Talk:Electron transport chain
| This It is of interest to the following WikiProjects: | |||||||||||||||||||||||||||
| |||||||||||||||||||||||||||
Wiki Education Foundation-supported course assignment
[edit]![]() This article is or was the subject of a Wiki Education Foundation-supported course assignment. Further details are available on the course page. Student editor(s): Nazir830, SubigyaKarmacharya. Peer reviewers: Nazir830, SubigyaKarmacharya.
This article is or was the subject of a Wiki Education Foundation-supported course assignment. Further details are available on the course page. Student editor(s): Nazir830, SubigyaKarmacharya. Peer reviewers: Nazir830, SubigyaKarmacharya.
Above undated message substituted from Template:Dashboard.wikiedu.org assignment by PrimeBOT (talk) 20:22, 16 January 2022 (UTC)
Untitled
[edit]I think the ETC also exists in plastids (chloroplasts) and respiring bacteria. AdamRetchless 22:04, 9 Apr 2005 (UTC)
Name: transfer? chain
[edit]I have always heard this subject referred to as the Electron Transport Chain. Is transfer more common?
The article states "In most organisms the majority of ATP is generated in electron transport chains, while only some obtain ATP by fermentation.[citation needed]" Do we have any in vivo in situ justification for this statement? We have lots of measurements made of ATP synthesized by isolated mitochondria, showing that they CAN make a lot of ATP, under certain conditions in a test tube (ie a reaction chamber equipped with an oxygen electrode and a few other things.) So we get values for P/O ratios under various conditions, and then extrapolate all the way back to the real world of whole organisms. What we're doing is taking these P/O ratios and treating them like they were yields of ATP in vivo! If that has ever been documented, fine. (Bearing in mind that I don't want to spend my time plowing through textbooks and articles that mindlessly repeat the same mantra as summarized here in the Wikipedia statement I just quoted, supra; in vivo, please, as in "real world"; real organisms, and no weasel words neither, dangnabbit!Richard8081 (talk) 16:19, 3 October 2012 (UTC))
Rename?
[edit]"Electron transport chain" has 90,000 hits, and "Electron transfer chain" has 11,800 hits. Does anybody object if I move the page? --Arcadian 5 July 2005 03:24 (UTC)
purpose of transferring electrons
[edit]why is it necessary to keep transferring electrons to so many carrier molecules? -- Bubbachuck 11:34, 23 December 2005 (UTC)
- I can't give a definite answer but it might be to spread the energy released as the electron goes from high energy to low energy over a number of steps, so that each step is a small release of energy that is more manageable to the organism. In addition to it being easier for all the energy to be picked up (increased efficiency), smaller redox potentials are less likely to cause the formation of reactive oxygen species that would be damaging to a mitochondria and the cell it's in. Just a guess. Philbradley 00:20, 15 June 2006 (UTC)
- My science textbook had an excellent way of putting it. Suppose you had a flow of water at 1,000 feet. If you had it fall those 1000 feet onto a waterwheel, you'd simply destroy your waterwheel. If instead you had the water go down a cliff pushing several wheels as it went, you'd get more energy out of it. Mathwhiz90601 03:14, 30 May 2007 (UTC)
- The problem is that none of these processes mentioned has sufficient Free energy to make one ATP. Therefore the work is divided into smallet steps and each step can pump a couple of protons and produce the pH gradient. However, the pH gradient is not sufficient to make one ATP either (because each step cannot make a large pH gradient by itself). Therefore the membrane potential comes into the picture. The membrane potential is made by pumping Na and K and other ions across the membrane. Now for each ATP syntheis, several protons and Na ions come back (down the concentraton gradient) and their total energy is used in making the ATP. This is one of the reasons that the ATP synthase is so complex (it looks and acts like a motor or generator). This will also explain why the number of ATP made per NADH or FADH2 is non-stochiometric (they simply work independently). Also Na ions need to be constantly pumped outside to maintain the membrane potential and this takes energy which is not included in the calculations.
I hope I am clear. chami 18:53, 30 May 2012 (UTC) — Preceding unsigned comment added by Ck.mitra (talk • contribs)
Image
[edit]I added this image that I made. Do I need to go back and change Kreb cycle to Krebs cycle or perhaps Citric acid cycle?
Please tell me if there are any more errors in this drawing.—The preceding unsigned comment was added by Rozzychan (talk • contribs) .
- I think the use of alternate names is not a problem. By the way, I enlarged the image so the small type would be easier to see. Hope no one minds. Thanks for putting in this picture, it really adds a lot to the page! delldot | talk 05:20, 10 March 2006 (UTC)
Possible Error in Table
[edit]Under the heading "Mitochondrial redox carriers" a diagramatic summary of the overall electron transport chain is given. From the Paragraphs give below, dealing with the four complexes, 'Complex II', in the diagram, should point at 'Q', instead of 'Cytochrome c'.
Incorrect diagram
[edit]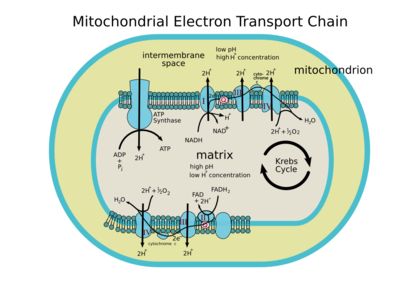
This illustration greatly improves the overall visual appearance of the article. Unfortunately, it is confusing, misleading, incomplete and factually incorrect. There are at least 10 problems:
1. It should be labeled “Mitochondrial electron transport chain” to distinguish it from bacterial electron transport chains and photosynthetic electron transport chains, which are also discussed in the article.
2. The upper membrane shows ATP synthase (so-called Complex V) off to the left. ATP synthase is not part of the electron transport chain. It is powered by a proton gradient, not by a series of redox reactions. Many diagrams of mitochondria include it, but “Complex V” is misleading when the subject is “electron transport chains.”
3. The upper membrane shows Complexes I and III connected by an arrow with an orange “II” floating overhead in the intermembrane space. Mitochondrial Complex II is a transmembrane complex just like I, III and IV and should be depicted as such.
4. Moreover, if this is intended to show the electron transport chain with NADH as the electron donor, “Complex II” does not belong here. Complex I should be connected to Complex III by ubiquinone (Q).
5. Complex IV (which is unlabeled in the diagram) is shown connected to III by “cyto chrome” [sic]. Why not “cytochorome c,” or “cyt c”?
6. If “2H+ + ½ O2 → H20” is intended to represent a reduction, the arrow (which represents electrons) should join with the reactants, not cross over them.
7. Moving onto the lower membrane, we see (in reverse logical order) the same bizarre “cross-reduction” diagram, Complex IV (unlabeled), “2e-” instead of “cytochrome c”, Complex III (unlabeled), and Complex I (unlabeled). Complex II is entirely missing.
If this is intended to represent the electron transport chain with FADH2 as the electron donor, Complex I should be omitted, II should be included and shown as connected to III by Q, “2e-” should be replaced by “Cyt c”, the final arrow should show a reduction, and the whole series should read left-to-right, not right-to-left.
8. Moving into the matrix, we find the “Kreb Cycle” (it’s “Krebs cycle”) floating off to the right between two arrows going around in a cycle. If the Krebs cycle is included in this diagram, it should be shown as feeding NADH and FADH2 into the electron transport chain.
9. A small point, but “high pH” and “low H+concentration” mean exactly the same thing. Do we really need both captions in the diagram, or can we assume a basic familiarity with chemistry on the part of the reader?
10. Finally, the statement that “FADH2 pumps 2 protons across the membrane and makes 2 ATP, NADH pumps 3 protons across the membrane and makes 3 ATP” is simply false. Complex I pumps 4 protons (per pair of electrons); Complex III pumps 4 protons, and Complex IV pumps 2 protons. This gives 10 protons per NADH, 6 protons per FADH2. ATP synthase needs 3 protons to produce one ATP; an additional proton is needed to transport the reactants (ADP and Pi) and products (ATP) across the membrane. The ratio of ATP molecules produced per NADH (or per FADH2) is not an integer.
This article could be vastly improved by the inclusion of graphics, but the graphics should reflect our current knowledge of electron transport chains. Excellent examples can be found in Lehninger and Voet. Too bad about those copyright restrictions.
However, Wikipedians can probably do better anyway. Btarski 18:26, 17 March 2006 (UTC)
- I thank you for your diligence in spelling out your concerns. It sounds like you are an expert on the subject -- if you could put together a quick-and-dirty diagram and upload it to the commons, you would be doing us a great favor. However, until/unless we have a better image, I have added User:Rozzychan's image back, with a caveat underneath it addressing the limitations of the diagram. This is the approach usually used on Wikipedia when there is no replacement image currently available. But I do agree that if/when another Wikipedian is kind enough to build or donate an improvement (possibly adapting one of the others that are found on the sister wikis) it would be appropriate to incorporate many of your suggestions. --Arcadian 01:25, 18 March 2006 (UTC)
Modified Image
[edit]I finally got around to editing this page. Thanks for the comments.
I addressed the listed points by number.
1. I thought that having the word Mitochondrion printed on the image would be sufficient to distinguish that this is the Mitochondrial ETC. I changed the title.
2. The ATP synthase is the main POINT of the ETC. I think that removing the ATP synthase confuses the student as to why the ETC exists at all. Also it was referenced in the article which is why I numbered it as I did, but since the article has been changed, I removed the V.
5. Writing cytochrome c without a space would be preferable, but then the font would be too small to read. I put a dash to make it more clear that this is a line wrap.
6. Done.
7. I disagree. Omitting complex I might confuse viewers. It is meant to be obvious that the complex shown on the bottom is the same one shown above for NADH.
As to it reading from left to right instead of right to left. I am sorry but a membrane makes no such distinction. Also, reading directions are not the same for all viewers.
8. Yes I know it's Krebs. I mentioned this in a previous comment. I don't want to go into detail for that cycle. How can I add this without needing to be accurate about the exact numbers that must feed into the cycle. I could remove the cycling arrows, but I don't think that would be clear.
9. No, I don't assume this familiarity by the reader. Beginning students are often confused on this point. I was trying to be clear to students who do not remember such facts immediately. This is a very important point. If I had to remove one, I would remove the pH.
10. This was a simplified note for beginning students, and I did not mean to leave it on this image.
Often we tell students inaccurate things because they are easier. When things do not come out as simple integers, students have a hard time understanding and remembering the point. Simple rules like this help a student understand. When they go on to more advanced studies, we tell them the earlier rule was slightly inaccurate.
However, since this is a dictionary entry, I have removed the text entirely and expect that you can mention the specifics of the reaction in the body of the article.
I changed the image to say that two protons were transferred due to the information shown on this website. https://www.elmhurst.edu/~chm/vchembook/596electransport.html
I have also included the source document (Etc2.svg). Feel free to fix any small errors that you see.
Rozzychan
- The link you have shown is simply wrong. One NADH oxidation, whatever way that may be carried out, does not have sufficient energy to make three ATPs.
Further, ATP synethesis is not coupled to the ET chain. ETC contributes to the pH gradient only.
ATP synthesis need both pH gradient and membrane potential. ATP synthase also carries Na ions downhill.
A table of standard free energies or redox potentials will be great help.
The article is too much simplified. The real process is somewhat complex.
chami 19:10, 30 May 2012 (UTC) — Preceding unsigned comment added by Ck.mitra (talk • contribs)
Basic View
[edit]A more basic, broad view would be appreciated, just tell us how it works in simple language. Thanks.
NADH
[edit]there is nothing about how it is transported across the mito inner mem. the shuttles i mean.--M siterman 09:22, 17 May 2006 (UTC)
Shuttles in Oxidative phosphorylation
[edit]I understand that explaining the shuttles would be useful in understanding the difference between the 36ATP and 38ATP yield for the aerobic breakdown of glucose, however this does not directly impact on the function of the mitochondrial electron transport chain.
I agree that this is an important point though. I did not know about the shuttles until I had to teach this point, and realized that the numbers did not add up. A little online research clarified the point, but I still did not tell this to my most recent class, as this detail will only confuse beginning students.
More advanced students, however, are more disturbed by numbers that don't add up when they expect them too.
Suggestions: 1. Add a sentence saying that NADH from glycolysis enters the mitochondria through either the Glycerol phosphate shuttle (LINK) or the malate-aspartate shuttle (link).
This sentence should probably go onto another page though, I'm not sure which. Perhaps Oxidative phosphorylation
2. Make two new pages for those shuttles.
3. Make a glossary page that links to all pages discussing Aerobic and Anaerobic respiration. Rozzychan 17:27, 23 June 2006 (UTC)
redundancy
[edit]This article seems to be redundant with many other articles in wikipedia. Since it is so long would it be wise to remove some of this information and rely on the other articles for more specific information? I am thinking of photosystems for one. David D. (Talk) 20:52, 16 June 2006 (UTC)
- I take this back. i was looking at the other articles and they are not as complete as I had imagined. However there is a distinct lack of cross referencing to related articles especially in the photosynthesis related pages. David D. (Talk) 20:57, 16 June 2006 (UTC)
Made a new index page Glucose_catabolism
[edit]To combat the feelings of redundancy and inadequate cross-referencing, I created a page to serve as an index to this subject called glucose catabolism.
Now we can add topics to this page. Rozzychan 20:07, 23 June 2006 (UTC)
- Wouldn't it be better to make a template? i think the page you have started could end up as a replicate of cellular respiration. David D. (Talk) 20:16, 23 June 2006 (UTC)
Recommend Keeping the Long Article
[edit]I think we should keep the long article.
The point of article, it seems to me, is to highlight the remarkable similarities in this process across all fields of biology, from the simplest organisms to the most complex. The discovery of these fundamental similarities was one of the crowning achievements of 20th century biology. The underlying unity of these seemingly unrelated processes (photosynthesis, mitochondrial ATP production, lithotrophy) motivates modern research in energy metabolism, one of the most exciting areas in biology.
The article now contains only background information and references. There is no discussion of electron transport chains themselves. The other four articles have no references, and they do not reference each other. Thus isolated and taken out of context, they are virtually meaningless to the average reader.
Moreover, three of them are now incomprehensible. Consider the article “Photosynthetic electron transport chains in bacteria.” It begins
- "PSII, PSI and b6f are found in chloroplasts. All plants and all photosynthetic algae contain chloroplasts, which produce NADPH and ATP by the mechanisms described above."
Note that (1) PSII, PSI, b6f, NADPH and ATP are all undefined; (2) there are no “mechanisms described above”; and (3) the article is about photosynthetic bacteria (which do not contain chloroplasts). I submit that most readers would find this incomprehensible.
I have no objection to expanding Wikipedia’s coverage of electron transport chains in longer, more specialized articles. However, I also think that the underlying unity of these processes is the main point. You can’t appreciate underlying unities if the article is split up into little bits and pieces.
I would strongly recommend restoring the pre-6/17/06 version of the article to this page. Btarski 05:35, 24 June 2006 (UTC)
- I like the idea of comparing and contrasting the different electron transport chains. i.e. location bacteria vs mitochondria with discussion relating to the evolutionary origin of mitochondria. Also contrasting the pH gradients between mitochondria and chloroplasts (pH1 vs pH4) would be of significant interst too. As well as the difference in the location of the compartment with low pH (inside for chloroplasts, outside for mitochondria.
- I think more focus on the central role of the ubiquinone would be interesting. i.e. both complex I and complex II feed their electrons into the ubiquinone pool. There are also many other electron sources such as glyceraldhyde dehydrogenase, one of the shuttling solutions to oxidase cytoplasmic NADH.
- In plants, the alternative oxidase allows the electron transport chain to function without oxygen. And so on.
- The old versionof the article seemed to focus too much on the various components rather than the big picture. It will require a lot of reorganisation but it would make it into a much more useful article. David D. (Talk) 15:27, 24 June 2006 (UTC)
Mediation
[edit]Hi everyone, I am an impartial "outsider" here to sort through the situation with splitting the article, and see if we can come to an acceptable compromise.
First off, I am going to assume that everyone involved has read and is familiar with the Wikipedia guideline Summary style, which addresses the need to break up articles longer than a certain length for readability.
- My understanding of the situation
- Arcadian noticed that the article was lengthy (around 36k) and decided to split it, making new articles out of what were rather large sections of Electron transport chain.
- The new articles are essentially the old sections from Electron transport chain, copied and pasted; no data was lost.
- The headers of the sections that were split were left in the article Electron transport chain, with notes to see the new articles.
- The current iteration removed those headers and simply left a section titled "See also" that points to the split articles.
- Community reactions
- Btarski is in favor of putting the article back the way it was before the split, believing that the new iteration makes the information presentation too confusing.
- It appears that David D. is in favor of the split, with the main article serving as an "big picture" overview.
- My observations
- The split was done with Summary style in mind, and the original article did seem too long to efficiently convey the information; I'm sure everyone is willing to assume good faith in this edit.
- It may have been better to seek consensus on the article talk page before performing the split, but the split was needed.
- The current iteration featuring the "See also" section is ineffective in conveying the depth of information offered; the article does not adequately follow Summary style.
- My initial proposal for compromise
- The headings for the sections that were split should be restored per Summary style, each featuring a brief synopsis of the material that was split and a proper link.
- The "Summary" section should be renamed to "Overview" and written to provide context for the split articles.
- Each article that was split should have a revised lead paragraph that more adequately introduces the topic.
Responses
[edit]Interested parties, please post your responses to my observations and compromise offer, and please remember to be civil and assume good faith in your peers. If you wish, simply add:
- Agree ~~~~
or if you disagree, please post constructive alternatives. --Aguerriero (talk) 18:20, 27 June 2006 (UTC)
- Agree --Arcadian 18:47, 27 June 2006 (UTC)
- Agree your synopsis and solution are both good. David D. (Talk) 19:05, 27 June 2006 (UTC)
- By the way why are we in mediation, I didn't know there was really anything happening here? Obviously there is a lot of rewriting required, which is one reason i have not got stuck into this yet. But is there actually a real dispute here? I thought we were just beginning? David D. (Talk) 19:26, 27 June 2006 (UTC)
- Btarski requested mediation. --Arcadian 19:40, 27 June 2006 (UTC)
- Oh I see. The only reason I'm surprised is that its normally mayhem when a mediator gets brought in. To date, i have just seen a bold move, that while not pretty, at least got the ball rolling on the fact that we need to get something done, i.e. rewite and reorganise. i do agree that the spin off articles were not articles, but i had assumed that they would be worked up to standard in due course. I think there is room to have very specific details in those four spin off pages, although, they too need to be rewritten now that they are out of context. This page will need to be rewritten to reflect the loss of the old material and highlight similarities and differences between the various transport chains. The challenge is to keep it simple while maintaining relevant links to encompase all the associated pages in wikipedia. David D. (Talk) 20:24, 27 June 2006 (UTC)
- It looks to me like this can be wrapped up quickly; I am just waiting for Btarski to respond. After the mediation, I will also make sure to communicate what other avenues of dispute resolution are available before going to mediation. Thanks for your quick responses, everyone! --Aguerriero (talk) 20:33, 27 June 2006 (UTC)
- Btarski requested mediation. --Arcadian 19:40, 27 June 2006 (UTC)
- By the way why are we in mediation, I didn't know there was really anything happening here? Obviously there is a lot of rewriting required, which is one reason i have not got stuck into this yet. But is there actually a real dispute here? I thought we were just beginning? David D. (Talk) 19:26, 27 June 2006 (UTC)
Btarski's proposal
[edit]Btarski has responded to the compromise offer here. Please review:
- His objections to the proposed compromise (namely that the work required may demand resources that may not be available)
- His offer of a different compromise (dividing the article into two pieces) which he has offered to perform if agreed upon.
Below, please indicate whether you Accept, Reject, or Counter Btarski's proposal. If you counter, please include a constructive alternative. Aguerriero (talk) 21:52, 28 June 2006 (UTC)
- There is already an article on photophosphorylation (Light-dependent reaction). That article cites the electron transport chain article here. The reason being that some of the specifics of the electron transport chain itself should be discussed in this article.
- We must not look at these two proposed articles as being independant of others that already exist. I agree this is a hard job, certainly one i do not have time to attack right now. However, i will make a real attempt in the future to bring these up to scratch and will make an effort to help whatever effort occurs before that time. i suspect an inital step should be to survey all the pages in wikipedia that discuss ETC's in a general way, as well as all the specific pages that deal with the complexes and proteins involved in electron transport. I expect there will be a lot of content already diluted throughtout wikipedia. To make this into a coherent set of articles will be well worth the trouble. David D. (Talk) 22:14, 28 June 2006 (UTC)
- Do I read that correctly as a reject of Btarski's proposal, in favor of the original proposal? Aguerriero (talk) 22:55, 28 June 2006 (UTC)
- Not a rejection. i just don't know how many related articles are out there. If we are going to rewrite this as two articles we have to have a plan of how they will complement the other articles. Photosynthesis, Photosynthetic_reaction_center, Photosystem and Light-dependent reaction articles already discuss the ETC from the photophosphorylation perspective (with varying degrees of detail). What angle will the new one take? i am not rejecting as I don't have all the details as to how this will dove tail with the other articles. I am happy for him to make a start and i will add input and constructive critiscism. David D. (Talk) 00:30, 29 June 2006 (UTC)
- Do I read that correctly as a reject of Btarski's proposal, in favor of the original proposal? Aguerriero (talk) 22:55, 28 June 2006 (UTC)
Can all interested parties please indicate their acceptance or rejection of Btarski's proposal based on his further clarification below? --Aguerriero (talk) 13:38, 29 June 2006 (UTC)
- It's fine with me. David D. (Talk) 14:54, 29 June 2006 (UTC)
- Agree. --Arcadian 15:13, 29 June 2006 (UTC)
Closing
[edit]Since everyone who has showed an interest has agreed on a solution, I am closing the mediation case. I suggest that you outline a plan for executing Btarski's proposal before carrying it out, perhaps as follows:
- Plan what content will end up on which page, based on the pre-split version.
- Revert Electron transport chain to the pre-split version.
- Perform the split into two articles as proposed.
- Arcadian blank the old split articles (now unused) and file requests for speedy deletion per G7.
Thank you for your cooperation and civility in this matter; carry on smartly. --Aguerriero (talk) 15:40, 29 June 2006 (UTC)
Proposed division of article
[edit]- Articles longer than 12 to 15 printed pages (more than 30 to 35 KB of readable text) take longer to read than the upper limit of the average adult's attention span—20 minutes. An important consideration is that attention span is lower for children, adults of below-average intelligence, and all those with attention deficit disorders.
- –Wikipedia content guideline: Summary style (https://wikipedia.org/wiki/WP:SS)
- Articles longer than 12 to 15 printed pages (more than 30 to 35 KB of readable text) take longer to read than the upper limit of the average adult's attention span—20 minutes. An important consideration is that attention span is lower for children, adults of below-average intelligence, and all those with attention deficit disorders.
It’s pretty hard to argue in favor of picking on ADHD victims, so let’s agree that the article should be subdivided.
I think that we can also agree that “the spin-off articles were not articles,” as David D. so nicely put it.
The proposed plan, as I understand it, it to:
- Split the article into five new articles: a main article serving as a “big picture” overview, and four new articles (Electron transport chains in mitochondria, Electron transport chains in bacteria, Photosynthetic electron transport chains in chloroplasts and Photosynthetic electron transport chains in bacteria).
- Write a brief synopsis of each of the new articles for inclusion in the new main article (per Summary style) along with appropriate links.
- Include an “Overview” section in the main article that puts the new articles into perspective. As David D. again so nicely put it, “This page will need to be rewritten to reflect the loss of the old material and highlight similarities and differences between various transport chains. The challenge is to keep it simple while maintaining relevant links to encompass all the associated pages.”
- Write a new lead paragraph for each of the four new articles. Each topic needs to be adequately introduced, and the relevant background and contextual information (such as the definition of “b6f”) must be included if the article is to stand by itself.
- Include references and links in each of the new articles.
This sounds like a pretty good plan to me. But I do have one question:
Exactly who is going to write all of this new material?
I can see two possibilities:
(1) The editor who implements this bold plan can write the new material himself. It is, after all, his idea, and therefore (one would presume) his responsibility.
(2) Or, we can put our trust in the magical powers of Wikipedia. We can just call the new articles “stubs” for the time being, and trust that at some unspecified time in the future, someone will come along and do all the work. This is the “eventualist” approach favored by Arcadian.
Since (1) is apparently out of the question, we are left with (2).
I do not think that (2) is a really good idea. Among other things, it leaves four unintelligible, virtually useless articles in Wikipedia, while at the same time removing an intelligible (albeit too long) article that Wikipedia readers might actually be able to use in the foreseeable future.
- When articles grow significantly past this amount of readable text, a plan to break up the article to improve readability and ease of editing should be explored.
- –Wikipedia content guideline: Summary style (https://wikipedia.org/wiki/WP:SS) [emphasis added]
- When articles grow significantly past this amount of readable text, a plan to break up the article to improve readability and ease of editing should be explored.
- Do not take precipitous action the very instant an article exceeds 32 kB. There is no need for haste. Discuss the overall topic structure with other editors. Determine whether the topic should be treated as several shorter articles and, if so, how best to organize them.
- –Wikipedia style guide: Article size (https://wikipedia.org/wiki/Article_size) [emphasis added]
- Do not take precipitous action the very instant an article exceeds 32 kB. There is no need for haste. Discuss the overall topic structure with other editors. Determine whether the topic should be treated as several shorter articles and, if so, how best to organize them.
If you will permit me (in the spirit of constructive criticism), I’d like to propose an alternate plan:
Why not follow the example of every standard textbook in this area and divide the subject into two articles, one on oxidative phosphorylation (the usual sense of “electron transport chain”), and the other on photophosphorylation. This division makes a certain amount of intuitive sense. Electron flow is driven by chemical energy in oxidative phosphorylation and by light energy in photophosphorylation; the former is a chemical process while the latter is a quantum process.
It would be relatively easy to write a short introductory paragraph for each of the new articles, linking them to each other. The cut-and-paste spin-off articles could then pretty much stand are, without extensive rewriting.
This plan would, of course, involve some additional writing in the “main” article if the unities discussed in the original article (the comparison of electron transport chains side-by-side) were to remain (in order to satisfy those readers who are interested in the “big picture”).
The similarities between oxidative phosphorylation and photophosphorylation are very deep. The creation of a transmembrane electrochemical potential, the synthesis of ATP, and even the proton pump (b6f, for God’s sake!) show fundamental similarities across all forms of life. I, for one, find this extremely fascinating, even beautiful. I agree with David D. that “I like the idea of comparing and contrasting the different electron transport chains.”
I propose that the article be split in two. I guess I could do this (if asked), although I’m not sure that the other members of this talk group would agree to such a plan, deeply offended as they are.
Nevertheless, the plan to leave the split-articles dangling in Wikispace, hoping that somebody else will fix them at some unspecified time in the future, strikes me as disservice to Wikipedia readers. The plan is to replaces an article that makes sense (even though it’s too long) with a set of articles that make no sense whatsoever. I do not think that this is an improvement in Wikipedia..
I disagree with this proposal.
Btarski 16:56, 28 June 2006 (UTC)
- Btarski, Please read David D.'s response to your proposal, and address his requests for clarification if you can. It sounds like your proposal may be duplicating some work and/or article topics. Aguerriero (talk) 02:51, 29 June 2006 (UTC)
Let me make my proposal more explict:
1. We agree that the article should be split, because it’s too long.
2. I volunteer to split it. Specifically, I volunteer to split it into two articles:
- Electron transport chain
- Photosynthetic electron transport chains
3. The first article will contain, more or less verbatim, the first parts of the original article. It will also contain a brief summary of photosynthetic electron transport chains, together with appropriate links. One link will be to the second part of the article. David D. mentions a number of other links. I think that all of these should be included, and I appreciate his input.
4. The second article will contain, essentially verbatim, the rest of the original article. It will require a new introduction (which, it seems to me, can be lifted for the most part from the first article). It will also contain a very brief discussion of non-photosynthetic electron transport chains, together with a link back to the first article.
5. Wikipedia’s coverage of photosynthetic electron transport chains is, as David points out, already quite large and quite diverse. I think that this is a perfect illustration of the power of Wikipedia. David’s list contains articles written from a number of different viewpoints and at a number of different educational levels. Isn’t this one of the main strengths of Wikipedia? There is no such thing as a single, perfect article on this or any other topic.
- Yes this is a strength. My point that is that by being aware of the articles that have already been written then we will not duplicate effort. In other words this new article should serve a different role and have its own niche. i looking forward to see how it develops. David D. (Talk) 15:20, 29 June 2006 (UTC)
6. I am not volunteering to review all existing Wikipedia articles on electron transport chains, oxidative phosphorylation and photosynthesis, much less to edit them, combine them, split them or delete them. I volunteer to split a single, overly-long article into two parts.
- To clarify, i was only suggesting that the context of these two 'new' articles should consider what is already in wikipedia. (See related comment above to point 5) David D. (Talk) 15:20, 29 June 2006 (UTC)
7. I strongly believe that this is not the time or place to revise, modify or otherwise change the article we are proposing to split. The Wikipedia community can be trusted to do this.
Btarski 04:13, 29 June 2006 (UTC)
The proposed split
[edit]In the interest of maintaining the momentum, the proposed split presented in full. The proposed new articles are Electron transport chain and Photophosphorylation. Their respective Tables of Contents will, hopefully, make more sense than the Table of the provisional 41 kilobyte article.
Some the material has been duplicated, and some of the material has been rearranged. New material (which is intended to summarize the stuff removed from Part A and transferred to Part B, and to properly introduce the new Part B) has been added. Nothing has been deleted.
I will leave it to your judgment whether to proceed with the split at this time. Arcadian has the authority to make it official.
It has been a pleasure to work with you on this issue. Thank you for all the time you took
Btarski 03:20, 1 July 2006 (UTC).
- Although I disagree that I have any special authority in this matter, in my personal opinion I do think that your work looks good, and I encourage you to move forward, and split the second half into Photophosphorylation. --Arcadian 04:38, 1 July 2006 (UTC)
Coupling with oxidative phosphorylation
[edit]The first sentence in the article is "Electron transport chains (also called electron transfer chains) are biochemical reactions that produce ATP". However, if you read the article you probably won't understand how this electron transport chain results in ATP synthesis. This is why i added the coupling with oxidative phosphorylation section. I also added a hint about uncouplers to clarify that in certain cases the electron transport chain doesn't result in ATP synthesis. I read somewhere in this talk page a debate about ATP synthase: whether it is complex V or not. Well, though ATP synthase is responsible for oxidative phosphorylation, it is regarded by many textbooks as complex V. I revised Lehninger. The fact that ATP synthase is also called complex V is mentioned in pages 697 and 708. You can view page 78 in Lippincott's illustrated reviews in biochemistry here(requires a google account). Notice that figure 6.13 contains complex V which is ATP synthase.--Wedian 16:37, 12 July 2006 (UTC)
I was part of the complex V discussion, but I think it is best to leave it as it is for now.
I think that we better stop talking and start writing or we'll have to split the talk page! Rozzychan 18:37, 14 July 2006 (UTC)
Number of ATP produced by NADH and FADH2
[edit]I've read that recent evidence shows that the P:O ratio for NADH is 2,5 and FADH2 is 1,5, instead of the earlier ~3 and ~2.
4/4/2 is the ammount of protons pumped out by the complexes and I've read in manuals (but these manuals use the P:O ratio 3 for NADH) that each complex can "synthesise" 1 ATP by it's own (by coupling to ox phospho of course) by means of experiments which use inhibitors and artificial electron carriers.
My question is if the first 2 can produce 1 each by pumping out 4 H+ how can then complex 4 also synthesise 1 ATP by just pumping out 2 protons? Mathematically it should be half a ATP because half the ammount of protons are pumped out since the proton gradient is the determining factor for ATP synthesis. Then 1+1+0,5 = 2,5 ? Is my logic right, is the new "2,5 ratio" not viedly accepted yet or is the 2,5 ratio due to other factors?
I have a biochem test soon so if someone could answer soon it would be appreciated.
Expanding on a related topic
[edit]I think the community would benefit if someone elaborated on the reverse electron flow briefly mentioned (perhaps creating a new page).
romunov 18:23, 12 January 2007 (UTC)
Intelligent design
[edit]Anonymous User 68.210.121.170 wants to replace
- In principle, it is possible to extrapolate backwards from primitive electron transport chains to the origins of life itself. Such scenarios become increasingly plausible as more details become available.
with the statement
- With the continuing advances in biochemistry as it relates to microbiological systems, it is becoming more difficult to extrapolate backwards from primitive electron transport chains to the origins of life itself, suggesting that a “template” was provided for life to evolve from.
This is an unscientific statement. 68.210.121.170 apparently believes that a “template” for life was “provided” by an intelligent designer.
Anonymous User 69.134.72.189 revises this to read
- . . . it is becoming more difficult to extrapolate backwards from primitive electron transport chains to the origins of life itself due to multiple systems of irreducible complexity.
“Irreducible complexity” is simply “intelligent design” under a different guise. It has no place in an article on biology. It is inappropriate to use this article as a forum for personal religious beliefs. These users violate Wikipedia’s neutral point of view policy. Btarski 18:09, 2 June 2007 (UTC)
- On the contrary, the term "irreducible complexity" may be used by intelligent design proponents but it has nothing to do with religion. There is nothing mentioned about anything metaphysical or a reference to a god or a designer or a creator. Your assumption that irreducible complexity is synonymous with intelligent design is absolutely incorrect. To dismiss irreducible complexity for this reason is more unscientific than full blown creationism. It is unscientific not to consider this as a possible alternative explanation for the complexity of basic biochemical life.
- When one remembers that the Earth did not always have an oxygen atmosphere, and that said oxygen atmosphere is in fact extremely toxic pollution to all life forms not evolved to dispose of free oxygen inside the cell, its a lot simpler to visualize how something so seemingly complex could be an emergent phenomenon. Anaerobes exist even today, as do mixed-type cells. Why is it so hard to accept that almost any development that enabled a cell to take a lethal waste product and dispose of it in a manner to get more energy out of food at the same time would provide a huge advantage? In fact, even such a huge advantage that billions of years later only the very most efficient designs remained. That, to me, doesn't seem like miracle. In fact, it seems like inevitable! With regards to creationism, there is simply no place for the heresy of intelligent design theory in the Christian bible. There is just no good way to bend the mythology present in the Old Testament to mesh with any of the modern ID proposals I have seen. This I find disturbing, because it suggests that much of the ID funding in the USA is from people seeking only to throw sand in the gears of science. However, it has recently come to my attention that ID theory and how eastern religion may mesh with it at least bears looking into, from a theological perspective. I am curious to learn more about this. Zaphraud 10:10, 4 August 2007 (UTC)
Unwarranted and Unscientific Speculation
[edit]The statement "It is possible to make an educated guess as to the type of electron transport processes that must have preceded the evolution of eukarya, bacteria and archaea as separate domains of life" has no place in this scientific discussion. Every scientist should know that guesswork and preemptive speculation as to what evidence you might or might not find is heresy to the scientific method. If there is no observable evidence for that certain claim, as in an organism found with that type of system, then it has no place in a scientific discussion. Don't do what the creationists do and make far reaching speculative assumptions based on evidence not seen.
Oxygen is reduced to water?
[edit]"Electrons from these donors are passed through an electron transport chain to oxygen, which is reduced to water." was stated under the "Electron transport chains in mitochondria" section. What? So electrons are passed to oxygen, O, and is somehow reduced(made smaller) to water, H2O. This doesn't make sense to me how a molecule getting bigger, is considered reduced. I know it probably made sense if I knew the details, but it doesn't include any details on it. Thanks in advance. --24.27.141.96 (talk) 22:04, 11 December 2007 (UTC)
- Good point. Would a wiki-link to redox be enough to clarify the usage of the word? David D. (Talk) 03:18, 14 December 2007 (UTC)
Source of energy for "all" life?
[edit]The article states in the Summary section at the end that "Electron transport chains are the source of energy for all known forms of life." While the ETC is a major component of most forms, I know that certain forms use only glycolysis as their energy source, anaerobic organisms in particular. While I would have normally just revise the offending material (and explain myself in the edit summary), I am merely an editor, with basic organic chemistry knowledge, who happened to stumble across this article. So I would like regular contributors to this article, who hopefully have a firmer grasp of organic chemistry than I do, to review the edit I made in case it too is incorrect. Typer525 Talk 04:16, 4 April 2008 (UTC)
I agree that to say that the ETC is "the" source of all life is misleading. As you mentioned, ATP is generated during glycolysis by phosphoglycerate kinase and pyruvate kinase. However, while it is true that some organisms are obligate anaerobes, as far as I know, all organisms have an ETC (although yeasts often prefer fermentation???). The obligate anaerobes use a different final electron acceptor than O2.
I think the article said it well in the Background section: "A small amount of ATP is available from substrate-level phosphorylation (for example, in glycolysis). Some organisms can obtain ATP exclusively by fermentation. In most organisms, however, the majority of ATP is generated by electron transport chains."
Maybe the "source of energy for all life" comment comes from the fact the glucose used in glycolysis comes from a photosynthetic electron transport chain. However, some bacteria oxidize inorganic molecules for energy (and then ship the generated reducing equivalents to their ETC.) Anyway, I guess the truth of this statement comes down to whether there are non-glucose oxidizing bacteria that solely use fermentation instead of an ETC to derive energy. I guess most agree that such organisms existed anceintly, but I don't think there are any extant organisms like this. As bacteria are not my specialty, I really don't know...
Either way, I think this statement is misleading, as most people will assume it means that ETCs are the ONLY way of producing ATP, which is incorrect.
Mahasanti (talk) 18:53, 20 June 2008 (UTC)
The above confusion comes from no one recognising that both plants and humans use photosynthesis. The 'two levels' (day/night) of plant power production and the oxymoronic 'dark photosynthesis' processes attempting to explain it, are exactly the same as all of you trying to explain in chemical (physical) terms what Peter Mitchell won the Nobel Prize for way back in 1978 when he showed that an electron flow across cellular lamellae signalled the onset of (plant) cellular power production. Human mitochondria contain lamellae and we know from our optic system that their function is to absorb light energy and create 'electrical excitation'. Plants and humans have low-level electromagnetic fields surrounding and permeating their physical form (as do all living things), and if you can ignore the West's aversion of Eastern medical/biological doctrines for just one moment (while you think and realise how perfectly and easily this explains everything) then Western research might progress at last. By considering that light energy is a factor in ALL of our 'unexplained phenomena', we can understand them. In the very near future the word 'aura', and the link between light energy and human biological processes (via our body's immense and STRATEGIC distribution of porphyrin compounds, cytochromes, bilichromes, carotenoids, and physical structures 'resonant' to specific light frequencies) will be well-established. I am in the process of freely releasing information into the public arena that will finally and irrefutably unite the East's and the West's 'Hippocratic Ideals', and mark the beginning of a far better undestanding of life processes. Bratkins41 (talk) 04:33, 6 May 2010 (UTC)
Where are the proteins for the electron transport chain located in prokaryotes since they don't have mitochondria? —Preceding unsigned comment added by 75.34.80.167 (talk) 22:42, 17 December 2008 (UTC)
- Inner cell membrane usually. Narayanese (talk) 20:30, 18 December 2008 (UTC)
I am a new user of Wikipedia and all the talk about ETC shows that no progress has been made since Peter Mitchell won the Nobel Prize for showing the ETC signalled the onset of cellular power production way back in 1978. Why not look for what causes the electron flow instead of looking among reactive (physical) processes for causative factors? The intense absorption of low level light energy (~5J/cm/cm) by the porphyrin-laden mitochondrial lamellae creates the ETC and power for all the physical reactions you observe. Remember lamellae perform this function in our optical system and surely mitochondrial lamellae don't somehow 'lose' this capability. It has been the West's xenophobia (of Eastern doctrine) that has stopped everyone from considering the possibility that auric light energy is in fact 'the missing link' the West needs to explain all of our 'unknown phenomena'. My name is Richard Bruce Atkins and I am willing to enlighten anyone who contacts me at bruce_atkins@hotmail.com
- Auric light energy? I'm afraid you chanced upon the wrong entry for your comments. This article deals with the features and mechanisms of the electron transport chain that have been uncovered by scientific methods. Researchers all over the world (i.e., in the east and west) are engaged in scientific research, most of whom are not particularly phobic of "doctrines" (if perhaps sceptical of some of them). Auras along with fairies and pixies are fictitious objects or beings not to be confused with observable reality. So your promise to "enlighten" anyone about your theories smacks of sad irony, considering that the Enlightenment provided the spark that ignited modern scientific research. It helped do away with a great many superstitions and plain humbug, such as, for example, bloodletting, phlogiston, and witchcraft that have held back rational reasoning and progress for a long time. But, as your comment suggests, this doesn't stop some people trying to reset the clock. Malljaja (talk) 21:25, 29 April 2010 (UTC)
Malljaja's comment precisely shows the point I tried to make. The 'knee-jerk' reaction to words like 'aura', and the immediate 'rejection' of everything else that was said is exactly why our research is faltering. The 'features and mechanisms' of the ETC mentioned by everyone since 1978 are REACTIVE and subsequent to light absorption by porphyrin-laden mitochondrial lamellae which causes the electron flow in our eyes, in plant cells, and in OUR cells. In future, for 'aura' please read "the well-established electromagnetic field that is visible with various radiological equipments (and by some people) and is the basis of all Eastern medical practises, but whose value has 'yet to be determined'. If you look at Dr. Liisa Laakso's work on the injection of LLLT (low-level light therapy) at miofascial trigger points for pain relief, you will find that she and her contemporaries (Spiros et al), have firmly established the existence of 'an unknown pathway' operating without any association to the neural or vascular systems. Since I first met Dr. Laakso (last millenium), I have devoted myself to showing how that (quantum) pathway works, and have done so! I have a terminal illness and am 'arranging' for my findings to be (freely) released into the public arena for the betterment of all. You are about to be surprised, Malljaja. Please try and be objective in future, look for the good (or informative) in all things and you will learn more that way. Bratkins41 (talk) 06:55, 1 May 2010 (UTC)
Discovery of ETC process?
[edit]It would be nice if some of our biologists here would add a section on how the ETC was discovered and by whom, when, etc. I remember being in school not so terribly long ago and learning that the Krebs cycle generated the ATP for the body, but it appears the vast majority of ATP derives from the ETC. A little history would not be unwelcome to the general reader, I think. --64.66.85.99 (talk) 13:13, 27 October 2010 (UTC)
- Krebs cycle as such does not produce any ATP. However, it produces sufficient NADH that are used for the ATP synthesis. The one step of the reaction, succinate dehydrogenase, is however directly linked to the ETC. ATP production is also distinct, not really a part of ETC and is explained by the chemiosmotic theory. chami 19:24, 30 May 2012 (UTC) — Preceding unsigned comment added by Ck.mitra (talk • contribs)
- The Krebs cycle does produce one GTP directly. AlphaHelical (talk) 14:22, 11 May 2013 (UTC)
Mechanical work
[edit]The article says
If protons flow back through the membrane, they enable mechanical work, such as rotating bacterial flagella. ATP synthase, an enzyme highly conserved among all domains of life, converts this mechanical work into chemical energy by producing ATP"
This seems confused. It seems to imply that mechanical work, such as rotating flagella, is part of ordinary ATP synthesis using a proton gradient. AlphaHelical (talk) 19:26, 20 April 2013 (UTC)
summary
[edit]I'm removing this as the last paragraph of the article:
The coupling of thermodynamically favorable to thermodynamically unfavorable biochemical reactions by biological macromolecules is an example of an emergent property – a property that could not have been predicted, even given full knowledge of the primitive geochemical systems from which these macromolecules evolved.[original research?] It is an open question whether such emergent properties evolve only by chance, or whether they necessarily evolve in any large biogeochemical system, given the underlying laws of physics.[citation needed]
It's out of place, speculative, and uninformative.
173.25.54.191 (talk) 05:00, 30 June 2013 (UTC)
Other source disagrees with "NADH → NAD+ + H+".
[edit]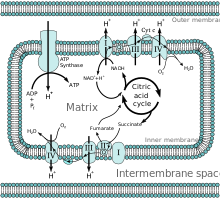
The current illustration (included here) indicates that the complex I reaction is NADH → NAD+ + H+. However, Biochemistry by Harvey and Ferrier says NADH + H+ → NAD+. Is there an error somewhere or am I misunderstanding things? —Bromskloss (talk) 22:14, 14 July 2013 (UTC)
- I thought about the same thing. Several other sources says that NADH + H+ → NAD+. I think 2 electrons are moved from NADH while the H+ from NADH and the free H+ goes into Coenzyme Q. 94.191.137.37 (talk) 17:48, 1 February 2023 (UTC)
Assessment comment
[edit]The comment(s) below were originally left at Talk:Electron transport chain/Comments, and are posted here for posterity. Following several discussions in past years, these subpages are now deprecated. The comments may be irrelevant or outdated; if so, please feel free to remove this section.
| Changed rating to "top" as this is high school/SAT biology content and an important mechanism in cell biology, both for respiration as well as photosynthesis. - tameeria 21:48, 18 February 2007 (UTC) |
Last edited at 21:48, 18 February 2007 (UTC). Substituted at 14:19, 29 April 2016 (UTC)
Out of place file
[edit]Currently there is an animated gif on the page that doesn't really fit the tone/style of the page, isn't really clear what information it conveys, and isn't encyclopedic. This file is also used exclusively on this page. I don't want to remove it without consulting the community. The file in question is

Dietcoke3.14 (talk) 19:13, 15 August 2019 (UTC)
Removed the image Elliot321 (talk | contribs) 08:36, 9 November 2019 (UTC)
Proposed edits
[edit]Hi I am Toby. A 3rd year biochemistry undergraduate at Imperial College London. The page, as pointed out by teachers and professors, is a little confusing with link cycles and a lack of clarity. Also, the page lacks a common theme and some good images for a few sections. Overall it is good but I believe it could be improved in some areas. Outlined below are some proposed changes on the general layout, content and diagrams of the page.
Examples of potential changes to article.
[edit]1. More accessible lead.
The article is so widely used due to being a key topic throughout all levels of teaching. It is classed as a Level 5 vital article by wikipedia. This may be GCSE students to Professors. At the moment the lead section assumes a large amount of knowledge already. Perhaps this is okay for an undergrad but due to the importance of the topic at a lower level than this I believe the lead should be more accessible. One example of this is in the second sentence of the article. It may not be necessary to include information on how ATP stores energy with no further link about strained bonds. "This creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP), a molecule that stores energy chemically in the form of highly strained bonds." A suitable change may involve moving this information to the ATP Synthase section and either linking to the adequate article to describe strained bonds and energy storage or addition of sufficient explanation for clarity.
2. Diagrams
Throughout the article there is a lack of consistent diagrams. One example of where a diagram could be produced for a clearer view of a process is the "Generalised electron transport chain in bacteria." Also, if there may be a new addition in these proposed edits that requires a diagram. One should be produced for this in accordance to others for consistency.
3. New additions
A few new additions to the page could be considered. A large topic only very briefly mentioned in the article is reverse electron flow in prokaryotic electron transport chains. This could have a new subsection under the bacterial heading.
4.Organisation
The article feels as if it could do with some cleaning. Some sentences in the lead article add don't add value and distract from key information. Also, in the later sections there is some ambiguity in what is stated, even if it is fundamentally correct. For example, "A small percentage of electrons do not complete the whole series and instead directly leak to oxygen, resulting in the formation of the free-radical superoxide, a highly reactive molecule that contributes to oxidative stress and has been implicated in a number of diseases and ageing." This statement is repeated in the lead. Either of these could be removed. In addition, the summary, at the end of the article, does not add value and likely would not be read by many. This could be removed as is not a general feature of good wikipedia articles. The need for an ending summary is negated by the lead.
If there are any reservations about any proposed changes please let me know and I'll gladly take them onboard. Tobyfensome (talk) 17:39, 16 April 2020 (UTC)
- B-Class level-5 vital articles
- Wikipedia level-5 vital articles in Biology and health sciences
- B-Class vital articles in Biology and health sciences
- B-Class Physiology articles
- Mid-importance Physiology articles
- Physiology articles about respiratory physiology
- WikiProject Physiology articles
- B-Class Molecular Biology articles
- Unknown-importance Molecular Biology articles
- B-Class MCB articles
- Top-importance MCB articles
- WikiProject Molecular and Cellular Biology articles
- All WikiProject Molecular Biology pages


