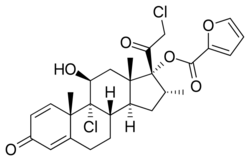
Chemistry:Mometasone furoate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Many[1] |
| Other names | 9α,21-Dichloro-11β,17α-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17α-(2-furoate) |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, inhalation (nasal spray) |
| Drug class | Corticosteroid; Glucocorticoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Nasal spray is virtually undetectable in plasma; but systemic availability is comparable to fluticasone[2] |
| Protein binding | 98% to 99% |
| Metabolism | Liver |
| Elimination half-life | 5.8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H28Cl2O4 for mometasone C27H30O6Cl2 as furoate |
| Molar mass | 427.361 g/mol (mometasone) 521.4 g/mol (furoate) |
| 3D model (JSmol) | |
| |
| |
| | |
Mometasone furoate is a glucocorticoid or corticosteroid used topically to reduce inflammation of the skin or in the airways. It is a prodrug of the free form mometasone (INN).[citation needed]
Medical uses
Mometasone furoate is used in the treatment of inflammatory skin disorders (such as eczema and psoriasis) (topical form), allergic rhinitis (such as hay fever) (topical form), asthma (inhalation form)[3][4] for patients unresponsive to less potent corticosteroids, and penile phimosis.[5] In terms of steroid strength, it is more potent than hydrocortisone, and less potent than dexamethasone.[6]
Some low-quality evidence suggests the use of mometasone for symptomatic improvement in children with adenoid hypertrophy.[7]
Mometasone is used to alleviate inflammation and itchiness in skin conditions that respond to treatment with glucocorticoids such as psoriasis and atopic dermatitis.[8][9]
Nasal mometasone is used in adults (including the elderly) and children over two years of age, to diminish the symptoms such as hay fever (seasonal allergic rhinitis) and other allergies (perennial rhinitis), including nasal congestion, discharge, pruritus, and sneezing and to treat nasal polyps.[10]
Asthma
Mometasone furoate can be used with formoterol for the treatment of asthma, due to its anti-inflammatory properties.[7][11]
Pharmacology
Pharmacodynamics
Mometasone furoate reduces inflammation by causing several effects:[10][12][13]
- Reversing the activation of inflammatory proteins
- Activating the secretion of anti-inflammatory proteins
- Stabilizing cell membranes
- Decreasing the influx of inflammatory cells
In addition to the glucocorticoid properties of mometasone furoate, it is a very potent agonist of the progesterone receptor as well as a partial agonist of the mineralocorticoid receptor.[14]
Mechanism of action
Mometasone, like other corticosteroids, possesses anti-inflammatory, antipruritic, and vasoconstrictive properties. For allergies, corticosteroids reduce the allergic reactions in various types of cells (mastocytes and eosinophils) that are responsible for allergic reactions. Mometasone and other corticosteroids circulate in the blood easily, crossing cellular membranes and binding with cytoplasmic receptors, resulting in the transcription and synthesis of proteins. It also inhibits the actions of the enzyme cytochrome P450 2C8 which participates in the activity of monooxygenase.[15]
The inflammation is reduced in decreasing the liberation of hydrolace acids of leukocytes, the prevention of the accumulation macrophages in the sites of inflammation, the interference with adhesion of leukocytes to capillary walls, the reduction of the permeability of the capillary membranes and consequently edema, the reduction of complementary components, inhibition of histamine and kinin liberation, and interference with scar tissue formation.[16] The proliferation of fibroblasts and collagen deposits is also reduced. It is believed that the action of corticosteroid anti-inflammatory agents is bound to inhibitive proteins of phospholipase A2, collectively called lipocortins. The lipocortins, in turn, control the biosynthesis of potent mediators of inflammation as the prostaglandins and leukotrienes, inhibiting the liberation of the molecular precursors of arachidonic acid. Intranasal mometasone alleviates symptoms such as rhinorrhea aquosa, nasal congestion, nasal drip, sneezing, and pharyngeal itching. Topical administration applied to skin reduces the inflammation associated with chronic or acute dermatosis.
Pharmacokinetics
Metabolism
Extensive metabolic hepatic metabolism of mometasone furoate to multiple metabolites occurs. No principal metabolites are detectable in plasma. After in vitro incubation, one of the minor metabolites formed is furoate 6β-hydroxymometasone. In human hepatic microsomes, the formation of these metabolites is regulated by CYP3A4.[10]
Society and culture
Brand names
As of 2016 mometasone furoate was available worldwide in formulations for nasal, oral inhalation, and topical administration, for human and for veterinary use, and in combinations with other drugs, under many brand names.[1]
The following combination drugs were available as of 2016:[1]
- Mometasone and azelastine as Nasaflex
- Mometasone and clotrimazole and gentamicin for veterinary use as Mometamax
- Mometasone and florfenicol and terbinafine for veterinary use as Claro
- Mometasone and formoterol as Dulera and Zenhale
- Mometasone and fusidic acid as Momate-F
- Mometasone and miconazole as Elica M and Sensicort-F
- Mometasone and mupirocin as Sensicort-B
- Mometasone and orbifloxacin and posaconazole for veterinary use as Posatex
- Mometasone and salicylic acid as Belosalic, Cortimax-S, Elicasal, Elocom Plus, Elosalic, Momate, Momesalic, Momtas, Monsalic, and Sensicort-S
- Mometasone and tazarotene as Tazasone Forte
- Mometasone and terbinafine as Cutizone-T
It was available as of 2016 as the single active agent in the following brands: Alcom, Altosone, Asmanex, Atozon, Aureox, Belloseta, Bioelementa, Biometasona, Bloctimo, Borgasone, Breso, Broner, Codermo, Cortynase, Cutimom, Cutizone, Cutticom, Dance, Demoson, Dergentil, Derimod, Dermacortine, Dermaten, Dermome, Dermosona, Dermotasone, Dermovel, Desdek, Ecelecort, Ecural, Edelan, Elica, Elisone, Elisox, Elitasone, Elna, Elocan, Elocom, Elocon, Elocortin, Elofute, Elomet, Elomox, Eloskin, Eloson, Elosone, Elovent, Elox, Etacid, Eversone, Eztom, F-Din, Fenisona, Flazcort, Flogocort, Fremomet, Frondava, Fu Mei Song, Fulmeta, Furo, Furoato de Mometasona, Furoderm, Gistan-H, Honmet, Iflacort, Intercon, Ivoxel, Kalmente, Konex, Ladexol, Lisoder, Logren, Loksin, Lomeane, M-Furo, Makiren, Mefurosan, Melocort, Mena, Mesone, Metacortil, Metactiv, Metaflam, Metagra, Metasafe, Metason, Metasone, Metaspray, Metatop, Metaz, Metmin, Metsone, Midermin, Mifusin, Minyear, Mofacort, Mofulex, Mofur, Mofuroate, Molison, Momate, Momax, Momecon, Momecort, Momecutan, Momederm, MomeGalen, Momegen, Momekort, Momelab, Momentum, Momeplus, Momerid, Momeson, Momesone, Momester, Momet, Mometa, Mometagen, Mometason, Mometasona, Mometasona Furoato, Mometasone Furoate, Mometasone Furoate Hydrate, Mometasonfuroaat, Mometasonfuroat, Mometasoni furoas, Mometasonum, Mometasyn, Mometasyn, Mometax, Mometazon, Mometazona, Mometazona Fuorat, Mometazonfuroat, Mometix-AQ, Momevate, Momexa, Mommex, Mommox, Momtas, Monaliz, Monez, Monovel, Monovo, Mosone, Motaderm, Motaneal, Movesan, Mtaz, Mundoson, Murozo, Myrey, Narinex, Nasamet, Nasehaler, Nasocure, Nasomet, Nasometin, Nasonex, Nassomet, Nazofix, Nazoster, Netonox, Nexomist, Novasone, Ovison, Ovixan, Oximax, Pharmecort, Pluster, Pronasal, Propel, Prospiril, Pydercon, Rinelon, Rinitek, Rino-Val, Rinobudex, Rinonex, Rinosal, Rinosona, Rinoval, Risonel, Sensicort, Septopic, Silkaren, Soneta, Suavicort, Suqi, Synaller, Tabunex, Topcort, Topison, Uniclar, Uniderm, Vizomet, Yperod, Zalconex, and Zynovate.[1]
References
- ↑ 1.0 1.1 1.2 1.3 "International brands for Mometasone". Drugs.com. https://www.drugs.com/international/mometasone.html. Retrieved 17 November 2016.
- ↑ Zia R. Tayab; Tom C. Fardon; Daniel K. C. Lee; Kay Haggart; Lesley C. McFarlane; Brian J. Lipworth; Günther Hochhaus (November 2007). "Pharmacokinetic/pharmacodynamic evaluation of urinary cortisol suppression after inhalation of fluticasone propionate and mometasone furoate". Br J Clin Pharmacol 64 (5): 698–705. doi:10.1111/j.1365-2125.2007.02919.x. PMID 17509041.
- ↑ "Mometasone furoate in the management of asthma: a review". Ther Clin Risk Manag 4 (6): 1201–8. December 2008. PMID 19337427.
- ↑ Bousquet J (May 2009). "Mometasone furoate: an effective anti-inflammatory with a well-defined safety and tolerability profile in the treatment of asthma". Int. J. Clin. Pract. 63 (5): 806–19. doi:10.1111/j.1742-1241.2009.02003.x. PMID 19392928. https://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=1368-5031&date=2009&volume=63&issue=5&spage=806.
- ↑ Khope S (March 2010). "Topical mometasone furoate for phimosis". Indian Pediatr 47 (3): 282. PMID 20371899.
- ↑ Williams, DM (2005). "What does potency actually mean for inhaled corticosteroids?". The Journal of asthma : official journal of the Association for the Care of Asthma 42 (6): 409–17. doi:10.1081/jas-57878. PMID 16293535.
- ↑ 7.0 7.1 Passali, D; Spinosi, MC; Crisanti, A; Bellussi, LM (2 May 2016). "Mometasone furoate nasal spray: a systematic review.". Multidisciplinary respiratory medicine 11: 18. doi:10.1186/s40248-016-0054-3. PMID 27141307.
- ↑ Green, C; Colquitt, JL; Kirby, J; Davidson, P; Payne, E (November 2004). "Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.". Health technology assessment (Winchester, England) 8 (47): iii, iv, 1–120. doi:10.3310/hta8470. PMID 15527669. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0015083/.
- ↑ Prakash, A; Benfield, P (January 1998). "Topical mometasone. A review of its pharmacological properties and therapeutic use in the treatment of dermatological disorders.". Drugs 55 (1): 145–63. doi:10.2165/00003495-199855010-00009. PMID 9463794.
- ↑ 10.0 10.1 10.2 "Nasonex label". FDA. January 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020762s044lbl.pdf.
- ↑ Bousquet, J (May 2009). "Mometasone furoate: an effective anti-inflammatory with a well-defined safety and tolerability profile in the treatment of asthma.". International journal of clinical practice 63 (5): 806–19. doi:10.1111/j.1742-1241.2009.02003.x. PMID 19392928.
- ↑ Publishers, Jones and Bartlett (2009-07-15). Nurse's Drug Handbook 2010. p. 677. ISBN 978-0-7637-7900-9. https://books.google.com/books?id=9b8JiegivA8C&pg=PA677
- ↑ Mani S. Kavuru (2007). "ch. 9 Anti-inflammatory agents". Diagnosis and Management of Asthma. ISBN 978-1-932610-38-3. https://books.google.com/books?id=Q1zF7xSRACIC
- ↑ Template:Vcite2 journal
- ↑ Walsky, R. L.; Gaman EA; Obach RS. (Jan 2005). Examination of 209 drugs for inhibition of cytochrome P450 2C8.. 45 (Pfizer Global Research and Development ed.). USA.: J Clin Pharmacol.. pp. 68–78.
- ↑ Blaiss, MS (2011). "Safety update regarding intranasal corticosteroids for the treatment of allergic rhinitis.". Allergy and Asthma Proceedings 32 (6): 413–8. doi:10.2500/aap.2011.32.3473. PMID 22221434.


