CN103205125A - Thermosetting Resin Composition For Semiconductor Encapsulation And Encapsulated Semiconductor Device - Google Patents
Thermosetting Resin Composition For Semiconductor Encapsulation And Encapsulated Semiconductor Device Download PDFInfo
- Publication number
- CN103205125A CN103205125A CN2013101138921A CN201310113892A CN103205125A CN 103205125 A CN103205125 A CN 103205125A CN 2013101138921 A CN2013101138921 A CN 2013101138921A CN 201310113892 A CN201310113892 A CN 201310113892A CN 103205125 A CN103205125 A CN 103205125A
- Authority
- CN
- China
- Prior art keywords
- group
- particle size
- composition
- resin
- integer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 0 CCCCCC*(C(*C(*1*)=O)=O)C1=O Chemical compound CCCCCC*(C(*C(*1*)=O)=O)C1=O 0.000 description 1
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
- H01L23/29—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the material, e.g. carbon
- H01L23/293—Organic, e.g. plastic
- H01L23/296—Organo-silicon compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L83/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon only; Compositions of derivatives of such polymers
- C08L83/04—Polysiloxanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L83/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon only; Compositions of derivatives of such polymers
- C08L83/04—Polysiloxanes
- C08L83/08—Polysiloxanes containing silicon bound to organic groups containing atoms other than carbon, hydrogen and oxygen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G77/00—Macromolecular compounds obtained by reactions forming a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon in the main chain of the macromolecule
- C08G77/04—Polysiloxanes
- C08G77/22—Polysiloxanes containing silicon bound to organic groups containing atoms other than carbon, hydrogen and oxygen
- C08G77/26—Polysiloxanes containing silicon bound to organic groups containing atoms other than carbon, hydrogen and oxygen nitrogen-containing groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/34—Silicon-containing compounds
- C08K3/36—Silica
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G77/00—Macromolecular compounds obtained by reactions forming a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon in the main chain of the macromolecule
- C08G77/04—Polysiloxanes
- C08G77/12—Polysiloxanes containing silicon bound to hydrogen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G77/00—Macromolecular compounds obtained by reactions forming a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon in the main chain of the macromolecule
- C08G77/04—Polysiloxanes
- C08G77/20—Polysiloxanes containing silicon bound to unsaturated aliphatic groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K7/00—Use of ingredients characterised by shape
- C08K7/16—Solid spheres
- C08K7/18—Solid spheres inorganic
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L2924/00—Indexing scheme for arrangements or methods for connecting or disconnecting semiconductor or solid-state bodies as covered by H01L24/00
- H01L2924/0001—Technical content checked by a classifier
- H01L2924/0002—Not covered by any one of groups H01L24/00, H01L24/00 and H01L2224/00
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- Computer Hardware Design (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Power Engineering (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Structures Or Materials For Encapsulating Or Coating Semiconductor Devices Or Solid State Devices (AREA)
- Silicon Polymers (AREA)
- Polyurethanes Or Polyureas (AREA)
Abstract
A thermosetting resin composition for semiconductor encapsulation contains a both end allyl isocyanurate ring-terminated organopolysiloxane polymer as a sole base polymer and an isocyanurate ring-containing organohydrogenpolysiloxane polymer as a sole curing agent or crosslinker. When a semiconductor element array having semiconductor elements mounted on a substrate with an adhesive is encapsulated with the thermosetting resin composition, warp-free semiconductor devices having improved heat resistance and moisture resistance are obtainable.
Description
Technical field
The present invention relates to for the compositions of thermosetting resin of semiconductor packages with the semiconducter device of its encapsulation.Be installed in semiconductor device array on inorganic substrate, metal base or the organic substrate by binding agent or chip join agent will having at least one semiconductor element, perhaps have the large size silicon wafer that formed semiconductor element therein by the thin compound of thermosetting resin after the solid state encapsulation, can obtain having the semiconducter device of thermotolerance and the essentially no warpage of moisture resistance properties of raising.This resin combination can once property encapsulation and the polished and section easily of this potting resin on this wafer-level.
Background technology
Semiconductor device can carry out resin package by various technology, comprises the silk screen printing of transfer mould, cast and liquid potting resin.Reduce the demand of electronics size and profile recently, require further with the semiconductor element microminiaturization.Even the slim packing that requires resin package comprises the multilayer silicon chip and has the thickness that is up to 500 μ m.
As having the wafer of about 8 inch diameters, current prior art is implemented encapsulation under no problem basically situation for the minor diameter base material.Yet, have base material greater than 12 inch diameters for those, because the string stress of encapsulation back Resins, epoxy, semiconductor element may separate from metal or other base materials, produces the problem that stops scale operation.
In order to overcome the problem that above-mentioned diameter about wafer and metal base increases, must be by with the filler loaded resin of 90wt% at least or the resin modulus is reduced the string stress of resin when solidifying reduced.
Under the current trend that a plurality of semiconductor elements pile up, the encapsulated layer thickening.Therefore, the main flow semiconducter device obtains by the potting resin layer is finished to thin profile.When the potting resin layer was polished, some problems can appear.If use and loaded the resin encapsulant of the filler of 90wt% at least, may make the breaks down of slice tool, need the frequent blade of changing, cause the increase of cost.In addition, polishing must be finished under high pressure, and this just has the destroyed or ruined risk of wafer of semiconductor element possibility.At the low modulus resin material, for example typically be under the situation of traditional silicoorganic compound, because their flexibility may produce the problem that resin stops up and breaks during polishing.
Comprising the polymkeric substance that contains the isocyanic ester ring and containing isocyanic ester ring hydrogen is that the composition of terminal polysiloxane polymer is known in this area.For example, patent document 1 discloses a kind of composition, and it comprises the polymkeric substance of the epoxy addition polymerization of the polysiloxane that diallyl list glycidyl isocyanuric acid ester and the addition reaction of hydrogeneous silyl polysiloxane obtain.Patent document 2 discloses a kind of composition, and it comprises and contains isocyanuric acid ester cyclopolysiloxane and hydrogeneous silyl polysiloxane.Patent document 3 discloses a kind of addition curable composition, and it comprises cyanacrylate and hydrogeneous silyl polysiloxane.Patent document 4-6 discloses the addition curable composition, and it comprises the polysiloxane that contains isocyanurate ring and hydrogen silyl and the solidifying agent that contains thiazolinyl.Patent document 1 and 2 this to contain the isocyanurate ring polymer composition be flexible, but incompatible with linking agent, this is because its basic components comprises siloxane bond.Patent document 1 and 2 this contain the isocyanuric acid ester cyclopolymer and be difficult to solidify by addition reaction, this is because the position of thiazolinyl group is uncertain, can't utilize hydrogenation silylation or addition reaction, that is, the advantage of fast setting reaction.High crosslink density is rigidity and less flexibility so this of patent document 3-6 contains the isocyanuric acid polymer composition.The term of Shi Yonging " hydrogen silyl " refers to Si-H herein.
A kind of cure polymer of current needs, it is to contain the isocyanuric acid ester cyclopolysiloxane and the addition reaction of hydrogeneous silyl polysiloxane obtains, and has flexibility, curing performance, consistency and water-fast vapour permeability.
The reference document tabulation
Patent document 1:JP-A 2008-143954
Patent document 2:JP-A 2008-150506
Patent document 3:JP-A H09-291214 (EP 0803529)
Patent document 4:JP 4073223
Patent document 5:JP-A 2006-291044
Patent document 6:JP-A 2007-009041
Summary of the invention
The purpose of this invention is to provide the thermoset composition for packaging semiconductor array or large size silicon wafer, this semiconductor array has at least one and uses binding agent or chip join agent to be installed in semiconductor element on inorganic substrate, metal base or the organic substrate, this large size silicon wafer has the semiconductor element that is formed on wherein, obtain having the semiconducter device of the essentially no warpage of improved thermotolerance and moisture resistance thus, with can be on wafer-level once the property encapsulation and can make polishing and section easily.Another purpose provides the semiconducter device that uses this compositions of thermosetting resin encapsulation.
