CN102335168A - 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗骨质疏松药物中的应用 - Google Patents
吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗骨质疏松药物中的应用 Download PDFInfo
- Publication number
- CN102335168A CN102335168A CN2011103267311A CN201110326731A CN102335168A CN 102335168 A CN102335168 A CN 102335168A CN 2011103267311 A CN2011103267311 A CN 2011103267311A CN 201110326731 A CN201110326731 A CN 201110326731A CN 102335168 A CN102335168 A CN 102335168A
- Authority
- CN
- China
- Prior art keywords
- indole
- carbinol
- osteoporosis
- methyl hydride
- dim
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- IVYPNXXAYMYVSP-UHFFFAOYSA-N Indole-3-carbinol Natural products C1=CC=C2C(CO)=CNC2=C1 IVYPNXXAYMYVSP-UHFFFAOYSA-N 0.000 title claims abstract description 58
- 235000002279 indole-3-carbinol Nutrition 0.000 title claims abstract description 30
- 208000001132 Osteoporosis Diseases 0.000 title claims abstract description 29
- 238000002360 preparation method Methods 0.000 title claims abstract description 27
- 239000003814 drug Substances 0.000 title claims abstract description 18
- RUMVKBSXRDGBGO-UHFFFAOYSA-N indole-3-carbinol Chemical compound C1=CC=C[C]2C(CO)=CN=C21 RUMVKBSXRDGBGO-UHFFFAOYSA-N 0.000 title claims 2
- TWJAXIHBWPVMIR-UHFFFAOYSA-N diindolylmethane Natural products C1=CC=C2NC(CC=3NC4=CC=CC=C4C=3)=CC2=C1 TWJAXIHBWPVMIR-UHFFFAOYSA-N 0.000 title abstract description 6
- VFTRKSBEFQDZKX-UHFFFAOYSA-N 3,3'-diindolylmethane Chemical compound C1=CC=C2C(CC=3C4=CC=CC=C4NC=3)=CNC2=C1 VFTRKSBEFQDZKX-UHFFFAOYSA-N 0.000 title abstract 5
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 claims description 16
- 125000003545 alkoxy group Chemical group 0.000 claims description 16
- 229910052739 hydrogen Inorganic materials 0.000 claims description 16
- 239000001257 hydrogen Substances 0.000 claims description 16
- XGDLHLISLBPXPR-UHFFFAOYSA-N C.C1=CC=C2NC=CC2=C1.C1=CC=C2NC=CC2=C1 Chemical compound C.C1=CC=C2NC=CC2=C1.C1=CC=C2NC=CC2=C1 XGDLHLISLBPXPR-UHFFFAOYSA-N 0.000 claims description 12
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 12
- 241001597008 Nomeidae Species 0.000 claims description 12
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 8
- 125000001424 substituent group Chemical group 0.000 claims description 6
- 125000005843 halogen group Chemical group 0.000 claims description 2
- 150000001875 compounds Chemical class 0.000 abstract description 21
- 210000002997 osteoclast Anatomy 0.000 abstract description 6
- 208000024891 symptom Diseases 0.000 abstract description 4
- 238000011633 osteoporosis animal model Methods 0.000 abstract description 3
- 230000001717 pathogenic effect Effects 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 43
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 39
- 229910052757 nitrogen Inorganic materials 0.000 description 24
