from importer import *
sN = slice(None)
now = datetime.now()
# results_uid = now.strftime("%Y%m%d-%H%M%S")
results_uid = '20240807-135247-postRebuttal2-Night2'
results_dir = get_root(f'results', results_uid)
figdir = results_dir.append('figures')
results_file = results_dir.append('result_dict.pkl')
restats_file = results_dir.append('result_and_stats_dict.pkl')
dl_aggregate_file = results_dir.append('aggregate_dict.pkl') # Stores models
os.makedirs(figdir, exist_ok=True)
print('Results stored at:', results_dir)Code supplement to:
Predicting Antidepressant Treatment Response from Cortical Structure on MRI: A Mega-Analysis from the ENIGMA-MDD Working Group
This README file is an export of main.ipynb, the primary report on the results presented in the article with this name. It is best to read this documentation in the original file in a notebook server like Jupyter. In this notebook export we will traverse through the data in the same order that things are discussed in the manuscript:
- Load, join and clean data
- Data inspection and exploration
- Preparation of Machine Learning Set Up
- Train Machine Learning Models
- Calculate and Print Statistics
- Interpretation of Results
Each partner in the ENIGMA consortium provided three types of data:
dbcDemographic, Behavioural and Clinical patient dataroiCortical measurements on thickness and surface area for each anatomical Region Of InterestmapA 2D projection of the surface data underlying to theroidata type. Let us start with loading and cleaning this data.
We first load the data and join sheets using the load_and_join_dfs method, which is not fancy only performs an inner join on the two number of data frames.
path_roi = get_root('data', 'ENIGMA-MDD_patients-included_data-subcortical.csv')
path_dbc = get_root('data', 'ENIGMA-MDD_patients-included_data-clinical_wk2.csv')
data = load_and_join_dfs(path_dbc, path_roi, index_col='SubjID', decimal=',')path_dbc_wk1 = get_root('data', 'ENIGMA-MDD_patients-included_data-clinical_wk1.csv')
wk1_subjs = read_any_csv(path_dbc_wk1, index_col='SubjID').index
print(f'Number of patients excluded if scan threshold is week 1 instead of week 2: {len([i for i in data.index if i not in wk1_subjs])}')Number of patients excluded if scan threshold is week 1 instead of week 2: 40
After loading the structured dbc and roi data, we now load the map 2D-projection data.
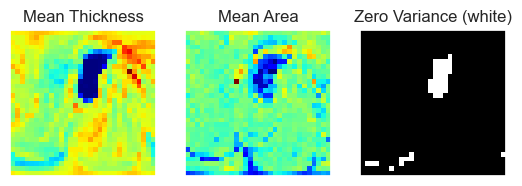
Briefly on the map data: it represents the thickness of an individual on the surface of a hemisphere of the brain. The brain hemisphere is represented as a 3D mesh, you can imagine this as a 3D point cloud, the points are called vertex, plural vertices. Thus, the original data was just a long list (164k) of thickness values (scalars) for each vertex in the mesh. By inflating the hemisphere we can find the position of each scalar on the surface of a sphere. The scalar values were then projected to a plane using stereographic projection using s12_sphere2place.py. This code makes sure that left an right hemispheres line up very well (Fig 2.). This scalar data was supplied to us as GIfTY file type. GIfTY (.gii) is the geometric equivalent to NIfTY (.nii) files, s12_sphere2place.py converts this into 2D NumPy arrays, which is the only thick we will be working with in this Notebook. We Clean the data right away by: removing borders, converting it to a 1D vector and dropping zero variance pixels.
# Remove edges and extra channel, and make it into a nice DataFrame with x,y pixel coordinates as column labels
dim = 32
X_thc, X_thc_arr = load_proj_df(data, 'Thickness', dim=dim)
X_are, X_are_arr = load_proj_df(data, 'Area', dim=dim)100%|██████████| 252/252 [00:00<00:00, 2708.94it/s]
100%|██████████| 252/252 [00:00<00:00, 2485.96it/s]
# Find zero variance (corpus callosum (CC))
bool_var = [var < 0.005 for _, var in X_thc.var().iteritems()]
zero_var = [col for col, var in X_thc.var().iteritems() if var < 0.002]
# Drop CC
X_thc = X_thc.drop(columns=zero_var)
X_are = X_are.drop(columns=zero_var)
# Visualization of loaded data
fig, axes = plt.subplots(1, 3)
img_arrays = X_thc_arr.mean(axis=0)[0], X_are_arr.mean(axis=0)[0], np.array(bool_var).reshape(dim - 2, dim - 2)
titles = 'Mean Thickness', 'Mean Area', 'Zero Variance (white)'
cmaps = 'jet', 'jet', 'gray'
for ax, arr, title, cmap in zip(axes, img_arrays, titles, cmaps):
ax.imshow(arr, cmap=cmap)
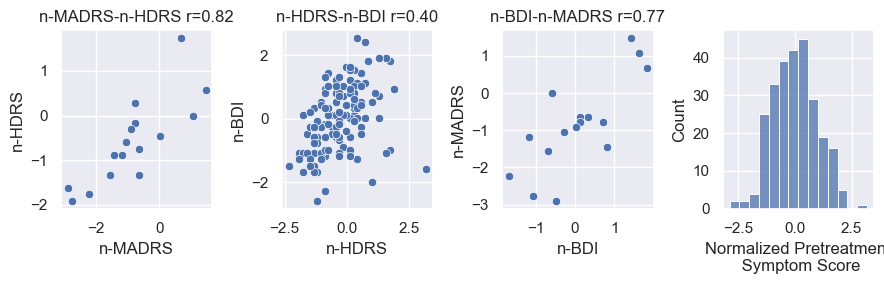
ax.set(title=title, xticks=[], yticks=[])Secondly we clean the joined data frame using the clean_data_df method. Although this method is mainly concerned with the sanitation of data, (e.g. renaming AO to Age_of_Onset) it also introduces Normalized_Pretreatment_Severity, a single numeric pretreatment symptom score. Briefly, we center all scoring methods around zero, and rescale them to their standard deviation. The normalized score we use is preferably MADRS, then HDRS, then BDI, depending on what is available. Since some subjects have more than one scoring we can check if different scoring lists are actually correlated. Correlations are fine (Fig.1)
scoring_methods = 'MADRS', 'HDRS', 'BDI'
data, norm_fig = clean_data_df(data, drop_old_symptom_scores=True)
norm_fig.savefig(figdir.append('NormalizedSeverityScores.png'))Three instruments to score treatment were used across all cohorts. The following scoring methods were used per site. The method used in order of preference is MADRS, HDRS then BDI.
score_df = pd.DataFrame(columns=scoring_methods)
for cohort in cohorts:
cohort_data = pd.DataFrame(data=data[data.cohort == cohort.key], columns=data.columns)
if len(cohort_data):
contains = lambda scoring_method: cohort_data[f'{scoring_method}_pre'].notna().sum()
score_df.loc[f'{cohort.name} (N={len(cohort_data)})'] = [
contains(score) if contains(score) else '' for score in scoring_methods]
print(score_df) MADRS HDRS BDI
AFFDIS (N=16) 16 16 16
DEP-ARREST-CLIN (N=57) 57
Hiroshima cohort (N=92) 92 92
Melbourne (N=49) 49
Minnesota (N=13) 13
Milano OSR (N=35) 34 26
Convert cells with treatment type information into column categories.
# Split up the treatment information into separate boolean columns
drug_types = set(flatten(data['Treatment_type_category'].values))
for drug_type in drug_types:
data[f'uses_{drug_type}'] = [drug_type in i for i in data['Treatment_type_category']]Let us also insert an is_extreme label, we will talk about the "Extremes subpopulation" later
q1, q3 = np.percentile(data['Response_percentage'], [25, 75])
data.insert(loc=0, column='is_extreme',
value=[(data['Response_percentage'] < q1) | (data['Response_percentage'] > q3)][0])
same_responders = 'AFFDIS', 'Hiroshima', 'Melb'
data.insert(loc=0, column='is_same_responder', value=[c in same_responders for c in data.cohort])Now that we know that there are substantial differences among sites in our full data set (total) we would like to define more homogeneous subgroups for future analyses. Due to the differences in both measured training data as primary outcomes we would like to initially define two subgroups. The first consists of just a single site, namely the Hiroshima cohort (Hiroshima), since it has the largest sample size and its population characteristics are reflective of the larger study. Second, we define a subpopulation as showing a similar treatment response rate under 50% (Same_resp). The three cohorts we chose we AFFDIS, Hiroshima cohort and Melbourne. Lastly, we define a third subgroup. These are subjects in the upper and lower qualtile in terms of treatment response. The hypothesis behind this subpopulation is that if there is a treatment effect in the data, one would expect this to be larger in patients showing more response.
Let us define subsets of our full data frame. Let us define the indices for each of our population subgroups that we can use to filter our data frame later:
long_treated = 'MOODS', 'Melb', 'Minnesota'
q1, q3 = np.percentile(data['Response_percentage'], [25, 75])
populations = {
'All': data.index,
'Hiroshima': data[data.cohort == 'Hiroshima'].index,
'SameResponders': pd.Index([i for i in data.index if i.split('_')[0] in same_responders]),
'Extremes': data[data.is_extreme].index,
'LongTreated': pd.Index([i for i in data.index if i.split('_')[0] in long_treated]),
}
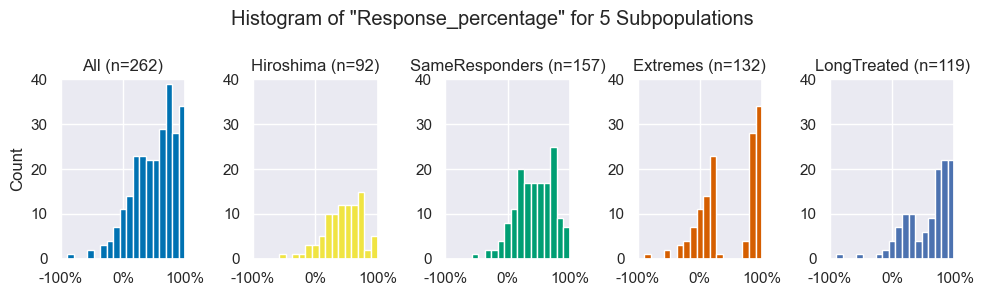
# Plot histograms of response percentage for each subpopulation
resp_fig = subpop_hists(data, populations)
resp_fig.savefig(figdir.append('ResponseHistogram.png'))
plt.show()Later on in this study, we present interesting findings in the Extremes subpopulation. An alternative explanation was that treatment duration could positively affect treatment outcome (longer follow up -> more chance to recover), thus that prediction in the Extremes cohort would primarily separate cohorts, and coincidentally, response and non-response. Our six cohorts do show an interesting difference among follow-up durations (see table below or table 1 in the manuscript). We roughly split them up in a group of Short treated ( < 6 weeks ) and LongTreated ( > 10 weeks ).
The hypothesis that the results in the Extremes population stems from a selection bias is weakened by the fact that the Extremes subpopulation consists of substantial (>37%) parts of each cohort. Additionally, the histogram of the Response_percentage in the LongTreated population does not have a clear trend to the right that supports the hypothesis that a longer follow-up leads to higher response rates. For details on this population see the table below.
However, interestingly, the LongTreated histogram shape looks like a blend between the All population and Extremes subpopulation, balanced accuracy in the LongTreated subpopulation as bad (49.7%) as in the All population (47.7%), especially when compared to the Extremes subpopulation (68.6%).
Adding the LongTreated subgroup will not affect our later analyses because we will tell the distiller object to look at the All population by default.
duration_based_cohort_printer = lambda in_only: '\n '.join([''.join([
c.name.ljust(20),
f'n={data.cohort.value_counts()[c.key]}'.ljust(7),
f'{data[data.cohort == c.key].is_responder.mean():.1%} ',
f'{str(c.treatment_duration_mu).rjust(4)}+{c.treatment_duration_sd}'
]) for c in cohorts if c.key in set(data.cohort) and (c.key in long_treated if in_only else c.key not in long_treated)])
print('Long treated:\n ', duration_based_cohort_printer(True), '\nShort treated:\n ',
duration_based_cohort_printer(False))Long treated:
DEP-ARREST-CLIN n=57 84.2% 12.0+0.0
Melbourne n=49 44.9% 12.0+0.0
Minnesota n=13 61.5% 9.9+2.0
Short treated:
AFFDIS n=16 37.5% 5.1+0.7
Hiroshima cohort n=92 48.9% 6.0+0.0
Milano OSR n=35 57.1% 4.2+0.7
expl = {
'All': '# samples that this cohort contributed to the entire population and the fraction of the entire population that comes from this sample.',
'All_Responders': '# Responders that this cohort contributed to the entire population.',
'F_of_All_Responders': "% all responders that comes from this cohort",
'Extremes': '# samples that this cohort contributed to the Extremes subpopulation and the fraction of the extremes population that comes from this sample.',
'Extreme_Responders': '# Responders that this cohort contributed to the Extremes subpopulation.',
'F_of_Extreme_Responders': "% Extreme responders that comes from this cohort",
}
extremes_contribution_df = pd.DataFrame(columns=list(expl.keys()))
extremes_contribution_df.loc['Total'] = [
f'{len(data)} (100%)',
f'{data.is_responder.sum()} ({data.is_responder.mean():.1%})',
'100%',
f'{len(data[data.is_extreme])} ({len(data[data.is_extreme]) / len(data):.1%})',
f'{data[data.is_extreme].is_responder.sum()} ({data[data.is_extreme].is_responder.mean():.1%})',
'100%',
]
for cohort in [c for c in cohorts if c.key in set(data.cohort)]:
cohort_data = data[data.cohort == cohort.key]
extreme_cohort_data = cohort_data[cohort_data.is_extreme]
values = [
f'{len(cohort_data)}' + f'({len(cohort_data) / len(data):.0%})'.rjust(7),
bool_fmt(cohort_data.is_responder),
f'{cohort_data.is_responder.sum() / data.is_responder.sum():.1%}',
f'{len(extreme_cohort_data)}' + f'({len(extreme_cohort_data) / data.is_extreme.sum():.0%})'.rjust(6),
bool_fmt(extreme_cohort_data.is_responder),
f'{cohort_data.is_responder.sum() / data.is_responder.sum():.1%}',
]
extremes_contribution_df.loc[cohort.name] = values
print(extremes_contribution_df)
print('\n'.join(['', 'Explained:'] + [f'{k}:'.ljust(np.max([len(i) for i in expl]) + 4) + v for k, v in expl.items()])) All All_Responders F_of_All_Responders Extremes Extreme_Responders F_of_Extreme_Responders
Total 262 (100%) 149 (56.9%) 100% 132 (50.4%) 66 (50.0%) 100%
AFFDIS 16 (6%) 6 (37.5%) 4.0% 8 (6%) 2 (25.0%) 4.0%
DEP-ARREST-CLIN 57 (22%) 48 (84.2%) 32.2% 36 (27%) 33 (91.7%) 32.2%
Hiroshima cohort 92 (35%) 45 (48.9%) 30.2% 33 (25%) 8 (24.2%) 30.2%
Melbourne 49 (19%) 22 (44.9%) 14.8% 26 (20%) 8 (30.8%) 14.8%
Minnesota 13 (5%) 8 (61.5%) 5.4% 11 (8%) 6 (54.5%) 5.4%
Milano OSR 35 (13%) 20 (57.1%) 13.4% 18 (14%) 9 (50.0%) 13.4%
Explained:
All: # samples that this cohort contributed to the entire population and the fraction of the entire population that comes from this sample.
