CN102964474A - Supported non-metallocene catalyst, preparation method and application - Google Patents
Supported non-metallocene catalyst, preparation method and application Download PDFInfo
- Publication number
- CN102964474A CN102964474A CN201110259258XA CN201110259258A CN102964474A CN 102964474 A CN102964474 A CN 102964474A CN 201110259258X A CN201110259258X A CN 201110259258XA CN 201110259258 A CN201110259258 A CN 201110259258A CN 102964474 A CN102964474 A CN 102964474A
- Authority
- CN
- China
- Prior art keywords
- group
- alkyl
- magnesium
- compound
- nitrogen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 0 C*C(C)(*(C1)C2)C11C2(C)*(*)/C(/*)=C(\*)/C(/*)=*/C1 Chemical compound C*C(C)(*(C1)C2)C11C2(C)*(*)/C(/*)=C(\*)/C(/*)=*/C1 0.000 description 4
Landscapes
- Transition And Organic Metals Composition Catalysts For Addition Polymerization (AREA)
Abstract
The invention relates to a supported non-metallocene catalyst and a preparation method. The supported non-metallocene catalyst is prepared by the steps of employing a magnesium compound, a porous carrier and a solvent to form a mixed slurry, adding a non-metallocene complex for a contact reaction and then adding a precipitating agent for completely precipitating, filtering, washing and drying, and then treating by a chemical treatment agent. The preparation method is simple and feasible, and the load capacity of the non-metallocene ligand is adjustable. The invention also relates to the application of the supported non-metallocene catalyst in olefin homopolymerisation/copolymerization. Compared with the prior art, the supported non-metallocene catalyst has the characteristics of less usage of a cocatalyst for catalyzing polymerization of alkene, high polymerization activity, substantial copolymerization effect, high bulk density of polymer, and high and adjustable viscosity average molecular weight of the prepared ultrahigh molecular weight polyethylene.
Description
The application based on " (national 11th Five-Year supporting plan problem " in the project of grinding.This project has obtained the great attention of the Ministry of Science and Technology and has supported energetically, its target is to form the polyolefin catalyst technology of new generation with independent intellectual property right, and improve domestic related products unification, improve China's polyolefine kind class, promote it to the future development of variation, seriation, customizations, high performance.
Technical field
The present invention relates to a kind of non-metallocene catalyst.Particularly, the present invention relates to a kind of load type non-metallocene catalyst, its preparation method and the application in alkene homopolymerization/copolymerization thereof.
Background technology
The non-metallocene catalyst that middle and later periods nineteen nineties occurs, claim again luxuriant rear catalyst, the central atom of Primary Catalysts has comprised nearly all transition metal, reach at some aspect of performance, even surpass metallocene catalyst, become after Ziegler, Ziegler-Natta and metallocene catalyst the 4th generation olefin polymerization catalysis.According to the difference of the central atom of Primary Catalysts, further can divide into again non-metallocene (IIIB family, IVB family, VB family, group vib, VIIB family) catalyzer and non-luxuriant rear transition metal (VIII family) catalyzer.By the excellent property of the polyolefin products of such catalyzer manufacturing, and low cost of manufacture.The non-metallocene catalyst ligating atom is oxygen, nitrogen, sulphur and phosphorus, do not contain cyclopentadienyl group or its deriveding group, such as indenyl and fluorenyl etc., it is characterized in that central ion has stronger Electron Affinities, and have cis alkyl or halogen metal division center, carry out easily alkene insertion and σ-key and shift, the easy alkylation of central metal is conducive to the generation at cation activity center; The title complex that forms has the geometric configuration of restriction, stereoselectivity, electronegativity and chirality controllability, and in addition, formed metal-carbon key polarizes easily, more is conducive to polymerization and the copolymerization of alkene.Therefore, even under higher polymeric reaction temperature, also can obtain the olefin polymer of higher molecular weight.
But homogeneous catalyst has been proved it in olefinic polyreaction has active duration short, easily sticking still, high methylaluminoxane consumption, and obtain the too low or too high weak point of polymericular weight, only can be used for solution polymerization process or high-pressure polymerization process, seriously limit its industrial applicability.
Patent ZL 01126323.7, ZL 02151294.9ZL 02110844.7 and WO 03/010207 disclose a kind of alkene homopolymerization/catalyst for copolymerization or catalyst system, has widely alkene homopolymerization/copolymerization performance, but need higher promotor consumption during in olefinic polymerization at the disclosed catalyzer of this patent or catalyst system, could obtain suitable olefin polymerizating activity, and it is short to exist active duration in the polymerization process, the phenomenons such as the sticking still of polymkeric substance.
Common way be with non-metallocene catalyst by certain load technology, make loaded catalyst, thereby improve the polymerization of alkene and the particle form of resulting polymers.It shows as the initial activity that has suitably reduced to a certain extent catalyzer, the polymerization activity life-span of extending catalyst, reduce even avoided caking or the cruelly poly-phenomenon in the polymerization process, improve the form of polymkeric substance, improve the apparent density of polymkeric substance, can make it satisfy more polymerization technique process, such as vapour phase polymerization or slurry polymerization etc.
Existing olefin polymerization catalysis patent is mostly based on metallocene catalyst, such as US 4808561, US 5240894, CN 1344749A, CN 1126480A, ZL94101358.8, CN 1307594A, CN 1103069A, CN1363537A, US6444604, EP0685494, US4871705 and EP0206794 etc., but these patents also all relate on the carrier after the metallocene catalyst that will contain transition metal is carried on processing.
For patent ZL 01126323.7, ZL02151294.9ZL 02110844.7 and the disclosed non-metallocene catalyst of WO03/010207, patent CN 1539855A, CN 1539856A, CN 1789291A, CN 1789292A, CN 1789290A, WO/2006/063501, patent ZL200510119401.x etc. provide various ways to carry out load obtaining load type non-metallocene catalyst, but these patents all relate on the carrier after the Nonmetallocene organic compound that will contain transition metal is carried on processing.
Chinese patent CN200910180602.9 discloses a kind of preparation method of load type non-metallocene catalyst, and it is that magnesium compound and Nonmetallocene title complex are dissolved in the solvent, obtains load type non-metallocene catalyst after the drying.Patent 200910180605.2 discloses a kind of preparation method of load type non-metallocene catalyst, and it is that magnesium compound and Nonmetallocene title complex are dissolved in the solvent, adds the precipitation agent precipitation, obtains load type non-metallocene catalyst after the filtration washing drying.What these two kinds of methods adopted is the magnesium compound carrier, the particle form of catalyzer is difficult to control, has limited the morphology that thus polymerization obtains.
Chinese patent CN200910180603.3, CN200910180604.8, CN200910210989.8, CN200910210986.4, the disclosed load type non-metallocene catalyst preparation method of CN200910210985.X, CN200910210990.0 and above-mentioned patent are similar, what all use is that magnesium compound is as carrier, still exist the particle form of catalyzer to be difficult to control, limited the morphology that thus polymerization obtains.
Catalyzer take Magnesium Chloride Anhydrous as carrier demonstrates higher catalytic activity in olefin polymerization process, but this type of catalyzer is highly brittle, and is broken easily in polymerization reactor, thereby causes polymer morphology bad.Silicon dioxide carried catalyzer has good flowability, can be used for gas fluidised bed polymerisation, but silicon dioxide carried metallocene and non-metallocene catalyst then show lower catalytic activity.Therefore if magnesium chloride and silicon-dioxide are well organically combined, just may prepare and have high catalytic activity, the catalyzer of the controlled and good abrasion strength resistance of globule size.
A kind of load method of non-metallocene catalyst of composite carrier load is disclosed such as CN1539856A, it is in accordance with the following steps: (1) will be as the porous support of carrier under 100-1000 ℃, inert atmosphere or reduced pressure, and drying or roasting 1~24h carry out thermal activation; (2) magnesium compound is dissolved in tetrahydrofuran (THF)-pure mixed system and forms solution, the porosu solid with thermal activation joins in this solution again, and fully reaction forms transparent system under 0~60 ℃ of agitation condition; Make complex carrier through filtration washing, drying with after draining; Perhaps this clear solution adding non-polar organic solutions is made it precipitation and fully separate out, then filtration washing, drying are drained and are made complex carrier; (3) non-metallocene olefin polymerization catalyst is dissolved in the solvent, then filtration washing, drying are drained into load type non-metallocene catalyst with complex carrier or after modifying complex carrier and contacting 12~72 hours.This method need to prepare first complex carrier, contacts with catalyst solution again.
CN1789290A discloses a kind of high activity loading method of load type non-metallocene catalyst, and it comprises the steps: carrier and chemical activating agent effect are obtained modifying carrier; Magnesium compound is dissolved in tetrahydrofuran (THF)-pure mixed system forms solution, will modify again carrier and join in this solution and react, after filtration washing, dry and drain and make complex carrier; Non-metallocene olefin polymerization catalyst is dissolved in the solvent, then drains with the rear washing and filtering of complex carrier reaction, drying, make load type non-metallocene catalyst.This method is to prepare first the modification carrier, obtains mixed carrier with the magnesium compound reaction again, contacts with catalyst solution again.
Patent CN101423574A discloses a kind of supported non-metallocene single site catalysis agent component and preparation method thereof, and the method comprises: the preparation of (1) magnesium chloride/silica-gel carrier; (2) preparation of the preparation of alkylaluminoxane/magnesium chloride/silica-gel carrier and (3) supported non-metallocene single site catalysts component.This method also is to prepare first complex carrier, with the alkylaluminoxane reaction, contacts with catalyst solution at last again.
EP260130 proposes loaded metallocene or non-luxuriant transition metal compound loaded on the silica supports that methylaluminoxane is processed, and the non-luxuriant transition metal here only refers to ZrCl
4, TiCl
4Perhaps VOCl
3, this patent think optimum be carrier surface through the mixture of organic-magnesium or magnesium compound and aluminum alkyls, but this process more complicated needs to pass through many preparation processes.
Patent CN200610026765.8 discloses a class single active center Z-N olefin polymerization catalysis.This catalyzer, obtains after processing through pretreated carrier (such as silica gel), metallic compound (such as titanium tetrachloride) and this electron donor by adding in magnesium compound (such as magnesium chloride)/tetrahydrofuran solution as electron donor with the salicylic alidehyde imine derivative of the salicylic alidehyde imine that contains coordinating group or replacement.
Patent CN200610026766.2 is similar with it, discloses a class and has contained heteroatomic organic compound and the application in Ziegler-Natta catalyst thereof.
Patent CN200910180100.6 and CN200910180607.1 disclose not to be had in the presence of the alcohol, the Nonmetallocene title complex is dissolved in the magnesium compound solution, then add porous support, convection drying or filtration washing drying are processed by IVB family chemical processing agent, thereby obtain preparation method and the polymeric applications of load type non-metallocene catalyst, although the Nonmetallocene title complex is present among the carrier uniformly, but because duct effect, contain in the magnesium compound solution of Nonmetallocene title complex Nonmetallocene title complex and the distribution of magnesium compound in the porous support duct greater than the duct outside, therefore follow-up when IVB family chemical processing agent contacts, Nonmetallocene title complex and magnesium compound touch opportunity are impartial, because the existence of magnesium compound, limited fully contacting of IVB family chemical processing agent and Nonmetallocene title complex, thereby reduced the synergy chance of catalysis in olefine polymerization, thereby the catalyzed ethylene polymerization activity is lower in an embodiment, and similarly patent CN200910180601.4 and the disclosed load type non-metallocene catalyst preparation method of CN200910180606.7 and polymeric applications, its main difference is to process without IVB family chemical processing agent, and the polymerization catalyst activity is also lower.
Patent CN200710162666.7 discloses loaded catalyst, load type non-metallocene catalyst and preparation method thereof, it is to have in the presence of the alcohol, magnesium compound is dissolved in the tetrahydrofuran solvent, add porous support, behind the convection drying with titanium tetrachloride reaction, last load non-metallocene metal complexes again, catalyst activity is higher, and the polymkeric substance that obtains of polymerization has high bulk density thus, but preparation process is comparatively complicated, chemical processing agent and carrier reaction can destroy in type carrier structure, then produce fine polymer powder in polymerization process.
Even so, the ubiquitous problem of the load type non-metallocene catalyst that exists in the prior art is, the load process is complicated, generally need to carry out the multistep of carrier and process afterwards again load non-metallocene metal complexes, olefin polymerizating activity is low and be difficult to regulate, and in order to improve its polymerization activity, when carrying out olefinic polymerization, must assist higher promotor consumption.
Therefore, still need a kind of load type non-metallocene catalyst, its preparation method is simple, is fit to suitability for industrialized production, and can overcomes to prepare those problems that exist in the prior art load type non-metallocene catalyst process.
Summary of the invention
The inventor passes through diligent research on the basis of existing technology, and finds through lot of experiments, by making described load type non-metallocene catalyst with a kind of specific preparation method, just can solve foregoing problems, and finish thus the present invention.
In the preparation method of load type non-metallocene catalyst of the present invention, do not add proton donor (such as conventional those that use in this area).In addition, in the preparation method of load type non-metallocene catalyst of the present invention, do not add electron donor (such as in this area for this reason and the conventional compounds such as monoesters class, di-esters, two ethers, diones and diol-lipid that use).Moreover, in the preparation method of load type non-metallocene catalyst of the present invention, also need not harsh reaction requirement and reaction conditions.Therefore, the preparation method of this loaded catalyst is simple, and is very suitable for suitability for industrialized production.
Particularly, the present invention relates to the content of following aspect:
1. the preparation method of a load type non-metallocene catalyst may further comprise the steps:
Magnesium compound is dissolved in the solvent, obtains the step of magnesium compound solution;
Optional porous support through thermal activation treatment is contacted with described magnesium compound solution, obtain the step of the first mixed serum;
The Nonmetallocene title complex is contacted with described the first mixed serum, obtain the step of the second mixed serum;
In described the second mixed serum, add precipitation agent, obtain to modify the step of complex carrier, and
Make the chemical processing agent and the reaction of described modification complex carrier that are selected from IVB family metallic compound, obtain the step of described load type non-metallocene catalyst.
2. according to the described preparation method of aforementioned either side, it is characterized in that, described porous support is selected from olefin homo or multipolymer, polyvinyl alcohol or its multipolymer, cyclodextrin, polyester or copolyesters, polymeric amide or copolyamide, ryuron or multipolymer, Voncoat R 3310 or multipolymer, methacrylic acid ester homopolymer or multipolymer, styrene homopolymers or multipolymer, the partial cross-linked form of these homopolymer or multipolymer, periodic table of elements IIA, IIIA, the refractory oxide of IVA or IVB family metal or infusibility composite oxides, clay, molecular sieve, mica, polynite, in wilkinite and the diatomite one or more, be preferably selected from partial cross-linked styrene polymer, silicon-dioxide, aluminum oxide, magnesium oxide, the oxidation sial, the oxidation magnalium, titanium dioxide, in molecular sieve and the polynite one or more more preferably are selected from silicon-dioxide.
3. according to the described preparation method of aforementioned either side, it is characterized in that, described magnesium compound is selected from one or more in magnesium halide, alkoxyl group magnesium halide, alkoxyl magnesium, alkyl magnesium, alkyl halide magnesium and the alkyl alkoxy magnesium, be preferably selected from the magnesium halide one or more, more preferably magnesium chloride.
4. according to the described preparation method of aforementioned either side, it is characterized in that described solvent is selected from C
6-12Aromatic hydrocarbon, halo C
6-12In aromatic hydrocarbon, ester and the ether one or more are preferably selected from C
6-12In aromatic hydrocarbon and the tetrahydrofuran (THF) one or more, most preferably tetrahydrofuran (THF).
