Chemistry:Siramesine
From HandWiki
Short description: Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
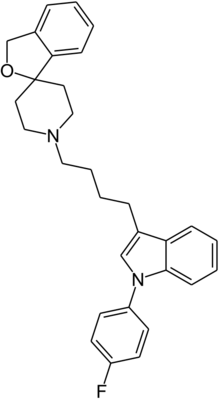
| Formula | C30H31FN2O |
| Molar mass | 454.589 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Siramesine (or Lu 28-179) is a sigma receptor agonist, selective for the σ2 subtype.[1] In animal studies, siramesine has been shown to produce anxiolytic[2] and antidepressant[3] effects. It was developed by the pharmaceutical company H Lundbeck for the treatment of anxiety,[4] although development was discontinued after clinical trials showed a lack of efficacy in humans. Siramesine has been shown to produce an enhanced antidepressant effect when co-administered with NMDA antagonists.[5] It has also been used to study the σ2 activity of cocaine,[6] and has been shown to produce anticancer properties both in vitro[7] and in vivo.[8]
References
- ↑ "Lu 28-179 labels a sigma(2)-site in rat and human brain". Neuropharmacology 43 (1): 95–100. July 2002. doi:10.1016/s0028-3908(02)00071-0. PMID 12213263.
- ↑ "The selective sigma2-ligand Lu 28-179 has potent anxiolytic-like effects in rodents". The Journal of Pharmacology and Experimental Therapeutics 283 (3): 1323–32. December 1997. PMID 9400007.
- ↑ "The selective sigma2 ligand Lu 28-179 has an antidepressant-like profile in the rat chronic mild stress model of depression". Behavioural Pharmacology 11 (2): 117–24. April 2000. doi:10.1097/00008877-200004000-00003. PMID 10877116.
- ↑ "Siramesine H Lundbeck". Current Opinion in Investigational Drugs 2 (2): 266–70. February 2001. PMID 11816842.
- ↑ "The synergistic effect of selective sigma receptor agonists and uncompetitive NMDA receptor antagonists in the forced swim test in rats.". Journal of Physiology and Pharmacology 57 (2): 217–29. June 2006. PMID 16845227. https://jpp.krakow.pl/journal/archive/06_06/pdf/217_06_06_article.pdf.
- ↑ "Sigma2 (sigma2) receptors as a target for cocaine action in the rat striatum". European Journal of Pharmacology 535 (1–3): 98–103. March 2006. doi:10.1016/j.ejphar.2005.12.077. PMID 16480713.
- ↑ "Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress". Cancer Research 65 (19): 8975–83. October 2005. doi:10.1158/0008-5472.CAN-05-0269. PMID 16204071.
- ↑ "Vincristine induces dramatic lysosomal changes and sensitizes cancer cells to lysosome-destabilizing siramesine". Cancer Research 67 (5): 2217–25. March 2007. doi:10.1158/0008-5472.CAN-06-3520. PMID 17332352.
External links
- Siramesine at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

