WO2023067246A1 - A coating composition, method for preparing such composition, a method of coating, a coated sheet and uses of the coating - Google Patents
A coating composition, method for preparing such composition, a method of coating, a coated sheet and uses of the coating Download PDFInfo
- Publication number
- WO2023067246A1 WO2023067246A1 PCT/FI2022/050697 FI2022050697W WO2023067246A1 WO 2023067246 A1 WO2023067246 A1 WO 2023067246A1 FI 2022050697 W FI2022050697 W FI 2022050697W WO 2023067246 A1 WO2023067246 A1 WO 2023067246A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cellulose
- coating
- water
- fibrous web
- coating composition
- Prior art date
Links
- 238000000576 coating method Methods 0.000 title claims abstract description 110
- 239000011248 coating agent Substances 0.000 title claims abstract description 96
- 239000008199 coating composition Substances 0.000 title claims abstract description 77
- 238000000034 method Methods 0.000 title claims abstract description 58
- 239000000203 mixture Substances 0.000 title claims abstract description 50
- 229920002678 cellulose Polymers 0.000 claims abstract description 91
- 239000001913 cellulose Substances 0.000 claims abstract description 90
- 102000004190 Enzymes Human genes 0.000 claims abstract description 64
- 108090000790 Enzymes Proteins 0.000 claims abstract description 64
- 239000000758 substrate Substances 0.000 claims abstract description 38
- 235000010980 cellulose Nutrition 0.000 claims description 89
- 229940088598 enzyme Drugs 0.000 claims description 62
- 230000004888 barrier function Effects 0.000 claims description 60
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 claims description 50
- 239000004354 Hydroxyethyl cellulose Substances 0.000 claims description 50
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 claims description 50
- 239000007787 solid Substances 0.000 claims description 45
- 108010059892 Cellulase Proteins 0.000 claims description 40
- 238000012360 testing method Methods 0.000 claims description 40
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 35
- 230000007515 enzymatic degradation Effects 0.000 claims description 26
- 229920002134 Carboxymethyl cellulose Polymers 0.000 claims description 22
- 229920000609 methyl cellulose Polymers 0.000 claims description 22
- 239000001923 methylcellulose Substances 0.000 claims description 22
- 235000010981 methylcellulose Nutrition 0.000 claims description 22
- 239000004519 grease Substances 0.000 claims description 20
- 239000002245 particle Substances 0.000 claims description 19
- 239000000049 pigment Substances 0.000 claims description 18
- 239000001768 carboxy methyl cellulose Substances 0.000 claims description 17
- 235000010948 carboxy methyl cellulose Nutrition 0.000 claims description 17
- 239000008112 carboxymethyl-cellulose Substances 0.000 claims description 17
- 230000000694 effects Effects 0.000 claims description 17
- 239000000123 paper Substances 0.000 claims description 17
- 229920013820 alkyl cellulose Polymers 0.000 claims description 16
- 239000004014 plasticizer Substances 0.000 claims description 16
- 230000000903 blocking effect Effects 0.000 claims description 15
- 229940106157 cellulase Drugs 0.000 claims description 15
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 14
- 229920001479 Hydroxyethyl methyl cellulose Polymers 0.000 claims description 13
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 claims description 13
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 13
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 claims description 13
- 229920002472 Starch Polymers 0.000 claims description 12
- 239000008107 starch Substances 0.000 claims description 12
- 235000019698 starch Nutrition 0.000 claims description 12
- 239000011230 binding agent Substances 0.000 claims description 11
- 229920003063 hydroxymethyl cellulose Polymers 0.000 claims description 10
- 229940031574 hydroxymethyl cellulose Drugs 0.000 claims description 10
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 claims description 9
- 230000003247 decreasing effect Effects 0.000 claims description 9
- 239000001863 hydroxypropyl cellulose Substances 0.000 claims description 9
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 claims description 9
- 239000000725 suspension Substances 0.000 claims description 9
- 239000003795 chemical substances by application Substances 0.000 claims description 8
- 239000000470 constituent Substances 0.000 claims description 8
- 230000001461 cytolytic effect Effects 0.000 claims description 8
- 230000000593 degrading effect Effects 0.000 claims description 8
- 229920013821 hydroxy alkyl cellulose Polymers 0.000 claims description 8
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims description 8
- 238000002360 preparation method Methods 0.000 claims description 8
- 239000004971 Cross linker Substances 0.000 claims description 7
- 239000002270 dispersing agent Substances 0.000 claims description 7
- 239000000314 lubricant Substances 0.000 claims description 7
- 239000002562 thickening agent Substances 0.000 claims description 7
- 239000007900 aqueous suspension Substances 0.000 claims description 4
- 230000005540 biological transmission Effects 0.000 claims description 4
- -1 hydroxypropyl Chemical group 0.000 claims description 4
- 239000011247 coating layer Substances 0.000 description 23
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 19
- 239000000047 product Substances 0.000 description 19
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 18
- 238000005336 cracking Methods 0.000 description 13
- 108010084185 Cellulases Proteins 0.000 description 11
- 102000005575 Cellulases Human genes 0.000 description 11
- 238000006731 degradation reaction Methods 0.000 description 11
- 230000015556 catabolic process Effects 0.000 description 10
- 239000004006 olive oil Substances 0.000 description 10
- 235000008390 olive oil Nutrition 0.000 description 10
- 238000010186 staining Methods 0.000 description 10
- 239000004372 Polyvinyl alcohol Substances 0.000 description 9
- 229910000019 calcium carbonate Inorganic materials 0.000 description 9
- 229920002451 polyvinyl alcohol Polymers 0.000 description 9
- 238000005259 measurement Methods 0.000 description 7
- 239000002480 mineral oil Substances 0.000 description 7
- 235000010446 mineral oil Nutrition 0.000 description 7
- 239000003921 oil Substances 0.000 description 7
- 235000019198 oils Nutrition 0.000 description 7
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 239000001023 inorganic pigment Substances 0.000 description 6
- 230000035515 penetration Effects 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 238000001542 size-exclusion chromatography Methods 0.000 description 6
- 230000007547 defect Effects 0.000 description 5
- 239000006185 dispersion Substances 0.000 description 5
- 238000004806 packaging method and process Methods 0.000 description 5
- 239000000454 talc Substances 0.000 description 5
- 229910052623 talc Inorganic materials 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 239000000835 fiber Substances 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 4
- 230000007062 hydrolysis Effects 0.000 description 4
- 238000006460 hydrolysis reaction Methods 0.000 description 4
- 229910052500 inorganic mineral Inorganic materials 0.000 description 4
- 239000010410 layer Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 238000010998 test method Methods 0.000 description 4
- 239000005995 Aluminium silicate Substances 0.000 description 3
- 229920000742 Cotton Polymers 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 229920002556 Polyethylene Glycol 300 Polymers 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 235000012211 aluminium silicate Nutrition 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 3
- 230000002255 enzymatic effect Effects 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 239000004744 fabric Substances 0.000 description 3
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- 241000549556 Nanos Species 0.000 description 2
- 239000004373 Pullulan Substances 0.000 description 2
- 229920001218 Pullulan Polymers 0.000 description 2
- 241000499912 Trichoderma reesei Species 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 238000011021 bench scale process Methods 0.000 description 2
- 239000011436 cob Substances 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 238000010227 cup method (microbiological evaluation) Methods 0.000 description 2
- 230000032798 delamination Effects 0.000 description 2
- 239000003480 eluent Substances 0.000 description 2
- 238000006911 enzymatic reaction Methods 0.000 description 2
- 235000013410 fast food Nutrition 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 150000004676 glycans Chemical class 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- CEQFOVLGLXCDCX-WUKNDPDISA-N methyl red Chemical compound C1=CC(N(C)C)=CC=C1\N=N\C1=CC=CC=C1C(O)=O CEQFOVLGLXCDCX-WUKNDPDISA-N 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 235000019423 pullulan Nutrition 0.000 description 2
- 238000004537 pulping Methods 0.000 description 2
- 238000001448 refractive index detection Methods 0.000 description 2
- 238000004062 sedimentation Methods 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 239000003643 water by type Substances 0.000 description 2
- 230000004580 weight loss Effects 0.000 description 2
- VOJUXHHACRXLTD-UHFFFAOYSA-N 1,4-dihydroxy-2-naphthoic acid Chemical compound C1=CC=CC2=C(O)C(C(=O)O)=CC(O)=C21 VOJUXHHACRXLTD-UHFFFAOYSA-N 0.000 description 1
- 241001134630 Acidothermus cellulolyticus Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- GAWIXWVDTYZWAW-UHFFFAOYSA-N C[CH]O Chemical group C[CH]O GAWIXWVDTYZWAW-UHFFFAOYSA-N 0.000 description 1
- QMGYPNKICQJHLN-UHFFFAOYSA-M Carboxymethylcellulose cellulose carboxymethyl ether Chemical compound [Na+].CC([O-])=O.OCC(O)C(O)C(O)C(O)C=O QMGYPNKICQJHLN-UHFFFAOYSA-M 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 229920001503 Glucan Polymers 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 241000845077 Iare Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- UWHCKJMYHZGTIT-UHFFFAOYSA-N Tetraethylene glycol, Natural products OCCOCCOCCOCCO UWHCKJMYHZGTIT-UHFFFAOYSA-N 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- 241000112708 Vates Species 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 235000015173 baked goods and baking mixes Nutrition 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 235000014510 cooky Nutrition 0.000 description 1
- 238000007766 curtain coating Methods 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 235000012489 doughnuts Nutrition 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000002657 fibrous material Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 239000008241 heterogeneous mixture Substances 0.000 description 1
- 230000005661 hydrophobic surface Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 235000019426 modified starch Nutrition 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 239000005022 packaging material Substances 0.000 description 1
- 239000011087 paperboard Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004804 polysaccharides Polymers 0.000 description 1
- 229940088417 precipitated calcium carbonate Drugs 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 235000011890 sandwich Nutrition 0.000 description 1
- 238000004513 sizing Methods 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000007655 standard test method Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 229940014800 succinic anhydride Drugs 0.000 description 1
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/21—Macromolecular organic compounds of natural origin; Derivatives thereof
- D21H17/24—Polysaccharides
- D21H17/25—Cellulose
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/005—Microorganisms or enzymes
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/21—Macromolecular organic compounds of natural origin; Derivatives thereof
- D21H17/24—Polysaccharides
- D21H17/25—Cellulose
- D21H17/26—Ethers thereof
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/20—Macromolecular organic compounds
- D21H17/21—Macromolecular organic compounds of natural origin; Derivatives thereof
- D21H17/24—Polysaccharides
- D21H17/25—Cellulose
- D21H17/27—Esters thereof
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H19/00—Coated paper; Coating material
- D21H19/10—Coatings without pigments
- D21H19/14—Coatings without pigments applied in a form other than the aqueous solution defined in group D21H19/12
- D21H19/34—Coatings without pigments applied in a form other than the aqueous solution defined in group D21H19/12 comprising cellulose or derivatives thereof
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H19/00—Coated paper; Coating material
- D21H19/36—Coatings with pigments
- D21H19/44—Coatings with pigments characterised by the other ingredients, e.g. the binder or dispersing agent
- D21H19/52—Cellulose; Derivatives thereof
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H21/00—Non-fibrous material added to the pulp, characterised by its function, form or properties; Paper-impregnating or coating material, characterised by its function, form or properties
- D21H21/14—Non-fibrous material added to the pulp, characterised by its function, form or properties; Paper-impregnating or coating material, characterised by its function, form or properties characterised by function or properties in or on the paper
- D21H21/16—Sizing or water-repelling agents
Definitions
- the present invention relates to a coating comprising one or more enzymatically hydrolysed water-soluble cellulose derivatives and a method of degrading one or more water-soluble cellulose derivatives or preparing a coating. Furthermore, the present invention relates to a method of preparing a coating composition by enzymatically degrading one or more water-soluble cellulose derivatives.
- a coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web substrate is also within the scope of the present invention as well as a method of coating and use of the coating composition here described.
- Various coatings can be applied on the surface of fibrous material such as paper or board in order to improve their properties.
- Grease, water and/or moisture barrier properties are particularly important for paper and board that are used for products for packaging purposes.
- Coatings applied on the surface of paper or board should provide an effective barrier against leakage from the goods inside the package and/or protect the packaged goods from contamination and/or contact with the surroundings. The barrier requirements are especially stringent for packaging materials used for foodstuff and consumable liquids.
- Coatings for packaging purposes should have good resistance for creasing and folding.
- the coating should not crack when the paper or board is folded into a box or wrapped around the product. Cracking may decrease or even completely destroy the barrier properties of the coating. They should not be tacky and have low blocking tendency but on the other hand heat sealability is desired.
