CN102496479B - Method for preparing titanium dioxide (TiO2)/niobium pentoxide (Nb2O5) core-shell structure nano-fiber film for dye-sensitized solar cell - Google Patents
Method for preparing titanium dioxide (TiO2)/niobium pentoxide (Nb2O5) core-shell structure nano-fiber film for dye-sensitized solar cell Download PDFInfo
- Publication number
- CN102496479B CN102496479B CN 201110399846 CN201110399846A CN102496479B CN 102496479 B CN102496479 B CN 102496479B CN 201110399846 CN201110399846 CN 201110399846 CN 201110399846 A CN201110399846 A CN 201110399846A CN 102496479 B CN102496479 B CN 102496479B
- Authority
- CN
- China
- Prior art keywords
- dye
- tio
- shell structure
- dmf solution
- pvac
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034 method Methods 0.000 title claims abstract description 18
- 239000002121 nanofiber Substances 0.000 title abstract description 9
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 title abstract description 5
- 239000011258 core-shell material Substances 0.000 title abstract description 4
- ZKATWMILCYLAPD-UHFFFAOYSA-N niobium pentoxide Chemical compound O=[Nb](=O)O[Nb](=O)=O ZKATWMILCYLAPD-UHFFFAOYSA-N 0.000 title abstract 10
- 238000006243 chemical reaction Methods 0.000 claims abstract description 11
- 239000000243 solution Substances 0.000 claims description 67
- 239000010955 niobium Substances 0.000 claims description 42
- 238000009987 spinning Methods 0.000 claims description 36
- 239000012528 membrane Substances 0.000 claims description 25
- 229920002689 polyvinyl acetate Polymers 0.000 claims description 22
- 238000002360 preparation method Methods 0.000 claims description 20
- 238000004528 spin coating Methods 0.000 claims description 20
- 239000002131 composite material Substances 0.000 claims description 16
- 238000013019 agitation Methods 0.000 claims description 15
- 239000011521 glass Substances 0.000 claims description 14
- 238000001125 extrusion Methods 0.000 claims description 13
- 239000000203 mixture Substances 0.000 claims description 13
- 230000000694 effects Effects 0.000 claims description 11
- 230000008569 process Effects 0.000 claims description 10
- 239000013504 Triton X-100 Substances 0.000 claims description 9
- 229920004890 Triton X-100 Polymers 0.000 claims description 9
- 238000000520 microinjection Methods 0.000 claims description 9
- 238000005245 sintering Methods 0.000 claims description 9
- FFTSTCGBNPNQGW-UHFFFAOYSA-M [O-2].[O-2].[O-2].[O-2].[OH-].O.O.[Ti+4].[Nb+5] Chemical compound [O-2].[O-2].[O-2].[O-2].[OH-].O.O.[Ti+4].[Nb+5] FFTSTCGBNPNQGW-UHFFFAOYSA-M 0.000 claims description 7
- 238000002347 injection Methods 0.000 claims description 7
- 239000007924 injection Substances 0.000 claims description 7
- 239000002243 precursor Substances 0.000 claims description 6
- 239000004094 surface-active agent Substances 0.000 claims description 6
- 238000001035 drying Methods 0.000 claims description 5
- 230000005684 electric field Effects 0.000 claims description 5
- 239000000835 fiber Substances 0.000 claims description 5
- 239000007788 liquid Substances 0.000 claims description 5
- 239000002904 solvent Substances 0.000 claims description 5
- 239000007921 spray Substances 0.000 claims description 5
- 238000010792 warming Methods 0.000 claims description 5
- 230000015572 biosynthetic process Effects 0.000 claims description 3
- 238000005516 engineering process Methods 0.000 abstract description 13
- 239000002086 nanomaterial Substances 0.000 abstract description 13
- 238000000576 coating method Methods 0.000 abstract description 12
- 239000011248 coating agent Substances 0.000 abstract description 11
- 230000006798 recombination Effects 0.000 abstract description 7
- 238000005215 recombination Methods 0.000 abstract description 7
- 238000010041 electrostatic spinning Methods 0.000 abstract description 4
- 238000007385 chemical modification Methods 0.000 abstract 1
- 230000002401 inhibitory effect Effects 0.000 abstract 1
- 239000004408 titanium dioxide Substances 0.000 abstract 1
- 229910010413 TiO 2 Inorganic materials 0.000 description 32
- 239000010408 film Substances 0.000 description 18
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 17
- 239000000975 dye Substances 0.000 description 13
- 239000003792 electrolyte Substances 0.000 description 9
- 238000011160 research Methods 0.000 description 7
- 230000001235 sensitizing effect Effects 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- 238000011161 development Methods 0.000 description 6
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 6
- 238000007747 plating Methods 0.000 description 5
- 238000001720 action spectrum Methods 0.000 description 4
- 238000012512 characterization method Methods 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- 230000003647 oxidation Effects 0.000 description 4
- 238000007254 oxidation reaction Methods 0.000 description 4
- 206010013786 Dry skin Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 230000005540 biological transmission Effects 0.000 description 3
- 238000005352 clarification Methods 0.000 description 3
- 239000002159 nanocrystal Substances 0.000 description 3
- 239000003960 organic solvent Substances 0.000 description 3
- 238000004506 ultrasonic cleaning Methods 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- 229910012820 LiCoO Inorganic materials 0.000 description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 2
- 230000005284 excitation Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000000051 modifying effect Effects 0.000 description 2