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 21
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 15
- 241000700159 Rattus Species 0.000 description 13
- WHOOUMGHGSPMGR-UHFFFAOYSA-N indol-3-ylacetaldehyde Chemical class C1=CC=C2C(CC=O)=CNC2=C1 WHOOUMGHGSPMGR-UHFFFAOYSA-N 0.000 description 12
- 235000011837 pasties Nutrition 0.000 description 12
- BZCGWAXQDLXLQM-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O.ClP(Cl)(Cl)=O BZCGWAXQDLXLQM-UHFFFAOYSA-N 0.000 description 12
- 239000000243 solution Substances 0.000 description 12
- 238000004809 thin layer chromatography Methods 0.000 description 12
- 238000001291 vacuum drying Methods 0.000 description 12
- 239000007787 solid Substances 0.000 description 10
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 8
- 238000000034 method Methods 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 241000283073 Equus caballus Species 0.000 description 7
- 230000037182 bone density Effects 0.000 description 7
- 230000001009 osteoporotic effect Effects 0.000 description 7
- 239000008363 phosphate buffer Substances 0.000 description 7
- 239000012279 sodium borohydride Substances 0.000 description 7
- 229910000033 sodium borohydride Inorganic materials 0.000 description 7
- 210000002700 urine Anatomy 0.000 description 7
- 238000007605 air drying Methods 0.000 description 6
- 239000007864 aqueous solution Substances 0.000 description 6
- 238000009835 boiling Methods 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 238000001816 cooling Methods 0.000 description 6
- 238000010438 heat treatment Methods 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 239000000376 reactant Substances 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 239000000725 suspension Substances 0.000 description 6
- 238000005406 washing Methods 0.000 description 6
- 210000000689 upper leg Anatomy 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 4
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 4
- 150000002431 hydrogen Chemical group 0.000 description 4
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 4
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 4
- MLLQRDYRRHXRTP-UHFFFAOYSA-N (1-butyl-2-methylindol-3-yl)methanol Chemical compound C(CCC)N1C(=C(C2=CC=CC=C12)CO)C MLLQRDYRRHXRTP-UHFFFAOYSA-N 0.000 description 3
- ALSCHTQXMJNCJS-UHFFFAOYSA-N (4-bromo-1h-indol-3-yl)methanol Chemical compound C1=CC(Br)=C2C(CO)=CNC2=C1 ALSCHTQXMJNCJS-UHFFFAOYSA-N 0.000 description 3
- DUSHSPLKAUXPEY-UHFFFAOYSA-N (5-chloro-1h-indol-3-yl)methanol Chemical compound C1=C(Cl)C=C2C(CO)=CNC2=C1 DUSHSPLKAUXPEY-UHFFFAOYSA-N 0.000 description 3
- 208000006386 Bone Resorption Diseases 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- JYWPLBCRBPLXBG-UHFFFAOYSA-N [N+](=O)([O-])C=1C=C2C(CNC2=CC1)CO Chemical compound [N+](=O)([O-])C=1C=C2C(CNC2=CC1)CO JYWPLBCRBPLXBG-UHFFFAOYSA-N 0.000 description 3
