CN101796420B - 分析用仪器和使用该分析用仪器的分析装置及分析方法 - Google Patents
分析用仪器和使用该分析用仪器的分析装置及分析方法 Download PDFInfo
- Publication number
- CN101796420B CN101796420B CN2008801022246A CN200880102224A CN101796420B CN 101796420 B CN101796420 B CN 101796420B CN 2008801022246 A CN2008801022246 A CN 2008801022246A CN 200880102224 A CN200880102224 A CN 200880102224A CN 101796420 B CN101796420 B CN 101796420B
- Authority
- CN
- China
- Prior art keywords
- instrument
- analysis
- mentioned
- chamber
- reagent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000004458 analytical method Methods 0.000 title claims description 235
- 239000007788 liquid Substances 0.000 claims abstract description 260
- 239000007787 solid Substances 0.000 claims abstract description 41
- 238000012360 testing method Methods 0.000 claims description 175
- 239000000203 mixture Substances 0.000 claims description 79
- 239000000470 constituent Substances 0.000 claims description 44
- 230000027455 binding Effects 0.000 claims description 36
- 238000009739 binding Methods 0.000 claims description 36
- 239000000376 reactant Substances 0.000 claims description 36
- 238000013459 approach Methods 0.000 claims description 13
- 239000000523 sample Substances 0.000 abstract description 106
- 239000012488 sample solution Substances 0.000 abstract description 59
- 230000005484 gravity Effects 0.000 abstract description 37
- 238000000926 separation method Methods 0.000 abstract description 28
- 239000003153 chemical reaction reagent Substances 0.000 description 219
- 239000000758 substrate Substances 0.000 description 137
- 239000012895 dilution Substances 0.000 description 130
- 238000010790 dilution Methods 0.000 description 130
- 238000006243 chemical reaction Methods 0.000 description 101
- 238000000034 method Methods 0.000 description 82
- 239000000243 solution Substances 0.000 description 75
- 108010014663 Glycated Hemoglobin A Proteins 0.000 description 63
- 102000017011 Glycated Hemoglobin A Human genes 0.000 description 63
- 238000003018 immunoassay Methods 0.000 description 61
- 230000003287 optical effect Effects 0.000 description 51
- 239000011259 mixed solution Substances 0.000 description 50
- 210000004369 blood Anatomy 0.000 description 48
- 239000008280 blood Substances 0.000 description 47
- 239000004816 latex Substances 0.000 description 36
- 229920000126 latex Polymers 0.000 description 36
- 230000007850 degeneration Effects 0.000 description 33
- 210000000601 blood cell Anatomy 0.000 description 32
- 230000004523 agglutinating effect Effects 0.000 description 29
- 230000008569 process Effects 0.000 description 29
- 230000009471 action Effects 0.000 description 27
- 230000008859 change Effects 0.000 description 27
- 210000002381 plasma Anatomy 0.000 description 26
- 238000010586 diagram Methods 0.000 description 23
- 239000000463 material Substances 0.000 description 22
- 238000002835 absorbance Methods 0.000 description 19
- 230000032258 transport Effects 0.000 description 18
- 238000003756 stirring Methods 0.000 description 16
- 230000001133 acceleration Effects 0.000 description 15
- 230000004520 agglutination Effects 0.000 description 15
- 238000002156 mixing Methods 0.000 description 15
- 230000002093 peripheral effect Effects 0.000 description 15
- 239000002245 particle Substances 0.000 description 14
- 238000011282 treatment Methods 0.000 description 14
- 210000004027 cell Anatomy 0.000 description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- 230000015572 biosynthetic process Effects 0.000 description 11