- -1 nanometer rods Substances 0.000 description 2
- 239000002071 nanotube Substances 0.000 description 2
- 239000002070 nanowire Substances 0.000 description 2
- 238000011056 performance test Methods 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000001172 regenerating effect Effects 0.000 description 2
- 239000004065 semiconductor Substances 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 239000012327 Ruthenium complex Substances 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 229910021417 amorphous silicon Inorganic materials 0.000 description 1
- 239000010405 anode material Substances 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 239000003245 coal Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 230000005518 electrochemistry Effects 0.000 description 1
- 238000001523 electrospinning Methods 0.000 description 1
- 238000002389 environmental scanning electron microscopy Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000007380 fibre production Methods 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 230000005283 ground state Effects 0.000 description 1
- ILHIHKRJJMKBEE-UHFFFAOYSA-N hydroperoxyethane Chemical compound CCOO ILHIHKRJJMKBEE-UHFFFAOYSA-N 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- URLJKFSTXLNXLG-UHFFFAOYSA-N niobium(5+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[Nb+5].[Nb+5] URLJKFSTXLNXLG-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000033116 oxidation-reduction process Effects 0.000 description 1
- 238000002161 passivation Methods 0.000 description 1
- 230000001699 photocatalysis Effects 0.000 description 1
- 238000007146 photocatalysis Methods 0.000 description 1
- 230000005622 photoelectricity Effects 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- VXUYXOFXAQZZMF-UHFFFAOYSA-N titanium(IV) isopropoxide Chemical compound CC(C)O[Ti](OC(C)C)(OC(C)C)OC(C)C VXUYXOFXAQZZMF-UHFFFAOYSA-N 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229910052724 xenon Inorganic materials 0.000 description 1
- FHNFHKCVQCLJFQ-UHFFFAOYSA-N xenon atom Chemical compound [Xe] FHNFHKCVQCLJFQ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/542—Dye sensitized solar cells
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/549—Organic PV cells
Landscapes
- Hybrid Cells (AREA)
Abstract
The invention discloses a method for preparing a titanium dioxide (TiO2)/niobium pentoxide (Nb2O5) core-shell structure nano-fiber film for a dye-sensitized solar cell. A photo-anode film is a core part of the dye-sensitized solar cell. The performance of the dye-sensitized solar cell can be improved by performing physical and chemical modification such as surface coating on the photo-anode film and developing a novel nano structure photo-anode film. The TiO2/Nb2O5 core-shell structure nano-fiber film for the photo-anode of the dye-sensitized solar cell is prepared by using a unique coaxial electrostatic spinning technology. By the method, a quasi-one-dimensional nano structure can be formed, and shell coating treatment for inhibiting charge recombination can be realized. A small quantity of grain boundaries having the quasi-one-dimensional nano structure is generated, charge recombination is inhibited, and a short-circuit current Isc of a battery is improved. By introduction of a shell, an open-circuit voltage Voc of the battery is improved. Correspondingly, the overall photoelectric conversion efficiency eta of the battery is improved by 24 percent to 33 percent.
Description
Technical field
The present invention relates to solar cell manufacturing technology field, particularly a kind of dye-sensitized cell preparation method of titanium dioxide-niobium pentaoxide nuclear shell structure nano tunica fibrosa.
Background technology
Along with society, sustainable development of economy, non-renewable energy resources such as oil, coal etc. are exhausted day by day, and human existence and development are being faced with unprecedented challenge.Searching cleaning, regenerative resource have become global problem.It is estimated that the energy that earth every year obtains from solar irradiation is about 100,000 times of human annual energy-output ratio, therefore, the development and use of solar energy are the key subjects of new energy field.The solar cell of based semiconductor silicon pn knot principle is the most ripe electrooptical device of present technology, and in recent years, the paces that the silicon solar cell scale is used are just progressively accelerated.Yet expensive still is the reality that can't avoid of photovoltaic generation.For this reason, the effort of development of new photovoltaic device does not stop all the time in the global range.
Last century end, along with the rise of nanometer technology, the novel solar cell of a class is that dye-sensitized solar cells (DSSC) arises at the historic moment.1991, Gr tzel reported first be the semiconductor nano thin film solar cell of sensitizer with the ruthenium complex dyestuff, its photoelectric conversion efficiency is higher than 7%, causes scientific circles' extensive concern, is regarded as third generation solar cell.Make great efforts after deliberation, the high conversion efficiency of small size DSSC has reached 11%, and with being on close level of amorphous silicon membrane battery, and cost is less than 1/5 of silion cell.Rely on tangible cost advantage and easy manufacture craft, DSSC or will in following photovoltaic industry development, become the strong competitor of silion cell, application potential is huge.
