WO2023013487A1 - Multicomponent curable composition and utilization thereof - Google Patents
Multicomponent curable composition and utilization thereof Download PDFInfo
- Publication number
- WO2023013487A1 WO2023013487A1 PCT/JP2022/028867 JP2022028867W WO2023013487A1 WO 2023013487 A1 WO2023013487 A1 WO 2023013487A1 JP 2022028867 W JP2022028867 W JP 2022028867W WO 2023013487 A1 WO2023013487 A1 WO 2023013487A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- curable composition
- epoxy resin
- polyoxyalkylene polymer
- weight
- Prior art date
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 117
- 229920000642 polymer Polymers 0.000 claims abstract description 138
- -1 mercaptan compound Chemical class 0.000 claims abstract description 114
- 239000003822 epoxy resin Substances 0.000 claims abstract description 88
- 229920000647 polyepoxide Polymers 0.000 claims abstract description 88
- 239000003795 chemical substances by application Substances 0.000 claims abstract description 82
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims abstract description 70
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical group [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims abstract description 59
- FZHAPNGMFPVSLP-UHFFFAOYSA-N silanamine Chemical compound [SiH3]N FZHAPNGMFPVSLP-UHFFFAOYSA-N 0.000 claims abstract description 23
- 239000000463 material Substances 0.000 claims description 36
- 125000004432 carbon atom Chemical group C* 0.000 claims description 35
- 239000011256 inorganic filler Substances 0.000 claims description 20
- 229910003475 inorganic filler Inorganic materials 0.000 claims description 20
- 238000000576 coating method Methods 0.000 claims description 16
- 239000011248 coating agent Substances 0.000 claims description 15
- 238000004519 manufacturing process Methods 0.000 claims description 13
- 239000007788 liquid Substances 0.000 claims description 10
- WNAHIZMDSQCWRP-UHFFFAOYSA-N dodecane-1-thiol Chemical compound CCCCCCCCCCCCS WNAHIZMDSQCWRP-UHFFFAOYSA-N 0.000 claims description 9
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 8
- 150000003512 tertiary amines Chemical class 0.000 claims description 8
- IKYAJDOSWUATPI-UHFFFAOYSA-N 3-[dimethoxy(methyl)silyl]propane-1-thiol Chemical compound CO[Si](C)(OC)CCCS IKYAJDOSWUATPI-UHFFFAOYSA-N 0.000 claims description 7
- 125000003700 epoxy group Chemical group 0.000 claims description 7
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 claims description 6
- 125000005842 heteroatom Chemical group 0.000 claims description 6
- WYTZZXDRDKSJID-UHFFFAOYSA-N (3-aminopropyl)triethoxysilane Chemical compound CCO[Si](OCC)(OCC)CCCN WYTZZXDRDKSJID-UHFFFAOYSA-N 0.000 claims description 5
- IMQFZQVZKBIPCQ-UHFFFAOYSA-N 2,2-bis(3-sulfanylpropanoyloxymethyl)butyl 3-sulfanylpropanoate Chemical compound SCCC(=O)OCC(CC)(COC(=O)CCS)COC(=O)CCS IMQFZQVZKBIPCQ-UHFFFAOYSA-N 0.000 claims description 5
- 125000004429 atom Chemical group 0.000 claims description 5
- 229910052799 carbon Inorganic materials 0.000 claims description 5
- QJOOZNCPHALTKK-UHFFFAOYSA-N trimethoxysilylmethanethiol Chemical compound CO[Si](CS)(OC)OC QJOOZNCPHALTKK-UHFFFAOYSA-N 0.000 claims description 5
- YAJYJWXEWKRTPO-UHFFFAOYSA-N 2,3,3,4,4,5-hexamethylhexane-2-thiol Chemical compound CC(C)C(C)(C)C(C)(C)C(C)(C)S YAJYJWXEWKRTPO-UHFFFAOYSA-N 0.000 claims description 4
- XZQIZFNSIXIEBH-UHFFFAOYSA-N 3-[chloromethyl(dimethoxy)silyl]propane-1-thiol Chemical compound CO[Si](CCl)(OC)CCCS XZQIZFNSIXIEBH-UHFFFAOYSA-N 0.000 claims description 4
- FWWKQFGBMPLAND-UHFFFAOYSA-N 3-[dimethoxy(methoxymethyl)silyl]propane-1-thiol Chemical compound COC[Si](OC)(OC)CCCS FWWKQFGBMPLAND-UHFFFAOYSA-N 0.000 claims description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 4
- JOBBTVPTPXRUBP-UHFFFAOYSA-N [3-(3-sulfanylpropanoyloxy)-2,2-bis(3-sulfanylpropanoyloxymethyl)propyl] 3-sulfanylpropanoate Chemical compound SCCC(=O)OCC(COC(=O)CCS)(COC(=O)CCS)COC(=O)CCS JOBBTVPTPXRUBP-UHFFFAOYSA-N 0.000 claims description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 4
- KTJUNCYQALUJRL-UHFFFAOYSA-N dimethoxymethylsilylmethanethiol Chemical compound COC(OC)[SiH2]CS KTJUNCYQALUJRL-UHFFFAOYSA-N 0.000 claims description 4
- 229910052739 hydrogen Inorganic materials 0.000 claims description 4
- 239000001257 hydrogen Substances 0.000 claims description 4
- 125000005647 linker group Chemical group 0.000 claims description 4
- 229910052757 nitrogen Inorganic materials 0.000 claims description 4
- 229910052760 oxygen Inorganic materials 0.000 claims description 4
- 239000001301 oxygen Substances 0.000 claims description 4
- YUYCVXFAYWRXLS-UHFFFAOYSA-N trimethoxysilane Chemical compound CO[SiH](OC)OC YUYCVXFAYWRXLS-UHFFFAOYSA-N 0.000 claims description 4
- 125000001183 hydrocarbyl group Chemical group 0.000 claims 5
- 239000000047 product Substances 0.000 description 51
- 238000001723 curing Methods 0.000 description 43
- 150000001875 compounds Chemical class 0.000 description 39
- 238000000034 method Methods 0.000 description 37
- 150000002430 hydrocarbons Chemical group 0.000 description 35
- 229920005989 resin Polymers 0.000 description 22
- 239000011347 resin Substances 0.000 description 22
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 20
- 239000003054 catalyst Substances 0.000 description 19
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 18
- 229920001451 polypropylene glycol Polymers 0.000 description 18
- CREMABGTGYGIQB-UHFFFAOYSA-N carbon carbon Chemical compound C.C CREMABGTGYGIQB-UHFFFAOYSA-N 0.000 description 15
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 14
- 230000008901 benefit Effects 0.000 description 14
- 239000004014 plasticizer Substances 0.000 description 14
- 229920000620 organic polymer Polymers 0.000 description 13
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 12
- 150000004756 silanes Chemical class 0.000 description 11
- 238000009826 distribution Methods 0.000 description 9
- 239000003566 sealing material Substances 0.000 description 9
- 238000004078 waterproofing Methods 0.000 description 9
- 239000004593 Epoxy Substances 0.000 description 8
- 239000012024 dehydrating agents Substances 0.000 description 8
- 239000000945 filler Substances 0.000 description 8
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 8
- UUEWCQRISZBELL-UHFFFAOYSA-N 3-trimethoxysilylpropane-1-thiol Chemical compound CO[Si](OC)(OC)CCCS UUEWCQRISZBELL-UHFFFAOYSA-N 0.000 description 7
- 239000003963 antioxidant agent Substances 0.000 description 7
- 229910000019 calcium carbonate Inorganic materials 0.000 description 7
- 150000008282 halocarbons Chemical class 0.000 description 7
- 239000011572 manganese Substances 0.000 description 7
- 238000007665 sagging Methods 0.000 description 7
- OSDWBNJEKMUWAV-UHFFFAOYSA-N Allyl chloride Chemical compound ClCC=C OSDWBNJEKMUWAV-UHFFFAOYSA-N 0.000 description 6
- 239000006087 Silane Coupling Agent Substances 0.000 description 6
- 239000000853 adhesive Substances 0.000 description 6
- 230000001070 adhesive effect Effects 0.000 description 6
- XYYQWMDBQFSCPB-UHFFFAOYSA-N dimethoxymethylsilane Chemical compound COC([SiH3])OC XYYQWMDBQFSCPB-UHFFFAOYSA-N 0.000 description 6
- 239000006260 foam Substances 0.000 description 6
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate group Chemical group [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 description 6
- 239000004611 light stabiliser Substances 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- 229910052710 silicon Inorganic materials 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- AHDSRXYHVZECER-UHFFFAOYSA-N 2,4,6-tris[(dimethylamino)methyl]phenol Chemical compound CN(C)CC1=CC(CN(C)C)=C(O)C(CN(C)C)=C1 AHDSRXYHVZECER-UHFFFAOYSA-N 0.000 description 5
- STMDPCBYJCIZOD-UHFFFAOYSA-N 2-(2,4-dinitroanilino)-4-methylpentanoic acid Chemical compound CC(C)CC(C(O)=O)NC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O STMDPCBYJCIZOD-UHFFFAOYSA-N 0.000 description 5
- OHXAOPZTJOUYKM-UHFFFAOYSA-N 3-Chloro-2-methylpropene Chemical compound CC(=C)CCl OHXAOPZTJOUYKM-UHFFFAOYSA-N 0.000 description 5
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 5
- 150000001412 amines Chemical class 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical class C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 239000007795 chemical reaction product Substances 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 125000000524 functional group Chemical group 0.000 description 5
- 230000006872 improvement Effects 0.000 description 5
- 239000003999 initiator Substances 0.000 description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 5
- 239000013500 performance material Substances 0.000 description 5
- 229920001568 phenolic resin Polymers 0.000 description 5
- 239000005011 phenolic resin Substances 0.000 description 5
- 238000006116 polymerization reaction Methods 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 230000009257 reactivity Effects 0.000 description 5
- 239000011342 resin composition Substances 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- 125000003396 thiol group Chemical group [H]S* 0.000 description 5
- 229910052725 zinc Inorganic materials 0.000 description 5
- 239000011701 zinc Substances 0.000 description 5
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 4
- FMGBDYLOANULLW-UHFFFAOYSA-N 3-isocyanatopropyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)CCCN=C=O FMGBDYLOANULLW-UHFFFAOYSA-N 0.000 description 4
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 4
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 4
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 4
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 4
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 4
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 4
- 229910052783 alkali metal Inorganic materials 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- WGQKYBSKWIADBV-UHFFFAOYSA-N benzylamine Chemical compound NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- PAFZNILMFXTMIY-UHFFFAOYSA-N cyclohexylamine Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 description 4
- AYOHIQLKSOJJQH-UHFFFAOYSA-N dibutyltin Chemical compound CCCC[Sn]CCCC AYOHIQLKSOJJQH-UHFFFAOYSA-N 0.000 description 4
- GYZLOYUZLJXAJU-UHFFFAOYSA-N diglycidyl ether Chemical class C1OC1COCC1CO1 GYZLOYUZLJXAJU-UHFFFAOYSA-N 0.000 description 4
- 238000007323 disproportionation reaction Methods 0.000 description 4
- 238000011156 evaluation Methods 0.000 description 4
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 4
- 239000012948 isocyanate Substances 0.000 description 4
- 150000002513 isocyanates Chemical class 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- PHQOGHDTIVQXHL-UHFFFAOYSA-N n'-(3-trimethoxysilylpropyl)ethane-1,2-diamine Chemical compound CO[Si](OC)(OC)CCCNCCN PHQOGHDTIVQXHL-UHFFFAOYSA-N 0.000 description 4
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 229920001228 polyisocyanate Polymers 0.000 description 4
- 239000005056 polyisocyanate Substances 0.000 description 4
- 238000003860 storage Methods 0.000 description 4
- 239000006097 ultraviolet radiation absorber Substances 0.000 description 4
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 3
- ZYAASQNKCWTPKI-UHFFFAOYSA-N 3-[dimethoxy(methyl)silyl]propan-1-amine Chemical compound CO[Si](C)(OC)CCCN ZYAASQNKCWTPKI-UHFFFAOYSA-N 0.000 description 3
- USEGQJLHQSTGHW-UHFFFAOYSA-N 3-bromo-2-methylprop-1-ene Chemical compound CC(=C)CBr USEGQJLHQSTGHW-UHFFFAOYSA-N 0.000 description 3
- SJECZPVISLOESU-UHFFFAOYSA-N 3-trimethoxysilylpropan-1-amine Chemical compound CO[Si](OC)(OC)CCCN SJECZPVISLOESU-UHFFFAOYSA-N 0.000 description 3
- RSWGJHLUYNHPMX-UHFFFAOYSA-N Abietic-Saeure Natural products C12CCC(C(C)C)=CC2=CCC2C1(C)CCCC2(C)C(O)=O RSWGJHLUYNHPMX-UHFFFAOYSA-N 0.000 description 3
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 3
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- KHPCPRHQVVSZAH-HUOMCSJISA-N Rosin Natural products O(C/C=C/c1ccccc1)[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 KHPCPRHQVVSZAH-HUOMCSJISA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- BHELZAPQIKSEDF-UHFFFAOYSA-N allyl bromide Chemical compound BrCC=C BHELZAPQIKSEDF-UHFFFAOYSA-N 0.000 description 3
- HFEHLDPGIKPNKL-UHFFFAOYSA-N allyl iodide Chemical compound ICC=C HFEHLDPGIKPNKL-UHFFFAOYSA-N 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 230000003078 antioxidant effect Effects 0.000 description 3
- TZCXTZWJZNENPQ-UHFFFAOYSA-L barium sulfate Chemical compound [Ba+2].[O-]S([O-])(=O)=O TZCXTZWJZNENPQ-UHFFFAOYSA-L 0.000 description 3
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 3
- 239000012964 benzotriazole Substances 0.000 description 3
- BJQHLKABXJIVAM-UHFFFAOYSA-N bis(2-ethylhexyl) phthalate Chemical compound CCCCC(CC)COC(=O)C1=CC=CC=C1C(=O)OCC(CC)CCCC BJQHLKABXJIVAM-UHFFFAOYSA-N 0.000 description 3
- INLLPKCGLOXCIV-UHFFFAOYSA-N bromoethene Chemical compound BrC=C INLLPKCGLOXCIV-UHFFFAOYSA-N 0.000 description 3
- 238000009833 condensation Methods 0.000 description 3
- 230000005494 condensation Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- UAOMVDZJSHZZME-UHFFFAOYSA-N diisopropylamine Chemical compound CC(C)NC(C)C UAOMVDZJSHZZME-UHFFFAOYSA-N 0.000 description 3
- YQGOWXYZDLJBFL-UHFFFAOYSA-N dimethoxysilane Chemical compound CO[SiH2]OC YQGOWXYZDLJBFL-UHFFFAOYSA-N 0.000 description 3
- JRBPAEWTRLWTQC-UHFFFAOYSA-N dodecylamine Chemical compound CCCCCCCCCCCCN JRBPAEWTRLWTQC-UHFFFAOYSA-N 0.000 description 3
- NKSJNEHGWDZZQF-UHFFFAOYSA-N ethenyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)C=C NKSJNEHGWDZZQF-UHFFFAOYSA-N 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- GHXZPUGJZVBLGC-UHFFFAOYSA-N iodoethene Chemical compound IC=C GHXZPUGJZVBLGC-UHFFFAOYSA-N 0.000 description 3
- 238000000691 measurement method Methods 0.000 description 3
- 125000004184 methoxymethyl group Chemical group [H]C([H])([H])OC([H])([H])* 0.000 description 3
- 239000003208 petroleum Substances 0.000 description 3
- 150000003254 radicals Chemical class 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 150000003505 terpenes Chemical class 0.000 description 3
- 235000007586 terpenes Nutrition 0.000 description 3
- 150000003606 tin compounds Chemical class 0.000 description 3
- KHPCPRHQVVSZAH-UHFFFAOYSA-N trans-cinnamyl beta-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OCC=CC1=CC=CC=C1 KHPCPRHQVVSZAH-UHFFFAOYSA-N 0.000 description 3
- UKRDPEFKFJNXQM-UHFFFAOYSA-N vinylsilane Chemical compound [SiH3]C=C UKRDPEFKFJNXQM-UHFFFAOYSA-N 0.000 description 3
- 239000011787 zinc oxide Substances 0.000 description 3
- 235000014692 zinc oxide Nutrition 0.000 description 3
- 229910052726 zirconium Inorganic materials 0.000 description 3
- QGLWBTPVKHMVHM-KTKRTIGZSA-N (z)-octadec-9-en-1-amine Chemical compound CCCCCCCC\C=C/CCCCCCCCN QGLWBTPVKHMVHM-KTKRTIGZSA-N 0.000 description 2
- KUBDPQJOLOUJRM-UHFFFAOYSA-N 2-(chloromethyl)oxirane;4-[2-(4-hydroxyphenyl)propan-2-yl]phenol Chemical compound ClCC1CO1.C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 KUBDPQJOLOUJRM-UHFFFAOYSA-N 0.000 description 2
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N 2-ethoxyethanol Chemical compound CCOCCO ZNQVEEAIQZEUHB-UHFFFAOYSA-N 0.000 description 2
- HXLAEGYMDGUSBD-UHFFFAOYSA-N 3-[diethoxy(methyl)silyl]propan-1-amine Chemical compound CCO[Si](C)(OCC)CCCN HXLAEGYMDGUSBD-UHFFFAOYSA-N 0.000 description 2
- CWLKGDAVCFYWJK-UHFFFAOYSA-N 3-aminophenol Chemical compound NC1=CC=CC(O)=C1 CWLKGDAVCFYWJK-UHFFFAOYSA-N 0.000 description 2
- HVSJHYFLAANPJS-UHFFFAOYSA-N 3-iodo-2-methylprop-1-ene Chemical compound CC(=C)CI HVSJHYFLAANPJS-UHFFFAOYSA-N 0.000 description 2
- WGCICQJXVYFFCA-UHFFFAOYSA-N 3-iodoprop-1-yne Chemical compound ICC#C WGCICQJXVYFFCA-UHFFFAOYSA-N 0.000 description 2
- NNTRMVRTACZZIO-UHFFFAOYSA-N 3-isocyanatopropyl-dimethoxy-methylsilane Chemical compound CO[Si](C)(OC)CCCN=C=O NNTRMVRTACZZIO-UHFFFAOYSA-N 0.000 description 2
- DCQBZYNUSLHVJC-UHFFFAOYSA-N 3-triethoxysilylpropane-1-thiol Chemical compound CCO[Si](OCC)(OCC)CCCS DCQBZYNUSLHVJC-UHFFFAOYSA-N 0.000 description 2
- VLJQDHDVZJXNQL-UHFFFAOYSA-N 4-methyl-n-(oxomethylidene)benzenesulfonamide Chemical compound CC1=CC=C(S(=O)(=O)N=C=O)C=C1 VLJQDHDVZJXNQL-UHFFFAOYSA-N 0.000 description 2
- XFXPMWWXUTWYJX-UHFFFAOYSA-N Cyanide Chemical compound N#[C-] XFXPMWWXUTWYJX-UHFFFAOYSA-N 0.000 description 2
- ZVFDTKUVRCTHQE-UHFFFAOYSA-N Diisodecyl phthalate Chemical compound CC(C)CCCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCCC(C)C ZVFDTKUVRCTHQE-UHFFFAOYSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- ZRALSGWEFCBTJO-UHFFFAOYSA-N Guanidine Chemical compound NC(N)=N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 2
- 239000013032 Hydrocarbon resin Substances 0.000 description 2
- LSDPWZHWYPCBBB-UHFFFAOYSA-N Methanethiol Chemical compound SC LSDPWZHWYPCBBB-UHFFFAOYSA-N 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- KWYHDKDOAIKMQN-UHFFFAOYSA-N N,N,N',N'-tetramethylethylenediamine Chemical compound CN(C)CCN(C)C KWYHDKDOAIKMQN-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 2
- 239000006096 absorbing agent Substances 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000002947 alkylene group Chemical group 0.000 description 2
- XXROGKLTLUQVRX-UHFFFAOYSA-N allyl alcohol Chemical compound OCC=C XXROGKLTLUQVRX-UHFFFAOYSA-N 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 150000004982 aromatic amines Chemical class 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- ZUMQLFHCCNIEAO-UHFFFAOYSA-N bis(ethenyl)-silyloxysilane platinum Chemical compound [Pt].[SiH3]O[SiH](C=C)C=C ZUMQLFHCCNIEAO-UHFFFAOYSA-N 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000006229 carbon black Substances 0.000 description 2
- 150000001735 carboxylic acids Chemical class 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 239000013522 chelant Substances 0.000 description 2
- 125000004218 chloromethyl group Chemical group [H]C([H])(Cl)* 0.000 description 2
- 239000004927 clay Substances 0.000 description 2
- 229910052570 clay Inorganic materials 0.000 description 2
- 229920006026 co-polymeric resin Polymers 0.000 description 2
- 238000004581 coalescence Methods 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 230000006866 deterioration Effects 0.000 description 2
- JQVDAXLFBXTEQA-UHFFFAOYSA-N dibutylamine Chemical compound CCCCNCCCC JQVDAXLFBXTEQA-UHFFFAOYSA-N 0.000 description 2
- BFQCAXFSZDLJSN-UHFFFAOYSA-N dimethylsilyloxy-dimethyl-(2-trimethoxysilylethyl)silane Chemical compound CO[Si](OC)(OC)CC[Si](C)(C)O[SiH](C)C BFQCAXFSZDLJSN-UHFFFAOYSA-N 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 2
- 239000003063 flame retardant Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- NAQMVNRVTILPCV-UHFFFAOYSA-N hexane-1,6-diamine Chemical compound NCCCCCCN NAQMVNRVTILPCV-UHFFFAOYSA-N 0.000 description 2
- 229920006270 hydrocarbon resin Polymers 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 2
- UIUXUFNYAYAMOE-UHFFFAOYSA-N methylsilane Chemical compound [SiH3]C UIUXUFNYAYAMOE-UHFFFAOYSA-N 0.000 description 2
- 239000011259 mixed solution Substances 0.000 description 2
- 239000003607 modifier Substances 0.000 description 2
- KBJFYLLAMSZSOG-UHFFFAOYSA-N n-(3-trimethoxysilylpropyl)aniline Chemical compound CO[Si](OC)(OC)CCCNC1=CC=CC=C1 KBJFYLLAMSZSOG-UHFFFAOYSA-N 0.000 description 2
- 229920003986 novolac Polymers 0.000 description 2
- 125000005702 oxyalkylene group Chemical group 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 229920000768 polyamine Polymers 0.000 description 2
- 229920002503 polyoxyethylene-polyoxypropylene Polymers 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- BDAWXSQJJCIFIK-UHFFFAOYSA-N potassium methoxide Chemical compound [K+].[O-]C BDAWXSQJJCIFIK-UHFFFAOYSA-N 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- YORCIIVHUBAYBQ-UHFFFAOYSA-N propargyl bromide Chemical compound BrCC#C YORCIIVHUBAYBQ-UHFFFAOYSA-N 0.000 description 2
- LJZPPWWHKPGCHS-UHFFFAOYSA-N propargyl chloride Chemical compound ClCC#C LJZPPWWHKPGCHS-UHFFFAOYSA-N 0.000 description 2
- 238000004080 punching Methods 0.000 description 2
- 239000000376 reactant Substances 0.000 description 2
- 230000035484 reaction time Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 229910000077 silane Inorganic materials 0.000 description 2
- SCPYDCQAZCOKTP-UHFFFAOYSA-N silanol Chemical compound [SiH3]O SCPYDCQAZCOKTP-UHFFFAOYSA-N 0.000 description 2
- 150000004760 silicates Chemical class 0.000 description 2
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 230000002194 synthesizing effect Effects 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- QQQSFSZALRVCSZ-UHFFFAOYSA-N triethoxysilane Chemical compound CCO[SiH](OCC)OCC QQQSFSZALRVCSZ-UHFFFAOYSA-N 0.000 description 2
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 2
- OBETXYAYXDNJHR-SSDOTTSWSA-M (2r)-2-ethylhexanoate Chemical compound CCCC[C@@H](CC)C([O-])=O OBETXYAYXDNJHR-SSDOTTSWSA-M 0.000 description 1
- YOBOXHGSEJBUPB-MTOQALJVSA-N (z)-4-hydroxypent-3-en-2-one;zirconium Chemical compound [Zr].C\C(O)=C\C(C)=O.C\C(O)=C\C(C)=O.C\C(O)=C\C(C)=O.C\C(O)=C\C(C)=O YOBOXHGSEJBUPB-MTOQALJVSA-N 0.000 description 1
- TYKCBTYOMAUNLH-MTOQALJVSA-J (z)-4-oxopent-2-en-2-olate;titanium(4+) Chemical compound [Ti+4].C\C([O-])=C\C(C)=O.C\C([O-])=C\C(C)=O.C\C([O-])=C\C(C)=O.C\C([O-])=C\C(C)=O TYKCBTYOMAUNLH-MTOQALJVSA-J 0.000 description 1
- ZBBLRPRYYSJUCZ-GRHBHMESSA-L (z)-but-2-enedioate;dibutyltin(2+) Chemical compound [O-]C(=O)\C=C/C([O-])=O.CCCC[Sn+2]CCCC ZBBLRPRYYSJUCZ-GRHBHMESSA-L 0.000 description 1
- OUPZKGBUJRBPGC-UHFFFAOYSA-N 1,3,5-tris(oxiran-2-ylmethyl)-1,3,5-triazinane-2,4,6-trione Chemical compound O=C1N(CC2OC2)C(=O)N(CC2OC2)C(=O)N1CC1CO1 OUPZKGBUJRBPGC-UHFFFAOYSA-N 0.000 description 1
- OWRCNXZUPFZXOS-UHFFFAOYSA-N 1,3-diphenylguanidine Chemical compound C=1C=CC=CC=1NC(=N)NC1=CC=CC=C1 OWRCNXZUPFZXOS-UHFFFAOYSA-N 0.000 description 1
- SAJMJXZROMTSEF-UHFFFAOYSA-N 1,4-dibromobut-2-yne Chemical compound BrCC#CCBr SAJMJXZROMTSEF-UHFFFAOYSA-N 0.000 description 1
- RCHDLEVSZBOHOS-UHFFFAOYSA-N 1,4-dichlorobut-2-yne Chemical compound ClCC#CCCl RCHDLEVSZBOHOS-UHFFFAOYSA-N 0.000 description 1
- COJDINJLLPTTSL-UHFFFAOYSA-N 1,4-diiodobut-2-yne Chemical compound ICC#CCI COJDINJLLPTTSL-UHFFFAOYSA-N 0.000 description 1
- FJLUATLTXUNBOT-UHFFFAOYSA-N 1-Hexadecylamine Chemical compound CCCCCCCCCCCCCCCCN FJLUATLTXUNBOT-UHFFFAOYSA-N 0.000 description 1
- AAQUUNGVNHQEHA-UHFFFAOYSA-N 1-[3-(dimethylamino)propyl-methylamino]ethanol Chemical compound CC(O)N(C)CCCN(C)C AAQUUNGVNHQEHA-UHFFFAOYSA-N 0.000 description 1
- JPZYXGPCHFZBHO-UHFFFAOYSA-N 1-aminopentadecane Chemical compound CCCCCCCCCCCCCCCN JPZYXGPCHFZBHO-UHFFFAOYSA-N 0.000 description 1
- KPAPHODVWOVUJL-UHFFFAOYSA-N 1-benzofuran;1h-indene Chemical compound C1=CC=C2CC=CC2=C1.C1=CC=C2OC=CC2=C1 KPAPHODVWOVUJL-UHFFFAOYSA-N 0.000 description 1
- LNNXOEHOXSYWLD-UHFFFAOYSA-N 1-bromobut-2-yne Chemical compound CC#CCBr LNNXOEHOXSYWLD-UHFFFAOYSA-N 0.000 description 1
- QKPBYOBXEXNWOA-UHFFFAOYSA-N 1-bromooct-2-yne Chemical compound CCCCCC#CCBr QKPBYOBXEXNWOA-UHFFFAOYSA-N 0.000 description 1
- VDHGRVFJBGRHMD-UHFFFAOYSA-N 1-bromopent-2-yne Chemical compound CCC#CCBr VDHGRVFJBGRHMD-UHFFFAOYSA-N 0.000 description 1
- OKWUYBGGPXXFLS-UHFFFAOYSA-N 1-chlorobut-2-yne Chemical compound CC#CCCl OKWUYBGGPXXFLS-UHFFFAOYSA-N 0.000 description 1
- OUMUOQQKEAGFCJ-UHFFFAOYSA-N 1-chlorooct-2-yne Chemical compound CCCCCC#CCCl OUMUOQQKEAGFCJ-UHFFFAOYSA-N 0.000 description 1
- JBKXDMAPOZZDCE-UHFFFAOYSA-N 1-chloropent-2-yne Chemical compound CCC#CCCl JBKXDMAPOZZDCE-UHFFFAOYSA-N 0.000 description 1
- LKAIOZOVAZYTRN-UHFFFAOYSA-N 1-iodobut-2-yne Chemical compound CC#CCI LKAIOZOVAZYTRN-UHFFFAOYSA-N 0.000 description 1
- ZGXTTXXIZMBGIL-UHFFFAOYSA-N 1-iodooct-2-yne Chemical compound CCCCCC#CCI ZGXTTXXIZMBGIL-UHFFFAOYSA-N 0.000 description 1
- BIXFMRXHWVNDBA-UHFFFAOYSA-N 1-iodopent-2-yne Chemical compound CCC#CCI BIXFMRXHWVNDBA-UHFFFAOYSA-N 0.000 description 1
- RAXXELZNTBOGNW-UHFFFAOYSA-N 1H-imidazole Chemical compound C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 1
- VILCJCGEZXAXTO-UHFFFAOYSA-N 2,2,2-tetramine Chemical compound NCCNCCNCCN VILCJCGEZXAXTO-UHFFFAOYSA-N 0.000 description 1
- RNFJDJUURJAICM-UHFFFAOYSA-N 2,2,4,4,6,6-hexaphenoxy-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyclohexa-1,3,5-triene Chemical compound N=1P(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP(OC=2C=CC=CC=2)(OC=2C=CC=CC=2)=NP=1(OC=1C=CC=CC=1)OC1=CC=CC=C1 RNFJDJUURJAICM-UHFFFAOYSA-N 0.000 description 1
- YOYAIZYFCNQIRF-UHFFFAOYSA-N 2,6-dichlorobenzonitrile Chemical compound ClC1=CC=CC(Cl)=C1C#N YOYAIZYFCNQIRF-UHFFFAOYSA-N 0.000 description 1
- LQZDDWKUQKQXGC-UHFFFAOYSA-N 2-(2-methylprop-2-enoxymethyl)oxirane Chemical compound CC(=C)COCC1CO1 LQZDDWKUQKQXGC-UHFFFAOYSA-N 0.000 description 1
- OLFNXLXEGXRUOI-UHFFFAOYSA-N 2-(benzotriazol-2-yl)-4,6-bis(2-phenylpropan-2-yl)phenol Chemical compound C=1C(N2N=C3C=CC=CC3=N2)=C(O)C(C(C)(C)C=2C=CC=CC=2)=CC=1C(C)(C)C1=CC=CC=C1 OLFNXLXEGXRUOI-UHFFFAOYSA-N 0.000 description 1
- IYAZLDLPUNDVAG-UHFFFAOYSA-N 2-(benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl)phenol Chemical compound CC(C)(C)CC(C)(C)C1=CC=C(O)C(N2N=C3C=CC=CC3=N2)=C1 IYAZLDLPUNDVAG-UHFFFAOYSA-N 0.000 description 1
- LKMJVFRMDSNFRT-UHFFFAOYSA-N 2-(methoxymethyl)oxirane Chemical compound COCC1CO1 LKMJVFRMDSNFRT-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- AOBIOSPNXBMOAT-UHFFFAOYSA-N 2-[2-(oxiran-2-ylmethoxy)ethoxymethyl]oxirane Chemical compound C1OC1COCCOCC1CO1 AOBIOSPNXBMOAT-UHFFFAOYSA-N 0.000 description 1
- 229940093475 2-ethoxyethanol Drugs 0.000 description 1
- OVEUFHOBGCSKSH-UHFFFAOYSA-N 2-methyl-n,n-bis(oxiran-2-ylmethyl)aniline Chemical compound CC1=CC=CC=C1N(CC1OC1)CC1OC1 OVEUFHOBGCSKSH-UHFFFAOYSA-N 0.000 description 1
- VEORPZCZECFIRK-UHFFFAOYSA-N 3,3',5,5'-tetrabromobisphenol A Chemical compound C=1C(Br)=C(O)C(Br)=CC=1C(C)(C)C1=CC(Br)=C(O)C(Br)=C1 VEORPZCZECFIRK-UHFFFAOYSA-N 0.000 description 1
- OZFSDOFRXYCNKS-UHFFFAOYSA-N 3-[diethoxy(1-phenylpropan-2-yloxy)silyl]-n-ethenylpropan-1-amine Chemical compound C=CNCCC[Si](OCC)(OCC)OC(C)CC1=CC=CC=C1 OZFSDOFRXYCNKS-UHFFFAOYSA-N 0.000 description 1
- MBNRBJNIYVXSQV-UHFFFAOYSA-N 3-[diethoxy(methyl)silyl]propane-1-thiol Chemical compound CCO[Si](C)(OCC)CCCS MBNRBJNIYVXSQV-UHFFFAOYSA-N 0.000 description 1
- 229940018563 3-aminophenol Drugs 0.000 description 1
- ATVJXMYDOSMEPO-UHFFFAOYSA-N 3-prop-2-enoxyprop-1-ene Chemical compound C=CCOCC=C ATVJXMYDOSMEPO-UHFFFAOYSA-N 0.000 description 1
- OXKAXHPVFLEQHV-UHFFFAOYSA-N 3-tri(propan-2-yloxy)silylpropan-1-amine Chemical compound CC(C)O[Si](OC(C)C)(OC(C)C)CCCN OXKAXHPVFLEQHV-UHFFFAOYSA-N 0.000 description 1
- LVACOMKKELLCHJ-UHFFFAOYSA-N 3-trimethoxysilylpropylurea Chemical compound CO[Si](OC)(OC)CCCNC(N)=O LVACOMKKELLCHJ-UHFFFAOYSA-N 0.000 description 1
- VPWNQTHUCYMVMZ-UHFFFAOYSA-N 4,4'-sulfonyldiphenol Chemical class C1=CC(O)=CC=C1S(=O)(=O)C1=CC=C(O)C=C1 VPWNQTHUCYMVMZ-UHFFFAOYSA-N 0.000 description 1
- VNGLVZLEUDIDQH-UHFFFAOYSA-N 4-[2-(4-hydroxyphenyl)propan-2-yl]phenol;2-methyloxirane Chemical class CC1CO1.C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 VNGLVZLEUDIDQH-UHFFFAOYSA-N 0.000 description 1
- XLYOGWXIKVUXCL-UHFFFAOYSA-N 4-bromobut-1-yne Chemical compound BrCCC#C XLYOGWXIKVUXCL-UHFFFAOYSA-N 0.000 description 1
- SCALDUUTBUBDKM-UHFFFAOYSA-N 4-chlorobut-1-yne Chemical compound ClCCC#C SCALDUUTBUBDKM-UHFFFAOYSA-N 0.000 description 1
- SGLSTFIXVDSVNV-UHFFFAOYSA-N 4-iodobut-1-yne Chemical compound ICCC#C SGLSTFIXVDSVNV-UHFFFAOYSA-N 0.000 description 1
- UWSMKYBKUPAEJQ-UHFFFAOYSA-N 5-Chloro-2-(3,5-di-tert-butyl-2-hydroxyphenyl)-2H-benzotriazole Chemical compound CC(C)(C)C1=CC(C(C)(C)C)=CC(N2N=C3C=C(Cl)C=CC3=N2)=C1O UWSMKYBKUPAEJQ-UHFFFAOYSA-N 0.000 description 1
- KEKBNXAJNJSILY-UHFFFAOYSA-N 5-bromopent-1-yne Chemical compound BrCCCC#C KEKBNXAJNJSILY-UHFFFAOYSA-N 0.000 description 1
- UXFIKVWAAMKFQE-UHFFFAOYSA-N 5-chloropent-1-yne Chemical compound ClCCCC#C UXFIKVWAAMKFQE-UHFFFAOYSA-N 0.000 description 1
- SEHFYZRHGUPLSY-UHFFFAOYSA-N 5-iodopent-1-yne Chemical compound ICCCC#C SEHFYZRHGUPLSY-UHFFFAOYSA-N 0.000 description 1
- ULKLGIFJWFIQFF-UHFFFAOYSA-N 5K8XI641G3 Chemical compound CCC1=NC=C(C)N1 ULKLGIFJWFIQFF-UHFFFAOYSA-N 0.000 description 1
- WDNFOERVYIUQPG-UHFFFAOYSA-N 6-bromohex-1-yne Chemical compound BrCCCCC#C WDNFOERVYIUQPG-UHFFFAOYSA-N 0.000 description 1
- ZUKOCGMVJUXIJA-UHFFFAOYSA-N 6-chlorohex-1-yne Chemical compound ClCCCCC#C ZUKOCGMVJUXIJA-UHFFFAOYSA-N 0.000 description 1
- YITSYYQPKJETAH-UHFFFAOYSA-N 6-iodohex-1-yne Chemical compound ICCCCC#C YITSYYQPKJETAH-UHFFFAOYSA-N 0.000 description 1
- YPIFGDQKSSMYHQ-UHFFFAOYSA-N 7,7-dimethyloctanoic acid Chemical compound CC(C)(C)CCCCCC(O)=O YPIFGDQKSSMYHQ-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- OAOABCKPVCUNKO-UHFFFAOYSA-N 8-methyl Nonanoic acid Chemical compound CC(C)CCCCCCC(O)=O OAOABCKPVCUNKO-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- SAIKULLUBZKPDA-UHFFFAOYSA-N Bis(2-ethylhexyl) amine Chemical compound CCCCC(CC)CNCC(CC)CCCC SAIKULLUBZKPDA-UHFFFAOYSA-N 0.000 description 1
- 229930185605 Bisphenol Natural products 0.000 description 1
- XXTJHEXYFBBGJV-UHFFFAOYSA-N C(C)OC(C(O)CC(=O)OCC)=O.C(CCC)[Sn]CCCC Chemical compound C(C)OC(C(O)CC(=O)OCC)=O.C(CCC)[Sn]CCCC XXTJHEXYFBBGJV-UHFFFAOYSA-N 0.000 description 1
- XEWDHRTWFOELEM-UHFFFAOYSA-N C(C1=CC=CC=C1)OC(C(O)CC(=O)OCC1=CC=CC=C1)=O.C(CCC)[Sn]CCCC Chemical compound C(C1=CC=CC=C1)OC(C(O)CC(=O)OCC1=CC=CC=C1)=O.C(CCC)[Sn]CCCC XEWDHRTWFOELEM-UHFFFAOYSA-N 0.000 description 1
- RSIOIDACLNBIHM-UHFFFAOYSA-N C(C=C/C(=O)OCC)(=O)OCC.C(CCCCCCC)[Sn]CCCCCCCC Chemical compound C(C=C/C(=O)OCC)(=O)OCC.C(CCCCCCC)[Sn]CCCCCCCC RSIOIDACLNBIHM-UHFFFAOYSA-N 0.000 description 1
- JZTYABBFHFUJSS-UHFFFAOYSA-N CC(C)OC([SiH3])OC(C)C Chemical compound CC(C)OC([SiH3])OC(C)C JZTYABBFHFUJSS-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- 239000004821 Contact adhesive Substances 0.000 description 1
- 102100035474 DNA polymerase kappa Human genes 0.000 description 1
- 101710108091 DNA polymerase kappa Proteins 0.000 description 1
- MQIUGAXCHLFZKX-UHFFFAOYSA-N Di-n-octyl phthalate Natural products CCCCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCCC MQIUGAXCHLFZKX-UHFFFAOYSA-N 0.000 description 1
- HECLRDQVFMWTQS-UHFFFAOYSA-N Dicyclopentadiene Chemical compound C1C2C3CC=CC3C1C=C2 HECLRDQVFMWTQS-UHFFFAOYSA-N 0.000 description 1
