PH scalePH scale determines the acidic, neutral, or basic nature of the substance, material, or solutions. pH stands for power of Hydrogen. It means that the pH is determined on basis of the hydrogen ions present in the solution. The range of pH scale lies between 0 and 14. The pH of 7 is considered neutral. The best example of a neutral substance is water.  The pH scale displays the range of acidic, neutral, and alkaline solutions, as shown above. Here, we will discuss the following topics: What is the pH scale? Significance of acidic, neutral, and basic Substances with different pH values Litmus paper Classification of soil and its pH ranges pH value in the living system Applications of the pH scale What is the pH scale?In chemistry, pH refers to power and hydrogen. It is used to determine the nature of the aqueous solution (acidic or basic). pH scale displays different colors for the range from 0 to 14. It means it shows 15 different colors that signify the nature of a substance. The pH scale is shown below:  pH below 7 is acidic, while pH above 7 is basic. As the value from 7 decreases, the acidic nature of the substance increases. Similarly, as the value from 7 increases, the basic nature of the substance increases. We can also say that pH 0 is highly acidic, and pH 14 is a strong alkali. Alkali is the basic salt of alkali metal or earth metal. It is also called a base dissolved in water. pH is generally measured at room temperature (25 degrees Celsius). The value of the pH scale can also exist less than 0 or more than 14, but those substances are powerful acids and bases. Significance of acidic, neutral, and basicThere are three substances that lie on the pH scale, namely, acidic, neutral, and base. Acidic and bases are the two ends on the pH scale. Most of the substances come under these two categories. Let' discuss the significance of these three substances. AcidicAny substance or material below pH 7 is considered as acidic. Water is a neutral substance, while rainwater is acidic. It is due to the dissolve acids in the rainwater from the atmosphere, such as carbon dioxide. Why acids have lower pH values? The acids are classified based on the presence of hydrogen ions. It easily provides hydrogen ions in the water. The stronger the acid, the more it donates the hydrogen ions (H+). It means that the higher concentration of hydrogen ions increases the acidic nature of the solution. Hence, acids have lower pH values. Properties The properties of acids are as follows:
NeutralThe substance with pH 7 is considered neutral. The most common example of a neutral substance is water. It is neither acidic nor basic. Distilled water is also a neutral substance. But, pure distilled water can sometimes be acidic as it absorbs carbon dioxide from the atmosphere. Properties
BaseSubstances with the pH above 7 are considered as bases. The bases produce hydroxide ions (OH-) when dissolved in water. Why bases have a pH range higher than 7? Bases are the substances that provide hydroxide ions, which are responsible for the lower pH value. It readily donates these hydroxide ions. When the hydroxide ions combine with hydrogen ions, it forms water, as shown in the below equation: 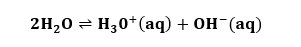 The formation of water increases the pH value. Thus, we can say that strong bases dissociate entirely in water. Hence, bases have higher pH ranges. Properties The properties of bases are as follows:
Substances with different pH valuesLet's discuss some popular substances with their pH scale. We will discuss the reason of the substance holding the corresponding pH value.