DSSC is made up of electrode nano structure membrane light anode, FTO electro-conductive glass matrix, sensitizing dyestuff, electrolyte and platinum plating.Its operation principle is as follows: sensitizing dyestuff absorbs luminous energy, electronics by ground state transition to excitation state; Anode film on the FTO is accepted the excitation state electronics as electron acceptor, and simultaneously, dye molecule loses electronics and is oxidation state; I
-/ I
3 -I in the electrolyte
-Provide electronics and with its reducing/regenerating as electron donor to the oxidation state dye molecule, I
3 -Be diffused into and electrode is obtained electronics be reduced, thereby finish the Optical Electro-Chemistry reaction cycle.
Wherein, photo-anode film is core and the primary study object of DSSC, and its effect is that the dye molecule electrons excited is received and transmits.Conventional photo-anode film is usually with titanium dioxide (TiO
2) nanocrystalline be raw material.Nearly 20 years, a large amount of researchers carried out continuing deep research to nanocrystalline photo-anode film, had formed ripe relatively Experiment Preparation technology gradually.But this photo-anode film also exists some inherent shortcomings, reason is that a large amount of crystal boundaries is arranged between nanocrystal, specific area is huge, the surface dangling bonds play the trap effect of capturing light induced electron, they can make the life-span of electronics and diffusion length reduce, recombination probability increases, and is restricting the raising of battery efficiency.Therefore, nanocrystalline photo-anode film is carried out physical chemistry modifying, and to come this skin effect of passivation be the important channel of improving the DSSC performance, mainly comprises doping, TiCl
4Methods such as processing, surface coating.
In above-mentioned three kinds of means, the above two are relatively easy.But, doping is more limited to the raising of battery efficiency.TiCl
4Though the effect of handling is subjected to certainly, it is ripe that technology is tending towards.And the coating method is in mechanism and technical all complicated, but the raising of performance is had more potentiality, and the selection of coating has diversity, thereby attracted more research.Because nano-crystal film surface density of states height causes TiO
2Electron acceptor is compound serious in conduction band electron and oxidation state dyestuff or the electrolyte.And charge recombination restricts the principal element that DSSC efficient improves just.For this reason, some research trials reduce compound in thin layer of metal oxide formation nuclear-shell (Core-shell) structure that the nanocrystal surface coating has higher conduction band position.Namely with TiO
2For nuclear, with other oxide (ZnO, Nb
2O
5, Al
2O
3, MgO etc.) be the structure of shell.Research is thought, can form energy barrier at shell after coating is handled and suppress TiO
2Conduction band electron and dye well are electrolytical compound, or form the injection efficiency that dipole layer improves electronics at the interface at nucleocapsid.In the above-mentioned coating, Nb
2O
5Be that effect is a kind of preferably.As A. Zaban etc. with immersion method to TiO
2The nanocrystalline Nb that is
2O
5Coat and handle, usefulness gel-sol methods such as A.F. Nogueira are carried out identical coating, and the efficient of DSSC all is improved significantly.
Meanwhile, the novel nano structure also is the important directions of DSSC photo-anode film research.If replace nanocrystalline with one-dimensional nano structures such as nano wire, nanometer rods, nanotubes, because their crystal boundary is less, can effectively reduce wherein surface state to the capturing of light induced electron, suppress charge recombination meanwhile, the novel nano structure also is the important directions of DSSC photo-anode film research.If replace nanocrystalline with one-dimensional nano structures such as nano wire, nanometer rods, nanotubes, because their crystal boundary is less, can effectively reduce wherein surface state trap to the capturing of light induced electron, suppress charge recombination, accelerate collection, the transmission rate of electronics, and then improve the performance of battery.
Except one-dimensional nano structure, the accurate one dimension TiO that is made by electrostatic spinning
2Nano fibrous membrane also begins in recent years for DSSC light anode.In theory, this photo-anode film will be obtained the effect similar with above-mentioned one-dimensional nano structure film.Related work mainly concentrates on the S. Ramakrishna of Singapore, several groups such as S. Shiratori of the D.Y. Kim of Korea S and Japan.Normally with TiO
2Precursor solution spin composite nano-fiber membrane directly be deposited on the FTO matrix, form the light anode through sintering.What deserves to be mentioned is to also have a special coaxial electrically spun technology in the electrostatic spinning technique, namely utilize coaxial spinneret orifice to prepare the different nuclear-shell of inside and outside composition (or claiming skin-core) structure nano fiber.At present, this technology more is the preparation for high polymer co-axial nano fiber.When it is used for the coaxial fiber production of inorganic matter, need consider two processes of spinning and sintering simultaneously, technology is more than the former complexity, thereby studies lessly, only has and counts few reports, typical as LiCoO that electrospinnings such as D.R. Chen prepare
2/ MgO co-axial nano fiber is namely realized anode material of lithium battery LiCoO with MgO
2Coating modification.And the TiO of their preparation
2/ SiO
2The co-axial nano fiber be used for photocatalysis research.Infer accordingly, by coaxial electrically spun and sintering, estimate also can form with TiO
2Be core, with Nb
2O
5Be the nanofiber of shell, realize surface coated effect, but present report without any this respect.
Summary of the invention
The purpose of this invention is to provide a kind of dye-sensitized cell preparation method of titanium dioxide-niobium pentaoxide nuclear shell structure nano tunica fibrosa.
