Caesium hexafluorocuprate is the inorganic compound with the chemical formula Cs2CuF6. It is a red solid that degrades upon contact with water. It was first prepared be heating CsCuCl
3 and caesium fluoride at 410°C under 350 atmospheres of fluorine:[2]
| |
| Names | |
|---|---|
| Other names
Cesium hexafluorocuprate; Dicesium hexafluorocuprate
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Cs2CuF6 | |
| Molar mass | 443.35 g/mol |
| Appearance | Red orange solid[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
- 2 CsCuCl3 + 2 CsF + 5 F2 → 2 Cs2CuF6 + 3 Cl2
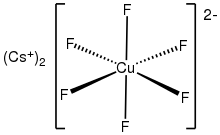
The anion [CuF6]2- is a rare example of a copper(IV) complex. In terms of its electronic structure, the anion has a low-spin d7 configuration. It is thus susceptible to Jahn-Teller distortion.[3]
References
edit- ^ Jane E. Macintyre, ed. (1992). Dictionary of Inorganic Compounds. CRC Press. p. 3100. ISBN 9780412301209.
- ^ Harnischmacher, Werner; Hoppe, Rudolf (1973). "Tetravalent Copper: Cs2[CuF6]". Angewandte Chemie International Edition in English. 12 (7): 582–583. doi:10.1002/anie.197305822.
- ^ Grannec, J.; Tressaud, A.; Hagenmuller, P. (1984). "Some physical properties of d-transition metal fluorides in unusual oxidation states". Journal of Fluorine Chemistry. 25 (1): 83–90. Bibcode:1984JFluC..25...83G. doi:10.1016/S0022-1139(00)81198-7.
Further reading
edit- Müller, Bernd G. (1987). "Fluoride mit Kupfer, Silber, Gold und Palladium". Angewandte Chemie. 99 (11): 1120–1135. Bibcode:1987AngCh..99.1120M. doi:10.1002/ange.19870991105.
- Popova, T. V.; Aksenova, N. V. (2003). "Complexes of Copper in Unstable Oxidation States". Russian Journal of Coordination Chemistry. 29 (11): 743. doi:10.1023/B:RUCO.0000003432.39025.cc. S2CID 93464243.