Litmus paperIt is a form of paper that is used as a pH indicator. It tests the acidic or basic nature of the aqueous solution. It is simply paper and can be safely used at home. It is also used for experimental purposes in the laboratories. Types of litmus paper There are three types of litmus paper. Yellow litmus paper Red litmus paper Blue litmus paper Yellow litmus paper Yellow litmus paper turns red when dipped in the acidic solution. Similarly, it turns blue when dipped in the basic solution. If the yellow litmus paper turns green or violet, it means that the corresponding solution is neutral. It is shown below:  Red litmus paper Red litmus paper remains red when dipped in the acidic solution and turns blue when dipped in the basic solution. It is shown below:  It does not appear exact red in color. It is generally used to test the basic or alkaline solution. Blue litmus paper Blue litmus paper turns red when dipped in the acidic solution and remains blue when dipped in the basic solution. It is shown below:  Blue litmus paper is generally used to test the presence of acidic solution. Otherwise, it remains blue. Thus, we can conclude that yellow litmus paper can test the acidic, neutral, and basic solutions. While the red litmus paper is used to test the presence of basic solutions, blue litmus paper is used to test only acidic solutions. How does it work?When the litmus paper is dipped in the aqueous solution, it changes its color. It means, The color of litmus paper, when dipped in acidic solution is: Red The color of litmus paper, when dipped in basic solution is: Blue The litmus paper can also indicate the presence of a neutral solution. It means it turns green or purple when dipped in the aqueous solution comprising of neutral substances. Calculation of pH valueThe pH is calculated as the logarithmic of the hydrogen ion concentration in the solution. It is given by:  Let's consider an example. Example: Find the pH of the solution that has the hydrogen ion activity of 5 x 10^-6? Solution: The pH can be calculated as: 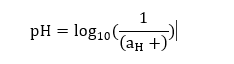 pH = log10 (1 / 5 x 10^-6) pH = log10 (2 x 10^5) pH = 5.3 Classification of soil and its pH rangesThere are various types of soils with different pH ranges. The classification of soil based on the pH ranges helps in the proper cultivation of different types of crops. The ideal pH range for soil lies between 6 and 7. The soils are also classified as acidic and basic. The pH value of acidic soil lies below 7, while pH value of alkaline soil lies above 7. According to the pH ranges, the soils are classified as:
The above table displays the types of soil, its pH values, and the example of the plants suitable to grow in that pH range. Most of the crops are ideal for growing in the pH range of 5 - 7.5. It includes apple, turnip, pumpkin, broccoli, cabbage, lettuce, potato, wheat, corn, etc. The high acidic nature of the soil leads to poor crop cultivation. Effect of addition of fertilizers to the soilThe addition of fertilizers to the soil results in a variation in pH. It also affects the number of nutrients present in the oil. It is generally added to increase the production of crops. Some fertilizers can even harden the soil and lead to various disadvantages, such as water pollution, decreasing soil fertility, etc. For example, Phosphoric acid: It increases the acidic nature of the soil. It also creates an adverse impact on the soil, such as poor plant growth. The phosphorus helps in the transfer of energy in the plant. Ammonia-based fertilizers: It is also added to lower the pH value of the soil. It also leads create direct damage to the leaves. Ammonia-based fertilizers produce fewer greenhouse gases as compared to other fertilizers. The nitrogen in ammonia also helps in plant growth. pH value in the living systemThe pH is the most important factor for survival on the Earth. The most important task of the human body is food digestion. The acid present in the stomach has a pH between 1.5 and 3. It helps in the breakdown of the food and enzymes. It also helps to kill harmful viruses and bacteria. Let's the pH value associated with different parts of the body. It is shown in the below table.
The pH of blood flowing in our body lies between the range 7.3 and 7.4. The pH at a defined scale is essential for the proper functioning of the body. Improper balance in the pH value can lead to various diseases. Let's discuss the pH imbalance in the human body in detail. pH Imbalance in the human bodyThe lungs and kidneys of the human body play an essential role in balancing pH. The acid-base balance is essential for the proper functioning of the body. Such imbalance requires medical attention cannot be quickly resolved with dietary changes.
Applications of the pH scale There are various applications of pH scales ranging from school practical to industrial use. Let's discuss some most common applications. MedicineThe medicines are created to treat the acidity in stomach, which has a pH between 1.5 and 3.0. It can cause heart burn, indigestion, and imbalance in the body's functioning. The medicine works as an antacid to lower the amount of acidity. Water treatmentA pH scale is used to measure the pH of the water. The water is basic if it contains more hydroxide ions. Similarly, the water is acidic if it contains more free hydrogen ions. It helps in determining the chemical nature of the water and removes further impurities. ChemistryIn chemistry, pH helps also helps to determine the acidic and basic nature of the aqueous solutions. It is also used in laboratories to perform experiments. AgronomyAgronomy is defined as the study of soil management. The ph scale is used to check the acid or basic nature of the soil. It helps in determining the optimal crop range for suitable cultivation. Hence, it is quite popular in agriculture. Industrial processesThe pH scale is also used to test the acid or basic nature of the food, products, etc. It helps to determine the taste and quality of different food products. For example, citric acid is added to the liquid package product as a preservative. Next TopicHow to Start a Blog |