Chemistry:Trimethyltrienolone
 | |
| Clinical data | |
|---|---|
| Other names | TMT; R-2956; RU-2956; 2α,2β,17α-Trimethyltrienolone; 2α,2β,17α-Trimethyltrenbolone; 2α,2β-Dimethylmetribolone; δ9,11-2α,2β,17α-trimethyl-19-nortestosterone; 2α,2β,17α-Trimethylestra-4,9,11-trien-17β-ol-3-one; 17β-Hydroxy-2α,2β,17α-trimethylestra-4,9,11-trien-3-one |
| Drug class | Steroidal antiandrogen |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trimethyltrienolone (TMT), also known by its developmental code name R-2956 or RU-2956, is an antiandrogen medication which was never introduced for medical use but has been used in scientific research.[1][2][3]
Side effects
Due to its close relation to metribolone (methyltrienolone), it is thought that TMT may produce hepatotoxicity.[4]
Pharmacology
Pharmacodynamics
TMT is a selective and highly potent competitive antagonist of the androgen receptor (AR) with very low intrinsic/partial androgenic activity and no estrogenic, antiestrogenic, progestogenic, or antimineralocorticoid activity.[5][6] The drug is a derivative of the extremely potent androgen/anabolic steroid metribolone (R-1881; 17α-methyltrenbolone),[6][7] and has been reported to possess only about 4-fold lower affinity for the AR in comparison.[8] In accordance, it has relatively high affinity for the AR among steroidal antiandrogens, and almost completely inhibits dihydrotestosterone (DHT) binding to the AR in vitro at a mere 10-fold molar excess.[9] The AR weak partial agonistic activity of TMT is comparable to that of cyproterone acetate.[4]
| Compound | PR | AR | ER | GR | MR |
|---|---|---|---|---|---|
| Testosterone | 1–3, 1–5 | 100 | <1 | <1, 1–5 | <1 |
| 5α-Dihydrotestosterone | <1, 1–3 | 100–125 | <1 | <1 | <1 |
| Metribolone (RU-1881) | 200–300, 250–600 | 200–300, 250–600 | <1 | 25–50 | 15–25 |
| Trimethyltrienolone (RU-2956) | ≤1 | 14 | <1 | <1 | <1 |
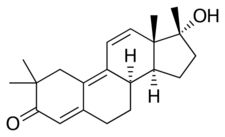
Chemistry
TMT, also known as 2α,2β,17α-trimethyltrienolone[10] or as δ9,11-2α,2β,17α-trimethyl-19-nortestosterone, as well as 2α,2β,17α-trimethylestra-4,9,11-trien-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone and 19-nortestosterone.[5][11][2] It is the 2α,2β,17α-trimethyl derivative of trenbolone (trienolone) and the 2α,2β-dimethyl derivative of metribolone (methyltrienolone), both of which are synthetic androgens/anabolic steroids.[11]
History
TMT was developed by Roussel Uclaf in France and was first known as early as 1969.[3][12][11] It was one of the earliest antiandrogens to be discovered and developed, along with others such as benorterone, BOMT, cyproterone, and cyproterone acetate.[5][13][14][15][16] The drug was under investigation by Roussel Uclaf for potential medical use, but was abandoned in favor of nonsteroidal antiandrogens like flutamide and nilutamide due to their comparative advantage of a complete lack of androgenicity.[1] Roussel Uclaf subsequently developed and introduced nilutamide for medical use.[17]
References
- ↑ 1.0 1.1 "The pure antiandrogen RU 23908 (Anandron), a candidate of choice for the combined antihormonal treatment of prostatic cancer: a review". The Prostate 5 (3): 299–311. 1984. doi:10.1002/pros.2990050307. PMID 6374639. "[...] flutamide but we soon abandoned the development of steroid derivatives such as RU 2956 because of inherent androgenicity [17], and focused on the nonsteroidal antiandrogens.".
- ↑ 2.0 2.1 Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. 2001. p. 2158. ISBN 978-3-527-30247-5. https://books.google.com/books?id=zmpqAAAAMAAJ. "10635 (8596) C21H28O2 23983-19-9 17β-Hydroxy-2,2,17-trimethylestra-4,9,11-trien-3-one : (17β)-17-Hydroxy-2,2,17-trimethylestra-4,9,11-trien-3-one (•) S R 2956 U Anti-androgen"
- ↑ 3.0 3.1 Androgens II and Antiandrogens / Androgene II und Antiandrogene. Springer Science & Business Media. 27 November 2013. pp. 1–. ISBN 978-3-642-80859-3. https://books.google.com/books?id=7JPsCAAAQBAJ&pg=PA1.
- ↑ 4.0 4.1 "The design and use of sex-steroid antagonists". Journal of Steroid Biochemistry 25 (5B): 811–833. November 1986. doi:10.1016/0022-4731(86)90313-4. PMID 3543501.
