WO2021057840A1 - Promédicaments anti-inflammatoires et anti-sénescence et leurs méthodes d'utilisation - Google Patents
Promédicaments anti-inflammatoires et anti-sénescence et leurs méthodes d'utilisation Download PDFInfo
- Publication number
- WO2021057840A1 WO2021057840A1 PCT/CN2020/117386 CN2020117386W WO2021057840A1 WO 2021057840 A1 WO2021057840 A1 WO 2021057840A1 CN 2020117386 W CN2020117386 W CN 2020117386W WO 2021057840 A1 WO2021057840 A1 WO 2021057840A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- substituted
- unsubstituted
- disease
- ssk1
- agent
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/549—Sugars, nucleosides, nucleotides or nucleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H15/00—Compounds containing hydrocarbon or substituted hydrocarbon radicals directly attached to hetero atoms of saccharide radicals
- C07H15/20—Carbocyclic rings
- C07H15/203—Monocyclic carbocyclic rings other than cyclohexane rings; Bicyclic carbocyclic ring systems
Definitions
- the present invention relates to compounds that are capable of selectively killing senescent cells over non-senescent cells, i.e. senolytic compounds, and methods of use thereof.
- Aging is the major risk factor for physiological degeneration, increased chronic morbidities, and age-specific mortality (Lopez-Otin, et al., Cell 153, 1194-1217 (2013) ) .
- senescent cells accumulate in multiple tissues and cause tissue dysfunction (van Deursen, et al., Nature 509, 439-446 (2014) ; McHugh, et al., J Cell Biol 217, 65-77 (2016) ) .
- Senescent cells also secrete a variety of pro-inflammatory factors, termed the senescence-associated secretory phenotype (SASP) , which leads to age-related physical decline (Coppe, et al., PLoS Biol 6, 2853-2868 (2008) ; Coppe, et al., Annu Rev Pathol 5, 99-118 (2010) ) . Elimination of senescent cells has emerged as an attractive potential method to ameliorate age-associated diseases and improve healthspan (Xu et al., Nat Med 24, 1246-1256 (2016) ; Baker et al., . Nature 479, 232-236 (2011) ; Baker et al., Nature 530, 184-189 (2016) . ) .
- SASP senescence-associated secretory phenotype
- senolytics Compounds that selectively kill senescent cells, known as ‘senolytics’ , have attracted considerable interest and revealed that anti-apoptotic pathways (Zhu et al., Aging Cell 14, 644-658 (2015) , Chang et al., Nat Med 22, 78-83 (2016) ) , HSP90 (Fuhrmann-Stroissnigg et al., Nat Commun 8, 422 (2017) ) , and FOXO4-p53 complex (Baar et al., Cell 169, 132-147 e116 (2017) ) could be targeted to achieve this goal.
- compositions for reducing inflammation in a subject in need thereof It is also an object of the present invention to provide compositions for reducing inflammation in a subject in need thereof.
- Prodrugs are provided which are senolytic and antiinflammatory.
- the prodrugs are designed from a cytotoxic agent (parent cytotoxic agent) , by chemically modifying the cytotoxic agent to incorporate a site cleavable by SA- ⁇ -gal (to release the active parent cytotoxic drug) following delivery of the prodrug in vivo, for preferentially killing senescent cells.
- the prodrug includes a galactose-based moiety, which is modified (herein, a modified galactose moiety) , preferably by acetylation, a benzyloxycarbonyl group and a cytotoxic moiety (provided by the parent cytotoxic agent) .
- the cytotoxic parent cytotoxic drug used to make the prodrug lacks a phenol group.
- a particularly preferred cytotoxic agent lacking a phenolic group is for example, gemcitabine, cytarabine, and 5'-Deoxy-5-fluorocytidine.
- the prodrug may be in a crystal form.
- a preferred acetylated galactose moiety is a D-galactose tetraacetate moiety, shown below.
- one or more (e.g., two, three, or four) of the acetyl (Ac) groups may be removed from the galactose based-moiety.
- a particualarly preferred benzyloxycarbonyl group is shown below.
- NO 2 may be removed from the above benzyloxycarbonyl group.
- a method of selectively killing one or more senescent cells in a subject in need thereof includes administering to the subject a therapeutically effective amount of one or more of the disclosed senolytic prodrugs.
- the agent selectively kills cells undergoing oncogene-induced senescence; cells undergoing drug-induced senescence; cells undergoing age-induced senescence; cells disease-associated senescence and/or cells undergoing irradiation-induced senescence.
- compositions can be used to ameliorate symptoms associated with proinflammation, for example, excessive activated macrophage accumulation (and reduction of the associated cytokines) .
- the disclosed compositions can be administered to a subject in need thereof, to reduce inflammatory responses associated with a viral infection, for example, a coronavirus (CoV) infection, and more particularly, a SARS-CoV or SARS-CoV2 infection.
- a viral infection for example, a coronavirus (CoV) infection, and more particularly, a SARS-CoV or SARS-CoV2 infection.
- the composition is administered in an effective amount to reduce one or more macrophages in the subject, preferably, SA- ⁇ -gal positive macrophages.
- compositions can be used to reduce one or more symptoms associated with a Senescence-associated disease or disorder in a subject and/or one or more inflammation-associated disorders in a subject, which contains a long list of other pathologies, including neurological (e.g. brain aneurysm, Alzheimer's and Parkinson) , pulmonary (e.g. idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease and cystic fibrosis) , ophthalmological (e.g. cataracts, glaucoma, macular degeneration) , musculoskeletal (e.g. sarcopenia, disc degeneration, osteoarthritis) , cardiovascular (e.g.
- neurological e.g. brain aneurysm, Alzheimer's and Parkinson
- pulmonary e.g. idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease and cystic fibrosis
- ophthalmological e.g. cataracts, glaucoma, macular de
- Atherosclerosis cardiac fibrosis, aorta aneurysm
- renal e.g. chronic kidney disease, transplant complications
- osteoarthritis of the knee and others such as diabetes, mucositis, hypertension, liver fibrosis and osteomyelofibrosis (OMF) .
- OMF osteomyelofibrosis
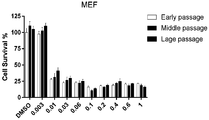
- FIG. 1A-B are graphs showing validation of its ability to selectively kill senescent cells.
- FIG. 1A Metabolism of SSK1 into gemcitabine in SSK1-treated (0.5 ⁇ M) , replication-induced senescent mouse embryonic fibroblasts (MEFs) and their non-senescent counterparts.
- FIGs. 1A Metabolism of SSK1 into gemcitabine in SSK1-treated (0.5 ⁇ M) , replication-induced senescent mouse embryonic fibroblasts (MEFs) and their non-senescent counterparts.
- FIG. 1B Quantification
- FIG. 1C and D show the effect of gemcitabine on senescent and non-senescent cells
- FIG. 2A shows the effect of SSK1 on senescent cells, driven by inducing apoptosis.
- FIG. 2A top panels
- FIGs., 2B and 2C show the effect of SSK1 p38 MAPK activation in senescent cells.
- FIG. 2B Western blot of phos-p38 MAPK and ⁇ H2AX in gemcitabine-or SSK1-treated senescent cells.
- FIG. 2F Quantification of viable non-senescent and senescent human embryonic fibroblasts (HEFs) induced by replication, a small molecule (etoposide, 10 ⁇ M) , or irradiation (10 Gy) after SSK1 treatment (n 3) .
- FIG. 2G Quantification of viability in non-senescent and replication-induced senescent human umbilical vein endothelial cells (HUVECs) incubated with increasing doses of SSK1 (n 4) .
- Data are means ⁇ s.e.m, unpaired t-test for (FIG. 2E) and (FIG. 2 G) , one-way annova test for (FIG. 2D) and (FIG.
- FIGs. 2H-L show studies on the effect of SSK1 on SA- ⁇ -gal-positive senescent cells and lung fibrosis in a bleomycin-induced lung injury model.
- FIG. 2H Experimental design for bleomycin-induced lung injury model.
- FIGs. 3A-J show studies on the effect of SSK1 on senescent cells and SASP in aged mice.
- FIG. 3A Experimental design for SSK1 treatment of aged mice.
- FIG. 3E and FIG. 3F Expression of p16, p21, IL1 ⁇ , TNF ⁇ , and other SASP factors analyzed by RT-qPCR in liver (FIG.
- FIG. 3G-J IL1 ⁇ (FIG. 3G) , IL6 (FIG. 3H) , CXCL1 (FIG. 3I) , and TNF ⁇ (FIG. 3J) protein levels in blood serum from old mice treated with SSK1 (0.5 mg/kg) or vehicle, as measured by ELISA.
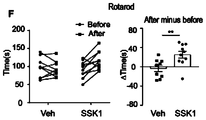
- FIGs. 4A-J show studies on the effect of SSK1 on physical functions of aged mice.
- FIGs. 4A to E Quantification of maximal rotarod time (FIG. 4A) , treadmill distance (FIG. 4B) , grip strength (FIG. 4C) , time to cross balance beam (FIG. 4D) , and rearing exploration times (FIG. 4E) for old female mice treated with vehicle or SSK1 (0.5 mg/kg) .
- Data are means ⁇ s.e.m, each data point represents an individual mouse, *P ⁇ 0.05, **P ⁇ 0.01, unpaired t-test.
- FIG. 4I Quantification of maximal rotarod time (left panel) , treadmill distance (middle panel) , and grip strength (right panel) in old male mice treated with vehicle or SSK1 (0.5 mg/kg) .
- Fig. 4J Treatment with SSK1 improved the rotarod, treadmill, and grip strength functions when compared to the other senolytic compounds.
- FIG. 5A shows the effect of SSK1 (0.5 mg/kg) treatment on the senescence associated GSEA gene set (Fridman senescence up) in the livers and in the kidneys from old mice compared with vehicle treatment.
- FIG. 5B shows the effect of SSK1 treatment on age-associated signatures in aged livers and kidneys.
- the data shows GSEA of a statistically significant gene set: Kyng Werner Syndyom and Normal Aging Up of livers downregulated in the old mice treated with SSK1 compared with vehicle; and GSEA of a statistically significant gene set: Rodwell Aging Kidney Up of kidneys downregulated in the old mice treated with SSK1 compared with vehicle.
- FIG. 5C and 5D show GSEA of statistically significant gene sets in liver (FIG.
- FIG. 5C shows GSEA of a statistically significant gene sets in liver: Hallmark inflammatory TNF ⁇ signaling via NF- ⁇ B, Hallmark IL6 Jak Stat3 signaling and Hallmark complement enriched in the livers from old mice compared with young mice, and downregulated in the old mice treated with SSK1 compared with vehicle.
- FIG. 5F shows GSEA of statistically significant gene sets in kidney. Hallmark inflammatory TNF ⁇ signaling via NF- ⁇ B, Hallmark IL6 Jak Stat3 signaling and Hallmark complement enriched in the livers from old mice compared with young mice, and downregulated in the old mice treated with SSK1 compared with vehicle.
- Scale bar 200 ⁇ m.
- FIGs. 6D-G GO Terms of up-regulated genes in the livers (FIG. D) and kidneys (FIG. F) of old mice compared with young mice; And GO terms for down-regulated genes in the livers (FIG. E) and kidneys (FIG. G) of SSK1-treated old mice relative to vehicle-treated old mice.
- FIG. 7A is a serum biochemical test, which shows the level of Alanine transaminase (ALT) , aspartate transaminase (AST) , creatinine (CREA) and uric acid (UA) in old mice after vehicle, SSK1 (0.5 mg/kg) or gemcitabine (Gem, 0.5 mg/kg) treatment for 8 weeks.
- FIG. 7B shows routine analysis of blood, showing the number of granulocytes, white blood cells, monocytes and red blood cells of old mice after vehicle, SSK1 (0.5 mg/kg) or gemcitabine (Gem, 0.5 mg/kg) treatment for 8 weeks.
- FIG. 7C shows serum biochemical test of mice treated with increasing dosed of SSK1.
- the level of Alanine transaminase (ALT) , aspartate transaminase (AST) , creatinine (CREA) and uric acid (UA) in old mice after vehicle and SSK1 (3, 10, 30, 60, 100 mg/kg) treatment for 5 weeks with 3 injections a week. (n 5, 5, 5, 4, 4, 4 for each group respectively) .
- FIG. 7D shows routine analysis of blood of mice treated with increasing dosed of SSK1.
- (D) H&E staining images show that SSK1 treatment improved the pneumonia of monkeys that were infected with SARS-CoV-2.
- FIG. 9 SSK1 Decreased Macrophage Infiltration and Reduced Inflammation during Pulmonary Recovery.
- A Immunohistochemistry (IHC) analysis for CD68 in lung tissues collected from SARS-CoV-2-infected monkeys treated with vehicle, 0.5 mg/kg or 2.0 mg/kg SSK1. Top, low magnification images; bottom, high magnification images of the boxed area in the top line. Scale bar, top: 100 ⁇ m; bottom: 25 ⁇ m.
- C Concentration change of inflammatory cytokines in serum samples from old monkeys between 21 and 28 dpi.
- D Concentration measurement for IL-18 in serum samples from SARS-CoV-2 infected monkeys before and after SSK1 treatment (2.0 mg/kg) . Arrow, starting point of administration.
- FIG. 10 SSK1 Reduces Clinical Signs in SARS-COV-2-infected Rhesus Macaques upon Early-time Administration
- A Scheme of the experimental design of early SSK1 intervention. Black dot, measurement of clinical signs, blood sample and X-ray at the indicated time points. Red dot, treatment days. Brown triangle, sample collections following euthanasia. Virus inoculation was performed at 0 dpi.
- B Body temperature monitoring after infection and during the treatment period.
- C Images of dorsal-ventral radiographs of the chest of animals at 0, 3, and 6 dpi. Red circles mark ground-glass opacification representing pulmonary interstitial infiltrates. R, right side of the animal.
- D Detection of viral RNA in nose, throat, anal swabs and blood samples by RT-qPCR at different time points after infection. L.O.D.: limit of detection. See also Figure 13.
- FIG. 11 Early SSK1 Intervention Reduces Inflammatory Damage to the Lungs Histopathology examination of necropsied lung tissues of rhesus macaques.
- A Left column, H&E staining indicates that SSK1 treatment recovers the thickened alveolar septum caused by SARS-CoV-2. Right column, high magnification images of the boxed area in the left column. Scale bar, left: 200 ⁇ m; right: 50 ⁇ m.
- B AB-PAS staining shows that SSK1 treatment recovers mucinous secretions in bronchioles. Scale bar, 100 ⁇ m.
- C Concentration of inflammatory cytokines in the lungs of rhesus macaques.
- FIG. 12 (Related to Figure 8) Low magnification images of H&E staining shows that SSK1 treatment improved the pneumonia of monkeys infected with SARS-CoV-2. Circle shows the area of interstitial pneumonia. Scale bar, 500 ⁇ m.
- FIG. 13 (Related to Figure 10)
- A Body weight changes of SARS-CoV-2-infected monkeys with vehicle and SSK1 between 0 and 7 dpi.
- B Hematological changes in SARS-CoV-2-infected rhesus macaques. M1 showed an abnormal high level of white blood cell (WBC) , lymphocyte and monocyte counts in the blood.
- C Viral loads in tissues of SARS-CoV-2-infected rhesus macaques collected at the time of necropsy. L.O.D.: limit of detection.
- D Viral load (top) and virus titer (bottom) were determined in BALF collected from SARS-CoV-2 infected rhesus macaques.
- Figure 14 (Related to Figure 11)
- a and B Gross pathology of the lungs and livers of SARS-CoV-2-infected rhesus macaques treated with SSK1. The arrows and circles represent gross lesions in the necropsied lungs or livers.
- C H&E staining shows that SSK1 treatment improved the pneumonia of monkeys infected with SARS-CoV-2. Circle shows the area of interstitial pneumonia. Scale bar, 500 ⁇ m.
- D H&E staining shows that M1 had local pulmonary hemorrhages. Scale bar, 200 ⁇ m.
- FIG. 15 SSK2 selectively eliminates senescent cells in human embryonic fibroblast in a dose-dependent manner after treatment for 72h.
- B) Quantification of viable cells in etoposide-induced senescent HEFs incubated with increasing doses of SSK2; Data were shown as mean ⁇ SD. N 3 per group. *P ⁇ 0.05, **P ⁇ 0.01.
- Figure 16 The effect of SSK1 or SSK2 on the viability of primary human chondrocytes isolated from Osteoarthritis patient.
- A) Apoptosis analysis of chondrocytes from human OA tissue by flow cytometry after treated with 10 ⁇ M SSK1, SSK2, or Vehicle for 72h.
- FIG. 1 Dose-dependent elimination of senescent cells in OA chondrocytes isolated from human OA tissue after SSK2 treatment for 48h. Quantification of viable human OA chondrocytes after treatment with increasing concentrations of SSK2 for 72 h. Data were shown as mean ⁇ SD.
- SA- ⁇ -gal is a major characteristic of senescence and a widely used senescent marker (Dimri, et al., PNAS, 92: 9363-9367 (1995) ; Lee et al., Aging Cell 5, 187-195 (2006) ) , whose activity is exploited herein to selectively metabolize small molecules in senescent cells (Lozano-Torres et al., J Am Chem Soc 139, 8808-8811 (2017) ) .
- the compositions and methods disclosed herein are based on the development of a prodrug strategy based on SA- ⁇ -gal to release the active parent drug for preferentially killing senescent cells.
- the disclosed compositions can be used to ameliorate symptoms associated with proinflammation, for example, excessive accumulation of activatd macrophages in response to an infection, for example, a viral infectrion.
- Cytotoxic moiety refers to the portion of the senolytic prodrug provided by the parent cytotoxic agent.
- Cytotoxic agent refers to a chemical or drug used to kill cells.
- Parenteral administration means administration by any method other than through the digestive tract or non-invasive topical or regional routes.
- Patient or “subject” to be treated as used herein refers to either a human or non-human animal.
- Parenteral administration means administration by any method other than through the digestive tract or non-invasive topical or regional routes.
- Patient or “subject” to be treated as used herein refers to either a human or non-human animal.
- “Senolytic” as used herein selectively killing one or more senescent cells over non-senescent cells.
- “Therapeutically effective” or “effective amount” as used herein means that the amount of the composition used is of sufficient quantity to ameliorate one or more causes or symptoms of a disease or disorder. Such amelioration only requires a reduction or alteration, not necessarily elimination.
- therapeutically effective amount “therapeutic amount” and “pharmaceutically effective amount” are synonymous.
- One of skill in the art can readily determine the proper therapeutic amount.
- treating includes abrogating, substantially inhibiting, slowing or reversing the progression of a disease or disorder, substantially ameliorating clinical symptoms of a disease or disorder or substantially preventing the appearance of clinical symptoms of a disease or disorder.
- the permissible substituents include acyclic and cyclic, branched and unbranched, carbocyclic and heterocyclic, aromatic and nonaromatic substituents of organic compounds.