- 210000000988 bone and bone Anatomy 0.000 description 3
- 230000024279 bone resorption Effects 0.000 description 3
- 239000007795 chemical reaction product Substances 0.000 description 3
- 238000009833 condensation Methods 0.000 description 3
- 230000005494 condensation Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 125000001041 indolyl group Chemical group 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 210000002303 tibia Anatomy 0.000 description 3
- OPAHUBUOHKIOOL-UHFFFAOYSA-N 1-butyl-2-methylindole Chemical compound C1=CC=C2N(CCCC)C(C)=CC2=C1 OPAHUBUOHKIOOL-UHFFFAOYSA-N 0.000 description 2
- IZSXLPLQAFHBKZ-UHFFFAOYSA-N 2-(1-butyl-2-methylindol-3-yl)acetaldehyde Chemical compound C(CCC)N1C(=C(C2=CC=CC=C12)CC=O)C IZSXLPLQAFHBKZ-UHFFFAOYSA-N 0.000 description 2
- QZBYXAGBNVQBGV-UHFFFAOYSA-N 2-(5-chloro-1H-indol-3-yl)acetaldehyde Chemical compound ClC=1C=C2C(=CNC2=CC=1)CC=O QZBYXAGBNVQBGV-UHFFFAOYSA-N 0.000 description 2
- WJQWYAJTPPYORB-UHFFFAOYSA-N 5-nitro-2,3-dihydro-1h-indole Chemical compound [O-][N+](=O)C1=CC=C2NCCC2=C1 WJQWYAJTPPYORB-UHFFFAOYSA-N 0.000 description 2
- KJNFUMMBZDMUDE-UHFFFAOYSA-N BrC1=CC=CC2=C1C(CC=O)=CN2 Chemical compound BrC1=CC=CC2=C1C(CC=O)=CN2 KJNFUMMBZDMUDE-UHFFFAOYSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 2
- YBCQFLPSHXYBRB-UHFFFAOYSA-N [N+](=O)([O-])C=1C=C2C(CNC2=CC1)CC=O Chemical compound [N+](=O)([O-])C=1C=C2C(CNC2=CC1)CC=O YBCQFLPSHXYBRB-UHFFFAOYSA-N 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 230000023852 carbohydrate metabolic process Effects 0.000 description 2
- 235000021256 carbohydrate metabolism Nutrition 0.000 description 2
- 229940109239 creatinine Drugs 0.000 description 2
- 229940000406 drug candidate Drugs 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 230000009245 menopause Effects 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 239000011574 phosphorus Substances 0.000 description 2
- 208000001685 postmenopausal osteoporosis Diseases 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 230000003449 preventive effect Effects 0.000 description 2
- 210000000582 semen Anatomy 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
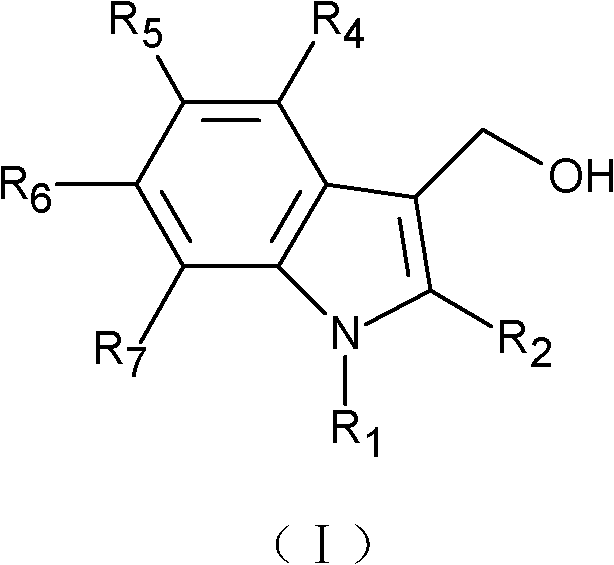
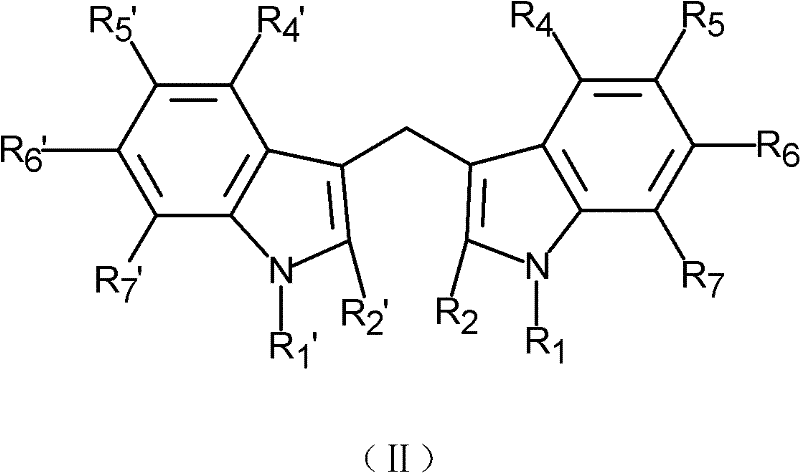
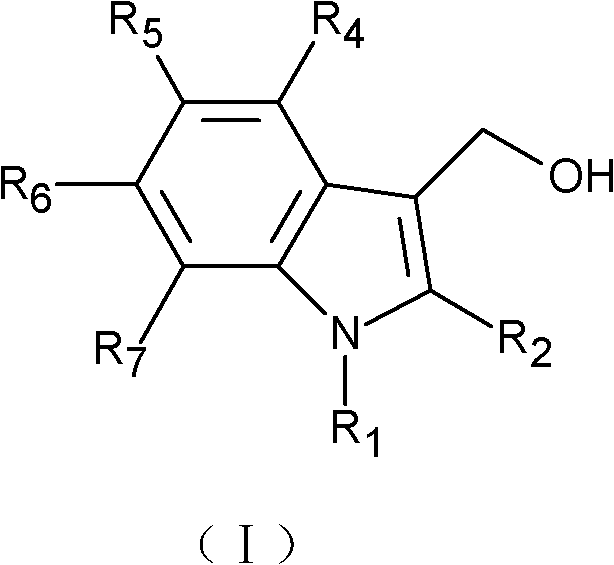
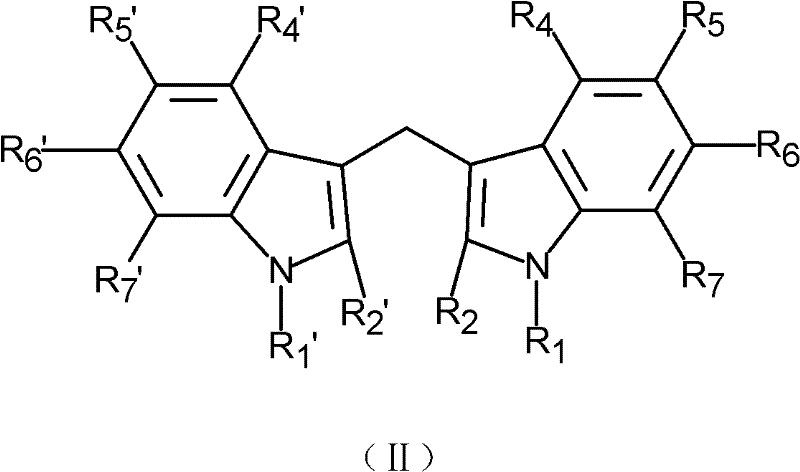
- 0 *c1c(CO)c(c(*)c(*)c(*)c2*)c2[n]1* Chemical compound *c1c(CO)c(c(*)c(*)c(*)c2*)c2[n]1* 0.000 description 1
- GRJZJFUBQYULKL-UHFFFAOYSA-N 4-bromo-1h-indole Chemical compound BrC1=CC=CC2=C1C=CN2 GRJZJFUBQYULKL-UHFFFAOYSA-N 0.000 description 1
- MYTGFBZJLDLWQG-UHFFFAOYSA-N 5-chloro-1h-indole Chemical compound ClC1=CC=C2NC=CC2=C1 MYTGFBZJLDLWQG-UHFFFAOYSA-N 0.000 description 1
- 108010005094 Advanced Glycation End Products Proteins 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 208000020084 Bone disease Diseases 0.000 description 1
