WO2019136309A1 - Anti-tissue factor antibodies, antibody-drug conjugates, and related methods - Google Patents
Anti-tissue factor antibodies, antibody-drug conjugates, and related methods Download PDFInfo
- Publication number
- WO2019136309A1 WO2019136309A1 PCT/US2019/012427 US2019012427W WO2019136309A1 WO 2019136309 A1 WO2019136309 A1 WO 2019136309A1 US 2019012427 W US2019012427 W US 2019012427W WO 2019136309 A1 WO2019136309 A1 WO 2019136309A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- antibody
- seq
- human
- extracellular domain
- binding
- Prior art date
Links
- 229940049595 antibody-drug conjugate Drugs 0.000 title claims abstract description 271
- 239000000611 antibody drug conjugate Substances 0.000 title claims abstract description 248
- 238000000034 method Methods 0.000 title claims abstract description 127
- 108010000499 Thromboplastin Proteins 0.000 claims abstract description 680
- 102000002262 Thromboplastin Human genes 0.000 claims abstract description 680
- 101000635804 Homo sapiens Tissue factor Proteins 0.000 claims abstract description 453
- 230000027455 binding Effects 0.000 claims description 843
- 125000000539 amino acid group Chemical group 0.000 claims description 568
- 108090000190 Thrombin Proteins 0.000 claims description 352
- 229960004072 thrombin Drugs 0.000 claims description 352
- 238000012032 thrombin generation assay Methods 0.000 claims description 296
- 238000003556 assay Methods 0.000 claims description 281
- 238000010186 staining Methods 0.000 claims description 231
- 101000635831 Rattus norvegicus Tissue factor Proteins 0.000 claims description 201
- 230000035772 mutation Effects 0.000 claims description 186
- 206010028980 Neoplasm Diseases 0.000 claims description 170
- 201000011510 cancer Diseases 0.000 claims description 149
- 229960003766 thrombin (human) Drugs 0.000 claims description 144
- 229940127089 cytotoxic agent Drugs 0.000 claims description 58
- 239000002254 cytotoxic agent Substances 0.000 claims description 58
- 231100000599 cytotoxic agent Toxicity 0.000 claims description 58
- 101001052793 Homo sapiens GDP-L-fucose synthase Proteins 0.000 claims description 48
- 102000050085 human TSTA3 Human genes 0.000 claims description 48
- 230000001419 dependent effect Effects 0.000 claims description 43
- 230000011664 signaling Effects 0.000 claims description 42
- 201000010099 disease Diseases 0.000 claims description 40
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 40
- 101000635833 Mus musculus Tissue factor Proteins 0.000 claims description 39
- 241000283973 Oryctolagus cuniculus Species 0.000 claims description 37
- 230000007423 decrease Effects 0.000 claims description 36
- 239000000427 antigen Substances 0.000 claims description 30
- 108091007433 antigens Proteins 0.000 claims description 30
- 102000036639 antigens Human genes 0.000 claims description 30
- 208000005590 Choroidal Neovascularization Diseases 0.000 claims description 28
- 206010060823 Choroidal neovascularisation Diseases 0.000 claims description 28
- 239000003814 drug Substances 0.000 claims description 27
- 101100261250 Sus scrofa TF gene Proteins 0.000 claims description 25
- 229940124597 therapeutic agent Drugs 0.000 claims description 24
- 239000008194 pharmaceutical composition Substances 0.000 claims description 20
- 230000000295 complement effect Effects 0.000 claims description 19
- HMQPEDMEOBLSQB-UITYFYQISA-N beta-D-Galp-(1->3)-D-GalpNAc Chemical compound CC(=O)N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HMQPEDMEOBLSQB-UITYFYQISA-N 0.000 claims description 16
- 230000003902 lesion Effects 0.000 claims description 16
- 241000282898 Sus scrofa Species 0.000 claims description 15
- 206010064930 age-related macular degeneration Diseases 0.000 claims description 13
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 claims description 13
- 208000002780 macular degeneration Diseases 0.000 claims description 13
- 230000004048 modification Effects 0.000 claims description 13
- 238000012986 modification Methods 0.000 claims description 13
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 claims description 12
- 206010029113 Neovascularisation Diseases 0.000 claims description 12
- 102000040430 polynucleotide Human genes 0.000 claims description 12
- 108091033319 polynucleotide Proteins 0.000 claims description 12
- 239000002157 polynucleotide Substances 0.000 claims description 12
- 206010033128 Ovarian cancer Diseases 0.000 claims description 11
- 201000010536 head and neck cancer Diseases 0.000 claims description 11
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 11
- 206010005003 Bladder cancer Diseases 0.000 claims description 10
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 10
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 10
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 claims description 10
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 10
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 10
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 10
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 10
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 10
- 206010060862 Prostate cancer Diseases 0.000 claims description 10
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 10
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 claims description 10
- 206010038389 Renal cancer Diseases 0.000 claims description 10
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 10
- 208000003721 Triple Negative Breast Neoplasms Diseases 0.000 claims description 10
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 10
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 10
- 201000010881 cervical cancer Diseases 0.000 claims description 10
- 201000004101 esophageal cancer Diseases 0.000 claims description 10
- 102000015694 estrogen receptors Human genes 0.000 claims description 10
- 108010038795 estrogen receptors Proteins 0.000 claims description 10
- 206010017758 gastric cancer Diseases 0.000 claims description 10
- 208000005017 glioblastoma Diseases 0.000 claims description 10
- 201000010982 kidney cancer Diseases 0.000 claims description 10
- 201000005202 lung cancer Diseases 0.000 claims description 10
- 208000020816 lung neoplasm Diseases 0.000 claims description 10
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 10
- 201000001441 melanoma Diseases 0.000 claims description 10
- 201000002528 pancreatic cancer Diseases 0.000 claims description 10
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 10
- 102000003998 progesterone receptors Human genes 0.000 claims description 10
- 108090000468 progesterone receptors Proteins 0.000 claims description 10
- 201000011549 stomach cancer Diseases 0.000 claims description 10
- 208000022679 triple-negative breast carcinoma Diseases 0.000 claims description 10
- 201000005112 urinary bladder cancer Diseases 0.000 claims description 10
- 230000005888 antibody-dependent cellular phagocytosis Effects 0.000 claims description 8
- 230000004540 complement-dependent cytotoxicity Effects 0.000 claims description 8
- 239000013598 vector Substances 0.000 claims description 8
- 206010012689 Diabetic retinopathy Diseases 0.000 claims description 7
- 239000003795 chemical substances by application Substances 0.000 claims description 7
- 229940088597 hormone Drugs 0.000 claims description 7
- 239000005556 hormone Substances 0.000 claims description 7
- 208000035868 Vascular inflammations Diseases 0.000 claims description 6
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 claims description 6
- -1 camptothecan Chemical compound 0.000 claims description 6
- 229960004562 carboplatin Drugs 0.000 claims description 6
- 229960004679 doxorubicin Drugs 0.000 claims description 6
- 230000000694 effects Effects 0.000 claims description 6
- 239000012636 effector Substances 0.000 claims description 5
- 102000004190 Enzymes Human genes 0.000 claims description 4
- 108090000790 Enzymes Proteins 0.000 claims description 4
- 108091005804 Peptidases Proteins 0.000 claims description 4
- 102000035195 Peptidases Human genes 0.000 claims description 4
- 230000003247 decreasing effect Effects 0.000 claims description 4
- 230000009977 dual effect Effects 0.000 claims description 4
- 229940088598 enzyme Drugs 0.000 claims description 4
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 4
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 claims description 3
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 claims description 3
- FJHBVJOVLFPMQE-QFIPXVFZSA-N 7-Ethyl-10-Hydroxy-Camptothecin Chemical compound C1=C(O)C=C2C(CC)=C(CN3C(C4=C([C@@](C(=O)OC4)(O)CC)C=C33)=O)C3=NC2=C1 FJHBVJOVLFPMQE-QFIPXVFZSA-N 0.000 claims description 3
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 claims description 3
- 102000019034 Chemokines Human genes 0.000 claims description 3
- 108010012236 Chemokines Proteins 0.000 claims description 3
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 claims description 3
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 claims description 3
- 108010092160 Dactinomycin Proteins 0.000 claims description 3
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 claims description 3
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 claims description 3
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 claims description 3
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 claims description 3
- 229940122803 Vinca alkaloid Drugs 0.000 claims description 3
- 239000002253 acid Substances 0.000 claims description 3
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 claims description 3
- 239000000556 agonist Substances 0.000 claims description 3
- 229930013930 alkaloid Natural products 0.000 claims description 3
- 150000003797 alkaloid derivatives Chemical class 0.000 claims description 3
- 229940100198 alkylating agent Drugs 0.000 claims description 3
- 239000002168 alkylating agent Substances 0.000 claims description 3
- 239000004037 angiogenesis inhibitor Substances 0.000 claims description 3
- 239000003242 anti bacterial agent Substances 0.000 claims description 3
- 230000000340 anti-metabolite Effects 0.000 claims description 3
- 229940100197 antimetabolite Drugs 0.000 claims description 3
- 239000002256 antimetabolite Substances 0.000 claims description 3
- 239000003080 antimitotic agent Substances 0.000 claims description 3
- 108010044540 auristatin Proteins 0.000 claims description 3
- 230000003115 biocidal effect Effects 0.000 claims description 3
- 229940127093 camptothecin Drugs 0.000 claims description 3
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 claims description 3
- 229960004397 cyclophosphamide Drugs 0.000 claims description 3
- 229960000684 cytarabine Drugs 0.000 claims description 3
- 229960003901 dacarbazine Drugs 0.000 claims description 3
- 229960000640 dactinomycin Drugs 0.000 claims description 3
- 229960000975 daunorubicin Drugs 0.000 claims description 3
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 claims description 3
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 claims description 3
- 229960003668 docetaxel Drugs 0.000 claims description 3
- AMRJKAQTDDKMCE-UHFFFAOYSA-N dolastatin Chemical compound CC(C)C(N(C)C)C(=O)NC(C(C)C)C(=O)N(C)C(C(C)C)C(OC)CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(C=1SC=CN=1)CC1=CC=CC=C1 AMRJKAQTDDKMCE-UHFFFAOYSA-N 0.000 claims description 3
- 229930188854 dolastatin Natural products 0.000 claims description 3
- 229940079593 drug Drugs 0.000 claims description 3
- VQNATVDKACXKTF-XELLLNAOSA-N duocarmycin Chemical compound COC1=C(OC)C(OC)=C2NC(C(=O)N3C4=CC(=O)C5=C([C@@]64C[C@@H]6C3)C=C(N5)C(=O)OC)=CC2=C1 VQNATVDKACXKTF-XELLLNAOSA-N 0.000 claims description 3
- 229960005501 duocarmycin Drugs 0.000 claims description 3
- 229930184221 duocarmycin Natural products 0.000 claims description 3
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 claims description 3
- 229960005420 etoposide Drugs 0.000 claims description 3
- 239000003667 hormone antagonist Substances 0.000 claims description 3
- 229960000908 idarubicin Drugs 0.000 claims description 3
- 229960004768 irinotecan Drugs 0.000 claims description 3
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 claims description 3
- 230000000861 pro-apoptotic effect Effects 0.000 claims description 3
- 229940002612 prodrug Drugs 0.000 claims description 3
- 239000000651 prodrug Substances 0.000 claims description 3
- 235000019833 protease Nutrition 0.000 claims description 3
- YUOCYTRGANSSRY-UHFFFAOYSA-N pyrrolo[2,3-i][1,2]benzodiazepine Chemical compound C1=CN=NC2=C3C=CN=C3C=CC2=C1 YUOCYTRGANSSRY-UHFFFAOYSA-N 0.000 claims description 3
- 230000002285 radioactive effect Effects 0.000 claims description 3
- 229960000303 topotecan Drugs 0.000 claims description 3
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 claims description 3
- 239000003053 toxin Substances 0.000 claims description 3
- 231100000765 toxin Toxicity 0.000 claims description 3
- 102220020471 rs397508335 Human genes 0.000 claims 8
- 190000008236 carboplatin Chemical compound 0.000 claims 2
- 102000053391 human F Human genes 0.000 claims 1
- 108700031895 human F Proteins 0.000 claims 1
- 239000000203 mixture Substances 0.000 abstract description 2
- 238000002405 diagnostic procedure Methods 0.000 abstract 1
- 230000001225 therapeutic effect Effects 0.000 abstract 1
- 238000002560 therapeutic procedure Methods 0.000 abstract 1
- 210000004027 cell Anatomy 0.000 description 182
- 238000004448 titration Methods 0.000 description 39
- 108010074860 Factor Xa Proteins 0.000 description 36
- 108010014173 Factor X Proteins 0.000 description 27
- 108091022873 acetoacetate decarboxylase Proteins 0.000 description 23
- 238000011282 treatment Methods 0.000 description 20
- 230000003833 cell viability Effects 0.000 description 17
- 108060003951 Immunoglobulin Proteins 0.000 description 12
- 239000012634 fragment Substances 0.000 description 12
- 102000018358 immunoglobulin Human genes 0.000 description 12
- 230000002998 immunogenetic effect Effects 0.000 description 11
- 238000011534 incubation Methods 0.000 description 11
- IEDXPSOJFSVCKU-HOKPPMCLSA-N [4-[[(2S)-5-(carbamoylamino)-2-[[(2S)-2-[6-(2,5-dioxopyrrolidin-1-yl)hexanoylamino]-3-methylbutanoyl]amino]pentanoyl]amino]phenyl]methyl N-[(2S)-1-[[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]-N-methylcarbamate Chemical compound CC[C@H](C)[C@@H]([C@@H](CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCc1ccc(NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@@H](NC(=O)CCCCCN2C(=O)CCC2=O)C(C)C)cc1)C(C)C IEDXPSOJFSVCKU-HOKPPMCLSA-N 0.000 description 10
- 108010093470 monomethyl auristatin E Proteins 0.000 description 10
- 238000011533 pre-incubation Methods 0.000 description 10
- 102000039446 nucleic acids Human genes 0.000 description 9
- 108020004707 nucleic acids Proteins 0.000 description 9
- 150000007523 nucleic acids Chemical class 0.000 description 9
- 102220471623 Interleukin-10 receptor subunit alpha_K68N_mutation Human genes 0.000 description 8
- MFRNYXJJRJQHNW-DEMKXPNLSA-N (2s)-2-[[(2r,3r)-3-methoxy-3-[(2s)-1-[(3r,4s,5s)-3-methoxy-5-methyl-4-[methyl-[(2s)-3-methyl-2-[[(2s)-3-methyl-2-(methylamino)butanoyl]amino]butanoyl]amino]heptanoyl]pyrrolidin-2-yl]-2-methylpropanoyl]amino]-3-phenylpropanoic acid Chemical compound CN[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MFRNYXJJRJQHNW-DEMKXPNLSA-N 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 238000000954 titration curve Methods 0.000 description 7
- 102000004127 Cytokines Human genes 0.000 description 6
- 108090000695 Cytokines Proteins 0.000 description 6
- 238000013534 fluorescein angiography Methods 0.000 description 6
- 239000011159 matrix material Substances 0.000 description 6
- 108010059074 monomethylauristatin F Proteins 0.000 description 6
- 235000018102 proteins Nutrition 0.000 description 6
- 102000004169 proteins and genes Human genes 0.000 description 6
- 108090000623 proteins and genes Proteins 0.000 description 6
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 5
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 5
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 5
- 238000004113 cell culture Methods 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 229940072221 immunoglobulins Drugs 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 230000003442 weekly effect Effects 0.000 description 5
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 4
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 4
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 4
- 241000700159 Rattus Species 0.000 description 4
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 4
- 125000003275 alpha amino acid group Chemical group 0.000 description 4
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 4
- 229910052791 calcium Inorganic materials 0.000 description 4
- 239000011575 calcium Substances 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 230000002757 inflammatory effect Effects 0.000 description 4
- 238000004020 luminiscence type Methods 0.000 description 4
- 108090000765 processed proteins & peptides Proteins 0.000 description 4
- 241000894007 species Species 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 3
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 3
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- 102000029749 Microtubule Human genes 0.000 description 3
- 108091022875 Microtubule Proteins 0.000 description 3
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 210000001072 colon Anatomy 0.000 description 3
- 239000000562 conjugate Substances 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 210000004688 microtubule Anatomy 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 102000004196 processed proteins & peptides Human genes 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 230000004614 tumor growth Effects 0.000 description 3
- 102000000844 Cell Surface Receptors Human genes 0.000 description 2
- 108010001857 Cell Surface Receptors Proteins 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- 108010087819 Fc receptors Proteins 0.000 description 2
- 102000009109 Fc receptors Human genes 0.000 description 2
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 108010022999 Serine Proteases Proteins 0.000 description 2
- 102000012479 Serine Proteases Human genes 0.000 description 2
- 108091008874 T cell receptors Proteins 0.000 description 2
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 2
- 230000033115 angiogenesis Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000023555 blood coagulation Effects 0.000 description 2
- 210000004899 c-terminal region Anatomy 0.000 description 2
- 230000030833 cell death Effects 0.000 description 2
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 2
- 230000015271 coagulation Effects 0.000 description 2
- 238000005345 coagulation Methods 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000010369 molecular cloning Methods 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 2
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 2
- 108700012359 toxins Proteins 0.000 description 2
- PGOHTUIFYSHAQG-LJSDBVFPSA-N (2S)-6-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-1-[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-4-methylsulfanylbutanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-5-carbamimidamidopentanoyl]amino]propanoyl]pyrrolidine-2-carbonyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]acetyl]amino]-3-hydroxypropanoyl]amino]-4-methylpentanoyl]amino]-3-sulfanylpropanoyl]amino]-4-methylsulfanylbutanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-hydroxybutanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-4-methylpentanoyl]amino]-3-hydroxybutanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-oxopentanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxypropanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]-5-oxopentanoyl]amino]-3-phenylpropanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]-4-oxobutanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-4-carboxybutanoyl]amino]-5-oxopentanoyl]amino]hexanoic acid Chemical compound CSCC[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O PGOHTUIFYSHAQG-LJSDBVFPSA-N 0.000 description 1
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 1
- 108010049777 Ankyrins Proteins 0.000 description 1
- 102000008102 Ankyrins Human genes 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 101000984722 Bos taurus Pancreatic trypsin inhibitor Proteins 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 1
- 108700022150 Designed Ankyrin Repeat Proteins Proteins 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 102100037362 Fibronectin Human genes 0.000 description 1
- 102000002090 Fibronectin type III Human genes 0.000 description 1
- 108050009401 Fibronectin type III Proteins 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 230000037059 G2/M phase arrest Effects 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 102100032518 Gamma-crystallin B Human genes 0.000 description 1
- 101710092798 Gamma-crystallin B Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 108050006654 Lipocalin Proteins 0.000 description 1
- 102000019298 Lipocalin Human genes 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 206010061309 Neoplasm progression Diseases 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 108010079855 Peptide Aptamers Proteins 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010094028 Prothrombin Proteins 0.000 description 1
- 102100027378 Prothrombin Human genes 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 101150025711 TF gene Proteins 0.000 description 1
- 102000002933 Thioredoxin Human genes 0.000 description 1
- 108090000848 Ubiquitin Proteins 0.000 description 1
- 102000044159 Ubiquitin Human genes 0.000 description 1
- 208000000208 Wet Macular Degeneration Diseases 0.000 description 1
- 230000009824 affinity maturation Effects 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 238000012575 bio-layer interferometry Methods 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 238000003570 cell viability assay Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 230000004154 complement system Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- RWYFURDDADFSHT-RBBHPAOJSA-N diane Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=C1.C1=C(Cl)C2=CC(=O)[C@@H]3CC3[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RWYFURDDADFSHT-RBBHPAOJSA-N 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- 201000006585 gastric adenocarcinoma Diseases 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 201000003911 head and neck carcinoma Diseases 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 230000009835 non selective interaction Effects 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 239000002953 phosphate buffered saline Substances 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 229940039716 prothrombin Drugs 0.000 description 1
- 108010014806 prothrombinase complex Proteins 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 108060008226 thioredoxin Proteins 0.000 description 1
- 229940094937 thioredoxin Drugs 0.000 description 1
- 230000005751 tumor progression Effects 0.000 description 1
- 210000003606 umbilical vein Anatomy 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/36—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against blood coagulation factors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/68031—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being an auristatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6843—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a material from animals or humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6849—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a receptor, a cell surface antigen or a cell surface determinant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/10—Cells modified by introduction of foreign genetic material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/77—Internalization into the cell
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/745—Assays involving non-enzymic blood coagulation factors
- G01N2333/7454—Tissue factor (tissue thromboplastin, Factor III)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
Definitions
- TF Tissue factor
- FVIIa serine protease factor Vila
- the TF/FVIIa complex catalyzes conversion of the inactive protease factor X (FX) into the active protease factor Xa (FXa).
- FXa and its co-factor FVa form the prothrombinase complex, which generates thrombin from prothrombin.
- Thrombin converts soluble fibrinogen into insoluble strands of fibrin and catalyzes many other coagulation-related processes.
- TF is over-expressed on multiple types of solid tumors.
- TF/FVIIa signaling can support angiogenesis, tumor progression, and metastasis.
- Increased TF expression can also induce inflammation and/or angiogenesis in many other diseases, including wet age- related macular degeneration (AMD) and diabetic retinopathy.
- AMD wet age- related macular degeneration
- TF Tissue Factor
- an isolated human antibody which binds to the extracellular domain of human Tissue Factor (TF), wherein the antibody binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa.
- TF Tissue Factor
- the isolated antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822, and (2) the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the isolated antibody inhibits human thrombin generation to a lesser extent as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822, and (2) the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the isolated antibody allows human thrombin generation to a greater extent as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822, and (2) the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the isolated antibody inhibits human thrombin generation by a lesser amount as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822, and (2) the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the isolated antibody allows human thrombin generation by a greater amount as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822, and (2) the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:779; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:780; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:78l; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:782; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:783; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:784.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:872; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:873; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:874; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:875; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:876; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:877.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:878; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:879; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:880; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:88l; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:882; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:883.
- the isolated antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the isolated antibody inhibits human thrombin generation to a lesser extent as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the isolated antibody allows human thrombin generation to a greater extent as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the isolated antibody inhibits human thrombin generation by a lesser amount as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the isolated antibody allows human thrombin generation by a greater amount as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA). In some embodiments, the antibody does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control. In some embodiments, the antibody does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control. In some embodiments, the antibody does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control.
- TGA thrombin generation assay
- the antibody allows human thrombin generation as determined by thrombin generation assay (TGA).
- TGA thrombin generation assay
- the antibody maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control.
- the antibody maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control.
- the antibody preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control.
- ETP endogenous thrombin potential
- the antibody binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX. In some embodiments, the antibody does not interfere with the ability of TF :FVIIa to convert FX into FXa.
- the antibody does not compete for binding to human TF with human FVIIa.
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA), allows human thrombin generation as determined by thrombin generation assay (TGA), binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX, does not interfere with the ability of TF :F Vila to convert FX into FXa, and does not compete for binding to human TF with FVIIa.
- TGA thrombin generation assay
- TGA allows human thrombin generation as determined by thrombin generation assay
- binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX does not interfere with the ability of TF :F Vila to convert FX into FXa, and does not compete for binding to human TF with FVIIa.
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA), does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control, allows human thrombin generation as determined by thrombin generation assay (TGA), preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control, binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX, does not interfere with the ability of TF :F Vila to convert FX into FXa, and does not compete for binding to human TF with FVIIa.
- TGA thrombin generation assay
- EDP endogenous thrombin potential
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA), does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control, does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control, does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control, allows human thrombin generation as determined by thrombin generation assay (TGA), maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control, maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control, preserves the endogenous thrombin potential
- the antibody inhibits FVIIa-dependent TF signaling.
- the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N.
- the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is K68N.
- the binding between the isolated antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
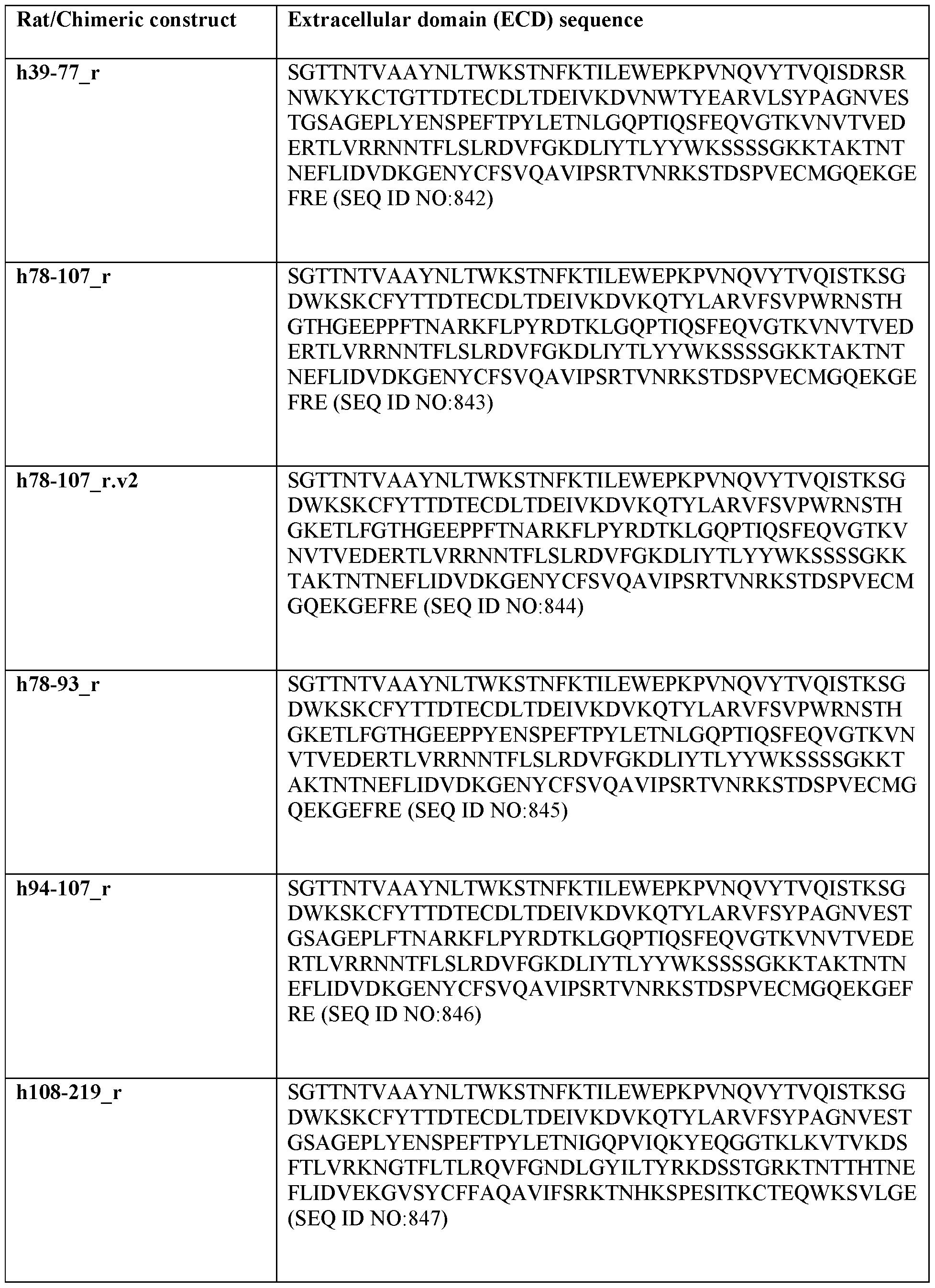
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 1-77 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 1-76 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 39-77 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 38-76 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 146-158 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 151-163 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 159-219 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-224 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 159-189 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-194 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 159-174 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-179 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 167-174 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 172-179 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a rat TF extracellular domain with amino acid residues 141-194 of the sequence shown in SEQ ID NO: 838 replaced by human TF extracellular domain amino acid residues 136-189 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- extracellular domain of TF of the sequence shown in SEQ ID NO: 810; the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 39-77 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 38-76 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO; the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 146-158 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 151-163 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO; and the binding between the isolated antibody and a rat TF extracellular domain with amino acid residues 141-194 of the sequence shown in SEQ ID NO: 838 replaced by human TF extracellular domain amino acid residues 136-189 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810; the binding between the isolated antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO; the binding between the isolated antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810; the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 1-77 of the sequence shown in SEQ
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO; the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 146-158 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 151-163 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810; the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 159-219 of the sequence shown in SEQ ID NO:8lO replaced by
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; the mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is K68N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody binds to cynomolgus TF. In some embodiments, the antibody binds to mouse TF. In some embodiments, the antibody binds to rabbit TF. In some embodiments, the antibody binds to pig TF.
- the antibody reduces lesion size in a swine choroidal neovascularization (CNV) model.
- CNV swine choroidal neovascularization
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); and (b) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) allows human thrombin generation as determined by thrombin generation assay (TGA); and(b) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and (c) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) allows human thrombin generation as determined by thrombin generation assay (TGA); (b) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and (c) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) binds to cynomolgus TF; (c) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and (d) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) allows human thrombin generation as determined by thrombin generation assay (TGA); (b) binds to cynomolgus TF; (c) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and (d) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay;
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) allows human thrombin generation as determined by thrombin generation assay (TGA); (c) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (d) does not interfere with the ability of TF :F Vila to convert FX into FXa; (e) does not compete for binding to human TF with FVIIa; (f) inhibits FVIIa-dependent TF signaling; (g) binds to cynomolgus TF; (h) binds to mouse TF; and (i) binds to rabbit TF.
- TGA thrombin generation assay
- TGA allows human thrombin generation as determined by thrombin generation assay
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (c) allows human thrombin generation as determined by thrombin generation assay (TGA); (d) preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (f) does not interfere with the ability of TF :F Vila to convert FX into FXa; (g) does not compete for binding to human TF with FVIIa; (h) inhibits FVIIa-dependent TF signaling; (i) binds to cynomolgus
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (c) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (d) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) allows human thrombin generation as determined by thrombin generation assay (TGA); (f) maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (g) maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPe
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) allows human thrombin generation as determined by thrombin generation assay (TGA); (c) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (d) does not interfere with the ability of TF :F Vila to convert FX into FXa; (e) does not compete for binding to human TF with FVIIa; (f) inhibits FVIIa-dependent TF signaling; (g) binds to cynomolgus TF; (h) binds to mouse TF; (i) binds to rabbit TF; (j) binds to pig TF; and (k) reduces lesion size in a swine choroidal neovascularization (CNV) model.
- TGA thrombin generation assay
- TGA allows human thrombin generation as determined by
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (c) allows human thrombin generation as determined by thrombin generation assay (TGA); (d) preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (f) does not interfere with the ability of TF :F Vila to convert FX into FXa; (g) does not compete for binding to human TF with FVIIa; (h) inhibits FVIIa-dependent TF signaling; (i) binds to cynomolgus
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (c) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (d) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) allows human thrombin generation as determined by thrombin generation assay (TGA); (f) maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (g) maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPe
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (c) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (d) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) allows human thrombin generation as determined by thrombin generation assay (TGA); (f) maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (g) maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPe
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (c) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (d) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) allows human thrombin generation as determined by thrombin generation assay (TGA); (f) maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (g) maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPe
- the antibody competes for binding to human TF with the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody competes for binding to human TF with the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody competes for binding to human TF with the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody binds to the same human TF epitope bound by the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody binds to the same human TF epitope bound by the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody binds to the same human TF epitope bound by the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody comprises all three heavy chain Complementary Determining Regions (CDRs) and all three light chain CDRs from: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the three heavy chain CDRs and the three light chain CDRs are determined using Rabat, Chothia, AbM, Contact, or IMGT numbering.
- the antibody comprises all three heavy chain Complementary Determining Regions (CDRs) and all three light chain CDRs from: the antibody designated 25A, the antibody designated 25A5-T, the antibody designated 25A3, the antibody designated 25 A5, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- CDRs Complementary Determining Regions
- the antibody comprises all three heavy chain Complementary Determining Regions (CDRs) and all three light chain CDRs from: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25A.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25 A3.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25 A5.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25A5-T. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G1. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G9. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B1.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B7. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D7. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D8. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43E. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43Ea.
- the antibody comprises a VH sequence of SEQ ID NO: 113 and a VL sequence of SEQ ID NO: 114. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO: 151 and a VL sequence of SEQ ID NO: 152. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO: 189 and a VL sequence of SEQ ID NO: 190. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:836 and a VL sequence of SEQ ID NO:837. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:227 and a VL sequence of SEQ ID NO:228.
- the antibody comprises a VH sequence of SEQ ID NO:265 and a VL sequence of SEQ ID NO:266. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:303 and a VL sequence of SEQ ID NO:304. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:455 and a VL sequence of SEQ ID NO:456. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:493 and a VL sequence of SEQ ID NO:494. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:53 l and a VL sequence of SEQ ID NO:532.
- the antibody comprises a VH sequence of SEQ ID NO:569 and a VL sequence of SEQ ID NO:570. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:607 and a VL sequence of SEQ ID NO:608. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:645 and a VL sequence of SEQ ID NO:646. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:683 and a VL sequence of SEQ ID NO:684. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:72l and a VL sequence of SEQ ID NO:722.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:779; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:780; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:78l; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:782; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:783; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:784.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:872; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:873; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:874; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:875; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:876; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:877.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:878; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:879; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:880; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:88l; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:882; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:883.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:797; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:798; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:799; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:800; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:80l; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:802.
- the antibody comprises a VH sequence of SEQ ID NO:763 and a VL sequence of SEQ ID NO:764. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:868 and a VL sequence of SEQ ID NO:869. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:870 and a VL sequence of SEQ ID NO:87l. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:769 and a VL sequence of SEQ ID NO:770.