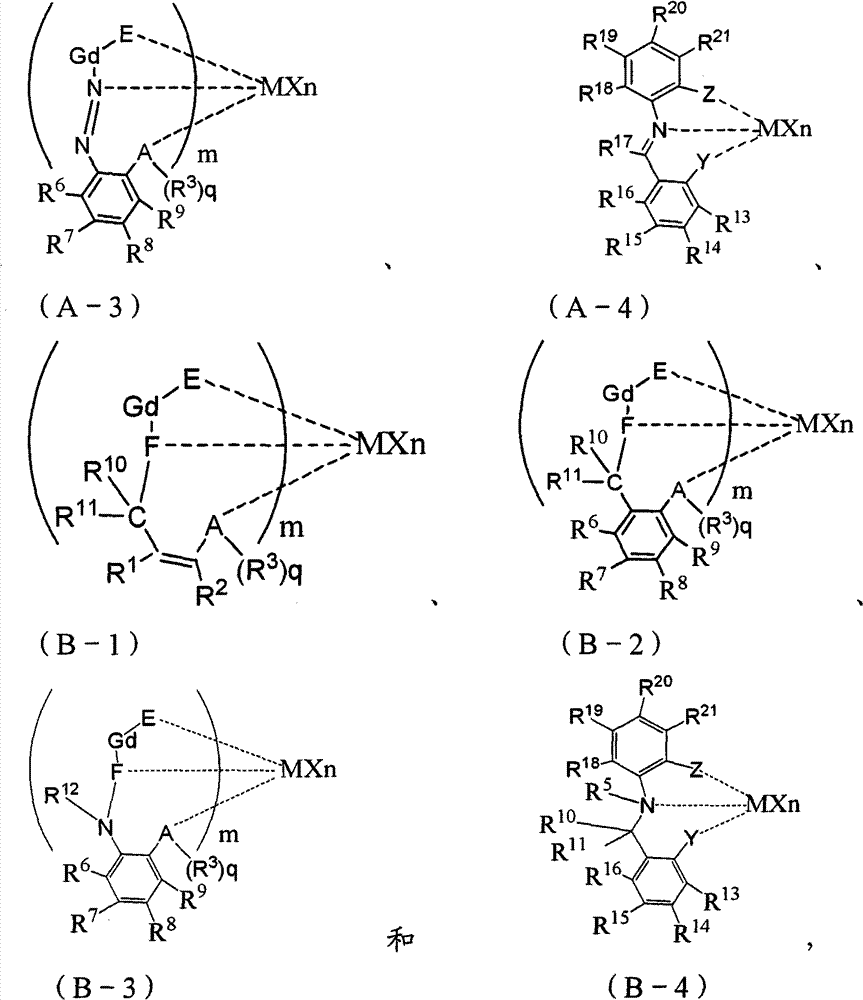
5. according to the described preparation method of aforementioned either side, it is characterized in that described Nonmetallocene title complex is selected from one or more in the compound with following chemical structural formula:
Be preferably selected from compound (A) with following chemical structural formula and in the compound (B) one or more:
More preferably be selected to compound (A-4) and compound (B-1) to compound (B-4) one or more of compound (A-1) with following chemical structural formula:
In above all chemical structural formulas,
Q is 0 or 1;
D is 0 or 1;
M is 1,2 or 3;
M is selected from periodic table of elements III-th family to XI family atoms metal, preferred IVB family atoms metal, more preferably Ti (IV) and Zr (IV);
N is 1,2,3 or 4, depends on the valence state of described central metal atom M;
X is selected from halogen, hydrogen atom, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl, oxy radical, nitrogen-containing group, sulfur-containing group, boron-containing group, contain aluminium base group, phosphorus-containing groups, silicon-containing group, germanic group or contain tin group, a plurality of X can be identical, also can be different, and can also be each other in key or Cheng Huan;
A be selected from Sauerstoffatom, sulphur atom, selenium atom,
-NR
23R
24,-N (O) R
25R
26,
-PR
28R
29,-P (O) R
30OR
31, sulfuryl, sulfoxide group or-Se (O) R
39, N, O, S, Se and the P coordination atom of respectively doing for oneself wherein;
B is selected from nitrogen-atoms, nitrogen-containing group, phosphorus-containing groups or C
1-C
30Alkyl;
D is selected from nitrogen-atoms, Sauerstoffatom, sulphur atom, selenium atom, phosphorus atom, nitrogen-containing group, phosphorus-containing groups, C
1-C
30Alkyl, sulfuryl, sulfoxide group,
-N (O) R
25R
26,
Or-P (O) R
32(OR
33), N, O, S, Se and the P coordination atom of respectively doing for oneself wherein;
E is selected from nitrogen-containing group, oxy radical, sulfur-containing group, contains seleno group, phosphorus-containing groups or cyano group, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
F is selected from nitrogen-atoms, nitrogen-containing group, oxy radical, sulfur-containing group, contain seleno group or phosphorus-containing groups, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
G is selected from C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or safing function group;
Y is selected from Sauerstoffatom, nitrogen-containing group, oxy radical, sulfur-containing group, contain seleno group or phosphorus-containing groups, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
Z is selected from nitrogen-containing group, oxy radical, sulfur-containing group, contains seleno group, phosphorus-containing groups or cyano group, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
→ represent singly-bound or two key;
-represent covalent linkage or ionic linkage;
---represent coordinate bond, covalent linkage or ionic linkage;
R
1To R
4, R
6To R
36, R
38And R
39Be selected from independently of one another hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or safing function group, above-mentioned group can be the same or different to each other, and wherein adjacent group can combine togather into key or Cheng Huan, is preferably formed aromatic ring, and
R
5Be selected from lone-pair electron on the nitrogen, hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl, oxy radical, sulfur-containing group, nitrogen-containing group, contain seleno group or phosphorus-containing groups; Work as R
5For oxy radical, sulfur-containing group, nitrogen-containing group, when containing seleno group or phosphorus-containing groups, R
5In N, O, S, P and Se can be used as coordination and carry out coordination with atom and described center IVB family atoms metal,
Described safing function group is selected from halogen, oxy radical, nitrogen-containing group, silicon-containing group, germanic group, sulfur-containing group, contains tin group, C
1-C
10Ester group or nitro,
Described Nonmetallocene title complex further is preferably selected from one or more in the compound with following chemical structural formula:
Most preferably be selected from the compound with following chemical structural formula one or more:
6. according to the described preparation method of aforementioned either side, it is characterized in that,
Described halogen is selected from F, Cl, Br or I;
Described phosphorus-containing groups is selected from
-PR
28R
29,-P (O) R
30R
31Or-P (O) R
32(OR
33);
Described oxy radical be selected from hydroxyl ,-OR
34With-T-OR
34
Described sulfur-containing group is selected from-SR
35,-T-SR
35,-S (O) R
36Or-T-SO
2R
37
The described seleno group that contains is selected from-SeR
38,-T-SeR
38,-Se (O) R
39Or-T-Se (O) R
39
Described group T is selected from C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or described safing function group;
Described R
37Be selected from hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or described safing function group;
Described C
1-C
30Alkyl is selected from C
1-C
30Alkyl, C
7-C
50Alkaryl, C
7-C
50Aralkyl, C
3-C
30Cyclic alkyl, C
2-C
30Thiazolinyl, C
2-C
30Alkynyl, C
6-C
30Aryl, C
8-C
30Condensed ring radical or C
4-C
30Heterocyclic radical, wherein said heterocyclic radical contain 1-3 heteroatoms that is selected from nitrogen-atoms, Sauerstoffatom or sulphur atom;
The C of described replacement
1-C
30Alkyl is selected from one or more described halogens and/or described C
1-C
30Alkyl is as substituent described C
1-C
30Alkyl;
Described boron-containing group is selected from BF
4 -, (C
6F
5)
4B
-Or (R
40BAr
3)
-
Describedly contain aluminium base group and be selected from aluminum alkyls, AlPh
4 -, AlF
4 -, AlCl
4 -, AlBr
4 -, AlI
4 -Or R
41AlAr
3 -
Described silicon-containing group is selected from-SiR
42R
43R
44Or-T-SiR
45
Described germanic group is selected from-GeR
46R
47R
48Or-T-GeR
49
Describedly contain tin group and be selected from-SnR
50R
51R
52,-T-SnR
53Or-T-Sn (O) R
54,
Described Ar represents C
6-C
30Aryl, and
R
40To R
54Be selected from independently of one another hydrogen, described C
1-C
30The C of alkyl, described replacement
1-C
30Alkyl or described safing function group, wherein these groups can be the same or different to each other, and wherein adjacent group can combine togather into key or Cheng Huan, and
Described group T defines with aforementioned either side.
7. according to the described preparation method of aforementioned either side, it is characterized in that, described IVB family metallic compound is selected from one or more in IVB family metal halide, IVB family metal alkyl compound, IVB family metal alkoxide compound, IVB family metal alkyl halides and the IVB family metal alkoxide halogenide, be preferably selected from the IVB family metal halide one or more, more preferably be selected from TiCl
4, TiBr
4, ZrCl
4, ZrBr
4, HfCl
4And HfBr
4In one or more, most preferably be selected from TiCl
4And ZrCl
4In one or more.
8. according to the described preparation method of aforementioned either side, it is characterized in that described precipitation agent is selected from C
5-12Alkane, C
5-12Naphthenic hydrocarbon, halo C
1-10Alkane and halo C
5-12In the naphthenic hydrocarbon one or more, be preferably selected from pentane, hexane, heptane, octane, nonane, decane, hexanaphthene, pentamethylene, suberane, cyclodecane, cyclononane, methylene dichloride, dichloro hexane, two chloroheptanes, trichloromethane, trichloroethane, three chlorobutanes, methylene bromide, ethylene dibromide, dibromo-heptane, methenyl bromide, tribromoethane, three n-butyl bromide, chlorocyclopentane, chlorocyclohexane, the chloro suberane, the chloro cyclooctane, the chloro cyclononane, the chloro cyclodecane, bromocyclopentane, bromocyclohexane, the bromo suberane, the bromo cyclooctane, in bromo cyclononane and the bromo cyclodecane one or more, further be preferably selected from hexane, heptane, in decane and the hexanaphthene one or more, most preferably hexane.
9. according to the described preparation method of aforementioned either side, it is characterized in that, take the mol ratio of the described magnesium compound of Mg element and described Nonmetallocene title complex as 1: 0.01-1, preferred 1: 0.04-0.4, more preferably 1: 0.08-0.2, the ratio of described magnesium compound and described solvent is 1mol: 75~400ml, preferred 1mol: 150~300ml, more preferably 1mol: 200~250ml, take the mass ratio of the described magnesium compound of magnesium compound solid and described porous support as 1: 0.1-20, preferred 1: 0.5-10, more preferably 1: 1-5, in the described magnesium compound of Mg element with take the mol ratio of the described chemical processing agent of IVB family metallic element as 1: 0.01-1, preferred 1: 0.01-0.50, more preferably 1: 0.10-0.30, and the volume ratio of described precipitation agent and described solvent is 1: 0.2~5, preferred 1: 0.5~2, more preferably 1: 0.8~1.5.
10. according to the described preparation method of aforementioned either side, also be included in and make before described chemical processing agent and the reaction of described modification complex carrier, with the step that helps the described modification complex carrier of chemical processing agent pre-treatment that is selected from aikyiaiurnirsoxan beta, aluminum alkyls or its arbitrary combination.
11. according to the described preparation method of aforementioned either side, it is characterized in that, described aikyiaiurnirsoxan beta is selected from methylaluminoxane, ethylaluminoxane, in isobutyl aluminium alkoxide and the normal-butyl alumina alkane one or more, more preferably be selected from methylaluminoxane and the isobutyl aluminium alkoxide one or more, and described aluminum alkyls is selected from trimethyl aluminium, triethyl aluminum, tri-n-n-propyl aluminum, triisobutyl aluminium, three n-butylaluminum, triisopentyl aluminium, three n-pentyl aluminium, tri-n-hexyl aluminum, three isohexyl aluminium, in diethylmethyl aluminium and the dimethyl ethyl aluminium one or more, be preferably selected from trimethyl aluminium, triethyl aluminum, in tri-n-n-propyl aluminum and the triisobutyl aluminium one or more most preferably are selected from triethyl aluminum and the triisobutyl aluminium one or more.
12. according to the described preparation method of aforementioned either side, it is characterized in that, in the described magnesium compound of Mg element and the mol ratio that helps chemical processing agent take Al element described as 1: 0-1.0, preferred 1: 0-0.5, more preferably 1: 0.1-0.5.
13. a load type non-metallocene catalyst, it is by making according to the described preparation method of aforementioned either side.
14. alkene homopolymerization/copolymerization process, it is characterized in that, take according to aspect 13 described load type non-metallocene catalysts as Primary Catalysts, to be selected from aikyiaiurnirsoxan beta, aluminum alkyls, haloalkyl aluminium, boron fluothane, boron alkyl and the boron alkyl ammonium salt one or more as promotor, make alkene homopolymerization or copolymerization.
15. an alkene homopolymerization/copolymerization process is characterized in that, may further comprise the steps:
Described preparation method makes load type non-metallocene catalyst according to aforementioned either side, and
Take described load type non-metallocene catalyst as Primary Catalysts, to be selected from aikyiaiurnirsoxan beta, aluminum alkyls, haloalkyl aluminium, boron fluothane, boron alkyl and the boron alkyl ammonium salt one or more as promotor, make alkene homopolymerization or copolymerization.
Technique effect
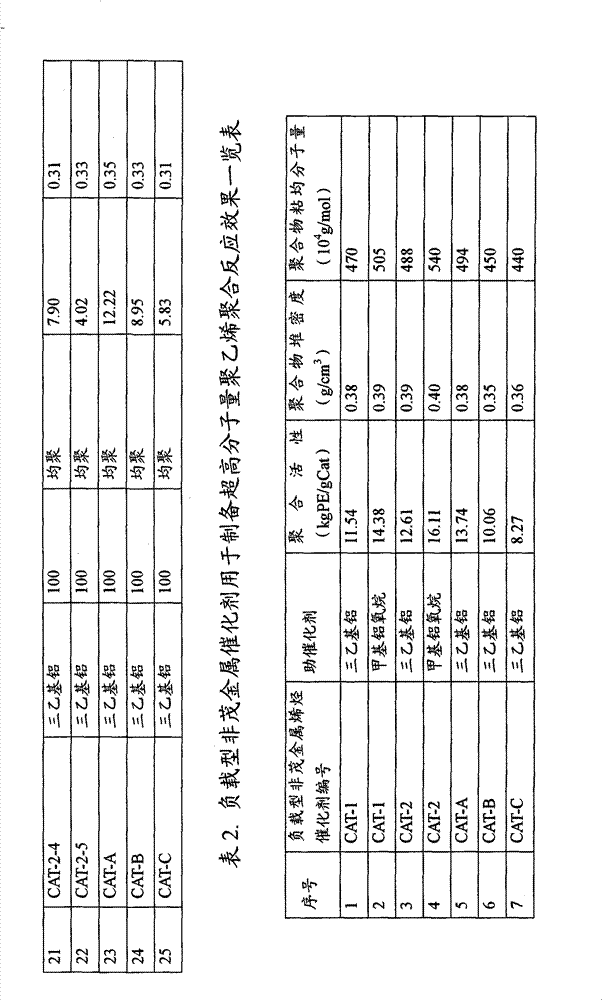
Preparation method's technique simple possible of load type non-metallocene catalyst of the present invention, and the charge capacity of Nonmetallocene title complex is adjustable, can give full play to it and obtain the performance of polyolefin product at catalysis in olefine polymerization, thereby and can regulate polymer performance and ultrahigh molecular weight polyethylene(UHMWPE) viscosity-average molecular weight by the difference of regulating add-on.
In addition, by adopting different chemical processing agent and chemical processing agent and the consumptions of helping, can obtain polymerization activity from low to high and adjustable load type non-metallocene catalyst, adapt to thus different olefinic polymerization requirements, thereby and can cooperate the preparation process of the add-on of Nonmetallocene title complex that catalyzer and polymer performance are regulated.
Adopt method for preparing catalyst provided by the invention, because load type non-metallocene catalyst is by the mixed serum of precipitation magnesium-containing compound, porous support, Nonmetallocene title complex and solvent, filtration washing drying, the mode of processing finally by chemical processing agent obtains, therefore each component is in conjunction with tight in the catalyzer, and the polymer stacks density that obtains thus is higher.
The present invention finds, jointly be dissolved in the solvent than the Nonmetallocene title complex prior to magnesium compound, add porous support and form slurry, adding precipitation agent fully precipitates, after the filtration washing drying, again with the dry prepared load type non-metallocene catalyst of the rear filtration washing of IVB family chemical processing agent reaction, magnesium compound provided by the present invention is dissolved in the solvent, the adding precipitation agent fully precipitates after adding porous support, after the filtration washing drying, again with the dry prepared load type non-metallocene catalyst of the rear filtration washing of IVB family chemical processing agent reaction, because duct effect, magnesium compound preferentially enters in the porous support, the Nonmetallocene title complex is distributed on the magnesium compound, therefore can be fully and IVB family chemical processing agent react, bring into play the synergy of both catalyzed alkenes, therefore catalyst activity and polymer stacks density are higher, and the ultrahigh molecular weight polyethylene(UHMWPE) viscosity-average molecular weight of preparation is also higher.
Also find simultaneously, when the load type non-metallocene catalyst that employing the present invention obtains and promotor consist of catalyst system, only need fewer promotor (such as methylaluminoxane or triethyl aluminum) consumption, just can obtain high olefin polymerizating activity, show significant comonomer effect during copolymerization, namely under relatively equal condition, Copolymerization activity is higher than the homopolymerization activity, and has good polymer morphology and high polymer bulk density by polymkeric substance such as catalyzed alkene homopolymerization or the resulting polyethylene of copolymerization.
Embodiment
The below is elaborated to the specific embodiment of the present invention, but it is pointed out that protection scope of the present invention is not subjected to the restriction of these embodiments, but is determined by claims of appendix.
In the context of the present invention, unless clearly definition is arranged in addition, perhaps this implication has exceeded those skilled in the art's understanding scope, and the hydrocarbon that 3 carbon atoms are above or hydrocarbon derivative group (such as propyl group, propoxy-, butyl, butane, butylene, butenyl, hexane etc.) all have implication identical with titled with prefix " just " time not titled with prefix " just " time.Such as, propyl group is generally understood as n-propyl, and butyl is generally understood as normal-butyl.
The present invention relates to a kind of preparation method of load type non-metallocene catalyst, may further comprise the steps: magnesium compound is dissolved in the solvent, obtains the step of magnesium compound solution; Optional porous support through thermal activation treatment is contacted with described magnesium compound solution, obtain the step of the first mixed serum; The Nonmetallocene title complex is contacted with described the first mixed serum, obtain the step of the second mixed serum; In described the second mixed serum, add precipitation agent, obtain to modify the step of complex carrier, and make chemical processing agent and the reaction of described modification complex carrier that is selected from IVB family metallic compound, obtain the step of described load type non-metallocene catalyst.
Below described magnesium compound is carried out specific description.
According to the present invention, term " magnesium compound " uses the common concept in this area, refers to as the conventional organic or inorganic solid water-free magnesium-containing compound that uses of the carrier of supported olefin polymerization catalyst.
According to the present invention, as described magnesium compound, such as enumerating magnesium halide, alkoxyl group magnesium halide, alkoxyl magnesium, alkyl magnesium, alkyl halide magnesium and alkyl alkoxy magnesium.
Particularly, as described magnesium halide, such as enumerating magnesium chloride (MgCl
2), magnesium bromide (MgBr
2), magnesium iodide (MgI
2) and magnesium fluoride (MgF
2) etc., preferred magnesium chloride wherein.
As described alkoxyl group magnesium halide, such as enumerating methoxyl group chlorination magnesium (Mg (OCH
3) Cl), oxyethyl group magnesium chloride (Mg (OC
2H
5) Cl), propoxy-magnesium chloride (Mg (OC
3H
7) Cl), n-butoxy magnesium chloride (Mg (OC
4H
9) Cl), isobutoxy magnesium chloride (Mg (i-OC
4H
9) Cl), methoxyl group magnesium bromide (Mg (OCH
3) Br), oxyethyl group magnesium bromide (Mg (OC
2H
5) Br), propoxy-magnesium bromide (Mg (OC
3H
7) Br), n-butoxy magnesium bromide (Mg (OC
4H
9) Br), isobutoxy magnesium bromide (Mg (i-OC
4H
9) Br), methoxyl group magnesium iodide (Mg (OCH
3) I), oxyethyl group magnesium iodide (Mg (OC
2H
5) I), propoxy-magnesium iodide (Mg (OC
3H
7) I), n-butoxy magnesium iodide (Mg (OC
4H
9) I) and isobutoxy magnesium iodide (Mg (i-OC
4H
9) I) etc., wherein preferred methoxyl group chlorination magnesium, oxyethyl group magnesium chloride and isobutoxy magnesium chloride.
As described alkoxyl magnesium, such as enumerating magnesium methylate (Mg (OCH
3)
2), magnesium ethylate (Mg (OC
2H
5)
2), propoxy-magnesium (Mg (OC
3H
7)
2), butoxy magnesium (Mg (OC
4H
9)
2), isobutoxy magnesium (Mg (i-OC
4H
9)
2) and 2-ethyl hexyl oxy magnesium (Mg (OCH
2CH (C
2H
5) C
4H)
2) etc., wherein preferred magnesium ethylate and isobutoxy magnesium.
As described alkyl magnesium, such as enumerating methyl magnesium (Mg (CH
3)
2), magnesium ethide (Mg (C
2H
5)
2), propyl group magnesium (Mg (C
3H
7)
2), normal-butyl magnesium (Mg (C
4H
9)
2) and isobutyl-magnesium (Mg (i-C
4H
9)
2) etc., wherein preferred magnesium ethide and normal-butyl magnesium.