- ↑ 5.0 5.1 5.2 "[17beta-hydroxy-2,2,17-trimethyl-estra-4, 9,11-trien-3-one). 1. Profil endocrinien. (Antiandrogenic activity of R2956 (17beta-hydroxy-2,2,17-trimethyl-estra-4,9,11-trien-3-one). 1. Endocrine profile) Activite anti-androgene du R 2956"] (in fr). Journal de Pharmacologie 5 (4): 509–520. 1974. https://www.popline.org/node/494155. Retrieved 12 August 2016. "R 2956 (17beta-hydroxy-2,2,17-trimethyl-estra-4,9,11-trien-3-one) was tested for antiandrogenic activity in rats (Dorfman test); in dogs; for androgenic activity in female rats (Hershberger); in male rats; for progestagenic activity in rabbits (Clauberg); for uterotrophic activity in mice (Rubin); and for antiestrogenic activity in mice (Dorfman). R 2956 significantly antagonized the hypertrophic effect of .05 mg testosterone propionate on rat seminal vesicles and ventral prostate in proportion to dose from .4-5 mg/day orally. In dogs R 2956 lowered prostate epithelial hyperplasia induced by androstanolone. R 2956 had no androgenic, estrogenic, progestational, or antiestrogenic activities and inhibited development of corpora lutea to an extent comparable with that of norethindrone.".
- ↑ 6.0 6.1 Proceedings of the Fourth International Congress on Hormonal Steroids: Mexico City, September 1974. Elsevier Science. 22 October 2013. pp. 618, 620. ISBN 978-1-4831-4566-2. https://books.google.com/books?id=Iq0aAwAAQBAJ&pg=PA620. "R-2956 [41-43], a dimethyl derivative of an extremely potent androgen, R 1881 [44], is a powerful testosterone antagonist with very low androgenic activity."
- ↑ "Steroid responsiveness of the human cell line NHIK 3025". Acta Endocrinologica 97 (4): 551–558. August 1981. doi:10.1530/acta.0.0970551. PMID 7270009.
- ↑ Innovative Approaches in Drug Research: Proceedings of the Third Noordwijkerhout Symposium on Medicinal Chemistry, Held in the Netherlands, September 3-6, 1985. Elsevier. 1 January 1986. ISBN 978-0-444-42606-2. https://books.google.com/books?id=PwhtAAAAMAAJ. "At this stage, RU 2956 exerts a competitive effect about 4 times less marked than metribolone may be because the steric hindrance of the dimethyl group in position C-2 interferes with H-bond formation between the C-3 oxygen and the receptor protein, i.e., with the recognition step, and consequently, with the association rate."
- ↑ "Receptor characteristics of the rat mammary carcinoma cell line 64-24". Cancer Research 41 (1): 42–48. January 1981. PMID 6256064.
- ↑ "Dissociating behavioral, autonomic, and neuroendocrine effects of androgen steroids in animal models". Psychiatric Disorders. Methods in Molecular Biology. 829. 2012. pp. 397–431. doi:10.1007/978-1-61779-458-2_26. ISBN 978-1-61779-457-5. "Administration of steroidal, blocking agents such as spironolactone, cyproterone acetate, or trimethyltrienolone, or nonsteroidal, such as flutamide, bicalutamide, blocking agents, can attain this result (169–171)."
- ↑ 11.0 11.1 11.2 Male Accessory Sex Organs: Structure and Function in Mammals. Elsevier. 2 December 2012. pp. 323–. ISBN 978-0-323-14666-1. https://books.google.com/books?id=TPfM6mwJozYC&pg=PA323.
- ↑ "A prostatic cytosol receptor". Biochemical and Biophysical Research Communications 38 (4): 599–606. February 1970. doi:10.1016/0006-291X(70)90623-6. PMID 5443703.
- ↑ "Anti-androgenic Activity of R 2956 (17beta-hydroxy-2,2,17alpha-trimethyl-estra-4,9,11-trien-3-one). 2. Mechanism Of Action". Journal de Pharmacologie 5 (4): 521–532. 1974.
- ↑ Androgenetic Alopecia: Modern Concepts of Pathogenesis and Treatment. Springer Science & Business Media. 14 March 2013. pp. 531–. ISBN 978-4-431-67038-4. https://books.google.com/books?id=RX7dBgAAQBAJ&pg=PT531.
- ↑ "Steroidal antiandrogens and 5alpha-reductase inhibitors". Current Medicinal Chemistry 6 (12): 1107–23. December 1999. doi:10.2174/0929867306666220401180500. PMID 10519917. "Several androstane derivatives have also demonstrated an antiandrogenic activity; 17a-methyl-B-nortestosterone 8 was prepared and tested in 1964 for antihormonal activity [43]. Within the next decade, several other androstane analogs were prepared and found to possess antiandrogenic activity [43, 44, 45, 46] including BOMT 9 "figure 2", R2956 10, SC9420 11, and oxendolone 12 "figure 3".".
- ↑ Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. 6 December 2012. pp. 112–. ISBN 978-94-009-8195-9. https://books.google.com/books?id=7IrpCAAAQBAJ&pg=PA112.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier. pp. 2935–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA2935-IA22.
 |