- RPNUMPOLZDHAAY-UHFFFAOYSA-N Diethylenetriamine Chemical compound NCCNCCN RPNUMPOLZDHAAY-UHFFFAOYSA-N 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 239000004831 Hot glue Substances 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- CHJJGSNFBQVOTG-UHFFFAOYSA-N N-methyl-guanidine Natural products CNC(N)=N CHJJGSNFBQVOTG-UHFFFAOYSA-N 0.000 description 1
- DFPAKSUCGFBDDF-UHFFFAOYSA-N Nicotinamide Chemical group NC(=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-UHFFFAOYSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- REYJJPSVUYRZGE-UHFFFAOYSA-N Octadecylamine Chemical compound CCCCCCCCCCCCCCCCCCN REYJJPSVUYRZGE-UHFFFAOYSA-N 0.000 description 1
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 239000004809 Teflon Substances 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical class [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- XSTXAVWGXDQKEL-UHFFFAOYSA-N Trichloroethylene Chemical compound ClC=C(Cl)Cl XSTXAVWGXDQKEL-UHFFFAOYSA-N 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 229910000004 White lead Inorganic materials 0.000 description 1
- 229910021536 Zeolite Inorganic materials 0.000 description 1
- 239000005083 Zinc sulfide Substances 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- GKXVJHDEWHKBFH-UHFFFAOYSA-N [2-(aminomethyl)phenyl]methanamine Chemical compound NCC1=CC=CC=C1CN GKXVJHDEWHKBFH-UHFFFAOYSA-N 0.000 description 1
- ISKQADXMHQSTHK-UHFFFAOYSA-N [4-(aminomethyl)phenyl]methanamine Chemical compound NCC1=CC=C(CN)C=C1 ISKQADXMHQSTHK-UHFFFAOYSA-N 0.000 description 1
- CQQXCSFSYHAZOO-UHFFFAOYSA-L [acetyloxy(dioctyl)stannyl] acetate Chemical compound CCCCCCCC[Sn](OC(C)=O)(OC(C)=O)CCCCCCCC CQQXCSFSYHAZOO-UHFFFAOYSA-L 0.000 description 1
- MZFBKHCHWCYNSO-UHFFFAOYSA-N [acetyloxy(phenyl)silyl] acetate Chemical compound CC(=O)O[SiH](OC(C)=O)C1=CC=CC=C1 MZFBKHCHWCYNSO-UHFFFAOYSA-N 0.000 description 1
- UKLDJPRMSDWDSL-UHFFFAOYSA-L [dibutyl(dodecanoyloxy)stannyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)O[Sn](CCCC)(CCCC)OC(=O)CCCCCCCCCCC UKLDJPRMSDWDSL-UHFFFAOYSA-L 0.000 description 1
- XQBCVRSTVUHIGH-UHFFFAOYSA-L [dodecanoyloxy(dioctyl)stannyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)O[Sn](CCCCCCCC)(CCCCCCCC)OC(=O)CCCCCCCCCCC XQBCVRSTVUHIGH-UHFFFAOYSA-L 0.000 description 1
- UZFVQGTYOXJWTF-UHFFFAOYSA-L [octadecanoyloxy(dioctyl)stannyl] octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Sn](CCCCCCCC)(CCCCCCCC)OC(=O)CCCCCCCCCCCCCCCCC UZFVQGTYOXJWTF-UHFFFAOYSA-L 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 238000007259 addition reaction Methods 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000002723 alicyclic group Chemical group 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 125000003302 alkenyloxy group Chemical group 0.000 description 1
- 150000004703 alkoxides Chemical class 0.000 description 1
- 229920000180 alkyd Polymers 0.000 description 1
- 150000001346 alkyl aryl ethers Chemical class 0.000 description 1
- 150000001343 alkyl silanes Chemical class 0.000 description 1
- OBETXYAYXDNJHR-UHFFFAOYSA-N alpha-ethylcaproic acid Natural products CCCCC(CC)C(O)=O OBETXYAYXDNJHR-UHFFFAOYSA-N 0.000 description 1
- CEGOLXSVJUTHNZ-UHFFFAOYSA-K aluminium tristearate Chemical compound [Al+3].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CEGOLXSVJUTHNZ-UHFFFAOYSA-K 0.000 description 1
- 229940063655 aluminum stearate Drugs 0.000 description 1
- IKHOZNOYZQPPCK-UHFFFAOYSA-K aluminum;4,4-diethyl-3-oxohexanoate Chemical compound [Al+3].CCC(CC)(CC)C(=O)CC([O-])=O.CCC(CC)(CC)C(=O)CC([O-])=O.CCC(CC)(CC)C(=O)CC([O-])=O IKHOZNOYZQPPCK-UHFFFAOYSA-K 0.000 description 1
- 125000003368 amide group Chemical group 0.000 description 1
- 125000002344 aminooxy group Chemical group [H]N([H])O[*] 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 125000002490 anilino group Chemical group [H]N(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 230000003712 anti-aging effect Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 239000002928 artificial marble Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- AGXUVMPSUKZYDT-UHFFFAOYSA-L barium(2+);octadecanoate Chemical compound [Ba+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O AGXUVMPSUKZYDT-UHFFFAOYSA-L 0.000 description 1
- AYJRCSIUFZENHW-DEQYMQKBSA-L barium(2+);oxomethanediolate Chemical compound [Ba+2].[O-][14C]([O-])=O AYJRCSIUFZENHW-DEQYMQKBSA-L 0.000 description 1
- 239000010428 baryte Substances 0.000 description 1
- 229910052601 baryte Inorganic materials 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 1
- 239000012965 benzophenone Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- BCPQQVJRGVNJMJ-JRYLAINFSA-N bis(6-methylheptyl) (Z)-but-2-enedioate dioctyltin Chemical compound CCCCCCCC[Sn]CCCCCCCC.CC(C)CCCCCOC(=O)\C=C/C(=O)OCCCCCC(C)C BCPQQVJRGVNJMJ-JRYLAINFSA-N 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- OCWYEMOEOGEQAN-UHFFFAOYSA-N bumetrizole Chemical compound CC(C)(C)C1=CC(C)=CC(N2N=C3C=C(Cl)C=CC3=N2)=C1O OCWYEMOEOGEQAN-UHFFFAOYSA-N 0.000 description 1
- VEGRTTZOXYUSTI-UHFFFAOYSA-N butyl-tri(propan-2-yloxy)stannane Chemical compound CCCC[Sn](OC(C)C)(OC(C)C)OC(C)C VEGRTTZOXYUSTI-UHFFFAOYSA-N 0.000 description 1
- LUZSPGQEISANPO-UHFFFAOYSA-N butyltin Chemical class CCCC[Sn] LUZSPGQEISANPO-UHFFFAOYSA-N 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 description 1
- 239000000292 calcium oxide Substances 0.000 description 1
- ODINCKMPIJJUCX-UHFFFAOYSA-N calcium oxide Inorganic materials [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 238000011088 calibration curve Methods 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 239000003518 caustics Substances 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- QABCGOSYZHCPGN-UHFFFAOYSA-N chloro(dimethyl)silicon Chemical compound C[Si](C)Cl QABCGOSYZHCPGN-UHFFFAOYSA-N 0.000 description 1
- HNEGQIOMVPPMNR-IHWYPQMZSA-N citraconic acid Chemical compound OC(=O)C(/C)=C\C(O)=O HNEGQIOMVPPMNR-IHWYPQMZSA-N 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 238000013016 damping Methods 0.000 description 1
- AONDIGWFVXEZGD-UHFFFAOYSA-N diacetyloxy(methyl)silicon Chemical compound CC(=O)O[Si](C)OC(C)=O AONDIGWFVXEZGD-UHFFFAOYSA-N 0.000 description 1
- OJLSGPFUCQBGSZ-UHFFFAOYSA-N dibutyl 2-hydroxybutanedioate dibutyltin Chemical compound C(CCC)OC(C(O)CC(=O)OCCCC)=O.C(CCC)[Sn]CCCC OJLSGPFUCQBGSZ-UHFFFAOYSA-N 0.000 description 1
- UROKUKHYYXBCQE-UHFFFAOYSA-L dibutyl(diphenoxy)stannane Chemical compound C=1C=CC=CC=1O[Sn](CCCC)(CCCC)OC1=CC=CC=C1 UROKUKHYYXBCQE-UHFFFAOYSA-L 0.000 description 1
- JGFBRKRYDCGYKD-UHFFFAOYSA-N dibutyl(oxo)tin Chemical compound CCCC[Sn](=O)CCCC JGFBRKRYDCGYKD-UHFFFAOYSA-N 0.000 description 1
- 239000012975 dibutyltin dilaurate Substances 0.000 description 1
- BZPQWSADDIRASD-UHFFFAOYSA-N dibutyltin dimethyl 2-hydroxybutanedioate Chemical compound COC(C(O)CC(=O)OC)=O.C(CCC)[Sn]CCCC BZPQWSADDIRASD-UHFFFAOYSA-N 0.000 description 1
- WEKQKOVUZKXNIT-UHFFFAOYSA-N dibutyltin ditridecyl 2-hydroxybutanedioate Chemical compound C(CCCCCCCCCCCC)OC(C(O)CC(=O)OCCCCCCCCCCCCC)=O.C(CCC)[Sn]CCCC WEKQKOVUZKXNIT-UHFFFAOYSA-N 0.000 description 1
- JFHKCZFPXJVELF-UHFFFAOYSA-L dibutyltin(2+);2,2-diethylhexanoate Chemical compound CCCC[Sn+2]CCCC.CCCCC(CC)(CC)C([O-])=O.CCCCC(CC)(CC)C([O-])=O JFHKCZFPXJVELF-UHFFFAOYSA-L 0.000 description 1
- PLTDXZXYBAXBSF-UHFFFAOYSA-L dibutyltin(2+);4-ethyl-3-oxohexanoate Chemical compound CCCC[Sn+2]CCCC.CCC(CC)C(=O)CC([O-])=O.CCC(CC)C(=O)CC([O-])=O PLTDXZXYBAXBSF-UHFFFAOYSA-L 0.000 description 1
- ZXDVQYBUEVYUCG-UHFFFAOYSA-N dibutyltin(2+);methanolate Chemical compound CCCC[Sn](OC)(OC)CCCC ZXDVQYBUEVYUCG-UHFFFAOYSA-N 0.000 description 1
- KTQYJQFGNYHXMB-UHFFFAOYSA-N dichloro(methyl)silicon Chemical compound C[Si](Cl)Cl KTQYJQFGNYHXMB-UHFFFAOYSA-N 0.000 description 1
- XNAFLNBULDHNJS-UHFFFAOYSA-N dichloro(phenyl)silicon Chemical compound Cl[Si](Cl)C1=CC=CC=C1 XNAFLNBULDHNJS-UHFFFAOYSA-N 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- PJIFJEUHCQYNHO-UHFFFAOYSA-N diethoxy-(3-isocyanatopropyl)-methylsilane Chemical compound CCO[Si](C)(OCC)CCCN=C=O PJIFJEUHCQYNHO-UHFFFAOYSA-N 0.000 description 1
- FGKBDYJRENOVOQ-UHFFFAOYSA-N diethoxymethyl(isocyanatomethyl)silane Chemical compound CCOC(OCC)[SiH2]CN=C=O FGKBDYJRENOVOQ-UHFFFAOYSA-N 0.000 description 1
- NBBQQQJUOYRZCA-UHFFFAOYSA-N diethoxymethylsilane Chemical compound CCOC([SiH3])OCC NBBQQQJUOYRZCA-UHFFFAOYSA-N 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- HBGGXOJOCNVPFY-UHFFFAOYSA-N diisononyl phthalate Chemical compound CC(C)CCCCCCOC(=O)C1=CC=CC=C1C(=O)OCCCCCCC(C)C HBGGXOJOCNVPFY-UHFFFAOYSA-N 0.000 description 1
- 229940043279 diisopropylamine Drugs 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- PLYDAAYVOHDGFT-UHFFFAOYSA-N dimethoxy(methoxymethyl)silane Chemical compound COC[SiH](OC)OC PLYDAAYVOHDGFT-UHFFFAOYSA-N 0.000 description 1
- CIQDYIQMZXESRD-UHFFFAOYSA-N dimethoxy(phenyl)silane Chemical compound CO[SiH](OC)C1=CC=CC=C1 CIQDYIQMZXESRD-UHFFFAOYSA-N 0.000 description 1
- SWSQBOPZIKWTGO-UHFFFAOYSA-N dimethylaminoamidine Natural products CN(C)C(N)=N SWSQBOPZIKWTGO-UHFFFAOYSA-N 0.000 description 1
- WJJCULODEKDFBL-UHFFFAOYSA-N dimethylsilyloxy-dimethyl-(2-trimethoxysilylhexyl)silane Chemical compound CCCCC([Si](OC)(OC)OC)C[Si](C)(C)O[SiH](C)C WJJCULODEKDFBL-UHFFFAOYSA-N 0.000 description 1
- ZDHORWFYHNQFOW-UHFFFAOYSA-N dimethylsilyloxy-dimethyl-(2-trimethoxysilylpropyl)silane Chemical compound CO[Si](OC)(OC)C(C)C[Si](C)(C)O[SiH](C)C ZDHORWFYHNQFOW-UHFFFAOYSA-N 0.000 description 1
- LQRUPWUPINJLMU-UHFFFAOYSA-N dioctyl(oxo)tin Chemical compound CCCCCCCC[Sn](=O)CCCCCCCC LQRUPWUPINJLMU-UHFFFAOYSA-N 0.000 description 1
- LAWOZCWGWDVVSG-UHFFFAOYSA-N dioctylamine Chemical compound CCCCCCCCNCCCCCCCC LAWOZCWGWDVVSG-UHFFFAOYSA-N 0.000 description 1
- HGQSXVKHVMGQRG-UHFFFAOYSA-N dioctyltin Chemical compound CCCCCCCC[Sn]CCCCCCCC HGQSXVKHVMGQRG-UHFFFAOYSA-N 0.000 description 1
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 1
- ZZTCPWRAHWXWCH-UHFFFAOYSA-N diphenylmethanediamine Chemical compound C=1C=CC=CC=1C(N)(N)C1=CC=CC=C1 ZZTCPWRAHWXWCH-UHFFFAOYSA-N 0.000 description 1
- WEHWNAOGRSTTBQ-UHFFFAOYSA-N dipropylamine Chemical compound CCCNCCC WEHWNAOGRSTTBQ-UHFFFAOYSA-N 0.000 description 1
- VPNOHCYAOXWMAR-UHFFFAOYSA-N docosan-1-amine Chemical compound CCCCCCCCCCCCCCCCCCCCCCN VPNOHCYAOXWMAR-UHFFFAOYSA-N 0.000 description 1
- MCPKSFINULVDNX-UHFFFAOYSA-N drometrizole Chemical compound CC1=CC=C(O)C(N2N=C3C=CC=CC3=N2)=C1 MCPKSFINULVDNX-UHFFFAOYSA-N 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- CWAFVXWRGIEBPL-UHFFFAOYSA-N ethoxysilane Chemical compound CCO[SiH3] CWAFVXWRGIEBPL-UHFFFAOYSA-N 0.000 description 1
- XYIBRDXRRQCHLP-UHFFFAOYSA-N ethyl acetoacetate Chemical compound CCOC(=O)CC(C)=O XYIBRDXRRQCHLP-UHFFFAOYSA-N 0.000 description 1
- 229940093858 ethyl acetoacetate Drugs 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- VOZRXNHHFUQHIL-UHFFFAOYSA-N glycidyl methacrylate Chemical compound CC(=C)C(=O)OCC1CO1 VOZRXNHHFUQHIL-UHFFFAOYSA-N 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 239000010440 gypsum Substances 0.000 description 1
- 229910052602 gypsum Inorganic materials 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 230000017525 heat dissipation Effects 0.000 description 1
- WJRBRSLFGCUECM-UHFFFAOYSA-N hydantoin Chemical compound O=C1CNC(=O)N1 WJRBRSLFGCUECM-UHFFFAOYSA-N 0.000 description 1
- 229940091173 hydantoin Drugs 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 238000006459 hydrosilylation reaction Methods 0.000 description 1
- BUHXFUSLEBPCEB-UHFFFAOYSA-N icosan-1-amine Chemical compound CCCCCCCCCCCCCCCCCCCCN BUHXFUSLEBPCEB-UHFFFAOYSA-N 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 238000009413 insulation Methods 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 238000003973 irrigation Methods 0.000 description 1
- 230000002262 irrigation Effects 0.000 description 1
- QRFPECUQGPJPMV-UHFFFAOYSA-N isocyanatomethyl(trimethoxy)silane Chemical compound CO[Si](OC)(OC)CN=C=O QRFPECUQGPJPMV-UHFFFAOYSA-N 0.000 description 1
- HENJUOQEQGBPSV-UHFFFAOYSA-N isocyanatomethyl-dimethoxy-methylsilane Chemical compound CO[Si](C)(OC)CN=C=O HENJUOQEQGBPSV-UHFFFAOYSA-N 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000005340 laminated glass Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- 239000006078 metal deactivator Substances 0.000 description 1
- 239000005048 methyldichlorosilane Substances 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 229910052618 mica group Inorganic materials 0.000 description 1
- 239000012778 molding material Substances 0.000 description 1
- QOHMWDJIBGVPIF-UHFFFAOYSA-N n',n'-diethylpropane-1,3-diamine Chemical compound CCN(CC)CCCN QOHMWDJIBGVPIF-UHFFFAOYSA-N 0.000 description 1
- INJVFBCDVXYHGQ-UHFFFAOYSA-N n'-(3-triethoxysilylpropyl)ethane-1,2-diamine Chemical compound CCO[Si](OCC)(OCC)CCCNCCN INJVFBCDVXYHGQ-UHFFFAOYSA-N 0.000 description 1
- CSNJSTXFSLBBPX-UHFFFAOYSA-N n'-(trimethoxysilylmethyl)ethane-1,2-diamine Chemical compound CO[Si](OC)(OC)CNCCN CSNJSTXFSLBBPX-UHFFFAOYSA-N 0.000 description 1
- MQWFLKHKWJMCEN-UHFFFAOYSA-N n'-[3-[dimethoxy(methyl)silyl]propyl]ethane-1,2-diamine Chemical compound CO[Si](C)(OC)CCCNCCN MQWFLKHKWJMCEN-UHFFFAOYSA-N 0.000 description 1
- JHIJIKGKJSBSKN-UHFFFAOYSA-N n'-[3-tri(propan-2-yloxy)silylpropyl]ethane-1,2-diamine Chemical compound CC(C)O[Si](OC(C)C)(OC(C)C)CCCNCCN JHIJIKGKJSBSKN-UHFFFAOYSA-N 0.000 description 1
- JAYXSROKFZAHRQ-UHFFFAOYSA-N n,n-bis(oxiran-2-ylmethyl)aniline Chemical compound C1OC1CN(C=1C=CC=CC=1)CC1CO1 JAYXSROKFZAHRQ-UHFFFAOYSA-N 0.000 description 1
- DIAIBWNEUYXDNL-UHFFFAOYSA-N n,n-dihexylhexan-1-amine Chemical compound CCCCCCN(CCCCCC)CCCCCC DIAIBWNEUYXDNL-UHFFFAOYSA-N 0.000 description 1
- UCAOGXRUJFKQAP-UHFFFAOYSA-N n,n-dimethyl-5-nitropyridin-2-amine Chemical compound CN(C)C1=CC=C([N+]([O-])=O)C=N1 UCAOGXRUJFKQAP-UHFFFAOYSA-N 0.000 description 1
- XTAZYLNFDRKIHJ-UHFFFAOYSA-N n,n-dioctyloctan-1-amine Chemical compound CCCCCCCCN(CCCCCCCC)CCCCCCCC XTAZYLNFDRKIHJ-UHFFFAOYSA-N 0.000 description 1
- OOHAUGDGCWURIT-UHFFFAOYSA-N n,n-dipentylpentan-1-amine Chemical compound CCCCCN(CCCCC)CCCCC OOHAUGDGCWURIT-UHFFFAOYSA-N 0.000 description 1
- CLYWMXVFAMGARU-UHFFFAOYSA-N n-benzyl-3-trimethoxysilylpropan-1-amine Chemical compound CO[Si](OC)(OC)CCCNCC1=CC=CC=C1 CLYWMXVFAMGARU-UHFFFAOYSA-N 0.000 description 1
- GMTCPFCMAHMEMT-UHFFFAOYSA-N n-decyldecan-1-amine Chemical compound CCCCCCCCCCNCCCCCCCCCC GMTCPFCMAHMEMT-UHFFFAOYSA-N 0.000 description 1
- LQKYCMRSWKQVBQ-UHFFFAOYSA-N n-dodecylaniline Chemical compound CCCCCCCCCCCCNC1=CC=CC=C1 LQKYCMRSWKQVBQ-UHFFFAOYSA-N 0.000 description 1
- MJCJUDJQDGGKOX-UHFFFAOYSA-N n-dodecyldodecan-1-amine Chemical compound CCCCCCCCCCCCNCCCCCCCCCCCC MJCJUDJQDGGKOX-UHFFFAOYSA-N 0.000 description 1
- NQYKSVOHDVVDOR-UHFFFAOYSA-N n-hexadecylhexadecan-1-amine Chemical compound CCCCCCCCCCCCCCCCNCCCCCCCCCCCCCCCC NQYKSVOHDVVDOR-UHFFFAOYSA-N 0.000 description 1
- SZEGKVHRCLBFKJ-UHFFFAOYSA-N n-methyloctadecan-1-amine Chemical compound CCCCCCCCCCCCCCCCCCNC SZEGKVHRCLBFKJ-UHFFFAOYSA-N 0.000 description 1
- UEKWTIYPDJLSKK-UHFFFAOYSA-N n-octadecylaniline Chemical compound CCCCCCCCCCCCCCCCCCNC1=CC=CC=C1 UEKWTIYPDJLSKK-UHFFFAOYSA-N 0.000 description 1
- HKUFIYBZNQSHQS-UHFFFAOYSA-N n-octadecyloctadecan-1-amine Chemical compound CCCCCCCCCCCCCCCCCCNCCCCCCCCCCCCCCCCCC HKUFIYBZNQSHQS-UHFFFAOYSA-N 0.000 description 1
- JACMPVXHEARCBO-UHFFFAOYSA-N n-pentylpentan-1-amine Chemical compound CCCCCNCCCCC JACMPVXHEARCBO-UHFFFAOYSA-N 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 1
- JFOJYGMDZRCSPA-UHFFFAOYSA-J octadecanoate;tin(4+) Chemical compound [Sn+4].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O JFOJYGMDZRCSPA-UHFFFAOYSA-J 0.000 description 1
- 125000005474 octanoate group Chemical group 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- ZMHZSHHZIKJFIR-UHFFFAOYSA-N octyltin Chemical class CCCCCCCC[Sn] ZMHZSHHZIKJFIR-UHFFFAOYSA-N 0.000 description 1
- 125000000962 organic group Chemical group 0.000 description 1
- RPQRDASANLAFCM-UHFFFAOYSA-N oxiran-2-ylmethyl prop-2-enoate Chemical compound C=CC(=O)OCC1CO1 RPQRDASANLAFCM-UHFFFAOYSA-N 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 1
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N phenol group Chemical group C1(=CC=CC=C1)O ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 150000004980 phosphorus peroxides Chemical class 0.000 description 1
- 238000000016 photochemical curing Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 229920001515 polyalkylene glycol Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920006122 polyamide resin Polymers 0.000 description 1
- 239000002952 polymeric resin Substances 0.000 description 1
- 239000003505 polymerization initiator Substances 0.000 description 1
- 230000000379 polymerizing effect Effects 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 150000008442 polyphenolic compounds Chemical class 0.000 description 1
- 235000013824 polyphenols Nutrition 0.000 description 1
- 150000007519 polyprotic acids Polymers 0.000 description 1
- 229920005990 polystyrene resin Polymers 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- RPDAUEIUDPHABB-UHFFFAOYSA-N potassium ethoxide Chemical compound [K+].CC[O-] RPDAUEIUDPHABB-UHFFFAOYSA-N 0.000 description 1
- 238000004382 potting Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 238000007342 radical addition reaction Methods 0.000 description 1
- 238000007142 ring opening reaction Methods 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 1
- 229920006132 styrene block copolymer Polymers 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- TXDNPSYEJHXKMK-UHFFFAOYSA-N sulfanylsilane Chemical compound S[SiH3] TXDNPSYEJHXKMK-UHFFFAOYSA-N 0.000 description 1
- MBSOYVMTSJAYPW-UHFFFAOYSA-N sulfanylsilane 3-trimethoxysilylpropane-1-thiol Chemical compound S[SiH3].CO[Si](OC)(OC)CCCS MBSOYVMTSJAYPW-UHFFFAOYSA-N 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 239000012974 tin catalyst Substances 0.000 description 1
- KSBAEPSJVUENNK-UHFFFAOYSA-L tin(ii) 2-ethylhexanoate Chemical compound [Sn+2].CCCCC(CC)C([O-])=O.CCCCC(CC)C([O-])=O KSBAEPSJVUENNK-UHFFFAOYSA-L 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- VPYJNCGUESNPMV-UHFFFAOYSA-N triallylamine Chemical compound C=CCN(CC=C)CC=C VPYJNCGUESNPMV-UHFFFAOYSA-N 0.000 description 1
- ZDHXKXAHOVTTAH-UHFFFAOYSA-N trichlorosilane Chemical compound Cl[SiH](Cl)Cl ZDHXKXAHOVTTAH-UHFFFAOYSA-N 0.000 description 1
- 239000005052 trichlorosilane Substances 0.000 description 1
- BOTMPGMIDPRZGP-UHFFFAOYSA-N triethoxy(isocyanatomethyl)silane Chemical compound CCO[Si](OCC)(OCC)CN=C=O BOTMPGMIDPRZGP-UHFFFAOYSA-N 0.000 description 1
- NBXZNTLFQLUFES-UHFFFAOYSA-N triethoxy(propyl)silane Chemical compound CCC[Si](OCC)(OCC)OCC NBXZNTLFQLUFES-UHFFFAOYSA-N 0.000 description 1
- XSIGLRIVXRKQRA-UHFFFAOYSA-N triethoxysilylmethanethiol Chemical compound CCO[Si](CS)(OCC)OCC XSIGLRIVXRKQRA-UHFFFAOYSA-N 0.000 description 1
- DQZNLOXENNXVAD-UHFFFAOYSA-N trimethoxy-[2-(7-oxabicyclo[4.1.0]heptan-4-yl)ethyl]silane Chemical compound C1C(CC[Si](OC)(OC)OC)CCC2OC21 DQZNLOXENNXVAD-UHFFFAOYSA-N 0.000 description 1
- BPSIOYPQMFLKFR-UHFFFAOYSA-N trimethoxy-[3-(oxiran-2-ylmethoxy)propyl]silane Chemical compound CO[Si](OC)(OC)CCCOCC1CO1 BPSIOYPQMFLKFR-UHFFFAOYSA-N 0.000 description 1
- PZJJKWKADRNWSW-UHFFFAOYSA-N trimethoxysilicon Chemical group CO[Si](OC)OC PZJJKWKADRNWSW-UHFFFAOYSA-N 0.000 description 1
- PHYFQTYBJUILEZ-IUPFWZBJSA-N triolein Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(OC(=O)CCCCCCC\C=C/CCCCCCCC)COC(=O)CCCCCCC\C=C/CCCCCCCC PHYFQTYBJUILEZ-IUPFWZBJSA-N 0.000 description 1
- ODHXBMXNKOYIBV-UHFFFAOYSA-N triphenylamine Chemical compound C1=CC=CC=C1N(C=1C=CC=CC=1)C1=CC=CC=C1 ODHXBMXNKOYIBV-UHFFFAOYSA-N 0.000 description 1
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
- 229910052984 zinc sulfide Inorganic materials 0.000 description 1
- DRDVZXDWVBGGMH-UHFFFAOYSA-N zinc;sulfide Chemical compound [S-2].[Zn+2] DRDVZXDWVBGGMH-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G59/00—Polycondensates containing more than one epoxy group per molecule; Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups
- C08G59/18—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing
- C08G59/40—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing characterised by the curing agents used
- C08G59/66—Mercaptans
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G65/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G65/02—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from cyclic ethers by opening of the heterocyclic ring
- C08G65/32—Polymers modified by chemical after-treatment
- C08G65/329—Polymers modified by chemical after-treatment with organic compounds
- C08G65/336—Polymers modified by chemical after-treatment with organic compounds containing silicon
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L71/00—Compositions of polyethers obtained by reactions forming an ether link in the main chain; Compositions of derivatives of such polymers
- C08L71/02—Polyalkylene oxides
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K3/00—Materials not provided for elsewhere
- C09K3/10—Materials in mouldable or extrudable form for sealing or packing joints or covers
Definitions
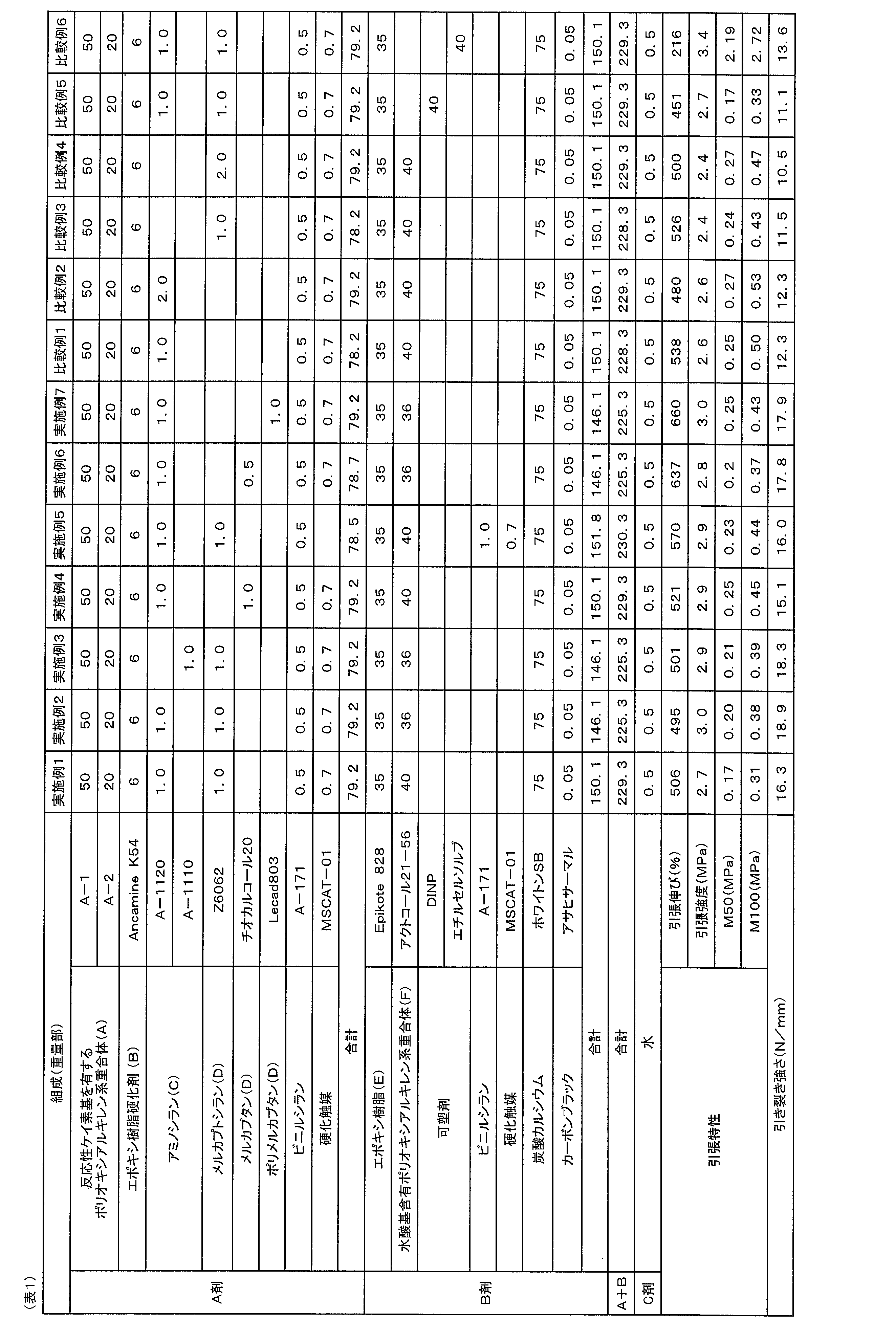
- the present invention relates to a multi-component curable composition, and a cured product and waterproof material using it.
- the present invention also relates to a method of manufacturing a waterproof structure using the multi-component curable composition.
- Patent Document 1 discloses a technique of blending a modified silicone compound containing two or more reactive silicon groups in one molecule, a mercaptosilane compound, and an epoxy resin. is disclosed.
- Patent Document 2 discloses a polyoxyalkylene polymer having a specific reactive silicon group, a (meth)acrylic acid ester polymer having a specific reactive silicon group, and an epoxy resin curing agent. and a B-part comprising an epoxy resin and a silanol condensation catalyst are disclosed.
- an object of the present invention is to provide a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength.
- the present inventors have made intensive studies to solve the above problems, and found that aminosilane (C), mercaptan It is the first time that a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength can be obtained by blending the compound (D) and the hydroxyl group-containing polyoxyalkylene polymer (F). This led to the completion of the present invention.
- one aspect of the present invention is a polyoxyalkylene polymer (A) having a reactive silicon group represented by general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D ) and a B agent containing an epoxy resin (E), wherein the A agent and / or the B agent contains a hydroxyl group-containing polyoxyalkylene polymer (F).
- A a reactive silicon group represented by general formula (1)
- an epoxy resin curing agent B
- an aminosilane C
- a mercaptan compound D
- E a B agent containing an epoxy resin
- F a hydroxyl group-containing polyoxyalkylene polymer
- composition —Si(R) 3-a (X) a
- each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms.
- the hydrocarbon group as R may be substitute
- a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength.
- a multi-component curable composition according to one embodiment of the present invention is a polyoxyalkylene having a reactive silicon group represented by general formula (1)
- each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms.
- the hydrocarbon group as R may be substituted and may have a hetero-containing group.
- X is each independently a hydroxyl group or a hydro is a degradable group.
- a is an integer of 1 to 3.
- Inexpensive two-liquid urethane products are commonly used for coating film waterproofing materials, but non-isocyanate products have been required due to environmental regulations.
- Patent Documents 1 and 2 for example, when a non-isocyanate curing agent is used, there is room for improvement in terms of tensile properties and tear strength.
- the curable composition contains a plasticizer and a low-cost filler in order to reduce the viscosity and cost, the above problems arise.
- ⁇ Aminosilane (C), mercaptan compound (D), and hydroxyl group-containing polyoxyalkylene polymer (F) are compounded into a multicomponent curable composition containing a specific polyoxyalkylene polymer and epoxy resin. By doing so, it is possible to obtain a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength.
- the multicomponent curable composition contains an inorganic filler (e.g., calcium carbonate), a plasticizer, etc., the aminosilane (C), the mercaptan compound (D), and the hydroxyl group-containing polyoxyalkylene polymer
- an inorganic filler e.g., calcium carbonate
- a plasticizer e.g., ethylene glycol dimethacrylate
- the aminosilane (C) e.g., calcium carbonate
- D mercaptan compound
- F hydroxyl group-containing polyoxyalkylene polymer
- the present multi-component curable composition that is environmentally friendly and has excellent tensile properties and tear strength.
- the present multi-component curable composition can be used by increasing the content of inexpensive materials such as calcium carbonate (inorganic filler), plasticizer, and the like, so the cost can be reduced. But it is advantageous.
- a waterproof material with excellent tensile properties and tear strength can be produced without using highly toxic isocyanate.
- sustainable development such as Goal 12 “Ensure sustainable consumption and production patterns” and Goal 14 “Conserve and sustainably use the seas, seas and marine resources for sustainable development”. can contribute to the achievement of various development goals (SDGs).
- SDGs development goals
- the multicomponent curable composition comprises a polyoxyalkylene polymer (A) having a specific reactive silicon group, an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D ) and a B agent containing an epoxy resin (E), and the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
- a polyoxyalkylene polymer (A) having a reactive silicon group has a reactive silicon group represented by general formula (1).
- —Si(R) 3-a (X) a (1) each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms.
- the hydrocarbon group as R may be substituted and may have a hetero-containing group.
- X is each independently a hydroxyl group or a hydro is a degradable group.
- a is an integer of 1 to 3.
- the number of carbon atoms in the hydrocarbon group of R is preferably 1 to 10, more preferably 1 to 5, even more preferably 1 to 3.
- Specific examples of R include, for example, methyl group, ethyl group, chloromethyl group, methoxymethyl group and N,N-diethylaminomethyl group, preferably methyl group, ethyl group and chloromethyl group. , a methoxymethyl group, more preferably a methyl group or a methoxymethyl group. According to the said structure, it has the advantage that it is easy to balance storage stability and reactivity.
- Examples of X include hydroxyl group, halogen, alkoxy group, acyloxy group, ketoximate group, amino group, amide group, acid amide group, aminooxy group, mercapto group, and alkenyloxy group.
- an alkoxy group such as a methoxy group and an ethoxy group is more preferred, and a methoxy group and an ethoxy group are particularly preferred, since they are moderately hydrolyzable and easy to handle.
- Specific examples of the reactive silicon group possessed by the polyoxyalkylene polymer (A) include a trimethoxysilyl group, a triethoxysilyl group, a tris(2-propenyloxy)silyl group, a triacetoxysilyl group, and dimethoxymethyl silyl group, diethoxymethylsilyl group, dimethoxyethylsilyl group, (chloromethyl)dimethoxysilyl group, (chloromethyl)diethoxysilyl group, (methoxymethyl)dimethoxysilyl group, (methoxymethyl)diethoxysilyl group, (N ,N-diethylaminomethyl)dimethoxysilyl group, (N,N-diethylaminomethyl)diethoxysilyl group, and the like, but are not limited thereto.
- methyldimethoxysilyl trimethoxysilyl, triethoxysilyl, (chloromethyl)dimethoxysilyl, (methoxymethyl)dimethoxysilyl, (methoxymethyl)diethoxysilyl, (N,N- Diethylaminomethyl)dimethoxysilyl group is preferred because it exhibits high activity and gives a cured product with good mechanical properties, and a trimethoxysilyl group and a triethoxysilyl group are more preferred because a cured product with high rigidity can be obtained. A trimethoxysilyl group is more preferred.
- the polyoxyalkylene-based polymer (A) may have more than one reactive silicon group on average at one terminal site. Having more than one reactive silicon group on average at one terminal site means that the polyoxyalkylene polymer (A) has 2 at one terminal site as represented by the following general formula (2) It shows that polyoxyalkylenes with one or more reactive silicon groups are included. That is, the polyoxyalkylene-based polymer (A) may contain only a polyoxyalkylene having two or more reactive silicon groups at one terminal site, or two or more at one terminal site. and polyoxyalkylenes having one reactive silicon group at one terminal site.
- the plurality of terminal sites possessed by one molecule of polyoxyalkylene may include both a terminal site having two or more reactive silicon groups and a terminal site having one reactive silicon group.
- the polyoxyalkylene polymer (A) as a whole has an average of more than one reactive silicon group at one terminal site, but a poly having a terminal site having no reactive silicon group It may contain oxyalkylene.
- the terminal portion of the polyoxyalkylene-based polymer (A) in this multi-component curable composition has the general formula (2):
- R 1 and R 3 are each independently a divalent C 1-6 bonding group, and the atoms bonded to the respective carbon atoms adjacent to R 1 and R 3 are carbon, oxygen , nitrogen, each of R 2 and R 4 is independently hydrogen or a hydrocarbon group having 1 to 10 carbon atoms, n is an integer of 1 to 10, R 5 is a substituted or an unsubstituted hydrocarbon group having 1 to 20 carbon atoms, X is a hydroxyl group or a hydrolyzable group, and c is an integer of 1 to 3.).
- R 1 and R 3 may be a divalent organic group having 1 to 6 carbon atoms, may contain an oxygen atom, or may be a hydrocarbon group.
- the number of carbon atoms in the hydrocarbon group is preferably 1-4, more preferably 1-3, even more preferably 1-2.
- Specific examples of R 1 include CH 2 OCH 2 , CH 2 O and CH 2 , preferably CH 2 OCH 2 .
- Specific examples of R 3 include CH 2 and CH 2 CH 2 , preferably CH 2 .
- the number of carbon atoms in the hydrocarbon groups of R 2 and R 4 is preferably 1-5, more preferably 1-3, even more preferably 1-2.
- Specific examples of R 2 and R 4 include a hydrogen atom, a methyl group and an ethyl group, preferably a hydrogen atom and a methyl group, more preferably a hydrogen atom.
- the terminal moiety represented by the general formula (2) is CH 2 OCH 2 for R 1 , CH 2 for R 3 , and hydrogen atoms for R 2 and R 4 .
- n is preferably an integer of 1 to 5, more preferably an integer of 1 to 3, and even more preferably 1 or 2.
- n is not limited to one value, and may be a mixture of multiple values.
- the number of reactive silicon groups possessed by the polyoxyalkylene polymer (A) is preferably more than 1.0 on average at one terminal site, more preferably 1.1 or more. , is more preferably 1.5 or more, and even more preferably 2.0 or more. Also, the number is preferably 5 or less, more preferably 3 or less.
- the number of terminal sites having more than one reactive silicon group contained in one molecule of the polyoxyalkylene polymer (A) is preferably 0.5 or more on average, and 1.0 It is more preferably 1 or more, still more preferably 1.1 or more, and even more preferably 1.5 or more. Also, the number is preferably 4 or less, more preferably 3 or less.
- the polyoxyalkylene polymer (A) may have reactive silicon groups in addition to the terminal sites, but having them only in the terminal sites yields a rubber-like cured product with high elongation and low elastic modulus. It is preferable because it becomes easy to be
- the average number of reactive silicon groups per molecule of the polyoxyalkylene polymer (A) is preferably more than 1.0, more preferably 1.2 or more, and further preferably 1.3 or more. It is preferably 1.5 or more, more preferably 1.7 or more. Also, the number is preferably 6.0 or less, more preferably 5.5 or less, and most preferably 5.0 or less. If the average number of reactive silicon groups per molecule is 1.0 or less, a cured product with high strength may not be obtained. If the average number of reactive silicon groups per molecule exceeds 6.0, it may not be possible to obtain a cured product with high elongation.
- the main chain skeleton of the polyoxyalkylene polymer (A) is not particularly limited, and examples include polyoxyethylene, polyoxypropylene, polyoxybutylene, polyoxytetramethylene, polyoxyethylene-polyoxypropylene copolymer, Examples include polyoxypropylene-polyoxybutylene copolymers. Among them, polyoxypropylene is preferred.
- the number average molecular weight of the polyoxyalkylene polymer (A) is 3,000 to 100,000, more preferably 3,000 to 50,000, and particularly preferably 3,000 to 30,000 in terms of polystyrene equivalent molecular weight in GPC. If the number average molecular weight is less than 3,000, the amount of reactive silicon groups to be introduced increases, which may be disadvantageous in terms of production costs. There is
- the organic polymer precursor before the introduction of the reactive silicon group was subjected to the hydroxyl value measurement method of JIS K 1557 and the iodine value of JIS K 0070.
- the terminal group concentration was measured by titration analysis based on the principle of the measurement method, and indicate the terminal group equivalent molecular weight obtained by considering the structure of the organic polymer (degree of branching determined by the polymerization initiator used).
- the terminal group-equivalent molecular weight of the polyoxyalkylene-based polymer (A) is obtained by preparing a calibration curve of the number average molecular weight obtained by general GPC measurement of the organic polymer precursor and the above-mentioned terminal-group-equivalent molecular weight. It is also possible to convert the number-average molecular weight of the polymer (A) obtained by GPC into a terminal group-equivalent molecular weight.
- the molecular weight distribution (Mw/Mn) of the polyoxyalkylene polymer (A) is not particularly limited, but is preferably narrow, preferably less than 2.0, more preferably 1.6 or less, and even more preferably 1.5 or less. , 1.4 or less is particularly preferred, and 1.2 or less is most preferred.
- the molecular weight distribution of the polyoxyalkylene polymer (A) can be obtained from the number average molecular weight and weight average molecular weight obtained by GPC measurement.
- main chain structure of the polyoxyalkylene polymer (A) of the present invention may be linear or branched.
- the polyoxyalkylene-based polymer (A) is a mixture of a high-viscosity polyoxyalkylene-based polymer (A-1) and a low-viscosity polyoxyalkylene-based polymer (A-2). It is preferable to use
- the viscosity of the polyoxyalkylene polymer (A-1) is not particularly limited, but is preferably 6.0 Pa s to 50 Pa s, more preferably 7.0 Pa s to 48 Pa s, and 8.0 Pa s. ⁇ 45 Pa ⁇ s is more preferable.
- This configuration has the advantage of improving the adhesiveness of the obtained multi-component curable composition and the tensile elongation of the cured product.
- the viscosity of the polyoxyalkylene polymer (A-2) is not particularly limited, but is preferably 1 Pa s to 5 Pa s, more preferably 1.5 Pa s to 4 Pa s, and 2 Pa s to 3 Pa s. is more preferred. This configuration has the advantage that the resulting multi-component curable composition has a low viscosity.
- the weight ratio (A-1):(A-2) of the polyoxyalkylene polymer (A-1) and the polyoxyalkylene polymer (A-2) is 95:5 to 50:50. is preferred. Within this range, a cured product exhibiting flexibility and high shear adhesive strength can be obtained. Furthermore, in order to achieve both high rigidity and flexibility, (A-1):(A-2) is preferably 80:20 to 50:50, more preferably 70:30 to 50:50. preferable.
- Introduction of a reactive silicon group to the main chain of the polyoxyalkylene polymer (A) may be performed by a known method. For example, the following methods are mentioned.
- Method I An organic polymer having a functional group such as a hydroxy group is reacted with a compound having an active group that exhibits reactivity with the functional group and an unsaturated group to obtain an organic polymer having an unsaturated group. Then, the resulting organic polymer having unsaturated groups is reacted with a hydrosilane compound having reactive silicon groups by hydrosilylation.
- Examples of compounds having a reactive active group and an unsaturated group that can be used in Method I include unsaturated group-containing epoxy compounds such as allyl glycidyl ether, allyl chloride, methallyl chloride, vinyl bromide, allyl bromide, Examples thereof include compounds having a carbon-carbon double bond such as methallyl bromide, vinyl iodide, allyl iodide and methallyl iodide.
- Examples of compounds having a carbon-carbon triple bond include propargyl chloride, 1-chloro-2-butyne, 4-chloro-1-butyne, 1-chloro-2-octyne, 1-chloro-2-pentyne, 1,4-dichloro-2-butyne, 5-chloro-1-pentyne, 6-chloro-1-hexyne, propargyl bromide, 1-bromo-2-butyne, 4-bromo-1-butyne, 1-bromo- 2-octyne, 1-bromo-2-pentyne, 1,4-dibromo-2-butyne, 5-bromo-1-pentyne, 6-bromo-1-hexyne, propargyl iodide, 1-iodo-2-butyne, 4-iodo-1-butyne, 1-iodo-2-octyne, 1-iodo-2-p
- Halogenated hydrocarbon compounds having a carbon-carbon triple bond as well as compounds such as vinyl chloride, allyl chloride, methallyl chloride, vinyl bromide, allyl bromide, methallyl bromide, vinyl iodide, allyl iodide, and methallyl iodide. Hydrocarbon compounds with unsaturated bonds other than halogenated hydrocarbons with carbon-carbon triple bonds may be used.
- hydrosilane compounds that can be used in method I include halogenated silanes, alkoxysilanes, acyloxysilanes, ketoximate silanes, and the like. Hydrosilane compounds are not limited to these.
- halogenated silanes include trichlorosilane, methyldichlorosilane, dimethylchlorosilane, and phenyldichlorosilane.
- Alkoxysilanes include, for example, trimethoxysilane, triethoxysilane, triisopropoxysilane, dimethoxymethylsilane, diethoxymethylsilane, diisopropoxymethylsilane, (methoxymethyl)dimethoxysilane, phenyldimethoxysilane, 1-[ 2-(Trimethoxysilyl)ethyl]-1,1,3,3-tetramethyldisiloxane and the like.
- acyloxysilanes include methyldiacetoxysilane and phenyldiacetoxysilane.
- ketoximate silanes include bis(dimethylketoximate)methylsilane and bis(cyclohexylketoximate)methylsilane.
- halogenated silanes and alkoxysilanes are particularly preferred.
- Alkoxysilanes are most preferred because they are mildly hydrolyzable and easy to handle.
- alkoxysilanes it is easy to obtain, it is easy to obtain a resin composition for foams with excellent curability and storage stability, and it is possible to produce foams with excellent tensile strength using the resin composition for foams.
- Dimethoxymethylsilane is preferred because it is easy to use.
- Trimethoxysilane and triethoxysilane are also preferable from the viewpoint of easily obtaining a resin composition for foam having excellent curability.
- Method II A compound having a mercapto group and a reactive silicon group is subjected to a radical addition reaction in the presence of a radical initiator and/or a radical generation source to obtain an organic polymer having an unsaturated group in the same manner as in Method I. Method of introducing into the unsaturated group site of.
- Compounds having a mercapto group and a reactive silicon group that can be used in method II include, for example, 3-mercapto-n-propyltrimethoxysilane, 3-mercapto-n-propylmethyldimethoxysilane, 3-mercapto-n-propyl triethoxysilane, 3-mercapto-n-propylmethyldiethoxysilane, mercaptomethyltrimethoxysilane, mercaptomethyltriethoxysilane and the like.
- Compounds having mercapto groups and reactive silicon groups are not limited to these.
- Method III A method of reacting an organic polymer having a functional group such as a hydroxyl group, an epoxy group, an isocyanate group, etc. in the molecule with a compound having a functional group showing reactivity to these functional groups and a reactive silicon group. .
- the method of reacting an organic polymer having a hydroxy group with a compound having an isocyanate group and a reactive silicon group, which can be employed in Method III, is not particularly limited. and the like.
- Compounds having isocyanate groups and reactive silicon groups that can be used in Method III include, for example, 3-isocyanato-n-propyltrimethoxysilane, 3-isocyanato-n-propylmethyldimethoxysilane, 3-isocyanato-n- Examples include propyltriethoxysilane, 3-isocyanato-n-propylmethyldiethoxysilane, isocyanatomethyltrimethoxysilane, isocyanatomethyltriethoxysilane, isocyanatomethyldimethoxymethylsilane, isocyanatomethyldiethoxymethylsilane and the like. Compounds having isocyanate groups and reactive silicon groups are not limited to these.
- Silane compounds such as trimethoxysilane, in which three hydrolyzable groups are bonded to one silicon atom, may undergo a disproportionation reaction. As the disproportionation reaction progresses, unstable compounds such as dimethoxysilane are produced, which can be difficult to handle. However, such a disproportionation reaction does not proceed with 3-mercapto-n-propyltrimethoxysilane and 3-isocyanato-n-propyltrimethoxysilane. Therefore, when a group in which three hydrolyzable groups are bonded to one silicon atom, such as a trimethoxysilyl group, is used as the silicon-containing group, method II or method III is preferably used.
- the disproportionation reaction does not proceed with the silane compound represented by the following formula (2a).
- X is the same as in formula (1a).
- 2m+2 R 2a are independently the same as R 1a in Formula (1a).
- R 3a represents a substituted or unsubstituted divalent hydrocarbon group having 1 to 20 carbon atoms.
- m represents an integer of 0 or more and 19 or less.
- R 2a are each independently preferably a hydrocarbon group having 1 to 20 carbon atoms, more preferably a hydrocarbon group having 1 to 8 carbon atoms. A hydrocarbon group having 1 or more and 4 or less atoms is more preferable.
- R 3a is preferably a divalent hydrocarbon group having 1 to 12 carbon atoms, more preferably a divalent hydrocarbon group having 2 to 8 carbon atoms, and a divalent hydrocarbon group having 2 carbon atoms. groups are more preferred.
- m is most preferably 1.
- Silane compounds represented by formula (2a) include, for example, 1-[2-(trimethoxysilyl)ethyl]-1,1,3,3-tetramethyldisiloxane, 1-[2-(trimethoxysilyl) propyl]-1,1,3,3-tetramethyldisiloxane, 1-[2-(trimethoxysilyl)hexyl]-1,1,3,3-tetramethyldisiloxane and the like.
- the method of reacting an organic polymer having a terminal hydroxy group with a compound having an isocyanate group and a reactive silicon group provides a high conversion rate in a relatively short reaction time.
- the organic polymer having a reactive silicon group obtained by Method I has a lower viscosity than the organic polymer having a reactive silicon group obtained by Method III, and the resin composition for foams has good workability. is obtained, and the organic polymer having a reactive silicon group obtained by Method II has a strong odor derived from mercaptosilane. Therefore, Method I is particularly preferred.
- polyoxyalkylene polymers having a reactive silicon group examples include, for example, JP-B-45-36319, JP-B-46-12154, JP-A-50-156599, and JP-A-54-6096. , JP-A-55-13767, JP-A-55-13468, JP-A-57-164123, JP-B-3-2450, US Pat. No. 3632557, US Pat. No. 4345053, US Pat. and US Pat. No. 4,960,844.
- JP-A-61-197631, JP-A-61-215622, JP-A-61-215623, JP-A-61-218632, JP-A-3-72527, JP-A-3- 47825 and JP-A-8-231707 the number average molecular weight is 6,000 or more and the molecular weight distribution (Mw/Mn) is 1.6 or less or 1.3 or less.
- Mw/Mn molecular weight distribution
- a polyoxyalkylene polymer having a narrow reactive silicon group is also preferred. Polyoxyalkylene polymers having such reactive silicon groups may be used alone, or two or more of them may be used in combination.
- a synthetic method for introducing an average of more than 1.0 reactive silicon groups to one terminal site of a polyoxyalkylene polymer will be described below.
- the polyoxyalkylene polymer (A) having an average of more than 1.0 reactive silicon groups at one terminal site has two at one terminal of the hydroxyl group-terminated polymer obtained by polymerization. After introducing the above carbon-carbon unsaturated bonds, it is preferable to react with a reactive silicon group-containing compound that reacts with the carbon-carbon unsaturated bonds.
- the polyoxyalkylene polymer (A) is preferably produced by polymerizing an epoxy compound with an initiator having a hydroxyl group using a double metal cyanide complex catalyst such as a zinc hexacyanocobaltate glyme complex.
- initiators having a hydroxyl group examples include ethylene glycol, propylene glycol, glycerin, pentaerythritol, low-molecular-weight polyoxypropylene glycol, polyoxypropylene triol, allyl alcohol, polypropylene monoallyl ether, polypropylene monoalkyl ether, and the like. Those having the above are mentioned.
- Epoxy compounds include alkylene oxides such as ethylene oxide and propylene oxide, and glycidyl ethers such as methyl glycidyl ether and allyl glycidyl ether. Among these, propylene oxide is preferred.
- alkali metal salt used in the present invention sodium hydroxide, sodium methoxide, sodium ethoxide, potassium hydroxide, potassium methoxide and potassium ethoxide are preferred, and sodium methoxide and potassium methoxide are more preferred.
- Sodium methoxide is particularly preferred because of its availability.
- the temperature at which the alkali metal salt is allowed to act is preferably 50°C or higher and 150°C or lower, more preferably 110°C or higher and 140°C or lower.
- the time for which the alkali metal salt is allowed to act is preferably 10 minutes or more and 5 hours or less, more preferably 30 minutes or more and 3 hours or less.
- epoxy compound having a carbon-carbon unsaturated bond used in the present invention, especially general formula (3):
- R 1 and R 2 in the formula are the same as above
- allyl glycidyl ether, methallyl glycidyl ether, glycidyl acrylate, glycidyl methacrylate, butadiene monoxide, and 1,4-cyclopentadiene monoepoxide are preferred from the viewpoint of reaction activity, and allyl glycidyl ether is particularly preferred.
- the amount of the epoxy compound having a carbon-carbon unsaturated bond used in the present invention can be any amount in consideration of the introduction amount and reactivity of the carbon-carbon unsaturated bond to the polymer.
- the molar ratio of the hydroxyl group-terminated polymer to the hydroxyl group is preferably 0.2 or more, more preferably 0.5 or more. Also, it is preferably 5.0 or less, more preferably 2.0 or less.
- the reaction temperature for the ring-opening addition reaction of an epoxy compound having a carbon-carbon unsaturated bond with a polymer containing a hydroxyl group is preferably 60° C. or higher and 150° C. or lower. It is more preferably 110° C. or higher and 140° C. or lower.
- the halogenated hydrocarbon compound having a carbon-carbon unsaturated bond used in the present invention includes vinyl chloride, allyl chloride, methallyl chloride, vinyl bromide, allyl bromide, methallyl bromide, vinyl iodide, allyl iodide, iodine, and methallyl chloride, and it is more preferable to use allyl chloride and methallyl chloride from the viewpoint of ease of handling.
- the amount of the halogenated hydrocarbon compound having a carbon-carbon unsaturated bond is not particularly limited, but the molar ratio to the hydroxyl group of the hydroxyl-terminated polymer is preferably 0.7 or more, and 1.0 or more. more preferred. Moreover, 5.0 or less is preferable and 2.0 or less is more preferable.
- the temperature at which the halogenated hydrocarbon compound having a carbon-carbon unsaturated bond is reacted is preferably 50°C or higher and 150°C or lower, more preferably 110°C or higher and 140°C or lower.
- the reaction time is preferably 10 minutes or more and 5 hours or less, more preferably 30 minutes or more and 3 hours or less.
- the method for introducing the reactive silicon group is not particularly limited, and the three methods described above can be used, and other known methods can be used.
- Epoxy resin curing agent (B) The epoxy resin curing agent (B) in the present multi-component curable composition is preferably an epoxy resin curing agent having a tertiary amine. This configuration has the advantage of improving the tear strength of the cured product.
- any compound having a tertiary amine can be used.
- Examples include benzylamine, N-methyl-N-(dimethylaminopropyl)aminoethanol, 2,4,6-tris(dimethylaminomethyl)phenol, tripropylamine, DBU, DBN, and salts of these tertiary amines can be, but are not limited to. These may be used alone or in combination of two or more, and a known epoxy resin curing agent other than component (B) may be further added.
- the epoxy resin curing agent (B) having a tertiary amine is preferably an aromatic amine, and more preferably has three or more amino groups. Specifically, 2,4,6-tris(dimethylaminomethyl)phenol can be exemplified.
- the content of the epoxy resin curing agent (B) is preferably 0.1 to 10 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is more preferably 0.5 to 9.0 parts by weight, even more preferably 1.0 to 8.0 parts by weight, and particularly preferably 2.0 to 7.0 parts by weight. If the content of the epoxy resin curing agent (B) is within the above range, there is an advantage that both improvement in tear strength of the cured product and cost reduction can be easily achieved.
- aminosilane (C) examples include ⁇ -aminopropyltriethoxysilane, N- ⁇ -(aminoethyl)- ⁇ -aminopropyltrimethoxysilane, N- ⁇ -(amino ethyl)- ⁇ -aminopropylmethyldimethoxysilane and the like. These may use only 1 type and may use 2 or more types together.
- Silquest A-1120 manufactured by Momentive Performance Materials Japan LLC
- Silquest A-1110 manufactured by Momentive Performance Materials Japan LLC
- KBM-602 Shin-Etsu Chemical Co., Ltd. (manufactured by Shin-Etsu Chemical Co., Ltd.)
- KBM-603 manufactured by Shin-Etsu Chemical Co., Ltd.
- KBM-903 manufactured by Shin-Etsu Chemical Co., Ltd.
- the content of the aminosilane (C) is preferably 0.1 to 5.0 parts by weight with respect to the total 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is more preferably 2 to 4.5 parts by weight, still more preferably 0.3 to 4.0 parts by weight, and particularly preferably 0.4 to 3.8 parts by weight. If the content of the aminosilane (C) is within the above range, there is an advantage that both improvement in tear strength of the cured product and cost reduction can easily be achieved.
- the mercaptan compound (D) in the present multi-component curable composition includes, for example, n-dodecylmercaptan, tert-dodecylmercaptan, laurylmercaptan, trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakis ( 3-mercaptopropionate), 3-mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropylchloromethyldimethoxysilane, 3-mercaptopropylmethoxymethyldimethoxysilane, (mercaptomethyl)dimethoxymethylsilane, (mercaptomethyl)trimethoxysilane and the like.
- Examples of commercially available products include Z6062 (manufactured by Dow Chemical Japan Co., Ltd.), Thiocalcol 20 (manufactured by Kao Corporation), KBM-802 (manufactured by Shin-Etsu Chemical Co., Ltd.), KBM-803 (manufactured by Shin-Etsu Chemical Co., Ltd.), etc. may be used.
- the content of the mercaptan compound (D) is preferably 0.2 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is more preferably 0.22 to 4.5 parts by weight, more preferably 0.24 to 4.0 parts by weight, and particularly preferably 0.26 to 3.8 parts by weight. If the content of the mercaptan compound (D) is within the above range, there is an advantage that both improvement in tear strength of the cured product and cost reduction are readily compatible.
- Epoxy resin (E) The epoxy resin (E) in the present multi-component curable composition is preferably an epoxy resin having at least two epoxy groups per molecule. This configuration has the advantage of improving the tear strength of the cured product.
- epoxy resin (E) examples include epichlorohydrin-bisphenol A type epoxy resin, epichlorohydrin-bisphenol F type epoxy resin, flame retardant epoxy resin such as glycidyl ether of tetrabromobisphenol A, novolac type epoxy resin, and hydrogenated bisphenol A type epoxy resin.
- Resins glycidyl ether type epoxy resins of bisphenol A propylene oxide adducts, p-oxybenzoic acid glycidyl ether ester type epoxy resins, m-aminophenol type epoxy resins, diaminodiphenylmethane type epoxy resins, urethane modified epoxy resins, various alicyclic Epoxy resin, N,N-diglycidylaniline, N,N-diglycidyl-o-toluidine, triglycidyl isocyanurate, polyalkylene glycol diglycidyl ether, glycidyl ether of polyhydric alcohol such as glycerin, hydantoin type epoxy resin, petroleum resin Examples include epoxidized unsaturated polymers such as, but not limited to, commonly used epoxy resins can be used. Epoxy resins having at least two epoxy groups per molecule are preferred because they have high reactivity during curing and the cured product can easily form a three-dimensional
- the weight ratio (A):(E) of the polyoxyalkylene polymer (A) and the epoxy resin (E) is preferably 90:10 to 50:50. . If the proportion of (A) is more than 90%, the strength will be reduced, and if it is less than 50%, the flexibility will be reduced and the material will become too hard. Furthermore, 80:20 to 60:40 is more preferable in terms of balance between flexibility and strength.
- This multi-component curable composition contains a hydroxyl group-containing polyoxyalkylene polymer (F) in the A agent and/or the B agent.
- the hydroxyl group-containing polyoxyalkylene polymer (F) functions as a plasticizer.
- the hydroxyl group-containing polyoxyalkylene polymer (F) is used as a plasticizer for the polyoxyalkylene polymer (A), it can be crosslinked at room temperature like the reactive silicon groups possessed by the oxyalkylene polymer (A). It is preferred that it does not contain a group capable of
- the main chain of the hydroxyl group-containing polyoxyalkylene polymer (F) has the general formula: -R f -O- (In the formula, R f is a divalent hydrocarbon group, most preferably an alkylene group having 3 or 4 carbon atoms). A specific example of R f is etc.
- the molecular chain of the hydroxyl group-containing polyoxyalkylene polymer (F) may consist of one type of repeating unit or may consist of two or more types of repeating units. is preferably represented by
- hydroxyl group-containing polyoxyalkylene polymer (F) are not particularly limited, but include, for example, polyoxyethylene, polyoxypropylene, polyoxybutylene, polyoxytetramethylene, polyoxyethylene-polyoxypropylene copolymer coalescence, polyoxypropylene-polyoxybutylene copolymer, and the like.
- polyoxypropylene is preferable from the viewpoint of suppressing bleeding that occurs with the lapse of time.
- the hydroxyl group-containing polyoxyalkylene polymer (F) acts as a plasticizer for the polyoxyalkylene polymer (A) having a reactive silicon group, its number average molecular weight is It must be less than coalesce (A).
- the number average molecular weight of the hydroxyl group-containing polyoxyalkylene polymer (F) is preferably 300 or more, more preferably 800 or more, even more preferably 1000 or more.
- the upper limit is preferably 15,000 or less, more preferably 10,000 or less, even more preferably 8,000 or less, and particularly preferably 5,000 or less.
- the number average molecular weight of the hydroxyl group-containing polyoxyalkylene polymer (F) is 300 or more, it is effective in that it is difficult to volatilize.
- the number average molecular weight of the hydroxyl group-containing polyoxyalkylene polymer (F) is 15,000 or less, the viscosity of the multi-component curable composition can be easily lowered.
- the number average molecular weight of the hydroxyl group-containing polyoxyalkylene polymer (F) is a molecular weight corresponding to the number average molecular weight obtained by terminal group analysis.
- Mw/Mn is 1.6 or less, preferably 1.5 or less.
- the molecular weight distribution (Mw/Mn) of the hydroxyl group-containing polyoxyalkylene polymer (F) is measured using GPC (converted to polystyrene).
- the content of the hydroxyl group-containing polyoxyalkylene polymer (F) is preferably 5 parts by weight or more, and 10 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). 1 part or more is more preferable, and 20 parts by weight or more is even more preferable.
- the upper limit is preferably 150 parts by weight or less, more preferably 100 parts by weight or less, and even more preferably 50 parts by weight or less.
- the cured product can have an appropriate mechanical strength.
- the hydroxyl group-containing polyoxyalkylene polymer (F) can also be blended during polymer production.
- the hydroxyl group-containing polyoxyalkylene polymer (F) may be produced by a polymerization method using a normal caustic alkali, but may be produced by a polymerization method using a double metal cyanide complex such as zinc hexacyanocobaltate as a catalyst. Others can also be used.
- the multi-component curable composition preferably further contains an inorganic filler.
- Inorganic fillers are inexpensive materials, so they enable cost reduction.
- inorganic fillers include, but are not limited to, calcium carbonate, magnesium carbonate, barium carbonate, barium sulfate, diatomaceous earth, calcined clay, clay, talc, barite, anhydride gypsum, titanium oxide, bentonite, organic bentonite, and dioxic oxide.
- calcium carbonate is preferable from the viewpoint of being cheaper.
- These inorganic fillers may be used alone or in combination of two or more.
- the content of the inorganic filler is, for example, preferably 50 to 300 parts by weight, preferably 50 to 200 parts by weight, with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). is more preferably 50 to 180 parts by weight, more preferably 60 to 160 parts by weight, even more preferably 70 to 150 parts by weight.
- the content of the inorganic filler is within the above range, there is an advantage that both low viscosity and low cost of the multi-component curable composition are easily achieved.
- the inorganic filler may be contained in agent A, agent B, or both agent A and agent B.
- the inorganic filler is preferably contained in liquid B (agent B) from the viewpoint of adsorbed water.
- the multi-component curable composition preferably further contains a curing catalyst.
- Any curing catalyst can be used without any particular limitation as long as it can be used as a condensation catalyst.
- Curing catalysts include, for example, dibutyltin dilaurate, dibutyltin diacetate, dibutyltin diethylhexanoate, dibutyltin dioctate, dibutyltin dimethylmalate, dibutyltin diethylmalate, dibutyltin dibutylmalate, dibutyltin diisooctyl Dialkyls such as maleate, dibutyltin ditridecylmalate, dibutyltin dibenzylmalate, dibutyltin maleate, dioctyltin diacetate, dioctyltin distearate, dioctyltin dilaurate, dioctyltin diethyl maleate, dioctyltin diisooctyl maleate, etc.
- Tin dicarboxylates dialkyltin alkoxides such as dibutyltin dimethoxide and dibutyltin diphenoxide, intramolecular coordinating derivatives of dialkyltin such as dibutyltin diacetylacetonate and dibutyltin diethylacetoacetate, such as , reactants of dialkyltin oxides such as dibutyltin oxide and dioctyltin oxide with ester compounds such as dioctyl phthalate, diisodecyl phthalate and methyl maleate; tin obtained by reacting dialkyltin oxides, carboxylic acids and alcohol compounds; compounds such as dibutyltin bistriethoxysilicate, dioctyltin bistriethoxysilicate, and other dialkyltin oxides and silicate compounds, and tetravalent tin compounds such as oxy derivatives of these dialkyltin compounds (stannoxane compounds);
- diethylenetriamine triethylenetetramine, oleylamine, cyclohexylamine, benzylamine, diethylaminopropylamine, xylylenediamine, ethylenediamine, hexamethylenediamine, triethylenediamine, guanidine, diphenylguanidine, 2,4,6-tris(dimethylaminomethyl)phenol , morpholine, N-methylmorpholine, 2-ethyl-4-methylimidazole, 1,8-diazabicyclo(5,4,0)undecene-7 (DBU) and other amine compounds, or calcium carbonate of these amine compounds.
- DBU 1,8-diazabicyclo(5,4,0)undecene-7
- silane coupling agents having an amino group such as amino-modified silyl polymers, silylated amino polymers, unsaturated aminosilane complexes, phenylamino long-chain alkylsilanes, and aminosilylated silicones, which are modified derivatives thereof;
- Catalysts further known silanol condensation catalysts such as fatty acids such as felzatic acid, other acidic catalysts such as organic acidic phosphoric acid ester compounds, basic catalysts and the like can be exemplified.
- These curing catalysts may be used alone or in combination of two or more.
- the content of the curing catalyst is preferably, for example, 0.1 to 5 parts by weight, preferably 0.2 parts by weight, with respect to the total 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is more preferably from 0.3 to 1 part by weight, more preferably from 0.3 to 1 part by weight. If the content of the curing catalyst is within the above range, there is an advantage that both curability and cost reduction can easily be achieved.
- the multi-component curable composition preferably further contains a dehydrating agent.
- a dehydrating agent By containing a dehydrating agent, the present multi-component curable composition has an advantage of excellent storage stability.
- dehydrating agents include, but are not limited to, vinylsilane, tosylisocyanate, vinyltrimethoxysilane, calcium oxide, zeolite, p-toluenesulfonylisocyanate, 3-ethyl-2-methyl-2-(3-methylbutyl)-1. , 3-oxazolidine and the like. These dehydrating agents may be used alone or in combination of two or more.
- the content of the dehydrating agent is, for example, 0.1 to 10 parts by weight, and 0.1 to 5.0 parts by weight, based on 100 parts by weight of the total of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is preferably 0 parts by weight, more preferably 1.0 to 3.0 parts by weight.
- the content of the dehydrating agent is 0.1 parts by weight or more with respect to 100 parts by weight of the polyoxyalkylene polymer (A)
- the polyoxyalkylene polymer (A) that reacts in the presence of water becomes excessive. It has the advantage of being able to prevent reactions.
- the content of the dehydrating agent is 3.0 parts by weight or less with respect to the total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E), storage stability and cost reduction are achieved. have the advantage of being compatible with each other.
- this multi-component curable composition contains fillers, adhesion imparting agents, anti-sagging agents, antioxidants, light stabilizers, ultraviolet absorbers, tackifying resins, low-molecular-weight plasticizers, and other additives. agents, other resins, etc. may be added.
- various additives may be added to the present multi-component curable composition as necessary for the purpose of adjusting various physical properties of the curable composition or cured product.
- Such additives include, for example, solvents, diluents, photo-curing substances, oxygen-curing substances, surface property modifiers, silicates, curability modifiers, radical inhibitors, metal deactivators, ozone deterioration inhibitors, agents, phosphorus peroxide decomposers, lubricants, pigments, antifungal agents, flame retardants, foaming agents, and the like.
- fillers other than the inorganic filler can be blended into the present multi-component curable composition.
- examples of fillers include PVC powder and PMMA powder.
- the amount of the filler used is preferably 0.5 to 100 parts by weight, more preferably 1 to 60 parts by weight, with respect to 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E) combined. .
- Organic balloons may be added for the purpose of reducing the weight (lowering the specific gravity) of the multi-component curable composition.
- Adhesion imparting agent An adhesion-imparting agent can be added to the present multi-component curable composition.
- a silane coupling agent or a reactant of the silane coupling agent can be added as an adhesion imparting agent.
- silane coupling agents include ⁇ -aminopropyltrimethoxysilane, ⁇ -aminopropylmethyldimethoxysilane, N- ⁇ -aminoethyl- ⁇ -aminopropyltrimethoxysilane, N- ⁇ -aminoethyl- ⁇ - Amino group-containing silanes such as aminopropylmethyldimethoxysilane, N-phenyl- ⁇ -aminopropyltrimethoxysilane, (2-aminoethyl)aminomethyltrimethoxysilane; ⁇ -isocyanatopropyltrimethoxysilane, ⁇ -isocyanatopropyltrimethoxysilane; isocyanate group-containing silanes such as ethoxysilane, ⁇ -isocyanatopropylmethyldimethoxysilane, ⁇ -isocyanatomethyltrimethoxysilane, ⁇ -isocyan
- the adhesiveness-imparting agent may be used alone or in combination of two or more. Reaction products of various silane coupling agents can also be used.
- the amount of the silane coupling agent used is preferably 0.1 to 20 parts by weight, particularly 0.5 to 10 parts by weight, based on 100 parts by weight of the total of the polyoxyalkylene polymer (A) and the epoxy resin (E). Parts by weight are preferred.
- an anti-sagging agent may be added to the multi-component curable composition to prevent sagging and improve workability.
- the anti-sagging agent is not particularly limited, but examples thereof include polyamide waxes; hydrogenated castor oil derivatives; metal soaps such as calcium stearate, aluminum stearate and barium stearate. These anti-sagging agents may be used alone or in combination of two or more.
- the amount of anti-sagging agent to be used is preferably 0.1 to 20 parts by weight with respect to 100 parts by weight in total of the polyoxyalkylene polymer (A) and the epoxy resin (E).
- Antioxidants can be used in the present multi-component curable composition.
- the use of an antioxidant can enhance the weather resistance of the cured product.
- antioxidants include hindered phenols, monophenols, bisphenols, and polyphenols. Specific examples of antioxidants are also described in JP-A-4-283259 and JP-A-9-194731.
- the amount of the antioxidant used is preferably 0.1 to 10 parts by weight, particularly 0.2 to 5 parts by weight, with respect to 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E) combined. part is preferred.
- a light stabilizer can be used in the present multi-part curable composition.
- the use of a light stabilizer can prevent photo-oxidative deterioration of the cured product.
- Benzotriazole-based, hindered amine-based, and benzoate-based compounds can be exemplified as light stabilizers, and hindered amine-based compounds are particularly preferred.
- the amount of the light stabilizer used is preferably 0.1 to 10 parts by weight, particularly 0.2 to 5 parts by weight, based on 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E) combined. part is preferred.
- An ultraviolet absorber can be used in the present multi-component curable composition.
- the use of an ultraviolet absorber can enhance the surface weather resistance of the cured product.
- Benzophenone-based, benzotriazole-based, salicylate-based, substituted tolyl-based and metal chelate-based compounds can be exemplified as ultraviolet absorbers, and benzotriazole-based compounds are particularly preferred, and commercially available names of Tinuvin P, Tinuvin 213, Tinuvin 234, Tinuvin 326, Tinuvin 327, Tinuvin 328, Tinuvin 329, and Tinuvin 571 (manufactured by BASF).
- the amount of the ultraviolet absorber to be used is preferably 0.1 to 10 parts by weight, particularly 0.2 to 5 parts by weight, per 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E) combined. part is preferred.
- a tackifying resin can be added to the present multi-component curable composition for the purpose of enhancing the adhesiveness or adhesion to a substrate, or for other purposes.
- the tackifier resin there is no particular limitation and any commonly used one can be used.
- terpene-based resins aromatic modified terpene resins, hydrogenated terpene resins, terpene-phenolic resins, phenolic resins, modified phenolic resins, xylene-phenolic resins, cyclopentadiene-phenolic resins, coumarone-indene resins, rosin-based Resins, rosin ester resins, hydrogenated rosin ester resins, xylene resins, low molecular weight polystyrene resins, styrene copolymer resins, styrene block copolymers and hydrogenated products thereof, petroleum resins (e.g., C5 hydrocarbon resins, C9 hydrocarbon resins, C5C9 hydrocarbon copolymer resins, etc.), hydrogenated petroleum resins, DCPD resins, and the like. These may be used alone or in combination of two or more.
- petroleum resins e.g., C5 hydrocarbon resins, C9 hydrocarbon resins, C
- the amount of the tackifying resin used is preferably 2 to 100 parts by weight, more preferably 5 to 50 parts by weight, with respect to the total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). More preferably, it is 5 to 30 parts by weight.
- a low molecular weight plasticizer can be added to the present multi-part curable composition. More specifically, diisonoyl phthalate, 2-ethoxyethanol, bis(2-ethylhexyl) phthalate, diisodecyl phthalate, and the like are preferable as the low-molecular-weight plasticizer. These low-molecular-weight plasticizers may be used alone or in combination of two or more.
- the content of the low-molecular-weight plasticizer in the present multi-liquid curable composition is not particularly limited. It is preferably 5 to 150 parts by weight, more preferably 20 to 100 parts by weight, even more preferably 30 to 80 parts by weight. This configuration has an advantage that it is easy to achieve both low viscosity of the multi-component curable composition and high tear strength of the cured product.
- This multi-liquid curable composition is suitable for construction and industrial sealing materials such as waterproofing materials, architectural elastic sealing materials, siding board sealing materials, double glazing sealing materials, vehicle sealing materials, back sealing materials for solar cells.
- Electrical and electronic component materials such as adhesives, electrical insulating materials such as insulation coating materials for electric wires and cables, adhesives, adhesives, elastic adhesives, contact adhesives, tile adhesives, reactive hot melt adhesives, paints , powder coatings, coating materials, foams, sealing materials for can lids, etc., heat dissipation sheets, potting agents for electrical and electronic devices, films, gaskets, marine deck caulking, casting materials, various molding materials, artificial marble, and netting Sealing materials for rust prevention and waterproofing of glass and laminated glass edges (cut parts), anti-vibration, damping, sound and seismic isolation materials used in automobiles, ships, home appliances, etc., automotive parts, electrical parts, various machine parts It can be used for various purposes such as a liquid sealing material used in such as.
- a cured product (hereinafter referred to as "main cured product") is provided by curing the present multi-component curable composition.
- the cured product is formed by curing the multi-component curable composition.
- the cured product contains component (A), component (B), component (C), and component A containing component (D), component (E), and component (F). It is formed by curing a multi-component curable composition containing a B agent at room temperature without heating.
- the cured product comprises an agent A containing component (A), component (B), component (C), component (D), and component (F), and component (E) and a multi-component curable composition containing and cured at room temperature without heating.
- the cured product comprises an agent A containing component (A), component (B), component (C), component (D), and component (F), and component (E) and a B agent containing component (F), and cured at room temperature without heating.
- the present multi-component curable composition contains a volatile component such as a solvent, the volatile component volatilizes during the curing period. Therefore, the present cured product does not substantially contain the volatile components contained in the present multi-component curable composition.
- waterproof material In one embodiment of the present invention, a waterproof material containing the cured product (hereinafter referred to as "the present waterproof material”) is provided.
- This waterproof material forms a seamless coating film and is highly reliable in waterproofing, so it is particularly useful as a moisture-permeable coating film waterproofing material for roofs that requires high waterproof performance.
- Moisture-permeable coating film waterproofing materials for roofs of buildings are waterproofing materials applied to base materials for roofs such as sheathing boards.
- the use of the present waterproof material is not particularly limited, but it is preferably used as an outdoor waterproof material for ceilings, rooftops, balconies, irrigation channels, garages and the like.
- the present waterproof material may contain, in addition to the present cured product, any component that a waterproof material may generally contain. 1 type may be sufficient as such a component, and 2 or more types may be sufficient as it. Also, the content of such components is not particularly limited, and can be appropriately set by those skilled in the art as long as the effects of the present invention are achieved.
- a method of making a waterproof structure comprising applying the multi-part curable composition to a building substrate.
- this multi-component curable composition can provide a cured product with excellent tensile properties and tear strength. It is possible to provide a waterproof structure excellent in
- the method of applying the multi-component curable composition to the base of the building is not particularly limited, but for example, the multi-component curable composition may be directly applied to the base of the building, or may be applied to the base. For example, it may be applied after a primer has been applied.
- a method for manufacturing a waterproof structure which includes the step of applying a topcoat onto a coating film formed by curing the multi-component curable composition.
- one aspect of the present invention includes the following. ⁇ 1> A agent containing a polyoxyalkylene polymer (A) having a reactive silicon group represented by the general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D) , and a B agent containing an epoxy resin (E), A multicomponent curable composition, wherein the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
- each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms.
- the hydrocarbon group as R may be substituted and may have a hetero-containing group.
- X is each independently a hydroxyl group or a hydro is a degradable group.
- a is an integer of 1 to 3.
- the content of the aminosilane (C) is 0.1 to 3.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E).
- the content of the mercaptan compound (D) is 0.1 to 3.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E).
- the content of the inorganic filler is 50 to 300 parts by weight with respect to the total 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E).
- the terminal portion of the polyoxyalkylene polymer (A) has the general formula (2):
- R 1 and R 3 are each independently a divalent C 1-6 bonding group, and the atoms bonded to the respective carbon atoms adjacent to R 1 and R 3 are carbon, oxygen , nitrogen, each of R 2 and R 4 is independently hydrogen or a hydrocarbon group having 1 to 10 carbon atoms, n is an integer of 1 to 10, R 5 is a substituted or an unsubstituted hydrocarbon group having 1 to 20 carbon atoms, X is a hydroxyl group or a hydrolyzable group, and c is an integer of 1 to 3.), represented by ⁇ 1> to ⁇ 5>, the multi-component curable composition according to any one of above.
- the aminosilane (C) is ⁇ -aminopropyltriethoxysilane, N- ⁇ -(aminoethyl)- ⁇ -aminopropyltrimethoxysilane, and N- ⁇ -(aminoethyl)- ⁇ -aminopropylmethyl
- the multi-component curable composition according to any one of ⁇ 1> to ⁇ 7> which is at least one selected from the group consisting of dimethoxysilane.
- the mercaptan compound (D) is n-dodecyl mercaptan, tert-dodecyl mercaptan, lauryl mercaptan, trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakis (3-mercaptopropionate), 3 - from mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropylchloromethyldimethoxysilane, 3-mercaptopropylmethoxymethyldimethoxysilane, (mercaptomethyl)dimethoxymethylsilane and (mercaptomethyl)trimethoxysilane.
- the multi-component curable composition according to any one of ⁇ 1> to ⁇ 8> which is at least one selected from the group consisting of: ⁇ 10>
- ⁇ 11> The hydroxyl group-containing polyoxyalkylene polymer (F) according to any one of ⁇ 1> to ⁇ 10>, wherein the hydroxyl group-containing polyoxyalkylene polymer (F) has a number average molecular weight of 300 or more.
- ⁇ 12> A cured product obtained by curing the multi-component curable composition according to any one of ⁇ 1> to ⁇ 11>.
- ⁇ 13> A waterproof material comprising the cured product according to ⁇ 12>.
- ⁇ 14> A method for producing a waterproof structure, comprising the step of applying the multi-component curable composition according to any one of ⁇ 1> to ⁇ 11> to a foundation of a building.
- a method for producing a waterproof structure comprising a step of applying a topcoat onto a coating film formed by curing the multi-component curable composition according to any one of ⁇ 1> to ⁇ 11>. .
- One aspect of the invention may include the following. ⁇ 16> A agent containing a polyoxyalkylene polymer (A) having a reactive silicon group represented by the general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D) , and a B agent containing an epoxy resin (E), A multicomponent curable composition, wherein the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
- each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms.
- the hydrocarbon group as R may be substituted and may have a hetero-containing group.
- X is each independently a hydroxyl group or a hydro is a degradable group.
- a is an integer of 1 to 3.
- the content of the mercaptan compound (D) is 0.2 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E).
- the content of the inorganic filler is 50 to 180 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E).
- the content of the aminosilane (C) is 0.1 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E).
- the terminal portion of the polyoxyalkylene polymer (A) has the general formula (2):
- R 1 and R 3 are each independently a divalent C 1-6 bonding group, and the atoms bonded to the respective carbon atoms adjacent to R 1 and R 3 are carbon, oxygen , nitrogen, each of R 2 and R 4 is independently hydrogen or a hydrocarbon group having 1 to 10 carbon atoms, n is an integer of 1 to 10, R 5 is a substituted or an unsubstituted hydrocarbon group having 1 to 20 carbon atoms, X is a hydroxyl group or a hydrolyzable group, and c is an integer of 1 to 3.), represented by ⁇ 16> to ⁇ 20>.
- the aminosilane (C) is ⁇ -aminopropyltriethoxysilane, N- ⁇ -(aminoethyl)- ⁇ -aminopropyltrimethoxysilane, and N- ⁇ -(aminoethyl)- ⁇ -aminopropylmethyl
- the multi-component curable composition according to any one of ⁇ 16> to ⁇ 22> which is at least one selected from the group consisting of dimethoxysilane.
- the mercaptan compound (D) is n-dodecylmercaptan, tert-dodecylmercaptan, laurylmercaptan, trimethylolpropane tris(3-mercaptopropionate), pentaerythritol tetrakis(3-mercaptopropionate), 3 - from mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropylchloromethyldimethoxysilane, 3-mercaptopropylmethoxymethyldimethoxysilane, (mercaptomethyl)dimethoxymethylsilane and (mercaptomethyl)trimethoxysilane
- the multi-component curable composition according to any one of ⁇ 16> to ⁇ 23> which is at least one selected from the group consisting of: ⁇ 25>
- ⁇ 26> The hydroxyl group-containing polyoxyalkylene polymer (F) according to any one of ⁇ 16> to ⁇ 25>, wherein the hydroxyl group-containing polyoxyalkylene polymer (F) has a number average molecular weight of 300 or more.
- a multi-component curable composition. ⁇ 27> A cured product obtained by curing the multi-component curable composition according to any one of ⁇ 16> to ⁇ 26>.
- ⁇ 28> A waterproof material comprising the cured product according to ⁇ 27>.
- ⁇ 29> A method for producing a waterproof structure, comprising the step of applying the multi-component curable composition according to any one of ⁇ 16> to ⁇ 26> to the foundation of a building.
- a method for producing a waterproof structure comprising the step of applying a topcoat onto a coating film formed by curing the multi-component curable composition according to any one of ⁇ 16> to ⁇ 26>. .
- a tensile strength test was performed at 23° C. and 50% RH using Autograph (AGS-J) manufactured by Shimadzu Corporation. Evaluation was carried out according to JIS A 6021 urethane-based high elongation type (former type 1). Specifically, tensile strength and tensile elongation were determined by punching according to JIS No.3. Tensile measurements were performed at 500 mm/min.
- Tear strength A tensile strength test was performed at 23° C. and 50% RH using Autograph (AGS-J) manufactured by Shimadzu Corporation. Evaluation was carried out according to JIS A 6021 urethane-based high elongation type (former type 1). The tear strength was obtained by punching with an angle-shaped dumbbell without cuts specified in JIS K 6252.
- Polyoxypropylene glycol having a number average molecular weight of about 4,800 is used as an initiator, and propylene oxide is polymerized with a zinc hexacyanocobaltate glyme complex catalyst, and a number average molecular weight of 28,000 having hydroxyl groups at both ends (terminal group equivalent molecular weight of 18,000 ) to obtain polyoxypropylene (P-1) having a molecular weight distribution Mw/Mn of 1.21. Subsequently, a 28% methanol solution of sodium methoxide was added in an amount of 1.0 molar equivalent to the hydroxyl group of this hydroxyl-terminated polyoxypropylene (P-1).
- Example 1 (A-1) and (A-2) as the polyoxyalkylene polymer (A) having a reactive silicon group, Ankamine K54 as the epoxy resin curing agent (B), and A-1120 as the aminosilane (C). , Z6062 as a mercaptan compound (D), vinylsilane A-171 as a dehydrating agent, and MSCAT-01 as a curing catalyst were added in the amounts shown in Table 1, respectively, and stirred by hand for 2 minutes to obtain solution A. (Agent A) was produced.
- Epikote 828 as the epoxy resin (E), Actcol 21-56 as the hydroxyl-containing polyoxyalkylene polymer (F), calcium carbonate (Whiten SB) as the inorganic filler, and carbon black (Asahi Thermal) as the filler. and were added in the respective amounts shown in Table 1, stirred by hand for 2 minutes, and then passed through three ceramic rolls three times to prepare liquid B (agent B).
- liquid A (agent A), liquid B (agent B), and liquid C (agent C) were sequentially added to a disposable cup and stirred by hand. After that, they were mixed and defoamed with a super mixer (ARE-250, manufactured by Thinky Co., Ltd.). Specifically, mixing and defoaming were performed by stirring at 500 rpm for 20 seconds, 1500 rpm for 20 seconds, and 2000 rpm for 40 seconds, followed by defoaming at 1500 rpm for 40 seconds. Then, the defoamed mixture was poured into a mold (Teflon (registered trademark) sheet + packer, thickness 2 mm).
- ARE-250 manufactured by Thinky Co., Ltd.
- Examples 2 to 16 Comparative Examples 1 to 6
- a curable resin composition and a cured product were prepared in the same manner as in Example 1, except that the amount of each component was changed as shown in Tables 1 and 2.
- Tensile properties and tear strength were evaluated for the resulting cured product. Results are shown in Tables 1 and 2.
- Tables 1 and 2 show that the curable compositions of Examples 1 to 16 are excellent in tensile properties and tear strength when cured. That is, according to one embodiment of the present invention, it was shown that a curable composition capable of providing a cured product having excellent tensile properties and tear strength can be provided.
- the curable compositions of Comparative Examples 1 to 6 are poor in tensile properties and tear strength. That is, it was shown that when the curable composition does not satisfy the configuration of the present multicomponent curable composition, the tensile properties and tear strength are poor.
- this multi-component curable composition can provide a cured product having excellent tensile properties and tear strength, it can be suitably used for waterproof materials and the like.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
The present invention addresses the problem of providing a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength. Provided is a multicomponent curable composition including: a component A, which includes a polyoxyalkylene polymer having a specific reactive silicon group (A), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D); and a component B, which includes an epoxy resin (E). The multicomponent curable composition includes a hydroxyl group-containing polyoxyalkylene polymer (F) in the component A and/or the component B.
Description
本発明は、多液型硬化性組成物、ならびにそれを用いた硬化物および防水材に関する。本発明はまた、多液型硬化性組成物を用いた防水構造物の製造方法に関する。
The present invention relates to a multi-component curable composition, and a cured product and waterproof material using it. The present invention also relates to a method of manufacturing a waterproof structure using the multi-component curable composition.
建築業界で使われている塗膜防水材において、主剤としてポリオール樹脂(樹脂成分)を、硬化剤(架橋剤)としてポリイソシアネートを用いた、2液型ウレタン塗膜防水材がよく使用されている。その一方で、ポリイソシアネートは毒性が強く、環境への悪影響も大きいことから、ポリイソシアネートに代わる材料が求められている。
Among the coating waterproofing materials used in the construction industry, two-part urethane coating waterproofing materials that use polyol resin (resin component) as the main agent and polyisocyanate as the curing agent (crosslinking agent) are often used. . On the other hand, polyisocyanate is highly toxic and has a great adverse effect on the environment, so there is a demand for alternative materials to polyisocyanate.
そこで、ポリイソシアネートに代わる材料として、例えば、特許文献1には、1分子内に2個以上の反応性ケイ素基を含有する変性シリコーン化合物と、メルカプトシラン化合物と、エポキシ樹脂と、を配合する技術が開示されている。
Therefore, as a material to replace polyisocyanate, for example, Patent Document 1 discloses a technique of blending a modified silicone compound containing two or more reactive silicon groups in one molecule, a mercaptosilane compound, and an epoxy resin. is disclosed.
また、特許文献2には、特定の反応性ケイ素基を有するポリオキシアルキレン系重合体、特定の反応性ケイ素基を有する(メタ)アクリル酸エステル系重合体、およびエポキシ樹脂硬化剤、を含むA剤と、エポキシ樹脂、およびシラノール縮合触媒、を含むB剤、を含む多液型の硬化性組成物が開示されている。
In addition, Patent Document 2 discloses a polyoxyalkylene polymer having a specific reactive silicon group, a (meth)acrylic acid ester polymer having a specific reactive silicon group, and an epoxy resin curing agent. and a B-part comprising an epoxy resin and a silanol condensation catalyst are disclosed.
しかしながら、上記の技術では、引張特性および引き裂き強さの両立の観点で、改善の余地があった。
However, with the above technology, there is room for improvement in terms of both tensile properties and tear strength.
そこで、本発明の目的は、引張特性と引き裂き強さとに優れる硬化物を提供し得る多液型硬化性組成物を提供することにある。
Accordingly, an object of the present invention is to provide a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength.
本発明者らは、上記課題を解決するために鋭意研究を重ねた結果、特定のポリオキシアルキレン系重合体およびエポキシ樹脂を含む多液型硬化性組成物に対して、アミノシラン(C)、メルカプタン化合物(D)、および水酸基含有ポリオキシアルキレン系重合体(F)を配合することにより、引張特性と引き裂き強さとに優れる硬化物を提供し得る多液型硬化性組成物が得られることを初めて見出し、本発明を完成させるに至った。
The present inventors have made intensive studies to solve the above problems, and found that aminosilane (C), mercaptan It is the first time that a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength can be obtained by blending the compound (D) and the hydroxyl group-containing polyoxyalkylene polymer (F). This led to the completion of the present invention.
したがって、本発明の一態様は、一般式(1)に示す反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む、多液型硬化性組成物である。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。) Therefore, one aspect of the present invention is a polyoxyalkylene polymer (A) having a reactive silicon group represented by general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D ) and a B agent containing an epoxy resin (E), wherein the A agent and / or the B agent contains a hydroxyl group-containing polyoxyalkylene polymer (F). composition.
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。) Therefore, one aspect of the present invention is a polyoxyalkylene polymer (A) having a reactive silicon group represented by general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D ) and a B agent containing an epoxy resin (E), wherein the A agent and / or the B agent contains a hydroxyl group-containing polyoxyalkylene polymer (F). composition.
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
本発明の一態様によれば、引張特性と引き裂き強さに優れる硬化物を提供し得る多液型硬化性組成物を提供することができる。
According to one aspect of the present invention, it is possible to provide a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength.
本発明の実施の一形態について、以下に詳細に説明する。なお、本明細書において特記しない限り、数値範囲を表す「A~B」は、「A以上、B以下」を意味する。また、本明細書中に記載された文献の全てが、本明細書中において参考文献として援用される。
One embodiment of the present invention will be described in detail below. In this specification, unless otherwise specified, "A to B" representing a numerical range means "A or more and B or less". Also, all of the documents mentioned in this specification are incorporated herein by reference.
〔1.本発明の概要〕
本発明の一実施形態に係る多液型硬化性組成物(以下、「本多液型硬化性組成物」と称する。)は、一般式(1)に示す反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。)
塗膜防水材では、安価な2液ウレタン製品(残存イソシアネート含有)が一般的に使用されているが、環境規制のために、非イソシアネート化が求められていた。しかし、例えば、特許文献1および2に示すように、非イソシアネート系の硬化剤を用いた場合には、引張特性と引き裂き強さとの点で改善の余地がある。特に、硬化性組成物を低粘度化かつ低コスト化するために、可塑剤および低価格のフィラーを含む場合には、上記の問題が大きく生じる。 [1. Overview of the present invention]
A multi-component curable composition according to one embodiment of the present invention (hereinafter referred to as "this multi-component curable composition") is a polyoxyalkylene having a reactive silicon group represented by general formula (1) A agent containing a system polymer (A), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D), and a B agent containing an epoxy resin (E), wherein the A agent and / Or the B agent contains a hydroxyl group-containing polyoxyalkylene polymer (F).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
Inexpensive two-liquid urethane products (containing residual isocyanate) are commonly used for coating film waterproofing materials, but non-isocyanate products have been required due to environmental regulations. However, as shown in Patent Documents 1 and 2, for example, when a non-isocyanate curing agent is used, there is room for improvement in terms of tensile properties and tear strength. In particular, when the curable composition contains a plasticizer and a low-cost filler in order to reduce the viscosity and cost, the above problems arise.
本発明の一実施形態に係る多液型硬化性組成物(以下、「本多液型硬化性組成物」と称する。)は、一般式(1)に示す反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。)
塗膜防水材では、安価な2液ウレタン製品(残存イソシアネート含有)が一般的に使用されているが、環境規制のために、非イソシアネート化が求められていた。しかし、例えば、特許文献1および2に示すように、非イソシアネート系の硬化剤を用いた場合には、引張特性と引き裂き強さとの点で改善の余地がある。特に、硬化性組成物を低粘度化かつ低コスト化するために、可塑剤および低価格のフィラーを含む場合には、上記の問題が大きく生じる。 [1. Overview of the present invention]
A multi-component curable composition according to one embodiment of the present invention (hereinafter referred to as "this multi-component curable composition") is a polyoxyalkylene having a reactive silicon group represented by general formula (1) A agent containing a system polymer (A), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D), and a B agent containing an epoxy resin (E), wherein the A agent and / Or the B agent contains a hydroxyl group-containing polyoxyalkylene polymer (F).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
Inexpensive two-liquid urethane products (containing residual isocyanate) are commonly used for coating film waterproofing materials, but non-isocyanate products have been required due to environmental regulations. However, as shown in Patent Documents 1 and 2, for example, when a non-isocyanate curing agent is used, there is room for improvement in terms of tensile properties and tear strength. In particular, when the curable composition contains a plasticizer and a low-cost filler in order to reduce the viscosity and cost, the above problems arise.
そこで、本発明者は、上記課題を解決し得る手段について鋭意検討を行った結果、以下の知見を得ることに成功した。
・特定のポリオキシアルキレン系重合体およびエポキシ樹脂を含む多液型硬化性組成物に対して、アミノシラン(C)、メルカプタン化合物(D)、および水酸基含有ポリオキシアルキレン系重合体(F)を配合することにより、引張特性および引き裂き強さに優れる硬化物を提供し得る多液型硬化性組成物が得られる。
・特に、上記多液型硬化性組成物が、無機フィラー(例えば、炭酸カルシウム)、可塑剤等を含む場合にも、アミノシラン(C)、メルカプタン化合物(D)、および水酸基含有ポリオキシアルキレン系重合体(F)を配合することにより、引張特性および引き裂き強さに優れる硬化物を提供し得る多液型硬化性組成物が得られる。 Therefore, the present inventors have made intensive studies on means for solving the above problems, and as a result, have succeeded in obtaining the following findings.
・Aminosilane (C), mercaptan compound (D), and hydroxyl group-containing polyoxyalkylene polymer (F) are compounded into a multicomponent curable composition containing a specific polyoxyalkylene polymer and epoxy resin. By doing so, it is possible to obtain a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength.
- In particular, even when the multicomponent curable composition contains an inorganic filler (e.g., calcium carbonate), a plasticizer, etc., the aminosilane (C), the mercaptan compound (D), and the hydroxyl group-containing polyoxyalkylene polymer By blending coalescence (F), a multicomponent curable composition can be obtained which can provide a cured product having excellent tensile properties and tear strength.
・特定のポリオキシアルキレン系重合体およびエポキシ樹脂を含む多液型硬化性組成物に対して、アミノシラン(C)、メルカプタン化合物(D)、および水酸基含有ポリオキシアルキレン系重合体(F)を配合することにより、引張特性および引き裂き強さに優れる硬化物を提供し得る多液型硬化性組成物が得られる。
・特に、上記多液型硬化性組成物が、無機フィラー(例えば、炭酸カルシウム)、可塑剤等を含む場合にも、アミノシラン(C)、メルカプタン化合物(D)、および水酸基含有ポリオキシアルキレン系重合体(F)を配合することにより、引張特性および引き裂き強さに優れる硬化物を提供し得る多液型硬化性組成物が得られる。 Therefore, the present inventors have made intensive studies on means for solving the above problems, and as a result, have succeeded in obtaining the following findings.
・Aminosilane (C), mercaptan compound (D), and hydroxyl group-containing polyoxyalkylene polymer (F) are compounded into a multicomponent curable composition containing a specific polyoxyalkylene polymer and epoxy resin. By doing so, it is possible to obtain a multicomponent curable composition capable of providing a cured product having excellent tensile properties and tear strength.
- In particular, even when the multicomponent curable composition contains an inorganic filler (e.g., calcium carbonate), a plasticizer, etc., the aminosilane (C), the mercaptan compound (D), and the hydroxyl group-containing polyoxyalkylene polymer By blending coalescence (F), a multicomponent curable composition can be obtained which can provide a cured product having excellent tensile properties and tear strength.
したがって、本発明の一態様によると、環境に対応した、引張特性および引き裂き強さに優れる本多液型硬化性組成物を提供することができる。また、本発明の一態様によると、本多液型硬化性組成物は、安価な材料である、炭酸カルシウム(無機フィラー)、可塑剤等の含有量を増やして利用できるため、コストダウンの観点でも有利である。
Therefore, according to one aspect of the present invention, it is possible to provide the present multi-component curable composition that is environmentally friendly and has excellent tensile properties and tear strength. In addition, according to one aspect of the present invention, the present multi-component curable composition can be used by increasing the content of inexpensive materials such as calcium carbonate (inorganic filler), plasticizer, and the like, so the cost can be reduced. But it is advantageous.
上述の通り、本発明の一態様による構成によれば、毒性の強いイソシアネートを使用することなく、引張特性および引き裂き強さが優れた防水材を製造することができる。これにより、例えば、目標12「持続可能な消費生産形態を確保する」や目標14「持続可能な開発のために、海・海洋資源を保全し、持続可能な形で利用する」等の持続可能な開発目標(SDGs)の達成に貢献できる。以下、本多液型硬化性組成物の構成について詳説する。
As described above, according to the configuration according to one aspect of the present invention, a waterproof material with excellent tensile properties and tear strength can be produced without using highly toxic isocyanate. This will enable, for example, sustainable development such as Goal 12 “Ensure sustainable consumption and production patterns” and Goal 14 “Conserve and sustainably use the seas, seas and marine resources for sustainable development”. can contribute to the achievement of various development goals (SDGs). The configuration of the present multi-component curable composition will be described in detail below.
〔2.多液型硬化性組成物〕
本多液型硬化性組成物は、上述の通り、特定の反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む。 [2. Multi-component curable composition]
As described above, the multicomponent curable composition comprises a polyoxyalkylene polymer (A) having a specific reactive silicon group, an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D ) and a B agent containing an epoxy resin (E), and the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
本多液型硬化性組成物は、上述の通り、特定の反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む。 [2. Multi-component curable composition]
As described above, the multicomponent curable composition comprises a polyoxyalkylene polymer (A) having a specific reactive silicon group, an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D ) and a B agent containing an epoxy resin (E), and the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
(2-1.反応性ケイ素基を有するポリオキシアルキレン系重合体(A))
本発明の一実施形態に係るポリオキシアルキレン系重合体(A)は、一般式(1)に示す反応性ケイ素基を有する。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。)
Rの炭化水素基の炭素数は1~10が好ましく、1~5がより好ましく、1~3がさらに好ましい。Rの具体例としては、例えば、メチル基、エチル基、クロロメチル基、メトキシメチル基、N,N-ジエチルアミノメチル基、を挙げることができるが、好ましくは、メチル基、エチル基、クロロメチル基、メトキシメチル基であり、より好ましくは、メチル基、メトキシメチル基である。当該構成によれば、貯蔵安定性と反応性とが両立しやすいという利点を有する。 (2-1. Polyoxyalkylene polymer (A) having a reactive silicon group)
A polyoxyalkylene polymer (A) according to one embodiment of the present invention has a reactive silicon group represented by general formula (1).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
The number of carbon atoms in the hydrocarbon group of R is preferably 1 to 10, more preferably 1 to 5, even more preferably 1 to 3. Specific examples of R include, for example, methyl group, ethyl group, chloromethyl group, methoxymethyl group and N,N-diethylaminomethyl group, preferably methyl group, ethyl group and chloromethyl group. , a methoxymethyl group, more preferably a methyl group or a methoxymethyl group. According to the said structure, it has the advantage that it is easy to balance storage stability and reactivity.
本発明の一実施形態に係るポリオキシアルキレン系重合体(A)は、一般式(1)に示す反応性ケイ素基を有する。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。)
Rの炭化水素基の炭素数は1~10が好ましく、1~5がより好ましく、1~3がさらに好ましい。Rの具体例としては、例えば、メチル基、エチル基、クロロメチル基、メトキシメチル基、N,N-ジエチルアミノメチル基、を挙げることができるが、好ましくは、メチル基、エチル基、クロロメチル基、メトキシメチル基であり、より好ましくは、メチル基、メトキシメチル基である。当該構成によれば、貯蔵安定性と反応性とが両立しやすいという利点を有する。 (2-1. Polyoxyalkylene polymer (A) having a reactive silicon group)
A polyoxyalkylene polymer (A) according to one embodiment of the present invention has a reactive silicon group represented by general formula (1).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
The number of carbon atoms in the hydrocarbon group of R is preferably 1 to 10, more preferably 1 to 5, even more preferably 1 to 3. Specific examples of R include, for example, methyl group, ethyl group, chloromethyl group, methoxymethyl group and N,N-diethylaminomethyl group, preferably methyl group, ethyl group and chloromethyl group. , a methoxymethyl group, more preferably a methyl group or a methoxymethyl group. According to the said structure, it has the advantage that it is easy to balance storage stability and reactivity.
Xとしては、例えば、水酸基、ハロゲン、アルコキシ基、アシルオキシ基、ケトキシメート基、アミノ基、アミド基、酸アミド基、アミノオキシ基、メルカプト基、アルケニルオキシ基などが挙げられる。これらの中では、加水分解性が穏やかで取扱いやすいことからメトキシ基、エトキシ基などのアルコキシ基がより好ましく、メトキシ基、エトキシ基が特に好ましい。
Examples of X include hydroxyl group, halogen, alkoxy group, acyloxy group, ketoximate group, amino group, amide group, acid amide group, aminooxy group, mercapto group, and alkenyloxy group. Among these, an alkoxy group such as a methoxy group and an ethoxy group is more preferred, and a methoxy group and an ethoxy group are particularly preferred, since they are moderately hydrolyzable and easy to handle.
ポリオキシアルキレン系重合体(A)が有する反応性ケイ素基としては、具体的には、トリメトキシシリル基、トリエトキシシリル基、トリス(2-プロペニルオキシ)シリル基、トリアセトキシシリル基、ジメトキシメチルシリル基、ジエトキシメチルシリル基、ジメトキシエチルシリル基、(クロロメチル)ジメトキシシリル基、(クロロメチル)ジエトキシシリル基、(メトキシメチル)ジメトキシシリル基、(メトキシメチル)ジエトキシシリル基、(N,N-ジエチルアミノメチル)ジメトキシシリル基、(N,N-ジエチルアミノメチル)ジエトキシシリル基などが挙げられるが、これらに限定されない。これらの中では、メチルジメトキシシリル基、トリメトキシシリル基、トリエトキシシリル基、(クロロメチル)ジメトキシシリル基、(メトキシメチル)ジメトキシシリル基、(メトキシメチル)ジエトキシシリル基、(N,N-ジエチルアミノメチル)ジメトキシシリル基が高い活性を示し、良好な機械物性を有する硬化物が得られるため好ましく、高剛性の硬化物が得られることから、トリメトキシシリル基、トリエトキシシリル基がより好ましく、トリメトキシシリル基がさらに好ましい。
Specific examples of the reactive silicon group possessed by the polyoxyalkylene polymer (A) include a trimethoxysilyl group, a triethoxysilyl group, a tris(2-propenyloxy)silyl group, a triacetoxysilyl group, and dimethoxymethyl silyl group, diethoxymethylsilyl group, dimethoxyethylsilyl group, (chloromethyl)dimethoxysilyl group, (chloromethyl)diethoxysilyl group, (methoxymethyl)dimethoxysilyl group, (methoxymethyl)diethoxysilyl group, (N ,N-diethylaminomethyl)dimethoxysilyl group, (N,N-diethylaminomethyl)diethoxysilyl group, and the like, but are not limited thereto. Among these are methyldimethoxysilyl, trimethoxysilyl, triethoxysilyl, (chloromethyl)dimethoxysilyl, (methoxymethyl)dimethoxysilyl, (methoxymethyl)diethoxysilyl, (N,N- Diethylaminomethyl)dimethoxysilyl group is preferred because it exhibits high activity and gives a cured product with good mechanical properties, and a trimethoxysilyl group and a triethoxysilyl group are more preferred because a cured product with high rigidity can be obtained. A trimethoxysilyl group is more preferred.
ポリオキシアルキレン系重合体(A)は、1つの末端部位に平均して1個より多い反応性ケイ素基を有していてもよい。1つの末端部位に平均して1個より多い反応性ケイ素基を有するとは、ポリオキシアルキレン系重合体(A)に、下記の一般式(2)で示されるような1つの末端部位に2個以上の反応性ケイ素基を有するポリオキシアルキレンが含まれていることを示している。すなわち、ポリオキシアルキレン系重合体(A)は、1つの末端部位に2個以上の反応性ケイ素基を有するポリオキシアルキレンのみを含むものであってもよいし、1つの末端部位に2個以上の反応性ケイ素基を有するポリオキシアルキレンと、1つの末端部位に1個の反応性ケイ素基を有するポリオキシアルキレンの両方を含むものであってもよい。また、1分子のポリオキシアルキレンが有する複数の末端部位として、2個以上の反応性ケイ素基を有する末端部位と、1個の反応性ケイ素基を有する末端部位の双方があってもよい。さらに、ポリオキシアルキレン系重合体(A)は、総体としては、1つの末端部位に平均して1個より多い反応性ケイ素基を有するが、反応性ケイ素基を有さない末端部位を有するポリオキシアルキレンを含むものであってもよい。
The polyoxyalkylene-based polymer (A) may have more than one reactive silicon group on average at one terminal site. Having more than one reactive silicon group on average at one terminal site means that the polyoxyalkylene polymer (A) has 2 at one terminal site as represented by the following general formula (2) It shows that polyoxyalkylenes with one or more reactive silicon groups are included. That is, the polyoxyalkylene-based polymer (A) may contain only a polyoxyalkylene having two or more reactive silicon groups at one terminal site, or two or more at one terminal site. and polyoxyalkylenes having one reactive silicon group at one terminal site. In addition, the plurality of terminal sites possessed by one molecule of polyoxyalkylene may include both a terminal site having two or more reactive silicon groups and a terminal site having one reactive silicon group. Furthermore, the polyoxyalkylene polymer (A) as a whole has an average of more than one reactive silicon group at one terminal site, but a poly having a terminal site having no reactive silicon group It may contain oxyalkylene.
本多液型硬化性組成物における、ポリオキシアルキレン系重合体(A)の末端部位は、一般式(2):
The terminal portion of the polyoxyalkylene-based polymer (A) in this multi-component curable composition has the general formula (2):
R1、R3としては、2価の炭素数1~6の有機基であってよく、酸素原子を含んでもよく、炭化水素基であってもよい。該炭化水素基の炭素数は1~4が好ましく、1~3がより好ましく、1~2がさらに好ましい。R1の具体例としては、例えば、CH2OCH2、CH2O、CH2を挙げることができるが、好ましくは、CH2OCH2である。R3の具体例としては、例えば、CH2、CH2CH2を挙げることができるが、好ましくは、CH2である。
R 1 and R 3 may be a divalent organic group having 1 to 6 carbon atoms, may contain an oxygen atom, or may be a hydrocarbon group. The number of carbon atoms in the hydrocarbon group is preferably 1-4, more preferably 1-3, even more preferably 1-2. Specific examples of R 1 include CH 2 OCH 2 , CH 2 O and CH 2 , preferably CH 2 OCH 2 . Specific examples of R 3 include CH 2 and CH 2 CH 2 , preferably CH 2 .
R2、R4の炭化水素基の炭素数としては1~5が好ましく、1~3がより好ましく、1~2がさらに好ましい。R2、R4の具体例としては、例えば、水素原子、メチル基、エチル基を挙げることができるが、好ましくは、水素原子、メチル基であり、より好ましくは水素原子である。
The number of carbon atoms in the hydrocarbon groups of R 2 and R 4 is preferably 1-5, more preferably 1-3, even more preferably 1-2. Specific examples of R 2 and R 4 include a hydrogen atom, a methyl group and an ethyl group, preferably a hydrogen atom and a methyl group, more preferably a hydrogen atom.
一般式(2)で表される末端部位は、特に好ましい態様によると、R1がCH2OCH2であり、R3がCH2であり、R2およびR4がそれぞれ水素原子である。nは1~5の整数が好ましく、1~3の整数がより好ましく、1または2がさらに好ましい。ただし、nは、1つの値に限定されるものではなく、複数の値が混在していてもよい。
According to a particularly preferred embodiment, the terminal moiety represented by the general formula (2) is CH 2 OCH 2 for R 1 , CH 2 for R 3 , and hydrogen atoms for R 2 and R 4 . n is preferably an integer of 1 to 5, more preferably an integer of 1 to 3, and even more preferably 1 or 2. However, n is not limited to one value, and may be a mixture of multiple values.
ポリオキシアルキレン系重合体(A)が有する反応性ケイ素基は、1つの末端部位に平均して1.0個より多く有していることが好ましく、1.1個以上であることがより好ましく、1.5個以上であることが更に好ましく、2.0個以上であることがより更に好ましい。また、5個以下であることが好ましく、3個以下であることがより好ましい。
The number of reactive silicon groups possessed by the polyoxyalkylene polymer (A) is preferably more than 1.0 on average at one terminal site, more preferably 1.1 or more. , is more preferably 1.5 or more, and even more preferably 2.0 or more. Also, the number is preferably 5 or less, more preferably 3 or less.
ポリオキシアルキレン系重合体(A)1分子中に含まれる、1個より多くの反応性ケイ素基を有する末端部位の数は、平均して0.5個以上であることが好ましく、1.0個以上であることがより好ましく、1.1個以上であることがさらに好ましく、1.5個以上であることがより更に好ましい。また、4個以下であることが好ましく、3個以下であることがより好ましい。
The number of terminal sites having more than one reactive silicon group contained in one molecule of the polyoxyalkylene polymer (A) is preferably 0.5 or more on average, and 1.0 It is more preferably 1 or more, still more preferably 1.1 or more, and even more preferably 1.5 or more. Also, the number is preferably 4 or less, more preferably 3 or less.
ポリオキシアルキレン系重合体(A)は、末端部位以外に反応性ケイ素基を有しても良いが、末端部位にのみ有することが、高伸びで、低弾性率を示すゴム状硬化物が得られやすくなるため好ましい。
The polyoxyalkylene polymer (A) may have reactive silicon groups in addition to the terminal sites, but having them only in the terminal sites yields a rubber-like cured product with high elongation and low elastic modulus. It is preferable because it becomes easy to be
ポリオキシアルキレン系重合体(A)が有する反応性ケイ素基の1分子当たりの平均個数は、1.0個より多いことが好ましく、1.2個以上がより好ましく、1.3個以上がさらに好ましく、1.5個以上がより更に好ましく、1.7個以上が特に好ましい。また、6.0個以下が好ましく、5.5個以下がより好ましく、5.0個以下が最も好ましい。1分子当たりの反応性ケイ素基の平均個数が1.0個以下では、高強度の硬化物が得られなくなる可能性がある。1分子当たりの反応性ケイ素基の平均個数が6.0個を超えると、高伸びの硬化物が得られなくなる可能性がある。
The average number of reactive silicon groups per molecule of the polyoxyalkylene polymer (A) is preferably more than 1.0, more preferably 1.2 or more, and further preferably 1.3 or more. It is preferably 1.5 or more, more preferably 1.7 or more. Also, the number is preferably 6.0 or less, more preferably 5.5 or less, and most preferably 5.0 or less. If the average number of reactive silicon groups per molecule is 1.0 or less, a cured product with high strength may not be obtained. If the average number of reactive silicon groups per molecule exceeds 6.0, it may not be possible to obtain a cured product with high elongation.
<主鎖構造>
ポリオキシアルキレン系重合体(A)の主鎖骨格には特に制限はなく、例えば、ポリオキシエチレン、ポリオキシプロピレン、ポリオキシブチレン、ポリオキシテトラメチレン、ポリオキシエチレン-ポリオキシプロピレン共重合体、ポリオキシプロピレン-ポリオキシブチレン共重合体などが挙げられる。その中でも、ポリオキシプロピレンが好ましい。 <Main chain structure>
The main chain skeleton of the polyoxyalkylene polymer (A) is not particularly limited, and examples include polyoxyethylene, polyoxypropylene, polyoxybutylene, polyoxytetramethylene, polyoxyethylene-polyoxypropylene copolymer, Examples include polyoxypropylene-polyoxybutylene copolymers. Among them, polyoxypropylene is preferred.
ポリオキシアルキレン系重合体(A)の主鎖骨格には特に制限はなく、例えば、ポリオキシエチレン、ポリオキシプロピレン、ポリオキシブチレン、ポリオキシテトラメチレン、ポリオキシエチレン-ポリオキシプロピレン共重合体、ポリオキシプロピレン-ポリオキシブチレン共重合体などが挙げられる。その中でも、ポリオキシプロピレンが好ましい。 <Main chain structure>
The main chain skeleton of the polyoxyalkylene polymer (A) is not particularly limited, and examples include polyoxyethylene, polyoxypropylene, polyoxybutylene, polyoxytetramethylene, polyoxyethylene-polyoxypropylene copolymer, Examples include polyoxypropylene-polyoxybutylene copolymers. Among them, polyoxypropylene is preferred.
ポリオキシアルキレン系重合体(A)の数平均分子量はGPCにおけるポリスチレン換算分子量において3000~100000、より好ましくは3000~50000であり、特に好ましくは3000~30000である。数平均分子量が3000未満では、反応性ケイ素基の導入量が多くなり、製造コストの点で不都合になる場合があり、100000を越えると、高粘度となる為に作業性の点で不都合な傾向がある。
The number average molecular weight of the polyoxyalkylene polymer (A) is 3,000 to 100,000, more preferably 3,000 to 50,000, and particularly preferably 3,000 to 30,000 in terms of polystyrene equivalent molecular weight in GPC. If the number average molecular weight is less than 3,000, the amount of reactive silicon groups to be introduced increases, which may be disadvantageous in terms of production costs. There is
ポリオキシアルキレン系重合体(A)の分子量としては、反応性ケイ素基導入前の有機重合体前駆体を、JIS K 1557の水酸基価の測定方法と、JIS K 0070に規定されたよう素価の測定方法の原理に基づいた滴定分析により、直接的に末端基濃度を測定し、有機重合体の構造(使用した重合開始剤によって定まる分岐度)を考慮して求めた末端基換算分子量で示すことも出来る。ポリオキシアルキレン系重合体(A)の末端基換算分子量は、有機重合体前駆体の一般的なGPC測定により求めた数平均分子量と上記末端基換算分子量の検量線を作成し、ポリオキシアルキレン系重合体(A)のGPCにより求めた数平均分子量を末端基換算分子量に換算して求めることも可能である。
As the molecular weight of the polyoxyalkylene polymer (A), the organic polymer precursor before the introduction of the reactive silicon group was subjected to the hydroxyl value measurement method of JIS K 1557 and the iodine value of JIS K 0070. Directly measure the terminal group concentration by titration analysis based on the principle of the measurement method, and indicate the terminal group equivalent molecular weight obtained by considering the structure of the organic polymer (degree of branching determined by the polymerization initiator used). can also The terminal group-equivalent molecular weight of the polyoxyalkylene-based polymer (A) is obtained by preparing a calibration curve of the number average molecular weight obtained by general GPC measurement of the organic polymer precursor and the above-mentioned terminal-group-equivalent molecular weight. It is also possible to convert the number-average molecular weight of the polymer (A) obtained by GPC into a terminal group-equivalent molecular weight.
ポリオキシアルキレン系重合体(A)の分子量分布(Mw/Mn)は特に限定されないが、狭いことが好ましく、2.0未満が好ましく、1.6以下がより好ましく、1.5以下がさらに好ましく、1.4以下が特に好ましく、1.2以下が最も好ましい。ポリオキシアルキレン系重合体(A)の分子量分布は、GPC測定により得られる数平均分子量と重量平均分子量から求めることが出来る。
The molecular weight distribution (Mw/Mn) of the polyoxyalkylene polymer (A) is not particularly limited, but is preferably narrow, preferably less than 2.0, more preferably 1.6 or less, and even more preferably 1.5 or less. , 1.4 or less is particularly preferred, and 1.2 or less is most preferred. The molecular weight distribution of the polyoxyalkylene polymer (A) can be obtained from the number average molecular weight and weight average molecular weight obtained by GPC measurement.
また、本発明のポリオキシアルキレン系重合体(A)の主鎖構造は直鎖状であっても分岐状であってもよい。
Further, the main chain structure of the polyoxyalkylene polymer (A) of the present invention may be linear or branched.
本発明の一実施形態に係るポリオキシアルキレン系重合体(A)は、高粘度のポリオキシアルキレン系重合体(A-1)および低粘度のポリオキシアルキレン系重合体(A-2)を混合して用いることが好ましい。
The polyoxyalkylene-based polymer (A) according to one embodiment of the present invention is a mixture of a high-viscosity polyoxyalkylene-based polymer (A-1) and a low-viscosity polyoxyalkylene-based polymer (A-2). It is preferable to use
ポリオキシアルキレン系重合体(A-1)の粘度は、特に限定されないが、6.0Pa・s~50Pa・sが好ましく、7.0Pa・s~48Pa・sがより好ましく、8.0Pa・s~45Pa・sがさらに好ましい。当該構成によれば、得られる多液型硬化性組成物の接着性、および硬化物の引っ張り伸びが向上するとの利点を有する。
The viscosity of the polyoxyalkylene polymer (A-1) is not particularly limited, but is preferably 6.0 Pa s to 50 Pa s, more preferably 7.0 Pa s to 48 Pa s, and 8.0 Pa s. ~45 Pa·s is more preferable. This configuration has the advantage of improving the adhesiveness of the obtained multi-component curable composition and the tensile elongation of the cured product.
ポリオキシアルキレン系重合体(A-2)の粘度は、特に限定されないが、1Pa・s~5Pa・sが好ましく、1.5Pa・s~4Pa・sがより好ましく、2Pa・s~3Pa・sがさらに好ましい。当該構成によれば、得られる多液型硬化性組成物が低粘度化するとの利点を有する。
The viscosity of the polyoxyalkylene polymer (A-2) is not particularly limited, but is preferably 1 Pa s to 5 Pa s, more preferably 1.5 Pa s to 4 Pa s, and 2 Pa s to 3 Pa s. is more preferred. This configuration has the advantage that the resulting multi-component curable composition has a low viscosity.
ポリオキシアルキレン系重合体(A-1)とポリオキシアルキレン系重合体(A-2)の重量比(A-1):(A-2)は、は95:5~50:50であることが好ましい。この範囲であると、柔軟性と高いせん断接着強度を示す硬化物を得ることができる。さらに、高剛性と柔軟性を両立する点で、(A-1):(A-2)は80:20~50:50であることが好ましく、70:30~50:50であることがより好ましい。
The weight ratio (A-1):(A-2) of the polyoxyalkylene polymer (A-1) and the polyoxyalkylene polymer (A-2) is 95:5 to 50:50. is preferred. Within this range, a cured product exhibiting flexibility and high shear adhesive strength can be obtained. Furthermore, in order to achieve both high rigidity and flexibility, (A-1):(A-2) is preferably 80:20 to 50:50, more preferably 70:30 to 50:50. preferable.
<ポリオキシアルキレン系重合体(A)の合成方法>
次に、ポリオキシアルキレン系重合体(A)の合成方法について説明する。 <Method for synthesizing polyoxyalkylene polymer (A)>
Next, a method for synthesizing the polyoxyalkylene polymer (A) will be described.
次に、ポリオキシアルキレン系重合体(A)の合成方法について説明する。 <Method for synthesizing polyoxyalkylene polymer (A)>
Next, a method for synthesizing the polyoxyalkylene polymer (A) will be described.
ポリオキシアルキレン系重合体(A)の主鎖への反応性ケイ素基の導入は、公知の方法で行えばよい。例えば、以下の方法が挙げられる。
Introduction of a reactive silicon group to the main chain of the polyoxyalkylene polymer (A) may be performed by a known method. For example, the following methods are mentioned.
方法I:ヒドロキシ基等の官能基を有する有機重合体に、この官能基に対して反応性を示す活性基および不飽和基を有する化合物を反応させ、不飽和基を有する有機重合体を得る。次いで、得られた不飽和基を有する有機重合体に、ヒドロシリル化によって、反応性ケイ素基を有するヒドロシラン化合物を反応させる。
Method I: An organic polymer having a functional group such as a hydroxy group is reacted with a compound having an active group that exhibits reactivity with the functional group and an unsaturated group to obtain an organic polymer having an unsaturated group. Then, the resulting organic polymer having unsaturated groups is reacted with a hydrosilane compound having reactive silicon groups by hydrosilylation.
方法Iにおいて使用し得る反応性を示す活性基および不飽和基を有する化合物としては、例えば、アリルグリシジルエーテル等の不飽和基含有エポキシ化合物、塩化アリル、塩化メタリル、臭化ビニル、臭化アリル、臭化メタリル、ヨウ化ビニル、ヨウ化アリル、ヨウ化メタリル等の炭素-炭素二重結合を有する化合物等が挙げられる。
Examples of compounds having a reactive active group and an unsaturated group that can be used in Method I include unsaturated group-containing epoxy compounds such as allyl glycidyl ether, allyl chloride, methallyl chloride, vinyl bromide, allyl bromide, Examples thereof include compounds having a carbon-carbon double bond such as methallyl bromide, vinyl iodide, allyl iodide and methallyl iodide.
また、炭素-炭素三重結合を有する化合物としては、例えば、塩化プロパルギル、1-クロロ-2-ブチン、4-クロロ-1-ブチン、1-クロロ-2-オクチン、1-クロロ-2-ペンチン、1,4-ジクロロ-2-ブチン、5-クロロ-1-ペンチン、6-クロロ-1-ヘキシン、臭化プロパルギル、1-ブロモ-2-ブチン、4-ブロモ-1-ブチン、1-ブロモ-2-オクチン、1-ブロモ-2-ペンチン、1,4-ジブロモ-2-ブチン、5-ブロモ-1-ペンチン、6-ブロモ-1-ヘキシン、ヨウ化プロパルギル、1-ヨード-2-ブチン、4-ヨード-1-ブチン、1-ヨード-2-オクチン、1-ヨード-2-ペンチン、1,4-ジヨード-2-ブチン、5-ヨード-1-ペンチン、6-ヨード-1-ヘキシン等の炭素-炭素三重結合を有するハロゲン化炭化水素化合物等が挙げられる。これらの中では、塩化プロパルギル、臭化プロパルギル、およびヨウ化プロパルギルがより好ましい。
Examples of compounds having a carbon-carbon triple bond include propargyl chloride, 1-chloro-2-butyne, 4-chloro-1-butyne, 1-chloro-2-octyne, 1-chloro-2-pentyne, 1,4-dichloro-2-butyne, 5-chloro-1-pentyne, 6-chloro-1-hexyne, propargyl bromide, 1-bromo-2-butyne, 4-bromo-1-butyne, 1-bromo- 2-octyne, 1-bromo-2-pentyne, 1,4-dibromo-2-butyne, 5-bromo-1-pentyne, 6-bromo-1-hexyne, propargyl iodide, 1-iodo-2-butyne, 4-iodo-1-butyne, 1-iodo-2-octyne, 1-iodo-2-pentyne, 1,4-diiodo-2-butyne, 5-iodo-1-pentyne, 6-iodo-1-hexyne, etc. and halogenated hydrocarbon compounds having a carbon-carbon triple bond of . Among these, propargyl chloride, propargyl bromide, and propargyl iodide are more preferred.
炭素-炭素三重結合を有するハロゲン化炭化水素化合物と同時に、塩化ビニル、塩化アリル、塩化メタリル、臭化ビニル、臭化アリル、臭化メタリル、ヨウ化ビニル、ヨウ化アリル、およびヨウ化メタリルなどの炭素-炭素三重結合を有するハロゲン化炭化水素以外の不飽和結合を有する炭化水素化合物を使用してもよい。
Halogenated hydrocarbon compounds having a carbon-carbon triple bond, as well as compounds such as vinyl chloride, allyl chloride, methallyl chloride, vinyl bromide, allyl bromide, methallyl bromide, vinyl iodide, allyl iodide, and methallyl iodide. Hydrocarbon compounds with unsaturated bonds other than halogenated hydrocarbons with carbon-carbon triple bonds may be used.
方法Iにおいて使用し得るヒドロシラン化合物としては、例えば、ハロゲン化シラン類、アルコキシシラン類、アシロキシシラン類、ケトキシメートシラン類等が挙げられる。ヒドロシラン化合物は、これらに限定されない。
Examples of hydrosilane compounds that can be used in method I include halogenated silanes, alkoxysilanes, acyloxysilanes, ketoximate silanes, and the like. Hydrosilane compounds are not limited to these.
ハロゲン化シラン類としては、例えば、トリクロロシラン、メチルジクロロシラン、ジメチルクロロシラン、フェニルジクロロシラン等が挙げられる。
Examples of halogenated silanes include trichlorosilane, methyldichlorosilane, dimethylchlorosilane, and phenyldichlorosilane.
アルコキシシラン類としては、例えば、トリメトキシシラン、トリエトキシシラン、トリイソプロポキシシラン、ジメトキシメチルシラン、ジエトキシメチルシラン、ジイソプロポキシメチルシラン、(メトキシメチル)ジメトキシシラン、フェニルジメトキシシラン、1-[2-(トリメトキシシリル)エチル]-1,1,3,3-テトラメチルジシロキサン等が挙げられる。
Alkoxysilanes include, for example, trimethoxysilane, triethoxysilane, triisopropoxysilane, dimethoxymethylsilane, diethoxymethylsilane, diisopropoxymethylsilane, (methoxymethyl)dimethoxysilane, phenyldimethoxysilane, 1-[ 2-(Trimethoxysilyl)ethyl]-1,1,3,3-tetramethyldisiloxane and the like.
アシロキシシラン類としては、例えば、メチルジアセトキシシラン、フェニルジアセトキシシラン等が挙げられる。
Examples of acyloxysilanes include methyldiacetoxysilane and phenyldiacetoxysilane.
ケトキシメートシラン類としては、例えば、ビス(ジメチルケトキシメート)メチルシラン、ビス(シクロヘキシルケトキシメート)メチルシラン等が挙げられる。
Examples of ketoximate silanes include bis(dimethylketoximate)methylsilane and bis(cyclohexylketoximate)methylsilane.
これらの中では、ハロゲン化シラン類、およびアルコキシシラン類が特に好ましい。アルコキシシラン類は、加水分解性が穏やかで取り扱いやすいために最も好ましい。
Among these, halogenated silanes and alkoxysilanes are particularly preferred. Alkoxysilanes are most preferred because they are mildly hydrolyzable and easy to handle.
アルコキシシラン類の中では、入手しやすい点、硬化性、および貯蔵安定性に優れる発泡体用樹脂組成物を得やすい点、発泡体用樹脂組成物を用いて引張強度に優れる発泡体を製造しやすい点等からジメトキシメチルシランが好ましい。また、硬化性に優れる発泡体用樹脂組成物を得やすい点から、トリメトキシシラン、およびトリエトキシシランも好ましい。
Among the alkoxysilanes, it is easy to obtain, it is easy to obtain a resin composition for foams with excellent curability and storage stability, and it is possible to produce foams with excellent tensile strength using the resin composition for foams. Dimethoxymethylsilane is preferred because it is easy to use. Trimethoxysilane and triethoxysilane are also preferable from the viewpoint of easily obtaining a resin composition for foam having excellent curability.
方法II:メルカプト基および反応性ケイ素基を有する化合物を、ラジカル開始剤および/またはラジカル発生源存在下でのラジカル付加反応によって、方法Iと同様にして得られた不飽和基を有する有機重合体の不飽和基部位に導入する方法。
Method II: A compound having a mercapto group and a reactive silicon group is subjected to a radical addition reaction in the presence of a radical initiator and/or a radical generation source to obtain an organic polymer having an unsaturated group in the same manner as in Method I. Method of introducing into the unsaturated group site of.
方法IIにおいて使用し得るメルカプト基および反応性ケイ素基を有する化合物としては、例えば、3-メルカプト-n-プロピルトリメトキシシラン、3-メルカプト-n-プロピルメチルジメトキシシラン、3-メルカプト-n-プロピルトリエトキシシラン、3-メルカプト-n-プロピルメチルジエトキシシラン、メルカプトメチルトリメトキシシラン、メルカプトメチルトリエトキシシラン等が挙げられる。メルカプト基および反応性ケイ素基を有する化合物は、これらに限定されない。
Compounds having a mercapto group and a reactive silicon group that can be used in method II include, for example, 3-mercapto-n-propyltrimethoxysilane, 3-mercapto-n-propylmethyldimethoxysilane, 3-mercapto-n-propyl triethoxysilane, 3-mercapto-n-propylmethyldiethoxysilane, mercaptomethyltrimethoxysilane, mercaptomethyltriethoxysilane and the like. Compounds having mercapto groups and reactive silicon groups are not limited to these.
方法III:分子中にヒドロキシ基、エポキシ基、イソシアネート基等の官能基を有する有機重合体に、これらの官能基に対して反応性を示す官能基および反応性ケイ素基を有する化合物を反応させる方法。
Method III: A method of reacting an organic polymer having a functional group such as a hydroxyl group, an epoxy group, an isocyanate group, etc. in the molecule with a compound having a functional group showing reactivity to these functional groups and a reactive silicon group. .
方法IIIにおいて採用し得る、ヒドロキシ基を有する有機重合体と、イソシアネート基および反応性ケイ素基を有する化合物とを反応させる方法としては、特に限定されないが、例えば、特開平3-47825号公報に示される方法等が挙げられる。
The method of reacting an organic polymer having a hydroxy group with a compound having an isocyanate group and a reactive silicon group, which can be employed in Method III, is not particularly limited. and the like.
方法IIIにおいて使用し得る、イソシアネート基および反応性ケイ素基を有する化合物としては、例えば、3-イソシアナト-n-プロピルトリメトキシシラン、3-イソシアナト-n-プロピルメチルジメトキシシラン、3-イソシアナト-n-プロピルトリエトキシシラン、3-イソシアナト-n-プロピルメチルジエトキシシラン、イソシアネトメチルトリメトキシシラン、イソシアナトメチルトリエトキシシラン、イソシアナトメチルジメトキシメチルシラン、イソシアナトメチルジエトキシメチルシラン等があげられる。イソシアネート基および反応性ケイ素基を有する化合物はこれらに限定されない。
Compounds having isocyanate groups and reactive silicon groups that can be used in Method III include, for example, 3-isocyanato-n-propyltrimethoxysilane, 3-isocyanato-n-propylmethyldimethoxysilane, 3-isocyanato-n- Examples include propyltriethoxysilane, 3-isocyanato-n-propylmethyldiethoxysilane, isocyanatomethyltrimethoxysilane, isocyanatomethyltriethoxysilane, isocyanatomethyldimethoxymethylsilane, isocyanatomethyldiethoxymethylsilane and the like. Compounds having isocyanate groups and reactive silicon groups are not limited to these.
トリメトキシシラン等の1つのケイ素原子に3個の加水分解性基が結合しているシラン化合物は不均化反応が進行する場合がある。不均化反応が進むと、ジメトキシシランのような不安定な化合物が生じ、取り扱いが困難となることがある。しかし、3-メルカプト-n-プロピルトリメトキシシランや3-イソシアナト-n-プロピルトリメトキシシランでは、このような不均化反応は進行しない。このため、ケイ素含有基としてトリメトキシシリル基等の3個の加水分解性基が1つのケイ素原子に結合している基を用いる場合には、方法IIまたは方法IIIの方法を用いることが好ましい。
Silane compounds such as trimethoxysilane, in which three hydrolyzable groups are bonded to one silicon atom, may undergo a disproportionation reaction. As the disproportionation reaction progresses, unstable compounds such as dimethoxysilane are produced, which can be difficult to handle. However, such a disproportionation reaction does not proceed with 3-mercapto-n-propyltrimethoxysilane and 3-isocyanato-n-propyltrimethoxysilane. Therefore, when a group in which three hydrolyzable groups are bonded to one silicon atom, such as a trimethoxysilyl group, is used as the silicon-containing group, method II or method III is preferably used.
一方、下記式(2a)で表されるシラン化合物は不均化反応が進まない。
H-(SiR2a 2O)mSiR2a 2-R3a-SiX3 (2a)
ここで、式(2a)において、Xは式(1a)と同じである。2m+2個のR2aはそれぞれ独立に式(1a)のR1aと同じである。R3aは、炭素原子数1以上20以下の置換または非置換の2価の炭化水素基を示す。mは0以上19以下の整数を示す。 On the other hand, the disproportionation reaction does not proceed with the silane compound represented by the following formula (2a).
H—(SiR 2a 2 O) m SiR 2a 2 —R 3a —SiX 3 (2a)
Here, in formula (2a), X is the same as in formula (1a). 2m+2 R 2a are independently the same as R 1a in Formula (1a). R 3a represents a substituted or unsubstituted divalent hydrocarbon group having 1 to 20 carbon atoms. m represents an integer of 0 or more and 19 or less.
H-(SiR2a 2O)mSiR2a 2-R3a-SiX3 (2a)
ここで、式(2a)において、Xは式(1a)と同じである。2m+2個のR2aはそれぞれ独立に式(1a)のR1aと同じである。R3aは、炭素原子数1以上20以下の置換または非置換の2価の炭化水素基を示す。mは0以上19以下の整数を示す。 On the other hand, the disproportionation reaction does not proceed with the silane compound represented by the following formula (2a).
H—(SiR 2a 2 O) m SiR 2a 2 —R 3a —SiX 3 (2a)
Here, in formula (2a), X is the same as in formula (1a). 2m+2 R 2a are independently the same as R 1a in Formula (1a). R 3a represents a substituted or unsubstituted divalent hydrocarbon group having 1 to 20 carbon atoms. m represents an integer of 0 or more and 19 or less.
このため、方法Iで、3個の加水分解性基が1つのケイ素原子に結合している基を導入する場合には、式(2a)で表されるシラン化合物を用いることが好ましい。入手性およびコストの点から、2m+2個のR2aとしては、それぞれ独立に、炭素原子数1以上20以下の炭化水素基が好ましく、炭素原子数1以上8以下の炭化水素基がより好ましく、炭素原子数1以上4以下の炭化水素基がさらに好ましい。R3aとしては、炭素原子数1以上12以下の2価の炭化水素基が好ましく、炭素原子数2以上8以下の2価の炭化水素基がより好ましく、炭素原子数2の2価の炭化水素基がさらに好ましい。mは1が最も好ましい。
Therefore, when introducing a group in which three hydrolyzable groups are bonded to one silicon atom in method I, it is preferable to use a silane compound represented by formula (2a). From the viewpoint of availability and cost, 2m+2 R 2a are each independently preferably a hydrocarbon group having 1 to 20 carbon atoms, more preferably a hydrocarbon group having 1 to 8 carbon atoms. A hydrocarbon group having 1 or more and 4 or less atoms is more preferable. R 3a is preferably a divalent hydrocarbon group having 1 to 12 carbon atoms, more preferably a divalent hydrocarbon group having 2 to 8 carbon atoms, and a divalent hydrocarbon group having 2 carbon atoms. groups are more preferred. m is most preferably 1.
式(2a)で示されるシラン化合物としては、例えば、1-[2-(トリメトキシシリル)エチル]-1,1,3,3-テトラメチルジシロキサン、1-[2-(トリメトキシシリル)プロピル]-1,1,3,3-テトラメチルジシロキサン、1-[2-(トリメトキシシリル)ヘキシル]-1,1,3,3-テトラメチルジシロキサン等が挙げられる。
Silane compounds represented by formula (2a) include, for example, 1-[2-(trimethoxysilyl)ethyl]-1,1,3,3-tetramethyldisiloxane, 1-[2-(trimethoxysilyl) propyl]-1,1,3,3-tetramethyldisiloxane, 1-[2-(trimethoxysilyl)hexyl]-1,1,3,3-tetramethyldisiloxane and the like.
上記の方法Iまたは方法IIIにおいて、末端にヒドロキシ基を有する有機重合体と、イソシアネート基および反応性ケイ素基を有する化合物とを反応させる方法は、比較的短い反応時間で高い転化率が得られるために好ましい。さらに、方法Iで得られた反応性ケイ素基を有する有機重合体は、方法IIIで得られる反応性ケイ素基を有する有機重合体よりも低粘度であり、作業性のよい発泡体用樹脂組成物が得られること、また、方法IIで得られる反応性ケイ素基を有する有機重合体は、メルカプトシランに基づく臭気が強いことから、方法Iが特に好ましい。
In the above method I or method III, the method of reacting an organic polymer having a terminal hydroxy group with a compound having an isocyanate group and a reactive silicon group provides a high conversion rate in a relatively short reaction time. preferred. Furthermore, the organic polymer having a reactive silicon group obtained by Method I has a lower viscosity than the organic polymer having a reactive silicon group obtained by Method III, and the resin composition for foams has good workability. is obtained, and the organic polymer having a reactive silicon group obtained by Method II has a strong odor derived from mercaptosilane. Therefore, Method I is particularly preferred.
反応性ケイ素基を有するポリオキシアルキレン系重合体としては、例えば、特公昭45-36319号公報、特公昭46-12154号公報、特開昭50-156599号公報、特開昭54-6096号公報、特開昭55-13767号公報、特開昭55-13468号公報、特開昭57-164123号公報、特公平3-2450号公報、米国特許3632557号、米国特許4345053号、米国特許4366307号、米国特許4960844号等の各公報に提案されている重合体が挙げられる。また、特開昭61-197631号公報、特開昭61-215622号公報、特開昭61-215623号公報、特開昭61-218632号公報、特開平3-72527号公報、特開平3-47825号公報、特開平8-231707号公報の各公報に提案されている数平均分子量6,000以上、分子量分布(Mw/Mn)が1.6以下や1.3以下の高分子量で分子量分布が狭い反応性ケイ素基を有するポリオキシアルキレン系重合体等も好ましい。このような反応性ケイ素基を有するポリオキシアルキレン系重合体は単独で使用してもよく、2種以上を併用してもよい。
Examples of polyoxyalkylene polymers having a reactive silicon group include, for example, JP-B-45-36319, JP-B-46-12154, JP-A-50-156599, and JP-A-54-6096. , JP-A-55-13767, JP-A-55-13468, JP-A-57-164123, JP-B-3-2450, US Pat. No. 3632557, US Pat. No. 4345053, US Pat. and US Pat. No. 4,960,844. In addition, JP-A-61-197631, JP-A-61-215622, JP-A-61-215623, JP-A-61-218632, JP-A-3-72527, JP-A-3- 47825 and JP-A-8-231707, the number average molecular weight is 6,000 or more and the molecular weight distribution (Mw/Mn) is 1.6 or less or 1.3 or less. A polyoxyalkylene polymer having a narrow reactive silicon group is also preferred. Polyoxyalkylene polymers having such reactive silicon groups may be used alone, or two or more of them may be used in combination.
ポリオキシアルキレン系重合体の1つの末端部位に平均して1.0個より多い反応性ケイ素基を導入する合成方法について、以下に説明する。
A synthetic method for introducing an average of more than 1.0 reactive silicon groups to one terminal site of a polyoxyalkylene polymer will be described below.
1つの末端部位に平均して1.0個より多い反応性ケイ素基を有しているポリオキシアルキレン系重合体(A)は、重合によって得られた水酸基末端重合体の1つの末端に2個以上の炭素-炭素不飽和結合を導入した後、炭素-炭素不飽和結合と反応する反応性ケイ素基含有化合物を反応させて得ることが好ましい。
The polyoxyalkylene polymer (A) having an average of more than 1.0 reactive silicon groups at one terminal site has two at one terminal of the hydroxyl group-terminated polymer obtained by polymerization. After introducing the above carbon-carbon unsaturated bonds, it is preferable to react with a reactive silicon group-containing compound that reacts with the carbon-carbon unsaturated bonds.
(重合)
ポリオキシアルキレン系重合体(A)は、亜鉛ヘキサシアノコバルテートグライム錯体等の複合金属シアン化物錯体触媒を用いた、水酸基を有する開始剤にエポキシ化合物を重合させる方法が好ましい。 (polymerization)
The polyoxyalkylene polymer (A) is preferably produced by polymerizing an epoxy compound with an initiator having a hydroxyl group using a double metal cyanide complex catalyst such as a zinc hexacyanocobaltate glyme complex.
ポリオキシアルキレン系重合体(A)は、亜鉛ヘキサシアノコバルテートグライム錯体等の複合金属シアン化物錯体触媒を用いた、水酸基を有する開始剤にエポキシ化合物を重合させる方法が好ましい。 (polymerization)
The polyoxyalkylene polymer (A) is preferably produced by polymerizing an epoxy compound with an initiator having a hydroxyl group using a double metal cyanide complex catalyst such as a zinc hexacyanocobaltate glyme complex.
水酸基を有する開始剤としては、エチレングリコール、プロピレングリコール、グリセリン、ペンタエリスリトール、低分子量のポリオキシプロピレングリコール、ポリオキシプロピレントリオール、アリルアルコール、ポリプロピレンモノアリルエーテル、ポリプロピレンモノアルキルエーテル等の水酸基を1個以上有するものが挙げられる。
Examples of initiators having a hydroxyl group include ethylene glycol, propylene glycol, glycerin, pentaerythritol, low-molecular-weight polyoxypropylene glycol, polyoxypropylene triol, allyl alcohol, polypropylene monoallyl ether, polypropylene monoalkyl ether, and the like. Those having the above are mentioned.
エポキシ化合物としては、エチレンオキサイド、プロピレンオキサイド、等のアルキレンオキサイド類、メチルグリシジルエーテル、アリルグリシジルエーテル、等のグリシジルエーテル類、等が挙げられる。このなかでもプロピレンオキサイドが好ましい。
Epoxy compounds include alkylene oxides such as ethylene oxide and propylene oxide, and glycidyl ethers such as methyl glycidyl ether and allyl glycidyl ether. Among these, propylene oxide is preferred.
(炭素-炭素不飽和結合の導入)
1つの末端に2個以上の炭素-炭素不飽和結合を導入する方法としては、水酸基末端重合体に、アルカリ金属塩を作用させた後、先ず炭素-炭素不飽和結合を有するエポキシ化合物を反応させ、次いで炭素-炭素不飽和結合を有するハロゲン化炭化水素化合物を反応させる方法を用いるのが好ましい。この方法を用いることで、重合体主鎖の分子量や分子量分布を重合条件によって制御しつつ、さらに反応性基の導入を効率的かつ安定的に行うことが可能となる。 (Introduction of carbon-carbon unsaturated bond)
As a method for introducing two or more carbon-carbon unsaturated bonds to one terminal, an alkali metal salt is allowed to act on a hydroxyl-terminated polymer, and then an epoxy compound having a carbon-carbon unsaturated bond is first reacted. and then reacting a halogenated hydrocarbon compound having a carbon-carbon unsaturated bond. By using this method, it is possible to efficiently and stably introduce reactive groups while controlling the molecular weight and molecular weight distribution of the polymer main chain by the polymerization conditions.
1つの末端に2個以上の炭素-炭素不飽和結合を導入する方法としては、水酸基末端重合体に、アルカリ金属塩を作用させた後、先ず炭素-炭素不飽和結合を有するエポキシ化合物を反応させ、次いで炭素-炭素不飽和結合を有するハロゲン化炭化水素化合物を反応させる方法を用いるのが好ましい。この方法を用いることで、重合体主鎖の分子量や分子量分布を重合条件によって制御しつつ、さらに反応性基の導入を効率的かつ安定的に行うことが可能となる。 (Introduction of carbon-carbon unsaturated bond)
As a method for introducing two or more carbon-carbon unsaturated bonds to one terminal, an alkali metal salt is allowed to act on a hydroxyl-terminated polymer, and then an epoxy compound having a carbon-carbon unsaturated bond is first reacted. and then reacting a halogenated hydrocarbon compound having a carbon-carbon unsaturated bond. By using this method, it is possible to efficiently and stably introduce reactive groups while controlling the molecular weight and molecular weight distribution of the polymer main chain by the polymerization conditions.
本発明で用いるアルカリ金属塩としては、水酸化ナトリウム、ナトリウムメトキシド、ナトリウムエトキシド、水酸化カリウム、カリウムメトキシド、カリウムエトキシドが好ましく、ナトリウムメトキシド、カリウムメトキシドがより好ましい。入手性の点でナトリウムメトキシドが特に好ましい。
As the alkali metal salt used in the present invention, sodium hydroxide, sodium methoxide, sodium ethoxide, potassium hydroxide, potassium methoxide and potassium ethoxide are preferred, and sodium methoxide and potassium methoxide are more preferred. Sodium methoxide is particularly preferred because of its availability.
アルカリ金属塩を作用させる際の温度としては、50℃以上150℃以下が好ましく、110℃以上140℃以下がより好ましい。アルカリ金属塩を作用させる際の時間としては、10分以上5時間以下が好ましく、30分以上3時間以下がより好ましい。
The temperature at which the alkali metal salt is allowed to act is preferably 50°C or higher and 150°C or lower, more preferably 110°C or higher and 140°C or lower. The time for which the alkali metal salt is allowed to act is preferably 10 minutes or more and 5 hours or less, more preferably 30 minutes or more and 3 hours or less.
本発明で用いる炭素-炭素不飽和結合を有するエポキシ化合物として、特に一般式(3):
As the epoxy compound having a carbon-carbon unsaturated bond used in the present invention, especially general formula (3):
本発明で用いる炭素-炭素不飽和結合を有するエポキシ化合物の添加量は、重合体に対する炭素-炭素不飽和結合の導入量や反応性を考慮して任意の量を使用できる。特に、水酸基末端重合体が有する水酸基に対するモル比は、0.2以上であることが好ましく、0.5以上がより好ましい。また、5.0以下であることが好ましく、2.0以下であることがより好ましい。
The amount of the epoxy compound having a carbon-carbon unsaturated bond used in the present invention can be any amount in consideration of the introduction amount and reactivity of the carbon-carbon unsaturated bond to the polymer. In particular, the molar ratio of the hydroxyl group-terminated polymer to the hydroxyl group is preferably 0.2 or more, more preferably 0.5 or more. Also, it is preferably 5.0 or less, more preferably 2.0 or less.
本発明において、水酸基を含有する重合体に対し炭素-炭素不飽和結合を有するエポキシ化合物を開環付加反応させる際の反応温度は、反応温度は60℃以上、150℃以下であることが好ましく、110℃以上、140℃以下であることがより好ましい。
In the present invention, the reaction temperature for the ring-opening addition reaction of an epoxy compound having a carbon-carbon unsaturated bond with a polymer containing a hydroxyl group is preferably 60° C. or higher and 150° C. or lower. It is more preferably 110° C. or higher and 140° C. or lower.
本発明で用いる炭素-炭素不飽和結合を有するハロゲン化炭化水素化合物としては、塩化ビニル、塩化アリル、塩化メタリル、臭化ビニル、臭化アリル、臭化メタリル、ヨウ化ビニル、ヨウ化アリル、ヨウ化メタリルなどが挙げられ、取り扱いの容易さから塩化アリル、塩化メタリルを用いることがより好ましい。
The halogenated hydrocarbon compound having a carbon-carbon unsaturated bond used in the present invention includes vinyl chloride, allyl chloride, methallyl chloride, vinyl bromide, allyl bromide, methallyl bromide, vinyl iodide, allyl iodide, iodine, and methallyl chloride, and it is more preferable to use allyl chloride and methallyl chloride from the viewpoint of ease of handling.
上記の炭素-炭素不飽和結合を有するハロゲン化炭化水素化合物の添加量は、特に制限はないが、水酸基末端重合体が有する水酸基に対するモル比は、0.7以上が好ましく、1.0以上がより好ましい。また、5.0以下が好ましく、2.0以下がより好ましい。
The amount of the halogenated hydrocarbon compound having a carbon-carbon unsaturated bond is not particularly limited, but the molar ratio to the hydroxyl group of the hydroxyl-terminated polymer is preferably 0.7 or more, and 1.0 or more. more preferred. Moreover, 5.0 or less is preferable and 2.0 or less is more preferable.
炭素-炭素不飽和結合を有するハロゲン化炭化水素化合物を反応させる際の温度としては、50℃以上150℃以下が好ましく、110℃以上140℃以下がより好ましい。反応時間としては、10分以上5時間以下が好ましく、30分以上3時間以下がより好ましい。
The temperature at which the halogenated hydrocarbon compound having a carbon-carbon unsaturated bond is reacted is preferably 50°C or higher and 150°C or lower, more preferably 110°C or higher and 140°C or lower. The reaction time is preferably 10 minutes or more and 5 hours or less, more preferably 30 minutes or more and 3 hours or less.
(反応性ケイ素基の導入)
反応性ケイ素基の導入方法は、上記で説明した3種類の方法が利用でき、特に限定されず、他の公知の方法を利用することができる。 (Introduction of reactive silicon group)
The method for introducing the reactive silicon group is not particularly limited, and the three methods described above can be used, and other known methods can be used.
反応性ケイ素基の導入方法は、上記で説明した3種類の方法が利用でき、特に限定されず、他の公知の方法を利用することができる。 (Introduction of reactive silicon group)
The method for introducing the reactive silicon group is not particularly limited, and the three methods described above can be used, and other known methods can be used.
(2-2.エポキシ樹脂硬化剤(B))
本多液型硬化性組成物における、エポキシ樹脂硬化剤(B)は、第三級アミンを有するエポキシ樹脂硬化剤であることが好ましい。当該構成によれば、硬化物の引き裂き強さが向上するという利点を有する。 (2-2. Epoxy resin curing agent (B))
The epoxy resin curing agent (B) in the present multi-component curable composition is preferably an epoxy resin curing agent having a tertiary amine. This configuration has the advantage of improving the tear strength of the cured product.
本多液型硬化性組成物における、エポキシ樹脂硬化剤(B)は、第三級アミンを有するエポキシ樹脂硬化剤であることが好ましい。当該構成によれば、硬化物の引き裂き強さが向上するという利点を有する。 (2-2. Epoxy resin curing agent (B))
The epoxy resin curing agent (B) in the present multi-component curable composition is preferably an epoxy resin curing agent having a tertiary amine. This configuration has the advantage of improving the tear strength of the cured product.
第三級アミンを有するエポキシ樹脂硬化剤(B)としては、第三級アミンを有する化合物であれば使用できる。具体的には、例えば、N,N,N’,N’-テトラメチル-1,3-ジミノプロパン、N,N,N’,N’-テトラメチル-1,6-ジミノヘキサン、N,N-ジメチルベンジルアミン、N-メチル-N-(ジメチルアミノプロピル)アミノエタノール、2,4,6-トリス(ジメチルアミノメチル)フェノール、トリプロピルアミン、DBU、DBN、およびこれら第三級アミン類の塩類を例示することができるが、これらに限定されるものではない。これらは、1種のみを使用してもよく、2種以上を併用してもよく、(B)成分以外の公知のエポキシ樹脂硬化剤をさらに添加してもよい。
As the epoxy resin curing agent (B) having a tertiary amine, any compound having a tertiary amine can be used. Specifically, for example, N,N,N',N'-tetramethyl-1,3-diminopropane, N,N,N',N'-tetramethyl-1,6-diminohexane, N,N-dimethyl Examples include benzylamine, N-methyl-N-(dimethylaminopropyl)aminoethanol, 2,4,6-tris(dimethylaminomethyl)phenol, tripropylamine, DBU, DBN, and salts of these tertiary amines can be, but are not limited to. These may be used alone or in combination of two or more, and a known epoxy resin curing agent other than component (B) may be further added.
第三級アミンを有するエポキシ樹脂硬化剤(B)は、芳香族アミンであることが好ましく、アミノ基を3つ以上有することがさらに好ましい。具体的には、2,4,6-トリス(ジメチルアミノメチル)フェノールを例示することができる。
The epoxy resin curing agent (B) having a tertiary amine is preferably an aromatic amine, and more preferably has three or more amino groups. Specifically, 2,4,6-tris(dimethylaminomethyl)phenol can be exemplified.
エポキシ樹脂硬化剤(B)の含有量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.1~10重量部であることが好ましく、0.5~9.0重量部であることがより好ましく、1.0~8.0重量部であることがさらに好ましく、2.0~7.0重量部であることが特に好ましい。エポキシ樹脂硬化剤(B)の含有量が上記の範囲内であれば、硬化物の引き裂き強さ向上と低コスト化とが両立し易いという利点を有する。
The content of the epoxy resin curing agent (B) is preferably 0.1 to 10 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is more preferably 0.5 to 9.0 parts by weight, even more preferably 1.0 to 8.0 parts by weight, and particularly preferably 2.0 to 7.0 parts by weight. If the content of the epoxy resin curing agent (B) is within the above range, there is an advantage that both improvement in tear strength of the cured product and cost reduction can be easily achieved.
(2-3.アミノシラン(C))
本多液型硬化性組成物における、アミノシラン(C)としては、例えば、γ-アミノプロピルトリエトキシシラン、N-β-(アミノエチル)-γ-アミノプロピルトリメトキシシラン、N-β-(アミノエチル)-γ-アミノプロピルメチルジメトキシシラン等が挙げられる。これらは、1種のみを使用してもよく、2種以上を併用してもよい。市販品として、例えば、Silquest A-1120(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製)、Silquest A-1110(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製)、KBM-602(信越化学工業株式会社製)、KBM-603(信越化学工業株式会社製)、KBM-903(信越化学工業株式会社製)等を用いてもよい。 (2-3. Aminosilane (C))
Examples of the aminosilane (C) in the multi-component curable composition include γ-aminopropyltriethoxysilane, N-β-(aminoethyl)-γ-aminopropyltrimethoxysilane, N-β-(amino ethyl)-γ-aminopropylmethyldimethoxysilane and the like. These may use only 1 type and may use 2 or more types together. Commercially available products include, for example, Silquest A-1120 (manufactured by Momentive Performance Materials Japan LLC), Silquest A-1110 (manufactured by Momentive Performance Materials Japan LLC), KBM-602 (Shin-Etsu Chemical Co., Ltd. (manufactured by Shin-Etsu Chemical Co., Ltd.), KBM-603 (manufactured by Shin-Etsu Chemical Co., Ltd.), KBM-903 (manufactured by Shin-Etsu Chemical Co., Ltd.), and the like may be used.
本多液型硬化性組成物における、アミノシラン(C)としては、例えば、γ-アミノプロピルトリエトキシシラン、N-β-(アミノエチル)-γ-アミノプロピルトリメトキシシラン、N-β-(アミノエチル)-γ-アミノプロピルメチルジメトキシシラン等が挙げられる。これらは、1種のみを使用してもよく、2種以上を併用してもよい。市販品として、例えば、Silquest A-1120(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製)、Silquest A-1110(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製)、KBM-602(信越化学工業株式会社製)、KBM-603(信越化学工業株式会社製)、KBM-903(信越化学工業株式会社製)等を用いてもよい。 (2-3. Aminosilane (C))
Examples of the aminosilane (C) in the multi-component curable composition include γ-aminopropyltriethoxysilane, N-β-(aminoethyl)-γ-aminopropyltrimethoxysilane, N-β-(amino ethyl)-γ-aminopropylmethyldimethoxysilane and the like. These may use only 1 type and may use 2 or more types together. Commercially available products include, for example, Silquest A-1120 (manufactured by Momentive Performance Materials Japan LLC), Silquest A-1110 (manufactured by Momentive Performance Materials Japan LLC), KBM-602 (Shin-Etsu Chemical Co., Ltd. (manufactured by Shin-Etsu Chemical Co., Ltd.), KBM-603 (manufactured by Shin-Etsu Chemical Co., Ltd.), KBM-903 (manufactured by Shin-Etsu Chemical Co., Ltd.), and the like may be used.
アミノシラン(C)の含有量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.1~5.0重量部であることが好ましく、0.2~4.5重量部であることがより好ましく、0.3~4.0重量部であることがさらに好ましく、0.4~3.8重量部であることが特に好ましい。アミノシラン(C)の含有量が上記の範囲内であれば、硬化物の引き裂き強さ向上と低コスト化とが両立し易いという利点を有する。
The content of the aminosilane (C) is preferably 0.1 to 5.0 parts by weight with respect to the total 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is more preferably 2 to 4.5 parts by weight, still more preferably 0.3 to 4.0 parts by weight, and particularly preferably 0.4 to 3.8 parts by weight. If the content of the aminosilane (C) is within the above range, there is an advantage that both improvement in tear strength of the cured product and cost reduction can easily be achieved.
(2-4.メルカプタン化合物(D))
本多液型硬化性組成物における、メルカプタン化合物(D)としては、例えば、n-ドデシルメルカプタン、tert-ドデシルメルカプタン、ラウリルメルカプタン、トリメチロールプロパントリス(3-メルカプトプロピオネート)、ペンタエリスリトールテトラキス(3-メルカプトプロピオネート)、3-メルカプトプロピルトリメトキシシラン、3-メルカプトプロピルメチルジメトキシシラン、3-メルカプトプロピルクロロメチルジメトキシシラン、3-メルカプトプロピルメトキシメチルジメトキシシラン、(メルカプトメチル)ジメトキシメチルシラン、(メルカプトメチル)トリメトキシシラン等が挙げられる。これらは、1種のみを使用してもよく、2種以上を併用してもよい。市販品として、例えば、Z6062(ダウ・ケミカル日本株式会社製)、チオカルコール20(花王株式会社製)、KBM-802(信越化学工業株式会社製)、KBM-803(信越化学工業株式会社製)等を用いてもよい。 (2-4. Mercaptan compound (D))
The mercaptan compound (D) in the present multi-component curable composition includes, for example, n-dodecylmercaptan, tert-dodecylmercaptan, laurylmercaptan, trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakis ( 3-mercaptopropionate), 3-mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropylchloromethyldimethoxysilane, 3-mercaptopropylmethoxymethyldimethoxysilane, (mercaptomethyl)dimethoxymethylsilane, (mercaptomethyl)trimethoxysilane and the like. These may use only 1 type and may use 2 or more types together. Examples of commercially available products include Z6062 (manufactured by Dow Chemical Japan Co., Ltd.), Thiocalcol 20 (manufactured by Kao Corporation), KBM-802 (manufactured by Shin-Etsu Chemical Co., Ltd.), KBM-803 (manufactured by Shin-Etsu Chemical Co., Ltd.), etc. may be used.
本多液型硬化性組成物における、メルカプタン化合物(D)としては、例えば、n-ドデシルメルカプタン、tert-ドデシルメルカプタン、ラウリルメルカプタン、トリメチロールプロパントリス(3-メルカプトプロピオネート)、ペンタエリスリトールテトラキス(3-メルカプトプロピオネート)、3-メルカプトプロピルトリメトキシシラン、3-メルカプトプロピルメチルジメトキシシラン、3-メルカプトプロピルクロロメチルジメトキシシラン、3-メルカプトプロピルメトキシメチルジメトキシシラン、(メルカプトメチル)ジメトキシメチルシラン、(メルカプトメチル)トリメトキシシラン等が挙げられる。これらは、1種のみを使用してもよく、2種以上を併用してもよい。市販品として、例えば、Z6062(ダウ・ケミカル日本株式会社製)、チオカルコール20(花王株式会社製)、KBM-802(信越化学工業株式会社製)、KBM-803(信越化学工業株式会社製)等を用いてもよい。 (2-4. Mercaptan compound (D))
The mercaptan compound (D) in the present multi-component curable composition includes, for example, n-dodecylmercaptan, tert-dodecylmercaptan, laurylmercaptan, trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakis ( 3-mercaptopropionate), 3-mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropylchloromethyldimethoxysilane, 3-mercaptopropylmethoxymethyldimethoxysilane, (mercaptomethyl)dimethoxymethylsilane, (mercaptomethyl)trimethoxysilane and the like. These may use only 1 type and may use 2 or more types together. Examples of commercially available products include Z6062 (manufactured by Dow Chemical Japan Co., Ltd.), Thiocalcol 20 (manufactured by Kao Corporation), KBM-802 (manufactured by Shin-Etsu Chemical Co., Ltd.), KBM-803 (manufactured by Shin-Etsu Chemical Co., Ltd.), etc. may be used.
メルカプタン化合物(D)の含有量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.2~5.0重量部であることが好ましく、0.22~4.5重量部であることがより好ましく、0.24~4.0重量部であることがより好ましく、0.26~3.8重量部であることが特に好ましい。メルカプタン化合物(D)の含有量が上記の範囲内であれば、硬化物の引き裂き強さ向上と低コスト化とが両立し易いという利点を有する。
The content of the mercaptan compound (D) is preferably 0.2 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is more preferably 0.22 to 4.5 parts by weight, more preferably 0.24 to 4.0 parts by weight, and particularly preferably 0.26 to 3.8 parts by weight. If the content of the mercaptan compound (D) is within the above range, there is an advantage that both improvement in tear strength of the cured product and cost reduction are readily compatible.
(2-5.エポキシ樹脂(E))
本多液型硬化性組成物における、前記エポキシ樹脂(E)は、エポキシ基を1分子中に少なくとも2個以上有するエポキシ樹脂であることが好ましい。当該構成によれば、硬化物の引き裂き強さが向上するという利点を有する。 (2-5. Epoxy resin (E))
The epoxy resin (E) in the present multi-component curable composition is preferably an epoxy resin having at least two epoxy groups per molecule. This configuration has the advantage of improving the tear strength of the cured product.
本多液型硬化性組成物における、前記エポキシ樹脂(E)は、エポキシ基を1分子中に少なくとも2個以上有するエポキシ樹脂であることが好ましい。当該構成によれば、硬化物の引き裂き強さが向上するという利点を有する。 (2-5. Epoxy resin (E))
The epoxy resin (E) in the present multi-component curable composition is preferably an epoxy resin having at least two epoxy groups per molecule. This configuration has the advantage of improving the tear strength of the cured product.
エポキシ樹脂(E)としては、エピクロルヒドリン-ビスフェノールA型エポキシ樹脂、エピクロルヒドリン-ビスフェノールF型エポキシ樹脂、テトラブロモビスフェノールAのグリシジルエーテル等の難燃型エポキシ樹脂、ノボラック型エポキシ樹脂、水添ビスフェノールA型エポキシ樹脂、ビスフェノールAプロピレンオキシド付加物のグリシジルエーテル型エポキシ樹脂、p-オキシ安息香酸グリシジルエーテルエステル型エポキシ樹脂、m-アミノフェノール系エポキシ樹脂、ジアミノジフェニルメタン系エポキシ樹脂、ウレタン変性エポキシ樹脂、各種脂環式エポキシ樹脂、N,N-ジグリシジルアニリン、N,N-ジグリシジル-o-トルイジン、トリグリシジルイソシアヌレート、ポリアルキレングリコールジグリシジルエーテル、グリセリン等の多価アルコールのグリシジルエーテル、ヒダントイン型エポキシ樹脂、石油樹脂等の不飽和重合体のエポキシ化物等が例示されるが、これらに限定されるものではなく、一般に使用されているエポキシ樹脂が使用され得る。エポキシ基を1分子中に少なくとも2個有するエポキシ樹脂が、硬化に際し反応性が高く、また硬化物が3次元的網目をつくりやすい等の点から好ましい。さらに好ましいものとしては、ビスフェノールA型エポキシ樹脂類、ノボラック型エポキシ樹脂等が挙げられる。
Examples of the epoxy resin (E) include epichlorohydrin-bisphenol A type epoxy resin, epichlorohydrin-bisphenol F type epoxy resin, flame retardant epoxy resin such as glycidyl ether of tetrabromobisphenol A, novolac type epoxy resin, and hydrogenated bisphenol A type epoxy resin. Resins, glycidyl ether type epoxy resins of bisphenol A propylene oxide adducts, p-oxybenzoic acid glycidyl ether ester type epoxy resins, m-aminophenol type epoxy resins, diaminodiphenylmethane type epoxy resins, urethane modified epoxy resins, various alicyclic Epoxy resin, N,N-diglycidylaniline, N,N-diglycidyl-o-toluidine, triglycidyl isocyanurate, polyalkylene glycol diglycidyl ether, glycidyl ether of polyhydric alcohol such as glycerin, hydantoin type epoxy resin, petroleum resin Examples include epoxidized unsaturated polymers such as, but not limited to, commonly used epoxy resins can be used. Epoxy resins having at least two epoxy groups per molecule are preferred because they have high reactivity during curing and the cured product can easily form a three-dimensional network. Bisphenol A type epoxy resins, novolac type epoxy resins and the like are more preferable.
エポキシ樹脂(E)の含有量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との重量比(A):(E)が、90:10~50:50であることが好ましい。(A)の割合が90%より大きくなると強度が低下し、50%より小さくなると柔軟性が低下し、硬くなりすぎる。さらに、80:20~60:40が柔軟性と強度のバランスの点でより好ましい。
As for the content of the epoxy resin (E), the weight ratio (A):(E) of the polyoxyalkylene polymer (A) and the epoxy resin (E) is preferably 90:10 to 50:50. . If the proportion of (A) is more than 90%, the strength will be reduced, and if it is less than 50%, the flexibility will be reduced and the material will become too hard. Furthermore, 80:20 to 60:40 is more preferable in terms of balance between flexibility and strength.
(2-6.水酸基含有ポリオキシアルキレン系重合体(F))
本多液型硬化性組成物は、前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む。本多液型硬化性組成物において、水酸基含有ポリオキシアルキレン系重合体(F)は、可塑剤として機能する。本多液型硬化性組成物が水酸基含有ポリオキシアルキレン系重合体(F)を含むことにより、当該組成物の粘度低下、コスト低下や、当該組成物からの硬化物の硬度、弾性率等の特性を調整するとともに、当該硬化物にアルキッド系塗料を塗装した場合の塗膜を乾燥させやすくすることができる。 (2-6. Hydroxyl Group-Containing Polyoxyalkylene Polymer (F))
This multi-component curable composition contains a hydroxyl group-containing polyoxyalkylene polymer (F) in the A agent and/or the B agent. In this multi-component curable composition, the hydroxyl group-containing polyoxyalkylene polymer (F) functions as a plasticizer. By including the hydroxyl group-containing polyoxyalkylene polymer (F) in the multicomponent curable composition, the viscosity of the composition is reduced, the cost is reduced, and the hardness and elastic modulus of the cured product from the composition are improved. In addition to adjusting the properties, it is possible to facilitate the drying of the coating film when the alkyd-based coating material is applied to the cured product.
本多液型硬化性組成物は、前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む。本多液型硬化性組成物において、水酸基含有ポリオキシアルキレン系重合体(F)は、可塑剤として機能する。本多液型硬化性組成物が水酸基含有ポリオキシアルキレン系重合体(F)を含むことにより、当該組成物の粘度低下、コスト低下や、当該組成物からの硬化物の硬度、弾性率等の特性を調整するとともに、当該硬化物にアルキッド系塗料を塗装した場合の塗膜を乾燥させやすくすることができる。 (2-6. Hydroxyl Group-Containing Polyoxyalkylene Polymer (F))
This multi-component curable composition contains a hydroxyl group-containing polyoxyalkylene polymer (F) in the A agent and/or the B agent. In this multi-component curable composition, the hydroxyl group-containing polyoxyalkylene polymer (F) functions as a plasticizer. By including the hydroxyl group-containing polyoxyalkylene polymer (F) in the multicomponent curable composition, the viscosity of the composition is reduced, the cost is reduced, and the hardness and elastic modulus of the cured product from the composition are improved. In addition to adjusting the properties, it is possible to facilitate the drying of the coating film when the alkyd-based coating material is applied to the cured product.
水酸基含有ポリオキシアルキレン系重合体(F)は、ポリオキシアルキレン系重合体(A)の可塑剤として用いられるため、オキシアルキレン系重合体(A)が有する反応性ケイ素基のような室温で架橋しうる基を含まないことが好ましい。
Since the hydroxyl group-containing polyoxyalkylene polymer (F) is used as a plasticizer for the polyoxyalkylene polymer (A), it can be crosslinked at room temperature like the reactive silicon groups possessed by the oxyalkylene polymer (A). It is preferred that it does not contain a group capable of
水酸基含有ポリオキシアルキレン系重合体(F)の主鎖は、主鎖を構成する単量体単位の60%以上、好ましくは80%以上が、一般式:
-Rf-O-
(式中、Rfは2価の炭化水素基であるが、その大部分が炭素数3または4のアルキレン基であるとき最も好ましい)で示される繰返し単位を有するものであるのが好ましい。Rfの具体例としては、
等が挙げられる。水酸基含有ポリオキシアルキレン系重合体(F)の分子鎖は1種だけの繰返し単位からなっていてもよいし、2種以上の繰返し単位からなっていてもよいが、Rfとしては特に、
で示されるものであるのが好ましい。
The main chain of the hydroxyl group-containing polyoxyalkylene polymer (F) has the general formula:
-R f -O-
(In the formula, R f is a divalent hydrocarbon group, most preferably an alkylene group having 3 or 4 carbon atoms). A specific example of R f is
etc. The molecular chain of the hydroxyl group-containing polyoxyalkylene polymer (F) may consist of one type of repeating unit or may consist of two or more types of repeating units.
is preferably represented by
-Rf-O-
(式中、Rfは2価の炭化水素基であるが、その大部分が炭素数3または4のアルキレン基であるとき最も好ましい)で示される繰返し単位を有するものであるのが好ましい。Rfの具体例としては、
-R f -O-
(In the formula, R f is a divalent hydrocarbon group, most preferably an alkylene group having 3 or 4 carbon atoms). A specific example of R f is
水酸基含有ポリオキシアルキレン系重合体(F)の具体例としては、特に限定されないが、例えば、ポリオキシエチレン、ポリオキシプロピレン、ポリオキシブチレン、ポリオキシテトラメチレン、ポリオキシエチレン-ポリオキシプロピレン共重合体、ポリオキシプロピレン-ポリオキシブチレン共重合体等が挙げられる。中でも、時間経過に伴って生じるブリードを抑制する観点から、ポリオキシプロピレンが好ましい。
Specific examples of the hydroxyl group-containing polyoxyalkylene polymer (F) are not particularly limited, but include, for example, polyoxyethylene, polyoxypropylene, polyoxybutylene, polyoxytetramethylene, polyoxyethylene-polyoxypropylene copolymer coalescence, polyoxypropylene-polyoxybutylene copolymer, and the like. Among them, polyoxypropylene is preferable from the viewpoint of suppressing bleeding that occurs with the lapse of time.
水酸基含有ポリオキシアルキレン系重合体(F)は、反応性ケイ素基を有するポリオキシアルキレン系重合体(A)の可塑剤として作用するものであるため、その数平均分子量は、ポリオキシアルキレン系重合体(A)より小さいことが必要である。水酸基含有ポリオキシアルキレン系重合体(F)の数平均分子量は、300以上が好ましく、800以上がより好ましく、1000以上がさらに好ましい。上限は、15000以下が好ましく、10000以下がより好ましく、8000以下がさらに好ましく、5000以下が特に好ましい。水酸基含有ポリオキシアルキレン系重合体(F)の数平均分子量が300以上であると、揮発し難いとの効果を奏する。また、水酸基含有ポリオキシアルキレン系重合体(F)の数平均分子量が15000以下であると、多液型硬化性組成物が低粘度化し易いとの効果を奏する。水酸基含有ポリオキシアルキレン系重合体(F)の数平均分子量は、末端基分析によって得られた数平均分子量に相当する分子量である。
Since the hydroxyl group-containing polyoxyalkylene polymer (F) acts as a plasticizer for the polyoxyalkylene polymer (A) having a reactive silicon group, its number average molecular weight is It must be less than coalesce (A). The number average molecular weight of the hydroxyl group-containing polyoxyalkylene polymer (F) is preferably 300 or more, more preferably 800 or more, even more preferably 1000 or more. The upper limit is preferably 15,000 or less, more preferably 10,000 or less, even more preferably 8,000 or less, and particularly preferably 5,000 or less. When the number average molecular weight of the hydroxyl group-containing polyoxyalkylene polymer (F) is 300 or more, it is effective in that it is difficult to volatilize. Moreover, when the number average molecular weight of the hydroxyl group-containing polyoxyalkylene polymer (F) is 15,000 or less, the viscosity of the multi-component curable composition can be easily lowered. The number average molecular weight of the hydroxyl group-containing polyoxyalkylene polymer (F) is a molecular weight corresponding to the number average molecular weight obtained by terminal group analysis.
水酸基含有ポリオキシアルキレン系重合体(F)の分子量分布は、小さい程粘度が低くなり、好ましい。水酸基含有ポリオキシアルキレン系重合体(F)の分子量分布は、例えば、Mw/Mnが1.6以下であり、好ましくは、1.5以下である。水酸基含有ポリオキシアルキレン系重合体(F)の分子量分布(Mw/Mn)は、GPC(ポリスチレン換算)を用いて測定する。
The smaller the molecular weight distribution of the hydroxyl group-containing polyoxyalkylene polymer (F), the lower the viscosity, which is preferable. As for the molecular weight distribution of the hydroxyl group-containing polyoxyalkylene polymer (F), for example, Mw/Mn is 1.6 or less, preferably 1.5 or less. The molecular weight distribution (Mw/Mn) of the hydroxyl group-containing polyoxyalkylene polymer (F) is measured using GPC (converted to polystyrene).
水酸基含有ポリオキシアルキレン系重合体(F)の含有量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、5重量部以上が好ましく、10重量部以上がより好ましく、20重量部以上がさらに好ましい。上限は、150重量部以下が好ましく、100重量部以下がより好ましく、50重量部以下がさらに好ましい。水酸基含有ポリオキシアルキレン系重合体(F)の含有量が5重量部以上であると、可塑剤としての効果が十分に発揮できる。また、水酸基含有ポリオキシアルキレン系重合体(F)の含有量が150重量部以下であると、硬化物の適度な機械強度が得られる。なお、水酸基含有ポリオキシアルキレン系重合体(F)は、重合体製造時に配合することも可能である。
The content of the hydroxyl group-containing polyoxyalkylene polymer (F) is preferably 5 parts by weight or more, and 10 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). 1 part or more is more preferable, and 20 parts by weight or more is even more preferable. The upper limit is preferably 150 parts by weight or less, more preferably 100 parts by weight or less, and even more preferably 50 parts by weight or less. When the content of the hydroxyl group-containing polyoxyalkylene polymer (F) is 5 parts by weight or more, the effect as a plasticizer can be sufficiently exhibited. Moreover, when the content of the hydroxyl group-containing polyoxyalkylene polymer (F) is 150 parts by weight or less, the cured product can have an appropriate mechanical strength. Incidentally, the hydroxyl group-containing polyoxyalkylene polymer (F) can also be blended during polymer production.
水酸基含有ポリオキシアルキレン系重合体(F)は、通常の苛性アルカリを用いる重合法によって製造されたものでよいが、亜鉛ヘキサシアノコバルテート等の複合金属シアン化物錯体を触媒として用いる重合法によって製造されたものも用いることができる。
The hydroxyl group-containing polyoxyalkylene polymer (F) may be produced by a polymerization method using a normal caustic alkali, but may be produced by a polymerization method using a double metal cyanide complex such as zinc hexacyanocobaltate as a catalyst. Others can also be used.
(2-7.無機フィラー)
本多液型硬化性組成物は、無機フィラーをさらに含むことが好ましい。無機フィラーは、安価な材料であるため、コストダウンを可能とする。 (2-7. Inorganic filler)
The multi-component curable composition preferably further contains an inorganic filler. Inorganic fillers are inexpensive materials, so they enable cost reduction.
本多液型硬化性組成物は、無機フィラーをさらに含むことが好ましい。無機フィラーは、安価な材料であるため、コストダウンを可能とする。 (2-7. Inorganic filler)
The multi-component curable composition preferably further contains an inorganic filler. Inorganic fillers are inexpensive materials, so they enable cost reduction.
無機フィラーとしては、特に限定されないが、例えば、炭酸カルシウム、炭酸マグネシウム、炭酸バリウム、硫酸バリウム、ケイソウ土、焼成クレー、クレー、タルク、バライト、無水石膏、酸化チタン、ベントナイト、有機ベントナイト、酸化第二鉄、アルミニウム微粉末、フリント粉末、酸化亜鉛、活性亜鉛華、マイカ、亜鉛華、鉛白、リトポン、硫化亜鉛、シラスバルーン、ガラスミクロバルーン等が挙げられる。中でも、より安価であるという観点から、炭酸カルシウムが好ましい。これら無機フィラーは、単独で使用してもよく、2種以上を組み合わせて使用してもよい。
Examples of inorganic fillers include, but are not limited to, calcium carbonate, magnesium carbonate, barium carbonate, barium sulfate, diatomaceous earth, calcined clay, clay, talc, barite, anhydride gypsum, titanium oxide, bentonite, organic bentonite, and dioxic oxide. iron, aluminum fine powder, flint powder, zinc oxide, active zinc white, mica, zinc white, white lead, lithopone, zinc sulfide, shirasu balloon, glass microballoon and the like. Among them, calcium carbonate is preferable from the viewpoint of being cheaper. These inorganic fillers may be used alone or in combination of two or more.
無機フィラーの含有量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、例えば、50~300重量部であることが好ましく、50~200重量部であることがより好ましく、50重量部~180重量部であることがより好ましく、60~160重量部であることがより好ましく、70~150重量部であることがさらに好ましい。無機フィラーの含有量が上記の範囲内であれば、多液型硬化性組成物の低粘度化と低コスト化とが両立しやすいという利点を有する。
The content of the inorganic filler is, for example, preferably 50 to 300 parts by weight, preferably 50 to 200 parts by weight, with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). is more preferably 50 to 180 parts by weight, more preferably 60 to 160 parts by weight, even more preferably 70 to 150 parts by weight. When the content of the inorganic filler is within the above range, there is an advantage that both low viscosity and low cost of the multi-component curable composition are easily achieved.
無機フィラーは、A剤に含まれてもよいし、B剤に含まれてもよいし、A剤およびB剤の両方に含まれてもよい。無機フィラーは、吸着水の観点から、好ましくは、B液(B剤)に含まれる。
The inorganic filler may be contained in agent A, agent B, or both agent A and agent B. The inorganic filler is preferably contained in liquid B (agent B) from the viewpoint of adsorbed water.
(2-8.硬化触媒)
本多液型硬化性組成物は、硬化触媒をさらに含むことが好ましい。硬化触媒としては、縮合触媒として使用し得るものである限り、特に制限はなく、任意のものを使用し得る。 (2-8. Curing catalyst)
The multi-component curable composition preferably further contains a curing catalyst. Any curing catalyst can be used without any particular limitation as long as it can be used as a condensation catalyst.
本多液型硬化性組成物は、硬化触媒をさらに含むことが好ましい。硬化触媒としては、縮合触媒として使用し得るものである限り、特に制限はなく、任意のものを使用し得る。 (2-8. Curing catalyst)
The multi-component curable composition preferably further contains a curing catalyst. Any curing catalyst can be used without any particular limitation as long as it can be used as a condensation catalyst.
硬化触媒としては、例えば、ジブチル錫ジラウレート、ジブチル錫ジアセテート、ジブチル錫ジエチルヘキサノエート、ジブチル錫ジオクテート、ジブチル錫ジメチルマレート、ジブチル錫ジエチルマレート、ジブチル錫ジブチルマレート、ジブチル錫ジイソオクチルマレート、ジブチル錫ジトリデシルマレート、ジブチル錫ジベンジルマレート、ジブチル錫マレエート、ジオクチル錫ジアセテート、ジオクチル錫ジステアレート、ジオクチル錫ジラウレート、ジオクチル錫ジエチルマレート、ジオクチル錫ジイソオクチルマレート等のジアルキル錫ジカルボキシレート類、例えば、ジブチル錫ジメトキシド、ジブチル錫ジフェノキシド等のジアルキル錫アルコキサイド類、例えば、ジブチル錫ジアセチルアセトナート、ジブチル錫ジエチルアセトアセテートなどのジアルキル錫の分子内配位性誘導体類、例えば、ジブチル錫オキサイドやジオクチル錫オキサイド等のジアルキル錫オキサイドと例えば、ジオクチルフタレート、ジイソデシルフタレート、メチルマレエート等のエステル化合物との反応物、ジアルキル錫オキサイド、カルボン酸およびアルコール化合物を反応させて得られる錫化合物、例えば、ジブチル錫ビストリエトキシシリケート、ジオクチル錫ビストリエトキシシリケート等のジアルキル錫オキサイドとシリケート化合物との反応物、およびこれらジアルキル錫化合物のオキシ誘導体(スタノキサン化合物)等の4価の錫化合物類;例えば、オクチル酸錫、ナフテン酸錫、ステアリン酸錫、フェルザチック酸錫等の2価の錫化合物類、あるいはこれらと後述のラウリルアミン等のアミン系化合物との反応物および混合物;例えば、モノブチル錫トリスオクトエートやモノブチル錫トリイソプロポキシド等のモノブチル錫化合物やモノオクチル錫化合物等のモノアルキル錫類;例えば、テトラブチルチタネート、テトラプロピルチタネート、テトラ(2-エチルヘキシル)チタネート、イソプロポキシチタンビス(エチルアセトアセテート)等のチタン酸エステル類;アルミニウムトリスアセチルアセトナート、アルミニウムトリスエチルアセトアセテート、ジ-イソプロポキシアルミニウムエチルアセトアセテート等の有機アルミニウム化合物類;カルボン酸ビスマス、カルボン酸鉄、カルボン酸チタニウム、カルボン酸鉛、カルボン酸バナジウム、カルボン酸ジルコニウム、カルボン酸カルシウム、カルボン酸カリウム、カルボン酸バリウム、カルボン酸マンガン、カルボン酸セリウム、カルボン酸ニッケル、カルボン酸コバルト、カルボン酸亜鉛、カルボン酸アルミニウム等のカルボン酸(2-エチルヘキサン酸、ネオデカン酸、バーサチック酸、オレイン酸、ナフテン酸等)金属塩、あるいはこれらと後述のラウリルアミン等のアミン系化合物との反応物および混合物;ジルコニウムテトラアセチルアセトナート、ジルコニウムトリブトキシアセチルアセトナート、ジブトキシジルコニウムジアセチルアセトナート、ジルコニウムアセチルアセトナートビス(エチルアセトアセテート)、チタンテトラアセチルアセトナート等のキレート化合物類;メチルアミン、エチルアミン、プロピルアミン、イソプロピルアミン、ブチルアミン、アミルアミン、ヘキシルアミン、オクチルアミン、2-エチルヘキシルアミン、ノニルアミン、デシルアミン、ラウリルアミン、ペンタデシルアミン、セチルアミン、ステアリルアミン、シクロヘキシルアミン等の脂肪族第一アミン類;ジメチルアミン、ジエチルアミン、ジプロピルアミン、ジイソプロピルアミン、ジブチルアミン、ジアミルアミン、ジオクチルアミン、ジ(2-エチルヘキシル)アミン、ジデシルアミン、ジラウリルアミン、ジセチルアミン、ジステアリルアミン、メチルステアリルアミン、エチルステアリルアミン、ブチルステアリルアミン等の脂肪族第二アミン類;トリアミルアミン、トリヘキシルアミン、トリオクチルアミン等の脂肪族第三アミン類;トリアリルアミン、オレイルアミン、などの脂肪族不飽和アミン類;ラウリルアニリン、ステアリルアニリン、トリフェニルアミン等の芳香族アミン類;および、その他のアミン類として、モノエタノールアミン、ジエタノールアミン、トリエタノールアミン、ジエチレントリアミン、トリエチレンテトラミン、オレイルアミン、シクロヘキシルアミン、ベンジルアミン、ジエチルアミノプロピルアミン、キシリレンジアミン、エチレンジアミン、ヘキサメチレンジアミン、トリエチレンジアミン、グアニジン、ジフェニルグアニジン、2,4,6-トリス(ジメチルアミノメチル)フェノール、モルホリン、N-メチルモルホリン、2-エチル-4-メチルイミダゾール、1,8-ジアザビシクロ(5,4,0)ウンデセン-7(DBU)等のアミン系化合物、あるいはこれらのアミン系化合物のカルボン酸等との塩;ラウリルアミンとオクチル酸錫の反応物あるいは混合物のようなアミン系化合物と有機錫化合物との反応物および混合物;過剰のポリアミンと多塩基酸とから得られる低分子量ポリアミド樹脂;過剰のポリアミンとエポキシ化合物との反応生成物;γ-アミノプロピルトリメトキシシラン、γ-アミノプロピルトリエトキシシラン、γ-アミノプロピルトリイソプロポキシシラン、γ-アミノプロピルメチルジメトキシシラン、γ-アミノプロピルメチルジエトキシシラン、N-(β-アミノエチル)アミノプロピルトリメトキシシラン、N-(β-アミノエチル)アミノプロピルメチルジメトキシシラン、N-(β-アミノエチル)アミノプロピルトリエトキシシラン、N-(β-アミノエチル)アミノプロピルメチルジエトキシシラン、N-(β-アミノエチル)アミノプロピルトリイソプロポキシシラン、γ-ウレイドプロピルトリメトキシシラン、N-フェニル-γ-アミノプロピルトリメトキシシラン、N-ベンジル-γ-アミノプロピルトリメトキシシラン、N-ビニルベンジル-γ-アミノプロピルトリエトキシシラン等を挙げることができる。また、これらを変性した誘導体である、アミノ変性シリルポリマー、シリル化アミノポリマー、不飽和アミノシラン錯体、フェニルアミノ長鎖アルキルシラン、アミノシリル化シリコーン等のアミノ基を有するシランカップリング剤;等のシラノール縮合触媒、さらにはフェルザチック酸等の脂肪酸や有機酸性リン酸エステル化合物等他の酸性触媒、塩基性触媒等の公知のシラノール縮合触媒等が例示できる。これら硬化触媒は、単独で使用してもよく、2種以上を組み合わせて使用してもよい。
Curing catalysts include, for example, dibutyltin dilaurate, dibutyltin diacetate, dibutyltin diethylhexanoate, dibutyltin dioctate, dibutyltin dimethylmalate, dibutyltin diethylmalate, dibutyltin dibutylmalate, dibutyltin diisooctyl Dialkyls such as maleate, dibutyltin ditridecylmalate, dibutyltin dibenzylmalate, dibutyltin maleate, dioctyltin diacetate, dioctyltin distearate, dioctyltin dilaurate, dioctyltin diethyl maleate, dioctyltin diisooctyl maleate, etc. Tin dicarboxylates, dialkyltin alkoxides such as dibutyltin dimethoxide and dibutyltin diphenoxide, intramolecular coordinating derivatives of dialkyltin such as dibutyltin diacetylacetonate and dibutyltin diethylacetoacetate, such as , reactants of dialkyltin oxides such as dibutyltin oxide and dioctyltin oxide with ester compounds such as dioctyl phthalate, diisodecyl phthalate and methyl maleate; tin obtained by reacting dialkyltin oxides, carboxylic acids and alcohol compounds; compounds such as dibutyltin bistriethoxysilicate, dioctyltin bistriethoxysilicate, and other dialkyltin oxides and silicate compounds, and tetravalent tin compounds such as oxy derivatives of these dialkyltin compounds (stannoxane compounds); , tin octoate, tin naphthenate, tin stearate, tin felzatate and other divalent tin compounds, or reaction products and mixtures thereof with amine compounds such as laurylamine described later; monoalkyltin compounds such as monobutyltin compounds such as octoate and monobutyltin triisopropoxide; monoalkyltin compounds such as monooctyltin compounds; Acetate) and other titanate esters; aluminum trisacetylacetonate, aluminum trisethylacetoacetate, di-isopropoxyaluminum ethylacetoacetate and other organoaluminum compounds; bismuth carboxylate, iron carboxylate, titanium carboxylate, carboxylic acid lead, vanadium carboxylate, zirconium carboxylate, calcium carboxylate , potassium carboxylate, barium carboxylate, manganese carboxylate, cerium carboxylate, nickel carboxylate, cobalt carboxylate, zinc carboxylate, aluminum carboxylate (2-ethylhexanoic acid, neodecanoic acid, versatic acid, olein zirconium tetraacetylacetonate, zirconium tributoxyacetylacetonate, dibutoxyzirconium diacetylacetonate, zirconium acetyl chelate compounds such as acetonatobis(ethylacetoacetate), titanium tetraacetylacetonate; Aliphatic primary amines such as laurylamine, pentadecylamine, cetylamine, stearylamine, cyclohexylamine; dimethylamine, diethylamine, dipropylamine, diisopropylamine, dibutylamine, diamylamine, dioctylamine, di(2-ethylhexyl)amine , didecylamine, dilaurylamine, dicetylamine, distearylamine, methylstearylamine, ethylstearylamine, butylstearylamine; aliphatic tertiary amines such as triamylamine, trihexylamine, trioctylamine; Amines; aliphatic unsaturated amines such as triallylamine and oleylamine; aromatic amines such as laurylaniline, stearylaniline and triphenylamine; and other amines such as monoethanolamine, diethanolamine and triethanolamine. , diethylenetriamine, triethylenetetramine, oleylamine, cyclohexylamine, benzylamine, diethylaminopropylamine, xylylenediamine, ethylenediamine, hexamethylenediamine, triethylenediamine, guanidine, diphenylguanidine, 2,4,6-tris(dimethylaminomethyl)phenol , morpholine, N-methylmorpholine, 2-ethyl-4-methylimidazole, 1,8-diazabicyclo(5,4,0)undecene-7 (DBU) and other amine compounds, or calcium carbonate of these amine compounds. salts with carboxylic acids, etc.; reaction products and mixtures of amine compounds and organic tin compounds, such as reaction products or mixtures of laurylamine and tin octylate; low molecular weight polyamide resins obtained from excess polyamines and polybasic acids Reaction products of excess polyamine and epoxy compounds; γ-aminopropyltrimethoxysilane, γ-aminopropyltriethoxysilane, γ-aminopropyltriisopropoxysilane, γ-aminopropylmethyldimethoxysilane, γ-aminopropyl methyldiethoxysilane, N-(β-aminoethyl)aminopropyltrimethoxysilane, N-(β-aminoethyl)aminopropylmethyldimethoxysilane, N-(β-aminoethyl)aminopropyltriethoxysilane, N-( β-aminoethyl)aminopropylmethyldiethoxysilane, N-(β-aminoethyl)aminopropyltriisopropoxysilane, γ-ureidopropyltrimethoxysilane, N-phenyl-γ-aminopropyltrimethoxysilane, N-benzyl -γ-aminopropyltrimethoxysilane, N-vinylbenzyl-γ-aminopropyltriethoxysilane and the like. In addition, silane coupling agents having an amino group, such as amino-modified silyl polymers, silylated amino polymers, unsaturated aminosilane complexes, phenylamino long-chain alkylsilanes, and aminosilylated silicones, which are modified derivatives thereof; Catalysts, further known silanol condensation catalysts such as fatty acids such as felzatic acid, other acidic catalysts such as organic acidic phosphoric acid ester compounds, basic catalysts and the like can be exemplified. These curing catalysts may be used alone or in combination of two or more.
硬化触媒の含有量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、例えば、0.1~5重量部であることが好ましく、0.2~2重量部であることがより好ましく、0.3~1重量部であることがさらに好ましい。硬化触媒の含有量が上記の範囲内であれば、硬化性と低コスト化とが両立しやすいという利点を有する。
The content of the curing catalyst is preferably, for example, 0.1 to 5 parts by weight, preferably 0.2 parts by weight, with respect to the total 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is more preferably from 0.3 to 1 part by weight, more preferably from 0.3 to 1 part by weight. If the content of the curing catalyst is within the above range, there is an advantage that both curability and cost reduction can easily be achieved.
(2-9.脱水剤)
本多液型硬化性組成物は、さらに、脱水剤を含むことが好ましい。本多液型硬化性組成物が脱水剤を含むことにより、貯蔵安定性に優れるという利点を有する。 (2-9. Dehydrating agent)
The multi-component curable composition preferably further contains a dehydrating agent. By containing a dehydrating agent, the present multi-component curable composition has an advantage of excellent storage stability.
本多液型硬化性組成物は、さらに、脱水剤を含むことが好ましい。本多液型硬化性組成物が脱水剤を含むことにより、貯蔵安定性に優れるという利点を有する。 (2-9. Dehydrating agent)
The multi-component curable composition preferably further contains a dehydrating agent. By containing a dehydrating agent, the present multi-component curable composition has an advantage of excellent storage stability.
脱水剤としては、特に限定されないが、例えば、ビニルシラン、トシルイソシアネート、ビニルトリメトキシシラン、酸化カルシウム、ゼオライト、p-トルエンスルホニルイソシアネート、3-エチル-2-メチル-2-(3-メチルブチル)-1,3-オキサゾリジン等が挙げられる。これら脱水剤は、単独で使用してもよく、2種以上を組み合わせて使用してもよい。
Examples of dehydrating agents include, but are not limited to, vinylsilane, tosylisocyanate, vinyltrimethoxysilane, calcium oxide, zeolite, p-toluenesulfonylisocyanate, 3-ethyl-2-methyl-2-(3-methylbutyl)-1. , 3-oxazolidine and the like. These dehydrating agents may be used alone or in combination of two or more.
脱水剤の含有量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、例えば、0.1~10重量部であり、0.1~5.0重量部であることが好ましく、1.0~3.0重量部であることがより好ましい。脱水剤の含有量がポリオキシアルキレン系重合体(A)100重量部に対して0.1重量部以上であると、水の存在下で反応するポリオキシアルキレン系重合体(A)が過剰に反応してしまうことを防ぐことができるという利点を有する。また、脱水剤の含有量がポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して3.0重量部以下であると、貯蔵安定性と低コスト化とが両立し易いという利点を有する。
The content of the dehydrating agent is, for example, 0.1 to 10 parts by weight, and 0.1 to 5.0 parts by weight, based on 100 parts by weight of the total of the polyoxyalkylene polymer (A) and the epoxy resin (E). It is preferably 0 parts by weight, more preferably 1.0 to 3.0 parts by weight. When the content of the dehydrating agent is 0.1 parts by weight or more with respect to 100 parts by weight of the polyoxyalkylene polymer (A), the polyoxyalkylene polymer (A) that reacts in the presence of water becomes excessive. It has the advantage of being able to prevent reactions. Further, when the content of the dehydrating agent is 3.0 parts by weight or less with respect to the total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E), storage stability and cost reduction are achieved. have the advantage of being compatible with each other.
(2-10.その他の成分)
本多液型硬化性組成物は、上記の他に、添加剤として、充填剤、接着性付与剤、タレ防止剤、酸化防止剤、光安定剤、紫外線吸収剤、粘着付与樹脂、低分子可塑剤、その他の樹脂等を添加してもよい。また、本多液型硬化性組成物には、硬化性組成物または硬化物の諸物性の調整を目的として、必要に応じて各種添加剤を添加してもよい。このような添加物としては、例えば、溶剤、希釈剤、光硬化性物質、酸素硬化性物質、表面性改良剤、シリケート、硬化性調整剤、ラジカル禁止剤、金属不活性化剤、オゾン劣化防止剤、リン系過酸化物分解剤、滑剤、顔料、防かび剤、難燃剤、発泡剤等が挙げられる。 (2-10. Other components)
In addition to the above, this multi-component curable composition contains fillers, adhesion imparting agents, anti-sagging agents, antioxidants, light stabilizers, ultraviolet absorbers, tackifying resins, low-molecular-weight plasticizers, and other additives. agents, other resins, etc. may be added. In addition, various additives may be added to the present multi-component curable composition as necessary for the purpose of adjusting various physical properties of the curable composition or cured product. Such additives include, for example, solvents, diluents, photo-curing substances, oxygen-curing substances, surface property modifiers, silicates, curability modifiers, radical inhibitors, metal deactivators, ozone deterioration inhibitors, agents, phosphorus peroxide decomposers, lubricants, pigments, antifungal agents, flame retardants, foaming agents, and the like.
本多液型硬化性組成物は、上記の他に、添加剤として、充填剤、接着性付与剤、タレ防止剤、酸化防止剤、光安定剤、紫外線吸収剤、粘着付与樹脂、低分子可塑剤、その他の樹脂等を添加してもよい。また、本多液型硬化性組成物には、硬化性組成物または硬化物の諸物性の調整を目的として、必要に応じて各種添加剤を添加してもよい。このような添加物としては、例えば、溶剤、希釈剤、光硬化性物質、酸素硬化性物質、表面性改良剤、シリケート、硬化性調整剤、ラジカル禁止剤、金属不活性化剤、オゾン劣化防止剤、リン系過酸化物分解剤、滑剤、顔料、防かび剤、難燃剤、発泡剤等が挙げられる。 (2-10. Other components)
In addition to the above, this multi-component curable composition contains fillers, adhesion imparting agents, anti-sagging agents, antioxidants, light stabilizers, ultraviolet absorbers, tackifying resins, low-molecular-weight plasticizers, and other additives. agents, other resins, etc. may be added. In addition, various additives may be added to the present multi-component curable composition as necessary for the purpose of adjusting various physical properties of the curable composition or cured product. Such additives include, for example, solvents, diluents, photo-curing substances, oxygen-curing substances, surface property modifiers, silicates, curability modifiers, radical inhibitors, metal deactivators, ozone deterioration inhibitors, agents, phosphorus peroxide decomposers, lubricants, pigments, antifungal agents, flame retardants, foaming agents, and the like.
<充填剤>
本多液型硬化性組成物には、無機フィラー以外の種々の充填剤を配合することができる。充填剤としては、例えば、PVC粉末、PMMA粉末等が挙げられる。 <Filler>
Various fillers other than the inorganic filler can be blended into the present multi-component curable composition. Examples of fillers include PVC powder and PMMA powder.
本多液型硬化性組成物には、無機フィラー以外の種々の充填剤を配合することができる。充填剤としては、例えば、PVC粉末、PMMA粉末等が挙げられる。 <Filler>
Various fillers other than the inorganic filler can be blended into the present multi-component curable composition. Examples of fillers include PVC powder and PMMA powder.
充填剤の使用量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.5~100重量部が好ましく、1~60重量部がより好ましい。
The amount of the filler used is preferably 0.5 to 100 parts by weight, more preferably 1 to 60 parts by weight, with respect to 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E) combined. .
本多液型硬化性組成物の軽量化(低比重化)の目的で、有機バルーンを添加してもよい。
Organic balloons may be added for the purpose of reducing the weight (lowering the specific gravity) of the multi-component curable composition.
<接着性付与剤>
本多液型硬化性組成物には、接着性付与剤を添加することができる。 <Adhesion imparting agent>
An adhesion-imparting agent can be added to the present multi-component curable composition.
本多液型硬化性組成物には、接着性付与剤を添加することができる。 <Adhesion imparting agent>
An adhesion-imparting agent can be added to the present multi-component curable composition.
接着性付与剤としては、シランカップリング剤、シランカップリング剤の反応物を添加することができる。
A silane coupling agent or a reactant of the silane coupling agent can be added as an adhesion imparting agent.
シランカップリング剤の具体例としては、γ-アミノプロピルトリメトキシシラン、γ-アミノプロピルメチルジメトキシシラン、N-β-アミノエチル-γ-アミノプロピルトリメトキシシラン、N-β-アミノエチル-γ-アミノプロピルメチルジメトキシシラン、N-フェニル-γ-アミノプロピルトリメトキシシラン、(2-アミノエチル)アミノメチルトリメトキシシランなどのアミノ基含有シラン類;γ-イソシアネートプロピルトリメトキシシラン、γ-イソシアネートプロピルトリエトキシシラン、γ-イソシアネートプロピルメチルジメトキシシラン、α-イソシアネートメチルトリメトキシシラン、α-イソシアネートメチルジメトキシメチルシラン等のイソシアネート基含有シラン類;γ-メルカプトプロピルトリメトキシシラン、γ-メルカプトプロピルトリエトキシシラン、γ-メルカプトプロピルメチルジメトキシシラン等のメルカプト基含有シラン類;γ-グリシドキシプロピルトリメトキシシラン、β-(3,4-エポキシシクロヘキシル)エチルトリメトキシシラン等のエポキシ基含有シラン類、が挙げられる。
Specific examples of silane coupling agents include γ-aminopropyltrimethoxysilane, γ-aminopropylmethyldimethoxysilane, N-β-aminoethyl-γ-aminopropyltrimethoxysilane, N-β-aminoethyl-γ- Amino group-containing silanes such as aminopropylmethyldimethoxysilane, N-phenyl-γ-aminopropyltrimethoxysilane, (2-aminoethyl)aminomethyltrimethoxysilane; γ-isocyanatopropyltrimethoxysilane, γ-isocyanatopropyltrimethoxysilane; isocyanate group-containing silanes such as ethoxysilane, γ-isocyanatopropylmethyldimethoxysilane, α-isocyanatomethyltrimethoxysilane, α-isocyanatomethyldimethoxymethylsilane; γ-mercaptopropyltrimethoxysilane, γ-mercaptopropyltriethoxysilane, mercapto group-containing silanes such as γ-mercaptopropylmethyldimethoxysilane; epoxy group-containing silanes such as γ-glycidoxypropyltrimethoxysilane and β-(3,4-epoxycyclohexyl)ethyltrimethoxysilane; .
上記接着性付与剤は1種類のみで使用しても良いし、2種類以上混合使用しても良い。また、各種シランカップリング剤の反応物も使用できる。
The adhesiveness-imparting agent may be used alone or in combination of two or more. Reaction products of various silane coupling agents can also be used.
シランカップリング剤の使用量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.1~20重量部が好ましく、特に0.5~10重量部が好ましい。
The amount of the silane coupling agent used is preferably 0.1 to 20 parts by weight, particularly 0.5 to 10 parts by weight, based on 100 parts by weight of the total of the polyoxyalkylene polymer (A) and the epoxy resin (E). Parts by weight are preferred.
<タレ防止剤>
本多液型硬化性組成物には、必要に応じてタレを防止し、作業性を良くするためにタレ防止剤を添加しても良い。タレ防止剤としては特に限定されないが、例えば、ポリアミドワックス類;水添ヒマシ油誘導体類;ステアリン酸カルシウム、ステアリン酸アルミニウム、ステアリン酸バリウム等の金属石鹸類等が挙げられる。これらタレ防止剤は単独で用いてもよく、2種以上併用してもよい。 <Anti-sagging agent>
If necessary, an anti-sagging agent may be added to the multi-component curable composition to prevent sagging and improve workability. The anti-sagging agent is not particularly limited, but examples thereof include polyamide waxes; hydrogenated castor oil derivatives; metal soaps such as calcium stearate, aluminum stearate and barium stearate. These anti-sagging agents may be used alone or in combination of two or more.
本多液型硬化性組成物には、必要に応じてタレを防止し、作業性を良くするためにタレ防止剤を添加しても良い。タレ防止剤としては特に限定されないが、例えば、ポリアミドワックス類;水添ヒマシ油誘導体類;ステアリン酸カルシウム、ステアリン酸アルミニウム、ステアリン酸バリウム等の金属石鹸類等が挙げられる。これらタレ防止剤は単独で用いてもよく、2種以上併用してもよい。 <Anti-sagging agent>
If necessary, an anti-sagging agent may be added to the multi-component curable composition to prevent sagging and improve workability. The anti-sagging agent is not particularly limited, but examples thereof include polyamide waxes; hydrogenated castor oil derivatives; metal soaps such as calcium stearate, aluminum stearate and barium stearate. These anti-sagging agents may be used alone or in combination of two or more.
タレ防止剤の使用量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.1~20重量部が好ましい。
The amount of anti-sagging agent to be used is preferably 0.1 to 20 parts by weight with respect to 100 parts by weight in total of the polyoxyalkylene polymer (A) and the epoxy resin (E).
<酸化防止剤>
本多液型硬化性組成物には、酸化防止剤(老化防止剤)を使用することができる。酸化防止剤を使用すると硬化物の耐候性を高めることができる。酸化防止剤としてはヒンダードフェノール系、モノフェノール系、ビスフェノール系、ポリフェノール系が例示できる。酸化防止剤の具体例は特開平4-283259号公報や特開平9-194731号公報にも記載されている。 <Antioxidant>
Antioxidants (antiaging agents) can be used in the present multi-component curable composition. The use of an antioxidant can enhance the weather resistance of the cured product. Examples of antioxidants include hindered phenols, monophenols, bisphenols, and polyphenols. Specific examples of antioxidants are also described in JP-A-4-283259 and JP-A-9-194731.
本多液型硬化性組成物には、酸化防止剤(老化防止剤)を使用することができる。酸化防止剤を使用すると硬化物の耐候性を高めることができる。酸化防止剤としてはヒンダードフェノール系、モノフェノール系、ビスフェノール系、ポリフェノール系が例示できる。酸化防止剤の具体例は特開平4-283259号公報や特開平9-194731号公報にも記載されている。 <Antioxidant>
Antioxidants (antiaging agents) can be used in the present multi-component curable composition. The use of an antioxidant can enhance the weather resistance of the cured product. Examples of antioxidants include hindered phenols, monophenols, bisphenols, and polyphenols. Specific examples of antioxidants are also described in JP-A-4-283259 and JP-A-9-194731.
酸化防止剤の使用量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.1~10重量部が好ましく、特に0.2~5重量部が好ましい。
The amount of the antioxidant used is preferably 0.1 to 10 parts by weight, particularly 0.2 to 5 parts by weight, with respect to 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E) combined. part is preferred.
<光安定剤>
本多液型硬化性組成物には、光安定剤を使用することができる。光安定剤を使用すると硬化物の光酸化劣化を防止できる。光安定剤としてベンゾトリアゾール系、ヒンダードアミン系、ベンゾエート系化合物等が例示できるが、特にヒンダードアミン系が好ましい。 <Light stabilizer>
A light stabilizer can be used in the present multi-part curable composition. The use of a light stabilizer can prevent photo-oxidative deterioration of the cured product. Benzotriazole-based, hindered amine-based, and benzoate-based compounds can be exemplified as light stabilizers, and hindered amine-based compounds are particularly preferred.
本多液型硬化性組成物には、光安定剤を使用することができる。光安定剤を使用すると硬化物の光酸化劣化を防止できる。光安定剤としてベンゾトリアゾール系、ヒンダードアミン系、ベンゾエート系化合物等が例示できるが、特にヒンダードアミン系が好ましい。 <Light stabilizer>
A light stabilizer can be used in the present multi-part curable composition. The use of a light stabilizer can prevent photo-oxidative deterioration of the cured product. Benzotriazole-based, hindered amine-based, and benzoate-based compounds can be exemplified as light stabilizers, and hindered amine-based compounds are particularly preferred.
光安定剤の使用量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.1~10重量部が好ましく、特に0.2~5重量部が好ましい。
The amount of the light stabilizer used is preferably 0.1 to 10 parts by weight, particularly 0.2 to 5 parts by weight, based on 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E) combined. part is preferred.
<紫外線吸収剤>
本多液型硬化性組成物には、紫外線吸収剤を使用することができる。紫外線吸収剤を使用すると硬化物の表面耐候性を高めることができる。紫外線吸収剤としてはベンゾフェノン系、ベンゾトリアゾール系、サリチレート系、置換トリル系および金属キレート系化合物等が例示できるが、特にベンゾトリアゾール系が好ましく、市販名チヌビンP、チヌビン213、チヌビン234、チヌビン326、チヌビン327、チヌビン328、チヌビン329、チヌビン571(以上、BASF製)が挙げられる。 <Ultraviolet absorber>
An ultraviolet absorber can be used in the present multi-component curable composition. The use of an ultraviolet absorber can enhance the surface weather resistance of the cured product. Benzophenone-based, benzotriazole-based, salicylate-based, substituted tolyl-based and metal chelate-based compounds can be exemplified as ultraviolet absorbers, and benzotriazole-based compounds are particularly preferred, and commercially available names of Tinuvin P, Tinuvin 213, Tinuvin 234, Tinuvin 326, Tinuvin 327, Tinuvin 328, Tinuvin 329, and Tinuvin 571 (manufactured by BASF).
本多液型硬化性組成物には、紫外線吸収剤を使用することができる。紫外線吸収剤を使用すると硬化物の表面耐候性を高めることができる。紫外線吸収剤としてはベンゾフェノン系、ベンゾトリアゾール系、サリチレート系、置換トリル系および金属キレート系化合物等が例示できるが、特にベンゾトリアゾール系が好ましく、市販名チヌビンP、チヌビン213、チヌビン234、チヌビン326、チヌビン327、チヌビン328、チヌビン329、チヌビン571(以上、BASF製)が挙げられる。 <Ultraviolet absorber>
An ultraviolet absorber can be used in the present multi-component curable composition. The use of an ultraviolet absorber can enhance the surface weather resistance of the cured product. Benzophenone-based, benzotriazole-based, salicylate-based, substituted tolyl-based and metal chelate-based compounds can be exemplified as ultraviolet absorbers, and benzotriazole-based compounds are particularly preferred, and commercially available names of Tinuvin P, Tinuvin 213, Tinuvin 234, Tinuvin 326, Tinuvin 327, Tinuvin 328, Tinuvin 329, and Tinuvin 571 (manufactured by BASF).
紫外線吸収剤の使用量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、0.1~10重量部が好ましく、特に0.2~5重量部が好ましい。
The amount of the ultraviolet absorber to be used is preferably 0.1 to 10 parts by weight, particularly 0.2 to 5 parts by weight, per 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E) combined. part is preferred.
<粘着付与樹脂>
本多液型硬化性組成物には、基材への接着性や密着性を高める目的、あるいはその他必要に応じて粘着付与樹脂を添加できる。粘着付与樹脂としては、特に制限はなく通常使用されているものを使うことができる。 <Tackifying resin>
A tackifying resin can be added to the present multi-component curable composition for the purpose of enhancing the adhesiveness or adhesion to a substrate, or for other purposes. As the tackifier resin, there is no particular limitation and any commonly used one can be used.
本多液型硬化性組成物には、基材への接着性や密着性を高める目的、あるいはその他必要に応じて粘着付与樹脂を添加できる。粘着付与樹脂としては、特に制限はなく通常使用されているものを使うことができる。 <Tackifying resin>
A tackifying resin can be added to the present multi-component curable composition for the purpose of enhancing the adhesiveness or adhesion to a substrate, or for other purposes. As the tackifier resin, there is no particular limitation and any commonly used one can be used.
具体例としては、テルペン系樹脂、芳香族変性テルペン樹脂、水素添加テルペン樹脂、テルペン-フェノール樹脂、フェノール樹脂、変性フェノール樹脂、キシレン-フェノール樹脂、シクロペンタジエン-フェノール樹脂、クマロンインデン樹脂、ロジン系樹脂、ロジンエステル樹脂、水添ロジンエステル樹脂、キシレン樹脂、低分子量ポリスチレン系樹脂、スチレン共重合体樹脂、スチレン系ブロック共重合体およびその水素添加物、石油樹脂(例えば、C5炭化水素樹脂、C9炭化水素樹脂、C5C9炭化水素共重合樹脂等)、水添石油樹脂、DCPD樹脂等が挙げられる。これらは単独で用いても良く、2種以上を併用しても良い。
Specific examples include terpene-based resins, aromatic modified terpene resins, hydrogenated terpene resins, terpene-phenolic resins, phenolic resins, modified phenolic resins, xylene-phenolic resins, cyclopentadiene-phenolic resins, coumarone-indene resins, rosin-based Resins, rosin ester resins, hydrogenated rosin ester resins, xylene resins, low molecular weight polystyrene resins, styrene copolymer resins, styrene block copolymers and hydrogenated products thereof, petroleum resins (e.g., C5 hydrocarbon resins, C9 hydrocarbon resins, C5C9 hydrocarbon copolymer resins, etc.), hydrogenated petroleum resins, DCPD resins, and the like. These may be used alone or in combination of two or more.
粘着付与樹脂の使用量は、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、2~100重量部が好ましく、5~50重量部であることがより好ましく、5~30重量部であることがさらに好ましい。
The amount of the tackifying resin used is preferably 2 to 100 parts by weight, more preferably 5 to 50 parts by weight, with respect to the total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). More preferably, it is 5 to 30 parts by weight.
<低分子可塑剤>
本多液型硬化性組成物には、低分子可塑剤を添加できる。低分子可塑剤として、より具体的には、フタル酸ジイソノイル、2-エトキシエタノール、フタル酸ビス(2-エチルヘキシル)、フタル酸ジイソデシル等が好ましい。これらの低分子可塑剤は、単独で使用してもよく、2種以上を組み合わせて使用してもよい。 <Low molecular weight plasticizer>
A low molecular weight plasticizer can be added to the present multi-part curable composition. More specifically, diisonoyl phthalate, 2-ethoxyethanol, bis(2-ethylhexyl) phthalate, diisodecyl phthalate, and the like are preferable as the low-molecular-weight plasticizer. These low-molecular-weight plasticizers may be used alone or in combination of two or more.
本多液型硬化性組成物には、低分子可塑剤を添加できる。低分子可塑剤として、より具体的には、フタル酸ジイソノイル、2-エトキシエタノール、フタル酸ビス(2-エチルヘキシル)、フタル酸ジイソデシル等が好ましい。これらの低分子可塑剤は、単独で使用してもよく、2種以上を組み合わせて使用してもよい。 <Low molecular weight plasticizer>
A low molecular weight plasticizer can be added to the present multi-part curable composition. More specifically, diisonoyl phthalate, 2-ethoxyethanol, bis(2-ethylhexyl) phthalate, diisodecyl phthalate, and the like are preferable as the low-molecular-weight plasticizer. These low-molecular-weight plasticizers may be used alone or in combination of two or more.
本多液型硬化性組成物における低分子可塑剤の含有量は、特に限定されないが、ポリオキシアルキレン系重合体(A)とエポキシ樹脂(E)との合計100重量部に対して、例えば、5~150重量部であることが好ましく、20~100重量部であることがより好ましく、30~80重量部であることがさらに好ましい。当該構成によれば、多液型硬化性組成物の低粘度化と、硬化物の引き裂き強さと、が両立し易いという利点を有する。
The content of the low-molecular-weight plasticizer in the present multi-liquid curable composition is not particularly limited. It is preferably 5 to 150 parts by weight, more preferably 20 to 100 parts by weight, even more preferably 30 to 80 parts by weight. This configuration has an advantage that it is easy to achieve both low viscosity of the multi-component curable composition and high tear strength of the cured product.
(2-11.用途)
本多液型硬化性組成物は、防水材、建築用弾性シーリング材、サイディングボード用シーリング材、複層ガラス用シーリング材、車両用シーリング材等建築用および工業用のシーリング材、太陽電池裏面封止剤などの電気・電子部品材料、電線・ケーブル用絶縁被覆材などの電気絶縁材料、粘着剤、接着剤、弾性接着剤、コンタクト接着剤、タイル用接着剤、反応性ホットメルト接着剤、塗料、粉体塗料、コーティング材、発泡体、缶蓋等のシール材、放熱シート、電気電子用ポッティング剤、フィルム、ガスケット、マリンデッキコーキング、注型材料、各種成形材料、人工大理石、および、網入りガラスや合わせガラス端面(切断部)の防錆・防水用封止材、自動車や船舶、家電等に使用される防振・制振・防音・免震材料、自動車部品、電機部品、各種機械部品などにおいて使用される液状シール材等の様々な用途に利用可能である。 (2-11. Application)
This multi-liquid curable composition is suitable for construction and industrial sealing materials such as waterproofing materials, architectural elastic sealing materials, siding board sealing materials, double glazing sealing materials, vehicle sealing materials, back sealing materials for solar cells. Electrical and electronic component materials such as adhesives, electrical insulating materials such as insulation coating materials for electric wires and cables, adhesives, adhesives, elastic adhesives, contact adhesives, tile adhesives, reactive hot melt adhesives, paints , powder coatings, coating materials, foams, sealing materials for can lids, etc., heat dissipation sheets, potting agents for electrical and electronic devices, films, gaskets, marine deck caulking, casting materials, various molding materials, artificial marble, and netting Sealing materials for rust prevention and waterproofing of glass and laminated glass edges (cut parts), anti-vibration, damping, sound and seismic isolation materials used in automobiles, ships, home appliances, etc., automotive parts, electrical parts, various machine parts It can be used for various purposes such as a liquid sealing material used in such as.
本多液型硬化性組成物は、防水材、建築用弾性シーリング材、サイディングボード用シーリング材、複層ガラス用シーリング材、車両用シーリング材等建築用および工業用のシーリング材、太陽電池裏面封止剤などの電気・電子部品材料、電線・ケーブル用絶縁被覆材などの電気絶縁材料、粘着剤、接着剤、弾性接着剤、コンタクト接着剤、タイル用接着剤、反応性ホットメルト接着剤、塗料、粉体塗料、コーティング材、発泡体、缶蓋等のシール材、放熱シート、電気電子用ポッティング剤、フィルム、ガスケット、マリンデッキコーキング、注型材料、各種成形材料、人工大理石、および、網入りガラスや合わせガラス端面(切断部)の防錆・防水用封止材、自動車や船舶、家電等に使用される防振・制振・防音・免震材料、自動車部品、電機部品、各種機械部品などにおいて使用される液状シール材等の様々な用途に利用可能である。 (2-11. Application)
This multi-liquid curable composition is suitable for construction and industrial sealing materials such as waterproofing materials, architectural elastic sealing materials, siding board sealing materials, double glazing sealing materials, vehicle sealing materials, back sealing materials for solar cells. Electrical and electronic component materials such as adhesives, electrical insulating materials such as insulation coating materials for electric wires and cables, adhesives, adhesives, elastic adhesives, contact adhesives, tile adhesives, reactive hot melt adhesives, paints , powder coatings, coating materials, foams, sealing materials for can lids, etc., heat dissipation sheets, potting agents for electrical and electronic devices, films, gaskets, marine deck caulking, casting materials, various molding materials, artificial marble, and netting Sealing materials for rust prevention and waterproofing of glass and laminated glass edges (cut parts), anti-vibration, damping, sound and seismic isolation materials used in automobiles, ships, home appliances, etc., automotive parts, electrical parts, various machine parts It can be used for various purposes such as a liquid sealing material used in such as.
〔3.硬化物〕
本発明の一実施形態において、本多液型硬化性組成物を硬化させてなる、硬化物(以下、「本硬化物」と称する。)を提供する。 [3. Cured material]
In one embodiment of the present invention, a cured product (hereinafter referred to as "main cured product") is provided by curing the present multi-component curable composition.
本発明の一実施形態において、本多液型硬化性組成物を硬化させてなる、硬化物(以下、「本硬化物」と称する。)を提供する。 [3. Cured material]
In one embodiment of the present invention, a cured product (hereinafter referred to as "main cured product") is provided by curing the present multi-component curable composition.
本硬化物は、本多液型硬化性組成物を硬化して形成されたものである。本発明の一実施形態において、本硬化物は、成分(A)、成分(B)、成分(C)、および成分(D)を含むA剤と、成分(E)および成分(F)を含むB剤と、を含む多液型硬化性組成物を、加熱せずに室温で硬化させることによって形成されたものである。また、本発明の一実施形態において、本硬化物は、成分(A)、成分(B)、成分(C)、成分(D)、および成分(F)を含むA剤と、成分(E)を含むB剤と、を含む多液型硬化性組成物を、加熱せずに室温で硬化させることによって形成されたものである。さらに、本発明の一実施形態において、本硬化物は、成分(A)、成分(B)、成分(C)、成分(D)、および成分(F)を含むA剤と、成分(E)および成分(F)を含むB剤と、を含む多液型硬化性組成物を、加熱せずに室温で硬化させることによって形成されたものである。なお、本多液型硬化性組成物が、溶剤等の揮発成分を含む場合、当該揮発成分は、養生期間に揮発する。したがって、本硬化物は、本多液型硬化性組成物に含まれる揮発成分を実質的に含まない。
The cured product is formed by curing the multi-component curable composition. In one embodiment of the present invention, the cured product contains component (A), component (B), component (C), and component A containing component (D), component (E), and component (F). It is formed by curing a multi-component curable composition containing a B agent at room temperature without heating. In one embodiment of the present invention, the cured product comprises an agent A containing component (A), component (B), component (C), component (D), and component (F), and component (E) and a multi-component curable composition containing and cured at room temperature without heating. Furthermore, in one embodiment of the present invention, the cured product comprises an agent A containing component (A), component (B), component (C), component (D), and component (F), and component (E) and a B agent containing component (F), and cured at room temperature without heating. In addition, when the present multi-component curable composition contains a volatile component such as a solvent, the volatile component volatilizes during the curing period. Therefore, the present cured product does not substantially contain the volatile components contained in the present multi-component curable composition.
〔4.防水材〕
本発明の一実施形態において、本硬化物を含む防水材(以下、「本防水材」と称する。)を提供する。 [4. waterproof material]
In one embodiment of the present invention, a waterproof material containing the cured product (hereinafter referred to as "the present waterproof material") is provided.
本発明の一実施形態において、本硬化物を含む防水材(以下、「本防水材」と称する。)を提供する。 [4. waterproof material]
In one embodiment of the present invention, a waterproof material containing the cured product (hereinafter referred to as "the present waterproof material") is provided.
本防水材は、継ぎ目がない塗膜を形成し、防水の信頼性が高いことから、高い防水性能を必要とする屋根用透湿性塗膜防水材として、特に有用である。建物の屋根用透湿性塗膜防水材は、野地板などの屋根用下地材に塗布する防水材である。本防水材の用途は、特に限定されないが、天井、屋上、ベランダ、用水路、車庫等の屋外の防水材として使用されることが好ましい。
This waterproof material forms a seamless coating film and is highly reliable in waterproofing, so it is particularly useful as a moisture-permeable coating film waterproofing material for roofs that requires high waterproof performance. Moisture-permeable coating film waterproofing materials for roofs of buildings are waterproofing materials applied to base materials for roofs such as sheathing boards. The use of the present waterproof material is not particularly limited, but it is preferably used as an outdoor waterproof material for ceilings, rooftops, balconies, irrigation channels, garages and the like.
本発明の一実施形態において、本防水材は、本硬化物の他に、防水材が一般に含み得る任意の成分を含んでいてもよい。そのような成分は、1種であってもよいし、2種以上であってもよい。また、そのような成分の含有量も特に限定されず、本発明の効果を奏する限り、当業者により適宜設定され得る。
In one embodiment of the present invention, the present waterproof material may contain, in addition to the present cured product, any component that a waterproof material may generally contain. 1 type may be sufficient as such a component, and 2 or more types may be sufficient as it. Also, the content of such components is not particularly limited, and can be appropriately set by those skilled in the art as long as the effects of the present invention are achieved.
〔5.防水構造物の製造方法〕
本発明の一実施形態において、本多液型硬化性組成物を建築物の下地に塗布する工程を含む、防水構造物の製造方法を提供する。本多液型硬化性組成物は、上述の通り、引張特性および引き裂き強さに優れる硬化物を提供できるため、本多液型硬化性組成物を建築物の下地に塗布することにより、防水機能に優れた防水構造物を提供できる。 [5. Manufacturing method of waterproof structure]
In one embodiment of the present invention, there is provided a method of making a waterproof structure comprising applying the multi-part curable composition to a building substrate. As described above, this multi-component curable composition can provide a cured product with excellent tensile properties and tear strength. It is possible to provide a waterproof structure excellent in
本発明の一実施形態において、本多液型硬化性組成物を建築物の下地に塗布する工程を含む、防水構造物の製造方法を提供する。本多液型硬化性組成物は、上述の通り、引張特性および引き裂き強さに優れる硬化物を提供できるため、本多液型硬化性組成物を建築物の下地に塗布することにより、防水機能に優れた防水構造物を提供できる。 [5. Manufacturing method of waterproof structure]
In one embodiment of the present invention, there is provided a method of making a waterproof structure comprising applying the multi-part curable composition to a building substrate. As described above, this multi-component curable composition can provide a cured product with excellent tensile properties and tear strength. It is possible to provide a waterproof structure excellent in
本多液型硬化性組成物を建築物の下地に塗布する方法としては、特に限定されないが、例えば、本多液型硬化性組成物を建築物の下地に直接塗布する場合の他、下地にプライマーが塗られた上に塗布すること等が挙げられる。
The method of applying the multi-component curable composition to the base of the building is not particularly limited, but for example, the multi-component curable composition may be directly applied to the base of the building, or may be applied to the base. For example, it may be applied after a primer has been applied.
また、本発明の一実施形態において、本多液型硬化性組成物を硬化させてなる塗膜の上に、トップコートを塗付する工程を含む、防水構造物の製造方法を提供する。本多液型硬化性組成物を硬化させてなる塗膜の上に、トップコートを塗付することにより、防水機能がより高まる。
In addition, in one embodiment of the present invention, there is provided a method for manufacturing a waterproof structure, which includes the step of applying a topcoat onto a coating film formed by curing the multi-component curable composition. By applying a topcoat on the coating film formed by curing the present multi-component curable composition, the waterproof function is further enhanced.
本発明は上述した各実施形態に限定されるものではなく、請求項に示した範囲で種々の変更が可能であり、異なる実施形態にそれぞれ開示された技術的手段を適宜組み合わせて得られる実施形態についても本発明の技術的範囲に含まれる。
The present invention is not limited to the above-described embodiments, but can be modified in various ways within the scope of the claims, and can be obtained by appropriately combining technical means disclosed in different embodiments. is also included in the technical scope of the present invention.
すなわち、本発明の一態様は、以下を含む。
<1>一般式(1)に示す反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、
前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む、多液型硬化性組成物。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。)
<2>前記アミノシラン(C)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.1~3.0重量部である、<1>に記載の多液型硬化性組成物。
<3>前記メルカプタン化合物(D)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.1~3.0重量部である、<1>または<2>に記載の多液型硬化性組成物。
<4>無機フィラーをさらに含む、<1>~<3>のいずれかに記載の多液型硬化性組成物。
<5>前記無機フィラーの含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、50~300重量部である、<4>に記載の多液型硬化性組成物。
<6>前記ポリオキシアルキレン系重合体(A)の末端部位が、一般式(2): That is, one aspect of the present invention includes the following.
<1> A agent containing a polyoxyalkylene polymer (A) having a reactive silicon group represented by the general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D) , and a B agent containing an epoxy resin (E),
A multicomponent curable composition, wherein the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
<2> The content of the aminosilane (C) is 0.1 to 3.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to <1>.
<3> The content of the mercaptan compound (D) is 0.1 to 3.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to <1> or <2>.
<4> The multi-component curable composition according to any one of <1> to <3>, further comprising an inorganic filler.
<5> In <4>, the content of the inorganic filler is 50 to 300 parts by weight with respect to the total 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). A multi-part curable composition as described.
<6> The terminal portion of the polyoxyalkylene polymer (A) has the general formula (2):
<1>一般式(1)に示す反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、
前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む、多液型硬化性組成物。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。)
<2>前記アミノシラン(C)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.1~3.0重量部である、<1>に記載の多液型硬化性組成物。
<3>前記メルカプタン化合物(D)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.1~3.0重量部である、<1>または<2>に記載の多液型硬化性組成物。
<4>無機フィラーをさらに含む、<1>~<3>のいずれかに記載の多液型硬化性組成物。
<5>前記無機フィラーの含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、50~300重量部である、<4>に記載の多液型硬化性組成物。
<6>前記ポリオキシアルキレン系重合体(A)の末端部位が、一般式(2): That is, one aspect of the present invention includes the following.
<1> A agent containing a polyoxyalkylene polymer (A) having a reactive silicon group represented by the general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D) , and a B agent containing an epoxy resin (E),
A multicomponent curable composition, wherein the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
<2> The content of the aminosilane (C) is 0.1 to 3.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to <1>.
<3> The content of the mercaptan compound (D) is 0.1 to 3.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to <1> or <2>.
<4> The multi-component curable composition according to any one of <1> to <3>, further comprising an inorganic filler.
<5> In <4>, the content of the inorganic filler is 50 to 300 parts by weight with respect to the total 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). A multi-part curable composition as described.
<6> The terminal portion of the polyoxyalkylene polymer (A) has the general formula (2):
<7>前記エポキシ樹脂硬化剤(B)が、第三級アミンを有するエポキシ樹脂硬化剤である、<1>~<6>のいずれかに記載の多液型硬化性組成物。
<8>前記アミノシラン(C)が、γ-アミノプロピルトリエトキシシラン、N-β-(アミノエチル)-γ-アミノプロピルトリメトキシシラン、およびN-β-(アミノエチル)-γ-アミノプロピルメチルジメトキシシランからなる群より選択される少なくとも1種である、<1>~<7>のいずれかに記載の多液型硬化性組成物。
<9>前記メルカプタン化合物(D)が、n-ドデシルメルカプタン、tert-ドデシルメルカプタン、ラウリルメルカプタン、トリメチロールプロパントリス(3-メルカプトプロピオネート)、ペンタエリスリトールテトラキス(3-メルカプトプロピオネート)、3-メルカプトプロピルトリメトキシシラン、3-メルカプトプロピルメチルジメトキシシラン、3-メルカプトプロピルクロロメチルジメトキシシラン、3-メルカプトプロピルメトキシメチルジメトキシシラン、(メルカプトメチル)ジメトキシメチルシラン、および(メルカプトメチル)トリメトキシシランからなる群より選択される少なくとも1種である、<1>~<8>のいずれかに記載の多液型硬化性組成物。
<10>前記エポキシ樹脂(E)が、エポキシ基を1分子中に少なくとも2個有するエポキシ樹脂である、<1>~<9>のいずれかに記載の多液型硬化性組成物。
<11>前記水酸基含有ポリオキシアルキレン系重合体(F)が、数平均分子量が300以上の水酸基を含有するポリオキシアルキレン系重合体である、<1>~<10>のいずれかに記載の多液型硬化性組成物。
<12><1>~<11>のいずれかに記載の多液型硬化性組成物を硬化させてなる、硬化物。
<13><12>に記載の硬化物を含む、防水材。
<14><1>~<11>のいずれかに記載の多液型硬化性組成物を建築物の下地に塗布する工程を含む、防水構造物の製造方法。
<15><1>~<11>のいずれかに記載の多液型硬化性組成物を硬化させてなる塗膜の上に、トップコートを塗付する工程を含む、防水構造物の製造方法。
<7> The multicomponent curable composition according to any one of <1> to <6>, wherein the epoxy resin curing agent (B) is a tertiary amine-containing epoxy resin curing agent.
<8> The aminosilane (C) is γ-aminopropyltriethoxysilane, N-β-(aminoethyl)-γ-aminopropyltrimethoxysilane, and N-β-(aminoethyl)-γ-aminopropylmethyl The multi-component curable composition according to any one of <1> to <7>, which is at least one selected from the group consisting of dimethoxysilane.
<9> The mercaptan compound (D) is n-dodecyl mercaptan, tert-dodecyl mercaptan, lauryl mercaptan, trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakis (3-mercaptopropionate), 3 - from mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropylchloromethyldimethoxysilane, 3-mercaptopropylmethoxymethyldimethoxysilane, (mercaptomethyl)dimethoxymethylsilane and (mercaptomethyl)trimethoxysilane The multi-component curable composition according to any one of <1> to <8>, which is at least one selected from the group consisting of:
<10> The multicomponent curable composition according to any one of <1> to <9>, wherein the epoxy resin (E) is an epoxy resin having at least two epoxy groups per molecule.
<11> The hydroxyl group-containing polyoxyalkylene polymer (F) according to any one of <1> to <10>, wherein the hydroxyl group-containing polyoxyalkylene polymer (F) has a number average molecular weight of 300 or more. A multi-component curable composition.
<12> A cured product obtained by curing the multi-component curable composition according to any one of <1> to <11>.
<13> A waterproof material comprising the cured product according to <12>.
<14> A method for producing a waterproof structure, comprising the step of applying the multi-component curable composition according to any one of <1> to <11> to a foundation of a building.
<15> A method for producing a waterproof structure, comprising a step of applying a topcoat onto a coating film formed by curing the multi-component curable composition according to any one of <1> to <11>. .
本発明の一態様は、以下を含んでもよい。
<16>一般式(1)に示す反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、
前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む、多液型硬化性組成物。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。)
<17>前記メルカプタン化合物(D)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.2~5.0重量部である、<16>に記載の多液型硬化性組成物。
<18>無機フィラーをさらに含む、<16>または<17>に記載の多液型硬化性組成物。
<19>前記無機フィラーの含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、50~180重量部である、<18>に記載の多液型硬化性組成物。
<20>前記アミノシラン(C)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.1~5.0重量部である、<16>~<19>のいずれかに記載の多液型硬化性組成物。
<21>前記ポリオキシアルキレン系重合体(A)の末端部位が、一般式(2): One aspect of the invention may include the following.
<16> A agent containing a polyoxyalkylene polymer (A) having a reactive silicon group represented by the general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D) , and a B agent containing an epoxy resin (E),
A multicomponent curable composition, wherein the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
<17> The content of the mercaptan compound (D) is 0.2 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to <16>.
<18> The multi-component curable composition according to <16> or <17>, further comprising an inorganic filler.
<19> In <18>, the content of the inorganic filler is 50 to 180 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). A multi-part curable composition as described.
<20> The content of the aminosilane (C) is 0.1 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to any one of <16> to <19>.
<21> The terminal portion of the polyoxyalkylene polymer (A) has the general formula (2):
<16>一般式(1)に示す反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、
前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む、多液型硬化性組成物。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。)
<17>前記メルカプタン化合物(D)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.2~5.0重量部である、<16>に記載の多液型硬化性組成物。
<18>無機フィラーをさらに含む、<16>または<17>に記載の多液型硬化性組成物。
<19>前記無機フィラーの含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、50~180重量部である、<18>に記載の多液型硬化性組成物。
<20>前記アミノシラン(C)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.1~5.0重量部である、<16>~<19>のいずれかに記載の多液型硬化性組成物。
<21>前記ポリオキシアルキレン系重合体(A)の末端部位が、一般式(2): One aspect of the invention may include the following.
<16> A agent containing a polyoxyalkylene polymer (A) having a reactive silicon group represented by the general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D) , and a B agent containing an epoxy resin (E),
A multicomponent curable composition, wherein the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.)
<17> The content of the mercaptan compound (D) is 0.2 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to <16>.
<18> The multi-component curable composition according to <16> or <17>, further comprising an inorganic filler.
<19> In <18>, the content of the inorganic filler is 50 to 180 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). A multi-part curable composition as described.
<20> The content of the aminosilane (C) is 0.1 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to any one of <16> to <19>.
<21> The terminal portion of the polyoxyalkylene polymer (A) has the general formula (2):
<22>前記エポキシ樹脂硬化剤(B)が、第三級アミンを有するエポキシ樹脂硬化剤である、<16>~<21>のいずれかに記載の多液型硬化性組成物。
<23>前記アミノシラン(C)が、γ-アミノプロピルトリエトキシシラン、N-β-(アミノエチル)-γ-アミノプロピルトリメトキシシラン、およびN-β-(アミノエチル)-γ-アミノプロピルメチルジメトキシシランからなる群より選択される少なくとも1種である、<16>~<22>のいずれかに記載の多液型硬化性組成物。
<24>前記メルカプタン化合物(D)が、n-ドデシルメルカプタン、tert-ドデシルメルカプタン、ラウリルメルカプタン、トリメチロールプロパントリス(3-メルカプトプロピオネート)、ペンタエリスリトールテトラキス(3-メルカプトプロピオネート)、3-メルカプトプロピルトリメトキシシラン、3-メルカプトプロピルメチルジメトキシシラン、3-メルカプトプロピルクロロメチルジメトキシシラン、3-メルカプトプロピルメトキシメチルジメトキシシラン、(メルカプトメチル)ジメトキシメチルシラン、および(メルカプトメチル)トリメトキシシランからなる群より選択される少なくとも1種である、<16>~<23>のいずれかに記載の多液型硬化性組成物。
<25>前記エポキシ樹脂(E)が、エポキシ基を1分子中に少なくとも2個有するエポキシ樹脂である、<16>~<24>のいずれかに記載の多液型硬化性組成物。
<26>前記水酸基含有ポリオキシアルキレン系重合体(F)が、数平均分子量が300以上の水酸基を含有するポリオキシアルキレン系重合体である、<16>~<25>のいずれかに記載の多液型硬化性組成物。
<27><16>~<26>のいずれかに記載の多液型硬化性組成物を硬化させてなる、硬化物。
<28><27>に記載の硬化物を含む、防水材。
<29><16>~<26>のいずれかに記載の多液型硬化性組成物を建築物の下地に塗布する工程を含む、防水構造物の製造方法。
<30><16>~<26>のいずれかに記載の多液型硬化性組成物を硬化させてなる塗膜の上に、トップコートを塗付する工程を含む、防水構造物の製造方法。
<22> The multi-component curable composition according to any one of <16> to <21>, wherein the epoxy resin curing agent (B) is a tertiary amine-containing epoxy resin curing agent.
<23> The aminosilane (C) is γ-aminopropyltriethoxysilane, N-β-(aminoethyl)-γ-aminopropyltrimethoxysilane, and N-β-(aminoethyl)-γ-aminopropylmethyl The multi-component curable composition according to any one of <16> to <22>, which is at least one selected from the group consisting of dimethoxysilane.
<24> The mercaptan compound (D) is n-dodecylmercaptan, tert-dodecylmercaptan, laurylmercaptan, trimethylolpropane tris(3-mercaptopropionate), pentaerythritol tetrakis(3-mercaptopropionate), 3 - from mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropylchloromethyldimethoxysilane, 3-mercaptopropylmethoxymethyldimethoxysilane, (mercaptomethyl)dimethoxymethylsilane and (mercaptomethyl)trimethoxysilane The multi-component curable composition according to any one of <16> to <23>, which is at least one selected from the group consisting of:
<25> The multicomponent curable composition according to any one of <16> to <24>, wherein the epoxy resin (E) is an epoxy resin having at least two epoxy groups per molecule.
<26> The hydroxyl group-containing polyoxyalkylene polymer (F) according to any one of <16> to <25>, wherein the hydroxyl group-containing polyoxyalkylene polymer (F) has a number average molecular weight of 300 or more. A multi-component curable composition.
<27> A cured product obtained by curing the multi-component curable composition according to any one of <16> to <26>.
<28> A waterproof material comprising the cured product according to <27>.
<29> A method for producing a waterproof structure, comprising the step of applying the multi-component curable composition according to any one of <16> to <26> to the foundation of a building.
<30> A method for producing a waterproof structure, comprising the step of applying a topcoat onto a coating film formed by curing the multi-component curable composition according to any one of <16> to <26>. .
以下、本発明を実施例に基づいてより詳細に説明するが、本発明はこれら実施例に限定されるものではない。
Hereinafter, the present invention will be described in more detail based on examples, but the present invention is not limited to these examples.
〔材料〕
実施例および比較例で使用した材料は、以下の通りである。 〔material〕
Materials used in Examples and Comparative Examples are as follows.
実施例および比較例で使用した材料は、以下の通りである。 〔material〕
Materials used in Examples and Comparative Examples are as follows.
<ポリオキシアルキレン系重合体(A)>
下記の合成例に基づき製造した反応性ケイ素基を有するポリオキシアルキレン系重合体(A-1)、および(A-2)を使用した。 <Polyoxyalkylene polymer (A)>
Polyoxyalkylene polymers (A-1) and (A-2) having reactive silicon groups produced according to the following synthesis examples were used.
下記の合成例に基づき製造した反応性ケイ素基を有するポリオキシアルキレン系重合体(A-1)、および(A-2)を使用した。 <Polyoxyalkylene polymer (A)>
Polyoxyalkylene polymers (A-1) and (A-2) having reactive silicon groups produced according to the following synthesis examples were used.
<エポキシ樹脂硬化剤(B)>
2,4,6-トリス(ジメチルアミノメチル)フェノール:(日新化成株式会社製の「Ancamine K54」)
<アミノシラン(C)>
N-(2-アミノエチル)-3-アミノプロピルトリメトキシシラン:(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製の「Silquest A-1120」)
γ-アミノプロピルトリメトキシシラン:(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製の「Silquest A-1110」)
<メルカプタン化合物(D)>
(メルカプトシラン)
3-メルカプトプロピルトリメトキシシラン:(ダウ・ケミカル日本株式会社製の「Z6062」)
(メルカプタン)
n-ドデシルメルカプタン:(花王株式会社製の「チオカルコール20」)
トリメチロールプロパントリス(3-メルカプトプロピオネート):(SC有機化学株式会社製の「Lecad803」
<エポキシ樹脂(E)>
ビスフェノールA型エポキシ樹脂:(三菱ケミカル株式会社製の「Epikote 828」)
<水酸基含有ポリオキシアルキレン系重合体(F)>
ポリプロピレングリコール樹脂:(三井化学株式会社製の「アクトコール21-56」)
<無機フィラー>
炭酸カルシウム:(白石カルシウム株式会社製の「ホワイトンSB」)
<硬化触媒>
錫触媒:(日本化学産業株式会社製の「MSCAT-01」)
<低分子可塑剤>
フタル酸ジイソノイル:(株式会社ジェイ・プラス製の「DINP」)
エチルセルソルブ:(ナカライテスク株式会社製)
<脱水剤>
ビニルシラン:(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製の「A-171」)
<その他の成分>
(充填剤)
カーボンブラック(旭カーボン株式会社製の「アサヒサーマル」)
〔測定および評価方法〕
実施例および比較例における測定および評価を、以下の方法で行った。 <Epoxy resin curing agent (B)>
2,4,6-tris (dimethylaminomethyl) phenol: ("Ancamine K54" manufactured by Nisshin Kasei Co., Ltd.)
<Aminosilane (C)>
N-(2-aminoethyl)-3-aminopropyltrimethoxysilane: ("Silquest A-1120" manufactured by Momentive Performance Materials Japan LLC)
γ-Aminopropyltrimethoxysilane: ("Silquest A-1110" manufactured by Momentive Performance Materials Japan LLC)
<Mercaptan compound (D)>
(Mercaptosilane)
3-Mercaptopropyltrimethoxysilane: ("Z6062" manufactured by Dow Chemical Japan Co., Ltd.)
(Mercaptan)
n-dodecyl mercaptan: ("Thiocalcol 20" manufactured by Kao Corporation)
Trimethylolpropane tris (3-mercaptopropionate): ("Lecad803" manufactured by SC Organic Chemical Co., Ltd.
<Epoxy resin (E)>
Bisphenol A type epoxy resin: ("Epikote 828" manufactured by Mitsubishi Chemical Corporation)
<Hydroxyl Group-Containing Polyoxyalkylene Polymer (F)>
Polypropylene glycol resin: ("Actocol 21-56" manufactured by Mitsui Chemicals, Inc.)
<Inorganic filler>
Calcium carbonate: (“Whiten SB” manufactured by Shiraishi Calcium Co., Ltd.)
<Curing catalyst>
Tin catalyst: ("MSCAT-01" manufactured by Nippon Kagaku Sangyo Co., Ltd.)
<Low molecular weight plasticizer>
Diisonoyl phthalate: (“DINP” manufactured by J-Plus Co., Ltd.)
Ethyl Cellosolve: (manufactured by Nacalai Tesque Co., Ltd.)
<Dehydrating agent>
Vinylsilane: ("A-171" manufactured by Momentive Performance Materials Japan LLC)
<Other ingredients>
(filler)
Carbon black ("Asahi Thermal" manufactured by Asahi Carbon Co., Ltd.)
[Measurement and evaluation method]
Measurements and evaluations in Examples and Comparative Examples were carried out by the following methods.
2,4,6-トリス(ジメチルアミノメチル)フェノール:(日新化成株式会社製の「Ancamine K54」)
<アミノシラン(C)>
N-(2-アミノエチル)-3-アミノプロピルトリメトキシシラン:(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製の「Silquest A-1120」)
γ-アミノプロピルトリメトキシシラン:(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製の「Silquest A-1110」)
<メルカプタン化合物(D)>
(メルカプトシラン)
3-メルカプトプロピルトリメトキシシラン:(ダウ・ケミカル日本株式会社製の「Z6062」)
(メルカプタン)
n-ドデシルメルカプタン:(花王株式会社製の「チオカルコール20」)
トリメチロールプロパントリス(3-メルカプトプロピオネート):(SC有機化学株式会社製の「Lecad803」
<エポキシ樹脂(E)>
ビスフェノールA型エポキシ樹脂:(三菱ケミカル株式会社製の「Epikote 828」)
<水酸基含有ポリオキシアルキレン系重合体(F)>
ポリプロピレングリコール樹脂:(三井化学株式会社製の「アクトコール21-56」)
<無機フィラー>
炭酸カルシウム:(白石カルシウム株式会社製の「ホワイトンSB」)
<硬化触媒>
錫触媒:(日本化学産業株式会社製の「MSCAT-01」)
<低分子可塑剤>
フタル酸ジイソノイル:(株式会社ジェイ・プラス製の「DINP」)
エチルセルソルブ:(ナカライテスク株式会社製)
<脱水剤>
ビニルシラン:(モメンティブ・パフォーマンス・マテリアルズ・ジャパン合同会社製の「A-171」)
<その他の成分>
(充填剤)
カーボンブラック(旭カーボン株式会社製の「アサヒサーマル」)
〔測定および評価方法〕
実施例および比較例における測定および評価を、以下の方法で行った。 <Epoxy resin curing agent (B)>
2,4,6-tris (dimethylaminomethyl) phenol: ("Ancamine K54" manufactured by Nisshin Kasei Co., Ltd.)
<Aminosilane (C)>
N-(2-aminoethyl)-3-aminopropyltrimethoxysilane: ("Silquest A-1120" manufactured by Momentive Performance Materials Japan LLC)
γ-Aminopropyltrimethoxysilane: ("Silquest A-1110" manufactured by Momentive Performance Materials Japan LLC)
<Mercaptan compound (D)>
(Mercaptosilane)
3-Mercaptopropyltrimethoxysilane: ("Z6062" manufactured by Dow Chemical Japan Co., Ltd.)
(Mercaptan)
n-dodecyl mercaptan: ("Thiocalcol 20" manufactured by Kao Corporation)
Trimethylolpropane tris (3-mercaptopropionate): ("Lecad803" manufactured by SC Organic Chemical Co., Ltd.
<Epoxy resin (E)>
Bisphenol A type epoxy resin: ("Epikote 828" manufactured by Mitsubishi Chemical Corporation)
<Hydroxyl Group-Containing Polyoxyalkylene Polymer (F)>
Polypropylene glycol resin: ("Actocol 21-56" manufactured by Mitsui Chemicals, Inc.)
<Inorganic filler>
Calcium carbonate: (“Whiten SB” manufactured by Shiraishi Calcium Co., Ltd.)
<Curing catalyst>
Tin catalyst: ("MSCAT-01" manufactured by Nippon Kagaku Sangyo Co., Ltd.)
<Low molecular weight plasticizer>
Diisonoyl phthalate: (“DINP” manufactured by J-Plus Co., Ltd.)
Ethyl Cellosolve: (manufactured by Nacalai Tesque Co., Ltd.)
<Dehydrating agent>
Vinylsilane: ("A-171" manufactured by Momentive Performance Materials Japan LLC)
<Other ingredients>
(filler)
Carbon black ("Asahi Thermal" manufactured by Asahi Carbon Co., Ltd.)
[Measurement and evaluation method]
Measurements and evaluations in Examples and Comparative Examples were carried out by the following methods.
(引張特性)
(株)島津製オートグラフ(AGS-J)を用い、23℃50%RHで、引張強度試験を行った。JIS A 6021のウレタン系高伸長型(旧1類)に準じた評価を実施した。具体的には、引張強度および引張伸びは、JIS3号で打ち抜いた。引張測定は、500mm/minで行った。 (tensile properties)
A tensile strength test was performed at 23° C. and 50% RH using Autograph (AGS-J) manufactured by Shimadzu Corporation. Evaluation was carried out according to JIS A 6021 urethane-based high elongation type (former type 1). Specifically, tensile strength and tensile elongation were determined by punching according to JIS No.3. Tensile measurements were performed at 500 mm/min.
(株)島津製オートグラフ(AGS-J)を用い、23℃50%RHで、引張強度試験を行った。JIS A 6021のウレタン系高伸長型(旧1類)に準じた評価を実施した。具体的には、引張強度および引張伸びは、JIS3号で打ち抜いた。引張測定は、500mm/minで行った。 (tensile properties)
A tensile strength test was performed at 23° C. and 50% RH using Autograph (AGS-J) manufactured by Shimadzu Corporation. Evaluation was carried out according to JIS A 6021 urethane-based high elongation type (former type 1). Specifically, tensile strength and tensile elongation were determined by punching according to JIS No.3. Tensile measurements were performed at 500 mm/min.
(引き裂き強さ)
(株)島津製オートグラフ(AGS-J)を用い、23℃50%RHで、引張強度試験を行った。JIS A 6021のウレタン系高伸長型(旧1類)に準じた評価を実施した。引き裂き強さは、JIS K 6252に規定する切込みなしアングル形ダンベルで打抜いたものを使用した。 (Tear strength)
A tensile strength test was performed at 23° C. and 50% RH using Autograph (AGS-J) manufactured by Shimadzu Corporation. Evaluation was carried out according to JIS A 6021 urethane-based high elongation type (former type 1). The tear strength was obtained by punching with an angle-shaped dumbbell without cuts specified in JIS K 6252.
(株)島津製オートグラフ(AGS-J)を用い、23℃50%RHで、引張強度試験を行った。JIS A 6021のウレタン系高伸長型(旧1類)に準じた評価を実施した。引き裂き強さは、JIS K 6252に規定する切込みなしアングル形ダンベルで打抜いたものを使用した。 (Tear strength)
A tensile strength test was performed at 23° C. and 50% RH using Autograph (AGS-J) manufactured by Shimadzu Corporation. Evaluation was carried out according to JIS A 6021 urethane-based high elongation type (former type 1). The tear strength was obtained by punching with an angle-shaped dumbbell without cuts specified in JIS K 6252.
〔合成例1〕
ポリオキシアルキレン系重合体(A-1)の合成例を以下に示す。 [Synthesis Example 1]
A synthesis example of the polyoxyalkylene polymer (A-1) is shown below.
ポリオキシアルキレン系重合体(A-1)の合成例を以下に示す。 [Synthesis Example 1]
A synthesis example of the polyoxyalkylene polymer (A-1) is shown below.
数平均分子量が約4800のポリオキシプロピレングリコールを開始剤とし、亜鉛ヘキサシアノコバルテートグライム錯体触媒にてプロピレンオキサイドの重合を行い、両末端に水酸基を有する数平均分子量28,000(末端基換算分子量18000)、分子量分布Mw/Mn=1.21のポリオキシプロピレン(P-1)を得た。続いてこの水酸基末端ポリオキシプロピレン(P-1)の水酸基に対して1.0モル当量のナトリウムメトキシドを28%メタノール溶液として添加した。真空脱揮によりメタノールを留去した後、重合体(P-1)の水酸基に対して、1.0モル当量のアリルグリシジルエーテルを添加して130℃で2時間反応を行った。その後、0.28モル当量のナトリウムメトキシドのメタノール溶液を添加してメタノールを除去し、さらに1.79モル当量の塩化アリルを添加して末端の水酸基をアリル基に変換した。得られた未精製のポリオキシプロピレンをn-ヘキサンと、水を混合攪拌した後、遠心分離により水を除去し、得られたヘキサン溶液からヘキサンを減圧脱揮することでポリマー中の金属塩を除去した。以上により、末端に複数の炭素-炭素不飽和結合を有するポリオキシプロピレン(Q-1)を得た。重合体(Q-1)は1つの末端に炭素-炭素不飽和結合が平均2.0個導入されていることがわかった。
Polyoxypropylene glycol having a number average molecular weight of about 4,800 is used as an initiator, and propylene oxide is polymerized with a zinc hexacyanocobaltate glyme complex catalyst, and a number average molecular weight of 28,000 having hydroxyl groups at both ends (terminal group equivalent molecular weight of 18,000 ) to obtain polyoxypropylene (P-1) having a molecular weight distribution Mw/Mn of 1.21. Subsequently, a 28% methanol solution of sodium methoxide was added in an amount of 1.0 molar equivalent to the hydroxyl group of this hydroxyl-terminated polyoxypropylene (P-1). After methanol was distilled off by vacuum devolatilization, 1.0 molar equivalent of allyl glycidyl ether was added to the hydroxyl groups of polymer (P-1), and reaction was carried out at 130° C. for 2 hours. Thereafter, 0.28 molar equivalent of sodium methoxide in methanol was added to remove the methanol, and 1.79 molar equivalent of allyl chloride was added to convert the terminal hydroxyl group to an allyl group. The unpurified polyoxypropylene thus obtained was mixed with n-hexane and water, and then the water was removed by centrifugation. Removed. As a result, polyoxypropylene (Q-1) having a plurality of carbon-carbon unsaturated bonds at the ends was obtained. Polymer (Q-1) was found to have an average of 2.0 carbon-carbon unsaturated bonds introduced at one end.
得られた(Q-1)500gに対し白金ジビニルジシロキサン錯体溶液(白金換算で3重量%のイソプロパノール溶液)50μlを加え、撹拌しながらジメトキシメチルシラン9.6gをゆっくりと滴下した。その混合溶液を100℃で2時間反応させた後、未反応のジメトキシメチルシランを減圧下留去する事により、末端に複数のジメトキシメチルシリル基を有する数平均分子量28,500のポリオキシプロピレン(A-1)を得た。ポリオキシアルキレン系重合体(A-1)はジメトキシメチルシリル基を1つの末端に平均1.7個、一分子中に平均3.4個有することが分かった。
To 500 g of the obtained (Q-1) was added 50 μl of a platinum divinyldisiloxane complex solution (3% by weight isopropanol solution in terms of platinum), and 9.6 g of dimethoxymethylsilane was slowly added dropwise while stirring. After the mixed solution was reacted at 100° C. for 2 hours, unreacted dimethoxymethylsilane was distilled off under reduced pressure to obtain a polyoxypropylene having a number average molecular weight of 28,500 and having a plurality of terminal dimethoxymethylsilyl groups ( A-1) was obtained. It was found that the polyoxyalkylene polymer (A-1) had an average of 1.7 dimethoxymethylsilyl groups at one terminal and an average of 3.4 dimethoxymethylsilyl groups per molecule.
〔合成例2〕
ポリオキシアルキレン系重合体(A-2)の合成例を以下に示す。 [Synthesis Example 2]
A synthesis example of the polyoxyalkylene polymer (A-2) is shown below.
ポリオキシアルキレン系重合体(A-2)の合成例を以下に示す。 [Synthesis Example 2]
A synthesis example of the polyoxyalkylene polymer (A-2) is shown below.
ブタノールを開始剤とし、亜鉛ヘキサシアノコバルテートグライム錯体触媒にてプロピレンオキサイドの重合を行い、片方の末端に水酸基を有する数平均分子量7800(末端基換算分子量5000)、分子量分布Mw/Mn=1.48のポリオキシプロピレン(P-2)を得た。続いてこの水酸基末端ポリオキシプロピレン(P-2)の水酸基に対して1.2モル当量のナトリウムメトキシドを28%メタノール溶液として添加した。真空脱揮によりメタノールを留去した後、重合体(P-2)の水酸基に対して、2.0モル当量の塩化アリルを添加して末端の水酸基をアリル基に変換し、未反応の塩化アリルを減圧脱揮により除去した。得られた未精製のポリオキシプロピレンをn-ヘキサンと、水を混合攪拌した後、遠心分離により水を除去し、得られたヘキサン溶液からヘキサンを減圧脱揮することでポリマー中の金属塩を除去した。以上により、アリル基を片末端のみに有するポリオキシプロピレン(Q-2)を得た。得られた(Q-2)500gに対し白金ジビニルジシロキサン錯体(白金換算で3重量%の2-プロパノール溶液)50μlを加え、撹拌しながら、ジメトキシメチルシラン9.5gをゆっくりと滴下した。その混合溶液を100℃で2時間反応させた後、未反応のジメトキシメチルシランを減圧下留去する事により、片末端のみにジメトキシメチルシリル基を有するポリオキシプロピレン(A-2)を得た。ポリオキシアルキレン系重合体(A-2)はジメトキシメチルシリル基を片末端のみに平均0.8個有することが分かった。
Using butanol as an initiator, propylene oxide is polymerized with a zinc hexacyanocobaltate glyme complex catalyst, and the number average molecular weight having a hydroxyl group at one end is 7800 (molecular weight in terms of terminal group: 5000), molecular weight distribution Mw/Mn = 1.48. of polyoxypropylene (P-2) was obtained. Subsequently, sodium methoxide was added as a 28% methanol solution in an amount of 1.2 molar equivalents relative to the hydroxyl groups of this hydroxyl-terminated polyoxypropylene (P-2). After methanol is distilled off by vacuum devolatilization, 2.0 molar equivalents of allyl chloride are added to the hydroxyl groups of the polymer (P-2) to convert terminal hydroxyl groups to allyl groups, and unreacted chlorides are added. Allyl was removed by vacuum devolatilization. The unpurified polyoxypropylene thus obtained was mixed with n-hexane and water, and then the water was removed by centrifugation. Removed. As a result, polyoxypropylene (Q-2) having an allyl group only at one end was obtained. To 500 g of the obtained (Q-2) was added 50 μl of a platinum divinyldisiloxane complex (2-propanol solution of 3% by weight in terms of platinum), and 9.5 g of dimethoxymethylsilane was slowly added dropwise while stirring. After reacting the mixed solution at 100° C. for 2 hours, unreacted dimethoxymethylsilane was distilled off under reduced pressure to obtain polyoxypropylene (A-2) having a dimethoxymethylsilyl group only at one end. . It was found that the polyoxyalkylene polymer (A-2) had an average of 0.8 dimethoxymethylsilyl groups only at one end.
〔実施例1〕
反応性ケイ素基を有するポリオキシアルキレン系重合体(A)として(A-1)および(A-2)と、エポキシ樹脂硬化剤(B)としてAnkamine K54と、アミノシラン(C)としてA-1120と、メルカプタン化合物(D)としてZ6062と、脱水剤としてビニルシランA-171と、硬化触媒としてMSCAT-01とを、それぞれ表1に記載の量で添加し、手撹拌で2分間攪拌して、A液(A剤)を作製した。 [Example 1]
(A-1) and (A-2) as the polyoxyalkylene polymer (A) having a reactive silicon group, Ankamine K54 as the epoxy resin curing agent (B), and A-1120 as the aminosilane (C). , Z6062 as a mercaptan compound (D), vinylsilane A-171 as a dehydrating agent, and MSCAT-01 as a curing catalyst were added in the amounts shown in Table 1, respectively, and stirred by hand for 2 minutes to obtain solution A. (Agent A) was produced.
反応性ケイ素基を有するポリオキシアルキレン系重合体(A)として(A-1)および(A-2)と、エポキシ樹脂硬化剤(B)としてAnkamine K54と、アミノシラン(C)としてA-1120と、メルカプタン化合物(D)としてZ6062と、脱水剤としてビニルシランA-171と、硬化触媒としてMSCAT-01とを、それぞれ表1に記載の量で添加し、手撹拌で2分間攪拌して、A液(A剤)を作製した。 [Example 1]
(A-1) and (A-2) as the polyoxyalkylene polymer (A) having a reactive silicon group, Ankamine K54 as the epoxy resin curing agent (B), and A-1120 as the aminosilane (C). , Z6062 as a mercaptan compound (D), vinylsilane A-171 as a dehydrating agent, and MSCAT-01 as a curing catalyst were added in the amounts shown in Table 1, respectively, and stirred by hand for 2 minutes to obtain solution A. (Agent A) was produced.
エポキシ樹脂(E)としてEpikote 828と、水酸基含有ポリオキシアルキレン系重合体(F)としてアクトコール21-56と、無機フィラーとして炭酸カルシウム(ホワイトンSB)と、充填剤としてカーボンブラック(アサヒサーマル)とを、それぞれ表1に記載の量で添加し、手撹拌で2分間攪拌し、その後、セラミック3本ロールを3回通過させて、B液(B剤)を作製した。
Epikote 828 as the epoxy resin (E), Actcol 21-56 as the hydroxyl-containing polyoxyalkylene polymer (F), calcium carbonate (Whiten SB) as the inorganic filler, and carbon black (Asahi Thermal) as the filler. and were added in the respective amounts shown in Table 1, stirred by hand for 2 minutes, and then passed through three ceramic rolls three times to prepare liquid B (agent B).
次いで、表1に記載の量で、A液(A剤)、B液(B剤)およびC液(C剤)を順次ディスポーザブルのカップに添加し、手撹拌した。その後、スーパーミキサー(株式会社シンキー製、ARE-250)で混合および脱泡した。混合および脱泡は、具体的には、500rpmで20秒間、1500rpmで20秒間、2000rpmで40秒間撹拌した後、1500rpmで40秒間脱泡することにより行った。次いで、脱泡後の混合物を、型枠(テフロン(登録商標)シート+パッカー、厚さ2mm)に流し込んだ。
Then, in the amounts shown in Table 1, liquid A (agent A), liquid B (agent B), and liquid C (agent C) were sequentially added to a disposable cup and stirred by hand. After that, they were mixed and defoamed with a super mixer (ARE-250, manufactured by Thinky Co., Ltd.). Specifically, mixing and defoaming were performed by stirring at 500 rpm for 20 seconds, 1500 rpm for 20 seconds, and 2000 rpm for 40 seconds, followed by defoaming at 1500 rpm for 40 seconds. Then, the defoamed mixture was poured into a mold (Teflon (registered trademark) sheet + packer, thickness 2 mm).
これらの操作は、2時間以内に終了するように実行した。
These operations were completed within 2 hours.
その後、23℃50%RH条件下で7日間の硬化養生を行った。得られた硬化物について、引張特性および引き裂き強さを評価した。結果を表1に示す。
After that, hardening was performed for 7 days under conditions of 23°C and 50% RH. Tensile properties and tear strength were evaluated for the resulting cured product. Table 1 shows the results.
〔実施例2~16、比較例1~6〕
各成分の配合量を表1および表2に記載の通りに変更したこと以外は実施例1と同様の手順により、硬化性樹脂組成物および硬化物を作製した。得られた硬化物について、引張特性および引き裂き強さを評価した。結果を表1および表2に示す。
[Examples 2 to 16, Comparative Examples 1 to 6]
A curable resin composition and a cured product were prepared in the same manner as in Example 1, except that the amount of each component was changed as shown in Tables 1 and 2. Tensile properties and tear strength were evaluated for the resulting cured product. Results are shown in Tables 1 and 2.
各成分の配合量を表1および表2に記載の通りに変更したこと以外は実施例1と同様の手順により、硬化性樹脂組成物および硬化物を作製した。得られた硬化物について、引張特性および引き裂き強さを評価した。結果を表1および表2に示す。
A curable resin composition and a cured product were prepared in the same manner as in Example 1, except that the amount of each component was changed as shown in Tables 1 and 2. Tensile properties and tear strength were evaluated for the resulting cured product. Results are shown in Tables 1 and 2.
表1および表2より、実施例1~16の硬化性組成物は、硬化物とした際に、引張特性および引き裂き強さに優れることが示された。すなわち、本発明の一実施形態によれば、引張特性および引き裂き強さに優れる硬化物を提供し得る硬化性組成物を提供できることが示された。
Tables 1 and 2 show that the curable compositions of Examples 1 to 16 are excellent in tensile properties and tear strength when cured. That is, according to one embodiment of the present invention, it was shown that a curable composition capable of providing a cured product having excellent tensile properties and tear strength can be provided.
一方、表1より、比較例1~6の硬化性組成物では、引張特性および引き裂き強さが不良となっている。すなわち、硬化性組成物が本多液型硬化性組成物の構成を満たさない場合、引張特性および引き裂き強さが不良となることが示された。
On the other hand, from Table 1, the curable compositions of Comparative Examples 1 to 6 are poor in tensile properties and tear strength. That is, it was shown that when the curable composition does not satisfy the configuration of the present multicomponent curable composition, the tensile properties and tear strength are poor.
本多液型硬化性組成物は、引張特性および引き裂き強さに優れる硬化物を提供できるため、防水材等に好適に利用することができる。
Since this multi-component curable composition can provide a cured product having excellent tensile properties and tear strength, it can be suitably used for waterproof materials and the like.
Since this multi-component curable composition can provide a cured product having excellent tensile properties and tear strength, it can be suitably used for waterproof materials and the like.
Claims (15)
- 一般式(1)に示す反応性ケイ素基を有するポリオキシアルキレン系重合体(A)、エポキシ樹脂硬化剤(B)、アミノシラン(C)、およびメルカプタン化合物(D)を含むA剤と、エポキシ樹脂(E)を含むB剤と、を含み、
前記A剤および/または前記B剤に水酸基含有ポリオキシアルキレン系重合体(F)を含む、多液型硬化性組成物。
-Si(R)3-a(X)a (1)
(式中、Rは、それぞれ独立に、炭素原子数1~20の炭化水素基、または-OSi(R’)3(R’は、それぞれ独立に、炭素原子数1~20の炭化水素基である。)で示されるトリオルガノシロキシ基であり、Rとしての炭化水素基は、置換されていてもよく、かつ、ヘテロ含有基を有してもよい。Xは、それぞれ独立に、水酸基または加水分解性基である。aは、1~3の整数である。) A agent containing a polyoxyalkylene polymer (A) having a reactive silicon group represented by general formula (1), an epoxy resin curing agent (B), an aminosilane (C), and a mercaptan compound (D), and an epoxy resin and a B agent containing (E),
A multicomponent curable composition, wherein the A agent and/or the B agent contain a hydroxyl group-containing polyoxyalkylene polymer (F).
—Si(R) 3-a (X) a (1)
(In the formula, each R is independently a hydrocarbon group having 1 to 20 carbon atoms, or —OSi(R′) 3 (R′ is each independently a hydrocarbon group having 1 to 20 carbon atoms. The hydrocarbon group as R may be substituted and may have a hetero-containing group.X is each independently a hydroxyl group or a hydro is a degradable group. a is an integer of 1 to 3.) - 前記メルカプタン化合物(D)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.2~5.0重量部である、請求項1に記載の多液型硬化性組成物。 The content of the mercaptan compound (D) is 0.2 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to claim 1.
- 無機フィラーをさらに含む、請求項1または2に記載の多液型硬化性組成物。 The multi-component curable composition according to claim 1 or 2, further comprising an inorganic filler.
- 前記無機フィラーの含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、50~180重量部である、請求項3に記載の多液型硬化性組成物。 The content of the inorganic filler is 50 to 180 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E), Liquid curable composition.
- 前記アミノシラン(C)の含有量が、前記ポリオキシアルキレン系重合体(A)と前記エポキシ樹脂(E)との合計100重量部に対して、0.1~5.0重量部である、請求項1~4のいずれか1項に記載の多液型硬化性組成物。 The content of the aminosilane (C) is 0.1 to 5.0 parts by weight with respect to a total of 100 parts by weight of the polyoxyalkylene polymer (A) and the epoxy resin (E). The multi-component curable composition according to any one of items 1 to 4.
- 前記ポリオキシアルキレン系重合体(A)の末端部位が、一般式(2):
- 前記エポキシ樹脂硬化剤(B)が、第三級アミンを有するエポキシ樹脂硬化剤である、請求項1~6のいずれか1項に記載の多液型硬化性組成物。 The multicomponent curable composition according to any one of claims 1 to 6, wherein the epoxy resin curing agent (B) is an epoxy resin curing agent having a tertiary amine.
- 前記アミノシラン(C)が、γ-アミノプロピルトリエトキシシラン、N-β-(アミノエチル)-γ-アミノプロピルトリメトキシシラン、およびN-β-(アミノエチル)-γ-アミノプロピルメチルジメトキシシランからなる群より選択される少なくとも1種である、請求項1~7のいずれか1項に記載の多液型硬化性組成物。 the aminosilane (C) is selected from γ-aminopropyltriethoxysilane, N-β-(aminoethyl)-γ-aminopropyltrimethoxysilane, and N-β-(aminoethyl)-γ-aminopropylmethyldimethoxysilane; The multi-component curable composition according to any one of claims 1 to 7, which is at least one selected from the group consisting of:
- 前記メルカプタン化合物(D)が、n-ドデシルメルカプタン、tert-ドデシルメルカプタン、ラウリルメルカプタン、トリメチロールプロパントリス(3-メルカプトプロピオネート)、ペンタエリスリトールテトラキス(3-メルカプトプロピオネート)、3-メルカプトプロピルトリメトキシシラン、3-メルカプトプロピルメチルジメトキシシラン、3-メルカプトプロピルクロロメチルジメトキシシラン、3-メルカプトプロピルメトキシメチルジメトキシシラン、(メルカプトメチル)ジメトキシメチルシラン、および(メルカプトメチル)トリメトキシシランからなる群より選択される少なくとも1種である、請求項1~8のいずれか1項に記載の多液型硬化性組成物。 The mercaptan compound (D) is n-dodecyl mercaptan, tert-dodecyl mercaptan, lauryl mercaptan, trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakis (3-mercaptopropionate), 3-mercaptopropyl from the group consisting of trimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropylchloromethyldimethoxysilane, 3-mercaptopropylmethoxymethyldimethoxysilane, (mercaptomethyl)dimethoxymethylsilane, and (mercaptomethyl)trimethoxysilane The multi-component curable composition according to any one of claims 1 to 8, which is at least one selected.
- 前記エポキシ樹脂(E)が、エポキシ基を1分子中に少なくとも2個有するエポキシ樹脂である、請求項1~9のいずれか1項に記載の多液型硬化性組成物。 The multicomponent curable composition according to any one of claims 1 to 9, wherein the epoxy resin (E) is an epoxy resin having at least two epoxy groups per molecule.
- 前記水酸基含有ポリオキシアルキレン系重合体(F)が、数平均分子量が300以上の水酸基を含有するポリオキシアルキレン系重合体である、請求項1~10のいずれか1項に記載の多液型硬化性組成物。 The multicomponent type according to any one of claims 1 to 10, wherein the hydroxyl group-containing polyoxyalkylene polymer (F) is a hydroxyl group-containing polyoxyalkylene polymer having a number average molecular weight of 300 or more. Curable composition.
- 請求項1~11のいずれか1項に記載の多液型硬化性組成物を硬化させてなる、硬化物。 A cured product obtained by curing the multi-component curable composition according to any one of claims 1 to 11.
- 請求項12に記載の硬化物を含む、防水材。 A waterproof material containing the cured product according to claim 12.
- 請求項1~11のいずれか1項に記載の多液型硬化性組成物を建築物の下地に塗布する工程を含む、防水構造物の製造方法。 A method for producing a waterproof structure, comprising the step of applying the multi-component curable composition according to any one of claims 1 to 11 to the foundation of a building.
- 請求項1~11のいずれか1項に記載の多液型硬化性組成物を硬化させてなる塗膜の上に、トップコートを塗付する工程を含む、防水構造物の製造方法。 A method for producing a waterproof structure, comprising the step of applying a topcoat onto the coating film formed by curing the multi-component curable composition according to any one of claims 1 to 11.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2023540281A JPWO2023013487A1 (en) | 2021-08-05 | 2022-07-27 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021-129259 | 2021-08-05 | ||
| JP2021129259 | 2021-08-05 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2023013487A1 true WO2023013487A1 (en) | 2023-02-09 |
Family
ID=85154561
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2022/028867 WO2023013487A1 (en) | 2021-08-05 | 2022-07-27 | Multicomponent curable composition and utilization thereof |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JPWO2023013487A1 (en) |
| WO (1) | WO2023013487A1 (en) |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09279047A (en) * | 1996-04-11 | 1997-10-28 | Sekisui Chem Co Ltd | Room-temperature curing two-package composition |
| JP2002309077A (en) * | 2001-04-16 | 2002-10-23 | Kanegafuchi Chem Ind Co Ltd | 2-pack type curable composition |
| WO2006075482A1 (en) * | 2005-01-11 | 2006-07-20 | Kaneka Corporation | Curable composition |
| JP2011256284A (en) * | 2010-06-09 | 2011-12-22 | Kaneka Corp | Two-liquid type curable composition having high initial bonding strength |
| WO2019069866A1 (en) * | 2017-10-06 | 2019-04-11 | 株式会社カネカ | Curable composition |
| WO2020170908A1 (en) * | 2019-02-18 | 2020-08-27 | 株式会社カネカ | Curable composition |
| WO2021024817A1 (en) * | 2019-08-06 | 2021-02-11 | 株式会社カネカ | Curable composition |
| WO2021059972A1 (en) * | 2019-09-25 | 2021-04-01 | 株式会社カネカ | Curable composition |
-
2022
- 2022-07-27 WO PCT/JP2022/028867 patent/WO2023013487A1/en active Application Filing
- 2022-07-27 JP JP2023540281A patent/JPWO2023013487A1/ja active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09279047A (en) * | 1996-04-11 | 1997-10-28 | Sekisui Chem Co Ltd | Room-temperature curing two-package composition |
| JP2002309077A (en) * | 2001-04-16 | 2002-10-23 | Kanegafuchi Chem Ind Co Ltd | 2-pack type curable composition |
| WO2006075482A1 (en) * | 2005-01-11 | 2006-07-20 | Kaneka Corporation | Curable composition |
| JP2011256284A (en) * | 2010-06-09 | 2011-12-22 | Kaneka Corp | Two-liquid type curable composition having high initial bonding strength |
| WO2019069866A1 (en) * | 2017-10-06 | 2019-04-11 | 株式会社カネカ | Curable composition |
| WO2020170908A1 (en) * | 2019-02-18 | 2020-08-27 | 株式会社カネカ | Curable composition |
| WO2021024817A1 (en) * | 2019-08-06 | 2021-02-11 | 株式会社カネカ | Curable composition |
| WO2021059972A1 (en) * | 2019-09-25 | 2021-04-01 | 株式会社カネカ | Curable composition |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2023013487A1 (en) | 2023-02-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6141910B2 (en) | Curable composition | |
| JP6527589B2 (en) | Curable composition | |
| JP5666904B2 (en) | Room temperature curable composition and cured product thereof | |
| KR20130008530A (en) | Curable composition | |
| WO2004031300A1 (en) | Curable composition | |
| JP5883439B2 (en) | Curable composition | |
| WO2015080067A1 (en) | Curable composition | |
| JP6290785B2 (en) | Moisture curable composition | |
| JP2014234396A (en) | Room temperature-curable composition and cured product thereof | |
| WO2012036109A1 (en) | Curable composition | |
| WO2022181545A1 (en) | Manufacturing method for polymer comprising hydrolyzable silyl group, and polymer, curable composition, and cured product | |
| JP5110957B2 (en) | Curable composition | |
| JP6716459B2 (en) | Curable composition and cured product thereof | |
| JP2024009271A (en) | Polyoxyalkylene-based polymer and curable composition | |
| WO2023013487A1 (en) | Multicomponent curable composition and utilization thereof | |
| JP7356247B2 (en) | Curable composition and cured product | |
| WO2023068083A1 (en) | Multicomponent curable composition and utilization thereof | |
| JP5560795B2 (en) | Curable composition | |
| JP4398123B2 (en) | Curable composition | |
| WO2023171425A1 (en) | Polyoxyalkylene-based polymer mixture and curable composition | |
| JP2023149911A (en) | Hydrolyzable silyl group-containing polyoxyalkylene polymer and method for producing the same, and composition thereof | |
| WO2024225204A1 (en) | Ruthenium complex, method for producing silyl group-containing compound, silyl group-containing polymer mixture, curable composition, and cured product | |
| JP2022134561A (en) | Polyoxyalkylene-based polymer and curable composition | |
| JP2022143083A (en) | Method for producing organic polymer, organic polymer, curable composition, and cured product | |
| WO2022202132A1 (en) | Silane-crosslinkable-polymer-containing composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22852910 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2023540281 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 22852910 Country of ref document: EP Kind code of ref document: A1 |