As described alkyl halide magnesium, such as enumerating methylmagnesium-chloride (Mg (CH
3) Cl), ethylmagnesium chloride (Mg (C
2H
5) Cl), propyl group magnesium chloride (Mg (C
3H
7) Cl), normal-butyl chlorination magnesium (Mg (C
4H
9) Cl), isobutyl-chlorination magnesium (Mg (i-C
4H
9) Cl), methyl-magnesium-bromide (Mg (CH
3) Br), ethylmagnesium bromide (Mg (C
2H
5) Br), propyl group magnesium bromide (Mg (C
3H
7) Br), normal-butyl bromination magnesium (Mg (C
4H
9) Br), selenium alkynide (Mg (i-C
4H
9) Br), methyl magnesium iodide (Mg (CH
3) I), ethyl magnesium iodide (Mg (C
2H
5) I), propyl group magnesium iodide (Mg (C
3H
7) I), normal-butyl iodate magnesium (Mg (C
4H
9) I) and isobutyl-iodate magnesium (Mg (i-C
4H
9) I) etc., wherein preferable methyl magnesium chloride, ethylmagnesium chloride and isobutyl-chlorination magnesium.
As described alkyl alkoxy magnesium, such as enumerating methyl methoxy base magnesium (Mg (OCH
3) (CH
3)), methyl ethoxy magnesium (Mg (OC
2H
5) (CH
3)), methyl propoxy-magnesium (Mg (OC
3H
7) (CH
3)), methyl n-butoxy magnesium (Mg (OC
4H
9) (CH
3)), methyl isobutoxy magnesium (Mg (i-OC
4H
9) (CH
3)), ethyl magnesium methylate (Mg (OCH
3) (C
2H
5)), ethyl magnesium ethylate (Mg (OC
2H
5) (C
2H
5)), ethyl propoxy-magnesium (Mg (OC
3H
7) (C
2H
5)), ethyl n-butoxy magnesium (Mg (OC
4H
9) (C
2H
5)), ethyl isobutoxy magnesium (Mg (i-OC
4H
9) (C
2H
5)), propyl group magnesium methylate (Mg (OCH
3) (C
3H
7)), propyl group magnesium ethylate (Mg (OC
2H
5) (C
3H
7)), propyl group propoxy-magnesium (Mg (OC
3H
7) (C
3H
7)), propyl group n-butoxy magnesium (Mg (OC
4H
9) (C
3H
7)), propyl group isobutoxy magnesium (Mg (i-OC
4H
9) (C
3H
7)), normal-butyl magnesium methylate (Mg (OCH
3) (C
4H
9)), normal-butyl magnesium ethylate (Mg (OC
2H
5) (C
4H
9)), normal-butyl propoxy-magnesium (Mg (OC
3H
7) (C
4H
9)), normal-butyl n-butoxy magnesium (Mg (OC
4H
9) (C
4H
9)), normal-butyl isobutoxy magnesium (Mg (i-OC
4H
9) (C
4H
9)), isobutyl-magnesium methylate (Mg (OCH
3) (i-C
4H
9)), isobutyl-magnesium ethylate (Mg (OC
2H
5) (i-C
4H
9)), isobutyl-propoxy-magnesium (Mg (OC
3H
7) (i-C
4H
9)), isobutyl-n-butoxy magnesium (Mg (OC
4H
9) (i-C
4H
9)) and isobutyl-isobutoxy magnesium (Mg (i-OC
4H
9) (i-C
4H
9)) etc., preferred butyl magnesium ethylate wherein.
These magnesium compounds can be used alone, and also can multiple mixing use, not special restriction.
When using with the form of multiple mixing, the mol ratio between two kinds of magnesium compounds in the described magnesium compound mixture is such as being 0.25~4: 1, preferred 0.5~3: 1, more preferably 1~2: 1.
According to the present invention, magnesium compound is dissolved in the solvent (not comprising alcoholic solvent), obtain magnesium compound solution.
Below the step that obtains described magnesium compound solution is carried out specific description.
Particularly, make described magnesium compound (solid) be dissolved in suitable solvent (below be sometimes referred to as for the solvent that dissolves described magnesium compound), thereby obtain described magnesium compound solution.
As described solvent, such as enumerating C
6-12Aromatic hydrocarbon, halo C
6-12Aromatic hydrocarbon, ester and ether equal solvent.
As described C
6-12Aromatic hydrocarbon is such as enumerating toluene, dimethylbenzene, trimethylbenzene, ethylbenzene, diethylbenzene.
As described halo C
6-12Aromatic hydrocarbon is such as enumerating chlorotoluene, chloro ethylbenzene, bromo toluene, bromo ethylbenzene etc.
As described ester, such as enumerating methyl-formiate, ethyl formate, propyl formate, butyl formate, methyl acetate, ethyl acetate, propyl acetate, butylacetate, methyl propionate, ethyl propionate, butyl propionate, butyl butyrate etc.
As described ether, such as enumerating ether, methyl ethyl ether, tetrahydrofuran (THF) etc.
Wherein, preferred C
6-12Aromatic hydrocarbon and tetrahydrofuran (THF), most preferably tetrahydrofuran (THF).
According to the present invention one preferred embodiment, described solvent preferably can dissolve described magnesium compound (solid) and Nonmetallocene title complex hereinafter described simultaneously.At this moment, as described solvent, such as enumerating described C
6-12Aromatic hydrocarbon, described halo C
6-12Aromatic hydrocarbon and tetrahydrofuran (THF) etc.
It is pointed out that the present invention in preparation during described load type non-metallocene catalyst, in any step, all do not use alcohols (such as aromatic alcohols such as the fatty alcohols such as ethanol, phenylcarbinols etc.).
These solvents can be used alone, and also can use with the arbitrarily multiple mixing of ratio.
In order to prepare described magnesium compound solution, the meterings such as described magnesium compound are added to dissolve in the described solvent to getting final product.
There is no particular limitation to the preparation time (being the dissolution time of described magnesium compound etc.) of described magnesium compound solution, but be generally 0.5~24h, preferred 4~24h.In this preparation process, can utilize and stir the dissolving promote described magnesium compound etc.This stirring can be adopted any form, such as stirring rake (rotating speed is generally 10~1000 rev/mins) etc.As required, sometimes can promote dissolving by suitable heating (but top temperature must be lower than the boiling point of described solvent).
Below described porous support is carried out specific description.
According to the present invention, as described porous support, such as can enumerate this area when making supported olefin polymerization catalyst as carrier and conventional those organic or inorganic porosu solids that use.
Particularly, as described Porous-Organic solid, such as enumerating olefin homo or multipolymer, polyvinyl alcohol or its multipolymer, cyclodextrin, (being total to) polyester, (being total to) polymeric amide, ryuron or multipolymer, Voncoat R 3310 or multipolymer, methacrylic acid ester homopolymer or multipolymer, and styrene homopolymers or multipolymer etc., and the partial cross-linked form of these homopolymer or multipolymer, wherein preferably partial cross-linked (such as degree of crosslinking be at least 2% but less than 100%) styrene polymer.
Preferred embodiment according to the present invention, preferably on the surface of described Porous-Organic solid with such as any one or the multiple active function groups that are selected from hydroxyl, primary amino, secondary amino group, sulfonic group, carboxyl, amide group, the mono-substituted amide group of N-, sulfoamido, the mono-substituted sulfoamido of N-, sulfydryl, acylimino and the hydrazide group, at least a in preferred carboxyl and the hydroxyl wherein.
According to an embodiment of the invention, before use described Porous-Organic solid is carried out thermal activation treatment.This thermal activation treatment can be carried out according to common mode, such as under reduced pressure or under the inert atmosphere described Porous-Organic solid being carried out heat treated.Here said inert atmosphere refer to only contain in the gas extremely trace or do not contain can with the component of described Porous-Organic solid reaction.As described inert atmosphere, such as enumerating nitrogen or rare gas atmosphere, preferred nitrogen atmosphere.Because the poor heat resistance of Porous-Organic solid, thus this thermal activation process with the structure of not destroying described Porous-Organic solid itself with basic composition is prerequisite.Usually, the temperature of this thermal activation is 50~400 ℃, preferred 100~250 ℃, and the thermal activation time is 1~24h, preferred 2~12h.After the thermal activation treatment, described Porous-Organic solid need to save backup in malleation under the inert atmosphere.
As described inorganic porous solid, such as the refractory oxide that can enumerate periodic table of elements IIA, IIIA, IVA or IVB family metal (such as silicon-dioxide (being called again silicon oxide or silica gel), aluminum oxide, magnesium oxide, titanium oxide, zirconium white or Thorotrast etc.), perhaps any infusibility composite oxides of these metals (such as oxidation sial, oxidation magnalium, titanium oxide silicon, titanium oxide magnesium and titanium oxide aluminium etc.), and clay, molecular sieve (such as ZSM-5 and MCM-41), mica, polynite, wilkinite and diatomite etc.As described inorganic porous solid, can also enumerate the oxide compound that is generated by pyrohydrolysis by gaseous metal halogenide or gaseous silicon compound, such as the silica gel that is obtained by the silicon tetrachloride pyrohydrolysis, the aluminum oxide that is perhaps obtained by the aluminum chloride pyrohydrolysis etc.
As described inorganic porous solid, preferred silicon-dioxide, aluminum oxide, magnesium oxide, oxidation sial, oxidation magnalium, titanium oxide silicon, titanium dioxide, molecular sieve and polynite etc., particularly preferably silicon-dioxide.
According to the present invention, suitable silicon-dioxide can be by the ordinary method manufacturing, it perhaps can be the commerical prod that to buy arbitrarily, such as the Grace 955 that can enumerate Grace company, Grace 948, Grace SP9-351, Grace SP9-485, Grace SP9-10046, Davsion Syloid 245 and Aerosil812, the ES70 of Ineos company, ES70X, ES70Y, ES70W, ES757, EP10X and EP11, and the CS-2133 of Pq Corp. and MS-3040.
Preferred embodiment according to the present invention, preferably on the surface of described inorganic porous solid with hydroxyl isoreactivity functional group.
According to the present invention, in one embodiment, before use described inorganic porous solid is carried out thermal activation treatment.This thermal activation treatment can be carried out according to common mode, such as under reduced pressure or under the inert atmosphere described inorganic porous solid being carried out heat treated.Here said inert atmosphere refer to only contain in the gas extremely trace or do not contain can with the component of described inorganic porous solid reaction.As described inert atmosphere, such as enumerating nitrogen or rare gas atmosphere, preferred nitrogen atmosphere.Usually, the temperature of this thermal activation is 200-800 ℃, and preferred 400~700 ℃, most preferably 400~650 ℃, heat-up time is such as being 0.5~24h, preferred 2~12h, most preferably 4~8h.After the thermal activation treatment, described inorganic porous solid need to save backup in malleation under the inert atmosphere.
According to the present invention, there is no particular limitation to the surface-area of described porous support, but be generally 10~1000m
2/ g (BET method mensuration), preferred 100~600m
2/ g; The pore volume of this porous support (determination of nitrogen adsorption) is generally 0.1~4cm
3/ g, preferred 0.2~2cm
3/ g, and preferred 1~500 μ m of its median size (laser particle analyzer mensuration), more preferably 1~100 μ m.
According to the present invention, described porous support can be form arbitrarily, such as micropowder, granular, spherical, aggregate or other form.
By making described optional porous support through thermal activation contact (mixing) with described magnesium compound solution, obtain thus the first mixed serum.
According to the present invention, the mixing process of described porous support and described magnesium compound solution can adopt usual method to carry out, and there is no particular limitation.Such as enumerating, at normal temperature to the preparation temperature of described magnesium compound solution, in described magnesium compound solution, be metered into described porous support, perhaps in described porous support, be metered into described magnesium compound solution, mix 0.1~8h, preferred 0.5~4h, optimum 1~2h (in case of necessity by stirring) gets final product.
According to the present invention, consumption as described porous support, so that the mass ratio of described magnesium compound (in the magnesium compound solid that contains in the described magnesium compound solution) and described porous support reaches 1: 0.1-20, preferred 1: 0.5-10, more preferably 1: 1-5.
At this moment, the first mixed serum that obtains is a kind of system of pulpous state.Although unessential, in order to ensure the homogeneity of system, this mixed serum preferably carries out certain hour (2~48h, preferred 4~24h, most preferably 6~18h) airtight leaving standstill afterwards in preparation.
According to the present invention, term " Nonmetallocene title complex " is a kind of single site olefin polymerization catalysts for metallocene catalyst, do not contain the cyclopentadienyl or derivatives thereofs such as luxuriant ring, fluorenes ring or indenes ring in the structure, and with promotor (such as hereinafter described those) combination the time, can demonstrate the organometallics (therefore described Nonmetallocene title complex is also sometimes referred to as the non-metallocene olefin polymerization title complex) of olefinic polymerization catalysis activity.This compound comprises the polydentate ligand (preferably tridentate ligand or more polydentate ligand) that central metal atom and at least one and described central metal atom are combined with coordinate bond, and term " Nonmetallocene part " is aforesaid polydentate ligand.
According to the present invention, described Nonmetallocene title complex is selected from the compound with following chemical structural formula:
According to this chemical structural formula, the part that forms coordinate bond with central metal atom M comprises n radicals X and m polydentate ligand (structural formula in the bracket).According to the chemical structural formula of described polydentate ligand, group A, D and E (coordination group) form coordinate bond with atom (such as heteroatomss such as N, O, S, Se and P) with described central metal atom M by the contained coordination of these groups.
According to the present invention, all parts (comprising described radicals X and described polydentate ligand) with the positively charged absolute value of absolute value and the described central metal atom M of negative charge sum identical.
At one more specifically in the embodiment, described Nonmetallocene title complex is selected from compound (A) and the compound (B) with following chemical structural formula.
At one more specifically in the embodiment, described Nonmetallocene title complex be selected from compound (A-1) with following chemical structural formula to compound (A-4) and compound (B-1) to compound (B-4).
In above all chemical structural formulas,
Q is 0 or 1;
D is 0 or 1;
M is 1,2 or 3;
M is selected from periodic table of elements III-th family to XI family atoms metal, preferred IVB family atoms metal is such as enumerating Ti (IV), Zr (IV), Hf (IV), Cr (III), Fe (III), Ni (II), Pd (II) or Co (II);
N is 1,2,3 or 4, depends on the valence state of described central metal atom M;
X is selected from halogen, hydrogen atom, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl, oxy radical, nitrogen-containing group, sulfur-containing group, boron-containing group, contain aluminium base group, phosphorus-containing groups, silicon-containing group, germanic group or contain tin group, a plurality of X can be identical, also can be different, and can also be each other in key or Cheng Huan;
A be selected from Sauerstoffatom, sulphur atom, selenium atom,
-NR
23R
24,-N (O) R
25R
26,
-PR
28R
29,-P (O) R
30OR
31, sulfuryl, sulfoxide group or-Se (O) R
39, N, O, S, Se and the P coordination atom of respectively doing for oneself wherein;
B is selected from nitrogen-atoms, nitrogen-containing group, phosphorus-containing groups or C
1-C
30Alkyl;
D is selected from nitrogen-atoms, Sauerstoffatom, sulphur atom, selenium atom, phosphorus atom, nitrogen-containing group, phosphorus-containing groups, C
1-C
30Alkyl, sulfuryl, sulfoxide group,
-N (O) R
25R
26,
Or-P (O) R
32(OR
33), N, O, S, Se and the P coordination atom of respectively doing for oneself wherein;
E is selected from nitrogen-containing group, oxy radical, sulfur-containing group, contains seleno group, phosphorus-containing groups or cyano group (CN), N, O, S, Se and the P coordination atom of respectively doing for oneself wherein;
F is selected from nitrogen-atoms, nitrogen-containing group, oxy radical, sulfur-containing group, contain seleno group or phosphorus-containing groups, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
G is selected from C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or safing function group;
Y is selected from Sauerstoffatom, nitrogen-containing group, oxy radical, sulfur-containing group, contain seleno group or phosphorus-containing groups, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
Z is selected from nitrogen-containing group, oxy radical, sulfur-containing group, contains seleno group, phosphorus-containing groups or cyano group (CN), such as enumerating-NR
23R
24,-N (O) R
25R
26,-PR
28R
29,-P (O) R
30R
31,-OR
34,-SR
35,-S (O) R
36,-SeR
38Or-Se (O) R
39, N, O, S, Se and the P coordination atom of respectively doing for oneself wherein;
→ represent singly-bound or two key;
-represent covalent linkage or ionic linkage;
---represent coordinate bond, covalent linkage or ionic linkage.
R
1To R
4, R
6To R
36, R
38And R
39Be selected from independently of one another hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl (preferred halo alkyl wherein, such as-CH
2Cl and-CH
2CH
2Cl) or the safing function group.Above-mentioned group can be the same or different to each other, and wherein adjacent group is such as R
1With R
2, R
6With R
7, R
7With R
8, R
8With R
9, R
13With R
14, R
14With R
15, R
15With R
16, R
18With R
19, R
19With R
20, R
20With R
21, R
23With R
24, perhaps R
25With R
26Deng combining togather into key or Cheng Huan, be preferably formed aromatic ring, such as unsubstituted phenyl ring or by 1-4 C
1-C
30The C of alkyl, replacement
1-C
30Alkyl (preferred halo alkyl wherein, such as-CH
2Cl and-CH
2CH
2Cl) or the phenyl ring that replaces of safing function group, and
R
5Be selected from lone-pair electron on the nitrogen, hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl, oxy radical, sulfur-containing group, nitrogen-containing group, contain seleno group or phosphorus-containing groups.Work as R
5For oxy radical, sulfur-containing group, nitrogen-containing group, when containing seleno group or phosphorus-containing groups, R
5In N, O, S, P and Se can be used as coordination and carry out coordination with atom and described center IVB family atoms metal.
According to the present invention, in aforementioned all chemical structural formulas, as the case may be, any adjacent two or more groups are such as R
21With group Z, perhaps R
13With group Y, can combine togather into ring, be preferably formed and comprise the heteroatomic C that comes from described group Z or Y
6-C
30Heteroaromatic, such as pyridine ring etc., wherein said heteroaromatic is optional to be selected from C by one or more
1-C
30The C of alkyl, replacement
1-C
30The substituting group of alkyl and safing function group replaces.
In the context of the present invention, described halogen is selected from F, Cl, Br or I.Described nitrogen-containing group is selected from
-NR
23R
24,-T-NR
23R
24Or-N (O) R
25R
26Described phosphorus-containing groups is selected from
-PR
28R
29,-P (O) R
30R
31Or-P (O) R
32(OR
33).Described oxy radical be selected from hydroxyl ,-OR
34With-T-OR
34Described sulfur-containing group is selected from-SR
35,-T-SR
35,-S (O) R
36Or-T-SO
2R
37The described seleno group that contains is selected from-SeR
38,-T-SeR
38,-Se (O) R
39Or-T-Se (O) R
39Described group T is selected from C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or safing function group.Described R
37Be selected from hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or safing function group.
