CN100584835C - 桂哌齐特的药用盐及其制备方法 - Google Patents
桂哌齐特的药用盐及其制备方法 Download PDFInfo
- Publication number
- CN100584835C CN100584835C CN200610110549A CN200610110549A CN100584835C CN 100584835 C CN100584835 C CN 100584835C CN 200610110549 A CN200610110549 A CN 200610110549A CN 200610110549 A CN200610110549 A CN 200610110549A CN 100584835 C CN100584835 C CN 100584835C
- Authority
- CN
- China
- Prior art keywords
- cinepazide
- pharmaceutical salts
- preparation
- injection
- acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 150000003839 salts Chemical class 0.000 title claims abstract description 56
- 229960004201 cinepazide Drugs 0.000 title claims description 173
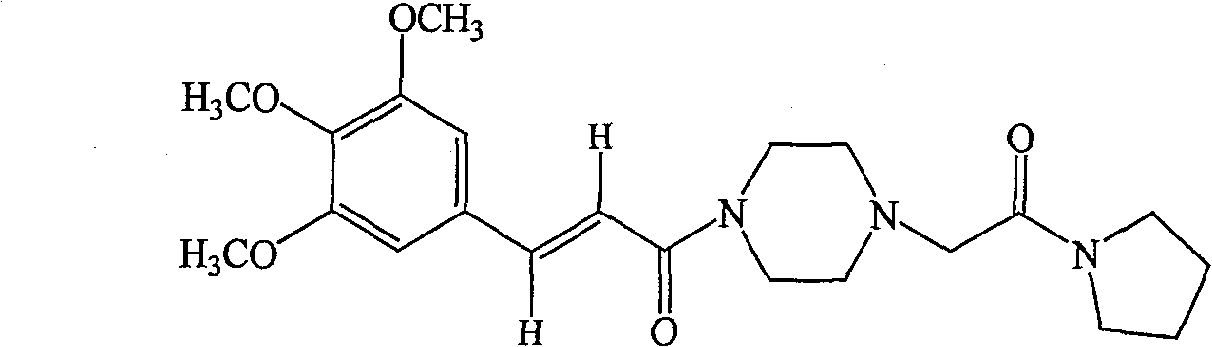
- RCUDFXMNPQNBDU-VOTSOKGWSA-N cinepazide Chemical compound COC1=C(OC)C(OC)=CC(\C=C\C(=O)N2CCN(CC(=O)N3CCCC3)CC2)=C1 RCUDFXMNPQNBDU-VOTSOKGWSA-N 0.000 title claims description 158
- 238000002360 preparation method Methods 0.000 title claims description 43
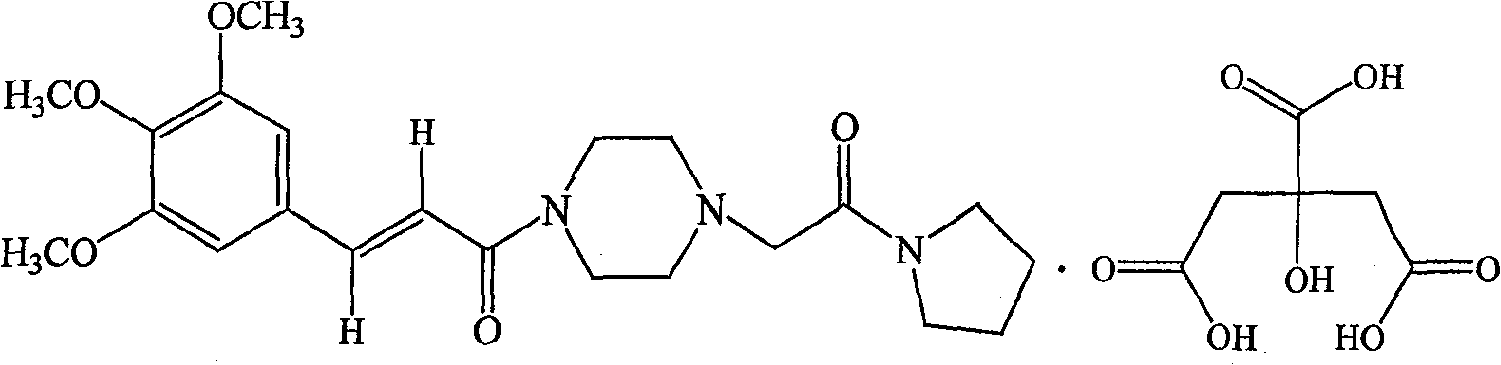
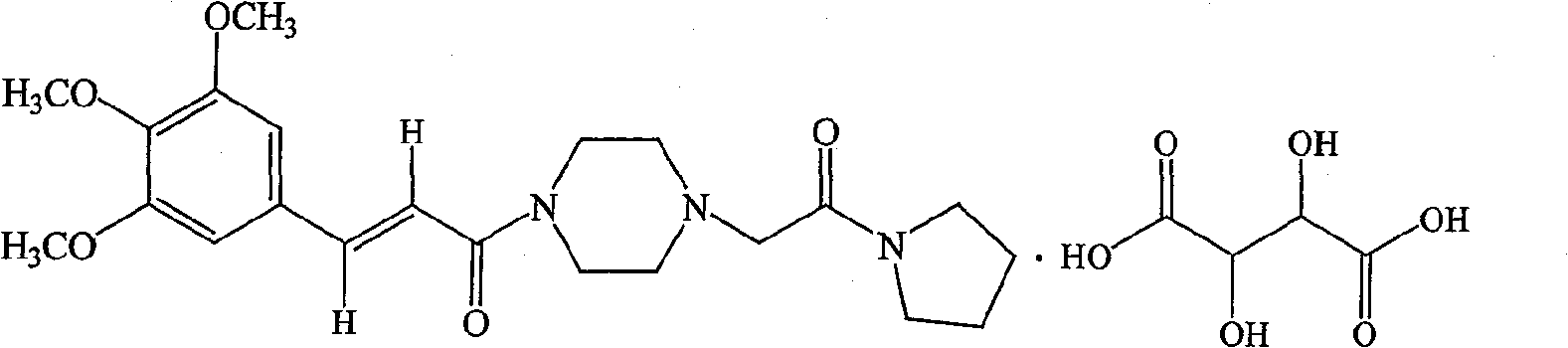
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims abstract description 30
- 229940095064 tartrate Drugs 0.000 claims abstract description 30
- 239000003814 drug Substances 0.000 claims abstract description 17
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 claims abstract description 15
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 claims abstract description 15
- 208000026106 cerebrovascular disease Diseases 0.000 claims abstract description 6
- 230000002526 effect on cardiovascular system Effects 0.000 claims abstract description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 120
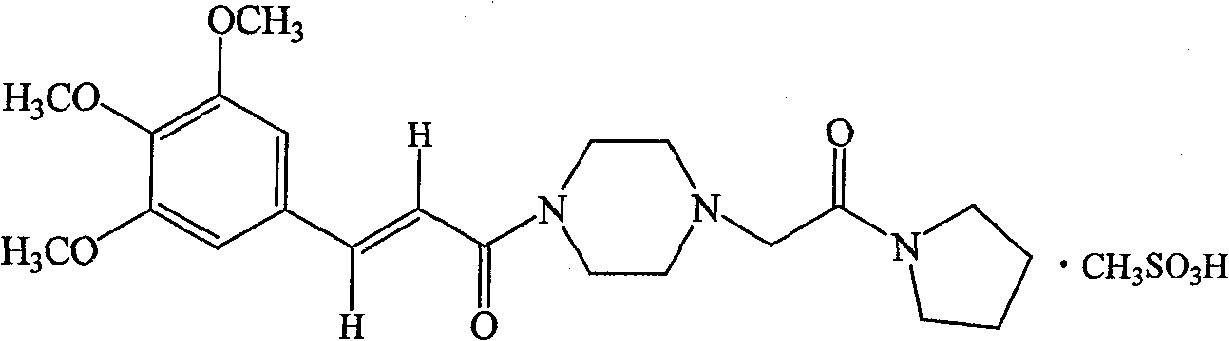
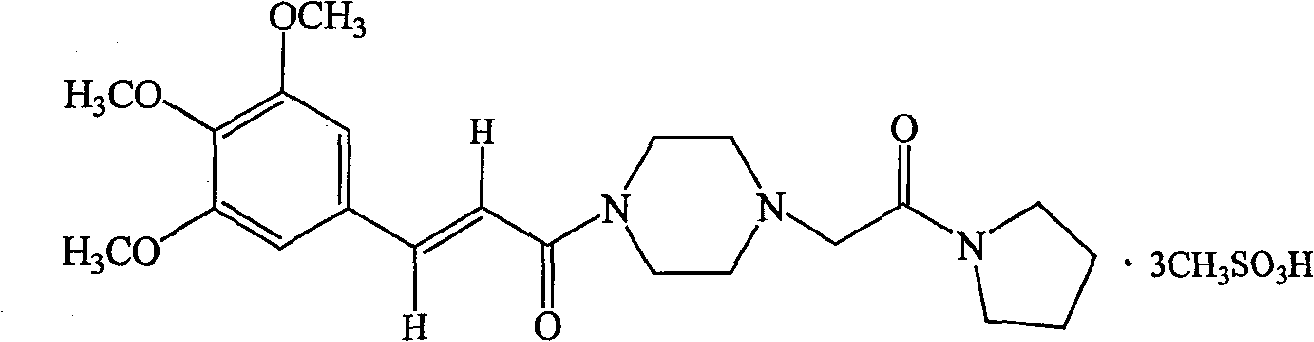
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 60
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 57
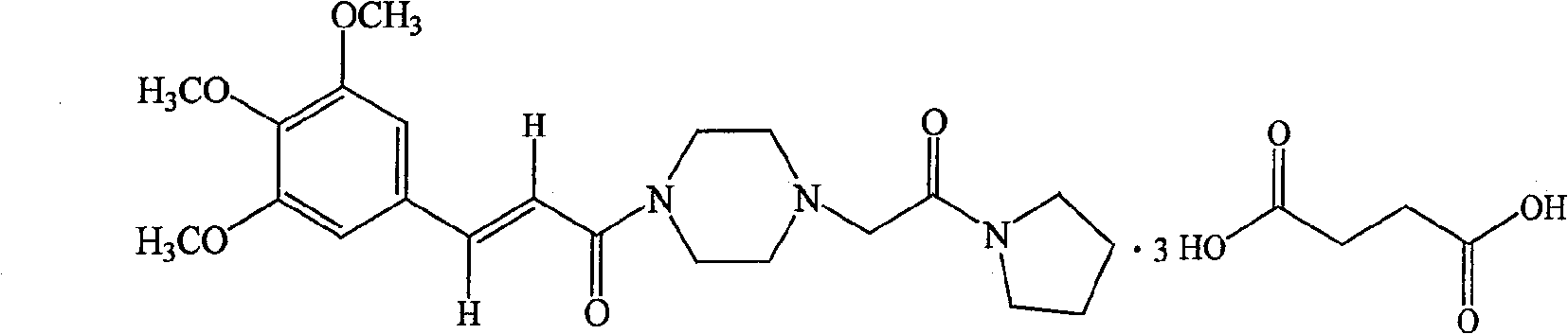
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 claims description 35
- 239000012043 crude product Substances 0.000 claims description 27
- 239000007924 injection Substances 0.000 claims description 18
- 238000002347 injection Methods 0.000 claims description 18
- 239000001384 succinic acid Substances 0.000 claims description 16
- 229940001468 citrate Drugs 0.000 claims description 14
- 229940086735 succinate Drugs 0.000 claims description 14
- 239000000047 product Substances 0.000 claims description 8
- 239000000203 mixture Substances 0.000 claims description 7
- 208000018262 Peripheral vascular disease Diseases 0.000 claims description 4
- 239000004480 active ingredient Substances 0.000 claims description 3
- 239000003937 drug carrier Substances 0.000 claims description 3
- 239000000376 reactant Substances 0.000 claims description 2
- 150000007524 organic acids Chemical class 0.000 claims 1
- 238000007670 refining Methods 0.000 claims 1
- 239000003795 chemical substances by application Substances 0.000 abstract description 13
- 201000010099 disease Diseases 0.000 abstract description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 5
- 238000000034 method Methods 0.000 abstract description 4
- 208000024172 Cardiovascular disease Diseases 0.000 abstract description 3
- 229940079593 drug Drugs 0.000 abstract description 2
- VIAYOUBFHWUVLW-FMIVXFBMSA-N 1-phenyl-3-[2-[[4-[(e)-3-phenylprop-2-enyl]piperazin-1-yl]methyl]benzimidazol-1-yl]propan-1-one Chemical compound C=1C=CC=CC=1C(=O)CCN(C1=CC=CC=C1N=1)C=1CN(CC1)CCN1C\C=C\C1=CC=CC=C1 VIAYOUBFHWUVLW-FMIVXFBMSA-N 0.000 abstract 1
- 241000723347 Cinnamomum Species 0.000 abstract 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-M Methanesulfonate Chemical compound CS([O-])(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-M 0.000 abstract 1
- 125000002252 acyl group Chemical group 0.000 abstract 1
- 235000017803 cinnamon Nutrition 0.000 abstract 1
- 229950009472 cinprazole Drugs 0.000 abstract 1
- 230000002093 peripheral effect Effects 0.000 abstract 1
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 36
- 239000000243 solution Substances 0.000 description 35
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 35
- 238000003756 stirring Methods 0.000 description 33
- 239000008215 water for injection Substances 0.000 description 29
- 238000006243 chemical reaction Methods 0.000 description 26
- 238000002425 crystallisation Methods 0.000 description 26
- 230000008025 crystallization Effects 0.000 description 26
- 229940098779 methanesulfonic acid Drugs 0.000 description 19
- 239000000843 powder Substances 0.000 description 18
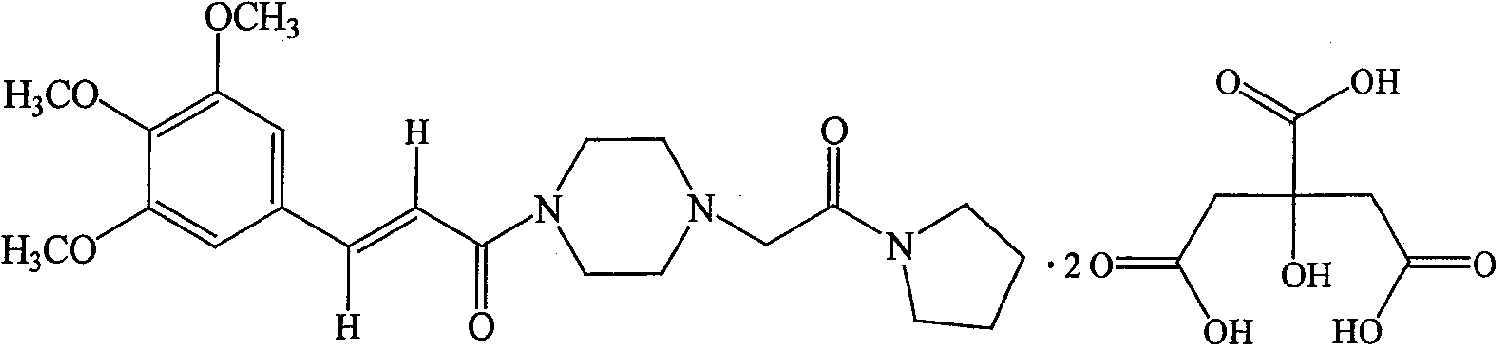
- XSTJTOKYCAJVMJ-GVTSEVKNSA-N (z)-but-2-enedioic acid;(e)-1-[4-(2-oxo-2-pyrrolidin-1-ylethyl)piperazin-1-yl]-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one Chemical compound OC(=O)\C=C/C(O)=O.COC1=C(OC)C(OC)=CC(\C=C\C(=O)N2CCN(CC(=O)N3CCCC3)CC2)=C1 XSTJTOKYCAJVMJ-GVTSEVKNSA-N 0.000 description 16
- 229940090044 injection Drugs 0.000 description 16
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 14
- DPDMMXDBJGCCQC-UHFFFAOYSA-N [Na].[Cl] Chemical compound [Na].[Cl] DPDMMXDBJGCCQC-UHFFFAOYSA-N 0.000 description 13
- 239000000463 material Substances 0.000 description 13
- 238000005160 1H NMR spectroscopy Methods 0.000 description 12
- 238000013019 agitation Methods 0.000 description 12
- 238000004458 analytical method Methods 0.000 description 12
- 238000001816 cooling Methods 0.000 description 12
- 238000007710 freezing Methods 0.000 description 12
- 230000008014 freezing Effects 0.000 description 12
- 239000007788 liquid Substances 0.000 description 12
- -1 cinnamoyl Chemical group 0.000 description 10
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 9
- 229920002472 Starch Polymers 0.000 description 9
- 239000002775 capsule Substances 0.000 description 9
- 239000006104 solid solution Substances 0.000 description 9
- 239000008107 starch Substances 0.000 description 9
- 235000019698 starch Nutrition 0.000 description 9
- 229940032147 starch Drugs 0.000 description 9
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 8
- 238000005516 engineering process Methods 0.000 description 8
- 239000008103 glucose Substances 0.000 description 8
- 239000002994 raw material Substances 0.000 description 8
- 235000014347 soups Nutrition 0.000 description 8
- 239000003826 tablet Substances 0.000 description 8
- 239000007864 aqueous solution Substances 0.000 description 7
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 7
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 7
- 238000007689 inspection Methods 0.000 description 7
- 235000019359 magnesium stearate Nutrition 0.000 description 7
- 239000002245 particle Substances 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- 229920002307 Dextran Polymers 0.000 description 6
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 6
- 239000003125 aqueous solvent Substances 0.000 description 6
- 239000008187 granular material Substances 0.000 description 6
- 238000010438 heat treatment Methods 0.000 description 6
- 229940016286 microcrystalline cellulose Drugs 0.000 description 6
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 6
- 239000008108 microcrystalline cellulose Substances 0.000 description 6
- 229940023488 pill Drugs 0.000 description 6
- 239000006187 pill Substances 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- LASHKOJDARUCTA-UHFFFAOYSA-N N1=CC=CC=C1.C(C)(=O)C=1NC=CC1 Chemical compound N1=CC=CC=C1.C(C)(=O)C=1NC=CC1 LASHKOJDARUCTA-UHFFFAOYSA-N 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 238000012856 packing Methods 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 241000196324 Embryophyta Species 0.000 description 4
- 239000003610 charcoal Substances 0.000 description 4
- 238000011082 depyrogenation Methods 0.000 description 4
- 238000001514 detection method Methods 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 238000004108 freeze drying Methods 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 239000011265 semifinished product Substances 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 238000005303 weighing Methods 0.000 description 4
- BLSQLHNBWJLIBQ-OZXSUGGESA-N (2R,4S)-terconazole Chemical compound C1CN(C(C)C)CCN1C(C=C1)=CC=C1OC[C@@H]1O[C@@](CN2N=CN=C2)(C=2C(=CC(Cl)=CC=2)Cl)OC1 BLSQLHNBWJLIBQ-OZXSUGGESA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 3
- 229960005305 adenosine Drugs 0.000 description 3
- 230000003750 conditioning effect Effects 0.000 description 3
- 238000013270 controlled release Methods 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 229940089256 fungistat Drugs 0.000 description 3
- 238000005286 illumination Methods 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 239000003112 inhibitor Substances 0.000 description 3
- 239000000314 lubricant Substances 0.000 description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 230000003204 osmotic effect Effects 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 239000008194 pharmaceutical composition Substances 0.000 description 3
- 230000001954 sterilising effect Effects 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- JGSARLDLIJGVTE-UHFFFAOYSA-N 3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Chemical compound O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-UHFFFAOYSA-N 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000008052 alkyl sulfonates Chemical class 0.000 description 2
- 239000003708 ampul Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 150000007942 carboxylates Chemical class 0.000 description 2
- 230000002490 cerebral effect Effects 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 238000005469 granulation Methods 0.000 description 2
- 230000003179 granulation Effects 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 229940100688 oral solution Drugs 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 239000007901 soft capsule Substances 0.000 description 2
- 239000008174 sterile solution Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- MEIRRNXMZYDVDW-MQQKCMAXSA-N (2E,4E)-2,4-hexadien-1-ol Chemical compound C\C=C\C=C\CO MEIRRNXMZYDVDW-MQQKCMAXSA-N 0.000 description 1
- 102000001707 3',5'-Cyclic-AMP Phosphodiesterases Human genes 0.000 description 1
- 108010054479 3',5'-Cyclic-AMP Phosphodiesterases Proteins 0.000 description 1
- 206010002383 Angina Pectoris Diseases 0.000 description 1
- 206010003210 Arteriosclerosis Diseases 0.000 description 1
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 1
- 208000033386 Buerger disease Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 206010065559 Cerebral arteriosclerosis Diseases 0.000 description 1
- 206010008088 Cerebral artery embolism Diseases 0.000 description 1
- 206010008132 Cerebral thrombosis Diseases 0.000 description 1
- 206010008190 Cerebrovascular accident Diseases 0.000 description 1
- FITPCXSHEGAMCJ-JJKGCWMISA-N ClC(=O)[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.[Na] Chemical compound ClC(=O)[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.[Na] FITPCXSHEGAMCJ-JJKGCWMISA-N 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 206010013786 Dry skin Diseases 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 201000001429 Intracranial Thrombosis Diseases 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- 206010067482 No adverse event Diseases 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 1
- 229920003081 Povidone K 30 Polymers 0.000 description 1
- 208000003782 Raynaud disease Diseases 0.000 description 1
- 208000012322 Raynaud phenomenon Diseases 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 239000004141 Sodium laurylsulphate Substances 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- 206010043540 Thromboangiitis obliterans Diseases 0.000 description 1
- 206010047163 Vasospasm Diseases 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 230000008485 antagonism Effects 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- 206010003230 arteritis Diseases 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- 229960004217 benzyl alcohol Drugs 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000001218 blood-brain barrier Anatomy 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- LRHPLDYGYMQRHN-UHFFFAOYSA-N butyl alcohol Substances CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 238000010523 cascade reaction Methods 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 239000007910 chewable tablet Substances 0.000 description 1
- 229940068682 chewable tablet Drugs 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- 239000003218 coronary vasodilator agent Substances 0.000 description 1
- 150000001896 cresols Chemical class 0.000 description 1
- 229960001681 croscarmellose sodium Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- FPAFDBFIGPHWGO-UHFFFAOYSA-N dioxosilane;oxomagnesium;hydrate Chemical compound O.[Mg]=O.[Mg]=O.[Mg]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O FPAFDBFIGPHWGO-UHFFFAOYSA-N 0.000 description 1
- 239000007919 dispersible tablet Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 239000007938 effervescent tablet Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 239000002662 enteric coated tablet Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 229940093181 glucose injection Drugs 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 229920000591 gum Polymers 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 229960003943 hypromellose Drugs 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 201000005851 intracranial arteriosclerosis Diseases 0.000 description 1
- 201000010849 intracranial embolism Diseases 0.000 description 1
- 230000004171 ischemic cascade Effects 0.000 description 1
- 230000000302 ischemic effect Effects 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 229940031703 low substituted hydroxypropyl cellulose Drugs 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 230000004089 microcirculation Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 230000036284 oxygen consumption Effects 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 239000001253 polyvinylpolypyrrolidone Substances 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000010298 pulverizing process Methods 0.000 description 1
- 230000033904 relaxation of vascular smooth muscle Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229960004249 sodium acetate Drugs 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000008354 sodium chloride injection Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 1
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 235000010262 sodium metabisulphite Nutrition 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 1
- 239000011122 softwood Substances 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000006190 sub-lingual tablet Substances 0.000 description 1
- 229940098466 sublingual tablet Drugs 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 230000024883 vasodilation Effects 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 235000020985 whole grains Nutrition 0.000 description 1
Landscapes
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Description
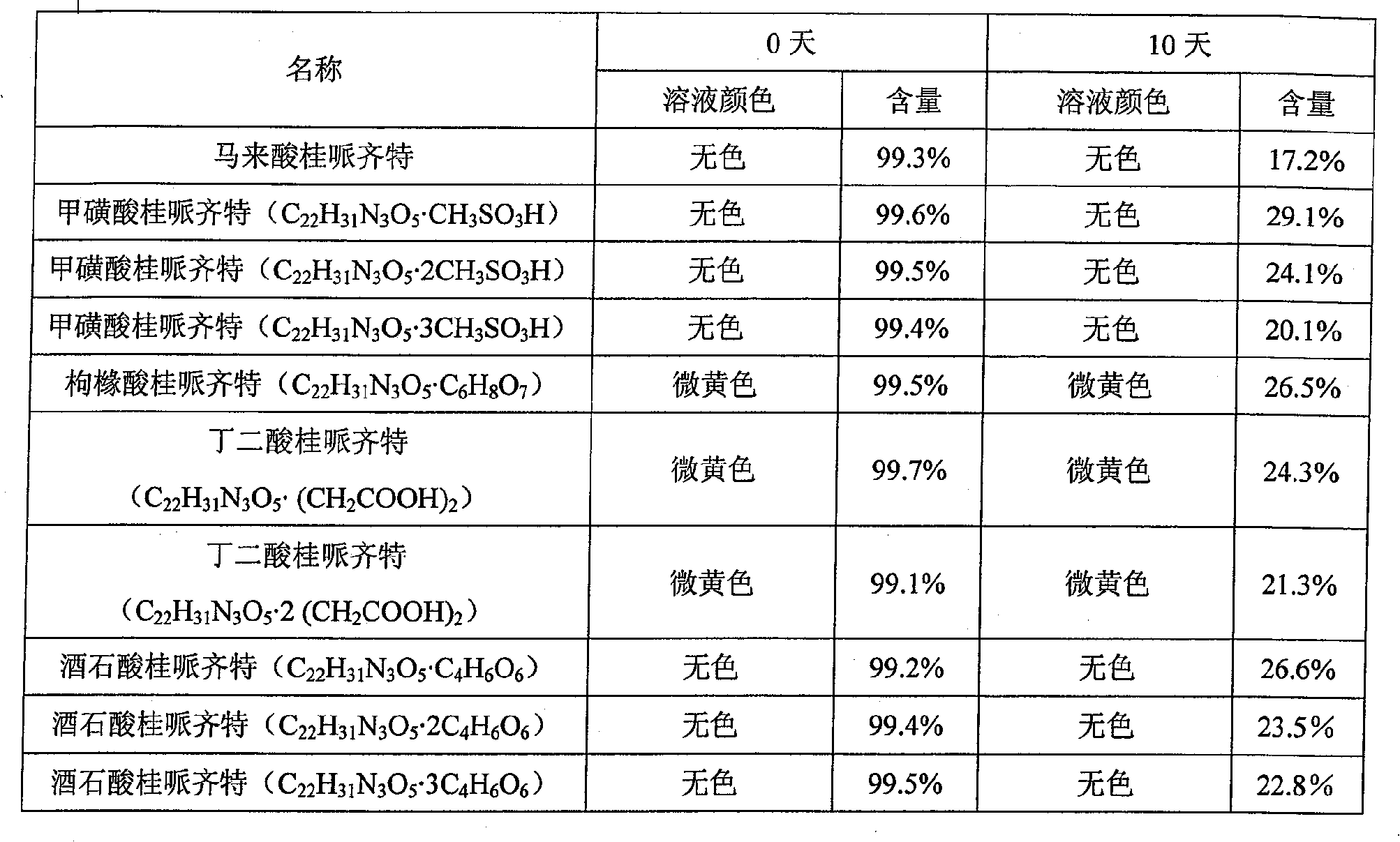
| 溶解度 | 水(25℃,mg/ml) |
| 马来酸桂哌齐特 | 485 |
| 甲磺酸桂哌齐特(C<sub>22</sub>H<sub>31</sub>N<sub>3</sub>O<sub>5</sub>·CH<sub>3</sub>SO<sub>3</sub>H) | 1320 |
| 甲磺酸桂哌齐特(C<sub>22</sub>H<sub>31</sub>N<sub>3</sub>O<sub>5</sub>·2CH<sub>3</sub>SO<sub>3</sub>H) | 1250 |
| 甲磺酸桂哌齐特(C<sub>22</sub>H<sub>31</sub>N<sub>3</sub>O<sub>5</sub>·3CH<sub>3</sub>SO<sub>3</sub>H) | 1200 |
| 枸橼酸桂哌齐特(C<sub>22</sub>H<sub>31</sub>N<sub>3</sub>O<sub>5</sub>·C<sub>6</sub>H<sub>8</sub>O<sub>7</sub>) | 1150 |
| 丁二酸桂哌齐特(C<sub>22</sub>H<sub>31</sub>N<sub>3</sub>O<sub>5</sub>·(CH<sub>2</sub>COOH)<sub>2</sub>) | 1080 |
| 酒石酸桂哌齐特(C<sub>22</sub>H<sub>31</sub>N<sub>3</sub>O<sub>5</sub>·C<sub>4</sub>H<sub>6</sub>O<sub>6</sub>) | 550 |
| 酒石酸桂哌齐特(C<sub>22</sub>H<sub>31</sub>N<sub>3</sub>O<sub>5</sub>·2C<sub>4</sub>H<sub>6</sub>O<sub>6</sub>) | 520 |
| 酒石酸桂哌齐特(C<sub>22</sub>H<sub>31</sub>N<sub>3</sub>O<sub>5</sub>·3C<sub>4</sub>H<sub>6</sub>O<sub>6</sub>) | 500 |
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200610110549A CN100584835C (zh) | 2006-04-21 | 2006-08-08 | 桂哌齐特的药用盐及其制备方法 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200610043843 | 2006-04-21 | ||
| CN200610043843.5 | 2006-04-21 | ||
| CN200610110549A CN100584835C (zh) | 2006-04-21 | 2006-08-08 | 桂哌齐特的药用盐及其制备方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101058566A CN101058566A (zh) | 2007-10-24 |
| CN100584835C true CN100584835C (zh) | 2010-01-27 |
Family
ID=38864885
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN200610110549A Active CN100584835C (zh) | 2006-04-21 | 2006-08-08 | 桂哌齐特的药用盐及其制备方法 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN100584835C (zh) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102391209B (zh) * | 2008-12-01 | 2013-09-25 | 北京四环制药有限公司 | 甲磺酸桂哌齐特晶型ii及其制备方法 |
| CN102351812B (zh) * | 2008-12-01 | 2013-12-18 | 北京四环制药有限公司 | 甲磺酸桂哌齐特晶型iii及其制备方法 |
| CN101747295B (zh) * | 2008-12-01 | 2012-04-25 | 北京四环制药有限公司 | 甲磺酸桂哌齐特晶型及其制备方法 |
| CN102060808B (zh) * | 2010-12-16 | 2013-06-26 | 沈阳亿灵医药科技有限公司 | 桂哌齐特酸加成盐及其制备方法 |
| CN102336724A (zh) * | 2011-06-03 | 2012-02-01 | 辽宁中海康生物药业有限公司 | 新的桂哌齐特盐及制备方法以及基于桂哌齐特盐的药物组合物 |
| CN107224569A (zh) * | 2016-03-26 | 2017-10-03 | 复旦大学 | 一种硼替佐米水溶性药用组合物及其制备方法和用途 |
-
2006
- 2006-08-08 CN CN200610110549A patent/CN100584835C/zh active Active
Non-Patent Citations (2)
| Title |
|---|
| 血管扩张药桂哌齐特的合成研究. 徐娟等.中国新药杂志,第12卷第8期. 2003 |
| 血管扩张药桂哌齐特的合成研究. 徐娟等.中国新药杂志,第12卷第8期. 2003 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN101058566A (zh) | 2007-10-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101092413B (zh) | 法舒地尔的药用盐的水合物 | |
| TWI296523B (en) | Formulations | |
| CN100584835C (zh) | 桂哌齐特的药用盐及其制备方法 | |
| CN102391250A (zh) | 一种达比加群酯化合物、制备方法及其药物组合物 | |
| CN101585859B (zh) | 一种新的灯盏花乙素衍生物、其制备方法及其药物组合物 | |
| CN108938626B (zh) | 一种稳定性好和安全性高的卡络磺钠药物组合物及其制备方法和应用 | |
| CN102481287B (zh) | 含维生素c或其衍生物的替莫唑胺药物组合物及其制备方法 | |
| CN101195582A (zh) | 肉桂酰胺衍生物 | |
| CN103214382B (zh) | 一种盐酸甲氯芬酯化合物及其药物组合物 | |
| KR20110026311A (ko) | 엔테카비어의 신규한 염 | |
| CN103980279B (zh) | 一种甲氨蝶呤化合物及注射用甲氨蝶呤 | |
| CN102106846A (zh) | 左旋盐酸艾司洛尔药物组合物及其制备方法 | |
| CN115124420B (zh) | 大黄酸与苦参碱共晶物水合物及制备方法和其组合物与用途 | |
| EP2292241A1 (en) | A scutellarin derivative, the preparing process, the pharmaceutical composition and the use thereof | |
| CN104706655A (zh) | 注射用环磷腺苷葡胺粉针剂药物组合物和制法 | |
| CN115124532B (zh) | 大黄酸与苦参碱共晶物及制备方法和其组合物与用途 | |
| CN102093234B (zh) | 一种二元酯酸的氨丁三醇盐化合物及其制备方法和药物应用 | |
| CN112778369B (zh) | 一种三唑类衍生物及其制备方法和用途 | |
| CN101195570B (zh) | 阿魏酸的氨基酸盐 | |
| CN100526293C (zh) | 2,5-二羟基苯磺酸镁及其水合物 | |
| CN102796156B (zh) | 环磷腺苷二葡甲胺化合物及其制备方法 | |
| CN104739776A (zh) | 左卡尼汀的固体分散体组合物及其制备方法和药物应用 | |
| CN113491668B (zh) | 注射用药物组合制剂及其制备方法与应用 | |
| CN101121696B (zh) | 吡硫醇的金属盐及其水合物 | |
| CN100534978C (zh) | 2,5-二羟基苯磺酸的药用复合盐及其水合物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| ASS | Succession or assignment of patent right |
Owner name: BEIJING SIHUAN PHARMACEUTICAL CO., LTD. Free format text: FORMER OWNER: CHE FENGSHENG Effective date: 20110422 |
|
| C41 | Transfer of patent application or patent right or utility model | ||
| COR | Change of bibliographic data |
Free format text: CORRECT: ADDRESS; FROM: 570125 31/F, XINDA BUSINESS BUILDING, NO. 48, GUOMAO AVENUE, HAIKOU CITY, HAINAN PROVINCE TO: 101114 EAST OF QISHANZHUANG VILLAGE, ZHANGJIAWAN TOWN, TONGZHOU DISTRICT, BEIJING |
|
| TR01 | Transfer of patent right |
Effective date of registration: 20110422 Address after: 101114 Beijing city Tongzhou District Zhangjiawan Town Qi Shanzhuang East Village Patentee after: Beijing Sihuan Pharmaceutical Co., Ltd. Address before: The new business building No. 48 570125 Hainan city of Haikou province China World Trade Center road 31 layer Patentee before: Che Fengsheng |
|
| ASS | Succession or assignment of patent right |
Owner name: TONGHUA SIHUAN PHARMACEUTICAL CO., LTD. |
|
| C41 | Transfer of patent application or patent right or utility model | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20111008 Address after: 101114 Beijing city Tongzhou District Zhangjiawan Town Qi Shanzhuang East Village Co-patentee after: Tonghua Sihuan Pharm Co., Ltd. Patentee after: Beijing Sihuan Pharmaceutical Co., Ltd. Address before: 101114 Beijing city Tongzhou District Zhangjiawan Town Qi Shanzhuang East Village Patentee before: Beijing Sihuan Pharmaceutical Co., Ltd. |
|
| C56 | Change in the name or address of the patentee | ||
| CP03 | Change of name, title or address |
Address after: 101114 Beijing city Tongzhou District Zhangjiawan Town Qi Shanzhuang East Village Co-patentee after: Tonghua Sihuan Pharm Co., Ltd. Patentee after: Beijing Sihuan Pharmaceutical Co., Ltd. Address before: 101114 Beijing city Tongzhou District Zhangjiawan Town Qi Shanzhuang East Village Co-patentee before: Tonghua Sihuan Pharm Co., Ltd. Patentee before: Beijing Sihuan Pharmaceutical Co., Ltd. |
|
| C56 | Change in the name or address of the patentee | ||
| CP01 | Change in the name or title of a patent holder |
Address after: 101114 Beijing city Tongzhou District Zhangjiawan Town Qi Shanzhuang East Village Patentee after: Beijing Sihuan Pharmaceutical Co., Ltd. Patentee after: Tonghua Jida Pharmaceutical Co., Ltd. Address before: 101114 Beijing city Tongzhou District Zhangjiawan Town Qi Shanzhuang East Village Patentee before: Beijing Sihuan Pharmaceutical Co., Ltd. Patentee before: Tonghua Sihuan Pharm Co., Ltd. |
|
| TR01 | Transfer of patent right |
Effective date of registration: 20181127 Address after: 101114 East Village of Qi Shan village, Zhangjia Bay, Tongzhou District, Beijing Co-patentee after: Tonghua Jida Pharmaceutical Co., Ltd. Patentee after: Beijing Sihuan Pharmaceutical Co., Ltd. Co-patentee after: Jilin Huikang Pharmaceutical Co., Ltd. Address before: 101114 East Village of Qi Shan village, Zhangjia Bay, Tongzhou District, Beijing Co-patentee before: Tonghua Jida Pharmaceutical Co., Ltd. Patentee before: Beijing Sihuan Pharmaceutical Co., Ltd. |
|
| TR01 | Transfer of patent right |