- 238000005755 formation reaction Methods 0.000 description 11
- 102000001554 Hemoglobins Human genes 0.000 description 10
- 108010054147 Hemoglobins Proteins 0.000 description 10
- 238000012423 maintenance Methods 0.000 description 10
- 239000011248 coating agent Substances 0.000 description 9
- 238000000576 coating method Methods 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 239000012530 fluid Substances 0.000 description 9
- 238000010521 absorption reaction Methods 0.000 description 7
- 238000007865 diluting Methods 0.000 description 7
- 235000018102 proteins Nutrition 0.000 description 7
- 102000004169 proteins and genes Human genes 0.000 description 7
- 108090000623 proteins and genes Proteins 0.000 description 7
- 239000000427 antigen Substances 0.000 description 6
- 102000036639 antigens Human genes 0.000 description 6
- 108091007433 antigens Proteins 0.000 description 6
- 238000012545 processing Methods 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 230000001681 protective effect Effects 0.000 description 6
- 206010070834 Sensitisation Diseases 0.000 description 5
- 239000000853 adhesive Substances 0.000 description 5
- 230000001070 adhesive effect Effects 0.000 description 5
- 230000033228 biological regulation Effects 0.000 description 5
- 230000005540 biological transmission Effects 0.000 description 5
- 210000003850 cellular structure Anatomy 0.000 description 5
- 238000009826 distribution Methods 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 238000001704 evaporation Methods 0.000 description 5
- 230000008020 evaporation Effects 0.000 description 5
- 238000004108 freeze drying Methods 0.000 description 5
- 230000028993 immune response Effects 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 238000007789 sealing Methods 0.000 description 5
- 230000008313 sensitization Effects 0.000 description 5
- 239000004743 Polypropylene Substances 0.000 description 4
- 239000012298 atmosphere Substances 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 238000000605 extraction Methods 0.000 description 4
- 230000036039 immunity Effects 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 238000007639 printing Methods 0.000 description 4
- 230000003068 static effect Effects 0.000 description 4
- 239000004094 surface-active agent Substances 0.000 description 4
- 238000002965 ELISA Methods 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 230000003321 amplification Effects 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 238000001514 detection method Methods 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 238000005194 fractionation Methods 0.000 description 3
- 239000008103 glucose Substances 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- -1 imino hexylyloxy Chemical group 0.000 description 3
- 238000002649 immunization Methods 0.000 description 3
- 230000003053 immunization Effects 0.000 description 3
- 230000002163 immunogen Effects 0.000 description 3
- 238000007689 inspection Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 238000003199 nucleic acid amplification method Methods 0.000 description 3
- 238000004321 preservation Methods 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 229920005989 resin Polymers 0.000 description 3
- 239000011347 resin Substances 0.000 description 3
- 238000005728 strengthening Methods 0.000 description 3
- 239000012749 thinning agent Substances 0.000 description 3