The technical solution used in the present invention:
1) preparation high polymer PVAc(polyvinyl acetate) the quality percentage composition is the DMF(N of 15wt.%, dinethylformamide) solution, be that the surfactant Triton X-100(song of DMF solution 25% draws logical with volume), 2 times of quality are to the TiP(of PVAc isopropyl titanate), and 1.5 times of quality are to the C of PVAc
10H
25NbO
5(ethoxy alcohol niobium) joins in the DMF solution, obtains TiO through magnetic agitation 8h
2/ Nb
2O
5Composite precursor spin coating liquid, and with the spin coating instrument it is spin-coated on the FTO electro-conductive glass spin-coated layer that forms one deck, spin coating instrument rotating speed is 2000rmin
-1, the spin coating time is 30s;
2) preparation PVAc quality percentage composition is the DMF solution of 11.5wt.%; Be the surfactant Triton X-100 of DMF solution 15% with volume, and 1.5 times of quality are to the C of PVAc
10H
25NbO
5Join in the DMF solution, obtain Nb through magnetic agitation 8h
2O
5(niobium pentaoxide) presoma spinning solution; Preparation PVAc quality preparation PVAc quality percentage composition is the DMF solution of 11.5wt.%, be the surfactant Triton X-100 of DMF solution 15% with volume, volume is the HAc(glacial acetic acid of DMF solution 10%), and 2 times of TiP to PVAc of quality join in the DMF solution, obtain TiO through magnetic agitation 8h
2The presoma spinning solution; With Nb
2O
5, TiO
2Two kinds of presoma spinning solutions are respectively charged in two syringes, between coaxial spinning head and receiving system, add high voltage, charged drop overcomes self under effect of electric field surface tension forms the injection thread, is controlled the rate of extrusion of two kinds of presoma spinning solutions respectively by two micro-injection pumps; Along with solvent evaporates, spray the fiber that thread solidify to form nucleocapsid structure, directly be collected on the FTO of spin-coat process electro-conductive glass with disordered state and form composite cellulosic membrane, FTO glass is fixed by receiving system;
3) (film thickness behind the corresponding sintering is about 10 μ m, the i.e. effective thickness of photo-anode film) behind the spinning 30min is with putting into the Muffle furnace sintering after the composite cellulosic membrane drying of collecting, with 1
°Cmin
-1Speed be warming up to 500
°Be incubated 1h behind the C, obtain the TiO of nucleocapsid structure
2/ Nb
2O
5Nano fibrous membrane.
Use TiO
2/ Nb
2O
5Nuclear shell structure nano tunica fibrosa and TiO
2Nano fibrous membrane, N719 sensitizing dyestuff, I
-/ I
3 -Relevant characterization and test are carried out in standard electrolyte and the electrode assembled battery of plating Pt, and compare the performance difference between two class batteries, and test mainly comprises
I-VCharacteristic curve and photoelectric current action spectrum.
I-VThe curve test macro is made up of the xenon lamp of simulated solar light source, digital source table etc.By
I-VCurve, can obtain battery open circuit voltage (
V Oc ), short circuit current (
I Sc ), fill factor, curve factor (
FF) and conversion efficiency (
η) four parameters, wherein, conversion efficiency
ηBe the basic parameter of estimating the solar cell performance, be defined as the battery peak power output (
P Max ) and input power (
P In ) ratio, can calculate by following formula:
η=
P Max /
P In =(
FF * I Sc * V Oc )/
P In The photoelectric current action spectrum is the relation curve between IPCE and the lambda1-wavelength, measures by the QE/IPCE measuring system.So-called IPCE is the monochromatic photon of incident-electronics transformation efficiency, is defined as the light induced electron number that produces in the unit interval
NeWith incident monochromatic light subnumber
NpRatio, the photoelectricity that is battery at the different wave length place of reflection transforms situation, also is to influence short circuit current
I Sc The most critical factor.IPCE can be expressed as the product of three factors, i.e. IPCE
(λ)=
LHE (λ) * Φ Inj * Φ c , wherein
LHE (λ)The capture rate of expression light,
Φ Inj The injection efficiency of expression electronics,
Φ c It then is the collection efficiency of electronics.
The beneficial effect that the present invention has is:
Address before the background technology part, photo-anode film is carried out physical chemistry modifyings such as surface coating, and the nanostructure anode film of development of new, all be the important channel of realizing that the DSSC performance improves.The present invention adopts unique coaxial electrically spun technology to prepare TiO for DSSC light anode
2/ Nb
2O
5The nuclear shell structure nano tunica fibrosa.This technology can realize two kinds of processes of shell coating processing that quasi-one dimensional nanostructure forms and suppresses charge recombination simultaneously.The crystal boundary of quasi-one dimensional nanostructure is less, less conduction band electron and oxidation state sensitizing dyestuff and electrolyte electron acceptor I
3 -Between compound, namely increased the collection efficiency of electronics
Φ c , improved IPCE and short circuit current
I Sc The open circuit voltage of battery
V Oc Depend on TiO
2Quasi-Fermi level and electrolyte oxidation reduction are poor to electromotive force, Nb
2O
5TiO has been raised in the introducing of shell
2The quasi-Fermi level of nano fibrous membrane means battery
V Oc Improved.Correspondingly, the total conversion efficiency of battery
ηAlso be improved.As seen, the synergy of the two can improve the performance of battery better.