All_Responders: # Responders that this cohort contributed to the entire population.
F_of_All_Responders: % all responders that comes from this cohort
Extremes: # samples that this cohort contributed to the Extremes subpopulation and the fraction of the extremes population that comes from this sample.
Extreme_Responders: # Responders that this cohort contributed to the Extremes subpopulation.
F_of_Extreme_Responders: % Extreme responders that comes from this cohort
The ENIGMA consortium provided is with a large amount of subjects from a number of consortia. It is crucial to have a good grasp of the properties of our population as well as differences among sites for the design of our methods and the interpretation of our results
We start off with a table that shows exclusion of patients. In the code below we go through two exclusion steps: First to select patients with follow-up data available for our classification target, second with complete data available for training. Thus, we go through three stages which we call: stg1, stg2 and stg3, of which the last one is equal to the cleaned data set data above.
# Load subjects in Stage 1
path_stg1 = get_root('data', 'ENIGMA-MDD_patients-all_data-clinical.csv')
df_stg1 = read_any_csv(path_stg1, index_col='SubjID').iloc[:, 1:]
# Load subjects in Stage 2
df_stg2 = read_any_csv(path_dbc, index_col='SubjID')
# We already have Stage 3
df_stg3 = data
# For Stage 4 we need to edit our projections data frame a bit
df_stg4 = X_thc.loc[[i for i in X_thc.index if 'LH' in i]]
df_stg4.index = df_stg4.index.map(lambda tag: tag.split('|')[0])
# Get the sites, and the data that are in each of the three steps
dfs = df_stg1, df_stg2, data, df_stg4,
sts = [sorted(set([i.split('_')[0] for i in df.index])) for df in dfs]
# Print a verbose overview of the subject selection process
print(f'Prior to subject selection (stage 1) we have {len(dfs[0])} subjects from {len(sts[0])} cohorts.')
print(f'After subject selection (stage 2) we have {len(dfs[1])} subjects from {len(sts[1])} cohorts.')
print(
f'After checking for completeness of data for ROI analysis (stage 3) we still have {len(dfs[2])} subjects from {len(sts[2])} cohorts.')
print(f'For Vec and 2D analysis, we have {len(dfs[3])} subjects from {len(sts[3])} cohorts')
# Print a table header
n_dfs = [
{'Total': len(df), '--': 0, **{f'{c.name} ({c.key})': len([i for i in df.index if c.key in i]) for c in cohorts}}
for df in dfs]
n_all, n_cov, n_dat, _ = n_dfs
# We can split this up further
n_series = [pd.Series(data=n_df, name=f'Stage {i}') for i, n_df in enumerate(n_dfs, 1)]
series_list = flatten([[ser, None] for ser in n_series])[:-1]
# Set the even numbered columns
for i, (df_before, df_after) in enumerate(zip(dfs[:-1], dfs[1:])):
series_list[1 + i * 2] = pd.Series(data={
'Total': len(df_before) - len(df_after),
'--': 0,
**{f'{cohorts[k].name} ({k})': len(
[i for i in df_before.index if i not in df_after.index and i.split('_')[0] == k]) for k in cohorts.keys()}
}, dtype=float, name=f'--excl-->')
print(pd.concat(series_list, axis=1).fillna(0).astype(int).replace(0, '').head(12))
# Uncomment this line if you would like to see which subjects do NOT have Cortical Data
# print("Subjects without Raw Cortigal data are:\n -", "\n - ".join([i for i in df_stg3.index if i not in df_stg4.index]))Prior to subject selection (stage 1) we have 795 subjects from 9 cohorts.
After subject selection (stage 2) we have 262 subjects from 6 cohorts.
After checking for completeness of data for ROI analysis (stage 3) we still have 262 subjects from 6 cohorts.
For Vec and 2D analysis, we have 252 subjects from 6 cohorts
Stage 1 --excl--> Stage 2 --excl--> Stage 3 --excl--> Stage 4
Total 795 533 262 262 10 252
--
AFFDIS (AFFDIS) 29 13 16 16 16
Cardiff (CARDIFF) 40 40
DEP-ARREST-CLIN (MOODS) 64 7 57 57 57
Hiroshima cohort (Hiroshima) 150 58 92 92 1 91
Melbourne (Melb) 156 107 49 49 49
Minnesota (Minnesota) 70 57 13 13 13
Milano OSR (SanRaffaele) 160 125 35 35 9 26
Stanford TIGER (TIGER) 48 48
UCSF Adolescent MDD (SF) 78 78
Before we move to the map data, let us take a look at the roi data that we received.
There are also a lot of non-cortext columns. Do not worry about them, later on we will mention which are included in the model as BDC features explicitly.
# Load structured data
dkt_atlas_features = [i for i in data.columns if any([j in i for j in dkt_atlas_lut])]
subcortical_rois = [i for i in pd.read_csv(path_roi, sep=';', index_col='SubjID').columns if
i[1] != '_' and not 'Site' in i][:-2]
subcortical_rois = [i for i in subcortical_rois if 'Thick' not in i and 'Surf' not in i]
print(
f'The number of cortical features we found is {len(dkt_atlas_features)} ({32 * 2 * 3} = 32 ROIs × 2 maps (Thickness & Sufrace) × 3 hemispheres (Left, Right, Mean). That means there are {len(data.columns) - len(dkt_atlas_features)} non-surface columns.')
subc_rois = subcortical_rois
subc_no_icv = subc_rois[:-1]
print(f'\nWe also have ICV and {len(subc_rois) - 1} subcortical_rois:', ', '.join(subc_rois[:-1]))
unique_subc_rois = sorted(set([i[1:].capitalize() if i[0] in 'LRM' else i for i in subc_rois]))
print(f'Of these {len(unique_subc_rois)} are unique:', ', '.join(unique_subc_rois),
'Vent, LatVent and ICV are not included, so 7 remain.')
# data[dkt_atlas_features].head() # You can take a look at the DKT Atlas features by uncommenting this line
data[subc_no_icv] = data[subc_no_icv].astype(float).div(data['ICV'], axis=0)The number of cortical features we found is 192 (192 = 32 ROIs × 2 maps (Thickness & Sufrace) × 3 hemispheres (Left, Right, Mean). That means there are 82 non-surface columns.
We also have ICV and 24 subcortical_rois: LLatVent, RLatVent, Lthal, Rthal, Lcaud, Rcaud, Lput, Rput, Lpal, Rpal, Lhippo, Rhippo, Lamyg, Ramyg, Laccumb, Raccumb, Mvent, Mthal, Mcaud, Mput, Mpal, Mhippo, Mamyg, Maccumb
Of these 10 are unique: Accumb, Amyg, Caud, Hippo, ICV, Latvent, Pal, Put, Thal, Vent Vent, LatVent and ICV are not included, so 7 remain.
Each data set was collected using different inclusion protocols. Thus, our the population per site can vary a lot. Let us start with group level statistics. We first create a data frame containing subject properties per site called site_stats using the collect_stats_per_site method. This data frame will be the input to the stacked_hist method. This method can read a column from the site_stats table and create a stacked histogram. In addition to site_stats we also receive a site_means table, which contains the same information but nicely formatted for printing, so let us start with that:
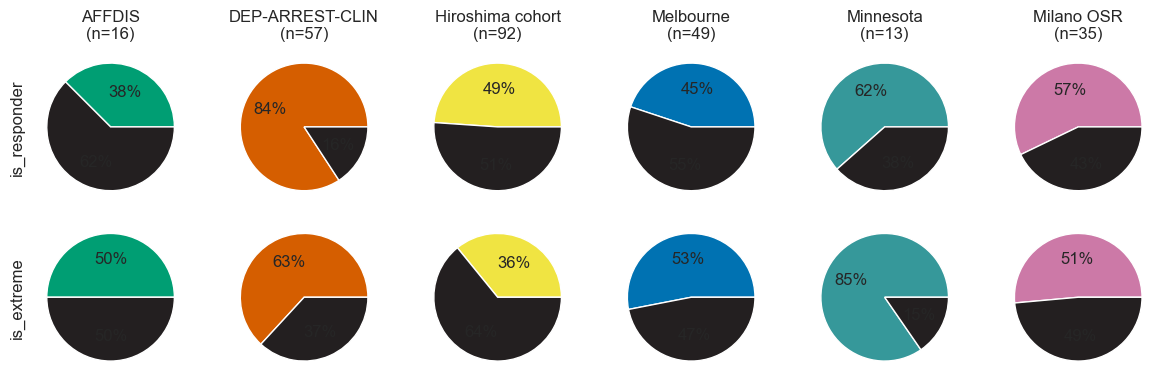
As you can see, the percentage of participants that is female (is_female) is more than twice as high in the Milano OSR cohort than in the AFFDIS cohor. Also, mean age of the participants included (Age) varies (15.0 for the Minnesota, and 50.3 for Milano OSR. Lastly, response rate varies from 37% at AFFDIS to 74% for DEP-ARREST-CLIN.
site_stats, site_means = collect_stats_per_site(data)
print(site_means.T)Cohort Total AFFDIS DEP-ARREST-CLIN Hiroshima cohort Melbourne Minnesota Milano OSR
Numel 262 16 57 92 49 13 35
Age 36.5 ± 15.3 44.1 ± 14.1 34.1 ± 12.7 43.0 ± 11.6 19.6 ± 3.0 15.0 ± 2.2 51.3 ± 9.6
Treatment_duration 8.3 ± 3.2 5.1 ± 0.7 12.0 ± 0.0 6.0 ± 0.0 12.0 ± 0.0 9.9 ± 1.9 4.3 ± 0.7
Normalized_Pretreatment_Severity 0.04 ± 0.99 -0.85 ± 1.20 0.79 ± 0.78 -0.31 ± 0.79 0.28 ± 0.72 0.15 ± 0.95 -0.25 ± 1.12
Age_of_Onset 30.3 ± 14.8 35.6 ± 15.7 28.0 ± 11.7 39.2 ± 13.3 15.7 ± 2.7 11.2 ± 2.6 36.5 ± 11.1
is_female 154 (58.8%) 5 (31.2%) 39 (68.4%) 47 (51.1%) 33 (67.3%) 9 (69.2%) 21 (60.0%)
is_recurrent 160 (61.1%) 15 (93.8%) 29 (50.9%) 46 (50.0%) 31 (63.3%) 9 (69.2%) 30 (85.7%)
is_responder 149 (56.9%) 6 (37.5%) 48 (84.2%) 45 (48.9%) 22 (44.9%) 8 (61.5%) 20 (57.1%)
uses_SSRI 194 (74.0%) 9 (56.2%) 0 (0.0%) 91 (98.9%) 49 (100.0%) 13 (100.0%) 32 (91.4%)
uses_SNRI 68 (26.0%) 3 (18.8%) 57 (100.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 8 (22.9%)
uses_ATYP 16 (6.1%) 8 (50.0%) 0 (0.0%) 1 (1.1%) 0 (0.0%) 0 (0.0%) 7 (20.0%)
is_same_responder 157 (59.9%) 16 (100.0%) 0 (0.0%) 92 (100.0%) 49 (100.0%) 0 (0.0%) 0 (0.0%)
is_extreme 132 (50.4%) 8 (50.0%) 36 (63.2%) 33 (35.9%) 26 (53.1%) 11 (84.6%) 18 (51.4%)
ADcur 1.5 ± 5.0 10.8 ± 14.4 0.0 ± 0.0 0.9 ± 0.6 nan ± nan nan ± nan 1.0 ± 0.5
Response_percentage 50.9% ± 34.9% 36.7% ± 35.5% 74.6% ± 23.1% 43.9% ± 30.1% 43.9% ± 30.0% 41.0% ± 60.6% 50.8% ± 37.7%
is_remitter 118 (45.0%) 7 (43.8%) 42 (73.7%) 31 (33.7%) 17 (34.7%) 7 (53.8%) 14 (40.0%)
responder_stats, responder_means = collect_stats_per_site(data.loc[data.is_responder])
non_responder_stats, non_responder_means = collect_stats_per_site(data.loc[~data.is_responder])
non_vs_responders = pd.concat([site_means.T.Total, responder_means.T.Total, non_responder_means.T.Total], axis=1)
non_vs_responders.columns = ['Total', 'Responders', 'Non-responders']
print(non_vs_responders) Total Responders Non-responders
Numel 262 149 113
Age 36.5 ± 15.3 35.6 ± 15.0 37.6 ± 15.6
Treatment_duration 8.3 ± 3.2 8.7 ± 3.3 7.8 ± 3.1
Normalized_Pretreatment_Severity 0.04 ± 0.99 0.18 ± 0.97 -0.15 ± 0.99
Age_of_Onset 30.3 ± 14.8 30.2 ± 14.5 30.3 ± 15.0
is_female 154 (58.8%) 84 (56.4%) 70 (61.9%)
is_recurrent 160 (61.1%) 83 (55.7%) 77 (68.1%)
is_responder 149 (56.9%) 149 (100.0%) 0 (0.0%)
uses_SSRI 194 (74.0%) 94 (63.1%) 100 (88.5%)
uses_SNRI 68 (26.0%) 53 (35.6%) 15 (13.3%)
uses_ATYP 16 (6.1%) 9 (6.0%) 7 (6.2%)
is_same_responder 157 (59.9%) 73 (49.0%) 84 (74.3%)
is_extreme 132 (50.4%) 66 (44.3%) 66 (58.4%)
ADcur 1.5 ± 5.0 0.7 ± 1.2 2.5 ± 7.5
Response_percentage 50.9% ± 34.9% 76.2% ± 14.5% 17.6% ± 24.2%
is_remitter 118 (45.0%) 115 (77.2%) 3 (2.7%)
We observe a large difference among sites in treatment response rate (37%-84%). Is this obviously related to any other properties, say age, treatment duration or medication?
