WO2022211573A1 - 단백질 키나아제 저해 활성을 갖는 피리미딘 유도체 및 이를 포함하는 치료용 약학 조성물 - Google Patents
단백질 키나아제 저해 활성을 갖는 피리미딘 유도체 및 이를 포함하는 치료용 약학 조성물 Download PDFInfo
- Publication number
- WO2022211573A1 WO2022211573A1 PCT/KR2022/004702 KR2022004702W WO2022211573A1 WO 2022211573 A1 WO2022211573 A1 WO 2022211573A1 KR 2022004702 W KR2022004702 W KR 2022004702W WO 2022211573 A1 WO2022211573 A1 WO 2022211573A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- amino
- chloro
- compound
- phenyl
- pyrimidin
- Prior art date
Links
- 239000008194 pharmaceutical composition Substances 0.000 title claims abstract description 52
- 102000001253 Protein Kinase Human genes 0.000 title abstract description 11
- 108060006633 protein kinase Proteins 0.000 title abstract description 11
- 230000002401 inhibitory effect Effects 0.000 title abstract description 7
- 150000003230 pyrimidines Chemical class 0.000 title abstract description 3
- 230000001225 therapeutic effect Effects 0.000 title description 6
- 150000001875 compounds Chemical class 0.000 claims abstract description 1136
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 69
- 150000003839 salts Chemical class 0.000 claims abstract description 46
- 239000004480 active ingredient Substances 0.000 claims abstract description 22
- 208000020816 lung neoplasm Diseases 0.000 claims abstract description 15
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims abstract description 14
- 201000005202 lung cancer Diseases 0.000 claims abstract description 14
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 claims abstract 9
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 claims abstract 9
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 claims abstract 9
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 814
- 125000004527 pyrimidin-4-yl group Chemical group N1=CN=C(C=C1)* 0.000 claims description 593
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 513
- HNQIVZYLYMDVSB-UHFFFAOYSA-N methanesulfonimidic acid Chemical compound CS(N)(=O)=O HNQIVZYLYMDVSB-UHFFFAOYSA-N 0.000 claims description 469
- -1 pyrimidine derivative compound Chemical class 0.000 claims description 257
- 239000001257 hydrogen Substances 0.000 claims description 79
- 229910052739 hydrogen Inorganic materials 0.000 claims description 79
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 71
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 claims description 55
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 54
- 230000035772 mutation Effects 0.000 claims description 50
- 201000011510 cancer Diseases 0.000 claims description 46
- 238000000034 method Methods 0.000 claims description 43
- 125000000217 alkyl group Chemical group 0.000 claims description 40
- 125000004553 quinoxalin-5-yl group Chemical group N1=CC=NC2=C(C=CC=C12)* 0.000 claims description 40
- WQAWEUZTDVWTDB-UHFFFAOYSA-N dimethyl(oxo)phosphanium Chemical compound C[P+](C)=O WQAWEUZTDVWTDB-UHFFFAOYSA-N 0.000 claims description 37
- 125000001072 heteroaryl group Chemical group 0.000 claims description 37
- 125000000623 heterocyclic group Chemical group 0.000 claims description 34
- METKIMKYRPQLGS-UHFFFAOYSA-N atenolol Chemical compound CC(C)NCC(O)COC1=CC=C(CC(N)=O)C=C1 METKIMKYRPQLGS-UHFFFAOYSA-N 0.000 claims description 31
- 125000000041 C6-C10 aryl group Chemical group 0.000 claims description 28
- 229910052799 carbon Inorganic materials 0.000 claims description 28
- 125000005843 halogen group Chemical group 0.000 claims description 28
- 229910052757 nitrogen Inorganic materials 0.000 claims description 28
- 125000003277 amino group Chemical group 0.000 claims description 27
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims description 26
- RWTNPBWLLIMQHL-UHFFFAOYSA-N fexofenadine Chemical group C1=CC(C(C)(C(O)=O)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 RWTNPBWLLIMQHL-UHFFFAOYSA-N 0.000 claims description 25
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 24
- 238000011282 treatment Methods 0.000 claims description 24
- 229920006395 saturated elastomer Polymers 0.000 claims description 23
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 claims description 21
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 21
- QDVBKXJMLILLLB-UHFFFAOYSA-N 1,4'-bipiperidine Chemical compound C1CCCCN1C1CCNCC1 QDVBKXJMLILLLB-UHFFFAOYSA-N 0.000 claims description 20
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 20
- 125000004122 cyclic group Chemical group 0.000 claims description 19
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 claims description 17
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 15
- 239000000460 chlorine Substances 0.000 claims description 14
- 229910052760 oxygen Inorganic materials 0.000 claims description 14
- 229910052717 sulfur Inorganic materials 0.000 claims description 14
- 125000000101 thioether group Chemical group 0.000 claims description 14
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 13
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 claims description 12
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 12
- 238000012217 deletion Methods 0.000 claims description 12
- 230000037430 deletion Effects 0.000 claims description 12
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 claims description 12
- 230000002265 prevention Effects 0.000 claims description 12
- 125000001424 substituent group Chemical group 0.000 claims description 11
- 125000001174 sulfone group Chemical group 0.000 claims description 11
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 claims description 10
- 229910052736 halogen Inorganic materials 0.000 claims description 10
- 150000002367 halogens Chemical class 0.000 claims description 10
- 125000001841 imino group Chemical group [H]N=* 0.000 claims description 10
- UHOVQNZJYSORNB-UHFFFAOYSA-N monobenzene Natural products C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 claims description 10
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 9
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 claims description 9
- 125000003368 amide group Chemical group 0.000 claims description 9
- 125000002843 carboxylic acid group Chemical group 0.000 claims description 9
- 125000002560 nitrile group Chemical group 0.000 claims description 9
- 125000000565 sulfonamide group Chemical group 0.000 claims description 9
- XSQUKJJJFZCRTK-UHFFFAOYSA-N urea group Chemical group NC(=O)N XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 claims description 9
- 206010009944 Colon cancer Diseases 0.000 claims description 8
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 claims description 8
- 150000001721 carbon Chemical group 0.000 claims description 8
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 8
- 150000004820 halides Chemical group 0.000 claims description 8
- 150000002431 hydrogen Chemical class 0.000 claims description 8
- QRSQJUGVQOHLTE-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=CC=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=CC=C1)=C1F QRSQJUGVQOHLTE-UHFFFAOYSA-N 0.000 claims description 6
- RMUKFIDHLHDYHU-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C1=CC=CC(OC)=N1 Chemical compound CN(CC1)CCN1C(CC1)CCN1C1=CC=CC(OC)=N1 RMUKFIDHLHDYHU-UHFFFAOYSA-N 0.000 claims description 6
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 claims description 6
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 claims description 6
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 claims description 6
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims description 6
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims description 6
- 125000005842 heteroatom Chemical group 0.000 claims description 6
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 claims description 6
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 claims description 6
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 6
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 claims description 5
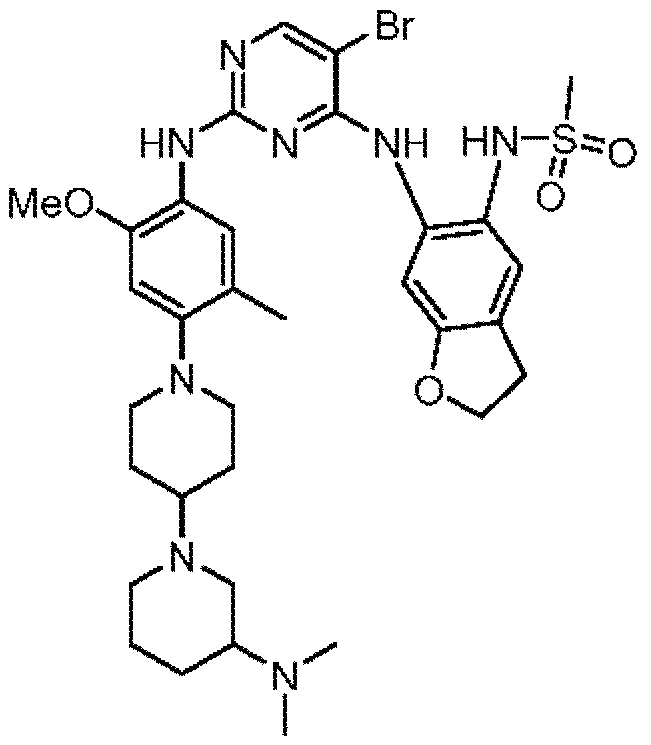
- GPQMBQNTNAAJBQ-UHFFFAOYSA-N CC(NC(C=CC=C1)=C1NC1=NC(NC(C=C2)=CC(F)=C2N(CC2)CCC2N2CCN(C)CC2)=NC=C1Cl)=O Chemical compound CC(NC(C=CC=C1)=C1NC1=NC(NC(C=C2)=CC(F)=C2N(CC2)CCC2N2CCN(C)CC2)=NC=C1Cl)=O GPQMBQNTNAAJBQ-UHFFFAOYSA-N 0.000 claims description 5
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 5
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 5
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 5
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 5
- 229910052794 bromium Inorganic materials 0.000 claims description 5
- 229910052801 chlorine Inorganic materials 0.000 claims description 5
- 206010017758 gastric cancer Diseases 0.000 claims description 5
- 201000007270 liver cancer Diseases 0.000 claims description 5
- 208000014018 liver neoplasm Diseases 0.000 claims description 5
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 claims description 5
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 claims description 5
- 201000011549 stomach cancer Diseases 0.000 claims description 5
- 201000002510 thyroid cancer Diseases 0.000 claims description 5
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical compound C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 claims description 4
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 claims description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 4
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 claims description 4
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 claims description 4
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 claims description 4
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 claims description 4
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 claims description 4
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 claims description 4
- 235000015165 citric acid Nutrition 0.000 claims description 4
- 125000001207 fluorophenyl group Chemical group 0.000 claims description 4
- 208000005017 glioblastoma Diseases 0.000 claims description 4
- 201000010536 head and neck cancer Diseases 0.000 claims description 4
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 4
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 claims description 4
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Natural products C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 claims description 4
- 150000007522 mineralic acids Chemical class 0.000 claims description 4
- 150000007524 organic acids Chemical class 0.000 claims description 4
- 229910052698 phosphorus Inorganic materials 0.000 claims description 4
- 239000011574 phosphorus Substances 0.000 claims description 4
- XSCHRSMBECNVNS-UHFFFAOYSA-N quinoxaline Chemical compound N1=CC=NC2=CC=CC=C21 XSCHRSMBECNVNS-UHFFFAOYSA-N 0.000 claims description 4
- DLFVBJFMPXGRIB-UHFFFAOYSA-N thioacetamide Natural products CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 claims description 4
- 125000006727 (C1-C6) alkenyl group Chemical group 0.000 claims description 3
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 3
- 125000006728 (C1-C6) alkynyl group Chemical group 0.000 claims description 3
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 claims description 3
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 claims description 3
- 239000005711 Benzoic acid Substances 0.000 claims description 3
- UPKXAIHEPNVHAP-UHFFFAOYSA-N CN1CCC(CC1)N2CCN(CC2)C3=NC(=CC=C3)OC Chemical compound CN1CCC(CC1)N2CCN(CC2)C3=NC(=CC=C3)OC UPKXAIHEPNVHAP-UHFFFAOYSA-N 0.000 claims description 3
- QSLXWTGOABYLPQ-UHFFFAOYSA-N CNS(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N3CCOCC3)N=C2)=NC=C1Cl)(=O)=O Chemical compound CNS(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N3CCOCC3)N=C2)=NC=C1Cl)(=O)=O QSLXWTGOABYLPQ-UHFFFAOYSA-N 0.000 claims description 3
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims description 3
- HKPJTERYTYBHLZ-UHFFFAOYSA-N FC(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N3CCOCC3)N=C2)=NC=C1Cl)(F)F Chemical compound FC(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N3CCOCC3)N=C2)=NC=C1Cl)(F)F HKPJTERYTYBHLZ-UHFFFAOYSA-N 0.000 claims description 3
- 208000034578 Multiple myelomas Diseases 0.000 claims description 3
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 claims description 3
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 3
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 claims description 3
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 claims description 3
- 208000003721 Triple Negative Breast Neoplasms Diseases 0.000 claims description 3
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 claims description 3
- 229910000147 aluminium phosphate Inorganic materials 0.000 claims description 3
- 235000010233 benzoic acid Nutrition 0.000 claims description 3
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 claims description 3
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims description 3
- 239000001530 fumaric acid Substances 0.000 claims description 3
- 239000004310 lactic acid Substances 0.000 claims description 3
- 235000014655 lactic acid Nutrition 0.000 claims description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 3
- 239000011976 maleic acid Substances 0.000 claims description 3
- 239000001630 malic acid Substances 0.000 claims description 3
- 235000011090 malic acid Nutrition 0.000 claims description 3
- 229940098779 methanesulfonic acid Drugs 0.000 claims description 3
- 229910017604 nitric acid Inorganic materials 0.000 claims description 3
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 3
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical compound C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 claims description 3
- 229960004889 salicylic acid Drugs 0.000 claims description 3
- SNOOUWRIMMFWNE-UHFFFAOYSA-M sodium;6-[(3,4,5-trimethoxybenzoyl)amino]hexanoate Chemical compound [Na+].COC1=CC(C(=O)NCCCCCC([O-])=O)=CC(OC)=C1OC SNOOUWRIMMFWNE-UHFFFAOYSA-M 0.000 claims description 3
- 229940124530 sulfonamide Drugs 0.000 claims description 3
- 235000002906 tartaric acid Nutrition 0.000 claims description 3
- 239000011975 tartaric acid Substances 0.000 claims description 3
- 150000003852 triazoles Chemical class 0.000 claims description 3
- 208000022679 triple-negative breast carcinoma Diseases 0.000 claims description 3
- UWYVPFMHMJIBHE-OWOJBTEDSA-N (e)-2-hydroxybut-2-enedioic acid Chemical compound OC(=O)\C=C(\O)C(O)=O UWYVPFMHMJIBHE-OWOJBTEDSA-N 0.000 claims description 2
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 claims description 2
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 claims description 2
- RTBFRGCFXZNCOE-UHFFFAOYSA-N 1-methylsulfonylpiperidin-4-one Chemical compound CS(=O)(=O)N1CCC(=O)CC1 RTBFRGCFXZNCOE-UHFFFAOYSA-N 0.000 claims description 2
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 claims description 2
- WLJVXDMOQOGPHL-PPJXEINESA-N 2-phenylacetic acid Chemical compound O[14C](=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-PPJXEINESA-N 0.000 claims description 2
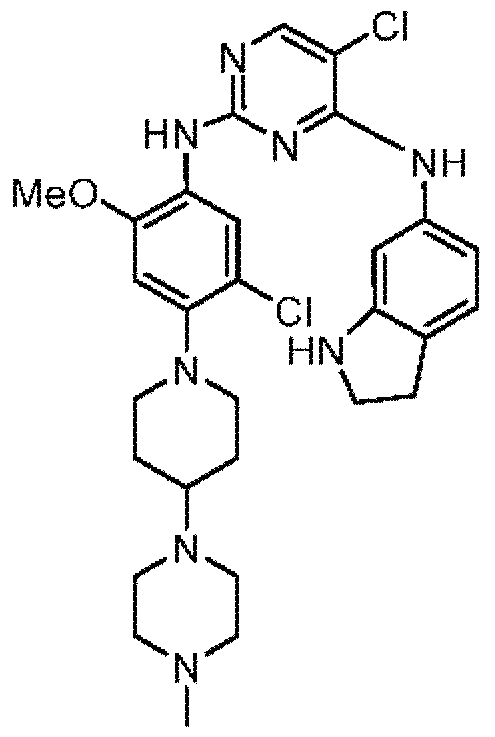
- XDZNKHQECGPOPS-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(C)CCN(C)C)=C1)=CC(NC(N=C2NC(C=C(C(Cl)=C3)Cl)=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N(C)CCN(C)C)=C1)=CC(NC(N=C2NC(C=C(C(Cl)=C3)Cl)=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC XDZNKHQECGPOPS-UHFFFAOYSA-N 0.000 claims description 2
- BRXJWODBSDXWIA-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC(C=C(C(F)(F)F)C=C3)=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC(C=C(C(F)(F)F)C=C3)=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC BRXJWODBSDXWIA-UHFFFAOYSA-N 0.000 claims description 2
- KNJQCCXMGTVWRL-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC(C=C(C=C3)OC)=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC(C=C(C=C3)OC)=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC KNJQCCXMGTVWRL-UHFFFAOYSA-N 0.000 claims description 2
- AGFBUXVYZHZQTF-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC(C=CC(F)=C3)=C3NS(C)(=O)=O)=NC=C2Br)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC(C=CC(F)=C3)=C3NS(C)(=O)=O)=NC=C2Br)=C1OC AGFBUXVYZHZQTF-UHFFFAOYSA-N 0.000 claims description 2
- LGDJWUKIZAVHRI-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3N(C)S(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3N(C)S(C)(=O)=O)=NC=C2Cl)=C1OC LGDJWUKIZAVHRI-UHFFFAOYSA-N 0.000 claims description 2
- BUKUOJWPYGKQRZ-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3NS(C)(=O)=O)=NC=C2Br)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3NS(C)(=O)=O)=NC=C2Br)=C1OC BUKUOJWPYGKQRZ-UHFFFAOYSA-N 0.000 claims description 2
- FQZKCZKTCBJWQW-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CC1)CCN1C1CC1)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC FQZKCZKTCBJWQW-UHFFFAOYSA-N 0.000 claims description 2
- KEWKOPFTLOKXKK-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CCC1)CC1N(C)C)=C1)=CC(NC(N=C2NC(C=C(C=C3)OC)=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CCC1)CC1N(C)C)=C1)=CC(NC(N=C2NC(C=C(C=C3)OC)=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC KEWKOPFTLOKXKK-UHFFFAOYSA-N 0.000 claims description 2
- REQFEYTZEHGVJA-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CCC1)CC1N(C)C)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3N(C)S(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CCC1)CC1N(C)C)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3N(C)S(C)(=O)=O)=NC=C2Cl)=C1OC REQFEYTZEHGVJA-UHFFFAOYSA-N 0.000 claims description 2
- ZPFTWQOAPIJYSM-UHFFFAOYSA-N CC(C(N(CC1)CCC1N(CCC1)CC1N(C)C)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N(CCC1)CC1N(C)C)=C1)=CC(NC(N=C2NC3=CC(OCC4)=C4C=C3NS(C)(=O)=O)=NC=C2Cl)=C1OC ZPFTWQOAPIJYSM-UHFFFAOYSA-N 0.000 claims description 2
- MSMANAZMDGXGQY-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2N(C(C=CC3=C4N=CS3)=C4NS(C)(=O)=O)S(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2N(C(C=CC3=C4N=CS3)=C4NS(C)(=O)=O)S(C)(=O)=O)=NC=C2Cl)=C1OC MSMANAZMDGXGQY-UHFFFAOYSA-N 0.000 claims description 2
- PIWXZHXXQRNWGE-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2N(C(C=CC=C3)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2N(C(C=CC=C3)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C2Cl)=C1OC PIWXZHXXQRNWGE-UHFFFAOYSA-N 0.000 claims description 2
- ABOZXRGIUSCOTI-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC(C=C(C=C3)Cl)=C3C3=CN=NN3)=NC=C2Br)=C1OC Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC(C=C(C=C3)Cl)=C3C3=CN=NN3)=NC=C2Br)=C1OC ABOZXRGIUSCOTI-UHFFFAOYSA-N 0.000 claims description 2
- TWTANZLORLPMRQ-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC(C=C(C=C3)Cl)=C3C3=CNN=N3)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC(C=C(C=C3)Cl)=C3C3=CNN=N3)=NC=C2Cl)=C1OC TWTANZLORLPMRQ-UHFFFAOYSA-N 0.000 claims description 2
- ZTNNUKCRXWOTAU-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=C(C(F)(F)F)C=CC=C3)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=C(C(F)(F)F)C=CC=C3)=NC=C2Cl)=C1OC ZTNNUKCRXWOTAU-UHFFFAOYSA-N 0.000 claims description 2
- JJIBRTPAJBIICQ-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=C(CS(C)(=O)=O)C=CC=C3)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=C(CS(C)(=O)=O)C=CC=C3)=NC=C2Cl)=C1OC JJIBRTPAJBIICQ-UHFFFAOYSA-N 0.000 claims description 2
- CWBPUCMGJSLKHE-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=CC=C(CCN4)C4=C3)=NC=C2Cl)=C1Cl Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=CC=C(CCN4)C4=C3)=NC=C2Cl)=C1Cl CWBPUCMGJSLKHE-UHFFFAOYSA-N 0.000 claims description 2
- MGWBRWPXOBNPQZ-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=CC=C(CCN4C)C4=C3)=NC=C2Cl)=C1Cl Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=CC=C(CCN4C)C4=C3)=NC=C2Cl)=C1Cl MGWBRWPXOBNPQZ-UHFFFAOYSA-N 0.000 claims description 2
- OAXWZXOEHCZREU-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=CC=C(CCN4C)C4=C3)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CC1)=C1)=CC(NC(N=C2NC3=CC=C(CCN4C)C4=C3)=NC=C2Cl)=C1OC OAXWZXOEHCZREU-UHFFFAOYSA-N 0.000 claims description 2
- DVDTUVSXORLUOE-UHFFFAOYSA-N CC(C(N(CC1)CCC1N1CCN(C)CCC1)=C1)=CC(NC(N=C2NC3=CC=C(CCN4C)C4=C3)=NC=C2Cl)=C1OC Chemical compound CC(C(N(CC1)CCC1N1CCN(C)CCC1)=C1)=CC(NC(N=C2NC3=CC=C(CCN4C)C4=C3)=NC=C2Cl)=C1OC DVDTUVSXORLUOE-UHFFFAOYSA-N 0.000 claims description 2
- NBAWYNZIHGONPJ-UHFFFAOYSA-N CC(C)(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br Chemical compound CC(C)(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br NBAWYNZIHGONPJ-UHFFFAOYSA-N 0.000 claims description 2
- QADMBSMAIZTWES-UHFFFAOYSA-N CC(C=C1NC(N=C2NC(C=CC=C3)=C3S(NC)(=O)=O)=NC=C2Cl)=C(N(CC2)CCC2N2CCN(C)CC2)N=C1OC Chemical compound CC(C=C1NC(N=C2NC(C=CC=C3)=C3S(NC)(=O)=O)=NC=C2Cl)=C(N(CC2)CCC2N2CCN(C)CC2)N=C1OC QADMBSMAIZTWES-UHFFFAOYSA-N 0.000 claims description 2
- SGZCREONNAPYFX-UHFFFAOYSA-N CC(N(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl)=O Chemical compound CC(N(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl)=O SGZCREONNAPYFX-UHFFFAOYSA-N 0.000 claims description 2
- LEBWMGUZZUIHFH-UHFFFAOYSA-N CC(NC(C=C(C(Cl)=C1)Cl)=C1NC1=NC(NC2=CC=C(N3CCOCC3)N=C2)=NC=C1Cl)=O Chemical compound CC(NC(C=C(C(Cl)=C1)Cl)=C1NC1=NC(NC2=CC=C(N3CCOCC3)N=C2)=NC=C1Cl)=O LEBWMGUZZUIHFH-UHFFFAOYSA-N 0.000 claims description 2
- IVSONFMXGLFFML-UHFFFAOYSA-N CCN(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC=C2)=C2S(NC)(=O)=O)=NC=C1Cl Chemical compound CCN(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC=C2)=C2S(NC)(=O)=O)=NC=C1Cl IVSONFMXGLFFML-UHFFFAOYSA-N 0.000 claims description 2
- ZIQOHYNAIZOPDD-UHFFFAOYSA-N CN(C)C(CC1)CN1C(CC1)CCN1C(C(Br)=C1)=CC(OC)=C1NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(C)C(CC1)CN1C(CC1)CCN1C(C(Br)=C1)=CC(OC)=C1NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl ZIQOHYNAIZOPDD-UHFFFAOYSA-N 0.000 claims description 2
- UOSNDMYMNXGDDA-UHFFFAOYSA-N CN(C)C(CC1)CN1C(N=C1OC)=CC=C1NC(N=C1N(C(C=CC=C2)=C2NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl Chemical compound CN(C)C(CC1)CN1C(N=C1OC)=CC=C1NC(N=C1N(C(C=CC=C2)=C2NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl UOSNDMYMNXGDDA-UHFFFAOYSA-N 0.000 claims description 2
- CCSBFWJEHAGGAV-UHFFFAOYSA-N CN(C)C(CC1)CN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(C)C(CC1)CN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl CCSBFWJEHAGGAV-UHFFFAOYSA-N 0.000 claims description 2
- JNZHCTBFCAGDQO-UHFFFAOYSA-N CN(C)C(CC1)CN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl Chemical compound CN(C)C(CC1)CN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl JNZHCTBFCAGDQO-UHFFFAOYSA-N 0.000 claims description 2
- JZQQARIRUVUPRT-UHFFFAOYSA-N CN(C)CCCN1N=CC(NC(N=C2NC(C=C(C=C3)Cl)=C3NS(C)(=O)=O)=NC=C2Cl)=C1 Chemical compound CN(C)CCCN1N=CC(NC(N=C2NC(C=C(C=C3)Cl)=C3NS(C)(=O)=O)=NC=C2Cl)=C1 JZQQARIRUVUPRT-UHFFFAOYSA-N 0.000 claims description 2
- KDEQYZKRDKPDDZ-UHFFFAOYSA-N CN(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=CC=C3N=CC=CC3=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=CC=C3N=CC=CC3=C2NS(C)(=O)=O)=NC=C1Cl KDEQYZKRDKPDDZ-UHFFFAOYSA-N 0.000 claims description 2
- PXXOZBVCHQZLEW-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C(Cl)=C1)=CC(OC)=C1NC(N=C1N(C(C=CC2=C3N=CS2)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C(Cl)=C1)=CC(OC)=C1NC(N=C1N(C(C=CC2=C3N=CS2)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl PXXOZBVCHQZLEW-UHFFFAOYSA-N 0.000 claims description 2
- XESPMJBKJASEKB-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C(Cl)=C1)=CC(OC)=C1NC(N=C1NC(C=C(C=C2)Cl)=C2C2=CN=NN2)=NC=C1Br Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C(Cl)=C1)=CC(OC)=C1NC(N=C1NC(C=C(C=C2)Cl)=C2C2=CN=NN2)=NC=C1Br XESPMJBKJASEKB-UHFFFAOYSA-N 0.000 claims description 2
- RRMPEYSQRWJPBQ-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C(Cl)=C1)=CC(OC)=C1NC(N=C1NC(C=C(C=C2)Cl)=C2C2=CN=NN2)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C(Cl)=C1)=CC(OC)=C1NC(N=C1NC(C=C(C=C2)Cl)=C2C2=CN=NN2)=NC=C1Cl RRMPEYSQRWJPBQ-UHFFFAOYSA-N 0.000 claims description 2
- OBWVXEGJYNOMHB-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C(Cl)=C1)=CC(OC)=C1NC(N=C1NC2=CC=C(CCN3)C3=C2)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C(Cl)=C1)=CC(OC)=C1NC(N=C1NC2=CC=C(CCN3)C3=C2)=NC=C1Cl OBWVXEGJYNOMHB-UHFFFAOYSA-N 0.000 claims description 2
- UWTSGUIJCDNWJD-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(Cl)=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(Cl)=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br UWTSGUIJCDNWJD-UHFFFAOYSA-N 0.000 claims description 2
- LJUUAHHVARWGIW-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(Cl)=C1NC(N=C1NC2=CC(OCC3)=C3C=C2CS(N)(=O)=O)=NC=C1Br Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(Cl)=C1NC(N=C1NC2=CC(OCC3)=C3C=C2CS(N)(=O)=O)=NC=C1Br LJUUAHHVARWGIW-UHFFFAOYSA-N 0.000 claims description 2
- DDWZIIQTHFAAMO-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(Cl)=C1NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(Cl)=C1NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl DDWZIIQTHFAAMO-UHFFFAOYSA-N 0.000 claims description 2
- QRZYJARPZQGCEL-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC(C=C(C=C2)OC)=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC(C=C(C=C2)OC)=C2NS(C)(=O)=O)=NC=C1Cl QRZYJARPZQGCEL-UHFFFAOYSA-N 0.000 claims description 2
- NVLGIHXFHDPOBT-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=C(C(F)(F)F)C=CC=C2)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=C(C(F)(F)F)C=CC=C2)=NC=C1Cl NVLGIHXFHDPOBT-UHFFFAOYSA-N 0.000 claims description 2
- QUIXCFVJARWZIF-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl QUIXCFVJARWZIF-UHFFFAOYSA-N 0.000 claims description 2
- OSWQLFISVQMAEG-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=CC=C(CCN3)C3=C2)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=CC=C(CCN3)C3=C2)=NC=C1Cl OSWQLFISVQMAEG-UHFFFAOYSA-N 0.000 claims description 2
- GEGCECOAVNNORL-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=C1)=CC(OC)=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2NS(C)(=O)=O)=NC=C1Cl GEGCECOAVNNORL-UHFFFAOYSA-N 0.000 claims description 2
- DQDRZBSTWSJHPV-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1N(C(C=CC2=C3N=CS2)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1N(C(C=CC2=C3N=CS2)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl)=C1)=C1F DQDRZBSTWSJHPV-UHFFFAOYSA-N 0.000 claims description 2
- DLFKQCWNUOMGNU-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1NC(C=CC=C2)=C2NS(C)(=O)=O)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1NC(C=CC=C2)=C2NS(C)(=O)=O)=NC=C1Cl)=C1)=C1F DLFKQCWNUOMGNU-UHFFFAOYSA-N 0.000 claims description 2
- ZIHPEQBZEUEGOB-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1NC(C=CC=C2)=C2S(NC2CC2)(=O)=O)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1NC(C=CC=C2)=C2S(NC2CC2)(=O)=O)=NC=C1Cl)=C1)=C1F ZIHPEQBZEUEGOB-UHFFFAOYSA-N 0.000 claims description 2
- GGPGDTCJAQWSRX-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1NC2=C(CS(C)(=O)=O)C=CC=C2)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1NC2=C(CS(C)(=O)=O)C=CC=C2)=NC=C1Cl)=C1)=C1F GGPGDTCJAQWSRX-UHFFFAOYSA-N 0.000 claims description 2
- FFGKFWOBINJRJP-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=CC(NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl)=C1)=C1F FFGKFWOBINJRJP-UHFFFAOYSA-N 0.000 claims description 2
- PZNXQLNWZWJAJV-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=CC1=C2)=NC1=CC=C2NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=CC1=C2)=NC1=CC=C2NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br PZNXQLNWZWJAJV-UHFFFAOYSA-N 0.000 claims description 2
- OHLMLQQJNRMKGW-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(C=CC1=C2)=NC1=CC=C2NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(C=CC1=C2)=NC1=CC=C2NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl OHLMLQQJNRMKGW-UHFFFAOYSA-N 0.000 claims description 2
- WDNKFJYLLHMAHI-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=C1)=CC=C1NC(N=C1NC(C=CC=C2)=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=C1)=CC=C1NC(N=C1NC(C=CC=C2)=C2NS(C)(=O)=O)=NC=C1Cl WDNKFJYLLHMAHI-UHFFFAOYSA-N 0.000 claims description 2
- MAKDHVIONOSOBT-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1N(C(C=CC2=C3N=CS2)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1N(C(C=CC2=C3N=CS2)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl MAKDHVIONOSOBT-UHFFFAOYSA-N 0.000 claims description 2
- AKZDKIAJGUKJSI-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1N(C(C=CC=C2)=C2NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1N(C(C=CC=C2)=C2NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl AKZDKIAJGUKJSI-UHFFFAOYSA-N 0.000 claims description 2
- NPOYUIBNASXXEV-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl NPOYUIBNASXXEV-UHFFFAOYSA-N 0.000 claims description 2
- LTUXMYOVDIKKMI-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC2=C(CS(C)(=O)=O)C=CC=C2)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC2=C(CS(C)(=O)=O)C=CC=C2)=NC=C1Cl LTUXMYOVDIKKMI-UHFFFAOYSA-N 0.000 claims description 2
- HRNNZUOBBLVCSV-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2NS(C)(=O)=O)=NC=C1Cl HRNNZUOBBLVCSV-UHFFFAOYSA-N 0.000 claims description 2
- GDQJIVJEAFYYGC-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl GDQJIVJEAFYYGC-UHFFFAOYSA-N 0.000 claims description 2
- FCYOFLGFEHUWGY-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=CC(NC(N=C1N(C(C=CC2=C3N=CS2)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=CC(NC(N=C1N(C(C=CC2=C3N=CS2)=C3NS(C)(=O)=O)S(C)(=O)=O)=NC=C1Cl)=C1)=C1F FCYOFLGFEHUWGY-UHFFFAOYSA-N 0.000 claims description 2
- GSHJZURXBKXZJA-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=CC(NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=CC(NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl)=C1)=C1F GSHJZURXBKXZJA-UHFFFAOYSA-N 0.000 claims description 2
- HOFBUMIVUPLXBM-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=CC(NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=CC(NC(N=C1NC2=CC(OCC3)=C3C=C2NS(C)(=O)=O)=NC=C1Cl)=C1)=C1F HOFBUMIVUPLXBM-UHFFFAOYSA-N 0.000 claims description 2
- HTJYAKUHKYUERH-UHFFFAOYSA-N CN(CC1)CCN1C(CC1)CCN1C(N=CC(NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl)=C1)=C1F Chemical compound CN(CC1)CCN1C(CC1)CCN1C(N=CC(NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl)=C1)=C1F HTJYAKUHKYUERH-UHFFFAOYSA-N 0.000 claims description 2
- ONAWBLIWTLUADC-UHFFFAOYSA-N CN(CCC1)CCC1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br Chemical compound CN(CCC1)CCC1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br ONAWBLIWTLUADC-UHFFFAOYSA-N 0.000 claims description 2
- ATCUTUUSOIFDOO-UHFFFAOYSA-N CN1C2=CC(NC3=NC(NC(C=C(C(N(CC4)CCC4N4CCN(C)CC4)=C4)Cl)=C4OC)=NC=C3Cl)=CC=C2CC1 Chemical compound CN1C2=CC(NC3=NC(NC(C=C(C(N(CC4)CCC4N4CCN(C)CC4)=C4)Cl)=C4OC)=NC=C3Cl)=CC=C2CC1 ATCUTUUSOIFDOO-UHFFFAOYSA-N 0.000 claims description 2
- UERIDSOCBXXNDU-UHFFFAOYSA-N CNS(C(C=CC=C1)=C1NC1=NC(NC(C=CC(N(CC2)CCC2N2CCN(C)CC2)=C2)=C2OC)=NC=C1Cl)(=O)=O Chemical compound CNS(C(C=CC=C1)=C1NC1=NC(NC(C=CC(N(CC2)CCC2N2CCN(C)CC2)=C2)=C2OC)=NC=C1Cl)(=O)=O UERIDSOCBXXNDU-UHFFFAOYSA-N 0.000 claims description 2
- ZVGLIODLPXICPZ-UHFFFAOYSA-N CNS(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N(CC3)CC3N(C)C)N=C2OC)=NC=C1Cl)(=O)=O Chemical compound CNS(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N(CC3)CC3N(C)C)N=C2OC)=NC=C1Cl)(=O)=O ZVGLIODLPXICPZ-UHFFFAOYSA-N 0.000 claims description 2
- BOCLNUCVVDSMOM-UHFFFAOYSA-N CNS(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N(CC3)CCC3N3CCN(C)CC3)N=C2)=NC=C1Cl)(=O)=O Chemical compound CNS(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N(CC3)CCC3N3CCN(C)CC3)N=C2)=NC=C1Cl)(=O)=O BOCLNUCVVDSMOM-UHFFFAOYSA-N 0.000 claims description 2
- DEWABPONBYIOQR-UHFFFAOYSA-N CNS(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N3CCN(CCO)CC3)N=C2OC)=NC=C1Cl)(=O)=O Chemical compound CNS(C(C=CC=C1)=C1NC1=NC(NC2=CC=C(N3CCN(CCO)CC3)N=C2OC)=NC=C1Cl)(=O)=O DEWABPONBYIOQR-UHFFFAOYSA-N 0.000 claims description 2
- NJQPRBHRPUCJEO-UHFFFAOYSA-N COC(N=C(C=C1)N(CC2)CCS2(=O)=O)=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl Chemical compound COC(N=C(C=C1)N(CC2)CCS2(=O)=O)=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl NJQPRBHRPUCJEO-UHFFFAOYSA-N 0.000 claims description 2
- PSKLQHHQEAGGOS-UHFFFAOYSA-N COC1=NC(N(CC2)CCC2N2CCOCC2)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br Chemical compound COC1=NC(N(CC2)CCC2N2CCOCC2)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br PSKLQHHQEAGGOS-UHFFFAOYSA-N 0.000 claims description 2
- FIYMCJOHNZJRPN-UHFFFAOYSA-N COC1=NC(N(CC2)CCC2N2CCOCC2)=CC=C1NC(N=C1NC2=CC=C3N=CC=CC3=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound COC1=NC(N(CC2)CCC2N2CCOCC2)=CC=C1NC(N=C1NC2=CC=C3N=CC=CC3=C2NS(C)(=O)=O)=NC=C1Cl FIYMCJOHNZJRPN-UHFFFAOYSA-N 0.000 claims description 2
- IDJWMEWNTABZKP-UHFFFAOYSA-N COC1=NC(N(CC2)CCC2O)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound COC1=NC(N(CC2)CCC2O)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl IDJWMEWNTABZKP-UHFFFAOYSA-N 0.000 claims description 2
- NMHIPYZBXRNJQB-UHFFFAOYSA-N COC1=NC(N(CC2)CCN2C2CC2)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br Chemical compound COC1=NC(N(CC2)CCN2C2CC2)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br NMHIPYZBXRNJQB-UHFFFAOYSA-N 0.000 claims description 2
- HBIXGLRYKVLZOO-UHFFFAOYSA-N COC1=NC(N(CC2)CCN2C2COC2)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound COC1=NC(N(CC2)CCN2C2COC2)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl HBIXGLRYKVLZOO-UHFFFAOYSA-N 0.000 claims description 2
- YCRLMVUPIQZMCH-UHFFFAOYSA-N COC1=NC(N2CCOCC2)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br Chemical compound COC1=NC(N2CCOCC2)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Br YCRLMVUPIQZMCH-UHFFFAOYSA-N 0.000 claims description 2
- OQQIJNPSLYAGRF-GASCZTMLSA-N C[C@H](C1)N(C)[C@@H](C)CN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound C[C@H](C1)N(C)[C@@H](C)CN1C(N=C1OC)=CC=C1NC(N=C1NC(C=CC(F)=C2)=C2NS(C)(=O)=O)=NC=C1Cl OQQIJNPSLYAGRF-GASCZTMLSA-N 0.000 claims description 2
- UJEUQCIJJMJPIR-IYBDPMFKSA-N C[C@H](C1)N(C)[C@@H](C)CN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2NS(C)(=O)=O)=NC=C1Cl Chemical compound C[C@H](C1)N(C)[C@@H](C)CN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2NS(C)(=O)=O)=NC=C1Cl UJEUQCIJJMJPIR-IYBDPMFKSA-N 0.000 claims description 2
- JHECWFWCPOJJRV-CALCHBBNSA-N C[C@H](C1)N(C)[C@@H](C)CN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl Chemical compound C[C@H](C1)N(C)[C@@H](C)CN1C(N=C1OC)=CC=C1NC(N=C1NC2=CC=C3N=CC=NC3=C2P(C)(C)=O)=NC=C1Cl JHECWFWCPOJJRV-CALCHBBNSA-N 0.000 claims description 2
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 claims description 2
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 claims description 2
- 235000021314 Palmitic acid Nutrition 0.000 claims description 2
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 claims description 2
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 claims description 2
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 claims description 2
- JFCQEDHGNNZCLN-UHFFFAOYSA-N anhydrous glutaric acid Natural products OC(=O)CCCC(O)=O JFCQEDHGNNZCLN-UHFFFAOYSA-N 0.000 claims description 2
- 235000010323 ascorbic acid Nutrition 0.000 claims description 2
- 239000011668 ascorbic acid Substances 0.000 claims description 2
- 229960005070 ascorbic acid Drugs 0.000 claims description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 claims description 2
- 229940092714 benzenesulfonic acid Drugs 0.000 claims description 2
- 235000013985 cinnamic acid Nutrition 0.000 claims description 2
- 229930016911 cinnamic acid Natural products 0.000 claims description 2
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 claims description 2
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 claims description 2
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 claims description 2
- 238000011321 prophylaxis Methods 0.000 claims description 2
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 claims description 2
- 229940107700 pyruvic acid Drugs 0.000 claims description 2
- 150000003536 tetrazoles Chemical class 0.000 claims description 2
- 229930192474 thiophene Natural products 0.000 claims description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 claims 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 claims 1
- 206010073478 Anaplastic large-cell lymphoma Diseases 0.000 claims 1
- 206010051066 Gastrointestinal stromal tumour Diseases 0.000 claims 1
- 208000032004 Large-Cell Anaplastic Lymphoma Diseases 0.000 claims 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 claims 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 claims 1
- 201000011243 gastrointestinal stromal tumor Diseases 0.000 claims 1
- 229960002510 mandelic acid Drugs 0.000 claims 1
- 230000002159 abnormal effect Effects 0.000 abstract description 10
- 230000010261 cell growth Effects 0.000 abstract description 6
- 239000003795 chemical substances by application Substances 0.000 abstract description 4
- 206010059866 Drug resistance Diseases 0.000 abstract 1
- 238000002360 preparation method Methods 0.000 description 345
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 292
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 114
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 111
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 96
- 230000002829 reductive effect Effects 0.000 description 91
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 87
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 64
- 238000006243 chemical reaction Methods 0.000 description 58
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 44
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 43
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 42
- 239000011541 reaction mixture Substances 0.000 description 42
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 39
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 39
- 239000000243 solution Substances 0.000 description 38
- 239000000203 mixture Substances 0.000 description 36
- 102000001301 EGF receptor Human genes 0.000 description 33
- 108060006698 EGF receptor Proteins 0.000 description 33
- 239000012044 organic layer Substances 0.000 description 32
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 31
- 238000004440 column chromatography Methods 0.000 description 27
- 239000012153 distilled water Substances 0.000 description 26
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 25
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 22
- 201000010099 disease Diseases 0.000 description 22
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 21
- MSQACBWWAIBWIC-UHFFFAOYSA-N hydron;piperazine;chloride Chemical compound Cl.C1CNCCN1 MSQACBWWAIBWIC-UHFFFAOYSA-N 0.000 description 21
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 21
- 235000017557 sodium bicarbonate Nutrition 0.000 description 21
- 238000003756 stirring Methods 0.000 description 21
- CKIFFDUKUXANNM-UHFFFAOYSA-N 1-chloro-5-methoxy-2-methyl-4-nitrobenzene Chemical compound COC1=CC(Cl)=C(C)C=C1[N+]([O-])=O CKIFFDUKUXANNM-UHFFFAOYSA-N 0.000 description 19
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 19
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 16
- 239000003814 drug Substances 0.000 description 15
- YAAWASYJIRZXSZ-UHFFFAOYSA-N pyrimidine-2,4-diamine Chemical compound NC1=CC=NC(N)=N1 YAAWASYJIRZXSZ-UHFFFAOYSA-N 0.000 description 13
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 12
- 230000005764 inhibitory process Effects 0.000 description 12
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 11
- 230000000694 effects Effects 0.000 description 11
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical class O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 11
- GIKMWFAAEIACRF-UHFFFAOYSA-N 2,4,5-trichloropyrimidine Chemical compound ClC1=NC=C(Cl)C(Cl)=N1 GIKMWFAAEIACRF-UHFFFAOYSA-N 0.000 description 10
- 238000005481 NMR spectroscopy Methods 0.000 description 10
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 10
- 125000003118 aryl group Chemical group 0.000 description 10
- 238000004587 chromatography analysis Methods 0.000 description 10
- 239000012312 sodium hydride Substances 0.000 description 10
- 229910000104 sodium hydride Inorganic materials 0.000 description 10
- 229940124597 therapeutic agent Drugs 0.000 description 10
- SIKXIUWKPGWBBF-UHFFFAOYSA-N 5-bromo-2,4-dichloropyrimidine Chemical compound ClC1=NC=C(Br)C(Cl)=N1 SIKXIUWKPGWBBF-UHFFFAOYSA-N 0.000 description 9
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 235000019270 ammonium chloride Nutrition 0.000 description 9
- 238000009472 formulation Methods 0.000 description 9
- 239000012453 solvate Substances 0.000 description 9
- 239000000126 substance Substances 0.000 description 9
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 8
- 239000012299 nitrogen atmosphere Substances 0.000 description 8
- 239000000546 pharmaceutical excipient Substances 0.000 description 8
- 230000009467 reduction Effects 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 7
- 229960003278 osimertinib Drugs 0.000 description 7
- DUYJMQONPNNFPI-UHFFFAOYSA-N osimertinib Chemical compound COC1=CC(N(C)CCN(C)C)=C(NC(=O)C=C)C=C1NC1=NC=CC(C=2C3=CC=CC=C3N(C)C=2)=N1 DUYJMQONPNNFPI-UHFFFAOYSA-N 0.000 description 7
- 239000003755 preservative agent Substances 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- 206010001233 Adenoma benign Diseases 0.000 description 6
- 241000124008 Mammalia Species 0.000 description 6
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 6
- 229930006000 Sucrose Natural products 0.000 description 6
- 239000003085 diluting agent Substances 0.000 description 6
- 150000004677 hydrates Chemical class 0.000 description 6
- QARBMVPHQWIHKH-UHFFFAOYSA-N methanesulfonyl chloride Chemical compound CS(Cl)(=O)=O QARBMVPHQWIHKH-UHFFFAOYSA-N 0.000 description 6
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 239000005720 sucrose Substances 0.000 description 6
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 6
- SRVXSISGYBMIHR-UHFFFAOYSA-N 3-[3-[3-(2-amino-2-oxoethyl)phenyl]-5-chlorophenyl]-3-(5-methyl-1,3-thiazol-2-yl)propanoic acid Chemical compound S1C(C)=CN=C1C(CC(O)=O)C1=CC(Cl)=CC(C=2C=C(CC(N)=O)C=CC=2)=C1 SRVXSISGYBMIHR-UHFFFAOYSA-N 0.000 description 5
- CYJRNFFLTBEQSQ-UHFFFAOYSA-N 8-(3-methyl-1-benzothiophen-5-yl)-N-(4-methylsulfonylpyridin-3-yl)quinoxalin-6-amine Chemical compound CS(=O)(=O)C1=C(C=NC=C1)NC=1C=C2N=CC=NC2=C(C=1)C=1C=CC2=C(C(=CS2)C)C=1 CYJRNFFLTBEQSQ-UHFFFAOYSA-N 0.000 description 5
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 5
- 208000017604 Hodgkin disease Diseases 0.000 description 5
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 5
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 5
- 206010025323 Lymphomas Diseases 0.000 description 5
- 239000012359 Methanesulfonyl chloride Substances 0.000 description 5
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 5
- 230000003213 activating effect Effects 0.000 description 5
- 239000002246 antineoplastic agent Substances 0.000 description 5
- 230000019522 cellular metabolic process Effects 0.000 description 5
- 238000002648 combination therapy Methods 0.000 description 5
- 239000003937 drug carrier Substances 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 5
- 239000008101 lactose Substances 0.000 description 5
- 239000002904 solvent Substances 0.000 description 5
- 125000006527 (C1-C5) alkyl group Chemical group 0.000 description 4
- 238000005160 1H NMR spectroscopy Methods 0.000 description 4
- 206010005003 Bladder cancer Diseases 0.000 description 4
- 208000003174 Brain Neoplasms Diseases 0.000 description 4
- 206010006187 Breast cancer Diseases 0.000 description 4
- 208000026310 Breast neoplasm Diseases 0.000 description 4
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 4
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 4
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 4
- 108010010803 Gelatin Proteins 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 208000008839 Kidney Neoplasms Diseases 0.000 description 4
- 229930195725 Mannitol Natural products 0.000 description 4
- 206010027476 Metastases Diseases 0.000 description 4
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 4
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 4
- 206010060862 Prostate cancer Diseases 0.000 description 4
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 4
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 4
- 206010038389 Renal cancer Diseases 0.000 description 4
- 206010039491 Sarcoma Diseases 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 4
- 125000003545 alkoxy group Chemical group 0.000 description 4
- 239000012062 aqueous buffer Substances 0.000 description 4
- 230000037396 body weight Effects 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 229940127089 cytotoxic agent Drugs 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 229940121647 egfr inhibitor Drugs 0.000 description 4
- 201000004101 esophageal cancer Diseases 0.000 description 4
- 239000008273 gelatin Substances 0.000 description 4
- 229920000159 gelatin Polymers 0.000 description 4
- 235000019322 gelatine Nutrition 0.000 description 4
- 235000011852 gelatine desserts Nutrition 0.000 description 4
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 201000010982 kidney cancer Diseases 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 235000019359 magnesium stearate Nutrition 0.000 description 4
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 4
- 239000000594 mannitol Substances 0.000 description 4
- 235000010355 mannitol Nutrition 0.000 description 4
- 201000001441 melanoma Diseases 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- 201000002528 pancreatic cancer Diseases 0.000 description 4
- 208000008443 pancreatic carcinoma Diseases 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 201000002314 small intestine cancer Diseases 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 239000000454 talc Substances 0.000 description 4
- 229910052623 talc Inorganic materials 0.000 description 4
- 235000012222 talc Nutrition 0.000 description 4
- 210000004881 tumor cell Anatomy 0.000 description 4
- 201000005112 urinary bladder cancer Diseases 0.000 description 4
- 239000000080 wetting agent Substances 0.000 description 4
- WNEODWDFDXWOLU-QHCPKHFHSA-N 3-[3-(hydroxymethyl)-4-[1-methyl-5-[[5-[(2s)-2-methyl-4-(oxetan-3-yl)piperazin-1-yl]pyridin-2-yl]amino]-6-oxopyridin-3-yl]pyridin-2-yl]-7,7-dimethyl-1,2,6,8-tetrahydrocyclopenta[3,4]pyrrolo[3,5-b]pyrazin-4-one Chemical compound C([C@@H](N(CC1)C=2C=NC(NC=3C(N(C)C=C(C=3)C=3C(=C(N4C(C5=CC=6CC(C)(C)CC=6N5CC4)=O)N=CC=3)CO)=O)=CC=2)C)N1C1COC1 WNEODWDFDXWOLU-QHCPKHFHSA-N 0.000 description 3
- 208000003200 Adenoma Diseases 0.000 description 3
- 206010005949 Bone cancer Diseases 0.000 description 3
- 208000018084 Bone neoplasm Diseases 0.000 description 3
- 208000007659 Fibroadenoma Diseases 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 208000000821 Parathyroid Neoplasms Diseases 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 206010046431 Urethral cancer Diseases 0.000 description 3
- 206010046458 Urethral neoplasms Diseases 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 125000003342 alkenyl group Chemical group 0.000 description 3
- 125000000304 alkynyl group Chemical group 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 3
- 201000003149 breast fibroadenoma Diseases 0.000 description 3
- BTANRVKWQNVYAZ-UHFFFAOYSA-N butan-2-ol Chemical compound CCC(C)O BTANRVKWQNVYAZ-UHFFFAOYSA-N 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 208000029742 colonic neoplasm Diseases 0.000 description 3
- 239000008121 dextrose Substances 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 3
- 230000014509 gene expression Effects 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 201000005787 hematologic cancer Diseases 0.000 description 3
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- 239000000314 lubricant Substances 0.000 description 3
- 208000026045 malignant tumor of parathyroid gland Diseases 0.000 description 3
- 208000030159 metabolic disease Diseases 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- RWXSHEPMWDRBNR-UHFFFAOYSA-N n-(2-amino-5-fluorophenyl)methanesulfonamide Chemical compound CS(=O)(=O)NC1=CC(F)=CC=C1N RWXSHEPMWDRBNR-UHFFFAOYSA-N 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 230000000069 prophylactic effect Effects 0.000 description 3
- 238000001959 radiotherapy Methods 0.000 description 3
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 3
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 3
- 238000009097 single-agent therapy Methods 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 239000006188 syrup Substances 0.000 description 3
- 235000020357 syrup Nutrition 0.000 description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- NXUBYBOIURHPJO-UHFFFAOYSA-N 2-chloro-6-methoxy-3-methyl-5-nitropyridine Chemical compound COc1nc(Cl)c(C)cc1[N+]([O-])=O NXUBYBOIURHPJO-UHFFFAOYSA-N 0.000 description 2
- YHKUFWFVZIIVDL-UHFFFAOYSA-N 3,5,6-trichloro-1,2,4-triazine Chemical compound ClC1=NN=C(Cl)C(Cl)=N1 YHKUFWFVZIIVDL-UHFFFAOYSA-N 0.000 description 2
- OVVBXVJFGCFEFK-UHFFFAOYSA-N 6-chloro-2-methoxy-3-nitropyridine Chemical compound COC1=NC(Cl)=CC=C1[N+]([O-])=O OVVBXVJFGCFEFK-UHFFFAOYSA-N 0.000 description 2
- BTYCNIIIWYDUOF-UHFFFAOYSA-N 6-nitro-2,3-dihydro-1-benzofuran-5-amine Chemical compound C1=C([N+]([O-])=O)C(N)=CC2=C1OCC2 BTYCNIIIWYDUOF-UHFFFAOYSA-N 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- BQTPDOMHZZVJEO-UHFFFAOYSA-N CC(C=C1)=C(N(CC2)CCC2N2CCN(C)CC2)N=C1OC Chemical compound CC(C=C1)=C(N(CC2)CCC2N2CCN(C)CC2)N=C1OC BQTPDOMHZZVJEO-UHFFFAOYSA-N 0.000 description 2
- BHGOQFFMHZHHCQ-UHFFFAOYSA-N CN(C(C=CC(Cl)=C1)=C1N)S(C)(=O)=O Chemical compound CN(C(C=CC(Cl)=C1)=C1N)S(C)(=O)=O BHGOQFFMHZHHCQ-UHFFFAOYSA-N 0.000 description 2
- RTBOEVLFYFWMNK-UHFFFAOYSA-N CS(NC(C=C(C(Cl)=C1)F)=C1N)(=O)=O Chemical compound CS(NC(C=C(C(Cl)=C1)F)=C1N)(=O)=O RTBOEVLFYFWMNK-UHFFFAOYSA-N 0.000 description 2
- 206010008342 Cervix carcinoma Diseases 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- 206010014733 Endometrial cancer Diseases 0.000 description 2
- 206010014759 Endometrial neoplasm Diseases 0.000 description 2
- 102000009024 Epidermal Growth Factor Human genes 0.000 description 2
- 101800003838 Epidermal growth factor Proteins 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- WTDHULULXKLSOZ-UHFFFAOYSA-N Hydroxylamine hydrochloride Chemical compound Cl.ON WTDHULULXKLSOZ-UHFFFAOYSA-N 0.000 description 2
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 208000003445 Mouth Neoplasms Diseases 0.000 description 2
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 2
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 2
- OLGIXZUEHWJIST-UHFFFAOYSA-N N-[2-[(2,5-dichloropyrimidin-4-yl)amino]phenyl]acetamide Chemical compound ClC1=NC=C(C(=N1)NC1=C(C=CC=C1)NC(C)=O)Cl OLGIXZUEHWJIST-UHFFFAOYSA-N 0.000 description 2
- IDRGFNPZDVBSSE-UHFFFAOYSA-N OCCN1CCN(CC1)c1ccc(Nc2ncc3cccc(-c4cccc(NC(=O)C=C)c4)c3n2)c(F)c1F Chemical compound OCCN1CCN(CC1)c1ccc(Nc2ncc3cccc(-c4cccc(NC(=O)C=C)c4)c3n2)c(F)c1F IDRGFNPZDVBSSE-UHFFFAOYSA-N 0.000 description 2
- 208000008589 Obesity Diseases 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 241000288906 Primates Species 0.000 description 2
- 108090000315 Protein Kinase C Proteins 0.000 description 2
- 102000003923 Protein Kinase C Human genes 0.000 description 2
- 208000015634 Rectal Neoplasms Diseases 0.000 description 2
- 208000000453 Skin Neoplasms Diseases 0.000 description 2
- 208000024313 Testicular Neoplasms Diseases 0.000 description 2
- 206010057644 Testis cancer Diseases 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 2
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 2
- 239000000370 acceptor Substances 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000008272 agar Substances 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 235000001014 amino acid Nutrition 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 229940041181 antineoplastic drug Drugs 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 235000010216 calcium carbonate Nutrition 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 2
- 230000032823 cell division Effects 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 201000010881 cervical cancer Diseases 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 239000002254 cytotoxic agent Substances 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 238000007865 diluting Methods 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 229940116977 epidermal growth factor Drugs 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 235000003599 food sweetener Nutrition 0.000 description 2
- 239000003205 fragrance Substances 0.000 description 2
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 2
- LEQAOMBKQFMDFZ-UHFFFAOYSA-N glyoxal Chemical compound O=CC=O LEQAOMBKQFMDFZ-UHFFFAOYSA-N 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 125000004404 heteroalkyl group Chemical group 0.000 description 2
- 150000002390 heteroarenes Chemical class 0.000 description 2
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 2
- 125000001041 indolyl group Chemical group 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 2
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 125000002950 monocyclic group Chemical group 0.000 description 2
- UNBRAXDPKYSNMN-UHFFFAOYSA-N n-(2-amino-4-chlorophenyl)methanesulfonamide Chemical compound CS(=O)(=O)NC1=CC=C(Cl)C=C1N UNBRAXDPKYSNMN-UHFFFAOYSA-N 0.000 description 2
- 102000037979 non-receptor tyrosine kinases Human genes 0.000 description 2
- 108091008046 non-receptor tyrosine kinases Proteins 0.000 description 2
- 235000020824 obesity Nutrition 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 150000007530 organic bases Chemical class 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- 239000003002 pH adjusting agent Substances 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- JYVLIDXNZAXMDK-UHFFFAOYSA-N pentan-2-ol Chemical compound CCCC(C)O JYVLIDXNZAXMDK-UHFFFAOYSA-N 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 2
- 125000003386 piperidinyl group Chemical group 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- 230000002335 preservative effect Effects 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 229960003415 propylparaben Drugs 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 125000000719 pyrrolidinyl group Chemical group 0.000 description 2
- 206010038038 rectal cancer Diseases 0.000 description 2
- 201000001275 rectum cancer Diseases 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 201000000849 skin cancer Diseases 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 239000003765 sweetening agent Substances 0.000 description 2
- 229940120982 tarceva Drugs 0.000 description 2
- 201000003120 testicular cancer Diseases 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- VCGRFBXVSFAGGA-UHFFFAOYSA-N (1,1-dioxo-1,4-thiazinan-4-yl)-[6-[[3-(4-fluorophenyl)-5-methyl-1,2-oxazol-4-yl]methoxy]pyridin-3-yl]methanone Chemical compound CC=1ON=C(C=2C=CC(F)=CC=2)C=1COC(N=C1)=CC=C1C(=O)N1CCS(=O)(=O)CC1 VCGRFBXVSFAGGA-UHFFFAOYSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- MAYZWDRUFKUGGP-VIFPVBQESA-N (3s)-1-[5-tert-butyl-3-[(1-methyltetrazol-5-yl)methyl]triazolo[4,5-d]pyrimidin-7-yl]pyrrolidin-3-ol Chemical compound CN1N=NN=C1CN1C2=NC(C(C)(C)C)=NC(N3C[C@@H](O)CC3)=C2N=N1 MAYZWDRUFKUGGP-VIFPVBQESA-N 0.000 description 1
- UKGJZDSUJSPAJL-YPUOHESYSA-N (e)-n-[(1r)-1-[3,5-difluoro-4-(methanesulfonamido)phenyl]ethyl]-3-[2-propyl-6-(trifluoromethyl)pyridin-3-yl]prop-2-enamide Chemical compound CCCC1=NC(C(F)(F)F)=CC=C1\C=C\C(=O)N[C@H](C)C1=CC(F)=C(NS(C)(=O)=O)C(F)=C1 UKGJZDSUJSPAJL-YPUOHESYSA-N 0.000 description 1
- ZGYIXVSQHOKQRZ-COIATFDQSA-N (e)-n-[4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-3-cyano-7-[(3s)-oxolan-3-yl]oxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide Chemical compound N#CC1=CN=C2C=C(O[C@@H]3COCC3)C(NC(=O)/C=C/CN(C)C)=CC2=C1NC(C=C1Cl)=CC=C1OCC1=CC=CC=N1 ZGYIXVSQHOKQRZ-COIATFDQSA-N 0.000 description 1
- MOWXJLUYGFNTAL-DEOSSOPVSA-N (s)-[2-chloro-4-fluoro-5-(7-morpholin-4-ylquinazolin-4-yl)phenyl]-(6-methoxypyridazin-3-yl)methanol Chemical compound N1=NC(OC)=CC=C1[C@@H](O)C1=CC(C=2C3=CC=C(C=C3N=CN=2)N2CCOCC2)=C(F)C=C1Cl MOWXJLUYGFNTAL-DEOSSOPVSA-N 0.000 description 1
- UGUHFDPGDQDVGX-UHFFFAOYSA-N 1,2,3-thiadiazole Chemical group C1=CSN=N1 UGUHFDPGDQDVGX-UHFFFAOYSA-N 0.000 description 1
- NOAVWRBHJBZYHD-UHFFFAOYSA-N 1,3-benzothiazole-4,5-diamine Chemical compound NC1=CC=C2SC=NC2=C1N NOAVWRBHJBZYHD-UHFFFAOYSA-N 0.000 description 1
- NDOVLWQBFFJETK-UHFFFAOYSA-N 1,4-thiazinane 1,1-dioxide Chemical compound O=S1(=O)CCNCC1 NDOVLWQBFFJETK-UHFFFAOYSA-N 0.000 description 1
- APWRZPQBPCAXFP-UHFFFAOYSA-N 1-(1-oxo-2H-isoquinolin-5-yl)-5-(trifluoromethyl)-N-[2-(trifluoromethyl)pyridin-4-yl]pyrazole-4-carboxamide Chemical compound O=C1NC=CC2=C(C=CC=C12)N1N=CC(=C1C(F)(F)F)C(=O)NC1=CC(=NC=C1)C(F)(F)F APWRZPQBPCAXFP-UHFFFAOYSA-N 0.000 description 1
- ABDDQTDRAHXHOC-QMMMGPOBSA-N 1-[(7s)-5,7-dihydro-4h-thieno[2,3-c]pyran-7-yl]-n-methylmethanamine Chemical compound CNC[C@@H]1OCCC2=C1SC=C2 ABDDQTDRAHXHOC-QMMMGPOBSA-N 0.000 description 1
- WGCYRFWNGRMRJA-UHFFFAOYSA-N 1-ethylpiperazine Chemical compound CCN1CCNCC1 WGCYRFWNGRMRJA-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- 125000001637 1-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C(*)=C([H])C([H])=C([H])C2=C1[H] 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- BTTNYQZNBZNDOR-UHFFFAOYSA-N 2,4-dichloropyrimidine Chemical compound ClC1=CC=NC(Cl)=N1 BTTNYQZNBZNDOR-UHFFFAOYSA-N 0.000 description 1
- GLDNZPLPEYLLNS-UHFFFAOYSA-N 2,5,6-trichloropyrimidin-4-amine Chemical compound NC1=NC(Cl)=NC(Cl)=C1Cl GLDNZPLPEYLLNS-UHFFFAOYSA-N 0.000 description 1
- PETWOVLVTBVMPD-UHFFFAOYSA-N 2,5-dichloro-N-[2-(methylsulfonylmethyl)phenyl]pyrimidin-4-amine Chemical compound CS(CC(C=CC=C1)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O PETWOVLVTBVMPD-UHFFFAOYSA-N 0.000 description 1
- SKLWXONCHDKFLJ-UHFFFAOYSA-N 2,5-dichloro-N-[2-(trifluoromethyl)phenyl]pyrimidin-4-amine Chemical compound FC(F)(F)C1=CC=CC=C1NC1=NC(Cl)=NC=C1Cl SKLWXONCHDKFLJ-UHFFFAOYSA-N 0.000 description 1
- VZSRBBMJRBPUNF-UHFFFAOYSA-N 2-(2,3-dihydro-1H-inden-2-ylamino)-N-[3-oxo-3-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propyl]pyrimidine-5-carboxamide Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)C(=O)NCCC(N1CC2=C(CC1)NN=N2)=O VZSRBBMJRBPUNF-UHFFFAOYSA-N 0.000 description 1
- FYOZRCYKUKGDHH-UHFFFAOYSA-N 2-(methylsulfonylmethyl)aniline Chemical compound CS(=O)(=O)CC1=CC=CC=C1N FYOZRCYKUKGDHH-UHFFFAOYSA-N 0.000 description 1
- VBLXCTYLWZJBKA-UHFFFAOYSA-N 2-(trifluoromethyl)aniline Chemical compound NC1=CC=CC=C1C(F)(F)F VBLXCTYLWZJBKA-UHFFFAOYSA-N 0.000 description 1
- CUBHDXYOAVGZNN-UHFFFAOYSA-N 2-[(2,5-dichloropyrimidin-4-yl)amino]-n-methylbenzenesulfonamide Chemical compound CNS(=O)(=O)C1=CC=CC=C1NC1=NC(Cl)=NC=C1Cl CUBHDXYOAVGZNN-UHFFFAOYSA-N 0.000 description 1
- OMLGXMQYBUUKRS-UHFFFAOYSA-N 2-amino-n-cyclopropylbenzenesulfonamide Chemical compound NC1=CC=CC=C1S(=O)(=O)NC1CC1 OMLGXMQYBUUKRS-UHFFFAOYSA-N 0.000 description 1
- SSEZSHJJNNLTQI-UHFFFAOYSA-N 2-amino-n-methylbenzenesulfonamide Chemical compound CNS(=O)(=O)C1=CC=CC=C1N SSEZSHJJNNLTQI-UHFFFAOYSA-N 0.000 description 1
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 description 1
- DLURHXYXQYMPLT-UHFFFAOYSA-N 2-nitro-p-toluidine Chemical compound CC1=CC=C(N)C([N+]([O-])=O)=C1 DLURHXYXQYMPLT-UHFFFAOYSA-N 0.000 description 1
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- HCDMJFOHIXMBOV-UHFFFAOYSA-N 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(morpholin-4-ylmethyl)-4,7-dihydropyrrolo[4,5]pyrido[1,2-d]pyrimidin-2-one Chemical compound C=1C2=C3N(CC)C(=O)N(C=4C(=C(OC)C=C(OC)C=4F)F)CC3=CN=C2NC=1CN1CCOCC1 HCDMJFOHIXMBOV-UHFFFAOYSA-N 0.000 description 1
- BYHQTRFJOGIQAO-GOSISDBHSA-N 3-(4-bromophenyl)-8-[(2R)-2-hydroxypropyl]-1-[(3-methoxyphenyl)methyl]-1,3,8-triazaspiro[4.5]decan-2-one Chemical compound C[C@H](CN1CCC2(CC1)CN(C(=O)N2CC3=CC(=CC=C3)OC)C4=CC=C(C=C4)Br)O BYHQTRFJOGIQAO-GOSISDBHSA-N 0.000 description 1
- YGYGASJNJTYNOL-CQSZACIVSA-N 3-[(4r)-2,2-dimethyl-1,1-dioxothian-4-yl]-5-(4-fluorophenyl)-1h-indole-7-carboxamide Chemical compound C1CS(=O)(=O)C(C)(C)C[C@@H]1C1=CNC2=C(C(N)=O)C=C(C=3C=CC(F)=CC=3)C=C12 YGYGASJNJTYNOL-CQSZACIVSA-N 0.000 description 1
- FSGTULQLEVAYRS-UHFFFAOYSA-N 4,5-dichloro-2-nitroaniline Chemical compound NC1=CC(Cl)=C(Cl)C=C1[N+]([O-])=O FSGTULQLEVAYRS-UHFFFAOYSA-N 0.000 description 1
- XJPZKYIHCLDXST-UHFFFAOYSA-N 4,6-dichloropyrimidine Chemical compound ClC1=CC(Cl)=NC=N1 XJPZKYIHCLDXST-UHFFFAOYSA-N 0.000 description 1
- VJPPLCNBDLZIFG-ZDUSSCGKSA-N 4-[(3S)-3-(but-2-ynoylamino)piperidin-1-yl]-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide Chemical compound C(C#CC)(=O)N[C@@H]1CN(CCC1)C1=C2C(=C(NC2=C(C=C1F)C(=O)N)C)C VJPPLCNBDLZIFG-ZDUSSCGKSA-N 0.000 description 1
- YFCIFWOJYYFDQP-PTWZRHHISA-N 4-[3-amino-6-[(1S,3S,4S)-3-fluoro-4-hydroxycyclohexyl]pyrazin-2-yl]-N-[(1S)-1-(3-bromo-5-fluorophenyl)-2-(methylamino)ethyl]-2-fluorobenzamide Chemical compound CNC[C@@H](NC(=O)c1ccc(cc1F)-c1nc(cnc1N)[C@H]1CC[C@H](O)[C@@H](F)C1)c1cc(F)cc(Br)c1 YFCIFWOJYYFDQP-PTWZRHHISA-N 0.000 description 1
- XYWIPYBIIRTJMM-IBGZPJMESA-N 4-[[(2S)-2-[4-[5-chloro-2-[4-(trifluoromethyl)triazol-1-yl]phenyl]-5-methoxy-2-oxopyridin-1-yl]butanoyl]amino]-2-fluorobenzamide Chemical compound CC[C@H](N1C=C(OC)C(=CC1=O)C1=C(C=CC(Cl)=C1)N1C=C(N=N1)C(F)(F)F)C(=O)NC1=CC(F)=C(C=C1)C(N)=O XYWIPYBIIRTJMM-IBGZPJMESA-N 0.000 description 1
- KVCQTKNUUQOELD-UHFFFAOYSA-N 4-amino-n-[1-(3-chloro-2-fluoroanilino)-6-methylisoquinolin-5-yl]thieno[3,2-d]pyrimidine-7-carboxamide Chemical compound N=1C=CC2=C(NC(=O)C=3C4=NC=NC(N)=C4SC=3)C(C)=CC=C2C=1NC1=CC=CC(Cl)=C1F KVCQTKNUUQOELD-UHFFFAOYSA-N 0.000 description 1
- PBGKNXWGYQPUJK-UHFFFAOYSA-N 4-chloro-2-nitroaniline Chemical compound NC1=CC=C(Cl)C=C1[N+]([O-])=O PBGKNXWGYQPUJK-UHFFFAOYSA-N 0.000 description 1
- QFMJFXFXQAFGBO-UHFFFAOYSA-N 4-methoxy-2-nitroaniline Chemical compound COC1=CC=C(N)C([N+]([O-])=O)=C1 QFMJFXFXQAFGBO-UHFFFAOYSA-N 0.000 description 1
- RAUWPNXIALNKQM-UHFFFAOYSA-N 4-nitro-1,2-phenylenediamine Chemical compound NC1=CC=C([N+]([O-])=O)C=C1N RAUWPNXIALNKQM-UHFFFAOYSA-N 0.000 description 1
- VIAMARGNYJIAPP-UHFFFAOYSA-N 4-nitro-1,3-benzothiazol-5-amine Chemical compound NC1=CC=C2SC=NC2=C1[N+]([O-])=O VIAMARGNYJIAPP-UHFFFAOYSA-N 0.000 description 1
- 125000001054 5 membered carbocyclic group Chemical group 0.000 description 1
- IRPVABHDSJVBNZ-RTHVDDQRSA-N 5-[1-(cyclopropylmethyl)-5-[(1R,5S)-3-(oxetan-3-yl)-3-azabicyclo[3.1.0]hexan-6-yl]pyrazol-3-yl]-3-(trifluoromethyl)pyridin-2-amine Chemical compound C1=C(C(F)(F)F)C(N)=NC=C1C1=NN(CC2CC2)C(C2[C@@H]3CN(C[C@@H]32)C2COC2)=C1 IRPVABHDSJVBNZ-RTHVDDQRSA-N 0.000 description 1
- GNWQNMHAPAJNPL-UHFFFAOYSA-N 5-[2-(methylsulfonylmethyl)phenyl]pyrimidine-2,4-diamine Chemical compound CS(=O)(=O)CC1=CC=CC=C1C1=CN=C(N)N=C1N GNWQNMHAPAJNPL-UHFFFAOYSA-N 0.000 description 1
- FEOMAFDDLHSVMO-UHFFFAOYSA-N 5-chloro-2-iodoaniline Chemical compound NC1=CC(Cl)=CC=C1I FEOMAFDDLHSVMO-UHFFFAOYSA-N 0.000 description 1
- HFPASWSTLSWIEI-UHFFFAOYSA-N 5-dimethylphosphorylquinoxalin-6-amine Chemical compound P(=O)(C)(C)C1=C(N)C=CC2=NC=CN=C12 HFPASWSTLSWIEI-UHFFFAOYSA-N 0.000 description 1
- KHNROOYMVZFPJF-UHFFFAOYSA-N 5-iodoquinoxalin-6-amine Chemical compound N1=CC=NC2=C(I)C(N)=CC=C21 KHNROOYMVZFPJF-UHFFFAOYSA-N 0.000 description 1
- 125000004008 6 membered carbocyclic group Chemical group 0.000 description 1
- KCBWAFJCKVKYHO-UHFFFAOYSA-N 6-(4-cyclopropyl-6-methoxypyrimidin-5-yl)-1-[[4-[1-propan-2-yl-4-(trifluoromethyl)imidazol-2-yl]phenyl]methyl]pyrazolo[3,4-d]pyrimidine Chemical compound C1(CC1)C1=NC=NC(=C1C1=NC=C2C(=N1)N(N=C2)CC1=CC=C(C=C1)C=1N(C=C(N=1)C(F)(F)F)C(C)C)OC KCBWAFJCKVKYHO-UHFFFAOYSA-N 0.000 description 1
- RJAULGAJRJUBOV-UHFFFAOYSA-N 6-(4-ethylpiperazin-1-yl)-2-methoxypyridin-3-amine Chemical compound C1CN(CC)CCN1C1=CC=C(N)C(OC)=N1 RJAULGAJRJUBOV-UHFFFAOYSA-N 0.000 description 1
- TYBYHEXFKFLRFT-UHFFFAOYSA-N 6-nitroquinolin-5-amine Chemical compound C1=CC=C2C(N)=C([N+]([O-])=O)C=CC2=N1 TYBYHEXFKFLRFT-UHFFFAOYSA-N 0.000 description 1
- CNCRRABYYAOEEV-UHFFFAOYSA-N 6-nitroquinoxalin-5-amine Chemical compound C1=CN=C2C(N)=C([N+]([O-])=O)C=CC2=N1 CNCRRABYYAOEEV-UHFFFAOYSA-N 0.000 description 1
- YLKFDRWBZAALPN-UHFFFAOYSA-N 6-nitroquinoxaline Chemical compound N1=CC=NC2=CC([N+](=O)[O-])=CC=C21 YLKFDRWBZAALPN-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- ZRPZPNYZFSJUPA-UHFFFAOYSA-N ARS-1620 Chemical compound Oc1cccc(F)c1-c1c(Cl)cc2c(ncnc2c1F)N1CCN(CC1)C(=O)C=C ZRPZPNYZFSJUPA-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 1
- ULXXDDBFHOBEHA-ONEGZZNKSA-N Afatinib Chemical compound N1=CN=C2C=C(OC3COCC3)C(NC(=O)/C=C/CN(C)C)=CC2=C1NC1=CC=C(F)C(Cl)=C1 ULXXDDBFHOBEHA-ONEGZZNKSA-N 0.000 description 1
- BMFMQGXDDJALKQ-BYPYZUCNSA-N Argininic acid Chemical compound NC(N)=NCCC[C@H](O)C(O)=O BMFMQGXDDJALKQ-BYPYZUCNSA-N 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 206010004593 Bile duct cancer Diseases 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- XRARLZWEGBIJBZ-UHFFFAOYSA-N C1=CC=CC2[N+](=O)ON=C21 Chemical compound C1=CC=CC2[N+](=O)ON=C21 XRARLZWEGBIJBZ-UHFFFAOYSA-N 0.000 description 1
- RFZNDJOGEKCGSS-UHFFFAOYSA-N CC(C)C(C=C1)=CC(N)=C1NS(C)(=O)=O Chemical compound CC(C)C(C=C1)=CC(N)=C1NS(C)(=O)=O RFZNDJOGEKCGSS-UHFFFAOYSA-N 0.000 description 1
- OAZWJZKLSJOXJZ-UHFFFAOYSA-N CC(C)C(C=C1)=CC(NC2=NC(Cl)=NC=C2Cl)=C1NS(C)(=O)=O Chemical compound CC(C)C(C=C1)=CC(NC2=NC(Cl)=NC=C2Cl)=C1NS(C)(=O)=O OAZWJZKLSJOXJZ-UHFFFAOYSA-N 0.000 description 1
- NVJVNRPAHGVUJQ-UHFFFAOYSA-N CC(C=C1)=CC(NC2=NC(Cl)=NC=C2Br)=C1NS(C)(=O)=O Chemical compound CC(C=C1)=CC(NC2=NC(Cl)=NC=C2Br)=C1NS(C)(=O)=O NVJVNRPAHGVUJQ-UHFFFAOYSA-N 0.000 description 1
- KORUXMIOYVURDE-UHFFFAOYSA-N CC(N(C)C(C=C(C(Cl)=C1)Cl)=C1N(C)C1=NC(Cl)=NC=C1Cl)=O Chemical compound CC(N(C)C(C=C(C(Cl)=C1)Cl)=C1N(C)C1=NC(Cl)=NC=C1Cl)=O KORUXMIOYVURDE-UHFFFAOYSA-N 0.000 description 1
- JPKHMPCCGGBYHP-UHFFFAOYSA-N CC(NC(C=CC(Cl)=C1)=C1NC1=NC(Cl)=NC=C1Cl)=O Chemical compound CC(NC(C=CC(Cl)=C1)=C1NC1=NC(Cl)=NC=C1Cl)=O JPKHMPCCGGBYHP-UHFFFAOYSA-N 0.000 description 1
- JNDUYOMSSSKESF-UHFFFAOYSA-N CN(C(C(N)=C1)=CC2=C1OCC2)S(=O)=O Chemical compound CN(C(C(N)=C1)=CC2=C1OCC2)S(=O)=O JNDUYOMSSSKESF-UHFFFAOYSA-N 0.000 description 1
- UXBRUHRIXNAIAG-UHFFFAOYSA-N CN(C(C(NC1=NC(Cl)=NC=C1Br)=C1)=CC2=C1OCC2)S(C)(=O)=O Chemical compound CN(C(C(NC1=NC(Cl)=NC=C1Br)=C1)=CC2=C1OCC2)S(C)(=O)=O UXBRUHRIXNAIAG-UHFFFAOYSA-N 0.000 description 1
- VEKVZDQUXYKLMP-UHFFFAOYSA-N CN(C(C(NC1=NC(Cl)=NC=C1Cl)=C1)=CC2=C1OCC2)S(C)(=O)=O Chemical compound CN(C(C(NC1=NC(Cl)=NC=C1Cl)=C1)=CC2=C1OCC2)S(C)(=O)=O VEKVZDQUXYKLMP-UHFFFAOYSA-N 0.000 description 1
- KPRGPKHUCUSGIV-UHFFFAOYSA-N CN(C(C([N+]([O-])=O)=C1)=CC2=C1OCC2)S(C)(=O)=O Chemical compound CN(C(C([N+]([O-])=O)=C1)=CC2=C1OCC2)S(C)(=O)=O KPRGPKHUCUSGIV-UHFFFAOYSA-N 0.000 description 1
- OBUINJQBEPXZCY-UHFFFAOYSA-N CN(C(C=CC(Cl)=C1)=C1NC1=NC(Cl)=NC=C1Br)S(C)(=O)=O Chemical compound CN(C(C=CC(Cl)=C1)=C1NC1=NC(Cl)=NC=C1Br)S(C)(=O)=O OBUINJQBEPXZCY-UHFFFAOYSA-N 0.000 description 1
- LEIUKAZTSRMJEW-UHFFFAOYSA-N CN(C(C=CC(Cl)=C1)=C1NC1=NC(Cl)=NC=C1Cl)S(C)(=O)=O Chemical compound CN(C(C=CC(Cl)=C1)=C1NC1=NC(Cl)=NC=C1Cl)S(C)(=O)=O LEIUKAZTSRMJEW-UHFFFAOYSA-N 0.000 description 1
- IPXYMAVFQHADCY-UHFFFAOYSA-N CN(C(C=CC=C1)=C1N(C)S(C)(=O)=O)C1=NC(Cl)=NC=C1Cl Chemical compound CN(C(C=CC=C1)=C1N(C)S(C)(=O)=O)C1=NC(Cl)=NC=C1Cl IPXYMAVFQHADCY-UHFFFAOYSA-N 0.000 description 1
- KTRZMKZEHKDMIY-UHFFFAOYSA-N CN(C(C=CC=C1)=C1NS(C)(=O)=O)C1=NC(Cl)=NC=C1Cl Chemical compound CN(C(C=CC=C1)=C1NS(C)(=O)=O)C1=NC(Cl)=NC=C1Cl KTRZMKZEHKDMIY-UHFFFAOYSA-N 0.000 description 1
- ZQVUGJJOKDRMGS-UHFFFAOYSA-N CN(C(C=CC=C1)=C1[N+]([O-])=O)C1=NC(Cl)=NC=C1Cl Chemical compound CN(C(C=CC=C1)=C1[N+]([O-])=O)C1=NC(Cl)=NC=C1Cl ZQVUGJJOKDRMGS-UHFFFAOYSA-N 0.000 description 1
- PQNFDOMGNGZFMJ-UHFFFAOYSA-N CN(C)C(CC1)CN1C(N=C1OC)=CC=C1NC(N=C1)=NC=C1Cl Chemical compound CN(C)C(CC1)CN1C(N=C1OC)=CC=C1NC(N=C1)=NC=C1Cl PQNFDOMGNGZFMJ-UHFFFAOYSA-N 0.000 description 1
- RKBCHAXRUSPOOZ-UHFFFAOYSA-N CP(C)(C(C=CC(Cl)=C1)=C1N)=O Chemical compound CP(C)(C(C=CC(Cl)=C1)=C1N)=O RKBCHAXRUSPOOZ-UHFFFAOYSA-N 0.000 description 1
- JIOWCRFAGRXWTH-UHFFFAOYSA-N CS(=O)(=O)NC1=C(NC2=NC(=NC=C2Br)Cl)C=CC(F)=C1 Chemical compound CS(=O)(=O)NC1=C(NC2=NC(=NC=C2Br)Cl)C=CC(F)=C1 JIOWCRFAGRXWTH-UHFFFAOYSA-N 0.000 description 1
- BCKIJNCTICKFQK-UHFFFAOYSA-N CS(N(C(C([N+]([O-])=O)=C1)=CC2=C1OCC2)S(C)(=O)=O)(=O)=O Chemical compound CS(N(C(C([N+]([O-])=O)=C1)=CC2=C1OCC2)S(C)(=O)=O)(=O)=O BCKIJNCTICKFQK-UHFFFAOYSA-N 0.000 description 1
- GRXZFOQSFPYLRM-UHFFFAOYSA-N CS(N(C1=C2N=CC=NC2=CC=C1N)S(C)(=O)=O)(=O)=O Chemical compound CS(N(C1=C2N=CC=NC2=CC=C1N)S(C)(=O)=O)(=O)=O GRXZFOQSFPYLRM-UHFFFAOYSA-N 0.000 description 1
- BMPJYXRFORSPOH-UHFFFAOYSA-N CS(N(C1=C2N=CC=NC2=CC=C1[N+]([O-])=O)S(C)(=O)=O)(=O)=O Chemical compound CS(N(C1=C2N=CC=NC2=CC=C1[N+]([O-])=O)S(C)(=O)=O)(=O)=O BMPJYXRFORSPOH-UHFFFAOYSA-N 0.000 description 1
- LYJHYVLCVFPVMA-UHFFFAOYSA-N CS(NC(C(N)=C1)=CC2=C1OCC2)(=O)=O Chemical compound CS(NC(C(N)=C1)=CC2=C1OCC2)(=O)=O LYJHYVLCVFPVMA-UHFFFAOYSA-N 0.000 description 1
- GFGSSEDZVYWDSU-UHFFFAOYSA-N CS(NC(C(NC1=NC(Cl)=NC=C1Br)=C1)=CC2=C1OCC2)(=O)=O Chemical compound CS(NC(C(NC1=NC(Cl)=NC=C1Br)=C1)=CC2=C1OCC2)(=O)=O GFGSSEDZVYWDSU-UHFFFAOYSA-N 0.000 description 1
- ACTMWSYRLJSBDC-UHFFFAOYSA-N CS(NC(C(NC1=NC(Cl)=NC=C1Cl)=C1)=CC2=C1OCC2)(=O)=O Chemical compound CS(NC(C(NC1=NC(Cl)=NC=C1Cl)=C1)=CC2=C1OCC2)(=O)=O ACTMWSYRLJSBDC-UHFFFAOYSA-N 0.000 description 1
- AYLBWYQNIJJHNZ-UHFFFAOYSA-N CS(NC(C1=C(C=C2)SC=N1)=C2N(C1=NC(Cl)=NC=C1Cl)S(C)(=O)=O)(=O)=O Chemical compound CS(NC(C1=C(C=C2)SC=N1)=C2N(C1=NC(Cl)=NC=C1Cl)S(C)(=O)=O)(=O)=O AYLBWYQNIJJHNZ-UHFFFAOYSA-N 0.000 description 1
- UNJFFBXSMMALGP-UHFFFAOYSA-N CS(NC(C=C(C(Cl)=C1)Cl)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O Chemical compound CS(NC(C=C(C(Cl)=C1)Cl)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O UNJFFBXSMMALGP-UHFFFAOYSA-N 0.000 description 1
- KMSJSBBIKORILT-UHFFFAOYSA-N CS(NC(C=C(C(Cl)=C1)F)=C1NC1=NC(Cl)=NC=C1Br)(=O)=O Chemical compound CS(NC(C=C(C(Cl)=C1)F)=C1NC1=NC(Cl)=NC=C1Br)(=O)=O KMSJSBBIKORILT-UHFFFAOYSA-N 0.000 description 1
- VWVDTBFNJSDJOD-UHFFFAOYSA-N CS(NC(C=C(C(Cl)=C1)F)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O Chemical compound CS(NC(C=C(C(Cl)=C1)F)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O VWVDTBFNJSDJOD-UHFFFAOYSA-N 0.000 description 1
- BITCZOABDGNSGN-UHFFFAOYSA-N CS(NC(C=C(C=C1)F)=C1NC(N=C(N=N1)Cl)=C1Cl)(=O)=O Chemical compound CS(NC(C=C(C=C1)F)=C1NC(N=C(N=N1)Cl)=C1Cl)(=O)=O BITCZOABDGNSGN-UHFFFAOYSA-N 0.000 description 1
- HKNNWLUVTOEZGX-UHFFFAOYSA-N CS(NC(C=CC(Cl)=C1)=C1NC(N=C(N=N1)Cl)=C1Cl)(=O)=O Chemical compound CS(NC(C=CC(Cl)=C1)=C1NC(N=C(N=N1)Cl)=C1Cl)(=O)=O HKNNWLUVTOEZGX-UHFFFAOYSA-N 0.000 description 1
- VDEXCOZZHDLDMS-UHFFFAOYSA-N CS(NC(C=CC(Cl)=C1)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O Chemical compound CS(NC(C=CC(Cl)=C1)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O VDEXCOZZHDLDMS-UHFFFAOYSA-N 0.000 description 1
- NRDZDKZZSPSLGO-UHFFFAOYSA-N CS(NC(C=CC=C1)=C1N(C1=NC(Cl)=NC=C1Cl)S(C)(=O)=O)(=O)=O Chemical compound CS(NC(C=CC=C1)=C1N(C1=NC(Cl)=NC=C1Cl)S(C)(=O)=O)(=O)=O NRDZDKZZSPSLGO-UHFFFAOYSA-N 0.000 description 1
- ITVDLOTUMKSDTI-UHFFFAOYSA-N CS(NC1=C(C=CC=N2)C2=CC=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O Chemical compound CS(NC1=C(C=CC=N2)C2=CC=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O ITVDLOTUMKSDTI-UHFFFAOYSA-N 0.000 description 1
- OJIHUKDEVDDVNQ-UHFFFAOYSA-N CS(NC1=C2N=CC=NC2=CC=C1[N+]([O-])=O)(=O)=O Chemical compound CS(NC1=C2N=CC=NC2=CC=C1[N+]([O-])=O)(=O)=O OJIHUKDEVDDVNQ-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- PTHCMJGKKRQCBF-UHFFFAOYSA-N Cellulose, microcrystalline Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC)C(CO)O1 PTHCMJGKKRQCBF-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- QELBTVGQXOZGEY-UHFFFAOYSA-N ClC(C=C1)=CC(NC2=NC(Cl)=NC=C2Cl)=C1I Chemical compound ClC(C=C1)=CC(NC2=NC(Cl)=NC=C2Cl)=C1I QELBTVGQXOZGEY-UHFFFAOYSA-N 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- KKUKTXOBAWVSHC-UHFFFAOYSA-N Dimethylphosphate Chemical compound COP(O)(=O)OC KKUKTXOBAWVSHC-UHFFFAOYSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 101150039808 Egfr gene Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 239000004386 Erythritol Substances 0.000 description 1
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 1
- 208000010201 Exanthema Diseases 0.000 description 1
- ONVOHEKUDDOTHX-UHFFFAOYSA-N FC(C1=C(C=CC=C1)C=1C(=NC(=NC=1)N)N)(F)F Chemical compound FC(C1=C(C=CC=C1)C=1C(=NC(=NC=1)N)N)(F)F ONVOHEKUDDOTHX-UHFFFAOYSA-N 0.000 description 1
- GISRWBROCYNDME-PELMWDNLSA-N F[C@H]1[C@H]([C@H](NC1=O)COC1=NC=CC2=CC(=C(C=C12)OC)C(=O)N)C Chemical compound F[C@H]1[C@H]([C@H](NC1=O)COC1=NC=CC2=CC(=C(C=C12)OC)C(=O)N)C GISRWBROCYNDME-PELMWDNLSA-N 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 208000004463 Follicular Adenocarcinoma Diseases 0.000 description 1
- 235000016623 Fragaria vesca Nutrition 0.000 description 1
- 240000009088 Fragaria x ananassa Species 0.000 description 1
- 235000011363 Fragaria x ananassa Nutrition 0.000 description 1
- 206010064571 Gene mutation Diseases 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 101001059454 Homo sapiens Serine/threonine-protein kinase MARK2 Proteins 0.000 description 1
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 1
- QZRGKCOWNLSUDK-UHFFFAOYSA-N Iodochlorine Chemical compound ICl QZRGKCOWNLSUDK-UHFFFAOYSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- 206010023825 Laryngeal cancer Diseases 0.000 description 1
- 108091054455 MAP kinase family Proteins 0.000 description 1
- 102000043136 MAP kinase family Human genes 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- AYCPARAPKDAOEN-LJQANCHMSA-N N-[(1S)-2-(dimethylamino)-1-phenylethyl]-6,6-dimethyl-3-[(2-methyl-4-thieno[3,2-d]pyrimidinyl)amino]-1,4-dihydropyrrolo[3,4-c]pyrazole-5-carboxamide Chemical compound C1([C@H](NC(=O)N2C(C=3NN=C(NC=4C=5SC=CC=5N=C(C)N=4)C=3C2)(C)C)CN(C)C)=CC=CC=C1 AYCPARAPKDAOEN-LJQANCHMSA-N 0.000 description 1
- HHEWWSNCPNXHJQ-UHFFFAOYSA-N NS(CC(C([N+]([O-])=O)=C1)=CC2=C1OCC2)(=O)=O Chemical compound NS(CC(C([N+]([O-])=O)=C1)=CC2=C1OCC2)(=O)=O HHEWWSNCPNXHJQ-UHFFFAOYSA-N 0.000 description 1
- JSMYDDKSWCEOMR-UHFFFAOYSA-N NS(CC(C=C(C=C1)F)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O Chemical compound NS(CC(C=C(C=C1)F)=C1NC1=NC(Cl)=NC=C1Cl)(=O)=O JSMYDDKSWCEOMR-UHFFFAOYSA-N 0.000 description 1
- PRSAQRISIOPFSI-UHFFFAOYSA-N O=S(C(C=CC=C1)=C1NC1=NC(Cl)=NC=C1Cl)(NC1CC1)=O Chemical compound O=S(C(C=CC=C1)=C1NC1=NC(Cl)=NC=C1Cl)(NC1CC1)=O PRSAQRISIOPFSI-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 229920002230 Pectic acid Polymers 0.000 description 1
- 102000004422 Phospholipase C gamma Human genes 0.000 description 1
- 108010056751 Phospholipase C gamma Proteins 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 201000010208 Seminoma Diseases 0.000 description 1
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 206010042033 Stevens-Johnson syndrome Diseases 0.000 description 1
- 231100000168 Stevens-Johnson syndrome Toxicity 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical group C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- 206010062129 Tongue neoplasm Diseases 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- LXRZVMYMQHNYJB-UNXOBOICSA-N [(1R,2S,4R)-4-[[5-[4-[(1R)-7-chloro-1,2,3,4-tetrahydroisoquinolin-1-yl]-5-methylthiophene-2-carbonyl]pyrimidin-4-yl]amino]-2-hydroxycyclopentyl]methyl sulfamate Chemical compound CC1=C(C=C(S1)C(=O)C1=C(N[C@H]2C[C@H](O)[C@@H](COS(N)(=O)=O)C2)N=CN=C1)[C@@H]1NCCC2=C1C=C(Cl)C=C2 LXRZVMYMQHNYJB-UNXOBOICSA-N 0.000 description 1
- ZXSDNYCJSHRMEQ-UHFFFAOYSA-N [O-][N+](C(C1=C(C=C2)SC=N1)=C2NC1=NC(Cl)=NC=C1Cl)=O Chemical compound [O-][N+](C(C1=C(C=C2)SC=N1)=C2NC1=NC(Cl)=NC=C1Cl)=O ZXSDNYCJSHRMEQ-UHFFFAOYSA-N 0.000 description 1
- XWNFSLZDKIGQLM-UHFFFAOYSA-N [SH4]=O Chemical compound [SH4]=O XWNFSLZDKIGQLM-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 208000009956 adenocarcinoma Diseases 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 229960001686 afatinib Drugs 0.000 description 1
- ULXXDDBFHOBEHA-CWDCEQMOSA-N afatinib Chemical compound N1=CN=C2C=C(O[C@@H]3COCC3)C(NC(=O)/C=C/CN(C)C)=CC2=C1NC1=CC=C(F)C(Cl)=C1 ULXXDDBFHOBEHA-CWDCEQMOSA-N 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 125000005233 alkylalcohol group Chemical group 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 description 1
- 230000006229 amino acid addition Effects 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000002744 anti-aggregatory effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 230000005975 antitumor immune response Effects 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000004603 benzisoxazolyl group Chemical group O1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- 208000026900 bile duct neoplasm Diseases 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- RFCBNSCSPXMEBK-INFSMZHSSA-N c-GMP-AMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]3[C@@H](O)[C@H](N4C5=NC=NC(N)=C5N=C4)O[C@@H]3COP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 RFCBNSCSPXMEBK-INFSMZHSSA-N 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- 235000012241 calcium silicate Nutrition 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000007726 cellular glucose metabolism Effects 0.000 description 1
- 201000007455 central nervous system cancer Diseases 0.000 description 1
- DGLFSNZWRYADFC-UHFFFAOYSA-N chembl2334586 Chemical compound C1CCC2=CN=C(N)N=C2C2=C1NC1=CC=C(C#CC(C)(O)C)C=C12 DGLFSNZWRYADFC-UHFFFAOYSA-N 0.000 description 1
- 239000007810 chemical reaction solvent Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 208000006990 cholangiocarcinoma Diseases 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000012050 conventional carrier Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229910000365 copper sulfate Inorganic materials 0.000 description 1
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 239000002577 cryoprotective agent Substances 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 229940043239 cytotoxic antineoplastic drug Drugs 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- VAYGXNSJCAHWJZ-UHFFFAOYSA-N dimethyl sulfate Chemical compound COS(=O)(=O)OC VAYGXNSJCAHWJZ-UHFFFAOYSA-N 0.000 description 1
- 208000016097 disease of metabolism Diseases 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 229940037395 electrolytes Drugs 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 108700021358 erbB-1 Genes Proteins 0.000 description 1
- 229960001433 erlotinib Drugs 0.000 description 1
- 235000019414 erythritol Nutrition 0.000 description 1
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 1
- 229940009714 erythritol Drugs 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 201000005884 exanthem Diseases 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 229940121645 first-generation egfr tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 238000005187 foaming Methods 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 125000004612 furopyridinyl group Chemical group O1C(=CC2=C1C=CC=N2)* 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- 229940087158 gilotrif Drugs 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 229940015043 glyoxal Drugs 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 210000002768 hair cell Anatomy 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 201000011066 hemangioma Diseases 0.000 description 1
- 231100000086 high toxicity Toxicity 0.000 description 1
- FUKUFMFMCZIRNT-UHFFFAOYSA-N hydron;methanol;chloride Chemical compound Cl.OC FUKUFMFMCZIRNT-UHFFFAOYSA-N 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 201000001371 inclusion conjunctivitis Diseases 0.000 description 1
- 125000003392 indanyl group Chemical group C1(CCC2=CC=CC=C12)* 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000004068 intracellular signaling Effects 0.000 description 1
- 230000002601 intratumoral effect Effects 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229940084651 iressa Drugs 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 208000003849 large cell carcinoma Diseases 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 201000005249 lung adenocarcinoma Diseases 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 235000010449 maltitol Nutrition 0.000 description 1
- 239000000845 maltitol Substances 0.000 description 1
- VQHSOMBJVWLPSR-WUJBLJFYSA-N maltitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-WUJBLJFYSA-N 0.000 description 1
- 229940035436 maltitol Drugs 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- PKAUVIXBZJUYRV-UHFFFAOYSA-N methane;hydroiodide Chemical compound C.I PKAUVIXBZJUYRV-UHFFFAOYSA-N 0.000 description 1
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 239000003226 mitogen Substances 0.000 description 1
- 230000011278 mitosis Effects 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- AVAWMINJNRAQFS-UHFFFAOYSA-N n,n-dimethylpyrrolidin-3-amine Chemical compound CN(C)C1CCNC1 AVAWMINJNRAQFS-UHFFFAOYSA-N 0.000 description 1
- RFAVPISRRMQCOS-UHFFFAOYSA-N n-(2-amino-4,5-dichlorophenyl)acetamide Chemical compound CC(=O)NC1=CC(Cl)=C(Cl)C=C1N RFAVPISRRMQCOS-UHFFFAOYSA-N 0.000 description 1
- FOIBPMNXVLDNPX-UHFFFAOYSA-N n-(2-amino-4-chlorophenyl)acetamide Chemical compound CC(=O)NC1=CC=C(Cl)C=C1N FOIBPMNXVLDNPX-UHFFFAOYSA-N 0.000 description 1
- KGWZUEMSCDAHEE-UHFFFAOYSA-N n-(2-amino-4-methoxyphenyl)methanesulfonamide Chemical compound COC1=CC=C(NS(C)(=O)=O)C(N)=C1 KGWZUEMSCDAHEE-UHFFFAOYSA-N 0.000 description 1
- MPXAYYWSDIKNTP-UHFFFAOYSA-N n-(2-aminophenyl)acetamide Chemical compound CC(=O)NC1=CC=CC=C1N MPXAYYWSDIKNTP-UHFFFAOYSA-N 0.000 description 1
- BGPNDOBOLHJJEE-UHFFFAOYSA-N n-(2-aminophenyl)methanesulfonamide Chemical compound CS(=O)(=O)NC1=CC=CC=C1N BGPNDOBOLHJJEE-UHFFFAOYSA-N 0.000 description 1
- MYVUAJLZCUJAOC-UHFFFAOYSA-N n-(4-chloro-2-nitrophenyl)methanesulfonamide Chemical compound CS(=O)(=O)NC1=CC=C(Cl)C=C1[N+]([O-])=O MYVUAJLZCUJAOC-UHFFFAOYSA-N 0.000 description 1
- YLNRCLFODARCHN-UHFFFAOYSA-N n-[2-[(2,5-dichloropyrimidin-4-yl)amino]phenyl]methanesulfonamide Chemical compound CS(=O)(=O)NC1=CC=CC=C1NC1=NC(Cl)=NC=C1Cl YLNRCLFODARCHN-UHFFFAOYSA-N 0.000 description 1
- QZLFTWBTXDWEFZ-UHFFFAOYSA-N n-[2-amino-4-(trifluoromethyl)phenyl]methanesulfonamide Chemical compound CS(=O)(=O)NC1=CC=C(C(F)(F)F)C=C1N QZLFTWBTXDWEFZ-UHFFFAOYSA-N 0.000 description 1
- KFBOUJZFFJDYTA-UHFFFAOYSA-N n-methyl-2-nitroaniline Chemical compound CNC1=CC=CC=C1[N+]([O-])=O KFBOUJZFFJDYTA-UHFFFAOYSA-N 0.000 description 1
- SVDVKEBISAOWJT-UHFFFAOYSA-N n-methylbenzenesulfonamide Chemical compound CNS(=O)(=O)C1=CC=CC=C1 SVDVKEBISAOWJT-UHFFFAOYSA-N 0.000 description 1
- 150000002828 nitro derivatives Chemical class 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 229940127084 other anti-cancer agent Drugs 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- KDLHZDBZIXYQEI-UHFFFAOYSA-N palladium Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 1
- LXNAVEXFUKBNMK-UHFFFAOYSA-N palladium(II) acetate Substances [Pd].CC(O)=O.CC(O)=O LXNAVEXFUKBNMK-UHFFFAOYSA-N 0.000 description 1
- YJVFFLUZDVXJQI-UHFFFAOYSA-L palladium(ii) acetate Chemical compound [Pd+2].CC([O-])=O.CC([O-])=O YJVFFLUZDVXJQI-UHFFFAOYSA-L 0.000 description 1
- WLJNZVDCPSBLRP-UHFFFAOYSA-N pamoic acid Chemical compound C1=CC=C2C(CC=3C4=CC=CC=C4C=C(C=3O)C(=O)O)=C(O)C(C(O)=O)=CC2=C1 WLJNZVDCPSBLRP-UHFFFAOYSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- LCLHHZYHLXDRQG-ZNKJPWOQSA-N pectic acid Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)O[C@H](C(O)=O)[C@@H]1OC1[C@H](O)[C@@H](O)[C@@H](OC2[C@@H]([C@@H](O)[C@@H](O)[C@H](O2)C(O)=O)O)[C@@H](C(O)=O)O1 LCLHHZYHLXDRQG-ZNKJPWOQSA-N 0.000 description 1
- 208000028169 periodontal disease Diseases 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 229940021222 peritoneal dialysis isotonic solution Drugs 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- ABOYDMHGKWRPFD-UHFFFAOYSA-N phenylmethanesulfonamide Chemical compound NS(=O)(=O)CC1=CC=CC=C1 ABOYDMHGKWRPFD-UHFFFAOYSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 230000000865 phosphorylative effect Effects 0.000 description 1
- XKJCHHZQLQNZHY-UHFFFAOYSA-N phthalimide Chemical compound C1=CC=C2C(=O)NC(=O)C2=C1 XKJCHHZQLQNZHY-UHFFFAOYSA-N 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000010318 polygalacturonic acid Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- LZMJNVRJMFMYQS-UHFFFAOYSA-N poseltinib Chemical compound C1CN(C)CCN1C(C=C1)=CC=C1NC1=NC(OC=2C=C(NC(=O)C=C)C=CC=2)=C(OC=C2)C2=N1 LZMJNVRJMFMYQS-UHFFFAOYSA-N 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 235000018102 proteins Nutrition 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- 229940083082 pyrimidine derivative acting on arteriolar smooth muscle Drugs 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 125000004550 quinolin-6-yl group Chemical group N1=CC=CC2=CC(=CC=C12)* 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- MSGRFBKVMUKEGZ-UHFFFAOYSA-N quinoxalin-6-amine Chemical compound N1=CC=NC2=CC(N)=CC=C21 MSGRFBKVMUKEGZ-UHFFFAOYSA-N 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 206010037844 rash Diseases 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 201000000306 sarcoidosis Diseases 0.000 description 1
- 229940121644 second-generation egfr tyrosine kinase inhibitor Drugs 0.000 description 1
- XIIOFHFUYBLOLW-UHFFFAOYSA-N selpercatinib Chemical compound OC(COC=1C=C(C=2N(C=1)N=CC=2C#N)C=1C=NC(=CC=1)N1CC2N(C(C1)C2)CC=1C=NC(=CC=1)OC)(C)C XIIOFHFUYBLOLW-UHFFFAOYSA-N 0.000 description 1
- 230000008054 signal transmission Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- XGVXKJKTISMIOW-ZDUSSCGKSA-N simurosertib Chemical compound N1N=CC(C=2SC=3C(=O)NC(=NC=3C=2)[C@H]2N3CCC(CC3)C2)=C1C XGVXKJKTISMIOW-ZDUSSCGKSA-N 0.000 description 1
- 208000000649 small cell carcinoma Diseases 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 239000000779 smoke Substances 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- PPASLZSBLFJQEF-RKJRWTFHSA-M sodium ascorbate Substances [Na+].OC[C@@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RKJRWTFHSA-M 0.000 description 1
- 235000010378 sodium ascorbate Nutrition 0.000 description 1
- 229960005055 sodium ascorbate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- PPASLZSBLFJQEF-RXSVEWSESA-M sodium-L-ascorbate Chemical compound [Na+].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RXSVEWSESA-M 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 230000002037 soft tissue calcification Effects 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000000528 statistical test Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000003206 sterilizing agent Substances 0.000 description 1
- 239000008362 succinate buffer Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- YBBRCQOCSYXUOC-UHFFFAOYSA-N sulfuryl dichloride Chemical compound ClS(Cl)(=O)=O YBBRCQOCSYXUOC-UHFFFAOYSA-N 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- DPKBAXPHAYBPRL-UHFFFAOYSA-M tetrabutylazanium;iodide Chemical compound [I-].CCCC[N+](CCCC)(CCCC)CCCC DPKBAXPHAYBPRL-UHFFFAOYSA-M 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- 229940121646 third-generation egfr tyrosine kinase inhibitor Drugs 0.000 description 1
- 208000030901 thyroid gland follicular carcinoma Diseases 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 201000006134 tongue cancer Diseases 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 206010044325 trachoma Diseases 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- SEDZOYHHAIAQIW-UHFFFAOYSA-N trimethylsilyl azide Chemical compound C[Si](C)(C)N=[N+]=[N-] SEDZOYHHAIAQIW-UHFFFAOYSA-N 0.000 description 1
- CWMFRHBXRUITQE-UHFFFAOYSA-N trimethylsilylacetylene Chemical group C[Si](C)(C)C#C CWMFRHBXRUITQE-UHFFFAOYSA-N 0.000 description 1
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 1
- 235000019798 tripotassium phosphate Nutrition 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 125000004417 unsaturated alkyl group Chemical group 0.000 description 1
- 239000008371 vanilla flavor Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/506—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/53—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with three nitrogens as the only ring hetero atoms, e.g. chlorazanil, melamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5377—1,4-Oxazines, e.g. morpholine not condensed and containing further heterocyclic rings, e.g. timolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/54—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame
- A61K31/541—Non-condensed thiazines containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/551—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having two nitrogen atoms, e.g. dilazep
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/675—Phosphorus compounds having nitrogen as a ring hetero atom, e.g. pyridoxal phosphate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/02—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
- A61K9/2018—Sugars, or sugar alcohols, e.g. lactose, mannitol; Derivatives thereof, e.g. polysorbates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/2027—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2059—Starch, including chemically or physically modified derivatives; Amylose; Amylopectin; Dextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4816—Wall or shell material
- A61K9/4825—Proteins, e.g. gelatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4858—Organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4866—Organic macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/46—Two or more oxygen, sulphur or nitrogen atoms
- C07D239/48—Two nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/547—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom
- C07F9/6558—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom containing at least two different or differently substituted hetero rings neither condensed among themselves nor condensed with a common carbocyclic ring or ring system
- C07F9/65583—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom containing at least two different or differently substituted hetero rings neither condensed among themselves nor condensed with a common carbocyclic ring or ring system each of the hetero rings containing nitrogen as ring hetero atom
Definitions
- the present invention provides a compound selected from novel trisubstituted pyrimidine derivatives having protein kinase inhibitory activity, a pharmaceutically acceptable salt thereof, a hydrate thereof or a stereoisomer thereof, a method for preparing the compound, and an active ingredient comprising the compound It relates to a pharmaceutical composition for preventing, alleviating or treating cancer diseases caused by abnormal cell growth comprising
- Protein kinase is an enzyme that catalyzes the phosphorylation reaction that transfers the gamma-phosphate group of ATP to the hydroxyl groups of tyrosine, serine and threonine of proteins, and is responsible for cell metabolism, gene expression, cell growth, differentiation, and cell division. It plays an important role in signal transmission. Protein kinases make up about 2% of the eukaryotic genome, and there are about 518 species in the human genome. Protein kinases are classified into tyrosine protein kinases that phosphorylate tyrosine and serine/threonine kinases that phosphorylate serine and threonine.
- Receptor tyrosine kinases is a membrane protein having a domain that can accommodate growth factors on the cell surface and an active site capable of phosphorylating tyrosine residues in the cytoplasm.
- Protein kinases are molecular switches, and the transition between active and inactive states in cells must be smoothly regulated. Abnormal regulation leads to excessive activation of intracellular signaling, leading to out-of-control cell division and proliferation.
- abnormal activation by gene mutation, amplification and overexpression of protein kinase is associated with the development and progression of various tumors, and thus plays a decisive role in the growth and metastasis of cancer cells.
- Lung cancer is a malignant tumor that occurs in the lungs, the respiratory tract, and is divided into non-small cell lung cancer and small cell lung cancer depending on the size and shape of cancer cells.
- Non-small cell lung cancer accounts for 80% of all lung cancers.
- Non-small cell lung cancer can be cured with surgery if detected early, but 20-50% of patients undergoing surgery have recurrence, and metastases to the brain, bone, liver and other lung are more common than other cancers.
- non-small cell lung cancer is a mutation in the epidermal growth factor receptor (EGFR) tyrosine kinase gene. 30-40% of non-small cell lung cancer patients in Asia and 10-15% in the United States and Europe have EGFR mutations, and 40-50% of Asian non-smokers have EGFR mutations. Mutations in the EGFR gene cause lung cancer regardless of whether or not you smoke. Of all EGFR mutations, 90% are activating mutations that can activate EGFR without epidermal growth factor (exon 19 deletion mutation 43%, L858R point mutation 41%).
- EGFR epidermal growth factor receptor
- EGFR a transmembrane tyrosine kinase
- Activated EGFR forms a condition for easy binding to sub-molecules and phosphorylates them in lower pathways (RAS-mitogen-activated protein kinase (MAPK), phosphotidylinosititol 3-kinase (PI3-K)-Akt, phospholipase-C ⁇ (PLC ⁇ ) / It activates protein kinase-C (PKC), signal transducer and activator of transcription (STAT), etc.) to inhibit apoptosis, promote cell growth and proliferation, and regulate gene expression.
- APK protein kinase-C
- STAT signal transducer and activator of transcription
- the first-generation EGFR tyrosine kinase inhibitors that can be administered orally, extend the survival period by 10 months compared to cytotoxic anticancer drugs, which are the existing standard anticancer drugs. Although superiority has been demonstrated, first-generation inhibitor resistance results in T790M mutations in 60% of patients.
- Jilotrif (afatinib, Gilotrif®, Boehringer Ingelheim), a second-generation EGFR tyrosine kinase inhibitor capable of inhibiting the T790M mutation, was released and demonstrated good efficacy in clinical practice, but did not secure a therapeutic window.
- Second-generation inhibitors have relatively narrow indications as they are only approved as first-line treatment for locally advanced or metastatic non-small cell lung cancer with EGFR mutations.
- Tagrisso (osimertinib, Targrisso®, AstraZeneca), a third-generation EGFR tyrosine kinase inhibitor, introduced a michael acceptor that can covalently bind to cysteine and was the first third-generation EGFR-targeted anticancer drug to be released after receiving FDA approval.
- the patient developed C797S mutation acquired resistance.
- the C797S mutation is a mutation in which the cysteine residue of the tagrisso binding site is changed to serine, and it disables the covalent binding of tagrisso.
- Tagrisso can be used when a T790M mutation occurs after treatment with first- and second-generation EGFR inhibitors
- patients who develop resistance to Tagrisso have an EGFR activating mutation (exon 19 deletion or L858R), and three mutations T790M and C797S. have all
- fourth-generation EGFR inhibitors have triple mutations (exon 19 deletion/T790M/C797S or L858R/T790M/C797S) as well as double mutations (exon 19 deletion/T790M or L858R/T790M) and activating mutations (exon 19 deletion or L858R), respectively. should also have a suitable effect.
- Lung cancer is one of the most lethal cancers, with more than 20,000 occurrences per year and the highest mortality rate in Korea. In particular, when it metastasizes to other organs, the 5-year survival rate is very low at 8.9%. In 2021, the number of lung cancer patients in Korea will be about 30,000, of which 30-40% are EGFR mutation-positive. In addition to lung cancer, EGFR mutations are also found in several carcinomas including glioblastoma (50%), triple-negative breast cancer (13% - 30%), and colorectal cancer (25% -77%). Clinical trials are in progress.
- the present inventors completed the present invention by conducting research to develop a novel material that can overcome the limitations of the third-generation EGFR inhibitor.
- Patent Document 1 PCT Patent Publication No. WO/2018/230934/Al
- Patent Document 2 US/2020/0207768/Al
- Patent Document 3 Korean Patent Laid-Open Patent No. 10-2019-0114910
- Patent Document 4 PCT Patent Publication No. WO/2019/112344/A1
- Patent Document 5 PCT Patent Publication No. WO/2020/147702/A1
- Non-Patent Document 1 J. Med. Chem. 2018, 61, 4290-4300
- Non-Patent Document 2 J. Med. Chem. 2019, 62, 10272-10293
- Another object of the present invention is a novel pyrimidine derivative compound, a pharmaceutically acceptable salt thereof, a hydrate thereof, a solvate thereof, or a pharmaceutical composition useful for the treatment, prevention and alleviation of cancer diseases containing a stereoisomer thereof as an active ingredient. Its purpose is to provide
- another object of the present invention is to provide a therapeutic agent for cancer diseases caused by EGFR mutation containing a novel pyrimidine derivative compound, a pharmaceutically acceptable salt thereof, a hydrate thereof, a solvate thereof or an isomer thereof as an active ingredient. for that purpose
- one aspect of the present invention is to provide a method for preparing the above-described novel compound.
- one aspect of the present invention is to provide a novel intermediate compound synthesized in the process of performing the above-described preparation method.
- one aspect of the present invention provides a compound selected from a pyrimidine derivative compound represented by the following Chemical Formula 10, 11 or 12, a pharmaceutically acceptable salt thereof, a hydrate thereof, and a stereoisomer thereof.
- R 1 is hydrogen; hydroxyl group; halogen group; C 1 -C 13 alkyl group; Or a C 1 -C 6 alkoxy group;
- R 2 is hydrogen; halogen group; C 1 -C 13 alkyl group; C 3 -C 10 cyclyl group; an amino group (-NR 8 R 9 ); or a nitro group (—N(O) 2 );
- R 3 and R 4 are each independently hydrogen; C 1 -C 13 alkyl group; C 3 -C 10 cyclyl group; sulfide group (-SR 8 ); a sulfone group (-S(O) 2 R 8 ); or a phospyryl group (-P(O)R 8 R 9 );
- X 1 , X 2 and X 3 are each independently carbon or nitrogen
- B is a C 6 -C 10 aryl group; C 3 -C 10 cyclyl group; C 3 -C 10 heteroaryl group; or a C 3 -C 10 heterocyclyl group; ego,
- the C 3 -C 10 heteroaryl group and the C 3 -C 10 heterocyclyl group include one or more heteroatoms selected from the group consisting of N, O, and S.
- Another aspect of the present invention provides a pharmaceutical composition for preventing, improving or treating diseases caused by EGFR protein kinase containing the compound as an active ingredient.
- the disease induced by the EGFR protein kinase is a metabolic disease induced by EGFR protein kinase; or tumor diseases caused by abnormal cell proliferation caused by EGFR protein kinase; Phosphorus, provides a pharmaceutical composition.
- the disease is lung cancer, liver cancer, esophageal cancer, stomach cancer, colorectal cancer, small intestine cancer, pancreatic cancer, melanoma, breast cancer, oral cancer, brain tumor, thyroid cancer, parathyroid cancer, kidney cancer, cervical cancer, sarcoma, prostate cancer , urethral cancer, bladder cancer, testicular cancer, blood cancer, lymphoma, skin cancer, may be a disease selected from the group consisting of psoriasis and fibroadenoma.
- the active ingredient provides a pharmaceutical composition for preventing, improving or treating the disease through inhibition of the activity of EGFR kinase or EGFR mutation.
- the EGFR mutation may be the activity of del19, L858R, T790M, C797S, or a combination thereof.
- the active ingredient provides a pharmaceutical composition that can be administered in combination with a cytotoxic therapeutic agent.
- the active ingredient and the cytotoxic agent provide a pharmaceutical composition that can be administered in combination in an amount of 10 to 1000 parts by weight of the cytotoxic agent based on 100 parts by weight of the active ingredient.
- the compound of the present invention Since the compound of the present invention has excellent ability to inhibit the activity of EGFR protein kinase, it can be used as an active ingredient in a pharmaceutical composition for the prevention and treatment of diseases caused by abnormal cell growth and metabolism.
- the compound according to the present invention is a disease caused by abnormal cell growth and metabolism, for example, metabolic diseases such as diabetes and obesity, endometrial cancer, bladder cancer, stomach cancer, lung cancer, liver cancer, colon cancer, small intestine cancer, pancreatic cancer, brain cancer , bone cancer, melanoma, breast cancer, sclerosing adenoma, head and neck cancer, esophageal cancer, thyroid cancer, parathyroid cancer, kidney cancer, sarcoma, prostate cancer, urethral cancer, leukemia, multiple myeloma, blood cancer such as myelodysplastic syndrome, Hodgkin's disease and non-Hodgkin's disease It can be used as a prophylactic and therapeutic agent for various tumor diseases selected from lymphoma, such as lymphoma, or fibroadenoma.
- metabolic diseases such as diabetes and obesity, endometrial cancer, bladder cancer, stomach cancer, lung cancer, liver cancer, colon cancer, small intestine cancer, pancreatic cancer, brain cancer ,
- variable when a range is described for a variable, the variable will be understood to include all values within the stated range including the stated endpoints of the range.
- a range of “5 to 10” includes the values of 5, 6, 7, 8, 9, and 10, as well as any subranges such as 6 to 10, 7 to 10, 6 to 9, 7 to 9, etc. It will be understood to include any value between integers that are appropriate for the scope of the recited range, such as 5.5, 6.5, 7.5, 5.5 to 8.5 and 6.5 to 9, and the like.
- a range of “10% to 30%” includes values such as 10%, 11%, 12%, 13%, and all integers up to and including 30%, as well as 10% to 15%, 12% to It will be understood to include any subrange, such as 18%, 20% to 30%, etc., as well as any value between reasonable integers within the scope of the recited range, such as 10.5%, 15.5%, 25.5%, and the like.
- the terms “individual(s),” “subject(s),” and “patient(s)” mean any mammal.
- the mammal is a human.
- the mammal is not a human. Neither term requires, or is limited to, a situation characterized by the supervision (e.g., regular or intermittent) of a health care practitioner (e.g., physician, regular nurse, apprentice nurse, physician's assistant, handyman or hospice worker) doesn't happen
- Treatment is an attempt made with the intention of preventing the development or alteration of a lesion of a disease. Accordingly, “treatment” refers to both therapeutic, therapeutic and prophylactic dimensions. Those that need to be treated include those already having the disease as well as those in which the disease is to be prevented. In the treatment of tumors, a therapeutic agent may mean directly reducing the pathology of the tumor cells or making the tumor cells more susceptible to treatment by other therapeutic agents, for example radiation and/or chemotherapy and/or immunotherapy. . As used herein, the term “relief” or “treated” refers to a symptom approaching normalized values measured by conventional statistical tests.
- a sign approaching a normalized value here is, for example, a value obtained from a healthy patient or individual, a value which differs from the normalized value by less than 50%, preferably a value which differs by less than 25%, more preferably It may be a value showing a difference of less than 10%, and more preferably a value not significantly different from a normalized value.
- Treatment of cancer means any one or more of the following effects; 1) i) inhibition of tumor growth, including slowing or ii) complete growth arrest; 2) reduction in the number of tumor cells; 3) maintenance of tumor size; 4) reduction in tumor size; 5) inhibition of tumor cell infiltration into peripheral organs comprising i) reduction or ii) blunting or iii) complete prophylaxis; 6) i) reduction or ii) slowing or iii) inhibition of metastasis, including complete prevention; 7) enhancement of the anti-tumor immune response, which may result in i) maintenance of tumor size or ii) reduction in tumor size or iii) slowing of growth of a tumor or iv) reduction, slowing or prevention of invasion. increase.
- an appropriate "effective" amount for any individual case is determined using techniques such as dose escalation studies.
- an "effective amount” is an amount of a disclosed compound in monotherapy or combination therapy, i.e., when administered in one or more doses, compared to EGFR activity in an individual not treated with the compound, or prior to treatment with the compound, or EGFR by about 20% (20% inhibition), at least about 30% (30% inhibition), at least about 40% (40% inhibition), at least about 50% (50% inhibition) when compared to EGFR activity in the subject later. , about 60% or more (60% inhibition), about 70% or more (70% inhibition), about 80% or more (80% inhibition), about 90% or more (90% inhibition).
- a “therapeutically effective amount” is an amount of a compound disclosed in monotherapy or combination therapy, i.e., when administered in one or more doses, compared to the tumor burden of a subject not treated with the compound, or to be treated with the compound. about 20%, about 30% or more, about 40% or more, about 50% or more, about 60% or more, about 70% or more, about 80% or more, when compared to the subject's tumor burden before or after , an amount effective to reduce by an amount greater than or equal to about 90%.
- tumor burden is the total mass of tumor tissue carried by a subject with cancer.
- a "therapeutically effective amount” is an amount of a compound disclosed in monotherapy or combination therapy, i.e., the dose of radiation therapy required to observe tumor shrinkage in subjects not treated with the compound when administered in one or more doses. compared to about 20%, about 30% or more, about 40% or more, about 50% or more, about 60% or more, about 70% or more, about 80% of the amount of radiotherapy required to observe tumor shrinkage in a subject. It is an effective amount to reduce more than, about 90% or more.
- compositions such as vehicles, adjuvants, carriers or diluents are readily available to those skilled in the art.
- Pharmaceutically acceptable auxiliary substances such as pH adjusting agents and buffers, isotonicity adjusting agents, stabilizers, wetting agents and the like are readily available to those skilled in the art.
- a compound of the invention is formulated in an aqueous buffer.
- Suitable aqueous buffers include, but are not limited to, acetate, succinate, citrate and phosphate buffers of varying strengths from 5 mM to 1000 mM.
- aqueous buffers include reagents that provide isotonic solutions. Such reagents include, but are not limited to, sodium chloride, sugars such as mannitol, dextrose, sucrose, and the like.
- the aqueous buffer further comprises a nonionic surfactant such as polysorbate 20 or 80.
- the formulation may further comprise a preservative.
- Suitable preservatives include, but are not limited to, benzyl alcohol, phenol, chlorobutanol, benzalkonium chloride, and the like.
- the formulation is stored at about 4°C.
- the formulations may also be lyophilized, which generally contain a cryoprotectant such as sucrose, trehalose, lactose, maltose, mannitol, and the like. Freeze-dried preparations can be stored for a long period of time even at room temperature.
- a pharmaceutical composition may comprise, or consist essentially of, a compound disclosed herein, or a pharmaceutically acceptable salt thereof, an isomer thereof, a tautomer thereof, and additionally the pharmaceutical composition comprises one or more additional active agents of interest. It provides a pharmaceutical composition comprising or consisting essentially of. Any convenient active agent may be used in the present methods with the compounds of the present invention.
- the compound of the invention and the checkpoint inhibitor, as well as the additional therapeutic agent as described herein for combination therapy may be administered orally, subcutaneously, intramuscularly, intranasally, parenterally or by other routes.
- a compound of the present invention and a chemotherapeutic agent are oral, subcutaneous, intramuscular , intranasal, parenteral or other routes.
- the compound of the invention and the second active agent may be administered by the same route of administration or by different routes of administration.
- the therapeutic agent may be administered by any suitable means including, but not limited to, oral, rectal, nasal, topical, vaginal, parenteral, intravenous, intranasal, intratumoral injection into the organ affected.
- the compounds of the present invention may be administered in unit dosage form and may be prepared by any method well known in the art. Such methods include the step of combining a compound of the present invention with a pharmaceutically acceptable carrier or diluent which constitutes one of one or more accessory ingredients.
- Pharmaceutically acceptable carriers are selected based on the chosen route of administration and standard pharmaceutical practice. Each carrier must be “pharmaceutically acceptable” in the sense of being compatible with the other ingredients of the formulation and not harming the subject or patient. This carrier may be solid or liquid, the type of which is generally selected according to the type of administration used.
- suitable solid carriers include lactose, sucrose, gelatin, agar and bulk powder.
- suitable liquid carriers include water, pharmaceutically acceptable fats and oils, alcohols or other organic solvents such as esters, emulsions, syrups or elixirs, suspensions and solutions reconstituted from non-foaming granules and/or including suspensions.
- Such liquid carriers may contain, for example, suitable solvents, preservatives, emulsifying agents, suspending agents, diluents, sweetening, thickening and melting agents.
- One aspect of the present invention provides a compound selected from a pyrimidine derivative compound represented by the following Chemical Formula 10, 11 or 12, a pharmaceutically acceptable salt thereof, a hydrate thereof, and a stereoisomer thereof.
- R 1 is hydrogen; hydroxyl group; halogen group; C 1 -C 13 alkyl group; Or a C 1 -C 6 alkoxy group;
- R 2 is hydrogen; halogen group; C 1 -C 13 alkyl group; C 3 -C 10 cyclyl group; an amino group (-NR 8 R 9 ); or a nitro group (—N(O) 2 );
- R 3 and R 4 are each independently hydrogen; C 1 -C 13 alkyl group; C 3 -C 10 cyclyl group; sulfide group (-SR 8 ); a sulfone group (-S(O) 2 R 8 ); or a phospyryl group (-P(O)R 8 R 9 );
- X 1 , X 2 and X 3 are each independently carbon or nitrogen
- B is a C 6 -C 10 aryl group; C 3 -C 10 cyclyl group; C 3 -C 10 heteroaryl group; or a C 3 -C 10 heterocyclyl group; ego,
- the C 3 -C 10 heteroaryl group and the C 3 -C 10 heterocyclyl group include one or more heteroatoms selected from the group consisting of N, O, and S.
- a pharmaceutically acceptable salt of the pyrimidine compound represented by Formula 10, 11 or 12 according to the present invention may be prepared by a conventional method in the art.
- Pharmaceutically acceptable salts should have low toxicity to the human body and should not adversely affect the biological activity and physicochemical properties of the parent compound.
- Free acids that can be used to prepare pharmaceutically acceptable salts can be divided into inorganic acids and organic acids.
- the inorganic acid may be hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid, perchloric acid, hydrobromic acid, and the like.
- Organic acids include acetic acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic acid, fumaric acid, maleic acid, malonic acid, phthalic acid, succinic acid, lactic acid, citric acid, citric acid, gluconic acid, tartaric acid, salicylic acid, malic acid, oxalic acid, Benzoic acid, embonic acid, aspartic acid, glutamic acid and the like can be used.
- Organic bases that can be used in the preparation of organic base addition salts are tris(hydroxymethyl)methylamine, dicyclohexylamine, and the like.
- Amino acids that can be used to prepare the amino acid addition base are natural amino acids such as alanine and glycine.
- the pyrimidine compound represented by Formula 10, 11 or 12 includes all hydrates and solvates in addition to the pharmaceutically acceptable salts described above. Hydrates and solvates are crystallized after the compound represented by Formula 10, 11 or 12 is dissolved in a water-miscible solvent such as methanol, ethanol, acetone, and 1,4-dioxane, and then a free acid or a free base is added. or recrystallize. In such cases, solvates (especially hydrates) may be formed. Accordingly, the compounds of the present invention include stoichiometric solvates including hydrates in addition to various amounts of water-containing compounds that can be prepared by methods such as freeze-drying.
- a water-miscible solvent such as methanol, ethanol, acetone, and 1,4-dioxane
- alkyl means an aliphatic hydrocarbon radical.
- Alkyl may be "saturated alkyl” containing no alkenyl or alkynyl moieties, or "unsaturated alkyl” containing at least one alkenyl or alkynyl moiety.
- Alkenyl means a group comprising at least one carbon-carbon double bond
- alkynyl means a group comprising at least one carbon-carbon triple bond.
- Alkyl when used alone or in combination, may each be cyclic, branched or straight-chain.
- 'aryl' refers to a carbocyclic containing 6 carbon atoms which may be further fused with a second 5 or 6 membered carbocyclic group which may be aromatic, saturated or unsaturated. aromatic monocyclic group.
- aryl may include, but are not limited to, phenyl, indanyl, 1-naphthyl, 2-naphthyl, teprahyadronaphthyl, and the like.
- Aryl may be linked with other groups at appropriate positions on the aromatic ring.
- alkoxy' refers to an alkyl group linked to another group through an oxygen atom (ie, -O-alkyl).
- Alkoxy groups may be unsubstituted or substituted with one or more suitable substituents.
- the alkoxy group include a (C1-C6)alkoxy group such as -O-methyl, -O-ethyl, -O-propyl, -O-isopropyl, -O-2-methyl-1-propyl, -O-2 -Methyl-2-propyl, -O-2-methyl-1-butyl, -O-3-methyl-1-butyl, -O-2-methyl-3-butyl, -O-2,2-dimethyl-1 -Propyl, -O-2-methyl-1-pentyl, 3-O-methyl-1-pentyl, -O-4-methyl-1-pentyl, -O-2-methyl-2-pentyl, -O-3 -Meth
- 'phenoxy' refers to a phenyl group linked to another group through an oxygen atom (ie, -O-aryl).
- the phenoxy group is unsubstituted or one or more halogen; an alkyl group; It may be substituted with an aryl group and a hetero aryl group, but is not limited thereto.
- amino group' refers to an alkyl group linked to another group through a nitrogen atom (ie -NH- or -N-alkyl).
- the amino group may be unsubstituted or substituted with one or more suitable substituents.
- amino group examples include a (C1-C6)amino group such as -NH-methyl, -NH-ethyl, -NH-propyl, -NH-isopropyl, -NH-2-methyl-1-propyl, -NH-2-methyl -2-propyl, -NH-2-methyl-1-butyl, -NH-3-methyl-1-butyl, -NH-2-methyl-3-butyl, -NH-2,2-dimethyl-1-propyl , -NH-2-methyl-1-pentyl, 3-NH-methyl-1-pentyl, -NH-4-methyl-1-pentyl, -NH-2-methyl-2-pentyl, -NH-3-methyl -2-pentyl, -NH-4-methyl-2-pentyl, -NH-2,2-dimethyl-1-butyl, -NH-3,3-dimethyl-butyl, -NH-2-ethyl-1-butyl , -NH
- 'halogen group means fluorine, chlorine, bromine or iodine.
- heterocycle group' refers to a heteroaromatic compound including at least one hetero atom selected from the group consisting of N, O, and S, unless otherwise stated.
- the heterocyclyl group may include a pyrrolidine group, a furan group, a morpholine group, a piperazine and a piperidine group, more preferably a pyrrolidine group, a piperidine group, a piperazine group, and a mol It may include a porine group, but is not limited thereto.
- heteroaryl group' refers to a heteroaromatic compound including at least one hetero atom selected from the group consisting of N, O, and S, unless otherwise stated.
- the heteroaryl group is a pyridine group, a pyrazine group, a pyrimidine group, a pyridazine group, a pyrazole group, an imidazole group, a triazole group, an indole group, an oxadiazole group, a thiadiazole group, a quinoline, an isoquinoline group, It may include, but is not limited to, an isoxazole group, an oxazole group, a thiazole group, and a pyrrole group.
- R 1 Is hydrogen; or a halogen group; or a C 1 -C 3 alkyl group; and R 2 is hydrogen; C 1 -C 3 alkyl group; or an amino group (-NR 8 R 9 ); and R 3 is hydrogen; C 1 -C 3 alkyl group; sulfide group (-SR 8 ); or a sulfone group (—S(O) 2 R 8 ); and R 4 is hydrogen; Or a C 1 -C 3 alkyl group; and R 5 is hydrogen; hydroxyl group; halogen group; C 1 -C 3 alkyl group; or a C 1 -C 6 alkoxy group; and R 6 is hydrogen; halogen group; or a C 1 -C 3 alkyl group; and R 7 is hydrogen; or a C 1 -C 3 alkyl group; phosphorus, a pyrimidine derivative compound represented by Formula 10, 11 or 12, a pharmaceutically acceptable salt thereof, a hydrate thereof, and
- the B is a C 6 -C 10 aryl group; or a C 3 -C 10 heteroaryl group; Provided is a compound selected from phosphorus, a pyrimidine derivative compound represented by Formula 10, 11 or 12, a pharmaceutically acceptable salt thereof, a hydrate thereof, and a stereoisomer thereof.
- R 1 Is a halogen group; and R 2 is hydrogen; or an amino group (-NR 8 R 9 ); and R 3 is hydrogen; C 1 -C 3 alkyl group; or a sulfone group (—S(O) 2 R 8 ); and R 4 is hydrogen; and R 5 is hydrogen; halogen group; or a C 1 -C 6 alkoxy group; and R 6 is hydrogen; halogen group; or a C 1 -C 3 alkyl group; and R 7 is hydrogen;
- A is any one selected from the group consisting of piperazine, piperidine, morpholine, pyrrolidine and imidazole
- B is benzene, cyazole, thiophene, pyrazole, benzothiophene, pyridazine, Any one selected from the group consisting of pyrazine, imidazole, oxadiazole, triazole, furan, pyrimidine, oxazole,
- R 1 is Cl or Br
- R 2 is hydrogen or —NH 2
- R 3 is hydrogen; -CH 3 ; or —S(O) 2 CH 3
- R 4 is hydrogen
- R 5 is hydrogen
- R 6 is hydrogen
- R 7 is hydrogen
- A is any one selected from the group consisting of piperazine, piperidine, morpholine and pyrrolidine
- B is any one selected from the group consisting of benzene, benzocyazole, quinoline and quinoxaline, Formula 10
- A is any one selected from the group consisting of piperazine, piperidine, morpholine and pyrrolidine
- B is any one selected from the group consisting of benzene, benzocyazole, quinoline and quinoxaline, Formula 10,
- A is a first organic compound having the same in one aspect of the present invention.
- B is
- R 1 is Cl or Br
- R 2 is hydrogen or —NH 2
- R 3 is hydrogen; -CH 3 ; or —S(O) 2 CH 3
- R 4 is hydrogen
- R 5 is hydrogen
- R 6 is hydrogen; Cl; Br; F; -CH 3 ; or -CF 3
- R 7 is hydrogen
- A is , , , and Any one selected from the group consisting of, B is , , , , and Provided is a compound selected from a pyrimidine derivative compound represented by Formula 10, 11 or 12, which is any one selected from the group consisting of, a pharmaceutically acceptable salt thereof, a hydrate thereof, and a stereoisomer thereof.
- the compound is a pyrimidine derivative compound represented by Formula 10, 11 or 12, characterized in that any one selected from the group consisting of the following compound numbers 1 to 283, a pharmaceutically acceptable A compound selected from salts, hydrates thereof and stereoisomers thereof is provided:
- Compound No. 16 N- (2-((5-chloro-2-((5-fluoro-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridine- 3-yl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide;
- Compound No. 38 N- (2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methyl-1,4-diazepan-1-yl)) piperidin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide;
- Compound No. 40 N- (2-((5-bromo-2-((4-(4-(3-(dimethylamino)pyrrolidin-1-yl)piperidin-1-yl)-) 2-methoxy-5-methylphenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide;
- Compound No. 65 N- (4-chloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperi) din-1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)acetamide;
- Compound No. 70 N- (2-((5-chloro-2-((3-fluoro-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) amino)pyrimidin-4-yl)amino)phenyl)acetamide;
- Compound No. 80 N- (4-chloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperi) din-1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)methanesulfonamide;
- Compound No. 110 N- (5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) amino)pyrimidin-4-yl)-N- ( 4-(methylsulfonamido)benzo[d]thiazol-5-yl)methanesulfonamide;
- Compound No. 128 (6-((5-chloro-2-((2-methoxy-6-(piperazin-1-yl)pyridin-3-yl)amino)pyrimidin-4-yl)amino)quinoc salin-5-yl)dimethylphosphine oxide;
- Compound No. 130 (6-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) )phenyl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide;
- Compound No. 150 N- (2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(trifluoromethyl)-[1,4'-bipiperi) diin]-1′-yl)phenyl)amino)pyrimidin-4-yl)amino)-4-chlorophenyl)methanesulfonamide;
- Compound No. 180 N- (4-chloro-2-((5-chloro-2-((2-chloro-5-methyl-4-(4-(4-methyl-1,4-diazepane-1-) yl)piperidin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl) -N -methylmethanesulfonamide;
- Compound No. 181 N- (4-chloro-2-((5-chloro-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperi) din-1-yl)phenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide;
- Compound No. 192 (6-((5-bromo-2-((5-ethyl-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) )phenyl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide;
- Compound No. 220 N- (6-((5-chloro-2-((2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) amino)pyrimidin-4-yl)amino)-2,3-dihydrobenzofuran-5-yl)methanesulfonamide;
- the pharmaceutically acceptable salts are hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid, nitric acid, acetic acid, glycolic acid, lactic acid, pyruvic acid, malonic acid, succinic acid, glutaric acid, fumaric acid, malic acid, but Delic acid, tartaric acid, citric acid, ascorbic acid, palmitic acid, maleic acid, hydroxymaleic acid, benzoic acid, hydroxybenzoic acid, phenylacetic acid, cinnamic acid, salicylic acid, methanesulfonic acid, benzenesulfonic acid and toluenesulfonic acid selected from the group consisting of Provided is a compound selected from a pyrimidine derivative compound represented by Formula 10, 11 or 12, which is a salt of an inorganic acid or an organic acid, a pharmaceutically acceptable salt thereof, a hydrate thereof, and a stereoisomer thereof.
- Another aspect of the present invention provides a pharmaceutical composition for preventing, alleviating or treating cancer, wherein the compound according to any one of the aspects of the present invention is included as an active ingredient.
- compositions for preventing, alleviating or treating cancer characterized in that the cancer is caused by EGFR mutation.
- the pharmaceutical composition provides a pharmaceutical composition for preventing, alleviating or treating cancer, which is applied to a patient having an EGFR mutation.
- the cancer is at least one selected from the group consisting of glioblastoma, triple-negative breast cancer, colorectal cancer, lung cancer and head and neck cancer, and provides a pharmaceutical composition for preventing, alleviating or treating cancer.
- the cancer is lung cancer, it provides a pharmaceutical composition for preventing, alleviating or treating cancer.
- the pharmaceutical composition is an EGFR-related mutation, exon 19 deletion / T790M / C797S triple mutation or L858R / T790M / C797S triple mutation of L858R / T790M / C797S It is characterized in that administered to a patient, prevention, alleviation of cancer Or it provides a pharmaceutical composition for treatment.
- the pharmaceutical composition is an EGFR-related mutation, exon 19 deletion / T790M double mutation or L858R / T790M double mutation, characterized in that administered to a patient carrying a, cancer prevention, alleviation or treatment pharmaceuticals A composition is provided.
- the pharmaceutical composition provides a pharmaceutical composition for preventing, alleviating or treating cancer, characterized in that it is administered to a patient having an exon 19 deletion mutation or L858R mutation, which is an EGFR-related mutation.
- the pharmaceutical composition may be applied to, but not limited to, experimental animals such as mice, rabbits, rats, guinea pigs, or hamsters, or primates including humans, preferably, primates including humans, more preferably humans.
- experimental animals such as mice, rabbits, rats, guinea pigs, or hamsters, or primates including humans, preferably, primates including humans, more preferably humans.
- treatment refers to alleviation or amelioration of symptoms, reduction of the scope of disease, delay or alleviation of disease progression, improvement, alleviation or stabilization of disease state, partial or complete recovery, prolongation of survival and other beneficial therapeutic results, etc. may be used in the meaning of including all of them.
- cancer refers to treatment of all cancer cells, and cancer includes angiogenesis of endothelial cells and mitosis (solid tumors, tumor metastases and benign tumors).
- cancer is breast cancer, ovarian cancer, cervical cancer, prostate cancer, testicular cancer, urogenital organ cancer, esophageal cancer, laryngeal cancer, glioblastoma, gastric cancer, skin cancer, keratoacytoma, lung cancer, squamous cell carcinoma, large cell carcinoma, Small cell carcinoma, lung adenocarcinoma, bone cancer, colon cancer, adenoma, pancreatic cancer, adenocarcinoma, thyroid cancer, follicular adenocarcinoma, undifferentiated cancer, papillary cancer, seminomas, melanoma, sarcoma, bladder cancer, liver and bile duct cancer, kidney cancer, myeloid disease, lymphoid Disease, Hodgkin's disease
- the content of the compound represented by Formula 10, 11 or 12, a pharmaceutically acceptable salt thereof, or a hydrate thereof as an active ingredient according to the mode and method of use of the pharmaceutical composition of the present invention may be appropriately adjusted according to the selection of those skilled in the art.
- the pharmaceutical composition may contain the compound represented by Formula 10, 11 or 12, a pharmaceutically acceptable salt, or hydrate thereof in an amount of 0.1 to 10% by weight, more preferably 0.5 to 5% by weight, based on the total weight of the total composition. It may be included in an amount of weight %.
- a pharmaceutically acceptable salt or hydrate thereof of the compound represented by Formula 10, 11 or 12 may be included alone in the pharmaceutical composition, or may be included together with other pharmaceutically acceptable carriers, excipients, diluents or subcomponents. may be
- Examples of the pharmaceutically acceptable carrier, excipient or diluent include lactose, dextrose, sucrose, sorbitol, mannitol, xylitol, erythritol, maltitol, starch, acacia gum, alginate, gelatin, calcium phosphate, calcium silicate, Cellulose, methyl cellulose, microcrystalline cellulose, polyvinyl pyrrolidone, water, methylhydroxybenzoate, propylhydroxybenzoate, talc, magnesium stearate and mineral oil, propylhydroxybenzoate, talc, magnesium stearate and mineral oil , dextrin, calcium carbonate, propylene glycol, liquid paraffin, and at least one selected from the group consisting of physiological saline, but is not limited thereto, and all conventional carriers, excipients or diluents may be used.
- the pharmaceutical composition may contain conventional fillers, extenders, binders, disintegrants, anti-aggregating agents, lubricants, wetting agents, pH adjusting agents, nutrients, vitamins, electrolytes, alginic acid and salts thereof, pectic acid and salts thereof, protective colors, glycerin , a fragrance, emulsifier or preservative may be further included.
- the compound of Formula 10, 11 or 12 or a pharmaceutically acceptable salt thereof according to the present invention may be administered in combination with other anticancer agents for treating cancer or tumors to enhance the therapeutic effect of the anticancer agent.
- the method of administering the pharmaceutical composition may be oral or parenteral.
- it may be administered through several routes including oral, transdermal, subcutaneous, intravenous or intramuscular.
- the formulation of the composition may vary depending on the method of use, and is formulated using a method well known in the art to provide rapid, sustained or delayed release of the active ingredient after administration to a mammal.
- solid preparations for oral administration include tablets (TABLETS), pills, soft or hard capsules (CAPSULES), pills (PILLS), powders (POWDERS), and granules (GRANULES), and such preparations include one or more An excipient, for example, may be prepared by mixing starch, calcium carbonate, sucrose or lactose, gelatin, and the like. In addition to simple excipients, lubricants such as magnesium stearate and talc may also be used.
- Liquid formulations for oral use include suspensions (SUSTESIONS), internal solutions, emulsions (EMULSIONS), and syrups (SYRUPS). Sweetening agents, flavoring agents, preservatives, and the like may be included.
- Forms for parenteral administration are creams, lotions, ointments, ONITMENTS, PLASTERS, LIQUIDS AND SOULTIONS, aerosols, FRUIDEXTRACTS, and elixirs.
- ELIXIR infusions
- INFUSIONS infusions
- SACHET sachets
- PATCH PATCH
- injections inJECTIONS
- the pharmaceutical composition may further contain adjuvants such as sterilizing agents, preservatives, stabilizers, wetting agents or emulsification accelerators, salts and/or buffers for regulating osmotic pressure, and other therapeutically useful substances, and conventional mixing, granulation Alternatively, it may be formulated according to a coating method, and in addition, it may be formulated using an appropriate method known in the art.
- adjuvants such as sterilizing agents, preservatives, stabilizers, wetting agents or emulsification accelerators, salts and/or buffers for regulating osmotic pressure, and other therapeutically useful substances, and conventional mixing, granulation
- it may be formulated according to a coating method, and in addition, it may be formulated using an appropriate method known in the art.
- the dosage of the pharmaceutical composition can be determined in consideration of the administration method, the age, sex, severity, condition of the patient, the absorption of the active ingredient in the body, the inactivation rate, and the drug to be used in combination, once or several times. It can be administered in divided doses.
- an active ingredient of the pharmaceutical composition preferably in an amount of 0.001 to 100 mg/kg body weight, preferably 0.01 to 35 mg/kg body weight, per day to mammals including humans, once a day or divided into oral or parenteral It may be administered by the oral route.
- Another embodiment of the present invention provides a method for treating cancer, comprising administering a therapeutically effective amount of a compound represented by the following Chemical Formulas 10, 11 or 12, a pharmaceutically acceptable salt thereof, or a hydrate thereof do.
- the treatment method may further include the step of identifying a patient in need of prevention or treatment of the cancer before the administering step.
- the "therapeutically effective amount” of the present invention means an amount of an active ingredient for a mammal that is effective for the prevention or treatment of cancer, and the therapeutically effective amount includes the type of disease, the severity of the disease, the active ingredient and other ingredients contained in the composition. It can be adjusted according to various factors including the type and content of the drug, the type of dosage form, and the patient's age, weight, general health, sex and diet, administration time, administration route and blood clearance of the composition, treatment period, and concurrently used drugs. However, preferably, as described above, in an amount of 0.001 to 100 mg/kg body weight, preferably 0.01 to 35 mg/kg body weight, once a day or divided into oral or parenteral routes. can do.
- the present invention is characterized by a method for preparing a compound represented by the following formula (1).
- R 1 is a hydrogen atom, -Cl, -Br, and
- R 2 is a hydrogen atom, a C- 1 -C 5 alkyl group, —SO 2 CH 3 , or —SO 2 CF 3 ;
- R 3 , R 4 , R 8 , and R 9 is each independently selected from a hydrogen atom, a halogen, an amino group, a C- 1 -C 5 haloalkyl group, an alkoxy group, a C- 1 -C 5 alkyl group,
- R 5 and R 6 are each independently a hydrogen atom, halogen, -CF 3, C- 1 -C 5 haloalkyl group, C- 1 -C 5 alkyl group, is selected from;
- R 7 is -CF 3, C- 1 -C 5 haloalkyl group, C- 1 -C 5 alkyl group, is selected from;
- A is a C 3 -C 10 heterocycloalkyl group; or a C 6 -C 13 heterobicycloalkyl group; or a C 3 -C 15 linear or branched heteroalkyl group, wherein the heterocycloalkyl group or heterobicycloalkyl group, and the linear or branched heteroalkyl group are halogen; hydroxyl group; an alkyl alcohol group; It may have one or more substituents selected from a C 1-5 alkyl group;
- X is carbon or nitrogen
- Y is -N-, -NH-, -CH 2 -, -CO-, is selected from;
- Z is -NH-, -SO- 2 -, -SO-(CH 3 )-- 2 , is selected from;
- B is a C 6 aryl group, a heteroaryl group, or a C 3 -C 10 -heterobicycloaryl group.
- B refers to a monocyclic aryl group or a bicyclic, substituted or unsubstituted, aromatic group containing one or more heteroatoms selected from N, S, and O.
- bicyclic heteroaryl include indolyl, benzothiophenyl, benzofuranyl, benzimidazolyl, benzoxazolyl, benzisoxazolyl, benzthiazolyl, benzthiadiazolyl, benztriazolyl, quinolinyl, iso quinolinyl, purinyl, furopyridinyl, oxochromene, dioxoisoindoline, pyrazolopyridinyl, pyrazolo[1,5-a]pyridinyl, isoquinoxaline and the like; it's not going to be
- the present invention combines a commercially available pyrimidine compound represented by Formula 2 with an amine represented by Formula 3 to prepare a pyrimidine compound represented by Formula 1 below. includes the process of
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , X, Y, Z, A and B are as defined in Formula I above.
- the preparation method according to Scheme 1 can obtain Chemical Formula 4 by combining commercially available Chemical Formula 3 from a commercially available pyrimidine compound (Formula 2). It entails stirring at a temperature of 60 ° C.
- the reaction solvent is usually tetrahydrofuran, dioxane, N,N -dimethylformamide, N,N -dimethylsulfoxide, 2-butanol, 2-pentanol, etc. of organic solvents can be used.
- a sulfone group or a methyl group is substituted by reacting the prepared formula (4) at room temperature in a solvent such as N,N -dimethylformamide under basic conditions such as sodium hydride using commercially available sulfonyl chloride or methane iodide.
- a solvent such as N,N -dimethylformamide
- basic conditions such as sodium hydride using commercially available sulfonyl chloride or methane iodide.
- Formula 6 can be obtained.
- Formula 1 can be obtained by stirring the prepared amines represented by Formula 4 or Formula 6 and Formula 5 at 120° C. in the presence of 1.25 M hydrochloric acid methanol solution and acetic acid.
- Scheme 2 is a reaction scheme for preparing an amine intermediate of Formula 5
- R 3 , R 4 , R 8 , X, and A are as defined in Formula 1 above, and R 8 is a fluoro group or a chloro group.
- iron and ammonia chloride are stirred at 85° C. in a mixed solvent of tetrahydrofuran, methanol, and water to reduce the nitro group to an amino group to obtain Formula 5.
- the present invention relates to a pharmaceutical composition
- a pharmaceutical composition comprising the pyrimidine compound represented by Formula 1, Formula 10, Formula 11 or Formula 12, a pharmaceutically acceptable salt thereof, a solvate thereof, a hydrate thereof, or an optical isomer thereof as an active ingredient.
- the compound of the present invention Since the compound of the present invention has excellent ability to inhibit the activity of EGFR protein kinase, it can be used as an active ingredient in a pharmaceutical composition for the prevention and treatment of diseases caused by abnormal cell metabolism and sugar metabolism. Therefore, the compound according to the present invention is a disease caused by abnormal cell metabolism and glucose metabolism, for example, metabolic diseases such as diabetes and obesity, endometrial cancer, bladder cancer, stomach cancer, lung cancer, liver cancer, colon cancer, small intestine cancer, pancreatic cancer, brain cancer , bone cancer, melanoma, breast cancer, sclerosing adenoma, head and neck cancer, esophageal cancer, thyroid cancer, parathyroid cancer, kidney cancer, sarcoma, prostate cancer, urethral cancer, leukemia, multiple myeloma, blood cancer such as myelodysplastic syndrome, Hodgkin's disease and non-Hodgkin's disease It can be used as a prophylactic and therapeutic agent for various tumor diseases selected from lymphom
- the present invention provides a pharmaceutical composition
- a pharmaceutical composition comprising the pyrimidine compound represented by Formula 1, Formula 10, Formula 11 or Formula 12, a pharmaceutically acceptable salt thereof, a solvate thereof, and a hydrate thereof as an active ingredient, and abnormal cells It is characterized as a preventive and therapeutic agent for diseases caused by metabolism and sugar metabolism.
- the pharmaceutical composition of the present invention contains at least one selected from the pyrimidine compound represented by Formula 1, Formula 10, Formula 11 or Formula 12, pharmaceutically acceptable salts thereof, solvates thereof, and hydrates thereof as an active ingredient. and a conventional non-toxic pharmaceutically acceptable carrier, adjuvant and excipient added thereto, for example, a formulation for oral administration such as tablets, capsules, troches, solutions, suspensions, etc. It can be formulated as a preparation for parenteral administration.
- Excipients that can be used in the pharmaceutical composition of the present invention include sweeteners, binders, solubilizers, solubilizers, wetting agents, emulsifiers, isotonic agents, adsorbents, disintegrants, antioxidants, preservatives, lubricants, fillers, fragrances, and the like.
- lactose for example, lactose, dextrose, sucrose, mannitol, sorbitol, cellulose, glycine, silica, talc, stearic acid, sterine, magnesium stearate, magnesium aluminum silicate, starch, gelatin, gum tragacanth, arginic acid, sodium Alginate, methylcellulose, sodium carboxymethylcellulose, agar, water, ethanol, polyethylene glycol, polyvinylpyrrolidone, sodium chloride, calcium chloride, orange essence, strawberry essence, vanilla flavor, and the like.
- the dosage of the compound according to the present invention to the human body may vary depending on the patient's age, weight, sex, dosage form, health status and disease level, and is generally 0.01 based on an adult patient weighing 70 kg. to 1,000 mg/day, and may be administered in divided doses once or several times a day at regular time intervals according to the judgment of a doctor or pharmacist.
- Example 8 N- (5-chloro-2-((6-(3-(dimethylamino)pyrrolidin-1-yl)-2-methoxypyridin-3-yl)amino)pyrimidine-4 Preparation of -yl) -N- (2-(methylsulfonamido)phenyl)methanesulfonamide
- Example 1 1.0 g (3.0 mmol) of the compound prepared in step 1) of Example 1 was diluted in 6 ml of N,N-dimethylformamide, and 138 mg (6.0 mmol) of 60% sodium hydride was slowly added at 0° C. at room temperature. was stirred for 30 min. To this, 0.27 ml (3.6 mmol) of methanesulfonyl chloride was slowly added at 0° C., followed by stirring at room temperature for 2 hours. Upon completion of the reaction, the resulting mixture was diluted with ethyl acetate and washed with distilled water and saturated brine. The resulting organic layer was dried over anhydrous sodium sulfate, filtered under reduced pressure, and distilled under reduced pressure. The resulting residue was purified by chromatography (50% ethyl acetate/hexane) to obtain 1.95 g (yield: 65%) of the title compound.
- Step 2 N- (5-chloro-2-((6-(3-(dimethylamino)pyrrolidin-1-yl)-2-methoxypyridin-3-yl)amino)pyrimidin-4- Preparation of yl) -N- (2-(methylsulfonamido)phenyl)methanesulfonamide
- Example 11 N- (2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidini-1-yl)piperidini-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)(methyl)amino)phenyl) -N -methylsulfonamide
- Example 1 1.0 g (3.0 mmol) of the compound prepared in step 1) of Example 1 was diluted in 6 ml of N,N-dimethylformamide, and 138 mg (6.0 mmol) of 60% sodium hydride was slowly added at 0° C. at room temperature. was stirred for 30 min. 0.22 ml (3.6 mmol) of methyl iodide was slowly added thereto at 0° C., followed by stirring at room temperature for 2 hours. Upon completion of the reaction, the resulting mixture was diluted with ethyl acetate and washed with distilled water and saturated brine. The resulting organic layer was dried over anhydrous sodium sulfate, filtered under reduced pressure, and distilled under reduced pressure. The resulting residue was purified by chromatography (50% ethyl acetate/hexanes) to obtain 600 mg of the title compound (Yield: 53%).
- Step 2) N -(2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) Preparation of )phenyl)amino)pyrimidin-4-yl)(methyl)amino)phenyl) -N -methylsulfonamide
- Example 12 N- (2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amido)-5-fluorophenyl)methanesulfonamide
- Step 2 N-(2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) Preparation of )phenyl)amino)pyrimidin-4-yl)amido)-5-fluorophenyl)methanesulfonamide
- step 1) the compound prepared in step 2) of Example 1, 1-chloro-5-methoxy-2-methyl-4-nitrobenzene, and 1-methyl-4-piperidin-4-yl -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 15 N- (2-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)pyridin-3-yl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 24 N- (2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1) Preparation of -yl)phenyl)amino)pyrimidin-4-yl)amido)-5-fluorophenyl)methanesulfonamide
- Step 1) of Example 1 using 5-bromo-2,4-dichloropyrimidine and N- (2-amino-5-fluorophenyl)methanesulfonamide in Step 1) of Example 1) The title compound was obtained in the same manner as described above.
- Step 2 N-(2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amido)-5-fluorophenyl)methanesulfonamide
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 25 N- (2-((5-bromo-2-((5-fluoro-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridine) Preparation of -3-yl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 26 N- (2-((5-bromo-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1) Preparation of -yl)phenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 28 N- (2-((5-bromo-2-((2-methoxy-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridine) Preparation of -3-yl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 34 N- (2-((5-bromo-2-((2-chloro-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)-5) Preparation of -(trifluoromethyl)pyridin-3-yl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 36 N- (2-((5-bromo-2-((2-methoxy-6-(4-(1-methylpiperidin-4-yl)piperazin-1-yl)pyridine) Preparation of -3-yl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 39 N- (2-((5-bromo-2-((4-(3-(dimethylamino)-[1,4'-bipiperidin]-1'-yl)-2) Preparation of -methoxy-5-methylphenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 41 N- (2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-((1-methyl-1H-pyrazol-5-yl)amino) Preparation of )piperidin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 42 N- (2-((5-bromo-2-((4-(4-(4-cyclopropylpiperazin-1-yl)piperidin-1-yl)-2-methoxy Preparation of -5-methylphenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 44 N- (2-((5-bromo-2-((2-chloro-5-methyl-4-(4-(4-methyl-1,4-diazepan-1-yl)p) Preparation of peridin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Step 1) of Example 1 was carried out in the same manner as in Step 1) of Example 1 using 2-(trifluoromethyl)aniline to obtain the title compound.
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 48 5-chloro- N 2 -(4-(4-ethylpiperazin-1-yl)phenyl) -N 4 -(2-(trifluoromethyl)phenyl)pyrimidine-2,4-dia manufacturing of min
- Example 46 The title compound was obtained in the same manner as in Example 1 using the compound prepared in step 1) of Example 46 and the corresponding aniline.
- Example 50 2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) Preparation of )amino)pyrimidin-4-yl)amino) -N -methylbenzenesulfonamide
- step 1) of Example 1 the title compound was obtained in the same manner as in step 1) of Example 1 using 2-amino- N -methylbenzenesulfonamide.
- Step 2 2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) Preparation of amino)pyrimidin-4-yl)amino) -N -methylbenzenesulfonamide
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 1 The title compound was obtained in the same manner as in Example 1 using the compound prepared in step 1) of Example 50 and the corresponding aniline.
- step 1) of Example 1 the title compound was obtained in the same manner as in step 1) of Example 1 using 2-amino- N -cyclopropylbenzenesulfonamide.
- Step 2 2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) Preparation of amino)pyrimidin-4-yl)amino) -N -cyclopropylbenzenesulfonamide
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 60 2-((5-chloro-2-((3-fluoro-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl)amino)pyri Preparation of midin-4-yl)amino) -N -cyclopropylbenzenesulfonamide
- Example 62 5-chloro- N 2 -(2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) -N 4 Preparation of -(2-((methylsulfonyl)methyl)phenyl)pyrimidine-2,4-diamine
- Step 2) 5-chloro- N 2 -(2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) -N 4 - Preparation of (2-((methylsulfonyl)methyl)phenyl)pyrimidine-2,4-diamine
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 64 5-chloro- N 2 -(2-methoxy-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridin-3-yl) -N 4 Preparation of -(2-((methylsulfonyl)methyl)phenyl)pyrimidine-2,4-diamine
- Example 65 N- (4-chloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperid) Preparation of diin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)acetamide
- Step 1) of Example 1 the title compound was obtained in the same manner as in Step 1) of Example 1 using N- (2-amino-4-chlorophenyl)acetamide.
- Step 2) N -(4-chloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidine) Preparation of -1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)acetamide
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 66 N- (4-chloro-2-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperi) Preparation of diin-1-yl)pyridin-3-yl)amino)pyrimidin-4-yl)amino)phenyl)acetamide
- Example 68 N- (2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl) phenyl) amino) pyrimidin-4-yl) amino) phenyl) acetamide
- Step 1) of Example 1 the title compound was obtained in the same manner as in Step 1) of Example 1 using N- (2-aminophenyl)acetamide.
- Step 2) N -(2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) Preparation of )phenyl)amino)pyrimidin-4-yl)amino)phenyl)acetamide
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 70 N- (2-((5-chloro-2-((3-fluoro-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) Preparation of amino) pyrimidin-4-yl) amino) phenyl) acetamide
- Step 2) N -(4,5-dichloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)) Preparation of piperidin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)acetami
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 72 N- (4,5-dichloro-2-((5-chloro-2-((2-methoxy-6-(4-(4-methylpiperazin-1-yl)piperidine) Preparation of -1-yl)pyridin-3-yl)amino)pyrimidin-4-yl)amino)phenyl)acetamide
- Example 71 The title compound was obtained in the same manner as in Example 1 using the following aniline corresponding to the compound prepared in step 1) of Example 71.
- Example 75 N- (4,5-dichloro-2-((5-chloro-2-((2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidine) Preparation of -1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)acetamide
- Example 78 N- (4,5-dichloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl) )piperidin-1-yl)phenyl)amino)pyrimidin-4-yl)(methyl)amino)phenyl) -N -methylacetamide
- Step 2) N -(4,5-dichloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)) Preparation of piperidin-1-yl)phenyl)amino)pyrimidin-4-yl)(methyl)amino)phenyl) -N -methylacetamide
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 79 N- (4,5-dichloro-2-((5-chloro-2-((2-methoxy-6-(4-(4-methylpiperazin-1-yl)piperidine) Preparation of -1-yl)pyridin-3-yl)amino)pyrimidin-4-yl)(methyl)amino)phenyl) -N -methylacetamide
- Example 78 The compound prepared in step 1) of Example 78 and 6-chloro-2-methoxy-3-nitropyridine and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 80 N- (4-chloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperi) Preparation of diin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)methanesulfonamide
- Step 4) N -(4-chloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidine) Preparation of -1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)methanesulfonamide
- Example 1 The compound prepared in step 3) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 81 N- (4-chloro-2-((5-chloro-2-((2-methoxy-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl) pyridin-3-yl) amino) pyrimidin-4-yl) aminophenyl) methanesulfonamide
- Example 80 The title compound was obtained in the same manner as in Example 1 using the compound prepared in step 3) of Example 80 and the corresponding aniline.
- Example 82 N- (4-chloro-2-((5-chloro-2-((2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)methanesulfonamide
- Example 80 The title compound was obtained in the same manner as in Example 1 using the compound prepared in step 3) of Example 80 and the corresponding aniline.
- Example 83 N- (4-chloro-2-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperi) Preparation of diin-1-yl)pyridin-3-yl)amino)pyrimidin-4-yl)amino)phenyl)methanesulfonamide
- Example 84 N- (4-chloro-2-((5-chloro-2-((3-fluoro-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl) phenyl) amino) pyrimidin-4-yl) amino) phenyl) methanesulfonamide
- Example 85 N- (2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1) Preparation of -yl)phenyl)amino)pyrimidin-4-yl)amino-4-chlorophenyl)methanesulfonamide
- Example 80 The title compound was obtained in the same manner as in step 3) of Example 25, except that 5-bromo-2,4-dichloropyrimidine was used in step 3) of Example 80.
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 86 N- (2-((5-bromo-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperidin-1) Preparation of -yl)pyridin-3-yl)amino)pyrimidin-4-yl)amino)-4-chlorophenyl)methanesulfonamide
- Example 88 N- (2-((5-bromo-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1) Preparation of -yl)phenyl)amino)pyrimidin-4-yl)amino)-4-chlorophenyl)methanesulfonamide
- Example 85 The title compound was obtained in the same manner as in Example 1 using the compound prepared in step 1) of Example 85 and the corresponding aniline.
- Example 90 N- (2-((5-bromo-2-((4-(3-(dimethylamino)-[1,4′-bipiperidin]-1′-yl)-2 Preparation of -methoxy-5-methylphenyl)amino)pyrimidin-4-yl)amino)-4-chlorophenyl)methanesulfonamide
- Example 93 N- (2-((5-bromo-2-((2-chloro-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)-4-chlorophenyl)methanesulfonamide
- Example 96 N- (2-((5-bromo-2-((2-chloro-4-(4-(3-(dimethylamino)pyrrolidin-1-yl)piperidin-1) Preparation of -yl)-5-methylphenyl)amino)pyrimidin-4-yl)amino)-4-chlorophenyl)methanesulfonamide
- Example 80 The title compound was obtained in the same manner as in step 3) of Example 80, except that 4,5-dichloro-2-nitroaniline was used in step 1) of Example 80.
- Step 2) N -(4,5-dichloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)) Preparation of piperidin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)methanesulfonamide
- Example 100 N- (4,5-dichloro-2-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl) Preparation of )piperidin-1-yl)pyridin-3-yl)amino)pyrimidin-4-yl)amino)phenylmethanesulfonamide
- Example 101 N- (4,5-dichloro-2-((5-chloro-2-((3-fluoro-4-(4-(4-methylpiperazin-1-yl)piperidine) Preparation of -1-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)methanesulfonamide
- Example 102 N- (4,5-dichloro-2-((5-chloro-2-((2-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)) Preparation of quinolin-6-yl)amineno)pyrimidin-4-yl)amino)phenyl)methanesulfonamide
- Example 103 N- (4,5-dichloro-2-((5-chloro-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl) Preparation of )piperidin-1-yl)phenyl)amino)pyrimidin-4-yl)aminophenyl)methanesulfonamide
- Example 104 N- (4,5-dichloro-2-((5-chloro-2-((4-(4-((2-(dimethylamino)ethyl)(methyl)amino)piperidine Preparation of -1-yl)-2-methoxy-5-methylphenyl)amino)pyrimidin-4-yl)amino)phenyl)methanesulfonamide
- Example 105 N- (4,5-dichloro-2-((5-chloro-2-((2-methoxy-5-methyl-4-(methyl(3-(4-methylpiperazine-1) Preparation of -yl) propyl) amino) phenyl) amino) pyrimidin-4-yl) amino) phenyl) methanesulfonamide
- Example 106 N- (2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1) Preparation of -yl)phenyl)amino)pyrimidin-4-yl)amino)-4-methylphenyl)methanesulfonamide
- Example 24 Except for using 4-methyl-2-nitroaniline in step 1) of Example 24 and using 5-bromo-2,4-dichloropyrimidine in step 3) of Example 24 was carried out in the same manner as in step 3) of Example 24 to obtain the title compound.
- Step 2) N- (2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)-4-methylphenyl)methanesulfonamide
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 107 N- (2-((5-bromo-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1) Preparation of -yl)phenyl)amino)pyrimidin-4-yl)amino)-4-methylphenyl)methanesulfonamide
- Example 108 N- (2-((5-bromo-2-((5-fluoro-2-methoxy-4-(4-(4-methoxy-1-yl)piperidine-1) Preparation of -yl)phenyl)amino)pyrimidin-4-yl)amino)-4-methylphenyl)methanesulfonamide
- Example 109 N- (2-((5-bromo-2-((2-chloro-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)-4-methylphenyl)methanesulfonamide
- Example 111 N- (5-chloro-2-((2-methoxy-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridin-3-yl) Preparation of amino)pyrimidin-4-yl)-N- ( 4-(methylsulfonamido)benzo[d]thiazol-5-yl)methanesulfonamide
- Example 112 N- (5-chloro-2-((3-fluoro-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl)amino)pyrimidine Preparation of -4-yl) -N- (4-(methylsulfonamido)benzo[d]thiazol-5-yl)methanesulfonamide
- Example 114 N- (5-chloro-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) Preparation of amino)pyrimidin-4-yl)-N- ( 4-(methylsulfonamido)benzo[d]thiazol-5-yl)methanesulfonamide
- Example 115 N- (6-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)quinolin-5-yl)methanesulfonamide
- Example 80 The title compound was obtained in the same manner as in Example 80, except that 6-nitroquinolin-5-amine and 2,4-dichloropyrimidine were used in step 1) of Example 80.
- Example 1 The compound prepared in step 1) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 116 N- (6-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl) pyridin-3-yl) amino) pyrimidin-4-yl) amino) quinolin-5-yl) methanesulfonamide
- Example 118 N- (6-((5-chloro-2-((5-chloro-2-methoxy-4-(4-(4-methoxypiperazin-1-yl)piperidin-1) Preparation of -yl)phenyl)amino)pyrimidin-4-yl)amino)quinolin-5-yl)methanesulfonamide
- Example 120 N- (2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl) phenyl) amino) pyrimidin-4-yl) (methyl) amino) phenyl) methanesulfonamide
- Example 1 The compound prepared in step 3) and 1-chloro-5-methoxy-2-methyl-4-nitrobenzene and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 121 N- (2-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)pyridin-3-yl)amino)pyrimidin-4-yl)(methyl)amino)phenyl)methanesulfonamide
- Example 120 The compound prepared in step 3) of Example 120 and 2-chloro-6-methoxy-3-methyl-5-nitropyridine and 1-methyl-4-piperidine in step 2) of Example 1
- the title compound was obtained in the same manner as in Example 1, except that -4-yl-piperazine hydrochloride was used.
- Example 122 (6-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)) Preparation of pyridin-3-yl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Step 6 (6-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridine) Preparation of -3-yl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Example 1 The compound prepared in step 5) and 2-chloro-6-methoxy-3-methyl-5-nitropyridine and 1-methyl-4-piperidin-4-yl in step 2) of Example 1 -
- the title compound was obtained in the same manner as in Example 1, except that piperazine hydrochloride was used.
- Example 123 (6-((5-chloro-2-((2-methoxy-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridin-3- Preparation of yl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Example 124 (6-((5-chloro-2-((5-fluoro-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridin-3- Preparation of yl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Example 125 (6-((5-chloro-2-((3-fluoro-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl)amino) Preparation of pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Example 126 (6-((5-chloro-2-((2-methoxy-6-((3S,5R)-3,4,5-trimethylpiperazin-1-yl)pyridin-3- Preparation of yl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Example 127 (6-((5-chloro-2-((6-(3-(dimethylamino)pyrrolidin-1-yl)-2-methoxypyridin-3-yl)amino)pyrimidine Preparation of -4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Example 129 4-(5-((5-chloro-4-((5-(dimethylphosphoryl)quinoxalin-6-yl)amino)pyrimidin-2-yl)amino)-6-methoxy Preparation of pyridin-2-yl)thiomorpholine 1,1-dioxide
- Example 130 (6-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) Preparation of )phenyl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Example 122 5.0 g (28.5 mmol) of the compound prepared in step 1) of Example 122 was diluted in 60 ml methanol, and sodium t-butoxide 5.8 g (60 mmol) and hydroxylamine hydrochloride 4.1 g (60 mmol) were added at 0 ° C. After addition, the mixture was stirred at 65 °C for 4 hours. After the reaction was completed, the reaction mixture was cooled to room temperature, extracted with dichloromethane, filtered under reduced pressure, and concentrated to obtain 3.2 g (yield: 60%) of the title compound.
- Step 4) (6-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl)amino Preparation of )pyrimidin-4-yl)amino)quinoxalin-5-yl)dimethylphosphine oxide
- Example 131 N- (6-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)methanesulfonamide
- Example 130 5.0 g (28.5 mmol) of the compound prepared in the same manner as in step 2) of Example 130 was diluted with 40 ml 2 N sodium hydroxide / tetrahydrofuran and stirred at room temperature for 4 hours. After completion of the reaction, the reaction mixture was cooled to room temperature, extracted with dichloromethane, filtered under reduced pressure, and concentrated to obtain 6.7 g (yield: 87%) of the title compound.
- Step 2) N -(6-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) Preparation of )phenyl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)methanesulfonamide
- Example 132 N- (6-((5-chloro-2-((2-methoxy-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridine- Preparation of 3-yl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)methanesulfonamide
- Example 133 N- (6-((5-chloro-2-((2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl) Preparation of amino) pyrimidin-4-yl) amino) quinoxalin-5-yl) methanesulfonamide
- Example 134 N- (6-((5-chloro-2-((2-methoxy-5-methyl-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl) pyridin-3-yl) amino) pyrimidin-4-yl) amino) quinoxalin-5-yl) methanesulfonamide
- Example 135 N- (6-((5-chloro-2-((2-methoxy-6-((3S,5R)-3,4,5-trimethylpiperazin-1-yl)pyridine- Preparation of 3-yl)amino)pyrimidin-4-yl)amino)quinoxalin-5-yl)methanesulfonamide
- Example 136 N- (6-((5-chloro-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yyl)amino)quinoxalin-5-yl)methanesulfonamide
- Example 137 N- (2-((5-chloro-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)-4-(trifluoromethyl)phenyl)methanesulfonamide
- Example 138 N- (2-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)-4-(trifluoromethyl)phenyl)methanesulfonamide
- Example 139 N- (2-((5-chloro-2-((4-(4-(4-cyclopropylpiperazin-1-yl)piperidin-1-yl)-2-methoxy- Preparation of 5-methylphenyl)amino)pyrimidin-4-yl)amino)-4-(trifluoromethyl)phenyl)methanesulfonamide
- Example 140 N- (6-((5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)-2,3-dihydrobenzofuran-5-yl)methanesulfonamide
- Example 141 N- (2-((6-amino-5-chloro-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperid) Preparation of diin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)-5-fluorophenyl)methanesulfonamide
- Example 142 ((2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)imino)dimethyl- ⁇ 6 -sulfanone
- Step 4) ((2-((5-bromo-2-((2-methoxy-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) ) phenyl) amino) pyrimidin-4-yl) amino) phenyl) imino) dimethyl- Preparation of ⁇ 6 -sulfanone
- Example 143 ((2-((5-bromo-2-((5-chloro-2-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)piperidin-1-yl) Preparation of yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)imino)dimethyl- ⁇ 6 -sulfanone
- Example 144 ((2-((5-bromo-2-((2-chloro-5-methyl-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl) Preparation of ) phenyl) amino) pyrimidin-4-yl) amino) phenyl) imino) dimethyl- ⁇ 6 -sulfanone sulfanone
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Inorganic Chemistry (AREA)
- Dermatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Description
| GI50 Ba/F3 (μM) | ||||||
| 실시예 | Parental | EGFR-wt | EGFR-DTC | EGFR-LTC | EGFR-DT | EGFR-LT |
| 1 | E | D | C | nd | nd | nd |
| 2 | D | C | A | nd | nd | nd |
| 3 | D | D | C | nd | nd | nd |
| 4 | E | E | D | nd | nd | nd |
| 5 | B | C | A | nd | nd | nd |
| 6 | F | D | D | D | D | D |
| 7 | D | C | C | C | nd | nd |
| 8 | E | E | C | D | nd | nd |
| 9 | D | C | C | B | nd | nd |
| 10 | E | D | D | D | nd | nd |
| 11 | D | E | D | D | nd | nd |
| 12 | E | C | B | nd | nd | nd |
| 13 | D | D | C | nd | nd | nd |
| 14 | E | F | E | nd | nd | nd |
| 15 | D | D | C | C | A | C |
| 16 | D | E | D | nd | nd | nd |
| 17 | E | E | D | nd | nd | nd |
| 18 | C | B | D | D | D | D |
| 19 | E | D | D | D | D | D |
| 20 | E | E | D | D | D | D |
| 21 | F | E | D | D | D | E |
| 22 | D | E | D | D | B | E |
| 23 | C | C | C | C | C | D |
| 24 | D | C | A | A | A | A |
| 25 | D | D | C | C | nd | nd |
| 26 | E | C | B | B | nd | nd |
| 27 | E | E | C | D | nd | nd |
| 28 | E | E | D | D | nd | nd |
| 29 | F | F | F | F | nd | nd |
| 30 | F | F | E | E | nd | nd |
| 31 | E | E | D | E | nd | nd |
| 32 | E | E | E | E | nd | nd |
| 33 | E | E | E | E | nd | nd |
| 34 | E | E | D | C | nd | nd |
| 35 | C | C | B | B | nd | nd |
| 36 | E | D | C | C | nd | nd |
| 37 | F | F | F | F | nd | nd |
| 38 | D | C | B | B | B | C |
| 39 | D | C | A | A | C | B |
| 40 | E | D | B | C | nd | nd |
| 41 | E | D | C | D | nd | nd |
| 42 | D | D | B | C | nd | nd |
| 43 | D | C | C | B | nd | nd |
| 44 | D | D | B | B | B | C |
| 45 | D | D | B | B | B | C |
| 46 | E | E | E | nd | nd | nd |
| 47 | E | E | D | nd | nd | nd |
| 48 | E | E | D | nd | nd | nd |
| 49 | E | E | D | nd | nd | nd |
| 50 | D | D | C | nd | nd | nd |
| 51 | E | E | C | nd | nd | nd |
| 52 | C | C | B | nd | nd | nd |
| 53 | C | C | C | nd | nd | nd |
| 54 | E | C | E | nd | nd | nd |
| 55 | C | D | D | nd | nd | nd |
| 56 | E | D | D | D | C | D |
| 57 | D | D | D | D | C | D |
| 58 | C | C | C | C | C | D |
| 59 | D | D | D | nd | nd | nd |
| 60 | C | C | B | nd | nd | nd |
| 61 | D | E | E | nd | nd | nd |
| 62 | D | C | B | B | nd | nd |
| 63 | B | C | B | B | nd | nd |
| 64 | E | E | D | D | nd | nd |
| 65 | D | D | C | C | nd | nd |
| 66 | E | D | D | C | nd | nd |
| 67 | E | C | B | B | C | C |
| 68 | E | E | D | E | nd | nd |
| 69 | A | B | A | A | nd | nd |
| 70 | D | C | B | B | B | B |
| 71 | D | C | C | C | nd | nd |
| 72 | E | D | D | D | nd | nd |
| 73 | E | D | D | D | nd | nd |
| 74 | F | F | E | E | nd | nd |
| 75 | C | C | C | C | nd | nd |
| 76 | C | C | C | C | nd | nd |
| 77 | D | D | D | D | nd | nd |
| 78 | E | E | E | E | nd | nd |
| 79 | D | E | D | D | nd | nd |
| 80 | E | C | A | A | A | A |
| 81 | D | E | D | D | nd | nd |
| 82 | D | D | C | C | nd | nd |
| 83 | E | D | C | C | nd | nd |
| 84 | D | C | C | C | nd | nd |
| 85 | D | C | A | A | A | A |
| 86 | E | D | B | A | A | B |
| 87 | C | C | B | B | B | B |
| 88 | D | C | A | A | A | A |
| 89 | D | C | A | A | B | A |
| 90 | D | C | A | A | A | A |
| 91 | E | C | A | B | nd | nd |
| 92 | E | C | A | B | nd | nd |
| 93 | E | D | A | B | nd | nd |
| 94 | D | B | A | A | A | A |
| 95 | D | C | A | A | A | A |
| 96 | F | D | D | D | nd | nd |
| 97 | D | D | B | A | B | B |
| 98 | D | C | B | B | B | C |
| 99 | F | D | B | B | nd | nd |
| 100 | E | D | C | C | nd | nd |
| 101 | E | C | C | C | nd | nd |
| 102 | E | D | D | C | nd | nd |
| 103 | E | D | C | B | nd | nd |
| 104 | D | D | D | D | nd | nd |
| 105 | F | F | F | F | nd | nd |
| 106 | D | D | A | B | nd | nd |
| 107 | D | D | B | C | nd | nd |
| 108 | E | D | B | C | nd | nd |
| 109 | D | C | B | A | nd | nd |
| 110 | D | C | C | B | nd | nd |
| 111 | E | E | D | nd | D | E |
| 112 | C | C | B | B | nd | nd |
| 113 | E | E | D | D | nd | nd |
| 114 | D | D | C | C | nd | nd |
| 115 | E | C | A | A | C | B |
| 116 | E | D | B | C | nd | nd |
| 117 | E | D | D | D | nd | nd |
| 118 | F | D | C | C | nd | nd |
| 119 | E | D | D | D | nd | nd |
| 120 | F | E | E | E | nd | nd |
| 121 | F | E | E | E | nd | nd |
| 122 | D | C | C | B | nd | nd |
| 123 | E | D | C | C | nd | nd |
| 124 | C | C | B | B | nd | nd |
| 125 | C | C | B | C | nd | nd |
| 126 | E | E | C | D | nd | nd |
| 127 | F | F | E | E | nd | nd |
| 128 | E | E | D | D | nd | nd |
| 129 | F | E | E | E | nd | nd |
| 130 | D | D | D | B | nd | nd |
| 131 | D | C | B | A | A | B |
| 132 | D | D | C | C | nd | nd |
| 133 | D | D | C | C | nd | nd |
| 134 | C | C | B | B | C | C |
| 135 | D | D | C | C | nd | nd |
| 136 | D | D | C | C | nd | nd |
| 137 | E | D | C | C | nd | nd |
| 138 | D | E | C | C | nd | nd |
| 139 | D | E | D | D | nd | nd |
| 140 | D | C | B | A | A | B |
| 141 | F | D | D | D | nd | nd |
| 142 | D | D | C | B | D | D |
| 143 | C | C | C | C | D | D |
| 144 | D | D | D | C | D | C |
| 145 | E | E | C | C | nd | nd |
| 146 | E | D | C | C | nd | nd |
| 147 | D | D | B | B | B | B |
| 148 | D | D | C | C | C | D |
| 149 | C | C | C | B | C | C |
| 150 | D | D | B | B | C | C |
| 151 | D | C | A | A | A | A |
| 152 | D | C | A | A | A | A |
| 153 | E | D | C | C | C | D |
| 154 | E | E | C | D | D | E |
| 155 | C | B | A | A | A | B |
| 156 | C | A | A | A | A | A |
| 157 | D | C | C | B | B | B |
| 158 | E | D | A | A | B | B |
| 159 | E | C | A | A | A | A |
| 160 | E | D | B | C | C | C |
| 161 | E | E | C | D | C | D |
| 162 | E | D | C | E | E | E |
| 163 | F | E | C | E | E | E |
| 164 | F | E | C | E | E | E |
| 165 | E | D | B | B | B | B |
| 166 | D | C | A | A | nd | nd |
| 167 | E | D | C | E | E | E |
| 168 | E | B | A | A | A | A |
| 169 | D | C | A | A | A | A |
| 170 | C | B | A | A | A | A |
| 171 | C | B | A | A | A | B |
| 172 | C | C | A | A | B | A |
| 173 | C | C | B | B | A | A |
| 174 | D | C | A | B | A | A |
| 175 | F | D | B | B | C | C |
| 176 | D | C | A | A | B | B |
| 177 | E | E | B | A | C | C |
| 178 | E | D | A | B | B | B |
| 179 | D | C | C | B | C | B |
| 180 | E | D | B | B | B | B |
| 181 | E | D | C | B | B | B |
| 182 | F | E | B | B | B | B |
| 183 | B | A | A | A | A | A |
| 184 | D | B | C | A | A | A |
| 185 | D | D | D | B | nd | nd |
| 186 | C | B | B | B | nd | nd |
| 187 | D | C | B | B | nd | nd |
| 188 | D | C | A | A | B | B |
| 189 | D | B | A | A | A | B |
| 190 | C | C | B | B | A | A |
| 191 | E | C | A | A | A | A |
| 192 | C | B | A | A | A | A |
| 193 | D | C | A | B | C | C |
| 194 | C | C | B | B | B | C |
| 195 | C | C | B | B | nd | nd |
| 196 | E | D | A | C | A | B |
| 197 | E | C | B | C | A | C |
| 198 | nd | nd | nd | nd | nd | nd |
| 199 | C | C | A | A | A | B |
| 200 | C | C | B | B | A | B |
| 201 | B | A | A | A | A | A |
| 202 | C | C | B | B | A | B |
| 203 | E | D | B | B | A | C |
| 204 | E | B | A | B | A | B |
| 205 | E | B | A | A | A | A |
| 206 | E | C | A | B | A | A |
| 207 | E | C | B | B | B | A |
| 208 | E | C | A | B | A | A |
| 209 | E | C | A | B | B | A |
| 210 | E | D | C | B | B | B |
| 211 | E | C | B | A | A | B |
| 212 | E | C | B | A | A | A |
| 213 | E | C | B | B | A | C |
| 214 | E | C | B | B | A | A |
| 215 | E | C | B | B | A | B |
| 216 | E | B | A | B | A | A |
| 217 | D | C | A | A | A | A |
| 218 | D | C | A | C | A | B |
| 219 | E | C | A | B | A | A |
| 220 | B | D | C | C | C | B |
| 221 | D | C | B | B | B | B |
| 222 | E | C | A | C | A | A |
| 223 | E | E | D | D | C | C |
| 224 | C | D | B | B | C | B |
| 225 | E | D | A | B | B | B |
| 226 | D | C | B | B | B | B |
| 227 | C | A | A | B | A | A |
| 228 | C | C | A | B | B | A |
| 229 | E | D | B | C | A | B |
| 230 | E | D | C | C | B | C |
| 231 | E | E | C | C | C | C |
| 232 | E | D | B | C | B | C |
| 233 | E | D | B | B | B | B |
| 234 | E | E | C | C | C | C |
| 235 | E | D | B | C | B | C |
| 236 | E | E | C | C | C | C |
| 237 | E | D | C | A | C | A |
| 238 | E | E | E | C | E | C |
| 239 | E | D | D | C | B | B |
| 240 | E | D | C | A | C | A |
| 241 | E | D | C | B | C | A |
| 242 | E | E | E | B | D | A |
| 243 | E | D | C | A | C | A |
| 244 | C | B | B | A | A | A |
| 245 | E | E | B | A | C | A |
| 246 | E | D | C | A | C | A |
| 247 | E | D | C | C | B | C |
| 248 | E | C | B | A | A | A |
| 249 | E | C | B | A | A | A |
| 250 | E | C | A | A | A | A |
| 251 | E | C | B | A | A | B |
| 252 | E | B | B | A | A | A |
| 253 | C | D | A | B | B | B |
| 254 | D | D | A | B | A | B |
| 255 | E | E | B | C | B | C |
| 256 | C | C | B | C | A | C |
| 257 | C | E | A | C | B | C |
| 258 | C | D | A | C | B | C |
| 259 | D | D | A | C | B | B |
| 260 | E | C | C | C | A | B |
| 261 | E | B | C | B | A | B |
| 262 | B | C | B | B | A | B |
| 263 | F | D | D | C | C | C |
| 264 | E | C | B | B | A | A |
| 265 | D | C | B | B | B | B |
| 266 | E | E | D | C | B | C |
| 267 | D | D | C | B | B | B |
| 268 | E | D | C | C | B | B |
| 269 | E | D | C | C | B | B |
| 270 | D | D | A | A | A | A |
| 271 | E | E | A | A | A | A |
| 272 | E | E | D | D | D | D |
| 273 | D | B | D | C | D | C |
| 274 | D | B | E | D | D | D |
| 275 | D | C | D | D | C | D |
| 276 | E | D | C | C | B | C |
| 277 | E | F | F | F | E | E |
| 278 | E | F | E | F | F | E |
| 279 | E | E | E | E | C | D |
| 280 | E | B | C | A | A | A |
| 281 | C | C | B | A | A | A |
| 282 | E | C | C | B | A | A |
| 283 | E | C | C | B | A | A |
| BBT-176 | D | D | C | C | B | C |
| TQB-3804 | D | D | B | B | C | C |
| [GI50의 분류] A: 0.001 μM ~ 0.025 μM, B: 0.025 μM ~ 0.100 μM, C: 0.100 μM ~ 0.500 μM, D: 0.500 μM ~ 2.0 μM, E: 2.0 μM ~ 10.0 μM, F: > 10.0 Μm, nd: not determined |
||||||
Claims (18)
- 하기 화학식 10, 11 또는 12로 표시되는 피리미딘 유도체 화합물, 이의 약학적으로 허용 가능한 염, 이의 수화물 및 이의 입체 이성질체로부터 선택된 화합물:[화학식 10][화학식 11][화학식 12]상기 화학식 10 내지 12에서,R1는 수소; 히드록시기; 할로겐기; C1-C13 알킬기; 또는 C1-C6 알콕시기;이고,R2는 수소; 할로겐기; C1-C13 알킬기; C3-C10 사이클릴기; 아미노기(-NR8R9); 또는 니트로기(-N(O)2);이고,R3는 및 R4는 각각 독립적으로 수소; C1-C13 알킬기; C3-C10 사이클릴기; 설피드기(-SR8); 술폰기(-S(O)2R8);또는 포스피릴기(-P(O)R8R9)이고;R5, R6 및 R7는 각각 독립적으로 수소; 히드록시기; 할로겐기; C1-C13 알킬기; C1-C6 알콕시기; C3-C10 사이클릴기; C3-C10 헤테로사이클릴기; -C(O)-(C1-C13 알킬); 아미노기(-NR8R9); 니트로기(-N(O)2); 아마이드기(-(C=O)NR8R9); 카르복실산기(-C(O)OH); 니트릴기(-CN); 유레아기(-NR8(C=O)NR9-); 술폰아미드기(-NHS(O)2R8); 설피드기(-SR8); 술폰기(-S(O)2R8);또는 포스피릴기(-P(O)R8R9)이고;X1, X2 및 X3 은 각각 독립적으로 탄소 또는 질소이고,A는 C1-C13 알킬기; 아미노기(-NR8R9); C6-C10 아릴기; C3-C10 사이클릴기; C3-C10 헤테로아릴기; C3-C10 헤테로사이클릴기; 또는 A는 R6과 연결된 탄소 원자와 함께 N, O, S, NH, C=N, C=O, -NHC(O)-, -NHC(O)NH-, -NHS(O)2-, 또는 SO2 중 적어도 1종을 임의로 포함할 수 있고, 수소, C1-C13 알킬기, C6-C10 아릴기, C3-C10 헤테로아릴기, 히드록실기, 할라이드기 및 시아노기 중 적어도 1종으로 임의로 치환될 수 있는 3 내지 7원(membered) 포화 고리를 형성하고;B는 C6-C10 아릴기; C3-C10 사이클릴기; C3-C10 헤테로아릴기; 또는 C3-C10 헤테로사이클릴기; 이고,X1 또는 X3가 각각 독립적으로 질소일 때, R2 또는 R7은 치환되지 않으며,상기 C1-C6 알콕시기, C1-C13 알킬기 또는 C3-C10 사이클릴기는, 수소; 히드록시기; 할로겐기; C1-C13 알킬기; C1-C6 알콕시기; 아미노기(-NR8R9); 니트로기(-N(O)2); 아마이드기(-(C=O)NR8R9); 카르복실산기(-C(O)OH); 니트릴기(-CN); 유레아기(-NR8(C=O)NR9-); 술폰아미드기(-NHS(O)2-); 설피드기(-S-); 술폰기(-S(O)2-); 포스피릴기(-P(O)R8R9); C6-C10 아릴기; C3-C10 헤테로아릴기; 및 C3-C10 헤테로사이클릴기로 이루어진 군으로부터 선택되는 1 이상의 치환기를 포함하며,상기 C6-C10 아릴기, C3-C10 헤테로아릴기 또는 C3-C10 헤테로사이클릴기는, 수소; 히드록시기; 할로겐기; 카보닐기(-(C=O)R8R9); 할로겐 또는 C3-C10 헤테로사이클릴기로 치환 또는 비치환된 C1-C3 알킬기; 할로겐 또는 C3-C10 헤테로사이클릴기로 치환 또는 비치환된 C1-C3 알콕시기; C6-C10 페녹시; 아미노기(-NR8R9); 니트로기(-N(O)2); 아마이드기(-(C=O)NR8R9); 카르복실산기(-C(O)OH); 니트릴기(-CN); 유레아기(-NR8(C=O)NR9-); 술폰아미드기(-NHS(O)2-); 설피드기(-S-); 술폰기(-S(O)2-); 포스피릴기(-P(O)R8R9); C6-C10 아릴기; C3-C10 헤테로아릴기 및 C3-C10 헤테로사이클릴기로 이루어진 군에서 선택되는 1 이상의 치환기를 포함하고,상기 R8 및 R9은 각각 독립적으로 수소; C1-C6 알킬기; C1-C6 알케닐기; C1-C6 알키닐기; C6-C10 아릴기; C3-C10 헤테로아릴기; C3-C10 헤테로사이클릴기; 또는 R8는 R9과 연결된 질소 또는 탄소 원자와 함께 N, O, S, NH, C=N, C=O, -NHC(O)-, -NHC(O)NH-, -NHS(O)2-, 또는 SO2 중 적어도 1종을 임의로 포함할 수 있고, 수소, C1-C13 알킬기, C6-C10 아릴기, C3-C10 헤테로아릴기, 히드록실기, 할라이드기 및 시아노기 중 적어도 1종으로 임의로 치환될 수 있는 3 내지 7원(membered) 포화 고리를 형성하고,상기 C3-C10 헤테로아릴기 및 C3-C10 헤테로사이클릴기는 N, O, 및 S로 이루어지는 군에서 선택된 1종 이상의 헤테로원자를 포함한다.
- 제 1항에 있어서,R1는 수소; 또는 할로겐기; 또는 C1-C3 알킬기; 이고,R2는 수소; C1-C3 알킬기; 또는 아미노기(-NR8R9); 이며,R3는 수소; C1-C3 알킬기; 설피드기(-SR8); 또는 술폰기(-S(O)2R8); 이고,R4는 수소; 또는 C1-C3 알킬기;이며,R5 는 수소; 히드록시기; 할로겐기; C1-C3 알킬기; 또는 C1-C6 알콕시기; 이고,R6 는 수소; 할로겐기; 또는 C1-C3 알킬기; 이며,R7는 수소; 또는 C1-C3 알킬기;인,화학식 10, 11 또는 12로 표시되는 피리미딘 유도체 화합물, 이의 약학적으로 허용 가능한 염, 이의 수화물 및 이의 입체 이성질체로부터 선택된 화합물.
- 제 1항에 있어서,상기 A는 아미노기(-NR8R9); C3-C10 헤테로아릴기; C3-C10 헤테로사이클릴기; 또는 A는 R6과 연결된 탄소 원자와 함께 N, O, S, NH, C=N, C=O, -NHC(O)-, -NHC(O)NH-, -NHS(O)2-, 또는 SO2 중 적어도 1종을 임의로 포함할 수 있고, 수소, C1-C13 알킬기, C6-C10 아릴기, C3-C10 헤테로아릴기, 히드록실기, 할라이드기 및 시아노기 중 적어도 1종으로 임의로 치환될 수 있는 3 내지 7원(membered) 포화 고리를 형성하고;상기 B는 C6-C10 아릴기; 또는 C3-C10 헤테로아릴기; 인,화학식 10, 11 또는 12로 표시되는 피리미딘 유도체 화합물, 이의 약학적으로 허용 가능한 염, 이의 수화물 및 이의 입체 이성질체로부터 선택된 화합물.
- 제 1항에 있어서,R1는 할로겐기; 이고,R2는 수소; 또는 아미노기(-NR8R9); 이며,R3는 수소; C1-C3 알킬기; 또는 술폰기(-S(O)2R8); 이고,R4는 수소; 이며,R5 는 수소; 할로겐기; 또는 C1-C6 알콕시기; 이고,R6 는 수소; 할로겐기; 또는 C1-C3 알킬기; 이며,R7는 수소; 이며,A는 피페라진, 피페리딘, 모르포린, 피롤리딘 및 이미다졸로 이루어준 군에서 선택된 어느 하나이며,B는 벤젠, 사이아졸, 사이오펜, 피라졸, 벤조사이오펜, 피리다진, 피라진, 이미다졸, 옥사디아졸, 트리아졸, 퓨란, 피리미딘, 옥사졸, 피롤, 피리딘, 옥사디아졸, 트리아진, 시아디아졸, 이속사졸, 및 테트라졸로 이루어진 군에서 선택된 어느 하나인,화학식 10, 11 또는 12로 표시되는 피리미딘 유도체 화합물, 이의 약학적으로 허용 가능한 염, 이의 수화물 및 이의 입체 이성질체로부터 선택된 화합물.
- 제 1항에 있어서,R1는 Cl 또는 Br 이고,R2는 수소 또는 -NH2 이며,R3는 수소; -CH3; 또는 -S(O)2CH3; 이고,R4는 수소; 이며,R5 는 수소; Cl; Br; -OCH3; -OCH2CH3 또는 -OCH2CF3 이고,R6 는 수소; Cl; Br; F; -CH3; 또는 -CF3; 이며,R7는 수소; 이며,A는 피페라진, 피페리딘, 모르포린 및 피롤리딘로 이루어진 군에서 선택된 어느 하나이며,B는 벤젠, 벤조사이아졸, 퀴놀린 및 퀴녹살린으로 이루어진 군에서 선택된 어느 하나인,화학식 10, 11 또는 12로 표시되는 피리미딘 유도체 화합물, 이의 약학적으로 허용 가능한 염, 이의 수화물 및 이의 입체 이성질체로부터 선택된 화합물.
- 제 1항에 있어서,R1는 Cl 또는 Br 이고,R2는 수소 또는 -NH2 이며,R3는 수소; -CH3; 또는 -S(O)2CH3; 이고,R4는 수소; 이며,R5 는 수소; Cl; Br; -OCH3; -OCH2CH3 또는 -OCH2CF3 이고,R6 는 수소; Cl; Br; F; -CH3; 또는 -CF3; 이며,R7는 수소; 이며,화학식 10, 11 또는 12로 표시되는 피리미딘 유도체 화합물, 이의 약학적으로 허용 가능한 염, 이의 수화물 및 이의 입체 이성질체로부터 선택된 화합물.
- 제 1항에 있어서,상기 화합물은 하기 화합물번호 1 내지 283로 이루어진 군으로부터 선택되는 어느 하나인 것을 특징으로 하는, 화학식 10, 11 또는 12로 표시되는 피리미딘 유도체 화합물, 이의 약학적으로 허용 가능한 염, 이의 수화물 및 이의 입체 이성질체로부터 선택된 화합물:화합물번호 1: N-(2-((5-클로로-2-((6-(4-에틸피페라진-1-일)-2-메톡시피리딘-3-일)아미노)-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 2: N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 3: N-(2-((5-클로로-2-((6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 4: N-(2-((5-클로로-2-((6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)-2-(2,2,2-트라이플루오로에톡시)피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 5: N-(2-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 6: N-(2-((5-클로로-2-((6-(1,1-다이옥시싸이오몰폴리노)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 7: N-(2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메설폰아마이드;화합물번호 8: N-(5-클로로-2-((6-(3-(다이메틸아미노)피롤리딘-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)-N-(2-(메틸설폰아마이도)페닐)메탄설폰아마이드;화합물번호 9: N-(5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)-N-(2-(메틸설폰아마이도)페닐)메탄설폰아마이드;화합물번호 10: N-(5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)-N-(2-(메틸설폰아마이도)페닐)메탄설폰아마이드;화합물번호 11: N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리디니-1-일)페닐)아미노)피리미딘-4-일)(메틸)아미노)페닐)-N-메틸설폰아마이드;화합물번호 12: N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아마이도)-5-플루오로페닐)메탄설폰아마이드;화합물번호 13: N-(2-((5-클로로-2-((6-몰폴리노피리딘-3-일)아미노)피리미딘-4-일)아마이도)-5-플루오로페닐)메탄설폰아마이드;화합물번호 14: N-(2-((5-클로로-2-((6-(4-(4-메틸피페라진-1-yl)피페리딘-1-일)피리딘-3-일)아마이도)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 15: N-(2-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 16: N-(2-((5-클로로-2-((5-플루오로-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 17: N-(2-((5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 18: N-(2-((5-클로로-2-((6-(4-(2-하이드록시메틸)피페라진-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 19: N-(2-((2-((6-(4-아세틸피페라진-1-일)-2-메톡시피리딘-3-일)아미노)-5-클로로피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 20: N-(2-((5-클로로-2-((2-메톡시-6-((3S,5R)-3,4,5-트라이메틸피페라진-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 21: N-(2-((5-클로로-((6-(4-하이드록시피페리딘-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 22: N-(2-((5-클로로-2-((2-메톡시-6-(4-(옥세탄-3-일)피페라진-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 23: N-(2-((5-클로로-2-((6-(3-(다이메틸아미노)피롤리딘-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 24: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아마이도)-5-플루오로페닐)메탄설폰아마이드;화합물번호 25: N-(2-((5-브로모-2-((5-플루오로-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 26: N-(2-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 27: N-(2-((5-브로모-2-((2-클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 28: N-(2-((5-브로모-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 29: N-(2-((5-브로모-2-((2-메톡시-6-몰폴리노피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 30: N-(2-((5-브로모-2-((6-(4-사이클로프로필피페라진-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 31: N-(2-((5-브로모-2-((2-메톡시-6-(4-몰폴리노피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 32: N-(2-((5-브로모-2-((6-(4,4-다이메틸피페리딘-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드 ;화합물번호 33: N-(2-((5-브로모-2-((6-(4,4-다이플루오로피페리딘-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 34: N-(2-((5-브로모-2-((2-클로로-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)-5-(트라이플루오로메틸)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 35: N-(2-((5-브로모-2-((2-(4-(4-메틸피페라진-1-일)피페리딘-1-일)퀴놀린-6-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드 ;화합물번호 36: N-(2-((5-브로모-2-((2-메톡시-6-(4-(1-메틸피페리딘-4-일)피페라진-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 37: N-(2-((5-브로모-2-((2-메톡시-6-(4-(1-메틸-4-일)피페라진-1-일)-5-(트라이플루오로메틸)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 38: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 39: N-(2-((5-브로모-2-((4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 40: N-(2-((5-브로모-2-((4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 41: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-((1-메틸-1H-피라졸-5-일)아미노)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 42: N-(2-((5-브로모-2-((4-(4-(4-사이클로프로필피페라진-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 43: N-(2-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸피페리딘-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 44: N-(2-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 45: N-(2-((5-브로모-2-((2-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)플루오로페닐)메탄설폰아마이드;화합물번호 46: 5-클로로-N 2-(2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(2-(트라이플루오로메틸)페닐)피리미딘-2,4-다이아민;화합물번호 47: 5-클로로-N 2-(2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(2-(트라이플루오로메틸)페닐)피리미딘-2,4-다이아민;화합물번호 48: 5-클로로-N 2-(4-(4-에틸피페라진-1-일)페닐)-N 4-(2-(트라이플루오로메틸)페닐)피리미딘-2,4-다이아민;화합물번호 49: 5-클로로-N 2-(6-몰폴리노피리딘-3-일)-N 4-(2-(트라이플루오로메틸)페닐)피리미딘-2,4-다이아민;화합물번호 50: 2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 51: 2-((5-클로로-2-((2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 52: 2-((5-클로로-2-((6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 53: 2-((5-클로로-2-((6-몰폴리노피리딘-3-일)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 54: 2-((5-클로로-2-((6-(4-에틸피페라진-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 55: 2-((5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 56: 2-((5-클로로-2-((6-(4-(2-하이드록시에틸)피페라진-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 57: 2-((5-클로로-2-((6-(3-(다이메틸아미노)피롤리딘-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 58: 2-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-N-메틸벤젠설폰아마이드;화합물번호 59: 2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-N-사이클로프로필벤젠설폰아마이드;화합물번호 60: 2-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-N-사이클로프로필벤젠설폰아마이드;화합물번호 61: 2-((5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페라딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-N-사이클로프로필벤젠설폰아마이드;화합물번호 62: 5-클로로-N 2-(2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(2-((메틸설포닐)메틸)페닐)피리미딘-2,4-다이아민;화합물번호 63: 5-클로로-N 2-(3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(2-((메틸설포닐)메틸)페닐)피리미딘-2,4-다이아민;화합물번호 64: 5-클로로-N 2-(2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)-N 4-(2-((메틸설포닐)메틸)페닐)피리미딘-2,4-다이아민;화합물번호 65: N-(4-클로로-2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 66: N-(4-클로로-2-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 67: N-(4-클로로-2-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 68: N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 69: N-(2-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 70: N-(2-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 71: N-(4,5-다이클로로-2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 72: N-(4,5-다이클로로-2-((5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 73: N-(4,5-다이클로로-2-((5-클로로-2-((5-플루오로-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 74: N-(4,5-다이클로로-2-((5-클로로-2-((6-몰폴리노피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 75: N-(4,5-다이클로로-2-((5-클로로-2-((2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 76: N-(4,5-다이클로로-2-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 77: N-(4,5-다이클로로-2-((5-클로로-2-((2-클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)아세타마이드;화합물번호 78: N-(4,5-다이클로로-2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)(메틸)아미노)페닐)-N-메틸아세타마이드;화합물번호 79: N-(4,5-다이클로로-2-((5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)(메틸)아미노)페닐)-N-메틸아세타마이드;화합물번호 80: N-(4-클로로-2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 81: N-(4-클로로-2-((5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노페닐)메탄설폰아마이드;화합물번호 82: N-(4-클로로-2-((5-클로로-2-((2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 83: N-(4-클로로-2-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 84: N-(4-클로로-2-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 85: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노-4-클로로페닐)메탄설폰아마이드;화합물번호 86: N-(2-((5-브로모-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 87: N-(2-((5-브로모-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 88: N-(2-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 89: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설포닐아마이드;화합물번호 90: N-(2-((5-브로모-2-((4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 91: N-(2-((5-브로모-2-((4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 92: N-(2-((5-브로모-2-((5-플루오로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 93: N-(2-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 94: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 95: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 96: N-(2-((5-브로모-2-((2-클로로-4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 97: N-(2-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 98: N-(2-((5-브로모-2-((2-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 99: N-(4,5-다이클로로-2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 100: N-(4,5-다이클로로-2-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)페닐메탄설폰아마이드;화합물번호 101: N-(4,5-다이클로로-2-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 102: N-(4,5-다이클로로-2-((5-클로로-2-((2-(4-(4-메틸피페라진-1-일)피페리딘-1-일)퀴놀린-6-일)아민노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 103: N-(4,5-다이클로로-2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노페닐)메탄설폰아마이드;화합물번호 104: N-(4,5-다이클로로-2-((5-클로로-2-((4-(4-((2-(다이메틸아미노)에틸)(메틸)아미노)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 105: N-(4,5-다이클로로-2-((5-클로로-2-((2-메톡시-5-메틸-4-(메틸(3-(4-메틸피페라진-1-일)프로필)아미노)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설포나마이드;화합물번호 106: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메틸페닐)메탄설폰아마이드;화합물번호 107: N-(2-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메틸페닐)메탄설폰아마이드;화합물번호 108: N-(2-((5-브로모-2-((5-플루오로-2-메톡시-4-(4-(4-메톡시-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메틸페닐)메탄설폰아마이드;화합물번호 109: N-(2-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메틸페닐)메탄설폰아마이드;화합물번호 110: N-(5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)-N-(4-(메틸설폰아마이도)벤조[d]싸이아졸-5-일)메탄설폰아마이드;화합물번호 111: N-(5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)-N-(4-(메틸설폰아마이도)벤조[d]싸이아졸-5-일)메탄설폰아마이드;화합물번호 112: N-(5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)-N-(4-(메틸설폰아마이도)벤조[d]싸이아졸-5-일)메탄설폰아마이드;화합물번호 113: N-(5-클로로-2-((5-플루오로-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)-N-(4-(메틸설폰아마이도)벤조[d]싸이아졸-5-일)메탄설폰아마이드;화합물번호 114: N-(5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)-N-(4-(메틸설폰아마이도)벤조[d]싸이아졸-5-일)메탄설폰아마이드;화합물번호 115: N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴놀린-5-일)메탄설폰아마이드;화합물번호 116: N-(6-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴놀린-5-일)메탄설폰아마이드;화합물번호 117: N-(6-((5-클로로-2-((2-메톡시-6-(4-몰폴리노피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴놀린-5-일)메탄설폰아마이드;화합물번호 118: N-(6-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메톡시피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴놀린-5-일)메탄설폰아마이드;화합물번호 119: N-(6-((5-클로로-2-((2-메톡시-4-(4-메틸피페라진-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴놀린-5-일)메탄설폰아마이드;화합물번호 120: N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)(메틸)아미노)페닐)메탄설폰아마이드;화합물번호 121: N-(2-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)(메틸)아미노)페닐)메탄설폰아마이드;화합물번호 122: (6-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 123: (6-((5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 124: (6-((5-클로로-2-((5-플루오로-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 125: (6-((5-클로로-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀옥사이드;화합물번호 126: (6-((5-클로로-2-((2-메톡시-6-((3S,5R)-3,4,5-트라이메틸피페라진-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 127: (6-((5-클로로-2-((6-(3-(다이메틸아미노)피롤리딘-1-일)-2-메톡시피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 128: (6-((5-클로로-2-((2-메톡시-6-(피페라진-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 129: 4-(5-((5-클로로-4-((5-(다이메틸포스포릴)퀴녹살린-6-일)아미노)피리미딘-2-일)아미노)-6-메톡시피리딘-2-일)싸이오몰폴린 1,1-다이옥사이드;화합물번호 130: (6-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 131: N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)메탄설폰아마이드;화합물번호 132: N-(6-((5-클로로-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)메탄설폰아마이드;화합물번호 133: N-(6-((5-클로로-2-((2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)메탄설폰아마이드;화합물번호 134: N-(6-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)메탄설폰아마이드;화합물번호 135: N-(6-((5-클로로-2-((2-메톡시-6-((3S,5R)-3,4,5-트라이메틸피페라진-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)메탄설폰아마이드;화합물번호 136: N-(6-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-y일)아미노)퀴녹살린-5-일)메탄설폰아마이드;화합물번호 137: N-(2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-(트라이플루오로메틸)페닐)메탄설폰아마이드;화합물번호 138: N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-(트라이플루오로메틸)페닐)메탄설폰아마이드;화합물번호 139: N-(2-((5-클로로-2-((4-(4-(4-사이클로프로필피페라진-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-(트라이플루오로메틸)페닐)메탄설폰아마이드;화합물번호 140: N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 141: N-(2-((6-아미노-5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 142: ((2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)이미노)다이메틸- λ 6-설파논;화합물번호 143: ((2-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)이미노)다이메틸- λ 6-설파논;화합물번호 144: ((2-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)이미노)다이메틸-λ 6-설파논;화합물번호 145: N-(2-((5-브로모-2-((2-메톡시-6-(1-메틸아제판-4-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 146: N-(2-((5-브로모-2-((5-플루오로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 147: N-(2-((5-브로모-2-((2-메톡시-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 148: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(트라이플루오로메틸)-[1,4'-바이피페리딘]-1'-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 149: N-(2-((5-브로모-2-((5-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 150: N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(트라이플루오로메틸)-[1,4'-바이피페리딘]-1'-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 151: N-(4-클로로-2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 152: N-(4-클로로-2-((6-클로로-3-(( -2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)-1,2,4-트리아진-5-일)아미노)페닐)메탄설폰아마이드;화합물번호 153 : (4-클로로-2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)다이메틸포스핀 옥사이드;화합물번호 154 : (4-클로로-2-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)다이메틸포스핀 옥사이드;화합물번호 155 : N-(2-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)-N-메틸메탄설폰아마이드;화합물번호 156 : N-(2-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)-N-메틸메탄설폰아마이드;화합물번호 157 : N-(4-클로로-2-((5-클로로-2-((2-클로로-4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 158 : N-(4-클로로-2-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 159 : N-(4-클로로-2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 159 : N-(4-클로로-2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 160 : N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-아이소프로필페닐)메탄설폰아마이드;화합물번호 161 : N-(2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-아이소프로필페닐)메탄설폰아마이드;화합물번호 162 : N-(4-클로로-2-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 163 : N-(4-클로로-2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 164 : N-(2-((6-클로로-3-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)-1,2,4-트리아진-5-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 165 : N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 166 : 5-클로로-N4-(5-클로로-2-(1H-1,2,3-트리아졸-4-일)페닐)-N2-(2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)피리미딘-2,4-다이아민;화합물번호 167 : N-(4-클로로-2-((5-클로로-2-((1-(3-(다이메틸아미노)프로필)-1H-피라졸-4-일)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 168 : N-(6-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 169 : N-(6-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 170 : N-(4-클로로-2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)-N-메틸메탄설폰아마이드;화합물번호 171 : N-(4-클로로-2-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)-N-메틸메탄설폰아마이드;화합물번호 172 : N-(6-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 173 : N-(6-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 174 : N-(6-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 175 : N-(6-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 176 : N-(4-클로로-2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 177 : N-(2-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로-5-플루오로페닐)메탄설폰아마이드;화합물번호 178 : N-(2-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로-5-플루오로페닐)메탄설폰아마이드;화합물번호 179 : N-(4-클로로-2-((5-클로로-2-((2-클로로-4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)페닐)-N-메틸메탄설폰아마이드;화합물번호 180 : N-(4-클로로-2-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)-N-메틸메탄설폰아마이드;화합물번호 181 : N-(4-클로로-2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 182 : N-(4-클로로-2-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-5-플루오로페닐)메탄설폰아마이드;화합물번호 183 : N-(6-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드.화합물번호 184 : N-(6-((5-브로모-2-((5-에틸-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 185 : 5-클로로-N4-(5-클로로-2-(1H-1,2,3-트라이졸-5-일)페닐)-N2-(2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)피리미딘-2,4-다이아민;화합물번호 186 : 5-클로로-N 4-(5-클로로-2-(1H-1,2,3-트리아졸-5-일)페닐)-N 2-(5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)피리미딘-2,4-다이아민;화합물번호 187 : 5-브로모-N 4-(5-클로로-2-(1H-1,2,3-트리아졸-5-일)페닐)-N 2-(2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)피리미딘-2,4-다이아민;화합물번호 188 : N-(6-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 189 : N-(6-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 190 : (6-((5-클로로-2-((5-에틸-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 191 : N-(2-((5-브로모-2-((5-에틸-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)메탄설폰아마이드;화합물번호 192 : (6-((5-브로모-2-((5-에틸-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드.화합물번호 193 : (6-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 194 : (6-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)퀴녹살린-5-일)다이메틸포스핀 옥사이드;화합물번호 195 : 5-브로모-N 4-(5-클로로-2-(1H-1,2,3-트리아졸-5-일)페닐)-N 2-(5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)피리미딘-2,4-다이아민;화합물번호 196 : N-(6-((5-브로모-2-((4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 197 : N-(6-((5-클로로-2-((2-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 198 : N-(4-클로로-2-((5-클로로-2-((2,5-다이클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)메탄설폰아마이드;화합물번호 199 : N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 200 : N-(2-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 201 : N-(2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드.화합물번호 202 : N-(2-((5-클로로-2-((2,5-다이클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 203 : N-(2-((5-클로로-2-((5-에틸-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 204 : N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 205 : N-(6-((5-클로로-2-((2,5-다이클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 206 : N-(6-((5-클로로-2-((5-에틸-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 207 : N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 208 : N-(6-((5-클로로-2-((4-(4-(4-사이클로프로필피페라진-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 209 : N-(6-((5-클로로-2-((4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 210 : N-(6-((5-클로로-2-((2-클로로-4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 211 : N-(6-((5-브로모-2-((4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 212 : N-(6-((5-브로모-2-((4-(4-(4-사이클로프로필피페라진-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 213 : N-(6-((5-브로모-2-((2-클로로-4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 214 : N-(6-((5-브로모-2-((2-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 215 : N-(6-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 216 : N-(6-((5-브로모-2-((5-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 217 : N-(6-((5-브로모-2-((2,5-다이클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 218 : N-(6-((5-클로로-2-((4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 219 : N-(6-((5-클로로-2-((5-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 220 : N-(6-((5-클로로-2-((2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 221 : N-(6-((5-클로로-2-((2-클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 222 : N-(6-((5-클로로-2-((5-플루오로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 223 : N-(6-((5-클로로-2-((2-(4-(4-메틸피페라진-1-일)피페리딘-1-일)퀴놀린-6-일)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 224 : N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-((1-메틸-1H-피라졸-5-일)아미노)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 225 : N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-(트라이플루오로메틸)피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 226 : N-(4-클로로-2-((5-클로로-2-((2,5-다이클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)페닐)-N-메틸메탄설폰아마이드;화합물번호 227 : N-(2-((5-브로모-2-((2-클로로-4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)-N-메틸메탄설폰아마이드;화합물번호 228 : N-(2-((5-브로모-2-((2,5-다이클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-클로로페닐)-N-메틸메탄설폰아마이드;화합물번호 229 : N-(2-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 230 : N-(2-((5-클로로-2-((2-클로로-4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 231 : N-(2-((5-클로로-2-((2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 232 : N-(2-((5-클로로-2-((4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 233 : N-(2-((5-클로로-2-((4-(4-(4-사이클로프로필피페라진-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 234 : N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(트라이플루오로메틸)-[1,4'-바이피페리딘]-1'-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 235 : N-(2-((5-클로로-2-((4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 236 : N-(2-((5-클로로-2-((2-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-5-메틸페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 237 : N-(2-((5-클로로-2-((5-클로로-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)메탄설폰아마이드;화합물번호 238 : N-(6-((5-브로모-2-((2-메톡시-6-(4-메틸-1,4-다이아제판-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 239 : N-(6-((5-브로모-2-((5-플루오로-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 240 : N-(6-((5-브로모-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 241 : N-(6-((5-클로로-2-((2-메톡시-5-메틸-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 242 : N-(6-((5-클로로-2-((5-플루오로-6-(4-(4-메틸피페라진-1-일)피페리딘-1-일)피리딘-3-일)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 243 : N-(6-((5-브로모-2-((2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 244 : N-(6-((5-브로모-2-((3-플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 245 : N-(6-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(트라이플루오로메틸)-[1,4'-바이피페리딘]-1'-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 246 : N-(6-((5-브로모-2-((2-클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설포닐아마이드;화합물번호 247 : N-(6-((5-브로모-2-((2-(4-(4-메틸피페라진-1-일)피페리딘-1-일)퀴놀린-6-일)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 248 : N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 249 : N-(6-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 250 : N-(6-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 251 : N-(6-((5-브로모-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 252 : N-(6-((5-브로모-2-((5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 253 : N-(6-((5-브로모-2-((2,5-다이클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 254 : N-(6-((5-브로모-2-((5-에틸-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 255 : N-(6-((5-브로모-2-((2-메톡시-5-메틸-4-(4-(4-(트라이플루오로메틸)피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 256 : N-(6-((5-브로모-2-((5-플루오로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 257 : N-(6-((5-브로모-2-((5-플루오로-2-메톡시-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 258 : N-(6-((5-브로모-2-((4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시-5-메톡시페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 259 : N-(6-((5-클로로-2-((2,5-다이클로로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 260 : N-(2-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-4-메톡시페닐)-N-메틸메탄설폰아마이드;화합물번호 261 : N-(6-((5-브로모-2-((4-(4-(4-사이클로프로필피페라진-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 262 : N-(6-((5-브로모-2-((4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 263 : N-(6-((5-클로로-2-((2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 264 : N-(6-((5-클로로-2-((5-클로로-2-메톡시-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 265 : N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 266 : N-(6-((5-클로로-2-((4-(4-(4-사이클로프로필피페라진-1-일)피페리딘-1-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 267 : N-(6-((5-클로로-2-((4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시-5-메틸페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)-N-메틸메탄설폰아마이드;화합물번호 268 : N-(6-((5-클로로-2-((2,5-다이플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 269 : N-(5-((5-브로모-2-((2,5-다이플루오로-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-4-일)메탄설폰아마이드;화합물번호 270 : N-(6-((2-((5-브로모-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)-5-클로로피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 271 : N-(6-((2-((5-브로모-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)-5-클로로피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 272 : 5-클로로-N 2-(2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(1-메틸인돌린-6-일)피리미딘-2,4-다이아민;화합물번호 273 : 5-클로로-N 2-(5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(1-메틸인돌린-6-일)피리미딘-2,4-다이아민;화합물번호 274 : 5-클로로-N 2-(2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(1-메틸인돌린-6-일)피리미딘-2,4-다이아민;화합물번호 275 : 5-클로로-N 2-(2-메톡시-5-메틸-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)-N 4-(1-메틸인돌린-6-일)피리미딘-2,4-다이아민;화합물번호 276 : N-(6-((5-클로로-2-((2-메톡시-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)아미노)피리미딘-4-일)아미노)-1-메틸인돌린-5-일)메탄설폰아마이드;화합물번호 277 : 5-클로로-N 2-(2-클로로-5-메틸-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(인돌린-6-일)피리미딘-2,4-다이아민;화합물번호 278 : 5-클로로-N 2-(5-클로로-2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)-N 4-(인돌린-6-일)피리미딘-2,4-다이아민;화합물번호 279 : 5-클로로-N 4-(인돌린-6-일)-N 2-(2-메톡시-4-(4-(4-메틸피페라진-1-일)피페리딘-1-일)페닐)피리미딘-2,4-다이아민;화합물번호 280 : N-(6-((2-((5-브로모-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시페닐)아미노)-5-클로로피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 281 : N-(6-((5-브로모-2-((5-브로모-4-(3-(다이메틸아미노)-[1,4'-바이피페리딘]-1'-일)-2-메톡시페닐)아미노)피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드;화합물번호 282 : N-(6-((2-((5-브로모-2-메톡시-4-(4-(4-메틸-1,4-다이아제판-1-일)피페리딘-1-일)페닐)아미노)-5-클로로피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드; 및화합물번호 283 : N-(6-((2-((5-브로모-4-(4-(3-(다이메틸아미노)피롤리딘-1-일)피페리딘-1-일)-2-메톡시페닐)아미노)-5-클로로피리미딘-4-일)아미노)-2,3-다이하이드로벤조퓨란-5-일)메탄설폰아마이드.
- 제1항에 있어서,상기 약학적으로 허용 가능한 염은 염산, 브롬화수소산, 황산, 인산, 질산, 아세트산, 글리콜산, 락트산, 피루브산, 말론산, 석신산, 글루타르산, 푸마르산, 말산, 만델산, 타타르산, 시트르산, 아스코빈산, 팔미트산, 말레인산, 하이드록시말레인산, 벤조산, 하이드록시벤조산, 페닐아세트산, 신남산, 살리실산, 메탄설폰산, 벤젠설폰산 및 톨루엔설폰산으로 구성된 군에서 선택되는 무기산 또는 유기산의 염인, 화학식 10, 11 또는 12로 표시되는 피리미딘 유도체 화합물, 이의 약학적으로 허용 가능한 염, 이의 수화물 및 이의 입체 이성질체로부터 선택된 화합물.
- 제1항 내지 제10항 중 어느 한 항의 화합물이 유효성분으로 포함되어 있는, 암 예방, 경감 또는 치료용 약학 조성물.
- 제11항에 있어서,상기 암이 EGFR 돌연변이로 유발되는 것을 특징으로 하는, 암 예방, 경감 또는 치료용 약학 조성물.
- 제11항에 있어서,상기 약학 조성물은 EGFR 돌연변이를 갖는 환자에 적용되는, 암 예방, 경감 또는 치료용 약학 조성물.
- 제11항에 있어서,상기 암은 교모세포종, 삼중음성 유방암, 대장암, 폐암, 역형성대세포림프종, 위장관 간질종양, 다발성골수종, 급성골수성백혈병, 간암, 위암, 갑상선암 및 두경부암으로 이루어진 군으로부터 선택되는 1 이상인, 암 예방, 경감 또는 치료용 약학 조성물.
- 제14항에 있어서,상기 암은 폐암인, 암 예방, 경감 또는 치료용 약학 조성물.
- 제11항에 있어서,상기 약학 조성물은 EGFR 관련 돌연변이인 엑손19결손/T790M/C797S 의 삼중 돌연변이 또는 L858R/T790M/C797S 의 삼중 돌연변이를 보유한 환자에게 투여되는 것을 특징으로 하는, 암의 예방, 경감 또는 치료용 약학 조성물.
- 제11항에 있어서,상기 약학 조성물은 EGFR 관련 돌연변이인 엑손19결손/T790M 의 이중 돌연변이 또는 L858R/T790M 의 이중 돌연변이를 보유한 환자에게 투여되는 것을 특징으로 하는, 암의 예방, 경감 또는 치료용 약학 조성물.
- 제11항에 있어서,상기 약학 조성물은 EGFR 관련 돌연변이인 엑손19결손 돌연변이 또는 L858R 돌연변이를 보유한 환자에게 투여되는 것을 특징으로 하는, 암의 예방, 경감 또는 치료용 약학 조성물.
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA3207015A CA3207015A1 (en) | 2021-04-01 | 2022-04-01 | Pyrimidine derivative with inhibitory activity against protein kinases and therapeutic pharmaceutical composition including the same |
| AU2022250976A AU2022250976A1 (en) | 2021-04-01 | 2022-04-01 | Pyrimidine derivative having protein kinase inhibitory activity, and therapeutic pharmaceutical composition comprising same |
| JP2023559714A JP2024511514A (ja) | 2021-04-01 | 2022-04-01 | タンパク質キナーゼに対する阻害活性を有するピリミジン誘導体及びそれを含む治療用医薬組成物 |
| CN202280025860.3A CN117120424A (zh) | 2021-04-01 | 2022-04-01 | 具有抑制蛋白激酶活性的嘧啶衍生物和包括其的治疗药物组合 |
| EP22781692.3A EP4286376A1 (en) | 2021-04-01 | 2022-04-01 | Pyrimidine derivative having protein kinase inhibitory activity, and therapeutic pharmaceutical composition comprising same |
| IL307327A IL307327A (en) | 2021-04-01 | 2022-04-01 | Inhibitors of protein kinase with a pyrimidine skeleton, and preparations for treatment containing them |
| US18/279,348 US20240228458A1 (en) | 2021-04-01 | 2022-04-01 | Pyrimidine derivative having protein kinase inhibitory activity, and therapeutic pharmaceutical composition comprising same |
| MX2023010016A MX2023010016A (es) | 2021-04-01 | 2022-04-01 | Derivado de pirimidina con actividad inhibidora contra las proteina cinasas y composicion farmaceutica terapeutica que incluye el mismo. |
| BR112023019370A BR112023019370A2 (pt) | 2021-04-01 | 2022-04-01 | Composto, e, composição farmacêutica para prevenir, melhorar ou tratar câncer |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR20210042821 | 2021-04-01 | ||
| KR10-2021-0042821 | 2021-04-01 | ||
| KR10-2022-0039968 | 2022-03-30 | ||
| KR1020220039968A KR20220136931A (ko) | 2021-04-01 | 2022-03-30 | 단백질 키나아제 저해 활성을 갖는 피리미딘 유도체 및 이를 포함하는 치료용 약학 조성물 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022211573A1 true WO2022211573A1 (ko) | 2022-10-06 |
Family
ID=83456614
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2022/004702 WO2022211573A1 (ko) | 2021-04-01 | 2022-04-01 | 단백질 키나아제 저해 활성을 갖는 피리미딘 유도체 및 이를 포함하는 치료용 약학 조성물 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20240228458A1 (ko) |
| EP (1) | EP4286376A1 (ko) |
| JP (1) | JP2024511514A (ko) |
| AU (1) | AU2022250976A1 (ko) |
| BR (1) | BR112023019370A2 (ko) |
| CA (1) | CA3207015A1 (ko) |
| IL (1) | IL307327A (ko) |
| MX (1) | MX2023010016A (ko) |
| WO (1) | WO2022211573A1 (ko) |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010146133A1 (en) * | 2009-06-18 | 2010-12-23 | Cellzome Limited | Heterocyclylaminopyrimidines as kinase inhibitors |
| WO2018230934A1 (ko) | 2017-06-13 | 2018-12-20 | 한국화학연구원 | N2,n4-디페닐피리미딘-2,4-디아민 유도체, 이의 제조방법 및 이를 유효성분으로 포함하는 암의 예방 또는 치료용 약학적 조성물 |
| WO2019112344A1 (ko) | 2017-12-07 | 2019-06-13 | 주식회사 온코빅스 | 암세포 성장 억제 효과를 나타내는 신규한 피리미딘 유도체 및 그를 포함하는 약제학적 조성물 |
| CN110305161A (zh) * | 2018-03-20 | 2019-10-08 | 暨南大学 | 2-氨基嘧啶类化合物及其应用 |
| KR20190114910A (ko) | 2018-03-30 | 2019-10-10 | 한미약품 주식회사 | 상피세포 성장인자 수용체 돌연변이 저해 효과를 갖는 신규 설폰아마이드 유도체 |
| US20200207768A1 (en) | 2017-07-19 | 2020-07-02 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Aryl-Phosphorus-Oxygen Compound As EGFR Kinase Inhibitor |
| WO2020147702A1 (en) | 2019-01-17 | 2020-07-23 | Betta Pharmaceuticals Co., Ltd | Egfr inhibitors, compositions and methods thereof |
| WO2020216371A1 (zh) * | 2019-04-26 | 2020-10-29 | 江苏先声药业有限公司 | Egfr抑制剂及其应用 |
| CN112538072A (zh) * | 2019-09-21 | 2021-03-23 | 齐鲁制药有限公司 | 新型氨基嘧啶类egfr抑制剂 |
-
2022
- 2022-04-01 WO PCT/KR2022/004702 patent/WO2022211573A1/ko active Application Filing
- 2022-04-01 BR BR112023019370A patent/BR112023019370A2/pt unknown
- 2022-04-01 US US18/279,348 patent/US20240228458A1/en active Pending
- 2022-04-01 IL IL307327A patent/IL307327A/en unknown
- 2022-04-01 AU AU2022250976A patent/AU2022250976A1/en active Pending
- 2022-04-01 CA CA3207015A patent/CA3207015A1/en active Pending
- 2022-04-01 MX MX2023010016A patent/MX2023010016A/es unknown
- 2022-04-01 EP EP22781692.3A patent/EP4286376A1/en active Pending
- 2022-04-01 JP JP2023559714A patent/JP2024511514A/ja active Pending
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010146133A1 (en) * | 2009-06-18 | 2010-12-23 | Cellzome Limited | Heterocyclylaminopyrimidines as kinase inhibitors |
| WO2018230934A1 (ko) | 2017-06-13 | 2018-12-20 | 한국화학연구원 | N2,n4-디페닐피리미딘-2,4-디아민 유도체, 이의 제조방법 및 이를 유효성분으로 포함하는 암의 예방 또는 치료용 약학적 조성물 |
| KR20180135781A (ko) * | 2017-06-13 | 2018-12-21 | 한국화학연구원 | N2,n4-디페닐피리미딘-2,4-디아민 유도체, 이의 제조방법 및 이를 유효성분으로 포함하는 암의 예방 또는 치료용 약학적 조성물 |
| US20200207768A1 (en) | 2017-07-19 | 2020-07-02 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | Aryl-Phosphorus-Oxygen Compound As EGFR Kinase Inhibitor |
| WO2019112344A1 (ko) | 2017-12-07 | 2019-06-13 | 주식회사 온코빅스 | 암세포 성장 억제 효과를 나타내는 신규한 피리미딘 유도체 및 그를 포함하는 약제학적 조성물 |
| CN110305161A (zh) * | 2018-03-20 | 2019-10-08 | 暨南大学 | 2-氨基嘧啶类化合物及其应用 |
| KR20190114910A (ko) | 2018-03-30 | 2019-10-10 | 한미약품 주식회사 | 상피세포 성장인자 수용체 돌연변이 저해 효과를 갖는 신규 설폰아마이드 유도체 |
| WO2020147702A1 (en) | 2019-01-17 | 2020-07-23 | Betta Pharmaceuticals Co., Ltd | Egfr inhibitors, compositions and methods thereof |
| WO2020216371A1 (zh) * | 2019-04-26 | 2020-10-29 | 江苏先声药业有限公司 | Egfr抑制剂及其应用 |
| CN112538072A (zh) * | 2019-09-21 | 2021-03-23 | 齐鲁制药有限公司 | 新型氨基嘧啶类egfr抑制剂 |
Non-Patent Citations (2)
| Title |
|---|
| J. MED. CHEM., vol. 61, 2018, pages 4290 - 4300 |
| J. MED. CHEM., vol. 62, 2019, pages 10272 - 10293 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2022250976A1 (en) | 2023-08-17 |
| BR112023019370A2 (pt) | 2023-12-26 |
| IL307327A (en) | 2023-11-01 |
| JP2024511514A (ja) | 2024-03-13 |
| CA3207015A1 (en) | 2022-10-06 |
| AU2022250976A9 (en) | 2024-07-11 |
| EP4286376A1 (en) | 2023-12-06 |
| US20240228458A1 (en) | 2024-07-11 |
| MX2023010016A (es) | 2023-09-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2014260605B2 (en) | Novel compounds for selective histone deacetylase inhibitors, and pharmaceutical composition comprising the same | |
| WO2018230934A1 (ko) | N2,n4-디페닐피리미딘-2,4-디아민 유도체, 이의 제조방법 및 이를 유효성분으로 포함하는 암의 예방 또는 치료용 약학적 조성물 | |
| WO2020171499A1 (ko) | 단백질 키나아제 저해 활성을 갖는 신규한 피리도[3,4-d]피리미딘-8-온 유도체 및 이를 포함하는 암의 예방, 개선 또는 치료용 약학 조성물 | |
| WO2019112344A1 (ko) | 암세포 성장 억제 효과를 나타내는 신규한 피리미딘 유도체 및 그를 포함하는 약제학적 조성물 | |
| WO2020190119A1 (ko) | 헤테로아릴 유도체, 이를 제조하는 방법, 및 이를 유효성분으로 포함하는 약학적 조성물 | |
| WO2017160116A9 (ko) | 니코틴아미드 포스포리보실트랜스퍼라제 억제용 신규 화합물 및 이를 포함하는 조성물 | |
| WO2021145521A1 (ko) | 피리도[3,4-d]피리미딘 유도체 및 이를 포함하는 치료용 약학 조성물 | |
| WO2016085221A2 (ko) | 단백질 키나아제 저해제로 유용한 헤테로아릴아민 유도체 | |
| AU2016317806B2 (en) | Heteroaryl compounds and their use as therapeutic drugs | |
| WO2018088749A1 (ko) | 신규한 피리미딘화합물, 이의 제조방법 및 이를 유효성분으로 함유하는 암 및 염증질환의 예방 또는 치료용 약학적 조성물 | |
| WO2021145520A1 (ko) | 단백질 키나아제 저해 활성을 갖는 7-아미노-3,4-디히드로피리미도피리미딘-2-온 유도체 및 이를 포함하는 치료용 약학 조성물 | |
| WO2021125803A1 (ko) | 신규한 피리미딘 유도체 및 이의 용도 | |
| WO2020149723A1 (ko) | 피롤로피리미딘 유도체 및 이를 유효성분으로 함유하는 단백질 키나아제 관련 질환의 예방 또는 치료용 약학적 조성물 | |
| WO2022139304A1 (ko) | Sos1 억제제로서의 신규한 퀴나졸린 유도체 화합물 및 이의 용도 | |
| WO2020036386A1 (ko) | 이소인돌린-1온 유도체, 이의 제조방법 및 이를 유효성분으로 포함하는 암의 예방 또는 치료용 약학적 조성물 | |
| EP3166945A2 (en) | Novel triazolopyrimidinone or triazolopyridinone derivatives, and use thereof | |
| WO2018169360A1 (ko) | TGase 2 억제제로서의 퀴놀린-5,8-디온 유도체 및 이를 포함하는 약제학적 조성물 | |
| WO2021096112A1 (ko) | 피롤로피리미딘, 피롤로피리딘, 인다졸 화합물 유도체 및 이를 포함하는 치료용 약학 조성물 | |
| WO2016006974A2 (en) | Novel triazolopyrimidinone or triazolopyridinone derivatives, and use thereof | |
| WO2020185044A1 (ko) | 헤테로아릴 유도체 및 이를 유효성분으로 포함하는 약학적 조성물 | |
| WO2023140629A1 (ko) | 단백질 키나아제 저해활성을 가지는 2, 7-치환된 피롤로[2,1-f][1,2,4]트라아진 화합물 | |
| WO2023153748A1 (ko) | Sos1 억제제 및 이의 유도체 | |
| WO2023195773A1 (ko) | 헤테로아릴 유도체 및 이의 용도 | |
| WO2022211573A1 (ko) | 단백질 키나아제 저해 활성을 갖는 피리미딘 유도체 및 이를 포함하는 치료용 약학 조성물 | |
| WO2022114812A1 (ko) | 다이아실글리세롤 키나아제 저해제로서 헤테로사이클 화합물 및 이의 용도 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22781692 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3207015 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2022250976 Country of ref document: AU Date of ref document: 20220401 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2023/010016 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 18279348 Country of ref document: US Ref document number: 2022781692 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2023559714 Country of ref document: JP Ref document number: 2023124692 Country of ref document: RU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202317065014 Country of ref document: IN Ref document number: 307327 Country of ref document: IL |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112023019370 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 2022781692 Country of ref document: EP Effective date: 20230829 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11202307416Y Country of ref document: SG |
|
| ENP | Entry into the national phase |
Ref document number: 112023019370 Country of ref document: BR Kind code of ref document: A2 Effective date: 20230922 |