The contriver finds when using the compositions of thermosetting resin encapsulated semiconductor device, said composition is compared with the silicoorganic compound that prior art is used, provide effective polishing, water tolerance and gas infiltrative advantage, this compositions of thermosetting resin comprises as the two ends of single base polymer and all is the organopolysiloxane polymkeric substance of terminal, is the polysiloxane polymer of terminal and the inorganic spherical filler with specified particle distribution of sizes as the isocyanurate ring hydrogen that contains of single curing agent with the allyl group isocyanurate ring.Also find even when using said composition encapsulation large-sized wafer, said composition provides the reduction warpage, the non-adhesive surface of curing composition and the advantage of high versatility.The present invention is found to be the basis with these.
On the one hand, the invention provides the compositions of thermosetting resin for semiconductor packages, comprise:
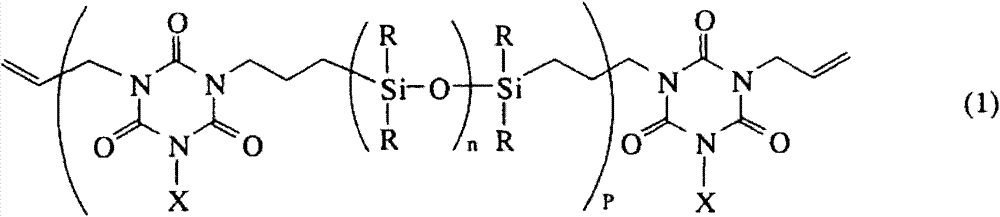
(A) the organopolysiloxane polymkeric substance that has allyl group isocyanic ester ring structure at the molecular chain two ends shown in general formula (1), as containing the thiazolinyl organopolysiloxane,
Wherein for not containing the univalence hydrocarbyl of aliphatic unsaturated link(age), R is alkyl or phenyl to X independently of one another independently of one another, and n is that integer and the P of 1-50 is the integer of 1-30,
(B) the organic radical hydrogen polysiloxanes polymkeric substance that contains isocyanurate ring shown in general formula (2), its terminal at siloxane chain have at least two hydrogen atoms (Si-H group) that are connected on the silicon, as the organic radical hydrogen polysiloxanes of no epoxy group(ing),
Wherein X is not independently of one another for containing aliphatic undersaturated monovalent hydrocarbon group, R is alkyl or phenyl independently of one another, n is the integer of 1-50, m is the integer of 0-5, with P be the integer of 1-30, wherein this repetition siloxane unit can randomly connect, its amount makes based on every mole of allyl group in the component (A), the Si-H group that has the 0.8-4.0 mole in the component (B)
(C) curing catalyst of catalytic amount and
(D) inorganic spherical filler, the part that is had 10.0 to 50.0 μ m average particle size particle size by (D-1), (D-2) has the part of 1.1-5.0 μ m average particle size particle size, (D-3) part of 0.1-1.0 μ m average particle size particle size is formed, (D-1): (D-2): weight ratio (D-3) is 95-70: 3-20: 2-10, and condition is (D-1), and total amount (D-2) and (D-3) is 100, the component of per 100 weight parts (A) and (B) sum, described amount of filler is the 30-900 weight part.Said composition should not contain in addition any of component (A) and contain the thiazolinyl organopolysiloxane and do not contain any component (B) no epoxy group(ing) organic radical hydrogen polysiloxanes in addition.
In preferred embodiments, this inorganic spherical filler (D) is preparing spherical SiO 2.Also preferred, this inorganic spherical filler (D) has used silane coupling agent to carry out surface treatment.This silane coupling agent is preferably selected from vinyltrimethoxy silane, vinyltriethoxysilane, 2-(3,4-epoxy group(ing) cyclohexyl) ethyl trimethoxy silane, 3-glycidoxypropyl methyl dimethoxysilane, the 3-glycidoxypropyltrimewasxysilane, 3-glycidoxypropyl methyldiethoxysilane, 3-glycidoxypropyl triethoxyl silane, right-the styryl Trimethoxy silane, 3-methacryloxypropyl methyl dimethoxysilane, 3-methacryloxypropyl trimethoxy silane, 3-methacryloxypropyl methyldiethoxysilane, 3-methacryloxypropyl triethoxyl silane, 3-acryloxy propyl-triethoxysilicane and 3-urea propyl-triethoxysilicane and their mixture.
On the other hand, the invention provides the semiconducter device of resin package, it is produced by the following method: a surface that the compositions of thermosetting resin of above-mentioned definition is coated on silicon wafer, this silicon wafer has at least one and be formed on wherein semiconductor element fully under pressure or under the decompression of vacuum environment, the thermofixation said composition encapsulates this wafer to use curing resin layer, polish curing resin layer and this wafer slice is become single device.
In a word, the compositions of thermosetting resin that should be used for semiconductor packages is characterised in that: as the formula (1) at the molecular chain two ends with the allyl group isocyanurate ring, typically with monomethyl allyl group isocyanurate ring be the organopolysiloxane polymkeric substance of terminal as single base polymer, be used as single curing agent or linking agent with as the formula (2) the isocyanurate ring organic radical hydrogen polysiloxanes polymkeric substance that contains that has at least two hydrogen atoms that are connected with silicon (Si-H group) at its siloxane chain two ends.
Beneficial effect of the present invention
The large size silicon wafer of the semiconductor element of formation will pass through with thermosetting resin of the present invention can obtain having the semiconducter device that does not have warpage substantially that has improved thermotolerance and wet fastness after the thin compound encapsulation by being installed in the semiconductor device array of the semiconductor element on inorganic substrate, metal base or the organic substrate with binding agent or chip join agent or will having therein having at least one.This compositions of thermosetting resin can be on this wafer-level once the property encapsulation and can make polishing and section easily.
Embodiment
In simple terms, the present invention relates to the compositions of thermosetting resin for semiconductor packages, comprising:
(A) the organopolysiloxane polymkeric substance conduct that has allyl group isocyanuric acid ester ring structure at its molecular chain two ends as the formula (1) contains the thiazolinyl organopolysiloxane,
(B) the organic radical hydrogen polysiloxanes polymkeric substance that contains isocyanurate ring as the formula (2) is as the organic radical hydrogen polysiloxanes of no epoxy group(ing), and its terminal at siloxane chain has at least two hydrogen atoms (Si-H group) that are connected on the silicon,
(C) curing catalyst and
(D) inorganic spherical filler, the part that is had 10.0 to 50.0 μ m average particle size particle size by (D-1), (D-2) has the part of 1.1-5.0 μ m average particle size particle size, (D-3) part of 0.1-1.0 μ m average particle size particle size is formed, (D-1): (D-2): weight ratio (D-3) is 95-70: 3-20: the 2-10 condition is (D-1), and total amount (D-2) and (D-3) is 100.Said composition does not comprise component (A) any organic radical hydrogen polysiloxanes that contains the thiazolinyl organopolysiloxane and do not contain component (B) any no epoxy group(ing) in addition in addition.
The compositions of thermosetting resin that should be used for semiconductor packages is characterised in that: have only as the formula (1) at molecular chain two ends allyl group isocyanurate ring, typically use the organopolysiloxane polymkeric substance of monomethyl allyl group isocyanurate ring end-blocking, the organopolysiloxane polymkeric substance that namely has thiazolinyl (typically allyl group) at its molecular chain two ends, contain the thiazolinyl organopolysiloxane as base polymer, with the organic radical hydrogen polysiloxanes polymkeric substance that contains isocyanurate ring that has at least two hydrogen atoms that are connected with silicon (Si-H group) at its siloxane chain two ends that has only as the formula (2), as the no epoxy group(ing) organic radical hydrogen polysiloxanes of solidifying agent or linking agent.Being used in combination of these polymkeric substance can guarantee to obtain to utilize the solidifying product of the advantage of hydrogenation silylation or addition reaction.
Component (A) is having the allyl group isocyanurate ring at its molecular chain two ends, typically have an organopolysiloxane polymkeric substance of monomethyl allyl group isocyanurate ring shown in general formula (1).In composition of the present invention, this thiazolinyl organopolysiloxane that contains as base polymer is made up of this organopolysiloxane polymkeric substance that has allyl group isocyanuric acid ester ring structure at its molecular chain two ends as the formula (1) and is not had other the thiazolinyl organopolysiloxane that contains to exist.