We find that this is the case, average age for responders is 35.1 and 37.8. Average treatment duration is 9 weeks for responders, and 7.9 for non-responders. Finally, we do find a large difference in the antidepressant used: 82% of SSRI-users does not respond, while 79% of SNRI-users does. However, these properties are strongly correlated with site, 57/66 SNRI users are from DEP-ARREST-CLIN the highest performing site with an average response rate of 84%.
covariates = 'Age', 'Treatment_duration', 'is_responder'
variables = ('is_responder',), ('is_responder',), ('uses_SSRI', 'uses_SNRI', 'uses_ATYP')
response_correlates = pd.DataFrame(columns=['Population', 'Property', '+', '-', 'n+', 'n-'])
for covariate, variable in zip(covariates, variables):
for var in variable:
group_a = data[data[var]]
group_b = data[~data[var]]
num_a = group_a[covariate].mean()
num_b = group_b[covariate].mean()
fmt = '{:.1%}' if num_a < 1 else '{:.1f}'
response_correlates.loc[len(response_correlates)] = var, covariate, fmt.format(num_a), fmt.format(num_b), len(
group_a), len(group_b)
print(response_correlates) Population Property + - n+ n-
0 is_responder Age 35.6 37.6 149 113
1 is_responder Treatment_duration 8.7 7.8 149 113
2 uses_SSRI is_responder 48.5% 80.9% 194 68
3 uses_SNRI is_responder 77.9% 49.5% 68 194
4 uses_ATYP is_responder 56.2% 56.9% 16 246
adolescent_df = pd.DataFrame(columns=('N', '%'))
for cohort in [c for c in cohorts if c.name in site_stats.index]:
adolescents = np.sum(site_stats.loc[cohort.name].Age < 20)
total = site_stats.loc[cohort.name].Numel
table_head = f'{cohort.name} (N={total})'
adolescent_df.loc[table_head] = (adolescents, f'{adolescents / total * 100:.0f}')
print(adolescent_df) N %
AFFDIS (N=16) 1 6
DEP-ARREST-CLIN (N=57) 6 11
Hiroshima cohort (N=92) 0 0
Melbourne (N=49) 23 47
Minnesota (N=13) 13 100
Milano OSR (N=35) 0 0
Let us see if treatment response varies by site. This will be important for harmonization. The first way to look at it is simply looking at remission rates per site:
outcomes = 'is_female', 'is_responder', 'is_remitter', 'is_extreme'
outcomes_to_plot = outcomes[1], outcomes[3]
n_otp = len(outcomes_to_plot)
pie_fig, axes = plt.subplots(n_otp, len(set(data.cohort)), figsize=(12, 2 * n_otp))
#axes = axes.reshape(-1)
for u, outcome in enumerate(outcomes_to_plot): # 'is_remitter']):
v = 0
for cohort in cohorts:
df_cohort = data[data.cohort == cohort.key]
if not any(df_cohort.index):
continue
axes[u, v].pie(
x=[df_cohort[outcome].sum(), len(df_cohort) - df_cohort[outcome].sum()],
colors=(cohort.color, colors.dark),
autopct='%.0f%%',
)
if not u:
axes[u, v].set_title(f'{cohort.name}\n(n={len(df_cohort)})')
if not v:
axes[u, v].set_ylabel(outcome)
v += 1
plt.tight_layout()
pie_fig.savefig(figdir.append('ResponseRateBySite.png'))
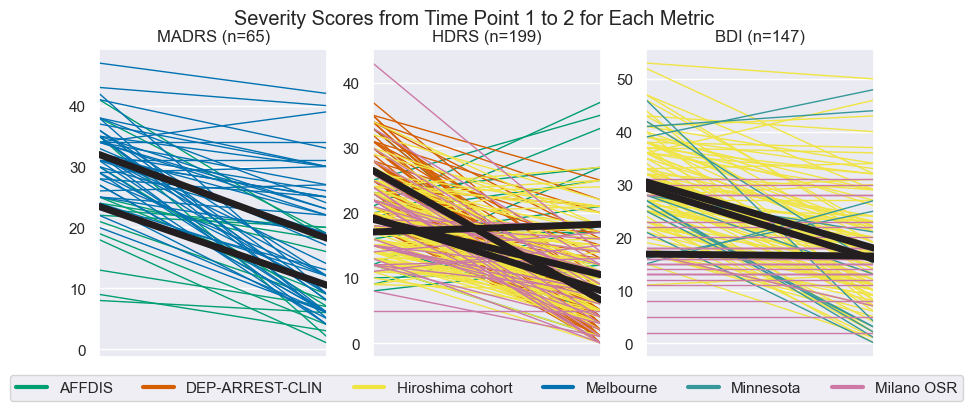
plt.show()Secondly, we look at the trends by plotting the change per site
trend_fig, axes = plt.subplots(1, 3, figsize=(10, 4))
for ax, score in zip(axes.reshape(-1), scoring_methods):
for cohort in cohorts:
# Get the values for a specific cohort and a specific symptom scoring method
cohort_data = pd.DataFrame(data=data[data.cohort == cohort.key], columns=data.columns)
pre = cohort_data[score + '_pre'].notna()
post = cohort_data[score + '_post'].notna()
subjs = cohort_data[(pre & post)]
if not len(subjs):
continue
vals = subjs[[score + '_pre', score + '_post']].to_numpy().astype(int).T
ax.plot(vals, color=cohort.color, lw=1) # Plot the found individual lines
ax.plot(vals.mean(axis=1), color=colors.dark, lw=5.0, zorder=3) # And plot the mean in bold
ax.set(xticks=(), xlim=(0, 1), title=f'{score} (n={data[score + "_pre"].notna().sum()})')
trend_fig.suptitle('Severity Scores from Time Point 1 to 2 for Each Metric')
# Format the legend
line_per_cohort = [Line2D([], [], color=c.color, label=c.name, lw=3) for c in cohorts if c.name in site_stats.index]
axes[1].legend(handles=line_per_cohort, ncol=len(site_stats.index), loc='center', bbox_to_anchor=(0.5, -.1))
trend_fig.savefig(figdir.append('ResponseTrends.png'))Later on in this work, we will find some interesting findings (good classification accuracy) for the "Extremes" subpopulation, so let us take a look at these characteristics as well.
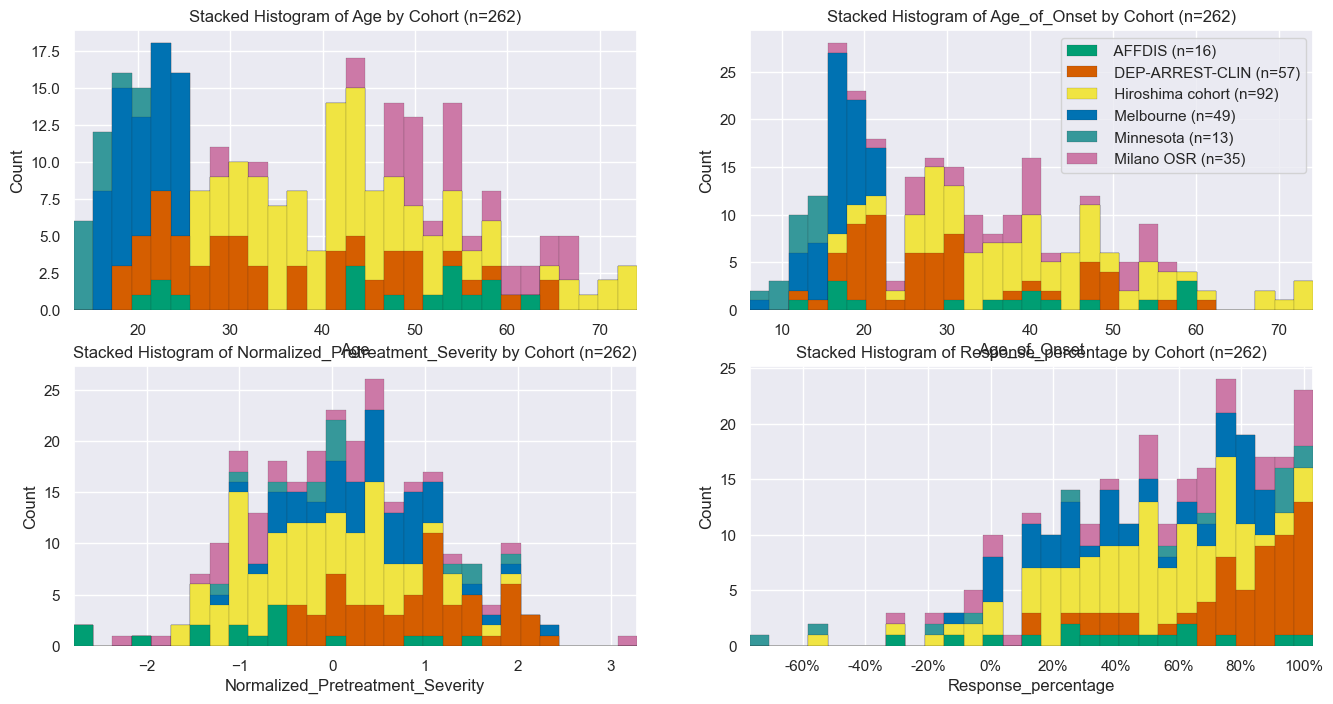
The underlying hypothesis for the formulation of this subpopulation was that - within MDD - there are different subpopulations with different a phenotype and different treatment response. However, simpler, alternative hypotheses, e.g. effects such as site, age or other clinical characteristics would be preferred. To verify the probability of the these hypotheses, we are interested in differences in population characteristics between the "Extremes" subpopulation and the total population, this is shown below. If anything stands out, this might weaken the belief in our primary hypothesis.
Under Numel we find that the Extremes subjects originate from all sites, and actually most sites also contributed about 50% to the Extremes subpopulation. In terms of Age or is_female, there are also no striking differences. This means that there is no clear evidence of the alternative hypotheses to our primary hypothesis.