In the context of the present invention, described C
1-C
30Alkyl is selected from C
1-C
30Alkyl (preferred C
1-C
6Alkyl is such as isobutyl-), C
7-C
50Alkaryl (such as tolyl, xylyl, diisobutyl phenyl etc.), C
7-C
50Aralkyl (such as benzyl), C
3-C
30Cyclic alkyl, C
2-C
30Thiazolinyl, C
2-C
30Alkynyl, C
6-C
30Aryl (such as phenyl, naphthyl, anthryl etc.), C
8-C
30Condensed ring radical or C
4-C
30Heterocyclic radical, wherein said heterocyclic radical contain 1-3 heteroatoms that is selected from nitrogen-atoms, Sauerstoffatom or sulphur atom, such as pyridyl, pyrryl, furyl or thienyl etc.
According to the present invention, in the context of the present invention, according to the particular case of the relevant group of its combination, described C
1-C
30Alkyl refers to C sometimes
1-C
30(divalent group perhaps is called C to hydrocarbon two bases
1-C
30Alkylene) or C
1-C
30Hydrocarbon three bases (trivalent group), this is obvious to those skilled in the art.
In the context of the present invention, the C of described replacement
1-C
30Alkyl refers to the aforementioned C with one or more inert substituents
1-C
30Alkyl.So-called inert substituent refers to these substituting groups aforementioned coordination (is referred to aforementioned group A, D, E, F, Y and Z, perhaps also chooses wantonly and comprise radicals R with group
5) there is not substantial interference with the coordination process of described central metal atom M; In other words, limit by the chemical structure of polydentate ligand of the present invention, these substituting groups do not have ability or have no chance (such as the impact that is subject to steric hindrance etc.) coordination reaction occurs and form coordinate bond with described central metal atom M.Generally speaking, described inert substituent is such as being selected from aforesaid halogen or C
1-C
30Alkyl (preferred C
1-C
6Alkyl is such as isobutyl-).
In the context of the present invention, described safing function group does not comprise aforesaid C
1-C
30The C of alkyl and aforesaid replacement
1-C
30Alkyl.As described safing function group, be selected from aforementioned halogen, aforementioned oxy radical, aforementioned nitrogen-containing group, silicon-containing group, germanic group, aforementioned sulfur-containing group such as enumerating, contain tin group, C
1-C
10Ester group or nitro (NO
2) at least a etc.
In the context of the present invention, limit by the chemical structure of polydentate ligand of the present invention, described safing function group has following characteristics:
(1) do not disturb the coordination process of described group A, D, E, F, Y or Z and described central metal atom M, and
(2) coordination ability with described central metal atom M is lower than described A, D, E, F, Y and Z group, and does not replace the existing coordination of these groups and described central metal atom M.
In the context of the present invention, described boron-containing group is selected from BF
4 -, (C
6F
5)
4B
-Or (R
40BAr
3)
-Describedly contain aluminium base group and be selected from aluminum alkyls, AlPh
4 -, AlF
4 -, AlCl
4 -, AlBr
4 -, AlI
4 -Or R
41AlAr
3 -Described silicon-containing group is selected from-SiR
42R
43R
44Or-T-SiR
45Described germanic group is selected from-GeR
46R
47R
48Or-T-GeR
49Describedly contain tin group and be selected from-SnR
50R
51R
52,-T-SnR
53Or-T-Sn (O) R
54, wherein Ar represents C
6-C
30Aryl.R
40To R
54Be selected from independently of one another hydrogen, aforesaid C
1-C
30The C of alkyl, aforesaid replacement
1-CX alkyl or aforesaid safing function group, above-mentioned group can be the same or different to each other, and wherein adjacent group can combine togather into key or Cheng Huan.Wherein, the definition of group T is the same.
As described Nonmetallocene title complex, such as enumerating following compound:
Described Nonmetallocene title complex is preferably selected from following compound:
Described Nonmetallocene title complex further is preferably selected from following compound:
Described Nonmetallocene title complex more preferably is selected from following compound:
These Nonmetallocene title complexs can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
According to the present invention, the described polydentate ligand in the described Nonmetallocene title complex is not as the normally used diether compounds of electronic donor compound capable in this area.
Described Nonmetallocene title complex or described polydentate ligand can be made according to any method well known by persons skilled in the art.About the particular content of its manufacture method, such as can be referring to WO03/010207 and Chinese patent ZL01126323.7 and ZL02110844.7 etc., the full text that this specification sheets is introduced these documents at this point as a reference.
According to the present invention, in order to measure with easy to operate, described Nonmetallocene title complex uses with the form of solution where necessary.
The preparation described Nonmetallocene title complex solution the time, to this moment used solvent there is no particular limitation, as long as can dissolve described Nonmetallocene title complex.As described solvent, such as can with described solvent phase be used to dissolving described magnesium compound with solvent etc.Wherein, preferred C
6-12Aromatic hydrocarbon and tetrahydrofuran (THF).
These solvents can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
When the described Nonmetallocene title complex of dissolving, can use as required stirring (rotating speed of this stirring is generally 10~500 rev/mins).
According to the present invention, be easily, described Nonmetallocene title complex is generally 0.01~0.25 grams per milliliter with respect to the ratio of described solvent, preferred 0.05~0.16 grams per milliliter, but sometimes be not limited to this.
According to the present invention, described Nonmetallocene title complex (or its solution) is contacted with described the first mixed serum, can obtain the second mixed serum.
When making described the second mixed serum, there is no particular limitation to the way of contact of described the first mixed serum and described Nonmetallocene title complex, directly measure the mode of adding described Nonmetallocene title complex such as enumerating in described the first mixed serum, perhaps with described Nonmetallocene title complex according to the aforementioned solution that is mixed with in advance, the mode etc. that then this complex solution is added in metering in described the first mixed serum.
In addition, in order to make described the second mixed serum, such as can be at normal temperature to the temperature of the boiling point that is lower than employed any solvent, make the contact reacts of described the first mixed serum and described Nonmetallocene title complex carry out 0.1~8h, preferred 0.5~4h, optimum 1~2h (in case of necessity by stirring) gets final product.
At this moment, the mixed serum that obtains is a kind of system of pulpous state.Although unessential, in order to ensure the homogeneity of system, this mixed serum preferably carries out certain hour (2~48h, preferred 4~24h, most preferably 6~18h) airtight leaving standstill afterwards in preparation.
Then, by in described the second mixed serum, being metered into precipitation agent, solid matter is precipitated out from this mixed serum, namely obtains to modify complex carrier.
Below described precipitation agent is carried out specific description.
According to the present invention, term " precipitation agent " uses the common concept in this area, refers to can reduce the solubleness of solid substance solute (such as described magnesium compound, porous support Nonmetallocene part or Nonmetallocene title complex etc.) in its solution also and then the unreactiveness liquid that it is separated out with solid form from described solution.
According to the present invention, as described precipitation agent, for solid substance solute to be precipitated (such as described magnesium compound, porous support Nonmetallocene part or Nonmetallocene title complex etc.), be poor solvent such as enumerating, and for the described solvent that is used for dissolving described solid substance solute (such as magnesium compound), be the solvent of good solvent, such as enumerating C
5-12Alkane, C
5-12Naphthenic hydrocarbon, halo C
1-10Alkane and halo C
5-12Naphthenic hydrocarbon.
As described C
5-12Alkane, such as enumerating pentane, hexane, heptane, octane, nonane and decane etc., wherein preferred hexane, heptane and decane, most preferably hexane.
As described C
5-12Naphthenic hydrocarbon is such as enumerating hexanaphthene, pentamethylene, suberane, cyclodecane and cyclononane etc., most preferably hexanaphthene.
As described halo C
1-10Alkane is such as enumerating methylene dichloride, dichloro hexane, two chloroheptanes, trichloromethane, trichloroethane, three chlorobutanes, methylene bromide, ethylene dibromide, dibromo-heptane, methenyl bromide, tribromoethane and three n-butyl bromide etc.
As described halo C
5-12Naphthenic hydrocarbon is such as enumerating chlorocyclopentane, chlorocyclohexane, chloro suberane, chloro cyclooctane, chloro cyclononane, chloro cyclodecane, bromocyclopentane, bromocyclohexane, bromo suberane, bromo cyclooctane, bromo cyclononane and bromo cyclodecane etc.
These precipitation agents can be used alone, and also can use with the arbitrarily multiple mixing of ratio.
The adding mode of precipitation agent can be disposable adding or dropping, preferred disposable adding.In this precipitation process, can utilize to stir to promote the dispersion of precipitation agent, and be conducive to the final precipitation of solid product.This stirring can be adopted any form (such as stirring rake), and rotating speed is generally 10~1000 rev/mins etc.
Also there is no particular limitation to the temperature of described precipitation agent, but general preferred normal temperature is to the temperature (preferred 20-80 ℃, more preferably 40-60 ℃) of the boiling point that is lower than employed any solvent and precipitation agent, but sometimes be not limited to this.And, this precipitation process general also preferably at normal temperature to (preferred 20-80 ℃ of the temperature of the boiling point that is lower than employed any solvent and precipitation agent, more preferably 40-60 ℃) under carry out 0.3-12 hour, but sometimes be not limited to this, and with solid product basically fully precipitation be as the criterion.
Fully after the precipitation, the solid product that obtains is filtered, washs and drying, obtain thus described modification complex carrier.
For described filtration, washing and the dry not special restriction of method, can use as required conventional those that use in this area.As required, described washing is generally carried out 1~6 time, preferred 3~4 times.Wherein, washer solvent preferably uses the solvent identical with precipitation agent, but also can be different.Described drying can adopt ordinary method to carry out, such as heat drying method under rare gas element desiccating method, boulton process or the vacuum, and heat drying method, most preferably heat drying method under the vacuum under preferred rare gas element desiccating method or the vacuum.The temperature range of described drying is generally normal temperature to 140 ℃.Be generally 2-20 hour time of drying, but also can be different according to the solvent situation for dissolving described magnesium compound of concrete use.Such as, when adopting the tetrahydrofuran (THF) conduct to be used for dissolving the solvent of described magnesium compound, drying temperature is generally about 80 ℃, under vacuum, got final product in dry 2~12 hours, and when adopting the toluene conduct to be used for dissolving the solvent of described magnesium compound, drying temperature is generally about 100 ℃, gets final product in dry 4~24 hours under vacuum.
Below described chemical processing agent is carried out specific description.
According to the present invention, with IVB family metallic compound as described chemical processing agent.
As described IVB family metallic compound, to be selected from IVB family metal halide, IVB family metal alkyl compound, IVB family metal alkoxide compound, IVB family metal alkyl halides and IVB family metal alkoxide halid at least a such as enumerating.
As described IVB family metal halide, described IVB family metal alkyl compound, described IVB family metal alkoxide compound, described IVB family's metal alkyl halides and described IVB family metal alkoxide halogenide, such as the compound that can enumerate following general formula (IV) structure:
M(OR
1)
mX
nR
2 4-m-n (IV)
Wherein:
M is 0,1,2,3 or 4;
N is 0,1,2,3 or 4;
M is IVB family metal in the periodic table of elements, such as titanium, zirconium and hafnium etc.;
X is halogen, such as F, Cl, Br and I etc.; And
R
1And R
2Be selected from independently of one another C
1-10Alkyl is such as methyl, ethyl, propyl group, normal-butyl, isobutyl-etc., R
1And R
2Can be identical, also can be different.
Particularly, as described IVB family metal halide, such as enumerating titanium tetrafluoride (TiF
4), titanium tetrachloride (TiCl
4), titanium tetrabromide (TiBr
4), titanium tetra iodide (TiI
4);
Zirconium tetrafluoride (ZrF
4), zirconium tetrachloride (ZrCl
4), tetrabormated zirconium (ZrBr
4), zirconium tetraiodide (ZrI
4);
Tetrafluoride hafnium (HfF
4), hafnium tetrachloride (HfCl
4), hafnium (HfBr
4), tetraiodide hafnium (HfI
4).
As described IVB family metal alkyl compound, such as enumerating tetramethyl-titanium (Ti (CH
3)
4), tetraethyl-titanium (Ti (CH
3CH
2)
4), four isobutyl-titanium (Ti (i-C
4H
9)
4), tetra-n-butyl titanium (Ti (C
4H
9)
4), triethyl methyltitanium (Ti (CH
3) (CH
3CH
2)
3), diethyl-dimethyl titanium (Ti (CH
3)
2(CH
3CH
2)
2), trimethylammonium ethyl titanium (Ti (CH
3)
3(CH
3CH
2)), triisobutyl methyltitanium (Ti (CH
3) (i-C
4H
9)
3), diisobutyl dimethyl titanium (Ti (CH
3)
2(i-C
4H
9)
2), trimethylammonium isobutyl-titanium (Ti (CH
3)
3(i-C
4H
9)), triisobutyl ethyl titanium (Ti (CH
3CH
2) (i-C
4H
9)
3), diisobutyl diethyl titanium (Ti (CH
3CH
2)
2(i-C
4H
9)
2), triethyl isobutyl-titanium (Ti (CH
3CH
2)
3(i-C
4H
9)), three normal-butyl methyltitanium (Ti (CH
3) (C
4H
9)
3), di-n-butyl dimethyl titanium (Ti (CH
3)
2(C
4H
9)
2), trimethylammonium normal-butyl titanium (Ti (CH
3)
3(C
4H
9)), three normal-butyl methyltitanium (Ti (CH
3CH
2) (C
4H
9)
3), di-n-butyl diethyl titanium (Ti (CH
3CH
2)
2(C
4H
9)
2), triethyl normal-butyl titanium (Ti (CH
3CH
2)
3(C
4H
9)) etc.;
Tetramethyl-zirconium (Zr (CH
3)
4), tetraethyl-zirconium (Zr (CH
3CH
2)
4), four isobutyl-zirconium (Zr (i-C
4H
9)
4), tetra-n-butyl zirconium (Zr (C
4H
9)
4), triethyl methylcyclopentadienyl zirconium (Zr (CH
3) (CH
3CH
2)
3), diethyl-dimethyl zirconium (Zr (CH
3)
2(CH
3CH
2)
2), trimethylammonium ethyl zirconium (Zr (CH
3)
3(CH
3CH
2)), triisobutyl methylcyclopentadienyl zirconium (Zr (CH
3) (i-C
4H
9)
3), diisobutyl zirconium dimethyl (Zr (CH
3)
2(i-C
4H
9)
2), trimethylammonium isobutyl-zirconium (Zr (CH
3)
3(i-C
4H
9)), triisobutyl ethyl zirconium (Zr (CH
3CH
2) (i-C
4H
9)
3), diisobutyl diethyl zirconium (Zr (CH
3CH
2)
2(i-C
4H
9)
2), triethyl isobutyl-zirconium (Zr (CH
3CH
2)
3(i-C
4H
9)), three normal-butyl methylcyclopentadienyl zirconium (Zr (CH
3) (C
4H
9)
3), di-n-butyl zirconium dimethyl (Zr (CH
3)
2(C
4H
9)
2), trimethylammonium normal-butyl zirconium (Zr (CH
3)
3(C
4H
9)), three normal-butyl methylcyclopentadienyl zirconium (Zr (CH
3CH
2) (C
4H
9)
3), di-n-butyl diethyl zirconium (Zr (CH
3CH
2)
2(C
4H
9)
2), triethyl normal-butyl zirconium (Zr (CH
3CH
2)
3(C
4H
9)) etc.;
Tetramethyl-hafnium (Hf (CH
3)
4), tetraethyl-hafnium (Hf (CH
3CH
2)
4), four isobutyl-hafnium (Hf (i-C
4H
9)
4), tetra-n-butyl hafnium (Hf (C
4H
9)
4), triethyl methylcyclopentadienyl hafnium (Hf (CH
3) (CH
3CH
2)
3), diethyl-dimethyl hafnium (Hf (CH
3)
2(CH
3CH
2)
2), trimethylammonium ethyl hafnium (Hf (CH
3)
3(CH
3CH
2)), triisobutyl methylcyclopentadienyl hafnium (Hf (CH
3) (i-C
4H
9)
3), diisobutyl dimethyl hafnium (Hf (CH
3)
2(i-C
4H
9)
2), trimethylammonium isobutyl-hafnium (Hf (CH
3)
3(i-C
4H
9)), triisobutyl ethyl hafnium (Hf (CH
3CH
2) (i-C
4H
9)
3), diisobutyl diethyl hafnium (Hf (CH
3CH
2)
2(i-C
4H
9)
2), triethyl isobutyl-hafnium (Hf (CH
3CH
2)
3(i-C
4H
9)), three normal-butyl methylcyclopentadienyl hafnium (Hf (CH
3) (C
4H
9)
3), di-n-butyl dimethyl hafnium (Hf (CH
3)
2(C
4H
9)
2), trimethylammonium normal-butyl hafnium (Hf (CH
3)
3(C
4H
9)), three normal-butyl methylcyclopentadienyl hafnium (Hf (CH
3CH
2) (C
4H
9)
3), di-n-butyl diethyl hafnium (Hf (CH
3CH
2)
2(C
4H
9)
2), triethyl normal-butyl hafnium (Hf (CH
3CH
2)
3(C
4H
9)) etc.