Herein, for not containing the monovalent hydrocarbon group of aliphatic unsaturated link(age), R is alkyl or phenyl to X independently of one another independently of one another, and n is that integer and the P of 1-50 is the integer of 1-30.
In formula (1), R is selected from the alkyl of 1-10 carbon atom independently of one another, as methyl, and ethyl and propyl group etc., and phenyl.Especially, for curing performance, flexible and easily synthetic preferable methyl.More preferably, methyl account for whole R group 50mol% at least (that is, 50-100mol%).
X is selected from the monovalent hydrocarbon group that does not contain aliphatic unsaturated link(age) independently of one another, for example, the alkyl of 1-10 carbon atom, as methyl, the aromatic yl group of ethyl and propyl group etc. and 6-10 carbon atom is as phenyl.Most preferably X is methyl.
Subscript n is that the integer of 1-50, preferred 1-30 and P are 1-30, preferred 1-10 and the more preferably integer of 1-8.
This organopolysiloxane polymkeric substance has the weight-average molecular weight (Mw) of 500-10000, preferred 600-5000 usually.It has usually at 25 ℃ of following 0.5-1000Pas, preferably in the viscosity of 25 ℃ of following 1-100Pas.It should be noted that Mw passes through gel permeation chromatography (GPC) use toluene or tetrahydrofuran (THF) is measured as developping agent and viscosity can typically have BL, BH, BS or awl/board-like measurement by rotational viscosimeter.
This contains isocyanurate ring organopolysiloxane polymkeric substance and can pass through as component (A), for example, the diallyl isocyanuric acid ester with general formula (3) is that hydrogenation silylation or the addition reaction of the organopolysiloxane of terminal prepares with what have general formula (4) with the hydrogen siloxy-.This addition reaction can be implemented by the technique known of prior art, typically carries out in room temperature (25 ℃) to 250 ℃, preferred 50-180 ℃ temperature, and 0.1-120 hour, preferred 1-10 hour.
Herein, X, R and n are as defined above.
This have the diallyl isocyanuric acid ester of formula (3) and have formula (4) in the hydrogen siloxy-be the organopolysiloxane of terminal with following quantitative response: make with the allyl group in every mole of formula (3), the amount of the Si-H group in the formula (4) is the 0.1-0.9 equivalent, more preferably 0.4-0.7 equivalent, that is, in the excessive system of allyl group.Obtain to have at two ends the organopolysiloxane polymkeric substance of diallyl isocyanurate ring then.
In reaction, can use catalyzer, for example comprise the compound of platinum metals such as platinum, rhodium and palladium.Especially, preferably contain platinic compound, for example, chloroplatinic acid salt (IV) hexahydrate, platinum-carbonyl ethylene ylmethyl complex compound, platinum-divinyl tetramethyl disiloxane complex compound, platinum-cyclic vinyl methylsiloxane complex compound, the platinum on platinum-octanal/octanol complex and the gac.The preferred consumption of this catalyzer is, based on the compound with formula (3), makes the metal that obtains 0.01-10000ppm, the more preferably amount of 0.1-100ppm metal.
When preparation contains the organopolysiloxane polymkeric substance of isocyanurate ring, can use solvent if desired.Suitable solvent comprises toluene, dimethylbenzene, sym-trimethylbenzene, diethylbenzene, tetrahydrofuran (THF), Anaesthetie Ether, 1,4-dioxane, and diphenyl ether.
Component (B) is the organic radical hydrogen polysiloxanes polymkeric substance that contains isocyanurate ring shown in general formula (2), has at least two hydrogen atoms that are connected with silicon (Si-H group) in the terminal of its siloxane chain.In composition of the present invention, this that is used as solidifying agent or linking agent do not have for the organic radical hydrogen polysiloxanes of the epoxide group of cohesiveness functional group to be made up of the organic radical hydrogen polysiloxanes polymkeric substance that contains the isocyanic ester ring with formula (2), and does not have other organic radical hydrogen polysiloxanes to exist.
Component (B) is characteristically by at its siloxane chain (namely, in the simple function siloxy units) terminal have at least two organic radical hydrogen polysiloxanes that are connected the hydrogen atom (Si-H group) on the Siliciumatom and form, refer to contain the polysiloxane polymer that isocyanurate ring hydrogen is terminal sometimes.Terminal at siloxane chain comprises at least two, preferred 2-50 hyperergy hydrogen atom (that is, (H) (R) that is connected with Siliciumatom
2Si0
1/2Si-H group in the unit), can guarantee with component (A) in quick hydrosilation or the addition reaction of thiazolinyl group (allyl group) at molecular chain two ends.
For not containing the monovalent hydrocarbon group of aliphatic unsaturated link(age), R is alkyl or phenyl to X independently of one another independently of one another herein, and n is the integer of 1-50, and m is that integer and the P of 0-5 is the integer of 1-30.This repetition siloxane unit can randomly connect.
In formula (2), R is selected from the alkyl of 1-10 carbon atom independently of one another, as methyl, and ethyl, and propyl group, and phenyl.Especially, for curing performance, flexible and synthetic and preferable methyl easily.More preferably, methyl account for whole R groups 50mol% at least (that is, 50-100mol%).
X is selected from the monovalent hydrocarbon group that does not contain aliphatic unsaturated link(age) independently of one another, for example, the alkyl of 1-10 carbon atom, as methyl, the aromatic yl group of an ethyl and propyl group and 6-10 carbon atom is as phenyl.Most preferably X is methyl.
Subscript n is the integer of 1-50, preferred 1-30, and m is that the integer of 0-5, preferred 0-2 and P are the integer of 1-30, preferred 1-10 and the more preferably integer of 1-8.
This organic radical hydrogen polysiloxanes polymkeric substance as component (B) has the weight-average molecular weight (Mw) of 500-10000, preferred 600-5000 usually.It has the viscosity of the 0.1-100Pas under 25 ℃ usually, preferably at 25 ℃ of following 0.5-10Pas.
This isocyanurate ring hydrogen that contains as component (B) is that the polysiloxane polymer of terminal can pass through, for example, the diallyl isocyanuric acid ester with general formula (5) is that hydrogenation silylation or the addition reaction of the organopolysiloxane of terminal prepares with what have general formula (6) with the hydrogen siloxy-.This addition reaction can be implemented by the known technology of prior art, typically the temperature of room temperature (25 ℃) to 250 ℃, preferred 50-180 ℃, 0.1-120 hour, preferred 1-10 hour.
Herein, X, R, n and m are as mentioned above.This repetition siloxane unit can randomly connect.
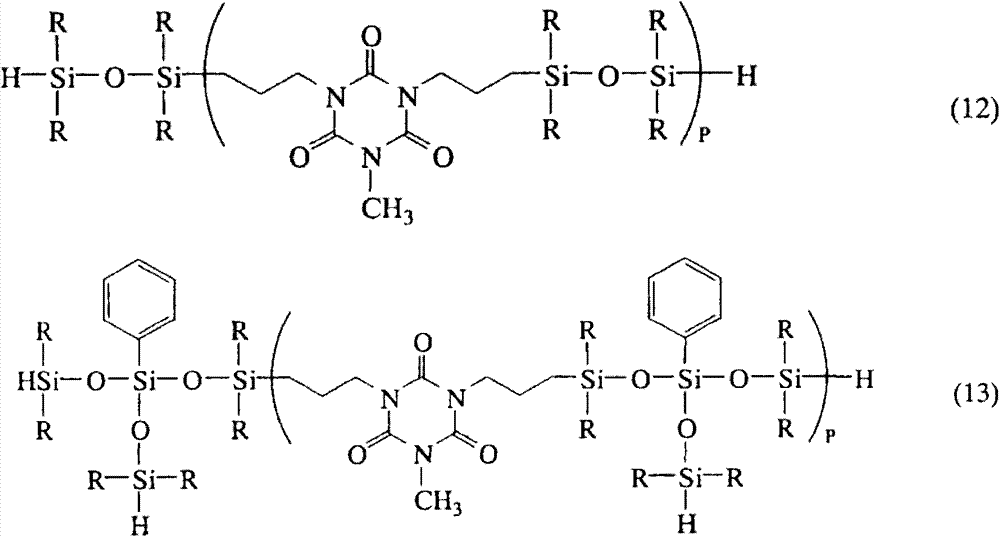
This have the diallyl isocyanuric acid ester of formula (5) and have formula (6) though the hydrogen siloxy-be that the organopolysiloxane of terminal is able to allyl group in every mole of formula (5) in such quantitative response, the amount of the Si-H group in the formula (6) is the 1.1-5.0 equivalent, more preferably 1.1-3.5 equivalent, that is, in the excessive system of Si-H.Obtain then to contain isocyanurate ring organic radical hydrogen polysiloxanes polymkeric substance what the siloxane chain terminal had at least two hydrogen siloxyies.