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 238000013019 agitation Methods 0.000 description 2
- 125000003275 alpha amino acid group Chemical group 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- 239000004411 aluminium Substances 0.000 description 2
- 238000011091 antibody purification Methods 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 238000005452 bending Methods 0.000 description 2
- 238000012742 biochemical analysis Methods 0.000 description 2
- 238000010241 blood sampling Methods 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 239000012141 concentrate Substances 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 229920006038 crystalline resin Polymers 0.000 description 2
- 239000003398 denaturant Substances 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 238000007599 discharging Methods 0.000 description 2
- 238000005553 drilling Methods 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 210000003743 erythrocyte Anatomy 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 238000009434 installation Methods 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 239000002736 nonionic surfactant Substances 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 238000007591 painting process Methods 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 238000004062 sedimentation Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- KZNICNPSHKQLFF-UHFFFAOYSA-N succinimide Chemical compound O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 208000030090 Acute Disease Diseases 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 206010003445 Ascites Diseases 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 108010044091 Globulins Proteins 0.000 description 1
- 102000006395 Globulins Human genes 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 239000004831 Hot glue Substances 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 241001597008 Nomeidae Species 0.000 description 1
- 206010034719 Personality change Diseases 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 238000005411 Van der Waals force Methods 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 239000002390 adhesive tape Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 230000005875 antibody response Effects 0.000 description 1
- 239000007767 bonding agent Substances 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 230000021235 carbamoylation Effects 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- HGAZMNJKRQFZKS-UHFFFAOYSA-N chloroethene;ethenyl acetate Chemical compound ClC=C.CC(=O)OC=C HGAZMNJKRQFZKS-UHFFFAOYSA-N 0.000 description 1
- 238000007398 colorimetric assay Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 108700042971 cyanomethemoglobin Proteins 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000003412 degenerative effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 238000004033 diameter control Methods 0.000 description 1
- 230000035622 drinking Effects 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 238000002875 fluorescence polarization Methods 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 108010074605 gamma-Globulins Proteins 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 102000018146 globin Human genes 0.000 description 1
- 108060003196 globin Proteins 0.000 description 1
- 239000003292 glue Substances 0.000 description 1
- 230000002641 glycemic effect Effects 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 150000003278 haem Chemical class 0.000 description 1
- 230000003760 hair shine Effects 0.000 description 1
- 230000009931 harmful effect Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 238000005534 hematocrit Methods 0.000 description 1
- 108010013766 hemoglobin A(0) Proteins 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 238000003317 immunochromatography Methods 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000004372 laser cladding Methods 0.000 description 1
- 159000000002 lithium salts Chemical group 0.000 description 1
- 230000002101 lytic effect Effects 0.000 description 1
- 239000008204 material by function Substances 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 230000012177 negative regulation of immune response Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000005375 photometry Methods 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 229920000151 polyglycol Polymers 0.000 description 1
- 239000010695 polyglycol Substances 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000002940 repellent Effects 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 229960002317 succinimide Drugs 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000007817 turbidimetric assay Methods 0.000 description 1
- 238000004879 turbidimetry Methods 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/483—Physical analysis of biological material
- G01N33/487—Physical analysis of biological material of liquid biological material
- G01N33/49—Blood
- G01N33/491—Blood by separating the blood components
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/544—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals the carrier being organic
- G01N33/545—Synthetic resin
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/72—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving blood pigments, e.g. haemoglobin, bilirubin or other porphyrins; involving occult blood
- G01N33/721—Haemoglobin
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Urology & Nephrology (AREA)
- Molecular Biology (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Pathology (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Ecology (AREA)
- Biophysics (AREA)
- Automatic Analysis And Handling Materials Therefor (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Centrifugal Separators (AREA)
Abstract
Description
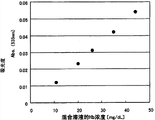
| 样本1 | 样本2 | 样本3 | |
| 糖化血红蛋白(%) | 4.8 | 7.6 | 11.2 |
| 样本1 | 样本2 | 样本3 | |
| 混合溶液中的Hb浓度(mg/dL) | 25.6 | 24.6 | 22.2 |
| 混合溶液中的糖化血红蛋白浓度(mg/dL) | 1.21 | 1.86 | 2.46 |
| 糖化血红蛋白(%) | 4.7 | 7.6 | 11.1 |
| 未稀释定量 | 稀释定量 | |
| 1 | 4.7% | 4.9% |
| 2 | 4.7% | 4.7% |
| 3 | 5.0% | 4.7% |
| 4 | 4.9% | 4.7% |
| 5 | 4.4% | 4.6% |
| 6 | 4.4% | 4.6% |
| 7 | 4.7% | 4.9% |
| 8 | 4.9% | 4.7% |
| 平均 | 4.7% | 4.7% |
| C.V. | 4.8% | 2.4% |
| 样本 | 样本Hb(g/dL) | 无浓缩工序混合液中的Hb浓度(mg/dL) | 浓缩工序后的混合液中的Hb浓度(mg/dL) |
| 样本A | 4 | 7.8 | 12.5 |
| 样本B | 7.1 | 14.1 | 28.4 |
| 样本C | 13.7 | 27.4 | 36.1 |
| 样本D | 20.9 | 41.6 | 45.3 |
| 样本 | 样本Hb(g/dL) | 无浓缩工序混合液中的糖化血红蛋白浓度(mg/dL) | 浓缩工序后的混合液中的糖化血红蛋白浓度(mg/dL) |
| 样本A | 4 | 0.30 | 0.60 |
| 样本B | 7.1 | 0.72 | 1.39 |
| 样本C | 13.7 | 1.34 | 1.73 |
| 样本D | 20.9 | 2.00 | 2.13 |
| 样本 | 样本Hb(g/dL) | 无浓缩工序混合液中的糖化血红蛋白浓度(%) | 浓缩工序后的混合液中的糖化血红蛋白浓度(%) |
| 样本A | 4 | 3.8 | 4.8 |
| 样本B | 7.1 | 5.1 | 4.9 |
| 样本C | 13.7 | 4.9 | 4.8 |
| 样本D | 20.9 | 4.8 | 4.7 |
Claims (3)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210356041.5A CN102879558B (zh) | 2007-10-04 | 2008-10-03 | 分析用仪器和使用该分析用仪器的分析装置及分析方法 |
Applications Claiming Priority (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007-260450 | 2007-10-04 | ||
| JP2007-260451 | 2007-10-04 | ||
| JP2007260450A JP4859804B2 (ja) | 2007-10-04 | 2007-10-04 | 分析用デバイスとこれを使用する分析装置および分析方法 |
| JP2007260451A JP4859805B2 (ja) | 2007-10-04 | 2007-10-04 | 分析用デバイスとこれを使用する分析装置および分析方法 |
| JP2007270764A JP5288768B2 (ja) | 2007-10-18 | 2007-10-18 | 分析容器と分析装置 |
| JP2007-270764 | 2007-10-18 | ||
| JP2007-278249 | 2007-10-26 | ||
| JP2007278249A JP5273986B2 (ja) | 2007-10-26 | 2007-10-26 | 分析容器と分析装置 |
| JP2007294983A JP5273990B2 (ja) | 2007-11-14 | 2007-11-14 | 分析用デバイスとこれを使用する分析装置および分析方法 |
| JP2007-294983 | 2007-11-14 | ||
| JP2008022195A JP5348901B2 (ja) | 2008-02-01 | 2008-02-01 | 分析用デバイスを用いた分析方法 |
| JP2008-022195 | 2008-02-01 | ||
| PCT/JP2008/002779 WO2009044552A1 (ja) | 2007-10-04 | 2008-10-03 | 分析用デバイスとこれを使用する分析装置および分析方法 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201210356041.5A Division CN102879558B (zh) | 2007-10-04 | 2008-10-03 | 分析用仪器和使用该分析用仪器的分析装置及分析方法 |
| CN201310374415.0A Division CN103424543B (zh) | 2007-10-04 | 2008-10-03 | 采用分析用仪器的分析方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101796420A CN101796420A (zh) | 2010-08-04 |
| CN101796420B true CN101796420B (zh) | 2013-09-11 |
Family
ID=40525984
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN2008801022246A Active CN101796420B (zh) | 2007-10-04 | 2008-10-03 | 分析用仪器和使用该分析用仪器的分析装置及分析方法 |
| CN201310374415.0A Active CN103424543B (zh) | 2007-10-04 | 2008-10-03 | 采用分析用仪器的分析方法 |
| CN201210356041.5A Active CN102879558B (zh) | 2007-10-04 | 2008-10-03 | 分析用仪器和使用该分析用仪器的分析装置及分析方法 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201310374415.0A Active CN103424543B (zh) | 2007-10-04 | 2008-10-03 | 采用分析用仪器的分析方法 |
| CN201210356041.5A Active CN102879558B (zh) | 2007-10-04 | 2008-10-03 | 分析用仪器和使用该分析用仪器的分析装置及分析方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US8415140B2 (zh) |
| EP (3) | EP2209008B1 (zh) |
| CN (3) | CN101796420B (zh) |
| WO (1) | WO2009044552A1 (zh) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8307988B2 (en) * | 2006-12-11 | 2012-11-13 | Samsung Electronics Co., Ltd. | Apparatus and method for separating components |
| CN101796420B (zh) * | 2007-10-04 | 2013-09-11 | 松下电器产业株式会社 | 分析用仪器和使用该分析用仪器的分析装置及分析方法 |
| ATE555386T1 (de) * | 2007-10-30 | 2012-05-15 | Panasonic Corp | Verfahren zur messung von hämoglobin und hämoglobin-derivat sowie messkit |
| JP2011133364A (ja) * | 2009-12-24 | 2011-07-07 | Beckman Coulter Inc | 血球凝集像判定方法及び血球凝集像判定装置 |
| JP5740264B2 (ja) * | 2011-09-20 | 2015-06-24 | 株式会社日立ハイテクノロジーズ | 自動分析装置及び分析方法 |
| PT106871B (pt) | 2013-04-08 | 2015-06-15 | Univ Do Minho | Dispositivo e método de análises de sangue por processamento de imagem |
| US10309976B2 (en) | 2014-06-30 | 2019-06-04 | Phc Holdings Corporation | Substrate for sample analysis, sample analysis device, sample analysis system, and program for sample analysis system |
| EP3163306A4 (en) | 2014-06-30 | 2018-01-24 | Panasonic Healthcare Holdings Co., Ltd. | Substrate for sample analysis, and sample analysis apparatus |
| CN106662595B (zh) | 2014-06-30 | 2019-10-15 | 普和希控股公司 | 试样分析用基板、试样分析装置、试样分析系统及从含磁性颗粒的液体中去除液体的方法 |
| WO2016002729A1 (ja) * | 2014-06-30 | 2016-01-07 | パナソニックヘルスケアホールディングス株式会社 | 試料分析用基板、試料分析装置、試料分析システムおよび試料分析システム用プログラム |
| JP6660305B2 (ja) | 2014-12-12 | 2020-03-11 | Phcホールディングス株式会社 | 試料分析用基板、試料分析装置、試料分析システムおよび試料分析システム用プログラム |
| JP6510251B2 (ja) * | 2015-01-30 | 2019-05-08 | 富士フイルム株式会社 | 計測装置 |
| WO2018077983A1 (en) | 2016-10-26 | 2018-05-03 | Radisens Diagnostics Limited | A point-of-care diagnostic assay cartridge |
| US11089979B2 (en) * | 2016-11-11 | 2021-08-17 | ELG Corporation | Device and method for measurement of glycated hemoglobin (A1c) |
| CN110291377B (zh) * | 2016-11-14 | 2023-09-05 | 巴布森诊断公司 | 样本制备装置 |
| WO2019116476A1 (ja) | 2017-12-13 | 2019-06-20 | 株式会社ニコン | 流体デバイス |
| CN109142705B (zh) * | 2018-05-05 | 2024-04-19 | 武汉芯生生物科技有限公司 | 生化免疫分析仪及其工作方法 |
| CN108776233A (zh) * | 2018-06-08 | 2018-11-09 | 上海贞元诊断用品科技有限公司 | 一种便携式全自动凝血分析仪及系统 |
| US10974240B2 (en) * | 2018-07-06 | 2021-04-13 | Qorvo Us, Inc. | Fluidic channel for a cartridge |
| GB201915499D0 (en) | 2019-10-25 | 2019-12-11 | Radisens Diagnostics Ltd | A point-of-care test cartridge |
| JPWO2023120159A1 (zh) * | 2021-12-20 | 2023-06-29 | ||
| CN115047181B (zh) * | 2022-08-15 | 2022-11-01 | 英飞凌生物科技(江苏)有限公司 | 一种定量滴样的免疫荧光定量分析仪及其使用方法 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1613542A (zh) * | 2003-03-21 | 2005-05-11 | Steag显微部件股份有限公司 | 从含有微粒的液体中分离出液态成分的微结构分离装置和微液方法 |
Family Cites Families (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3901658A (en) * | 1974-07-30 | 1975-08-26 | Us Energy | Whole blood analysis rotor assembly having removable cellular sedimentation bowl |
| US4035156A (en) * | 1977-01-21 | 1977-07-12 | The United States Of America As Represented By The United States Energy Research And Development Administration | Filter type rotor for multistation photometer |
| US4605686A (en) * | 1984-03-13 | 1986-08-12 | Sekisui Kagaku Kogyo Kabushiki Kaisha | Latex for immunoserological tests and a method for the production of the same |
| EP0413758A1 (en) | 1988-05-04 | 1991-02-27 | Cambridge Biotech Corporation | Capillary flow device and double capture assay method |
| IL94408A0 (en) | 1989-07-11 | 1991-03-10 | Miles Inc | Method,reaction cassette and kit for performing analytical assays |
| IL94724A (en) | 1989-07-13 | 1994-02-27 | Miles Inc | Decomposition of red blood cells and denaturation of hemoglobin by the use of lithium salts |
| WO1991018656A1 (en) | 1990-06-04 | 1991-12-12 | Abaxis, Inc. | Analytical rotors and methods for analysis of biological fluids |
| US5372948A (en) | 1993-03-17 | 1994-12-13 | Miles Inc. | Assay and reaction vessel with a compartmentalized solubilization chamber |
| WO1997021090A1 (en) * | 1995-12-05 | 1997-06-12 | Gamera Bioscience | Devices and methods for using centripetal acceleration to drive fluid movement in a microfluidics system with on-board informatics |
| US6063589A (en) * | 1997-05-23 | 2000-05-16 | Gamera Bioscience Corporation | Devices and methods for using centripetal acceleration to drive fluid movement on a microfluidics system |
| US6162400A (en) * | 1998-08-12 | 2000-12-19 | Agilent Technologies, Inc. | Apparatus for controlling reactions |
| ATE345868T1 (de) * | 2000-05-15 | 2006-12-15 | Tecan Trading Ag | Zweirichtungs-durchfluss-zentrifugalmikrofluid- vorrichtungen |
| AU2002227509A1 (en) | 2000-07-27 | 2002-02-13 | Kyowa Medex Co., Ltd. | Method of immunity examination with insoluble carrier particle and reagent therefor |
| JP2002221485A (ja) | 2000-11-22 | 2002-08-09 | Minolta Co Ltd | マイクロチップ |
| CN100549696C (zh) * | 2001-12-14 | 2009-10-14 | 爱科来株式会社 | 样品测量器具 |
| WO2003096008A1 (fr) * | 2002-05-08 | 2003-11-20 | Hitachi High-Technologies Corporation | Analyseur chimique et appareil de diagnostic genique |
| US7220593B2 (en) * | 2002-10-03 | 2007-05-22 | Battelle Memorial Institute | Buffy coat separator float system and method |
| JP4262466B2 (ja) | 2002-10-28 | 2009-05-13 | アークレイ株式会社 | 分析用具および分析装置 |
| WO2004050247A1 (en) * | 2002-12-02 | 2004-06-17 | Gyros Ab | Parallel processing of microfluidic devices |
| US7094354B2 (en) * | 2002-12-19 | 2006-08-22 | Bayer Healthcare Llc | Method and apparatus for separation of particles in a microfluidic device |
| CN1517709A (zh) * | 2003-01-14 | 2004-08-04 | 周中人 | 新型竞争法微孔膜介质免疫层析反应检测抗原 |
| US20040166551A1 (en) * | 2003-02-24 | 2004-08-26 | John Moulds | Detection of agglutination of assays |
| JP4026002B2 (ja) | 2003-03-31 | 2007-12-26 | 東亜ディーケーケー株式会社 | 分析装置および分析方法 |
| US20040265171A1 (en) * | 2003-06-27 | 2004-12-30 | Pugia Michael J. | Method for uniform application of fluid into a reactive reagent area |
| JP4617654B2 (ja) | 2003-10-06 | 2011-01-26 | ソニー株式会社 | 親媒性加工を用いるバイオアッセイ用基板の製造方法及びバイオアッセイ用基板 |
| DE102004005193B4 (de) | 2004-02-02 | 2006-08-24 | Medion Diagnostics Gmbh | Vorrichtung zur Separation einzelner Partikel von Partikel-Agglutinationen |
| JP2005345160A (ja) | 2004-05-31 | 2005-12-15 | Advance Co Ltd | 生体情報分析ユニット |
| EP1830189B1 (en) | 2004-12-24 | 2012-09-05 | Sekisui Medical Co., Ltd. | Reagent and method for assaying prostate specific antigen |
| WO2006070772A1 (en) * | 2004-12-28 | 2006-07-06 | Matsushita Electric Industrial Co., Ltd. | Testing device and blood mixing and diluting method |
| CN103382434B (zh) * | 2005-01-18 | 2016-05-25 | 生物概念股份有限公司 | 利用含排列成图案的立柱的微通道分离细胞 |
| JP2005181350A (ja) | 2005-03-22 | 2005-07-07 | Tohoku Techno Arch Co Ltd | 凸状領域を用いた分析方法 |
| JP2006292410A (ja) | 2005-04-06 | 2006-10-26 | Matsushita Electric Ind Co Ltd | 分析装置およびそれに使用する分析デバイス |
| EP1873522B1 (en) | 2005-04-14 | 2014-08-13 | Panasonic Healthcare Co., Ltd. | Method for determination of hemoglobin derivative, and reagent composition, assay kit, analysis device and analysis system for use in the method |
| US7935318B2 (en) * | 2005-06-13 | 2011-05-03 | Hewlett-Packard Development Company, L.P. | Microfluidic centrifugation systems |
| JP2007040833A (ja) | 2005-08-03 | 2007-02-15 | Advance Co Ltd | 生化学分析装置 |
| JP4673149B2 (ja) | 2005-06-29 | 2011-04-20 | ローム株式会社 | マイクロチップの使用方法、マイクロ流路及びマイクロチップ |
| JP4546889B2 (ja) | 2005-07-08 | 2010-09-22 | ローム株式会社 | 計量部を有するチップ |
| JP2007021450A (ja) | 2005-07-21 | 2007-02-01 | Matsushita Electric Ind Co Ltd | 遠心力による分離装置 |
| JP4802925B2 (ja) | 2005-08-19 | 2011-10-26 | パナソニック株式会社 | 分析用デバイス、およびこれを使用する分析装置 |
| JP4531677B2 (ja) | 2005-10-24 | 2010-08-25 | パナソニック株式会社 | アッセイ方法、それを実施するためのセンサデバイス、およびセンサデバイス用測定装置 |
| JP4775039B2 (ja) | 2006-03-03 | 2011-09-21 | パナソニック株式会社 | マイクロ流体チップ |
| JP4322956B2 (ja) * | 2006-07-11 | 2009-09-02 | パナソニック株式会社 | 送液装置及び送液方法 |
| JP2008082896A (ja) * | 2006-09-27 | 2008-04-10 | Fujifilm Corp | 血漿回収方法及び器具 |
| CN101796420B (zh) * | 2007-10-04 | 2013-09-11 | 松下电器产业株式会社 | 分析用仪器和使用该分析用仪器的分析装置及分析方法 |
-
2008
- 2008-10-03 CN CN2008801022246A patent/CN101796420B/zh active Active
- 2008-10-03 CN CN201310374415.0A patent/CN103424543B/zh active Active
- 2008-10-03 EP EP08836397.3A patent/EP2209008B1/en active Active
- 2008-10-03 CN CN201210356041.5A patent/CN102879558B/zh active Active
- 2008-10-03 EP EP16195057.1A patent/EP3141901B1/en active Active
- 2008-10-03 WO PCT/JP2008/002779 patent/WO2009044552A1/ja active Application Filing
- 2008-10-03 EP EP18188222.6A patent/EP3447494B1/en active Active
- 2008-10-03 US US12/681,493 patent/US8415140B2/en active Active
-
2013
- 2013-02-19 US US13/770,499 patent/US8956879B2/en active Active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1613542A (zh) * | 2003-03-21 | 2005-05-11 | Steag显微部件股份有限公司 | 从含有微粒的液体中分离出液态成分的微结构分离装置和微液方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20130164763A1 (en) | 2013-06-27 |
| EP3447494A1 (en) | 2019-02-27 |
| US20100221741A1 (en) | 2010-09-02 |
| EP2209008A4 (en) | 2014-11-26 |
| EP3141901B1 (en) | 2018-12-12 |
| WO2009044552A1 (ja) | 2009-04-09 |
| US8956879B2 (en) | 2015-02-17 |
| CN103424543B (zh) | 2015-04-29 |
| EP3447494B1 (en) | 2020-07-15 |
| EP3141901A1 (en) | 2017-03-15 |
| CN103424543A (zh) | 2013-12-04 |
| EP2209008A1 (en) | 2010-07-21 |
| CN102879558B (zh) | 2015-09-16 |
| CN101796420A (zh) | 2010-08-04 |
| US8415140B2 (en) | 2013-04-09 |
| EP2209008B1 (en) | 2016-12-21 |
| CN102879558A (zh) | 2013-01-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101796420B (zh) | 分析用仪器和使用该分析用仪器的分析装置及分析方法 | |
| JP5348901B2 (ja) | 分析用デバイスを用いた分析方法 | |
| US7125711B2 (en) | Method and apparatus for splitting of specimens into multiple channels of a microfluidic device | |
| EP2219034B1 (en) | Analyzing device and analyzing method using same | |
| US5798215A (en) | Device for use in analyte detection assays | |
| KR101431769B1 (ko) | 당화 혈색소 측정용 원심력 기반의 미세유동 구조물, 당화 혈색소 측정용 원심력 기반 미세유동 장치 및 당화 혈색소의 측정방법 | |
| US8337775B2 (en) | Apparatus for precise transfer and manipulation of fluids by centrifugal and or capillary forces | |
| US7087203B2 (en) | Methods and apparatus for blood typing with optical bio-disc | |
| CN101680906B (zh) | 分析用仪器和使用该分析用仪器的分析装置及分析方法 | |
| US20130041236A1 (en) | Sample analysis system and method of use | |
| EP2211175B1 (en) | Method for measurement of hemoglobin and hemoglobin derivative, and measurement kit | |
| EP3485973A1 (en) | Microfluidic reagent card and detection method and application thereof | |
| JP2007047170A (ja) | 一体化した液体区画を有するサンプル採取・計量デバイス | |
| JP2010243373A (ja) | 分析用デバイスにおける分析方法と分析装置 | |
| EP1437597A1 (en) | Protein measurement method in protein production plant by cell culture and apparatus thereof | |
| JP5125680B2 (ja) | 分離チップおよび分離方法 | |
| EP3368220B1 (en) | Hematology test slide |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| ASS | Succession or assignment of patent right |
Owner name: PANASONIC HEALTHCARE + MEDICAL EQUIPMENT CO., LTD. Free format text: FORMER OWNER: MATSUSHITA ELECTRIC INDUSTRIAL CO, LTD. Effective date: 20140523 |
|
| C41 | Transfer of patent application or patent right or utility model | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20140523 Address after: Ehime Prefecture, Japan Patentee after: Panasonic Healthcare Co., Ltd Address before: Osaka Japan Patentee before: Matsushita Electric Industrial Co., Ltd. |
|
| ASS | Succession or assignment of patent right |
Owner name: PANASONIC HEALTHCARE HOLDINGS CO., LTD. Free format text: FORMER OWNER: PANASONIC HEALTHCARE + MEDICAL EQUIPMENT CO., LTD. Effective date: 20150402 |
|
| TR01 | Transfer of patent right |
Effective date of registration: 20150402 Address after: Tokyo, Japan Patentee after: Panasonic's health medical treatment is controlled interest Co., Ltd. Address before: Ehime Prefecture, Japan Patentee before: Panasonic Healthcare Co., Ltd |
|
| CP01 | Change in the name or title of a patent holder |
Address after: Tokyo, Japan Patentee after: Pu Hei holding company Address before: Tokyo, Japan Patentee before: Panasonic's health medical treatment is controlled interest Co., Ltd. |
|
| CP01 | Change in the name or title of a patent holder |