- Illustrative substituents include, but are not limited to, halogens, hydroxyl groups, or any other organic groupings containing any number of carbon atoms, preferably 1-14 carbon atoms, and optionally include one or more heteroatoms such as oxygen, sulfur, or nitrogen grouping in linear, branched, or cyclic structural formats.
- substituents include alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, phenyl, substituted phenyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, halo, hydroxyl, alkoxy, substituted alkoxy, phenoxy, substituted phenoxy, aroxy, substituted aroxy, alkylthio, substituted alkylthio, phenylthio, substituted phenylthio, arylthio, substituted arylthio, cyano, isocyano, substituted isocyano, carbonyl, substituted carbonyl, carboxyl, substituted carboxyl, amino, substituted amino, amido, substituted amido, sulfonyl, substituted sulfonyl, sulfonic acid, phosphoryl, substituted phosphoryl, phosphonyl, substituted phosphonyl, polyaryl
- Heteroatoms such as nitrogen may have hydrogen substituents and/or any permissible substituents of organic compounds described herein which satisfy the valences of the heteroatoms. It is understood that “substitution” or “substituted” includes the implicit proviso that such substitution is in accordance with permitted valence of the substituted atom and the substituent, and that the substitution results in a stable compound, i.e. a compound that does not spontaneously undergo transformation such as by rearrangement, cyclization, elimination, etc.
- substituted refers to a structure, e.g., a chemical compound or a moiety on a larger chemical compound, regardless of how the strucuture was formed.
- the structure is not limited to a structure made by any specific method.
- Aryl, ” as used herein, refers to C 5 -C 26 -membered aromatic, fused aromatic, fused heterocyclic, or biaromatic ring systems.
- aryl, ” as used herein, includes 5-, 6-, 7-, 8-, 9-, 10-, 14-, 18-, and 24-membered single-ring aromatic groups that may include from zero to four heteroatoms, for example, benzene, naphthalene, anthracene, phenanthrene, chrysene, pyrene, corannulene, coronene, etc.
- Aryl further encompasses polycyclic ring systems having two or more cyclic rings in which two or more carbons are common to two adjoining rings (i.e., “fused rings” ) wherein at least one of the rings is aromatic, e.g., the other cyclic ring or rings can be cycloalkyls, cycloalkenyls, cycloalkynyls, aryls and/or heterocycles.
- substituted aryl refers to an aryl group, wherein one or more hydrogen atoms on one or more aromatic rings are substituted with one or more substituents including, but not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, alkoxy, carbonyl (such as a ketone, aldehyde, carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, imino, alkylthio,
- Heterocycle, ” “heterocyclic” and “heterocyclyl” are used interchangeably, and refer to a cyclic radical attached via a ring carbon or nitrogen atom of a monocyclic or bicyclic ring containing 3-10 ring atoms, and preferably from 5-6 ring atoms, consisting of carbon and one to four heteroatoms each selected from the group consisting of non-peroxide oxygen, sulfur, and N (Y) wherein Y is absent or is H, O, C 1 -C 10 alkyl, phenyl or benzyl, and optionally containing 1-3 double bonds and optionally substituted with one or more substituents. Heterocyclyl are distinguished from heteroaryl bydefinition.
- heterocycles include, but are not limited to piperazinyl, piperidinyl, piperidonyl, 4-piperidonyl, dihydrofuro [2, 3-b] tetrahydrofuran, morpholinyl, piperazinyl, piperidinyl, piperidonyl, 4-piperidonyl, piperonyl, pyranyl, 2H-pyrrolyl, 4H-quinolizinyl, quinuclidinyl, tetrahydrofuranyl, 6H-1, 2, 5-thiadiazinyl.
- Heterocyclic groups can optionally be substituted with one or more substituents as defined above for alkyl and aryl.
- heteroaryl refers to C 5 -C 26 -membered aromatic, fused aromatic, biaromatic ring systems, or combinations thereof, in which one or more carbon atoms on one or more aromatic ring structures have been substituted with a heteroatom.
- Suitable heteroatoms include, but are not limited to, oxygen, sulfur, and nitrogen.
- heteroaryl includes 5-, 6-, 7-, 8-, 9-, 10-, 14-, 18-, and 24-membered single-ring aromatic groups that may include from one to four heteroatoms, for example, pyrrole, furan, thiophene, imidazole, oxazole, thiazole, triazole, tetrazole, pyrazole, pyridine, pyrazine, pyridazine and pyrimidine, and the like.
- the heteroaryl group may also be referred to as “aryl heterocycles” or “heteroaromatics” .
- Heteroaryl further encompasses polycyclic ring systems having two or more rings in which two or more carbons are common to two adjoining rings (i.e., “fused rings” ) wherein at least one of the rings is heteroaromatic, e.g., the other cyclic ring or rings can be cycloalkyls, cycloalkenyls, cycloalkynyls, aryls, heterocycles, or combinations thereof.
- heteroaryl rings include, but are not limited to, benzimidazolyl, benzofuranyl, benzothiofuranyl, benzothiophenyl, benzoxazolyl, benzoxazolinyl, benzthiazolyl, benztriazolyl, benztetrazolyl, benzisoxazolyl, benzisothiazolyl, benzimidazolinyl, carbazolyl, 4aH-carbazolyl, carbolinyl, chromanyl, chromenyl, cinnolinyl, decahydroquinolinyl, 2H, 6H-1, 5, 2-dithiazinyl, furanyl, furazanyl, imidazolidinyl, imidazolinyl, imidazolyl, 1H-indazolyl, indolenyl, indolinyl, indolizinyl, indolyl, 3H-indolyl, is
- substituted heteroaryl refers to a heteroaryl group in which one or more hydrogen atoms on one or more heteroaromatic rings are substituted with one or more substituents including, but not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, alkoxy, carbonyl (such as a ketone, aldehyde, carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, imino, alkyl
- Alkyl refers to the radical of saturated aliphatic groups, including straight-chain alkyl, branched-chain alkyl, cycloalkyl (alicyclic) , alkyl substituted cycloalkyl groups, and cycloalkyl substituted alkyl.
- a straight chain or branched chain alkyl has 30 or fewer carbon atoms in its backbone (e.g., C 1 -C 30 for straight chains, C 3 -C 30 for branched chains) , preferably 20 or fewer, more preferably 15 or fewer, most preferably 10 or fewer.
- preferred cycloalkyls have from 3-10 carbon atoms in their ring structure, and more preferably have 5, 6 or 7 carbons in the ring structure.
- alkyl (or “lower alkyl” ) as used throughout the specification, examples, and claims is intended to include both “unsubstituted alkyls” and “substituted alkyls, ” the latter of which refers to alkyl moieties having one or more substituents replacing a hydrogen on one or more carbons of the hydrocarbon backbone.
- substituents include, but are not limited to, halogen, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, a hosphinate, amino, amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, sulfonate, sulfamoyl, sulfoxide, sulfonamido, sulfonyl, heterocyclyl, aralkyl, or an aromatic or heteroaromatic moiety.
- lower alkyl as used herein means an alkyl group, as defined above, but having from one to ten carbons, more preferably from one to six carbon atoms in its backbone structure. Likewise, “lower alkenyl” and “lower alkynyl” have similar chain lengths. Throughout the application, preferred alkyl groups are lower alkyls. In preferred embodiments, a substituent designated herein as alkyl is a lower alkyl.
- Alkyl includes one or more substitutions at one or more carbon atoms of the hydrocarbon radical as well as heteroalkyls. Suitable substituents include, but are not limited to, halogens, such as fluorine, chlorine, bromine, or iodine; hydroxyl; -NRR’, wherein R and R’ are independently hydrogen, alkyl, or aryl, and wherein the nitrogen atom is optionally quaternized; -SR, wherein R is hydrogen, alkyl, or aryl; -CN; -NO 2 ; -COOH; carboxylate; –COR, -COOR, or -CON (R) 2 , wherein R is hydrogen, alkyl, or aryl; azide, aralkyl, alkoxyl, imino, phosphonate, phosphinate, silyl, ether, sulfonyl, sulfonamido, heterocyclyl, aromatic or heteroaromatic moieties,
- the moieties substituted on the hydrocarbon chain can themselves be substituted, if appropriate.
- the substituents of a substituted alkyl may include halogen, hydroxy, nitro, thiols, amino, azido, imino, amido, phosphoryl (including phosphonate and phosphinate) , sulfonyl (including sulfate, sulfonamido, sulfamoyl, sulfoxide and sulfonate) , and silyl groups, as well as ethers, alkylthios, carbonyls (including ketones, aldehydes, carboxylates, and esters) , haloalkyls, -CN and the like. Cycloalkyls can be substituted in the same manner.
- alkenyl and alkynyl refer to unsaturated aliphatic groups analogous in length and possible substitution to the alkyls described above, but that contain at least one double or triple bond respectively.
- substituted alkenyl refers to alkenyl moieties having one or more substituents replacing one or more hydrogen atoms on one or more carbons of the hydrocarbon backbone.
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, s
- substituted alkynyl refers to alkynyl moieties having one or more substituents replacing one or more hydrogen atoms on one or more carbons of the hydrocarbon backbone.
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate,
- Amino and “Amine, ” as used herein, are art-recognized and refer to both substituted and unsubstituted amines, e.g., a moiety that can be represented by the general formula:
- R, R’, and R each independently represent a hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted carbonyl, - (CH 2 ) m -R”’, or R and R’ taken together with the N atom to which they are attached complete a heterocycle having from 3 to 14 atoms in the ring structure;
- R”’ represents a hydroxy group, substituted or unsubstituted carbonyl group, an aryl, a cycloalkyl ring, a cycloalkenyl ring, a heterocycle, or a polycycle; and m is zero or an integer ranging from 1 to 8.
- R and R’ can be a carbonyl, e.g., R and R’ together with the nitrogen do not form an imide.
- R and R’ (and optionally R”) each independently represent a hydrogen atom, substituted or unsubstituted alkyl, a substituted or unsubstituted alkenyl, or - (CH 2 ) m -R”’.
- alkylamine refers to an amine group, as defined above, having a substituted or unsubstituted alkyl attached thereto (i.e. at least one of R, R’, or R” is an alkyl group) .
- Carbonyl, ” as used herein, is art-recognized and includes such moieties as can be represented by the general formula:
- R represents a hydrogen, a substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted alkylaryl, substituted or unsubstituted arylalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, - (CH 2 ) m -R”, or a pharmaceutical acceptable salt
- R’ represents a hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl,
- X is oxygen and R is defined as above, the moiety is also referred to as a carboxyl group.
- the formula represents a ‘carboxylic acid. ’ Where X is oxygen and R’ is hydrogen, the formula represents a ‘formate. ’ Where X is oxygen and R or R’ is not hydrogen, the formula represents an "ester” .
- the oxygen atom of the above formula is replaced by a sulfur atom, the formula represents a ‘thiocarbonyl’ group. Where X is sulfur and R or R’ is not hydrogen, the formula represents a ‘thioester. ’ Where X is sulfur and R is hydrogen, the formula represents a ‘thiocarboxylic acid.
- substituted carbonyl refers to a carbonyl, as defined above, wherein one or more hydrogen atoms in R, R’ or a group to which the moiety
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, sulfonate, sulfamoyl, sulfoxide, sulfonamido, sulfonyl, heterocyclyl, al
- R iv is an alkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, alkylaryl, arylalkyl, aryl, or heteroaryl.
- a straight chain or branched chain alkyl, alkenyl, and alkynyl have 30 or fewer carbon atoms in its backbone (e.g., C 1 -C 30 for straight chain alkyl, C 3 -C 30 for branched chain alkyl, C 2 -C 30 for straight chain alkenyl and alkynyl, C 3 -C 30 for branched chain alkenyl and alkynyl) , preferably 20 or fewer, more preferably 15 or fewer, most preferably 10 or fewer.
- preferred cycloalkyls, heterocyclyls, aryls and heteroaryls have from 3-10 carbon atoms in their ring structure, and more preferably have 5, 6 or 7 carbons in the ring structure.
- substituted carboxyl refers to a carboxyl, as defined above, wherein one or more hydrogen atoms in R iv are substituted.
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, sulfonate, sul
- Heteroalkyl refers to straight or branched chain, or cyclic carbon-containing radicals, or combinations thereof, containing at least one heteroatom. Suitable heteroatoms include, but are not limited to, O, N, Si, P and S, wherein the nitrogen, phosphorous and sulfur atoms are optionally oxidized, and the nitrogen heteroatom is optionally quaternized.
- saturated hydrocarbon radicals include, but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, t-butyl, isobutyl, sec-butyl, cyclohexyl, (cyclohexyl) methyl, cyclopropylmethyl, and homologs and isomers of, for example, n-pentyl, n-hexyl, n-heptyl, n-octyl.
- unsaturated alkyl groups include, but are not limited to, vinyl, 2-propenyl, crotyl, 2-isopentenyl, 2- (butadienyl) , 2, 4-pentadienyl, 3- (1, 4-pentadienyl) , ethynyl, 1-and 3-propynyl, and 3-butynyl.
- alkoxyl or “alkoxy, ” “aroxy” or “aryloxy, ” generally describe compounds represented by the formula -OR v , wherein R v includes, but is not limited to, substituted or unsubstituted alkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, cycloalkenyl, heterocycloalkenyl, aryl, heteroaryl, arylalkyl, heteroalkyls, alkylaryl, alkylheteroaryl.
- alkoxyl or "alkoxy” as used herein refer to an alkyl group, as defined above, having an oxygen radical attached thereto.
- Representative alkoxyl groups include methoxy, ethoxy, propyloxy, tert-butoxy and the like.
- An "ether” is two hydrocarbons covalently linked by an oxygen. Accordingly, the substituent of an alkyl that renders that alkyl an ether is or resembles an alkoxyl, such as can be represented by one of -O-alkyl, -O-alkenyl, and -O-alkynyl.
- alkoxy also includes cycloalkyl, heterocyclyl, cycloalkenyl, heterocycloalkenyl, and arylalkyl having an oxygen radical attached to at least one of the carbon atoms, as valency permits.
- substituted alkoxy refers to an alkoxy group having one or more substituents replacing one or more hydrogen atoms on one or more carbons of the alkoxy backbone.
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, sulf
- alkylthio refers to an alkyl group, as defined above, having a sulfur radical attached thereto.
- the "alkylthio" moiety is represented by -S-alkyl.
- Representative alkylthio groups include methylthio, ethylthio, and the like.
- alkylthio also encompasses cycloalkyl groups having a sulfur radical attached thereto.
- substituted alkylthio refers to an alkylthio group having one or more substituents replacing one or more hydrogen atoms on one or more carbon atoms of the alkylthio backbone.
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, s
- Arylthio refers to -S-aryl or -S-heteroaryl groups, wherein aryl and heteroaryl as as defined herein.
- substituted arylthio represents -S-aryl or -S-heteroaryl, having one or more substituents replacing a hydrogen atom on one or more ring atoms of the aryl and heteroaryl rings as defined herein.
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, sulfonate, sulfamoyl, sulfoxide, sulfonamido, sulfonyl, heterocyclyl, alkylaryl, haloal
- Arylalkyl refers to an alkyl group that is substituted with a substituted or unsubstituted aryl or heteroaryl group.
- Alkylaryl refers to an aryl group (e.g., an aromatic or hetero aromatic group) , substituted with a substituted or unsubstituted alkyl group.
- amide or “amido” are used interchangeably, refer to both “unsubstituted amido” and “substituted amido” and are represented by the general formula:
- E is absent, or E is substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted aralkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocyclyl, wherein independently of E, R and R’ each independently represent a hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted carbonyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted alkylaryl, substituted or unsubstituted arylalkyl,
- R and R’ can be a carbonyl, e.g., R and R’ together with the nitrogen do not form an imide.
- R and R’ each independently represent a hydrogen atom, substituted or unsubstituted alkyl, a substituted or unsubstituted alkenyl, or - (CH 2 ) m -R”’.
- E oxygen
- a carbamate is formed. The carbamate cannot be attached to another chemical species, such as to form an oxygen-oxygen bond, or other unstable bonds, as understood by one of ordinary skill in the art.
- E is absent, or E is alkyl, alkenyl, alkynyl, aralkyl, alkylaryl, cycloalkyl, aryl, heteroaryl, heterocyclyl, wherein independently of E, R represents a hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted amine, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted alkylaryl, substituted or unsubstituted arylalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, - (CH 2 ) m -R”’, or E and R taken together with the S atom to which they are attached complete a heterocycle having from 3 to 14 atoms in the atom
- only one of E and R can be substituted or unsubstituted amine, to form a “sulfonamide” or “sulfonamido. ”
- the substituted or unsubstituted amine is as defined above.
- substituted sulfonyl represents a sulfonyl in which E and R are independently substituted.
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, sulfonate, sulfamoyl,
- sulfonic acid refers to a sulfonyl, as defined above, wherein R is hydroxyl, and E is absent, or E is substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted alkylaryl, substituted or unsubstituted arylalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl.
- sulfate refers to a sulfonyl, as defined above, wherein E is absent, oxygen, alkoxy, aroxy, substituted alkoxy or substituted aroxy, as defined above, and R is independently hydroxyl, alkoxy, aroxy, substituted alkoxy or substituted aroxy, as defined above.
- E oxygen
- the sulfate cannot be attached to another chemical species, such as to form an oxygen-oxygen bond, or other unstable bonds, as understood by one of ordinary skill in the art.
- sulfonate refers to a sulfonyl, as defined above, wherein E is oxygen, alkoxy, aroxy, substituted alkoxy or substituted aroxy, as defined above, and R is independently hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted amine, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted alkylaryl, substituted or unsubstituted arylalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, - (CH 2 ) m -R”’, R”’ represents a hydroxy group, substituted or unsubstituted carbonyl group, an aryl, a cycloalkyl ring
- sulfamoyl refers to a sulfonamide or sulfonamide represented by the formula
- E is absent, or E is substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted aralkyl, substituted or unsubstituted alkylaryl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocyclyl, wherein independently of E, R and R’ each independently represent a hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted carbonyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted alkylaryl, substituted or
- E is absent, or E is alkyl, alkenyl, alkynyl, aralkyl, alkylaryl, cycloalkyl, aryl, heteroaryl, heterocyclyl, wherein independently of E, R represents a hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted amine, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted alkylaryl, substituted or unsubstituted arylalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, - (CH 2 ) m -R”’, or E and R taken together with the S atom to which they are attached complete a heterocycle having from 3 to 14 atoms in the atom
- E is absent, or E is substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted aralkyl, substituted or unsubstituted alkylaryl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocyclyl, , wherein, independently of E, R vi and R vii are independently hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted carbonyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted alkylaryl, substitute
- substituted phosphonyl represents a phosphonyl in which E, R vi and R vii are independently substituted.
- substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, sulfonate, sulfamoyl,
- phosphoryl defines a phoshonyl in which E is absent, oxygen, alkoxy, aroxy, substituted alkoxy or substituted aroxy, as defined above, and independently of E, R vi and R vii are independently hydroxyl, alkoxy, aroxy, substituted alkoxy or substituted aroxy, as defined above.