Description of drawings
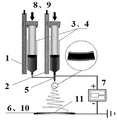
Fig. 1 is the schematic diagram of electrostatic spinning process.Among the figure: 1, TiO
2The presoma spinning solution, 2, Nb
2O
5The presoma spinning solution, 3, syringe, 4, syringe, 5, coaxial spinning head, 6, receiving system, 7, high voltage source, 8, micro-injection pump, 9, micro-injection pump, 10, electro-conductive glass, 11, composite cellulosic membrane.
The TiO that Fig. 2 makes for embodiment 1
2/ Nb
2O
5The SEM(ESEM of nuclear shell structure nano tunica fibrosa) photo.
Fig. 3 executes the TiO that example 1 makes
2/ Nb
2O
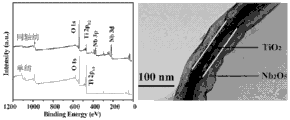
5The xps energy spectrum figure of nuclear shell structure nano tunica fibrosa and TEM(transmission electron microscope) photo.
Fig. 4 executes the TiO that example 1 makes
2/ Nb
2O
5Nuclear shell structure nano tunica fibrosa and TiO
2The IPCE photoelectric current action spectrum contrast of the DSSC of nano fibrous membrane assembling.
Fig. 5 executes the TiO that example 1 makes
2/ Nb
2O
5Nuclear shell structure nano tunica fibrosa and TiO
2The DSSC's of nano fibrous membrane assembling
I-VThe characteristic curve contrast.
Embodiment
The invention will be further described below in conjunction with drawings and Examples.
Embodiment 1:
0.68g PVAc joined in the 4ml DMF organic solvent to form the quality percentage composition be the solution of 15wt.%, in this solution, add 1ml Triton X-100,1.36g TiP, and 1.02g C
10H
25NbO
5, through the TiO of magnetic agitation 8h acquisition clarification, thickness
2/ Nb
2O
5Composite precursor spin coating liquid; And with the spin coating instrument it being spin-coated on the FTO electro-conductive glass of acetone and absolute ethyl alcohol ultrasonic cleaning the spin-coated layer that forms one deck, spin coating instrument rotating speed is 2000rmin
-1, the used spin coating time is 30s.
0.52g PVAc joined among the 4ml DMF to form the quality percentage composition be the solution of 11.5wt.%, two parts of same solution preparations, ie in solution I and solution II; In the solution I, add 0.6ml Triton X-100 and 0.78g C
10H
25NbO
5, magnetic agitation 8h obtains Nb
2O
5Presoma spinning solution 1.In the solution II, add 0.6ml Triton X-100,0.4ml HAc, and 1.04g TiP, magnetic agitation 8h obtains TiO
2Presoma spinning solution 2.With Nb
2O
5Presoma spinning solution 1 and TiO
2 Presoma spinning solution 2 is respectively charged in two syringes 3 and 4, at coaxial spinning head 5 and 6 high voltages that add 16KV of receiving system, spinning head is 12cm to the distance of dash receiver, and charged drop overcomes self under effect of electric field surface tension forms the injection thread.Control the rate of extrusion of two syringes respectively by two micro-injection pumps 8 and 9, wherein the shell rate of extrusion is 0.7ml/h, and the rate of extrusion of stratum nucleare is 0.4ml/h; Along with solvent evaporates, spray the fiber that thread solidify to form nucleocapsid structure, directly be collected in disordered state and on the FTO of spin-coat process electro-conductive glass 10, form composite cellulosic membrane 11, as shown in Figure 1; Behind the spinning 30min, with putting into the Muffle furnace sintering after composite cellulosic membrane 11 dryings of collecting, with 1
°Cmin
-1Speed be warming up to 500
°Be incubated 1h behind the C, obtain TiO
2/ Nb
2O
5The nuclear shell structure nano tunica fibrosa.The TiO that Fig. 2 makes for this embodiment
2/ Nb
2O
5The stereoscan photograph of nuclear shell structure nano tunica fibrosa.
Use TiO
2/ Nb
2O
5Nuclear shell structure nano tunica fibrosa and TiO
2Nano fibrous membrane, N719 sensitizing dyestuff, I
-/ I
3 -Relevant characterization and test are carried out in standard electrolyte and the electrode assembled battery of plating Pt, and compare the performance difference between two class batteries.Fig. 3 is xps energy spectrum figure and the transmission electron microscope photo of gained nano fibrous membrane, has confirmed TiO
2/ Nb
2O
5The formation of nucleocapsid structure; Fig. 4 is for the contrast of the photoelectric current action spectrum of two class DSSC of assembling, based on TiO
2/ Nb
2O
5The IPCE of the DSSC of anode film is higher than pure TiO
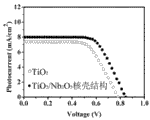
2The DSSC of film; Fig. 5 is two class DSSC of assembling
I-VThe characteristic curve contrast.Pure TiO
2Film DSSC's
V Oc =0.78V,
I Sc =7.4mAcm
-2,
FF=61%,
η=3.5%; And Nb
2O
5After shell coats
V Oc =0.85V,
I Sc =8 mAcm
-2,
FF=66%,
η=4.5%.Wherein, the photoelectric conversion efficiency of most critical brings up to 4.5% from 3.5%, has improved 28%.