As described IVB family metal alkoxide compound, such as enumerating tetramethoxy titanium (Ti (OCH
3)
4), purity titanium tetraethoxide (Ti (OCH
3CH
2)
4), four isobutoxy titanium (Ti (i-OC
4H
9)
4), four titanium n-butoxide (Ti (OC
4H
9)
4), triethoxy methoxyl group titanium (Ti (OCH
3) (OCH
3CH
2)
3), diethoxy dimethoxy titanium (Ti (OCH
3)
2(OCH
3CH
2)
2), trimethoxy ethanolato-titanium (Ti (OCH
3)
3(OCH
3CH
2)), three isobutoxy methoxyl group titanium (Ti (OCH
3) (i-OC
4H
9)
3), two isobutoxy dimethoxy titanium (Ti (OCH
3)
2(i-OC
4H
9)
2), trimethoxy isobutoxy titanium (Ti (OCH
3)
3(i-OC
4H
9)), three isobutoxy ethanolato-titanium (Ti (OCH
3CH
2) (i-OC
4H
9)
3), two isobutoxy diethoxy titanium (Ti (OCH
3CH
2)
2(i-OC
4H
9)
2), triethoxy isobutoxy titanium (Ti (OCH
3CH
2)
3(i-OC
4H
9)), three n-butoxy methoxyl group titanium (Ti (OCH
3) (OC
4H
9)
3), two n-butoxy dimethoxy titanium (Ti (OCH
3)
2(OC
4H
9)
2), trimethoxy titanium n-butoxide (Ti (OCH
3)
3(OC
4H
9)), three n-butoxy methoxyl group titanium (Ti (OCH
3CH
2) (OC
4H
9)
3), two n-butoxy diethoxy titanium (Ti (OCH
3CH
2)
2(OC
4H
9)
2), triethoxy titanium n-butoxide (Ti (OCH
3CH
2)
3(OC
4H
9)) etc.;
Tetramethoxy zirconium (Zr (OCH
3)
4), tetraethoxy zirconium (Zr (OCH
3CH
2)
4), four isobutoxy zirconium (Zr (i-OC
4H
9)
4), four n-butoxy zirconium (Zr (OC
4H
9)
4), triethoxy methoxyl group zirconium (Zr (OCH
3) (OCH
3CH
2)
3), diethoxy dimethoxy zirconium (Zr (OCH
3)
2(OCH
3CH
2)
2), trimethoxy oxyethyl group zirconium (Zr (OCH
3)
3(OCH
3CH
2)), three isobutoxy methoxyl group zirconium (Zr (OCH
3) (i-OC
4H
9)
3), two isobutoxy dimethoxy zirconium (Zr (OCH
3)
2(i-OC
4H
9)
2), trimethoxy isobutoxy zirconium (Zr (OCH
3)
3(i-C
4H
9)), three isobutoxy oxyethyl group zirconium (Zr (OCH
3CH
2) (i-OC
4H
9)
3), two isobutoxy diethoxy zirconium (Zr (OCH
3CH
2)
2(i-OC
4H
9)
2), triethoxy isobutoxy zirconium (Zr (OCH
3CH
2)
3(i-OC
4H
9)), three n-butoxy methoxyl group zirconium (Zr (OCH
3) (OC
4H
9)
3), two n-butoxy dimethoxy zirconium (Zr (OCH
3)
2(OC
4H
9)
2), trimethoxy n-butoxy zirconium (Zr (OCH
3)
3(OC
4H
9)), three n-butoxy methoxyl group zirconium (Zr (OCH
3CH
2) (OC
4H
9)
3), two n-butoxy diethoxy zirconium (Zr (OCH
3CH
2)
2(OC
4H
9)
2), triethoxy n-butoxy zirconium (Zr (OCH
3CH
2)
3(OC
4H
9)) etc.;
Tetramethoxy hafnium (Hf (OCH
3)
4), tetraethoxy hafnium (Hf (OCH
3CH
2)
4), four isobutoxy hafnium (Hf (i-OC
4H
9)
4), four n-butoxy hafnium (Hf (OC
4H
9)
4), triethoxy methoxyl group hafnium (Hf (OCH
3) (OCH
3CH
2)
3), diethoxy dimethoxy hafnium (Hf (OCH
3)
2(OCH
3CH
2)
2), trimethoxy oxyethyl group hafnium (Hf (OCH
3)
3(OCH
3CH
2)), three isobutoxy methoxyl group hafnium (Hf (OCH
3) (i-OC
4H
9)
3), two isobutoxy dimethoxy hafnium (Hf (OCH
3)
2(i-OC
4H
9)
2), trimethoxy isobutoxy hafnium (Hf (OCH
3)
3(i-OC
4H
9)), three isobutoxy oxyethyl group hafnium (Hf (OCH
3CH
2) (i-OC
4H
9)
3), two isobutoxy diethoxy hafnium (Hf (OCH
3CH
2)
2(i-OC
4H
9)
2), triethoxy isobutoxy hafnium (Hf (OCH
3CH
2)
3(i-C
4H
9)), three n-butoxy methoxyl group hafnium (Hf (OCH
3) (OC
4H
9)
3), two n-butoxy dimethoxy hafnium (Hf (OCH
3)
2(OC
4H
9)
2), trimethoxy n-butoxy hafnium (Hf (OCH
3)
3(OC
4H
9)), three n-butoxy methoxyl group hafnium (Hf (OCH
3CH
2) (OC
4H
9)
3), two n-butoxy diethoxy hafnium (Hf (OCH
3CH
2)
2(OC
4H
9)
2), triethoxy n-butoxy hafnium (Hf (OCH
3CH
2)
3(OC
4H
9)) etc.
As described IVB family metal alkyl halides, such as enumerating trimethylammonium titanium chloride (TiCl (CH
3)
3), triethyl titanium chloride (TiCl (CH
3CH
2)
3), triisobutyl titanium chloride (TiCl (i-C
4H
9)
3), three normal-butyl chlorination titanium (TiCl (C
4H
9)
3), dimethyl titanium dichloride (TiCl
2(CH
3)
2), diethyl titanium dichloride (TiCl
2(CH
3CH
2)
2), diisobutyl titanium dichloride (TiCl
2(i-C
4H
9)
2), three normal-butyl chlorination titanium (TiCl (C
4H
9)
3), methyl titanous chloride (Ti (CH
3) Cl
3), ethyl titanous chloride (Ti (CH
3CH
2) Cl
3), isobutyl-titanous chloride (Ti (i-C
4H
9) Cl
3), normal-butyl titanous chloride (Ti (C
4H
9) Cl
3);
Trimethylammonium titanium bromide (TiBr (CH
3)
3), triethyl titanium bromide (TiBr (CH
3CH
2)
3), triisobutyl titanium bromide (TiBr (i-C
4H
9)
3), three normal-butyl bromination titanium (TiBr (C
4H
9)
3), dimethyl dibrominated titanium (TiBr
2(CH
3)
2), diethyl dibrominated titanium (TiBr
2(CH
3CH
2)
2), diisobutyl dibrominated titanium (TiBr
2(i-C
4H
9)
2), three normal-butyl bromination titanium (TiBr (C
4H
9)
3), methyl titanium tribromide (Ti (CH
3) Br
3), ethyl titanium tribromide (Ti (CH
3CH
2) Br
3), isobutyl-titanium tribromide (Ti (i-C
4H
9) Br
3), normal-butyl titanium tribromide (Ti (C
4H
9) Br
3);
Trimethylammonium zirconium chloride (ZrCl (CH
3)
3), triethyl zirconium chloride (ZrCl (CH
3CH
2)
3), triisobutyl zirconium chloride (ZrCl (i-C
4H
9)
3), three normal-butyl chlorination zirconium (ZrCl (C
4H
9)
3), dimethyl zirconium dichloride (ZrCl
2(CH
3)
2), diethyl zirconium dichloride (ZrCl
2(CH
3CH
2)
2), diisobutyl zirconium dichloride (ZrCl
2(i-C
4H
9)
2), three normal-butyl chlorination zirconium (ZrCl (C
4H
9)
3), methyl tri-chlorination zirconium (Zr (CH
3) Cl
3), ethyl tri-chlorination zirconium (Zr (CH
3CH
2) Cl
3), isobutyl-tri-chlorination zirconium (Zr (i-C
4H
9) Cl
3), normal-butyl tri-chlorination zirconium (Zr (C
4H
9) Cl
3);
Trimethylammonium zirconium bromide (ZrBr (CH
3)
3), triethyl zirconium bromide (ZrBr (CH
3CH
2)
3), triisobutyl zirconium bromide (ZrBr (i-C
4H
9)
3), three normal-butyl bromination zirconium (ZrBr (C
4H
9)
3), dimethyl dibrominated zirconium (ZrBr
2(CH
3)
2), diethyl dibrominated zirconium (ZrBr
2(CH
3CH
2)
2), diisobutyl dibrominated zirconium (ZrBr
2(i-C
4H
9)
2), three normal-butyl bromination zirconium (ZrBr (C
4H
9)
3), methyl tribromide zirconium (Zr (CH
3) Br
3), ethyl tribromide zirconium (Zr (CH
3CH
2) Br
3), isobutyl-tribromide zirconium (Zr (i-C
4H
9) Br
3), normal-butyl tribromide zirconium (Zr (C
4H
9) Br
3);
Trimethylammonium hafnium chloride (HfCl (CH
3)
3), triethyl hafnium chloride (HfCl (CH
3CH
2)
3), triisobutyl hafnium chloride (HfCl (i-C
4H
9)
3), three normal-butyl chlorination hafnium (HfCl (C
4H
9)
3), dimethyl hafnium dichloride (HfCl
2(CH
3)
2), diethyl hafnium dichloride (HfCl
2(CH
3CH
2)
2), diisobutyl hafnium dichloride (HfCl
2(i-C
4H
9)
2), three normal-butyl chlorination hafnium (HfCl (C
4H
9)
3), methyl tri-chlorination hafnium (Hf (CH
3) Cl
3), ethyl tri-chlorination hafnium (Hf (CH
3CH
2) Cl
3), isobutyl-tri-chlorination hafnium (Hf (i-C
4H
9) Cl
3), normal-butyl tri-chlorination hafnium (Hf (C
4H
9) Cl
3);
Trimethylammonium bromination hafnium (HfBr (CH
3)
3), triethyl bromination hafnium (HfBr (CH
3CH
2)
3), triisobutyl bromination hafnium (HfBr (i-C
4H
9)
3), three normal-butyl bromination hafnium (HfBr (C
4H
9)
3), dimethyl dibrominated hafnium (HfBr
2(CH
3)
2), diethyl dibrominated hafnium (HfBr
2(CH
3CH
2)
2), diisobutyl dibrominated hafnium (HfBr
2(i-C
4H
9)
2), three normal-butyl bromination hafnium (HfBr (C
4H
9)
3), methyl tribromide hafnium (Hf (CH
3) Br
3), ethyl tribromide hafnium (Hf (CH
3CH
2) Br
3), isobutyl-tribromide hafnium (Hf (i-C
4H
9) Br
3), normal-butyl tribromide hafnium (Hf (C
4H
9) Br
3).
As described IVB family metal alkoxide halogenide, such as enumerating trimethoxy titanium chloride (TiCl (OCH
3)
3), triethoxy titanium chloride (TiCl (OCH
3CH
2)
3), three isobutoxy titanium chloride (TiCl (i-OC
4H
9)
3), three n-Butoxyl titanium-chlorides (TiCl (OC
4H
9)
3), dimethoxy titanium dichloride (TiCl
2(OCH
3)
2), diethoxy titanium dichloride (TiCl
2(OCH
3CH
2)
2), two isobutoxy titanium dichloride (TiCl
2(i-OC
4H
9)
2), three n-Butoxyl titanium-chlorides (TiCl (OC
4H
9)
3), methoxyl group titanous chloride (Ti (OCH
3) Cl
3), oxyethyl group titanous chloride (Ti (OCH
3CH
2) Cl
3), isobutoxy titanous chloride (Ti (i-C
4H
9) Cl
3), n-butoxy titanous chloride (Ti (OC
4H
9) Cl
3);
Trimethoxy titanium bromide (TiBr (OCH
3)
3), triethoxy titanium bromide (TiBr (OCH
3CH
2)
3), three isobutoxy titanium bromide (TiBr (i-OC
4H
9)
3), three n-butoxy titanium bromide (TiBr (OC
4H
9)
3), dimethoxy dibrominated titanium (TiBr
2(OCH
3)
2), diethoxy dibrominated titanium (TiBr
2(OCH
3CH
2)
2), two isobutoxy dibrominated titanium (TiBr
2(i-OC
4H
9)
2), three n-butoxy titanium bromide (TiBr (OC
4H
9)
3), methoxyl group titanium tribromide (Ti (OCH
3) Br
3), oxyethyl group titanium tribromide (Ti (OCH
3CH
2) Br
3), isobutoxy titanium tribromide (Ti (i-C
4H
9) Br
3), n-butoxy titanium tribromide (Ti (OC
4H
9) Br
3);
Trimethoxy zirconium chloride (ZrCl (OCH
3)
3), triethoxy zirconium chloride (ZrCl (OCH
3CH
2)
3), three isobutoxy zirconium chloride (ZrCl (i-OC
4H
9)
3), three n-butoxy zirconium chloride (ZrCl (OC
4H
9)
3), dimethoxy zirconium dichloride (ZrCl
2(OCH
3)
2), diethoxy zirconium dichloride (ZrCl
2(OCH
3CH
2)
2), two isobutoxy zirconium dichloride (ZrCl
2(i-OC
4H
9)
2), three n-butoxy zirconium chloride (ZrCl (OC
4H
9)
3), methoxyl group tri-chlorination zirconium (Zr (OCH
3) Cl
3), oxyethyl group tri-chlorination zirconium (Zr (OCH
3CH
2) Cl
3), isobutoxy tri-chlorination zirconium (Zr (i-C
4H
9) Cl
3), n-butoxy tri-chlorination zirconium (Zr (OC
4H
9) Cl
3);
Trimethoxy zirconium bromide (ZrBr (OCH
3)
3), triethoxy zirconium bromide (ZrBr (OCH
3CH
2)
3), three isobutoxy zirconium bromide (ZrBr (i-OC
4H
9)
3), three n-butoxy zirconium bromide (ZrBr (OC
4H
9)
3), dimethoxy dibrominated zirconium (ZrBr
2(OCH
3)
2), diethoxy dibrominated zirconium (ZrBr
2(OCH
3CH
2)
2), two isobutoxy dibrominated zirconium (ZrBr
2(i-OC
4H
9)
2), three n-butoxy zirconium bromide (ZrBr (OC
4H
9)
3), methoxyl group tribromide zirconium (Zr (OCH
3) Br
3), oxyethyl group tribromide zirconium (Zr (OCH
3CH
2) Br
3), isobutoxy tribromide zirconium (Zr (i-C
4H
9) Br
3), n-butoxy tribromide zirconium (Zr (OC
4H
9) Br
3);
Trimethoxy hafnium chloride (HfCl (OCH
3)
3), triethoxy hafnium chloride (HfCl (OCH
3CH
2)
3), three isobutoxy hafnium chloride (HfCl (i-OC
4H
9)
3), three n-butoxy hafnium chloride (HfCl (OC
4H
9)
3), dimethoxy hafnium dichloride (HfCl
2(OCH
3)
2), diethoxy hafnium dichloride (HfCl
2(OCH
3CH
2)
2), two isobutoxy hafnium dichloride (HfCl
2(i-OC
4H
9)
2), three n-butoxy hafnium chloride (HfCl (OC
4H
9)
3), methoxyl group tri-chlorination hafnium (Hf (OCH
3) Cl
3), oxyethyl group tri-chlorination hafnium (Hf (OCH
3CH
2) Cl
3), isobutoxy tri-chlorination hafnium (Hf (i-C
4H
9) Cl
3), n-butoxy tri-chlorination hafnium (Hf (OC
4H
9) Cl
3);
Trimethoxy bromination hafnium (HfBr (OCH
3)
3), triethoxy bromination hafnium (HfBr (OCH
3CH
2)
3), three isobutoxy bromination hafnium (HfBr (i-OC
4H
9)
3), three n-butoxy bromination hafnium (HfBr (OC
4H
9)
3), dimethoxy dibrominated hafnium (HfBr
2(OCH
3)
2), diethoxy dibrominated hafnium (HfBr
2(OCH
3CH
2)
2), two isobutoxy dibrominated hafnium (HfBr
2(i-OC
4H
9)
2), three n-butoxy bromination hafnium (HfBr (OC
4H
9)
3), methoxyl group tribromide hafnium (Hf (OCH
3) Br
3), oxyethyl group tribromide hafnium (Hf (OCH
3CH
2) Br
3), isobutoxy tribromide hafnium (Hf (i-C
4H
9) Br
3), n-butoxy tribromide hafnium (Hf (OC
4H
9) Br
3).
As described IVB family metallic compound, preferred described IVB family metal halide, more preferably TiCl
4, TiBr
4, ZrCl
4, ZrBr
4, HfCl
4And HfBr
4, TiCl most preferably
4And ZrCl
4
These IVB family metallic compounds can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
When described chemical processing agent is liquid state at normal temperatures, can use described chemical processing agent by the mode that in the reaction object (such as aforesaid modification complex carrier) that remains to utilize this chemical processing agent to process, directly drips the described chemical processing agent of predetermined amount.
When described chemical processing agent when being solid-state at normal temperatures, for measure with easy to operate for the purpose of, preferably use described chemical processing agent with the form of solution.Certainly, when described chemical processing agent is liquid state at normal temperatures, sometimes also can use described chemical processing agent with the form of solution as required, not special the restriction.
When the solution of the described chemical processing agent of preparation, to this moment employed solvent there is no particular limitation, as long as it can dissolve this chemical processing agent.
Particularly, can enumerate C
5-12Alkane, C
5-12Naphthenic hydrocarbon, halo C
5-12Alkane, halo C
5-12Naphthenic hydrocarbon, C
6-12Aromatic hydrocarbons or halo C
6-12Aromatic hydrocarbons etc., such as enumerating pentane, hexane, heptane, octane, nonane, decane, undecane, dodecane, pentamethylene, hexanaphthene, suberane, cyclooctane, toluene, ethylbenzene, dimethylbenzene, chloro-pentane, chloro-hexane, chloro heptane, chloro octane, chloro nonane, chloro decane, chloro undecane, chlorinated dodecane, chlorocyclohexane, chlorotoluene, chloro ethylbenzene and xylene monochloride etc., wherein preferred pentane, hexane, decane, hexanaphthene and toluene, most preferably hexane and toluene.
These solvents can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
In addition, there is no particular limitation to the concentration of described chemical processing agent in its solution, can suitably select as required, as long as it can realize implementing described chemical treatment with the described chemical processing agent of predetermined amount.As previously mentioned, if chemical processing agent is liquid, can directly carry out described processing with chemical processing agent, but use after also it can being modulated into the chemical treatment agent solution.
That the volumetric molar concentration of described chemical processing agent in its solution generally is set as 0.01~1.0mol/L, but is not limited to this easily.
According to the present invention, make the reaction of described chemical processing agent and described modification complex carrier, can obtain load type non-metallocene catalyst of the present invention.
As carrying out described chemically treated method, such as enumerating, be in the situation of solid-state (such as zirconium tetrachloride) at chemical processing agent, the solution that at first prepares described chemical processing agent, then in pending reaction object (such as aforesaid modification complex carrier), add the described solution that (the preferred dropping) contains the described chemical processing agent of predetermined amount, to carry out the chemical treatment reaction.Be in the situation of liquid (such as titanium tetrachloride) at chemical processing agent, directly in described chemical processing agent adding (the preferred dropping) the described pending reaction object (such as aforesaid modification complex carrier) with predetermined amount, to carry out the chemical treatment reaction, perhaps after this chemical processing agent is prepared into solution, in described pending reaction object (such as aforesaid modification complex carrier), add the described solution that (the preferred dropping) contains the described chemical processing agent of predetermined amount, to carry out the chemical treatment reaction.
Generally speaking, chemical treatment reaction (in case of necessity by stirring) was carried out 0.5~24 hour, preferred 1~8 hour, more preferably got final product in 2~6 hours.
After the chemical treatment reaction finishes, by filtering, wash and drying, can obtain through chemically treated product, i.e. load type non-metallocene catalyst of the present invention.
According to the present invention, described filtration, washing and drying can adopt ordinary method to carry out, and wherein washer solvent can adopt used identical solvent when dissolving described chemical processing agent.As required, this washing is generally carried out 1~8 time, and preferred 2~6 times, most preferably 2~4 times.