- the antibody comprises: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody comprises: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody comprises: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody consists of: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody consists of: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody consists of: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- an isolated antibody comprising all three heavy chain Complementary Determining Regions (CDRs) and all three light chain CDRs from: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- CDRs Complementary Determining Regions
- the antibody is human, humanized, or chimeric.
- the three heavy chain CDRs and the three light chain CDRs are determined using Rabat, Chothia, AbM, Contact, or IMGT numbering.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25A. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25 A3. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25 A5. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25A5-T. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G1. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G9.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B1. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B7. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D7. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D8. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43E. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43Ea.
- the antibody comprises a VH sequence of SEQ ID NO: 113 and a VL sequence of SEQ ID NO: 114. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO: 151 and a VL sequence of SEQ ID NO: 152. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO: 189 and a VL sequence of SEQ ID NO: 190. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:836 and a VL sequence of SEQ ID NO:837. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:227 and a VL sequence of SEQ ID NO:228.
- the antibody comprises a VH sequence of SEQ ID NO:265 and a VL sequence of SEQ ID NO:266. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:303 and a VL sequence of SEQ ID NO:304. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:455 and a VL sequence of SEQ ID NO:456. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:493 and a VL sequence of SEQ ID NO:494. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:53 l and a VL sequence of SEQ ID NO:532.
- the antibody comprises a VH sequence of SEQ ID NO:569 and a VL sequence of SEQ ID NO:570. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:607 and a VL sequence of SEQ ID NO:608. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:645 and a VL sequence of SEQ ID NO:646. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:683 and a VL sequence of SEQ ID NO:684. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:72l and a VL sequence of SEQ ID NO:722.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:779; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:780; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:78l; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:782; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:783; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:784.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:872; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:873; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:874; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:875; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:876; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:877.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:878; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:879; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:880; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:88l; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:882; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:883.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:797; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:798; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:799; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:800; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:80l; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:802.
- the antibody comprises a VH sequence of SEQ ID NO:763 and a VL sequence of SEQ ID NO:764. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:868 and a VL sequence of SEQ ID NO:869. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:870 and a VL sequence of SEQ ID NO:87l. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:769 and a VL sequence of SEQ ID NO:770.
- the antibody comprises: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody comprises: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody comprises: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody consists of: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody consists: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody consists: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- an isolated antibody that competes for binding to human TF with: the antibody designated 1F, the antibody designated 1G, the antibody designated 29D, the antibody designated 29E, the antibody designated 39 A, or the antibody designated 54E.
- the antibody is human, humanized, or chimeric.
- the antibody inhibits FVIIa-dependent TF signaling.
- the antibody binds to cynomolgus TF.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 78-93 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-98 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 78-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 78-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-85 and 92-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810; and the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 78-93 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-98 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the isolated antibody relative to an isotype control in a live cell staining assay.
- the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810; the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 78-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the isolated antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO; and wherein the binding between the isolated antibody and a human TF extracellular domain with amino acid residues 78-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino
- the antibody comprises all three heavy chain
- Complementary Determining Regions and all three light chain CDRs from: the antibody designated 1F, the antibody designated 1G, the antibody designated 29D, the antibody designated 29E, the antibody designated 39 A, the antibody designated 43Ea, or the antibody designated 54E.
- the three heavy chain CDRs and the three light chain CDRs are determined using Rabat, Chothia, AbM, Contact, or IMGT numbering.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 1F. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 1G. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 29D. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 29E. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 39 A. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 1G. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 29D. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 29E. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 54E.
- the antibody comprises a VH sequence of SEQ ID NO:37 and a VL sequence of SEQ ID NO:38. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:75 and a VL sequence of SEQ ID NO:76. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:34l and a VL sequence of SEQ ID
- the antibody comprises a VH sequence of SEQ ID NO:379 and a VL sequence of SEQ ID NO:380. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:4l7 and a VL sequence of SEQ ID NO:4l8. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:759 and a VL sequence of SEQ ID NO:760.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:773; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:774; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:775; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:776; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:777; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:778.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:785; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:786; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:787; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:788; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:789; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:790.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:79l; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:792; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:793; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:794; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:795; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:796.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:803; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:804; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:805; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:806; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:807; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:808.
- the antibody comprises a VH sequence of SEQ ID NO:76l and a VL sequence of SEQ ID NO:762. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:765 and a VL sequence of SEQ ID NO:766. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:767 and a VL sequence of SEQ ID NO:768. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:77l and a VL sequence of SEQ ID NO:772.
- the antibody comprises: the antibody designated 1F, the antibody designated 1G, the antibody designated 29D, the antibody designated 29E, the antibody designated 39A, or the antibody designated 54E. [00108] In some embodiments, the antibody consists of: the antibody designated 1F, the antibody designated 1G, the antibody designated 29D, the antibody designated 29E, the antibody designated 39A, or the antibody designated 54E.
- an isolated antibody comprising: a VH- CDR1 comprising the sequence set forth in SEQ ID NO:773; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:774; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:775; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:776; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:777; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:778.
- an isolated antibody comprising: a VH- CDR1 comprising the sequence set forth in SEQ ID NO:779; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:780; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:78l; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:782; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:783; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:784.
- an isolated antibody comprising: a VH- CDR1 comprising the sequence set forth in SEQ ID NO: 785; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:786; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:787; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:788; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:789; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:790.
- an isolated antibody comprising: a VH- CDR1 comprising the sequence set forth in SEQ ID NO: 791; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:792; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:793; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:794; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:795; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:796.
- an isolated antibody comprising: a VH- CDR1 comprising the sequence set forth in SEQ ID NO: 797; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:798; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:799; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:800; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:80l; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:802.
- an isolated antibody comprising: a VH- CDR1 comprising the sequence set forth in SEQ ID NO:803; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:804; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:805; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:806; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:807; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO: 808.
- an isolated antibody comprising: a VH- CDR1 comprising the sequence set forth in SEQ ID NO:872; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:873; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:874; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:875; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:876; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:877.
- an isolated antibody comprising: a VH- CDR1 comprising the sequence set forth in SEQ ID NO:878; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:879; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:880; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:88l; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:882; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:883.
- the antibody binds to human TF with a KD of less than or equal to 50 nM, 10 nM, 5 nM, 1 nM, 0.5 nM or 0.1 nM, as measured by Octet QK384 or
- the antibody is a monoclonal antibody.
- the antibody is multispecific.
- the antibody is a Fab, Fab', F(ab')2 , Fv, scFv, (scFv)2, single chain antibody molecule, dual variable domain antibody, single variable domain antibody, linear antibody, or V domain antibody.
- the antibody comprises a scaffold, optionally wherein the scaffold is Fc, optionally human Fc.
- the antibody comprises a heavy chain constant region of a class selected from IgG, IgA, IgD, IgE, and IgM.
- the antibody comprises a heavy chain constant region of the class IgG and a subclass selected from IgGl, IgG2, IgG3, and IgG4.
- the antibody comprises a heavy chain constant region of IgGl.
- the Fc comprises one or more modifications, wherein the one or more modifications result in increased half-life, increased antibody-dependent cellular cytotoxicity (ADCC), increased antibody-dependent cellular phagocytosis (ADCP), increased complement-dependent cytotoxicity (CDC), or decreased effector function, compared with the Fc without the one or more modifications.
- ADCC antibody-dependent cellular cytotoxicity
- ADCP antibody-dependent cellular phagocytosis
- CDC complement-dependent cytotoxicity
- an isolated antibody that competes for binding to human TF with any antibody above.
- an isolated antibody that binds the human TF epitope bound by any antibody above.
- a vector or set of vectors comprising the polynucleotide or set of polynucleotides above.
- a host cell comprising the polynucleotide or set of polynucleotides above or the vector or set of vectors above.
- a method of producing an antibody comprising expressing the antibody with the host cell above and isolating the expressed antibody.
- composition comprising any antibody above and a pharmaceutically acceptable excipient.
- a method of treating or preventing a disease or condition in a subject in need thereof comprising administering to the subject an effective amount of any antibody above or the pharmaceutical composition above.
- the disease or condition is cancer.
- the cancer is head and neck cancer.
- the cancer is ovarian cancer.
- the cancer is gastric cancer.
- the cancer is esophageal cancer.
- the cancer is cervical cancer.
- the cancer is prostate cancer. In some embodiments, the cancer is pancreatic cancer. In some embodiments, the cancer is estrogen receptors negative (ER-), progesterone receptors negative (PR-), and HER2 negative (HER2-) triple negative breast cancer. In some embodiments, the cancer is glioblastoma. In some embodiments, the cancer is lung cancer. In some embodiments, the cancer is bladder cancer. In some embodiments, the cancer is melanoma. In some embodiments, the cancer is kidney cancer.
- the disease or condition involves neovascularization.
- the disease or condition involving neovascularization is age-related macular degeneration (AMD), diabetic retinopathy, or cancer.
- AMD age-related macular degeneration
- the disease or condition involves vascular inflammation.
- the method further comprises administering one or more additional therapeutic agents to the subject.
- the additional therapeutic agent is formulated in the same pharmaceutical composition as the antibody.
- the additional therapeutic agent is formulated in a different pharmaceutical composition from the antibody.
- the additional therapeutic agent is administered prior to administering the antibody.
- the additional therapeutic agent is administered after administering the antibody.
- the additional therapeutic agent is administered contemporaneously with the antibody.
- a method of detecting TF in a subject having or suspected of having a disease or condition comprising: (a) receiving a sample from the subject; and (b) detecting the presence or the level of TF in the sample by contacting the sample with any antibody above.
- the disease or condition is cancer.
- the cancer is head and neck cancer.
- the cancer is ovarian cancer.
- the cancer is gastric cancer.
- the cancer is esophageal cancer.
- the cancer is cervical cancer.
- the cancer is prostate cancer. In some embodiments, the cancer is pancreatic cancer. In some embodiments, the cancer is estrogen receptors negative (ER-), progesterone receptors negative (PR-), and HER2 negative (HER2-) triple negative breast cancer. In some embodiments, the cancer is glioblastoma. In some embodiments, the cancer is lung cancer. In some embodiments, the cancer is bladder cancer. In some embodiments, the cancer is melanoma. In some embodiments, the cancer is kidney cancer.
- the disease or condition involves neovascularization.
- the disease or condition involving neovascularization is age-related macular degeneration (AMD), diabetic retinopathy, or cancer.
- AMD age-related macular degeneration
- the disease or condition involves vascular inflammation.
- a method of detecting TF in a subject having or suspected of having a disease or condition comprising: (a) administering to the subject any antibody above; and (b) detecting the presence or the level of TF in the subject.
- the disease or condition is cancer.
- the cancer is head and neck cancer.
- the cancer is ovarian cancer.
- the cancer is gastric cancer.
- the cancer is esophageal cancer.
- the cancer is cervical cancer.
- the cancer is prostate cancer.
- the cancer is pancreatic cancer.
- the cancer is estrogen receptors negative (ER-), progesterone receptors negative (PR-), and HER2 negative (HER2-) triple negative breast cancer.
- the cancer is glioblastoma.
- the cancer is lung cancer.
- the cancer is bladder cancer.
- the cancer is melanoma.
- the cancer is kidney cancer.
- the disease or condition involves neovascularization.
- the disease or condition involving neovascularization is age-related macular degeneration (AMD), diabetic retinopathy, or cancer.
- AMD age-related macular degeneration
- the disease or condition involves vascular inflammation.
- kits comprising any antibody above or the pharmaceutical composition above and instructions for use.
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody binds to the extracellular domain of human Tissue Factor (TF) at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa.
- anti-hTF anti-human Tissue Factor
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO: 822, and (2) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the antibody inhibits human thrombin generation to a lesser extent as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO: 822, and (2) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the antibody allows human thrombin generation to a greater extent as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO: 822, and (2) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the antibody inhibits human thrombin generation by a lesser amount as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO: 822, and (2) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the antibody allows human thrombin generation by a greater amount as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO: 822, and (2) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:779; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:780; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:78l; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:782; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:783; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:784.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:872; a VH-CDR2 HIcomprising the sequence set forth in SEQ ID NO:873; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:874; a VL- CDR1 comprising the sequence set forth in SEQ ID NO:875; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:876; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:877.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:878; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:879; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:880; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:88l; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:882; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:883.
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the antibody inhibits human thrombin generation to a lesser extent as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the antibody allows human thrombin generation to a greater extent as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the antibody inhibits human thrombin generation by a lesser amount as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the antibody allows human thrombin generation by a greater amount as determined by thrombin generation assay (TGA) compared to a reference antibody comprising a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- TGA thrombin generation assay
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA). In some embodiments, the antibody does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control. In some embodiments, the antibody does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control. In some embodiments, the antibody does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control.
- TGA thrombin generation assay
- the antibody allows human thrombin generation as determined by thrombin generation assay (TGA).
- TGA thrombin generation assay
- the antibody maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control.
- the antibody maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control.
- the antibody preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control.
- ETP endogenous thrombin potential
- the antibody binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX. In some embodiments, the antibody does not interfere with the ability of TF :FVIIa to convert FX into FXa.
- the antibody does not compete for binding to human TF with human FVIIa.
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA), allows human thrombin generation as determined by thrombin generation assay (TGA), binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX, does not interfere with the ability of TF :F Vila to convert FX into FXa, and does not compete for binding to human TF with FVIIa.
- TGA thrombin generation assay
- TGA thrombin generation assay
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA), does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control, allows human thrombin generation as determined by thrombin generation assay (TGA), preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control, binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX, does not interfere with the ability of TF :F Vila to convert FX into FXa, and does not compete for binding to human TF with FVIIa.
- TGA thrombin generation assay
- EDP endogenous thrombin potential
- the antibody does not inhibit human thrombin generation as determined by thrombin generation assay (TGA), does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control, does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control, does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control, allows human thrombin generation as determined by thrombin generation assay (TGA), maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control, maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control, preserves the endogenous thrombin potential
- the antibody inhibits FVIIa-dependent TF signaling.
- the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N.
- the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is K68N.
- the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 39-77 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 38-76 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 146-158 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 151-163 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 159-219 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-224 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 159-189 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-194 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 159-174 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-179 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 167-174 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 172-179 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a rat TF extracellular domain with amino acid residues 141-194 of the sequence shown in SEQ ID NO:838 replaced by human TF extracellular domain amino acid residues 136-189 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810
- the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810
- the binding between the antibody and a human TF extracellular domain with amino acid residues 1-77 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 1-76 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810
- extracellular domain amino acid residues 38-76 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, the binding between the antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, the binding between the antibody and a human TF extracellular domain with amino acid residues 146-158 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 151-163 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, the binding between the antibody and a rat
- the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810
- the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810
- the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; the mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is K68N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody binds to cynomolgus TF. In some embodiments, the antibody binds to mouse TF. In some embodiments, the antibody binds to rabbit TF. In some embodiments, the antibody binds to pig TF.
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); and(b) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) allows human thrombin generation as determined by thrombin generation assay (TGA); and(b) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and (c) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) allows human thrombin generation as determined by thrombin generation assay (TGA); (b) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and (c) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- TGA thrombin generation assay
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) binds to cynomolgus TF; (c) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and (d) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) allows human thrombin generation as determined by thrombin generation assay (TGA); (b) binds to cynomolgus TF; (c) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and (d) the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay;
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) allows human thrombin generation as determined by thrombin generation assay (TGA); (c) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (d) does not interfere with the ability of TF :F Vila to convert FX into FXa; (e) does not compete for binding to human TF with F Vila; (f) inhibits F Vila-dependent TF signaling; (g) binds to cynomolgus TF; (h) binds to mouse TF; and (i) binds to rabbit TF.
- TGA thrombin generation assay
- TGA allows human thrombin generation as determined by thrombin generation assay
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (c) allows human thrombin generation as determined by thrombin generation assay (TGA); (d) preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (f) does not interfere with the ability of TF :F Vila to convert FX into FXa; (g) does not compete for binding to human TF with FVIIa; (h) inhibits FVIIa-dependent TF signaling; (i) binds to cynomolgus
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (c) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (d) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) allows human thrombin generation as determined by thrombin generation assay (TGA); (f) maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (g) maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPe
- (h) preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (i) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (j) does not interfere with the ability of TF:FVIIa to convert FX into FXa; (k) does not compete for binding to human TF with FVIIa; (1) inhibits FVIIa-dependent TF signaling; (m) binds to cynomolgus TF; (n) binds to mouse TF; and (o) binds to rabbit TF.
- ETP endogenous thrombin potential
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) allows human thrombin generation as determined by thrombin generation assay (TGA); (c) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (d) does not interfere with the ability of TF :F Vila to convert FX into FXa; (e) does not compete for binding to human TF with FVIIa; (f) inhibits FVIIa-dependent TF signaling; (g) binds to cynomolgus TF; (h) binds to mouse TF; (i) binds to rabbit TF; and (j) binds to pig TF.
- TGA thrombin generation assay
- TGA allows human thrombin generation as determined by thrombin generation assay
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (c) allows human thrombin generation as determined by thrombin generation assay (TGA); (d) preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (f) does not interfere with the ability of TF :F Vila to convert FX into FXa; (g) does not compete for binding to human TF with FVIIa; (h) inhibits FVIIa-dependent TF signaling; (i) binds to cynomolgus
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (c) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (d) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) allows human thrombin generation as determined by thrombin generation assay (TGA); (f) maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (g) maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPe
- (h) preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (i) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (j) does not interfere with the ability of TF:FVIIa to convert FX into FXa; (k) does not compete for binding to human TF with FVIIa; (1) inhibits FVIIa-dependent TF signaling; (m) binds to cynomolgus TF; (n) binds to mouse TF; (o) binds to rabbit TF; and (p) binds to pig TF.
- ETP endogenous thrombin potential
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (c) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (d) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) allows human thrombin generation as determined by thrombin generation assay (TGA); (f) maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (g) maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPe
- (h) preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (i) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (j) does not interfere with the ability of TF:FVIIa to convert FX into FXa; (k) does not compete for binding to human TF with FVIIa; (1) inhibits FVIIa-dependent TF signaling; (m) binds to cynomolgus TF; (n) binds to mouse TF; (o) binds to rabbit TF; (p) binds to pig TF; (q) the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO:
- the antibody (a) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (b) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (c) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (d) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (e) allows human thrombin generation as determined by thrombin generation assay (TGA); (f) maintains the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (g) maintains the time from the assay start to the thrombin peak on a thrombin generation curve (ttPe
- (h) preserves the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (i) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX; (j) does not interfere with the ability of TF:FVIIa to convert FX into FXa; (k) does not compete for binding to human TF with FVIIa; (1) inhibits FVIIa-dependent TF signaling; (m) binds to cynomolgus TF; (n) binds to mouse TF; (o) binds to rabbit TF; (p) binds to pig TF; (q) the binding between the antibody and a variant TF extracellular domain comprising a mutation K149N of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810,
- the antibody competes for binding to human TF with the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody competes for binding to human TF with the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody competes for binding to human TF with the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody binds to the same human TF epitope bound by the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody binds to the same human TF epitope bound by the antibody designated 25 A, the antibody designated 25 A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody binds to the same human TF epitope bound by the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody comprises all three heavy chain
- Complementary Determining Regions and all three light chain CDRs from: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the three heavy chain CDRs and the three light chain CDRs are determined using Rabat, Chothia, AbM, Contact, or IMGT numbering.
- the antibody comprises all three heavy chain
- Complementary Determining Regions and all three light chain CDRs from: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody comprises all three heavy chain
- Complementary Determining Regions and all three light chain CDRs from: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25A. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25 A3. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25 A5. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25A5-T. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G1.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 25G9. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B1. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43B7. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D7.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43D8. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43E. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 43Ea.
- the antibody comprises a VH sequence of SEQ ID NO:l 13 and a VL sequence of SEQ ID NO: 114. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO: 151 and a VL sequence of SEQ ID NO: 152. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO: 189 and a VL sequence of SEQ ID NO: 190. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:836 and a VL sequence of SEQ ID NO:837. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:227 and a VL sequence of SEQ ID NO:228.
- the antibody comprises a VH sequence of SEQ ID NO:265 and a VL sequence of SEQ ID NO:266. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:303 and a VL sequence of SEQ ID NO:304. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:455 and a VL sequence of SEQ ID NO:456. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:493 and a VL sequence of SEQ ID NO:494. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:53 l and a VL sequence of SEQ ID NO:532.
- the antibody comprises a VH sequence of SEQ ID NO:569 and a VL sequence of SEQ ID NO:570. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:607 and a VL sequence of SEQ ID NO:608. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:645 and a VL sequence of SEQ ID NO:646. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:683 and a VL sequence of SEQ ID NO:684. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:72l and a VL sequence of SEQ ID NO:722.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:779; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:780; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:78l; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:782; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:783; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:784.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:872; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:873; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:874; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:875; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:876; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:877.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:878; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:879; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:880; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:88l; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:882; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:883.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:797; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:798; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:799; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:800; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:80l; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:802.
- the antibody comprises a VH sequence of SEQ ID NO:763 and a VL sequence of SEQ ID NO:764. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:868 and a VL sequence of SEQ ID NO:869. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:870 and a VL sequence of SEQ ID NO:87l. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:769 and a VL sequence of SEQ ID NO:770.
- the antibody comprises: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody comprises: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody comprises: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody consists of: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, the antibody designated 25G9, the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- the antibody consists of: the antibody designated 25A, the antibody designated 25A3, the antibody designated 25A5, the antibody designated 25A5-T, the antibody designated 25G, the antibody designated 25G1, or the antibody designated 25G9.
- the antibody consists of: the antibody designated 43B, the antibody designated 43B1, the antibody designated 43B7, the antibody designated 43D, the antibody designated 43D7, the antibody designated 43D8, the antibody designated 43E, or the antibody designated 43Ea.
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody competes for binding to human TF with: the antibody designated 1F, the antibody designated 1G, the antibody designated 29D, the antibody designated 29E, the antibody designated 39 A, or the antibody designated 54E.
- anti-hTF anti-human Tissue Factor
- the antibody inhibits FVIIa-dependent TF signaling.
- the antibody binds to cynomolgus TF.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 78-93 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-98 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 78-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 78-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-85 and 92-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810; and the binding between the antibody and a human TF extracellular domain with amino acid residues 78-93 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-98 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between the antibody and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810; the binding between the antibody and a human TF extracellular domain with amino acid residues 78-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810; and the binding between the antibody and a human TF extracellular domain with amino acid residues 78-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 77-85 and
- the antibody comprises all three heavy chain
- Complementary Determining Regions and all three light chain CDRs from: the antibody designated 1F, the antibody designated 1G, the antibody designated 29D, the antibody designated 29E, the antibody designated 39 A, the antibody designated 43Ea, or the antibody designated 54E.
- the three heavy chain CDRs and the three light chain CDRs are determined using Rabat, Chothia, AbM, Contact, or IMGT numbering.
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 1F. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 1G. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 29D. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 29E. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 39 A. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 1F. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 1G. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 29D. In some embodiments, the antibody comprises all three heavy chain CDRs and all three light
- the antibody comprises all three heavy chain CDRs and all three light chain CDRs from the antibody designated 54E.
- the antibody comprises a VH sequence of SEQ ID NO:37 and a VL sequence of SEQ ID NO:38. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:75 and a VL sequence of SEQ ID NO:76. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:34l and a VL sequence of SEQ ID NO:342. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:379 and a VL sequence of SEQ ID NO:380. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:4l7 and a VL sequence of SEQ ID NO:4l8. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:759 and a VL sequence of SEQ ID NO:760.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:773; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:774; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:775; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:776; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:777; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:778.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:785; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:786; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:787; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:788; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:789; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:790.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:79l; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:792; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:793; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:794; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:795; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:796.
- the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:803; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:804; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:805; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:806; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:807; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:808.
- the antibody comprises a VH sequence of SEQ ID NO:76l and a VL sequence of SEQ ID NO:762. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:765 and a VL sequence of SEQ ID NO:766. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:767 and a VL sequence of SEQ ID NO:768. In some embodiments, the antibody comprises a VH sequence of SEQ ID NO:77l and a VL sequence of SEQ ID NO:772.
- the antibody comprises: the antibody designated 1F, the antibody designated 1G, the antibody designated 29D, the antibody designated 29E, the antibody designated 39A, or the antibody designated 54E. In some embodiments, the antibody consists of: the antibody designated 1F, the antibody designated 1G, the antibody designated 29D, the antibody designated 29E, the antibody designated 39 A, or the antibody designated 54E.
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:773; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:774; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:775; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:776; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:777; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:778.
- anti-hTF anti-human Tissue Factor
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:779; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:780; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:78l; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:782; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:783; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:784.
- anti-hTF anti-human Tissue Factor
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:785; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:786; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:787; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:788; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:789; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:790.
- anti-hTF anti-human Tissue Factor
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:79l; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:792; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:793; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:794; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:795; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:796.
- anti-hTF anti-human Tissue Factor
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:797; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:798; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:799; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:800; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:80l; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:802.
- anti-hTF anti-human Tissue Factor
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:803; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:804; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:805; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:806; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:807; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:808.
- anti-hTF anti-human Tissue Factor
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:872; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:873; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:874; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:875; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:876; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:877.
- anti-hTF anti-human Tissue Factor
- an antibody-drug conjugate comprising: antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody comprises: a VH-CDR1 comprising the sequence set forth in SEQ ID NO:878; a VH-CDR2 comprising the sequence set forth in SEQ ID NO:879; a VH-CDR3 comprising the sequence set forth in SEQ ID NO:880; a VL-CDR1 comprising the sequence set forth in SEQ ID NO:88l; a VL-CDR2 comprising the sequence set forth in SEQ ID NO:882; and a VL-CDR3 comprising the sequence set forth in SEQ ID NO:883.
- anti-hTF anti-human Tissue Factor
- the antibody is human, humanized, or chimeric.
- the antibody binds to human TF with a KD of less than or equal to 50 nM, 10 nM, 5 nM, 1 nM, 0.5 nM or 0.1 nM, as measured by Octet QK384 or
- the antibody is a monoclonal antibody.
- the antibody is multispecific.
- the antibody is a Fab, Fab', F(ab')2 , Fv, scFv, (scFv)2, single chain antibody molecule, dual variable domain antibody, single variable domain antibody, linear antibody, or V domain antibody.
- the antibody comprises a scaffold, optionally wherein the scaffold is Fc, optionally human Fc.
- the antibody comprises a heavy chain constant region of a class selected from IgG, IgA, IgD, IgE, and IgM.
- the antibody comprises a heavy chain constant region of the class IgG and a subclass selected from IgGl, IgG2, IgG3, and IgG4.
- the antibody comprises a heavy chain constant region of IgGl.
- the Fc comprises one or more modifications, wherein the one or more modifications result in increased half- life, increased antibody-dependent cellular cytotoxicity (ADCC), increased antibody- dependent cellular phagocytosis (ADCP), increased complement-dependent cytotoxicity (CDC), or decreased effector function, compared with the Fc without the one or more modifications.
- ADCC antibody-dependent cellular cytotoxicity
- ADCP antibody- dependent cellular phagocytosis
- CDC complement-dependent cytotoxicity
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody competes for binding to human TF with any antibody above.
- anti-hTF anti-human Tissue Factor
- an antibody-drug conjugate comprising: an anti-human Tissue Factor (anti-hTF) antibody, a cytotoxic agent linked to the antibody, and optionally a linker that links the antibody to the cytotoxic agent, wherein the antibody binds the human TF epitope bound by any antibody above.
- anti-hTF anti-human Tissue Factor
- the cytotoxic agent comprises an anti-angiogenic agent, a pro-apoptotic agent, an anti-mitotic agent, an anti-kinase agent, an alkylating agent, a hormone, a hormone agonist, a hormone antagonist, a chemokine, a drug, a prodrug, a toxin, an enzyme, an antimetabolite, an antibiotic, an alkaloid, or a radioactive isotope.
- the cytotoxic agent comprises at least one of: calicheamycin, camptothecin, carboplatin, irinotecan, SN-38, carboplatin, camptothecan, cyclophosphamide, cytarabine, dacarbazine, docetaxel, dactinomycin, daunorubicin, doxorubicin, doxorubicin, etoposide, idarubicin, topotecan, vinca alkaloid, maytansinoid, maytansinoid analog,
- the linker comprises a labile linker, an acid labile linker, a photolabile linker, a charged linker, a disulfide-containing linker, a peptidase-sensitive linker, a b-glucuronide-linker, a dimethyl linker, a thio-ether linker, or a hydrophilic linker.
- the linker is a cleavable linker. In some embodiments, the linker is a non- cleavable linker.
- a pharmaceutical composition comprising any antibody-drug conjugate above and a pharmaceutically acceptable excipient.
- a method of treating or preventing a disease or condition in a subject in need thereof comprising administering to the subject an effective amount of any antibody-drug conjugate above or the pharmaceutical composition above.
- the disease or condition is cancer.
- the cancer is head and neck cancer.
- the cancer is ovarian cancer.
- the cancer is gastric cancer.
- the cancer is esophageal cancer.
- the cancer is cervical cancer.
- the cancer is prostate cancer. In some embodiments, the cancer is pancreatic cancer. In some embodiments, the cancer is estrogen receptors negative (ER-), progesterone receptors negative (PR-), and HER2 negative (HER2-) triple negative breast cancer. In some embodiments, the cancer is glioblastoma. In some embodiments, the cancer is lung cancer. In some embodiments, the cancer is bladder cancer. In some embodiments, the cancer is melanoma. In some embodiments, the cancer is kidney cancer.
- the method futher comprises administering one or more additional therapeutic agents to the subject.
- the additional therapeutic agent is formulated in the same pharmaceutical composition as the antibody-drug conjugate.
- the additional therapeutic agent is formulated in a different pharmaceutical composition from the antibody-drug conjugate.
- the additional therapeutic agent is administered prior to administering the antibody-drug conjugate.
- the additional therapeutic agent is administered after administering the antibody-drug conjugate.
- the additional therapeutic agent is administered contemporaneously with the antibody-drug conjugate.
- a method of detecting TF in a subject having or suspected of having a disease or condition comprising: (a) administering to the subject any antibody-drug conjugate above; and (b) detecting the presence or the level of TF in the subject.
- the disease or condition is cancer.
- the cancer is head and neck cancer.
- the cancer is ovarian cancer.
- the cancer is gastric cancer.
- the cancer is esophageal cancer.
- the cancer is cervical cancer.
- the cancer is prostate cancer. In some embodiments, the cancer is pancreatic cancer. In some embodiments, the cancer is estrogen receptors negative (ER-), progesterone receptors negative (PR-), and HER2 negative (HER2-) triple negative breast cancer. In some embodiments, the cancer is glioblastoma. In some embodiments, the cancer is lung cancer. In some embodiments, the cancer is bladder cancer. In some embodiments, the cancer is melanoma. In some embodiments, the cancer is kidney cancer.
- kits comprising any antibody-drug conjugate above or the pharmaceutical composition above and instructions for use.
- Figures 1A and IB show binding of anti-TF antibodies to human TF-positive cells.
- Figure 1A shows the median fluorescence intensity (MFI) of antibody bound to HCT- 116 cells plotted against concentrations of antibodies from groups 1, 25, and 29 and the reportable cell ECso.
- Figure IB shows the median fluorescence intensity of antibody bound to HCT-l 16 cells plotted against concentrations of antibodies from groups 39, 43, and 54 and the reportable cell ECso.
- the isotype control in Figure IB applies to both Figures 1A and IB
- Figures 2A and 2B show binding of anti-TF antibodies to mouse TF-positive cells.
- Figure 2A shows the median fluorescence intensity (MFI) of antibody bound to CHO cells recombinantly expressing mouse TF (CHO-mTF) plotted against concentrations of antibodies from groups 1, 25, and 29 and the reportable cell ECso.
- Figure 2B shows the median fluorescence intensity of antibody bound to CHO-mTF cells plotted against concentrations of antibodies from groups 39, 43, and 54 and the reportable cell ECso.
- the isotype control in Figure 2B applies to both Figures 2A and 2B.
- Figures 3A and 3B show thrombin generation in the presence of anti-TF antibody.
- Figure 3A shows Peak Ila/Thrombin generation (% Peak Ila) as measured by the Thrombin Generation Assay (TGA) without antibody incubation prior to addition of calcium and thrombin substrate in the presence of titrations of anti-TF antibodies from groups 1, 25, 29, 39, 43, and 54.
- Figure 3B shows Peak Ila/Thrombin generation (% Peak Ila) as measured by the Thrombin Generation Assay (TGA) with a lO-min antibody incubation prior to addition of calcium and thrombin substrate in the presence of titrations of anti-TF antibodies from groups 1, 25, 29, 39, 43, and 54.
- Figures 4A and 4B show FXa conversion in the presence of anti-TF antibody.
- Figure 4A shows the percentage of FXa conversion (% FXa) by TF:FVIIa in MDA-MB-231 cells in the presence of titrations of anti-TF antibodies from groups 1, 25, and 29.
- Figure 4B shows the percentage of FXa conversion (% FXa) by TF:FVIIa in MDA-MB-231 cells in the presence of titrations of anti-TF antibodies from groups 39, 43, and 54.
- % FXa conversion at a reported concentration is calculated relative to an antibody-free FXa conversion reaction.
- the isotype control in Figure 4B applies to both Figures 4A and 4B.
- Figures 5A and 5B show FVIIa binding in the presence of anti-TF antibody.
- Figure 5 A shows the percentage of FVIIa binding (% FVIIa) in TF-positive MDA-MB-231 cells in the presence of titrations of anti-TF antibodies from groups 1, 25, and 29.
- Figure 5B shows the percentage of FVIIa binding (% FVIIa) in MDA-MB-231 cells in the presence of titrations of anti-TF antibodies from groups 39, 43, and 54.
- % FVIIa binding at a reported concentration is calculated relative to antibody-free FVIIa binding.
- the isotype control in Figure 5B applies to both Figures 5A and 5B.
- Figures 6A, 6B, 6C, and 6D show FVIIa-dependent TF signaling in the presence of anti-TF antibody.
- Figure 6A shows the concentration of IL8 (IL8 cone) in MDA-MB-231 cells in the presence of titrations of anti-TF antibodies from groups 1, 25, and 29.
- Figure 6B shows the concentration of IL8 (IL8 cone) in MDA-MB-231 cells in the presence of titrations of anti-TF antibodies from groups 39, 43, and 54.
- the control in Figure 6B applies to both Figures 6A and 6B.
- Figure 6C shows the concentration of GM-CSF (GM-CSF cone) in MDA-MB-231 cells in the presence of titrations of anti-TF antibodies from groups 1, 25, and 29.
- Figure 6D shows the concentration of GM-CSF (GM-CSF cone) in MDA-MB-231 cells in the presence of titrations of anti-TF antibodies from groups 39, 43, and 54.
- the control in Figure 6D applies to both Figures 6C and 6D.
- Figures 7 A and 7B show internalization of anti-TF antibody by TF-positive cells.
- Figure 7A shows the cell viability of TF-positive A431 cell cultures after the addition of an anti-TF antibody from groups 1, 25, and 29 and a secondary antibody against the human Fc conjugated to mono-methyl auristatin F (MMAF).
- Figure 7B shows the cell viability of TF- positive A431 cell cultures after the addition of an anti-TF antibody from groups 39, 43, and 54 and a secondary antibody against the human Fc conjugated to mono-methyl auristatin F (MMAF).
- the isotype control in Figure 7B applies to both Figures 7A and 7B.
- Figures 8A and 8B show thrombin generation in the presence of anti-TF antibody.
- Figure 8A shows Peak Ila/Thrombin generation (% Peak Ila) as measured by the Thrombin Generation Assay (TGA) without antibody incubation prior to addition of calcium and thrombin substrate in the presence of titrations of anti-TF antibodies from groups 25, 39, 43, and anti-TF Ml 593.
- Figure 8B shows Peak Ila/Thrombin generation (% Peak Ila) as measured by the Thrombin Generation Assay (TGA) with a lO-min antibody incubation prior to addition of calcium and thrombin substrate in the presence of titrations of anti-TF antibodies from groups 25, 39, 43, and anti-TF Ml 593.
- Figures 9A and 9B show anti-TF ADC-induced cell death in TF-positive cells.
- Figure 9A shows cell viability of TF-positive A431 cells after a 3-day incubation with titrations of anti-TF antibodies conjugated to MC-vc-PAB-MMAE (DAR of 3-4).
- Figure 9B shows cell viability of TF-positive HPAF-II cells after a 4-day incubation with titrations of anti-TF antibodies conjugated to MC-vc-PAB-MMAE (DAR of 3-4).
- Figures 10A and 10B show the effect of anti-TF ADCs on tumor size in xenograft models.
- Figure 10A shows the efficacy of anti-TF ADCs in the A431 xenograft model.
- Figure 10B shows the efficacy of anti-TF ADCs in the HPAF-II xenograft model.
- the arrows indicate treatments with ADC or vehicle (PBS) dosed at 5 mg/kg once per week for 3 weeks.
- Figure 11 shows the effect of anti-TF ADCs on tumor size in a head and neck cancer patient-derived xenograft model.
- the arrows indicate treatments with anti-TF ADC or IgGl control ADC dosed at 5 mg/kg once per week for 2 weeks.
- Figures 12A and 12B show binding of anti-TF antibodies to human TF-positive cancer cells.
- Figure 12A shows the median fluorescence intensity (MFI) of antibody bound to A431 cells plotted against concentrations of antibodies from groups 1, 25, 29, 39, 43, and 54. Reportable cell ECso’s and their 95% confidence intervals are listed.
- Figure 12B shows the median fluorescence intensity of antibody bound to MDA-MB-231 cells plotted against concentrations of antibodies from groups 25, 29, 39, and 43. Reportable cell ECso’s and their 95% confidence intervals are listed.
- Figures 13A, 13B and 13C show thrombin generation in the presence of anti-TF antibody.
- Figure 13A shows the thrombin generation curves in the absence or presence of 100 nM anti-TF antibodies from groups 1, 25, and 29 and previously generated anti-TF antibodies TF-011, 5G9, and 10H10 (samples on plate 1 of Table 44).
- Figure 13B shows the thrombin generation curves in the absence or presence of 100 nM anti-TF antibodies from groups 39, 43, and 54 (samples on plate 2 of Table 44).
- Figure 13C shows the peak thrombin concentration in the absence or presence of titrations of anti-TF antibodies. The mean of a triplicate data set is shown. The standard deviation of the mean is listed in Table 44.
- Figures 14A and 14B show TFT Vila-dependent FXa Conversion and FVII binding in the presence of anti-TF antibodies TF-011, 5G9, and 10H10.
- Figure 14A shows TFT Vila-dependent conversion of FX into FXa on the cell surface of MDA-MB-231 cells in the absence or presence of titrations of anti-TF antibodies TF-011, 5G9 and 10H10.
- Figure 14B shows FVII binding in the absence or presence of titrations of anti-TF antibodies TF- 011, 5G9 and 10H10 after pre-incubation of MDA-MB-231 cells with the anti-TF antibodies. For antibodies that exhibited no less than 25% competition with FVII, the ICso is reported.
- Figures 15A and 15B show percent binding (% Binding) of A488-conjugated 25 A3 anti-TF antibody to MDA-MB-231 cells after pre-incubation of the cells with titrations of unlabeled competitor antibodies.
- Figure 15A shows percent binding of 25A3 after pre incubation with unlabeled competitor antibodies from groups 1, 25, 29, 39, 43, and 54.
- Figure 15B shows percent binding of 25A3 after pre-incubation with unlabeled competitor antibodies TF-011, 5G9, and 10H10. The ICso value of antibodies that compete with 25A3 is listed.
- Figures 16A and 16B show percent binding (% Binding) of A488-conjugated 43D7 anti-TF antibody to MDA-MB-231 cells after pre-incubation of the cells with titrations of unlabeled competitor antibodies.
- Figure 16A shows percent binding of 43D7 after pre incubation with unlabeled competitor antibodies from groups 1, 25, 29, 39, 43, and 54.
- Figure 16B shows percent binding of 43D7 after pre-incubation with unlabeled competitor antibodies TF-011, 5G9, and 10H10. The ICso value of antibodies that compete with 43D7 is listed.
- Figures 17A and 17B show percent binding (% Binding) of A488-conjugated 39A anti-TF antibody to MDA-MB-231 cells after pre-incubation of the cells with titrations of unlabeled competitor antibodies.
- Figure 17A shows percent binding of 39A after pre- incubation with unlabeled competitor antibodies from groups 1, 25, 29, 39, 43, and 54.
- Figure 17B shows percent binding of 39A after pre-incubation with unlabeled competitor antibodies TF-011, 5G9, and 10H10. The ICso value of antibodies that compete with 39A is listed.
- Figures 18A, 18B, and 18C show the internalization of anti-TF antibodies as measured by cell viability assay and internalization assay.
- Figure 18A shows cell viability of TF-positive A431 cell cultures three days after titrations of anti-TF antibodies.
- Figure 18B shows cell viability of TF-positive A431 cell cultures three days after titrations of anti-TF antibodies complexed with a Fab fragment against the human Fc conjugated to mono-methyl auristatin F (Fab:MMAF). The ICso of the anti-TF antibody Fab:MMAF complexes is listed.
- Figure 18C shows internalization of anti-TF antibodies conjugated to A488. Percent internalization of A488-conjugated anti-TF antibodies at 4 h is listed.
- Figures 19A, 19B, and 19C show the binding of anti-TF antibodies and ADCs to human TF-positive HCT-l 16 cells.
- Figure 19A shows the binding of anti-TF antibodies HCT-l 16 cells.
- Figure 19B shows the binding of anti-TF ADCs to HCT-l 16 cells.
- Figure 19C lists reportable cell ECso’s and their 95% confidence intervals.
- Figures 20A, 20B, and 20C show cell viability of A431 cells after titrations of anti-TF ADCs.
- Figure 20A shows the cell viability after titrations of anti-TF ADCs with a continuous 72 h incubation.
- Figure 20B shows the cell viability after titrations of anti-TF ADCs with a 4 h incubation followed by removal of excess ADC and culture for another 68 h.
- Figure 20C lists the reportable ICso values of ADCs.
- Figures 21A, 21B, and 21C show the effect of FVIIa on the in vitro efficacy of anti-TF ADCs.
- Figure 21A shows the cell viability after titrations of anti-TF ADCs with a 4 h incubation followed by removal of excess ADC and culture for another 68 h in the absence of FVIIa.
- Figure 21B shows the cell viability after titrations of anti-TF ADCs with a 4 h incubation followed by removal of excess ADC and culture for another 68 h in the presence of FVIIa.
- Figure 21C lists the reportable ICso values.
- Figures 22A, 22B, 22C, 22D, and 22E show cell viability of additional cancer cell lines after titrations of anti-TF ADCs.
- Figure 22A shows the TF copy number in various cell lines with the anti-TF antibody 5G9. The standard error of the mean and the number of measurements (n) are also presented.
- Figure 22B shows the cell viability of HCT-l 16 cells after 72 h culture in the absence or presence of titrations of anti-TF MMAE ADCs.
- Figure 22C shows the cell viability of CHO cells after 72 h culture in the absence or presence of titrations of anti-TF MMAE ADCs.
- Figure 22D shows the cell viability of MDA-MB-231 cells after 5-day culture in the absence or presence of titrations of anti-TF MMAE ADCs.
- Figure 22E shows the cell viability of HPAF-II cells after 5-day culture in the absence or presence of titrations of anti-TF MMAE ADCs.
- Figures 23A and 23B show staining of the microtubule network after treatment with anti-TF 25A3 MMAE ADC (25A3-vc-MMAE) or isotype control MMAE ADC (isotype ctrl-vc-MMAE).
- Figure 23A shows staining of the microtubule network of A431 cells after treatment.
- Figure 23B shows staining of the microtubule network of HPAF-II cells after treatment. Scale bar, 10 microns.
- Figures 24A and 24B show the TF expression after cytokine treatment and the effect of anti-TF ADCs on the viability of cytokine-treated human umbilical vein endothelial cells (HUVECs).
- Figure 24A shows copy numbers of surface TF on HUVECs treated with or without an inflammatory cytokine cocktail for 3, 6, or 20 h prior to analysis.
- Figure 24B shows cell viability of inflammatory cytokine-treated HUVEC cultures after 4 days of culture in the presence of titrations of anti-TF or isotype-control MMAE ADCs.
- Figures 25A, 25B, and 25C show the quantitation of the G2/M arrest in HUVECs or HCT-l 16 cells treated for 24 h with titrations of anti-TF ADCs.
- Figure 25A shows the percentage of pH3-positive cells (% pH3) with titrations of anti-TF ADCs of HUVECs in the absence of inflammatory cytokines.
- Figure 25B shows the percentage of pH3-positive cells (% pH3) with titrations of anti-TF ADCs of HUVECs in the presence of inflammatory cytokines.
- Figure 25C shows the percentage of pH3-positive cells (% pH3) with titrations of anti-TF ADCs of HCT-l 16 cells.
- Figures 26A and 26B show the percentage of pH3-positive HCT-l 16 cells analyzed by flow cytometry with or without anti-TF ADC treatment.
- Figure 26A shows the pH3 versus DNA content dot plot after treatment of 10 nM Isotype-vc-MMAE.
- Figure 26B shows the pH3 versus DNA content dot plot after treatment of 10 nM 25A-vc-MMAE.
- Figures 27A and 27B show the sensitivity of HUVECs and HCT-l 16 cells to MMAE.
- Figure 27A shows the percentage of pH3-positive HUVECs (% pH3) in the absence or presence of 24 h of MMAE treatment.
- Figure 27B shows the percentage of pH3 -positive HCT-l 16 cells (% pH3) in the absence or presence of 24 h of MMAE treatment.
- Figure 28 shows the analysis of Erk phosphorylation by Western blotting with an anti-phospho-Erkl/2 antibody and an anti-Erkl/2 antibody. The values of pErk induction are listed.
- Figures 29A, 29B, and 29C show antibody-dependent cellular cytotoxicity (ADCC) reporter luminescence after a 6 h incubation of the reporter Jurkat cell line with TF- positive A431 cells.
- Figure 29A shows the ADCC reporter luminescence in the absence or presence of titrations anti-TF antibodies.
- Figure 29B shows the ADCC reporter luminescence in the absence or presence of titrations anti-TF ADCs.
- Figure 29C lists the ADCC reporter luminescence EC so values for each anti-TF antibody or ADC.
- Figures 30A and 30B show in vivo efficacy of anti-TF ADCs in HPAF-II xenograft model.
- Figure 30 A shows the mean tumor volume after weekly treatment of an anti-TF ADC at 5 mg/kg for 3 weeks.
- Figure 30B shows the mean tumor volume after weekly treatment of an anti-TF ADC at 2 mg/kg for 2 weeks.
- the mean tumor volumes (Mean) and tumor growth inhibition (TGI) percentages on day 21 are listed.
- the /’-values for the mean tumor volume comparison between each ADC and the vehicle control are also listed.
- the number of partial regression (PR), complete regression (CR), and tumor-free survivor (TFS) animals at the end of the study (day 59 for Figure 30A and day 39 for Figure 30B) are also listed.
- Figures 31A and 31B show in vivo efficacy of anti-TF ADCs in MDA-MB-231 xenograft model.
- Figure 31 A shows the mean tumor volume after weekly treatment of an anti-TF ADC at 4 mg/kg for 2 weeks.
- Figure 31B shows the mean tumor volume after weekly treatment of an anti-TF ADC at 2 mg/kg for 2 weeks.
- the mean tumor volume and tumor growth inhibition on day 25 ( Figure 31A) and day 27 ( Figure 31B) are listed.
- the P- values for the mean tumor volume comparison between each ADC and the vehicle control are also listed.
- the number of partial regression (PR), complete regression (CR), and tumor-free survivor (TFS) animals at the end of the study (day 49 for Figure 31 A and day 41 for Figure 31B) are also listed.
- Figure 32 shows the mean tumor volume after weekly treatment of unconjugated anti-TF antibodies at 10 mg/kg for 2 weeks in the HPAF-II xenograft model. The mean tumor volume on day 15 is listed.
- Figures 33A, 33B, and 33C show in vivo efficacy of anti-TF ADCs in patient- derived xenograft (PDX) models.
- Figure 33A shows the mean tumor volume in the PDX model of a head and neck carcinoma after treatment of an anti-TF ADC.
- Figure 33B shows the mean tumor volume in the PDX model of an ovarian carcinoma after treatment of an anti- TF ADC.
- Figure 33C shows the mean tumor volume in the PDX model of a gastric adenocarcinoma after treatment of an anti-TF ADC.
- the mean tumor volume and tumor growth inhibition on day 44 ( Figure 33 A), day 15 ( Figure 33B), and day 25 ( Figure 33C) are listed.
- the /-’-values for the mean tumor volume comparison between each ADC and the isotype control are also listed.
- the number of patial responder (PR), complete responder (CR), and tumor free survivor (TFS) animals at the end of the study are also listed.
- Figures 34A and 34B show the change in lesion size after administration of anti- TF antibody in a swine choroidal neovascularization (CNV) model.
- Figure 34A shows the percentage change in lesion size from day 7 (baseline) to day 14 as measured by Fluorescein Angiography (FA) after intravitreal administration of anti-TF antibodies 25G9, 43D8, 1G, and 29D respectively.
- Figure 34B shows the percentage change in lesion size from day 7 (baseline) to day 28 as measured by Fluorescein Angiography (FA) after intravitreal administration of anti-TF antibodies 25G9, 43D8, 1G, and 29D respectively.
- Figure 35 shows the change in lesion size in a swine choroidal neovascularization (CNV) model from day 7 (baseline) to day 28 as measured by Fluorescein Angiography (FA) after intravitreal administration of anti-TF antibodies 25G9 at 600ug, 2mg and 4mg respectively.
- CNV swine choroidal neovascularization
- Figures 39A-F show the titration curves of anti-TF antibodies from lineages 25 and 43, h5G9, and 10H10 on select TF constructs.
- Figure 39A shows the titration curves of anti-TF antibodies on human TF construct.
- Figure 39B shows the titration curves of anti-TF antibodies on rat TF construct.
- Figure 39C shows the titration curves of anti-TF antibodies on chimeric human-rat TF construct hTF_K68N.
- Figure 39D shows the titration curves of anti-TF antibodies on chimeric human-rat TF construct hTF_KT49N.
- Figure 39E shows the titration curves of anti-TF antibodies on chimeric human-rat TF construct
- Figure 39F shows the titration curves of anti-TF antibodies on chimeric rat-human TF construct rl4l-l94_h.
- the term“comprising” also specifically includes embodiments “consisting of’ and“consisting essentially of’ the recited elements, unless specifically indicated otherwise.
- the term“about” indicates and encompasses an indicated value and a range above and below that value. In certain embodiments, the term“about” indicates the designated value ⁇ 10%, ⁇ 5%, or ⁇ 1%. In certain embodiments, where applicable, the term“about” indicates the designated value(s) ⁇ one standard deviation of that value(s).
- thromboplastin and“CD 142” are used interchangeably herein to refer to TF, or any variants ( e.g ., splice variants and allelic variants), isoforms, and species homologs of TF that are naturally expressed by cells, or that are expressed by cells transfected with a TF gene.
- the TF protein is a TF protein naturally expressed by a primate (e.g., a monkey or a human), a rodent (e.g, a mouse or a rat), a dog, a camel, a cat, a cow, a goat, a horse, a pig or a sheep.
- the TF protein is human TF (hTF; SEQ ID NO:809). In some aspects, the TF protein is cynomolgus TF (cTF; SEQ ID NO:8l3). In some aspects, the TF protein is mouse TF (mTF; SEQ ID NO:8l7). In some aspects, the TF protein is pig TF (pTF; SEQ ID NO:824). TF is a cell surface receptor for the serine protease factor Vila. It is often times constitutively expressed by certain cells surrounding blood vessels and in some disease settings.
- the term“antibody-drug conjugate” or“ADC” refers to a conjugate comprising an antibody conjugated to one or more cytotoxic agents, optionally through one or more linkers.
- the term“anti-TF antibody-drug conjugate” or“anti-TF ADC” refers to a conjugate comprising an anti-TF antibody conjugated to one or more cytotoxic agents, optionally through one or more linkers.
- cytotoxic agent refers to a substance that inhibits or prevents a cellular function and/or causes cell death or destruction.
- the cytotoxic agent can be an anti-angiogenic agent, a pro-apoptotic agent, an anti-mitotic agent, an anti-kinase agent, an alkylating agent, a hormone, a hormone agonist, a hormone antagonist, a chemokine, a drug, a prodrug, a toxin, an enzyme, an antimetabolite, an antibiotic, an alkaloid, or a radioactive isotope.
- cytotoxic agents include calicheamycin, camptothecin, carboplatin, irinotecan, SN-38, carboplatin, camptothecan, cyclophosphamide, cytarabine, dacarbazine, docetaxel, dactinomycin, daunorubicin, doxorubicin, doxorubicin, etoposide, idarubicin, topotecan, vinca alkaloid, maytansinoid, maytansinoid analog,
- pyrrolobenzodiazepine taxoid, duocarmycin, dolastatin, auristatin, and derivatives thereof.
- A“linker” refers to a molecule that connects one composition to another, e.g., an antibody to an agent.
- Linkers described herein can conjugate an antibody to a cytotoxic agent.
- Exemplary linkers include a labile linker, an acid labile linker, a photolabile linker, a charged linker, a disulfide-containing linker, a peptidase-sensitive linker, a ⁇ -glucuronide-linker, a dimethyl linker, a thio-ether linker, and a hydrophilic linker.
- a linker can be cleavable or non-cleavable.
- immunoglobulin refers to a class of structurally related proteins generally comprising two pairs of polypeptide chains: one pair of light (L) chains and one pair of heavy (H) chains. In an“intact immunoglobulin,” all four of these chains are interconnected by disulfide bonds.
- the structure of immunoglobulins has been well characterized. See, e.g., Paul, Fundamental Immunology 7th ed., Ch. 5 (2013) Lippincott Williams & Wilkins, Philadelphia, PA. Briefly, each heavy chain typically comprises a heavy chain variable region (VH) and a heavy chain constant region (CH).
- the heavy chain constant region typically comprises three domains, abbreviated CHI, Cm, and Cm.
- Each light chain typically comprises a light chain variable region (VL) and a light chain constant region.
- the light chain constant region typically comprises one domain, abbreviated CL.
- antibody is used herein in its broadest sense and includes certain types of immunoglobulin molecules comprising one or more antigen-binding domains that specifically bind to an antigen or epitope.
- An antibody specifically includes intact antibodies (e.g, intact immunoglobulins), antibody fragments, and multi-specific antibodies.
- alternative scaffold refers to a molecule in which one or more regions may be diversified to produce one or more antigen-binding domains that specifically bind to an antigen or epitope.
- the antigen-binding domain binds the antigen or epitope with specificity and affinity similar to that of an antibody.
- Exemplary alternative scaffolds include those derived from fibronectin (e.g, AdnectinsTM), the b-sandwich (e.g, iMab), lipocalin (e.g, Anticalins ® ), EETI-IEAGRP, BPTI/LACI-D1/ITI-D2 (e.g, Kunitz domains), thioredoxin peptide aptamers, protein A (e.g, Affibody ® ), ankyrin repeats (e.g, DARPins), gamma-B-crystallin/ubiquitin (e.g, Affilins), CTLD3 (e.g, Tetranectins), Fynomers, and (LDLR-A module) (e.g, Avimers).
- fibronectin e.g, AdnectinsTM
- the b-sandwich e.g, iMab
- lipocalin e.g, Anticalins ®
- antigen-binding domain means the portion of an antibody that is capable of specifically binding to an antigen or epitope.
- an antigen-binding domain is an antigen-binding domain formed by a VH -VL dimer of an antibody.
- Another example of an antigen-binding domain is an antigen-binding domain formed by
- Antigen-binding domains can be found in various contexts including antibodies and chimeric antigen receptors (CARs), for example CARs derived from antibodies or antibody fragments such as scFvs.
- CARs chimeric antigen receptors
- full length antibody “intact antibody,” and“whole antibody” are used herein interchangeably to refer to an antibody having a structure substantially similar to a naturally occurring antibody structure and having heavy chains that comprise an Fc region.
- a“full length antibody” is an antibody that comprises two heavy chains and two light chains.
- Fc region means the C-terminal region of an immunoglobulin heavy chain that, in naturally occurring antibodies, interacts with Fc receptors and certain proteins of the complement system.
- the structures of the Fc regions of various immunoglobulins, and the glycosylation sites contained therein, are known in the art. See Schroeder and Cavacini, ./. Allergy Clin. Immunol ., 2010, l25:S4l-52, incorporated by reference in its entirety.
- the Fc region may be a naturally occurring Fc region, or an Fc region modified as described in the art or elsewhere in this disclosure.
- the VH and VL regions may be further subdivided into regions of hypervariability (“hypervariable regions (HVRs);” also called“complementarity determining regions” (CDRs)) interspersed with regions that are more conserved.
- the more conserved regions are called framework regions (FRs).
- Each VH and VL generally comprises three CDRs and four FRs, arranged in the following order (from N-terminus to C-terminus): FR1 - CDR1 - FR2 - CDR2 - FR3 - CDR3 - FR4.
- the CDRs are involved in antigen binding, and influence antigen specificity and binding affinity of the antibody. See Rabat et al., Sequences of Proteins of Immunological Interest 5th ed. (1991) Public Health Service, National Institutes of Health, Bethesda, MD, incorporated by reference in its entirety.
- CDR Complementary Determining Region
- hypervariable regions within the non-framework region of the
- CDRs are variable region sequences interspersed within the framework region sequences.
- CDRs are well recognized in the art and have been defined by, for example, Rabat as the regions of most hypervariability within the antibody variable (V) domains. See Rabat et al., J Biol Chem , 1977, 252:6609-6616 and Rabat, Adv Protein Chem , 1978, 32: 1-75, each of which is incorporated by reference in its entirety.
- CDRs have also been defined
- a number of hypervariable region delineations are in use and are included herein.
- the Kabat CDRs are based on sequence variability and are the most commonly used. See Kabat et al. (1992) Sequences of Proteins of Immunological Interest , DIANE Publishing: 2719, incorporated by reference in its entirety. Chothia refers instead to the location of the structural loops (Chothia and Lesk, supra).
- the AbM hypervariable regions represent a compromise between the Kabat CDRs and Chothia structural loops, and are used by Oxford Molecular's AbM antibody modeling software.
- the Contact hypervariable regions are based on an analysis of the available complex crystal structures. The residues from each of these hypervariable regions are noted in Table 1.
- IMGT immunoglobulins
- TR T cell receptors
- MHC major histocompatibility complex
- immunoglobulin variable domain is conserved between species and present in structures called loops, by using numbering systems that align variable domain sequences according to structural features, CDR and framework residues are readily identified.
- CDR and framework residues are readily identified.
- Correspondence between the Kabat, Chothia and IMGT numbering is also well known in the art (Lefranc et al., supra).
- An Exemplary system, shown herein, combines Kabat and Chothia CDR definitions. Table 1
- the light chain from any vertebrate species can be assigned to one of two types, called kappa (K) and lambda (l), based on the sequence of its constant domain.
- the heavy chain from any vertebrate species can be assigned to one of five different classes (or isotypes): IgA, IgD, IgE, IgG, and IgM. These classes are also designated a, d, e, g, and m, respectively.
- the IgG and IgA classes are further divided into subclasses on the basis of differences in sequence and function. Humans express the following subclasses: IgGl, IgG2, IgG3, IgG4, IgAl, and IgA2.
- constant region refers to a carboxy terminal portion of the light and heavy chain which is not directly involved in binding of the antibody to antigen but exhibits various effector function, such as interaction with the Fc receptor.
- the terms refer to the portion of an immunoglobulin molecule having a more conserved amino acid sequence relative to the other portion of the immunoglobulin, the variable domain, which contains the antigen-binding site.
- the constant domain contains the CHI, Cm and Cm domains of the heavy chain and the CL domain of the light chain.
- The“EU numbering scheme” is generally used when referring to a residue in an antibody heavy chain constant region ( e.g ., as reported in Kabat et al., supra). ETnless stated otherwise, the EEG numbering scheme is used to refer to residues in antibody heavy chain constant regions described herein.
- An“antibody fragment” comprises a portion of an intact antibody, such as the antigen-binding or variable region of an intact antibody.
- Antibody fragments include, for example, Fv fragments, Fab fragments, F(ab’)2 fragments, Fab’ fragments, scFv (sFv) fragments, and scFv-Fc fragments.
- “Fv” fragments comprise a non-covalently-linked dimer of one heavy chain variable domain and one light chain variable domain.
- “Fab” fragments comprise, in addition to the heavy and light chain variable domains, the constant domain of the light chain and the first constant domain (Cm) of the heavy chain. Fab fragments may be generated, for example, by recombinant methods or by papain digestion of a full-length antibody.
- F(ab’)2 fragments contain two Fab’ fragments joined, near the hinge region, by disulfide bonds.
- F(ab’)2 fragments may be generated, for example, by recombinant methods or by pepsin digestion of an intact antibody.
- the F(ab’) fragments can be dissociated, for example, by treatment with B-mercaptoethanol.
- “Single-chain Fv” or“sFv” or“scFv” antibody fragments comprise a VH domain and a VL domain in a single polypeptide chain.
- the VH and VL are generally linked by a peptide linker.
- Any suitable linker may be used.
- the linker is a (GGGGS)n(SEQ ID NO:823).
- n 1, 2,
- scFv-Fc fragments comprise an scFv attached to an Fc domain.
- an Fc domain For example, an Fc domain
- Fc domain may be attached to the C-terminal of the scFv.
- the Fc domain may follow the VH or VL, depending on the orientation of the variable domains in the scFv (i.e., VH -VL or VL - VH). Any suitable Fc domain known in the art or described herein may be used.
- single domain antibody refers to a molecule in which one variable domain of an antibody specifically binds to an antigen without the presence of the other variable domain.
- Single domain antibodies, and fragments thereof, are described in Arabi Ghahroudi et ak, FEBS Letters , 1998, 414:521-526 and Muyldermans et ah, Trends in Biochem. Sci., 2001, 26:230-245, each of which is incorporated by reference in its entirety.
- Single domain antibodies are also known as sdAbs or nanobodies.
- A“multispecific antibody” is an antibody that comprises two or more different antigen-binding domains that collectively specifically bind two or more different epitopes.
- the two or more different epitopes may be epitopes on the same antigen (e.g ., a single TF molecule expressed by a cell) or on different antigens (e.g., a TF molecule and a non-TF molecule).
- a multi-specific antibody binds two different epitopes (i.e., a “bispecific antibody”).
- a multi-specific antibody binds three different epitopes (i.e., a“trispecific antibody”).
- a multi-specific antibody binds four different epitopes (i.e., a“quadspecific antibody”). In some aspects, a multi-specific antibody binds five different epitopes (i.e., a“quintspecific antibody”). In some aspects, a multi- specific antibody binds 6, 7, 8, or more different epitopes. Each binding specificity may be present in any suitable valency. Examples of multispecific antibodies are provided elsewhere in this disclosure.
- A“monospecific antibody” is an antibody that comprises one or more binding sites that specifically bind to a single epitope.
- An example of a monospecific antibody is a naturally occurring IgG molecule which, while divalent (z.e., having two antigen-binding domains), recognizes the same epitope at each of the two antigen-binding domains.
- the binding specificity may be present in any suitable valency.
- the term“monoclonal antibody” refers to an antibody from a population of substantially homogeneous antibodies.
- a population of substantially homogeneous antibodies comprises antibodies that are substantially similar and that bind the same epitope(s), except for variants that may normally arise during production of the monoclonal antibody. Such variants are generally present in only minor amounts.
- a monoclonal antibody is typically obtained by a process that includes the selection of a single antibody from a plurality of antibodies.
- the selection process can be the selection of a unique clone from a plurality of clones, such as a pool of hybridoma clones, phage clones, yeast clones, bacterial clones, or other recombinant DNA clones.
- the selected antibody can be further altered, for example, to improve affinity for the target (“affinity maturation”), to humanize the antibody, to improve its production in cell culture, and/or to reduce its immunogenicity in a subject.
- chimeric antibody refers to an antibody in which a portion of the heavy and/or light chain is derived from a particular source or species, while the remainder of the heavy and/or light chain is derived from a different source or species.
- “Humanized” forms of non-human antibodies are chimeric antibodies that contain minimal sequence derived from the non-human antibody.
- a humanized antibody is generally a human antibody (recipient antibody) in which residues from one or more CDRs are replaced by residues from one or more CDRs of a non-human antibody (donor antibody).
- the donor antibody can be any suitable non-human antibody, such as a mouse, rat, rabbit, chicken, or non-human primate antibody having a desired specificity, affinity, or biological effect.
- selected framework region residues of the recipient antibody are replaced by the corresponding framework region residues from the donor antibody.
- Humanized antibodies may also comprise residues that are not found in either the recipient antibody or the donor antibody. Such modifications may be made to further refine antibody function. For further details, see Jones et ah, Nature , 1986, 321 :522-525; Riechmann et ah, Nature , 1988, 332:323-329; and Presta, Curr. Op. Struct. Biol., 1992, 2:593-596, each of which is incorporated by reference in its entirety.
- A“human antibody” is one which possesses an amino acid sequence
- Human antibodies specifically exclude humanized antibodies.
- An“isolated antibody” or“isolated nucleic acid” is an antibody or nucleic acid that has been separated and/or recovered from a component of its natural environment.
- Components of the natural environment may include enzymes, hormones, and other proteinaceous or nonproteinaceous materials.
- an isolated antibody is purified to a degree sufficient to obtain at least 15 residues of N-terminal or internal amino acid sequence, for example by use of a spinning cup sequenator.
- an isolated antibody is purified to homogeneity by gel electrophoresis (e.g., SDS-PAGE) under reducing or nonreducing conditions, with detection by Coomassie blue or silver stain.
- an isolated antibody may include an antibody in situ within recombinant cells, since at least one component of the antibody’s natural environment is not present.
- an isolated antibody or isolated nucleic acid is prepared by at least one purification step.
- an isolated antibody or isolated nucleic acid is purified to at least 80%, 85%, 90%, 95%, or 99% by weight. In some embodiments, an isolated antibody or isolated nucleic acid is purified to at least 80%, 85%, 90%, 95%, or 99% by volume. In some embodiments, an isolated antibody or isolated nucleic acid is provided as a solution comprising at least 85%, 90%, 95%, 98%, 99% to 100% antibody or nucleic acid by weight. In some embodiments, an isolated antibody or isolated nucleic acid is provided as a solution comprising at least 85%, 90%, 95%, 98%, 99% to 100% antibody or nucleic acid by volume.
- affinity refers to the strength of the sum total of non-covalent interactions between a single binding site of a molecule (e.g, an antibody) and its binding partner (e.g, an antigen or epitope).
- affinity refers to intrinsic binding affinity, which reflects a 1 : 1 interaction between members of a binding pair (e.g, antibody and antigen or epitope).
- the affinity of a molecule X for its partner Y can be represented by the dissociation equilibrium constant (KD). The kinetic components that contribute to the dissociation equilibrium constant are described in more detail below.
- Affinity can be measured by common methods known in the art, including those described herein, such as surface plasmon resonance (SPR) technology (e.g ., BIACORE ® ) or biolayer interferometry (e.g., FORTEBIO ® ).
- SPR surface plasmon resonance
- BIACORE ® BIACORE ®
- biolayer interferometry e.g., FORTEBIO ®
- the terms“bind,” “specific binding,”“specifically binds to,”“specific for,”“selectively binds,” and“selective for” a particular antigen (e.g, a polypeptide target) or an epitope on a particular antigen mean binding that is measurably different from a non-specific or non-selective interaction (e.g, with a non-target molecule).
- Specific binding can be measured, for example, by measuring binding to a target molecule and comparing it to binding to a non-target molecule.
- Specific binding can also be determined by competition with a control molecule that mimics the epitope recognized on the target molecule.
- the affinity of a TF antibody for a non-target molecule is less than about 50% of the affinity for TF. In some aspects, the affinity of a TF antibody for a non target molecule is less than about 40% of the affinity for TF. In some aspects, the affinity of a TF antibody for a non-target molecule is less than about 30% of the affinity for TF. In some aspects, the affinity of a TF antibody for a non-target molecule is less than about 20% of the affinity for TF. In some aspects, the affinity of a TF antibody for a non-target molecule is less than about 10% of the affinity for TF.
- the affinity of a TF antibody for a non-target molecule is less than about 1% of the affinity for TF. In some aspects, the affinity of a TF antibody for a non-target molecule is less than about 0.1% of the affinity for TF.
- the term“kd” (sec -1 ), as used herein, refers to the dissociation rate constant of a particular antibody-antigen interaction. This value is also referred to as the k 0ff value.
- k a (M - 1 /sec - 1 ), as used herein, refers to the association rate constant of a particular antibody-antigen interaction. This value is also referred to as the k 0 n value.
- KD KD
- M dissociation equilibrium constant of a particular antibody-antigen interaction.
- KD kd/k a.
- affinity of an antibody is described in terms of the KD for an interaction between such antibody and its antigen. For clarity, as known in the art, a smaller KD value indicates a higher affinity interaction, while a larger KD value indicates a lower affinity interaction.
- An“affinity matured” antibody is an antibody with one or more alterations (e.g, in one or more CDRs or FRs) relative to a parent antibody (i.e., an antibody from which the altered antibody is derived or designed) that result in an improvement in the affinity of the antibody for its antigen, compared to the parent antibody which does not possess the alteration(s).
- a parent antibody i.e., an antibody from which the altered antibody is derived or designed
- an affinity matured antibody has nanomolar or picomolar affinity for the target antigen.
- Affinity matured antibodies may be produced using a variety of methods known in the art. For example, Marks et al. ( Bio/Technology , 1992, 10:779-783, incorporated by reference in its entirety) describes affinity maturation by VH and VL domain shuffling.
- Random mutagenesis of CDR and/or framework residues is described by, for example, Barbas et al., Proc. Nat. Acad. Sci. U.S.A., 1994, 91 :3809-3813; Schier et al., Gene , 1995, 169: 147-155; Yelton et al., J. Immunol ., 1995, 155: 1994-2004; Jackson et al., J.
- Fc effector functions refer to those biological activities mediated by the Fc region of an antibody, which activities may vary depending on the antibody isotype.
- antibody effector functions include Clq binding to activate complement dependent cytotoxicity (CDC), Fc receptor binding to activate antibody-dependent cellular cytotoxicity (ADCC), and antibody dependent cellular phagocytosis (ADCP).
- the term“competes with” or“cross-competes with” indicates that the two or more antibodies compete for binding to an antigen (e.g ., TF).
- TF is coated on a surface and contacted with a first TF antibody, after which a second TF antibody is added.
- first a TF antibody is coated on a surface and contacted with TF, and then a second TF antibody is added. If the presence of the first TF antibody reduces binding of the second TF antibody, in either assay, then the antibodies compete with each other.
- the term“competes with” also includes combinations of antibodies where one antibody reduces binding of another antibody, but where no competition is observed when the antibodies are added in the reverse order.
- the first and second antibodies inhibit binding of each other, regardless of the order in which they are added.
- one antibody reduces binding of another antibody to its antigen by at least 25%, at least 50%, at least 60%, at least 70%, at least 80%, at least 85%, at least 90%, or at least 95%.
- concentrations of the antibodies used in the competition assays based on the affinities of the antibodies for TF and the valency of the antibodies.
- the assays described in this definition are illustrative, and a skilled artisan can utilize any suitable assay to determine if antibodies compete with each other. Suitable assays are described, for example, in Cox et al.,“Immunoassay Methods,” in Assay Guidance Manual [Internet], Updated December 24, 2014 (www.ncbi.nlm.nih.gov/books/NBK92434/; accessed September 29, 2015); Silman et al., Cytometry , 2001, 44:30-37; and Finco et al., J. Pharm. Biomed. Anal., 2011, 54:351-358; each of which is incorporated by reference in its entirety. As provided in Example 8, antibodies of group 25 and antibodies of group 43 compete with each other for binding to human TF, while antibodies from groups 1, 29, 39, and 54 do not compete for binding to human TF with antibodies of groups 25 and 43.
- an antibody that binds specifically to a human antigen is considered to bind the same antigen of mouse origin when a KD value can be measured on a ForteBio Octet with the mouse antigen.
- An antibody that binds specifically to a human antigen is considered to be“cross-reactive” with the same antigen of mouse origin when the KD value for the mouse antigen is no greater than 20 times the corresponding KD value for the respective human antigen.
- the antibody Ml 593 described in U.S Pat. Nos. 8,722,044, 8,951,525, and 8,999,333 each of which is herein incorporated by reference for all purposes, the humanized 5G9 antibody described in Ngo et al., 2007, Int J Cancer,
- TF antibodies from groups 25 and 43 bind to mouse TF, e.g., the TF antibodies 25G, 25G1, 25G9, and 43D8 are cross-reactive with mouse TF.
- an antibody that binds specifically to a human antigen is considered to be“cross-reactive” with the same antigen of cynomolgus monkey origin when the KD value for the cynomolgus monkey antigen is no greater than 15 times the
- epitope means a portion of an antigen that is specifically bound by an antibody.
- Epitopes frequently include surface-accessible amino acid residues and/or sugar side chains and may have specific three dimensional structural characteristics, as well as specific charge characteristics. Conformational and non-conformational epitopes are distinguished in that the binding to the former but not the latter may be lost in the presence of denaturing solvents.
- An epitope may comprise amino acid residues that are directly involved in the binding, and other amino acid residues, which are not directly involved in the binding.
- the epitope to which an antibody binds can be determined using known techniques for epitope determination such as, for example, testing for antibody binding to TF variants with different point-mutations, or to chimeric TF variants.
- Percent“identity” between a polypeptide sequence and a reference sequence is defined as the percentage of amino acid residues in the polypeptide sequence that are identical to the amino acid residues in the reference sequence, after aligning the sequences and introducing gaps, if necessary, to achieve the maximum percent sequence identity.
- Alignment for purposes of determining percent amino acid sequence identity can be achieved in various ways that are within the skill in the art, for instance, using publicly available computer software such as BLAST, BLAST-2, ALIGN, MEGALIGN (DNASTAR), CLUSTALW, CLUSTAL OMEGA, or MUSCLE software. Those skilled in the art can determine appropriate parameters for aligning sequences, including any algorithms needed to achieve maximal alignment over the full length of the sequences being compared.
- A“conservative substitution” or a“conservative amino acid substitution,” refers to the substitution of an amino acid with a chemically or functionally similar amino acid.
- Conservative substitution tables providing similar amino acids are well known in the art.
- the groups of amino acids provided in Tables 2-4 are, in some
- Table 2 Selected groups of amino acids that are considered conservative substitutions for one another, in certain embodiments.
- Table 3 Additional selected groups of amino acids that are considered conservative substitutions for one another, in certain embodiments.
- amino acid refers to the twenty common naturally occurring amino acids.
- Naturally occurring amino acids include alanine (Ala; A), arginine (Arg; R), asparagine (Asn; N), aspartic acid (Asp; D), cysteine (Cys; C); glutamic acid (Glu; E), glutamine (Gln; Q), Glycine (Gly; G); histidine (His; H), isoleucine (Ile; I), leucine (Leu; L), lysine (Lys; K), methionine (Met; M), phenylalanine (Phe; F), proline (Pro; P), serine (Ser;
- vector refers to a nucleic acid molecule capable of propagating another nucleic acid to which it is linked.
- the term includes the vector as a self- replicating nucleic acid structure as well as the vector incorporated into the genome of a host cell into which it has been introduced.
- Certain vectors are capable of directing the expression of nucleic acids to which they are operatively linked. Such vectors are referred to herein as “expression vectors.”
- Host cells include“transformants” (or“transformed cells”) and “transfectants” (or“transfected cells”), which each include the primary transformed or transfected cell and progeny derived therefrom.
- Such progeny may not be completely identical in nucleic acid content to a parent cell, and may contain mutations.
- the term“treating” refers to clinical intervention in an attempt to alter the natural course of a disease or condition in a subject in need thereof. Treatment can be performed both for prophylaxis and during the course of clinical pathology. Desirable effects of treatment include preventing occurrence or recurrence of disease, alleviation of symptoms, diminishment of any direct or indirect pathological consequences of the disease, preventing metastasis, decreasing the rate of disease progression, amelioration or palliation of the disease state, and remission or improved prognosis.
- the term“therapeutically effective amount” or“effective amount” refers to an amount of an antibody or pharmaceutical composition provided herein that, when administered to a subject, is effective to treat a disease or disorder.
- the term“subject” means a mammalian subject.
- exemplary subjects include humans, monkeys, dogs, cats, mice, rats, cows, horses, camels, goats, rabbits, pigs and sheep.
- the subject is a human.
- the subject has a disease or condition that can be treated with an antibody provided herein.
- the disease or condition is a cancer.
- the disease or condition involves neovascularization or vascular inflammation.
- the disease or condition involving neovascularization is age-related macular degeneration (AMD), diabetic retinopathy, or cancer.
- package insert is used to refer to instructions customarily included in commercial packages of therapeutic or diagnostic products (e.g ., kits) that contain
- A“chemotherapeutic agent” refers to a chemical compound useful in the treatment of cancer.
- Chemotherapeutic agents include“anti -hormonal agents” or“endocrine therapeutics” which act to regulate, reduce, block, or inhibit the effects of hormones that can promote the growth of cancer.
- cytostatic agent refers to a compound or composition which arrests growth of a cell either in vitro or in vivo.
- a cytostatic agent is an agent that reduces the percentage of cells in S phase.
- a cytostatic agent reduces the percentage of cells in S phase by at least about 20%, at least about 40%, at least about 60%, or at least about 80%.
- composition refers to a preparation which is in such form as to permit the biological activity of an active ingredient contained therein to be effective in treating a subject, and which contains no additional components which are unacceptably toxic to the subject in the amounts provided in the pharmaceutical composition.
- modulate and“modulation” refer to reducing or inhibiting or, alternatively, activating or increasing, a recited variable.
- the terms“increase” and“activate” refer to an increase of 10%, 20%, 30%, 40%, 50%, 60%, 70%, 75%, 80%, 85%, 90%, 95%, 100%, 2-fold, 3-fold, 4-fold, 5-fold, lO-fold, 20-fold, 50-fold, lOO-fold, or greater in a recited variable.
- the terms“reduce” and“inhibit” refer to a decrease of 10%, 20%, 30%, 40%, 50%, 60%, 70%, 75%, 80%, 85%, 90%, 95%, 2-fold, 3-fold, 4-fold, 5-fold, lO-fold, 20-fold, 50-fold, lOO-fold, or greater in a recited variable.
- the term“agonize” refers to the activation of receptor signaling to induce a biological response associated with activation of the receptor.
- An“agonist” is an entity that binds to and agonizes a receptor.
- the term“antagonize” refers to the inhibition of receptor signaling to inhibit a biological response associated with activation of the receptor.
- An“antagonist” is an entity that binds to and antagonizes a receptor.
- the TF is hTF (SEQ ID NO:809).
- the TF is cTF (SEQ ID NO:8l3).
- the TF is mTF (SEQ ID NO:8l7).
- the TF is rabbit TF (SEQ ID NO:832).
- the TF is pTF (SEQ ID NO:824).
- the antibodies provided herein specifically bind to hTF (SEQ ID NO: 809), cTF (SEQ ID NO:
- the antibodies provided herein specifically bind to hTF (SEQ ID NO:809), cTF (SEQ ID NO:8l3), mTF (SEQ ID NO:8l7), and pTF (SEQ ID NO:824). In some embodiments, the antibodies provided herein specifically bind to hTF (SEQ ID NO:809), cTF (SEQ ID NO:8l3), mTF (SEQ ID NO:8l7), and pTF (SEQ ID NO:824). In some embodiments, the antibodies provided herein specifically bind to hTF (SEQ ID NO:809), cTF (SEQ ID NO:8l3), mTF (SEQ ID NO:8l7), and pTF (SEQ ID NO:824). In some embodiments, the antibodies provided herein specifically bind to hTF (SEQ ID NO:809), cTF (SEQ ID NO:8l3), mTF (SEQ ID NO:8l7), and pTF (SEQ ID NO:824)
- the antibodies provided herein specifically bind to hTF (SEQ ID NO: 809) and cTF (SEQ ID NO:8l3). In some embodiments, the antibodies provided herein do not bind mTF (SEQ ID NO:8l7). In some embodiments, the antibodies provided herein do not bind pTF (SEQ ID NO:824). In some embodiments, the antibodies provided herein do not bind rabbit TF (SEQ ID NO:832).
- the antibodies provided herein specifically bind to the extracellular domain of human TF (SEQ ID NO:8lO).
- the binding between an antibody provided herein and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody provided herein and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N.
- the binding between an antibody provided herein and a variant TF extracellular domain comprising a mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the antibody provided herein and the extracellular domain of TF of the sequence shown in SEQ ID NO:
- the mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is K68N.
- the binding between an antibody provided herein and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody provided herein and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO
- the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the binding between an antibody provided herein and a human TF extracellular domain with amino acid residues 1-77 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 1-76 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a human TF extracellular domain with amino acid residues 39-77 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 38-76 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a human TF extracellular domain with amino acid residues 94-107 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 99-112 of the sequence shown in SEQ ID NO:838 is greater than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a human TF extracellular domain with amino acid residues 146-158 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 151-163 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a human TF extracellular domain with amino acid residues 159-219 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-224 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a human TF extracellular domain with amino acid residues 159-189 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-194 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a human TF extracellular domain with amino acid residues 159-174 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 164-179 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a human TF extracellular domain with amino acid residues 167-174 of the sequence shown in SEQ ID NO:8lO replaced by rat TF extracellular domain amino acid residues 172-179 of the sequence shown in SEQ ID NO:838 is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a rat TF extracellular domain with amino acid residues 141-194 of the sequence shown in SEQ ID NO: 838 replaced by human TF extracellular domain amino acid residues 136-189 of the sequence shown in SEQ ID NO:8lO is greater than 50% of the binding between the antibody provided herein and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- the binding between an antibody provided herein and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody provided herein and the extracellular domain of TF of the sequence shown in SEQ ID NO:8lO
- the mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is K149N; the mutation at amino acid residue 68 of the sequence shown in SEQ ID NO:8lO is K68N; and the mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO are N171H and T197K.
- the antibodies provided herein are inert in inhibiting human thrombin generation as determined by thrombin generation assay (TGA) compared to a reference antibody Ml 593, wherein the reference antibody Ml 593 comprises a VH sequence of SEQ ID NO:82l and a VL sequence of SEQ ID NO:822.
- the antibodies provided herein do not inhibit human thrombin generation as determined by thrombin generation assay (TGA). In certain embodiments, the antibodies provided herein allow human thrombin generation as determined by thrombin generation assay (TGA).
- the antibodies provided herein bind human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX. In certain embodiments, the antibodies provided herein do not interfere with the ability of TF :FVIIa to convert FX into FXa.
- the antibodies provided herein bind human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa. In certain embodiments, the antibodies provided herein do not compete for binding to human TF with human FVIIa.
- the antibodies provided herein bind to the extracellular domain of human TF, bind human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa, bind human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX, and allow human thrombin generation as determined by thrombin generation assay (TGA).
- TGA thrombin generation assay
- the antibodies provided herein bind to the extracellular domain of human TF, do not inhibit human thrombin generation as determined by thrombin generation assay (TGA), do not interfere with the ability of TF:FVIIa to convert FX into FXa, and do not compete for binding to human TF with human FVIIa.
- TGA thrombin generation assay
- the antibodies provided herein bind to the extracellular domain of human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa, do not inhibit human thrombin generation as determined by thrombin generation assay (TGA), allow human thrombin generation as determined by thrombin generation assay (TGA), bind to human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX, do not interfere with the ability of TF :F Vila to convert FX into FXa, and do not compete for binding to human TF with human FVIIa.
- the antibodies provided herein inhibit FVIIa-dependent TF signaling.
- the antibodies provided herein reduce lesion size in a swine choroidal neovascularization (CNV) model.
- the antibodies provided herein bind to the extracellular domain of human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa, do not inhibit human thrombin generation as determined by thrombin generation assay (TGA), allow human thrombin generation as determined by thrombin generation assay (TGA), bind to human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX, do not interfere with the ability of TF :F Vila to convert FX into FXa, do not compete for binding to human TF with human FVIIa, and bind to cynomolgus and mouse TF.
- TGA thrombin generation assay
- TGA thrombin generation assay
- the antibodies provided herein bind to the extracellular domain of human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa, do not inhibit human thrombin generation as determined by thrombin generation assay (TGA), allow human thrombin generation as determined by thrombin generation assay (TGA), bind to human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX, do not interfere with the ability of TF :F Vila to convert FX into FXa, do not compete for binding to human TF with human FVIIa, bind to cynomolgus, mouse, and pig TF, and reduce lesion size in a swine choroidal
- CNV neovascularization
- the antibodies provided herein bind to the extracellular domain of human TF, inhibit FVIIa-dependent TF signaling, and bind to cynomolgus TF.
- an antibody provided herein comprises a VH sequence selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759.
- an antibody provided herein comprises a VH sequence of SEQ ID NO:37.
- an antibody provided herein comprises a VH sequence of SEQ ID NO:75.
- an antibody provided herein comprises a VH sequence of SEQ ID NO: 113.
- an antibody provided herein comprises a VH sequence of SEQ ID NO: 151.
- an antibody provided herein comprises a VH sequence of SEQ ID NO: 189. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:836. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:227. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:265. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:303. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:34l. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:379. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:4l7. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:455. In some embodiments,
- an antibody provided herein comprises a VH sequence of SEQ ID NO:493. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:53 l. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:569. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:607. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO: 645. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:683. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:72l. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:759.
- an antibody provided herein comprises a VH sequence having at least about 50%, 60%, 70%, 80%, 90%, 95%, or 99% identity to an illustrative VH sequence provided in SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759.
- an antibody provided herein comprises a VH sequence provided in SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759, with up to 1, 2, 3, 4, 5,
- the amino acid substitutions are conservative amino acid substitutions.
- the antibodies described in this paragraph are referred to herein as“variants.” In some embodiments, such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein. In some
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- V L Domains are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a VL sequence selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760.
- an antibody provided herein comprises a VL sequence of SEQ ID NO:38.
- an antibody provided herein comprises a VL sequence of SEQ ID NO:76.
- an antibody provided herein comprises a VL sequence of SEQ ID NO: 114.
- an antibody provided herein comprises a VL sequence of SEQ ID NO: 152.
- an antibody provided herein comprises a VL sequence of SEQ ID NO: 190. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:837. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:228. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:266. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:304. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:342. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:380. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:4l8. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:456. In some embodiments,
- an antibody provided herein comprises a VL sequence of SEQ ID NO:494. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:532. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:570. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO: 608. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:646. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:684. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:722. In some embodiments, an antibody provided herein comprises a VL sequence of SEQ ID NO:760.
- an antibody provided herein comprises a VL sequence having at least about 50%, 60%, 70%, 80%, 90%, 95%, or 99% identity to an illustrative VL sequence provided in SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760.
- an antibody provided herein comprises a VL sequence provided in SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760, with up to 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 amino acid substitutions.
- the amino acid substitutions are conservative amino acid substitutions.
- the antibodies described in this paragraph are referred to herein as“variants.”
- variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a VH sequence selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759 and a VL sequence selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760.
- an antibody provided herein comprises a VH sequence of SEQ ID NO:37 and a VL sequence of SEQ ID NO:38. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:75 and a VL sequence of SEQ ID NO:76. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO: 113 and a VL sequence of SEQ ID NO: 114. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO: 151 and a VL sequence of SEQ ID NO: 152.
- an antibody provided herein comprises a VH sequence of SEQ ID NO: 189 and a VL sequence of SEQ ID NO: 190. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:836 and a VL sequence of SEQ ID NO:837. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:227 and a VL sequence of SEQ ID NO:228. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:265 and a VL sequence of SEQ ID NO:266.
- an antibody provided herein comprises a VH sequence of SEQ ID NO:303 and a VL sequence of SEQ ID NO:304. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:34l and a VL sequence of SEQ ID NO:342. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:379 and a VL sequence of SEQ ID NO:380. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:4l7 and a VL sequence of SEQ ID NO:4l8.
- an antibody provided herein comprises a VH sequence of SEQ ID NO:455 and a VL sequence of SEQ ID NO:456. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:493 and a VL sequence of SEQ ID NO:494. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:53 l and a VL sequence of SEQ ID NO:532. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:569 and a VL sequence of SEQ ID NO:570. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO: 607 and a VL sequence of SEQ ID NO: 608.
- an antibody provided herein comprises a VH sequence of SEQ ID NO:645 and a VL sequence of SEQ ID NO:646. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:683 and a VL sequence of SEQ ID NO:684. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:72l and a VL sequence of SEQ ID NO:722. In some embodiments, an antibody provided herein comprises a VH sequence of SEQ ID NO:759 and a VL sequence of SEQ ID NO:760.
- an antibody provided herein comprises a VH sequence having at least about 50%, 60%, 70%, 80%, 90%, 95%, or 99% identity to an illustrative VH sequence provided in SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759, and a VL sequence having at least about 50%, 60%, 70%, 80%, 90%, 95%, or 99% identity to an illustrative VL sequence provided in SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646,
- an antibody provided herein comprises a VH sequence provided in SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759, with up to 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 amino acid substitutions, and a VL sequence provided in SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760, with up to 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 amino acid substitutions.
- the amino acid substitutions are conservative amino acid substitutions.
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies. 2.2.4. CDRs
- an antibody provided herein comprises one to three CDRs of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759.
- an antibody provided herein comprises two to three CDRs of a VH domain selected from SEQ ID NOs:
- an antibody provided herein comprises three CDRs of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759.
- the CDRs are Exemplary CDRs.
- the CDRs are Rabat CDRs.
- the CDRs are Chothia CDRs.
- the CDRs are AbM CDRs. In some aspects, the CDRs are Contact CDRs. In some aspects, the CDRs are IMGT CDRs.
- the CDRs are CDRs having at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H1, CDR-H2, or CDR-H3 of SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759.
- the CDR-H1 is a CDR-H1 of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759, with up to 1, 2, 3, 4, or 5 amino acid substitutions.
- the CDR-H2 is a CDR-H2 of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759, with up to 1,
- the CDR-H3 is a CDR- H3 of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759, with up to 1, 2, 3, 4, 5, 6, 7, or 8 amino acid substitutions.
- the amino acid substitutions are conservative amino acid substitutions.
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises one to three CDRs of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760. In some embodiments, an antibody provided herein comprises two to three CDRs of a VL domain selected from SEQ ID NOs:
- an antibody provided herein comprises three CDRs of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760.
- the CDRs are Exemplary CDRs.
- the CDRs are Rabat CDRs.
- the CDRs are Chothia CDRs.
- the CDRs are AbM CDRs. In some aspects, the CDRs are Contact CDRs. In some aspects, the CDRs are IMGT CDRs.
- the CDRs are CDRs having at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L1, CDR-L2, or CDR-L3 of SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760.
- the CDR-L1 is a CDR-L1 of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760, with up to 1, 2, 3, 4, or 5 amino acid substitutions.
- the CDR- L2 is a CDR-L2 of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760, with up to 1, 2, 3, 4, 5,
- the CDR-L3 is a CDR-L3 of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760, with up to 1, 2, 3, 4, 5, 6, 7, or 8 amino acid substitutions.
- the amino acid substitutions are conservative amino acid substitutions.
- the antibodies described in this paragraph are referred to herein as“variants.”
- variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises one to three CDRs of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759 and one to three CDRs of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760.
- an antibody provided herein comprises two to three CDRs of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759 and two to three CDRs of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760.
- an antibody provided herein comprises three CDRs of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759 and three CDRs of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152,
- the CDRs are Exemplary CDRs.
- the CDRs are Rabat CDRs.
- the CDRs are Chothia CDRs.
- the CDRs are AbM CDRs.
- the CDRs are Contact CDRs.
- the CDRs are IMGT CDRs.
- the CDRs are CDRs having at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H1, CDR-H2, or CDR-H3 of SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759 and at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L1, CDR-L2, or CDR-L3 of SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760.
- the CDR-H1 is a CDR-H1 of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455, 493, 531, 569, 607, 645, 683, 721, and 759, with up to 1, 2, 3, 4, or 5 amino acid
- the CDR-H2 is a CDR-H2 of a VH domain selected from SEQ ID NOs: 37, 75,
- the CDR-H3 is a CDR-H3 of a VH domain selected from SEQ ID NOs: 37, 75, 113, 151, 189, 227, 265, 303, 341, 379, 417, 455,
- the CDR-L1 is a CDR-L1 of a VL domain selected from SEQ ID NOs: 38, 76,
- the CDR-L2 is a CDR-L2 of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456,
- the CDR-L3 is a CDR-L3 of a VL domain selected from SEQ ID NOs: 38, 76, 114, 152, 190, 228, 266, 304, 342, 380, 418, 456, 494, 532, 570, 608, 646, 684, 722, and 760, with up to 1, 2, 3, 4, or 5 amino acid substitutions.
- the amino acid substitutions are conservative amino acid substitutions.
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-H3 selected from SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535,
- the CDR-H3 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H3 of SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535, 573, 611, 649, 687, and 725.
- the CDR-H3 is a CDR-H3 selected from SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535,
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-H2 selected from SEQ ID NOs: 2, 40, 78, 116, 154, 192, 230, 268, 306, 344, 382, 420, 458, 496, 534,
- the CDR-H2 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H2 of SEQ ID NOs: 2, 40, 78, 116, 154, 192, 230, 268, 306, 344, 382, 420, 458, 496, 534, 572, 610, 648, 686, and 724.
- the CDR-H2 is a CDR-H2 selected from SEQ ID NOs: 2, 40, 78, 116, 154, 192, 230, 268, 306, 344, 382, 420, 458, 496, 534,
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-H1 selected from SEQ ID NOs: 1, 39, 77, 115, 153, 191, 229, 267, 305, 343, 381, 419, 457, 495, 533,
- the CDR-H1 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H1 of SEQ ID NOs: 1, 39, 77, 115, 153, 191, 229, 267, 305, 343, 381, 419, 457, 495, 533, 571, 609, 647, 685, and 723.
- the CDR-H1 is a CDR-H1 selected from SEQ ID NOs: 1, 39, 77, 115, 153, 191, 229, 267, 305, 343, 381, 419, 457, 495, 533,
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-H3 selected from SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535,
- an antibody provided herein comprises a CDR-H3 selected from SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535, 573, 611, 649, 687, and 725, a CDR-H2 selected from SEQ ID NOs: 2, 40, 78, 116, 154, 192, 230, 268, 306, 344,
- the CDR-H3 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H3 of SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535, 573, 611, 649, 687, and 725
- the CDR-H2 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H2 of SEQ ID NOs: 2, 40,
- the CDR-H1 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR- Hl of SEQ ID NOs: 1, 39, 77, 115, 153, 191, 229, 267, 305, 343, 381, 419, 457, 495, 533, 571, 609, 647, 685, and 723.
- the CDR-H3 is a CDR-H3 selected from SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535, 573, 611, 649, 687, and 725, with up to 1, 2, 3, 4, 5, 6, 7, or 8 amino acid substitutions;
- the CDR-H2 is a CDR-H2 selected from SEQ ID NOs: 2, 40, 78, 116, 154, 192, 230, 268, 306, 344, 382,
- the CDR-H1 is a CDR-H1 selected from SEQ ID NOs: 1, 39, 77, 115, 153, 191, 229, 267, 305, 343, 381, 419, 457, 495, 533, 571, 609, 647, 685, and 723, with up to 1,
- variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein. In some embodiments, such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-L3 selected from SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538,
- the CDR-L3 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L3 of SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538, 576, 614, 652, 690, and 728.
- the CDR-L3 is a CDR-L3 selected from SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538,
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-L2 selected from SEQ ID NOs: 5, 43, 81, 119, 157, 195, 233, 271, 309, 347, 385, 423, 461, 499, 537,
- the CDR-L2 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L2 of SEQ ID NOs: 5, 43, 81, 119, 157, 195, 233, 271, 309, 347, 385, 423, 461, 499, 537, 575, 613, 651, 689, and 727.
- the CDR-L2 is a CDR-L2 selected from SEQ ID NOs: 5, 43, 81, 119, 157, 195, 233, 271, 309, 347, 385, 423, 461, 499, 537, 575, 613, 651, 689, and 727, with up to 1, 2, 3, 4, 5, 6, 7, or 8 amino acid substitutions.
- the amino acid substitutions are conservative amino acid substitutions.
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-L1 selected from SEQ ID NOs: 4, 42, 80, 118, 156, 194, 232, 270, 308, 346, 384, 422, 460, 498, 536,
- the CDR-L1 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L1 of SEQ ID NOs: 4, 42, 80, 118, 156, 194, 232, 270, 308, 346, 384, 422, 460, 498, 536, 574, 612, 650, 688, and 726.
- the CDR-L1 is a CDR-L1 selected from SEQ ID NOs: 4, 42, 80, 118, 156, 194, 232, 270, 308, 346, 384, 422, 460, 498, 536,
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-L3 selected from SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538,
- an antibody provided herein comprises a CDR-L3 selected from SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538, 576, 614, 652, 690, and 728, a CDR-L2 selected from SEQ ID NOs: 5, 43, 81, 119, 157, 195, 233, 271, 309, 347,
- the CDR-L3 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L3 of SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538, 576, 614, 652, 690, and 728, the CDR-L2 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L2 of SEQ ID NOs: 5, 43,
- the CDR-L1 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR- Ll of SEQ ID NOs: 4, 42, 80, 118, 156, 194, 232, 270, 308, 346, 384, 422, 460, 498, 536,
- the CDR-L3 is a CDR-L3 selected from SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538, 576, 614, 652, 690, and 728, with up to 1, 2, 3, 4, or 5 amino acid substitutions
- the CDR-L2 is a CDR- L2 selected from SEQ ID NOs: 5, 43, 81, 119, 157, 195, 233, 271, 309, 347, 385, 423, 461, 499, 537, 575, 613, 651, 689, and 727, with up to 1, 2, 3, or 4 amino acid substitutions
- the CDR-L1 is a CDR-L1 selected from SEQ ID NOs: 4, 42, 80, 118, 156, 194, 232, 270,
- the antibodies described in this paragraph are referred to herein as“variants.”
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein.
- such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-H3 selected from SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535,
- a CDR-H2 selected from SEQ ID NOs: 2, 40, 78, 116, 154, 192, 230, 268, 306, 344, 382, 420, 458, 496, 534, 572, 610, 648, 686, and 724, a CDR-H1 selected from SEQ ID NOs: 1, 39, 77, 115, 153, 191, 229, 267, 305, 343, 381, 419, 457, 495, 533,
- a CDR-L3 selected from SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538, 576, 614, 652, 690, and 728
- a CDR-L2 selected from SEQ ID NOs: 5, 43, 81, 119, 157, 195, 233, 271, 309, 347, 385, 423, 461, 499, 537,
- CDR-L1 selected from SEQ ID NOs: 4, 42, 80, 118, 156, 194, 232, 270, 308, 346, 384, 422, 460, 498, 536, 574, 612, 650, 688, and 726.
- the CDR-H3 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H3 of SEQ ID NOs: 3, 41, 79, 117, 155, 193, 231, 269, 307, 345, 383, 421, 459, 497, 535, 573, 611, 649, 687, and 725
- the CDR-H2 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H2 of SEQ ID NOs: 2, 40, 78, 116, 154, 192, 230, 268, 306, 344, 382, 420, 458, 496, 534, 572, 610, 648, 686, and 724
- the CDR-H1 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-H1 of SEQ ID NOs: 1, 39,
- the CDR-L3 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L3 of SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538, 576, 614, 652, 690, and 728
- the CDR-L2 has at least about 50%, 75%, 80%, 85%, 90%, or 95% identity with a CDR-L2 of SEQ ID NOs: 5, 43, 81, 119, 157, 195, 233, 271, 309, 347, 385, 423, 461, 499, 537, 575, 613, 651, 689, and 727
- the CDR-L1 has at least about 50%,
- the CDR-H2 is a CDR-H2 selected from SEQ ID NOs: 2, 40, 78, 116, 154, 192, 230, 268, 306, 344, 382, 420, 458, 496, 534, 572, 610, 648, 686, and 724, with up to 1, 2, 3, 4, 5, 6, 7, or 8 amino acid substitutions
- the CDR-H1 is a CDR-H1 selected from SEQ ID NOs: 1, 39, 77, 115, 153, 191, 229, 267, 305, 343, 381, 419, 457, 495, 533, 571, 609, 647, 685, and 723, with up to 1, 2, 3, 4, or 5 amino acid
- the CDR-L3 is a CDR-L3 selected from SEQ ID NOs: 6, 44, 82, 120, 158, 196, 234, 272, 310, 348, 386, 424, 462, 500, 538, 576, 614, 652, 690, and 728, with up to 1, 2, 3,
- the CDR-L2 is a CDR-L2 selected from SEQ ID NOs: 5, 43, 81, 119, 157, 195, 233, 271, 309, 347, 385, 423, 461, 499, 537, 575, 613, 651, 689, and 727, with up to 1, 2, 3, or 4 amino acid substitutions
- the CDR-L1 is a CDR-L1 selected from SEQ ID NOs: 4, 42, 80, 118, 156, 194, 232, 270, 308, 346, 384, 422, 460, 498, 536, 574, 612, 650, 688, and 726, with up to 1, 2, 3, 4, 5, or 6 amino acid substitutions.
- the amino acid substitutions are conservative amino acid substitutions.
- the antibodies described in this paragraph are referred to herein as“variants.” In some embodiments, the antibodies described in this paragraph are referred to herein as“variants.” In some embodiments, the antibodies described in this paragraph are referred to herein
- such variants are derived from a sequence provided herein, for example, by affinity maturation, site directed mutagenesis, random mutagenesis, or any other method known in the art or described herein. In some embodiments, such variants are not derived from a sequence provided herein and may, for example, be isolated de novo according to the methods provided herein for obtaining antibodies.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO: 1, a CDR-H2 of SEQ ID NO:2, a CDR-H3 of SEQ ID NO:3, a CDR-L1 of SEQ ID NO:4, a CDR-L2 of SEQ ID NO:5, and a CDR-L1 of SEQ ID NO:6, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:39, a CDR-H2 of SEQ ID NO:40, a CDR-H3 of SEQ ID NO:4l, a CDR-L1 of SEQ ID NO:42, a CDR-L2 of SEQ ID NO:43, and a CDR-L1 of SEQ ID NO:44, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:77, a CDR-H2 of SEQ ID NO:78, a CDR-H3 of SEQ ID NO:79, a CDR-L1 of SEQ ID NO: 80, a CDR-L2 of SEQ ID NO: 81, and a CDR-L1 of SEQ ID NO: 82, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO: 115, a CDR-H2 of SEQ ID NO: l 16, a CDR-H3 of SEQ ID NO: l l7, a CDR-L1 of SEQ ID NO: 118, a CDR-L2 of SEQ ID NO: 119, and a CDR-L1 of SEQ ID NO: 120, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO: 153, a CDR-H2 of SEQ ID NO: l54, a CDR-H3 of SEQ ID NO: l55, a CDR-L1 of SEQ ID NO: 156, a CDR-L2 of SEQ ID NO: 157, and a CDR-L1 of SEQ ID NO: 158, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:884, a CDR-H2 of SEQ ID NO:885, a CDR-H3 of SEQ ID NO:886, a CDR-L1 of SEQ ID NO:887, a CDR-L2 of SEQ ID NO:888, and a CDR-L1 of SEQ ID NO:889, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO : 191 , a CDR-H2 of SEQ ID NO : 192, a CDR-H3 of SEQ ID NO : 193 , a CDR-L 1 of SEQ ID NO: 194, a CDR-L2 of SEQ ID NO: 195, and a CDR-L 1 of SEQ ID NO: 196, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:229, a CDR-H2 of SEQ ID NO:230, a CDR-H3 of SEQ ID NO:231, a CDR-L 1 of SEQ ID NO:232, a CDR-L2 of SEQ ID NO:233, and a CDR-L 1 of SEQ ID NO:234, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:267, a CDR-H2 of SEQ ID NO:268, a CDR-H3 of SEQ ID NO:269, a CDR-L 1 of SEQ ID NO:270, a CDR-L2 of SEQ ID NO:27l, and a CDR-L 1 of SEQ ID NO:272, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:305, a CDR-H2 of SEQ ID NO:306, a CDR-H3 of SEQ ID NO:307, a CDR-L1 of SEQ ID NO: 308, a CDR-L2 of SEQ ID NO: 309, and a CDR-L1 of SEQ ID NO: 310, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:343, a CDR-H2 of SEQ ID NO:344, a CDR-H3 of SEQ ID NO:345, a CDR-L1 of SEQ ID NO:346, a CDR-L2 of SEQ ID NO:347, and a CDR-L1 of SEQ ID NO:348, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:38l, a CDR-H2 of SEQ ID NO:382, a CDR-H3 of SEQ ID NO:383, a CDR-L1 of SEQ ID NO:384, a CDR-L2 of SEQ ID NO:385, and a CDR-L1 of SEQ ID NO:386, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:4l9, a CDR-H2 of SEQ ID NO:420, a CDR-H3 of SEQ ID NO:42l, a CDR-L1 of SEQ ID NO:422, a CDR-L2 of SEQ ID NO:423, and a CDR-L1 of SEQ ID NO:424, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:457, a CDR-H2 of SEQ ID NO:458, a CDR-H3 of SEQ ID NO:459, a CDR-L1 of SEQ ID NO:460, a CDR-L2 of SEQ ID NO:46l, and a CDR-L1 of SEQ ID NO:462, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:495, a CDR-H2 of SEQ ID NO:496, a CDR-H3 of SEQ ID NO:497, a CDR-L1 of SEQ ID NO:498, a CDR-L2 of SEQ ID NO:499, and a CDR-L1 of SEQ ID NO:500, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:533, a CDR-H2 of SEQ ID NO:534, a CDR-H3 of SEQ ID NO:535, a CDR-L1 of SEQ ID NO:536, a CDR-L2 of SEQ ID NO:537, and a CDR-L1 of SEQ ID NO:538, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:57l, a CDR-H2 of SEQ ID NO:572, a CDR-H3 of SEQ ID NO:573, a CDR-L1 of SEQ ID NO:574, a CDR-L2 of SEQ ID NO:575, and a CDR-L1 of SEQ ID NO:576, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:609, a CDR-H2 of SEQ ID NO:6lO, a CDR-H3 of SEQ ID NO:6l 1, a CDR-L1 of SEQ ID NO:6l2, a CDR-L2 of SEQ ID NO:6l3, and a CDR-L1 of SEQ ID NO:6l4, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:647, a CDR-H2 of SEQ ID NO:648, a CDR-H3 of SEQ ID NO:649, a CDR-L1 of SEQ ID NO:650, a CDR-L2 of SEQ ID NO:65 l, and a CDR-L1 of SEQ ID NO:652, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:685, a CDR-H2 of SEQ ID NO:686, a CDR-H3 of SEQ ID NO:687, a CDR-L1 of SEQ ID NO:688, a CDR-L2 of SEQ ID NO:689, and a CDR-L1 of SEQ ID NO:690, as determined by the Exemplary numbering system.
- an antibody provided herein comprises a CDR-H1 of SEQ ID NO:723, a CDR-H2 of SEQ ID NO:724, a CDR-H3 of SEQ ID NO:725, a CDR-L1 of SEQ ID NO: 726, a CDR-L2 of SEQ ID NO: 727, and a CDR-L1 of SEQ ID NO: 728, as determined by the Exemplary numbering system.
- a first family of antibodies comprising the following six CDR sequences: (a) a CDR-H1 having the sequence G-F-T-F-S-X1-Y-A-M-X2, wherein Xi is D or S and X2 is A or G (SEQ ID NO:773); (b) a CDR-H2 having the sequence X3-I-S-G-S-G-G-L-T-Y-Y-A-D-S-V-K-G, wherein X3 is A or T (SEQ ID NO:774); (c) a CDR-H3 having the sequence APYGYYMDV (SEQ ID NO:775); (d) a CDR-L1 having the sequence RASQSISSWLA (SEQ ID NO:776); (e) a CDR-L2 having the sequence KASSLES (SEQ ID NO:777); and (f) a CDR sequences: (a) a CDR-H1 having the sequence G-F-
- an antibody of such family comprises the following six CDR sequences: (a) a CDR-H1 having the sequence G-Y-T-F-X1-X2-Y-G-I-S, wherein Xi is D or R and X2 is S or V (SEQ ID NO: 779); (b) a CDR-H2 having the sequence W-X3-A-P-Y-X4-G-N-T-N-Y-A-Q-K-L-Q- G, wherein X3 is I or V and X 4 is S or N (SEQ ID NO:780); (c) a CDR-H3 having the sequence D-A-G-T-Y-S-P-Xs-G-Y-G-M-D-V, wherein X 5 is F or Y (SEQ ID NO:78l); (d) a CDR-L1 having the sequence X6-A-S-X
- an antibody of such family comprises the following six CDR sequences: (a) a CDR-H1 having the sequence G-F-T-F-X1-S-X2-G-M-H, wherein Xi is H or R and X2 is R or Y (SEQ ID NO:785); (b) a CDR-H2 having the sequence VITYDGINKYYADSVEG (SEQ ID NO:786); (c) a CDR-H3 having the sequence DGVYYGVYDY (SEQ ID NO: 787); (d) a CDR-L1 having the sequence K S S Q S VLF S SNNKN YL A (SEQ ID NO:788); (e) a CDR-L2 having the sequence WASTRES (SEQ ID NO:789); and (f) a CDR-L3 having the sequence
- an antibody of such family comprises a VH sequence of SEQ ID NO:765 and a VL sequence of SEQ ID NO:766. In some embodiments, provided herein is an antibody within such third family.
- a fourth family of antibodies wherein an antibody of such family comprises the following six CDR sequences: (a) a CDR-H1 having the sequence GGTFSSNAIG (SEQ ID NO:79l); (b) a CDR-H2 having the sequence SIIPIIGF ANY AQKFQG (SEQ ID NO: 792); (c) a CDR-H3 having the sequence
- DSGYYYGASSFGMDV (SEQ ID NO:793); (d) a CDR-L1 having the sequence
- an antibody of such family comprises a VH sequence of SEQ ID NO:767 and a VL sequence of SEQ ID NO:768. In some embodiments, provided herein is an antibody within such fourth family.
- an antibody of such family comprises the following six CDR sequences: (a) a CDR-H1 having the sequence G-G-S-X1-S-S-G-X2-Y-W-S, wherein Xi is I or L and X2 is Q or Y (SEQ ID NO:797); (b) a CDR-H2 having the sequence E-I-X3-X4-S-G-S-T-R-Y-N-P-S-L-K-S, wherein X3 is Y or G and X4 is Y or A (SEQ ID NO:798); (c) a CDR-H3 having the sequence D-Xs-P-Y-Y-Y-Xe-G-G-Y-Y-Y-Y-M-D-V, wherein X 5 is T or A and Xe is E, G, or D (SEQ ID NO:799); (d)
- a sixth family of antibodies wherein an antibody of such family comprises the following six CDR sequences: (a) a CDR-H1 having the sequence GYTFANYYMH (SEQ ID NO:803); (b) a CDR-H2 having the sequence IINPSGGITVYAQKFQG (SEQ ID NO:804); (c) a CDR-H3 having the sequence
- an antibody of such family comprises a VH sequence of SEQ ID NO:77l and a VL sequence of SEQ ID NO:772. In some embodiments, provided herein is an antibody within such sixth family.
- a seventh family of antibodies wherein an antibody of such family comprises the following six CDR sequences: (a) a CDR-H1 having the sequence G-Y-T-F-D-Xi-Y-G-I-S, wherein Xi is V or A (SEQ ID NO:872); (b) a CDR-H2 having the sequence W-I-A-P-Y-X2-G-N-T-N-Y-A-Q-K-L-Q-G, wherein X2 is N or S (SEQ ID NO:873); (c) a CDR-H3 having the sequence D-A-G-T-Y-S-P-F-G-Y-G-M-D-V (SEQ ID NO:874); (d) a CDR-L1 having the sequence X3-A-S-X4-S-I-X5-X6-W-L-A, wherein X3 is R or Q, X
- an antibody of such family comprises the following six CDR sequences: (a) a CDR-H1 having the sequence G-Y-T-F-R-S-Y-G-I-S (SEQ ID NO:878); (b) a CDR-H2 having the sequence W-V-A-P-Y-Xi-G-N-T-N-Y-A-Q-K-L-Q-G, wherein Xi is S or N (SEQ ID NO:878); (b) a CDR-H2 having the sequence W-V-A-P-Y-Xi-G-N-T-N-Y-A-Q-K-L-Q-G, wherein Xi is S or N (SEQ ID NO:878); (b) a CDR-H2 having the sequence W-V-A-P-Y-Xi-G-N-T-N-Y-A-Q-K-L-Q-G, wherein Xi is S or N (SEQ ID NO:878); (
- an antibody of such family comprises a VH sequence
- antibody variants defined based on percent identity to an illustrative antibody sequence provided herein, or substitution of amino acid residues in comparison to an illustrative antibody sequence provided herein.
- a variant of an antibody provided herein has specificity for hTF. In some embodiments, a variant of an antibody provided herein has specificity for cTF. In some embodiments, a variant of an antibody provided herein has specificity for mTF. In some embodiments, a variant of an antibody provided herein has specificity for hTF and cTF. In some embodiments, a variant of an antibody provided herein has specificity for hTF and mTF. In some embodiments, a variant of an antibody provided herein has specificity for cTF and mTF. In some embodiments, a variant of an antibody provided herein has specificity for hTF, cTF and mTF.
- a variant of an antibody that is derived from an illustrative antibody sequence provided herein retains affinity, as measured by KD, for hTF that is within about 1.5-fold, about 2-fold, about 3-fold, about 4-fold, about 5-fold, about 6-fold, about 7- fold, about 8-fold, about 9-fold or about lO-fold the affinity of such illustrative antibody.
- a variant of an antibody that is derived from an illustrative antibody sequence provided herein retains affinity, as measured by KD, for cTF that is within about 1.5-fold, about 2-fold, about 3-fold, about 4-fold, about 5-fold, about 6-fold, about 7-fold, about 8-fold, about 9-fold or about lO-fold the affinity of such illustrative antibody.
- a variant of an antibody that is derived from an illustrative antibody sequence provided herein retains affinity, as measured by KD, for mTF that is within about 1.5-fold, about 2-fold, about 3-fold, about 4-fold, about 5-fold, about 6-fold, about 7-fold, about 8- fold, about 9-fold or about lO-fold the affinity of such illustrative antibody.
- a variant of an antibody that is derived from an illustrative antibody sequence provided herein retains affinity, as measured by KD, for both hTF and cTF that is within about 1.5-fold, about 2-fold, about 3-fold, about 4-fold, about 5-fold, about 6-fold, about 7- fold, about 8-fold, about 9-fold or about lO-fold the affinity of such illustrative antibody.
- a variant of an antibody that is derived from an illustrative antibody sequence provided herein retains affinity, as measured by KD, for both hTF and mTF that is within about 1.5-fold, about 2-fold, about 3-fold, about 4-fold, about 5-fold, about 6-fold, about 7-fold, about 8-fold, about 9-fold or about lO-fold the affinity of such illustrative antibody.
- a variant of an antibody that is derived from an illustrative antibody sequence provided herein retains affinity, as measured by KD, for both cTF and mTF that is within about 1.5-fold, about 2-fold, about 3-fold, about 4-fold, about 5-fold, about 6-fold, about 7-fold, about 8-fold, about 9-fold or about lO-fold the affinity of such illustrative antibody.
- a variant of an antibody that is derived from an illustrative antibody sequence provided herein retains affinity, as measured by KD, for all three of hTF, cTF and mTF that is within about 1.5-fold, about 2-fold, about 3-fold, about 4- fold, about 5-fold, about 6-fold, about 7-fold, about 8-fold, about 9-fold or about lO-fold the affinity of such illustrative antibody.
- a variant of an antibody provided herein retains the ability to inhibit TF signaling, as measured by one or more assays or biological effects described herein. In some embodiments, a variant of an antibody provided herein retains the normal function of TF in the blood coagulation processes.
- a variant of an antibody provided herein competes for binding to TF with an antibody selected from 1F, 1G, 25A, 25A3, 25A5, 25A5-T, 25G,
- a variant of an antibody provided herein competes for binding to TF with an antibody selected from 25 A, 25 A3,
- a variant of an antibody provided herein competes for binding to TF with an antibody selected from 43B, 43B1,
- a variant of an antibody provided herein competes for binding to TF with an antibody selected from 25 A, 25 A3,
- a variant of an antibody provided herein competes for binding to TF with an antibody selected from 1F, 1G, 29D, 29E, 39A, or 54E.
- a variant of an antibody provided herein allows human thrombin generation as determined by thrombin generation assay (TGA). In some embodiments, a variant of an antibody provided herein does not inhibit human thrombin generation as determined by thrombin generation assay (TGA).
- a variant of an antibody provided herein binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX. In some embodiments, a variant of an antibody provided herein does not interfere with the ability of TF :FVIIa to convert FX into FXa.
- a variant of an antibody provided herein binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa. In some embodiments, a variant of an antibody provided herein does not compete for binding to human TF with human FVIIa.
- a variant of an antibody provided herein inhibits FVIIa-dependent TF signaling.
- a variant of an antibody provided herein binds mouse TF (SEQ ID NO:8l7). In some embodiments, a variant of an antibody provided herein binds mouse TF with an affinity lower (as indicated by higher KD) than the affinity of the antibody for hTF. In some embodiments, a variant of an antibody provided herein does not bind mTF.
- a variant of an antibody provided herein binds pig TF (SEQ ID NO:824). In some embodiments, a variant of an antibody provided herein binds pig TF with an affinity lower (as indicated by higher KD) than the affinity of the antibody for hTF. In some embodiments, a variant of an antibody provided herein does not bind pTF.
- a variant of an antibody provided herein binds the same epitope of TF as such antibody.
- an antibody provided herein has one or more of the characteristics listed in the following (a)-(dd): (a) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; (b) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (c) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (d) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (e) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (f) allows human thrombin generation as determined by thrombin generation assay (TGA); (g) maintains the thrombin
- an antibody provided herein has two or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has three or more of the characteristics listed in the foregoing (a)- (dd). In some embodiments, an antibody provided herein has four or more of the
- an antibody provided herein has five or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has six or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has seven or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has eight or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has nine or more of the characteristics listed in the foregoing (a)-(dd).
- an antibody provided herein has ten or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has eleven or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twelve or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has thirteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has fourteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has fifteen or more of the characteristics listed in the foregoing (a)-(dd).
- an antibody provided herein has sixteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has seventeen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has eighteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has nineteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-one or more of the characteristics listed in the foregoing (a)-(dd).
- an antibody provided herein has twenty-two or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-three of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-four of the characteristics listed in the foregoing (a)- (dd). In some embodiments, an antibody provided herein has twenty-five of the
- an antibody provided herein has twenty-six of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-seven of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-eight of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-nine of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has all thirty of the characteristics listed in the foregoing (a)-(dd).
- an antibody provided herein has one or more of the characteristics listed in the following (a)-(dd): (a) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; (b) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (c) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (d) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (e) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (f) allows human thrombin generation as determined by thrombin generation assay (TGA); (g) maintains the thrombin
- an antibody provided herein has two or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has three or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has four or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has five or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has six or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has seven or more of the characteristics listed in the foregoing (a)-(dd).
- an antibody provided herein has eight or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has nine or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has ten or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has eleven or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twelve or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has thirteen or more of the characteristics listed in the foregoing (a)-(dd).
- an antibody provided herein has fourteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has fifteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has sixteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has seventeen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has eighteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has nineteen or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-one or more of the
- an antibody provided herein has twenty-two or more of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-three of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-four of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-five of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-six of the characteristics listed in the foregoing (a)-(dd).
- an antibody provided herein has twenty-seven of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-eight of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has twenty-nine of the characteristics listed in the foregoing (a)-(dd). In some embodiments, an antibody provided herein has all thirty of the characteristics listed in the foregoing (a)-(dd).
- an antibody provided herein exhibits a combination of characteristics comprising two or more of characteristics listed in the following (a)-(dd): (a) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; (b) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (c) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (d) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (e) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (f) allows human thrombin generation as determined by thrombin generation assay (TGA); (g)
- an antibody provided herein exhibits a combination of characteristics comprising two or more of characteristics listed in the following (a)-(dd): (a) binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; (b) does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); (c) does not reduce the thrombin peak on a thrombin generation curve (Peak Ila) compared to an isotype control; (d) does not increase the time from the assay start to the thrombin peak on a thrombin generation curve (ttPeak) compared to an isotype control; (e) does not decrease the endogenous thrombin potential (ETP) as determined by the area under a thrombin generation curve compared to an isotype control; (f) allows human thrombin generation as determined by thrombin generation assay (TGA); (g)
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); and the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); and the binding between the antibody and a variant TF extracellular domain comprising mutations Nl 71H and T197K of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; allows human thrombin generation as determined by thrombin generation assay (TGA); and the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; allows human thrombin generation as determined by thrombin generation assay (TGA); and the binding between the antibody and a variant TF extracellular domain comprising mutations Nl 71H and T197K of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 8
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); the binding between the antibody and a variant TF extracellular domain comprising a mutation K149N of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and the binding between the antibody and a variant TF extracellular domain comprising mutations Nl 71H and T197K of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; allows human thrombin generation as determined by thrombin generation assay (TGA); the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810,
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; allows human thrombin generation as determined by thrombin generation assay (TGA); the binding between the antibody and a variant TF extracellular domain comprising a mutation K149N of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and the binding between the antibody and a variant TF extracellular domain comprising mutations Nl 71H and T197K of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); binds to
- the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; does not inhibit human thrombin generation as determined by thrombin generation assay (TGA); binds to
- the binding between the antibody and a variant TF extracellular domain comprising a mutation K149N of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and the binding between the antibody and a variant TF extracellular domain comprising mutations Nl 71H and T197K of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay.
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; allows human thrombin generation as determined by thrombin generation assay (TGA); binds to cynomolgus TF; the binding between the antibody and a variant TF extracellular domain comprising a mutation at amino acid residue 149 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and the binding between the antibody and a variant TF extracellular domain comprising mutations at amino acid residues 171 and 197 of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of
- an antibody provided herein exhibits a combination of the characteristics listed in the following: binds human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa; allows human thrombin generation as determined by thrombin generation assay (TGA); binds to cynomolgus TF; the binding between the antibody and a variant TF extracellular domain comprising a mutation K149N of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the sequence shown in SEQ ID NO: 810, as determined by the median fluorescence intensity value of the antibody relative to an isotype control in a live cell staining assay; and the binding between the antibody and a variant TF extracellular domain comprising mutations Nl 71H and T197K of the sequence shown in SEQ ID NO:8lO is less than 50% of the binding between the antibody and the extracellular domain of TF of the
- the affinity of an antibody provided herein for TF as indicated by KD is less than about 10 -5 M, less than about 10 -6 M, less than about 10 -7 M, less than about 10 -8 M, less than about 10 -9 M, less than about 10 -10 M, less than about 10 -11 M, or less than about 10 -12 M. In some embodiments, the affinity of the antibody is between about 10 -7 M and 10 -12 M. In some embodiments, the affinity of the antibody is between about 10 -7 M and 10 -11 M. In some embodiments, the affinity of the antibody is between about 10 -7 M and 10 -10 M. In some embodiments, the affinity of the antibody is between about 10 -7 M and 10 -9 M. In some embodiments, the affinity of the antibody is between about 10 -7 M and 10 -8 M. In some embodiments, the affinity of the antibody is between about 10 -8 M and 10 -12 M.
- the affinity of the antibody is between about 10 -8 M and 10 -11 M. In some embodiments, the affinity of the antibody is between about 10 -9 M and 10 -11 M. In some embodiments, the affinity of the antibody is between about 10 -10 M and 10 -11 M.
- the KD value of an antibody provided herein for cTF is no more than 15 c of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for cTF is no more than 10* of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for cTF is no more than 8x of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for cTF is no more than 5x of the KD value of the antibody for hTF.
- the KD value of an antibody provided herein for cTF is no more than 3x of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for cTF is no more than 2x of the KD value of the antibody for hTF.
- the KD value of an antibody provided herein for mTF is no more than 20 x of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for mTF is no more than 15 c of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for mTF is no more than 10 c of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for mTF is no more than 5x of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for mTF is no more than 2x of the KD value of the antibody for hTF.
- the affinity of an antibody provided herein for hTF as indicated by KD measured by Biacore, as set forth in Table 5 is selected from about 0.31 nM, about 6.20 nM, about 0.36 nM, about 0.08 nM, about 23.0 nM, about 0.94 nM, about 13.3 nM, about 0.47 nM, about 0.09 nM, about 1.75 nM, about 0.07 nM, about 0.14 nM, about 2.09 nM, about 0.06 nM, about 0.15 nM, about 1.46 nM, about 1.60 nM, and about 0.42 nM.
- such affinity as indicated by KD ranges from about 23.0 nM to about 0.06 nM. In some embodiments, such is about 23.0 nM or less.
- the affinity of an antibody provided herein for hTF as indicated by KD measured by ForteBio, as set forth in Table 5 is selected from about 1.28 nM, about 2.20 nM, about 8.45 nM, about 1.67 nM, about 0.64 nM, about 21.9 nM, about 3.97 nM, about 35.8 nM, about 3.30 nM, about 2.32 nM, about 0.83 nM, about 2.40 nM, about 0.96 nM, about 0.86 nM, about 3.84 nM, about 1.02 nM, about 1.61 nM, about 2.52 nM, about 2.28 nM, and about 1.59 nM.
- such affinity as indicated by KD ranges from about 35.8 nM to about 0.64 nM. In some embodiments, such KD is about 35.8 nM or less.
- the affinity of an antibody provided herein for cTF as indicated by KD measured by Biacore, as set forth in Table 5 is selected from about 0.26 nM, about 5.42 nM, about 0.21 nM, about 0.04 nM, about 18.0 nM, about 0.78 nM, about 16.4 nM, about 5.06 nM, about 0.08 nM, about 5.64 nM, about 0.12 nM, about 0.24 nM, about 5.66 nM, about 0.39 nM, about 5.69 nM, about 6.42 nM, and about 1.83 nM.
- such affinity as indicated by KD ranges from about 18.0 nM to about 0.04 nM. In some embodiments, such KD is about 18.0 nM or less.
- the affinity of an antibody provided herein for cTF as indicated by KD measured by ForteBio, as set forth in Table 5 is selected from about 1.43 nM, about 2.70 nM, about 7.65 nM, about 1.36 nM, about 0.76 nM, about 17.5 nM, about 4.99 nM, about 42.9 nM, about 12.0 nM, about 15.0 nM, about 0.57 nM, about 3.40 nM, about 1.05 nM, about 0.94 nM, about 4.12 nM, about 1.11 nM, about 1.96 nM, about 4.07 nM, about 2.71 nM, and about 4.16 nM.
- such affinity as indicated by KD ranges from about 42.9 nM to about 0.57 nM. In some embodiments, such KD is about 42.9 nM or less.
- the affinity of an antibody provided herein for mTF as indicated by KD measured by Biacore, as set forth in Table 5 is selected from about 5.4 nM, about 2.9 nM, about 21 nM, and about 2.4 nM. In some embodiments, such affinity as indicated by KD ranges from about 21 nM to about 2.4 nM. In some embodiments, such KD is about 21 nM or less.
- the affinity of an antibody provided herein for mTF as indicated by KD measured by ForteBio, as set forth in Table 5 is selected from about 263 nM, about 131 nM, about 188 nM, about 114 nM, about 34.2 nM, about 9.16 nM, about 161 nM, about 72.1 nM, about 360 nM, about 281 nM, about 41.4 nM, about 6.12 nM, about 121 nM, and about 140 nM.
- such affinity as indicated by KD ranges from about 360 nM to about 6.12 nM. In some embodiments, such KD is about 360 nM or less.
- the affinity of an antibody provided herein for hTF as indicated by ECso measured with human TF-positive HCT-l 16 cells, as set forth in Figures 1A and IB is selected from about 50 pM, about 58 pM, about 169 pM, about 77 pM, about 88 pM, about 134 pM, about 85 pM, about 237 pM, about 152 pM, about 39 pM, about 559 pM, about 280 pM, about 255 pM, about 147 pM, about 94 pM, about 117 pM, about 687 pM, about 532 pM, and about 239 pM.
- such affinity ranges from about 687 pM to about 39 pM.
- such ECso is about 687 pM or less.
- the affinity of an antibody provided herein for mTF as indicated by ECso measured with mouse TF-positive CHO cells, as set forth in Figures 2A and 2B is selected from about 455 nM, about 87 nM, about 11 nM, about 3.9 nM, about 3.0 nM, about 3.4 nM, about 255 nM, about 2.9 nM, about 3.6 nM, and about 4.0 nM. In some embodiments, such affinity ranges from about 455 nM to about 2.9 nM. In some
- such ECso is about 455 pM or less.
- the KD value of an antibody provided herein for pTF is no more than 20 x of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for pTF is no more than 15 c of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for pTF is no more than 10* of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for pTF is no more than 5x of the KD value of the antibody for hTF. In some embodiments, the KD value of an antibody provided herein for pTF is no more than 2x of the KD value of the antibody for hTF.
- the affinity of an antibody provided herein for pTF as indicated by KD measured by Biacore, as set forth in Table 40 is 3.31 nM or 12.9 nM.
- the TF antibodies provided herein do not inhibit human thrombin generation as determined by thrombin generation assay (TGA). In certain embodiments, the TF antibodies provided herein allow human thrombin generation as determined by thrombin generation assay (TGA).
- the percent peak thrombin generation is at least 40% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 50% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 60% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % Peak Ila is at least 70% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 80% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 90% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % Peak Ila is at least 95% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 99% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % Peak Ila is at least 40% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 50% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 60% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % Peak Ila is at least 70% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 80% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 90% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % Peak Ila is at least 95% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 99% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % Peak Ila is at least 60% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 70% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 80% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % Peak Ila is at least 90% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 95% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % Peak Ila is at least 99% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % Peak Ila in the presence of 100 nM TF antibody, as set forth in Table 6 and Table 37 is selected from about 99%, about 100%, about 103%, about 64%, about 52%, about 87%, about 96%, about 98%, and about 53% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) without antibody pre-incubation.
- TGA thrombin generation assay
- such % Peak Ila ranges from about 52% to about 103%.
- such % Peak Ila is about 52% or more.
- the % Peak Ila in the presence of 50 nM TF antibody is selected from about 99%, about 100%, about 103%, about 67%, about 58%, about 89%, about 96%, about 98%, about 68%, about 62%, and about 88% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) without antibody pre-incubation. In some embodiments, such %
- Peak Ila ranges from about 58% to about 103%. In some embodiments, such % Peak Ila is about 58% or more.
- the % Peak Ila in the presence of 10 nM TF antibody, as set forth in Table 6 and Table 37 is selected from about 100%, about 99%, about 103%, about 87%, about 83%, about 95%, about 98%, about 86%, and about 96% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) without antibody pre-incubation.
- TGA thrombin generation assay
- such % Peak Ila ranges from about 83% to about 103%.
- such % Peak Ila is about 83% or more.
- the % Peak Ila in the presence of 100 nM TF antibody, as set forth in Table 7 and Table 38 is selected from about 108%, about 105%, about 111%, about 58%, about 47%, about 91%, about 103%, about 109%, about 107%, and about 45% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) with 10 min antibody pre-incubation.
- TGA thrombin generation assay
- such % Peak Ila ranges from about 45% to about 111%.
- such % Peak Ila is about 45% or more.
- the % Peak Ila in the presence of 50 nM TF antibody is selected from about 107%, about 104%, about 114%, about 62%, about 49%, about 87%, about 105%, about 109%, about 55%, and about 92% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) with 10 min antibody pre-incubation.
- TGA thrombin generation assay
- such % Peak Ila ranges from about 49% to about 114%.
- such % Peak Ila is about 49% or more.
- the % Peak Ila in the presence of 10 nM TF antibody is selected from about 105%, about 114%, about 76%, about 68%, about 94%, about 108%, about 104%, about 74%, and about 93% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) with 10 min antibody pre-incubation.
- TGA thrombin generation assay
- such % Peak Ila ranges from about 68% to about 114%.
- such % Peak Ila is about 68% or more.
- the percent endogenous thrombin potential is at least 80% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 90% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 95% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 99% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % ETP is at least 80% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 90% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 95% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 99% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % ETP is at least 80% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 90% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 95% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA). In some embodiments, the % ETP is at least 99% in the presence of no less than 10 nM TF antibody compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA).
- the % ETP in the presence of 100 nM TF antibody, as set forth in Table 6 and Table 37 is selected from about 108%, about 103%, about 109%, about 100%, about 96%, about 102%, about 105%, and about 92% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) without antibody pre-incubation.
- TGA thrombin generation assay
- such % ETP ranges from about 92% to about 109%.
- such % ETP is about 92% or more.
- the % ETP in the presence of 50 nM TF antibody, as set forth in Table 6 and Table 37 is selected from about 108%, about 103%, about 111%, about 101%, about 97%, about 104%, about 106%, about 93%, about 96%, and about 105% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) without antibody pre-incubation.
- TGA thrombin generation assay
- such % ETP ranges from about 93% to about 111%.
- such % ETP is about 93% or more.
- the % ETP in the presence of 10 nM TF antibody, as set forth in Table 6 and Table 37 is selected from about 106%, about 109%, about 105%, about 104%, about 107%, about 99%, about 101%, and about 102% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) without antibody pre-incubation.
- TGA thrombin generation assay
- such % ETP ranges from about 99% to about 109%. In some embodiments, such % ETP is about 99% or more.
- the % ETP in the presence of 100 nM TF antibody, as set forth in Table 7 and Table 38 is selected from about 110%, about 104%, about 106%, about 98%, about 95%, about 108%, about 107%, about 96%, about 92%, and about 103% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) with 10 min antibody pre-incubation.
- TGA thrombin generation assay
- such % ETP ranges from about 92% to about 110%. In some embodiments, such % ETP is about 92% or more.
- the % ETP in the presence of 50 nM TF antibody is selected from about 110%, about 106%, about 108%, about 103%, about 96%, about 109%, about 102%, about 104%, about 94%, and about 98% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) with 10 min antibody pre-incubation.
- TGA thrombin generation assay
- such % ETP ranges from about 94% to about 110%.
- such % ETP is about 94% or more.
- the % ETP in the presence of 10 nM TF antibody, as set forth in Table 7 and Table 38 is selected from about 107%, about 106%, about 110%, about 103%, about 100%, about 105%, about 102%, and about 101% compared to the control conditions without the antibody, as determined by thrombin generation assay (TGA) with 10 min antibody pre-incubation.
- TGA thrombin generation assay
- such % ETP ranges from about 100% to about 110%. In some embodiments, such % ETP is about 100% or more.
- the antibodies provided herein bind human TF at a human TF binding site that is distinct from a human TF binding site bound by human FX. In certain embodiments, the antibodies provided herein do not interfere with the ability of TF :FVIIa to convert FX into FXa.
- the percentage of FXa conversion is at least 75% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 80% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 85% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 90% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 95% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody.
- the % FXa is at least 75% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 80% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 85% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 90% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 95% in the presence of no less than 50 nM TF antibody compared to the control conditions without the antibody.
- the % FXa is at least 75% in the presence of no less than 25 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 80% in the presence of no less than 25 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 85% in the presence of no less than 25 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 90% in the presence of no less than 25 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 95% in the presence of no less than 25 nM TF antibody compared to the control conditions without the antibody.
- the % FXa is at least 75% in the presence of no less than 12.5 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 80% in the presence of no less than 12.5 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 85% in the presence of no less than 12.5 nM TF antibody compared to the control conditions without the antibody. In some embodiments, % FXa is at least 90% in the presence of no less than 12.5 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FXa is at least 95% in the presence of no less than 12.5 nM TF antibody compared to the control conditions without the antibody.
- the % FXa in the presence of 100 nM TF antibody, as set forth in Table 8 is selected from about 89%, about 96%, about 116%, about 108%, about 117%, about 105%, about 112%, about 106%, about 103%, about 111%, about 98%, and about 101% compared to the control conditions without the antibody. In some embodiments, such % FXa ranges from about 89% to about 117%. In some embodiments, such % FXa is about 89% or more.
- the % FXa in the presence of 50 nM TF antibody, as set forth in Table 8 is selected from about 94%, about 93%, about 78%, about 102%, about 99%, about 104%, about 105%, about 108%, about 107%, about 97%, and about 106% compared to the control conditions without the antibody. In some embodiments, such % FXa ranges from about 78% to about 108%. In some embodiments, such % FXa is about 78% or more.
- the % FXa in the presence of 25 nM TF antibody, as set forth in Table 8 is selected from about 81%, about 89%, about 85%, about 109%, about 96%, about 97%, about 108%, about 104%, about 103%, about 112%, and about 89% compared to the control conditions without the antibody. In some embodiments, such % FXa ranges from about 81% to about 112%. In some embodiments, such % FXa is about 81% or more.
- the % FXa in the presence of 12.5 nM TF antibody, as set forth in Table 8 is selected from about 87%, about 89%, about 82%, about 99%, about 101%, about 98%, about 113%, about 106%, about 115%, about 110%, about 120%, about 85%, and about 108% compared to the control conditions without the antibody.
- such % FXa ranges from about 82% to about 120%. In some embodiments, such % FXa is about 82% or more.
- the antibodies provided herein bind human TF at a human TF binding site that is distinct from a human TF binding site bound by human FVIIa. In certain embodiments, the antibodies provided herein do not compete for binding to human TF with human FVIIa.
- the percentage of FVIIa binding is at least 75% in the presence of no less than 250 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 80% in the presence of no less than 250 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 85% in the presence of no less than 250 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 90% in the presence of no less than 250 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 95% in the presence of no less than 250 nM TF antibody compared to the control conditions without the antibody.
- the % FVIIa is at least 75% in the presence of no less than 83 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 80% in the presence of no less than 83 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 85% in the presence of no less than 83 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 90% in the presence of no less than 83 nM TF antibody compared to the control conditions without the antibody.
- the % FVIIa is at least 95% in the presence of no less than 83 nM TF antibody compared to the control conditions without the antibody. [00503] In some embodiments, the % FVIIa is at least 75% in the presence of no less than 28 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 80% in the presence of no less than 28 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 85% in the presence of no less than 28 nM TF antibody compared to the control conditions without the antibody.
- the % FVIIa is at least 90% in the presence of no less than 28 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 95% in the presence of no less than 28 nM TF antibody compared to the control conditions without the antibody.
- the % FVIIa is at least 75% in the presence of no less than 9.25 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 80% in the presence of no less than 9.25 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 85% in the presence of no less than 9.25 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 90% in the presence of no less than 9.25 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the % FVIIa is at least 95% in the presence of no less than 9.25 nM TF antibody compared to the control conditions without the antibody.
- the % FVIIa in the presence of 250 nM TF antibody, as set forth in Table 9 is selected from about 98%, about 87%, about 80%, about 92%, about 95%, about 89%, about 91%, about 97%, about 94%, about 101%, and about 96% compared to the control conditions without the antibody. In some embodiments, such % FVIIa ranges from about 80% to about 101%. In some embodiments, such % FVIIa is about 80% or more.
- the % FVIIa in the presence of 83 nM TF antibody, as set forth in Table 9 is selected from about 97%, about 88%, about 77%, about 93%, about 94%, about 91%, about 98%, about 100%, and about 92% compared to the control conditions without the antibody. In some embodiments, such % FVIIa ranges from about 77% to about 100%. In some embodiments, such % FVIIa is about 77% or more.
- the % FVIIa in the presence of 28 nM TF antibody, as set forth in Table 9 is selected from about 101%, about 87%, about 79%, about 96%, about 93%, about 95%, about 98%, about 100%, about 102%, about 99%, about 92%, and about 91% compared to the control conditions without the antibody. In some embodiments, such % FVIIa ranges from about 79% to about 102%. In some embodiments, such % FVIIa is about 79% or more.
- the % FVIIa in the presence of 9.25 nM TF antibody, as set forth in Table 9 is selected from about 100%, about 90%, about 76%, about 97%, about 93%, about 99%, about 98%, about 102%, about 101%, and about 95% compared to the control conditions without the antibody. In some embodiments, such % FVIIa ranges from about 76% to about 102%. In some embodiments, such % FVIIa is about 76% or more.
- the antibodies provided herein inhibit FVIIa-dependent TF signaling.
- the inhibition of FVIIa-dependent TF signaling is measured by the reduction of IL8.
- the inhibition of FVIIa-dependent TF signaling is measured by the reduction of GM-CSF.
- the Interleukin 8 concentration is reduced by at least 70% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 80% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 90% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody.
- the IL8 cone is reduced by at least 70% in the presence of no less than 40 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 80% in the presence of no less than 40 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 90% in the presence of no less than 40 nM TF antibody compared to the control conditions without the antibody.
- the IL8 cone is reduced by at least 60% in the presence of no less than 16 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 70% in the presence of no less than 16 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 80% in the presence of no less than 16 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 90% in the presence of no less than 16 nM TF antibody compared to the control conditions without the antibody.
- the IL8 cone is reduced by at least 50% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 60% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 70% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 80% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the IL8 cone is reduced by at least 90% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody.
- the Granulocyte-Macrophage Colony-Stimulating Factor concentration is reduced by at least 70% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 80% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 90% in the presence of no less than 100 nM TF antibody compared to the control conditions without the antibody.
- the GM-CSF cone is reduced by at least 70% in the presence of no less than 40 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 80% in the presence of no less than 40 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 90% in the presence of no less than 40 nM TF antibody compared to the control conditions without the antibody.
- the GM-CSF cone is reduced by at least 60% in the presence of no less than 16 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 70% in the presence of no less than 16 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 80% in the presence of no less than 16 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 90% in the presence of no less than 16 nM TF antibody compared to the control conditions without the antibody.
- the GM-CSF cone is reduced by at least 50% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 60% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 70% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 80% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody. In some embodiments, the GM-CSF cone is reduced by at least 90% in the presence of no less than 6.4 nM TF antibody compared to the control conditions without the antibody.
- the percentage of Interleukin 8 (% IL8) in the presence of 100 nM TF antibody, as set forth in Table 10 is selected from about 2%, about 9%, about 8%, about 6%, about 13%, about 1%, about 3%, about 4%, and about 5% compared to the control conditions without the antibody.
- % IL8 ranges from about 1% to about 13%. In some embodiments, such % IL8 is about 13% or less.
- the % IL8 in the presence of 40 nM TF antibody, as set forth in Table 10 is selected from about 2%, about 8%, about 7%, about 10%, about 14%, about 4%, about 5%, and about 6% compared to the control conditions without the antibody. In some embodiments, such % IL8 ranges from about 2% to about 14%. In some
- such % IL8 is about 14% or less.
- the % IL8 in the presence of 16 nM TF antibody, as set forth in Table 10 is selected from about 2%, about 3%, about 10%, about 8%, about 7%, about 16%, about 9%, about 15%, about 5%, and about 6% compared to the control conditions without the antibody. In some embodiments, such % IL8 ranges from about 2% to about 16%. In some embodiments, such % IL8 is about 16% or less.
- the % IL8 in the presence of 6.4 nM TF antibody, as set forth in Table 10 is selected from about 3%, about 4%, about 11%, about 9%, about 14%, about 22%, about 12%, about 6%, about 5%, about 15%, about 21%, and about 8% compared to the control conditions without the antibody.
- such % IL8 ranges from about 3% to about 22%. In some embodiments, such % IL8 is about 22% or less.
- % GM-CSF Granulocyte-Macrophage Colony- Stimulating Factor
- Table 11 is selected from about 6%, about 7%, about 22%, about 20%, about 12%, about 19%, about 17%, about 25%, about 5%, about 14%, about 11%, and about 10% compared to the control conditions without the antibody.
- % GM-CSF ranges from about 5% to about 25%. In some embodiments, such % GM-CSF is about 25% or less.
- the % GM-CSF in the presence of 40 nM TF antibody, as set forth in Table 11 is selected from about 6%, about 7%, about 19%, about 15%, about 18%, about 16%, about 26%, about 5%, about 13%, about 11%, and about 10% compared to the control conditions without the antibody. In some embodiments, such % GM-CSF ranges from about 5% to about 26%. In some embodiments, such % GM-CSF is about 26% or less.
- the % GM-CSF in the presence of 16 nM TF antibody, as set forth in Table 11 is selected from about 6%, about 7%, about 22%, about 19%, about 14%, about 32%, about 17%, about 26%, about 5%, about 12%, about 13%, about 9%, about 11%, and about 15% compared to the control conditions without the antibody.
- such % GM-CSF ranges from about 5% to about 32%. In some embodiments, such % GM-CSF is about 32% or less.
- the % GM-CSF in the presence of 6.4 nM TF antibody, as set forth in Table 11 is selected from about 8%, about 9%, about 24%, about 20%, about 18%, about 39%, about 34%, about 15%, about 21%, about 16%, about 17%, and about 10% compared to the control conditions without the antibody.
- such % GM- CSF ranges from about 8% to about 39%. In some embodiments, such % GM-CSF is about 39% or less.
- the antibodies provided herein reduce lesion size in a swine choroidal neovascularization (CNV) model.
- the reduction in lesion size is measured by Fluorescein Angiography (FA).
- the lesion size in a swine CNV model is reduced by at least 5% 7 days after administration of the anti-TF antibody. In some embodiments, the lesion size in a swine CNV model is reduced by at least 10% 7 days after administration of the anti-TF antibody. In some embodiments, the lesion size in a swine CNV model is reduced by at least 20% 7 days after administration of the anti-TF antibody. In some embodiments, the lesion size in a swine CNV model is reduced by at least 40% 7 days after administration of the anti- TF antibody. In some embodiments, the lesion size in a swine CNV model is reduced by at least 60% 7 days after administration of the anti-TF antibody.
- the lesion size in a swine CNV model is reduced by at least 10% 21 days after administration of the anti-TF antibody. In some embodiments, the lesion size in a swine CNV model is reduced by at least 20% 21 days after administration of the anti-TF antibody. In some embodiments, the lesion size in a swine CNV model is reduced by at least 40% 21 days after administration of the anti-TF antibody. In some embodiments, the lesion size in a swine CNV model is reduced by at least 60% 21 days after administration of the anti-TF antibody. In some embodiments, the lesion size in a swine CNV model is reduced by at least 80% 21 days after administration of the anti-TF antibody.
- the antibodies provided herein may comprise any suitable VH and VL germline sequences.
- the VH region of an antibody provided herein is from the VH3 germline. In some embodiments, the VH region of an antibody provided herein is from the VHl germline. In some embodiments, the VH region of an antibody provided herein is from the VH4 germline.
- the VH region of an antibody provided herein is from the VH3-23 germline. In some embodiments, the VH region of an antibody provided herein is from the VHl -18 germline. In some embodiments, the VH region of an antibody provided herein is from the VH3-30 germline. In some embodiments, the VH region of an antibody provided herein is from the VHl -69 germline. In some embodiments, the VH region of an antibody provided herein is from the VH4-31 germline. In some embodiments, the VH region of an antibody provided herein is from the VH4-34 germline. In some embodiments, the VH region of an antibody provided herein is from the VHl -46 germline.
- the VL region of an antibody provided herein is from the VK1 germline. In some embodiments, the VL region of an antibody provided herein is from the VK4 germline. In some embodiments, the VL region of an antibody provided herein is from the VK3 germline
- the VL region of an antibody provided herein is from the VK1-05 germline. In some embodiments, the VL region of an antibody provided herein is from the VK4-01 germline. In some embodiments, the VL region of an antibody provided herein is from the VK3-15 germline. In some embodiments, the VL region of an antibody provided herein is from the VK3-20 germline. In some embodiments, the VL region of an antibody provided herein is from the VK1-33 germline.
- the antibodies provided herein are monospecific antibodies.
- the antibodies provided herein are multispecific antibodies.
- a multispecific antibody provided herein binds more than one antigen.
- a multispecific antibody binds two antigens.
- a multispecific antibody binds three antigens.
- a multispecific antibody binds four antigens.
- a multispecific antibody binds five antigens.
- a multispecific antibody provided herein binds more than one epitope on a TF antigen. In some embodiments, a multispecific antibody binds two epitopes on a TF antigen. In some embodiments, a multispecific antibody binds three epitopes on a TF antigen.
- the multispecific antibody comprises an immunoglobulin comprising at least two different heavy chain variable regions each paired with a common light chain variable region (i.e., a“common light chain antibody”).
- the common light chain variable region forms a distinct antigen-binding domain with each of the two different heavy chain variable regions.
- the multispecific antibody comprises an immunoglobulin comprising an antibody or fragment thereof attached to one or more of the N- or C-termini of the heavy or light chains of such immunoglobulin. See Coloma and Morrison, Nature Biotechnol. , 1997, 15: 159-163, incorporated by reference in its entirety. In some aspects, such antibody comprises a tetravalent bispecific antibody.
- the multispecific antibody comprises a hybrid
- immunoglobulin comprising at least two different heavy chain variable regions and at least two different light chain variable regions. See Milstein and Cuello, Nature , 1983, 305:537- 540; and Staerz and Bevan, Proc. Natl. Acad. Sci. USA , 1986, 83:1453-1457; each of which is incorporated by reference in its entirety.
- the multispecific antibody comprises immunoglobulin chains with alterations to reduce the formation of side products that do not have
- the antibodies comprise one or more“knobs-into-holes” modifications as described in U.S. Pat. No. 5,731,168, incorporated by reference in its entirety.
- the multispecific antibody comprises immunoglobulin chains with one or more electrostatic modifications to promote the assembly of Fc hetero- multimers. See WO 2009/089004, incorporated by reference in its entirety.
- the multispecific antibody comprises a bispecific single chain molecule. See Traunecker et al., EMBO J , 1991, 10:3655-3659; and Gruber et al., J Immunol ., 1994, 152:5368-5374; each of which is incorporated by reference in its entirety.
- the multispecific antibody comprises a heavy chain variable domain and a light chain variable domain connected by a polypeptide linker, where the length of the linker is selected to promote assembly of multispecific antibodies with the desired multispecificity.
- monospecific scFvs generally form when a heavy chain variable domain and light chain variable domain are connected by a polypeptide linker of more than 12 amino acid residues. See U.S. Pat. Nos. 4,946,778 and 5,132,405, each of which is incorporated by reference in its entirety.
- reduction of the polypeptide linker length to less than 12 amino acid residues prevents pairing of heavy and light chain variable domains on the same polypeptide chain, thereby allowing pairing of heavy and light chain variable domains from one chain with the complementary domains on another chain.
- the resulting antibodies therefore have multispecificity, with the specificity of each binding site contributed by more than one polypeptide chain.
- Polypeptide chains comprising heavy and light chain variable domains that are joined by linkers between 3 and 12 amino acid residues form predominantly dimers (termed diabodies). With linkers between 0 and 2 amino acid residues, trimers (termed triabodies) and tetramers (termed tetrabodies) are favored.
- the multispecific antibody comprises a diabody. See Hollinger et al., Proc. Natl. Acad. Sci. USA , 1993, 90:6444-6448, incorporated by reference in its entirety. In some embodiments, the multispecific antibody comprises a triabody. See Todorovska et al., J. Immunol. Methods , 2001, 248:47-66, incorporated by reference in its entirety. In some embodiments, the multispecific antibody comprises a tetrabody. See id., incorporated by reference in its entirety.
- the multispecific antibody comprises a trispecific F(ab’)3 derivative. See Tutt et al. J. Immunol., 1991, 147:60-69, incorporated by reference in its entirety. [00548] In some embodiments, the multispecific antibody comprises a cross-linked antibody. See U.S. Patent No. 4,676,980; Brennan et al., Science , 1985, 229:81-83; Staerz, et al. Nature , 1985, 314:628-631; and EP 0453082; each of which is incorporated by reference in its entirety.
- the multispecific antibody comprises antigen-binding domains assembled by leucine zippers. See Kostelny et al., J Immunol ., 1992, 148: 1547- 1553, incorporated by reference in its entirety.
- the multispecific antibody comprises complementary protein domains.
- the complementary protein domains comprise an anchoring domain (AD) and a dimerization and docking domain (DDD).
- AD and DDD bind to each other and thereby enable assembly of multispecific antibody structures via the“dock and lock” (DNL) approach.
- DNL dimerization and docking domain
- Multispecific antibodies comprising complementary protein domains are described, for example, in U.S. Pat. Nos. 7,521,056; 7,550,143; 7,534,866; and 7,527,787; each of which is incorporated by reference in its entirety.
- the multispecific antibody comprises a dual action Fab (DAF) antibody as described in U.S. Pat. Pub. No. 2008/0069820, incorporated by reference in its entirety.
- DAF dual action Fab
- the multispecific antibody comprises an antibody formed by reduction of two parental molecules followed by mixing of the two parental molecules and reoxidation to assembly a hybrid structure. See Carlring et al., PLoS One , 2011, 6:e22533, incorporated by reference in its entirety.
- the multispecific antibody comprises a DVD-IgTM.
- a DVD-IgTM is a dual variable domain immunoglobulin that can bind to two or more antigens. DVD-IgsTM are described in U.S. Pat. No. 7,612,181, incorporated by reference in its entirety.
- the multispecific antibody comprises a DARTTM.
- DARTsTM are described in Moore et al., Blood , 2011, 117:454-451, incorporated by reference in its entirety.
- the multispecific antibody comprises a DuoBody ® .
- the multispecific antibody comprises an antibody fragment attached to another antibody or fragment.
- the attachment can be covalent or non-covalent. When the attachment is covalent, it may be in the form of a fusion protein or via a chemical linker.
- multispecific antibodies comprising antibody fragments attached to other antibodies
- tetravalent bispecific antibodies where an scFv is fused to the C-terminus of the Cro from an IgG. See Coloma and Morrison, Nature Biotechnol. , 1997, 15: 159-163.
- Other examples include antibodies in which a Fab molecule is attached to the constant region of an immunoglobulin. See Miler et al., J. Immunol ., 2003, 170:4854- 4861, incorporated by reference in its entirety. Any suitable fragment may be used, including any of the fragments described herein or known in the art.
- the multispecific antibody comprises a CovX-Body.
- CovX-Bodies are described, for example, in Doppalapudi et al., Proc. Natl. Acad. Sci. USA , 2010, 107:22611-22616, incorporated by reference in its entirety.
- the multispecific antibody comprises an Fcab antibody, where one or more antigen-binding domains are introduced into an Fc region.
- Fcab antibodies are described in Wozniak-Knopp et al., Protein Eng. Des. Sel., 2010, 23:289-297, incorporated by reference in its entirety.
- the multispecific antibody comprises a TandAb ® antibody.
- TandAb ® antibodies are described in Kipriyanov et al., J. Mol. Biol ., 1999, 293:41-56 and Zhukovsky et al., Blood , 2013, 122:5116, each of which is incorporated by reference in its entirety.
- the multispecific antibody comprises a tandem Fab.
- Tandem Fabs are described in WO 2015/103072, incorporated by reference in its entirety.
- the multispecific antibody comprises a ZybodyTM.
- ZybodiesTM are described in LaFleur et al., mAbs , 2013, 5:208-218, incorporated by reference in its entirety.
- an antibody provided herein may be altered to increase, decrease or eliminate the extent to which it is glycosylated. Glycosylation of polypeptides is typically either“N-linked” or“O-linked.”
- N-linked glycosylation refers to the attachment of a carbohydrate moiety to the side chain of an asparagine residue.
- the tripeptide sequences asparagine-X-serine and asparagine-X-threonine, where X is any amino acid except proline, are the recognition sequences for enzymatic attachment of the carbohydrate moiety to the asparagine side chain.
- X is any amino acid except proline
- O-linked glycosylation refers to the attachment of one of the sugars N- acetylgalactosamine, galactose, or xylose to a hydroxyamino acid, most commonly serine or threonine, although 5-hydroxyproline or 5-hydroxylysine may also be used.
- Addition or deletion of N-linked glycosylation sites to or from an antibody provided herein may be accomplished by altering the amino acid sequence such that one or more of the above-described tripeptide sequences is created or removed.
- Addition or deletion of O-linked glycosylation sites may be accomplished by addition, deletion, or substitution of one or more serine or threonine residues in or to (as the case may be) the sequence of an antibody.
- an antibody provided herein comprises a glycosylation motif that is different from a naturally occurring antibody. Any suitable naturally occurring glycosylation motif can be modified in the antibodies provided herein.
- the structural and glycosylation properties of immunoglobulins, for example, are known in the art and summarized, for example, in Schroeder and Cavacini, J. Allergy Clin. Immunol ., 2010, l25:S4l-52, incorporated by reference in its entirety.
- an antibody provided herein comprises an IgGl Fc region with modification to the oligosaccharide attached to asparagine 297 (Asn 297).
- Naturally occurring IgGl antibodies produced by mammalian cells typically comprise a branched, biantennary oligosaccharide that is generally attached by an N-linkage to Asn 297 of the Cm domain of the Fc region. See Wright et ak, TIBTECH , 1997, 15:26-32, incorporated by reference in its entirety.
- the oligosaccharide attached to Asn 297 may include various carbohydrates such as mannose, N-acetyl glucosamine (GlcNAc), galactose, and sialic acid, as well as a fucose attached to a GlcNAc in the“stem” of the biantennary oligosaccharide structure.
- various carbohydrates such as mannose, N-acetyl glucosamine (GlcNAc), galactose, and sialic acid, as well as a fucose attached to a GlcNAc in the“stem” of the biantennary oligosaccharide structure.
- the oligosaccharide attached to Asn 297 is modified to create antibodies having altered ADCC. In some embodiments, the oligosaccharide is altered to improve ADCC. In some embodiments, the oligosaccharide is altered to reduce ADCC.
- an antibody provided herein comprises an IgGl domain with reduced fucose content at position Asn 297 compared to a naturally occurring IgGl domain.
- Fc domains are known to have improved ADCC. See Shields et ak, J. Biol. Chem ., 2002, 277:26733-26740, incorporated by reference in its entirety.
- such antibodies do not comprise any fucose at position Asn 297. The amount of fucose may be determined using any suitable method, for example as described in WO 2008/077546, incorporated by reference in its entirety.
- an antibody provided herein comprises a bisected oligosaccharide, such as a biantennary oligosaccharide attached to the Fc region of the antibody that is bisected by GlcNAc.
- a bisected oligosaccharide such as a biantennary oligosaccharide attached to the Fc region of the antibody that is bisected by GlcNAc.
- Such antibody variants may have reduced fucosylation and/or improved ADCC function. Examples of such antibody variants are described, for example, in WO 2003/011878; U.S. Pat. No. 6,602,684; and U.S. Pat. Pub. No.
- an antibody provided herein comprises an Fc region with at least one galactose residue in the oligosaccharide attached to the Fc region.
- Such antibody variants may have improved CDC function. Examples of such antibody variants are described, for example, in WO 1997/30087; WO 1998/58964; and WO 1999/22764; each of which is incorporated by reference in its entirety.
- Examples of cell lines capable of producing defucosylated antibodies include Lecl3 CHO cells, which are deficient in protein fucosylation (see Ripka et ak, Arch.
- an antibody provided herein is an aglycosylated antibody.
- An aglycosylated antibody can be produced using any method known in the art or described herein.
- an aglycosylated antibody is produced by modifying the antibody to remove all glycosylation sites.
- the glycosylation sites are removed only from the Fc region of the antibody.
- an aglycosylated antibody is produced by expressing the antibody in an organism that is not capable of glycosylation, such as E. coli , or by expressing the antibody in a cell-free reaction mixture.
- an antibody provided herein has a constant region with reduced effector function compared to a native IgGl antibody.
- the affinity of a constant region of an Fc region of an antibody provided herein for Fc receptor is less than the affinity of a native IgGl constant region for such Fc receptor.
- an antibody provided herein comprises an Fc region with one or more amino acid substitutions, insertions, or deletions in comparison to a naturally occurring Fc region.
- substitutions, insertions, or deletions yield antibodies with altered stability, glycosylation, or other characteristics.
- substitutions, insertions, or deletions yield aglycosylated antibodies.
- the Fc region of an antibody provided herein is modified to yield an antibody with altered affinity for an Fc receptor, or an antibody that is more
- the antibody variants provided herein possess some, but not all, effector functions. Such antibodies may be useful, for example, when the half-life of the antibody is important in vivo , but when certain effector functions (e.g, complement activation and ADCC) are unnecessary or deleterious.
- effector functions e.g, complement activation and ADCC
- the Fc region of an antibody provided herein is a human IgG4 Fc region comprising one or more of the hinge stabilizing mutations S228P and L235E. See Aalberse et al., Immunology, 2002, 105:9-19, incorporated by reference in its entirety.
- the IgG4 Fc region comprises one or more of the following mutations: E233P, F234V, and L235A. See Armour et al , Mol. Immunol., 2003, 40:585-593, incorporated by reference in its entirety.
- the IgG4 Fc region comprises a deletion at position G236.
- the Fc region of an antibody provided herein is a human IgGl Fc region comprising one or more mutations to reduce Fc receptor binding.
- the one or more mutations are in residues selected from S228 (e.g, S228A), L234 (e.g, L234A), L235 (e.g, L235A), D265 (e.g, D265A), and N297 (e.g, N297A).
- the antibody comprises a PVA236 mutation.
- PVA236 means that the amino acid sequence ELLG, from amino acid position 233 to 236 of IgGl or EFLG of IgG4, is replaced by PVA. See ET.S. Pat. No.
- the Fc region of an antibody provided herein is modified as described in Armour et al., Eur. J Immunol ., 1999, 29:2613-2624; WO 1999/058572; and/or U.K. Pat. App. No. 98099518; each of which is incorporated by reference in its entirety.
- the Fc region of an antibody provided herein is a human IgG2 Fc region comprising one or more of mutations A330S and P331S.
- the Fc region of an antibody provided herein has an amino acid substitution at one or more positions selected from 238, 265, 269, 270, 297, 327 and 329. See U.S. Pat. No. 6,737,056, incorporated by reference in its entirety.
- Such Fc mutants include Fc mutants with substitutions at two or more of amino acid positions 265, 269, 270, 297 and 327, including the so-called“DANA” Fc mutant with substitution of residues 265 and 297 with alanine. See U.S. Pat. No. 7,332,581, incorporated by reference in its entirety.
- the antibody comprises an alanine at amino acid position 265. In some embodiments, the antibody comprises an alanine at amino acid position 297.
- an antibody provided herein comprises an Fc region with one or more amino acid substitutions which improve ADCC, such as a substitution at one or more of positions 298, 333, and 334 of the Fc region.
- an antibody provided herein comprises an Fc region with one or more amino acid substitutions at positions 239, 332, and 330, as described in Lazar et al., Proc. Natl. Acad. Sci. USA ,
- an antibody provided herein comprises one or more alterations that improves or diminishes Clq binding and/or CDC. See U.S. Pat. No.
- an antibody provided herein comprises one or more alterations to increase half-life.
- Antibodies with increased half-lives and improved binding to the neonatal Fc receptor (FcRn) are described, for example, in Hinton et al., J. Immunol., 2006, 176:346-356; and U.S. Pat. Pub. No. 2005/0014934; each of which is incorporated by reference in its entirety.
- Such Fc variants include those with substitutions at one or more of Fc region residues: 238, 250, 256, 265, 272, 286, 303, 305, 307, 311, 312, 314, 317, 340,
- an antibody provided herein comprises one or more Fc region variants as described in U.S. Pat. Nos. 7,371,826, 5,648,260, and 5,624,821; Duncan and Winter, Nature, 1988, 322:738-740; and WO 94/29351; each of which is incorporated by reference in its entirety. 2.8. Pyroglutamate
- antibodies comprising a polypeptide sequence having a pE residue at the N-terminal position. In some embodiments, provided herein are antibodies comprising a polypeptide sequence in which the N-terminal residue has been converted from Q to pE. In some embodiments, provided herein are antibodies comprising a polypeptide sequence in which the N-terminal residue has been converted from E to pE.
- cysteine engineered antibodies also known as“thioMAbs,” in which one or more residues of the antibody are substituted with cysteine residues.
- the substituted residues occur at solvent accessible sites of the antibody.
- reactive thiol groups are introduced at solvent accessible sites of the antibody and may be used to conjugate the antibody to other moieties, such as drug moieties or linker-drug moieties, for example, to create an immunoconjugate.
- any one or more of the following residues may be substituted with cysteine: V205 of the light chain; Al 18 of the heavy chain Fc region; and S400 of the heavy chain Fc region.
- Cysteine engineered antibodies may be generated as described, for example, in ET.S. Pat. No. 7,521,541, which is incorporated by reference in its entirety.
- ADCs antibody-drug conjugates
- the cytotoxic agent is linked directly to the anti-TF antibody. In some embodiments, the cytotoxic agent is linked indirectly to the anti-TF antibody.
- the ADCs further comprise a linker.
- the linker links the anti-TF antibody to the cytotoxic agent.
- the ADCs provided herein have a drug-antibody ratio (DAR) of 1.
- the ADCs provided herein have a DAR of 2.
- the ADCs provided herein have a DAR of 3.
- the ADCs provided herein have a DAR of 4.
- the ADCs provided herein have a DAR of 5.
- the ADCs provided herein have a DAR of 1-2, 1-3, 1-4, 1- 5, 2-3, 2-4, 2-5, 3-4, 3-5, 4-5, 1, 2, 3, 4, or 5. In some embodiments, the ADCs provided herein have a DAR greater than 5. In some embodiments, the DAR is measured by UV/vis spectroscopy, hydrophobic interaction chromatography (HIC), and/or reverse phase liquid chromatography separation with time-of-flight detection and mass characterization (RP- UPLC/Mass spectrometry).
- HIC hydrophobic interaction chromatography
- RP- UPLC/Mass spectrometry reverse phase liquid chromatography separation with time-of-flight detection and mass characterization
- the TF antigen used for isolation of the antibodies provided herein may be intact TF or a fragment of TF.
- the TF antigen may be, for example, in the form of an isolated protein or a protein expressed on the surface of a cell.
- the TF antigen is a non-naturally occurring variant of TF, such as a TF protein having an amino acid sequence or post-translational modification that does not occur in nature.
- the TF antigen is truncated by removal of, for example, intracellular or membrane-spanning sequences, or signal sequences.
- the TF antigen is fused at its C-terminus to a human IgGl Fc domain or a polyhistidine tag.
- Monoclonal antibodies may be obtained, for example, using the hybridoma method first described by Kohler et al., Nature , 1975, 256:495-497 (incorporated by reference in its entirety), and/or by recombinant DNA methods ( see e.g., U.S. Patent No. 4,816,567, incorporated by reference in its entirety). Monoclonal antibodies may also be obtained, for example, using phage-display libraries (see e.g. , U.S. Patent No. 8,258,082, which is incorporated by reference in its entirety) or, alternatively, using yeast-based libraries (see e.g, U.S. Patent No. 8,691,730, which is incorporated by reference in its entirety).
- lymphocytes that produce or are capable of producing antibodies that will specifically bind to the protein used for immunization.
- lymphocytes may be immunized in vitro. Lymphocytes are then fused with myeloma cells using a suitable fusing agent, such as polyethylene glycol, to form a hybridoma cell.
- a suitable fusing agent such as polyethylene glycol
- the hybridoma cells are seeded and grown in a suitable culture medium that contains one or more substances that inhibit the growth or survival of the unfused, parental myeloma cells.
- a suitable culture medium that contains one or more substances that inhibit the growth or survival of the unfused, parental myeloma cells.
- the culture medium for the hybridomas typically will include hypoxanthine, aminopterin, and thymidine (HAT medium), which substances prevent the growth of HGPRT-deficient cells.
- Useful myeloma cells are those that fuse efficiently, support stable high-level production of antibody by the selected antibody-producing cells, and are sensitive media conditions, such as the presence or absence of HAT medium.
- preferred myeloma cell lines are murine myeloma lines, such as those derived from MOP-21 and MC- 11 mouse tumors (available from the Salk Institute Cell Distribution Center, San Diego, CA), and SP-2 or X63-Ag8-653 cells (available from the American Type Culture Collection, Rockville, MD).
- Human myeloma and mouse-human heteromyeloma cell lines also have been described for the production of human monoclonal antibodies. See e.g., Kozbor, J. Immunol ., 1984, 133:3001, incorporated by reference in its entirety.
- hybridoma cells After the identification of hybridoma cells that produce antibodies of the desired specificity, affinity, and/or biological activity, selected clones may be subcloned by limiting dilution procedures and grown by standard methods. See Goding, supra. Suitable culture media for this purpose include, for example, D-MEM or RPMI-1640 medium. In addition, the hybridoma cells may be grown in vivo as ascites tumors in an animal.
- DNA encoding the monoclonal antibodies may be readily isolated and sequenced using conventional procedures (e.g, by using oligonucleotide probes that are capable of binding specifically to genes encoding the heavy and light chains of the monoclonal antibodies).
- the hybridoma cells can serve as a useful source of DNA encoding antibodies with the desired properties.
- the DNA may be placed into expression vectors, which are then transfected into host cells such as bacteria (e.g, E. coli ), yeast (e.g, Saccharomyces or Pichia sp.), COS cells, Chinese hamster ovary (CHO) cells, or myeloma cells that do not otherwise produce antibody, to produce the monoclonal antibodies.
- a chimeric antibody is made by using recombinant techniques to combine a non-human variable region (e.g ., a variable region derived from a mouse, rat, hamster, rabbit, or non human primate, such as a monkey) with a human constant region.
- a non-human variable region e.g ., a variable region derived from a mouse, rat, hamster, rabbit, or non human primate, such as a monkey
- Humanized antibodies may be generated by replacing most, or all, of the structural portions of a non-human monoclonal antibody with corresponding human antibody sequences. Consequently, a hybrid molecule is generated in which only the antigen-specific variable, or CDR, is composed of non-human sequence.
- Methods to obtain humanized antibodies include those described in, for example, Winter and Milstein, Nature , 1991, 349:293-299; Rader et al., Proc. Nat. Acad. Sci. U.S.A., 1998, 95:8910-8915; Steinberger et al., J. Biol. Chem., 2000, 275:36073-36078; Queen et al., Proc. Natl. Acad. Sci. U.S. A., 1989, 86: 10029-10033; and U.S. Patent Nos. 5,585,089, 5,693,761, 5,693,762, and 6,180,370; each of which is incorporated by reference in its entirety.
- Human antibodies can be generated by a variety of techniques known in the art, for example by using transgenic animals (e.g., humanized mice). See, e.g, Jakobovits et al., Proc. Natl. Acad. Sci. U.S.A., 1993, 90:2551; Jakobovits et al., Nature, 1993, 362:255-258; Bruggermann et ak, Year in Immuno., 1993, 7:33; and U.S. Patent Nos. 5,591,669, 5,589,369 and 5,545,807; each of which is incorporated by reference in its entirety.
- Human antibodies can also be derived from phage-display libraries (see e.g, Hoogenboom et al., J. Mol. Biol., 1991, 227:381-388; Marks et al., J. Mol. Biol., 1991, 222:581-597; and U.S. Pat. Nos.
- Human antibodies may also be generated by in vitro activated B cells (see e.g, U.S. Patent. Nos. 5,567,610 and 5,229,275, each of which is incorporated by reference in its entirety). Human antibodies may also be derived from yeast-based libraries (see e.g, U.S. Patent No.
- the antibody fragments provided herein may be made by any suitable method, including the illustrative methods described herein or those known in the art. Suitable methods include recombinant techniques and proteolytic digestion of whole antibodies. Illustrative methods of making antibody fragments are described, for example, in Hudson et al., Nat. Med., 2003, 9: 129-134, incorporated by reference in its entirety. Methods of making scFv antibodies are described, for example, in Pluckthun, in The Pharmacology of
- the alternative scaffolds provided herein may be made by any suitable method, including the illustrative methods described herein or those known in the art.
- methods of preparing AdnectinsTM are described in Emanuel et al., mAbs, 2011, 3:38-48, incorporated by reference in its entirety.
- Methods of preparing iMabs are described in U.S. Pat. Pub. No. 2003/0215914, incorporated by reference in its entirety.
- Methods of preparing Anticalins ® are described in Vogt and Skerra, Chem. Biochem., 2004, 5:191-199,
- the multispecific antibodies provided herein may be made by any suitable method, including the illustrative methods described herein or those known in the art.
- DARTsTM Methods of making DARTsTM are described in Moore et al., Blood, 2011, 117:454-451, incorporated by reference in its entirety. Methods of making DuoBodies ® are described in Labrijn et al., Proc. Natl. Acad. Sci. USA, 2013, 110:5145-5150; Gramer et al., mAbs, 2013, 5:962-972; and Labrijn et al., Nature Protocols, 2014, 9:2450-2463; each of which is incorporated by reference in its entirety.
- tandem Fabs are described in WO 2015/103072, incorporated by reference in its entirety.
- Methods of making ZybodiesTM are described in LaFleur et al., mAbs, 2013, 5:208-218, incorporated by reference in its entirety.
- an antibody provided herein is an affinity matured variant of a parent antibody, which may be generated, for example, using phage display-based affinity maturation techniques. Briefly, one or more CDR residues may be mutated and the variant antibodies, or portions thereof, displayed on phage and screened for affinity. Such alterations may be made in CDR“hotspots,” or residues encoded by codons that undergo mutation at high frequency during the somatic maturation process (see Chowdhury, Methods Mol. Biol., 2008, 207: 179-196, incorporated by reference in its entirety), and/or residues that contact the antigen.
- Any suitable method can be used to introduce variability into a polynucleotide sequence(s) encoding an antibody, including error-prone PCR, chain shuffling, and oligonucleotide-directed mutagenesis such as trinucleotide-directed mutagenesis (TRIM).
- TAM trinucleotide-directed mutagenesis
- CDR residues e.g ., 4-6 residues at a time
- CDR residues involved in antigen binding may be specifically identified, for example, using alanine scanning mutagenesis or modeling.
- CDR-H3 and CDR-L3 in particular are often targeted for mutation.
- variable regions and/or CDRs can be used to produce a secondary library.
- the secondary library is then screened to identify antibody variants with improved affinity.
- Affinity maturation by constructing and reselecting from secondary libraries has been described, for example, in Hoogenboom et al., Methods in Molecular Biology, 2001, 178:1-37, incorporated by reference in its entirety.
- nucleic acids encoding TF antibodies
- vectors comprising the nucleic acids
- host cells comprising the vectors and nucleic acids, as well as recombinant techniques for the production of the antibodies.
- the nucleic acid(s) encoding it may be isolated and inserted into a replicable vector for further cloning (i.e ., amplification of the DNA) or expression.
- the nucleic acid may be produced by homologous recombination, for example as described in U.S. Patent No. 5,204,244, incorporated by reference in its entirety.
- the vector components generally include one or more of the following: a signal sequence, an origin of replication, one or more marker genes, an enhancer element, a promoter, and a transcription termination sequence, for example as described in U.S. Patent No. 5,534,615, incorporated by reference in its entirety.
- Suitable host cells are provided below. These host cells are not meant to be limiting, and any suitable host cell may be used to produce the antibodies provided herein.
- Suitable host cells include any prokaryotic (e.g, bacterial), lower eukaryotic (e.g, yeast), or higher eukaryotic (e.g, mammalian) cells.
- Suitable prokaryotes include eubacteria, such as Gram-negative or Gram-positive organisms, for example, Enterobacteriaceae such as Escherichia (E. coli), Enterobacter, Erwinia, Klebsiella, Proteus, Salmonella (S.
- E. coli cloning host is E. coli 294, although other strains such as E. coli B, E. coli X1776, and E. coli W3110 are also suitable.
- eukaryotic microbes such as filamentous fungi or yeast are also suitable cloning or expression hosts for TF antibody-encoding vectors.
- Saccharomyces cerevisiae or common baker’s yeast
- Saccharomyces cerevisiae is a commonly used lower eukaryotic host microorganism.
- a number of other genera, species, and strains are available and useful, such as Schizosaccharomyces pombe , Kluyveromyces (K. lactis , K. fragilis, K. bulgaricus K. wickeramii , K. waltii , K. drosophilarum , K. thermotolerans , and K.
- Neurospora crassa Schwanniomyces ( S . occidentals), and filamentous fungi such as, for example Penicillium, Tolypocladium , and Aspergillus (A. nidulans and A. nigef).
- filamentous fungi such as, for example Penicillium, Tolypocladium , and Aspergillus (A. nidulans and A. nigef).
- Useful mammalian host cells include COS-7 cells, HEK293 cells, baby hamster kidney (BHK) cells, Chinese hamster ovary (CHO), mouse sertoli cells, African green monkey kidney cells (VERO-76), and the like.
- the host cells used to produce the TF antibody of this invention may be cultured in a variety of media.
- Commercially available media such as, for example, Ham’s F10, Minimal Essential Medium (MEM), RPMI-1640, and Dulbecco’s Modified Eagle’s Medium (DMEM) are suitable for culturing the host cells.
- MEM Minimal Essential Medium
- RPMI-1640 RPMI-1640
- DMEM Dulbecco’s Modified Eagle’s Medium
- any of these media may be supplemented as necessary with hormones and/or other growth factors (such as insulin, transferrin, or epidermal growth factor), salts (such as sodium chloride, calcium, magnesium, and phosphate), buffers (such as HEPES), nucleotides (such as adenosine and thymidine), antibiotics, trace elements (defined as inorganic compounds usually present at final concentrations in the micromolar range), and glucose or an equivalent energy source. Any other necessary supplements may also be included at appropriate concentrations that would be known to those skilled in the art.
- growth factors such as insulin, transferrin, or epidermal growth factor
- salts such as sodium chloride, calcium, magnesium, and phosphate
- buffers such as HEPES
- nucleotides such as adenosine and thymidine
- antibiotics such as adenosine and thymidine
- trace elements defined as inorganic compounds usually present at final concentrations in the micromolar range
- glucose or an equivalent energy source
- the culture conditions such as temperature, pH, and the like, are those previously used with the host cell selected for expression, and will be apparent to the ordinarily skilled artisan.
- the antibody can be produced intracellularly, in the periplasmic space, or directly secreted into the medium. If the antibody is produced intracellularly, as a first step, the particulate debris, either host cells or lysed fragments, is removed, for example, by centrifugation or ultrafiltration. For example, Carter et al.
- the antibody is produced in a cell-free system.
- the cell-free system is an in vitro transcription and translation system as described in Yin et al., mAbs , 2012, 4:217-225, incorporated by reference in its entirety.
- the cell-free system utilizes a cell-free extract from a eukaryotic cell or from a prokaryotic cell.
- the prokaryotic cell is E. coli.
- Cell-free expression of the antibody may be useful, for example, where the antibody accumulates in a cell as an insoluble aggregate, or where yields from periplasmic expression are low.
- supernatants from such expression systems are generally first concentrated using a commercially available protein concentration filter, for example, an Amicon ® or Millipore ® Pellcon ® ultrafiltration unit.
- a protease inhibitor such as PMSF may be included in any of the foregoing steps to inhibit proteolysis and antibiotics may be included to prevent the growth of adventitious
- the antibody composition prepared from the cells can be purified using, for example, hydroxylapatite chromatography, gel electrophoresis, dialysis, and affinity chromatography, with affinity chromatography being a particularly useful purification technique.
- the suitability of protein A as an affinity ligand depends on the species and isotype of any immunoglobulin Fc domain that is present in the antibody.
- Protein A can be used to purify antibodies that comprise human g ⁇ , g2, or g4 heavy chains (Lindmark et al., ./. Immunol. Meth ., 1983, 62: 1-13, incorporated by reference in its entirety).
- Protein G is useful for all mouse isotypes and for human g3 (Guss et al., EMBO J, 1986, 5: 1567-1575, incorporated by reference in its entirety).
- the matrix to which the affinity ligand is attached is most often agarose, but other matrices are available. Mechanically stable matrices such as controlled pore glass or poly(styrenedivinyl)benzene allow for faster flow rates and shorter processing times than can be achieved with agarose. Where the antibody comprises a Cm domain, the BakerBond ABX ® resin is useful for purification. [00628] Other techniques for protein purification, such as fractionation on an ion-exchange column, ethanol precipitation, Reverse Phase HPLC, chromatography on silica,
- the mixture comprising the antibody of interest and contaminants may be subjected to low pH hydrophobic interaction chromatography using an elution buffer at a pH between about 2.5 to about 4.5, generally performed at low salt concentrations (e.g ., from about 0 to about 0.25 M salt).
- ADCs provided herein comprise a cytotoxic agent.
- the cytotoxic agents provided herein include various anti-tumor or anti-cancer agents known in the art.
- the cytotoxic agents cause destruction of cancer cells.
- the cytotoxic agents inhibit the growth or proliferation of cancer cells.
- Suitable cytotoxic agents include anti-angiogenic agents, pro-apoptotic agents, anti-mitotic agents, anti-kinase agents, alkylating agents, hormones, hormone agonists, hormone antagonists, chemokines, drugs, prodrugs, toxins, enzymes, antimetabolites, antibiotics, alkaloids, and radioactive isotopes.
- the cytotoxic agent comprises at least one of:
- the cytotoxic agent is monomethyl auristatin E (MMAE).
- the cytotoxic agent is a diagnostic agent, such as a radioactive isotope, a metal chelator, an enzyme, a fluorescent compound, a bioluminescent compound, or a chemiluminescent compound.
- the cytotoxic agent is a cytotoxic payload improved safety profile, for example XMT-1267 and other cytotoxic payloads described in Trail et ah, Pharmacol Ther , 2018, 181 : 126-142.
- ADCs provided herein comprise a linker.
- an unbound linker comprises two reactive termini: an antibody conjugation reactive termini and an cytotoxic agent conjugation reactive termini.
- the antibody conjugation reactive terminus of the linker can be conjugated to the antibody through a cysteine thiol or lysine amine group on the antibody, typically a thiol-reactive group such as a double bond, a leaving group such as a chloro, bromo or iodo, an R-sulfanyl group or sulfonyl group, or an amine-reactive group such as a carboxyl group.
- the cytotoxic agent conjugation reactive terminus of the linker can be conjugated to the cytotoxic agent through formation of an amide bond with a basic amine or carboxyl group on the cytotoxin, typically a carboxyl or basic amine group.
- the linker is a non-cleavable linker. In some embodiments, the linker is a cleavable linker. In some embodiments, the cytotoxic agent is released from the ADC in a cell.
- Suitable linkers of ADCs include labile linkers, acid labile linkers (e.g ., hydrazone linkers), photolabile linkers, charged linkers, disulfide-containing linkers, peptidase-sensitive linkers (e.g., peptide linkers comprising amino acids, for example, valine and/or citrulline such as citrulline-valine or phenylalanine-lysine), b-glucuronide-linkers ( See e.g, Graaf et ah, Curr Pharm Des, 2002, 8: 1391-1403), dimethyl linkers (See e.g., Chari et ah, Cancer Research, 1992, 52: 127-131; U.S.
- the cytotoxic agent is conjugated to the antibody using a valine-citrulline (vc) linker.
- vc valine-citrulline
- the antibody-drug conjugates (ADCs) provided herein can be made using a variety of bifunctional protein coupling agents such as BMPS, EMCS, GMBS, HBVS, LC- SMCC, MBS, MPBH, SBAP, SIA, SIAB, SMCC, SMPB, SMPH, sulfo-EMCS, sulfo- GMBS, sulfo-KMUS, sulfo-MBS, sulfoSIAB, sulfo-SMCC, and sulfo-SMPB, and SVSB (succinimidyl-(4-vinylsulfone )benzoate )).
- bifunctional protein coupling agents such as BMPS, EMCS, GMBS, HBVS, LC- SMCC, MBS, MPBH, SBAP, SIA, SIAB, SMCC, SMPB, SMPH, sulfo-EMCS, sulfo- GMBS
- a ricin immunotoxin can be prepared as described in Vitetta et ah, Science , 1987, 238: 1098.
- the ADCs can be prepared using any suitable methods as disclosed in the art, e.g, in Bioconjugate
- the ADCs are made with site-specific conjugation techniques, resulting in homogeneous drug loading and avoiding ADC subpopulations with altered antigen-binding or pharmacokinetics.
- "thiomabs" comprising cysteine substitutions at positions on the heavy and light chains are engineered to provide reactive thiol groups that do not disrupt immunoglobulin folding and assembly or alter antigen binding (Junutula et al., J. Immunol. Meth ., 2008, 332: 41-52; Junutula et al., Nat. Biotechnol. , 2008, 26: 925-932, ).
- selenocysteine is co-translationally inserted into an antibody sequence by recoding the stop codon UGA from termination to selenocysteine insertion, allowing site specific covalent conjugation at the nucleophilic selenol group of selenocysteine in the presence of the other natural amino acids ( See e.g. , Hofer et al., Proc. Natl. Acad. Sci. USA, 2008, 105: 12451-12456; Hofer et al., Biochemistry, 2009, 48(50): 12047-12057).
- ADCs were synthesized as described in Behrens et al , Mol Pharm, 2015, 12:3986-98.
- a variety of assays known in the art may be used to identify and characterize anti- TF antibodies and anti-TF ADCs provided herein.
- antigen-binding activity of the antibodies provided herein may be evaluated by any suitable method, including using SPR, BLI, RIA and MSD-SET, as described elsewhere in this disclosure. Additionally, antigen-binding activity may be evaluated by ELISA assays and Western blot assays.
- the epitope is determined by peptide competition.
- the epitope is determined by mass spectrometry. In some embodiments, the epitope is determined by crystallography.
- Thrombin generation in the presence of the antibodies provided herein can be determined by the Thrombin Generation Assay (TGA), as described elsewhere in this disclosure.
- Inhibition of TF signaling can be determined by measuring the production of a cytokine regulated by the TF signaling, such as IL8 and GM-CSF. Assays for determining the IL8 and/or GM-CSF level are provided elsewhere in this disclosure and, for example, in Hjortoe et al., Blood , 2004, 103:3029-3037.
- Effector function following treatment with the antibodies provided herein may be evaluated using a variety of in vitro and in vivo assays known in the art, including those described in Ravetch and Kinet, Annu. Rev. Immunol ., 1991, 9:457-492; U.S. Pat. Nos.
- ADCs antibody-drug conjugates
- Immunohistochemistry (IHC) assays for evaluating the TF expression in patient samples are described elsewhere in this disclosure.
- the antibodies or ADCs provided herein can be formulated in any appropriate pharmaceutical composition and administered by any suitable route of administration.
- Suitable routes of administration include, but are not limited to, the intravitreal, intraarterial, intradermal, intramuscular, intraperitoneal, intravenous, nasal, parenteral, pulmonary, and subcutaneous routes.
- the pharmaceutical composition may comprise one or more pharmaceutical excipients. Any suitable pharmaceutical excipient may be used, and one of ordinary skill in the art is capable of selecting suitable pharmaceutical excipients. Accordingly, the
- pharmaceutical excipients provided below are intended to be illustrative, and not limiting. Additional pharmaceutical excipients include, for example, those described in the Handbook of Pharmaceutical Excipients , Rowe et al. (Eds.) 6th Ed. (2009), incorporated by reference in its entirety.
- parenteral dosage forms can be administered to subjects by various routes including, but not limited to, subcutaneous, intravenous (including infusions and bolus injections), intramuscular, and intraarterial. Because their administration typically bypasses subjects’ natural defenses against contaminants, parenteral dosage forms are typically, sterile or capable of being sterilized prior to administration to a subject. Examples of parenteral dosage forms include, but are not limited to, solutions ready for injection, dry ( e.g ., lyophilized) products ready to be dissolved or suspended in a pharmaceutically acceptable vehicle for injection, suspensions ready for injection, and emulsions.
- the doctor will determine the posology which he considers most appropriate according to a preventive or curative treatment and according to the age, weight, condition and other factors specific to the subject to be treated.
- compositions provided herein is a pharmaceutical composition or a single unit dosage form.
- Pharmaceutical compositions and single unit dosage forms provided herein comprise a prophylactically or therapeutically effective amount of one or more prophylactic or therapeutic antibodies or ADCs.
- the amount of the antibody/ ADC or composition which will be effective in the prevention or treatment of a disorder or one or more symptoms thereof can vary with the nature and severity of the disease or condition, and the route by which the antibody/ ADC is administered.
- the frequency and dosage can also vary according to factors specific for each subject depending on the specific therapy (e.g ., therapeutic or prophylactic agents) administered, the severity of the disorder, disease, or condition, the route of administration, as well as age, body, weight, response, and the past medical history of the subject.
- Effective doses may be extrapolated from dose-response curves derived from in vitro or animal model test systems.
- amounts sufficient to prevent, manage, treat or ameliorate such disorders, but insufficient to cause, or sufficient to reduce, adverse effects associated with the antibodies or ADCs provided herein are also encompassed by the dosage amounts and dose frequency schedules provided herein.
- the dosage administered to the subject may be increased to improve the prophylactic or therapeutic effect of the composition or it may be decreased to reduce one or more side effects that a particular subject is experiencing.
- an antibody or ADC provided herein may optionally be administered with one or more additional agents useful to prevent or treat a disease or disorder.
- the effective amount of such additional agents may depend on the amount of ADC present in the formulation, the type of disorder or treatment, and the other factors known in the art or described herein.
- the antibodies or ADCs of the invention are administered to a mammal, generally a human, in a pharmaceutically acceptable dosage form such as those known in the art and those discussed above.
- the antibodies or ADCs of the invention may be administered to a human intravenously as a bolus or by continuous infusion over a period of time, by intravitreal, intramuscular, intraperitoneal, intra-cerebrospinal, subcutaneous, intra-articular, intrasynovial, intrathecal, or intratumoral routes.
- the antibodies or ADCs also are suitably administered by peritumoral, intralesional, or perilesional routes, to exert local as well as systemic therapeutic effects.
- intraperitoneal route may be particularly useful, for example, in the treatment of ovarian tumors.
- the antibodies or ADCs provided herein may be useful for the treatment of any disease or condition involving TF.
- the disease or condition is a disease or condition that can benefit from treatment with an anti-TF antibody or ADC.
- the antibodies or ADCs provided herein are provided for use as a medicament. In some embodiments, the antibodies or ADCs provided herein are provided for use in the manufacture or preparation of a medicament. In some embodiments, the medicament is for the treatment of a disease or condition that can benefit from an anti-TF antibody or ADC.
- provided herein is a method of treating a disease or condition in a subject in need thereof by administering an effective amount of an anti-TF antibody or ADC provided herein to the subject.
- the disease or condition that can benefit from treatment with an anti-TF antibody or ADC is cancer.
- the anti-TF antibodies or ADCs provided herein are provided for use as a medicament for the treatment of cancer.
- the anti-TF antibodies or ADCs provided herein are provided for use in the manufacture or preparation of a medicament for the treatment of cancer.
- provided herein is a method of treating cancer in a subject in need thereof by administering an effective amount of an anti-TF antibody or ADC provided herein to the subject.
- TF is involved in thrombosis, metastasis, tumor growth, and/or tumor
- angiogenesis of various types of cancers such as ovarian cancer ( See Sakurai et al., hit .1 Gynecol Cancer , 2017, 27:37-43; Koizume et al., Biomark Cancer , 2015, 7: 1-13; each of which is incorporated by reference in its entirety), cervical cancer ( See Cocco et al., BMC Cancer , 2011, 11 :263, incorporated by reference in its entirety), head and neck cancer ( See Christensen et al., BMC Cancer , 2017, 17:572, incorporated by reference in its entirety), prostate cancer ⁇ See Yao et al., Cancer Invest., 2009, 27:430-434; Abdulkadir et al., Hum Pathol ., 2009, 31 :443-447; each of which is incorporated by reference in its entirety), pancreatic cancer ⁇ See Zhang et al., Oncotarget, 2017, 8:59086-59102, incorporated by reference in its entirety), triple negative breast cancer ⁇ See Zhang et
- bladder cancer ⁇ See Patry et al., Int J Cancer., 2008, 122:1592-1597, incorporated by reference in its entirety
- melanoma See Bromberg et al., Proc Natl Acad Sci U SA., 1995, 92:8205-8209, incorporated by reference in its entirety
- kidney cancer See Silva et al., IntBraz J Urol., 2014, 40:499-506, incorporated by reference in its entirety).
- the cancer is head and neck cancer. In some embodiments, the cancer is ovarian cancer. In some embodiments, the cancer is gastric cancer. In some embodiments, the cancer is esophageal cancer. In some embodiments, the cancer is cervical cancer. In some embodiments, the cancer is prostate cancer. In some embodiments, the cancer is pancreatic cancer. In some embodiments, the cancer is estrogen receptors negative (ER-), progesterone receptors negative (PR-), and HER2 negative (HER2-) triple negative breast cancer. In some embodiments, the cancer is glioblastoma. In some embodiments, the cancer is lung cancer. In some embodiments, the cancer is bladder cancer.
- the cancer is melanoma. In some embodiments, the cancer is kidney cancer. Additional information on the types of cancers that can be treated with anti-TF antibodies or ADCs is provided in van den Berg et al., Blood, 2012, 119:924-932, which is incorporated by reference in its entirety.
- provided herein is a method of delaying the onset of a cancer in a subject in need thereof by administering an effective amount of an antibody or ADC provided herein to the subject.
- provided herein is a method of preventing the onset of a cancer in a subject in need thereof by administering an effective amount of an antibody or ADC provided herein to the subject.
- provided herein is a method of reducing the size of a tumor in a subject in need thereof by administering an effective amount of an antibody or ADC provided herein to the subject.
- provided herein is a method of reducing the number of metastases in a subject in need thereof by administering an effective amount of an antibody or ADC provided herein to the subject.
- provided herein is a method for treating a subject who has become resistant to a standard of care therapeutic by administering an effective amount of an antibody or ADC provided herein to the subject.
- the disease or condition that can benefit from treatment with an anti-TF antibody is a disease or condition involving neovascularization.
- the disease or condition involving neovascularization is age-related macular degeneration (AMD).
- AMD age-related macular degeneration
- the disease or condition involving neovascularization is age-related macular degeneration (AMD).
- neovascularization is diabetic retinopathy.
- the disease or condition involving neovascularization is cancer.
- the disease or condition that can benefit from treatment with an anti-TF antibody is a disease or condition involving vascular inflammation.
- the anti-TF antibodies provided herein are provided for use as a medicament for the treatment of a disease or condition involving neovascularization. In some embodiments, the anti-TF antibodies provided herein are provided for use in the manufacture or preparation of a medicament for the treatment of a disease or condition involving neovascularization. In certain embodiments, the disease or condition involving neovascularization is age-related macular degeneration (AMD). In certain embodiments, the disease or condition involving neovascularization is diabetic retinopathy. In certain embodiments, the disease or condition involving neovascularization is cancer.
- AMD age-related macular degeneration
- the disease or condition involving neovascularization is diabetic retinopathy. In certain embodiments, the disease or condition involving neovascularization is cancer.
- the anti-TF antibodies provided herein are provided for use as a medicament for the treatment of a disease or condition involving vascular inflammation. In some embodiments, the anti-TF antibodies provided herein are provided for use in the manufacture or preparation of a medicament for the treatment of a disease or condition involving vascular inflammation.
- provided herein is a method of treating a disease or condition involving neovascularization in a subject in need thereof by administering an effective amount of an anti-TF antibody provided herein to the subject.
- the disease or condition involving neovascularization is age-related macular degeneration (AMD). In certain embodiments, the disease or condition involving AMD.
- AMD age-related macular degeneration
- neovascularization is diabetic retinopathy.
- the disease or condition involving neovascularization is cancer.
- provided herein is a method of treating a disease or condition involving vascular inflammation in a subject in need thereof by administering an effective amount of an anti-TF antibody provided herein to the subject.
- provided herein is a method of delaying the onset of a disease or condition involving neovascularization in a subject in need thereof by
- provided herein is a method of preventing the onset of a disease or condition involving neovascularization in a subject in need thereof by
- provided herein is a method of delaying the onset of age-related macular degeneration (AMD) in a subject in need thereof by administering an effective amount of an antibody provided herein to the subject.
- AMD age-related macular degeneration
- provided herein is a method of preventing the onset of age-related macular degeneration (AMD) in a subject in need thereof by administering an effective amount of an antibody provided herein to the subject.
- AMD age-related macular degeneration
- provided herein is a method of delaying the onset of diabetic retinopathy in a subject in need thereof by administering an effective amount of an antibody provided herein to the subject.
- provided herein is a method of preventing the onset of diabetic retinopathy in a subject in need thereof by administering an effective amount of an antibody provided herein to the subject.
- provided herein is a method of delaying the onset of a disease or condition involving vascular inflammation in a subject in need thereof by administering an effective amount of an antibody provided herein to the subject.
- provided herein is a method of preventing the onset of a disease or condition involving vascular inflammation in a subject in need thereof by administering an effective amount of an antibody provided herein to the subject.
- an antibody or ADC provided herein is administered with at least one additional therapeutic agent.
- Any suitable additional therapeutic agent may be administered with an antibody or ADC provided herein.
- the additional therapeutic agent is selected from radiation, a cytotoxic agent, a chemotherapeutic agent, a cytostatic agent, an anti-hormonal agent, an immunostimulatory agent, an anti-angiogenic agent, and combinations thereof.
- the additional therapeutic agent may be administered by any suitable means.
- an antibody or ADC provided herein and the additional therapeutic agent are included in the same pharmaceutical composition.
- an antibody or ADC provided herein and the additional therapeutic agent are included in different pharmaceutical compositions.
- administering can occur prior to, simultaneously, and/or following, administration of the additional therapeutic agent.
- the method can be used to detect TF in a subject having or suspected of having a disease or condition.
- the methods comprise (a) receiving a sample from the subject; and (b) detecting the presence or the level of TF in the sample by contacting the sample with the antibody provided herein.
- the methods comprise (a) administering to the subject the antibody provided herein; and (b) detecting the presence or the level of TF in the subject.
- the disease or condition is a cancer.
- the cancer is head and neck cancer.
- the cancer is ovarian cancer.
- the cancer is gastric cancer.
- the cancer is esophageal cancer.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Epidemiology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Zoology (AREA)
- Biophysics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Wood Science & Technology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Microbiology (AREA)
- Urology & Nephrology (AREA)
- General Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Ophthalmology & Optometry (AREA)
- General Physics & Mathematics (AREA)
- Oncology (AREA)
- Hospice & Palliative Care (AREA)
- Food Science & Technology (AREA)
- Analytical Chemistry (AREA)
- Pathology (AREA)
- Plant Pathology (AREA)
Abstract
Description
Claims
Priority Applications (19)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA3087537A CA3087537A1 (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| CN201980016636.6A CN111818943B (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| EA202091631A EA202091631A1 (en) | 2018-08-02 | 2019-01-04 | ANTIBODIES TO TISSUE FACTOR, ANTIBODY-DRUG CONJUGATES AND RELATED METHODS |
| KR1020247029236A KR20240134250A (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| EP19735874.0A EP3735271A4 (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| AU2019205330A AU2019205330A1 (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| MX2020007077A MX2020007077A (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods. |
| SG11202006400UA SG11202006400UA (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| BR112020013679-4A BR112020013679A2 (en) | 2018-01-04 | 2019-01-04 | antitecid factor antibodies, antibody-drug conjugates and related methods |
| KR1020207022435A KR20200118029A (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| JP2020557125A JP2021509823A (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibody, antibody drug conjugate, and related methods |
| IL315325A IL315325A (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| US16/959,652 US20220153871A1 (en) | 2018-01-04 | 2019-01-04 | Anti-Tissue Factor Antibodies, Antibody-Drug Conjugates, and Related Methods |
| IL275765A IL275765A (en) | 2018-01-04 | 2020-06-30 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| PH12020551037A PH12020551037A1 (en) | 2018-01-04 | 2020-07-02 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| US17/458,507 US11447566B2 (en) | 2018-01-04 | 2021-08-26 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| US17/877,853 US20230159657A1 (en) | 2018-01-04 | 2022-07-29 | Anti-Tissue Factor Antibodies, Antibody-Drug Conjugates, and Related Methods |
| JP2023110621A JP2023134565A (en) | 2018-01-04 | 2023-07-05 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| JP2023142243A JP2023175726A (en) | 2018-01-04 | 2023-09-01 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
Applications Claiming Priority (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201862613564P | 2018-01-04 | 2018-01-04 | |
| US201862613545P | 2018-01-04 | 2018-01-04 | |
| US62/613,564 | 2018-01-04 | ||
| US62/613,545 | 2018-01-04 | ||
| US201862646788P | 2018-03-22 | 2018-03-22 | |
| US62/646,788 | 2018-03-22 | ||
| US201862713797P | 2018-08-02 | 2018-08-02 | |
| US201862713804P | 2018-08-02 | 2018-08-02 | |
| US62/713,804 | 2018-08-02 | ||
| US62/713,797 | 2018-08-02 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/959,652 A-371-Of-International US20220153871A1 (en) | 2018-01-04 | 2019-01-04 | Anti-Tissue Factor Antibodies, Antibody-Drug Conjugates, and Related Methods |
| US17/458,507 Continuation US11447566B2 (en) | 2018-01-04 | 2021-08-26 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019136309A1 true WO2019136309A1 (en) | 2019-07-11 |
Family
ID=67143785
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2019/012427 WO2019136309A1 (en) | 2018-01-04 | 2019-01-04 | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
Country Status (13)
| Country | Link |
|---|---|
| US (3) | US20220153871A1 (en) |
| EP (1) | EP3735271A4 (en) |
| JP (3) | JP2021509823A (en) |
| KR (2) | KR20240134250A (en) |
| CN (1) | CN111818943B (en) |
| AU (1) | AU2019205330A1 (en) |
| BR (1) | BR112020013679A2 (en) |
| CA (1) | CA3087537A1 (en) |
| IL (2) | IL315325A (en) |
| MX (2) | MX2020007077A (en) |
| PH (1) | PH12020551037A1 (en) |
| SG (1) | SG11202006400UA (en) |
| WO (1) | WO2019136309A1 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021003399A1 (en) * | 2019-07-03 | 2021-01-07 | Iconic Therapeutics, Inc. | Anti-tissue factor antibody-drug conjugates and related methods |
| WO2021188736A1 (en) * | 2020-03-17 | 2021-09-23 | Systimmune, Inc. | MINIATURE GUIDANCE AND NAVIGATION CONTROL (miniGNC) ANTIBODY-LIKE PROTEINS AND METHODS OF MAKING AND USING THEREOF |
| US20210308208A1 (en) * | 2018-08-16 | 2021-10-07 | Genmab A/S | Anti-tissue factor antibody-drug conjugates and their use in the treatment of cancer |
| US11447566B2 (en) | 2018-01-04 | 2022-09-20 | Iconic Therapeutics, Inc. | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| CN116322713A (en) * | 2020-07-10 | 2023-06-23 | 伊科尼克治疗公司 | Treatment of inflammatory diseases using anti-tissue factor antibodies |
| WO2024054821A3 (en) * | 2022-09-07 | 2024-04-11 | Exelixis, Inc. | Tissue factor antibody-drug conjugates and uses thereof |
| EP4130036A4 (en) * | 2020-03-30 | 2024-05-15 | National Cancer Center | Antibody drug conjugate |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023172951A1 (en) * | 2022-03-09 | 2023-09-14 | Exelixis, Inc. | Methods of treating solid tumors with anti-tissue factor antibody-drug conjugates |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5622931A (en) * | 1987-03-31 | 1997-04-22 | The Scripps Research Institute | Human tissue factor related DNA segments, polypeptides and antibodies |
| US20090081200A1 (en) * | 2003-04-22 | 2009-03-26 | Purdue Pharma L.P. | Tissue Factor Antibodies and Uses Thereof |
| US7605235B2 (en) * | 2003-05-30 | 2009-10-20 | Centocor, Inc. | Anti-tissue factor antibodies and compositions |
| US20140234304A1 (en) * | 2011-03-15 | 2014-08-21 | Janssen Biotech, Inc. | Human tissue factor antibody and uses thereof |
Family Cites Families (156)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4235871A (en) | 1978-02-24 | 1980-11-25 | Papahadjopoulos Demetrios P | Method of encapsulating biologically active materials in lipid vesicles |
| US4722848A (en) | 1982-12-08 | 1988-02-02 | Health Research, Incorporated | Method for immunizing animals with synthetically modified vaccinia virus |
| US4501728A (en) | 1983-01-06 | 1985-02-26 | Technology Unlimited, Inc. | Masking of liposomes from RES recognition |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| GB8311018D0 (en) | 1983-04-22 | 1983-05-25 | Amersham Int Plc | Detecting mutations in dna |
| US6090406A (en) | 1984-04-12 | 2000-07-18 | The Liposome Company, Inc. | Potentiation of immune responses with liposomal adjuvants |
| US5019369A (en) | 1984-10-22 | 1991-05-28 | Vestar, Inc. | Method of targeting tumors in humans |
| US4676980A (en) | 1985-09-23 | 1987-06-30 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Target specific cross-linked heteroantibodies |
| US4946778A (en) | 1987-09-21 | 1990-08-07 | Genex Corporation | Single polypeptide chain binding molecules |
| US5567610A (en) | 1986-09-04 | 1996-10-22 | Bioinvent International Ab | Method of producing human monoclonal antibodies and kit therefor |
| US4837028A (en) | 1986-12-24 | 1989-06-06 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5132405A (en) | 1987-05-21 | 1992-07-21 | Creative Biomolecules, Inc. | Biosynthetic antibody binding sites |
| GB8823869D0 (en) | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| US5175384A (en) | 1988-12-05 | 1992-12-29 | Genpharm International | Transgenic mice depleted in mature t-cells and methods for making transgenic mice |
| US5217879A (en) | 1989-01-12 | 1993-06-08 | Washington University | Infectious Sindbis virus vectors |
| US5703055A (en) | 1989-03-21 | 1997-12-30 | Wisconsin Alumni Research Foundation | Generation of antibodies through lipid mediated DNA delivery |
| FR2650840B1 (en) | 1989-08-11 | 1991-11-29 | Bertin & Cie | RAPID DETECTION AND / OR IDENTIFICATION OF A SINGLE BASED ON A NUCLEIC ACID SEQUENCE, AND ITS APPLICATIONS |
| US5208020A (en) | 1989-10-25 | 1993-05-04 | Immunogen Inc. | Cytotoxic agents comprising maytansinoids and their therapeutic use |
| EP0800830A3 (en) | 1989-11-03 | 1999-03-17 | Vanderbilt University | Method of in vivo delivery of functioning foreign genes |
| IL97459A0 (en) | 1990-03-09 | 1992-06-21 | Hybritech Inc | Trifunctional antibody-like compounds as a combined diagnostic and therapeutic agent |
| US5279833A (en) | 1990-04-04 | 1994-01-18 | Yale University | Liposomal transfection of nucleic acids into animal cells |
| US5229275A (en) | 1990-04-26 | 1993-07-20 | Akzo N.V. | In-vitro method for producing antigen-specific human monoclonal antibodies |
| US5204253A (en) | 1990-05-29 | 1993-04-20 | E. I. Du Pont De Nemours And Company | Method and apparatus for introducing biological substances into living cells |
| US6004744A (en) | 1991-03-05 | 1999-12-21 | Molecular Tool, Inc. | Method for determining nucleotide identity through extension of immobilized primer |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| US6037135A (en) | 1992-08-07 | 2000-03-14 | Epimmune Inc. | Methods for making HLA binding peptides and their uses |
| WO1993016177A1 (en) | 1992-02-11 | 1993-08-19 | Cell Genesys, Inc. | Homogenotization of gene-targeting events |
| US5573905A (en) | 1992-03-30 | 1996-11-12 | The Scripps Research Institute | Encoded combinatorial chemical libraries |
| EP0646178A1 (en) | 1992-06-04 | 1995-04-05 | The Regents Of The University Of California | expression cassette with regularoty regions functional in the mammmlian host |
| US9340577B2 (en) | 1992-08-07 | 2016-05-17 | Epimmune Inc. | HLA binding motifs and peptides and their uses |
| US5662907A (en) | 1992-08-07 | 1997-09-02 | Cytel Corporation | Induction of anti-tumor cytotoxic T lymphocytes in humans using synthetic peptide epitopes |
| WO1994005328A1 (en) * | 1992-08-28 | 1994-03-17 | The Scripps Research Institute | Inhibition of tumor metastasis via neutralization of tissue factor function |
| US6413935B1 (en) | 1993-09-14 | 2002-07-02 | Epimmune Inc. | Induction of immune response against desired determinants |
| AU698962B2 (en) | 1993-09-14 | 1998-11-12 | Epimmune, Inc. | Alteration of immune response using pan DR-binding peptides |
| US6015686A (en) | 1993-09-15 | 2000-01-18 | Chiron Viagene, Inc. | Eukaryotic layered vector initiation systems |
| JP3952312B2 (en) | 1993-11-09 | 2007-08-01 | メディカル カレッジ オブ オハイオ | A stable cell line capable of expressing adeno-associated virus replication genes |
| FR2726285B1 (en) | 1994-10-28 | 1996-11-29 | Centre Nat Rech Scient | ADENOVIRUSES CONTAINING VIABLE CONTAMINANT PARTICLES, PREPARATION AND USE |
| DE69534166T2 (en) | 1994-10-28 | 2006-03-09 | Trustees Of The University Of Pennsylvania | RECOMBINANT ADENOVIRUS AND METHODS OF USE THEREOF |
| US6071890A (en) | 1994-12-09 | 2000-06-06 | Genzyme Corporation | Organ-specific targeting of cationic amphiphile/DNA complexes for gene therapy |
| US5849589A (en) | 1996-03-11 | 1998-12-15 | Duke University | Culturing monocytes with IL-4, TNF-α and GM-CSF TO induce differentiation to dendric cells |
| CA2259179C (en) | 1996-07-03 | 2008-09-23 | University Of Pittsburgh | Emulsion formulations for hydrophilic active agents |
| AU4255397A (en) | 1996-09-06 | 1998-03-26 | Trustees Of The University Of Pennsylvania, The | Chimpanzee adenovirus vectors |
| US7732129B1 (en) | 1998-12-01 | 2010-06-08 | Crucell Holland B.V. | Method for the production and purification of adenoviral vectors |
| US5849561A (en) | 1997-05-22 | 1998-12-15 | Cornell Research Foundation, Inc. | Method for the production of non-group C adenoviral vectors |
| US6194551B1 (en) | 1998-04-02 | 2001-02-27 | Genentech, Inc. | Polypeptide variants |
| ATE375365T1 (en) | 1998-04-02 | 2007-10-15 | Genentech Inc | ANTIBODIES VARIANTS AND FRAGMENTS THEREOF |
| PT1069185E (en) * | 1998-04-03 | 2011-08-11 | Chugai Pharmaceutical Co Ltd | Humanized antibody against human tissue factor (tf) and process for constructing humanized antibody |
| US20030072767A1 (en) | 1998-09-30 | 2003-04-17 | Alexander Gaiger | Compositions and methods for WT1 specific immunotherapy |
| US20080050393A1 (en) | 1998-12-03 | 2008-02-28 | Tang Y Tom | Novel nucleic acids and polypeptides |
| EP1137759A2 (en) | 1998-12-07 | 2001-10-04 | U.S. Medical Research Institute of Infectious Diseases | Live attenuated venezuelan equine encephalitis vaccine |
| US6737056B1 (en) | 1999-01-15 | 2004-05-18 | Genentech, Inc. | Polypeptide variants with altered effector function |
| AU4077100A (en) | 1999-04-08 | 2000-11-14 | Chiron Corporation | Enhancement of the immune response for vaccine and gene therapy applications |
| EP1175497B1 (en) | 1999-04-14 | 2010-04-07 | Novartis Vaccines and Diagnostics, Inc. | Compositions and methods for generating an immune response utilizing alphavirus-based vector systems |
| US8647864B2 (en) | 1999-04-14 | 2014-02-11 | Novartis Ag | Compositions and methods for generating an immune response utilizing alphavirus-based vector systems |
| US6365394B1 (en) | 1999-09-29 | 2002-04-02 | The Trustees Of The University Of Pennsylvania | Cell lines and constructs useful in production of E1-deleted adenoviruses in absence of replication competent adenovirus |
| US6703494B2 (en) * | 2000-03-16 | 2004-03-09 | Genentech, Inc. | Anti-tissue factor antibodies with enhanced anticoagulant potency |
| WO2001073027A2 (en) | 2000-03-24 | 2001-10-04 | Corixa Corporation | Compositions and methods for therapy and diagnosis of colon cancer |
| US6436703B1 (en) | 2000-03-31 | 2002-08-20 | Hyseq, Inc. | Nucleic acids and polypeptides |
| US20040115625A1 (en) | 2000-10-02 | 2004-06-17 | Reinhard Ebner | Cancer gene determination and therapeutic screening using signature gene sets |
| US6783939B2 (en) | 2000-07-07 | 2004-08-31 | Alphavax, Inc. | Alphavirus vectors and virosomes with modified HIV genes for use in vaccines |
| US7820441B2 (en) | 2000-09-25 | 2010-10-26 | The Regents Of The University Of Michigan | Production of viral vectors |
| ATE498718T1 (en) | 2000-12-18 | 2011-03-15 | Dyax Corp | DIRECTED LIBRARIES THAT ARE GENETICALLY PACKAGED |
| US20020137081A1 (en) | 2001-01-08 | 2002-09-26 | Olga Bandman | Genes differentially expressed in vascular tissue activation |
| WO2002061113A2 (en) | 2001-02-01 | 2002-08-08 | The Johns Hopkins University | Nucleic acid derived vaccine that encodes an antigen linked to a polypeptide that promotes antigen presentation |
| US20030232324A1 (en) | 2001-05-31 | 2003-12-18 | Chiron Corporation | Chimeric alphavirus replicon particles |
| ES2345876T3 (en) | 2001-05-31 | 2010-10-05 | Novartis Vaccines And Diagnostics, Inc. | PARTICLES OF CHEMICAL ALFAVIRUS REPLICATIONS. |
| EP1409748B1 (en) | 2001-06-22 | 2011-10-26 | The Trustees of The University of Pennsylvania | Recombinant Adenoviruses comprising simian adenovirus proteins and uses thereof. |
| PL213948B1 (en) | 2001-10-25 | 2013-05-31 | Genentech Inc | Glycoprotein compositions |
| KR20050000380A (en) | 2002-04-09 | 2005-01-03 | 교와 핫꼬 고교 가부시끼가이샤 | Cells with modified genome |
| AU2003271738C1 (en) | 2002-04-25 | 2008-04-17 | Crucell Holland B.V. | Stable adenoviral vectors and methods for propagation thereof |
| PT1497438E (en) | 2002-04-25 | 2010-02-04 | Crucell Holland Bv | Means and methods for the production of adenovirus vectors |
| WO2004023973A2 (en) | 2002-09-12 | 2004-03-25 | Incyte Corporation | Molecules for diagnostics and therapeutics |
| CA2500687A1 (en) | 2002-10-02 | 2004-04-15 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| EP3263596A1 (en) | 2002-12-16 | 2018-01-03 | Genentech, Inc. | Immunoglobulin variants and uses thereof |
| GB2398300A (en) | 2003-02-17 | 2004-08-18 | Isis Innovation | Method and compositions for boosting immune response |
| PT1629004E (en) | 2003-06-05 | 2008-11-14 | Wyeth Corp | Immunogenic compositions comprising venezuelan equine encephalitis virus replicon vectors and paramyxovirus protein antigens |
| US7291498B2 (en) | 2003-06-20 | 2007-11-06 | The Trustees Of The University Of Pennsylvania | Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses |
| DE10347710B4 (en) | 2003-10-14 | 2006-03-30 | Johannes-Gutenberg-Universität Mainz | Recombinant vaccines and their use |
| ES2337374T3 (en) | 2004-01-23 | 2010-04-23 | Istituto Di Richerche Di Biologia Molecolare P. Angeletti S.P.A. | CHINPANCE ADENOVIRUS VACCINE CARRIERS. |
| US8119336B2 (en) | 2004-03-03 | 2012-02-21 | Ibis Biosciences, Inc. | Compositions for use in identification of alphaviruses |
| US20050221414A1 (en) | 2004-03-31 | 2005-10-06 | Katalin Varadi | Kit for measuring the thrombin generation in a sample of a patient's blood or plasma |
| PT1751290E (en) | 2004-05-25 | 2015-02-03 | Novartis Vaccines & Diagnostic | Alphavirus replicon packaging constructs |
| US20060198854A1 (en) | 2004-12-28 | 2006-09-07 | Peter Pushko | Vector platforms derived from the alphavirus vaccines |
| CA2609142C (en) | 2005-05-27 | 2016-02-09 | Fondazione Centro San Raffaele Del Monte Tabor | Therapeutic gene vectors comprising mirna target sequences |
| ES2735531T3 (en) | 2005-08-23 | 2019-12-19 | Univ Pennsylvania | RNA containing modified nucleosides and methods of use thereof |
| EP2357000A1 (en) | 2005-10-18 | 2011-08-17 | Novartis Vaccines and Diagnostics, Inc. | Mucosal and systemic immunizations with alphavirus replicon particles |
| US20100028358A1 (en) * | 2005-11-07 | 2010-02-04 | Wolfram Ruf | Compositions and Methods for Controlling Tissue Factor Signaling Specificity |
| JP5538729B2 (en) | 2006-02-27 | 2014-07-02 | ザ ボード オブ リージェンツ オブ ザ ユニバーシティー オブ テキサス システム | Mock infectious flaviviruses and their use |
| US9085638B2 (en) | 2007-03-07 | 2015-07-21 | The Johns Hopkins University | DNA vaccine enhancement with MHC class II activators |
| GB0706914D0 (en) | 2007-04-10 | 2007-05-16 | Isis Innovation | Novel adenovirus vectors |
| AU2008262490B2 (en) | 2007-05-22 | 2011-11-17 | Amgen Inc. | Compositions and methods for producing bioactive fusion proteins |
| US8969542B2 (en) | 2007-05-31 | 2015-03-03 | Genimmune N.V. | HPV polyepitope constructs and uses thereof |
| US20100286070A1 (en) | 2007-09-14 | 2010-11-11 | Gert Verheyden | Affinity tag |
| MX2010002661A (en) | 2007-09-14 | 2010-05-20 | Adimab Inc | Rationally designed, synthetic antibody libraries and uses therefor. |
| US8877688B2 (en) | 2007-09-14 | 2014-11-04 | Adimab, Llc | Rationally designed, synthetic antibody libraries and uses therefor |
| GB0719526D0 (en) | 2007-10-05 | 2007-11-14 | Isis Innovation | Compositions and methods |
| US20100285050A1 (en) | 2007-10-05 | 2010-11-11 | Isis Innovation Limited | Compositions and Methods |
| JP5627464B2 (en) | 2007-11-26 | 2014-11-19 | ノバルティス アーゲー | How to generate alphavirus particles |
| WO2009089004A1 (en) | 2008-01-07 | 2009-07-16 | Amgen Inc. | Method for making antibody fc-heterodimeric molecules using electrostatic steering effects |
| US20090253184A1 (en) | 2008-01-23 | 2009-10-08 | Introgen Therapeutics, Inc. | Compositions and methods related to an adenoviral trans-complementing cell line |
| WO2010005704A2 (en) | 2008-06-13 | 2010-01-14 | New York University | Novel helper plasmid, defective sindbis viral vectors and methods of use thereof |
| EP3992296A1 (en) | 2008-07-17 | 2022-05-04 | Medigen, Inc. | Idna vaccines and methods for using the same |
| CN107574156A (en) | 2008-11-26 | 2018-01-12 | 美国国有健康与人类服务部 | Virus like particle compositions and its application method |
| UA109633C2 (en) | 2008-12-09 | 2015-09-25 | HUMAN ANTIBODY AGAINST TISSUE FACTOR | |
| DE102008061522A1 (en) | 2008-12-10 | 2010-06-17 | Biontech Ag | Use of Flt3 ligand to enhance immune responses in RNA immunization |
| EP2516460B1 (en) | 2009-12-22 | 2015-03-04 | F.Hoffmann-La Roche Ag | Sequence dependent aggregation |
| WO2011082388A2 (en) | 2009-12-31 | 2011-07-07 | Medigen, Inc . | Infectious dna vaccines against chikungunya virus |
| GB201006405D0 (en) | 2010-04-16 | 2010-06-02 | Isis Innovation | Poxvirus expression system |
| CN105648056A (en) | 2010-05-14 | 2016-06-08 | 综合医院公司 | Composite and method for detecting tumor specific novel antigen |
| PL2582728T3 (en) | 2010-06-15 | 2018-01-31 | Genmab As | Human antibody drug conjugates against tissue factor |
| US9192661B2 (en) | 2010-07-06 | 2015-11-24 | Novartis Ag | Delivery of self-replicating RNA using biodegradable polymer particles |
| PL3243526T3 (en) | 2010-07-06 | 2020-05-18 | Glaxosmithkline Biologicals S.A. | Delivery of rna to trigger multiple immune pathways |
| MX343410B (en) | 2010-07-06 | 2016-11-04 | Novartis Ag * | Cationic oil-in-water emulsions. |
| WO2012006378A1 (en) | 2010-07-06 | 2012-01-12 | Novartis Ag | Liposomes with lipids having an advantageous pka- value for rna delivery |
| EP4005592B1 (en) | 2010-07-06 | 2022-10-12 | GlaxoSmithKline Biologicals S.A. | Virion-like delivery particles for self-replicating rna molecules |
| US9770463B2 (en) | 2010-07-06 | 2017-09-26 | Glaxosmithkline Biologicals Sa | Delivery of RNA to different cell types |
| CA2804492A1 (en) | 2010-07-06 | 2012-01-12 | Novartis Ag | Immunisation of large mammals with low doses of rna |
| KR102024922B1 (en) | 2010-07-16 | 2019-09-25 | 아디맵 엘엘씨 | Antibody libraries |
| LT4008357T (en) | 2010-08-31 | 2023-01-25 | Glaxosmithkline Biologicals Sa | Small liposomes for delivery of immunogen-encoding rna |
| SI2611461T1 (en) | 2010-08-31 | 2022-08-31 | Glaxosmithkline Biologicals Sa | Pegylated liposomes for delivery of immunogen-encoding rna |
| RU2577983C2 (en) | 2010-08-31 | 2016-03-20 | Новартис Аг | Lipids suitable for liposomal delivery of rna encoding protein |
| WO2012106356A2 (en) | 2011-01-31 | 2012-08-09 | GOVERNMENT OF THE USA, as represented by THE SECRETARY, DEPARTMENT OF HEALTH & HUMAN SERVICES | Virus-like particles and methods of use |
| RS56748B1 (en) | 2011-05-13 | 2018-03-30 | Glaxosmithkline Biologicals Sa | Pre-fusion rsv f antigens |
| HRP20230443T1 (en) | 2011-05-24 | 2023-09-15 | BioNTech SE | Individualized vaccines for cancer |
| GB201108879D0 (en) | 2011-05-25 | 2011-07-06 | Isis Innovation | Vector |
| WO2012171541A1 (en) | 2011-06-15 | 2012-12-20 | Scil Proteins Gmbh | Human fusion proteins comprising interferons and hetero-dimeric modified ubiquitin proteins |
| CA2840989A1 (en) | 2011-07-06 | 2013-01-10 | Novartis Ag | Immunogenic combination compositions and uses thereof |
| CA2841047A1 (en) | 2011-07-06 | 2013-01-10 | Novartis Ag | Immunogenic compositions and uses thereof |
| US9636410B2 (en) | 2011-07-06 | 2017-05-02 | Glaxosmithkline Biologicals Sa | Cationic oil-in-water emulsions |
| US20140141070A1 (en) | 2011-07-06 | 2014-05-22 | Andrew Geall | Liposomes having useful n:p ratio for delivery of rna molecules |
| EP2559441B1 (en) | 2011-08-16 | 2021-07-21 | Samsung Electronics Co., Ltd. | Protein complex for intracellular delivery and uses thereof |
| US20140255472A1 (en) | 2011-08-31 | 2014-09-11 | Andrew Geall | Pegylated liposomes for delivery of immunogen-encoding rna |
| US20140271829A1 (en) | 2011-10-11 | 2014-09-18 | Anders Lilja | Recombinant self-replicating polycistronic rna molecules |
| NZ628206A (en) | 2012-02-16 | 2016-03-31 | Vlp Therapeutics Llc | Virus like particle composition |
| US20160289674A1 (en) | 2012-04-02 | 2016-10-06 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of membrane proteins |
| US9283287B2 (en) | 2012-04-02 | 2016-03-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of nuclear proteins |
| US9217159B2 (en) | 2012-05-18 | 2015-12-22 | The Trustees Of The University Of Pennsylvania | Subfamily E simian adenoviruses A1302, A1320, A1331 and A1337 and uses thereof |
| WO2014006146A1 (en) | 2012-07-04 | 2014-01-09 | Sirion Biotech Gmbh | Means and methods to increase adenovirus production |
| EP2869842A1 (en) | 2012-07-06 | 2015-05-13 | Novartis AG | Immunogenic compositions and uses thereof |
| WO2014058163A1 (en) | 2012-10-11 | 2014-04-17 | (주) 엘지화학 | Alkyl acrylate-vinyl aromatic compound-vinyl cyanide compound copolymer having improved low temperature impact strength, and polycarbonate composition comprising same |
| GB201220119D0 (en) | 2012-11-08 | 2012-12-26 | Univ Cork | Vector |
| US10156574B2 (en) | 2013-04-29 | 2018-12-18 | Adimab, Llc | Polyspecificity reagents, methods for their preparation and use |
| EP3004348B1 (en) | 2013-06-03 | 2023-03-08 | VLP Therapeutics, Inc. | Malaria vaccine |
| KR102006527B1 (en) | 2013-11-01 | 2019-08-02 | 화이자 인코포레이티드 | Vectors for expression of prostate-associated antigens |
| GB201319446D0 (en) | 2013-11-04 | 2013-12-18 | Immatics Biotechnologies Gmbh | Personalized immunotherapy against several neuronal and brain tumors |
| WO2015075201A1 (en) | 2013-11-21 | 2015-05-28 | Genmab A/S | Antibody-drug conjugate lyophilised formulation |
| EP3103811A4 (en) * | 2014-02-03 | 2017-11-29 | National Cancer Center | Anti-tissue factor monoclonal antibody |
| SG11201702143PA (en) | 2014-09-17 | 2017-04-27 | Zymeworks Inc | Cytotoxic and anti-mitotic compounds, and methods of using the same |
| EP3253795B1 (en) | 2015-02-06 | 2019-04-17 | Navigo Proteins Gmbh | Novel binding proteins comprising a ubiquitin mutein and antibodies or antibody fragments |
| PL3280441T3 (en) | 2015-04-07 | 2022-02-21 | Alector Llc | Anti-sortilin antibodies and methods of use thereof |
| HUE055109T2 (en) | 2015-09-11 | 2021-11-29 | Genmab As | Dosing regimens for anti-tf-antibody drug-conjugates |
| TWI765875B (en) | 2015-12-16 | 2022-06-01 | 美商磨石生物公司 | Neoantigen identification, manufacture, and use |
| US20210113673A1 (en) | 2017-04-19 | 2021-04-22 | Gritstone Oncology, Inc. | Neoantigen Identification, Manufacture, and Use |
| JP7437301B2 (en) | 2017-08-25 | 2024-02-22 | ファイヴ プライム セラピューティクス インク | B7-H4 antibody and its usage |
| AU2018371271A1 (en) | 2017-11-27 | 2020-06-11 | Purdue Pharma L.P. | Humanized antibodies targeting human tissue factor |
| EP3735271A4 (en) | 2018-01-04 | 2022-06-15 | Iconic Therapeutics, Inc. | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
-
2019
- 2019-01-04 EP EP19735874.0A patent/EP3735271A4/en active Pending
- 2019-01-04 JP JP2020557125A patent/JP2021509823A/en active Pending
- 2019-01-04 CN CN201980016636.6A patent/CN111818943B/en active Active
- 2019-01-04 MX MX2020007077A patent/MX2020007077A/en unknown
- 2019-01-04 CA CA3087537A patent/CA3087537A1/en active Pending
- 2019-01-04 AU AU2019205330A patent/AU2019205330A1/en active Pending
- 2019-01-04 US US16/959,652 patent/US20220153871A1/en not_active Abandoned
- 2019-01-04 KR KR1020247029236A patent/KR20240134250A/en active Search and Examination
- 2019-01-04 BR BR112020013679-4A patent/BR112020013679A2/en unknown
- 2019-01-04 WO PCT/US2019/012427 patent/WO2019136309A1/en unknown
- 2019-01-04 KR KR1020207022435A patent/KR20200118029A/en not_active Application Discontinuation
- 2019-01-04 SG SG11202006400UA patent/SG11202006400UA/en unknown
- 2019-01-04 IL IL315325A patent/IL315325A/en unknown
-
2020
- 2020-06-30 IL IL275765A patent/IL275765A/en unknown
- 2020-07-02 PH PH12020551037A patent/PH12020551037A1/en unknown
- 2020-07-13 MX MX2024010630A patent/MX2024010630A/en unknown
-
2021
- 2021-08-26 US US17/458,507 patent/US11447566B2/en active Active
-
2022
- 2022-07-29 US US17/877,853 patent/US20230159657A1/en active Pending
-
2023
- 2023-07-05 JP JP2023110621A patent/JP2023134565A/en active Pending
- 2023-09-01 JP JP2023142243A patent/JP2023175726A/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5622931A (en) * | 1987-03-31 | 1997-04-22 | The Scripps Research Institute | Human tissue factor related DNA segments, polypeptides and antibodies |
| US20090081200A1 (en) * | 2003-04-22 | 2009-03-26 | Purdue Pharma L.P. | Tissue Factor Antibodies and Uses Thereof |
| US7605235B2 (en) * | 2003-05-30 | 2009-10-20 | Centocor, Inc. | Anti-tissue factor antibodies and compositions |
| US20140234304A1 (en) * | 2011-03-15 | 2014-08-21 | Janssen Biotech, Inc. | Human tissue factor antibody and uses thereof |
Non-Patent Citations (2)
| Title |
|---|
| TEPLYAKOV ET AL.: "Crystal structure of tissue factor in complex with antibody 10H10 reveals the signaling epitope", CELLULAR SIGNALING, vol. 36, 31 August 2017 (2017-08-31), pages 139 - 144, XP085043691, doi:10.1016/j.cellsig.2017.05.004 * |
| TRIPISCIANO ET AL.: "Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin", SCIENTIFIC REPORTS, vol. 7, 26 July 2017 (2017-07-26), XP055622219 * |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11447566B2 (en) | 2018-01-04 | 2022-09-20 | Iconic Therapeutics, Inc. | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods |
| US20210308208A1 (en) * | 2018-08-16 | 2021-10-07 | Genmab A/S | Anti-tissue factor antibody-drug conjugates and their use in the treatment of cancer |
| WO2021003399A1 (en) * | 2019-07-03 | 2021-01-07 | Iconic Therapeutics, Inc. | Anti-tissue factor antibody-drug conjugates and related methods |
| CN114222752A (en) * | 2019-07-03 | 2022-03-22 | 伊科尼克治疗公司 | Anti-tissue factor antibody-drug conjugates and related methods |
| WO2021188736A1 (en) * | 2020-03-17 | 2021-09-23 | Systimmune, Inc. | MINIATURE GUIDANCE AND NAVIGATION CONTROL (miniGNC) ANTIBODY-LIKE PROTEINS AND METHODS OF MAKING AND USING THEREOF |
| EP4130036A4 (en) * | 2020-03-30 | 2024-05-15 | National Cancer Center | Antibody drug conjugate |
| CN116322713A (en) * | 2020-07-10 | 2023-06-23 | 伊科尼克治疗公司 | Treatment of inflammatory diseases using anti-tissue factor antibodies |
| EP4178608A4 (en) * | 2020-07-10 | 2024-07-24 | Iconic Therapeutics Llc | Inflammatory disease treatment using anti-tissue factor antibodies |
| WO2024054821A3 (en) * | 2022-09-07 | 2024-04-11 | Exelixis, Inc. | Tissue factor antibody-drug conjugates and uses thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2023134565A (en) | 2023-09-27 |
| IL275765A (en) | 2020-08-31 |
| KR20240134250A (en) | 2024-09-06 |
| EP3735271A4 (en) | 2022-06-15 |
| CN111818943A (en) | 2020-10-23 |
| PH12020551037A1 (en) | 2021-09-06 |
| US20230159657A1 (en) | 2023-05-25 |
| MX2024010630A (en) | 2024-09-06 |
| BR112020013679A2 (en) | 2020-12-01 |
| JP2023175726A (en) | 2023-12-12 |
| EP3735271A1 (en) | 2020-11-11 |
| IL315325A (en) | 2024-10-01 |
| MX2020007077A (en) | 2020-10-28 |
| US11447566B2 (en) | 2022-09-20 |
| JP2021509823A (en) | 2021-04-08 |
| US20220153871A1 (en) | 2022-05-19 |
| KR20200118029A (en) | 2020-10-14 |
| AU2019205330A1 (en) | 2020-08-27 |
| CN111818943B (en) | 2024-09-03 |
| US20220056151A1 (en) | 2022-02-24 |
| CA3087537A1 (en) | 2019-07-11 |
| SG11202006400UA (en) | 2020-08-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11447566B2 (en) | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods | |
| US20210403558A1 (en) | Anti-tim-3 antibodies, compositions comprising anti-tim-3 antibodies and methods of making and using anti-tim-3 antibodies | |
| US11186644B2 (en) | Anti-neuropilin antigen-binding proteins and methods of use thereof | |
| CN111448210A (en) | anti-SIRP- α antibodies and related methods | |
| US20220257789A1 (en) | Anti-tissue factor antibody-drug conjugates and related methods | |
| WO2015007727A1 (en) | Antibodies that bind urokinase plasminogen activator | |
| US20220106401A1 (en) | ANTI-EpCAM ANTIBODIES, COMPOSITIONS COMPRISING ANTI-EpCAM ANTIBODIES AND METHODS OF MAKING AND USING ANTI-EpCAM ANTIBODIES | |
| US20240166765A1 (en) | Inflammatory disease treatment using anti-tissue factor antibodies | |
| WO2022011323A1 (en) | Ocular disease treatment using anti-tissue factor antibodies | |
| CN118955721A (en) | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods | |
| CN118955720A (en) | Anti-tissue factor antibodies, antibody-drug conjugates, and related methods | |
| WO2023137399A2 (en) | Inflammatory disease treatment using anti-tissue factor antibodies |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19735874 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3087537 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2020557125 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2019735874 Country of ref document: EP Effective date: 20200804 |
|
| ENP | Entry into the national phase |
Ref document number: 2019205330 Country of ref document: AU Date of ref document: 20190104 Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112020013679 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112020013679 Country of ref document: BR Kind code of ref document: A2 Effective date: 20200703 |