Embodiment 2:
0.68g PVAc joined in the 4ml DMF organic solvent to form the quality percentage composition be the solution of 15wt.%, in this solution, add 1ml Triton X-100,1.36g TiP, and 1.02g C
10H
25NbO
5, through the TiO of magnetic agitation 8h acquisition clarification, thickness
2/ Nb
2O
5Composite precursor spin coating liquid; And with the spin coating instrument it being spin-coated on the FTO electro-conductive glass of acetone and absolute ethyl alcohol ultrasonic cleaning the spin-coated layer that forms one deck, spin coating instrument rotating speed is 2000rmin
-1, the used spin coating time is 30s.
0.52g PVAc joined among the 4ml DMF to form the quality percentage composition be the solution of 11.5wt.%, two parts of same solution preparations, ie in solution I and solution II; In the solution I, add 0.6ml Triton X-100 and 0.78g C
10H
25NbO
5, magnetic agitation 8h obtains Nb
2O
5Presoma spinning solution 1.In the solution II, add 0.6ml Triton X-100,0.4ml HAc, and 1.04g TiP, magnetic agitation 8h obtains TiO
2Presoma spinning solution 2.With Nb
2O
5Presoma spinning solution 1 and TiO
2 Presoma spinning solution 2 is respectively charged in two syringes 3 and 4, at coaxial spinning head 5 and 6 high voltages that add 16KV of receiving system, spinning head is 12cm to the distance of dash receiver, and charged drop overcomes self under effect of electric field surface tension forms the injection thread.Control the rate of extrusion of two syringes respectively by two micro-injection pumps 8 and 9, wherein the shell rate of extrusion is 0.6ml/h, and the rate of extrusion of stratum nucleare is 0.5ml/h; Along with solvent evaporates, spray the fiber that thread solidify to form nucleocapsid structure, directly be collected in disordered state and on the FTO of spin-coat process electro-conductive glass 10, form composite cellulosic membrane 11, as shown in Figure 1; Behind the spinning 30min, with putting into the Muffle furnace sintering after composite cellulosic membrane 11 dryings of collecting, with 1
°Cmin
-1Speed be warming up to 500
°Be incubated 1h behind the C, obtain TiO
2/ Nb
2O
5The nuclear shell structure nano tunica fibrosa.The TiO that Fig. 2 makes for this embodiment
2/ Nb
2O
5The stereoscan photograph of nuclear shell structure nano tunica fibrosa.
Use TiO
2/ Nb
2O
5Nuclear shell structure nano tunica fibrosa and TiO
2Nano fibrous membrane, N719 sensitizing dyestuff, I
-/ I
3 -Relevant characterization and test are carried out in standard electrolyte and the electrode assembled battery of plating Pt, and compare the performance difference between two class batteries.Characterize to the process of performance test and result and embodiment 1 in similar, related data and picture are not listed as.Wherein, the photoelectric conversion efficiency of most critical brings up to 4.66% from 3.5%, has improved 33%.
Embodiment 3:
0.68g PVAc joined in the 4ml DMF organic solvent to form the quality percentage composition be the solution of 15wt.%, in this solution, add 1ml Triton X-100,1.36g TiP, and 1.02g C
10H
25NbO
5, through the TiO of magnetic agitation 8h acquisition clarification, thickness
2/ Nb
2O
5Composite precursor spin coating liquid; And with the spin coating instrument it being spin-coated on the FTO electro-conductive glass of acetone and absolute ethyl alcohol ultrasonic cleaning the spin-coated layer that forms one deck, spin coating instrument rotating speed is 2000rmin
-1, the used spin coating time is 30s.
0.52g PVAc joined among the 4ml DMF to form the quality percentage composition be the solution of 11.5wt.%, two parts of same solution preparations, ie in solution I and solution II; In the solution I, add 0.6ml Triton X-100 and 0.78g C
10H
25NbO
5, magnetic agitation 8h obtains Nb
2O
5Presoma spinning solution 1.In the solution II, add 0.6ml Triton X-100,0.4ml HAc, and 1.04g TiP, magnetic agitation 8h obtains TiO
2Presoma spinning solution 2.With Nb
2O
5Presoma spinning solution 1 and TiO
2 Presoma spinning solution 2 is respectively charged in two syringes 3 and 4, at coaxial spinning head 5 and 6 high voltages that add 16KV of receiving system, spinning head is 12cm to the distance of dash receiver, and charged drop overcomes self under effect of electric field surface tension forms the injection thread.Control the rate of extrusion of two syringes respectively by two micro-injection pumps 8 and 9, wherein the shell rate of extrusion is 0.5ml/h, and the rate of extrusion of stratum nucleare is 0.6ml/h; Along with solvent evaporates, spray the fiber that thread solidify to form nucleocapsid structure, directly be collected in disordered state and on the FTO of spin-coat process electro-conductive glass 10, form composite cellulosic membrane 11, as shown in Figure 1; Behind the spinning 30min, with putting into the Muffle furnace sintering after composite cellulosic membrane 11 dryings of collecting, with 1
°Cmin
-1Speed be warming up to 500
°Be incubated 1h behind the C, obtain TiO
2/ Nb
2O
5The nuclear shell structure nano tunica fibrosa.The TiO that Fig. 2 makes for this embodiment
2/ Nb
2O
5The stereoscan photograph of nuclear shell structure nano tunica fibrosa.