Described drying can adopt ordinary method to carry out, such as heat drying method under rare gas element desiccating method, boulton process or the vacuum, and heat drying method, most preferably heat drying method under the vacuum under preferred rare gas element desiccating method or the vacuum.The temperature range of described drying is generally normal temperature to 140 ℃, is generally 2-20 hour time of drying, but is not limited to this.
Special embodiment according to the present invention, the preparation method of load type non-metallocene catalyst of the present invention also is included in and makes before described chemical processing agent and the reaction of described modification complex carrier, with the step that helps the described modification complex carrier of chemical processing agent pre-treatment (pre-treatment step) that is selected from aikyiaiurnirsoxan beta, aluminum alkyls or its arbitrary combination.
Below the described chemical processing agent that helps is carried out specific description.
According to the present invention, as the described chemical processing agent that helps, such as enumerating aikyiaiurnirsoxan beta and aluminum alkyls.
As described aikyiaiurnirsoxan beta, such as enumerating the line style aikyiaiurnirsoxan beta shown in the following general formula (I): (R) (R) Al-(Al (R)-O)
n-O-Al (R) (R), and the ring-type aikyiaiurnirsoxan beta shown in the following general formula (II) :-(Al (R)-O-)
N+2-.
In aforementioned formula, radicals R is same to each other or different to each other (preferably identical), is selected from independently of one another C
1-C
8Alkyl, preferable methyl, ethyl and isobutyl-, most preferable; N is the arbitrary integer in the 1-50 scope, the arbitrary integer in preferred 10~30 scopes.
As described aikyiaiurnirsoxan beta, preferable methyl aikyiaiurnirsoxan beta, ethylaluminoxane, isobutyl aluminium alkoxide and normal-butyl alumina alkane, further preferable methyl aikyiaiurnirsoxan beta and isobutyl aluminium alkoxide.
These aikyiaiurnirsoxan beta can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
As described aluminum alkyls, such as enumerating the compound shown in the following general formula:
Al(R)
3
Wherein, radicals R is same to each other or different to each other (preferably identical), and is selected from independently of one another C
1-C
8Alkyl, preferable methyl, ethyl and isobutyl-, most preferable.
Particularly, as described aluminum alkyls, such as enumerating trimethyl aluminium (Al (CH
3)
3), triethyl aluminum (Al (CH
3CH
2)
3), tri-n-n-propyl aluminum (Al (C
3H
7)
3), triisopropylaluminiuand (Al (i-C
3H
7)
3), triisobutyl aluminium (Al (i-C
4H
9)
3), three n-butylaluminum (Al (C
4H
9)
3), triisopentyl aluminium (Al (i-C
5H
11)
3), three n-pentyl aluminium (Al (C
5H
11)
3), tri-n-hexyl aluminum (Al (C
6H
13)
3), three isohexyl aluminium (Al (i-C
6H
13)
3), diethylmethyl aluminium (Al (CH
3) (CH
3CH
2)
2) and dimethyl ethyl aluminium (Al (CH
3CH
2) (CH
3)
2) etc., wherein preferred trimethyl aluminium, triethyl aluminum, tri-propyl aluminum and triisobutyl aluminium, most preferably triethyl aluminum and triisobutyl aluminium.
These aluminum alkylss can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
According to the present invention, as the described chemical processing agent that helps, can only adopt described aikyiaiurnirsoxan beta, also can only adopt described aluminum alkyls, but also can adopt any mixture of described aikyiaiurnirsoxan beta and described aluminum alkyls.And there is no particular limitation to the ratio of each component in this mixture, can select arbitrarily as required.
According to the present invention, the described chemical processing agent that helps generally is to use with the form of solution.When the described solution that helps chemical processing agent of preparation, to this moment employed solvent there is no particular limitation, as long as it can dissolve this and help chemical processing agent.
Particularly, can enumerate C
5-12Alkane, C
5-12Naphthenic hydrocarbon, halo C
5-12Alkane, halo C
5-12Naphthenic hydrocarbon, C
6-12Aromatic hydrocarbons or halo C
6-12Aromatic hydrocarbons etc., such as enumerating pentane, hexane, heptane, octane, nonane, decane, undecane, dodecane, pentamethylene, hexanaphthene, suberane, cyclooctane, toluene, ethylbenzene, dimethylbenzene, chloro-pentane, chloro-hexane, chloro heptane, chloro octane, chloro nonane, chloro decane, chloro undecane, chlorinated dodecane, chlorocyclohexane, chlorotoluene, chloro ethylbenzene and xylene monochloride etc., wherein preferred pentane, hexane, decane, hexanaphthene and toluene, most preferably hexane and toluene.
These solvents can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
It in addition, helps the concentration of chemical processing agent in its solution there is no particular limitation described, can suitably select as required, as long as can realize carrying out described pre-treatment with the described chemical processing agent that helps of predetermined amount.
Through described pre-treatment step, obtain through pretreated modification complex carrier thus.Then, according to carrying out and aforementioned same chemical treatment reaction with described chemical processing agent with aforementioned identical mode, just described modification complex carrier is replaced with the pretreated modification complex carrier of described process and get final product again.
That is, by with aforementioned same chemical treatment reaction, the chemical processing agent that is selected from described IVB family metallic compound is reacted with the pretreated modification complex carrier of described process, similarly obtain load type non-metallocene catalyst of the present invention.
As the method for carrying out described pre-treatment step, such as enumerating, at first prepare the described solution that helps chemical processing agent, then to intending being metered into (the preferred dropping) described chemical treatment agent solution (the described chemical processing agent that helps that wherein contains predetermined amount) that helps with described helping in the pretreated described modification complex carrier of chemical processing agent, perhaps add described modification complex carrier to the described chemical treatment agent solution amount of falling into a trap that helps, form thus reaction mixture.At this moment, temperature of reaction is generally-40~60 ℃, and preferred-30~30 ℃, the reaction times is generally 1~8h, preferred 2~6h, most preferably 3~4h (in case of necessity by stirring).Then, by filtration, washing and optionally drying, from this reaction mixture, isolate the pre-treatment product.
Perhaps, according to circumstances, also can be without this separation and be directly used in follow-up reactions steps with the form of mixed solution.At this moment, owing to contained a certain amount of solvent in the described mixed solution, so the solvent load that relates in can the described subsequent reactions step of corresponding minimizing.
According to the present invention, described filtration, washing and drying can adopt ordinary method to carry out, and wherein washer solvent can adopt and dissolve described used identical solvent when helping chemical processing agent.As required, this washing is generally carried out 1~8 time, and preferred 2~6 times, most preferably 2~4 times.Described drying can adopt ordinary method to carry out, such as heat drying method under rare gas element desiccating method, boulton process or the vacuum, and heat drying method, most preferably heat drying method under the vacuum under preferred rare gas element desiccating method or the vacuum.The temperature range of described drying is generally normal temperature to 140 ℃, is generally 2-20 hour time of drying, but is not limited to this.
According to the present invention, as the consumption of described Nonmetallocene title complex, so that reach 1 in the mol ratio of the described magnesium compound (solid) of Mg element and described Nonmetallocene title complex: 0.01-1, preferred 1: 0.04-0.4, more preferably 1: 0.08-0.2.
According to the present invention, consumption as described solvent be used to dissolving described magnesium compound, so that the ratio of described magnesium compound (solid) and described solvent reaches 1mol: 75~400ml, preferred 1mol: 150~300ml, more preferably 1mol: 200~250ml.
According to the present invention, as the consumption of described porous support, so that reach 1 in the described magnesium compound of magnesium compound solid and the mass ratio of described porous support: 0.1-20, preferred 1: 0.5-10, more preferably 1: 1-5.
According to the present invention, as the consumption of described precipitation agent, so that the volume ratio of described precipitation agent and described solvent be used to dissolving described magnesium compound is 1: 0.2~5, preferred 1: 0.5~2, more preferably 1: 0.8~1.5.
According to the present invention, consumption as described chemical processing agent, so that reach 1 in the described magnesium compound (solid) of Mg element and mol ratio in the described chemical processing agent of IVB family metal (such as Ti) element: 0.01-1, preferred 1: 0.01-0.50, more preferably 1: 0.10-0.30.
According to the present invention, as the described consumption that helps chemical processing agent, so that reach 1 in the described magnesium compound (solid) of Mg element and the described mol ratio of chemical processing agent that helps in the Al element: 0-1.0, preferred 1: 0-0.5, more preferably 1: 0.1-0.5.
Known to those skilled in the artly be that aforementioned all method steps all preferably carries out under the condition of anhydrous anaerobic basically.Here the said basically anhydrous anaerobic content that refers to water and oxygen in the system continues less than 100ppm.And load type non-metallocene catalyst of the present invention needs to save backup in the presence of the pressure-fired rare gas element (such as nitrogen, argon gas, helium etc.) in confined conditions in preparation afterwards usually.
In one embodiment, the invention still further relates to the load type non-metallocene catalyst (sometimes being also referred to as carry type non-metallocene calalyst for polymerization of olefine) of being made by the preparation method of aforesaid load type non-metallocene catalyst.
In a further embodiment, the present invention relates to a kind of alkene homopolymerization/copolymerization process, wherein with load type non-metallocene catalyst of the present invention as catalyst for olefines polymerizing, make alkene homopolymerization or copolymerization.
With regard to this alkene homopolymerization/copolymerization process involved in the present invention, except the following content that particularly points out, other contents of not explaining (such as polymerization with the addition manner of reactor, alkene consumption, catalyzer and alkene etc.), can directly be suitable for conventional known those in this area, not special restriction, the description thereof will be omitted at this.
According to homopolymerization/copolymerization process of the present invention, take load type non-metallocene catalyst of the present invention as Primary Catalysts, to be selected from aikyiaiurnirsoxan beta, aluminum alkyls, haloalkyl aluminium, boron fluothane, boron alkyl and the boron alkyl ammonium salt one or more as promotor, make alkene homopolymerization or copolymerization.
Primary Catalysts and promotor can be to add first Primary Catalysts to the adding mode in the polymerization reaction system, and then the adding promotor, perhaps add first promotor, and then add Primary Catalysts, or both contact first after the mixing and add together, perhaps add simultaneously respectively.Primary Catalysts and promotor added respectively fashionablely both can in same reinforced pipeline, add successively, also can in the reinforced pipeline of multichannel, add successively, and both add simultaneously respectively and fashionablely should select the multichannel pipeline that feeds in raw material.For the continous way polyreaction, the reinforced pipeline of preferred multichannel adds simultaneously continuously, and for the intermittence type polymerization reaction, adds together in same reinforced pipeline after preferably both mix first, perhaps in same reinforced pipeline, add first promotor, and then add Primary Catalysts.
According to the present invention, there is no particular limitation to the reactive mode of described alkene homopolymerization/copolymerization process, can adopt well known in the art those, such as enumerating slurry process, substance law and vapor phase process etc., wherein preferred slurries method and vapor phase process.
According to the present invention, as described alkene, such as enumerating C
2~C
10Monoolefine, diolefin, cyclic olefin and other ethylenically unsaturated compounds.
Particularly, as described C
2~C
12Monoolefine is such as enumerating ethene, propylene, 1-butylene, 1-hexene, 1-heptene, 4-methyl-1-pentene, 1-octene, 1-decene, 1-hendecene, 1-laurylene and vinylbenzene etc.; As described cyclic olefin, such as enumerating 1-cyclopentenes and norbornylene etc.; As described diolefin, such as enumerating Isosorbide-5-Nitrae-divinyl, 2,5-pentadiene, 1,6-hexadiene, norbornadiene and 1,7-octadiene etc.; And as described other ethylenically unsaturated compounds, such as enumerating vinyl acetate and (methyl) acrylate etc.Wherein, the homopolymerization of optimal ethylene, the perhaps copolymerization of ethene and propylene, 1-butylene or 1-hexene.
According to the present invention, homopolymerization refers to only a kind of polymerization of described alkene, and copolymerization refers to the polymerization between the two or more described alkene.
According to the present invention, described promotor is selected from aikyiaiurnirsoxan beta, aluminum alkyls, haloalkyl aluminium, boron fluothane, boron alkyl and boron alkyl ammonium salt, wherein preferred aikyiaiurnirsoxan beta and aluminum alkyls.
As described aikyiaiurnirsoxan beta, such as enumerating the line style aikyiaiurnirsoxan beta shown in the following general formula (I-1): (R) (R) Al-(Al (R)-O)
n-O-Al (R) (R), and the ring-type aikyiaiurnirsoxan beta shown in the following general formula (II-1) :-(Al (R)-O-)
N+2-.
In aforementioned formula, radicals R is same to each other or different to each other (preferably identical), is selected from independently of one another C
1-C
8Alkyl, preferable methyl, ethyl and isobutyl-, most preferable.N is the arbitrary integer in the 1-50 scope, the arbitrary integer in preferred 10~30 scopes.
As described aikyiaiurnirsoxan beta, preferable methyl aikyiaiurnirsoxan beta, ethylaluminoxane, isobutyl aluminium alkoxide and normal-butyl alumina alkane, further preferable methyl aikyiaiurnirsoxan beta and isobutyl aluminium alkoxide, and most preferable aikyiaiurnirsoxan beta.
These aikyiaiurnirsoxan beta can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
As described aluminum alkyls, such as enumerating the compound shown in the following general formula (III):
Al(R)
3 (III)
Wherein, radicals R is same to each other or different to each other (preferably identical), and is selected from independently of one another C
1-C
8Alkyl, preferable methyl, ethyl and isobutyl-, most preferable.
Particularly, as described aluminum alkyls, such as enumerating trimethyl aluminium (Al (CH
3)
3), triethyl aluminum (Al (CH
3CH
2)
3), tri-n-n-propyl aluminum (Al (C
3H
7)
3), triisobutyl aluminium (Al (i-C
4H
9)
3), three n-butylaluminum (Al (C
4H
9)
3), triisopentyl aluminium (Al (i-C
5H
11)
3), three n-pentyl aluminium (Al (C
5H
11)
3), tri-n-hexyl aluminum (Al (C
6H
13)
3), three isohexyl aluminium (Al (i-C
6H
13)
3), diethylmethyl aluminium (Al (CH
3) (CH
3CH
2)
2) and dimethyl ethyl aluminium (Al (CH
3CH
2) (CH
3)
2) etc., wherein preferred trimethyl aluminium, triethyl aluminum, tri-n-n-propyl aluminum and triisobutyl aluminium, further preferred triethyl aluminum and triisobutyl aluminium, and triethyl aluminum most preferably.
These aluminum alkylss can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
As described haloalkyl aluminium, such as enumerating the compound shown in the following general formula (IV):
Al(R)
nX
3-n (IV)
Wherein, radicals R is same to each other or different to each other (preferably identical), and is selected from independently of one another C
1-C
8Alkyl, preferable methyl, ethyl and isobutyl-, most preferable.Radicals X is halogen, preferred chlorine.N is 1 or 2.
Particularly, as described haloalkyl aluminium, such as enumerating a Chlorodimethyl aluminium (Al (CH
3)
2Cl), dichloromethyl aluminium (Al (CH
3) Cl
2)), aluminium diethyl monochloride (Al (CH
3CH
2)
2Cl), ethyl aluminum dichloride (Al (CH
3CH
2) Cl
2), a chlorine dipropyl aluminium (Al (C
3H
7)
2Cl), two chloropropyl aluminium (Al (C
3H
7) Cl
2)), a chlorine di-n-butyl aluminium (Al (C
4H
9)
2Cl), dichloro n-butylaluminum (Al (C
4H
9) Cl
2), a chloro-di-isobutyl aluminum (Al (i-C
4H
9)
2Cl), dichloro aluminium isobutyl (Al (i-C
4H
9) Cl
2), a chlorine two n-pentyl aluminium (Al (C
5H
11)
2Cl), dichloro n-pentyl aluminium (Al (C
5H
11) Cl
2), a chlorine diisoamyl aluminium (Al (i-C
5H
11)
2Cl), dichloro isopentyl aluminium (Al (i-C
5H
11) Cl
2), a chlorine di-n-hexyl aluminium (Al (C
6H
13)
2Cl), dichloro n-hexyl aluminium (Al (C
6H
13) Cl
2), a chlorine two isohexyl aluminium (Al (i-C
6H
13)
2Cl), dichloro isohexyl aluminium (Al (i-C
6H
13) Cl
2),
Chloromethyl aluminium triethyl (Al (CH
3) (CH
3CH
2) Cl), chloromethyl propyl group aluminium (Al (CH
3) (C
3H
7) Cl), chloromethyl n-butylaluminum (Al (CH
3) (C
4H
9) Cl), chloromethyl aluminium isobutyl (Al (CH
3) (i-C
4H
9) Cl), a chloroethyl propyl group aluminium (Al (CH
2CH
3) (C
3H
7) Cl), a chloroethyl n-butylaluminum (AlCH
2CH
3) (C
4H
9) Cl), chloromethyl aluminium isobutyl (AlCH
2CH
3) (i-C
4H
9) Cl) etc., wherein preferred aluminium diethyl monochloride, ethyl aluminum dichloride, a chlorine di-n-butyl aluminium, dichloro n-butylaluminum, a chloro-di-isobutyl aluminum, dichloro aluminium isobutyl, a chlorine di-n-hexyl aluminium, dichloro n-hexyl aluminium, further preferred chlorodiethyl aluminium, ethyl aluminum dichloride and a chlorine di-n-hexyl aluminium, and aluminium diethyl monochloride most preferably.
These haloalkyl aluminium can be used alone, and perhaps are used in combination multiple with ratio arbitrarily.
As described boron fluothane, described boron alkyl and described boron alkyl ammonium salt, can directly use conventional those that use in this area, not special restriction.
In addition, according to the present invention, described promotor can be used alone, and also can be as required be used in combination multiple aforesaid promotor, not special restriction with ratio arbitrarily.
According to the present invention, the difference (such as slurry polymerization) according to the reactive mode of described alkene homopolymerization/copolymerization process needs to use the polymerization solvent sometimes.
As described polymerization solvent, can use this area conventional those that use when carrying out alkene homopolymerization/copolymerization, not special restriction.
As described polymerization solvent, such as enumerating C
4-10Alkane (such as butane, pentane, hexane, heptane, octane, nonane or decane etc.), halo C
1-10Alkane (such as methylene dichloride), C
6-12Naphthenic hydrocarbon (hexanaphthene, suberane, cyclooctane, cyclononane or cyclodecane), C
6-20Aromatic hydrocarbon (such as toluene and dimethylbenzene) etc.Wherein, preferably using pentane, hexane, heptane and cyclohexane give is described polymerization solvent, most preferably hexane.
These polymerizations can be used alone with solvent, perhaps are used in combination multiple with ratio arbitrarily.
According to the present invention, the polymerization pressure of described alkene homopolymerization/copolymerization process is generally 0.1~10MPa, preferred 0.1~4MPa, and more preferably 0.4~3MPa, but sometimes be not limited to this.According to the present invention, polymeric reaction temperature is generally-40 ℃~200 ℃, and preferred 10 ℃~100 ℃, more preferably 40 ℃~95 ℃, but sometimes be not limited to this.