- barrier coatings used for packages should preferably also satisfy the recyclability requirements, for example, they should not disturb the repulping process.
- bio-based components used in coating compositions such as starch, often do not perform well in barriers coatings. Furthermore, the bio-based component should preferably originate from non-food chain sources, which requirement is not fulfilled e.g. by starch and starch derivatives. Cellulose or cellulose derivatives have been tested earlier in barrier applications. Drawbacks of the prior art include e.g. low solids content of the cellulose or cellulose derivatives coating products as dissolved in water and/or too high viscosity. Increased solids content and/or lower viscosity of coating compositions/formulations are needed for more effective and profitable coatings as well as easier coating methods. Barrier coatings with improved performance are desired.
- the present invention provides sustainable and renewable bio-based sources for coating compositions, method for their preparation, coatings made therefrom and coated product.
- Such coatings are easily biodegradable and/or repulpable e.g., together with paper or board.
- Defects of the prior art including but not limited to undesired blocking properties of the paper or board products, low solids content of the cellulose or cellulose derivatives when dissolved in water in coating compositions/ and/or too high viscosity of such compositions, can be overcome with the methods or products of the present invention.
- moisture and/or grease barrier properties of coatings can be improved with the present invention.
- the first aspect of the invention is a coating composition for a sheet like cellulosic fibrous web. Characteristic features of said composition are depicted in claim 1 .
- the second aspect of the invention is method of preparing a coating composition by enzymatically degrading one or more water-soluble cellulose derivatives. Characteristic steps of said method are depicted in claim 8.
- the third aspect in the invention is a coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web substrate, Characteristic features of said fibrous web or a product comprising it are depicted in claim 13.
- the fourth aspect of this invention is a method of coating a sheet like cellulosic fibrous web, wherein the method comprises applying the coating composition of any here described on said fibrous web.
- Enzymatic degradation of water-soluble cellulose derivatives leads to decreased weight average molecular weight MW of said derivatives and thus viscosity of the composition can be decreased, and higher solids content of the composition can be achieved. This enables higher coat weights applied for the barrier coating.
- the coating of the present invention still withstands cracking when creased and/or folded, e.g. on a substrate such as a paper or board.
- Hydrolysed cellulose derivates’ and ‘degraded cellulose derivates’ as used here both refer to cellulose derivates undergone an enzymatic treatment.
- the fifth aspect of the invention is use of the coating composition of the present invention for coating a sheet like cellulosic fibrous web for obtaining water, moisture and/or grease barrier properties.
- Figure 1 shows staining test images for samples coated with barrier formulates 1 -3 in Table 2. Samples with coat weight ca. 8 g/m 2 are shown on the top row and samples with coat weight over 17 g/m 2 are shown on the bottom row.
- Figure 2 shows olive oil grease barrier test images for samples coated with barrier formulates 1 -3.
- the top row reveals samples with coat weight ca. 8 g/m 2 and the bottom row shows samples with coat weight over 17 g/m 2 .
- Figure 3 shows staining test results for all coating formulations with enzymatically hydrolysed HEC.
- Figure 4 shows olive oil test results. On the top row there are samples with coating formulations 1 -3 and on the bottom row with formulations 4- 6.
- Figure 5 shows staining test images for coated samples using enzymatically hydrolysed cellulose derivatives.
- the present invention relates to a coating composition for a sheet like cellulosic fibrous web comprising one or more water-soluble cellulose derivatives selected from the group consisting of alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose, methyl cellulose (MC), and carboxymethyl cellulose hydrolyzed with an enzyme having cellulolytic activity, and having a final weight average molecular weight MW is 20 000 Da - 120 000 Da, such as, 30 000 - 100 000 Da.
- a final weight average molecular weight refers to molecular weight after enzyme hydrolysis.
- Weight average molecular weight MW’s of the enzymatically hydrolysed celluloses of the present invention are clearly above the oligomer limit 1000 Da.
- Enzymatic degradation (hydrolysis) of water-soluble cellulose derivatives leads to decreased weight average molecular weight MW of said derivatives and thus viscosity of the composition can be decreased, and higher solids content of the composition can be achieved. This enables higher coat weights applied for the barrier coating.
- the coating of the present invention still withstands cracking when creased and/or folded, e.g. on a substrate such as a paper or board.
- the cellulase hydrolysis provides the above advantages. With endoglucanase hydrolysis (endoglucanase or endoglucanase enriched enzyme product) more uniform average MW is achieved resulting in better performance and application, e.g., low tendency to stack to the roller.
- At least 20 weight-% of the water-soluble cellulose derivatives of the coating are hydrolysed water-soluble cellulose derivatives.
- water soluble cellulose derivates means that the cellulose deri- vates are individually dispersed throughout the water solution and they do not settle out or separate from the water solvent.
- said coating composition comprises water-soluble cellulose derivatives in amount 20-100 weight-%, preferably 30-90 weight-%, more preferably 40-70 weight-% of the total solids content of the coating composition or the coating.
- the coating composition may comprise water-soluble cellulose derivatives in amount of 20 - 99 weight-%, 30 - 99 weight-%, 40 - 99 weight-%, 50 - 99 weight-%, or 60 - 97 weight-%, calculated from total solids content of the coating composition, respectively.
- the coating composition further comprises one or more constituents) selected from the group comprising a plasticizer, pigment particle, starch, binder, thickener, cross-linker, lubricant, and dispersing agent; or the method of any of the previous claims, wherein one or more agents are selected from the group comprising a plasticizer, inorganic pigment, starch, binder, thickener, crosslinker, lubricant, and dispersing agent.
- one or more constituents to the composition comprising hydrolysed water-soluble cellulose derivatives enables obtaining an optimal coating.
- one or more constituents are selected from the group comprising a plasticizer, inorganic pigment, starch, binder, thickener, cross-linker, lubricant, and dispersing agent.
- the composition of the present invention can comprise one or more constituents selected from the group comprising a plasticizer, pigment particle, starch, binder, thickener, cross-linker, lubricant, and dispersing agent. Respective agents may be contained in a coating composition discussed above.
- the plasticizer may be selected from a group comprising polyol, such as sorbitol, mannitol, ethylene glycol, diethylene glycol, triethylene glycol, tetraethylene glycol, propylene glycol or polyethylene glycol; fatty acids; monosaccharides, ethanolamine; triethanolamine; urea; lecitin and glycerol.
- the plasticizer may be selected from sorbitol, polyethylene glycol or glycerol. Often the incorporation of a plasticizer into a barrier coating makes the barrier coating layer too tacky which may easily lead to blocking problems.
- the tackiness of the barrier coating layer comprising plasticizer is significantly decreased when the barrier coating layer comprises the enzymatically hydrolysed water-soluble cellulose derivatives. This means that it is possible to obtain a coating layer where the cracking and blocking resistance properties can be balanced with the flexibility in order to obtain a coating with optimal properties for the desired purpose.
- the composition may comprise plasticizer in amount of 1 - 50 weight-%, 3 - 40 weight-%, 5 - 30 weight-%, 10 - 20 weight-%, weight-% calculated from total solids content of the composition, respectively.
- the pigment particle can be e.g. an inorganic mineral pigment which may optionally be selected from kaolin, talc, calcium carbonate (e.g. ground calcium carbonate or precipitated calcium carbonate) or any mixture thereof. Calcium carbonate is one preferred embodiment.
- the particle size D50 of the pigment particles or inorganic pigment particles may be ⁇ 5 pm.
- the composition may comprise inorganic mineral particles, wherein at least 45% of the inorganic mineral particles has particle size ⁇ 2 pm. Addition of inorganic mineral pigment may further improve the obtained barrier properties. It has been unexpectedly found that the enzymatically hydrolysed water-soluble cellulose derivatives increase the flexibility of the coating in a manner that allows incorporation of high amounts of inorganic pigment particles to the coating. This may not only improve the barrier properties, but also makes the coating more economic to produce.
- the coating composition may comprise pigment particles or inorganic pigment particles in an amount of 5 - 40 weight-%, 10 - 35 weight- %, or 15 - 30 weight-% from the total dry solids content of the composition, respectively.
- the coating composition (ready for use) may comprise 40 - 90 weight- %, 50 - 90 weight-% or 60 - 90 weight-% of water-soluble cellulose derivatives, 5 - 40 weight-%, 10 - 35 weight-%, or 15 - 30 weight-% of pigment particles or inorganic pigment particles, and 1 - 50 weight-%, 3 - 40 weight-%, 5 - 30 weight-%, 5 - 20 weight-%, or 5 - 10 weight-% of plasticizer, 0 - 30 weight-% binder, such as polyvinyl alcohol (PVA), and up-to 1 weight-% buffering agent and residues of an enzyme composition calculated from the total dry solids content of the coating composition, respectively, the total amount of the components adding up to 100%.
- PVA polyvinyl alcohol
- the coating composition may further comprise an additional coating binder, e.g. polyvinyl alcohol.
- the weight average molecular weight of the polyvinyl alcohol may be ⁇ 100 000 g/mol, such as ⁇ 90 000 g/mol.
- Polyvinyl alcohol that is especially suitable for use as an additional coating binder may have a weight average molecular weight of ⁇ 70 000 g/mol, such as 13 000 - 70 000 g/mol.
- Polyvinyl alcohol may be at least partially hydrolysed.
- Polyvinyl alcohol when used as an additional coating binder, may improve the film formation and both water vapour and mineral oil barrier properties of the coating. Polyvinyl alcohol may also reduce blocking tendency of the obtained coating structure. In one embodiment the composition or coating is free of an additional coating binder such as polyvinyl alcohol.
- the coating composition layer may comprise one or more of the following additive agents: thickener(s), cross-linker(s), lubricant(s), alkyl ketene dimer(s), alkenyl succinic anhydride(s), and dispersing agent(s).
- the molecular weight can be determined by size-exclusion chromatography (SEC) using Viscotek GPCmax TDA 302 SEC equipment. Eluent was 0.1 M NaNOs. Column set consisted of three columns (Waters Ultrahydrogel 2000, 500 and 120) and a guard column. Pullulan standards (Polymer Standards Service) with molecular weights between 342 - 708 000 Da were used for conventional calibration with refractive index detection.
- the coating composition has viscosity of the coating is 50 - 3000 mPas or cP measured at 23 °C using 100 rpm and Brookfield DV-E viscometer.
- the coating composition may be in form of a dispersion, suspension or semi-solid or solid composition comprising enzymatically hydrolysed water-soluble cellulose derivatives and optionally further constituents up to 50 wt.-% calculated from total dry matter.
- the final coating may be in solid form.
- a dispersion refers to a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter.
- a suspension refers to a dispersion of particles sufficiently large for sedimentation. In one embodiment a suspension is a heterogeneous mixture of a fluid that contains solid particles sufficiently large for sedimentation.
- the coating composition may be a coating composition ready to be applied on a cellulosic fibrous web.
- a composition comprising one or more enzyme hydrolysed water-soluble cellulose derivatives having a weight average molecular weight MW 20 000 Da- 120 000 Da, such as 30 000 Da- 100 000 Da or 30 000 Da and 95 000 Da may be in form of dispersion, suspension, semi-solid or solid powder like form. Preferred range depends e.g. on the application. It may be supplemented with a diluent (such as water) before application on a substrate, or a coating layer.
- a diluent such as water
- said one or more water-soluble cellulose derivatives is/are selected from the group comprising or consisting of an alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose; and any combination thereof.
- said one or more water-soluble cellulose derivatives is/are selected from the group comprising or consisting of methyl cellulose (MC), carboxymethyl cellulose (CMC), hydroxymethyl cellulose (HMC), hydroxyethyl cellulose (HEC), methyl hydroxyethyl cellulose (MHEC), hydroxypropyl cellulose (HPC), hydroxypropylmethyl cellulose (HPMC), and any combination thereof.
- MC methyl cellulose
- CMC carboxymethyl cellulose
- HMC hydroxymethyl cellulose
- HEC hydroxyethyl cellulose
- MHEC methyl hydroxyethyl cellulose
- HPMC hydroxypropylmethyl cellulose
- one of said water-soluble cellulose derivatives is HEC or CMC, especially HEC.
- CMC is readily degradable by cellulase degrading enzymes.
- HEC provides a durable coating.
- the present invention also concerns method of preparing a coating composition by enzymatically degrading one or more water-soluble cellulose derivatives, wherein the method comprises
- step (c) may be continued until said water-soluble cellulose derivatives have having a weight average molecular weight MW is 20 000 Da - 120 000 Da, such as, 30 000 -100 000 Da.
- reaction time of item (c) is continued until a desired viscosity in view of dry matter is obtained.
- enzymatic reaction is followed by viscosity measurements.
- the enzyme activity is killed by heating the water-soluble cellulose derivative composition, optionally up to at least 85°C, 90°C, 95°C or 100°C, optionally after obtaining suitable viscosity.
- the coating or the coating composition may comprise inactivated enzymes or residues of enzyme composition.
- the incubation time contact time with enzymes
- activity of the enzyme preparation and reaction conditions are adapted to allow degradation until a desired viscosity is achieved.
- a person skilled in the art is able to select conditions, such as temperature, consistency and pH, conductive for activity of the enzyme preparation(s).
- the enzymatic reaction may be performed in lower consistency (dry matter content) than dry matter of the final coating composition.
- the dry matter content may be increased by removal of water and/or by adding further constituent to the composition after the enzymatic treatment.
- the hydrolysed water-soluble cellulosic derivatives may be subjected for removal of water for obtaining a higher solid content of even drying the hydrolysed water-soluble cellulosic derivatives in order to modify the consistency of the coating or enhancing the transportation.
- a water-soluble cellulose derivative refers to any water-soluble cellulose derivative which is optionally selected from the group comprising or consisting of an alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose and any of their mixture.
- the water-soluble cellulose derivative is selected from the group comprising or consisting of methyl cellulose (MC), carboxymethyl cellulose (CMC), hydroxymethyl cellulose (HMC), hydroxyethyl cellulose (HEC), methyl hydroxyethyl cellulose (MHEC; (hydroxyethyl)methyl cellulose), hydroxypropyl cellulose (HPC), hydroxypropylmethyl cellulose (HPMC; (hydroxypropyl)- methyl cellulose), and any combination or mixture thereof.
- MC methyl cellulose
- CMC carboxymethyl cellulose
- HMC hydroxymethyl cellulose
- HEC hydroxyethyl cellulose
- MHEC methyl hydroxyethyl cellulose
- HPMC hydroxypropylmethyl cellulose
- HPMC hydroxypropylmethyl cellulose
- said derivate is selected from methyl cellulose (MC), carboxymethyl cellulose (CMC), hydroxymethyl cellulose (HMC), hydroxyethyl cellulose (HEC), methyl hydroxyethyl cellulose (MHEC), hydroxypropyl cellulose (HPC), hydroxypropylmethyl cellulose (HPMC). In one embodiment said derivate is selected from HEC and CMC.
- Enzymatically hydrolysed water-soluble cellulose derivatives are able to effectively function as a grease and/or moisture barrier, especially against liquid grease or oil, and sometimes even as a mineral oil barrier. It has been found that when at least one of the hydrolysed water-soluble cellulose derivatives is present in the coating, the coating is more flexible and does not crack so easily at creasing or folding. Moreover, the hydrolysed water-soluble cellulose derivatives provide increased dry solid content for the composition formulation, thus making it easier to apply on the surface of the substrate to be coated and providing for a better film forming properties.
- the present invention it is possible to produce coatings where the content of bio-based components is high while maintaining the essential barrier and crack resistance properties on an excellent, good or at least acceptable level.
- the shares of the coating composition to be prepared are discussed in connection of the coating composition and evidently applicable to the process also.
- the method makes it possible to produce coating compositions with high solid content and a viscosity allowing easy application of the coating composition.
- viscosity of the composition decreases.
- water-soluble cellulose derivatives are further added to the composition during or after allowing the enzyme(s) to contact with the water-soluble cellulose derivatives in order to increase the solids content and viscosity of the composition.
- the coating composition of the coating may be obtained by enzymatically degrading water-soluble cellulose derivatives with one or more cellulases (e.g. cellulases of two or more different types), one or more endoglucanases (such as endo-1 ,4-0-D- glucanase), or a combination comprising one or more cellulases and one or more endoglucanases.
- the combination comprising one or more cellulases and one or more endoglucanases comprises at least 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90 or 95% cellulases or endoglucanases (% based on the number or activity of enzymes).
- the combination comprising one or more cellulases and one or more endoglucanases comprises at least 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90 or 95% endoglucanases (% based on the number or activity of enzymes).
- a cellulase is an enzyme produced e.g. by fungi, bacteria, and protozoans that catalyzes cellulolysis i.e. the decomposition of cellulose and optionally some related polysaccharides. Cellulases degrade cellulose and consequently produce glucose as the end product.
- the group of cellulases comprises 0-1 ,4-endoglucanases, p-1 ,4-exoglucanases, and p-1 ,4-glucosidases.
- Endoglucanases act randomly to hydrolyze interior p-1 ,4-glucan linkages of the cellulose chain thereby breaking the cellulose chain into smaller units, thus producing either oligosaccharides or smaller polysaccharide units.
- one or more endoglucanases (such as a combination of endoglucanases of two or more different types) give higher weight average molecular weight MW degradation products of cellulose derivatives than one or more cellulases.
- degradation of cellulose derivative chains by one or more endoglucanases is preferred.
- An expression “endoglucanase enriched cellulase” as used here means that the amount of endocellulase activity is increased compared to a cellulase activity produced by a host in which the cellulase profile has not been modified. This can be a cause by regulating activity of genes encoding cellulolytic enzymes or by expressing endocellulases in a recombinant production host.
- endoglucanase activity is the main activity of the cellulase preparation.
- the activity of a cellulase and/or an endoglucanase to degrade water-soluble cellulose derivatives can be determined by an enzyme assay wherein said enzyme(s) is(are) allowed to contact with water-soluble cellulose derivatives.
- the activity of an enzyme to degrade water-soluble cellulose derivatives can be determined e.g. by detecting the weight average molecular weight MW or the amount of hydrolysed water-soluble cellulose derivatives (e.g. as shown in examples of the present disclosure).
- the presence, absence or level of hydrolysed water-soluble cellulose derivatives can be detected or measured by any suitable method known in the art after allowing the enzymes(s) to contact with a composition comprising water-soluble cellulose derivatives.
- Non-limiting examples of suitable detection and/or measuring methods include but are not limited to filtration, solvent extraction, centrifugation, electrophoresis, liquid chromatography, gas chromatography, affinity chromatography, ion exchange chromatography, mass spectrometry or any combination thereof.
- the degradation process may also be followed by viscosity measurements.
- the incubation is continued until the desired viscosity or average molecular weight distribution for the water-soluble cellulose derivative composition to be contacted with the enzyme(s) for 10 seconds - 20 minutes (such as 15, 20, 25, 30, 35, 40, 45, 50, or 55 seconds, or one, two, three, four, five, six, seven, eight, nine, ten, 11 , 12, 13, 14, 15, 16, 17, 18, or 19 minutes) or even longer at a temperature below 90°C such as 30 - 80°C, 40 - 65°C or 50 - 60°C.
- water-soluble cellulose derivatives may be present 2 - 15 weight-% (such as 5 - 10% or 7%), e.g. the composition water-soluble cellulose derivatives are present 2 - 15% (such as 5 - 10% or 7%).
- the solids content of the composition comprising one or more water soluble cellulose derivatives to be treated with the enzyme having cellulolytic activity derivatives is 1 - 5% (weight-%, e.g. 2, 3 or 4%).
- viscosity of the composition treated with the enzyme is less than 5000, 4500, 4000, 3500, 3000, 2500, 2000, 1900, 1800, 1700, 1600, 1500, 1400, 1300, 1200, 1100 or 1000 mPas or cps (e.g. at room temperature, about. 23 °C), and/or more than 60, 100, 200, 300, 400, 500, 600, 700, 800, 900 or 1000 mPas or cps (e.g. at room temperature).
- the viscosity of the coating treated with the enzyme and ready to be applied on a surface is less than 5000, 4500, 4000, 3500, 3000, 2500, 2000, 1900, 1800, 1700, 1600, 1500, 1400, 1300, 1200, 1100 or 1000 mPas or cps (e.g. at room temperature, about 23 °C), and/or more than 60, 100, 200, 300, 400, 500, 600, 700, 800, 900 or 1000 mPas or cps (e.g. at room temperature about 23 °C).
- Viscosity of the coating or composition can be measured e.g., with a viscometer such as s Brookfield DV-E (Brookfield GmbH, Lorch, Germany) viscometer, e.g. at 100 rpm.
- the composition of hydrolysed water-soluble cellulose derivates may be concentrated by removing water by e.g. evaporation until desired consistency or even to dryness for the coating composition is achieved. At this stage, it may be necessary to dissolve powder like composition with water, or dilute a composition to desired consistency. Also further agents discussed above in connection of the coating composition, may be added to obtain a ready to be applied coting composition. The final constituents of the coating composition ready to be applied and thus also the coating, have been discussed above.
- the coating or composition is in a suspension, dispersion, semi-solid or solid form; or in a powder or paste form. As discussed, the consistency may be modified before application by adding water and/or additives.
- the coating composition may be delivered as a hydrolysed water soluble derivates, or a premix or a ready to be applied coating composition.
- the cellulose derivatives suitable for the present invention are water-soluble at least at room temperature (+ 21 °C).
- methyl cellulose and hydroxypropyl methyl cellulose become water insoluble in hot water (about + 75 °C), but they are still suitable for use for the present invention, and may even provide limited water barrier properties, especially against hot liquids, for the coating layer in addition the grease barrier properties.
- the present invention also concerns a coated cellulosic sheet like fibrous web or a product comprising a coated fibrous web, wherein the fibrous web has been coated with the coating composition disclosed here.
- the web comprises cellulosic or lignocellulosic fibres, or the substrate is a fibrous web, such as a paper, board, tissue or the like.
- the substate is a paper or board.
- the cellulosic or lignocellulosic fibres may have been obtained by any conventional pulping process, including chemical, mechanical, chemi-mechanical pulping processes.
- the cellulosic fibrous web (the substrate) may also comprise or consist of recycled fibres.
- the substrate has a grammage of 25 - 800 g/m 2 , 30 - 700 g/m 2 , or 40 - 500 g/m 2 .
- the coated substrate or the product has KIT test value of at least 7, water vapor transmission rate (WVTR) less than 1 10 g/m 2 /d (e.g. at 23 °C and 50 % relative humidity); and/or increased grease barrier properties compared to a substrate or product with a coating obtained without enzymatic degradation.
- WVTR water vapor transmission rate
- the KIT test value measures the repellency of the coating to oil and grease.
- the measurements can be performed according to standard TAPPI method T-559 pm- 96.
- the coated substrate or the product has decreased blocking tendency compared to a coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web with a coating obtained without enzymatic degradation.
- Grease barrier properties can be measured e.g. after oil treatment (such as olive oil treatment) followed by ASTM F119-82 test method. For example, oil can be dropped on cotton fabric placed on the coated side of the substrate and 50 grams weight can be placed on top of the substrate. Then said coated substrates can be placed at 40 °C oven for 120 minutes and photographed for evaluation of grease penetration.
- oil treatment such as olive oil treatment
- ASTM F119-82 test method For example, oil can be dropped on cotton fabric placed on the coated side of the substrate and 50 grams weight can be placed on top of the substrate. Then said coated substrates can be placed at 40 °C oven for 120 minutes and photographed for evaluation of grease penetration.
- WVTR value can be obtained e.g. by using Systech Permeation Analyzers M7002 instrument. WVTR value can be measured by using any standard method such as ASTM F-1249, ISO 15105-2, ISO 15106-3, and/or DIN 53122-2.
- Water resistance can be tested e.g. by using Cobb60 test (standard test method ISO 535).
- HVTR Hexane vapor transmission rate
- Method correlates with migration of mineral oil from recycled fiber based packaging to foodstuff.
- a coated substrate can be placed against a top side coating and treated e.g. at 40 °C temperature and 150 bar pressure for four hours.
- the coat weight of one coating layer or all coating layers together can be freely chosen depending on the desired end use and desired barrier properties of the substrate or product.
- the coating e.g. consisting of one or two or more coating layers
- the first coating layer may have a higher coat weight than the second coating layer, or vice versa.
- the coat weights of different coating layers may vary. Indeed, the substrate may comprise one, two or more coating layers.
- the substrate comprises a plurality of first coating layers and/or plurality of second coating layers, wherein the first coating layers are preferably chemically identical with each other, and the second coating layers are preferably chemically identical with each other.
- Chemically identical means that the coating layers are made from same components in identical amounts, i.e. coating layers are made by using identical coating formulation.
- a coating may be applied in one or both sides of the substate.
- the present invention further concerns a method of coating a sheet like cellulosic fibrous web, wherein the method comprises applying the coating of the present invention on a substrate.
- the coating may be applied on the surface of the substrate by using any conventional coating techniques, such as rod coating, blade coating, spray coating or curtain coating.
- the coating of the present invention can be applied directly on the surface of a substrate, such as a sheet-like substrate.
- the surface of the substrate may be surface sized, e.g. with a layer of hydrophobic surface size, before application of the coating layer, but preferably the coating layer is applied directly on the surface of a substrate which is free from any pre-existing treatment layers, such as surface sizing layers.
- the sheet-like substrate may comprise an internal size.
- the sheet like cellulosic web formed substrate coated with the coating of the present invention can be used e.g. for making a foodservice package or for liquid packaging.
- Typical examples of foodservice packages are packages for fast food, ready-to-eat meals, sandwiches, bakery products, such as cookies, doughnuts, or the like.
- the coating of the present invention can be used for coating a substrate for obtaining e.g. water, moisture and/or grease barrier properties, and/or better creasing or blocking results.
- the present invention concerns use of the enzyme hydrolysed water-soluble cellulose derivatives having a weight average molecular weight MW 20 000 Da- 120 000 Da, preferably 30 000 Da - 100 000 Da or 30 000 Da - 95 000 Da as a coating composition for a sheet like cellulosic fibrous web.
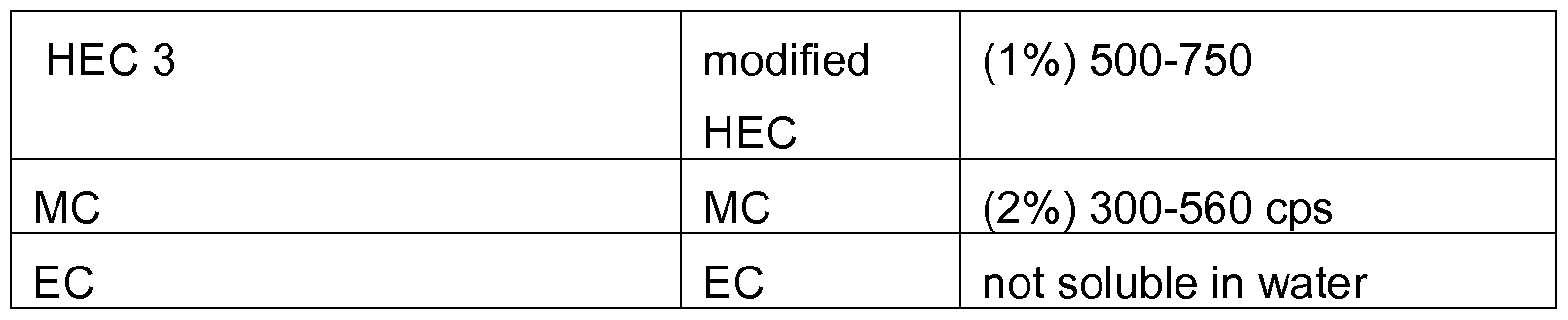
- HEC 1 Hydroxyethylcellulose 1 (HEC 1 )having Mw 220000 Da Hydxoxyethylcellulose 2 (HEC 2) having Mw 232000 Da Hydroxyethycellulose 3 (HEC 3) , modified HEC having Mw 160000 Da Enzymes used in the tests were Cellulase from Trichoderma reesei and endo-1 ,4- P-D-glucanase from Acidothermus cellulolyticus from Sigma Aldrich and commercial enzymes from Novozymes; Fibercare R and U, which have endoglucanase as main activity.
- HEC products were dissolved in water by mixing with magnetic stirrer and heated up to 60°C. Enzymatic degradation was tested for HEC 1 (Hydroxyethylcellulose 1 ) by dissolving it at 7% solids content and keeping the HEC solution at temperature 50-60°C. 0.2 grams of the enzymes were added per 100 grams of HEC solution. As viscosity decreased dry HEC was added in order to increase the solids content. Target solids content was 20% and target viscosity was around 1000 cPas. Enzyme activity was killed by heating the sample up to 90°C when suitable viscosity was obtained.
- Enzymatic degradation was also tested for other cellulose derivatives. Used products are listed in table 1 . Fibercare R was used for degradation.
- a Brookfield DV-E (Brookfield GmbH, Lorch, Germany) viscometer was used for measurement of the coating colors’ bulk viscosity immediately after preparation. Different spindles were used in accordance with the respective samples’ viscosity range. The measurements were performed at 100 rpm.
- Coat weight was determined by weighting the coated samples and uncoated base papers and coat weight was obtained by the weight difference. Simple converting test was done for the samples including sample creasing using Cyklos CPM 450 creasing and perforation unit. Creasing and folding was done in machine and cross directions. Staining test was done for the creased samples by using methyl red dissolved in ethanol. For folding Cobb roller was used to give uniform folding pressure. Water resistance was tested using Cobb60 test. Water vapor barrier properties were measured using Systech Permeation Analyzers M7002 instrument. Grease barrier properties were tested using olive oil following ASTM F119-82 test method. Oil was dropped on cotton fabric placed on the barrier coated side of the folded sample and 50 gram weight was placed on top of the sample.
- Cellulase mixture is a cellulase from Trichoderma reesei (Sigma Aldrich). An enzyme preparation has been added to HEC.
- KIT values are as matter of fact poorer for the enzymatically hydrolysed samples.
- Higher coat weight samples were achieved for enzymatically hydrolysed HEC samples and for those samples it can be seen that endoglucanase gives better KIT values.
- Sample 5 with Fibercare R as enzyme showed best water vapor barrier properties, KIT values and good blocking resistance. Staining test results are shown in figure 3 and olive oil test results in figure 4.
- coating formulations 5 and 6 using Fibercare enzymes for degradation showed coating layers without pinholes and no cracking at crease.
- Method 1 to carry out the enzymatic degradation was to add enzyme into 7% HEC solution and the solution was heated to 60 °C and 0.2 grams enzyme is added per 100 ml of cellulose derivative solution. Dry HEC powder is added when viscosity decreases. When similar viscosity level to original HEC solution is obtained for enzymatically hydrolysed HEC, the solution was heated to ca 90 °C in order to kill the enzyme, to get stable sample.
- Molecular weights and solids contents of the hydrolysed HEC prepared using different enzymes according to Method 1 are presented in Table 7:
- Method 2 Second way (Method 2) to carry out the enzymatic treatment was to add dry HEC powder in cold water (solids content ca. 50%) and adding 0.2 grams of enzyme was added per 100 ml of cellulose derivative solution. Sample was heated to 60°C and diluted if viscosity increased too high. After completing the enzymatic degradation, sample is heated to ca 90°C in order to kill the enzyme.
- Table 8 The obtained molecular weight and solids content according to Method 2 are presented in Table 8.
- Enzymatic degradation was carried out for two CMC grades; CMC 1 and CMC 2, having molecular weights Mw 261 and 338 kDa, respectively.
- the enzymatic degradation was carried out by adding enzyme into 10% or 7% CMC solution for CMC 1 and CMC 2, respectively. Samples were heated to 60°C. 0.2 grams of enzyme was added per 100 ml of CMC solution. Solids content was increased as more dry CMC powder was added when viscosity decreases. When similar viscosity level to original CMC solution was obtained for enzymatically hydrolysed product, the sample was heated to ca 90°C in order to kill the enzyme, in order to get stable sample. Obtained molecular weights and solids contents by using different enzymes are presented in Table 10. Table 10
- Enzymatic degradation was carried out in laboratory scale in a glass beaker and magnetic stirrer + heating. In bench scale reaction was carried out in glass reactor with heating and anchor stirrer.
- a Brookfield DV-E viscometer was used for measurement of the coating colors’ bulk viscosity immediately after preparation at room temperature (23°C). Different spindles were used in accordance with the respective samples’ viscosity range. The measurements were performed at 100 rpm.
- Coat weight was determined by weighting the coated samples and uncoated base papers and coat weight was obtained by the weight difference.
- Simple converting test was done for the samples including sample creasing using Cyklos CPM 450 creasing and perforation unit. Creasing and folding was done in machine and cross directions. Staining test was done for the creased samples by using methyl red dissolved in ethanol. For folding Cobb roller was used to give uniform folding pressure.
- Water resistance was tested using Cobb60 test. Water vapor barrier properties were measured using Systech Permeation Analyzers M7002 instrument. Grease barrier properties were tested using olive oil following ASTM F119-82 test method. Oil was dropped on cotton fabric placed on the barrier coated side of the folded sample and 50 gram weight was placed on top of the sample. Samples were placed at 40 °C oven for 120 minutes and photographed for evaluation of grease penetration. Blocking tests were carried out at 40 °C temperature and 150 bar pressure for four hours. The barrier coated sample was placed against the top side coating.
- Used scale for blocking test results is following; 1 : Samples did not adhere together, 2: Noise can be heard when pulling the sample strips apart, 3: Coating defect ⁇ 50 % of the surface area, 4: Coating defect >50 % of the surface area, 5: Base paper delamination.
- the molecular weight was determined by size-exclusion chromatography (SEC) using Viscotek GPCmax TDA 302 SEC equipment. Eluent was 0.1 M NaNOs. Column set consisted of three columns (Waters Ultrahydrogel 2000, 500 and 120) and a guard column. Pullulan standards (Polymer Standards Service) with molecular weights between 342 - 708 000 Da were used for conventional calibration with refractive index detection.
- Barrier coating was prepared by mixing the different HEC samples with calcium carbonate pigment and polyethylene glycol as plasticizer as presented in Table 11 .
- Comparison of different degradation protocols using Fibercare R was carried out using a reference coating formulation containing 30% calcium carbonate and 20% PEG 300, in Table 15.
- HEC-FCR was prepared by method 1 , HEC- high solids degrading by method 2 and HEC - pilot test by method 3.
- Hexane vapor transmission rate was determined by using a cup method. In the method, 20 grams of hexane was placed in a metal cup. Barrier sample was placed on top of the cup between two gaskets, coated side down. Metal frame was used to tighten the sample to the cup. Weight loss of hexane was recorded for 24 hours and calculated per area g/(m2*d).
- Enzymatic degradation of HEC was done by using Fibercare R enzyme.
- Base substrate used was folding boxboard, grammage 247 g/m 2 . Double coating was applied for all samples.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Microbiology (AREA)
- Paper (AREA)
Abstract
The present invention relates to a coating composition for a sheet like cellulosic fibrous web comprising one or more enzyme hydrolysed water-soluble cellulose derivatives and to a method of preparing such a composition. The present invention further relates to a coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web substrate and also to a method of coating a sheet like cellulosic fibrous web. Still further the invention relates to use of the enzyme hydrolysed water-soluble cellulose derivatives as a coating composition for a sheet like cellulosic fibrous web.
Description
A COATING COMPOSITION, METHOD FOR PREPARING SUCH COMPOSITION, A METHOD OF COATING, A COATED SHEET AND USES OF THE COATING
FIELD OF THE INVENTION
The present invention relates to a coating comprising one or more enzymatically hydrolysed water-soluble cellulose derivatives and a method of degrading one or more water-soluble cellulose derivatives or preparing a coating. Furthermore, the present invention relates to a method of preparing a coating composition by enzymatically degrading one or more water-soluble cellulose derivatives. A coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web substrate is also within the scope of the present invention as well as a method of coating and use of the coating composition here described.
BACKGROUND OF THE INVENTION
Various coatings can be applied on the surface of fibrous material such as paper or board in order to improve their properties. Grease, water and/or moisture barrier properties are particularly important for paper and board that are used for products for packaging purposes. Coatings applied on the surface of paper or board should provide an effective barrier against leakage from the goods inside the package and/or protect the packaged goods from contamination and/or contact with the surroundings. The barrier requirements are especially stringent for packaging materials used for foodstuff and consumable liquids.
Coatings for packaging purposes should have good resistance for creasing and folding. The coating should not crack when the paper or board is folded into a box or wrapped around the product. Cracking may decrease or even completely destroy the barrier properties of the coating. They should not be tacky and have low blocking tendency but on the other hand heat sealability is desired.
For environmental reasons it would be desirable to use sustainable and renewable bio-based sources for coatings. Furthermore, the barrier coatings used for packages should preferably also satisfy the recyclability requirements, for example, they should not disturb the repulping process.
Conventional bio-based components used in coating compositions, such as starch, often do not perform well in barriers coatings. Furthermore, the bio-based component should preferably originate from non-food chain sources, which requirement is
not fulfilled e.g. by starch and starch derivatives. Cellulose or cellulose derivatives have been tested earlier in barrier applications. Drawbacks of the prior art include e.g. low solids content of the cellulose or cellulose derivatives coating products as dissolved in water and/or too high viscosity. Increased solids content and/or lower viscosity of coating compositions/formulations are needed for more effective and profitable coatings as well as easier coating methods. Barrier coatings with improved performance are desired.
Indeed, there is a need for new coating formulations and methods related thereto that would solve the problems presently encountered.
BRIEF DESCRIPTION OF THE INVENTION
The present invention provides sustainable and renewable bio-based sources for coating compositions, method for their preparation, coatings made therefrom and coated product. Such coatings are easily biodegradable and/or repulpable e.g., together with paper or board.
Defects of the prior art including but not limited to undesired blocking properties of the paper or board products, low solids content of the cellulose or cellulose derivatives when dissolved in water in coating compositions/ and/or too high viscosity of such compositions, can be overcome with the methods or products of the present invention. Surprisingly, also moisture and/or grease barrier properties of coatings can be improved with the present invention.
It has now been surprisingly found that a decreased weight average molecular weight MW or a specific weight average molecular weight MW range of the hydrolysed water-soluble cellulose derivatives can be used for obtaining very effective coating compositions for example for paper or board.
The first aspect of the invention is a coating composition for a sheet like cellulosic fibrous web. Characteristic features of said composition are depicted in claim 1 .
The second aspect of the invention is method of preparing a coating composition by enzymatically degrading one or more water-soluble cellulose derivatives. Characteristic steps of said method are depicted in claim 8.
The third aspect in the invention is a coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web substrate, Characteristic features of said fibrous web or a product comprising it are depicted in claim 13.
The fourth aspect of this invention is a method of coating a sheet like cellulosic fibrous web, wherein the method comprises applying the coating composition of any here described on said fibrous web.
Enzymatic degradation of water-soluble cellulose derivatives leads to decreased weight average molecular weight MW of said derivatives and thus viscosity of the composition can be decreased, and higher solids content of the composition can be achieved. This enables higher coat weights applied for the barrier coating. However, the coating of the present invention still withstands cracking when creased and/or folded, e.g. on a substrate such as a paper or board. Terms ‘hydrolysed cellulose derivates’ and ‘degraded cellulose derivates’ as used here both refer to cellulose derivates undergone an enzymatic treatment.
The fifth aspect of the invention is use of the coating composition of the present invention for coating a sheet like cellulosic fibrous web for obtaining water, moisture and/or grease barrier properties.
Other objects, details and advantages of the present invention will become apparent from the following drawings, detailed description and examples.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows staining test images for samples coated with barrier formulates 1 -3 in Table 2. Samples with coat weight ca. 8 g/m2 are shown on the top row and samples with coat weight over 17 g/m2 are shown on the bottom row.
Figure 2 shows olive oil grease barrier test images for samples coated with barrier formulates 1 -3. The top row reveals samples with coat weight ca. 8 g/m2 and the bottom row shows samples with coat weight over 17 g/m2.
Figure 3 shows staining test results for all coating formulations with enzymatically hydrolysed HEC.
Figure 4 shows olive oil test results. On the top row there are samples with coating formulations 1 -3 and on the bottom row with formulations 4- 6.
Figure 5 shows staining test images for coated samples using enzymatically hydrolysed cellulose derivatives.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to a coating composition for a sheet like cellulosic fibrous web comprising one or more water-soluble cellulose derivatives selected from the group consisting of alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose, methyl cellulose (MC), and carboxymethyl cellulose hydrolyzed with an enzyme having cellulolytic activity, and having a final weight average molecular weight MW is 20 000 Da - 120 000 Da, such as, 30 000 - 100 000 Da. Here a final weight average molecular weight refers to molecular weight after enzyme hydrolysis. Weight average molecular weight MW’s of the enzymatically hydrolysed celluloses of the present invention are clearly above the oligomer limit 1000 Da.
Enzymatic degradation (hydrolysis) of water-soluble cellulose derivatives leads to decreased weight average molecular weight MW of said derivatives and thus viscosity of the composition can be decreased, and higher solids content of the composition can be achieved. This enables higher coat weights applied for the barrier coating. However, the coating of the present invention still withstands cracking when creased and/or folded, e.g. on a substrate such as a paper or board.
The cellulase hydrolysis provides the above advantages. With endoglucanase hydrolysis (endoglucanase or endoglucanase enriched enzyme product) more uniform average MW is achieved resulting in better performance and application, e.g., low tendency to stack to the roller.
In one embodiment at least 20 weight-% of the water-soluble cellulose derivatives of the coating are hydrolysed water-soluble cellulose derivatives. In this connection an expression “water soluble cellulose derivates” means that the cellulose deri- vates are individually dispersed throughout the water solution and they do not settle out or separate from the water solvent.
In one embodiment said coating composition comprises water-soluble cellulose derivatives in amount 20-100 weight-%, preferably 30-90 weight-%, more preferably
40-70 weight-% of the total solids content of the coating composition or the coating. For example, the coating composition may comprise water-soluble cellulose derivatives in amount of 20 - 99 weight-%, 30 - 99 weight-%, 40 - 99 weight-%, 50 - 99 weight-%, or 60 - 97 weight-%, calculated from total solids content of the coating composition, respectively.
In one embodiment the coating composition further comprises one or more constituents) selected from the group comprising a plasticizer, pigment particle, starch, binder, thickener, cross-linker, lubricant, and dispersing agent; or the method of any of the previous claims, wherein one or more agents are selected from the group comprising a plasticizer, inorganic pigment, starch, binder, thickener, crosslinker, lubricant, and dispersing agent.
In one embodiment one or more constituents to the composition comprising hydrolysed water-soluble cellulose derivatives enables obtaining an optimal coating. In one embodiment one or more constituents are selected from the group comprising a plasticizer, inorganic pigment, starch, binder, thickener, cross-linker, lubricant, and dispersing agent. Indeed, the composition of the present invention can comprise one or more constituents selected from the group comprising a plasticizer, pigment particle, starch, binder, thickener, cross-linker, lubricant, and dispersing agent. Respective agents may be contained in a coating composition discussed above.
The plasticizer may be selected from a group comprising polyol, such as sorbitol, mannitol, ethylene glycol, diethylene glycol, triethylene glycol, tetraethylene glycol, propylene glycol or polyethylene glycol; fatty acids; monosaccharides, ethanolamine; triethanolamine; urea; lecitin and glycerol. According to one embodiment the plasticizer may be selected from sorbitol, polyethylene glycol or glycerol. Often the incorporation of a plasticizer into a barrier coating makes the barrier coating layer too tacky which may easily lead to blocking problems. Now it has been unexpectedly found that the tackiness of the barrier coating layer comprising plasticizer is significantly decreased when the barrier coating layer comprises the enzymatically hydrolysed water-soluble cellulose derivatives. This means that it is possible to obtain a coating layer where the cracking and blocking resistance properties can be balanced with the flexibility in order to obtain a coating with optimal properties for the desired purpose.
In one embodiment the composition may comprise plasticizer in amount of 1 - 50 weight-%, 3 - 40 weight-%, 5 - 30 weight-%, 10 - 20 weight-%, weight-% calculated from total solids content of the composition, respectively.
The pigment particle can be e.g. an inorganic mineral pigment which may optionally be selected from kaolin, talc, calcium carbonate (e.g. ground calcium carbonate or precipitated calcium carbonate) or any mixture thereof. Calcium carbonate is one preferred embodiment. The particle size D50 of the pigment particles or inorganic pigment particles may be <5 pm. According to one embodiment the composition may comprise inorganic mineral particles, wherein at least 45% of the inorganic mineral particles has particle size <2 pm. Addition of inorganic mineral pigment may further improve the obtained barrier properties. It has been unexpectedly found that the enzymatically hydrolysed water-soluble cellulose derivatives increase the flexibility of the coating in a manner that allows incorporation of high amounts of inorganic pigment particles to the coating. This may not only improve the barrier properties, but also makes the coating more economic to produce.
According to one embodiment the coating composition may comprise pigment particles or inorganic pigment particles in an amount of 5 - 40 weight-%, 10 - 35 weight- %, or 15 - 30 weight-% from the total dry solids content of the composition, respectively.
For example, the coating composition (ready for use) may comprise 40 - 90 weight- %, 50 - 90 weight-% or 60 - 90 weight-% of water-soluble cellulose derivatives, 5 - 40 weight-%, 10 - 35 weight-%, or 15 - 30 weight-% of pigment particles or inorganic pigment particles, and 1 - 50 weight-%, 3 - 40 weight-%, 5 - 30 weight-%, 5 - 20 weight-%, or 5 - 10 weight-% of plasticizer, 0 - 30 weight-% binder, such as polyvinyl alcohol (PVA), and up-to 1 weight-% buffering agent and residues of an enzyme composition calculated from the total dry solids content of the coating composition, respectively, the total amount of the components adding up to 100%.
Starch may increase the brittleness of the coating and reduce the resistance for cracking. Therefore, starch is not needed in the composition of the present invention or the amount of starch can be minimised. In one embodiment the composition is free of starch.
In one embodiment of the invention the coating composition may further comprise an additional coating binder, e.g. polyvinyl alcohol. The weight average molecular weight of the polyvinyl alcohol may be < 100 000 g/mol, such as < 90 000 g/mol. Polyvinyl alcohol that is especially suitable for use as an additional coating binder may have a weight average molecular weight of <70 000 g/mol, such as 13 000 - 70 000 g/mol. Polyvinyl alcohol may be at least partially hydrolysed. Polyvinyl alcohol, when used as an additional coating binder, may improve the film formation and both water vapour and mineral oil barrier properties of the coating. Polyvinyl alcohol may also reduce blocking tendency of the obtained coating structure. In one embodiment the composition or coating is free of an additional coating binder such as polyvinyl alcohol.
According to a further embodiment the coating composition layer may comprise one or more of the following additive agents: thickener(s), cross-linker(s), lubricant(s), alkyl ketene dimer(s), alkenyl succinic anhydride(s), and dispersing agent(s).
The molecular weight can be determined by size-exclusion chromatography (SEC) using Viscotek GPCmax TDA 302 SEC equipment. Eluent was 0.1 M NaNOs. Column set consisted of three columns (Waters Ultrahydrogel 2000, 500 and 120) and a guard column. Pullulan standards (Polymer Standards Service) with molecular weights between 342 - 708 000 Da were used for conventional calibration with refractive index detection.
In one embodiment of the coating composition has viscosity of the coating is 50 - 3000 mPas or cP measured at 23 °C using 100 rpm and Brookfield DV-E viscometer.
The coating composition may be in form of a dispersion, suspension or semi-solid or solid composition comprising enzymatically hydrolysed water-soluble cellulose derivatives and optionally further constituents up to 50 wt.-% calculated from total dry matter. The final coating may be in solid form.
As used herein “a dispersion” refers to a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter.
As used herein “a suspension” refers to a dispersion of particles sufficiently large for sedimentation. In one embodiment a suspension is a heterogeneous mixture of a fluid that contains solid particles sufficiently large for sedimentation.
In one embodiment the coating composition may be a coating composition ready to be applied on a cellulosic fibrous web. A composition comprising one or more enzyme hydrolysed water-soluble cellulose derivatives having a weight average molecular weight MW 20 000 Da- 120 000 Da, such as 30 000 Da- 100 000 Da or 30 000 Da and 95 000 Da may be in form of dispersion, suspension, semi-solid or solid powder like form. Preferred range depends e.g. on the application. It may be supplemented with a diluent (such as water) before application on a substrate, or a coating layer.
In one embodiment said one or more water-soluble cellulose derivatives is/are selected from the group comprising or consisting of an alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose; and any combination thereof.
In one embodiment said one or more water-soluble cellulose derivatives is/are selected from the group comprising or consisting of methyl cellulose (MC), carboxymethyl cellulose (CMC), hydroxymethyl cellulose (HMC), hydroxyethyl cellulose (HEC), methyl hydroxyethyl cellulose (MHEC), hydroxypropyl cellulose (HPC), hydroxypropylmethyl cellulose (HPMC), and any combination thereof.
In one embodiment one of said water-soluble cellulose derivatives is HEC or CMC, especially HEC. CMC is readily degradable by cellulase degrading enzymes. HEC provides a durable coating.
The present invention also concerns method of preparing a coating composition by enzymatically degrading one or more water-soluble cellulose derivatives, wherein the method comprises
(a) providing an aqueous suspension of one or more water-soluble cellulose derivatives selected from alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose, methyl cellulose (MC), and carboxymethyl cellulose; and
(b) contacting said suspension with an enzyme having cellulolytic activity;
(c) allowing to react at conditions conductive for activity of said enzyme;
(d) optionally removing excess of water; and
(e) optionally adding one or more agents to the composition comprising degraded water-soluble cellulose derivatives.
The reaction of step (c) may be continued until said water-soluble cellulose derivatives have having a weight average molecular weight MW is 20 000 Da - 120 000 Da, such as, 30 000 -100 000 Da.
In one embodiment the reaction time of item (c) is continued until a desired viscosity in view of dry matter is obtained. Thus, in one embodiment the enzymatic reaction is followed by viscosity measurements.
In one embodiment the enzyme activity is killed by heating the water-soluble cellulose derivative composition, optionally up to at least 85°C, 90°C, 95°C or 100°C, optionally after obtaining suitable viscosity. The coating or the coating composition may comprise inactivated enzymes or residues of enzyme composition.
The incubation time (contact time with enzymes), activity of the enzyme preparation and reaction conditions are adapted to allow degradation until a desired viscosity is achieved. A person skilled in the art is able to select conditions, such as temperature, consistency and pH, conductive for activity of the enzyme preparation(s).
It is to be noted that the enzymatic reaction may be performed in lower consistency (dry matter content) than dry matter of the final coating composition. The dry matter content may be increased by removal of water and/or by adding further constituent to the composition after the enzymatic treatment. The hydrolysed water-soluble cellulosic derivatives may be subjected for removal of water for obtaining a higher solid content of even drying the hydrolysed water-soluble cellulosic derivatives in order to modify the consistency of the coating or enhancing the transportation.
As used herein “a water-soluble cellulose derivative” refers to any water-soluble cellulose derivative which is optionally selected from the group comprising or consisting of an alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose and any of their mixture. In one embodiment the water-soluble cellulose derivative is selected from the group comprising or consisting of methyl cellulose (MC), carboxymethyl cellulose (CMC), hydroxymethyl cellulose (HMC), hydroxyethyl cellulose (HEC), methyl hydroxyethyl cellulose (MHEC; (hydroxyethyl)methyl cellulose), hydroxypropyl cellulose (HPC), hydroxypropylmethyl cellulose (HPMC; (hydroxypropyl)-
methyl cellulose), and any combination or mixture thereof. CLM 10 In one embodiment said derivate is selected from methyl cellulose (MC), carboxymethyl cellulose (CMC), hydroxymethyl cellulose (HMC), hydroxyethyl cellulose (HEC), methyl hydroxyethyl cellulose (MHEC), hydroxypropyl cellulose (HPC), hydroxypropylmethyl cellulose (HPMC). In one embodiment said derivate is selected from HEC and CMC.
Enzymatically hydrolysed water-soluble cellulose derivatives are able to effectively function as a grease and/or moisture barrier, especially against liquid grease or oil, and sometimes even as a mineral oil barrier. It has been found that when at least one of the hydrolysed water-soluble cellulose derivatives is present in the coating, the coating is more flexible and does not crack so easily at creasing or folding. Moreover, the hydrolysed water-soluble cellulose derivatives provide increased dry solid content for the composition formulation, thus making it easier to apply on the surface of the substrate to be coated and providing for a better film forming properties.
With the present invention it is possible to produce coatings where the content of bio-based components is high while maintaining the essential barrier and crack resistance properties on an excellent, good or at least acceptable level. The shares of the coating composition to be prepared are discussed in connection of the coating composition and evidently applicable to the process also. The method makes it possible to produce coating compositions with high solid content and a viscosity allowing easy application of the coating composition.
In the method of the present invention when the enzyme(s) are allowed to contact with the water-soluble cellulose derivatives viscosity of the composition decreases. In one embodiment water-soluble cellulose derivatives are further added to the composition during or after allowing the enzyme(s) to contact with the water-soluble cellulose derivatives in order to increase the solids content and viscosity of the composition.
The coating composition of the coating may be obtained by enzymatically degrading water-soluble cellulose derivatives with one or more cellulases (e.g. cellulases of two or more different types), one or more endoglucanases (such as endo-1 ,4-0-D- glucanase), or a combination comprising one or more cellulases and one or more endoglucanases. In one embodiment the combination comprising one or more cellulases and one or more endoglucanases comprises at least 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90 or 95% cellulases or endoglucanases (% based on
the number or activity of enzymes). In one embodiment the combination comprising one or more cellulases and one or more endoglucanases comprises at least 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90 or 95% endoglucanases (% based on the number or activity of enzymes). A cellulase is an enzyme produced e.g. by fungi, bacteria, and protozoans that catalyzes cellulolysis i.e. the decomposition of cellulose and optionally some related polysaccharides. Cellulases degrade cellulose and consequently produce glucose as the end product. The group of cellulases comprises 0-1 ,4-endoglucanases, p-1 ,4-exoglucanases, and p-1 ,4-glucosidases. Endoglucanases act randomly to hydrolyze interior p-1 ,4-glucan linkages of the cellulose chain thereby breaking the cellulose chain into smaller units, thus producing either oligosaccharides or smaller polysaccharide units. In one embodiment one or more endoglucanases (such as a combination of endoglucanases of two or more different types) give higher weight average molecular weight MW degradation products of cellulose derivatives than one or more cellulases. Indeed, in one embodiment degradation of cellulose derivative chains by one or more endoglucanases is preferred. An expression “endoglucanase enriched cellulase” as used here means that the amount of endocellulase activity is increased compared to a cellulase activity produced by a host in which the cellulase profile has not been modified. This can be a cause by regulating activity of genes encoding cellulolytic enzymes or by expressing endocellulases in a recombinant production host. In one embodiment endoglucanase activity is the main activity of the cellulase preparation.
The activity of a cellulase and/or an endoglucanase to degrade water-soluble cellulose derivatives can be determined by an enzyme assay wherein said enzyme(s) is(are) allowed to contact with water-soluble cellulose derivatives. The activity of an enzyme to degrade water-soluble cellulose derivatives can be determined e.g. by detecting the weight average molecular weight MW or the amount of hydrolysed water-soluble cellulose derivatives (e.g. as shown in examples of the present disclosure). The presence, absence or level of hydrolysed water-soluble cellulose derivatives can be detected or measured by any suitable method known in the art after allowing the enzymes(s) to contact with a composition comprising water-soluble cellulose derivatives. Non-limiting examples of suitable detection and/or measuring methods include but are not limited to filtration, solvent extraction, centrifugation, electrophoresis, liquid chromatography, gas chromatography, affinity chromatography, ion exchange chromatography, mass spectrometry or any combination thereof. The degradation process may also be followed by viscosity measurements.
In one embodiment the incubation is continued until the desired viscosity or average molecular weight distribution for the water-soluble cellulose derivative composition to be contacted with the enzyme(s) for 10 seconds - 20 minutes (such as 15, 20, 25, 30, 35, 40, 45, 50, or 55 seconds, or one, two, three, four, five, six, seven, eight, nine, ten, 11 , 12, 13, 14, 15, 16, 17, 18, or 19 minutes) or even longer at a temperature below 90°C such as 30 - 80°C, 40 - 65°C or 50 - 60°C.
In the water-soluble cellulose derivative composition water-soluble cellulose derivatives may be present 2 - 15 weight-% (such as 5 - 10% or 7%), e.g. the composition water-soluble cellulose derivatives are present 2 - 15% (such as 5 - 10% or 7%).
In one embodiment, the solids content of the composition comprising one or more water soluble cellulose derivatives to be treated with the enzyme having cellulolytic activity derivatives is 1 - 5% (weight-%, e.g. 2, 3 or 4%).
In one embodiment, viscosity of the composition treated with the enzyme is less than 5000, 4500, 4000, 3500, 3000, 2500, 2000, 1900, 1800, 1700, 1600, 1500, 1400, 1300, 1200, 1100 or 1000 mPas or cps (e.g. at room temperature, about. 23 °C), and/or more than 60, 100, 200, 300, 400, 500, 600, 700, 800, 900 or 1000 mPas or cps (e.g. at room temperature).
The viscosity of the coating treated with the enzyme and ready to be applied on a surface is less than 5000, 4500, 4000, 3500, 3000, 2500, 2000, 1900, 1800, 1700, 1600, 1500, 1400, 1300, 1200, 1100 or 1000 mPas or cps (e.g. at room temperature, about 23 °C), and/or more than 60, 100, 200, 300, 400, 500, 600, 700, 800, 900 or 1000 mPas or cps (e.g. at room temperature about 23 °C). Viscosity of the coating or composition can be measured e.g., with a viscometer such as s Brookfield DV-E (Brookfield GmbH, Lorch, Germany) viscometer, e.g. at 100 rpm.
The composition of hydrolysed water-soluble cellulose derivates may be concentrated by removing water by e.g. evaporation until desired consistency or even to dryness for the coating composition is achieved. At this stage, it may be necessary to dissolve powder like composition with water, or dilute a composition to desired consistency. Also further agents discussed above in connection of the coating composition, may be added to obtain a ready to be applied coting composition. The final constituents of the coating composition ready to be applied and thus also the coating, have been discussed above.
In one embodiment of the coating or method of the present invention, the coating or composition is in a suspension, dispersion, semi-solid or solid form; or in a powder or paste form. As discussed, the consistency may be modified before application by adding water and/or additives. The coating composition may be delivered as a hydrolysed water soluble derivates, or a premix or a ready to be applied coating composition.
In one embodiment the cellulose derivatives suitable for the present invention are water-soluble at least at room temperature (+ 21 °C). For example methyl cellulose and hydroxypropyl methyl cellulose become water insoluble in hot water (about + 75 °C), but they are still suitable for use for the present invention, and may even provide limited water barrier properties, especially against hot liquids, for the coating layer in addition the grease barrier properties.
The present invention also concerns a coated cellulosic sheet like fibrous web or a product comprising a coated fibrous web, wherein the fibrous web has been coated with the coating composition disclosed here. In one embodiment the web comprises cellulosic or lignocellulosic fibres, or the substrate is a fibrous web, such as a paper, board, tissue or the like. In one embodiment the substate is a paper or board. The cellulosic or lignocellulosic fibres may have been obtained by any conventional pulping process, including chemical, mechanical, chemi-mechanical pulping processes. The cellulosic fibrous web (the substrate) may also comprise or consist of recycled fibres. In one embodiment the substrate has a grammage of 25 - 800 g/m2, 30 - 700 g/m2, or 40 - 500 g/m2.
In one embodiment the coated substrate or the product has KIT test value of at least 7, water vapor transmission rate (WVTR) less than 1 10 g/m2/d (e.g. at 23 °C and 50 % relative humidity); and/or increased grease barrier properties compared to a substrate or product with a coating obtained without enzymatic degradation.
The KIT test value measures the repellency of the coating to oil and grease. The measurements can be performed according to standard TAPPI method T-559 pm- 96.
In one embodiment the coated substrate or the product has decreased blocking tendency compared to a coated sheet like cellulosic fibrous web or a product
comprising said coated sheet like cellulosic fibrous web with a coating obtained without enzymatic degradation.
Grease barrier properties can be measured e.g. after oil treatment (such as olive oil treatment) followed by ASTM F119-82 test method. For example, oil can be dropped on cotton fabric placed on the coated side of the substrate and 50 grams weight can be placed on top of the substrate. Then said coated substrates can be placed at 40 °C oven for 120 minutes and photographed for evaluation of grease penetration.
WVTR value can be obtained e.g. by using Systech Permeation Analyzers M7002 instrument. WVTR value can be measured by using any standard method such as ASTM F-1249, ISO 15105-2, ISO 15106-3, and/or DIN 53122-2.
Water resistance can be tested e.g. by using Cobb60 test (standard test method ISO 535).
Hexane vapor transmission rate (HVTR) was determined by using a cup method.
In the method, 20 grams of hexane was placed in a metal cup. Barrier sample was placed on top of the cup between two gaskets, coated side down. Metal frame was used to tighten the sample to the cup. Weight loss of hexane was recorded for 24 hours and calculated per area g/(m2*d).
Method correlates with migration of mineral oil from recycled fiber based packaging to foodstuff.
For a blocking test a coated substrate can be placed against a top side coating and treated e.g. at 40 °C temperature and 150 bar pressure for four hours.
The coat weight of one coating layer or all coating layers together can be freely chosen depending on the desired end use and desired barrier properties of the substrate or product. In one embodiment the coating (e.g. consisting of one or two or more coating layers) has a coat weight 0.5 - 25 g/m2, 3 - 25 g/m2, or at least 3, 4, 5, 6, 7, 10, 15, or 17 g/m2. If there is more than one coating layer on a substrate in one embodiment the first coating layer may have a higher coat weight than the second coating layer, or vice versa. The coat weights of different coating layers may vary.
Indeed, the substrate may comprise one, two or more coating layers. In one embodiment, the substrate comprises a plurality of first coating layers and/or plurality of second coating layers, wherein the first coating layers are preferably chemically identical with each other, and the second coating layers are preferably chemically identical with each other. Chemically identical means that the coating layers are made from same components in identical amounts, i.e. coating layers are made by using identical coating formulation. A coating may be applied in one or both sides of the substate.
The present invention further concerns a method of coating a sheet like cellulosic fibrous web, wherein the method comprises applying the coating of the present invention on a substrate. The coating may be applied on the surface of the substrate by using any conventional coating techniques, such as rod coating, blade coating, spray coating or curtain coating. The coating of the present invention can be applied directly on the surface of a substrate, such as a sheet-like substrate. In some embodiments the surface of the substrate may be surface sized, e.g. with a layer of hydrophobic surface size, before application of the coating layer, but preferably the coating layer is applied directly on the surface of a substrate which is free from any pre-existing treatment layers, such as surface sizing layers. The sheet-like substrate may comprise an internal size.
The sheet like cellulosic web formed substrate coated with the coating of the present invention can be used e.g. for making a foodservice package or for liquid packaging. Typical examples of foodservice packages are packages for fast food, ready-to-eat meals, sandwiches, bakery products, such as cookies, doughnuts, or the like. The coating of the present invention can be used for coating a substrate for obtaining e.g. water, moisture and/or grease barrier properties, and/or better creasing or blocking results.
Thus, the present invention concerns use of the enzyme hydrolysed water-soluble cellulose derivatives having a weight average molecular weight MW 20 000 Da- 120 000 Da, preferably 30 000 Da - 100 000 Da or 30 000 Da - 95 000 Da as a coating composition for a sheet like cellulosic fibrous web.
In the present context, if not otherwise stated, all weight-% values given for the various components are calculated from the total dry solids content of the composition.
It will be obvious to a person skilled in the art that, as the technology advances, the inventive concept can be implemented in various ways. The invention and its embodiments are not limited to the examples described below but may vary within the scope of the claims.
EXAMPLES
Materials and methods
Several hydroxyethyl cellulose grades have been tested
Hydroxyethylcellulose 1 (HEC 1 )having Mw 220000 Da Hydxoxyethylcellulose 2 (HEC 2) having Mw 232000 Da Hydroxyethycellulose 3 (HEC 3) , modified HEC having Mw 160000 Da Enzymes used in the tests were Cellulase from Trichoderma reesei and endo-1 ,4- P-D-glucanase from Acidothermus cellulolyticus from Sigma Aldrich and commercial enzymes from Novozymes; Fibercare R and U, which have endoglucanase as main activity.
HEC products were dissolved in water by mixing with magnetic stirrer and heated up to 60°C. Enzymatic degradation was tested for HEC 1 (Hydroxyethylcellulose 1 ) by dissolving it at 7% solids content and keeping the HEC solution at temperature 50-60°C. 0.2 grams of the enzymes were added per 100 grams of HEC solution. As viscosity decreased dry HEC was added in order to increase the solids content. Target solids content was 20% and target viscosity was around 1000 cPas. Enzyme activity was killed by heating the sample up to 90°C when suitable viscosity was obtained.
Enzymatic degradation was also tested for other cellulose derivatives. Used products are listed in table 1 . Fibercare R was used for degradation.
Barrier coating tests were carried out for the enzymatically hydrolysed HEC samples. Used coating formulations are summarized in table 2.
A Brookfield DV-E (Brookfield GmbH, Lorch, Germany) viscometer was used for measurement of the coating colors’ bulk viscosity immediately after preparation. Different spindles were used in accordance with the respective samples’ viscosity range. The measurements were performed at 100 rpm.
Laboratory coating tests were carried out by using draw down coater K control coater (RK Print Coat Instruments, Litlington, UK) with different wound rods and coating speeds. Samples were dried using InfraRR IR dryers for 60 seconds directly after coating. Single and double coated samples were prepared. The used substrate in coating tests was virgin fiber based cartonboard (Avanta Prima) with basis weight of 265 g/m2. Barrier coating was applied on the uncoated bottom side.
Coat weight was determined by weighting the coated samples and uncoated base papers and coat weight was obtained by the weight difference. Simple converting test was done for the samples including sample creasing using Cyklos CPM 450 creasing and perforation unit. Creasing and folding was done in machine and cross directions. Staining test was done for the creased samples by using methyl red dissolved in ethanol. For folding Cobb roller was used to give uniform folding pressure.
Water resistance was tested using Cobb60 test. Water vapor barrier properties were measured using Systech Permeation Analyzers M7002 instrument. Grease barrier properties were tested using olive oil following ASTM F119-82 test method. Oil was dropped on cotton fabric placed on the barrier coated side of the folded sample and 50 gram weight was placed on top of the sample. Samples were placed at 40 °C oven for 120 minutes and photographed for evaluation of grease penetration. Blocking tests were carried out at 40 °C temperature and 150 bar pressure for four hours. The barrier coated sample was placed against the top side coating. Used scale for blocking test results is following; 1 : Samples did not adhere together, 2: Noise can be heard when pulling the sample strips apart, 3: Coating defect <50 % of the surface area, 4: Coating defect >50 % of the surface area, 5: Base paper delamination.
Results and discussion
Enzymatic degradation
Obtained molecular weights for HEC 1 by using different enzymes are summarized in table 3.
Table 3. Molecular weight results for enzymatically hydrolysed hydroxyethyl cellulose samples. Cellulase mixture is a cellulase from Trichoderma reesei (Sigma Aldrich). An enzyme preparation has been added to HEC.
Cellulase degradation gave lower weight average molecular weight MW for HEC than endoglucanase. Pure endoglucanase treatment showed lower average molecular weight than the commercial Fibercare enzymes. It can be seen that weight average molecular weight MWis very similar at lower solids content in the beginning of the degradation test and at high solids content in the end of the degradation. All samples showed average molecular weight clearly above oligomer limit (1000 Da).
Coating results
Hydroxyethyl cellulose
In the first set of samples coating formulations 1 -3 were tested and the results are shown in table 4. Sample 1 was reference without enzymatic degradation, sample 2 was hydrolysed using cellulase and sample 3 with endoglucanase. Staining test images are summarized in figure 1 and olive oil test results in figure 2.
At low coat weight enzymatically hydrolysed samples with higher solids content did not improve barrier properties, KIT values are as matter of fact poorer for the enzymatically hydrolysed samples. Higher coat weight samples were achieved for enzymatically hydrolysed HEC samples and for those samples it can be seen that endoglucanase gives better KIT values.
Lower weight average molecular weight samples show better creasing properties compared to the original HEC grade at coat weight around 8 g/m2. Sample with endoglucanase used for degradation showed best folding properties. Minor cracking can still be seen on the samples with coat weight up to 20 g/m2.
In the second test series commercial Fibercare enzymes were used. Coat weight and barrier results are summarized in table 5. able 5. Coat weigh and barrier data for coated samp es using formulations 1 -6.
Sample 5 with Fibercare R as enzyme showed best water vapor barrier properties, KIT values and good blocking resistance. Staining test results are shown in figure 3 and olive oil test results in figure 4.
In the staining test images coating formulations 5 and 6 using Fibercare enzymes for degradation showed coating layers without pinholes and no cracking at crease.
In the olive oil test reference samples P1 and P4 showed crearly more oil penetration than the enzymatically hydrolysed samples.
Other cellulose derivatives
Obtained solids contents, maximum coat weights for double coated samples and KIT values for the samples with good film formation are summarized in table 6 and staining test results for the different enzymatically hydrolysed cellulose derivatives are shown in figure 5.
Table 6. Solids contents before and after enzymatical degradation, maximum coat weights and KIT values for best samples.
Highest solids contents were obtained for HEC, MHEC, methyl cellulose and HPMC. The higher the solids content the higher the obtained coat weight. Only with HEC, MHEC and HPMC good enough film formation was obtained in order to measure KIT values. HEC and HPMS showed as flat surface good KIT values but creasing caused some cracking and lowered KIT value and showed stain penetration in figure 5.
Enzymatic degradation, Hydroxyethyl cellulose
Hydroxyethyl cellulose 1 , having a molecular weight of Mw = 220000 Da was selected for enzymatic degradation studies. Highest solids content applicable for it in paper coating application is about 7%.
First way (Method 1 ) to carry out the enzymatic degradation was to add enzyme into 7% HEC solution and the solution was heated to 60 °C and 0.2 grams enzyme is added per 100 ml of cellulose derivative solution. Dry HEC powder is added when viscosity decreases. When similar viscosity level to original HEC solution is obtained for enzymatically hydrolysed HEC, the solution was heated to ca 90 °C in order to kill the enzyme, to get stable sample. Molecular weights and solids contents of the hydrolysed HEC prepared using different enzymes according to Method 1 are presented in Table 7:
Second way (Method 2) to carry out the enzymatic treatment was to add dry HEC powder in cold water (solids content ca. 50%) and adding 0.2 grams of enzyme was added per 100 ml of cellulose derivative solution. Sample was heated to 60°C and diluted if viscosity increased too high. After completing the enzymatic degradation,
sample is heated to ca 90°C in order to kill the enzyme. The obtained molecular weight and solids content according to Method 2 are presented in Table 8.
In bench scale reactor (Method 3) enzymatic degradation was carried as follows: Cold water and enzyme was placed in reactor and heated to 60°C. Dry HEC powder was added in 4 batches. Calculated solids content was 17%. After completing the enzymatic degradation, the sample was heated to ca 90°C in order to kill the enzyme. The obtained molecular weight and solids according to Method 3 iare presented s in Table 9.
Enzymatic degradation, Carboxymethyl cellulose
Enzymatic degradation was carried out for two CMC grades; CMC 1 and CMC 2, having molecular weights Mw 261 and 338 kDa, respectively. The enzymatic degradation was carried out by adding enzyme into 10% or 7% CMC solution for CMC 1 and CMC 2, respectively. Samples were heated to 60°C. 0.2 grams of enzyme was added per 100 ml of CMC solution. Solids content was increased as more dry CMC powder was added when viscosity decreases. When similar viscosity level to original CMC solution was obtained for enzymatically hydrolysed product, the sample was heated to ca 90°C in order to kill the enzyme, in order to get stable sample. Obtained molecular weights and solids contents by using different enzymes are presented in Table 10.
Table 10
Enzymatic degradation, Test methods
Enzymatic degradation was carried out in laboratory scale in a glass beaker and magnetic stirrer + heating. In bench scale reaction was carried out in glass reactor with heating and anchor stirrer.
A Brookfield DV-E viscometer was used for measurement of the coating colors’ bulk viscosity immediately after preparation at room temperature (23°C). Different spindles were used in accordance with the respective samples’ viscosity range. The measurements were performed at 100 rpm.
Laboratory coating tests were carried out by using draw down coater K control coater (RK Print Coat Instruments, Litlington, UK) with different wound rods and coating speeds. Samples were dried using InfraRR IR dryers for 60 seconds directly after coating. Double coated samples were prepared. The used substrate was virgin fiber based cartonboard with basis weight of 265 or 247(mentioned separately when used) g/m2. Barrier coating was applied on the uncoated bottom side.
Coat weight was determined by weighting the coated samples and uncoated base papers and coat weight was obtained by the weight difference. Simple converting test was done for the samples including sample creasing using Cyklos CPM 450 creasing and perforation unit. Creasing and folding was done in machine and cross directions. Staining test was done for the creased samples by using methyl red dissolved in ethanol. For folding Cobb roller was used to give uniform folding pressure.
Water resistance was tested using Cobb60 test. Water vapor barrier properties were measured using Systech Permeation Analyzers M7002 instrument. Grease barrier
properties were tested using olive oil following ASTM F119-82 test method. Oil was dropped on cotton fabric placed on the barrier coated side of the folded sample and 50 gram weight was placed on top of the sample. Samples were placed at 40 °C oven for 120 minutes and photographed for evaluation of grease penetration. Blocking tests were carried out at 40 °C temperature and 150 bar pressure for four hours. The barrier coated sample was placed against the top side coating. Used scale for blocking test results is following; 1 : Samples did not adhere together, 2: Noise can be heard when pulling the sample strips apart, 3: Coating defect <50 % of the surface area, 4: Coating defect >50 % of the surface area, 5: Base paper delamination.
The molecular weight was determined by size-exclusion chromatography (SEC) using Viscotek GPCmax TDA 302 SEC equipment. Eluent was 0.1 M NaNOs. Column set consisted of three columns (Waters Ultrahydrogel 2000, 500 and 120) and a guard column. Pullulan standards (Polymer Standards Service) with molecular weights between 342 - 708 000 Da were used for conventional calibration with refractive index detection.
COATIN G/BARRIER TESTS
With calcium carbonate as coating pigment
Using pure enzymes was tested first. Barrier coating was prepared by mixing the different HEC samples with calcium carbonate pigment and polyethylene glycol as plasticizer as presented in Table 11 .
If comparing samples 1 , 2 and 3 at same coat weight (ca. 8 g/m2), it can be seen that grease barrier properties for creased samples improved due to less cracking. Higher coat weights can be obtained as the solids content is higher for the enzymatically hydrolysed HEC. For the higher coat weight samples better KIT values and blocking properties were obtained by using endoglucanase for enzymatic degradation. Also water vapor barrier properties were improved by the enzymatic degradation. Commercial enzymes with endoglucanase as main activity (Fibercare R and U) were tested next. Similar coating formulations, Table 13, were prepared as in the first part.
Similar improvement in WVTR was seen by using Fibercare enzymes that was ob- served with pure enzymes, Table 14. With Fibercare R best KIT values were obtained for coat weight of 8 g/m2. Only minor olive oil penetration was detected for Fibercare samples.
Comparison of different degradation protocols using Fibercare R was carried out using a reference coating formulation containing 30% calcium carbonate and 20% PEG 300, in Table 15. HEC-FCR was prepared by method 1 , HEC- high solids degrading by method 2 and HEC - pilot test by method 3.
Hexane vapor transmission rate (HVTR) was determined by using a cup method. In the method, 20 grams of hexane was placed in a metal cup. Barrier sample was placed on top of the cup between two gaskets, coated side down. Metal frame was used to tighten the sample to the cup. Weight loss of hexane was recorded for 24 hours and calculated per area g/(m2*d).
Method correlates with migration of mineral oil from recycled fiber based packaging to foodstuff
Similar barrier results were obtained with all enzymatic degradation methods. All samples showed good folding properties and good grease, and mineral oil barrier properties (HVTR).
Calcium carbonate as coating pigment + amount of plasticizer
Table 16; 20% plasticizer
The coating formulations with calcium carbonate and 20% of PEG 300 showed no cracking at crease up to 30% pigment content. The same samples showed also good mineral oil barrier properties, Table 16.
The coating formulations with calcium carbonate and 10% of PEG 300 showed only very minor cracking at crease up to 30% pigment content. The same samples showed also good mineral oil barrier properties. The best KIT values for the folded samples were obtained when the pigment content was 20% or lower. Table 17. COATING/BARRIER TESTS; Kaolin and talc as coating pigment
Enzymatic degradation of HEC was done by using Fibercare R enzyme. Base substrate used was folding boxboard, grammage 247 g/m2. Double coating was applied for all samples.
Talc containing coating formulations, Table 18, showed more cracking at fold in staining test but the olive oil test results were still good up to 40% pigment content. HVTR results were at good level for samples containing 10 and 20% talc. Table 19; Kaolin
Claims
1. A coating composition for a sheet like cellulosic fibrous web comprising one or more water-soluble cellulose derivatives selected from the group consisting of alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose, methyl cellulose (MC), and carboxymethyl cellulose hydrolyzed with an enzyme having cellulolytic activity, and having a weight average molecular weight MW is 20 000 Da - 120 000 Da, such as, 30 000 - 100 000 Da.
2. The coating composition of claim 1 , wherein solids content of the coating composition is at least 20 weight-%.
3. The coating composition of claim 1 or 2, wherein viscosity of the coating composition is 50 - 3000 mPas measured at 23 °C using 100 rpm and Brookfield DV- E viscometer.
4. The coating composition of any of claims 1 to 3 or the coating of claim 1 , wherein said coating composition comprises water-soluble cellulose derivatives in amount 20-100 weight-%, preferably 30-90 weight-%, more preferably 40-70 weight-% of the total solids content of the coating composition or the coating.
5. The coating composition of any of claims 1 to 4, wherein the coating composition further comprises one or more constituent(s) selected from the group comprising a plasticizer, pigment particle, starch, binder, thickener, cross-linker, lubricant, and dispersing agent; or the method of any of the previous claims, wherein one or more agents are selected from the group comprising a plasticizer, pigment particle, starch, binder, thickener, cross-linker, lubricant, and dispersing agent.
6. The coating composition of any of claims 1 to 5, wherein one or more water- soluble cellulose derivatives is/are selected from the group comprising or consisting of an alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose; and any combination thereof.
7. The coating composition of claim 6, wherein one or more water-soluble cellulose derivatives is/are selected from the group comprising or consisting of methyl cellulose (MC), carboxymethyl cellulose (CMC), hydroxymethyl cellulose (HMC), hydroxyethyl cellulose (HEC), methyl hydroxyethyl cellulose (MHEC), hydroxypropyl
33 cellulose (HPC), hydroxypropylmethyl cellulose (HPMC), and any combination thereof.
8. A method of preparing a coating composition for a sheet like cellulosic fibrous web by enzymatically degrading one or more water-soluble cellulose derivatives, wherein the method comprises
(a) providing an aqueous suspension of one or more water-soluble cellulose derivatives selected from alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose, methyl cellulose (MC), and carboxymethyl cellulose; and
(b) contacting said suspension with an enzyme having cellulolytic activity;
(c) allowing to react at conditions conductive for activity of said enzyme; and
(d) optionally removing excess of water; and
(e) optionally adding one or more agents to the composition comprising degraded water-soluble cellulose derivatives.
9. The method of claim 8, wherein said one or more water-soluble cellulose derivatives is/are selected from the group comprising or consisting of methyl cellulose (MC), carboxymethyl cellulose (CMC), hydroxymethyl cellulose (HMC), hydroxyethyl cellulose (HEC), methyl hydroxyethyl cellulose (MHEC), hydroxypropyl cellulose (HPC) and hydroxypropylmethyl cellulose (HPMC) and any combination thereof.
10. The method of any of claim 8 to 9, wherein said enzyme having cellulolytic activity is a cellulase such as an endoglucanase enriched cellulase preparation.
11 . The method of any of claims 8 to 11 , wherein the aqueous suspension of one or more water-soluble cellulose derivatives during ncubating / allowing to react at phase_has a consistency of 2 - 20 wt.-% dry matter from the total weight of the total weight of said suspension.
12. A coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web substrate, wherein the substrate has been coated with the coating composition of any of claims 1 to 7 or obtained by method of any of claims 8 to 11 .
13. The coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web of claim 12, wherein the substrate comprises
cellulosic or lignocellulosic fibres, or the substrate is a fibrous web, such as a paper, board, tissue or the like.
14. The coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web of claim 12 or 13, wherein the coated substrate or the product has KIT test value of at least 7, water vapor transmission rate (WVTR) less than 110 g/m2/d; decreased blocking tendency compared to a coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web with a coating obtained without enzymatic degradation; and/or increased grease barrier properties compared to a coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web with a coating obtained without enzymatic degradation.
15. The coated sheet like cellulosic fibrous web or a product comprising said coated sheet like cellulosic fibrous web of any of claims 13 to 15, wherein the coating has a coat weight 3 - 25 g/m2.
16. A method of coating a sheet like cellulosic fibrous web, wherein the method comprises applying the coating composition of any of claims 1 - 7 on said web.
17. A method of improving grease barrier properties of coating for a sheet like cellulosic fibrous web, wherein the method comprises
(a) providing an aqueous suspension of one or more water-soluble cellulose derivatives selected from alkyl cellulose, hydroxyalkyl cellulose, hydroxyalkyl alkyl cellulose, methyl cellulose (MC) and carboxymethyl cellulose; and
(b) contacting said suspension with an enzyme having cellulolytic activity; and
(c) allowing to react at conditions conductive for activity of said enzyme until said water-soluble cellulose derivatives have having a weight average molecular weight MW is 20 000 Da - 120 000 Da, such as, 30 000 -100 000 Da; and
(d) optionally removing excess of water; and
(e) optionally adding one or more agents to the composition comprising hydrolysed water-soluble cellulose derivatives.
18. Use of the enzyme hydrolysed water-soluble cellulose derivatives having a weight average molecular weight MW is 20 000 Da- 120 000 Da, such as 30 000 Da - 100 000 Da as a coating composition for a sheet like cellulosic fibrous web.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FI20216090 | 2021-10-21 | ||
| FI20216090 | 2021-10-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2023067246A1 true WO2023067246A1 (en) | 2023-04-27 |
Family
ID=84043922
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/FI2022/050697 WO2023067246A1 (en) | 2021-10-21 | 2022-10-20 | A coating composition, method for preparing such composition, a method of coating, a coated sheet and uses of the coating |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2023067246A1 (en) |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0382578A2 (en) * | 1989-02-10 | 1990-08-16 | Alko Ltd. | Substitution of degraded cellulose derivatives for high calorie ingredients in foods |
| WO1994028117A1 (en) * | 1993-06-02 | 1994-12-08 | Oy Alko Ab | Novel endoglucanase enzyme |
| EP0894834A2 (en) * | 1997-07-28 | 1999-02-03 | Hercules Incorporated | Biostable water-borne paints and processes for their preparation |
| WO2008036315A2 (en) * | 2006-09-20 | 2008-03-27 | Nanopaper, Llc | Grease resistant formulations |
| WO2008112419A2 (en) * | 2007-03-13 | 2008-09-18 | Cp Kelco U.S., Inc. | Improved paint formulations comprising cellulose ether/network building polymer fluid gel thickeners |
| KR101039637B1 (en) * | 2010-10-06 | 2011-06-08 | 김관수 | Water soluble coating materials for paper coating and manufacturing method thereof |
| EP2399980A1 (en) * | 2010-06-24 | 2011-12-28 | The Procter & Gamble Company | Stable compositions comprising cationic cellulose polymer and cellulase |
| US20130165555A1 (en) * | 2010-09-08 | 2013-06-27 | Sakura Color Products Corporation | Aqueous painting material composition |
-
2022
- 2022-10-20 WO PCT/FI2022/050697 patent/WO2023067246A1/en active Application Filing
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0382578A2 (en) * | 1989-02-10 | 1990-08-16 | Alko Ltd. | Substitution of degraded cellulose derivatives for high calorie ingredients in foods |
| WO1994028117A1 (en) * | 1993-06-02 | 1994-12-08 | Oy Alko Ab | Novel endoglucanase enzyme |
| EP0894834A2 (en) * | 1997-07-28 | 1999-02-03 | Hercules Incorporated | Biostable water-borne paints and processes for their preparation |
| WO2008036315A2 (en) * | 2006-09-20 | 2008-03-27 | Nanopaper, Llc | Grease resistant formulations |
| WO2008112419A2 (en) * | 2007-03-13 | 2008-09-18 | Cp Kelco U.S., Inc. | Improved paint formulations comprising cellulose ether/network building polymer fluid gel thickeners |
| EP2399980A1 (en) * | 2010-06-24 | 2011-12-28 | The Procter & Gamble Company | Stable compositions comprising cationic cellulose polymer and cellulase |
| US20130165555A1 (en) * | 2010-09-08 | 2013-06-27 | Sakura Color Products Corporation | Aqueous painting material composition |
| KR101039637B1 (en) * | 2010-10-06 | 2011-06-08 | 김관수 | Water soluble coating materials for paper coating and manufacturing method thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8241756B2 (en) | Composition for coating of printing paper | |
| JP2024519934A (en) | Coated paper for use as packaging material | |
| US20230383468A1 (en) | Oil Resistant Article | |
| CN112342839A (en) | Trace coating food packaging paper and preparation method thereof | |
| DE212012000194U1 (en) | A migration barrier film or coating comprising hemicellulose | |
| US20140230691A1 (en) | Coating and a method for preparing thereof | |
| CN116670043A (en) | Aqueous ethylcellulose dispersion | |
| EP3810856B1 (en) | Coating structure, sheet-like product and its use | |
| WO2023067246A1 (en) | A coating composition, method for preparing such composition, a method of coating, a coated sheet and uses of the coating | |
| EP4172280A1 (en) | Barrier coating for paper and paperboard | |
| WO2021255334A1 (en) | Coating structure, sheet-like product and its use | |
| CN114585694A (en) | Coating for reducing oil absorption of a cellulosic web | |
| WO2024218669A1 (en) | Barrier coating for paper and paperboard | |
| US20220243402A1 (en) | Method for manufacturing a coated cellulosic substrate and a coated cellulosic substrate | |
| US20240271368A1 (en) | Barrier coating for paper and paperboard | |
| GB2617573A (en) | Material having a barrier layer comprising lignosulfonate | |
| Fithriyah et al. | Mechanical properties of paper sheets coated with chitosan nanoparticle | |
| SE544664C2 (en) | Flourocarbon free and biobased oil and water barrier materials |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22797406 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 22797406 Country of ref document: EP Kind code of ref document: A1 |