Use TiO
2/ Nb
2O
5Nuclear shell structure nano tunica fibrosa and TiO
2Nano fibrous membrane, N719 sensitizing dyestuff, I
-/ I
3 -Relevant characterization and test are carried out in standard electrolyte and the electrode assembled battery of plating Pt, and compare the performance difference between two class batteries.Characterize to the process of performance test and result and embodiment 1 in similar, related data and picture are unlisted.Wherein, the photoelectric conversion efficiency of most critical brings up to 4.34% from 3.5%, has improved 24%.
Claims (4)
1. a dye-sensitized cell is characterized in that the step of this method is as follows with the preparation method of titanium dioxide-niobium pentaoxide nuclear shell structure nano tunica fibrosa:
1) preparation PVAc quality percentage composition is the DMF solution of 15wt.%, is the surfactant Triton X-100 of DMF solution 25% with volume, and 2 times of quality are to the TiP of PVAc, and 1.5 times of quality are to the C of PVAc
10H
25NbO
5Join in the DMF solution; Obtain TiO through magnetic agitation 8h
2/ Nb
2O
5Composite precursor spin coating liquid, and with the spin coating instrument it is spin-coated on the FTO electro-conductive glass spin-coated layer that forms one deck;
2) preparation PVAc quality percentage composition is the DMF solution of 11.5wt.%, is the surfactant Triton X-100 of DMF solution 15% with volume, and 1.5 times of quality are to the C of PVAc
10H
25NbO
5Join in the DMF solution, obtain Nb through magnetic agitation 8h
2O
5Presoma spinning solution (1); Preparation PVAc quality percentage composition is the DMF solution of 11.5wt.%, be the surfactant Triton X-100 of DMF solution 15% with volume, volume is the HAc of DMF solution 10%, and 2 times of TiP to PVAc of quality join in the DMF solution, obtains TiO through magnetic agitation 8h
2Presoma spinning solution (2); Presoma spinning solution (1) and (2) are respectively charged in syringe (3) and (4), between coaxial spinning head (5) and receiving system (6), add high voltage (7), charged drop overcomes self under effect of electric field surface tension forms the injection thread, is controlled the rate of extrusion of (3) and (4) respectively by micro-injection pump (8) and (9); Along with solvent evaporates, spray the fiber that thread solidify to form nucleocapsid structure, directly be collected in through the FTO of spin-coat process electro-conductive glass (10) with disordered state and go up formation composite cellulosic membrane (11), FTO glass (10) is fixing by receiving system (6); 3) behind the spinning 30min, with putting into the Muffle furnace sintering after tunica fibrosa (11) drying of collecting, with 1
°Cmin
-1Speed be warming up to 500
°Be incubated 1h behind the C, obtain the TiO of nucleocapsid structure
2/ Nb
2O
5Nano fibrous membrane.
2. a kind of dye-sensitized cell according to claim 1 is with the preparation method of titanium dioxide-niobium pentaoxide nuclear shell structure nano tunica fibrosa, and it is characterized in that: the spin coating instrument rotating speed that adopts in the described step 1) is 2000rmin
-1, the spin coating time is 30s.
3. a kind of dye-sensitized cell according to claim 1 is with the preparation method of titanium dioxide-niobium pentaoxide nuclear shell structure nano tunica fibrosa, it is characterized in that: micro-injection pump (8) is that the rate of extrusion of shell is 0.5 ~ 0.7 ml/h described step 2), and micro-injection pump (9) is that the rate of extrusion of stratum nucleare is 0.4 ~ 0.6 ml/h.
4. a kind of dye-sensitized cell according to claim 1 is characterized in that: TiO with the preparation method of titanium dioxide-niobium pentaoxide nuclear shell structure nano tunica fibrosa
2/ Nb
2O
5DSSC and the TiO of the assembling of nuclear shell structure nano tunica fibrosa
2The DSSC of nano fibrous membrane assembling compares photoelectric conversion efficiency
ηImproved 24% ~ 33%.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN 201110399846 CN102496479B (en) | 2011-12-06 | 2011-12-06 | Method for preparing titanium dioxide (TiO2)/niobium pentoxide (Nb2O5) core-shell structure nano-fiber film for dye-sensitized solar cell |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN 201110399846 CN102496479B (en) | 2011-12-06 | 2011-12-06 | Method for preparing titanium dioxide (TiO2)/niobium pentoxide (Nb2O5) core-shell structure nano-fiber film for dye-sensitized solar cell |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN102496479A CN102496479A (en) | 2012-06-13 |
| CN102496479B true CN102496479B (en) | 2013-07-17 |
Family
ID=46188290
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN 201110399846 Expired - Fee Related CN102496479B (en) | 2011-12-06 | 2011-12-06 | Method for preparing titanium dioxide (TiO2)/niobium pentoxide (Nb2O5) core-shell structure nano-fiber film for dye-sensitized solar cell |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN102496479B (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101244381A (en) * | 2008-03-07 | 2008-08-20 | 东南大学 | Process for producing TiO2-Al2O3composite nano-powder body material |
| CN101582332A (en) * | 2009-06-29 | 2009-11-18 | 中国科学院等离子体物理研究所 | Application of down-conversion luminescent material on dye-sensitized solar cells |
| CN101834068A (en) * | 2009-03-13 | 2010-09-15 | 中国科学院福建物质结构研究所 | Core-shell structure positive electrode for dye sensitization solar battery and preparation method thereof |
| CN102082031A (en) * | 2009-11-27 | 2011-06-01 | 济南大学 | Novel dye-sensitized solar cell photoanode |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006078281A2 (en) * | 2004-07-07 | 2006-07-27 | Nanosys, Inc. | Systems and methods for harvesting and integrating nanowires |
-
2011
- 2011-12-06 CN CN 201110399846 patent/CN102496479B/en not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101244381A (en) * | 2008-03-07 | 2008-08-20 | 东南大学 | Process for producing TiO2-Al2O3composite nano-powder body material |
| CN101834068A (en) * | 2009-03-13 | 2010-09-15 | 中国科学院福建物质结构研究所 | Core-shell structure positive electrode for dye sensitization solar battery and preparation method thereof |
| CN101582332A (en) * | 2009-06-29 | 2009-11-18 | 中国科学院等离子体物理研究所 | Application of down-conversion luminescent material on dye-sensitized solar cells |
| CN102082031A (en) * | 2009-11-27 | 2011-06-01 | 济南大学 | Novel dye-sensitized solar cell photoanode |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102496479A (en) | 2012-06-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102496471B (en) | Method for preparing titanium dioxide-zinc oxide nuclear shell structure nanometer fiber membrane for dye sensitized battery | |
| US9368287B2 (en) | Dye-sensitized solar cell with metal oxide layer containing metal oxide nanoparticles produced by electrospinning and method for manufacturing same | |
| CN101901693B (en) | Graphene composite dye-sensitized solar cell light anode and preparation method thereof | |
| Li et al. | Detachment and transfer of ordered TiO 2 nanotube arrays for front-illuminated dye-sensitized solar cells | |
| CN101728083B (en) | Heterostructure photoanode for dye-sensitized solar cell and manufacturing method thereof | |
| US20150144199A1 (en) | Dye-sensitized solar cell having carbon nano-web coated with graphene and method for manufacturing same | |
| Yeh et al. | Preparing core–shell structure of ZnO@ TiO2 nanowires through a simple dipping–rinse–hydrolyzation process as the photoanode for dye-sensitized solar cells | |
| CN102496485B (en) | Method for improving firmness of combination of dye-sensitized cell nanometer fibrous membrane and conductive glass | |
| Zhu et al. | Facile fabrication of open-ended TiO2 nanotube arrays with large area for efficient dye-sensitized solar cells | |
| Wu et al. | Anodic TiO2 nanotube arrays for dye-sensitized solar cells characterized by electrochemical impedance spectroscopy | |
| Kim et al. | Influence of TiO 2 coating thickness on energy conversion efficiency of dye-sensitized solar cells | |
| JP2015185836A (en) | Composite self-supporting film, solar cell, method of producing composite self-supporting film | |
| CN109065724B (en) | Mo-titanium dioxide-AgNWs flexible perovskite solar cell and preparation method thereof | |
| CN104310794A (en) | Porous TiO2 nanocrystalline thin film having three-dimensional nanorod floral structure as well as preparation method and application of porous TiO2 nanocrystalline thin film | |
| Wang et al. | Effects of low pressure plasma treatments on DSSCs based on rutile TiO2 array photoanodes | |
| KR101431817B1 (en) | Double device merged tandem solar cell and its production method | |
| CN101697320B (en) | Dye-sensitized solar cell photoanode and preparation method thereof | |
| CN105702472A (en) | Solar cell electrode, preparation method therefor, and solar cell | |
| CN102496478A (en) | Method for improving performance of nanofiber membrane of dye-sensitized battery by synergistic action of carbon nanotubes and titanium tetrachloride | |
| CN102751096B (en) | A kind of transparent two sides dye-sensitized solar cell anode | |
| CN102832051B (en) | A kind of preparation method of dye-sensitized solar cell anode | |
| CN102496479B (en) | Method for preparing titanium dioxide (TiO2)/niobium pentoxide (Nb2O5) core-shell structure nano-fiber film for dye-sensitized solar cell | |
| Arifin et al. | Characteristics of ZnO nanofiber in double Layer (TiO2/ZnO) DSSC results of direct deposition electrospinning manufacturing: Variation of tip to collector distance | |
| Khusaini et al. | The influence of electrospinning flow rate parameter on ZnO nanofiber as photoanode of dye-sensitized solar cell | |
| CN103972398A (en) | Organic and inorganic hybridization solar cell and manufacturing method of organic and inorganic hybridization solar cell |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20130717 Termination date: 20151206 |
|
| EXPY | Termination of patent right or utility model |