In addition, according to the present invention, described alkene homopolymerization/copolymerization process can carry out under the condition that has hydrogen to exist, and also can carry out under the condition that does not have hydrogen to exist.In situation about existing, the dividing potential drop of hydrogen can be 0.01%~99% of described polymerization pressure, and is preferred 0.01%~50%, but sometimes is not limited to this.
According to the present invention, when carrying out described alkene homopolymerization/copolymerization process, be generally 1~1000 in the described promotor of aluminium or boron and mol ratio in the described load type non-metallocene catalyst of described central metal atom: 1, preferred 10~500: 1, more preferably 15~300: 1, but sometimes be not limited to this.
Embodiment
Below adopt embodiment that the present invention is described in further detail, but the present invention is not limited to these embodiment.
(unit is g/cm to polymer stacks density
3) mensuration carry out with reference to CNS GB 1636-79.
The content of IVB family metal (such as Ti) and Mg element adopts the ICP-AES method to measure in the load type non-metallocene catalyst, and the content of Nonmetallocene part or title complex adopts analyses.
The polymerization activity of catalyzer calculates in accordance with the following methods: after polyreaction finishes, polymerisate in the reactor is filtered and drying, then the quality of this polymerisate of weighing represents that divided by the ratio of the quality of used load type non-metallocene catalyst (unit is kg polymkeric substance/g catalyzer or kg polymkeric substance/gCat) for the polymerization activity of this catalyzer with this polymerisate quality.
Molecular weight Mw, the Mn of polymkeric substance and molecular weight distribution (Mw/Mn) adopt the GPC V2000 type gel chromatography analyser of U.S. WATERS company to measure, and are solvent with 1,2,4-trichlorobenzene, and the temperature during mensuration is 150 ℃.
The viscosity-average molecular weight of polymkeric substance is calculated in accordance with the following methods: according to standard A STM D4020-00, (capillary inner diameter is 0.44mm to adopt high temperature dilution type Ubbelohde viscometer method, the thermostatic bath medium is No. 300 silicone oil, dilution is perhydronaphthalene with solvent, measuring temperature is 135 ℃) measure the limiting viscosity of described polymkeric substance, then calculate the viscosity-average molecular weight Mv of described polymkeric substance according to following formula.
Mv=5.37×10
4×[η]
1.37
Wherein, η is limiting viscosity.
Embodiment 1
Magnesium compound adopts Magnesium Chloride Anhydrous, and solvent adopts tetrahydrofuran (THF), and porous support adopts silicon-dioxide, i.e. silica gel, and model is the ES757 of Ineos company, before use silica gel is continued roasting 4h and thermal activation under 600 ℃, nitrogen atmosphere.The Nonmetallocene title complex adopts structure to be
Compound, precipitation agent adopts hexane, the chemical processing agent of IVB family metallic compound adopts titanium tetrachloride, chemical processing agent adopts hexane with solvent.
Take by weighing the 5g Magnesium Chloride Anhydrous, fully dissolving under the normal temperature obtains magnesium compound solution behind the adding solvent, then add the silica gel through thermal activation, stir under the normal temperature and form the first mixed serum after 2 hours, then add the Nonmetallocene title complex, stir at normal temperatures and obtained the second mixed serum in 6 hours, then add precipitation agent and make it abundant precipitation, stir after 2 hours, filter, adopt precipitation agent washing 3 times, each 60ml, vacuum-drying obtains modifying complex carrier under the last normal temperature.
The modification complex carrier that makes is joined chemical processing agent with in the solvent hexane, normal temperature is added dropwise to chemical processing agent in lower 30 minutes, then homogeneous heating to 60 ℃ isothermal reaction is after 2 hours, filter, chemical processing agent solvent wash 3 times, each 60ml vacuumizes drying at last under 60 ℃, obtain load type non-metallocene catalyst.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 210ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.08; The mass ratio of magnesium compound and porous support is 1: 2; The volume proportion of precipitation agent and solvent is 1: 1; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.15.
Load type non-metallocene catalyst is designated as CAT-1.
Embodiment 1-1
Substantially the same manner as Example 1, but following change is arranged:
Porous support is changed into 955 of Grace company, continues roasting 8h and thermal activation under 400 ℃, nitrogen atmosphere.
Solvent is changed into toluene, and the Nonmetallocene title complex adopts
, the chemical processing agent of IVB family metallic compound is changed into zirconium tetrachloride (ZrCl
4), precipitation agent changes hexanaphthene into, and chemical processing agent is changed into hexanaphthene with solvent.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 150ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.15; The mass ratio of magnesium compound and porous support is 1: 4; The volume proportion of precipitation agent and solvent is 1: 2; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.20.
Load type non-metallocene catalyst is designated as CAT-1-1.
Embodiment 1-2
Substantially the same manner as Example 1, but following change is arranged:
Porous support adopts aluminium sesquioxide.Aluminium sesquioxide is continued roasting 6h under 700 ℃, nitrogen atmosphere.
Magnesium compound is changed into anhydrous magnesium bromide (MgBr
2), the Nonmetallocene title complex adopts
Solvent is changed into ethylbenzene, and precipitation agent is changed into heptane, and the chemical processing agent of IVB family metallic compound is changed into titanium tetrabromide (TiBr
4), chemical processing agent is changed into heptane with solvent.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 250ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.20; The mass ratio of magnesium compound and porous support is 1: 1; The volume proportion of precipitation agent and solvent is 1: 0.7; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.30.
Load type non-metallocene catalyst is designated as CAT-1-2.
Embodiment 1-3
Substantially the same manner as Example 1, but following change is arranged:
Porous support adopts silica-magnesia mixed oxide (mass ratio 1: 1).The silica-magnesia mixed oxide is continued roasting 4h under 600 ℃, argon gas atmosphere.
Magnesium compound is changed into oxyethyl group magnesium chloride (MgCl (OC
2H
5)), the Nonmetallocene title complex adopts
Solvent is changed into dimethylbenzene, and precipitation agent is changed into decane, and the chemical processing agent of IVB family metallic compound is changed into tetraethyl-titanium (Ti (CH
3CH
2)
4), chemical processing agent is changed into decane with solvent.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 300ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.04; The mass ratio of magnesium compound and porous support is 1: 3; The volume proportion of precipitation agent and solvent is 1: 1.5; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.05.
Load type non-metallocene catalyst is designated as CAT-1-3.
Embodiment 1-4
Substantially the same manner as Example 1, but following change is arranged:
The porous support adopting montmorillonite.Polynite is continued roasting 8h under 400 ℃, nitrogen atmosphere.
Magnesium compound is changed into magnesium ethylate (Mg (OC
2H
5)
2), the Nonmetallocene title complex adopts
Solvent is changed into diethylbenzene, and precipitation agent is changed into pentane, and the chemical processing agent of IVB family metallic compound is changed into tetra-n-butyl titanium (Ti (C
4H
9)
4), chemical processing agent is changed into pentane with solvent.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 400ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.30; The mass ratio of magnesium compound and porous support is 1: 5; The volume proportion of precipitation agent and solvent is 1: 0.5; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.50.
Load type non-metallocene catalyst is designated as CAT-1-4.
Embodiment 1-5
Substantially the same manner as Example 1, but following change is arranged:
Porous support adopts the polystyrene of partial cross-linked (degree of crosslinking is 30%).This polystyrene is continued oven dry 12h under 100 ℃, nitrogen atmosphere.
Magnesium compound is changed into methylmagnesium-chloride (Mg (CH
3) Cl), the Nonmetallocene title complex adopts
Solvent is changed into chlorotoluene.
Wherein proportioning is, magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.10; The mass ratio of magnesium compound and porous support is 1: 10; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.10.
Load type non-metallocene catalyst is designated as CAT-1-5.
Embodiment 1-6
Substantially the same manner as Example 1, but following change is arranged:
Porous support adopts diatomite.Diatomite is continued roasting 8h under 500 ℃, nitrogen atmosphere.
Magnesium compound is changed into ethylmagnesium chloride (Mg (C
2H
5) Cl), solvent is changed into bromo ethylbenzene, and the Nonmetallocene title complex adopts
Precipitation agent is changed into suberane, and the chemical processing agent of IVB family metallic compound is changed into purity titanium tetraethoxide (Ti (OCH
3CH
2)
4), chemical processing agent is changed into suberane with solvent.
Wherein proportioning is, the mass ratio of magnesium compound and porous support is 1: 0.5; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 1.
Load type non-metallocene catalyst is designated as CAT-1-6.
Embodiment 1-7
Substantially the same manner as Example 1, but following change is arranged:
Porous support adopts titanium dioxide.Titanium dioxide is continued roasting 6h under 300 ℃, nitrogen atmosphere.
Magnesium compound is changed into magnesium ethide (Mg (C
2H
5)
2), the Nonmetallocene title complex adopts
The chemical processing agent of IVB family metallic compound is changed into isobutyl-titanous chloride (Ti (i-C
4H
9) Cl
3).
Load type non-metallocene catalyst is designated as CAT-1-7.
Embodiment 1-8
Substantially the same manner as Example 1, but following change is arranged:
Magnesium compound is changed into methyl ethoxy magnesium (Mg (OC
2H
5) (CH
3)), precipitation agent is changed into chlorocyclohexane, and the chemical processing agent of IVB family metallic compound is changed into three isobutoxy titanium chloride (TiCl (i-OC
4H
9)
3), chemical processing agent is changed into chlorocyclohexane with solvent.
Load type non-metallocene catalyst is designated as CAT-1-8.
Embodiment 1-9
Substantially the same manner as Example 1, but following change is arranged:
Magnesium compound is changed into butyl magnesium ethylate (Mg (OC
2H
5) (C
4H
9)), precipitation agent is changed into the bromo suberane, and the chemical processing agent of IVB family metallic compound is changed into dimethoxy zirconium dichloride (ZrCl
2(OCH
3)
2).
Load type non-metallocene catalyst is designated as CAT-1-9.
Embodiment 2
Magnesium compound adopts Magnesium Chloride Anhydrous, and solvent adopts tetrahydrofuran (THF), and porous support adopts silicon-dioxide, i.e. silica gel, and model is the ES757 of Ineos company, before use silica gel is continued roasting 4h and thermal activation under 600 ℃, nitrogen atmosphere.The Nonmetallocene title complex adopts structure to be
Compound, precipitation agent adopts hexane, helps chemical processing agent to adopt triethyl aluminum, the chemical processing agent of IVB family metallic compound adopts titanium tetrachloride, chemical processing agent adopts hexane with solvent.
Take by weighing the 5g Magnesium Chloride Anhydrous, fully dissolving under the normal temperature obtains magnesium compound solution behind the adding solvent, then add the silica gel through thermal activation, stir under the normal temperature and form the first mixed serum after 2 hours, then add the Nonmetallocene title complex, stir at normal temperatures and obtained the second mixed serum in 6 hours, then add precipitation agent and make it abundant precipitation, stir after 2 hours, filter, adopt precipitation agent washing 3 times, each 60ml, vacuum-drying obtains modifying complex carrier under the last normal temperature.
Then in the modification complex carrier that obtains, add the 60ml hexane, under agitation condition, adopt triethyl aluminum (concentration is the hexane solution of 15wt%) to help chemical processing agent to process and modify complex carrier, with 30 minutes dropping triethyl aluminums, 60 ℃ of lower stirring reactions are after 4 hours, filter, hexane washing 2 times, each hexane consumption 60ml, vacuum-drying obtains pretreated modification complex carrier under the normal temperature.
Described pretreated modification complex carrier is joined chemical processing agent with in the solvent hexane, normal temperature is added dropwise to chemical processing agent in lower 30 minutes, then homogeneous heating to 60 ℃ isothermal reaction is after 2 hours, filter, chemical processing agent solvent wash 3 times, each 60ml vacuumizes drying at last under 60 ℃, obtain load type non-metallocene catalyst.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 210ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.08; The mass ratio of magnesium compound and porous support is 1: 2; Magnesium compound with help the chemical processing agent mol ratio as 1: 0.15 take the Al element; The volume proportion of precipitation agent and solvent is 1: 1; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.15.
Load type non-metallocene catalyst is designated as CAT-2.
Embodiment 2-1
Substantially the same manner as Example 2, but following change is arranged:
Porous support is changed into 955 of Grace company, continues roasting 8h and thermal activation under 400 ℃, nitrogen atmosphere.
Solvent is changed into toluene, and the Nonmetallocene title complex adopts
Precipitation agent changes hexanaphthene into, helps chemical processing agent to change into methylaluminoxane (MAO, the toluene solution of 10wt%), and the chemical processing agent of IVB family metallic compound is changed into zirconium tetrachloride (ZrCl
4), chemical processing agent is changed into hexanaphthene with solvent.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 150ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.15; The mass ratio of magnesium compound and porous support is 1: 4; The volume proportion of precipitation agent and solvent is 1: 2; Magnesium compound with help the chemical processing agent mol ratio as 1: 0.20 take the Al element; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.20.
Load type non-metallocene catalyst is designated as CAT-2-1.
Embodiment 2-2
Substantially the same manner as Example 2, but following change is arranged:
Porous support adopts aluminium sesquioxide.Aluminium sesquioxide is continued roasting 6h under 700 ℃, nitrogen atmosphere.
Magnesium compound is changed into anhydrous magnesium bromide (MgBr
2), the Nonmetallocene title complex adopts
Solvent is changed into ethylbenzene, and precipitation agent is changed into heptane, helps chemical processing agent to change into trimethyl aluminium (Al (CH
3)
3), the chemical processing agent of IVB family metallic compound is changed into titanium tetrabromide (TiBr
4), chemical processing agent is changed into heptane with solvent.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 250ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.20; The mass ratio of magnesium compound and porous support is 1: 1; The volume proportion of precipitation agent and solvent is 1: 0.7; Magnesium compound with help the chemical processing agent mol ratio as 1: 0.30 take the Al element; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.30.
Load type non-metallocene catalyst is designated as CAT-2-2.
Embodiment 2-3
Substantially the same manner as Example 2, but following change is arranged:
Porous support adopts silica-magnesia mixed oxide (mass ratio 1: 1).The silica-magnesia mixed oxide is continued roasting 4h under 600 ℃, argon gas atmosphere.
Magnesium compound is changed into oxyethyl group magnesium chloride (MgCl (OC
2H
5)), the Nonmetallocene title complex adopts
Solvent is changed into dimethylbenzene, and precipitation agent is changed into decane, helps chemical processing agent to change into triisobutyl aluminium (Al (i-C
4H
9)
3), the chemical processing agent of IVB family metallic compound is changed into tetraethyl-titanium (Ti (CH
3CH
2)
4), chemical processing agent is changed into decane with solvent.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 300ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.04; The mass ratio of magnesium compound and porous support is 1: 3; The volume proportion of precipitation agent and solvent is 1: 1.5; Magnesium compound with help the chemical processing agent mol ratio as 1: 0.05 take the Al element; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.05.
Load type non-metallocene catalyst is designated as CAT-2-3.
Embodiment 2-4
Substantially the same manner as Example 2, but following change is arranged:
The porous support adopting montmorillonite.Polynite is continued roasting 8h under 400 ℃, nitrogen atmosphere.
Magnesium compound is changed into magnesium ethylate (Mg (OC
2H
5)
2), the Nonmetallocene title complex adopts
Solvent is changed into diethylbenzene, and precipitation agent is changed into pentane, helps chemical processing agent to change into isobutyl aluminium alkoxide, and the chemical processing agent of IVB family metallic compound is changed into tetra-n-butyl titanium (Ti (C
4H
9)
4), chemical processing agent is changed into pentane with solvent.
Wherein proportioning is, magnesium compound and solvent burden ratio are 1mol: 400ml; Magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.30; The mass ratio of magnesium compound and porous support is 1: 5; The volume proportion of precipitation agent and solvent is 1: 0.5; Magnesium compound with help the chemical processing agent mol ratio as 1: 0.50 take the Al element; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.50.
Load type non-metallocene catalyst is designated as CAT-2-4.
Embodiment 2-5
Substantially the same manner as Example 1, but following change is arranged:
Porous support adopts the polystyrene of partial cross-linked (degree of crosslinking is 30%).This polystyrene is continued oven dry 12h under 100 ℃, nitrogen atmosphere.
Magnesium compound is changed into methylmagnesium-chloride (Mg (CH
3) Cl), the Nonmetallocene title complex adopts
Solvent is changed into chlorotoluene.
Wherein proportioning is, magnesium compound and Nonmetallocene title complex mol ratio are 1: 0.10; The mass ratio of magnesium compound and porous support is 1: 10; Magnesium compound with help the chemical processing agent mol ratio as 1: 0.40 take the Al element; Magnesium compound with take the chemical processing agent mol ratio of IVB family metallic element as 1: 0.10.
Load type non-metallocene catalyst is designated as CAT-2-5.
The comparative example A
Substantially the same manner as Example 1, but following change is arranged:
Magnesium compound and Nonmetallocene title complex mol ratio are changed into 1: 0.16;
Catalyzer is designated as CAT-A.
Comparative Examples B
Substantially the same manner as Example 1, but following change is arranged:
Magnesium compound and Nonmetallocene title complex mol ratio are changed into 1: 0.04;
Catalyzer is designated as CAT-B.
Comparative Examples C
Substantially the same manner as Example 1, but following change is arranged:
The Nonmetallocene title complex forms solution with magnesium compound first in solvent, the porous support that adds again thermal activation treatment forms mixed serum, fully precipitate rear filtration washing drying to wherein adding precipitation agent, with the chemical processing agent reaction of IVB family metallic compound, obtain load type non-metallocene catalyst after the filtration washing drying again.
Catalyzer is designated as CAT-C.
Embodiment 3 (Application Example)
With the catalyzer CAT-1~CAT-2, the CAT-1-1 that make in the embodiment of the invention~9, CAT-2-1~5, CAT-A~C, carry out in accordance with the following methods under the following conditions homopolymerization, copolymerization and the preparation ultrahigh molecular weight polyethylene(UHMWPE) of ethene respectively.
Homopolymerization is: 5 liters of polymerization autoclaves, slurry polymerization processes, 2.5 liters of hexane solvents, polymerization stagnation pressure 0.8MPa, 85 ℃ of polymerization temperatures, hydrogen partial pressure 0.2MPa, 2 hours reaction times.At first 2.5 liters of hexanes are joined in the polymerization autoclave, open and stir, then add 50mg load type non-metallocene catalyst and catalyst mixture, add again hydrogen to 0.2MPa, continue at last to pass into ethene and make the polymerization stagnation pressure constant in 0.8MPa.Reaction with gas reactor emptying, is emitted the still interpolymer after finishing, dry rear weighing quality.The particular case of this polyreaction and polymerization evaluation result are as shown in table 1.