The example that this hydrogen siloxy-with formula (6) is the organopolysiloxane of terminal comprises following formula
(7)-(11) those in:
In reaction, can use catalyzer, for example, comprise the compound of platinum, rhodium and palladium.Especially, preferably contain platinic compound, for example, chloroplatinic acid salt (IV) hexahydrate, platinum-carbonyl ethylene ylmethyl complex compound, platinum-divinyl tetramethyl disiloxane complex compound, platinum-cyclic vinyl methylsiloxane complex compound, the platinum on platinum-octanal/octanol complex and the gac.The preferred consumption of this catalyzer is for making based on the compound with formula (5) to have this metal of 0.01-10000ppm, more preferably 0.1-100ppm.
When preparation organic radical hydrogen polysiloxanes polymkeric substance, more can add solvent if need.Suitable solvent comprises toluene, dimethylbenzene, sym-trimethylbenzene, diethylbenzene, tetrahydrofuran (THF), Anaesthetie Ether, 1,4-dioxane, and diphenyl ether.
The example of the organic radical hydrogen polysiloxanes polymkeric substance by method for preparing comprises those in general formula (12) and (13):
R and P such as above-mentioned definition herein defines.
Be that the polysiloxane polymer of terminal uses in such amount and makes that with the allyl group in every molar constituent (A) amount of the Si-H group in the component (B) is the 0.8-4.0 mole as the organopolysiloxane polymkeric substance of the two ends allyl group isocyanurate ring end-blocking of component (A) with as the isocyanurate ring hydrogen that contains of component (B).The molar ratio of preferred Si-H group and allyl group is 1.0-3.0.If the allylic ratio of Si-H/ is less than 0.8 or more than 4.0, said composition may be solidified deficiency so, or resin surface may spottiness behind the composition molding.
Have low modulus and the infiltrative solidifying product of favorable mechanical performance, thermotolerance, electrical insulating property, chemical resistant properties, water tolerance and gas as crosslinked the providing between component (A) and the organopolysiloxane polymkeric substance that contains isocyanurate ring (B).
Purpose for the semiconductor element encapsulation, as the organopolysiloxane polymkeric substance of the two ends allyl group isocyanurate ring end-blocking of base polymer and as solidifying agent or linking agent contain isocyanurate ring hydrogen be the polysiloxane polymer of terminal preferably should have minimum content such as the halide-ions of chlorine etc. with such as the basic ion of sodium etc., the extraction quantity of typical every kind of ion under 120 ℃ is at most 10ppm.
Component (C) is curing catalyst or curing catalysts, and it can be for being used for the catalyzer of hydrogenation silylation or addition reaction.Appropriate catalyst comprises platinum metal catalysts, as platinum and platinum based catalyst and ferric oxide etc.Especially, platinum metal catalysts is preferred, and it comprises platinum, palladium and rhodium-series catalysts.From viewpoints such as costs, preferred platinum group catalyst, as platinum, platinum black, and Platinic chloride.Typical example comprises H
2PtCl
6-xH
2O, K
2PtCl
6, KHPtCl
6-xH
2O, K
2PtCl
4, K
2PtCl
4-xH
2O, and PtO
2-xH
2O wherein x is positive integer and they and hydrocarbon such as alkene, alcohol or the complex compound that contains vinyl organopolysiloxane etc.These catalyzer can use separately or two or more mix use.
This curing catalyst uses with catalytic amount,, enough promotes the amount of solidifying that is.This platinum metal catalysts uses with such amount and makes that based on component (A) and weight sum (B) amount of platinum metals is 0.1-500ppm.Consumption is outside this numerical range the time, and said composition may be solidified deficiency, or because the accumulation of viscosity rapidly that short shelf lives and fast setting cause may cause the said composition processing difficulties.
Segmentation (D) is the inorganic spherical filler.This filler is not particularly limited, as long as it is spherical.Preparing spherical SiO 2 is preferred.This inorganic spherical filler is had the large size part of 10.0 to 50.0 μ m average particle size particle size by (D-1), (D-2) has the middle size part of 1.1-5.0 μ m average particle size particle size, (D-3) small size of 0.1-1.0 μ m average particle size particle size is partly formed, it is with combinations of states and the use of closestpacking, especially at (D-1), (D-2) and total amount (D-3) be under 100 the condition, (D-1): (D-2): weight ratio (D-3) is 95-70: 3-20: 2-10, preferred 92-78: 5-15: 3-7.Closestpacking mixture ratio (weight ratio) for example is large size part (D-1): middle size part (D-2): small size part (D-3)=85: 10: 5, but be not limited to this.
Before adding and being mixed into composition, as the inorganic spherical filler of component (D), preparing spherical SiO 2 can carry out surface treatment with silane coupling agent typically.Suitable silane coupling agent comprises vinyltrimethoxy silane, vinyltriethoxysilane, 2-(3,4-epoxy group(ing) cyclohexyl) ethyl trimethoxy silane, 3-glycidoxypropyl methyl dimethoxysilane, the 3-glycidoxypropyltrimewasxysilane, 3-glycidoxypropyl methyldiethoxysilane, 3-glycidoxypropyl triethoxyl silane, right-the styryl Trimethoxy silane, 3-methacryloxypropyl methyl dimethoxysilane, 3-methacryloxypropyl trimethoxy silane, 3-methacryloxypropyl methyldiethoxysilane, 3-methacryloxypropyl triethoxyl silane, 3-acryloxy propyl-triethoxysilicane and 3-urea propyl-triethoxysilicane, it can use or mix use separately.Especially, preferred 3-methacryloxypropyl trimethoxy silane.
Amount and surface treatment method to the coupling agent that uses are not particularly limited.The preferred 0.5-2.0wt% of amount of the coupling agent that uses, more preferably 0.5-1.0wt% is based on the weight as the inorganic spherical filler of component (D).
When will be as the inorganic spherical filler of component (D), when preparing spherical SiO 2 adds into compositions of thermosetting resin typically, the amount of component (D), namely, (D-1) total amount to (D-3) part is the 30-900 weight part, preferred 40-600 weight part is the organopolysiloxane polymkeric substance of terminal and is that the polysiloxane polymer of terminal adds up to as the isocyanurate ring hydrogen that contains of solidifying agent (B) with the two ends allyl group isocyanurate ring as base polymer (A) of per 100 weight parts.With respect to per 100 parts by weight of component (A) and (B) total amount meter, if the consumption of filler is less than 30 weight parts, just can not obtain enough intensity, if consumption is greater than 900 weight parts, the viscosity accumulation, cause mobile lacking, lack of fill is incomplete with the encapsulation that is arranged in the semiconductor element on the packing base.
If desired, can add any additives to this compositions of thermosetting resin.Suitable additive comprises the bonding improving agent, for example, has the silicoorganic compound of epoxide group, as the 3-glycidoxypropyltrimewasxysilane or as follows have a structural formula (14) contain epoxy group(ing) organic radical hydrogen siloxane; Solidify slow dose, for example, ethynyl methyl decyl methyl alcohol, organo phosphorous compounds, triphenylphosphine for example, nitrogen-containing organic compound, tributylamine for example, Tetramethyl Ethylene Diamine and benzotriazole; And tinting material, for example, carbon black is as acetylene black and furnace black.Can add these additives, only otherwise harm the object of the invention.
This compositions of thermosetting resin can prepare by using the even mixing said ingredients of ordinary method.This compositions of thermosetting resin can solidify by heating.Suitable condition of cure comprises 110-200 ℃, 120-180 ℃ temperature and 1-6 hour, time of 2-3 hour especially especially.