hist_fig, axes = plt.subplots(2, 2, figsize=(16, 8))
stacked_hist(data, site_stats, ax=axes[0, 0], make_legend=False, name='Age')
stacked_hist(data, site_stats, ax=axes[0, 1], make_legend=False, name='Age_of_Onset')
stacked_hist(data, site_stats, ax=axes[1, 0], make_legend=False, name='Normalized_Pretreatment_Severity')
stacked_hist(data, site_stats, ax=axes[1, 1], make_legend=True, name='Response_percentage')
axes[1, 1].set_xticklabels([f'{t:.0%}' for t in axes[1, 1].get_xticks()])
hist_fig.savefig(figdir.append('ResponseHistogram.png'))
plt.show()_, ss_means = collect_stats_per_site(data.loc[populations['Hiroshima']])
print(ss_means.T)Cohort Total Hiroshima cohort
Numel 92 92
Age 43.0 ± 11.6 43.0 ± 11.6
Treatment_duration 6.0 ± 0.0 6.0 ± 0.0
Normalized_Pretreatment_Severity -0.31 ± 0.79 -0.31 ± 0.79
Age_of_Onset 39.2 ± 13.3 39.2 ± 13.3
is_female 47 (51.1%) 47 (51.1%)
is_recurrent 46 (50.0%) 46 (50.0%)
is_responder 45 (48.9%) 45 (48.9%)
uses_SSRI 91 (98.9%) 91 (98.9%)
uses_SNRI 0 (0.0%) 0 (0.0%)
uses_ATYP 1 (1.1%) 1 (1.1%)
is_same_responder 92 (100.0%) 92 (100.0%)
is_extreme 33 (35.9%) 33 (35.9%)
ADcur 0.9 ± 0.6 0.9 ± 0.6
Response_percentage 43.9% ± 30.1% 43.9% ± 30.1%
is_remitter 31 (33.7%) 31 (33.7%)
_, rrs_means = collect_stats_per_site(data.loc[populations['SameResponders']])
print(rrs_means.T)Cohort Total AFFDIS Hiroshima cohort Melbourne
Numel 157 16 92 49
Age 35.8 ± 14.9 44.1 ± 14.1 43.0 ± 11.6 19.6 ± 3.0
Treatment_duration 7.8 ± 2.9 5.1 ± 0.7 6.0 ± 0.0 12.0 ± 0.0
Normalized_Pretreatment_Severity -0.18 ± 0.89 -0.85 ± 1.20 -0.31 ± 0.79 0.28 ± 0.72
Age_of_Onset 31.4 ± 15.7 35.6 ± 15.7 39.2 ± 13.3 15.7 ± 2.7
is_female 85 (54.1%) 5 (31.2%) 47 (51.1%) 33 (67.3%)
is_recurrent 92 (58.6%) 15 (93.8%) 46 (50.0%) 31 (63.3%)
is_responder 73 (46.5%) 6 (37.5%) 45 (48.9%) 22 (44.9%)
uses_SSRI 149 (94.9%) 9 (56.2%) 91 (98.9%) 49 (100.0%)
uses_SNRI 3 (1.9%) 3 (18.8%) 0 (0.0%) 0 (0.0%)
uses_ATYP 9 (5.7%) 8 (50.0%) 1 (1.1%) 0 (0.0%)
is_same_responder 157 (100.0%) 16 (100.0%) 92 (100.0%) 49 (100.0%)
is_extreme 67 (42.7%) 8 (50.0%) 33 (35.9%) 26 (53.1%)
ADcur 2.4 ± 6.6 10.8 ± 14.4 0.9 ± 0.6 nan ± nan
Response_percentage 43.2% ± 30.7% 36.7% ± 35.5% 43.9% ± 30.1% 43.9% ± 30.0%
is_remitter 55 (35.0%) 7 (43.8%) 31 (33.7%) 17 (34.7%)
extr_stats, extr_means = collect_stats_per_site(data.loc[populations['Extremes']])
print(extr_means.T)Cohort Total AFFDIS DEP-ARREST-CLIN Hiroshima cohort Melbourne Minnesota Milano OSR
Numel 132 8 36 33 26 11 18
Age 34.3 ± 14.8 43.4 ± 13.1 32.4 ± 11.0 42.8 ± 10.4 19.8 ± 3.1 15.3 ± 2.3 50.9 ± 10.4
Treatment_duration 8.9 ± 3.3 5.0 ± 0.7 12.0 ± 0.0 6.0 ± 0.0 12.0 ± 0.0 10.3 ± 1.9 4.3 ± 0.7
Normalized_Pretreatment_Severity 0.03 ± 0.98 -0.68 ± 1.02 0.72 ± 0.75 -0.54 ± 0.73 0.43 ± 0.76 0.05 ± 0.95 -0.58 ± 0.85
Age_of_Onset 27.2 ± 13.8 31.1 ± 14.3 25.8 ± 10.1 39.1 ± 13.7 15.6 ± 3.0 11.7 ± 2.0 34.3 ± 10.3
is_female 81 (61.4%) 5 (62.5%) 20 (55.6%) 19 (57.6%) 18 (69.2%) 7 (63.6%) 12 (66.7%)
is_recurrent 83 (62.9%) 7 (87.5%) 19 (52.8%) 18 (54.5%) 16 (61.5%) 7 (63.6%) 16 (88.9%)
is_responder 66 (50.0%) 2 (25.0%) 33 (91.7%) 8 (24.2%) 8 (30.8%) 6 (54.5%) 9 (50.0%)
uses_SSRI 91 (68.9%) 4 (50.0%) 0 (0.0%) 33 (100.0%) 26 (100.0%) 11 (100.0%) 17 (94.4%)
uses_SNRI 42 (31.8%) 2 (25.0%) 36 (100.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 4 (22.2%)
uses_ATYP 5 (3.8%) 3 (37.5%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 2 (11.1%)
is_same_responder 67 (50.8%) 8 (100.0%) 0 (0.0%) 33 (100.0%) 26 (100.0%) 0 (0.0%) 0 (0.0%)
is_extreme 132 (100.0%) 8 (100.0%) 36 (100.0%) 33 (100.0%) 26 (100.0%) 11 (100.0%) 18 (100.0%)
ADcur 1.5 ± 5.7 12.0 ± 16.0 0.0 ± 0.0 0.8 ± 0.4 nan ± nan nan ± nan 0.9 ± 0.5
Response_percentage 46.4% ± 46.3% 22.9% ± 44.1% 83.6% ± 21.6% 25.6% ± 40.2% 32.9% ± 34.4% 37.7% ± 65.3% 45.3% ± 50.2%
is_remitter 67 (50.8%) 3 (37.5%) 33 (91.7%) 8 (24.2%) 8 (30.8%) 6 (54.5%) 9 (50.0%)
extremes = data.loc[populations['Extremes']]
extr_lo_stats, extr_lo_means = collect_stats_per_site(extremes[~extremes.is_responder])
extr_hi_stats, extr_hi_means = collect_stats_per_site(extremes[extremes.is_responder])
extr_non_vs_responders = pd.concat([extr_means.T.Total, extr_lo_means.T.Total, extr_hi_means.T.Total], axis=1)
extr_non_vs_responders.columns = ['Extremes Total', 'Extreme Non-responders', 'Extreme Responders']
print(extr_non_vs_responders) Extremes Total Extreme Non-responders Extreme Responders
Numel 132 66 66
Age 34.3 ± 14.8 35.9 ± 16.0 32.6 ± 13.3
Treatment_duration 8.9 ± 3.3 7.9 ± 3.2 9.8 ± 3.2
Normalized_Pretreatment_Severity 0.03 ± 0.98 -0.29 ± 0.95 0.35 ± 0.91
Age_of_Onset 27.2 ± 13.8 28.3 ± 14.8 26.2 ± 12.7
is_female 81 (61.4%) 41 (62.1%) 40 (60.6%)
is_recurrent 83 (62.9%) 44 (66.7%) 39 (59.1%)
is_responder 66 (50.0%) 0 (0.0%) 66 (100.0%)
uses_SSRI 91 (68.9%) 59 (89.4%) 32 (48.5%)
uses_SNRI 42 (31.8%) 8 (12.1%) 34 (51.5%)
uses_ATYP 5 (3.8%) 2 (3.0%) 3 (4.5%)
is_same_responder 67 (50.8%) 49 (74.2%) 18 (27.3%)
is_extreme 132 (100.0%) 66 (100.0%) 66 (100.0%)
ADcur 1.5 ± 5.7 2.8 ± 8.2 0.4 ± 0.8
Response_percentage 46.4% ± 46.3% 2.9% ± 21.3% 89.9% ± 6.8%
is_remitter 67 (50.8%) 1 (1.5%) 66 (100.0%)
print('Mean ± 1 SD treatment duration for the following populations:')
relevant_cohorts = [c for c in cohorts if c.name in site_stats.index]
for stats_desc, stats in zip(
['All', 'Responders', 'Non-responders', 'Extremes', 'Responding Extremes', 'Non-responding Extremes'],
[site_stats, responder_stats, non_responder_stats, extr_stats, extr_hi_stats, extr_lo_stats]):
print(stats_desc.ljust(24),
f'{np.sum([stats.loc[c.name].Numel * c.treatment_duration_mu for c in relevant_cohorts if c.name in stats.index]) / stats.Numel.sum():.2f} ± '
f'{np.sum([stats.loc[c.name].Numel * c.treatment_duration_sd for c in relevant_cohorts if c.name in stats.index]) / stats.Numel.sum():.2f}')Mean ± 1 SD treatment duration for the following populations:
All 8.33 ± 0.24
Responders 8.75 ± 0.23
Non-responders 7.77 ± 0.24
Extremes 8.84 ± 0.30
Responding Extremes 9.81 ± 0.30
Non-responding Extremes 7.88 ± 0.31
print('\n'.join([f'Extremes in {c.name} with ADcur == 0:'.ljust(50) + str(
(data[(data.cohort == c.key) & data.is_extreme].ADcur == 0).sum()) for c in relevant_cohorts]))Extremes in AFFDIS with ADcur == 0: 1
Extremes in DEP-ARREST-CLIN with ADcur == 0: 36
Extremes in Hiroshima cohort with ADcur == 0: 2
Extremes in Melbourne with ADcur == 0: 0
Extremes in Minnesota with ADcur == 0: 0
Extremes in Milano OSR with ADcur == 0: 0
Our primary goal is to predict treatment response. However, we also have several sub questions for which we create five dimensions of comparisons:
data_typeDo we use theroidata, or themapdata?dbc_presenceDo we adddbcdata to our chosen data type?populationDo we train on the entire population (All) or a subpopulation (i.e. Hiroshima, Extremes or Same_responders).cv_methodWhich cross-validation method do we use? K-fold (Fold) or leave-site-out (Site)classifierWhich classifier do we use? Logistic regression, Support Vector Classifier (SVC) or a Gradient boosting classifier.target_labelWhat the label is that we are trying to predict. Although this is usuallyis_responder, there is the option to classifyis_femaleas a sanity check, and alsois_remitter, and out of curiosity we can also predictis_extreme.
Furthermore, we perform LASSO feature selection using an meta transformer on an L1-penalized support vector classifier. Data harmonization is performed using ComBat. The ComBat wrapper stores our data such that it can access covariates from withing the sklearn pipeline. Features are centered around the mean and scaled to unit variance. We report accuracy, balanced accuracy (bAcc) and model Effect Size and Bayes Factor.
roicortical thickness and the surface area for each of the 34 ROIs per hemisphere, resulting in 136 predictors (a. ROI average).vecthe voxel-wise cortical surface area and cortical thickness measurements as a single one-dimensional (1D) vector generated by downsampling using spatial linear interpolation, resulting in 900 predictors (b. cortical vector).2DTcortical data representations by projecting the cortical surface thickness measurements to two-dimensional (2D) planes of 64×64 pixels using stereographic projection (c. cortical thickness projection).2DAcortical data representations by projecting the cortical surface area measurements to two-dimensional (2D) planes of 64×64 pixels using stereographic projection (d. surface area projection).
Here is a list of what the different dbc_options mean:
dbc_noadd nothing to cortical datadbc_yesadd DBC data to cortical data (6:is_ad,is_recurrent,is_female,Age,Normalized_Pretreatment_Severity,Age_of_Onset)sub_addadd subcortical data to cortical data (25:ICV,LLatVent, ...,Rput,Rthal)sub_nlyadd nothing to subcortical datasub_dbcadd DBC data to subcortical datasub_alladd subcortical and DBC data to cortical datadbc_nlyonly use DBC data
Here is a list of the populations with explaination:
AllAll eligible patients from all six cohorts (n=265).Hiroshimathe single largest cohort to ascertain if inter-cohort variance played a role in our prediction outcomes (I. single cohort) (n=103).SameResponderscohorts with a mean response rate below 50% (II. response rate selected cohorts) (n-168).Extremesa subpopulation consisting of the extreme subgroups of responders and non-responders, i.e., the 25% of patients showing the lowest percentage changes in depression severity and 25% responding the largest percentage changes to antidepressant treatment (III. extreme (non-)responders) (n=132).LongTreatedT
Fold10 times repeated stratified 10-fold cross-validation implemented throughsklearn.model_selection.RepeatedStratifiedKFold.Siteleave site out cross-validation implemented throughsklearn.model_selection.LeaveOneGroupOut.
LogisticRegressionSVCGradientBoostingClassifier
is_responderprimary outcome.is_remitteralternative outcome.is_femaleused as a sanity check.is_extremebriefly explored since treatment response prediction in this group works, if you can predict this group beforehand, this might still be useful. But as we will find in analysis 6.3.3.2, we can't predict it.
So the code is set up to be able to run all the analyses. This will mean that about 2560 models would have to be trained. To make life a bit quicker, we drop options that we will not use in the later analyses. This operation in optional, and can be undone in later runs (i.e., you could first run the essential runs, and because these are loaded automatically and then skipped, in a later stage add these unused options for future applications.)
# Preallocate dictionary for storage
if os.path.isfile(results_file):
results_dict = pickle_in(results_file)
print(f'Loaded results file: {results_file}.\n The file contained {len(nested_dict_to_df(results_dict))} results')
dl_aggregates = pickle_in(dl_aggregate_file)
print(
f'Loaded aggregates file: {results_file}.\n The file contained {len(nested_dict_to_df(dl_aggregates))} trained Deep Learning models')
else:
results_dict, dl_aggregates = {}, {}
print(f'Created new results file: {results_file}')Loaded results file: D:\repositories\ENIGMA_lean\results\20240807-135247-postRebuttal2-Night2\result_dict.pkl.
The file contained 352 results
Loaded aggregates file: D:\repositories\ENIGMA_lean\results\20240807-135247-postRebuttal2-Night2\result_dict.pkl.
The file contained 140 trained Deep Learning models
# Some settings to our machine learning method:
target_labels = 'is_responder', # 'is_remitter', 'is_female', 'is_extreme',
n_splits = 10
n_repeats = 1
n_permutations = 100
n_iter = 25
n_epochs = 20
# 1. Define data type for full population we need :X = train data, y = label data, c = stratification data
get_y_for_target = lambda tl: pd.Series(data=[data[tl].loc[sub.split('|')[0]] for sub in X_thc.index],
index=X_thc.index, name=tl)
dtypes = {'vec': (X_thc,
pd.DataFrame.from_dict(
data={target_label: get_y_for_target(target_label) for target_label in target_labels}),
pd.Series(data={sub: data.loc[sub.split('|')[0]].cohort_idx for sub in X_thc.index},
index=X_thc.index, name='Stratification Label'),),
'roi': (data[dkt_atlas_features],
pd.DataFrame.from_dict(data={target_label: data[target_label] for target_label in target_labels}),
data.cohort_idx,),
}
# 2.1 Presence of Demographic, behavioural and clinical (DBC) data:
dbc_cols = ['is_ad', 'is_recurrent', 'is_female', 'Age', 'Normalized_Pretreatment_Severity', 'Age_of_Onset']
dbc_prj = data[dbc_cols].loc[[s.split('|')[0] for s in X_thc.index]]
sub_prj = data[dbc_cols].loc[[s.split('|')[0] for s in X_thc.index]]
dbc_prj.insert(loc=0, value=X_thc.index, column='SubjID|Hem')
sub_prj.insert(loc=0, value=X_thc.index, column='SubjID|Hem')
dbc_prj = dbc_prj.set_index('SubjID|Hem')
dbc_options = {
'dbc_no': {'roi': pd.DataFrame(), 'vec': pd.DataFrame()}, # add nothing to cortical data
'dbc_yes': {'roi': data[dbc_cols], 'vec': dbc_prj}, # add DBC data to cortical data
'sub_add': {'roi': data[subc_rois], 'vec': None}, # add subcortical data to cortical data
'sub_nly': {'roi': pd.DataFrame(), 'vec': None}, # add nothing to subcortical data
'sub_dbc': {'roi': data[dbc_cols], 'vec': None}, # add DBC data to subcortical data
'sub_all': {'roi': data[subc_rois + dbc_cols], 'vec': None}, # add subcortical and DBC data to cortical data
'dbc_nly': {'roi': pd.DataFrame(), 'vec': None}, # only use DBC
}
# 2.2 Get indices each population, both for our roi data frame (which we already defined at 2.3) but also for projection data
get_indices = lambda key, indices: {'roi': indices, 'vec': [i for i in X_thc.index if i.split('|')[0] in indices]}
population_indices = {k: get_indices(k, i) for k, i in populations.items()}
# 3. Define the data for each of our previously defined populations
data_dict = make_empty_nest(proj_type=dtypes, dbc_presence=dbc_options, population=populations)
for dtype_name, dtype_values in dtypes.items():
X, y, c = dtype_values
for dbc_option, dbc_values in dbc_options.items():
# These conditionals are a bit hacky because we replace the cortical
# ROIs with something else (subc or dbc data), but it save a ton of code
if dbc_option in ('sub_nly', 'sub_dbc',):
X = data[subc_rois]
elif dbc_option in ('dbc_nly',):
X = data[dbc_cols]
if dbc_values[dtype_name] is None:
del data_dict[dtype_name][dbc_option]
continue
X_cort_and_dbc = pd.concat([dbc_values[dtype_name], X], axis=1)
for pop_key, pop_idx in population_indices.items():
get_subpop_subjs = lambda sub_df: sub_df.loc[pop_idx[dtype_name]]
data_dict[dtype_name][dbc_option][pop_key] = [get_subpop_subjs(sub_df) for sub_df in (X_cort_and_dbc, y, c)]
# 4. Define our cross-validation methods:
cv_schemes = {'Fold': (RepeatedStratifiedKFold(n_splits=n_splits, n_repeats=n_repeats, random_state=0),
CombatWrapper(data=data, discrete_covariates=['is_female'],
continuous_covariates=['Age', 'Age2']),),
'Site': (LeaveOneGroupOut(), None,)}
# 5. Define classifiers and hyperparameter search space to use:
from hyper_space import svc_space, gbc_space, log_space, rfc_space
scorer = Scorer({'accuracy': accuracy_score, 'balanced_accuracy': balanced_accuracy_score})
classifiers = \
(SVC(), svc_space), \
(GradientBoostingClassifier(), gbc_space), \
(LogisticRegression(max_iter=500), log_space), \
(RandomForestClassifier(), rfc_space)
# Define other pipeline components
imputer = PipeWrapper(KNNImputer)
regressor = RegressorWrapper(data=data, continuous_covariates=['Age', 'Age2'])
selector = SelectFromModel(LinearSVC(penalty="l1", dual=False, max_iter=20000), threshold=0)
scaler = PipeWrapper(StandardScaler)
# Define Deep Learning model
torch_scorer = {'accuracy': torch_accuracy, 'balanced_accuracy': torch_balanced_accuracy, }
resnet_model = resnet18(weights=ResNet18_Weights.IMAGENET1K_V1)
resnet_model.fc = nn.Sequential(nn.Linear(in_features=512, out_features=2, bias=True), Squeeze())
model = TorchTrainer(
subestimator=resnet_model,
batch_size=32,
epochs=n_epochs,
verbose=False,
random_state=0,
metrics=torch_scorer
)
# Sensitivity testing
subj_classes = {'wk_1': wk1_subjs, 'wk_2': data.index}We have set up an impressive nested dictionary of six levels, with at the deepest level data (X), labels(y) and a stratification variable (c). To train all machine learning models we loop over each item. At the deepest loop level you will find two chucks of code: the first for classical machine learning, and the second for deep learning (see 1) and 2)). Although it might have been nice to also create a loop for these two options, they do not share data or models, so that would not have made a lot of sense.