- 206010065687 Bone loss Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 241000219193 Brassicaceae Species 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 206010015719 Exsanguination Diseases 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 206010027336 Menstruation delayed Diseases 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- -1 aldehyde radical Chemical class 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000002146 bilateral effect Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 201000008275 breast carcinoma Diseases 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 230000002308 calcification Effects 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000006482 condensation reaction Methods 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000009513 drug distribution Methods 0.000 description 1
- 230000036267 drug metabolism Effects 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 201000003914 endometrial carcinoma Diseases 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 210000001105 femoral artery Anatomy 0.000 description 1
- 210000004211 gastric acid Anatomy 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 238000002657 hormone replacement therapy Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 206010025482 malaise Diseases 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 210000003928 nasal cavity Anatomy 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 230000011164 ossification Effects 0.000 description 1
- 210000000963 osteoblast Anatomy 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011552 rat model Methods 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229940126586 small molecule drug Drugs 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
Landscapes
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
| 组别 | 例数(只) | 股骨(g/cm2) | 胫骨(g/cm2) |
| 假手术组 | 13 | 0.264±0.021 | 0.237±0.015 |
| 卵巢切除组 | 13 | 0.214±0.005 | 0.208±0.009 |
| I3C | 13 | 0.244±0.019 | 0.223±0.016 |
| DIM | 13 | 0.249±0.002 | 0.221±0.015 |
| 5-Cl-I3C | 13 | 0.248±0.007 | 0.226±0.004 |
| 5,5’-Cl-DIM | 13 | 0.250±0.011 | 0.227±0.024 |
| 5-C5-I3C | 13 | 0.241±0.015 | 0.228±0.018 |
| 5,5’-C5-DIM | 13 | 0.243±0.016 | 0.222±0.024 |
| 5-MOE-I3C | 13 | 0.247±0.012 | 0.229±0.021 |
| 5,5’-MOE-DIM | 13 | 0.244±0.021 | 0.228±0.014 |
| 5-NO-I3C | 13 | 0.251±0.010 | 0.226±0.008 |
| 5,5’-NO-DIM | 13 | 0.245±0.012 | 0.227±0.014 |
| N-Me-I3C | 13 | 0.246±0.014 | 0.228±0.017 |
| N,N’-Me-DIM | 13 | 0.243±0.002 | 0.225±0.021 |
| N-MOE-I3C | 13 | 0.241±0.009 | 0.225±0.013 |
| N,N’-MOE-DIM | 13 | 0.249±0.013 | 0.226±0.002 |
| 2-C5-I3C | 13 | 0.248±0.017 | 0.226±0.015 |
| 2,2’-C5-DIM | 13 | 0.242±0.014 | 0.229±0.005 |
| 2-MOE-I3C | 13 | 0.242±0.004 | 0.224±0.019 |
| 2,2’-MOE-DIM | 13 | 0.242±0.013 | 0.227±0.013 |
