Solvent DefinitionA solvent is a substance capable of dissolving other substances (solutes) to produce a solution.  They are mostly in liquid form, but they can also be in solid and gas forms. The word solute referred to here is that particle dissolved into a great substance. The attractive forces in the solvent and solute are equal to those developed in the solution. Common examples of solvents include a brass saxophone, a filled tooth cavity, and water. A solution is a composition of a homogeneous mixture of substances. The term solution is also interpreted as all the substances being uniformly mixed. For example, your initial sip of tea will be the same as your last sip. A solution is composed of solute (a substance that is being dissolved) and solvent (it is the substance that is present in the largest quantity in which the solute is going to be dissolved) Solute + Solvent = Solution List of Common SolventsSome of the common solvents that are used frequently are:
Classifications of Solvents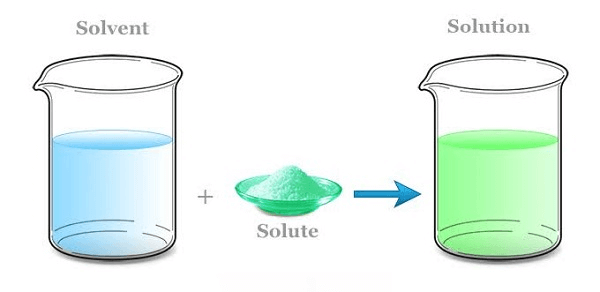 The two major types of solvents include Organic and Inorganic Solvents. The inorganic substance does not possess carbon elements. Some of the most common solvents include liquid Ammonia and water. Organic Solvents comprise glycol ethers composed of carbon and oxygen in the composition and alcohol. They have mainly classified into two categories: Polar and Non-Polar Solvents. There is a unique case of Mercury where the solution is called amalgam. The dielectric constant of the solvents gives solvent polarity. 1. Polar SolventIt is a solvent form containing dipole moments and huge partial charges. The bond present between the atoms are very different but quantifiable electronegativities. A polar solvent can polar compounds and ions. They are strong polar molecules and develop hydrogen bonding at the interface form. Polar solvents operate by disintegrating the covalent bond of the solute and inducing the ionization of the solute. Polar solvents are widely used in drug delivery systems. Examples of the polar solvent include sugar ketones, alcohols, and aldehydes compounds containing -OH groups are general solutes. 2. Non-Polar SolventsIt contains minimal or no dipolar character. They utilize induced dipole-induced dipole interactions for the dissolving of pertinent solutes. Non-polar solvents possess dielectric constant between 1 and 20 and consist of chloroform, oils, and carbon tetrachloride. The solute dissolved in the non-polar solvents includes fatty acids, fats, and oils. 3. Aprotic SolventsThese solvents do not emit protons but may behave as a simple solvents, where polarity may be calculated by the dielectric constant. They may behave as a proton acceptor, also known as Aprotic Basic. Aprotic solvents are polar compounds of liquid that possess no dissociable atoms of hydrogen. Some of the bonds absent in such solvents include O-H and N-H. Groups that are absent from the aprotic solvents include the amine group (-N and hydroxyl groups (-OH). They are not able to develop hydrogen bonds. Aprotic solvent share with protic solvents the ability to ion dissolving. It has a deficiency of acidic hydrogen in these solvents; therefore, it does not see a major release of hydrogen ions. Polar aprotic solvents possess a dielectric constant that is moderate or minimal. A moderate polarity is observed in such solvents. 4. Protic SolventsIt is composed of molecules that may act as donors of hydrogen bonds. Carboxylic acid, water, and alcohol are examples of protic solvents. Aprotic solvents are substances that cannot act as donors to hydrogen bonds. Hexane, ether, and methylene chloride are examples of aprotic solvents. The general formula ROH describes the compound that is a polar protic solvent. The polarity of the polar protic solvent happens from the O-H bond dipole. Merged with the small size of the hydrogen atoms, the big difference in the oxygen and hydrogen electronegativity causes the isolation of molecules of OH group compounds that do not. Properties of Universal Solvent: WaterIt is a polar and protic Solvent and is denoted with the formula . It. is capable of dissolving a wide variety of materials into it. Therefore, it is considered a better solvent than other liquids and a universal solvent. Water is very significant for the living organism that is present on the earth. Water helps in the transportation of crucial important minerals and nutrients in the Body. Some Other SolventsAcetone Acetone can dissolve both compounds: Polar and Non- Polar compounds. Therefore, it is regarded as a good solvent because others can dissolve only one, either a non-polar or polar compound. Since Acetone is a miscible material, it is a strong solvent. It presents a chance to fuse some quantity of water into it. It has been used in numerous significant organic reactions. The usage of Acetone becomes important in the Jones Oxidation reaction. It is a crucial name reaction that turns secondary alcohols into ketones. It should be remembered that this compound does not give azeotrope with water. Ethanol It is regarded as a very versatile solvent. The solvent gives miscible mixtures and provides organic solvents like carbon tetrachloride, diethyl ether, ethylene glycol, toluene, benzene, pyridine, chloroform, and Acetone. It is miscible with aliphatic hydrocarbons; some are hexane and pentane. Compounds miscible with ethanol are tetrachlorethylene (An Aliphatic chloride). Frequently Asked QuestionsQuestion: What polar solvents dissolve? Answer: In general, polar solvents dissolve polar solutes, and non-polar solvents dissolve non-polar solute. This principle is termed "Like dissolves like ."Water is a universal solvent because so many liquids are dissolved in water. Question: How is water a polar solvent? Answer: Water is a polar solvent due to the polarity present of its ions; water meets differently with polar and charged substances compared to non-polar material. Water molecules contain the polar character and comprise partial hydrogen positive charge, partial o sub 2negative charge, and a twisted overall shape. Next TopicSpectroscopy Definition |