WO2019210835A1 - Diaryl macrocyclic compound as protein kinase modulator - Google Patents
Diaryl macrocyclic compound as protein kinase modulator Download PDFInfo
- Publication number
- WO2019210835A1 WO2019210835A1 PCT/CN2019/085090 CN2019085090W WO2019210835A1 WO 2019210835 A1 WO2019210835 A1 WO 2019210835A1 CN 2019085090 W CN2019085090 W CN 2019085090W WO 2019210835 A1 WO2019210835 A1 WO 2019210835A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- reaction
- compound
- group
- added
- Prior art date
Links
- 0 CC1(*)CCCCC(C)(*C(**)c2****(*)c2*)CCCCC1 Chemical compound CC1(*)CCCCC(C)(*C(**)c2****(*)c2*)CCCCC1 0.000 description 8
- ZWIDHXJJUGELRQ-SECBINFHSA-N CCOC(c(cn[n]1cc2)c1nc2N[C@H](C)c(cc(cn1)F)c1O)=O Chemical compound CCOC(c(cn[n]1cc2)c1nc2N[C@H](C)c(cc(cn1)F)c1O)=O ZWIDHXJJUGELRQ-SECBINFHSA-N 0.000 description 2
- GPJIAUAWUHDWJC-PFRQMTDMSA-N CC(C)(C)OC(N[C@H](CC1)C[C@H]1Oc(c([C@@H](C[C@@H](C1)F)N1c(cc[n]1nc2)nc1c2C(O)=O)c1)ncc1F)=O Chemical compound CC(C)(C)OC(N[C@H](CC1)C[C@H]1Oc(c([C@@H](C[C@@H](C1)F)N1c(cc[n]1nc2)nc1c2C(O)=O)c1)ncc1F)=O GPJIAUAWUHDWJC-PFRQMTDMSA-N 0.000 description 1
- XMHBHMXIFGLWLM-SMDDNHRTSA-N CCOC(c(cn[n]1cc2)c1nc2N(C[C@H](C1)F)[C@H]1c(cc(cn1)F)c1O)=O Chemical compound CCOC(c(cn[n]1cc2)c1nc2N(C[C@H](C1)F)[C@H]1c(cc(cn1)F)c1O)=O XMHBHMXIFGLWLM-SMDDNHRTSA-N 0.000 description 1
- NNUXIUZQZCJRIY-LMVRJCEZSA-N CCOC(c(cn[n]1cc2)c1nc2N(C[C@H](C1)F)[C@H]1c1cc(F)cnc1O[C@@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)=O Chemical compound CCOC(c(cn[n]1cc2)c1nc2N(C[C@H](C1)F)[C@H]1c1cc(F)cnc1O[C@@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)=O NNUXIUZQZCJRIY-LMVRJCEZSA-N 0.000 description 1
- DZVGWXUYGOAVIC-QRQLOZEOSA-N CCOC(c(cn[n]1cc2)c1nc2N[C@H](C)c1cc(F)ccc1O[C@@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)=O Chemical compound CCOC(c(cn[n]1cc2)c1nc2N[C@H](C)c1cc(F)ccc1O[C@@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)=O DZVGWXUYGOAVIC-QRQLOZEOSA-N 0.000 description 1
- NGXMOVNLGNTSET-FAEJEUNOSA-N CCOC(c(cn[n]1cc2)c1nc2N[C@H](C)c1cc(F)cnc1OC(CC1)CC1NC(OC(C)(C)C)=O)=O Chemical compound CCOC(c(cn[n]1cc2)c1nc2N[C@H](C)c1cc(F)cnc1OC(CC1)CC1NC(OC(C)(C)C)=O)=O NGXMOVNLGNTSET-FAEJEUNOSA-N 0.000 description 1
- NGXMOVNLGNTSET-KBAYOESNSA-N CCOC(c(cn[n]1cc2)c1nc2N[C@H](C)c1cc(F)cnc1O[C@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)=O Chemical compound CCOC(c(cn[n]1cc2)c1nc2N[C@H](C)c1cc(F)cnc1O[C@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)=O NGXMOVNLGNTSET-KBAYOESNSA-N 0.000 description 1
- XJHUWFMYOJXOEE-BNOWGMLFSA-N C[C@H](c(cc(cc1)F)c1O[C@@H](CC1)C[C@@H]1N1)Nc(cc[n]2nc3)nc2c3C1=O Chemical compound C[C@H](c(cc(cc1)F)c1O[C@@H](CC1)C[C@@H]1N1)Nc(cc[n]2nc3)nc2c3C1=O XJHUWFMYOJXOEE-BNOWGMLFSA-N 0.000 description 1
- DGJKHJUHFGFGIE-OIISXLGYSA-N C[C@H](c(cc(cc1)F)c1O[C@@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)Nc(cc[n]1nc2)nc1c2C(O)=O Chemical compound C[C@H](c(cc(cc1)F)c1O[C@@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)Nc(cc[n]1nc2)nc1c2C(O)=O DGJKHJUHFGFGIE-OIISXLGYSA-N 0.000 description 1
- DESJPXLUMKKPEG-QFWMXSHPSA-N C[C@H](c(cc(cn1)F)c1OC(CC1)CC1N)Nc(cc[n]1nc2)nc1c2C(O)=O Chemical compound C[C@H](c(cc(cn1)F)c1OC(CC1)CC1N)Nc(cc[n]1nc2)nc1c2C(O)=O DESJPXLUMKKPEG-QFWMXSHPSA-N 0.000 description 1
- FICARSDAQRSAOZ-QFWMXSHPSA-N C[C@H](c(cc(cn1)F)c1OC(CC1)CC1N1)Nc(cc[n]2nc3)nc2c3C1=O Chemical compound C[C@H](c(cc(cn1)F)c1OC(CC1)CC1N1)Nc(cc[n]2nc3)nc2c3C1=O FICARSDAQRSAOZ-QFWMXSHPSA-N 0.000 description 1
- FICARSDAQRSAOZ-RTXFEEFZSA-N C[C@H](c(cc(cn1)F)c1O[C@@H](CC1)C[C@@H]1N1)Nc(cc[n]2nc3)nc2c3C1=O Chemical compound C[C@H](c(cc(cn1)F)c1O[C@@H](CC1)C[C@@H]1N1)Nc(cc[n]2nc3)nc2c3C1=O FICARSDAQRSAOZ-RTXFEEFZSA-N 0.000 description 1
- FICARSDAQRSAOZ-KGYLQXTDSA-N C[C@H](c(cc(cn1)F)c1O[C@H](CC1)C[C@H]1N1)Nc(cc[n]2nc3)nc2c3C1=O Chemical compound C[C@H](c(cc(cn1)F)c1O[C@H](CC1)C[C@H]1N1)Nc(cc[n]2nc3)nc2c3C1=O FICARSDAQRSAOZ-KGYLQXTDSA-N 0.000 description 1
- BHZZWQXOQRGARB-RBHXEPJQSA-N C[C@H](c1cc(F)ccc1O)N[S@](C(C)(C)C)=O Chemical compound C[C@H](c1cc(F)ccc1O)N[S@](C(C)(C)C)=O BHZZWQXOQRGARB-RBHXEPJQSA-N 0.000 description 1
- JYRMSMXPYDGPTN-LLVKDONJSA-N C[C@H](c1cc(F)ccc1OC1CCC1)Nc(cc[n]1nc2)nc1c2C(N)=O Chemical compound C[C@H](c1cc(F)ccc1OC1CCC1)Nc(cc[n]1nc2)nc1c2C(N)=O JYRMSMXPYDGPTN-LLVKDONJSA-N 0.000 description 1
- AYFRWKSBUPMXJH-BNOWGMLFSA-N C[C@H](c1cc(F)ccc1O[C@@H](CC1)C[C@@H]1N)Nc(cc[n]1nc2)nc1c2C(O)=O Chemical compound C[C@H](c1cc(F)ccc1O[C@@H](CC1)C[C@@H]1N)Nc(cc[n]1nc2)nc1c2C(O)=O AYFRWKSBUPMXJH-BNOWGMLFSA-N 0.000 description 1
- RKAGFEKGFBGQFG-BNOWGMLFSA-N C[C@H](c1cc(F)ccc1O[C@@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)N Chemical compound C[C@H](c1cc(F)ccc1O[C@@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)N RKAGFEKGFBGQFG-BNOWGMLFSA-N 0.000 description 1
- YXUVLHSXZXGYIV-IUDNXUCKSA-N C[C@H](c1cc(F)cnc1OC(CC1)CC1NC(OC(C)(C)C)=O)Nc(cc[n]1nc2)nc1c2C(O)=O Chemical compound C[C@H](c1cc(F)cnc1OC(CC1)CC1NC(OC(C)(C)C)=O)Nc(cc[n]1nc2)nc1c2C(O)=O YXUVLHSXZXGYIV-IUDNXUCKSA-N 0.000 description 1
- DESJPXLUMKKPEG-RAIGVLPGSA-N C[C@H](c1cc(F)cnc1O[C@H](CC1)C[C@@H]1N)Nc(cc[n]1nc2)nc1c2C(O)=O Chemical compound C[C@H](c1cc(F)cnc1O[C@H](CC1)C[C@@H]1N)Nc(cc[n]1nc2)nc1c2C(O)=O DESJPXLUMKKPEG-RAIGVLPGSA-N 0.000 description 1
- FICARSDAQRSAOZ-RAIGVLPGSA-N C[C@H](c1cc(F)cnc1O[C@H](CC1)C[C@@H]1N1)Nc(cc[n]2nc3)nc2c3C1=O Chemical compound C[C@H](c1cc(F)cnc1O[C@H](CC1)C[C@@H]1N1)Nc(cc[n]2nc3)nc2c3C1=O FICARSDAQRSAOZ-RAIGVLPGSA-N 0.000 description 1
- YXUVLHSXZXGYIV-FVQBIDKESA-N C[C@H](c1cc(F)cnc1O[C@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)Nc(cc[n]1nc2)nc1c2C(O)=O Chemical compound C[C@H](c1cc(F)cnc1O[C@H](CC1)C[C@@H]1NC(OC(C)(C)C)=O)Nc(cc[n]1nc2)nc1c2C(O)=O YXUVLHSXZXGYIV-FVQBIDKESA-N 0.000 description 1
- SUNXKQBMPCZRND-QDEZUTFSSA-N O=C(c(cn[n]1cc2)c1nc2N(C[C@H](C1)F)[C@H]1c1c2)N[C@H](CC3)C[C@H]3Oc1ncc2F Chemical compound O=C(c(cn[n]1cc2)c1nc2N(C[C@H](C1)F)[C@H]1c1c2)N[C@H](CC3)C[C@H]3Oc1ncc2F SUNXKQBMPCZRND-QDEZUTFSSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D498/08—Bridged systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D519/00—Heterocyclic compounds containing more than one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system not provided for in groups C07D453/00 or C07D455/00
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
Definitions
- the present application relates to a diaryl macrocyclic compound as a protein kinase modulator, a process for the preparation thereof, a pharmaceutical composition containing the same, and use thereof in the treatment of cancer, pain, neurological diseases, autoimmune diseases and inflammation.
- Protein kinases are key regulators of cell growth, proliferation, and survival, acting on specific proteins and altering their activity. These kinases play a wide-ranging role in cell signaling and its complex life activities. Genetic and epigenetic changes accumulate in cancer cells, leading to abnormal activation of the signal transduction pathway leading to the process of deterioration. Pharmacological inhibition of these signaling pathways provides a promise for targeted cancer therapy.
- ALK Anaplasticlymphoma kinase
- ALK is found in a subtype of anaplastic large cell lymphoma (ALCL).
- NSCLC non-small cell lung cancer
- IMT inflammatory myofibroblastic tumor
- ALK and leukocyte tyrosine kinase (LTK) are the insulin receptor (IR) superfamily of receptor tyrosine kinases that can be fused to a variety of genes, such as NPM-ALK, TPM3-ALK, TFG-ALK, ATIC- ALK, CLTC-ALK, etc.
- IR insulin receptor
- ALK is primarily expressed in the central and peripheral nervous systems and has a potential role in the normal development and function of the nervous system. More than 20 different ALK translocation partners have been discovered in many cancers.
- the EML4-ALK fusion gene can be found in a variety of tumors, such as anaplastic large cell lymphoma, inflammatory myofibroblastoma, neuroblastoma, and NSCLC, which are caused by short arm insertion of chromosome 2.
- the EML4 gene mainly maintains the basic morphology of the cell, while the ALK gene can activate and promote cell proliferation.
- the 5' end of the EML4 gene is translocated with the 3' end of the ALK gene.
- the EML4-ALK fusion gene is at least There are 10 kinds.
- the EML4-ALK fusion gene forms an intricate signal transduction network, which affects cell proliferation, differentiation and apoptosis through the activation and transmission of downstream substrate molecules, and the transduction and overlapping of each transduction pathway.
- ALK small molecule kinase inhibitors include Crizotinib, Lorlatinib and the like.
- ALK kinase inhibitors have achieved great success in the treatment of patients with ALK abnormal gene lung cancer.
- Drug resistance mechanisms typically include target gene amplification, acquired resistance mutations, bypass signaling, epithelial-mesenchymal transition (EMT), and metastasis.
- Trks The tropomyosin-related receptor tyrosine kinase (Trks), including TrkA/B/C encoded by the NTRK1/2/3 gene, is a high-affinity receptor for neurotrophic factors (NTs). Trk family members highly express Trks (TrkA, TrkB and TrkC) in neurogenic cells through their preferential neurotrophic factors (NGF to TrkA, brain-derived neurotrophic factor (BDNF) and NT4/5 to TrkB and NT3 to TrkC) It mediates the survival and differentiation of neurons during development.
- the NT/Trk signaling pathway acts as an endogenous system to protect neurons after biochemical damage, transient ischemia or physical injury.
- Trk was originally cloned in the extracellular domain as an oncogene fused to the tropomyosin gene. Activating mutations caused by chromosomal rearrangements or mutations in NTRK1 (TrkA) have been identified in thyroid papillary and thyroid cancer and more recently in NSCLC. Because Trk plays an important role in pain perception and tumor cell growth and survival signals, Trk receptor kinase inhibitors are thought to have great potential for the treatment of pain and cancer. At present, Trk small molecule kinase inhibitors include Larotrectinib and LOXO-195.
- TrkA G595R and TrkC G623R both similar to ALK G1202R
- TrkA G595R and TrkC G623R both similar to ALK G1202R
- a new generation of Trk inhibitors against wild-type and mutant Trks is essential for the effective treatment of patients with fusion Trk.
- ROS1 kinase is a receptor tyrosine kinase with an unknown ligand.
- the chimeric protein of ROS1 gene fusion has strong proliferative activity.
- ROS1 kinase has been reported to undergo genetic rearrangement to produce constitutively active fusion in various human cancers. Proteins, including glioblastoma, NSCLC cholangiocarcinoma, ovarian cancer, gastric adenocarcinoma, colorectal cancer, inflammatory myofibroblastic tumor, angiosarcoma, and epithelioid hemangioendothelioma.
- Crizotinib showed a median PFS nearly double that of ALK-positive patients in NSCLC patients positive for ROS1 fusion mutations, reaching 18.3 months with an ORR of 66%. However, acquired resistance mutations have been observed in patients treated with Crizotinib, so there is an urgent need to develop second-generation ROS1 inhibitors that overcome the resistance of Crizotinib ROS1.
- the present application relates to a compound of formula I or a pharmaceutically acceptable salt thereof,
- Ring G is selected from
- X is selected from O or NR 3 ;
- R 1 is selected from hydrogen, C 1-6 alkyl, C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl, said C 1-6 alkyl C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl is optionally substituted by halogen, hydroxy, cyano, -OC 1-6 Alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -NHC(O)C 1-6 alkyl, -NHC(O)NH 2 , -CO 2 H, -C(O)OC 1-6 alkyl, -C(O)NH 2 , -C(O)NH(C 1-6 alkyl), -C(O)N (C 1- 6 alkyl) 2 , -SH, -SC 1-6 alkyl, -S(O
- R 3 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl, 3-7 membered heterocycloalkyl, 6-10 membered aryl Or a 6-10 membered heteroaryl group, said C 1-6 alkyl group, C 2-6 alkenyl group, C 2-6 alkynyl group, C 3-6 cycloalkyl group, 3-7 membered heterocycloalkyl group, 6 a -10 membered aryl or a 6-10 membered heteroaryl group is optionally substituted with a halogen or -OC 1-6 alkyl group; or
- R 1 and R 3 together with the atom to which they are attached form a 3-6 membered heterocycloalkyl group, which is optionally substituted by a halogen, C 1-6 alkyl group, Hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl ;
- T 1 , T 2 , T 3 or T 4 are independently selected from CR b or N;
- R b is independently selected from the group consisting of hydrogen, halogen, C 1-6 alkyl, hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NHC 1-6 alkyl, -N(C 1-6 alkane Base) 2 or -CF 3 ;
- L 1 is selected from -O-, -NR a -, C 1-6 alkylene, -OC 1-6 alkylene- or -C 1-6 alkylene O-, said alkylene optionally Substituted by: halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH Or -SC 1-6 alkyl;
- L 2 is selected from -NHC 1-6 alkylene-, -C 1-6 alkylene NH-, -C 1-6 alkylene-NR a CO-, -NR a C(O)- or -C (O)N(R a )-, the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 Alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl;
- R a is independently selected from hydrogen or C 1-6 alkyl
- n is independently selected from 1, 2 or 3;
- q is selected from 0-4;
- R 2 is independently selected from halogen, cyano, hydroxy, C 1-6 alkyl optionally substituted by halogen or hydroxy, -OC 1-6 alkyl, -NH 2 , -NHC 1-6 alkyl, -N (C 1-6 alkyl) 2 .
- the X is selected from NR 3.
- R 1 is selected from the group consisting of hydrogen, C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl, or 6-10 membered aryl, C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl is optionally substituted by halogen, hydroxy, cyano , -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -NHC(O)C 1-3 alkyl, -NHC (O)NH 2 , -CO 2 H, -C(O)OC 1-3 alkyl, -C(O)NH 2 , -C(O)NH(C 1-3 alkyl), -C(O N(C 1-3 alkyl) 2 , -SH, -SC 1-3 alkyl
- R 1 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl, or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl is optionally substituted by halogen, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1- 3 alkyl) 2 , -NHC(O)C 1-3 alkyl, -NHC(O)NH 2 , -CO 2 H, -C(O)OC 1-3 alkyl, -C(O)NH 2 , -C(O)NH(C 1-3 alkyl), -C(O)N(C 1-3 alkyl) 2 , -SH, -SC 1-3 alkyl, -S(O)C 1 -3 alkyl, -S(O) 2 C 1-3 alkyl, -S(O)NH(C 1-3 alkyl, -
- R 1 is selected from hydrogen or C 1-3 alkyl optionally substituted with fluorine, chlorine, bromine, hydroxy, cyano or -NH 2 . In some embodiments, R 1 is selected from methyl optionally substituted with fluorine.
- R 3 is selected from the group consisting of hydrogen, C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl, 3-6 membered heterocycloalkyl, 6-10 membered aryl or 6-10 membered heteroaryl, said C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl, 3-6 Heterocycloalkyl, 6-10 membered aryl or 6-10 membered heteroaryl is optionally substituted by halogen or -OC 1-3 alkyl.
- R 3 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl, or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C The 2-3 alkynyl group is optionally substituted with a halogen or -OC 1-3 alkyl group. In some embodiments, R 3 is selected from hydrogen or C 1-3 alkyl optionally substituted with fluorine, chlorine or bromine. In some embodiments, R 3 is selected from hydrogen.
- R 1 and R 3 and the atoms to which they are attached form a 4-5 membered heterocycloalkyl, which is optionally substituted with the following: halogen, C 1-6 alkyl, hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or - SC 1-6 alkyl.
- R 1 and R 3 and the atoms to which they are attached form a 5-membered heterocycloalkyl group, which is optionally substituted with a halogen, C 1-3 alkane Base, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl.
- R 1 and R 3 and the atom to which they are attached together form a tetrahydropyrrolyl group, which is optionally substituted with a fluoro, chloro, bromo, hydroxy, cyano or -NH 2 .
- R 1 and R 3 and the atoms to which they are attached form a tetrahydropyrrole group, which is optionally substituted with fluorine.
- R b is independently selected from the group consisting of hydrogen, halogen, C 1-3 alkyl, hydroxy, cyano, —OC 1-3 alkyl, —NH 2 , —NHC 1-3 alkyl, —N (C 1-3 alkyl) 2 or -CF 3 .
- R b is independently selected from hydrogen, halo, hydroxy, cyano, -NH 2 or -CF 3 .
- R b is independently selected from hydrogen, fluoro, chloro or bromo.
- R b is independently selected from hydrogen or fluoro.
- T 1 is selected from CH, N or CF.
- T 1 or T 4 are each independently selected from CH or N.
- T 2 is selected from CH or N.
- T 3 is selected from CF or N.
- L 1 is selected from the group consisting of -O-, -NR a -, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-,
- the alkylene group is optionally substituted with a halogen, a cyano group, a hydroxyl group, a -OC 1-3 alkyl group, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkane Base) 2 , -SH or -SC 1-3 alkyl.
- L 1 is selected from the group consisting of -O-, -NR a -, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-, The alkylene group is optionally substituted by a halogen. In some embodiments, L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O- is optionally substituted by F. In some embodiments, L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 -, -OCF 2 -, -CH 2 O-, or -CF 2 O-.
- L 1 is selected from -O-, C 1-6 alkylene, -OC 1-6 alkylene- or -C 1-6 alkylene O-, said alkylene optionally The ground is replaced by the following groups: halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 ,- SH or -SC 1-6 alkyl.
- L 1 is selected from -O-, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-, the alkylene optionally The ground is replaced by the following groups: halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 ,- SH or -SC 1-3 alkyl.
- L 1 is selected from -O-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O - optionally replaced by F.
- L 1 is selected from the group consisting of -O-, -CH 2 CH 2 -, -OCH 2 -, -OCF 2 -, -CH 2 O-, or -CF 2 O-.
- L 2 is selected from the group consisting of -NHC 1-3 alkylene-, -C 1-3 alkylene NH-, -C 1-3 alkylene-NR a CO-, -NR a C ( O)- or -C(O)N(R a )-, the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , - NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl.
- L 2 is selected from the group consisting of -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)-, or -C(O)N(R a )-
- the methylene group in the -NHCH 2 -, -CH 2 NH- or -CH 2 -NR a CO- is optionally substituted with a halogen, a cyano group, a hydroxyl group, a -OC 1-3 alkyl group.
- L 2 is selected from the group consisting of -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)-, or -C(O)N(R a )- .
- L 2 is selected from the group consisting of -NHC 1-6 alkylene-, -C 1-6 alkylene NH-, -NR a C(O)-, or -C(O)N(R a ) - the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl.
- L 2 is selected from the group consisting of -NHC 1-3 alkylene-, -C 1-3 alkylene NH-, -NR a C(O)-, or -C(O)N(R a ) - the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl.
- L 2 is selected from -NHCH 2 -, -CH 2 NH-, -NR a C(O)- or -C(O)N(R a )-, said -NHCH 2 - or -
- the methylene group in CH 2 NH- is optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N (C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl.
- L 2 is selected from the group consisting of -NHCH 2 -, -CH 2 NH-, -NR a C(O)-, or -C(O)N(R a )-.
- R a is independently selected from hydrogen or C 1-3 alkyl. In some embodiments, R a is independently selected from hydrogen or methyl.
- m, n are independently selected from 1 or 2. In some embodiments, m is selected from the group consisting of 1, and n is selected from 1 or 2. In some embodiments, m is selected from the group consisting of 1, and n is selected from the group consisting of 1.
- q is selected from 0 or 1. In some embodiments q is selected from zero.
- R 2 is independently selected from halo, cyano, hydroxy, or C 1-3 alkyl, optionally substituted by halogen or hydroxy. In some embodiments, R 2 is independently selected from C 1-3 alkyl. In some embodiments, R 2 is independently selected from methyl.
- the structural unit Selected from In some embodiments, the structural unit Selected from In some embodiments, the structural unit Selected from In some embodiments, the structural unit Selected from
- the invention provides a compound of Formula I, or a pharmaceutically acceptable salt thereof, of the present application, wherein
- Ring G is selected from
- X is selected from O or NR 3 ;
- R 1 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl
- the ground is replaced by the following groups: halogen, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -NHC(O)C 1-3 alkyl, -NHC(O)NH 2 , -CO 2 H, -C(O)OC 1-3 alkyl, -C(O)NH 2 , -C(O) NH(C 1-3 alkyl) or -C(O)N(C 1-3 alkyl) 2 ;
- R 3 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl The ground is replaced by the following group: halogen or -OC 1-3 alkyl; or,
- R 1 and R 3 and the atom to which they are attached form a 4-5 membered heterocycloalkyl group, which is optionally substituted by a halogen, C 1-6 alkyl group, Hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl ;
- T 1 , T 2 , T 3 or T 4 are each independently selected from CR b or N;
- R b is independently selected from the group consisting of hydrogen, halogen, hydroxy, cyano, -NH 2 or -CF 3 ;
- L 1 is selected from -O-, -NR a -, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-, said alkylene optionally Substituted by: halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH Or -SC 1-3 alkyl;
- L 2 is selected from -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)- or -C(O)N(R a )-, the -NHCH 2
- the methylene group in -, -CH 2 NH- or -CH 2 -NR a CO- is optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , - NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl;
- R a is independently selected from hydrogen or C 1-3 alkyl
- n is independently selected from 1 or 2;
- q is selected from 0 or 1;
- R 2 is independently selected from halogen, cyano, hydroxy or C 1-3 alkyl optionally substituted by halogen or hydroxy.
- the invention provides a compound of Formula I, or a pharmaceutically acceptable salt thereof, of the present application, wherein
- Ring G is selected from
- X is selected from O or NR 3 ;
- R 1 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl The ground is replaced by the following groups: halogen, hydroxy, cyano or -NH 2 ;
- R 3 is selected from hydrogen or a C 1-3 alkyl group optionally substituted by halogen; or
- R 1 and R 3 together with the atom to which they are attached form a 5-membered heterocycloalkyl group which is optionally substituted by a halogen, a C 1-3 alkyl group, a hydroxyl group, a cyano group. Or -NH 2 ;
- T 1 , T 2 , T 3 or T 4 are each independently selected from CH, N or CF;
- L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-
- the ground is replaced by a halogen
- L 2 is selected from -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)- or -C(O)N(R a )-, the -NHCH 2
- the methylene group in -, -CH 2 NH- or -CH 2 -NR a CO- is optionally substituted by halogen;
- R a is independently selected from hydrogen, methyl or ethyl
- n is selected from 1 or 2;
- q is selected from 0 or 1;
- R 2 is independently selected from halogen or C 1-3 alkyl optionally substituted by halogen or hydroxy.
- the invention provides a compound of Formula I, or a pharmaceutically acceptable salt thereof, of the present application, wherein
- Ring G is selected from
- X is selected from O or NR 3 ;
- R 1 is selected from hydrogen or C 1-3 alkyl, and the C 1-3 alkyl group is optionally substituted with fluorine;
- R 3 is selected from hydrogen or C 1-3 alkyl; or,
- R 1 and R 3 together with the atom to which they are attached form a tetrahydropyrrolyl group optionally substituted by a fluorine or a C 1-3 alkyl group;
- T 1 , T 2 , T 3 or T 4 are each independently selected from CH, N or CF;
- L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-
- the ground is replaced by fluorine;
- L 2 is selected from -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)- or -C(O)N(R a )-;
- R a is independently selected from hydrogen, methyl or ethyl
- n is selected from 1 or 2;
- q is selected from 0 or 1;
- R 2 is independently selected from C 1-3 alkyl.
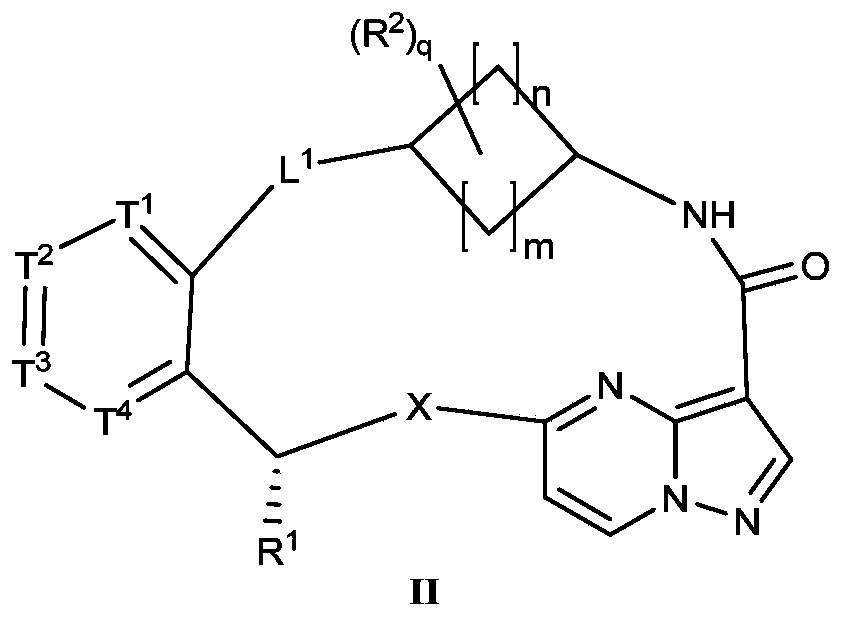
- a compound of Formula I of the present application is selected from a compound of Formula II or a pharmaceutically acceptable salt thereof:
- X, R 1 , T 1 , T 2 , T 3 , T 4 , L 1 , m, n, q or R 2 are as defined in the compound of formula I.
- a compound of Formula I of the present application is selected from Formula III or a pharmaceutically acceptable salt thereof:
- a compound of Formula I of the present application is selected from a compound of Formula IV or a pharmaceutically acceptable salt thereof:
- a compound of Formula I of the present application is selected from the group consisting of: or a pharmaceutically acceptable salt thereof:
- the present application is directed to a pharmaceutical composition comprising a compound of formula I, or a pharmaceutically acceptable salt thereof, of the present application.
- the pharmaceutical compositions of the present application also include pharmaceutically acceptable excipients.
- the application describes a method of treating or inhibiting cell proliferation, cell invasion, metastasis, apoptosis, or angiogenesis-related diseases in a mammal, comprising administering a therapeutically effective amount to a mammal, preferably a human, in need of such treatment.
- a mammal preferably a human
- a compound or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof is administered to a mammal, preferably a human.
- the application describes a compound of Formula I, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof, for use in the manufacture of a medicament for preventing or treating a cell proliferation, cell invasion, metastasis, apoptosis or angiogenesis-related disease in a mammal Use in.
- the application describes the use of a compound of Formula I, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof, for preventing or treating a cell proliferation, cell invasion, metastasis, apoptosis, or angiogenesis-related disease in a mammal .
- the application features a compound of formula I, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof, for use in preventing or treating a cell proliferation, cell invasion, metastasis, apoptosis or angiogenesis-related disease in a mammal.
- the cell proliferation, cell invasion, metastasis, apoptosis, or angiogenesis is by ALK, ALK-EML-4 fusion protein, AXL, Aur B&C, mutant BCR-ABL, BLK, Eph6B, HPK, IRAK1&3 , LCK, LTK, various MEKKs, RON, ROS1, SLK, STK10, TIE1&2 or TRKs1-3.
- the cell proliferation, cell invasion, metastasis, apoptosis or angiogenesis-related diseases are selected from the group consisting of cancer, pain, neurological diseases, autoimmune diseases, and inflammation.
- substituted means that any one or more hydrogen atoms on a particular atom are replaced by a substituent as long as the valence of the particular atom is normal and the substituted compound is stable.
- it means that two hydrogen atoms are substituted and the oxo does not occur on the aryl group.
- an ethyl group “optionally” substituted with halo refers to an ethyl group may be unsubstituted (-CH 2 CH 3), monosubstituted (e.g., -CH 2 CH 2 F), polysubstituted (e.g., -CHFCH 2 F, -CH 2 CHF 2 , etc.) or completely substituted (-CF 2 CF 3 ). It will be understood by those skilled in the art that for any group containing one or more substituents, no substitution or substitution pattern that is sterically impossible to exist and/or which cannot be synthesized is introduced.
- C mn herein is that the moiety has an integer number of carbon atoms in a given range.
- C1-6 means that the group may have 1 carbon atom, 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms or 6 carbon atoms.
- any variable eg, R
- its definition in each case is independent.
- each R has an independent option.
- linking group When the number of one linking group is 0, such as -(CH 2 ) 0 -, it means that the linking group is a bond.
- one of the variables is selected from a covalent bond, it means that the two groups to which it is attached are directly linked.
- L represents a bond in A-L-Z
- the structure is actually A-Z.
- the substituent When a bond of a substituent is cross-linked to two atoms on a ring, the substituent may be bonded to any atom on the ring.
- a structural unit It is indicated that it can be substituted at any position on the cyclohexyl or cyclohexadiene.
- L 1 is selected from -OCH 2 -
- L 2 is selected from -NR a C(O)-
- the structural unit is In another example, when L 1 is selected from -CH 2 O-, and L 2 is selected from -NHCH 2 -, the structural unit is
- halo or halogen refers to fluoro, chloro, bromo and iodo.
- hydroxy refers to an -OH group.
- cyano refers to a -CN group.
- amino means -NH 2 group.
- alkyl refers to a hydrocarbon group of the formula C n H 2n +.
- the alkyl group can be straight or branched.
- C 1 - 6 alkyl refers to (e.g., methyl, ethyl, n-propyl, isopropyl, alkyl containing 1 to 6 carbon atoms, n-butyl, isobutyl, sec-butyl, Tert-butyl, n-pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl, neopentyl, hexyl, 2-methylpentyl, etc.).
- alkyl moiety i.e., alkyl
- an alkoxy group an alkylamino group, a dialkylamino group, an alkylsulfonyl group, and an alkylthio group
- alkyl i.e., alkyl
- alkylene refers to a divalent group formed by the removal of one hydrogen at any position of the alkyl group.
- C1-6 alkylene include, but are not limited to, methylene, ethylene, methylmethylene, dimethylmethylene, 1,3-propylene, and the like. .
- alkenyl refers to a straight or branched unsaturated aliphatic hydrocarbon group having at least one double bond consisting of a carbon atom and a hydrogen atom.
- alkenyl groups include, but are not limited to, ethenyl, 1-propenyl, 2-propenyl, 1-butenyl, isobutenyl, 1,3-butadienyl, and the like.
- alkynyl means a straight or branched unsaturated aliphatic hydrocarbon group having at least one triple bond composed of a carbon atom and a hydrogen atom.
- alkynyl groups include, but are not limited to, ethynyl (-C ⁇ CH), 1-propynyl (-C ⁇ C-CH 3 ), 2-propynyl (-CH 2 -C ⁇ CH), 1,3-butadiynyl (-C ⁇ CC ⁇ CH) or the like.
- cycloalkyl refers to a carbocyclic group that is fully saturated and can exist as a single ring, bridged ring or spiro ring. Unless otherwise indicated, the carbocyclic ring is typically a 3 to 10 membered ring.
- Non-limiting examples of cycloalkyl groups include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, norbornyl (bicyclo[2.2.1]heptyl), bicyclo[2.2.2]octyl, diamond Alkyl and the like.
- heterocycloalkyl refers to a cyclic group that is fully saturated and can exist as a monocyclic, bridged or spiro ring. Unless otherwise indicated, the heterocyclic ring is typically a 3 to 7 membered ring containing from 1 to 3 heteroatoms (preferably 1 or 2 heteroatoms) independently selected from sulfur, oxygen and/or nitrogen.
- 3-membered heterocycloalkyl groups include, but are not limited to, oxiranyl, cyclohexylethane, cycloalkylethane, non-limiting examples of 4-membered heterocycloalkyl including, but not limited to, azetidinyl, acetophenan
- Examples of a cyclic group, a thibutyl group, a 5-membered heterocycloalkyl group include, but are not limited to, tetrahydrofuranyl, tetrahydrothiophenyl, pyrrolidinyl, isoxazolidinyl, oxazolidinyl, isothiazolidinyl, thiazolidine
- Examples of the group, imidazolidinyl group, tetrahydropyrazolyl group, 6-membered heterocycloalkyl group include, but are not limited to, piperidinyl, tetrahydropyranyl, tetrahydrothio
- aryl refers to an all-carbon monocyclic or fused polycyclic aromatic ring group having a conjugated ⁇ -electron system.
- an aryl group can have 6 to 20 carbon atoms, 6 to 14 carbon atoms, or 6 to 12 carbon atoms.
- Non-limiting examples of aryl groups include, but are not limited to, phenyl, naphthyl, anthracenyl, 1,2,3,4-tetrahydronaphthalene, and the like.
- heteroaryl refers to a monocyclic or fused polycyclic ring system containing at least one ring atom selected from N, O, S, the remaining ring atoms being C, and having at least one aromatic ring.
- Preferred heteroaryl groups have a single 4 to 8 membered ring, especially a 5 to 8 membered ring, or a plurality of fused rings containing from 6 to 14, especially from 6 to 10 ring atoms.
- heteroaryl groups include, but are not limited to, pyrrolyl, furyl, thienyl, imidazolyl, oxazolyl, pyrazolyl, pyridyl, pyrimidinyl, pyrazinyl, quinolinyl, isoquinolinyl , tetrazolyl, triazolyl, triazinyl, benzofuranyl, benzothienyl, fluorenyl, isodecyl and the like.
- treating means administering a compound or formulation described herein to prevent, ameliorate or eliminate a disease or one or more symptoms associated with the disease, and includes:
- terapéuticaally effective amount means (i) treating or preventing a particular disease, condition or disorder, (ii) alleviating, ameliorating or eliminating one or more symptoms of a particular disease, condition or disorder, or (iii) preventing or delaying The amount of a compound of the present application in which one or more symptoms of a particular disease, condition, or disorder are described herein.
- the amount of a compound of the present application which constitutes a “therapeutically effective amount” will vary depending on the compound, the condition and severity thereof, the mode of administration, and the age of the mammal to be treated, but can be routinely determined by those skilled in the art It is determined by its own knowledge and the present disclosure.
- pharmaceutically acceptable is intended to mean that those compounds, materials, compositions and/or dosage forms are within the scope of sound medical judgment and are suitable for use in contact with human and animal tissues without Many toxic, irritating, allergic reactions or other problems or complications are commensurate with a reasonable benefit/risk ratio.
- a metal salt, an ammonium salt, a salt with an organic base, a salt with an inorganic acid, a salt with an organic acid, a salt with a basic or acidic amino acid, or the like can be mentioned.
- pharmaceutical composition refers to a mixture of one or more compounds of the present application or a salt thereof and a pharmaceutically acceptable adjuvant.
- the purpose of the pharmaceutical composition is to facilitate administration of the compounds of the present application to an organism.
- pharmaceutically acceptable excipient refers to those excipients which have no significant irritating effect on the organism and which do not impair the biological activity and properties of the active compound. Suitable excipients are well known to those skilled in the art, such as carbohydrates, waxes, water soluble and/or water swellable polymers, hydrophilic or hydrophobic materials, gelatin, oils, solvents, water, and the like.
- tautomer or "tautomeric form” refers to structural isomers of different energies that are interconvertible via a low energy barrier.
- proton tautomers also known as proton transfer tautomers
- proton transfer tautomers include interconversions via proton transfer, such as keto-enol and imine-enamine isomerization.
- a specific example of a proton tautomer is an imidazole moiety in which a proton can migrate between two ring nitrogens. Tautomers include recombination through some of the bonding electrons.
- the present application also includes isotopically labeled compounds of the present application that are identical to those described herein, but in which one or more atoms are replaced by an atomic weight or mass number different from the atomic mass or mass number normally found in nature.
- isotopes that may be incorporated into the compounds of the present application include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, fluorine, iodine, and chlorine, such as 2 H, 3 H, 11 C, 13 C, 14 C, 13 respectively.
- isotopically-labeled compounds of the present application can be used in compound and/or substrate tissue distribution assays.
- Deuterated (i.e., 3 H) and carbon-14 (i.e., 14 C) isotopes are especially preferred due to ease of preparation and detectability.
- Positron emitting isotopes such as 15 O, 13 N, 11 C and 18 F can be used in positron emission tomography (PET) studies to determine substrate occupancy.
- Isotopically labeled compounds of the present application can generally be prepared by substituting an isotopically labeled reagent for an unisotopically labeled reagent by procedures similar to those disclosed in the schemes and/or examples disclosed below.
- substitution with heavier isotopes such as deuterium can provide certain therapeutic advantages resulting from higher metabolic stability (eg, increased in vivo half-life or reduced dosage requirements), and thus It may be preferred in some cases where the hydrazine substitution may be partial or complete and the partial hydrazine substitution means that at least one hydrogen is replaced by at least one hydrazine.
- the alkylene group referred to in the present application may be a partially or fully deuterated alkylene group.
- the compounds of the present application may be asymmetric, for example, having one or more stereoisomers. Unless otherwise stated, all stereoisomers are included in the present application, such as enantiomers and diastereomers.
- the asymmetric carbon atom-containing compounds of the present application can be isolated in optically active pure form or in racemic form. The optically active pure form can be resolved from the racemic mixture or synthesized by using a chiral starting material or a chiral reagent.
- the compounds of the present application may exist in specific geometric or stereoisomeric forms. All such compounds are contemplated by the present application, including tautomers, cis and trans isomers, (-)- and (+)-enantiomers, (R)- and (S)-enantiomers , diastereomers, (D)-isomers, (L)-isomers, and racemic mixtures thereof, and other mixtures, such as enantiomerically or diastereomeric enriched mixtures, All of these are within the scope of this application. Additional asymmetric carbon atoms may be present in the substituent such as an alkyl group. All such isomers, as well as mixtures thereof, are included within the scope of the present application. Structural unit Cis isomer form And trans isomer form
- compositions of the present application can be prepared by combining the compounds of the present application with suitable pharmaceutically acceptable excipients, for example, as solid, semi-solid, liquid or gaseous preparations, such as tablets, pills, capsules, powders. , granules, ointments, emulsions, suspensions, suppositories, injections, inhalants, gels, microspheres and aerosols.
- suitable pharmaceutically acceptable excipients for example, as solid, semi-solid, liquid or gaseous preparations, such as tablets, pills, capsules, powders. , granules, ointments, emulsions, suspensions, suppositories, injections, inhalants, gels, microspheres and aerosols.
- Typical routes of administration of a compound of the present application, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof include, but are not limited to, oral, rectal, topical, inhalation, parenteral, sublingual, intravaginal, intranasal, intraocular, intraperitoneal, Intramuscular, subcutaneous, intravenous administration.
- the pharmaceutical composition of the present application can be produced by a method well known in the art, such as a conventional mixing method, a dissolution method, a granulation method, a sugar-coating method, a grinding method, an emulsification method, a freeze-drying method, and the like.
- the pharmaceutical composition is in oral form.
- the pharmaceutical composition can be formulated by admixing the active compound with pharmaceutically acceptable excipients which are well known in the art. These excipients enable the compounds of the present application to be formulated into tablets, pills, troches, dragees, capsules, liquids, gels, slurries, suspensions and the like for oral administration to a patient.
- Solid oral compositions can be prepared by conventional methods of mixing, filling or tabletting. For example, it can be obtained by mixing the active compound with a solid adjuvant, optionally milling the resulting mixture, adding other suitable excipients if necessary, and then processing the mixture into granules to give tablets. Or the core of the sugar coating. Suitable excipients include, but are not limited to, binders, diluents, disintegrants, lubricants, glidants, sweeteners or flavoring agents, and the like.
- compositions may also be suitable for parenteral administration, such as sterile solutions, suspensions or lyophilized products in a suitable unit dosage form.
- the daily dose is from 0.01 to 200 mg/kg body weight, either alone or in divided doses.
- the compounds of the present application can be prepared by a variety of synthetic methods well known to those skilled in the art, including the specific embodiments listed below, combinations thereof with other chemical synthesis methods, and equivalents well known to those skilled in the art. Alternatively, preferred embodiments include, but are not limited to, embodiments of the present application.
- the compounds of Formula II herein can be prepared by one of ordinary skill in the art of organic synthesis by conventional methods in the art using Route 1:
- T 1 , T 2 , T 3 , T 4 , L 1 , X, R 1 , R 2 , q, m, n are as defined for the compound of formula I; R represents hydrogen, and Pg represents a protecting group such as Boc, etc., R 4 Represents a C 1-6 alkyl group.
- EA stands for ethyl acetate; DCM stands for dichloromethane; HATU stands for 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate; DIPEA stands for N , N-diisopropylethylamine; DMF stands for N,N-dimethylformamide; DEAD stands for diethyl azodicarboxylate; NBS stands for N-bromosuccinimide; DMA stands for N,N- Dimethylacetamide; FDPP stands for pentafluorophenyl diphenyl phosphate; MeOH stands for methanol; TEA stands for triethylamine; T3P stands for 1-propylphosphoric acid anhydride; MsCl stands for methylsulfonyl chloride; DIAD stands for azodicarboxylic acid Diisopropyl ester; Boc represents tert-butoxycarbonyl;
- Example 1 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-phenyl-3(1,3)-cyclobutylcaprolactam-5-one
- Step 1 (R,E)-N-(5-fluoro-2-hydroxyphenylalkenyl)-2-methylpropyl-2-sulfinamide
- Step 2 (R)-N-((R)-1-(5-fluoro-2-hydroxyphenyl)ethyl)-2-methylpropyl-2-sulfenamide
- Step 3 ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl carboxylate
- Step 4 ((1S,3S)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
- Step 5 5-(((R)-1-(2-((1s,3S)-3-(tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl)amino) Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 6 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl)amino Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 7 5-((R)-1-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazolo[1,5 -a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 8 (3 1 S, 3 3 S, 6 3 E, 6 4 E, 8R) -1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6 (3, 5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-phenyl-3(1,3)-cyclobutylcaprolactam-5-one (Compound I-1)
- Example 2 (3 1 R, 3 3 R, 6 3 E, 6 4 E, 8S)-1 4 -fluoro-8-methyl-2-oxo-4,7-diaza-6 (3, 5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-phenyl-3(1,3)-cyclobutanol cyclooctanolac-5-one
- Step 1 ((1s,3s)-3-(2-Acetyl-4-fluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
- Step 2 ((1S,3s)-3-(2-((Z)-1-((R)-tert-butylsulfinyl)imide)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl carbamate
- Step 3 ((1R,3s)-3-(2-((S)-1-(((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl formate
- Step 4 ((1R,3s)-3-(2-((S)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
- Step 5 5-((S)-1-(2-((1s,3R)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl)amino Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 6 5-(((S)-1-(2-((1),3R)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 7 5-((S)-1-(2-((1s,3R)-3-Aminocyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazolo[1,5- a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 8 (3 1 R,3 3 R,6 3 E,6 4 E,8S)-1 4 -fluoro-8-methyl-2-oxo-4,7-diaza-6 (3,5 )-pyrazolo[1,5-a]pyrimidin-1(1,2)-phenyl-3(1,3)-cyclobutanol cyclooctanolac-5-one (Compound I-2)
- Example 3 (3 1 R,3 3 R,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidine-l-1(1,2)-benzene-3(1,3)-cyclobutane heterocyclic caprolactam-5-one
- Step 1 (1s, 3s)-3-((tert-Butoxycarbonyl)amino)cyclobutylmethanesulfonate
- Step 2 ((1R,3r)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl carbamate
- Step 3 ((1R,3r)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
- Step 4 (((R)-1-(2-((1r,3R)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl)amino) Pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 5 5-(((R)-1-(2-((1r,3R)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 6 5-((R)-1-(2-((1r,3R)-3-Aminocyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazolo[1,5 -a]pyrimidine-3-carboxylic acid
- Step 7 (3 1 R,3 3 R,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutane heterocyclic caprolactam-5-one (Compound I-3) )
- Example 4 (1 3 E, 1 4 E, 2 2 R, 5 1 S, 5 3 S)-3 5 -fluoro-4-oxa-6-aza-1(5,3)-pyrazole And [1,5- ⁇ ]pyrimidinium-2(1,2)-pyrrole-3(1,2)-benzene-5(1,3)-cyclobutanol cyclooctanolactam-7-one
- Step 2 (R)-5-(2-(5-Fluoro-2-hydroxyphenyl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 3 5-(R)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)pyrrolidine-1- Ethyl pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 4 5-((R)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)pyrrolidine-1 -yl)pyrazolo[1,5- ⁇ ]pyrimidine-3-carboxylic acid
- Step 5 5-((R)-2-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluorophenyl)pyrrolidin-1-yl)pyrazolo[1, 5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 6 (1 3 E, 1 4 E, 2 2 R, 5 1 S, 5 3 S)-3 5 -fluoro-4-oxa-6-aza-1(5,3)-pyrazole [1,5- ⁇ ]pyrimidine-hetero-2(1,2)-pyrrole-3(1,2)-benzene-5(1,3)-cyclobutanol cyclooctanolactam-7-one (compound) I-4)
- Example 5 (1 3 E, 1 4 E, 2 2 R, 5 1 S, 5 3 S)-3 5 -fluoro-4-oxa-6-aza-1(5,3)-pyrazole And [1,5-a]pyrimidin-3(3,2)-pyridine-2(1,2)-pyrrole-5(1,3)-cyclobutylcycloheptanolac-7-one
- Step 1 (R)-5-(2-(5-Fluoro-2-methoxypyridin-3-yl)pyrrolyl-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate Ethyl acetate
- Step 2 (R)-5-(2-(5-Fluoro-2-hydroxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 3 5-((R)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl)pyrrole Ethyl-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 4 5-((R)-2-(2-((1s,3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl)pyrrole Alkan-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 5 5-((R)-2-(2-((1s,3S)-3-Aminocyclobutyloxa)-5-fluoropyridin-3-yl)pyrrolidin-1-yl)pyrazole And [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 6 (1 3 E, 1 4 E, 2 2 R, 5 1 S, 5 3 S)-3 5 -fluoro-4-oxa-6-aza-1(5,3)-pyrazole [1,5-a]pyrimidine-3(3,2)-pyridine-2(1,2)-pyrrolidin-5(1,3)-cyclobutylcycloheptanolac-7-one (I-5)
- Example 6 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5- ⁇ ]pyrimidine-l(2,3)-pyridin-3(1,3)-cyclobutanol-5-one
- Step 1 (R,E)-N((5-fluoro-2-methoxypyridin-3-yl)methylene)-2-methylpropane-2-sulfinamide
- Step 2 (R)-N-((R)-1-(5-fluoro-2-methoxypyridin-3-yl)ethyl)-2-methylpropane-2-sulfinamide
- Step 4 (R)-5-((1-(5-fluoro-2-methoxypyridin-3-yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ester
- Step 5 (R)-5-((1-(5-Fluoro-2-hydroxypyridin-3-yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 6 5-((R)-1-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl)ethyl Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 7 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl)) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 8 5-((R)-1-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluoropyridin-3-yl)ethyl)amino)pyrazolo[1 ,5-a]pyrimidine-3-carboxylic acid
- Step 9 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrazolo[1,5- ⁇ ]pyrimidin-1(2,3)-pyridin-3(1,3)-cyclobutanolactam-5-one (Compound I-6)
- Example 7 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutylcaprolactam-5-one
- Step 1 ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl (meth)carbamate
- Step 2 ((1S,3s)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)(methyl)carbamic acid tert-butyl ester
- Step 3 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)(methyl)amino)cyclobutyloxy)-5-fluorobenzene) Ethyl)amino)pyrrolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 4 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)(methyl)amino)cyclobutyloxy)-5-fluorobenzene) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 5 5-((R)-1-(5-fluoro-2-((1s,3S)-3-(methylamino)cyclobutoxy)phenyl)ethyl)amino)pyrazole [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 6 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-4,8-dimethyl-2-oxa-4,7-diaza-6 (3,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctyllactam-5-one (Compound 1-7 )
- Example 8 (1 3 E,1 4 E,7 1 S,7 3 S,3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1(5 ,3)-pyrazolo[1,5-a]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutyllactam-9-one
- Step 1 ((1s, 3s)-3-(hydroxymethyl)cyclobutyl)carbamic acid tert-butyl ester
- Step 2 ((1S,3s)-3-((2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)) Tert-butyl)carbamic acid tert-butyl ester
- Step 3 ((1S,3s)-3-((2-((R)-1-aminoethyl)-4-fluorophenoxy)methyl)cyclobutyl)carbamic acid tert-butyl ester
- Step 4 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)methoxy)-5-fluorophenoxy Ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 5 5-(((R)-1-(2-(((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)methoxy)-5-fluorophenoxy) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 6 5-(((R)-1-(2-((1(3S))-3-aminocyclobutyl)methoxy)-5-fluorophenyl)ethyl)amino)pyrazole [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride salt
- Compound 23F 164 mg was added to a reaction flask, and 10 mL of 4N hydrogen chloride in dioxane was added thereto under ice water, and the mixture was reacted at room temperature. After completion of the reaction, the solvent was removed by concentration, and then acetonitrile was added to the concentrate, and a large solid was precipitated, which was filtered to give a solid and dried to give compound 23G (130 mg).
- Step 7 (1 3 E, 1 4 E, 7 1 S, 7 3 S, 3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1 (5, 3)-pyrazolo[1,5-a]pyrimidin-4(1,2)-benzo-7(1,3)-cyclobutyllactam-9-one (Compound I-8)
- Example 9 (1 3 E, 1 4 E, 7 1 R, 7 3 R, 3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza- 1 (5 ,3)-pyrazolo[1,5- ⁇ ]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutanol-9-one
- Step 1 ((1R,3r)-3-((2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)) Tert-butyl)carbamic acid tert-butyl ester
- Step 2 ((1R,3r)-3-((2-((R)-1-Aminoethyl)-4-fluorophenoxy)methyl)cyclobutyl)carbamic acid tert-butyl ester
- Step 3 5-((R)-1-(2-((1r,3R)-3-((tert-Butoxycarbonyl)amino)cyclobutyl)methoxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 4 5-(((R)-1-(2-((1r,3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)methoxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 5 5-((R)-1-(2-(((1r,3R)-3-Aminocyclobutyl)methoxy)-5-fluorophenyl)ethyl)amino)pyrazolo[ 1,5-a]pyrimidine-3-carboxylic acid
- Step 6 (1 3 E, 1 4 E, 7 1 R, 7 3 R, 3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza- 1 (5, 3)-pyrazolo[1,5- ⁇ ]pyrimidin-4(1,2)-benzo-7(1,3)-cyclobutanlactam-9-one (Compound I-9)
- Example 10 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-5,7-diaza- 6 (3) ,5)-pyrazolo[1,5-a]pyrimidine-l-(1,2)-benzene-3(1,3)-cyclobutanol cyclooctanolactam-4-one
- Step 1 (1S,3s)-3-(2-((R)-1-(((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Methyl-1-carboxylate
- Step 2 Preparation of (1S,3s)-3-(2-((R)-1-aminoethyl)-4-fluorophenoxy)cyclobutane-1-carboxylic acid methyl ester
- Step 3 (1S,3s)-3-(4-fluoro-2-((R)-1-((3-nitropyrazolo[1,5-a]pyrimidin-5-yl)amino)) Methyl phenoxy)-formate
- Step 4 (1S,3s)-3-(2-((R)-1-((3-Aminopyrazolo[1,5-a]pyrimidin-5-yl)amino)ethyl)-4- Methyl fluorophenoxy)carboxylate
- Step 5 (1S,3s)-3-(2-((R)-1-((3-aminopyrazolo[1,5-a]pyrimidin-5-yl)amino)ethyl)-4- Fluorophenoxy)cyclobutane-1-carboxylic acid
- Step 6 (3 1 S, 3 3 S, 6 3 E, 6 4 E, 8R) -1 4 - fluoro-8-methyl-2-oxa-5,7-diazepin-6 (3, 5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutanolcyclooctanolactam-4-one (Compound I-10)
- Example 11 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidine-l-1(1,2)-benzene-3(1,3)-cyclopentyloctanolactam-5-one
- Step 1 ((1R,3R)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclopentane Tert-butyl carbamate
- Step 2 ((1R,3R)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclopentyl)carbamic acid tert-butyl ester
- Step 3 5-(((R)-1-(2-((1(R)))) ((tert-butyloxycarbonyl)amino)cyclopentyl)oxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 4 5-(((R)-1-(2-((1(R)))) ((tert-butyloxycarbonyl)amino)cyclopentyl)oxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 6 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrazolo[1,5-a]pyrimidine-l-1(1,2)-benzene-3(1,3)-cyclopentylcaprolactam-5-one (Compound I-11)
- Example 12 (3 1 s, 3 3 s, 6 3 Z, 6 4 E, 8R) -1 4 - fluoro-8-methyl-2-oxa-4,7-diazepin-6 (3 ,5)-pyrazolo[1,5- ⁇ ]pyrimidine-l-1(1,2)-benzene-3(1,3)-cyclobutane heterocyclic octanolactam
- Step 1 (3 1 s, 3 3 s, 6 3 Z, 6 4 E, 8R) -1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6 (3, 5)-pyrazolo[1,5- ⁇ ]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutane heterocyclic octanolactam (Compound I-12)
- Example 13 (3 1 S,3 3 S,8R,E)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3,6)-imidazole [1,2-b]pyridin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctanolac-5-one
- Step 1 6-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)imidazo[1,2-b]pyridine-3-carboxylic acid ethyl ester
- Step 2 6-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)imidazo[1,2-b]pyridine-3-carboxylic acid
- Step 3 6-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)imidazo[1,2-b]pyridine-3-carboxylic acid trihydrochloride
- Step 4 (3 1 S,3 3 S,8R,E)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3,6)-imidazo[ 1,2-b]pyridin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctanolactam-5-one (Compound I-13)
- Example 14 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-tetrahydropyrrole-5(1,3)-cyclobutane Alkane heterocyclic heptanolactam-7-one
- Step 1 (R)-N-((R)-1-(5-fluoro-2-methoxypyridin-3-yl)but-3-en-1-yl)-2-methylpropane-2 - sulfenamide
- Step 4 (5R)-5-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidin-3-yl acetate
- Step 5 (2R)-4-acetoxy-2-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidine-1-carboxylic acid tert-butyl ester
- Step 6 (2R)-2-(5-Fluoro-2-methoxypyridin-3-yl)-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester
- Step 7 (R)-2-(5-Fluoro-2-methoxypyridin-3-yl)-4-oxopyrrolidine-1-carboxylic acid tert-butyl ester
- Step 8 (2R,4R)-2-(5-fluoro-2-methoxypyridin-3-yl)-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester
- Step 9 (2R,4S)-4-fluoro-2-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidine-1-carboxylic acid tert-butyl ester
- Step 10 5-Fluoro-3((2R,4S)-4-fluoropyrrolidin-2-yl)-2-methoxypyridine hydrochloride
- Step 11 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a Pyrimidine-3-carboxylate
- Step 12 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine Ethyl 3-carboxylate
- Step 13 5-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl) Ethyl 4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 14 5-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl) )-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 15 5-(2R,4S)-2-(2-((1s,3S)-3-Aminocyclobutyloxy)-5-fluoropyridin-3-yl)-4-fluoropyrrolidine-1 -yl)pyrazolo[1,5a]pyrimidine-3-carboxylic acid trihydrochloride (Compound 48O)
- Step 16 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclobutane Cycloheptamamide-7-one (Compound I-14)
- Example 15 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza- 1 (5,3)-pyrazolo[1,5- ⁇ ]pyrimidine hetero-2(1,2)-tetrahydropyrrole-3(1,2)-benzene-5(1,3)-cyclobutane Alkane heterocyclic heptanolactam-7-one
- Tetraethyl titanate (35.5 g) was slowly added dropwise at 0 ° C to 5-fluoro-2-methoxy-benzaldehyde (20 g) and (R)-(+)-tert-butylsulfinamide (17.30 g). The mixture was stirred in tetrahydrofuran (150 mL), and the reaction was stirred at 50 °C. After the reaction was completed, saturated brine (200 mL) was added and filtered. The filter cake was washed with EA, and the filtrate was separated. Filtration and concentration of the filtrate gave compound 15A (37.039 g). It was used in the next reaction without purification. MS (ESI) m / z: 258.4 [M+H] + .
- Step 2 (R)-N-((R)-1-(5-fluoro-2-methoxyphenyl)-3-buten-1-yl)-2-methylpropane-2-sulfin Amide
- Step 6 (2R)-4-acetoxy-2-(5-fluoro-2-methoxyphenyl)pyrrolidine-1-carboxylic acid tert-butyl ester
- Step 7 (2R)-2-(5-Fluoro-2-methoxyphenyl)-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester
- Step 8 (R)-2-(5-Fluoro-2-methoxyphenyl)-4-oxopyrrolidine-1-carboxylic acid tert-butyl ester
- Step 9 (2R,4R)-2-(5-fluoro-2-methoxyphenyl)-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester
- Step 10 (2R,4S)-4-fluoro-2-(5-fluoro-2-methoxyphenyl)pyrrolidine-1-carboxylic acid tert-butyl ester
- Step 11 4-Fluoro-2-((2R,4S)-4-fluoropyrrolidin-2-yl)phenol hydrobromide
- Step 12 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxyphenyl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3- Ethyl carboxylate
- Step 13 5-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)-4 -fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 14 5-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)-4 -fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 15 5-((2R,4S)-2-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluorophenyl)-4-fluoropyrrolidin-1-yl) Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 16 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5- ⁇ ]pyrimidine hetero-2(1,2-)-tetrahydropyrrole-3(1,2)-benzene-5(1,3)-cyclobutane Alkane heterocyclic heptanolactam-7-one
- Example 16 (3 1 R,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrrolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclopentylcyclooctanolac-5-one
- Step 1 ((1R,3S)-3-(2-((S)-1-((())-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclopentane Tert-butyl carbamate
- Step 2 ((1S,3R)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclopentyl)carbamic acid tert-butyl ester
- Step 3 5-(((R)-1-(2-((1(R))))((tert-butyloxycarbonyl)amino)cyclopentyl)oxa)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 4 5-(((R)-1-(2-((1(R))))((tert-butyloxycarbonyl)amino)cyclopentyl)oxa)-5-fluorophenyl Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 6 (3 1 R,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrrolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclopentylcyclooctanolac-5-one
- Example 17 (1 3 E,1 4 E,7 1 S,7 3 R,3R)-4 5 -fluoro-3-methyl-2,8-diaza-1(5,3)-pyridyl Zizo[1,5- ⁇ ]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutane heterocyclic lactam-9-one
- Step 1 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)ethynyl)-5-fluorophenyl) Ethyl)amino)pyrazole [1,5-a]pyrimidine-3-carboxylate
- Step 2 5-(((R)-1-(2-(2-((1),3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)ethyl)-5-fluorobenzene Ethyl)ethyl)amino)pyrazole [1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 3 5-(((R)-1-(2-(2-((1),3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)ethyl)-5-fluorobenzene Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 4 5-(((R)-1-(2-(2-((1),3R)-3-aminocyclobutyl)ethyl)-5-fluorophenyl)ethyl)amino)pyrazole And [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 5 (1 3 E,1 4 E,7 1 S,7 3 R,3R)-4 5 -fluoro-3-methyl-2,8-diaza-1(5,3)-pyrazole And [1,5- ⁇ ]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutane heterocyclic lactam-9-one
- Example 18 (1 3 E,1 4 E,7 1 S,7 3 R,3R)-4 5 -fluoro-3-methyl-2,8-diaza- 1(5,3)-pyridyl Zoxao[1,5- ⁇ ]pyrimidin-4(3,2)-pyridin-7(1,3)-cyclobutanelactam-9-one
- Step 1 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)ethynyl)-5-fluoropyridine-3 -ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 2 5-(((R)-1-(2-(2-((1),3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)ethyl)-5-fluoropyridine Ethyl 3-ethyl)ethyl)aminoethyl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 3 5-(((R)-1-(2-(2-((1),3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)ethyl)-5-fluoropyridine -3-yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 4 5-((R)-1-(2-(2-((1s,3R)-3-Aminocyclobutyl)ethyl)-5-fluoropyridin-3-yl)ethyl)amino Pyrazolo[1,5- ⁇ ]pyrimidine-3-carboxylic acid trihydrochloride
- Step 5 (1 3 E,1 4 E,7 1 S,7 3 R,3R)-4 5 -fluoro-3-methyl-2,8-diaza-1(5,3)-pyrazole And [1,5- ⁇ ]pyrimidine hetero-4(3,2)-pyridin-7(1,3)-cyclobutane heterocyclic lactam-9-one
- Example 19 (3 1 S,3 3 S,8R,E)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3,6)-imidazole [1,2-b]pyridazine-1(2,3)-pyridin-3(1,3)-cyclobutylcyclooctanolac-5-one
- Step 1 (R)-6-((1-(5-Fluoro-2-methoxypyridin-3-yl)ethyl)amino)imidazo[1,2-b]pyridazine-3-carboxylic acid Ethyl ester
- Step 2 (R)-6-((1-(5-Fluoro-2-hydroxypyridin-3-yl)ethyl)amino)imidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester
- Step 3 6-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutyloxy)-5-fluoropyridin-3-yl) Ethyl)amino)imidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester
- Step 4 6-(((R)-1-(2-((1s,3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl) Ethyl)amino)imidazo[1,2-b]pyridazine-3-carboxylic acid
- Step 5 6-(((R)-1-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluoropyridin-3-yl)ethyl)amino)imidazo[1 ,2-b]pyridazine-3-carboxylic acid trihydrochloride
- Step 6 (3 1 S,3 3 S,8R,E)-1 5 -Fluoro-8-methyl-2-oxa-4,7-diaza-6(3,6)-imidazo[ 1,2-b]pyridazine-1(2,3)-pyridin-3(1,3)-cyclobutylcyclooctanolac-5-one
- Example 20 (1 3 E,1 4 E,7 1 S,7 3 S,3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1(5 ,3)-pyrazolo[1,5- ⁇ ]pyrimidin-4(3,2)-pyridin-7(1,3)-cyclobutanecyclolactam-9-one
- Step 1 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)cyclobutyl)methoxy)-5-fluoropyridine-3 -ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 2 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)methoxy)-5-fluoropyridine- 3-yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 3 5-(((R)-1-(2-(((1),3S)-3-aminocyclobutyl)methoxy)-5-fluoropyridin-3-yl)ethyl)amino) Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 4 (1 3 E, 1 4 E, 7 1 S, 7 3 S, 3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1 (5, 3)-pyrazolo[1,5- ⁇ ]pyrimidine-4(3,2)-pyridin-7(1,3)-cyclobutanecyclolactam-9-one
- Example 21 (3 1 S,3 3 R,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrrolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclopentylcyclooctanolac-5-one
- Step 1 ((1R,3S)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclopentyl Tert-butyl carbonate
- Step 2 ((1R,3S)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclopentyl)carbonate tert-butyl ester
- Step 3 5-(((R)-1-(2-(((()))))))))))))))))) Ethyl)amino)pyrrolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 4 5-(((R)-1-(2-(((()))))))) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 6 (3 1 S,3 3 R,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrrolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclopentylcyclooctanolac-5-one
- Example 22 (3 1 R,3 3 R,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one
- Step 1 5-(((R)-1-(2-((1(R)))))((tert-butyloxycarbonyl)amino)cyclopentyl)oxa)-5-fluoropyridine-3 -ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 2 5-(((R)-1-(2-((1(R)))))((tert-butyloxycarbonyl)amino)cyclopentyl)oxa)-5-fluoropyridine-3 -yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 4 (3 1 R, 3 3 R, 6 3 E, 6 4 E, 8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6 (3, 5)-pyrazolo[1,5-a]pyrimidin-1(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolac-5-one
- Example 23 (1 3 E,1 4 E,7 1 S,7 3 S,3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1(5 ,3)-pyrazolo[1,5- ⁇ ]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutane heterocyclic lactam-9-one-6,6 -d 2
- Step 1 (1s, 3s)-3-((tert-Butoxycarbonyl)amino)cyclobutane-1-carboxylic acid methyl ester
- Step 2 ((1s,3s)-3-(Hydroxymethyl-d 2 )cyclobutyl)carbamic acid tert-butyl ester
- Step 3 5-(((R)-1-(2-(((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)methoxy-d 2 )-5-) Ethyl fluorophenyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 4 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)methoxy-d 2 )-5- Fluorophenyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 5 5 - (((R ) -1- (2 - (((1s, 3S) -3- amino-cyclobutyl) methoxy -d 2) -5- fluorophenyl) ethyl) amino) Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 6 (1 3 E, 1 4 E, 7 1 S, 7 3 S, 3R) -4 5 - fluoro-3-methyl-5-oxa-2,8-diaza-1 (5 3)-pyrazolo[1,5- ⁇ ]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutane heterocyclic lactam-9-one-6,6- d 2
- Example 24 (1 1 S,1 3 S,6 3 E,6 4 E,4R)-3 4 -fluoro-4-methyl-2-oxa-5,8-diaza-6(5 ,3)-pyrazolo[1,5-a]pyrimidin-3(1,2)-benzene-1(1,3)-cyclobutanlactam-7-one
- Step 1 ((1r,3r)-3-Hydroxycyclobutyl)methyl)carbamic acid tert-butyl ester
- Step 2 ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl)methyl)carbamate
- Step 3 ((1S,3s)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)methyl)carbamic acid tert-butyl ester
- Step 4 5-(((R)-1-(2-((1),3S)-3-(((tert-Butoxycarbonyl)amino)methyl)cyclobutoxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 5 5-(((R)-1-(2-((1),3S)-3-(((tert-Butoxycarbonyl)amino)methyl)cyclobutoxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 6 5-((R)-1-(2-((1s,3S)-3-(Aminomethyl)cyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazolo [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 7 (1 1 S,1 3 S,6 3 E,6 4 E,4R)-3 4 -fluoro-4-methyl-2-oxa-5,8-diaza-6(5, 3)-pyrazolo[1,5-a]pyrimidin-3(1,2)-benzene-1(1,3)-cyclobutanol-7-one
- Example 25 (3 1 S,3 3 R,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6 (3 ,5)-pyrazolo[1,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one (Compound I-25 -1)
- Step 1 5-(((1R)-1-(2-((3-((tert-Butyloxycarbonyl))amino)cyclopentyl)oxy)-5-fluoropyridin-3-yl)ethyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 2 5-(((1R)-1-(2-((3-((tert-butyloxycarbonyl))amino)cyclopentyl)oxy)-5-fluoropyridin-3-yl)ethyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 3 5-(((1R)-1-(2-((3-Aminocyclopentyl)oxy)-5-fluoropyridin-3-yl)ethyl)amino)pyrazolo[1,5 -a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 4 (6 3 E,6 4 E,8R)-1 5 -Fluoro-8-methyl-2-oxa-4,7-diaza-6(3,5)-pyrazolo[1 ,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one
- Step 5 (3 1 S,3 3 R,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrazolo[1,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one (I-25-1 )
- Compound 25E was separated and purified by high pressure SFC (CHIRAL ART Cellulose-SC (30 ⁇ 250 mm, 5 ⁇ m, YMC) column, eluent anhydrous ethanol/n-hexane (20/80) was eluted isocratically, flow rate 35 mL/min) Compound I-25-1 (30 mg) and I-25-2 (31 mg) were obtained.
- Example 26 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro- 3 3 ,8-dimethyl-2-oxa-4,7-diaza -6(3,5)-pyrazolo[1,5- ⁇ ]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutanecyclooctanolac-5-one
- Step 1 ((1r,3r)-3-Hydroxy-1-methylcyclobutyl)carbamic acid tert-butyl ester
- Step 2 ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)-1 -methylcyclobutyl)carbamic acid tert-butyl ester
- Step 3 ((1S,3s)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)-1-methylcyclobutyl)carbamic acid tert-butyl ester
- Step 4 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)-3-methylcyclobutoxy)-5-fluorobenzene) Ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 5 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)-3-methylcyclobutoxy)-5-fluorobenzene) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 6 5-((R)-1-(2-((1s,3S)-3-Amino-3-methylcyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazole And [1,5-a]pyridine-3-carboxylic acid trihydrochloride
- Step 7 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro- 3 3 ,8-dimethyl-2-oxa-4,7-diaza – 6(3,5)-pyrazolo[1,5- ⁇ ]pyrimidine-1(1,2)-benzene-3(1,3)-cyclobutanecyclooctanolac-5-one
- Pentafluorophenyl phosphate (30 mg) was added to a solution containing 26G (37 mg), DIPEA (57 mg), DCM (40 mL), DMF (8 mL). The reaction was stirred at room temperature. After the reaction was completed, the mixture was evaporated. ethyl acetate (50 mL) was evaporated. Filter and concentrate the filtrate. Purification by column chromatography (MeOH: EtOAc:EtOAc:EtOAc
- Example 27 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 ,1 6 -difluoro-8-methyl-2-oxa-4,7-diaza -6(3,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctanolac-5-one
- Step 1 ((1s,3s)-3-(2-acetyl-4,6-difluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
- Step 2 ((1S,3s)-3-(2-((E)-1-(((R)-tert-butylsulfinyl)imide)ethyl)-4,6-difluorophenoxy Tert-butyl)carbamic acid tert-butyl ester
- Step 3 ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4,6-difluorophenoxy Cyclobutyl)carbamic acid tert-butyl ester
- Step 4 ((1S,3s)-3-(2-((R)-1-Aminoethyl)-4,6-difluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
- Step 5 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-3,5-difluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 6 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-3,5-difluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 7 5-((R)-1-(2-((1s,3S)-3-Aminocyclobutoxy)-3,5-difluorophenyl)ethyl)amino)pyrazolo[ 1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 8 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 ,1 6 -difluoro-8-methyl-2-oxa-4,7-diaza- 6(3,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctanolac-5-one
- Example 28 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 R)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidinium-2(1,2)-pyrrolidinyl-3(1,2)-benzene-5(1,3)-cyclobutylcyclooctine Lactam-5-one
- Step 1 5-((2R,4S)-2-(2-((1S,3R)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluorophenyl) Ethyl 4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 2 5-((2R,4S)-2-(2-((1S,3R)-3-((tert-Butyloxycarbonyl)amino)cyclopentyl)oxy)-5-fluorophenyl) )-4-fluoropyrrolidin-1-yl)pyrrolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 3 5-((2R,4S)-2-(2-((1S,3R)-3-Aminocyclopentyl)oxy)-5-fluorophenyl)-4-fluoropyrrolidine-1 -yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 4 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 R) - 2 4 , 3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidinium-2(1,2)-pyrrolidinyl-3(1,2)-benzene-5(1,3)-cyclobutylcyclooctene Amide-5-one
- Example 29 (2 2 R, 2 4 S, 5 1 S, 5 3 S, E)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1(6,3)- Imidazo[1,2-b]pyridazine-2(1,2)-pyrrolidinyl-3(1,2)-benzene-5(1,3)-cyclobutylcyclooctanolactam-7-one
- Step 1 4-Fluoro-2-((2R,4S)-4-fluoropyrrolidin-2-yl)phenol hydrobromide
- Step 2 2,2,2-Trifluoro-1-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxyphenyl)pyrrolidin-1-yl)ethan-1-one
- Step 3 ((1S,3s)-3-(4-fluoro-2-((2R,4S)-4-fluoro-1-(2,2,2-trifluoroacetyl)pyrrolidin-2-yl) Phenyloxy)cyclobutyl)carbamic acid tert-butyl ester
- Step 4 ((1S,3s)-3-(4-Fluoro-2-((2R,4S)-4-fluoropyrrolidin-2-yl)phenoxy)cyclobutyl)carbamic acid tert-butyl ester
- Step 5 6-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)-4 -fluoropyrrolidin-1-yl)imidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester
- Step 6 6-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)-4 -fluoropyrrolidin-1-yl)imidazo[1,2-b]pyridazine-3-carboxylic acid
- Step 7 6-((2R,4S)-2-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluorophenyl)-4-fluoropyrrolidin-1-yl) Imidazo[1,2-b]pyridazine-3-carboxylic acid trihydrochloride
- Step 8 (2 2 R, 2 4 S, 5 1 S, 5 3 S, E) - 2 4 , 3 5 -difluoro-4-oxa-6-aza-1(6,3)-imidazole And [1,2-b]pyridazine-2(1,2)-pyrrolidinyl-3(1,2)-benzene-5(1,3)-cyclobutylcyclooctanolactam-7-one
- Example 30 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 R, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclopentyl ring Geptanolactam-7-one
- Step 1 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a Pyrimidine-3-carboxylic acid ethyl ester
- Step 2 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine Ethyl-3-carboxylate
- Step 3 5-((2R,4S)-2-(2-((1R,3S)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluoropyridine- Ethyl 3-yl)-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 4 5-((2R,4S)-2-(2-((1R,3S)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluoropyridine- 3-yl)-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 5 5-((2R,4S)-2-(2-((1R,3S)-3-Aminocyclopentyl)oxy)-5-fluoropyridin-3-yl)-4-fluoropyrrole Alkan-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 6 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 R, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclopentylcycloheptane Lactam-7-one
- Example 31 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 R)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclopentyl Cycloheptamamide-7-one
- Step 1 5-((2R,4S)-2-(2-((1S,3R)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluoropyridine- Ethyl 3-yl)-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 2 5-((2R,4S)-2-(2-((1S,3R)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluoropyridine- 3-yl)-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 3 5-((2R,4S)-2-(2-((1S,3R)-3-Aminocyclopentyl)oxy)-5-fluoropyridin-3-yl)-4-fluoropyrrole Alkan-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 4 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 R) - 2 4 , 3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclopentyl ring Geptanolactam-7-one
- Example 32 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 R, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidinium-2(1,2)-pyrrolidin-3(1,2)-benzene-5(1,3)-cyclopentyl Cycloheptamamide-7-one
- Step 1 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxyphenyl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidin-3- Ethyl carboxylate
- Step 2 5-((2R,4S)-2-(2-((1R,3S)-3-((tert-Butoxycarbonyl))amino)cyclopentyl)oxy)-5-fluorophenyl Ethyl 4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 3 5-((2R,4S)-2-(2-((1R,3S)-3-((tert-Butoxycarbonyl))amino)cyclopentyl)oxy)-5-fluorophenyl )-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 4 5-((2R,4S)-2-(2-((1R,3S)-3-Aminocyclopentyl)oxy)-5-fluorophenyl)-4-fluoropyrrolidine-1 -yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
- Step 5 (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 R, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidinium-2(1,2)-pyrrolidin-3(1,2)-benzene-5(1,3)-cyclopentyl ring Geptanolactam-7-one
- Example 33 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2,4,7-triaza-6 (3,5) -pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutanooctanoic acid-5-one
- Step 1 (R,Z)-N-(1-(2-Bromo-5-fluorophenyl)ethylidene)-2-methylpropane-2-sulfinamide
- Step 2 (R)-N-((R)-1-(2-bromo-5-fluorophenyl)ethyl)-2-methylpropane-2-sulfinamide
- Step 4 (R)-5-((1-(2-Bromo-5-fluorophenyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
- Step 5 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)amino)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
- Step 6 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)amino)-5-fluorophenyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
- Step 8 (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -Fluoro-8-methyl-2,4,7-triaza-6(3,5)- Pyrazolo[1,5-a]pyrimidine-1(1,2)-benzene-3(1,3)-cyclobutanooctanoic acid-5-one
- Test Example 1 In vitro enzyme activity
- EML4-ALK mother solution 50 ng/ ⁇ L of EML4-ALK mother solution was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 ⁇ L of 1.67 ⁇ 0.4 ng/ ⁇ L working solution was added per well. (final concentration was 0.24 ng/ ⁇ L), and different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.24nM, 4 times gradient, a total of 7 concentrations, and a blank control Hole (without enzyme) and negative control well (containing enzyme, plus solvent DMSO), set 2 duplicate wells.
- kinase buffer 50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20
- the 8 nM detection reagent (final concentration of 2 nM, Eu-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature, and the Envision instrument was read (excitation 320 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 1.
- ALK (G1202R) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 ⁇ L of 1.67 ⁇ 0.167 ng/uL was added per well.
- Liquid (final concentration 0.1 ng / ⁇ L) the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.24nM, 4 times gradient, a total of 7 concentrations, and set a blank Control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- the 8 nM detection reagent (final concentration 2 nM, Eu-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; Envision instrument read plate (excitation 320 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 2.
- ALK (C1156Y) mother solution was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 ⁇ L of 1.67 ⁇ 0.167 ng/ ⁇ L was added per well.
- Liquid final concentration 0.1 ng / ⁇ L
- the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.24nM, 4 times gradient, a total of 7 concentrations, and set a blank Control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- the 8 nM detection reagent (final concentration 2 nM, Eu-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; Envision instrument read plate (excitation 320 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 2.
- ALK (G1269A) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 ⁇ L of 1.67 ⁇ 0.005 ng/ ⁇ L was added per well.
- the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and at the same time Blank control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- ALK (F1174L) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 ⁇ L of 1.67 ⁇ 0.005 ng/ ⁇ L was added per well.
- the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and at the same time Blank control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- ALK (R1275Q) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 ⁇ L of 1.67 ⁇ 0.01 ng/ ⁇ L was added per well.
- the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and at the same time Blank control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- kinase buffer 50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20
- 6 ⁇ L of 1.67 ⁇ 0.00668 ng/ ⁇ L was added per well.
- the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and at the same time Blank control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- kinase buffer 50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20
- 6 ⁇ L of 1.67 ⁇ 0.0334 ng/ ⁇ L working solution was added per well (final The concentration was 0.02 ng/ ⁇ L.
- the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and blank control wells. (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- the 8 nM detection reagent (final concentration 2 nM, E ⁇ -anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; the Envision instrument was read (excitation 320 s 340 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 3.
- kinase buffer 50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20
- 6 ⁇ L of 1.67 ⁇ 0.001 ng/ ⁇ L working solution was added per well (final The concentration was 0.0006 ng/ ⁇ L.
- the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and blank control wells. (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- TRKC stock solution 50 ng/ ⁇ L was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 ⁇ L of 1.67 ⁇ 0.1336 ng/ ⁇ L working solution was added per well (final The concentration was 0.08 ng/ ⁇ L), and the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and blank control wells. (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- kinase buffer 50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20
- 6 ⁇ L of 1.67 ⁇ 0.1336 ng/ ⁇ L working solution was added per well (
- the 8 nM detection reagent (final concentration 2 nM, E ⁇ -anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; the Envision instrument was read (excitation 320 or 340 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fit. The results are shown in Table 3.
- kinase buffer 50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20
- 6 ⁇ L of 1.67 ⁇ 0.05 ng/ ⁇ L working solution was added per well (final At a concentration of 0.03 ng/ ⁇ L, different compounds dissolved in DMSO were added to the wells using a nanoliter sampler to give a final concentration of 1000 nM-0.244 nM, a total of 7 concentration gradients, 4 fold dilutions, and blank control wells. (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO).
- the 8 nM detection reagent (final concentration 2 nM, E ⁇ -anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; the Envision instrument was read (excitation 320 s 340 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 4.
- A in the table indicates that the compound has an IC 50 ⁇ 15 nM for EML4-ALK activity;
- B indicates 15 ⁇ IC 50 ⁇ 50 nM;
- C indicates 50 ⁇ IC 50 ⁇ 500 nM;
- D indicates an IC 50 > 500 nM.
- A in the table indicates that the compound has an IC 50 ⁇ 5 nM for ALK G1202R, ALK C1156Y, ALK R1275Q, ALK F1174L, ALK L1196M and ALK G1269A;
- B means 5 ⁇ IC 50 ⁇ 50 nM;
- C means 50 ⁇ IC 50 ⁇ 500 nM;
- D indicates an IC 50 > 500 nM.
- TRKA TRKB TRKC Compound TRKA TRKB TRKC I-1 B B B I-19 B A B I-2 C C C I-20 A A A I-3 B B B I-21 A A A I-4 A A I-22 B B I-5 A A B I-23 B A B
- A in the table indicates that the compound has an activity IC 50 ⁇ 0.25 nM for TRKA, TRKB and TRKC;
- B indicates 0.25 ⁇ IC 50 ⁇ 10 nM;
- C indicates 10 ⁇ IC 50 ⁇ 500 nM;
- D indicates IC 50 > 500nM.
- A in the table indicates that the compound has an IC 50 ⁇ 10 nM for ROS1 activity;
- B indicates 10 ⁇ IC 50 ⁇ 50 nM;
- C indicates 50 ⁇ IC 50 ⁇ 500 nM;
- D indicates an IC 50 > 500 nM.
- NCI-H2228 cells in good exponential growth phase were taken, washed once with 3 mL of PBS, and 2 mL of trypsin was added. Digestion is carried out in a cell culture incubator, and it is observed under a microscope from time to time. When the cells have just fallen off, 3 mL of complete medium is added to terminate the digestion. Low speed bench top centrifuge, 1500 rpm, centrifuged for 3 min. The cell digest was drained, and the cells were resuspended by pipetting with 5 mL of plate medium (RPMI medium + sodium pyruvate + 5% FBS).
- plate medium RPMI medium + sodium pyruvate + 5% FBS
- the cells were counted using a cytometer, diluted with a seed plate medium, and the cell density was adjusted to 6 ⁇ 10 4 cells/mL.
- the cells were seeded in a 96-well plate using a lance, 100 ⁇ L/well, and cultured in a constant temperature CO 2 incubator for 24 hours.
- the compound was added using a nanoliter sampler. After 72 hours, CCK-8 was added, 10 ⁇ L/well. After 4 hours, the absorbance was measured at 450 nm from the Envision plate reader, and the inhibition rate was calculated.
- the inhibition rate (%) (negative control group average) Value—average of experimental group)/(average of negative control group—average of blank group) ⁇ 100%, with logarithm of compound concentration as abscissa, inhibition rate as ordinate, four-parameter analysis, fitting dose-effect curve, calculation IC 50 , the results are shown in Table 5-1.
- EML4-ALK L1196M BaF3 cells Take a bottle of EML4-ALK L1196M BaF3 cells in good exponential growth phase, collect the cells into a centrifuge tube, centrifuge at 1000 rpm, centrifuge for 3 min, discard the supernatant, and use plate medium (RPMI medium + 10% FBS) Suspended cells. The cells were counted using a cytometer, diluted with a seed plate medium, and the cell density was adjusted to 1 ⁇ 10 4 /mL. The cells were seeded in a 96-well plate using a lance, 100 ⁇ L/well, and cultured in a cell incubator at 37 ° C, 5% CO 2 saturated humidity. After 24 hours of culture, compound loading was performed using a nanoliter sampler.
- plate medium RPMI medium + 10% FBS
- TFG-NTRK1 BaF3 cells Take a bottle of TFG-NTRK1 BaF3 cells in good exponential growth phase, collect the cells into a centrifuge tube, centrifuge at 1000 rpm, centrifuge for 3 min, discard the supernatant, and resuspend with plate medium (RPMI medium + 10% FBS). cell.
- the cells were counted using a cytometer, diluted with a seed plate medium, and the cell density was adjusted to 5 ⁇ 10 4 /mL.
- the cells were seeded in a 96-well plate using a lance, 100 ⁇ L/well, and cultured in a cell incubator at 37 ° C, 5% CO 2 saturated humidity. After 24 hours of culture, compound loading was performed using a nanoliter sampler.
- A in the table indicates that the compound has an IC 50 ⁇ 0.5 nM against TFG-NTRK1 BaF3 activity;
- B indicates 0.5 ⁇ IC 50 ⁇ 1 nM;
- C indicates 1 ⁇ IC 50 ⁇ 5 nM.
- 300 ⁇ L of the final incubation system contained 30 ⁇ L of liver microsomes (protein concentration: 0.15 mg/mL), 30 ⁇ L of NADPH + MgCl 2 , 3 ⁇ L of test compound, and 237 ⁇ L of PBS buffer (pH 7.4).
- the ratio of the organic solvent (acetonitrile) was 1%. 2 copies of each species, 0.3 mL per serving.
- Each tube was first prepared with a total volume of 270 ⁇ L of substrate and enzyme mixture, and NADPH was preincubated at 37 ° C for 5 min, then 30 ⁇ L of NADPH + MgCl 2 was added and mixed at 0 min, 15 min, 30 min, 60 min.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
A diaryl macrocyclic compound as a protein kinase modulator. Specifically, the present invention relates to a compound in formula I or a pharmaceutically acceptable salt thereof, a preparation method therefor and a pharmaceutical composition containing the compound, and use of the compound in treatment of cancers, pains, neurologic diseases, autoimmune diseases, and inflammations.
Description
相关申请的交叉引用Cross-reference to related applications
本申请要求于2018年5月4日向中国国家知识产权局提交的第201810421414.X号中国专利申请的优先权和权益及2019年3月15日向中国国家知识产权局提交的第201910198329.6号中国专利申请的优先权和权益,所述申请公开的内容通过引用整体并入本文中。This application claims the priority and benefit of Chinese Patent Application No. 201810421414.X filed with the Chinese State Intellectual Property Office on May 4, 2018, and Chinese Patent Application No. 201910198329.6 submitted to the State Intellectual Property Office of China on March 15, 2019. The priority and benefits of the disclosure are hereby incorporated by reference in its entirety.
本申请涉及作为蛋白激酶调节剂的二芳基大环化合物、其制备方法、含有该化合物的药物组合物,以及其在治疗癌症、疼痛、神经疾病、自体免疫疾病及炎症中的用途。The present application relates to a diaryl macrocyclic compound as a protein kinase modulator, a process for the preparation thereof, a pharmaceutical composition containing the same, and use thereof in the treatment of cancer, pain, neurological diseases, autoimmune diseases and inflammation.
蛋白激酶是细胞生长、增殖和存活的关键调控因子,作用于特定的蛋白质,并改变其活性。这些激酶在细胞的信号传导及其复杂的生命活动中起到了广泛作用。遗传和表观遗传改变蓄积在癌细胞中,导致信号转导途径的异常激活从而驱动了恶化过程。这些信号通路的药理学抑制为靶向治疗癌症提供了希望。Protein kinases are key regulators of cell growth, proliferation, and survival, acting on specific proteins and altering their activity. These kinases play a wide-ranging role in cell signaling and its complex life activities. Genetic and epigenetic changes accumulate in cancer cells, leading to abnormal activation of the signal transduction pathway leading to the process of deterioration. Pharmacological inhibition of these signaling pathways provides a promise for targeted cancer therapy.
ALK(Anaplasticlymphoma kinase)是在间变性大细胞淋巴瘤(ALCL)的一个亚型中被发现的。在非小细胞肺癌(NSCLC)、弥漫性大B细胞淋巴瘤和炎症性肌纤维母细胞瘤(IMT)中分别发现了有多种类型的ALK基因重排,至此证明ALK是强力致癌驱动基因。ALK与白细胞酪氨酸激酶(LTK)是受体酪氨酸激酶的胰岛素受体(IR)超家族,能与多种基因发生融合,如NPM-ALK、TPM3-ALK、TFG-ALK、ATIC-ALK、CLTC-ALK等。ALK主要在中枢和外周神经系统中表达,在神经系统的正常发育和功能中具有潜在作用。在许多癌症中已经发现了20多种不同的ALK易位配偶体。ALK (Anaplasticlymphoma kinase) is found in a subtype of anaplastic large cell lymphoma (ALCL). Multiple types of ALK gene rearrangements have been found in non-small cell lung cancer (NSCLC), diffuse large B-cell lymphoma, and inflammatory myofibroblastic tumor (IMT), respectively, and ALK has been shown to be a potent carcinogenic driver. ALK and leukocyte tyrosine kinase (LTK) are the insulin receptor (IR) superfamily of receptor tyrosine kinases that can be fused to a variety of genes, such as NPM-ALK, TPM3-ALK, TFG-ALK, ATIC- ALK, CLTC-ALK, etc. ALK is primarily expressed in the central and peripheral nervous systems and has a potential role in the normal development and function of the nervous system. More than 20 different ALK translocation partners have been discovered in many cancers.
EML4-ALK融合基因可见于多种肿瘤,例如间变性大细胞淋巴瘤、炎性成肌纤维细胞瘤、成神经细胞瘤和NSCLC等,它是由第2号染色体短臂插入引起。EML4基因主要维持细胞的基本形态,而ALK基因能够激活和促进细胞增殖,EML4基因的5’端与ALK基因的3’端易位融合,根据EML4基因断裂点的不同,EML4-ALK融合基因至少有10种。EML4-ALK融合基因通过下游底物分子的激活、传递,各转导途径的相互交叉、重合,形成了一个错综复杂的信号转导网络,影响细胞增殖、分化和凋亡。The EML4-ALK fusion gene can be found in a variety of tumors, such as anaplastic large cell lymphoma, inflammatory myofibroblastoma, neuroblastoma, and NSCLC, which are caused by short arm insertion of chromosome 2. The EML4 gene mainly maintains the basic morphology of the cell, while the ALK gene can activate and promote cell proliferation. The 5' end of the EML4 gene is translocated with the 3' end of the ALK gene. According to the EML4 gene breakpoint, the EML4-ALK fusion gene is at least There are 10 kinds. The EML4-ALK fusion gene forms an intricate signal transduction network, which affects cell proliferation, differentiation and apoptosis through the activation and transmission of downstream substrate molecules, and the transduction and overlapping of each transduction pathway.
目前,ALK小分子激酶抑制剂有Crizotinib、Lorlatinib等。ALK激酶抑制剂在ALK异常基因肺癌患者的治疗中取得了巨大的成功。但是,耐药性的出现限制了其长期的临床应用。耐药机制通常包括靶基因扩增、获得性耐药突变、旁路信号传导、上皮-间质转化(EMT)和转移。Currently, ALK small molecule kinase inhibitors include Crizotinib, Lorlatinib and the like. ALK kinase inhibitors have achieved great success in the treatment of patients with ALK abnormal gene lung cancer. However, the emergence of drug resistance limits its long-term clinical application. Drug resistance mechanisms typically include target gene amplification, acquired resistance mutations, bypass signaling, epithelial-mesenchymal transition (EMT), and metastasis.
原肌球蛋白相关受体酪氨酸激酶(Trks),包括由NTRK1/2/3基因编码的TrkA/B/C,是神经营养因子(NTs)的高亲和力受体。Trk家族成员在神经起源细胞中高度表达Trks(TrkA、TrkB和TrkC),通过其优先的神经营养因子(NGF至TrkA,脑源性神经营养因子(BDNF)和NT4/5至TrkB以及NT3至TrkC)介导神经元在发育过程中的存活和分化。NT/Trk信号通路起到内源性系统的作用,在生物化学损伤,短暂性缺血或物理损伤后保护神经元。然而,Trk最初是作为与原肌球蛋白基因融合的癌基因在细胞外结构域中克隆的。已经在甲状腺乳头状和甲状腺癌中以及最近在NSCLC中鉴定了由染色体重排或NTRK1(TrkA)中的突变引起的激活突变。因为Trk在疼痛感觉以及肿瘤细胞生长和存活信号中起重要作用,所以Trk受体激酶抑制剂被认为在治疗疼痛和癌症方面有着巨大的潜力。目前Trk小分子激酶抑制剂有Larotrectinib及LOXO-195等。The tropomyosin-related receptor tyrosine kinase (Trks), including TrkA/B/C encoded by the NTRK1/2/3 gene, is a high-affinity receptor for neurotrophic factors (NTs). Trk family members highly express Trks (TrkA, TrkB and TrkC) in neurogenic cells through their preferential neurotrophic factors (NGF to TrkA, brain-derived neurotrophic factor (BDNF) and NT4/5 to TrkB and NT3 to TrkC) It mediates the survival and differentiation of neurons during development. The NT/Trk signaling pathway acts as an endogenous system to protect neurons after biochemical damage, transient ischemia or physical injury. However, Trk was originally cloned in the extracellular domain as an oncogene fused to the tropomyosin gene. Activating mutations caused by chromosomal rearrangements or mutations in NTRK1 (TrkA) have been identified in thyroid papillary and thyroid cancer and more recently in NSCLC. Because Trk plays an important role in pain perception and tumor cell growth and survival signals, Trk receptor kinase inhibitors are thought to have great potential for the treatment of pain and cancer. At present, Trk small molecule kinase inhibitors include Larotrectinib and LOXO-195.
已经在许多实体恶性肿瘤中发现了NTRK1、NTRK2和NTRK3的致癌重排。与ALK和ROS1抑制剂治疗类似,前突变TrkA G595R和TrkC G623R(均与ALK G1202R类似)已经在Larotrectinib等难治性患者的临床中报道。针对野生型和突变Trks的新一代Trk抑制剂对于有效治疗携带融合Trk的患者非常有必要。Carcinogenic rearrangements of NTRK1, NTRK2 and NTRK3 have been found in many solid malignancies. Similar to the treatment of ALK and ROS1 inhibitors, the pre-mutant TrkA G595R and TrkC G623R (both similar to ALK G1202R) have been reported in the clinical trials of refractory patients such as Larotrectinib. A new generation of Trk inhibitors against wild-type and mutant Trks is essential for the effective treatment of patients with fusion Trk.
ROS1激酶是具有未知配体的受体酪氨酸激酶,ROS1基因融合生存的嵌合蛋白具有较强的增殖活性,已经报道ROS1激酶经历遗传重排以在多种人类癌症中产生组成型活性融合蛋白,所述癌症包括成胶质细胞瘤、NSCLC胆管癌、卵巢癌、胃腺癌、结肠直肠癌、炎性成肌纤维细胞瘤、血管肉瘤和上皮样血管内皮瘤。Crizotinib在ROS1融合突变呈阳性的NSCLC患者中间表现出比在ALK阳性的患者几乎高一倍的中位PFS,达到18.3个月,ORR为66%。但是在Crizotinib治疗患者中观察到获得性耐药突变,因此迫切 需要开发用于克服Crizotinib ROS1抗性的第二代ROS1抑制剂。ROS1 kinase is a receptor tyrosine kinase with an unknown ligand. The chimeric protein of ROS1 gene fusion has strong proliferative activity. ROS1 kinase has been reported to undergo genetic rearrangement to produce constitutively active fusion in various human cancers. Proteins, including glioblastoma, NSCLC cholangiocarcinoma, ovarian cancer, gastric adenocarcinoma, colorectal cancer, inflammatory myofibroblastic tumor, angiosarcoma, and epithelioid hemangioendothelioma. Crizotinib showed a median PFS nearly double that of ALK-positive patients in NSCLC patients positive for ROS1 fusion mutations, reaching 18.3 months with an ORR of 66%. However, acquired resistance mutations have been observed in patients treated with Crizotinib, so there is an urgent need to develop second-generation ROS1 inhibitors that overcome the resistance of Crizotinib ROS1.
目前,临床需要更多具有适当的药理学特征的多蛋白或酪氨酸激酶靶标的小分子抑制剂,以及需要其具有药效、选择性、药代动力学、穿透血脑屏障的能力和持续作用时间方面的优势。具体而言,需要抑制具有ALK,TrkA/B/C和ROS1等耐药突变的小分子抑制剂。本发明合成了一系列作为蛋白激酶调节剂的二芳基大环化合物。Currently, there is a need for more small molecule inhibitors of polyprotein or tyrosine kinase targets with appropriate pharmacological characteristics, as well as their ability to be pharmacologically selective, pharmacokinetic, and penetrating the blood-brain barrier. The advantage of continuous action time. Specifically, it is desirable to inhibit small molecule inhibitors having resistant mutations such as ALK, TrkA/B/C and ROS1. The present invention synthesizes a series of diaryl macrocycles as protein kinase modulators.
发明详述Detailed description of the invention
本申请涉及式I化合物或其药学上可接受的盐,The present application relates to a compound of formula I or a pharmaceutically acceptable salt thereof,
其中,among them,
X选自O或NR
3;
X is selected from O or NR 3 ;
R
1选自氢、C
1-6烷基、C
2-6烯基、C
2-6炔基、C
3-6环烷基或6-10元芳基,所述C
1-6烷基、C
2-6烯基、C
2-6炔基、C
3-6环烷基或6-10元芳基任选地被以下基团取代:卤素、羟基、氰基、-OC
1-6烷基、-NH
2、-NH(C
1-6烷基)、-N(C
1-6烷基)
2、-NHC(O)C
1-6烷基、-NHC(O)NH
2、-CO
2H、-C(O)OC
1-6烷基、-C(O)NH
2、-C(O)NH(C
1-6烷基)、-C(O)N(C
1-6烷基)
2、-SH、-SC
1-6烷基、-S(O)C
1-6烷基、-S(O)
2C
1-6烷基、-S(O)NH(C
1-6烷基)、-S(O)
2NH(C
1-6烷基)、-S(O)N(C
1-6烷基)
2或-S(O)
2N(C
1-6烷基)
2;
R 1 is selected from hydrogen, C 1-6 alkyl, C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl, said C 1-6 alkyl C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl is optionally substituted by halogen, hydroxy, cyano, -OC 1-6 Alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -NHC(O)C 1-6 alkyl, -NHC(O)NH 2 , -CO 2 H, -C(O)OC 1-6 alkyl, -C(O)NH 2 , -C(O)NH(C 1-6 alkyl), -C(O)N (C 1- 6 alkyl) 2 , -SH, -SC 1-6 alkyl, -S(O)C 1-6 alkyl, -S(O) 2 C 1-6 alkyl, -S(O)NH(C 1-6 alkyl), -S(O) 2 NH(C 1-6 alkyl), -S(O)N(C 1-6 alkyl) 2 or -S(O) 2 N (C 1- 6 alkyl) 2 ;
R
3选自氢、C
1-6烷基、C
2-6烯基、C
2-6炔基、C
3-6环烷基、3-7元杂环烷基、6-10元芳基或6-10元杂芳基,所述C
1-6烷基、C
2-6烯基、C
2-6炔基、C
3-6环烷基、3-7元杂环烷基、6-10元芳基或6-10元杂芳基任选地被以下基团取代:卤素或-OC
1-6烷基;或者,
R 3 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl, 3-7 membered heterocycloalkyl, 6-10 membered aryl Or a 6-10 membered heteroaryl group, said C 1-6 alkyl group, C 2-6 alkenyl group, C 2-6 alkynyl group, C 3-6 cycloalkyl group, 3-7 membered heterocycloalkyl group, 6 a -10 membered aryl or a 6-10 membered heteroaryl group is optionally substituted with a halogen or -OC 1-6 alkyl group; or
R
1与R
3及其所连接的原子共同形成3-6元杂环烷基,所述3-6元杂环烷基任选地被以下基团取代:卤素、C
1-6烷基、羟基、氰基、-OC
1-6烷基、-NH
2、-NH(C
1-6烷基)、-N(C
1-6烷基)
2、-SH或-SC
1-6烷基;
R 1 and R 3 together with the atom to which they are attached form a 3-6 membered heterocycloalkyl group, which is optionally substituted by a halogen, C 1-6 alkyl group, Hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl ;
T
1、T
2、T
3或T
4独立地选自CR
b或N;
T 1 , T 2 , T 3 or T 4 are independently selected from CR b or N;
R
b独立地选自氢、卤素、C
1-6烷基、羟基、氰基、-OC
1-6烷基、-NH
2、-NHC
1-6烷基、-N(C
1-6烷基)
2或-CF
3;
R b is independently selected from the group consisting of hydrogen, halogen, C 1-6 alkyl, hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NHC 1-6 alkyl, -N(C 1-6 alkane Base) 2 or -CF 3 ;
L
1选自-O-、-NR
a-、C
1-6亚烷基、-OC
1-6亚烷基-或-C
1-6亚烷基O-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-6烷基、-NH
2、-NH(C
1-6烷基)、-N(C
1-6烷基)
2、-SH或-SC
1-6烷基;
L 1 is selected from -O-, -NR a -, C 1-6 alkylene, -OC 1-6 alkylene- or -C 1-6 alkylene O-, said alkylene optionally Substituted by: halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH Or -SC 1-6 alkyl;
L
2选自-NHC
1-6亚烷基-、-C
1-6亚烷基NH-、-C
1-6亚烷基-NR
aCO-、-NR
aC(O)-或-C(O)N(R
a)-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-6烷基、-NH
2、-NH(C
1-6烷基)、-N(C
1-6烷基)
2、-SH或-SC
1-6烷基;
L 2 is selected from -NHC 1-6 alkylene-, -C 1-6 alkylene NH-, -C 1-6 alkylene-NR a CO-, -NR a C(O)- or -C (O)N(R a )-, the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 Alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl;
R
a独立地选自氢或C
1-6烷基;
R a is independently selected from hydrogen or C 1-6 alkyl;
m或n独立地选自1、2或3;m or n is independently selected from 1, 2 or 3;
q选自0-4;q is selected from 0-4;
R
2独立地选自卤素,氰基,羟基,任选被卤素或羟基取代的C
1-6烷基、-OC
1-6烷基、-NH
2、-NHC
1-6 烷基、-N(C
1-6烷基)
2。
R 2 is independently selected from halogen, cyano, hydroxy, C 1-6 alkyl optionally substituted by halogen or hydroxy, -OC 1-6 alkyl, -NH 2 , -NHC 1-6 alkyl, -N (C 1-6 alkyl) 2 .
在一些实施方案中,上述X选自NR
3。
In some embodiments, the X is selected from NR 3.
在一些实施方案中,R
1选自氢、C
1-3烷基、C
2-3烯基、C
2-3炔基、C
3-6环烷基或6-10元芳基,所述C
1-3烷基、C
2-3烯基、C
2-3炔基、C
3-6环烷基或6-10元芳基任选地被以下基团取代:卤素、羟基、氰基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-NHC(O)C
1-3烷基、-NHC(O)NH
2、-CO
2H、-C(O)OC
1-3烷基、-C(O)NH
2、-C(O)NH(C
1-3烷基)、-C(O)N(C
1-3烷基)
2、-SH、-SC
1-3烷基、-S(O)C
1-3烷基、-S(O)
2C
1-3烷基、-S(O)NH(C
1-3烷基)、-S(O)
2NH(C
1-3烷基)、-S(O)N(C
1-3烷基)
2或-S(O)
2N(C
1-3烷基)
2。在一些实施方案中,R
1选自氢、C
1-3烷基、C
2-3烯基或C
2-3炔基,所述C
1-3烷基、C
2-3烯基或C
2-3炔基任选地被以下基团取代:卤素、羟基、氰基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-NHC(O)C
1-3烷基、-NHC(O)NH
2、-CO
2H、-C(O)OC
1-3烷基、-C(O)NH
2、-C(O)NH(C
1-3烷基)、-C(O)N(C
1-3烷基)
2、-SH、-SC
1-3烷基、-S(O)C
1-3烷基、-S(O)
2C
1-3烷基、-S(O)NH(C
1-3烷基)、-S(O)
2NH(C
1-3烷基)、-S(O)N(C
1-3烷基)
2或-S(O)
2N(C
1-3烷基)
2。在一些实施方案中,R
1选自氢或任选地被氟、氯、溴、羟基、氰基或-NH
2取代的C
1-3烷基。在一些实施方案中,R
1选自任选地被氟取代的甲基。
In some embodiments, R 1 is selected from the group consisting of hydrogen, C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl, or 6-10 membered aryl, C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl is optionally substituted by halogen, hydroxy, cyano , -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -NHC(O)C 1-3 alkyl, -NHC (O)NH 2 , -CO 2 H, -C(O)OC 1-3 alkyl, -C(O)NH 2 , -C(O)NH(C 1-3 alkyl), -C(O N(C 1-3 alkyl) 2 , -SH, -SC 1-3 alkyl, -S(O)C 1-3 alkyl, -S(O) 2 C 1-3 alkyl, -S (O) NH(C 1-3 alkyl), -S(O) 2 NH(C 1-3 alkyl), -S(O)N(C 1-3 alkyl) 2 or -S(O) 2 N(C 1-3 alkyl) 2 . In some embodiments, R 1 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl, or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl is optionally substituted by halogen, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1- 3 alkyl) 2 , -NHC(O)C 1-3 alkyl, -NHC(O)NH 2 , -CO 2 H, -C(O)OC 1-3 alkyl, -C(O)NH 2 , -C(O)NH(C 1-3 alkyl), -C(O)N(C 1-3 alkyl) 2 , -SH, -SC 1-3 alkyl, -S(O)C 1 -3 alkyl, -S(O) 2 C 1-3 alkyl, -S(O)NH(C 1-3 alkyl), -S(O) 2 NH(C 1-3 alkyl), - S(O)N(C 1-3 alkyl) 2 or -S(O) 2 N(C 1-3 alkyl) 2 . In some embodiments, R 1 is selected from hydrogen or C 1-3 alkyl optionally substituted with fluorine, chlorine, bromine, hydroxy, cyano or -NH 2 . In some embodiments, R 1 is selected from methyl optionally substituted with fluorine.
在一些实施方案中,R
3选自氢、C
1-3烷基、C
2-3烯基、C
2-3炔基、C
3-6环烷基、3-6元杂环烷基、6-10元芳基或6-10元杂芳基,所述C
1-3烷基、C
2-3烯基、C
2-3炔基、C
3-6环烷基、3-6元杂环烷基、6-10元芳基或6-10元杂芳基任选地被以下基团取代:卤素或-OC
1-3烷基。在一些实施方案中,R
3选自氢、C
1-3烷基、C
2-3烯基或C
2-3炔基,所述C
1-3烷基、C
2-3烯基或C
2-3炔基任选地被以下基团取代:卤素或-OC
1-3烷基。在一些实施方案中,R
3选自氢或任选地被氟、氯或溴取代的C
1-3烷基。在一些实施方案中,R
3选自氢。
In some embodiments, R 3 is selected from the group consisting of hydrogen, C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl, 3-6 membered heterocycloalkyl, 6-10 membered aryl or 6-10 membered heteroaryl, said C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl, 3-6 Heterocycloalkyl, 6-10 membered aryl or 6-10 membered heteroaryl is optionally substituted by halogen or -OC 1-3 alkyl. In some embodiments, R 3 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl, or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C The 2-3 alkynyl group is optionally substituted with a halogen or -OC 1-3 alkyl group. In some embodiments, R 3 is selected from hydrogen or C 1-3 alkyl optionally substituted with fluorine, chlorine or bromine. In some embodiments, R 3 is selected from hydrogen.
在一些实施方案中,R
1与R
3及其所连接的原子共同形成4-5元杂环烷基,所述4-5元杂环烷基任选地被以下基团取代:卤素、C
1-6烷基、羟基、氰基、-OC
1-6烷基、-NH
2、-NH(C
1-6烷基)、-N(C
1-6烷基)
2、-SH或-SC
1-6烷基。在一些实施方案中,R
1与R
3及其所连接的原子共同形成5元杂环烷基,所述5元杂环烷基任选地被以下基团取代:卤素、C
1-3烷基、羟基、氰基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基。在一些实施方案中,R
1与R
3及其所连接的原子共同形成四氢吡咯基,所述四氢吡咯基任选地被以下基团取代:氟、氯、溴、羟基、氰基或-NH
2。在一些实施方案中,R
1与R
3及其所连接的原子共同形成四氢吡咯基,所述四氢吡咯基任选地被氟取代。
In some embodiments, R 1 and R 3 and the atoms to which they are attached form a 4-5 membered heterocycloalkyl, which is optionally substituted with the following: halogen, C 1-6 alkyl, hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or - SC 1-6 alkyl. In some embodiments, R 1 and R 3 and the atoms to which they are attached form a 5-membered heterocycloalkyl group, which is optionally substituted with a halogen, C 1-3 alkane Base, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl. In some embodiments, R 1 and R 3 and the atom to which they are attached, together form a tetrahydropyrrolyl group, which is optionally substituted with a fluoro, chloro, bromo, hydroxy, cyano or -NH 2 . In some embodiments, R 1 and R 3 and the atoms to which they are attached form a tetrahydropyrrole group, which is optionally substituted with fluorine.
在一些实施方案中,R
b独立地选自氢、卤素、C
1-3烷基、羟基、氰基、-OC
1-3烷基、-NH
2、-NHC
1-3烷基、-N(C
1-3烷基)
2或-CF
3。在一些实施方案中,R
b独立地选自氢、卤素、羟基、氰基、-NH
2或-CF
3。在一些实施方案中,R
b独立地选自氢、氟、氯或溴。在一些实施方案中,R
b独立地选自氢或氟。
In some embodiments, R b is independently selected from the group consisting of hydrogen, halogen, C 1-3 alkyl, hydroxy, cyano, —OC 1-3 alkyl, —NH 2 , —NHC 1-3 alkyl, —N (C 1-3 alkyl) 2 or -CF 3 . In some embodiments, R b is independently selected from hydrogen, halo, hydroxy, cyano, -NH 2 or -CF 3 . In some embodiments, R b is independently selected from hydrogen, fluoro, chloro or bromo. In some embodiments, R b is independently selected from hydrogen or fluoro.
在一些实施方案中,T
1选自CH、N或CF。
In some embodiments, T 1 is selected from CH, N or CF.
在一些实施方案中,T
1或T
4分别独立地选自CH或N。
In some embodiments, T 1 or T 4 are each independently selected from CH or N.
在一些实施方案中,T
2选自CH或N。
In some embodiments, T 2 is selected from CH or N.
在一些实施方案中,T
3选自CF或N。
In some embodiments, T 3 is selected from CF or N.
在一些实施方案中,L
1选自-O-、-NR
a-、C
1-3亚烷基、-OC
1-3亚烷基-或-C
1-3亚烷基O-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基。在一些实施方案中,L
1选自-O-、-NR
a-、C
1-3亚烷基、-OC
1-3亚烷基-或-C
1-3亚烷基O-,所述亚烷基任选地被卤素取代。在一些实施方案中,L
1选自-O-、-NH-、-CH
2CH
2-、-OCH
2-或-CH
2O-,所述-CH
2CH
2-、-OCH
2-或-CH
2O-任选地被F取代。在一些实施方案中,L
1选自-O-、-NH-、-CH
2CH
2-、-OCH
2-、-OCF
2-、-CH
2O-或-CF
2O-。
In some embodiments, L 1 is selected from the group consisting of -O-, -NR a -, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-, The alkylene group is optionally substituted with a halogen, a cyano group, a hydroxyl group, a -OC 1-3 alkyl group, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkane Base) 2 , -SH or -SC 1-3 alkyl. In some embodiments, L 1 is selected from the group consisting of -O-, -NR a -, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-, The alkylene group is optionally substituted by a halogen. In some embodiments, L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O- is optionally substituted by F. In some embodiments, L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 -, -OCF 2 -, -CH 2 O-, or -CF 2 O-.
在一些实施方案中,L
1选自-O-、C
1-6亚烷基、-OC
1-6亚烷基-或-C
1-6亚烷基O-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-6烷基、-NH
2、-NH(C
1-6烷基)、-N(C
1-6烷基)
2、-SH或-SC
1-6烷基。在一些实施方案中,L
1选自-O-、C
1-3亚烷基、-OC
1-3亚烷基-或-C
1-3亚烷基O-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基。在一些实施方案中,L
1选自-O-、-CH
2CH
2-、-OCH
2-或-CH
2O-,所述-CH
2CH
2-、-OCH
2-或-CH
2O-任选地被F取代。在一些实施方案中,L
1选自-O-、-CH
2CH
2-、-OCH
2-、-OCF
2-、-CH
2O-或-CF
2O-。
In some embodiments, L 1 is selected from -O-, C 1-6 alkylene, -OC 1-6 alkylene- or -C 1-6 alkylene O-, said alkylene optionally The ground is replaced by the following groups: halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 ,- SH or -SC 1-6 alkyl. In some embodiments, L 1 is selected from -O-, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-, the alkylene optionally The ground is replaced by the following groups: halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 ,- SH or -SC 1-3 alkyl. In some embodiments, L 1 is selected from -O-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O - optionally replaced by F. In some embodiments, L 1 is selected from the group consisting of -O-, -CH 2 CH 2 -, -OCH 2 -, -OCF 2 -, -CH 2 O-, or -CF 2 O-.
在一些实施方案中,L
2选自-NHC
1-3亚烷基-、-C
1-3亚烷基NH-、-C
1-3亚烷基-NR
aCO-、-NR
aC(O)-或-C(O)N(R
a)-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基。在一些实施方案中,L
2选自-NHCH
2-、-CH
2NH-、-CH
2-NR
aCO-、-NR
aC(O)-或-C(O)N(R
a)-,所述-NHCH
2-、-CH
2NH-或-CH
2-NR
aCO-中的亚甲基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基。在一些实施方案中,L
2选自-NHCH
2-、-CH
2NH-、-CH
2-NR
aCO-、-NR
aC(O)-或-C(O)N(R
a)-。
In some embodiments, L 2 is selected from the group consisting of -NHC 1-3 alkylene-, -C 1-3 alkylene NH-, -C 1-3 alkylene-NR a CO-, -NR a C ( O)- or -C(O)N(R a )-, the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , - NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl. In some embodiments, L 2 is selected from the group consisting of -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)-, or -C(O)N(R a )- The methylene group in the -NHCH 2 -, -CH 2 NH- or -CH 2 -NR a CO- is optionally substituted with a halogen, a cyano group, a hydroxyl group, a -OC 1-3 alkyl group. , -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl. In some embodiments, L 2 is selected from the group consisting of -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)-, or -C(O)N(R a )- .
在一些实施方案中,L
2选自-NHC
1-6亚烷基-、-C
1-6亚烷基NH-、-NR
aC(O)-或-C(O)N(R
a)-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-6烷基、-NH
2、-NH(C
1-6烷基)、-N(C
1-6烷基)
2、-SH或-SC
1-6烷基。在一些实施方案中,L
2选自-NHC
1-3亚烷基-、-C
1-3亚烷基NH-、-NR
aC(O)-或-C(O)N(R
a)-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基。在一些实施方案中,L
2选自-NHCH
2-、-CH
2NH-、-NR
aC(O)-或-C(O)N(R
a)-,所述-NHCH
2-或-CH
2NH-中的亚甲基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基。在一些实施方案中,L
2选自-NHCH
2-、-CH
2NH-、-NR
aC(O)-或-C(O)N(R
a)-。
In some embodiments, L 2 is selected from the group consisting of -NHC 1-6 alkylene-, -C 1-6 alkylene NH-, -NR a C(O)-, or -C(O)N(R a ) - the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl. In some embodiments, L 2 is selected from the group consisting of -NHC 1-3 alkylene-, -C 1-3 alkylene NH-, -NR a C(O)-, or -C(O)N(R a ) - the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl. In some embodiments, L 2 is selected from -NHCH 2 -, -CH 2 NH-, -NR a C(O)- or -C(O)N(R a )-, said -NHCH 2 - or - The methylene group in CH 2 NH- is optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N (C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl. In some embodiments, L 2 is selected from the group consisting of -NHCH 2 -, -CH 2 NH-, -NR a C(O)-, or -C(O)N(R a )-.
在一些实施方案中,R
a独立地选自氢或C
1-3烷基。在一些实施方案中,R
a独立地选自氢或甲基。
In some embodiments, R a is independently selected from hydrogen or C 1-3 alkyl. In some embodiments, R a is independently selected from hydrogen or methyl.
在一些实施方案中,m、n独立地选自1或2。在一些实施方案中,m选自1,n选自1或2。在一些实施方案中,m选自1,n选自1。In some embodiments, m, n are independently selected from 1 or 2. In some embodiments, m is selected from the group consisting of 1, and n is selected from 1 or 2. In some embodiments, m is selected from the group consisting of 1, and n is selected from the group consisting of 1.
在一些实施方案中,q选自0或1。在一些实施方案中q选自0。In some embodiments, q is selected from 0 or 1. In some embodiments q is selected from zero.
在一些实施方案中,R
2独立地选自卤素、氰基、羟基或任选被卤素或羟基取代的C
1-3烷基。在一些实施方案中,R
2独立地选自C
1-3烷基。在一些实施方案中,R
2独立地选自甲基。
In some embodiments, R 2 is independently selected from halo, cyano, hydroxy, or C 1-3 alkyl, optionally substituted by halogen or hydroxy. In some embodiments, R 2 is independently selected from C 1-3 alkyl. In some embodiments, R 2 is independently selected from methyl.
在一些实施方案中,结构单元
选自
在一些实施方案中,结构单元
选自
在一些实施方案中,结 构单元
选自
在一些实施方案中,结构单元
选自
In some embodiments, the structural unit Selected from In some embodiments, the structural unit Selected from In some embodiments, the structural unit Selected from In some embodiments, the structural unit Selected from
在一些实施方案中,本发明提供了本申请的式I化合物或其药学上可接受的盐,其中In some embodiments, the invention provides a compound of Formula I, or a pharmaceutically acceptable salt thereof, of the present application, wherein
X选自O或NR
3;
X is selected from O or NR 3 ;
R
1选自氢、C
1-3烷基、C
2-3烯基或C
2-3炔基,所述C
1-3烷基、C
2-3烯基或C
2-3炔基任选地被以下基团取代:卤素、羟基、氰基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-NHC(O)C
1-3烷基、-NHC(O)NH
2、-CO
2H、-C(O)OC
1-3烷基、-C(O)NH
2、-C(O)NH(C
1-3烷基)或-C(O)N(C
1-3烷基)
2;
R 1 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl The ground is replaced by the following groups: halogen, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -NHC(O)C 1-3 alkyl, -NHC(O)NH 2 , -CO 2 H, -C(O)OC 1-3 alkyl, -C(O)NH 2 , -C(O) NH(C 1-3 alkyl) or -C(O)N(C 1-3 alkyl) 2 ;
R
3选自氢、C
1-3烷基、C
2-3烯基或C
2-3炔基,所述C
1-3烷基、C
2-3烯基或C
2-3炔基任选地被以下基团取代:卤素或-OC
1-3烷基;或者,
R 3 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl The ground is replaced by the following group: halogen or -OC 1-3 alkyl; or,
R
1与R
3及其所连接的原子共同形成4-5元杂环烷基,所述4-5元杂环烷基任选地被以下基团取代:卤素、C
1-6烷基、羟基、氰基、-OC
1-6烷基、-NH
2、-NH(C
1-6烷基)、-N(C
1-6烷基)
2、-SH或-SC
1-6烷基;
R 1 and R 3 and the atom to which they are attached form a 4-5 membered heterocycloalkyl group, which is optionally substituted by a halogen, C 1-6 alkyl group, Hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl ;
T
1、T
2、T
3或T
4各自独立地选自CR
b或N;
T 1 , T 2 , T 3 or T 4 are each independently selected from CR b or N;
R
b独立地选自氢、卤素、羟基、氰基、-NH
2或-CF
3;
R b is independently selected from the group consisting of hydrogen, halogen, hydroxy, cyano, -NH 2 or -CF 3 ;
L
1选自-O-、-NR
a-、C
1-3亚烷基、-OC
1-3亚烷基-或-C
1-3亚烷基O-,所述亚烷基任选地被以下基团取代: 卤素、氰基、羟基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基;
L 1 is selected from -O-, -NR a -, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-, said alkylene optionally Substituted by: halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH Or -SC 1-3 alkyl;
L
2选自-NHCH
2-、-CH
2NH-、-CH
2-NR
aCO-、-NR
aC(O)-或-C(O)N(R
a)-,所述-NHCH
2-、-CH
2NH-或-CH
2-NR
aCO-中的亚甲基任选地被以下基团取代:卤素、氰基、羟基、-OC
1-3烷基、-NH
2、-NH(C
1-3烷基)、-N(C
1-3烷基)
2、-SH或-SC
1-3烷基;
L 2 is selected from -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)- or -C(O)N(R a )-, the -NHCH 2 The methylene group in -, -CH 2 NH- or -CH 2 -NR a CO- is optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , - NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl;
R
a独立地选自氢或C
1-3烷基;
R a is independently selected from hydrogen or C 1-3 alkyl;
m或n独立地选自1或2;m or n is independently selected from 1 or 2;
q选自0或1;q is selected from 0 or 1;
R
2独立地选自卤素、氰基、羟基或任选被卤素或羟基取代的C
1-3烷基。
R 2 is independently selected from halogen, cyano, hydroxy or C 1-3 alkyl optionally substituted by halogen or hydroxy.
在一些实施方案中,本发明提供了本申请的式I化合物或其药学上可接受的盐,其中In some embodiments, the invention provides a compound of Formula I, or a pharmaceutically acceptable salt thereof, of the present application, wherein
X选自O或NR
3;
X is selected from O or NR 3 ;
R
1选自氢、C
1-3烷基、C
2-3烯基或C
2-3炔基,所述C
1-3烷基、C
2-3烯基或C
2-3炔基任选地被以下基团取代:卤素、羟基、氰基或-NH
2;
R 1 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl The ground is replaced by the following groups: halogen, hydroxy, cyano or -NH 2 ;
R
3选自氢或任选地被卤素取代的C
1-3烷基;或者,
R 3 is selected from hydrogen or a C 1-3 alkyl group optionally substituted by halogen; or
R
1与R
3及其所连接的原子共同形成5元杂环烷基,所述5元杂环烷基任选地被以下基团取代:卤素、C
1-3烷基、羟基、氰基或-NH
2;
R 1 and R 3 together with the atom to which they are attached form a 5-membered heterocycloalkyl group which is optionally substituted by a halogen, a C 1-3 alkyl group, a hydroxyl group, a cyano group. Or -NH 2 ;
T
1、T
2、T
3或T
4各自独立地选自CH、N或CF;
T 1 , T 2 , T 3 or T 4 are each independently selected from CH, N or CF;
L
1选自-O-、-NH-、-CH
2CH
2-、-OCH
2-或-CH
2O-,所述-CH
2CH
2-、-OCH
2-或-CH
2O-任选地被卤素取代;
L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O- The ground is replaced by a halogen;
L
2选自-NHCH
2-、-CH
2NH-、-CH
2-NR
aCO-、-NR
aC(O)-或-C(O)N(R
a)-,所述-NHCH
2-、-CH
2NH-或-CH
2-NR
aCO-中的亚甲基任选地被卤素取代;
L 2 is selected from -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)- or -C(O)N(R a )-, the -NHCH 2 The methylene group in -, -CH 2 NH- or -CH 2 -NR a CO- is optionally substituted by halogen;
R
a独立地选自氢、甲基或乙基;
R a is independently selected from hydrogen, methyl or ethyl;
m选自1,n选自1或2;m is selected from 1, and n is selected from 1 or 2;
q选自0或1;q is selected from 0 or 1;
R
2独立地选自卤素或任选被卤素或羟基取代的C
1-3烷基。
R 2 is independently selected from halogen or C 1-3 alkyl optionally substituted by halogen or hydroxy.
在一些实施方案中,本发明提供了本申请的式I化合物或其药学上可接受的盐,其中In some embodiments, the invention provides a compound of Formula I, or a pharmaceutically acceptable salt thereof, of the present application, wherein
X选自O或NR
3;
X is selected from O or NR 3 ;
R
1选自氢或C
1-3烷基,所述C
1-3烷基任选地被氟取代;
R 1 is selected from hydrogen or C 1-3 alkyl, and the C 1-3 alkyl group is optionally substituted with fluorine;
R
3选自氢或C
1-3烷基;或者,
R 3 is selected from hydrogen or C 1-3 alkyl; or,
R
1与R
3及其所连接的原子共同形成任选地被以下基团取代的四氢吡咯基:氟或C
1-3烷基;
R 1 and R 3 together with the atom to which they are attached form a tetrahydropyrrolyl group optionally substituted by a fluorine or a C 1-3 alkyl group;
T
1、T
2、T
3或T
4各自独立地选自CH、N或CF;
T 1 , T 2 , T 3 or T 4 are each independently selected from CH, N or CF;
L
1选自-O-、-NH-、-CH
2CH
2-、-OCH
2-或-CH
2O-,所述-CH
2CH
2-、-OCH
2-或-CH
2O-任选地被氟取代;
L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O- The ground is replaced by fluorine;
L
2选自-NHCH
2-、-CH
2NH-、-CH
2-NR
aCO-、-NR
aC(O)-或-C(O)N(R
a)-;
L 2 is selected from -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)- or -C(O)N(R a )-;
R
a独立地选自氢、甲基或乙基;
R a is independently selected from hydrogen, methyl or ethyl;
m选自1,n选自1或2;m is selected from 1, and n is selected from 1 or 2;
q选自0或1;q is selected from 0 or 1;
R
2独立地选自C
1-3烷基。
R 2 is independently selected from C 1-3 alkyl.
在一些实施方案中,本申请的式I化合物或其药学上可接受的盐选自式II化合物或其药学上可接受的盐:In some embodiments, a compound of Formula I of the present application, or a pharmaceutically acceptable salt thereof, is selected from a compound of Formula II or a pharmaceutically acceptable salt thereof:
其中,among them,
X、R
1、T
1、T
2、T
3、T
4、L
1、m、n、q或R
2同式I化合物中的定义。
X, R 1 , T 1 , T 2 , T 3 , T 4 , L 1 , m, n, q or R 2 are as defined in the compound of formula I.
在一些实施方案中,本申请的式I化合物或其药学上可接受的盐选自式III或其药学上可接受的盐:In some embodiments, a compound of Formula I of the present application, or a pharmaceutically acceptable salt thereof, is selected from Formula III or a pharmaceutically acceptable salt thereof:
其中,among them,
环G、X、R
b、R
1、R
2、T
1、L
1、L
2、m、n或q同式I化合物中的定义。
The definition of the ring G, X, R b , R 1 , R 2 , T 1 , L 1 , L 2 , m, n or q as in the compound of the formula I.
在一些实施方案中,本申请的式I化合物或其药学上可接受的盐选自式IV化合物或其药学上可接受的盐:In some embodiments, a compound of Formula I of the present application, or a pharmaceutically acceptable salt thereof, is selected from a compound of Formula IV or a pharmaceutically acceptable salt thereof:
其中,among them,
环G、T
1、T
2、T
3、T
4、L
1、m、n、q或R
2同式I化合物中的定义。
The definition of the ring G, T 1 , T 2 , T 3 , T 4 , L 1 , m, n, q or R 2 is the same as in the compound of the formula I.
在一些实施方案中,本申请的式I化合物或其药学上可接受的盐选自以下化合物或其药学上可接受的盐:In some embodiments, a compound of Formula I of the present application, or a pharmaceutically acceptable salt thereof, is selected from the group consisting of: or a pharmaceutically acceptable salt thereof:
另一方面,本申请涉及药物组合物,其包含本申请的式I化合物或其药学上可接受的盐。在一些实施方案中,本申请的药物组合物还包括药学上可接受的辅料。In another aspect, the present application is directed to a pharmaceutical composition comprising a compound of formula I, or a pharmaceutically acceptable salt thereof, of the present application. In some embodiments, the pharmaceutical compositions of the present application also include pharmaceutically acceptable excipients.
另一方面,本申请描述了治疗或抑制哺乳动物中细胞增殖、细胞侵袭、转移、凋亡或血管生成相关疾病的方法,包括对需要该治疗的哺乳动物、优选人类,给予治疗有效量的式I化合物或其药学上可接受的盐、或其药物组合物。In another aspect, the application describes a method of treating or inhibiting cell proliferation, cell invasion, metastasis, apoptosis, or angiogenesis-related diseases in a mammal, comprising administering a therapeutically effective amount to a mammal, preferably a human, in need of such treatment. A compound or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof.
另一方面,本申请描述了式I化合物或其药学上可接受的盐、或其药物组合物在制备预防或者治疗哺乳动物中细胞增殖、细胞侵袭、转移、凋亡或血管生成相关疾病的药物中的用途。In another aspect, the application describes a compound of Formula I, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof, for use in the manufacture of a medicament for preventing or treating a cell proliferation, cell invasion, metastasis, apoptosis or angiogenesis-related disease in a mammal Use in.
另一方面,本申请描述了式I化合物或其药学上可接受的盐、或其药物组合物在预防或者治疗哺乳动物中细胞增殖、细胞侵袭、转移、凋亡或血管生成相关疾病中的用途。In another aspect, the application describes the use of a compound of Formula I, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof, for preventing or treating a cell proliferation, cell invasion, metastasis, apoptosis, or angiogenesis-related disease in a mammal .
另一方面,本申请描述了用于预防或者治疗哺乳动物中细胞增殖、细胞侵袭、转移、凋亡或血管生成相关疾病的式Ⅰ化合物或其药学上可接受的盐、或其药物组合物。In another aspect, the application features a compound of formula I, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof, for use in preventing or treating a cell proliferation, cell invasion, metastasis, apoptosis or angiogenesis-related disease in a mammal.
在一些实施方案中,所述细胞增殖、细胞侵袭、转移、凋亡或血管生成由ALK、ALK-EML-4融合蛋白、AXL、Aur B&C、突变体BCR-ABL、BLK、Eph6B、HPK、IRAK1&3、LCK、LTK、各种MEKKs、RON、ROS1、SLK、STK10、TIE1&2或TRKs1-3介导。In some embodiments, the cell proliferation, cell invasion, metastasis, apoptosis, or angiogenesis is by ALK, ALK-EML-4 fusion protein, AXL, Aur B&C, mutant BCR-ABL, BLK, Eph6B, HPK, IRAK1&3 , LCK, LTK, various MEKKs, RON, ROS1, SLK, STK10, TIE1&2 or TRKs1-3.
在另一实施方案中,所述细胞增殖、细胞侵袭、转移、凋亡或血管生成相关的疾病选自癌症、疼痛、神经疾病、自体免疫疾病及炎症等。In another embodiment, the cell proliferation, cell invasion, metastasis, apoptosis or angiogenesis-related diseases are selected from the group consisting of cancer, pain, neurological diseases, autoimmune diseases, and inflammation.
定义definition
除非另有说明,本申请中所用的下列术语具有下列含义。一个特定的术语在没有特别定义的情况下不应该被认为是不确定的或不清楚的,而应该按照本领域普通的含义去理解。当本文中出现商品名时,意在指代其对应的商品或其活性成分。Unless otherwise stated, the following terms as used in this application have the following meanings. A particular term should not be considered as undefined or unclear without a particular definition, and should be understood in the ordinary meaning of the art. When a trade name appears in this document, it is intended to refer to its corresponding commodity or its active ingredient.
术语“被取代”是指特定原子上的任意一个或多个氢原子被取代基取代,只要特定原子的价态是正常的并且取代后的化合物是稳定的。当取代基为氧代(即=O)时,意味着两个氢原子被取代,氧代不会发生在芳香基上。The term "substituted" means that any one or more hydrogen atoms on a particular atom are replaced by a substituent as long as the valence of the particular atom is normal and the substituted compound is stable. When the substituent is oxo (ie, =0), it means that two hydrogen atoms are substituted and the oxo does not occur on the aryl group.
术语“任选”或“任选地”是指随后描述的事件或情况可以发生或不发生,该描述包括发生所述事件或情况和不发生所述事件或情况。例如,乙基“任选”被卤素取代,指乙基可以是未被取代的(-CH
2CH
3)、单取代的(如-CH
2CH
2F)、多取代的(如-CHFCH
2F、-CH
2CHF
2等)或完全被取代的(-CF
2CF
3)。本领域技术人员可理解,对于包含一个或多个取代基的任何基团,不会引入任何在空间上不可能存在和/或不能合成的取代或取代模式。
The term "optional" or "optionally" means that the subsequently described event or circumstance may or may not occur, the description including the occurrence of the event or condition and the occurrence of the event or condition. For example, an ethyl group "optionally" substituted with halo, refers to an ethyl group may be unsubstituted (-CH 2 CH 3), monosubstituted (e.g., -CH 2 CH 2 F), polysubstituted (e.g., -CHFCH 2 F, -CH 2 CHF 2 , etc.) or completely substituted (-CF 2 CF 3 ). It will be understood by those skilled in the art that for any group containing one or more substituents, no substitution or substitution pattern that is sterically impossible to exist and/or which cannot be synthesized is introduced.
本文中的C
m-n,是该部分具有给定范围中的整数个碳原子。例如“C
1-6”是指该基团可具有1个碳原子、2个碳原子、3个碳原子、4个碳原子、5个碳原子或6个碳原子。
C mn herein is that the moiety has an integer number of carbon atoms in a given range. For example, " C1-6 " means that the group may have 1 carbon atom, 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms or 6 carbon atoms.
当任何变量(例如R)在化合物的组成或结构中出现一次以上时,其在每一种情况下的定义都是独立的。因此,例如,如果一个基团被2个R所取代,则每个R都有独立的选项。When any variable (eg, R) occurs more than once in the composition or structure of a compound, its definition in each case is independent. Thus, for example, if a group is replaced by 2 R, then each R has an independent option.
当一个连接基团的数量为0时,比如-(CH
2)
0-,表示该连接基团为键。
When the number of one linking group is 0, such as -(CH 2 ) 0 -, it means that the linking group is a bond.
当其中一个变量选自共价键时,表示其连接的两个基团直接相连,比如A-L-Z中L代表键时表示该结构实际上是A-Z。When one of the variables is selected from a covalent bond, it means that the two groups to which it is attached are directly linked. For example, when L represents a bond in A-L-Z, the structure is actually A-Z.
当一个取代基的键交叉连接到一个环上的两个原子时,这种取代基可以与这个环上的任意原子相键合。例如,结构单元
表示其可在环己基或者环己二烯上的任意一个位置发生取代。
When a bond of a substituent is cross-linked to two atoms on a ring, the substituent may be bonded to any atom on the ring. For example, a structural unit It is indicated that it can be substituted at any position on the cyclohexyl or cyclohexadiene.
对于结构单元
当L
1选自-OCH
2-,L
2选自-NR
aC(O)-,所述结构单元为
又如当L
1选自-CH
2O-,L
2选自-NHCH
2-,所述结构单元为
For structural units When L 1 is selected from -OCH 2 -, L 2 is selected from -NR a C(O)-, and the structural unit is In another example, when L 1 is selected from -CH 2 O-, and L 2 is selected from -NHCH 2 -, the structural unit is
术语“卤”或“卤素”是指氟、氯、溴和碘。The term "halo" or "halogen" refers to fluoro, chloro, bromo and iodo.
术语“羟基”指-OH基团。The term "hydroxy" refers to an -OH group.
术语“氰基”指-CN基团。The term "cyano" refers to a -CN group.
术语“氨基”指-NH
2基团。
The term "amino" means -NH 2 group.
术语“烷基”是指通式为C
nH
2n+1的烃基。该烷基可以是直链或支链的。例如,术语“C
1-
6烷基”指含有1至6个碳原子的烷基(例如甲基、乙基、正丙基、异丙基、正丁基、异丁基、仲丁基、叔丁基、正戊基、1-甲基丁基、2-甲基丁基、3-甲基丁基、新戊基、己基、2-甲基戊基等)。类似地,烷氧基、烷基氨基、二烷基氨基、烷基磺酰基和烷硫基的烷基部分(即烷基)具有上述相同定义。
The term "alkyl" refers to a hydrocarbon group of the formula C n H 2n +. The alkyl group can be straight or branched. For example, the term "C 1 - 6 alkyl" refers to (e.g., methyl, ethyl, n-propyl, isopropyl, alkyl containing 1 to 6 carbon atoms, n-butyl, isobutyl, sec-butyl, Tert-butyl, n-pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl, neopentyl, hexyl, 2-methylpentyl, etc.). Similarly, the alkyl moiety (i.e., alkyl) of an alkoxy group, an alkylamino group, a dialkylamino group, an alkylsulfonyl group, and an alkylthio group has the same definition as defined above.
术语“亚烷基”是指烷基的任意位置除去1个氢而形成的二价基团。例如,术语“C
1-6亚烷基”的非限制性实例包括但不限于亚甲基、亚乙基、甲基亚甲基、二甲基亚甲基、1,3-亚丙基等。
The term "alkylene" refers to a divalent group formed by the removal of one hydrogen at any position of the alkyl group. For example, non-limiting examples of the term " C1-6 alkylene" include, but are not limited to, methylene, ethylene, methylmethylene, dimethylmethylene, 1,3-propylene, and the like. .
术语“烯基”是指由碳原子和氢原子组成的直链或支链的具有至少一个双键的不饱和脂肪族烃基。烯基的非限制性实例包括但不限于乙烯基、1-丙烯基、2-丙烯基、1-丁烯基、异丁烯基、1,3-丁二烯基等。The term "alkenyl" refers to a straight or branched unsaturated aliphatic hydrocarbon group having at least one double bond consisting of a carbon atom and a hydrogen atom. Non-limiting examples of alkenyl groups include, but are not limited to, ethenyl, 1-propenyl, 2-propenyl, 1-butenyl, isobutenyl, 1,3-butadienyl, and the like.
术语“炔基”是指由碳原子和氢原子组成的直链或支链的具有至少一个三键的不饱和脂肪族烃基。炔基的非限制性实例包括但不限于乙炔基(-C≡CH)、1-丙炔基(-C≡C-CH
3)、2-丙炔基(-CH
2-C≡CH)、1,3-丁二炔基(-C≡C-C≡CH)等。
The term "alkynyl" means a straight or branched unsaturated aliphatic hydrocarbon group having at least one triple bond composed of a carbon atom and a hydrogen atom. Non-limiting examples of alkynyl groups include, but are not limited to, ethynyl (-C≡CH), 1-propynyl (-C≡C-CH 3 ), 2-propynyl (-CH 2 -C≡CH), 1,3-butadiynyl (-C≡CC≡CH) or the like.
术语“环烷基”指完全饱和的并且可以以呈单环、桥环或螺环存在的碳环基团。除非另有指示,该碳环通常为3至10元环。环烷基非限制性实例包括但不限于环丙基、环丁基、环戊基、环己基、降冰片基(双环[2.2.1]庚基)、双环[2.2.2]辛基、金刚烷基等。The term "cycloalkyl" refers to a carbocyclic group that is fully saturated and can exist as a single ring, bridged ring or spiro ring. Unless otherwise indicated, the carbocyclic ring is typically a 3 to 10 membered ring. Non-limiting examples of cycloalkyl groups include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, norbornyl (bicyclo[2.2.1]heptyl), bicyclo[2.2.2]octyl, diamond Alkyl and the like.
术语“杂环烷基”是指完全饱和的并且可以以单环、桥环或螺环存在的环状基团。除非另有指示,该杂环通常为含有1至3个独立地选自硫、氧和/或氮的杂原子(优选1或2个杂原子)的3至7元环。3元杂环烷基的实例包括但不限于环氧乙烷基、环硫乙烷基、环氮乙烷基,4元杂环烷基的非限制性实例包括但不限于吖丁啶基、噁丁环基、噻丁环基,5元杂环烷基的实例包括但不限于四氢呋喃基、四氢噻吩基、吡咯烷基、异噁唑烷基、噁唑烷基、异噻唑烷基、噻唑烷基、咪唑烷基、四氢吡唑基,6元杂环烷基的实例包括但不限于哌啶基、四氢吡喃基、四氢噻喃基、吗啉基、哌嗪基、1,4-噻噁烷基、1,4-二氧六环基、硫代吗啉基、1,3-二噻烷基、1,4-二噻烷基,7元杂环烷基的实例包括但不限于氮杂环庚烷基、氧杂环庚烷基、硫杂环庚烷基。优选为具有5或6个环原子的单环杂环烷基。The term "heterocycloalkyl" refers to a cyclic group that is fully saturated and can exist as a monocyclic, bridged or spiro ring. Unless otherwise indicated, the heterocyclic ring is typically a 3 to 7 membered ring containing from 1 to 3 heteroatoms (preferably 1 or 2 heteroatoms) independently selected from sulfur, oxygen and/or nitrogen. Examples of 3-membered heterocycloalkyl groups include, but are not limited to, oxiranyl, cyclohexylethane, cycloalkylethane, non-limiting examples of 4-membered heterocycloalkyl including, but not limited to, azetidinyl, acetophenan Examples of a cyclic group, a thibutyl group, a 5-membered heterocycloalkyl group include, but are not limited to, tetrahydrofuranyl, tetrahydrothiophenyl, pyrrolidinyl, isoxazolidinyl, oxazolidinyl, isothiazolidinyl, thiazolidine Examples of the group, imidazolidinyl group, tetrahydropyrazolyl group, 6-membered heterocycloalkyl group include, but are not limited to, piperidinyl, tetrahydropyranyl, tetrahydrothiopyranyl, morpholinyl, piperazinyl, 1, Examples of 4-thiaxyl, 1,4-dioxacyclyl, thiomorpholinyl, 1,3-dithiaalkyl, 1,4-dithiaalkyl, 7-membered heterocycloalkyl include However, it is not limited to azacycloheptyl, oxetanyl or thiaheptyl. Preferred is a monocyclic heterocycloalkyl group having 5 or 6 ring atoms.
术语“芳基”是指具有共轭的π电子体系的全碳单环或稠合多环的芳香环基团。例如,芳基可以具有6-20个碳原子、6-14个碳原子或6-12个碳原子。芳基的非限制性实例包括但不限于苯基、萘基、蒽基和1,2,3,4-四氢化萘等。The term "aryl" refers to an all-carbon monocyclic or fused polycyclic aromatic ring group having a conjugated π-electron system. For example, an aryl group can have 6 to 20 carbon atoms, 6 to 14 carbon atoms, or 6 to 12 carbon atoms. Non-limiting examples of aryl groups include, but are not limited to, phenyl, naphthyl, anthracenyl, 1,2,3,4-tetrahydronaphthalene, and the like.
术语“杂芳基”是指单环或稠合多环体系,其中含有至少一个选自N、O、S的环原子,其余环原子为C,并且具有至少一个芳香环。优选的杂芳基具有单个4至8元环、尤其是5至8元环,或包含6至14个、尤其是6至10个环原子的多个稠合环。杂芳基的非限制性实例包括但不限于吡咯基、呋喃基、噻吩基、咪唑基、噁唑基、吡唑基、吡啶基、嘧啶基、吡嗪基、喹啉基、异喹啉基、四唑基、三唑基、三嗪基、苯并呋喃基、苯并噻吩基、吲哚基、异吲哚基等。The term "heteroaryl" refers to a monocyclic or fused polycyclic ring system containing at least one ring atom selected from N, O, S, the remaining ring atoms being C, and having at least one aromatic ring. Preferred heteroaryl groups have a single 4 to 8 membered ring, especially a 5 to 8 membered ring, or a plurality of fused rings containing from 6 to 14, especially from 6 to 10 ring atoms. Non-limiting examples of heteroaryl groups include, but are not limited to, pyrrolyl, furyl, thienyl, imidazolyl, oxazolyl, pyrazolyl, pyridyl, pyrimidinyl, pyrazinyl, quinolinyl, isoquinolinyl , tetrazolyl, triazolyl, triazinyl, benzofuranyl, benzothienyl, fluorenyl, isodecyl and the like.
术语“治疗”意为将本申请所述化合物或制剂进行给药以预防、改善或消除疾病或与所述疾病相关的一个或多个症状,且包括:The term "treating" means administering a compound or formulation described herein to prevent, ameliorate or eliminate a disease or one or more symptoms associated with the disease, and includes:
(i)预防疾病或疾病状态在哺乳动物中出现,特别是当这类哺乳动物易患有该疾病状态,但尚未被诊断为已患有该疾病状态时;(i) preventing a disease or disease state from occurring in a mammal, particularly when such a mammal is susceptible to the disease state but has not been diagnosed as having the disease state;
(ii)抑制疾病或疾病状态,即遏制其发展;(ii) inhibiting the disease or disease state, ie, curbing its development;
(iii)缓解疾病或疾病状态,即使该疾病或疾病状态消退。(iii) alleviating the disease or condition, even if the disease or condition resolves.
术语“治疗有效量”意指(i)治疗或预防特定疾病、病况或障碍,(ii)减轻、改善或消除特定疾病、病况或障碍的一种或多种症状,或(iii)预防或延迟本文中所述的特定疾病、病况或障碍的一种或多种症状发作的本申请化合物的用量。构成“治疗有效量”的本申请化合物的量取决于该化合物、疾病状态及其严重性、给药方式以及待被治疗的哺乳动物的年龄而改变,但可例行性地由本领域技术人员根据其自身的知识及本公开内容而确定。The term "therapeutically effective amount" means (i) treating or preventing a particular disease, condition or disorder, (ii) alleviating, ameliorating or eliminating one or more symptoms of a particular disease, condition or disorder, or (iii) preventing or delaying The amount of a compound of the present application in which one or more symptoms of a particular disease, condition, or disorder are described herein. The amount of a compound of the present application which constitutes a "therapeutically effective amount" will vary depending on the compound, the condition and severity thereof, the mode of administration, and the age of the mammal to be treated, but can be routinely determined by those skilled in the art It is determined by its own knowledge and the present disclosure.
术语“药学上可接受的”是指针对那些化合物、材料、组合物和/或剂型而言,它们在可靠的医学判断的范围之内,适用于与人类和动物的组织接触使用,而没有过多的毒性、刺激性、过敏性反应或其它问题或并发症,与合理的利益/风险比相称。The term "pharmaceutically acceptable" is intended to mean that those compounds, materials, compositions and/or dosage forms are within the scope of sound medical judgment and are suitable for use in contact with human and animal tissues without Many toxic, irritating, allergic reactions or other problems or complications are commensurate with a reasonable benefit/risk ratio.
作为药学上可接受的盐,例如,可以提及金属盐、铵盐、与有机碱形成的盐、与无机酸形成的盐、与有机酸形成的盐、与碱性或者酸性氨基酸形成的盐等。As the pharmaceutically acceptable salt, for example, a metal salt, an ammonium salt, a salt with an organic base, a salt with an inorganic acid, a salt with an organic acid, a salt with a basic or acidic amino acid, or the like can be mentioned. .
术语“药物组合物”是指一种或多种本申请的化合物或其盐与药学上可接受的辅料组成的混合物。药物组合物的目的是有利于对有机体给予本申请的化合物。The term "pharmaceutical composition" refers to a mixture of one or more compounds of the present application or a salt thereof and a pharmaceutically acceptable adjuvant. The purpose of the pharmaceutical composition is to facilitate administration of the compounds of the present application to an organism.
术语“药学上可接受的辅料”是指对有机体无明显刺激作用,而且不会损害该活性化合物的生物活性及性能的那些辅料。合适的辅料是本领域技术人员熟知的,例如碳水化合物、蜡、水溶性和/或水可膨胀的聚合物、亲水性或疏水性材料、明胶、油、溶剂、水等。The term "pharmaceutically acceptable excipient" refers to those excipients which have no significant irritating effect on the organism and which do not impair the biological activity and properties of the active compound. Suitable excipients are well known to those skilled in the art, such as carbohydrates, waxes, water soluble and/or water swellable polymers, hydrophilic or hydrophobic materials, gelatin, oils, solvents, water, and the like.
词语“包括(comprise)”或“包含(comprise)”及其英文变体例如comprises或comprising应理解为开放的、非排他性的意义,即“包括但不限于”。The word "comprise" or "comprise" and its English variants such as "comprises" or "comprising" shall be understood to mean an open, non-exclusive meaning, ie "including but not limited to".
本申请的化合物和中间体还可以以不同的互变异构体形式存在,并且所有这样的形式包含于本申请的范围内。术语“互变异构体”或“互变异构体形式”是指可经由低能垒互变的不同能量的结构异构体。例如,质子互变异构体(也称为质子转移互变异构体)包括经由质子迁移的互变,如酮-烯醇及亚胺-烯胺异构化。质子互变异构体的具体实例是咪唑部分,其中质子可在两个环氮间迁移。互变异构体包括通过一些成键电子的重组的互变。The compounds and intermediates of the present application may also exist in different tautomeric forms, and all such forms are embraced within the scope of the present application. The term "tautomer" or "tautomeric form" refers to structural isomers of different energies that are interconvertible via a low energy barrier. For example, proton tautomers (also known as proton transfer tautomers) include interconversions via proton transfer, such as keto-enol and imine-enamine isomerization. A specific example of a proton tautomer is an imidazole moiety in which a proton can migrate between two ring nitrogens. Tautomers include recombination through some of the bonding electrons.
本申请还包括与本文中记载的那些相同的,但一个或多个原子被原子量或质量数不同于自然中通常发现的原子量或质量数的原子置换的同位素标记的本申请化合物。可结合到本申请化合物的同位素的实例包括氢、碳、氮、氧、磷、硫、氟、碘和氯的同位素,诸如分别为
2H、
3H、
11C、
13C、
14C、
13N、
15N、
15O、
17O、
18O、
31P、
32P、
35S、
18F、
123I、
125I和
36Cl等。例如,
The present application also includes isotopically labeled compounds of the present application that are identical to those described herein, but in which one or more atoms are replaced by an atomic weight or mass number different from the atomic mass or mass number normally found in nature. Examples of isotopes that may be incorporated into the compounds of the present application include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, fluorine, iodine, and chlorine, such as 2 H, 3 H, 11 C, 13 C, 14 C, 13 respectively. N, 15 N, 15 O, 17 O, 18 O, 31 P, 32 P, 35 S, 18 F, 123 I, 125 I and 36 Cl, and the like. E.g,
某些同位素标记的本申请化合物(例如用
3H及
14C标记的那些)可用于化合物和/或底物组织分布分析中。氚化(即
3H)和碳-14(即
14C)同位素由于易于制备和可检测性是尤其优选的。正电子发射同位素,诸如
15O、
13N、
11C和
18F可用于正电子发射断层扫描(PET)研究以测定底物占有率。通常可以通过与公开于下文的方案和/或实施例中的那些类似的下列程序,通过同位素标记试剂取代未经同位素标记的试剂来制备同位素标记的本申请化合物。
Certain isotopically-labeled compounds of the present application (such as those labeled with 3 H and 14 C) can be used in compound and/or substrate tissue distribution assays. Deuterated (i.e., 3 H) and carbon-14 (i.e., 14 C) isotopes are especially preferred due to ease of preparation and detectability. Positron emitting isotopes such as 15 O, 13 N, 11 C and 18 F can be used in positron emission tomography (PET) studies to determine substrate occupancy. Isotopically labeled compounds of the present application can generally be prepared by substituting an isotopically labeled reagent for an unisotopically labeled reagent by procedures similar to those disclosed in the schemes and/or examples disclosed below.
此外,用较重同位素(诸如氘(即
2H/D))取代可以提供某些由更高的代谢稳定性产生的治疗优点(例如增加的体内半衰期或降低的剂量需求),并且因此在某些情形下可能是优选的,其中氘取代可以是部分或完全的,部分氘取代是指至少一个氢被至少一个氘取代。例如,本申请中涉及的亚烷基可为部分或完全氘代的亚烷基。
In addition, substitution with heavier isotopes such as deuterium (ie, 2 H/D) can provide certain therapeutic advantages resulting from higher metabolic stability (eg, increased in vivo half-life or reduced dosage requirements), and thus It may be preferred in some cases where the hydrazine substitution may be partial or complete and the partial hydrazine substitution means that at least one hydrogen is replaced by at least one hydrazine. For example, the alkylene group referred to in the present application may be a partially or fully deuterated alkylene group.
本申请化合物可以是不对称的,例如,具有一个或多个立体异构体。除非另有说明,所有立体异构体都包括在本申请中,如对映异构体和非对映异构体。本申请的含有不对称碳原子的化合物可以以光学活性纯的形式或外消旋形式被分离出来。光学活性纯的形式可以从外消旋混合物拆分,或通过使用手性原料或手性试剂合成。The compounds of the present application may be asymmetric, for example, having one or more stereoisomers. Unless otherwise stated, all stereoisomers are included in the present application, such as enantiomers and diastereomers. The asymmetric carbon atom-containing compounds of the present application can be isolated in optically active pure form or in racemic form. The optically active pure form can be resolved from the racemic mixture or synthesized by using a chiral starting material or a chiral reagent.
本申请的化合物可以存在特定的几何异构体或立体异构体形式。本申请设想所有的这类化合物,包括互变异构体、顺式和反式异构体、(-)-和(+)-对映体、(R)-和(S)-对映体、非对映异构体、(D)-异构体、(L)-异构体,及其外消旋混合物和其他混合物,例如对映异构体或非对映体富集的混合物,所有这些都属于本申请的范围之内。烷基等取代基中可以存在另外的不对称碳原子。所有这些异构体以及它们的混合物均包括在本申请的范围之内。例如结构单元
包括顺式异构体形式
及反式异构体形式
The compounds of the present application may exist in specific geometric or stereoisomeric forms. All such compounds are contemplated by the present application, including tautomers, cis and trans isomers, (-)- and (+)-enantiomers, (R)- and (S)-enantiomers , diastereomers, (D)-isomers, (L)-isomers, and racemic mixtures thereof, and other mixtures, such as enantiomerically or diastereomeric enriched mixtures, All of these are within the scope of this application. Additional asymmetric carbon atoms may be present in the substituent such as an alkyl group. All such isomers, as well as mixtures thereof, are included within the scope of the present application. Structural unit Cis isomer form And trans isomer form
本申请的药物组合物可通过将本申请的化合物与适宜的药学上可接受的辅料组合而制备,例如可配制成固态、半固态、液态或气态制剂,如片剂、丸剂、胶囊剂、粉剂、颗粒剂、膏剂、乳剂、悬浮剂、栓剂、注射剂、吸入剂、凝胶剂、微球及气溶胶等。The pharmaceutical compositions of the present application can be prepared by combining the compounds of the present application with suitable pharmaceutically acceptable excipients, for example, as solid, semi-solid, liquid or gaseous preparations, such as tablets, pills, capsules, powders. , granules, ointments, emulsions, suspensions, suppositories, injections, inhalants, gels, microspheres and aerosols.
给予本申请化合物或其药学上可接受的盐或其药物组合物的典型途径包括但不限于口服、直肠、局部、吸入、肠胃外、舌下、阴道内、鼻内、眼内、腹膜内、肌内、皮下、静脉内给药。Typical routes of administration of a compound of the present application, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof include, but are not limited to, oral, rectal, topical, inhalation, parenteral, sublingual, intravaginal, intranasal, intraocular, intraperitoneal, Intramuscular, subcutaneous, intravenous administration.
本申请的药物组合物可以采用本领域众所周知的方法制造,如常规的混合法、溶解法、制粒法、制糖衣药丸法、磨细法、乳化法、冷冻干燥法等。The pharmaceutical composition of the present application can be produced by a method well known in the art, such as a conventional mixing method, a dissolution method, a granulation method, a sugar-coating method, a grinding method, an emulsification method, a freeze-drying method, and the like.
在一些实施方案中,药物组合物是口服形式。对于口服给药,可以通过将活性化合物与本领域熟知的药学上可接受的辅料混合,来配制该药物组合物。这些辅料能使本申请的化合物被配制成片剂、丸剂、锭剂、糖衣剂、胶囊剂、液体、凝胶剂、浆剂、悬浮剂等,用于对患者的口服给药。In some embodiments, the pharmaceutical composition is in oral form. For oral administration, the pharmaceutical composition can be formulated by admixing the active compound with pharmaceutically acceptable excipients which are well known in the art. These excipients enable the compounds of the present application to be formulated into tablets, pills, troches, dragees, capsules, liquids, gels, slurries, suspensions and the like for oral administration to a patient.
可以通过常规的混合、填充或压片方法来制备固体口服组合物。例如,可通过下述方法获得:将所述的活性化合物与固体辅料混合,任选地碾磨所得的混合物,如果需要则加入其它合适的辅料,然后将该混合物加工成颗粒,得到了片剂或糖衣剂的核心。适合的辅料包括但不限于:粘合剂、稀释剂、崩解剂、润滑剂、助流剂、甜味剂或矫味剂等。Solid oral compositions can be prepared by conventional methods of mixing, filling or tabletting. For example, it can be obtained by mixing the active compound with a solid adjuvant, optionally milling the resulting mixture, adding other suitable excipients if necessary, and then processing the mixture into granules to give tablets. Or the core of the sugar coating. Suitable excipients include, but are not limited to, binders, diluents, disintegrants, lubricants, glidants, sweeteners or flavoring agents, and the like.
药物组合物还可适用于肠胃外给药,如合适的单位剂型的无菌溶液剂、混悬剂或冻干产品等。The pharmaceutical compositions may also be suitable for parenteral administration, such as sterile solutions, suspensions or lyophilized products in a suitable unit dosage form.
本文所述的通式I化合物的所有施用方法中,每天给药的剂量为0.01到200mg/kg体重,以单独或分开剂量的形式。In all methods of administration of the compounds of formula I described herein, the daily dose is from 0.01 to 200 mg/kg body weight, either alone or in divided doses.
本申请的化合物可以通过本领域技术人员所熟知的多种合成方法来制备,包括下面列举的具体实施方式、其与其他化学合成方法的结合所形成的实施方式以及本领域技术人员所熟知的等同替换方式,优选的实施方式包括但不限于本申请的实施例。The compounds of the present application can be prepared by a variety of synthetic methods well known to those skilled in the art, including the specific embodiments listed below, combinations thereof with other chemical synthesis methods, and equivalents well known to those skilled in the art. Alternatively, preferred embodiments include, but are not limited to, embodiments of the present application.
本申请具体实施方式的化学反应是在合适的溶剂中完成的,所述的溶剂须适合于本申请的化学变化及其所需的试剂和物料。为了获得本申请的化合物,有时需要本领域技术人员在已有实施方式的基础上对合成步骤或者反应流程进行修改或选择。The chemical reactions of the specific embodiments of the present application are accomplished in a suitable solvent which is suitable for the chemical changes of the present application and the reagents and materials required thereof. In order to obtain the compounds of the present application, it is sometimes necessary for those skilled in the art to modify or select the synthetic steps or reaction schemes based on the prior embodiments.
本领域合成路线规划中的一个重要考量因素是为反应性官能团(如本申请中的氨基)选择合适的保护基,例如,可参考Greene's Protective Groups in Organic Synthesis(4th Ed).Hoboken,New Jersey:John Wiley&Sons,Inc.本申请引用的所有参考文献整体上并入本申请。An important consideration in the art of synthetic route planning is the selection of suitable protecting groups for reactive functional groups (such as amino groups in the present application), for example, reference to Greene's Protective Groups in Organic Synthesis (4th Ed). Hoboken, New Jersey: John Wiley & Sons, Inc. All references cited herein are hereby incorporated by reference in their entirety.
在一些实施方案中,本申请式II的化合物可以由有机合成领域技术人员通过路线1用本领域的常规方法来制备:In some embodiments, the compounds of Formula II herein can be prepared by one of ordinary skill in the art of organic synthesis by conventional methods in the art using Route 1:
路线1Route 1
其中T
1、T
2、T
3、T
4、L
1、X、R
1、R
2、q、m、n同式I化合物的定义;R表示氢,Pg表示保护基例如Boc等,R
4表示C
1-6烷基。
Wherein T 1 , T 2 , T 3 , T 4 , L 1 , X, R 1 , R 2 , q, m, n are as defined for the compound of formula I; R represents hydrogen, and Pg represents a protecting group such as Boc, etc., R 4 Represents a C 1-6 alkyl group.
本申请采用下述缩略词:This application uses the following abbreviations:
EA代表乙酸乙酯;DCM代表二氯甲烷;HATU代表2-(7-偶氮苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯;DIPEA代表N,N-二异丙基乙胺;DMF代表N,N-二甲基甲酰胺;DEAD代表偶氮二甲酸二乙酯;NBS代表N-溴代丁二酰亚胺;DMA代表N,N-二甲基乙酰胺;FDPP代表五氟苯基二苯基磷酸酯;MeOH代表甲醇;TEA代表三乙胺;T3P代表1-丙基磷酸酐;MsCl代表甲基磺酰氯;DIAD代表偶氮二甲酸二异丙酯;Boc表示叔丁氧羰基;HEPES代表4-(2-羟乙基)-1-哌嗪乙磺酸;DTT代表二硫苏糖醇;EGTA代表乙二醇双(2-氨基乙基醚)四乙酸。EA stands for ethyl acetate; DCM stands for dichloromethane; HATU stands for 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate; DIPEA stands for N , N-diisopropylethylamine; DMF stands for N,N-dimethylformamide; DEAD stands for diethyl azodicarboxylate; NBS stands for N-bromosuccinimide; DMA stands for N,N- Dimethylacetamide; FDPP stands for pentafluorophenyl diphenyl phosphate; MeOH stands for methanol; TEA stands for triethylamine; T3P stands for 1-propylphosphoric acid anhydride; MsCl stands for methylsulfonyl chloride; DIAD stands for azodicarboxylic acid Diisopropyl ester; Boc represents tert-butoxycarbonyl; HEPES stands for 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; DTT stands for dithiothreitol; EGTA stands for ethylene glycol bis(2-amino Ethyl ether) tetraacetic acid.
为清楚起见,进一步用实施例来阐述本发明,但是实施例并非限制本申请的范围。本申请所使用的所有试剂是市售的,无需进一步纯化即可使用。The invention is further illustrated by the following examples, but the examples are not intended to limit the scope of the application. All reagents used in this application are commercially available and can be used without further purification.
实施例1:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶-1(1,2)-苯基-3(1,3)-环丁基辛内酰胺-5-酮
Example 1: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-phenyl-3(1,3)-cyclobutylcaprolactam-5-one
步骤1:(R,E)-N-(5-氟-2-羟基苯基烯基)-2-甲基丙基-2-亚磺酰胺Step 1: (R,E)-N-(5-fluoro-2-hydroxyphenylalkenyl)-2-methylpropyl-2-sulfinamide
室温下,向反应瓶中加入化合物1A(20g)和(R)-(+)-叔丁基亚磺酰胺(19.03g)和二氯甲烷(300mL),冰水浴下,分批加入碳酸铯(74.4g),氮气保护及冰水浴下搅拌30分钟后,移至室温。反应完全,移至冰水浴中,向反应液中加水;用二氯甲烷萃取,有机相用饱和食盐水洗,加入无水硫酸钠干燥,过滤、浓缩,浓缩物利用石油醚打浆,得化合物1B(33.84g)。Compound 1A (20 g) and (R)-(+)-tert-butylsulfinamide (19.03 g) and dichloromethane (300 mL) were added to the reaction flask at room temperature, and cesium carbonate was added in portions under ice-water bath. 74.4 g), stirred under nitrogen for 30 minutes in an ice water bath, and then transferred to room temperature. The reaction was completed, and the mixture was transferred to an ice-water bath, and water was added to the mixture. The mixture was extracted with dichloromethane. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated. 33.84g).
1H NMR(500MHz,DMSO-d
6):δ10.60(br,1H),8.81~8.81(d,J=2.0Hz,1H),7.53~7.56(dd,J=9.0Hz,3.5Hz,1H),7.27~7.31(m,1H),6.99~7.02(dd,J=9.0Hz,4.5Hz,1H),1.17(s,9H).LC-MS m/z:242.3,[M-H]
-。
1 H NMR (500MHz, DMSO- d 6): δ10.60 (br, 1H), 8.81 ~ 8.81 (d, J = 2.0Hz, 1H), 7.53 ~ 7.56 (dd, J = 9.0Hz, 3.5Hz, 1H ), 7.27 to 7.31 (m, 1H), 6.99 to 7.02 (dd, J = 9.0 Hz, 4.5 Hz, 1H), 1.17 (s, 9H). LC-MS m/z: 242.3, [MH] - .
步骤2:(R)-N-((R)-1-(5-氟-2-羟基苯基)乙基)-2-甲基丙基-2-亚磺酰胺Step 2: (R)-N-((R)-1-(5-fluoro-2-hydroxyphenyl)ethyl)-2-methylpropyl-2-sulfenamide
向反应瓶中加入化合物1B(1.0g)和四氢呋喃(15mL),-70℃及氮气保护下,将甲基溴化镁(2.45g)(1.0M的四氢呋喃溶液)缓慢加入化合物1B的搅拌液中,滴加完毕,恢复至室温反应。反应完全,冰水浴下,缓慢向反应液中加水。利用乙酸乙酯萃取,加入无水硫酸钠干燥,过滤浓缩,柱层析(石油醚:乙酸乙酯=75:25)纯化得到化合物1C(0.66g)。Compound 1B (1.0 g) and tetrahydrofuran (15 mL) were added to the reaction flask, and methylmagnesium bromide (2.45 g) (1.0 M in tetrahydrofuran solution) was slowly added to the stirred solution of compound 1B under a nitrogen atmosphere at -70 °C. After the addition is completed, the reaction is returned to room temperature. The reaction was completed, and water was slowly added to the reaction solution under ice water bath. It was extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, and evaporated.
1H NMR(500MHz,CDCl
3):δ8.89(s,1H),6.79~6.81(dd,J=9.0Hz,3.0Hz,1H),6.52~6.65(m,1H),6.49~6.52(dd,J=8.5Hz,4.5Hz,1H),4.89~4.90(d,J=7.0Hz,1H),4.39~4.45(m,1H),1.53~1.55(d,J=7.0Hz,3H),1.28(s,9H).LCMS:m/z:258.3,[M-H]
-。
1 H NMR (500 MHz, CDCl 3 ): δ 8.89 (s, 1H), 6.79 to 6.81 (dd, J = 9.0 Hz, 3.0 Hz, 1H), 6.52 to 6.65 (m, 1H), 6.49 to 6.52 (dd , J = 8.5 Hz, 4.5 Hz, 1H), 4.89 - 4.90 (d, J = 7.0 Hz, 1H), 4.39 - 4.45 (m, 1H), 1.53 - 1.55 (d, J = 7.0 Hz, 3H), 1.28 (s, 9H). LCMS: m/z: 258.3, [MH] - .
步骤3:((1S,3s)-3-(2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环丁基)羧酸叔丁基酯Step 3: ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl carboxylate
向反应瓶中加入化合物1C(6g)和((1r,3r)-3-羟基环丁基)氨基甲酸叔丁酯(4.33g)、三苯基磷(9.10g)和四氢呋喃(100mL),溶解,冰水浴和氮气保护下,缓慢加入DEAD(6.45g)的四氢呋喃(6mL)溶液,反应完全后,浓缩反应液,柱层析纯化(石油醚:乙酸乙酯=85:15)得到化合物1D(5.68g)。LC-MS m/z:451.4,[M+Na]
+。
To the reaction flask were added compound 1C (6 g) and ((1r, 3r)-3-hydroxycyclobutyl)carbamic acid tert-butyl ester (4.33 g), triphenylphosphine (9.10 g) and tetrahydrofuran (100 mL), dissolved. Under ice water and nitrogen, a solution of DEAD (6.45 g) in tetrahydrofuran (6 mL) was added slowly. After the reaction was completed, the reaction mixture was concentrated and purified by column chromatography ( petroleum ether: ethyl acetate = 85:15) 5.68g). LC-MS m/z: 451.4, [M+Na] + .
步骤4:((1S,3S)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)环丁基)氨基甲酸叔丁酯Step 4: ((1S,3S)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶加入化合物1D(3.58g)、四氢呋喃(60mL)、水(6mL)及碘(424mg),氮气保护、50℃下反应。反应完全后,向反应液加入10wt%硫代硫酸钠水溶液,乙酸乙酯萃取,无水硫酸钠干燥,过滤浓缩得到化合物1E(3.48g)。LC-MS m/z:325.4[M+H]
+。
Compound 1D (3.58 g), tetrahydrofuran (60 mL), water (6 mL) and iodine (424 mg) were added to the reaction flask, and the mixture was reacted under nitrogen atmosphere at 50 °C. After the completion of the reaction, a 10 wt% aqueous sodium thiosulfate solution was added to the reaction mixture, and ethyl acetate was evaporated, dried over anhydrous sodium sulfate and filtered to give Compound 1E (3.48 g). LC-MS m/z: 325.4 [M+H] + .
步骤5:5-(((R)-1-(2-((1s,3S)-3-(叔丁氧羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 5: 5-(((R)-1-(2-((1s,3S)-3-(tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl)amino) Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中加入化合物1E(3.08g)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(2.24g)、DIPEA(8.20g)及正丁醇(60ml),氮气保护下,加热至140℃反应。反应完全后,冷至室温,向反应液中加水、乙酸乙酯萃取,无水硫酸钠干燥过滤,浓缩,柱层析纯化得到化合物1F(1.92g)。To the reaction flask were added compound 1E (3.08 g), ethyl 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (2.24 g), DIPEA (8.20 g) and n-butanol (60 ml) Under nitrogen protection, the reaction was heated to 140 ° C. After completion of the reaction, the mixture was cooled to room temperature, and then the mixture was evaporated.
1H NMR(500MHz,CDCl
3):δ8.26~8.29(br,s,2H),7.07~7.08(d,J=6.5Hz,1H),6.89~6.90(ddd,1H),6.68~6.70(dd,J=4.0,8.5Hz,1H),6.24(s,1H),4.46~4.50(m,3H),4.12~4.17(dd,J=7.0Hz,14.0Hz,1H),3.90~3.91(m,1H),2.94~2.99(m,2H),2.32~2.33(m,2H),2.23~2.26(m,2H),1.46(s,9H),1.40(m,3H),1.28(m,3H).LC-MS 514.4,[M+H]
+。
1 H NMR (500 MHz, CDCl 3 ): δ 8.26 to 8.29 (br, s, 2H), 7.07 to 7.08 (d, J = 6.5 Hz, 1H), 6.89 to 6.90 (ddd, 1H), 6.68 to 6.70 ( Dd, J=4.0, 8.5 Hz, 1H), 6.24 (s, 1H), 4.46 to 4.50 (m, 3H), 4.12 to 4.17 (dd, J = 7.0 Hz, 14.0 Hz, 1H), 3.90 to 3.91 (m) , 1H), 2.94 to 2.99 (m, 2H), 2.32 to 2.33 (m, 2H), 2.23 to 2.26 (m, 2H), 1.46 (s, 9H), 1.40 (m, 3H), 1.28 (m, 3H) ). LC-MS 514.4, [M+H] + .
步骤6:5-(((R)-1-(2-((1s,3S)-3-((叔丁氧羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 6: 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl)amino Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中加入化合物1F(1.8g)、甲醇(20mL)、四氢呋喃(7mL)、2M氢氧化锂的水溶液(12ml),氮气保护下加热至70℃。反应完全后,滴加2.0M的盐酸pH调至小于5,用二氯甲烷萃取,无水硫酸钠干燥;过滤浓缩得到化合物1G(1.57g).LC-MS m/z:484.3,[M-H]
-。
A 1F (1.8 g), methanol (20 mL), tetrahydrofuran (7 mL), 2M aqueous lithium hydroxide solution (12 ml) was added to the reaction mixture and heated to 70 ° C under nitrogen atmosphere. After completion of the reaction, the pH of 2.0 M hydrochloric acid was added dropwise to less than 5, extracted with dichloromethane, dried over anhydrous sodium sulfate and concentrated by filtration to give compound 1G (1.57 g). LC-MS m/z: 484.3, [MH] - .
步骤7:5-(((R)-1-(2-((1s,3S)-3-氨基环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 7: 5-((R)-1-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazolo[1,5 -a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中加入化合物1G(1.48g)和二氯甲烷(15mL),冰水浴下加入4N氯化氢的二氧六环溶液7.0mL,在室温下反应。反应完成后,过滤并干燥所得固体,得到化合物1H(1.478g)。LC-MS m/z:384.2,[M-H]
-。
Compound 1G (1.48 g) and dichloromethane (15 mL) were added to a reaction flask, and 7.0 mL of 4N hydrogen chloride in dioxane solution was added thereto under ice water, and the mixture was reacted at room temperature. After completion of the reaction, the obtained solid was filtered and dried to give Compound 1H (1.478 g). LC-MS m / z: 384.2 , [MH] -.
步骤8:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶-1(1,2)-苯基-3(1,3)-环丁基辛内酰胺-5-酮(化合物I-1)
Step 8: (3 1 S, 3 3 S, 6 3 E, 6 4 E, 8R) -1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6 (3, 5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-phenyl-3(1,3)-cyclobutylcaprolactam-5-one (Compound I-1)
向反应瓶中加入DIPEA(3.039g)、DMF(40mL)及二氯甲烷(90mL),氮气保护下,室温下搅拌,将化合物1H(1.45g)的DMF溶液分批加入反应液中,然后加入五氟苯基二磷酸酯(1.19g)。反应完全,向反应液中滴加2M碳酸钠水溶液(60mL),二氯甲烷萃取,无水硫酸钠干燥,过滤浓缩有机相,所得浓缩物经柱层析纯化(二氯甲烷:甲醇=97:3)得到化合物I-1(880mg)。DIPEA (3.039g), DMF (40mL) and dichloromethane (90mL) were added to the reaction flask, and the mixture was stirred at room temperature under nitrogen atmosphere. The compound 1H (1.45g) DMF solution was added to the reaction solution in batches, then added Pentafluorophenyl diphosphate (1.19 g). The reaction was completed, and a 2M aqueous solution of sodium carbonate (60 mL) was evaporated. 3) Compound I-1 (880 mg) was obtained.
化合物I-1Compound I-1
1H NMR(500MHz,DMSO-d6):δ9.305(d,J=10.5Hz 1H),8.746(d,J=7.0Hz 1H),8.544(d,J=7.5Hz 1H),8.030(s,1H),7.179(d,J=9.5Hz,1H),6.970(t,J=7.5Hz,1H),6.578(t,J=4.5Hz,1H),6.379(d,J=7.0Hz 1H),5.787(t,J=7.0Hz 1H),4.193(s,1H),4.699-4.689(m,1H),3.087(t,J=7.0Hz,1H),2.842(t,J=7.0Hz,1H),2.145-2.104(dd,J=7.0,13.0Hz,1H),1.666-1.625(dd,J=7.0,13.0Hz,1H),1.441(d,J=6.5Hz,3H)。
1 H NMR (500MHz, DMSO- d6): δ9.305 (d, J = 10.5Hz 1H), 8.746 (d, J = 7.0Hz 1H), 8.544 (d, J = 7.5Hz 1H), 8.030 (s, 1H), 7.179 (d, J = 9.5 Hz, 1H), 6.970 (t, J = 7.5 Hz, 1H), 6.558 (t, J = 4.5 Hz, 1H), 6.379 (d, J = 7.0 Hz 1H), 5.787 (t, J = 7.0 Hz 1H), 4.193 (s, 1H), 4.699-4.689 (m, 1H), 3.087 (t, J = 7.0 Hz, 1H), 2.842 (t, J = 7.0 Hz, 1H) , 2.145-2.104 (dd, J = 7.0, 13.0 Hz, 1H), 1.666-1.625 (dd, J = 7.0, 13.0 Hz, 1H), 1.441 (d, J = 6.5 Hz, 3H).
13C NMR(125MHz,DMSO-d6):δ161.21,158.01,156.13,155.50,149.15,146.57,144.17,136.54,136.18,114.14,113.78,101.30,79.42,74.32,43.85,42.92,37.19,36.13,23.14.LC-MS m/z:368.3,[M+H]
+。
13 C NMR (125 MHz, DMSO-d6): δ 161.21, 158.01, 156.13, 155.50, 149.15, 146.57, 144.17, 136.54, 136.18, 114.14, 113.78, 101.30, 79.42, 74.32, 43.85, 42.92, 37.19, 36.13, 23.14. - MS m/z: 368.3, [M+H] + .
实施例2:(3
1R,3
3R,6
3E,6
4E,8S)-1
4-氟-8-甲基-2-氧-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶-1(1,2)-苯基-3(1,3)-环丁醇环辛内酰胺-5-酮
Example 2: (3 1 R, 3 3 R, 6 3 E, 6 4 E, 8S)-1 4 -fluoro-8-methyl-2-oxo-4,7-diaza-6 (3, 5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-phenyl-3(1,3)-cyclobutanol cyclooctanolac-5-one
步骤1:((1s,3s)-3-(2-乙酰基-4-氟苯氧基)环丁基)氨基甲酸叔丁酯Step 1: ((1s,3s)-3-(2-Acetyl-4-fluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中加入化合物1J(37g)、((1r,3r)-3-羟基环丁基)氨基甲酸叔丁酯(40g)、三苯基膦(84g)和四氢呋喃(400mL),冰水浴下,分次加入DEAD(59.5g),氮气保护下,反应在冰水浴下搅拌30分钟后移至室温。反应完全,冰水浴条件下,滴加碳酸钠水溶液淬灭反应;用乙酸乙酯萃取,有机相用饱和食盐水洗,无水硫酸钠干燥,过滤浓缩得到化合物1K(33.8g)。Compound 1J (37 g), ((1r, 3r)-3-hydroxycyclobutyl)carbamic acid tert-butyl ester (40 g), triphenylphosphine (84 g) and tetrahydrofuran (400 mL) were added to the reaction flask under ice-water bath DEAD (59.5 g) was added in portions, and the reaction was stirred under ice-cooling for 30 minutes and then transferred to room temperature. The reaction was completed, and the mixture was evaporated.
1H NMR(500MHz,DMSO-d
6):δ7.375-7.328(m,2H),7.214(d,J=8.0Hz,1H),7.030-7.004(dd,J=4.5,9.0Hz,1H),4.527(m,J=7.0Hz,1H),3.747-3.700(m,1H),2.814-2.782(m,2H),2.574(s,3H),2.061-2.025(m,2H),1.382(s,9H).
1 H NMR (500MHz, DMSO- d 6): δ7.375-7.328 (m, 2H), 7.214 (d, J = 8.0Hz, 1H), 7.030-7.004 (dd, J = 4.5,9.0Hz, 1H) , 4.527 (m, J = 7.0 Hz, 1H), 3.747-3.700 (m, 1H), 2.814-2.782 (m, 2H), 2.574 (s, 3H), 2.061-2.025 (m, 2H), 1.382 (s) , 9H).
步骤2:((1S,3s)-3-(2-((Z)-1-(((R)叔丁基亚磺酰基)亚胺)乙基)-4-氟苯氧基)环丁基)氨基甲酸叔丁酯Step 2: ((1S,3s)-3-(2-((Z)-1-((R)-tert-butylsulfinyl)imide)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl carbamate
向反应瓶中加入化合物1K(16g)、(R)-(+)-叔丁基亚磺酰胺(9.0g)、二乙二醇二甲醚(6.6g)、四氢呋喃(70mL)及2-甲基四氢呋喃(70.0mL),氮气置换3次后,加入钛酸四乙酯(56.5g),室温搅拌溶清后,加热60℃反应。反应完全后,将反应液倒入碎冰中,加入乙酸乙酯剧烈搅拌,析出固体,过滤,滤液使用乙酸乙酯萃取,后用饱和食盐水洗有机相,有机相浓缩后得到化合物1L(22.3g)。LC-MS m/z=449.4,[M+Na]
+。
Compound 1K (16 g), (R)-(+)-tert-butylsulfinamide (9.0 g), diethylene glycol dimethyl ether (6.6 g), tetrahydrofuran (70 mL) and 2-A were added to the reaction flask. After the tetrahydrofuran (70.0 mL) was replaced with nitrogen three times, tetraethyl titanate (56.5 g) was added, and the mixture was stirred at room temperature, and then heated at 60 ° C to react. After the reaction was completed, the reaction mixture was poured into crushed ice, and the mixture was stirred vigorously with ethyl acetate. The solid was separated and filtered, and the filtrate was extracted with ethyl acetate. The organic phase was washed with saturated brine and the organic phase was concentrated to give compound 1L (22.3 g) ). LC-MS m/z = 449.4, [M+Na] + .
步骤3:((1R,3s)-3-(2-((S)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环丁基)甲酸叔丁酯Step 3: ((1R,3s)-3-(2-((S)-1-(((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl formate
向反应瓶加入化合物1L(26.7g)、四氢呋喃(180mL)和水(3mL),搅拌溶清,所得反应液置于低温反应仪(-50℃)中,缓慢加入硼氢化钠(7.1g),反应完全,用乙酸乙酯萃取,饱和食盐水洗,无水硫酸钠干燥有机相,过滤浓缩有机相,柱层析纯化得化合物1M(11.6g)。LC-MS m/z:451.4,[M+Na]+。Compound 1 L (26.7 g), tetrahydrofuran (180 mL) and water (3 mL) were added to the reaction flask, and the mixture was stirred and dissolved. The obtained reaction solution was placed in a low temperature reactor (-50 ° C), and sodium borohydride (7.1 g) was slowly added. The reaction was completed, extracted with EtOAc (EtOAc)EtOAc. LC-MS m/z: 451.4, [M+Na]+.
步骤4:((1R,3s)-3-(2-((S)-1-氨基乙基)-4-氟苯氧基)环丁基)氨基甲酸叔丁酯Step 4: ((1R,3s)-3-(2-((S)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中加入化合物1M(1.6g)、碘单质(0.19g)、四氢呋喃(15mL)和水(1.5mL),氮气下,反应混合物在室温下搅拌过夜。反应完成后,加入10wt%硫代硫酸钠水溶液(15mL),再用EA萃取(20mL×3);合并有机相,无水硫酸钠干燥,抽滤,旋干,干燥得化合物9A的粗品(1.9g),未进一步纯化直接用于下一步反应。Compound 1M (1.6 g), iodine (0.19 g), tetrahydrofuran (15 mL) and water (1.5 mL) were added to the reaction mixture, and the mixture was stirred at room temperature overnight. After completion of the reaction, a 10 wt% aqueous sodium thiosulfate solution (15 mL) was added, followed by extraction with EA (20 mL × 3); the organic phase was combined, dried over anhydrous sodium sulfate, filtered, dried, dried g) was used in the next reaction without further purification.
1H NMR(500MHz,DMSO-d
6):δ8.12(br,s,3H),7.28~7.31(dd,J=3.0,9.5Hz,1H),7.15~7.19(ddd,2H),6.92~6.95(dd,J=4.5,13.5Hz,1H),4.58~4.60(m,1H),4.44~4.47(m,1H),3.68(m,1H),2.75~2.88(m,1H),1.99~2.06(m,2H),1.46~1.47(d,J=6.5Hz,3H),1.38(s,9H).LC-MS:m/z 325.4[M+H]
+。
1 H NMR (500 MHz, DMSO-d 6 ): δ 8.12 (br, s, 3H), 7.28 - 7.31 (dd, J = 3.0, 9.5 Hz, 1H), 7.15 - 7.19 (ddd, 2H), 6.92 6.95 (dd, J=4.5, 13.5 Hz, 1H), 4.58 to 4.60 (m, 1H), 4.44 to 4.47 (m, 1H), 3.68 (m, 1H), 2.75 to 2.88 (m, 1H), 1.99~ 2.06 (m, 2H), 1.46 - 1.47 (d, J = 6.5 Hz, 3H), 1.38 (s, 9H). LC-MS: m/z 325.4 [M+H] + .
步骤5:5-((S)-1-(2-((1s,3R)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 5: 5-((S)-1-(2-((1s,3R)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl)amino Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中加入化合物9A(0.43g)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(0.3g)、DIPEA(1.15g)和正丁醇(8mL),微波条件下,100瓦特下,加热至120℃,反应30分钟。反应完成后,向反应混合物中加入饱和碳酸氢钠水溶液(15mL),用EA萃取(25mL×3),合并有机相,无水硫酸钠干燥,抽滤,旋干,浓缩物通过柱层析纯化(DCM:MeOH=100:0~95:5)得化合物9B的粗品(0.77g)。To the reaction flask were added compound 9A (0.43 g), ethyl 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (0.3 g), DIPEA (1.15 g) and n-butanol (8 mL). Under microwave conditions, at 100 watts, heat to 120 ° C and react for 30 minutes. After completion of the reaction, a saturated aqueous solution of sodium hydrogencarbonate (15 mL) was evaporated. (DCM: MeOH = 100:0 - 95:5)yield of Compound 9B (0.77 g).
1H NMR(500MHz,DMSO-d
6):δ8.54~8.56(dd,J=7.5Hz,1H),8.19~8.21(dd,J=7.5Hz,1H),8.13(s,1H),7.12~7.14(dd,J=2.5,9.5Hz,2H),6.96~7.00(ddd,1H),6.83~6.86(dd,J=4.5,9.0Hz,1H),6.50~6.51(d,J=7.5Hz,1H),5.59(m,1H),4.45~4.48(t,J=6.5Hz,2H),4.15~4.19(m,1H),3.75(m,1H),2.76~2.81(m,2H),2.10~2.11(d,J=8.5Hz,1H),1.96~1.98(d,J=8.5Hz,1H),1.45~1.47(d,J=7.0Hz,3H),1.38(s,9H),1.23~1.26(d,J=7.0Hz,3H).LC-MS:m/z 514.4[M+H]
+.
1 H NMR (500MHz, DMSO- d 6): δ8.54 ~ 8.56 (dd, J = 7.5Hz, 1H), 8.19 ~ 8.21 (dd, J = 7.5Hz, 1H), 8.13 (s, 1H), 7.12 ~ 7.14 (dd, J = 2.5, 9.5 Hz, 2H), 6.96 to 7.00 (ddd, 1H), 6.83 to 6.86 (dd, J = 4.5, 9.0 Hz, 1H), 6.50 to 6.51 (d, J = 7.5 Hz) , 1H), 5.59 (m, 1H), 4.45 to 4.48 (t, J = 6.5 Hz, 2H), 4.15 to 4.19 (m, 1H), 3.75 (m, 1H), 2.76 to 2.81 (m, 2H), 2.10 to 2.11 (d, J = 8.5 Hz, 1H), 1.96 to 1.98 (d, J = 8.5 Hz, 1H), 1.45 to 1.47 (d, J = 7.0 Hz, 3H), 1.38 (s, 9H), 1.23 ~1.26 (d, J=7.0 Hz, 3H). LC-MS: m/z 514.4 [M+H] + .
步骤6:5-(((S)-1-(2-((1s,3R)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 6: 5-(((S)-1-(2-((1),3R)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中加入化合物9B(0.35g)、一水合氢氧化锂(0.17g,4.09mmol)、甲醇(10mL)、四氢呋喃(4mL)和水(1mL),氮气保护下,反应混合物加热至70℃下反应。反应完成后,用2.0M的HCl水溶液调至pH小于5,再加入水(10mL)稀释反应,用DCM萃取(20mL×3),合并有机相,加入无水硫酸钠干燥;抽滤,旋干,最后通过减压干燥得化合物9C的粗品(0.35g)。Compound 9B (0.35 g), lithium hydroxide monohydrate (0.17 g, 4.09 mmol), methanol (10 mL), tetrahydrofuran (4 mL) and water (1 mL) were added to the reaction flask, and the mixture was heated to 70 ° C under nitrogen. The next reaction. After completion of the reaction, the pH was adjusted to less than 5 with aq. EtOAc (aq.), and then the mixture was diluted with water (10 mL). Finally, the crude product (0.35 g) of Compound 9C was obtained by drying under reduced pressure.
1H NMR(500MHz,DMSO-d
6):δ11.35(br,1H),8.55~8.57(dd,J=7.0Hz,1H),8.31~8.32(d,J=7.0Hz,1H),8.11(s,1H),7.11~7.14(dd,J=2.5,9.0Hz,2H),6.96~6.99(ddd,1H),6.83~6.86(dd,J=4.5,9.0Hz,1H),6.48~6.49(d,J=6.5Hz,1H),5.53(m,1H),4.41~4.43(d,J=6.5Hz,1H),3.69~3.71(m,1H),2.77~2.79(m,2H),1.98~2.07(m,2H),1.44~1.46(d,J=6.5Hz,3H),1.39(s,9H).LC-MS:m/z 486.4[M+H]
+。
1 H NMR (500MHz, DMSO- d 6): δ11.35 (br, 1H), 8.55 ~ 8.57 (dd, J = 7.0Hz, 1H), 8.31 ~ 8.32 (d, J = 7.0Hz, 1H), 8.11 (s, 1H), 7.11 to 7.14 (dd, J = 2.5, 9.0 Hz, 2H), 6.96 to 6.99 (ddd, 1H), 6.83 to 6.86 (dd, J = 4.5, 9.0 Hz, 1H), 6.48 to 6.49 (d, J = 6.5 Hz, 1H), 5.53 (m, 1H), 4.41 to 4.43 (d, J = 6.5 Hz, 1H), 3.69 to 3.71 (m, 1H), 2.77 to 2.79 (m, 2H), 1.98-2.07 (m, 2H), 1.44 to 1.46 (d, J = 6.5 Hz, 3H), 1.39 (s, 9H). LC-MS: m/z 486.4 [M+H] + .
步骤7:5-((S)-1-(2-((1s,3R)-3-氨基环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸三盐酸盐Step 7: 5-((S)-1-(2-((1s,3R)-3-Aminocyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazolo[1,5- a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中加入化合物9C(0.33g),4N盐酸的1,4-二氧六环溶液(5mL),混合物在室温下反应。反应完成后,停止搅拌,旋干,再加入乙腈得到白色混悬物,抽滤得到的滤饼再通过减压干燥得化合物9D(0.30g)。LC-MS:m/z 408.3[M+Na]
+。
Compound 9C (0.33 g), 4N hydrochloric acid in 1,4-dioxane solution (5 mL) was added to the reaction mixture, and the mixture was reacted at room temperature. After the completion of the reaction, the stirring was stopped, the mixture was dried, and then acetonitrile was added to give a white suspension. The filter cake obtained by suction filtration was dried under reduced pressure to give compound 9D (0.30 g). LC-MS: m/z 408.3 [M+Na] + .
步骤8:(3
1R,3
3R,6
3E,6
4E,8S)-1
4-氟-8-甲基-2-氧-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶-1(1,2)-苯基-3(1,3)-环丁醇环辛内酰胺-5-酮(化合物I-2)
Step 8: (3 1 R,3 3 R,6 3 E,6 4 E,8S)-1 4 -fluoro-8-methyl-2-oxo-4,7-diaza-6 (3,5 )-pyrazolo[1,5-a]pyrimidin-1(1,2)-phenyl-3(1,3)-cyclobutanol cyclooctanolac-5-one (Compound I-2)
向反应瓶中加入化合物9D(0.30g),DIPEA(0.63g),DMF(4mL)及二氯甲烷(16mL),N
2保护下,混合物在室温下搅拌30分钟后,五氟苯酚二苯基磷酸酯(0.25g)加入反应体系中,混合物在室温下搅拌反应。反应完成后,向反应中滴加2M碳酸钠水溶液(20mL),分液,水相再用DCM萃取(20mL×3),合并有机相,加入无水硫酸钠干燥,抽滤,旋干。浓缩物通过柱层析纯化(DCM:MeOH=100:0~90:10)得化合物I-2(0.11g)。
After the compound was added to the reaction flask 9D (0.30g), DIPEA (0.63g ), under DMF (4mL) and methylene chloride (16mL), N 2 protection, and the mixture was stirred at room temperature for 30 minutes, diphenyl pentafluorophenol Phosphate ester (0.25 g) was added to the reaction system, and the mixture was stirred at room temperature. After completion of the reaction, a 2M aqueous solution of sodium carbonate (20 mL) was added dropwise, and the mixture was evaporated. The concentrate was purified by column chromatography (DCM: MeOH = 100:0 to 90:10) to yield Compound 1-2 (0.11 g).
化合物I-2:
1H NMR(500MHz,DMSO-d
6):δ9.29~9.31(d,J=11.0Hz,1H),8.75~8.77(d,J=7.5Hz,1H),8.56~8.57(d,J=8.0Hz,1H),8.04(s,1H),7.17~7.20(dd,J=3.0,9.0Hz,1H),6.97~7.01(ddd,1H),6.58~6.61(dd,J=4.5,9.0Hz,1H),6.38~6.39(d,J=8.0Hz,1H),5.76~5.80(t,J=6.0,13.0Hz,1H),4.92~4.93(d,J=4.0Hz,1H),4.68~4.70(m,1H),3.08~3.09(m,1H),2.83~2.85(m,1H),2.10~2.14(m,1H),1.61~1.66(m,1H),1.43~1.45(d,J=7.0Hz,3H).
Compound I-2: 1 H NMR (500 MHz, DMSO-d 6 ): δ 9.29 - 9.31 (d, J = 11.0 Hz, 1H), 8.75 - 8.77 (d, J = 7.5 Hz, 1H), 8.56 - 8.57 (d, J = 8.0 Hz, 1H), 8.04 (s, 1H), 7.17 to 7.20 (dd, J = 3.0, 9.0 Hz, 1H), 6.97 to 7.01 (ddd, 1H), 6.58 to 6.61 (dd, J = 4.5, 9.0 Hz, 1H), 6.38 to 6.39 (d, J = 8.0 Hz, 1H), 5.76 to 5.80 (t, J = 6.0, 13.0 Hz, 1H), 4.92 to 4.93 (d, J = 4.0 Hz, 1H), 4.68 to 4.70 (m, 1H), 3.08 to 3.09 (m, 1H), 2.83 to 2.85 (m, 1H), 2.10 to 2.14 (m, 1H), 1.61 to 1.66 (m, 1H), 1.43 1.45 (d, J = 7.0 Hz, 3H).
13C NMR(125MHz,DMSO-d
6):161.18,158.01,156.13,155.51,149.18,146.55,144.21,136.61,114.37,101.67,100.93,74.35,55.37,43.85,42.93,37.19,36.11,23.15。LC-MS:m/z 368.3[M+H]
+.
13 C NMR (125 MHz, DMSO-d 6 ): 161.18, 150.01, 156.13, 155.51, 149.18, 146.55, 144.21, 136.61, 114.37, 101.67, 100.93, 74.35, 55.37, 43.85, 42.93, 37.19, 36.11, 23.15. LC-MS: m/z 368.3 [M+H] + .
实施例3:(3
1R,3
3R,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁烷杂环辛内酰胺-5-酮
Example 3: (3 1 R,3 3 R,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidine-l-1(1,2)-benzene-3(1,3)-cyclobutane heterocyclic caprolactam-5-one
步骤1:(1s,3s)-3-((叔丁氧基羰基)氨基)环丁基甲磺酸酯Step 1: (1s, 3s)-3-((tert-Butoxycarbonyl)amino)cyclobutylmethanesulfonate
-30℃下,将甲基磺酰氯(3.67g)(溶液于25mL二氯甲烷中)缓慢滴入((1s,3s)-3-羟基环丁基)氨基甲酸叔丁酯(5g)及三乙胺(8.11g)的二氯甲烷(50mL)溶液中,滴加完毕,混合物在剧烈搅拌下由-30℃缓慢升至室温并搅拌过夜。反应完成,反应液分别用水(100mL)、5wt%柠檬酸水溶液(50mL)及浓盐水(100mL)洗涤,干燥。过滤,浓缩得到黄色固体,石油醚:乙酸乙酯=44mL:22mL室温打浆半小时,过滤干燥得到化合物12A(6.32g)。Methanesulfonyl chloride (3.67g) (solution in 25mL of dichloromethane) was slowly added dropwise ((1s, 3s)-3-hydroxycyclobutyl)carbamic acid tert-butyl ester (5g) and three at -30 °C A solution of ethylamine (8.11 g) in dichloromethane (50 mL) was applied dropwise, and the mixture was slowly warmed from -30 ° C to room temperature and stirred overnight. The reaction was completed, and the reaction mixture was washed with water (100 mL), 5 wt% aqueous citric acid (50 mL) and concentrated brine (100 mL) and dried. Filtration and concentration gave a yellow solid. EtOAc (EtOAc:EtOAc)
1H NMR(500MHz,CDCl
3)δ4.83-4.60(m,2H),3.81(br s,1H),2.98(s,3H),2.94-2.85(m,2H),2.22-2.14(m,2H),1.43(s,9H).
1 H NMR (500MHz, CDCl 3 ) δ4.83-4.60 (m, 2H), 3.81 (br s, 1H), 2.98 (s, 3H), 2.94-2.85 (m, 2H), 2.22-2.14 (m, 2H), 1.43 (s, 9H).
步骤2:((1R,3r)-3-(2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环丁基)氨基甲酸叔丁酯Step 2: ((1R,3r)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl carbamate
向反应瓶中依次加入化合物1C(3.23g)、碳酸铯(7.37g)、N,N-二甲基甲酰胺(80mL),混合物室温搅拌0.5小时。将混合物加热至40℃,向反应液中加入化合物12A(3g)的DMF(25mL)溶液,将混合物加热至120℃反应7小时。反应完成,减压浓缩得到残余物,加入乙酸乙酯300mL,硅藻土助虑,滤液用饱和食盐水洗两次(100mL×2),干燥。过滤,所得滤液减压浓缩得到的残余物经柱层析(石油醚/乙酸乙酯=4/1)得到化合物12B(1.89g)。Compound 1C (3.23 g), cesium carbonate (7.37 g), and N,N-dimethylformamide (80 mL) were sequentially added to the reaction mixture, and the mixture was stirred at room temperature for 0.5 hr. The mixture was heated to 40 ° C, and a solution of Compound 12A (3 g) in DMF (25 mL) was added to the reaction mixture, and the mixture was heated to 120 ° C for 7 hours. The reaction was completed, and the residue was evaporated to dryness crystals crystals crystals crystals Filtration and the resulting residue were evaporated to dryness crystalljjjjjjjjjj
1H NMR(500MHz,CDCl
3)δ7.04(dd,J=9.1,2.9Hz,1H),6.87(td,J=8.5,3.0Hz,1H),6.55(dd,J=8.9,4.3Hz,1H),4.88–4.75(m,3H),4.29(br s,1H),3.82(d,J=4.4Hz,1H),2.63-2.55(m,2H),2.47-2.38(m,2H),1.49(d,J=6.7Hz,3H),1.47(s,9H),1.24(s,9H).MS:m/z=451.4[M+Na]
+.
1 H NMR (500 MHz, CDCl 3 ) δ 7.04 (dd, J = 9.1, 2.9 Hz, 1H), 6.87 (td, J = 8.5, 3.0 Hz, 1H), 6.55 (dd, J = 8.9, 4.3 Hz, 1H), 4.88–4.75 (m, 3H), 4.29 (br s, 1H), 3.82 (d, J = 4.4 Hz, 1H), 2.63-2.55 (m, 2H), 2.47-2.38 (m, 2H), 1.49 (d, J = 6.7 Hz, 3H), 1.47 (s, 9H), 1.24 (s, 9H). MS: m/z = 451.4 [M+Na] + .
步骤3:((1R,3r)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)环丁基)氨基甲酸叔丁酯Step 3: ((1R,3r)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中依次加入化合物12B(1.80g)、四氢呋喃(25mL)、水(1mL)及碘单质(0.21g),混合物50℃反应3小时。反应完成,冷却至室温,加入水(55mL)、乙酸乙酯(20mL),剧烈搅拌下向其中加入无水亚硫酸钠固体,再加入固体碳酸氢钠调节体系pH=7~8。加入50mL水稀释,乙酸乙酯萃取(40mL×3),食盐水(100mL)洗涤一次,干燥。过滤,所得滤液减压浓缩得到化合物12C(1.66g)。未经纯化直接用于下一步反应。Compound 12B (1.80 g), tetrahydrofuran (25 mL), water (1 mL) and iodine (0.21 g) were sequentially added to the reaction mixture, and the mixture was reacted at 50 ° C for 3 hours. After the reaction was completed, it was cooled to room temperature, water (55 mL) and ethyl acetate (20 mL) were added, and anhydrous sodium sulfite solid was added thereto with vigorous stirring, and solid sodium hydrogencarbonate was added to adjust the pH of the system to 7-8. It was diluted with 50 mL of water, extracted with ethyl acetate (40 mL × 3), washed once with brine (100 mL) and dried. Filtration and concentration of the filtrate were evaporated under reduced pressure to give compound 12C (1.66 g). It was used in the next reaction without purification.
1H NMR(500MHz,CDCl
3)δ7.20(dd,J=8.9,2.7Hz,1H),6.92(td,J=8.5,3.0Hz,1H),6.56(dd,J=9.0,4.3Hz,1H),4.94(d,J=6.4Hz,1H),4.85-4.78(m,1H),4.67(q,J=6.7Hz,1H),4.35-4.26(m,1H),2.73-2.59(m,2H),2.45-2.38(m,2H),1.49-1.44(m,12H).MS:m/z=347.4[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ) δ7.20 (dd, J = 8.9,2.7Hz, 1H), 6.92 (td, J = 8.5,3.0Hz, 1H), 6.56 (dd, J = 9.0,4.3Hz, 1H), 4.94 (d, J = 6.4 Hz, 1H), 4.85-4.78 (m, 1H), 4.67 (q, J = 6.7 Hz, 1H), 4.35 - 4.26 (m, 1H), 2.73 - 2.59 (m , 2H), 2.45-2.38 (m, 2H), 1.49-1.44 (m, 12H). MS: m/z = 347.4 [M+Na] + .
步骤4:(((R)-1-(2-((1r,3R)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 4: (((R)-1-(2-((1r,3R)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl)amino) Pyrazolo[1,5-a]pyrimidine-3-carboxylate
向35mL微波管中依次加入化合物12C(1.65g)、5-氯吡唑并[1,5-a]嘧啶-3-甲酸乙酯(1.14g)、正丁醇(20mL)和DIPEA(3.56g),搅拌1分钟后,120℃微波反应35分钟。反应完成,减压浓缩得到残余物,加入乙酸乙酯(100mL)和饱和碳酸氢钠水溶液(200mL)。分离有机相,水相用乙酸乙酯(2*100mL)萃取,饱和食盐水(100mL)洗,干燥。过滤,所得滤液减压浓缩得到残余物,经柱层析(石油醚/乙酸乙酯=7/3)得到化合物12D(0.99g)。Compound 12C (1.65 g), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (1.14 g), n-butanol (20 mL) and DIPEA (3.56 g) were sequentially added to a 35 mL microwave tube. After stirring for 1 minute, microwave reaction at 120 ° C for 35 minutes. The reaction was completed and the residue was evaporated. The organic phase was separated and the aqueous extracted with ethyl acetate (2*100 mL) Filtration and concentration of the filtrate were evaporated. mjjjjjjjjj
1H NMR(500MHz,CDCl
3)δ8.27(s,2H),7.10(dd,J=8.7,2.3Hz,1H),6.88(td,J=8.5,2.9Hz,1H),6.60(dd,J=8.9,4.3Hz,1H),6.14(d,J=3.8Hz,1H),5.01-4.83(m,2H),4.45-4.32(m,3H),2.65-2.54(m,2H),2.51-2.42(m,2H),1.62(d,J=6.7Hz,3H),1.47(s,9H),1.42(t,J=7.1Hz,3H).MS:m/z=536.3[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ) δ8.27 (s, 2H), 7.10 (dd, J = 8.7,2.3Hz, 1H), 6.88 (td, J = 8.5,2.9Hz, 1H), 6.60 (dd, J=8.9, 4.3 Hz, 1H), 6.14 (d, J=3.8 Hz, 1H), 5.01-4.83 (m, 2H), 4.45-4.32 (m, 3H), 2.65-2.54 (m, 2H), 2.51 -2.42 (m, 2H), 1.62 (d, J = 6.7 Hz, 3H), 1.47 (s, 9H), 1.42 (t, J = 7.1 Hz, 3H). MS: m/z = 536.3 [M+Na ] + .
步骤5:5-(((R)-1-(2-((1r,3R)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 5: 5-(((R)-1-(2-((1r,3R)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入化合物12D(0.97g)、氢氧化锂一水合物(0.476g)、水(1mL)、四氢呋喃(2mL)及甲醇(4mL),混合物加热至70℃反应1.5小时。反应完成,倒入150mL冰水中,稀盐酸调节pH=5,二氯甲烷萃取(40mL×4),饱和食盐水(100mL)洗涤,干燥。过滤,所得滤液减压浓缩得到化合物12E(913mg)。未经纯化直接用于下一步反应。To the reaction flask, a compound 12D (0.97 g), lithium hydroxide monohydrate (0.476 g), water (1 mL), tetrahydrofuran (2 mL) and methanol (4 mL) were sequentially added, and the mixture was heated to 70 ° C for 1.5 hours. The reaction was completed, poured into 150 mL of ice water, diluted with hydrochloric acid to adjust pH = 5, extracted with dichloromethane (40 mL × 4), washed with brine (100 mL) and dried. Filtration and concentration of the filtrate were evaporated under reduced pressure to give Compound 12E (913mg). It was used in the next reaction without purification.
1H NMR(500MHz,CDCl
3)δ8.24(br s,2H),7.07-6.98(m,1H),6.93-6.75(m,2H),6.60(dd,J=8.1,3.5Hz,1H),6.45(br s,1H),5.49(s,1H),5.03-4.81(m,2H),4.40(s,1H),2.72-2.40(m,4H),1.64-1.56(m,3H),1.47(s,9H).MS:m/z=508.4[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ) δ8.24 (br s, 2H), 7.07-6.98 (m, 1H), 6.93-6.75 (m, 2H), 6.60 (dd, J = 8.1,3.5Hz, 1H) , 6.45 (br s, 1H), 5.49 (s, 1H), 5.03-4.81 (m, 2H), 4.40 (s, 1H), 2.72-2.40 (m, 4H), 1.64-1.56 (m, 3H), 1.47 (s, 9H). MS: m / z = 508.4 [M + Na] + .
步骤6:5-(((R)-1-(2-((1r,3R)-3-氨基环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸Step 6: 5-((R)-1-(2-((1r,3R)-3-Aminocyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazolo[1,5 -a]pyrimidine-3-carboxylic acid
向反应瓶中加入化合物12E(900mg),加入二氯甲烷(10mL),搅拌下加入氯化氢的1,4-二氧六环溶液(4M,10.00mL),混合物50℃搅拌1小时。反应完成,减压浓缩得到固体,加入乙腈10mL打浆。过滤干燥得到化合物12F(738mg)。未经纯化直接用于下一步反应。MS:m/z=408.2[M+Na]
+.
Compound 12E (900 mg) was added to a reaction mixture, dichloromethane (10 mL) was added, and a solution of hydrogen chloride in 1,4-dioxane (4M, 10.00 mL) was added, and the mixture was stirred at 50 ° C for 1 hour. The reaction was completed and concentrated under reduced pressure to give a solid. Filtration and drying gave Compound 12F (738 mg). It was used in the next reaction without purification. MS: m/z = 408.2 [M+Na] + .
步骤7:(3
1R,3
3R,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁烷杂环辛内酰胺-5-酮(化合物I-3)
Step 7: (3 1 R,3 3 R,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutane heterocyclic caprolactam-5-one (Compound I-3) )
向反应瓶中加入化合物12F(300mg)、DMF(12mL)、二氯甲烷(20mL)及DIPEA(735mg),室温搅拌下向其中加入FDPP(130mg),继续室温搅拌30分钟,向其中加入剩余的FDPP(157mg),继续室温搅拌2.5小时,停止反应,向体系中加入乙酸乙酯(300mL),饱和碳酸氢钠水溶液(100mL)、食盐水(100mL×2)洗涤有机相,干燥。过滤,所得滤液减压浓缩得到残余物,经柱层析纯化(二氯甲烷/甲醇=96/4)得到化合物I-3(77mg)。Compound 12F (300 mg), DMF (12 mL), dichloromethane (20 mL), and DIPEA (735 mg) were added to the reaction flask, and FDPP (130 mg) was added thereto at room temperature under stirring, and the mixture was stirred at room temperature for 30 minutes, and the remainder was added thereto. FDPP (157 mg) was stirred at room temperature for 2.5 hours, the reaction was stopped, ethyl acetate (300 mL) was added to the mixture, and the organic phase was washed with saturated aqueous sodium hydrogen carbonate (100 mL) and brine (100 mL×2) and dried. Filtration, the obtained filtrate was concentrated under reduced vacuo. mjjjjjjj
化合物I-3:
1H NMR(500MHz,DMSO)δ8.46(t,J=7.8Hz,2H),8.23(s,1H),7.62(d,J=9.1Hz,1H),7.25(dd,J=9.6,2.0Hz,1H),7.00-6.93(m,2H),6.88-6.79(m,1H),6.33(d,J=7.6Hz,1H),5.91-5.84(m,1H),4.98(t,J=4.6Hz,1H),2.49-2.40(m,2H),1.85-1.78(m,1H),1.72-1.65(m,1H),1.38(d,J=7.0Hz,3H).MS:m/z=368.4[M+H]
+.
Compound I-3: 1 H NMR (500 MHz, DMSO) δ 8.46 (t, J = 7.8 Hz, 2H), 8.23 (s, 1H), 7.62 (d, J = 9.1 Hz, 1H), 7.25 (dd, J=9.6, 2.0 Hz, 1H), 7.00-6.93 (m, 2H), 6.88-6.79 (m, 1H), 6.33 (d, J = 7.6 Hz, 1H), 5.91-5.84 (m, 1H), 4.98 (t, J = 4.6 Hz, 1H), 2.49-2.40 (m, 2H), 1.85-1.78 (m, 1H), 1.72-1.65 (m, 1H), 1.38 (d, J = 7.0 Hz, 3H). MS: m/z = 368.4 [M+H] + .
实施例4:(1
3E,1
4E,2
2R,5
1S,5
3S)-3
5-氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-α]嘧啶杂-2(1,2)-吡咯杂-3(1,2)-苯杂-5(1,3)-环丁醇环辛内酰胺-7-酮
Example 4: (1 3 E, 1 4 E, 2 2 R, 5 1 S, 5 3 S)-3 5 -fluoro-4-oxa-6-aza-1(5,3)-pyrazole And [1,5-α]pyrimidinium-2(1,2)-pyrrole-3(1,2)-benzene-5(1,3)-cyclobutanol cyclooctanolactam-7-one
步骤1:(R)-4-氟-2-(吡咯烷-2-基)苯酚Step 1: (R)-4-fluoro-2-(pyrrolidin-2-yl)phenol
N
2保护及-40℃下,将1M三溴化硼的二氯甲烷溶液(5.19mL)缓慢滴加至化合物14A(0.40g)的二氯甲烷(15mL)的搅拌液中。反应恢复至室温,反应完全后,冰水浴下缓慢向反应中滴加饱和碳酸氢钠水溶液淬灭反应;然后通过二氯甲烷萃取(30mL×3),合并有机相,加入无水硫酸钠干燥,抽滤,浓缩,干燥得到化合物14B(0.28g)。
A 1 M solution of boron tribromide in dichloromethane (5.19 mL) was slowly added dropwise to a stirred solution of compound 14A (0.40 g) in dichloromethane (15 mL). The reaction was returned to room temperature. After the reaction was completed, a saturated aqueous solution of sodium hydrogencarbonate was added dropwise to the reaction mixture, and the mixture was evaporated to methylene chloride (30 mL × 3), and the organic phase was combined and dried over anhydrous sodium sulfate. Filtration, concentration and drying gave compound 14B (0.28 g).
1H NMR(500MHz,CDCl
3):δ6.84(ddd,1H),6.81(ddd,1H),6.71(dd,1H),4.31(t,1H),3.24(m,1H),3.13(m,1H),2.24(m,1H),2.03(m,1H),1.91(m,2H).LC-MS:m/z 182.4[M+H]
+.
1 H NMR (500MHz, CDCl 3 ): δ6.84 (ddd, 1H), 6.81 (ddd, 1H), 6.71 (dd, 1H), 4.31 (t, 1H), 3.24 (m, 1H), 3.13 (m , 1H), 2.24 (m, 1H), 2.03 (m, 1H), 1.91 (m, 2H). LC-MS: m/z 182.4 [M+H] + .
步骤2:(R)-5-(2-(5-氟-2-羟基苯基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 2: (R)-5-(2-(5-Fluoro-2-hydroxyphenyl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向微波反应瓶中加入化合物14B(0.25g)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(0.26g)、DIPEA(0.60g)和正丁醇(8mL),微波条件下,100瓦特下,加热至120℃,反应1小时。反应完成后,向反应混合物中加热饱和碳酸氢钠水溶液,再通过乙酸乙酯萃取(20mL×2),合并有机相,加入无水硫酸钠干燥,抽滤,浓缩。浓缩物通过柱层析纯化(DCM:MeOH=100:0~95:5)得到化合物14C(0.37g)。LC-MS:m/z 371.5[M+H]
+.
To the microwave reaction flask was added compound 14B (0.25 g), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (0.26 g), DIPEA (0.60 g) and n-butanol (8 mL) Under microwave conditions, at 100 watts, it was heated to 120 ° C and reacted for 1 hour. After the reaction was completed, a saturated aqueous solution of sodium hydrogen sulfate was evaporated. The concentrate was purified by column chromatography (DCM: MeOH = 100: EtOAc: EtOAc) LC-MS: m/z 371.5 [M+H] + .
步骤3:5-(R)-2-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟苯基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 3: 5-(R)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)pyrrolidine-1- Ethyl pyrazolo[1,5-a]pyrimidine-3-carboxylate
N
2保护及0℃下,将DIAD(0.29g)缓慢滴加至化合物14C(0.33g)、反式-3-羟基环丁基氨基甲酸叔丁酯(0.20g)、三苯基膦(0.37g)的四氢呋喃(5mL)溶液中,然后恢复至室温反应。反应完全后,浓缩,浓缩物通过柱层析分离(PE:EA=50:1~1:1)得到化合物14D(0.45g)。LC-MS:m/z 540.5[M+H]
+.
N 2 protection and DIAD (0.29 g) were slowly added dropwise to compound 14C (0.33 g), trans-3-hydroxycyclobutylcarbamic acid tert-butyl ester (0.20 g), triphenylphosphine (0.37) at 0 °C. g) in tetrahydrofuran (5 mL) solution, and then returned to room temperature for reaction. After completion of the reaction, the mixture was concentrated, and the concentrate was separated by column chromatography (PE: EA = 50:1 to 1:1) to afford compound 14D (0.45 g). LC-MS: m/z 540.5 [M+H] + .
步骤4:5-((R)-2-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟苯基)吡咯烷-1-基)吡唑并[1,5-α]嘧啶-3-羧酸Step 4: 5-((R)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)pyrrolidine-1 -yl)pyrazolo[1,5-α]pyrimidine-3-carboxylic acid
依次向反应瓶中加入化合物14D(0.44g)、甲醇(10mL)、四氢呋喃(4mL)、水(1mL)及氢氧化锂的一水合物(0.21g),N
2保护下,将反应混合物加热至70℃。反应完全后,缓慢向反应混合物中滴加2M的盐酸水溶液调至pH小于5,加水稀释,再用二氯甲烷萃取(20mL×3),合并有机相,加入无水硫酸钠干燥,抽滤,浓缩,干燥得到化合物14E(0.36g)。LC-MS:m/z 534.5[M+Na]
+.
Compound 14D (0.44g), methanol (10mL), tetrahydrofuran (4mL), water (1mL) and lithium hydroxide monohydrate (0.21g) were added to the reaction flask, and the reaction mixture was heated under N 2 protection. 70 ° C. After the reaction was completed, 2M aqueous hydrochloric acid solution was added dropwise to the reaction mixture to adjust to pH less than 5, diluted with water, and extracted with dichloromethane (20 mL×3), the organic phase was combined, dried over anhydrous sodium sulfate and filtered. Concentration and drying gave compound 14E (0.36 g). LC-MS: m/z 534.5 [M+Na] + .
步骤5:5-((R)-2-(2-((1s,3S)-3-氨基环丁氧基)-5-氟苯基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸三盐酸盐Step 5: 5-((R)-2-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluorophenyl)pyrrolidin-1-yl)pyrazolo[1, 5-a]pyrimidine-3-carboxylic acid trihydrochloride
依次向反应瓶中加入化合物14E(0.36g)、4M盐酸的1,4-二氧六环溶液(3mL),N
2保护下,反应混合物在室温下搅拌反应。反应完全后,浓缩干燥得到化合物14F(0.30g)。LC-MS:m/z 410.4[M-H]
-.
Compounds were added at 14E (0.36g) added to the reaction flask, 1,4-dioxane solution of 4M hydrochloric acid (3mL), N 2 protection, reaction mixture was stirred at room temperature. After completion of the reaction, it was concentrated to dryness to give Compound 14F (0.30 g). LC-MS: m/z 410.4 [MH] - .
步骤6:(1
3E,1
4E,2
2R,5
1S,5
3S)-3
5-氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-α]嘧啶杂-2(1,2)-吡咯杂-3(1,2)-苯杂-5(1,3)-环丁醇环辛内酰胺-7-酮(化合物I-4)
Step 6: (1 3 E, 1 4 E, 2 2 R, 5 1 S, 5 3 S)-3 5 -fluoro-4-oxa-6-aza-1(5,3)-pyrazole [1,5-α]pyrimidine-hetero-2(1,2)-pyrrole-3(1,2)-benzene-5(1,3)-cyclobutanol cyclooctanolactam-7-one (compound) I-4)
向反应瓶中加入化合物14F(60mg)、DIPEA(15mg)、DMF(2mL)及二氯甲烷(8mL),N
2保护下,在室温下搅拌30分钟后,加入五氟苯基二苯基磷酸酯(46mg),混合物在室温下搅拌1小时。反应完成后,向反应中滴加2M碳酸钠水溶液(20mL),分出有机相,水相再用DCM萃取(20mL×3),合并有机相,加入无水硫酸钠干燥,抽滤,浓缩。浓缩物通过柱层析分离纯化(DCM:MeOH=100:0~90:10)得化合物I-4(40mg)。
Compound 14F (60 mg), DIPEA (15 mg), DMF (2 mL), and dichloromethane (8 mL) were added to the reaction flask, and the mixture was stirred under N 2 and stirred at room temperature for 30 minutes, then pentafluorophenyldiphenylphosphoric acid was added. The ester (46 mg) was stirred at room temperature for 1 hour. After the reaction was completed, a 2M aqueous solution of sodium carbonate (20 mL) was added dropwise, and the organic phase was separated, and the aqueous phase was extracted with DCM (20 mL×3). The concentrate was separated and purified by column chromatography (DCM: MeOH = 100:0 to 90:10) to yield Compound 1-4 (40 mg).
1H NMR(500MHz,DMSO-d
6):δ9.04~9.06(d,J=10.5Hz,1H),8.73~8.75(d,J=7.5Hz,1H),8.08(s,1H),7.10~7.12(d,J=8.5Hz,1H),6.98~7.02(t,J=8.5Hz,1H),6.58~6.62(m,2H),6.02~6.03(d,J=6.0Hz,1H),4.95~4.96(d,J=3.5Hz,1H),4.68~4.70(d,J=6.5Hz,1H),4.14(s,br,1H),3.64~3.68(t,J=8.0Hz,1H),3.06~3.09(t,J=7.5Hz,1H),2.81~2.84(t,J=6.5Hz,1H),2.37~2.39(m,2H),2.08~2.12(m,2H),1.63~1.68(m,2H).LC-MS:m/z 394.4[M+H]
+.
1 H NMR (500 MHz, DMSO-d 6 ): δ 9.04 - 9.06 (d, J = 10.5 Hz, 1H), 8.73 - 8.75 (d, J = 7.5 Hz, 1H), 8.08 (s, 1H), 7.10 ~7.12 (d, J=8.5 Hz, 1H), 6.98 to 7.02 (t, J=8.5 Hz, 1H), 6.58 to 6.62 (m, 2H), 6.02 to 6.03 (d, J = 6.0 Hz, 1H), 4.95 to 4.96 (d, J = 3.5 Hz, 1H), 4.68 to 4.70 (d, J = 6.5 Hz, 1H), 4.14 (s, br, 1H), 3.64 to 3.68 (t, J = 8.0 Hz, 1H) , 3.06 to 3.09 (t, J = 7.5 Hz, 1H), 2.81 to 2.84 (t, J = 6.5 Hz, 1H), 2.37 to 2.39 (m, 2H), 2.08 to 2.12 (m, 2H), 1.63 to 1.68 (m, 2H). LC-MS: m/z 394.4 [M+H] + .
实施例5:(1
3E,1
4E,2
2R,5
1S,5
3S)-3
5-氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶-3(3,2)-吡啶-2(1,2)-吡咯-5(1,3)-环丁基环庚内酰胺-7-酮
Example 5: (1 3 E, 1 4 E, 2 2 R, 5 1 S, 5 3 S)-3 5 -fluoro-4-oxa-6-aza-1(5,3)-pyrazole And [1,5-a]pyrimidin-3(3,2)-pyridine-2(1,2)-pyrrole-5(1,3)-cyclobutylcycloheptanolac-7-one
步骤1:(R)-5-(2-(5-氟-2-甲氧基吡啶-3-基)吡咯基-1-基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 1: (R)-5-(2-(5-Fluoro-2-methoxypyridin-3-yl)pyrrolyl-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate Ethyl acetate
35mL反应管中依次加入化合物18A(477mg)、化合物5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(400mg)、DIPEA(917mg)和正丁醇(5mL),搅拌1分钟后,放入微波反应器中,100℃反应2小时。反应完全,冷至室温,加入饱和碳酸氢钠水溶液,再通过乙酸乙酯萃取,合并有机相,加入无水硫酸钠干燥,除去干燥剂,柱层析分离(石油醚:乙酸乙酯=40:60)浓缩得到化合物18B(0.76g)。LC-MS:m/z 408.3[M+Na]
+.
Compound 18A (477 mg), compound 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (400 mg), DIPEA (917 mg) and n-butanol (5 mL) were sequentially added to a 35 mL reaction tube and stirred. After 1 minute, it was placed in a microwave reactor and reacted at 100 ° C for 2 hours. The reaction was completed, and the mixture was cooled to room temperature. EtOAc was evaporated. 60) Concentration gave compound 18B (0.76 g). LC-MS: m/z 408.3 [M+Na] + .
步骤2:(R)-5-(2-(5-氟-2-羟基吡啶-3-基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 2: (R)-5-(2-(5-Fluoro-2-hydroxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向反应瓶中加入化合物18B(0.76g)、氯化氢的1,4-二氧六环溶液(4M,10.00mL),50℃搅拌反应。反应完成,用饱和碳酸氢钠水溶液使pH为弱碱性,然后再用DCM萃取(30mL×3),合并有机相,加人无水硫酸钠干燥,抽滤,浓缩干燥得化合物18C(0.70g)。MS:m/z=372.1[M+H]
+.
A compound 18B (0.76 g) and a hydrogen chloride solution of 1,4-dioxane (4M, 10.00 mL) were added to the reaction mixture, and the mixture was stirred at 50 °C. The reaction was completed, the pH was made to be weakly basic with saturated aqueous sodium hydrogen carbonate, and then extracted with DCM (30mL×3). The organic phase was combined, dried over anhydrous sodium sulfate, filtered and concentrated to give compound 18C (0.70 g) ). MS: m/z = 372.1 [M + H] + .
步骤3:5-((R)-2-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟吡啶-3-基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 3: 5-((R)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl)pyrrole Ethyl-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
N
2保护及0℃下,将DIAD(0.38g)缓慢滴加至化合物18C(0.70g)、反式-3-羟基环丁基氨基甲酸叔丁酯(0.35g)、三苯基膦(0.49g)的四氢呋喃(6mL)溶液中,然后恢复至室温反应。反应完全后,浓缩,浓缩物通过柱层析分离(PE:EA=50:1~1:1)得到化合物18D(0.57g)。LC-MS:m/z 541.6[M+H]
+.
N 2 protection and DIAD (0.38 g) were slowly added dropwise to compound 18C (0.70 g), trans-3-hydroxycyclobutylcarbamic acid tert-butyl ester (0.35 g), triphenylphosphine (0.49) at 0 °C. g) in tetrahydrofuran (6 mL) and then returned to room temperature for reaction. After completion of the reaction, the mixture was concentrated, and the concentrate was separated by column chromatography (PE: EA = 50:1 to 1:1) to afford compound 18D (0.57 g). LC-MS: m / z 541.6 [M + H] +.
步骤4:5-((R)-2-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧杂)-5-氟吡啶-3-基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸Step 4: 5-((R)-2-(2-((1s,3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl)pyrrole Alkan-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入化合物18D(566mg)、氢氧化锂一水合物(264mg)、甲醇(10mL)、四氢呋喃(5mL)、水(2mL),氮气保护下,将混合物置于70℃反应。反应完全,冷却至室温,向反应液加入 2N HCl调节溶液pH至小于5,加入乙酸乙酯萃取,饱和氯化钠水洗涤有机相,无水硫酸钠干燥,过滤,浓缩得到化合物18E(610mg)。LC-MS m/z:535.4,[M+Na]+。Into a reaction flask, a compound 18D (566 mg), lithium hydroxide monohydrate (264 mg), methanol (10 mL), tetrahydrofuran (5 mL), and water (2 mL) were sequentially added, and the mixture was allowed to react at 70 ° C under nitrogen atmosphere. The reaction was completed, and the mixture was cooled to room temperature. To the reaction mixture was added 2N HCl to adjust the pH of the solution to less than 5, ethyl acetate was added, and the organic phase was washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered and concentrated to give compound 18E (610 mg) . LC-MS m/z: 535.4, [M+Na]+.
步骤5:5-((R)-2-(2-((1s,3S)-3-氨基环丁基氧杂)-5-氟吡啶-3-基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 5: 5-((R)-2-(2-((1s,3S)-3-Aminocyclobutyloxa)-5-fluoropyridin-3-yl)pyrrolidin-1-yl)pyrazole And [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中加入化合物18E(610mg),冰水浴下加入4N氯化氢的二氧六环溶液12mL,室温下反应。反应完全,浓缩反应液,然后向浓缩液中加入乙腈,有大量的固体析出,过滤干燥得到化合物18F(120mg)。LC-MS m/z:435.2,[M+Na]+。Compound 18E (610 mg) was added to the reaction flask, and 12 mL of 4N hydrogen chloride in dioxane solution was added thereto under ice water, and the mixture was reacted at room temperature. The reaction was completed, the reaction mixture was concentrated, and then acetonitrile was added to the concentrate, and a large amount of solid was precipitated and dried by filtration to give compound 18F (120 mg). LC-MS m/z: 435.2, [M+Na]+.
步骤6:(1
3E,1
4E,2
2R,5
1S,5
3S)-3
5-氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶-3(3,2)-吡啶-2(1,2)-吡咯烷-5(1,3)-环丁基环庚内酰胺-7-酮(I-5)
Step 6: (1 3 E, 1 4 E, 2 2 R, 5 1 S, 5 3 S)-3 5 -fluoro-4-oxa-6-aza-1(5,3)-pyrazole [1,5-a]pyrimidine-3(3,2)-pyridine-2(1,2)-pyrrolidin-5(1,3)-cyclobutylcycloheptanolac-7-one (I-5)
向反应瓶中依次加入DIPEA(276mg)、二氯甲烷(60mL)、DMF(10.0mL),氮气保护下,将化合物18F(122mg)加入到上述反应液中,将化合物五氟苯基二苯基磷酸酯(90mg)配制成DMF(2mL)的溶液,然后加到上述反应液中,室温搅拌反应。反应完全。柱层析(二氯甲烷:甲醇=97:3),得到化合物1-5(41mg)。DIPEA (276 mg), dichloromethane (60 mL), and DMF (10.0 mL) were sequentially added to the reaction flask, and the compound 18F (122 mg) was added to the above reaction solution under nitrogen atmosphere to give the compound pentafluorophenyl diphenyl. Phosphate (90 mg) was made into a solution of DMF (2 mL), and then added to the above reaction mixture, and the mixture was stirred at room temperature. The reaction is complete. Column chromatography (dichloromethane:methanol = 97:3) gave Compound 1-5 (41 mg).
1H NMR(500MHz,CDCl
3):δ9.11(d,J=5.5Hz,1H),8.35(s,1H),8.32(d,J=8.0Hz,1H),7.90(s,1H),7.28-7.26(m,1H),6.32(d,J=7.5Hz,1H),6.04(d,J=7.5Hz,1H),5.20(q,J=4.5Hz,1H),4.93-4.92(m,1H),4.01(m,1H),3.71-3.70(m,1H),3.07(t,J=6.5Hz,1H),2.94-2.91(m,1H),2.51-2.47(m,2H),2.31-2.27(m,2H),1.98-1.92(m,2H)。
1 H NMR (500MHz, CDCl 3 ): δ9.11 (d, J = 5.5Hz, 1H), 8.35 (s, 1H), 8.32 (d, J = 8.0Hz, 1H), 7.90 (s, 1H), 7.28-7.26 (m, 1H), 6.32 (d, J = 7.5 Hz, 1H), 6.04 (d, J = 7.5 Hz, 1H), 5.20 (q, J = 4.5 Hz, 1H), 4.93-4.92 (m) , 1H), 4.01 (m, 1H), 3.71-3.70 (m, 1H), 3.07 (t, J = 6.5 Hz, 1H), 2.94 - 2.91 (m, 1H), 2.51-2.47 (m, 2H), 2.31-2.27 (m, 2H), 1.98-1.92 (m, 2H).
13C NMR(125MHz,CDCl
3):δ161.82,156.49,155.44,154.52,154.43,146.11,132.20,128.95,123.41,102.16,98.09,75.48,54.62,48.96,44.62,38.43,36.11,33.56,24.23。LC-MS m/z:395.3,[M+H]
+。
13 C NMR (125 MHz, CDCl 3 ): δ 161.82, 156.49, 155.44, 154.52, 154.43, 146.11, 132.20, 128.95, 123.41, 102.16, 98.09, 75.48, 54.62, 48.96, 44.62, 38.43, 36.11, 33.56, 24.23. LC-MS m/z: 395.3, [M+H] + .
实施例6:(3
1S,3
3S,6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-α]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环丁杂壬内酰胺-5-酮
Example 6: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-α]pyrimidine-l(2,3)-pyridin-3(1,3)-cyclobutanol-5-one
步骤1:(R,E)-N((5-氟-2-甲氧基吡啶-3-基)亚甲基)-2-甲基丙烷-2-亚磺酰胺Step 1: (R,E)-N((5-fluoro-2-methoxypyridin-3-yl)methylene)-2-methylpropane-2-sulfinamide
向反应瓶中,加入化合物20A(6g)、(R)-2-甲基丙烷-2-亚磺酰胺(5.16g)及碳酸铯(20.16g)及二氯甲烷(120mL),氮气保护下,将混合物加热至30℃,反应完全后,浓缩反应液,向残留物中加入水(40 mL)。用乙酸乙酯(60mL)萃取,有机相合并后使用水(60mL)、饱和食盐水(60mL)洗涤,无水硫酸钠干燥,过滤,浓缩,得化合物20B(9.47g)。MS(ESI)m/z:259.3[M+H]
+.
To the reaction flask, compound 20A (6g), (R)-2-methylpropane-2-sulfinamide (5.16g) and cesium carbonate (20.16g) and dichloromethane (120mL) were added, The mixture was heated to 30 ° C. After the reaction was completed, the mixture was concentrated, and water (40 mL) was added to the residue. The mixture was extracted with EtOAc (EtOAc) (EtOAc)EtOAc. MS (ESI) m / z: 259.3 [M + H] +.
步骤2:(R)-N-((R)-1-(5-氟-2-甲氧基吡啶-3-基)乙基)-2-甲基丙烷-2-亚磺酰胺Step 2: (R)-N-((R)-1-(5-fluoro-2-methoxypyridin-3-yl)ethyl)-2-methylpropane-2-sulfinamide
-50℃及N
2保护下,将3N甲基溴化镁的2-甲基四氢呋喃溶液(12.23mL)滴入到化合物20B(9.47g)的THF(120mL)搅拌液中,加毕,混合物在-50℃反应1小时后,将反应液升至室温搅拌。反应完全后,冰水浴下,向反应液中滴加饱和氯化铵水溶液(40mL)。使用乙酸乙酯(60mL)萃取,有机相合并后使用饱和食盐水(60mL)洗涤,无水硫酸钠干燥,过滤,浓缩,柱层析纯化(石油醚:乙酸乙酯=85:15)得化合物20C(787mg)。
And N 2 at -50 ℃ protected, 2-methyl-tetrahydrofuran solution of 3N methylmagnesium bromide (12.23mL) was added dropwise to compound 20B (9.47g) in THF (120mL) was stirred, addition was complete, the mixture After reacting at -50 ° C for 1 hour, the reaction solution was stirred to room temperature and stirred. After completion of the reaction, a saturated aqueous ammonium chloride solution (40 mL) was added dropwise to the mixture. The mixture was extracted with EtOAc (EtOAc) (EtOAc) (EtOAc) 20C (787mg).
1H NMR(500MHz,DMSO-d
6):δ8.040(d,J=3.0Hz,1H),7.759(dd,J=2.5,9.0Hz,1H),5.805(d,J=9.0Hz,1H),4.543(t,J=7.5Hz,1H),3.892(s,3H),1.340(d,J=6.5Hz,3H),1.117(s,9H).MS(ESI)m/z:275.3[M+H]
+.
1 H NMR (500MHz, DMSO- d 6): δ8.040 (d, J = 3.0Hz, 1H), 7.759 (dd, J = 2.5,9.0Hz, 1H), 5.805 (d, J = 9.0Hz, 1H ), 4.543 (t, J = 7.5 Hz, 1H), 3.892 (s, 3H), 1.340 (d, J = 6.5 Hz, 3H), 1.17 (s, 9H). MS (ESI) m/z: 275.3 [ M+H] + .
步骤3:(R)-1-(5-氟-2-甲氧基吡啶-3-基)乙基-1-胺Step 3: (R)-1-(5-fluoro-2-methoxypyridin-3-yl)ethyl-1-amine
向反应瓶中,加入化合物20C(1.60g)、正丁醇(25mL)和水(5mL),搅拌溶解后加入碘粒(0.30g)。将混合物加热50℃,反应完全后,浓缩反应液至干后加入甲苯(60mL),浓缩,然后加入苯(50mL),浓缩,得化合物20D(1.84g)。To the reaction flask, a compound 20C (1.60 g), n-butanol (25 mL) and water (5 mL) were added, stirred and dissolved, and then iodine (0.30 g) was added. The mixture was heated to 50 ° C. After the reaction was completed, the mixture was concentrated to dryness, then toluene (60 <RTIgt; </RTI> <RTIgt; </RTI> <RTIgt;
步骤4:(R)-5-((1-(5-氟-2-甲氧基吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 4: (R)-5-((1-(5-fluoro-2-methoxypyridin-3-yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ester
向含有化合物20D(1.84g)的反应瓶中,加入5-氯吡唑并[1,5-a]嘧啶-3-甲酸乙酯(3.14g)、乙醇(50mL)及DIPEA(12.00g),将混合物加热80℃,反应完全后。浓缩反应液,向残留物中加入乙酸乙酯(200mL),用水(30mL)、饱和食盐水(30mL)洗涤,无水硫酸钠干燥。过滤,浓缩,柱层析纯化(乙酸乙酯:石油醚=0:100~42:58)得化合物20E(1.25g)。To a reaction flask containing compound 20D (1.84 g), ethyl 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (3.14 g), ethanol (50 mL) and DIPEA (12.00 g) were added. The mixture was heated to 80 ° C and the reaction was completed. The reaction mixture was concentrated, and ethyl acetate (200 mL) was evaporated. Filtration, concentration and purification by column chromatography (ethyl acetate: petroleum ether = 0: 100 to 42: 58) gave Compound 20E (1.25 g).
1H NMR(500MHz,CDCl
3):δ8.56(d,J=7.5Hz,1H),8.28(d,J=7.5Hz,1H),8.13(s,1H),8.03(s,1H),7.62(d,J=9.0Hz,1H),6.49(d,J=7.0Hz,1H),5.41(brs,1H),4.15(dd,J=3.5and 6.0Hz,2H),3.94(s,3H),1.48(d,J=7.0Hz,3H),1.23(t,J=7.0Hz,3H).MS(ESI)m/z:382.4[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ): δ8.56 (d, J = 7.5Hz, 1H), 8.28 (d, J = 7.5Hz, 1H), 8.13 (s, 1H), 8.03 (s, 1H), 7.62 (d, J = 9.0 Hz, 1H), 6.49 (d, J = 7.0 Hz, 1H), 5.41 (brs, 1H), 4.15 (dd, J = 3.5 and 6.0 Hz, 2H), 3.94 (s, 3H) ), 1.48 (d, J = 7.0 Hz, 3H), 1.23 (t, J = 7.0 Hz, 3H). MS (ESI) m/z: 382.4 [M+Na] + .
步骤5:(R)-5-((1-(5-氟-2-羟基吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 5: (R)-5-((1-(5-Fluoro-2-hydroxypyridin-3-yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向反应瓶中,加入化合物20E(870mg)、乙醇(15mL)、4N氯化氢的1,4-二氧六环溶液(15.00mL)。将反应液加热80℃。反应完全后,浓缩反应液得化合物20F(850mg)。To the reaction flask, a compound 1,4-E (870 mg), ethanol (15 mL), and 4N hydrogen chloride in 1,4-dioxane (15.00 mL) was added. The reaction solution was heated to 80 °C. After the reaction was completed, the reaction mixture was concentrated to give Compound 20F (850 mg).
1H NMR(500MHz,CDCl
3):δ8.54(d,J=7.0Hz,1H),8.38(brs,1H),8.12(s,1H),7.54(d,J=7.5Hz,1H),7.48(s,1H),6.55(d,J=7.5Hz,1H),5.26(brs,1H),4.17(d,J=5.5Hz,2H),1.46(d,J=7.0Hz,3H),1.26(t,J=7.0Hz,3H).MS(ESI)m/z:368.3[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ): δ8.54 (d, J = 7.0Hz, 1H), 8.38 (brs, 1H), 8.12 (s, 1H), 7.54 (d, J = 7.5Hz, 1H), 7.48 (s, 1H), 6.55 (d, J = 7.5 Hz, 1H), 5.26 (brs, 1H), 4.17 (d, J = 5.5 Hz, 2H), 1.46 (d, J = 7.0 Hz, 3H), 1.26 (t, J = 7.0 Hz, 3H). MS (ESI) m/z: 368.3 [M+Na] + .
步骤6:5-((R)-1-(2-((1s,3S)-3-((叔丁氧羰基)氨基)环丁氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 6: 5-((R)-1-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl)ethyl Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中,加入化合物20F(400mg)、((1r,3r)-3-羟基环丁基)氨基甲酸叔丁酯(217mg)、三苯基膦(516mg)和四氢呋喃(5mL),加毕,冰乙醇浴及氮气保护下搅拌,滴加DEAD(343mg)的THF(2mL)溶液,加毕室温反应。反应完全后,浓缩反应液,柱层析纯化(乙酸乙酯:石油醚=0:100~30:70)得化合物20G(452.1mg)。To the reaction flask, compound 20F (400 mg), ((1r, 3r)-3-hydroxycyclobutyl)carbamic acid tert-butyl ester (217 mg), triphenylphosphine (516 mg) and tetrahydrofuran (5 mL) were added. The mixture was stirred with ice-cold ethanol and nitrogen, and a solution of DEAD (343 mg) in THF (2 mL) was added dropwise. After the reaction was completed, the reaction mixture was concentrated and purified by ethylamine (ethyl acetate: petroleum ether = 0: 100 to 30: 70) to give Compound 20G (452.1mg).
1H NMR(500MHz,CDCl
3):δ8.57(d,J=6.5Hz,1H),8.26(d,J=7.0Hz,1H),8.13(s,1H),7.98(s,1H),7.13(d,J=5.5Hz,1H),6.51(d,J=6.5Hz,1H),5.40(brs,1H),4.89(t,d,J=7.0Hz,1H),4.15(t,J=6.0Hz,2H),3.72(m,1H),2.74(t,d,J=7.5Hz,2H),2.12(m,1H),2.00(m,2H),1.51(d,J=6.5Hz,3H),1.38(s,9H),1.22(m,3H).MS(ESI)m/z:515.4[M+H]
+.
1 H NMR (500MHz, CDCl 3 ): δ8.57 (d, J = 6.5Hz, 1H), 8.26 (d, J = 7.0Hz, 1H), 8.13 (s, 1H), 7.98 (s, 1H), 7.13 (d, J = 5.5 Hz, 1H), 6.51 (d, J = 6.5 Hz, 1H), 5.40 (brs, 1H), 4.89 (t, d, J = 7.0 Hz, 1H), 4.15 (t, J =6.0 Hz, 2H), 3.72 (m, 1H), 2.74 (t, d, J = 7.5 Hz, 2H), 2.12 (m, 1H), 2.00 (m, 2H), 1.51 (d, J = 6.5 Hz) , 3H), 1.38 (s, 9H), 1.22 (m, 3H). MS (ESI) m/z: 515.4 [M+H] + .
步骤7:5-(((R)-1-(2-((1s,3S)-3-((叔丁氧羰基)氨基)环丁氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸Step 7: 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl)) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向含有化合物20G(432.7mg)的反应瓶中,加入甲醇(3.5mL)和四氢呋喃(2mL),溶解后,加入一水合氢氧化锂水溶液(212mg溶于1mL水)。加毕将混合物加热70℃。反应完全后,将反应液倒入冰水(100mL)中,缓慢加入0.5M盐酸调节pH至6,用二氯甲烷萃取,合并二氯甲烷相,无水硫酸钠干燥。过滤,滤液浓缩得化合物20H(412mg)。To a reaction flask containing the compound 20G (432.7 mg), methanol (3.5 mL) and tetrahydrofuran (2 mL) were added, and after dissolved, an aqueous lithium hydroxide monohydrate solution (212 mg dissolved in 1 mL of water) was added. The mixture was heated to 70 ° C after the addition. After completion of the reaction, the reaction mixture was poured into ice water (100 mL). Filtration and concentration of the filtrate gave compound 20H (412 mg).
1H NMR(500MHz,CDCl
3):δ11.46(brs,1H),8.57(s,1H),8.34(d,J=5.0Hz,1H),8.11(s,1H),7.97(s,1H),7.14(s,1H),6.49(s,1H),5.37(brs,1H),4.87(m,1H),3.71(m,1H),2.73(m,2H),2.03(m,3H),1.50(d,J=5.5Hz,3H),1.39(s,9H).MS(ESI)m/z:509.4[M+H]
+,485.4[M-H]
-.
1 H NMR (500MHz, CDCl 3 ): δ11.46 (brs, 1H), 8.57 (s, 1H), 8.34 (d, J = 5.0Hz, 1H), 8.11 (s, 1H), 7.97 (s, 1H ), 7.14 (s, 1H), 6.49 (s, 1H), 5.37 (brs, 1H), 4.87 (m, 1H), 3.71 (m, 1H), 2.73 (m, 2H), 2.03 (m, 3H) , 1.50 (d, J = 5.5 Hz, 3H), 1.39 (s, 9H). MS (ESI) m/z: 509.4 [M+H] + , 485.4 [MH] - .
步骤8:5-((R)-1-(2-((1s,3S)-3-氨基环丁氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 8: 5-((R)-1-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluoropyridin-3-yl)ethyl)amino)pyrazolo[1 ,5-a]pyrimidine-3-carboxylic acid
向含有化合物20H(409mg)的反应瓶中加入4N盐酸的1,4-二氧六环溶液(5.00mL),将混合物加热70℃。反应完全后,将反应液浓缩至干后,向残留物中加入乙腈(3mL),室温打浆。过滤,滤饼用乙腈(1mL)洗涤,干燥得化合物20I(127.9mg)。MS(ESI)m/z:387.3[M+H]
+.
To a reaction flask containing the compound 20H (409 mg), a 4N hydrochloric acid 1,4-dioxane solution (5.00 mL) was added, and the mixture was heated at 70 °C. After the reaction was completed, the reaction mixture was concentrated to dryness. After filtration, the filter cake was washed with EtOAc (1 mL). MS (ESI) m/z: 387.3 [M+H] + .
步骤9:(3
1S,3
3S,6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-α]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环丁杂壬内酰胺-5-酮(化合物I-6)
Step 9: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrazolo[1,5-α]pyrimidin-1(2,3)-pyridin-3(1,3)-cyclobutanolactam-5-one (Compound I-6)
向反应瓶中,加入化合物20I(127.1mg)、二氯甲烷(30mL)和DIPEA(311mg),室温搅拌下,加入五氟苯基二苯基膦酸酯(115mg)的DMF(2mL)溶液。加毕,室温反应。反应完全后,浓缩反应液,向残留物中加入乙酸乙酯(100mL),依次用2M碳酸钠水溶液、水洗涤,饱和食盐水(30mL)洗涤,无水硫酸钠干燥有机相。过滤,浓缩,柱层析纯化(甲醇:二氯甲烷=0:100~4:96)得化合物I-6(64.6mg)。To the reaction flask, a compound 20I (127.1 mg), dichloromethane (30 mL), and DIPEA (311 mg) was added, and a solution of pentafluorophenyldiphenylphosphonate (115 mg) in DMF (2 mL) was added. Addition, reaction at room temperature. After the reaction was completed, the reaction mixture was evaporated, evaporated, evaporated, evaporated Filtration, concentration and purification by column chromatography (methanol: methylene chloride = 0: 100 to 4:96) gave Compound I-6 (64.6mg).
1H NMR(500MHz,CDCl
3)δ9.23(d,J=10.5Hz,1H),8.75(d,J=7.5Hz,1H),8.58(d,J=7.5Hz,1H),8.05(s,1H),8.02(d,J=3.0Hz,1H),7.68(dd,J=3.0and 8.5Hz,1H),6.40(d,J=8.0Hz,1H),5.62(dd,J=5.5and 7.0Hz,1H),5.12(dd,J=4.5and 9.0Hz,1H),4.67(t,J=3.0Hz,1H),3.06(t,J=6.5Hz,1H),2.88(m,1H),2.14(m,1H),1.67(m,1H),1.48(d,J=7.0Hz,3H).
13C NMR(125MHz,CDCl
3):δ161.23,156.86,155.64,146.44,144.33,136.70,132.35,130.5,124.46,101.88,100.99,75.07,44.30,43.43,38.91,35.79,22.69.MS(ESI)e/z:369.3[M+H]
+.
1 H NMR (500MHz, CDCl 3 ) δ9.23 (d, J = 10.5Hz, 1H), 8.75 (d, J = 7.5Hz, 1H), 8.58 (d, J = 7.5Hz, 1H), 8.05 (s , 1H), 8.02 (d, J = 3.0 Hz, 1H), 7.68 (dd, J = 3.0 and 8.5 Hz, 1H), 6.40 (d, J = 8.0 Hz, 1H), 5.62 (dd, J = 5.5 and 7.0 Hz, 1H), 5.12 (dd, J = 4.5 and 9.0 Hz, 1H), 4.67 (t, J = 3.0 Hz, 1H), 3.06 (t, J = 6.5 Hz, 1H), 2.88 (m, 1H) , 2.14 (m, 1H), 1.67 (m, 1H), 1.48 (d, J = 7.0 Hz, 3H). 13 C NMR (125MHz, CDCl 3 ): δ 161.23, 156.86, 155.64, 146.44, 144.33, 136.70, 132.35 , 130.5, 124.46, 101.88, 100.99, 75.07, 44.30, 43.43, 38.91, 35.79, 22.69. MS (ESI) e/z: 369.3 [M+H] + .
实施例7:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶-1(1,2)-苯杂-3(1,3)-环丁基辛内酰胺-5-酮
Example 7: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutylcaprolactam-5-one
步骤1:((1S,3s)-3-(2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环丁基)(甲基)氨基甲酸叔丁酯Step 1: ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl (meth)carbamate
向反应瓶中,依次加入化合物1C(303mg)、化合物((1s,3s)-3-羟基环丁基)(甲基)氨基甲酸叔丁酯(300mg)、三丁基磷(426mg),N
2保护及冰水浴下,将化合物偶氮二甲酰胺(362mg)的四氢呋喃(10mL)溶液滴加至上述反应液中。反应完全,反应液滤去不溶物,使用乙酸乙酯萃取母液,然后使用饱和食盐水(10mL)水洗有机相,无水硫酸钠干燥,过滤浓缩得到化合物21A(450mg),未经分离纯化直接用于下一步反应。
To the reaction flask, a compound 1C (303 mg), a compound ((1s, 3s)-3-hydroxycyclobutyl) (methyl)carbamic acid tert-butyl ester (300 mg), and tributylphosphine (426 mg), N an ice-water bath and the protective, azodicarbonamide compound (362 mg) in tetrahydrofuran (10mL) was added dropwise to the reaction solution. The reaction was completed, and the insoluble material was filtered, and the mixture was evaporated to ethyl acetate. The organic phase was washed with brine (10 mL), dried over anhydrous sodium sulfate and filtered to give Compound 21A (450 mg). In the next step.
步骤2:((1S,3s)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)环丁基)(甲基)氨基甲酸叔丁酯Step 2: ((1S,3s)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)(methyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入化合物21A(180mg)、碘(20.64mg)、四氢呋喃(5mL)及水(0.5mL),N
2保护下,将混合物加热至70℃。反应完全后,向残留物中加入10wt%Na
2S
2O
3水溶液(10mL)淬灭反应, 然后使用乙酸乙酯萃取,合并有机相并使用饱和食盐水洗涤,无水硫酸钠干燥,过滤浓缩,得化合物21B(288mg)。LC-MS m/z:339.5,[M+H]
+。
To the reaction flask, Compound 21A (180 mg), iodine (20.64 mg), tetrahydrofuran (5 mL) and water (0.5 mL) were sequentially added, and the mixture was heated to 70 ° C under N 2 . After completion of the reaction, to the residue was added 10wt% Na 2 S 2 O 3 solution (10 mL) quenched the reaction and then extracted with ethyl acetate, the organic phases were combined and washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated Compound 21B (288 mg) was obtained. LC-MS m/z: 339.5, [M+H] + .
步骤3:5-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)(甲基)氨基)环丁基氧基)-5-氟苯)乙基)氨基)吡咯并[1,5-a]嘧啶-3-羧酸乙酯Step 3: 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)(methyl)amino)cyclobutyloxy)-5-fluorobenzene) Ethyl)amino)pyrrolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向微波反应管中,依次加入化合物21B(288mg)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(192mg)、DIPEA(825mg)及正丁醇(3mL),N
2保护及120℃下,微波条件下反应。反应完全,向残留物中加入乙酸乙酯萃取,分离有机相,然后用饱和食盐水洗涤有机相,无水硫酸钠干燥,过滤浓缩,柱层析(石油醚:乙酸乙酯=60:40)得到化合物21C(81mg)。
To the microwave reaction tube, Compound 21B (288 mg), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (192 mg), DIPEA (825 mg) and n-butanol (3 mL) were sequentially added. , N 2 protection and reaction at 120 ° C under microwave conditions. The reaction was completed, and ethyl acetate was added to the residue. EtOAc was evaporated. Compound 21C (81 mg) was obtained.
1H NMR(500MHz,DMSO-d
6):δ8.543(d,J=7.0Hz,1H),8.178(d,J=7.0Hz,1H),8.123(s,1H),7.175-7.148(dd,J=3.5,10.0Hz,1H),7.020-6.980(dt,J=3.0,8.0Hz,1H),6.884-6.857(dd,J=3.5,9.0Hz,1H),6.478(d,J=7.5Hz,1H),5.626(s,1H),4.492(s,1H),4.183-4.141(m,3H),2.728(s,3H),2.695(br,2H),2.225-2.206(m,1H),2.127-2.110(m,1H),1.472(d,J=7.0Hz,3H),1.398(s,9H),1.254(d,J=7.0Hz,3H)。LC-MS m/z:528.5,[M+H]
+。
1 H NMR (500MHz, DMSO- d 6): δ8.543 (d, J = 7.0Hz, 1H), 8.178 (d, J = 7.0Hz, 1H), 8.123 (s, 1H), 7.175-7.148 (dd , J=3.5, 10.0 Hz, 1H), 7.020-6.980 (dt, J=3.0, 8.0 Hz, 1H), 6.884-6.857 (dd, J=3.5, 9.0 Hz, 1H), 6.478 (d, J=7.5) Hz, 1H), 5.626 (s, 1H), 4.492 (s, 1H), 4.183-4.141 (m, 3H), 2.728 (s, 3H), 2.695 (br, 2H), 2.225-2.206 (m, 1H) , 2.127-2.110 (m, 1H), 1.472 (d, J = 7.0 Hz, 3H), 1.398 (s, 9H), 1.254 (d, J = 7.0 Hz, 3H). LC-MS m/z: 528.5, [M+H] + .
步骤4:5-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)(甲基)氨基)环丁基氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 4: 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)(methyl)amino)cyclobutyloxy)-5-fluorobenzene) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入氢氧化锂一水合物(29.0mg)、化合物21C(365mg)、甲醇(10mL)、四氢呋喃(4mL)及水(2mL),氮气保护下,将混合物加热70℃反应。反应完全,将反应液冷却至室温,向反应液加入2N盐酸调节溶液pH小于5,向反应瓶加入乙酸乙酯萃取,有机相使用饱和氯化钠水溶液洗涤,无水硫酸钠干燥,过滤浓缩,得到化合物21D(409mg)。LC-MS m/z:500.4,[M+H]
+。
To the reaction flask, lithium hydroxide monohydrate (29.0 mg), compound 21C (365 mg), methanol (10 mL), tetrahydrofuran (4 mL) and water (2 mL) were sequentially added, and the mixture was heated at 70 ° C under a nitrogen atmosphere. The reaction was completed, the reaction solution was cooled to room temperature, and the pH of the solution was adjusted to less than 5 by adding 2N hydrochloric acid to the reaction mixture. The mixture was extracted with ethyl acetate. The organic phase was washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate Compound 21D (409 mg) was obtained. LC-MS m/z: 500.4, [M+H] + .
步骤5:5-(((R)-1-(5-氟-2-((1s,3S)-3-(甲基氨基)环丁氧基)苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 5: 5-((R)-1-(5-fluoro-2-((1s,3S)-3-(methylamino)cyclobutoxy)phenyl)ethyl)amino)pyrazole [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中加入化合物21D(409mg),冰水浴下加入4M氯化氢的二氧六环溶液10mL,室温下反应。反应完全后,浓缩除去溶剂,后向浓缩物中加入乙腈,有大量的固体析出,过滤得固体,干燥后得化合物21E(285mg)。LC-MS m/z:398.3,[M-H]
-。
Compound 21D (409 mg) was added to the reaction flask, and 10 mL of a 4 M hydrogen chloride in dioxane solution was added thereto under ice water, and the mixture was reacted at room temperature. After completion of the reaction, the solvent was removed by concentration, and then acetonitrile was added to the concentrate, and a large amount of solid was precipitated, and the solid was filtered to give the compound 21E (285 mg). LC-MS m / z: 398.3 , [MH] -.
步骤6:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-4,8-二甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶-1(1,2)-苯杂-3(1,3)-环丁基环辛基内酰胺-5-酮(化合物1-7)
Step 6: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-4,8-dimethyl-2-oxa-4,7-diaza-6 (3,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctyllactam-5-one (Compound 1-7 )
向反应瓶中依次加入化合物21E(284mg)、DMF(6mL)、二氯甲烷(20mL)、DIPEA(780mg)及五氟苯基二苯基磷酸酯(231mg),氮气保护及室温下搅拌;反应完全后,将反应液浓缩,向浓缩物中滴加2M碳酸钠水溶液(10mL),乙酸乙酯萃取,合并有机相,无水硫酸钠干燥,过滤浓缩。柱层析纯化(二氯甲烷:甲醇=96:4)得到化合物1-7(160mg)。Compound 21E (284 mg), DMF (6 mL), dichloromethane (20 mL), DIPEA (780 mg) and pentafluorophenyldiphenyl phosphate (231 mg) were added to the reaction flask, and the mixture was stirred under nitrogen and stirred at room temperature; After the reaction mixture was concentrated, EtOAc (EtOAc m. Purification by column chromatography (dichloromethane:methanol = 96:4)
1H NMR(500MHz,DMSO-d
6):δ8.475(d,J=7.5Hz,1H),8.387(d,J=7.0Hz,1H),7.899(s,1H),7.088(d,J=9.0Hz,1H),6.879(t,J=8.0Hz,1H),6.465-6.439(dd,J=4.5,9.0Hz,1H),6.313(d,J=7.5Hz,1H),5.641(t,J=7.0Hz,1H),4.831(s,1H),4.203(s,1H),3.199(s,3H),3.176-2.826(m,1H),2.782-2.690(m,1H),2.678-2.649(m,1H),1.689-1.661(dd,J=6.0Hz,13.5Hz,1H),1.413(d,J=7.0Hz,3H)。
1 H NMR (500MHz, DMSO- d 6): δ8.475 (d, J = 7.5Hz, 1H), 8.387 (d, J = 7.0Hz, 1H), 7.899 (s, 1H), 7.088 (d, J = 9.0 Hz, 1H), 6.789 (t, J = 8.0 Hz, 1H), 6.465-6.439 (dd, J = 4.5, 9.0 Hz, 1H), 6.313 (d, J = 7.5 Hz, 1H), 5.641 (t , J=7.0 Hz, 1H), 4.831 (s, 1H), 4.203 (s, 1H), 3.199 (s, 3H), 3.176-2.826 (m, 1H), 2.782-2.690 (m, 1H), 2.678- 2.649 (m, 1H), 1.689-1.661 (dd, J = 6.0 Hz, 13.5 Hz, 1H), 1.413 (d, J = 7.0 Hz, 3H).
13C NMR(125MHz,DMSO-d
6):δ165.14,157.72,155.85,149.83,146.01,144.03,137.50,137.46,135.92,113.74,113.25,104.05,100.39,72.31,55.36,51.49,43.25,36.02,29.70,25.54。LC-MS m/z:404.3,[M+Na]
+
13 C NMR (125 MHz, DMSO-d 6 ): δ 165.14, 157.72, 155.85, 149.83, 146.01, 144.03, 137.50, 137.46, 135.92, 113.74, 113.25, 104.05, 100.39, 72.31, 55.36, 51.49, 43.25, 36.02, 29.70, 25.54. LC-MS m/z: 404.3, [M+Na] +
实施例8:(1
3E,1
4E,7
1S,7
3S,3R)-4
5-氟-3-甲基-5-氧杂-2,8-二氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-4(1,2)-苯杂-7(1,3)-环丁基壬内酰胺-9-酮
Example 8: (1 3 E,1 4 E,7 1 S,7 3 S,3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1(5 ,3)-pyrazolo[1,5-a]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutyllactam-9-one
步骤1:((1s,3s)-3-(羟甲基)环丁基)氨基甲酸叔丁酯Step 1: ((1s, 3s)-3-(hydroxymethyl)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中,加入化合物23A(505mg)、三乙胺(557mg)、四氢呋喃(10mL)及水(2mL),冰水浴搅拌,加入二碳酸二叔丁酯(841mg)。后室温反应。反应完成后,将反应液倒入乙酸乙酯(200mL)中,搅拌后依次用水(50mL)、饱和食盐水(50mL)洗涤,无水硫酸钠干燥,过滤、浓缩,得到化合物23B(791mg),未经分离纯化直接用于下一步反应。To the reaction flask, Compound 23A (505 mg), triethylamine (557 mg), tetrahydrofuran (10 mL) and water (2 mL) were added, and the mixture was stirred in ice water, and di-tert-butyl dicarbonate (841 mg) was added. After the reaction at room temperature. After completion of the reaction, the reaction mixture was poured into EtOAc EtOAc (EtOAc) It was used in the next reaction without isolation and purification.
步骤2:((1S,3s)-3-((2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)甲基)环丁基)氨基甲酸叔丁酯Step 2: ((1S,3s)-3-((2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)) Tert-butyl)carbamic acid tert-butyl ester
向反应瓶中依次加入化合物23B(853mg)、化合物1C(791mg)及三苯基磷(1296mg),N
2保护及冰水浴下,将DEAD(974mg)的四氢呋喃(2mL)溶液滴加至上述反应液中。反应完全后,向反应体系加入石油醚,滤去不溶物,使用乙酸乙酯萃取滤液,使用饱和食盐水洗涤有机相,无水硫酸钠干燥、过滤、浓缩,得到化合物23C(3.6g)。粗品不再纯化直接下一步反应。LC-MS m/z:465.4,[M+Na]
+。
Compound 23B (853 mg), Compound 1C (791 mg) and triphenylphosphine (1296 mg) were sequentially added to the reaction flask, and the solution of DEAD (974 mg) in tetrahydrofuran (2 mL) was added dropwise to the above reaction under N 2 and ice water bath. In the liquid. After the completion of the reaction, petroleum ether was added to the reaction mixture, the insoluble material was filtered, and the filtrate was extracted with ethyl acetate. The organic phase was washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated to afford compound 23C (3.6 g). The crude product was no longer purified directly to the next reaction. LC-MS m/z: 465.4, [M+Na] + .
步骤3:((1S,3s)-3-((2-((R)-1-氨基乙基)-4-氟苯氧基)甲基)环丁基)氨基甲酸叔丁酯Step 3: ((1S,3s)-3-((2-((R)-1-aminoethyl)-4-fluorophenoxy)methyl)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中加入化合物23C(3.6g)、碘(2.064g)、四氢呋喃(10mL)及水(0.147g),N
2保护下,将混合物加热至70℃。反应完全,向反应液中加入10wt%Na
2S
2O
3的水溶液(10mL),使用乙酸乙酯萃取,合并有机相,用15mL饱和食盐水洗涤有机相,无水硫酸钠干燥,过滤浓缩,得到化合物23D(2.8g)。LC-MS m/z:361.5,[M+Na]
+。
Compound 23C (3.6 g), iodine (2.064 g), tetrahydrofuran (10 mL) and water (0.147 g) were added to the reaction flask, and the mixture was heated to 70 ° C under N 2 . The reaction was completed, and a 10 wt% aqueous solution of Na 2 S 2 O 3 (10 mL) was added to the mixture, and the mixture was combined with ethyl acetate. Compound 23D (2.8 g) was obtained. LC-MS m/z: 361.5, [M+Na] + .
步骤4:5-(((R)-1-(2-(((1s,3S)-3-((叔丁基氧羰基)氨基)环丁基)甲氧基)-5-氟苯氧基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 4: 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)methoxy)-5-fluorophenoxy Ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中,加入化合物23D(3.2g)、化合物7(361mg)、正丁醇(12mL)及DIPEA(1.447g),N
2保护下,加热120℃。反应完全,向残留物中加入乙酸乙酯萃取,分离有机相,用饱和食盐水洗涤有机相,无水硫酸钠干燥,过滤浓缩,柱层析分离(石油醚:乙酸乙酯=50:50)得到化合物23E(200mg)。LC-MS m/z:528.5,[M+H]
+。
To the reaction flask, compound 23D (3.2 g), compound 7 (361 mg), n-butanol (12 mL) and DIPEA (1.447 g) were added, and the mixture was heated to 120 ° C under N 2 . The reaction was completed, and ethyl acetate was added to the residue, and the organic layer was separated, and the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and evaporated. Compound 23E (200 mg) was obtained. LC-MS m/z: 528.5, [M+H] + .
步骤5:5-(((R)-1-(2-(((1s,3S)-3-((叔丁基氧羰基)氨基)环丁基)甲氧基)-5-氟苯氧)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 5: 5-(((R)-1-(2-(((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)methoxy)-5-fluorophenoxy) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,加入氢氧化锂一水合物(96mg)、化合物23E(190mg)、甲醇(2mL)、四氢呋喃(0.5mL)及水(1mL),氮气保护下,将混合物加热70℃反应。反应完全后,将反应液冷却至室温,向反应 液加入2N HCl调节溶液pH小于5,向反应瓶加入乙酸乙酯萃取,有机相使用饱和食盐水洗涤,无水硫酸钠干燥,过滤浓缩,得到化合物23F(164mg)。LC-MS m/z:522.4,[M+Na]
+
Lithium hydroxide monohydrate (96 mg), compound 23E (190 mg), methanol (2 mL), tetrahydrofuran (0.5 mL) and water (1 mL) were added to the reaction flask, and the mixture was heated at 70 ° C under a nitrogen atmosphere. After the reaction was completed, the reaction mixture was cooled to room temperature, and 2N HCl was added to the reaction mixture to adjust the pH of the solution to less than 5, and ethyl acetate was added to the reaction mixture. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate Compound 23F (164 mg). LC-MS m/z: 522.4, [M+Na] +
步骤6:5-(((R)-1-(2-(((1s,3S)-3-氨基环丁基)甲氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐向反应瓶中加入化合物23F(164mg),冰水浴下加入4N氯化氢的二氧六环10mL,在室温下反应。反应完全后,浓缩除去溶剂,后向浓缩物中加入乙腈,有大量的固体析出,过滤得到固体,干燥得化合物23G(130mg)。LC-MS m/z:422.3,[M+Na]
+。
Step 6: 5-(((R)-1-(2-((1(3S))-3-aminocyclobutyl)methoxy)-5-fluorophenyl)ethyl)amino)pyrazole [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride salt Compound 23F (164 mg) was added to a reaction flask, and 10 mL of 4N hydrogen chloride in dioxane was added thereto under ice water, and the mixture was reacted at room temperature. After completion of the reaction, the solvent was removed by concentration, and then acetonitrile was added to the concentrate, and a large solid was precipitated, which was filtered to give a solid and dried to give compound 23G (130 mg). LC-MS m/z: 422.3, [M+Na] + .
步骤7:(1
3E,1
4E,7
1S,7
3S,3R)-4
5-氟-3-甲基-5-氧杂-2,8-二氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-4(1,2)-苯杂-7(1,3)-环丁基壬内酰胺-9-酮(化合物I-8)
Step 7: (1 3 E, 1 4 E, 7 1 S, 7 3 S, 3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1 (5, 3)-pyrazolo[1,5-a]pyrimidin-4(1,2)-benzo-7(1,3)-cyclobutyllactam-9-one (Compound I-8)
向反应瓶中加入化合物23G(130mg)、DIPEA(0.2g)、DMF(2mL)及二氯甲烷(10mL),氮气保护下,将五氟苯基二苯基磷酸酯(80mg)的DMF(2mL)溶液滴加到上述反应液中,滴加完毕,室温搅拌反应。反应完全,将反应液浓缩,向浓缩物中缓慢滴加2M碳酸钠水溶液(10mL),乙酸乙酯萃取,合并有机相,加入无水硫酸钠干燥,柱层析分离(二氯甲烷:甲醇=96:4)得到化合物I-8(17mg)。Compound 23G (130 mg), DIPEA (0.2 g), DMF (2 mL) and dichloromethane (10 mL) were added to the reaction flask, and pentafluorophenyl diphenyl phosphate (80 mg) in DMF (2 mL) The solution was added dropwise to the above reaction solution, and the dropwise addition was completed, and the reaction was stirred at room temperature. The reaction was completed, the reaction mixture was concentrated, and 2M aqueous sodium carbonate (10 mL) was slowly added dropwise to the concentrate, ethyl acetate was extracted, and the organic phase was combined, dried over anhydrous sodium sulfate and separated by column chromatography (dichloromethane: methanol = 96:4) Compound I-8 (17 mg) was obtained.
1H NMR(500MHz,DMSO-d
6):δ8.734(d,J=7.5Hz,2H),8.583(d,J=6.5Hz,1H),8.021(s,1H),7.147(s,2H),6.980(s,1H),6.432(d,J=7.0Hz,1H),5.852(s,1H),4.526-4.470(m,2H),3.973-3.951(m,1H),2.805-2.785(m,1H),2.666-2.626(m,2H),2.271(d,J=6.0Hz,1H),1.703(d,J=5.5Hz,1H),1.450(d,J=4.5Hz,3H)。
1 H NMR (500MHz, DMSO- d 6): δ8.734 (d, J = 7.5Hz, 2H), 8.583 (d, J = 6.5Hz, 1H), 8.021 (s, 1H), 7.147 (s, 2H ), 6.980 (s, 1H), 6.432 (d, J = 7.0 Hz, 1H), 5.852 (s, 1H), 4.526-4.470 (m, 2H), 3.973-3.951 (m, 1H), 2.805-2.785 ( m, 1H), 2.666-2.626 (m, 2H), 2.271 (d, J = 6.0 Hz, 1H), 1.703 (d, J = 5.5 Hz, 1H), 1.450 (d, J = 4.5 Hz, 3H).
13C NMR(125MHz,DMSO-d
6):δ160.46,157.94,155.55,152.67,146.36,144.25,136.55,114.34,112.84,101.52,101.05,69.91,43.80,38.24,33.32,32.11,29.48,29.13,28.89,22.86。LC-MS m/z:404.3,[M+Na]
+。
13 C NMR (125 MHz, DMSO-d 6 ): δ 160.46, 157.94, 155.55, 152.67, 146.36, 144.25, 136.55, 114.34, 112.84, 101.52, 101.05, 69.91, 43.80, 38.24, 33.32, 32.11, 29.48, 29.13, 28.89, 22.86. LC-MS m/z: 404.3, [M+Na] + .
实施例9:(1
3E,1
4E,7
1R,7
3R,3R)-4
5-氟-3-甲基-5-氧杂-2,8-二氮杂–1(5,3)-吡唑并[1,5-α]嘧啶杂-4(1,2)-苯杂-7(1,3)-环丁杂癸内酰胺-9-酮
Example 9: (1 3 E, 1 4 E, 7 1 R, 7 3 R, 3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza- 1 (5 ,3)-pyrazolo[1,5-α]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutanol-9-one
步骤1:((1R,3r)-3-((2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)甲基)环丁基)氨基甲酸叔丁酯Step 1: ((1R,3r)-3-((2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)) Tert-butyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入化合物1C(410mg)、((1r,3r)-3-(羟基甲基)环丁基)氨基甲酸叔丁酯(318mg)、三苯基膦(705mg,2.69mmol)和四氢呋喃(10mL),冰/乙醇浴冷却。DEAD(468mg)的四氢呋喃(2mL)溶液滴加到反应液中,反应完全后,浓缩反应液,柱层析纯化(乙酸乙酯:石油醚=0:100~20:80),得到化合物24A(1.89g)。MS(ESI)e/z:465.4[M+Na]
+.
To the reaction flask, Compound 1C (410 mg), ((1r, 3r)-3-(hydroxymethyl)cyclobutyl)carbamic acid tert-butyl ester (318 mg), triphenylphosphine (705 mg, 2.69 mmol) were added in order. It was cooled with tetrahydrofuran (10 mL) in an ice/ethanol bath. A solution of DEAD (468 mg) in tetrahydrofuran (2 mL) was added dropwise to the reaction mixture. After the reaction was completed, the reaction mixture was concentrated and purified by column chromatography (ethyl acetate: petroleum ether = 0: 100 to 20: 80) to afford compound 24A ( 1.89g). MS (ESI) e / z: 465.4 [M+Na] + .
步骤2:((1R,3r)-3-((2-((R)-1-氨基乙基)-4-氟苯氧基)甲基)环丁基)氨基甲酸叔丁酯Step 2: ((1R,3r)-3-((2-((R)-1-Aminoethyl)-4-fluorophenoxy)methyl)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入中间体24A(1.05g)、四氢呋喃(5mL)和水(0.5mL),溶解后,加入碘(250mg),将反应液加热70℃。反应完全后,反应液倒入乙酸乙酯(200mL)中,加入10wt%硫代硫酸钠水溶液(15mL),分离有机相,有机相用水(50mL)、饱和食盐水(30mL)洗涤,无水硫酸钠干燥。过滤,浓缩,得到化合物24B(866.0mg)。To the reaction flask, Intermediate 24A (1.05 g), tetrahydrofuran (5 mL) and water (0.5 mL) were successively added, and after dissolved, iodine (250 mg) was added, and the reaction mixture was heated at 70 °C. After the reaction was completed, the reaction mixture was poured into ethyl acetate (200 mL), EtOAc (EtOAc, EtOAc (EtOAc) Sodium is dry. Filtration and concentration gave Compound 24B (866.0 mg).
步骤3:5-((R)-1-(2-(((1r,3R)-3-((叔丁氧羰基)氨基)环丁基)甲氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 3: 5-((R)-1-(2-((1r,3R)-3-((tert-Butoxycarbonyl)amino)cyclobutyl)methoxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向微波反应管中,依次加入化合物24B(866mg)、5-氯吡唑并[1,5-a]嘧啶-3-甲酸乙酯(577mg)、DIPEA(992mg)和正丁醇(8mL),放入微波反应器中,微波120℃反应。反应完全后,浓缩反应液,残留物溶于乙酸乙酯(200mL)中,过滤,滤液依次用水(50mL,2次)、饱和食盐水(30mL)洗涤。无水硫酸钠干燥。过滤,浓缩,柱层析纯化(乙酸乙酯:石油醚=0:100~28:72),得到化合物24C(114.2g)。MS(ESI)e/z:528.4[M+H]
+。
To the microwave reaction tube, compound 24B (866 mg), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester (577 mg), DIPEA (992 mg) and n-butanol (8 mL) were added in that order. Into the microwave reactor, the microwave was reacted at 120 °C. After the reaction was completed, the reaction mixture was evaporated. mjjjjjjjjjjjj Dry over anhydrous sodium sulfate. Filtration, concentration and purification by column chromatography (ethyl acetate: petroleum ether = 0:100 - 28:72) gave Compound 24C (114.2 g). MS (ESI) e / z: 528.4 [M+H] + .
步骤4:5-(((R)-1-(2-(((1r,3R)-3-((叔丁氧羰基)氨基)环丁基)甲氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸Step 4: 5-(((R)-1-(2-((1r,3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)methoxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向含有化合物24C(104mg)的25mL圆底烧瓶中,依次加入甲醇(2mL)和四氢呋喃(1mL),溶解后,加入一水合氢氧化锂的水溶液(66.2mg溶于0.5mL)。将反应液加热70℃,反应完全,停止加热,将反应液倒入冰/水(100mL)中,缓慢加入0.5M盐酸调节pH至6,用二氯甲烷萃取,合并有机相,无水硫酸钠干燥。过滤,浓缩,得到化合物24D(100mg)。MS(ESI)m/z:500.1[M+H]
+.
To a 25 mL round bottom flask containing the compound 24C (104 mg), methanol (2 mL) and tetrahydrofuran (1 mL) were successively added, and after dissolved, an aqueous solution of lithium hydroxide monohydrate (66.2 mg dissolved in 0.5 mL) was added. The reaction solution was heated to 70 ° C, the reaction was completed, the heating was stopped, the reaction solution was poured into ice/water (100 mL), and the pH was adjusted to 6 by slowly adding 0.5 M hydrochloric acid, and the organic phase was combined with anhydrous sodium sulfate. dry. Filtration and concentration gave Compound 24D (100 mg). MS (ESI) m/z: 500.1 [M+H] + .
步骤5:5-((R)-1-(2-(((1r,3R)-3-氨基环丁基)甲氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸Step 5: 5-((R)-1-(2-(((1r,3R)-3-Aminocyclobutyl)methoxy)-5-fluorophenyl)ethyl)amino)pyrazolo[ 1,5-a]pyrimidine-3-carboxylic acid
向含有化合物24D(100mg)的反应瓶中加入4N盐酸的1,4-二氧六环溶液(5mL),混合物加热70℃。反应完全后,浓缩反应液,得到化合物24E(116.3mg)。MS(ESI)e/z:422.3[M+Na]
+.
To a reaction flask containing Compound 24D (100 mg) was added 4N hydrochloric acid in 1,4-dioxane (5 mL), and the mixture was heated at 70 °C. After the reaction was completed, the reaction mixture was concentrated to give Compound 24E (116.3mg). MS (ESI) e / z: 422.3 [M+Na] + .
步骤6:(1
3E,1
4E,7
1R,7
3R,3R)-4
5-氟-3-甲基-5-氧杂-2,8-二氮杂–1(5,3)-吡唑并[1,5-α]嘧啶杂-4(1,2)-苯杂-7(1,3)-环丁杂癸内酰胺-9-酮(化合物I-9)
Step 6: (1 3 E, 1 4 E, 7 1 R, 7 3 R, 3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza- 1 (5, 3)-pyrazolo[1,5-α]pyrimidin-4(1,2)-benzo-7(1,3)-cyclobutanlactam-9-one (Compound I-9)
在含有化合物24E(116.3mg)的反应瓶中依次加入二氯甲烷(20mL)和DIPEA(236mg),混合物室温搅拌下,加入五氟苯基二苯基膦酸酯(88mg)的DMF(2mL)溶液。室温反应,反应完全后,浓缩反应液,向浓缩物中加入乙酸乙酯(50mL),有机相依次用2M碳酸钠水溶液(20mL)、水、饱和食盐水(30mL)洗涤后,无水硫酸钠干燥。过滤,浓缩,柱层析纯化(甲醇:二氯甲烷=0:100~4:96),得到化合物I-9(22.2mg)。MS(ESI)e/z:382.4[M+H]
+.
Dichloromethane (20 mL) and DIPEA (236 mg) were added successively to a reaction flask containing compound 24E (116.3 mg), and the mixture was stirred at room temperature, and then added to the mixture of pentafluorophenyldiphenylphosphonate (88 mg) in DMF (2 mL) Solution. The reaction was carried out at room temperature. After the reaction was completed, the reaction mixture was concentrated, ethyl acetate (50 mL) was added to the concentrate, and the organic phase was washed sequentially with 2M aqueous sodium carbonate (20 mL), water, and brine (30 mL) dry. Filtration, concentration and purification by column chromatography (methanol: methylene chloride = 0: 100 to 4:96) gave Compound I-9 (22.2 mg). MS (ESI) e / z: 382.4 [M+H] + .
实施例10:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-5,7-二氮杂–6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁醇环辛内酰胺-4-酮
Example 10: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-5,7-diaza- 6 (3) ,5)-pyrazolo[1,5-a]pyrimidine-l-(1,2)-benzene-3(1,3)-cyclobutanol cyclooctanolactam-4-one
步骤1:(1S,3s)-3-(2–((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环丁烷-1-甲酸甲酯Step 1: (1S,3s)-3-(2-((R)-1-(((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Methyl-1-carboxylate
向反应瓶中加入化合物1C(1.60g)、反式-3-羟基环丁烷甲酸甲酯(0.80g)和四氢呋喃(3mL),再向反应溶液中加入三苯基膦(2.43g),冰水浴及N
2保护下,加入DEAD(1.72g)的四氢呋喃溶液(2mL)于搅拌液中,混合物在室温下反应。反应完成后,浓缩反应液,浓缩物通过柱层析纯化(PE:EA=50:1~1:1)得到化合物30A(1.78g)。
To the reaction flask were added Compound 1C (1.60 g), trans-3-hydroxycyclobutanecarboxylic acid methyl ester (0.80 g) and tetrahydrofuran (3 mL), and then triphenylphosphine (2.43 g) was added to the reaction solution, ice water bath and N 2 protection, tetrahydrofuran was added DEAD (1.72g) (2mL) was stirred in solution, and the mixture was reacted at room temperature. After completion of the reaction, the reaction mixture was concentrated, and the purified material was purified by column chromatography (PE: EA=50:1 to 1:1) to give Compound 30A (1.78 g).
1H NMR(500MHz,DMSO-d
6):δ8.97(br,s,1H),7.24~7.27(dd,J=3.5,10.0Hz,1H),6.97~6.99(ddd,1H),6.79~6.82(dd,J=4.5,9.0Hz,1H),5.64~5.66(d,J=8.0Hz,1H),4.64~4.68(m,1H),2.85~2.88(m,1H),2.70~2.76(m,2H),2.19~2.23(m,2H),1.32~1.33(d,J=7.0Hz,3H),1.11(s,9H).LC-MS:m/z 372.4[M+H]
+.
1 H NMR (500 MHz, DMSO-d 6 ): δ 8.97 (br, s, 1H), 7.24 to 7.27 (dd, J = 3.5, 10.0 Hz, 1H), 6.97 to 6.99 (ddd, 1H), 6.79 6.82 (dd, J=4.5, 9.0 Hz, 1H), 5.64 to 5.66 (d, J=8.0 Hz, 1H), 4.64 to 4.68 (m, 1H), 2.85 to 2.88 (m, 1H), 2.70 to 2.76 ( m, 2H), 2.19 to 2.23 (m, 2H), 1.32 to 1.33 (d, J = 7.0 Hz, 3H), 1.11 (s, 9H). LC-MS: m/z 372.4 [M+H] + .
步骤2:(1S,3s)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)环丁烷-1-甲酸甲酯的制备Step 2: Preparation of (1S,3s)-3-(2-((R)-1-aminoethyl)-4-fluorophenoxy)cyclobutane-1-carboxylic acid methyl ester
向反应瓶中加入化合物30A(1.70g)、碘单质(0.20g)、四氢呋喃(15mL)和水(1.5mL),氮气保护下,反应混合物在40℃下反应。反应完成后,加入10wt%硫代硫酸钠水溶液(15mL),再用EA萃取(20mL×3);合并有机相,无水硫酸钠干燥,过滤,浓缩,干燥得化合物30B(1.71g),未进一步纯化直接用于下一步反应。LC-MS:m/z 290.3[M+Na]
+.
Compound 30A (1.70 g), iodine simple substance (0.20 g), tetrahydrofuran (15 mL) and water (1.5 mL) were added to the reaction mixture, and the reaction mixture was reacted at 40 ° C under nitrogen. After completion of the reaction, a 10% by weight aqueous solution of sodium thiosulfate (15 mL) was added, and then extracted with EA (20 mL×3). The organic phase was combined, dried over anhydrous sodium sulfate, filtered, concentrated and dried to give compound 30B (1.71 g) Further purification was used directly for the next reaction. LC-MS: m/z 290.3 [M+Na] + .
步骤3:(1S,3s)-3-(4-氟-2-((R)-1-((3-硝基吡唑并[1,5-a]嘧啶-5-基)氨基)乙基)苯氧基)-甲酸甲酯Step 3: (1S,3s)-3-(4-fluoro-2-((R)-1-((3-nitropyrazolo[1,5-a]pyrimidin-5-yl)amino)) Methyl phenoxy)-formate
向微波反应瓶中加入化合物30B(0.97g)、5-氯-3-硝基吡唑并[1,5-a]嘧啶(0.60g)、DIPEA(2.34g)和正丁醇(8mL),N
2保护下,将混合物加热至120℃反应。反应完成后,加入饱和碳酸氢钠水溶液(30mL),再通过EA萃取(30mL×3),合并有机相,加入无水硫酸钠干燥,过滤,浓缩,浓缩物通过柱层析纯化分离(DCM:MeOH=100:0~95:5)得到化合物30C(1.09g)。
To the microwave reaction flask were added compound 30B (0.97 g), 5-chloro-3-nitropyrazolo[1,5-a]pyrimidine (0.60 g), DIPEA (2.34 g) and n-butanol (8 mL), N Under 2 protection, the mixture was heated to 120 ° C for reaction. After completion of the reaction, a saturated aqueous solution of sodium hydrogencarbonate (30 mL) was added, and then EtOAc (30 mL, 3), and the organic phase was combined, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography (DCM: MeOH = 100:0 to 95:5) Compound 30C (1.09 g) was obtained.
1H NMR(500MHz,DMSO-d
6):δ8.69~8.71(d,J=8.0Hz,1H),8.61~8.62(d,J=8.0Hz,1H),8.52(s,1H),7.15~7.17(dd,J=3.0,9.5Hz,1H),6.99~7.01(ddd,1H),6.86~6.88(dd,J=4.5,9.0Hz,1H),6.57~6.59(d,J=8.0Hz,1H),5.64~5.67(m,1H),4.70~4.75(m,1H),3.62(s,3H),2.82~2.86(m,1H),2.68~2.73(m,2H),2.23~2.26(m,2H),1.47~1.48(d,J=7.0Hz,3H).LC-MS:m/z 430.3[M+H]
+。
1 H NMR (500MHz, DMSO- d 6): δ8.69 ~ 8.71 (d, J = 8.0Hz, 1H), 8.61 ~ 8.62 (d, J = 8.0Hz, 1H), 8.52 (s, 1H), 7.15 ~7.17 (dd, J=3.0, 9.5 Hz, 1H), 6.99 to 7.01 (ddd, 1H), 6.86 to 6.88 (dd, J=4.5, 9.0 Hz, 1H), 6.57 to 6.59 (d, J=8.0 Hz) , 1H), 5.64 to 5.67 (m, 1H), 4.70 to 4.75 (m, 1H), 3.62 (s, 3H), 2.82 to 2.86 (m, 1H), 2.68 to 2.73 (m, 2H), 2.23 to 2.26 (m, 2H), 1.47 to 1.48 (d, J = 7.0 Hz, 3H). LC-MS: m/z 430.3 [M+H] + .
步骤4:(1S,3s)-3-(2–((R)-1-((3-氨基吡唑并[1,5-a]嘧啶-5-基)氨基)乙基)-4-氟苯氧基)羧酸甲酯Step 4: (1S,3s)-3-(2-((R)-1-((3-Aminopyrazolo[1,5-a]pyrimidin-5-yl)amino)ethyl)-4- Methyl fluorophenoxy)carboxylate
向反应瓶中加入化合物30C(1.08g)、二水合二氯化锡(2.27g)和乙醇(25mL),氮气保护,加热至回流反应。反应完成后,加入水(50mL)稀释反应,用6N NaOH水溶液调pH至碱性(大于等于14),再用DCM萃取(50mL×3),合并有机相,加入无水硫酸钠干燥,过滤,浓缩,浓缩物通过柱层析分离纯化(DCM:MeOH=100:0~90:10得到化合物30D(0.14g)。Compound 30C (1.08 g), tin dichloride dihydrate (2.27 g) and ethanol (25 mL) were added to the reaction flask, and the mixture was filtered and evaporated to reflux. After the reaction was completed, the reaction was diluted with water (50 mL), and the mixture was adjusted to basic (yield: 14) with 6N NaOH aqueous solution, and then extracted with DCM (50mL×3), the organic phase was combined, dried over anhydrous sodium sulfate and filtered. Concentration and purification of the concentrate by column chromatography (DCM:MeOH = 100:0 - 90:10) Compound 30D (0.14 g).
1H NMR(500MHz,DMSO-d
6):δ8.26~8.27(d,J=7.5Hz,1H),7.55~7.57(d,J=8.0Hz,1H),7.40(s,1H),7.09~7.12(d,J=3.0,9.5Hz,1H),6.95~6.97(ddd,1H),6.84~6.87(dd,J=4.5,9.0Hz,1H),6.15~6.17(d,J=7.5Hz,1H),4.73~4.75(m,1H),2.83~2.85(m,1H),2.71~2.76(m,2H),2.22~2.76(m,2H),1.39~1.40(d,J=7.0Hz,3H),1.18~1.21(t,J=7.0Hz,3H).LC-MS:m/z 400.4[M+H]
+.
1 H NMR (500 MHz, DMSO-d 6 ): δ 8.26 - 8.27 (d, J = 7.5 Hz, 1H), 7.55 - 7.57 (d, J = 8.0 Hz, 1H), 7.40 (s, 1H), 7.09 ~7.12 (d, J=3.0, 9.5 Hz, 1H), 6.95 to 6.97 (ddd, 1H), 6.84 to 6.87 (dd, J=4.5, 9.0 Hz, 1H), 6.15 to 6.17 (d, J=7.5 Hz) , 1H), 4.73 to 4.75 (m, 1H), 2.83 to 2.85 (m, 1H), 2.71 to 2.76 (m, 2H), 2.22 to 2.76 (m, 2H), 1.39 to 1.40 (d, J = 7.0 Hz) , 3H), 1.18 to 1.21 (t, J = 7.0 Hz, 3H). LC-MS: m/z 400.4 [M+H] + .
步骤5:(1S,3s)-3-(2–((R)-1-((3-氨基吡唑并[1,5-a]嘧啶-5-基)氨基)乙基)-4-氟苯氧基)环丁烷-1-甲酸Step 5: (1S,3s)-3-(2-((R)-1-((3-aminopyrazolo[1,5-a]pyrimidin-5-yl)amino)ethyl)-4- Fluorophenoxy)cyclobutane-1-carboxylic acid
向反应瓶中加入化合物30D(0.13g)、一水合氢氧化锂(0.082g)、甲醇(8mL)、四氢呋喃(3mL)和水(0.5mL),氮气保护下,反应混合物加热至70℃下反应。反应完成后,浓缩反应液至干,然后加入乙腈然后浓缩,重复三次,干燥得化合物30E(0.20g),未进一步纯化直接用于下一步反应。LC-MS:m/z 384.2[M-H]
-.
Compound 30D (0.13g), lithium hydroxide monohydrate (0.082g), methanol (8mL), tetrahydrofuran (3mL) and water (0.5mL) were added to the reaction flask. The reaction mixture was heated to 70 ° C under nitrogen. . After the reaction was completed, the reaction mixture was evaporated to dryness. LC-MS: m/z 384.2 [MH] - .
步骤6:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-5,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁醇环辛内酰胺-4-酮(化合物I-10)
Step 6: (3 1 S, 3 3 S, 6 3 E, 6 4 E, 8R) -1 4 - fluoro-8-methyl-2-oxa-5,7-diazepin-6 (3, 5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutanolcyclooctanolactam-4-one (Compound I-10)
向反应瓶中加入化合物30E(0.19g)、DIPEA(0.51g)、DMF(4mL)及二氯甲烷(16mL),N
2保护下,室温下搅拌30分钟后,将五氟苯酚二苯基磷酸酯(0.20g)加入反应体系中,室温下反应。反应完成后,向反应液中滴加2M碳酸钠水溶液(20mL),分出有机相,水相用DCM萃取(20mL×3),合并有机相,加入无水硫酸钠干燥,过滤,浓缩,浓缩物通过柱层析分离纯化(DCM:MeOH=100:0~90:10)得到化合物I-10(19.4mg)。
Compound 30E (0.19g), DIPEA (0.51g), DMF (4mL) and dichloromethane (16mL) were added to the reaction flask under N 2 and stirred at room temperature for 30 minutes, then pentafluorophenol diphenyl phosphate The ester (0.20 g) was added to the reaction system and allowed to react at room temperature. After the completion of the reaction, a 2M aqueous solution of sodium carbonate (20 mL) was added dropwise, and the organic phase was separated, and the aqueous phase was extracted with DCM (20 mL×3), the organic phase was combined, dried over anhydrous sodium sulfate, filtered, concentrated and concentrated. Purification by column chromatography (DCM:MeOH = 100:0 - 90:10) gave Compound I-10 (19.4mg).
1H NMR(500MHz,DMSO-d
6):δ8.38~8.39(d,J=7.5Hz,1H),8.29(s,1H),8.04~8.05(d,J=6.0Hz,1H),7.63(s,1H),7.04~7.06(dd,J=3.0,9.5Hz,1H),6.89~6.93(ddd,1H),6.55~6.58(dd,J=4.5,9.0Hz,1H),6.25~6.26(d.J=7.5Hz,1H),5.43~5.46(m,1H),4.86(s,1H),3.06~3.07(m,1H),2.73~2.87(m,3H),2.17~2.20(m,1H),1.33~1.35(d,J=7.0Hz,3H).
1 H NMR (500 MHz, DMSO-d 6 ): δ 8.38 - 8.39 (d, J = 7.5 Hz, 1H), 8.29 (s, 1H), 8.04 to 8.05 (d, J = 6.0 Hz, 1H), 7.63 (s, 1H), 7.04 to 7.06 (dd, J = 3.0, 9.5 Hz, 1H), 6.89 to 6.93 (ddd, 1H), 6.55 to 6.58 (dd, J = 4.5, 9.0 Hz, 1H), 6.25 to 6.26 (dJ=7.5 Hz, 1H), 5.43 to 5.46 (m, 1H), 4.86 (s, 1H), 3.06 to 3.07 (m, 1H), 2.73 to 2.87 (m, 3H), 2.17 to 2.20 (m, 1H) ), 1.33 ~ 1.35 (d, J = 7.0Hz, 3H).
13C NMR(125MHz,DMSO-d
6):176.18,157.88,156.01,153.78,149.78,142.98,140.49,138.63,135.39,114.27,113.52,106.24,100.20,71.68,43.63,35.32,33.73,30.25,22.80.LC-MS:m/z 368.4[M+H]
+.
13 C NMR (125 MHz, DMSO-d 6 ): 176.18, 157.88, 156.01, 153.78, 149.78, 142.98, 140.49, 138.63, 135.39, 114.27, 113.52, 106.24, 100.20, 71.68, 43.63, 35.32, 33.73, 30.25, 22.80. LC-MS: m/z 368.4 [M+H] + .
实施例11:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环戊基辛内酰胺-5-酮
Example 11: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidine-l-1(1,2)-benzene-3(1,3)-cyclopentyloctanolactam-5-one
步骤1:((1R,3R)-3-(2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环戊基)氨基甲酸叔丁酯Step 1: ((1R,3R)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclopentane Tert-butyl carbamate
冰水浴及氮气保护下,将DEAD(1.39g)缓慢加入化合物1C(1.30g)、化合物31A(1.20g)及三苯基膦(1.96g)的四氢呋喃(0.3mL)溶液中,加毕,恢复至室温反应。反应完全后,加入5mL石油醚,析出固体,过滤,将所得滤液浓缩,柱层析纯化(石油醚:乙酸乙酯=80:20),得到化合物31B(2.88g)。LCMS:m/z:465.4[M+Na]
+.
DEAD (1.39 g) was slowly added to a solution of compound 1C (1.30 g), compound 31A (1.20 g) and triphenylphosphine (1.96 g) in tetrahydrofuran (0.3 mL) in an ice water bath under a nitrogen atmosphere. React to room temperature. After the completion of the reaction, 5 mL of petroleum ether was added, and the solid was separated, filtered, and the obtained filtrate was concentrated and purified by column chromatography ( petroleum ether: ethyl acetate = 80: 20) to give Compound 31B (2.88 g). LCMS: m/z: 465.4 [M+Na] + .
步骤2:((1R,3R)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)环戊基)氨基甲酸叔丁酯Step 2: ((1R,3R)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclopentyl)carbamic acid tert-butyl ester
向反应瓶中加入化合物31B(5.88g)、碘(0.14g)、四氢呋喃(20mL)及水(2mL),氮气保护下,将混合物加热至50℃反应。反应完全后冷却至室温,向反应液加入10mL 10wt%硫代硫酸钠溶液,用乙酸乙酯萃取,饱和食盐水洗涤有机相,无水硫酸钠干燥,过滤浓缩得到化合物31C(3.1g)。LCMS:m/z:361.5[M+Na]
+
Compound 31B (5.88 g), iodine (0.14 g), tetrahydrofuran (20 mL) and water (2 mL) were added to the reaction mixture, and the mixture was heated to 50 ° C under nitrogen. After the completion of the reaction, the mixture was cooled to room temperature, and 10 mL of a 10% by weight of sodium thiosulfate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. LCMS: m/z: 361.5 [M+Na] +
步骤3:5-(((R)-1-(2-(((1R,3R)-3-((叔丁基氧羰基)氨基)环戊基)氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 3: 5-(((R)-1-(2-((1(R))))) ((tert-butyloxycarbonyl)amino)cyclopentyl)oxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向反应瓶中加入化合物31C(3.00g)、5-吡唑并[1,5-a]嘧啶-3-羧酸乙酯(1.35g)、DIPEA(3.87g)及正丁醇(25mL),氮气保护下,加热至120℃反应。反应完全后冷至室温,浓缩反应液,向浓缩物中加入乙酸乙酯萃取,饱和食盐水洗涤后无水硫酸钠干燥,过滤,浓缩后柱层析纯化(石油醚:乙酸乙酯=55:45),得到化合物31D(1.59g)。LCMS:m/z:528.5([M+H]
+)。
Compound 31C (3.00 g), 5-pyrazolo[1,5-a]pyrimidine-3-carboxylate (1.35 g), DIPEA (3.87 g) and n-butanol (25 mL) were added to the reaction flask. The reaction was heated to 120 ° C under nitrogen. After the reaction was completed, the mixture was cooled to room temperature. The mixture was evaporated, evaporated, evaporated, evaporated, evaporated. 45), Compound 31D (1.59 g) was obtained. LCMS: m/z: 528.5 ([M+H] + ).
步骤4:5-(((R)-1-(2-(((1R,3R)-3-((叔丁基氧羰基)氨基)环戊基)氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 4: 5-(((R)-1-(2-((1(R))))) ((tert-butyloxycarbonyl)amino)cyclopentyl)oxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中加入化合物31D(1.59g)、甲醇(15mL)、四氢呋喃(6mL)及一水合氢氧化锂(2M水溶液)水溶液9mL,氮气保护下,加热至70℃反应。反应完全,冷却至室温,向反应液加入2N盐酸调节溶液pH至小于5,乙酸乙酯萃取,水洗、饱和氯化钠水溶液洗涤有机相,无水硫酸钠干燥,过滤浓缩,干燥得到化合物31E(1.40g)。LC-MS:m/z:500.5[M+H]
+.
To the reaction flask were added 9 mL of an aqueous solution of Compound 31D (1.59 g), methanol (15 mL), tetrahydrofuran (6 mL) and lithium hydroxide monohydrate (2M aqueous solution), and the mixture was heated to 70 ° C under nitrogen. The reaction was completed, and the mixture was cooled to room temperature. To the reaction mixture was added 2N hydrochloric acid to adjust the pH of the solution to less than 5, ethyl acetate was extracted, and the organic phase was washed with water and saturated aqueous sodium chloride, dried over anhydrous sodium sulfate 1.40g). LC-MS: m/z: 500.5 [M+H] + .
步骤5:5-(((R)-1-(2-(((1R,3R)-3-氨基环戊基)氧杂)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 5: 5-(((R)-1-(2-(((1(R)))))))))))))))))) 1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中加入化合物31E(1.40g)、二氯甲烷(25mL),冰水浴下加入4N的氯化氢的二氧六环溶液7.0mL,加毕转移至室温反应,反应完全,浓缩,然后加入乙腈,析出固体,过滤,所得滤饼减压干燥得到化合物31F(694mg)。LCMS:m/z:422.4[M+H]
+。
Add compound 31E (1.40g), dichloromethane (25mL) to the reaction flask, add 7.0N of 4N hydrogen chloride in dioxane solution in ice water bath, transfer to room temperature, complete the reaction, concentrate, then add acetonitrile The solid was precipitated, filtered, and the obtained cake was dried under reduced pressure to give compound 31F (694mg). LCMS: m / z: 422.4 [ M + H] +.
步骤6:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环戊基辛内酰胺-5-酮(化合物I-11)
Step 6: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrazolo[1,5-a]pyrimidine-l-1(1,2)-benzene-3(1,3)-cyclopentylcaprolactam-5-one (Compound I-11)
向反应瓶中加入DIPEA(1.03g)、DMF(15mL)及二氯甲烷(30mL),氮气保护下,加入化合物31F(694mg),然后加入五氟苯基二苯基磷酸酯(0.435g),加毕,室温反应,反应完全,向反应中滴加2M碳酸钠水溶液(10mL),分液,水相用乙酸乙酯萃取,合并有机相,无水硫酸钠干燥,过滤浓缩,所得浓缩物柱层析分离(二氯甲烷:甲醇=96:4)得到化合物I-11(372mg)。DIPEA (1.03 g), DMF (15 mL) and dichloromethane (30 mL) were added to the reaction flask, and under the protection of nitrogen, compound 31F (694 mg) was added, followed by pentafluorophenyldiphenyl phosphate (0.435 g). After the addition, the reaction was carried out at room temperature, and the reaction was completed. A 2M aqueous solution of sodium carbonate (10 mL) was added dropwise to the mixture, and the mixture was separated, and the aqueous phase was extracted with ethyl acetate. Chromatography (dichloromethane:methanol = 96:4) gave Compound I-11
1H NMR(500MHz,DMSO-d
6):δ8.578(d,J=7.5Hz,1H),8.472(d,J=6.5Hz,1H),7.933(s,1H),7.560(d,J=7.5Hz,1H),7.097-7.093(m,1H),7.076-6.934(m,1H),6.483(d,J=8.0Hz,1H),5.330(d,J=6.5Hz,1H),4.178(s,1H),2.248-2.232(m,1H),2.109-1.991(m,1H),1.726-1.697(m,2H),1.520-1.491(m,2H),1.419(d,J=7.0Hz,3H).
1 H NMR (500 MHz, DMSO-d 6 ): δ 8.548 (d, J = 7.5 Hz, 1H), 8.472 (d, J = 6.5 Hz, 1H), 7.933 (s, 1H), 7.560 (d, J) = 7.5 Hz, 1H), 7.097-7.093 (m, 1H), 7.076-6.934 (m, 1H), 6.383 (d, J = 8.0 Hz, 1H), 5.330 (d, J = 6.5 Hz, 1H), 4.178 (s, 1H), 2.248-2.232 (m, 1H), 2.109-1.991 (m, 1H), 1.726-1.697 (m, 2H), 1.520-1.491 (m, 2H), 1.419 (d, J = 7.0 Hz) , 3H).
13C NMR(125MHz,DMSO-d
6):δ170.81,165.87,155.80,146.87,143.65,141.25,136.55,125.57,114.66,104.35,81.77,60.23,49.75,30.50,27.54,27.44,23.92,23.27,21.22.LCMS:m/z:382.4[M+H]
+.
13 C NMR (125 MHz, DMSO-d 6 ): δ 170.81, 165.87, 155.80, 146.87, 143.65, 141.25, 136.55, 125.57, 114.66, 104.35, 81.77, 60.23, 49.75, 30.50, 27.54, 27.44, 23.92, 23.27, 21.22. LCMS: m/z: 382.4 [M+H] + .
实施例12:(3
1s,3
3s,6
3Z,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-α]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁烷杂环辛内酰胺
Example 12: (3 1 s, 3 3 s, 6 3 Z, 6 4 E, 8R) -1 4 - fluoro-8-methyl-2-oxa-4,7-diazepin-6 (3 ,5)-pyrazolo[1,5-α]pyrimidine-l-1(1,2)-benzene-3(1,3)-cyclobutane heterocyclic octanolactam
步骤1:(3
1s,3
3s,6
3Z,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-α]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁烷杂环辛内酰胺(化合物I-12)
Step 1: (3 1 s, 3 3 s, 6 3 Z, 6 4 E, 8R) -1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6 (3, 5)-pyrazolo[1,5-α]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutane heterocyclic octanolactam (Compound I-12)
冰浴下,向含有四氢锂铝(207mg)及四氢呋喃(20mL)的反应瓶中加入化合物I-1(250mg,悬浮于20mLTHF中),加热70℃反应。反应完成,冷却至室温。将反应液倒入200mL冰水中,搅拌下向其中加入2M氢氧化钠水溶液(100mL),过滤,DCM萃取(100mL×3),干燥,过滤,所得滤液浓缩。残余物经柱层析(DCM/MeOH=85/15)得到化合物I-12(14mg)。Under ice bath, Compound I-1 (250 mg, suspended in 20 mL of THF) was added to a reaction flask containing lithium aluminum hydride (207 mg) and tetrahydrofuran (20 mL), and the mixture was heated at 70 ° C. The reaction was completed and cooled to room temperature. The reaction solution was poured into 200 mL of ice water, and a 2M aqueous sodium hydroxide solution (100 mL) was added to the mixture, and the mixture was filtered. The residue was subjected to EtOAc EtOAc EtOAc (EtOAc:
1H NMR(500MHz,DMSO-d
6)δ8.47(d,J=7.3Hz,1H),7.74(s,1H),7.18(d,J=9.5Hz,1H),7.03-6.96(m,1H),6.76-6.69(m,1H),6.32(d,J=7.4Hz,1H),5.61-5.53(m,1H),4.97(s,1H),4.06-3.85(m,2H),3.57(s,1H),3.04-2.94(m,1H),2.7-2.61(m,1H),2.44-2.34(m,1H),2.17-2.09(m,1H),2.05-1.92(m,1H),1.49-1.32(m,4H).
1 H NMR (500MHz, DMSO- d 6) δ8.47 (d, J = 7.3Hz, 1H), 7.74 (s, 1H), 7.18 (d, J = 9.5Hz, 1H), 7.03-6.96 (m, 1H), 6.76-6.69 (m, 1H), 6.32 (d, J = 7.4 Hz, 1H), 5.61-5.53 (m, 1H), 4.97 (s, 1H), 4.06-3.85 (m, 2H), 3.57 (s, 1H), 3.04-2.94 (m, 1H), 2.7-2.61 (m, 1H), 2.44-2.34 (m, 1H), 2.17-2.09 (m, 1H), 2.05-1.92 (m, 1H) , 1.49-1.32 (m, 4H).
13C NMR(125MHz,DMSO-d
6)δ156.49,154.10,148.88,146.22,142.77,135.89,130.11,114.67,114.09,113.66,100.70,71.74,51.27,42.95,33.32,31.89,22.90,14.40.MS:m/z=354.5[M+H]
+.
13 C NMR (125MHz, DMSO-d 6 ) δ 156.49, 154.10, 148.88, 146.22, 142.77, 135.89, 130.11, 114.67, 114.09, 113.66, 100.70, 71.74, 51.27, 42.95, 33.32, 31.89, 22.90, 14.40.MS:m /z=354.5[M+H] + .
实施例13:(3
1S,3
3S,8R,E)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,6)-咪唑并[1,2-b]吡啶杂-1(1,2)-苯杂-3(1,3)-环丁基环辛内酰胺-5-酮
Example 13: (3 1 S,3 3 S,8R,E)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3,6)-imidazole [1,2-b]pyridin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctanolac-5-one
步骤1:6-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)咪唑并[1,2-b]吡啶-3-羧酸乙酯Step 1: 6-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)imidazo[1,2-b]pyridine-3-carboxylic acid ethyl ester
向微波反应管中,依次加入化合物1E(537mg)、6-氯咪唑并[1,2-b]吡啶-3-羧酸乙酯(270mg)、氟化钾(813mg)及DMSO(1mL),N
2保护,将混合物置于微波反应仪140℃反应。反应完全,将反应液倒入20mL水中,用乙酸乙酯萃取。饱和食盐水洗涤有机相,无水硫酸钠干燥,过滤,柱层析(二氯甲烷:甲醇=97:3)得到化合物44A(463mg)。LC-MS m/z:536.5[M+Na]
+。
To the microwave reaction tube, Compound 1E (537 mg), 6-chloroimidazo[1,2-b]pyridine-3-carboxylic acid ethyl ester (270 mg), potassium fluoride (813 mg) and DMSO (1 mL) were sequentially added. N 2 protection, the mixture was placed in a microwave reactor at 140 ° C for reaction. The reaction was completed, and the mixture was poured into water (20 mL) and ethyl acetate. The organic layer was washed with brine, dried over anhydrous sodium sulfate LC-MS m/z: 536.5 [M+Na] + .
步骤2:6-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)咪唑并[1,2-b]吡啶-3-羧酸Step 2: 6-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)imidazo[1,2-b]pyridine-3-carboxylic acid
向反应瓶中,依次加入化合物44A(463mg)、一水合氢氧化锂(227mg)、甲醇(10mL)、四氢呋喃(4mL)及水(2mL),N
2保护下,将混合物加热至70℃反应。反应完全,使用盐酸调节pH至小于5,加入乙酸乙酯萃取,饱和食盐水洗涤有机相,洗涤后无水硫酸钠干燥,过滤,浓缩得到化合物44B(392mg)。LC-MS m/z:484.2[M-H]
-。
To the reaction flask, followed by addition of compound 44A (463mg), lithium hydroxide monohydrate (227 mg of), methanol (10 mL), tetrahydrofuran (4mL) and water (2 mL), under N 2, the reaction mixture was heated to 70 deg.] C. The reaction was completed, the pH was adjusted to less than 5 with hydrochloric acid, ethyl acetate was added, and the organic layer was washed with saturated brine, washed with anhydrous sodium sulfate, filtered and concentrated to give Compound 44B (392mg). LC-MS m / z: 484.2 [MH] -.
步骤3:6-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧基)-5-氟苯基)乙基)氨基)咪唑并[1,2-b]吡啶-3-羧酸三盐酸盐Step 3: 6-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)ethyl) Amino)imidazo[1,2-b]pyridine-3-carboxylic acid trihydrochloride
向反应瓶中加入44B(392mg),冰水浴下加入4M氯化氢的二氧六环(2917mg),在室温下反应。反应完全,浓缩反应液,向浓缩物中加入20mL MeCN,有固体析出,过滤,干燥滤饼得到化合物44C(270mg)。LC-MS m/z:384.2[M-H]
-。
44B (392 mg) was added to the reaction flask, and 4 M hydrogen chloride in dioxane (2917 mg) was added under ice-water bath, and the mixture was reacted at room temperature. The reaction was completed, the reaction mixture was concentrated, then 20 mL of MeCN was added to the concentrate, and solid was precipitated, filtered, and dried to give Compound 44C (270 mg). LC-MS m / z: 384.2 [MH] -.
步骤4:(3
1S,3
3S,8R,E)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,6)-咪唑并[1,2-b]吡啶杂-1(1,2)-苯杂-3(1,3)-环丁基环辛内酰胺-5-酮(化合物I-13)
Step 4: (3 1 S,3 3 S,8R,E)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3,6)-imidazo[ 1,2-b]pyridin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctanolactam-5-one (Compound I-13)
向反应瓶中加入化合物44C(270mg)、DIPEA(282mg)、DMF(6mL)及二氯甲烷(60mL),氮气保护下,将五氟苯酚二磷酸酯(723mg)的DMF(2mL)溶液滴加到上述反应液中,室温反应。反应完完全,柱层析(二氯甲烷:甲醇=96:4)得到化合物I-13(111mg)。LC-MS m/z:368.4[M+H]
+。
Compound 44C (270 mg), DIPEA (282 mg), DMF (6 mL) and dichloromethane (60 mL) were added to the reaction flask, and a solution of pentafluorophenol diphosphate (723 mg) in DMF (2 mL) was added dropwise under a nitrogen atmosphere. The reaction mixture was allowed to react at room temperature. After completion of the reaction, column chromatography (dichloromethane:methanol = <RTI ID=0.0> LC-MS m/z: 368.4 [M+H] + .
实施例14:(1
3E,1
4E,2
2R,2
4S,5
1S,5
3S)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-3(3,2)-吡啶杂-2(1,2)-四氢吡咯杂-5(1,3)-环丁烷杂环庚内酰胺-7-酮
Example 14: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-tetrahydropyrrole-5(1,3)-cyclobutane Alkane heterocyclic heptanolactam-7-one
步骤1:(R)-N-((R)-1-(5-氟-2-甲氧基吡啶-3-基)丁-3-烯-1-基)-2-甲基丙烷-2-亚磺酰胺Step 1: (R)-N-((R)-1-(5-fluoro-2-methoxypyridin-3-yl)but-3-en-1-yl)-2-methylpropane-2 - sulfenamide
冰浴下,向反应瓶中依次加入(R,E)-N-((5-氟-2-甲氧基吡啶-3-基)亚甲基)-2-甲基丙烷-2-亚磺酰胺(36g)、六甲基磷酰三胺(100mL)、锌粉(18.22g)、3-溴丙烯(33.7g)及水(2.51g),室温反应。反应完成,向反应液中加入100mL水,室温搅拌30分钟。再向其中加入30mL甲基叔丁基醚、60mL 10wt%柠檬酸水溶液,室温搅拌30分钟。过滤,分离有机相,用200mL 10wt%柠檬酸水溶液、盐水(200mL×2)洗涤有机相,干燥,过滤,浓缩,残留物经柱层析(PE/EA=70/30)得到化合物48A(9.72g)。(R,E)-N-((5-fluoro-2-methoxypyridin-3-yl)methylene)-2-methylpropane-2-sulfinate was added to the reaction flask in an ice bath. Amide (36 g), hexamethylphosphoric triamide (100 mL), zinc powder (18.22 g), 3-bromopropene (33.7 g) and water (2.51 g) were reacted at room temperature. The reaction was completed, 100 mL of water was added to the reaction mixture, and the mixture was stirred at room temperature for 30 minutes. Further, 30 mL of methyl t-butyl ether and 60 mL of a 10 wt% aqueous citric acid solution were added thereto, and the mixture was stirred at room temperature for 30 minutes. Filtration, separation of the organic phase, washing the organic phase with 200 mL of 10 wt% aqueous citric acid, brine (200 mL × 2), dried, filtered, concentrated, and the residue obtained by column chromatography (PE/EA=70/30) to give compound 48A (9.72) g).
1H NMR(500MHz,CDCl
3)δ7.91(d,J=2.9Hz,1H),7.34(dd,J=8.1,2.9Hz,1H),5.66(ddt,J=17.2,10.4,7.1Hz,1H),5.07(dd,J=13.6,6.4Hz,2H),4.50(dd,J=14.5,7.0Hz,1H),4.08(d,J=8.2Hz,1H),3.98(s,3H),2.67-2.52(m,2H),1.22(s,9H).MS:m/z=323.4[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ) δ7.91 (d, J = 2.9Hz, 1H), 7.34 (dd, J = 8.1,2.9Hz, 1H), 5.66 (ddt, J = 17.2,10.4,7.1Hz, 1H), 5.07 (dd, J = 13.6, 6.4 Hz, 2H), 4.50 (dd, J = 14.5, 7.0 Hz, 1H), 4.08 (d, J = 8.2 Hz, 1H), 3.98 (s, 3H), 2.67-2.52 (m, 2H), 1.22 (s, 9H) .MS: m / z = 323.4 [m + Na] +.
步骤2:(R)-1-(5-氟-2-甲氧基吡啶-3-基)丁-3-烯-1-胺Step 2: (R)-1-(5-fluoro-2-methoxypyridin-3-yl)but-3-en-1-amine
向反应瓶中,依次加化合物48A(9g)、MeOH(15mL)、HCl的二氧六环溶液(4M,40mL),室温反应。反应完成,浓缩反应液,浓缩物加入200mL二氯甲烷,150mL饱和碳酸氢钠水溶液洗,干燥。过滤,浓缩得到化合物48B(8.65g),不经纯化直接用于下一步反应。To the reaction flask, a solution of Compound 48A (9 g), MeOH (15 mL), EtOAc (EtOAc) After completion of the reaction, the reaction mixture was concentrated, and the mixture was evaporated and evaporated. Filtration and concentration gave Compound 48B (8.65 g).
步骤3:(R)-N-(1-(5-氟-2-甲氧基吡啶-3-基)丁-3-烯-1-基)乙酰胺Step 3: (R)-N-(1-(5-fluoro-2-methoxypyridin-3-yl)but-3-en-1-yl)acetamide
冰浴下,向反应瓶中依次加入化合物48B(8.65g)、二氯甲烷(150mL)、三乙胺(4.51g)及乙酸酐(3.80g),冰浴下反应。反应完成,向反应液中加入200mL饱和碳酸氢钠水溶液,分离有机相,用100mL二氯甲烷萃取水相。合并有机相,干燥。过滤,浓缩,残留物经柱层析(PE/EA=3/7)得到化合物48C(8.56g)。Under an ice bath, Compound 48B (8.65 g), dichloromethane (150 mL), triethylamine (4.51 g), and acetic anhydride (3.80 g) were sequentially added to the reaction mixture, and the mixture was reacted in an ice bath. After completion of the reaction, 200 mL of a saturated aqueous sodium hydrogencarbonate solution was added and the organic phase was separated, and the aqueous phase was extracted with 100 mL of dichloromethane. The organic phases were combined and dried. Filtration, concentration, and mp EtOAc (EtOAc/EtOAc)
1H NMR(500MHz,CDCl
3)δ7.91(d,J=2.7Hz,1H),7.25(dd,J=8.0,2.7Hz,1H),6.29(d,J=7.2Hz,1H),5.65(dt,J=16.8,7.2Hz,1H),5.15-5.04(m,3H),4.01(s,3H),2.61-2.50(m,2H),2.03(s,3H).MS:m/z=239.4[M+H]
+。
1 H NMR (500 MHz, CDCl 3 ) δ 7.91 (d, J = 2.7 Hz, 1H), 7.25 (dd, J = 8.0, 2.7 Hz, 1H), 6.29 (d, J = 7.2 Hz, 1H), 5.65 (dt, J = 16.8, 7.2 Hz, 1H), 5.15-5.04 (m, 3H), 4.01 (s, 3H), 2.61-2.50 (m, 2H), 2.03 (s, 3H). MS: m/z =239.4[M+H] + .
步骤4:(5R)-5-(5-氟-2-甲氧基吡啶-3-基)吡咯烷-3-基乙酸酯Step 4: (5R)-5-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidin-3-yl acetate
向反应瓶中,依次加入化合物48C(7.00g)、四氢呋喃(100mL)、水(16mL)及碘单质(22.37g),室温反应。反应完成,向反应液中加入水300mL,剧烈搅拌下向其中加入亚硫酸氢钠与碳酸钠调节pH=10,DCM萃取(200mL×4),水洗有机相(300mL),干燥。过滤,浓缩得到化合物48D(6.53g),不经纯化直接用于下一步反应。MS:m/z=255.4[M+H]
+。
Into a reaction flask, a compound 48C (7.00 g), tetrahydrofuran (100 mL), water (16 mL) and iodine (22.37 g) were sequentially added and reacted at room temperature. After completion of the reaction, 300 mL of water was added to the reaction mixture, and sodium hydrogensulfite and sodium carbonate were added thereto under vigorous stirring to adjust pH = 10, DCM was extracted (200 mL × 4), and the organic phase (300 mL) was washed with water and dried. Filtration and concentration gave Compound 48D (6.53 g). MS: m/z = 255.4 [M + H] + .
步骤5:(2R)-4-乙酰氧基-2-(5-氟-2-甲氧基吡啶-3-基)吡咯烷-1-甲酸叔丁酯Step 5: (2R)-4-acetoxy-2-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidine-1-carboxylic acid tert-butyl ester
向反应瓶中,依次加入化合物48D(6.00g)、四氢呋喃(50mL)、水(10mL)及三乙胺(4.78g),冰浴下向上述混合物中加入二碳酸二叔丁酯(6.18g),室温反应。反应完成,向反应液中加入300mL乙酸乙酯,饱和食盐水洗涤(150mL×2),干燥。过滤,浓缩,浓缩物经柱层析(PE/EA=80/20)得到化合物48E(6.09g)。To the reaction flask, a compound 48D (6.00 g), tetrahydrofuran (50 mL), water (10 mL) and triethylamine (4.78 g) were successively added, and di-tert-butyl dicarbonate (6.18 g) was added to the mixture under ice bath. , reaction at room temperature. After completion of the reaction, 300 mL of ethyl acetate was added to the reaction mixture, and brine (150 mL × 2) was evaporated. Filtration, concentration and concentration of EtOAc (EtOAc/EtOAc)
1H NMR(500MHz,CDCl
3)δ7.89(d,J=8.8Hz,1H),7.40-7.10(m,1H),5.33-4.95(m,2H),3.95(d,J=3.7Hz,3H),3.90-3.79(m,1H),3.78-3.72(m,1H),2.54(dd,J=13.2,7.7Hz,1H),2.07(d,J=21.8Hz,3H),1.83(s,1H),1.48(d,J=12.6Hz,4H),1.29-1.18(m,5H).MS:m/z=355.5[M+H]
+.
1 H NMR (500MHz, CDCl 3 ) δ7.89 (d, J = 8.8Hz, 1H), 7.40-7.10 (m, 1H), 5.33-4.95 (m, 2H), 3.95 (d, J = 3.7Hz, 3H), 3.90-3.79 (m, 1H), 3.78-3.72 (m, 1H), 2.54 (dd, J = 13.2, 7.7 Hz, 1H), 2.07 (d, J = 21.8 Hz, 3H), 1.83 (s) , 1H), 1.48 (d, J = 12.6 Hz, 4H), 1.29-1.18 (m, 5H). MS: m/z = 355.5 [M+H] + .
步骤6:(2R)-2-(5-氟-2-甲氧基吡啶-3-基)-4-羟基吡咯烷-1-羧酸叔丁酯Step 6: (2R)-2-(5-Fluoro-2-methoxypyridin-3-yl)-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester
向反应瓶中,依次加入化合物48E(6.00g)、甲醇(80mL)及2M氢氧化钠水溶液(9.3mL),室温反应。反应完成,浓缩反应液,向残留物中加入水200mL,搅拌下向其中加入3N HCl水溶液调节pH=7,乙酸乙酯萃取(150mL×3),干燥有机相。过滤,浓缩得到化合物48F(5.15g),未经纯化直接用于下一步反应。To the reaction flask, a compound 48E (6.00 g), methanol (80 mL) and a 2M aqueous sodium hydroxide solution (9.3 mL) were sequentially added, and the mixture was reacted at room temperature. After completion of the reaction, the reaction mixture was concentrated, and water (200 mL) was added to the residue, and 3N HCl aqueous solution was added thereto under stirring to adjust pH = 7 and ethyl acetate (150 mL × 3), and the organic phase was dried. Filtration and concentration gave Compound 48F (5.15 g).
1H NMR(500MHz,CDCl
3)δ7.87(d,J=10.2Hz,1H),7.45-7.07(m,1H),5.19-4.90(m,1H),4.50-4.35(m,1H),3.95(d,J=12.3Hz,3H),3.83-3.69(m,1H),3.68-3.58(m,1H),2.96-2.38(m,2H),2.00-1.86(m,1H),1.52-1.13(m,9H).MS:m/z=313.4[M+H]
+.
1 H NMR (500MHz, CDCl 3 ) δ7.87 (d, J = 10.2Hz, 1H), 7.45-7.07 (m, 1H), 5.19-4.90 (m, 1H), 4.50-4.35 (m, 1H), 3.95 (d, J = 12.3 Hz, 3H), 3.83 - 3.69 (m, 1H), 3.68-3.58 (m, 1H), 2.96-2.38 (m, 2H), 2.00-1.86 (m, 1H), 1.52- 1.13(m,9H).MS: m/z = 313.4 [M+H] + .
步骤7:(R)-2-(5-氟-2-甲氧基吡啶-3-基)-4-氧代吡咯烷-1-羧酸叔丁酯Step 7: (R)-2-(5-Fluoro-2-methoxypyridin-3-yl)-4-oxopyrrolidine-1-carboxylic acid tert-butyl ester
向反应瓶中,依次加入化合物48F(5.10g)、二氯甲烷(100mL)、碳酸氢钠(1.372g)及戴斯-马丁氧化剂(20.78g),室温反应。反应完成,向反应液中加入饱和碳酸氢钠水溶液调节至pH=7,二氯甲烷萃取(150mL×3),有机相干燥。过滤,浓缩,残余物经柱层析(PE/EA=9/1)得到化合物48G(4.78g)。To the reaction flask, Compound 48F (5.10 g), dichloromethane (100 mL), sodium hydrogencarbonate (1.372 g) and Dess-Martin oxidant (20.78 g) were sequentially added and reacted at room temperature. After completion of the reaction, a saturated aqueous solution of sodium hydrogencarbonate was added to the mixture and the mixture was adjusted to pH=7, dichloromethane (150mL×3), and the organic phase was dried. Filtration, concentration and residue were purified by column chromatography (PE/EA=9/1).
1H NMR(500MHz,CDCl
3)δ7.94(s,1H),7.29(s,1H),5.24(d,J=54.3Hz,1H),4.07-3.77(m,5H),3.07(dd,J=17.7,10.6Hz,1H),2.57(d,J=18.3Hz,1H),1.40(s,9H).MS:m/z=311.4[M+H]
+.
1 H NMR (500 MHz, CDCl 3 ) δ 7.94 (s, 1H), 7.29 (s, 1H), 5.24 (d, J = 54.3 Hz, 1H), 4.07 - 3.77 (m, 5H), 3.07 (dd, J = 17.7, 10.6 Hz, 1H), 2.57 (d, J = 18.3 Hz, 1H), 1.40 (s, 9H). MS: m/z = 311.4 [M+H] + .
步骤8:(2R,4R)-2-(5-氟-2-甲氧基吡啶-3-基)-4-羟基吡咯烷-1-羧酸叔丁酯Step 8: (2R,4R)-2-(5-fluoro-2-methoxypyridin-3-yl)-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester
冰浴下,将硼氢化钠(0.21g)加入到化合物48G(3.50g)的乙醇(100mL)中,冰浴下反应。反应完成,冰浴下,向反应液中缓慢加入100mL饱和氯化铵水溶液,再升至室温。二氯甲烷萃取(150mL×3),干燥有机相。过滤,浓缩,残留物经柱层析(PE/EA=4/1)得到化合物48H(3.23g)。Sodium borohydride (0.21 g) was added to a compound 48G (3.50 g) in ethanol (100 mL). After completion of the reaction, 100 mL of a saturated aqueous ammonium chloride solution was slowly added to the reaction solution under ice-cooling, and the mixture was further warmed to room temperature. Extract with dichloromethane (150 mL x 3) and dry the organic phase. Filtration, concentration, and mp EtOAc (EtOAc/EtOAc)
1H NMR(500MHz,CDCl
3)δ7.85(s,1H),7.44-7.24(m,1H),5.02(d,J=40.5Hz,1H),4.51-4.41(m,1H),3.95(s,3H),3.79-3.69(m,1H),3.59(d,J=11.8Hz,1H),2.60-2.46(m,1H),2.12-1.86(m,2H),1.59-1.12(m,9H).MS:m/z=313.4[M+H]
+。
1 H NMR (500 MHz, CDCl 3 ) δ 7.85 (s, 1H), 7.44 - 7.24 (m, 1H), 5.02 (d, J = 40.5 Hz, 1H), 4.51-4.41 (m, 1H), 3.95 ( s, 3H), 3.79-3.69 (m, 1H), 3.59 (d, J = 11.8 Hz, 1H), 2.60-2.46 (m, 1H), 2.12-1.86 (m, 2H), 1.59-1.12 (m, 9H). MS: m/z = 313.4 [M+H] + .
步骤9:(2R,4S)-4-氟-2-(5-氟-2-甲氧基吡啶-3-基)吡咯烷-1-羧酸叔丁酯Step 9: (2R,4S)-4-fluoro-2-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidine-1-carboxylic acid tert-butyl ester
-78℃下,将二乙胺基三氟化硫(17.94g)缓慢加入化合物48H(3.16g)的二氯甲烷(100mL)溶液中,加毕,将混合物在-78℃搅拌2小时。将混合物升至室温搅拌2小时。反应完成,冰浴下,滴加饱和碳酸氢钠水溶液。二氯甲烷萃取(100mL×3),干燥。过滤,浓缩,残余物经柱层析(PE/EA=9/1)得到化合物48I(1.98g)。Diethylaminosulfur trifluoride (17.94 g) was slowly added to a solution of compound 48H (3.16 g) in dichloromethane (100 mL), and the mixture was stirred at -78 ° C for 2 hours. The mixture was allowed to warm to room temperature and stirred for 2 hours. The reaction was completed, and a saturated aqueous solution of sodium hydrogencarbonate was added dropwise under ice bath. Extract with dichloromethane (100 mL x 3) and dry. Filtration, concentration and residue were purified by column chromatography (PE/EA=9/1).
1H NMR(500MHz,CDCl
3)δ7.91(s,1H),7.23(s,1H),5.32-5.01(m,1H),4.20-4.01(m,1H),3.96(s,3H),3.67(dd,J=38.2,12.0Hz,1H),2.87-2.68(m,1H),2.13-1.80(m,2H),1.60-1.14(m,9H)。MS:m/z=315.4[M+H]
+。
1 H NMR (500MHz, CDCl 3 ) δ7.91 (s, 1H), 7.23 (s, 1H), 5.32-5.01 (m, 1H), 4.20-4.01 (m, 1H), 3.96 (s, 3H), 3.67 (dd, J = 38.2, 12.0 Hz, 1H), 2.87-2.68 (m, 1H), 2.13-1.80 (m, 2H), 1.60-1.14 (m, 9H). MS: m/z = 315.4 [M+H] + .
步骤10:5-氟-3–((2R,4S)-4-氟吡咯烷-2-基)-2-甲氧基吡啶盐酸盐Step 10: 5-Fluoro-3((2R,4S)-4-fluoropyrrolidin-2-yl)-2-methoxypyridine hydrochloride
冰浴下,HCl的二氧六环溶液(4M,40.0mL)缓慢加入化合物48I(1.94g)的乙酸乙酯(20mL)溶液中,加毕,室温反应。反应完成,浓缩得到化合物48J(1.72g)。A solution of the compound 48I (1.94 g) in ethyl acetate (20 mL) was slowly evaporated. The reaction was completed and concentrated to give compound 48J (1.72 g).
1H NMR(500MHz,DMSO-d
6)δ10.56(s,1H),9.88(s,1H),8.23(s,1H),8.04(d,J=8.7Hz,1H),5.57(d,J=52.4Hz,1H),4.88(dd,J=11.3,5.8Hz,1H),3.93(s,3H),3.77-3.62(m,1H),3.59-3.47(m,1H),2.64-2.54(m,1H),2.49-2.36(m,1H)。
1 H NMR (500MHz, DMSO- d 6) δ10.56 (s, 1H), 9.88 (s, 1H), 8.23 (s, 1H), 8.04 (d, J = 8.7Hz, 1H), 5.57 (d, J=52.4 Hz, 1H), 4.88 (dd, J=11.3, 5.8 Hz, 1H), 3.93 (s, 3H), 3.77-3.62 (m, 1H), 3.59-3.47 (m, 1H), 2.64-2.54 (m, 1H), 2.49-2.36 (m, 1H).
步骤11:5-((2R,4S)-4-氟-2-(5-氟-2-甲氧基吡啶-3-基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 11: 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a Pyrimidine-3-carboxylate
向微波管中依次加入化合物48J(1.00g)、5-氯吡唑并[1,5-a]嘧啶-3-甲酸乙酯(0.99g)及DIPEA(2.06g),100℃条件下微波反应。反应完成,将反应液加入100mL饱和碳酸氢钠水溶液中,乙酸乙酯萃取(30mL×3),干燥有机相。过滤,浓缩,残余物经柱层析(石油醚:乙酸乙酯=65:35)得到化合物48K(1.22g)。MS:m/z=426.4[M+Na]
+.
Compound 48J (1.00 g), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester (0.99 g) and DIPEA (2.06 g) were sequentially added to the microwave tube, and the microwave reaction was carried out at 100 ° C. . After completion of the reaction, the reaction mixture was poured into 100 mL of saturated aqueous sodium hydrogen sulfate and ethyl acetate (30 mL×3) Filtration, concentrating, and EtOAc (EtOAc:EtOAc) MS: m/z = 426.4 [M+Na] + .
步骤12:5-((2R,4S)-4-氟-2-(5-氟-2-羟基吡啶-3-基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 12: 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine Ethyl 3-carboxylate
向反应瓶中,依次加入化合物48K、乙醇(30mL)及HCl的二氧六环溶液(4M,40mL),N
2氛围下,75℃反应。反应完成,浓缩反应液。残余物加入水(150mL)及三乙胺(5mL),二氯甲烷萃取(100mL×3),干燥。过滤,浓缩,残余物经柱层析(石油醚:乙酸乙酯=1:1)得到化合物48L(0.97g)。
To the reaction flask, a compound 48K, ethanol (30 mL) and a HCl solution of dioxane (4M, 40 mL) were sequentially added, and reacted at 75 ° C under N 2 atmosphere. The reaction was completed and the reaction mixture was concentrated. The residue was taken up in water (150 mL) and triethylamine (5 mL). Filtration, concentrating, and EtOAc (EtOAc:EtOAc)
1H NMR(500MHz,DMSO-d
6)δ11.61(s,1H),8.68(d,J=35.0Hz,1H),8.19(d,J=13.8Hz,1H),7.64(d,J=8.0Hz,1H),7.50(d,J=66.5Hz,1H),6.43(dd,J=238.3,0.6Hz,1H),5.51(dd,J=54.4,9.6Hz,1H),5.23(d,J=75.7Hz,1H),4.21(s,2H),4.14-3.94(m,2H),2.85-2.55(m,1H),2.45-2.13(m,1H),1.33-1.21(m,3H)。MS:m/z=412.4[M+Na]
+。
1 H NMR (500MHz, DMSO- d 6) δ11.61 (s, 1H), 8.68 (d, J = 35.0Hz, 1H), 8.19 (d, J = 13.8Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.50 (d, J = 66.5 Hz, 1H), 6.43 (dd, J = 238.3, 0.6 Hz, 1H), 5.51 (dd, J = 54.4, 9.6 Hz, 1H), 5.23 (d, J=75.7 Hz, 1H), 4.21 (s, 2H), 4.14-3.94 (m, 2H), 2.85-2.55 (m, 1H), 2.45-2.13 (m, 1H), 1.33-1.21 (m, 3H) . MS: m/z = 412.4 [M + Na] + .
步骤13:5-((2R,4S)-2-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 13: 5-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl) Ethyl 4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
冰浴及N
2保护下,将DIAD(353mg,溶解于1.5mL无水四氢呋喃中)缓慢加入含有化合物48L(400mg)、(1r,3r)-3-羟基环丁基)氨基甲酸叔丁酯(231mg)及三苯基膦(404mg)的四氢呋喃(2.5mL)溶液中,滴加完毕,室温反应。反应完成,浓缩得到黄色残余物,经柱层析(石油醚:乙酸乙酯=65:35)得到化合物48M(737mg)。
In an ice bath and N 2 protection, will DIAD (353mg, was dissolved in 1.5mL of anhydrous tetrahydrofuran) was slowly added to a compound containing 48L (400mg), (1r, 3r) -3- hydroxy-cyclobutyl) carbamate ( 231 mg) and a solution of triphenylphosphine (404 mg) in tetrahydrofuran (2.5 mL) were added dropwise and allowed to react at room temperature. The reaction was completed and concentrated to give a white crystallite.
1H NMR(500MHz,DMSO-d
6)δ8.70(d,J=55Hz,1H),8.21(d,J=30Hz,1H),8.02(d,J=75Hz,1H),7.23-7.04(m,1H),6.78-6.09(m,1H),5.69-5.21(m,2H),4.99-4.85(m,1H),4.29-4.85(m,4H),3.79-3.67(m,1H),3.01-2.61(m,3H),2.43-2.20(m,1H),2.11-1.88(m,3H),1.40-1.12(m,12H)。MS:m/z=581.5[M+Na]
+。
1 H NMR (500MHz, DMSO- d 6) δ8.70 (d, J = 55Hz, 1H), 8.21 (d, J = 30Hz, 1H), 8.02 (d, J = 75Hz, 1H), 7.23-7.04 ( m,1H), 6.78-6.09 (m, 1H), 5.69-5.21 (m, 2H), 4.99-4.85 (m, 1H), 4.29-4.85 (m, 4H), 3.79-3.67 (m, 1H), 3.01-2.61 (m, 3H), 2.43-2.20 (m, 1H), 2.11-1.88 (m, 3H), 1.40-1.12 (m, 12H). MS: m/z = 581.5 [M + Na] + .
步骤14:5-((2R,4S)-2-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸Step 14: 5-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl) )-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入化合物48M(700mg)、甲醇(4mL)、四氢呋喃(2mL)、水(1mL)及氢氧化锂一水合物(358mg),70℃下反应。反应完成,倒入100mL冰水中,缓慢滴加2N氯化氢水溶液至pH=4,二氯甲烷萃取(50mL×3),干燥。过滤,浓缩得到化合物48N(685mg)。To the reaction flask, a compound 48M (700 mg), methanol (4 mL), tetrahydrofuran (2 mL), water (1 mL), and lithium hydroxide monohydrate (358 mg) were sequentially added and reacted at 70 °C. After the reaction was completed, it was poured into 100 mL of ice water, and 2N aqueous hydrogen chloride solution was slowly added dropwise to pH = 4, extracted with dichloromethane (50 mL × 3), and dried. Filtration and concentration gave compound 48N (685 mg).
1H NMR(500MHz,DMSO-d
6)δ11.61(s,1H),8.69(d,J=51.3Hz,1H),8.17(d,J=34.3Hz,1H),8.00(d,J=74.6Hz,1H),7.26-7.03(m,1H),6.38(d,J=303.6Hz,1H),5.71-5.20(m,2H),4.89(s,1H),4.26-3.92(m,2H),3.77-3.64(m,1H),3.62-3.57(m,1H),2.81-2.61(m,2H),2.10-1.88(m,3H),1.75-1.72(m,1H),1.37(d,J=8.8Hz,9H)。MS:m/z=529.5[M-H]
-。
1 H NMR (500 MHz, DMSO-d 6 ) δ 11.61 (s, 1H), 8.69 (d, J = 51.3 Hz, 1H), 8.17 (d, J = 34.3 Hz, 1H), 8.00 (d, J = 74.6 Hz, 1H), 7.26-7.03 (m, 1H), 6.38 (d, J = 303.6 Hz, 1H), 5.71-5.20 (m, 2H), 4.89 (s, 1H), 4.26-3.92 (m, 2H) ), 3.77-3.64(m,1H), 3.62-3.57(m,1H),2.81-2.61(m,2H),2.10-1.88(m,3H),1.75-1.72(m,1H), 1.37(d , J = 8.8 Hz, 9H). MS: m/z = 529.5 [MH] - .
步骤15:5-(2R,4S)-2-(2-((1s,3S)-3-氨基环丁基氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5a]嘧啶-3-甲酸三盐酸盐(化合物48O)Step 15: 5-(2R,4S)-2-(2-((1s,3S)-3-Aminocyclobutyloxy)-5-fluoropyridin-3-yl)-4-fluoropyrrolidine-1 -yl)pyrazolo[1,5a]pyrimidine-3-carboxylic acid trihydrochloride (Compound 48O)
向反应瓶中,依次加入化合物48N(680mg)、二氯甲烷(10mL)及氯化氢二氧六环溶液(4M,15mL),室温反应。反应完成,浓缩。残余物利用二氯甲烷/二氧六环=20mL/20mL打浆,过滤,干燥得到化合物48O(446mg),不经纯化直接用于下一步反应。MS:m/z=429.3[M-H]
-。
To the reaction flask, a compound 48N (680 mg), dichloromethane (10 mL), and a hydrogen chloride dioxane solution (4M, 15 mL) were sequentially added and reacted at room temperature. The reaction was completed and concentrated. The residue was triturated with dichloromethane / dioxane = 20 mL / 20 mL, filtered and dried to afford compound 48O (446mg). MS: m/z = 429.3 [MH] - .
步骤16:(1
3E,1
4E,2
2R,2
4S,5
1S,5
3S)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-3(3,2)-吡啶杂-2(1,2)-吡咯烷杂-5(1,3)-环丁烷杂环庚内酰胺-7-酮(化合物I-14)
Step 16: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclobutane Cycloheptamamide-7-one (Compound I-14)
向反应瓶中依次加入化合物48O(440mg)、DIPEA(843mg)、二氯甲烷(50mL)、DMF(5mL)及五氟苯基二苯基磷酸酯(329mg),室温反应。反应完成,浓缩,残余物加入饱和碳酸氢钠水溶液(100mL),乙酸乙酯萃取(50mL×3),干燥。过滤,浓缩,残余物经柱层析(二氯甲烷:甲醇=97:3)得到化合物I-14(50mg)。Compound 48O (440 mg), DIPEA (843 mg), dichloromethane (50 mL), DMF (5 mL) and pentafluorophenyldiphenyl phosphate (329 mg) were sequentially added to the reaction mixture and reacted at room temperature. The reaction was completed, EtOAc EtOAc m. Filtration, concentrating, and EtOAc EtOAc (EtOAc)
实施例15:(1
3E,1
4E,2
2R,2
4S,5
1S,5
3S)-2
4,3
5-二氟-4-氧杂-6-氮杂–1(5,3)-吡唑并[1,5-α]嘧啶杂-2(1,2)-四氢吡咯杂-3(1,2)-苯杂-5(1,3)-环丁烷杂环庚内酰胺-7-酮
Example 15: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza- 1 (5,3)-pyrazolo[1,5-α]pyrimidine hetero-2(1,2)-tetrahydropyrrole-3(1,2)-benzene-5(1,3)-cyclobutane Alkane heterocyclic heptanolactam-7-one
步骤1:(R,E)-N-(5-氟-2-甲氧基亚苄基)-2-甲基丙基-2-亚磺酰胺Step 1: (R,E)-N-(5-fluoro-2-methoxybenzylidene)-2-methylpropyl-2-sulfinamide
0℃下,将钛酸四乙酯(35.5g)缓慢滴入5-氟-2-甲氧基-苯甲醛(20g)与(R)-(+)-叔丁基亚磺酰胺(17.30g)的四氢呋喃(150mL)搅拌液中,加毕,50℃搅拌反应。反应完全后,向反应液中加入饱和食盐水(200mL),过滤。滤饼用EA洗涤,滤液分相,有机相用饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物15A(37.039g)。不经纯化直接用于下一步反应。MS(ESI)m/z:258.4[M+H]
+.
Tetraethyl titanate (35.5 g) was slowly added dropwise at 0 ° C to 5-fluoro-2-methoxy-benzaldehyde (20 g) and (R)-(+)-tert-butylsulfinamide (17.30 g). The mixture was stirred in tetrahydrofuran (150 mL), and the reaction was stirred at 50 °C. After the reaction was completed, saturated brine (200 mL) was added and filtered. The filter cake was washed with EA, and the filtrate was separated. Filtration and concentration of the filtrate gave compound 15A (37.039 g). It was used in the next reaction without purification. MS (ESI) m / z: 258.4 [M+H] + .
步骤2:(R)-N-((R)-1-(5-氟-2-甲氧基苯基)-3-丁烯-1-基)-2-甲基丙烷-2-亚磺酰胺Step 2: (R)-N-((R)-1-(5-fluoro-2-methoxyphenyl)-3-buten-1-yl)-2-methylpropane-2-sulfin Amide
向反应瓶依次加入15A(37g)、无水氯化锂(12.19g)、锌粉(18.80g)、DMF(50mL)、3-溴-1-丙烯(34.8g),加毕氮气保护下室温搅拌反应。反应完全后,过滤,滤饼用EA(200mL)洗涤,滤液合并后倒入饱和食盐水(600mL)中,分相,水相用EA萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=4/1)得到化合物15B(30.478g)。Add 15A (37g), anhydrous lithium chloride (12.19g), zinc powder (18.80g), DMF (50mL), 3-bromo-1-propene (34.8g) to the reaction flask, and add room temperature under nitrogen protection. Stir the reaction. After the reaction was completed, the mixture was filtered, and the filter cake was washed with EA (200 mL). The filtrate was combined and poured into saturated brine (600 mL), and the phases were separated, and the aqueous phase was extracted with EA. dry. Filter and concentrate the filtrate. Column chromatography purification (petroleum ether / ethyl acetate = 4 / 1) gave Compound 15B (30.478 g).
1H NMR(500MHz,CDCl
3)δ6.97(dd,J=9.0,2.9Hz,1H),6.91(td,J=8.5,3.0Hz,1H),6.80(dd,J=8.9,4.4Hz,1H),5.66(ddt,J=14.2,10.7,7.1Hz,1H),5.04(d,J=5.9Hz,1H),5.01(s,1H),4.62(q,J=6.9Hz,1H),3.93(d,J=7.2Hz,1H),3.83(s,3H),2.64(dt,J=14.0,6.9Hz,1H),2.53(dt,J=14.1,7.0Hz,1H),1.20(s,9H).MS(ESI)m/z:322.4[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ) δ6.97 (dd, J = 9.0,2.9Hz, 1H), 6.91 (td, J = 8.5,3.0Hz, 1H), 6.80 (dd, J = 8.9,4.4Hz, 1H), 5.66 (ddt, J = 14.2, 10.7, 7.1 Hz, 1H), 5.04 (d, J = 5.9 Hz, 1H), 5.01 (s, 1H), 4.62 (q, J = 6.9 Hz, 1H), 3.93 (d, J = 7.2 Hz, 1H), 3.83 (s, 3H), 2.64 (dt, J = 14.0, 6.9 Hz, 1H), 2.53 (dt, J = 14.1, 7.0 Hz, 1H), 1.20 (s) , 9H). MS (ESI) m / z: 322.4 [M+Na] + .
步骤3:(R)-1-(5-氟-2-甲氧基苯基)-3-丁烯-1-胺Step 3: (R)-1-(5-fluoro-2-methoxyphenyl)-3-buten-1-amine
向反应瓶中,依次加入15B(30.4g)、MeOH(100mL)、氯化氢的1,4-二氧六环溶液(4M,250mL),加毕室温搅拌反应。反应完全后,浓缩反应液,残留物用DCM(500mL)溶解后,用饱和碳酸氢钠水溶液洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物15C(31.43g),不经纯化直接用于下一步反应。To the reaction flask, a solution of 15B (30.4 g), MeOH (100 mL) and hydrogen chloride in 1,4-dioxane (4M, 250 mL) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated. Filtration and concentration of the filtrate gave compound 15C (31.43 g).
步骤4:(R)-N-(1-(5-氟-2-甲氧基苯基)-3-丁烯-1-基)乙酰胺Step 4: (R)-N-(1-(5-Fluoro-2-methoxyphenyl)-3-buten-1-yl)acetamide
冰浴下,向反应瓶中依次加入15C(19.82g)、DCM(300mL)、TEA(12.33g)及乙酸酐(10.36g),加毕冰浴搅拌反应。反应完全后,向反应液中加入饱和碳酸氢钠水溶液(300mL),分相,水相再用DCM萃取。合并有机相,无水硫酸钠干燥。过滤,滤液浓缩。残留物用(石油醚/乙酸乙酯=200mL/20mL)打浆,过滤干燥得到化合物15D(22.98g)。Under ice cooling, 15 C (19.82 g), DCM (300 mL), TEA (12.33 g) and acetic anhydride (10.36 g) were sequentially added to the reaction flask, and the reaction was stirred with an ice bath. After the reaction was completed, a saturated aqueous solution of sodium bicarbonate (300 mL) was then evaporated. The organic phases were combined and dried over anhydrous sodium sulfate. Filter and concentrate the filtrate. The residue was slurried (petroleum ether / ethyl acetate = 200 mL / 20mL) and filtered to afford compound 15D (22.98 g).
1H NMR(500MHz,CDCl
3)δ6.93-6.85(m,2H),6.81(dd,J=8.8,4.3Hz,1H),6.25(d,J=7.5Hz,1H),5.65(dq,J=10.0,7.1Hz,1H),5.20(dd,J=15.4,7.3Hz,1H),5.07-5.01(m,2H),3.85(s,3H),2.52(t,J=7.0Hz,2H),1.99(s,3H).MS(ESI)m/z:260.4[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ) δ6.93-6.85 (m, 2H), 6.81 (dd, J = 8.8,4.3Hz, 1H), 6.25 (d, J = 7.5Hz, 1H), 5.65 (dq, J = 10.0, 7.1 Hz, 1H), 5.20 (dd, J = 15.4, 7.3 Hz, 1H), 5.07-5.01 (m, 2H), 3.85 (s, 3H), 2.52 (t, J = 7.0 Hz, 2H) ), 1.99 (s, 3H). MS (ESI) m/z: 260.4 [M+Na] + .
步骤5:(5R)-5-(5-氟-2-甲氧基苯基)吡咯烷基-3-乙酸酯Step 5: (5R)-5-(5-fluoro-2-methoxyphenyl)pyrrolidin-3-acetate
向反应瓶中,依次加入15D(7g)、四氢呋喃(300mL)、水(52mL)、碘单质(51g),加毕室温搅拌反应。反应完全后,向反应液中加入水(300mL),剧烈搅拌下向其中慢慢加入亚硫酸氢钠与碳酸钠调节pH至10,EA萃取,有机相用水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物15E(22.57g)。不经纯化直接用于下一步反应。MS(ESI)m/z:254.6[M+Na]
+.
To the reaction flask, 15D (7 g), tetrahydrofuran (300 mL), water (52 mL), and iodine (51 g) were sequentially added, and the mixture was stirred at room temperature. After the reaction was completed, water (300 mL) was added to the reaction mixture, and sodium hydrogensulfite and sodium carbonate were slowly added thereto under vigorous stirring to adjust the pH to 10, EA was extracted, and the organic phase was washed with water and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 15E (22.57 g). It was used in the next reaction without purification. MS (ESI) m / z: 254.6 [M+Na] + .
步骤6:(2R)-4-乙酰氧基-2-(5-氟-2-甲氧基苯基)吡咯烷-1-羧酸叔丁酯Step 6: (2R)-4-acetoxy-2-(5-fluoro-2-methoxyphenyl)pyrrolidine-1-carboxylic acid tert-butyl ester
向反应瓶中,依次加入15E(22.5g)、四氢呋喃(180mL)、水(37.5mL)及TEA(17.98g),冰浴下向上述混合物中加入二碳酸二叔丁酯(23.27g),加毕室温搅拌反应。反应完全后,向反应液中加入乙酸乙酯(500mL),用饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩,柱层析纯化(石油醚/乙酸乙酯=7/3)得到化合物15F(29.22g)。MS(ESI)m/z:376.5[M+Na]
+.
To the reaction flask, 15E (22.5g), tetrahydrofuran (180mL), water (37.5mL) and TEA (17.98g) were added, and di-tert-butyl dicarbonate (23.27g) was added to the above mixture under ice bath. The reaction was stirred at room temperature. After the reaction was completed, ethyl acetate (500 mL) was evaporated. Filtration, concentration of the filtrate and purification by column chromatography ( petroleum ether / ethyl acetate = 7 / 3) gave Compound 15F (29.22 g). MS (ESI) m / z: 376.5 [M+Na] + .
步骤7:(2R)-2-(5-氟-2-甲氧基苯基)-4-羟基吡咯烷-1-羧酸叔丁酯Step 7: (2R)-2-(5-Fluoro-2-methoxyphenyl)-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester
向反应瓶中,依次加入15F(29.2g)、MeOH(300mL)、氢氧化钠水溶液(2M,49.5mL),加毕室温搅拌反应。反应完全后,浓缩反应液,向残留物中加入水(500mL),搅拌下向其中加入稀盐酸(3N)调节pH至7,EA萃取,无水硫酸钠干燥。过滤,滤液浓缩得到化合物15G(24.78g),未经纯化直接用于下一步反应。MS(ESI)m/z:334.5[M+Na]
+.
To the reaction flask, 15F (29.2 g), MeOH (300 mL), and aqueous sodium hydroxide (2M, 49.5 mL) were successively added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated, and water (500 mL) was added to the residue, and then diluted with hydrochloric acid (3N) was added thereto to adjust the pH to 7 and extracted with EA and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 15G (24.78 g). MS (ESI) m/z: 334.5 [M+Na] + .
步骤8:(R)-2-(5-氟-2-甲氧基苯基)-4-氧代吡咯烷-1-羧酸叔丁酯Step 8: (R)-2-(5-Fluoro-2-methoxyphenyl)-4-oxopyrrolidine-1-carboxylic acid tert-butyl ester
向反应瓶中,依次加入15G(9.66g)、DCM(200mL)、碳酸氢钠(2.61g)及戴斯-马丁氧化剂(39.5g),加毕室温搅拌反应。反应完全后,向反应液中加入饱和碳酸氢钠水溶液调节pH至7,DCM萃取,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=4/1)得到化合物15H(9.19g)。To the reaction flask, 15 G (9.66 g), DCM (200 mL), sodium hydrogencarbonate (2.61 g) and Dess-Martin oxidant (39.5 g) were successively added, and the reaction was stirred at room temperature. After the reaction was completed, a saturated aqueous solution of sodium hydrogencarbonate was added to the mixture, and the mixture was adjusted to pH 7. Filter and concentrate the filtrate. Purification by column chromatography (petrole ether / ethyl acetate = 4 / 1) gave Compound 15H (9.19 g).
1H NMR(500MHz,CDCl
3)δ6.93(d,J=6.2Hz,2H),6.79(dd,J=8.9,4.2Hz,1H),5.26(d,J=96.7Hz,1H),3.97(d,J=18.7Hz,1H),3.92-3.81(m,1H),3.74(s,3H),3.04(dd,J=18.4,10.6Hz,1H),2.55(d,J=16.9Hz,1H),1.40(d,J=47.1Hz,9H).
1 H NMR (500MHz, CDCl 3 ) δ6.93 (d, J = 6.2Hz, 2H), 6.79 (dd, J = 8.9,4.2Hz, 1H), 5.26 (d, J = 96.7Hz, 1H), 3.97 (d, J = 18.7 Hz, 1H), 3.92-3.81 (m, 1H), 3.74 (s, 3H), 3.04 (dd, J = 18.4, 10.6 Hz, 1H), 2.55 (d, J = 16.9 Hz, 1H), 1.40 (d, J = 47.1 Hz, 9H).
步骤9:(2R,4R)-2-(5-氟-2-甲氧基苯基)-4-羟基吡咯烷-1-羧酸叔丁酯Step 9: (2R,4R)-2-(5-fluoro-2-methoxyphenyl)-4-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester
冰浴下,将硼氢化钠(0.556g)加入到15H(9.1g)的乙醇(300mL)搅拌液中,加毕冰浴下搅拌反应。反应完全后,冰浴下,向反应液中缓慢滴加饱和氯化铵水溶液(400mL)淬灭反应,再升至室温。DCM萃取,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=7/3)得到化合物15I(8.21g)。Under an ice bath, sodium borohydride (0.556 g) was added to a stirred solution of 15H (9.1 g) of ethanol (300 mL), and the mixture was stirred under ice bath. After completion of the reaction, the reaction mixture was slowly added dropwise with a saturated aqueous solution of ammonium chloride (400 mL), and the mixture was evaporated to room temperature. It was extracted with DCM and dried over anhydrous sodium sulfate. Filter and concentrate the filtrate. Column chromatography purification (petroleum ether / ethyl acetate = 7 / 3) gave Compound 15I (8.21 g).
1H NMR(500MHz,CDCl
3)δ7.02-6.90(m,1H),6.86(t,J=7.2Hz,1H),6.76(dd,J=8.7,4.2Hz,1H),5.11(d,J=60.8Hz,1H),4.47-4.39(m,1H),3.87-3.74(m,4H),3.58(d,J=11.7Hz,1H),2.62-2.47(s,1H),1.93(dd,J=13.7,3.3Hz,1H),1.63(s,1H),1.34(d,J=127.3Hz,9H).MS(ESI)m/z=334.6[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ) δ7.02-6.90 (m, 1H), 6.86 (t, J = 7.2Hz, 1H), 6.76 (dd, J = 8.7,4.2Hz, 1H), 5.11 (d, J=60.8 Hz, 1H), 4.47-4.39 (m, 1H), 3.87-3.74 (m, 4H), 3.58 (d, J = 11.7 Hz, 1H), 2.62-2.47 (s, 1H), 1.93 (dd , J = 13.7, 3.3 Hz, 1H), 1.63 (s, 1H), 1.34 (d, J = 127.3 Hz, 9H). MS (ESI) m/z = 334.6 [M+Na] + .
步骤10:(2R,4S)-4-氟-2-(5-氟-2-甲氧基苯基)吡咯烷-1-羧酸叔丁酯Step 10: (2R,4S)-4-fluoro-2-(5-fluoro-2-methoxyphenyl)pyrrolidine-1-carboxylic acid tert-butyl ester
-78℃下,将二乙胺基三氟化硫(46.7g)缓慢滴入15I(8.2g)的DCM(250mL)搅拌液中,加毕,混合物在-78℃搅拌2小时。将混合物升至室温搅拌反应。反应完全后,冰浴冷却,缓慢滴加饱和碳酸氢钠水溶液至不再有气泡。DCM萃取,无水硫酸钠干燥。过滤,减压浓缩,柱层析纯化(石油醚/乙酸乙酯=9/1)得到化合物15J(4.69g)。Diethylaminosulfur trifluoride (46.7 g) was slowly added dropwise to a stirred solution of 15 I (8.2 g) of DCM (250 mL), and the mixture was stirred at -78 ° C for 2 hours. The mixture was allowed to warm to room temperature and the reaction was stirred. After the reaction was completed, the ice bath was cooled, and a saturated aqueous solution of sodium hydrogencarbonate was slowly added dropwise until no more air bubbles. It was extracted with DCM and dried over anhydrous sodium sulfate. Filtration, concentration under reduced pressure and purification by column chromatography (EtOAc /EtOAcEtOAc
1H NMR(500MHz,CDCl
3)δ6.96-6.74(m,3H),5.19(dd,J=48.4,15.3Hz,2H),4.15-3.96(m,1H),3.80(s,3H),3.66(dd,J=37.7,13.4Hz,1H),2.83-2.62(m,1H),2.10-1.87(m,1H),1.53-1.11(m,9H).
1 H NMR (500MHz, CDCl 3 ) δ6.96-6.74 (m, 3H), 5.19 (dd, J = 48.4,15.3Hz, 2H), 4.15-3.96 (m, 1H), 3.80 (s, 3H), 3.66 (dd, J = 37.7, 13.4 Hz, 1H), 2.83 - 2.62 (m, 1H), 2.10 - 1.87 (m, 1H), 1.53-1.11 (m, 9H).
步骤11:4-氟-2-((2R,4S)-4-氟吡咯烷-2-基)苯酚氢溴酸盐Step 11: 4-Fluoro-2-((2R,4S)-4-fluoropyrrolidin-2-yl)phenol hydrobromide
向反应瓶中加入15J(1.23g)、氢溴酸水溶液(100mL,48%w/w)、四丁基溴化铵(1g),120℃加热搅拌反应。反应完全后,浓缩反应液得到化合物15K(5.3g)。未经纯化直接用于下一步反应。MS(ESI)m/z:200.4[M+H]
+.
15 J (1.23 g), a hydrobromic acid aqueous solution (100 mL, 48% w/w), and tetrabutylammonium bromide (1 g) were added to the reaction flask, and the reaction was stirred with heating at 120 °C. After completion of the reaction, the reaction mixture was concentrated to give compound 15K (5.3 g). It was used in the next reaction without purification. MS (ESI) m/z: 200.4 [M+H] + .
步骤12:5-((2R,4S)-4-氟-2-(5-氟-2-羟基苯基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 12: 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxyphenyl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3- Ethyl carboxylate
向反应瓶中依次加入15K(1.2g)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(1.06g)、正丁醇(100mL),加毕105℃搅拌反应。反应完全后,浓缩反应液,向残留物中加入饱和碳酸氢钠水溶液(200mL),EA萃取,合并有机相,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=65/35)得到化合物15L(1.17g)。15K (1.2g), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester (1.06g), n-butanol (100mL) were added to the reaction flask, and stirred at 105 ° C. reaction. After the reaction was completed, the reaction mixture was evaporated. Filter and concentrate the filtrate. Purification by column chromatography (petrole ether / ethyl acetate = 65/35) gave Compound 15L (1.17 g).
1H NMR(500MHz,CDCl
3):δ8.26(s,2H),6.97(s,1H),6.87-6.83(m,1H),6.77(dd,J=2.5,9.0Hz,1H),6.21(d,J=6.5Hz,1H),4.44-4.40(m,2H),4.15-4.02(m,2H),2.94-2.92(m,1H),2.52-2.32(m,1H),1.71(s,2H),1.42(t,J=7.0Hz,3H).MS(ESI)m/z:411.2[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ): δ8.26 (s, 2H), 6.97 (s, 1H), 6.87-6.83 (m, 1H), 6.77 (dd, J = 2.5,9.0Hz, 1H), 6.21 (d, J = 6.5 Hz, 1H), 4.44 - 4.40 (m, 2H), 4.15 - 4.02 (m, 2H), 2.94 - 2.92 (m, 1H), 2.52 - 2.32 (m, 1H), 1.71 (s) , 2H), 1.42 (t, J = 7.0 Hz, 3H). MS (ESI) m/z: 411.2 [M+Na] + .
步骤13:5-((2R,4S)-2-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 13: 5-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)-4 -fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
冰浴及氮气保护下,DIAD(1.22g)的无水四氢呋喃(4mL)溶液滴入到15L(1.17g)、(1r,3r)-3-羟基环丁基)氨基甲酸叔丁酯(678mg)、三苯基膦(1.58g)的无水四氢呋喃(6mL)搅拌液中,加毕,混合物室温搅拌反应。反应完全后,浓缩反应液,柱层析纯化(石油醚/乙酸乙酯=65/35)得化合物15M(4.76g)。MS(ESI)m/z:580.6[M+Na]
+.
Under ice bath and nitrogen atmosphere, DIAD (1.22g) in anhydrous tetrahydrofuran (4mL) was added dropwise to 15L (1.17g), tert-butyl (1r,3r)-3-hydroxycyclobutyl)carbamate (678mg) The mixture of triphenylphosphine (1.58 g) in anhydrous tetrahydrofuran (6 mL) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated, mjjjjjj MS (ESI) m/z: 580.6 [M+Na] + .
步骤14:5-((2R,4S)-2-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)环丁氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸Step 14: 5-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butoxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)-4 -fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入15M(4.00g)、MeOH(10mL)、四氢呋喃(5mL)、水(2.5mL)及氢氧化锂一水合物(2.05g),加毕70℃搅拌反应。反应完全后,将反应液倒入冰水(120mL)中,缓慢滴加 稀盐酸(2N)调节pH至4,DCM萃取,无水硫酸钠干燥。过滤,滤液浓缩得到化合物15N(3.42g)。未经纯化直接用于下一步反应。MS(ESI)m/z:552.6[M+Na]
+.
15 M (4.00 g), MeOH (10 mL), tetrahydrofuran (5 mL), water (2.5 mL) and lithium hydroxide monohydrate (2.05 g) were successively added to the reaction flask, and the reaction was stirred at 70 °C. After the reaction was completed, the reaction mixture was poured into ice water (120 mL), and then diluted with hydrochloric acid (2N) to adjust to pH 4, extracted with DCM and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 15N (3.42 g). It was used in the next reaction without purification. MS (ESI) m / z: 552.6 [M+Na] + .
步骤15:5-((2R,4S)-2-(2-((1s,3S)-3-氨基环丁氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 15: 5-((2R,4S)-2-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluorophenyl)-4-fluoropyrrolidin-1-yl) Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入15N(3.40g)、DCM(20mL)、氯化氢的1,4-二氧六环溶液(4M,30mL),加毕室温搅拌反应。反应完全后,浓缩反应液得到化合物15O(3.56g),未经纯化直接用于下一步反应。To the reaction flask, a solution of 15 N (3.40 g), DCM (20 mL), hydrogen chloride in 1,4-dioxane (4M, 30 mL) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated to give Compound 15O (3.56 g).
步骤16:(1
3E,1
4E,2
2R,2
4S,5
1S,5
3S)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-α]嘧啶杂-2(1,2-)-四氢吡咯杂-3(1,2)-苯杂-5(1,3)-环丁烷杂环庚内酰胺-7-酮
Step 16: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-α]pyrimidine hetero-2(1,2-)-tetrahydropyrrole-3(1,2)-benzene-5(1,3)-cyclobutane Alkane heterocyclic heptanolactam-7-one
向反应瓶中依次加入15O(440mg)、DIPEA(3.11g)、DCM(150mL)、DMF(15mL)、五氟苯基二苯基磷酸酯(1.21g),加毕室温搅拌反应。反应完全后,浓缩反应液,残留物中加入饱和碳酸氢钠水溶液(200mL),EA萃取,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM/MeOH=97/3)得到化合物I-15(79mg)。15O (440 mg), DIPEA (3.11 g), DCM (150 mL), DMF (15 mL), and pentafluorophenyldiphenyl phosphate (1.21 g) were sequentially added to the reaction flask, and the reaction was stirred at room temperature. After the reaction was completed, the mixture was evaporated. Filter and concentrate the filtrate. Purification by column chromatography (DCM / MeOH = EtOAc)
1H NMR(500MHz,DMSO-d
6)δ8.96(d,J=10.7Hz,1H),8.80(d,J=7.8Hz,1H),8.12(s,1H),7.24(dd,J=9.5,2.9Hz,1H),7.00(td,J=8.6,3.0Hz,1H),6.72(d,J=7.8Hz,1H),6.58(dd,J=9.0,4.5Hz,1H),6.03(t,J=8.2Hz,1H),5.56(d,J=52.7Hz,1H),4.97-4.90(m,1H),4.73-4.65(m,1H),4.48-4.33(m,1H),4.16(dd,J=20.5,13.0Hz,1H),3.10-3.02(m,1H),2.86-2.71(m,2H),2.24-2.06(m,2H),1.69(dd,J=13.4,7.7Hz,1H).
13C NMR(126MHz,DMSO-d
6)δ161.15,158.19,149.57,145.88,144.90,137.39,134.49,131.85,129.28,114.84,114.66,114.24,101.74,93.93,74.89,56.97,52.81,44.27,42.68,36.81.MS(ESI)m/z=412.5[M+H]
+.
1 H NMR (500MHz, DMSO- d 6) δ8.96 (d, J = 10.7Hz, 1H), 8.80 (d, J = 7.8Hz, 1H), 8.12 (s, 1H), 7.24 (dd, J = 9.5, 2.9 Hz, 1H), 7.00 (td, J = 8.6, 3.0 Hz, 1H), 6.72 (d, J = 7.8 Hz, 1H), 6.58 (dd, J = 9.0, 4.5 Hz, 1H), 6.03 ( t, J = 8.2 Hz, 1H), 5.56 (d, J = 52.7 Hz, 1H), 4.97 - 4.90 (m, 1H), 4.73-4.65 (m, 1H), 4.48-4.33 (m, 1H), 4.16 (dd, J=20.5, 13.0 Hz, 1H), 3.10-3.02 (m, 1H), 2.86-2.71 (m, 2H), 2.24-2.06 (m, 2H), 1.69 (dd, J = 13.4, 7.7 Hz) , 1H). 13 C NMR ( 126MHz, DMSO-d 6) δ161.15,158.19,149.57,145.88,144.90,137.39,134.49,131.85,129.28,114.84,114.66,114.24,101.74,93.93,74.89,56.97,52.81,44.27 , 42.68, 36.81. MS (ESI) m/z = 412.5 [M+H] + .
实施例16:(3
1R,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡咯并[1,5-a]嘧啶-1(1,2)-苯杂-3(1,3)-环戊基环辛内酰胺-5-酮
Example 16: (3 1 R,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrrolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclopentylcyclooctanolac-5-one
步骤1:((1R,3S)-3-(2-((S)-1-(((S)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环戊基)氨基甲酸叔丁基酯Step 1: ((1R,3S)-3-(2-((S)-1-((())-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclopentane Tert-butyl carbamate
氮气保护及冰水浴条件下,将偶氮二甲酸二乙酯(557mg)缓慢加入到((1S,3S)-3-羟基环戊基)氨基甲酸叔丁酯(400mg)、16A(518mg)、三苯基磷(786mg)及四氢呋喃(2mL)溶液中,加毕室温搅拌20分钟后,50℃搅拌反应。反应完全后,反应液冷却至室温后,向反应液中加入石油醚,过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=80/20)得到化合物16B(580mg)。MS(ESI)m/z:465.6[M+Na]
+.
Under nitrogen protection and ice water bath, diethyl azodicarboxylate (557 mg) was slowly added to ((1S,3S)-3-hydroxycyclopentyl)carbamic acid tert-butyl ester (400 mg), 16A (518 mg), In a solution of triphenylphosphine (786 mg) and tetrahydrofuran (2 mL), the mixture was stirred at room temperature for 20 minutes, and then stirred at 50 ° C. After completion of the reaction, the reaction solution was cooled to room temperature, then petroleum ether was added to the reaction mixture, and the filtrate was concentrated. Purification by column chromatography (petrole ether / ethyl acetate = 80 / 20) gave Compound 16B (580mg). MS (ESI) m / z: 465.6 [M+Na] + .
步骤2:((1S,3R)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)环戊基)氨基甲酸叔丁酯Step 2: ((1S,3R)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclopentyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入16B(580mg)、碘(66.5mg)、四氢呋喃(12mL)及水(2g),加毕氮气保护下,60℃搅拌反应。反应完全后,将反应液冷至室温,向反应液中加入饱和硫代硫酸钠溶液,EA萃取, 有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物16C(317mg)。MS(ESI)m/z:361.5[M+Na]
+.
To the reaction flask, 16B (580 mg), iodine (66.5 mg), tetrahydrofuran (12 mL) and water (2 g) were successively added, and the reaction was stirred at 60 ° C under a nitrogen atmosphere. After the reaction was completed, the reaction mixture was cooled to room temperature, and a saturated aqueous solution of sodium thiosulfate was added to the mixture. Filtration and concentration of the filtrate gave compound 16C (317 mg). MS (ESI) m / z: 361.5 [M+Na] + .
步骤3:5-(((R)-1-(2-(((1R,3S)-3-((叔丁基氧羰基)氨基)环戊基)氧杂)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 3: 5-(((R)-1-(2-((1(R))))((tert-butyloxycarbonyl)amino)cyclopentyl)oxa)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中,依次加入16C(317mg)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(280mg)、DIPEA(870mg)及正丁醇(15mL),加毕氮气保护下,120℃搅拌反应。反应完全后,反应液冷至室温。向反应液中加入乙酸乙酯,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=60/40)得化合物16D(114mg)。MS(ESI)m/z:550.6[M+Na]
+.
To the reaction flask, 16 C (317 mg), ethyl 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (280 mg), DIPEA (870 mg) and n-butanol (15 mL) were added in that order. The reaction was stirred at 120 ° C under nitrogen protection. After the reaction was completed, the reaction solution was cooled to room temperature. Ethyl acetate was added to the reaction mixture, and the mixture was washed with saturated brine Filter and concentrate the filtrate. Purification by column chromatography (peel ether / ethyl acetate = 60 / 40) gave Compound 16D (114mg). MS (ESI) m / z: 550.6 [M+Na] + .
步骤4:5-(((R)-1-(2-(((1R,3S)-3-((叔丁基氧羰基)氨基)环戊基)氧杂)-5-氟苯基乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 4: 5-(((R)-1-(2-((1(R)))))((tert-butyloxycarbonyl)amino)cyclopentyl)oxa)-5-fluorophenyl Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入16D(114mg)、氢氧化锂一水合物(94mg)、水(800mg)、四氢呋喃(2mL)和MeOH(4mL),加毕氮气保护下,60℃搅拌反应。反应完全后,将反应液冷至室温,向反应液加入稀盐酸(0.5M)调节溶液pH至5-7,EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得化合物16E(110mg)。MS(ESI)m/z:498.4[M-H]
+.
To the reaction flask, 16D (114 mg), lithium hydroxide monohydrate (94 mg), water (800 mg), tetrahydrofuran (2 mL) and MeOH (4 mL) were sequentially added, and the reaction was stirred at 60 ° C under nitrogen atmosphere. After the reaction was completed, the reaction mixture was cooled to room temperature. To the reaction mixture was added dilute hydrochloric acid (0.5M) to adjust the pH of the solution to 5-7, EA was extracted, the organic phase was combined, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 16E (110 mg). MS (ESI) m/z: 498.4 [MH] + .
步骤5:5-(((R)-1-(2-(((1R,3S)-3-氨基环戊基)氧杂)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 5: 5-(((R)-1-(2-(((1(R)))))))))))))))))) 1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入16E(110mg)、氯化氢的1,4-二氧六环溶液(4M,5mL),氮气保护下,加毕室温搅拌反应。反应完全后,浓缩反应液,得到化合物16F(466mg)。MS(ESI)m/z:398.3[M-H]
+.
To the reaction flask, 16E (110 mg), hydrogen chloride in 1,4-dioxane solution (4M, 5 mL) was added, and the mixture was stirred at room temperature under nitrogen. After completion of the reaction, the reaction mixture was concentrated to give Compound 16F (466 mg). MS (ESI) m/z: 398.3 [MH] + .
步骤6:(3
1R,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡咯并[1,5-a]嘧啶-1(1,2)-苯杂-3(1,3)-环戊基环辛内酰胺-5-酮
Step 6: (3 1 R,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrrolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclopentylcyclooctanolac-5-one
向反应瓶,依次加入16F(466mg)、DIPEA(175mg)、DCM(30mL)及DMF(5mL),加毕氮气保护下,将五氟苯基二苯基磷酸酯(91mg)分两次缓慢加入到上述反应液中,加毕室温搅拌反应。反应完全后,浓缩反应液。将残留物溶于EA后,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM/MeOH=96/4),得到化合物I-16(40mg)。To the reaction flask, 16F (466mg), DIPEA (175mg), DCM (30mL) and DMF (5mL) were added, and the pentafluorophenyl diphenyl phosphate (91mg) was added slowly twice under the protection of nitrogen. Into the above reaction solution, the reaction was stirred at room temperature. After the reaction was completed, the reaction solution was concentrated. The residue was dissolved in EA, washed with brine and dried over anhydrous sodium sulfate. Filter and concentrate the filtrate. Purification by column chromatography (DCM / MeOH = EtOAc /EtOAc)
1H NMR(500MHz,DMSO-d
6):δ8.64(t,J=9.0Hz,2H),8.52(d,J=7.5Hz,1H),8.06(s,1H),7.22(d,J=9.0Hz,1H),7.00(t,J=8.5Hz,1H),6.85(t,J=4.0Hz,1H),6.29(d,J=8.5Hz,1H),5.90(d,J=7.0Hz,1H),4.95(s,1H),4.42(s,1H),2.42-2.36(m,1H),2.14(d,J=15.0Hz,2H),1.85(t,J=10.0Hz,1H),1.67-1.56(m,2H),1.41(d,J=7.0Hz,3H).
13C NMR(125MHz,DMSO-d
6):161.93,157.78,155.90,155.58,149.28,146.49,144.49,136.52,135.56,114.61,101.69,100.77,79.00,50.71,41.61,34.21,30.64,23.26.MS(ESI)m/z:382.6[M+H]
+.
1 H NMR (500MHz, DMSO- d 6): δ8.64 (t, J = 9.0Hz, 2H), 8.52 (d, J = 7.5Hz, 1H), 8.06 (s, 1H), 7.22 (d, J = 9.0 Hz, 1H), 7.00 (t, J = 8.5 Hz, 1H), 6.85 (t, J = 4.0 Hz, 1H), 6.29 (d, J = 8.5 Hz, 1H), 5.90 (d, J = 7.0) Hz, 1H), 4.95 (s, 1H), 4.42 (s, 1H), 2.42-2.36 (m, 1H), 2.14 (d, J = 15.0 Hz, 2H), 1.85 (t, J = 10.0 Hz, 1H) ), 1.67-1.56 (m, 2H), 1.41 (d, J = 7.0 Hz, 3H). 13 C NMR (125MHz, DMSO-d 6 ): 161.93, 157.78, 155.90, 155.58, 149.28, 146.49, 144.49, 136.52 , 135.56, 114.61, 101.69, 100.77, 79.00, 50.71, 41.61, 34.21, 30.64, 23.26. MS (ESI) m/z: 382.6 [M+H] + .
实施例17:(1
3E,1
4E,7
1S,7
3R,3R)-4
5-氟-3-甲基-2,8-二氮杂-1(5,3)-吡唑并[1,5-α]嘧啶杂-4(1,2)-苯杂-7(1,3)-环丁烷杂环壬内酰胺-9-酮
Example 17: (1 3 E,1 4 E,7 1 S,7 3 R,3R)-4 5 -fluoro-3-methyl-2,8-diaza-1(5,3)-pyridyl Zizo[1,5-α]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutane heterocyclic lactam-9-one
步骤1:5-(((R)-1-(2-(((1s,3S)-3-((叔丁氧基羰基)氨基)环丁基)乙炔基)-5-氟苯基)乙基)氨基)吡唑[1,5-a]嘧啶-3-甲酸乙酯Step 1: 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)ethynyl)-5-fluorophenyl) Ethyl)amino)pyrazole [1,5-a]pyrimidine-3-carboxylate
向反应瓶中,依次加入(R)-5-((1-(5-氟-2–(((三氟甲基)磺酰基)氧基)苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯(600mg)、DMF(10mL)、叔丁基((1s,3s)-3-乙炔基环丁基)氨基甲酸酯(1.0g)、碘化亚铜(96mg)、双三苯基磷二氯化钯(354mg)和DIPEA(326mg),加毕氮气保护下,70℃搅拌反应。反应完全后,浓缩反应液。柱层析纯化(石油醚/乙酸乙酯=1/1)纯化得到化合物17A(673mg)。MS(ESI)m/z=544.5[M+Na]
+.
To the reaction flask, (R)-5-((1-(5-fluoro-2-(((trifluoromethyl))sulfonyl)oxy)phenyl)ethyl)amino)pyrazolo[ 1,5-a]pyrimidine-3-carboxylic acid ethyl ester (600 mg), DMF (10 mL), tert-butyl ((1s, 3s)-3-ethynylcyclobutyl)carbamate (1.0 g), Cuprous iodide (96 mg), bistriphenylphosphine palladium dichloride (354 mg) and DIPEA (326 mg) were stirred under nitrogen and the reaction was stirred at 70 °C. After the reaction was completed, the reaction solution was concentrated. Purification by column chromatography (petroleum ether / ethyl acetate = 1 / 1) afforded Compound 17A (673mg). MS (ESI) m/z = 544.5 [M+Na] + .
步骤2:5-(((R)-1-(2-(2-((1s,3R)-3-((叔丁氧基羰基)氨基)环丁基)乙基)-5-氟苯基)乙基)氨基)吡唑[1,5-a]嘧啶-3-甲酸乙酯Step 2: 5-(((R)-1-(2-(2-((1),3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)ethyl)-5-fluorobenzene Ethyl)ethyl)amino)pyrazole [1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向高压釜中,依次加入17A(670mg)、MeOH(50mL)和氢氧化钯(361mg),氢气置换三次后,充入氢气至54bar,加毕66℃搅拌反应。反应完全后,过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=60/40)得到化合物17B(170mg)。To the autoclave, 17A (670 mg), MeOH (50 mL) and palladium hydroxide (361 mg) were successively added, and after hydrogen was replaced three times, hydrogen gas was charged to 54 bar, and the reaction was stirred at 66 ° C. After the reaction was completed, it was filtered and the filtrate was concentrated. Purification by column chromatography (petrole ether / ethyl acetate = 60 / 40) gave Compound 17B (170 mg).
1H NMR(500MHz,CDCl
3)δ8.27(s,1H),8.23(d,J=7.6Hz,1H),7.12(ddd,J=12.6,9.2,4.2Hz,2H),6.91(td,J=8.2,2.5Hz,1H),6.04(d,J=6.5Hz,1H),5.56-5.29(m,1H),4.44-4.29(m,2H),4.02-3.90(m,1H),2.78-2.68(m,1H),2.64-2.55(m,1H),2.55-2.43(m,2H),2.06-1.93(m,2H),1.79-1.65(m,3H),1.61(d,J=6.6Hz,3H),1.58-1.52(m,2H),1.45(s,9H),1.40(t,J=7.1Hz,3H).MS(ESI)m/z:548.6[M+Na]
+.
1 H NMR (500MHz, CDCl 3 ) δ8.27 (s, 1H), 8.23 (d, J = 7.6Hz, 1H), 7.12 (ddd, J = 12.6,9.2,4.2Hz, 2H), 6.91 (td, J=8.2, 2.5 Hz, 1H), 6.04 (d, J=6.5 Hz, 1H), 5.56-5.29 (m, 1H), 4.44-4.29 (m, 2H), 4.02-3.90 (m, 1H), 2.78 -2.68 (m, 1H), 2.64-2.55 (m, 1H), 2.55-2.43 (m, 2H), 2.06-1.93 (m, 2H), 1.79-1.65 (m, 3H), 1.61 (d, J = 6.6 Hz, 3H), 1.58-1.52 (m, 2H), 1.45 (s, 9H), 1.40 (t, J = 7.1 Hz, 3H). MS (ESI) m/z: 548.6 [M+Na] + .
步骤3:5-(((R)-1-(2-(2-((1s,3R)-3-((叔丁氧基羰基)氨基)环丁基)乙基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 3: 5-(((R)-1-(2-(2-((1),3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)ethyl)-5-fluorobenzene Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入17B(160mg)、MeOH(4mL)、四氢呋喃(2mL)、水(1mL)和氢氧化锂一水合物(89mg),加毕80℃搅拌反应。反应完全后,将反应液倒入冰水(100mL)中,缓慢滴加稀盐酸(2N)调节pH至4,DCM萃取,无水硫酸钠干燥。过滤,滤液浓缩得到化合物17C(162mg),不经纯化直接用于下一步反应。17B (160 mg), MeOH (4 mL), tetrahydrofuran (2 mL), water (1 mL) and lithium hydroxide monohydrate (89 mg) were successively added to the reaction flask, and the reaction was stirred at 80 ° C. After the reaction was completed, the reaction mixture was poured into ice water (100 mL), and then diluted with hydrochloric acid (2N) to adjust to pH 4, extracted with DCM and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 17C (162 mg).
1H NMR(500MHz,DMSO-d
6)δ11.69(brs,1H),8.52(d,J=7.5Hz,1H),8.39(d,J=7.8Hz,1H),8.09(s,1H),7.20(dd,J=10.6,2.7Hz,1H),7.13(dd,J=7.9,6.5Hz,1H),6.95(td,J=8.2,2.7Hz,2H),6.39(d,J=7.4Hz,1H),5.62-5.45(m,1H),3.79-3.69(m,1H),3.12-2.98(m,1H),2.50-2.45(m,1H),2.38-2.29(m,1H),2.28-2.19(m,1H),2.04-1.96(m,1H),1.95-1.81(m,1H),1.66-1.58(m,2H),1.46(d,J=6.8Hz,3H),1.36(s,9H).MS(ESI)m/z:520.5[M+Na]
+.
1 H NMR (500MHz, DMSO- d 6) δ11.69 (brs, 1H), 8.52 (d, J = 7.5Hz, 1H), 8.39 (d, J = 7.8Hz, 1H), 8.09 (s, 1H) , 7.20 (dd, J = 10.6, 2.7 Hz, 1H), 7.13 (dd, J = 7.9, 6.5 Hz, 1H), 6.95 (td, J = 8.2, 2.7 Hz, 2H), 6.39 (d, J = 7.4) Hz, 1H), 5.62-5.45 (m, 1H), 3.79-3.69 (m, 1H), 3.12-2.98 (m, 1H), 2.50-2.45 (m, 1H), 2.38-2.29 (m, 1H), 2.28-2.19 (m, 1H), 2.04-1.96 (m, 1H), 1.95-1.81 (m, 1H), 1.66-1.58 (m, 2H), 1.46 (d, J = 6.8 Hz, 3H), 1.36 ( s, 9H). MS (ESI) m/z: 520.5 [M+Na] + .
步骤4:5-(((R)-1-(2-(2-((1s,3R)-3-氨基环丁基)乙基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 4: 5-(((R)-1-(2-(2-((1),3R)-3-aminocyclobutyl)ethyl)-5-fluorophenyl)ethyl)amino)pyrazole And [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入17C(162mg)、DCM(10mL)、氯化氢的1,4-二氧六环溶液(4M,7.47mL),加毕室温搅拌反应。反应完全后,浓缩反应液得到化合物17D(173mg),不经纯化直接用于下一步反应。MS(ESI)m/z=420.2[M+Na]
+.
To the reaction flask, a solution of 17 C (162 mg), DCM (10 mL) and hydrogen chloride in 1,4-dioxane (4M, 7.47 mL) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated to mjjjjjd MS (ESI) m / z = 420.2 [M+Na] + .
步骤5:(1
3E,1
4E,7
1S,7
3R,3R)-4
5-氟-3-甲基-2,8-二氮杂-1(5,3)-吡唑并[1,5-α]嘧啶杂-4(1,2)-苯杂-7(1,3)-环丁烷杂环壬内酰胺-9-酮
Step 5: (1 3 E,1 4 E,7 1 S,7 3 R,3R)-4 5 -fluoro-3-methyl-2,8-diaza-1(5,3)-pyrazole And [1,5-α]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutane heterocyclic lactam-9-one
向反应瓶中,依次加入17D(170mg)、DCM(40mL)、DMF(5mL)及DIPEA(347mg),冰浴下加入五氟苯基二苯基膦酸酯(129mg),加毕混合物室温搅拌反应。反应完全后,浓缩反应液。向残留物中加入饱和碳酸钠水溶液(100mL),EA萃取,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM/MeOH=96/4)得到化合物I-17(56mg)。To the reaction flask, 17D (170 mg), DCM (40 mL), DMF (5 mL) and DIPEA (347 mg) were added successively, and pentafluorophenyldiphenylphosphonate (129 mg) was added under ice-cooling, and the mixture was stirred at room temperature. reaction. After the reaction was completed, the reaction solution was concentrated. A saturated aqueous solution of sodium carbonate (100 mL) was added toEtOAc. Filter and concentrate the filtrate. Purification by column chromatography (DCM /MeOH = EtOAc /
1H NMR(500MHz,DMSO-d
6)δ8.74(d,J=9.2Hz,1H),8.61(d,J=7.6Hz,1H),8.57(d,J=7.7Hz,1H),8.05(s,1H),7.35(dd,J=10.7,2.6Hz,1H),7.22(dd,J=8.3,6.1Hz,1H),6.98(td,J=8.4,2.7Hz,1H),6.47(d,J=7.6Hz,1H),5.82-5.73(m,1H),4.26(dd,J=13.3,6.5Hz,1H),2.87-2.76(m,2H),2.59(dt,J=12.2,7.6Hz,1H),2.42-2.28(m,2H),2.18(ddd,J=15.6,13.0,3.6Hz,1H),2.03-1.97(m,1H),1.85-1.76(m,2H),1.45(d,J=6.8Hz,3H).MS(ESI)m/z=402.4[M+Na]
+.
1 H NMR (500MHz, DMSO- d 6) δ8.74 (d, J = 9.2Hz, 1H), 8.61 (d, J = 7.6Hz, 1H), 8.57 (d, J = 7.7Hz, 1H), 8.05 (s, 1H), 7.35 (dd, J = 10.7, 2.6 Hz, 1H), 7.22 (dd, J = 8.3, 6.1 Hz, 1H), 6.98 (td, J = 8.4, 2.7 Hz, 1H), 6.47 ( d, J = 7.6 Hz, 1H), 5.82-5.73 (m, 1H), 4.26 (dd, J = 13.3, 6.5 Hz, 1H), 2.87-2.76 (m, 2H), 2.59 (dt, J = 12.2, 7.6 Hz, 1H), 2.42 - 2.28 (m, 2H), 2.18 (ddd, J = 15.6, 13.0, 3.6 Hz, 1H), 2.03-1.97 (m, 1H), 1.85-1.76 (m, 2H), 1.45 (d, J = 6.8 Hz, 3H). MS (ESI) m/z = 402.4 [M+Na] + .
实施例18:(1
3E,1
4E,7
1S,7
3R,3R)-4
5-氟-3-甲基-2,8-二氮杂–1(5,3)-吡唑并[1,5-α]嘧啶杂-4(3,2)-吡啶杂-7(1,3)-环丁烷杂壬内酰胺-9-酮
Example 18: (1 3 E,1 4 E,7 1 S,7 3 R,3R)-4 5 -fluoro-3-methyl-2,8-diaza- 1(5,3)-pyridyl Zoxao[1,5-α]pyrimidin-4(3,2)-pyridin-7(1,3)-cyclobutanelactam-9-one
步骤1:5-(((R)-1-(2-(((1s,3S)-3-((叔丁氧基羰基)氨基)环丁基)乙炔基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 1: 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)ethynyl)-5-fluoropyridine-3 -ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向反应瓶中,依次加入(R)-5-((1-(5-氟-2-(((三氟甲基)磺酰基)氧基)吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯(487mg)、DMF(10mL)、叔丁基((1s,3s)-3-乙炔基环丁基)氨基甲酸酯(797mg)、碘化亚铜(78mg)及双三苯基磷二氯化钯(286mg)、TEA(206mg),加毕氮气保护下,70℃搅拌反应。反应完全后,浓缩反应液。柱层析纯化(石油醚/乙酸乙酯=1/1)得到化合物18A-1(426mg)。MS(ESI)m/z:545.5[M+Na]
+.
To the reaction flask, (R)-5-((1-(5-fluoro-2-(((trifluoromethyl))sulfonyl)oxy)pyridin-3-yl)ethyl)amino)pyridyl Ethylzolo[1,5-a]pyrimidine-3-carboxylate (487 mg), DMF (10 mL), tert-butyl((1s,3s)-3-ethynylcyclobutyl)carbamate (797 mg) ), cuprous iodide (78 mg) and bistriphenylphosphine palladium dichloride (286 mg), TEA (206 mg), and the reaction was stirred at 70 ° C under a nitrogen atmosphere. After the reaction was completed, the reaction solution was concentrated. Purification by column chromatography (petrole ether / ethyl acetate = 1 / 1) gave Compound 18A-1 (426mg). MS (ESI) m/z: 545.5 [M+Na] + .
步骤2:5-(((R)-1-(2-(2-((1s,3R)-3-((叔丁氧基羰基)氨基)环丁基)乙基)-5-氟吡啶-3-基)乙基)氨基乙基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 2: 5-(((R)-1-(2-(2-((1),3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)ethyl)-5-fluoropyridine Ethyl 3-ethyl)ethyl)aminoethyl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向高压釜中,依次加入18A-1(426mg)、MeOH(50mL)、氢氧化钯(229mg),氢气置换三次,充入氢气至54bar,66℃加热搅拌反应。反应完全后,过滤,滤饼用甲醇洗涤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=60/40)得到化合物18B-1(284mg)。Into the autoclave, 18A-1 (426 mg), MeOH (50 mL) and palladium hydroxide (229 mg) were successively added, three times of hydrogen was charged, and hydrogen gas was charged to 54 bar, and the reaction was stirred with heating at 66 °C. After completion of the reaction, it was filtered, and the filter cake was washed with methanol, and the filtrate was concentrated. Purification by column chromatography (petrole ether / ethyl acetate = 60 / 40) gave Compound 18B-1 (284mg).
1H NMR(500MHz,CDCl3)δ8.30(d,J=2.7Hz,1H),8.26(s,1H),8.23(d,J=7.5Hz,1H),7.71-7.65(m,1H),7.61-7.46(m,2H),6.15(d,J=5.3Hz,1H),6.04(s,1H),5.65-5.39(m,1H),5.02-4.84(m,1H),4.40-4.30(m,2H),3.04-2.84(m,2H),2.58-2.41(m,2H),2.10-2.00(m,2H),1.97-1.91(m,1H),1.89-1.83(m,1H),1.61(d,J=6.8Hz,3H),1.44(s,9H),1.38(t,J=7.1Hz,3H).MS(ESI)m/z:549.5[M+Na]
+.
1 H NMR (500MHz, CDCl3) δ8.30 (d, J = 2.7Hz, 1H), 8.26 (s, 1H), 8.23 (d, J = 7.5Hz, 1H), 7.71-7.65 (m, 1H), 7.61-7.46 (m, 2H), 6.15 (d, J = 5.3 Hz, 1H), 6.04 (s, 1H), 5.65-5.39 (m, 1H), 5.02-4.84 (m, 1H), 4.40-4.30 ( m, 2H), 3.04-2.84 (m, 2H), 2.58-2.41 (m, 2H), 2.10-2.00 (m, 2H), 1.97-1.91 (m, 1H), 1.89-1.83 (m, 1H), 1.61 (d, J = 6.8 Hz, 3H), 1.44 (s, 9H), 1.38 (t, J = 7.1 Hz, 3H). MS (ESI) m/z: 549.5 [M+Na] + .
步骤3:5-(((R)-1-(2-(2-((1s,3R)-3-((叔丁氧基羰基)氨基)环丁基)乙基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 3: 5-(((R)-1-(2-(2-((1),3R)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)ethyl)-5-fluoropyridine -3-yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入18B-1(300mg)、MeOH(4mL)、四氢呋喃(2mL)、水(1mL)、氢氧化锂一水合物(163mg),80℃加热搅拌反应。反应完全后,将反应液倒入冰水(100mL)中,缓慢滴加稀盐酸(2N)至反应液pH为4,DCM萃取,无水硫酸钠干燥。过滤,滤液浓缩得到化合物18C-1(359mg),不经纯化直接用于下一步反应。MS(ESI)m/z:521.6[M+Na]
+.
Into a reaction flask, 18B-1 (300 mg), MeOH (4 mL), tetrahydrofuran (2 mL), water (1 mL), and lithium hydroxide monohydrate (163 mg) were successively added, and the mixture was stirred under heating at 80 °C. After the reaction was completed, the reaction mixture was poured into ice water (100 mL), and diluted hydrochloric acid (2N) was slowly added dropwise to pH 4, extracted with DCM and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 18C-1 (359 mg). MS (ESI) m / z: 521.6 [M+Na] + .
步骤4:5-(((R)-1-(2-(2-((1s,3R)-3-氨基环丁基)乙基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-α]嘧啶-3-羧酸三盐酸盐Step 4: 5-((R)-1-(2-(2-((1s,3R)-3-Aminocyclobutyl)ethyl)-5-fluoropyridin-3-yl)ethyl)amino Pyrazolo[1,5-α]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入18C-1(350mg)、DCM(10mL)、氯化氢的1,4-二氧六环溶液(4M,10.00mL),加毕室温搅拌反应。反应完全后,反应液过滤,滤饼干燥得到化合物18D-1(355mg)。MS(ESI)m/z:421.6[M+Na]
+.
To the reaction flask, a solution of 18C-1 (350 mg), DCM (10 mL) and hydrogen chloride in 1,4-dioxane (4M, 10.00 mL) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was filtered, and then filtered, and then dried to give Compound 18D-1 (355 mg). MS (ESI) m / z: 421.6 [M+Na] + .
步骤5:(1
3E,1
4E,7
1S,7
3R,3R)-4
5-氟-3-甲基-2,8-二氮杂-1(5,3)-吡唑并[1,5-α]嘧啶杂-4(3,2)-吡啶杂-7(1,3)-环丁烷杂环壬内酰胺-9-酮
Step 5: (1 3 E,1 4 E,7 1 S,7 3 R,3R)-4 5 -fluoro-3-methyl-2,8-diaza-1(5,3)-pyrazole And [1,5-α]pyrimidine hetero-4(3,2)-pyridin-7(1,3)-cyclobutane heterocyclic lactam-9-one
向反应瓶中,依次加入18D-1(340mg)、DCM(40mL)、DMF(5mL)及DIPEA(692mg),冰浴下加入五氟苯基二苯基膦酸酯(257mg),加毕室温搅拌反应。反应完全后,浓缩反应液。向残留物中加入饱和碳酸钠水溶液(100mL),EA萃取,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM/MeOH=96/4)得到化合物I-18(74mg)。To the reaction flask, 18D-1 (340mg), DCM (40mL), DMF (5mL) and DIPEA (692mg) were added in sequence, and pentafluorophenyldiphenylphosphonate (257mg) was added to the ice bath, and the room temperature was added. Stir the reaction. After the reaction was completed, the reaction solution was concentrated. A saturated aqueous solution of sodium carbonate (100 mL) was added toEtOAc. Filter and concentrate the filtrate. Purification by column chromatography (DCM / MeOH = 96 / 4)
1H NMR(500MHz,DMSO-d
6)δ8.75(d,J=8.4Hz,1H),8.62(d,J=7.6Hz,1H),8.41(d,J=8.1Hz,1H),8.35(d,J=2.7Hz,1H),8.05(s,1H),7.73(dd,J=10.1,2.6Hz,1H),6.47(d,J=7.6Hz,1H),5.77-5.65(m,1H),4.25(d,J=6.4Hz,1H),3.18-3.09(m,1H),2.77-2.69(m,1H),2.64-2.55(m,1H),2.54-2.45(m,2H),2.41-2.25(m,2H),1.93-1.79(m,2H),1.48(d,J=6.8Hz,3H).
13C NMR(126MHz,DMSO-d
6)δ160.95,159.69,157.69,155.80,154.68,146.37,144.23,140.19,136.99,135.53,120.37,101.81,45.93,43.06,36.08,35.91,32.52,31.41,31.30,23.88.MS(ESI)m/z:403.4[M+Na]
+.
1 H NMR (500MHz, DMSO- d 6) δ8.75 (d, J = 8.4Hz, 1H), 8.62 (d, J = 7.6Hz, 1H), 8.41 (d, J = 8.1Hz, 1H), 8.35 (d, J = 2.7 Hz, 1H), 8.05 (s, 1H), 7.73 (dd, J = 10.1, 2.6 Hz, 1H), 6.47 (d, J = 7.6 Hz, 1H), 5.77-5.65 (m, 1H), 4.25 (d, J = 6.4 Hz, 1H), 3.18-3.09 (m, 1H), 2.77-2.69 (m, 1H), 2.64-2.55 (m, 1H), 2.54-2.45 (m, 2H) , 2.41-2.25 (m, 2H), 1.93-1.79 (m, 2H), 1.48 (d, J = 6.8 Hz, 3H). 13 C NMR (126 MHz, DMSO-d 6 ) δ 160.95, 159.69, 157.69, 155.80, 154.68, 146.37, 144.23, 140.19, 136.99, 135.53, 120.37, 101.81, 45.93, 43.06, 36.08, 35.91, 32.52, 31.41, 31.30, 23.88. MS (ESI) m/z: 403.4 [M+Na] + .
实施例19:(3
1S,3
3S,8R,E)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,6)-咪唑并[1,2-b]哒嗪-1(2,3)-吡啶杂-3(1,3)-环丁基环辛内酰胺-5-酮
Example 19: (3 1 S,3 3 S,8R,E)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3,6)-imidazole [1,2-b]pyridazine-1(2,3)-pyridin-3(1,3)-cyclobutylcyclooctanolac-5-one
步骤1:(R)-6-((1-(5-氟-2-甲氧基吡啶-3-基)乙基)氨基)咪唑并[1,2-b]哒嗪-3-羧酸乙酯Step 1: (R)-6-((1-(5-Fluoro-2-methoxypyridin-3-yl)ethyl)amino)imidazo[1,2-b]pyridazine-3-carboxylic acid Ethyl ester
向反应瓶中依次加入19A(2.04g)、6-氯咪唑并[1,2-b]哒嗪-3-甲酸乙酯(2.28g)、氟化钾(5.8g)和二甲亚砜(20mL),氮气保护下,140℃搅拌反应。反应完全后,浓缩反应液,将残留物溶于乙酸乙酯后,饱和食盐水洗涤,无水硫酸干燥,过滤,滤液浓缩。柱层析纯化(石油醚:乙酸乙酯=40:60),得到化合物19B(3.1681g)。不经纯化直接用于下一步反应。MS(ESI)m/z:360.5[M+H]
+
To the reaction flask were successively added 19A (2.04 g), 6-chloroimidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester (2.28 g), potassium fluoride (5.8 g) and dimethyl sulfoxide ( 20 mL), the reaction was stirred at 140 ° C under nitrogen. After the reaction was completed, the reaction mixture was evaporated, evaporated, evaporated, evaporated Purification by column chromatography (petrole ether: ethyl acetate = 40: 60) gave Compound 19B (3.1681 g). It was used in the next reaction without purification. MS (ESI) m/z: 360.5 [M+H] +
步骤2:(R)-6-((1-(5-氟-2-羟基吡啶-3-基)乙基)氨基)咪唑并[1,2-b]哒嗪-3-羧酸乙酯Step 2: (R)-6-((1-(5-Fluoro-2-hydroxypyridin-3-yl)ethyl)amino)imidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester
向反应瓶中依次加入19B(3.16g)、氯化氢的1,4-二氧六环溶液(4M,4.38g),氮气保护下,75℃搅拌反应。反应完全后,冰水浴冷却下用饱和碳酸氢钠水溶液调节反应液pH至弱碱性。EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物19C(2.541g)。不经纯化直接用于下一步反应。MS(ESI)m/z:346.5[M+H]
+.
To the reaction flask, a solution of 19B (3.16 g) and hydrogen chloride in 1,4-dioxane (4M, 4.38 g) was sequentially added, and the mixture was stirred at 75 ° C under nitrogen atmosphere. After the reaction was completed, the pH of the reaction mixture was adjusted to be weakly basic with a saturated aqueous solution of sodium hydrogencarbonate under ice-cooling. The organic phase was combined, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 19C (2.541 g). It was used in the next reaction without purification. MS (ESI) m / z: 346.5 [M+H] + .
步骤3:6-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁基氧基)-5-氟吡啶-3-基)乙基)氨基)咪唑并[1,2-b]哒嗪-3-羧酸乙酯Step 3: 6-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutyloxy)-5-fluoropyridin-3-yl) Ethyl)amino)imidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester
向反应瓶中,依次加入19C(2.54g)、((1r,3r)-3-羟基环丁基)氨基甲酸叔丁酯(1.67g)、三苯基磷(2.95g)及四氢呋喃(15mL),氮气保护下室温搅拌10分钟后,冰水浴冷却下将DEAD(2.08g)缓慢滴加至上述反应液中,加毕50℃搅拌反应。反应完全后,反应液中加入石油醚,大量的白色固体析出,过滤,滤液浓缩。柱层析纯化(石油醚:乙酸乙酯=55:45),得到化合物19D(2.01g)。MS(ESI)m/z:515.6[M+H]
+.
To the reaction flask, 19C (2.54 g), ((1r, 3r)-3-hydroxycyclobutyl)carbamic acid tert-butyl ester (1.67 g), triphenylphosphine (2.95 g) and tetrahydrofuran (15 mL) were added in order. After stirring at room temperature for 10 minutes under nitrogen atmosphere, DEAD (2.08 g) was slowly added dropwise to the above reaction liquid under ice-cooling, and the reaction was stirred at 50 °C. After the reaction was completed, petroleum ether was added to the reaction mixture, and a large white solid was precipitated, filtered, and the filtrate was concentrated. Purification by column chromatography (petrole ether: ethyl acetate = 55:45) gave Compound 19D (2.01 g). MS (ESI) m / z: 515.6 [M+H] + .
步骤4:6-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧基)-5-氟吡啶-3-基)乙基)氨基)咪唑并[1,2-b]哒嗪-3-羧酸Step 4: 6-(((R)-1-(2-((1s,3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluoropyridin-3-yl) Ethyl)amino)imidazo[1,2-b]pyridazine-3-carboxylic acid
向反应瓶中,依次加入19D(2.01g)、氢氧化锂一水合物(1.65g)、四氢呋喃(10.00mL)、乙醇(10.00mL)和水(10mL),氮气保护下,70℃搅拌反应。反应完全后,冰水浴冷却下向反应液加入稀盐酸(2N)调节pH为弱酸性,EA萃取,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物19E(1.95g)。MS(ESI)m/z:485.4[M-H]
-.
To the reaction flask, 19D (2.01 g), lithium hydroxide monohydrate (1.65 g), tetrahydrofuran (10.00 mL), ethanol (10.00 mL), and water (10 mL) were sequentially added, and the mixture was stirred at 70 ° C under nitrogen atmosphere. After the reaction was completed, dilute hydrochloric acid (2N) was added to the reaction mixture under cooling in an ice water bath to adjust the pH to be weakly acidic, extracted with EA, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 19E (1.95 g). MS (ESI) m/z: 485.4 [MH] - .
步骤5:6-(((R)-1-(2-((1s,3S)-3-氨基环丁氧基)-5-氟吡啶-3-基)乙基)氨基)咪唑并[1,2-b]哒嗪-3-羧酸三盐酸盐Step 5: 6-(((R)-1-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluoropyridin-3-yl)ethyl)amino)imidazo[1 ,2-b]pyridazine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入19E(1.95g)和氯化氢的1,4-二氧六环溶液(2.33g),加毕室温搅拌反应。反应完全后,浓缩反应液得到化合物19F(1.86g)。MS(ESI)m/z:385.0[M-H]
-.
To the reaction flask, a solution of 19E (1.95 g) and hydrogen chloride in 1,4-dioxane (2.33 g) was added in that order, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated to give Compound 19F (1.86 g). MS (ESI) m/z: 385.0 [MH] - .
步骤6:(3
1S,3
3S,8R,E)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,6)-咪唑并[1,2-b]哒嗪-1(2,3)-吡啶杂-3(1,3)-环丁基环辛内酰胺-5-酮
Step 6: (3 1 S,3 3 S,8R,E)-1 5 -Fluoro-8-methyl-2-oxa-4,7-diaza-6(3,6)-imidazo[ 1,2-b]pyridazine-1(2,3)-pyridin-3(1,3)-cyclobutylcyclooctanolac-5-one
向反应瓶中,依次加入19F(1.863g)、DIPEA(3.01g)、DCM(60mL)及DMF(10mL),加毕氮气保护下,将五氟苯基二苯基磷酸酯(1.49g)分两批加入到上述反应液中,加毕室温搅拌反应。反应完全后,浓缩反应液,向残留物中加入水(100mL),EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM:MeOH=94:6)得到化合物I-19(760mg)。To the reaction flask, 19F (1.863 g), DIPEA (3.01 g), DCM (60 mL) and DMF (10 mL) were added, and the pentafluorophenyl diphenyl phosphate (1.49 g) was added under nitrogen protection. Two batches were added to the above reaction solution, and the reaction was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated, evaporated, evaporated, evaporated Filter and concentrate the filtrate. Column chromatography (DCM: MeOH = 94: 6) gave Compound I-19 (760 mg).
1H NMR(500MHz,DMSO-d
6):δ9.62(d,J=10.5Hz,1H),8.22(d,J=10.5Hz,1H),8.04(d,J=3.0Hz,1H),7.87-7.75(m,2H),7.74(dd,J=8.5Hz,3.0Hz,1H),6.84(d,J=9.5Hz,1H),5.44(m,J=7.0Hz,1H),5.15-5.13(m,1H),4.79(t,J=3.5Hz,1H),3.06(m,,J=7.5Hz,1H),2.90(m,,J=6.5Hz,1H),2.19(dd,,J=13.0Hz,7.5Hz,1H),1.73(dd,,J=13.0Hz,7.5Hz,1H),1.47(d,J=7.0Hz,3H).
13C NMR(125MHz,DMSO-d
6):δ157.47,156.98,155.33,152.88,136.21,136.02,132.50,130.58,127.17,124.18,122.53,114.42,75.08,44.60,43.43,38.43,35.63,23.32.MS(ESI)m/z:369.5[M+H]
+.
1 H NMR (500MHz, DMSO- d 6): δ9.62 (d, J = 10.5Hz, 1H), 8.22 (d, J = 10.5Hz, 1H), 8.04 (d, J = 3.0Hz, 1H), 7.87-7.75 (m, 2H), 7.74 (dd, J = 8.5 Hz, 3.0 Hz, 1H), 6.84 (d, J = 9.5 Hz, 1H), 5.44 (m, J = 7.0 Hz, 1H), 5.15- 5.13 (m, 1H), 4.79 (t, J = 3.5 Hz, 1H), 3.06 (m, J = 7.5 Hz, 1H), 2.90 (m, J = 6.5 Hz, 1H), 2.19 (dd,, J = 13.0 Hz, 7.5 Hz, 1H), 1.73 (dd, J = 13.0 Hz, 7.5 Hz, 1H), 1.47 (d, J = 7.0 Hz, 3H). 13 C NMR (125 MHz, DMSO-d 6 ) : δ 157.47, 156.98, 155.33, 152.88, 136.21, 136.02, 132.50, 130.58, 127.17, 124.18, 122.53, 114.42, 75.08, 44.60, 43.43, 38.43, 35.63, 23.32. MS (ESI) m/z: 369.5 [M+H ] + .
实施例20:(1
3E,1
4E,7
1S,7
3S,3R)-4
5-氟-3-甲基-5-氧杂-2,8-二氮杂-1(5,3)-吡唑并[1,5-α]嘧啶-4(3,2)-吡啶杂-7(1,3)-环丁烷环壬内酰胺-9-酮
Example 20: (1 3 E,1 4 E,7 1 S,7 3 S,3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1(5 ,3)-pyrazolo[1,5-α]pyrimidin-4(3,2)-pyridin-7(1,3)-cyclobutanecyclolactam-9-one
步骤1:5-(((R)-1-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)环丁基)甲氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 1: 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)cyclobutyl)methoxy)-5-fluoropyridine-3 -ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向反应瓶中,加入20A-1(400mg)、((1s,3s)-3-羟甲基环丁基)氨基甲酸叔丁酯(256mg)、三苯基膦(486mg)和四氢呋喃(4mL),氮气保护下,冰水浴冷却下滴加DEAD(323mg)的四氢呋喃(2mL)溶液,加毕室温搅拌反应。反应完全后,浓缩反应液,柱层析纯化(乙酸乙酯:石油醚=0:100~60:40)得化合物20B-1(383mg)。MS(ESI)m/z:529.8[M+H]
+.
To the reaction flask, 20A-1 (400 mg), ((1s, 3s)-3-hydroxymethylcyclobutyl)carbamic acid tert-butyl ester (256 mg), triphenylphosphine (486 mg) and tetrahydrofuran (4 mL) were added. Under a nitrogen atmosphere, a solution of DEAD (323 mg) in tetrahydrofuran (2 mL) was added dropwise under ice-cooling, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated and purified by ethylamine (ethyl acetate: petroleum ether = 0:100 to 60:40) to give Compound 20B-1 (383mg). MS (ESI) m/z: 529.8 [M+H] + .
步骤2:5-(((R)-1-(2-(((1s,3S)-3-((叔丁氧基羰基)氨基)环丁基)甲氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 2: 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)methoxy)-5-fluoropyridine- 3-yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向含有20B-1(360mg)的反应瓶中,加入MeOH(10mL)和四氢呋喃(4mL)使其溶解,加入氢氧化锂一水合物(171mg)的水(1mL)溶液。加毕70℃搅拌反应。反应完全后,将反应液倒入冰水(100mL)中,缓慢加入稀盐酸(0.5N)调节pH至5,用DCM萃取,合并有机相,无水硫酸钠干燥。过滤,滤液浓缩得到化合物20C-1(372mg),未经纯化直接用于下一步反应。MS(ESI)m/z:523.4[M+Na]
+.
To a reaction flask containing 20B-1 (360 mg), MeOH (10 mL) and tetrahydrofuran (4 mL) were added and dissolved, and a solution of lithium hydroxide monohydrate (171 mg) in water (1 mL) was added. The reaction was stirred at 70 ° C. After the reaction was completed, the reaction mixture was poured into ice water (100 mL), EtOAc (EtOAc) Filtration and concentration of the filtrate gave Compound 20C-1 (372 mg). MS (ESI) m / z: 523.4 [M+Na] + .
步骤3:5-(((R)-1-(2-(((1s,3S)-3-氨基环丁基)甲氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 3: 5-(((R)-1-(2-(((1),3S)-3-aminocyclobutyl)methoxy)-5-fluoropyridin-3-yl)ethyl)amino) Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向含有20C-1(370mg)的反应瓶中加入氯化氢的1,4-二氧六环溶液(4N,5.00mL),混合物室温搅拌反应。反应完全后,浓缩反应液得到化合物20D-1(413.7mg),未经纯化直接用于下一步反应。MS(ESI)m/z:401.5[M+H]
+.
A 1,4-dioxane solution (4N, 5.00 mL) of hydrogen chloride was added to a reaction flask containing 20C-1 (370 mg), and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated to give Compound 20D-1 (413.7mg). MS (ESI) m / z: 401.5 [M+H] + .
步骤4:(1
3E,1
4E,7
1S,7
3S,3R)-4
5-氟-3-甲基-5-氧杂-2,8-二氮杂-1(5,3)-吡唑并[1,5-α]嘧啶-4(3,2)-吡啶杂-7(1,3)-环丁烷环壬内酰胺-9-酮
Step 4: (1 3 E, 1 4 E, 7 1 S, 7 3 S, 3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1 (5, 3)-pyrazolo[1,5-α]pyrimidine-4(3,2)-pyridin-7(1,3)-cyclobutanecyclolactam-9-one
向反应瓶中,加入20D-1(380mg)、DCM(100mL)、DMF(10mL)和DIPEA(771mg),室温搅拌下,加入五氟苯基二苯基膦酸酯(301mg)的DMF(5mL)溶液。加毕,加毕室温搅拌反应。反应完全后,浓缩反应液,向残留物中加入乙酸乙酯(100mL),依次用碳酸钠水溶液(2M)、水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(MeOH:DCM=0:100~4:96)得到化合物I-20(110mg)。To the reaction flask, 20D-1 (380 mg), DCM (100 mL), DMF (10 mL) and DIPEA (771 mg) were added, and, under stirring, pentafluorophenyldiphenylphosphonate (301 mg) in DMF (5 mL) ) solution. After the addition, the reaction was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated. EtOAcjjjjjjjjjjj Filter and concentrate the filtrate. Purification by column chromatography (MeOH: DCM = EtOAc: EtOAc)
1H NMR(500MHz,DMSO-d6):δ8.78~8.80(d,J=7.0Hz,1H),8.62~8.64(d,J=7.5Hz,1H),8.48~8.50(d,J=10.5Hz,1H),8.03(s,1H),7.98~7.99(d,J=3.0Hz,1H),7.57~7.60(dd,J=3.0,8.5Hz,1H),6.48~6.50(d,J=7.5Hz,1H),5.58~5.61(t,J=7.0Hz,1H),5.46~5.48(d,J=11.5Hz,1H),4.45~4.46(m,1H),3.85~3.88(d,J=11.0Hz,1H),2.74~2.76(m,1H),2.56~2.57(m,2H),2.29~2.33(m,1H),1.46~1.51(m,4H).13C NMR(125MHz,DMSO-d6):160.28,157.34,156.63,155.56,154.69,146.22,144.25,136.85,121.24,130.01,122.94,101.54,65.64,44.64,37.72,33.35,32.08,28.20,22.06.MS(ESI)e/z:383.5[M+H]
+
1H NMR (500MHz, DMSO-d6): δ 8.78~8.80 (d, J=7.0Hz, 1H), 8.62~8.64 (d, J=7.5Hz, 1H), 8.48~8.50(d, J=10.5Hz , 1H), 8.03 (s, 1H), 7.98 to 7.99 (d, J = 3.0 Hz, 1H), 7.57 to 7.60 (dd, J = 3.0, 8.5 Hz, 1H), 6.48 to 6.50 (d, J = 7.5) Hz, 1H), 5.58 to 5.61 (t, J = 7.0 Hz, 1H), 5.46 to 5.48 (d, J = 11.5 Hz, 1H), 4.45 to 4.46 (m, 1H), 3.85 to 3.88 (d, J = 11.0 Hz, 1H), 2.74 to 2.76 (m, 1H), 2.56 to 2.57 (m, 2H), 2.29 to 2.33 (m, 1H), 1.46 to 1.51 (m, 4H). 13C NMR (125 MHz, DMSO-d6) ): 160.28, 157.34, 156.63, 155.56, 154.69, 146.22, 144.25, 136.85, 121.24, 130.01, 122.94, 101.54, 65.64, 44.64, 37.72, 33.35, 32.08, 28.20, 22.06. MS (ESI) e/z: 383.5 [ M+H] +
实施例21:(3
1S,3
3R,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡咯并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环戊基环辛内酰胺-5-酮
Example 21: (3 1 S,3 3 R,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrrolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclopentylcyclooctanolac-5-one
步骤1:((1R,3S)-3-(2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环戊基)碳酸叔丁酯Step 1: ((1R,3S)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclopentyl Tert-butyl carbonate
向反应瓶中,依次加入((1R,3R)-3-羟基环戊基)氨基甲酸叔丁酯(400mg)、21-A-1(520mg)、三苯基膦(786mg)及四氢呋喃(2mL),冰水浴氮气保护下,将偶氮二甲酸二乙酯(558mg)缓慢加入到上述 反应液,加毕将反应液转移至室温反应,室温搅拌20min,然后将混合物加热至50℃反应。反应完全,向反应液中加入石油醚,过滤,母液浓缩,柱层析纯化(石油醚:乙酸乙酯=85:15),得到21-B-1(688mg)。To the reaction flask, ((1R,3R)-3-hydroxycyclopentyl)carbamic acid tert-butyl ester (400 mg), 21-A-1 (520 mg), triphenylphosphine (786 mg) and tetrahydrofuran (2 mL) were added in order. Under ice protection with ice water, diethyl azodicarboxylate (558 mg) was slowly added to the above reaction solution. After the addition, the reaction solution was transferred to room temperature, stirred at room temperature for 20 min, and then the mixture was heated to 50 ° C to react. The reaction was completed, and petroleum ether was added to the reaction mixture, which was filtered, and the residue was evaporated and purified by column chromatography (ethyl ether: ethyl acetate = 85:15) to afford 21-B-1 (688mg).
1H NMR(500MHz,DMSO-d6):δ9.00(s,1H),7.24(dd,J=3.0Hz,10.0Hz,1H),6.95(dd,J=3.0Hz,8.5Hz,1H),6.88-6.86(m,1H),5.58(dd,J=8.5Hz,,1H),4.75(br,1H),4.67(br,1H),4.04(d,J=7.5Hz,1H),2.39-2.37(m,1H),1.90-1.84(m,3H),1.81-1.60(m,2H),1.36(s,9H),1.32(d,J=6.5Hz,3H),1.12(s,9H)。
1 H NMR (500MHz, DMSO- d6): δ9.00 (s, 1H), 7.24 (dd, J = 3.0Hz, 10.0Hz, 1H), 6.95 (dd, J = 3.0Hz, 8.5Hz, 1H), 6.88-6.86 (m, 1H), 5.58 (dd, J = 8.5 Hz, 1H), 4.75 (br, 1H), 4.67 (br, 1H), 4.04 (d, J = 7.5 Hz, 1H), 2.39- 2.37 (m, 1H), 1.90- 1.84 (m, 3H), 1.81-1.60 (m, 2H), 1.36 (s, 9H), 1.32 (d, J = 6.5 Hz, 3H), 1.12 (s, 9H) .
13C NMR(125MHz,DMSO-d6):157.49,155.62,150.39,136.23,130.14,114.50,77.86,60.93,55.70,50.77,49.19,39.15,313.31,28.71,23.28,15.00。LC-MS:m/z=465.6[M+Na]
+.
13 C NMR (125 MHz, DMSO-d6): 157.49, 155.22, 150.39, 136.23, 130.14, 114.50, 77.86, 60.93, 55.70, 50.77, 49.19, 39.15, 313.31, 28.71, 23.28, 15.00. LC-MS: m/z = 465.6 [M+Na] + .
步骤2:((1R,3S)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)环戊基)碳酸叔丁酯Step 2: ((1R,3S)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclopentyl)carbonate tert-butyl ester
向反应瓶中,依次加入21-B-1(678mg)、四氢呋喃(10mL)、碘(85mg)、水(500mg),氮气保护下,将混合物加热至60℃反应。反应完全,停止加热,向反应液加入乙腈,除去溶剂得到21-C-1(642mg)。未经纯化直接进行下一步反应。To the reaction flask, 21-B-1 (678 mg), tetrahydrofuran (10 mL), iodine (85 mg), and water (500 mg) were sequentially added, and the mixture was heated to 60 ° C under a nitrogen atmosphere. The reaction was completed, heating was stopped, acetonitrile was added to the reaction mixture, and the solvent was removed to give 21-C-1 (642 mg). The next reaction was carried out without purification.
步骤3:5-(((R)-1-(2-(((1S,3R)-3-((叔丁基氧羰基)氨基)环戊基)氧杂)-5-氟苯基)乙基)氨基)吡咯并[1,5-a]嘧啶-3-羧酸乙酯Step 3: 5-(((R)-1-(2-(((())))))))) Ethyl)amino)pyrrolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中,依次加入21-C-1(642mg)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(355mg)、N,N-二异丙基乙基胺(1.16g)及正丁醇(6mL),氮气保护下,将混合物加热至120℃反应。反应完全,停止搅拌,乙酸乙酯萃取反应液,有机相合并,饱和食盐水洗涤有机相,无水硫酸钠干燥,过滤,浓缩。柱层析得到21-D-1(442mg)。To the reaction flask, 21-C-1 (642 mg), ethyl 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (355 mg), N,N-diisopropyl The amine (1.16 g) and n-butanol (6 mL) were heated to 120 ° C under nitrogen. The reaction was completed, the stirring was stopped, and the mixture was evaporated to ethyl ether. Column chromatography gave 21-D-1 (442 mg).
1H NMR(500MHz,DMSO-d6):δ8.54(d,J=7.5Hz,1H),8.17(d,J=7.5Hz,1H),8.12(s,1H),7.13(d,J=7.5Hz,1H),6.99-6.97(m,1H),6.95-6.94(br,1H),6.74(s,1H),6.49(d,J=7.5Hz,1H),5.55(s,1H),4.80(br,1H),4.19-4.17(m,2H),3.82-3.81(m,1H),2.46-2.43(m,1H),1.91-1.82(m,3H),1.70-1.65(m,2H),1.46(d,J=6.5Hz,1H),1.34(s,9H),1.26-1.24(m,3H)
1 H NMR (500MHz, DMSO- d6): δ8.54 (d, J = 7.5Hz, 1H), 8.17 (d, J = 7.5Hz, 1H), 8.12 (s, 1H), 7.13 (d, J = 7.5 Hz, 1H), 6.99-6.97 (m, 1H), 6.95-6.94 (br, 1H), 6.74 (s, 1H), 6.49 (d, J = 7.5 Hz, 1H), 5.55 (s, 1H), 4.80(br,1H), 4.19-4.17(m,2H),3.82-3.81(m,1H),2.46-2.43(m,1H),1.91-1.82(m,3H),1.70-1.65(m,2H) ), 1.46 (d, J = 6.5 Hz, 1H), 1.34 (s, 9H), 1.26-1.24 (m, 3H)
13C NMR(125MHz,DMSO-d6):162.74,157.60,156.75,155.73,155.57,151.32,148.53,146.19,136.27,135.50,114.60,101.60,98.28,77.97,60.22,50.88,45.60,31.00,28.65,21.40,14.85。LC-MS m/z=528.5[M+H]
+.
13 C NMR (125 MHz, DMSO-d6): 162.74, 157.60, 156.75, 155.73, 155.57, 151.32, 148.53, 146.19, 136.27, 135.50, 114.60, 101.60, 98.28, 77.97, 60.22, 50.88, 45.60, 31.00, 28.65, 21.40 , 14.85. LC-MS m/z = 528.5 [M+H] + .
步骤4:5-(((R)-1-(2-(((1S,3R)-3-((叔丁基氧羰基)氨基)环戊基)氧杂)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 4: 5-(((R)-1-(2-(((()))))))) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入21-D-1(442mg)、一水合氢氧化锂(246mg)、甲醇(5mL)、四氢呋喃(2mL)及水(4mL),氮气保护下,将混合物加热至60℃反应。原料反应完全,停止加热和搅拌,反应液冷至室温,向反应液加入稀盐酸调节反应液pH在5-7,乙酸乙酯萃取,有机相合并,饱和食盐水洗涤有机相,无水硫酸钠干燥,过滤,浓缩,得到21-E-1(430mg)。To the reaction flask, 21-D-1 (442 mg), lithium hydroxide monohydrate (246 mg), methanol (5 mL), tetrahydrofuran (2 mL) and water (4 mL) were added, and the mixture was heated to 60 ° C under nitrogen. reaction. The reaction of the starting material is complete, the heating and stirring are stopped, the reaction liquid is cooled to room temperature, the diluted hydrochloric acid is added to the reaction liquid, the pH of the reaction liquid is adjusted to 5-7, the ethyl acetate is extracted, the organic phase is combined, and the organic phase is washed with saturated brine, anhydrous sodium sulfate Dry, filter and concentrate to give 21-E-1 (430 mg).
LC-MS m/z=522.6[M+Na]
+。
LC-MS m/z = 522.6 [M+Na] + .
步骤5:5-(((R)-1-(2-(((1S,3R)-3-氨基环戊基)氧杂)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐向反应瓶中,依次加入21-E-1(430mg)、氯化氢的二氧六环溶液(1.458g),氮气保护下,室温搅拌反应。减压浓缩,向浓缩物种加入乙腈,浓缩,浓缩物过滤,滤液减压干燥得到21-F-1(330mg)。LC-MS m/z=400.4[M+H]
+。
Step 5: 5-(((R)-1-(2-(((()))))))))))))))) 1,5-a]pyrimidine-3-carboxylic acid trihydrochloride salt To the reaction flask, 21-E-1 (430 mg), hydrogen chloride in dioxane solution (1.458 g) was added in sequence, and the mixture was stirred at room temperature under nitrogen. reaction. The organic layer was concentrated under reduced pressure. EtOAc (EtOAc) was evaporated. LC-MS m/z = 400.4 [M+H] + .
步骤6:(3
1S,3
3R,6
3E,6
4E,8R)-1
4-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡咯并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环戊基环辛内酰胺-5-酮
Step 6: (3 1 S,3 3 R,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrrolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclopentylcyclooctanolac-5-one
反应瓶中,依次加入21-F-1(322mg)、N,N-二异丙基乙基胺(650mg)、二氯甲烷(60mL)及二甲基甲酰胺(20mL),室温搅拌20min,将五氟苯基二苯基磷酸酯(280mg)分批缓慢加入至上述反应液中,氮气保护下,室温搅拌反应。反应完全。减压蒸馏除去溶剂,向反应液加入饱和碳酸钠水溶液淬灭反应,乙酸乙酯萃取,有机相合并,饱和食盐水洗涤有机相,无水硫酸钠干燥有机相,过滤,浓缩,柱层析(二氯甲烷:甲醇=96:4)纯化,得到化合物I-21(111mg)。21-F-1 (322 mg), N,N-diisopropylethylamine (650 mg), dichloromethane (60 mL) and dimethylformamide (20 mL) were added to the reaction mixture, and stirred at room temperature for 20 min. Pentafluorophenyl diphenyl phosphate (280 mg) was slowly added portionwise to the above reaction solution, and the reaction was stirred at room temperature under a nitrogen atmosphere. The reaction is complete. The solvent was evaporated under reduced pressure, and the mixture was evaporated, evaporated, evaporated, evaporated, evaporated. Purification with dichloromethane:methanol = 96:4) gave Compound I-21 (111 mg).
1H NMR(500MHz,DMSO-d6):δ8.74(d,J=7.0Hz,1H),8.66(d,J=10.0Hz,1H),8.54(d,J=8.0Hz,1H),8.02(s,1H),7.12-7.09(dd,J=3.0Hz,9.5Hz,1H),6.98-6.96(m,2H),6.37(d,J=7.5Hz,1H),5.54(t,J=7.0Hz,1H), 4.99(t,J=9.0Hz,1H),4.77(d,J=7.5Hz,1H),2.26(m,1H),2.16(m,1H),2.04-1.91(m,2H),1.90-1.81(m,1H)1.79-1.78(m,1H),1.40(d,J=7.0Hz,3H)。
13C NMR(125MHz,DMSO-d6):160.47,157.75,,150.30,146.21,144.26,136.51,114.15,101.54,100.84,79.39,55.36,46.55,43.30,38.68,33.50,32.27,22.65。LC-MS m/z=382.5[M+H]
+。
1 H NMR (500MHz, DMSO- d6): δ8.74 (d, J = 7.0Hz, 1H), 8.66 (d, J = 10.0Hz, 1H), 8.54 (d, J = 8.0Hz, 1H), 8.02 (s, 1H), 7.12-7.09 (dd, J = 3.0 Hz, 9.5 Hz, 1H), 6.98-6.96 (m, 2H), 6.37 (d, J = 7.5 Hz, 1H), 5.54 (t, J = 7.0 Hz, 1H), 4.99 (t, J = 9.0 Hz, 1H), 4.77 (d, J = 7.5 Hz, 1H), 2.26 (m, 1H), 2.16 (m, 1H), 2.04-1.91 (m, 2H), 1.90-1.81 (m, 1H) 1.79-1.78 (m, 1H), 1.40 (d, J = 7.0 Hz, 3H). 13 C NMR (125 MHz, DMSO-d6): 160.47, 157.75, 150.30, 146.21, 144.26, 136.51, 114.15, 101.54, 100.84, 79.39, 55.36, 46.55, 43.30, 38.68, 33.50, 32.27, 22.65. LC-MS m/z = 382.5 [M+H] + .
实施例22:(3
1R,3
3R,6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环戊基环辛内酰胺-5-酮
Example 22: (3 1 R,3 3 R,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3 ,5)-pyrazolo[1,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one
步骤1:5-(((R)-1-(2-(((1R,3R)-3-((叔丁基氧羰基)氨基)环戊基)氧杂)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 1: 5-(((R)-1-(2-((1(R)))))((tert-butyloxycarbonyl)amino)cyclopentyl)oxa)-5-fluoropyridine-3 -ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
氮气保护及冰水浴冷却下,将DEAD(260mg)缓慢加入到22A(345mg)、((1R,3S)-3-羟基环戊基)氨基甲酸叔丁酯(221mg)、三苯基磷(393mg)的四氢呋喃(2mL)溶液中,将反应转移至室温反应30分钟,然后将反应液加热至50℃搅拌反应。反应完全后,EA萃取反应液,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,浓缩柱层析纯化(石油醚/乙酸乙酯=85/15)得到化合物22B(459mg)。MS(ESI)m/z:551.6[M+Na]
+.
Under nitrogen protection and ice water cooling, DEAD (260 mg) was slowly added to 22A (345 mg), (1R,3S)-3-hydroxycyclopentyl)carbamic acid tert-butyl ester (221 mg), triphenylphosphine (393 mg). In a solution of tetrahydrofuran (2 mL), the reaction was transferred to room temperature for 30 minutes, and then the reaction solution was heated to 50 ° C to stir the reaction. After the reaction was completed, the reaction mixture was extracted with EtOAc. Filtration and purification by column chromatography (EtOAc/EtOAcEtOAcEtOAc MS (ESI) m / z: 551.6 [M+Na] + .
步骤2:5-(((R)-1-(2-(((1R,3R)-3-((叔丁基氧羰基)氨基)环戊基)氧杂)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 2: 5-(((R)-1-(2-((1(R)))))((tert-butyloxycarbonyl)amino)cyclopentyl)oxa)-5-fluoropyridine-3 -yl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入22B(430mg)、氢氧化锂一水合物(513mg)、MeOH(20mL)、四氢呋喃(5mL)和水(5mL),氮气保护下,70℃搅拌反应。反应完全后,冰水浴冷却下向反应液中加入稀盐酸(0.5N)调节pH至5-7,EA萃取,有机相相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物22C(429mg)。MS(ESI)m/z:523.6[M+Na]
+.
To the reaction flask, 22B (430 mg), lithium hydroxide monohydrate (513 mg), MeOH (20 mL), tetrahydrofuran (5 mL) and water (5 mL) were sequentially added, and the mixture was stirred at 70 ° C under nitrogen atmosphere. After the reaction was completed, dilute hydrochloric acid (0.5 N) was added to the reaction mixture under ice-cooling to adjust the pH to 5-7, EA was extracted, the organic phase was combined, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 22C (429 mg). MS (ESI) m / z: 523.6 [M+Na] + .
步骤3:5-(((R)-1-(2-(((1R,3R)-3-氨基环戊基)氧杂)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 3: 5-(((R)-1-(2-(((1(R))))))))))))))))) Zindro[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
冰水浴氮气保护下,氯化氢的1,4-二氧六环溶液(4M,1.6g)缓慢滴入22C(429mg)中。加毕室温搅拌反应。反应完全后,过滤,滤饼干燥得化合物22D(180mg)。MS(ESI)m/z:423.3[M+Na]
+.
A solution of hydrogen chloride in 1,4-dioxane (4M, 1.6 g) was slowly added dropwise to 22C (429 mg) under ice-cooling. The reaction was stirred at room temperature. After completion of the reaction, the mixture was filtered and dried to give compound 22D (180 mg). MS (ESI) m / z: 423.3 [M+Na] + .
步骤4:(3
1R,3
3R,6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环戊基环辛内酰胺-5-酮
Step 4: (3 1 R, 3 3 R, 6 3 E, 6 4 E, 8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6 (3, 5)-pyrazolo[1,5-a]pyrimidin-1(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolac-5-one
向反应瓶中,依次加入22D(180mg)、DIPEA(457mg))、DMF(10mL)、DCM(80mL),加毕氮气保护下,将五氟苯基二苯基磷酸酯(140mg)分两批加入到上述反应液中,加毕室温搅拌反应。反应完全后,浓缩反应液,残留物溶于EA后,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩,柱层析纯化(DCM/MeOH=96/4),得到化合物I-22(55mg)。To the reaction flask, 22D (180 mg), DIPEA (457 mg), DMF (10 mL), DCM (80 mL) were added, and the pentafluorophenyl diphenyl phosphate (140 mg) was divided into two batches under the protection of nitrogen. It was added to the above reaction liquid, and the reaction was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated. Filtration, concentration of the filtrate, and purification by column chromatography (DCM / MeOH = 96 / 4)
1H NMR(500MHz,DMSO-d6):δ8.47(d,J=7.5Hz,1H),8.20(d,J=7.5Hz,1H),8.06(d,J=3.0Hz,1H),7.79(dd,J=3.0Hz,9.0Hz,1H),7.62(d,J=8.5Hz,2H),6.48(br,1H),6.20(d,J=7.5Hz,1H),6.12(m,1H),5.14-5.12(m,1H),2.15(s,1H),1.99-1.88(m,1H),1.81-1.78(m,1H),1.56-1.55(m,3H),1.44-1.43(m,1H),1.37-1.35(m,1H),1.19-1.16(m,1H).
13C NMR(125MHz,DMSO-d6):170.80,165.90,156.52,155.61,149.21,143.61,136.85,132.71,130.23,124.91,103.40,100.65,77.15,60.22,49.74,40.85,29.26,22.56,14.55.MS(ESI)m/z:405.4[M+Na]
+.
1 H NMR (500MHz, DMSO- d6): δ8.47 (d, J = 7.5Hz, 1H), 8.20 (d, J = 7.5Hz, 1H), 8.06 (d, J = 3.0Hz, 1H), 7.79 (dd, J = 3.0 Hz, 9.0 Hz, 1H), 7.62 (d, J = 8.5 Hz, 2H), 6.48 (br, 1H), 6.20 (d, J = 7.5 Hz, 1H), 6.12 (m, 1H) ), 5.14 - 5.12 (m, 1H), 2.15 (s, 1H), 1.99-1.88 (m, 1H), 1.81-1.78 (m, 1H), 1.56-1.55 (m, 3H), 1.44-1.43 (m , 1H), 1.37-1.35 (m, 1H), 1.19-1.16 (m, 1H). 13 C NMR (125MHz, DMSO-d6): 170.80, 165.90, 156.52, 155.61, 149.21, 143.61, 136.85, 132.71, 130.23 , 124.91, 103.40, 100.65, 77.15, 60.22, 49.74, 40.85, 29.26, 22.56, 14.55. MS (ESI) m/z: 405.4 [M+Na] + .
实施例23:(1
3E,1
4E,7
1S,7
3S,3R)-4
5-氟-3-甲基-5-氧杂-2,8-二氮杂-1(5,3)-吡唑并[1,5-α]嘧啶杂-4(1,2)-苯杂-7(1,3)-环丁烷杂环壬内酰胺-9-酮-6,6-d
2
Example 23: (1 3 E,1 4 E,7 1 S,7 3 S,3R)-4 5 -fluoro-3-methyl-5-oxa-2,8-diaza-1(5 ,3)-pyrazolo[1,5-α]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutane heterocyclic lactam-9-one-6,6 -d 2
步骤1:(1s,3s)-3-((叔丁氧基羰基)氨基)环丁烷-1-羧酸甲酯Step 1: (1s, 3s)-3-((tert-Butoxycarbonyl)amino)cyclobutane-1-carboxylic acid methyl ester
0℃下,碘甲烷(2.97g)缓慢滴入23A-1(3g)与碳酸钾(2.89g)的DMF(10mL)搅拌液中,加毕,混合物室温搅反应。反应完全后,向反应液中加入水(200mL),EA萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物23B-1(3.42g)。Methyl iodide (2.97 g) was slowly added dropwise to a stirred solution of 23A-1 (3 g) and potassium carbonate (2.89 g) in DMF (10 mL) at 0 ° C, and the mixture was stirred at room temperature. After the reaction was completed, water (200 mL) was added to the mixture and the mixture was evaporated. Filtration and concentration of the filtrate gave compound 23B-1 (3.42 g).
1H NMR(500MHz,CDCl
3)δ4.94(s,1H),4.16-3.91(m,1H),3.63(s,3H),2.78–2.68(m,1H),2.63–2.48(m,2H),2.12–2.02(m,2H),1.39(s,9H).
1 H NMR (500MHz, CDCl 3 ) δ4.94 (s, 1H), 4.16-3.91 (m, 1H), 3.63 (s, 3H), 2.78-2.68 (m, 1H), 2.63-2.48 (m, 2H ), 2.12–2.02 (m, 2H), 1.39 (s, 9H).
步骤2:((1s,3s)-3-(羟甲基-d
2)环丁基)氨基甲酸叔丁酯
Step 2: ((1s,3s)-3-(Hydroxymethyl-d 2 )cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入23B-1(700mg)、硼氢化钠-d4(383mg)、氯化锂(388mg)、四氢呋喃(15mL)及甲醇-d1(10.00mL),加毕氮气保护下,加毕室温搅拌反应。反应完全后,向反应液中加入5wt%柠檬酸重水溶液(60mL)淬灭反应,用DCM萃取。合并DCM相,依次用5wt%碳酸氢钠重水溶液、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物23C-1(592mg)。To the reaction flask, 23B-1 (700mg), sodium borohydride-d4 (383mg), lithium chloride (388mg), tetrahydrofuran (15mL) and methanol-d1 (10.00mL) were added in sequence, and added under nitrogen protection. The reaction was stirred at room temperature. After the reaction was completed, a 5 wt% aqueous citric acid solution (60 mL) was added to the reaction mixture, and the mixture was evaporated. The combined DCM phases were washed successively with a 5 wt% aqueous sodium hydrogencarbonate solution and brine, and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 23C-1 (592 mg).
1H NMR(500MHz,CDCl
3)δ4.74(s,1H),4.03(d,J=6.3Hz,1H),2.49-2.38(m,2H),2.21-2.12(m,1H),1.69-1.58(m,3H),1.45(s,9H).
1 H NMR (500MHz, CDCl 3 ) δ4.74 (s, 1H), 4.03 (d, J = 6.3Hz, 1H), 2.49-2.38 (m, 2H), 2.21-2.12 (m, 1H), 1.69- 1.58 (m, 3H), 1.45 (s, 9H).
步骤3:5-(((R)-1-(2-(((1s,3S)-3-((叔丁氧基羰基)氨基)环丁基)甲氧基-d
2)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯
Step 3: 5-(((R)-1-(2-(((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutyl)methoxy-d 2 )-5-) Ethyl fluorophenyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
0℃下,氮气保护下,将DIAD(446mg)的无水四氢呋喃(1.5mL)溶液缓慢滴入23C-1(280mg)、(R)-5-((1-(5-氟-2-羟基苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯(474mg)、三苯基膦(578mg)的四氢呋喃(2.5mL)搅拌液中,50℃搅拌反应。反应完全后,浓缩反应液。柱层析纯化(石油醚/乙酸乙酯=7/3)得到化合物23D-1(875mg)。MS(ESI)m/z:552.6[M+Na]
+.
A solution of DIAD (446 mg) in anhydrous tetrahydrofuran (1.5 mL) was slowly added dropwise to a solution of 23C-1 (280 mg), (R)-5-((1-(5-fluoro-2-hydroxy) at 0 ° C under N2. Stirring at 50 ° C with a stirred solution of ethyl phenyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate (474 mg), triphenylphosphine (578 mg) in tetrahydrofuran (2.5 mL) reaction. After the reaction was completed, the reaction solution was concentrated. Purification by column chromatography (petrole ether / ethyl acetate = 7 / 3) gave Compound 23D-1 (875mg). MS (ESI) m / z: 552.6 [M+Na] + .
步骤4:5-(((R)-1-(2-(((1s,3S)-3-((叔丁氧基羰基)氨基)环丁基)甲氧基-d
2)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸
Step 4: 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)methoxy-d 2 )-5- Fluorophenyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入23D-1(875mg)、MeOH(4mL)、四氢呋喃(2mL)、水(1mL)和氢氧化锂一水合物(471mg),80℃搅拌反应。反应完全后,将反应液倒入冰水(100mL)中,缓慢滴加稀盐 酸(2N)调节pH至4,DCM萃取,无水硫酸钠干燥。过滤,滤液浓缩得到化合物23E-1(786mg),不经纯化直接用于下一步反应。MS(ESI)m/z:500.5[M-H]
-.
Into a reaction flask, 23D-1 (875 mg), MeOH (4 mL), tetrahydrofuran (2 mL), water (1 mL) and lithium hydroxide monohydrate (471 mg) were sequentially added, and the mixture was stirred at 80 °C. After the reaction was completed, the reaction mixture was poured into ice water (100 mL), and then diluted with hydrochloric acid (2N) to adjust to pH 4, extracted with DCM and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 23E-1 (786 mg). MS (ESI) m/z: 500.5 [MH] - .
步骤5:5-(((R)-1-(2-(((1s,3S)-3-氨基环丁基)甲氧基-d
2)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐
Step 5: 5 - (((R ) -1- (2 - (((1s, 3S) -3- amino-cyclobutyl) methoxy -d 2) -5- fluorophenyl) ethyl) amino) Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入23E-1(786mg)、DCM(10mL)、氯化氢的1,4-二氧六环溶液(4M,20mL),加毕室温搅拌反应。反应完全后,浓缩反应液得到化合物23F-1(453mg),不经纯化直接用于下一步反应。MS(ESI)m/z:400.3[M-H]
-.
To the reaction flask, a solution of 23E-1 (786 mg), DCM (10 mL) and hydrogen chloride in 1,4-dioxane (4M, 20 mL) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated to give Compound 23F-1 (453 mg). MS (ESI) m/z: 400.3 [MH] - .
步骤6:(1
3E,1
4E,7
1S,7
3S,3R)-4
5-氟-3-甲基-5-氧杂-2,8-二氮杂–1(5,3)-吡唑并[1,5-α]嘧啶杂-4(1,2)-苯杂-7(1,3)-环丁烷杂环壬内酰胺-9-酮-6,6-d
2
Step 6: (1 3 E, 1 4 E, 7 1 S, 7 3 S, 3R) -4 5 - fluoro-3-methyl-5-oxa-2,8-diaza-1 (5 3)-pyrazolo[1,5-α]pyrimidin-4(1,2)-benzene-7(1,3)-cyclobutane heterocyclic lactam-9-one-6,6- d 2
向反应瓶中,依次加入23F-1(453mg)、DCM(70mL)、DMF(7mL)及DIPEA(917mg),冰浴下加入五氟苯基二苯基磷酸酯(341mg),室温搅拌反应。反应完全后,浓缩反应液。向残留物加入饱和碳酸钠水溶液(100mL),EA萃取,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM/MeOH=96/4)得化合物I-23(140mg)。To the reaction flask, 23F-1 (453 mg), DCM (70 mL), DMF (7 mL) and DIPEA (917 mg) were sequentially added, and pentafluorophenyldiphenyl phosphate (341 mg) was added thereto under ice-cooling, and the reaction was stirred at room temperature. After the reaction was completed, the reaction solution was concentrated. A saturated aqueous solution of sodium carbonate (100 mL) was added toEtOAc. Filter and concentrate the filtrate. Purification by column chromatography (DCM / MeOH = EtOAc)
1H NMR(500MHz,DMSO-d
6)δ8.74(t,J=8.3Hz,2H),8.59(d,J=7.6Hz,1H),8.03(s,1H),7.17-7.12(m,2H),6.98(td,J=8.6,3.1Hz,1H),6.43(d,J=7.6Hz,1H),5.84(p,J=7.0Hz,1H),4.52-4.42(m,1H),2.79(dd,J=21.6,9.8Hz,1H),2.65(dd,J=21.1,9.3Hz,1H),2.61-2.54(m,1H),2.30-2.23(m,1H),1.73-1.66(m,1H),1.45(d,J=6.9Hz,3H).
13C NMR(126MHz,DMSO-d
6)δ160.45,157.92,156.04,155.55,152.68,146.36,144.25,136.74,136.35,114.46,114.33,112.90,101.50,43.83,38.23,33.25,32.05,28.67,22.84.MS(ESI)m/z:[M+H]
+=384.1784.
1 H NMR (500MHz, DMSO- d 6) δ8.74 (t, J = 8.3Hz, 2H), 8.59 (d, J = 7.6Hz, 1H), 8.03 (s, 1H), 7.17-7.12 (m, 2H), 6.98 (td, J = 8.6, 3.1 Hz, 1H), 6.43 (d, J = 7.6 Hz, 1H), 5.84 (p, J = 7.0 Hz, 1H), 4.52-4.42 (m, 1H), 2.79 (dd, J=21.6, 9.8 Hz, 1H), 2.65 (dd, J=21.1, 9.3 Hz, 1H), 2.61-2.54 (m, 1H), 2.30-2.23 (m, 1H), 1.73-1.66 ( m,1H), 1.45 (d, J = 6.9 Hz, 3H). 13 C NMR (126 MHz, DMSO-d 6 ) δ 160.45, 157.92, 156.04, 155.55, 152.68, 146.36, 144.25, 136.74, 136.35, 114.46, 114.33, 112.90,101.50,43.83,38.23,33.25,32.05,28.67,22.84.MS(ESI) m/z:[M+H] + =384.1784.
实施例24:(1
1S,1
3S,6
3E,6
4E,4R)-3
4-氟-4-甲基-2-氧杂-5,8-二氮杂-6(5,3)-吡唑并[1,5-a]嘧啶杂-3(1,2)-苯杂-1(1,3)-环丁杂壬内酰胺-7-酮
Example 24: (1 1 S,1 3 S,6 3 E,6 4 E,4R)-3 4 -fluoro-4-methyl-2-oxa-5,8-diaza-6(5 ,3)-pyrazolo[1,5-a]pyrimidin-3(1,2)-benzene-1(1,3)-cyclobutanlactam-7-one
步骤1:((1r,3r)-3-羟基环丁基)甲基)氨基甲酸叔丁酯Step 1: ((1r,3r)-3-Hydroxycyclobutyl)methyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入24A-1(500mg)、四氢呋喃(10mL)、水(2mL)、TEA(1103mg)和二碳酸二叔丁酯(872mg),加毕室温搅拌反应。反应完全后,将反应液倒入EA(100mL)中,依次用水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物24B-1(727.8mg)。To the reaction flask, 24A-1 (500 mg), tetrahydrofuran (10 mL), water (2 mL), TEA (1103 mg) and di-tert-butyl dicarbonate (872 mg) were sequentially added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was poured into EA (100 mL). Filtration and concentration of the filtrate gave compound 24B-1 (727.8 mg).
1H NMR(500MHz,DMSO-d
6)δ6.81(t,J=5.0Hz,1H),4.89(d,J=6.5Hz,1H),4.14(dd,J=7.0,14.0Hz,1H),2.94(t,J=7.0Hz,2H),2.13-2.09(m,1H),1.96-1.92(m,2H),1.85-1.79(m,2H),1.37(s,9H).
13C NMR(125MHz,DMSO-d
6)δ156.26,77.81,64.57,45.04,35.74,28.72,27.27.
1 H NMR (500MHz, DMSO- d 6) δ6.81 (t, J = 5.0Hz, 1H), 4.89 (d, J = 6.5Hz, 1H), 4.14 (dd, J = 7.0,14.0Hz, 1H) , 2.94 (t, J = 7.0 Hz, 2H), 2.13 - 2.09 (m, 1H), 1.96-1.92 (m, 2H), 1.85-1.79 (m, 2H), 1.37 (s, 9H). 13 C NMR (125MHz, DMSO-d 6 ) δ156.26, 77.81, 64.57, 45.04, 35.74, 28.72, 27.27.
步骤2:((1S,3s)-3-(2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)环丁基)甲基)氨基甲酸叔丁酯Step 2: ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)cyclobutane Tert-butyl)methyl)carbamate
向反应瓶中,加入24B-1(727.8mg)、16A(750mg)、三苯基膦(1518mg)和四氢呋喃(10mL),加毕,冰乙醇浴及氮气保护下搅拌,滴加DEAD(1008mg),加毕室温搅拌反应。反应完全后,浓缩反应液,柱层析纯化(乙酸乙酯:石油醚=0:100~30:70)得到化合物24C-1(1328mg)。MS(ESI)m/z:465.5[M+Na]
+.
To the reaction flask, 24B-1 (727.8mg), 16A (750mg), triphenylphosphine (1518mg) and tetrahydrofuran (10mL) were added, added, stirred under ice-cold bath and nitrogen, and DEAD (1008mg) was added dropwise. The reaction was stirred at room temperature. After completion of the reaction, the reaction mixture was concentrated and purified by ethylamine (ethyl acetate: petroleum ether = 0: 100 to 30: 70) to afford compound 24C-1 (1328 mg). MS (ESI) m / z: 465.5 [M+Na] + .
步骤3:((1S,3s)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)环丁基)甲基)氨基甲酸叔丁酯Step 3: ((1S,3s)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)cyclobutyl)methyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入24C-1(1328mg)、碘粒(300mg)和四氢呋喃(20mL)、水(2mL),加毕50℃搅拌反应。反应完全后,将反应液倒入乙酸乙酯(200mL),搅拌下加入10wt%硫代硫酸钠水溶液(20mL),搅拌,分相,有机相依次用水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物24D-1(1021mg)。To the reaction flask, 24C-1 (1328 mg), iodine (300 mg), tetrahydrofuran (20 mL), and water (2 mL) were successively added, and the mixture was stirred at 50 ° C. After the completion of the reaction, the reaction mixture was poured into ethyl acetate (200 mL), and then, 10% by weight aqueous sodium thiosulfate solution (20 mL) was added and stirred, and the phases were separated. The organic phase was washed successively with water and brine, dried over anhydrous sodium sulfate . Filtration and concentration of the filtrate gave compound 24D-1 (1021 mg).
步骤4:5-(((R)-1-(2-((1s,3S)-3-(((叔丁氧基羰基)氨基)甲基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 4: 5-(((R)-1-(2-((1),3S)-3-(((tert-Butoxycarbonyl)amino)methyl)cyclobutoxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中,依次加入24D-1(1021mg)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(817mg)、乙醇(30mL)和DIPEA(3.90g),加毕80℃搅拌反应。反应完全后,浓缩反应液,残留物溶于乙酸乙酯(100mL),依次用水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。制砂,柱层析纯化(乙酸乙酯:石油醚=0:100~60:40)得到化合物24E-1(323mg)。To the reaction flask, 24D-1 (1021 mg), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester (817 mg), ethanol (30 mL) and DIPEA (3.90 g) were sequentially added. The reaction was stirred at 80 ° C. After the reaction was completed, the reaction mixture was evaporated, evaporated, evaporated Filter and concentrate the filtrate. Sanding and column chromatography (ethyl acetate: petroleum ether = 0: 100 - 60: 40) gave Compound 24E-1 (323 mg).
1H NMR(500MHz,DMSO-d
6)δ8.54(d,J=7.0Hz,1H),8.19(d,J=6.5Hz,1H),8.12(s,1H),7.13(d,J=9.5Hz,1H),6.98(t,J=8.0Hz,1H),6.86-6.83(m,2H),6.50(d,J=6.5Hz,1H)5.59-5.57(m,1H),4.61-4.60(m,1H),4.17-4.15(m,2H),3.02-3.01(m,2H),2.07-1.68(m,4H),1.47(d,J=6.5Hz,3H),1.37(s,9H),1.25(t,J=7.0Hz,3H).MS(ESI)m/z:528.6[M+H]
+.
1 H NMR (500 MHz, DMSO-d 6 ) δ 8.54 (d, J = 7.0 Hz, 1H), 8.19 (d, J = 6.5 Hz, 1H), 8.12 (s, 1H), 7.13 (d, J = 9.5 Hz, 1H), 6.98 (t, J = 8.0 Hz, 1H), 6.86-6.83 (m, 2H), 6.50 (d, J = 6.5 Hz, 1H) 5.59 - 5.57 (m, 1H), 4.61-4.60 (m, 1H), 4.17-4.15 (m, 2H), 3.02-3.01 (m, 2H), 2.07-1.68 (m, 4H), 1.47 (d, J = 6.5 Hz, 3H), 1.37 (s, 9H) ), 1.25 (t, J = 7.0 Hz, 3H). MS (ESI) m/z: 528.6 [M+H] + .
步骤5:5-(((R)-1-(2-((1s,3S)-3-(((叔丁氧基羰基)氨基)甲基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 5: 5-(((R)-1-(2-((1),3S)-3-(((tert-Butoxycarbonyl)amino)methyl)cyclobutoxy)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向含有24E-1(308.9mg)的反应瓶中,依次加入MeOH(5mL)、四氢呋喃(2mL)和水(1mL),搅拌溶解后,加入氢氧化锂一水合物(147mg),加毕80℃搅拌反应。反应完全后,将反应液倒入冰/水(200mL)中,缓慢加入0.5M盐酸调节pH至4-5,用DCM萃取,合并DCM相,无水硫酸钠干燥。过滤,滤液浓缩得到化合物24F-1(302.9mg)。MS(ESI)m/z:498.5[M-H]
-.
To a reaction flask containing 24E-1 (308.9 mg), MeOH (5 mL), tetrahydrofuran (2 mL) and water (1 mL) were successively added, and the mixture was stirred and dissolved, and then lithium hydroxide monohydrate (147 mg) was added thereto, and 80 ° C was added thereto. Stir the reaction. After the reaction was completed, the reaction mixture was poured into ice-water (200 mL). Filtration and concentration of the filtrate gave compound 24F-1 (302.9 mg). MS (ESI) m/z: 498.5 [MH] - .
步骤6:5-(((R)-1-(2-((1s,3S)-3-(氨基甲基)环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 6: 5-((R)-1-(2-((1s,3S)-3-(Aminomethyl)cyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazolo [1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向含有24F-1(292mg)的反应瓶中加入氯化氢的1,4-二氧六环溶液(4N,20mL),加毕50℃搅拌反应。反应完全后,浓缩反应液,得到化合物24G-1(399mg)。MS(ESI)m/z:400.4[M+H]
+.
To a reaction flask containing 24F-1 (292 mg), a 1,4-dioxane solution (4N, 20 mL) of hydrogen chloride was added, and the reaction was stirred at 50 °C. After completion of the reaction, the reaction mixture was concentrated to give Compound 24G-1 (399 mg). MS (ESI) m/z: 400.4 [M+H] + .
步骤7:(1
1S,1
3S,6
3E,6
4E,4R)-3
4-氟-4-甲基-2-氧杂-5,8-二氮杂-6(5,3)-吡唑并[1,5-a]嘧啶杂-3(1,2)-苯杂-1(1,3)-环丁杂壬内酰胺-7-酮
Step 7: (1 1 S,1 3 S,6 3 E,6 4 E,4R)-3 4 -fluoro-4-methyl-2-oxa-5,8-diaza-6(5, 3)-pyrazolo[1,5-a]pyrimidin-3(1,2)-benzene-1(1,3)-cyclobutanol-7-one
室温搅拌下,向含有24G-1(399mg)、DCM(80mL)、DMF(15mL)和DIPEA(905mg)的反应瓶中,分3次加入五氟苯基二苯基膦酸酯(224mg)的DMF(5mL)溶液。加毕室温搅拌反应。反应完全后,浓缩反应液,向残留物中加入乙酸乙酯(200mL),依次用2M碳酸钠水溶液、水洗涤,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(MeOH:DCM=0:100~2.5:97.5)得到化合物I-24(125mg)。To a reaction flask containing 24G-1 (399mg), DCM (80mL), DMF (15mL), and DIPEA (905mg), pentafluorophenyldiphenylphosphonate (224mg) was added in three portions at room temperature with stirring. DMF (5 mL) solution. The reaction was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated. Filter and concentrate the filtrate. Purification by column chromatography (MeOH: EtOAc: EtOAc:EtOAc:
1H NMR(500MHz,DMSO-d
6)δ8.61(d,J=8.5Hz,1H),8.55(d,J=7.5Hz,1H),8.07(s,1H),7.26(d,J=5.0Hz,1H),7.13(dd,J=3.0,9.5Hz,1H),6.98-6.94(m,1H),6.73(dd,J=4.5,9.0Hz,1H),6.42(d,J=7.5Hz,1H),5.80-5.76(m,1H),4.91-4.88(m,1H),3.67-3.64(m,1H),3.07(d,J=14.0Hz,1H),2.72-2.70(m,1H),2.58-2.57(m,1H),2.34-2.35(m,1H),2.23-2.22(m,1H),1.71-1.70(m,1H),1.37(d,J=7.0Hz,3H).
13C NMR(125MHz,DMSO-d
6)δ163.03,158.37,156.32,148.96,146.19,145.35,138.14,136.47,116.70,114.22,112.41,102.19,101.41,68.34,43.43,41.33,32.62,29.20,24.09,23.05.MS(ESI)m/z:382.5[M+H]
+.
1 H NMR (500 MHz, DMSO-d 6 ) δ 8.61 (d, J = 8.5 Hz, 1H), 8.85 (d, J = 7.5 Hz, 1H), 8.07 (s, 1H), 7.26 (d, J = 5.0 Hz, 1H), 7.13 (dd, J=3.0, 9.5 Hz, 1H), 6.98-6.94 (m, 1H), 6.73 (dd, J=4.5, 9.0 Hz, 1H), 6.42 (d, J=7.5) Hz, 1H), 5.80-5.76 (m, 1H), 4.91-4.88 (m, 1H), 3.67-3.64 (m, 1H), 3.07 (d, J = 14.0 Hz, 1H), 2.72-2.70 (m, 1H), 2.58-2.57 (m, 1H), 2.34 - 2.35 (m, 1H), 2.23 - 2.22 (m, 1H), 1.71-1.70 (m, 1H), 1.37 (d, J = 7.0 Hz, 3H) 13 C NMR (125MHz, DMSO-d 6 ) δ163.03, 158.37, 156.32, 148.96, 146.19, 145.35, 138.14, 136.47, 116.70, 114.22, 112.41, 102.19, 101.41, 68.34, 43.43, 41.33, 32.62, 29.20, 24.09, 23.05. MS (ESI) m/z: 382.5 [M+H] + .
实施例25:(3
1S,3
3R,6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环戊基环辛内酰胺-5-酮(化合物I-25-1)
Example 25: (3 1 S,3 3 R,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6 (3 ,5)-pyrazolo[1,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one (Compound I-25 -1)
(3
1R,3
3S,6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环戊基环辛内酰胺-5-酮(化合物I-25-2)
(3 1 R,3 3 S,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3,5)- Pyrazolo[1,5-a]pyrimidin-1(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolac-5-one (Compound I-25-2)
步骤1:5-(((1R)-1-(2-((3-((叔丁基氧羰基)氨基)环戊基)氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 1: 5-(((1R)-1-(2-((3-((tert-Butyloxycarbonyl))amino)cyclopentyl)oxy)-5-fluoropyridin-3-yl)ethyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
氮气保护冰水浴冷却下,将DEAD(326mg)缓慢加入含有25A(405mg)、(1r,3r)(3-羟基环戊基)氨基甲酸叔丁酯(260mg)、三苯基磷(460mg)、四氢呋喃(2mL)的反应液中,加毕室温搅拌20分钟后,50℃搅拌反应。反应完全后,浓缩反应液,柱层析纯化(石油醚:乙酸乙酯=70:30),得到化合物25B(434mg)。MS(ESI)m/z=551.5[M+Na]
+.
Under a nitrogen-protected ice water bath, DEAD (326 mg) was slowly added to contain 25A (405 mg), (1r, 3r) (3-hydroxycyclopentyl)carbamic acid tert-butyl ester (260 mg), triphenylphosphine (460 mg), The reaction mixture of tetrahydrofuran (2 mL) was stirred at room temperature for 20 minutes, and then stirred at 50 °C. After completion of the reaction, the reaction mixture was concentrated and purified mjjjjjjjjj MS (ESI) m / z = 551.5 [M+Na] + .
步骤2:5-(((1R)-1-(2-((3-((叔丁基氧羰基)氨基)环戊基)氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 2: 5-(((1R)-1-(2-((3-((tert-butyloxycarbonyl))amino)cyclopentyl)oxy)-5-fluoropyridin-3-yl)ethyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入25B(420mg)、氢氧化锂一水合物(334mg)、MeOH(15mL)、四氢呋喃(6mL)及水(6mL),氮气保护下,70℃搅拌反应。冰水浴冷却下向反应液中加入稀盐酸(2N)调节PH至5-7,EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物25C(420mg)。MS(ESI)m/z:523.6[M+Na]
+.
To the reaction flask, 25B (420 mg), lithium hydroxide monohydrate (334 mg), MeOH (15 mL), tetrahydrofuran (6 mL) and water (6 mL) were sequentially added, and the mixture was stirred at 70 ° C under nitrogen atmosphere. To the reaction mixture, dilute hydrochloric acid (2N) was added to the mixture to adjust the pH to 5-7, EA was extracted, and the organic phase was combined, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 25C (420 mg). MS (ESI) m / z: 523.6 [M+Na] + .
步骤3:5-(((1R)-1-(2-((3-氨基环戊基)氧基)-5-氟吡啶-3-基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 3: 5-(((1R)-1-(2-((3-Aminocyclopentyl)oxy)-5-fluoropyridin-3-yl)ethyl)amino)pyrazolo[1,5 -a]pyrimidine-3-carboxylic acid trihydrochloride
冰水浴冷却下,将氯化氢的1,4二氧六环溶液(4M,1.88g)缓慢滴入25C(420mg)中,加毕,室温搅拌反应。反应完全后,浓缩反应液,残留物用无水乙腈打浆,过滤,滤饼干燥后得到化合物25D(202mg)。MS(ESI)m/z:423.3[M+Na]
+.
Under ice cooling, a 1,4 dioxane solution (4M, 1.88 g) of hydrogen chloride was slowly dropped into 25 C (420 mg), and the reaction was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated. mjjjjjjjj MS (ESI) m / z: 423.3 [M+Na] + .
步骤4:(6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环戊基环辛内酰胺-5-酮
Step 4: (6 3 E,6 4 E,8R)-1 5 -Fluoro-8-methyl-2-oxa-4,7-diaza-6(3,5)-pyrazolo[1 ,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one
氮气保护下,将五氟苯基磷酸酯(170mg)分批缓慢加入到含有DIPEA(560mg)、DMF(8mL)、DCM(80mL)和25D(202mg)的反应液中,室温搅拌反应,反应完全后,浓缩反应液,向残留物加入EA,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM:MeOH=96:4)得到化合物25E(140mg)。Under a nitrogen atmosphere, pentafluorophenyl phosphate (170 mg) was slowly added in portions to a reaction solution containing DIPEA (560 mg), DMF (8 mL), DCM (80 mL) and 25D (202 mg). After the reaction mixture was concentrated, EA was added and the mixture was evaporated and evaporated Filter and concentrate the filtrate. Column chromatography (DCM: MeOH = 96: 4) gave Compound 25E (140 mg).
步骤5:(3
1S,3
3R,6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环戊基环辛内酰胺-5-酮(I-25-1)
Step 5: (3 1 S,3 3 R,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3, 5)-pyrazolo[1,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one (I-25-1 )
(3
1R,3
3S,6
3E,6
4E,8R)-1
5-氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(2,3)-吡啶杂-3(1,3)-环戊基环辛内酰胺-5-酮(I-25-2)
(3 1 R,3 3 S,6 3 E,6 4 E,8R)-1 5 -fluoro-8-methyl-2-oxa-4,7-diaza-6(3,5)- Pyrazolo[1,5-a]pyrimidine-l-(2,3)-pyridin-3(1,3)-cyclopentylcyclooctanolactam-5-one (I-25-2)
将化合物25E,采用高压SFC分离纯化(CHIRAL ART Cellulose-SC(30×250mm,5μm,YMC)柱,洗脱剂无水乙醇/正己烷(20/80)等度洗脱,流速35mL/min)得到化合物I-25-1(30mg)及I-25-2(31mg)。Compound 25E was separated and purified by high pressure SFC (CHIRAL ART Cellulose-SC (30×250 mm, 5 μm, YMC) column, eluent anhydrous ethanol/n-hexane (20/80) was eluted isocratically, flow rate 35 mL/min) Compound I-25-1 (30 mg) and I-25-2 (31 mg) were obtained.
I-25-1:
1H NMR(500MHz,MeOD):δ8.96(d,J=10.5Hz,1H),8.33(d,J=7.5Hz,1H),8.11(s,1H),7.88(s,1H),7.52(dd,J=3.0Hz,8.5Hz,1H),6.39(d,J=7.5Hz 1H),5.51-5.45(m,2H),4.88-4.78(m,1H),2.46-2.44(m,1H),2.28-2.24(m,2H),2.11-1.99(m,3H),1.50(d,J=7.0Hz,3H).
13C NMR(125MHz,CDCl
3):162.22,156.86,155.53,154.91,146.52,143.84,135.37,131.20,129.90,122.77,100.76,79.26,47.77,43.56,39.59,32.79,31.13,20.64.MS(ESI)m/z:383.5[M+H]
+.
I-25-1: 1 H NMR (500MHz, MeOD): δ 8.96 (d, J = 10.5 Hz, 1H), 8.33 (d, J = 7.5 Hz, 1H), 8.11 (s, 1H), 7.88 ( s, 1H), 7.52 (dd, J = 3.0 Hz, 8.5 Hz, 1H), 6.39 (d, J = 7.5 Hz 1H), 5.51-5.45 (m, 2H), 4.88-4.78 (m, 1H), 2.46 -2.44 (m, 1H), 2.28-2.24 (m, 2H), 2.11-1.99 (m, 3H), 1.50 (d, J = 7.0 Hz, 3H). 13 C NMR (125 MHz, CDCl 3 ): 162.22, 156.86, 155.53, 154.91, 146.52, 143.84, 135.37, 131.20, 129.90, 122.77, 100.76, 79.26, 47.77, 43.56, 39.59, 32.79, 31.13, 20.64. MS (ESI) m/z: 383.5 [M+H] + .
I-25-2:
1H NMR(500MHz,MeOD):δ8.98(d,J=7.5Hz,1H),8.32(d,J=8.0Hz,1H),8.162(s,1H),7.91(s,1H),7.61-7.58(dd,J=3.0Hz,7.5Hz,1H),6.32(d,J=8.0Hz 1H),5.85-5.84(q,J=6.5Hz,1H),5.32-5.28(m,1H),4.57-4.55(m,1H),2.54-2.51(m,1H),2.32-2.32(m,1H),2.24-2.23(m,1H),2.21-2.21(m,1H),1.97-1.77(m,2H),1.76-1.50(m,3H).MS(ESI)m/z:383.5[M+H]
+.
I-25-2: 1 H NMR (500MHz, MeOD): δ 8.98 (d, J = 7.5 Hz, 1H), 8.32 (d, J = 8.0 Hz, 1H), 8.162 (s, 1H), 7.91 ( s,1H), 7.61-7.58 (dd, J=3.0 Hz, 7.5 Hz, 1H), 6.32 (d, J=8.0 Hz 1H), 5.85-5.84 (q, J=6.5 Hz, 1H), 5.32-5.28 (m, 1H), 4.57-4.55 (m, 1H), 2.54-2.51 (m, 1H), 2.32-2.32 (m, 1H), 2.24-2.23 (m, 1H), 2.21-2.21 (m, 1H) , 1.97-1.77 (m, 2H), 1.76-1.50 (m, 3H). MS (ESI) m/z: 383.5 [M+H] + .
实施例26:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-3
3,8-二甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-α]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁烷环辛内酰胺-5-酮
Example 26: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro- 3 3 ,8-dimethyl-2-oxa-4,7-diaza -6(3,5)-pyrazolo[1,5-α]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutanecyclooctanolac-5-one
步骤1:((1r,3r)-3-羟基-1-甲基环丁基)氨基甲酸叔丁酯Step 1: ((1r,3r)-3-Hydroxy-1-methylcyclobutyl)carbamic acid tert-butyl ester
冰水浴冷却下,将二碳酸二叔丁酯(349mg)滴加入26A(220mg)和TEA(162mg)的DCM(10mL)反应液中。室温搅拌反应。反应完全后,向反应液中加入饱和碳酸氢钠水溶液(20mL),分液,有机相用饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得化合物26B(457mg),未经纯化直接用于下一步反应。Di-tert-butyl dicarbonate (349 mg) was added dropwise to a reaction mixture of 26A (220 mg) and TEA (162 mg) in DCM (10 mL). The reaction was stirred at room temperature. After the reaction was completed, a saturated aqueous solution of sodium hydrogencarbonate (20 mL) was evaporated. Filtration and concentration of the filtrate gave Compound 26B (457 mg).
1H NMR(500MHz,CDCl
3):δ4.57(br,1H),4.46-4.42(m,1H),2.64(br,2H),1.94-1.90(m,2H),1.46-1.44(m,12H).
1 H NMR (500MHz, CDCl 3 ): δ4.57 (br, 1H), 4.46-4.42 (m, 1H), 2.64 (br, 2H), 1.94-1.90 (m, 2H), 1.46-1.44 (m, 12H).
步骤2:((1S,3s)-3-(2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4-氟苯氧基)-1-甲基环丁基)氨基甲酸叔丁酯Step 2: ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4-fluorophenoxy)-1 -methylcyclobutyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入26B(400mg)、16A(310mg)、三苯基膦(607mg)和四氢呋喃(2mL)。冰水浴冷却下,将DEAD(430mg)的四氢呋喃(2mL)溶液滴加到反应液中,反应完全后,浓缩反应液,柱层析纯化(乙酸乙酯:石油醚=0:100~50:50),得到化合物26C(225mg)。MS(ESI)e/z:465.6[M+Na]
+.
To the reaction flask, 26B (400 mg), 16A (310 mg), triphenylphosphine (607 mg) and tetrahydrofuran (2 mL) were sequentially added. Under ice cooling, a solution of DEAD (430 mg) in tetrahydrofuran (2 mL) was added dropwise to the reaction mixture. After the reaction was completed, the reaction mixture was concentrated and purified by column chromatography (ethyl acetate: petroleum ether = 0:100 to 50:50) ), Compound 26C (225 mg) was obtained. MS (ESI) e / z: 465.6 [M+Na] + .
步骤3:((1S,3s)-3-(2-((R)-1-氨基乙基)-4-氟苯氧基)-1-甲基环丁基)氨基甲酸叔丁酯Step 3: ((1S,3s)-3-(2-((R)-1-Aminoethyl)-4-fluorophenoxy)-1-methylcyclobutyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入26C(210mg)、四氢呋喃(8mL)、水(1mL)和碘(30mg),加毕室温搅拌反应。反应完全后,浓缩反应液得到化合物26D(241mg),未经纯化直接用于下一步反应。MS(ESI)e/z:361.5[M+Na]
+.
To the reaction flask, 26 C (210 mg), tetrahydrofuran (8 mL), water (1 mL) and iodine (30 mg) were sequentially added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated to give Compound 26D (241 mg). MS (ESI) e / z: 361.5 [M+Na] + .
步骤4:5-(((R)-1-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)-3-甲基环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 4: 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)-3-methylcyclobutoxy)-5-fluorobenzene) Ethyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中,依次加入26D(200mg)、5-氯吡唑并[1,5-a]嘧啶-3-甲酸乙酯(160mg)、DIPEA(92mg)和正丁醇(8mL),加毕氮气保护下,120℃搅拌反应。反应完全后,浓缩反应液,残留物溶于乙酸乙酯(30mL)中,依次用水、饱和食盐水洗涤。无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(乙酸乙酯:石油醚=0:100~50:50),得到化合物26E(62mg)。MS(ESI)e/z:528.7[M+H]
+.
To the reaction flask, 26D (200 mg), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester (160 mg), DIPEA (92 mg) and n-butanol (8 mL) were added, and nitrogen was added. The reaction was stirred at 120 ° C under protection. After the reaction was completed, the reaction mixture was evaporated. Dry over anhydrous sodium sulfate. Filter and concentrate the filtrate. Purification by column chromatography (ethyl acetate: petroleum ether = 0: 100 to 50: 50) gave Compound 26E (62mg). MS (ESI) e / z: 528.7 [M+H] + .
步骤5:5-(((R)-1-(2-((1s,3S)-3-((叔丁氧基羰基)氨基)-3-甲基环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 5: 5-(((R)-1-(2-((1),3S)-3-((tert-Butoxycarbonyl)amino)-3-methylcyclobutoxy)-5-fluorobenzene) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入26E(60mg)、氢氧化锂一水合物(19mg)、MeOH(10mL)、四氢呋喃(4mL)和水(1mL),70℃搅拌反应。反应完全后,冰水浴冷却下,缓慢加入稀盐酸(0.5M)调节pH至小于5,用DCM萃取,合并有机相,无水硫酸钠干燥。过滤,滤液浓缩得到化合物26F(36mg)。MS(ESI)m/z:498.4[M-H]
-.
Into a reaction flask, 26E (60 mg), lithium hydroxide monohydrate (19 mg), MeOH (10 mL), tetrahydrofuran (4 mL) and water (1 mL) were sequentially added, and the mixture was stirred at 70 °C. After the reaction was completed, the mixture was cooled with ice water, and then diluted with hydrochloric acid (0.5M) to adjust to pH to less than 5, and extracted with DCM. Filtration and concentration of the filtrate gave compound 26F (36 mg). MS (ESI) m/z: 498.4 [MH] - .
步骤6:5-(((R)-1-(2-((1s,3S)-3-氨基-3-甲基环丁氧基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]吡啶-3-羧酸三盐酸盐Step 6: 5-((R)-1-(2-((1s,3S)-3-Amino-3-methylcyclobutoxy)-5-fluorophenyl)ethyl)amino)pyrazole And [1,5-a]pyridine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入26F(36mg)和氯化氢的1,4-二氧六环溶液(4N,5mL),加毕40℃搅拌反应。反应完全后,浓缩反应液得到化合物26G(37mg),未经纯化直接用于下一步反应。MS(ESI)e/z:398.1[M-H]
-.
To the reaction flask, a solution of 26F (36 mg) and hydrogen chloride in 1,4-dioxane (4N, 5 mL) was sequentially added, and the mixture was stirred at 40 ° C. After the reaction was completed, the reaction mixture was concentrated to give Compound 26G (37 mg). MS (ESI) e/z: 398.1 [MH] - .
步骤7:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-3
3,8-二甲基-2-氧杂-4,7-二氮杂–6(3,5)-吡唑并[1,5-α]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁烷环辛内酰胺-5-酮
Step 7: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro- 3 3 ,8-dimethyl-2-oxa-4,7-diaza – 6(3,5)-pyrazolo[1,5-α]pyrimidine-1(1,2)-benzene-3(1,3)-cyclobutanecyclooctanolac-5-one
将五氟苯基磷酸酯(30mg)加入到含有26G(37mg)、DIPEA(57mg)、DCM(40mL)、DMF(8mL)的溶液中。室温搅拌反应,反应完全后,浓缩反应液,向浓缩物中加入乙酸乙酯(50mL),有机相依次用2M碳酸钠水溶液、水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(MeOH:DCM=0:100~4:96)得化合物I-26(22mg)。Pentafluorophenyl phosphate (30 mg) was added to a solution containing 26G (37 mg), DIPEA (57 mg), DCM (40 mL), DMF (8 mL). The reaction was stirred at room temperature. After the reaction was completed, the mixture was evaporated. ethyl acetate (50 mL) was evaporated. Filter and concentrate the filtrate. Purification by column chromatography (MeOH: EtOAc:EtOAc:EtOAc
1H NMR(500MHz,DMSO-d
6):δ8.61(d,J=7.0Hz,1H),8.52(d,J=8.0Hz,1H),8.17(s,1H),7.96(s,1H),7.13(dd,J=2.5,9.0Hz,1H),6.96-6.94(m,1H),6.52(dd,J=4.5,9.0Hz,1H),6.34(d,J=7.5Hz,1H),5.78-5.73(m,1H),4.82-4.80(m,1H),2.69(dd,J=6.0,13.5Hz,1H),2.60(dd,J=6.0,13.5Hz,1H),2.42(dd,J=6.0,13.5Hz,1H),2.00(dd,J=6.0,13.5Hz,1H),1.83(s,3H),1.42(d,J=7.0Hz,3H).
13C NMR(125MHz,DMSO-d
6):163.09,157.92,156.04,149.36,146.08,144.71,136.37,114.23,113.01,103.41,100.93,69.65,55.80,44.77,43.09,42.56,27.69,22.65。MS(ESI)e/z:382.5[M+H]
+.
1 H NMR (500MHz, DMSO- d 6): δ8.61 (d, J = 7.0Hz, 1H), 8.52 (d, J = 8.0Hz, 1H), 8.17 (s, 1H), 7.96 (s, 1H ), 7.13 (dd, J = 2.5, 9.0 Hz, 1H), 6.96-6.94 (m, 1H), 6.52 (dd, J = 4.5, 9.0 Hz, 1H), 6.34 (d, J = 7.5 Hz, 1H) , 5.78-5.73 (m, 1H), 4.82-4.80 (m, 1H), 2.69 (dd, J = 6.0, 13.5 Hz, 1H), 2.60 (dd, J = 6.0, 13.5 Hz, 1H), 2.42 (dd , J = 6.0, 13.5 Hz, 1H), 2.00 (dd, J = 6.0, 13.5 Hz, 1H), 1.83 (s, 3H), 1.42 (d, J = 7.0 Hz, 3H). 13 C NMR (125 MHz, DMSO-d 6 ): 163.09, 157.92, 156.04, 149.36, 146.08, 144.71, 136.37, 114.23, 113.01, 103.41, 100.93, 69.65, 55.80, 44.77, 43.09, 42.56, 27.69, 22.65. MS (ESI) e / z: 382.5 [M+H] + .
实施例27:(3
1S,3
3S,6
3E,6
4E,8R)-1
4,1
6-二氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁基环辛内酰胺-5-酮
Example 27: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 ,1 6 -difluoro-8-methyl-2-oxa-4,7-diaza -6(3,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctanolac-5-one
步骤1:((1s,3s)-3-(2-乙酰基-4,6-二氟苯氧基)环丁基)氨基甲酸叔丁酯Step 1: ((1s,3s)-3-(2-acetyl-4,6-difluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入27A(2.56g)、((1r,3r)-3-羟基环丁基)氨基甲酸叔丁酯(3.34g)、三苯基膦(5.84g)和四氢呋喃(20mL),氮气保护冰水浴冷却下,将DEAD(4.15g)缓慢滴加至上述反应液中,加毕室温搅拌10分钟后,50℃搅拌反应。反应完全后,向反应液加入石油醚。过滤,滤液浓缩。柱层析纯化(石油醚:乙酸乙酯=85:15),得到化合物27B(3.23g)。MS(ESI)m/z:364.5[M+Na]
+.
To the reaction flask, 27A (2.56 g), ((1r, 3r)-3-hydroxycyclobutyl)carbamic acid tert-butyl ester (3.34 g), triphenylphosphine (5.84 g) and tetrahydrofuran (20 mL) were sequentially added. Under a nitrogen-protected ice water bath, DEAD (4.15 g) was slowly added dropwise to the above reaction solution, stirred at room temperature for 10 minutes, and then stirred at 50 ° C. After the reaction was completed, petroleum ether was added to the reaction liquid. Filter and concentrate the filtrate. Purification by column chromatography (petrole ether: ethyl acetate = 85: 15) gave Compound 27B (3.23 g). MS (ESI) m/z: 364.5 [M+Na] + .
步骤2:((1S,3s)-3-(2-((E)-1-(((R)-叔丁基亚磺酰基)亚胺)乙基)-4,6-二氟苯氧基)环丁基)氨基甲酸叔丁酯Step 2: ((1S,3s)-3-(2-((E)-1-(((R)-tert-butylsulfinyl)imide)ethyl)-4,6-difluorophenoxy Tert-butyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入27B(3.2g)、(R)-2-甲基丙基-2-磺酰亚胺(1.24g)、1-甲氧基-2-(2-甲氧基乙氧基)乙烷(1.258g)、四氢呋喃(10mL)和2-甲基四氢呋喃(10mL),加毕室温搅拌溶清后。冰水浴冷却下向反应液缓慢滴加钛酸四乙酯(24.26g),加毕65℃搅拌反应。反应完全后,将反应液直接倒入冰水中,过滤,滤液使用EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚:乙酸乙酯=90:10)得到化合物27C(2.852g)。MS(ESI)m/z:467.5[M+Na]
+.
To the reaction flask, 27B (3.2g), (R)-2-methylpropyl-2-sulfonimide (1.24g), 1-methoxy-2-(2-methoxyB) were added in order. Oxy)ethane (1.258 g), tetrahydrofuran (10 mL) and 2-methyltetrahydrofuran (10 mL) were added and stirred at room temperature. To the reaction liquid, tetraethyl titanate (24.26 g) was slowly added dropwise under ice cooling, and the reaction was stirred at 65 ° C. After the completion of the reaction, the reaction mixture was poured into ice water, filtered, and the filtrate was extracted with EtOAc. Filter and concentrate the filtrate. Column chromatography purification (petroleum ether: ethyl acetate = 90: 10) gave Compound 27C (2.852 g). MS (ESI) m / z: 467.5 [M+Na] + .
步骤3:((1S,3s)-3-(2-((R)-1-(((R)-叔丁基亚磺酰基)氨基)乙基)-4,6-二氟苯氧基)环丁基)氨基甲酸叔丁酯Step 3: ((1S,3s)-3-(2-((R)-1-((R)-tert-butylsulfinyl)amino)ethyl)-4,6-difluorophenoxy Cyclobutyl)carbamic acid tert-butyl ester
-78℃,氮气保护下,硼氢化钠(0.72g)缓慢加入含有27C(2.8g)的四氢呋喃(100mL)搅拌液中,搅拌反应,然后将反应液缓慢升温至-30℃。反应完全后,缓慢向反应液加入MeOH和饱和食盐水淬灭反应,使用EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚:乙酸乙酯=75:25),得到化合物27D(1.24g)。MS(ESI)m/z=469.5[M+Na]
+.
Under a nitrogen atmosphere, sodium borohydride (0.72 g) was slowly added to a stirred solution of 27 C (2.8 g) in tetrahydrofuran (100 mL), and the reaction was stirred, and then the mixture was slowly warmed to -30 °C. After the completion of the reaction, the reaction mixture was stirred and evaporated to drynessnessness Filter and concentrate the filtrate. Purification by column chromatography (petrole ether: ethyl acetate = 75:25) gave Compound 27D (1.24 g). MS (ESI) m/z = 469.5 [M+Na] + .
步骤4:((1S,3s)-3-(2-((R)-1-氨基乙基)-4,6-二氟苯氧基)环丁基)氨基甲酸叔丁酯Step 4: ((1S,3s)-3-(2-((R)-1-Aminoethyl)-4,6-difluorophenoxy)cyclobutyl)carbamic acid tert-butyl ester
向反应瓶中,依次加入27D(1.22g)、碘(0.139g)、四氢呋喃(25mL)和水(2mL),氮气保护下,70℃搅拌反应。反应完全后,浓缩反应液得到化合物27E(1.14g)。MS(ESI)m/z:365.5[M+Na]
+.
To the reaction flask, 27D (1.22 g), iodine (0.139 g), tetrahydrofuran (25 mL) and water (2 mL) were sequentially added, and the reaction was stirred at 70 ° C under nitrogen atmosphere. After the reaction was completed, the reaction mixture was concentrated to give Compound 27E (1.14 g). MS (ESI) m/z: 365.5 [M+Na] + .
步骤5:5-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧基)-3,5-二氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 5: 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-3,5-difluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向反应瓶中,依次加入27E(1.14g)、5-氯吡唑并[1,5-a]嘧啶-3-甲酸乙酯(689mg)、DIPEA(3.6g)及乙醇(15mL),加毕氮气保护下,80℃搅拌反应。反应完全后,浓缩反应液,向残留物溶于EA后,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=65/35)得到化合物27F(812mg)。To the reaction flask, 27E (1.14g), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (689mg), DIPEA (3.6g) and ethanol (15mL) were added in order. The reaction was stirred at 80 ° C under a nitrogen atmosphere. After the completion of the reaction, the reaction mixture was concentrated. Filter and concentrate the filtrate. Purification by column chromatography (petrole ether / ethyl acetate = 65/35) gave Compound 27F (812mg).
1H NMR(500MHz,DMSO-d
6):δ8.58(d,J=7.5Hz,1H),8.30(dd,J=7.5Hz,1H),7.65(t,J=8.0Hz,1H),7.07(d,J=7.5Hz,1H),7.01(d,J=7.5Hz,1H),6.49(d,J=7.5Hz,1H),5.55(br,1H),4.51(br,1H),4.50-4.49(m, 2H),3.72-3.61(m,1H),2.69(d,J=4.0Hz,1H),2.63(t,J=4.0Hz,1H),2.21-2.16(m,1H),1.43(d,J=6.5Hz,3H),1.37(s,9H),1.471.22(m,5H).MS(ESI)m/z:554.6[M+Na]
+.
1 H NMR (500MHz, DMSO- d 6): δ8.58 (d, J = 7.5Hz, 1H), 8.30 (dd, J = 7.5Hz, 1H), 7.65 (t, J = 8.0Hz, 1H), 7.07 (d, J = 7.5 Hz, 1H), 7.01 (d, J = 7.5 Hz, 1H), 6.49 (d, J = 7.5 Hz, 1H), 5.55 (br, 1H), 4.51 (br, 1H), 4.50-4.49 (m, 2H), 3.72-3.61 (m, 1H), 2.69 (d, J = 4.0 Hz, 1H), 2.63 (t, J = 4.0 Hz, 1H), 2.21-2.16 (m, 1H) , 1.43 (d, J = 6.5 Hz, 3H), 1.37 (s, 9H), 1.471.22 (m, 5H). MS (ESI) m/z: 554.6 [M+Na] + .
步骤6:5-(((R)-1-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧基)-3,5-二氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 6: 5-(((R)-1-(2-((1),3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-3,5-difluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入27F(800mg)、氢氧化锂一水合物(632mg)、四氢呋喃(15mL)和水(2.000mL),加毕氮气保护下,70℃搅拌反应。反应完全后,冰水浴冷却下向反应液加入浓盐酸调节pH至5-7,EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物27G(750mg)。MS(ESI)m/z:526.5[M+Na]
+.
To the reaction flask, 27F (800 mg), lithium hydroxide monohydrate (632 mg), tetrahydrofuran (15 mL) and water (2.000 mL) were successively added, and the reaction was stirred at 70 ° C under nitrogen atmosphere. After the reaction was completed, concentrated hydrochloric acid was added to the reaction mixture under ice-cooled water to adjust the pH to 5-7, EA was extracted, and the organic phases were combined, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave Compound 27G (750 mg). MS (ESI) m / z: 526.5 [M+Na] + .
步骤7:5-(((R)-1-(2-((1s,3S)-3-氨基环丁氧基)-3,5-二氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 7: 5-((R)-1-(2-((1s,3S)-3-Aminocyclobutoxy)-3,5-difluorophenyl)ethyl)amino)pyrazolo[ 1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入27G(750mg)、氯化氢的1,4-二氧六环溶液(4M,2.19g),加毕室温搅拌反应。反应完全后,浓缩反应液得到化合物27H(1.54g)。未经进一步分离纯化直接下一步反应。To the reaction flask, a solution of 27 G (750 mg) of hydrogen chloride in 1,4-dioxane (4M, 2.19 g) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated to give Compound 27H (l. The next reaction was carried out without further separation and purification.
步骤8:(3
1S,3
3S,6
3E,6
4E,8R)-1
4,1
6-二氟-8-甲基-2-氧杂-4,7-二氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁基环辛内酰胺-5-酮
Step 8: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 ,1 6 -difluoro-8-methyl-2-oxa-4,7-diaza- 6(3,5)-pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutylcyclooctanolac-5-one
向反应瓶中,依次加入27H(1.5g)、DIPEA(1.16g)、DCM(80mL)及DMF(20mL),室温搅拌20分钟后。将五氟苯基二苯基磷酸酯(576mg)分批加至上述反应液中,室温搅拌反应。反应完全后,浓缩反应液,向残留物中加EA,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM:MeOH=94:6)得化合物I-27(460mg)。Into a reaction flask, 27H (1.5 g), DIPEA (1.16 g), DCM (80 mL), and DMF (20 mL) were added, and the mixture was stirred at room temperature for 20 minutes. Pentafluorophenyl diphenyl phosphate (576 mg) was added portionwise to the above reaction solution, and the reaction was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated, EtOAc was evaporated. Filter and concentrate the filtrate. Purification by column chromatography (DCM:MeOH = EtOAc)
1H NMR(500MHz,DMSO-d
6):δ9.33(d,J=10.5Hz,1H),8.85(d,J=7.5Hz,1H),8.61(d,J=7.5Hz,1H),8.06(s,1H),7.12-7.03(m,2H),6.43(d,J=7.5Hz,1H),5.73(t,J=7.5Hz,1H),5.22(t,J=2.5Hz 1H),4.62(d,J=7.5Hz,1H),3.10(m,1H),2.93-2.90(m,1H),2.12-2.08(m,1H),1.78-1.62(m,1H),1.41(d,J=7.5Hz,3H).
13C NMR(125MHz,DMSO-d
6):δ160.94,157.24,155.34,151.73,149.78,146.47,144.01,140.00,136.86,109.25,104.34,101.58,77.71,55.37,44.27,42.00,37.77,23.08.MS(ESI)m/z:386.4[M+H]
+.
1 H NMR (500MHz, DMSO- d 6): δ9.33 (d, J = 10.5Hz, 1H), 8.85 (d, J = 7.5Hz, 1H), 8.61 (d, J = 7.5Hz, 1H), 8.06(s,1H),7.12-7.03(m,2H),6.43(d,J=7.5Hz,1H), 5.73(t,J=7.5Hz,1H),5.22(t,J=2.5Hz 1H) , 4.62 (d, J = 7.5 Hz, 1H), 3.10 (m, 1H), 2.93-2.90 (m, 1H), 2.12-2.08 (m, 1H), 1.78-1.62 (m, 1H), 1.41 (d) , J = 7.5 Hz, 3H). 13 C NMR (125MHz, DMSO-d 6 ): δ160.94, 157.24, 155.34, 151.73, 149.78, 146.47, 144.01, 140.00, 136.86, 109.25, 104.34, 101.58, 77.71, 55.37, 44.27 , 42.00, 37.77, 23.08. MS (ESI) m/z: 386.4 [M+H] + .
实施例28:(1
3E,1
4E,2
2R,2
4S,5
1S,5
3R)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-2(1,2)-吡咯烷基-3(1,2)-苯杂-5(1,3)-环丁基环辛内酰胺-5-酮
Example 28: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 R)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidinium-2(1,2)-pyrrolidinyl-3(1,2)-benzene-5(1,3)-cyclobutylcyclooctine Lactam-5-one
步骤1:5-((2R,4S)-2-(2-(((1S,3R)-3-((叔丁氧基羰基)氨基)环戊基)氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 1: 5-((2R,4S)-2-(2-((1S,3R)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluorophenyl) Ethyl 4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
向反应瓶中,依次加入28A(776mg)、((1R,3R)-3-羟基环戊基)氨基甲酸叔丁酯(480mg)、三苯基磷(786mg)及四氢呋喃(1mL),氮气保护及冰水浴冷却下将DEAD(560mg)缓慢滴加至反应液中,室温搅拌20分钟后,50℃搅拌反应。反应完全后,柱层析纯化(石油醚/乙酸乙酯=60/40)得到化合物28B(1.04g)。MS(ESI)m/z:594.5[M+Na]
+.
To the reaction flask, 28A (776 mg), ((1R,3R)-3-hydroxycyclopentyl)carbamic acid tert-butyl ester (480 mg), triphenylphosphine (786 mg) and tetrahydrofuran (1 mL) were added in this order, and nitrogen-protected DEAD (560 mg) was slowly added dropwise to the reaction mixture under ice cooling, and the mixture was stirred at room temperature for 20 minutes, and then stirred at 50 ° C. After completion of the reaction, purification by column chromatography ( petroleum ether / ethyl acetate = 60/40) gave Compound 28B (1.04 g). MS (ESI) m / z: 594.5 [M+Na] + .
步骤2:5-((2R,4S)-2-(2-(((1S,3R)-3-((叔丁基氧羰基)氨基)环戊基)氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡咯并[1,5-a]嘧啶-3-羧酸Step 2: 5-((2R,4S)-2-(2-((1S,3R)-3-((tert-Butyloxycarbonyl)amino)cyclopentyl)oxy)-5-fluorophenyl) )-4-fluoropyrrolidin-1-yl)pyrrolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入28B(998mg)、氢氧化锂一水合物(735mg)、MeOH(30mL)、四氢呋喃(5mL)和水(5mL),加毕氮气保护下60℃搅拌反应。反应完全后,冰水浴冷却下向反应液加入稀盐酸(0.5M)调节pH至5-7,EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩,得到化合物28C(872mg)。MS(ESI)m/z:566.5[M+Na]
+.
To the reaction flask, 28B (998 mg), lithium hydroxide monohydrate (735 mg), MeOH (30 mL), tetrahydrofuran (5 mL) and water (5 mL) were sequentially added, and the mixture was stirred at 60 ° C under nitrogen atmosphere. After the completion of the reaction, the reaction mixture was diluted with ice water (0.5M) to adjust to pH 5-7, EA was extracted, the organic phase was combined, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave Compound 28C (872 mg). MS (ESI) m / z: 566.5 [M+Na] + .
步骤3:5-((2R,4S)-2-(2-(((1S,3R)-3-氨基环戊基)氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 3: 5-((2R,4S)-2-(2-((1S,3R)-3-Aminocyclopentyl)oxy)-5-fluorophenyl)-4-fluoropyrrolidine-1 -yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中,依次加入28C(845mg)和氯化氢的1,4-二氧六环溶液(2.92g),加毕氮气保护下,加毕室温搅拌反应。反应完全后,停止搅拌。浓缩反应液得到化合物28D(764mg)。MS(ESI)m/z:466.2[M+Na]
+.
To the reaction flask, 28 C (845 mg) and a hydrogen chloride solution of 1,4-dioxane (2.92 g) were added in that order, and the reaction was stirred at room temperature under a nitrogen atmosphere. After the reaction was completed, the stirring was stopped. The reaction mixture was concentrated to give Compound 28D ( 264 g). MS (ESI) m/z: 466.2 [M+Na] + .
步骤4:(1
3E,1
4E,2
2R,2
4S,5
1S,5
3R)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-2(1,2)-吡咯烷基-3(1,2)-苯杂-5(1,3)-环丁基环辛内酰胺-5-酮
Step 4: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 R) - 2 4 , 3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidinium-2(1,2)-pyrrolidinyl-3(1,2)-benzene-5(1,3)-cyclobutylcyclooctene Amide-5-one
向反应瓶中,依次加入28D(380mg)、DIPEA(750mg)、DCM(10mL)及DMF(40.00mL),室温搅拌10分钟,将五氟苯基二苯基磷酸酯(240mg)分批加入到上述反应液中,室温搅拌反应。反应完全后,浓缩反应液,向残留物溶于EA后,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM:MeOH=97:3)得化合物I-28(149mg)。To the reaction flask, 28D (380 mg), DIPEA (750 mg), DCM (10 mL) and DMF (40.00 mL) were sequentially added, and stirred at room temperature for 10 minutes, and pentafluorophenyl diphenyl phosphate (240 mg) was added in portions. In the above reaction liquid, the reaction was stirred at room temperature. After the completion of the reaction, the reaction mixture was concentrated. Filter and concentrate the filtrate. Purification by column chromatography (DCM:MeOH =EtOAc:
1H NMR(500MHz,DMSO-d
6):δ8.78(d,J=8.0Hz,1H),8.43(d,J=10.5Hz,1H),8.09(s,1H),7.15(d,J=10.5Hz,1H),6.99(d,J=6.0Hz,2H),6.67(t,J=8.0Hz,1H),5.79(t,J=7.5Hz,1H),5.61-5.51(m,1H),4.99(s,1H),4.81(d,J=7.5Hz,1H),4.44-4.34(m,1H),4.11(dd,J=13.0Hz,21Hz,1H),2.76(m,1H),2.32-2.29(m,1H),2.19-2.17(m,J=4.5H,1H),2.07-1.97(m,4H),1.79-1.76(m,J=6.0Hz,1H).
13C NMR(125MHz,DMSO-d
6):δ160.22,157.96,156.09,154.74,150.56,145.61,137.26,134.23,114.64,101.54,99.99,93.97,92.57,79.63,56.43,55.37,53.07,46.17,42.26,38.50,32.24,32.22.MS(ESI)m/z:426.4[M+H]
+.
1 H NMR (500MHz, DMSO- d 6): δ8.78 (d, J = 8.0Hz, 1H), 8.43 (d, J = 10.5Hz, 1H), 8.09 (s, 1H), 7.15 (d, J =10.5 Hz, 1H), 6.99 (d, J = 6.0 Hz, 2H), 6.67 (t, J = 8.0 Hz, 1H), 5.79 (t, J = 7.5 Hz, 1H), 5.61-5.51 (m, 1H) ), 4.99 (s, 1H), 4.81 (d, J = 7.5 Hz, 1H), 4.44 - 4.34 (m, 1H), 4.11 (dd, J = 13.0 Hz, 21 Hz, 1H), 2.76 (m, 1H) , 2.32-2.29 (m, 1H), 2.19-2.17 (m, J = 4.5H, 1H), 2.07-1.97 (m, 4H), 1.79-1.76 (m, J = 6.0 Hz, 1H). 13 C NMR (125MHz, DMSO-d 6 ): δ160.22,157.96,156.09,154.74,150.56,145.61,137.26,134.23,114.64,101.54,99.99,93.97,92.57,79.63,56.43,55.37,53.07,46.17,42.26,38.50,32.24 , 32.22. MS (ESI) m/z: 426.4 [M+H] + .
实施例29:(2
2R,2
4S,5
1S,5
3S,E)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(6,3)-咪唑并[1,2-b]哒嗪-2(1,2)-吡咯烷基-3(1,2)-苯杂-5(1,3)-环丁基环辛内酰胺-7-酮
Example 29: (2 2 R, 2 4 S, 5 1 S, 5 3 S, E)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1(6,3)- Imidazo[1,2-b]pyridazine-2(1,2)-pyrrolidinyl-3(1,2)-benzene-5(1,3)-cyclobutylcyclooctanolactam-7-one
步骤1:4-氟-2-((2R,4S)-4-氟吡咯烷-2-基)苯酚氢溴酸盐Step 1: 4-Fluoro-2-((2R,4S)-4-fluoropyrrolidin-2-yl)phenol hydrobromide
在反应瓶中,依次加入29A(5.34g)、四丁基溴化铵(0.352g)及溴化氢水溶液(71.7g),120℃搅拌反应。反应完全后,浓缩反应液得到化合物29B(5.44g)。未纯化直接投料下一步。MS(ESI)m/z:200.14[M+H]
+.
In a reaction flask, 29A (5.34 g), tetrabutylammonium bromide (0.352 g) and an aqueous hydrogen bromide solution (71.7 g) were successively added, and the mixture was stirred at 120 °C. After the reaction was completed, the reaction mixture was concentrated to give Compound 29B (5.44 g). The next step was directly fed without purification. MS (ESI) m/z:21.21.[M+H] + .
步骤2:2,2,2-三氟-1-((2R,4S)-4-氟-2-(5-氟-2-羟基苯基)吡咯烷-1-基)乙-1-酮Step 2: 2,2,2-Trifluoro-1-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxyphenyl)pyrrolidin-1-yl)ethan-1-one
反应瓶中,氮气保护及冰水浴冷却下,缓慢将三氟乙酸酐(474mg)滴加到29B(300mg)、TEA(457mg)和DCM(10mL)混合物中,加毕室温搅拌反应。反应完全后,浓缩反应液,柱层析纯化(石油醚/乙酸乙酯=80/20)得到化合物29C(142mg)。In a reaction flask, under a nitrogen atmosphere and ice-water bath cooling, trifluoroacetic acid anhydride (474 mg) was slowly added dropwise to a mixture of 29B (300 mg), TEA (457 mg) and DCM (10 mL), and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated to mjjjjjjjj
1H NMR(500MHz,DMSO-d
6):δ9.71(s,1H),6.96-6.89(m,2H),6.79(dd,J=3.0Hz,6.5Hz,1H),5.53-5.42(m,1H),5.24(t,J=8.5Hz,1H),4.18-4.03(m,2H),2.65(m,1H),2.18-2.05(m,1H).MS(ESI)m/z:318.5[M+Na]
+.
1 H NMR (500MHz, DMSO- d 6): δ9.71 (s, 1H), 6.96-6.89 (m, 2H), 6.79 (dd, J = 3.0Hz, 6.5Hz, 1H), 5.53-5.42 (m , 1H), 5.24 (t, J = 8.5 Hz, 1H), 4.18-4.03 (m, 2H), 2.65 (m, 1H), 2.18-2.05 (m, 1H). MS (ESI) m/z: 318.5 [M+Na] + .
步骤3:((1S,3s)-3-(4-氟-2-((2R,4S)-4-氟-1-(2,2,2-三氟乙酰基)吡咯烷-2-基)苯氧基)环丁基)氨基甲酸叔丁酯Step 3: ((1S,3s)-3-(4-fluoro-2-((2R,4S)-4-fluoro-1-(2,2,2-trifluoroacetyl)pyrrolidin-2-yl) Phenyloxy)cyclobutyl)carbamic acid tert-butyl ester
反应瓶中,氮气保护及冰水浴冷却下,将DEAD(77mg)缓慢滴加到29C(142mg)、((1r,3r)-3-羟基环戊胺)氨基甲酸叔丁酯(66.5mg)、三苯基磷(124mg)和四氢呋喃(2mL)混合物中,加毕室温搅拌30分钟后,50℃搅拌反应。反应完全后,直接向反应液中加入石油醚,过滤,滤液浓缩。柱层析纯化(石油醚:乙酸乙酯=85:15)得到化合物29D(82mg)。In the reaction flask, under nitrogen protection and cooling in an ice water bath, DEAD (77 mg) was slowly added dropwise to 29C (142 mg), ((1r, 3r)-3-hydroxycyclopentylamine) t-butyl carbamate (66.5 mg), The mixture of triphenylphosphine (124 mg) and tetrahydrofuran (2 mL) was stirred at room temperature for 30 minutes, and then stirred at 50 °C. After the reaction was completed, petroleum ether was directly added to the reaction mixture, and the filtrate was concentrated. Column chromatography purification (petroleum ether: ethyl acetate = 85: 15) gave Compound 29D (82mg).
1H NMR(500MHz,DMSO-d
6):δ7.20(s,1H),7.19-7.02(m,2H),6.86(t,J=4.5Hz,1H),5.55-5.44(m,1H),5.26(t,J=7.5Hz,1H),4.43(t,J=6.5Hz,1H),4.14-4.08(m,2H),3.68(d,J=7.0Hz,1H),2.75(br,3H),2.17-1.96(m,3H),1.38(s,9H).
13C NMR(125MHz,DMSO-d
6):δ157.66,155.78,155.03,150.62,130.49,127.41,117.49,93.55,92.16,78.21,65.79,60.22,54.59,38.65,28.69.MS(ESI)m/z:487.4[M+Na]
+.
1 H NMR (500MHz, DMSO- d 6): δ7.20 (s, 1H), 7.19-7.02 (m, 2H), 6.86 (t, J = 4.5Hz, 1H), 5.55-5.44 (m, 1H) , 5.26 (t, J = 7.5 Hz, 1H), 4.43 (t, J = 6.5 Hz, 1H), 4.14 - 4.08 (m, 2H), 3.68 (d, J = 7.0 Hz, 1H), 2.75 (br, 3H), 2.17-1.96 (m, 3H), 1.38 (s, 9H). 13 C NMR (125MHz, DMSO-d 6 ): δ 157.66, 155.78, 155.03, 150.62, 130.49, 127.41, 117.49, 93.55, 92.16, 78.21 , 65.79, 60.22, 54.59, 38.65, 28.69. MS (ESI) m/z: 487.4 [M+Na] + .
步骤4:((1S,3s)-3-(4-氟-2-((2R,4S)-4-氟吡咯烷-2-基)苯氧基)环丁基)氨基甲酸叔丁酯Step 4: ((1S,3s)-3-(4-Fluoro-2-((2R,4S)-4-fluoropyrrolidin-2-yl)phenoxy)cyclobutyl)carbamic acid tert-butyl ester
反应瓶中,依次加入29D(82mg,)、氢氧化锂一水合物(44.5mg)、MeOH(4mL)、四氢呋喃(0.5mL)和水(0.5mL),加毕氮气保护下,60℃搅拌反应。反应完全后,冰水浴冷却下向反应液中加入稀盐酸(0.5M)调节pH至5-7,EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸干燥。过滤,滤液浓缩,得到化合物29E(54mg)。未纯化直接投料下一步。MS(ESI)m/z:369.5[M+H]
+.
In the reaction flask, 29D (82 mg,), lithium hydroxide monohydrate (44.5 mg), MeOH (4 mL), tetrahydrofuran (0.5 mL) and water (0.5 mL) were added, and the reaction was stirred at 60 ° C under nitrogen. . After the reaction was completed, dilute hydrochloric acid (0.5 M) was added to the reaction mixture under ice-cooling to adjust the pH to 5-7, EA was extracted, and the organic phases were combined, washed with saturated brine and dried over anhydrous sulfate. Filtration and concentration of the filtrate gave compound 29E (54 mg). The next step was directly fed without purification. MS (ESI) m/z: 369.5 [M+H] + .
步骤5:6-((2R,4S)-2-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧基)-5-氟苯基)-4-氟吡咯烷-1-基)咪唑并[1,2-b]哒嗪-3-羧酸乙酯Step 5: 6-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)-4 -fluoropyrrolidin-1-yl)imidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester
反应瓶中,依次加入29E(410mg)、6-氯咪唑并[1,2-b]哒嗪3-甲酸乙酯(251mg)、氟化钾(388mg)及二甲基亚砜(6mL),氮气保护及120℃搅拌反应。反应完全后,将反应液倒入EA中,饱和食盐水洗涤,无水硫酸干燥,过滤,滤液浓缩。柱层析纯化(石油醚:乙酸乙酯=50:50),得到化合物29F(156mg)。MS(ESI)m/z:580.5[M+Na]
+.
In the reaction flask, 29E (410 mg), 6-chloroimidazo[1,2-b]pyridazine 3-carboxylic acid ethyl ester (251 mg), potassium fluoride (388 mg) and dimethyl sulfoxide (6 mL) were sequentially added. Nitrogen protection and stirring reaction at 120 °C. After the reaction was completed, the reaction mixture was poured into EA, washed with brine, dried over anhydrous Purification by column chromatography (petrole ether: ethyl acetate = 50: 50) gave Compound 29F (156mg). MS (ESI) m / z: 580.5 [M+Na] + .
步骤6:6-((2R,4S)-2-(2-((1s,3S)-3-((叔丁基氧羰基)氨基)环丁氧基)-5-氟苯基)-4-氟吡咯烷-1-基)咪唑并[1,2-b]哒嗪-3-羧酸Step 6: 6-((2R,4S)-2-(2-((1s,3S)-3-((tert-Butyloxycarbonyl)amino)cyclobutoxy)-5-fluorophenyl)-4 -fluoropyrrolidin-1-yl)imidazo[1,2-b]pyridazine-3-carboxylic acid
反应瓶中,依次加入29F(156mg)、氢氧化锂一水合物(70.4mg)、MeOH(6mL)、四氢呋喃(2mL)及水(0.5mL),加毕氮气保护下,60℃搅拌反应。反应完全后,冰水浴冷却下向反应液中加入稀盐酸(0.5M)调节pH至5-7,EA萃取,有机相合并,饱和食盐水洗涤,无水硫酸干燥。过滤,滤液浓缩,得到化合物29G(146mg)。MS(ESI)m/z:552.5[M+Na]
+.
29F (156 mg), lithium hydroxide monohydrate (70.4 mg), MeOH (6 mL), tetrahydrofuran (2 mL) and water (0.5 mL) were successively added to the reaction flask, and the reaction was stirred at 60 ° C under a nitrogen atmosphere. After the reaction was completed, dilute hydrochloric acid (0.5 M) was added to the reaction mixture under ice-cooling to adjust the pH to 5-7, EA was extracted, and the organic phases were combined, washed with saturated brine and dried over anhydrous sulfate. Filtration and concentration of the filtrate gave Compound 29G (146 mg). MS (ESI) m / z: 552.5 [M+Na] + .
步骤7:6-((2R,4S)-2-(2-((1s,3S)-3-氨基环丁氧基)-5-氟苯基)-4-氟吡咯烷-1-基)咪唑并[1,2-b]哒嗪-3-羧酸三盐酸盐Step 7: 6-((2R,4S)-2-(2-((1s,3S)-3-Aminocyclobutoxy)-5-fluorophenyl)-4-fluoropyrrolidin-1-yl) Imidazo[1,2-b]pyridazine-3-carboxylic acid trihydrochloride
反应瓶中,依次加入29G(146mg)、氯化氢的1,4-二氧六环溶液(4M,10mL),室温搅拌反应。反应完全后,浓缩反应液得到化合物29H(184mg)。MS(ESI)m/z:427.9[M-H]
-.
In a reaction flask, a solution of 29 G (146 mg) of hydrogen chloride in 1,4-dioxane (4M, 10 mL) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated to give Compound 29H (184 mg). MS (ESI) m/z: 427.9 [MH] - .
步骤8:(2
2R,2
4S,5
1S,5
3S,E)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(6,3)-咪唑并[1,2-b]哒嗪-2(1,2)-吡咯烷基-3(1,2)-苯杂-5(1,3)-环丁基环辛内酰胺-7-酮
Step 8: (2 2 R, 2 4 S, 5 1 S, 5 3 S, E) - 2 4 , 3 5 -difluoro-4-oxa-6-aza-1(6,3)-imidazole And [1,2-b]pyridazine-2(1,2)-pyrrolidinyl-3(1,2)-benzene-5(1,3)-cyclobutylcyclooctanolactam-7-one
反应瓶中,依次加入29H(184mg)、DIPEA(265mg)、DCM(60mL)和DMF(10.0mL),加毕室温搅拌10分钟。将五氟苯基二苯基磷酸酯(131mg)分次加入到上述反应液中,室温搅拌反应。反应完全后,将反应液倒入EA,有机相合并,饱和食盐水洗涤,无水硫酸干燥。过滤,滤液浓缩。柱层析纯化(DCM/MeOH=96/4)得化合物I-29(15mg)。In a reaction flask, 29H (184 mg), DIPEA (265 mg), DCM (60 mL) and DMF (10.0 mL) were sequentially added, and the mixture was stirred at room temperature for 10 minutes. Pentafluorophenyl diphenyl phosphate (131 mg) was added to the above reaction solution in portions, and the reaction was stirred at room temperature. After the reaction was completed, the reaction mixture was poured into EA. Filter and concentrate the filtrate. Purification by column chromatography (DCM / MeOH = EtOAc)
1H NMR(500MHz,DMSO-d
6):δ9.30(d,J=10.5Hz,1H),8.04(d,J=10.0Hz,1H),7.96(s,1H),7.27(d,J=7.5Hz,1H),7.18(d,J=10.0Hz,1H),7.02(t,J=7.5Hz,1H),6.61(t,J=4.5Hz,1H),5.89(t,J=7.5Hz,1H),5.60-5.49(m,1H),4.99(s,1H),4.81(d,J=4.5Hz,1H),4.47-4.39(m,1H),4.13-4.08(m,1H),3.08(t,J=6.5Hz,1H),2.85-2.77(m,2H),2.18(q,J=7.5Hz,2H),1.76(t,J=7.5Hz,1H).
13C NMR(125MHz,DMSO-d
6):δ158.30,157.72,156.42,153.59,149.17,138.33,136.81,134.33,127.61,122.46,114.91,93.83,92.44,74.88,58.01,52.53,44.72,43.08,36.37,35.44.MS(ESI)m/z:412.5[M+H]
+.
1 H NMR (500MHz, DMSO- d 6): δ9.30 (d, J = 10.5Hz, 1H), 8.04 (d, J = 10.0Hz, 1H), 7.96 (s, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.18 (d, J = 10.0 Hz, 1H), 7.02 (t, J = 7.5 Hz, 1H), 6.61 (t, J = 4.5 Hz, 1H), 5.89 (t, J = 7.5) Hz, 1H), 5.60-5.49 (m, 1H), 4.99 (s, 1H), 4.81 (d, J = 4.5 Hz, 1H), 4.47-4.39 (m, 1H), 4.13-4.08 (m, 1H) , 3.08 (t, J = 6.5 Hz, 1H), 2.85-2.77 (m, 2H), 2.18 (q, J = 7.5 Hz, 2H), 1.76 (t, J = 7.5 Hz, 1H). 13 C NMR ( 125 MHz, DMSO-d 6 ): δ 158.30, 157.72, 156.42, 153.59, 149.17, 138.33, 136.81, 134.33, 127.61, 122.46, 114.91, 93.83, 92.44, 74.88, 58.01, 52.53, 44.72, 43.08, 36.37, 35.44. ESI) m/z: 412.5 [M+H] + .
实施例30:(1
3E,1
4E,2
2R,2
4S,5
1R,5
3S)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-3(3,2)-吡啶杂-2(1,2)-吡咯烷-5(1,3)-环戊基环庚内酰胺-7-酮
Example 30: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 R, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclopentyl ring Geptanolactam-7-one
步骤1:5-((2R,4S)-4-氟-2-(5-氟-2-甲氧基吡啶-3-基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 1: 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-methoxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a Pyrimidine-3-carboxylic acid ethyl ester
向微波管中依次加入30A-1(0.64g)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(0.634g)、DIPEA(1.650g)和正丁醇(15mL),搅拌1分钟后,微波120℃搅拌反应。反应完全后,浓缩反应液,残留物中加入乙酸乙酯,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚:乙酸乙酯=60:40)得到化合物30B-1(0.676g)。MS(ESI)m/z:426.4[M+Na]
+.
Add 30A-1 (0.64g), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester (0.634g), DIPEA (1.650g) and n-butanol (15mL) to the microwave tube. After stirring for 1 minute, the reaction was stirred at 120 ° C in a microwave. After the reaction was completed, the reaction mixture was evaporated. Filter and concentrate the filtrate. Column chromatography purification (petroleum ether: ethyl acetate = 60: 40) gave Compound 30B-1 (0.676 g). MS (ESI) m / z: 426.4 [M+Na] + .
步骤2:5-((2R,4S)-4-氟-2-(5-氟-2-羟基吡啶-3-基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 2: 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxypyridin-3-yl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine Ethyl-3-carboxylate
向反应瓶中依次加入30B-1(0.676g)、无水乙醇(25mL)、氯化氢的1,4-二氧六环溶液(4M,45mL),75℃搅拌反应。反应完全后,浓缩反应液,残留物中加入饱和碳酸氢钠水溶液调节pH至7-8,用DCM萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物30C-1(0.64g)。MS(ESI)m/z:412.4[M+Na]
+.
To the reaction flask were successively added 30B-1 (0.676 g), absolute ethanol (25 mL), hydrogen chloride in 1,4-dioxane solution (4M, 45 mL), and the mixture was stirred at 75 °C. After the reaction was completed, the reaction mixture was evaporated. Filtration and concentration of the filtrate gave compound 30C-1 (0.64 g). MS (ESI) m / z: 412.4 [M+Na] + .
步骤3:5-((2R,4S)-2-(2-(((1R,3S)-3-((叔丁氧基羰基)氨基)环戊基)氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 3: 5-((2R,4S)-2-(2-((1R,3S)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluoropyridine- Ethyl 3-yl)-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
在0℃及氮气保护下,将DEAD(0.23g)缓慢滴加至30C-1(0.32g)、((1S,3S)-3-羟基环戊基)氨基甲酸叔丁酯(0.20g)和三苯基膦(0.32g)的四氢呋喃(15mL)溶液中,50℃搅拌反应。反应完全后,浓缩反应液,柱层析纯化(石油醚/乙酸乙酯=60/40)得到化合物30D-1(0.31g)。MS(ESI)m/z:595.6[M+Na]
+.
DEAD (0.23g) was slowly added dropwise to 30C-1 (0.32g), ((1S,3S)-3-hydroxycyclopentyl)carbamic acid tert-butyl ester (0.20g) at 0 ° C under nitrogen and A solution of triphenylphosphine (0.32 g) in tetrahydrofuran (15 mL) was stirred at 50 °C. After completion of the reaction, the reaction mixture was concentrated and purified mjjjjjjjj MS (ESI) m/z: 595.6 [M+Na] + .
步骤4:5-((2R,4S)-2-(2-(((1R,3S)-3-((叔丁氧基羰基)氨基)环戊基)氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸Step 4: 5-((2R,4S)-2-(2-((1R,3S)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluoropyridine- 3-yl)-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入30D-1(0.31g)、氢氧化锂一水合物(0.23g)、乙醇(5mL)、四氢呋喃(2.5mL)和水(2mL),氮气保护下,70℃搅拌反应。反应完全后,冷却至室温,向反应液加入稀盐酸(2N)调节pH至小于5,EA萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物30E-1(0.26g)。MS(ESI)m/z:567.6[M+Na]
+.
To the reaction flask, 30D-1 (0.31g), lithium hydroxide monohydrate (0.23g), ethanol (5mL), tetrahydrofuran (2.5mL) and water (2mL) were added in sequence, and the reaction was stirred at 70 ° C under nitrogen atmosphere. . After the reaction was completed, it was cooled to room temperature, and the mixture was diluted with hydrochloric acid (2N) to adjust the pH to less than 5, and extracted with EA. Filtration and concentration of the filtrate gave compound 30E-1 (0.26 g). MS (ESI) m/z: 567.6 [M+Na] + .
步骤5:5-((2R,4S)-2-(2-(((1R,3S)-3-氨基环戊基)氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 5: 5-((2R,4S)-2-(2-((1R,3S)-3-Aminocyclopentyl)oxy)-5-fluoropyridin-3-yl)-4-fluoropyrrole Alkan-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向反应瓶中加入30E-1(255mg),冰水浴冷却下加入乙醇(2mL)和氯化氢的1,4-二氧六环溶液(4M,8mL),加毕室温搅拌反应。反应完全后,浓缩反应液,残留物用乙腈打浆,过滤,滤饼干燥得到化合物30F-1(248mg)。MS(ESI)m/z:467.6[M+Na]
+.
30E-1 (255 mg) was added to the reaction flask, and a solution of ethanol (2 mL) and hydrogen chloride in 1,4-dioxane (4M, 8 mL) was added under ice-cooling, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated. mjjjjjjj MS (ESI) m/z: 467.6 [M+Na] + .
步骤6:(1
3E,1
4E,2
2R,2
4S,5
1R,5
3S)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-3(3,2)-吡啶杂-2(1,2)-吡咯烷-5(1,3)-环戊基环庚内酰胺-7-酮
Step 6: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 R, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclopentylcycloheptane Lactam-7-one
将五氟苯基二苯基磷酸酯(181mg)分批加入到30F-1(248mg)、DIPEA(579mg)、DMF(10mL)和DCM(50mL)的混合物中,室温搅拌反应。反应完全后,浓缩反应液,残留物中加入饱和碳酸钠水溶液,EA萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM:MeOH=97:3)得到化合物I-30(71mg)。Pentafluorophenyl diphenyl phosphate (181 mg) was added portionwise to a mixture of 30F-1 (248 mg), DIPEA (579 mg), DMF (10 mL) and DCM (50 mL). After the reaction was completed, the reaction mixture was evaporated, evaporated, evaporated, evaporated Filter and concentrate the filtrate. Purification by column chromatography (DCM:MeOH =EtOAc:EtOAc)
1H NMR(500MHz,DMSO-d
6)δ8.79(d,J=7.6Hz,1H),8.38(d,J=7.2Hz,1H),8.17(d,J=2.5Hz,1H),8.05(d,J=2.9Hz,1H),7.88(dd,J=9.1,2.9Hz,1H),6.70(d,J=7.8Hz,1H),6.03–5.93(m,1H),5.63–5.52(m,1H),5.21(td,J=6.4,2.8Hz,1H),4.49–4.32(m,2H),4.24–4.10(m,1H),2.85–2.76(m,1H),2.43–2.37(m,1H),2.34–2.15(m,2H),2.13–2.07(m,1H),1.90–1.86(m,1H),1.81–1.74(m,1H),1.64–1.57(m,1H).
13C NMR(126MHz,DMSO-d
6)δ161.64,156.43,155.73,145.51,137.40,132.59,126.18,102.05,100.17,94.10,92.71,79.48,57.15,52.11,51.30,42.60,42.43,38.49,33.60,31.62.MS(ESI)m/z:449.4[M+Na]
+.
1 H NMR (500 MHz, DMSO-d 6 ) δ 8.79 (d, J = 7.6 Hz, 1H), 8.38 (d, J = 7.2 Hz, 1H), 8.17 (d, J = 2.5 Hz, 1H), 8.05 (d, J = 2.9 Hz, 1H), 7.88 (dd, J = 9.1, 2.9 Hz, 1H), 6.70 (d, J = 7.8 Hz, 1H), 6.03 - 5.93 (m, 1H), 5.63 - 5.52 ( m,1H), 5.21 (td, J=6.4, 2.8 Hz, 1H), 4.49–4.32 (m, 2H), 4.24–4.10 (m, 1H), 2.85–2.76 (m, 1H), 2.43–2.37 ( m,1H), 2.34–2.15 (m, 2H), 2.13–2.07 (m, 1H), 1.90–1.86 (m, 1H), 1.81–1.74 (m, 1H), 1.64–1.57 (m, 1H). 13 C NMR (126 MHz, DMSO-d 6 ) δ 161.64, 156.43, 155.73, 145.51, 137.40, 132.59, 126.18, 102.05, 100.17, 94.10, 92.71, 79.48, 57.15, 52.11, 51.30, 42.60, 42.43, 38.49, 33.60, 31.62 .MS (ESI) m/z: 449.4 [M+Na] + .
实施例31:(1
3E,1
4E,2
2R,2
4S,5
1S,5
3R)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-3(3,2)-吡啶杂-2(1,2)-吡咯烷杂-5(1,3)-环戊基环庚内酰胺-7-酮
Example 31: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 R)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclopentyl Cycloheptamamide-7-one
步骤1:5-((2R,4S)-2-(2-(((1S,3R)-3-((叔丁氧基羰基)氨基)环戊基)氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 1: 5-((2R,4S)-2-(2-((1S,3R)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluoropyridine- Ethyl 3-yl)-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
在0℃及氮气保护下,将DEAD(248mg)缓慢滴加至30C-1(346mg)、((1R,3R)-3-羟基环戊基)氨基甲酸叔丁酯(233mg)、三苯基膦(350mg)的四氢呋喃(15mL)溶液中,50℃搅拌反应。反应完全后,浓缩反应液,柱层析纯化(石油醚:乙酸乙酯=60:40)得到化合物31A-1(402mg)。MS(ESI)m/z:595.6[M+Na]
+.
DEAD (248 mg) was slowly added dropwise to 30C-1 (346 mg), ((1R,3R)-3-hydroxycyclopentyl)carbamic acid tert-butyl ester (233 mg), triphenyl at 0 ° C under a nitrogen atmosphere. A solution of phosphine (350 mg) in tetrahydrofuran (15 mL) was stirred at 50 °C. After the reaction was completed, the reaction mixture was evaporated to mjjjjjjjjjj MS (ESI) m/z: 595.6 [M+Na] + .
步骤2:5-((2R,4S)-2-(2-(((1S,3R)-3-((叔丁氧基羰基)氨基)环戊基)氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸Step 2: 5-((2R,4S)-2-(2-((1S,3R)-3-((tert-Butoxycarbonyl)amino)cyclopentyl)oxy)-5-fluoropyridine- 3-yl)-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
反应瓶中,依次加入31A-1(400mg)、氢氧化锂一水合物(493mg)、乙醇(5mL)、四氢呋喃(2.5mL)及水(2mL),氮气保护下,70℃搅拌反应。反应完全后,向反应液中加入稀盐酸(2N)调节pH至小于5,EA萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得到化合物31B-1(357mg)。MS(ESI)m/z:543.6[M-H]
+.
In the reaction flask, 31A-1 (400 mg), lithium hydroxide monohydrate (493 mg), ethanol (5 mL), tetrahydrofuran (2.5 mL) and water (2 mL) were successively added, and the mixture was stirred at 70 ° C under nitrogen atmosphere. After the completion of the reaction, dilute hydrochloric acid (2N) was added to the reaction mixture to adjust the pH to less than 5, EA was extracted, and the organic phase was combined, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave Compound 31B-1 (357 mg). MS (ESI) m/z: 543.6 [MH] + .
步骤3:5-((2R,4S)-2-(2-(((1S,3R)-3-氨基环戊基)氧基)-5-氟吡啶-3-基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸Step 3: 5-((2R,4S)-2-(2-((1S,3R)-3-Aminocyclopentyl)oxy)-5-fluoropyridin-3-yl)-4-fluoropyrrole Alkan-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
冰浴冷却下,向含有31B-1(357mg)的反应瓶中,加入乙醇(2mL)和氯化氢的1,4-二氧六环溶液(4M,8mL),室温搅拌反应。反应完全后,浓缩反应液,残留物用乙腈打浆,过滤,滤饼干燥得到化合物31C-1(377mg)。MS(ESI)m/z:443.1[M-H]
+.
Under ice-cooling, to a reaction flask containing 31B-1 (357 mg), ethanol (2 mL) and hydrogen chloride in 1,4-dioxane solution (4M, 8 mL) were added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was evaporated. mjjjjjjjj MS (ESI) m/z: 443.1 [MH] + .
步骤4:(1
3E,1
4E,2
2R,2
4S,5
1S,5
3R)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-3(3,2)-吡啶杂-2(1,2)-吡咯烷杂-5(1,3)-环戊基环庚内酰胺-7-酮
Step 4: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 S, 5 3 R) - 2 4 , 3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidin-3(3,2)-pyridin-2(1,2)-pyrrolidin-5(1,3)-cyclopentyl ring Geptanolactam-7-one
将五氟苯基二苯基磷酸酯(275mg)分批加入到31C-1(377mg)、DIPEA(880mg)、DMF(10mL)及DCM(50mL)溶液中,室温搅拌反应。反应完全后,浓缩反应液,残留物中加入饱和碳酸钠水溶液,EA萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM/MeOH=97/3)得到化合物I-31(135mg)。Pentafluorophenyl diphenyl phosphate (275 mg) was added portionwise to a solution of 31C-1 (377 mg), DIPEA (880 mg), DMF (10 mL) and DCM (50 mL). After the reaction was completed, the reaction mixture was evaporated, evaporated, evaporated, evaporated Filter and concentrate the filtrate. Purification by column chromatography (DCM / MeOH = EtOAc / EtOAc)
1H NMR(500MHz,DMSO-d6)δ8.79(dd,J=7.8,2.3Hz,1H),8.35(d,J=10.5Hz,1H),8.10(d,J=2.2Hz,1H),8.03(t,J=2.6Hz,1H),7.77(dd,J=9.1,3.1Hz,1H),6.75–6.63(m,1H),5.72–5.50(m,2H),5.33(t,J=3.8Hz,1H),4.82–4.77(m,1H),4.47–4.37(m,1H),4.15–4.08(m,1H),2.84–2.76(m,1H),2.40–2.28(m,1H),2.27–2.08(m,3H),2.07–1.86(m,2H),1.83–1.77(m,1H).
13C NMR(126MHz,DMSO-d6)δ160.15,156.39,154.73,145.49,145.04,137.37,132.23,128.58,124.71,124.54,101.69,99.98,93.89,92.49,79.82,56.28,53.22,45.88,41.91,33.27,31.52.MS(ESI)m/z:449.4[M+Na]
+.
1 H NMR (500MHz, DMSO- d6) δ8.79 (dd, J = 7.8,2.3Hz, 1H), 8.35 (d, J = 10.5Hz, 1H), 8.10 (d, J = 2.2Hz, 1H), 8.03 (t, J = 2.6 Hz, 1H), 7.77 (dd, J = 9.1, 3.1 Hz, 1H), 6.75 - 6.63 (m, 1H), 5.72 - 5.50 (m, 2H), 5.33 (t, J = 3.8 Hz, 1H), 4.82–4.77 (m, 1H), 4.47–4.37 (m, 1H), 4.15–4.08 (m, 1H), 2.84–2.76 (m, 1H), 2.40–2.28 (m, 1H) , 2.27–2.08 (m, 3H), 2.07–1.86 (m, 2H), 1.83–1.77 (m, 1H). 13 C NMR (126 MHz, DMSO-d6) δ 160.15, 156.39, 154.73, 145.49, 145.04, 137.37, 132.23,128.58,124.71,124.54,101.69,99.98,93.89,92.49,79.82,56.28,53.22,45.88,41.91,33.27,31.52.MS(ESI) m/z:449.4[M+Na] +.
实施例32:(1
3E,1
4E,2
2R,2
4S,5
1R,5
3S)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-2(1,2)-吡咯烷杂-3(1,2)-苯杂-5(1,3)-环戊基环庚内酰胺-7-酮
Example 32: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 R, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 (5,3)-pyrazolo[1,5-a]pyrimidinium-2(1,2)-pyrrolidin-3(1,2)-benzene-5(1,3)-cyclopentyl Cycloheptamamide-7-one
步骤1:5-((2R,4S)-4-氟-2-(5-氟-2-羟基苯基)吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 1: 5-((2R,4S)-4-fluoro-2-(5-fluoro-2-hydroxyphenyl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidin-3- Ethyl carboxylate
微波管中依次加入32A(1.25g)、5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(1.11g)、DIPEA(2.88g)和正丁醇(20mL),搅拌1分钟后,微波120℃搅拌反应。反应完全后,浓缩反应液,残留物溶于乙酸乙酯(200mL),用饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(石油醚/乙酸乙酯=60/40)得到化合物32B(1.01g)。MS(ESI)m/z:411.4[M+Na]
+.
32A (1.25g), 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester (1.11g), DIPEA (2.88g) and n-butanol (20mL) were added to the microwave tube and stirred. After 1 minute, the reaction was stirred at 120 ° C in a microwave. After the reaction was completed, the reaction mixture was evaporated. Filter and concentrate the filtrate. Purification by column chromatography (petrole ether / ethyl acetate = 60 / 40) gave Compound 32B (1.01 g). MS (ESI) m / z: 411.4 [M+Na] + .
步骤2:5-((2R,4S)-2-(2-(((1R,3S)-3-((叔丁氧基羰基)氨基)环戊基)氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 2: 5-((2R,4S)-2-(2-((1R,3S)-3-((tert-Butoxycarbonyl))amino)cyclopentyl)oxy)-5-fluorophenyl Ethyl 4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylate
0℃,氮气保护下,将DEAD(359mg)缓慢加至32B(500mg)、((1S,3S)-3-羟基环戊基)氨基甲酸叔丁酯(311mg)、三苯基膦(507mg)的四氢呋喃(15mL)溶液中,50℃搅拌反应。反应完全后,浓缩反应液,柱层析纯化(石油醚/乙酸乙酯=60/40)得到化合物32C(687mg)。MS(ESI)m/z:594.5[M+Na]
+.
DEAD (359 mg) was slowly added to 32B (500 mg), ((1S,3S)-3-hydroxycyclopentyl)carbamic acid tert-butyl ester (311 mg), triphenylphosphine (507 mg) at 0 ° C under nitrogen. The solution was stirred at 50 ° C in a solution of tetrahydrofuran (15 mL). After the reaction was completed, the reaction mixture was evaporated, mjjjjjjj MS (ESI) m / z: 594.5 [M+Na] + .
步骤3:5-((2R,4S)-2-(2–(((1R,3S)-3-((叔丁氧基羰基)氨基)环戊基)氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸Step 3: 5-((2R,4S)-2-(2-((1R,3S)-3-((tert-Butoxycarbonyl))amino)cyclopentyl)oxy)-5-fluorophenyl )-4-fluoropyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向反应瓶中,依次加入32C(500mg)、氢氧化锂一水合物(367mg)、乙醇(5mL)、四氢呋喃(2.5mL)及水(2mL),氮气保护下,70℃搅拌反应。反应完全后,冰水浴冷却下,向反应液加入稀盐酸(2N)调节溶液pH至小于5,EA萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩得化合物32D(369mg)。MS(ESI)m/z:566.5[M+Na]
+.
To the reaction flask, 32 C (500 mg), lithium hydroxide monohydrate (367 mg), ethanol (5 mL), tetrahydrofuran (2.5 mL) and water (2 mL) were sequentially added, and the mixture was stirred at 70 ° C under nitrogen atmosphere. After the reaction was completed, the reaction mixture was diluted with ice water (2N) to adjust the pH of the solution to less than 5, and extracted with EA. The organic phase was combined, washed with saturated brine and dried over anhydrous sodium sulfate. Filtration and concentration of the filtrate gave compound 32D (369 mg). MS (ESI) m / z: 566.5 [M+Na] + .
步骤4:5-((2R,4S)-2-(2–(((1R,3S)-3-氨基环戊基)氧基)-5-氟苯基)-4-氟吡咯烷-1-基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 4: 5-((2R,4S)-2-(2-((1R,3S)-3-Aminocyclopentyl)oxy)-5-fluorophenyl)-4-fluoropyrrolidine-1 -yl)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid trihydrochloride
向含有32D(369mg)的反应瓶中,冰水浴冷却下加入乙醇(2mL)和氯化氢的1,4-二氧六环溶液(4M,8mL),室温搅拌反应。反应完全后,浓缩反应液得到化合物32E(333mg)。MS(ESI)m/z:466.3[M+Na]
+.
To a reaction flask containing 32D (369 mg), a solution of ethanol (2 mL) and hydrogen chloride in 1,4-dioxane (4M, 8 mL) was added, and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was concentrated to give Compound 32E (333mg). MS (ESI) m / z: 466.3 [M+Na] + .
步骤5:(1
3E,1
4E,2
2R,2
4S,5
1R,5
3S)-2
4,3
5-二氟-4-氧杂-6-氮杂-1(5,3)-吡唑并[1,5-a]嘧啶杂-2(1,2)-吡咯烷杂-3(1,2)-苯杂-5(1,3)-环戊基环庚内酰胺-7-酮
Step 5: (1 3 E, 1 4 E, 2 2 R, 2 4 S, 5 1 R, 5 3 S)-2 4 ,3 5 -difluoro-4-oxa-6-aza-1 ( 5,3)-pyrazolo[1,5-a]pyrimidinium-2(1,2)-pyrrolidin-3(1,2)-benzene-5(1,3)-cyclopentyl ring Geptanolactam-7-one
向反应瓶中依次加入32E(333mg)、DIPEA(467mg)、DMF(10mL)及DCM(50mL),将五氟苯基二苯基磷酸酯(231mg)分批加入到上述反应液中,室温搅拌反应。反应完全后,浓缩反应液,残留物中加入饱和碳酸钠水溶液,EA萃取,合并有机相,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(DCM/MeOH=97/3)得到化合物I-32(45mg)。32E (333 mg), DIPEA (467 mg), DMF (10 mL) and DCM (50 mL) were added to the reaction flask, and pentafluorophenyldiphenyl phosphate (231 mg) was added portionwise to the above reaction mixture, and stirred at room temperature. reaction. After the reaction was completed, the reaction mixture was evaporated, evaporated, evaporated, evaporated Filter and concentrate the filtrate. Purification by column chromatography (DCM / MeOH = EtOAc)
1H NMR(500MHz,DMSO-d
6)δ8.77(d,J=7.7Hz,1H),8.41(d,J=7.2Hz,1H),8.16(s,1H),7.26(dd,J=9.9,2.4Hz,1H),7.08–6.94(m,1H),6.83(dd,J=9.5,4.4Hz,1H),6.69(d,J=7.9Hz,1H),6.14(t,J=8.3Hz,1H),5.56(d,J=53.0Hz,1H),4.96(t,J=6.4Hz,1H),4.46–4.30(m,2H),4.19–4.13(m,1H),2.77–2.73(m,1H),2.42–2.35(m,1H),2.27–2.09(m,3H),1.98–1.90(m,1H),1.68–1.62(m,2H).
13C NMR(126MHz,DMSO-d6)δ161.68,156.43,149.70,145.30,137.49,115.60,114.82,114.16,101.90,100.23,94.24,92.85,79.19,57.41,57.24,51.78,51.13,42.86,42.69,39.49,33.92,30.38.MS(ESI)m/z:448.4[M+Na]
+.
1 H NMR (500MHz, DMSO- d 6) δ8.77 (d, J = 7.7Hz, 1H), 8.41 (d, J = 7.2Hz, 1H), 8.16 (s, 1H), 7.26 (dd, J = 9.9, 2.4 Hz, 1H), 7.08–6.94 (m, 1H), 6.83 (dd, J=9.5, 4.4 Hz, 1H), 6.69 (d, J=7.9 Hz, 1H), 6.14 (t, J=8.3) Hz, 1H), 5.56 (d, J = 53.0 Hz, 1H), 4.96 (t, J = 6.4 Hz, 1H), 4.46 - 4.30 (m, 2H), 4.19 - 4.13 (m, 1H), 2.77 - 2.73 (m, 1H), 2.42–2.35 (m, 1H), 2.27–2.09 (m, 3H), 1.98–1.90 (m, 1H), 1.68–1.62 (m, 2H). 13 C NMR (126 MHz, DMSO- D6) δ 161.68, 156.43, 149.70, 145.30, 137.49, 115.60, 114.82, 114.16, 101.90, 100.23, 94.24, 92.85, 79.19, 57.41, 57.24, 51.78, 51.13, 42.86, 42.69, 39.49, 33.92, 30.38. MS (ESI) m/z: 448.4 [M+Na] + .
实施例33:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2,4,7-三氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁杂辛内酰胺-5-酮
Example 33: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -fluoro-8-methyl-2,4,7-triaza-6 (3,5) -pyrazolo[1,5-a]pyrimidin-1(1,2)-benzene-3(1,3)-cyclobutanooctanoic acid-5-one
步骤1:(R,Z)-N-(1-(2-溴-5-氟苯基)亚乙基)-2-甲基丙烷-2-亚磺酰胺Step 1: (R,Z)-N-(1-(2-Bromo-5-fluorophenyl)ethylidene)-2-methylpropane-2-sulfinamide
反应瓶中,依次加入33A(20.00g)、(R)-(+)-叔丁基亚磺酰胺(16.75g)、二乙二醇二甲醚(12.36g)、四氢呋喃(150mL)及2-甲基四氢呋喃(150mL)。冰水浴冷却下,加入钛酸四乙酯(105g),加毕60℃搅拌反应。反应完全后,将反应液倒入冰水(2L)中,加入EA(1L)剧烈搅拌。过滤,滤饼用EA洗涤,滤液分相,水相用EA萃取,合并有机相,依次用水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(乙酸乙酯:石油醚=0:100~10:90)得到化合物33B(20.80g)。In the reaction flask, 33A (20.00 g), (R)-(+)-tert-butylsulfinamide (16.75 g), diethylene glycol dimethyl ether (12.36 g), tetrahydrofuran (150 mL) and 2- were successively added. Methyltetrahydrofuran (150 mL). Under ice cooling, a tetraethyl titanate (105 g) was added, and the reaction was stirred at 60 ° C. After the reaction was completed, the reaction mixture was poured into ice water (2 L), and EA (1 L) was stirred vigorously. After filtration, the filter cake was washed with EA, the filtrate was separated, and the aqueous phase was extracted with EtOAc. Filter and concentrate the filtrate. Purification by column chromatography (ethyl acetate: petroleum ether = 0: 100 to 10: 90) gave Compound 33B (20.80 g).
步骤2:(R)-N-((R)-1-(2-溴-5-氟苯基)乙基)-2-甲基丙烷-2-亚磺酰胺Step 2: (R)-N-((R)-1-(2-bromo-5-fluorophenyl)ethyl)-2-methylpropane-2-sulfinamide
-15℃下,向反应瓶中,依次加入33B(20.80g)、四氢呋喃(300mL)和水(6mL),搅拌溶解后,分批加入硼氢化钠(7.47g)。加毕室温搅拌反应。反应完全后,将反应液倒入冰水(2L)中,剧烈搅拌45分钟后,加入EA(1L),分相,水相用EA萃取,合并有机相,依次用水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。选用Chiral ART Cellulose-SC柱手性制备(流动性体系:正己烷93%,DCM/乙醇(3:1)7%等度洗脱)得到化合物33C(7.44g)。To the reaction flask, 33B (20.80 g), tetrahydrofuran (300 mL) and water (6 mL) were successively added at -15 ° C, and the mixture was stirred and dissolved, and sodium borohydride (7.47 g) was added portionwise. The reaction was stirred at room temperature. After the reaction was completed, the reaction mixture was poured into ice water (2 L). After stirring for 45 minutes, EA (1 L) was added, and the phases were separated, the aqueous phase was extracted with EA, and the organic phases were combined and washed sequentially with water and brine. Dry with sodium sulfate. Filter and concentrate the filtrate. Chiral ART Cellulose-SC column chiral preparation (mobile system: n-hexane 93%, DCM/ethanol (3:1) 7% isocratic elution) afforded compound 33C (7.44 g).
1H NMR(500MHz,DMSO-d
6)δ7.62(dd,J=5.5,8.5Hz,1H),7.47(dd,J=3.0,10.0Hz,1H),7.11-7.07(m,1H),6.03(d,J=8.0Hz,1H),4.70-4.65(m,1H),1.35(d,J=6.5Hz,3H),1.11(s,9H).UPC
2MS(ESI)m/z:344.07[M+Na]
+.
1 H NMR (500MHz, DMSO- d 6) δ7.62 (dd, J = 5.5,8.5Hz, 1H), 7.47 (dd, J = 3.0,10.0Hz, 1H), 7.11-7.07 (m, 1H), 6.03 (d, J = 8.0 Hz, 1H), 4.70 - 4.65 (m, 1H), 1.35 (d, J = 6.5 Hz, 3H), 1.11 (s, 9H). UPC 2 MS (ESI) m/z: 344.07[M+Na] + .
步骤3:(R)-1-(2-溴-5-氟苯基)乙-1-胺Step 3: (R)-1-(2-bromo-5-fluorophenyl)ethan-1-amine
向反应瓶中,依次加入33C(4.4274g)、碘(1g)、四氢呋喃(80mL)和水(8mL),50℃搅拌反应。反应完全后,浓缩反应液,得到化合物33D(5.88g)。未纯化直接投料下一步。To the reaction flask, 33 C (4.4274 g), iodine (1 g), tetrahydrofuran (80 mL) and water (8 mL) were sequentially added, and the mixture was stirred at 50 °C. After completion of the reaction, the reaction mixture was concentrated to give Compound 33D (5.88 g). The next step was directly fed without purification.
步骤4:(R)-5-((1-(2-溴-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸乙酯Step 4: (R)-5-((1-(2-Bromo-5-fluorophenyl)ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid ethyl ester
向含有33D(5.88g)的反应瓶中,依次加入5-氯吡唑并[1,5-a]嘧啶-3-羧酸乙酯(3.41g)、正丁醇(40mL)、DIPEA(8.89g),加毕,微波120℃搅拌反应。反应完全后,浓缩反应液,残留物用EA(300mL)溶解后,依次用水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(乙酸乙酯:石油醚=0:100~50:50)得到化合物33E(2.82g)。To a reaction flask containing 33D (5.88 g), ethyl 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (3.41 g), n-butanol (40 mL), DIPEA (8.89) were sequentially added. g), after the addition, the reaction was stirred at 120 ° C in a microwave. After the completion of the reaction, the reaction mixture was concentrated, and the residue was evaporated. Filter and concentrate the filtrate. Purification by column chromatography (ethyl acetate: petroleum ether = 0: 100 to 50: 50) gave Compound 33E (2.82 g).
1H NMR(400MHz,DMSO-d
6)8.59(d,J=6.0Hz,1H),8.45(d,J=4.8Hz,1H),8.14(s,1H),7.66(t,J=5.6Hz,1H),7.30(d,J=7.2Hz,1H),7.09(d,J=6.0Hz,1H),6.50(d,J=5.2Hz,1H),5.52(s,1H),4.20(t,J= 5.6Hz,1H),1.48(d,J=5.2Hz,3H),1.24(t,J=5.6Hz,3H).
13C NMR(100MHz,DMSO)163.2,162.8,156.6,148.0,146.8,136.5,134.9,117.1,116.4,114.3,101.5,98.8,59.2,50.0,21.4,15.1.MS(ESI)m/z:429.3[M+Na]
+.
1 H NMR (400 MHz, DMSO-d 6 ) 8.59 (d, J = 6.0 Hz, 1H), 8.45 (d, J = 4.8 Hz, 1H), 8.14 (s, 1H), 7.66 (t, J = 5.6 Hz) , 1H), 7.30 (d, J = 7.2 Hz, 1H), 7.09 (d, J = 6.0 Hz, 1H), 6.50 (d, J = 5.2 Hz, 1H), 5.52 (s, 1H), 4.20 (t , J = 5.6 Hz, 1H), 1.48 (d, J = 5.2 Hz, 3H), 1.24 (t, J = 5.6 Hz, 3H). 13 C NMR (100 MHz, DMSO) 163.2, 162.8, 156.6, 148.0, 146.8 , 136.5, 134.9, 117.1, 116.4, 114.3, 101.5, 98.8, 59.2, 50.0, 21.4, 15.1. MS (ESI) m/z: 429.3 [M+Na] + .
步骤5:5-(((R)-1-(2-(((1s,3S)-3-((叔丁氧基羰基)氨基)环丁基)氨基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-甲酸乙酯Step 5: 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)amino)-5-fluorophenyl) Ethyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylate
反应瓶中,依次加入33E(700mg)、顺式-3-氨基-1-环丁基氨基甲酸叔丁酯(480mg)、碘化亚铜(65.5mg)、N,N-二乙基水杨酰胺(133mg)、超干DMF(30mL)和磷酸三钾(1095mg),110℃搅拌反应。反应完全后,将反应液倒入乙酸乙酯(300mL)中,依次用水、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(乙酸乙酯:石油醚=0:100~35:65)得到化合物33F(0.236g)。In the reaction flask, 33E (700 mg), cis-3-amino-1-cyclobutylcarbamic acid tert-butyl ester (480 mg), cuprous iodide (65.5 mg), N,N-diethyl salicylate were sequentially added. The amide (133 mg), ultra dry DMF (30 mL) and tripotassium phosphate (1095 mg) were stirred at 110 °C. After the reaction was completed, the reaction mixture was poured into ethyl acetate (300 mL). Filter and concentrate the filtrate. Purification by column chromatography (ethyl acetate: petroleum ether = 0: 100 to 35: 65) gave Compound 33F (0.236 g).
1H NMR(500MHz,DMSO)δ8.54(d,J=7.5Hz,1H),8.21(d,J=8.0Hz,1H),8.15(s,1H),7.06(d,J=9.0Hz,1H),6.91-6.88(m,1H),8.80(d,J=5.0Hz,1H),6.45-6.44(m,1H),6.37(d,J=7Hz,1H),5.50(s,1H),5.10(s,1H),4.22(t,J=6.0Hz,2H),3.67-3.65(m,1H),3.42-3.41(m,1H),2.60-2.54(m,2H),1.65-1.63(m,2H),1.49(d,J=6.0Hz,3H),1.36(s,9H),1.27(t,J=7.0Hz,3H).MS(ESI)m/z:513.8[M+H]
+.
1 H NMR (500MHz, DMSO) δ8.54 (d, J = 7.5Hz, 1H), 8.21 (d, J = 8.0Hz, 1H), 8.15 (s, 1H), 7.06 (d, J = 9.0Hz, 1H), 6.91-6.88 (m, 1H), 8.80 (d, J = 5.0 Hz, 1H), 6.45-6.44 (m, 1H), 6.37 (d, J = 7 Hz, 1H), 5.50 (s, 1H) , 5.10 (s, 1H), 4.22 (t, J = 6.0 Hz, 2H), 3.67-3.65 (m, 1H), 3.42-3.41 (m, 1H), 2.60-2.54 (m, 2H), 1.65-1.63 (m, 2H), 1.49 (d, J = 6.0 Hz, 3H), 1.36 (s, 9H), 1.27 (t, J = 7.0 Hz, 3H). MS (ESI) m/z: 513.8 [M+H ] + .
步骤6:5-(((R)-1-(2-(((1s,3S)-3-((叔丁氧基羰基)氨基)环丁基)氨基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸Step 6: 5-(((R)-1-(2-(((s)))))((tert-butoxycarbonyl)amino)cyclobutyl)amino)-5-fluorophenyl) Amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
向含有33F(226mg)的反应瓶中,依次加入乙醇(2mL)、四氢呋喃(2mL)、水(0.5mL)和氢氧化锂一水合物(185mg),80℃搅拌反应。反应完全后,将反应液倒入冰水(100mL)中,缓慢加入稀盐酸(0.5M)调节pH至3-4,用DCM萃取,合并DCM相,无水硫酸钠干燥。过滤,滤液浓缩得到化合物33G(201mg)。未纯化直接下一步反应。To a reaction flask containing 33F (226 mg), ethanol (2 mL), tetrahydrofuran (2 mL), water (0.5 mL) and lithium hydroxide monohydrate (185 mg) were sequentially added, and the mixture was stirred at 80 °C. After the reaction was completed, the reaction mixture was poured into ice water (100 mL), EtOAc (EtOAc) Filtration and concentration of the filtrate gave Compound 33G (201 mg). The next reaction was not directly purified.
步骤7:5-(((R)-1-(2-(((1s,3S)-3-氨基环丁基)氨基)-5-氟苯基)乙基)氨基)吡唑并[1,5-a]嘧啶-3-羧酸三盐酸盐Step 7: 5-(((R)-1-(2-(((s))))))))))))))) , 5-a]pyrimidine-3-carboxylic acid trihydrochloride
向含有33G(201mg)的反应瓶中加入氯化氢的1,4-二氧六环溶液(4N,30mL),50℃搅拌反应。反应完全后,浓缩反应液,得到化合物33H(205mg)。未纯化直接下一步反应。To a reaction flask containing 33 G (201 mg), a 1,4-dioxane solution (4N, 30 mL) of hydrogen chloride was added, and the mixture was stirred at 50 °C. After the reaction was completed, the reaction mixture was concentrated to give Compound 33H (205mg). The next reaction was not directly purified.
步骤8:(3
1S,3
3S,6
3E,6
4E,8R)-1
4-氟-8-甲基-2,4,7-三氮杂-6(3,5)-吡唑并[1,5-a]嘧啶杂-1(1,2)-苯杂-3(1,3)-环丁杂辛内酰胺-5-酮
Step 8: (3 1 S,3 3 S,6 3 E,6 4 E,8R)-1 4 -Fluoro-8-methyl-2,4,7-triaza-6(3,5)- Pyrazolo[1,5-a]pyrimidine-1(1,2)-benzene-3(1,3)-cyclobutanooctanoic acid-5-one
向含有33H(205mg)的反应瓶中,依次加入DCM(60mL)、DMF(6mL)和DIPEA(449mg),室温搅拌下,加入五氟苯基二苯基膦酸酯(175mg)。加毕室温搅拌反应。反应完全后,浓缩反应液,向残留物溶于EA后(200mL),依次用2M碳酸钠水溶液、水洗涤,饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液浓缩。柱层析纯化(MeOH:DCM=0:100~2.5:97.5)得化合物I-33(52mg)。To a reaction flask containing 33H (205 mg), DCM (60 mL), DMF (6 mL) and DIPEA (449 mg) were successively added, and, under stirring, pentafluorophenyldiphenylphosphonate (175 mg) was added. The reaction was stirred at room temperature. After the completion of the reaction, the reaction mixture was evaporated, evaporated, evaporated, evaporated Filter and concentrate the filtrate. Purification by column chromatography (MeOH: EtOAc:EtOAc:EtOAc:
1H NMR(500MHz,DMSO-d
6)δ9.37(d,J=7.5Hz,1H),8.72(d,J=7.0Hz,1H),8.53(d,J=7.5Hz,1H),8.02(s,1H),7.06(dd,J=2.0,10.0Hz,1H),6.88-6.85(m,1H),6.36(d,J=7.5Hz,1H),6.20(dd,J=5.0,9.0Hz,1H),5.86(s,1H),5.54-5.52(m,1H),4.68-4.67(m,1H),3.96(d,J=3.5Hz,1H),2.89-2.84(m,1H),2.60-2.56(m,1H),2.09-2.05(m,1H),1.45(d,J=6.5Hz,3H).
13C NMR(125MHz,DMSO-d
6)δ160.89,156.86,155.55,146.44,144.48,139.67,136.36,135.12,114.37,113.76,113.33,101.85,100.92,48.62,44.49,43.54,35.57,35.04,23.27.MS(ESI)m/z:367.19[M+H]
+.
1 H NMR (500MHz, DMSO- d 6) δ9.37 (d, J = 7.5Hz, 1H), 8.72 (d, J = 7.0Hz, 1H), 8.53 (d, J = 7.5Hz, 1H), 8.02 (s, 1H), 7.06 (dd, J = 2.0, 10.0 Hz, 1H), 6.88-6.85 (m, 1H), 6.36 (d, J = 7.5 Hz, 1H), 6.20 (dd, J = 5.0, 9.0 Hz, 1H), 5.86 (s, 1H), 5.54-5.52 (m, 1H), 4.68-4.67 (m, 1H), 3.96 (d, J = 3.5 Hz, 1H), 2.89-2.84 (m, 1H) , 2.60-2.56 (m, 1H), 2.09-2.05 (m, 1H), 1.45 (d, J = 6.5 Hz, 3H). 13 C NMR (125 MHz, DMSO-d 6 ) δ 160.89, 156.86, 155.55, 146.44, 144.48, 139.67, 136.36, 135.12, 114.37, 113.76, 113.33, 101.85, 100.92, 48.62, 44.49, 43.54, 35.57, 35.04, 23.27. MS (ESI) m/z: 367.19 [M+H] + .
试验例1:体外酶活性Test Example 1: In vitro enzyme activity
1.1 EML4-ALK抑制活性1.1 EML4-ALK inhibitory activity
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的EML4-ALK母液进行稀释,按每孔加入6μL 1.67×的0.4ng/μL的工作液(终浓度为0.24ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.24nM,4倍梯度,共7个浓度,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO),设2个复孔。酶与化合物或溶媒反应60min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,ULight-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL 4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eu-anti-phospho-tyrosine antibody),室温孵育1小时,Envision仪器读板(激发320nm,发射665nm),结果经四参数拟合计算IC
50。结果如表1所示。
50 ng/μL of EML4-ALK mother solution was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.4 ng/μL working solution was added per well. (final concentration was 0.24 ng/μL), and different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.24nM, 4 times gradient, a total of 7 concentrations, and a blank control Hole (without enzyme) and negative control well (containing enzyme, plus solvent DMSO), set 2 duplicate wells. After reacting the enzyme with the compound or vehicle for 60 min, 5×50 μM ATP (final concentration 10 μM) prepared with kinase buffer and 5×0.5 μM substrate (final concentration 0.1 μM, ULight-poly GT) were pressed. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration 10 mM) to each well, room temperature for 5 min, and add 5 μL per well. The 8 nM detection reagent (final concentration of 2 nM, Eu-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature, and the Envision instrument was read (excitation 320 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 1.
1.2 ALK(G1202R)抑制活性1.2 ALK (G1202R) inhibitory activity
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的ALK(G1202R)母液进行稀释,按每孔加入6μL 1.67×的0.167ng/uL的工作液(终浓度为0.1ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.24nM,4倍梯度,共7个浓度,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应60min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,ULight-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL 4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eu-anti-phospho-tyrosine antibody),室温孵育1小时;Envision仪器读板(激发320nm,发射665nm),结果经四参数拟合计算IC
50。结果如表2所示。
50 ng/μL of ALK (G1202R) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.167 ng/uL was added per well. Liquid (final concentration 0.1 ng / μL), the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.24nM, 4 times gradient, a total of 7 concentrations, and set a blank Control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 60 min, 5×50 μM ATP (final concentration 10 μM) prepared with kinase buffer and 5×0.5 μM substrate (final concentration 0.1 μM, ULight-poly GT) were pressed. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration 10 mM) to each well, room temperature for 5 min, and add 5 μL per well. The 8 nM detection reagent (final concentration 2 nM, Eu-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; Envision instrument read plate (excitation 320 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 2.
1.3 ALK(C1156Y)抑制活性1.3 ALK (C1156Y) inhibitory activity
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的ALK(C1156Y)母液进行稀释,按每孔加入6μL 1.67×的0.167ng/μL的工作液(终浓度为0.1ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.24nM,4倍梯度,共7个浓度,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应60min后,将用激酶缓冲液配制好的5×的10μM ATP(终浓度为2μM)与5×的0.5μM底物(终浓度为0.1μM,ULight-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL 4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eu-anti-phospho-tyrosine antibody),室温孵育1小时;Envision仪器读板(激发320nm,发射665nm),结果经四参数拟合计算IC
50。结果如表2所示。
50 ng/μL of ALK (C1156Y) mother solution was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.167 ng/μL was added per well. Liquid (final concentration 0.1 ng / μL), the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.24nM, 4 times gradient, a total of 7 concentrations, and set a blank Control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 60 min, 5×10 μM ATP (final concentration 2 μM) prepared with kinase buffer and 5×0.5 μM substrate (final concentration 0.1 μM, ULight-poly GT) were pressed. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration 10 mM) to each well, room temperature for 5 min, and add 5 μL per well. The 8 nM detection reagent (final concentration 2 nM, Eu-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; Envision instrument read plate (excitation 320 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 2.
1.4 ALK(G1269A)抑制活性筛选1.4 ALK (G1269A) inhibition activity screening
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的ALK(G1269A)母液进行稀释,按每孔加入6μL 1.67×的0.005ng/μL的工作液(终浓度为0.003ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.244nM,共7个浓度梯度,4倍稀释,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应30min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,U Light-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL 4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eμ-anti-phospho-tyrosine antibody),室温孵育1小时;PE Envision多功能酶标仪进行读板(激发320nm,发射665nm),采用四参数拟合,计算IC
50。结果如表2所示。
50 ng/μL of ALK (G1269A) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.005 ng/μL was added per well. The liquid (final concentration is 0.003 ng / μL), the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and at the same time Blank control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 30 min, 5×50 μM ATP (final concentration 10 μM) and 5×0.5 μM substrate (final concentration 0.1 μM, U Light-poly GT) prepared in kinase buffer were used. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration 10 mM) to each well, room temperature for 5 min, then add 5 μL per well. 4× 8nM detection reagent (final concentration 2nM, Eμ-anti-phospho-tyrosine antibody), incubate for 1 hour at room temperature; PE Envision multi-function microplate reader for reading plate (excitation 320nm, emission 665nm), four-parameter fit , calculate the IC 50 . The results are shown in Table 2.
1.5 ALK(F1174L)抑制活性筛选1.5 ALK (F1174L) inhibition activity screening
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的ALK(F1174L)母液进行稀释,按每孔加入6μL 1.67×的0.005ng/μL的工作液(终浓度为0.003ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.244nM,共7个浓度梯度,4倍稀释,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应30min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,U Light-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL 4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eμ-anti-phospho-tyrosine antibody),室温孵育1小时;PE Envision多功能酶标仪进行读板(激发320nm,发射665nm),采用四参数拟合,计算IC
50。结果如表2所示。
50 ng/μL of ALK (F1174L) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.005 ng/μL was added per well. The liquid (final concentration is 0.003 ng / μL), the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and at the same time Blank control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 30 min, 5×50 μM ATP (final concentration 10 μM) and 5×0.5 μM substrate (final concentration 0.1 μM, U Light-poly GT) prepared in kinase buffer were used. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration 10 mM) to each well, room temperature for 5 min, then add 5 μL per well. 4× 8nM detection reagent (final concentration 2nM, Eμ-anti-phospho-tyrosine antibody), incubate for 1 hour at room temperature; PE Envision multi-function microplate reader for reading plate (excitation 320nm, emission 665nm), four-parameter fit , calculate the IC 50 . The results are shown in Table 2.
1.6 ALK(R1275Q)抑制活性筛选1.6 ALK (R1275Q) inhibition activity screening
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的ALK(R1275Q)母液进行稀释,按每孔加入6μL 1.67×的0.01ng/μL的工作液(终浓度为0.006ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.244nM,共7个浓度梯度,4倍稀释,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或 溶媒反应30min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,U Light-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL 4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eμ-anti-phospho-tyrosine antibody),室温孵育1小时;PE Envision多功能酶标仪进行读板(激发320nm,发射665nm),采用四参数拟合,计算IC
50。结果如表2所示。
50 ng/μL of ALK (R1275Q) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.01 ng/μL was added per well. The liquid (final concentration is 0.006 ng/μL), the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and at the same time Blank control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 30 min, 5×50 μM ATP (final concentration 10 μM) and 5×0.5 μM substrate (final concentration 0.1 μM, U Light-poly GT) prepared in kinase buffer were used. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration 10 mM) to each well, room temperature for 5 min, then add 5 μL per well. 4× 8nM detection reagent (final concentration 2nM, Eμ-anti-phospho-tyrosine antibody), incubate for 1 hour at room temperature; PE Envision multi-function microplate reader for reading plate (excitation 320nm, emission 665nm), four-parameter fit , calculate the IC 50 . The results are shown in Table 2.
1.7 ALK(L1196M)抑制活性筛选1.7 ALK (L1196M) inhibition activity screening
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的ALK(L1196M)母液进行稀释,按每孔加入6μL 1.67×的0.00668ng/μL的工作液(终浓度为0.004ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.244nM,共7个浓度梯度,4倍稀释,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应30min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,U Light-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL 4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eμ-anti-phospho-tyrosine antibody),室温孵育1小时;PE Envision多功能酶标仪进行读板(激发320nm,发射665nm),采用四参数拟合,计算IC
50。结果如表2所示。
50 ng/μL of ALK (L1196M) mother liquor was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.00668 ng/μL was added per well. The liquid (final concentration is 0.004 ng/μL), the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and at the same time Blank control wells (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 30 min, 5×50 μM ATP (final concentration 10 μM) and 5×0.5 μM substrate (final concentration 0.1 μM, U Light-poly GT) prepared in kinase buffer were used. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration 10 mM) to each well, room temperature for 5 min, then add 5 μL per well. 4× 8nM detection reagent (final concentration 2nM, Eμ-anti-phospho-tyrosine antibody), incubate for 1 hour at room temperature; PE Envision multi-function microplate reader for reading plate (excitation 320nm, emission 665nm), four-parameter fit , calculate the IC 50 . The results are shown in Table 2.
1.8 TRKA抑制活性1.8 TRKA inhibitory activity
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的TRKA母液进行稀释,按每孔加入6μL 1.67×的0.0334ng/μL的工作液(终浓度为0.02ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.244nM,共7个浓度梯度,4倍稀释,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应60min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,U Light-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eμ-anti-phospho-tyrosine antibody),室温孵育1小时;Envision仪器读板(激发320or 340nm,发射665nm),结果经四参数拟合计算IC
50。结果如表3所示。
50 ng/μL of the TRKA stock solution was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.0334 ng/μL working solution was added per well (final The concentration was 0.02 ng/μL. The different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and blank control wells. (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 60 min, 5×50 μM ATP (final concentration 10 μM) and 5×0.5 μM substrate (final concentration 0.1 μM, U Light-poly GT) prepared in kinase buffer were used. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after reacting for 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration of 10 mM) to each well, room temperature for 5 min, and add 5 μL per well. The 8 nM detection reagent (final concentration 2 nM, Eμ-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; the Envision instrument was read (excitation 320 s 340 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 3.
1.9 TRKB抑制活性1.9 TRKB inhibitory activity
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的TRKB母液进行稀释,按每孔加入6μL 1.67×的0.001ng/μL的工作液(终浓度为0.0006ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.244nM,共7个浓度梯度,4倍稀释,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应60min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,U Light-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL 4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eμ-anti-phospho-tyrosine antibody),室温孵育1小时;Envision仪器读板(激发320or340nm,发射665nm),结果经四参数拟合计算IC
50。结果如表3所示。
50 ng/μL of the TRKB mother solution was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67× 0.001 ng/μL working solution was added per well (final The concentration was 0.0006 ng/μL. The different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and blank control wells. (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 60 min, 5×50 μM ATP (final concentration 10 μM) and 5×0.5 μM substrate (final concentration 0.1 μM, U Light-poly GT) prepared in kinase buffer were used. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration 10 mM) to each well, room temperature for 5 min, then add 5 μL per well. 4× 8 nM detection reagent (final concentration 2 nM, Eμ-anti-phospho-tyrosine antibody), incubate for 1 hour at room temperature; Envision instrument reads plate (excitation 320 340 nm, emission 665 nm), and the IC 50 is calculated by four-parameter fitting. The results are shown in Table 3.
1.10 TRKC抑制活性1.10 TRKC inhibitory activity
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的TRKC母液进行稀释,按每孔加入6μL 1.67×的0.1336ng/μL的工作液(终浓度为0.08ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.244nM,共7个浓度梯度,4倍稀释,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应60min后,将用激酶缓冲液配制好的5×的50μM ATP(终浓度为10μM)与5×的0.5μM底物(终浓度为0.1μM,U Light-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eμ-anti-phospho-tyrosine antibody),室温孵育1小时;Envision仪器读板(激发320或340nm,发射665nm),结果经四参数拟合计算IC
50。结果如表3所示。
50 ng/μL of TRKC stock solution was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.1336 ng/μL working solution was added per well (final The concentration was 0.08 ng/μL), and the different compounds dissolved in DMSO were added to the wells with a nanoliter sampler to make the final concentration of the compound 1000nM-0.244nM, a total of 7 concentration gradients, 4 times dilution, and blank control wells. (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 60 min, 5×50 μM ATP (final concentration 10 μM) and 5×0.5 μM substrate (final concentration 0.1 μM, U Light-poly GT) prepared in kinase buffer were used. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after reacting for 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration of 10 mM) to each well, room temperature for 5 min, and add 5 μL per well. The 8 nM detection reagent (final concentration 2 nM, Eμ-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; the Envision instrument was read (excitation 320 or 340 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fit. The results are shown in Table 3.
1.11 ROS1抑制活性1.11 ROS1 inhibitory activity
用激酶缓冲液(50mM HEPES、10mM MgCl
2、2mM DTT、1mM EGTA、0.01%Tween 20)对50ng/μL的ROS1母液进行稀释,按每孔加入6μL 1.67×的0.05ng/μL的工作液(终浓度为0.03ng/μL),用纳升加样仪将DMSO溶解的不同化合物加入到孔中,使化合物终浓度为1000nM-0.244nM,共7个浓度梯度,4倍稀释,同时设空白对照孔(不含酶)与阴性对照孔(含酶,加溶媒DMSO)。酶与化合物或溶媒反应30min后,将用激酶缓冲液配制好的5×的250μM ATP(终浓度为50μM)与5×的0.5μM底物(终浓度为0.1μM,U Light-poly GT),按1:1混合后按每孔4μL加入孔中;封板膜封板以后,室温反应2h后,每孔加入5μL4×的40mM EDTA(终浓度为10mM),室温5min,再每孔加入5μL 4×的8nM检测试剂(终浓度为2nM,Eμ-anti-phospho-tyrosine antibody),室温孵育1小时;Envision仪器读板(激发320or 340nm,发射665nm),结果经四参数拟合计算IC
50。结果如表4所示。
50 ng/μL of ROS1 stock solution was diluted with kinase buffer (50 mM HEPES, 10 mM MgCl 2 , 2 mM DTT, 1 mM EGTA, 0.01% Tween 20), and 6 μL of 1.67×0.05 ng/μL working solution was added per well (final At a concentration of 0.03 ng/μL, different compounds dissolved in DMSO were added to the wells using a nanoliter sampler to give a final concentration of 1000 nM-0.244 nM, a total of 7 concentration gradients, 4 fold dilutions, and blank control wells. (without enzyme) and negative control wells (containing enzyme, plus vehicle DMSO). After reacting the enzyme with the compound or vehicle for 30 min, 5×250 μM ATP (final concentration 50 μM) and 5×0.5 μM substrate (final concentration 0.1 μM, U Light-poly GT) prepared with kinase buffer were used. After mixing 1:1, add 4 μL per well to the wells; after sealing the membrane, after reacting for 2 h at room temperature, add 5 μL of 4×40 mM EDTA (final concentration of 10 mM) to each well, room temperature for 5 min, and add 5 μL per well. The 8 nM detection reagent (final concentration 2 nM, Eμ-anti-phospho-tyrosine antibody) was incubated for 1 hour at room temperature; the Envision instrument was read (excitation 320 s 340 nm, emission 665 nm), and the IC 50 was calculated by four-parameter fitting. The results are shown in Table 4.
表1 体外酶活性IC
50(nM)
Table 1 In vitro enzyme activity IC 50 (nM)
| 化合物Compound | EML4-ALKEML4-ALK | 化合物Compound | EML4-ALKEML4-ALK | 化合物Compound | EML4-ALKEML4-ALK |
| I-1I-1 | AA | I-7I-7 | CC | I-25-1I-25-1 | AA |
| I-2I-2 | CC | I-8I-8 | AA | I-25-2I-25-2 | AA |
| I-3I-3 | BB | I-9I-9 | CC | I-26I-26 | AA |
| I-5I-5 | BB | I-11I-11 | CC | I-27I-27 | AA |
| I-6I-6 | AA | I-18I-18 | CC | I-28I-28 | AA |
| I-4I-4 | AA | I-19I-19 | AA | I-29I-29 | AA |
| I-13I-13 | AA | I-20I-20 | AA | I-30I-30 | AA |
| I-14I-14 | AA | I-21I-21 | AA | I-31I-31 | AA |
| I-15I-15 | AA | I-22I-22 | CC | I-32I-32 | AA |
| I-16I-16 | AA | I-23I-23 | AA | I-33I-33 | AA |
| I-17I-17 | BB | I-24I-24 | BB |
注:表中“A”表示化合物针对EML4-ALK活性IC
50≤15nM;“B”表示15<IC
50≤50nM;“C”表示50<IC
50≤500nM;“D”表示IC
50>500nM。
Note: "A" in the table indicates that the compound has an IC 50 ≤ 15 nM for EML4-ALK activity; "B" indicates 15 < IC 50 ≤ 50 nM; "C" indicates 50 < IC 50 ≤ 500 nM; "D" indicates an IC 50 > 500 nM.
表2 体外酶活性IC
50(nM)
Table 2 In vitro enzyme activity IC 50 (nM)
| 化合物Compound | ALK G1202RALK G1202R | ALK C1156YALK C1156Y | ALK R1275QALK R1275Q | ALK F1174LALK F1174L | ALK L1196MALK L1196M | ALK G1269AALK G1269A |
| I-1I-1 | AA | AA | AA | AA | AA | AA |
| I-2I-2 | CC | |||||
| I-3I-3 | BB | BB | BB | |||
| I-4I-4 | CC | BB | ||||
| I-5I-5 | CC | CC | BB | BB | BB | CC |
| I-6I-6 | BB | BB | AA | AA | AA | BB |
| I-7I-7 | BB | |||||
| I-8I-8 | BB | BB | AA | |||
| I-9I-9 | BB |
| I-11I-11 | CC | |||||
| I-13I-13 | AA | AA | AA | AA | AA | BB |
| I-14I-14 | AA | AA | AA | AA | AA | BB |
| I-15I-15 | AA | AA | AA | AA | AA | |
| I-16I-16 | AA | AA | AA | BB | BB | |
| I-17I-17 | CC | |||||
| I-18I-18 | CC | |||||
| I-19I-19 | AA | AA | BB | BB | BB | BB |
| I-20I-20 | AA | AA | AA | AA | AA | BB |
| I-21I-21 | AA | AA | AA | AA | AA | BB |
| I-22I-22 | CC | |||||
| I-23I-23 | AA | AA | AA | AA | BB | |
| I-24I-24 | BB | BB | BB | BB | BB | |
| I-25-1I-25-1 | AA | AA | AA | AA | BB | |
| I-25-2I-25-2 | AA | AA | AA | BB | BB | |
| I-26I-26 | BB | AA | AA | BB | BB | BB |
| I-27I-27 | AA | AA | AA | AA | AA | BB |
| I-28I-28 | AA | AA | AA | AA | AA | BB |
| I-29I-29 | BB | AA | AA | BB | AA | BB |
| I-30I-30 | AA | AA | AA | BB | AA | BB |
| I-31I-31 | AA | AA | AA | AA | AA | BB |
| I-32I-32 | AA | AA | AA | BB | BB | BB |
| I-33I-33 | BB | BB | BB |
注:表中“A”表示化合物针对ALK G1202R、ALK C1156Y、ALK R1275Q、ALK F1174L、ALK L1196M及ALK G1269A活性IC
50≤5nM;“B”表示5<IC
50≤50nM;“C”表示50<IC
50≤500nM;“D”表示IC
50>500nM。
Note: "A" in the table indicates that the compound has an IC 50 ≤ 5 nM for ALK G1202R, ALK C1156Y, ALK R1275Q, ALK F1174L, ALK L1196M and ALK G1269A; "B" means 5 < IC 50 ≤ 50 nM; "C" means 50 < IC 50 ≤ 500 nM; "D" indicates an IC 50 > 500 nM.
表3 体外酶活性IC
50(nM)
Table 3 In vitro enzyme activity IC 50 (nM)
| 化合物Compound | TRKATRKA | TRKBTRKB | TRKCTRKC | 化合物Compound | TRKATRKA | TRKBTRKB | TRKCTRKC |
| I-1I-1 | BB | BB | BB | I-19I-19 | BB | AA | BB |
| I-2I-2 | CC | CC | CC | I-20I-20 | AA | AA | AA |
| I-3I-3 | BB | BB | BB | I-21I-21 | AA | AA | AA |
| I-4I-4 | AA | AA | I-22I-22 | BB | BB | ||
| I-5I-5 | AA | AA | BB | I-23I-23 | BB | AA | BB |
| I-6I-6 | BB | AA | BB | I-24I-24 | BB | AA | BB |
| I-7I-7 | CC | CC | CC | I-25-1I-25-1 | AA | AA | BB |
| I-8I-8 | BB | AA | BB | I-25-2I-25-2 | BB | AA | BB |
| I-9I-9 | BB | BB | CC | I-26I-26 | AA | AA | BB |
| I-11I-11 | BB | BB | CC | I-27I-27 | AA | AA | BB |
| I-13I-13 | AA | AA | AA | I-28I-28 | AA | AA | AA |
| I-14I-14 | AA | AA | AA | I-29I-29 | AA | AA | AA |
| I-15I-15 | AA | AA | AA | I-30I-30 | AA | AA | AA |
| I-16I-16 | BB | BB | BB | I-31I-31 | AA | AA | AA |
| I-17I-17 | BB | BB | I-32I-32 | AA | AA | BB | |
| I-18I-18 | CC | BB |
注:表中“A”表示化合物针对TRKA、TRKB及TRKC的活性IC
50≤0.25nM;“B”表示0.25<IC
50≤10nM;“C”表示10<IC
50≤500nM;“D”表示IC
50>500nM。
Note: "A" in the table indicates that the compound has an activity IC 50 ≤ 0.25 nM for TRKA, TRKB and TRKC; "B" indicates 0.25 < IC 50 ≤ 10 nM; "C" indicates 10 < IC 50 ≤ 500 nM; "D" indicates IC 50 > 500nM.
表4 体外酶活性IC
50(nM)
Table 4 In vitro enzyme activity IC 50 (nM)
| 化合物Compound | ROS1ROS1 |
| I-1I-1 | AA |
| I-3I-3 | CC |
注:表中“A”表示化合物针对ROS1活性IC
50≤10nM;“B”表示10<IC
50≤50nM;“C”表示50<IC
50≤500nM;“D”表示IC
50>500nM。
Note: "A" in the table indicates that the compound has an IC 50 ≤ 10 nM for ROS1 activity; "B" indicates 10 < IC 50 ≤ 50 nM; "C" indicates 50 < IC 50 ≤ 500 nM; "D" indicates an IC 50 > 500 nM.
试验例2:体外细胞活性Test Example 2: In vitro cell viability
2.1 对NCI-H2228细胞的增殖抑制作用2.1 inhibition of proliferation of NCI-H2228 cells
取处于指数生长期状态良好的NCI-H2228细胞,加入3mL PBS清洗一遍,加入2mL胰酶。放入细胞培养箱中进行消化,不时取出显微镜下观察,待细胞刚脱落时,加入3mL完全培养基终止消化。低速台式离心机,1500转/min,离心3min。倒掉细胞消化液,用移液器加入5mL种板培养基(RPMI培养基+丙酮酸钠+5%FBS)进行细胞重悬。使用细胞计数仪计数,用种板培养基进行稀释,调整细胞密度至6×10
4cells/mL。使用排枪接种于96孔板上,100μL/孔,置恒温CO
2培养箱中培养24小时。使用纳升加样仪加化合物,72h后,加CCK-8,10μL/孔,4小时后Envision酶标仪450nm处检测其吸光值,计算抑制率,抑制率(%)=(阴性对照组平均值—实验组平均值)/(阴性对照组平均值—空白组平均值)×100%,以化合物浓度对数为横坐标,抑制率为纵坐标,四参数分析,拟合量效曲线,计算IC
50,结果见表5-1。
NCI-H2228 cells in good exponential growth phase were taken, washed once with 3 mL of PBS, and 2 mL of trypsin was added. Digestion is carried out in a cell culture incubator, and it is observed under a microscope from time to time. When the cells have just fallen off, 3 mL of complete medium is added to terminate the digestion. Low speed bench top centrifuge, 1500 rpm, centrifuged for 3 min. The cell digest was drained, and the cells were resuspended by pipetting with 5 mL of plate medium (RPMI medium + sodium pyruvate + 5% FBS). The cells were counted using a cytometer, diluted with a seed plate medium, and the cell density was adjusted to 6 × 10 4 cells/mL. The cells were seeded in a 96-well plate using a lance, 100 μL/well, and cultured in a constant temperature CO 2 incubator for 24 hours. The compound was added using a nanoliter sampler. After 72 hours, CCK-8 was added, 10 μL/well. After 4 hours, the absorbance was measured at 450 nm from the Envision plate reader, and the inhibition rate was calculated. The inhibition rate (%) = (negative control group average) Value—average of experimental group)/(average of negative control group—average of blank group)×100%, with logarithm of compound concentration as abscissa, inhibition rate as ordinate, four-parameter analysis, fitting dose-effect curve, calculation IC 50 , the results are shown in Table 5-1.
2.2 对Karpas299细胞的增殖抑制作用2.2 inhibition of proliferation of Karpas299 cells
取处于指数生长期状态良好的Karpas299细胞一皿,收集细胞至离心管,低速台式离心机,1500转/min,离心3min,弃上清,用移液器加入10mL种板培养基(RPMI培养基+5%FBS)进行细胞重悬。使用细胞计数仪计数,用种板培养基进行稀释,调整细胞密度至6×10
4个/mL。使用排枪接种于96孔板上,100μL/孔,置于37℃、含5%CO
2饱和湿度的细胞培养箱中培养。培养24h后,使用纳升加样仪进行化合物加样,每一浓度设置2个复孔,以不加化合物的细胞作为阴性对照,72小时后加CCK-8,10μL/孔,4小时后Envision酶标仪450nm处检测其吸光值,计算抑制率,抑制率(%)=(阴性对照组平均值—实验组平均值)/(阴性对照组平均值—空白组平均值)×100%,以化合物浓度对数为横坐标,抑制率为纵坐标,四参数分析,拟合量效曲线,计算IC
50,结果见表5-2。
Take a dish of Karpas299 cells in good exponential growth phase, collect the cells into a centrifuge tube, centrifuge at 1500 rpm, centrifuge for 3 min, discard the supernatant, and add 10 mL of plate medium (RPMI medium) with a pipette. +5% FBS) was used to resuspend the cells. The cells were counted using a cytometer, diluted with a seed plate medium, and the cell density was adjusted to 6 × 10 4 /mL. The cells were seeded in a 96-well plate using a lance, 100 μL/well, and cultured in a cell incubator at 37 ° C, 5% CO 2 saturated humidity. After 24 hours of culture, compound loading was performed using a nanoliter sampler. Two replicate wells were set at each concentration, and cells without compound were used as a negative control. After 72 hours, CCK-8, 10 μL/well was added, and Envision was added 4 hours later. The absorbance value was measured at 450 nm, and the inhibition rate was calculated. The inhibition rate (%) = (negative control group mean - experimental group average) / (negative control group mean - blank group mean) × 100%, The logarithm of the compound concentration is the abscissa, the inhibition rate is the ordinate, the four-parameter analysis, the fitting dose-effect curve, and the IC 50 is calculated. The results are shown in Table 5-2.
2.3 对EML4-ALK L1196M BaF3细胞的增殖抑制作用2.3 Proliferation inhibition of EML4-ALK L1196M BaF3 cells
取处于指数生长期状态良好的EML4-ALK L1196M BaF3细胞一瓶,收集细胞至离心管,1000转/min,离心3min,弃上清,用种板培养基(RPMI培养基+10%FBS)重悬细胞。使用细胞计数仪计数,用种板培养基进行稀释,调整细胞密度至1×10
4个/mL。使用排枪接种于96孔板上,100μL/孔,置于37℃、含5%CO
2饱和湿度的细胞培养箱中培养。培养24h后,使用纳升加样仪进行化合物加样,每一浓度设置2个复孔,以不加化合物的细胞作为阴性对照,72小时后加CCK-8,10μL/孔,2小时后Envision酶标仪450nm处检测其吸光值,计算抑制率,抑制率(%)=(阴性对照组平均值-实验组平均值)/(阴性对照组平均值)×100%,以化合物浓度对数为横坐标,抑制率为纵坐标,四参数分析,拟合量效曲线,计算IC
50,结果见表5-3。
Take a bottle of EML4-ALK L1196M BaF3 cells in good exponential growth phase, collect the cells into a centrifuge tube, centrifuge at 1000 rpm, centrifuge for 3 min, discard the supernatant, and use plate medium (RPMI medium + 10% FBS) Suspended cells. The cells were counted using a cytometer, diluted with a seed plate medium, and the cell density was adjusted to 1 × 10 4 /mL. The cells were seeded in a 96-well plate using a lance, 100 μL/well, and cultured in a cell incubator at 37 ° C, 5% CO 2 saturated humidity. After 24 hours of culture, compound loading was performed using a nanoliter sampler. Two replicate wells were set at each concentration, and cells without compound were used as a negative control. After 72 hours, CCK-8, 10 μL/well was added, and Envision was added 2 hours later. The absorbance value was measured at 450 nm, and the inhibition rate was calculated. The inhibition rate (%) = (negative control group mean - experimental group mean) / (negative control group mean) × 100%, with the compound concentration logarithm The abscissa, the inhibition rate is the ordinate, the four-parameter analysis, the fitting dose-effect curve, and the IC 50 is calculated. The results are shown in Table 5-3.
2.4 对TFG-NTRK1 BaF3细胞的增殖抑制作用2.4 Proliferation inhibition of TFG-NTRK1 BaF3 cells
取处于指数生长期状态良好的TFG-NTRK1 BaF3细胞一瓶,收集细胞至离心管,1000转/min,离心3min,弃上清,用种板培养基(RPMI培养基+10%FBS)重悬细胞。使用细胞计数仪计数,用种板培养基进行稀释,调整细胞密度至5×10
4个/mL。使用排枪接种于96孔板上,100μL/孔,置于37℃、含5%CO
2饱和湿度的细胞培养箱中培养。培养24h后,使用纳升加样仪进行化合物加样,每一浓度设置2个复孔,以不加化合物的细胞作为阴性对照,72小时后加CCK-8,10μL/孔,2小时后Envision酶标仪450nm处检测其吸光值,计算抑制率,抑制率(%)=(阴性对照组平均值-实验组平均值)/(阴性对照组平均值)×100%,以化合物浓度对数为横坐标,抑制率为纵坐标,四参数分析,拟合量效曲线,计算IC
50,结果见表5-4。
Take a bottle of TFG-NTRK1 BaF3 cells in good exponential growth phase, collect the cells into a centrifuge tube, centrifuge at 1000 rpm, centrifuge for 3 min, discard the supernatant, and resuspend with plate medium (RPMI medium + 10% FBS). cell. The cells were counted using a cytometer, diluted with a seed plate medium, and the cell density was adjusted to 5 × 10 4 /mL. The cells were seeded in a 96-well plate using a lance, 100 μL/well, and cultured in a cell incubator at 37 ° C, 5% CO 2 saturated humidity. After 24 hours of culture, compound loading was performed using a nanoliter sampler. Two replicate wells were set at each concentration, and cells without compound were used as a negative control. After 72 hours, CCK-8, 10 μL/well was added, and Envision was added 2 hours later. The absorbance value was measured at 450 nm, and the inhibition rate was calculated. The inhibition rate (%) = (negative control group mean - experimental group mean) / (negative control group mean) × 100%, with the compound concentration logarithm The abscissa, the inhibition rate is the ordinate, the four-parameter analysis, the fitting dose-effect curve, and the IC 50 is calculated. The results are shown in Table 5-4.
表5-1 体外细胞活性IC
50(nM)
Table 5-1 In vitro cell activity IC 50 (nM)
| 化合物Compound | NCI-H2228NCI-H2228 | 化合物Compound | NCI-H2228NCI-H2228 | 化合物Compound | NCI-H2228NCI-H2228 |
| I-13I-13 | AA | I-19I-19 | AA | I-23I-23 | BB |
| I-14I-14 | AA | I-20I-20 | AA | I-25-1I-25-1 | BB |
| I-16I-16 | BB | I-21I-21 | AA | I-25-2I-25-2 | BB |
注:表中“A”表示化合物针对NCI-H2228活性IC
50≤100nM;“B”表示100<IC
50≤200nM。
Note: "A" in the table indicates that the compound has an IC 50 ≤ 100 nM for NCI-H2228 activity; "B" indicates 100 < IC 50 ≤ 200 nM.
表5-2 体外细胞活性IC
50(nM)
Table 5-2 In vitro cell activity IC 50 (nM)
| 化合物Compound | Karpas299Karpas299 | 化合物Compound | Karpas299Karpas299 | 化合物Compound | Karpas299Karpas299 |
| I-6I-6 | BB | I-15I-15 | AA | I-21I-21 | AA |
| I-8I-8 | AA | I-16I-16 | AA | I-23I-23 | AA |
| I-13I-13 | AA | I-19I-19 | BB | I-25-1I-25-1 | AA |
| I-14I-14 | AA | I-20I-20 | AA | I-25-2I-25-2 | BB |
注:表中“A”表示化合物针对Karpas299活性IC
50≤100nM;“B”表示100<IC
50≤150nM。
Note: "A" in the table indicates that the compound has an IC 50 ≤ 100 nM for Karpas 299; "B" indicates 100 < IC 50 ≤ 150 nM.
表5-3 体外细胞活性IC
50(nM)
Table 5-3 In vitro cell activity IC 50 (nM)
注:表中“A”表示化合物针对EML4-ALK L1196M BaF3活性IC
50≤100nM;“B”表示100<IC
50≤250nM。
Note: "A" in the table indicates that the compound has an IC 50 ≤ 100 nM for EML4-ALK L1196M BaF3 activity; "B" indicates 100 < IC 50 ≤ 250 nM.
表5-4 体外细胞活性IC
50(nM)
Table 5-4 In vitro cell activity IC 50 (nM)
注:表中“A”表示化合物针对TFG-NTRK1 BaF3活性IC
50≤0.5nM;“B”表示0.5<IC
50≤1nM;“C”表示1<IC
50≤5nM。
Note: "A" in the table indicates that the compound has an IC 50 ≤ 0.5 nM against TFG-NTRK1 BaF3 activity; "B" indicates 0.5 < IC 50 ≤ 1 nM; "C" indicates 1 < IC 50 ≤ 5 nM.
试验例3:体外肝微粒体稳定性Test Example 3: In vitro liver microsome stability
300μL最终的温孵体系中,含30μL肝微粒体(蛋白浓度:0.15mg/mL),30μL NADPH+MgCl
2,3μL受试化合物,237μL PBS缓冲液(PH7.4)。其中有机溶剂(乙腈)的比例为1%。每个种属做2份,每份0.3mL。每管先配好总体积为270μL的底物及酶的混匀液,和NADPH分别在37℃预温孵5min后,加入30μL NADPH+MgCl
2混合,分别于0min、15min、30min、60min后取出50μL温孵样品,加入300μL含内标(地西泮20ng/mL)的冰乙腈沉淀,涡旋震荡5min后,离心(12000rpm,4℃)10min。吸取上清液75μL,加入75μL超纯水稀释混匀,0.5μL进样分析。相关化合物在人、大鼠和小鼠肝微粒体中消除参数见表6-1、6-2和6-1。
300 μL of the final incubation system contained 30 μL of liver microsomes (protein concentration: 0.15 mg/mL), 30 μL of NADPH + MgCl 2 , 3 μL of test compound, and 237 μL of PBS buffer (pH 7.4). The ratio of the organic solvent (acetonitrile) was 1%. 2 copies of each species, 0.3 mL per serving. Each tube was first prepared with a total volume of 270 μL of substrate and enzyme mixture, and NADPH was preincubated at 37 ° C for 5 min, then 30 μL of NADPH + MgCl 2 was added and mixed at 0 min, 15 min, 30 min, 60 min. 50 μL of the incubation sample was added to 300 μL of ice acetonitrile containing an internal standard (diazepam 20 ng/mL), vortexed for 5 min, and centrifuged (12,000 rpm, 4 ° C) for 10 min. Aspirate 75 μL of the supernatant, add 75 μL of ultrapure water, dilute and mix, and 0.5 μL for injection analysis. The relevant compounds were eliminated in human, rat and mouse liver microsomes as shown in Tables 6-1, 6-2 and 6-1.
表6-1 人肝微粒体稳定性Table 6-1 Human liver microsome stability
表6-2 大鼠肝微粒体稳定性Table 6-2 Rat liver microsome stability
表6-3 小鼠肝微粒体稳定性Table 6-3 Mouse liver microsome stability
Claims (20)
- 式I化合物或其药学上可接受的盐,a compound of formula I or a pharmaceutically acceptable salt thereof,其中,among them,X选自O或NR 3; X is selected from O or NR 3 ;R 1选自氢、C 1-6烷基、C 2-6烯基、C 2-6炔基、C 3-6环烷基或6-10元芳基,所述C 1-6烷基、C 2-6烯基、C 2-6炔基、C 3-6环烷基或6-10元芳基任选地被以下基团取代:卤素、羟基、氰基、-OC 1-6烷基、-NH 2、-NH(C 1-6烷基)、-N(C 1-6烷基) 2、-NHC(O)C 1-6烷基、-NHC(O)NH 2、-CO 2H、-C(O)OC 1-6烷基、-C(O)NH 2、-C(O)NH(C 1-6烷基)、-C(O)N(C 1-6烷基) 2、-SH、-SC 1-6烷基、-S(O)C 1-6烷基、-S(O) 2C 1-6烷基、-S(O)NH(C 1-6烷基)、-S(O) 2NH(C 1-6烷基)、-S(O)N(C 1-6烷基) 2或-S(O) 2N(C 1-6烷基) 2; R 1 is selected from hydrogen, C 1-6 alkyl, C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl, said C 1-6 alkyl C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl is optionally substituted by halogen, hydroxy, cyano, -OC 1-6 Alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -NHC(O)C 1-6 alkyl, -NHC(O)NH 2 , -CO 2 H, -C(O)OC 1-6 alkyl, -C(O)NH 2 , -C(O)NH(C 1-6 alkyl), -C(O)N (C 1- 6 alkyl) 2 , -SH, -SC 1-6 alkyl, -S(O)C 1-6 alkyl, -S(O) 2 C 1-6 alkyl, -S(O)NH(C 1-6 alkyl), -S(O) 2 NH(C 1-6 alkyl), -S(O)N(C 1-6 alkyl) 2 or -S(O) 2 N (C 1- 6 alkyl) 2 ;R 3选自氢、C 1-6烷基、C 2-6烯基、C 2-6炔基、C 3-6环烷基、3-7元杂环烷基、6-10元芳基或6-10元杂芳基,所述C 1-6烷基、C 2-6烯基、C 2-6炔基、C 3-6环烷基、3-7元杂环烷基、6-10元芳基或6-10元杂芳基任选地被以下基团取代:卤素或-OC 1-6烷基;或者, R 3 is selected from the group consisting of hydrogen, C 1-6 alkyl, C 2-6 alkenyl, C 2-6 alkynyl, C 3-6 cycloalkyl, 3-7 membered heterocycloalkyl, 6-10 membered aryl Or a 6-10 membered heteroaryl group, said C 1-6 alkyl group, C 2-6 alkenyl group, C 2-6 alkynyl group, C 3-6 cycloalkyl group, 3-7 membered heterocycloalkyl group, 6 a -10 membered aryl or a 6-10 membered heteroaryl group is optionally substituted with a halogen or -OC 1-6 alkyl group; orR 1与R 3及其所连接的原子共同形成3-6元杂环烷基,所述3-6元杂环烷基任选地被以下基团取代:卤素、C 1-6烷基、羟基、氰基、-OC 1-6烷基、-NH 2、-NH(C 1-6烷基)、-N(C 1-6烷基) 2、-SH或-SC 1-6烷基; R 1 and R 3 together with the atom to which they are attached form a 3-6 membered heterocycloalkyl group, which is optionally substituted by a halogen, C 1-6 alkyl group, Hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl ;T 1、T 2、T 3或T 4独立地选自CR b或N; T 1 , T 2 , T 3 or T 4 are independently selected from CR b or N;R b独立地选自氢、卤素、C 1-6烷基、羟基、氰基、-OC 1-6烷基、-NH 2、-NHC 1-6烷基、-N(C 1-6烷基) 2或-CF 3; R b is independently selected from the group consisting of hydrogen, halogen, C 1-6 alkyl, hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NHC 1-6 alkyl, -N(C 1-6 alkane Base) 2 or -CF 3 ;L 1选自-O-、-NR a-、C 1-6亚烷基、-OC 1-6亚烷基-或-C 1-6亚烷基O-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC 1-6烷基、-NH 2、-NH(C 1-6烷基)、-N(C 1-6烷基) 2、-SH或-SC 1-6烷基; L 1 is selected from -O-, -NR a -, C 1-6 alkylene, -OC 1-6 alkylene- or -C 1-6 alkylene O-, said alkylene optionally Substituted by: halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH Or -SC 1-6 alkyl;L 2选自-NHC 1-6亚烷基-、-C 1-6亚烷基NH-、-C 1-6亚烷基-NR aCO-、-NR aC(O)-或-C(O)N(R a)-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC 1-6烷基、-NH 2、-NH(C 1-6烷基)、-N(C 1-6烷基) 2、-SH或-SC 1-6烷基; L 2 is selected from -NHC 1-6 alkylene-, -C 1-6 alkylene NH-, -C 1-6 alkylene-NR a CO-, -NR a C(O)- or -C (O)N(R a )-, the alkylene group is optionally substituted by halogen, cyano, hydroxy, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 Alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl;R a独立地选自氢或C 1-6烷基; R a is independently selected from hydrogen or C 1-6 alkyl;m或n独立地选自1、2或3;m or n is independently selected from 1, 2 or 3;q选自0-4;q is selected from 0-4;R 2独立地选自卤素,氰基,羟基,任选被卤素或羟基取代的C 1-6烷基、-OC 1-6烷基、-NH 2、-NHC 1-6烷基、-N(C 1-6烷基) 2。 R 2 is independently selected from halogen, cyano, hydroxy, C 1-6 alkyl optionally substituted by halogen or hydroxy, -OC 1-6 alkyl, -NH 2 , -NHC 1-6 alkyl, -N (C 1-6 alkyl) 2 .
- 如权利要求1所述的式I化合物或其药学上可接受的盐,其中X选自NR 3。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to claim 1, wherein X is selected from NR 3 .
- 如权利要求1或2所述的式I化合物或其药学上可接受的盐,其中R 1选自氢、C 1-3烷基、C 2-3烯基、C 2-3炔基、C 3-6环烷基或6-10元芳基,所述C 1-3烷基、C 2-3烯基、C 2-3炔基、C 3-6环烷基或6-10元芳基,任选地被以下基团取代:卤素、羟基、氰基、-OC 1-3烷基、-NH 2、-NH(C 1-3烷基)、-N(C 1-3烷基) 2、-NHC(O)C 1-3烷基、-NHC(O)NH 2、-CO 2H、-C(O)OC 1-3烷基、-C(O)NH 2、-C(O)NH(C 1-3烷基)、-C(O)N(C 1-3烷基) 2、-SH、-SC 1-3烷基、-S(O)C 1-3烷基、-S(O) 2C 1-3烷基、-S(O)NH(C 1-3烷基)、-S(O) 2NH(C 1-3烷基)、-S(O)N(C 1-3烷基) 2或-S(O) 2N(C 1-3烷基) 2;优选地,R 1选自氢、C 1-3烷基、C 2-3烯基或C 2-3炔基,所述C 1-3烷基、C 2-3烯基或C 2-3炔基,任选地被以下基团取代:卤素、羟基、氰基、-OC 1-3烷基、-NH 2、-NH(C 1-3烷基)、-N(C 1-3烷基) 2、-NHC(O)C 1-3烷基、-NHC(O)NH 2、-CO 2H、-C(O)OC 1-3烷基、-C(O)NH 2、-C(O)NH(C 1-3烷基)、-C(O)N(C 1-3烷基) 2、-SH、-SC 1-3烷基、-S(O)C 1-3烷基、-S(O) 2C 1-3烷基、-S(O)NH(C 1-3烷基)、-S(O) 2NH(C 1-3烷基)、-S(O)N(C 1-3烷基) 2、-S(O) 2N(C 1-3烷基) 2;更优选地,R 1选自氢或任选地被氟、氯、溴、羟基、氰基或-NH 2取代的C 1-3烷基;最优选地,R 1选自任选地被氟取代的甲基。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to claim 1 or 2, wherein R 1 is selected from the group consisting of hydrogen, C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl or 6-10 membered aryl, said C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkynyl, C 3-6 cycloalkyl or 6-10 member aryl Substituents, optionally substituted by halogen, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl 2 , -NHC(O)C 1-3 alkyl, -NHC(O)NH 2 , -CO 2 H, -C(O)OC 1-3 alkyl, -C(O)NH 2 , -C (O) NH(C 1-3 alkyl), -C(O)N(C 1-3 alkyl) 2 , -SH, -SC 1-3 alkyl, -S(O)C 1-3 alkane , -S(O) 2 C 1-3 alkyl, -S(O)NH(C 1-3 alkyl), -S(O) 2 NH(C 1-3 alkyl), -S(O N(C 1-3 alkyl) 2 or -S(O) 2 N(C 1-3 alkyl) 2 ; preferably, R 1 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkene Or a C 2-3 alkynyl group, said C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl, optionally substituted by halogen, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -NHC(O)C 1-3 alkyl, -NHC(O) NH 2 , -CO 2 H, -C(O)OC 1-3 alkyl, -C(O)NH 2 , -C(O)NH(C 1 -3 alkyl), -C(O)N(C 1-3 alkyl) 2 , -SH, -SC 1-3 alkyl, -S(O)C 1-3 alkyl, -S(O) 2 C 1-3 alkyl, -S(O)NH(C 1-3 alkyl), -S(O) 2 NH(C 1-3 alkyl), -S(O)N (C 1-3 Alkyl) 2 , -S(O) 2 N(C 1-3 alkyl) 2 ; more preferably, R 1 is selected from hydrogen or optionally by fluorine, chlorine, bromine, hydroxyl, cyano or -NH 2 Substituted C 1-3 alkyl; most preferably, R 1 is selected from methyl optionally substituted with fluorine.
- 如权利要求1-3任一项所述的式I化合物或其药学上可接受的盐,其中R 3选自氢、C 1-3烷基、C 2-3烯基、C 2-3炔基、C 3-6环烷基、3-6元杂环烷基、6-10元芳基或6-10元杂芳基,所述C 1-3烷基、C 2-3烯基、C 2-3炔基、C 3-6环烷基、3-6元杂环烷基、6-10元芳基或6-10元杂芳基,任选地被以下基团取代:卤素或-OC 1-3烷基;优选地,R 3选自氢、C 1-3烷基、C 2-3烯基或C 2-3炔基,所述C 1-3烷基、C 2-3烯基或C 2-3炔基,任选地被以下基团取代:卤素或-OC 1-3烷基;进一步优选地,R 3选自氢或任选地被氟、氯或溴取代的C 1-3烷基;最优选地,R 3选自氢。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any one of claims 1 to 3, wherein R 3 is selected from the group consisting of hydrogen, C 1-3 alkyl, C 2-3 alkenyl, C 2-3 alkyne a C 3-6 cycloalkyl group, a 3-6 membered heterocycloalkyl group, a 6-10 membered aryl group or a 6-10 membered heteroaryl group, said C 1-3 alkyl group, C 2-3 alkenyl group, C 2-3 alkynyl, C 3-6 cycloalkyl, 3-6 membered heterocycloalkyl, 6-10 membered aryl or 6-10 membered heteroaryl, optionally substituted by halogen or -OC 1-3 alkyl; preferably, R 3 is selected from hydrogen, C 1-3 alkyl, C 2-3 alkenyl or C 2-3 alkynyl, said C 1-3 alkyl, C 2- a 3 alkenyl or C 2-3 alkynyl group, optionally substituted by a halogen or -OC 1-3 alkyl group; further preferably, R 3 is selected from hydrogen or optionally substituted by fluorine, chlorine or bromine C 1-3 alkyl; most preferably, R 3 is selected from hydrogen.
- 如权利要求1或2所述的式I化合物或其药学上可接受的盐,其中R 1与R 3及其所连接的原子共同形成4-5元杂环烷基,所述4-5元杂环烷基任选地被以下基团取代:卤素、C 1-6烷基、羟基、氰基、-OC 1-6烷基、-NH 2、-NH(C 1-6烷基)、-N(C 1-6烷基) 2、-SH或-SC 1-6烷基;优选地,R 1与R 3及其所连接的原子共同形成5元杂环烷基,所述5元杂环烷基任选地被以下基团取代:卤素、C 1-3烷基、羟基、氰基、-OC 1-3烷基、-NH 2、-NH(C 1-3烷基)、-N(C 1-3烷基) 2、-SH或-SC 1-3烷基;更优选地,R 1与R 3及其所连接的原子共同形成四氢吡咯基,所述四氢吡咯基任选地被以下基团取代:氟、氯、溴、羟基、氰基或-NH 2;最优选地,R 1与R 3及其所连接的原子共同形成四氢吡咯基,所述四氢吡咯基任选地被氟取代。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to claim 1 or 2, wherein R 1 and R 3 and the atoms to which they are attached form a 4-5 membered heterocycloalkyl group, said 4-5 members. The heterocycloalkyl group is optionally substituted by halogen, C 1-6 alkyl, hydroxy, cyano, -OC 1-6 alkyl, -NH 2 , -NH(C 1-6 alkyl), -N(C 1-6 alkyl) 2 , -SH or -SC 1-6 alkyl; preferably, R 1 and R 3 together with the atom to which they are attached form a 5-membered heterocycloalkyl group, said 5 member Heterocycloalkyl is optionally substituted by halogen, C 1-3 alkyl, hydroxy, cyano, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl; more preferably, R 1 and R 3 and the atoms to which they are attached form a tetrahydropyrrole group, said tetrahydropyrrole The base is optionally substituted with fluorine, chlorine, bromine, hydroxyl, cyano or -NH 2 ; most preferably, R 1 and R 3 and the atoms to which they are attached form a tetrahydropyrrolyl group, said four The hydropyrrolyl group is optionally substituted with fluorine.
- 如权利要求1-5任一项所述的式I化合物或其药学上可接受的盐,其中R b独立地选自氢、卤素、C 1-3烷基、羟基、氰基、-OC 1-3烷基、-NH 2、-NHC 1-3烷基、-N(C 1-3烷基) 2或-CF 3;优选地,R b独立地选自氢、卤素、羟基、氰基、-NH 2或-CF 3;更优选地,R b独立地选自氢、氟、氯或溴;进一步优选地,R b独立地选自氢或氟。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any one of claims 1 to 5, wherein R b is independently selected from the group consisting of hydrogen, halogen, C 1-3 alkyl, hydroxy, cyano, -OC 1 -3 alkyl, -NH 2 , -NHC 1-3 alkyl, -N(C 1-3 alkyl) 2 or -CF 3 ; preferably, R b is independently selected from hydrogen, halogen, hydroxy, cyano , -NH 2 or -CF 3 ; more preferably, R b is independently selected from hydrogen, fluorine, chlorine or bromine; further preferably, R b is independently selected from hydrogen or fluorine.
- 如权利要求1-6任一项所述的式I化合物或其药学上可接受的盐,其中T 1选自CH、N或CF;其中T 2选自CH或N;其中T 3选自CF或N;其中T 4选自CH或N。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any one of claims 1 to 6, wherein T 1 is selected from CH, N or CF; wherein T 2 is selected from CH or N; wherein T 3 is selected from CF Or N; wherein T 4 is selected from CH or N.
- 如权利要求1-7任一项所述的式I化合物或其药学上可接受的盐,其中L 1选自-O-、-NR a-、C 1-3亚烷基、-OC 1-3亚烷基-或-C 1-3亚烷基O-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC 1-3烷基、-NH 2、-NH(C 1-3烷基)、-N(C 1-3烷基) 2、-SH或-SC 1-3烷基;优选地,L 1选自-O-、-NR a-、C 1-3亚烷基、-OC 1-3亚烷基-或-C 1-3亚烷基O-,所述亚烷基任选地被卤素取代;更优选地,L 1选自-O-、-NH-、-CH 2CH 2-、-OCH 2-或-CH 2O-,所述-CH 2CH 2-、-OCH 2-或-CH 2O-任选地被F取代;进一步优选地,L 1选自-O-、-NH-、-CH 2CH 2-、-OCH 2-、-OCF 2-、-CH 2O-或-CF 2O-。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any one of claims 1 to 7, wherein L 1 is selected from the group consisting of -O-, -NR a -, C 1-3 alkylene, -OC 1- 3 alkylene- or -C 1-3 alkylene O-, the alkylene group optionally substituted by halogen, cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl; preferably, L 1 is selected from -O-, -NR a -, C 1-3 alkylene, -OC 1-3 alkylene- or -C 1-3 alkylene O-, said alkylene group being optionally substituted by halogen; more preferably, L 1 is selected from - O-, -NH-, -CH 2 CH 2 -, -OCH 2 - or -CH 2 O-, the -CH 2 CH 2 -, -OCH 2 - or -CH 2 O- is optionally substituted by F Further preferably, L 1 is selected from -O-, -NH-, -CH 2 CH 2 -, -OCH 2 -, -OCF 2 -, -CH 2 O- or -CF 2 O-.
- 如权利要求1-8任一项所述的式I化合物或其药学上可接受的盐,其中L 2选自-NHC 1-3亚烷基-、-C 1-3亚烷基NH-、-C 1-3亚烷基-NR aCO-、-NR aC(O)-或-C(O)N(R a)-,所述亚烷基任选地被以下基团取代:卤素、氰基、羟基、-OC 1-3烷基、-NH 2、-NH(C 1-3烷基)、-N(C 1-3烷基) 2、-SH或-SC 1-3烷基;优选地,L 2选自-NHCH 2-、-CH 2NH-、-CH 2-NR aCO-、-NR aC(O)-或-C(O)N(R a)-,所述-NHCH 2-或-CH 2NH-中的亚甲基任选地被以下基团取代:卤素、氰基、羟基、-OC 1-3烷基、-NH 2、-NH(C 1-3烷基)、-N(C 1-3烷基) 2、-SH或-SC 1-3烷基;进一步优选地,L 2选自-NHCH 2-、-CH 2NH-、-CH 2-NR aCO-、-NR aC(O)-或-C(O)N(R a)-。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any one of claims 1-8, wherein L 2 is selected from the group consisting of -NHC 1-3 alkylene-, -C 1-3 alkylene NH-, -C 1-3 alkylene-NR a CO-, -NR a C(O)- or -C(O)N(R a )-, the alkylene group optionally substituted by a halogen group: halogen , cyano, hydroxy, -OC 1-3 alkyl, -NH 2 , -NH(C 1-3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkane Preferably, L 2 is selected from the group consisting of -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)- or -C(O)N(R a )-, The methylene group in the -NHCH 2 - or -CH 2 NH- is optionally substituted with a halogen, a cyano group, a hydroxyl group, -OC 1-3 alkyl group, -NH 2 , -NH (C 1 -3 alkyl), -N(C 1-3 alkyl) 2 , -SH or -SC 1-3 alkyl; further preferably, L 2 is selected from -NHCH 2 -, -CH 2 NH-, -CH 2 -NR a CO-, -NR a C(O)- or -C(O)N(R a )-.
- 如权利要求1-9任一项所述的式I化合物或其药学上可接受的盐,其中R a独立地选自氢或C 1-3烷基;优选地,R a独立地选自氢或甲基。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any one of claims 1-9, wherein R a is independently selected from hydrogen or C 1-3 alkyl; preferably, R a is independently selected from hydrogen Or methyl.
- 如权利要求1-10任一项所述的式I化合物或其药学上可接受的盐,其中m、n独立地选自1或2;优选地,m选自1,n选自1或2;更优选地,m选自1,n选自1。A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any one of claims 1 to 10, wherein m, n are independently selected from 1 or 2; preferably, m is selected from 1, and n is selected from 1 or 2 More preferably, m is selected from the group consisting of 1, and n is selected from 1.
- 如权利要求1-11任一项所述的式I化合物或其药学上可接受的盐,其中q选自0或1;优选地,q选自0。A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any of claims 1-11, wherein q is selected from 0 or 1; preferably, q is selected from 0.
- 如权利要求1-12任一项所述的式I化合物或其药学上可接受的盐,其中R 2独立地选自卤素、氰基、羟基或任选被卤素或羟基取代的C 1-3烷基;优选地,R 2独立地选自C 1-3烷基;更优选地,R 2独立地选自甲基。 A compound of formula I, or a pharmaceutically acceptable salt thereof, according to any one of claims 1 to 12, wherein R 2 is independently selected from halogen, cyano, hydroxy or C 1-3 optionally substituted by halogen or hydroxy Alkyl; preferably, R 2 is independently selected from C 1-3 alkyl; more preferably, R 2 is independently selected from methyl.
- 权利要求1-13任一项所述的式I化合物或其药学上可接受的盐,选自式II化合物或其药学上可接受的盐:A compound of formula I according to any one of claims 1 to 13, or a pharmaceutically acceptable salt thereof, selected from the group consisting of a compound of formula II or a pharmaceutically acceptable salt thereof:其中,X、R 1、T 1、T 2、T 3、T 4、L 1、m、n、q或R 2同权利要求1-13任一项中式I化合物中的定义。 Wherein X, R 1 , T 1 , T 2 , T 3 , T 4 , L 1 , m, n, q or R 2 are as defined in the compound of formula I in any one of claims 1-13.
- 权利要求1-13任一项所述的式I化合物或其药学上可接受的盐,选自式III或其药学上可接受的盐:A compound of formula I according to any one of claims 1 to 13, or a pharmaceutically acceptable salt thereof, selected from formula III or a pharmaceutically acceptable salt thereof:其中,环G、X、R b、R 1、R 2、T 1、L 1、L 2、m、n或q同权利要求1-13任一项中式I化合物中的定义。 Wherein the ring G, X, R b , R 1 , R 2 , T 1 , L 1 , L 2 , m, n or q is as defined in the compound of the formula I in any one of claims 1 to 13.
- 权利要求1-13任一项所述的式I化合物或其药学上可接受的盐,选自式IV化合物或其药学上可接受的盐:A compound of formula I according to any one of claims 1 to 13, or a pharmaceutically acceptable salt thereof, selected from the group consisting of a compound of formula IV or a pharmaceutically acceptable salt thereof:其中,环G、T 1、T 2、T 3、T 4、L 1、m、n、q或R 2同权利要求1-13任一项中式I化合物中的定义。 Wherein ring G, T 1 , T 2 , T 3 , T 4 , L 1 , m, n, q or R 2 is as defined in the compound of formula I according to any one of claims 1-13.
- 一种药物组合物,其包含权利要求1-17任一项所述的化合物或其药学上可接受的盐;优选地,还包括药学上可接受的辅料。A pharmaceutical composition comprising the compound of any one of claims 1-17, or a pharmaceutically acceptable salt thereof; preferably, further comprising a pharmaceutically acceptable excipient.
- 权利要求1-17任一项所述的化合物或其药学上可接受的盐、或权利要求18所述的药物组合物在制备治疗哺乳动物细胞增殖、细胞侵袭、转移、凋亡或血管生成相关的疾病的药物中的用途。A compound according to any one of claims 1 to 17, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition according to claim 18, which is related to the preparation of a mammalian cell proliferation, cell invasion, metastasis, apoptosis or angiogenesis The use of the disease in medicine.
- 用于预防或者治疗哺乳动物细胞增殖、细胞侵袭、转移、凋亡或血管生成相关疾病的权利要求1-17任一项所述的化合物或其药学上可接受的盐、或权利要求18所述的药物组合物。A compound according to any one of claims 1 to 17, or a pharmaceutically acceptable salt thereof, or a method according to claim 18, for use in the prevention or treatment of a mammalian cell proliferation, cell invasion, metastasis, apoptosis or angiogenesis-related disease Pharmaceutical composition.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201980025145.8A CN111918868B (en) | 2018-05-04 | 2019-04-30 | Diaryl macrocycles as protein kinase modulators |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201810421414 | 2018-05-04 | ||
| CN201810421414.X | 2018-05-04 | ||
| CN201910198329 | 2019-03-15 | ||
| CN201910198329.6 | 2019-03-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019210835A1 true WO2019210835A1 (en) | 2019-11-07 |
Family
ID=68386965
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2019/085090 WO2019210835A1 (en) | 2018-05-04 | 2019-04-30 | Diaryl macrocyclic compound as protein kinase modulator |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN111918868B (en) |
| WO (1) | WO2019210835A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021042890A1 (en) * | 2019-09-04 | 2021-03-11 | 罗欣药业(上海)有限公司 | Heterocyclic compound and application thereof as trk kinase inhibitor |
| WO2021115401A1 (en) | 2019-12-13 | 2021-06-17 | 成都倍特药业股份有限公司 | Fluorine-containing heterocyclic derivatives with macrocyclic structure and use thereof |
| CN113045575A (en) * | 2019-12-27 | 2021-06-29 | 成都倍特药业股份有限公司 | Preparation method of compound, intermediate thereof and preparation method of intermediate |
| CN113045587A (en) * | 2019-12-27 | 2021-06-29 | 成都倍特药业股份有限公司 | Crystal form of macrocyclic compound and preparation method thereof |
| CN113121568A (en) * | 2019-12-31 | 2021-07-16 | 成都倍特药业股份有限公司 | Salt of macrocyclic compound and preparation method thereof |
| CN113563341A (en) * | 2020-04-29 | 2021-10-29 | 南京雷正医药科技有限公司 | Substituted pyrazolo [1,5-a ] pyrimidine compounds as Trk inhibitors |
| WO2022033575A1 (en) * | 2020-08-14 | 2022-02-17 | 赛诺哈勃药业(成都)有限公司 | Application of fluorine-containing heterocyclic derivative having macrocyclic structure |
| WO2022218276A1 (en) * | 2021-04-12 | 2022-10-20 | 成都倍特药业股份有限公司 | Solid form of fluorine-containing macrocyclic structure compound, preparation method, and application |
| WO2024032390A1 (en) * | 2022-08-09 | 2024-02-15 | 苏州朗睿生物医药有限公司 | Macrocyclic triazole derivative and preparation method therefor and use thereof |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118724916A (en) * | 2023-03-30 | 2024-10-01 | 浙江养生堂天然药物研究所有限公司 | Polyaryl-containing macrocyclic compounds and uses thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102971322A (en) * | 2010-05-20 | 2013-03-13 | 阵列生物制药公司 | Macrocyclic compounds as TRK kinase inhibitors |
| CN104169286A (en) * | 2012-03-06 | 2014-11-26 | 辉瑞大药厂 | Macrocyclic derivatives for the treatment of proliferative diseases |
| CN106170289A (en) * | 2014-01-24 | 2016-11-30 | Tp生物医药公司 | The huge ring of diaryl as the regulator of protein kinase |
| WO2017015367A1 (en) * | 2015-07-21 | 2017-01-26 | Tp Therapeutics, Inc. | Chiral diaryl macrocycles and uses thereof |
| CN107735399A (en) * | 2015-07-02 | 2018-02-23 | Tp生物医药公司 | The big ring of chiral diaryl as the conditioning agent of protein kinase |
-
2019
- 2019-04-30 WO PCT/CN2019/085090 patent/WO2019210835A1/en active Application Filing
- 2019-04-30 CN CN201980025145.8A patent/CN111918868B/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102971322A (en) * | 2010-05-20 | 2013-03-13 | 阵列生物制药公司 | Macrocyclic compounds as TRK kinase inhibitors |
| CN104169286A (en) * | 2012-03-06 | 2014-11-26 | 辉瑞大药厂 | Macrocyclic derivatives for the treatment of proliferative diseases |
| CN106170289A (en) * | 2014-01-24 | 2016-11-30 | Tp生物医药公司 | The huge ring of diaryl as the regulator of protein kinase |
| CN107735399A (en) * | 2015-07-02 | 2018-02-23 | Tp生物医药公司 | The big ring of chiral diaryl as the conditioning agent of protein kinase |
| WO2017015367A1 (en) * | 2015-07-21 | 2017-01-26 | Tp Therapeutics, Inc. | Chiral diaryl macrocycles and uses thereof |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021042890A1 (en) * | 2019-09-04 | 2021-03-11 | 罗欣药业(上海)有限公司 | Heterocyclic compound and application thereof as trk kinase inhibitor |
| JP2023504866A (en) * | 2019-12-13 | 2023-02-07 | ▲賽▼▲諾▼哈勃▲薬▼▲業▼(成都)有限公司 | Fluorine-containing heterocyclic derivative having macrocyclic structure and use thereof |
| WO2021115401A1 (en) | 2019-12-13 | 2021-06-17 | 成都倍特药业股份有限公司 | Fluorine-containing heterocyclic derivatives with macrocyclic structure and use thereof |
| CN112979679A (en) * | 2019-12-13 | 2021-06-18 | 成都倍特药业股份有限公司 | Fluoroheterocyclic derivative having macrocyclic structure and use thereof |
| AU2020402942B2 (en) * | 2019-12-13 | 2023-05-11 | Scinnohub Pharmaceutical Co., Ltd | Fluorine-containing heterocyclic derivatives with macrocyclic structure and use thereof |
| TWI760005B (en) * | 2019-12-13 | 2022-04-01 | 大陸商賽諾哈勃藥業(成都)有限公司 | Fluorinated heterocyclic derivatives with macrocyclic structure and uses thereof |
| CN113045575A (en) * | 2019-12-27 | 2021-06-29 | 成都倍特药业股份有限公司 | Preparation method of compound, intermediate thereof and preparation method of intermediate |
| CN113045587A (en) * | 2019-12-27 | 2021-06-29 | 成都倍特药业股份有限公司 | Crystal form of macrocyclic compound and preparation method thereof |
| CN113045575B (en) * | 2019-12-27 | 2022-06-17 | 成都倍特药业股份有限公司 | Preparation method of compound, intermediate thereof and preparation method of intermediate |
| CN113121568A (en) * | 2019-12-31 | 2021-07-16 | 成都倍特药业股份有限公司 | Salt of macrocyclic compound and preparation method thereof |
| CN113563341A (en) * | 2020-04-29 | 2021-10-29 | 南京雷正医药科技有限公司 | Substituted pyrazolo [1,5-a ] pyrimidine compounds as Trk inhibitors |
| CN113563341B (en) * | 2020-04-29 | 2022-07-12 | 山东轩硕医药科技有限公司 | Substituted pyrazolo [1,5-a ] pyrimidine compounds as Trk inhibitors |
| CN114073704A (en) * | 2020-08-14 | 2022-02-22 | 赛诺哈勃药业(成都)有限公司 | Use of fluoro-heterocyclic derivatives having macrocyclic structure |
| WO2022033575A1 (en) * | 2020-08-14 | 2022-02-17 | 赛诺哈勃药业(成都)有限公司 | Application of fluorine-containing heterocyclic derivative having macrocyclic structure |
| CN114073704B (en) * | 2020-08-14 | 2023-08-11 | 赛诺哈勃药业(成都)有限公司 | Use of fluorine-containing heterocyclic derivative with macrocyclic structure |
| WO2022218276A1 (en) * | 2021-04-12 | 2022-10-20 | 成都倍特药业股份有限公司 | Solid form of fluorine-containing macrocyclic structure compound, preparation method, and application |
| WO2024032390A1 (en) * | 2022-08-09 | 2024-02-15 | 苏州朗睿生物医药有限公司 | Macrocyclic triazole derivative and preparation method therefor and use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN111918868B (en) | 2022-12-30 |
| CN111918868A (en) | 2020-11-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2019210835A1 (en) | Diaryl macrocyclic compound as protein kinase modulator | |
| CN107735399B (en) | Chiral diaryl macrocycles as modulators of protein kinases | |
| WO2021169990A1 (en) | Kras inhibitors for treating cancers | |
| CN111606908B (en) | JAK inhibitor compounds and uses thereof | |
| JP5832524B2 (en) | Pyridone and azapyridone compounds and methods for their use | |
| CN112585139A (en) | Nalidinone compounds as T cell activators | |
| WO2022268051A1 (en) | Fused tetracyclic compound, preparation method therefor and application thereof in medicine | |
| CN113795483A (en) | Carboxamide-pyrimidine derivatives as SHP2 antagonists | |
| KR101469334B1 (en) | N-9-substituted purine compounds, compositions and methods of use | |
| WO2023217230A1 (en) | Kinesin kif18a inhibitor and use thereof | |
| WO2021115457A9 (en) | Pyrazolo[1,5-a]pyridine compound, preparation method therefor and use thereof | |
| WO2019157879A1 (en) | Heterocyclic compound which acts as trk inhibitor | |
| CN111032641A (en) | Substituted 5-cyanoindole compounds and uses thereof | |
| WO2021042890A1 (en) | Heterocyclic compound and application thereof as trk kinase inhibitor | |
| WO2019037761A1 (en) | Macrocycle containing aminopyrazole and pyrimidine and pharmaceutical composition and use thereof | |
| CN110950876B (en) | Furanolactam compounds, preparation method and application | |
| WO2023179600A1 (en) | Novel substituted macroheterocyclic compounds and use thereof | |
| CN112979655A (en) | Triazolopyridazine derivative, preparation method, pharmaceutical composition and application thereof | |
| CN113166156A (en) | Tyrosine kinase inhibitors, compositions and methods thereof | |
| WO2020015735A1 (en) | Bruton tyrosine kinase inhibitors | |
| WO2024041606A1 (en) | Compound with anti-kras mutant tumor activity | |
| WO2022002142A1 (en) | Tetrahydroisoquinoline compounds and use thereof | |
| WO2019233457A1 (en) | Erk inhibitor and use thereof | |
| WO2023151621A1 (en) | Compound having anti-kras mutant tumor activity | |
| WO2020052628A1 (en) | Furo[3,4-b]pyrrole-containing btk inhibitor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19797049 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19797049 Country of ref document: EP Kind code of ref document: A1 |