| 1Bu-2Me-I3C | 13 | 0.246±0.023 | 0.227±0.018 |
| 1,1’Bu-2,2’Me-DIM | 13 | 0.243±0.008 | 0.221±0.011 |
| 4-Br-I3C | 13 | 0.245±0.015 | 0.223±0.017 |
| 4,4’-Br-DIM | 13 | 0.242±0.016 | 0.221±0.014 |
Claims (8)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201110326731.1A CN102335168B (zh) | 2011-10-25 | 2011-10-25 | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗骨质疏松药物中的应用 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201110326731.1A CN102335168B (zh) | 2011-10-25 | 2011-10-25 | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗骨质疏松药物中的应用 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN102335168A true CN102335168A (zh) | 2012-02-01 |
| CN102335168B CN102335168B (zh) | 2014-04-02 |
Family
ID=45511271
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201110326731.1A Active CN102335168B (zh) | 2011-10-25 | 2011-10-25 | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗骨质疏松药物中的应用 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN102335168B (zh) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102895227A (zh) * | 2012-09-28 | 2013-01-30 | 达瑞医药香港有限公司 | 3,3’-二吲哚甲烷在制备治疗乳痛症的药物中的应用 |
| CN105963294A (zh) * | 2016-06-18 | 2016-09-28 | 张阳康 | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗红斑狼疮药物中的应用 |
| CN106074505A (zh) * | 2016-06-18 | 2016-11-09 | 张阳康 | 吲哚‑3‑甲醇、二吲哚甲烷及其衍生物在制备治疗毛囊角化症药物中的应用 |
| CN106074504A (zh) * | 2016-06-18 | 2016-11-09 | 张阳康 | 吲哚‑3‑甲醇、二吲哚甲烷及其衍生物在制备治疗前列腺肥大药物中的应用 |
| CN113248472A (zh) * | 2020-02-12 | 2021-08-13 | 中国药科大学 | 抗骨质疏松化合物及其衍生物,药物组合物、制备方法和应用 |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1398264A (zh) * | 2000-02-11 | 2003-02-19 | 默克专利股份有限公司 | 吲哚-3-基衍生物 |
| WO2004052854A2 (en) * | 2002-12-10 | 2004-06-24 | Wyeth | Aryl, aryloxy, and alkyloxy substituted 1h-indol-3-yl glyoxylic acid derivatives as inhibitors of plasminogen activator inhibitor-1 (pai-1) |
| CN1686115A (zh) * | 2005-04-06 | 2005-10-26 | 黄晶 | 吲哚-3-甲醇及其二聚体在制备防治增殖性血管疾病药物中的应用 |
| WO2006105196A2 (en) * | 2005-03-28 | 2006-10-05 | Bioresponse, L.L.C. | Diindolylmethane-based compositions and methods of use thereof for promoting oral mucosal and bone health |
| KR20090042689A (ko) * | 2007-10-27 | 2009-04-30 | 한림대학교 산학협력단 | 3,3’-디인돌릴메탄을 유효성분으로 함유하는 염증 질환의예방 및 치료용 조성물 |
| CN101428016A (zh) * | 2008-12-23 | 2009-05-13 | 南京大学 | 二吲哚甲烷及其衍生物在类风湿关节炎治疗中的应用 |
| KR20100070664A (ko) * | 2008-12-18 | 2010-06-28 | 연세대학교 산학협력단 | 인돌-3-카비놀 유도체를 유효성분으로 포함하는 비만, 고지혈증, 지방간 또는 당뇨의 예방 또는 치료용 조성물 |
| CN101940568A (zh) * | 2010-08-17 | 2011-01-12 | 合肥博太医药生物技术发展有限公司 | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗蒽环抗癌药引起的心脏衰竭药物中的应用 |
-
2011
- 2011-10-25 CN CN201110326731.1A patent/CN102335168B/zh active Active
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1398264A (zh) * | 2000-02-11 | 2003-02-19 | 默克专利股份有限公司 | 吲哚-3-基衍生物 |