Because this compositions of thermosetting resin is solidified into the infiltrative product of mechanical property, thermotolerance, electrical insulating property, chemical resistant properties, water tolerance and gas that has low modulus, is satisfied with, so it is well suited for the encapsulants as semiconductor element, typically be used for total encapsulation of sealing large-sized wafer and base material.
Use binding agent or chip join agent to be installed in the semiconductor device array of the semiconductor element on inorganic substrate, metal base or the organic substrate or large size silicon wafer with the semiconductor element that is formed on wherein during by this compositions of thermosetting resin encapsulation when having at least one, can obtain to have the semiconducter device of the essentially no warpage of improved thermotolerance and wet fastness.This resin combination can be on wafer-level once property encapsulation and the resin that is in this encapsulation of solid state can polish and cut into slices easily.
Another embodiment provides the semiconducter device of resin package, and it is in following state: have the silicon wafer of at least one semiconductor element that forms therein or disposable whole encapsulation of compositions of thermosetting resin that base material is in solid state.The semiconducter device of this resin package obtains by the following method: under pressure or at the compositions of thermosetting resin that applies above-mentioned definition under the decompression of vacuum environment to a surface of this silicon wafer, the thermofixation said composition encapsulates this wafer to use curing resin layer, polish curing resin layer and this wafer slice is become single device.
Embodiment
Provide embodiments of the invention and be not the method for it being made restriction by the method that describes below.In an embodiment, room temperature is 25 ℃, and all umbers all are that weight part and Vi represent vinyl.Viscosity is by the observed value of rotational viscosimeter under 25 ℃ as defining in the testing method.
Synthesis example 1
In the separable flask of 2L, pack into 400g (1.79 moles) monomethyl diallyl isocyanuric acid ester, 400g toluene and 0.32g Platinic chloride toluene solution (Pt that comprises 0.5wt%).This solution is heated to 100 ℃, and 120g's (0.89 mole) has 1,1,3 of a following formula (15) then, and the 3-tetramethyl disiloxane dropwise adds this solution, stirs 8 hours down at 100 ℃ then.Distill out toluene in a vacuum, obtain achromaticity and clarification liquid.
By
1H-NMR spectrum analysis, near the peak (4.6ppm) that represents the Si-H proton disappear and near the peak (5.0-5.4 and the 5.7-6.0ppm) that represents the vinyl proton is held.Can confirm that reaction has taken place for allyl group and 1,1,3,3-tetramethyl disiloxane in some monomethyl diallyl isocyanuric acid esters.Product has 1200 weight-average molecular weight by gpc analysis.Mean polymerisation degree 2.6, ethene base value 2.48mmol/g, 25 ℃ of following viscosity 3.0Pas.
Product is unreacted reactant and the mixture with reactor product of different polymerization degree.GPC and NMR analyze this product of explanation, are appointed as mixing material A, mainly are made up of unreacted reactant (monomethyl diallyl isocyanuric acid ester) and compound with following formula (16).
Pass through gpc analysis, mixing material A is by the unreacted reactant (monomethyl diallyl isocyanuric acid ester) of 18.5wt%, the compound of the P=1 of 27.3wt% (16), the compound of the P=2 of 22.3wt% (16), the compound of the P=3 of 14.8wt% (16), the compound (16) of P 〉=5 of the compound of the P=4 of 8.1wt% (16) and 9.0wt% is formed.
The weight-average molecular weight of the compound by removing the formula (16) that unreacted reactant (that is the formula of P=0 (16)) separates from mixing material A is 1468 (mean P=3.3).
(mixing material A)
Synthesis example 2
The hydrogen that the 900g (2.73 moles) that packs in the separable flask of 3L has formula (17) is the siloxanes of terminal, that is, and and three (dimethyl hydrogen siloxy-) phenyl silane and 900g toluene.This solution is heated to 100 ℃, thus 0.71g Platinic chloride toluene solution (Pt that comprises 0.5wt%) is dropwise added.Drip monomethyl diallyl isocyanuric acid ester and the 300g toluene of 300g (1.34mol) then in this solution, stirred 8 hours down at 100 ℃ thereafter.Distill out toluene in a vacuum, obtain achromaticity and clarification liquid.
By
1The H-NMR spectrum analysis, near the peak (5.0-5.4 and the 5.7-6.0ppm) that represents the vinyl proton disappears, and shows that the completely consumed of reactant monomethyl diallyl isocyanuric acid ester falls.Near the peak (4.6ppm) that represents the Si-H proton keeps, and the hydrogen that shows allyl group on monomethyl diallyl isocyanuric acid ester and formula (17) is that reaction has taken place the Si-H group of the siloxanes end of terminal.Product has 2470 weight-average molecular weight by gpc analysis.Si-H value 4.54mmol/g, 25 ℃ of following viscosity 0.4Pas.
Product is the mixture with reactor product of different polymerization degree.GPC and NMR analyze this product of explanation, are appointed as mixing material B, mainly are made up of the compound with following formula (18).Especially, mixing material B is by the compound (18) of the P=1 of 12.4wt%, the compound of the P=2 of 23.3wt% (18), the compound of the P=3 of 20.0wt% (18), the compound of the P=4 of 14.6wt% (18), the compound (18) of P 〉=6 of the compound of the P=5 of 11.8wt% (18) and 17.9wt% is formed, mean P=3.8.
(mixing material B)
Example 1
Comprise mixing material A as single base polymer and mixing material B as the Si-H/ allyl group ratio of single curing agent be 1.0 resin combination by following formulation, this resin combination has the silica filler loadings of 60wt%.
* preparing spherical SiO 2 is by large size silicon-dioxide (average particle size particle size d=10 μ m), middle size silicon-dioxide (d=2 μ m) and small size silicon-dioxide (d=0.8 μ m) are formed, weight ratio is 85: 10: 5, and its 3-methacryloxypropyl trimethoxy silane with 1wt% is at room temperature carried out surface treatment 240 seconds in the Henschel mixing machine.
Liquid resin composition prepares by the following method: stir and blending ingredients #1 to #7 in planetary-type mixer in the three-roll mill of 80 μ m roll spacings mixing for three times and mix in planetary-type mixer under vacuum.
Embodiment 2
Comprise mixing material A as single base polymer and mixing material B as the Si-H/ allyl group ratio of single curing agent be 1.8 resin combination by following formulation, this resin combination has the silica filler loadings of 60wt%.
* with embodiment 1 in identical
Liquid resin composition prepares by the following method: stir and blending ingredients #1 to #7 in planetary-type mixer in the three-roll mill of 80 μ m roll spacings mixing for three times and mix in planetary-type mixer under vacuum.
Embodiment 3
Comprise mixing material A as single base polymer and mixing material B as the Si-H/ allyl group ratio of single curing agent be 2.2 resin combination by following formulation, this resin combination has the silica filler loadings of 60wt%.
* with embodiment 1 in identical
Liquid resin composition prepares by the following method: stir and blending ingredients #1 to #7 in planetary-type mixer in the three-roll mill of 80 μ m roll spacings mixing for three times and mix in planetary-type mixer under vacuum.
Embodiment 4
Comprise mixing material A as single base polymer and mixing material B as the Si-H/ allyl group ratio of single curing agent be 2.2 resin combination by following formulation, this resin combination has the silica filler loadings of 65wt%.
* with embodiment 1 in identical
Liquid resin composition prepares by the following method: stir and blending ingredients #1 to #7 in planetary-type mixer in the three-roll mill of 80 μ m roll spacings mixing for three times and mix in planetary-type mixer under vacuum.
Comparative example 1
Comprise that vinyl polysiloxane and mixing material A are as base polymer, mixing material B and branching organic radical hydrogen polysiloxanes as the Si-H/Vi ratio of solidifying agent be 2.0 resin combination by following formulation, this resin combination has the silica filler loadings of 64wt%.
The * preparing spherical SiO 2 is by large size silicon-dioxide (average particle size particle size d=10 μ m), middle size silicon-dioxide (d=2 μ m) and small size silicon-dioxide (d=0.8 μ m) are formed, weight ratio is 85: 10: 5, and its 3-glycidoxypropyltrimewasxysilane with 1wt% is at room temperature carried out surface treatment 240 seconds in the Henschel mixing machine.
Liquid resin composition prepares by the following method: stir and blending ingredients #1 to #10 in planetary-type mixer in the three-roll mill of 80 μ m roll spacings mixing for three times and mix in planetary-type mixer under vacuum.
Comparative example 2
Comprise the vinyl polysiloxane as base polymer and branching organic radical hydrogen polysiloxanes as the Si-H/Si-Vi ratio of solidifying agent be 2.0 resin combination by following formulation, this resin combination has the silica filler loadings of 82wt% (high loadings).
Identical in * and the comparing embodiment 1
Liquid resin composition prepares by the following method: stir and blending ingredients #1 to #7 in planetary-type mixer in the three-roll mill of 80 μ m roll spacings mixing for three times and mix in planetary-type mixer under vacuum.
Testing method
The liquid resin composition of embodiment and comparing embodiment by method tested viscosity as described below, thixotropy index, DSC, cohesive strength, tensile strength, elongation, tensile modulus, curing after warpage, current mark, do not fill the space, peel off, polishing and reliability.The result is as shown in table 1.
I) viscosity and thixotropy index
Viscosity at room temperature uses Brookfield rheology instrument able to programme DV-IIIUltra type viscometer (bell-shaped rotor CP-51) to measure under 1.0rpm.The thixotropy index calculates except the viscosity under the 0.1rpm by the viscosity under the 1.0rpm that measures with viscometer.
Ii) dsc measurement
Carrying out the differential scanning colorimetry by DSC821e type (METTLER-TOLEDO International Inc.) measures.
Iii) cohesive strength
The cohesive strength of the resin combination of chip rear surface is determined by following method: the using liquid resin combination is cut to the minute surface of the imitative wafer of 10 millimeters square silicon to 725 μ m with minute surface are thick, make thick the cutting to the minute surface of the imitative wafer of 2 millimeters square silicon and 10 millimeters square silicon of 725 μ m with minute surface imitate the combination that matches of resin coating on the wafer, with this resin coating of interlayer between the imitative wafer of silicon, heated this assembly 2 hours down at 150 ℃, to solidify this resin combination, obtain specimen.This cohesive strength is by use test device (Dage Series4000 bond tstr), keeps these samples in hot plate last 40 second down at 260 ℃, and application level power to chip side is brought in measurement then.
Iv) tensile strength
Liquid resin composition was shaped to the thick plate of 1.0mm 150 ℃ of lower mould in 2 hours with being heating and curing, and struck out #2 dumbbell shape sample.Record (autograph) Loadcell type SBL-5KN (Shimadzu Corp.) measures under two ends clamp distance 100.0mm and rate of extension 2.0mm/min the tensile strength of this sample by using automatically.
V) elongation
Liquid resin composition was shaped to the thick plate of 1.0mm 150 ℃ of lower mould in 2 hours with being heating and curing, and struck out #2 dumbbell shape sample.Record (autograph) Loadcell type SBL-5KN (Shimadzu Corp.) measures under two ends clamp distance 100.0mm and rate of extension 2.0mm/min the percentage ratio elongation of this sample by using automatically.
Vi) tensile modulus
Liquid resin composition was shaped to the thick plate of 1.0mm 150 ℃ of lower mould in 2 hours with being heating and curing, and struck out #2 dumbbell shape sample.Record (autograph) Loadcell type SBL-5KN (Shimadzu Corp.) measures under two ends clamp distance 100.0mm and rate of extension 2.0mm/min the tensile modulus of this sample by using automatically.
Vii) warpage
Liquid resin composition to moulded section is measured warpage.Use 8 inches thick wafers of 200 μ m.Wafer die MZ407-1 (Apic Yamada Corp.) is arranged to the resin thickness of 400 μ m.This liquid resin composition is 110 ℃ of dip mold moulding 600 seconds with 150 ℃ of following after fixing (or fully solidify) 2 hours.This sample is used for the warpage test.
Viii) current mark and do not fill the space
Use 8 inches thick wafers of 200 μ m.Wafer die MZ407-1 (Apic Yamada Corp.) is arranged to the resin thickness of 400 μ m.This liquid resin composition is 110 ℃ of dip mold moulding 600 seconds with 150 ℃ of following after fixing (or fully solidify) 2 hours, to form the thick resin sample of 400 μ m.This sample visual inspection current mark and whether exist and do not fill the space.To be white waviness vestige extend radially outwardly from the center of resin-formed body current mark.The appearance of current mark shows defective outward appearance, because non-homogeneous dispersion, the variation of the physicals of curing and the reliability variation of following of silicon-dioxide.Do not fill the space and refer to lacking along the external margin resin of wafer.Under unfilled situation, indentation may be regarded mistakenly by sensor in any space when wafer is sent to next position, and causes misalignment.
Ix) polishing
Use 8 inches thick wafers of 200 μ m.Wafer die MZ407-1 (Apic Yamada Corp.) is arranged to the resin thickness of 400 μ m.This liquid resin composition is 110 ℃ of dip mold moulding 600 seconds with 150 ℃ of following after fixing (or fully solidify) 2 hours, to form the thick resin sample of 400 μ m.This sample is by the grinding rate of operation automatic surface mill DAG810 (DISCO Corp.) at 1.0 μ m/s, and test is polished under the branch stage speed of the rotor speed of 4800rpm and 300rpm.The sample polishing of possible 600 meshes and stabilized power supply during polishing polish and are rated as poor (*) being rated as (zero) and 600 inhomogeneous meshes below 8.0 amperes.
X) reliability
Use thick film scanner/printer (thick film printer model MC212), (Shin-Etsu Chemical Co. Ltd) is printed to 20 μ m thickness at 8 inches thick wafers of 200 μ m to chip join material SFX-513M1.B-rank wafer by slice tool section be 7 millimeters square, obtain semi-conductor chip.
Under the following conditions: 10N, 150 ℃ and 1.0 seconds, use flip-chip bond machine NM-SB50A (Panasonic), the semi-conductor chip of and chip join coated materials that 220 μ ms thick square with 7 millimeters is with on the thick 8 inches wafers of chip join to 200 μ m.Acquisition has the thick wafer of 200 μ m of semi-conductor chip mounted thereto.
The filling of moulded section machine has the thick wafer of 200 μ m of semi-conductor chip mounted thereto, distributes the liquid resin composition of appropriate amount on it, at the peak pressure compacted under of 30MPa-15MPa, solidifies 10 minutes down at 110 ℃ then.The amount of liquid resin composition is adjusted into the resin that makes after the moulding and has the amount of 400 ± 10 μ m thickness.Wafer after this moulding under 150 ℃ in baking oven thermal treatment 2 hours with after fixing.Re-use slice tool, with wafer slice become 7.1 millimeters square, obtain to have the single resin encapsulated semiconductor chip of 400 μ m resin thicknesses.
Under the following conditions: 10N, 150 ℃ and 1.5 seconds, use flip-over type jointing machine NM-SB50A (Panasonic) and chip join material SFX-513S (Shin-Etsu Chemical Co., Ltd), with single resin encapsulated semiconductor chip join to the BT base material.Then under 150 ℃ in baking oven 4 hours after fixing of thermal treatment, obtain having the BT base material of resin encapsulated semiconductor chip mounted thereto.
Under the following conditions: 175 ℃, 90 seconds and 90MPa, to the thickness of 1.5mm, use transfer moIding machine G-Line Press (Apic Yamada Corp.), with moulding mixing material transfer mould on the BT base material with resin encapsulated semiconductor chip mounted thereto.Re-use slice tool, with base material be sliced into 10.0 millimeters square, obtain the bearing semiconductor chip BT base material (that is semiconducter device) of single moulding mixing material resin package.
This single semiconducter device stands moist test 168 hours that absorb under 85 ℃ and 85% relative humidity.Make them pass through 260 ℃ of default top temperatures and in 30 ± 3 seconds 255-260 ℃ following hold-times reflow stove three times, as the test of welding thermotolerance power, check any peeling off by visual inspection thus.
Xi) thermal cycling test (TCT)
Use low profile thermal loop test instrument TSE-11 (ESPEC Corp.), this single moulding mixing material resin encapsulated semiconductor chip bearing BT base material-55 ℃/15 minutes and+carry out automatic thermal cycling between 125 ℃/15 minutes.In initial (0 circulation), peeling off by loseless method of any semi-conductor chip uses ultrasonic Flaw Detection device QUANTUM350 (Sonix Co.) to detect under the probe of 75MHz.Similar detection is in 250 circulations, and implement 500 circulations and 750 circulation backs.The result is presented in the table 1.
The 5% up-to-date style product that are less than the semi-conductor chip zone when whole stripping areas are assessed as " nothing is peeled off " (OK), and the 5% up-to-date style product that are equal to or greater than the semi-conductor chip zone when whole stripping areas are assessed as " peeling off " (NG).
Table 1
Embodiment 1-4 for the resin that only uses those organopolysiloxane polymkeric substance derived from two ends allyl group isocyanurate ring end-blocking (mixing material A) and contain isocyanurate ring hydrogen terminated polysiloxane polymkeric substance (mixing material B) respectively as the liquid resin composition of base polymer and solidifying agent (or linking agent).Though Si-H/ allyl group ratio is changed to 1.8 or 2.2 from 1.0, the semiconducter device of making by the pressing mold liquid resin composition had not both demonstrated small peeling off yet and had not peeled off in peeling off test.Anti-stripping semiconductor device is provided.
Be compression molded into type and after 150 ℃ of following after fixing 2 hours at the liquid resin composition of embodiment 1-4, both do not found after the visual inspection outward appearance that current mark do not fill the space yet.Polishing does not produce any problem.Do not peel off because after thermal cycling test 750 circulations, both detected small peeling off yet, so reliability is acceptable.
On the contrary, the liquid resin composition when comparing embodiment 1 and 2 is compression molded into type and in the time of 2 hours, detects current mark at 150 ℃ of following after fixing.Especially, the position of resin before the moulded section and near have white current mark to take place.Do not detect and do not fill the space.Polishing has problem.Reliability testing becomes improper after 750 circulations.
Claims (5)
1. compositions of thermosetting resin that is used for semiconductor packages, it comprises
(A) the organopolysiloxane polymkeric substance conduct that has allyl group isocyanuric acid ester ring structure at the molecular chain two ends shown in general formula (1) contains the thiazolinyl organopolysiloxane,
Wherein for not containing the monovalent hydrocarbon group of aliphatic unsaturated link(age), R is alkyl or phenyl to X independently of one another independently of one another, and n is that integer and the P of 1-50 is the integer of 1-30,
(B) the organic radical hydrogen polysiloxanes polymkeric substance that contains isocyanurate ring shown in general formula (2) is as the organic radical hydrogen polysiloxanes of no epoxy group(ing), has at least two hydrogen atoms (Si-H group) that are connected on the silicon in the terminal of its siloxane chain,
Wherein X is not independently of one another for containing the monovalent hydrocarbon group of aliphatic unsaturated link(age), R is alkyl or phenyl independently of one another, n is the integer of 1-50, m is the integer of 0-5, with P be the integer of 1-30, wherein this repetition siloxane unit can randomly connect, its amount makes based on every mole of allyl group in the component (A), the Si-H group that has the 0.8-4.0 mole in the component (B)
(C) curing catalyst of catalytic amount and
(D) inorganic spherical filler, the part that is had 10.0 to 50.0 μ m average particle size particle size by (D-1), (D-2) has the part of 1.1-5.0 μ m average particle size particle size, (D-3) part of 0.1-1.0 μ m average particle size particle size is formed, and (D-1): (D-2): weight ratio (D-3) is 95-70: 3-20: 2-10, and condition is (D-1), (D-2) and total amount (D-3) be 100, in the component (A) of per 100 weight parts and (B) total amount, described amount of filler is the 30-900 weight part
Said composition does not contain in addition any of component (A) and contains the thiazolinyl organopolysiloxane and do not contain component (B) any no epoxy group(ing) organic radical hydrogen polysiloxanes in addition.
2. the composition of claim 1, wherein inorganic spherical filler (D) is preparing spherical SiO 2.
3. the composition of claim 1, wherein inorganic spherical filler (D) carries out surface treatment with silane coupling agent.
4. the composition of claim 3, wherein silane coupling agent is for being selected from by vinyltrimethoxy silane, vinyltriethoxysilane, 2-(3,4-epoxy group(ing) cyclohexyl) ethyl trimethoxy silane, 3-glycidoxypropyl methyl dimethoxysilane, the 3-glycidoxypropyltrimewasxysilane, 3-glycidoxypropyl methyldiethoxysilane, 3-glycidoxypropyl triethoxyl silane, right-the styryl Trimethoxy silane, 3-methacryloxypropyl methyl dimethoxysilane, 3-methacryloxypropyl trimethoxy silane, 3-methacryloxypropyl methyldiethoxysilane, 3-methacryloxypropyl triethoxyl silane, at least a in the group that 3-acryloxy propyl-triethoxysilicane and 3-urea propyl-triethoxysilicane are formed.
5. semiconductor element sealed with resin, it is coated on a surface of silicon wafer by the compositions of thermosetting resin with claim 1, this silicon wafer has at least one and be formed on wherein semiconductor element fully under pressure or under the decompression of vacuum environment, the thermofixation said composition encapsulates this wafer to use curing resin layer, polishing curing resin layer and be that single device is produced with this wafer slice.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012006254A JP2013144763A (en) | 2012-01-16 | 2012-01-16 | Thermosetting resin composition for sealing semiconductor and semiconductor device sealed by the composition |
| JP2012-006254 | 2012-03-19 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN103205125A true CN103205125A (en) | 2013-07-17 |
Family
ID=47623925
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN2013101138921A Pending CN103205125A (en) | 2012-01-16 | 2013-01-16 | Thermosetting Resin Composition For Semiconductor Encapsulation And Encapsulated Semiconductor Device |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20130181361A1 (en) |
| EP (1) | EP2615141A3 (en) |
| JP (1) | JP2013144763A (en) |
| KR (1) | KR20130084259A (en) |
| CN (1) | CN103205125A (en) |
| TW (1) | TW201341474A (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105566913A (en) * | 2014-10-30 | 2016-05-11 | 信越化学工业株式会社 | Silicone resin, resin composition, resin film, semiconductor device, and making method |
| CN105623271A (en) * | 2014-11-20 | 2016-06-01 | 爱克工业株式会社 | Addition reaction cured resin composition and photosemiconductor device |
| CN110760067A (en) * | 2019-10-22 | 2020-02-07 | 广东万木新材料科技有限公司 | Organic silicon oligomer and synthesis method and application thereof |
| WO2020168784A1 (en) * | 2019-02-22 | 2020-08-27 | 湖州五爻硅基材料研究院有限公司 | Preparation method for spherical or angular powder filler, spherical or angular powder filler obtained thereby, and application thereof |
| CN111801808A (en) * | 2019-02-22 | 2020-10-20 | 浙江三时纪新材科技有限公司 | Preparation method of spherical or angular powder filler, spherical or angular powder filler obtained by preparation method and application of spherical or angular powder filler |
| CN112812304A (en) * | 2021-01-07 | 2021-05-18 | 天津德高化成光电科技有限责任公司 | Prepolymer, packaging resin containing prepolymer and application of packaging resin |
| CN113773501A (en) * | 2021-09-08 | 2021-12-10 | 广东致格纳米科技有限公司 | Preparation method of curable silicon-based hybrid resin |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015093942A (en) * | 2013-11-13 | 2015-05-18 | 信越化学工業株式会社 | Method for producing thermosetting resin composition and semiconductor device |
| JP2015203105A (en) * | 2014-04-16 | 2015-11-16 | 信越化学工業株式会社 | Thermosetting resin composition and method for manufacturing semiconductor device |
| CN105199397B (en) * | 2014-06-17 | 2018-05-08 | 广州慧谷化学有限公司 | A kind of curable organopolysiloxane composition and semiconductor devices |
| JP2018503638A (en) * | 2015-01-13 | 2018-02-08 | ヘンケル・アクチェンゲゼルシャフト・ウント・コムパニー・コマンディットゲゼルシャフト・アウフ・アクチェンHenkel AG & Co.KGaA | Organopolysiloxane prepolymer and curable organopolysiloxane composition containing the same |
| US10788621B2 (en) * | 2015-07-07 | 2020-09-29 | Ofs Fitel, Llc | UV-transparent optical fiber coating for high temperature application, and fibers made therefrom |
| WO2019230969A1 (en) * | 2018-05-31 | 2019-12-05 | 積水化学工業株式会社 | Heat dissipation composition, heat dissipation member, and filler aggregate for heat dissipation member |
| EP3617252A1 (en) * | 2018-08-29 | 2020-03-04 | Rhodia Operations | Polysiloxanes bearing isocyanuric acid or barbituric acid moieties and compositions comprising them |
| EP3617251A1 (en) * | 2018-08-29 | 2020-03-04 | Rhodia Operations | Thermoplastic composition comprising a polysiloxane bearing diaminotriazine moieties |
| WO2020002558A1 (en) * | 2018-06-29 | 2020-01-02 | Rhodia Operations | Polysiloxanes bearing isocyanuric acid or barbituric acid moieties and compositions comprising them |
| WO2020002556A1 (en) * | 2018-06-29 | 2020-01-02 | Rhodia Operations | Thermoplastic composition comprising a polysiloxane bearing diaminotriazine moieties |
| JP7033047B2 (en) * | 2018-10-26 | 2022-03-09 | 信越化学工業株式会社 | Thermally conductive silicone composition and its cured product |
| WO2020087196A1 (en) * | 2018-10-29 | 2020-05-07 | Henkel Ag & Co. Kgaa | Thermal conductive potting composition |
| JP7490255B2 (en) * | 2019-02-22 | 2024-05-27 | 浙江三時紀新材科技有限公司 | Method for producing spherical or angular powder filler, spherical or angular powder filler obtained by the method and its application |
| JP7172805B2 (en) * | 2019-04-02 | 2022-11-16 | 信越化学工業株式会社 | Addition-curable silicone adhesive composition |
| JP7531472B2 (en) | 2021-11-01 | 2024-08-09 | 信越化学工業株式会社 | Modified silicone composition and optical semiconductor device |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0645740B2 (en) * | 1989-03-01 | 1994-06-15 | 信越化学工業株式会社 | Epoxy resin composition for semiconductor encapsulation |
| TW200502372A (en) * | 2003-02-25 | 2005-01-16 | Kaneka Corp | Curing composition and method for preparing same, light-shielding paste, light-shielding resin and method for producing same, package for light-emitting diode, and semiconductor device |
| JP2008143954A (en) * | 2006-12-06 | 2008-06-26 | Jsr Corp | Isocyanuric ring-containing polymer, method for producing the same, and composition containing the same |
| JP2008150506A (en) * | 2006-12-18 | 2008-07-03 | Jsr Corp | Curable resin composition and its use |
| KR101174971B1 (en) * | 2007-09-05 | 2012-08-17 | 세키스이가가쿠 고교가부시키가이샤 | Insulating sheet and multilayer structure |
| TWI433875B (en) * | 2008-01-28 | 2014-04-11 | Shinetsu Chemical Co | Diglygidylisocyanurylmodified organopolysiloxane and composition including the organopolysiloxane |
| KR20100109241A (en) * | 2009-03-31 | 2010-10-08 | 삼성전자주식회사 | Chip stack package and fabrication method thereof |
| JP5571326B2 (en) * | 2009-05-26 | 2014-08-13 | 株式会社カネカ | Curable composition and cured product thereof |
| JP5539690B2 (en) * | 2009-09-16 | 2014-07-02 | 株式会社カネカ | Curable composition |
-
2012
- 2012-01-16 JP JP2012006254A patent/JP2013144763A/en active Pending
- 2012-12-26 TW TW101150107A patent/TW201341474A/en unknown
-
2013
- 2013-01-15 EP EP13151253.5A patent/EP2615141A3/en not_active Withdrawn
- 2013-01-15 KR KR1020130004488A patent/KR20130084259A/en not_active Application Discontinuation
- 2013-01-15 US US13/741,441 patent/US20130181361A1/en not_active Abandoned
- 2013-01-16 CN CN2013101138921A patent/CN103205125A/en active Pending
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105566913A (en) * | 2014-10-30 | 2016-05-11 | 信越化学工业株式会社 | Silicone resin, resin composition, resin film, semiconductor device, and making method |
| CN105623271A (en) * | 2014-11-20 | 2016-06-01 | 爱克工业株式会社 | Addition reaction cured resin composition and photosemiconductor device |
| WO2020168784A1 (en) * | 2019-02-22 | 2020-08-27 | 湖州五爻硅基材料研究院有限公司 | Preparation method for spherical or angular powder filler, spherical or angular powder filler obtained thereby, and application thereof |
| CN111801808A (en) * | 2019-02-22 | 2020-10-20 | 浙江三时纪新材科技有限公司 | Preparation method of spherical or angular powder filler, spherical or angular powder filler obtained by preparation method and application of spherical or angular powder filler |
| CN111801808B (en) * | 2019-02-22 | 2021-04-23 | 浙江三时纪新材科技有限公司 | Preparation method of spherical or angular powder filler, spherical or angular powder filler obtained by preparation method and application of spherical or angular powder filler |
| CN110760067A (en) * | 2019-10-22 | 2020-02-07 | 广东万木新材料科技有限公司 | Organic silicon oligomer and synthesis method and application thereof |
| CN110760067B (en) * | 2019-10-22 | 2021-09-24 | 广东万木新材料科技有限公司 | Organic silicon oligomer and synthesis method and application thereof |
| CN112812304A (en) * | 2021-01-07 | 2021-05-18 | 天津德高化成光电科技有限责任公司 | Prepolymer, packaging resin containing prepolymer and application of packaging resin |
| CN113773501A (en) * | 2021-09-08 | 2021-12-10 | 广东致格纳米科技有限公司 | Preparation method of curable silicon-based hybrid resin |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20130084259A (en) | 2013-07-24 |
| EP2615141A2 (en) | 2013-07-17 |
| EP2615141A3 (en) | 2014-01-15 |
| TW201341474A (en) | 2013-10-16 |
| JP2013144763A (en) | 2013-07-25 |
| US20130181361A1 (en) | 2013-07-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103205125A (en) | Thermosetting Resin Composition For Semiconductor Encapsulation And Encapsulated Semiconductor Device | |
| CN102532915B (en) | Low gas permeable silicone resin composition and photoelectric device | |
| JP4636275B2 (en) | Semiconductor device sealed with silicone resin composition and silicone resin tablet for sealing semiconductor device | |
| EP2392607B1 (en) | Composition for thermosetting silicone resin | |
| CN103173020B (en) | High reliability curable silicone resin composition and the luminescent semiconductor device using said composition | |
| JP5827834B2 (en) | Silicone resin composition, silicone resin sheet, method for producing silicone resin sheet, and optical semiconductor device | |
| EP2912128B1 (en) | Organopolysiloxane, curable silicone composition, cured product thereof, and optical semiconductor device | |
| WO2013005858A1 (en) | Curable silicon composition, cured product thereof, and optical semiconductor device | |
| CN103009780B (en) | Silicone resin sheet, its manufacture method, case chip and Light-Emitting Diode device | |
| EP2196503A1 (en) | Thermosetting silicone resin composition, silicone resin, silicone resin sheet and use thereof | |
| EP3587498B1 (en) | Curable organopolysiloxane composition and semiconductor device | |
| JP2011219597A (en) | Silicone resin sheet | |
| US8470952B2 (en) | Composition for thermosetting silicone resin | |
| US8822351B2 (en) | Composition for thermosetting silcone resin | |
| US9153755B2 (en) | Silicone resin sheet, cured sheet, and light emitting diode device and producing method thereof | |
| JP5882729B2 (en) | Silicone resin sheet, cured sheet, light-emitting diode device, and manufacturing method thereof | |
| CN110088207A (en) | Curable silicone composition and the optical semiconductor device for using it | |
| TW202214783A (en) | Curable silicone composition, encapsulant, and optical semiconductor device | |
| CN117916322A (en) | Addition-curable silicone composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C05 | Deemed withdrawal (patent law before 1993) | ||
| WD01 | Invention patent application deemed withdrawn after publication |
Application publication date: 20130717 |