- E oxygen
- the phosphoryl cannot be attached to another chemical species, such as to form an oxygen-oxygen bond, or other unstable bonds, as understood by one of ordinary skill in the art.
- the substituents include, but are not limited to, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, carbonyl (such as a carboxyl, alkoxycarbonyl, formyl, or an acyl) , silyl, ether, ester, thiocarbonyl (such as a thioester, a thioacetate, or a thioformate) , alkoxyl, phosphoryl, phosphate, phosphonate, phosphinate, amino (or quarternized amino) , amido, amidine, imine, cyano, nitro, azido, sulfhydryl, alkylthio, sulfate, sulfonate, sulfamoyl, sulfoxide, sulfonamido, sulfonyl, hetero
- C 3 -C 20 cyclic refers to a substituted or unsubstituted cycloalkyl, substituted or unsubstituted cycloalkenyl, substituted or unsubstituted cycloalkynyl, substituted or unsubstituted heterocyclyl that have from three to 20 carbon atoms, as geometric constraints permit.
- the cyclic structures are formed from single or fused ring systems.
- the substituted cycloalkyls, cycloalkenyls, cycloalkynyls and heterocyclyls are substituted as defined above for the alkyls, alkenyls, alkynyls and heterocyclyls, respectively.
- Senolytic prodrugs disclosed herein are based on a selective design of cytotoxic agents to introduce a site cleavable by SA- ⁇ -gal following delivery of the prodrug in vivo, to release the active parent cytotoxic agent for preferentially killing senescent cells.
- the cytotoxic agent lacks a phenol group.
- a particularly preferred cytotoxic agent is gemcitabine.
- the prodrug compounds disclosed therein include, a cytotoxic agent, exemplified herein with gemcitabine modified for cleavage by SA- ⁇ -gal.
- the cytotoxic agent is modified to include a galactose-based moiety, which is preferably acetylated and a benzyloxycarbonyl group as exemplified below for SSK1.
- the prodrug does not include free hydroxyl groups (-OH) on the galactose-based moiety.
- the prodrug may include modification (s) (e.g., removal of nitro group (s) (such as -NO 2 ) on the aryl or heteroaryl (e.g., phenyl) of the benzyloxycarbonyl moiety or addition of other functional group (s) thereon) to reduce its potential immunogenicity.
- the prodrug may include nitro group (s) (such as -NO 2 ) on the aryl or heteroaryl (e.g., phenyl) of the benzyloxycarbonyl moiety, which may, e.g., affect efficiency of the prodrug to produce the cytotoxic agent after activation by ⁇ -galactosidase.
- the prodrug may include further modification (s) (e.g., removal of one or more of the protecting groups (e.g., acetyl (Ac) groups) from the galactose based-moiety) to increase its water solubility, which may, e.g., facilitate formation of a stable crystal form, simplify the metabolic pathway in vivo, and/or obtain better drug-like properties.
- further modification e.g., removal of one or more of the protecting groups (e.g., acetyl (Ac) groups) from the galactose based-moiety
- the protecting groups e.g., acetyl (Ac) groups
- benzyloxycarbonyl moiety has the structure shown below:
- X is aryl or heteroaryl, preferably C 6 aryl, such as phenyl,
- V is O, S, or NR 1 ’, wherein R 1 ’ is hydrogen, substituted C 1 -C 5 alkyl, unsubstituted C 1 -C 5 alkyl, substituted C 6 -C 10 aryl, or unsubstituted C 6 -C 10 aryl, wherein preferably, V is O,
- Y is substituted C 1 -C 5 alkylene, unsubstituted C 1 -C 5 alkylene, unsubstituted C 1 alkylene, substituted C 1 alkylene, unsubstituted C 2 alkylene, substituted C 2 alkylene, unsubstituted C 3 alkylene, substituted C 3 alkylene, unsubstituted C 4 alkylene, substituted C 4 alkylene, unsubstituted C 1 alkylene, substituted C 5 alkylene, wherein preferably, Y is methylene,
- Z is O, S, or NR 2 ’, wherein R 2 ’ is hydrogen, substituted C 1 -C 5 alkyl, unsubstituted C 1 -C 5 alkyl, substituted C 6 -C 10 aryl, or unsubstituted C 6 -C 10 aryl, wherein preferably, Z is O,
- W is O, S, or NR 3 ’, wherein R 3 ’ is hydrogen, substituted C 1 -C 5 alkyl, unsubstituted C 1 -C 5 alkyl, substituted C 6 -C 10 aryl, or unsubstituted C 6 -C 10 aryl, wherein preferably, W is O,
- each U is independently nitro- (such as -NO 2 ) , cyano (such as -CN) , amino (such as -NH 2 ) , hydroxy (-OH) , thiol (-SH) , halogen (e.g. F, Cl, I, Br) , alkoxy, alkylamino, dialkylamino, substituted alkoxy, carboxyl, carbonyl, substituted carbonyl, amido, sulfonyl, sulfonic acid, phosphoryl, phosphonyl, phosphonyl, hydrogen, alkyl, substituted alkyl, wherein preferably, U is -NO 2 or hydrogen, and
- n is an integer between 0 and 10, inclusive, such as 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10, as valency permits.
- X is C 6 aryl.
- U is -NO 2 .
- U is hydrogen.
- V is O.
- W is O.
- Y is substituted C 1 alkylene.
- Y is substituted C 1 alkylene.
- Z is O.
- X is C 6 aryl
- Y is substituted C 1 alkylene or substituted C 1 alkylene.
- X is C 6 aryl
- Y is substituted C 1 alkylene or substituted C 1 alkylene
- V and W are O.
- X is C 6 aryl
- Y is substituted C 1 alkylene or substituted C 1 alkylene
- V, Z, and W are O.
- the benzyloxycarbonyl is Formula II, shown below:
- each R 4 ’ is independently nitro- (such as -NO 2 ) , cyano (such as -CN) , amino (such as -NH 2 ) , hydroxy (-OH) , thiol (-SH) , halogen (e.g. F, Cl, I, Br) , alkoxy, alkylamino, dialkylamino, substituted alkoxy, carboxyl, carbonyl, substituted carbonyl, amido, sulfonyl, sulfonic acid, phosphoryl, phosphonyl, phosphonyl, hydrogen, alkyl, substituted alkyl, wherein preferably, R 4 ’ is -NO 2 or hydrogen,
- R 5 ’ is hydrogen, nitro- (such as -NO 2 ) , cyano (such as -CN) , isocyano (such as ) , amino (such as -NH 2 ) , hydroxy (-OH) , thiol (-SH) , halogen (e.g. F, Cl, I, Br) , alkoxy, alkylamino, dialkylamino, substituted alkoxy, carboxyl, carbonyl, substituted carbonyl, amido, sulfonyl, sulfonic acid, phosphoryl, phosphonyl, phosphonyl, hydrogen, alkyl, substituted alkyl, wherein preferably, R 5 ’ is hydrogen, and
- n is an integer between 0 and 4, inclusive, such as 0, 1, 2, 3, and 4, as valency permits.
- At least one R 4 ’ is nitro- (such as -NO 2 ) , cyano (such as -CN) , amino (such as -NH 2 ) , hydroxy (-OH) , thiol (-SH) , halogen (e.g. F, Cl, I, Br) , alkoxy, alkylamino, dialkylamino, substituted alkoxy, carboxyl, carbonyl, substituted carbonyl, amido, sulfonyl, sulfonic acid, phosphoryl, phosphonyl, or phosphonyl.
- halogen e.g. F, Cl, I, Br
- At least one R 4 ’ is nitro- (such as -NO 2 ) , cyano (such as -CN) , isocyano (such as ) , amino (such as -NH 2 ) , hydroxy (-OH) , thiol (-SH) , hydrogen, or halogen (e.g. F, Cl, I, Br) , .
- at least one R 4 ’ is nitro- (such as -NO 2 ) .
- at least one R 4 ’ is nitro- (such as -NO 2 ) , which may e.g., increase efficiency to produce a cytotoxic agent after activation.
- at least one R 4 ’ is hydrogen (instend of a nitro group (such as -NO 2 ) ) , which may e.g., reduce potential immunogenicity.
- R 5 ’ is hydrogen, alkyl, or substituted alkyl. In some forms of Formula II, R 5 ’ is hydrogen.
- the compound includes a galactose based-moiety of the structure shown below.
- R 1 , R 2 , R 3 , and R 4 could each/independently be H or any substituent that could be hydrolyzed inside the cell.
- R 1 , R 2 , R 3 , and R 4 are each hydrogen or substituents such that -OR 1 , -OR 2 , -OR 3 , and/or -OR 4 can be hydrolyzed inside a cell.
- R 1 , R 2 , R 3 , and R 4 are independently H, R 5 C (O) -, R 6 OC (O) -, R 7 R 8 NC (O) -, or R 9 PO 3 - -, such that -OR x is independently an ester, a carbonate, a carbamate, or a phosphodiester group, respectively, where x is 1, 2, 3, or 4.
- R 1 , R 2 , R 3 , and R 4 are independently R 5 C (O) -, R 6 OC (O) -, R 7 R 8 NC (O) -.
- R 5 , R 6 , R 7 , R 8 , and R 9 are independently hydrogen, substituted alkyl, unsubstituted alkyl, substituted aryl, unsubstituted aryl, substituted heteroaryl, unsubstituted heteroaryl, substituted C 3 -C 10 cyclyl, unsubstituted C 3 -C 10 cyclyl, substituted C 3 -C 10 heterocyclyl, unsubstituted C 3 -C 10 heterocyclyl.
- R 5 , R 6 , R 7 , R 8 , and R 9 are independently hydrogen, substituted C 1 -C 5 alkyl, unsubstituted C 1 -C 5 alkyl, substituted C 6 -C 10 aryl, unsubstituted C 6 -C 10 aryl, substituted C 6 -C 10 heteroaryl, unsubstituted C 6 -C 10 heteroaryl, substituted C 3 -C 10 cyclyl, unsubstituted C 3 -C 10 cyclyl, or substituted C 3 -C 10 heterocyclyl, unsubstituted C 3 -C 10 heterocyclyl.
- R 5 , R 6 , R 7 , R 8 , and R 9 are independently hydrogen, substituted C 1 -C 3 alkyl, unsubstituted C 1 -C 3 alkyl, substituted C 6 aryl, unsubstituted C 6 aryl, substituted C 6 heteroaryl, unsubstituted C 6 heteroaryl, substituted C 6 cyclyl, unsubstituted C 6 cyclyl, or substituted C 6 heterocyclyl, unsubstituted C 6 heterocyclyl.
- one or more (or all) of R 1 , R 2 , R 3 , and R 4 could be H, which may, e.g., increase water solubility, facilitate formation of a stable crystal form, simplify the metabolic pathway in vivo, and/or obtain better drug-like properties.
- one or more (or all) of R 1 , R 2 , R 3 , and R 4 are H.
- R 1 , R 2 , R 3 , and R 4 are R 5 C (O) -.
- R 5 is independently substituted C 1 -C 3 alkyl or unsubstituted C 1 -C 3 alkyl.
- R 5 is, preferably, methyl.
- a preferred galactose-based moiety is a D-galactose tetraacetate moiety, shown below.
- one or more (e.g., two, three, or four) of the protecting groups may be removed from the galactose based-moiety.
- removel of one or more (e.g., two, three, or four) of the protecting groups may increase water solubility, facilitate formation of a stable crystal form, simplify the metabolic pathway in vivo, and/or obtain better drug-like properties.
- the prodrug may be in a crystal form, which may, e.g., do not contain protecting groups (e.g., acetyl (Ac) groups) or comprises a reduced number of protecting groups (e.g., acetyl (Ac) groups) on the galactose based-moiety.
- protecting groups e.g., acetyl (Ac) groups
- acetyl (Ac) groups e.g., acetyl (Ac) groups
- R 1 , R 2 , R 3 , and R 4 are H.
- the agent is represented by
- D comprises a cytotoxic agent.
- one or more (e.g., two, three, or four) of the protecting groups e.g., acetyl (Ac) groups
- the protecting groups e.g., acetyl (Ac) groups
- R 1 , R 2 , R 3 , and R 4 are H.
- R 4 ’ may be nitro- (such as -NO 2 ) .
- R 4 ’ may be hydrogen (instend of e.g., a nitro group (such as -NO 2 ) ) .
- cytotoxic agents are chemotherapeutic agents/drugs which are known in the art.
- chemotherqpeutic agents include, but are not limited to gemcitabine, cytarabine, 5'-Deoxy-5-fluorocytidine, mercaptopurine, Sapacitabine, nelarabine, clofarabine, decitabine, and azacitidine, methotrexate, vinblastine, doxorubicin, ifosfamide, pemetrexed, cisplatin, carboplatin, and paclitaxel.
- Chemetherapeutic agents with short plasma circulation times such as gemcitabine, are preferred.
- derivatives of parent pharmaceutcals which have been modified to prolong their plasma circulation time are not preferred, for example, pegylated derivatives.
- Particularly preferred are chemotherapeutic agents lacking a phenolic group.
- cytotoxic agents that can be used to make the senolytic prodrugs disclosed herein include, but are not limited to gemcitabine, cytarabine, and 5'-Deoxy-5-fluorocytidine, mercaptopurine, Sapacitabine, nelarabine, clofarabine, decitabine, and azacitidine.
- the xytotoxic agent is not quercetin or panobinostat.
- R 1 , R 2 , R 3 , and R 4 are as described above for Formula III, and
- R 4 ’ and R 5 ’ are as described above for Formula II.
- a particularly preferred benzyloxycarbonyl group is shown below.
- NO 2 may be removed from the above group.
- one or more (e.g., two, three, or four) of the protecting groups may be removed from the galactose based-moiety.
- the protecting groups e.g., acetyl (Ac) groups
- another preferred compound is shown below.
- NO 2 may be removed and another preferred compound is shown below.
- both NO 2 and one or more (e.g., two, three, or four) of the protecting groups may be removed and another preferred compound is shown below.
- cytotoxic agents such as gemcitabine, cytarabine, and 5'-Deoxy-5-fluorocytidine which lack the phenolic or hydroxyl functional group are not amenable to direct modification with a galactose modification for at least the reason that direct attachment of a galactose moiety to amine groups for example may result either in an unstable compound or a prodrug that is not cleaved or is poorly cleaved by SA- ⁇ -gal.
- the prodrugs disclosed herein do not include a direct conjugation of a galactose moiety to any group on the cytotoxic agent.
- prodrug compounds are based on a selective and specific design of preferably, non-phenolic cytotoxic agents for (i) increased cellular permeability and (ii) improved cleavage by SA- ⁇ -gal.
- a proposed scheme is release of the active agent from the prodrug is exemplified below for SSK1.
- the compounds described herein can be formulated for enteral, parenteral, topical, or pulmonary administration.
- the compounds can be combined with one or more pharmaceutically acceptable carriers and/or excipients that are considered safe and effective and may be administered to an individual without causing undesirable biological side effects or unwanted interactions.
- the carrier is all components present in the pharmaceutical formulation other than the active ingredient or ingredients.
- the compounds are included in the formulations in a therapeutically effect amount.
- a therapeutically effective amount can be estimated initially from cell culture assays. For example, a dose can be formulated in animal models to achieve a circulating concentration range that includes the IC50 or the IC10o as determined in cell culture. Such information can be used to more accurately determine useful doses in humans. Initial dosages can also be estimated from in vivo data. Using these initial guidelines one of ordinary skill in the art could determine an effective dosage in humans.
- the compounds described herein can be formulated for parenteral administration.
- parenteral administration may include administration to a patient intravenously, intradermally, intraarterially, intraperitoneally, intralesionally, intracranially, intraarticularly, intraprostatically, intrapleurally, intratracheally, intravitreally, intratumorally, intramuscularly, subcutaneously, subconjunctivally, intravesicularly, intrapericardially, intraumbilically, by injection, and by infusion.
- Parenteral formulations can be prepared as aqueous compositions using techniques is known in the art.
- such compositions can be prepared as injectable formulations, for example, solutions or suspensions; solid forms suitable for using to prepare solutions or suspensions upon the addition of a reconstitution medium prior to injection; emulsions, such as water-in-oil (w/o) emulsions, oil-in-water (o/w) emulsions, and microemulsions thereof, liposomes, or emulsomes.
- injectable formulations for example, solutions or suspensions
- solid forms suitable for using to prepare solutions or suspensions upon the addition of a reconstitution medium prior to injection emulsions, such as water-in-oil (w/o) emulsions, oil-in-water (o/w) emulsions, and microemulsions thereof, liposomes, or emulsomes.
- emulsions such as water-in-oil (w/o) emulsions
- the carrier can be a solvent or dispersion medium containing, for example, water, ethanol, one or more polyols (e.g., glycerol, propylene glycol, and liquid polyethylene glycol) , oils, such as vegetable oils (e.g., peanut oil, corn oil, sesame oil, etc. ) , and combinations thereof.
- polyols e.g., glycerol, propylene glycol, and liquid polyethylene glycol
- oils such as vegetable oils (e.g., peanut oil, corn oil, sesame oil, etc. )
- the proper fluidity can be maintained, for example, by the use of a coating, such as lecithin, by the maintenance of the required particle size in the case of dispersion and/or by the use of surfactants.
- isotonic agents for example, sugars or sodium chloride.
- Solutions and dispersions of the active compounds as the free acid or base or pharmacologically acceptable salts thereof can be prepared in water or another solvent or dispersing medium suitably mixed with one or more pharmaceutically acceptable excipients including, but not limited to, surfactants, dispersants, emulsifiers, pH modifying agents, viscosity modifying agents, and combination thereof.
- Suitable surfactants may be anionic, cationic, amphoteric or nonionic surface-active agents.
- Suitable anionic surfactants include, but are not limited to, those containing carboxylate, sulfonate and sulfate ions.
- anionic surfactants include sodium, potassium, ammonium of long chain alkyl sulfonates and alkyl aryl sulfonates such as sodium dodecylbenzene sulfonate; dialkyl sodium sulfosuccinates, such as sodium dodecylbenzene sulfonate; dialkyl sodium sulfosuccinates, such as sodium bis- (2-ethylthioxyl) -sulfosuccinate; and alkyl sulfates such as sodium lauryl sulfate.
- Cationic surfactants include, but are not limited to, quaternary ammonium compounds such as benzalkonium chloride, benzethonium chloride, cetrimonium bromide, stearyl dimethylbenzyl ammonium chloride, polyoxyethylene and coconut amine.
- nonionic surfactants include ethylene glycol monostearate, propylene glycol myristate, glyceryl monostearate, glyceryl stearate, polyglyceryl-4-oleate, sorbitan acylate, sucrose acylate, PEG-150 laurate, PEG-400 monolaurate, polyoxyethylene monolaurate, polysorbates, polyoxyethylene octylphenylether, PEG-1000 cetyl ether, polyoxyethylene tridecyl ether, polypropylene glycol butyl ether, 401, stearoyl monoisopropanolamide, and polyoxyethylene hydrogenated tallow amide.
- amphoteric surfactants include sodium N-dodecyl-. beta. -alanine, sodium N-lauryl-. beta. -iminodipropionate, myristoamphoacetate, lauryl betaine and lauryl sulfobetaine.
- the formulation can contain a preservative to prevent the growth of microorganisms. Suitable preservatives include, but are not limited to, parabens, chlorobutanol, phenol, sorbic acid, and thimerosal.
- the formulation may also contain an antioxidant to prevent degradation of the active agent (s) .
- the formulation is typically buffered to a pH of 3-8 for parenteral administration upon reconstitution.
- Suitable buffers include, but are not limited to, phosphate buffers, acetate buffers, and citrate buffers.
- Water-soluble polymers are often used in formulations for parenteral administration. Suitable water-soluble polymers include, but are not limited to, polyvinylpyrrolidone, dextran, carboxymethylcellulose, and polyethylene glycol.
- Sterile injectable solutions can be prepared by incorporating the active compounds in the required amount in the appropriate solvent or dispersion medium with one or more of the excipients listed above, as required, followed by filtered sterilization.
- dispersions are prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those listed above.
- the preferred methods of preparation are vacuum-drying and freeze-drying techniques which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile-filtered solution thereof.
- the powders can be prepared in such a manner that the particles are porous in nature, which can increase dissolution of the particles. Methods for making porous particles are well known in the art.
- parenteral formulations described herein can be formulated for controlled release including immediate release, delayed release, extended release, pulsatile release, and combinations thereof.
- the one or more compounds, and optional one or more additional active agents can be incorporated into microparticles, nanoparticles, or combinations thereof that provide controlled release of the compounds and/or one or more additional active agents.
- the formulations contains two or more drugs
- the drugs can be formulated for the same type of controlled release (e.g., delayed, extended, immediate, or pulsatile) or the drugs can be independently formulated for different types of release (e.g., immediate and delayed, immediate and extended, delayed and extended, delayed and pulsatile, etc. ) .
- the compounds and/or one or more additional active agents can be incorporated into polymeric microparticles, which provide controlled release of the drug (s) . Release of the drug (s) is controlled by diffusion of the drug (s) out of the microparticles and/or degradation of the polymeric particles by hydrolysis and/or enzymatic degradation.
- Suitable polymers include ethylcellulose and other natural or synthetic cellulose derivatives.
- Polymers which are slowly soluble and form a gel in an aqueous environment, such as hydroxypropyl methylcellulose or polyethylene oxide, can also be suitable as materials for drug containing microparticles.
- Other polymers include, but are not limited to, polyanhydrides, poly (ester anhydrides) , polyhydroxy acids, such as polylactide (PLA) , polyglycolide (PGA) , poly (lactide-co-glycolide) (PLGA) , poly-3-hydroxybutyrate (PHB) and copolymers thereof, poly-4-hydroxybutyrate (P4HB) and copolymers thereof, polycaprolactone and copolymers thereof, and combinations thereof.
- PLA polylactide
- PGA polyglycolide
- P4HB poly-4-hydroxybutyrate
- the drug (s) can be incorporated into microparticles prepared from materials which are insoluble in aqueous solution or slowly soluble in aqueous solution, but are capable of degrading within the GI tract by means including enzymatic degradation, surfactant action of bile acids, and/or mechanical erosion.
- slowly soluble in water refers to materials that are not dissolved in water within a period of 30 minutes. Preferred examples include fats, fatty substances, waxes, wax-like substances and mixtures thereof.
- Suitable fats and fatty substances include fatty alcohols (such as lauryl, myristyl stearyl, cetyl or cetostearyl alcohol) , fatty acids and derivatives, including but not limited to fatty acid esters, fatty acid glycerides (mono-, di-and tri-glycerides) , and hydrogenated fats. Specific examples include, but are not limited to hydrogenated vegetable oil, hydrogenated cottonseed oil, hydrogenated castor oil, hydrogenated oils available under the trade name stearic acid, cocoa butter, and stearyl alcohol. Suitable waxes and wax-like materials include natural or synthetic waxes, hydrocarbons, and normal waxes.
- waxes include beeswax, glycowax, castor wax, carnauba wax, paraffins and candelilla wax.
- a wax-like material is defined as any material, which is normally solid at room temperature and has a melting point of from about 30 to 300°C.
- rate-controlling (wicking) agents can be formulated along with the fats or waxes listed above.
- rate-controlling materials include certain starch derivatives (e.g., waxy maltodextrin and drum dried corn starch) , cellulose derivatives (e.g., hydroxypropylmethyl-cellulose, hydroxypropylcellulose, methylcellulose, and carboxymethyl-cellulose) , alginic acid, lactose and talc.
- a pharmaceutically acceptable surfactant for example, lecithin may be added to facilitate the degradation of such microparticles.
- Proteins which are water insoluble, such as zein, can also be used as materials for the formation of drug containing microparticles. Additionally, proteins, polysaccharides and combinations thereof, which are water-soluble, can be formulated with drug into microparticles and subsequently cross-linked to form an insoluble network. For example, cyclodextrins can be complexed with individual drug molecules and subsequently cross-linked.
- Encapsulation or incorporation of drug into carrier materials to produce drug-containing microparticles can be achieved through known pharmaceutical formulation techniques.
- the carrier material is typically heated above its melting temperature and the drug is added to form a mixture comprising drug particles suspended in the carrier material, drug dissolved in the carrier material, or a mixture thereof.
- Microparticles can be subsequently formulated through several methods including, but not limited to, the processes of congealing, extrusion, spray chilling or aqueous dispersion.
- wax is heated above its melting temperature, drug is added, and the molten wax-drug mixture is congealed under constant stirring as the mixture cools.
- the molten wax-drug mixture can be extruded and spheronized to form pellets or beads.
- a solvent evaporation technique to produce drug-containing microparticles.
- drug and carrier material are co-dissolved in a mutual solvent and microparticles can subsequently be produced by several techniques including, but not limited to, forming an emulsion in water or other appropriate media, spray drying or by evaporating off the solvent from the bulk solution and milling the resulting material.
- drug in a particulate form is homogeneously dispersed in a water-insoluble or slowly water soluble material.
- the drug powder itself may be milled to generate fine particles prior to formulation. The process of jet milling, known in the pharmaceutical art, can be used for this purpose.
- drug in a particulate form is homogeneously dispersed in a wax or wax like substance by heating the wax or wax like substance above its melting point and adding the drug particles while stirring the mixture.
- a pharmaceutically acceptable surfactant may be added to the mixture to facilitate the dispersion of the drug particles.
- the particles can also be coated with one or more modified release coatings.
- Solid esters of fatty acids which are hydrolyzed by lipases, can be spray coated onto microparticles or drug particles.
- Zein is an example of a naturally water-insoluble protein. It can be coated onto drug containing microparticles or drug particles by spray coating or by wet granulation techniques.
- some substrates of digestive enzymes can be treated with cross-linking procedures, resulting in the formation of non-soluble networks.
- Many methods of cross-linking proteins initiated by both chemical and physical means, have been reported. One of the most common methods to obtain cross-linking is the use of chemical cross-linking agents.
- cross-linking agents examples include aldehydes (gluteraldehyde and formaldehyde) , epoxy compounds, carbodiimides, and genipin.
- aldehydes gluteraldehyde and formaldehyde
- epoxy compounds carbodiimides
- genipin examples include aldehydes (gluteraldehyde and formaldehyde) , epoxy compounds, carbodiimides, and genipin.
- oxidized and native sugars have been used to cross-link gelatin.
- Cross-linking can also be accomplished using enzymatic means; for example, transglutaminase has been approved as a GRAS substance for cross-linking seafood products.
- cross-linking can be initiated by physical means such as thermal treatment, UV irradiation and gamma irradiation.
- a water-soluble protein can be spray coated onto the microparticles and subsequently cross-linked by the one of the methods described above.
- drug-containing microparticles can be microencapsulated within protein by coacervation-phase separation (for example, by the addition of salts) and subsequently cross-linked.
- suitable proteins for this purpose include gelatin, albumin, casein, and gluten.
- Polysaccharides can also be cross-linked to form a water-insoluble network. For many polysaccharides, this can be accomplished by reaction with calcium salts or multivalent cations, which cross-link the main polymer chains. Pectin, alginate, dextran, amylose and guar gum are subject to cross-linking in the presence of multivalent cations. Complexes between oppositely charged polysaccharides can also be formed; pectin and chitosan, for example, can be complexed via electrostatic interactions.
- the compounds described herein can be incorporated into injectable/implantable solid or semi-solid implants, such as polymeric implants.
- the compounds are incorporated into a polymer that is a liquid or paste at room temperature, but upon contact with aqueous medium, such as physiological fluids, exhibits an increase in viscosity to form a semi-solid or solid material.
- exemplary polymers include, but are not limited to, hydroxyalkanoic acid polyesters derived from the copolymerization of at least one unsaturated hydroxy fatty acid copolymerized with hydroxyalkanoic acids. The polymer can be melted, mixed with the active substance and cast or injection molded into a device.
- melt fabrication require polymers having a melting point that is below the temperature at which the substance to be delivered and polymer degrade or become reactive.
- the device can also be prepared by solvent casting where the polymer is dissolved in a solvent and the drug dissolved or dispersed in the polymer solution and the solvent is then evaporated. Solvent processes require that the polymer be soluble in organic solvents.
- Another method is compression molding of a mixed powder of the polymer and the drug or polymer particles loaded with the active agent.
- the compounds can be incorporated into a polymer matrix and molded, compressed, or extruded into a device that is a solid at room temperature.
- the compounds can be incorporated into a biodegradable polymer, such as polyanhydrides, polyhydroalkanoic acids (PHAs) , PLA, PGA, PLGA, polycaprolactone, polyesters, polyamides, polyorthoesters, polyphosphazenes, proteins and polysaccharides such as collagen, hyaluronic acid, albumin and gelatin, and combinations thereof and compressed into solid device, such as disks, or extruded into a device, such as rods.
- PHAs polyhydroalkanoic acids
- PLA polyhydroalkanoic acids
- PGA PGA
- PLGA polycaprolactone
- polyesters polyamides
- polyorthoesters polyphosphazenes
- proteins and polysaccharides such as collagen, hyaluronic acid, albumin and ge
- the release of the one or more compounds from the implant can be varied by selection of the polymer, the molecular weight of the polymer, and/or modification of the polymer to increase degradation, such as the formation of pores and/or incorporation of hydrolyzable linkages.
- Methods for modifying the properties of biodegradable polymers to vary the release profile of the compounds from the implant are well known in the art.
- Suitable oral dosage forms include tablets, capsules, solutions, suspensions, syrups, and lozenges. Tablets can be made using compression or molding techniques well known in the art. Gelatin or non-gelatin capsules can be prepared as hard or soft capsule shells, which can encapsulate liquid, solid, and semi-solid fill materials, using techniques well known in the art.
- Formulations may be prepared using a pharmaceutically acceptable carrier.
- carrier includes, but is not limited to, diluents, preservatives, binders, lubricants, disintegrators, swelling agents, fillers, stabilizers, and combinations thereof.
- Carrier also includes all components of the coating composition, which may include plasticizers, pigments, colorants, stabilizing agents, and glidants.
- suitable coating materials include, but are not limited to, cellulose polymers such as cellulose acetate phthalate, hydroxypropyl cellulose, hydroxypropyl methylcellulose, hydroxypropyl methylcellulose phthalate and hydroxypropyl methylcellulose acetate succinate; polyvinyl acetate phthalate, acrylic acid polymers and copolymers, and methacrylic resins that are commercially available under the trade name (Roth Pharma, Westerstadt, Germany) , zein, shellac, and polysaccharides.
- cellulose polymers such as cellulose acetate phthalate, hydroxypropyl cellulose, hydroxypropyl methylcellulose, hydroxypropyl methylcellulose phthalate and hydroxypropyl methylcellulose acetate succinate
- polyvinyl acetate phthalate acrylic acid polymers and copolymers
- methacrylic resins that are commercially available under the trade name (Roth Pharma, Westerstadt, Germany) , zein, shellac,
- the coating material may contain conventional carriers such as plasticizers, pigments, colorants, glidants, stabilization agents, pore formers and surfactants.
- “Diluents” are typically necessary to increase the bulk of a solid dosage form so that a practical size is provided for compression of tablets or formation of beads and granules.
- Suitable diluents include, but are not limited to, dicalcium phosphate dihydrate, calcium sulfate, lactose, sucrose, mannitol, sorbitol, cellulose, microcrystalline cellulose, kaolin, sodium chloride, dry starch, hydrolyzed starches, pregelatinized starch, silicone dioxide, titanium oxide, magnesium aluminum silicate and powdered sugar.
- Binders are used to impart cohesive qualities to a solid dosage formulation, and thus ensure that a tablet or bead or granule remains intact after the formation of the dosage forms.
- Suitable binder materials include, but are not limited to, starch, pregelatinized starch, gelatin, sugars (including sucrose, glucose, dextrose, lactose and sorbitol) , polyethylene glycol, waxes, natural and synthetic gums such as acacia, tragacanth, sodium alginate, cellulose, including hydroxypropylmethylcellulose, hydroxypropylcellulose, ethylcellulose, and veegum, and synthetic polymers such as acrylic acid and methacrylic acid copolymers, methacrylic acid copolymers, methyl methacrylate copolymers, aminoalkyl methacrylate copolymers, polyacrylic acid/polymethacrylic acid and polyvinylpyrrolidone.
- Lubricants are used to facilitate tablet manufacture.
- suitable lubricants include, but are not limited to, magnesium stearate, calcium stearate, stearic acid, glycerol behenate, polyethylene glycol, talc, and mineral oil.
- Disintegrants are used to facilitate dosage form disintegration or "breakup" after administration, and generally include, but are not limited to, starch, sodium starch glycolate, sodium carboxymethyl starch, sodium carboxymethylcellulose, hydroxypropyl cellulose, pregelatinized starch, clays, cellulose, alginine, gums or cross linked polymers, such as cross-linked PVP ( XL from GAF Chemical Corp) .
- Stabilizers are used to inhibit or retard drug decomposition reactions, which include, by way of example, oxidative reactions.
- Suitable stabilizers include, but are not limited to, antioxidants, butylated hydroxytoluene (BHT) ; ascorbic acid, its salts and esters; Vitamin E, tocopherol and its salts; sulfites such as sodium metabisulphite; cysteine and its derivatives; citric acid; propyl gallate, and butylated hydroxyanisole (BHA) .
- Oral dosage forms such as capsules, tablets, solutions, and suspensions, can for formulated for controlled release.
- the one or more compounds and optional one or more additional active agents can be formulated into nanoparticles, microparticles, and combinations thereof, and encapsulated in a soft or hard gelatin or non-gelatin capsule or dispersed in a dispersing medium to form an oral suspension or syrup.
- the particles can be formed of the drug and a controlled release polymer or matrix.
- the drug particles can be coated with one or more controlled release coatings prior to incorporation in to the finished dosage form.
- the one or more compounds and optional one or more additional active agents are dispersed in a matrix material, which gels or emulsifies upon contact with an aqueous medium, such as physiological fluids.
- aqueous medium such as physiological fluids.
- the matrix swells entrapping the active agents, which are released slowly over time by diffusion and/or degradation of the matrix material.
- Such matrices can be formulated as tablets or as fill materials for hard and soft capsules.
- the one or more compounds, and optional one or more additional active agents are formulated into a sold oral dosage form, such as a tablet or capsule, and the solid dosage form is coated with one or more controlled release coatings, such as a delayed release coatings or extended release coatings.
- the coating or coatings may also contain the compounds and/or additional active agents.
- the extended release formulations are generally prepared as diffusion or osmotic systems, which are known in the art.
- a diffusion system typically consists of two types of devices, a reservoir and a matrix, and is well known and described in the art.
- the matrix devices are generally prepared by compressing the drug with a slowly dissolving polymer carrier into a tablet form.
- the three major types of materials used in the preparation of matrix devices are insoluble plastics, hydrophilic polymers, and fatty compounds.
- Plastic matrices include, but are not limited to, methyl acrylate-methyl methacrylate, polyvinyl chloride, and polyethylene.
- Hydrophilic polymers include, but are not limited to, cellulosic polymers such as methyl and ethyl cellulose, hydroxyalkylcelluloses such as hydroxypropyl-cellulose, hydroxypropylmethylcellulose, sodium carboxymethylcellulose, and 934, polyethylene oxides and mixtures thereof.
- Fatty compounds include, but are not limited to, various waxes such as carnauba wax and glyceryl tristearate and wax-type substances including hydrogenated castor oil or hydrogenated vegetable oil, or mixtures thereof.
- the plastic material is a pharmaceutically acceptable acrylic polymer, including but not limited to, acrylic acid and methacrylic acid copolymers, methyl methacrylate, methyl methacrylate copolymers, ethoxyethyl methacrylates, cyanoethyl methacrylate, aminoalkyl methacrylate copolymer, poly (acrylic acid) , poly (methacrylic acid) , methacrylic acid alkylamine copolymer poly (methyl methacrylate) , poly (methacrylic acid) (anhydride) , polymethacrylate, polyacrylamide, poly (methacrylic acid anhydride) , and glycidyl methacrylate copolymers.
- acrylic acid and methacrylic acid copolymers including but not limited to, acrylic acid and methacrylic acid copolymers, methyl methacrylate, methyl methacrylate copolymers, ethoxyethyl methacrylates, cyanoethyl
- the acrylic polymer is comprised of one or more ammonio methacrylate copolymers.
- Ammonio methacrylate copolymers are well known in the art, and are described in NF XVII as fully polymerized copolymers of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups.
- the acrylic polymer is an acrylic resin lacquer such as that which is commercially available from Rohm Pharma under the tradename
- the acrylic polymer comprises a mixture of two acrylic resin lacquers commercially available from Rohm Pharma under the tradenames RL30D and RS30D, respectively.
- RL30D and RS30D are copolymers of acrylic and methacrylic esters with a low content of quaternary ammonium groups, the molar ratio of ammonium groups to the remaining neutral (meth) acrylic esters being 1: 20 in RL30D and 1: 40 in RS30D.
- the mean molecular weight is about 150,000.
- S-100 and L-100 are also preferred.
- RL high permeability
- RS low permeability
- RL/RS may be mixed together in any desired ratio in order to ultimately obtain a sustained-release formulation having a desirable dissolution profile.
- Desirable sustained-release multiparticulate systems may be obtained, for instance, from 100% RL, 50% RL and 50% RS, and 10% RL and 90% RS.
- acrylic polymers may also be used, such as, for example, L.
- extended release formulations can be prepared using osmotic systems or by applying a semi-permeable coating to the dosage form.
- the desired drug release profile can be achieved by combining low permeable and high permeable coating materials in suitable proportion.
- the devices with different drug release mechanisms described above can be combined in a final dosage form comprising single or multiple units.
- multiple units include, but are not limited to, multilayer tablets and capsules containing tablets, beads, or granules
- An immediate release portion can be added to the extended release system by means of either applying an immediate release layer on top of the extended release core using a coating or compression process or in a multiple unit system such as a capsule containing extended and immediate release beads.
- Extended release tablets containing hydrophilic polymers are prepared by techniques commonly known in the art such as direct compression, wet granulation, or dry granulation. Their formulations usually incorporate polymers, diluents, binders, and lubricants as well as the active pharmaceutical ingredient.
- the usual diluents include inert powdered substances such as starches, powdered cellulose, especially crystalline and microcrystalline cellulose, sugars such as fructose, mannitol and sucrose, grain flours and similar edible powders.
- Typical diluents include, for example, various types of starch, lactose, mannitol, kaolin, calcium phosphate or sulfate, inorganic salts such as sodium chloride and powdered sugar.
- Powdered cellulose derivatives are also useful.
- Typical tablet binders include substances such as starch, gelatin and sugars such as lactose, fructose, and glucose.
- Natural and synthetic gums including acacia, alginates, methylcellulose, and polyvinylpyrrolidone can also be used.
- Polyethylene glycol, hydrophilic polymers, ethylcellulose and waxes can also serve as binders.
- a lubricant is necessary in a tablet formulation to prevent the tablet and punches from sticking in the die.
- the lubricant is chosen from such slippery solids as talc, magnesium and calcium stearate, stearic acid and hydrogenated vegetable oils.
- Extended release tablets containing wax materials are generally prepared using methods known in the art such as a direct blend method, a congealing method, and an aqueous dispersion method.
- the congealing method the drug is mixed with a wax material and either spray-congealed or congealed and screened and processed.
- Delayed release formulations can be created by coating a solid dosage form with a polymer film, which is insoluble in the acidic environment of the stomach, and soluble in the neutral environment of the small intestine.
- the delayed release dosage units can be prepared, for example, by coating a drug or a drug-containing composition with a selected coating material.
- the drug-containing composition may be, e.g., a tablet for incorporation into a capsule, a tablet for use as an inner core in a "coated core” dosage form, or a plurality of drug-containing beads, particles or granules, for incorporation into either a tablet or capsule.
- Preferred coating materials include bioerodible, gradually hydrolyzable, gradually water-soluble, and/or enzymatically degradable polymers, and may be conventional "enteric" polymers.
- Enteric polymers become soluble in the higher pH environment of the lower gastrointestinal tract or slowly erode as the dosage form passes through the gastrointestinal tract, while enzymatically degradable polymers are degraded by bacterial enzymes present in the lower gastrointestinal tract, particularly in the colon.
- Suitable coating materials for effecting delayed release include, but are not limited to, cellulosic polymers such as hydroxypropyl cellulose, hydroxyethyl cellulose, hydroxymethyl cellulose, hydroxypropyl methyl cellulose, hydroxypropyl methyl cellulose acetate succinate, hydroxypropylmethyl cellulose phthalate, methylcellulose, ethyl cellulose, cellulose acetate, cellulose acetate phthalate, cellulose acetate trimellitate and carboxymethylcellulose sodium; acrylic acid polymers and copolymers, preferably formed from acrylic acid, methacrylic acid, methyl acrylate, ethyl acrylate, methyl methacrylate and/or ethyl methacrylate, and other methacrylic resins that are commercially available under the tradename (Rohm Pharma; Westerstadt, Germany) , including L30D-55 and L100-55 (soluble at pH 5.5 and above) , L-100 (soluble at pH 6.0 and above) , S
- the preferred coating weights for particular coating materials may be readily determined by those skilled in the art by evaluating individual release profiles for tablets, beads and granules prepared with different quantities of various coating materials. It is the combination of materials, method and form of application that produce the desired release characteristics, which one can determine only from the clinical studies.
- the coating composition may include conventional additives, such as plasticizers, pigments, colorants, stabilizing agents, glidants, etc.
- a plasticizer is normally present to reduce the fragility of the coating, and will generally represent about 10 wt. %to 50 wt. %relative to the dry weight of the polymer.
- typical plasticizers include polyethylene glycol, propylene glycol, triacetin, dimethyl phthalate, diethyl phthalate, dibutyl phthalate, dibutyl sebacate, triethyl citrate, tributyl citrate, triethyl acetyl citrate, castor oil and acetylated monoglycerides.
- a stabilizing agent is preferably used to stabilize particles in the dispersion.
- Typical stabilizing agents are nonionic emulsifiers such as sorbitan esters, polysorbates and polyvinylpyrrolidone. Glidants are recommended to reduce sticking effects during film formation and drying, and will generally represent approximately 25 wt. %to 100 wt. %of the polymer weight in the coating solution.
- One effective glidant is talc.
- Other glidants such as magnesium stearate and glycerol monostearates may also be used.
- Pigments such as titanium dioxide may also be used.
- Small quantities of an anti-foaming agent such as a silicone (e.g., simethicone) , may also be added to the coating composition.
- Suitable dosage forms for topical administration include creams, ointments, salves, sprays, gels, lotions, emulsions, and transdermal patches.
- the formulation may be formulated for transmucosal, transepithelial, transendothelial, or transdermal administration.
- the compounds can also be formulated for intranasal delivery, pulmonary delivery, or inhalation.
- the compositions may further contain one or more chemical penetration enhancers, membrane permeability agents, membrane transport agents, emollients, surfactants, stabilizers, buffers, and combination thereof.
- repeated application can be done or a patch can be used to provide continuous administration of the compounds over an extended period of time
- Buffers are used to control pH of a composition.
- the buffers buffer the composition from a pH of about 4 to a pH of about 7.5, more preferably from a pH of about 4 to a pH of about 7, and most preferably from a pH of about 5 to a pH of about 7.
- the buffer is triethanolamine.
- “Emollients” are an externally applied agent that softens or soothes skin and are generally known in the art and listed in compendia, such as the “Handbook of Pharmaceutical Excipients” , 4 th Ed., Pharmaceutical Press, 2003. These include, without limitation, almond oil, castor oil, ceratonia extract, cetostearoyl alcohol, cetyl alcohol, cetyl esters wax, cholesterol, cottonseed oil, cyclomethicone, ethylene glycol palmitostearate, glycerin, glycerin monostearate, glyceryl monooleate, isopropyl myristate, isopropyl palmitate, lanolin, lecithin, light mineral oil, medium-chain triglycerides, mineral oil and lanolin alcohols, petrolatum, petrolatum and lanolin alcohols, soybean oil, starch, stearyl alcohol, sunflower oil, xylitol and combinations thereof. In one embodiment, the emollients are e
- Emmulsifiers are surface active substances which promote the suspension of one liquid in another and promote the formation of a stable mixture, or emulsion, of oil and water. Common emulsifiers are: metallic soaps, certain animal and vegetable oils, and various polar compounds.
- Suitable emulsifiers include acacia, anionic emulsifying wax, calcium stearate, carbomers, cetostearyl alcohol, cetyl alcohol, cholesterol, diethanolamine, ethylene glycol palmitostearate, glycerin monostearate, glyceryl monooleate, hydroxpropyl cellulose, hypromellose, lanolin, hydrous, lanolin alcohols, lecithin, medium-chain triglycerides, methylcellulose, mineral oil and lanolin alcohols, monobasic sodium phosphate, monoethanolamine, nonionic emulsifying wax, oleic acid, poloxamer, poloxamers, polyoxyethylene alkyl ethers, polyoxyethylene castor oil derivatives, polyoxyethylene sorbitan fatty acid esters, polyoxyethylene stearates, propylene glycol alginate, self-emulsifying glyceryl monostearate, sodium citrate dehydrate, sodium lauryl sulf
- “Penetration enhancers” include, but are not limited to, fatty alcohols, fatty acid esters, fatty acids, fatty alcohol ethers, amino acids, phospholipids, lecithins, cholate salts, enzymes, amines and amides, complexing agents (liposomes, cyclodextrins, modified celluloses, and diimides) , macrocyclics, such as macrocylic lactones, ketones, and anhydrides and cyclic ureas, surfactants, N-methyl pyrrolidones and derivatives thereof, DMSO and related compounds, ionic compounds, azone and related compounds, and solvents, such as alcohols, ketones, amides, polyols (e.g., glycols) . Examples of these classes are known in the art.
- Preservatives can be used to prevent the growth of fungi and microorganisms.
- Suitable antifungal and antimicrobial agents include, but are not limited to, benzoic acid, butylparaben, ethyl paraben, methyl paraben, propylparaben, sodium benzoate, sodium propionate, benzalkonium chloride, benzethonium chloride, benzyl alcohol, cetylpyridinium chloride, chlorobutanol, phenol, phenylethyl alcohol, and thimerosal.
- “Surfactants” are surface-active agents that lower surface tension and thereby increase the emulsifying, foaming, dispersing, spreading and wetting properties of a product.
- Suitable non-ionic surfactants include emulsifying wax, glyceryl monooleate, polyoxyethylene alkyl ethers, polyoxyethylene castor oil derivatives, polysorbate, sorbitan esters, benzyl alcohol, benzyl benzoate, cyclodextrins, glycerin monostearate, poloxamer, povidone and combinations thereof.
- the non-ionic surfactant is stearyl alcohol.
- An emulsion is a preparation of one liquid distributed in small globules throughout the body of a second liquid.
- the non-miscible components of the emulsion include a lipophilic component and an aqueous component.
- the dispersed liquid is the discontinuous phase, and the dispersion medium is the continuous phase.
- oil is the dispersed liquid and an aqueous solution is the continuous phase, it is known as an oil-in-water emulsion
- water or aqueous solution is the dispersed phase and oil or oleaginous substance is the continuous phase
- water-in-oil emulsion water-in-oil emulsion.
- Either or both of the oil phase and the aqueous phase may contain one or more surfactants, emulsifiers, emulsion stabilizers, buffers, and other excipients.
- Preferred excipients include surfactants, especially non-ionic surfactants; emulsifying agents, especially emulsifying waxes; and liquid non-volatile non-aqueous materials, particularly glycols such as propylene glycol.
- the oil phase may contain other oily pharmaceutically approved excipients. For example, materials such as hydroxylated castor oil or sesame oil may be used in the oil phase as surfactants or emulsifiers.
- the oil phase may consist at least in part of a propellant, such as an HFA propellant.
- a propellant such as an HFA propellant.
- Either or both of the oil phase and the aqueous phase may contain one or more surfactants, emulsifiers, emulsion stabilizers, buffers, and other excipients.
- Preferred excipients include surfactants, especially non-ionic surfactants; emulsifying agents, especially emulsifying waxes; and liquid non-volatile non-aqueous materials, particularly glycols such as propylene glycol.
- the oil phase may contain other oily pharmaceutically approved excipients. For example, materials such as hydroxylated castor oil or sesame oil may be used in the oil phase as surfactants or emulsifiers.
- a sub-set of emulsions are the self-emulsifying systems.
- These drug delivery systems are typically capsules (hard shell or soft shell) comprised of the drug dispersed or dissolved in a mixture of surfactant (s) and lipophilic liquids such as oils or other water immiscible liquids.
- s surfactant
- lipophilic liquids such as oils or other water immiscible liquids.
- a lotion can contain finely powdered substances that are in soluble in the dispersion medium through the use of suspending agents and dispersing agents.
- lotions can have as the dispersed phase liquid substances that are immiscible with the vehicle and are usually dispersed by means of emulsifying agents or other suitable stabilizers.
- the lotion is in the form of an emulsion having a viscosity of between 100 and 1000 centistokes. The fluidity of lotions permits rapid and uniform application over a wide surface area. Lotions are typically intended to dry on the skin leaving a thin coat of their medicinal components on the skin’s surface.
- Creams may contain emulsifying agents and/or other stabilizing agents.
- the formulation is in the form of a cream having a viscosity of greater than 1000 centistokes, typically in the range of 20,000-50,000 centistokes. Creams are often time preferred over ointments, as they are generally easier to spread and easier to remove.
- creams are typically thicker than lotions, may have various uses and often one uses more varied oils/butters, depending upon the desired effect upon the skin.
- the water-base percentage is about 60-75 %and the oil-base is about 20-30 %of the total, with the other percentages being the emulsifier agent, preservatives and additives for a total of 100 %.
- ointment bases examples include hydrocarbon bases (e.g., petrolatum, white petrolatum, yellow ointment, and mineral oil) ; absorption bases (hydrophilic petrolatum, anhydrous lanolin, lanolin, and cold cream) ; water-removable bases (e.g., hydrophilic ointment) , and water-soluble bases (e.g., polyethylene glycol ointments) .
- Pastes typically differ from ointments in that they contain a larger percentage of solids. Pastes are typically more absorptive and less greasy that ointments prepared with the same components.
- Gels are semisolid systems containing dispersions of small or large molecules in a liquid vehicle that is rendered semisolid by the action of a thickening agent or polymeric material dissolved or suspended in the liquid vehicle.
- the liquid may include a lipophilic component, an aqueous component or both.
- Some emulsions may be gels or otherwise include a gel component.
- Some gels, however, are not emulsions because they do not contain a homogenized blend of immiscible components.
- Suitable gelling agents include, but are not limited to, modified celluloses, such as hydroxypropyl cellulose and hydroxyethyl cellulose; Carbopol homopolymers and copolymers; and combinations thereof.
- Suitable solvents in the liquid vehicle include, but are not limited to, diglycol monoethyl ether; alklene glycols, such as propylene glycol; dimethyl isosorbide; alcohols, such as isopropyl alcohol and ethanol.
- the solvents are typically selected for their ability to dissolve the drug.
- Other additives, which improve the skin feel and/or emolliency of the formulation, may also be incorporated. Examples of such additives include, but are not limited, isopropyl myristate, ethyl acetate, C 12 -C 15 alkyl benzoates, mineral oil, squalane, cyclomethicone, capric/caprylic triglycerides, and combinations thereof.
- Foams consist of an emulsion in combination with a gaseous propellant.
- the gaseous propellant consists primarily of hydrofluoroalkanes (HFAs) .
- Suitable propellants include HFAs such as 1, 1, 1, 2-tetrafluoroethane (HFA 134a) and 1, 1, 1, 2, 3, 3, 3-heptafluoropropane (HFA 227) .
- the propellants preferably are not hydrocarbon propellant gases, which can produce flammable or explosive vapors during spraying.
- the compositions preferably contain no volatile alcohols, which can produce flammable or explosive vapors during use.
- compositions can be used to treat senescence-associated disorders and disorders associated with aberrant inflammation, in a subject in need thereof.
- the subject is a mammal, more preferably a human.
- senescence-associated disorders or diseases include disorders or diseases associated with, or caused by cellular senescence, including age-related diseases and disorders.
- a senescence-associated disease or disorder may also be called a senescent cell-associated disease or disorder.
- Senescence is a cellular response characterized by a stable growth arrest and other phenotypic alterations that include a proinflammatory secretome. Senescence plays roles in normal development, maintains tissue homeostasis, and limits tumor progression. However, senescence has also been implicated as a major cause of age-related disease. Senescent cells accumulate during age and are associated with many diseases, including cancer, fibrosis and many age-related pathologies. Recent evidence suggests that senescent cells are detrimental in multiple pathologies and their elimination confers many advantages, ameliorating multiple pathologies and increasing healthspan and lifespan.
- Senescent cells are present in many pre-neoplastic lesions, fibrotic tissues (e.g. in the liver, kidney, heart, pancreas) and old tissues.
- SA- ⁇ -gal positive macrophages can be harmful and have been found to accumulate in injured and aged tissues contributing to chronic inflammation (Hall et al., Aging (Albany NY) 8, 1294-1315 (2016) ; Oishi &Manabe, NPJ Aging Mech Dis 2, 16018 (2016) ) .
- the data in this application shows that SSK1 decreases the number of SA- ⁇ -gal positive macrophages in injured lungs and aged livers, which is consistent with the observation of reduced secretion of chronic inflammation-related cytokines. Therefore, eliminating macrophage accumulation by modulating SSK1 might reduce chronic inflammation and benefit aged organisms.
- activated macrophages have crucial roles in acute inflammation and cytokine storm (Hamidzadeh et al., Annu Rev Physiol 79, 567-592 (2017) ) , especially those induced by virus infection.
- macrophages produce pro-inflammatory factors and trigger initiation of cytokine storms (Hamidzadeh et al., Annu Rev Physiol 79, 567-592 (2017) ) .
- the depletion of macrophages could protect mice from coronavirus-induced lethal infection (Channappanavar et al., Cell Host Microbe 19, 181-193 (2016) ) .
- activated macrophages can be targeted using the compounds and formulatons disclosed herein, to treating acute inflammation and cytokine storms via SSK1.
- the compositions are administered in an effective reduce the amount of macrophages in the subject, particularly, SA- ⁇ -gal positive macrophages.
- compositions can be used to ameliorate the symptoms associated with abberrant cytokine production, as a result of virus-induced inflammation.
- viral infections during which a subject can benefit from administration of effective amounts of the formulations disclosed herein include coronaviruses, for example, coronaviruses, for example, Severe Acute Respiratory Syndrome (SARS) coronaviruses (CoV) , SARS-CoV-2 (which causes COVID-19 (Coronavirus Disease 2019) ) , and a Middle East respiratory syndrome (MERS) -CoV.
- SARS-CoV-2 virus is a betacoronavirus, like MERS-CoV and SARS-CoV.
- compositions are administered in an effective amount to reduce the levels of one or more macrophages in the subject.
- the disclosed compositions are administered to a subject infected with a coronavirus, preferably, SARS-CoV or SARS Coronavirus 2 (Sars-CoV-2) .
- Senescent cells can contribute to tumor progression by enhancing the proliferative potential of cancer cells or contributing to epithelial to mesenchymal transition. Therefore, the increased numbers of senescent cells present in aged tissues could contribute to the increased incidence of cancer with age. Supporting this, a delayed onset in tumor formation is observed when senescent cells are eliminated. Senolytic therapy also reduces the incidence of metastasis, the leading cause of cancer-related deaths. (Reviewed in McHugh, et al., J. Cell Biol., 217 (1) : 65-77 (2016) .
- compositions disclosed herein can be used in combination with cancer therapeutics.
- the subject being treated has been diagnosed with cancer.
- cancers which may be treated according to this aspect of the invention include: adenocarcinoma, adrenal gland tumor, ameloblastoma, anaplastic, anaplastic carcinoma of the thyroid, angiofibroma, angioma, angiosarcoma, apudoma, argentaffmoma, arrhenob!
- astoma ascites tumor cell, ascitic tumor, astroblastoma, astrocytoma, ataxia-telangiectasia, atrial myxoma, a basal cell carcinoma cell, bone cancer, brainstem glioma, brain tumor, breast cancer, Burkitt's lymphoma, cerebellar astrocytoma, cervical cancer, cherry angioma, cholangiocarcinoma, cholangioma, chondroblastoma, chondroma, chondrosarcoma, chorioblastoma, choriocarcinoma, colon cancer, common acute lymphoblastic leukemia, craniopharyngioma, cystocarcinoma, cystofbroma, cystoma, ductal carcinoma in situ, ductal papilloma, dysgerminoma, encephaloma, endometrial carcinoma, endothelioma, ependymom
- the disclosed senolytic prodrugs are used in conjunction with other treatments for cancer that induce senescence, such as irradiation or chemotherapy (for example, treatment with Palbociclib, ribociclib or abemaciclib, or other chemotherapeutic agents) .
- the agent can: (a) eliminate cancer cells that have been pushed to senescence; and /or eliminate or reduce certain side effects produced by senescent cells such as inflammation, promotion of cancer growth, promotion of metastasis and other side effects of chemotherapy or radiotherapy; and/or (b) reduce or eliminate precancerous lesions; and/or (c) eliminate or reduce cells that Undergo senescence by treatment with CDK4/6 inhibitors.
- the disclosed agents can reduce or eliminate precancerous (or preneoplastic) lesions. Senescent cells exist in premalignant tumors. In this regard, it is understood that a substantial number of cells in premalignant tumors undergo oncogene-induced senescence. Thus, the disclosed agents can be used to reduce or eliminate precancerous (or pre-neoplastic) lesions.
- the disclosed senolytic prodrugs are administered to eliminate or reduce chemotherapy-induced senescence, for example, before, during or after treatment with a chemotherapeutic agent.
- the agent can be used in combination treatment with a chemotherapeutic agent, where the agent is administered separately, sequentially or concomitantly with the chemotherapeutic agent.
- chemotherapeutic agents include, but are not limited to, anthracyclines, doxorubicin, etoposide, daunorubicin, taxols, paclitaxel, gemcitabine, pomaiidomide, and lenaiidomide.
- the disclosed senolytic prodrugs can eliminate or reduce senescence induced by treatment with a CDK inhibitor, for example, a CDK4 or CDK6 inhibitor.
- a CDK inhibitor for example, a CDK4 or CDK6 inhibitor.
- the disclosed senolytic prodrugs can be used in combination treatment with a CDK4 or CDK6 inhibitor, where the agent is administered separately, sequentially or concomitantly with the CDK4 or CDK6 inhibitor.
- the disclosed senolytic prodrugs can eliminate or reduce Palbociclib-induced senescence. In the context of eliminating or reducing Palbociclib-induced senescence, administration of the agent can potentially prevent cancer remission as cells reenter the cell cycle.
- the agent can eliminate cancer cells that have been pushed to senescence.
- the agent delays tumorigenesis.
- the disclosed senolytic prodrugs can reduce certain side effects produced by senescent cells such as inflammation, promotion of cancer growth, promotion of metastasis and other side effects of chemotherapy or radiotherapy.
- Chemotherapy-induced side effects or radiotherapy-induced side effects include, but art not limited to, weight loss, endocrine changes, hormone imbalance, changes in hormone signaling, changes is cardiotoxicity, body composition, reduced ability to be physically active, gastrointestinal toxicity, nausea, vomiting, constipation, anorexia, diarrhea, peripheral neuropathy, fatigue, malaise, low physical activity, hematological toxicity, anemia, hepatotoxicity, alopecia, pain, infection, mucositis, fluid retention, dermatological toxicity, rashes, dermatitis, hyperpigmentation, urticaria, photosensitivity, nail changes, mouth, gum or throat problems, and any toxic side effect caused by a chemotherapy or radiotherapy.
- the agent can be used in combination treatment with a chemotherapeutic agent, where the agent is administered separately, sequentially or concomitantly with the chemotherapeutic agent.
- the agent can be used in combination treatment with radiotherapy, where the agent is administered before, during or after radiotherapy.
- Another embodiment of the invention relates to an agent as described herein for use in reducing or alleviating one or more chemotherapy-induced or radiotherapy-induced side effects.
- methods for treating or reducing the likelihood of metastasis comprising administering an agent described herein during an off-chemotherapy or off-radiotherapy time interval or after the chemotherapy or radiotherapy treatment regimen has been completed, are provided.
- Another embodiment of the invention relates to an agent as described herein for use in treating or reducing the likelihood of metastasis.
- the disclosed senolytic prodrugs can be used in the treatment of chronic or long-term chemotherapy-induced or radiotherapy-induced side effects.
- Certain toxic effects can appear long after treatment and can result from damage to an organ or system by the therapy.
- Organ dysfunction for example, neurological, pulmonary, cardiovascular, and endocrine dysfunction, can be observed in subjects who were treated for cancers during childhood.
- Chronic or late toxic side effects that occur in subjects who received chemotherapy or radiation therapy include, for example, cardiomyopathy, congestive heart disease, inflammation, early menopause, osteoporosis, infertility, impaired cognitive function, peripheral neuropathy, secondary cancers, cataracts and other vision problems, hearing loss, chronic fatigue, reduced lung capacity, and lung disease.
- the subject has not been diagnosed with cancer and is not being treated for cancer.
- the disclosed compositions are administered to decrease the senescent cell population in a subject in need thereof.
- Senescent cells are associated with a long list of other pathologies, including neurological (e.g. brain aneurysm, Alzheimer's and Parkinson) , pulmonary (e.g. idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease and cystic fibrosis) , ophthalmological (e.g. cataracts, glaucoma, macular degeneration) , musculoskeletal (e.g. sarcopenia, disc degeneration, osteoarthritis) , cardiovascular (e.g. atherosclerosis, cardiac fibrosis, aorta aneurysm) , renal (e.g. chronic kidney disease, transplant complications) , osteoarthritis of the knee and others such as diabetes, mucositis, hypertension, liver fibrosis and osteomyelofibrosis (OMF) .
- neurological e.g. brain aneurysm, Alzheimer's and Parkinson
- pulmonary e.g. idiopathic pulmonary
- a prominent feature of aging is a gradual loss of function, or degeneration that occurs at the molecular, cellular, tissue, and organismal levels.
- Age-related degeneration gives rise to well-recognized pathologies such as sarcopenia, atherosclerosis and heart failure, osteoporosis, pulmonary insufficiency, renal failure, neurodegeneration (including macular degeneration, Alzheimer's disease, and Parkinson's disease) , and many others.
- Senescence-associated diseases and disorders include, but are not limited to, cardiovascular diseases and disorders, inflammatory diseases and disorders, autoimmune diseases and disorders, pulmonary diseases and disorders, eye diseases and disorders, metabolic diseases and disorders, neurological diseases and disorders (e.g., neurodegenerative diseases and disorders) ; age-related diseases and disorders induced by senescence; skin conditions; age-related diseases; dermatological diseases and disorders; and transplant related diseases and disorders.
- the agent can be used in the treatment of a disease or disorder which correlates, or is associated with, elevated ⁇ -galactosidase activity.
- the elevated ⁇ -galactosidase activity is a result of elevated expression of ⁇ -galactosidase or overexpression of ⁇ -galactosidase activity relative to baseline levels which can be determined by standard methods.
- Expression of ⁇ -galactosidase can be detected in cells by histochemical or immunohistochemcal methods.
- SA-beta galactosidase SA-beta galactosidase (SA ⁇ -Gal) can be detected by known methods (Dimri et al, Proc. Natl. Acad. Sci. USA 92: 9363-9367 (1995) ) .
- the disease is selected from the Wiedemann-Rautenstrauch syndrome of neonatal progeria, the Werner syndrome of adult progeria, Hutchinson-Gilford syndrome, Rothmund Thompson syndrome, Mulviii-Smith syndrome, Cockayne syndrome, Dyskeratosis Congenita, idiopathic pulmonary fibrosis, aplastic anaemia, emphysema, type 2 diabetes, and degeneration of cartilage.
- Hutchinson-Gilford Progeria Syndrome ( “Progeria” , or “HGPS” ) a rare, fatal genetic condition characterized by an appearance of accelerated aging in children. Although they are born looking healthy, children with Progeria begin to display many characteristics of accelerated aging within the first two years of life. Progeria signs include growth failure, loss of body fat and hair, aged-looking skin, stiffness of joints, hip dislocation, generalized atherosclerosis, cardiovascular (heart) disease and stroke. Other progeroid syndromes include Werner’s syndrome, also known as “adult progeria” which does not have an onset until the late teen years. There is no cure for progeria, but occupational and physical therapy can help the child keep moving if their joints are stiff.
- compositions and methods can ameliorate the accelerated ageing symptoms associated with Progeria Syndrome.
- the examples below demonstrate that show that depletion of L1 RNA in cells obtained from HGPS mouse model (LAKI) using antisense oligonucleotides (AON) restored the levels of epigenetic marks and reduced the expression of senescent-associated genes, and increased life span.
- the senescence-associated disease or disorder is a cardiovascular disease.
- cardiovascular disease examples include, but are not limited to, atherosclerosis, angina, arrhythmia, cardiomyopathy, congestive heart failure, coronary artery disease, carotid artery disease, endocarditis, coronary thrombosis, myocardial infarction, hypertension, aortic aneurysm, cardiac diastolic dysfunction, hypercholesterolemia, hyperiipidemia, mitral valve prolapsed, peripheral vascular disease, cardiac stress resistance, cardiac fibrosis, brain aneurysm and stroke.
- the senescence-associated disease or disorder is associated with or caused by atherosclerosis (i.e. hardening of the arteries) .
- Atherosclerosis is characterized by patchy intimal plaques (atheromas) that encroach on the lumen of medium-sized and large arteries.
- Administration of an agent according to the invention can reduce the lipid content of an atherosclerotic plaque in a blood vessel of the subject and/or increase the fibrous cap thickness.
- the senescence-associated disease or disorder is an inflammatory or autoimmune disease or disorder.
- autoimmune diseases include osteoporosis, osteoarthritis, psoriasis, oral mucositis, rheumatoid arthritis, inflammatory bowel disease, eczema, kyphosis (curvature of the spinal column) , herniated intervertebral disc, and the pulmonary diseases, COPD and idiopathic pulmonary fibrosis.
- the senescence-associated disease or disorder is chronic inflammation.
- the agents of the invention can reduce or inhibit loss or erosion of proteoglycan layers in a joint, reduce inflammation in the affected joint, and promote production of collagen. Removal of senescent cells causes a reduction in the amount of inflammatory cytokines, such as IL-6, produced in a joint and inflammation is reduced.
- the senescence-associated disease or disorder can in some embodiments be rheumatoid arthritis.
- Rheumatoid arthritis is a chronic inflammatory disorder that typically affects the joints in hands and feet.
- the senescence-associated disease or disorder is osteoporosis.
- Osteoporosis is a progressive bone disease that is characterized by a decrease in bone mass and density that may lead to an increased risk of fracture. Bone mineral density (BMD) is reduced, bone microarchitecture deteriorates, and the amount and variety of proteins in bone are altered.
- the disclosed agents are used in treating herniated intervertebral discs. Subjects with herniated discs exhibit elevated presence of cell senescence in the blood and in vessel walls (Roberts et al. (2006) Eur. Spine J. 15 Suppl 3: S312-316) .
- the senescence-associated disease or disorder is a neurological disease or disorder selected from Alzheimer's disease (and other dementias) , Parkinson's disease, Huntington's disease, dementia, mild cognitive impairment (MCI) , macular degeneration and motor neuron dysfunction (MND) , and diseases and disorders of the eyes, such as age-related macular degeneration.
- a neurological disease or disorder selected from Alzheimer's disease (and other dementias) , Parkinson's disease, Huntington's disease, dementia, mild cognitive impairment (MCI) , macular degeneration and motor neuron dysfunction (MND) , and diseases and disorders of the eyes, such as age-related macular degeneration.
- Parkinson's disease is a disabling condition of the brain characterized by slowness of movement (bradykinesia) , shaking, stiffness, postural instability and loss of balance. Many of these symptoms are due to the loss of certain nerves in the brain, which results in a lack of dopamine. Senescence of dopamine-producing neurons is thought to contribute to the observed ceil death in PD through the production of reactive oxygen species.
- AD Alzheimer's disease
- Age is the single greatest predisposing risk factor for developing AD, which is the leading cause of dementia in the elderly (Hebert, et al., Arch. Neurol. 60: 1 19-1122 (2003) ) .
- Early clinical symptoms show remarkable similarity to mild cognitive impairment.
- Mild Cognitive Impairment is a brain-function syndrome involving the onset and evolution of cognitive impairments beyond those expected based on age and education of the individual, but which are not significant enough to interfere with the individual's daily activities.
- MCI is an aspect of cognitive aging that is considered to be a transitional state between normal aging and the dementia into which it may convert (Pepeu, Dialogues in Clinical Neuroscience, 6: 369-377, 2004) .
- MCI that primarily affects memory is known as “amnestic MCI” , which is frequently seen as prodromal stage of Alzheimer's disease.
- MCI that affects thinking skills other than memory is known as "non-amnestic MCI. "
- MND is a group of progressive neurological disorders that destroy motor neurons, the cells that control voluntary muscle activities such as speaking, walking, breathing and swallowing.
- MNDs include Amyotrophic Lateral Sclerosis (ALS) , also known as Lou Gehrig's Disease, progressive bulbar palsy, pseudobulbar palsy, primary lateral sclerosis, progressive muscular atrophy, lower motor neuron disease, and spinal muscular atrophy (SMA) (e.g., SMA1 (also known as Werdnig-Hoffmann Disease) , Kugelberg-Welander syndrome, and Kennedy's disease, post-polio syndrome, and hereditary spastic paraplegia.
- ALS Amyotrophic Lateral Sclerosis
- SMA spinal muscular atrophy
- the senescence-associated disease or disorder is an ocular disease, disorder, or condition.
- ocular disease disorders, or condition. Examples include, but are not limited to, presbyopia, macular degeneration, cataracts and glaucoma.
- Macular degeneration is a neurodegenerative disease that causes the loss of photoreceptor cells in the central part of retina, called the macula.
- the senescence-associated disease or disorder is a metabolic disease selected from diabetes, diabetic ulcer, metabolic syndrome, and obesity.
- Senescent cells are understood to play a role in metabolic diseases, such as obesity and type 2 diabetes.
- metabolic diseases such as obesity and type 2 diabetes.
- Induction of senescent cells in obesity has potential clinical implications because pro-inflammatory SASP components are also believed to contribute to type 2 diabetes.
- a similar pattern of up-regulation of senescence markers and SASP components are associated with diabetes, both in mice and in humans. Accordingly, the agents described herein have potential applications in treating or preventing type 2 diabetes, obesity and metabolic syndrome.
- the senescence-associated disease or disorder is a pulmonary disease.
- pulmonary disease examples include, but are not limited to, pulmonary fibrosis, chronic obstructive pulmonary disease (COPD) , asthma, cystic fibrosis, emphysema, bronchiectasis, and age-related loss of pulmonary function.
- COPD chronic obstructive pulmonary disease
- COPD chronic and progressive lung disease characterized by stiffening and scarring of the lung, which may lead to respiratory failure, lung cancer, and heart failure.
- the agents of the invention can also be used for treating a subject who is aging and has loss (or degeneration) of pulmonary function (i.e., declining or impaired pulmonary function compared with a younger subject) and/or degeneration of pulmonary tissue.
- the senescence-associated disease or disorder being treated is an age-related disorder selected from renal disease, renal failure, frailty, hearing loss, muscle fatigue, skin conditions, skin wound healing, Liver fibrosis, pancreatic fibrosis, oral submucosa fibrosis, and sarcopenia.
- the senescence-associated disease or disorder is a dermatological disease or disorder
- dermatological disease or disorder examples include, but are not limited to, eczema, psoriasis, hyperpigmentation, nevi, rashes, atopic dermatitis (a form of eczema and associated with inflammation) , urticaria, diseases and disorders related to photosensitivity or photoaging, rhytides (wrinkles due to aging) ; pruritis (linked to diabetes and aging) ; dysesthesia (chemotherapy side effect linked to diabetes and multiple sclerosis) ; eczematous eruptions (often observed in aging patients and linked to side effects of certain drugs) ; eosinophilic dermatosis (linked to certain kinds of hemotologic cancers) ; reactive neutrophilic dermatosis (associated with underlying diseases such as inflammatory bowel syndrome) ; pemphigus; pemphigoid; immunobullous dermatosis (autoimmune
- the agent described herein can be used to extend the lifespan of a subject by selectively killing senescent cells over non-senescent cells. In some embodiments, extending the lifespan of the subject comprises delaying onset or progression of an age-related disease or condition.
- the age-related disease or condition is selected from atherosclerosis, cardiovascular disease, cancer, arthritis, dementia, cataract, osteoporosis, diabetes, hypertension, age-related fat loss, lipodystrophy, and kidney disease.
- the age-related disease or condition is geriatric anxiety disorder.
- the age-related disease or condition is age-related inactivity.
- the age-related disease or condition is reduction of spontaneous activity.
- the age-related disease or condition is reduction of exploratory behavior.
- Mass spectrometric data were obtained using a Brüker Apex IV FTMS using ESI (electrospray ionization) .
- Mouse embryonic fibroblasts were isolated from E13.5 embryos as described previously (Zhao et al., Cell Stem Cell 23, 31-45 e37 (2008) ) .
- Newborn mouse skin fibroblasts were isolated from the skin of day 1-3 newborn mice.
- Adult mouse lung fibroblasts were isolated from 2-month-old and 23-month-old mice. Briefly, mouse embryonic tissues, skin and lung were obtained from described donor mice. Then, these tissues were minced with forceps and incubated in 2 mg/ml collagenase IV (Gibco) for 2-4 hours at 37 °C.
- mice After enzyme treatment, cells were collected by centrifugation and resuspended in high-glucose DMEM (Gibco) supplemented with 10%fetal bovine serum (FBS) and 1%penicillin-streptomycin (Gibco) . The three types of mouse primary cells were all cultured in a humidified incubator at 37°C and 5%CO 2 .
- HEFs Human embryonic skin fibroblasts
- human embryonic skin tissues obtained from aborted tissue with informed patient consent, were minced with forceps and incubated in 1 mg/ml collagenase IV for 1-2 hours at 37°C. After enzyme treatment, cells were collected by centrifugation and resuspended in HEF medium (Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10%fetal bovine serum (FBS, Ausbian) , 1%GlutaMAX (Gibco) , 1%Non-Essential Amino Acids (NEAA, Gibco) and 1%penicillin/streptomycin (Gibco) . Cells were plated on 10 cm tissue culture dishes and grown in 10%FBS DMEM.
- low-passage proliferative NBFs ⁇ 3 passages
- MEFs ⁇ 3 passages
- mouse lung fibroblasts ⁇ 3 passages
- HEFs ⁇ 10 passages
- HUVECs ⁇ 6 passages
- NBFs were used as senescent cells after approximately 8-9 passages or 12 population doublings.
- HEFs were used after approximately 35 passages or 50 population doublings.
- HUVECs were senescent after approximately 18 passages or 24 population doublings.
- MEFs and HEFs were exposed to 10 Gy of ionizing irradiation in an RS 2000 X-ray Biological Irradiator (Rad Source Technologies, Inc. ) at a dose rate of 1.205 Gy/min. The day after irradiation, MEFs or HEFs were passaged at a 1: 3 dilution and cultured for another three days. Then these cells were plated to carry out further experiments.
- MEFs were treated with 2 ⁇ M etoposide for 20 hours, and HEFs were treated with 10 ⁇ M etoposide for 12-18 hours.
- H 2 O 2 peroxide hydrogen
- shRNAs short hairpin RNAs
- Lentivirus production, collection, and infection were as described (Zhao et al., Cell Stem Cell 3, 475-479 (2008) ) .
- the metabolism analysis was performed as previous reported (Karampelas et al., Mol Pharmaceut 14, 674-685 (2017) ) . Briefly, the senescent and non-senescent cells were incubated with 0.5 ⁇ M SSK1 in DMEM medium containing 10%FBS and 1%penicillin-streptomycin for the indicated time. Then the cells slightly washed with water for twice and then harvested. Cold methanol used to extract the compounds and samples were prepared after centrifugation. The concentration of gemcitabine was determined by LC-MS/MS analysis.
- Fibroblasts were digested into single cell suspensions by incubation with 0.25%trypsin-EDTA (Invitrogen) at 37°C for 3-5 min or with accutase at 37°C for 10 min. Cells were then stained with FITC annexin V and propidium iodide (PI) according to the manufacturer’s protocol (FITC Annexin V Apoptosis Detection Kit I, BD Pharmingen TM ) . For chondrocytes Annexin V/7-AAD analysis, the trypsinized live chondrocytes and floating cells were collected, centrifuged and washed with PBS.
- trypsinized live chondrocytes and floating cells were collected, centrifuged and washed with PBS.
- Senescent NBF cells were plated and incubated overnight at 37°C. The cells were treated with 0.05 ⁇ M gemcitabine or 0.5 ⁇ M SSK1 prodrug for the indicated time. Before harvest, cells were washed twice with pre-cooled PBS buffer.
- Total protein was extracted with lysis buffer (50 mM Tris-HCl (pH 7.5) , 137 mM sodium chloride, 1 mM EDTA, 1%Nonidet P-40, 10%glycerol, 0.1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 20 mM ⁇ -glycerophosphate, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride) , and the protein concentrations were normalized using the BCA Protein Assay Kit (Pierce) . Protein samples were mixed with protein loading buffer and incubated at 95°C for 5 min.
- lysis buffer 50 mM Tris-HCl (pH 7.5) , 137 mM sodium chloride, 1 mM EDTA, 1%Nonidet P-40, 10%glycerol, 0.1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 20 mM ⁇ -glycero
- Western blotting was performed by using the following antibodies: phospho-specific p38 MAPK (Thr180/Tyr182) (Cell Signaling Technology, CST, catalog number 4511) and p38 (CST, catalog number 8690) ; phospho-histone H2A.
- X Ser139
- CST catalog number 9718
- histone H2A X
- CST catalog number 7631
- the ⁇ -tubulin protein level was also determined as the loading control by using the ⁇ -tubulin antibody (CST, catalog number 2128) . All the antibodies used in western blotting are listed in Table 2.
- mice were obtained from Beijing Vital River Laboratory Animal Technology Co, Ltd. and maintained under specific pathogen-free facility (SPF) conditions with a 12 light/12 dark cycle and free access to food and water. Old male mice were caged individually, and female mice and young male littermates were maintained with no more than five mice per cage.
- SPF pathogen-free facility
- All drugs were mixed in 90%PBS, 5%Tween-80 (P1754, Sigma) , and 5%polyethylene glycol (PEG) (81172, Sigma) and administered to mice by intraperitoneal (i.p. ) injection.
- SSK1 0.5 mg/kg or vehicle were given consecutive two days every week for five weeks.
- SSK1 0.5 mg/kg
- gemcitabine 0.5 mg/kg or vehicle were administrated for continued 3 days every two weeks.
- Gemcitabine was purchased from MCE.
- Bleomycin was purchased from Selleck. To induce lung senescence and fibrosis, young male and female mice (3-month-old) were subjected to transtracheal injection of bleomycin (1 mg/kg) as previously reported (Bivas-Benita et al., Eur J Pharm Biopharm 61, 214-218 (2005) )
- ⁇ -galactosidase staining of frozen sections the frozen sections were dried at 37°C for 20-30 min and then fixed in ⁇ -galactosidase staining fix solution for 15 min at room temperature. The frozen sections were washed three times with PBS and incubated with ⁇ -galactosidase staining solution overnight at 37°C. After completion of ⁇ -galactosidase staining, the sections were stained with eosin for 1-2 min, rinsed under running water for 1 min, differentiated in 1%acid alcohol for 10-20 sec, and washed again under running water for 1 min. Sections were dehydrated in increasing concentrations of alcohols and cleared in xylene. Excess xylene was drained and a coverslip was placed over the section. After drying, the sample was observed under a microscope.
- Mouse liver tissues were fixed in 4%paraformaldehyde (DingGuo, AR-0211) for 24 h at room temperature and dehydrated with graded sucrose solution (20%and 30%respectively 24 h) before embedded in OCT compound (Sakura) for cryosection.
- the embedded tissues were cut and affixed on slides.
- the sections were fixed in 4%paraformaldehyde (DingGuo, AR-0211) at room temperature for 15 min and blocked with PBS containing 0.25%Triton X-100 (Sigma-Aldrich, T8787) and 2%normal donkey serum (Jackson ImmunoResearch Laboratories, 017-000-121) at room temperature for 1 h.
- RNA was treated with DNase and converted to cDNA using TransScript First -Strand cDNA Synthesis SuperMix (TransGen Biotech, AT311-03) .
- qPCR was performed using Kapa FAST qPCR Kit Master Mix (Kapa Biosystems, KM4101) on a CFX Connect TM Real-Time System or CFX96 TM Real-Time System (Bio-Rad) . Data were analyzed using the 2 ⁇ (- ⁇ Ct) method. Gapdh was used as a control to normalize the expression of target genes.
- the primers for RT-qPCR are listed in Table 3.
- IL1 ⁇ AAGTCTCCAGGGCAGAGAGG (SEQ ID NO: 10) IL1 ⁇ AAAAGCCTCGTGCTGTCG (SEQ ID NO: 12) IL6 GTTCTCTGGGAAATCGTGGA (SEQ ID NO: 14) CXCL1 ACCGAAGTCATAGCCACACTC (SEQ ID NO: 16) TNF ⁇ GCCTCTTCTCATTCCTGCTT (SEQ ID NO: 18) GLB1 GGATGGACAGCCATTCCGAT (SEQ ID NO: 20) GAPDH CTTTGTCAAGCTCATTTCCTGG (SEQ ID NO: 22) Fibronectin CCACCCCCATAAGGCATAGG (SEQ ID NO: 24) Collagen I TGCCGTGACCTCAAGATGTG (SEQ ID NO: 26) Collagen III GCGGAATTCCTGGACCAAAAGGTGATGCTG (SEQ ID NO: 28) Gene Reverse Primer (5’ to 3’) p16 GCGACGTTCCCAGCGGTACACA (SEQ ID NO: 5)
- senescent and non-senescent cells treated with SSK1 cells were stained with a mixture of Hoechst 33342 and propidium iodide (PI) (CA1120, Solarbio) to distinguish living and dead cells. After treatment with the small molecules, the cell culture medium was removed, and the cells were washed once with PBS. Then, the cells were stained according to the manufacturer’s protocol, where the final concentrations of Hoechst 33342 and PI were 5 ⁇ g/ml and 2 ⁇ g/ml in PBS buffer. The plate was incubated at 4°C for 30 min and observed on a fluorescence microscope or automatic cell imaging system. Viable cells were quantified using a MD Image Xpress Micro XL (Molecular Devices) .
- PI propidium iodide
- Cell Counting Kit–8 (CCK-8) analysis was also used to assess cell viability. Senescent and non-senescent cells or chondrocytes were inoculated into 96-well plates and cultured overnight. Then cells were treated with vehicle (0.05%DMSO) or different concentrations of SSK2. After 48h or 72h, CCK-8 solution (C0041, Beyotime) was added to each well as 1: 10 ratio and inoculated for another 2 h. The absorbance was measured at 450 nm wavelength.
- Mouse blood samples were collected, stewed 2 hours at room temperature or overnight at 4°C, and then centrifuged (3000rpm, 10min) to gain serum.
- Secretion of mouse IL1 ⁇ , IL6, and TNF ⁇ was measured using the Mouse Interleukin 1 ⁇ (IL1 ⁇ ) ELISA Kit (CSB-E04621m, CUSABIO) , the Mouse Tumor Necrosis Factor ⁇ (TNF ⁇ ) ELISA Kit (CSB-E04741m, CUSABIO) , and the IL-6 (Interleukin-6) Mouse ELISA Kit (ab100712, Abcam) according to the manufacturers’ instructions.
- IL1 ⁇ Mouse Interleukin 1 ⁇
- TNF ⁇ Mouse Tumor Necrosis Factor ⁇
- IL-6 Interleukin-6 Mouse ELISA Kit
- the rotarod test was used to evaluate motor coordination and balance with an accelerating RotaRod system (SANS Bio Instrument, China, SA102) . Mice were placed in separate lanes on the rod rotating at an initial speed of 4 rpm/min. The apparatus was set to accelerate from 4 to 44 rpm/min in 300 s. A timer was used to record when each mouse fell or clung to the rod and completed a full passive rotation. Mice were trained at least two times on days 1 and 2 and tested on days 3, 4, and 5. Results were the averaged over 3 trials.
- Treadmill exhaustion tests were used to evaluate exercise capacity and endurance.
- a motorized treadmill was used at an incline of 5°with 0.5 mA electrical stimulation (SANS Bio Instrument, China, SA102) .
- Mice were trained for three days, starting at an initial speed at 5 m/min for 2 min and accelerating to 7 m/min for 2 min and then 9 m/min for 1 min. After three training sessions and one day of rest, mice were tested on the fifth day at an initial speed of 5 m/min, which increased by 2 m/min every 2 min until mice were unable to return to the treadmill. The distance (m) traveled before exhaustion was recorded for each mouse.
- mice were placed on the top of a grid (Grip strength meter, Columbus Instruments) so they grasped the grid with all four paws.
- the meter was set to the Peak Tension (T-PK) mode and recorded the grip strength over seven trials.
- the grip strength (N) was averaged, with the maximum and minimum data points excluded.
- mice were placed on a 1-m long, 6-mm wide beam resting 60 cm above the floor. A black box full of nesting material from the home cage was placed at the end of the beam apparatus as the end point. At the first day, mice were trained three times on the first day to walk across the beam to the safe box successfully without hesitation or observation. On the test day, the time (s) to cross the center 80 cm mark was measured by two motion detectors: one at 0 cm that starts a timer and one at 80 cm that stops the timer
- mice were taken from the housing room into the testing room and allowed to acclimate to the new environment for a minimum of 30 min before the test. Mice were carefully placed in a 14-cm high and 11-cm diameter transparent cylinder and recorded for 5 min by video camera. The resulting video was analyzed to measure the rearing frequency (when mice stand only on hind legs, raise forelimbs off the ground, and stand upright for over 1 sec) .
- RNA sequencing libraries were constructed using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB England BioLabs) . Fragmented and randomly primed 2 ⁇ 150 bp paired-end libraries were sequenced using Illumina HiSeq X Ten. RNA sequencing and raw data quality control were performed by Novogene Co., Ltd.
- Gene ontology (GO) term enrichment analyses were performed using DAVID 6.8 functional annotation tool.
- the gene lists were selected by comparing gene expression between old and young mice or SSK1 treated and vehicle treated old mice with t-test statistics: fold changes >2 and P-values ⁇ 0.05.
- xCell was used to perform cell type enrichment analysis from expression data (Aran et al., Genome Biol 18, 220 (2017) ) .
- xCell can decompose tissue expression profile into 64 immune and stroma cell types enrichment score by using gene signatures learned from thousands of pure cell types from various source.
- Each mouse gene symbols were converted human homologous gene symbols according to the “Human and Mouse Homology Classes with Sequence information” table from MGI database (http: //www. informatics. jax. org/downloads/reports/HOM_MouseHumanSequence. rpt) . Converted gene symbols with highest expression levels were selected for duplicate gene symbols. log2 (FPKM+1) values were used as expression levels.
- the homology converted expression matrix was uploaded to xCell online version ( https: //xcell. ucsf. edu/ ) to compute cell type enrichment score.
- ALT Alanine transaminase
- AST aspartate transaminase
- U uric acid
- CREA creatinine
- P values were calculated by t-test (when comparing only two groups) or one-way ANOVA (when comparing more than two groups) in Excel or GraphPad Prism 8 with default parameters. All results are expressed as the mean ⁇ s.e.m., and n indicates the number of the number of mice. P values are as follows: *P ⁇ 0.05; **P ⁇ 0.01; ***P ⁇ 0.001; ****P ⁇ 0.0001.
- a panel of FDA-approved drugs were tested to select a compound with potent cytotoxicity for senescent cells.
- gemcitabine has a 4-amino group which is suitable site for prodrug development (Moysan, et al. Molecular Pharmaceutics 10, 430-444 (2013) ) .
- Gemcitabine was used to synthesize s enescence s pecific k illing compound 1 (SSK1, shown below) by introducing an SA- ⁇ -gal-responsive moiety into its backbone (Ghosh, et al. Tetrahedron Letters 41, 4871-4874 (2000) ) , which included acetyl groups, for improved the compound’s cellular permeability (Sarkar, et al. Proceedings of the National Academy of Sciences of the United States of America 92, 3323-3327 (1995) ) .
- SSK1 activated p38 MAPK by phosphorylation and induced apoptosis in senescent cells (Fig. 2A and 2B) .
- the p38 MAPK inhibitors SB203580, Birb796, and SB202190 impaired SSK1’s ability to specifically kill senescent cells (Fig. 2C) .
- SSK1 specificity of SSK1 for mouse and human senescent cells was further tested.
- senescent mouse fibroblasts induced by ionizing irradiation, oncogene overexpression, or genotoxic stress (etoposide treatment) were treated with SSK1. These senescent cells induced by various stimuli were selectively killed by SSK1, while non-senescent cells were largely unaffected (Fig. 2D) .
- senescent lung cells from 23-month-old mice were treated with SSK1 and the data showed that SSK1 specifically eliminated these senescent cells when compared to non-senescent lung cells from young (3 months) mice (Fig. 2E) .
- SSK1 The effect of SSK1 on senescent cells was tested in vivo.
- Two independent in vivo senescent models were employed: stress-induced premature senescence and naturally occurring senescence in aged mice.
- stress-induced senescence DNA strand breakage and cellular senescence were induced in mouse lungs by intratracheal instillation of bleomycin as a model of idiopathic pulmonary fibrosis (Moeller, et al. International Journal of Biochemistry &Cell Biology 40, 362-382 (2008) ) .
- SSK1 or vehicle was administrated by intraperitoneal injection for five weeks after five days of bleomycin induction (Fig. 2H) .
- SASP contributes to local and systemic low-grade inflammation during aging, age-related degenerative phenotypes, and impairment of physical function (5) .
- SSK1 decreased several inflammatory factors, including IL1 ⁇ and IL1 ⁇ in aged liver and IL6 in aged kidneys (Fig. 3E and F) .
- SSKI was next compared with other reported senolytic compounds in cultured senescent cells and aged mice.
- ABT263 selectively killed hydroperoxide-induced senescent human fibroblasts but not replication-induced cells.
- irradiation-and etoposide-induced senescent cells were more sensitive to the combination of dasatinib and quercetin, while non-senescent and replication-induced senescent cells remained viable.
- SSK1 treatment in aged mice could downregulate the gene signature associated with senescence (Fig. 5A) by gene set enrichment analysis (GSEA) in both livers and kidneys.
- GSEA gene set enrichment analysis
- SSK1 reduced naturally accumulated senescent cells and decreased senescence markers in mice.
- GSEA analysis also showed that SSK1 down regulated genes associated with inflammatory response, TNF ⁇ signaling via NF- ⁇ B, IL6, JAK Stat3 signaling and complement trending toward the level of their young counterparts (Fig. 5C to F) . Similar results were also shown by gene ontology (GO) enrichment analysis (Fig. 6D to G) .
- macrophages are also reported to cause age-associated chronic inflammation. Since the accumulated macrophages are tend to display senescence features such as high level of SA- ⁇ -gal and p16 expression, we also studied the effect of SSK1 on the macrophages. We performed immunofluorescence analysis of F4/80 positive macrophages, and found that these cells accumulated in livers with aging and SSK1 could delete macrophages infiltration (Fig. 6A, and data not shown) , which could partially account for the decreased inflammatory cytokines in aged mice. The decline of macrophage accumulation was further confirmed by our RNA-seq data of livers analyzed by the xCell approach (Fig. 6B) .
- prodrug design approach as demonstrated here to target SA- ⁇ -gal can improve selective elimination senescent cells in a species-, cell type-and senescence stimulus-independent manner.
- SSK1 cleared stress-induced and naturally occurring senescent cells, decreased SASP, and improved global physical function in aged mice.
- prodrug design can improve the use SA- ⁇ -gal as a target for prodrug design and clearance of senescent cells both in vitro and in vivo.
- Effective clearance of SA- ⁇ -gal-positive senescent cells by SSK1 provides a prospective approach to delay aging.
- the demonstrated prodrug design strategy provides a foundation to generate new compounds that directly target senescence for anti-aging therapy.
- Gemcitabine is a nucleoside analogue that potently affects rapidly dividing cells and the off-target effects of Gemcitabine could be shown in different proliferating cell types (Bocci et al., Eur J Pharmacol 498, 9-18 (2004) ; Rettig et al., Int J Cancer 129, 832-838 (2011) ) .
- SSK1 targets only ⁇ -gal positive cells.
- the data above shows that the majority of non-senescent ⁇ -gal positive cells are rapidly dividing epithelial cells.
- the potential types of proliferating cells with SSK1 sensitivity are greatly narrowed relative to Gemcitabine.
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a pandemic of coronavirus disease 2019 (COVID-19) .
- SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2
- the transformations from mild to severe life-threatening situations during SARS-CoV-2 infection can be fast (Huang et al., 2020; Wang et al., 2020) .
- Approximately 15%of patients progressed to severe pneumonia, resulting in respiratory failure and high hospital mortality, especially in elderly patients (Grasselli et al., 2020; Yang et al., 2020) . Accordingly, this situation has led to an urgent demand for effective therapeutic strategies for COVID-19.
- Hyperinflammation has emerged as a critical driver of COVID-19’s disease severity based on clinical immunopathology.
- acute immune responses induced by SARS-CoV-2 infection including intensive immune cell infiltration and elevated proinflammatory cytokine release, are associated with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (Tay et al., 2020; Moore and June, 2020) .
- ARDS acute respiratory distress syndrome
- MERS MERS
- SARS de Wit et al., 2016; Channappanavar and Perlman, 2017
- anti-inflammatory treatment interfering with JAK/STAT3 pathways may dysregulate type I interferon-mediated antiviral innate immunity (Boor et al., 2017) .
- new clinical treatments of COVID-19 are needed that can target the inflammatory characteristics of severe patients without interrupting antiviral activity.
- scRNA-seq single-cell RNA sequencing
- SSK1 a small molecule that reduces ⁇ -galactosidase-positive macrophages, effectively ameliorated pneumonia caused by SARS-CoV-2 infection in nonhuman primates.
- SSK1 efficiently mitigated clinical symptoms and pathologically reduced pneumonia progression at both early and late stages of SARS-CoV-2 infection.
- an anti-inflammatory effect with a reduction in macrophage infiltration in the lungs of SSK1-treated animals, which was accompanied by decreased levels of cytokines in the peripheral blood and lungs.
- Our results demonstrate the therapeutic benefit of SSK1 treatment in nonhuman primates to SARS-CoV-2 infection, leading to the development of effective treatments for COVID-19.
- the final grouping is presented as following: the vehicle-treated group, RM1 (young) , RM2 (adult) and RM3 (old) ; 0.5 mg/kg SSK1-treated group, RM4 (young) , RM5 (adult) and RM6 (old) ; 2.0 mg/kg SSK1-treated group, RM7 (young) , RM8 (adult) and RM9 (old) .
- vehicle (DMSO) low dosage SSK1 (0.5 mg/kg) and high dosage SSK1 (2.0 mg/kg) were administrated intravenously (i.v. ) once a day from 22 to 28 dpi.
- the monkeys were treated immediately with 2.0 mg/kg SSK1 (SSK1-M1, SSK1-M2) or vehicle solution (Vehicle-M3, Vehicle-M4) after SARS-CoV-2 inoculation once daily for 7 consecutive days.
- Clinical examinations were performed daily and we recorded body weight and anal temperature. Blood samples for hematological analysis and swabs drawn from the nose, throat and rectum were collected for quantitative virus detection. Chest X-ray was performed as indicated time point. Animals were euthanized and necropsied at the indicated time points after treatment.
- BALF bronchoalveolar lavage fluid
- Viral stock of SARS-CoV-2 was obtained from the Center of Diseases Control, Guangdongzhou China. Viruses were amplified on Vero-E6 cells and concentrated by ultrafilter system via 300 kDa module (Millipore) . Amplified SARS-CoV-2 were confirmed via RT-PCR, sequencing and transmission electronic microscopy, and titrated via plaque assay (10 6 pfu/ml) .
- Vero-E6 cells were well cultured in high-glucose DMEM (Gibco, Cat#11995500BT) supplemented with 10%fetal bovine serum (FBS, Gibco, Cat#10099-141C) and 1%penicillin-streptomycin (Solarbio, Cat#P1400) in a humidified incubator at 37°C and 5%CO 2 . Trypsin-EDTA (0.05%) phenol red (Gibco, Cat#25200) was used for cell dissociation.
- the monkey Before image taken, the monkey was anaesthetized with 10 mg/kg ketamine hydrochloride (Beikang, Cat#100761663) . Then mobile digital medical X-ray photography system (MobileCooper, Browiner China) was used to take the chest X-ray image of the monkey as the indicated time point. To analyze the X-ray data, radiographs were evaluated by two experienced doctors blinded to the group assignment of the monkeys.
- the blood samples were collected from saphenous vein of hind limb of anaesthetized monkeys using 5 ml blood collection tube containing sodium citrate anti-coagulant for further analysis.
- CBC Complete Blood Count
- ALT alanine transaminase
- AST aspartate transaminase
- ALP alkaline phosphatase
- U uric acid
- CRE-E creatinine
- Half lung from each monkey was used for BALF collection and the other half was used for homogenate and histopathology examination.
- the following tissues were collected: trachea, bronchus, lung, submandibular gland, intestinal lymph node, hilar lymph node, spleen, stomach, duodenum, colon, rectum, liver, pancreas, kidney, heart, muscle, testis, bladder, penis, spinal cord, brain and cerebrospinal fluid.
- One gram of tissue was homogenized in 3 ml PBS. The homogenates were centrifuged at 3,000 rpm for 10 min and the supernatant was collected and used for analysis.
- BALF Broncheoalveolar lavage fluid
- G-CSF granulocyte colony-stimulating factor
- GM-CSF granulocyte-macrophage colony-stimulating factor
- IFN interferon
- IL interleukin
- IL-12/23 p40
- MCP monocyte chemotactic protein
- MIP macrophage inflammatory protein
- sCD40L soluble CD40-ligand
- TGF tumor necrosis factor
- TNF tumor necrosis factor
- VEGF vascular endothelial growth factor
- RNA extraction and quantitative real-time reverse transcription–polymerase chain reaction were performed on nasal/throat/anal swab, blood and necropsied tissue samples collected from infected monkeys.
- the virus RNA was extracted from inactivated samples using Trizol LS Reagent (Invitrogen, Cat#10296010) according to the Direct-zolTM RNA MiniPrep protocol (ZYMO RESEARCH CORP, US) . 50 ⁇ l of DNase/RNase-Free Water was used to elute RNA.
- Real time RT-PCR was used to quantify viral genome in samples using TaqMan Fast Virus 1-Step Master Mix (ThermoFisher, Cat#4444434) and purified viral RNA of SARS-CoV-2 as a standard curve, performed on CFX384 Touch Real-Time PCR Detection System (Bio-Rad, US) .
- Conditions for RT-PCR were used as follows: 25°C for 2 min, 50°C for 15 min, 95°C for 2 min, then 40 cycles at 95°C 5 sec and 58°C 31 sec.
- Primers and probe, specific for NP gene was synthesized according to sequences reported by Chinese Center for Disease Control and Prevention (CDC) .
- Target-2-F GGGGAACTTCTCCTGCTAGAAT (SEQ ID NO: 30) ,
- Target-2-R CAGACATTTTGCTCTCAAGCTG (SEQ ID NO: 31) ,
- Target-2-P 5'-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3' (SEQ ID NO: 32) .
- ABSPAS staining Alcian blue-periodic acid-Schiff (AB-PAS) staining was performed by the AB-PAS staining kit (Servicebio, Cat#G1049) .
- the anti-CD68 primary antibody (abcam, Cat#ab213098) was used.
- the corresponding second antibody used is HRP conjugated Goat Anti-Mouse IgG (H+L) (Servicebio, Cat#GB23301) .
- H+L Goat Anti-Mouse IgG
- the whole slide images were collected using Pannoramic DESK (3D HISTECH) and analyzed with Caseviewer C. V 2.3 and Image Pro plus 6.0.
- P values were calculated by t-test (when comparing only two groups) or one-way ANOVA (when comparing more than two groups) using GraphPad Prism 8. with default parameters. Error bars represent SEM and P ⁇ 0.05 was considered statistically significant (ns, not significant, *P ⁇ 0.05, **P ⁇ 0.01, ***P ⁇ 0.001, ****P ⁇ 0.0001) .
- rhesus macaques were divided into three groups: Vehicle (RM1-RM3) , 0.5 mg/kg SSK1 (RM4-RM6) and 2.0 mg/kg SSK1 (RM7-RM9) , each group contains one young, one adult, and one elderly monkey (Table 4) .
- Treatment was started from 22 dpi by intravenous injection for 7 consecutive days, and clinical sign data were collected for further analysis (Figure 8A) . Animals were euthanized for pathological analysis 5 days after the treatments stopped.
- Senescent cell accumulation plays a causative role in the development of osteoarthritis (OA) and selectively elimination of senescent cell could be an effective treatment strategy for OA patients (Jeon O, Kim C, Laberge R M, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment [J] . Nature Medicine, 2017, 23 (6) : 775-781) . Therefore, we tested the ability of SSK2 to kill OA chondrocytes and found that SSK2 can efficiently eliminate primary human chondrocytes isolated from Osteoarthritis patient (Fig. 16A, B and Fig. 17) .
- SSK1 showed efficient therapeutic efficacy in elderly monkeys infected with SARS-CoV-2.
- Body weight loss, elevation of multiple inflammatory cytokines and macrophage infiltration in the aged infection group were significantly reduced by SSK1 treatment ( Figures 1 and 2) .
- the reduction of inflammatory factors by SSK1 treatment was also observed in aged mice in our previous study (Cai et al., 2020) .
- the anti-inflammatory effects of SSK1 in aged individuals are of great importance in treating COVID-19 because this disease has higher mortality in senior populations who also have basal chronic inflammation (Zhou et al., 2020) .
- SSK1 may have a better therapeutic effect for the elderly population which has a higher rate of severe COVID-19.
- SSK1 is the first anti-inflammatory treatment that has been studied for COVID-19 using nonhuman primate models.
- SSK1 showed the benefits of anti-inflammation without affecting antiviral effects ( Figures 3D and S3C) , and SSK1 treatment also led to a reduction in macrophage infiltration in the lung ( Figures 2A and 2B) .
- macrophages In addition to their important roles in regulating inflammation, macrophages have also been shown to play a key role in antibody-dependent enhancement (ADE) and complement-mediated thrombosis (Yip et al., 2014; Risitano et al., 2020) .
- ADE antibody-dependent enhancement
- Yip et al., 2014 Risitano et al., 2020
- the complement system has been found to be overactivated in the lungs of COVID-19 patients with aggravated lung injuries (Magro et al., 2020) , and previous studies noted that coronavirus infection, such as SARS-CoV, can activate complement C3, leading to an overactivated immune response through macrophages and neutrophils and contributing to thrombotic microangiopathies (Gralinski et al., 2018) . Accordingly, transiently targeting macrophages by SSK1 may also have beneficial effects in reducing complement-associated inflammation and thrombosis during SARS-CoV-2 infection.
- Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH-and cysteine protease-independent FcgammaR pathway. J VIROL 85, 10582-10597.
- Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI insight 4.
- Interleukin-37 Ameliorates Influenza Pneumonia by Attenuating Macrophage Cytokine Production in a MAPK-Dependent Manner. FRONT MICROBIOL 10.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Organic Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Rheumatology (AREA)
- Pain & Pain Management (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Toxicology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
L'invention concerne des promédicaments anti-inflammatoires et anti-sénescence, qui sont conçus à partir d'un agent cytotoxique, en modifiant chimiquement l'agent cytotoxique pour incorporer un site clivable par SA-β-gal après l'administration du promédicament in vivo, pour libérer le médicament parent actif. Le promédicament comprend une fraction à base de galactose, qui est de préférence acétylée, et un groupe benzyloxycarboxy et une fraction d'agent cytotoxique. Le promédicament anti-sénescence est utilisé pour tuer sélectivement une ou plusieurs cellules sénescentes et/ou réduire une réponse inflammatoire aiguë ou chronique chez un sujet qui en a besoin, par l'administration au sujet d'une quantité thérapeutiquement efficace des promédicaments anti-sénescence. Les compositions peuvent être utilisées pour réduire chez un sujet un ou plusieurs symptômes associés à une maladie ou à un trouble associé à la sénescence ou un trouble inflammatoire, par exemple, une inflammation médiée par un virus.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202080058263.1A CN114341148B (zh) | 2019-09-25 | 2020-09-24 | 抗衰老和抗炎前药及其使用方法 |
| US17/763,127 US20220362387A1 (en) | 2019-09-25 | 2020-09-24 | Senolytic and antiinflammatory prodrugs and methods of use thereof |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/CN2019/107887 WO2021056270A1 (fr) | 2019-09-25 | 2019-09-25 | Promédicaments sénolytiques et leurs procédés d'utilisation |
| CNPCT/CN2019/107887 | 2019-09-25 | ||
| CNPCT/CN2020/081133 | 2020-03-25 | ||
| PCT/CN2020/081133 WO2021056996A1 (fr) | 2019-09-25 | 2020-03-25 | Promédicaments anti-sénescence et anti-inflammatoires et leurs méthodes d'utilisation |
| PCT/CN2020/092933 WO2021057061A1 (fr) | 2019-09-25 | 2020-05-28 | Promédicaments sénolytiques et anti-inflammatoires et procédés d'utilisation de ces derniers |
| CNPCT/CN2020/092933 | 2020-05-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021057840A1 true WO2021057840A1 (fr) | 2021-04-01 |
Family
ID=75165555
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2020/117386 WO2021057840A1 (fr) | 2019-09-25 | 2020-09-24 | Promédicaments anti-inflammatoires et anti-sénescence et leurs méthodes d'utilisation |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2021057840A1 (fr) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114085256A (zh) * | 2021-11-23 | 2022-02-25 | 江南大学 | 一种β-半乳糖苷酶响应的糖类衍生物及其应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5561119A (en) * | 1991-04-30 | 1996-10-01 | Laboratoires Hoechst | Glycosylated prodrugs, their method of preparation and their uses |
| WO2009016647A1 (fr) * | 2007-08-01 | 2009-02-05 | Council Of Scientific & Industrial Research | Promédicaments pyrrolo [2,1-c][1,4] benzodiazépine-glycosides utiles comme agent anti-tumoral sélectif |
| WO2017189553A1 (fr) * | 2016-04-25 | 2017-11-02 | Everon Biosciences, Inc. | Élimination de macrophages associés à la sénescence |
| US20180094015A1 (en) * | 2016-09-30 | 2018-04-05 | Washington State University | Enzyme-directed immunostimulant and uses thereof cross-reference to related application(s) |
-
2020
- 2020-09-24 WO PCT/CN2020/117386 patent/WO2021057840A1/fr active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5561119A (en) * | 1991-04-30 | 1996-10-01 | Laboratoires Hoechst | Glycosylated prodrugs, their method of preparation and their uses |
| WO2009016647A1 (fr) * | 2007-08-01 | 2009-02-05 | Council Of Scientific & Industrial Research | Promédicaments pyrrolo [2,1-c][1,4] benzodiazépine-glycosides utiles comme agent anti-tumoral sélectif |
| WO2017189553A1 (fr) * | 2016-04-25 | 2017-11-02 | Everon Biosciences, Inc. | Élimination de macrophages associés à la sénescence |
| US20180094015A1 (en) * | 2016-09-30 | 2018-04-05 | Washington State University | Enzyme-directed immunostimulant and uses thereof cross-reference to related application(s) |
Non-Patent Citations (6)
| Title |
|---|
| GHOSH, A. K. ET AL.: "A daunorubicin β-galactoside prodrug for use in conjunction with gene-directed enzyme prodrug therapy.", TETRAHEDRON LETTERS., vol. 41, 31 December 2000 (2000-12-31), pages 4871 - 4874, XP004205673 * |
| HANTHO JOSEPH D., STRAYER TIMOTHY A., NIELSEN AMY E., MANCINI ROCK J.: "An Enzyme-Directed Imidazoquinoline for Cancer Immunotherapy", CHEM.MED.CHEM, WILEY-VCH, DE, vol. 11, no. 22, 11 October 2016 (2016-10-11), DE, pages 2496 - 2500, XP055793846, ISSN: 1860-7179, DOI: 10.1002/cmdc.201600443 * |
| HEIKO J. SCHUSTER, BIRGIT KREWER, J. MARIAN VON HOF, KIANGA SCHMUCK, INGRID SCHUBERTH, FRAUKE ALVES, LUTZ F. TIETZE: "Synthesis of the first spacer containing prodrug of a duocarmycin analogue and determination of its biological activity", ORGANIC & BIOMOLECULAR CHEMISTRY, ROYAL SOCIETY OF CHEMISTRY, vol. 8, no. 8, 17 February 2010 (2010-02-17), pages 1833 - 1842, XP055019053, ISSN: 1477-0520, DOI: 10.1039/b925070k * |
| KAMAL, A. ET AL.: "Development of pyrrolo[2,1-c] [1,4] benzodiazepine β-galactoside prodrugs for selective therapy of cancer by ADEPT and PMT.", CHEMMEDCHEM., vol. 3, 5 February 2008 (2008-02-05), pages 794 - 802, XP002651397, DOI: 20201207154348X * |
| SARKAR, A.K. ET AL.: "Disaccharide uptake and priming in animal cells: Inhibition of sialyl Lewis X by acetylated Galβ1-4G1cNAcβ-O-naphthalenemethanol Proc.", NATL. ACAD. SCI.USA., vol. 92, 30 April 1995 (1995-04-30), pages 3323 - 3327, XP055793938 * |
| SHARMA, A. ET AL.: "Development of a theranostic prodrug for colon cancer therapy by combining ligand-targeted delivery and enzyme-stimulated activation.", BIOMATERIALS., vol. 155, 20 November 2017 (2017-11-20), pages 145 - 151, XP085320144 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114085256A (zh) * | 2021-11-23 | 2022-02-25 | 江南大学 | 一种β-半乳糖苷酶响应的糖类衍生物及其应用 |
| CN114085256B (zh) * | 2021-11-23 | 2024-03-19 | 江南大学 | 一种β-半乳糖苷酶响应的糖类衍生物及其应用 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Zhang et al. | STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress | |
| TWI723017B (zh) | 奧貝膽酸組成物及使用方法 | |
| EP2968347B1 (fr) | Modulateurs de la voie eif2 alpha | |
| KR101208016B1 (ko) | 미토콘드리아 질환 치료용 조성물 및 치료방법 | |
| JP7053478B2 (ja) | Fxrアゴニストを使用するための方法 | |
| Vilahur et al. | Silybum marianum provides cardioprotection and limits adverse remodeling post-myocardial infarction by mitigating oxidative stress and reactive fibrosis | |
| EP3277675B1 (fr) | Modulateurs hétérocycles de la synthèse des lipides | |
| Zhuge et al. | Microbiota-induced lipid peroxidation impairs obeticholic acid-mediated antifibrotic effect towards nonalcoholic steatohepatitis in mice | |
| CN112739346A (zh) | 大麻素及其用途 | |
| Sun et al. | Liposomes encapsulated dimethyl curcumin regulates dipeptidyl peptidase I activity, gelatinase release and cell cycle of spleen lymphocytes in-vivo to attenuate collagen induced arthritis in rats | |
| WO2021057840A1 (fr) | Promédicaments anti-inflammatoires et anti-sénescence et leurs méthodes d'utilisation | |
| JP2011525194A (ja) | 線維性疾患または病態を治療するための組成物 | |
| US20220362387A1 (en) | Senolytic and antiinflammatory prodrugs and methods of use thereof | |
| WO2021057061A1 (fr) | Promédicaments sénolytiques et anti-inflammatoires et procédés d'utilisation de ces derniers | |
| US12103946B2 (en) | Steroid compounds as Treg modulators and uses thereof | |
| KR102352729B1 (ko) | 간 질환의 치료 방법 | |
| WO2014208354A1 (fr) | Composition pharmaceutique pour le traitement ou la prophylaxie de maladies inflammatoires | |
| Ljungblad et al. | Omega-3 fatty acids decrease CRYAB, production of oncogenic prostaglandin E2 and suppress tumor growth in medulloblastoma | |
| CN108697663A (zh) | 在肝病治疗中使用胱天蛋白酶抑制剂的方法 | |
| AU2019333119A1 (en) | Small molecule stimulators of steroid receptor coactivator-3 and methods of their use as cardioprotective and/or vascular regenerative agents | |
| JP2019507116A (ja) | エストロゲン受容体を活性化する新規化合物ならびにその組成物および使用方法 | |
| WO2022076742A1 (fr) | Méthodes et compositions de traitement d'infections virales | |
| WO2024036961A1 (fr) | Promédicament contre des maladies associées au vieillissement et son procédé d'utilisation | |
| JPWO2020166662A1 (ja) | 骨疾患の予防または治療用の医薬組成物 | |
| Bai et al. | Serine Supports Epithelial and Immune Cell Function in Colitis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20869521 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 20869521 Country of ref document: EP Kind code of ref document: A1 |