Copolymerization is: 5 liters of polymerization autoclaves, slurry polymerization processes, 2.5 liters of hexane solvents, polymerization stagnation pressure 0.8MPa, 85 ℃ of polymerization temperatures, hydrogen partial pressure 0.2MPa, 2 hours reaction times.At first 2.5 liters of hexanes are joined in the polymerization autoclave, open and stir, then add 50mg load type non-metallocene catalyst and catalyst mixture, disposable adding hexene-1 comonomer 50g, add again hydrogen to 0.2MPa, continue at last to pass into ethene and make the polymerization stagnation pressure constant in 0.8MPa.Reaction with gas reactor emptying, is emitted the still interpolymer after finishing, dry rear weighing quality.The particular case of this polyreaction and polymerization evaluation result are as shown in table 1.
The preparation ultrahigh molecular weight polyethylene(UHMWPE) is polymerized to: 5 liters of polymerization autoclaves, slurry polymerization processes, 2.5 liters of hexane solvents, polymerization stagnation pressure 0.5MPa, 70 ℃ of polymerization temperatures, 2 hours reaction times.At first 2.5 liters of hexanes are joined in the polymerization autoclave, open and stir, then add 50mg load type non-metallocene catalyst and catalyst mixture, promotor is 100 with catalyst activity metal molar ratio, continues at last to pass into ethene and makes the polymerization stagnation pressure constant in 0.5MPa.Reaction with gas reactor emptying, is emitted the still interpolymer after finishing, dry rear weighing quality.The particular case of this polyreaction and polymerization evaluation result are as shown in table 2.
Test-results data by sequence number in the table 13 and 4 and 16 and 17 increase the consumption of promotor as can be known, namely improve promotor and catalyst activity metal molar than the time, the impact of, polymer stacks density active on polymerization catalyst and molecular weight distribution is not remarkable.It can be said that brightly, adopt the load type non-metallocene catalyst of method provided by the invention preparation only to need fewer promotor consumption just can obtain high olefin polymerizating activity; And the polymkeric substance such as resulting polyethylene has good polymer morphology and high polymer bulk density thus.
In the contrast table 1 sequence number 1 and 3 test-results data as can be known, after the copolymerization, catalyst activity has greatly to be increased, thus explanation adopts the load type non-metallocene catalyst of method preparation provided by the invention to have comparatively significant comonomer effect.
Test-results data by sequence number 1 in the contrast table 1 and Comparative Examples sequence number 23~24 reduce or increase the add-on of Nonmetallocene title complex as can be known in the catalyzer, its activity decreases or increases.Thereby illustrate that Nonmetallocene title complex content plays an important role to catalyst activity.
By as seen from Table 2, adopt catalyzer provided by the present invention, can prepare ultrahigh molecular weight polyethylene(UHMWPE), its bulk density all increases to some extent, and contrast sequence number 1 and 2 and 3 and 4 as seen, adopts methylaluminoxane can increase the viscosity-average molecular weight of polymkeric substance as promotor.Sequence number 1 and Comparative Examples 5 in the contrast table 2,6 test-results data reduce in the catalyzer or increase the Nonmetallocene title complex as can be known, and the polymkeric substance viscosity-average molecular weight reduces thereupon or increases.Thereby the effect that the Nonmetallocene title complex also has increases the polymkeric substance viscosity-average molecular weight is described.
Sequence number 1~9 and 15~22 in the contrast table 1, sequence number 1~2 and 3~4 as seen in the table 2, before chemical processing agent is processed the modification complex carrier, adopt first the promotor pre-treatment to process and modify complex carrier, the load type non-metallocene catalyst that obtains after the last vacuum-drying, with only process with chemical processing agent, but not processing the resulting load type non-metallocene catalyst of modification complex carrier through promotor compares, catalytic activity and polymer stacks density are higher, and the ultrahigh molecular weight polyethylene(UHMWPE) viscosity-average molecular weight is higher.
Sequence number 1 and contrast sequence number 25 in the contrast table 1,1 and 7 test-results as seen in the table 2, jointly be dissolved in the solvent than the Nonmetallocene title complex prior to magnesium compound, add porous support and form slurries, after adding the rear filtration washing drying of precipitation agent precipitation, again with the dry prepared load type non-metallocene catalyst of the rear filtration washing of IVB family chemical processing agent reaction, magnesium compound provided by the present invention is dissolved in the solvent, add again the Nonmetallocene title complex after adding porous support, after adding the rear filtration washing drying of precipitation agent precipitation, again with the dry prepared load type non-metallocene catalyst of the rear filtration washing of IVB family chemical processing agent reaction, catalyst activity and polymer stacks density are higher, and the ultrahigh molecular weight polyethylene(UHMWPE) viscosity-average molecular weight of preparation is also higher.
Although more than in conjunction with the embodiments the specific embodiment of the present invention is had been described in detail, it is pointed out that protection scope of the present invention is not subjected to the restriction of these embodiments, but determined by claims of appendix.Those skilled in the art can carry out suitable change to these embodiments in the scope that does not break away from technological thought of the present invention and purport, and these embodiments after changing obviously are also included within protection scope of the present invention.
Claims (11)
1. the preparation method of a load type non-metallocene catalyst may further comprise the steps:
Magnesium compound is dissolved in the solvent, obtains the step of magnesium compound solution;
Optional porous support through thermal activation treatment is contacted with described magnesium compound solution, obtain the step of the first mixed serum;
The Nonmetallocene title complex is contacted with described the first mixed serum, obtain the step of the second mixed serum;
In described the second mixed serum, add precipitation agent, obtain to modify the step of complex carrier, know
Make the chemical processing agent and the reaction of described modification complex carrier that are selected from IVB family metallic compound, obtain the step of described load type non-metallocene catalyst,
Optional also being included in of wherein said preparation method makes before described chemical processing agent and the reaction of described modification complex carrier, with the step that helps the described modification complex carrier of chemical processing agent pre-treatment that is selected from aikyiaiurnirsoxan beta, aluminum alkyls or its arbitrary combination.
2. according to preparation method claimed in claim 1, it is characterized in that, described porous support is selected from olefin homo or multipolymer, polyvinyl alcohol or its multipolymer, cyclodextrin, polyester or copolyesters, polymeric amide or copolyamide, ryuron or multipolymer, Voncoat R 3310 or multipolymer, methacrylic acid ester homopolymer or multipolymer, styrene homopolymers or multipolymer, the partial cross-linked form of these homopolymer or multipolymer, periodic table of elements IIA, IIIA, the refractory oxide of IVA or IVB family metal or infusibility composite oxides, clay, molecular sieve, mica, polynite, in wilkinite and the diatomite one or more, be preferably selected from partial cross-linked styrene polymer, silicon-dioxide, aluminum oxide, magnesium oxide, the oxidation sial, the oxidation magnalium, titanium dioxide, in molecular sieve and the polynite one or more more preferably are selected from silicon-dioxide.
3. according to preparation method claimed in claim 1, it is characterized in that, described magnesium compound is selected from one or more in magnesium halide, alkoxyl group magnesium halide, alkoxyl magnesium, alkyl magnesium, alkyl halide magnesium and the alkyl alkoxy magnesium, is preferably selected from the magnesium halide one or more, more preferably magnesium chloride.
4. according to preparation method claimed in claim 1, it is characterized in that described solvent is selected from C
6-12Aromatic hydrocarbon, halo C
6-12In aromatic hydrocarbon, ester and the ether one or more are preferably selected from C
6-12In aromatic hydrocarbon and the tetrahydrofuran (THF) one or more, most preferably tetrahydrofuran (THF).
5. according to preparation method claimed in claim 1, it is characterized in that described Nonmetallocene title complex is selected from one or more in the compound with following chemical structural formula:
Be preferably selected from compound (A) with following chemical structural formula and in the compound (B) one or more:
More preferably be selected to compound (A-4) and compound (B-1) to compound (B-4) one or more of compound (A-1) with following chemical structural formula:
In above all chemical structural formulas,
Q is 0 or 1;
D is 0 or 1;
M is 1,2 or 3;
M is selected from periodic table of elements III-th family to XI family atoms metal, preferred IVB family atoms metal, more preferably Ti (IV) and Zr (IV);
N is 1,2,3 or 4, depends on the valence state of described central metal atom M;
X is selected from halogen, hydrogen atom, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl, oxy radical, nitrogen-containing group, sulfur-containing group, boron-containing group, contain aluminium base group, phosphorus-containing groups, silicon-containing group, germanic group or contain tin group, a plurality of X can be identical, also can be different, and can also be each other in key or Cheng Huan;
A be selected from Sauerstoffatom, sulphur atom, selenium atom,
-NR
23R
24,-N (O) R
25R
26,
-PR
28R
29,-P (O) R
30OR
31, sulfuryl, sulfoxide group or-Se (O) R
39, N, O, S, Se and the P coordination atom of respectively doing for oneself wherein;
B is selected from nitrogen-atoms, nitrogen-containing group, phosphorus-containing groups or C
1-C
30Alkyl;
D is selected from nitrogen-atoms, Sauerstoffatom, sulphur atom, selenium atom, phosphorus atom, nitrogen-containing group, phosphorus-containing groups, C
1-C
30Alkyl, sulfuryl, sulfoxide group,
-N (O) R
25R
26,
Or-P (O) R
32(OR
33), N, O, S, Se and the P coordination atom of respectively doing for oneself wherein;
E is selected from nitrogen-containing group, oxy radical, sulfur-containing group, contains seleno group, phosphorus-containing groups or cyano group, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
F is selected from nitrogen-atoms, nitrogen-containing group, oxy radical, sulfur-containing group, contain seleno group or phosphorus-containing groups, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
G is selected from C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or safing function group;
Y is selected from Sauerstoffatom, nitrogen-containing group, oxy radical, sulfur-containing group, contain seleno group or phosphorus-containing groups, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
Z is selected from nitrogen-containing group, oxy radical, sulfur-containing group, contains seleno group, phosphorus-containing groups or cyano group, wherein N, O, S, Se and the P coordination atom of respectively doing for oneself;
→ represent singly-bound or two key;
-represent covalent linkage or ionic linkage;
---represent coordinate bond, covalent linkage or ionic linkage;
R
1To R
4, R
6To R
36, R
38And R
39Be selected from independently of one another hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or safing function group, above-mentioned group can be the same or different to each other, and wherein adjacent group can combine togather into key or Cheng Huan, is preferably formed aromatic ring, and
R
5Be selected from lone-pair electron on the nitrogen, hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl, oxy radical, sulfur-containing group, nitrogen-containing group, contain seleno group or phosphorus-containing groups; Work as R
5For oxy radical, sulfur-containing group, nitrogen-containing group, when containing seleno group or phosphorus-containing groups, R
5In N, O, S, P and Se can be used as coordination and carry out coordination with atom and described center IVB family atoms metal,
Described safing function group is selected from halogen, oxy radical, nitrogen-containing group, silicon-containing group, germanic group, sulfur-containing group, contains tin group, C
1-C
10Ester group or nitro,
Described Nonmetallocene title complex further is preferably selected from one or more in the compound with following chemical structural formula:
Most preferably be selected from the compound with following chemical structural formula one or more:
6. according to preparation method claimed in claim 5, it is characterized in that,
Described halogen is selected from F, Cl, Br or I;
Described phosphorus-containing groups is selected from
-PR
28R
29,-P (O) R
30R
31Or-P (O) R
32(OR
33);
Described oxy radical be selected from hydroxyl ,-OR
34With-T-OR
34
Described sulfur-containing group is selected from-SR
35,-T-SR
35,-S (O) R
36Or-T-SO
2R
37
The described seleno group that contains is selected from-SeR
38,-T-SeR
38,-Se (O) R
39Or-T-Se (O) R
39
Described group T is selected from C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or described safing function group;
Described R
37Be selected from hydrogen, C
1-C
30The C of alkyl, replacement
1-C
30Alkyl or described safing function group;
Described C
1-C
30Alkyl is selected from C
1-C
30Alkyl, C
7-C
50Alkaryl, C
7-C
50Aralkyl, C
3-C
30Cyclic alkyl, C
2-C
30Thiazolinyl, C
2-C
30Alkynyl, C
6-C
30Aryl, C
8-C
30Condensed ring radical or C
4-C
30Heterocyclic radical, wherein said heterocyclic radical contain 1-3 heteroatoms that is selected from nitrogen-atoms, Sauerstoffatom or sulphur atom;
The C of described replacement
1-C
30Alkyl is selected from one or more described halogens and/or described C
1-C
30Alkyl is as substituent described C
1-C
30Alkyl;
Described boron-containing group is selected from BF
4 -, (C
6F
5)
4B
-Territory (R
40BAr
3)
-
Describedly contain aluminium base group and be selected from aluminum alkyls, AlPh
4 -, AlF
4 -, AlCl
4 -, AlBr
4 -, AlI
4 -Or R
41AlAr
3 -
Described silicon-containing group is selected from-SiR
42R
43R
44Or-T-SiR
45
Described germanic group is selected from-GeR
46R
47R
48Or-T-GeR
49
Describedly contain tin group and be selected from-SnR
50R
51R
52,-T-SnR
53Or-T-Sn (O) R
54,
Described Ar represents C
6-C
30Aryl, and
R
40To R
54Be selected from independently of one another hydrogen, described C
1-C
30The C of alkyl, described replacement
1-C
30Alkyl or described safing function group, wherein these groups can be the same or different to each other, and wherein adjacent group can combine togather into key or Cheng Huan, and
Described group T defines with claim 5.
7. according to preparation method claimed in claim 1, it is characterized in that, described IVB family metallic compound is selected from one or more in IVB family metal halide, IVB family metal alkyl compound, IVB family metal alkoxide compound, IVB family metal alkyl halides and the IVB family metal alkoxide halogenide, be preferably selected from the IVB family metal halide one or more, more preferably be selected from TiCl
4, TiBr
4, ZrCl
4, ZrBr
4, HfCl
4And HfBr
4In one or more, most preferably be selected from TiCl
4And ZrCl
4In one or more.
8. according to preparation method claimed in claim 1, it is characterized in that described precipitation agent is selected from C
5-12Alkane, C
5-12Naphthenic hydrocarbon, halo C
1-10Alkane and halo C
5-12In the naphthenic hydrocarbon one or more, be preferably selected from pentane, hexane, heptane, octane, nonane, decane, hexanaphthene, pentamethylene, suberane, cyclodecane, cyclononane, methylene dichloride, dichloro hexane, two chloroheptanes, trichloromethane, trichloroethane, three chlorobutanes, methylene bromide, ethylene dibromide, dibromo-heptane, methenyl bromide, tribromoethane, three n-butyl bromide, chlorocyclopentane, chlorocyclohexane, the chloro suberane, the chloro cyclooctane, the chloro cyclononane, the chloro cyclodecane, bromocyclopentane, bromocyclohexane, the bromo suberane, the bromo cyclooctane, in bromo cyclononane and the bromo cyclodecane one or more, further be preferably selected from hexane, heptane, in decane and the hexanaphthene one or more, most preferably hexane.
9. according to preparation method claimed in claim 1, it is characterized in that, take the mol ratio of the described magnesium compound of Mg element and described Nonmetallocene title complex as 1: 0.01-1, preferred 1: 0.04-0.4, more preferably 1: 0.08-0.2, the ratio of described magnesium compound and described solvent is 1mol: 75~400ml, preferred 1mol: 150~300ml, more preferably 1mol: 200~250ml, take the mass ratio of the described magnesium compound of magnesium compound solid and described porous support as 1: 0.1-20, preferred 1: 0.5-10, more preferably 1: 1-5, in the described magnesium compound of Mg element with take the mol ratio of the described chemical processing agent of IVB family metallic element as 1: 0.01-1, preferred 1: 0.01-0.50, more preferably 1: 0.10-0.30, in the described magnesium compound of Mg element and the mol ratio that helps chemical processing agent take Al element described as 1: 0-1.0, preferred 1: 0-0.5, more preferably 1: 0.1-0.5, and the volume ratio of described precipitation agent and described solvent is 1: 0.2~5, preferred 1: 0.5~2, more preferably 1: 0.8~1.5.
10. load type non-metallocene catalyst, it is by making according to each described preparation method of claim 1-9.
11. alkene homopolymerization/copolymerization process, it is characterized in that, take according to load type non-metallocene catalyst claimed in claim 10 as Primary Catalysts, to be selected from aikyiaiurnirsoxan beta, aluminum alkyls, haloalkyl aluminium, boron fluothane, boron alkyl and the boron alkyl ammonium salt one or more as promotor, make alkene homopolymerization or copolymerization.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201110259258.XA CN102964474B (en) | 2011-08-31 | 2011-08-31 | Supported non-metallocene catalyst, preparation method and application |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201110259258.XA CN102964474B (en) | 2011-08-31 | 2011-08-31 | Supported non-metallocene catalyst, preparation method and application |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN102964474A true CN102964474A (en) | 2013-03-13 |
| CN102964474B CN102964474B (en) | 2015-01-14 |
Family
ID=47794930
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201110259258.XA Active CN102964474B (en) | 2011-08-31 | 2011-08-31 | Supported non-metallocene catalyst, preparation method and application |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN102964474B (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0260130A1 (en) * | 1986-09-09 | 1988-03-16 | Exxon Chemical Patents Inc. | New supported polymerization catalyst |
| CN102039191A (en) * | 2009-10-26 | 2011-05-04 | 中国石油化工股份有限公司 | Load type non-metallocene catalyst, preparation method and application thereof |
-
2011
- 2011-08-31 CN CN201110259258.XA patent/CN102964474B/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0260130A1 (en) * | 1986-09-09 | 1988-03-16 | Exxon Chemical Patents Inc. | New supported polymerization catalyst |
| CN102039191A (en) * | 2009-10-26 | 2011-05-04 | 中国石油化工股份有限公司 | Load type non-metallocene catalyst, preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102964474B (en) | 2015-01-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102039184B (en) | Supported non-metallocene catalyst as well as preparation method and application thereof | |
| CN102964476B (en) | Load type non-metallocene catalyst, its preparation method and application thereof | |
| CN102039191B (en) | Load type non-metallocene catalyst, preparation method and application thereof | |
| CN102059152B (en) | Loaded non-metallocene catalyst and preparation method and application thereof | |
| CN102964479B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964471A (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964489B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102059148B (en) | Loaded non-metallocene catalyst and preparation method and application thereof | |
| CN102039188B (en) | Supported non-metallocene catalyst, its preparation method and uses | |
| CN102964485B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964472B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964484B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964483B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102059150B (en) | Loaded non-metallocene catalyst and preparation method and application thereof | |
| CN102964477B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964478A (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102399314B (en) | Supported non-metallocene catalyst and preparation method and application thereof | |
| CN102964482B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964490B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN103304703A (en) | Loaded non-metallocene catalyst, its preparation method and application | |
| CN102964473A (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964475B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964474B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964486B (en) | Supported non-metallocene catalyst, preparation method and application | |
| CN102964481B (en) | Supported non-metallocene catalyst, preparation method and application |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant |