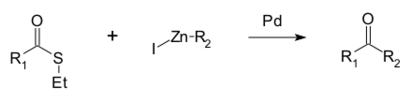Biology:Thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure R–C(=O)–S–R’. They are analogous to carboxylate esters (R–C(=O)–O–R’) with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid (R–C(=O)–O–H) with a thiol (R'–S–H). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.[1] The R and R' represent organyl groups, or H in the case of R.
Synthesis
The most typical route to thioester involves the reaction of an acid chloride with an alkali metal salt of a thiol:[1]
- [math]\ce{ RSNa + R'COCl -> R'COSR + NaCl }[/math]
Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate:[1]
- [math]\ce{ CH3COSK + RX -> CH3COSR + KX }[/math]
The analogous alkylation of an acetate salt is rarely practiced. The alkylation can be conducted using Mannich bases and the thiocarboxylic acid:
- [math]\ce{ CH3COSH + R'_2NCH2OH -> CH3COSCH2NR'_2 + H2O }[/math]
Thioesters can be prepared by condensation of thiols and carboxylic acids in the presence of dehydrating agents:[2][3]
- [math]\ce{ RSH + R'CO2H -> RSC(O)R' + H2O }[/math]
A typical dehydration agent is DCC.[4] Efforts to improve the sustainability of thioester synthesis have also been reported utilising safer coupling reagent T3P and greener solvent cyclopentanone.[5] Acid anhydrides and some lactones also give thioesters upon treatment with thiols in the presence of a base.
Thioesters can be conveniently prepared from alcohols by the Mitsunobu reaction, using thioacetic acid.[6]
They also arise via carbonylation of alkynes and alkenes in the presence of thiols.[7]
Reactions
Thioesters hydrolyze to thiols and the carboxylic acid:
- RC(O)SR' + H2O → RCO2H + RSH
The carbonyl center in thioesters is more reactive toward amine nucleophiles to give amides:
In a related reaction, but using a soft-metal to capture the thiolate, thioesters are converted into esters.[8] Thioesters provide useful chemoselectivity in the synthesis of biomolecules.[9]
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an organozinc halide by a palladium catalyst to give a ketone.
Biochemistry

Thioesters are common intermediates in many biosynthetic reactions, including the formation and degradation of fatty acids and mevalonate, precursor to steroids. Examples include malonyl-CoA, acetoacetyl-CoA, propionyl-CoA, cinnamoyl-CoA, and acyl carrier protein (ACP) thioesters. Acetogenesis proceeds via the formation of acetyl-CoA. The biosynthesis of lignin, which comprises a large fraction of the Earth's land biomass, proceeds via a thioester derivative of caffeic acid.[10] These thioesters arise analogously to those prepared synthetically, the difference being that the dehydration agent is ATP. In addition, thioesters play an important role in the tagging of proteins with ubiquitin, which tags the protein for degradation.
Oxidation of the sulfur atom in thioesters (thiolactones) is postulated in the bioactivation of the antithrombotic prodrugs ticlopidine, clopidogrel, and prasugrel.[11][12]
Thioesters and the origin of life
As posited in a "Thioester World", thioesters are possible precursors to life.[13] As Christian de Duve explains:
It is revealing that thioesters are obligatory intermediates in several key processes in which ATP is either used or regenerated. Thioesters are involved in the synthesis of all esters, including those found in complex lipids. They also participate in the synthesis of a number of other cellular components, including peptides, fatty acids, sterols, terpenes, porphyrins, and others. In addition, thioesters are formed as key intermediates in several particularly ancient processes that result in the assembly of ATP. In both these instances, the thioester is closer than ATP to the process that uses or yields energy. In other words, thioesters could have actually played the role of ATP in a "thioester world" initially devoid of ATP. Eventually, [these] thioesters could have served to usher in ATP through its ability to support the formation of bonds between phosphate groups.
However, due to the high free energy change of thioester's hydrolysis and correspondingly their low equilibrium constants, it is unlikely that these compounds could have accumulated abiotically to any significant extent especially in hydrothermal vent conditions.[14]
Thionoesters

Thionoesters are isomeric with thioesters. In a thionoester, sulfur replaces the carbonyl oxygen in an ester. Methyl thionobenzoate is C6H5C(S)OCH3. Such compounds are typically prepared by the reaction of the thioacyl chloride with an alcohol.[15]
They can also be made by the reaction of Lawesson's reagent with esters or by treating pinner salts with hydrogen sulphide. An alternatively, various thionoesters may be prepared through the transesterification of an existing methyl thionoester with an alcohol under base-catalyzed conditions.[16]
Xanthates[17] and thioamides[18] can be transformed to thionoesters under metal-catalyzed cross-coupling conditions.
See also
- Thiocarboxylic acid
- Thiocarbonate
- Liebeskind-Srogl coupling
- Aldrithiol-2
References
- ↑ 1.0 1.1 1.2 Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. doi:10.1002/9780470771099.ch15
- ↑ Fujiwara, S.; Kambe, N. (2005). "Thio-, Seleno-, and Telluro-Carboxylic Acid Esters". Topics in Current Chemistry. 251. Berlin / Heidelberg: Springer. pp. 87–140. doi:10.1007/b101007. ISBN 978-3-540-23012-0.
- ↑ "Synthesis of thioesters". Organic Chemistry Portal. https://www.organic-chemistry.org/synthesis/C1S/thioesters.shtm.
- ↑ Mori, Y.; Seki, M. (2007). "Synthesis of Multifunctionalized Ketones Through the Fukuyama Coupling Reaction Catalyzed by Pearlman's Catalyst: Preparation of Ethyl 6-oxotridecanoate". Organic Syntheses 84: 285. https://www.orgsyn.org/demo.aspx?prep=V84P0285.; Collective Volume, 11, pp. 281
- ↑ Jordan, Andrew; Sneddon, Helen F. (2019). "Development of a solvent-reagent selection guide for the formation of thioesters". Green Chemistry 21 (8): 1900–1906. doi:10.1039/C9GC00355J.
- ↑ Volante, R. (1981). "A new, highly efficient method for the conversion of alcohols to thiolesters and thiols". Tetrahedron Letters 22 (33): 3119–3122. doi:10.1016/S0040-4039(01)81842-6.
- ↑ Bertleff, W.; Roeper, M.; Sava, X.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_217.pub2.
- ↑ Wan Kit Chan; S. Masamune; Gary O. Spessard (1983). "Preparation Of O-esters From The Corresponding Thiol Esters: Tert-butyl Cyclohexanecarboxylate". Organic Syntheses 61: 48. doi:10.15227/orgsyn.061.0048.
- ↑ McGrath, N. A.; Raines, R. T. (2011). "Chemoselectivity in chemical biology: Acyl transfer reactions with sulfur and selenium". Acc. Chem. Res. 44 (9): 752–761. doi:10.1021/ar200081s. PMID 21639109.
- ↑ Lehninger, A. L.; Nelson, D. L.; Cox, M. M. (2000). Principles of Biochemistry (3rd ed.). New York: Worth Publishing. ISBN 1-57259-153-6. https://archive.org/details/lehningerprincip01lehn.
- ↑ Mansuy, D.; Dansette, P. M. (2011). "Sulfenic acids as reactive intermediates in xenobiotic metabolism". Archives of Biochemistry and Biophysics 507 (1): 174–185. doi:10.1016/j.abb.2010.09.015. PMID 20869346. https://zenodo.org/record/898058.
- ↑ Dansette, P. M.; Rosi, J.; Debernardi, J.; Bertho, G.; Mansuy, D. (2012). "Metabolic Activation of Prasugrel: Nature of the Two Competitive Pathways Resulting in the Opening of Its Thiophene Ring". Chemical Research in Toxicology 25 (5): 1058–1065. doi:10.1021/tx3000279. PMID 22482514.
- ↑ de Duve, C. (1995). "The Beginnings of Life on Earth". American Scientist 83 (5): 428–437. https://www.jstor.org/stable/29775520.
- ↑ Chandru, Kuhan; Gilbert, Alexis; Butch, Christopher; Aono, Masashi; Cleaves, Henderson James II (21 July 2016). "The Abiotic Chemistry of Thiolated Acetate Derivatives and the Origin of Life". Scientific Reports 6 (29883): 29883. doi:10.1038/srep29883. PMID 27443234. Bibcode: 2016NatSR...629883C.
- ↑ Cremlyn, R. J. (1996). An Introduction to Organosulfur Chemistry. Chichester: John Wiley and Sons. ISBN 0-471-95512-4.
- ↑ Newton, Josiah J.; Britton, Robert; Friesen, Chadron M. (4 October 2018). "Base-Catalyzed Transesterification of Thionoesters". The Journal of Organic Chemistry 83 (20): 12784–12792. doi:10.1021/acs.joc.8b02260. PMID 30235418.
- ↑ Monteith, John J.; Scotchburn, Katerina; Mills, L. Reginald; Rousseaux, Sophie A. L. (2022). "Ni-Catalyzed Synthesis of Thiocarboxylic Acid Derivatives". Organic Letters 24 (2): 619–624. doi:10.1021/acs.orglett.1c04074. PMID 34978834. https://doi.org/10.1021/acs.orglett.1c04074.
- ↑ Liu, Yinbo; Mo, Xiaofeng; Majeed, Irfan; Zhang, Mei; Wang, Hui; Zeng, Zhuo (2022). "An efficient and straightforward approach for accessing thionoesters via palladium-catalyzed C–N cleavage of thioamides". Organic & Biomolecular Chemistry 20 (7): 1532–1537. doi:10.1039/d1ob02349g. ISSN 1477-0520. PMID 35129563. https://dx.doi.org/10.1039/d1ob02349g.
 |