Most calulations are performed in the cross_validate and torch_val_score functions, which output a list of n_splits × n_repeats of test outcome values. These results are stored in the results_dict. For the exact storage location in this dictionary we use the same experiment_specifier that we used to get the data from the data_dict. Finally, we can simply convert this nested dictionary to a multi index Pandas DataFrame.
By default, the training loop checks if results are preexisting, to allow for continuation of training and prevent overwriting.
# Print progress
n_tasks = count_values(data_dict) * len(cv_schemes) * len(classifiers) * len(target_labels)
# n_tasks = 1512
n_tasks = 412
pb = ProgressBar(n_tasks, desc='models trained')
# For explanation on these six nested loops please see the list at 3.
with Timer():
for subj_class, subj_idxs in subj_classes.items():
for target_label in target_labels:
for dtype, data_a in data_dict.items():
for dbc_presence, data_b in data_a.items():
for population_name, (X, ys, c) in data_b.items():
subj_incl = [i for i in X.index if i.split('|')[0] in subj_idxs]
# Define True label
X_safe = X.loc[subj_incl].drop(columns=[target_label], errors='ignore')
y = ys.loc[subj_incl][target_label]
c = c.loc[subj_incl]
for cv_name, (cv, harmonizer) in cv_schemes.items():
n_groups = (1, len(set(c))) if cv_name == 'Site' else (n_repeats, n_splits)
for classifier, param_space in classifiers:
if population_name == 'Extremes' and target_label == 'is_extreme' or \
len(c.unique()) == 1 and cv_name == 'Site':
# Can't predict on own population or do LSO-CV on one site
continue
if subj_class == 'wk_1' and (
dbc_presence != 'dbc_no' or
population_name != 'All' or
cv_name != 'Fold' or
target_label != 'is_responder'):
# We only want to run the sensitivity analysis of the weeks of cutoff
# for runs that are relevant to the primary analysis
continue
# 1) Classical Machine Learning: Specify which Classical ML experiment we are going to run
experiment_specifier = dtype, dbc_presence, population_name, cv_name, classifier.__class__.__name__, target_label, subj_class, 'done'
previous_result = safe_dict_get(results_dict, *experiment_specifier)
if previous_result['done'] is None: # Skip if results exist
pipeline = make_pipeline(imputer, regressor, harmonizer, scaler, imputer, selector, classifier, )
opt = BayesSearchCV(estimator=pipeline, search_spaces=param_space, n_iter=n_iter, cv=5, n_jobs=-1)
try:
_, _, pvalue = permutation_test_score(opt, X_safe, y, groups=c, cv=cv, n_permutations=n_permutations, scoring=scorer)
previous_result['pvalue'] = pvalue
for score_name, score_value in scorer.items():
score_tensor = np.reshape(score_value, [1 + n_permutations, *n_groups])
previous_result[score_name] = score_tensor[0]
previous_result[f'null_{score_name}'] = score_tensor[1:]
finally:
scorer.reset() # Clear the scorer when we are done
# Save results
previous_result['done'] = True
pickle_out(results_dict, results_file)
pb()
if dbc_presence == 'dbc_no' and dtype == 'vec':
for X_arr, letter in zip((X_thc_arr, X_are_arr), 'TA'):
# 2) Deep Learning: Specify which Deep Learning experiment we are going to run
dl_specifier = f'2D{letter}', dbc_presence, population_name, cv_name, 'ResNet', target_label, subj_class, 'done'
previous_dl_result = safe_dict_get(results_dict, *dl_specifier)
if previous_dl_result['done'] is None: # Skip if results exist
pop_idx = [idx for idx, subj_id in enumerate(X_thc.index) if
subj_id in y.index]
# Get CV-scores, score and a priori chance
previous_dl_result.update({''.join(null_metric): [] for null_metric in
product(('null_', ''), torch_scorer)})
for do_permute in range(1 + n_permutations):
tag = 'null_' if do_permute else ''
torch_results = torch_val_score(model, X_arr[pop_idx], y, cv,
groups=c, verbose=False,
return_pipeline='aggregate',
do_permute=bool(do_permute))
for metric in torch_scorer:
dl_score_matrix = np.reshape(
[r[metric] for r in torch_results['test']], n_groups)
previous_dl_result[tag + metric].append(dl_score_matrix)
# Convert the list of dl_permutations into a properly formatted array
for metric, tag in product(torch_scorer, ('', 'null_')):
permutes = n_permutations if tag else 1
sc = previous_dl_result[tag + metric]
sc = np.reshape(sc, (permutes, *sc[0].shape))
previous_dl_result[tag + metric] = sc
# Computation of permutation p-value, copied from scipy (see permutation_test)
dl_pvalue = ((previous_dl_result[f'null_accuracy'].mean(2).mean(1) >=
previous_dl_result[f'accuracy'].mean(2).mean(
1)).sum() + 1.0) / (n_permutations + 1)
previous_dl_result['pvalue'] = dl_pvalue
dl_aggregate = safe_dict_get(dl_aggregates, *dl_specifier[:-1])
dl_aggregate[dl_specifier[-2]] = torch_results['pipeline']
# Save results
previous_dl_result['done'] = True
pickle_out(results_dict, results_file)
pickle_out(dl_aggregates, dl_aggregate_file)
pb()VBox(children=(IntProgress(value=0, bar_style='info', description='Progress:', layout=Layout(width='50%'), max…
Elapsed: 0.4226229190826416
results_table = nested_dict_to_df(results_dict)
# The table is ordered by the order of the nesting of the calculation.
# Reorder the index to be consistent with the order described in the manuscript.
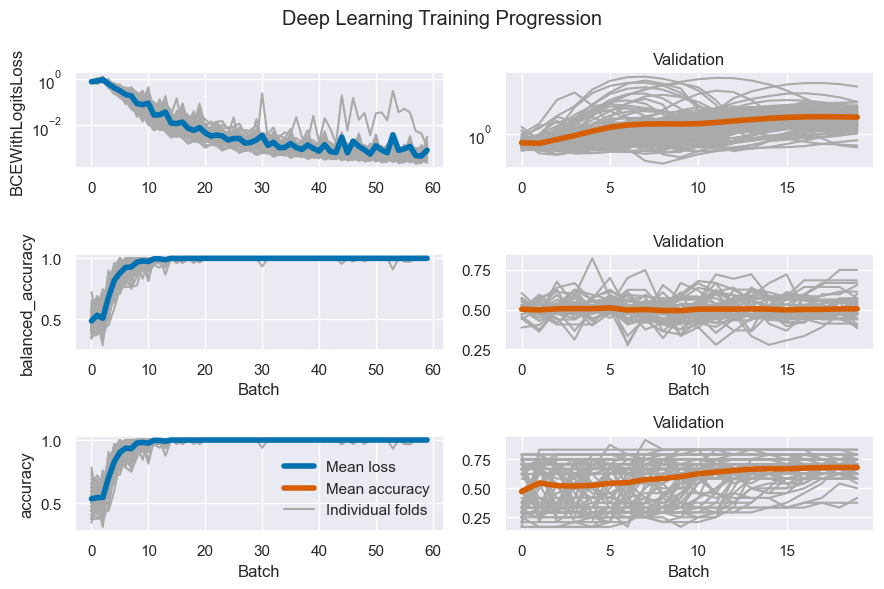
results_table = results_table.reorder_levels([5, 0, 1, 4, 3, 2, 6])Display an example of the training process of a deep learning model as a check.
# Specify which DL result to look at as an deep learning configuration:
a_dl_res = dl_aggregates['2DA']['dbc_no']['Extremes']['Fold']['ResNet']['is_responder'][
'wk_2'] # A Deep Learning Result
# Show it
lw = 4
dl_fig, axes = plt.subplots(3, 2, figsize=(9, 6))
line_colors = colors.blue, colors.orange,
for u, (partition_name, partition) in enumerate(a_dl_res.items()):
for v, (metric_name, metric_values) in enumerate(partition.items()):
# Plot all learning curves
axes[v, u].plot(metric_values.T, color='#aaa')
# Plot the mean learning curve
axes[v, u].plot(metric_values.mean(0), color=line_colors[u], lw=lw)
axes[v, u].set(
title=f'{partition_name.capitalize()}' if u else '',
ylabel=metric_name if not u else None,
xlabel='Batch' if v else None,
yscale='log' if 'Log' in metric_name else 'linear',
)
# Only draw a legend once
if not u and v == 2:
axes[v, u].legend(frameon=False, handles=[
Line2D([], [], color=line_colors[0], label='Mean loss', linewidth=lw, ),
Line2D([], [], color=line_colors[1], label='Mean accuracy', linewidth=lw, ),
Line2D([], [], color='#aaaaaa', label='Individual folds')],
)
dl_fig.suptitle('Deep Learning Training Progression')
dl_fig.tight_layout()
dl_fig.savefig(figdir.append(f'dl_training.png'))
dl_fig.show()Let us drop the LRC since it was only for development purposes, and RandomForestClassifier because it is not included in the main analyses.
results_table_with_alternate_classifiers = deepcopy(results_table)
results_table = results_table[~(results_table.index.get_level_values(3) == 'LogisticRegression')]
results_table = results_table[~(results_table.index.get_level_values(3) == 'RandomForestClassifier')]From our results we will compute statistics and add them to the data frame to create a data frame aptly called: results_and_stats
These statistics are: population size, T-statisic, p-value, Bayes factor and Effect size. Then combine statistics and results tables, and separate cortical (primary) from subcortical (exploratory) findings.
statistics_calculator = calc_stats_wrapper(population_indices)
stats_table = statistics_calculator(results_table)
# Horizontal concat the statistics table to the results table
results_and_stats = pd.concat((results_table, stats_table), axis=1)
pickle_out(results_and_stats, restats_file)
# Split our results for primary and exploratory analyses
cortical_results = results_and_stats.loc[(sN, sN, ['dbc_no', 'dbc_yes']), :] # Limit primary analysis to cortical dataThis line means: present the average K-Fold cross-validation result for predicting response in the entire population. These results include multiple data types (ROI, 1D-vector and 2D projection; multiple ML models (GBC, SVC, ResNet) and with and without behavioural, clinical and demographic data.
is_strict means that we limit our selected data frame to the value specified for the level on which a comparison is requested. i.e.: The level we are requesting a comparison for (6) is considered with predicted outcomes. Here we only want to see the value that we specified (is_responder). When is_strict were False (default), we would also see the other possible predicted values is_female, is_remitter and is_extreme. It would not have affected the printed value.
distiller = TableDistiller(cortical_results, 'is_responder', None, 'dbc_no', None, 'Fold', 'All', 'wk_2',
is_strict=True, verbose=True, n_splits=n_splits)
distiller(1)(1) 'is_responder'
(2) 'vec', '2DT', '2DA', 'roi'
(3) 'dbc_no', 'dbc_yes'
(4) 'GradientBoostingClassifier', 'ResNet', 'SVC'
(5) 'Site', 'Fold'
(6) 'LongTreated', 'SameResponders', 'Extremes', 'Hiroshima', 'All'
(7) 'wk_1', 'wk_2'
| bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
1.is_responder| 50.5% | 5.9% | 53.6% | 7.2% | 50.4% | 5.3% | 53.2% | 6.8% | 0.657 |
This line means: present the average K-Fold cross-validation result for predicting response in the entire population. Here, we split the results out three times, based on data type, based on the inclusion of clinical data and based on the machine learning method used. is_strict does not have an effect here, since we do not specify any value for any level we are requesting(1, 2 and 5 are None).
