, M)+H+):547.7/549.7/551.7
Compounds 1-20: melting point 186-188 deg.C; mass Spectrometry ESI (M/z, M + H)+):545.7/547.7/549.7
Compounds 1-21: melting point 211-213 ℃; mass Spectrometry ESI (M/z, M + H)+):559.7/561.7/563.7
Compounds 1-37: melting point 210 ℃ and 212 ℃; mass Spectrometry ESI (M/z, M + H)+):589.7/591.7/593.7
Compounds 1-39: melting point 223-; mass Spectrometry ESI (M/z, M + H)+):630.7/632.7/634.7
Compound 31-1: melting point 145-147 deg.C; mass Spectrometry ESI (M/z, M + H)+):521.7/523.7/525.7
Compound 31-2: melting point 240-242 ℃; mass Spectrometry ESI (M/z, M + H)+):535.7/537.7/539.7
Compound 31-3: melting point 227-; mass Spectrometry ESI (M/z, M + H)+):549.7/551.7/553.7
Compounds 31-10: melting point 122-124 ℃; mass Spectrometry ESI (M/z, M + H)+):563.7/565.7/567.7
Compounds 31-20: melting point 223-; mass Spectrometry ESI (M/z, M + H)+):561.7/563.7/565.7
Compounds 31-37: melting point 250 ℃ and 252 ℃; mass Spectrometry ESI (M/z, M + H)+):605.7/607.7/609.7
Compounds 31-39: melting point 214-; mass Spectrometry ESI (M/z, M + H)+):648.7/650.7/652.7
Compound 91-2: melting point 230-; mass Spectrometry ESI (M/z, M + H)+):581.6/583.6/585.6
Compound 91-10: melting point 127-; mass Spectrometry ESI (M/z, M + H)+):609.7/611.7/613.7
Compound 111-2: melting point 198 and 200 ℃; mass Spectrometry ESI (M/z, M + H)+):515.7/517.7/519.7
Compounds 111-10: melting point 125-127 ℃; mass Spectrometry ESI (M/z, M + H)+):543.8/545.8/547.8
Formulation examples (the amounts of the components added are in weight percent, and the active compound is metered in after being reduced to a hundred)
Example 4 30% wettable powder
Mixing the compounds 1-152, various surfactants and solid diluents, pulverizing with a superfine pulverizer to obtain 30% wettable powder
Example 5 20% suspending agent
Adding compound 1-218, dispersant, suspending agent and antifreeze agent into a sand mill by using water as a medium, and grinding to prepare the suspending agent.
Example 6 60% Water dispersible granules
Mixing and crushing the compounds 1-220, powdery solid diluent, wetting and spreading agent, adhesive and the like, adding water, kneading, adding into a granulator with a 10-100 mesh screen for granulation, drying, and screening (according to the range of the screen).
Example 7 10% aqueous emulsion
The compound 1-264, solvent and emulsifier are added together to dissolve into a uniform oil phase. Mixing water and antifreeze agent to obtain uniform water phase. Under high-speed stirring, mixing the water phase and the oil phase to form the aqueous emulsion with good dispersibility.
Example 8 25% suspending agent
Taking water as a medium, adding the compound 1-257, a dispersing agent, a suspending agent, an anti-freezing agent and the like into a sand mill for grinding, and preparing the suspending agent.
Example 9 20% wettable powder
The compound 1-271, various surfactants, solid diluents and the like are fully mixed and crushed by a superfine pulverizer to obtain a 20 percent wettable powder product.
Examples of measurement of biological Activity
EXAMPLE 10 insecticidal and acaricidal Activity assay
The compounds of the invention can be used to control the following pests:
lepidoptera (moths and butterflies): plutella xylostella, beet armyworm (beet armyworm), cnaphalocrocis medinalis, Nanmei corn seedling spotted borer, brown banded moth species, lychee yao-filiformis, diamond-back diamond-like diamond-back moth, cotton brown banded cabbage moth, Egypt diamond-back moth, cutworm species (cutworm), rice leaf roller (meadow leaf roller), cutworm (black cutworm), diamond-back moth species (cotton bollworm), mango transverse line tail moth, southwestern corn stalk borer, cotton leaf looper (cotton leaf moth), oriental fruit borer (oriental fruit borer), sugarcane borer, cotton bud, butterfly species, Juglans sylvestris, ostrinia (borer), aromatic wood moth (wood cudweed moth), velvet bean moth, silk moth species (borer), fruit tree leaf roller moth, codling moth (apple leaf moth), rose leaf roller, pea pod moth, green fruit seed (yellow pod moth), yellow pod moth (yellow knot moth), yellow knot moth (yellow knot moth), yellow moth (yellow moth), yellow moth seed (yellow moth), yellow moth (yellow moth), yellow moth) species (yellow moth, yellow rice moth, the present invention relates to a method for preventing and treating trichoderma serpentinatum, cabbage looper, cotton leafminer, cutworm (root cutting insect), tobacco budworm, light brown apple moth, plum fruit moth (fruit moth), tenebrio molitor, thin leaf moth (leaf miner), fruit moth (noctuid), primary root cutter, stem borer, peach fruit moth (peach fruit moth), oil palm bag moth, eggplant white wing moth, corn fruit moth (corn borer moth/cotton bollworm), cotton bollworm, rose leaf moth, bean white line root cutter, tomato white moth, fine moth, moth (common grape leaf moth), oriental moth (peach (apricot) fruit borer), cabbage caterpillar, small leaf moth, tomato leaf moth (European corn borer), European corn borer (European corn borer), bean leaf borer, cabbage moth (cabbage moth), cabbage leaf borer (cabbage moth), cabbage leaf moth (cabbage moth), cabbage looper (cabbage looper), cabbage looper (cabbage leaf moth), cabbage looper (cabbage moth, cabbage leaf moth), cabbage looper (cabbage moth, cabbage leaf moth), cabbage leaf moth (cabbage leaf moth ), cabbage leaf moth, armyworm species (cutworm), hydropiper punctiferalis (cnaphalocrocis medinalis), rice stem moth, winter looper, Indian rice leaf spot moth, pink bollworm (pink bollworm), black cutworm, grape leaf spot moth, grape berry moth, african dalbergia, gypsy moth, pink spot moth species (pink borer), mediterranean pink borer, coffee leaf miner, peach leaf miner (apple leaf miner), pink spot moth, wheat moth, marchantia, potato tuber moth, yellow stem borer, pink stem borer, phomopsis species (root borer), soybean looper, glossy privet leaf looper (grape berry moth), spodoptera species (armyworm), grape leaf curl moth, leopard moth, fruit moth, meadow armyworm (fall armyworm), olive oil moth, cabbage caterpillar (imported cabbage caterpillar), Loxatis (looper), looper (spodoptera), looper, armyworm, cabbage moth, grass moth, cabbage looper, grass moth, etc Chlamydomonas, armyworm (noctuid), armyworm, southern spodoptera littoralis (southern armyworm), mythic fungus, pink borer, stem-eating nocturnal species (stem borer), tobacco pink borer (tobacco borer), coffea dull moth, Epimeces species, hornet noctuid species (stem borer), noctuid, inchworm, looper, telangiecta, cupra fusca.
Homoptera (whitefly, aphid, leafhopper, scale): aphid species (aphid), cereal sipunculid, bipolaris avenae (bipolaris mylittae), corn leaf aphid (corn aphid), sonophorus species (aphid), greenhouse whitefly (greenhouse whitefly), eleusine lecanii (black scale), arrowhead clam (arrowhead), barley tube aphid (England wheat aphid), variegated aphid species (aphid), white wing whitefly, ceroplastes species (scale), whitefly species (whitefly), white back planthopper, cupula lecanii (scale), Erysipelothrix striata species (scale), black tail leafhopper (green leafhopper), ebony black sandalwood scale, pyricularia pyricularis (holy jorda), quadlike (skimmit), field cicada species (blowshell), amanita avena (rose aphid), mealybug species (mealybug), green peach leaf, black leaf aphid (seph), black leaf aphid (black mealybug), white tail (mealybug), white tail leaf mealybug) and white leaf, Pythium mythidae, Rhizomucor vitis, Nippon zeae planthopper, Pipis spp, Tocopyrium peltatum (scale), Nilaparvata lugens, Blastomya gossypii, Asparagus pipiens (Asparagus), Coccinum spp, Rosa longula (Rosa), Pink Aphis pomonella, Trionyx brassicae (whitefly), Myzus avenae (Engraulis praecox), Triplophora albonella, Gossypium gossypii, Trionyx lecanii, Ammopsis cerealis (Russian), Cerasirus spp (scale), Trialeurodes spirochaeta, Trialeuroptera (whitefly), Brassica oleracea (cabbage aphid), Phymatomorus major (potato aphid), Trialeurodes filiformica (Sophora gossypii), mango yellow leaf, Cicada (cicada), Cicadae, Coccinum, Lepidium spp, Aleuritis indica (Carpesium), Psidium amanus, Lepidium spp), Pseudocerotis (Ipompholus, Lepidium spp), Pseudocercosphacida (Ipompholus), Aleurotis (Ipompholus), Pseudoceros pellus, Aleurotis (Ipompholus), Aleur, Apple aphid, Lecanicillium lecanii, and eggplant Nepalosiphum lecanii.
Several insects were tested for insecticidal activity using the compounds of the present invention. The method of measurement is as follows:
after the test compound was dissolved in N, N-dimethylformamide, it was diluted to the desired concentration with water containing 0.1% tween 80.
1. The method takes diamondback moth and beet armyworm as targets and adopts an immersion method to determine the insecticidal activity. Mortality of the target was investigated 2-3 days after treatment. Some of the test results are as follows:
when the concentration of the liquid medicine is 20ppm, the death rate of the compound reaches 100 percent for diamondback moth, wherein the compound accounts for 1-2, 1-10, 1-37, 31-2, 31-10, 31-37, 91-2, 91-10, 111-2 and 111-10; the death rate of the compound 1-2, 1-10, 1-37, 31-2, 31-10, 91-2, 91-10, 111-2 and 111-10 to beet armyworm reaches 100%.
When the concentration of the liquid medicine is 4ppm, the death rate of the compound reaches 100% for diamondback moth, wherein the compound accounts for 1-2, 1-10, 1-37, 31-2, 31-10, 31-37, 91-2, 91-10 and 111-10; the mortality rate of the compounds 1-2, 1-10, 31-2, 31-10, 91-2, 91-10 and 111-10 to beet armyworm reaches 100 percent.
When the concentration of the liquid medicine is 1ppm, the death rate of the compound 1-2, 1-10, 31-2, 31-10, 91-2, 91-10 and 111-10 to the diamondback moth reaches 100 percent; the mortality rate of the compounds 1-2, 1-10, 31-2, 31-10, 91-2 and 91-10 to beet armyworm reaches 100 percent.
When the concentration of the liquid medicine is 0.5ppm, the death rate of the compounds 1-2, 1-10, 31-2, 31-10 and 91-2 to the plutella xylostella reaches 100 percent; the mortality rate of the compounds 31-2 and 31-10 to beet armyworm reaches 100 percent.
When the concentration of the liquid medicine is 0.25ppm, the death rate of the compounds 1-2, 1-10, 31-2, 31-10 and 91-2 to the plutella xylostella reaches 100 percent;
when the concentration of the liquid medicine is 0.05ppm, the mortality rate of the compounds 1-2, 1-10, 31-2 and 31-10 to the plutella xylostella reaches more than 80 percent;
according to the method, the compound 1-2 and known compounds chlorantraniliprole and cyantraniliprole are selected for carrying out parallel determination on the diamondback moth killing activity. The results of the experiment are shown in the following table:
table for assaying the activity of killing plutella xylostella:
| compound numbering | Concentration (ppm) | Mortality (%) |
| 1-2 | 0.025 | 90 |
| Chlorantraniliprole | 0.025 | 28 |
| Cyantraniliprole | 0.025 | 30 |
2. Taking aphids as targets, adopting a Potter spray tower spraying method to carry out insecticidal activity determination, and investigating the mortality of the targets 2-3 days after treatment. Some of the test results are as follows:
when the concentration of the liquid medicine is 4ppm, the death rate of the compounds 1-2, 1-10, 31-2 and 31-10 to aphids reaches more than 90 percent;
3. the insecticidal activity is determined by taking chilo suppressalis as a target and adopting an immersion method, and the mortality of the target is investigated 2-3 days after treatment. Some of the test results are as follows:
when the concentration of the liquid medicine is 4ppm, the death rate of the compounds 1-2, 1-10 and 31-10 to chilo suppressalis reaches 100%.
According to the method, the compounds 1-2, 1-10 and 31-10 are selected to be used for carrying out the chilo suppressalis killing activity parallel determination with the known compounds chlorantraniliprole and cyantraniliprole. The results of the experiment are shown in the following table:
striped rice borer killing activity assay table:
| compound numbering | Concentration (ppm) | Mortality (%) |
| 1-2 | 4 | 100 |
| 1-10 | 4 | 100 |
| 31-10 | 4 | 100 |
| Chlorantraniliprole | 4 | 50 |
| Cyantraniliprole | 4 | 20 |
Claims (8)
1. A substituted pyrazole amide compound is shown as a general formula I:
in the formula:
a and B are O;
x is H;
z is N;
R1selected from F, Cl, Br, CH3;
R2,R3Can be the same or different and is respectively selected from H, F, Cl, Br, CN, CH3、CH2CH2CH3、CH2F、CF2H、CF3、CH2Cl、CCl2H、CCl3、CFClH;
R4Selected from F, Cl, Br, CN;
R5, R6can be the same or different and is respectively selected from H, F, Cl and Br;
R7selected from H, CH3、CH2CH3、CH(CH3)2;
R9Is selected from H or CH3。
2. The substituted pyrazole amide compound according to claim 1 wherein in formula I,
a and B are O;
x is H;
z is N;
R1selected from F, Cl, Br, CH3;
R2,R3Can be the same or different and is respectively selected from F, Cl, Br and CN;
R4selected from F, Cl, Br;
R5, R6can be the same or different and is respectively selected from H, F, Cl and Br;
R7selected from H, CH3;
R9Is selected from H.
4. The substituted pyrazole amide compound of claim 1, wherein formula I is specifically:
a is O, B is O, X is H, Z is N, R1Is F, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH3,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is F, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH (CH3)2, R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is F, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is composed of,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Cl, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is from CH3,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Cl, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH (CH)3)2,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Cl, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is composed of,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Br, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH3,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Br, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH (CH)3)2,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is CH3,R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH3,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is CH3,R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH (CH3)2, R9Is H.
5. The substituted pyrazole amide compound of claim 4 wherein formula I is specifically:
a is O, B is O, X is H, Z is N, R1Is F, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH3,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is F, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH (CH)3)2,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Cl, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is from CH3,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Cl, R2Is a compound of the formula Cl,R3is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH (CH)3)2,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Br, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH3,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is Br, R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH (CH)3)2,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is CH3,R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH3,R9Is H; or the like, or, alternatively,
a is O, B is O, X is H, Z is N, R1Is CH3,R2Is Cl, R3Is Cl, R4Is Br, R5Is Cl, R6Is H, R7Is H, R8Is CH (CH)3)2,R9Is H.
6. The use of a compound of the general formula I according to claim 1 as a pesticide in the agricultural field.
7. A pesticidal composition comprising a biologically effective amount of a compound of formula I according to claim 1 and at least one additional component selected from the group consisting of a surfactant, a solid diluent or a liquid diluent.
8. An insecticidal and bactericidal composition, which is characterized in that: comprising a compound of formula I according to claim 1 and an agriculturally acceptable carrier, the active ingredients in the composition being present in an amount of 0.1 to 99% by weight.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201610028679.4A CN106977494B (en) | 2016-01-16 | 2016-01-16 | Substituted pyrazole amide compounds and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201610028679.4A CN106977494B (en) | 2016-01-16 | 2016-01-16 | Substituted pyrazole amide compounds and application thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN106977494A CN106977494A (en) | 2017-07-25 |
| CN106977494B true CN106977494B (en) | 2021-04-30 |
Family
ID=59340023
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201610028679.4A Active CN106977494B (en) | 2016-01-16 | 2016-01-16 | Substituted pyrazole amide compounds and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN106977494B (en) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110066270B (en) * | 2018-01-24 | 2021-02-26 | 湖南化工研究院有限公司 | Polyamide compound and preparation method and application thereof |
| CN110835330A (en) * | 2018-08-15 | 2020-02-25 | 海利尔药业集团股份有限公司 | Preparation method of substituted pyrazole amide compound with insecticidal activity |
| BR112023019788A2 (en) | 2021-03-30 | 2023-11-07 | Bayer Ag | 3-(HETERO)ARYL-5-CHLORODIFLOROMETHYL-1,2,4-OXADIAZOLE AS A FUNGICIDE |
| BR112023019400A2 (en) | 2021-03-30 | 2023-12-05 | Bayer Ag | 3-(HETERO)ARYL-5-CHLORODIFLOROMETHYL-1,2,4-OXADIAZOLE AS A FUNGICIDE |
| EP4334315A1 (en) | 2021-05-06 | 2024-03-13 | Bayer Aktiengesellschaft | Alkylamide substituted, annulated imidazoles and use thereof as insecticides |
| WO2022238391A1 (en) | 2021-05-12 | 2022-11-17 | Bayer Aktiengesellschaft | 2-(het)aryl-substituted condensed heterocycle derivatives as pest control agents |
| CN113331198B (en) * | 2021-06-28 | 2023-12-12 | 海利尔药业集团股份有限公司 | Use of flucloxapyroxad for improving plant quality |
| WO2023280999A1 (en) | 2021-07-07 | 2023-01-12 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| JP2024528271A (en) | 2021-08-05 | 2024-07-26 | シンジェンタ クロップ プロテクション アクチェンゲゼルシャフト | Method for controlling diamide-resistant pests and compounds therefor |
| JP2024529148A (en) | 2021-08-13 | 2024-08-01 | バイエル、アクチエンゲゼルシャフト | Active compound combinations and antifungal compositions containing them - Patents.com |
| CA3229873A1 (en) | 2021-08-25 | 2023-03-02 | Bayer Aktiengesellschaft | Novel pyrazinyl-triazole compounds as pesticides |
| CN113693070B (en) * | 2021-09-06 | 2024-03-12 | 海利尔药业集团股份有限公司 | Use of flucloxapyroxad for increasing plant reproductive activity |
| EP4404753A1 (en) | 2021-09-23 | 2024-07-31 | Syngenta Crop Protection AG | Insect, acarina and nematode pest control |
| WO2023105065A1 (en) | 2021-12-10 | 2023-06-15 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023105064A1 (en) | 2021-12-10 | 2023-06-15 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023152340A1 (en) | 2022-02-10 | 2023-08-17 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023203038A1 (en) | 2022-04-19 | 2023-10-26 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| AU2023264210A1 (en) | 2022-05-03 | 2024-10-31 | Bayer Aktiengesellschaft | Use of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine for controlling unwanted microorganisms |
| WO2023213670A1 (en) | 2022-05-03 | 2023-11-09 | Bayer Aktiengesellschaft | Crystalline forms of (5s)-3-[3-(3-chloro-2-fluorophenoxy)-6-methylpyridazin-4-yl]-5-(2-chloro-4-methylbenzyl)-5,6-dihydro-4h-1,2,4-oxadiazine |
| EP4295683A1 (en) | 2022-06-21 | 2023-12-27 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| WO2023237444A1 (en) | 2022-06-06 | 2023-12-14 | Bayer Aktiengesellschaft | Agrochemical formulations comprising crystalline form a of 4-[(6-chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5h)-one |
| WO2024104643A1 (en) | 2022-11-17 | 2024-05-23 | Bayer Aktiengesellschaft | Use of isotianil for controlling plasmodiophora brassica |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1541063A (en) * | 2001-08-13 | 2004-10-27 | ��Ļ���Ű˾ | Method for controlling particular insects by applying anthranilamide compounds |
| CN1549811A (en) * | 2001-08-15 | 2004-11-24 | ��Ļ���Ű˾ | Ortho-substituted aryl amides for controlling invertebrate pests |
| CN1678192A (en) * | 2001-08-13 | 2005-10-05 | 纳幕尔杜邦公司 | Arthropodicidal anthranilamides |
| CN1918144A (en) * | 2004-02-18 | 2007-02-21 | 石原产业株式会社 | Anthranilamide-based compound, method for producing the same, and pest-controlling agent containing the same |
| WO2008155990A1 (en) * | 2007-06-20 | 2008-12-24 | Ishihara Sangyo Kaisha, Ltd. | Method for producing anthranilamide compound |
| CN102617551A (en) * | 2012-03-08 | 2012-08-01 | 华东理工大学 | Anthranilic diamide compound containing difluoro (methylene) methyl |
-
2016
- 2016-01-16 CN CN201610028679.4A patent/CN106977494B/en active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1541063A (en) * | 2001-08-13 | 2004-10-27 | ��Ļ���Ű˾ | Method for controlling particular insects by applying anthranilamide compounds |
| CN1678192A (en) * | 2001-08-13 | 2005-10-05 | 纳幕尔杜邦公司 | Arthropodicidal anthranilamides |
| CN1549811A (en) * | 2001-08-15 | 2004-11-24 | ��Ļ���Ű˾ | Ortho-substituted aryl amides for controlling invertebrate pests |
| CN1918144A (en) * | 2004-02-18 | 2007-02-21 | 石原产业株式会社 | Anthranilamide-based compound, method for producing the same, and pest-controlling agent containing the same |
| WO2008155990A1 (en) * | 2007-06-20 | 2008-12-24 | Ishihara Sangyo Kaisha, Ltd. | Method for producing anthranilamide compound |
| CN102617551A (en) * | 2012-03-08 | 2012-08-01 | 华东理工大学 | Anthranilic diamide compound containing difluoro (methylene) methyl |
Also Published As
| Publication number | Publication date |
|---|---|
| CN106977494A (en) | 2017-07-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN106977494B (en) | Substituted pyrazole amide compounds and application thereof | |
| US11540516B2 (en) | M-diamide compound and preparation method therefor and use thereof | |
| CN106588870B (en) | Substituted salicylamide compound and application thereof | |
| ES2370534T3 (en) | PEST CONTROL AGENT CONTAINING A NEW PIRIDIL-METHANAMINE DERIVATIVE OR ONE OF ITS SALTS. | |
| JP5183735B2 (en) | Substituted pyrimidine ether compounds and their use | |
| MX2010013705A (en) | Novel heteroaromatic amides and thioamides as pesticides. | |
| CN111662269B (en) | 1-pyridyl pyrazole amide compound and preparation method and application thereof | |
| CS327891A3 (en) | 1-arylimidazoles, process of their preparation and pesticidal compositions comprising said 1-arylimidazoles | |
| CN112661665B (en) | Amide compound and preparation method and application thereof | |
| US4725589A (en) | Insecticidal composition for agricultural and horticultural use | |
| JPH0686439B2 (en) | Novel substituted 3-isocyanato-1,2,5-oxadiazoles | |
| TW201934545A (en) | Novel anthranilamides, their use as insecticide and processes for preparing the same | |
| TW202114999A (en) | Isoxazoline compounds and their use as pest control agents | |
| US10781177B2 (en) | Pyridine compound and use thereof | |
| CA1234819A (en) | N-(thio)carbamoylaryl(thio)carboximidic acid esters, process for their preparation, agents containing them and their use as pest control agents | |
| TWI252228B (en) | Pesticidal pyrimidine-derivatives | |
| CN107001325B (en) | O-formamido benzamide compound and application thereof | |
| US5981438A (en) | 1-phenyl-5-anilinotetrazoles | |
| WO2008134970A1 (en) | Anthranilamide compounds and the use thereof | |
| DE19605903A1 (en) | Pyridyl phenyl and benzyl ethers, processes and intermediates for their preparation and their use | |
| CN108069973B (en) | Substituted six-membered heterocyclic compound containing pyrimido ring and preparation method and application thereof | |
| CN111285861B (en) | Trifluoromethyl oxadiazole substituted pyrimidinamine compound, and preparation method and application thereof | |
| JPH05507727A (en) | Nematicide pyrimidine derivatives | |
| CN114516868B (en) | N-heteroaryl methyl difluoromethyl pyrimidine amine compound, and preparation method and application thereof | |
| JPH05509334A (en) | Nematicidal pyrimidine derivatives |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |