Chemistry:Mebicar
This article needs more medical references for verification or relies too heavily on primary sources. (June 2014) |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 3h[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
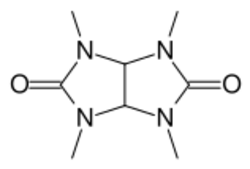
| Formula | C8H14N4O2 |
| Molar mass | 198.226 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mebicar (or tetramethylglycoluril) is an anxiolytic medication produced by Latvian pharmaceutical company Olainfarm and sold in Latvia and Russia under the brand name Adaptol.[2]
Mebicar has an effect on the structure of limbic-reticular activity, particularly on hypothalamus emotional zone, as well as on all 4 basic neuromediator systems – γ aminobutyric acid (GABA), choline, serotonin and adrenergic activity. Mebicar decreases the brain noradrenaline level, exerts no effect on the dopaminergic systems, increases the brain serotonin level, and does not elicit cholinolytic action.[3]
Mebicar purportedly has anti-anxiety (anxiolytic) properties.[3][4][5][6][7] It is also used to aid smoking cessation.[2] In addition, mebicar may be useful in the treatment of ADHD symptoms.[8] In contrast with typical anxiolytic medications such as benzodiazepines, mebicar is non-habit forming, non-sedating and does not impair motor function.[4][2]
It can be prepared by condensation of dimethyl urea (which can be made by n, n methylating urea using paraformaldehyde+oxalic acid dihydrate) with glyoxal. One recent publication described an elegant variation in which the two reactants are combined in an aqueous solution with phosphoric anhydride as the catalyst; the reaction takes place at room temperature, is fast, with essentially quantitative yield, and the procedure is easy and cost efficient because the product precipitates as it is formed, and after removal of the solids by filtration, the leftover solution can be used one or more times with no additional catalyst (with a longer reaction time).
Availability
Mebicar has not been evaluated by the U.S. medical system. It cannot be given to a patient in the context of any licensed (or self ascribed) medical practice. Although independently obtaining it for personal use is technically not illegal, it is discouraged. Self-prescribed, unapproved treatments (in general) can be dangerous; people usually are not all that knowledgeable and likely to misdiagnose and mistreat their symptoms; and any incidence of side effect or medical emergency is less likely to be effectively treated if the causative agent is unknown to the emergency treatment team.
See also
- Fabomotizole
- Phenibut
- Selank
- Validol
References
- ↑ Schwarz J, Weisspapir M, "Sustained release pharmaceutical composition containing mebicar", US patent 20110070305
- ↑ 2.0 2.1 2.2 "Adaptol product summary". https://olainfarm.lv/en/prescription-drugs/adaptol-3.
- ↑ 3.0 3.1 "[Characteristics of the psychotropic spectrum of action of mebicar]". Biulleten' Eksperimental'noi Biologii I Meditsiny 89 (5): 568–70. May 1980. PMID 6104993.
- ↑ 4.0 4.1 "A study of the spectrum of psychotropic action of mebicar". Bulletin of Experimental Biology and Medicine 89 (5): 621–624. 1980. doi:10.1007/BF00835799.
- ↑ "[Adaptol--verges of possible]". Likars'ka Sprava (5): 125–33. 2012. PMID 23534281.
- ↑ "[Generalized anxiety disorder: psychosomatic aspects and treatment approaches]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 112 (1): 40–4. 2012. PMID 22678674.
- ↑ "[Asthenic disorders in children]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 110 (11 Pt 1): 26–9. 2010. PMID 21183919.
- ↑ "[Adaptol in the treatment of ADHD]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 109 (8): 45–8. 2009. PMID 19738569.