distiller.is_strict = False
distiller(2, 3, 4) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
2.No Significant difference (p_val: 0.101, eta-sqr=0.121 F=2.56e+00) among:
-roi | 50.6% | 5.4% | 54.6% | 5.6% | 50.9% | 5.2% | 54.6% | 5.1% | 0.612 |
-2DA | 49.8% | 2.1% | 53.9% | 9.1% | 50.1% | 4.2% | 53.7% | 8.2% | 0.436 |
-2DT | 50.3% | 5.0% | 48.1% | 6.1% | 49.0% | 5.2% | 46.6% | 6.4% | 0.273 |
-vec | 50.9% | 7.7% | 55.1% | 6.6% | 50.7% | 5.7% | 54.9% | 5.7% | 0.630 |
3.No Significant difference (p_val: 0.703, ) between:
-dbc_no | 51.0% | 5.5% | 54.4% | 6.3% | 50.3% | 5.8% | 54.2% | 5.7% | 0.617 |
-dbc_yes | 50.5% | 5.9% | 53.6% | 7.2% | 50.4% | 5.3% | 53.2% | 6.8% | 0.657 |
4.No Significant difference (p_val: 0.153, eta-sqr=0.078 F=2.41e+00) among:
-ResNet | 50.0% | 3.9% | 51.0% | 8.3% | 49.5% | 4.7% | 50.1% | 8.2% | 0.372 |
-GradientBoos| 51.0% | 8.3% | 53.8% | 7.4% | 51.8% | 6.7% | 53.7% | 6.5% | 0.701 |
-SVC | 50.5% | 4.3% | 55.9% | 4.3% | 49.9% | 3.7% | 55.8% | 3.8% | 0.545 |
This line means: present the average results for predicting response in the entire population using either K-Fold os Leave-Site-Out cross validation. is_strict does not have an effect here, since we do not specify any value for the level we are requesting (4 is None)
distiller(5) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
5.No Significant difference (p_val: 0.727, ) between:
-Site | 52.3% | 5.5% | 51.1% | 10.4% | 50.0% | 7.1% | 48.7% | 11.5% | 0.235 |
-Fold | 50.5% | 5.9% | 53.6% | 7.2% | 50.4% | 5.3% | 53.2% | 6.8% | 0.657 |
Compared to previous analyses, we do not specify a population (level 3 is None). Thus, this line means: present the average results for predicting response in each of the subpopulations using K-Fold cross-validation and any type of data, machine learning method or inclusion of clinical data. Like earlier, is_strict does not have an effect here, since we do not specify any value for the level we are requesting (3 is None)
added_data = results_and_stats.index.get_level_values(2)
subc_compare = results_and_stats[np.logical_or(added_data == 'dbc_no', added_data == 'sub_add')]
distiller = TableDistiller(subc_compare, 'is_responder', None, 'dbc_no', None, 'Fold', None, 'wk_2', n_splits=n_splits)
distiller(6) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
6.Significant difference (p_val: 0.002, eta-sqr=0.326 F=3.56e+01) among:
-LongTreated | 50.5% | 5.2% | 62.6% | 9.1% | 51.1% | 6.9% | 62.1% | 9.9% | 0.757 |
-SameResponde| 50.1% | 7.2% | 52.1% | 10.0% | 50.5% | 8.0% | 52.1% | 9.3% | 0.732 |
-Extremes | 63.9% | 10.6% | 63.6% | 8.7% | 52.3% | 9.7% | 52.1% | 10.3% | 0.001* |
-Hiroshima | 49.6% | 7.1% | 48.4% | 8.0% | 49.1% | 9.2% | 47.5% | 10.6% | 0.666 |
-All | 50.5% | 5.9% | 53.6% | 7.2% | 50.4% | 5.3% | 53.2% | 6.8% | 0.657 |
distiller = TableDistiller(subc_compare, 'is_responder', None, None, None, 'Fold', 'All', 'wk_2', n_splits=n_splits)
distiller(3) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
3.No Significant difference (p_val: 0.703, ) between:
-sub_no | 51.6% | 3.7% | 55.3% | 3.8% | 51.4% | 5.9% | 55.4% | 5.7% | 0.587 |
-dbc_yes | 50.5% | 5.9% | 53.6% | 7.2% | 50.4% | 5.3% | 53.2% | 6.8% | 0.657 |
distiller = TableDistiller(cortical_results, 'is_responder', None, 'dbc_no', None, 'Fold', 'Extremes', 'wk_2',
is_strict=True, spacing=15, n_splits=n_splits)
distiller(1)
distiller.is_strict = False
distiller(2, 3, 4, 5) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
1.is_responder| 63.9% | 10.6% | 63.6% | 8.7% | 52.3% | 9.7% | 52.1% | 10.3% | 0.001* |
| bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
2.No Significant difference (p_val: 0.839, eta-sqr=0.036 F=6.98e-01) among:
-vec | 63.6% | 16.3% | 63.9% | 12.2% | 53.8% | 12.6% | 53.8% | 12.6% | 0.002* |
-2DT | 63.1% | 2.4% | 60.5% | 5.2% | 50.1% | 2.0% | 48.1% | 5.7% | 0.010* |
-2DA | 63.1% | 2.7% | 62.8% | 5.0% | 49.6% | 4.5% | 50.1% | 6.9% | 0.010* |
-roi | 65.1% | 8.1% | 65.3% | 6.7% | 53.3% | 10.3% | 53.3% | 10.3% | 0.003* |
3.No Significant difference (p_val: 0.703, ) between:
-dbc_no | 65.4% | 13.2% | 65.1% | 9.7% | 53.6% | 11.6% | 53.6% | 11.6% | 0.001* |
-dbc_yes | 63.9% | 10.6% | 63.6% | 8.7% | 52.3% | 9.7% | 52.1% | 10.3% | 0.001* |
4.No Significant difference (p_val: 0.300, eta-sqr=0.055 F=1.66e+00) among:
-ResNet | 63.1% | 2.6% | 61.7% | 5.2% | 49.9% | 3.5% | 49.1% | 6.4% | 0.001* |
-GradientBoos| 65.9% | 11.6% | 66.5% | 8.6% | 55.2% | 10.3% | 55.3% | 10.3% | 0.002* |
-SVC | 62.8% | 13.8% | 62.8% | 10.7% | 51.8% | 12.3% | 51.8% | 12.4% | 0.003* |
5.No Significant difference (p_val: 0.703, ) between:
-Fold | 63.9% | 10.6% | 63.6% | 8.7% | 52.3% | 9.7% | 52.1% | 10.3% | 0.001* |
-Site | 65.0% | 7.6% | 56.7% | 14.5% | 50.2% | 10.3% | 41.0% | 18.2% | 0.001* |
distiller = TableDistiller(results_and_stats, 'is_responder', None, None, None, 'Fold', 'All', 'wk_2')
distiller(3) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
3.No Significant difference (p_val: 0.306, eta-sqr=0.043 F=1.46e+00) among:
-dbc_no | 50.5% | 5.9% | 53.6% | 7.2% | 50.4% | 5.3% | 53.2% | 6.8% | 0.657 |
-dbc_nly | 53.6% | 4.8% | 55.9% | 4.2% | 51.3% | 6.8% | 54.3% | 6.2% | 0.289 |
-sub_all | 55.6% | 5.0% | 57.6% | 5.4% | 52.6% | 5.6% | 55.9% | 5.6% | 0.275 |
-sub_nly | 54.1% | 4.4% | 56.0% | 5.3% | 51.5% | 6.2% | 54.5% | 6.2% | 0.217 |
-sub_dbc | 53.4% | 5.0% | 55.7% | 5.8% | 52.1% | 6.2% | 55.2% | 5.9% | 0.554 |
-sub_add | 51.6% | 3.7% | 55.3% | 3.8% | 51.4% | 5.9% | 55.4% | 5.7% | 0.587 |
-dbc_yes | 51.0% | 5.5% | 54.4% | 6.3% | 50.3% | 5.8% | 54.2% | 5.7% | 0.617 |
distiller = TableDistiller(results_and_stats, 'is_responder', None, None, None, 'Fold', 'Extremes', 'wk_2')
distiller(3) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
3.Significant difference (p_val: 0.017, eta-sqr=0.092 F=3.25e+00) among:
-dbc_no | 63.9% | 10.6% | 63.6% | 8.7% | 52.3% | 9.7% | 52.1% | 10.3% | 0.001* |
-dbc_nly | 67.4% | 8.6% | 67.2% | 6.8% | 53.0% | 10.6% | 52.9% | 10.5% | 0.002* |
-sub_all | 71.0% | 10.2% | 70.9% | 7.6% | 53.1% | 9.7% | 53.0% | 9.7% | 0.001* |
-sub_nly | 68.3% | 9.6% | 68.0% | 7.3% | 52.8% | 10.4% | 52.8% | 10.3% | 0.001* |
-sub_dbc | 69.2% | 9.6% | 69.6% | 7.3% | 53.1% | 9.9% | 53.0% | 9.8% | 0.001* |
-sub_add | 63.0% | 12.3% | 62.8% | 8.7% | 53.6% | 10.0% | 53.6% | 10.0% | 0.009* |
-dbc_yes | 65.4% | 13.2% | 65.1% | 9.7% | 53.6% | 11.6% | 53.6% | 11.6% | 0.001* |
distiller = TableDistiller(cortical_results, 'is_responder', None, 'dbc_no', None, 'Fold', 'LongTreated', 'wk_2',
is_strict=True, verbose=False, spacing=15, n_splits=n_splits)
distiller(1)
distiller.is_strict = False
distiller(2, 3, 4, 5, 6) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
1.is_responder| 50.5% | 5.2% | 62.6% | 9.1% | 51.1% | 6.9% | 62.1% | 9.9% | 0.757 |
| bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
2.Significant difference (p_val: 0.003, eta-sqr=0.241 F=5.91e+00) among:
-vec | 51.4% | 6.5% | 65.0% | 7.3% | 52.5% | 6.2% | 65.0% | 5.4% | 0.913 |
-2DT | 49.4% | 3.6% | 53.0% | 13.5% | 50.2% | 6.9% | 52.8% | 15.5% | 0.406 |
-2DA | 51.6% | 3.9% | 66.6% | 5.9% | 49.1% | 4.0% | 63.9% | 7.2% | 0.182 |
-roi | 49.6% | 4.7% | 62.9% | 5.2% | 51.2% | 8.1% | 62.8% | 8.0% | 0.706 |
3.No Significant difference (p_val: 0.703, ) between:
-dbc_no | 50.5% | 5.2% | 62.6% | 9.1% | 51.1% | 6.9% | 62.1% | 9.9% | 0.757 |
-dbc_yes | 52.5% | 7.3% | 65.1% | 6.9% | 52.2% | 7.3% | 64.1% | 6.6% | 0.572 |
4.No Significant difference (p_val: 0.180, eta-sqr=0.067 F=2.06e+00) among:
-GradientBoos| 50.8% | 8.1% | 62.3% | 8.5% | 53.6% | 9.6% | 62.2% | 9.0% | 0.701 |
-ResNet | 50.5% | 3.9% | 59.8% | 12.4% | 49.7% | 5.7% | 58.4% | 13.3% | 0.266 |
-SVC | 50.2% | 1.1% | 65.6% | 2.2% | 50.0% | 2.7% | 65.6% | 3.0% | 0.918 |
5.No Significant difference (p_val: 0.727, ) between:
-Site | 52.7% | 4.6% | 49.0% | 15.8% | 50.3% | 6.1% | 48.5% | 18.0% | 0.565 |
-Fold | 50.5% | 5.2% | 62.6% | 9.1% | 51.1% | 6.9% | 62.1% | 9.9% | 0.757 |
6.Significant difference (p_val: 0.002, eta-sqr=0.326 F=3.56e+01) among:
-LongTreated | 50.5% | 5.2% | 62.6% | 9.1% | 51.1% | 6.9% | 62.1% | 9.9% | 0.757 |
-SameResponde| 50.1% | 7.2% | 52.1% | 10.0% | 50.5% | 8.0% | 52.1% | 9.3% | 0.732 |
-Extremes | 63.9% | 10.6% | 63.6% | 8.7% | 52.3% | 9.7% | 52.1% | 10.3% | 0.001* |
-Hiroshima | 49.6% | 7.1% | 48.4% | 8.0% | 49.1% | 9.2% | 47.5% | 10.6% | 0.666 |
-All | 50.5% | 5.9% | 53.6% | 7.2% | 50.4% | 5.3% | 53.2% | 6.8% | 0.657 |
all_clf_distiller = TableDistiller(pd.concat(
(results_table_with_alternate_classifiers, statistics_calculator(results_table_with_alternate_classifiers)),
axis=1), 'is_responder', None, 'dbc_no', 'RandomForestClassifier', 'Fold', 'All', 'wk_2', is_strict=True,
n_splits=n_splits)
all_clf_distiller(1)
all_clf_distiller.is_strict = False
all_clf_distiller(4) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
1.is_responder| 52.1% | 4.8% | 55.3% | 5.2% | 51.3% | 6.5% | 54.3% | 6.4% | 0.463 |
| bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
4.No Significant difference (p_val: 0.220, eta-sqr=0.070 F=1.80e+00) among:
-RandomForest| 52.1% | 4.8% | 55.3% | 5.2% | 51.3% | 6.5% | 54.3% | 6.4% | 0.463 |
-ResNet | 50.0% | 3.9% | 51.0% | 8.3% | 49.5% | 4.7% | 50.1% | 8.2% | 0.372 |
-LogisticRegr| 51.5% | 6.3% | 52.7% | 6.2% | 48.8% | 6.1% | 49.9% | 6.1% | 0.199 |
-SVC | 50.5% | 4.3% | 55.9% | 4.3% | 49.9% | 3.7% | 55.8% | 3.8% | 0.545 |
-GradientBoos| 51.0% | 8.3% | 53.8% | 7.4% | 51.8% | 6.7% | 53.7% | 6.5% | 0.701 |
distiller = TableDistiller(cortical_results, 'is_responder', None, 'dbc_no', None, 'Fold', 'All', 'wk_1', is_strict=False,
n_splits=n_splits)
distiller(7) | bAcc | sbAc | Acc | sAcc | nbAc | snbA | nAcc | snAc | p_val |
7.No Significant difference (p_val: 0.953, ) between:
-wk_1 | 50.5% | 5.7% | 53.8% | 7.2% | 50.4% | 5.8% | 53.2% | 6.8% | 0.657 |
-wk_2 | 50.5% | 5.9% | 53.6% | 7.2% | 50.4% | 5.3% | 53.2% | 6.8% | 0.657 |
# Human-readable Title Dictionaries
hr_data = OrderedDict({
'dbc_no': 'cortical',
'dbc_nly': 'clinical',
'sub_nly': 'sub-cortical',
'dbc_yes': 'cortical and clinical',
'sub_dbc': 'sub-cortical and clinical',
'sub_add': '(sub-)cortical',
'sub_all': '(sub-)cortical and clinical',
})
hr_pop = {
'All': 'Full\nPopulation',
'Extremes': 'Extremes\nSubpopulation',
}
bins = np.linspace(-80, 80, 17)
queries = flatten(
[[('is_responder', 'roi', d, 'GradientBoostingClassifier', 'Fold', p, 'wk_2',) for d in hr_data] for p in hr_pop])
n_rows = 2
n_cols = len(queries) // n_rows
xlim = 80
ylim = 0.05 # max(ax.get_ylim()) # example.population.mean() #
cs = [colors.blue] * n_cols + [colors.orange] * n_cols
bacc_fig, axes = plt.subplots(n_rows, n_cols, figsize=(14, 6))
for ax_n, query, ax, c in zip(range(n_rows * n_cols), queries, axes.reshape(-1), cs):
# Get data
example = results_and_stats.loc[query]
score, null = example['balanced_accuracy'], example['null_balanced_accuracy']
score, null = score.ravel(), null.ravel()
# Plot
ax.hist((score.mean() - null) * 100, bins, density=True, color=c, label=hr_pop[query[-2]])
ax.plot([0, 0], [0, ylim], c='k')
acc_explainer = '' if ax_n else '(bAcc)'
if not ax_n % n_cols:
ax.legend(frameon=False)
ax.text(-xlim, ylim, f'{np.mean(score):.1%}\n{acc_explainer}', va='top', size=14)
# Format the plot
ax.set(
xlim=(-xlim, xlim),
ylim=(0, ylim),
title=hr_data[query[2]].capitalize(),
)
if not ax_n % n_cols:
ax.set_ylabel('Normalized frequency')
ax.set_xlabel('bAcc Δ to null')
if ax_n < n_cols:
ax.get_xaxis().set_visible(False)
ax.xaxis.set_major_formatter(mtick.PercentFormatter())
plt.suptitle('Predicting Response Using Various Sets of ROI Data in two populations', fontsize=18)
bacc_fig.tight_layout()
plt.show()
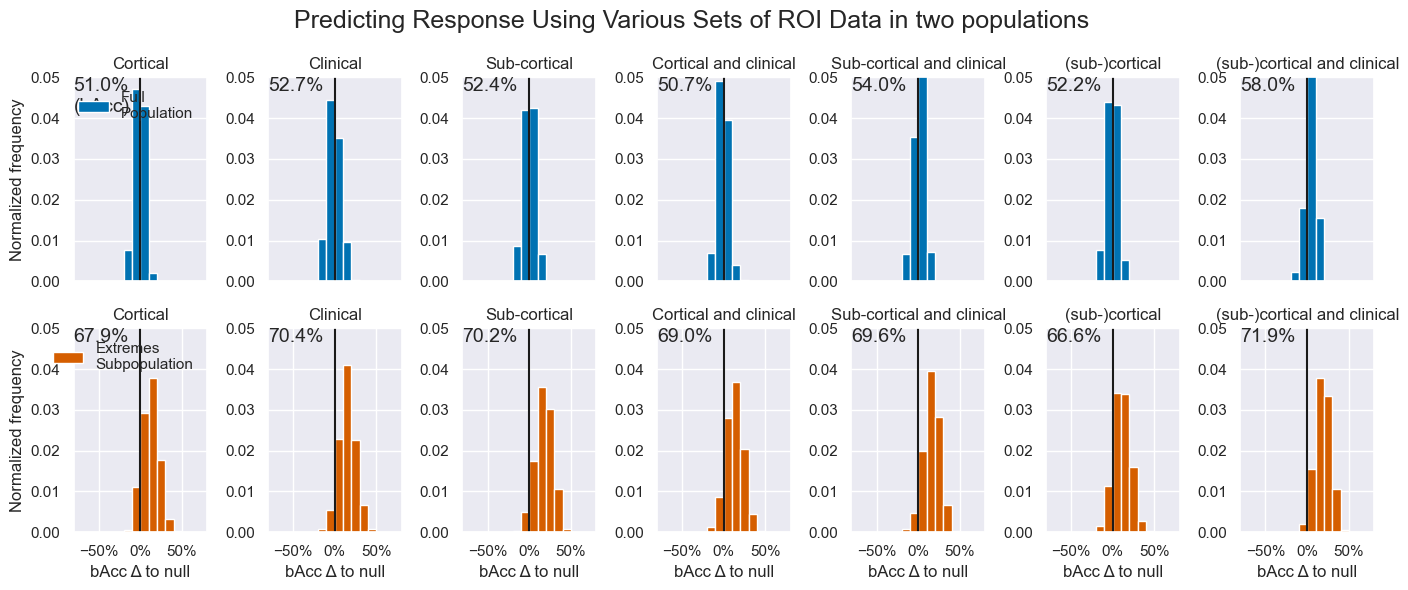
bacc_fig.savefig(figdir.append(f'bAcc_histograms.png'))Three things are worth mentioning about these results:
- Why the null score for
Siteis < 50% - Why the null score for
Extremesis < 50% - How the bAcc > 50% when Acc < Null
The reasons are:
- Because sites present varying a-priori response rates, but the mean of all sites is around 50%. This implies that, when a site with dominant class A is in the test set, the dominant class in the train set will likely be B. Thus we often see a mismatch between dominant class in the train set and the test set.
- The classes in the entire data set are balanced 50%/50%. When we use (even stratified) cross-validation, whenever there are more samples of class A in the train set, there will be more samples of B in the test set. This will incur a deviation from the 50% balance of size ~
1/(sample_size/n_splits)(deviates due to rounding of test set sizes). - Because the "mean of balanced accuracies" != "the balanced accuracy of the means"
Based on simple calculations using the subject samples and labels we can provide an estimate of the expected null score and compare this with the measured null score.
# Retrieve the accuracy score from our results
queries = ('Fold', 'Extremes'), ('Site', 'All')
measured_sites_accs = [np.mean(flatten(results_and_stats.loc[('is_responder', sN, sN, sN, *q)]['null_accuracy'])) for q in
queries]
print('We can say the following about the null accuracy score:')
for i, ((cv_name, (cv_scheme, _)), example_population, measured_sites_acc) in enumerate(
zip(cv_schemes.items(), ('Extremes', 'All'), measured_sites_accs), 1):
# Simulate simple
Xt, yt, ct, = data_dict['roi']['dbc_no'][example_population]
yt = yt.is_responder
sites_acc = []
dominant_class_mismatches, n_splits = 0, 0
for train_idx, test_idx in cv_scheme.split(yt, y=yt, groups=ct):
n_splits += 1
dominant_class_in_train = yt.iloc[train_idx].mode()[0].item()
dominant_class_in_test = yt.iloc[test_idx].mode()[0].item()
if dominant_class_in_train != dominant_class_in_test:
dominant_class_mismatches += 1
test_samples = yt.iloc[test_idx]
sites_acc.append(np.mean(test_samples == dominant_class_in_train))
print(
f'{i}. In Leave-{cv_name}-Out CV of {example_population} the dominant class was mismatched {dominant_class_mismatches}/{n_splits} times:\n'
f' - Expected: {np.mean(sites_acc):.1%}\n'
f' - Measured: {measured_sites_acc:.1%}\n')
s = results_and_stats.loc['is_responder', '2DT', 'dbc_no', 'ResNet', 'Fold', 'All', 'wk_2']['accuracy']
n = results_and_stats.loc['is_responder', '2DT', 'dbc_no', 'ResNet', 'Fold', 'All', 'wk_2']['null_accuracy']
print(f'3. The mean-balanced-accuracy (bAcc) is not the same as balanced-accuracy of the means:\n'
f' Mean accuracy score: {np.mean(s):.1%} ± {np.std(s):.1%}, Mean null score: {np.mean(n):.1%} ± {np.std(n):.1%}\n'
f' - Balanced accuracy of means: {np.mean(s) / np.mean(n) / 2:.1%}\n'
f' - Mean of balanced accuracies: {np.mean([i / j / 2 for i, j in zip(s, n)]):.1%}\n')We can say the following about the null accuracy score:
1. In Leave-Fold-Out CV of Extremes the dominant class was mismatched 8/10 times:
- Expected: 46.9%
- Measured: 52.9%
2. In Leave-Site-Out CV of All the dominant class was mismatched 4/6 times:
- Expected: 44.3%
- Measured: 48.7%
3. The mean-balanced-accuracy (bAcc) is not the same as balanced-accuracy of the means:
Mean accuracy score: 48.1% ± 6.1%, Mean null score: 46.6% ± 6.4%
- Balanced accuracy of means: 51.6%
- Mean of balanced accuracies: 51.8%
Now previously, I have attempted to fix this by replacing in this notebook:
previous_result['null_accuracy'] = [np.mean(y[test] == y[train].mode()[0]) for train, test in list(split_null)]
with
previous_result['null_accuracy'] = np.array([np.mean(y[test]) if y.mean() == 0.5 else np.mean(y[test] == y[train].mode()[0]) for train, test in list(split_null)])
And adding a special case in the torch_val_score method when if y.mean() == 0.5. But creates an overestimation of the performance and only makes the results harder to interpret, so I have since removed them.
We run simulations for two populations All and Extremes and two data types, . We store the coefficients in a dictionary called coef_dict.
# Parameters of our simulation
n_splits = 40
n_repeats = 2
# Define the machine learning pipeline
combat = list(cv_schemes.values())[-1][-1]
pipeline = make_pipeline(imputer, combat, scaler, selector, LogisticRegression, )
# Preallocate two dictionaries: One to store the simulation bAccs, one to store results
coef_bacc = make_empty_nest(['All', 'Extremes'], ['dbc_no', 'sub_all'], bottom=[])
coef_dict = deepcopy(coef_bacc)
# We use a ROS to balanced training data. Test data is balanced through RSKF-CV.
ros = RandomOverSampler()
for population_name, coef_dict_b in coef_dict.items():
for data_type in coef_dict_b:
X, y, _ = data_dict['roi'][data_type][population_name]
y = y['is_responder']
cv = RepeatedStratifiedKFold(n_splits=n_splits, n_repeats=n_repeats, random_state=0)
coef_df = pd.DataFrame(columns=X.columns)
b_accs = []
for train_index, test_idx in tqdm(cv.split(X, y)):
X_train, y_train = X.iloc[train_index], y.iloc[train_index]
X_train, y_train, = ros.fit_resample(X_train, y_train)
X_test, y_test = X.iloc[test_idx], y.iloc[test_idx]
X_test, y_test = ros.fit_resample(X_test, y_test)
pipeline = make_pipeline(imputer, scaler, selector, classifiers[-1], )
pipeline.fit(X_train, y_train)
clf = pipeline[-1]
y_pred = pipeline.predict(X_test)
# We have already used ROS, so Acc = bAcc, but just for good measure:
bacc = balanced_accuracy_score(y_test, y_pred)
coef_df.loc[len(coef_df)] = clf.feature_importances_
b_accs.append(bacc)
coef_dict[population_name][data_type] = coef_df.mean()
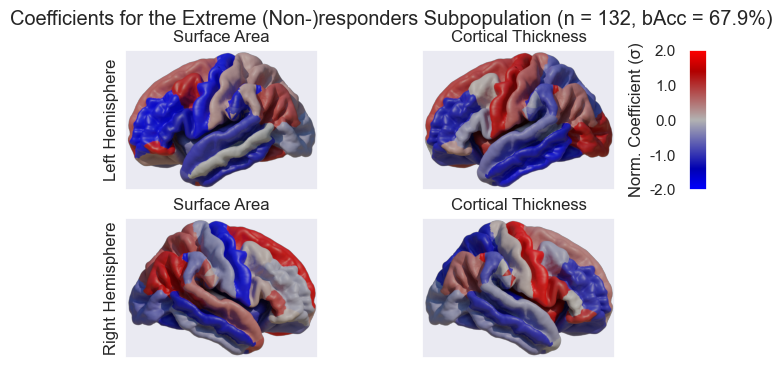
coef_bacc[population_name][data_type] = np.mean(b_accs)We can then visualize the coefficients in Blender. To export them we will store them in a Look-Up Table (LUT)
# Flip the atlas that matches regions to indices
inv_dkt_atlas_lut = {v: k for k, v in dkt_atlas_lut.items()}
# Assign storage location
lut_dir = get_root('data', 'fsaverage', 'manual_labels')
os.makedirs(lut_dir, exist_ok=True)
dfs = {'All': [], 'Extremes': []}
# pp stands for projection_property: area or thickness
for pop, hemi, map_prop in product(('All', 'Extremes'), ("Left", "Right"), ('surf', 'thick')):
coef_means = coef_dict[pop]['dbc_no']
lut_fname = lut_dir.append(f'{hemi[0]}H-LRC_{map_prop}-coefs-{pop}.pkl')
if not os.path.isfile(lut_fname):
idx2coef = make_coef_lut(coef_means, hm=hemi[0], property=map_prop, sd=True)
pickle_out(idx2coef, lut_fname)
lut_status = 'created'
else:
idx2coef = pickle_in(lut_fname)
lut_status = 'loaded'
dfs[pop].append(pd.Series({inv_dkt_atlas_lut[k]: v for k, v in idx2coef.items()}, name=f'{hemi}_{map_prop}'))
print(f'Coefficient Look-Up-Table {lut_status} for {map_prop} in {hemi} hemisphere. ({lut_fname})')
pd.concat(dfs['Extremes'], axis=1)Coefficient Look-Up-Table loaded for surf in Left hemisphere. (D:\repositories\ENIGMA_lean\data\fsaverage\manual_labels\LH-LRC_surf-coefs-All.pkl)
Coefficient Look-Up-Table loaded for thick in Left hemisphere. (D:\repositories\ENIGMA_lean\data\fsaverage\manual_labels\LH-LRC_thick-coefs-All.pkl)
Coefficient Look-Up-Table loaded for surf in Right hemisphere. (D:\repositories\ENIGMA_lean\data\fsaverage\manual_labels\RH-LRC_surf-coefs-All.pkl)
Coefficient Look-Up-Table loaded for thick in Right hemisphere. (D:\repositories\ENIGMA_lean\data\fsaverage\manual_labels\RH-LRC_thick-coefs-All.pkl)
Coefficient Look-Up-Table loaded for surf in Left hemisphere. (D:\repositories\ENIGMA_lean\data\fsaverage\manual_labels\LH-LRC_surf-coefs-Extremes.pkl)
Coefficient Look-Up-Table loaded for thick in Left hemisphere. (D:\repositories\ENIGMA_lean\data\fsaverage\manual_labels\LH-LRC_thick-coefs-Extremes.pkl)
Coefficient Look-Up-Table loaded for surf in Right hemisphere. (D:\repositories\ENIGMA_lean\data\fsaverage\manual_labels\RH-LRC_surf-coefs-Extremes.pkl)
Coefficient Look-Up-Table loaded for thick in Right hemisphere. (D:\repositories\ENIGMA_lean\data\fsaverage\manual_labels\RH-LRC_thick-coefs-Extremes.pkl)
.dataframe tbody tr th {
vertical-align: top;
}
.dataframe thead th {
text-align: right;
}
| Left_surf | Left_thick | Right_surf | Right_thick | |
|---|---|---|---|---|
| bankssts | 0.225564 | 0.400976 | 0.725218 | 0.318618 |
| caudalanteriorcingulate | 0.714526 | 0.733274 | 0.236932 | 0.532695 |
| caudalmiddlefrontal | 0.716545 | 0.559250 | 0.547786 | 0.429713 |
| cuneus | 0.553615 | 0.519163 | 0.594030 | 0.218352 |
| fusiform | 0.694217 | 0.114038 | 0.173524 | 0.370372 |
| inferiorparietal | 0.622145 | 0.666933 | 0.952800 | 0.644766 |
| inferiortemporal | 0.262894 | 0.000000 | 0.310763 | 0.412891 |
| isthmuscingulate | 0.348311 | 0.566538 | 0.361238 | 0.514188 |
| lateraloccipital | 0.528657 | 0.814262 | 0.290009 | 0.444446 |
| lateralorbitofrontal | 0.617164 | 0.346796 | 0.384433 | 0.207585 |
| lingual | 0.745644 | 0.606157 | 0.585818 | 0.394041 |
| medialorbitofrontal | 0.354821 | 0.682403 | 1.000000 | 0.420663 |
| middletemporal | 0.418054 | 0.170677 | 0.234334 | 0.277385 |
| parahippocampal | 0.968004 | 0.117933 | 0.101126 | 0.127951 |
| paracentral | 0.407474 | 0.131782 | 0.209960 | 0.545614 |
| parsopercularis | 0.114000 | 0.474552 | 0.617022 | 0.745656 |
| parsorbitalis | 0.818598 | 0.682201 | 0.570766 | 0.843756 |
| parstriangularis | 0.171510 | 0.408061 | 0.700506 | 0.448635 |
| pericalcarine | 0.212133 | 0.468999 | 0.196210 | 0.336493 |
| postcentral | 0.617473 | 0.655423 | 0.469110 | 0.574932 |
| posteriorcingulate | 0.643339 | 0.641265 | 0.297176 | 0.464945 |
| precentral | -0.550000 | 0.848927 | -0.550000 | 1.000000 |
| precuneus | 0.764550 | 0.486661 | 0.892318 | 0.585712 |
| rostralanteriorcingulate | 0.764884 | 0.772472 | 0.501688 | 0.288294 |
| rostralmiddlefrontal | 0.000000 | 0.261614 | 0.466002 | 0.255178 |
| superiorfrontal | 0.768010 | 0.655299 | 0.838486 | 0.690202 |
| superiorparietal | 0.543888 | 0.385158 | 0.779848 | 0.256075 |
| superiortemporal | 0.208468 | 0.434077 | 0.567895 | 0.493174 |
| frontalpole | 0.716716 | 0.514380 | 0.505318 | 0.592433 |
| temporalpole | 0.209870 | 0.399616 | 0.663377 | 0.543110 |
| transversetemporal | 0.279125 | 0.472147 | 0.543926 | 0.157032 |
| insula | 0.434086 | 0.656469 | 0.184733 | 0.817245 |
# inverse the dkt_atlas
population = 'Extremes'
dkt_atlas_lut_ud = {v: k for k, v in dkt_atlas_lut.items()}
colnames = []
feature_importances = []
for hm in 'LR':
for letter in 'surf', 'thick':
# load the LUT
lut_fname = lut_dir.append(f'{hm}H-LRC_{letter}-coefs-{population}.pkl')
feature_importances.append({dkt_atlas_lut_ud[k]: v for k, v in pickle_in(lut_fname).items()})
colnames.append(f'{hm}H_{letter}')
print(f'Coefficients as provided for imaging of the {population} population:')
print(pd.DataFrame(feature_importances, index=colnames).T)Coefficients as provided for imaging of the Extremes population:
LH_surf LH_thick RH_surf RH_thick
bankssts 0.225564 0.400976 0.725218 0.318618
caudalanteriorcingulate 0.714526 0.733274 0.236932 0.532695
caudalmiddlefrontal 0.716545 0.559250 0.547786 0.429713
cuneus 0.553615 0.519163 0.594030 0.218352
fusiform 0.694217 0.114038 0.173524 0.370372
inferiorparietal 0.622145 0.666933 0.952800 0.644766
inferiortemporal 0.262894 0.000000 0.310763 0.412891
isthmuscingulate 0.348311 0.566538 0.361238 0.514188
lateraloccipital 0.528657 0.814262 0.290009 0.444446
lateralorbitofrontal 0.617164 0.346796 0.384433 0.207585
lingual 0.745644 0.606157 0.585818 0.394041
medialorbitofrontal 0.354821 0.682403 1.000000 0.420663
middletemporal 0.418054 0.170677 0.234334 0.277385
parahippocampal 0.968004 0.117933 0.101126 0.127951
paracentral 0.407474 0.131782 0.209960 0.545614
parsopercularis 0.114000 0.474552 0.617022 0.745656
parsorbitalis 0.818598 0.682201 0.570766 0.843756
parstriangularis 0.171510 0.408061 0.700506 0.448635
pericalcarine 0.212133 0.468999 0.196210 0.336493
postcentral 0.617473 0.655423 0.469110 0.574932
posteriorcingulate 0.643339 0.641265 0.297176 0.464945
precentral -0.550000 0.848927 -0.550000 1.000000
precuneus 0.764550 0.486661 0.892318 0.585712
rostralanteriorcingulate 0.764884 0.772472 0.501688 0.288294
rostralmiddlefrontal 0.000000 0.261614 0.466002 0.255178
superiorfrontal 0.768010 0.655299 0.838486 0.690202
superiorparietal 0.543888 0.385158 0.779848 0.256075
superiortemporal 0.208468 0.434077 0.567895 0.493174
frontalpole 0.716716 0.514380 0.505318 0.592433
temporalpole 0.209870 0.399616 0.663377 0.543110
transversetemporal 0.279125 0.472147 0.543926 0.157032
insula 0.434086 0.656469 0.184733 0.817245
#show colormap
n_shades = 200
cbar_lim = -2, 2
colorbar = get_rgb_cbar(n_shades=n_shades)
# #Plot with label brain (annot)
# imgs = 'lh_annot', 'lh_surf', 'lh_thick', 'colorbar', 'rh_annot', 'rh_surf', 'rh_thick',
# imgs_fpath = r"D:\repositories\ENIGMA\documents\graphics\Blender\{}.png"
# img_titles = 'Labels', 'Surface Coefficients', 'Thickness Coefficients', '',\
# 'Labels', 'Surface Coefficients', 'Thickness Coefficients',
#Plot without label brain (annot)
imgs = 'lh_surf', 'lh_thick', 'colorbar', 'rh_surf', 'rh_thick',
imgs_fpath = figdir.append('{}.png')
img_titles = 'Surface Area', 'Cortical Thickness', '', \
'Surface Area', 'Cortical Thickness',
n_rows = 2
n_cols = len(imgs) // n_rows
for img_pop in ('Extremes',):
brain_fig, axes = plt.subplots(nrows=n_rows,
ncols=len(imgs) // n_rows + 1,
figsize=(n_cols * 3 + 2, 2 * n_rows),
gridspec_kw={'width_ratios': n_cols * [1] + [0.1]})
axes = axes.reshape(-1)
for ax, title, img_path in zip(axes, img_titles, imgs):
# Select and display image
formatted_img_path = imgs_fpath.format(img_path if 'annot' in img_path else f'{img_path}_{img_pop}')
img = colorbar if img_path == 'colorbar' else mpimg.imread(formatted_img_path)
ax.imshow(img)
# Set axes properties
ax.set(xticks=[], title=title)
if img_path == 'colorbar':
ax.set_yticks(np.linspace(0, n_shades, 5))
ax.grid(False)
ax.set_yticklabels(np.linspace(cbar_lim[-1], cbar_lim[0], 5))
ax.set_ylabel('Norm. Coefficient (σ)')
else:
ax.set_yticks([])
axes[-1].axis('off')
axes[0].set_ylabel('Left Hemisphere')
axes[n_cols + 1].set_ylabel('Right Hemisphere')
perf = np.mean(results_dict['roi']['dbc_no'][img_pop]['Fold']['GradientBoostingClassifier']['is_responder']['wk_2'][
'balanced_accuracy'])
n_part = len(data_dict['roi']['dbc_no'][img_pop][0])
# plt.suptitle(f'Coefficients for {img_pop} Population (n = {n_part:.0f}, bAcc = {bacc:.1%})')
plt.suptitle(f'Coefficients for the Extreme (Non-)responders Subpopulation (n = {n_part:.0f}, bAcc = {perf:.1%})')
brain_fig.savefig(figdir.append(f'{img_pop}-3Dbrains.tiff'))
plt.show()# We never ended up using this because it was hard to retrace
# which "pixels" were dropped in preprocessing, so they might
# not be placed correctly (23 out of 900 were dropped)
X, y, _ = data_dict['vec']['dbc_no']['Extremes']
clf = LogisticRegression()
clf.fit(X, y)
coef = np.squeeze(clf.coef_)
X_thc2, _ = load_proj_df(data, 'Thickness')
imps = np.zeros(30 * 30)
counter = 0
for i, pixel in enumerate(X_thc2.var().to_numpy().ravel()):
if pixel >= 0.002:
imps[i] = clf.coef_[0][counter]
counter += 1
titles = 'Median Imporance', 'Median thickness', 'aparc.a2009s.annot'
map_imp = np.reshape(imps, (30, 30))
map_thk = np.reshape(np.median(X_thc2, axis=0), (30, 30))
map_img = np.load(results_dir.append('aggregated_label_annot_res-32.npy'))
fig, axes = plt.subplots(1, len(titles), figsize=(10, 4))
h_thk = axes[0].imshow(map_imp, cmap='viridis', clim=(-map_imp.std() * 2, map_imp.std() * 2), )
h_imp = axes[1].imshow(map_thk, cmap='jet')
h_img = axes[2].imshow(map_img)
for ax, mappable, title in zip(axes, [h_thk, h_imp, None], titles):
ax.grid(False)
ax.set(title=title, xticks=[], yticks=[])
if mappable:
fig.colorbar(mappable, ax=ax)
fig.tight_layout()
fig.show()100%|██████████| 252/252 [00:00<00:00, 2483.71it/s]
Note that the labeling we were provided in the ROI analysis (aparc.DKTatlas40.annot) is different from the labeling we were provided in the projection analysis (aparc.a2009s.annot) which is shown in the figure above. Also, one is not simply as subset of the other.
I Wish we would have the DKTatlas for the projection data so we could see if the importance found in the projection analysis lines up with the importance found in the ROI analysis.
Show a table
# A function to strip subcortical ROIs from their hemisphere information
rm_hem = lambda x: x[1:] if x[0] in hems else x
# Get hemisphere options, and ROI options so we can strip them from column names
hems = 'LRM'
# Features of interest, pick one
feature_set = ['sub_all', 'dbc_no'][0]
imp_dfs = {}
for population, population_coef_dict in coef_dict.items():
bacc = np.mean(coef_bacc[population][feature_set])
# Subcortical importances
subc = {k: v for k, v in population_coef_dict[feature_set].items() if k in subcortical_rois}
# Apply the subcortical strip function to the index to get all unique ROIs
no_hem_subc_rois = set(population_coef_dict[feature_set].index.map(rm_hem))
# Preallocate a numpty array to store our
subc_coefs = np.empty((len(no_hem_subc_rois), len(hems)))
subc_coefs[:] = np.nan
# Collect coef by ROI and hemisphere, and appoint directly into a numpy array
for i, v in subc.items():
roi_name = rm_hem(i)
hemi = i.replace(roi_name, '')
hemi = hemi if hemi else 'M'
x = list(no_hem_subc_rois).index(roi_name)
y = hems.index(hemi)
subc_coefs[x, y] = v
subc_coefs = np.concatenate([subc_coefs, np.nansum(np.abs(subc_coefs), 1)[:, np.newaxis]], axis=1)
# Convert the array to a table, and do formatting
subc_relevance_table = pd.DataFrame(data=subc_coefs, columns=list(hems) + ['SAR'],
index=no_hem_subc_rois) # We use Sum of Absolute Relevances
subc_relevance_table = subc_relevance_table.sort_values(by='SAR', ascending=False) # for sorting
subc_relevance_table = subc_relevance_table.drop(columns=['SAR']) # and drop it after.
subc_relevance_table = subc_relevance_table.replace(np.nan, pd.NA)
subc_relevance_table = subc_relevance_table.rename(columns={'L': 'Left', 'R': 'Right', 'M': 'Middle / NA'})
# Store the table
imp_dfs[population] = subc_relevance_tableprint('start time:', results_uid)
print('finish time:', datetime.now().strftime("%Y%m%d-%H%M%S"))start time: 20240807-135247-postRebuttal2-Night2
finish time: 20240810-015510