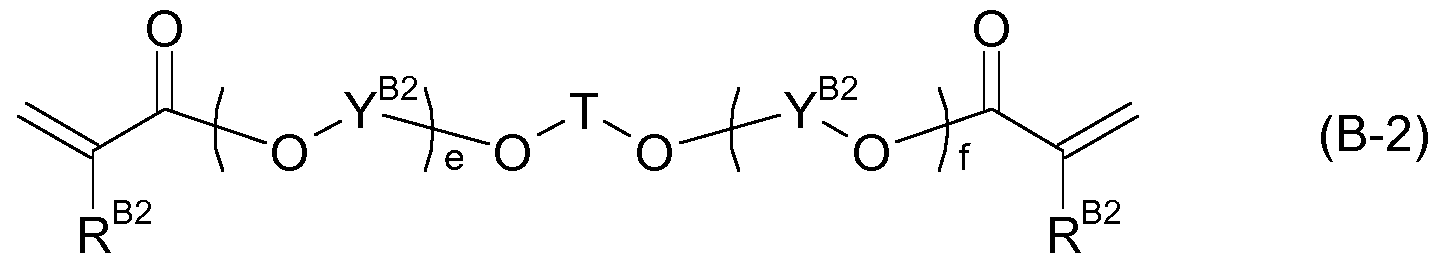WO2018146259A1 - Acrylate-based monomers for use as reactive diluents in printing formulations - Google Patents
Acrylate-based monomers for use as reactive diluents in printing formulations Download PDFInfo
- Publication number
- WO2018146259A1 WO2018146259A1 PCT/EP2018/053304 EP2018053304W WO2018146259A1 WO 2018146259 A1 WO2018146259 A1 WO 2018146259A1 EP 2018053304 W EP2018053304 W EP 2018053304W WO 2018146259 A1 WO2018146259 A1 WO 2018146259A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- component
- meth
- acrylate
- alkyl
- Prior art date
Links
- GOXNUYXRIQJIEF-UHFFFAOYSA-N OCCN(CCO1)C1=O Chemical compound OCCN(CCO1)C1=O GOXNUYXRIQJIEF-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/02—Printing inks
- C09D11/10—Printing inks based on artificial resins
- C09D11/101—Inks specially adapted for printing processes involving curing by wave energy or particle radiation, e.g. with UV-curing following the printing
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41J—TYPEWRITERS; SELECTIVE PRINTING MECHANISMS, i.e. MECHANISMS PRINTING OTHERWISE THAN FROM A FORME; CORRECTION OF TYPOGRAPHICAL ERRORS
- B41J11/00—Devices or arrangements of selective printing mechanisms, e.g. ink-jet printers or thermal printers, for supporting or handling copy material in sheet or web form
- B41J11/0015—Devices or arrangements of selective printing mechanisms, e.g. ink-jet printers or thermal printers, for supporting or handling copy material in sheet or web form for treating before, during or after printing or for uniform coating or laminating the copy material before or after printing
- B41J11/002—Curing or drying the ink on the copy materials, e.g. by heating or irradiating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M5/00—Duplicating or marking methods; Sheet materials for use therein
- B41M5/0023—Digital printing methods characterised by the inks used
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M5/00—Duplicating or marking methods; Sheet materials for use therein
- B41M5/0041—Digital printing on surfaces other than ordinary paper
- B41M5/0047—Digital printing on surfaces other than ordinary paper by ink-jet printing
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/34—Esters containing nitrogen, e.g. N,N-dimethylaminoethyl (meth)acrylate
- C08F220/36—Esters containing nitrogen, e.g. N,N-dimethylaminoethyl (meth)acrylate containing oxygen in addition to the carboxy oxygen, e.g. 2-N-morpholinoethyl (meth)acrylate or 2-isocyanatoethyl (meth)acrylate
- C08F220/365—Esters containing nitrogen, e.g. N,N-dimethylaminoethyl (meth)acrylate containing oxygen in addition to the carboxy oxygen, e.g. 2-N-morpholinoethyl (meth)acrylate or 2-isocyanatoethyl (meth)acrylate containing further carboxylic moieties
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/1006—Esters of polyhydric alcohols or polyhydric phenols
- C08F222/102—Esters of polyhydric alcohols or polyhydric phenols of dialcohols, e.g. ethylene glycol di(meth)acrylate or 1,4-butanediol dimethacrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/14—Homopolymers or copolymers of esters of esters containing halogen, nitrogen, sulfur, or oxygen atoms in addition to the carboxy oxygen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L67/00—Compositions of polyesters obtained by reactions forming a carboxylic ester link in the main chain; Compositions of derivatives of such polymers
- C08L67/06—Unsaturated polyesters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L75/00—Compositions of polyureas or polyurethanes; Compositions of derivatives of such polymers
- C08L75/04—Polyurethanes
- C08L75/14—Polyurethanes having carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/02—Printing inks
- C09D11/10—Printing inks based on artificial resins
- C09D11/106—Printing inks based on artificial resins containing macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C09D11/107—Printing inks based on artificial resins containing macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds from unsaturated acids or derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/30—Inkjet printing inks
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D4/00—Coating compositions, e.g. paints, varnishes or lacquers, based on organic non-macromolecular compounds having at least one polymerisable carbon-to-carbon unsaturated bond ; Coating compositions, based on monomers of macromolecular compounds of groups C09D183/00 - C09D183/16
Definitions
- the invention relates to compositions, comprising a particular (meth)acrylate monomer, as well as the use of these compositions as printing inks, preferably inkjet printing inks. Furthermore, the invention relates to a method for printing, preferably inkjet printing, which uses these compositions.
- Radiation curable compositions are commonly used as printing inks, in particular inkjet printing inks. Recently developed systems are disclosed in, e.g., GB 2517592 A, WO 2015/140538, WO 2015/140539, WO 2015/140540, WO 2015/140541 , WO 2015/148094, and WO 2015/022228. However, there is an ongoing need for curable compositions which combine low viscosity, high reactivity, and good adhesion on the huge manifold of plastic substrates.
- N-vinyl-pyrrolidone (NVP) and N-vinyl-caprolactam (NVC) are well-known reactive diluents.
- NVP N-vinyl-pyrrolidone
- NVC N-vinyl-caprolactam
- compositions comprising, and preferably consisting of,
- R 1 , R 2 , R 3 , R 4 are each independently H, Ci-C6-alkyl, Ci-C6-alkoxy, or Ci-C6-alkoxy-Ci-C6- alkyl;
- R 5 is H or d-Ce-alkyl
- X is CR 6 R 7 , O, or N R 8 ;
- R 6 , R 7 are each independently H, Ci-C6-alkyl, Ci-C6-alkoxy, or Ci-C6-alkoxy-Ci-C6-alkyl;
- R 8 is H, d-Ce-alkyl, or Ci-C 6 -alkoxy-Ci-C 6 -alkyl;
- k 1 , 2, 3, 4 or 5
- component A b) 1 .00 to 60.00% by weight of at least one monomer having two (meth)acrylate groups and having a molecular weight no more than 500 Dalton, as component B;
- component C 0 to 25% by weight of at least one monomer having at least three (meth)acrylate groups and having a molecular weight of no more than 600 Dalton, as component C;
- component D 1 .00 to 30.00% by weight of at least one polymer having at least two (meth)acrylate groups and having a molecular weight of at least 700 Dalton, as component D;
- component F 0 to 10.00% by weight of one or more colorants, as component F;
- g 0 to 2.00% by weight of one or more stabilizers, as component G;
- component H 0 to 50.00% by weight of one or more further monomers, as component H;
- component J 0 to 10.00% by weight of one or more further additives, as component J;
- the amount of components A) plus B) is at least 50% by weight, based on the sum of components A to J, and that in all cases the amounts of components A to J add up to 100% by weight.
- a particularly preferred compound of formula (I) is the compound of formula (la)
- PEA PEA
- compositions of the invention combine low viscosity, high reactivity, and good adhesion on the huge manifold of plastic substrates. Furthermore, the compositions of the invention do not require the presence of N-vinyl-pyrrolidone (NVP) and/or N-vinyl-caprolactam (NVC).
- NVP N-vinyl-pyrrolidone
- NVC N-vinyl-caprolactam
- the cured compositions have good mechanical and chemical resistance properties.
- PEA is an excellent monofunctional monomer acrylate with an outstanding performance profile hardly found for any commercially available monofunctional monomer acrylate in UV inkjet. It combines very low viscosity as pure substance as well as in UV inkjet ink formulations with very high cure speed and very good adhesion on various substrates, such as plastic films. This well- balanced performance package is only matched by NVC known to be under severe pressure on the market due to its toxicity problems.
- (meth)acrylate stands for "acrylate or methacrylate”. In one embodiment the (meth)acrylate is an acrylate. In another embodiment the (meth)acrylate is a methacrylate. Preferably, the (meth)acrylate is an acrylate.
- Ethylene refers to -CH 2 -CH 2 -.
- Propylene refers to -CH 2 -CH 2 -CH 2 -, -CH 2 -CH(CH 3 )-, or -CH(CH 3 )- CH2-.
- propylene refers to -CH2-CH(CH3)- or -CH(CH3)-CH2-.
- propylene refers to -CH2-CH2-CH2-.
- Butylene refers to linear or branched C4H8, preferably branched C4H8.
- Ethyleneoxy refers to -O-CH2-CH2-.
- Propyleneoxy refers to -O-CH2-CH2-CH2-,
- propyleneoxy refers to -0-CH2-CH(CH3)- or -0-CH(CH3)-CH2-. In another embodiment propyleneoxy refers to
- butyleneoxy refers to linear or branched OC4H8, preferably branched OC4H8.
- molecular weight refers to the weight average molecular weight M w given in Dalton (if not specified otherwise).
- composition of the invention comprises, as component A, at least one, preferably one to three, more preferably one or two, even more preferably one, compound of formula (I),
- R 1 , R 2 , R 3 , R 4 are each independently H, Ci-C6-alkyl, Ci-C6-alkoxy, or Ci-C6-alkoxy-Ci-C6- alkyl;
- R 5 is H or d-Ce-alkyl
- X is CR 6 R 7 , O, or NR 8 ;
- R 6 , R 7 are each independently H, Ci-C6-alkyl, Ci-C6-alkoxy, or Ci-C6-alkoxy-Ci-C6- alkyl;
- R 8 is H, Ci-Ce-alkyl, or Ci-C 6 -alkoxy-Ci-C 6 -alkyl;
- k is 1 , 2, 3, 4 or 5.
- R 1 , R 3 are each independently H, Ci-C4-alkyl, Ci-C4-alkoxy, or Ci-C2-alkoxy-Ci-C2-alkyl.
- R 2 , R 4 are each independently H, Ci-C4-alkyl, or Ci-C2-alkoxy-Ci-C2-alkyl.
- R 7 is H, Ci-C4-alkyl, or Ci-C2-alkoxy-Ci-C2- alkyl.
- R 1 , R 2 , R 3 , R 4 are each independently H or Ci-C4-alkyl.
- R 6 , R 7 are each independently H or Ci-C 4 -alkyl.
- R 1 , R 3 are each independently H, Ci-C4-alkyl, Ci-C4-alkoxy, or Ci-C2-alkoxy-Ci-C2- alkyl;
- R 2 , R 4 are each independently H, Ci-C4-alkyl, or Ci-C2-alkoxy-Ci-C2-alkyl;
- R 5 is H or Ci-C 4 -alkyl
- X is CR 6 R 7 , O, or NR 8 ;
- R 6 is H, Ci-C 4 -alkyl, Ci-C 4 -alkoxy, or Ci-C 2 -alkoxy-Ci-C 2 -alkyl;
- R 7 is H, Ci-C 4 -alkyl, or Ci-C 2 -alkoxy-Ci-C 2 -alkyl;
- R 8 is H or Ci-C 4 -alkyl
- k 1 , 2 or 3.
- R 1 , R 2 , R 3 , R 4 are each independently H or Ci-C 4 -alkyl
- R 5 is H or CH 3 ;
- X is CR 6 R 7 or O
- R 6 , R 7 are each independently H or Ci-C4-alkyl
- R , R 2 , R 3 , R 4 are H
- R 5 is H
- X is Chb or O
- a particularly preferred compound of formula (I) is PEA (la)
- Another particularly preferred compound of formula (I) is a compound of formula (lb):
- the compound of formula (lb) is herein referred to as heonon acrylate.
- the compounds of formula (I) can be prepared according to methods known in the art.
- the compounds of formula (I) can be prepared by reacting a compound of formula (II),
- R 1 , R 2 , R 3 , R 4 , X, and k are defined as in formula (I), with a compound of formula
- R 5 is defined as in formula (I), and
- R 9 is d-Ce-alkyl, preferably in the presence of a catalyst.
- Suitable catalysts for the reaction of the compound of formula (II) with the compound of formula (III) include Lewis acids, such as titanium tetraisopropoxide.
- the reaction of the compound of formula (II) with the compound of formula (III) can be carried out in the presence of further addi- tives, such as stabilizers and/or inhibitors.
- further additives for the reaction of the compound of formula (II) with the compound of formula (III) include methylhydroquinone and/or phenothiazine.
- composition of the invention comprises, as component B, at least one monomer having two (meth)acrylate groups and having a molecular weight of no more than 500 Dalton.
- the composition of the invention comprises, as component B, one to five, preferably one to four, more preferably one to three, also more preferably two to four, even more preferably two or three, particularly preferably two, also particularly preferably three monomers) having two (meth)acrylate groups and having a molecular weight of no more than 500 Dalton.
- Preferred monomers having two (meth)acrylate groups have a molecular weight of no more than 500 Dalton, more preferably no more than 400 Dalton, even more preferably no more than 350 Dalton.
- Preferred monomers having two (meth)acrylate groups have a molecular weight in the range of from 150 to 500 Dalton, more preferably from 150 to 400 Dalton, even more preferably from 150 to 350 Dalton.
- molecular weight refers to the weight average molecular weight M w .
- Preferred monomers having two (meth)acrylate groups have a dynamic viscosity at 23°C in the range of from 3 to 400 mPas, more preferably from 3 to 150 mPas, even more preferably from 3 to 50 mPas.
- a typical shear rate is 100 s _1 .
- a typical method for determining viscosities is given in the experimental part of this application. This method can be applied in all cases in the context of the invention where dynamic viscosities are determined.
- the at least one monomer having two (meth)acrylate groups of component B has a molecular weight in the range of 150 to 400 Dalton and a dynamic viscosity at 23°C in the range of from 3 to 150 mPas.
- Preferred monomers having two (meth)acrylate groups also have at least one group Y which is selected from -O-CH2-CH2-, -O-CH2-CH2-CH2-, -0-CH 2 -CH(CH 3 )-, and -O- CH(CH3)-CH2- and which is attached to at least one of the (meth)acrylate groups.
- Said group Y is attached via a carbon atom to an oxygen atom of said (meth)acrylate group.
- Preferred monomers having two (meth)acrylate groups are di(meth)acrylates of alkoxylated diols.
- the alkoxylated diol is selected from ethoxylated, propoxylated, and butoxylated diols. More preferably, the alkoxylated diol is selected from ethoxylated and propoxylated diols. Even more preferably, the alkoxylated diol is an ethoxylated diol. Also even more preferably, the alkoxylated diol is a propoxylated diol.
- Preferred di(meth)acrylates of alkoxylated diols have an average of 1 to 20 , more preferably 2 to 15, even more preferably 2 to 10 alkyleneoxy groups per molecule.
- the alkylene- oxy groups are selected from ethyleneoxy, propyleneoxy, and butyleneoxy groups. More preferably, the alkyleneoxy groups are selected from ethyleneoxy and propyleneoxy groups. Even more preferably, the alkyleneoxy groups are selected from -O-CH2-CH2-, -0-CH2-CH(CH3)-, and -0-CH(CH3)-CH2- groups. Particularly preferably, the alkyleneoxy groups are -O-CH2-CH2- groups. Also particularly preferably, the alkyleneoxy groups are selected from -0-CH2-CH(CH3)- and -0-CH(CH 3 )-CH 2 - groups.
- Preferred diols are ethylene glycol, diethylene glycol, triethylene glycol, propylene glycol, dipro- pylene glycol, tripropylene glycol, 1 ,3-propanediol, 1 ,4-butanediol, 1 ,5-pentanediol, neopentyl glycol, 1 ,6-hexanediol, 2,2-diethyl-1 ,3-propanediol and 3-methyl-1 ,5-pentanediol
- More preferred diols are neopentyl glycol, dipropylene glycol, tripropylene glycol and 3-methyl- 1 ,5-pentanediol.
- Preferred monomers having two (meth)acrylate groups are monomers of formula (B-1 ),
- each R B1 is independently H or CH3;
- each Y B1 is independently ethylene, propylene, or butylene;
- p is a number from 1 to 15.
- each R B1 is independently H or CH3;
- each Y B1 is independently ethylene or propylene
- p is a number from 1 .5 to 10.
- each R B1 is independently H or CH3;
- each Y B is independently -CH2-CH2-, -CH 2 -CH(CH 3 )-, or -CH(CH 3 )-CH 2 -;
- p is a number from 1 .8 to 2.4.
- each R B1 is H
- each Y B is independently -CH 2 -CH(CH 3 )- or -CH(CH 3 )-CH 2 -;
- a particularly preferred compound having two (meth)acrylate groups is dipropy- leneglycol diacrylate, which is commercially available as Laromer® DPGDA from BASF.
- the compounds of formula (B-1 ) can be prepared according to methods known in the art.
- the compounds of formula (B-1 ) can be prepared by reacting a diol of the formula HO(Y B1 0)pH with, e.g., (meth)acrylic acid or an alkyl (meth)acrylate, optionally in the presence of a catalyst.
- Further preferred monomers having two (meth)acrylate groups (component B) are monomers of formula (B-2),
- T is Ci-Cio-alkylene
- each R B2 is independently H or CH3;
- each Y B2 is independently ethylene, propylene, or butylene;
- e and f are numbers, with the proviso that e + f is a number from 1 to 10.
- T is Cs-Cs-alkylene
- each R B2 is independently H or CH3;
- each Y B2 is independently ethylene or propylene
- e and f are numbers, with the proviso that e + f is a number from 1 .5 to 5.
- T is C4-C6-alkylene
- each R B2 is independently H or CH3;
- each Y B2 is independently -CH2-CH2-, -CH 2 -CH(CH 3 )-, or -CH(CH 3 )-CH 2 -;
- e and f are numbers, with the proviso that e + f is a number from 1 .8 to 2.4.
- T is -CH 2 -C(CH 3 ) 2 -CH 2 -;
- each R B2 is H
- each Y B2 is independently -CH 2 -CH(CH 3 )- or -CH(CH 3 )-CH 2 -;
- a particularly preferred monomer having two (meth)acrylate groups is a propox- ylated neopentyl glycol diacrylate having an average of 2 propyleneoxy groups per molecule, i.e. propoxylated (2.0) neopentyl glycol diacrylate:
- component B is selected from the group consisting of mers of formula (B-1 ) and monomers of formula (B-2).
- the monomers of formula (B-2) can be prepared according to methods known in the art.
- the monomers of formula (B-2) can be prepared by reacting a diol of the formula 1-1(0 Y B2 )eOTO(Y B2 0)fH with, e.g., (meth)acrylic acid or an alkyl (meth)acrylate, optionally in the presence of a catalyst.
- composition of the invention comprises, as component C, at least one monomer having at least three (meth)acrylate groups and having a molecular weight of no more than 600 Dalton.
- the composition of the invention comprises, as component C, one to four, preferably one to three, more preferably one or two, even more preferably one, also even more preferably two monomer(s) having at least three (meth)acrylate groups and having a molecular weight of no more than 600 Dalton.
- Preferred monomers having at least three (meth)acrylate groups are monomers having three to eight (meth)acrylate groups. More preferred are monomers having three to six (meth)acrylate groups. Even more preferred are monomers having three or four (meth)acrylate groups. Particularly preferred are monomers having three (meth)acrylate groups. Also particu- larly preferred are monomers having four (meth)acrylate groups.
- Preferred monomers having at least three (meth)acrylate groups have a molecular weight of at most 600 g/mol, more preferably at most 550 Dalton, even more preferably at most 500 Dalton.
- Preferred monomers having at least three (meth)acrylate groups have a molecu- lar weight in the range of from 200 to 600 Dalton, more preferably from 200 to 550 Dalton, even more preferably from 200 to 500 Dalton.
- molecular weight refers to the weight average molecular weight M w .
- Preferred monomers having at least three (meth)acrylate groups have a dynam- ic viscosity at 23°C in the range of from 10 to 400 mPas, more preferably from 10 to 200 mPas, even more preferably from 10 to 100 mPas.
- the at least one monomer having at least three (meth)acry- late groups of component C has a molecular weight in the range of 200 to 550 Dalton and a dynamic viscosity at 23°C in the range of from 10 to 200 mPas.
- Preferred monomers having at least three (meth)acrylate groups also have at least one group Y which is selected from -O-CH2-CH2-, -O-CH2-CH2-CH2-, -0-CH 2 -CH(CH 3 )-, and -0-CH(CH3)-CH2- and which is attached to at least one of the (meth)acrylate groups.
- Said group Y is attached via a carbon atom to an oxygen atom of said (meth)acrylate group.
- Preferred monomers having at least three (meth)acrylate groups (component C) are (meth)acry- lates of alkoxylated polyhydric alcohols.
- the alkoxylated polyhydric alcohol is selected from ethoxylated, propoxylated, and butoxylated polyhydric alcohols. More preferably, the alkoxylated polyhydric alcohol is selected from ethoxylated and propoxylated polyhydric alcohols. Even more preferably, the alkoxylated polyhydric alcohol is an ethoxylated polyhydric alcohol. Also even more preferably, the alkoxylated polyhydric alcohol is a propoxylated polyhydric alcohol.
- Preferred (meth)acrylates of alkoxylated polyhydric alcohols have an average of 3 to 20, more preferably 3to 15, even more preferably 3 to 10 alkyleneoxy groups per molecule.
- the alkyleneoxy groups are selected from ethyleneoxy, propyleneoxy, and butyleneoxy groups. More preferably, the alkyleneoxy groups are selected from ethyleneoxy and propyleneoxy groups. Even more preferably, the alkyleneoxy groups are selected from -O-CH2-CH2-, -O-CH2- CH(CH3)-, and -0-CH(CH3)-CH2- groups. Particularly preferably, the alkyleneoxy groups are -O-CH2-CH2- groups. Also particularly preferably, the alkyleneoxy groups are selected from -0-CH 2 -CH(CH 3 )- and -0-CH(CH 3 )-CH 2 - groups.
- the polyhydric alcohol is selected from triols, tetraols, pentaols, and hexaols. More preferably, the polyhydric alcohol is selected from triols and tetraols. Even more preferably, the polyhydric alcohol is a triol. Also even more preferably, the polyhydric alcohol is a tetraol.
- triols are trimethylolmethane, trimethylolethane, trimethylolpropane, glycerol.
- triols are trimethylolpropane and glycerol.
- Preferred tetraols are pentaerythritol and di(trimethylolpropane)
- More preferred tetraols are pentaerythritol
- a particularly preferred tetraol is pentaerythritol.
- Preferred hexanols are dipentaerythritol
- the number of (meth)acrylate groups in the molecule corresponds to the number of hydroxy groups in the polyhydric alcohol which the molecule is based on.
- the number of (meth)acrylate groups preferably is three.
- the number of (meth)acrylate groups preferably is four.
- the polyhydric alcohol is a pentaol, the number of (meth)acrylate groups preferably is five.
- the polyhydric alcohol is a hexaol, the number of (meth)acrylate groups preferably is six.
- Preferred (meth)acrylates of alkoxylated polyhydric alcohols are selected from tri(meth)acrylates of alkoxylated triols, tetra(meth)acrylates of alkoxylated tetraols, penta(meth)acrylates of alkoxylated pentaols, and hexa(meth)acrylates of alkoxylated hexaols. More preferred (meth)acrylates of alkoxylated polyhydric alcohols are selected from tri(meth)acrylates of alkoxylated triols and tetra(meth)acrylates of alkoxylated tetraols. Even more preferred are tri(meth)acrylates of alkoxylated triols. Also even more preferred are tetra(meth)acrylates of alkoxylated tetraols.
- Preferred monomers having at least three (meth)acrylate groups are compounds of formula (C-1 ),
- each R C1 is independently H or CH3;
- each Y C1 is independently ethylene, propylene, or butylene;
- a, b, c, and d are numbers, with the proviso that a + b + c + d is a number from 1 to 15.
- Preferred are monomers of formula (C-1 ) wherein
- each R C1 is independently H or CH3;
- each Y C1 is independently ethylene or propylene
- a, b, c, and d are numbers, with the proviso that a + b + c + d is a number from 2 to 10. More preferred are monomers of formula (C-1 ) wherein
- each R C1 is independently H or CH3;
- each Y C is independently -CH2-CH2-, -CH 2 -CH(CH 3 )-, or -CH(CH 3 )-CH 2 -;
- a, b, c, and d are numbers, with the proviso that a + b + c + d is a number from 3 to 8. Even more preferred are monomers of formula (C-1 ) wherein
- each R C1 is H
- each Y C is -CH 2 -CH 2 -;
- a particularly preferred monomer having at least three (meth)acrylate groups (component C) is an ethoxylated pentaerythritol tetraacrylate having an average of 5 ethyleneoxy groups per molecule.
- an "ethoxylated pentaerythritol tetraacrylate having an average of 5 ethyleneoxy groups per molecule” is a tetraacrylate of ethoxylated pentaerythritol which has an average of 5 ethyleneoxy groups per molecule (ethoxylated (5.0) pentaerythrol tetraacrylate), which is commercial- ly available as Laromer® PPTTA from BASF.
- the compounds of formula (C-1 ) can be prepared according to methods known in the art.
- the compounds of formula (C-1 ) can be prepared by reacting the corresponding alkoxylated (e.g., ethoxylated, propoxylated, or butoxylated) pentaerythritol with, e.g.,
- composition of the invention comprises, as component D, at least one polymer having at least two (meth)acrylate groups and having a molecular weight of at least 700 Dalton.
- Preferred polymers (component D) have a molecular weight of at least 700 Dalton, more preferably at least 1000 Dalton, even more preferably at least 1500 Dalton.
- Preferred polymers have a molecular weight in the range of from 700 to 2000 Dalton. In cases where the molecular weight is distributed around an average value, the term "molecular weight” refers to the weight average molecular weight M w .
- Preferred polymers (component D) have a dynamic viscosity at 23°C in the range of from 100 to 5000 mPas, more preferably from 100 to 2500 mPas, even more preferably from 100 to 1000 mPas.
- the at least one polymer having at least two (meth)acrylate groups of component D has a molecular weight in the range of 1000 to 2000 Dalton and a dynamic viscosity at 23°C in the range of from 100 to 2500 mPas.
- Suitable polymers as component D show low to medium viscosity, good film forming properties and good adhesion on paper, plastics and other substrates. Such polymers are known to those skilled in the art and are commercially available.
- component D are: a) amine modified polyether acrylates, which are commercially available under various tradenames, such as
- Laromer® PO 94 F (BASF SE, viscosity at 23.0°C, 300-600 mPas)
- Laromer® PO 9103 (BASF SE, viscosity at 23.0°C, 2500-4000 mPas)
- Laromer® PO 9106 (BASF SE, viscosity at 23.0°C, 2500-3500 mPas)
- Laromer® LR 8997 (BASF SE, viscosity at 23.0°C, 300-500 mPas);
- polyether acrylates (not amine modified), which are commercially available under various tradenames such as SR415 (Sartomer, ethoxylated (20) trimethylolpropane triacrylate, viscosity at 25°C, 150-300 mPas), SR 9035 (Sartomer, ethoxylated (15) trimethylolpropane triacrylate, viscosity at 25°C, 100-240 mPas);
- polyesteracrylates which are available under various tradenames such as
- Laromer® PE 9105 (BASF SE, tetrafunctional polyester acrylate, viscosity at 23°C,
- Genomer® 3485 (Rahn AG, polyester acrylate, viscocity at 25°C, 500 mPas),
- CN 2305 (Sartomer, hyperbranched polyester acrylate, viscosity at 25°C, 250-400 mPas), CN 2505 (Sartomer, polyester acrylate, viscosity at 25°C, 400-1000 mPas);
- CN 925 (Sartomer, viscosity at 25°C, 2500 mPas),
- CN 9251 (Sartomer, viscosity at 20°C, 450 mPas).
- polymer (component D) also has amino groups.
- the polymer (component D) is an amine-modified polyether acrylate.
- Suitable amine-modified polyether acrylates are known to a person skilled in the art.
- the polymer (component D) is an amine-modified (meth)acry- late of an alkoxylated polyhydric alcohol.
- Suitable amine-modified (meth)acrylates of alkoxylated polyhydric alcohols are known to a person skilled in the art.
- the composition of the invention comprises, as component E, one or more, preferably one to five, more preferably one to four, even more preferably two to four photoinitiators.
- Suitable photoinitiators are known to a person skilled in the art. Examples of suitable photoinitiators include alpha-hydroxyketones, alpha-aminoketones, acyl- phosphine oxides, benzoin and benzoin derivatives, and benzil derivatives, acetophenone and acetophenone derivatives, benzophenone, and benzophenone derivatives, thioxanthone and thioxanthone derivatives.
- Examples of preferred photoinitiators include alpha-hydroxyketones and acylphosphine oxides.
- Examples of particularly preferred photoinitiators include 2-hydroxy-1 - ⁇ 4-[4-(2-hydroxy-2-methyl- propionyl)-benzyl]-phenyl ⁇ -2-methyl-propan-1 -one, bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide, or diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide.
- composition of the invention comprises, as photo- initiators, Irgacure® 127, Irgacure® 819, and/or Irgacure® TPO, which are commercially available from IGM Resins.
- the photoinitiator component E may comprise up to 50 % by weight (based on the total of component E) of one or more synergists.
- synergists are aliphatic tertiary amines like triethylamine, triethanolamine or N-methyldiethanolamine and aromatic amines like esters of 4-dimethylaminobenzoic acid.
- compositions of the invention that do not comprise a photoinitiator can be used, e.g., in electron beam curing process.
- Component F (Colorant)
- the composition of the invention comprises, as component F, one or more, preferably one to five, more preferably one to four, even more preferably one to three colorants.
- Suitable colorants are known to a person skilled in the art.
- Preferred colorants are pigments and dyes. More preferred colorants are pigments.
- Suitable dyes include azo dyes, anthraquinone dyes, xanthene dyes, or azine dyes.
- Suitable pigments include phthalocyanine pigments, quinacridone pigments, benzimidazolone pigments, carbon black and titanium dioxides.
- Examples of preferred pigments include phthalocyanine pigments, quinacridone pigments, benzimidazolone pigments and carbon black.
- composition of the invention comprises, as a colorant/pigment, Microlith® Blue 7080 J, Microlith® Magenta 4500 J, Microlith® Yellow 1061 J, or Microlith® Black 0066 J, which are commercially available.
- Microlith® Blue 7080 J is a pigment preparation which contains a phthalocyanine pigment (about 70% by weight) predispersed in an acrylic copolymer binder.
- Microlith® Magenta 4500 J is a pigment preparation which contains a quinacridone pigment (about 70% by weight) predispersed in an acrylic copolymer binder.
- Microlith® Yellow 1061 J is a pigment preparation which contains a benzimidazolone pigment (about 70% by weight) predispersed in an acrylic copolymer binder.
- Microlith® Black 0066 J is a pigment preparation which contains carbon black (about 65% by weight) predispersed in an acrylic copolymer binder.
- compositions of the invention that do not comprise a colorant can be used, e.g., as overprint varnishes.
- Component G Stabilizer
- the composition of the invention comprises, as component G, one or more, preferably one to five, more preferably one to four, even more preferably one to three in-can stabilizers.
- Suitable in-can stabilizers are known to a person skilled in the art.
- the stabilizers are in-can stabilizers.
- in-can stabilizer is meant a stabilizer that improves the long term storage stability.
- suitable stabilizers include nitroxyl compounds, such as 1 -oxyl-2,2,6,6-tetramethyl- piperidine or 4-hydroxy-1 -oxyl-2,2,6,6-tetramethylpiperidine, phenol derivatives, such as 2,6-di- tert-butyl-4-methylphenol, tocopherols, quinones, benzoquinones, quinone methide derivatives, such as 4-benzylidene-2,6-ditert-butyl-cyclohexa-2,5-dien-1 -one, hydroquinones, such as hy- droquinone monomethyl ether, N-oxyl compounds, aromatic amines, phenylenediamines, imines, sulfonamides, oximes, hydroxylamines, urea derivatives, phosphorus-containing compounds, such as triphenylphosphine, trip
- particularly preferred stabilizers are methylhydroquinone or phenothiazine.
- Another example of a particularly preferred stabilizer is 4-benzylidene-2,6-ditert-butyl-cyclohexa-2,5- dien-1 -one.
- composition of the invention comprises, as a stabilizer, Irgastab® UV 25, which is commercially available from BASF.
- Component H Frther monomers
- the composition of the invention comprises, as component H, one or more further monomers.
- the further monomers (component H) are different from components A to C. Suitable further monomers are known to a person skilled in the art.
- N-vinyl compounds such as
- N-vinyl-pyrrolidone N-vinyl-pyrrolidone
- NVC N-vinyl-caprolactam
- O-vinyl compounds such as
- ODVE octadecyl vinyl ether
- DVE-2 diethyleneglycol divinyl ether
- HBVE hydroxybutyl vinyl ether
- VEEA 2-(2-vinyloxyethoxy)ethyl acrylate
- VEEM 2-(2-vinyloxyethoxy)ethyl methacrylate
- Component J Frther additives
- composition of the invention comprises, as component J, one or more further additives.
- the further additives (component J) are different from components A to H.
- component J examples include dispersants, fillers, rheological aids, slip agents, leveling agents, substrate wetting agents, antifoaming agents, antistatic agents and antioxidants.
- component J Preferred as one class of further additives (component J) are dispersants.
- Suitable dispersants are known to those skilled in the art.
- Preferred as dispersants are high molecular weight modified polyacrylates, such as Efka® PA 4400 (BASF) and Efka® PX 4733 (BASF), and high molecular weight acrylic block copolymers, such as Efka® PX 4701 (BASF) and Efka® PX 4320.
- an organically modified polysiloxane is used as a further additive, for example as a slip, leveling, and/or substrate wetting agent.
- Efka® SL 3210 which is commercially available from BASF, is used as a further additive, for example as a slip, leveling, and/or substrate wetting agent.
- composition of the invention comprises, and preferably consists of, (all percentages are by weight): a) from 1 .00 %, preferably 3.00 %, more preferably 3.00 %, in particular 5.00 % to 65.00 %, preferably 50 %, more preferably 40.00 %, in particular 30.00 % of component A;
- component B from 1 .00 %, preferably 5.00 %, more preferably 10.00 %, in particular 20 % to 60 %, preferably 55 %, more preferably 40.00 %, in particular 35.00 % of component B;
- component J i) from 0.00 %, preferably 0.10 %, more preferably 0.20 %, in particular 0.25 % to 10 %, preferably 7.50 %, more preferably 5.00 %, in particular 3.00 % of component J, wherein in one embodiment, the amount of component J is 0.00 %, and wherein in another em- bodiment, the amount of component J is at least 0.10 %,
- the amount of components A + B is at least 50, and the amounts of components A to J add up to 100 %.
- composition comprises and preferably consists of a) from 3.00% by weight to 50% by weight of component A;
- component B from 5.00% by weight to 55% by weight of component B;
- component C from 0.50% by weight to 22.50% by weight of component C;
- component F from 0.10% by weight to 7.50% by weight of component F;
- component G from 0.01 % by weight to 1 .50% by weight of component G;
- component H from 0.00% by weight or from 1 .00% by weight to 40.00% by weight of component H; i) from 0.10% by weight to 7.50% by weight of component J; wherein in all cases, the amount of components A + B is at least 50% by weight, and the amounts of components A to J add up to 100% by weight.
- the composition of the invention preferably has a water content of less than 2.00% by weight, more preferred less than 0.50% by weight, even more preferred less than 0.10% by weight.
- a typical water content due to traces of water in the various components is from 0.10 to 0.40% by weight.
- composition of the invention preferably comprises less than 2.00% by weight, more preferred less than 0.50% by weight, even more preferred less than 0.10% by weight of one or more inert organic solvents.
- a typical content of inert organic solvents due to traces from the synthesis of the various components is from 0.10 to 0.04% by weight.
- the composition of the invention is free of N-vinyl-pyrrolidone (NVP), which means that the composition comprises less than 1.00% by weight, more preferably less than 0.50% by weight, even more preferably less than 0.10% by weight, particularly preferably less than 0.01 % by weight of N-vinyl-pyrrolidone (NVP), based on the total weight of the composition.
- NVP N-vinyl-pyrrolidone
- the composition of the invention is free of N-vinyl-caprolactam (NVC), which means that the composition comprises less than 1.00% by weight, more preferably less than 0.50% by weight, even more preferably less than 0.10% by weight, particularly preferably less than 0.01 % by weight of N-vinyl-caprolactam (NVC), based on the total weight of the composition.
- NVC N-vinyl-caprolactam
- composition of the invention is free of N-vinyl-pyrrolidone (NVP) and free of N-vinyl-caprolactam (NVC).
- NVP N-vinyl-pyrrolidone
- NVC N-vinyl-caprolactam
- composition of the invention is a printing ink.
- composition of the invention is an inkjet printing ink.
- the composition of the invention has a viscosity (dynamic viscosity, 25°C, shear rate
- composition of the invention can be prepared by methods known in the art.
- composition of the invention can be prepared by adding and mixing the components of the composition in any order.
- composition of the invention as a printing ink.
- the composition is used as an inkjet printing ink.
- a composition of the invention as an inkjet printing ink.
- a method for printing comprising the steps of: a) applying a composition of the invention onto a substrate;
- Preferred printing techniques are inkjet printing, flexographic printing (flexo printing, flexo- graphy), gravure printing, screen printing, lithographic printing (litho printing, lithography), offset printing, or letterpress printing.
- a particularly preferred printing technique is inkjet printing.
- inkjet printers can be used.
- suitable inkjet printers include single-pass and multi-pass inkjet printers.
- composition of the invention can be applied onto various substrates.
- Preferred substrates are paper, carton, cardboard, corrugated board, glass, plastic films, or metallized films. More preferred substrates are plastic films.
- plastic films are polyethylene terephthalate films, polyamide films, polystyrene films, polyvinylchloride films, polycarbonate films, or polyolefin (e.g., polyethylene or polypropylene) films.
- plastic films are polyethylene terephthalate films, polyamide films, polystyrene films, polyvinylchloride films, polycarbonate films, or polyolefin (e.g., polyethylene or polypropylene) films.
- more preferred plastic films are polyethylene terephthalate films, polystyrene films, polyvinylchloride films, polyethylene films, or polypropylene films.
- the substrates for example the plastic films, can be pretreated, for example, corona-pretreated.
- the composition of the invention can be cured by methods known in the art.
- the composition of the invention is cured by exposure to actinic radiation.
- the actinic radiation is preferably UV radiation and preferably has a wavelength in the range of from 200 to 500nm, more preferably from 250 to 450 nm.
- Suitable radiation sources include halogen lamps, medium pressure mercury lamps, low pressure mercury lamps, UV LEDs, excimer lamps, or lasers.
- a medium pressure mercury/gallium lamp is used to cure the composition of the invention.
- composition of the invention is cured by electron beam.
- the composition of the invention is cured at a temperature under air in the range of from 15 to 40°C, more preferably from 20 to 40°C, even more preferably from 20 to 35°C.
- composition of the invention can be cured in an inert atmosphere, such as a nitrogen atmosphere or a carbon dioxide atmosphere.
- VIMOX Monofunctional monomer vinyl amide N-vinyl-5-methyl-oxazolidinone from BASF SE
- NVC Monofunctional monomer vinyl amide N-vinyl-caprolactam from BASF SE
- ACMO Monofunctional monomer acryl amide
- Laromer ® DPGDA dipropylene glycol diacrylate from BASF SE (DPGDA); molecular weight: 242 g/mol.
- Laromer ® PO 9102 (propoxylated (2.0) neopentylglycol diacrylate) from BASF SE (PONPGDA).
- Laromer ® HDDA (1 ,6-hexanediol diacrylate) from BASF SE (HDDA).
- Laromer ® PPTTA ethoxylated (5.0) pentaerythritol tetraacrylate
- BASF SE BASF SE
- molecular weight 572 g/mol (calculated).
- - MeHQ monomethyl ether of hydroquinone or hydroquinone monomethyl ether, also known as 4-methoxyphenol or 4-hydroxyanisole.
- Laromer® PPTTA an ethoxylated pentaerythritol tetraacrylate having an average of 5 eth- yleneoxy groups per molecule.
- Irgastab® UV 25 4-benzylidene-2,6-ditert-butyl-cyclohexa-2,5-dien-1 -one (14% by weight) Laromer ® POEA.
- Irgacure® 127 2-hydroxy-1 - ⁇ 4-[4-(2-hydroxy-2-methyl-propionyl)-benzyl]-phenyl ⁇ -2-methyl- propan-1 -one.
- Irgacure® 819 bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide.
- Irgacure® TPO diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide.
- - PC Cyan a pigment concentrate which comprises Microlith® Blue 7080 J (12% by weight, based on the pigment concentrate), Laromer ® POEA (48% by weight, based on the pigment concentrate), and Laromer® PO 9102 (40% by weight, based on the pigment concentrate).
- - PC Black a pigment concentrate which comprises Microlith® Black 0066 J (14% by weight, based on the pigment concentrate), Laromer ® POEA (46% by weight, based on the pigment concentrate), and Laromer® PO 9102 (40% by weight, based on the pigment concentrate).
- Laromer® PO 9102 a propoxylated neopentyl glycol diacrylate having an average of 2 propyl- eneoxy groups per molecule.
- Bicor ® MB400 clear biaxially oriented polypropylene (boPP) film with a thickness of 30 ⁇ from Jindal Films
- Viscosity was measured at 23.0°C for different shear rates with ramping up the shear rate from
- Reactivity was determined radiometrically as energy density in mJ/cm 2 for 12 ⁇ drawdowns on Melinex ® 506.
- the corresponding drawdowns were prepared on the automatic coater and then immediately UV cured on the UV dryer by varying the conveyor belt speed and with that the energy density, until the UV ink film could not be damaged anymore by the thumb twist test. For this the thumb was twisted under pressure clockwise and subsequently anti-clockwise under pressure on the UV ink film surface, until no impression on the UV ink film could be observed anymore.
- the energy density at this point was defined as the reactivity.
- Acetone resistance was determined for 12 ⁇ drawdowns on Melinex ® 506 prepared on the automatic coater and then immediately UV cured on the UV dryer with an energy density 10% higher than that determined for the reactivity. After 24 h the number of double rubs was recorded for a cotton pad soaked with acetone causing no visible damage of the UV ink film surface anymore; the maximum number of double rubs applied was 100.
- ethyl acrylate (2550 g), MeHQ (1 .28 g), pheno- thiazine (128 mg) and hydroxy ethyl pyrrolidin-2-one (1 100 g) were added. The mixture was heated up while stirring and lean air was introduced. Ethyl acrylate was distilled off to remove water traces and fresh ethyl acrylate was added. Titanium tetra isopropoxylate (33.3 g) was added at a sump temperature of 67°C and further heated to 93°C with a vacuum of 800 mbar.
- IR (KBr) v (cm- 1 ) 2958, 1725, 1689, 1635, 1620, 1495, 1463, 1409, 1289, 1 187, 1 1 10, 1068, 988, 81 1 , 654, 570.
- Ethyl acrylate was dosed to the reactor in equal amount to the distillate. After 5.5 h 20 g of catalyst were added. The sump temperature raised to 104°C. Sump and distillate samples were taken regularly to monitor the course of the reaction. After 17 h GC shows a con- tent of 98.8% (area%) of heonon acrylate and 1 .2% of residual alcohol (ethyl acrylate taken out in the calculation). 150 ml. of water were added, the reaction mixture was filtered over a sand filled frit and concentrated in vacuo. The product was obtained after a clear filtration in 830 g yield and a purity of 96% (GC area%).
- IR (KBr) v (cm- 1 ) 3482, 2918, 1751 , 1635, 1619, 1485, 1438, 1410, 1363, 1272, 1 188, 1 1 19, 1054, 985, 914, 81 1 , 764, 697, 628, 460.
- the pigment concentrates were prepared by adding the solid, already pre-dispersed nanoscale Microlith ® J pigment preparations slowly to Laromer ® POEA and Laromer ® PO 9102 in a disper- sion vessel with continuous stirring followed then by high speed mixing with the dissolver at 3200 rpm for 30 minutes (all concentrations are given in weight percent).
- the resulting liquid pigment concentrates were used for the preparation of the corresponding UV inkjet inks without further characterization.
- Cyan 1 Cyan 2 Cyan 3 Cyan 4 Cyan 5 Cyan 6 Cyan 7 Component (comp.) (inv.) (comp.) (inv.) (comp.) (comp.) (comp.) (comp.)
- Laromer ® PE 1 1.00% 1 1.00% 1 1.00% 1 1.00% 1 1.00% 1 1.00% 1 1.00% 1 1.00% 9105
- UV Inkjet Black All colorless formulation compounds were gently mixed with continuous stirring in a dispersion vessel that was then heated to 50°C on a hotplate to accomplish a complete dissolution of the difficult to solubilize photoinitiators Irgacure ® 127 and Irgacure ® 819. Afterwards the pigment concentrate PC Black was added and the resulting UV inkjet inks were homogenized by mixing for 5 minutes at 600 rpm with the dissolver (all concentrations are given in weight percent).
- Laromer ® PE 1 1.00% 1 1.00% 1 1.00% 1 1.00% 1 1.00% 1 1.00% 1 1.00% 1 1.00% 9105
- the UV inkjet inks with PEA are more at the upper end of the typical UV inkjet viscosity window of 25 - 50 mPa-s at 23°C, but still showing nearly Newtonian flow behavior. These ink viscosity data run parallel to that of the pure monomers.
- PEA is a fast curing monomer being more reactive than both difunctional monomer acrylates HDDA and DPGDA.
- PEA is on the same high reactivity level as the other nitrogen containing heterocyclic monomers carrying each an unsaturated, polymerizable group.
- Adhesion and Acetone Resistance PEA is characterized by outstanding adhesion and acetone resistance properties and as least as good as the other monomer acrylates, vinyl amides and acryl amides; vinyl amides and acryl amides are known to show the best adhesion performance of all radiation curable monomers on plastic films difficult to adhere to.
- the UV inkjet inks with PEA are in the middle of the typical UV inkjet viscosity window of 25 - 50 mPa-s at 23°C and showing excellent Newtonian flow behavior. These ink viscosity data run parallel to that of the pure monomers. For gloss and colour strength there are no significant differences between the monomer acrylates, vinyl amides and acryl amides being evaluated.
- PEA is a fast curing monomer being more reactive than both difunctional monomer acrylates HDDA and DPGDA.
- PEA is on a similar high reactivity level as the other nitrogen containing heterocyclic monomers carrying each an unsaturated, polymerizable group.
- PEA Adhesion and Acetone Resistance
- PEA is characterized by outstanding adhesion and very good acetone resistance properties and outperforms the other monomer acrylates, vinyl amides and acryl amides in terms of adhesion with vinyl amides and acryl amides are known to show the best adhesion performance of all radiation curable monomers on plastic films difficult to adhere to.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Inks, Pencil-Leads, Or Crayons (AREA)
- Macromonomer-Based Addition Polymer (AREA)
Abstract
Description
Claims
Priority Applications (14)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| MX2019009563A MX2019009563A (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing formulations. |
| RU2019128254A RU2769444C2 (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing compositions |
| JP2019543247A JP7206204B2 (en) | 2017-02-10 | 2018-02-09 | Acrylate-containing monomers used as reactive diluents in printing formulations |
| US16/484,896 US10745576B2 (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing formulations |
| MYPI2019004420A MY193850A (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing formulations |
| SG11201906757PA SG11201906757PA (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing formulations |
| CA3053037A CA3053037A1 (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing formulations |
| ES18703337T ES2926235T3 (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive thinners in printing formulations |
| CN201880010929.9A CN110291162A (en) | 2017-02-10 | 2018-02-09 | Acrylate base monomer as the reactive diluent in printable formulation |
| EP18703337.8A EP3580287B1 (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing formulations |
| KR1020197026073A KR102575388B1 (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomer for use as a reactive diluent in printing formulations |
| AU2018219661A AU2018219661B2 (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing formulations |
| BR112019015727-1A BR112019015727B1 (en) | 2017-02-10 | 2018-02-09 | COMPOSITION, USE OF A COMPOSITION, AND METHOD FOR PRINTING |
| ZA2019/05835A ZA201905835B (en) | 2017-02-10 | 2019-09-04 | Acrylate-based monomers for use as reactive diluents in printing formulations |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP17155623 | 2017-02-10 | ||
| EP17155623.6 | 2017-02-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018146259A1 true WO2018146259A1 (en) | 2018-08-16 |
Family
ID=58016626
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2018/053304 WO2018146259A1 (en) | 2017-02-10 | 2018-02-09 | Acrylate-based monomers for use as reactive diluents in printing formulations |
Country Status (16)
| Country | Link |
|---|---|
| US (1) | US10745576B2 (en) |
| EP (1) | EP3580287B1 (en) |
| JP (1) | JP7206204B2 (en) |
| KR (1) | KR102575388B1 (en) |
| CN (1) | CN110291162A (en) |
| AU (1) | AU2018219661B2 (en) |
| BR (1) | BR112019015727B1 (en) |
| CA (1) | CA3053037A1 (en) |
| ES (1) | ES2926235T3 (en) |
| MX (1) | MX2019009563A (en) |
| MY (1) | MY193850A (en) |
| RU (1) | RU2769444C2 (en) |
| SG (1) | SG11201906757PA (en) |
| TW (1) | TWI815805B (en) |
| WO (1) | WO2018146259A1 (en) |
| ZA (1) | ZA201905835B (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019077364A1 (en) * | 2017-10-20 | 2019-04-25 | Fujifilm Speciality Ink Systems Limited | A printing ink |
| WO2020064523A1 (en) | 2018-09-24 | 2020-04-02 | Basf Se | Uv curable composition for use in 3d printing |
| WO2020179155A1 (en) * | 2019-03-06 | 2020-09-10 | 富士フイルム株式会社 | Ink-jet ink composition, method for recording image, and object with recorded image |
| WO2022055625A1 (en) * | 2020-09-10 | 2022-03-17 | Sun Chemical Corporation | Led energy curable ink compositions |
| EP4050073A1 (en) | 2021-02-26 | 2022-08-31 | Agfa Nv | Ink set and inkjet printing methods |
| EP4050072A1 (en) | 2021-02-26 | 2022-08-31 | Agfa Nv | Manufacturing methods of decorative surfaces |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MY193750A (en) * | 2017-02-10 | 2022-10-27 | Basf Se | Acrylate-based monomers for use as reactive diluents in printing formulations |
| EP3724174B1 (en) | 2017-12-15 | 2022-02-09 | Basf Se | Method for the production of glycerine carbonate methacrylate |
| JP7443704B2 (en) * | 2019-09-12 | 2024-03-06 | セイコーエプソン株式会社 | Radiation-curable inkjet composition and inkjet method |
| JP2021042322A (en) * | 2019-09-12 | 2021-03-18 | セイコーエプソン株式会社 | Radiation-curable inkjet composition and inkjet method |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0425441A2 (en) * | 1989-10-27 | 1991-05-02 | Ciba-Geigy Ag | Photosensitive mixture |
| US5629359A (en) | 1994-05-31 | 1997-05-13 | U C B S.A. | Radiation curable compositions |
| JP2010248310A (en) * | 2009-04-13 | 2010-11-04 | Toagosei Co Ltd | Composition for use in active energy ray-curable-type covering material, containing unsaturated compound with nitrogen-containing heterocycle |
| JP2011178863A (en) | 2010-02-26 | 2011-09-15 | Nippon Shokubai Co Ltd | Active energy ray-curable composition, curable resin composition and cured product |
| WO2015022228A1 (en) | 2013-08-12 | 2015-02-19 | Basf Se | Ink-jet printing ink comprising n-vinyloxazolidinone |
| GB2517592A (en) | 2013-08-05 | 2015-02-25 | Sericol Ltd | Method for designing inks |
| WO2015140539A1 (en) | 2014-03-17 | 2015-09-24 | Fujifilm Speciality Ink Systems Limited | Printing ink |
| WO2015140541A1 (en) | 2014-03-20 | 2015-09-24 | Fujifilm Speciality Ink Systems Limited | Printing ink |
| WO2015140540A1 (en) | 2014-03-20 | 2015-09-24 | Fujifilm Speciality Ink Systems Limited | Printing ink |
| WO2015140538A1 (en) | 2014-03-17 | 2015-09-24 | Fujifilm Speciality Ink Systems Limited | Printing ink |
| WO2015148094A1 (en) | 2014-03-28 | 2015-10-01 | Sun Chemical Corporation | Low migration radiation curable inks |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6916855B2 (en) * | 2000-11-22 | 2005-07-12 | Dsm Ip Assets B.V. | Radiation curable compositions |
| JP4204333B2 (en) | 2003-01-20 | 2009-01-07 | 株式会社日本触媒 | Active energy ray-curable composition and inkjet ink |
| JP4526056B2 (en) | 2003-08-26 | 2010-08-18 | 三菱レイヨン株式会社 | Active energy ray-curable composition |
| DE102004040419A1 (en) * | 2004-08-19 | 2006-02-23 | Basf Ag | Water soluble radiation curable products and their use |
| EP1803784B3 (en) | 2005-12-28 | 2018-02-14 | Fujifilm Corporation | Inkjet recording composition, inkjet recording method, method for producing planographic printing plate, and planographic printing plate |
| JP2009067826A (en) | 2007-09-10 | 2009-04-02 | Nippon Shokubai Co Ltd | Curable composition |
| ES2361861T3 (en) * | 2007-10-24 | 2011-06-22 | Agfa Graphics N.V. | CURABLE LIQUIDS AND INKS FOR APPLICATIONS TO FOOD TOYS AND PACKAGING. |
| JP2010132787A (en) | 2008-12-05 | 2010-06-17 | Seiko Epson Corp | Ink composition for inkjet recording, ink set, ink cartridge, and apparatus for inkjet recording |
| RU2561095C2 (en) * | 2009-08-21 | 2015-08-20 | Серикол Лимитед | Ink, printing device and method |
| WO2011084833A1 (en) | 2010-01-11 | 2011-07-14 | Isp Investments Inc. | Compositions comprising a reactive monomer and uses thereof |
| JP2011241323A (en) | 2010-05-19 | 2011-12-01 | Seiko Epson Corp | Ink composition set, ink recording method using the ink composition set, and recorded matter |
| JP5632670B2 (en) | 2010-07-27 | 2014-11-26 | 富士フイルム株式会社 | Inkjet recording method |
| JP5650049B2 (en) | 2010-07-29 | 2015-01-07 | 富士フイルム株式会社 | Inkjet recording method and printed matter |
| JP5613042B2 (en) | 2010-12-28 | 2014-10-22 | 富士フイルム株式会社 | Ink set for inkjet recording and inkjet recording method |
| JP6209934B2 (en) | 2013-10-23 | 2017-10-11 | 株式会社リコー | Photopolymerizable inkjet ink, ink cartridge, and method for forming image or cured product |
-
2018
- 2018-02-09 SG SG11201906757PA patent/SG11201906757PA/en unknown
- 2018-02-09 AU AU2018219661A patent/AU2018219661B2/en active Active
- 2018-02-09 WO PCT/EP2018/053304 patent/WO2018146259A1/en active Search and Examination
- 2018-02-09 JP JP2019543247A patent/JP7206204B2/en active Active
- 2018-02-09 KR KR1020197026073A patent/KR102575388B1/en active IP Right Grant
- 2018-02-09 US US16/484,896 patent/US10745576B2/en active Active
- 2018-02-09 EP EP18703337.8A patent/EP3580287B1/en active Active
- 2018-02-09 BR BR112019015727-1A patent/BR112019015727B1/en active IP Right Grant
- 2018-02-09 MY MYPI2019004420A patent/MY193850A/en unknown
- 2018-02-09 TW TW107104609A patent/TWI815805B/en active
- 2018-02-09 ES ES18703337T patent/ES2926235T3/en active Active
- 2018-02-09 RU RU2019128254A patent/RU2769444C2/en active
- 2018-02-09 CA CA3053037A patent/CA3053037A1/en active Pending
- 2018-02-09 CN CN201880010929.9A patent/CN110291162A/en active Pending
- 2018-02-09 MX MX2019009563A patent/MX2019009563A/en unknown
-
2019
- 2019-09-04 ZA ZA2019/05835A patent/ZA201905835B/en unknown
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0425441A2 (en) * | 1989-10-27 | 1991-05-02 | Ciba-Geigy Ag | Photosensitive mixture |
| US5629359A (en) | 1994-05-31 | 1997-05-13 | U C B S.A. | Radiation curable compositions |
| JP2010248310A (en) * | 2009-04-13 | 2010-11-04 | Toagosei Co Ltd | Composition for use in active energy ray-curable-type covering material, containing unsaturated compound with nitrogen-containing heterocycle |
| JP2011178863A (en) | 2010-02-26 | 2011-09-15 | Nippon Shokubai Co Ltd | Active energy ray-curable composition, curable resin composition and cured product |
| GB2517592A (en) | 2013-08-05 | 2015-02-25 | Sericol Ltd | Method for designing inks |
| WO2015022228A1 (en) | 2013-08-12 | 2015-02-19 | Basf Se | Ink-jet printing ink comprising n-vinyloxazolidinone |
| WO2015140539A1 (en) | 2014-03-17 | 2015-09-24 | Fujifilm Speciality Ink Systems Limited | Printing ink |
| WO2015140538A1 (en) | 2014-03-17 | 2015-09-24 | Fujifilm Speciality Ink Systems Limited | Printing ink |
| WO2015140541A1 (en) | 2014-03-20 | 2015-09-24 | Fujifilm Speciality Ink Systems Limited | Printing ink |
| WO2015140540A1 (en) | 2014-03-20 | 2015-09-24 | Fujifilm Speciality Ink Systems Limited | Printing ink |
| WO2015148094A1 (en) | 2014-03-28 | 2015-10-01 | Sun Chemical Corporation | Low migration radiation curable inks |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019077364A1 (en) * | 2017-10-20 | 2019-04-25 | Fujifilm Speciality Ink Systems Limited | A printing ink |
| WO2020064523A1 (en) | 2018-09-24 | 2020-04-02 | Basf Se | Uv curable composition for use in 3d printing |
| CN112639034A (en) * | 2018-09-24 | 2021-04-09 | 巴斯夫欧洲公司 | UV curable composition for 3D printing |
| US11999865B2 (en) | 2018-09-24 | 2024-06-04 | Basf Se | UV curable composition for use in 3D printing |
| JP7256592B2 (en) | 2019-03-06 | 2023-04-12 | 富士フイルム株式会社 | Inkjet ink composition, image recording method and image recorded matter |
| WO2020179155A1 (en) * | 2019-03-06 | 2020-09-10 | 富士フイルム株式会社 | Ink-jet ink composition, method for recording image, and object with recorded image |
| CN113544224A (en) * | 2019-03-06 | 2021-10-22 | 富士胶片株式会社 | Inkjet ink composition, image recording method, and image recorded matter |
| JPWO2020179155A1 (en) * | 2019-03-06 | 2021-12-23 | 富士フイルム株式会社 | Inkjet ink composition, image recording method and image recording material |
| WO2022055625A1 (en) * | 2020-09-10 | 2022-03-17 | Sun Chemical Corporation | Led energy curable ink compositions |
| US20230303868A1 (en) * | 2020-09-10 | 2023-09-28 | Sun Chemical Corporation | Led energy curable ink compositions |
| US11993721B2 (en) | 2020-09-10 | 2024-05-28 | Sun Chemical Corporation | LED energy curable ink compositions |
| WO2022179880A1 (en) | 2021-02-26 | 2022-09-01 | Agfa Nv | Ink set and inkjet printing methods |
| WO2022179879A1 (en) | 2021-02-26 | 2022-09-01 | Agfa Nv | Manufacturing methods of decorative surfaces |
| EP4050072A1 (en) | 2021-02-26 | 2022-08-31 | Agfa Nv | Manufacturing methods of decorative surfaces |
| EP4050073A1 (en) | 2021-02-26 | 2022-08-31 | Agfa Nv | Ink set and inkjet printing methods |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2020507658A (en) | 2020-03-12 |
| MY193850A (en) | 2022-10-28 |
| CN110291162A (en) | 2019-09-27 |
| RU2019128254A3 (en) | 2021-05-18 |
| AU2018219661B2 (en) | 2022-12-01 |
| MX2019009563A (en) | 2019-10-30 |
| KR20190112120A (en) | 2019-10-02 |
| TWI815805B (en) | 2023-09-21 |
| JP7206204B2 (en) | 2023-01-17 |
| CA3053037A1 (en) | 2018-08-16 |
| RU2769444C2 (en) | 2022-03-31 |
| AU2018219661A1 (en) | 2019-08-22 |
| BR112019015727B1 (en) | 2023-03-28 |
| TW201835244A (en) | 2018-10-01 |
| EP3580287B1 (en) | 2022-06-22 |
| ZA201905835B (en) | 2022-05-25 |
| KR102575388B1 (en) | 2023-09-08 |
| US20190375954A1 (en) | 2019-12-12 |
| RU2019128254A (en) | 2021-03-10 |
| EP3580287A1 (en) | 2019-12-18 |
| BR112019015727A2 (en) | 2020-03-24 |
| ES2926235T3 (en) | 2022-10-24 |
| SG11201906757PA (en) | 2019-08-27 |
| US10745576B2 (en) | 2020-08-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2018219661B2 (en) | Acrylate-based monomers for use as reactive diluents in printing formulations | |
| AU2018219660B2 (en) | Acrylate-based monomers for use as reactive diluents in printing formulations | |
| TWI847957B (en) | Energy curable compositions comprising polymeric aminoacrylates | |
| TWI783919B (en) | Polymeric aminoacrylates | |
| WO2007125273A1 (en) | A printing ink | |
| EP2010615A1 (en) | A printing ink | |
| JP2021098770A (en) | Active energy ray-curable ink composition and printed matter of the same | |
| BR112019015888B1 (en) | COMPOSITION, USE OF A COMPOSITION, AND METHOD FOR PRINTING | |
| JP7236578B1 (en) | Actinic energy ray-curable offset printing ink, printed matter using the same, and method for producing printed matter | |
| DE202020001249U1 (en) | Hydroxyalkyl acrylate based monomers for use as reactive diluents in printing formulations |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18703337 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112019015727 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 3053037 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2019543247 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2018219661 Country of ref document: AU Date of ref document: 20180209 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 20197026073 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2018703337 Country of ref document: EP Effective date: 20190910 |
|
| ENP | Entry into the national phase |
Ref document number: 112019015727 Country of ref document: BR Kind code of ref document: A2 Effective date: 20190730 |