| WO2004052854A2 (en) * | 2002-12-10 | 2004-06-24 | Wyeth | Aryl, aryloxy, and alkyloxy substituted 1h-indol-3-yl glyoxylic acid derivatives as inhibitors of plasminogen activator inhibitor-1 (pai-1) |
| WO2006105196A2 (en) * | 2005-03-28 | 2006-10-05 | Bioresponse, L.L.C. | Diindolylmethane-based compositions and methods of use thereof for promoting oral mucosal and bone health |
| CN1686115A (zh) * | 2005-04-06 | 2005-10-26 | 黄晶 | 吲哚-3-甲醇及其二聚体在制备防治增殖性血管疾病药物中的应用 |
| KR20090042689A (ko) * | 2007-10-27 | 2009-04-30 | 한림대학교 산학협력단 | 3,3’-디인돌릴메탄을 유효성분으로 함유하는 염증 질환의예방 및 치료용 조성물 |
| KR20100070664A (ko) * | 2008-12-18 | 2010-06-28 | 연세대학교 산학협력단 | 인돌-3-카비놀 유도체를 유효성분으로 포함하는 비만, 고지혈증, 지방간 또는 당뇨의 예방 또는 치료용 조성물 |
| CN101428016A (zh) * | 2008-12-23 | 2009-05-13 | 南京大学 | 二吲哚甲烷及其衍生物在类风湿关节炎治疗中的应用 |
| CN101940568A (zh) * | 2010-08-17 | 2011-01-12 | 合肥博太医药生物技术发展有限公司 | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗蒽环抗癌药引起的心脏衰竭药物中的应用 |
Non-Patent Citations (2)
| Title |
|---|
| PING JIE ET AL: "Effect of indole-3-carbinol on activation,proliferation and collagen secretion of rat hepatic stellate cells", 《中国药理学与毒理学杂志》 * |
| 张春等: "吲哚-3-原醇对乙醇损伤性大鼠肝切片的保护作用", 《中国药理学通报》 * |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102895227A (zh) * | 2012-09-28 | 2013-01-30 | 达瑞医药香港有限公司 | 3,3’-二吲哚甲烷在制备治疗乳痛症的药物中的应用 |
| CN105963294A (zh) * | 2016-06-18 | 2016-09-28 | 张阳康 | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗红斑狼疮药物中的应用 |
| CN106074505A (zh) * | 2016-06-18 | 2016-11-09 | 张阳康 | 吲哚‑3‑甲醇、二吲哚甲烷及其衍生物在制备治疗毛囊角化症药物中的应用 |
| CN106074504A (zh) * | 2016-06-18 | 2016-11-09 | 张阳康 | 吲哚‑3‑甲醇、二吲哚甲烷及其衍生物在制备治疗前列腺肥大药物中的应用 |
| CN113248472A (zh) * | 2020-02-12 | 2021-08-13 | 中国药科大学 | 抗骨质疏松化合物及其衍生物,药物组合物、制备方法和应用 |
| CN113248472B (zh) * | 2020-02-12 | 2022-06-28 | 中国药科大学 | 抗骨质疏松化合物及其衍生物,药物组合物、制备方法和应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102335168B (zh) | 2014-04-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102335168B (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗骨质疏松药物中的应用 | |
| CN101940568B (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗蒽环抗癌药引起的心脏衰竭药物中的应用 | |
| CN102389419A (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备防治动脉粥样硬化药物中的应用 | |
| CN101947234A (zh) | 含有氨基葡萄糖制剂的制备方法及其应用 | |
| CN102370638B (zh) | 3,3’-二吲哚甲烷及衍生物在制备治疗肝脏疾病药物中的应用 | |
| CN101810627B (zh) | 复方磺胺间甲氧嘧啶或复方磺胺间甲氧嘧啶钠注射液及制备方法 | |
| US3968249A (en) | Method of treating malignant neoplastic disease | |
| CN102327262A (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗糖尿病药物中的应用 | |
| EP4324836A1 (en) | Novel boron carrier, preparation method and pharmaceutical formulation thereof | |
| CN101279967B (zh) | 一种治疗癌症的三甲基呫吨酮-4-乙酸药物组合物及其用途 | |
| CN102429901B (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备防治肾纤维化药物中的应用 | |
| AU2003285351B2 (en) | Agent having a destructive effect on malignant tumors and method for the production thereof | |
| CN102335169B (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备治疗老年痴呆药物中的应用 | |
| CN105012297A (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗接触性皮炎药物的应用 | |
| CN102389420A (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物在制备防治肺纤维化药物中的应用 | |
| CN102526038A (zh) | 替莫唑胺的脑靶向药物组合物及其应用 | |
| CN106565718A (zh) | 含硒化合物及其用途 | |
| CN106265644A (zh) | 吲哚‑3‑甲醇、二吲哚甲烷及其衍生物在制备治疗脂溢性脱发药物中的应用 | |
| NO329746B1 (no) | Anvendelse av metoksymorfolinodoksorubicin for behandling av levertumor og en farmasoytisk sammensetning inneholdende denne | |
| Ajagun-Ogunleye et al. | Hypoglycemic and high dosage effects of bidens pilosa in type-1 diabetes mellitus | |
| CN106265643A (zh) | 吲哚‑3‑甲醇、二吲哚甲烷及其衍生物在制备治疗前列腺肥大药物中的应用 | |
| CN1078462C (zh) | 棉酚和/或其衍生物作为有效成分在制备用于治疗前列腺炎的栓剂中的应用 | |
| CN102008461A (zh) | 一种注射用布洛芬药物组合物 | |
| CN104906086A (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗肾小球肾炎药物的应用 | |
| CN105030766A (zh) | 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗过敏性咽炎药物的应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20170523 Address after: 230088, Hefei province high tech Zone Innovation Avenue 2800, innovation industry park two, E District 1, B, building 2, Anhui Patentee after: HEFEI SHUOJIAN PHARMACEUTICAL TECHNOLOGY CO.,LTD. Address before: 230088 No. 26, phreatic East Road, hi tech Zone, Anhui, Hefei Patentee before: Hefei Boltec Biopharm Co.,Ltd. |
|
| TR01 | Transfer of patent right | ||
| TR01 | Transfer of patent right | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20170815 Address after: 242300 Anhui province Ningguo harbor eco industrial park road and road intersection Pik conch Patentee after: NINGGUO WUYUE MEDICAL TECHNOLOGY CO.,LTD. Address before: 230088, Hefei province high tech Zone Innovation Avenue 2800, innovation industry park two, E District 1, B, building 2, Anhui Patentee before: HEFEI SHUOJIAN PHARMACEUTICAL TECHNOLOGY CO.,LTD. |
|
| TR01 | Transfer of patent right | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20231102 Address after: 230601 First Floor, Building 2-A, Furong Road North, Economic and Technological Development Zone, Hefei City, Anhui Province Patentee after: HEFEI DAMI MEDICAL SCIENCE & TECHNOLOGY Co.,Ltd. Address before: 242300 intersection of Hailuo road and Biyun road in Ningguo port eco industrial park, Anhui Province Patentee before: NINGGUO WUYUE MEDICAL TECHNOLOGY CO.,LTD. |
|
| TR01 | Transfer of patent right | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20231225 Address after: No. 88 Dong'an Road, Anzhen Street, Xishan District, Wuxi City, Jiangsu Province, 214104 Patentee after: Wuxi Kanghe Qingyuan Biotechnology Co.,Ltd. Address before: 230601 First Floor, Building 2-A, Furong Road North, Economic and Technological Development Zone, Hefei City, Anhui Province Patentee before: HEFEI DAMI MEDICAL SCIENCE & TECHNOLOGY Co.,Ltd. |